
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci., 08 January 2025
Sec. Cellular Neuropathology
Volume 18 - 2024 | https://doi.org/10.3389/fncel.2024.1495224
This article is part of the Research TopicRole of Microbiota in Neurocognitive Disorders: A Developmental Origin PerspectiveView all 9 articles
 Ines Hadrich1
Ines Hadrich1 Mariem Turki2,3
Mariem Turki2,3 Imen Chaari2,3
Imen Chaari2,3 Balkiss Abdelmoula4
Balkiss Abdelmoula4 Rahma Gargouri5
Rahma Gargouri5 Nahed Khemakhem1
Nahed Khemakhem1 Dhawia Elatoui3
Dhawia Elatoui3 Fatma Abid4
Fatma Abid4 Sonda Kammoun4,6
Sonda Kammoun4,6 Mona Rekik4,6
Mona Rekik4,6 Samir Aloulou4,7
Samir Aloulou4,7 Mariem Sehli4,6
Mariem Sehli4,6 Aymen Ben Mrad4,6
Aymen Ben Mrad4,6 Sourour Neji1
Sourour Neji1 Fabian M. Feiguin8
Fabian M. Feiguin8 Jihene Aloulou2,3
Jihene Aloulou2,3 Nouha Bouayed Abdelmoula4
Nouha Bouayed Abdelmoula4 Hayet Sellami3,9*
Hayet Sellami3,9*Background: The human gut mycobiome, a minor but integral component of the gut microbiome, has emerged as a significant player in host homeostasis and disease development. While bacteria have traditionally been the focus of gut microbiome studies, recent evidence suggests that fungal communities (mycobiota) may also play a crucial role in modulating health, particularly in neuropsychiatric disorders.
Objective: This review aims to provide a comprehensive overview of current knowledge on the relationship between the gut mycobiome and neuropsychiatric disorders, exploring the potential of targeting fungal communities as a novel therapeutic strategy.
Methods: We summarized recent findings from metagenomic analyses that characterize the diversity and composition of gut mycobiota and discuss how these communities interact with the host and other microorganisms via the gut-brain axis. Key methodologies for studying mycobiota, such as high-throughout sequencing and bioinformatics approaches, were also reviewed to highlight advances in the field.
Results: Emerging research links gut mycobiota dysbiosis to conditions such as schizophrenia, Alzheimer’s disease, autism spectrum disorders, bipolar disorder, and depression. Studies indicate that specific fungal populations, such as Candida and Saccharomyces, may influence neuroinflammation, gut permeability and immune responses, thereby affecting mental health outcomes.
Conclusion: Understanding the gut mycobiome’s role in neuropsychiatric disorders opens new avenues for therapeutic interventions, including antifungal treatments, probiotics, and dietary modifications. Future research should integrate multi-omics approaches to unravel the complex interkingdom interactions within the gut ecosystem, paving the way for personalized medicine in mental health care.
Over the past decade, the microbiota and its effect on health has been largely studied. Gut microbiota have been recognized as a crucial key regulator of health status, and, consequently, its alterations can lead to several diseases (D’Argenio et al., 2022). Researchers have primarily focused on gut bacterial microbiota, often overlooking the role of the fungal, the viruses and parasites fractions. In fact, despite the small fraction of fungi approximately 0.1% of the total microbial population in the gut (Chin et al., 2020), the secondary metabolites produced during interactions with bacteria and host cells are essential for immunological control and metabolic homeostasis.
A growing body of research highlighted the close relationship between gut microbiota and psychiatric disorders, with dysbiosis playing a pivotal role in conditions like depression, anxiety, Autism spectrum disorder (ASD), Attention-deficit/hyperactivity disorder (ADHD), and schizophrenia (SCZ) (Ahmed et al., 2024). In fact, dysbiosis of the normal gut microbiota can have negative consequences for humans, particularly during critical stages of life, as the gut microbes change with age in both phenotype and number of bacterial species. Alterations in the metabolites produced by the gut microbiota can impact neurological disorders, mental health conditions, and even euthymic states (Jones et al., 2019). Emerging evidence highlights that the gut mycobiome, like its bacterial counterpart, influences the microbiota-gut-brain axis and mental health outcomes, with shifts in fungal communities linked to conditions such as SCZ, mood disorders, and ASD.
In this review, we discuss the technological limitations in studying the gut mycobiota and summarized the recent findings on links between gut mycobiome and neuropsychiatric disorders.
Analysis of fungal (mycobiota) diversity over the past decade has provided significant insights into the role of microbial communities in human diseases (Cui et al., 2013; Kumpitsch et al., 2019; Tiew et al., 2020). The results of microbial diversity studies are heavily influenced by the methods. Innovative technologies such as next-generation sequencing methods, and metagenomic and amplicon sequencing, have revolutionized our ability to identify and characterize fungal species in the human body.
However, data on fungal communities are still limited, partly because fungi are more challenging to manipulate and analyze (Angebault et al., 2020).
Metagenomic study of mycobiota in human fecal samples involves several steps, including sample collection, nucleic acid extraction, library preparation, sequencing and bioinformatics analysis. Samples must be handled in sterile environments, with rapid refrigeration at -80°C to preserve microbiota diversity (Choo et al., 2015; Chiappori et al., 2022; Wang L.-J. et al., 2023). Nucleic acid extraction must effectively capture all fungi, despite the challenge of breaking the tough fungal cell wall, often requiring mechanical or enzymatic disruption (Nguyen et al., 2015; Halwachs et al., 2017; Vesty et al., 2017). For fungal profiling, both internal transcribed spacer (ITS), ITS1 and ITS2 sequencing are commonly used. Some studies have compared ITS1 and ITS2 for fungal profiling (Bazzicalupo et al., 2013; Blaalid et al., 2013; Monard et al., 2013; Wang et al., 2015; Yang et al., 2018). While some researchers found ITS2 to be more suitable for revealing richness (Bazzicalupo et al., 2013), others reported that ITS1 was likely the better choice for studying fungal and eukaryotic species (Yang et al., 2018).
Functional classification of metagenomics data is vital for investigating the functional and metabolic roles of microbiome member species, as well as their variations under different conditions/treatments. Overall, tools for functional classification share common features with tools used for whole genome analyses. Bioinformatics tools are essential for analyzing metagenomic data, including pre-processing, taxonomic classification, and statistical analysis. Various approaches, such as OTU clustering, can affect the results, and improving fungal databases is key to enhancing data accuracy. Tools like QIIME and mothur are commonly used, with specialized pipelines like LotuS and FindFungi improving fungal sequence classification (Donovan et al., 2018). Newer pipelines like DAnIEL support ITS analysis and allow for comparison with publicly available datasets (Loos et al., 2021).
These advancements in methodology and technology are crucial for studying the mycobiota and its potential links to brain health and behavior.
A summary of the key studies on the gut mycobiome’s role in neuropsychiatric disorders, including their methodologies and main findings, is presented in Table 1.
Schizophrenia (SCZ) is a chronic and severe mental disorder characterized by relapsing episodes, prolonged course, and residual symptoms. Symptoms of SCZ fall into three main categories: psychotic, negative, and cognitive. Psychotic symptoms include hallucinations—sensory experiences such as seeing, hearing, or feeling things that are not present—and delusions, involving strong, often irrational beliefs. Negative symptoms encompass reduced motivation, diminished interest in daily activities, social withdrawal, and challenges in emotional expression and daily functioning. Cognitive symptoms typically include difficulties with attention, concentration, and memory.
Prior studies have increasingly focused on the microbiome–gut–brain axis, a complex communication system linking the gastrointestinal (GI) tract to the brain, in relation to SCZ (Ju et al., 2023). While considerable attention has been directed at the bacterial components of the gut microbiota in SCZ, the fungal aspect, or mycobiome, remains less explored. Nonetheless, emerging evidence suggests a potential link between gut mycobiome dysbiosis and SCZ development.
Yuan et al. (2024) conducted an analysis of gut mycobiota in 109 chronic SCZ patients and 77 controls, identifying significant reductions in fungal diversity and an overrepresentation of Candida species linked to inflammatory processes. This study used ITS sequencing to profile fungal diversity. They observed significant alterations in the mycobiota composition in SCZ patients, marked by a reduced diversity of fungal species compared to healthy controls. This dysbiosis was characterized by a depletion of fungi, such as Saccharomyces cerevisiae, known for its immune-modulatory effects, potentially impacting gut homeostasis and immune regulation in these patients. On the other hand, Candida albicans, an opportunistic fungal species commonly found in the human GI tract, plays a central role in this fungal dysbiosis. The overgrowth of Candida albicans has been linked to chronic inflammatory states and increased gut permeability, promoting neuroinflammatory responses through the production of pro-inflammatory molecules such as β-glucans (Huffnagle and Noverr, 2013; Huang et al., 2024; Kearns, 2024; Villavicencio-Tejo et al., 2023; Yuan et al., 2024). Yuan et al. (2024) demonstrated that a Candida-dominant enterotype correlates with more severe psychotic symptoms and heightened systemic inflammation. In another study, Yuan et al. (2023) investigated the combined effects of genetic risk and mycobiome dysbiosis on SCZ risk among 137 drug-naïve, first-episode SCZ patients and 76 healthy controls. Patients with a higher Polygenic Risk Score (PRS), a measure quantifying the cumulative genetic predisposition to SCZ, exhibited a Candida-dominant enterotype (Yuan et al., 2023). These findings highlight the interplay between genetic predispositions, fungal dysbiosis, and disease severity. For a summary of observed dysbiosis patterns and their clinical implications, see Table 2.
Further research by Yuan et al. (2022) explored changes in mycobiome composition and fungi-bacteria interaction networks in 205 SCZ patients compared to 125 healthy controls, using 18S ribosomal RNA gene sequencing. The study found reduced fungal α-diversity in SCZ patients, along with an altered fungi-to-bacteria diversity ratio. Notably, higher levels of Purpureocillium were associated with more severe psychiatric symptoms and poorer cognitive function in SCZ patients, emphasizing the potential impact of specific fungal species on disease progression (Yuan et al., 2022).
In an earlier study, Zhang et al. (2020) examined gut mycobiota dysbiosis in drug-naïve SCZ patients using ITS1-based DNA sequencing, comparing 10 SCZ patients to 16 healthy controls. The results revealed reduced fungal alpha diversity and altered composition, with higher levels of Chaetomium and lower levels of Trichoderma in SCZ patients. Additionally, SCZ patients exhibited an intensified bacteria-fungi interaction network, suggesting disrupted interkingdom interactions within the gut microbiome, which may play a role in SCZ pathophysiology (Zhang et al., 2020).
Given the observed overrepresentation of Candida albicans and its role in systemic inflammation, therapeutic strategies such as antifungal agents or probiotics targeting fungal dysbiosis could help restore gut homeostasis. Early evidence suggests that probiotics like Saccharomyces boulardii may reduce fungal overgrowth and improve gut barrier integrity, potentially mitigating neuroinflammatory pathways linked to SCZ (Kelesidis and Pothoulakis, 2012). These findings underscore the potential of therapeutic strategies targeting fungal dysbiosis, such as probiotics, to mitigate inflammation and improve gut-brain axis health in SCZ.
A detailed overview of SCZ-related findings, methodologies, and main fungal communities identified is provided in Tables 1, 2.
Alzheimer’s disease (AD) is a chronic neurodegenerative condition characterized by amyloid beta plaques, neurofibrillary tangles, and both central and systemic inflammation, primarily affecting the medial temporal lobe and associative neocortical regions. Recently, the gut microbiome has gained attention as a modifiable risk factor for AD due to its bidirectional communication with the brain. Dysbiosis of the gut microbiota has been associated with the initiation and progression of neuroinflammatory processes that underlie AD (Seo and Holtzman, 2024). Emerging research suggests that the fungal component of the gut microbiome, or mycobiome, may also play a significant role in AD pathology (Seo and Holtzman, 2024).
A study by D’Argenio et al. (2022) analyzed the mycobiome of 3xTg-AD mice, a widely used model for AD, revealing a notable increase in fungi from the Dipodascaceae family compared to wild-type mice. To analyze the fungal composition, D’Argenio et al. (2022) utilized ITS sequencing in their assessment of the mycobiome within AD mouse models, revealing significant fungal shifts related to AD pathology. This family has been linked to metabolic disruptions, as it is also elevated in obese individuals and correlates with adiposity and cholesterol levels (Mar Rodríguez et al., 2015). Given the metabolic dysregulation observed in AD, D’Argenio et al. (2022) hypothesized that Dipodascaceae overgrowth may contribute to AD’s metabolic profile. However, further studies are needed to confirm these findings and elucidate the specific mechanisms involved (D’Argenio et al., 2022).
Dietary adjustments have shown potential in modulating the gut microbiome, which could impact AD progression. Nagpal et al. (2020) conducted a study on older adults with mild cognitive impairment (MCI) who followed either a modified Mediterranean ketogenic diet (MMKD) or the American Heart Association Diet (AHAD) for 6 weeks. Mycobiome analysis through fungal rRNA ITS1 gene sequencing revealed that MCI patients had higher proportions of fungal families and genera, including Sclerotiniaceae, Phaffomycetaceae, and Botrytis, compared to cognitively normal (CN) individuals. Notably, the MMKD intervention had a more substantial impact on the mycobiome than the AHAD, particularly in reducing levels of Botrytis, which was elevated in MCI patients at baseline. Additionally, the MMKD intervention led to an increase in Geotrichum abundance and a reduction in Saccharomyces, Zygosaccharomyces, and Aureobasidium, changes that may contribute to an improved gut environment. This suggests that diet-based interventions could modulate the gut mycobiome in ways that may benefit cognitive health. The study also highlighted correlations between specific fungal genera and Alzheimer’s biomarkers in cerebrospinal fluid (CSF). For example, Aspergillus and Meyerozyma were negatively correlated with amyloid-beta 40 and positively with tau protein levels in both CN and MCI patients, while Wallemia showed a negative correlation with tau markers in MCI but not CN subjects. These fungal biomarkers could help stratify patients based on their mycobiome profiles, enabling personalized dietary or pharmacological interventions (Nagpal et al., 2020).
While biomarkers derived from the gut mycobiome offer insights into systemic changes, the detection of fungal species directly within AD brains highlights a more localized impact, further implicating fungal dysbiosis in AD pathology. Phuna and Madhavan detected Candida spp., Malassezia spp., Cladosporium spp., and Alternaria spp. in AD brains, suggesting that these fungi may inadvertently reach the brain, especially in immunocompromised individuals, through a compromised epithelial barrier (Phuna and Madhavan, 2022). Candida species, for instance, may induce fungal glial granulomas with accumulated amyloid precursor protein, while Malassezia species can activate neuroinflammatory Th1 and Th17 immune responses. The roles of Cladosporium and Alternaria in AD are less understood, but these fungi may contribute to neuroinflammation and could potentially be involved in acetylcholinesterase inhibition. The presence of these fungi may collectively drive chronic neuroinflammation and neurodegeneration in AD. Additionally, targeting specific fungal biomarkers could provide a novel approach for early diagnosis and monitoring of disease progression (Nagpal et al., 2020).
Overall, these studies underscore the complex relationship between the gut mycobiome and AD, highlighting potential avenues for therapeutic interventions. Diet-based strategies, particularly those resembling the Mediterranean ketogenic diet, may offer benefits by modulating mycobiome composition. Additionally, mycobiome biomarkers could serve as tools for monitoring AD progression and treatment efficacy. As research progresses, a deeper understanding of these fungal contributions could lead to targeted approaches that address both bacterial and fungal dysbiosis in AD.
Key findings on fungal dysbiosis in AD, including methodologies and clinical implications, are summarized in Tables 1, 2.
Autism Spectrum Disorders (ASD) are complex neurodevelopmental conditions characterized by repetitive behaviors, restricted interests, and significant challenges in communication and social interaction (CDC, 2024). Despite extensive research, the exact causes of ASD remain elusive, though it is widely accepted that both genetic and environmental factors contribute to its development.
Recent studies have underscored a strong link between gastrointestinal (GI) issues and the severity of ASD symptoms. Individuals with ASD frequently experience a higher prevalence of GI disturbances, prompting researchers to explore the gut-brain axis and its potential role in ASD pathophysiology (Adams et al., 2011; Wang et al., 2011). Specifically, the gut microbiota has become a focal point of interest. Emerging evidence suggests that gut dysbiosis may correlate with the severity of ASD symptoms. This dysbiosis often manifests as reduced bacterial diversity and a decrease in beneficial commensal bacteria (Hughes et al., 2018). The mycobiome, the fungal component of the microbiome, has also gained attention, despite being previously overlooked in gut-brain axis research (Iovene et al., 2017; Strati et al., 2017).
In ASD, the fungal families Saccharomycetaceae and Debaryomycetaceae have been observed at altered levels. Saccharomycetaceae, which includes common yeast species, may play a role in GI issues frequently reported in ASD, potentially through interactions that influence gut motility and permeability. The presence of Debaryomycetaceae may contribute to neurobehavioral symptoms by affecting microbial metabolites that impact neurodevelopmental pathways (Strati et al., 2017; Zou et al., 2021; Alharthi et al., 2022)?. These alterations suggest that fungal communities may play a role in the severity of ASD-related GI issues and behavioral symptoms. This study used next-generation sequencing (NGS) techniques to evaluate fungal diversity, identifying associations between specific fungal taxa, like Saccharomycetaceae, and autism symptom severity (Strati et al., 2017).
The role of Candida species in ASD appears limited compared to other taxa. In ASD, although no significant differences in overall Candida abundance were observed compared to typically developing children, Candida albicans exacerbated GI symptoms in some cases (Strati et al., 2017). Therefore, targeting the mycobiome could represent a promising therapeutic approach for alleviating GI symptoms in children with ASD, potentially reducing symptom severity and enhancing quality of life (Strati et al., 2017). For ASD, targeted therapies such as probiotics or carefully monitored antifungal agents could help alleviate symptoms. However, their application requires caution due to potential toxicity and antifungal resistance (Alookaran et al., 2022). Emerging research suggests that restoring fungal balance through diet or fecal microbiota transplantation (FMT) may also hold promise in improving ASD outcomes (Strati et al., 2017; Wang L.-J. et al., 2023). Developing improved research methodologies will be crucial to optimizing these treatments and ensuring their efficacy and safety in managing ASD symptoms (Lewandowska-Pietruszka et al., 2023). A summary of ASD-associated fungal species and their potential neurobehavioral impacts can be found in Table 2.
In a broader point of view, recent research has highlighted the complex interactions between the gut microbiota and mycobiota, suggesting that these interactions might contribute significantly to ASD symptom severity. For instance, imbalances between bacterial and fungal populations in the gut may affect host microRNA expression, which in turn could regulate bacterial growth and contribute to ASD development (Yap et al., 2021). Additionally, small non-coding RNAs (sncRNAs), including microRNAs (miRNAs) and piwi-interacting RNAs (piRNAs), have been detected in fecal samples from individuals with ASD, suggesting a potential mechanism by which gut dysbiosis could exacerbate ASD symptoms through transcriptional regulation and inflammatory processes (Chiappori et al., 2022). These findings suggest that targeting gut dysbiosis may indirectly modulate RNA-mediated inflammatory pathways, offering a novel avenue for therapeutic research.
Dysbiosis within the gut mycobiome has been associated with neuroinflammation and neurotransmitter disruptions (specifically disruptions in serotonin and dopamine pathways), which are relevant to the pathophysiology of mood disorders such as depression and bipolar disorder (McGuinness et al., 2024). For instance, increased levels of Candida and reduced diversity of other beneficial fungi have been observed in mood disorder patients, which may exacerbate mood dysregulation. Therapeutic strategies that target these fungal imbalances, such as probiotics or antifungal agents, could help restore a healthier microbiome balance and offer new avenues for managing mood disorder symptoms (McGuinness et al., 2024). These interventions may work by reducing systemic inflammation and restoring neurotransmitter homeostasis, thus alleviating mood disorder symptoms.
Key findings and fungal species associated with mood disorders are listed in Table 1 and summarized by clinical implications in Table 2.
Bipolar disorder (BD), previously known as manic-depressive illness, is a mental health condition characterized by extreme mood swings, changes in energy levels, activity, and concentration, significantly impacting daily functioning. Emerging research on the gut-brain axis suggests a potential role of the gut mycobiome in the development of BD.
While most research on BD and the gut-brain axis has concentrated on bacterial communities, this section emphasizes the emerging role of fungal dysbiosis, particularly involving Candida albicans, in mood stabilization and neuroinflammation. Candida albicans, discussed as a key species in fungal dysbiosis, contributes to chronic inflammation and mood dysregulation in BD. This specific dysbiosis, characterized by reduced fungal diversity, highlights Candida albicans as a key species potentially contributing to chronic inflammation and mood dysregulation in affected patients (McGuinness et al., 2022). Current literature suggests a possible relationship between mycobiome composition changes and BD symptoms. Additionally, as the gut mycobiome may influence the absorption of psychopharmacological treatments, adjusting its composition could improve BD symptoms (Lai et al., 2022). Emerging evidence also indicates that mycobiome-based biomarkers could be valuable for diagnosing BD and predicting treatment responses. From a therapeutic perspective, interventions targeting the mycobiome, such as probiotics and FMT, have shown potential for stabilizing mood and reducing BD symptoms. Modulating the mycobiome presents a promising strategy for managing BD (McGuinness et al., 2022). Additionally, studies have explored the role of Candida albicans in cognitive deficits and inflammation, which are common in both BD and SCZ. These findings suggest that fungal dysbiosis contributes to neuroinflammation and neurotransmitter disruptions, potentially aggravating BD symptoms (Musumeci et al., 2022). Also, mycobiome-specific probiotics could complement psychopharmacological treatments by addressing gut inflammation and improving drug absorption (Nagpal et al., 2019). Finally, causal analyses have identified a genetic-level link between gut microbiota and BD risk, emphasizing the need to explore fungal contributions within this framework (Xu et al., 2023).
Dysbiosis has been described in patients experiencing current depressive episodes (CDE). Metagenomic and metabolomic analyses have been employed to better understand the structure and potential functions of the gut mycobiome and its impact on CDE development. In patients with CDE, the gut mycobiota is marked by a relative reduction in alpha diversity and altered composition, particularly higher levels of Candida and lower levels of Penicillium compared to healthy controls. The gut microbiota in these patients also shows significant disruptions in the bacteria-fungi correlation network, indicating altered interkingdom interactions (Jiang et al., 2020). Additionally, the potential of microbial alterations as biomarkers for diagnosing depressive episodes and predicting treatment responses has been highlighted (Jiang et al., 2020). Further research has shown that the intricate interactions between bacteria and fungi in the gut are crucial for maintaining overall gut health, and disruptions in these interactions are common in depressive disorders. These disturbances can exacerbate depressive symptoms and contribute to the systemic inflammation often observed in depression (Jiang et al., 2020).
However, the bidirectional nature of these alterations raises important questions. It remains unclear whether fungal dysbiosis actively drives depressive pathophysiology or arises as a secondary effect of depression-related parameters such as altered diet, medication, and stress. Additionally, while Candida has been implicated in neuroinflammatory processes, the functional roles of other fungi, such as Penicillium, in gut-brain axis regulation remain speculative and warrant further investigation.
Attention-Deficit/Hyperactivity Disorder (ADHD) is a common childhood mental disorder with still poorly understood pathophysiological mechanisms. ADHD is characterized by persistent patterns of inattention and/or hyperactivity-impulsivity that interfere with daily functioning and development. Recent studies suggest that gut mycobiome and immune dysfunction may influence brain functions and social behaviors associated with ADHD.
Research exploring the gut mycobiome in ADHD using next-generation sequencing (NGS) platforms has highlighted potential links between fungal dysbiosis and ADHD susceptibility. At the phylum level, ADHD patients showed a significantly higher abundance of Ascomycota and a lower abundance of Basidiomycota compared to controls. At the genus level, Candida, particularly Candida albicans, was notably more abundant in ADHD patients (Wang L.-J. et al., 2023). Higher levels of Candida albicans have been associated with increased intestinal permeability, potentially influencing neurodevelopmental processes through systemic inflammation (Wang L.-J. et al., 2023). Such dysbiosis in the fungal mycobiome may contribute to ADHD-related behavioral symptoms through gut-brain axis interactions. Consequently, interventions targeting fungal dysbiosis could prove beneficial. Probiotics or synbiotics tailored to reduce fungal overgrowth and enhance gut barrier function warrant further investigation (Kelesidis and Pothoulakis, 2012).
Overall, this fungal imbalance may influence neurodevelopmental pathways relevant to ADHD. This underscores the potential of gut-targeted therapies, such as probiotics or antifungal agents, to address gut permeability issues and support neurodevelopmental health in ADHD patients.
Details on fungal dysbiosis in ADHD are consolidated in Tables 1, 2.
The fungal dysbiosis associated with alcohol dependence is still under investigation, but it is hypothesized that fungi can exploit disrupted bacterial homeostasis, often leading to fungal overgrowth following bacterial dysbiosis (Kakiyama et al., 2014; Bajaj et al., 2017; Yan et al., 2017; Chu et al., 2019; Lang et al., 2020). In alcohol dependence, fungal genera such as Candida and Pichia are frequently overrepresented. Candida, particularly Candida albicans, is known to disrupt gut barrier function, leading to increased gut permeability and systemic inflammation that may exacerbate alcohol cravings. Pichia species are thought to thrive in the altered gut environments found in alcohol-dependent individuals, further disturbing microbial homeostasis and potentially impacting addiction pathways via inflammatory and neurochemical changes (Rosenbach et al., 2010; Wang et al., 2014; Yang et al., 2017). Recent experiments suggest that gut dysbiosis in alcohol-dependent patients can promote anxiety-like and depression-like behaviors in animal models, potentially facilitating alcohol dependence by influencing cholecystokinin (CCK) and related receptors (Wang C. et al., 2023). Altered gut microbiome composition in alcoholism, along with compromised intestinal barrier integrity, may also worsen central nervous system symptoms, leading to depression, anxiety, and alcohol cravings (Leclercq et al., 2014). Additionally, the role of fungal dysbiosis in alcoholic liver disease (ALD) has gained attention. Studies have found that Candida and Pichia are dominant in the mycobiomes of patients with ALD, with these fungi accumulating β-glucan in the liver, potentially driving inflammation and exacerbating liver disease (Yang et al., 2017). Furthermore, Candida albicans exotoxin, Candida lysin, has been shown to damage liver cells and increase inflammation in alcoholic mouse models, worsening liver disease (Chu et al., 2019). FMT or antifungal therapies targeting these fungal species could complement current addiction treatments by addressing gut-derived inflammation (Bibbò et al., 2020; Chu et al., 2020).
These findings highlight the potential of gut mycobiome modulation as an adjunct to existing treatments, offering a comprehensive approach to managing both the physiological and behavioral aspects of alcohol dependence. For a summary of mycobiome findings in alcohol dependence, including clinical implications of fungal dysbiosis, refer to Tables 1, 2.
The gut mycobiome is emerging as a critical component of the gut-brain axis, with mounting evidence linking fungal dysbiosis to neuropsychiatric disorders such as SCZ, depression, and BD, as depicted in Figure 1. While bacterial microbiota has been extensively studied, fungi exhibit distinct biological properties, including the ability to persist in hostile environments, produce pro-inflammatory molecules like β-glucans, and interact directly with host epithelial and immune cells (Brown et al., 2003; Romani, 2011). These unique fungal traits may explain their role in modulating chronic inflammation and neurotransmitter pathways, differentiating their contributions from those of bacterial communities.
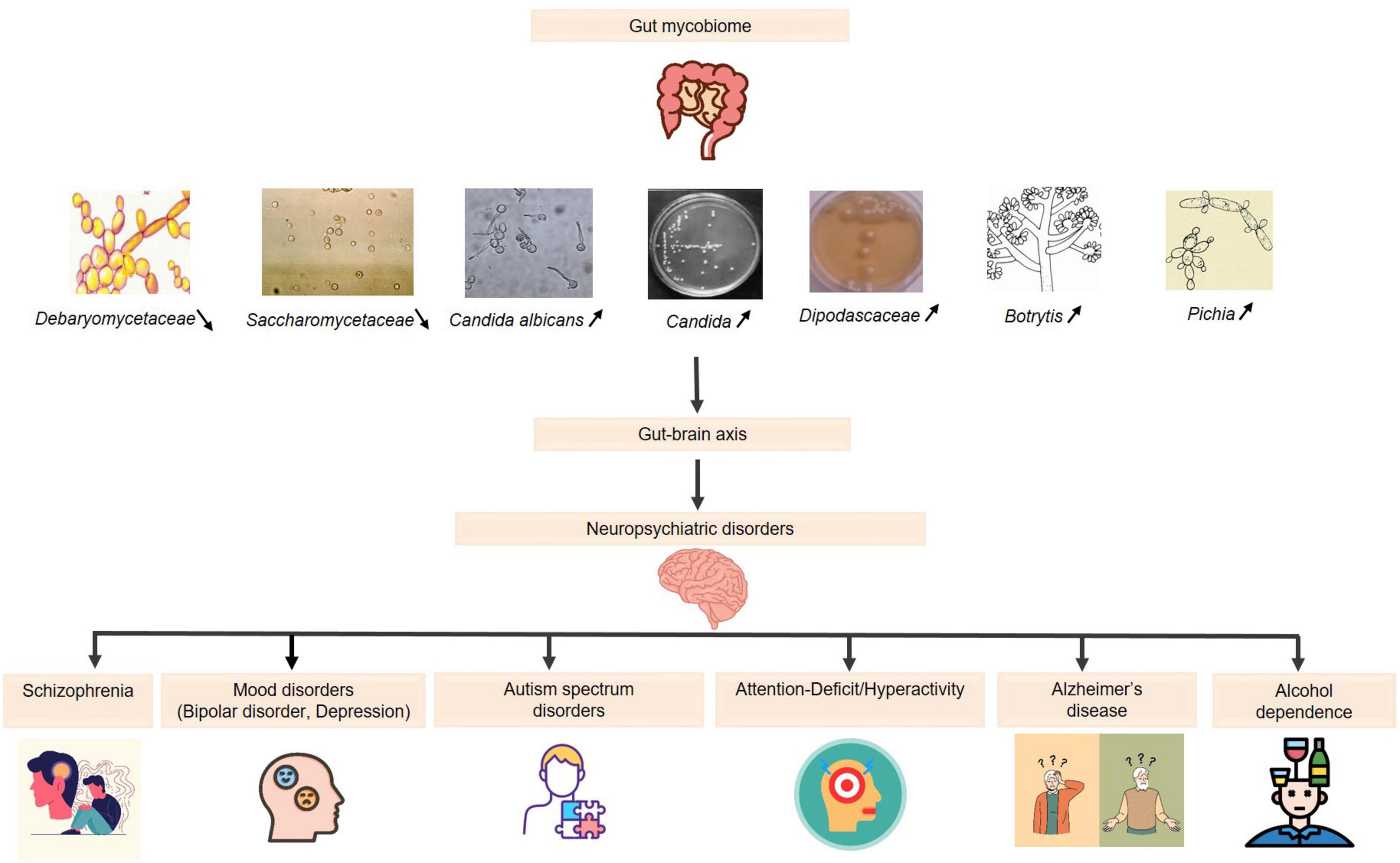
Figure 1. The role of the gut mycobiome in neuropsychiatric disorders via the gut-brain axis. Increased levels  : (Candida, Candida albicans, Dipodascaceae, Botrytis, Pichia): reduction in the diversity of beneficial fungi: immune system imbalance and disruption of neurotransmitter pathways → effectiveness of psychopharmacological treatments. Decreased levels
: (Candida, Candida albicans, Dipodascaceae, Botrytis, Pichia): reduction in the diversity of beneficial fungi: immune system imbalance and disruption of neurotransmitter pathways → effectiveness of psychopharmacological treatments. Decreased levels  : (Saccharomycetaceae, Debaryomycetaceae): affecting gut homeostasis → potential neurobehavioral consequences.
: (Saccharomycetaceae, Debaryomycetaceae): affecting gut homeostasis → potential neurobehavioral consequences.
Current studies reveal a connection between reduced fungal diversity and mood-related disorders, suggesting that specific fungal taxa may influence disease progression. However, the causal relationships between fungal dysbiosis and neuropsychiatric outcomes remain unclear. Additionally, the interplay between fungal and bacterial communities in shaping the gut-brain axis is underexplored, limiting our ability to fully understand the synergistic effects.
While biomarkers derived from the gut mycobiome show promise for early diagnosis and treatment monitoring, their clinical utility remains unproven. Longitudinal studies and multi-omic approaches are urgently needed to validate these findings and establish the mechanistic pathways underlying these associations.
Therapeutic strategies addressing fungal dysbiosis—such as probiotics, dietary interventions, or antifungal therapies—show promise in mitigating the impact of the gut mycobiome on neuropsychiatric disorders. These approaches, discussed in the specific sections, underscore the need for further clinical validation and personalized interventions. Incorporating the mycobiome as a biomarker, as highlighted in public health studies (Buytaers et al., 2024), could also pave the way for stratified therapies and better health monitoring in neuropsychiatric populations.
Advancing our understanding of the gut mycobiome in neuropsychiatric disorders will require comprehensive, interdisciplinary research. Multi-omic frameworks combining metagenomics, metabolomics, and transcriptomics should be prioritized to capture the complexity of fungal-bacterial interactions. Moreover, linking genetic predispositions, such as polygenic risk scores (PRS), with mycobiome data could enhance our understanding of disease susceptibility and progression. Finally, identifying reliable fungal biomarkers remains a critical objective for advancing diagnostic and therapeutic innovations in neuropsychiatry.
The gut mycobiome is an underexplored but critical aspect of the gut-brain axis, offering unique insights into the pathophysiology of neuropsychiatric disorders such as SCZ, depression, and BD. Recent studies have illuminated the potential role of fungal dysbiosis, particularly involving Candida albicans, in driving neuroinflammatory pathways, neurotransmitter imbalances, and mood dysregulation. These findings underscore the importance of integrating fungal communities into the broader microbiome research framework, which has historically focused on bacteria. Targeted modulation of the mycobiome holds significant promise for the development of innovative therapeutic approaches, ranging from antifungal agents and probiotics to FMT. However, these interventions require rigorous validation through clinical trials, and their mechanisms of action must be better understood. The identification of fungal biomarkers could revolutionize the diagnosis, monitoring, and treatment of neuropsychiatric disorders, bringing us closer to the era of precision psychiatry. Despite these advances, substantial gaps in our understanding persist. The complex interplay between fungal and bacterial communities, their interactions with host genetics, and their combined impact on neuropsychiatric outcomes remain largely unexplored. Addressing these challenges will require interdisciplinary research efforts that incorporate multi-omic approaches, longitudinal studies, and robust clinical trials. In conclusion, the gut mycobiome represents a new frontier in neuropsychiatric research, with the potential to unlock transformative insights into disease mechanisms and therapeutic strategies. By bridging existing knowledge gaps, future studies could establish the mycobiome as a cornerstone of personalized medicine in psychiatry, improving outcomes for millions of patients worldwide.
IH: Conceptualization, Validation, Writing – review and editing, Methodology, Supervision. MT: Conceptualization, Validation, Writing – original draft, Writing – review and editing. IC: Writing – original draft, Writing – review and editing. BA: Methodology, Project administration, Writing – review and editing. RG: Methodology, Writing – review and editing. NK: Writing – original draft, Writing – review and editing. DE: Formal Analysis, Writing – original draft. FA: Formal Analysis, Writing – review and editing. SK: Methodology, Writing – review and editing. MR: Writing – review and editing. SA: Writing – review and editing. MS: Writing – review and editing. AM: Writing – review and editing. SN: Writing – review and editing, Validation, Conceptualization. FF: Validation, Writing – review and editing. JA: Supervision, Validation, Writing – review and editing, Conceptualization. NA: Methodology, Project administration, Validation, Writing – review and editing. HS: Conceptualization, Methodology, Validation, Writing – review and editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, J. B., Johansen, L. J., Powell, L. D., Quig, D., and Rubin, R. A. (2011). Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11:22. doi: 10.1186/1471-230X-11-22
Ahmed, G. K., Ramadan, H. K.-A., Elbeh, K., and Haridy, N. A. (2024). Bridging the gap: Associations between gut microbiota and psychiatric disorders. Middle East Curr. Psychiatry 31:2. doi: 10.1186/s43045-024-00395-9
Alharthi, A., Alhazmi, S., Alburae, N., and Bahieldin, A. (2022). The human gut microbiome as a potential factor in autism spectrum disorder. Int. J. Mol. Sci. 23:1363. doi: 10.3390/ijms23031363
Alookaran, J., Liu, Y., Auchtung, T. A., Tahanan, A., Hessabi, M., Asgarisabet, P., et al. (2022). Fungi: Friend or foe? A mycobiome evaluation in children with autism and gastrointestinal symptoms. J. Pediatr. Gastroenterol. Nutr. 74, 377–382. doi: 10.1097/MPG.0000000000003349
Angebault, C., Payen, M., Woerther, P.-L., Rodriguez, C., and Botterel, F. (2020). Combined bacterial and fungal targeted amplicon sequencing of respiratory samples: Does the DNA extraction method matter? PLoS One 15:e0232215. doi: 10.1371/journal.pone.0232215
Bajaj, J. S., Kassam, Z., Fagan, A., Gavis, E. A., Liu, E., Cox, I. J., et al. (2017). Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 66, 1727–1738. doi: 10.1002/hep.29306
Bazzicalupo, A. L., Bálint, M., and Schmitt, I. (2013). Comparison of ITS1 and ITS2 rDNA in 454 sequencing of hyperdiverse fungal communities. Fung. Ecol. 6, 102–109. doi: 10.1016/j.funeco.2012.09.003
Bibbò, S., Settanni, C. R., Porcari, S., Bocchino, E., Ianiro, G., Cammarota, G., et al. (2020). Fecal microbiota transplantation: Screening and selection to choose the optimal donor. J. Clin. Med. 9:1757. doi: 10.3390/jcm9061757
Blaalid, R., Kumar, S., Nilsson, R. H., Abarenkov, K., Kirk, P. M., and Kauserud, H. (2013). ITS1 versus ITS2 as DNA metabarcodes for fungi. Mol. Ecol. Resour. 13, 218–224. doi: 10.1111/1755-0998.12065
Brown, G. D., Herre, J., Williams, D. L., Willment, J. A., Marshall, A. S. J., and Gordon, S. (2003). Dectin-1 mediates the biological effects of beta-glucans. J. Exp. Med. 197, 1119–1124. doi: 10.1084/jem.20021890
Buytaers, F. E., Berger, N., Van der Heyden, J., Roosens, N. H. C., and De Keersmaecker, S. C. J. (2024). The potential of including the microbiome as biomarker in population-based health studies: Methods and benefits. Front. Public Health 12:1467121. doi: 10.3389/fpubh.2024.1467121
CDC (2024). Clinical testing and diagnosis for autism spectrum disorder. Autism spectrum disorder (ASD). Available online at: https://www.cdc.gov/autism/hcp/diagnosis/index.html (accessed August 28, 2024).
Chiappori, F., Cupaioli, F. A., Consiglio, A., Di Nanni, N., Mosca, E., Licciulli, V. F., et al. (2022). Analysis of faecal microbiota and small ncRNAs in autism: Detection of miRNAs and piRNAs with possible implications in host–gut microbiota cross-talk. Nutrients 14:1340. doi: 10.3390/nu14071340
Chin, V. K., Yong, V. C., Chong, P. P., Amin Nordin, S., Basir, R., and Abdullah, M. (2020). Mycobiome in the gut: A multiperspective review. Mediators Inflamm. 2020:9560684. doi: 10.1155/2020/9560684
Choo, J. M., Leong, L. E., and Rogers, G. B. (2015). Sample storage conditions significantly influence faecal microbiome profiles. Sci. Rep. 5:16350. doi: 10.1038/srep16350
Chu, H., Duan, Y., Lang, S., Jiang, L., Wang, Y., Llorente, C., et al. (2020). The Candida albicans exotoxin candidalysin promotes alcohol-associated liver disease. J. Hepatol. 72, 391–400. doi: 10.1016/j.jhep.2019.09.029
Chu, H., Duan, Y., Yang, L., and Schnabl, B. (2019). Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut 68, 359–370. doi: 10.1136/gutjnl-2018-316307
Cui, L., Morris, A., and Ghedin, E. (2013). The human mycobiome in health and disease. Genome Med. 5:63. doi: 10.1186/gm467
D’Argenio, V., Veneruso, I., Gong, C., Cecarini, V., Bonfili, L., and Eleuteri, A. M. (2022). Gut microbiome and mycobiome alterations in an in vivo model of Alzheimer’s disease. Genes (Basel) 13:1564. doi: 10.3390/genes13091564
Donovan, P. D., Gonzalez, G., Higgins, D. G., Butler, G., and Ito, K. (2018). Identification of fungi in shotgun metagenomics datasets. PLoS One 13:e0192898. doi: 10.1371/journal.pone.0192898
Halwachs, B., Madhusudhan, N., Krause, R., Nilsson, R. H., Moissl-Eichinger, C., Högenauer, C., et al. (2017). Critical issues in mycobiota analysis. Front. Microbiol. 8:180. doi: 10.3389/fmicb.2017.00180
Huang, Y., Wang, Y., Huang, X., and Yu, X. (2024). Unveiling the overlooked fungi: The vital of gut fungi in inflammatory bowel disease and colorectal cancer. Gut Pathogens 16:59. doi: 10.1186/s13099-024-00651-7
Huffnagle, G. B., and Noverr, M. C. (2013). The emerging world of the fungal microbiome. Trends Microbiol. 21, 334–341. doi: 10.1016/j.tim.2013.04.002
Hughes, H. K., Rose, D., and Ashwood, P. (2018). The gut microbiota and dysbiosis in autism spectrum disorders. Curr. Neurol. Neurosci. Rep. 18:81. doi: 10.1007/s11910-018-0887-6
Iovene, M. R., Bombace, F., Maresca, R., Sapone, A., Iardino, P., Picardi, A., et al. (2017). Intestinal dysbiosis and yeast isolation in stool of subjects with autism spectrum disorders. Mycopathologia 182, 349–363. doi: 10.1007/s11046-016-0068-6
Jiang, H., Pan, L., Zhang, X., Zhang, Z., Zhou, Y., and Ruan, B. (2020). Altered gut bacterial–fungal interkingdom networks in patients with current depressive episode. Brain Behav. 10:e01677. doi: 10.1002/brb3.1677
Jones, L., Kumar, J., Mistry, A., Sankar Chittoor Mana, T., Perry, G., Reddy, V. P., et al. (2019). The transformative possibilities of the microbiota and mycobiota for health, disease, aging, and technological innovation. Biomedicines 7:24. doi: 10.3390/biomedicines7020024
Ju, S., Shin, Y., Han, S., Kwon, J., Choi, T. G., Kang, I., et al. (2023). the gut–brain axis in schizophrenia: The implications of the gut microbiome and SCFA production. Nutrients 15:4391. doi: 10.3390/nu15204391
Kakiyama, G., Muto, A., Takei, H., Nittono, H., Murai, T., Kurosawa, T., et al. (2014). A simple and accurate HPLC method for fecal bile acid profile in healthy and cirrhotic subjects: Validation by GC-MS and LC-MS. J. Lipid Res. 55, 978–990. doi: 10.1194/jlr.D047506
Kearns, R. (2024). Gut–brain axis and neuroinflammation: The role of gut permeability and the kynurenine pathway in neurological disorders. Cell. Mol. Neurobiol. 44:64. doi: 10.1007/s10571-024-01496-z
Kelesidis, T., and Pothoulakis, C. (2012). Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Ther. Adv. Gastroenterol. 5, 111–125. doi: 10.1177/1756283X11428502
Kumpitsch, C., Koskinen, K., Schöpf, V., and Moissl-Eichinger, C. (2019). The microbiome of the upper respiratory tract in health and disease. BMC Biol. 17:87. doi: 10.1186/s12915-019-0703-z
Lai, J., Li, A., Jiang, J., Yuan, X., Zhang, P., Xi, C., et al. (2022). Metagenomic analysis reveals gut bacterial signatures for diagnosis and treatment outcome prediction in bipolar depression. Psychiatry Res. 307:114326. doi: 10.1016/j.psychres.2021.114326
Lang, S., Duan, Y., Liu, J., Torralba, M. G., Kuelbs, C., Ventura-Cots, M., et al. (2020). Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology 71, 522–538. doi: 10.1002/hep.30832
Leclercq, S., Matamoros, S., Cani, P. D., Neyrinck, A. M., Jamar, F., Stärkel, P., et al. (2014). Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. U.S.A 111, E4485–E4493. doi: 10.1073/pnas.1415174111
Lewandowska-Pietruszka, Z., Figlerowicz, M., and Mazur-Melewska, K. (2023). Microbiota in autism spectrum disorder: A systematic review. IJMS 24:16660. doi: 10.3390/ijms242316660
Loos, D., Zhang, L., Beemelmanns, C., Kurzai, O., and Panagiotou, G. (2021). DAnIEL: A user-friendly web server for fungal ITS amplicon sequencing data. Front. Microbiol. 12:720513. doi: 10.3389/fmicb.2021.720513
Mar Rodríguez, M., Pérez, D., Javier Chaves, F., Esteve, E., Marin-Garcia, P., Xifra, G., et al. (2015). Obesity changes the human gut mycobiome. Sci. Rep. 5:14600. doi: 10.1038/srep14600
McGuinness, A. J., Davis, J. A., Dawson, S. L., Loughman, A., Collier, F., O’Hely, M., et al. (2022). A systematic review of gut microbiota composition in observational studies of major depressive disorder, bipolar disorder and schizophrenia. Mol. Psychiatry 27, 1920–1935. doi: 10.1038/s41380-022-01456-3
McGuinness, A. J., Loughman, A., Foster, J. A., and Jacka, F. (2024). Mood disorders: The gut bacteriome and beyond. Biol. Psychiatry 95, 319–328. doi: 10.1016/j.biopsych.2023.08.020
Monard, C., Gantner, S., and Stenlid, J. (2013). Utilizing ITS1 and ITS2 to study environmental fungal diversity using pyrosequencing. FEMS Microbiol. Ecol. 84, 165–175. doi: 10.1111/1574-6941.12046
Musumeci, S., Coen, M., Leidi, A., and Schrenzel, J. (2022). The human gut mycobiome and the specific role of Candida albicans: Where do we stand, as clinicians? Clin Microbiol. Infect. 28, 58–63. doi: 10.1016/j.cmi.2021.07.034
Nagpal, R., Neth, B. J., Wang, S., Craft, S., and Yadav, H. (2019). Modified Mediterranean-ketogenic diet modulates gut microbiome and short-chain fatty acids in association with Alzheimer’s disease markers in subjects with mild cognitive impairment. EBioMedicine 47, 529–542. doi: 10.1016/j.ebiom.2019.08.032
Nagpal, R., Neth, B. J., Wang, S., Mishra, S. P., Craft, S., and Yadav, H. (2020). Gut mycobiome and its interaction with diet, gut bacteria and alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. EBioMedicine 59:102950. doi: 10.1016/j.ebiom.2020.102950
Nguyen, L. D. N., Viscogliosi, E., and Delhaes, L. (2015). The lung mycobiome: An emerging field of the human respiratory microbiome. Front. Microbiol. 6:89. doi: 10.3389/fmicb.2015.00089
Phuna, Z. X., and Madhavan, P. (2022). A closer look at the mycobiome in Alzheimer’s disease: Fungal species, pathogenesis and transmission. Eur. J. Neurosci. 55, 1291–1321. doi: 10.1111/ejn.15599
Romani, L. (2011). Immunity to fungal infections. Nat. Rev. Immunol. 11, 275–288. doi: 10.1038/nri2939
Rosenbach, A., Dignard, D., Pierce, J. V., Whiteway, M., and Kumamoto, C. A. (2010). Adaptations of Candida albicans for growth in the mammalian intestinal tract. Eukaryot. Cell 9, 1075–1086. doi: 10.1128/EC.00034-10
Seo, D.-O., and Holtzman, D. M. (2024). Current understanding of the Alzheimer’s disease-associated microbiome and therapeutic strategies. Exp. Mol. Med. 56, 86–94. doi: 10.1038/s12276-023-01146-2
Strati, F., Cavalieri, D., Albanese, D., De Felice, C., Donati, C., Hayek, J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5:24. doi: 10.1186/s40168-017-0242-1
Tiew, P. Y., Mac Aogain, M., Ali, N. A. B. M., Thng, K. X., Goh, K., Lau, K. J. X., et al. (2020). The mycobiome in health and disease: Emerging concepts, methodologies and challenges. Mycopathologia 185, 207–231. doi: 10.1007/s11046-019-00413-z
Vesty, A., Biswas, K., Taylor, M. W., Gear, K., and Douglas, R. G. (2017). Evaluating the impact of DNA extraction method on the representation of human oral bacterial and fungal communities. PLoS One 12:e0169877. doi: 10.1371/journal.pone.0169877
Villavicencio-Tejo, F., Olesen, M. A., Navarro, L., Calisto, N., Iribarren, C., García, K., et al. (2023). Gut-brain axis deregulation and its possible contribution to neurodegenerative disorders. Neurotoxicity Res. 42:4. doi: 10.1007/s12640-023-00681-0
Wang, C., Yan, J., Du, K., Liu, S., Wang, J., Wang, Q., et al. (2023). Intestinal microbiome dysbiosis in alcohol-dependent patients and its effect on rat behaviors. mBio 14, e2392–23. doi: 10.1128/mbio.02392-23
Wang, L. W., Tancredi, D. J., and Thomas, D. W. (2011). The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. J. Dev. Behav. Pediatr. 32, 351–360. doi: 10.1097/DBP.0b013e31821bd06a
Wang, L.-J., Li, S.-C., Yeh, Y.-M., Lee, S.-Y., Kuo, H.-C., and Yang, C.-Y. (2023). Gut mycobiome dysbiosis and its impact on intestinal permeability in attention-deficit/hyperactivity disorder. J. Child Psychol. Psychiatry 64, 1280–1291. doi: 10.1111/jcpp.13779
Wang, X.-C., Liu, C., Huang, L., Bengtsson-Palme, J., Chen, H., Zhang, J.-H., et al. (2015). ITS1: A DNA barcode better than ITS2 in eukaryotes? Mol. Ecol. Resour. 15, 573–586. doi: 10.1111/1755-0998.12325
Wang, Z. K., Yang, Y. S., Stefka, A. T., Sun, G., and Peng, L. H. (2014). Review article: Fungal microbiota and digestive diseases. Aliment Pharmacol. Ther. 39, 751–766. doi: 10.1111/apt.12665
Xu, R., Liu, S., Li, L., Zhang, Y., Luo, G., Fang, B., et al. (2023). Causal effects of gut microbiota on the risk of bipolar disorder: A Mendelian randomization study. Front. Microbiomes 2:1249518. doi: 10.3389/frmbi.2023.1249518
Yan, Q., Gu, Y., Li, X., Yang, W., Jia, L., Chen, C., et al. (2017). Alterations of the gut microbiome in hypertension. Front. Cell. Infect. Microbiol. 7:381. doi: 10.3389/fcimb.2017.00381
Yang, A.-M., Inamine, T., Hochrath, K., Chen, P., Wang, L., Llorente, C., et al. (2017). Intestinal fungi contribute to development of alcoholic liver disease. J. Clin. Invest. 127, 2829–2841. doi: 10.1172/JCI90562
Yang, R.-H., Su, J.-H., Shang, J.-J., Wu, Y.-Y., Li, Y., Bao, D.-P., et al. (2018). Evaluation of the ribosomal DNA internal transcribed spacer (ITS), specifically ITS1 and ITS2, for the analysis of fungal diversity by deep sequencing. PLoS One 13:e0206428. doi: 10.1371/journal.pone.0206428
Yap, C. X., Henders, A. K., Alvares, G. A., Wood, D. L. A., Krause, L., Tyson, G. W., et al. (2021). Autism-related dietary preferences mediate autism-gut microbiome associations. Cell 184, 5916–5931.e17. doi: 10.1016/j.cell.2021.10.015
Yuan, X. X., Sun, Y., Pang, L. J., Li, X., Hei, G. R., Zhang, X. Y., et al. (2023). [The influence of interaction between polygenic risk score and intestinal fungal microbiota on the risk of schizophrenia]. Zhonghua Yi Xue Za Zhi 103, 2324–2329. doi: 10.3760/cma.j.cn112137-20221110-02365
Yuan, X., Li, X., Hei, G., Zhang, X., and Song, X. (2024). Intestinal mycobiota dysbiosis associated inflammation activation in chronic schizophrenia. Behav. Brain Res. 472:115149. doi: 10.1016/j.bbr.2024.115149
Yuan, X., Li, X., Kang, Y., Pang, L., Hei, G., Zhang, X., et al. (2022). Gut mycobiota dysbiosis in drug-naïve, first-episode schizophrenia. Schizophr. Res. 250, 76–86. doi: 10.1016/j.schres.2022.10.011
Zhang, X., Pan, L.-Y., Zhang, Z., Zhou, Y.-Y., Jiang, H.-Y., and Ruan, B. (2020). Analysis of gut mycobiota in first-episode, drug-naïve Chinese patients with schizophrenia: A pilot study. Behav. Brain Res. 379:112374. doi: 10.1016/j.bbr.2019.112374
Keywords: gut mycobiome, neuropsychiatric disorders, dysbiosis, gut-brain axis, fungi, innovative therapy
Citation: Hadrich I, Turki M, Chaari I, Abdelmoula B, Gargouri R, Khemakhem N, Elatoui D, Abid F, Kammoun S, Rekik M, Aloulou S, Sehli M, Mrad AB, Neji S, Feiguin FM, Aloulou J, Abdelmoula NB and Sellami H (2025) Gut mycobiome and neuropsychiatric disorders: insights and therapeutic potential. Front. Cell. Neurosci. 18:1495224. doi: 10.3389/fncel.2024.1495224
Received: 12 September 2024; Accepted: 16 December 2024;
Published: 08 January 2025.
Edited by:
Matthew McMillin, Baylor College of Medicine, United StatesReviewed by:
Daniel Erny, University Medical Center Freiburg, GermanyCopyright © 2025 Hadrich, Turki, Chaari, Abdelmoula, Gargouri, Khemakhem, Elatoui, Abid, Kammoun, Rekik, Aloulou, Sehli, Mrad, Neji, Feiguin, Aloulou, Abdelmoula and Sellami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hayet Sellami, aGF5ZXRzZWxsYW1pMUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.