- 1Department of Radiation Oncology, University of Tübingen, Tübingen, Germany
- 2Department of Radiation Oncology, Medical University Vienna, Vienna, Austria
- 3Department of Pharmacology, Toxicology and Clinical Pharmacy, Institute of Pharmacy, University of Tübingen, Tübingen, Germany
Therapies with weak, non-ionizing electromagnetic fields comprise FDA-approved treatments such as Tumor Treating Fields (TTFields) that are used for adjuvant therapy of glioblastoma. In vitro data and animal models suggest a variety of biological TTFields effects. In particular, effects ranging from direct tumoricidal, radio- or chemotherapy-sensitizing, metastatic spread-inhibiting, up to immunostimulation have been described. Diverse underlying molecular mechanisms, such as dielectrophoresis of cellular compounds during cytokinesis, disturbing the formation of the spindle apparatus during mitosis, and perforating the plasma membrane have been proposed. Little attention, however, has been paid to molecular structures that are predestinated to percept electromagnetic fields—the voltage sensors of voltage-gated ion channels. The present review article briefly summarizes the mode of action of voltage sensing by ion channels. Moreover, it introduces into the perception of ultra-weak electric fields by specific organs of fishes with voltage-gated ion channels as key functional units therein. Finally, this article provides an overview of the published data on modulation of ion channel function by diverse external electromagnetic field protocols. Combined, these data strongly point to a function of voltage-gated ion channels as transducers between electricity and biology and, hence, to voltage-gated ion channels as primary targets of electrotherapy.
1. Introduction
Tumor cells express an ion channel toolkit that differs from that of the non-transformed parental cells. Few ion channel types are upregulated by several tumor entities of different origin (carcinomas, leukemias, gliomas). These so-called “oncochannels” have been ascribed specific functions in tumor biology. For instance, glioblastoma, a primary brain tumor with poor patient prognosis, over-expresses TRPM8 (member 8 of the melastatin sub-family of transient receptor potential) unselective cation channels, intermediate conductance IKCa (KCNN4) and high conductance BKCa (KCNMA1) K+ channels. These channels reportedly contribute to glioblastoma stem cell properties, program and execute cell migration and brain invasion, regulate cell cycle, or confer therapy resistance (for review see Huber, 2013; Roth and Huber, 2022). Unexpectedly, ionizing radiation in a clinical relevant dose may induce hypermigration of human glioblastoma cells in vitro (Steinle et al., 2011) and brain invasion in an orthotopic glioma xenograft mouse model (Edalat et al., 2016). Notably, radiation-induced BKCa K+ channel activation has been identified as a key event herein (Steinle et al., 2011; Edalat et al., 2016). This example illustrates that modulation of oncochannel function by direct therapeutical targeting or as an off-target effect might exert beyond intended but also unintended, detrimental effects, due to the versatile functions of oncochannels (Huber, 2013). In this context, some electrotherapies applying weak external electromagnetic fields (EMF) have been proposed to affect ion channel function (e.g., Li et al., 2020a). The present review article, therefore, aims to summarize our current knowledge on ion channel modulation by EMF with a particular focus on tumor treating fields (TTFields), an adjuvant electrotherapy in glioblastoma.
In vitro (e.g., Rozek et al., 1987; García-Sancho et al., 1994; Morgado-Valle et al., 1998; Aldinucci et al., 2000; Djamgoz et al., 2001; Verdugo-Díiaz et al., 2002; Rosen, 2003; Grassi et al., 2004; Giladi et al., 2014a, 2017; Bertagna et al., 2022; Toda et al., 2022) and preclinical in vivo studies (e.g., Kirson et al., 2007; 2009a; Giladi et al., 2014a, b; Buckner et al., 2015; Castellví et al., 2015; Li et al., 2016; Voloshin et al., 2016, Jo et al., 2018; Kim et al., 2018; Voloshin et al., 2020a; Barati et al., 2021; Wu et al., 2021; Davidi et al., 2022; Huegel et al., 2022), as well as randomized controlled clinical trials (e.g., Mooney, 1990; Linovitz et al., 2002; Foley et al., 2008; Stupp et al., 2017), have accumulated some evidence for a responsiveness of physiological processes to weak external EMF. As a consequence, the US Food and Drug Administration (FDA) has approved electrotherapies in the low frequency to radiofrequency band and the non-hyperthermia-inducing, non-electroporating, non-ionizing energy spectrum. Among those are TTFields, repetitive transcranial magnetic stimulation (rTMS), deep brain stimulation (DBS), or pulsed electromagnetic fields (PEMFs). The latter are applied to improve the healing of bone fractures. PEMF and further EMF therapies used for orthopedic applications have been hypothesized to mimic the endogenous environmental electromagnetic fields occurring during mechanical stress of joints/bones. By doing so, they might exert pro-anabolic effects resulting in an increase of the structural integrity of bone and cartilage extracellular matrix (Cadossi et al., 2020). A Cochrane review of electromagnetic stimulation, in contrast, found only inconclusive evidence regarding its efficacy on bone fracture healing (Griffin et al., 2011). Similarly, early results of DBS in patients with treatment-resistant depression were highly promising (Mayberg et al., 2005), whereas a subsequent large sham-controlled randomized trial, could not delineate significant antidepressant effects of DBS (Holtzheimer et al., 2017). In a renewed attempt to utilize DBS for treating depressive symptoms, individualizing types of electrical stimulation is increasingly tested in new trials (Drew, 2022; Sheth et al., 2022).
Due to their limited (or even un-proven) stand-alone effects, weak EMF electrotherapies could not replace conventional therapies but have been implemented in the clinical routine as modalities adjuvant or complementary to the conventional treatments (for review see Mattsson and Simkó, 2019). For the different established electrotherapies, a huge spectrum of biological responses has been reported in preclinical studies. This is not surprising considering the diversity in frequency (ranging from static magnetic or electric fields, i.e., 0 Hz, to, e.g., 100 kHz of TTFields), pulse form (sinus, square, etc.), application (pulsed or continuous), or intensity of the individual EMF treatments. Since these therapies often operate with very low intensities, the molecular mode of action is mostly ill-defined and the physics underlying the electrobiological interaction difficult to deduce (for review see Mattsson and Simkó, 2019). As an example, weak magnetic fields with low frequency have been reported in several in vitro studies to exert maximal biological effects when their frequencies match the “cyclotron resonance” of certain ions, such as Ca2+, Mg2+, or K+ (for review see Liboff, 2018). At the cyclotron resonance frequency, the ratio of the applied alternating magnetic field frequency to the static magnetic field (e.g., the geomagnetism) equals the ratio between the mass and the charge of the respective ion. However, as the authors of the aforementioned review article discuss, it has been hypothesized that EMF in cyclotron resonance frequency alter the physiological activity of the targeted ion. From a physics point of view, however, increasing the kinetic energy of a magnetic field-experiencing ion by its cyclotron frequency can only occur in vacuum or low-pressure gases. In biological microenvironments, by sharp contrast, EMF-induced acceleration of the ion is prevented by damping. Moreover, the restricted dimension of the cellular subcompartment is several thousand orders of magnitude lower than the radius of cyclotron resonance-accelerated ions in vacuum. In addition, the cyclotron frequency refers to dehydrated ion masses (for review see Liboff, 2018). In biological systems, free ions carry a hydration envelope most of the time that may be only shortly shed, e.g., by the selective filter of an ion channel pore during transmembrane transport. Hence, as shown by this example, the molecular basis of the electrobiological energy transfer of weak EMF therapy often remains obscure. In contrast, a more mechanistic understanding has been postulated for the TTFields electrotherapy of cancer as introduced in the second section of this article.
2. Tumor treating fields (TTFields), a superweapon against glioblastoma?
TTFields, which have been developed by the company Novocure in Haifa, Israel, are alternating electric fields with intermediate frequency and low intensity. Besides glioblastoma, TTFields therapy is FDA-approved for mesothelioma. In a randomized controlled (but not sham-controlled) clinical trial in newly diagnosed glioblastoma patients (Stupp et al., 2017), patients benefitted from TTFields irrespective of MGMT methylation status of patients. In this trial, TTFields were applied concurrently to temozolomide maintenance therapy (following surgery and adjuvant concurrent temozolomide/fractionated radiation therapy) and compared to temozolomide maintenance therapy alone.
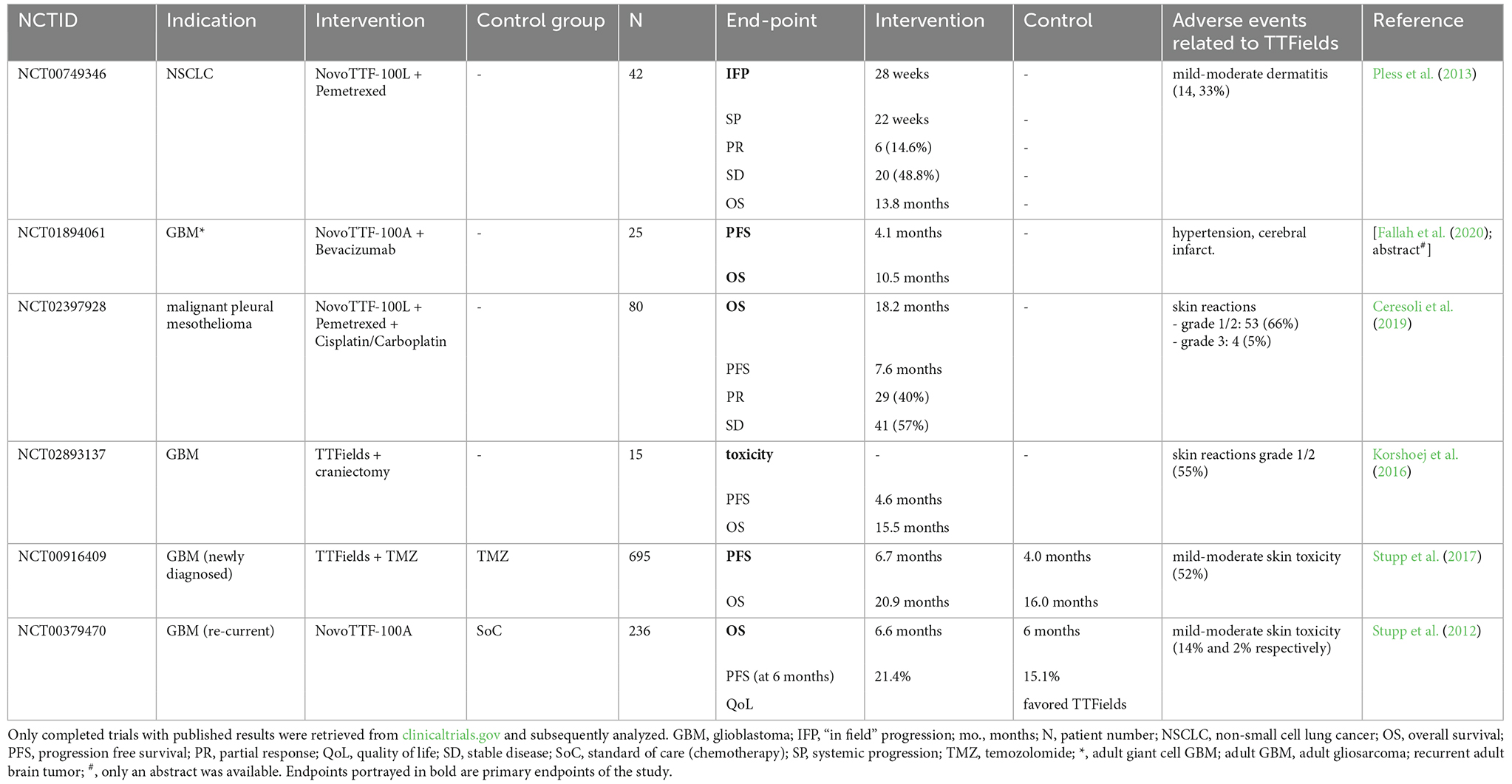
For glioblastoma, alternating electric fields with a frequency of 200 kHz are applied capacitively via ceramic electrode arrays placed onto the shaved scalp of the patient. Modeling the field intensity with due consideration of pre-specified conductivity and relative permittivity of skin, skull and brain tissues has predicted electric field strengths in the range of 1–3 V/cm in the brain tumor (Lok et al., 2019). Table 1 gives an overview of all completed clinical trials of TTFields applications in different cancer entities as of December 12th, 2022. Notably, only two trials had active control arms, and among these only NCT00916409 (Stupp et al., 2017) could establish prolonged survival for patients in the TTFields group. Hence, most completed trials so far are not well-versed to inform about the efficacy of TTFields therapy. Further trials should compare TTFields to the current standard of care (SoC) to establish efficacy in other cancer entities as well, which several of the ongoing trials are doing (e.g., NCT03940196 or NCT02973789).
On the other hand, most studies published so far report few TTFields-specific adverse events, with the exception of mild to moderate skin reactions. Whether this is due to specific electrotherapy effects or due to other effects due to the application itself (frequent scalp shaving, electrode gel, frequent sweating underneath the electrodes) is hard to differentiate without running sham-controlled trials. Last, several trials currently underway analyze TTFields’ efficacy in other tumor entities, such as brain metastases (NCT04967027), gastric- (NCT04281576), pancreatic- (NCT03377491; Rivera et al., 2019), and ovarian cancer (NCT02244502; Vergote et al., 2018).
Preclinical in vitro studies have suggested multiple modes of action for the interference of TTFields with tumor biology. TTFields reportedly impair the formation of the spindle apparatus, mitosis and cytokinesis of cancer cells (Kirson et al., 2004; 2009b; Gera et al., 2015; Giladi et al., 2015). Moreover, TTFields have been demonstrated to delay DNA repair (Giladi et al., 2017; Karanam et al., 2017), to inhibit tumor cell motility (Voloshin et al., 2020b) and to permeabilize the plasma membrane of cancer cells (Chang et al., 2018; Aguilar et al., 2021) and the blood brain barrier (Salvador et al., 2023). Furthermore, TTFields reportedly do not impair ex vivo viability and function of peripheral blood or glioblastoma-infiltrating T cells and of CAR T-cells (Simchony et al., 2019; Diamant et al., 2021). The tumor preference of these effects has been explained by the proliferation rate that differs between transformed and parental cells. The most effective frequency of the alternating electric fields has been proposed to be determined by the tumor cell geometry (Kirson et al., 2004). TTFields have also been applied to animal tumor models. These preclinical in vivo studies confirm TTFields-mediated inhibition of tumor growth (e.g., Grassi et al., 2004), chemotherapy-sensitizing effects (e.g., Giladi et al., 2017), and permeabilization of the blood-brain barrier (Salvador et al., 2022). In addition, TTFields reportedly delay formation of tumor metastases (Giladi et al., 2014a), support the anti-tumor immune response (Voloshin et al., 2020a; Chen D. et al., 2022), and mitigate tumor angiogenesis (Jo et al., 2018).
Mechanistically, TTFields have been proposed to restrict molecular motility of macromolecular dipoles such as tubulin dimers (Kirson et al., 2007; 2009a) and septins (Gera et al., 2015) inhibiting mitotic spindle assembly and correct positioning of the cytokinetic cleavage furrow, respectively. Moreover, condensation of the electric field lines at the cytokinetic furrow and associated dielectrophoresis (i.e., migration of dipoles or charged particles towards higher fields intensities) has been postulated to inhibit symmetric segregation of cellular material to the daughter cells during cytokinesis (Kirson et al., 2004, 2007). As a consequence of these modes of action, TTFields efficacy is dependent on the relative orientation of the cleavage axis of dividing cells to the applied electrical field. Likewise, interference with actin and microtubule dynamics has been suggested to underlie the anti-migratory action of TTFields in tumor cells (Voloshin et al., 2020b) and inhibition of ciliogenesis (Shi et al., 2022). Beyond that, further cell biological effects such as TTFields-triggered replication stress (Karanam et al., 2020) or AMPK-mediated ER stress and autophagy (Shteingauz et al., 2018), have been reported more recently. Combined, these studies point to a highly complex interference of TTFields with several cellular processes.
Notably, the plethora of cellular effects described by in vitro experiments might be overestimated due to a misfeature of the inovitroTM system provided by Novocure to study TTFields effects in vitro. This device uses ceramic dishes with integrated electrodes for the capacitive injection of the TTFields. The electric fields are delivered parallelly to the cell layer interchangeably from two perpendicular directions in order to increase the number of dividing cells with matching directions of electric field and mitotic cleavage axis. Applying TTFields by the inovitroTM system heats cells and cell culture medium. Field strength, therefore, has to be regulated indirectly: Decreasing the ambient temperature of the incubator below 37°C results by feed-back in an increase in generator power output and TTFields intensity until the cell culture medium and the ceramic dish reach 37°C as controlled by two temperature probes. This indirect control of field intensity, however, harbors the problem that the water vapor pressure in the air atmosphere of the TTFields-heated, 37°C-adjusted ceramic dish (in the better situation sealed with parafilm, in the worst case loosely covered by a plastic lid) exceeds that of the incubator air with lower temperature, resulting in continuous evaporation of water from the culture medium (Figure 1). Drying-out of the cells is prevented by replenishing the lost water volume by adding culture medium. Water evaporation increases and volume replenishment with medium eventually lead to an increase in the osmolarity of the cell culture medium. Since controls are run in 37°C incubators with iso-osmotic cell culture medium, the inovitroTM system in our opinion cannot distinguish between hyperosmotic stress- (Burg et al., 2007; Zhou et al., 2016) and TTFields-mediated cellular effects and hence, the conclusions drawn from the data are limited (discussed also in Slangen et al., 2022). Unfortunately, the majority of the TTFields in vitro data were obtained with the inovitroTM system and published without mentioning the system-inherent weakness of the experimental setup (e.g., Voloshin et al., 2016; Chang et al., 2018; Voloshin et al., 2020b; Chen et al., 2022).
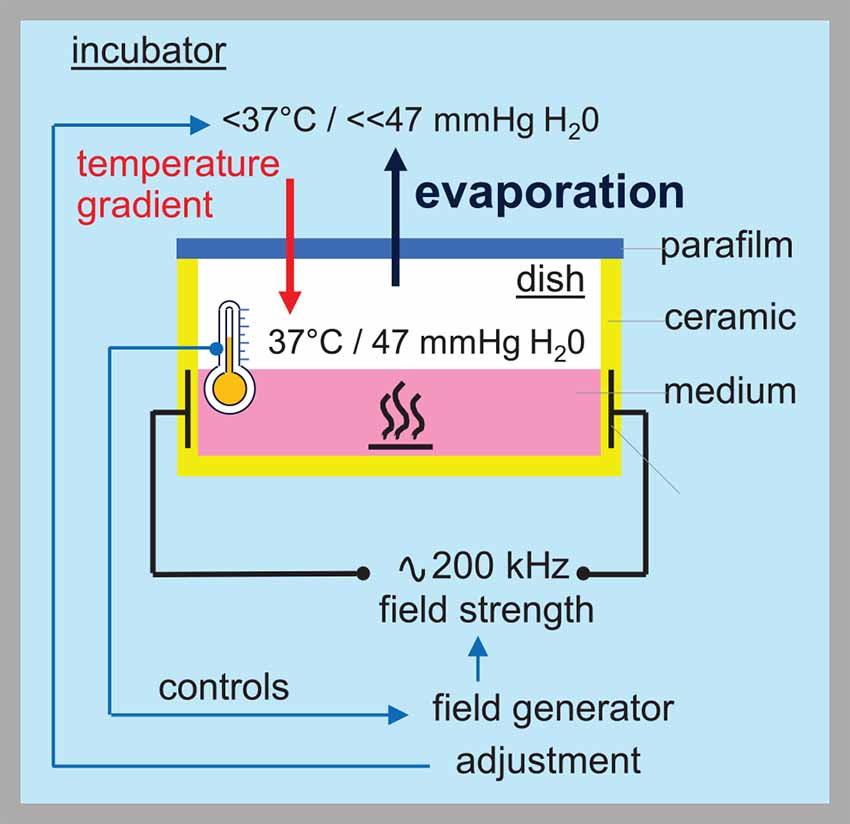
Figure 1. TTFields applied by the Novocure inovitroTM system increase the osmolarity of the medium. Thermal discharge of the inovitroTM system is counter-regulated by decreasing the ambient temperature of the incubator. The field strength is regulated indirectly by adjusting the ambient incubator temperature. Thereby, the temperature of the culture dish is continuously measured and the field strength increased until the dish temperature is equilibrated at 37°C. Different temperatures in incubator and culture dish, however, causes a lower water vapor pressure in the incubator than in the dish, resulting in continuous evaporation from the culture medium.
Further complicating the picture, a recent theoretical study by Li et al. (2020a) questioned the proposed mechanisms of electrobiological TTFields interactions. The authors concluded that the torsional moment induced by TTFields on dipoles such as tubulin dimers is several orders of magnitude lower than the Brownian energy of the molecules suggesting that TTFields are probably not able to disrupt mitotic spindle assembly. In addition, they estimated for the velocity of particles undergoing dielectrophoresis towards the cytokinetic furrow a value (0.003 μm/s) that is too slow to become biologically relevant during normally ongoing telophase. The same group (Li et al., 2020b) calculated the field distribution for spherical and spindle-shaped cell geometries. These geometries were then used to simulate the TTFields (2 V/cm)-associated temperature distributions for cell models resulting in a negligible TTFields-caused temperature rise. Please note, that this notion is in sharp contrast to the large heat production by the electronically elaborated, high-capacitively field-coupling inovitroTM system. This lack of a significant computed TTFields-mediated temperature rise was directly confirmed experimentally (Li et al., 2020b) suggesting that TTFields effects may not be conferred by hyperthermia.
Consistently, direct temperature measurements of TTFields-treated skin in a melanoma mouse model did not observe TTFields-elicited temperature effects (Li et al., 2016). Vice versa, TTFields effect on tumor growth in an ectopic mouse model of pancreatic carcinoma could not be mimicked by 41°C hyperthermia (Castellví et al., 2015). Instead, Li et al. (2020a) proposed a new mechanism for the electrobiological TTFields interaction. Their mathematical modeling predicts a significant TTFields-induced change of membrane potential and the authors hypothesized that this alteration in membrane potential modifies the activity of ion channels. Taken together, the mechanism of electrobiological TTFields transduction still seems to be elusive and modulation of voltage-gated ion channels might be an attractive alternative mechanism. The next paragraph introduces into the biochemistry of voltage sensors, the centerpiece of voltage-gating.
3. Molecular bases of voltage-sensing
Voltage-gated ion channels such as the shaker Kv K+- or the L type Cav Ca2+ channel activate upon depolarization of the membrane potential. The general construction principle of these channels is composed of four times six lipophilic transmembrane alpha helices (S1–S6) either realized by tetramers of four alpha subunits (Kv; Jiang et al., 2003) or a single alpha subunit with 24 transmembrane domains (Cav; Catterall et al., 2005). The four S5 and S6 helices together with the inter-connecting P loops form the ion-conducting pore and determine the ion specificity while the four S1-S4 helices around the S5–S6 helix bundle create the voltage sensor. The S4 helix contains several (typically four) positively charged amino acid residues (mostly arginine) separated from each other by two uncharged residues. These positively charged residues that are referred to as gating charges, are located within the membrane and become electrostatically stabilized, e.g., by negatively charged amino acids (i.e., countercharges) in the other (S1–S3) helixes of the voltage sensor (for review see Catacuzzeno and Franciolini, 2022; Figure 2A).
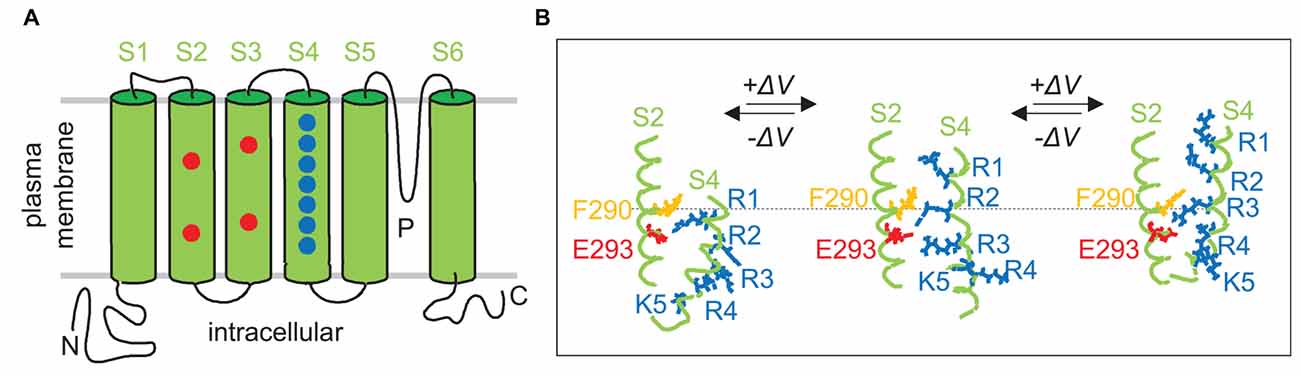
Figure 2. Voltage-sensing at a molecular level. (A) Membrane topology of a Shaker voltage-gated (Kv) K+ channel. Drawn is a Kv channel alpha subunit with the pore-forming domain (S5, S6, P-loop), the voltage sensor domain with the S4 helix containing basic amino acid residues, and the S2 and S3 helixes with acidic amino acid residues that serve in the voltage sensor as stabilizing counter ions. (B) Intramolecular relative movement of voltage sensor during gating. According to the “gating charge transfer center (GCTC) hypothesis”, the aromatic phenylalanine (F290) and the two negative residues E293 and D316 (not shown) of the S2 helix form a GCTC that binds transiently the positive charged residues (blue) of the S4 voltage sensor when consecutively crossing the channel pore area of trans-membrane voltage decline during gating. Scheme shows the deep channel close state at high negative potential (left), and the depolarization of membrane potential-caused successive upward “stepping” of the voltage sensor (middle and right). Transmembrane alpha helixes in (A,B) are shown in green, positively charged basic-, negatively charged acidic-, and aromatic (phenylalanine F290) amino acid residues in blue, red, and orange, respectively (redrawn and modified from Catacuzzeno and Franciolini, 2022).
The voltage sensor (S1–S4) can be envisaged as a gating channel with the S4 helix inside this channel. According to the sliding helix model of the voltage sensor action (Yarov-Yarovoy et al., 2012; Catterall et al., 2017), the S4 helix is pulled in towards the cytoplasmic membrane face at negative resting membrane potential. Membrane depolarization decreases this electrostatic force and the S4 helix is dragged out by a few Å towards the external membrane face thereby initiating structural changes in the S5–S6 helix bundle eventually leading to ion channel activation. Structurally, the gating channel has been proposed to be shaped as an hourglass-like pore with two crevices reaching from in- and outside, respectively, deeply in the membrane. A hydrophobic, by a phenylalanine residue-built, central plug (hydrophobic constriction site) isolates the two crevices electrically from each other. As a result, the whole membrane voltage drops across the central plug along a few Å membrane distance (for review see Catacuzzeno and Franciolini, 2022).
Multiexponential fitting and noise analysis of gating currents, structural information obtained by, e.g., X-ray crystallography or cryo-electron microscopy, simulation of molecular dynamics and other approaches suggest that the voltage-dependent sliding of the S4 helix sequentially proceeds by intermediate steps “between free energy basins separated by rate-limiting free energy barriers” (Delemotte et al., 2017). The former result from ion pair or hydrogen-bridge bonds of the gating charges with their respective countercharges or polar phospholipid head groups and other ionic or lipophilic interactions within the three-dimensional molecular environment of the voltage sensor. By consecutively passing the central plug, the gating charges stride through the voltage gradient thereby generating a gating current (12–14 qe elementary charges) that can be directly analyzed electrophysiologically (when the subsequently gated ion currents through the four S5-S6 helix bundle-built ion channel pore are prevented experimentally; for review see Catacuzzeno and Franciolini, 2022). A slightly modified model of voltage gating, the gating charge transfer center (GCTC) hypothesis, is described in Figure 2B in more detail.
Notably, cryo-electron microscopy and molecular dynamic simulation have revealed structural-functional differences between the four voltage sensors of the rabbit L-type Cav 1.1 Ca2+ channel. While, for instance, the S4 helix of the voltage sensor domain-I sequentially proceeds through three macrostates (resting 1–2 and activated state) with long transition times in the high μs to low ms timescale, that of the voltage sensor domain-IV steps through four macrostates (resting 1–3 and activated state) with transition times in the sub-μs to low μs timescale (Fernández-Quintero et al., 2021). Combined, these data suggest complex transitions between conformational macrostates of the Cav1.1 voltage sensor domains that exhibit differing kinetics and energy barriers. As a consequence thereof, one might assume that: (i) channel activation can not only be triggered by intrinsic membrane depolarization (due to, e.g., inactivation of K+-permeable- or activation of Na+-permeable ion channels) but also by external low intensity EMFs, and (ii) because of the multi-kinetics process of gating, transfer of activation energy from external EMFs may occur at several EMF frequencies (see also section 6 “Are 200 kHz TTFields too high-frequency for ion channel modulation in glioblastoma cells?” for a more detailed discussion on this issue). As a matter of fact, simulation of molecular dynamics unraveled that voltage sensors of voltage gated ion channels are prone to be directly affected by external electromagnetic fields. In particular, very short pulsed electric fields have been forecasted to activate voltage-gated ion channels while longer pulses, as used for electroporation of the plasma membrane, are predicted to convert the gating channels of the voltage sensor domain into a transient or a more complex phospholipid headgroup-stabilized long-living pore (Rems et al., 2020). Strong evidence for such a high susceptibility of voltage-gated ion channels to low intensity EMFs can be found in several fish species, which are capable of sensing very low electric fields with voltage-gated ion channels as signal transducers, as illustrated in the next paragraphs.
4. Biological voltmeters
Passive or active electrolocation is realized in species of cartilaginous and bony fishes. In passive electrolocation, fishes perceive weak electric fields that are generated by the neuronal, muscle, or gills ion pump activity of other animals and that are conductively coupled to specialized organs such as the ampullae of Lorenzini in elasmobranch fishes (Figure 3). The elephantfish (Mormyridae), an example of a species that is capable of active electrolocation, generates weak alternating electric fields with its electroorgan in the tail and analyzes distortions of the field that is coupled capacitively to tuberous organs (knollenorgans) in the skin (Figure 3). The ampullae of Lorenzini consist of electrosensory receptor- and auxiliary supporting cells lining a cavity beneath the skin that is conductively connected to the skin surface by canals. These canals are filled with seawater-containing jelly, have electrically resistant luminal cell surfaces and cell-cell connections, and thus, act as electrical wires that conduct the electric fields to the apical membrane of the electrosensory receptor cells. The epithelium of the cavity may contain thousands of these receptor cells that are surrounded by supporting cells. Epithelial conductivity of supporting cells, as well as paracellular shunts between supporting cells or supporting and receptor cells, seems to be lower than that of the receptor cells. The latter are cone-shaped with a large basal cell pole and a very small apical membrane area that projects a central kinocilium into the cavity lumen. In receptor cells, the apical membrane exhibits a severalfold higher electrical resistance than the basal membrane leading to a steeper potential drop of transepithelial voltage across the apical than basal membrane (Clusin and Bennett, 1977a, b). Electric fields have been shown to activate voltage-gated Ca2+ channels in the apical membrane which lead to Ca2+-influx-mediated membrane depolarization and subsequent activation of adjacent Ca2+- (skate) or voltage-activated (catshark) K+ channels in the apical membrane (Leitch and Julius, 2019; Figure 4).
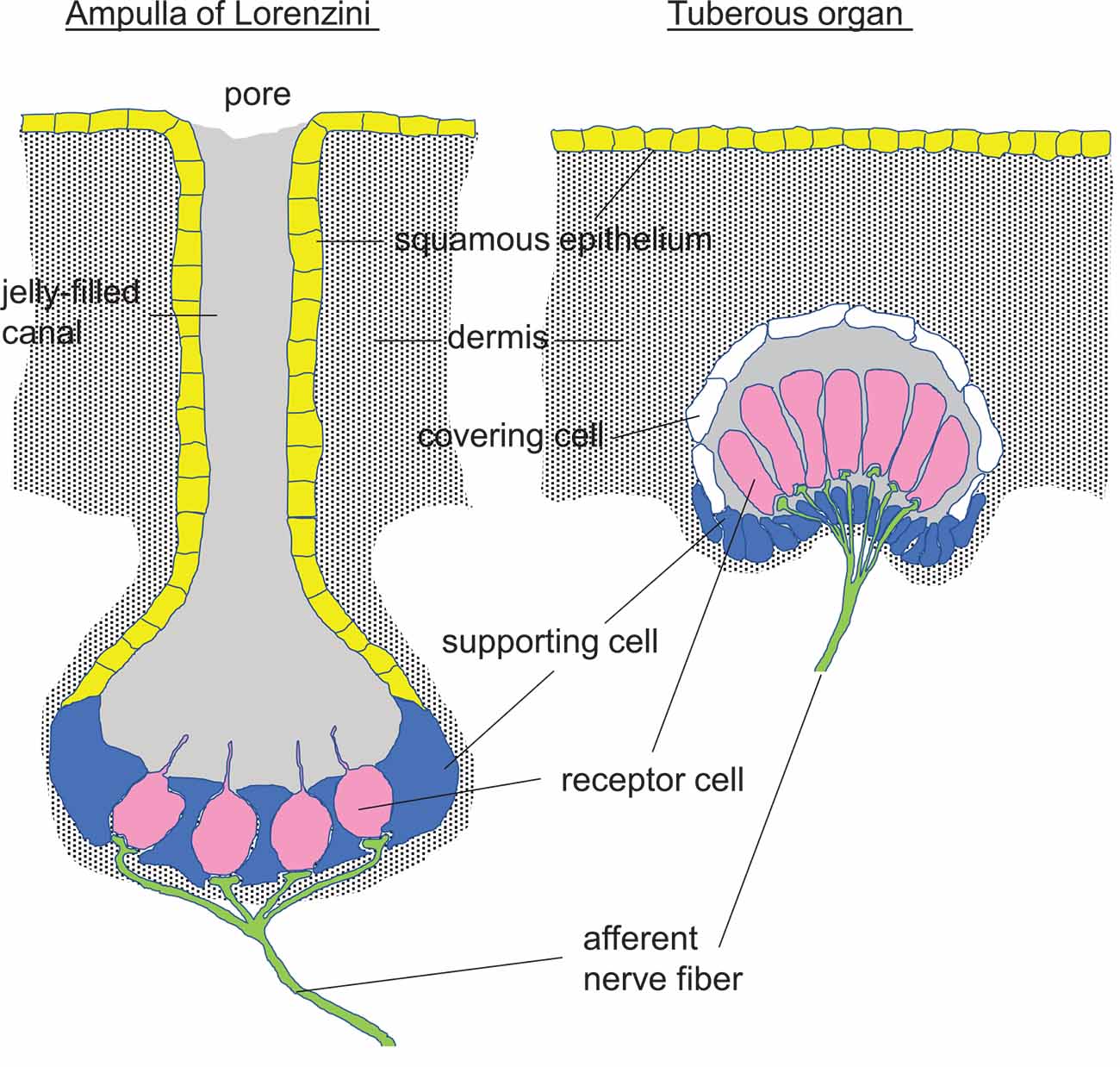
Figure 3. Perception of electric fields by fishes conductively and capacitively coupled to the electrosensory receptor cells (pink) in an Ampulla of Lorenzini and a Tuberous organ, respectively [redrawn and modified from Helfman et al. (2009) and Baker (2019)].
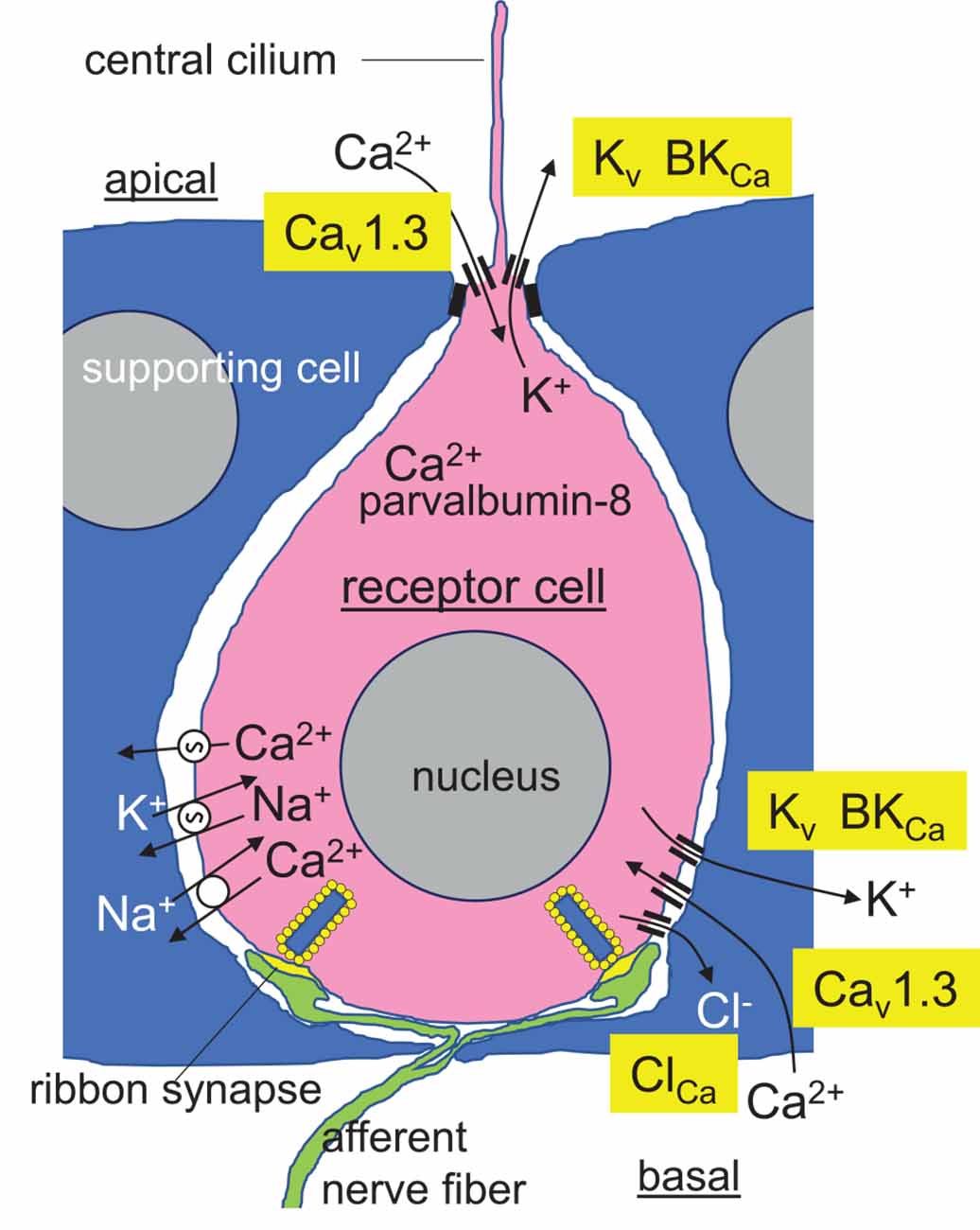
Figure 4. Hypothetical model of a receptor cell (pink) in the epithelium of the skate ampullae of Lorenzini. Tight junctions of the encompassing supporting cells (blue) with the receptor cells electrically isolate the high-resistance apical membrane of the receptor cells from the basolateral interstitial space. Low intensity electric fields modify the activity of Cav1.3 L-type voltage-gated Ca2+ channel in the apical membrane and central cilium resulting in membrane depolarization of the apical membrane and apical Ca2+ entry. Depolarization is conducted to the basal membrane followed by activation of probably basal Cav1.3 Ca2+ channels, basal Ca2+ entry, amplification of the membrane depolarization and Cav1.3 activity by Ca2+-activated Cl− channels, and transmitter release from the ribbon synapses. Apically entered Ca2+ is buffered by the Ca2+-binding protein parvalbumin-8, limiting the activity of apical Ca2+-activated BK K+ channels, which repolarize the basal membrane and maintain it close to the activation voltage threshold of the Cav1.3 channels. Alternating activity of Cav1.3 and basolateral BK- or/and voltage-activated Kv K+ channels programs oscillation of basal membrane potential and free cytosolic Ca2+ concentration with synchronized transmitter release. Ca2+ is extruded across the basal membrane by Ca-ATPase and Na/Ca-antiporter (from Clusin and Bennett, 1977a, b; Clusin and Bennett, 1979a, b; Lu and Fishman, 1995; Graydon et al., 2011; Bellono et al., 2017; Baker, 2019; Clusin et al., 2019; modified).
Electrophysiological and receptor cell transcriptome analyses in skate receptor cells have demonstrated high expression of orthologs of the mammalian L-type Cav1.3 (KACNA1D) voltage-gated Ca2+ channel and the BKCa Ca2+-activated K+ channel in the apical membrane and kinocilium of the electrosensory receptor cells [Bellono et al., 2017; and in preprint (Chen A. L. et al., 2022)]. Cav1.3 of skate receptor cells reportedly exhibits a lower threshold (i.e., the activation occurs at more negative voltages), a steeper activity/voltage relationship (i.e., during membrane depolarization maximal activity is immediately reached after passing the threshold of activation), and a slower inactivation kinetic as compared to its mammalian counterpart. Moreover, Cav1.3-mediated Ca2+ entry and subsequent activation of adjacent Ca2+-activated BKCa K+ channels do not result in strong membrane hyperpolarization (and complete deactivation of Cav1.3) of receptor cells since receptor cells express high amounts of Ca2+ buffering parvalbumin-8 protein and Ca2+-ATPase that, both, instantaneously decrease cytosolic free Ca2+ concentration, and thus, dampen BKCa channel activity (Figure 4). As a consequence, apical membrane potential of receptor cells is maintained around the activation threshold of Cav1.3 generating an extremely sensitive system that transduces external electric fields in Ca2+ action potentials (Bellono et al., 2017). Apical membrane depolarizations are conducted to the basal membranes of these secondary sensory receptor cells that form ribbon synapses with afferent fibers. Ca2+-activated Cl− channels and consecutive Cl− efflux probably amplify the depolarizing signals at the basal membrane (Lu and Fishman, 1995) triggering activation of basal L-type voltage-gated Ca2+ channels such as basally located Cav1.3 channels (Chen A. L. et al., 2022) followed by activation of basal K+ channels including BKCa channels (Clusin et al., 2019; Chen A. L. et al., 2022). Ca2+ is extruded across the basal membrane by Ca-ATPase and Na/Ca-antiporter. The latter is powered by the inwardly directed electrochemical gradient of Na+ as maintained by the Na/K-ATPase (Figure 4). The interplay of these channels/transporters/pumps results in oscillation of the basal membrane voltage and accompanying oscillation of the cytosolic free Ca2+ concentration (Clusin et al., 2019). Under resting conditions, these oscillations are thought to trigger basal transmitter release and afferent neural activity in a synchronized manner (Bellono et al., 2017). Convergent connectivity of the afferent neurons further adds sensitivity to the system (Peters et al., 1997).
Reportedly, electric field strengths as low as 1 nV/cm can be perceived. The ampullae of Lorenzini have been proposed to detect slow changes (frequencies between 0.1 and 50 Hz) in electric field strength (for review see Crampton, 2019) with probably highest sensitivity at the resonator frequency of the receptor cell (see also section 6 “Are 200 kHz TTFields too high-frequency for ion channel modulation in glioblastoma cells?”). In addition to Cav1.3 and BKCa, high expression of the orthologs of the mammalian Kv1.1 and Kv1.5 voltage-gated K+ channels in the receptor cells has been deduced from skate transcriptome data (Clusin et al., 2019), suggesting a more complex interplay of voltage-dependent ion channels in the voltage/Ca2+ transduction process (Figure 4).
Likewise, the orthologs of mammalian Kv1.x-like voltage-gated K+ channels are reportedly expressed by tuberous organs that are used for active electrolocation of, e.g., elephantfish (Smith et al., 2006). These tuberous electroreceptors are electrically isolated from the environmental fresh water. External electric fields, therefore, are capacitively coupled to the receptor cells that respond to AC electric organ discharges in the stimulus range of 20 Hz-18 kHz (25 kHz; for review see Kramer, 2009; Crampton, 2019; Figure 3). To the best of our knowledge, the molecular mechanism of electroreception in tuberous organ cells has not been defined, yet. Although tuberous organ and ampullae of Lorenzini evolved in a non-homologous manner in phylogenesis, it is tempting to speculate that tuberous organs also employ voltage-gated ion channels for electro/biochemical transduction.
In summary, specialized organs are capable of detecting electric field strengths that are several orders of magnitude lower than the fields applied in, e.g., anti-cancer electrotherapy. Moreover, voltage-gated ion channels have been identified at least in the ampulla of Lorenzini to act as signal transducers. Given the tight voltage reliance of voltage gated-ion channels, which has been phylogenetically tuned up to an ultra-high voltage sensitivity in electroreceptors of, e.g., fishes as compared to the very high field strengths applied in electrotherapy, it is intriguing to speculate that externally applied electromagnetic fields, such as in electrotherapies, indeed directly modify the conformational transitions of the voltage sensor domains in voltage-gated ion channels resulting in modified ion channel activity. Actually, several studies reported ion channel modulation by external electromagnetic fields as summarized in the next paragraphs.
5. Ion channels as targets of low frequency (<1 kHz) electrotherapy
Many carcinoma entities (Brackenbury, 2012; Djamgoz et al., 2019), as well as gliomas (Xing et al., 2014), upregulate voltage-gated Na+ (NaV) channels that are required for the function of excitable cells in normal tissue, such as neurons or muscle cells. In carcinoma resection specimens, Nav channel abundance reportedly is associated with formation of distant metastases and poorer patient survival. Proposed cellular mechanisms comprise Nav activity-regulated H+ extrusion via allosterically coupling to Na/H exchangers (NHE) at the lamellipodium of tissue-invading cancer cells. Local extracellular acidification, in turn, increases activity of cathepsins and metalloproteinases and promotes tissue invasion (Luo et al., 2020). Static (direct current, 0 Hz) electric fields (DC-EF, 0.1–4 V/cm) have been shown in vitro to direct migration of highly migratory (but not of weakly migratory) prostate cancer cells towards the cathode. This “galvanotaxis” was blocked by the Nav blocker tetrodotoxin suggesting a function of Nav channels in the electrobiological transduction. The epithelia of the prostate gland generate a transepithelial voltage of about -10 mV measured at the luminal as compared to the earthed basolateral side. The Nav-dependent “galvanotaxis” alongside this physiological transepithelial voltage gradient that corresponds to an electric field intensity of 5 V/cm has been hypothesized to promote invasion of prostate cancer cells into the gland ducts and metastasis (Djamgoz et al., 2001). Moreover, DC-EF (1–3 V/cm for 15 min) reportedly stimulates Ca2+ entry, firing rate, and insulin secretion in mouse insulinoma (bTC-6) β cells (Liebman et al., 2021). This DC-EF effect was sensitive to verapamil, a blocker of L- and T-type voltage-gated Ca2+ Cav channels (Bergson et al., 2011) suggesting an involvement of Cav channels in the transduction of the static electric field into Ca2+ signaling.
Pulsed EMF (PEMF) have been demonstrated in vitro to stimulate osteogenesis (Petecchia et al., 2015; Benya et al., 2021; Kar et al., 2021; He et al., 2022). Polycystin-2 (TRPP2, PKD-2), a voltage-dependent Ca2+-permeable cation channel of the polycystic subfamily of the transient receptor proteins (TRP), harbors a voltage sensor domain similar to other voltage-gated ion channels (Shen et al., 2016) and is expressed in rat osteoblasts. In these cells, TRPP2 is located on the primary cilium where it reportedly transduces PEMFs (0.6 mT, 50 Hz) into biological signaling that promotes osteogenic differentiation as evident from TRPP2 knock-down and pharmacological targeting (He et al., 2022). Consistent with a pivotal role of Ca2+ signaling in PEMF-stimulated osteogenesis, early osteogenic differentiation of human bone marrow stroma-derived mesenchymal stem cells by PEMF (2 mT, 1.3 ms pulses at 75 Hz for 10 min/day and several weeks) was paralleled by a continuously increasing steady state free cytosolic Ca2+ concentration and upregulation of L-type Cav channel expression (Petecchia et al., 2015). Similarly, PEMF (1 mT, 50 Hz)-induced neuronal differentiation of cultured dorsal root ganglion neurons has been shown to depend on L-type Cav channel-triggered Ca2+ signaling (Li et al., 2014). Along those lines, ELF-EMF (100 Hz for 120 h) co-administered with temozolomide has been demonstrated, beyond increasing Ca2+, to stimulate downregulation of stem cell and upregulation of differentiation markers in human glioblastoma U87 cells (Ahmadi-Zeidabadi et al., 2019). An increase in steady state free cytosolic Ca2+ concentration following PEMF (3 mT, 50 Hz) treatment has also been demonstrated in a human astrocyte cell line (Aldinucci et al., 2000). Finally, nano- to picoseconds-pulsed PEMF using electric fields of high (kV/cm) voltage have been demonstrated in vitro to activate Cav and other channels in various cell types (Craviso et al., 2010; Semenov et al., 2015; Burke et al., 2017; Azarov et al., 2019). In two of these studies, Cav channel activation secondarily to electroporation-mediated membrane depolarization was the most likely mechanism (Azarov et al., 2019) and could not be excluded (Craviso et al., 2010), respectively.
Extremely low-frequency electromagnetic fields (ELF-EMF, 8 Hz for 48 h) reportedly stimulate Ca2+ influx in B16-F10 melanoma cells that is blunted by L- and T-type Cav channel blockers (Wang et al., 2021). Functionally, ELF-EMF (1 mT, 100 Hz 1.3 ms, 2 h/day for 5 days) have been demonstrated to induce cell death in MC4-L2 breast cancer cells in a verapamil-sensitive manner (Barati et al., 2021). Likewise, a growth-impairing action of ELF-EMF (5–10 μT, frequency-modulated in a 25–6 Hz and 6–25 Hz frequency pattern, 1 h/day for 3–5 days) has been demonstrated in HeLa (cervix carcinoma), MDA-MB-231, and MCF-7 (both breast carcinoma), as well as B16-BL6 (mouse melanoma) cells but not in three non-malignant cell lines (Buckner et al., 2015). In this study, ELF-EMF (>15 min) induced in HeLa, MDA-MB-231, MCF-7, and B16-BL6 an increase in cytosolic free Ca2+ that was prevented by preincubating the cells with the Cav blockers bepridil or mibefradil. In the only cell line tested (B16-BL6), T-type Cav channel inhibition abolished the ELF-EMF-induced impairment of proliferation (Buckner et al., 2015). In sharp contrast, pro-proliferative and anti-apoptotic actions of ELF-EMF (50 Hz, 1 mT) have been shown in human neuroblastoma IMR32 and rat pituitary GH3 cells, which were sensitive to the L-type Cav blocker nifedipine. Here, ELF-EMF induced Cav expression but not activity (Grassi et al., 2004) suggesting that Cav channels are ineligible for electrobiological signal transduction. Besides growth modulation, ELF-EMF (0.7 mT, 60 Hz, 2 × 2 h/day for 7 days) reportedly promote neurite outgrowth of cultured rat chromaffin cells that is blocked by nifedipine (Verdugo-Díiaz et al., 2002).
Beyond Ca2+ channels, ELF-EMF (268 μT and 902 μT, 20 Hz) have been demonstrated to stimulate voltage-gated Kv1.3 (KCNA3) K+ currents of Kv1.3-expressing Chinese hamster ovary (CHO) cells in patch-clamp-whole-cell recording. Here, Kv1.3 activation occurs few seconds after switching-on ELF-EMF exposure and lasted several minutes after field removal (Cecchetto et al., 2020). Combined, these data strongly suggest that external low intensity, low frequency EMFs exert at least part of their biological in vitro effects by modulation of voltage-gated ion channels. That similar mechanisms are also applying for external low intensity EMF therapies in the intermediate and high frequency range (such as TTFields application in glioblastoma), in contrast, is much harder to imagine. The obvious reason for that is the kinetics of biological excitability which seems to be not fast enough to respond to intermediate/high EMF frequencies. As introduced in the next paragraphs, auditory hair cells are well suited to estimate the kinetics and frequency limitations of biological sensory responses and to get some insights into possible mechanisms of TTFields effects in glioblastoma.
6. Are 200 kHz TTFields too high-frequency for ion channel modulation in glioblastoma cells?
In frogs, turtles, lizards or birds, hair cells may exhibit electrical auditory tuning that contributes to the spectrum analysis of the acoustic frequency band. Thereby, individual hair cells differ in their electrical resonance frequency, i.e., in the acoustic (or experimentally applied electrical stimulus) frequency where they are responding best with synchronous oscillation of the membrane potential and transmitter release. Mechanistically, the acoustic activation of transducer channels in the apical membrane of the hair cell induces a membrane depolarization that stimulates in the basolateral membrane voltage-gated Cav Ca2+ channels. This results in further membrane depolarization, Ca2+ entry, and subsequent activation of adjacent BKCa Ca2+-activated and/or voltage-gated Kv channels very similar to the situation in the receptor cells of the ampullae of Lorenzini (see section 4 “Biological voltmeters”). Activation of K+ channels re(hyper)polarizes the membrane potential leading to deactivation of the Cav Ca2+ and Kv K+ channels, to a decrease of the cytosolic free Ca2+ concentration, and consequently to deactivation of the BKCa K+ channels. The transducer channel-triggered consecutive activation and deactivation of Ca2+ and K+ channels oscillate the membrane potential in an acoustic frequency-synchronous manner (for review see Fettiplace and Fuchs, 1999).
In electrically tuned hair cells, this frequency is constrained to a narrow resonance frequency band which is determined by the size and interconnected membrane capacitance of the hair cell and the number and kinetics of basolateral Ca2+ and K+ channels. Especially the kinetics of the expressed BKCa splice variants and auxiliary beta sub-units limit the maximal frequency a hair cell is electrically tuned at. In poikilothermic animals, this frequency range, as measured at room temperature or below, is reportedly limited to 1 kHz [with a minimal documented value of 250 Hz for the bull-frog Rana catesbeiana (Hudspeth and Lewis, 1988)]. In birds, the maximal resonance frequency increases with a Q10 = 2 at higher temperatures suggesting a maximal frequency of membrane potential oscillation up to 4 kHz in electrically tuned avian hair cells (for review see Fettiplace and Fuchs, 1999). Likewise, mammalian auditory inner hair cells, which do not show electrical tuning, may generate membrane oscillations synchronous to the acoustic stimulus with frequencies up to 2–3 kHz as demonstrated among others in guinea pig (Palmer and Russell, 1986).
As already mentioned, acoustically elicited oscillation of membrane potential encompasses several serially occurring time-dependent processes. These comprise beyond serial activation/deactivation of the involved channel variants, cyclic re-charging of the apical and basolateral membrane according to intrinsic capacitance-determined time- and resistance-dependent length constants. As a consequence thereof, the actual time constants of the subsequently occurring sub-processes, such as activation of the Cav Ca2+ channels in the basolateral membrane, must be profoundly shorter than an entire oscillation period of the membrane potential. As a matter of fact, in a patch-clamp study on auditory hair cells of chicken, activation of L-type voltage-gated Cav Ca2+ channel-generated Ba2+ currents occurred with a (voltage step-dependent) time constant down to τ = 100 μs (Zidanic and Fuchs, 1995). One has to bear in mind that this experimentally deduced time constant reflects also “non-biological” time-consuming processes. The latter includes patch-clamp amplifier-intrinsic, patch-pipette resistance-, as well as pipette and membrane capacitance-caused time delays of voltage clamping (Zidanic and Fuchs, 1995). Thus, the “real” activation kinetics of the studied Cav Ca2+ channel might be even faster. Along those lines, ion channels involved in fish electric organ discharge frequencies in the 20 kHz range (Kramer, 2009, Crampton, 2019; see section 4 “Biological voltmeters”) must operate with faster activation time constants than 100 μs. Notwithstanding, the presumed kinetics of voltage-gated ion channels in those very fast biological systems (auditory hair cells, electric organs, etc.) still seems to be too slow to be “in resonance” with the 200 kHz EMF of TTFields applied in glioblastoma therapy.
RT-PCR data of two human glioblastoma cell lines (T98G and U251; Neuhaus et al., 2019) as well as transcriptome data of glioblastoma resection specimens deposited in the TCGA database (Vatter et al., 2020) suggest high expression of Cav1.2 (CACNA1C) and Cav1.3 (CACNA1D) L-type voltage-gated Ca2+ channels. Moreover, patch-clamp recordings in human glioblastoma cell lines T98G (Steinle et al., 2011; Stegen et al., 2015), U-87MG (Edalat et al., 2016), U251 (Stegen et al., 2016) and RT-PCR data from primary cultures of mesenchymal glioblastoma stem cells suggest high (functional surface) expression of BKCa K+ channels (Ganser et al., 2022). In this way, glioblastoma cells resemble strikingly auditory hair cells or receptor cells in the Ampullae of Lorenzini. It is, therefore, intriguing to speculate that glioblastoma cells might show a certain degree of electrical “excitability” and resultant vulnerability to external EMFs. Along those lines, glioblastoma cells have been demonstrated to neuro-mimic neurogenesis programs (Venkataramani et al., 2022) and to integrate into neuro-glial networks by synaptogenesis (Venkatesh et al., 2019). In addition, they have been proposed to pursue oscillation of cytosolic Ca2+ and membrane potential to execute programs of brain invasion (Catacuzzeno and Franciolini, 2018).
Our previous study on single glioblastoma cells (human T98G and U251 cell lines, Neuhaus et al., 2019) demonstrated that TTFields (200 kHz, 0.25–2.5 Vpp/cm) induce a field strength-dependent increase in cytosolic free Ca2+ concentration in Fura-2 Ca2+ imaging experiments. Notably, this increase was not due to simple membrane electroporation since Cav Ca2+ channel blockers abolished TTFields-stimulated increase in cytosolic free Ca2+ reversibly. Moreover, the knock-down of Cav1.2 blunted this effect (Neuhaus et al., 2019). Furthermore, in cell-attached patch-clamp experiments with 2.5 Vpp/cm TTFields, this Ca2+ increase was paralleled by activation of BKCa K+ channels. Both, TTFields-stimulated BKCa and Cav channel activities were long-lasting and outlasted withdrawal of TTFields application by several minutes (Neuhaus et al., 2019). Notably, an identical time course has been observed for ELF-EMF-stimulated activation of voltage-gated Kv1.3 K+ currents in CHO cells (Cecchetto et al., 2020), as already mentioned above in Section 5 “Ion channels as targets of low frequency (<1 kHz) electrotherapy.”
Furthermore, we found that TTFields (200 kHz, 1 Vpp/cm) induced slight perturbations of cell cycle progression and dissipation of inner mitochondrial membrane potential. This resulted in small but significant cell death and a decrease in clonogenic survival in one (T98G) of the two glioblastoma cell lines tested (Neuhaus et al., 2019). Unexpectedly, inhibiting Cav channels by benidipine was unable to revert these TTFields effects suggesting that Cav channel activity did not contribute to the apparent tumoricidal action of the EMF in T98G cells. Rather, benidipine exerted effects slightly additive to those of the TTFields (Neuhaus et al., 2019). This might suggest that the TTFields-stimulated Cav channel activity and subsequent rise in cytosolic free Ca2+ concentration not necessarily must have detrimental consequences for the affected tumor cell.
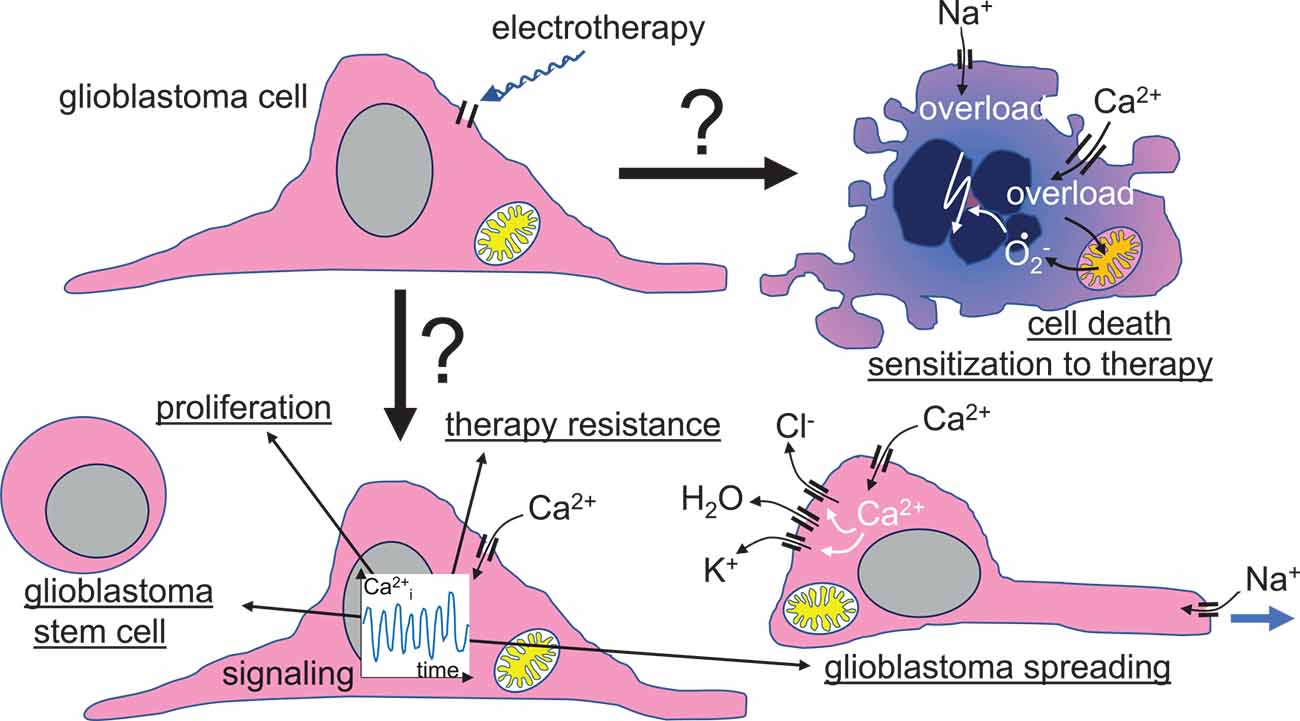
Figure 5. Speculative scenario of a primary voltage-gated ion channel targeting by glioblastoma electrotherapy. Beyond tumoricidal or therapy-sensitizing effects, electrotherapy-elicited modulation of ion channel activities might also boost malignant progression of glioblastoma cells by inducing transition in cancer stem cell phenotypes, cell proliferation, therapy resistance, and/or cell migration, glioblastoma spreading, and brain invasion.
Combined, the data described in sections 5 “Ion channels as targets of low frequency (<1 kHz) electrotherapy” and 6 “Are 200 kHz TTFields too high-frequency for ion channel modulation in glioblastoma cells?” demonstrate that low intensity EMF may interfere with cellular Ca2+ signaling in a wide range of frequencies including the 200 kHz frequency of the TTFields therapy. In the case of TTFields, one might speculate that the putative discrepancy between the reported kinetics of voltage-gated channels and the 200 kHz of TTFields might be reconciled by considering the multistep nature of voltage gating as described in section 3 “Molecular bases of voltage-sensing”: TTFields might interfere with sub-stepping between conformational macro states of the activating/deactivating channels that occur with much faster kinetics than the complete activation/deactivation process. Following the “ion channel hypothesis”, the reported tumor specificity of TTFields therapy (Kirson et al., 2007; Chang et al., 2018) must then arise from a tumor-specific expression/upregulation of TTFields-vulnerable types or variants of voltage-gated channels. As an example, glioblastoma cells have been demonstrated to express a specific splice variant (gliomaBKCa) of BKCa channel that exhibited highest Ca2+ sensitivity among the (at that time) known BKCa variants (Liu et al., 2002).
Another conclusion of the above-described studies is that the reported cellular EMF responses are cell type-specific and may span anti-proliferative and pro-apoptotic, pro-proliferative and anti-apoptotic, or phenotype-(de)differentiating effects. Moreover, the majority of the above-mentioned studies have identified voltage-gated ion channels and, in particular, Cav channels as primary EMF targets, strongly suggesting these channels as biological antennas of weak external electromagnetic fields.
Concluding remarks
In accordance with the tenor of the present review article, several authors have proposed voltage-gated (dependent) ion channels as molecular transducers of weak external EMF fields into biological signals (e.g., Pall, 2013, 2015, Pall, 2016; Li et al., 2020a; Georgiou and Margaritis, 2021; Panagopoulos et al., 2021; Wust et al., 2021). Identification of voltage-gated ion channels as central functional units in electrolocation of fishes (see section 4 “Biological voltmeters” of this article) may further support this “ion channel hypothesis” of external EMF perception. The wide range of EMF frequencies that comprise static fields (0 Hz) as well as radio frequency (MHz) and that reportedly all evoke activity changes of ion channels might be explained by the multi-step kinetics of ion channel gating with several “resonance” frequencies in this process (see section 3 “Molecular bases of voltage-sensing” of this article).
Although biological effects of weak external EMF have been demonstrated under controllable in vitro and preclinical in vivo conditions, their clinical relevance remains still highly debatable. Biological organisms are electrically active and life evolved in an ever-changing electromagnetic microenvironment due to the switch-on/switch-off activity of muscles or neurons in higher animals or locomotion-caused re-orientation within the terrestrial magnetic field. Bearing that in mind, one can assume that cells are well adapted to weak external EMF. This point is underlined since artificial EMF emitted by our electric devices of daily use (e.g., 50–60 Hz power supply, WiFi, cell phone, radio, TV, microwave, etc.), when used according to the guidelines and within the administrative limits of field strengths, seem not to elicit gross biological responses (Vijayalaxmi and Scarfi, 2014). Hence, strong effects of EMF in electrotherapies should not be expected. Nevertheless, electrotherapies have raised large hopes, especially in cancer entities with bad prognoses, such as glioblastoma. In this case, the hope is fueled by case reports on TTFields-associated complete tumor remission (Kessler et al., 2020; Stein et al., 2020) and one randomized controlled clinical trial (Stupp et al., 2017). As described above, more randomized controlled trials with proper control arms are underway to prove the replicability and generalizability of TTField’s effects against cancer.
Neoplastic transformation and malignant progression have been shown to rely on a remodeling of the ion channel toolkit of the tumor cell (Huber, 2013). In particular, many tumor entities upregulate voltage-gated channels like Nav (Martin et al., 2015), Kv (Pardo and Sühmer, 2008; Lastraioli et al., 2015), or Cav (Morrone et al., 2016) channels. Since these “oncochannels” reportedly play pivotal roles in tumor biology, electrotherapy may also entail risks when altering activities of these channels. Human glioblastoma cell lines, for example, have been shown to respond to weak DC-EFs with voltage-gated channel-dependent electrotaxis (Tsai et al., 2020) which might hint at the possibility that EF stimulates glioblastoma brain spreading. Moreover, EF reportedly induce a Cav-dependent proliferation and neuronal differentiation of neural stem cells (Wang et al., 2023) indicative of potential beneficial effects of EF therapies on neural tissue. EMF may also induce resistance against apoptosis in brain tumor cells (Grassi et al., 2004). Along those lines, in our previous work, TTFields-induced activation of L-type voltage-gated Cav1.2 Ca2+ channels in one human glioblastoma cell line exerted rather pro-survival (Neuhaus et al., 2019) than tumoricidal effects (Figure 5).
Therefore, a better understanding of the functional significance of potential electrobiological transducer channels in tumor biology and therapy resistance (for reviews see Huber, 2013; Klumpp et al., 2016; Roth and Huber, 2022) is inevitable for the development of further strategies in cancer electrotherapy. Such future strategies might aim to augment electrotherapy-induced cellular stress or to suppress cellular resistance mechanisms by concomitant targeting of potential resistance-mediating transducer channels. Moreover, once the electrobiological transduction process and the involved channel subtypes/splice variants are disclosed, electrotherapy protocols (waveform/frequency/intensity/duration/pulsed vs. continuous application) might become easily optimized in e.g., heterologous expression systems. Finally, the identification of potential electrotherapy transducer channels in a certain tumor entity may be utilized for personalization of electrotherapy, e.g., by stratification of patients according to the abundance of the transducer channels in the tumor resection specimens. However, to ultimately achieve this, much more joint efforts of electrotherapy developers, oncologists, physiologists and physicists are required.
Author contributions
TA: conception and design. All authors: literature search, writing, and review of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
FE and SH received funding from the German Research Foundation (DFG EC 575/2-1 and HU 781/7-1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aguilar, A. A., Ho, M. C., Chang, E., Carlson, K. W., Natarajan, A., Marciano, T., et al. (2021). Permeabilizing Cell membranes with electric fields. Cancers (Basel) 13:2283. doi: 10.3390/cancers13092283
Ahmadi-Zeidabadi, M., Akbarnejad, Z., Esmaeeli, M., Masoumi-Ardakani, Y., Mohammadipoor-Ghasemabad, L., and Eskandary, H. (2019). Impact of extremely low-frequency electromagnetic field (100 Hz, 100 G) exposure on human glioblastoma U87 cells during Temozolomide administration. Electromagn. Biol. Med. 38, 198–209. doi: 10.1080/15368378.2019.1625784
Aldinucci, C., Palmi, M., Sgaragli, G., Benocci, A., Meini, A., Pessina, F., et al. (2000). The effect of pulsed electromagnetic fields on the physiologic behaviour of a human astrocytoma cell line. Biochim. Biophys. Acta 1499, 101–108. doi: 10.1016/s0167-4889(00)00111-7
Azarov, J. E., Semenov, I., Casciola, M., and Pakhomov, A. G. (2019). Excitation of murine cardiac myocytes by nanosecond pulsed electric field. J. Cardiovasc. Electrophysiol. 30, 392–401. doi: 10.1111/jce.13834
Baker, C. V. H. (2019). “The development and evolution of lateral line electroreceptors: insights from comparative molecular approaches,” in Electroreception: Fundamental Insights from Comparative Approaches, Eds. B. A. Carlson, J. A. Sisneros, A. N. Popper and R. R. Fay (Cham: Springer International Publishing), 25–62. doi: 10.1007/978-3-030-29105-1_2
Barati, M., Javidi, M. A., Darvishi, B., Shariatpanahi, S. P., Mesbah Moosavi, Z. S., Ghadirian, R., et al. (2021). Necroptosis triggered by ROS accumulation and Ca2+ overload, partly explains the inflammatory responses and anti-cancer effects associated with 1Hz, 100mT ELF-MF in vivo. Free Radic. Biol. Med. 169, 84–98. doi: 10.1016/j.freeradbiomed.2021.04.002
Bellono, N. W., Leitch, D. B., and Julius, D. (2017). Molecular basis of ancestral vertebrate electroreception. Nature 543, 391–396. doi: 10.1038/nature21401
Benya, P. D., Kavanaugh, A., Zakarian, M., Söderlind, P., Jashashvili, T., Zhang, N., et al. (2021). Pulsed electromagnetic field (PEMF) transiently stimulates the rate of mineralization in a 3-dimensional ring culture model of osteogenesis. PLoS One 16:e0244223. doi: 10.1371/journal.pone.0244223
Bergson, P., Lipkind, G., Lee, S. P., Duban, M. E., and Hanck, D. A. (2011). Verapamil block of T-type calcium channels. Mol. Pharmacol. 79, 411–419. doi: 10.1124/mol.110.069492
Bertagna, F., Lewis, R., Silva, S. R. P., McFadden, J., and Jeevaratnam, K. (2022). Thapsigargin blocks electromagnetic field-elicited intracellular Ca2+ increase in HEK 293 cells. Physiol. Rep. 10:e15189. doi: 10.14814/phy2.15189
Brackenbury, W. J. (2012). Voltage-gated sodium channels and metastatic disease. Channels (Austin) 6, 352–361. doi: 10.4161/chan.21910
Buckner, C. A., Buckner, A. L., Koren, S. A., Persinger, M. A., and Lafrenie, R. M. (2015). Inhibition of cancer cell growth by exposure to a specific time-varying electromagnetic field involves T-type calcium channels. PLoS One 10:e0124136. doi: 10.1371/journal.pone.0124136
Burg, M. B., Ferraris, J. D., and Dmitrieva, N. I. (2007). Cellular response to hyperosmotic stresses. Physiol. Rev. 87, 1441–1474. doi: 10.1152/physrev.00056.2006
Burke, R. C., Bardet, S. M., Carr, L., Romanenko, S., Arnaud-Cormos, D., Leveque, P., et al. (2017). Nanosecond pulsed electric fields depolarize transmembrane potential via voltage-gated K+, Ca2+ and TRPM8 channels in U87 glioblastoma cells. Biochim. Biophys. Acta Biomembr. 1859, 2040–2050. doi: 10.1016/j.bbamem.2017.07.004
Cadossi, R., Massari, L., Racine-Avila, J., and Aaron, R. K. (2020). Pulsed electromagnetic field stimulation of bone healing and joint preservation: cellular mechanisms of skeletal response. J. Am. Acad. Orthop. Surg. Glob. Res. Rev. 4:e1900155. doi: 10.5435/JAAOSGlobal-D-19-00155
Castellví, Q., Ginestà, M. M., Capellà, G., and Ivorra, A. (2015). Tumor growth delay by adjuvant alternating electric fields which appears non-thermally mediated. Bioelectrochemistry 105, 16–24. doi: 10.1016/j.bioelechem.2015.04.006
Catacuzzeno, L., and Franciolini, F. (2018). Role of KCa3.1 channels in modulating Ca2+ oscillations during glioblastoma cell migration and invasion. Int. J. Mol. Sci. 19:2970. doi: 10.3390/ijms19102970
Catacuzzeno, L., and Franciolini, F. (2022). The 70-year search for the voltage-sensing mechanism of ion channels. J. Physiol. 600, 3227–3247. doi: 10.1113/JP282780
Catterall, W. A., Perez-Reyes, E., Snutch, T. P., and Striessnig, J. (2005). International union of pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57, 411–425. doi: 10.1124/pr.57.4.5
Catterall, W. A., Wisedchaisri, G., and Zheng, N. (2017). The chemical basis for electrical signaling. Nat. Chem. Biol. 13, 455–463. doi: 10.1038/nchembio.2353
Cecchetto, C., Maschietto, M., Boccaccio, P., and Vassanelli, S. (2020). Electromagnetic field affects the voltage-dependent potassium channel Kv1.3. Electromagn. Biol. Med. 39, 316–322. doi: 10.1080/15368378.2020.1799386
Ceresoli, G. L., Aerts, J. G., Dziadziuszko, R., Ramlau, R., Cedres, S., van Meerbeeck, J. P., et al. (2019). Tumour Treating Fields in combination with pemetrexed and cisplatin or carboplatin as first-line treatment for unresectable malignant pleural mesothelioma (STELLAR): a multicentre, single-arm phase 2 trial. Lancet Oncol. 20, 1702–1709. doi: 10.1016/S1470-2045(19)30532-7
Chang, E., Patel, C. B., Pohling, C., Young, C., Song, J., Flores, T. A., et al. (2018). Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 4:113. doi: 10.1038/s41420-018-0130-x
Chen, D., Le, S. B., Hutchinson, T. E., Calinescu, A. A., Sebastian, M., Jin, D., et al. (2022). Tumor Treating Fields dually activate STING and AIM2 inflammasomes to induce adjuvant immunity in glioblastoma. J. Clin. Invest. 132:e149258. doi: 10.1172/JCI149258
Chen, A. L., Wu, T.-H., Shi, L., Clusin, W. T., and Kao, P. N. (2022). Calcium-activated big conductance (BK) potassium channels traffic through nuclear envelopes into kinocilia in skate electrosensory cells. bioRxiv [Preprint]. doi: 10.1101/2022.05.10.491388
Clusin, W. T., and Bennett, M. V. (1977a). Calcium-activated conductance in skate electroreceptors: current clamp experiments. J. Gen. Physiol. 69, 121–143. doi: 10.1085/jgp.69.2.121
Clusin, W. T., and Bennett, M. V. (1977b). Calcium-activated conductance in skate electroreceptors: voltage clamp experiments. J. Gen. Physiol. 69, 145–182. doi: 10.1085/jgp.69.2.145
Clusin, W. T., and Bennett, M. V. (1979a). The ionic basis of oscillatory responses of skate electroreceptors. J. Gen. Physiol. 73, 703–723. doi: 10.1085/jgp.73.6.703
Clusin, W. T., and Bennett, M. V. (1979b). The oscillatory responses of skate electroreceptors to small voltage stimuli. J. Gen. Physiol. 73, 685–702. doi: 10.1085/jgp.73.6.685
Clusin, W. T., Wu, T. H., Shi, L. F., and Kao, P. N. (2019). Further studies of ion channels in the electroreceptor of the skate through deep sequencing, cloning and cross species comparisons. Gene 718:143989. doi: 10.1016/j.gene.2019.143989
Crampton, W. G. R. (2019). Electroreception, electrogenesis and electric signal evolution. J. Fish Biol. 95, 92–134. doi: 10.1111/jfb.13922
Craviso, G. L., Choe, S., Chatterjee, P., Chatterjee, I., and Vernier, P. T. (2010). Nanosecond electric pulses: a novel stimulus for triggering Ca2+ influx into chromaffin cells via voltage-gated Ca2+ channels. Cell. Mol. Neurobiol. 30, 1259–1265. doi: 10.1007/s10571-010-9573-1
Davidi, S., Jacobovitch, S., Shteingauz, A., Martinez-Conde, A., Braten, O., Tempel-Brami, C., et al. (2022). Tumor treating fields (TTFields) concomitant with sorafenib inhibit hepatocellular carcinoma in vitro and in vivo. Cancers (Basel) 14:2959. doi: 10.3390/cancers14122959
Delemotte, L., Kasimova, M. A., Sigg, D., Klein, M. L., Carnevale, V., and Tarek, M. (2017). Exploring the complex dynamics of an ion channel voltage sensor domain via computation. bioRxiv [Preprint]. doi: 10.1101/108217
Diamant, G., Simchony Goldman, H., Gasri Plotnitsky, L., Roitman, M., Shiloach, T., Globerson-Levin, A., et al. (2021). T cells retain pivotal antitumoral functions under tumor-treating electric fields. J. Immunol. 207, 709–719. doi: 10.4049/jimmunol.2100100
Djamgoz, M. B. A., Fraser, S. P., and Brackenbury, W. J. (2019). in vivo evidence for voltage-gated sodium channel expression in carcinomas and potentiation of metastasis. Cancers (Basel) 11:1675. doi: 10.3390/cancers11111675
Djamgoz, M. B. A., Mycielska, M., Madeja, Z., Fraser, S. P., and Korohoda, W. (2001). Directional movement of rat prostate cancer cells in direct-current electric field: involvement of voltagegated Na+ channel activity. J. Cell Sci. 114, 2697–2705. doi: 10.1242/jcs.114.14.2697
Drew, L. (2022). Wiring up the brain to beat depression. Nature 608, S46–S47. doi: 10.1038/d41586-022-02209-6
Edalat, L., Stegen, B., Klumpp, L., Haehl, E., Schilbach, K., Lukowski, R., et al. (2016). BK K+ channel blockade inhibits radiation-induced migration/brain infiltration of glioblastoma cells. Oncotarget 7, 14259–14278. doi: 10.18632/oncotarget.7423
Fallah, J. C., Rogers, R. T., Wei, L. R., Brewer, W., Peereboom, C. J., and Ahluwalia, D. M. (2020). Clinical outcomes of the combination of bevacizumab and ttfields in patients with recurrent glioblastoma: results of a phase II clinical trial. J. Clin. Oncol. 38:2537. doi: 10.1200/JCO.2020.38.15_suppl.2537
Fernández-Quintero, M. L., El Ghaleb, Y., Tuluc, P., Campiglio, M., Liedl, K. R., and Flucher, B. E. (2021). Structural determinants of voltage-gating properties in calcium channels. eLife 10:e64087. doi: 10.7554/eLife.64087
Fettiplace, R., and Fuchs, P. A. (1999). Mechanisms of hair cell tuning. Annu. Rev. Physiol. 61, 809–834. doi: 10.1146/annurev.physiol.61.1.809
Foley, K. T., Mroz, T. E., Arnold, P. M., Chandler, H. C., Jr., Dixon, R. A., Girasole, G. J., et al. (2008). Randomized, prospective and controlled clinical trial of pulsed electromagnetic field stimulation for cervical fusion. Spine J. 8, 436–442. doi: 10.1016/j.spinee.2007.06.006
Ganser, K., Eckert, F., Riedel, A., Stransky, N., Paulsen, F., Noell, S., et al. (2022). Patient-individual phenotypes of glioblastoma stem cells are conserved in culture and associate with radioresistance, brain infiltration and patient prognosis. Int. J. Cancer 150, 1722–1733. doi: 10.1002/ijc.33950
García-Sancho, J., Montero, M., Alvarez, J., Fonteriz, R. I., and Sanchez, A. (1994). Effects of extremely-low-frequency electromagnetic fields on ion transport in several mammalian cells. Bioelectromagnetics 15, 579–588. doi: 10.1002/bem.2250150611
Georgiou, C. D., and Margaritis, L. H. (2021). Oxidative stress and NADPH oxidase: connecting electromagnetic fields, cation channels and biological effects. Int. J. Mol. Sci. 22:10041. doi: 10.3390/ijms221810041
Gera, N., Yang, A., Holtzman, T. S., Lee, S. X., Wong, E. T., and Swanson, K. D. (2015). Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One 10:e0125269. doi: 10.1371/journal.pone.0125269
Giladi, M., Munster, M., Schneiderman, R. S., Voloshin, T., Porat, Y., Blat, R., et al. (2017). Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat. Oncol. 12:206. doi: 10.1186/s13014-017-0941-6
Giladi, M., Schneiderman, R. S., Porat, Y., Munster, M., Itzhaki, A., Mordechovich, D., et al. (2014a). Mitotic disruption and reduced clonogenicity of pancreatic cancer cells in vitro and in vivo by tumor treating fields. Pancreatology 14, 54–63. doi: 10.1016/j.pan.2013.11.009
Giladi, M., Weinberg, U., Schneiderman, R. S., Porat, Y., Munster, M., Voloshin, T., et al. (2014b). Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin. Oncol. 41, S35–S41. doi: 10.1053/j.seminoncol.2014.09.006
Giladi, M., Schneiderman, R. S., Voloshin, T., Porat, Y., Munster, M., Blat, R., et al. (2015). Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci. Rep. 5:18046. doi: 10.1038/srep18046
Grassi, C., D’Ascenzo, M., Torsello, A., Martinotti, G., Wolf, F., Cittadini, A., et al. (2004). Effects of 50 Hz electromagnetic fields on voltage-gated Ca2+ channels and their role in modulation of neuroendocrine cell proliferation and death. Cell Calcium 35, 307–315. doi: 10.1016/j.ceca.2003.09.001
Graydon, C. W., Cho, S., Li, G. L., Kachar, B., and von Gersdorff, H. (2011). Sharp Ca2+ nanodomains beneath the ribbon promote highly synchronous multivesicular release at hair cell synapses. J. Neurosci. 31, 16637–16650. doi: 10.1523/JNEUROSCI.1866-11.2011
Griffin, X. L., Costa, M. L., Parsons, N., and Smith, N. (2011). Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults. Cochrane Database Syst. Rev. 35:Cd008471. doi: 10.1002/14651858.CD008471.pub2
He, Y., Yang, M., Chen, Z., Wei, P., Qin, K., Xie, G., et al. (2022). [Effect of polycystin2 on differentiation and maturation of osteoblasts promoted by low-frequency pulsed electromagnetic fields]. Sheng Wu Gong Cheng Xue Bao 38, 1159–1172. doi: 10.13345/j.cjb.210356
Helfman, G. S. C., B, B., Facey, D. E., and Bowen, B. W. (2009). The Diversity of Fishes: Biology, Evolution and Ecology. Hoboken, NJ: Wiley-Blackwell.
Holtzheimer, P. E., Husain, M. M., Lisanby, S. H., Taylor, S. F., Whitworth, L. A., McClintock, S., et al. (2017). Subcallosal cingulate deep brain stimulation for treatment-resistant depression: a multisite, randomised, sham-controlled trial. Lancet Psychiatry 4, 839–849. doi: 10.1016/S2215-0366(17)30371-1
Hudspeth, A. J., and Lewis, R. S. (1988). A model for electrical resonance and frequency tuning in saccular hair cells of the bull-frog, Rana catesbeiana. J. Physiol. 400, 275–297. doi: 10.1113/jphysiol.1988.sp017120
Huegel, J., Chan, P. Y. W., Weiss, S. N., Nuss, C. A., Raja, H., Waldorff, E. I., et al. (2022). Pulsed electromagnetic field therapy alters early healing in a rat model of rotator cuff injury and repair: potential mechanisms. J. Orthop. Res. 40, 1593–1603. doi: 10.1002/jor.25185
Jiang, Y., Lee, A., Chen, J., Ruta, V., Cadene, M., Chait, B. T., et al. (2003). X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41. doi: 10.1038/nature01580
Jo, Y., Kim, E. H., Sai, S., Kim, J. S., Cho, J. M., Kim, H., et al. (2018). Functional biological activity of sorafenib as a tumor-treating field sensitizer for glioblastoma therapy. Int. J. Mol. Sci. 19:3684. doi: 10.3390/ijms19113684
Kar, N. S., Ferguson, D., Zhang, N., Waldorff, E. I., Ryaby, J. T., and DiDonato, J. A. (2021). Pulsed-electromagnetic-field induced osteoblast differentiation requires activation of genes downstream of adenosine receptors A2A and A3. PLoS One 16:e0247659. doi: 10.1371/journal.pone.0247659
Karanam, N. K., Ding, L., Aroumougame, A., and Story, M. D. (2020). Tumor treating fields cause replication stress and interfere with DNA replication fork maintenance: implications for cancer therapy. Transl. Res. 217, 33–46. doi: 10.1016/j.trsl.2019.10.003
Karanam, N. K., Srinivasan, K., Ding, L., Sishc, B., Saha, D., and Story, M. D. (2017). Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis. 8:e2711. doi: 10.1038/cddis.2017.136
Kessler, A. F., Linsenmann, T., Westermaier, T., Wolber, W., Weiland, J., Monoranu, C. M., et al. (2020). Complete radiological response following subtotal resection in three glioblastoma patients under treatment with Tumor Treating Fields. Oncol. Lett. 19, 557–561. doi: 10.3892/ol.2019.11110
Kim, J. H., Sohn, U. D., Kim, H. G., and Kim, H. R. (2018). Exposure to 835 MHz RF-EMF decreases the expression of calcium channels, inhibits apoptosis, but induces autophagy in the mouse hippocampus. Korean J. Physiol. Pharmacol. 22, 277–289. doi: 10.4196/kjpp.2018.22.3.277
Kirson, E. D., Dbalý, V., Tovarys, F., Vymazal, J., Soustiel, J. F., Itzhaki, A., et al. (2007). Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc. Natl. Acad. Sci. U S A 104, 10152–10157. doi: 10.1073/pnas.0702916104
Kirson, E. D., Giladi, M., Gurvich, Z., Itzhaki, A., Mordechovich, D., Schneiderman, R. S., et al. (2009a). Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin. Exp. Metastasis 26, 633–640. doi: 10.1007/s10585-009-9262-y
Kirson, E. D., Schneiderman, R. S., Dbalý, V., Tovarys, F., Vymazal, J., Itzhaki, A., et al. (2009b). Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med. Phys. 9:1. doi: 10.1186/1756-6649-9-
Kirson, E. D., Gurvich, Z., Schneiderman, R., Dekel, E., Itzhaki, A., Wasserman, Y., et al. (2004). Disruption of cancer cell replication by alternating electric fields. Cancer Res. 64, 3288–3295. doi: 10.1158/0008-5472.can-04-0083
Klumpp, L., Sezgin, E. C., Eckert, F., and Huber, S. M. (2016). Ion channels in brain metastasis. Int. J. Mol. Sci. 17:1513. doi: 10.3390/ijms17091513
Korshoej, A. R., Saturnino, G. B., Rasmussen, L. K., von Oettingen, G., Sørensen, J. C. H., and Thielscher, A. (2016). Enhancing predicted efficacy of tumor treating fields therapy of glioblastoma using targeted surgical craniectomy: a computer modeling study. PLoS One 11:e0164051. doi: 10.1371/journal.pone.0164051
Kramer, B. (2009). “Electric organ discharge,” in Encyclopedia of Neuroscience, Eds. M. D. Binder, N. Hirokawa, and U. Widhorst (Berlin: Springer), 1050–1056. doi: 10.1007/978-3-540-29678-2_2917
Lastraioli, E., Lottini, T., Bencini, L., Bernini, M., and Arcangeli, A. (2015). hERG1 potassium channels: novel biomarkers in human solid cancers. Biomed. Res. Int. 2015:896432. doi: 10.1155/2015/896432
Leitch, D. B., and Julius, D. (2019). “Electrosensory transduction: comparisons across structure, afferent response properties and cellular physiology,” in Electroreception: Fundamental Insights From Comparative Approaches (Vol. 70), Eds. B. Carlson, J. Sisneros, A. Popper and R. Fay (Cham: Springer International Publishing), 63–90. doi: 10.1007/978-3-030-29105-1_3
Li, J., Guo, C., Wang, Z., Gao, K., Shi, X., and Liu, J. (2016). Electrical stimulation towards melanoma therapy via liquid metal printed electronics on skin. Clin. Transl. Med. 5:21. doi: 10.1186/s40169-016-0102-9
Li, X., Yang, F., and Rubinsky, B. (2020a). A theoretical study on the biophysical mechanisms by which tumor treating fields affect tumor cells during mitosis. IEEE Trans. Biomed. Eng. 67, 2594–2602. doi: 10.1109/TBME.2020.2965883
Li, X., Yang, F., Gao, B., Yu, X., and Rubinsky, B. (2020b). A Theoretical analysis of the effects of tumor-treating electric fields on single cells. Bioelectromagnetics 41, 438–446. doi: 10.1002/bem.22274
Li, Y., Yan, X., Liu, J., Li, L., Hu, X., Sun, H., et al. (2014). Pulsed electromagnetic field enhances brain-derived neurotrophic factor expression through L-type voltage-gated calcium channel- and Erk-dependent signaling pathways in neonatal rat dorsal root ganglion neurons. Neurochem. Int. 75, 96–104. doi: 10.1016/j.neuint.2014.06.004
Liboff, A. R. (2018). “The ion cyclotron resonance hypothesis,” in Bioengineering and Biophysical Aspects of Electromagnetic Fields, Eds. F. S. Barnes and B. Greenebaum (Boca Raton: Tayler & Francis Group).
Liebman, C., Vu, T. M., Phillips, A., Chen, B., and Cho, M. (2021). Altered β-cell calcium dynamics via electric field exposure. Ann. Biomed. Eng. 49, 106–114. doi: 10.1007/s10439-020-02517-w
Linovitz, R. J., Pathria, M., Bernhardt, M., Green, D., Law, M. D., McGuire, R. A., et al. (2002). Combined magnetic fields accelerate and increase spine fusion: a double-blind, randomized, placebo controlled study. Spine (Phila Pa 1976) 27, 1383–1389. doi: 10.1097/00007632-200207010-00002
Liu, X., Chang, Y., Reinhart, P. H., Sontheimer, H., and Chang, Y. (2002). Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J. Neurosci. 22, 1840–1849. doi: 10.1523/JNEUROSCI.22-05-01840.2002
Lok, E., San, P., and Wong, E. T. (2019). “Insights from computer modeling: analysis of physical characteristics of glioblastoma in patients treated with tumor-treating fields,” in Brain and Human Body Modeling: Computational Human Modeling at EMBC 2018, Eds. S. Makarov, M. Horner, and G. Noetscher (Cham: Springer), 155–161. doi: 10.1007/978-3-030-21293-3_8
Lu, J., and Fishman, H. M. (1995). Ion channels and transporters in the electroreceptive ampullary epithelium from skates. Biophys. J. 69, 2467–2475. doi: 10.1016/S0006-3495(95)80117-7
Luo, Q., Wu, T., Wu, W., Chen, G., Luo, X., Jiang, L., et al. (2020). The functional role of voltage-gated sodium channel Nav1.5 in metastatic breast cancer. Front. Pharmacol. 11:1111. doi: 10.3389/fphar.2020.01111
Martin, F., Ufodiama, C., Watt, I., Bland, M., and Brackenbury, W. J. (2015). Therapeutic value of voltage-gated sodium channel inhibitors in breast, colorectal and prostate cancer: a systematic review. Front. Pharmacol. 6:273. doi: 10.3389/fphar.2015.00273
Mattsson, M.-O., and Simkó, M. (2019). Emerging medical applications based on non-ionizing electromagnetic fields from 0 Hz to 10 THz. Med. Devices (Auckl) 12, 347–368. doi: 10.2147/MDER.S214152
Mayberg, H. S., Lozano, A. M., Voon, V., McNeely, H. E., Seminowicz, D., Hamani, C., et al. (2005). Deep brain stimulation for treatment-resistant depression. Neuron 45, 651–660. doi: 10.1016/j.neuron.2005.02.014
Mooney, V. (1990). A randomized double-blind prospective study of the efficacy of pulsed electromagnetic fields for interbody lumbar fusions. Spine (Phila Pa 1976) 15, 708–712. doi: 10.1097/00007632-199007000-00016
Morgado-Valle, C., Verdugo-Diaz, L., Garcia, D. E., Morales-Orozco, C., and Drucker-Colin, R. (1998). The role of voltage-gated Ca2+ channels in neurite growth of cultured chromaffin cells induced by extremely low frequency (ELF) magnetic field stimulation. Cell Tissue Res. 291, 217–230. doi: 10.1007/s004410050992
Morrone, F. B., Gehring, M. P., and Nicoletti, N. F. (2016). Calcium channels and associated receptors in malignant brain tumor therapy. Mol. Pharmacol. 90, 403–409. doi: 10.1124/mol.116.103770
Neuhaus, E., Zirjacks, L., Ganser, K., Klumpp, L., Schuler, U., Zips, D., et al. (2019). Alternating electric fields (TTFields) activate Cav1.2 channels in human glioblastoma cells. Cancers (Basel) 11:110. doi: 10.3390/cancers11010110
Pall, M. L. (2013). Electromagnetic fields act via activation of voltage-gated calcium channels to produce beneficial or adverse effects. J. Cell. Mol. Med. 17, 958–965. doi: 10.1111/jcmm.12088
Pall, M. L. (2015). Scientific evidence contradicts findings and assumptions of Canadian safety panel 6: microwaves act through voltage-gated calcium channel activation to induce biological impacts at non-thermal levels, supporting a paradigm shift for microwave/lower frequency electromagnetic field action. Rev. Environ. Health 30, 99–116. doi: 10.1515/reveh-2015-0001
Pall, M. L. (2016). Microwave frequency electromagnetic fields (EMFs) produce widespread neuropsychiatric effects including depression. J. Chem. Neuroanat. 75, 43–51. doi: 10.1016/j.jchemneu.2015.08.001
Palmer, A. R., and Russell, I. J. (1986). Phase-locking in the cochlear nerve of the guinea-pig and its relation to the receptor potential of inner hair-cells. Hear. Res. 24, 1–15. doi: 10.1016/0378-5955(86)90002-x
Panagopoulos, D. J., Karabarbounis, A., Yakymenko, I., and Chrousos, G. P. (2021). Huma-made electromagnetic fields: ion forced-oscillation and voltage-gated ion channel dysfunction, oxidative stress and DNA damage (Review). Int. J. Oncol. 59:92. doi: 10.3892/ijo.2021.5272
Pardo, L. A., and Sühmer, W. (2008). Eag1 as a cancer target. Expert. Opin. Ther. Targets 12, 837–843. doi: 10.1517/14728222.12.7.837
Petecchia, L., Sbrana, F., Utzeri, R., Vercellino, M., Usai, C., Visai, L., et al. (2015). Electro-magnetic field promotes osteogenic differentiation of BM-hMSCs through a selective action on Ca2+-related mechanisms. Sci. Rep. 5:13856. doi: 10.1038/srep13856
Peters, R. C., Brans, R. J., Bretschneider, F., Versteeg, E., and Went, A. (1997). Converging electroreceptor cells improve sensitivity and tuning. Neuroscience 81, 297–301. doi: 10.1016/s0306-4522(97)00190-5
Pless, M., Droege, C., von Moos, R., Salzberg, M., and Betticher, D. (2013). A phase I/II trial of tumor treating fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Cancer 81, 445–450. doi: 10.1016/j.lungcan.2013.06.025
Rems, L., Kasimova, M. A., Testa, I., and Delemotte, L. (2020). Pulsed electric fields can create pores in the voltage sensors of voltage-gated ion channels. Biophys. J. 119, 190–205. doi: 10.1016/j.bpj.2020.05.030
Rivera, F., Benavides, M., Gallego, J., Guillen-Ponce, C., Lopez-Martin, J., and Küng, M. (2019). Tumor treating fields in combination with gemcitabine or gemcitabine plus nab-paclitaxel in pancreatic cancer: results of the PANOVA phase 2 study. Pancreatology 19, 64–72. doi: 10.1016/j.pan.2018.10.004
Rosen, A. D. (2003). Effect of a 125 mT static magnetic field on the kinetics of voltage activated Na+ channels in GH3 cells. Bioelectromagnetics 24, 517–523. doi: 10.1002/bem.10124
Roth, B., and Huber, S. M. (2022). Ion transport and radioresistance. Rev. Physiol. Biochem. Pharmacol. 183, 217–249. doi: 10.1007/112_2020_33
Rozek, R. J., Sherman, M. L., Liboff, A. R., McLeod, B. R., and Smith, S. D. (1987). Nifedipine is an antagonist to cyclotron resonance enhancement of 45Ca incorporation in human lymphocytes. Cell Calcium 8, 413–427. doi: 10.1016/0143-4160(87)90025-x
Salvador, E., Kessler, A. F., Domröse, D., Hörmann, J., Schaeffer, C., Giniunaite, A., et al. (2022). Tumor treating fields (TTFields) reversibly permeabilize the blood-brain barrier in vitro and in vivo. Biomolecules 12:1348. doi: 10.3390/biom12101348
Salvador, E., Köppl, T., Hörmann, J., Schönhärl, S., Bugaeva, P., Kessler, A. F., et al. (2023). Tumor treating fields (TTFields) induce cell junction alterations in a human 3D < model of the blood-brain barrier. Pharmaceutics 15:185. doi: 10.3390/pharmaceutics15010185
Semenov, I., Xiao, S., Kang, D., Schoenbach, K. H., and Pakhomov, A. G. (2015). Cell stimulation and calcium mobilization by picosecond electric pulses. Bioelectrochemistry 105, 65–71. doi: 10.1016/j.bioelechem.2015.05.013
Shen, P. S., Yang, X., DeCaen, P. G., Liu, X., Bulkley, D., Clapham, D. E., et al. (2016). The structure of the polycystic kidney disease channel PKD2 in lipid nanodiscs. Cell 167, 763–773.e11. doi: 10.1016/j.cell.2016.09.048
Sheth, S. A., Bijanki, K. R., Metzger, B., Allawala, A., Pirtle, V., Adkinson, J. A., et al. (2022). Deep brain stimulation for depression informed by intracranial recordings. Biol. Psychiatry 92, 246–251. doi: 10.1016/j.biopsych.2021.11.007
Shi, P., Tian, J., Ulm, B. S., Mallinger, J. C., Khoshbouei, H., Deleyrolle, L. P., et al. (2022). Tumor treating fields suppression of ciliogenesis enhances temozolomide toxicity. Front. Oncol. 12:837589. doi: 10.3389/fonc.2022.837589
Shteingauz, A., Porat, Y., Voloshin, T., Schneiderman, R. S., Munster, M., Zeevi, E., et al. (2018). AMPK-dependent autophagy upregulation serves as a survival mechanism in response to tumor treating fields (TTFields). Cell Death Dis. 9:1074. doi: 10.1038/s41419-018-1085-9
Simchony, H., Diamant, D., Ram, Z., and Volovitz, I. (2019). Evaluation of the compatibility of electric tumor treating fields with key anti-tumoral T-cell functions. Isr. Med. Assoc. J. 21:503.
Slangen, P. L. G., Porat, Y., Mertz, M., van den Broek, B., Jalink, K., de Gooijer, M. C., et al. (2022). Protocol for live-cell imaging during tumor treating fields treatment with inovitro live. STAR Protoc. 3:101246. doi: 10.1016/j.xpro.2022.101246
Smith, G. T., Unguez, G. A., and Weber, C. M. (2006). Distribution of Kv1-like potassium channels in the electromotor and electrosensory systems of the weakly electric fish Apteronotus leptorhynchus. J. Neurobiol. 66, 1011–1031. doi: 10.1002/neu.20283
Stegen, B., Butz, L., Klumpp, L., Zips, D., Dittmann, K., Ruth, P., et al. (2015). Ca2+-activated IK K+ channel blockade radiosensitizes glioblastoma cells. Mol. Cancer Res. 13, 1283–1295. doi: 10.1158/1541-7786.MCR-15-0075
Stegen, B., Klumpp, L., Misovic, M., Edalat, L., Eckert, M., Klumpp, D., et al. (2016). K+ channel signaling in irradiated tumor cells. Eur. Biophys. J. 45, 585–598. doi: 10.1007/s00249-016-1136-z
Stein, M., Dohmen, H., Wölk, B., Eberle, F., Kolodziej, M., Acker, T., et al. (2020). Case report of complete radiological response of a thalamic glioblastoma after treatment with proton therapy followed by temozolomide and tumor-treating fields. Front. Oncol. 10:477. doi: 10.3389/fonc.2020.00477
Steinle, M., Palme, D., Misovic, M., Rudner, J., Dittmann, K., Lukowski, R., et al. (2011). Ionizing radiation induces migration of glioblastoma cells by activating BK K+ channels. Radiother. Oncol. 101, 122–126. doi: 10.1016/j.radonc.2011.05.069
Stupp, R., Taillibert, S., Kanner, A., Read, W., Steinberg, D., Lhermitte, B., et al. (2017). Effect of tumor-treating fields plus maintenance temozolomide vs. maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA 318, 2306–2316. doi: 10.1001/jama.2017.18718
Stupp, R., Wong, E. T., Kanner, A. A., Steinberg, D., Engelhard, H., Heidecke, V., et al. (2012). NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur. J. Cancer 48, 2192–2202. doi: 10.1016/j.ejca.2012.04.011
Toda, T., Ito, M., Takeda, J. I., Masuda, A., Mino, H., Hattori, N., et al. (2022). Extremely low-frequency pulses of faint magnetic field induce mitophagy to rejuvenate mitochondria. Commun. Biol. 5:453. doi: 10.1038/s42003-022-03389-7
Tsai, H.-F., IJspeert, C., and Shen, A. Q. (2020). Voltage-gated ion channels mediate the electrotaxis of glioblastoma cells in a hybrid PMMA/PDMS microdevice. APL Bioeng. 4:036102. doi: 10.1063/5.0004893
Vatter, T., Klumpp, L., Ganser, K., Stransky, N., Zips, D., Eckert, F., et al. (2020). Against repurposing methadone for glioblastoma therapy. Biomolecules 10:917. doi: 10.3390/biom10060917
Venkataramani, V., Yang, Y., Schubert, M. C., Reyhan, E., Tetzlaff, S. K., Wißmann, N., et al. (2022). Glioblastoma hijacks neuronal mechanisms for brain invasion. Cell 185, 2899–2917.e31. doi: 10.1016/j.cell.2022.06.054
Venkatesh, H. S., Morishita, W., Geraghty, A. C., Silverbush, D., Gillespie, S. M., Arzt, M., et al. (2019). Electrical and synaptic integration of glioma into neural circuits. Nature 573, 539–545. doi: 10.1038/s41586-019-1563-y
Verdugo-Díiaz, L., Olivares-Bañnuelos, T., Navarro, L., and Drucker-Colíin, R. (2002). Effects of extremely low frequency electromagnetic field stimulation on cultured chromaffin cells. Ann. N Y Acad. Sci. 971, 266–268. doi: 10.1111/j.1749-6632.2002.tb04474.x
Vergote, I., von Moos, R., Manso, L., Van Nieuwenhuysen, E., Concin, N., and Sessa, C. (2018). Tumor treating fields in combination with paclitaxel in recurrent ovarian carcinoma: results of the INNOVATE pilot study. Gynecol. Oncol. 150, 471–477. doi: 10.1016/j.ygyno.2018.07.018
Vijayalaxmi, and Scarfi, M. R. (2014). International and national expert group evaluations: biological/health effects of radiofrequency fields. Int. J. Environ. Res. Public Health 11, 9376–9408. doi: 10.3390/ijerph110909376
Voloshin, T., Kaynan, N., Davidi, S., Porat, Y., Shteingauz, A., Schneiderman, R. S., et al. (2020a). Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol. Immunother. 69, 1191–1204. doi: 10.1007/s00262-020-02534-7
Voloshin, T., Schneiderman, R. S., Volodin, A., Shamir, R. R., Kaynan, N., Zeevi, E., et al. (2020b). Tumor treating fields (TTFields) hinder cancer cell motility through regulation of microtubule and acting dynamics. Cancers (Basel) 12:3016. doi: 10.3390/cancers12103016
Voloshin, T., Munster, M., Blatt, R., Shteingauz, A., Roberts, P. C., Schmelz, E. M., et al. (2016). Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. Int. J. Cancer 139, 2850–2858. doi: 10.1002/ijc.30406
Wang, S., Guan, S., Sun, C., Liu, H., Liu, T., and Ma, X. (2023). Electrical stimulation enhances the neuronal differentiation of neural stem cells in three-dimensional conductive scaffolds through the voltage-gated calcium ion channel. Brain Res. 1798:148163. doi: 10.1016/j.brainres.2022.148163
Wang, M. H., Jian, M. W., Tai, Y. H., Jang, L. S., and Chen, C. H. (2021). Inhibition of B16F10 cancer cell growth by exposure to the square Wave with 7.83+/–0.3Hz involves L- and T-type calcium channels. Electromagn. Biol. Med. 40, 150–157. doi: 10.1080/15368378.2020.1839491
Wu, H., Yang, L., Liu, H., Zhou, D., Chen, D., Zheng, X., et al. (2021). Exploring the efficacy of tumor electric field therapy against glioblastoma: an in vivo and in vitro study. CNS Neurosci. Ther. 27, 1587–1604. doi: 10.1111/cns.13750
Wust, P., Stein, U., and Ghadjar, P. (2021). Non-thermal membrane effects of electromagnetic fields and therapeutic applications in oncology. Int. J. Hyperthermia 38, 715–731. doi: 10.1080/02656736.2021.1914354
Xing, D., Wang, J., Ou, S., Wang, Y., Qiu, B., Ding, D., et al. (2014). Expression of neonatal Nav1.5 in human brain astrocytoma and its effect on proliferation, invasion and apoptosis of astrocytoma cells. Oncol. Rep. 31, 2692–2700. doi: 10.3892/or.2014.3143
Yarov-Yarovoy, V., DeCaen, P. G., Westenbroek, R. E., Pan, C. Y., Scheuer, T., Baker, D., et al. (2012). Structural basis for gating charge movement in the voltage sensor of a sodium channel. Proc. Natl. Acad. Sci. U S A 109, E93–E102. doi: 10.1073/pnas.1118434109
Zhou, X., Naguro, I., Ichijo, H., and Watanabe, K. (2016). Mitogen-activated protein kinases as key players in osmotic stress signaling. Biochim. Biophys. Acta 1860, 2037–2052. doi: 10.1016/j.bbagen.2016.05.032
Keywords: alternating electric fields, EMF, electrolocation, ampullae of Lorenzini, tuberous organs, voltage sensor
Citation: Abed T, Ganser K, Eckert F, Stransky N and Huber SM (2023) Ion channels as molecular targets of glioblastoma electrotherapy. Front. Cell. Neurosci. 17:1133984. doi: 10.3389/fncel.2023.1133984
Received: 29 December 2022; Accepted: 10 February 2023;
Published: 17 March 2023.
Edited by:
Dieter Wicher, Max Planck Institute for Chemical Ecology, GermanyReviewed by:
Bernard Fioretti, University of Perugia, ItalyRobert C. Wykes, University College London, United Kingdom
Copyright © 2023 Abed, Ganser, Eckert, Stransky and Huber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephan M. Huber, c3RlcGhhbi5odWJlckB1bmktdHVlYmluZ2VuLmRl
 Tayeb Abed
Tayeb Abed Katrin Ganser1
Katrin Ganser1 Nicolai Stransky
Nicolai Stransky Stephan M. Huber
Stephan M. Huber