
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Neurosci. , 02 June 2022
Sec. Cellular Neuropathology
Volume 16 - 2022 | https://doi.org/10.3389/fncel.2022.894886
 Cuola Deji
Cuola Deji Peng Yan
Peng Yan Yuanyuan Ji
Yuanyuan Ji Xinyue Yan
Xinyue Yan Yue Feng
Yue Feng Jincen Liu
Jincen Liu Yige Liu
Yige Liu Shuguang Wei
Shuguang Wei Yongsheng Zhu*
Yongsheng Zhu* Jianghua Lai*
Jianghua Lai*Anxiety is one of the most common comorbid conditions reported in people with opioid dependence. The basolateral amygdala (BLA) and ventral hippocampus (vHip) are critical brain regions for fear and anxiety. The kappa opioid receptor (KOR) is present in the mesolimbic regions involved in emotions and addiction. However, the precise circuits and molecular basis underlying anxiety associated with chronic opioid use are poorly understood. Using a mouse model, we demonstrated that anxiety-like behaviors appeared in the first 2 weeks after morphine withdrawal. Furthermore, the BLA and vHip were activated in mice experiencing anxiety after morphine withdrawal (Mor-A). KORs in the BLA to vHip projections were significantly increased in the Mor-A group. Optogenetic/chemogenetic inhibition of BLA inputs ameliorated anxiety-like behaviors and facilitated conditioned place preference (CPP) extinction in Mor-A mice. Knockdown of the BLA to vHip circuit KOR alleviated the anxiety-like behaviors but did not affect CPP extinction or reinstatement. Furthermore, combined treatment of inhibition of the BLA to vHip circuit and KOR antagonists mitigated anxiety-like behaviors and prevented stress-induced CPP reinstatement after morphine withdrawal. These results revealed a previously unknown circuit associated with the emotional component of opioid withdrawal and indicated that restoration of synaptic deficits with KOR antagonists might be effective in the treatment of anxiety associated with morphine withdrawal.
Opioid drugs are potent analgesics, but they also are exceedingly addictive (powerful euphoria). Opioid abuse relapse occurs with a frequency of more than 85.6% (Vuong et al., 2021). Addiction to opioids depends not only on their positive reinforcing effects but also on avoiding the negative, aversive consequences associated with withdrawal. Early withdrawal symptoms in opioid abusers include diarrhea, yawning, dysphoria, irritability, loss of appetite, severe abdominal pain, and nausea that emerge after drug abstinence (Heishman et al., 1989; Spanagel and Weiss, 1999). Furthermore, numerous psychological symptoms, including anxiety and major depression, gradually increase with the intensity of the drug craving as the withdrawal time lengthens (Goeldner et al., 2011; Radke and Gewirtz, 2012). Notably, heroin-addicted persons with anxiety have higher relapse rates and poor long-term treatment outcomes than heroin-addicted persons who do not have anxiety (Butelman et al., 2012; McKendrick et al., 2020).
The basolateral amygdala (BLA) is a primary site that orchestrates reward-related and emotional processes. Thus, BLA dysfunction is thought to be directly involved in anxiety-like responses and addictive behaviors (Sharp, 2017; Daviu et al., 2019). The BLA is necessary to promote responses to natural rewards, respond to second-order drug-conditioned cues, express stress-enhanced reacquisition of drug intake, and reinstate cue-dependent drug seeking (Sharp, 2017). The BLA mediates fear learning, and the expression of fear as a conditioned response also has been implicated in the genesis and perhaps maintenance of anxiety-like behaviors (Sah, 2017; Sun et al., 2020). The hippocampus has been profoundly implicated in forming addiction-related memories and drug reward experiences (Nestler, 2001a; Dong et al., 2006). The opioidergic system in the ventral hippocampus (vHip) has been demonstrated to be involved in reward-related memory and anxiety-like behaviors. The vHip is a critical site of action for the anxiolytic properties of morphine (Zarrindast et al., 2008; Alvandi et al., 2017). Furthermore, the vHip is a distal BLA projection target implicated in anxiety-related behaviors (McHugh et al., 2004). Previous studies have concluded that the vHip, and not the dorsal hippocampus, is required to express anxiety-related behaviors in the elevated plus maze (EPM) and open field test (OFT) (Bertoglio et al., 2006). The EPM and OFT are the principal behavior tests used to assess anxiety-like behavior in rodents (Carola et al., 2002). However, the functional contribution of BLA inputs to the vHip has not been directly investigated during the withdrawal period after repeated drug administration.
Mu, kappa, and delta are the primary opioid receptor subtypes in brain circuits that share common analgesic effects. This is consistent with the concept that kappa opioid receptor (KOR) activation in animals and humans produces negative affective states and drug-seeking behavioral responses (McLaughlin et al., 2003; Land et al., 2008; Bruijnzeel, 2009; Carroll and Carlezon, 2013). KOR activation exerts anti-reward effects throughout the process of addiction and has the opposite effect of mu opioid receptor (MOR) activation (reward). As addiction develops, intensified stress enhances KOR functions contributing to a dysphoric mood during withdrawal and leading to relapse (Wang, 2019). In humans, selective KOR agonists produce negative mood states, including dysphoria and anxiety (Pfeiffer et al., 1986). Microinfusion of KOR antagonists into the BLA in rodents reduces conditioned fear responses and anxiolytic-like effects in the EPM (Knoll et al., 2011). KOR antagonism prevents morphine stress-induced reinstatement of extinguished and cocaine-conditioned place preference (CPP) (Ross et al., 2012; Brice-Tutt et al., 2020). However, the mechanisms underlying KOR-dependent behaviors have not been clarified, especially at the level of distinct neural circuits and animal models.
We hypothesized that interactions of the BLA to vHip inputs with KORs might be linked to anxiety-like behaviors after morphine withdrawal. We examined anxiety-like behaviors and stress-induced CPP reinstatement during morphine withdrawal in mice. We explored the function of the distal projections from the BLA to the vHip and KORs expression within the projections. We identified a functional role for the BLA to vHip pathway that interacted with KORs in modulating anxiety-like behaviors after morphine withdrawal.
Adult male C57BL/6J mice (8 weeks, 22 ± 2 g) were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd. The loxP-flanked KOR transgenic mice (KORloxp/loxp mice) were bought from The Jackson Laboratory. All animals were housed in groups of three to four per cage and kept on a 12-h light/dark cycle (lights on at 7:00 p.m., off at 7:00 a.m.) at a stable temperature (22 ± 3°C) and humidity (50 ± 5%). Experiments were conducted during the light cycle. All animal procedures were approved by the Institutional Animal Care and Use Committee of Xi'an Jiaotong University.
The HCl-morphine (The Third Research Institute of The Ministry of Public Security, Shanghai, China) and naloxone (N822820, MACKLIN) were dissolved in saline. Morphine was administered at an escalating dose (from 10 to 50 mg/kg) via intraperitoneal (i.p.) injection two times daily for 6 consecutive days. Control animals received two times daily i.p. injections of saline for 6 consecutive days accordingly. Naloxone was injected subcutaneously (s.c.) into the morphine group mice and the saline group mice at a dose of 2 mg/kg. CNO (3 mg/kg, Sigma, C0832) and nor-BNI (10 mg/kg, 113158-34-2, MCE) were dissolved in the 0.5% DMSO. The DMSO was diluted to 0.5% with saline. KOR antagonist nor-BNI is a long-lasting antagonist (Horan et al., 1992), is delayed in its onset of action, and produces peak effects after 24 h (Endoh et al., 1992; Butelman et al., 1993; Metcalf and Coop, 2005). In addition, nor-BNI is most selective for KORs 24 h after administration (Endoh et al., 1992; Valdez and Harshberger, 2012). Thus, we injected 10 mg/kg nor-BNI intraperitoneally 24 h before the behavior test.
Naloxone, μ opioid receptor antagonist, was used to induce the somatic symptoms (Boyle et al., 2009). Mice received naloxone (2 mg/kg, s.c.) before the observation of somatic symptoms and were placed in a white opaque cylinder (32.0 cm height × 10.0 cm diameter); signs of withdrawal syndrome were monitored for 20 min. For the evaluation of behavioral signs of withdrawal, nine parameters were evaluated (The number of wet dog shakes, front paw tremors, scratches, jumping, and sniffing was counted. Body tremor, ptosis, teeth chattering, and piloerection were scored 1 for appearance or 0 for non-appearance within 5 min bins.). A global withdrawal score was calculated for each animal by giving each somatic sign a relative weight: 0.5 for each episode of wet dog shake, paw tremor, scratching, sniffing, and jumping; and 1 for the presence of body tremor, ptosis, mastication, and piloerection during each 5-min observation period. Each mouse was scored individually. Data were analyzed in a double-blinded manner. The results were assessed such that the higher the score, the more severe the withdrawal symptoms.
The CPP procedure was performed according to our previous study (Qiao et al., 2021). During the conditioning test, an escalating morphine was administrated to induce the morphine-paired side preference. On the test day, mice were allowed to freely explore the chambers for 15 min without injections. The time spent in each chamber was determined using a video tracking system. During the extinction training, all mice were given saline (i.p., 10 ml/kg) once daily and were immediately confined to the chambers for 45 min. The day after every 2 rounds (4 days) of extinction training, the place preference of the mice was tested for 15 min until the mice exhibited no preference for the morphine-paired side. The mice that showed CPP extinction were subjected to stress-induced reinstatement. On the next day, mice were shuttled from the CPP testing room to an intentionally different adjacent room with the shock apparatus, put in the shock box for 5 min of habituation, and then exposed to 15 min of random shocks (0.8 mA) that lasted 0.5 s each with an intershock interval from 10 to 70 s (mean of 40 s) (Nygard et al., 2016). After the 20-min foot shock stress, the stress-induced reinstatement was tested by allowing the mice to freely explore the CPP chambers for 15 min.
The EPM consists of two open arms (33 cm × 6 cm) and two closed arms (33 cm × 6 cm) intersecting at 90 degrees in the form of a plus, with a central area (6 cm × 6 cm). The maze was elevated 50 cm from the floor. Each mouse was placed in the center of the apparatus for a test for 5 min. The number of entries and the time spent in the open arm were recorded by ANY-maze software (Stoelting Company, Wood Dale, IL, USA) as a measure of anxiety. Between each trial, the maze was cleaned with 50% ethanol.
Mice were placed into an open-field box (45 × 45 × 30 cm) under dim light (80 lx) for 15 min. The ANY-maze software was used to record the movement trail and analyze the locomotor activity of mice. The total time spent in the central field (30 × 30 cm) was measured as an index of anxiety.
Mice were fixed in a stereotaxic frame (RWD, Shenzhen, China) under isoflurane anesthesia. Two holes were drilled in the skull of each mouse above the intended site of injection (BLA: AP – 1.4 mm, ML ± 3.4 mm, DV – 4.7 mm; vHip: AP – 3.1 mm, ML ± 3.3 mm, DV – 4.2 mm); 150–200 nl of the virus was delivered by 40 nl/min at each intended site through a Hamilton microsyringe with a microinjection pump (RWD, Shenzhen, China). After each injection, the needle was left in place for >10 min to allow for the diffusion of the virus and then slowly withdrawn.
For the optical experiment, animals were implanted bilaterally with optical fibers (200 mm core, numerical aperture = 0.37) held in a ceramic ferrule (Fibers, Shanghai, PRC) in vHip (−3.1 AP, ± 3.3 ML, and −3.7 DV), and the implants were secured to the skull with dental cement. All mice were handled for 3 days before behavioral assays for 5 min per day to reduce stress introduced by contact with the experimenter; 1–5 min were allowed for recovery in the home cage from handling. The EPM test consisted of a 9-min session divided into three 3-min epochs: the pre-stimulation light-off epoch, the light-on epoch, and the post-stimulation light-off epoch, in order (off-on-off epochs) (Felix-Ortiz et al., 2013). The OFT consisted of a 15-min session in which there were three 5-min epochs (off-on-off epochs). For optogenetic inhibition of BLA to vHip inputs, we used a constant illumination of yellow light stimulation (5 mw, NewDoon Aurora 220).
Mor-A mice and saline control mice were used to analyze c-Fos expression. Mor-A mice and saline control mice were tested for anxiety-like behavior using the EPM 2w after morphine withdrawal. These mice were returned directly to their home cages after testing. After 90 min, mice were perfused transcardially with saline, followed by 4% (w/v) paraformaldehyde in 0.1 M phosphate buffer. Brains were post-fixed in 4% paraformaldehyde for 3 h and then transferred to 30% sucrose for 24 h. Twenty micrometer thick floating sections were obtained using a freezing microtome (CM1950, Leica). The sections were rinsed in 0.01 M PBS three times and blocked in 0.01 M PBS containing 10% normal donkey serum and 0.3% (v/v) Triton X-100 for 1 h at room temperature. The blocked sections were then incubated overnight at room temperature with the mouse anti-c-Fos (1:200; ab208942, Abcam) in PBS containing 0.3% (v/v) Triton X-100, 0.25% (w/v) λ-carrageenan, and 5% (v/v) donkey serum (PBS-XCD). Sections were incubated for 5 h at room temperature with Alexa594-conjugated donkey anti-mouse IgG (1:200; A11055, Invitrogen). The sections were observed with a Zen microscope (ZEN 3.2, ZEISS). The c-Fos+ cell number per mm2 and the percent of c-Fos+ + FG+/FG+ cell number were calculated.
Mor-A mice and saline control mice were used for KOR analysis after the behavior test. We synthesized the digoxigenin (DIG)-labeled antisense single-strand RNA probes of KOR (http://mouse.brain-map.org/) with a DIG RNA labeling kit (11277073910, Roche Diagnostic). Target sections were treated with 2% H2O2 in 0.1 M of DEPC-PB for 10 min. After rinsing with 0.1 M DEPC-PB and reacting in acetylation solution, the sections were pre-hybridized for 1 h at 58°C in hybridization buffer. Then, 1 μg/ml KOR RNA probe was added and hybridized at 58°C for 20 h. After rinsing in wash buffer for 20 min two times at 58°C, the hybridized sections were incubated with 20 μg/ml ribonuclease A for 30 min at 37°C. The sections were incubated overnight with 0.5 μg/ml peroxidase-conjugated anti-digoxigenin sheep antibody (11-207-733-910; Roche Diagnostics). We performed the biotinylated tyramine (BT)-glucose oxidase (GO) amplification method to amplify the KOR hybridization signals. The sections were subsequently treated with 10 μg/ml Fluorescein Avidin D (A2901, Sigma) for 5 h. Then, the sections were observed with a Zen microscope (ZEN 3.2, ZEISS).
The brains were removed, and BLA and vHip were carefully dissected. The Western blotting procedure was conducted as described in our previous study (Qiao et al., 2021). The dilutions of primary antibodies were as follows: KOR (1:1,000, ab183825, Abcam) and GAPDH (internal control, 1:2,000, ab8245, Abcam). All species-appropriate horseradish peroxidase-conjugated secondary antibodies were used at a dilution of 1:10,000. The KOR protein expression level was normalized to GAPDH expression and presented as relative quantifications.
The RNA isolation, reverse transcription, and quantitative real-time PCR were carried out as described previously (Qiao et al., 2021). The level of KOR mRNA expression was analyzed by the fold change relative to GAPDH expression. The relative mRNA level was analyzed as the difference from the experimental relative to the control condition. KOR primer sequences were designed by Takara Bio Inc. (Beijing, China) and are described as follows: Oprk: forward (5′-3′): CATTTGGCTCCTGGCATCATC, reverse (5′-3′): AGGAGCATTCAATGACATCCACA; Gapdh: forward (5′-3′): TGTGTCCGTCGTGGATCTGA, reverse (5′-3′): TTGCTGTTGAAGTCGCAGGAG.
Statistical analyses were conducted using GraphPad Prism 8.0 (GraphPad Software Inc., La Jolla, CA). The data passed the normality and homogeneity of variance test. The behavior and withdrawal syndrome test were analyzed by unpaired Student's t-test. Chemogenetics and KOR knockdown experiments were analyzed by two-way ANOVA. Between-group comparisons were done only when there was a statistical interaction (Sidak's post-hoc test). Parameters of CPP and optogenetic experiments were analyzed by the repeated measure two-way ANOVA (RM-ANOVA). Multiple comparison was done by Sidak's post-hoc test. Others were analyzed by unpaired Student's t-test. The results are presented as the mean ± standard error of the mean (SEM) (Supplementary Table 1). Differences were considered significant at p < 0.05. Investigators were blinded to the allocation of groups and outcome assessment for all experiments.
Population studies have revealed that opioid addicts suffer from anxiety after withdrawal (Williams et al., 2001; Chu et al., 2009). This study utilized a chronic morphine regimen to develop a mouse morphine-withdrawal anxiety model (Figure 1A). Mice were treated with naloxone (2 mg/kg, s.c.) 2 h after the last morphine injection. We observed a robust somatic syndrome in the morphine-withdrawn mice (p = 0.0030, Figure 1B), while this syndrome was not present 1 week after morphine withdrawal. Due to the administration of naloxone, these groups were not used to test the withdrawal anxiety-like behavior. Next, we used another group to assess the negative emotional signs of morphine withdrawal. Anxiety-like behaviors were assessed using the EPM and OFT at 1, 2, and 4 weeks after withdrawal. After 1 week of withdrawal, mice spent less time in the open arms of the EPM (p = 0.0476, Figure 1C) and the central area of the OFT (p = 0.0397, Figure 1D) compared with saline control mice. Intriguingly, anxiety-like behavior was more pronounced after 2 weeks of withdrawal from morphine. After 2 weeks of morphine withdrawal, mice spent significantly less time in the open arms (p = 0.0254, Figure 1E) and exhibited decreased numbers of open-arm entries (p = 0.0230, Figure 1E). In addition, the differences in the central time and central distances in the OFT were statistically significant (p = 0.0062, p = 0.0181, Figure 1F). However, after 4 weeks of morphine withdrawal, the mice did not exhibit any anxiety-like behaviors (p > 0.05, Figures 1G,H). Also, the total distances were not different between these two groups, indicating that morphine withdrawal did not alter locomotion in the mice. Therefore, mice withdrawn from morphine for 2 weeks that showed anxiety (Mor-A) were used for subsequent experiments.
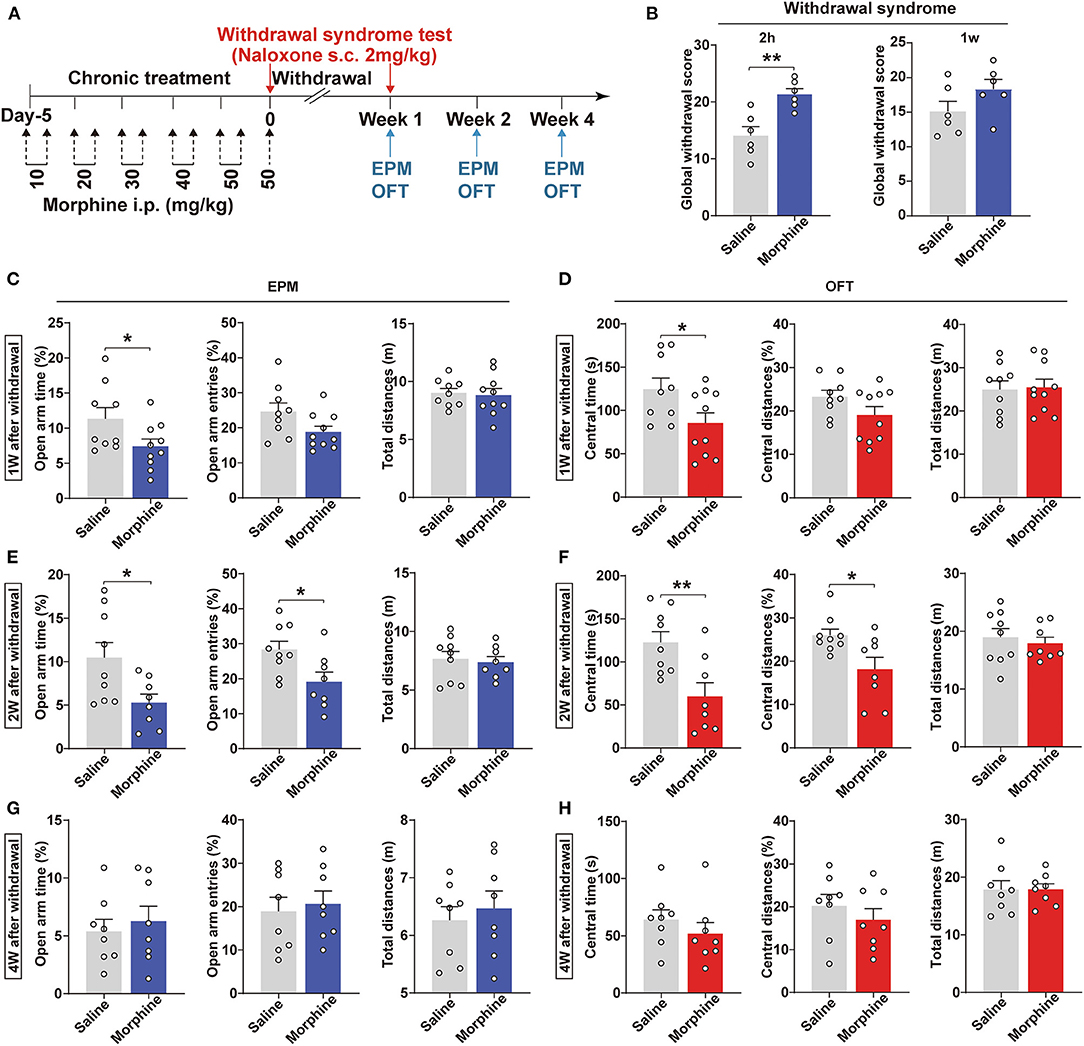
Figure 1. Morphine-withdrawn mice show anxiety-like behaviors. (A) Schematics of the experiment. (B) The global withdrawal score of morphine was higher than saline after withdrawal 2 h from morphine; 2 mg/kg of naloxone was injected subcutaneously (s.c.) into morphine and saline mice. Anxiety-like behavioral tests using the EPM and OFT at 1 week (C,D), 2 weeks (E,F), and 4 weeks (G,H) of withdrawal. Data were expressed as the mean ± SEM. (B) n = 6/group, (C–F) n = 8–10/group *P < 0.05, **P < 0.01, compared with saline group.
Previous studies suggested that BLA excitability was positively associated with increased anxiety-like responses and addiction (Sharp, 2017; Daviu et al., 2019). One distal BLA projection target implicated in anxiety-related behaviors was the vHip. The BLA provides glutamatergic inputs to the vHip (Felix-Ortiz et al., 2013). We confirmed the projections from the BLA to vHip and investigated whether the BLA and vHip were activated. We used virus-delivered trackers to delineate the circuit to map the connection between BLA and vHip. By injecting the anterograde tracker (AAV2/9-hSyn-eGFP, titer: 1.91 × 1013 vg/ml, OBiO) into the BLA, we observed robust expression of mCherry in the vHip (Figure 2A, top). Conversely, a retro adeno-associated virus (retroAAV-hSyn-mCherry, titer: 6.33 × 1013 vg/ml, OBiO) was injected into the vHip retrogradely labeled neurons in the BLA (Figure 2A, bottom). These results demonstrated that the BLA shared projections to the vHip. To confirm neuronal activation in both the BLA and vHip in Mor-A mice and the saline control mice, we measured the expression of an immediate-early gene product, c-Fos, which is a surrogate molecular marker of neuronal activity. We stained for c-Fos in the BLA and vHip using immunofluorescence. Robust c-Fos signals were detected in the BLA (p = 0.0008, Figure 2B) and vHip (p = 0.0001, Figure 2C) of Mor-A mice. To further determine that the BLA to vHip projections were activated in the Mor-A mice, we injected the retrograde tracer fluorogold (FG) into the vHip and performed FG/c-Fos double labeling in the BLA of the Mor-A mice and control mice (Figure 2D). Our results revealed an increased percentage of c-Fos+ + FG+ cells (calculated as c-Fos+FG+ double-labeled cells/total FG positive cells) in the BLA of Mor-A mice compared with the saline control mice (p = 0.0020, Figure 2E), verifying that the BLA to vHip projections were involved in the process of anxiety-like behaviors following morphine withdrawal.
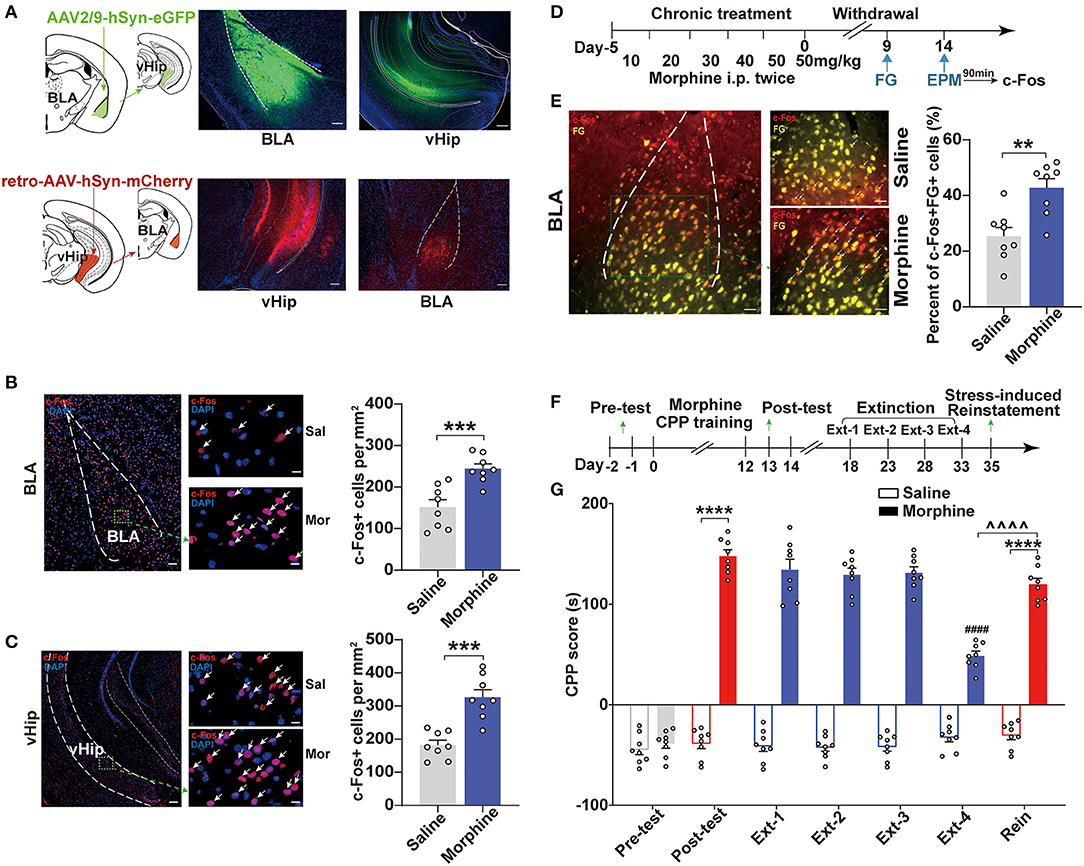
Figure 2. BLA to vHip projections are involved in the anxiety-like behaviors induced by morphine withdrawal. (A) Schematic for tracing projections from the BLA to the vHip in wild-type mice. Representative coronal images of the injection site and tracing terminals. (Top) The anterograde virus AAV2/9-hSyn-eGFP was delivered in the BLA. (Bottom) The retrograde virus retro-AAV-hSyn-mCherry was delivered in the vHip. Scale bar: 200 μm. Representative immunofluorescence images of c-Fos (red) in the BLA [(B), left] and vHip [(C), left]. The nuclei were stained with DAPI (blue). Scale bar: 200 μm. Arrows indicate neurons co-labeled with c-Fos. Regions enclosed in a green box are shown at higher magnification in the images to the right. Scale bars: 50 μm. The numbers of c-Fos-positive neurons per mm2 in the BLA [(B), right] and vHip [(C), right]. (D) Timeline of c-Fos and fluorogold (FG) co-expression in BLA of Mor-A mice. [(E), Left] Representative coronal image of c-Fos (red) and fluorogold (FG, yellow) immunostaining in the BLA. Scale bar: 100 μm. Regions enclosed by a green box are shown at higher magnification in the images to the right. Arrows indicate neurons co-labeled with c-Fos and FG. Scale bars: 50 μm. (Right) The percentage of neurons co-labeled with c-Fos+ and FG+ relative to FG in the BLA. (F) Schematic of the experimental design for the training and testing of morphine-CPP acquisition, extinction, and reinstatement. (G) CPP scores in the morphine-CPP expression, extinction, and reinstatement tests. Morphine-induced CPP, extinction at Ext-4 and reinstatement induced by stress. In the CPP results, #: comparison between the Extinction-4 of morphine group and the post-test of morphine group, ∧: comparison between the reinstatement of morphine group and the Extinction-4 of morphine group; *: comparison between the 2 indicated groups. BLA, basolateral amygdala. vHip, ventral hippocampus. Data are expressed as the mean ± SEM. **P < 0.01, ***P < 0.001, ****P < 0.0001, ∧∧∧∧P < 0.0001, ####P < 0.0001. n = 6–8/group.
Individuals with stress or anxiety disorders are particularly vulnerable to opioid addiction (Conway et al., 2006). In this study, the morphine group showed a significant preference for the morphine-paired chamber (p < 0.0001, Figures 2F,G). After 4 rounds of extinction (~2 weeks of withdrawal), the morphine-paired preference was significantly diminished in the Mor-A mice (p < 0.0001, Figure 2G). However, the morphine-paired preference of the Mor-A mice was restored by foot shock (p < 0.0001, Figure 2G). Furthermore, the reinstatement CPP score of the Mor-A mice significantly differed from the Ext-4 CPP score of the Mor-A mice (p < 0.0001, Figure 2G). This observation indicated that the preference in the morphine CPP mice was diminished after 4 rounds of extinction training in this study. Taken together, the BLA to vHip projections were involved in the anxiety-like behaviors induced by morphine withdrawal, and the Mor-A mice were prone to stress-induced reinstatement.
Population studies have shown that increased amygdala: hippocampus volume ratios are associated with increased anxiety severity (MacMillan et al., 2003). Animal studies have demonstrated that activation of the BLA-vHip inputs robustly increased anxiety-related behaviors (Felix-Ortiz et al., 2013). To explore whether BLA to vHip projections regulated anxiety-like behaviors following morphine withdrawal, we used a combination of retrograde viral and chemogenetic approaches to silence the activity of neurons involved in the BLA to vHip projections. Specifically, we selectively expressed the inhibitory designer Gi-coupled human muscarinic receptor 4 (hM4Di) in BLA to vHip projections and assessed anxiety-like behaviors in the presence of the selective exogenous ligand clozapine-N-oxide (CNO) that inhibited BLA to vHip projections (Figure 3A). The hM4Di-receptor was expressed exclusively in the glutamatergic projections from the BLA to vHip using a Cre-dependent approach [AAV9-EFIa-DIO-hM4Di (Gi)-mCherry, titer: 5.80 × 1013 vg/ml, OBiO; retroAAV-CaMK2-GFP-2A-Cre, titer: 4.36 × 1012 vg/ml, OBiO] (Figure 3B). Two weeks later, the mice were intraperitoneally injected with 3 mg/kg CNO or DMSO, and behavioral tests were conducted 30 min later. Two-way ANOVA showed significant main effects of morphine × CNO interactions [OFT: central time: F(1,29) = 12.40, p = 0.0014, central distances%: F(1,29) = 12.66, p = 0.0013; EPM: open-arm time%: F(1,25) = 7.876, p = 0.0096, open arm entries%: F(1,25) =16.16, p = 0.0005]. Compared with the Mor-A × DMSO mice, post-hoc analysis revealed that inhibition of the BLA to vHip projections in morphine-withdrawn mice (Mor-A × CNO) prevented the decreased time (p = 0.0085, Figure 3C) and decreased traveled distances (p = 0.0224, Figure 3C) in the central zone of the OFT. The decreased open-arm time (p = 0.0019, Figure 3D) and decreased entries (p = 0.0065, Figure 3D) in the EPM were also prevented. Chemogenetic inhibition of the BLA to vHip projections did not affect locomotor activity in Mor-A mice (p > 0.05, Figure 3C). Then, we used RM-ANOVA to analyze whether the inhibition of BLA to vHip inputs affected the morphine CPP process. The Mor-A × CNO mice showed a diminished morphine-paired preference at Ext 3 (p = 0.0216, Figure 3E), but the inhibition did not prevent the stress-induced reinstatement (p < 0.0001, Figure 3E). These results indicated that the bilateral inhibition of BLA terminals in the vHip ameliorated morphine-withdrawal-induced anxiety-like behaviors and accelerated the decreased rate of morphine-paired preference in morphine CPP.
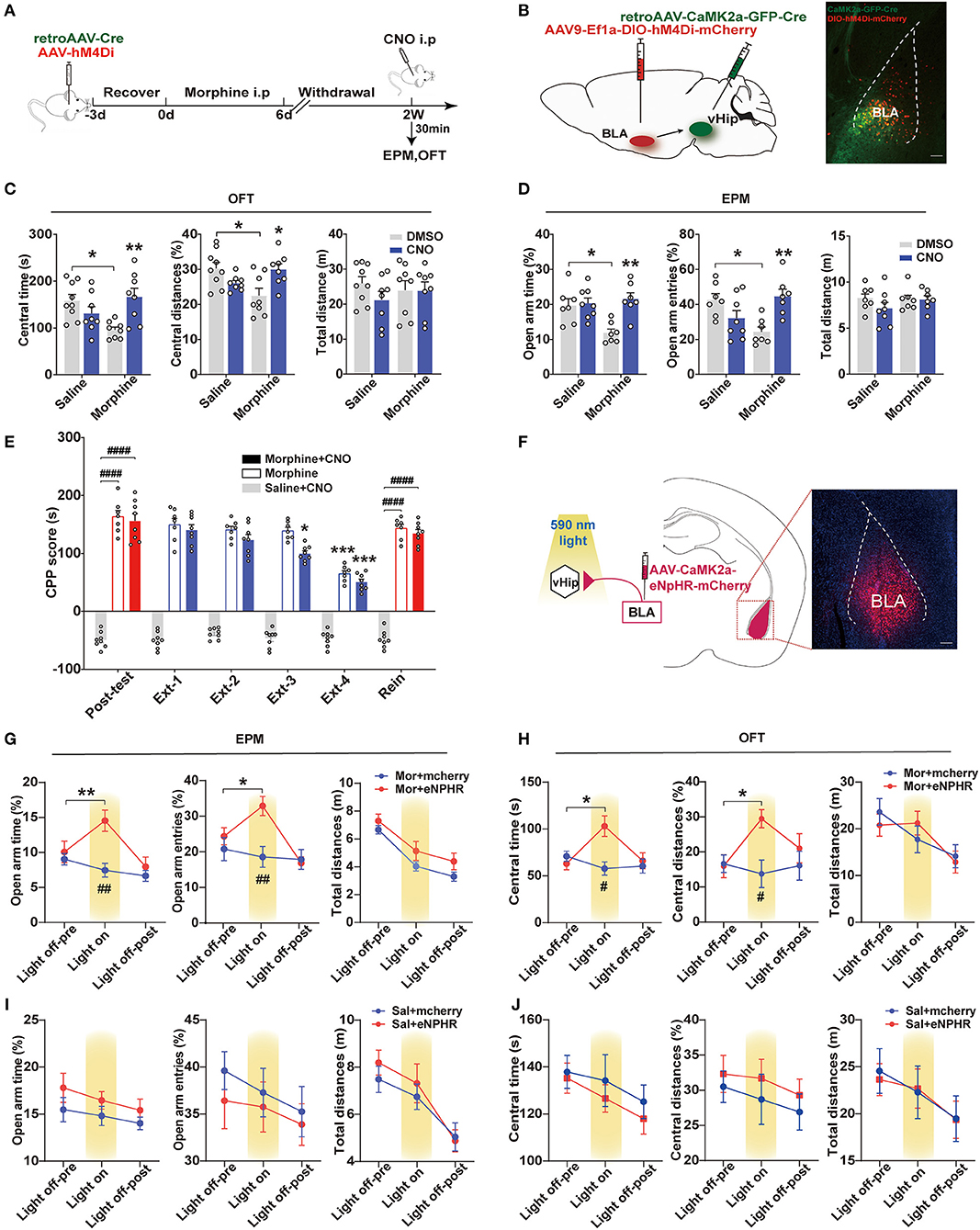
Figure 3. BLA to vHip projections regulate anxiety-like behavior in morphine-withdrawn mice. (A–E) Chemogenetic experiment. (A) Workflow for the chemogenetic experiment. (B) Schematic (left) and representative image (right) of chemogenetic virus injection. Scale bar: 200 μm. (C) The Mor-A×CNO mice exhibited significantly increased central time and central distances in the OFT. (D) The Mor-A × CNO mice exhibited significantly increased open-arm times and entries in the EPM. (E) The hM4Di inhibition accelerated the decreased rate of morphine-paired preference in the Mor-A mice. (F–H) Optogenetic experiment. (F) Schematic of the virus injection site in the BLA and optical fiber implantation site in the vHip. (Right) Image of a coronal brain slice showing the expression of eNpHR-mCherry in the BLA. Scale bar: 200 μm. (G) Increased open-arm entries and time spent during the light-on epoch. (H) Increased time spent and distances traveled in the central area of the OFT during the eNpHR illumination epoch. The EPM (I) and OFT (J) tests of the saline + eNpHR group and the saline + mCherry group. Data are expressed as the mean ± SEM. In the chemogenetic experiment, morphine CNO-treated mice were compared with morphine DMSO-treated mice. In the CPP results, *: comparison between the extinction group and the post-test group, #: comparison between the 2 indicated groups. In the optogenetic results, #: comparison between the Mor+mcherry and the Mor+eNPHR group during the light on epoch. n = 8–10/group.*P < 0.05, **P < 0.01, ***P < 0.001, #P < 0.05, ##P < 0.01, ####P < 0.0001.
To further confirm these results, an optogenetic approach inhibiting BLA to vHip projections was used to assess changes in anxiety-like behaviors. AAVs carrying either the inhibitory opsin, inhibitory natronomonas pharaonis halorhodopsin (eNpHR3.0), or mCherry fluorescent protein (red) under the control of the CamKIIα promoter were injected bilaterally into the BLA (AAV-CaMK2-eNpHR-mCherry, titer: 2.63 × 1012 vg/ml; AAV-CaMK2-mCherry, titer: 2.79 × 1012 vg/ml BrainVTA), and optical fibers were implanted in the vHip of mice (Figure 3F). The Mor-A mice were tested in the EPM for 9 min, with alternating 3-min periods of no illumination, illumination, and no illumination. RM-ANOVA revealed a significant main effect of light × morphine interactions on EPM [open-arm time%: F(2,28) = 17.07, p < 0.0001, open-arm entries%: F(2,26) = 8.749, p = 0.0012]. Mor-A mice expressing eNpHR (Mor-A × eNpHR) exhibited decreased open-arm avoidance, as evidenced by more entries (p = 0.0253, Figure 3G) and time spent in the open arms (p = 0.0077, Figure 3G). The mice were also tested in the OFT for 15 min (5 min off−5 min on−5 min off). RM-ANOVA revealed a significant main effect of light × morphine interactions on OFT [central time: F(2,28) = 8.633, p = 0.0012, central distances%: F(2,28) = 5.491, p = 0.0097]. Bilateral inhibition of BLA terminals in the vHip decreased center avoidance in the Mor-A mice with more spent time (p = 0.0290, Figure 3H) and increased traveled distances (p = 0.0101, Figure 3H) in the central area of the OFT. Locomotor behavior was not affected by illumination in either the mCherry or eNpHR group (p > 0.05, Figures 3H–J). These optogenetic results suggested that specific inhibition of this pathway ameliorated anxiety-like behaviors in the Mor-A mice. Collectively, the chemogenetic and optogenetic inhibition results suggested that the BLA to vHip projections were important for anxiety-like behavior induced by morphine withdrawal.
Evidence has suggested that KORs in the BLA modulate anxiety-like behaviors (Knoll et al., 2011). Therefore, we assessed the expression levels of KORs in the BLA. Western blot analysis revealed that the Mor-A mice exhibited a higher relative level of KOR in the BLA (p = 0.0372, Figure 4A). These results were confirmed by in situ hybridization. The KOR mRNA expression levels were significantly higher in the BLA of Mor-A mice (p = 0.0012, Figure 4B). Then, we injected fluorogold (FG) into the vHip and performed KOR mRNA in situ hybridization in the BLA. We observed increased numbers of FG+ and KOR mRNA double-labeled cells in the BLA of the Mor-A mice (p = 0.0191, Figure 4C), indicating that the KOR mRNA expression level in BLA to vHip projection neurons was also increased. We further determined whether KORs in the BLA to vHip projections modulated anxiety-like behaviors induced by morphine withdrawal. We used KORloxp/loxp mice to knock down KORs in the BLA to vHip projections conditionally. Specifically, the KORloxp/loxp mice were stereotaxically injected with AAV-Ef1a-fDIO-EGFP-2A-Cre (titer: 1.12 × 1013 vg/ml, OBiO) into the BLA and retro-AAV-CaMK2-mCherry-Flpo (titer: 4.37 × 1012 vg/ml, OBiO) into the vHip for widespread Cre-recombinase expression (Figure 4D). Two-way ANOVA revealed a significant main effect of Cre-recombinase expression on the KOR expression level [mRNA: F(1,23) = 0.3128, p = 0.5814; protein: F(1,17) = 0.3308, p = 0.5727]. The post-hoc test indicated that the KOR mRNA (p < 0.0001, p < 0.0001; Figure 4E) and protein expression levels (p < 0.0001, p < 0.0001; Figure 4F) were dramatically decreased in the KORloxp/loxp mice expressing Cre-recombinase, confirming that KOR transgene mice were an effective tool for manipulating KOR expression. Two-way ANOVA showed a significant main effect of KOR knockdown × morphine interaction on anxiety-like behavior induced by morphine withdrawal [EPM: open-arm time%: F(1,27) = 7.792, p = 0.0095, open-arm entries%: F(1,27) = 8.367, p = 0.0075; OFT: central time: F(1,23) = 4.928, p = 0.0366; central distances%: F(1,23) = 10.26, p = 0.0039]. The KORloxp/loxp Mor-A mice expressing Cre-recombinase spent more time (p = 0.0226, Figure 4G) and exhibited more entries (p = 0.0323, Figure 4G) into the open arms of the EPM, and spent more time (p = 0.0301, Figure 4H) and traveled a greater distance (p = 0.0078, Figure 4H) in the central area of the OFT. These results indicated that knocking down KORs in the BLA to vHip projections ameliorated the anxiety-like behaviors but did not affect CPP extinction or stress-induced reinstatement in the Mor-A mice (p > 0.05, Figure 4I). Taken together, these results revealed that KORs in the BLA to vHip projections maintained the basal level of anxiety-like behaviors induced by morphine withdrawal.
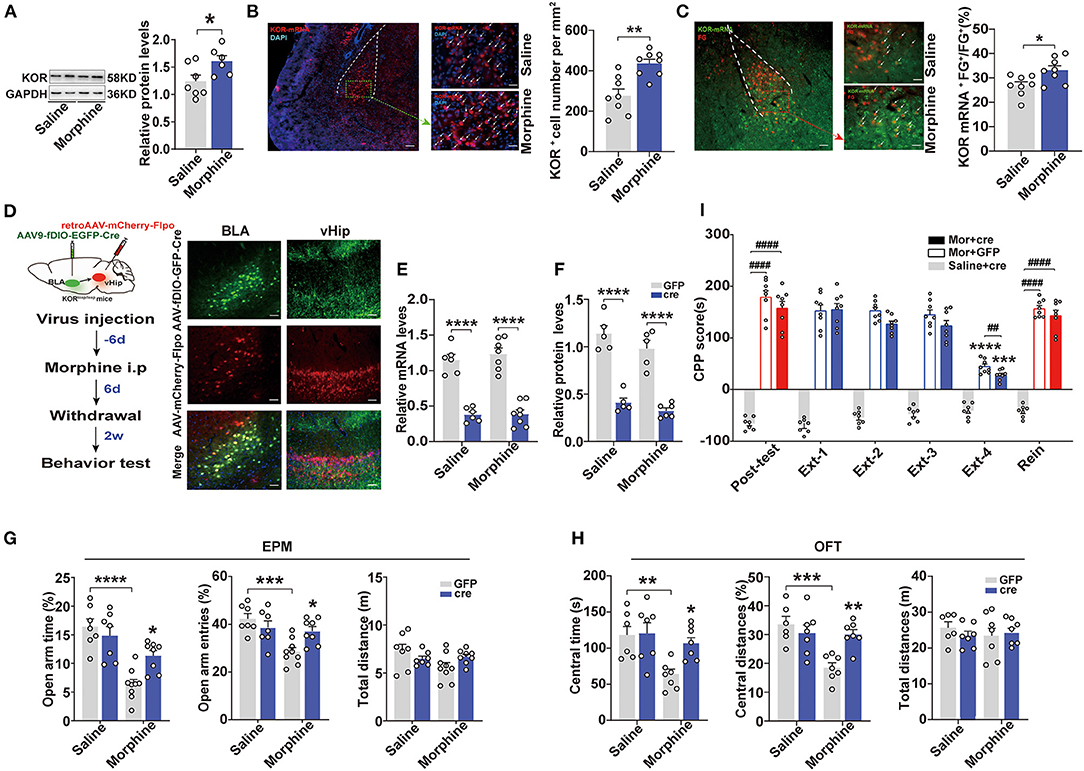
Figure 4. Specific knockdown of the kappa opioid receptor in the BLA to vHip projections ameliorate anxiety-like behaviors in morphine-withdrawn mice. (A) Immunoblots and quantification analysis of KOR levels in the BLA. KOR protein expression levels increased in the BLA of the Mor-A mice. (B) (Left) Representative images of KOR mRNA (red) in the BLA of the Mor-A mice. The nuclei were stained with DAPI (blue). Scale bar: 200 μm. BLA regions enclosed by a green box are shown at higher magnification in images to the right. Scale bars: 50 μm. (Right) KOR+ cell numbers per mm2 in the BLA of the Mor-A mice were increased. (C) Co-labeled neurons for KOR mRNA and FG in the BLA. Retrograde FG was injected into the vHip. (Left) Representative images of KOR mRNA (green) and fluorogold (FG; red) immunostaining in the BLA. Scale bar: 200 μm. BLA regions enclosed by a red box are shown at higher magnification in the images to the right. Arrows indicate neurons co-labeled with FG and KOR mRNA. Scale bars: 50 μm. (Right) The percentage of neurons co-labeled with KOR mRNA and FG relative to FG in the BLA. (D) (Left) Schematic of the KOR knockdown from the BLA to vHip projections in the KORloxp/loxp mice. (Right) Representative coronal images of virus injection in the BLA and vHip. Scale bar: 100 μm (E) KOR mRNA and (F) KOR protein expression levels in the BLA of the KORloxp/loxp mice with Cre-recombinase expression were decreased. (G) The Mor-A mice with KOR knockdown exhibited increases in open-arm time and entries in the EPM. (H) The Mor-A mice with KOR knockdown exhibited increases in central time and central distances in the OFT. (I) Knockdown of KOR in the BLA to vHip projections did not affect the morphine CPP. Data are expressed as the mean ± SEM. The KOR knockdown experiment compared the Mor-A mice expressing Cre-recombinase with Mor-A mice expressing GFP. In the CPP results, *: comparison between the extinction group and the post-test group. #: comparison between the 2 indicated groups. n = 6–8/group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001; ##P < 0.01, ####P < 0.0001.
Generally, KORs inhibit adenylate cyclase activity by interacting with inhibitory Gα subunits to decrease cell excitability and neurotransmitter release (Crowley and Kash, 2015). Therefore, we hypothesized that the BLA to vHip projections and KORs co-regulated the reinstatement of morphine CPP. To test this hypothesis, we combined chemogenetic approaches with in vivo pharmacological manipulations (nor-BNI, a long-lasting KOR antagonist, 10 mg/kg) and assessed anxiety-like behaviors and morphine CPP (Figure 5A). Interestingly, a two-way ANOVA revealed that the combined approach improved anxiety-like behaviors and prevented the stress-induced reinstatement of morphine CPP (p < 0.0001, Figures 5B–D). Systemic injection of the nor-BNI alone may prevent the stress-induced morphine CPP reinstatement independent of the BLA to vHip projection, but a synergistic action of the projection and the KORs is possible.
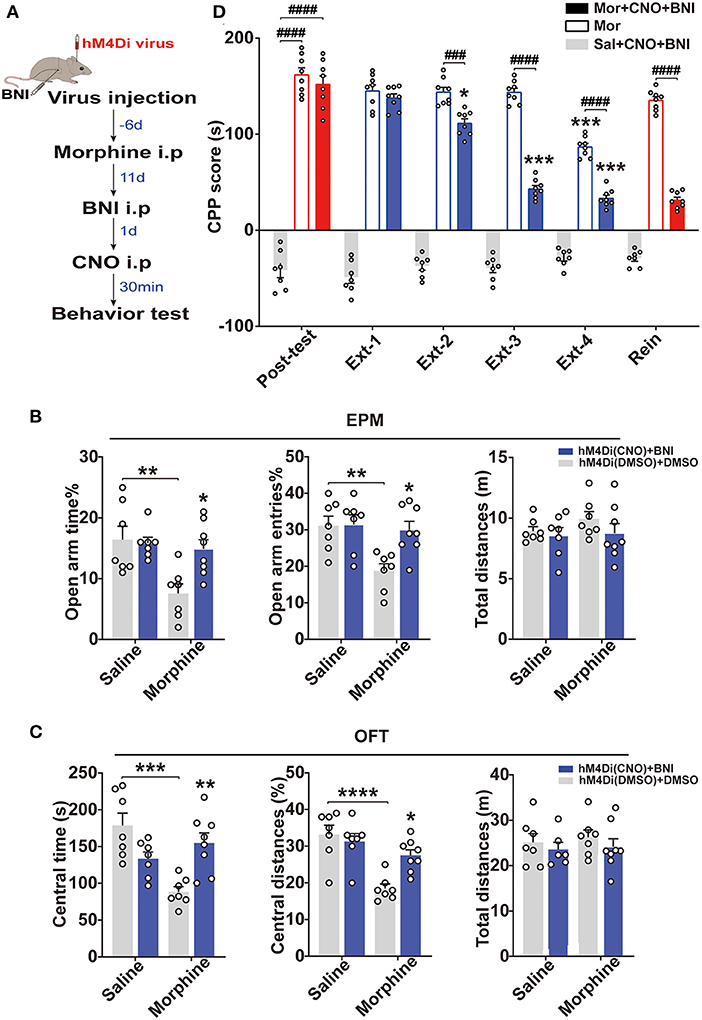
Figure 5. The simultaneous intervention of KORs and the BLA to vHip projections prevented the reinstatement of morphine CPP and anxiety-like behaviors in morphine-withdrawn mice. (A) Timeline of experiment. (B) The inhibition of the BLA to vHip projections combined with nor-BNI administration increased the open-arm time in and entries into the EPM by the Mor-A mice. (C) The inhibition of the BLA to vHip projections combined with nor-BNI administration increased the time spent and distances traveled in the central area of the OFT by the Mor-A mice. In (B,C), the blue histogram represents the hM4Di (CNO) + BNI group, in which the mice were first injected with the chemogenetic virus (hM4Di). Then, the mice morphine withdrawal anxiety model was constructed, and BNI was injected 2 weeks after withdrawal. The next day, the mice were injected with CNO and tested for anxiety-like behavior 30 min later. CNO and BNI were dissolved in 0.5% DMSO. The white histogram represents the hM4Di (DMSO) + DMSO group. (D) The inhibition of the BLA to vHip projections combined with nor-BNI administration prevented the reinstatement of morphine CPP. nor-BNI: a long-lasting KOR antagonist. Data are expressed as the mean ± SEM. In the CPP results, *: comparison between the extinction group and the post-test group. #: comparison between the 2 indicated groups. n = 7–10/group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, ###P < 0.001, ####P < 0.0001.
We demonstrated that inhibition of the BLA to vHip projections in mice represented an essential neural substrate for anxiety-like behaviors during morphine withdrawal. KORs in the BLA inputs to the vHip were involved in the anxiety-like behaviors after morphine withdrawal. The morphine-withdrawn mice with obvious anxiety-like behaviors were particularly prone to stress-induced reinstatement of morphine CPP. Furthermore, combined treatment of inhibition of BLA to vHip projections and administration of a KOR antagonist ameliorated anxiety-like behaviors and prevented stress-induced CPP reinstatement after morphine withdrawal.
Anxiety is a critical negative emotional state that emerges during drug withdrawal. In rodents, the EPM is one of the most common protocols used to screen the anxiolytic effects of drugs (Lister, 1987). The EPM is also useful for investigating the biological basis of addiction and withdrawal (Schulteis et al., 1998; Zanos et al., 2016; Masukawa et al., 2020). In our study, anxiety-like behaviors appeared during the first 2 weeks after morphine withdrawal and affected stress-induced reinstatement to drug seeking. This observation indicated that reinstatement could be caused by negative affective states that drive motivated behaviors (Koob and Le Moal, 2008; Koob and Volkow, 2010; Martins et al., 2012). Immunostaining in this study revealed significantly increased numbers of c-Fos-positive cells within the BLA to vHip projections of the Mor-A mice. The BLA is a central component of the neural circuitry governing anxiety-related information and is involved in alcohol (Harper et al., 2019), cocaine (Ladron de Guevara-Miranda et al., 2016), amphetamine (Navarro et al., 2004), and morphine (Niu et al., 2017) withdrawal-related behaviors. One region downstream of the BLA that has been implicated in anxiety-like behaviors in rodents is the vHip (Felix-Ortiz et al., 2013). The vHip contributes to increased anxiety-like behaviors during morphine (Zarrindast et al., 2010) and amphetamine withdrawal (Bray et al., 2016). Although these regions have been associated with addiction and anxiety, there is no previously published evidence demonstrating how these brain areas interact to maintain anxiety-like behaviors during addiction.
In vivo optogenetic/chemogenetic inhibition of BLA terminals in the vHip reduced anxiety-like behaviors and accelerated the decreased rate of morphine-paired preference, suggesting that BLA inputs to the vHip were required to maintain basal levels of anxiety-related behaviors after morphine withdrawal. Previous reports revealed that photostimulation of BLA projection neurons targeting the vHip increased anxiety-like behaviors, whereas photosilencing this pathway had the opposite effect (Felix-Ortiz et al., 2013; Namburi et al., 2015). The BLA controls numerous behaviors, including anxiety and reward seeking, via the activity of glutamatergic principal neurons. A previous study showed that intra-vHip glutamate receptor antagonism attenuated the effects of optogenetic stimulation, demonstrating that glutamatergic transmission from the BLA to the vHip was critical for mediating light-induced changes in social behaviors (Allsop et al., 2014). These findings demonstrated that excitatory projections from the BLA to the vHip were sufficient to mediate anxiety (Felix-Ortiz et al., 2013). The amygdala also has been central to concepts involving addiction, where it has been proposed to mediate craving and the abnormal attribution of motivational significance to drug-associated cues and contexts (Torregrossa et al., 2011). The hippocampus plays a critical role not only in learning and memory but also in the acquisition and expression of reward-related learning in the process of drug abuse and addiction (Farr et al., 2000; Nestler, 2001b; Moron et al., 2010). We found that inhibition of the BLA to vHip circuit accelerated the decreased rate of morphine-paired preference but did not prevent reinstatement in morphine CPP. Therefore, this study identified that the BLA to vHip circuit governs anxiety-like behaviors after morphine withdrawal.
The KORs in the BLA are an attractive target for neural influence over stress-related behaviors and emotional regulation (Knoll et al., 2011). Evidence has indicated that KORs regulate the neuronal activity of BLA outputs (Knoll et al., 2011), suggesting an important role of KOR within the BLA in opioid withdrawal-related behaviors. Based on the KOR mRNA in situ hybridization analysis, the KORs in the BLA afferents onto the vHip were increased in the Mor-A mice. We further found that the knockdown of KORs in the BLA to vHip projections prevented anxiety-like behaviors in the Mor-A mice. Indeed, BLA KOR signaling was necessary and sufficient for KOR-mediated anxiety (Knoll et al., 2011; Tejeda et al., 2015). Furthermore, KOR antagonism in the BLA produced anxiolytic effects (Bruchas et al., 2009; Carroll and Carlezon, 2013). Thus, KORs might mediate negative affective states by modulating neuronal activity in the BLA to vHip projections. We provided functional evidence that KOR knockdown in the BLA synapses in the vHip modulated anxiety-like behaviors in the morphine-withdrawn mice.
The KOR antagonists might have therapeutic benefits for treating mood disorders and drug addiction by promoting stress resilience (Carroll and Carlezon, 2013). However, less is known about the effect on opioid relapse when KOR antagonists are administered in combination with circuit inhibition. Our results indicated that BLA inputs to the vHip were capable of modulating anxiety-like behaviors after morphine withdrawal. Therefore, we used a novel combined approach (inhibition of BLA to vHip projections and KOR antagonism) to prevent stress-induced CPP reinstatement in the Mor-A mice. The results demonstrated that at the system level, projection inhibition (chemogenetic) combined with nor-BNI injections (i.p., 10 mg/kg) ameliorated anxiety-like behaviors and prevented stress-induced reinstatement of morphine CPP. Systemic injection of the nor-BNI alone may prevent the stress-induced morphine CPP reinstatement independent of the BLA to vHip projection, but a synergistic action of the projection and the KORs is possible. Thus, our novel strategy of pathway-specific inhibition with KOR antagonism might prove beneficial in treating anxiety symptoms and relapse during opioid withdrawal.
Collectively, our findings provided a greater understanding of the pathway specificity that underlies emotional valence following chronic morphine cessation and the function of KORs in the generation of negative affective states. The results established a combined protocol that used a chemogenetically inspired approach and KOR antagonist administration that effectively controlled the anxiety-like behavioral phenotype and stress-induced reinstatement of morphine CPP. Our findings suggested novel targets for treating comorbidities of anxiety and opioid addiction.
The BLA sends projections to several limbic, cortical, and thalamic regions, including the ventral hippocampus (vHip) (Felix-Ortiz et al., 2013), the medial prefrontal cortex (mPFC) (Felix-Ortiz et al., 2016), and the lateral hypothalamic area (LHA) (Jimenez et al., 2018). Although the BLA to vHip projections were studied in this study, other BLA projections required additional investigation. In addition, naloxone, an opioid antagonist, was used to induce the morphine somatic withdrawal syndrome (Laschka et al., 1976; Koob et al., 1992; Iyer et al., 2020). Thus, the effect of naloxone on the anxiety-like behavior after morphine withdrawal should also be studied. Furthermore, the specific synaptic plasticity mechanism of the BLA to vHip projections and the molecular mechanism of KORs activation need to be further investigated.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by the Institutional Animal Care and Use Committee of Xi'an Jiaotong University.
YZ and JLa designed this study, revised the manuscript, and suggestions for the manuscript. CD, YJ, and XY conducted behavior tests and molecular experiments. CD, YF, and YL conducted chemogenetic and optogenetic experiments. PY, JLi, and SW did the statistical analysis. All the authors reviewed and approved the final version of the publication.
This work was supported by grants from the National Natural Science Foundation of China (No. 82001999) and the Natural Science Foundation of Shaanxi Province (Nos. 2020SF-132 and 2020JM-007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors would like to thank Pei Leilei from the Public Health School, Xi'an Jiaotong University, for the statistical analysis. The authors would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2022.894886/full#supplementary-material
Allsop, S. A., Vander Weele, C. M., Wichmann, R., and Tye, K. M. (2014). Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front. Behav. Neurosci. 8, 241. doi: 10.3389/fnbeh.2014.00241
Alvandi, M. S., Bourmpoula, M., Homberg, J. R., and Fathollahi, Y. (2017). Association of contextual cues with morphine reward increases neural and synaptic plasticity in the ventral hippocampus of rats. Addict. Biol. 22, 1883–1894. doi: 10.1111/adb.12547
Bertoglio, L. J., Joca, S. R., and Guimaraes, F. S. (2006). Further evidence that anxiety and memory are regionally dissociated within the hippocampus. Behav. Brain Res. 175, 183–188. doi: 10.1016/j.bbr.2006.08.021
Boyle, J. S., Bechtel, L. K., and Holstege, C. P. (2009). Management of the critically poisoned patient. Scand. J. Trauma Resusc. Emerg. Med. 17, 29. doi: 10.1186/1757-7241-17-29
Bray, B., Scholl, J. L., Tu, W., Watt, M. J., Renner, K. J., and Forster, G. L. (2016). Amphetamine withdrawal differentially affects hippocampal and peripheral corticosterone levels in response to stress. Brain Res. 1644, 278–287. doi: 10.1016/j.brainres.2016.05.030
Brice-Tutt, A. C., Wilson, L. L., Eans, S. O., Stacy, H. M., Simons, C. A., Simpson, G. G., et al. (2020). Multifunctional opioid receptor agonism and antagonism by a novel macrocyclic tetrapeptide prevents reinstatement of morphine-seeking behaviour. Br. J. Pharmacol. 177, 4209–4222. doi: 10.1111/bph.15165
Bruchas, M. R., Land, B. B., Lemos, J. C., and Chavkin, C. (2009). CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS ONE 4, e8528. doi: 10.1371/journal.pone.0008528
Bruijnzeel, A. W. (2009). kappa-Opioid receptor signaling and brain reward function. Brain Res. Rev. 62, 127–146. doi: 10.1016/j.brainresrev.2009.09.008
Butelman, E. R., Negus, S. S., Ai, Y., de Costa, B. R., and Woods, J. H. (1993). Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J. Pharmacol. Exp. Ther. 267, 1269–1276.
Butelman, E. R., Yuferov, V., and Kreek, M. J. (2012). kappa-opioid receptor/dynorphin system: genetic and pharmacotherapeutic implications for addiction. Trends Neurosci. 35, 587–596. doi: 10.1016/j.tins.2012.05.005
Carola, V., D'Olimpio, F., Brunamonti, E., Mangia, F., and Renzi, P. (2002). Evaluation of the elevated plus-maze and open-field tests for the assessment of anxiety-related behaviour in inbred mice. Behav. Brain Res. 134, 49–57. doi: 10.1016/s0166-4328(01)00452-1
Carroll, F. I., and Carlezon, W. A. Jr. (2013). Development of kappa opioid receptor antagonists. J. Med. Chem. 56, 2178–2195. doi: 10.1021/jm301783x
Chu, L. F., Liang, D. Y., Li, X., Sahbaie, P., D'Arcy, N., Liao, G., et al. (2009). From mouse to man: the 5-HT3 receptor modulates physical dependence on opioid narcotics. Pharmacogenet. Genomics 19, 193–205. doi: 10.1097/FPC.0b013e328322e73d
Conway, K. P., Compton, W., Stinson, F. S., and Grant, B. F. (2006). Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J. Clin. Psychiatry 67, 247–257. doi: 10.4088/jcp.v67n0211
Crowley, N. A., and Kash, T. L. (2015). Kappa opioid receptor signaling in the brain: circuitry and implications for treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 62, 51–60. doi: 10.1016/j.pnpbp.2015.01.001
Daviu, N., Bruchas, M. R., Moghaddam, B., Sandi, C., and Beyeler, A. (2019). Neurobiological links between stress and anxiety. Neurobiol. Stress 11, 100191. doi: 10.1016/j.ynstr.2019.100191
Dong, Z., Han, H., Wang, M., Xu, L., Hao, W., and Cao, J. (2006). Morphine conditioned place preference depends on glucocorticoid receptors in both hippocampus and nucleus accumbens. Hippocampus 16, 809–813. doi: 10.1002/hipo.20216
Endoh, T., Matsuura, H., Tanaka, C., and Nagase, H. (1992). Nor-binaltorphimine: a potent and selective kappa-opioid receptor antagonist with long-lasting activity in vivo. Arch. Int. Pharmacodyn. Ther. 316, 30–42.
Farr, S. A., Flood, J. F., and Morley, J. E. (2000). The effect of cholinergic, GABAergic, serotonergic, and glutamatergic receptor modulation on posttrial memory processing in the hippocampus. Neurobiol. Learn. Mem. 73, 150–167. doi: 10.1006/nlme.1999.3927
Felix-Ortiz, A. C., Beyeler, A., Seo, C., Leppla, C. A., Wildes, C. P., and Tye, K. M. (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron 79, 658–664. doi: 10.1016/j.neuron.2013.06.016
Felix-Ortiz, A. C., Burgos-Robles, A., Bhagat, N. D., Leppla, C. A., and Tye, K. M. (2016). Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 321, 197–209. doi: 10.1016/j.neuroscience.2015.07.041
Goeldner, C., Lutz, P. E., Darcq, E., Halter, T., Clesse, D., Ouagazzal, A. M., et al. (2011). Impaired emotional-like behavior and serotonergic function during protracted abstinence from chronic morphine. Biol. Psychiatry 69, 236–244. doi: 10.1016/j.biopsych.2010.08.021
Harper, K. M., Knapp, D. J., Butler, R. K., Cook, C. A., Criswell, H. E., Stuber, G. D., et al. (2019). Amygdala arginine vasopressin modulates chronic ethanol withdrawal anxiety-like behavior in the social interaction task. Alcohol. Clin. Exp. Res. 43, 2134–2143. doi: 10.1111/acer.14163
Heishman, S. J., Stitzer, M. L., Bigelow, G. E., and Liebson, I. A. (1989). Acute opioid physical dependence in humans: effect of varying the morphine-naloxone interval. I. J. Pharmacol. Exp. Ther. 250, 485–491.
Horan, P., Taylor, J., Yamamura, H. I., and Porreca, F. (1992). Extremely long-lasting antagonistic actions of nor-binaltorphimine (nor-BNI) in the mouse tail-flick test. J. Pharmacol. Exp. Ther. 260, 1237–1243.
Iyer, V., Slivicki, R. A., Thomaz, A. C., Crystal, J. D., Mackie, K., and Hohmann, A. G. (2020). The cannabinoid CB2 receptor agonist LY2828360 synergizes with morphine to suppress neuropathic nociception and attenuates morphine reward and physical dependence. Eur. J. Pharmacol. 886, 173544. doi: 10.1016/j.ejphar.2020.173544
Jimenez, J. C., Su, K., Goldberg, A. R., Luna, V. M., Biane, J. S., Ordek, G., et al. (2018). Anxiety cells in a hippocampal-hypothalamic circuit. Neuron 97, 670–683.e676. doi: 10.1016/j.neuron.2018.01.016
Knoll, A. T., Muschamp, J. W., Sillivan, S. E., Ferguson, D., Dietz, D. M., Meloni, E. G., et al. (2011). Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol. Psychiatry 70, 425–433. doi: 10.1016/j.biopsych.2011.03.017
Koob, G. F., and Le Moal, M. (2008). Addiction and the brain antireward system. Annu. Rev. Psychol. 59, 29–53. doi: 10.1146/annurev.psych.59.103006.093548
Koob, G. F., Maldonado, R., and Stinus, L. (1992). Neural substrates of opiate withdrawal. Trends Neurosci. 15, 186–191. doi: 10.1016/0166-2236(92)90171-4
Koob, G. F., and Volkow, N. D. (2010). Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238. doi: 10.1038/npp.2009.110
Ladron de Guevara-Miranda, D., Pavon, F. J., Serrano, A., Rivera, P., Estivill-Torrus, G., Suarez, J., et al. (2016). Cocaine-conditioned place preference is predicted by previous anxiety-like behavior and is related to an increased number of neurons in the basolateral amygdala. Behav. Brain Res. 298(Pt B), 35–43. doi: 10.1016/j.bbr.2015.10.048
Land, B. B., Bruchas, M. R., Lemos, J. C., Xu, M., Melief, E. J., and Chavkin, C. (2008). The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J. Neurosci. 28, 407–414. doi: 10.1523/JNEUROSCI.4458-07.2008
Laschka, E., Teschemacher, H., Mehraein, P., and Herz, A. (1976). Sites of action of morphine involved in the development of physical dependence in rats. II. Morphine withdrawal precipitated by application of morphine antagonists into restricted parts of the ventricular system and by microinjection into various brain areas. Psychopharmacologia 46, 141–147. doi: 10.1007/BF00421383
Lister, R. G. (1987). The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology 92, 180–185. doi: 10.1007/BF00177912
MacMillan, S., Szeszko, P. R., Moore, G. J., Madden, R., Lorch, E., Ivey, J., et al. (2003). Increased amygdala: hippocampal volume ratios associated with severity of anxiety in pediatric major depression. J. Child Adolesc. Psychopharmacol. 13, 65–73. doi: 10.1089/104454603321666207
Martins, S. S., Fenton, M. C., Keyes, K. M., Blanco, C., Zhu, H., and Storr, C. L. (2012). Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol. Med. 42, 1261–1272. doi: 10.1017/S0033291711002145
Masukawa, M. Y., Correa-Netto, N. F., Silva-Gomes, A. M., Linardi, A., and Santos-Junior, J. G. (2020). Anxiety-like behavior in acute and protracted withdrawal after morphine-induced locomotor sensitization in C57BL/6 male mice: the role of context. Pharmacol. Biochem. Behav. 194, 172941. doi: 10.1016/j.pbb.2020.172941
McHugh, S. B., Deacon, R. M., Rawlins, J. N., and Bannerman, D. M. (2004). Amygdala and ventral hippocampus contribute differentially to mechanisms of fear and anxiety. Behav. Neurosci. 118, 63–78. doi: 10.1037/0735-7044.118.1.63
McKendrick, G., Garrett, H., Jones, H. E., McDevitt, D. S., Sharma, S., Silberman, Y., et al. (2020). Ketamine Blocks Morphine-Induced Conditioned Place Preference and Anxiety-Like Behaviors in Mice. Front. Behav. Neurosci. 14, 75. doi: 10.3389/fnbeh.2020.00075
McLaughlin, J. P., Marton-Popovici, M., and Chavkin, C. (2003). Kappa opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J. Neurosci. 23, 5674–5683.
Metcalf, M. D., and Coop, A. (2005). Kappa opioid antagonists: past successes and future prospects. AAPS J. 7, E704–722. doi: 10.1208/aapsj070371
Moron, J. A., Gullapalli, S., Taylor, C., Gupta, A., Gomes, I., and Devi, L. A. (2010). Modulation of opiate-related signaling molecules in morphine-dependent conditioned behavior: conditioned place preference to morphine induces CREB phosphorylation. Neuropsychopharmacology 35, 955–966. doi: 10.1038/npp.2009.199
Namburi, P., Beyeler, A., Yorozu, S., Calhoon, G. G., Halbert, S. A., Wichmann, R., et al. (2015). A circuit mechanism for differentiating positive and negative associations. Nature 520, 675–678. doi: 10.1038/nature14366
Navarro, J. F., Rivera, A., Maldonado, E., Cavas, M., and de la Calle, A. (2004). Anxiogenic-like activity of 3,4-methylenedioxy-methamphetamine (“Ecstasy”) in the social interaction test is accompanied by an increase of c-fos expression in mice amygdala. Prog. Neuropsychopharmacol. Biol. Psychiatry 28, 249–254. doi: 10.1016/j.pnpbp.2003.10.016
Nestler, E. J. (2001a). Molecular basis of long-term plasticity underlying addiction. Nat. Rev. Neurosci. 2, 119–128. doi: 10.1038/35053570
Nestler, E. J. (2001b). Neurobiology. Total recall-the memory of addiction. Science 292, 2266–2267. doi: 10.1126/science.1063024
Niu, H., Zhang, G., Li, H., Zhang, Q., Li, T., Ding, S., et al. (2017). Multi-system state shifts and cognitive deficits induced by chronic morphine during abstinence. Neurosci. Lett. 640, 144–151. doi: 10.1016/j.neulet.2016.10.057
Nygard, S. K., Hourguettes, N. J., Sobczak, G. G., Carlezon, W. A., and Bruchas, M. R. (2016). Stress-induced reinstatement of nicotine preference requires dynorphin/kappa opioid activity in the basolateral amygdala. J. Neurosci. 36, 9937–9948. doi: 10.1523/JNEUROSCI.0953-16.2016
Pfeiffer, A., Brantl, V., Herz, A., and Emrich, H. M. (1986). Psychotomimesis mediated by kappa opiate receptors. Science 233, 774–776. doi: 10.1126/science.3016896
Qiao, X., Zhu, Y., Dang, W., Wang, R., Sun, M., Chen, Y., et al. (2021). Dual-specificity phosphatase 15 (DUSP15) in the nucleus accumbens is a novel negative regulator of morphine-associated contextual memory. Addict. Biol. 26, e12884. doi: 10.1111/adb.12884
Radke, A. K., and Gewirtz, J. C. (2012). Increased dopamine receptor activity in the nucleus accumbens shell ameliorates anxiety during drug withdrawal. Neuropsychopharmacology 37, 2405–2415. doi: 10.1038/npp.2012.97
Ross, N. C., Reilley, K. J., Murray, T. F., Aldrich, J. V., and McLaughlin, J. P. (2012). Novel opioid cyclic tetrapeptides: Trp isomers of CJ-15,208 exhibit distinct opioid receptor agonism and short-acting kappa opioid receptor antagonism. Br. J. Pharmacol. 165, 1097–1108. doi: 10.1111/j.1476-5381.2011.01544.x
Schulteis, G., Yackey, M., Risbrough, V., and Koob, G. F. (1998). Anxiogenic-like effects of spontaneous and naloxone-precipitated opiate withdrawal in the elevated plus-maze. Pharmacol. Biochem. Behav. 60, 727–731. doi: 10.1016/s0091-3057(98)00034-3
Sharp, B. M. (2017). Basolateral amygdala and stress-induced hyperexcitability affect motivated behaviors and addiction. Transl. Psychiatry 7, e1194. doi: 10.1038/tp.2017.161
Spanagel, R., and Weiss, F. (1999). The dopamine hypothesis of reward: past and current status. Trends Neurosci. 22, 521–527. doi: 10.1016/s0166-2236(99)01447-2
Sun, Y., Gooch, H., and Sah, P. (2020). Fear conditioning and the basolateral amygdala. F1000Research 9. doi: 10.12688/f1000research.21201.1
Tejeda, H. A., Hanks, A. N., Scott, L., Mejias-Aponte, C., Hughes, Z. A., and O'Donnell, P. (2015). Prefrontal cortical kappa opioid receptors attenuate responses to amygdala inputs. Neuropsychopharmacology 40, 2856–2864. doi: 10.1038/npp.2015.138
Torregrossa, M. M., Corlett, P. R., and Taylor, J. R. (2011). Aberrant learning and memory in addiction. Neurobiol. Learn. Mem. 96, 609–623. doi: 10.1016/j.nlm.2011.02.014
Valdez, G. R., and Harshberger, E. (2012). kappa opioid regulation of anxiety-like behavior during acute ethanol withdrawal. Pharmacol. Biochem. Behav. 102, 44–47. doi: 10.1016/j.pbb.2012.03.019
Vuong, T., Ritter, A., Shanahan, M., Ali, R., Nguyen, N., and Minh, K. P. (2021). Quality of life as a predictor of time to heroin relapse among male residents following release from compulsory rehabilitation centres in Vietnam. Drug Alcohol Rev. 40, 296–306. doi: 10.1111/dar.13176
Wang, S. (2019). Historical review: opiate addiction and opioid receptors. Cell Transplant. 28, 233–238. doi: 10.1177/0963689718811060
Williams, J. T., Christie, M. J., and Manzoni, O. (2001). Cellular and synaptic adaptations mediating opioid dependence. Physiol. Rev. 81, 299–343. doi: 10.1152/physrev.2001.81.1.299
Zanos, P., Georgiou, P., Gonzalez, L. R., Hourani, S., Chen, Y., Kitchen, I., et al. (2016). Emotional impairment and persistent upregulation of mGlu5 receptor following morphine abstinence: implications of an mGlu5-MOPr interaction. Int. J. Neuropsychopharmacol. 19. doi: 10.1093/ijnp/pyw011
Zarrindast, M. R., Babapoor-Farrokhran, S., Babapoor-Farrokhran, S., and Rezayof, A. (2008). Involvement of opioidergic system of the ventral hippocampus, the nucleus accumbens or the central amygdala in anxiety-related behavior. Life Sci. 82, 1175–1181. doi: 10.1016/j.lfs.2008.03.020
Keywords: morphine, anxiety, basolateral amygdala (BLA), ventral hippocampus (vHip), kappa opioid receptor (KOR)
Citation: Deji C, Yan P, Ji Y, Yan X, Feng Y, Liu J, Liu Y, Wei S, Zhu Y and Lai J (2022) The Basolateral Amygdala to Ventral Hippocampus Circuit Controls Anxiety-Like Behaviors Induced by Morphine Withdrawal. Front. Cell. Neurosci. 16:894886. doi: 10.3389/fncel.2022.894886
Received: 12 March 2022; Accepted: 26 April 2022;
Published: 02 June 2022.
Edited by:
Fu-Chin Liu, National Yang Ming Chiao Tung University, TaiwanReviewed by:
Yonatan M. Kupchik, Hebrew University of Jerusalem, IsraelCopyright © 2022 Deji, Yan, Ji, Yan, Feng, Liu, Liu, Wei, Zhu and Lai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongsheng Zhu, enlzMzAwMEB4anR1LmVkdS5jbg==; Jianghua Lai, bGFpamgxMDExQG1haWwueGp0dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.