
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci., 17 May 2022
Sec. Cellular Neuropathology
Volume 16 - 2022 | https://doi.org/10.3389/fncel.2022.871720
This article is part of the Research TopicBrain Injury and Repair Following Cerebrovascular Diseases: From Bench to BedsideView all 14 articles
Ischemic stroke is the most common type of stroke with limited treatment options. Although the pathological mechanisms and potential therapeutic targets of ischemic stroke have been comprehensively studied, no effective therapies were translated into clinical practice. Gut microbiota is a complex and diverse dynamic metabolic ecological balance network in the body, including a large number of bacteria, archaea, and eukaryotes. The composition, quantity and distribution in gut microbiota are found to be associated with the pathogenesis of many diseases, such as individual immune abnormalities, metabolic disorders, and neurodegeneration. New insight suggests that ischemic stroke may lead to changes in the gut microbiota and the alterations of gut microbiota may determine stroke outcomes in turn. The link between gut microbiota and stroke is expected to provide new perspectives for ischemic stroke treatment. In this review, we discuss the gut microbiota alterations during ischemic stroke and gut microbiota-related stroke pathophysiology and complications. Finally, we highlight the role of the gut microbiota as a potential therapeutic target for ischemic stroke and summarize the microbiome-based treatment options that can improve the recovery of stroke patients.
Ischemic stroke accounts for about 70–80% of all stroke patients (Feigin et al., 2003). The main cause of ischemic stroke is insufficient blood and oxygen supply to the brain. Embolus or thrombus forms could block cerebrovascular, which make blood supply of local brain tissue decrease, thereby causing brain tissue damage (Dirnagl et al., 1999). Currently, there are two main therapies for ischemic stroke: thrombolysis and thrombectomy. However, their applications in clinical practice are still very limited due to the short treatment window (Fisher and Saver, 2015). In recent years, the pathological mechanisms and potential therapeutic targets of ischemic stroke have been comprehensively studied, including excitotoxicity, oxidative stress, neuroinflammation, apoptosis, and blood-brain barrier (BBB) disruption (Zhang Z. Y. et al., 2019). However, no effective therapies were translated into clinical practice. Therefore, it still needs our great attention to find new therapies to prevent or reduce neuronal injury after ischemic stroke.
Gut microbiota is a complex and diverse dynamic metabolic ecological balance network, including a large number of bacteria, archaea, and eukaryotes. Gut microbiota is formed at birth and retains maternal characteristics. After exposure to a complex microbiome, babies develop a largely stable gut microbiota by the time they are 1–3 years old (Mackie et al., 1999; Palmer et al., 2007). But it can also change due to the host’s dietary habits, stress, antibiotic use, and aging (Claesson et al., 2011; Wu and Hui, 2011; Shimizu, 2018). The composition, quantity and distribution in gut microbiota are associated with the pathogenesis of a wide variety of diseases, such as individual immune abnormalities, metabolic disorders, and neurodegeneration (Duvallet et al., 2017). At present, biphase associations between gut microbiota and many body organs have been identified, including gut-cardiac axis, gut-thyroid axis, and gut-liver axis (Koszewicz et al., 2021). The brain and gut microbiota can interact with each other not only through neuronal pathways but also through microbial metabolites, hormones, and the immune system, termed the gut-brain-microbiota axis (GBMAx) (El Aidy et al., 2015; Durgan et al., 2019). Ischemic stroke may lead to changes in the gut microbiota, which can affect surrounding or distant tissues and organs, causing serious damages to liver, kidney, lung, gastrointestinal tract, cardiovascular system, and so on. In turn, changes in the gut microbiota may be one of the risk factors for ischemic stroke and determine stroke outcomes (de Jong et al., 2016). The link between gut microbiota and stroke is expected to provide new perspectives for ischemic stroke treatment.
This article reviews the gut microbiota alterations during ischemic stroke, gut microbiota-related stroke pathophysiology and complications, as well as potential therapeutic strategies targeting gut microbiota for ischemic stroke.
Multiple clinical and animal studies have revealed the changes in gut microbiota following ischemic stroke. One case-control study showed that the gut microbiota was significantly disrupted in patients with ischemic stroke and transient ischemic attack compared to controls. The main manifestations were the increase of opportunistic pathogens and the decrease of commensal or beneficial genera (Yin et al., 2015). In another study, the gut microbiota of ischemic stroke patients had more short chain fatty acids producer compared to healthy controls. In addition, it was found that the genus Enterobacter was significantly correlated with good outcomes (Li et al., 2019). An animal experiment based on the mouse middle cerebral artery occlusion (MCAO) model showed that ischemic stroke resulted in reduced species diversity and bacterial overgrowth of Bacteroidetes in the gut (Singh et al., 2016). Another study found that the levels of Bacteroidetes phylum and Prevotella genus were significantly increased in the gut of cynomolgus monkeys after MCAO, while Firmicutes phylum as well as Faecalibacterium, Oscillospira, and Lactobacillus genera were decreased, Oscillobacter, and Lactobacillus were decreased. In addition, intestinal mucosal damage was also observed (Chen et al., 2019c).
In addition to causing gut microbiota dysbiosis, ischemic stroke may also facilitate the translocation and dissemination of selective strains of bacteria that originated from the host gut microbiota. Infection is usually more likely to be observed after an ischemic stroke. Stanley et al. (2016) demonstrated that the microbial community in the lungs of post-stroke mice were derived from the small intestine of the host using high-throughput 16S rRNA gene amplicon sequencing and bioinformatics analyses.
Changes in gut bacteria can also be a factor in ischemic stroke. Significant microbiological disorders have been detected in inflammatory bowel disease (including Crohn’s disease and ulcerative colitis) and chronic kidney disease, all of which were found to be risk factors for ischemic stroke (Lee et al., 2010; Kristensen et al., 2014; Xiao et al., 2015). In addition, the composition of gut bacteria of people at high risk of stroke is also different from that of the normal population. Compared with the low-risk group of stroke, the levels of opportunistic pathogens among the people of high-risk group were found to be higher, and the difference of enterobacteriaceae was the most obvious. The people of low-risk group had higher concentration of butyrate-producing bacteria, such as Lachnospiraceae and Ruminococcaceae (Zeng et al., 2019). These findings may imply that disruption of microbial homeostasis in gut may precede the development of stroke. Therefore, it is feasible to predict and prevent stroke in advance by observing changes in intestinal flora.
There are also differences in gut microbiota among stroke patients of different ages. The incidence of stroke is closely related to age, with about 70–80% of ischemic strokes occurring in people over 65 years of age (Ovbiagele and Nguyen-Huynh, 2011), and age plays an important role in the development and prognosis of stroke (Yager et al., 2006; Manwani et al., 2011). Some pathophysiological processes are associated with aging, such as chronic inflammation and decreased immune function, can affect functional recovery after stroke and lead to poor prognosis in the elderly (Crapser et al., 2016; Ritzel et al., 2018). And On the other hand, the composition of gut microbes can be influenced by environment, disease and eating habits, as well as age and gender differences (Coman and Vodnar, 2020). The composition of the gut microbiota changes and the diversity diminishes as we get older. When the gut microbiome disorders, it has a detrimental effect on normal physiological activity and is also thought to affect age-related neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and Huntington’s disease (Mulak and Bonaz, 2015; Wasser et al., 2020; Escobar et al., 2022). Studies have shown that age plays an important role in the interaction between gut microbiota and stroke. Bacteroidetes and Firmicutes dominated the gut microbiota of both young and old adults. In older adults, the relative abundance of Firmicutes increased, and the content of SCFAs-producing bacteria and butyrate level decreased significantly (Biagi et al., 2010; Claesson et al., 2011), and intestinal permeability of the elderly was significantly higher than that of the young (Lee et al., 2020), which makes the older more susceptible to inflammatory response. And according to another study in mice, stroke outcomes can be improved in older mice by transplanting microbiota from younger mice. In contrast, after acquiring the microbiome of the older mice, the younger mice increased functional impairment after stroke (Spychala et al., 2018). In addition, age is an independent risk factor for post-stroke infection, the frequency and severity of infection after stroke were higher in the elderly. This may be related to the impaired integrity of the intestinal barrier, the entry of intestinal bacteria into peripheral tissues through the damaged barrier. And another possible explanation is intestinal inflammation. Higher levels of pro-inflammatory cytokines were detected in older patients than in younger patients (Crapser et al., 2016; Spychala et al., 2018; Blasco et al., 2020).
In addition, differences in the performance of gut microbiota after stroke also exist between genders. As two common intestinal bacteria, Bacteroidetes and Firmicutes, there are more Firmicutes detected in the males’ gut when they had a BMI of less than 33 compared with females. And when BMI is greater than 33, males have an advantage over females in the abundance of Bacteroidetes. In addition, the abundance of Lactobacilli in female is much higher than that in male (Haro et al., 2016). And there are also gender differences in post-stroke outcomes. In some studies, adult females have better recovery outcomes than males after stroke (Toung et al., 1998; Branyan and Sohrabji, 2020). And in middle age (45–55 years), male stroke patients have a higher mortality rate than female stroke patients (Redon et al., 2011). The prognosis of senile stroke women is proved significantly worse. This suggests that estrogen may play a protective role in the development of stroke. In addition, there were gender differences in the expression of bacterial metabolites after stroke. Fecal butyrate levels in male were significantly lower than in female after stroke (Ahnstedt et al., 2020), but LPS was found to be higher in male. After induced stroke, the male mouse model had greater intestinal permeability (Ahnstedt et al., 2020; El-Hakim et al., 2021). This suggests that male patients are more susceptible to intestinal microbiota translocation and post-stroke infection after stroke. There were also differences between male and female in inflammatory responses after stroke. Females expressed more Treg cells, while males had higher concentrations of CD8 + T cells (Jackson et al., 2019; Ahnstedt et al., 2020; Blasco et al., 2020). However, no more studies have clearly proved that gender can cause changes in the composition of intestinal microbiota in stroke patients, so the association between gender and stroke and intestinal microbiota needs further exploration.
The interaction between gut microbiota and ischemic stroke plays an important role in the occurrence, development and outcomes of stroke. We summarize the relevant pathophysiological mechanisms, including neuroendocrine pathways, bacterial metabolite, and immune response (Figure 1). The studies exploring this interaction and relevant mechanisms are listed in Table 1.
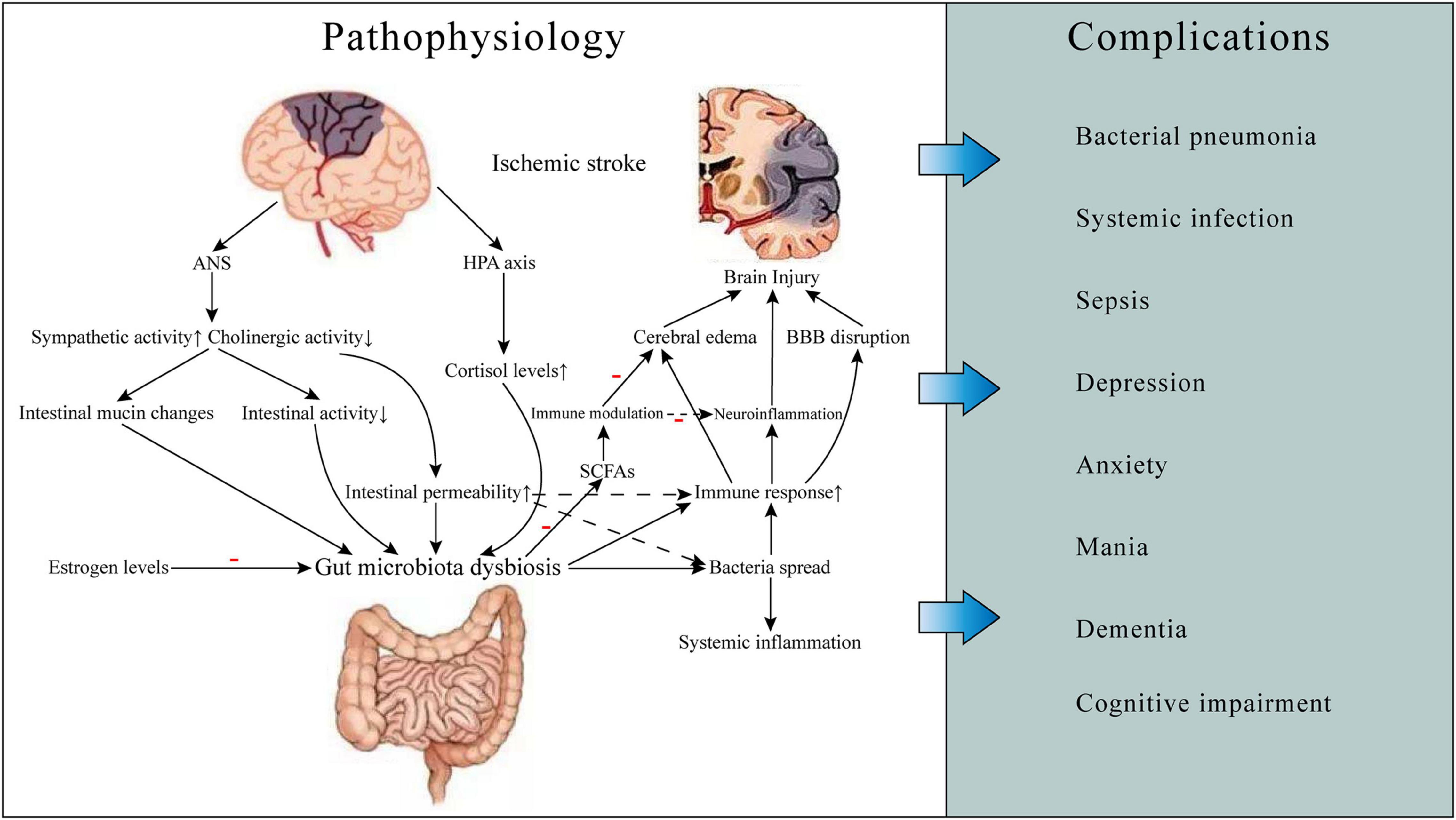
Figure 1. Gut microbiota-related ischemic stroke pathophysiology and complications. Ischemic stroke can cause gut microbiota dysbiosis, which may result in increased gut permeability and worsening brain injury, thereby leading to some complications such as infections and neuropsychiatric disorders and poor prognosis. The mechanisms involved include neuroendocrine pathways, bacterial metabolite, and immune response. ANS, autonomic nervous system; HPA, hypothalamic-pituitary-adrenal; BBB, blood-brain barrier; SCFAs, short-chain fatty acids.
Gut-brain-microbiota axis plays an important role in gut microbiota-related stroke pathophysiology. There are several neural pathways for GBMAx communication, such as spinal and vagal pathways, autonomic nervous system (ANS), enteric nervous system (ENS), and hypothalamic-pituitary-adrenal (HPA) axis (Foster and McVey Neufeld, 2013; Carabotti et al., 2015). The function of gastrointestinal ANS will change after ischemic stroke. The production and release of norepinephrine is increased, and cholinergic activity is decreased, which results in altered intestinal mucin production, inhibiting intestinal activity and increasing intestinal permeability (Caso et al., 2009). This can affect the size and quality of the intestinal mucus layer. As the habitat of most intestinal microbiota, the change of the status of the mucus layer can affect the composition and function of the microbiota. Changes in intestinal permeability induced by stress would lead to the activation of glial cells and mast cells, increased production of interferon, and morphological changes of colonic epithelium. These changes are caused by the expression of reduced tight junction protein 2 and the occlusion of an important component of the intestinal tight junction (Demaude et al., 2006). Increased permeability of the intestinal epithelium will cause bacterial antigens to cross the intestinal epithelium and trigger an immune response, resulting in changes in intestinal flora and systemic effects (Yates et al., 2001). Thus, stroke-induced increases in intestinal permeability can be ameliorated after inhibition of β-adrenergic activity with β -blockers. It can also reduce the risk of bacteria spreading to surrounding organs (Stanley et al., 2016). HPA axis is also an important part of GBMAx and plays a role in enter-brain regulation. HPA axis regulates the body through the interaction of three endocrine glands, including hypothalamus, pituitary and adrenal, which can stimulate the release of steroid hormones such as cortisol under stress. Long-term elevation of serum cortisol can have toxic effects on the nervous system. Serum cortisol levels were found to be associated with stroke severity and post-stroke mortality (Christensen et al., 2004; Barugh et al., 2014). In addition, one study suggested that cortisol levels may be associated with gut microbiota diversity (Keskitalo et al., 2021). Interactions of gut microbiota and HPA axis may explain some severe mental disorders after ischemic stroke (Misiak et al., 2020).
Hormone levels are also thought to play a role in ischemic stroke. Deficiency of estrogen and other ovarian hormones was found to be a risk factor for ischemic stroke in post-menopausal women (Reeves et al., 2008). In animal experiments, different effects of estrogen on the prognosis and recovery of stroke rats were closely related to the age of the rats. Many studies demonstrated that neurological function in young female animals was less affected than in older animals after ischemic stroke. And in the same age groups, female animals showed better post-stroke neurological performance than males (Hall et al., 1991; Alkayed et al., 1998; Manwani et al., 2013). Estrogen can effectively prevent the growth of pathogenic bacteria, promote the growth and reproduction of beneficial bacteria, and maintain a reasonable composition of intestinal microbiota (Chen and Madak-Erdogan, 2016; Baker et al., 2017). There are significant differences in intestinal microbiome and metabolites between young and old women. Akkermansia muciniphila, for example, is a bacterium that can affect energy regulation, metabolism, and cardiovascular function (Everard et al., 2013; Plovier et al., 2017). In healthy mice, levels of Akkermansia muciniphila were found to be lower in female mice than in male mice. But after a stroke, its levels were significantly elevated in male mice (Stanley et al., 2018; Park et al., 2020). Estrogen therapy in stroke patients can reduce lipopolysaccharide (LPS) production and increase short-chain fatty acids (SCFAs) levels, which helps to inhibit inflammatory response and reduce brain tissue damage after ischemic stroke (Blasco-Baque et al., 2012; Wu et al., 2021). Transplanting fecal microbiome from young women undergoing estrogen therapy to older women with stroke effectively improved their functional outcomes (Park et al., 2020). However, estrogen therapy, which has a protective effect in younger women, increased the risk and severity of stroke in post-menopausal women (Wassertheil-Smoller et al., 2003; Selvamani and Sohrabji, 2010a,b). These studies suggested that estrogen levels could regulate intestinal microbiome homeostasis after ischemic stroke and influence stroke outcomes. But more research is needed to explore the relationship between hormones, age, gut microbiota, and ischemic stroke.
Gut microbiota plays an important role in the production and secretion of over 100 metabolites. However, the role of these metabolites in neurological function after ischemic stroke has not been fully studied (Bostanciklioğlu, 2019). SCFAs are the main product that produced by gut microbiota through fermentation of dietary fiber, including acetate, propionate, and butyrate. Recent studies suggested that anaerobic bacteria, such as Firmicutes, produced a great amount of SCFAs by fermenting dietary fiber (Nakamura et al., 2017). SCFAs can also be produced by fermentation of proteins and amino acid, and about 1% of E. coli bacteria can produce branched SCFAs (such as isobutyric and isovaleric acids) through this pathway (Smith and Macfarlane, 1997; Louis and Flint, 2017). In addition, acetyl-CoA formed by glycolysis can also be converted to butyric acid by the action of Butyryl-CoA: Acetate-CoA transferase (Duncan et al., 2002). Moreover, SCFAs produced by different species of bacteria also varies. For instance, acetate is metabolized by enteric and acetogenic commensal bacteria (Maa et al., 2010). Propionate is the main metabolite of bacteroides and Firmicutes (Zapolska-Downar and Naruszewicz, 2009). Eubacterium, anaerobe and Faecalis are the main bacteria which produce butyrate (Zapolska-Downar et al., 2004).
Short-chain fatty acids have positive effects on human intestinal function, such as enhancing intestinal motility, reducing inflammatory cell level, and regulating intestinal hormones and neuropeptide levels (Silva et al., 2020). SCFAs are also important for cerebral development and maintenance of normal function of the central nervous system (CNS). They can cross the BBB, and are essential for several processes, such as microglial maturation, intestinal neuron stimulation of ANS, and mucosal serotonin secretion (Braniste et al., 2014; Erny et al., 2015). In addition, SCFAs also play an immunomodulatory role. They can induce T cells to differentiate into effector cells and regulatory cells according to the immune environment (Park et al., 2015).
In the early stage of ischemic stroke, the levels of acetic acid and propionic acid were found to be significantly lower than normal. The concentrations of isobutyric acid and isovaleric acid were increased in the early stage but low in the later stage. Butyric acid and valeric acid were deficient in both early and later stages of ischemic stroke (Chen et al., 2019b). This difference may be related to different metabolic pathways required to produce different SCFAs. Studies have demonstrated that SCFAs have a neuroprotective effect. The lower the concentration of acetic acid, valeric acid, especially butyric acid, in stroke patients, the greater the volume of cerebral infarction and the worse the neurological function score (Chen et al., 2019b). Oral infusion of SCFAs-producing bacteria and inulin reduced neurological deficits and improved post-stroke depression-like behavior in elderly mice (Lee et al., 2020). Butyrate has the function of reducing neurotoxicity, alleviating neuroinflammation, and relieving behavioral disorders. Intestinal butyrate supplementation can improve the level of neurological recovery after brain injury and reduce the volume of cerebral infarction. It was also effective in reducing cerebral edema, lowering blood lipid levels, and reducing the risk of thrombosis (Sharma et al., 2015; Patnala et al., 2017). Butyrate can regulate immune function by inhibiting histone deacetylase (HDAC) and mammalian target of rapamycin (mTOR) signal in circulating leukocytes. Studies have shown that higher concentrations of butyrate in feces or intravenous butyrate solution can enhance the antimicrobial activity of monocytes and macrophages and increase the body’s resistance to pathogens (Chakraborty et al., 2017; Haak et al., 2018).
Trimethylamine N-oxide (TMAO) is a kind of metabolic product of gut microbiota, mainly derived from the dietary nutrients rich in phosphatidylcholine, choline, and L-carnitine. First, Gut microbes metabolize foods such as eggs and beef to produce the intermediate trimethylamine by the activity of trimethylamine (TMA) lyases. In the second step, TMA is oxidized to TMAO by hepatic flavin-containing monooxygenases (Bennett et al., 2013). TMAO can induce atherosclerosis by increasing uptake of cholesterol in macrophages and promoting foam cell formation (Wang et al., 2011), enhance platelet hyperresponsiveness and increases the risk of thrombosis by changing stimulus-dependent calcium signal (Zhu et al., 2016). Higher concentrations of TMAO were found to be associated with an increased risk of cardiovascular events (Wang et al., 2011; Koeth et al., 2014; Tang et al., 2014). In addition, high levels of plasma TMAO have been shown to reduce long-term survival in patients with chronic kidney disease (Tang et al., 2015). Notably, a study of TMAO and cardiovascular disease risk in hemodialysis patients showed a significantly higher risk of death in white patients than in blacks (Shafi et al., 2017). This suggests that the effects of TMAO may differ across racial and ethnic groups.
According to the current study, TMAO can aggravate brain injury after ischemic stroke through a variety of pathophysiological processes. In addition to accelerating atherosclerosis and enhancing thrombogenesis potential, it can also promote vascular inflammation and endothelial dysfunction (Seldin et al., 2016; Boini et al., 2017; Li T. et al., 2017). TMAO also increases oxidative stress, enhances mitochondrial damage, and inhibits mTOR signaling, thereby impairing neural function (Chen et al., 2017; Li D. et al., 2018). TMAO is also a risk factor for hypertension and diabetes, which are linked to ischemic stroke. TMAO levels will rise and then decrease gradually over time after stroke onset. High concentrations of TMAO in patients with early onset are often associated with poor prognosis. Therefore, the measurement of plasma concentrations of gut microbial TMAO in stroke patients is helpful for us to predict the prognosis of patients (Tan et al., 2020; Zhang J. et al., 2019).
As a gathering place of immune cells, gastrointestinal tract affects the growth and development of immune cells and plays an important role in regulating immune response (Li et al., 2019). After ischemic stroke, both local neuroinflammatory responses and peripheral immune responses can be activated (Chamorro et al., 2012). It is found that different kinds of immune cells can aggravate the injury or protect the damaged brain tissue, respectively.
After the occurrence of stroke, activation of cerebral resident immune cells such as microglia, astrocytes, neutrophils and macrophages increase the production of pro-inflammatory cytokines, chemokines, proteases, and adhesive proteins (Iadecola and Anrather, 2011; Yang et al., 2019). The activation of inflammatory cells destroys the integrity of BBB, increases the chemotaxis of inflammatory cytokines in cerebral ischemia area, aggravates the damage of brain tissue. Experimental studies have shown that endotoxins metabolized by microbiota, such as LPS, can exacerbate neuroinflammation either directly or by inducing migration of peripheral immune cells to the brain (Lukiw et al., 2018). And raising LPS levels in stroke mice can promote the production of inflammatory factors like interleukin (IL)-6 and tumor necrosis factor (TNF)-α, affects BBB function, increase the neurological impairment, aggravating cerebral edema and reduce life expectancy of the mice (Dénes et al., 2011). It also led to increased plasma levels of pro-inflammatory cytokines that may promote dysregulation of the gut microbiome (Yamashiro et al., 2017). The dysbiosis of intestinal microflora can further increase the production of peripheral inflammatory cytokines. These cytokines can cross the BBB and exacerbate brain ischemic injury (Liu et al., 2020). Rapid dysregulation of intestinal flora in the first 24 h after stroke can promote cerebral infarction through inflammatory response. By inhibiting the overgrowth of Enterobacteriaceae and other opportunistic pathogens in stroke patients, systemic inflammation and cerebral infarction can be effectively reduced (Xu et al., 2021).
Peripheral immune inflammatory cells are involved in the cerebral immune inflammatory response following ischemic stroke and play an important role in the process of brain injury and tissue repair. The main cells involved in the human immune system are B lymphocytes, T lymphocytes, MHC and effector cells (Flajnik and Kasahara, 2010). In ischemic stroke, impaired BBB promotes T infiltration and interferon (IFN)-γ accumulation (Kleinschnitz et al., 2010; Liesz et al., 2011). In animal stroke models, T cell and B cell counts in Peyer’s patches decreased within 24 h, and activated T lymphocytes migrate from the Peyer patches of the small intestine or from the intestinal lamina propria to the brain within 2–3 days after stroke, where they primarily located in is leptomeninges (Schulte-Herbruggen et al., 2009). T cells can affect the secretion of cytokines IL-17 and IL-23 (Fan et al., 2020), lead to chemokine production and increased infiltration of cytotoxic cells (neutrophils and monocytes) into brain tissues, and then results in neurotoxic effects on ischemic lesions, resulting in increased infarct volume. Experiments have shown that inhibition of T lymphocyte invasion can effectively reduce the infarct size after stroke (Liesz et al., 2011). Conversely, upregulation of T regulates cell (Treg) level or increases IL-10 concentration can inhibit the production of proinflammatory mediators, thereby reducing the volume of cerebral infarction (Wei et al., 2011; Bodhankar et al., 2015). Similarly, regulatory B lymphocytes may also play a protective role in ischemic stroke by regulating anti-inflammatory factors such as IL-10 and transforming growth factor (TGF)-β (Doyle et al., 2015). Increasing B cell concentration in the brain can reduce infarct volume after stroke (Chen et al., 2012).
Intestinal microbiome dysregulation can reduce systemic anti-inflammatory cytokines such as TGF-β and IL-10 (Yan et al., 2009; Benakis et al., 2016). As a bacterial metabolite, SCFAs can act on immune cells by inhibiting histone deacetylase (HDAC) or by acting as a ligand for G-protein-coupled receptors. After stroke, SCFAs also stimulates the production of colonic Treg cells by producing IL-10 cytokines and TGF-β, and expressing Foxp3 and cell surface markers CD4 and CD25, thereby reducing infarct size (Sadler et al., 2020). In addition, monocytes/macrophages in the intestinal tract of stroke patients can be activated by intestinal flora. Intrusions of intestinal monocytes into the brain can be detected during the acute phase of stroke. Therefore, monocytes/macrophages also play a role in microbiome mediated stroke prognosis (Singh et al., 2016).
Post-stroke infection is an important factor causing worsen outcomes of stroke patients, and more than one-third of patient’s condition and treatment are complicated by post-stroke infection complications (Emsley and Hopkins, 2008). Increased susceptibility to infection after ischemic stroke is associated with activation of feedback activity between the CNS and peripheral immune organs (Chamorro et al., 2012). And according to current studies, the increased permeability and dysfunction of the intestinal barrier after stroke can cause bacterial migration and spread of the intestinal microbiome, which may be one of the mechanisms of post-stroke infection (Figure 1; Stanley et al., 2016).
After ischemic stroke, the sympathetic nervous system is activated, the intestinal permeability is increased, the intestinal barrier is damaged, and the antibacterial function of the body is reduced. These changes promote the transfer of bacteria to extra-intestinal organs, blood or lymph, participates in local and systemic immunity, and may lead to organ infections and even sepsis (Hagiwara et al., 2014). The β-adrenergic signaling pathway may play an important role (Wong et al., 2011). When β-adrenergic receptors are blocked, the integrity of the intestinal barrier can be inhibited. For example, feeding stroke mice with propranolol can’t completely avoid the occurrence of infection, but can obviously reduce the serious situation of systemic tissue infection after ischemic stroke (Stanley et al., 2016).
In an mice experimental study, germ-free mice were modeled by two methods. The results showed that the intestinal microbiota of mice with post-filament middle cerebral artery occlusion model (fMCAo) had significantly imbalance and the diversity was decreased while those in mice with permanent distal middle cerebral artery occlusion model (cMCAo) had relatively little influence. This suggests that the changes in microbiome are secondary to the stroke and the degree of disturbance is affected by the severity of stroke (Singh et al., 2016).
In turn, gut microbiota disturbance may be one of the important causes of enterogenic infection, sepsis even multiple-organ dysfunction syndromes (MODS) (Lyons et al., 2016; Haak and Wiersinga, 2017). After stroke, the destruction of the integrity of the intestinal barrier provides conditions for bacterial migration. Although in healthy individuals a variety of gut microbiomes are also found in blood and lung tissue (Potgieter et al., 2015; Dickson et al., 2016; Li Q. et al., 2018). However, due to the destruction of the integrity of the intestinal barrier, pathologic translocation of intestinal bacteria in stroke patients increases, leading to an increase in the incidence of post-stroke infection (Tascilar et al., 2010).
Bacterial pneumonia is the most common complication of ischemic stroke patients and one of the early nosocomial infections (Langhorne et al., 2000; Hannawi et al., 2013). Blood or sputum culture samples taken from patients with stroke complicated with pneumonia are often absent of the common pathogens that cause pneumonia, or have much less than those found in patients with common pneumonia (Marik, 2001). In one study, prophylaxis with antibiotics in ischemic stroke patients did not reduce the incidence of pneumonia or death compared with untreated patients (Kalra et al., 2015). In contrast, evidence from several studies suggests that post-stroke pneumonia is associated with the transmission of certain bacteria from the patient’s gut microbiota. These bacteria, when translocated, become pathogenic strains (Stanley et al., 2016). This suggests that endogenous factors play a more important role in the onset of post-stroke pneumonia than exogenous infection. Changes in intestinal permeability after ischemic stroke can induce bacterial migration and infection. In addition to direct transmission through the small intestine after, gut bacteria can also travel through the portal vein to the liver, where they can spread indirectly to the lungs after filtering through the blood.
Neuropsychiatric disorders are also common complications of stroke, includes depression, anxiety, mania, dementia, and cognitive impairment (Hackett et al., 2014; Hackett and Pickles, 2014). About one-third of patients experience cognitive impairment within a year of a stroke (Li X. et al., 2017). Gut microbiota dysbiosis can be found in many neurological disorders such as Alzheimer’s disease and depression (Cho et al., 2021). The high-abundance Prevotella group expressed more negative emotions and reduced hippocampal functional activation than the group with higher levels of bacteroides (Tillisch et al., 2017). In addition, bacterial metabolites have been linked to cognitive function. Ischemic stroke patients who detect higher levels of TMAO experience more severe cognitive impairment (Zhu et al., 2020). SCFAs producing bacteria (such as Lachnospiraceae and Ruminococcus) were significantly reduced in patients with amnestic cognitive impairment (Liu et al., 2021). Another study found similar results. The abundance of Lachnospiraceae, Clostridiaceae, and Ruminococcus was reduced in the high-risk group compared with the low-risk group (Huang Q. et al., 2021). Gut microbiota may aggravate neuropsychiatric symptoms by common pathogenesis, like neuroinflammatory response. These results suggest that it is feasible to predict the occurrence of cognitive impairment after ischemic stroke by intestinal flora.
Current studies on the gut-brain axis mainly focus on patients with ischemic stroke. Compared with ischemic stroke, there are fewer clinical and experimental studies on the association between hemorrhagic stroke and intestinal flora. The occurrence probability of intracranial hemorrhage (ICH) and subarachnoid hemorrhage (SAH) are less than that of cerebral infarction, but the mortality and disability rate of ICH and SAH are not low. Although the pathogenesis and clinical manifestations of the two types of stroke patients are different, similar results of microbiome disruption were found between the two stroke patients. Some studies have even shown that the stability disruptions of gut microbiota in patients with hemorrhagic stroke or high NIHSS scores are more severe than those with ischemic stroke and TIA (Zeng et al., 2019; Haak et al., 2021). Like ischemic stroke, some mechanisms of action are also at work in ICH patients. According to a case-control study of hypertension patients in China, intestinal bacterial metabolite TMAO levels are strongly associated with stroke. The association between TMAO and hemorrhagic stroke was significantly higher than that of ischemic stroke (Nie et al., 2018). TMAO has also been shown to be closely associated with the prognosis of ICH patients (Zhai et al., 2021). Inflammation has also been found to play an important role in the brain-gut axis in patients with intracerebral hemorrhage. An animal study has demonstrated that dysregulation of gut flora is associated with dysregulation of pro-inflammatory T cell differentiation in mice after intracerebral hemorrhage, which exacerbates neuroinflammatory responses and causes secondary damage to brain tissue. Neuroinflammation was reduced in ICH mice after intestinal transplantation with fecal gut microbiota from healthy mice (Yu et al., 2021). Intestinal disruption also occurred after ICH. Persistent ileal mucosal injury and increased intestinal permeability were observed in ICH mice. This permeability reached its highest level on the 7th day after intracerebral hemorrhage. Intestinal disruption also occurred after ICH. Persistent ileal mucosal injury and increased intestinal permeability were observed in ICH mice. This permeability reached its highest level on day 7 of intracerebral hemorrhage. Intestinal bacteria can enter the blood circulation through the broken intestinal mechanical barrier, and lead to systemic inflammation, especially pneumonia (Zhang H. et al., 2021).
As the most common cause of SAH, intracranial aneurysms have a prevalence of about 3% in the population and are associated with 80–85% of non-traumatic SAH (Kassell et al., 1990; Vlak et al., 2011). The current study reveals a partial link between intracranial aneurysms and gut microbiota. Destruction of the gut microbiota by antibiotics can reduce the incidence of intracranial aneurysms in mice (Shikata et al., 2019). And in another study, intracranial aneurysm formation can be induced in normal mice after transplantation of feces from patients with an intracranial aneurysm. Further studies revealed a relationship between aneurysms and the abundance of H. hathewayi in the gut (Li H. et al., 2020). This is a group of anaerobic bacteria that can maintain stable levels of serum taurine, which reduces the risk of aneurysm formation and rupture by inhibiting systemic inflammation. Meanwhile, artificial taurine supplementation also reversed the progression of intracranial aneurysms (Li H. et al., 2020). On the other hand, intestinal microbiota can also play an important role in the rupture of aneurysms. Compare the gut microbiota of patients with ruptured aneurysms (RA) and unruptured aneurysms (URA), researchers found that the genus Campylobacter and C. ureolyticus were significantly increased in the RA group patients (Kawabata et al., 2022). This may be related to the more intense inflammatory response and the remodeling or destruction of blood vessel walls induced by Campylobacter (Nilsson et al., 2018; Kushamae et al., 2020).
Cavernous hemangioma (CA) is also a kind of common vascular disorder will cause cerebral hemorrhage. More abundance Gram-negative bacteria O. splanchnicus and lower levels of gram-positive bacteria F. prausnitzii and B. adolescentis can be found in CA patients, compared with the non-CA patients. However, the most valuable combination of bacteria for diagnosing disease and assessing its severity was not found in this experiment. This indicates that the influence of bacteria on CA is not independent but may play a role together with other factors.
Currently, it has been proved that there is a correlation between intestinal microbiota dysbiosis after stroke and the incidence and progression of stroke. Therefore, it is feasible to predict the disease recovery of patients by indicators related to intestinal microbiome. By comparing samples from stroke patients and control group, several studies got the similar conclusions that stroke patients had a reduction in Firmicutes and Bacteroidetes, while the abundance of Proteobacteria was increased. And the difference of composition ratio in the microbiome was correlated with the severity of the disease. The abundance of TMA-producing bacteria was significantly higher, and the levels of intestinal butyrate-producing bacteria decreased in severe patients compared with mild patients. And the metabolites like TMA or butyrate of these bacterias also had a similar situation (Gu et al., 2021; Haak et al., 2021; Xia et al., 2021).
By examining the difference in intestinal microbiota distribution between acute ischemic stroke patients and healthy participants, a study established a Stroke Dysbiosis Index (SDI), as an independent predictor of severe disease (NIHSS > 8) and poor prognosis (MRS > 2) (Xia et al., 2019). Increased abundance of Enterobacteriaceae and Parabacteroides have a correlation with a higher SDI, while the abundance of fecalibacterium, Clostridiaceae, and Lachnospira decreased. In addition, animal studies have shown that mice that received fecal transplants from patients with a high SDI index experienced severe brain damage, increased levels of IL-17 and T cells, and a significantly higher risk of stroke than mice that received normal fecal transplants.
Dietary regulation is an important measure to improve the prognosis of stroke (Figure 2). At the same time, diet, smoking cessation and blood pressure control are also three important interventions to prevent stroke (Hackam and Spence, 2007). A low-fat diet is recommended to reduce the risk of cerebrovascular disease. Many of the guidelines recommend a diet that reduces saturated fat and cholesterol and increases fruit and vegetables. Specifically, it includes vegetables, grains, poultry, fish and nuts, and cuts out red meat, candy and sugary drinks (Juraschek et al., 2017). While meat from different animals has roughly the same amount of cholesterol, red meat is higher in saturated fat and has about four times as much carnitine as chicken and fish. Carnitine and choline can be converted to TMAO by intestinal bacteria, affecting stroke outcomes. Therefore, stroke patients and high-risk patients should avoid foods such as red meat and egg yolks.
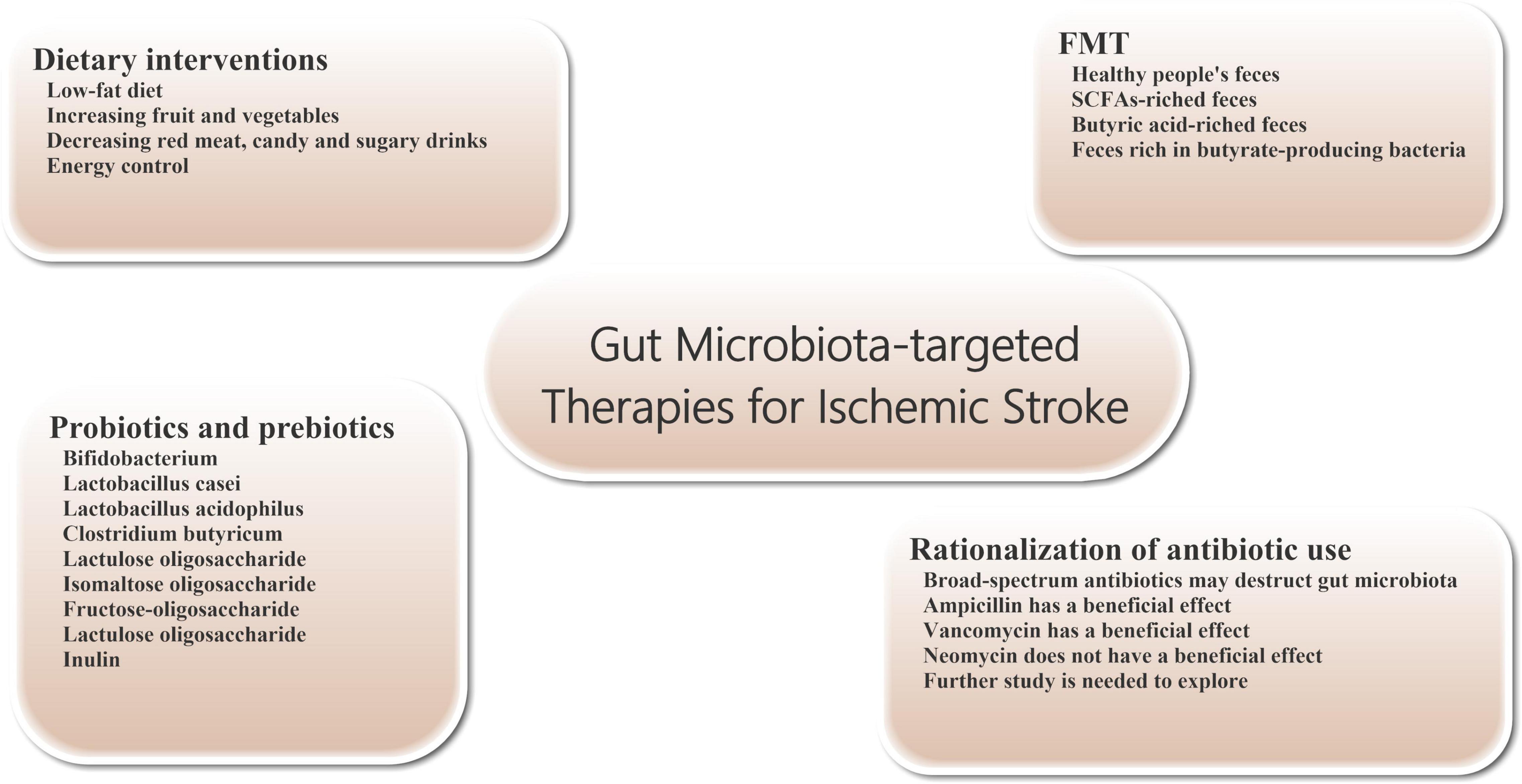
Figure 2. Gut microbiota-targeted treatments and managements for ischemic stroke. Gut microbiota-targeted treatments and managements can be considered for patients with ischemic stroke, including dietary interventions, probiotics and prebiotics supplementation, FMT, and rationalization of antibiotic use. FMT, fecal microbiome transplantation.
In addition, increasing the consumption of fruits and vegetables can increase fiber intake, which can increase the level of SCFAs production. For example, resistant starches (such as whole grains and legumes) and fructo-oligosaccharides (such as bananas, Onions and asparagus), as metabolic food sources for butyric acid producing bacteria, can increase butyric acid production in the gut (Le Blay et al., 1999).
Energy control is an effective way to promote good health and reshape the intestinal symbiotic microbiome. Some studies suggest that energy restriction to 60–70% of the recommended intake is protective against ischemic stroke (Mitchell et al., 2019). The protective effect of energy control on brain injury after stroke may be realized by promoting glycogen metabolism and adiponectin expression (Ciobanu et al., 2017; Zhang J. et al., 2019). And long-term energy control resulted in significant changes in the composition of intestinal flora in mice experiments, especially the enrichment of bifidobacterial (Huang J. T. et al., 2021).
Probiotics are a group of living gut microorganisms that are widely believed to be beneficial to the host. Probiotics can affect brain function by altering brain neurochemistry. Current studies have shown that probiotics supplementation can effectively reduce or prevent brain tissue damage in stroke patients. Probiotics may protect tissue from damage by reducing the production of oxygen free radicals and inflammatory cytokines. For example, probiotics can inhibit the production of TNF-α in vivo, promote the generation of anti-inflammatory cytokines, and improve the activity of antioxidant enzymes (Luo et al., 2014; Abhari et al., 2016). The severity of brain tissue damage in mice after focal cerebral ischemia was significantly reduced by 2 weeks of daily intake of probiotics such as bifidobacterium, Lactobacillus casei, Lactobacillus bulgaricus and Lactobacillus acidophilus (Akhoundzadeh et al., 2018). Pretreatment with Clostridium butyricum can effectively inhibit apoptosis and enhance antioxidant enzyme activity in rat cerebral ischemia model, thereby improving prognosis (Sun et al., 2016). In addition, regular consumption of lactobacillus probiotics can also alter the expression of brain-derived neurotrophic factor (BDNF) receptors and increase BDNF levels in the brain. There is evidence that elevated levels of BDNF in the brain have a protective effect on ischemic stroke (Bercik et al., 2011; Liang et al., 2015).
Prebiotics are oligosaccharides with no biological activity, such as lactulose oligosaccharide, isomaltose oligosaccharide, fructose-oligosaccharide, lactulose oligosaccharide and inulin, which can stimulate the growth and reproduction of beneficial bacteria in the intestine without being digested by intestinal metabolism. After entering the intestinal tract (mainly the lower digestive tract or colon), prebiotics can be hydrolyzed and used as nutrients by the beneficial bacteria in the intestinal tract, such as bifidobacterium, and promoting the reproduction and growth of these bacteria. In addition, prebiotics can also affect the production of SCFAs and regulate the production of mucin, thus enhancing the phagocytosis of macrophages (Markowiak and Śliżewska, 2017). In one study, prebiotics effectively reduced the incidence and severity of pneumonia during hospitalization in critically ill patients. Therefore, we believe that the use of prebiotics can play a certain role in alleviating ischemic stroke patients’ condition and the onset of infectious complications (Barraud et al., 2013).
The transfer of the entire microbiome from the stool of a healthy donor to the patient’s gastrointestinal tract is known as fecal microbiome transplantation (FMT). The technique is already being used to treat patients with severe infections, such as refractory bronchiolitis and pseudomembranous colitis (Eiseman et al., 1958; van Nood et al., 2013). In addition, FMT intervention can also relieve symptoms in patients with Parkinson’s disease and reduce autism in children with autism disorder (Aroniadis and Brandt, 2013; Kang et al., 2017). However, because the gut microbiome also has the potential to cause disease, it is important to select suitable healthy people as FMT donors. Transplantation SCFAs-riched feces (particularly butyric acid) can regulate the composition of intestinal microbes, increase lactobacillus species and enhance microbial activity, maintain intestinal wall integrity and reduce intestinal wall permeability, thereby reducing intestinal leakage in patients with ischemic stroke. These positive effects are beneficial to maintain the integrity of BBB and improve the functional status of brain tissue in ischemic stroke patients (Singh et al., 2016; Chen et al., 2019a,b). For example, transplanting gut microbiota from young mice can improve stroke outcomes in older mice (Spychala et al., 2018). Transplantation of feces rich in butyrate-producing bacteria has also been shown to reduce ischemic stroke injury in diabetic mice (Wang et al., 2021).
In clinical work, about 30% of patients with stroke will have bacterial infection within 1 week of onset (Westendorp et al., 2011), so a significant number of patients receive prophylactic anti-infective therapy with antibiotics, which often include a combination of broad-spectrum antibacterial drugs. However, to date, there is no clear evidence that prophylactic use of antibiotics after stroke benefits patient outcomes. Compared with standard treatment regimen, prophylactic use of antibiotics in stroke patients did not improve the long-term neurological status or mortality and had no significant effects on the incidence of post-stroke complications such as pneumonia. In some patients, intestinal microbiota damage can promote immune suppression, which increases the probability of facultative or mandatory bacterial re-invasion and increases the risk of infection (especially pneumonia) (Kalra et al., 2015; Westendorp et al., 2015). Further studies have shown that extensive destruction of the gut microbiome by untargeted use of broad-spectrum antibiotics after ischemic stroke can worsen stroke outcomes (Benakis et al., 2016; Winek et al., 2016). Studies have shown neuroprotective effects on brain tissue in stroke mice treated with ampicillin or vancomycin. A similar neuroprotective effect was not observed with neomycin (Benakis et al., 2020a,b). These different effects may be related to changes in the composition of intestinal flora. Therefore, further work is needed to explore whether specific antibiotics can have a beneficial effect on the prognosis of patients with ischemic stroke.
As the most common type of stroke, treatment options for ischemic stroke remain limited despite extensive research. New insights have highlighted the role of gut microbiota in the pathophysiology of ischemic stroke. Ischemic stroke could cause gut microbiota dysbiosis as well as translocation and dissemination of gut microbiota-derived selective strains of bacteria. In turn, changes in gut microbiota affect ischemic stroke-induced brain injury and determine stroke outcomes through multiple mechanisms, including neuroendocrine pathways, bacterial metabolite, and immune response. Gut microbiota dysbiosis may also contribute to some stroke complications such as pneumonia, sepsis, and neuropsychiatric disorders. Some gut microbiota-targeted therapies have shown potential in the treatment and management of ischemic stroke, including dietary interventions, probiotics supplementation, FMT, and rationalization of antibiotic use. Gut microbiota is expected to provide new perspectives for ischemic stroke treatment. However, the efficacy and safety of this treatment strategy for ischemic stroke have not been verified in large scale clinical trials. In addition, it must be recognized that gut microbiota dysbiosis is only one component of the multifactorial brain injury mechanisms of ischemic stroke. Further studies are necessary to broaden our knowledge of the role of gut microbiota in the pathogenesis of ischemic stroke and to facilitate the development of novel therapeutic strategies for ischemic stroke.
ZB wrote the manuscript. ZZ directed the writing of the manuscript, revised the manuscript, and made the figures and the table. GZ and AZ checked the manuscript. AS proposed the idea. FZ supervised the writing of the manuscript. All authors approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abhari, K., Shekarforoush, S. S., Hosseinzadeh, S., Nazifi, S., Sajedianfard, J., and Eskandari, M. H. (2016). The effects of orally administered Bacillus coagulans and inulin on prevention and progression of rheumatoid arthritis in rats. Food Nutr. Res. 60:30876. doi: 10.3402/fnr.v60.30876
Ahnstedt, H., Patrizz, A., Chauhan, A., Roy-O’reilly, M., Furr, J. W., Spychala, M. S., et al. (2020). Sex differences in T cell immune responses, gut permeability and outcome after ischemic stroke in aged mice. Brain Behav. Immun. 87, 556–567. doi: 10.1016/j.bbi.2020.02.001
Akhoundzadeh, K., Vakili, A., Shadnoush, M., and Sadeghzadeh, J. (2018). Effects of the oral ingestion of probiotics on brain damage in a transient model of focal cerebral ischemia in mice. Iran. J. Med. Sci. 43, 32–40.
Alkayed, N. J., Harukuni, I., Kimes, A. S., London, E. D., Traystman, R. J., and Hurn, P. D. (1998). Gender-linked brain injury in experimental stroke. Stroke 29, 159–165; discussion 166. doi: 10.1161/01.str.29.1.159
Aroniadis, O. C., and Brandt, L. J. (2013). Fecal microbiota transplantation: past, present and future. Curr. Opin. Gastroenterol. 29, 79–84. doi: 10.1097/mog.0b013e32835a4b3e
Baker, J. M., Al-Nakkash, L., and Herbst-Kralovetz, M. M. (2017). Estrogen-gut microbiome axis: physiological and clinical implications. Maturitas 103, 45–53. doi: 10.1016/j.maturitas.2017.06.025
Barraud, D., Bollaert, P. E., and Gibot, S. (2013). Impact of the administration of probiotics on mortality in critically ill adult patients: a meta-analysis of randomized controlled trials. Chest 143, 646–655. doi: 10.1378/chest.12-1745
Barugh, A. J., Gray, P., Shenkin, S. D., Maclullich, A. M., and Mead, G. E. (2014). Cortisol levels and the severity and outcomes of acute stroke: a systematic review. J. Neurol. 261, 533–545. doi: 10.1007/s00415-013-7231-5
Benakis, C., Brea, D., Caballero, S., Faraco, G., Moore, J., Murphy, M., et al. (2016). Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat. Med. 22, 516–523. doi: 10.1038/nm.4068
Benakis, C., Martin-Gallausiaux, C., Trezzi, J. P., Melton, P., Liesz, A., and Wilmes, P. (2020a). The microbiome-gut-brain axis in acute and chronic brain diseases. Curr. Opin. Neurobiol. 61, 1–9. doi: 10.1016/j.conb.2019.11.009
Benakis, C., Poon, C., Lane, D., Brea, D., Sita, G., Moore, J., et al. (2020b). Distinct commensal bacterial signature in the gut is associated with acute and long-term protection from ischemic stroke. Stroke 51, 1844–1854. doi: 10.1161/STROKEAHA.120.029262
Bennett, B. J., De Aguiar Vallim, T. Q., Wang, Z., Shih, D. M., Meng, Y., Gregory, J., et al. (2013). Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 17, 49–60. doi: 10.1016/j.cmet.2012.12.011
Bercik, P., Denou, E., Collins, J., Jackson, W., Lu, J., Jury, J., et al. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology 141, 599–609.e3. doi: 10.1053/j.gastro.2011.04.052
Biagi, E., Nylund, L., Candela, M., Ostan, R., Bucci, L., Pini, E., et al. (2010). Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5:e10667. doi: 10.1371/journal.pone.0010667
Blasco, M. P., Chauhan, A., Honarpisheh, P., Ahnstedt, H., D’aigle, J., Ganesan, A., et al. (2020). Age-dependent involvement of gut mast cells and histamine in post-stroke inflammation. J. Neuroinflammation 17:160. doi: 10.1186/s12974-020-01833-1
Blasco-Baque, V., Serino, M., Vergnes, J. N., Riant, E., Loubieres, P., Arnal, J. F., et al. (2012). High-fat diet induces periodontitis in mice through lipopolysaccharides (LPS) receptor signaling: protective action of estrogens. PLoS One 7:e48220. doi: 10.1371/journal.pone.0048220
Bodhankar, S., Chen, Y., Lapato, A., Vandenbark, A. A., Murphy, S. J., Saugstad, J. A., et al. (2015). Regulatory CD8(+)CD122 (+) T-cells predominate in CNS after treatment of experimental stroke in male mice with IL-10-secreting B-cells. Metab. Brain Dis. 30, 911–924. doi: 10.1007/s11011-014-9639-8
Boini, K. M., Hussain, T., Li, P. L., and Koka, S. (2017). Trimethylamine-N-oxide instigates NLRP3 inflammasome activation and endothelial dysfunction. Cell. Physiol. Biochem. 44, 152–162. doi: 10.1159/000484623
Bostanciklioğlu, M. (2019). The role of gut microbiota in pathogenesis of Alzheimer’s disease. J. Appl. Microbiol. 127, 954–967. doi: 10.1111/jam.14264
Braniste, V., Al-Asmakh, M., Kowal, C., Anuar, F., Abbaspour, A., Tóth, M., et al. (2014). The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 6:263ra158. doi: 10.1126/scitranslmed.3009759
Branyan, T. E., and Sohrabji, F. (2020). Sex differences in stroke co-morbidities. Exp. Neurol. 332:113384. doi: 10.1016/j.expneurol.2020.113384
Carabotti, M., Scirocco, A., Maselli, M. A., and Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209.
Caso, J. R., Hurtado, O., Pereira, M. P., Garcia-Bueno, B., Menchen, L., Alou, L., et al. (2009). Colonic bacterial translocation as a possible factor in stress-worsening experimental stroke outcome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R979–R985. doi: 10.1152/ajpregu.90825.2008
Chakraborty, K., Raundhal, M., Chen, B. B., Morse, C., Tyurina, Y. Y., Khare, A., et al. (2017). The mito-DAMP cardiolipin blocks IL-10 production causing persistent inflammation during bacterial pneumonia. Nat. Commun. 8:13944. doi: 10.1038/ncomms13944
Chamorro, Á., Meisel, A., Planas, A. M., Urra, X., Van De Beek, D., and Veltkamp, R. (2012). The immunology of acute stroke. Nat. Rev. Neurol. 8, 401–410. doi: 10.1038/nrneurol.2012.98
Chen, K. L., and Madak-Erdogan, Z. (2016). Estrogen and microbiota crosstalk: should we pay attention? Trends Endocrinol. Metab. 27, 752–755. doi: 10.1016/j.tem.2016.08.001
Chen, M. L., Zhu, X. H., Ran, L., Lang, H. D., Yi, L., and Mi, M. T. (2017). Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J. Am. Heart Assoc. 6:e006347.
Chen, Y., Liang, J., Ouyang, F., Chen, X., Lu, T., Jiang, Z., et al. (2019c). Persistence of gut microbiota dysbiosis and chronic systemic inflammation after cerebral infarction in cynomolgus monkeys. Front. Neurol. 10:661. doi: 10.3389/fneur.2019.00661
Chen, R., Xu, Y., Wu, P., Zhou, H., Lasanajak, Y., Fang, Y., et al. (2019b). Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 148:104403. doi: 10.1016/j.phrs.2019.104403
Chen, R., Wu, P., Cai, Z., Fang, Y., Zhou, H., Lasanajak, Y., et al. (2019a). Puerariae Lobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J. Nutr. Biochem. 65, 101–114. doi: 10.1016/j.jnutbio.2018.12.004
Chen, Y., Bodhankar, S., Murphy, S. J., Vandenbark, A. A., Alkayed, N. J., and Offner, H. (2012). Intrastriatal B-cell administration limits infarct size after stroke in B-cell deficient mice. Metab. Brain Dis. 27, 487–493. doi: 10.1007/s11011-012-9317-7
Cho, J., Park, Y. J., Gonzales-Portillo, B., Saft, M., Cozene, B., Sadanandan, N., et al. (2021). Gut dysbiosis in stroke and its implications on Alzheimer’s disease-like cognitive dysfunction. CNS Neurosci. Ther. 27, 505–514. doi: 10.1111/cns.13613
Christensen, H., Boysen, G., and Johannesen, H. H. (2004). Serum-cortisol reflects severity and mortality in acute stroke. J. Neurol. Sci. 217, 175–180. doi: 10.1016/j.jns.2003.09.013
Ciobanu, O., Elena Sandu, R., Tudor Balseanu, A., Zavaleanu, A., Gresita, A., Petcu, E. B., et al. (2017). Caloric restriction stabilizes body weight and accelerates behavioral recovery in aged rats after focal ischemia. Aging Cell 16, 1394–1403. doi: 10.1111/acel.12678
Claesson, M. J., Cusack, S., O’sullivan, O., Greene-Diniz, R., De Weerd, H., Flannery, E., et al. (2011). Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. U.S.A. 108, (Suppl. 1), 4586–4591. doi: 10.1073/pnas.1000097107
Coman, V., and Vodnar, D. C. (2020). Gut microbiota and old age: modulating factors and interventions for healthy longevity. Exp. Gerontol. 141:111095. doi: 10.1016/j.exger.2020.111095
Crapser, J., Ritzel, R., Verma, R., Venna, V. R., Liu, F., Chauhan, A., et al. (2016). Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging 8, 1049–1063. doi: 10.18632/aging.100952
de Jong, P. R., González-Navajas, J. M., and Jansen, N. J. (2016). The digestive tract as the origin of systemic inflammation. Crit. Care 20:279. doi: 10.1186/s13054-016-1458-3
Demaude, J., Salvador-Cartier, C., Fioramonti, J., Ferrier, L., and Bueno, L. (2006). Phenotypic changes in colonocytes following acute stress or activation of mast cells in mice: implications for delayed epithelial barrier dysfunction. Gut 55, 655–661. doi: 10.1136/gut.2005.078675
Dénes, A., Ferenczi, S., and Kovács, K. J. (2011). Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J. Neuroinflammation 8:164. doi: 10.1186/1742-2094-8-164
Dickson, R. P., Singer, B. H., Newstead, M. W., Falkowski, N. R., Erb-Downward, J. R., Standiford, T. J., et al. (2016). Enrichment of the lung microbiome with gut bacteria in sepsis and the acute respiratory distress syndrome. Nat. Microbiol. 1:16113. doi: 10.1038/nmicrobiol.2016.113
Dirnagl, U., Iadecola, C., and Moskowitz, M. A. (1999). Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 22, 391–397. doi: 10.1016/s0166-2236(99)01401-0
Doyle, K. P., Quach, L. N., Solé, M., Axtell, R. C., Nguyen, T. V., Soler-Llavina, G. J., et al. (2015). B-lymphocyte-mediated delayed cognitive impairment following stroke. J. Neurosci. 35, 2133–2145. doi: 10.1523/JNEUROSCI.4098-14.2015
Duncan, S. H., Barcenilla, A., Stewart, C. S., Pryde, S. E., and Flint, H. J. (2002). Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 68, 5186–5190. doi: 10.1128/AEM.68.10.5186-5190.2002
Durgan, D. J., Lee, J., Mccullough, L. D., and Bryan, R. M. Jr. (2019). Examining the role of the microbiota-gut-brain axis in stroke. Stroke 50, 2270–2277. doi: 10.1161/strokeaha.119.025140
Duvallet, C., Gibbons, S. M., Gurry, T., Irizarry, R. A., and Alm, E. J. (2017). Meta-analysis of gut microbiome studies identifies disease-specific and shared responses. Nat. Commun. 8:1784. doi: 10.1038/s41467-017-01973-8
Eiseman, B., Silen, W., Bascom, G. S., and Kauvar, A. J. (1958). Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 44, 854–859.
El Aidy, S., Dinan, T. G., and Cryan, J. F. (2015). Gut microbiota: the conductor in the orchestra of immune-neuroendocrine communication. Clin. Ther. 37, 954–967. doi: 10.1016/j.clinthera.2015.03.002
El-Hakim, Y., Mani, K. K., Eldouh, A., Pandey, S., Grimaldo, M. T., Dabney, A., et al. (2021). Sex differences in stroke outcome correspond to rapid and severe changes in gut permeability in adult Sprague-Dawley rats. Biol. Sex Differ. 12:14. doi: 10.1186/s13293-020-00352-1
Emsley, H. C., and Hopkins, S. J. (2008). Acute ischaemic stroke and infection: recent and emerging concepts. Lancet Neurol. 7, 341–353. doi: 10.1016/S1474-4422(08)70061-9
Erny, D., Hrabì De Angelis, A. L., Jaitin, D., Wieghofer, P., Staszewski, O., David, E., et al. (2015). Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977. doi: 10.1038/nn.4030
Escobar, Y. H., O’piela, D., Wold, L. E., and Mackos, A. R. (2022). Influence of the microbiota-gut-brain axis on cognition in Alzheimer’s disease. J. Alzheimers Dis. [Epub ahead of print]. doi: 10.3233/JAD-215290
Everard, A., Belzer, C., Geurts, L., Ouwerkerk, J. P., Druart, C., Bindels, L. B., et al. (2013). Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. U.S.A. 110, 9066–9071. doi: 10.1073/pnas.1219451110
Fan, L., Qu, X., Yi, T., Peng, Y., Jiang, M., Miao, J., et al. (2020). Metabolomics of the protective effect of Ampelopsis grossedentata and its major active compound dihydromyricetin on the liver of high-fat diet hamster. Evid. Based Complement. Alternat. Med. 2020:3472578. doi: 10.1155/2020/3472578
Feigin, V. L., Lawes, C. M., Bennett, D. A., and Anderson, C. S. (2003). Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2, 43–53. doi: 10.1016/s1474-4422(03)00266-7
Fisher, M., and Saver, J. L. (2015). Future directions of acute ischaemic stroke therapy. Lancet Neurol. 14, 758–767. doi: 10.1016/s1474-4422(15)00054-x
Flajnik, M. F., and Kasahara, M. (2010). Origin and evolution of the adaptive immune system: genetic events and selective pressures. Nat. Rev. Genet. 11, 47–59. doi: 10.1038/nrg2703
Foster, J. A., and McVey Neufeld, K. A. (2013). Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 36, 305–312. doi: 10.1016/j.tins.2013.01.005
Gu, M., Chen, N., Sun, H., Li, Z., Chen, X., Zhou, J., et al. (2021). Roseburia abundance associates with severity, evolution and outcome of acute ischemic stroke. Front. Cell. Infect. Microbiol. 11:669322. doi: 10.3389/fcimb.2021.669322
Haak, B. W., Littmann, E. R., Chaubard, J. L., Pickard, A. J., Fontana, E., Adhi, F., et al. (2018). Impact of gut colonization with butyrate-producing microbiota on respiratory viral infection following allo-HCT. Blood 131, 2978–2986. doi: 10.1182/blood-2018-01-828996
Haak, B. W., Westendorp, W. F., Van Engelen, T. S. R., Brands, X., Brouwer, M. C., Vermeij, J. D., et al. (2021). Disruptions of anaerobic gut bacteria are associated with stroke and post-stroke infection: a prospective case-control study. Transl. Stroke Res. 12, 581–592. doi: 10.1007/s12975-020-00863-4
Haak, B. W., and Wiersinga, W. J. (2017). The role of the gut microbiota in sepsis. Lancet Gastroenterol. Hepatol. 2, 135–143. doi: 10.1016/s2468-1253(16)30119-4
Hackam, D. G., and Spence, J. D. (2007). Combining multiple approaches for the secondary prevention of vascular events after stroke: a quantitative modeling study. Stroke 38, 1881–1885. doi: 10.1161/STROKEAHA.106.475525
Hackett, M. L., Köhler, S., O’brien, J. T., and Mead, G. E. (2014). Neuropsychiatric outcomes of stroke. Lancet Neurol. 13, 525–534. doi: 10.1016/s1474-4422(14)70016-x
Hackett, M. L., and Pickles, K. (2014). Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int. J. Stroke 9, 1017–1025. doi: 10.1111/ijs.12357
Hagiwara, S., Yoshida, A., Omata, Y., Tsukada, Y., Takahashi, H., Kamewada, H., et al. (2014). Desulfovibrio desulfuricans bacteremia in a patient hospitalized with acute cerebral infarction: case report and review. J. Infect. Chemother. 20, 274–277. doi: 10.1016/j.jiac.2013.10.009
Hall, E. D., Pazara, K. E., and Linseman, K. L. (1991). Sex differences in postischemic neuronal necrosis in gerbils. J. Cereb. Blood Flow Metab. 11, 292–298. doi: 10.1038/jcbfm.1991.61
Hannawi, Y., Hannawi, B., Rao, C. P., Suarez, J. I., and Bershad, E. M. (2013). Stroke-associated pneumonia: major advances and obstacles. Cerebrovasc. Dis. 35, 430–443. doi: 10.1159/000350199
Haro, C., Rangel-Zúñiga, O. A., Alcalá-Díaz, J. F., Gómez-Delgado, F., Pérez-Martínez, P., Delgado-Lista, J., et al. (2016). Intestinal microbiota is influenced by gender and body mass index. PLoS One 11:e0154090. doi: 10.1371/journal.pone.0154090
Huang, J. T., Mao, Y. Q., Han, B., Zhang, Z. Y., Chen, H. L., Li, Z. M., et al. (2021). Calorie restriction conferred improvement effect on long-term rehabilitation of ischemic stroke via gut microbiota. Pharmacol. Res. 170:105726. doi: 10.1016/j.phrs.2021.105726
Huang, Q., Di, L., Yu, F., Feng, X., Liu, Z., Wei, M., et al. (2021). Alterations in the gut microbiome with hemorrhagic transformation in experimental stroke. CNS Neurosci Ther 28, 77–91. doi: 10.1111/cns.13736
Iadecola, C., and Anrather, J. (2011). The immunology of stroke: from mechanisms to translation. Nat. Med. 17, 796–808. doi: 10.1038/nm.2399
Jackson, L., Li, W., Abdul, Y., Dong, G., Baban, B., and Ergul, A. (2019). Diabetic stroke promotes a sexually dimorphic expansion of T cells. Neuromolecular Med. 21, 445–453. doi: 10.1007/s12017-019-08554-6
Juraschek, S. P., Miller, E. R. III, Weaver, C. M., and Appel, L. J. (2017). Effects of sodium reduction and the DASH diet in relation to baseline blood pressure. J. Am. Coll. Cardiol. 70, 2841–2848. doi: 10.1016/j.jacc.2017.10.011
Kalra, L., Irshad, S., Hodsoll, J., Simpson, M., Gulliford, M., Smithard, D., et al. (2015). Prophylactic antibiotics after acute stroke for reducing pneumonia in patients with dysphagia (STROKE-INF): a prospective, cluster-randomised, open-label, masked endpoint, controlled clinical trial. Lancet 386, 1835–1844. doi: 10.1016/S0140-6736(15)00126-9
Kang, D. W., Adams, J. B., Gregory, A. C., Borody, T., Chittick, L., Fasano, A., et al. (2017). Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: an open-label study. Microbiome 5:10. doi: 10.1186/s40168-016-0225-7
Kassell, N. F., Torner, J. C., Jane, J. A., Haley, E. C. Jr., and Adams, H. P. (1990). The international cooperative study on the timing of aneurysm surgery. Part 2: surgical results. J. Neurosurg. 73, 37–47. doi: 10.3171/jns.1990.73.1.0037
Kawabata, S., Takagaki, M., Nakamura, H., Oki, H., Motooka, D., Nakamura, S., et al. (2022). Dysbiosis of gut microbiome is associated with rupture of cerebral aneurysms. Stroke 53, 895–903. doi: 10.1161/STROKEAHA.121.034792
Keskitalo, A., Aatsinki, A. K., Kortesluoma, S., Pelto, J., Korhonen, L., Lahti, L., et al. (2021). Gut microbiota diversity but not composition is related to saliva cortisol stress response at the age of 2.5 months. Stress 24, 551–560. doi: 10.1080/10253890.2021.1895110
Kleinschnitz, C., Schwab, N., Kraft, P., Hagedorn, I., Dreykluft, A., Schwarz, T., et al. (2010). Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood 115, 3835–3842. doi: 10.1182/blood-2009-10-249078
Koeth, R. A., Levison, B. S., Culley, M. K., Buffa, J. A., Wang, Z., Gregory, J. C., et al. (2014). γ-Butyrobetaine is a proatherogenic intermediate in gut microbial metabolism of L-carnitine to TMAO. Cell Metab. 20, 799–812. doi: 10.1016/j.cmet.2014.10.006
Koszewicz, M., Jaroch, J., Brzecka, A., Ejma, M., Budrewicz, S., Mikhaleva, L. M., et al. (2021). Dysbiosis is one of the risk factor for stroke and cognitive impairment and potential target for treatment. Pharmacol. Res. 164:105277. doi: 10.1016/j.phrs.2020.105277
Kristensen, S. L., Lindhardsen, J., Ahlehoff, O., Erichsen, R., Lamberts, M., Khalid, U., et al. (2014). Increased risk of atrial fibrillation and stroke during active stages of inflammatory bowel disease: a nationwide study. Europace 16, 477–484. doi: 10.1093/europace/eut312
Kushamae, M., Miyata, H., Shirai, M., Shimizu, K., Oka, M., Koseki, H., et al. (2020). Involvement of neutrophils in machineries underlying the rupture of intracranial aneurysms in rats. Sci. Rep. 10:20004. doi: 10.1038/s41598-020-74594-9
Langhorne, P., Stott, D. J., Robertson, L., Macdonald, J., Jones, L., Mcalpine, C., et al. (2000). Medical complications after stroke: a multicenter study. Stroke 31, 1223–1229. doi: 10.1161/01.str.31.6.1223
Le Blay, G., Michel, C., Blottière, H. M., and Cherbut, C. (1999). Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 129, 2231–2235. doi: 10.1093/jn/129.12.2231
Lee, J., D’aigle, J., Atadja, L., Quaicoe, V., Honarpisheh, P., Ganesh, B. P., et al. (2020). Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ. Res. 127, 453–465. doi: 10.1161/CIRCRESAHA.119.316448
Lee, M., Saver, J. L., Chang, K. H., Liao, H. W., Chang, S. C., and Ovbiagele, B. (2010). Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ 341:c4249. doi: 10.1136/bmj.c4249
Li, D., Ke, Y., Zhan, R., Liu, C., Zhao, M., Zeng, A., et al. (2018). Trimethylamine-N-oxide promotes brain aging and cognitive impairment in mice. Aging Cell 17:e12768. doi: 10.1111/acel.12768
Li, Q., Wang, C., Tang, C., Zhao, X., He, Q., and Li, J. (2018). Identification and characterization of blood and neutrophil-associated microbiomes in patients with severe acute pancreatitis using next-generation sequencing. Front. Cell. Infect. Microbiol. 8:5. doi: 10.3389/fcimb.2018.00005
Li, H., Xu, H., Li, Y., Jiang, Y., Hu, Y., Liu, T., et al. (2020). Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat. Commun. 11:3218. doi: 10.1038/s41467-020-16990-3
Li, N., Wang, X., Sun, C., Wu, X., Lu, M., Si, Y., et al. (2019). Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 19:191. doi: 10.1186/s12866-019-1552-1
Li, T., Chen, Y., Gua, C., and Li, X. (2017). Elevated circulating trimethylamine N-oxide levels contribute to endothelial dysfunction in aged rats through vascular inflammation and oxidative stress. Front. Physiol. 8:350. doi: 10.3389/fphys.2017.00350
Li, X., Ma, X., Lin, J., He, X., Tian, F., and Kong, D. (2017). Severe carotid artery stenosis evaluated by ultrasound is associated with post stroke vascular cognitive impairment. Brain Behav. 7:e00606. doi: 10.1002/brb3.606
Liang, S., Wang, T., Hu, X., Luo, J., Li, W., Wu, X., et al. (2015). Administration of Lactobacillus helveticus NS8 improves behavioral, cognitive, and biochemical aberrations caused by chronic restraint stress. Neuroscience 310, 561–577. doi: 10.1016/j.neuroscience.2015.09.033
Liesz, A., Zhou, W., Mracskó, E., Karcher, S., Bauer, H., Schwarting, S., et al. (2011). Inhibition of lymphocyte trafficking shields the brain against deleterious neuroinflammation after stroke. Brain 134, 704–720. doi: 10.1093/brain/awr008
Liu, F., Cheng, X., Zhong, S., Liu, C., Jolkkonen, J., Zhang, X., et al. (2020). Communications between peripheral and the brain-resident immune system in neuronal regeneration after stroke. Front Immunol 11:1931. doi: 10.3389/fimmu.2020.01931
Liu, P., Jia, X. Z., Chen, Y., Yu, Y., Zhang, K., Lin, Y. J., et al. (2021). Gut microbiota interacts with intrinsic brain activity of patients with amnestic mild cognitive impairment. CNS Neurosci. Ther. 27, 163–173. doi: 10.1111/cns.13451
Louis, P., and Flint, H. J. (2017). Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 19, 29–41. doi: 10.1111/1462-2920.13589
Lukiw, W. J., Cong, L., Jaber, V., and Zhao, Y. (2018). Microbiome-derived lipopolysaccharide (LPS) selectively inhibits neurofilament light chain (NF-L) gene expression in human neuronal-glial (HNG) cells in primary culture. Front. Neurosci. 12:896. doi: 10.3389/fnins.2018.00896
Luo, J., Wang, T., Liang, S., Hu, X., Li, W., and Jin, F. (2014). Ingestion of Lactobacillus strain reduces anxiety and improves cognitive function in the hyperammonemia rat. Sci. China Life Sci. 57, 327–335. doi: 10.1007/s11427-014-4615-4
Lyons, J. D., Ford, M. L., and Coopersmith, C. M. (2016). The microbiome in critical Illness: firm conclusions or bact to square one? Dig. Dis. Sci. 61, 1420–1421. doi: 10.1007/s10620-016-4092-7
Maa, M. C., Chang, M. Y., Hsieh, M. Y., Chen, Y. J., Yang, C. J., Chen, Z. C., et al. (2010). Butyrate reduced lipopolysaccharide-mediated macrophage migration by suppression of Src enhancement and focal adhesion kinase activity. J. Nutr. Biochem. 21, 1186–1192. doi: 10.1016/j.jnutbio.2009.10.004
Mackie, R. I., Sghir, A., and Gaskins, H. R. (1999). Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 69, 1035s–1045s. doi: 10.1093/ajcn/69.5.1035s
Manwani, B., Liu, F., Scranton, V., Hammond, M. D., Sansing, L. H., and Mccullough, L. D. (2013). Differential effects of aging and sex on stroke induced inflammation across the lifespan. Exp. Neurol. 249, 120–131. doi: 10.1016/j.expneurol.2013.08.011
Manwani, B., Liu, F., Xu, Y., Persky, R., Li, J., and Mccullough, L. D. (2011). Functional recovery in aging mice after experimental stroke. Brain Behav. Immun. 25, 1689–1700. doi: 10.1016/j.bbi.2011.06.015
Marik, P. E. (2001). Aspiration pneumonitis and aspiration pneumonia. N. Engl. J. Med. 344, 665–671. doi: 10.1056/nejm200103013440908
Markowiak, P., and Śliżewska, K. (2017). Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 9:1021. doi: 10.3390/nu9091021
Misiak, B., Loniewski, I., Marlicz, W., Frydecka, D., Szulc, A., Rudzki, L., et al. (2020). The HPA axis dysregulation in severe mental illness: can we shift the blame to gut microbiota? Prog. Neuropsychopharmacol. Biol. Psychiatry 102:109951. doi: 10.1016/j.pnpbp.2020.109951
Mitchell, S. J., Bernier, M., Mattison, J. A., Aon, M. A., Kaiser, T. A., Anson, R. M., et al. (2019). Daily fasting improves health and survival in male mice independent of diet composition and calories. Cell Metab. 29, 221–228.e3. doi: 10.1016/j.cmet.2018.08.011
Mulak, A., and Bonaz, B. (2015). Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 21, 10609–10620.
Nakamura, Y. K., Janowitz, C., Metea, C., Asquith, M., Karstens, L., Rosenbaum, J. T., et al. (2017). Short chain fatty acids ameliorate immune-mediated uveitis partially by altering migration of lymphocytes from the intestine. Sci. Rep. 7:11745. doi: 10.1038/s41598-017-12163-3
Nie, J., Xie, L., Zhao, B. X., Li, Y., Qiu, B., Zhu, F., et al. (2018). Serum trimethylamine N-oxide concentration is positively associated with first stroke in hypertensive patients. Stroke 49, 2021–2028. doi: 10.1161/STROKEAHA.118.021997
Nilsson, A., Tervahartiala, T., Lennebratt, D., Lannergård, A., Sorsa, T., and Rautelin, H. (2018). Enhanced systemic response of matrix metalloproteinases and their regulators in Campylobacter and Salmonella patients. Diagnostics 8:82. doi: 10.3390/diagnostics8040082
Ovbiagele, B., and Nguyen-Huynh, M. N. (2011). Stroke epidemiology: advancing our understanding of disease mechanism and therapy. Neurotherapeutics 8, 319–329. doi: 10.1007/s13311-011-0053-1
Palmer, C., Bik, E. M., Digiulio, D. B., Relman, D. A., and Brown, P. O. (2007). Development of the human infant intestinal microbiota. PLoS Biol. 5:e177. doi: 10.1371/journal.pbio.0050177
Park, J., Kim, M., Kang, S. G., Jannasch, A. H., Cooper, B., Patterson, J., et al. (2015). Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93. doi: 10.1038/mi.2014.44
Park, M. J., Pilla, R., Panta, A., Pandey, S., Sarawichitr, B., Suchodolski, J., et al. (2020). Reproductive senescence and ischemic stroke remodel the gut microbiome and modulate the effects of Estrogen treatment in female rats. Transl. Stroke Res. 11, 812–830. doi: 10.1007/s12975-019-00760-5
Patnala, R., Arumugam, T. V., Gupta, N., and Dheen, S. T. (2017). HDAC inhibitor sodium butyrate-mediated epigenetic regulation enhances neuroprotective function of microglia during ischemic stroke. Mol. Neurobiol. 54, 6391–6411. doi: 10.1007/s12035-016-0149-z
Plovier, H., Everard, A., Druart, C., Depommier, C., Van Hul, M., Geurts, L., et al. (2017). A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113. doi: 10.1038/nm.4236
Potgieter, M., Bester, J., Kell, D. B., and Pretorius, E. (2015). The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol. Rev. 39, 567–591. doi: 10.1093/femsre/fuv013
Redon, J., Olsen, M. H., Cooper, R. S., Zurriaga, O., Martinez-Beneito, M. A., Laurent, S., et al. (2011). Stroke mortality and trends from 1990 to 2006 in 39 countries from Europe and Central Asia: implications for control of high blood pressure. Eur. Heart J. 32, 1424–1431. doi: 10.1093/eurheartj/ehr045
Reeves, M. J., Bushnell, C. D., Howard, G., Gargano, J. W., Duncan, P. W., Lynch, G., et al. (2008). Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol. 7, 915–926. doi: 10.1016/S1474-4422(08)70193-5
Ritzel, R. M., Lai, Y. J., Crapser, J. D., Patel, A. R., Schrecengost, A., Grenier, J. M., et al. (2018). Aging alters the immunological response to ischemic stroke. Acta Neuropathol. 136, 89–110. doi: 10.1007/s00401-018-1859-2
Sadler, R., Cramer, J. V., Heindl, S., Kostidis, S., Betz, D., Zuurbier, K. R., et al. (2020). Short-chain fatty acids improve poststroke recovery via immunological mechanisms. J. Neurosci. 40, 1162–1173. doi: 10.1523/JNEUROSCI.1359-19.2019
Schulte-Herbruggen, O., Quarcoo, D., Meisel, A., and Meisel, C. (2009). Differential affection of intestinal immune cell populations after cerebral ischemia in mice. Neuroimmunomodulation 16, 213–218. doi: 10.1159/000205514
Seldin, M. M., Meng, Y., Qi, H., Zhu, W., Wang, Z., Hazen, S. L., et al. (2016). Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Heart Assoc. 5:e002767. doi: 10.1161/JAHA.115.002767
Selvamani, A., and Sohrabji, F. (2010a). The neurotoxic effects of Estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J. Neurosci. 30, 6852–6861. doi: 10.1523/JNEUROSCI.0761-10.2010
Selvamani, A., and Sohrabji, F. (2010b). Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of Estrogen treatment in female rats. Neurobiol. Aging 31, 1618–1628. doi: 10.1016/j.neurobiolaging.2008.08.014
Shafi, T., Powe, N. R., Meyer, T. W., Hwang, S., Hai, X., Melamed, M. L., et al. (2017). Trimethylamine N-oxide and cardiovascular events in hemodialysis patients. J. Am. Soc. Nephrol. 28, 321–331. doi: 10.1681/asn.2016030374
Sharma, S., Taliyan, R., and Singh, S. (2015). Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: modulation of histone deacetylase activity. Behav. Brain Res. 291, 306–314. doi: 10.1016/j.bbr.2015.05.052
Shikata, F., Shimada, K., Sato, H., Ikedo, T., Kuwabara, A., Furukawa, H., et al. (2019). Potential influences of gut microbiota on the formation of intracranial aneurysm. Hypertension 73, 491–496. doi: 10.1161/hypertensionaha.118.11804
Shimizu, Y. (2018). Gut microbiota in common elderly diseases affecting activities of daily living. World J. Gastroenterol. 24, 4750–4758. doi: 10.3748/wjg.v24.i42.4750
Silva, Y. P., Bernardi, A., and Frozza, R. L. (2020). The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front. Endocrinol. 11:25. doi: 10.3389/fendo.2020.00025
Singh, V., Roth, S., Llovera, G., Sadler, R., Garzetti, D., Stecher, B., et al. (2016). Microbiota dysbiosis controls the neuroinflammatory response after stroke. J. Neurosci. 36, 7428–7440. doi: 10.1523/JNEUROSCI.1114-16.2016
Smith, E. A., and Macfarlane, G. T. (1997). Dissimilatory amino acid metabolism in human colonic bacteria. Anaerobe 3, 327–337. doi: 10.1006/anae.1997.0121
Spychala, M. S., Venna, V. R., Jandzinski, M., Doran, S. J., Durgan, D. J., Ganesh, B. P., et al. (2018). Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 84, 23–36. doi: 10.1002/ana.25250
Stanley, D., Mason, L. J., Mackin, K. E., Srikhanta, Y. N., Lyras, D., Prakash, M. D., et al. (2016). Translocation and dissemination of commensal bacteria in post-stroke infection. Nat. Med. 22, 1277–1284. doi: 10.1038/nm.4194
Stanley, D., Moore, R. J., and Wong, C. H. Y. (2018). An insight into intestinal mucosal microbiota disruption after stroke. Sci. Rep. 8:568. doi: 10.1038/s41598-017-18904-8
Sun, J., Ling, Z., Wang, F., Chen, W., Li, H., Jin, J., et al. (2016). Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci. Lett. 613, 30–35. doi: 10.1016/j.neulet.2015.12.047
Tan, C., Wang, H., Gao, X., Xu, R., Zeng, X., Cui, Z., et al. (2020). Dynamic changes and prognostic value of gut microbiota-dependent trimethylamine-N-oxide in acute ischemic stroke. Front. Neurol. 11:29. doi: 10.3389/fneur.2020.00029
Tang, W. H., Wang, Z., Fan, Y., Levison, B., Hazen, J. E., Donahue, L. M., et al. (2014). Prognostic value of elevated levels of intestinal microbe-generated metabolite trimethylamine-N-oxide in patients with heart failure: refining the gut hypothesis. J. Am. Coll. Cardiol. 64, 1908–1914. doi: 10.1016/j.jacc.2014.02.617
Tang, W. H., Wang, Z., Kennedy, D. J., Wu, Y., Buffa, J. A., Agatisa-Boyle, B., et al. (2015). Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ. Res. 116, 448–455. doi: 10.1161/CIRCRESAHA.116.305360
Tascilar, N., Irkorucu, O., Tascilar, O., Comert, F., Eroglu, O., Bahadir, B., et al. (2010). Bacterial translocation in experimental stroke: what happens to the gut barrier? Bratisl. Lek. Listy 111, 194–199.
Tillisch, K., Mayer, E. A., Gupta, A., Gill, Z., Brazeilles, R., Le Nevé, B., et al. (2017). Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom. Med. 79, 905–913. doi: 10.1097/PSY.0000000000000493
Toung, T. J., Traystman, R. J., and Hurn, P. D. (1998). Estrogen-mediated neuroprotection after experimental stroke in male rats. Stroke 29, 1666–1670. doi: 10.1161/01.str.29.8.1666
van Nood, E., Vrieze, A., Nieuwdorp, M., Fuentes, S., Zoetendal, E. G., De Vos, W. M., et al. (2013). Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415. doi: 10.1056/NEJMoa1205037
Vlak, M. H., Algra, A., Brandenburg, R., and Rinkel, G. J. (2011). Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 10, 626–636. doi: 10.1016/S1474-4422(11)70109-0
Wang, H., Song, W., Wu, Q., Gao, X., Li, J., Tan, C., et al. (2021). Fecal transplantation from db/db mice treated with sodium butyrate attenuates ischemic stroke injury. Microbiol. Spectr. 9:e0004221. doi: 10.1128/Spectrum.00042-21
Wang, Z., Klipfell, E., Bennett, B. J., Koeth, R., Levison, B. S., Dugar, B., et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63. doi: 10.1038/nature09922
Wasser, C. I., Mercieca, E. C., Kong, G., Hannan, A. J., Mckeown, S. J., Glikmann-Johnston, Y., et al. (2020). Gut dysbiosis in Huntington’s disease: associations among gut microbiota, cognitive performance and clinical outcomes. Brain Commun. 2:fcaa110. doi: 10.1093/braincomms/fcaa110
Wassertheil-Smoller, S., Hendrix, S. L., Limacher, M., Heiss, G., Kooperberg, C., Baird, A., et al. (2003). Effect of Estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA 289, 2673–2684. doi: 10.1001/jama.289.20.2673
Wei, Y., Yemisci, M., Kim, H. H., Yung, L. M., Shin, H. K., Hwang, S. K., et al. (2011). Fingolimod provides long-term protection in rodent models of cerebral ischemia. Ann. Neurol. 69, 119–129. doi: 10.1002/ana.22186
Westendorp, W. F., Nederkoorn, P. J., Vermeij, J. D., Dijkgraaf, M. G., and Van De Beek, D. (2011). Post-stroke infection: a systematic review and meta-analysis. BMC Neurol. 11:110. doi: 10.1186/1471-2377-11-110
Westendorp, W. F., Vermeij, J. D., Zock, E., Hooijenga, I. J., Kruyt, N. D., Bosboom, H. J., et al. (2015). The preventive antibiotics in stroke study (PASS): a pragmatic randomised open-label masked endpoint clinical trial. Lancet 385, 1519–1526. doi: 10.1016/s0140-6736(14)62456-9
Winek, K., Engel, O., Koduah, P., Heimesaat, M. M., Fischer, A., Bereswill, S., et al. (2016). Depletion of cultivatable gut microbiota by broad-spectrum antibiotic pretreatment worsens outcome after murine stroke. Stroke 47, 1354–1363. doi: 10.1161/STROKEAHA.115.011800
Wong, C. H., Jenne, C. N., Lee, W. Y., Léger, C., and Kubes, P. (2011). Functional innervation of hepatic iNKT cells is immunosuppressive following stroke. Science 334, 101–105. doi: 10.1126/science.1210301
Wu, S. V., and Hui, H. (2011). Treat your bug right. Front. Physiol. 2:9. doi: 10.3389/fphys.2011.00009
Wu, X., Kim, M. J., Yang, H. J., and Park, S. (2021). Chitosan alleviated menopausal symptoms and modulated the gut microbiota in Estrogen-deficient rats. Eur. J. Nutr. 60, 1907–1919. doi: 10.1007/s00394-020-02382-2
Xia, G. H., You, C., Gao, X. X., Zeng, X. L., Zhu, J. J., Xu, K. Y., et al. (2019). Stroke dysbiosis index (SDI) in gut microbiome are associated with brain injury and prognosis of stroke. Front. Neurol. 10:397. doi: 10.3389/fneur.2019.00397
Xia, G. H., Zhang, M. S., Wu, Q. H., Wang, H. D., Zhou, H. W., He, Y., et al. (2021). Dysbiosis of gut microbiota is an independent risk factor of stroke-associated pneumonia: a Chinese pilot study. Front. Cell. Infect. Microbiol. 11:715475. doi: 10.3389/fcimb.2021.715475
Xiao, Z., Pei, Z., Yuan, M., Li, X., Chen, S., and Xu, L. (2015). Risk of stroke in patients with inflammatory bowel disease: a systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 24, 2774–2780. doi: 10.1016/j.jstrokecerebrovasdis.2015.08.008
Xu, K., Gao, X., Xia, G., Chen, M., Zeng, N., Wang, S., et al. (2021). Rapid gut dysbiosis induced by stroke exacerbates brain infarction in turn. Gut [Epub ahead of print]. doi: 10.1136/gutjnl-2020-323263
Yager, J. Y., Wright, S., Armstrong, E. A., Jahraus, C. M., and Saucier, D. M. (2006). The influence of aging on recovery following ischemic brain damage. Behav. Brain Res. 173, 171–180. doi: 10.1016/j.bbr.2006.06.019
Yamashiro, K., Tanaka, R., Urabe, T., Ueno, Y., Yamashiro, Y., Nomoto, K., et al. (2017). Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One 12:e0171521. doi: 10.1371/journal.pone.0171521
Yan, J., Greer, J. M., Etherington, K., Cadigan, G. P., Cavanagh, H., Henderson, R. D., et al. (2009). Immune activation in the peripheral blood of patients with acute ischemic stroke. J. Neuroimmunol. 206, 112–117. doi: 10.1016/j.jneuroim.2008.11.001
Yang, C., Hawkins, K. E., Doré, S., and Candelario-Jalil, E. (2019). Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 316, C135–C153. doi: 10.1152/ajpcell.00136.2018
Yates, D. A., Santos, J., Söderholm, J. D., and Perdue, M. H. (2001). Adaptation of stress-induced mucosal pathophysiology in rat colon involves opioid pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G124–G128. doi: 10.1152/ajpgi.2001.281.1.G124
Yin, J., Liao, S. X., He, Y., Wang, S., Xia, G. H., Liu, F. T., et al. (2015). Dysbiosis of gut microbiota with reduced trimethylamine-N-oxide level in patients with large-artery atherosclerotic stroke or transient ischemic attack. J. Am. Heart Assoc. 4:e002699. doi: 10.1161/JAHA.115.002699
Yu, X., Zhou, G., Shao, B., Zhou, H., Xu, C., Yan, F., et al. (2021). Gut microbiota dysbiosis induced by intracerebral hemorrhage aggravates neuroinflammation in mice. Front. Microbiol. 12:647304. doi: 10.3389/fmicb.2021.647304
Zapolska-Downar, D., and Naruszewicz, M. (2009). Propionate reduces the cytokine-induced VCAM-1 and ICAM-1 expression by inhibiting nuclear factor-kappa B (NF-kappaB) activation. J. Physiol. Pharmacol. 60, 123–131.
Zapolska-Downar, D., Siennicka, A., Kaczmarczyk, M., Kołodziej, B., and Naruszewicz, M. (2004). Butyrate inhibits cytokine-induced VCAM-1 and ICAM-1 expression in cultured endothelial cells: the role of NF-kappaB and PPARalpha. J. Nutr. Biochem. 15, 220–228. doi: 10.1016/j.jnutbio.2003.11.008
Zeng, X., Gao, X., Peng, Y., Wu, Q., Zhu, J., Tan, C., et al. (2019). Higher risk of stroke is correlated with increased opportunistic pathogen load and reduced levels of butyrate-producing bacteria in the gut. Front. Cell. Infect. Microbiol. 9:4. doi: 10.3389/fcimb.2019.00004
Zhai, Q., Sun, T., Sun, C., Yan, L., Wang, X., Wang, Y., et al. (2021). High plasma levels of trimethylamine N-oxide are associated with poor outcome in intracerebral hemorrhage patients. Neurol. Sci. 42, 1009–1016. doi: 10.1007/s10072-020-04618-9
Zhang, H., Huang, Y., Li, X., Han, X., Hu, J., Wang, B., et al. (2021). Dynamic process of secondary pulmonary infection in mice with intracerebral hemorrhage. Front. Immunol. 12:767155. doi: 10.3389/fimmu.2021.767155
Zhang, J., Wang, L., Cai, J., Lei, A., Liu, C., Lin, R., et al. (2021). Gut microbial metabolite TMAO portends prognosis in acute ischemic stroke. J. Neuroimmunol. 354:577526. doi: 10.1016/j.jneuroim.2021.577526
Zhang, J., Zhang, W., Gao, X., Zhao, Y., Chen, D., Xu, N., et al. (2019). Preconditioning with partial caloric restriction confers long-term protection against grey and white matter injury after transient focal ischemia. J. Cereb. Blood Flow Metab. 39, 1394–1409. doi: 10.1177/0271678X18785480
Zhang, Z. Y., Fang, Y. J., Luo, Y. J., Lenahan, C., Zhang, J. M., and Chen, S. (2019). The role of medical gas in stroke: an updated review. Med. Gas Res. 9, 221–228. doi: 10.4103/2045-9912.273960
Zhu, C., Li, G., Lv, Z., Li, J., Wang, X., Kang, J., et al. (2020). Association of plasma trimethylamine-N-oxide levels with post-stroke cognitive impairment: a 1-year longitudinal study. Neurol. Sci. 41, 57–63. doi: 10.1007/s10072-019-04040-w
Keywords: ischemic stroke, gut microbiota, microbiome, mechanism, target, treatment
Citation: Bao Z, Zhang Z, Zhou G, Zhang A, Shao A and Zhou F (2022) Novel Mechanisms and Therapeutic Targets for Ischemic Stroke: A Focus on Gut Microbiota. Front. Cell. Neurosci. 16:871720. doi: 10.3389/fncel.2022.871720
Received: 08 February 2022; Accepted: 19 April 2022;
Published: 17 May 2022.
Edited by:
Aurel Popa-Wagner, University of Medicine and Pharmacy of Craiova, RomaniaReviewed by:
Justyna Agier, Medical University of Łódź, PolandCopyright © 2022 Bao, Zhang, Zhou, Zhang, Shao and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anwen Shao, c2hhb2Fud2VuQHpqdS5lZHUuY24=; Feng Zhou, emhvdWZlbmdkQGhvdG1haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.