
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci. , 17 November 2022
Sec. Non-Neuronal Cells
Volume 16 - 2022 | https://doi.org/10.3389/fncel.2022.1005399
This article is part of the Research Topic New Challenges and Future Perspectives in Cellular Neuroscience View all 8 articles
Spinal cord injury (SCI) is a serious neurological trauma that is challenging to treat. After SCI, many neurons in the injured area die due to necrosis or apoptosis, and astrocytes, oligodendrocytes, microglia and other non-neuronal cells become dysfunctional, hindering the repair of the injured spinal cord. Corrective surgery and biological, physical and pharmacological therapies are commonly used treatment modalities for SCI; however, no current therapeutic strategies can achieve complete recovery. Somatic cell reprogramming is a promising technology that has gradually become a feasible therapeutic approach for repairing the injured spinal cord. This revolutionary technology can reprogram fibroblasts, astrocytes, NG2 cells and neural progenitor cells into neurons or oligodendrocytes for spinal cord repair. In this review, we provide an overview of the transcription factors, genes, microRNAs (miRNAs), small molecules and combinations of these factors that can mediate somatic cell reprogramming to repair the injured spinal cord. Although many challenges and questions related to this technique remain, we believe that the beneficial effect of somatic cell reprogramming provides new ideas for achieving functional recovery after SCI and a direction for the development of treatments for SCI.
Spinal cord injury (SCI), a type of central nervous system (CNS) injury, can affect motor and sensory functions in the innervated area in mild cases and lead to paraplegia in worst-case scenarios. The incidence of traumatic spinal injury is 10.5 cases per 100,000 individuals worldwide (Kumar et al., 2018). SCI significantly affects the physical and mental health of patients, poses a substantial challenge to health care workers, and imposes a very large economic burden on the healthcare system (Algahtany et al., 2021). Therefore, efforts are required to improve patients’ status and quality of life and reduce the burden on families and society (Savic et al., 2018). SCI can be accompanied by complications such as respiratory and heart diseases, which are associated with a high mortality rate (Barbiellini Amidei et al., 2022). In the clinic, there are no effective methods for SCI treatment (de Araujo et al., 2022; Lee and Jeong, 2022).
Cell-based regenerative therapy is a promising treatment strategy capable of modulating inflammatory responses and regenerating lost neural circuits (Papa et al., 2020). Researchers have integrated host circuits to aid the recovery of injured nerves in animals by transplanting autologous or homologous totipotent or multipotent cell-derived neurons (Lee and Jeong, 2022). Embryonic and neural stem cells are multipotent; the latter are more likely to differentiate into neural cells, but ethical concerns and limited supply restrict their clinical use. Although stromal stem cells can be easily used for autologous transplantation, their rate of transdifferentiation is low because these cells normally differentiate into mesodermal-lineage cells and must cross the germ cell layer to differentiate into neural lineage cells. In addition, there are some challenges related to mesenchymal stem cell use, such as a lack of standardized isolation protocols, the effect of cell heterogeneity on in vitro expansion, and the potential loss of multipotency during in vitro expansion (Hernandez et al., 2020). Induced pluripotent stem cells (iPSCs), the embryonic-like stem cells, can be induced from somatic cells treated with a cocktail of cytokines. iPSCs are convenient to use, have substantial developmental plasticity, can be used for autotransplantation, and can be induced to differentiate into neural stem cells. However, the clinical implementation of iPSCs remains complicated, and issues related to timing, influencing factors and the potential risk for teratoma formation need to be addressed (Csobonyeiova et al., 2019). As shown in Figure 1, mouse-derived somatic cells take 30 days to become iPSCs, 36 days to become embryoid bodies, 43 days to form immature neurospheres and 57 days to form mature neurospheres. It may take up to 180 days for human-derived somatic cells to become mature neurospheres (Matsui et al., 2014). Generally, the best time point for transplanting neural stem cells, the cells that derive from neurospheres, into SCI model animals is approximately 14 days after injury. However, the practicality of such a long-term experiment is unknown, and it seems doubtful that transplanted iPSC-derived neural stem cells can achieve the required rate of transdifferentiation (Kojima et al., 2019; Ahmadian-Moghadam et al., 2020; Giallongo et al., 2021).
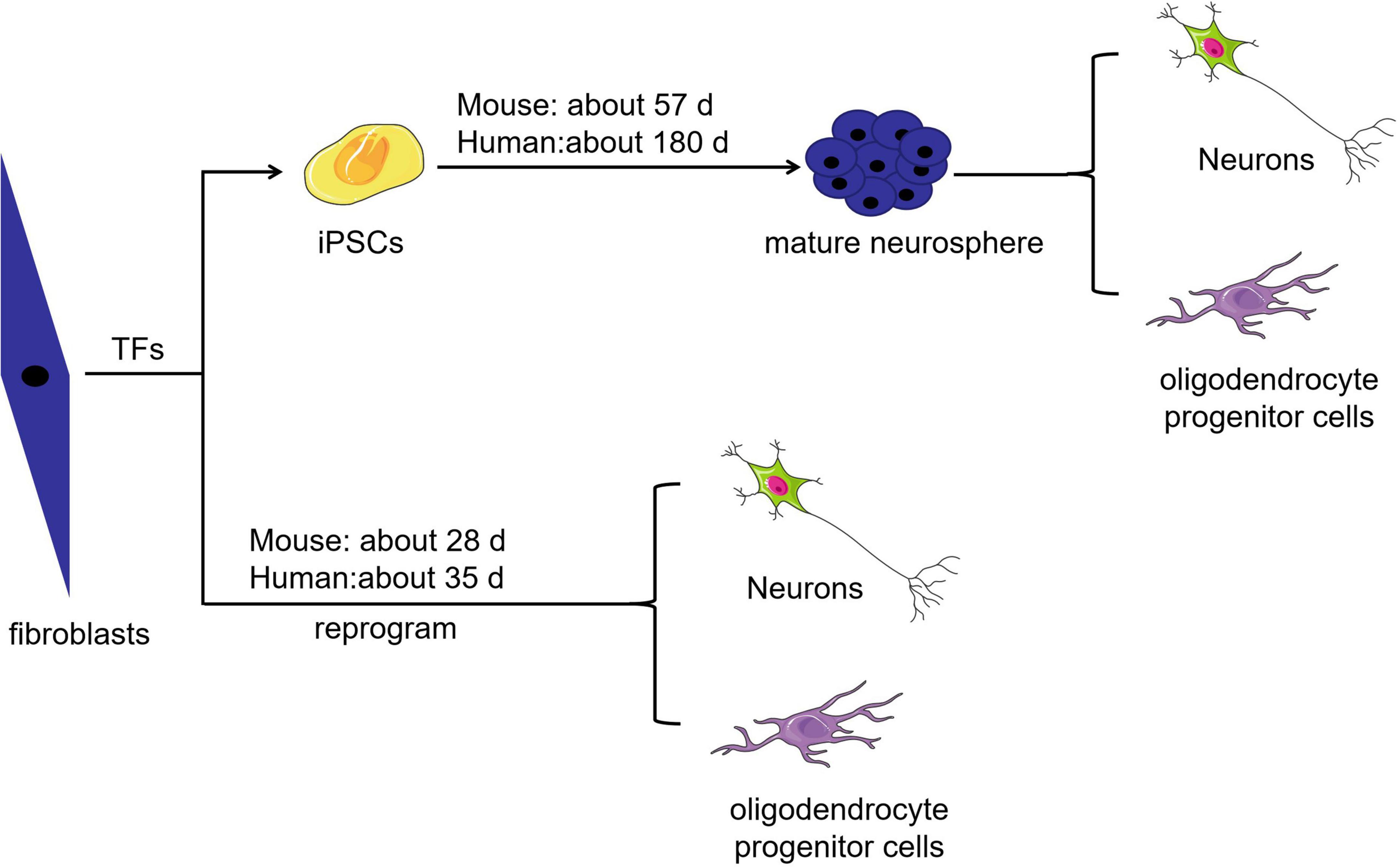
Figure 1. Comparison of the time required to obtain different target cells. The time required to obtain neurons and oligodendrocyte progenitor cells by somatic cell direct reprogramming is shorter than that required to obtain these target cells from iPSCs.
Advances in iPSC- and transdifferentiation-related techniques have led to the development of somatic cell reprogramming approaches (Velychko et al., 2019). Somatic cell reprogramming skips the iPSC stage, reducing the time of transdifferentiation and the risk of teratoma development (Csobonyeiova et al., 2019). Some factors influence the efficiency of somatic reprogramming (Heinrich et al., 2015). For instance, studies have shown that reprogramming cells with the same embryological origin as neurons (i.e., the ectoderm) is an effective strategy for obtaining neurons (Zhou and Melton, 2008; Mattugini et al., 2019). Cell cycle status has also been shown to impact reprogramming. With advancements in biotechnology, somatic cell reprogramming has gradually become a feasible and promising strategy for neural repair, and it has been confirmed that neurons generated via this method can survive for a long time in the CNS. In vivo, reprogrammed neurons can form synaptic connections with primitive neurons and integrate into neural circuits, both locally and globally, ultimately facilitating the recovery of neural function (Wang F. et al., 2021).
Due to challenges related to CNS regeneration, there are still many difficulties to overcome before somatic cell reprogramming can be widely used in clinical practice. Currently, somatic cell reprogramming is the most appealing method for treating SCI. Several studies have revealed that the direct reprogramming of somatic cells into neurons and oligodendrocytes can effectively promote the recovery of motor function in SCI mice. In this review, we discuss the challenges of spinal cord repair and related advances in the direct reprogramming of somatic cells into neurons and oligodendrocytes using somatic cell reprogramming techniques.
The interactions of many cell types, especially neurons, astrocytes, oligodendrocytes, and microglia, play an important role in spinal cord function. In SCI, these cellular interactions are disrupted, causing cell dysfunction and hindering the repair of the injured spinal cord (Anjum et al., 2020). SCI typically results from physical trauma, such as shearing, transection, and acute stretching/compression. SCI can cause a range of neurological symptoms leading to motor/sensory dysfunction, neuropathic pain, autonomic deficits, bowel/bladder dysfunction, and autonomic dysreflexia depending on the level and severity of the injury (Furlan et al., 2016).
SCI can be divided into four phases according to the time since the onset of injury, including the acute phase (< 48 h), subacute phase (48 h to 14 days), intermediate phase (14 days to 3 months), and chronic phase (> 3 months) (Tica et al., 2018). Acute SCI occurs when the spine is fractured and dislocated due to sudden trauma. The initial phase is the primary phase and is characterized by the presence of bone fragments and the tearing of spinal cord ligaments (Katoh et al., 2019). Regardless of the type of initial trauma, damage to the spinal cord blocks neural pathways, injures blood vessels, disrupts cell membranes, and leads to a range of pathophysiological consequences, including systemic hypotension, spinal shock, ischemia, vasospasm, neurotransmitter accumulation, and ionic imbalance (Ham and Leipzig, 2018; Chio et al., 2021). The severity of the initial damage and duration of spinal cord compression determines the consequence of SCI. After the primary injury occurs, a series of secondary events leading to chemical and mechanical injury in the spinal cord is initiated. In addition, loss of ion homeostasis following SCI causes an increase in the intracellular calcium ion concentration, and calcium overload eventually leads to cell death. Following SCI, the increased release of excitatory amino acids, such as glutamate, causes excitotoxicity, leading to neurological dysfunction (Vargas Luna et al., 2021). Secondary injury, the pathological process following primary injury, involves multiple phenomena. The initial clinical manifestations of secondary injury are hemorrhage and rapid death of necrotic cells, followed by regulated biochemical and molecular events, including vascular insufficiency, ischemia, neuronal excitotoxicity, the production of toxic free radicals, edema, dysregulation of ion homeostasis, lipid peroxidation, inflammatory reactions, immune reactions, astrocyte proliferation, demyelination, neuronal and glial apoptosis, glial scarring, and cyst formation (Anjum et al., 2020).
Cell death is a major mechanism of secondary injury (Karova et al., 2019). Initially, neurons become necrotic due to mechanical injury in the acute and subacute stages of SCI (Tang et al., 2021). Necrosis is caused by various factors, including the accumulation of toxic blood components, glutamate excitotoxicity, sudden loss of ion homeostasis, rapid loss of adenosine triphosphate (ATP), the production of proinflammatory cytokines by lymphocytes and neutrophils, and free radical formation (Alizadeh et al., 2019). Astrocytes are the most abundant cells in the CNS and are associated with SCI pathology. Primary injury induces astrocyte proliferation, which causes them to become reactive, leading to scarring (Yoshizaki et al., 2021). Subsequently, reactive astrocytes enter the injury epicenter, isolating inflammatory cells and preventing their spread (Gu et al., 2019), allowing fibroblasts and microglia to form fibrotic scars in the injury epicenter. Elongated reactive astrocytes and fibroblast-like pericytes interact with fibrotic scars to form glial scars (Dias et al., 2018). During spinal cord repair, dense glial scars act as barriers that inhibit nerve regeneration (Yokota et al., 2017). The persistence of systemic and local inflammatory responses from the acute to the chronic phase of SCI causes significant damage (Li et al., 2022). The death of cells that comprise neural circuits leads to the formation of cystic cavities known as spinal cavities (Berliner et al., 2020). These cystic cavities contain extracellular fluid, connective tissue, and infiltrating monocytes/macrophages (He et al., 2019), and an increase in cerebrospinal fluid pressure increases the volume of cystic cavities and hinders neural regeneration. These pathological and physiological responses are interrelated and interact with each other, making it challenging to alleviate SCI (Tran et al., 2018).
There are three major challenges in the treatment of SCI. First, reactive glial cells respond rapidly, and because axons take time to regrow, scars formed by reactive glial cells can obstruct the growth of severed axons. Second, many injured neurons are lost due to neuronal death or apoptosis, and residual neural stem cells are insufficient to replenish these lost neurons. Third, reactive glia have both positive and negative effects, as they stimulate the release of neurotrophic factors, as well as cytokines that inhibit axonal regeneration (Chen et al., 2020). Various inhibitory factors interact at the injury site to form a chemical microenvironment unfavorable for axonal regeneration. As spinal cord repair is complex and affected by several factors, many challenges must be overcome to achieve neuronal replacement after nerve injury (Sofroniew, 2018; Carpenter et al., 2020; Li et al., 2020; Liu et al., 2020). Below, we discuss the classical methods for SCI treatment.
Researchers have used strategies involving identifying the type and site of specific injuries, removal of bone fragments and foreign bodies, and physical stabilization and decompression of the spine to treat SCI (Furlan et al., 2011). Additional approaches, including surgical decompression and administration of anti-inflammatory drugs, are needed to prevent, and treat complications (Oderich, 2021). Furthermore, physical rehabilitation therapy, such as exercise and gait training, is beneficial for repair of the injured spinal cord (Gomara-Toldra et al., 2014). The abovementioned therapies are used in clinics. Currently, the ability of non-pharmacologic approaches, including hypothermia treatment (Tracy et al., 2015) and cerebrospinal fluid drainage (Kwon et al., 2009), to reduce post-SCI inflammation is being investigated in clinical trials with promising early results (Ashammakhi et al., 2019b).
Some studies have demonstrated that moderate inhibition of glial scar formation can promote functional recovery after SCI (Bradbury and Burnside, 2019; Yang et al., 2020b). For example, researchers have used multiple genetic loss-of-function strategies to decrease the number of scar-forming astrocytes and promote neuronal regeneration in adult mice after SCI (Rodriguez et al., 2014; Wu et al., 2021).
Stem cell-based therapies, including embryonic stem cell, stromal stem cell, neural stem cell and iPSC therapies, are promising approaches for spinal cord repair (Cusimano et al., 2018; Ashammakhi et al., 2019b; Shang et al., 2022). For instance, the transplantation of iPSC-derived neural stem cells into damaged tissues after SCI was found to have neuroprotective effects and promote neural regeneration (Shang et al., 2022). In a rodent model of SCI, transplantation of human iPSC-derived neural stem cells was found to promote the expression of synapse-related genes and proteins in the area surrounding the injured site and prevent atrophy of the injured spinal cord, thus promoting the recovery of motor function (Kawai et al., 2021). However, this strategy has limitations, such as a low cell survival rate and the possibility of uncontrolled differentiation after stem cell transplantation (Duncan et al., 2020).
Somatic cell reprogramming therapy mainly involves reprogramming somatic cells that can be easily isolated or proliferate extensively upon nerve injury (e.g., fibroblasts or reactive astrocytes) into target cells such as neurons by biotechnological means in vitro or in vivo (Figure 2). There are two potential approaches for utilizing somatic cell reprogramming for the treatment of SCI: endogenous repair and exogenous repair. Somatic cell reprogramming can generate cells for transplantation from endogenous or exogenous cell sources. Many recent studies have revealed that fibroblasts and astrocytes can transform into motor neurons in vitro (Son et al., 2011; Abernathy et al., 2017; Qin et al., 2018; Zhou et al., 2021). Some scholars have successfully transplanted these reprogrammed motor neurons into SCI model animals; others have reprogrammed somatic cells directly into neurons in situ using biotechnological means, all with excellent results (Zarei-Kheirabadi et al., 2019; Lee et al., 2020; Yang et al., 2020b).
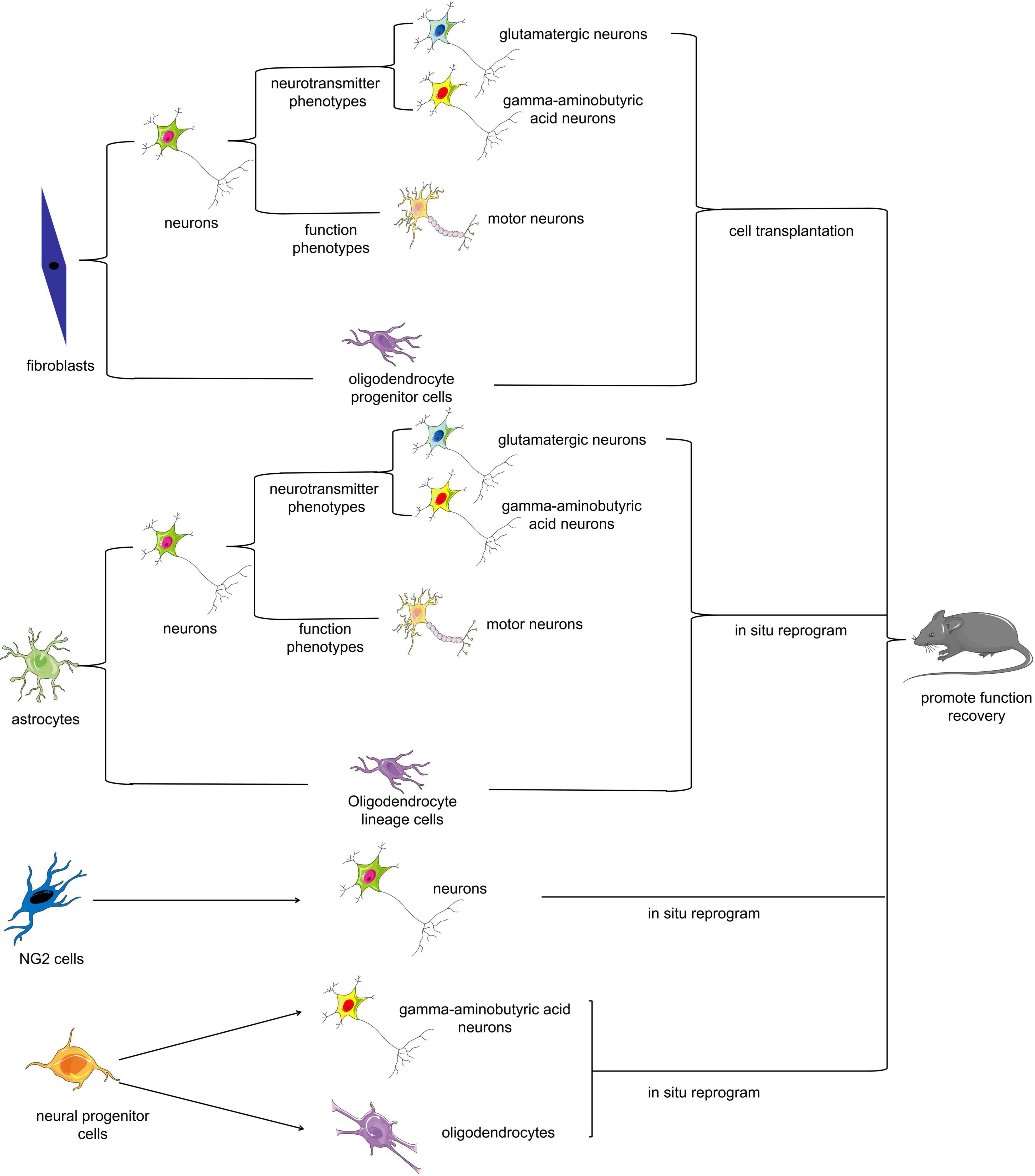
Figure 2. Cell fate reprogramming in the mammalian spinal cord. Multiple cell types can be reprogrammed either in vitro or in vivo. Fibroblasts, astrocytes, NG2 cells, and neural progenitor cells can be reprogrammed into neurons or oligodendrocytes, which can ultimately improve functional recovery after SCI.
Conduits, scaffolds, fibers, sheets, hydrogels and particles can be used as biomaterial therapies for SCI (Ashammakhi et al., 2019b). The use of scaffolds (Guest et al., 2018), conduits (Ziemba and Gilbert, 2017), and fibers (Li et al., 2015) can guide axon regeneration. Implanting the Neuro-spinal Scaffold™ comprising poly(lactic-co-glycolic acid)-b-poly(L-lysine) into a thoracic SCI in rats and minipigs diminished inflammation and reduced the lesion cavitation volume in preclinical studies (Guest et al., 2018). The Neuro-spinal Scaffold™ is currently being evaluated in clinical trials (Theodore et al., 2016). Small-scale clinical trials showed promising results after Neuro-spinal Scaffold™ therapy for thoracic SCI (Theodore et al., 2016). The biomaterial polyethylene glycol (PEG) has been approved by the US Food and Drug Administration for many applications. PEG hydrogels have been found to decrease glial scar invasion and support and guide axon regeneration in SCI repair (Kong et al., 2017; Dumont et al., 2019). Intravenous injection of ferulic acid-glycol chitosan nanoparticles could promote functional recovery in SCI rates (Wu et al., 2014). Three-dimensional (3D) magnetic hyaluronic acid hydrogels with biochemical and biophysical properties similar to those of the spinal extracellular matrix have been developed to promote the growth of functional neurons and the expression of excitatory and inhibitory ion channels (Tay et al., 2018). Hyaluronic acid hydrogels containing extracellular matrix from mouse embryonic stem cell-derived astrocytes were shown to decrease the size of the glial scar after SCI and promote axonal growth through the injury site, demonstrating their therapeutic potential (Thompson et al., 2018). A low-elastic hydrogel with an aligned nanofiber structure was found to promote axonal regeneration and motor function recovery in SCI rats (Yao et al., 2018).
Combinatorial treatments have been developed based on the complex pathophysiological changes after SCI. These approaches involve the delivery of a combination of biomaterials and cells or soluble molecules to the site of injury (Hou et al., 2022). The combination of biomaterial scaffolds and the transplantation of mesenchymal stem cells promotes the formation of connections between multiple cells during the pathological process after SCI (Papa et al., 2020). Hydrogels comprising hyaluronic acid mixed with methylcellulose can deliver cells to the injured spinal cord. Studies have shown that using such carriers for transplanting neural stem/progenitor cells effectively promotes behavioral recovery in SCI rats (Mothe et al., 2013). Hydrogels can be used as slow-release small-molecule drug reservoirs (Wang et al., 2022). For instance, serpin delivered by a chitosan-collagen hydrogel demonstrated a prolonged therapeutic effect and facilitated functional recovery in SCI rats (Kwiecien et al., 2020). Combinatorial therapies have also shown promise in promoting neurotrophic factor expression, optimizing the microenvironment after traumatic injury, improving cell survival after implantation, and promoting neuronal differentiation, neurite extension and linear axonal regeneration (Lin et al., 2016; Ashammakhi et al., 2019a).
Overall, significant preclinical advances related to spinal cord repair have been made, but these strategies should be further validated before widespread clinical application. Compared with other therapies, the advantage of somatic cell reprogramming therapy is that it cannot only provide cells for the transplant of exogenous somatic cell-derived neurons or oligodendrocytes for SCI repair but also replenish new neurons or oligodendrocytes at injury sites from neighboring endogenous glial cells (Li and Chen, 2016). Moreover, astrocytes can be targeted in vivo using viral vectors with specific promoters (Wang and Zhang, 2018). It is well known that glial scars play both inhibitory and protective roles in SCI repair (Yang et al., 2020a). Reducing the number of reactive astrocytes that proliferate and induce negative effects after SCI while effectively supplementing the number of neurons (including motor neurons with corresponding functions) at the site of injury is a promising method to address the challenges of nerve regeneration after SCI. This approach could appropriately eliminate glial scars and replenish lost neurons in situ while also improving the neural regeneration microenvironment. In conclusion, the potential of somatic cell reprogramming therapy in SCI provides new ideas for the development of cell supplementation and personalized regenerative medicine strategies.
Multiple somatic cell types can be reprogrammed in vitro or in vivo (Wang and Zhang, 2018). These somatic cells, including fibroblasts, astrocytes, NG2 cells, and neural progenitor cells, can all be reprogrammed into neurons or oligodendrocytes for spinal cord repair. Fibroblasts are widely distributed in connective tissues (Xu and Yao, 2021) and throughout the body and perform various functions, such as producing cytokines, growth factors and extracellular matrix components (Derk et al., 2021). Fibroblasts are easy to obtain, which is an important advantage for reprogramming strategies. Although fibroblast-derived neurons or oligodendrocytes are not used for in situ reprogramming, these cells can be used as a source of cells for transplantation for SCI repair (Heinrich et al., 2015). Astrocytes rapidly activate and proliferate after SCI, leading to the formation of a glial scar around the injured area. Reactive astrocytes display high expression levels of the neural stem cell markers Nestin and Vimentin (Sofroniew, 2015). After SCI, the reprogramming of reactive astrocytes in situ not only replenishes neurons or oligodendrocytes in the injured spinal cord but also modestly reduces the density of the glial scar (Ding et al., 2021; Yang et al., 2023). These characteristics of astrocytes make them appealing targets for reprogramming strategies for SCI repair (Chen and Li, 2022). NG2 cells, also known as oligodendrocyte precursor cells, are the fourth type of glial cells in the CNS, in addition to astrocytes, oligodendrocytes, and microglia. NG2 cells constitute a circulating population of glial cells in the adult CNS. These cells can rapidly proliferate and differentiate and are readily replaced after glial cell injury (Kirdajova and Anderova, 2020). NG2 cells are a good source of cells for reprogramming due to their proliferative capacity (Wang and Zhang, 2018). Neural progenitor cells are a heterogeneous population of adult cells with different differentiation capacities and multidifferentiation potentials. Although the number of resident neural progenitor cells is small after SCI, the multidifferentiation potential of these cells indicates their potential as a cell source for reprogramming.
In summary, multiple somatic cell types can be used for reprogramming for SCI repair. However, it is critical to consider the use of somatic cell reprogramming for the functional recovery of SCI. In the following sections, we discuss the application of somatic cell reprogramming strategies for repair of the injured spinal cord.
Fibroblasts can be reprogrammed into other cell types, including adipocytes and chondrocytes (Dorrier et al., 2022). Thus, fibroblasts have an important advantage for reprogramming because they are easy to obtain and demonstrate multidifferentiation potential. Researchers have successfully transformed fibroblasts into cardiomyocytes (Bektik and Fu, 2021), hematopoietic progenitor cells (Silverio-Alves et al., 2019), neurons, motor neurons (Son et al., 2011; Qin et al., 2018; Hu et al., 2019), dopaminergic neurons (Li et al., 2019), glutamatergic neurons, GABAergic neurons (Vierbuchen et al., 2010) and oligodendrocyte progenitor cells (Lee et al., 2018). However, fibroblasts have been found to be involved in fibrotic scar formation following acute CNS injury (Derk et al., 2021), indicating their importance in injured spinal cord repair. In the following section, we elaborate on the effectiveness of fibroblast reprogramming strategies in spinal cord repair (Table 1).
The CFLSSVY combination of small molecules [CHIR99021, forskolin, LDN193189, SB431542, SP600125, valproic acid (VPA), and Y-27632] can induce the conversion of rat dermal fibroblasts into neurons in vitro. The induced neurons were grown on a 3D porous silk fiber scaffold to form a neural network and functional neural scaffold. Transplantation of this neural scaffold into rats with transection SCI reduced the size of the cavity, promoted axonal regeneration and myelination, repaired injured tissue, promoted motor nerve conduction, and improved hindlimb motor function (Hu et al., 2019).
Glutamatergic neurons and GABAergic neurons play important roles in the transmission of neurotransmitters in the spinal cord and influence spinal cord repair (Flynn et al., 2011; Bertels et al., 2022). The alternating addition of two combinations of small molecules, CLRDPAI (CHIR99021, LDN193189, RG108, dorsomorphin, P7C3-A20, A83-01, and ISX9) and FYDPAPP [forskolin, Y27632, DAPT, PD0325901, A83-01, purmorphamine (PMA), and P7C3-A20], to the induction medium could induce the conversion of human fibroblasts to glutamatergic neuron-like cells in vitro. The fibroblast-derived glutamatergic neuron-like cells had neuron-specific transcriptional profiles, displayed action potentials and could form functional synapses. Moreover, after transplantation into the brains of postnatal mice, fibroblast-derived glutamatergic neuron-like cells integrated into local neuronal circuits (Yang et al., 2019).
In vitro, the combination of three neuron-specific transcription factors, achaete-scute family bHLH transcription factor 1 (Ascl1), POU class 3 homeobox 2 (Pou3f2) and myelin transcription factor 1 like (Myt1l), is sufficient to reprogram mouse embryonic fibroblasts and postnatal tail-tip fibroblasts into neurons, including some glutamatergic neurons and GABAergic neurons. These fibroblast-derived neurons generate action potentials and form functional synapses (Vierbuchen et al., 2010). In addition, human postnatal fibroblasts can be reprogrammed into glutamatergic neurons in vitro using four transcription factors, Ascl1, Pou3f2, Myt1l, and neuronal differentiation 1 (Neurod1) (Pang et al., 2011).
Combinations of brain-enriched neurogenic microRNAs (miRNAs), such as miR-124 and miR-9/9*, significantly mediate the direct transformation of adult fibroblasts into functional neurons and can increase the efficiency of this transformation. During the direct conversion of mature fibroblasts into neurons, this combination can contribute to neurogenesis and inhibit fibroblast progression by altering the chromatin structure of cells (Abernathy et al., 2017) or maintaining fibroblasts in a neurogenic state (Lu and Yoo, 2018; Habekost et al., 2020). The combination of miR-124, miR-9/9* and Ascl1 can reprogram porcine fibroblasts into glutamatergic neurons and GABAergic neurons in vitro. During this reprogramming process, fibroblast-specific genes are silenced, whereas neuron-specific genes are activated (Habekost et al., 2020).
Motor neurons are specialized neurons located in the anterior horn of the spinal cord that innervate the skeletal muscle and control its movement. Degeneration or loss of motor neurons is a typical feature of SCI (Qin et al., 2018; Rajpurohit et al., 2020).
Neuron-specific transcription factors, including Ascl1, Pou3f2, and Myt1l, and the motor neuron-specific transcription factors LIM homeobox 3 (Lhx3), motor neuron and pancreas homeobox 1 (Hb9), ISL LIM homeobox 1 (Isl1) and transcription factor neurogenin 2 (Ngn2) can directly transform mouse fibroblasts into motor neurons with high efficiency in vitro. Similarly, human-derived fibroblasts can be converted into motor neurons by these seven factors and Neurod1. When treated with these 7 factors, mouse fibroblast-derived motor neurons skip the neural progenitor phase. The resulting cells not only exhibit the gene expression profiles of motoneurons but can also form functional synapses with muscles and generate repetitive action potentials. In vivo, these reprogrammed motor neurons can efficiently integrate into the spinal cord when transplanted into the ventral cavity of the neural tube in chick embryos (Son et al., 2011).
Mouse embryonic fibroblasts treated with the transcription factors Ngn2 and Isl1 can be directly reprogrammed into motor neurons with spontaneous postsynaptic currents and repetitive action potentials in vitro (Zhou et al., 2021). In addition to these two factors, the sequential addition of organic cation/carnitine transporter 4 (Oct4) and Lhx3 can convert human-derived fibroblasts into functional motor neurons in vitro. Transplantation of Oct4- and Lhx3-treated motor neurons into rodents resulted in significant recovery of motor function following SCI (Lee et al., 2020). Both of these methods use only two transcription factors to induce somatic cell reprogramming, reducing the chance of viral mutation and simplifying the process (An et al., 2019).
In vitro, miR-9/9* and miR-124 combine with the motor neuron transcription factors Isl1 and Lhx3 and can directly transform fibroblasts into functionally mature motor neurons (Abernathy et al., 2017). Notably, the motor neuron-specific transcription factors Isl1 and Lhx3 were used in the four abovementioned studies (Son et al., 2011; Abernathy et al., 2017; Lee et al., 2020; Zhou et al., 2021), suggesting a key role for these two transcription factors in the conversion of fibroblasts into motor neurons (Erb et al., 2017).
In addition to the above methods, small molecule-mediated cellular reprogramming has been shown to be effective. The small molecule combination KFYPR, comprising kenpaulone, forskolin, Y-27632, PMA, and retinoic acid (RA), was found to effectively downregulate the expression of fibroblast-specific genes and upregulate the expression of neuron-specific genes, thereby converting mouse and human fibroblasts into motor neurons and bypassing the neural progenitor stage in vitro. Moreover, the transplantation of highly concentrated KFYPR-treated AG1-X2 beads into the dorsal subcutis of adult mice can result in the production of motor neurons in percutaneous tissue, suggesting that the small molecule combination KFYPR can induce motor neurogenesis in vivo (Qin et al., 2018).
Important progress has been made in the development of transcription factor- or small molecule-mediated fibroblast reprogramming strategies for repairing the injured spinal cord. However, it remains unclear whether combining transcription factors and small molecules could be more effective for fibroblast reprogramming. The combination of the transcription factors Ngn2, SRY-box transcription factor 11 (Sox11), Isl1, and Lhx3 and the small molecules forskolin and dorsomorphin has been found to directly transform adult skin fibroblasts into motor neurons. The transformed cells were shown to have the electrophysiological characteristics of mature spinal motor neurons, form neuromuscular junctions with skeletal muscle and control muscle activity. These researchers also used the transcription factors Isl1 and Lhx3, indicating that these factors may be key for reprogramming fibroblasts into motor neurons (Liu et al., 2016).
SCI causes demyelination of myelinated nerve fibers, creating an obstacle to spinal cord repair, for which myelin regeneration is critical. Oligodendrocytes are mainly involved in the production and maintenance of myelin sheaths in the CNS (Chanoumidou et al., 2021). Myelinated oligodendrocytes in the CNS are generated from oligodendrocyte progenitor cells (Butt et al., 2019). Therefore, oligodendrocyte progenitor cells are ideal therapeutic cells for treating SCI and demyelination-related diseases.
Inducing the expression of factors such as oligodendrocyte transcription factor 1 (Olig1), oligodendrocyte transcription factor 2 (Olig2), NK2 homeobox 2 (Nkx2.2), NK6 homeobox 2 (Nkx6.2), SRY box transcription factor 10 (Sox10), ST18 C2H2C-type zinc finger transcription factor (ST18), myelin regulatory factor (Gm98), myelin transcription factor 1 (Myt1) or the combination of the transcription factors Nkx6.2, Sox10, and Olig2 can transform mouse embryos and lung fibroblasts into oligodendrocyte progenitor cells in vitro. These oligodendrocyte progenitor cells exhibit an oligodendrocyte-like bipolar morphology and gene expression profile and could be cultured to the fifth generation. Furthermore, following transplantation into hypomyelinated mice, these cells can survive and wrap around host axons to further produce dense myelin sheaths (Najm et al., 2013).
It has also been reported that ectopic expression of Oct4 can directly transform adult mouse fibroblasts into self-renewing, amplifiable bipotent oligodendrocyte progenitor cells in vitro under specific conditions. Oct4-induced oligodendrocyte progenitor cells propagate in vitro with more than 31-fold higher efficiency. Transplantation of these cells into mice effectively promotes functional recovery after SCI and reduces tumorigenic risk by reducing host genome modification (Kim et al., 2015).
The combination of the transcription factors POU class 3 homeobox 4 (Pou3f4), SRY-box transcription factor 2 (Sox2), Kruppel-like factor 4 (Klf4), Myc-like transcriptional regulator Myc-like (c-Myc), and transcription factor 3 (Tcf3) with the small molecule PDGF-AA has been shown to reprogram mouse fibroblasts into oligodendrocyte progenitor cells via a two-step process. This approach first induces the direct transformation of fibroblasts into neural stem cells; subsequently, these cells are differentiated into oligodendrocyte progenitor cells that proliferate and efficiently differentiate into oligodendrocytes. Moreover, transplantation of these induced oligodendrocyte progenitor cells into SCI rats can promote the expression of myelin basic protein in the spinal cord and reduce the inflammatory response after SCI to some extent, ultimately promoting the recovery of motor function (Lee et al., 2018).
In summary, fibroblast reprogramming strategies are important approaches for spinal cord repair. Although most studies on fibroblast reprogramming into motor neurons were conducted in vitro, the results have established a solid foundation for using fibroblast reprogramming strategies in vivo. With further advancements, this technique could be used to overcome more challenges in spinal cord repair. In the abovementioned in vivo transplanted studies, neurons, motor neurons, and oligodendrocyte progenitor cells obtained from fibroblasts contributed to spinal cord repair and promoted functional recovery, indicating that transplantation of fibroblast-derived cells is a potential and promising therapy for SCI (Kim et al., 2015; Hu et al., 2019; Lee et al., 2020). However, the mechanism of fibroblast reprogramming has not been thoroughly discussed in the above reports. Single-cell-based deep sequencing technology may promote the exploration of the mechanisms of fibroblast reprogramming in the future (Shin et al., 2015). Moreover, more research is needed to determine the optimal number of transplanted fibroblast-derived cells, improve the survival rate of transplanted cells and enhance their functional integration with host circuits.
After SCI, astrocytes, fibroblasts, microglia, NG2 cells, ventricular canal cells and pericytes are activated, and they proliferate, migrate and generate fibroglial scars in and around the damaged area. Irreversible neuronal loss occurs at the injury site (Yates et al., 2019; Dias et al., 2021). Glial scars, of which reactive astrocytes are a major component, are thought to play a dual role in spinal cord repair (Adams and Gallo, 2018; Yang et al., 2020a; Gilbert et al., 2022), and it is now believed that a modest reduction in glial scar density is important for repair of the injured spinal cord (Hackett et al., 2018; Hesp et al., 2018; Yang et al., 2020b).
Astrocytes can be activated to form neurospheres when isolated and cultured in vitro. Several studies have found that astrocytes can be successfully reprogrammed into neurons by specific transcription factors or small molecule combinations (Wang et al., 2020). For instance, forced expression of the transcription factor Ngn2 and distal-free homolog 2 (Dlx2) can reprogram reactive astrocytes into glutamatergic neurons and GABAergic neurons, respectively (Heinrich et al., 2010), illustrating the differentiation potential of astrocytes and suggesting that endogenous astrocytes are a good choice for in vivo reprogramming studies. Astrocytes are widely distributed throughout the spinal cord, undergo reactive proliferation after SCI and are highly plastic. Therefore, the transformation of astrocytes into target cells, including neurons, motor neurons, other subtypes of neurons and oligodendrocytes, seems to be a promising approach for spinal cord repair (Chen and Li, 2022; Table 2).
Sox2 is an important transcription factor for astrocyte reprogramming. Doublecortin (Dcx), a microtubule-associated protein, is abundantly expressed in neuronal cells (Zarei-Kheirabadi et al., 2019). The overexpression of Sox2 was sufficient to reprogram endogenous spinal cord astrocytes into Dcx-positive neuroblasts. Such neuroblasts can be further transformed into synaptogenic neurons in situ (Su et al., 2014). When combined with other small molecules, Sox2 further induces the reprogramming of astrocytes into specific neuronal subtypes. Histone deacetylases play an important role in chromatin remodeling and epigenetic regulation, and their inhibitors can access chromatin to promote the transcription of silenced genes (Zhao et al., 2020). VPA, a histone deacetylase inhibitor, can increase cell reprogramming efficiency and promote normal neurogenesis and the maturation of neuroblasts (Yoshida et al., 2021). Studies have shown that applying the transcription factor Sox2 combined with VPA can further convert neurons into GABAergic-like neurons in vivo (Su et al., 2014).
Sox2 combined with shRNA-P53 was found to induce the conversion of astrocytes into glutamatergic neuron-dominated neurons in situ, significantly increasing the number of neurons in the ventral part of the mouse spinal cord. The researchers further applied this combination in mice with contusion SCI and found that it could significantly increase the number of neurons around the lesion site (Wang et al., 2016). This is because Sox2-mediated intrinsic neurogenesis in the adult mouse spinal cord occurs via glial cell reprogramming and is dependent on the p53 pathway, while silencing of p53 significantly promotes Sox2-mediated astrocytic reprogramming.
Induction of Sox2 expression combined with rehabilitation can significantly improve functional recovery in SCI mice (Yang et al., 2020b). These findings suggest that astrocyte reprogramming combined with rehabilitation could be an effective strategy to promote spinal cord repair.
In addition to Sox2, many other factors are involved in astrocyte reprogramming. It has been reported that the transcription factor zinc finger protein 521 (Zfp521) can reprogram astrocytes into neurons through the progenitor cell stage in situ in the spinal cords of rats with contusion SCI. These reprogrammed astrocytes were found to significantly improve motor performance and exhibit motor evoked potentials in vivo (Zarei-Kheirabadi et al., 2019). In vitro, the transcription factors Oct4 and Kruppel-like factor 4 (Klf4) were shown to transform primitive astrocytes into neural stem-like cells. In a contusion SCI mouse model, the combination of these two transcription factors effectively increased the porosity of glial scar tissue at the injury site, promoting myelin regeneration in axons after injury and improving the recovery of motor function (Huang et al., 2020).
Ngn2 is an essential transcription factor for the expression of glutamatergic neurotransmitter phenotypes in the embryonic neocortex and is critical for cell reprogramming (Falco et al., 2021). Ngn2 reprogrammed astrocytes into glutamatergic neurons and GABAergic neurons in situ in the dorsal spinal cords of adult mice. Reprogrammed neurons can integrate into local neural circuits and can respond to different afferent inputs from the dorsal root ganglia. Ngn2 has also been found to reprogram astrocytes into neurons at the site of injury in SCI models (Liu et al., 2021). Neurod1, a downstream target of Ngn2, can reprogram astrocytes in the dorsal horn of the spinal cord into glutamatergic neurons at the injury site in situ in a mouse model of SCI resulting from stab injury. These reprogrammed neurons can generate repetitive action potentials and spontaneous synaptic responses and thereby integrate into local spinal circuits. In addition, Neurod1 combined with the transcription factor Dlx2 significantly increases the proportion of astrocytes that are reprogrammed into GABAergic neurons at the site of SCI (Puls et al., 2020).
Because of the abundance of astrocytes in the spinal cord, the direct conversion of astrocytes to motor neurons in situ is important for nerve regeneration and functional recovery after SCI. CRISPR-mediated activation of the endogenous genes Ngn2 and Isl1 was shown to enable in vitro the reprogramming of mouse spinal cord astrocytes into motor neurons with spontaneous synapses and repetitive action potentials. In vivo experiments also revealed that an increase in Ngn2 and Isl1 expression can reprogram spinal astrocytes into motor neurons and that the axons of these motor neurons can project to the sciatic nerve (Zhou et al., 2021).
Extensive motor neuron loss occurs at the lesion site after SCI and astrocytes proliferate and form dense glial scars. Regulating the density of glial scars and replenishing motor neurons in the injured area is essential for spinal cord repair. Polypyrimidine tract binding protein (PTB) is a ribonucleic acid-binding protein involved in neurogenesis. To date, several laboratories have demonstrated that PTB silencing can effectively reprogram brain-derived astrocytes into functional neurons in vitro (Qian et al., 2020; Maimon et al., 2021). Moreover, in mouse models of Parkinson’s disease and aging, the knockdown of PTB can replenish dopaminergic and new neurons in the brain, respectively, ultimately promoting functional recovery in mice. Furthermore, silencing PTB can replenish ganglion cells in the mouse retina and promote the recovery of visual responses in mice with visual impairment (Zhou et al., 2020).
However, an interesting challenge was recently noted in this field (Wang L. L. et al., 2021). It has been argued that silencing PTB (Qian et al., 2020) cannot induce the rapid reprogramming of brain astrocytes into neurons in situ. It was concluded that the replenished neurons observed in vivo originated from endogenous neurons and were not reprogrammed brain astrocytes. Similarly, some researchers believe that astrocyte reprogramming is a long and gradual developmental process in vivo and that the conversion rate might be related to virus concentration and type. The origin of the replenished neurons cannot be determined through currently available biotechnological tools. However, it is clear that these neurons can promote functional recovery in disease models. Therefore, we recently explored the role of PTB silencing in spinal cord repair.
We discovered for the first time that mouse spinal cord reactive astrocytes can be successfully reprogrammed into motoneuron-like cells by knocking down the expression of PTB using lentivirus-expressing shRNA and antisense oligonucleotides in vitro. In a mouse model of compression SCI, PTB knockdown moderately reduced the density of the glial scar without destroying its overall structure while increasing the number of motoneuron-like cells around the injured area, effectively reducing cell apoptosis around the injured area and ultimately promoting the recovery of motor function in SCI mice (Yang et al., 2023). Based on the current literature, we hypothesize that the replenished motoneuron-like cells observed in the spinal cords of SCI mice after PTB silencing may either be induced by in situ astrocytic reprogramming, originate from endogenous neurons due to the protective effect of PTB silencing on neurons, or are derived from PTB silencing-mediated resident neural stem cells differentiation. Although the source of the replenished neurons needs to be confirmed in further studies, it is clear that PTB silencing promotes motor function recovery in SCI mice. These results suggest that PTB silencing may be a promising therapeutic approach for SCI.
SCI can lead to neuronal necrosis and axonal demyelination (Zhou P. et al., 2019), hindering myelin regeneration and the replacement of lost neurons and motor neurons. Oligodendrocytes, the main cells forming myelin sheaths around axons (Xia and Fancy, 2021), also undergo degeneration after SCI, further leading to axonal demyelination (Pukos et al., 2019). Therefore, replenishing new oligodendrocytes is an effective strategy to promote remyelination after SCI (Plemel et al., 2014; Huntemer-Silveira et al., 2020). Conversion of astrocytes to oligodendrocytes could be an innovative therapeutic approach for SCI repair. The protein neuregulin-1 (Nrg1) was reported to directly reprogram tumor necrosis factor-alpha (TNF-α)-expressing reactive astrocytes into oligodendrocyte lineage cells in vitro. In animal models, intrathecal injection of Nrg1 was also found to convert reactive astrocytes into oligodendrocyte lineage cells, promote axonal remyelination and effectively inhibit astrocyte proliferation. This method was demonstrated to protect axons and further promote the recovery of motor function following injury (Ding et al., 2021). The study also suggested that converting astrocytes into oligodendrocytes facilitated myelin regeneration and effectively promoted spinal cord repair.
These above astrocyte reprogramming strategies convert astrocytes into mainly glutamatergic, GABAergic, or mature neurons. Only two groups have reported that astrocytes can be reprogrammed into motor neurons (Zhou et al., 2021; Yang et al., 2023). The identification of new key transcription factors or genes involved in astrocyte-derived motor neuron reprogramming is expected to be important in motor function recovery after SCI.
The region-specific status of astrocytes influences the fate of neuronal subtypes in cell reprogramming. Astrocytes in specific regions have specific transcriptional and proteomic environments that lead to reprogramming into different subtype-specific neurons (Mattugini et al., 2019; Herrero-Navarro et al., 2021; Kempf et al., 2021), but the specific mechanisms are unclear and require further exploration. The functional recovery of SCI by astrocyte reprogramming depends on how many functional neurons are replenished in situ, whether they are the appropriate neuronal subtypes, and whether they can integrate into the right neural circuits.
Recent research has shown that NG2 cells act as a progenitor cell pool and can be reprogrammed into oligodendrocytes, astrocytes and even neurons under specific treatment conditions (Valny et al., 2017; Sanchez-Gonzalez et al., 2020; Chen et al., 2021). In brain injury and neurodegenerative disease models, retroviral infection of NG2 cells with the single transcription factor Neurod1 reprograms these cells in situ into functional neurons, such as glutamatergic and GABAergic neurons, promoting functional recovery (Guo et al., 2014). These results illustrate the strong multidifferentiation potential and plasticity of NG2 cells in spinal cord repair (Table 3).
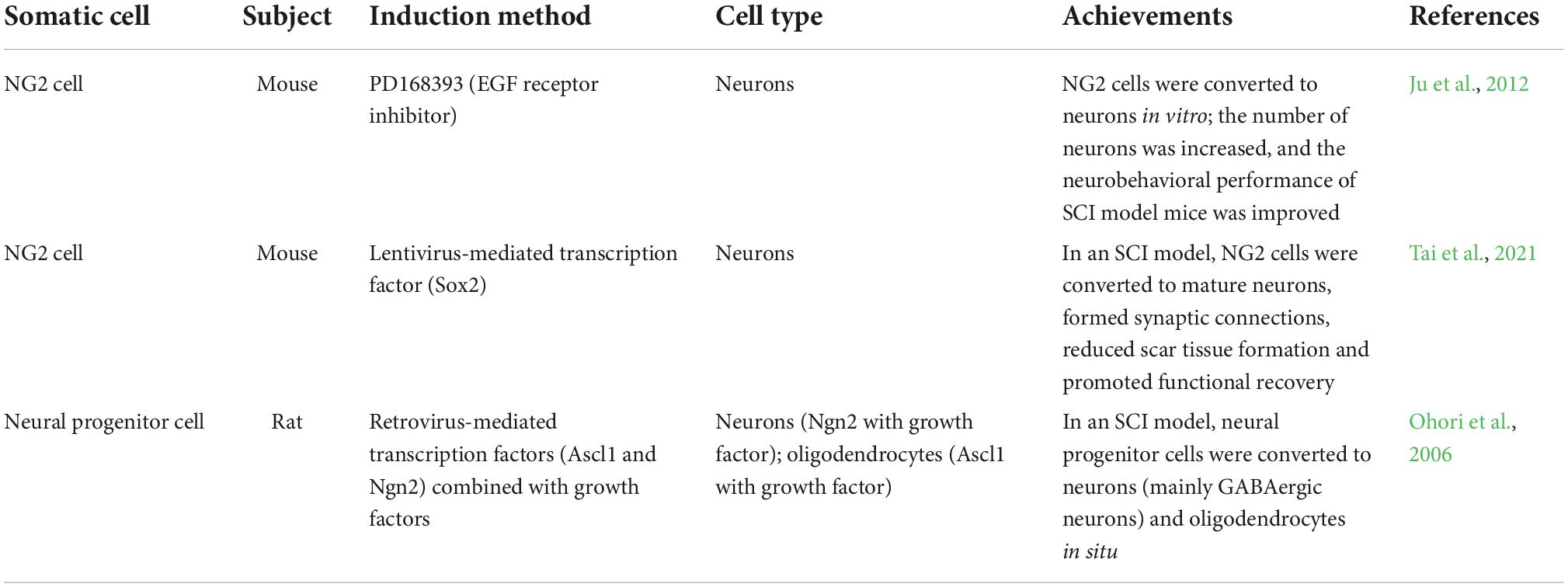
Table 3. Studies on the therapeutic effect of in vivo NG2 cell and neural progenitor cell reprogramming on SCI.
The epidermal growth factor (EGF) receptor inhibitor PD168393 has been reported to cause NG2 cells to acquire a neuronal phenotype. PD16839 was shown to reprogram NG2 cells directly into neurons in a contusion SCI mouse model, significantly increasing the number of neurons at the injury site and effectively improving neurobehavioral performance after injury (Ju et al., 2012).
High ectopic expression of Sox2 can lead to the reprogramming of NG2 cells into mature spinal cord neurons that can form synaptic connections with endogenous neurons in the spinal cord in SCI mouse models. These induced spinal cord neurons undergo neuronal conversion around the lesion site and form connections to achieve spontaneous functional recovery. Moreover, they effectively reduce the formation of glial scars after SCI and promote functional recovery in mice (Tai et al., 2021).
The above studies show the substantial potential of NG2 cell reprogramming in spinal cord repair. It has been demonstrated previously that inhibition of NG2 cells can promote the repair of SCI (Rodriguez et al., 2014; Hesp et al., 2018). Therefore, we wondered whether reprogramming with NG2 cells as target cells leads to a decrease in NG2 cells, further promoting SCI repair. These questions deserve further study.
Numerous studies have revealed that neural progenitor cells transplanted into various animal models can be induced to differentiate into target cells using specific approaches and that these cells promote functional recovery (Xue et al., 2021). Although the differentiation of endogenous neural progenitor cells into neurons and oligodendrocytes is strictly regulated by the cellular environment (Asrican et al., 2020), these cells can be applied for spinal cord repair (Table 3).
Some studies in rat SCI models have demonstrated that viral-mediated delivery of the transcription factor Ngn2 into the injury site, combined with EGF and fibroblast growth factor 2 (FGF2) can transform spinal endogenous neural progenitor cells into mainly GABAergic neurons in situ. The transcription factor Ascl1 combined with growth factors was shown to convert spinal cord endogenous neural progenitor cells into oligodendrocytes in situ (Ohori et al., 2006). However, in this study, the number of neural progenitor cell-derived neurons or oligodendrocytes was insufficient for functional repair in SCI.
Currently, SCI treatment is limited by the restricted differentiation of endogenous neural progenitors in vivo. Thus, the transformation of endogenous neural progenitor cells into neurons could be a novel and effective treatment strategy for spinal cord repair if these cells meet the requirements of specific neuronal subtypes and oligodendrocytes after injury (Voronova et al., 2020a).
In previous studies, fibroblast-derived neurons or oligodendrocyte progenitor cells were transplanted into the spinal cord for spinal cord repair (Kim et al., 2015; Lee et al., 2020). The use of external cells for fibroblast reprogramming therapy for spinal cord repair poses some difficult challenges. Reprogrammed cell transplantation is also complicated by immune rejection and poor cell purity and survival (Chen et al., 2015). Moreover, the process of cell transplantation itself causes damage to nerve tissue. Compared with fibroblast reprogramming, astrocyte reprogramming for spinal cord repair has the important advantage of converting spinal cord astrocytes into neurons in situ. Astrocyte reprogramming makes use of endogenous activated astrocytes that are adjacent to the lost neurons to regenerate functional cells in the injured area after SCI (Zarei-Kheirabadi et al., 2019; Puls et al., 2020). In addition to astrocytes, NG2 cells and neural progenitors can be reprogrammed into neurons in situ. However, there are fewer residual NG2 cells and neural progenitors than astrocytes around the injured site after SCI; thus, NG2 cell and neural progenitor reprogramming-mediated spinal cord repair is relatively difficult (Chen and Li, 2022). Reactive astrocytes are an abundant cell source for endogenous somatic reprogramming due to their ability to proliferate after SCI (Li and Chen, 2016). The graft-free somatic cell reprogramming strategy holds substantial promise for clinical SCI repair therapies.
Although recent progress has been made in the application of somatic cell reprogramming technology for spinal cord repair in preclinical studies, there are still some challenges in translating this strategy to clinical settings for SCI treatment. First, safety remains the most important issue related to somatic cell reprogramming for SCI treatment. Some transcription factors that induce somatic reprogramming are also highly expressed in some tumors. For instance, many studies have revealed that Sox2 is overexpressed in squamous cell carcinomas in many tissues, including the paranasal sinuses, hypopharynx, larynx, and lung (Zhou Y. X. et al., 2019). Second, safer and more effective methods for delivering reprogramming factors should be validated. Transcription factor delivery is currently achieved via viral infection, in which viral vectors integrate into the host cell genome (Vignoles et al., 2019). Despite the efficacy of viral vectors, this approach is associated with risks, including insertional mutagenesis, transgene integration, cellular senescence, strong immunogenicity, and viral infection (Gurumoorthy et al., 2022). Third, in the small molecule-mediated conversion approach in vivo, cell type specificity is difficult to achieve, and toxicity is a challenge (Hu et al., 2018). Fourth, our mechanistic knowledge of the somatic cell reprogramming process is rather rudimentary. More research is required to explore the molecular and cellular mechanisms of somatic cell reprogramming (Wang and Zhang, 2018).
Although skin fibroblasts from patients can be used for fibroblast reprogramming strategies, which is a promising strategy for autologous transplantation, research is mostly limited to cellular studies and animal experiments. Therefore, the clinical application of fibroblast-derived cells for therapy may involve many challenges. How to determine the optimal number of transplanted cells, balance efficacy and safety, and improve the survival rate of transplanted cells and their functional integration with host circuits requires further exploration. Extensive studies are required in the future to improve the efficacy of fibroblast-derived cell transplantation for the clinical treatment of SCI. In vivo reprogramming is a very promising approach for treating SCI in clinical settings because it utilizes endogenous cells. The development of AAV-mediated gene therapy involving cell-type-restricted promoters makes it possible to target resident cells in the spinal cord, which is the advantage of in situ cell reprogramming. AAV-mediated therapies have been successfully translated to the clinic in some cases; for example, an AAV9 vector has been used to induce the expression of SMN1 in motor neurons for treating spinal muscular atrophy (Vasan et al., 2021). NG2 cells and neural progenitor cells can differentiate into glial scar-forming astrocytes after SCI (Zhu et al., 2008); thus, there are fewer residual NG2 cells and neural progenitor cells than astrocytes. Astrocytes are activated and proliferate after SCI; thus, these cells are an ideal cell source for in vivo reprogramming in clinical settings (Wang and Zhang, 2018).
Recently, a different view related to astrocyte reprogramming has emerged (Wang L. L. et al., 2021). In addition to the controversy associated with PTB silencing discussed above (Qian et al., 2020), it has been argued that overexpression of the transcription factor Neurod1 (Guo et al., 2014) cannot reprogram brain astrocytes into neurons in situ. The opposing view is that in vivo replenished neurons are derived from endogenous neurons rather than reprogrammed brain astrocytes. Although the origin of replenished neurons in vivo following PTB silencing or Neurod1 overexpression has not yet been conclusively determined, these neurons promote functional recovery in disease-model mice. Therefore, from the perspective of promoting functional recovery, somatic cell reprogramming technology still has substantial potential for promoting spinal cord repair.
The efficacy of somatic cell reprogramming treatment strategies in human clinical trials could be limited due to the lack of control groups, and improvements in function observed in the trials could be related to decompression surgery at the time of cell transplantation or natural recovery after SCI rather than therapeutic effects (Bartlett et al., 2020). Future studies are needed to confirm the efficacy of somatic cell reprogramming in human clinical trials to promote its clinical application for SCI repair (Voronova et al., 2020b). Moreover, the severity of SCI is determined by multiple factors that must be considered when determining the appropriate treatment. Using biomaterials as carriers of reprogrammed cells, viral vectors and/or small molecules can improve somatic cell reprogramming strategies to achieve optimal spinal cord repair (Pinelli et al., 2022). Future work is required to devise clinical treatments for somatic cell reprogramming combined with biomaterials in vivo.
The development of somatic cell reprogramming approaches for spinal cord repair has progressed rapidly in recent years. However, due to the complexity of this process, the specific mechanisms require further investigation (Havelikova et al., 2022). Bypassing the iPSC phase during somatic cell reprogramming could avoid the potential risk of tumor formation. Furthermore, the direct transformation of somatic cells into neurons, functional neuronal subtypes or even oligodendrocytes is highly efficient, can be achieved quickly and is not limited by ethical issues, indicating that this approach shows promise as a potential new treatment for SCI. Somatic cell reprogramming technology is still in the early stages of development, and considerable investigation remains to be conducted before these approaches can be translated to clinical settings. Despite the limited data on functional recovery after SCI, available findings indicate that somatic cell reprogramming techniques have good prospects for spinal cord repair, cell regeneration, tissue repair, and functional recovery. We believe that developments in biotechnology and regenerative medicine and breakthroughs in somatic cell reprogramming technology will eventually lead to improvements in the efficacy of spinal cord repair methods.
GC designed the review. RY wrote and drafted the manuscript. All authors contributed to the revision of the manuscript and approved the final version.
This work was supported by the National Natural Science Foundation of China (82101455, 31872773, and 82001168), the Key Research and Development Program (Social Development) of Jiangsu Province (BE2020667), the Foundation of Jiangsu Province “333 Project High-level Talents” (BRA2020076), the Nantong Civic Science and Technology Project of China (JC2020028), and the Natural Science Research of Jiangsu Higher Education Institutions of China (19KJB310012) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and the Higher School in Jiangsu Province College Students’ Practice Innovation Training Programs (202210304042Z).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abernathy, D. G., Kim, W. K., McCoy, M. J., Lake, A. M., Ouwenga, R., Lee, S. W., et al. (2017). MicroRNAs induce a permissive chromatin environment that enables neuronal subtype-specific reprogramming of adult human fibroblasts. Cell Stem Cell 21, 332–348.e9. doi: 10.1016/j.stem.2017.08.002
Adams, K. L., and Gallo, V. (2018). The diversity and disparity of the glial scar. Nat. Neurosci. 21, 9–15. doi: 10.1038/s41593-017-0033-9
Ahmadian-Moghadam, H., Sadat-Shirazi, M. S., and Zarrindast, M. R. (2020). Therapeutic potential of stem cells for treatment of neurodegenerative diseases. Biotechnol. Lett. 42, 1073–1101. doi: 10.1007/s10529-020-02886-1
Algahtany, M., McFaull, S., Chen, L., Zhang, S., Saarela, O., Alqahtani, F., et al. (2021). The changing etiology and epidemiology of traumatic spinal injury: A population-based study. World Neurosurg. 149, e116–e127. doi: 10.1016/j.wneu.2021.02.066
Alizadeh, A., Dyck, S. M., and Karimi-Abdolrezaee, S. (2019). Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 10:282. doi: 10.3389/fneur.2019.00282
An, Z., Liu, P., Zheng, J., Si, C., Li, T., Chen, Y., et al. (2019). Sox2 and Klf4 as the functional core in pluripotency induction without exogenous Oct4. Cell Rep. 29, 1986–2000.e8. doi: 10.1016/j.celrep.2019.10.026
Anjum, A., Yazid, M. D., Fauzi Daud, M., Idris, J., Ng, A. M. H., Selvi Naicker, A., et al. (2020). Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. Int. J. Mol. Sci. 21:7533. doi: 10.3390/ijms21207533
Ashammakhi, N., Ahadian, S., Darabi, M. A., El Tahchi, M., Lee, J., Suthiwanich, K., et al. (2019a). Minimally invasive and regenerative therapeutics. Adv. Mater. 31:e1804041. doi: 10.1002/adma.201804041
Ashammakhi, N., Kim, H. J., Ehsanipour, A., Bierman, R. D., Kaarela, O., Xue, C., et al. (2019b). Regenerative therapies for spinal cord injury. Tissue Eng. Part B Rev. 25, 471–491. doi: 10.1089/ten.TEB.2019.0182
Asrican, B., Wooten, J., Li, Y. D., Quintanilla, L., Zhang, F., Wander, C., et al. (2020). Neuropeptides modulate local astrocytes to regulate adult hippocampal neural stem cells. Neuron 108, 349–366.e6. doi: 10.1016/j.neuron.2020.07.039
Barbiellini Amidei, C., Salmaso, L., Bellio, S., and Saia, M. (2022). Epidemiology of traumatic spinal cord injury: A large population-based study. Spinal Cord 60, 812–819. doi: 10.1038/s41393-022-00795-w
Bartlett, R. D., Burley, S., Ip, M., Phillips, J. B., and Choi, D. (2020). Cell therapies for spinal cord injury: Trends and challenges of current clinical trials. Neurosurgery 87, E456–E472. doi: 10.1093/neuros/nyaa149
Bektik, E., and Fu, J. D. (2021). Production of cardiomyocyte-like cells by fibroblast reprogramming with defined factors. Methods Mol. Biol. 2239, 33–46. doi: 10.1007/978-1-0716-1084-8_3
Berliner, J., Hemley, S., Najafi, E., Bilston, L., Stoodley, M., and Lam, M. (2020). Abnormalities in spinal cord ultrastructure in a rat model of post-traumatic syringomyelia. Fluids Barriers CNS 17:11. doi: 10.1186/s12987-020-0171-4
Bertels, H., Vicente-Ortiz, G., El Kanbi, K., and Takeoka, A. (2022). Neurotransmitter phenotype switching by spinal excitatory interneurons regulates locomotor recovery after spinal cord injury. Nat. Neurosci. 25, 617–629. doi: 10.1038/s41593-022-01067-9
Bradbury, E. J., and Burnside, E. R. (2019). Moving beyond the glial scar for spinal cord repair. Nat. Commun. 10:3879. doi: 10.1038/s41467-019-11707-7
Butt, A. M., Papanikolaou, M., and Rivera, A. (2019). Physiology of oligodendroglia. Adv. Exp. Med. Biol. 1175, 117–128. doi: 10.1007/978-981-13-9913-8_5
Carpenter, R. S., Marbourg, J. M., Brennan, F. H., Mifflin, K. A., Hall, J. C. E., Jiang, R. R., et al. (2020). Spinal cord injury causes chronic bone marrow failure. Nat. Commun. 11:3702. doi: 10.1038/s41467-020-17564-z
Chanoumidou, K., Hernandez-Rodriguez, B., Windener, F., Thomas, C., Stehling, M., Mozafari, S., et al. (2021). One-step reprogramming of human fibroblasts into oligodendrocyte-like cells by SOX10, OLIG2, and NKX6.2. Stem Cell Rep. 16, 771–783. doi: 10.1016/j.stemcr.2021.03.001
Chen, C. Z., Neumann, B., Forster, S., and Franklin, R. J. M. (2021). Schwann cell remyelination of the central nervous system: Why does it happen and what are the benefits? Open Biol. 11:200352. doi: 10.1098/rsob.200352
Chen, G., Wernig, M., Berninger, B., Nakafuku, M., Parmar, M., and Zhang, C. L. (2015). In vivo reprogramming for brain and spinal cord repair. eNeuro 2:ENEURO.0106-15.2015. doi: 10.1523/ENEURO.0106-15.2015
Chen, X., and Li, H. (2022). Neuronal reprogramming in treating spinal cord injury. Neural Regen. Res. 17, 1440–1445. doi: 10.4103/1673-5374.330590
Chen, Y., Qin, C., Huang, J., Tang, X., Liu, C., Huang, K., et al. (2020). The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 53:e12781. doi: 10.1111/cpr.12781
Chio, J. C. T., Wang, J., Surendran, V., Li, L., Zavvarian, M. M., Pieczonka, K., et al. (2021). Delayed administration of high dose human immunoglobulin G enhances recovery after traumatic cervical spinal cord injury by modulation of neuroinflammation and protection of the blood spinal cord barrier. Neurobiol. Dis. 148:105187. doi: 10.1016/j.nbd.2020.105187
Csobonyeiova, M., Polak, S., Zamborsky, R., and Danisovic, L. (2019). Recent progress in the regeneration of spinal cord injuries by induced pluripotent stem cells. Int. J. Mol. Sci. 20:3838. doi: 10.3390/ijms20153838
Cusimano, M., Brambilla, E., Capotondo, A., De Feo, D., Tomasso, A., Comi, G., et al. (2018). Selective killing of spinal cord neural stem cells impairs locomotor recovery in a mouse model of spinal cord injury. J. Neuroinflammation 15:58. doi: 10.1186/s12974-018-1085-9
de Araujo, L. T., Macedo, C. T., Damasceno, P. K. F., das Neves, I. G. C., de Lima, C. S., Santos, G. C., et al. (2022). Clinical trials using mesenchymal stem cells for spinal cord injury: Challenges in generating evidence. Cells 11:1019. doi: 10.3390/cells11061019
Derk, J., Jones, H. E., Como, C., Pawlikowski, B., and Siegenthaler, J. A. (2021). Living on the edge of the CNS: Meninges cell diversity in health and disease. Front. Cell. Neurosci. 15:703944. doi: 10.3389/fncel.2021.703944
Dias, D. O., Kalkitsas, J., Kelahmetoglu, Y., Estrada, C. P., Tatarishvili, J., Holl, D., et al. (2021). Pericyte-derived fibrotic scarring is conserved across diverse central nervous system lesions. Nat. Commun. 12:5501. doi: 10.1038/s41467-021-25585-5
Dias, D. O., Kim, H., Holl, D., Werne Solnestam, B., Lundeberg, J., Carlen, M., et al. (2018). Reducing pericyte-derived scarring promotes recovery after spinal cord injury. Cell 173, 153–165.e22. doi: 10.1016/j.cell.2018.02.004
Ding, Z., Dai, C., Zhong, L., Liu, R., Gao, W., Zhang, H., et al. (2021). Neuregulin-1 converts reactive astrocytes toward oligodendrocyte lineage cells via upregulating the PI3K-AKT-mTOR pathway to repair spinal cord injury. Biomed. Pharmacother. 134:111168. doi: 10.1016/j.biopha.2020.111168
Dorrier, C. E., Jones, H. E., Pintaric, L., Siegenthaler, J. A., and Daneman, R. (2022). Emerging roles for CNS fibroblasts in health, injury and disease. Nat. Rev. Neurosci. 23, 23–34. doi: 10.1038/s41583-021-00525-w
Dumont, C. M., Carlson, M. A., Munsell, M. K., Ciciriello, A. J., Strnadova, K., Park, J., et al. (2019). Aligned hydrogel tubes guide regeneration following spinal cord injury. Acta Biomater. 86, 312–322. doi: 10.1016/j.actbio.2018.12.052
Duncan, G. J., Manesh, S. B., Hilton, B. J., Assinck, P., Plemel, J. R., and Tetzlaff, W. (2020). The fate and function of oligodendrocyte progenitor cells after traumatic spinal cord injury. Glia 68, 227–245. doi: 10.1002/glia.23706
Erb, M., Lee, B., Yeon Seo, S., Lee, J. W., Lee, S., and Lee, S. K. (2017). The Isl1-Lhx3 complex promotes motor neuron specification by activating transcriptional pathways that enhance its own expression and formation. eNeuro 4:ENEURO.0349-16.2017. doi: 10.1523/ENEURO.0349-16.2017
Falco, A., Bartolome-Cabrero, R., and Gascon, S. (2021). Bcl-2-assisted reprogramming of mouse astrocytes and human fibroblasts into induced neurons. Methods Mol. Biol. 2352, 57–71. doi: 10.1007/978-1-0716-1601-7_5
Flynn, J. R., Graham, B. A., Galea, M. P., and Callister, R. J. (2011). The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology 60, 809–822. doi: 10.1016/j.neuropharm.2011.01.016
Furlan, J. C., Noonan, V., Cadotte, D. W., and Fehlings, M. G. (2011). Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: An evidence-based examination of pre-clinical and clinical studies. J. Neurotrauma 28, 1371–1399. doi: 10.1089/neu.2009.1147
Furlan, J. C., Verocai, F., Palmares, X., and Fehlings, M. G. (2016). Electrocardiographic abnormalities in the early stage following traumatic spinal cord injury. Spinal Cord 54, 872–877. doi: 10.1038/sc.2016.11
Giallongo, S., Rehakova, D., Raffaele, M., Lo Re, O., Koutna, I., and Vinciguerra, M. (2021). Redox and epigenetics in human pluripotent stem cells differentiation. Antioxid. Redox Signal. 34, 335–349. doi: 10.1089/ars.2019.7983
Gilbert, E. A. B., Lakshman, N., Lau, K. S. K., and Morshead, C. M. (2022). Regulating endogenous neural stem cell activation to promote spinal cord injury repair. Cells 11:846. doi: 10.3390/cells11050846
Gomara-Toldra, N., Sliwinski, M., and Dijkers, M. P. (2014). Physical therapy after spinal cord injury: A systematic review of treatments focused on participation. J. Spinal Cord Med. 37, 371–379. doi: 10.1179/2045772314Y.0000000194
Gu, Y., Cheng, X., Huang, X., Yuan, Y., Qin, S., Tan, Z., et al. (2019). Conditional ablation of reactive astrocytes to dissect their roles in spinal cord injury and repair. Brain Behav. Immun. 80, 394–405. doi: 10.1016/j.bbi.2019.04.016
Guest, J. D., Moore, S. W., Aimetti, A. A., Kutikov, A. B., Santamaria, A. J., Hofstetter, C. P., et al. (2018). Internal decompression of the acutely contused spinal cord: Differential effects of irrigation only versus biodegradable scaffold implantation. Biomaterials 185, 284–300. doi: 10.1016/j.biomaterials.2018.09.025
Guo, Z., Zhang, L., Wu, Z., Chen, Y., Wang, F., and Chen, G. (2014). In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer’s disease model. Cell Stem Cell 14, 188–202. doi: 10.1016/j.stem.2013.12.001
Gurumoorthy, N., Nordin, F., Tye, G. J., Wan Kamarul Zaman, W. S., and Ng, M. H. (2022). Non-integrating lentiviral vectors in clinical applications: A glance through. Biomedicines 10:107. doi: 10.3390/biomedicines10010107
Habekost, M., Jorgensen, A. L., Qvist, P., and Denham, M. (2020). MicroRNAs and Ascl1 facilitate direct conversion of porcine fibroblasts into induced neurons. Stem Cell Res. 48:101984. doi: 10.1016/j.scr.2020.101984
Hackett, A. R., Yahn, S. L., Lyapichev, K., Dajnoki, A., Lee, D. H., Rodriguez, M., et al. (2018). Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes. Exp. Neurol. 308, 72–79. doi: 10.1016/j.expneurol.2018.07.001
Ham, T. R., and Leipzig, N. D. (2018). Biomaterial strategies for limiting the impact of secondary events following spinal cord injury. Biomed. Mater. 13:024105. doi: 10.1088/1748-605X/aa9bbb
Havelikova, K., Smejkalova, B., and Jendelova, P. (2022). Neurogenesis as a tool for spinal cord injury. Int. J. Mol. Sci. 23:3728. doi: 10.3390/ijms23073728
He, Z., Zang, H., Zhu, L., Huang, K., Yi, T., Zhang, S., et al. (2019). An anti-inflammatory peptide and brain-derived neurotrophic factor-modified hyaluronan-methylcellulose hydrogel promotes nerve regeneration in rats with spinal cord injury. Int. J. Nanomedicine 14, 721–732. doi: 10.2147/IJN.S187854
Heinrich, C., Blum, R., Gascon, S., Masserdotti, G., Tripathi, P., Sanchez, R., et al. (2010). Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 8:e1000373. doi: 10.1371/journal.pbio.1000373
Heinrich, C., Spagnoli, F. M., and Berninger, B. (2015). In vivo reprogramming for tissue repair. Nat. Cell Biol. 17, 204–211. doi: 10.1038/ncb3108
Hernandez, R., Jimenez-Luna, C., Perales-Adan, J., Perazzoli, G., Melguizo, C., and Prados, J. (2020). Differentiation of human mesenchymal stem cells towards neuronal lineage: Clinical trials in nervous system disorders. Biomol. Ther. 28, 34–44. doi: 10.4062/biomolther.2019.065
Herrero-Navarro, A., Puche-Aroca, L., Moreno-Juan, V., Sempere-Ferrandez, A., Espinosa, A., Susin, R., et al. (2021). Astrocytes and neurons share region-specific transcriptional signatures that confer regional identity to neuronal reprogramming. Sci. Adv. 7:eabe8978. doi: 10.1126/sciadv.abe8978
Hesp, Z. C., Yoseph, R. Y., Suzuki, R., Jukkola, P., Wilson, C., Nishiyama, A., et al. (2018). Proliferating NG2-cell-dependent angiogenesis and scar formation alter axon growth and functional recovery after spinal cord injury in mice. J. Neurosci. 38, 1366–1382. doi: 10.1523/JNEUROSCI.3953-16.2017
Hou, Y., Liu, X., Guo, Y., Liu, D., Guo, P., and Liu, J. (2022). Strategies for effective neural circuit reconstruction after spinal cord injury: Use of stem cells and biomaterials. World Neurosurg. 161, 82–89. doi: 10.1016/j.wneu.2022.02.012
Hu, J., Qian, H., Xue, Y., and Fu, X. D. (2018). PTB/nPTB: Master regulators of neuronal fate in mammals. Biophys. Rep. 4, 204–214. doi: 10.1007/s41048-018-0066-y
Hu, Y., Zhang, F., Zhong, W., Liu, Y., He, Q., Yang, M., et al. (2019). Transplantation of neural scaffolds consisting of dermal fibroblast-reprogrammed neurons and 3D silk fibrous materials promotes the repair of spinal cord injury. J. Mater. Chem. B 7, 7525–7539. doi: 10.1039/c9tb01929d
Huang, X., Wang, C., Zhou, X., Wang, J., Xia, K., Yang, B., et al. (2020). Overexpression of the transcription factors OCT4 and KLF4 improves motor function after spinal cord injury. CNS Neurosci. Ther. 26, 940–951. doi: 10.1111/cns.13390
Huntemer-Silveira, A., Patil, N., Brickner, M. A., and Parr, A. M. (2020). Strategies for oligodendrocyte and myelin repair in traumatic CNS injury. Front. Cell. Neurosci. 14:619707. doi: 10.3389/fncel.2020.619707
Ju, P., Zhang, S., Yeap, Y., and Feng, Z. (2012). Induction of neuronal phenotypes from NG2+ glial progenitors by inhibiting epidermal growth factor receptor in mouse spinal cord injury. Glia 60, 1801–1814. doi: 10.1002/glia.22398
Karova, K., Wainwright, J. V., Machova-Urdzikova, L., Pisal, R. V., Schmidt, M., Jendelova, P., et al. (2019). Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-kappaB pathway inhibition. J. Neuroinflammation 16:12. doi: 10.1186/s12974-019-1394-7
Katoh, H., Yokota, K., and Fehlings, M. G. (2019). Regeneration of spinal cord connectivity through stem cell transplantation and biomaterial scaffolds. Front. Cell. Neurosci. 13:248. doi: 10.3389/fncel.2019.00248
Kawai, M., Imaizumi, K., Ishikawa, M., Shibata, S., Shinozaki, M., Shibata, T., et al. (2021). Long-term selective stimulation of transplanted neural stem/progenitor cells for spinal cord injury improves locomotor function. Cell Rep. 37:110019. doi: 10.1016/j.celrep.2021.110019
Kempf, J., Knelles, K., Hersbach, B. A., Petrik, D., Riedemann, T., Bednarova, V., et al. (2021). Heterogeneity of neurons reprogrammed from spinal cord astrocytes by the proneural factors Ascl1 and Neurogenin2. Cell Rep. 36:109409. doi: 10.1016/j.celrep.2021.109409
Kim, J. B., Lee, H., Arauzo-Bravo, M. J., Hwang, K., Nam, D., Park, M. R., et al. (2015). Oct4-induced oligodendrocyte progenitor cells enhance functional recovery in spinal cord injury model. EMBO J. 34, 2971–2983. doi: 10.15252/embj.201592652
Kirdajova, D., and Anderova, M. (2020). NG2 cells and their neurogenic potential. Curr. Opin. Pharmacol. 50, 53–60. doi: 10.1016/j.coph.2019.11.005
Kojima, K., Miyoshi, H., Nagoshi, N., Kohyama, J., Itakura, G., Kawabata, S., et al. (2019). Selective ablation of tumorigenic cells following human induced pluripotent stem cell-derived neural stem/progenitor cell transplantation in spinal cord injury. Stem Cells Transl. Med. 8, 260–270. doi: 10.1002/sctm.18-0096
Kong, X. B., Tang, Q. Y., Chen, X. Y., Tu, Y., Sun, S. Z., and Sun, Z. L. (2017). Polyethylene glycol as a promising synthetic material for repair of spinal cord injury. Neural Regen. Res. 12, 1003–1008. doi: 10.4103/1673-5374.208597
Kumar, R., Lim, J., Mekary, R. A., Rattani, A., Dewan, M. C., Sharif, S. Y., et al. (2018). Traumatic spinal injury: Global epidemiology and worldwide volume. World Neurosurg. 113, e345–e363. doi: 10.1016/j.wneu.2018.02.033
Kwiecien, J. M., Zhang, L., Yaron, J. R., Schutz, L. N., Kwiecien-Delaney, C. J., Awo, E. A., et al. (2020). Local serpin treatment via chitosan-collagen hydrogel after spinal cord injury reduces tissue damage and improves neurologic function. J. Clin. Med. 9:1221. doi: 10.3390/jcm9041221
Kwon, B. K., Curt, A., Belanger, L. M., Bernardo, A., Chan, D., Markez, J. A., et al. (2009). Intrathecal pressure monitoring and cerebrospinal fluid drainage in acute spinal cord injury: A prospective randomized trial. J. Neurosurg. Spine 10, 181–193. doi: 10.3171/2008.10.SPINE08217
Lee, B. J., and Jeong, J. H. (2022). Review: Steroid use in patients with acute spinal cord injury and guideline update. Korean J. Neurotrauma 18, 22–30. doi: 10.13004/kjnt.2022.18.e21
Lee, H., Lee, H. Y., Lee, B. E., Gerovska, D., Park, S. Y., Zaehres, H., et al. (2020). Sequentially induced motor neurons from human fibroblasts facilitate locomotor recovery in a rodent spinal cord injury model. eLife 9:e52069. doi: 10.7554/eLife.52069
Lee, Y., Kim, C. Y., Lee, H. J., Kim, J. G., Han, D. W., Ko, K., et al. (2018). Two-step generation of oligodendrocyte progenitor cells from mouse fibroblasts for spinal cord injury. Front. Cell. Neurosci. 12:198. doi: 10.3389/fncel.2018.00198
Li, H., and Chen, G. (2016). In vivo reprogramming for CNS repair: Regenerating neurons from endogenous glial cells. Neuron 91, 728–738. doi: 10.1016/j.neuron.2016.08.004
Li, S., Shi, Y., Yao, X., Wang, X., Shen, L., Rao, Z., et al. (2019). Conversion of astrocytes and fibroblasts into functional noradrenergic neurons. Cell Rep. 28, 682–697.e7. doi: 10.1016/j.celrep.2019.06.042
Li, X., Yang, C., Li, L., Xiong, J., Xie, L., Yang, B., et al. (2015). A therapeutic strategy for spinal cord defect: Human dental follicle cells combined with aligned PCL/PLGA electrospun material. Biomed Res. Int. 2015:197183. doi: 10.1155/2015/197183
Li, Y., He, X., Kawaguchi, R., Zhang, Y., Wang, Q., Monavarfeshani, A., et al. (2020). Microglia-organized scar-free spinal cord repair in neonatal mice. Nature 587, 613–618. doi: 10.1038/s41586-020-2795-6
Li, Z., Yu, S., Liu, Y., Hu, X., Li, Y., Xiao, Z., et al. (2022). SU16f inhibits fibrotic scar formation and facilitates axon regeneration and locomotor function recovery after spinal cord injury by blocking the PDGFRbeta pathway. J. Neuroinflammation 19:95. doi: 10.1186/s12974-022-02449-3
Lin, X. Y., Lai, B. Q., Zeng, X., Che, M. T., Ling, E. A., Wu, W., et al. (2016). Cell transplantation and neuroengineering approach for spinal cord injury treatment: A summary of current laboratory findings and review of literature. Cell Transplant. 25, 1425–1438. doi: 10.3727/096368916X690836
Liu, F., Zhang, Y., Chen, F., Yuan, J., Li, S., Han, S., et al. (2021). Neurog2 directly converts astrocytes into functional neurons in midbrain and spinal cord. Cell Death Dis. 12:225. doi: 10.1038/s41419-021-03498-x
Liu, J., Li, K., Huang, K., Yang, C., Huang, Z., Zhao, X., et al. (2020). Acellularized spinal cord scaffolds incorporating bpV(pic)/PLGA microspheres promote axonal regeneration and functional recovery after spinal cord injury. RSC Adv. 10, 18677–18686. doi: 10.1039/d0ra02661a
Liu, M. L., Zang, T., and Zhang, C. L. (2016). Direct lineage reprogramming reveals disease-specific phenotypes of motor neurons from human ALS patients. Cell Rep. 14, 115–128. doi: 10.1016/j.celrep.2015.12.018
Lu, Y. L., and Yoo, A. S. (2018). Mechanistic insights into MicroRNA-induced neuronal reprogramming of human adult fibroblasts. Front. Neurosci. 12:522. doi: 10.3389/fnins.2018.00522
Maimon, R., Chillon-Marinas, C., Snethlage, C. E., Singhal, S. M., McAlonis-Downes, M., Ling, K., et al. (2021). Therapeutically viable generation of neurons with antisense oligonucleotide suppression of PTB. Nat. Neurosci. 24, 1089–1099. doi: 10.1038/s41593-021-00864-y
Matsui, T., Akamatsu, W., Nakamura, M., and Okano, H. (2014). Regeneration of the damaged central nervous system through reprogramming technology: Basic concepts and potential application for cell replacement therapy. Exp. Neurol. 260, 12–18. doi: 10.1016/j.expneurol.2012.09.016
Mattugini, N., Bocchi, R., Scheuss, V., Russo, G. L., Torper, O., Lao, C. L., et al. (2019). Inducing different neuronal subtypes from astrocytes in the injured mouse cerebral cortex. Neuron 103, 1086–1095.e5. doi: 10.1016/j.neuron.2019.08.009
Mothe, A. J., Tam, R. Y., Zahir, T., Tator, C. H., and Shoichet, M. S. (2013). Repair of the injured spinal cord by transplantation of neural stem cells in a hyaluronan-based hydrogel. Biomaterials 34, 3775–3783. doi: 10.1016/j.biomaterials.2013.02.002
Najm, F. J., Lager, A. M., Zaremba, A., Wyatt, K., Caprariello, A. V., Factor, D. C., et al. (2013). Transcription factor-mediated reprogramming of fibroblasts to expandable, myelinogenic oligodendrocyte progenitor cells. Nat. Biotechnol. 31, 426–433. doi: 10.1038/nbt.2561
Oderich, G. S. (2021). The quest to lower spinal cord injuries continues. J. Vasc. Surg. 74, 1079–1080. doi: 10.1016/j.jvs.2021.03.022
Ohori, Y., Yamamoto, S., Nagao, M., Sugimori, M., Yamamoto, N., Nakamura, K., et al. (2006). Growth factor treatment and genetic manipulation stimulate neurogenesis and oligodendrogenesis by endogenous neural progenitors in the injured adult spinal cord. J. Neurosci. 26, 11948–11960. doi: 10.1523/JNEUROSCI.3127-06.2006
Pang, Z. P., Yang, N., Vierbuchen, T., Ostermeier, A., Fuentes, D. R., Yang, T. Q., et al. (2011). Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223. doi: 10.1038/nature10202
Papa, S., Pizzetti, F., Perale, G., Veglianese, P., and Rossi, F. (2020). Regenerative medicine for spinal cord injury: Focus on stem cells and biomaterials. Expert Opin. Biol. Ther. 20, 1203–1213. doi: 10.1080/14712598.2020.1770725
Pinelli, F., Pizzetti, F., Veneruso, V., Petillo, E., Raghunath, M., Perale, G., et al. (2022). Biomaterial-mediated factor delivery for spinal cord injury treatment. Biomedicines 10:1673. doi: 10.3390/biomedicines10071673
Plemel, J. R., Keough, M. B., Duncan, G. J., Sparling, J. S., Yong, V. W., Stys, P. K., et al. (2014). Remyelination after spinal cord injury: Is it a target for repair? Prog. Neurobiol. 117, 54–72. doi: 10.1016/j.pneurobio.2014.02.006
Pukos, N., Goodus, M. T., Sahinkaya, F. R., and McTigue, D. M. (2019). Myelin status and oligodendrocyte lineage cells over time after spinal cord injury: What do we know and what still needs to be unwrapped? Glia 67, 2178–2202. doi: 10.1002/glia.23702
Puls, B., Ding, Y., Zhang, F., Pan, M., Lei, Z., Pei, Z., et al. (2020). Regeneration of functional neurons after spinal cord injury via in situ NeuroD1-mediated astrocyte-to-neuron conversion. Front. Cell. Dev. Biol. 8:591883. doi: 10.3389/fcell.2020.591883
Qian, H., Kang, X., Hu, J., Zhang, D., Liang, Z., Meng, F., et al. (2020). Reversing a model of Parkinson’s disease with in situ converted nigral neurons. Nature 582, 550–556. doi: 10.1038/s41586-020-2388-4
Qin, H., Zhao, A., Ma, K., and Fu, X. (2018). Chemical conversion of human and mouse fibroblasts into motor neurons. Sci. China Life Sci. 61, 1151–1167. doi: 10.1007/s11427-018-9359-8
Rajpurohit, C. S., Kumar, V., Cheffer, A., Oliveira, D., Ulrich, H., Okamoto, O. K., et al. (2020). Mechanistic insights of astrocyte-mediated hyperactive autophagy and loss of motor neuron function in SOD1(L39R) linked amyotrophic lateral sclerosis. Mol. Neurobiol. 57, 4117–4133. doi: 10.1007/s12035-020-02006-0
Rodriguez, J. P., Coulter, M., Miotke, J., Meyer, R. L., Takemaru, K., and Levine, J. M. (2014). Abrogation of beta-catenin signaling in oligodendrocyte precursor cells reduces glial scarring and promotes axon regeneration after CNS injury. J. Neurosci. 34, 10285–10297. doi: 10.1523/JNEUROSCI.4915-13.2014
Sanchez-Gonzalez, R., Bribian, A., and Lopez-Mascaraque, L. (2020). Cell fate potential of NG2 progenitors. Sci. Rep. 10:9876. doi: 10.1038/s41598-020-66753-9
Savic, G., DeVivo, M. J., Frankel, H. L., Jamous, M. A., Soni, B. M., and Charlifue, S. (2018). Suicide and traumatic spinal cord injury-a cohort study. Spinal Cord 56, 2–6. doi: 10.1038/sc.2017.98
Shang, Z., Wang, R., Li, D., Chen, J., Zhang, B., Wang, M., et al. (2022). Spinal cord injury: A systematic review and network meta-analysis of therapeutic strategies based on 15 types of stem cells in animal models. Front. Pharmacol. 13:819861. doi: 10.3389/fphar.2022.819861
Shin, J., Berg, D. A., Zhu, Y., Shin, J. Y., Song, J., Bonaguidi, M. A., et al. (2015). Single-cell RNA-Seq with waterfall reveals molecular cascades underlying adult neurogenesis. Cell Stem Cell 17, 360–372. doi: 10.1016/j.stem.2015.07.013
Silverio-Alves, R., Gomes, A. M., Kurochkin, I., Moore, K. A., and Pereira, C. F. (2019). Hemogenic reprogramming of human fibroblasts by enforced expression of transcription factors. J. Vis. Exp. 153:e60112. doi: 10.3791/60112
Sofroniew, M. V. (2015). Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 16, 249–263. doi: 10.1038/nrn3898
Sofroniew, M. V. (2018). Dissecting spinal cord regeneration. Nature 557, 343–350. doi: 10.1038/s41586-018-0068-4
Son, E. Y., Ichida, J. K., Wainger, B. J., Toma, J. S., Rafuse, V. F., Woolf, C. J., et al. (2011). Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell 9, 205–218. doi: 10.1016/j.stem.2011.07.014
Su, Z., Niu, W., Liu, M. L., Zou, Y., and Zhang, C. L. (2014). In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 5:3338. doi: 10.1038/ncomms4338
Tai, W., Wu, W., Wang, L. L., Ni, H., Chen, C., Yang, J., et al. (2021). In vivo reprogramming of NG2 glia enables adult neurogenesis and functional recovery following spinal cord injury. Cell Stem Cell 28, 923–937.e4. doi: 10.1016/j.stem.2021.02.009
Tang, H., Song, Y., Li, T., Zheng, J., Jiang, P., Zhao, L., et al. (2021). [Necrostatin-1 promotes locomotor recovery after spinal cord injury through inhibiting apoptosis and M1 polarization of microglia/macrophage in mice]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 37, 775–780.
Tay, A., Sohrabi, A., Poole, K., Seidlits, S., and Di Carlo, D. (2018). A 3D magnetic hyaluronic acid hydrogel for magnetomechanical neuromodulation of primary dorsal root ganglion neurons. Adv. Mater. [Epub ahead of print]. doi: 10.1002/adma.201800927
Theodore, N., Hlubek, R., Danielson, J., Neff, K., Vaickus, L., Ulich, T. R., et al. (2016). First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: A clinical pilot study for safety and feasibility. Neurosurgery 79, E305–E312. doi: 10.1227/NEU.0000000000001283
Thompson, R. E., Pardieck, J., Smith, L., Kenny, P., Crawford, L., Shoichet, M., et al. (2018). Effect of hyaluronic acid hydrogels containing astrocyte-derived extracellular matrix and/or V2a interneurons on histologic outcomes following spinal cord injury. Biomaterials 162, 208–223. doi: 10.1016/j.biomaterials.2018.02.013
Tica, J., Bradbury, E. J., and Didangelos, A. (2018). Combined transcriptomics, proteomics and bioinformatics identify drug targets in spinal cord injury. Int. J. Mol. Sci. 19:1461. doi: 10.3390/ijms19051461
Tracy, B., Armola, R., and Micham, J. (2015). The “cold cord”: A review of therapeutic hypothermia for traumatic spinal cord injuries. Am. J. Crit. Care 24, 540–543. doi: 10.4037/ajcc2015879
Tran, A. P., Warren, P. M., and Silver, J. (2018). The biology of regeneration failure and success after spinal cord injury. Physiol. Rev. 98, 881–917. doi: 10.1152/physrev.00017.2017
Valny, M., Honsa, P., Kriska, J., and Anderova, M. (2017). Multipotency and therapeutic potential of NG2 cells. Biochem. Pharmacol. 141, 42–55. doi: 10.1016/j.bcp.2017.05.008
Vargas Luna, J. L., Brown, J., Krenn, M. J., McKay, B., Mayr, W., Rothwell, J. C., et al. (2021). Neurophysiology of epidurally evoked spinal cord reflexes in clinically motor-complete posttraumatic spinal cord injury. Exp. Brain Res. 239, 2605–2620. doi: 10.1007/s00221-021-06153-1
Vasan, L., Park, E., David, L. A., Fleming, T., and Schuurmans, C. (2021). Direct neuronal reprogramming: Bridging the gap between basic science and clinical application. Front. Cell. Dev. Biol. 9:681087. doi: 10.3389/fcell.2021.681087
Velychko, S., Kang, K., Kim, S. M., Kwak, T. H., Kim, K. P., Park, C., et al. (2019). Fusion of reprogramming factors alters the trajectory of somatic lineage conversion. Cell Rep. 27, 30–39.e4. doi: 10.1016/j.celrep.2019.03.023
Vierbuchen, T., Ostermeier, A., Pang, Z. P., Kokubu, Y., Sudhof, T. C., and Wernig, M. (2010). Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041. doi: 10.1038/nature08797
Vignoles, R., Lentini, C., d’Orange, M., and Heinrich, C. (2019). Direct lineage reprogramming for brain repair: Breakthroughs and challenges. Trends Mol. Med. 25, 897–914. doi: 10.1016/j.molmed.2019.06.006
Voronova, A. D., Stepanova, O. V., Valikhov, M. P., Chadin, A. V., Semkina, A. S., and Chekhonin, V. P. (2020a). Neural stem/progenitor cells of human olfactory mucosa for the treatment of chronic spinal cord injuries. Bull. Exp. Biol. Med. 168, 538–541. doi: 10.1007/s10517-020-04749-1
Voronova, A. D., Stepanova, O. V., Valikhov, M. P., Chadin, A. V., Semkina, A. S., Karsuntseva, E. K., et al. (2020b). Combined preparation of human olfactory ensheathing cells in the therapy of post-traumatic cysts of the spinal cord. Bull. Exp. Biol. Med. 169, 539–543. doi: 10.1007/s10517-020-04925-3
Wang, F., Cheng, L., and Zhang, X. (2021). Reprogramming glial cells into functional neurons for neuro-regeneration: Challenges and promise. Neurosci. Bull. 37, 1625–1636. doi: 10.1007/s12264-021-00751-3
Wang, J., Sareddy, G. R., Lu, Y., Pratap, U. P., Tang, F., Greene, K. M., et al. (2020). Astrocyte-derived estrogen regulates reactive astrogliosis and is neuroprotective following ischemic brain injury. J. Neurosci. 40, 9751–9771. doi: 10.1523/JNEUROSCI.0888-20.2020
Wang, L. L., Serrano, C., Zhong, X., Ma, S., Zou, Y., and Zhang, C. L. (2021). Revisiting astrocyte to neuron conversion with lineage tracing in vivo. Cell 184, 5465–5481.e16. doi: 10.1016/j.cell.2021.09.005
Wang, L. L., Su, Z., Tai, W., Zou, Y., Xu, X. M., and Zhang, C. L. (2016). The p53 pathway controls SOX2-mediated reprogramming in the adult mouse spinal cord. Cell Rep. 17, 891–903. doi: 10.1016/j.celrep.2016.09.038
Wang, L. L., and Zhang, C. L. (2018). Engineering new neurons: In vivo reprogramming in mammalian brain and spinal cord. Cell Tissue Res. 371, 201–212. doi: 10.1007/s00441-017-2729-2
Wang, Y., Lv, H. Q., Chao, X., Xu, W. X., Liu, Y., Ling, G. X., et al. (2022). Multimodal therapy strategies based on hydrogels for the repair of spinal cord injury. Mil. Med. Res. 9:16. doi: 10.1186/s40779-022-00376-1
Wu, J., Lu, B., Yang, R., Chen, Y., Chen, X., and Li, Y. (2021). EphB2 knockdown decreases the formation of astroglial-fibrotic scars to promote nerve regeneration after spinal cord injury in rats. CNS Neurosci. Ther. 27, 714–724. doi: 10.1111/cns.13641
Wu, W., Lee, S. Y., Wu, X., Tyler, J. Y., Wang, H., Ouyang, Z., et al. (2014). Neuroprotective ferulic acid (FA)-glycol chitosan (GC) nanoparticles for functional restoration of traumatically injured spinal cord. Biomaterials 35, 2355–2364. doi: 10.1016/j.biomaterials.2013.11.074
Xia, W., and Fancy, S. P. J. (2021). Mechanisms of oligodendrocyte progenitor developmental migration. Dev. Neurobiol. 81, 985–996. doi: 10.1002/dneu.22856
Xu, L., and Yao, Y. (2021). Central nervous system fibroblast-like cells in stroke and other neurological disorders. Stroke 52, 2456–2464. doi: 10.1161/STROKEAHA.120.033431
Xue, W., Fan, C., Chen, B., Zhao, Y., Xiao, Z., and Dai, J. (2021). Direct neuronal differentiation of neural stem cells for spinal cord injury repair. Stem Cells 39, 1025–1032. doi: 10.1002/stem.3366
Yang, R. Y., Chai, R., Pan, J. Y., Bao, J. Y., Xia, P. H., Wang, Y. K., et al. (2023). Knockdown of polypyrimidine tract binding protein facilitates motor function recovery after spinal cord injury. Neural Regen. Res. 18, 396–403. doi: 10.4103/1673-5374.346463
Yang, T., Dai, Y., Chen, G., and Cui, S. (2020a). Dissecting the dual role of the glial scar and scar-forming astrocytes in spinal cord injury. Front. Cell. Neurosci. 14:78. doi: 10.3389/fncel.2020.00078
Yang, T., Xing, L., Yu, W., Cai, Y., Cui, S., and Chen, G. (2020b). Astrocytic reprogramming combined with rehabilitation strategy improves recovery from spinal cord injury. FASEB J. 34, 15504–15515. doi: 10.1096/fj.202001657RR
Yang, Y., Chen, R., Wu, X., Zhao, Y., Fan, Y., Xiao, Z., et al. (2019). Rapid and efficient conversion of human fibroblasts into functional neurons by small molecules. Stem Cell Rep. 13, 862–876. doi: 10.1016/j.stemcr.2019.09.007
Yao, S., Yu, S., Cao, Z., Yang, Y., Yu, X., Mao, H. Q., et al. (2018). Hierarchically aligned fibrin nanofiber hydrogel accelerated axonal regrowth and locomotor function recovery in rat spinal cord injury. Int. J. Nanomedicine 13, 2883–2895. doi: 10.2147/IJN.S159356
Yates, A. G., Anthony, D. C., Ruitenberg, M. J., and Couch, Y. (2019). Systemic immune response to traumatic CNS injuries-are extracellular vesicles the missing link? Front. Immunol. 10:2723. doi: 10.3389/fimmu.2019.02723
Yokota, K., Kobayakawa, K., Saito, T., Hara, M., Kijima, K., Ohkawa, Y., et al. (2017). Periostin promotes scar formation through the interaction between pericytes and infiltrating monocytes/macrophages after spinal cord injury. Am. J. Pathol. 187, 639–653. doi: 10.1016/j.ajpath.2016.11.010
Yoshida, K., Uehara, O., Kurashige, Y., Paudel, D., Onishi, A., Neopane, P., et al. (2021). Direct reprogramming of epithelial cell rests of malassez into mesenchymal-like cells by epigenetic agents. Sci. Rep. 11:1852. doi: 10.1038/s41598-020-79426-4
Yoshizaki, S., Tamaru, T., Hara, M., Kijima, K., Tanaka, M., Konno, D. J., et al. (2021). Microglial inflammation after chronic spinal cord injury is enhanced by reactive astrocytes via the fibronectin/beta1 integrin pathway. J. Neuroinflammation 18:12. doi: 10.1186/s12974-020-02059-x
Zarei-Kheirabadi, M., Hesaraki, M., Kiani, S., and Baharvand, H. (2019). In vivo conversion of rat astrocytes into neuronal cells through neural stem cells in injured spinal cord with a single zinc-finger transcription factor. Stem Cell Res. Ther. 10:380. doi: 10.1186/s13287-019-1448-x
Zhao, C., Dong, H., Xu, Q., and Zhang, Y. (2020). Histone deacetylase (HDAC) inhibitors in cancer: A patent review (2017-present). Expert Opin. Ther. Pat. 30, 263–274. doi: 10.1080/13543776.2020.1725470
Zhou, H., Su, J., Hu, X., Zhou, C., Li, H., Chen, Z., et al. (2020). Glia-to-neuron conversion by CRISPR-CasRx alleviates symptoms of neurological disease in mice. Cell 181, 590–603.e16. doi: 10.1016/j.cell.2020.03.024
Zhou, M., Tao, X., Sui, M., Cui, M., Liu, D., Wang, B., et al. (2021). Reprogramming astrocytes to motor neurons by activation of endogenous Ngn2 and Isl1. Stem Cell Rep. 16, 1777–1791. doi: 10.1016/j.stemcr.2021.05.020
Zhou, P., Guan, J., Xu, P., Zhao, J., Zhang, C., Zhang, B., et al. (2019). Cell therapeutic strategies for spinal cord injury. Adv. Wound Care 8, 585–605. doi: 10.1089/wound.2019.1046
Zhou, Q., and Melton, D. A. (2008). Extreme makeover: Converting one cell into another. Cell Stem Cell 3, 382–388. doi: 10.1016/j.stem.2008.09.015
Zhou, Y. X., Liu, Q., Wang, H., Ding, F., and Ma, Y. Q. (2019). The expression and prognostic value of SOX2, beta-catenin and survivin in esophageal squamous cell carcinoma. Future Oncol. 15, 4181–4195. doi: 10.2217/fon-2018-0884
Zhu, X., Bergles, D. E., and Nishiyama, A. (2008). NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 135, 145–157. doi: 10.1242/dev.004895
Keywords: spinal cord injury repair, somatic cell reprogramming, fibroblasts, astrocytes, NG2 cells, neural progenitor cells, functional recovery
Citation: Yang R, Pan J, Wang Y, Xia P, Tai M, Jiang Z and Chen G (2022) Application and prospects of somatic cell reprogramming technology for spinal cord injury treatment. Front. Cell. Neurosci. 16:1005399. doi: 10.3389/fncel.2022.1005399
Received: 28 July 2022; Accepted: 02 November 2022;
Published: 17 November 2022.
Edited by:
Natalia Arias, King’s College London, United KingdomReviewed by:
Gemma Palazzolo, Italian Institute of Technology (IIT), ItalyCopyright © 2022 Yang, Pan, Wang, Xia, Tai, Jiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gang Chen, Y2hlbmdhbmc2NjI2QG50dS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.