
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci. , 18 December 2020
Sec. Non-Neuronal Cells
Volume 14 - 2020 | https://doi.org/10.3389/fncel.2020.601324
This article is part of the Research Topic Involvement of Blood Brain Barrier Efficacy, Neurovascular Coupling and Angiogenesis in the Healthy and Diseased Brain View all 19 articles
Brain pericytes reside on the abluminal surface of capillaries, and their processes cover ~90% of the length of the capillary bed. These cells were first described almost 150 years ago (Eberth, 1871; Rouget, 1873) and have been the subject of intense experimental scrutiny in recent years, but their physiological roles remain uncertain and little is known of the complement of signaling elements that they employ to carry out their functions. In this review, we synthesize functional data with single-cell RNAseq screens to explore the ion channel and G protein-coupled receptor (GPCR) toolkit of mesh and thin-strand pericytes of the brain, with the aim of providing a framework for deeper explorations of the molecular mechanisms that govern pericyte physiology. We argue that their complement of channels and receptors ideally positions capillary pericytes to play a central role in adapting blood flow to meet the challenge of satisfying neuronal energy requirements from deep within the capillary bed, by enabling dynamic regulation of their membrane potential to influence the electrical output of the cell. In particular, we outline how genetic and functional evidence suggest an important role for Gs-coupled GPCRs and ATP-sensitive potassium (KATP) channels in this context. We put forth a predictive model for long-range hyperpolarizing electrical signaling from pericytes to upstream arterioles, and detail the TRP and Ca2+ channels and Gq, Gi/o, and G12/13 signaling processes that counterbalance this. We underscore critical questions that need to be addressed to further advance our understanding of the signaling topology of capillary pericytes, and how this contributes to their physiological roles and their dysfunction in disease.
A combination of autonomic signaling (Cipolla et al., 2004; Hamel, 2006) and intrinsic pressure sensing and metabolic autoregulatory mechanisms (Bayliss, 1902; Paulson et al., 1990) drives continual adjustments in global and local blood flow in the brain. Importantly, as the brain lacks substantial energy stores it must be able to rapidly adapt local blood flow to fluctuating neuronal metabolic needs to provide adequate oxygen and glucose delivery. This is achieved through the on-demand process of functional hyperemia (FH), where increases in neural activity—which can span orders of magnitude in milliseconds—are met with an increase in local blood flow within seconds. This call-and-response phenomenon is underlain by a complex range of stratified mechanisms, collectively termed neurovascular coupling (NVC), which have inbuilt redundancy to ensure the fidelity of the blood flow response.
Significant inroads toward a full understanding of these NVC mechanisms have been made in recent years (Iadecola, 2017), and in particular ion channel and GPCR signaling networks within and between the cells of the neurovascular unit [NVU; neurons, astrocytes, smooth muscle cells (SMCs), endothelial cells (ECs), and pericytes] are emerging as major contributors (Longden et al., 2016). However, capillary pericytes represent a relative blind spot in our knowledge, and our understanding of their involvement in brain blood flow control is less well-developed than that for other cells of the NVU. Accordingly, the purpose of this review is to survey the signaling toolkit that mesh and thin-strand pericytes may employ to contribute to the control of blood flow throughout the brain. To this end, we leverage data from recent brain single-cell RNAseq (scRNAseq) screens (He et al., 2018; Vanlandewijck et al., 2018; Zeisel et al., 2018) to profile the expression of ion channels (Table 1) and GPCRs (Table 2) in brain capillary pericytes which, when synthesized with functional results, may aid in delineating their physiological roles.
An important caveat with this approach is that mRNA expression does not necessarily predict protein levels (Liu et al., 2016), and we thus stress that it is essential that the hypotheses generated by transcriptomic data be subject to further experimental scrutiny. Accordingly, while the following discussion is based on robust mRNA expression data, we highlight where there is question of whether gene expression translates into functional channels or receptors. A second putative caveat relates to the quality of the scRNAseq data. Specifically, it is important to ask if low-level mRNA counts reflect true and physiologically meaningful expression or artifacts such as contamination of the pericyte transcriptomes by mRNA from other cell types. Pericytes in particular are sensitive to endothelial contamination because of the tight physical association between these two cell types. With these caveats in mind, to arrive at a list of genes with reasonable likelihood of pericyte expression we first selected genes detected at levels >1 average count per cell in the 1,088 adult brain pericytes present in the Vanlandewijck et al. dataset (http://betsholtzlab.org/VascularSingleCells/database.html; He et al., 2018; Vanlandewijck et al., 2018) and compared this to their expression in the Zeisel dataset (http://mousebrain.org; Zeisel et al., 2018). In the latter, three pericyte clusters are provided (PER1, PER2, PER3) of which PER1 and PER2 are endothelial cell contaminated, whereas PER3 appears pure. After manually checking for signs of contamination by comparing the expression level in pericytes with expression in other brain cell types, we selected the following criteria as qualifying: (i) expression in >3% of the pericytes in the Vanlandewijck dataset and; (ii) detectable expression (>0) in the Zeisel et al. PER3 dataset (Figure 1).
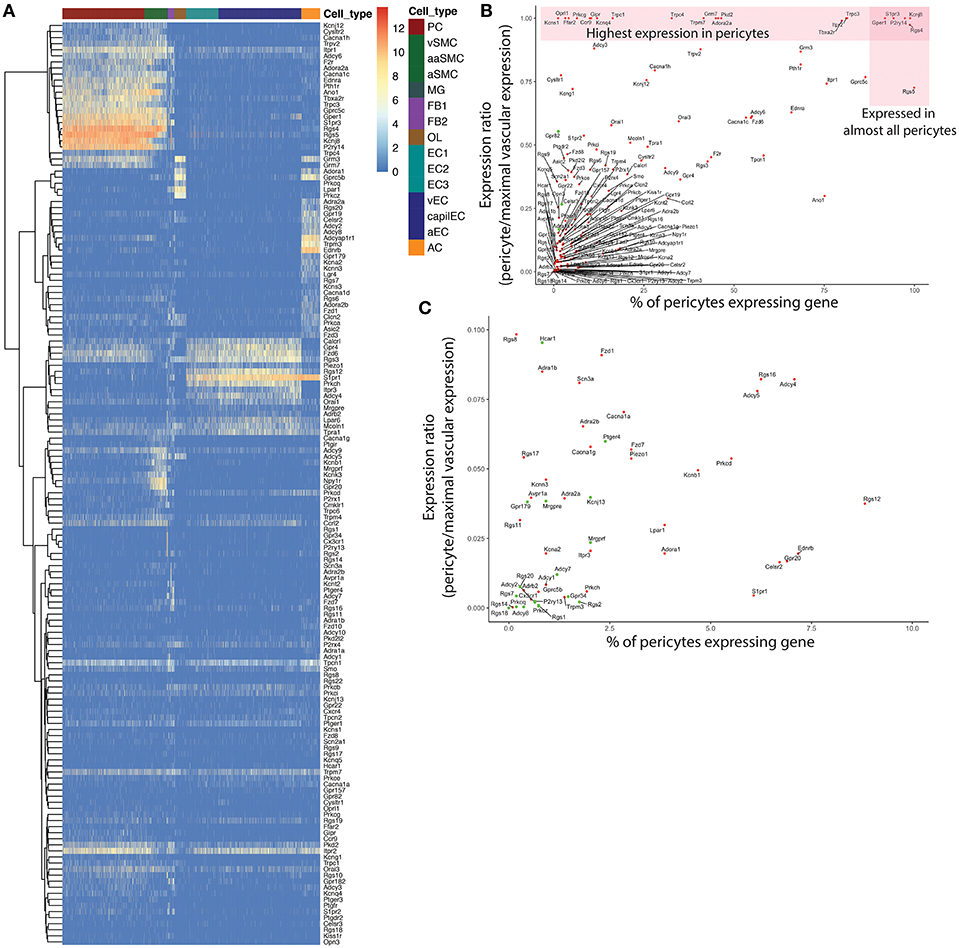
Figure 1. Overview of gene qualification process for pericyte ion channels and GPCRs and other genes of interest. An initial filter of 1 average count/cell was applied to exclude genes with extremely low expression. (A) Heatmap of expression of the remaining genes throughout the neurovascular unit. A small subset of these genes were highly enriched in pericytes (top left), while many showed higher expression in other cell types. To filter out potential contamination, genes that were expressed in <3% of pericytes, and were absent from the PER3 cluster of Zeisel et al. (2018) were excluded. (B) Relationship between pericyte-specificity of expression and fraction of pericytes expressing each gene considered. Genes represented by green circles were excluded according to the above criteria. (C) High resolution view of genes with a <0.1 expression ratio in pericytes, that were expressed in fewer than 10% of pericytes, corresponding to the bottom left corner in (B). Genes represented by green circles were excluded from further consideration as potential contamination.
Below, we focus our discussion on the ion channels and GPCRs that are likely to be most pertinent to blood flow control. We center our discussion on studies using acute and in vivo preparations, as cultured pericytes may exhibit phenotypic drift which confounds interpretation. Accordingly, we note instances in which we refer to cultured pericytes. We begin by briefly reviewing the key features of the brain vasculature and pericytes before exploring their ion channel and GPCR complement in detail.
From pial arteries on the brains surface, penetrating arterioles branch orthogonally and dive into the parenchyma (Duvernoy et al., 1981; Cipolla, 2009; Figure 2). Arteries and arterioles are composed of a lumen lined by electrically-coupled cobblestone–morphology ECs (Haas and Duling, 1997) that directly interface with the blood. These ECs are surrounded by a fenestrated internal elastic lamina (IEL), composed mainly of elastin and collagen (Schwartz et al., 1981), through which they extend projections to directly contact overlying contractile smooth muscle cells (SMCs) (Aydin et al., 1991).
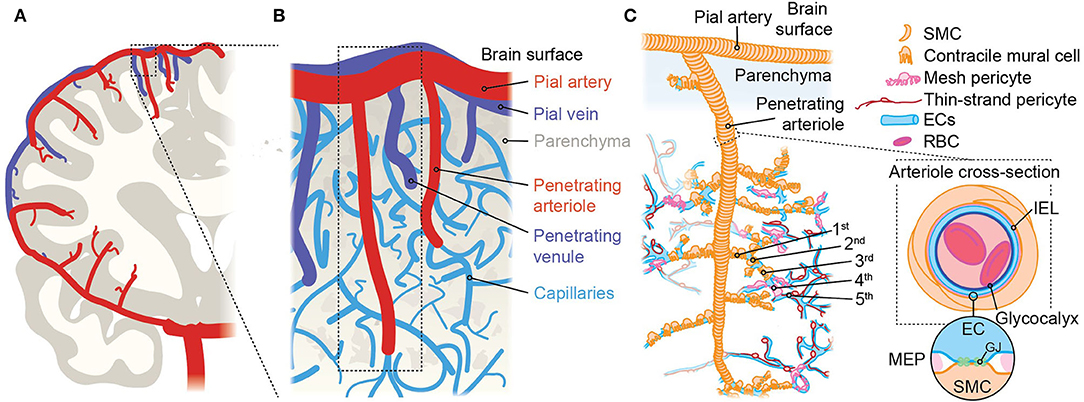
Figure 2. An overview of brain angioarchitecture. (A) Cross-section of one brain hemisphere illustrating macroscopic vascular architecture. The carotid artery joins the circle of Willis at the base of the brain, then gives rise to major pial arteries which course over the brain surface, from which multiple penetrating arterioles arise and dive into the tissue. (B) Close up view of the components of the vascular network approximating the area in the boxed region in A showing the interconnected organization of pial arteries, penetrating arterioles, the dense capillary network, and venules. The vessel labeling system we use takes the penetrating arteriole as the 0-order vessel and primary reference point, and vessels are numbered sequentially with regard to this. Vessel number automatically increases each time a vessel branches and thus, after vessel n branches, the daughter branches—regardless of diameter or orientation—are labeled vessel n + 1. (C) Illustration approximating the boxed region in (B), showing the cellular elements that make up the arteriolar side of the brain vasculature. Arteries and arterioles consist of SMCs surrounding ECs, which are in direct contact with the blood. The first 3–4 vessels emanating from the penetrating arteriole are a transitional zone and are covered with contractile mural cells that are positive for α-SMA and can change diameter abruptly. Immediately after the α-actin terminus are capillaries covered by mesh pericytes, following which are capillaries where thin-strand pericytes reside. The cross-section at right shows a section through an artery/arteriole and illustrates the presence of the internal elastic lamina (IEL) which separates ECs and SMCs. Occasional fenestrations dot the IEL, through which ECs and SMCs make direct contact via myoendothelial projections (MEPs, circular inset). These are sites of gap junctions (GJs) permitting chemical and electrical cell-cell communication.
As the penetrating arteriole extends deeper into the tissue, further vessels sprout from its length at regular intervals (Blinder et al., 2013). These initial branch points are sites of precapillary sphincters which are regulated over short time scales to control blood flowing into the capillary bed (Grubb et al., 2020). From this point, extensive ramification of the vascular bed greatly expands the surface area of the network, facilitating efficient exchange of nutrients and waste to rapidly satisfy the intense metabolic requirements of every neuron. The capillary bed—consisting of capillary ECs (cECs; Garcia and Longden, 2020) and overlying pericytes (see below) embedded in the basement membrane (a dense network of glycoproteins, collagens and secreted factors; Pozzi et al., 2017)—is incredibly dense, and each microliter of cortex holds approximately 1 m of blood vessels (Shih et al., 2015). Of these, around 90% by volume are capillaries (Gould et al., 2017). Accordingly, ECs are estimated to comprise around 30% of the non-neuronal cell mass in the gray matter, forming a network of 20–25 billion ECs throughout the entire human brain (von Bartheld et al., 2016). This places cECs in close apposition with all neurons, with each neuronal cell body lying within ~15 μm of a vessel (Tsai et al., 2009). Red blood cells (RBCs) traverse this network, releasing oxygen to diffuse down its concentration gradient into the tissue, while glucose is transported by ECs from the blood plasma into the parenchyma. After negotiating the capillary bed, oxygen-depleted RBCs eventually reach a vertically-oriented venule, which drain to veins at the cortical surface on the path back to the heart.
As the vascular bed ramifies from the penetrating arteriole, there is gradation in the morphology and functional characteristics of the mural cells associated with vessels. The first 3–4 branches of the vascular network (1st to 4th order) originating from the penetrating arteriole constitute a “transitional zone” (Ratelade et al., 2020). These vessels are covered by cells expressing high levels of α-smooth muscle actin (α-SMA) with ovoid cell bodies and multiple broad processes that almost completely ensheathe the underlying vessel (Grant et al., 2019; Figure 3A). Given that the identity of these cells is unresolved, and that they have been referred to as both pericytes (Peppiatt et al., 2006; Hall et al., 2014; Attwell et al., 2016; Grant et al., 2019) and SMCs (Hill et al., 2015; Grutzendler and Nedergaard, 2019), we refer to these cells here as “contractile mural cells” and to the segments of the vasculature that they cover as “vessels.” Expression of α-SMA permits these cells to rapidly regulate the diameter of the underlying vessel and therefore blood flow. Indeed, multiple studies have illustrated the importance of contractile mural cells in mediating dilation (of ~10–30%) in response to neuronal stimulation (Hill et al., 2015; Mishra et al., 2016; Kisler et al., 2017; Cai et al., 2018; Rungta et al., 2018).
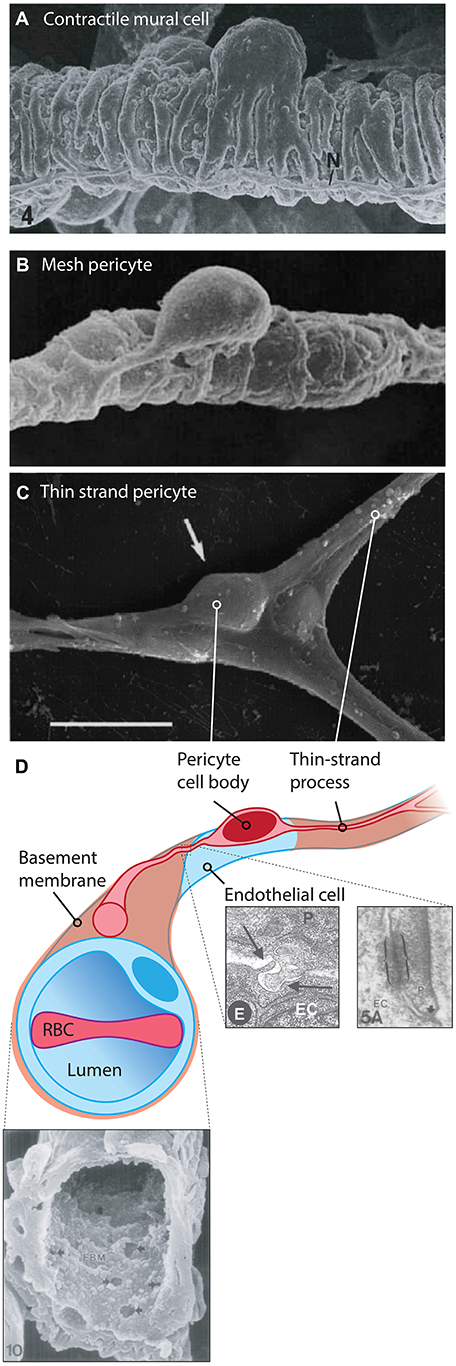
Figure 3. Cytoarchitecture and microenvironment of pericytes. (A) Mural cells with a ‘bump-on-a-log’ cell body, with multiple contractile processes that almost completely encase the underlying vessel. 6,000x, rat mammary gland vasculature. Reproduced with permission from Fujiwara and Uehara (1984). (B) A 4,400x magnification scanning electron micrograph of a putative mesh pericyte of the rat mammary gland. Multiple sparse processes enwrap the underlying capillary. Reproduced with permission from Fujiwara and Uehara (1984). (C) A thin-strand pericyte atop a rat retinal capillary, extending fine processes away from the ovoid cell body. Adapted with permission from Sakagami et al. (1999). Scale bar: 10 μm. (D) Illustration of a thin-strand pericyte. The bulk of the volume of the cell body is occupied by the nucleus. The pericyte is prevented from making direct contact with the underlying EC by the basement membrane, shown in the SEM at bottom left, reproduced with permission from Carlson (1989). Multiple small fenestrations are seen in this structure, allowing for pericyte and endothelial projections to make direct contact with one another, forming so-called ‘peg-socket junctions’ which are also sites of gap junction formation. At bottom right electron micrographs depicting a peg-socket junction (left) and a pericyte-endothelial gap junction (right) are shown, reproduced with permission from Díaz-Flores et al. (2009) and Carlson (1989). Abbreviations in micrographs: EC, endothelial cell; N, nerve; P, pericyte.
Beyond this point in the vasculature, mural cells do not express high levels of α-SMA, although one recent study suggested that retinal mural cells retain expression of a low level of this protein (Alarcon-Martinez et al., 2018) and they do express very low levels of the Acta2 gene in the brain (He et al., 2018; Vanlandewijck et al., 2018). As a result, these cells are not equipped to regulate vessel diameter over abrupt time scales, but there is clear evidence that they may contract slowly under certain circumstances (reducing the diameter of the underlying vessel by up to ~25%; Fernández-Klett et al., 2010; Gonzales et al., 2020). Thus, we consider the relatively static diameter vessels downstream of the α-SMA terminus (which typically occurs between the 1st and 4th order branch in immunostaining experiments; Grant et al., 2019) to be capillaries. The identity of mural cells on these so-defined capillaries is unambiguous, and there is consensus that these cells are pericytes.
The pericytes residing on capillaries display at least two distinct morphologies: (i) Immediately adjacent to the α-SMA terminus, pericytes take on a mesh-like appearance, and are thus known as “mesh pericytes” (Figure 3B); (ii) beyond these are cells that project long, thin processes along the vasculature, and accordingly these are referred to as “thin-strand pericytes” (Grant et al., 2019; Figures 3C,D).
Despite differing morphologies (Figure 3), mesh and thin-strand pericytes are indistinguishable at the level of single-cell transcriptomics, possibly due to the fact that mesh pericytes represent only a small fraction of capillary pericytes (Chasseigneaux et al., 2018). Pericyte cell bodies have a highly stereotyped shape, appearing as a large ovoid that protrudes from the wall of the capillary, which is often referred to as a “bump-on-a-log” (Grant et al., 2019). Mesh pericytes are few in number relative to thin-strand pericytes and have fewer, shorter longitudinal processes (their primary trunks averaging 40 μm in length; Hartmann et al., 2015) that cover ~70% of the underlying capillary. This contrasts with upstream contractile mural cells which cover 95% of the underlying vessel (Grant et al., 2019). Thin-strand pericytes extend long, thin, strand-like processes that are ~1.5 μm in diameter and cover on average around 250 μm in total capillary distance, in some instances exceeding 300 μm (Berthiaume et al., 2018). Together, the thin-strand pericyte cell body and its processes cover between one third (Mathiisen et al., 2010) and one half (Grant et al., 2019) of the abluminal surface area of the endothelium. A typical thin-strand process has a stable “non-terminal core” of ~50 μm in length that bifurcates into slightly shorter, dynamic terminal processes that may extend or retract up to 20 μm over the course of days to weeks (Berthiaume et al., 2018). At their terminal ends, thin-strand processes appear to come into close proximity with those of neighboring pericytes (Berthiaume et al., 2018), possibly allowing for direct contact between adjacent pericytes, although this awaits direct experimental confirmation. Changes in the length of processes of one cell appear to evoke opposite changes in the length of adjacent pericyte processes, preventing the formation of substantial gaps (Berthiaume et al., 2018).
These processes are for the most part prevented from making direct contact with the underlying endothelium by the basement membrane. However, electron microscopy has revealed that—similar to the IEL of arteries and arterioles—the capillary basement membrane is dotted with many fenestrations, with an average area of 1.5 μm2, ranging from 100 to 450 nm in diameter (Carlson, 1989; Figure 3D). In arteries, similar fenestrations are the sites of myoendothelial junctions, optimized for EC-SMC communication by the presence of a number of key enzymes, ion channels, and gap junction (GJ) proteins (Straub et al., 2014). In the capillary bed, these fenestrations are the site of “peg-socket” interdigitations where either the pericyte or the EC sends a projection to make contact with the adjacent cell (Tilton et al., 1979; Cuevas et al., 1984; Armulik et al., 2005). These contact points are thought to be the sites of GJ communication between the two cell types (see Box 1), and may be the location of key signaling events, such as local calcium (Ca2+) or cyclic adenosine monophosphate (cAMP) elevations. Moreover, they may be sites of macromolecular signaling complex assembly, containing ion channels, and GPCRs positioned to facilitate cell-cell communication.
Box 1. Potential gap junction configurations between capillary pericytes and cECs.
According to expression data (He et al., 2018; Vanlandewijck et al., 2018), pericytes predominantly express mRNA for connexin (Cx)37 and Cx45, along with much lower expression of Cx26 and Cx43. Capillary ECs, on the other hand, robustly express Cx43 and Cx45, with low levels of Cx37, whereas Cx26 is undetectable (see Figure). Electron microscopy has been used to visualize putative GJ sites between pericytes and ECs at peg-socket interdigitations. In contrast, similar sites between the processes of neighboring pericytes have yet to be clearly demonstrated. Nonetheless, a recent dye transfer study (Kovacs-Oller et al., 2020), has shown that the cells of the capillary bed form a syncytium. Accordingly, two configurations for cell-cell communication can be postulated: (i) Pericyte-EC GJs alone permit bidirectional transfer of intracellular materials and charge between cells of the capillary wall; (ii) both pericyte-EC GJs and pericyte-pericyte GJs permit intercellular communication along two parallel, closely adjacent paths. The latter configuration would provide redundancy in the event of cell-cell communication failing in one cell type.
GJs are homo- or hetero-dodecameric assemblies of Cx subunits (Koval et al., 2014), formed from two hexameric hemichannels that dock to yield intercellular channels. GJs can be homotypic, with both hemichannels composed of the same Cx isoform(s), or heterotypic, with each hemichannel consisting of a distinct assembly of 6 Cx subunits. Moreover, a given hemichannel may be homomeric (composed Cx monomers of the same isoform) or heteromeric (consisting of multiple Cx isoforms), a property that depends on the propensity of the locally expressed Cxs to co-assemble. These complexities yield channels with distinct attributes, which may further oligomerize into large GJ plaques with discrete population characteristics.
Considering pericyte connexins in isolation, α-class Cxs 37 and 45 are not known to assemble into heteromers, but both of these will heteromerize with the much more modestly expressed α Cx43. The β Cx26, on the other hand, is not compatible with α Cx isoforms. Thus, the available data suggest that the typical pericyte hemichannel is most likely to be a homomeric assembly of Cx37 or Cx45, with perhaps a low level of heteromerization involving Cx43. Similarly, the EC-expressed Cx43 will form heteromers with Cx37 and Cx45, but again the latter are not compatible with one another. Thus, the possibility of heteromerization appears to be higher for ECs. In terms of heterotypic compatibility in the formation of GJs, Cx37, Cx43, and Cx45 are known to readily assemble together, whereas Cx26 hemichannels will not dock with any of these.
Taken together, this complexity underscores the great deal of further work needed to firmly establish the nature and properties of GJs in the capillary wall.
A cursory review of the brain capillary pericyte ion channel expression data provided by He et al. (2018) and Vanlandewijck et al. (2018) reveals that potassium (K+) channels are the dominant ion channel species in pericytes. Remarkably, this is due to the adenosine triphosphate (ATP)-sensitive K+ (KATP) channel inward rectifier (Kir) subunit, Kir6.1, accounting for nearly half of the total ion channel gene expression in these cells. Transient receptor potential (TRP), Ca2+, and chloride (Cl−) channels make up the remaining half, along with lower expression of a handful of other channel subunits including two-pore channels (TPCs), voltage-gated sodium (Na+; Nav) channels, P2X receptors, acid sensing ion channels (ASICs), and Piezo1 (Table 1 and Figure 4).
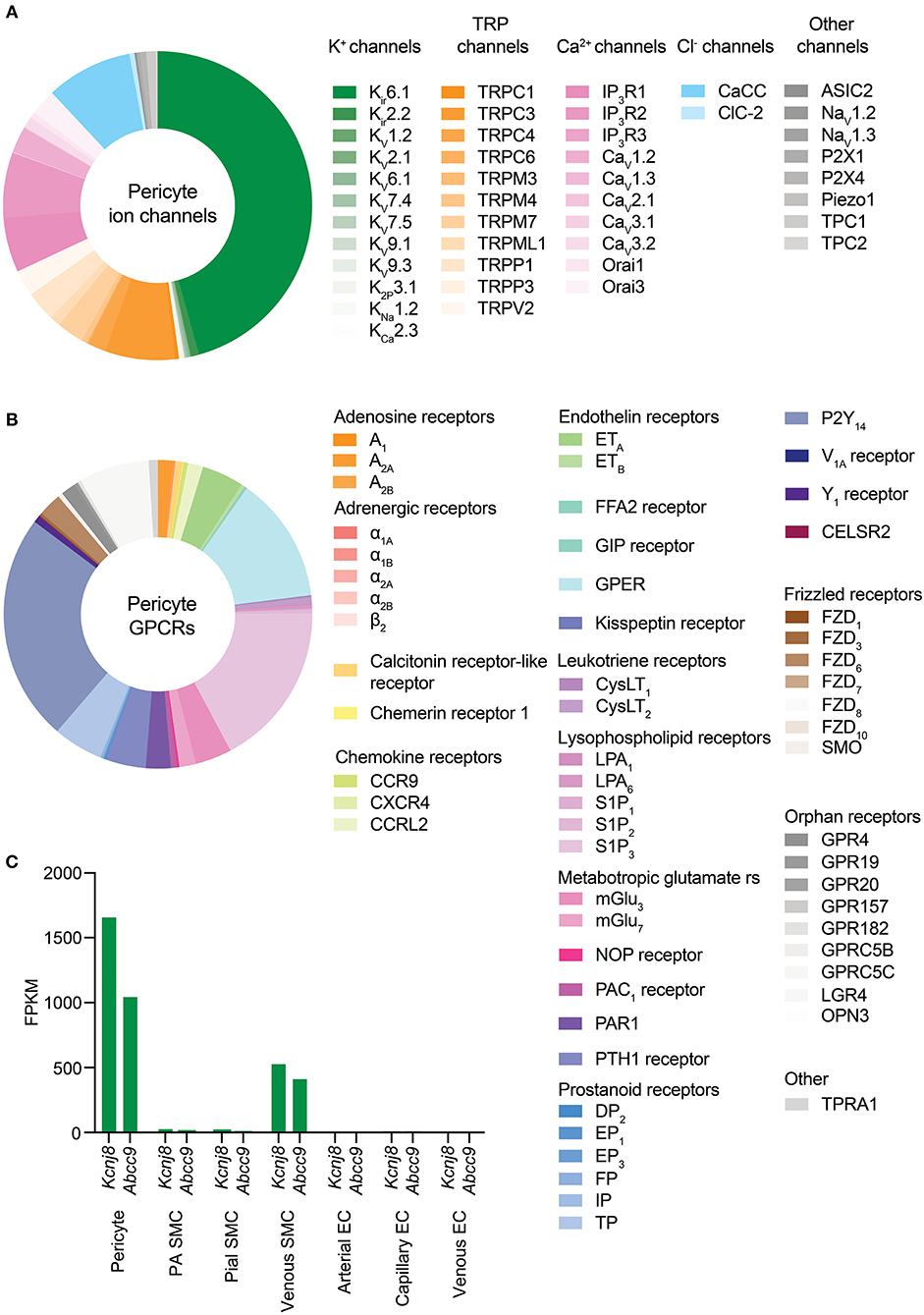
Figure 4. Overview of CNS pericyte ion channel and GPCR expression. (A) Relative abundance of mRNA for all ion channel subunits meeting our inclusion criteria. The size of each segment represents the relative expression of the underlying gene. Channels are clustered on the basis of the ion species that the corresponding functional channel conducts (denoted by shading of the same color) and are then grouped by family/subfamily. K+ channels are the predominant ion channel class due to extremely high expression of Kcnj8 which forms the pore of vascular KATP channels. The non-selective TRP channels are the next highest expressed, followed by Ca2+ channels, Cl− channels, and lower expression of other channels. (B) Relative expression of pericyte GPCRs. Here, receptors are organized by ligand sensitivity or class. (C) Expression of the KATP channel genes Kcnj8 and Abcc9 throughout the brain vasculature. Pericytes express both genes at much higher levels than arterial SMCs or ECs. However, venous SMCs also express high levels of KATP channel-forming genes.
Focusing initially on the K+ channel superfamily, capillary pericytes express Kir, two-pore domain (K2P), voltage-gated (Kv), Na+-activated (KNa), and Ca2+-activated (KCa) K+ channel genes.
Kir channels have the defining biophysical property of inward rectification, preferentially conducting large currents into the cell at voltages negative to the K+ equilibrium potential (EK), the magnitude of which depend on the electrochemical gradient for K+ [i.e., the difference between Vm and EK] (Katz, 1949; Hibino et al., 2010). At potentials positive to EK some degree of rectification occurs, ranging from strong—in which almost no current passes from the interior of the cell to the exterior—to weak, in which rectification is only seen at very positive potentials. Accordingly, Kir channels can be classified by their degree of rectification as strongly-rectifying (Kir2.x, Kir3.x), intermediately-rectifying (Kir4.x) or weakly-rectifying (Kir1.1, Kir6.x, Kir7.x). Alternatively, this group of channels can be classified according to function into classic (Kir2.x), G-protein sensitive (Kir3.x), KATP (Kir6.x), or K+ transport (Kir1.x, Kir4.x, Kir5.x, Kir7.x) channels (Hibino et al., 2010). Of the Kir channel family, capillary pericytes express extremely high levels of Kir6.1—far exceeding that of any other ion channel gene expressed by brain pericytes—and to a lesser extent Kir2.2 (Bondjers et al., 2006; He et al., 2018; Vanlandewijck et al., 2018).
As Kir6.1 is a component of KATP channels, this suggests that the two key roles of these channels—providing membrane hyperpolarization and coupling metabolism to membrane electrical activity—could be major contributors to pericyte physiology. Functional KATP channels are hetero-octameric assemblies of four two-transmembrane spanning pore–forming Kir6.x subunits (either Kir6.1 or Kir6.2, encoded by Kcnj8 and Kcnj11, respectively), each associated with a regulatory 17-transmembrane spanning ATP-binding cassette subfamily sulfonylurea subunit (SUR1 or SUR2, respectively encoded by Abcc8 and Abcc9—the latter of which is also highly expressed in brain pericytes; Figure 5A; Seino and Miki, 2003; Li et al., 2017). SURs are required for membrane trafficking of the channel (Burke et al., 2008) and impart sensitivity to KATP agonists and antagonists and intracellular nucleotides. Alternative splicing yields a number of SUR2 variants with SUR2A and SUR2B as the major forms, differing by just 42 amino acids in their C-terminal domains (Seino and Miki, 2003). Thus, the available expression data suggest that KATP channels native to brain pericytes are composed of Kir6.1 and SUR2—often referred to as the “vascular” form of KATP–and indicates that these are expressed much more highly in pericytes than they are in cerebral SMCs and ECs (Figure 4C).
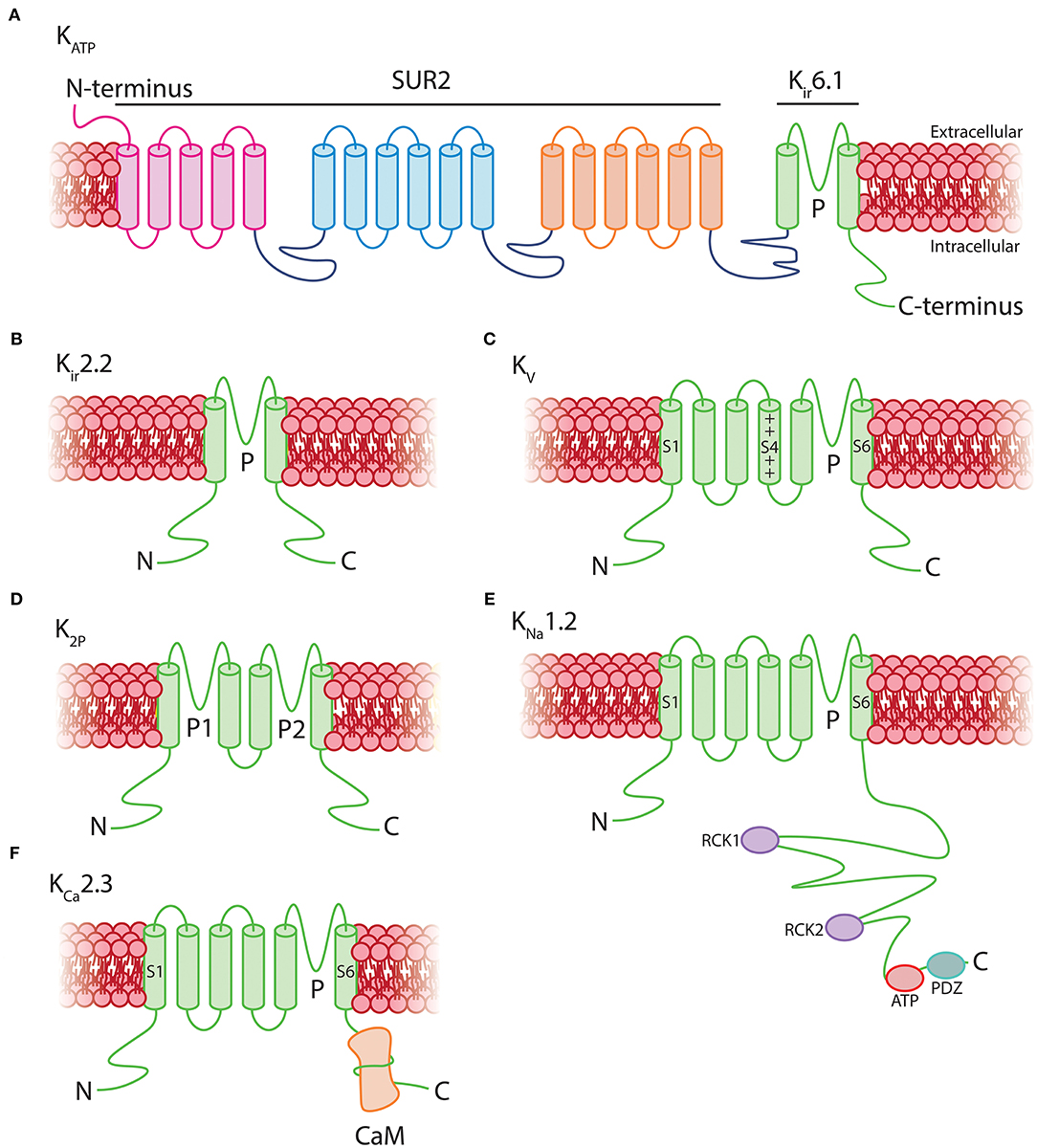
Figure 5. Structural topology of K+ channels expressed by pericytes. (A) Vascular KATP channels are octamers consisting of four 17-transmembrane SUR2 subunits associated with four 2-transmembrane pore-forming Kir6.1 subunits. (B) Kir2.2 channels consist of homo or heteromeric assemblies of four 2-transmembrane subunits. (C) Kv channels are composed of four 6-transmembrane alpha subunits with a positively charged voltage sensor at S4 which transduces changes in Vm into conformational alterations. (D) K2P channels are tetramers of two-pore domain four-transmembrane subunits. (E) KNa channels have a 6-transmembrane structure that lacks a voltage sensor, with multiple regulatory sites in the long intracellular COOH-terminus including two RCK domains, an ATP binding site, and a PDZ domain. (F) KCa2.3 channels consist of four 6-transmembrane domains which lack a voltage-sensor at S4. The COOH-terminus of each is associated with a calmodulin monomer, which imparts Ca2+ sensitivity to the channel.
K+ currents through KATP channels are weakly rectifying at potentials very positive to EK–the result of voltage-dependent intracellular magnesium (Mg2+) block (Findlay, 1987). The defining biophysical feature of KATP channels is that open probability (Po) decreases with increasing intracellular ATP levels, with ATP stabilizing the closed state of the channel (Enkvetchakul and Nichols, 2003). Thus, when cellular ATP demands are low and free cytosolic ATP is high, the channel is closed. In contrast, when cell activity increases or metabolism drops, the ADP:ATP ratio rises and the channel may open to hyperpolarize the membrane (Quayle et al., 1997). Consistent with these channels being saturated by ATP to keep them closed under resting conditions, the KATP channel blocker glibenclamide has no effects on resting CBF but levcromakalim, a KATP channel opener, increases global CBF by 14% (Al-Karagholi et al., 2020).
Nucleotide regulation of KATP channels is complex and has been best characterized for Kir6.2/SUR1-containing channels, which we review briefly here. Intracellular nucleotides are sensed by an array of sites throughout the channel complex: ATP has been shown to bind to an inhibitory site of the Kir6.2 subunit (Tucker et al., 1997; Tanabe et al., 2000) with just one of four subunits of the channel needing to bind ATP to effect closure (Markworth et al., 2000). The SUR1 subunit has two nucleotide binding domains (Li et al., 2017), where Mg2+-bound adenosine diphosphate (MgADP) occupancy increases channel activity (Tung and Kurachi, 1991; Gribble et al., 1997; Shyng et al., 1997). MgATP also has a stimulatory effect here, likely through hydrolysis to MgADP, although this is normally masked by the much more potent inhibitory effect of free ATP (Gribble et al., 1998; Proks et al., 2010). Thus, as might be expected, increasing intracellular Mg2+ antagonizes the inhibitory effect of free ATP (Gribble et al., 1998). Conversely, in the absence of Mg2+, ADP may have an inhibitory effect (Findlay, 1988). Comparatively less is known about the fine details of nucleotide regulation of Kir6.1/SUR2B channels, which have a smaller conductance than their Kir6.2-containing counterparts (~15–30 pS for Kir6.1/SUR2B-containing channels vs. ~50–90 pS for the Kir6.2/SUR2A form, for example; Hibino et al., 2010). However, it is clear that the presence of a nucleotide diphosphate and Mg2+ is a requirement for channel activity, and that these channels are also sensitive to ATP inhibition (Kajioka et al., 1991; Kovacs and Nelson, 1991; Beech et al., 1993; Kamouchi and Kitamura, 1994; Nelson and Quayle, 1995; Zhang and Bolton, 1996; Yamada et al., 1997).
One of the consequences of the nucleotide sensitivity of KATP channels is that they may act as sensors of the metabolic state of the cell and transduce changes in this parameter into adjustments of membrane voltage. This is perhaps best characterized in pancreatic β cells, where KATP channels composed of Kir6.2 and SUR1 subunits couple glucose concentration with insulin secretion (Tarasov et al., 2004). Here, elevated glucose leads to an increase in intracellular ATP due to increased glucose metabolism. This closes KATP channels, which depolarizes the cell and drives Ca2+-mediated insulin secretion through the activation of L-type voltage-dependent Ca2+ channels (VDCCs; MacDonald et al., 2005). Conversely, if glucose concentrations decrease the channel opens, hyperpolarizing the membrane to prevent insulin release. In an analogous situation, KATP channels composed of Kir6.2 and SUR1 are involved in glucose sensing and glucagon secretion in the ventromedial hypothalamic neurons of the hypothalamus (Miki et al., 2001).
Like many other channels (Hille et al., 2015; Dickson and Hille, 2019), KATP channels containing Kir6.2 pore-forming subunits are also influenced by the concentration of intracellular phosphoinositides, such as phosphoinositol-4,5-bisphosphate (PIP2; Fan and Makielski, 1997). In Kir6.2-containing channels, ATP and PIP2 compete for residues on overlapping binding sites on the pore forming subunit, each subtly altering channel conformation to stabilize closed or open states, respectively (Enkvetchakul and Nichols, 2003), with PIP2 additionally uncoupling the pore-forming subunit from its SUR companion (Li et al., 2017). Exposure of these KATP channels to PIP2 decreases ATP affinity (K0.5) in excess of two orders of magnitude from ~10 μM to ~3.5 mM, and furthermore in the absence of ATP increases channel Po (Shyng and Nichols, 1998). As the abundance of PIP2 thus regulates Po, this raises the possibility that cell signaling that impinges upon PIP2 levels may subsequently affect channel activity. Kir6.1/SUR2B channels, in contrast, appear to have a much higher affinity for PIP2 than Kir6.2 channels. Accordingly, PIP2 is thought to bind so tightly here as to be saturating, and thus physiological fluctuations of this phospholipid do not influence channel activity (Quinn et al., 2003; Harraz et al., 2020). However, a number of intracellular signaling pathways have been established to dramatically influence vascular KATP activity. Indeed, phosphorylation by protein kinase C (PKC), lying downstream of DAG, decreases the Po of Kir6.1/SUR2B channels (Bonev and Nelson, 1996; Shi et al., 2008b) and in stark contrast, protein kinase A (PKA), which is stimulated as a result of Gs-coupled GPCR engagement, phosphorylates KATP to increase Po (Kleppisch and Nelson, 1995; Bonev and Nelson, 1996; Quinn et al., 2004; Shi et al., 2007, 2008a).
Accordingly, there appear to be two major possible avenues through which vascular KATP channels could be engaged in pericytes:
i) Changes in metabolism may couple KATP channel activity to membrane hyperpolarization.
It is possible that brain pericyte KATP channels act as sensors of the metabolic state of the cell and adjust membrane potential in response to perturbations in energy supply. Notably, the expression of the glucose transporter GLUT1 is incredibly high in astrocytes and brain ECs compared to pericytes, which express much lower levels of GLUTs 1, 3 and 4 (He et al., 2018; Vanlandewijck et al., 2018). Therefore, while astrocytes and capillary endothelial cells are well equipped for glucose import, the comparatively lower expression of GLUTs in the pericytes situated between them could make them more sensitive to subtle changes in glucose levels, such as local depletions that occur during neural activity (Hu and Wilson, 1997; Paulson et al., 2010; Li and Freeman, 2015; Pearson-Leary and McNay, 2016). Such decreases in glucose could impact pericyte metabolism, increasing the ADP:ATP ratio to open KATP channels and hyperpolarize the membrane.
However, as glucose can be transmitted via gap junctions (Rouach et al., 2008) it is possible that pericyte glucose needs are instead satisfied directly by the underlying ECs, enabling them to continually maintain a high level of cytosolic ATP. This latter possibility, coupled with evidence that metabolic regulation of vascular KATP channels in arteriolar SMCs requires either anoxia or extreme ATP consumption (Quayle et al., 2006)—circumstances of energetic compromise that are unlikely to be seen under physiological conditions (Quayle et al., 1997)—suggests that KATP metabolism-electrical coupling may be primarily relevant in pathological situations (e.g., stroke). In this context, metabo-electrical coupling may represent a last-ditch effort to stimulate blood flow and therefore replenish O2 and glucose to regions in deep metabolic crisis. Further studies are needed to understand metabolic contributions to the control of pericyte KATP channels.
ii) Molecules that stimulate Gs signaling may engage pericyte KATP channels.
Pericytes express a broad repertoire of receptors that couple to the Gs signaling pathway, including those for purines, polyadenylate cyclase activating peptide (PACAP), parathyroid hormone (PTH) and prostaglandins (discussed in detail below, see Table 2). The release of these molecules into the paravascular space during neuronal activity could thus engage Gs signaling in local pericytes, culminating in the phosphorylation of KATP and channel opening. Indeed, in the retina (often used as a model of the NVU; see Box 2) the inhibitory neurotransmitter and metabolic byproduct adenosine hyperpolarizes the rat retinal pericyte membrane potential by ~30 mV through KATP channel engagement resulting from A1 and A2a adenosine receptor activation (Li and Puro, 2001), likely through engagement of cAMP and PKA.
Box 2. A brief comparison of retinal and brain vasculatures.
The retinal vasculature consists of two vascular beds—the outer layer of retinal photoreceptors is nourished by the choroidal vasculature, and the multilayered inner retinal vasculature provides oxygen and nutrients to the inner cell layers. The latter has a tightly regulated blood-retinal barrier, akin to the BBB, which pericytes help to maintain (Trost et al., 2016). Vascular density in the cerebral cortex varies according to the metabolic demand of the brain region it supplies (e.g., white vs. gray matter), whereas in the retina, capillary density tends to be greater in the center of the tissue and decreases toward the periphery (Patton et al., 2005). Both retinal and cerebral vascular cells have identical embryological origins: pericytes and SMCs derive from neuroectodermal neural crest cells and ECs derive from mesodermal hemangioblasts (Kurz, 2009; Dyer and Patterson, 2010). Structurally, the cortical and inner retinal vascular beds share a similar overall architecture, with a post-arteriolar transitional zone of 3–4 branches that are covered by contractile mural cells, leading to thin strand pericyte-covered deep capillaries (Ratelade et al., 2020). A distinction between these vascular beds is that the retinal vasculature is highly organized into two parallel plexi (Ramos et al., 2013), whereas cerebral capillaries form more elaborate three-dimensional geometries (Blinder et al., 2013). These structural differences could dictate differences in the flow of blood through each circulation and may necessitate distinctions in the signaling mechanisms that are utilized to direct blood flow through either bed. However, the vasculatures in both retina and cortex respond similarly to neuronal activity with elevations in blood flow (Newman, 2013), and similar mechanisms underpinning these responses appear to be at play in either bed. K+, PGE2, and EETs, for example, have been implicated in control of blood flow in both circulations (Newman, 2013; Longden et al., 2017; Gonzales et al., 2020). Recent studies have also indicated the utility of non-invasive examinations of the retinal vasculature as a marker for detecting cerebrovascular diseases, due to a similar susceptibility of both circulations to vascular risk factors such as hypertension or diabetes (Patton et al., 2005; van de Kreeke et al., 2018; McGrory et al., 2019; Querques et al., 2019). Data on gene expression in vascular cells of the retina are currently lacking, but would provide a useful standpoint for deeper comparisons of the similarities and differences between these vascular beds.
Studies on retinal pericytes (Li and Puro, 2001; Kawamura et al., 2002, 2003; Wu et al., 2003; Matsushita and Puro, 2006), on cerebral pericytes (Peppiatt et al., 2006; Fernández-Klett et al., 2010; Hill et al., 2015; Rungta et al., 2018), or both (Gonzales et al., 2020; Kovacs-Oller et al., 2020) have thus informed our current understanding of blood flow control and pericyte physiology. Although it is clear that a high degree of similarity exists between these vascular beds, the possibility of yet-to-be-identified differences between these networks should be borne in mind when attempting to draw generalizations from data from both vascular beds. To this end, we note explicitly where data on pericytes in this review were drawn from studies performed in retina.
What would be the physiological consequence of such profound membrane hyperpolarization in pericytes? It has been proposed that KATP-generated hyperpolarization of pericytes in the retinal vasculature could be transmitted over long distances to close VDCCs in the mural cells of upstream vessels, thereby causing vasorelaxation and an increase in blood flow (Ishizaki et al., 2009). Such a mechanism could be enabled by transmission of hyperpolarizing signals either between pericytes themselves, or between pericytes and ECs. Indeed, hyperpolarizations transmitted to cECs are predicted to engage Kir2.1 channels, which we have recently shown to rapidly propagate hyperpolarizing signals over long distances through the brain endothelium to upstream arterioles, causing their dilation and an increase in blood flow (Longden and Nelson, 2015; Longden et al., 2017). A similar mechanism involving both KATP and Kir2.1 channels has also recently been shown to be critical for control of blood flow in the heart (Zhao et al., 2020). In the brain, connexin (Cx)37, and Cx45 are highly expressed in pericytes (He et al., 2018; Vanlandewijck et al., 2018; see Box 1), and thus these likely form cell-cell GJs that facilitate long-range transmission of KATP-mediated electrical signals (Figure 6).
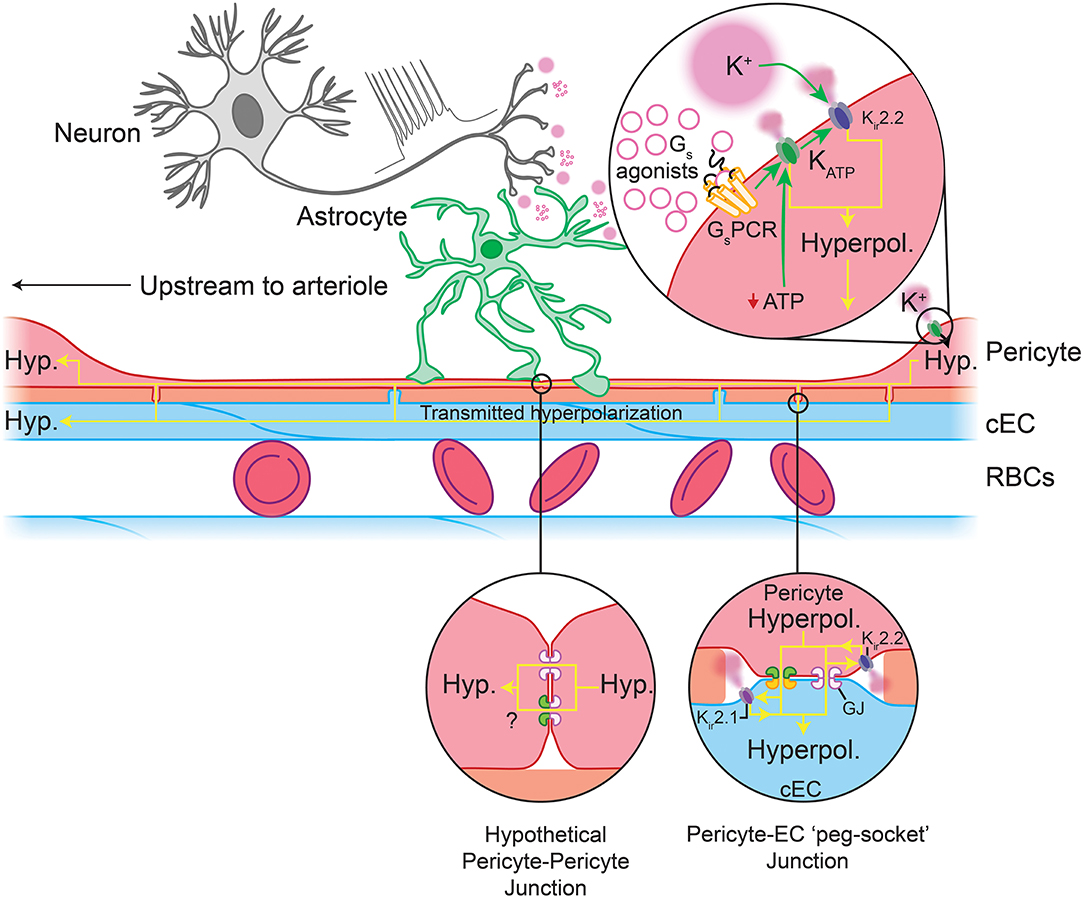
Figure 6. Predicted capillary pericyte-EC interactions to control local blood flow. Neuronal activity drives the release of K+ and GsPCR agonists. Top inset: These are predicted to engage pericyte Kir2.2 and their cognate GPCRs, respectively. GsPCR activity activates KATP channels, the hyperpolarization by which may feed forward to evoke further Kir2.2 activity (a sufficient fall in ATP:ADP would also engage KATP channels). The hyperpolarization generated by these channels may then be passed via gap junctions to cECs (bottom right inset) or possibly to adjacent pericytes, though direct pericyte-pericyte gap junctions have not been observed to date. In cECs, the incoming hyperpolarization will engage Kir2.1 channels to amplify hyperpolarization to a sufficient level to pass to adjacent cECs and pericytes. Hyperpolarization-mediated activation of Kir2.1 and Kir2.2 in these cells will rapidly regenerate the current so that it can be passed to the next cell, and so on upstream to the arteriole. Upon arrival at the arteriole and its first few offshoots, hyperpolarization will be passed via GJs at MEPs to SMCs and to contractile mural cells, which will close VDCCs, leading to a fall in intracellular Ca2+, relaxation of their actin-myosin contractile machinery, vasodilation, and an increase in blood flow.
Kir2 channels are activated not only by membrane hyperpolarization, but also by external K+, which is an important mediator of NVC (Filosa et al., 2006; Longden and Nelson, 2015; Longden et al., 2017). Neurons or astrocytes release K+ into the perivascular space during NVC, and its concentration can reach ~10 mM during concerted activity (Orkand et al., 1966; Newman, 1986; Ballanyi et al., 1996; Kofuji and Newman, 2004). Interestingly, Kir2.2 channels are expressed in pericytes (Table 1 and Figure 5B) and Kir currents with the expected biophysical characteristics and sensitivity to micromolar barium (Ba2+) have been reported in cultured retinal and heart pericytes (von Beckerath et al., 2000; Quignard et al., 2003), and retinal and kidney pericytes from microvessels (Cao et al., 2006; Matsushita and Puro, 2006). Strong rectification in Kir2 channels results from intracellular polyamine and Mg2+ block of the channel pore at depolarized membrane potentials, limiting outward current. This block is relieved by elevating external K+ to levels that are typically seen during neuronal activity, initiating rapid and self-perpetuating hyperpolarization that drives Vm toward EK (Longden and Nelson, 2015). Thus, pericyte Kir2.2 channels could contribute to transmitted hyperpolarizations in several ways. On one hand, K+ elevations resulting from neural activity may directly activate Kir2.2 channels on pericytes (Figure 6). Alternatively, engagement of pericyte KATP channels could cause a K+ or hyperpolarization-mediated recruitment of Kir2.2 channels, which would serve to amplify hyperpolarization. Kir2.2 channels could then propagate hyperpolarizing signals from capillary pericytes to upstream vessels by means of pericyte-pericyte communication through their thin-strand processes or by passing hyperpolarization to neighboring ECs via pericyte-endothelial GJs. PIP2 is also central to Kir2 channel function (D'Avanzo et al., 2010; Hansen et al., 2011), and its depletion via GqPCR signaling has recently been shown to play an important role in regulating Kir2.1 channel activity in cECs (Harraz et al., 2018). Accordingly, signaling processes that influence PIP2 levels are anticipated to factor in to Kir2.2 channel activity in pericytes.
Collectively, genetic and functional data to date argue for an important role of KATP and Kir2.2 channels in regulating pericyte electrical activity, and we thus propose that the activity of these channels plays a central role in the control of capillary blood flow (Figure 6).
Kv channels are formed by 4 identical subunits that surround a central pore. Each subunit is composed of six transmembrane segments (S1–S6) of which four form the voltage sensor domain (S1–S4) with several regularly spaced positively-charged amino acids in the S4 helix playing a central role in transducing voltage into conformational changes that gate the channel. The remaining two transmembrane regions line the K+-selective pore (S5–S6; Figure 5C; Jiang et al., 2003; Chen et al., 2010).
In order of mRNA abundance, cerebral pericytes express modest to low levels of genes encoding: Kv6.1, Kv7.4, Kv2.1, Kv9.3, Kv9.1, Kv7.5, and Kv1.2, in the absence of Kv beta subunits (Table 1). Outward K+ currents attributable to Kv channels have been measured in these cells, for example in guinea pig cochlear stria vascularis and cultured bovine retinal pericytes (von Beckerath et al., 2000; Quignard et al., 2003; Liu et al., 2018). Kv channels are crucial for negative feedback regulation of Vm, their Po and unitary currents increasing with membrane depolarization to provide a counterbalancing hyperpolarizing influence (Nelson and Quayle, 1995; Koide et al., 2018). Their activity can also be modulated by a range of intracellular signaling cascades that engage varied effectors such as PKC, c-SRC or Rho-kinase (which inhibit Kv channels) or cAMP-PKA and cyclic guanosine monophosphate(cGMP)-protein kinase G (PKG) signaling pathways (which promote channel activity) (Jackson, 2018). Of note, nitric oxide (NO) can exert major signaling effects via soluble guanylate cyclase (sGC) and cGMP-PKG in pericytes (Denninger and Marletta, 1999). As adjacent cECs are a major source of local NO (Longden et al., 2019), its elevation may be sufficient to engage pericyte PKG signaling to promote activity of KV and other PKG-sensitive channels.
Cerebral arteriolar SMCs are each estimated to express ~3,000 Kv channels/cell (Dabertrand et al., 2015) composed principally of Kv1.2 and Kv1.5 (Straub et al., 2009) with activation initially detectable above −40 mV and increasing e-fold per 11-13 mV, exhibiting half-activation between approximately −10 and 0 mV (Robertson and Nelson, 1994; Straub et al., 2009). These channels also exhibit substantial steady-state inactivation over the physiological voltage range (Robertson and Nelson, 1994). Kv currents with similar characteristics have been described in cultured retinal pericytes (Quignard et al., 2003), whereas the half-maximal activation of Kv channels recorded in cultured coronary pericytes is substantially more negative at −40.9 mV, along with a steeper voltage-dependence of activation (e-fold per 4.6 mV) and only modest inactivation at physiological membrane potentials (von Beckerath et al., 2000). Thus, Kv current characteristics in pericytes appear to be regionally dependent, likely a result of differential expression and assembly of distinct Kv isoforms. Direct characterization of Kv currents in native brain pericytes is therefore critical to furthering our understanding of their role in the control of pericyte Vm, where these channels are anticipated to provide negative feedback to limit depolarization effected by the activity of depolarizing ion channels in pericytes, such as those of the TRP family.
K2P channels contribute to maintenance of resting membrane potential due to steady outward K+ “leak” at potentials positive to EK. They comprise a family of 15 members, and are composed of two identical subunits, each with four transmembrane domains with two pore-forming loops making up a central K+-conducting pore (Figure 5D; Miller and Long, 2012; Lolicato et al., 2014). K2P3.1, also known as the two-pore domain weakly inwardly-rectifying K+ channel (TWIK)-related acid-sensitive K+ (TASK)-1 channel (Duprat et al., 1997), is the only K2P isoform expressed in capillary pericytes, and is also expressed in cerebral SMCs (He et al., 2018; Vanlandewijck et al., 2018). In SMCs, its steady current contributes to maintaining a relatively negative Vm by counterbalancing depolarizing influences (Gurney et al., 2003).
Perhaps the most well-studied characteristic of TASK-1 is its sensitivity to pH within the range of ~6.5–8. Acidic pH inhibits channel activity while alkaline pH increases it, with half-maximal activation occurring at pH 7.4 and ~90% of maximal TASK-1 current recorded at pH 7.7 (Duprat et al., 1997). Synchronous neuronal activity can cause rapid changes in pH. For example, alkalization in extracellular pH has been observed in the hippocampus, cerebellum and some cortical areas, by up to 0.2 units (Chesler and Kaila, 1992; Makani and Chesler, 2010). Thus, it is possible that in addition to setting resting Vm, K2P3.1 imparts sensitivity to pericytes in these regions to such shifts, which could hyperpolarize Vm to modulate blood flow through the mechanisms described above.
Capillary pericytes also express low levels of genes encoding the Na+-activated KNa1.2 channel and the Ca2+-activated KCa2.3 channel (Table 1). KNa1.2 channels (Figure 5E) are sensitive to intracellular Na+ and Cl−, and are dramatically stimulated by cell swelling and inhibited by a decrease in cell volume (Bhattacharjee et al., 2003; Tejada et al., 2014). Thus, they could impart sensitivity to pericyte volume changes, and may respond to fluctuations in intracellular ion concentrations or metabolic state.
KCa2.3 (also known as SK3) belongs to the family of small-conductance Ca2+-activated K+ (SK) channels that share overall transmembrane topology with Kv channels, yet lack a functional voltage-sensor at S4 (Figure 5F; Adelman et al., 2012). Each subunit in the tetrameric channel is associated with a calmodulin (CaM) monomer via a CaM binding domain in the C-terminal region. Ca2+ binding to CaM induces a conformational change which leads to rapid channel opening, with an EC50 for Ca2+ of 300–500 nM (Ledoux et al., 2006; Adelman et al., 2012). If functional SK channels in native pericytes are confirmed, they are expected to facilitate coupling between Ca2+ elevations and membrane hyperpolarization.
The TRP channel family mediates cellular responses to a wide range of stimuli (Clapham, 2003). These are non-selective cation channels that depolarize the membrane upon activation and, in many cases, conduct significant amounts of Ca2+. In mammals there are six subfamilies of TRP channels encoded by 28 genes, 11 of which are expressed by capillary pericytes. These are canonical (TRPC1, TRPC3, TRPC4, TRPC6), melastatin (TRPM3, TRPM4, TRPM7), mucolipin (TRPML1), poly-cystin (TRPP1, TRPP3), and vanilloid (TRPV2) channels (Earley and Brayden, 2010; He et al., 2018; Vanlandewijck et al., 2018). Functional TRP channels are tetramers of subunits with a common six transmembrane structure, which can assemble into homomeric or heteromeric functional channels. Their tendency to heteromerize, generally with closely related members, can give rise to channels with unique sensing capabilities and biophysical properties (Venkatachalam and Montell, 2007). Overall, subfamily members share ~35% amino acid sequence homology, with the majority of this diversity arising from differences in their cytoplasmic domains (Figure 7; Clapham, 2003; Nilius and Owsianik, 2011). While they have been traditionally described as “non-selective,” the pattern of ion selectivity for different cations varies between subfamilies (Hill-Eubanks et al., 2014; see Table 1).
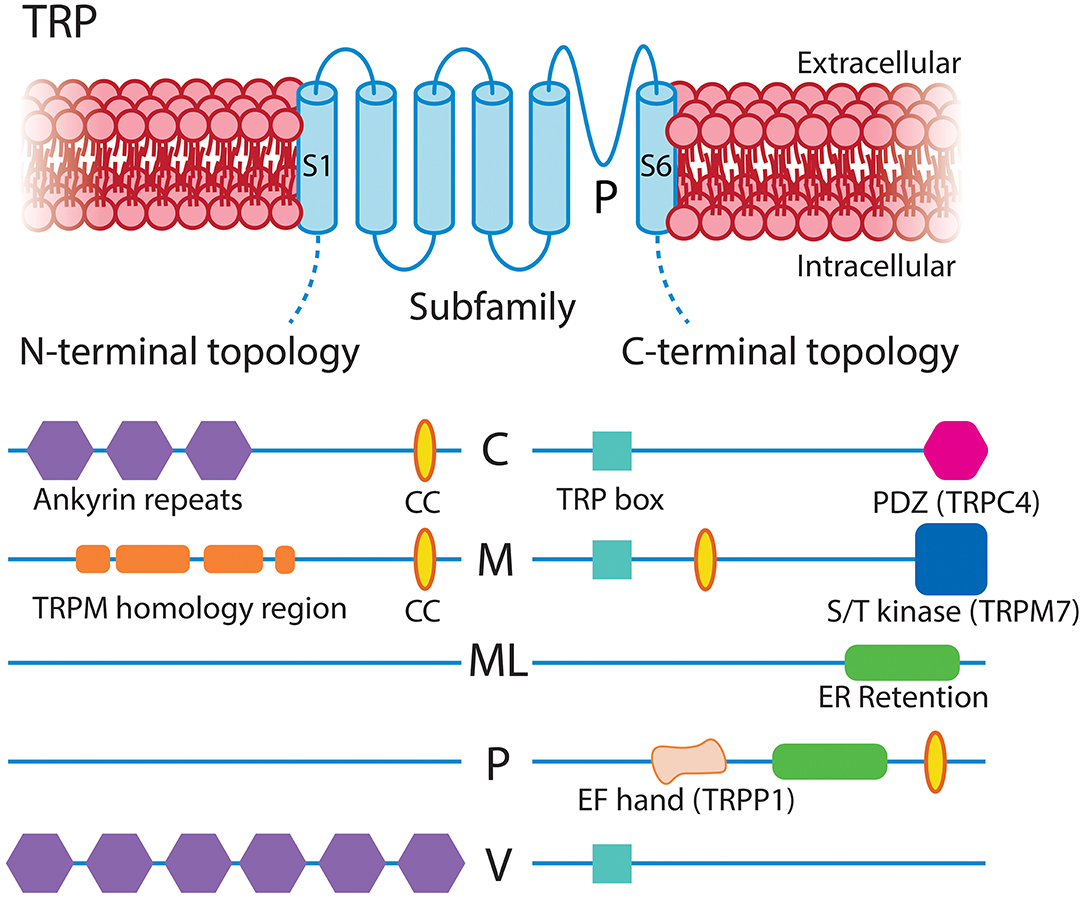
Figure 7. Structural overview of the TRP families expressed in CNS capillary pericytes, adapted with permission from Clapham (2003). All TRP channels share a common and typical 6-transmembrane structure with profoundly varying intracellular N- and C-terminal domains, the major features of which are illustrated. CC, coiled-coil domain.
Broadly speaking, TRP channels are major downstream effectors for GPCR signaling (Clapham, 2003; Veldhuis et al., 2015), with particular second messenger systems both activating or sensitizing some TRP channels, and decreasing the activity of others. TRPC channels are Ca2+ permeable and typically activated by plasmalemmal GPCRs or tyrosine kinase receptors that activate PLC isoforms (Albert, 2011). TRPC3/6 channels are directly activated by DAG, which is liberated by Gq signaling, and inhibited by PIP2, which decreases during Gq activity (Hofmann et al., 1999; Albert, 2011). The activation mechanisms of TRPC4 are less clear, whereas TRPC1-containing channels are unresponsive to DAG and are instead gated by PIP2 in a PKC-dependent manner (Hofmann et al., 1999; Albert, 2011), although heteromultimerization with TRPC3 can convey DAG sensitivity (Lintschinger et al., 2000). TRPC3 is the most robustly expressed TRP channel in capillary pericytes (Table 1) and is thus likely to be engaged during GqPCR-DAG signaling. This channel permits robust Ca2+ entry, although it has relatively low selectivity for Ca2+ over Na+ (pCa2+:pNa+ ~1.5; Pedersen et al., 2005). At the arteriolar level, TRPC3 has been implicated in mediating vasodilation through elevations of EC Ca2+ leading to KCa2.3 activation (Kochukov et al., 2014), whereas its activation in SMCs mediates arteriolar constriction through a mechanism involving an IP3R-activated (sarcoplasmic reticulum (SR) Ca2+ release independent) TRPC3-dependent Na+ current that depolarizes Vm and activates VDCCs (Xi et al., 2009). Similar couplings may occur in capillary pericytes, likely depending on the macromolecular organization of TRPC3 with other local signaling elements.
Members of the TRPC subfamily, in particular TRPC1, have also been suggested to participate in store-operated Ca2+ entry (SOCE)—an event activated by the depletion of endoplasmic reticulum (ER) Ca2+ stores that depends on Orai1 and the ER-Ca2+ status sensing protein stromal interaction molecule 1 (STIM1; Huang et al., 2006; Soboloff et al., 2006; Cheng et al., 2008, 2013). Capillary pericytes express STIM1 and Orai1 and 3 (Table 1), and thus a functional interaction between TRPC1 and these proteins could be important for SOCE in pericytes. Recent work also shows TRPM7 activation, although not essential, can positively modulate SOCE (Souza Bomfim et al., 2020).
The melastatin channel TRPM4 is unique in its exclusive permeability to monovalent cations. Na+ currents through TRPM4 are voltage-dependent and activated by intracellular Ca2+ (EC50 ~20 μM) with the Ca2+ sensitivity of the channel regulated by multiple factors including cytosolic ATP, PKC-dependent phosphorylation and calmodulin (Nilius et al., 2005; Ullrich et al., 2005). In cerebral SMCs, membrane stretch indirectly activates TRPM4 (and TRPC6) current through angiotensin II AT1 receptor activation and a resultant IP3-mediated Ca2+ elevation (Gonzales et al., 2014). Pericytes also express the AT1 receptor, and thus a similar mechanism may be present in capillary pericytes which could contribute to the mild, slow constrictions these cells are capable of Fernández-Klett et al. (2010). In contrast to the monovalent conductance of TRPM4, the closely related TRPM3 and TRPM7 channels are also permeable to Ca2+ and Mg2+ (Pedersen et al., 2005). TRPM3 is activated by cell swelling, the neurosteroid pregnenolone sulfate, and the metabolite D-erythro-sphingosine and related sphingosine analogs and thus may impart sensitivity to steroid and lipid signals to pericytes (Grimm et al., 2005; Wagner et al., 2008). As pericytes also robustly express the S1P3 receptor (discussed below), it is likely that TRPM3 and S1P3 respond in concert to locally released lipids, such as those released constitutively by ECs and RBCs (Selim et al., 2011; Ksiazek et al., 2015). TRPM7, in contrast, is ubiquitously expressed and plays a major role in Mg2+ homeostasis (Schlingmann et al., 2007).
Functional TRPP1 channels (encoded by the Pkd2 gene) have a large conductance and conduct a significant amount of Ca2+ (Earley and Brayden, 2015). This channel has been implicated in mechanosensation when expressed alongside polycystic kidney disease (PKD)1 (Giamarchi and Delmas, 2007; Sharif-Naeini et al., 2009; Narayanan et al., 2013). As PKD1 is also present in pericytes, these channels may aid in the detection of local mechanical forces, such as paravascular fluid shear from the glymphatic system (Mestre et al., 2018), or those imparted through the very thin endothelium by changes in blood flow during neuronal activity, or through subtle changes in diameter of the underlying capillary. Similarly, the vanilloid family member TRPV2, also expressed in SMCs throughout the vasculature (Muraki et al., 2003), has been suggested to play a role in mechanosensation-evoked Ca2+ entry (Perálvarez-Marín et al., 2013). Continuing this theme, mechanosensory contributions have also been reported for TRPC1, TRPC6, and TRPM4 (Yin and Kuebler, 2010). Combined with the fact that pericytes also express Piezo1 (see below), this represents a broad mechanosensing repertoire, suggesting that pericytes may be exquisitely sensitive to a range of mechanical perturbations. The resultant Ca2+ elevation and depolarizing currents through the activity of these channels could couple to a number of processes, including driving further Ca2+ release from stores, and activation of VDCCs, KCa2.3 channels, or Ca2+-activated Cl− channels (CaCCs; discussed below). As recent work demonstrates that pericytes can subtly influence tone throughout the capillary bed (Fernández-Klett et al., 2010), mechanosensing and Ca2+-mediated mechanisms may play an important role in influencing this process.
The overall expression level of Ca2+ channels is similar to that of TRP channels in pericytes, composed of message for IP3R subtypes and a range of VDCCs.
The vast majority of intracellular Ca2+ signals arise from either Ca2+ influx across the plasmalemma, or release from the SR/ER via IP3Rs or ryanodine receptors (RyRs). IP3Rs are enormous proteins (~1.3 MDa) formed by four IP3R subunits. Three subunit isoforms—IP3R1-3—exist, which are able to homo- or heterotetramize. Each individual subunit has six transmembrane segments: The fifth and sixth segments form a central ion-conducting pore that is connected via a linker to the peripheral bundle formed by transmembrane domains 1-4. The large cytoplasmic N-terminal domain contains the IP3 binding site and a putative Ca2+ sensor region, and binding of IP3 and Ca2+ leads to conformational changes which are transmitted to the pore to gate the channel (Figure 8; Fan et al., 2015; Baker et al., 2017; Hamada et al., 2017). IP3R subtypes share ~70% homology and differ in their affinity for IP3, with IP3R2 being more sensitive than IP3R1, and both of these subtypes being more sensitive than IP3R3 (Tu et al., 2005; Iwai et al., 2007). Brain capillary pericytes express the genes encoding IP3Rs 1 and 2 robustly, and a much lower level of IP3R3, whereas RyRs are not appreciably expressed by these cells (He et al., 2018; Vanlandewijck et al., 2018; Table 1).
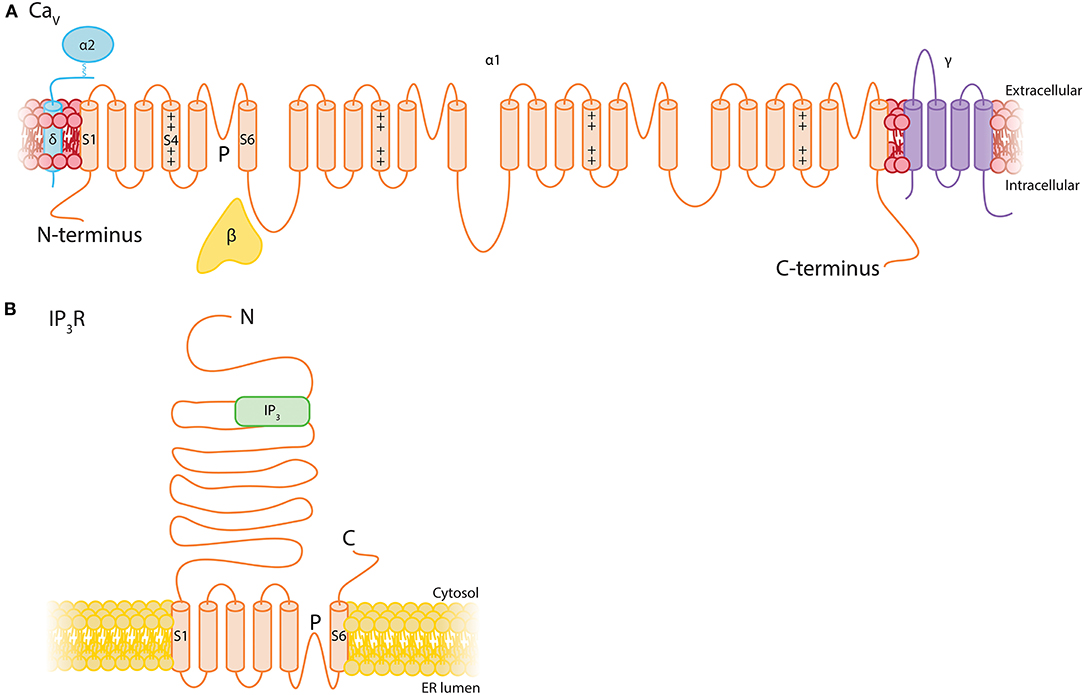
Figure 8. Structural topology of Ca2+ channels expressed by pericytes. (A) The general structure of Cav channels consists of a single 24-transmembrane α subunit which is a repeat of a 6-transmembrane motif with an embedded voltage sensor connected by intracellular loops. This is accompanied by associated β, γ, and α2δ subunits. (B) IP3Rs consist of a tetrameric assembly of 6- transmembrane subunits with a large N-terminal domain that contains the IP3 binding site.
As described briefly above, GqPCRs activating phospholipase Cβ (PLCβ) (Fisher et al., 2020), or receptor tyrosine kinases (RTKs) activating PLCγ, can mediate the formation of IP3 and DAG from PIP2. IP3 then binds to IP3Rs on the ER membrane, leading to Ca2+ release from the ER lumen (where Ca2+ is maintained between 100 and 800 μM; Burdakov et al., 2005) down its electrochemical gradient into the cytosol (<100 nM basal Ca2+; Berridge, 2016). IP3 and Ca2+ act as co-agonists at IP3Rs (Bezprozvanny et al., 1991; Finch et al., 1991; Foskett et al., 2007) and channels display a biphasic sensitivity to Ca2+, resulting in a characteristic bell-shaped concentration-response curve. In the presence of very low IP3 levels, IP3Rs are extremely sensitive to Ca2+ inhibition. However, a small increase in IP3 concentration (to ~100 nM) profoundly reduces the sensitivity of the channel to Ca2+ inhibition, permitting dramatic increases in activity (Iino, 1990; Bezprozvanny et al., 1991; Finch et al., 1991; Foskett et al., 2007).
The resultant release of stored Ca2+ can take on a broad range of spatiotemporal profiles, which depend on many factors. To name just a few, these include the concentration of local IP3 and Ca2+, ER Ca2+ load, the type, and number of IP3Rs expressed, their splice variation, whether they are homomers or heteromers, and the topology of the local microenvironment. Such intricacies provide the versatility to potentially generate a huge variety of Ca2+ signals that encode information through their amplitudes, durations, frequencies, and spatial characteristics (Bootman and Bultynck, 2020). Despite these inherent complexities, a range of stereotyped IP3R-mediated Ca2+ signals typically emerge. These range from the opening of single IP3R (termed a “blip”), to the coordinated, weakly cooperative openings of a cluster of around 6 IP3Rs within a release site (a “puff”), to finally—with sufficient IP3–a long-range regenerative Ca2+ “wave” arising due to the recruitment of successive sites through the process of Ca2+-induced Ca2+ release (CICR) (Berridge et al., 2000; Smith and Parker, 2009; Lock and Parker, 2020).
Store-mediated Ca2+ release has been observed in pericytes in a range of contexts. For example, pericytes of the ureter display long-duration IP3R-mediated Ca2+ transients in response to the GqPCR agonists endothelin-1 and arginine vasopressin. These signals are suppressed by elevations of Ca2+ in adjacent cECs, which are suggested to inhibit IP3R activity through a NO-dependent mechanism (Borysova et al., 2013). Spontaneous ER Ca2+ release-dependent Ca2+ transients have also been observed in suburothelial capillary pericytes, which activate CaCCs to depolarize the membrane, subsequently recruiting VDCCs (Hashitani et al., 2018).
In the brain, recent studies have revealed that capillary pericytes generate microdomain Ca2+ oscillations under ambient conditions, and that neural activity evoked by odor leads to a transient cessation of these signals and a decrease in basal Ca2+, which correlates with an increase in RBC velocity (Hill et al., 2015; Rungta et al., 2018). However, it is worthy of note that a decrease was not observed in similar experiments in which whisker stimulation was used to drive activity (Hill et al., 2015), suggesting the possibility of heterogeneity in the Ca2+ signaling machinery deployed by pericytes in different regions of the cortex. The specific ion channels and broader mechanisms that underlie these ambient signals have not yet been delineated, but IP3Rs are obvious potential candidates. Elucidation of the mechanistic basis and roles of these Ca2+ signals in brain capillaries is critical, and awaits further experimentation.
VDCCs are composed of four to five distinct subunits (α1, β, α2δ, and γ; Figure 7). The α1 subunits are pore forming and responsible for the pharmacological diversity of different VDCC subtypes. These are associated with an intracellular β subunit, a disulphide-linked α2δ subunit, and in some cases a transmembrane γ subunit, each of which regulate surface expression and tune the biophysical properties of the channel (Catterall et al., 2005). The large α1 subunit is organized into four homologous domains, each comprising six transmembrane segments (S1-S6) with intracellular N- and C- termini. Similar to Kv channels, the S4 segment of each of these domains comprises the voltage sensor and the S5-S6 regions form the ion conducting pore (Catterall et al., 2005). Capillary pericytes express genes encoding the α subunits for L-type (Cav1.2, Cav1.3), P/Q-type (Cav2.1), and T-type (Cav3.1, Cav3.2) channels and thus we briefly review the salient properties of these here. They also express low levels of several genes encoding β and α2δ auxiliary subunits (He et al., 2018; Vanlandewijck et al., 2018).
As with Kv channels, VDCC activity depends on membrane potential: Po steeply increases with depolarization, balanced by multiple feedback mechanisms that act to limit Ca2+ entry at depolarized potentials. Prominent among these are voltage- and Ca2+-dependent inactivation. Voltage-dependent inactivation (VDI) is inherent to the α1 subunit but is modulated by the ancillary β subunit and others, whereas Ca2+-dependent inactivation (CDI) is conferred by a CaM monomer associated with the α1 carboxy tail (Peterson et al., 1999; An and Zamponi, 2005; Dick et al., 2008; Tadross and Yue, 2010; Tadross et al., 2010). Regulation is additionally complicated by the panoply of alternative splice variants that can be expressed, which impact the biophysical properties of the functional channel, including sensitivity to CDI and VDI.
L-type channels are widely expressed, including in the heart, in skeletal and smooth muscle, and in neurons (Zamponi et al., 2015). Cav1.2 and Cav1.3 have distinct biophysical and pharmacological differences (Lipscombe et al., 2004)—Cav1.3 channels open and close on faster timescales than Cav1.2 (Helton et al., 2005), and are less sensitive to inhibition by dihydropyridines (Xu and Lipscombe, 2001). A C-terminal modulatory (CTM) domain can structurally interfere with CaM binding to decrease Po and reduce CDI, an effect that is more pronounced in Cav1.3 than Cav1.2 (Striessnig et al., 2014). Moreover, in alternatively spliced Cav1.3 channels, the absence of a CTM domain can shift the voltage of half-maximal activation by ~+10 mV by decreasing the slope factor of the activation curve without any effects on activation threshold (Singh et al., 2008). At physiological extracellular Ca2+ levels, the activation threshold of Cav1.3 is much more negative (-55 mV) than Cav1.2 (-25 to −30 mV) (Xu and Lipscombe, 2001). Thus, at pericyte resting Vm of around −45 mV, as measured in the retina (Zhang et al., 2011), Cav1.3 channels could be active and contribute to Ca2+ entry.
In addition to voltage- and Ca2+-dependent inhibition, L-type VDCC activity is heavily regulated by GPCR signaling. Prominent among these, Gs-cAMP-PKA signaling has long been known to play an important role in stimulating channel activity, and has been studied extensively in the heart. Here, it was recently shown that the target of PKA phosphorylation is not the core channel itself, as mutation of all PKA consensus phosphorylation sites to alanine resulted in channels that retained PKA regulation. Rather, PKA acts via the small G protein Rad, a constitutive inhibitor of VDCCs. Phosphorylation of Rad relieves its interaction with β subunits, and allows channel activity (Liu et al., 2020). Further regulation of L-type channels by PKC, stimulated by DAG liberated as a result of GqPCR activity, is also a possibility, with both inhibitory and potentiating effects having been observed (Kamp and Hell, 2000).
P- and Q-type currents are both attributable to Cav2.1, with the β subunit accompanying the pore-forming subunit thought to account for their differences (Zamponi et al., 2015). These channels have been best characterized in the nerve terminals and dendrites of neurons where they couple Ca2+ entry with neurotransmitter release (Zamponi et al., 2015) and also play a role in coupling Ca2+ entry to gene transcription via engagement of CaM kinase II (Wheeler et al., 2012). They open in response to similar depolarization levels as Cav1.2 channels, with an activation threshold of approximately −40 mV (Adams et al., 2009). Upon repetitive/tetanic stimulation, as occurs during neuronal activity, CaM can bind to two adjacent sites on the Cav2.1 α1 subunit to mediate an initial Ca2+-dependent facilitation (CDF) of P/Q-type current, followed by progressive CDI, with a relatively slow (30 s−1 min) recovery from this (Lee et al., 1999, 2000). While CDI of Cav2.1 requires a global Ca2+ increase, CDF can be promoted by Ca2+ entry through an individual Cav2.1 channel and results in an enhancement of channel Po, enabling stimulation-evoked increases in amplitude and duration of Ca2+ currents (Chaudhuri et al., 2007). Slow and fast modes of Cav2.1 gating have been proposed. The slow mode exhibits longer mean closed times and latency to first opening, slower kinetics of inactivation, and necessitates larger depolarizations to open the channel. Inactivation also occurs at more depolarized potentials in the slow compared to fast mode (Luvisetto et al., 2004). The type of β subunit modulates the prevalence of these modes, with fast and slow gating mediated by β3a and β4a subunits, respectively (Luvisetto et al., 2004), the latter of which is expressed more robustly by brain pericytes (He et al., 2018; Vanlandewijck et al., 2018). Cav2.1 channels are inhibited by GPCR activity through several distinct mechanisms—direct binding of the G protein βγ dimer can augment VDI, while voltage-independent mechanisms such as phosphorylation, depletion of essential lipids, and trafficking mechanisms also play important roles (Zamponi and Currie, 2013).
T-type (Cav3.1 and Cav3.2) channels are activated at more negative potentials, around −60 mV, with rapid gating kinetics and small single channel amplitudes (Iftinca and Zamponi, 2009; Rossier, 2016). At membrane potentials of −65 to −55 mV, these channels exhibit window currents in which the channels open but do not inactivate completely, permitting ongoing Ca2+ entry (Perez-Reyes, 2003). These channels can be modulated by the activity of a broad range of GPCRs, including those with Gα subunits that couple to PKA, PKC, and PKG, along with direct effects of Gβγ subunits (Iftinca and Zamponi, 2009).
Both L- and T-type VDCCs are expressed in cerebral SMCs (Hill-Eubanks et al., 2011; Harraz and Welsh, 2013; Harraz et al., 2014). Here, L-type channels provide Ca2+ for contraction (Nelson et al., 1990), whereas T-type channels provide negative feedback by coupling Ca2+ entry to RyR activity. Subsequent Ca2+ release via RyRs in turn activates large-conductance Ca2+-activated K+ (BK) channels to hyperpolarize the membrane (Harraz and Welsh, 2013; Harraz et al., 2014). T- and P/Q-type channel currents have not yet been observed in native pericytes, but L-type VDCC currents have been measured in the retina (Sakagami et al., 1999). Variance in the magnitude of L-type VDCC Ca2+ currents across the microvascular network has functional consequences for the degree of Ca2+ entry via these channels (Matsushita et al., 2010; Burdyga and Borysova, 2014). In the retina, L-type VDCC currents are 7.5-fold higher in SMCs as compared to capillary pericytes, suggesting that Vm changes influence intracellular Ca2+ levels to a greater degree at the level of arterioles (Matsushita et al., 2010). Indeed, extracellular K+ at 10 mM (a concentration that evokes Kir-mediated hyperpolarization) and 97.5 mM (which depolarizes the membrane to drive VDCC activity) significantly decreased and increased intracellular Ca2+ in arteriolar SMCs, respectively, but had only a marginal effect on capillary pericyte Ca2+ (Matsushita et al., 2010). Thorough characterization of native brain capillary pericyte VDCC currents and their densities is needed to advance our understanding of the contribution of these channels to pericyte Ca2+ handling.
Cl− channels are found in the plasma membrane and that of intracellular organelles and have been implicated in the regulation of cell excitability and volume, acidification of intracellular organelles, control of muscle tone, and synaptic transmission (Jentsch et al., 1999; Nilius and Droogmans, 2003). While they are permeable to other anions (such as iodide, bromide, or nitrate), they are referred to as Cl− channels since this is the most abundant permeating anion species (Jentsch et al., 2002). Capillary pericytes express the CaCC formerly known as TMEM16A or anoctamin (Ano)1, and several members of the voltage-dependent chloride channel (ClC) family—ClC-2,−3,−4,−6, and−7 (He et al., 2018; Vanlandewijck et al., 2018). The latter four of these are Cl−/H+ antiporters and are not considered further here. Capillary pericytes also express other anoctamins that have been implicated in lipid scrambling: Ano4 and Ano6, as well as the poorly understood Ano10 (He et al., 2018; Vanlandewijck et al., 2018). Reports indicate that Ano6 may act as a Ca2+-activated Cl− and non-selective cation channel with scramblase activity (Suzuki et al., 2010; Yang et al., 2012; Grubb et al., 2013) and Ano4 was recently shown to be a Ca2+-dependent non-specific cation channel with similar scrambling capabilities (Reichhart et al., 2019).
The CaCC TMEM16A is a homodimer of two pores and ten transmembrane domains, cytosolic N- and C-termini, and an extracellular domain (Dang et al., 2017; Paulino et al., 2017). Ca2+ binding to a transmembrane region of the pore induces a conformational rearrangement that gates the channel and leads to Cl− permeation, generating a current that is outwardly rectifying with a slope conductance of ~8 pS (Yang et al., 2008; Xiao et al., 2011; Paulino et al., 2017). Ca2+ and voltage gating are closely coupled, with a stretch of 8 amino acids controlling both Ca2+ sensitivity and voltage-dependence of the channel (Xiao et al., 2011). Indeed, a remarkable feature of this channel is the voltage-dependence of Ca2+ sensitivity, with an EC50 of 2.6 μM at −60 mV and 400 nM at +60 mV. At physiological voltages, the channel is maximally activated by around 10 μM intracellular Ca2+ but concentrations exceeding this lower activation. Strong depolarization (above ~100 mV), in contrast, opens the channel even in the absence of Ca2+, despite the lack of a classic voltage sensor in the CaCC structure (Yang et al., 2008; Xiao et al., 2011). The kinetics of activation are slow at positive potentials, but are sharpened by an elevation of Ca2+, and at negative potentials channels display deactivation (Nilius and Droogmans, 2003). This interplay between Vm and intracellular Ca2+ makes the CaCC an attractive candidate for regulation of Vm in response to elevations intracellular Ca2+.
Since CaCC is sensitive to micromolar-range intracellular Ca2+ at typical resting potentials, it seems plausible that it is stimulated by local Ca2+ elevations (as opposed to global increases) such as those occurring through nearby TRPs, VDCCs, Orai channels, or IP3Rs. In keeping with this notion, cerebral SMC CaCCs are activated by TRPC6-mediated Ca2+ entry which drives vasoconstriction (Wang et al., 2016). Coupling of IP3R activity to CaCCs has also been reported in response to purinergic receptor activation, wherein CaCC-containing membrane domains are closely localized with ER regions via a physical linkage between this protein and IP3R1, facilitating exclusive communication between the two and exposing the CaCC to high Ca2+ concentrations during its release from the ER (Jin et al., 2013; Cabrita et al., 2017).
Underscoring their important role in the vasculature, targeted disruption of CaCCs from contractile vascular SMCs, mural cells and pericytes lowers systemic blood pressure (Heinze et al., 2014), whereas conversely CaCC overexpression drives hypertension (Wang et al., 2015). In vascular SMCs, the driving force for depolarizing Cl− currents comes from Cl−/HCO3− exchange and Na+/K+/Cl− cotransport which enable high intracellular Cl− concentrations (30–50 mM; Owen, 1984; Chipperfield and Harper, 2000; Kitamura and Yamazaki, 2001). Capillary pericytes in the brain express mRNA for genes encoding two of the SLC4 family Cl−/HCO3− exchangers (Slc4a2, Slc4a3) and the NKCC1 Na+/K+/Cl− cotransporter (Slc12a2) (He et al., 2018; Vanlandewijck et al., 2018), which raise the potential for similarly high intracellular Cl− concentrations. ECl with 30–50 mM intracellular Cl− and 133 mM extracellular Cl− (Longden et al., 2016) is between approximately −35 and −25 mV—more positive than resting Vm of pericytes (~-45 mV, as measured in the retina; Zhang et al., 2011), therefore under these conditions activation of CaCC would cause Cl− efflux and membrane depolarization, as seen in SMCs (Kitamura and Yamazaki, 2001; Bulley and Jaggar, 2014). While direct evidence for CaCCs in cortical capillary pericytes is currently lacking, in bladder pericytes ER Ca2+ release activates CaCCs and the resulting depolarization propagates to upstream SMCs of pre-capillary arterioles via gap junctions, where they depolarize the membrane to activate L-type VDCCs (Hashitani et al., 2018). In the pericytes of descending vasa recta, angiotensin II causes cytoplasmic Ca2+ oscillations that activate CaCC channels and depolarize Vm to approximately −30 mV (Zhang et al., 2008; Lin et al., 2010). CaCC current and membrane depolarization have also been recorded in retinal pericytes, where CaCC activation depends on unidentified non-selective cation channels (Sakagami et al., 1999) and can be evoked by GqPCR stimulation with endothelin (Kawamura et al., 2002). Thus, CaCCs in brain pericytes are predicted to depolarize Vm by coupling to a number of potential Ca2+ sources, including IP3Rs and TRP channels.
ClCs are double-barreled homodimeric channels with one ion conduction pore per monomer (Dutzler et al., 2002). Each subunit is made up of 18 α-helices which display an interesting internal anti-parallel architecture, and many of these helices are shortened and tilted which permits disparate parts of the polypeptide to come together to form the Cl− selectivity filter of the pore (Dutzler et al., 2002). The C-terminus also contains two cystathione-β-synthase domains, which regulate gating by binding ATP and ADP to decelerate the kinetics of activation and deactivation (Estévez et al., 2004; Stölting et al., 2013). ClC-2 has a unitary conductance of 2-3 pS and displays strong inward rectification. A remarkable biophysical characteristic of this channel is its slow hyperpolarization-mediated activation at potentials negative to around−40 mV, giving rise to currents that are only very slowly inactivating (Nilius and Droogmans, 2003; Bi et al., 2014). In addition to its hyperpolarization activation, it is sensitive to changes in cell volume and extracellular pH and is also activated by PKA (Nilius and Droogmans, 2003; Bi et al., 2014). As we have suggested previously for hyperpolarizing electrical signals generated in cECs, ClC-2 is an attractive candidate for mediating membrane repolarization (Garcia and Longden, 2020), in that its slow activation kinetics would enable Kir-mediated electrical signals to be generated and sent upstream before ClC-2 mediated Cl− current fully develops to repolarize the membrane. Accordingly, ClC-2 may fulfill a similar role in pericytes to initiate membrane repolarization in the wake of electrical signals generated by KATP and Kir channels.
Capillary pericytes express an array of other ion channels, including the ubiquitous two-pore channels (TPCs), voltage-gated Na+ (Nav) channels, P2X receptors, and acid-sensing ion channels (ASICs; Table 1 and Figure 4). Due to their lower expression and dearth of functional data in capillary pericytes, detailed discussion of these channels is beyond the scope of this review, although we touch briefly upon the function of Piezo1 channels and P2X receptors.
The ubiquitous purine ATP has received attention as a putative gliotransmitter (Pelligrino et al., 2011) and acts as an endogenous agonist at P2Y GPCRs and the cation-selective ionotropic P2X receptors, permeable to Na+, K+, and Ca2+ (Khakh et al., 2001). P2X receptors are trimmers consisting of intracellular N- and C-termini, a large extracellular domain containing the ATP binding site, and two transmembrane segments that line an integral ion pore (Kawate et al., 2009). Capillary pericytes express mRNA for P2X1 and P2X4 receptors (Table 1), which have a pCa2+/pNa+ of ~5 and 4.2, respectively (Khakh et al., 2001). Thus, pericyte P2X receptors could function as sensors transducing ATP released into the local environment into Ca2+ elevations. Several studies have also suggested P2X7 receptors are functionally expressed in cultured human and freshly isolated rat retinal pericytes (Kawamura et al., 2003; Sugiyama et al., 2005; Platania et al., 2017), though it should be noted that our expression data do not unambiguously support the expression of this P2X isoform in CNS pericytes.
Piezo1 is a large (2,521 amino acids in humans) mechanosensitive cation channel, with three identical subunits, thought to have 38 transmembrane segments, that form a central ion conduction pore with surrounding peripheral domains shaped like propeller blades (Coste et al., 2010; Zhao et al., 2016, 2018; Wu et al., 2017). Functional channels have a single channel conductance of 29 pS and a current that rapidly activates and then decays on a millisecond timescale (Coste et al., 2010, 2015; Zhao et al., 2018). In ECs, piezo1 can be activated by fluid shear stress, and has been implicated in blood flow regulation, vascular development and remodeling, and permeability (Li et al., 2014; Ranade et al., 2014; Friedrich et al., 2019). Piezo1 may play similar roles in capillary pericytes to mechanosensitive TRP channels in detecting changes in blood flow, vessel diameter, or paravascular fluid shear stress.
Pericytes express a huge variety of GPCRs (Table 2 and Figure 4) enabling them to transduce a vast array of extracellular stimuli into intracellular responses. As outlined above, many of the signaling pathways triggered by GPCR signaling impinge upon ion channel activity and thus regulate pericyte Vm and intracellular Ca2+.
Assessment of the general characteristics of the list of GPCRs expressed by pericytes is revealing. The majority of pericyte GPCRs primarily interact with Gi/o α subunits. This is closely followed by Gq-coupled GPCRs, then those that are Gs-coupled, and the remainder couple primarily to G12/13. Perhaps tellingly, expression of the Gnas gene, encoding the Gs α subunit, is ~5 times higher than those collectively encoding Gq/11 α subunits, more than double that of Gi/o α subunit genes, and more than 12 times in excess of G12/13 genes (He et al., 2018; Vanlandewijck et al., 2018). Thus, while a wider variety of pericyte receptors may couple to depolarizing, Ca2+-elevating processes, it appears that hyperpolarizing Gs signaling may be a favored intracellular transduction pathway.
Around 12% of the receptor subtypes expressed by pericytes are promiscuous/pleiotropic in their G-protein coupling, the degree of which will depend on the expression levels of the signaling elements involved and their local densities and organization within GPCR signaling platforms. One such example is the highly-expressed A2A adenosine receptor which couples primarily to Gs, but also interacts with Gq and others (Olah, 1997; Fresco et al., 2004). Such promiscuity could represent an inbuilt feedback mechanism to prevent Vm being locked at hyperpolarized potentials by K+ channel activity, by facilitating recruitment of additional transduction pathways to promote repolarization. In contrast, the promiscuity in signaling exhibited among receptors that couple to Gq, Gi/o, and G12/13 would serve to reinforce depolarization. For example, the highly expressed S1P3 and PAR1 receptors frequently exhibit coupling to not just Gi/o, but also to both Gq and G12/13 α subunits (Tobo et al., 2007; Means and Brown, 2009; Yue et al., 2012).
At the time of writing, a significant portion of GPCRs expressed by pericytes (Table 2) remain orphan receptors with little functional data available. Strikingly, one such orphan, GPRC5C, is the 4th most robustly expressed GPCR in these cells. Given this lack of data, we omit this group from further discussion.
The GPCR family represents the largest family of mammalian proteins (Lagerström and Schiöth, 2008; Katritch et al., 2014) sharing a common 7-transmembrane topology with an extracellular N-terminus and intracellular C-terminus. G-protein heterotrimers are organized into four principal categories based on the similarity of function and homology in their α subunits: Gs, Gi/o, Gq/11, and G12/13 (Simon et al., 1991; Dupré et al., 2009). Broadly, the roles of these Gα subunits are to stimulate/inhibit production of cAMP by adenylate cyclase (AC; Gs and Gi/o, respectively), to activate PLC (Gq/11), and to activate Rho guanine nucleotide exchange factors (GEFs) (G12/13) (Hanlon and Andrew, 2015). The Gβγ subunit also activates downstream signaling elements and plays a role in GPCR mediated intracellular signaling (Dupré et al., 2009). Below, we outline how signaling through these pathways may modulate the activity of pericyte ion channel activity and consequently Vm and Ca2+ signaling, and we explore what the GPCRs expressed by pericytes might be able to tell us about NVC mechanisms.
In pericytes, Gs stimulation and subsequent PKA engagement is likely to drive phosphorylation of a number of ion channel targets including KATP, a range of TRP channels, VDCCs, and IP3Rs—modulating their activity and thus Vm and cellular behavior (Figure 9). GsPCR activation leads to association of the Gαs subunit with a cleft in the C2 domain of AC, catalyzing the conversion of ATP to cAMP (Sadana and Dessauer, 2009). cAMP then activates PKA by binding to its two regulatory subunits, inducing the dissociation of two catalytic subunits, enabling their subsequent phosphorylation of downstream targets (Sassone-Corsi, 2012). In contrast, Gi/o activation inhibits AC, opposing GsPCR activity. Here, Gαi/o binds to the C1 domain of AC to inhibit enzymatic activity, although this is limited to the AC-I, -V, and -VI isoforms (Sadana and Dessauer, 2009).
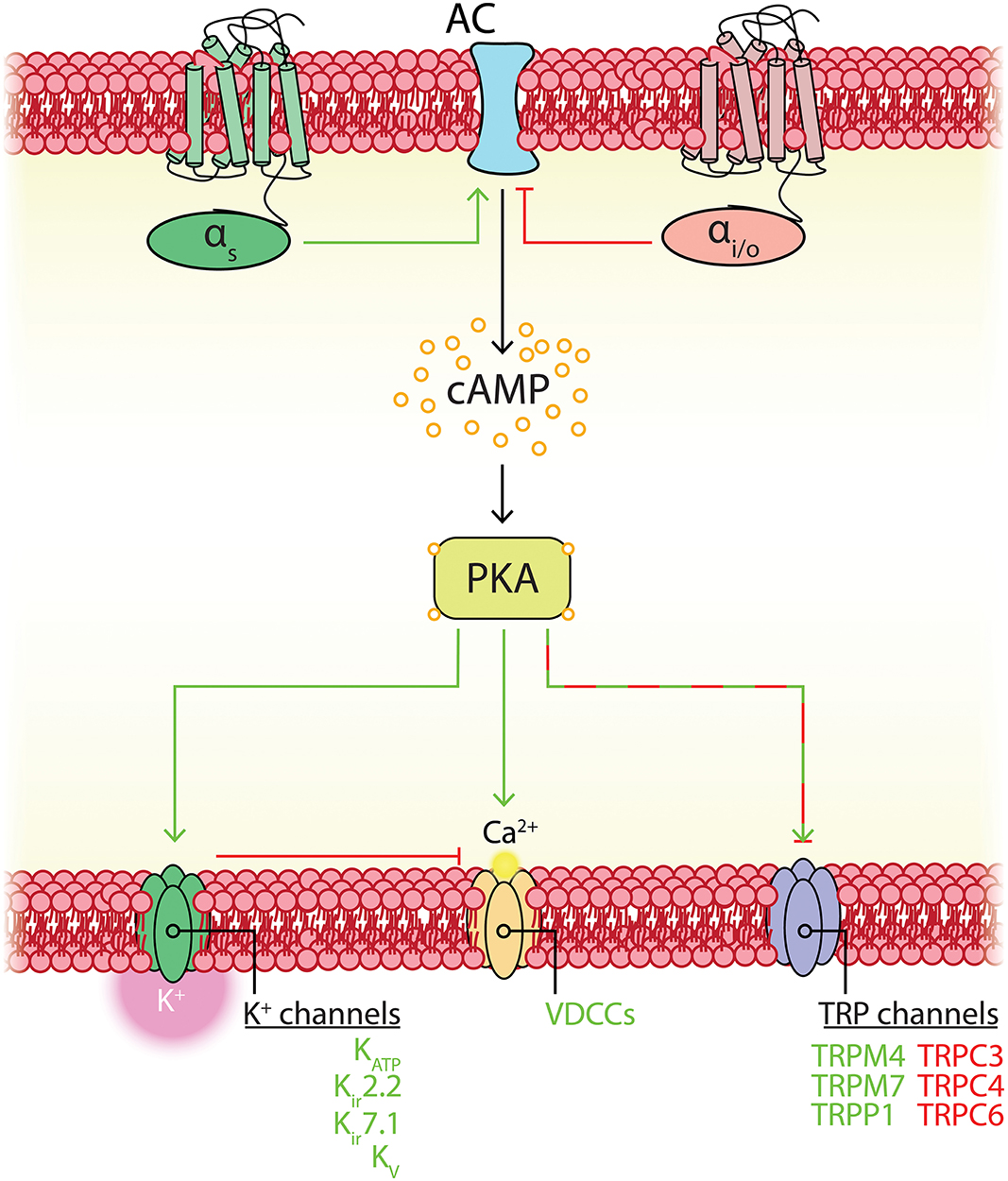
Figure 9. Potential Gs- and Gi/o-coupled GPCR–ion channel interactions in capillary pericytes. GsPCR activation promotes (green) adenylate cyclase (AC) activity, whereas Gi/oPCR activation inhibits (red) AC. AC in turn generates cAMP from ATP, which stimulates PKA activity. PKA interacts with a broad range of ion channels. In pericytes, its activity is expected to couple to plasma membrane K+ and VDCC activity, with mixed effects on TRP channel activity. K+ channel hyperpolarization will oppose VDCC activity and thus the overall effect of Gs stimulation is membrane hyperpolarization.
Kir channels are likely key determinants of pericyte Vm, and as noted previously KATP channel activity is bidirectionally modulated by cAMP levels. At tonic, low concentrations of cAMP, PKA increases vascular KATP channel activity by phosphorylating multiple sites on the pore-forming and regulatory subunits (Quinn et al., 2004; Shi et al., 2007, 2008b). At higher concentrations, cAMP conversely inhibits KATP channel activity in a Ca2+-dependent manner via engagement of the ubiquitous exchange protein activated by cAMP (Epac)-1 (Purves et al., 2009). PKA is preferentially activated by cAMP over Epac1, exhibiting a 30-fold lower EC50 (~1 vs. 30 μM; Purves et al., 2009). Accordingly, it seems that Gs activity will preferentially favor membrane hyperpolarization through KATP engagement. Consistent with this, activation of Gs-coupled adenosine receptors leads to a dramatic increase in retinal pericyte K+ currents (Li and Puro, 2001). High-level accumulation of cAMP might in turn be expected to act as an inbuilt concentration-based feedback mechanism to inhibit the channel through Epac1 engagement.
In addition to such concentration-dependent regulation of channel activity, spatial considerations are important in determining the functional outcome of cAMP elevations. The assembly of ACs and phosphodiesterases into membrane-bound scaffolds organized around A-kinase anchoring proteins (AKAPs) has been suggested to facilitate the generation of microdomains of cAMP (Arora et al., 2013; Lefkimmiatis and Zaccolo, 2014). Such compartmentalization may facilitate specific, local adjustment of, for example, KATP channel activity in a select part of the cell (e.g., a thin-strand process or around a peg-socket junction in the case of pericytes) without impacting ion channels in other regions.
Complementary to the activation by PKA that KATP channels exhibit, Kir2.2 is also positively regulated by PKA (Zitron et al., 2004). Moreover, several Kv isoforms expressed by pericytes exhibit PKA sensitivity, in that the activity of Kv7.4/7.5 heteromers or Kv7.5 homomers is potentiated by PKA activation (Mani et al., 2016). Kv2.1 membrane trafficking is also controlled by a PKA-dependent mechanism (Wu et al., 2015). Collectively, these data suggest a key stimulatory role for Gs-cAMP-PKA signaling in the regulation of pericyte K+ channels, along with potential negative feedback mechanisms to prevent over-activation.
TRP channels are extensively regulated by Gs activity, and in contrast to K+ channels this typically leads to a decrease in activity. Focusing on the TRP isoforms expressed by pericytes, TRPC3, TRPC4, TRPC6, and TRPML1 are all inhibited by PKA phosphorylation (Vergarajauregui et al., 2008; Nishioka et al., 2011; Sung et al., 2011). In contrast, TRPM4 exhibits activation as a result of Gs stimulation in an Epac1-and IP3R-mediated Ca2+ release-dependent manner (Mironov and Skorova, 2011), and TRPM7 can also be potentiated by PKA (Takezawa et al., 2004). Phosphorylation of TRPP1 by PKA also increases channel Po (Cantero del Rocío et al., 2015).
Thus, regulation of TRP channels via PKA is complex but it appears that this will to lean toward PKA-dependent inhibition of currents in pericytes. This reinforces the notion that engagement of PKA will shift the balance of ion channel activity to favor membrane hyperpolarization via K+ channel activity, while reducing Na+ and Ca2+ influx via TRP channels.
As noted, augmentation of Cav1.2 is primarily dependent on PKA phosphorylation of Rad to relieve channel inhibition (Liu et al., 2020). PKA phosphoregulation of Cav1.2 is also dependent on the AKAP isoform present in the macromolecular environment of the channel: AKAP15 permits sensitization of the channel whereas calcineurin associated with AKAP79 suppresses PKA-mediated increases in Cav1.2 activity via dephosphorylation (Fuller et al., 2014). scRNAseq data (He et al., 2018; Vanlandewijck et al., 2018) indicate that pericytes express AKAP79 at low levels whilst expressing high levels of AKAP15, suggesting Gs-stimulation in pericytes will favor increases in Cav1.2 channel activity. Along similar lines, an increase in PKA activity induces sensitization of Cav1.3 (Mahapatra et al., 2012), and Cav3.1 currents are augmented in a cAMP/PKA-dependent manner (Li et al., 2012). Moreover, the current of Cav3.2 is increased by cAMP, an effect that depends upon AKAP79/150, and its gene expression is also up-regulated by Gs-signaling, suggesting a mechanism for long term T-type VDCC regulation (Liu et al., 2010; Sekiguchi and Kawabata, 2013). Accordingly, PKA activity should increase VDCC channel activity but, due to its voltage-dependence, in the broader context of the pericyte ion channel repertoire this must be weighed against simultaneous increases in activity of multiple K+ channels which will hyperpolarize Vm and keep VDCCs closed.
IP3Rs also possess phosphorylation sites for PKA (Ferris et al., 1991a; Vanderheyden et al., 2009) and can also be directly influenced by cAMP (Tovey et al., 2010), allowing for direct crosstalk between cAMP and Ca2+ release pathways. Indeed, phosphorylation by PKA induces an increase in sensitivity of the receptor for IP3, promoting IP3-induced Ca2+ release, while Epac1 activation also potentiates Ca2+ release (Vanderheyden et al., 2009; Mironov and Skorova, 2011).
Drawing all of these threads together, the complement of PKA targets and their relative expression levels in pericytes suggests that the Gs-coupled receptors here likely primarily transduce stimuli into Vm hyperpolarization, but may in some cases also elevate intracellular Ca2+ via release from stores.
Pericytes express a range of receptors that couple to Gs–of particular note are the adenosine A2A receptor, the PACAP receptor, PAC1, the prostacyclin IP receptor and the PTH-type 1 receptor (PTHR1). The expression of these suggests the possibility that their endogenous agonists could be released onto pericytes during neuronal activity to evoke membrane hyperpolarization and electrical signaling to increase blood flow (Figure 6).
The vasodilatory effects of adenosine, an abundant metabolic byproduct, have long been appreciated (Drury and Szent-Györgyi, 1929). In the brain, adenosine is released into the extracellular space by widely-expressed nucleoside transporters, or more commonly accumulates through the extracellular catabolism of ATP by ectonucleotidases (Cunha, 2016). Recent in vivo work showing a reliable correlation between extracellular adenosine accumulation and rapid increases in local O2 suggest that adenosine is capable of acting as a neurovascular coupling mediator (Wang and Venton, 2017), and clear links have been established between sensory stimulation, adenosine receptor engagement, and increases in cerebral blood flow (Ko et al., 1990; Dirnagl et al., 1994). The precise cellular and molecular mechanisms underlying this linkage remain to be determined, and actions through pericyte adenosine receptors are a strong candidate for mediating these effects.
Considering prostanoids also, blockade of Gs-coupled IP receptors impairs neuronal activity-evoked vasodilation (Lacroix et al., 2015), which suggests a role for the classic vasodilator prostacyclin—produced in the same metabolic pathway as PGE2—in NVC. This possibility remains little explored, but the expression of IP receptors in pericytes provides a potential target for capillary endothelium-generated prostacyclin.
PACAP is a 27- or 38-amino acid neuropeptide that is an extremely potent vasodilatory agent (Koide et al., 2014). PACAP polypeptides are produced throughout the brain where they act as neurotransmitters and also have neurotrophic effects. These peptides are released by both neurons and astrocytes during activity and thus PACAP accumulation in the paravascular space could feasibly activate pericyte Gs-coupled PAC1 receptors (Johnson et al., 2020), warranting further exploration of their potential involvement in NVC.
Finally, PTHR1 binds the endocrine ligand PTH and the paracrine ligand PTH-related protein-1 (PTHrP-1) (Vilardaga et al., 2011). Intriguingly, PTH binding to PTHR1 triggers sustained and prolonged cAMP production by retaining the intact ligand-receptor complex even after endocytosis (Ferrandon et al., 2009). This could have important implications for pericyte Gs signaling if PTH is released during neuronal activity.
The purinergic family P2Y14 receptor is the most robustly expressed GPCR in pericytes. This receptor signals through Gi/o and is activated by uridine diphosphate (UDP) and nucleotide sugars—most potently by UDP-glucose (Harden et al., 2010). UDP-glucose is synthesized from glucose and acts as a glucose donor in the synthesis of glycogen, which is present at modest levels in the brain (Leloir et al., 1959; Breckenridge and Crawford, 1960; Öz et al., 2015). This and related nucleotide sugars also act as donors for glycosylation in the ER lumen and Golgi apparatus (Berninsone and Hirschberg, 1998), and as a consequence these molecules are thought to be released under basal and simulated conditions from a broad range of cells, primarily through vesicular transport accompanying glycoconjugate delivery to the cell membrane (Harden et al., 2010; Lazarowski, 2012). The released nucleotide sugars have been hypothesized to act in an autocrine or paracrine manner on local P2Y14 receptors (Lazarowski and Harden, 2015), and as the hydrolyzation of UDP-glucose is three times slower than that of ATP, this has been suggested to result in long-duration signaling (Lazarowski, 2006). As its synthesis is dependent on glucose, we speculate that UDP-glucose signaling through P2Y14 may function to notify pericytes of local energy substrate availability: in conditions of ample glucose, UDP-glucose maintains activity of P2Y14, which through Gi/o signaling would counterbalance cAMP generation and prevent PKA activation of KATP and other K+ channels. In the event that glucose levels fall, such as during neuronal activity (Hu and Wilson, 1997; Paulson et al., 2010; Li and Freeman, 2015; Pearson-Leary and McNay, 2016) or in situations of metabolic stress, the loss of this negative feedback could be relieved, leading to cAMP elevations and engagement of KATP and other K+ channels to increase blood flow and replenish local glucose.
The Gi/o-coupled metabotropic glutamate receptors mGluR3 and mGluR7 are both localized in presynaptic terminals of GABAergic and glutamatergic synapses, and mGluR3 is also found in glia (Harrison et al., 2008; Palazzo et al., 2016). Like other mGluRs, these receptors contain a large N-terminal venus flytrap domain with a glutamate binding site that dimerizes with that of neighboring mGluRs. mGluR7 has a comparatively low affinity for glutamate and is thus activated only by its accumulation at high extracellular concentrations, but is also activated by elevations of intracellular Ca2+ through CaM interactions with its C-terminal tail. In neurons activity of these receptors exerts a hyperpolarizing influence that depresses synaptic activity through the lowering of cAMP, activation of G protein-coupled Kir (GIRK) channels and the inhibition of VDCCs (Niswender and Conn, 2010). Pericytes do not express GIRKs, but they do express a range of VDCCs (Table 1). Thus, although the physiological roles of mGluRs in pericytes remain to be ascertained, their expression here implies that any glutamate elevations in the vicinity of pericytes could drive cAMP inhibition via mGluR3 and mGluR7 activation, and a reduction in Ca2+ entry via VDCCs.
Activation of the Gq α subunit stimulates phospholipase C (PLC), which mediates the conversion of membrane phospholipids to DAG and IP3, inducing PKC activation and Ca2+ release, respectively, which may affect a broad range of ion channels (Figure 10). We focus below on the ramifications of PKC signaling.
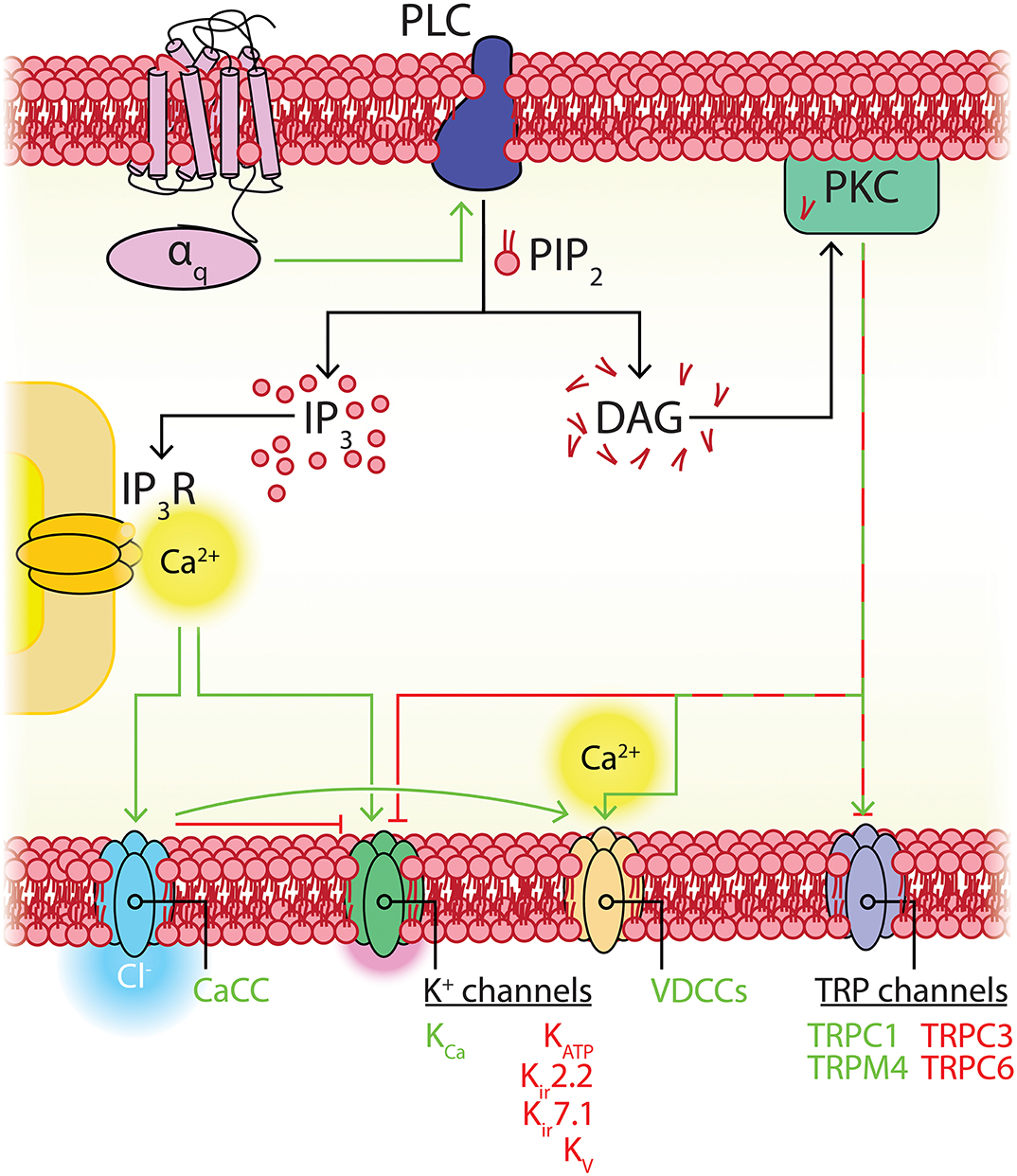
Figure 10. Potential GqPCR-ion channel interactions in capillary pericytes. GqPCR activation engages PLC, leading to the hydrolysis of PIP2 into IP3 and DAG. IP3 evokes Ca2+ release from the ER via resident IP3Rs, which may engage CaCCs and KCa channels. DAG stimulates PKC which has mixed effects on the TRP channels expressed by pericytes, promotes VDCC activity, and inhibits KATP, Kir, and Kv channels. The net effect of engagement of GqPCRs is thus membrane depolarization and intracellular Ca2+ elevation.
Activated PKC phosphorylates a diverse range of ion channels and is thus capable of exerting considerable influence on Vm. PKCs are divided into three subfamilies depending on their activation requirements: conventional PKCs require DAG, Ca2+ and a phospholipid for activation; novel PKCs require DAG but are independent of Ca2+; atypical PKCs require neither of these (Newton, 2010). CNS capillary pericytes express PKC isoforms from each of these subfamilies (Table 3).
Table 3. Expression of PKC isoforms in brain capillary pericytes, and their modes of activation and regulation.
All three IP3R isoforms can be phosphorylated by PKC. PKC phosphorylation of IP3R1 is potentiated by prior phosphorylation by PKA and increases Ca2+ release (Ferris et al., 1991a,b; Vermassen et al., 2004; Vanderheyden et al., 2009). In contrast, IP3R2 and IP3R3 are each inhibited by Ca2+-sensitive, conventional PKCs (Arguin et al., 2007; Caron et al., 2007).
Kir channels are also extensively regulated by PKC, where phosphorylation inhibits Kir6.1-containing KATP channels, contrasting starkly with the stimulatory effects of PKA. This phosphorylation is graded—multiple serine residues (ser-354,−379,−385,−397, and−397 in the Kir6.1 C-terminal domain) can be phosphorylated, and the degree of inhibition is proportional to the number of these sites that receive a phosphoryl group from PKC (Shi et al., 2008b). In pericytes this graded response to PKC for the highly expressed KATP channel could provide a means to fine tune activity, by permitting the degree of local Gq signaling to oppose the stimulatory effects or PKA or ATP depletion. PKC also regulates the membrane density of Kir6.1, in that the PKCε isoform induces internalization of the receptor in a caveolin-dependent manner (Jiao et al., 2008), providing another avenue to decrease KATP channel activity. Likewise, Kir2.2 has multiple sites that inhibit channel current upon phosphorylation by PKC, but the graded PKC phosphorylation observed for Kir6.1 is absent (Kim et al., 2015; Scherer et al., 2016).
TRP channels are subject to complex regulation by Gq activity, with important roles for DAG, detailed above, and PKC. TRPC3 and TRPC6 in particular are inhibited by PKC despite activation by other elements of the Gq signaling cascade (Bousquet et al., 2010; Earley and Brayden, 2015), and TRPC1 is in contrast activated by PKC (Xiao et al., 2017). TRPM4 can be phosphorylated by PKC to sensitize the receptor to Ca2+ (Nilius et al., 2005), which augments Na+ entry in response to subsequent local Ca2+ elevations.
Cav1.2 currents are enhanced by phosphorylation at Ser1928 by PKC isoforms from each subfamily (PKCα, PKCε, and PKCζ), permitting a broad range of conditions to regulate VDCC activity (Yang et al., 2005). As pericytes express members of all three subfamilies of PKC, regulation of Cav1.2 activity may be similarly robust in these cells. Cav1.2 surface expression is also increased within minutes of Gq stimulation via a PKC-dependent increase in channel trafficking to the plasma membrane (Raifman et al., 2017). In contrast, Cav1.3 is negatively regulated by both conventional and atypical PKC isoforms (PKCβ2 and the PKCε, respectively), both of which are expressed in CNS pericytes (Table 3). As for T-type channels, Cav3.1 activity is stimulated by PKC phosphorylation, independently of trafficking (Park et al., 2006), and Cav3.2 is negatively regulated by Ca2+-independent PKCη phosphorylation (Zhang Y. et al., 2018), although PKCη is absent in pericytes.
PKCα also activates CaCCs to promote Cl− efflux, where phosphorylation shifts the EC50 of intracellular Ca2+ from 349 to 63 nM for channel activation at −80 mV (Dutta et al., 2016).
Pulling these threads together, it seems that PKC activation as a result of Gq activity in pericytes will contrast with the effects of Gs-cAMP-PKA signaling by enhancing activity of depolarizing ion channels such as VDCCs, TRP channels, and CaCCs while inhibiting hyperpolarizing channels such as KATP and Kir. Given that Gq activity also induces the release of Ca2+ from intracellular stores via IP3Rs, Ca2+-sensitive PKC activation may act as a further amplification loop to increase the signal:noise ratio of Gq signaling and promote Ca2+ accumulation and depolarization.
The Gq-coupled thromboxane (TP) receptor is well-known to induce vasoconstriction by SMCs (Dorn and Becker, 1993) and contractile mural cells of 1st−4th order vessels (Mishra et al., 2016). The TP receptor's endogenous agonists include a range of eicosanoid lipids that are generated from arachidonic acid (AA), which is initially mobilized from membrane phospholipid pools by the action of Ca2+-dependent phospholipase A2 (PLA2; Balsinde et al., 2002). Subsequently, cyclooxygenase or prostaglandin H2 (PGH2) synthase enzymes convert AA to PGH2, a potent agonist of the TP receptor. Further processing of PGH2 yields thromboxane-A2 (TxA2), a still more potent agonist (Bos et al., 2004; Woodward et al., 2011). Alternatively, AA can be shuttled down a cytochrome P450 ω-hydroxylase pathway to generate the TP agonist 20-HETE (Miyata and Roman, 2005). The contractile influence of 20-HETE has been suggested to play a major role in determining the diameter of cerebral arterioles and thus controlling brain blood flow (Attwell et al., 2010), and the activation of TP receptors has also been suggested to cause mild, slow contractions of capillary pericytes (Fernández-Klett et al., 2010). It is unknown whether pericyte TP receptors are basally active to produce this effect in vivo, but subtle changes in capillary diameter induced by this process could regulate local blood flow over the long term, dependent on the local levels of these agonists.
The ETA receptor shares broad similarities with the TP receptor. Its principal transduction pathway is also Gq—although coupling to other G proteins such as G12/13 has been noted—and similar to the TP receptor, its activation evokes robust SMC contractions (Sokolovsky, 1995; Horinouchi et al., 2013; Davenport et al., 2016). The agonist of the ETA receptor, Endothelin-1, is constitutively released by ECs, SMCs, neurons and astrocytes (Russell and Davenport, 1999; Thorin and Webb, 2010; Freeman et al., 2014). In culture, release of endothelin-1 from ECs has been noted to drive changes in pericyte morphology through reorganization of F-actin and intermediate filaments (Dehouck et al., 1997), suggesting that ECs could regulate their coverage by pericyte processes through ETA signaling. In the context of Alzheimer's disease, aberrant ETA signaling caused by amyloid β accumulation results in capillary constriction by overlying pericytes which may limit oxygen and glucose delivery to the parenchyma (Nortley et al., 2019).
As described above, signaling through these receptors is expected to oppose Gs-cAMP-PKA signaling while promoting membrane depolarization and elevation of Ca2+.
The preceding discussion illustrates that many channels expressed by pericytes are differentially regulated by PKA and PKC phosphorylation, and thus their activity will depend in part on the balance of activity between these pathways. Crosstalk between these pathways also occurs at the level of effectors, in addition to ultimate phosphorylation targets. For example, the Gq and Gi/o pathways oppose the Gs pathway at the level of AC, which can be Ca2+ sensitive and modulated by PKC, dependent on isoform (Chern, 2000). Indeed, the most highly expressed AC isoform in brain pericytes is ACVI (Table 4), which is regulated by PKC, Gi/o, Ca2+, and Gβγ (Chern, 2000; Sadana and Dessauer, 2009). This regulation is mirrored for Gs acting on the Gq pathway, where PKA can directly inhibit the activity of PLC via phosphorylation (Nalli et al., 2014). Accordingly, Gs- and Gq-coupled receptors functionally oppose one another at multiple levels of their transduction pathways, which will help push the membrane potential toward either hyperpolarization or depolarization, respectively.
Another layer of control is provided by regulators of GPCR signaling (RGS)—small proteins that regulate the duration and intensity of GPCR signaling by driving GTPase activity of the Gα subunit and accelerating the hydrolysis of GTP, thereby inactivating their target (Ross and Wilkie, 2000; Kach et al., 2012). Capillary pericytes express high levels of RGS4 and 5 (Bondjers et al., 2003; He et al., 2018; Vanlandewijck et al., 2018) that act as GTPase activating proteins for Gi/o and Gq/11 subunits, while seemingly sparing Gs (Berman et al., 1996; Watson et al., 1996; Hepler et al., 1997; Huang et al., 1997; Cho et al., 2003; Gunaje et al., 2011). Intriguingly, RGS4 is known to be phosphorylated by PKA and PKG, which stimulate its activity, accelerating the deactivation of Gq/11 and inhibiting the hydrolysis of phosphoinositide to IP3 (Huang et al., 2007). Therefore, RGS engagement in pericytes may complement and amplify the hyperpolarizing effects of Gs signaling by stifling the depolarizing influences of Gi/o and Gq/11.
Capillary pericytes express several G12/13-coupled receptors, including a range of lysophospholipid receptors with important roles in lipid signaling, the promiscuous protease activated receptor PAR1, and several orphan receptors (Table 2). G12/13 activation couples to a number of interacting partners including cadherins, AKAPs, non-receptor tyrosine kinases and protein phosphatases, though its interaction with Ras homolog family member A (RhoA) is the best characterized (Worzfeld et al., 2008). In SMCs, RhoA engagement of its downstream effector Rho-associated kinase is known to contribute to a range of receptor-mediated contractile responses (Swärd et al., 2003).
RhoA is also frequently observed to be activated downstream of ion channel engagement, including TRPC6 and TRPM7 channels (Canales et al., 2019) and VDCCs (Fernández-Tenorio et al., 2011). RhoA modulating ion channel activity is less frequently reported, but RhoA may indirectly modulate Vm on slow time scales by promoting the endocytosis and translocation of channels such as Kv1.2, IP3Rs, and TRPC1 (Mehta et al., 2003; Mayor and Pagano, 2007; Stirling et al., 2009) and possibly KATP channels (Foster and Coetzee, 2015). Effects of RhoA on Kir2.1 channel activity have also been reported, although the mechanistic details of this interaction have not been fully clarified (Jones, 2003).
Initially, the Gβγ subunit was viewed as a negative regulator of the Gα subunit, serving to increase signal:noise ratio and specificity of signaling by preventing aberrant Gα activity in the absence of an agonist, but has since been found to be an active effector in its own right (Dupré et al., 2009), and may play important roles in pericyte physiology. Gβγ interacts with a range of canonical effectors (for example PLCβ, AC, GIRKs; Chern, 2000; Smrcka, 2008) along with a growing list of non-canonical effectors such as mitochondrial ATP synthase, a range of nuclear transcription factors, cytoskeletal regulators involved in motility, and constituents of the extracellular signal regulated kinase (ERK) pathway. These interactions implicate Gβγ in signaling roles as diverse as regulation of transcriptional activity, modulation of mRNA processing, control of nuclear import/export, cell motility, and oxidative phosphorylation (Khan et al., 2016). In addition to regulation of AC VI (Sadana and Dessauer, 2009)—the most highly expressed pericyte AC isoform (Table 4)—Gβγ signaling may also exert direct effects on pericyte Vm through activation of Kv7.4 (Stott et al., 2015). In contrast Cav2.1, Cav3.2, and TRPM3 can be inhibited through Gβγ-dependent mechanisms (Hu et al., 2009; Zamponi and Currie, 2013; Alkhatib et al., 2019).
The previously discussed GPCRs are largely selective in their G protein coupling, allowing for precise intracellular signaling in response to a range of stimuli. However, many GPCRs that are highly expressed in pericytes are capable of signaling through multiple G proteins. This may represent pleiotropy—physiological activation of different G proteins in response to differing signals—or promiscuity, i.e., engaging in non-preferred G protein interactions due to high levels of receptor expression or excessive stimulation (Maudsley et al., 2005). Here, we review examples of highly-expressed pericyte GPCRs with a tendency to couple to multiple G proteins.
Sphingosine-1-phosphate (S1P) is a lipid mediator formed through the action of ceramidase on lipids of the plasma membrane (Ksiazek et al., 2015). S1P is constitutively released by erythrocytes and its plasma concentration strongly correlates with hematocrit (Selim et al., 2011; Ksiazek et al., 2015). The transporter-mediated release of S1P from ECs has also been documented (Kerage et al., 2014) along with the export of the enzyme that catalyzes its formation, sphingosine kinase (Ancellin et al., 2002). This leads to S1P signaling in the vasculature, which is particularly important for maintenance of the BBB (Janiurek et al., 2019), vasoconstriction (Salomone et al., 2010), angiogenesis, and regulation of vascular tone at the level of arterioles (Kerage et al., 2014).
Pericytes are ideally positioned to sense the release of S1P from local ECs. The actions of S1P are mediated through a family of receptors that act through Gi/o, Gq, and G12/13 signaling, with S1P2 and the robustly expressed S1P3 coupling to each of these (Means and Brown, 2009). Accordingly, S1P sensed by pericytes is expected to promote PLC engagement, Ca2+ elevations, a fall in cAMP, and depolarization, but further information as to the physiological roles of signaling through these receptors awaits experimental attention. As pericytes are critical for the maintenance of blood-brain barrier tightness (Armulik et al., 2010), it is possible that S1P signaling contributes to this process. S1P signaling also strengthens contact between ECs and pericytes in culture through a mechanism involving the trafficking and activation of the adhesion molecule N-cadherin by ECs (Paik et al., 2004), and it is thus possible that this is mirrored in pericytes to contribute to this interaction and maintain peg-socket junctions.
Protease-activated receptor (PAR) 1 is a member of the PAR family and is stimulated by external proteases such as thrombin and trypsin. The proteolytic action of these enzymes on the extracellular domain of the receptor reveal an N-terminal tethered ligand sequence, exposure of which results in irreversible activity of the receptor that is halted only by its internalization (Soh et al., 2010). PARs are broadly expressed in the neurovascular unit, found in neurons, glia, ECs and SMCs, as well as pericytes. PAR1 couples to Gq, Gi/o, and G12/13, and while the release or activation of agonists for these receptors is typically associated with injury or inflammatory responses (Ma and Dorling, 2012; Yue et al., 2012), they have also been implicated in cell proliferation and differentiation, synaptic plasticity (Noorbakhsh et al., 2003), and driving vasodilation (Villari et al., 2017). Interestingly, thrombin signaling regulates morphology of fine processes in astrocytes through RhoA, and similar effects have been noted in neurons (Noorbakhsh et al., 2003). In line with this, it is possible that PAR1 signaling regulates the dynamics of pericyte process extension and retraction on capillaries (Berthiaume et al., 2018).
Finally, pericytes also express a range of members of the frizzled family of GPCRs. These are receptors for Wnt proteins, and G-protein coupling is of less importance in this group. Instead, canonical frizzled signaling occurs through the β-catenin pathway (MacDonald et al., 2009), but G protein coupling through signaling platforms assembled around the FZD-associated phosphoprotein Disheveled is also possible. The latter facilitates activation of Gq- and Gi/o-proteins to produce Ca2+ elevations and PKC engagement (Schulte, 2010; Kilander et al., 2014). Further research is required to infer the functional implications of pericyte expression of frizzled receptors, but developmental and homeostatic roles seem likely, as these are major aspects of Wnt signaling (Yang, 2012). Low levels of the adhesion class cadherin EGF lag seven pass receptors (CELSR)2 are also seen in pericytes.
The ion channels and GPCRs expressed by capillary pericytes represent a toolkit for the dynamic control of pericyte membrane potential and function. Among a panoply of roles for these signaling elements, the robust expression of genes encoding K+ channels and GsPCRs and their second messenger components implies an important role for pericyte membrane hyperpolarization, which we suggest contributes to long-range electrical signaling to control blood flow (Figure 6). Importantly, disturbances in blood flow and the processes that regulate it are increasingly appreciated to play a key role in a variety of pathological conditions. These include dementias such as Alzheimer's disease (AD) (Alsop et al., 2000; Iadecola, 2004; Nicolakakis and Hamel, 2011; Iturria-Medina et al., 2016), small vessel disease of the brain (Dabertrand et al., 2015; Capone et al., 2016; Huneau et al., 2018), psychological conditions such as schizophrenia (Mathew et al., 1988; Zhu et al., 2017) and chronic stress (Longden et al., 2014; Han et al., 2019), plus diabetes (Mogi and Horiuchi, 2011; Vetri et al., 2012), hypertension (Girouard and Iadecola, 2006; Capone et al., 2012), and stroke (Girouard and Iadecola, 2006; Koide et al., 2012; Balbi et al., 2017), and pericytes appear to be exceptionally sensitive to pathological perturbations (Winkler et al., 2011).
The ion channels and GPCRs that are highly expressed by brain pericytes thus have the potential to be pharmacological targets for vascular disorders, metabolic diseases, and neurodegenerative and neurological disorders (wherein for example KATP channels, IP3Rs, VDCCs, TRP channels, and GPCRs such as A2A and ETA receptors have been implicated, to name but a few; Hübner and Jentsch, 2002; Jacobson and Gao, 2006; Nilius et al., 2007; Ohkita et al., 2012; Aziz et al., 2014; Mikoshiba, 2015). Thus, furthering our understanding of the mechanisms through which pericytes contribute to blood flow control in the brain is a critical step in the search for ways in which to prevent decline or restore function in these disease contexts. The data we have discussed underscore that we are at an early stage in our understanding of how pericyte ion channels and GPCRs contribute to these functions, and warrant further studies to reveal novel mechanisms and therapeutic targets.
In the future, it will be important to determine the precise effects of both hyperpolarization and depolarization on pericyte functional outputs, for which optogenetic technologies or traditional electrophysiological approaches (Zhang et al., 2011) can be leveraged. At a deeper level, questions regarding the organization of pericyte ion channels and GPCRs await exploration—are these organized into macromolecular signaling complexes to facilitate privileged communication between complementary molecular players? Are these elements concentrated at sites to optimize cell-cell communication, such as peg-socket junctions, or distributed more broadly throughout the cell? What are the mechanisms that modulate the fidelity and gain of signaling (control of gene expression, protein trafficking, cell surface expression levels, and so on) and how are these affected in cerebrovascular disorders? The present survey of pericyte ion channels and GPCRs provides a map that can be used to guide these deeper explorations.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Support for this work was provided by the NIH National Institute on Aging and National Institute of Neurological Disorders and Stroke (1R01AG066645 and 1DP2NS121347, to TL), the American Heart Association (17SDG33670237 and 19IPLOI34660108 to TL), and the Swedish Cancer Society (to CB).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors thank B. Huang for data organization and proofreading the manuscript.
Adams, P. J., Garcia, E., David, L. S., Mulatz, K. J., Spacey, S. D., and Snutch, T. P. (2009). Cav2.1 P/Q-type calcium channel alternative splicing affects the functional impact of familial hemiplegic migraine mutations: implications for calcium channelopathies. Channels 3, 110–121. doi: 10.4161/chan.3.2.7932
Adelman, J. P., Maylie, J., and Sah, P. (2012). Small-conductance Ca2+-activated K+ channels: form and function. Annu. Rev. Physiol. 74, 245–269. doi: 10.1146/annurev-physiol-020911-153336
Alarcon-Martinez, L., Yilmaz-Ozcan, S., Yemisci, M., Schallek, J., Kil,iç, K., Can, A., et al. (2018). Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife 7:e34861. doi: 10.7554/eLife.34861.017
Albert, A. P. (2011). “Gating mechanisms of canonical transient receptor potential channel proteins: role of phosphoinositols and diacylglycerol,” in Transient Receptor Potential Channels. Advances in Experimental Medicine and Biology, ed M. Islam (Dordrecht: Springer), 391–411. doi: 10.1007/978-94-007-0265-3_22
Alexander, S. P. H., Christopoulos, A., Davenport, A. P., Kelly, E., Mathie, A., Peters, J. A., et al. (2019). The concise guide to pharmacology 2019/20: G protein-coupled receptors. Br. J. Pharmacol. 176, S21–S141. doi: 10.1111/bph.14748
Al-Karagholi, M. A.-M., Ghanizada, H., Nielsen, C. A. W., Ansari, A., Gram, C., Younis, S., et al. (2020). Cerebrovascular effects of glibenclamide investigated using high-resolution magnetic resonance imaging in healthy volunteers. J. Cereb. Blood Flow Metab. doi: 10.1177/0271678X20959294. [Epub ahead of print].
Alkhatib, O., da Costa, R., Gentry, C., Quallo, T., Bevan, S., and Andersson, D. A. (2019). Promiscuous G-protein-coupled receptor inhibition of transient receptor potential melastatin 3 ion channels by Gβγ subunits. J. Neurosci. 39, 7840–7852. doi: 10.1523/JNEUROSCI.0882-19.2019
Alsop, D. C., Detre, J. A., and Grossman, M. (2000). Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann. Neurol. 47, 93–100. doi: 10.1002/1531-8249(200001)47:1<93::AID-ANA15>3.0.CO;2-8
An, M. T., and Zamponi, G. W. (2005). “Voltage-dependent inactivation of voltage gated calcium channels,” in Voltage-Gated Calcium Channels. Molecular Biology Intelligence Unit, ed G. W. Zamponi (Boston, MA: Springer), 194–204. doi: 10.1007/0-387-27526-6_12
Ancellin, N., Colmont, C., Su, J., Li, Q., Mittereder, N., Chae, S. S., et al. (2002). Extracellular export of sphingosine kinase-1 enzyme. Sphingosine 1-phosphate generation and the induction of angiogenic vascular maturation. J. Biol. Chem. 277, 6667–6675. doi: 10.1074/jbc.M102841200
Aoki, M., Aoki, H., Ramanathan, R., Hait, N. C., and Takabe, K. (2016). Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediators Inflamm. 2016:8606878. doi: 10.1155/2016/8606878
Arguin, G., Regimbald-Dumas, Y., Fregeau, M. O., Caron, A. Z., and Guillemette, G. (2007). Protein kinase C phosphorylates the inositol 1,4,5-trisphosphate receptor type 2 and decreases the mobilization of Ca2+ in pancreatoma AR4-2J cells. J. Endocrinol. 192, 659–668. doi: 10.1677/JOE-06-0179
Armstrong, J. F., Faccenda, E., Harding, S. D., Pawson, A. J., Southan, C., Sharman, J. L., et al. (2020). The iuphar/bps guide to pharmacology in 2020: extending immunopharmacology content and introducing the iuphar/mmv guide to malaria pharmacology. Nucleic Acids Res. 48, D1006–D1021. doi: 10.1093/nar/gkz951
Armulik, A., Abramsson, A., and Betsholtz, C. (2005). Endothelial/pericyte interactions. Circ. Res. 97, 512–523. doi: 10.1161/01.RES.0000182903.16652.d7
Armulik, A., Genové, G., Mäe, M., Nisancioglu, M. H., Wallgard, E., Niaudet, C., et al. (2010). Pericytes regulate the blood-brain barrier. Nature 468, 557–561. doi: 10.1038/nature09522
Arora, K., Sinha, C., Zhang, W., Ren, A., Moon, C. S., Yarlagadda, S., et al. (2013). Compartmentalization of cyclic nucleotide signaling: a question of when, where, and why? Pflugers Arch. 465, 1397–1407. doi: 10.1007/s00424-013-1280-6
Asmar, M., Asmar, A., Simonsen, L., Dela, F., Holst, J. J., and Bülow, J. (2019). GIP-induced vasodilation in human adipose tissue involves capillary recruitment. Endocr. Connect. 8, 806–813. doi: 10.1530/EC-19-0144
Attwell, D., Buchan, A. M., Charpak, S., Lauritzen, M., MacVicar, B. A., and Newman, E. A. (2010). Glial and neuronal control of brain blood flow. Nature 468, 232–243. doi: 10.1038/nature09613
Attwell, D., Mishra, A., Hall, C. N., O'Farrell, F. M., and Dalkara, T. (2016). What is a pericyte? J. Cereb. Blood Flow Metab. 36, 451–455. doi: 10.1177/0271678X15610340
Aydin, F., Rosenblum, W. I., and Povlishock, J. T. (1991). Myoendothelial junctions in human brain arterioles. Stroke 22, 1592–1597. doi: 10.1161/01.STR.22.12.1592
Aziz, Q., Thomas, A. M., Gomes, J., Ang, R., Sones, W. R., Li, Y., et al. (2014). The ATP-sensitive potassium channel subunit, Kir6.1, in vascular smooth muscle plays a major role in blood pressure control. Hypertension 64, 523–529. doi: 10.1161/HYPERTENSIONAHA.114.03116
Baker, M. R., Fan, G., and Serysheva, I. I. (2017). Structure of IP3R channel: high-resolution insights from cryo-EM. Curr. Opin. Struct. Biol. 46, 38–47. doi: 10.1016/j.sbi.2017.05.014
Balbi, M., Koide, M., Wellman, G. C., and Plesnila, N. (2017). Inversion of neurovascular coupling after subarachnoid hemorrhage in vivo. J. Cereb. Blood Flow Metab. 37, 3625–3634. doi: 10.1177/0271678X16686595
Ballanyi, K., Doutheil, J., and Brockhaus, J. (1996). Membrane potentials and microenvironment of rat dorsal vagal cells in vitro during energy depletion. J. Physiol. 495, 769–784. doi: 10.1113/jphysiol.1996.sp021632
Balsinde, J., Winstead, M. V., and Dennis, E. A. (2002). Phospholipase A2 regulation of arachidonic acid mobilization. FEBS Lett. 531, 2–6. doi: 10.1016/S0014-5793(02)03413-0
Bayliss, W. M. (1902). On the local reactions of the arterial wall to changes of internal pressure. J. Physiol. 28, 220–231. doi: 10.1113/jphysiol.1902.sp000911
Beech, D. J., Zhang, H., Nakao, K., and Bolton, T. B. (1993). K channel activation by nucleotide diphosphates and its inhibition by glibenclamide in vascular smooth muscle cells. Br. J. Pharmacol. 110, 573–582. doi: 10.1111/j.1476-5381.1993.tb13849.x
Berman, D. M., Wilkie, T. M., and Gilman, A. G. (1996). GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell 86, 445–452. doi: 10.1016/S0092-8674(00)80117-8
Berninsone, P., and Hirschberg, C. B. (1998). Nucleotide sugars, nucleotide sulfate, and ATP transporters of the endoplasmic reticulum and Golgi apparatus. Ann. N. Y. Acad. Sci. 842, 91–99. doi: 10.1111/j.1749-6632.1998.tb09636.x
Berridge, M. (2016). The inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 96, 1261–1296. doi: 10.1152/physrev.00006.2016
Berridge, M. J., Lipp, P., and Bootman, M. D. (2000). The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21. doi: 10.1038/35036035
Berthiaume, A. A., Grant, R. I., McDowell, K. P., Underly, R. G., Hartmann, D. A., Levy, M., et al. (2018). Dynamic remodeling of pericytes in vivo maintains capillary coverage in the adult mouse brain. Cell Rep. 22, 8–16. doi: 10.1016/j.celrep.2017.12.016
Bezprozvanny, L., Watras, J., and Ehrlich, B. E. (1991). Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351, 751–754. doi: 10.1038/351751a0
Bhattacharjee, A., Joiner, W. J., Wu, M., Yang, Y., Sigworth, F. J., and Kaczmarek, L. K. (2003). Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J. Neurosci. 23, 11681–11691. doi: 10.1523/JNEUROSCI.23-37-11681.2003
Bi, M. M., Hong, S., Zhou, H. Y., Wang, H. W., Wang, L. N., and Zheng, Y. J. (2014). Chloride channelopathies of ClC-2. Int. J. Mol. Sci. 15, 218–249. doi: 10.3390/ijms15010218
Blinder, P., Tsai, P. S., Kaufhold, J. P., Knutsen, P. M., Suhl, H., and Kleinfeld, D. (2013). The cortical angiome: an interconnected vascular network with noncolumnar patterns of blood flow. Nat. Neurosci. 16, 889–897. doi: 10.1038/nn.3426
Bondjers, C., He, L., Takemoto, M., Norlin, J., Asker, N., Hellström, M., et al. (2006). Microarray analysis of blood microvessels from PDGF-B and PDGF-Rβ mutant mice identifies novel markers for brain pericytes. FASEB J. 20, E1005–E1013. doi: 10.1096/fj.05-4944fje
Bondjers, C., Kalén, M., Hellström, M., Scheidl, S. J., Abramsson, A., Renner, O., et al. (2003). Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am. J. Pathol. 162, 721–729. doi: 10.1016/S0002-9440(10)63868-0
Bonev, A. D., and Nelson, M. T. (1996). Vasoconstrictors inhibit ATP-sensitive K+ channels in arterial smooth muscle through protein kinase C. J. Gen. Physiol. 108, 315–323. doi: 10.1085/jgp.108.4.315
Bootman, M. D., and Bultynck, G. (2020). Fundamentals of cellular calcium signaling: a primer. Cold Spring Harb. Perspect. Biol. 12:a038802. doi: 10.1101/cshperspect.a038802
Borea, P. A., Gessi, S., Merighi, S., Vincenzi, F., and Varani, K. (2018). Pharmacology of adenosine receptors: the state of the art. Physiol. Rev. 98, 1591–1625. doi: 10.1152/physrev.00049.2017
Borysova, L., Wray, S., Eisner, D. A., and Burdyga, T. (2013). How calcium signals in myocytes and pericytes are integrated across in situ microvascular networks and control microvascular tone. Cell Calcium 54, 163–174. doi: 10.1016/j.ceca.2013.06.001
Bos, C. L., Richel, D. J., Ritsema, T., Peppelenbosch, M. P., and Versteeg, H. H. (2004). Prostanoids and prostanoid receptors in signal transduction. Int. J. Biochem. Cell Biol. 36, 1187–1205. doi: 10.1016/j.biocel.2003.08.006
Bousquet, S. M., Monet, M., and Boulay, G. (2010). Protein kinase C-dependent phosphorylation of transient receptor potential canonical 6 (TRPC6) on serine 448 causes channel inhibition. J. Biol. Chem. 285, 40534–40543. doi: 10.1074/jbc.M110.160051
Breckenridge, B. M., and Crawford, E. J. (1960). Glycogen synthesis from uridine diphosphate glucose in brain. J. Biol. Chem. 235, 3054–3057.
Bulley, S., and Jaggar, J. H. (2014). Cl− channels in smooth muscle cells. Pflügers Arch. 466, 861–872. doi: 10.1007/s00424-013-1357-2
Burdakov, D., Petersen, O. H., and Verkhratsky, A. (2005). Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium 38, 303–310. doi: 10.1016/j.ceca.2005.06.010
Burdyga, T., and Borysova, L. (2014). Calcium signalling in pericytes. J. Vasc. Res. 51, 190–199. doi: 10.1159/000362687
Burke, M. A., Mutharasan, R. K., and Ardehali, H. (2008). The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ. Res. 102, 164–176. doi: 10.1161/CIRCRESAHA.107.165324
Cabrita, I., Benedetto, R., Fonseca, A., Wanitchakool, P., Sirianant, L., Skryabin, B. V., et al. (2017). Differential effects of anoctamins on intracellular calcium signals. FASEB J. 31, 2123–2134. doi: 10.1096/fj.201600797RR
Cai, C., Fordsmann, J. C., Jensen, S. H., Gesslein, B., Lønstrup, M., Hald, B. O., et al. (2018). Stimulation-induced increases in cerebral blood flow and local capillary vasoconstriction depend on conducted vascular responses. Proc. Natl. Acad. Sci. U.S.A. 115, E5796–E5804. doi: 10.1073/pnas.1707702115
Calcraft, P. J., Arredouani, A., Ruas, M., Pan, Z., Cheng, X., Hao, X., et al. (2009). NAADP mobilizes calcium from acidic organelles through two-pore channel. Nature 459, 596–600. doi: 10.1038/nature08030
Canales, J., Morales, D., Blanco, C., Rivas, J., Diaz, N., Angelopoulos, I., et al. (2019). A tr(i)p to cell migration: new roles of trp channels in mechanotransduction and cancer. Front. Physiol. 10:757. doi: 10.3389/fphys.2019.00757
Cantero del Rocío, M., Velázquez, I. F., Streets, A. J., Ong, A. C. M., and Cantiello, H. F. (2015). The cAMP signaling pathway and direct protein kinase a phosphorylation regulate polycystin-2 (TRPP2) channel function. J. Biol. Chem. 290, 23888–23896. doi: 10.1074/jbc.M115.661082
Cao, C., Goo, J. H., Lee-Kwon, W., and Pallone, T. L. (2006). Vasa recta pericytes express a strong inward rectifier K+ conductance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1601–R1607. doi: 10.1152/ajpregu.00877.2005
Capone, C., Dabertrand, F., Baron-Menguy, C., Chalaris, A., Ghezali, L., Domenga-Denier, V., et al. (2016). Mechanistic insights into a TIMP3-sensitive pathway constitutively engaged in the regulation of cerebral hemodynamics. Elife 5:e17536. doi: 10.7554/eLife.17536.042
Capone, C., Faraco, G., Peterson, J. R., Coleman, C., Anrather, J., Milner, T. A., et al. (2012). Central cardiovascular circuits contribute to the neurovascular dysfunction in angiotensin II hypertension. J. Neurosci. 32, 4878–4886. doi: 10.1523/JNEUROSCI.6262-11.2012
Carlson, E. (1989). Fenestrated subendothelial basement membranes in human retinal capillaries. Investig. Opthalmology Vis. Sci. 30, 1923–1932.
Caron, A. Z., Chaloux, B., Arguin, G., and Guillemette, G. (2007). Protein kinase C decreases the apparent affinity of the inositol 1,4,5-trisphosphate receptor type 3 in RINm5F cells. Cell Calcium 42, 323–331. doi: 10.1016/j.ceca.2007.01.002
Carvalho, J., Chennupati, R., Li, R., Günther, S., Kaur, H., Zhao, W., et al. (2020). Orphan G protein-coupled receptor GPRC5B controls smooth muscle contractility and differentiation by inhibiting prostacyclin receptor signaling. Circulation 141, 1168–1183. doi: 10.1161/CIRCULATIONAHA.119.043703
Catterall, W. A., Perez-Reyes, E., Snutch, T. P., and Striessnig, J. (2005). International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol. Rev. 57, 411–425. doi: 10.1124/pr.57.4.5
Chasseigneaux, S., Moraca, Y., Cochois-Guégan, V., Boulay, A. C., Gilbert, A., Crom, S., et al. (2018). Isolation and differential transcriptome of vascular smooth muscle cells and mid-capillary pericytes from the rat brain. Sci. Rep. 8:12272. doi: 10.1038/s41598-018-30739-5
Chaudhuri, D., Issa, J. B., and Yue, D. T. (2007). Elementary mechanisms producing facilitation of Cav2.1 (P/Q-type) channels. J. Gen. Physiol. 129, 385–401. doi: 10.1085/jgp.200709749
Chen, X., Wang, Q., Ni, F., and Ma, J. (2010). Structure of the full-length Shaker potassium channel Kv1.2 by normal-mode-based X-ray crystallographic refinement. Proc. Natl. Acad. Sci. U.S.A. 107, 11352–11357. doi: 10.1073/pnas.1000142107
Cheng, H. Y., Dong, A., Panchatcharam, M., Mueller, P., Yang, F., Li, Z., et al. (2012). Lysophosphatidic acid signaling protects pulmonary vasculature from hypoxia-induced remodeling. Arterioscler. Thromb. Vasc. Biol. 32, 24–32. doi: 10.1161/ATVBAHA.111.234708
Cheng, K. T., Liu, X., Ong, H. L., and Ambudkar, I. S. (2008). Functional requirement for Orai1 in store-operated TRPC1-STIM1 channels. J. Biol. Chem. 283, 12935–12940. doi: 10.1074/jbc.C800008200
Cheng, K. T., Ong, H. L., Liu, X., and Ambudkar, I. S. (2013). Contribution and regulation of TRPC channels in store-operated Ca2+ entry. Curr. Top. Membr. 71, 149–179. doi: 10.1016/B978-0-12-407870-3.00007-X
Chern, Y. (2000). Regulation of adenylyl cyclase in the central nervous system. Cell. Signal. 12, 195–204. doi: 10.1016/S0898-6568(99)00084-4
Chesler, M., and Kaila, K. (1992). Modulation of pH by neuronal activity. Trends Neurosci. 15, 396–402. doi: 10.1016/0166-2236(92)90191-A
Cheung, W., Andrade-Gordon, P., Derian, C. K., and Damiano, B. P. (1998). Receptor-activating peptides distinguish thrombin receptor (PAR-1) and protease activated receptor 2 (PAR-2) mediated hemodynamic responses in vivo. Can. J. Physiol. Pharmacol. 76, 16–25. doi: 10.1139/y97-176
Chipperfield, A. R., and Harper, A. A. (2000). Chloride in smooth muscle. Prog. Biophys. Mol. Biol. 74, 175–221. doi: 10.1016/S0079-6107(00)00024-9
Cho, H., Kozasa, T., Bondjers, C., Betsholtz, C., and Kehrl, J. H. (2003). Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J. 17, 440–442. doi: 10.1096/fj.02-0340fje
Cipolla, M. (2009). “Chapter 2: Anatomy and Ultrastructure,” in The Cerebral Circulation (San Rafael, CA: Morgan & Claypool Life Sciences).
Cipolla, M., Li, R., and Vitullo, L. (2004). Perivascular innervation of penetrating brain parenchymal arterioles. J. Cardiovasc. Pharmacol. 44, 1–8. doi: 10.1097/00005344-200407000-00001
Clapham, D. E. (2003). TRP channels as cellular sensors. Nature 426, 517–524. doi: 10.1038/nature02196
Corda, G., and Sala, A. (2017). Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogenesis 6:e364. doi: 10.1038/oncsis.2017.69
Cortijo, C., Gouzi, M., Tissir, F., and Grapin-Botton, A. (2012). Planar cell polarity controls pancreatic beta cell differentiation and glucose homeostasis. Cell Rep. 2, 1593–1606. doi: 10.1016/j.celrep.2012.10.016
Coste, B., Mathur, J., Schmidt, M., Earley, T. J., Ranade, S., Petrus, M. J., et al. (2010). Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. doi: 10.1126/science.1193270
Coste, B., Murthy, S. E., Mathur, J., Schmidt, M., Mechioukhi, Y., Delmas, P., et al. (2015). Piezo1 ion channel pore properties are dictated by C-terminal region. Nat. Commun. 6:7223. doi: 10.1038/ncomms8223
Crnkovic, S., Egemnazarov, B., Jain, P., Seay, U., Gattinger, N., Marsh, L. M., et al. (2014). NPY/Y1 receptor-mediated vasoconstrictory and proliferative effects in pulmonary hypertension. Br. J. Pharmacol. 171, 3895–3907. doi: 10.1111/bph.12751
Cuevas, P., Gutierrez-Diaz, J. A., Reimers, D., Dujovny, M., Diaz, F. G., and Ausman, J. I. (1984). Pericyte endothelial gap junctions in human cerebral capillaries. Anat. Embryol. 170, 155–159. doi: 10.1007/BF00319000
Cunha, R. A. (2016). How does adenosine control neuronal dysfunction and neurodegeneration? J. Neurochem. 139, 1019–1055. doi: 10.1111/jnc.13724
Cvetković, D., Babwah, A. V., and Bhattacharya, M. (2013). Kisspeptin/KISSIR system in breast cancer. J. Cancer 4, 653–661. doi: 10.7150/jca.7626
Dabertrand, F., Krøigaard, C., Bonev, A. D., Cognat, E., Dalsgaard, T., Domenga-Denier, V., et al. (2015). Potassium channelopathy-like defect underlies early-stage cerebrovascular dysfunction in a genetic model of small vessel disease. Proc. Natl. Acad. Sci. U.S.A. 112, E796–E805. doi: 10.1073/pnas.1420765112
Dang, S., Feng, S., Tien, J., Peters, C. J., Bulkley, D., Lolicato, M., et al. (2017). Cryo-EM structures of the TMEM16A calcium-activated chloride channel. Nature 552, 426–429. doi: 10.1038/nature25024
D'Avanzo, N., Cheng, W. W. L., Doyle, D. A., and Nichols, C. G. (2010). Direct and specific activation of human inward rectifier K+ channels by membrane phosphatidylinositol 4,5-Bisphosphate. J. Biol. Chem. 285, 37129–37132. doi: 10.1074/jbc.C110.186692
Davenport, A. P., Hyndman, K. A., Dhaun, N., Southan, C., Kohan, D. E., Pollock, J. S., et al. (2016). Endothelin. Pharmacol. Rev. 68, 357–418. doi: 10.1124/pr.115.011833
De Henau, O., Degroot, G. N., Imbault, V., Robert, V., De Poorter, C., McHeik, S., et al. (2016). Signaling properties of chemerin receptors CMKLR1, GPR1 and CCRL2. PLoS ONE 11:e0164179. doi: 10.1371/journal.pone.0164179
de Oliveira, P. G., Ramos, M. L. S., Amaro, A. J., Dias, R. A., and Vieira, S. I. (2019). Gi/o-protein coupled receptors in the aging brain. Front. Aging Neurosci. 11:89. doi: 10.3389/fnagi.2019.00089
Dehouck, M. P., Vigne, P., Torpier, G., Breittmayer, J. P., Cecchelli, R., and Frelin, C. (1997). Endothelin-1 as a mediator of endothelial cell-pericyte interactions in bovine brain capillaries. J. Cereb. Blood Flow Metab. 17, 464–469. doi: 10.1097/00004647-199704000-00012
Denninger, J. W., and Marletta, M. A. (1999). Guanylate cyclase and the NO/cGMP signaling pathway. Biochim. Biophys. Acta Bioenerg. 1411, 334–350. doi: 10.1016/S0005-2728(99)00024-9
Díaz-Flores, L., Gutiérrez, R., Madrid, J. F., Varela, H., Valladares, F., Acosta, E., et al. (2009). Pericytes. Morphofunction, interactions and pathology in a quiescent and activated mesenchymal cell niche. Histol. Histopathol. 24, 909–969. doi: 10.14670/HH-24.909
Dick, I. E., Tadross, M. R., Liang, H., Tay, L. H., Yang, W., and Yue, D. T. (2008). A modular switch for spatial Ca2+ selectivity in the calmodulin regulation of Cav channels. Nature 451, 830–834. doi: 10.1038/nature06529
Dickson, E. J., and Hille, B. (2019). Understanding phosphoinositides: rare, dynamic, and essential membrane phospholipids. Biochem. J. 476, 1–23. doi: 10.1042/BCJ20180022
Dijksterhuis, J. P., Petersen, J., and Schulte, G. (2014). WNT/Frizzled signalling: Receptor-ligand selectivity with focus on FZD-G protein signalling and its physiological relevance: IUPHAR review 3. Br. J. Pharmacol. 171, 1195–1209. doi: 10.1111/bph.12364
Dirnagl, U., Niwa, K., Lindauer, U., and Villringer, A. (1994). Coupling of cerebral blood flow to neuronal activation: role of adenosine and nitric oxide. Am. J. Physiol. Hear. Circ. Physiol. 267, H296–H301. doi: 10.1152/ajpheart.1994.267.1.H296
Dorn, G. W. II., and Becker, M. W. (1993). Thromboxane A2 stimulated signal transduction in vascular smooth muscle. J. Pharmacol. Exp. Ther. 265, 447–456.
Drury, A. N., and Szent-Györgyi, A. (1929). The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 68, 213–237. doi: 10.1113/jphysiol.1929.sp002608
Duprat, F., Lesage, F., Fink, M., Reyes, R., Heurteaux, C., and Lazdunski, M. (1997). TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J. 16, 5464–5471. doi: 10.1093/emboj/16.17.5464
Dupré, D. J., Robitaille, M., Rebois, R. V., and Hébert, T. E. (2009). The role of Gβγ subunits in the organization, assembly, and function of GPCR signaling complexes. Annu. Rev. Pharmacol. Toxicol. 49, 31–56. doi: 10.1146/annurev-pharmtox-061008-103038
Dutta, A. K., Khimji, A. K., Liu, S., Karamysheva, Z., Fujita, A., Kresge, C., et al. (2016). PKCα regulates TMEM16A-mediated Cl− secretion in human biliary cells. Am. J. Physiol. Gastrointest. Liver Physiol. 310, G34–G42. doi: 10.1152/ajpgi.00146.2015
Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T., and MacKinnon, R. (2002). X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature 415, 287–294. doi: 10.1038/415287a
Duvernoy, H. M., Delon, S., and Vannson, J. L. (1981). Cortical blood vessels of the human brain. Brain Res. Bull. 7, 519–579. doi: 10.1016/0361-9230(81)90007-1
Dyer, L. A., and Patterson, C. (2010). Development of the endothelium: An emphasis on heterogeneity. Semin. Thromb. Hemost. 36, 227–235. doi: 10.1055/s-0030-1253446
Earley, S., and Brayden, J. E. (2010). Transient receptor potential channels and vascular function. Clin. Sci. 119, 19–36. doi: 10.1042/CS20090641
Earley, S., and Brayden, J. E. (2015). Transient receptor potential channels in the vasculature. Physiol. Rev. 95, 645–690. doi: 10.1152/physrev.00026.2014
Eberth, C. (1871). Handbuch der lehre von der gewegen des menschen und der tiere Vol. 1. Leipzig: Engelmann
Enkvetchakul, D., and Nichols, C. G. (2003). Gating mechanism of KATP channels: function fits form. J. Gen. Physiol. 122, 471–480. doi: 10.1085/jgp.200308878
Estévez, R., Pusch, M., Ferrer-Costa, C., Orozco, M., and Jentsch, T. J. (2004). Functional and structural conservational of CBS domains from CLC chloride channels. J. Physiol. 557, 363–378. doi: 10.1113/jphysiol.2003.058453
Evans, N. J., Bayliss, A. L., Reale, V., and Evans, P. D. (2016). Characterisation of signalling by the endogenous GPER1 (GPR30) receptor in an embryonic mouse hippocampal cell line (mHippoE-18). PLoS ONE 11:e0152138. doi: 10.1371/journal.pone.0152138
Fan, G., Baker, M. L., Wang, Z., Baker, M. R., Sinyagovskiy, P. A., Chiu, W., et al. (2015). Gating machinery of InsP3R channels revealed by electron cryomicroscopy. Nature 527, 336–341. doi: 10.1038/nature15249
Fan, Z., and Makielski, J. C. (1997). Anionic phospholipids activate ATP-sensitive potassium channels. J. Biol. Chem. 272, 5388–5395. doi: 10.1074/jbc.272.9.5388
Fernández-Klett, F., Offenhauser, N., Dirnagl, U., Priller, J., and Lindauer, U. (2010). Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 107, 22290–22295. doi: 10.1073/pnas.1011321108
Fernández-Tenorio, M., Porras-González, C., Castellano, A., Del Valle-Rodríguez, A., López-Barneo, J., and Ureña, J. (2011). Metabotropic regulation of RhoA/Rho-associated kinase by L-type Ca2+ channels: new mechanism for depolarization-evoked mammalian arterial contraction. Circ. Res. 108, 1348–1357. doi: 10.1161/CIRCRESAHA.111.240127
Ferrandon, S., Feinstein, T. N., Castro, M., Wang, B., Bouley, R., Potts, J. T., et al. (2009). Sustained cyclic AMP production by parathyroid hormone receptor endocytosis. Nat. Chem. Biol. 5, 734–742. doi: 10.1038/nchembio.206
Ferris, C. D., Cameron, A. M., Bredt, D. S., Huganir, R. L., and Snyder, S. H. (1991a). Inositol 1,4,5-trisphosphate receptor is phosphorylated by cyclic AMP-dependent protein kinase at serines 1755 and 1589. Biochem. Biophys. Res. Commun. 175, 192–198. doi: 10.1016/S0006-291X(05)81219-7
Ferris, C. D., Huganir, R. L., Bredt, D. S., Cameron, A. M., and Snyder, S. H. (1991b). Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc. Natl. Acad. Sci. U.S.A. 88, 2232–2235. doi: 10.1073/pnas.88.6.2232
Filosa, J. A., Bonev, A. D., Straub, S. V., Meredith, A. L., Wilkerson, M. K., Aldrich, R. W., et al. (2006). Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 9, 1397–1403. doi: 10.1038/nn1779
Finch, E., Turner, T., and Goldin, S. (1991). Calcium as a coagonist of inositol 1,4,5-trisphosphate-induced calcium release. Science 252, 443–446. doi: 10.1126/science.2017683
Findlay, I. (1987). The effects of magnesium upon adenosine triphosphate-sensitive potassium channels in a rat insulin-secreting cell line. J. Physiol. 391, 611–629. doi: 10.1113/jphysiol.1987.sp016759
Findlay, I. (1988). Effects of ADP upon the ATP-sensitive K+ channel in rat ventricular myocytes. J. Membr. Biol. 101, 83–92. doi: 10.1007/BF01872823
Fisher, I., Jenkins, M. L., Tall, G. G., Burke, J. E., and Smrcka, A. V. (2020). Activation of phospholipase C β by Gβγ and Gαq involves C-terminal rearrangement to release auto-inhibition. Structure 28, 1–10. doi: 10.1101/810994
Foskett, J. K., White, C., Cheung, K. H., and Mak, D. O. (2007). Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87, 593–658. doi: 10.1152/physrev.00035.2006
Foster, M. N., and Coetzee, W. A. (2015). KATP channels in the cardiovascular system. Physiol. Rev. 96, 177–252. doi: 10.1152/physrev.00003.2015
Freeman, B. D., Machado, F. S., Tanowitz, H. B., and Desruisseaux, M. S. (2014). Endothelin-1 and its role in the pathogenesis of infectious diseases. Life Sci. 118, 110–119. doi: 10.1016/j.lfs.2014.04.021
Fresco, P., Diniz, C., and Gonçalves, J. (2004). Facilitation of noradrenaline release by activation of adenosine A2A receptors triggers both phospholipase C and adenylate cyclase pathways in rat tail artery. Cardiovasc. Res. 63, 739–746. doi: 10.1016/j.cardiores.2004.05.015
Friedrich, E. E., Hong, Z., Xiong, S., Zhong, M., Di, A., Rehman, J., et al. (2019). Endothelial cell Piezo1 mediates pressure-induced lung vascular hyperpermeability via disruption of adherens junctions. Proc. Natl. Acad. Sci. U.S.A. 116, 12980–12985. doi: 10.1073/pnas.1902165116
Fujiwara, T., and Uehara, Y. (1984). The cytoarchitecture of the wall and the innervation pattern of the microvessels in the rat mammary gland: A scanning electron microscopic observation. Am. J. Anat. 170, 39–54. doi: 10.1002/aja.1001700104
Fuller, M. D., Fu, Y., Scheuer, T., and Catterall, W. A. (2014). Differential regulation of Cav1.2 channels by cAMP-dependent protein kinase bound to A-kinase anchoring proteins 15 and 79/150. J. Gen. Physiol. 143, 315–324. doi: 10.1085/jgp.201311075
Gannon, K. P., Vanlandingham, L. G., Jernigan, N. L., Grifoni, S. C., Hamilton, G., and Drummond, H. A. (2008). Impaired pressure-induced constriction in mouse middle cerebral arteries of ASIC2 knockout mice. Am. J. Physiol. Hear. Circ. Physiol. 294, H1793–H1803. doi: 10.1152/ajpheart.01380.2007
Garcia, D. C. G., and Longden, T. A. (2020). “Ion channels in capillary endothelium,” in Current Topics in Membranes. Ion Channels and Calcium Signaling in the Microcirculation, ed W. F. Jackson (Cambridge, MA: Academic Press), 261–300. doi: 10.1016/bs.ctm.2020.01.005
Giamarchi, A., and Delmas, P. (2007). “Activation mechanisms and functional roles of TRPP2 cation channels,” in TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades, eds W. Liedtke and S. Heller (Boca Raton FL: CRC Press/Taylor & Francis).
Girouard, H., and Iadecola, C. (2006). Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J. Appl. Physiol. 100, 328–335. doi: 10.1152/japplphysiol.00966.2005
Gonzales, A. L., Klug, N. R., Moshkforoush, A., Lee, J. C., Lee, F. K., Shui, B., et al. (2020). Contractile pericytes determine the direction of blood flow at capillary junctions. Proc. Natl. Acad. Sci. U.S.A. 117, 27022–27033. doi: 10.1073/pnas.1922755117
Gonzales, A. L., Yang, Y., Sullivan, M. N., Sanders, L., Dabertrand, F., Hill-Eubanks, D. C., et al. (2014). A PLCγ1-dependent, force-sensitive signaling network in the myogenic constriction of cerebral arteries. Sci. Signal. 7:ra49. doi: 10.1126/scisignal.2004732
Gould, I. G., Tsai, P., Kleinfeld, D., and Linninger, A. (2017). The capillary bed offers the largest hemodynamic resistance to the cortical blood supply. J. Cereb. Blood Flow Metab. 37, 52–68. doi: 10.1177/0271678X16671146
Grant, R. I., Hartmann, D. A., Underly, R. G., Berthiaume, A. A., Bhat, N. R., and Shih, A. Y. (2019). Organizational hierarchy and structural diversity of microvascular pericytes in adult mouse cortex. J. Cereb. Blood Flow Metab. 39, 411–425. doi: 10.1177/0271678X17732229
Gribble, F. M., Tucker, S. J., and Ashcroft, F. M. (1997). The essential role of the walker A motifs of SUR1 in K-ATP channel activation by Mg-ADP and diazoxide. EMBO J. 16, 1145–1152. doi: 10.1093/emboj/16.6.1145
Gribble, F. M., Tucker, S. J., Haug, T., and Ashcroft, F. M. (1998). MgATP activates the β cell KATP channel by interaction with its SUR1 subunit. Proc. Natl. Acad. Sci. U.S.A. 95, 7185–7190. doi: 10.1073/pnas.95.12.7185
Grimm, C., Kraft, R., Schultz, G., and Harteneck, C. (2005). Activation of the melastatin-related cation channel TRPM3 by D-erythro-sphingosine [Corrected]. Mol. Pharmacol. 67, 798–805. doi: 10.1124/mol.104.006734
Grubb, S., Cai, C., Hald, B. O., Khennouf, L., Murmu, R. P., Jensen, A. G. K., et al. (2020). Precapillary sphincters maintain perfusion in the cerebral cortex. Nat. Commun. 11:395. doi: 10.1038/s41467-020-14330-z
Grubb, S., Poulsen, K. A., Juul, C. A., Kyed, T., Klausen, T. K., Larsen, E. H., et al. (2013). TMEM16F (Anoctamin 6), an anion channel of delayed Ca2+ activation. J. Gen. Physiol. 141, 585–600. doi: 10.1085/jgp.201210861
Grutzendler, J., and Nedergaard, M. (2019). Cellular control of brain capillary blood flow: in vivo imaging veritas. Trends Neurosci. 42, 528–536. doi: 10.1016/j.tins.2019.05.009
Guimarães, S., and Moura, D. (2001). Vascular adrenoceptors: an update. Pharmacol. Rev. 53, 319–356.
Gunaje, J. J., Bahrami, A. J., Schwartz, S. M., Daum, G., and Mahoney, W. M. (2011). PDGF-dependent regulation of regulator of G protein signaling-5 expression and vascular smooth muscle cell functionality. Am. J. Physiol. Cell Physiol. 301, C478–C489. doi: 10.1152/ajpcell.00348.2010
Gurney, A. M., Osipenko, O. N., MacMillan, D., McFarlane, K. M., Tate, R. J., and Kempsill, F. E. J. (2003). Two-pore domain K channel, TASK-1, in pulmonary artery smooth muscle cells. Circ. Res. 93, 957–964. doi: 10.1161/01.RES.0000099883.68414.61
Haas, T. L., and Duling, B. R. (1997). Morphology favors an endothelial cell pathway for longitudinal conduction within arterioles. Microvasc. Res. 53, 113–120. doi: 10.1006/mvre.1996.1999
Hall, C. N., Reynell, C., Gesslein, B., Hamilton, N. B., Mishra, A., Sutherland, B. A., et al. (2014). Capillary pericytes regulate cerebral blood flow in health and disease. Nature 508, 55–60. doi: 10.1038/nature13165
Hamada, K., Miyatake, H., Terauchi, A., and Mikoshiba, K. (2017). IP3-mediated gating mechanism of the IP3 receptor revealed by mutagenesis and X-ray crystallography. Proc. Natl. Acad. Sci. U.S.A. 114, 4661–4666. doi: 10.1073/pnas.1701420114
Hamel, E. (2006). Perivascular nerves and the regulation of cerebrovascular tone. J. Appl. Physiol. 100, 1059–1064. doi: 10.1152/japplphysiol.00954.2005
Han, K., Min, J., Lee, M., Kang, B.-M., Park, T., Hahn, J., et al. (2019). Neurovascular coupling under chronic stress is modified by altered GABAergic interneuron activity. J. Neurosci. 39, 10081–10095. doi: 10.1523/JNEUROSCI.1357-19.2019
Hanlon, C. D., and Andrew, D. J. (2015). Outside-in signaling - a brief review of GPCR signaling with a focus on the Drosophila GPCR family. J. Cell Sci. 128, 3533–3542. doi: 10.1242/jcs.175158
Hansen, S. B., Tao, X., and MacKinnon, R. (2011). Structural basis of PIP2 activation of the classical inward rectifier K+ channel Kir2.2. Nature 477, 495–498. doi: 10.1038/nature10370
Harden, T. K., Sesma, J. I., Fricks, I. P., and Lazarowski, E. R. (2010). Signalling and pharmacological properties of the P2Y14 receptor. Acta Physiol. 199, 149–160. doi: 10.1111/j.1748-1716.2010.02116.x
Harraz, O. F., Abd El-Rahman, R. R., Bigdely-Shamloo, K., Wilson, S. M., Brett, S. E., Romero, M., et al. (2014). Cav3.2 channels and the induction of negative feedback in cerebral arteries. Circ. Res. 115, 650–661. doi: 10.1161/CIRCRESAHA.114.304056
Harraz, O. F., Hill-Eubanks, D., and Nelson, M. T. (2020). PIP2: a critical regulator of vascular ion channels hiding in plain sight. Proc. Natl. Acad. Sci. U.S.A. doi: 10.1073/pnas.2006737117. [Epub ahead of print].
Harraz, O. F., Longden, T. A., Dabertrand, F., Hill-Eubanks, D., and Nelson, M. T. (2018). Endothelial GqPCR activity controls capillary electrical signaling and brain blood flow through PIP2 depletion. Proc. Natl. Acad. Sci. U.S.A. 115, E3569–E3577. doi: 10.1073/pnas.1800201115
Harraz, O. F., and Welsh, D. G. (2013). T-Type Ca2+ channels in cerebral arteries: approaches, hypotheses, and speculation. Microcirculation 20, 299–306. doi: 10.1111/micc.12038
Harrison, P. J., Lyon, L., Sartorius, L. J., Burnet, P. W. J., and Lane, T. A. (2008). The group II metabotropic glutamate receptor 3 (mGluR3, mGlu3, GRM3): expression, function and involvement in schizophrenia. J. Psychopharmacol. 22, 308–322. doi: 10.1177/0269881108089818
Hartmann, D. A., Underly, R. G., Grant, R. I., Watson, A. N., Lindner, V., and Shih, A. Y. (2015). Pericyte structure and distribution in the cerebral cortex revealed by high-resolution imaging of transgenic mice. Neurophotonics 2:041402. doi: 10.1117/1.NPh.2.4.041402
Hashitani, H., Mitsui, R., Miwa-Nishimura, K., and Lam, M. (2018). Role of capillary pericytes in the integration of spontaneous Ca2+ transients in the suburothelial microvasculature in situ of the mouse bladder. J. Physiol. 596, 3531–3552. doi: 10.1113/JP275845
He, L., Vanlandewijck, M., Mäe, M., Andrae, J., Ando, K., Gaudio, F., et al. (2018). Single cell RNAseq of mouse brain and lung vascular and vessel-associated cell types. Sci. Data 5:180160. doi: 10.1038/sdata.2018.160
Heinze, C., Seniuk, A., Sokolov, M. V., Huebner, A. K., Klementowicz, A. E., Szijárt,ó, I. A., et al. (2014). Disruption of vascular Ca2+-activated chloride currents lowers blood pressure. J. Clin. Invest. 124, 675–686. doi: 10.1172/JCI70025
Helton, T. D., Xu, W., and Lipscombe, D. (2005). Neuronal L-type calcium channels open quickly and are inhibited slowly. J. Neurosci. 25, 10247–10251. doi: 10.1523/JNEUROSCI.1089-05.2005
Henno, P., Grassin-Delyle, S., Belle, E., Brollo, M., Naline, E., Sage, E., et al. (2017). In smokers, Sonic hedgehog modulates pulmonary endothelial function through vascular endothelial growth factor. Respir. Res. 18:102. doi: 10.1186/s12931-017-0590-1
Hepler, J. R., Berman, D. M., Gilman, A. G., and Kozasa, T. (1997). RGS4 and GAIP are GTPase-activating proteins for Gqα and block activation of phospholipase Cβ by γ-thio-GTP-Gqα. Proc. Natl. Acad. Sci. U.S.A. 94, 428–432. doi: 10.1073/pnas.94.2.428
Hibino, H., Inanobe, A., Furutani, K., Murakami, S., Findlay, I., and Kurachi, Y. (2010). Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol. Rev. 90, 291–366. doi: 10.1152/physrev.00021.2009
Hieble, J. P., and Ruffolo, R. R. Jr. (1997). Recent advances in the identification of α1- and α2-adrenoceptor subtypes: therapeutic implications. Expert Opin. Investig. Drugs 6, 367–387. doi: 10.1517/13543784.6.4.367
Hill, R. A., Tong, L., Yuan, P., Murikinati, S., Gupta, S., and Grutzendler, J. (2015). Regional blood flow in the normal and ischemic brain is controlled by arteriolar smooth muscle cell contractility and not by capillary pericytes. Neuron 87, 95–110. doi: 10.1016/j.neuron.2015.06.001
Hille, B., Dickson, E. J., Kruse, M., Vivas, O., and Suh, B. C. (2015). Phosphoinositides regulate ion channels. Biochim. Biophys. Acta 1851, 844–856. doi: 10.1016/j.bbalip.2014.09.010
Hill-Eubanks, D. C., Gonzales, A. L., Sonkusare, S. K., and Nelson, M. T. (2014). Vascular TRP channels: performing under pressure and going with the flow. Physiology 29, 343–360. doi: 10.1152/physiol.00009.2014
Hill-Eubanks, D. C., Werner, M. E., Heppner, T. J., and Nelson, M. T. (2011). Calcium signaling in smooth muscle. Cold Spring Harb. Perspect. Biol. 3:a004549. doi: 10.1101/cshperspect.a004549
Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T., and Schultz, G. (1999). Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature 397, 259–263. doi: 10.1038/16711
Horinouchi, T., Terada, K., Higashi, T., and Miwa, S. (2013). Endothelin receptor signaling: new insight into its regulatory mechanisms. J. Pharmacol. Sci. 123, 85–101. doi: 10.1254/jphs.13R02CR
Hot, B., Valnohova, J., Arthofer, E., Simon, K., Shin, J., Uhlén, M., et al. (2017). FZD10-Gα13 signalling axis points to a role of FZD10 in CNS angiogenesis. Cell. Signal. 32, 93–103. doi: 10.1016/j.cellsig.2017.01.023
Hu, C., DePuy, S. D., Yao, J., McIntire, W. E., and Barrett, P. Q. (2009). Protein kinase A activity controls the regulation of T-type Cav3.2 channels by Gβγ dimers. J. Biol. Chem. 284, 7465–7473. doi: 10.1074/jbc.M808049200
Hu, Y., and Wilson, G. S. (1997). Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J. Neurochem. 68, 1745–1752. doi: 10.1046/j.1471-4159.1997.68041745.x
Huang, C., Hepler, J. R., Gilman, A. G., and Mumby, S. M. (1997). Attenuation of Gi- and Gq-mediated signaling by expression of RGS4 or GAIP in mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 94, 6159–6163. doi: 10.1073/pnas.94.12.6159
Huang, G. N., Zeng, W., Kim, J. Y., Yuan, J. P., Han, L., Muallem, S., et al. (2006). STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat. Cell Biol. 8, 1003–1010. doi: 10.1038/ncb1454
Huang, J., Zhou, H., Mahavadi, S., Sriwai, W., and Murthy, K. S. (2007). Inhibition of Gαq-dependent PLC-β1 activity by PKG and PKA is mediated by phosphorylation of RGS4 and GRK2. Am. J. Physiol. Cell Physiol. 292, C200–C208. doi: 10.1152/ajpcell.00103.2006
Huang, Y., and Thathiah, A. (2015). Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett. 589, 1607–1619. doi: 10.1016/j.febslet.2015.05.007
Hübner, C. A., and Jentsch, T. J. (2002). Ion channel diseases. Hum. Mol. Genet. 11, 2435–2445. doi: 10.1093/hmg/11.20.2435
Huneau, C., Houot, M., Joutel, A., Béranger, B., Giroux, C., Benali, H., et al. (2018). Altered dynamics of neurovascular coupling in cadasil. Ann. Clin. Transl. Neurol. 5, 788–802. doi: 10.1002/acn3.574
Iadecola, C. (2004). Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat. Rev. Neurosci. 5, 347–360. doi: 10.1038/nrn1387
Iadecola, C. (2017). The neurovascular unit coming of age: a journey through neurovascular coupling in health and disease. Neuron 96, 17–42. doi: 10.1016/j.neuron.2017.07.030
Iftinca, M. C., and Zamponi, G. W. (2009). Regulation of neuronal T-type calcium channels. Trends Pharmacol. Sci. 30, 32–40. doi: 10.1016/j.tips.2008.10.004
Iino, M. (1990). Biphasic Ca2+ dependence of inositol 1,4,5-trisphosphate-induced Ca release in smooth muscle cells of the guinea pig taenia caeci. J. Gen. Physiol. 95, 1103–1122. doi: 10.1085/jgp.95.6.1103
Inada, H., Kawabata, F., Ishimaru, Y., Fushiki, T., Matsunami, H., and Tominaga, M. (2008). Off-response property of an acid-activated cation channel complex PKD1L3-PKD2L1. EMBO Rep. 9, 690–697. doi: 10.1038/embor.2008.89
Ishizaki, E., Fukumoto, M., and Puro, D. G. (2009). Functional KATP channels in the rat retinal microvasculature: topographical distribution, redox regulation, spermine modulation and diabetic alteration. J. Physiol. 587, 2233–2253. doi: 10.1113/jphysiol.2009.169003
Iturria-Medina, Y., Sotero, R. C., Toussaint, P. J., Mateos-Pérez, J. M., Evans, A. C., and Alzheimer's Disease Neuroimaging Initiative (2016). Early role of vascular dysregulation on late-onset Alzheimer's disease based on multifactorial data-driven analysis. Nat. Commun. 21:11934. doi: 10.1038/ncomms11934
Iwai, M., Michikawa, T., Bosanac, I., Ikura, M., and Mikoshiba, K. (2007). Molecular basis of the isoform-specific ligand-binding affinity of inositol 1,4,5-trisphosphate receptors. J. Biol. Chem. 282, 12755–12764. doi: 10.1074/jbc.M609833200
Jackson, W. F. (2018). Kv channels and the regulation of vascular smooth muscle tone. Microcirculation 25:e12421. doi: 10.1111/micc.12421
Jacobson, K. A., and Gao, Z. G. (2006). Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 5, 247–264. doi: 10.1038/nrd1983
Janiurek, M. M., Soylu-Kucharz, R., Christoffersen, C., Kucharz, K., and Lauritzen, M. (2019). Apolipoprotein M-bound sphingosine-1-phosphate regulates blood–brain barrier paracellular permeability and transcytosis. Elife 8:e49405. doi: 10.1101/684894
Jentsch, T. J., Friedrich, T., Schriever, A., and Yamada, H. (1999). The CLC chloride channel family. Pflügers Arch. 437, 783–795. doi: 10.1007/s004240050847
Jentsch, T. J., Stein, V., Weinreich, F., and Zdebik, A. A. (2002). Molecular structure and physiological function of chloride channels. Physiol. Rev. 82, 503–568. doi: 10.1152/physrev.00029.2001
Jeon, J. P., Hong, C., Park, E. J., Jeon, J. H., Cho, N. H., Kim, I. G., et al. (2012). Selective Gαi subunits as novel direct activators of transient receptor potential canonical (TRPC)4 and TRPC5 channels. J. Biol. Chem. 287, 17029–17039. doi: 10.1074/jbc.M111.326553
Jiang, Y., Lee, A., Chen, J., Ruta, V., Cadene, M., Chait, B. T., et al. (2003). X-ray structure of a voltage-dependent K+ channel. Nature 423, 33–41. doi: 10.1038/nature01580
Jiao, J., Garg, V., Yang, B., Elton, T. S., and Hu, K. (2008). Protein kinase C-ε; induces caveolin-dependent internalization of vascular adenosine 5′-triphosphate-sensitive K+ channels. Hypertension 52, 499–506. doi: 10.1161/HYPERTENSIONAHA.108.110817
Jin, X., Shah, S., Liu, Y., Zhang, H., Lees, M., Fu, Z., et al. (2013). Activation of the Cl− channel ANO1 by localized calcium signals in nociceptive sensory neurons requires coupling with the IP3 receptor. Sci. Signal. 6, ra73. doi: 10.1126/scisignal.2004184
Johnson, G. C., Parsons, R., May, V., and Hammack, S. E. (2020). The role of pituitary adenylate cyclase-activating polypeptide (PACAP) signaling in the hippocampal dentate gyrus. Front. Cell. Neurosci. 14:111. doi: 10.3389/fncel.2020.00111
Jones, S. V. P. (2003). Role of the small GTPase Rho in modulation of the inwardly rectifying potassium channel Kir2.1. Mol. Pharmacol. 64, 987–993. doi: 10.1124/mol.64.4.987
Kach, J., Sethakorn, N., and Dulin, N. O. (2012). A finer tuning of G-protein signaling through regulated control of RGS proteins. Am. J. Physiol. Hear. Circ. Physiol. 303, H19–H35. doi: 10.1152/ajpheart.00764.2011
Kaczynski, P., Kowalewski, M. P., and Waclawik, A. (2016). Prostaglandin F2α promotes angiogenesis and embryo-maternal interactions during implantation. Reproduction 151, 539–552. doi: 10.1530/REP-15-0496
Kajioka, S., Kitamura, K., and Kuriyama, H. (1991). Guanosine diphosphate activates an adenosine 5'-triphosphate-sensitive K+ channel in the rabbit portal vein. J. Physiol. 444, 397–418. doi: 10.1113/jphysiol.1991.sp018885
Kamouchi, M., and Kitamura, K. (1994). Regulation of ATP-sensitive K+ channels by ATP and nucleotide diphosphate in rabbit portal vein. Am. J. Physiol. Hear. Circ. Physiol. 266, H1687–H1698. doi: 10.1152/ajpheart.1994.266.5.H1687
Kamp, T. J., and Hell, J. W. (2000). Regulation of Cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 87, 1095–1102. doi: 10.1161/01.RES.87.12.1095
Kapusta, D. R., Dayan, L. A., and Kenigs, V. A. (2002). Nociceptin/orphanin FQ modulates the cardiovascular, but not renal, responses to stress in spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 29, 254–259. doi: 10.1046/j.1440-1681.2002.03639.x
Katritch, V., Fenalti, G., Abola, E. E., Roth, B. L., Cherezov, V., and Stevens, R. C. (2014). Allosteric sodium in class A GPCR signaling. Trends Biochem. Sci. 39, 233–244. doi: 10.1016/j.tibs.2014.03.002
Katz, B. (1949). Les constantes electriques de la membrane du muscle. Arch. Sci. Physiol. 3, 285–299.
Kawamura, H., Sugiyama, T., Wu, D. M., Kobayashi, M., Yamanishi, S., Katsumura, K., et al. (2003). ATP: a vasoactive signal in the pericyte-containing microvasculature of the rat retina. J. Physiol. 551, 787–799. doi: 10.1113/jphysiol.2003.047977
Kawamura, H., Oku, H., Li, Q., Sakagami, K., and Puro, D. G. (2002). Endothelin-induced changes in the physiology of retinal pericytes. Invest. Opthalmol. Vis. Sci. 43, 882–888. Available online at: https://iovs.arvojournals.org/article.aspx?articleid=2200149
Kawate, T., Michel, J. C., Birdsong, W. T., and Gouaux, E. (2009). Crystal structure of the ATP-gated P2X4 ion channel in the closed state. Nature 460, 592–598. doi: 10.1038/nature08198
Kennedy, A. J., Yang, P., Read, C., Kuc, R. E., Yang, L., Taylor, E. J. A., et al. (2016). Chemerin elicits potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein-coupled receptor 1 (GPR1), in human and rat vasculature. J. Am. Heart Assoc. 5:e004421. doi: 10.1161/JAHA.116.004421
Kerage, D., Brindley, D. N., and Hemmings, D. G. (2014). Review: Novel insights into the regulation of vascular tone by sphingosine 1-phosphate. Placenta 35, S86–S92. doi: 10.1016/j.placenta.2013.12.006
Khakh, B. S., Burnstock, G., Kennedy, C., King, B. F., North, R. A., Séguéla, P., et al. (2001). International union of pharmacology. XXIV. Current status of the nomenclature and properties of P2X receptors and their subunits. Pharmacol. Rev. 53, 107–118.
Khan, S. M., Sung, J. Y., and Hébert, T. E. (2016). Gβγ subunits—different spaces, different faces. Pharmacol. Res. 111, 434–441. doi: 10.1016/j.phrs.2016.06.026
Kilander, M. B. C., Petersen, J., Andressen, K. W., Ganji, R. S., Levy, F. O., Schuster, J., et al. (2014). Disheveled regulates precoupling of heterotrimeric G proteins to Frizzled 6. FASEB J. 28, 2293–2305. doi: 10.1096/fj.13-246363
Kim, K. S., Jang, J. H., Lin, H., Choi, S. W., Kim, H. R., Shin, D. H., et al. (2015). Rise and fall of Kir2.2 current by TLR4 signaling in human monocytes: PKC-dependent trafficking and PI3K-mediated PIP2 decrease. J. Immunol. 195, 3345–3354. doi: 10.4049/jimmunol.1500056
Kisler, K., Nelson, A. R., Rege, S. V., Ramanathan, A., Wang, Y., Ahuja, A., et al. (2017). Pericyte degeneration leads to neurovascular uncoupling and limits oxygen supply to brain. Nat. Neurosci. 20, 406–441. doi: 10.1038/nn.4489
Kitamura, K., and Yamazaki, J. (2001). Chloride channels and their functional roles in smooth muscle tone in the vasculature. Jpn. J. Pharmacol. 85, 351–357. doi: 10.1254/jjp.85.351
Kleppisch, T., and Nelson, M. T. (1995). Adenosine activates ATP-sensitive potassium channels in arterial myocytes via A2 receptors and cAMP-dependent protein kinase. Proc. Natl. Acad. Sci. U.S.A. 92, 12441–12445. doi: 10.1073/pnas.92.26.12441
Ko, K. R., Ngai, A. C., and Winn, H. R. (1990). Role of adenosine in regulation of regional cerebral blood flow in sensory cortex. Am. J. Physiol. Hear. Circ. Physiol. 259, H1703-H1708. doi: 10.1152/ajpheart.1990.259.6.H1703
Kochukov, M. Y., Balasubramanian, A., Abramowitz, J., Birnbaumer, L., and Marrelli, S. P. (2014). Activation of endothelial transient receptor potential C3 channel is required for small conductance calcium-activated potassium channel activation and sustained endothelial hyperpolarization and vasodilation of cerebral artery. J. Am. Heart Assoc. 3:e000913. doi: 10.1161/JAHA.114.000913
Kofuji, P., and Newman, E. A. (2004). Potassium buffering in the central nervous system. Neuroscience 129, 1045–1056. doi: 10.1016/j.neuroscience.2004.06.008
Koide, M., Bonev, A. D., Nelson, M. T., and Wellman, G. C. (2012). Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc. Natl. Acad. Sci. U.S.A. 109, E1387–E1395. doi: 10.1073/pnas.1121359109
Koide, M., Syed, A. U., Braas, K. M., May, V., and Wellman, G. C. (2014). Pituitary adenylate cyclase activating polypeptide (PACAP) dilates cerebellar arteries through activation of large-conductance Ca2+-activated (BK) and ATP-sensitive (KATP) K+ channels. J. Mol. Neurosci. 54, 443–450. doi: 10.1007/s12031-014-0301-z
Koide, M., Moshkforoush, A., Tsoukias, N. M., Hill-Eubanks, D. C., Wellman, G. C., Nelson, M. T., et al. (2018). The yin and yang of Kv channels in cerebral small vessel pathologies. Microcirculation 25, 1–10. doi: 10.1111/micc.12436
Kovacs, R. J., and Nelson, M. T. (1991). ATP-sensitive K+ channels from aortic smooth muscle incorporated into planar lipid bilayers. Am. J. Physiol. Hear. Circ. Physiol. 261, H604–H609. doi: 10.1152/ajpheart.1991.261.2.H604
Kovacs-Oller, T., Ivanova, E., Bianchimano, P., and Sagdullaev, B. T. (2020). The pericyte connectome: spatial precision of neurovascular coupling is driven by selective connectivity maps of pericytes and endothelial cells and is disrupted in diabetes. Cell Discov. 6:39. doi: 10.1038/s41421-020-0180-0
Koval, M., Molina, S. A., and Burt, J. M. (2014). Mix and match: investigating heteromeric and heterotypic gap junction channels in model systems and native tissues. FEBS Lett. 588, 1193–1204. doi: 10.1016/j.febslet.2014.02.025
Kozielewicz, P., Turku, A., and Schulte, G. (2020). Molecular pharmacology of class F receptor activation. Mol. Pharmacol. 97, 62–71. doi: 10.1124/mol.119.117986
Ksiazek, M., Chacińska, M., Chabowski, A., and Baranowski, M. (2015). Sources, metabolism, and regulation of circulating sphingosine-1-phosphate. J. Lipid Res. 56, 1271–1281. doi: 10.1194/jlr.R059543
Kurz, H. (2009). Cell lineages and early patterns of embryonic CNS vascularization. Cell Adhes. Migr. 3, 205–210. doi: 10.4161/cam.3.2.7855
Lacroix, A., Toussay, X., Anenberg, E., Lecrux, C., Ferreirós, N., Karagiannis, A., et al. (2015). COX-2-derived prostaglandin E2 produced by pyramidal neurons contributes to neurovascular coupling in the rodent cerebral cortex. J. Neurosci. 35, 11791–11810. doi: 10.1523/JNEUROSCI.0651-15.2015
Lagerström, M. C., and Schiöth, H. B. (2008). Structural diversity of G protein coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7, 339–357. doi: 10.1038/nrd2518
Lazarowski, E. (2006). Regulated release of nucleotides and UDP sugars from astrocytoma cells. Novartis Found. Symp. 276, 73–84. doi: 10.1002/9780470032244.ch7
Lazarowski, E. R. (2012). Vesicular and conductive mechanisms of nucleotide release. Purinergic Signal. 8, 359–373. doi: 10.1007/s11302-012-9304-9
Lazarowski, E. R., and Harden, T. K. (2015). UDP-sugars as extracellular signaling molecules: cellular and physiologic consequences of P2Y14 receptor activation. Mol. Pharmacol. 88, 151–160. doi: 10.1124/mol.115.098756
Ledoux, J., Werner, M. E., Brayden, J. E., and Nelson, M. T. (2006). Calcium-activated potassium channels and the regulation of vascular tone. Physiology 21, 69–78. doi: 10.1152/physiol.00040.2005
Lee, A., Scheuer, T., and Catterall, W. A. (2000). Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J. Neurosci. 20, 6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000
Lee, A., Wong, S. T., Gallagher, D., Li, B., Storm, D. R., Scheuer, T., et al. (1999). Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature 399, 155–159. doi: 10.1038/20194
Lee-Kwon, W., Goo, J. H., Zhang, Z., Silldorff, E. P., and Pallone, T. L. (2007). Vasa recta voltage-gated Na+ channel Nav1.3 is regulated by calmodulin. Am. J. Physiol. Ren. Physiol. 292, F404–F414. doi: 10.1152/ajprenal.00070.2006
Lefkimmiatis, K., and Zaccolo, M. (2014). cAMP signaling in subcellular compartments. Pharmacol. Ther. 143, 295–304. doi: 10.1016/j.pharmthera.2014.03.008
Leloir, L. F., Olavarría, J. M., Goldemberg, S. H., and Carminatti, H. (1959). Biosynthesis of glycogen from uridine diphosphate glucose. Arch. Biochem. Biophys. 81, 508–520. doi: 10.1016/0003-9861(59)90232-2
Li, B., and Freeman, R. D. (2015). Neurometabolic coupling between neural activity, glucose and lactate in activated visual cortex. J. Neurochem. 135, 742–754. doi: 10.1111/jnc.13143
Li, J., Hou, B., Tumova, S., Muraki, K., Bruns, A., Ludlow, M. J., et al. (2014). Piezo1 integration of vascular architecture with physiological force. Nature 515, 279–282. doi: 10.1038/nature13701
Li, M., van Esch, B. C. A. M., Henricks, P. A. J., Folkerts, G., and Garssen, J. (2018). The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide- or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 9:533. doi: 10.3389/fphar.2018.00533
Li, N., Wu, J. X., Ding, D., Cheng, J., Gao, N., and Chen, L. (2017). Structure of a pancreatic ATP-sensitive potassium channel. Cell 168, 101–110. doi: 10.1016/j.cell.2016.12.028
Li, Q., and Puro, D. G. (2001). Adenosine activates ATP-sensitive K+ currents in pericytes of rat retinal microvessels: role of A1 and A2a receptors. Brain Res. 907, 93–99. doi: 10.1016/S0006-8993(01)02607-5
Li, Y., Wang, F., Zhang, X., Qi, Z., Tang, M., Szeto, C., et al. (2012). β-adrenergic stimulation increases Cav3.1 activity in cardiac myocytes through protein kinase A. PLoS ONE 7:e39965. doi: 10.1371/journal.pone.0039965
Li, Z., Zhang, W., and Mulholland, M. W. (2015). LGR4 and its role in intestinal protection and energy metabolism. Front. Endocrinol. 6:131. doi: 10.3389/fendo.2015.00131
Lin, H., Pallone, T. L., and Cao, C. (2010). Murine vasa recta pericyte chloride conductance is controlled by calcium, depolarization, and kinase activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 299, R1317–R1325. doi: 10.1152/ajpregu.00129.2010
Lintschinger, B., Balzer-Geldsetzer, M., Baskaran, T., Graier, W. F., Christoph, R., Zhu, M. X., et al. (2000). Coassembly of Trp1 and Trp3 proteins generates diacylglycerol- and Ca2+-sensitive cation channels. J. Biol. Chem. 275, 27799–27805. doi: 10.1074/jbc.M002705200
Lipscombe, D., Helton, T. D., and Xu, W. (2004). L-type calcium channels: the low down. J. Neurophysiol. 92, 2633–2641. doi: 10.1152/jn.00486.2004
Liu, G., Papa, A., Katchman, A. N., Zakharov, S. I., Roybal, D., Hennessey, J. A., et al. (2020). Mechanism of adrenergic Cav1.2 stimulation revealed by proximity proteomics. Nature 577, 695–700. doi: 10.1038/s41586-020-1947-z
Liu, H., Enyeart, J. A., and Enyeart, J. J. (2010). ACTH induces Cav3.2 current and mRNA by cAMP-dependent and cAMP-independent mechanisms. J. Biol. Chem. 285, 20040–20050. doi: 10.1074/jbc.M110.104190
Liu, Y., Beyer, A., and Aebersold, R. (2016). On the dependency of cellular protein levels on mRNA abundance. Cell 165, 535–550. doi: 10.1016/j.cell.2016.03.014
Liu, Y., Zhang, Z., Wang, Y., Song, J., Ma, K., Si, J., et al. (2018). Electrophysiological properties of strial pericytes and the effect of aspirin on pericyte K+ channels. Mol. Med. Rep. 17, 2861–2868. doi: 10.3892/mmr.2017.8194
Lock, J. T., and Parker, I. (2020). IP3 mediated global Ca2+ signals arise through two temporally and spatially distinct modes of Ca2+ release. Elife 9:e55008. doi: 10.7554/eLife.55008.sa2
Lolicato, M., Riegelhaupt, P. M., Arrigoni, C., Clark, K. A., and Minor Jr., D. L. (2014). Transmembrane helix straightening and buckling underlies activation of mechanosensitive and thermosensitive K2P channels. Neuron 84, 1198–1212. doi: 10.1016/j.neuron.2014.11.017
Longden, T., Harraz, O., Hennig, G., Shui, B., Lee, F., Lee, J., et al. (2019). Neural activity drives dynamic Ca2+ signals in capillary endothelial cells that shape local brain blood flow. FASEB J. 33:688. doi: 10.1096/fasebj.2019.33.1_supplement.688.8
Longden, T. A., Dabertrand, F., Hill-Eubanks, D. C., Hammack, S. E., and Nelson, M. T. (2014). Stress-induced glucocorticoid signaling remodels neurovascular coupling through impairment of cerebrovascular inwardly rectifying K+ channel function. Proc. Natl. Acad. Sci. U.S.A. 111, 7462–7467. doi: 10.1073/pnas.1401811111
Longden, T. A., Dabertrand, F., Koide, M., Gonzales, A. L., Tykocki, N. R., Brayden, J. E., et al. (2017). Capillary K+-sensing initiates retrograde hyperpolarization to locally increase cerebral blood flow. Nat. Neurosci. 20, 717–726. doi: 10.1038/nn.4533
Longden, T. A., Hill-Eubanks, D. C., and Nelson, M. T. (2016). Ion channel networks in the control of cerebral blood flow. J. Cereb. Blood Flow Metab. 36, 492–512. doi: 10.1177/0271678X15616138
Longden, T. A., and Nelson, M. T. (2015). Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 22, 183–196. doi: 10.1111/micc.12190
Luvisetto, S., Fellin, T., Spagnolo, M., Hivert, B., Brust, P. F., Harpold, M. M., et al. (2004). Modal gating of human Cav2.1 (P/Q-type) calcium channels: I. The slow and the fast gating modes and their modulation by β subunits. J. Gen. Physiol. 124, 445–461. doi: 10.1085/jgp.200409034
Ma, L., and Dorling, A. (2012). The roles of thrombin and protease-activated receptors in inflammation. Semin. Immunopathol. 34, 63–72. doi: 10.1007/s00281-011-0281-9
MacDonald, B., Tamai, K., and He, X. (2009). Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell 17, 9–26. doi: 10.1016/j.devcel.2009.06.016
MacDonald, P. E., Joseph, J. W., and Rorsman, P. (2005). Glucose-sensing mechanisms in pancreatic β-cells. Philos. Trans. R. Soc. London. Ser. B, Biol. Sci. 360, 2211–2225. doi: 10.1098/rstb.2005.1762
Maguire, J. J., and Davenport, A. P. (2015). Endothelin receptors and their antagonists. Semin. Nephrol. 35, 125–136. doi: 10.1016/j.semnephrol.2015.02.002
Mahapatra, S., Marcantoni, A., Zuccotti, A., Carabelli, V., and Carbone, E. (2012). Equal sensitivity of Cav1.2 and Cav1.3 channels to the opposing modulations of PKA and PKG in mouse chromaffin cells. J. Physiol. 590, 5053–5073. doi: 10.1113/jphysiol.2012.236729
Mahon, M. J. (2012). The parathyroid hormone receptorsome and the potential for therapeutic intervention. Curr. Drug Targets 13, 116–128. doi: 10.2174/138945012798868416
Makani, S., and Chesler, M. (2010). Rapid rise of extracellular pH evoked by neural activity is generated by the plasma membrane calcium ATPase. J. Neurophysiol. 103, 667–676. doi: 10.1152/jn.00948.2009
Mani, B. K., Robakowski, C., Brueggemann, L. I., Cribbs, L. L., Tripathi, A., Majetschak, M., et al. (2016). Kv7.5 potassium channel subunits are the primary targets for PKA-Dependent enhancement of vascular smooth muscle Kv7 currents. Mol. Pharmacol. 89, 323–334. doi: 10.1124/mol.115.101758
Markworth, E., Schwanstecher, C., and Schwanstecher, M. (2000). ATP4- mediates closure of pancreatic beta-cell ATP-sensitive potassium channels by interaction with 1 of 4 identical sites. Diabetes 49, 1413–1418. doi: 10.2337/diabetes.49.9.1413
Masago, K., Kihara, Y., Yanagida, K., Hamano, F., Nakagawa, S., Niwa, M., et al. (2018). Lysophosphatidic acid receptor, LPA6, regulates endothelial blood-brain barrier function: implication for hepatic encephalopathy. Biochem. Biophys. Res. Commun. 501, 1048–1054. doi: 10.1016/j.bbrc.2018.05.106
Mathew, R. J., Wilson, W. H., Tant, S. R., Robinson, L., and Prakash, R. (1988). Abnormal resting regional cerebral blood flow patterns and their correlates in schizophrenia. Arch. Gen. Psychiatry 45, 542–549. doi: 10.1001/archpsyc.1988.01800300038004
Mathiisen, T. M., Lehre, K. P., Danbolt, N. C., and Ottersen, O. P. (2010). The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia 58, 1094–1103. doi: 10.1002/glia.20990
Matsushita, K., Fukumoto, M., Kobayashi, T., Kobayashi, M., Ishizaki, E., Minami, M., et al. (2010). Diabetes-induced inhibition of voltage-dependent calcium channels in the retinal microvasculature: role of spermine. Investig. Opthalmology Vis. Sci. 51, 5979–5990. doi: 10.1167/iovs.10-5377
Matsushita, K., and Puro, D. G. (2006). Topographical heterogeneity of Kir currents in pericyte-containing microvessels of the rat retina: effect of diabetes. J. Physiol. 573, 483–495. doi: 10.1113/jphysiol.2006.107102
Maudsley, S., Martin, B., and Luttrell, L. M. (2005). The origins of diversity and specificity in G protein-coupled receptor signaling. J. Pharmacol. Exp. Ther. 314, 485–494. doi: 10.1124/jpet.105.083121
May, V., Lutz, E., MacKenzie, C., Schutz, K. C., Dozark, K., and Braas, K. M. (2010). Pituitary adenylate cyclase-activating polypeptide (PACAP)/PAC 1HOP1 receptor activation coordinates multiple neurotrophic signaling pathways: akt activation through phosphatidylinositol 3-kinase γ and vesicle endocytosis for neuronal survival. J. Biol. Chem. 285, 9749–9761. doi: 10.1074/jbc.M109.043117
Mayor, S., and Pagano, R. E. (2007). Pathways of clathrin-independent endocytosis. Nat. Rev. Mol. Cell Biol. 8, 603–612. doi: 10.1038/nrm2216
Mazzotti, C., Gagliostro, V, Bosisio, D., Del Prete, A., Tiberio, L., Thelen, M. M., et al. (2017). The atypical receptor CCRL2 (C-C Chemokine Receptor-Like 2) does not act as a decoy receptor in endothelial cells. Front. Immunol. 8:1233. doi: 10.3389/fimmu.2017.01233
McGrory, S., Ballerini, L., Doubal, F. N., Staals, J., Allerhand, M., Valdes-Hernandez, M., et al. (2019). Retinal microvasculature and cerebral small vessel disease in the lothian birth cohort 1936 and mild stroke study. Sci. Rep. 9:6320. doi: 10.1038/s41598-019-42534-x
Means, C. K., and Brown, J. H. (2009). Sphingosine-1-phosphate receptor signalling in the heart. Cardiovasc. Res. 82, 193–200. doi: 10.1093/cvr/cvp086
Mehta, D., Ahmmed, G. U., Paria, B. C., Holinstat, M., Voyno-Yasenetskaya, T., Tiruppathi, C., et al. (2003). RhoA interaction with inositol 1,4,5-trisphosphate receptor and transient receptor potential channel-1 regulates Ca2+ entry: role in signaling increased endothelial permeability. J. Biol. Chem. 278, 33492–33500. doi: 10.1074/jbc.M302401200
Mestre, H., Tithof, J., Du, T., Song, W., Peng, W., Sweeney, A. M., et al. (2018). Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat. Commun. 9:4878. doi: 10.1038/s41467-018-07318-3
Miki, T., Liss, B., Minami, K., Shiuchi, T., Saraya, A., Kashima, Y., et al. (2001). ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat. Neurosci. 4, 507–512. doi: 10.1038/87455
Mikoshiba, K. (2015). Role of IP3 receptor signaling in cell functions and diseases. Adv. Biol. Regul. 57, 217–227. doi: 10.1016/j.jbior.2014.10.001
Miller, A. N., and Long, S. B. (2012). Crystal structure of the human two-pore domain potassium channel K2P1. Science 335, 432–436. doi: 10.1126/science.1213274
Mironov, S. L., and Skorova, E. Y. (2011). Stimulation of bursting in pre-Bötzinger neurons by Epac through calcium release and modulation of TRPM4 and K-ATP channels. J. Neurochem. 117, 295–308. doi: 10.1111/j.1471-4159.2011.07202.x
Mishra, A., Reynolds, J. P., Chen, Y., Gourine, A. V., Rusakov, D. A., and Attwell, D. (2016). Astrocytes mediate neurovascular signaling to capillary pericytes but not to arterioles. Nat. Neurosci. 19, 1619–1627. doi: 10.1038/nn.4428
Miyata, N., and Roman, R. J. (2005). Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J. Smooth Muscle Res. 41, 175–193. doi: 10.1540/jsmr.41.175
Mogi, M., and Horiuchi, M. (2011). Neurovascular coupling in cognitive impairment associated with diabetes mellitus. Circ. J. 75, 1042–1048. doi: 10.1253/circj.CJ-11-0121
Muraki, K., Iwata, Y., Katanosaka, Y., Ito, T., Ohya, S., Shigekawa, M., et al. (2003). TRPV2 is a component of osmotically sensitive cation channels in murine aortic myocytes. Circ. Res. 93, 829–838. doi: 10.1161/01.RES.0000097263.10220.0C
Muszkat, M., Kurnik, D., Solus, J., Sofowora, G. G., Xie, H. G., Jiang, L., et al. (2005). Variation in the α2B-adrenergic receptor gene (ADRA2B) and its relationship to vascular response in vivo. Pharmacogenet. Genomics 15, 407–414. doi: 10.1097/01213011-200506000-00006
Nalli, A. D., Kumar, D. P., Al-Shboul, O., Mahavadi, S., Kuemmerle, J. F., Grider, J. R., et al. (2014). Regulation of Gβγi-dependent PLC-β3 activity in smooth muscle: inhibitory phosphorylation of PLC-β3 by PKA and PKG and stimulatory phosphorylation of Gαi-GTPase-activating protein RGS2 by PKG. Cell Biochem. Biophys. 70, 867–880. doi: 10.1007/s12013-014-9992-6
Narayanan, D., Bulley, S., Leo, M. D., Burris, S. K., Gabrick, K. S., Boop, F. A., et al. (2013). Smooth muscle cell transient receptor potential polycystin-2 (TRPP2) channels contribute to the myogenic response in cerebral arteries. J. Physiol. 591, 5031–5046. doi: 10.1113/jphysiol.2013.258319
Nelson, M. T., Patlak, J. B., Worley, J. F., and Standen, N. B. (1990). Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am. J. Physiol. Cell Physiol. 259, C3–C18. doi: 10.1152/ajpcell.1990.259.1.C3
Nelson, M. T., and Quayle, J. M. (1995). Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. Cell Physiol. 268, C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799
Newman, E. (1986). High potassium conductance in astrocyte endfeet. Science 233, 453–454. doi: 10.1126/science.3726539
Newman, E. A. (2013). Functional hyperemia and mechanisms of neurovascular coupling in the retinal vasculature. J. Cereb. Blood Flow Metab. 33, 1685–1695. doi: 10.1038/jcbfm.2013.145
Newton, A. C. (2010). Protein kinase C: poised to signal. Am. J. Physiol. Endocrinol. Metab. 298, E395–E402. doi: 10.1152/ajpendo.00477.2009
Nichols, A. S., Floyd, D. H., Bruinsma, S. P., Narzinski, K., and Baranski, T. J. (2013). Frizzled receptors signal through G proteins. Cell. Signal. 25, 1468–1475. doi: 10.1016/j.cellsig.2013.03.009
Nicolakakis, N., and Hamel, E. (2011). Neurovascular function in Alzheimer's disease patients and experimental models. J. Cereb. Blood Flow Metab. 31, 1354–1370. doi: 10.1038/jcbfm.2011.43
Nilius, B., and Droogmans, G. (2003). Amazing chloride channels: an overview. Acta Physiol. Scand. 177, 119–147. doi: 10.1046/j.1365-201X.2003.01060.x
Nilius, B., and Owsianik, G. (2011). The transient receptor potential family of ion channels. Genome Biol. 12:218. doi: 10.1186/gb-2011-12-3-218
Nilius, B., Owsianik, G., Voets, T., and Peters, J. A. (2007). Transient receptor potential cation channels in disease. Physiol. Rev. 87, 165–217. doi: 10.1152/physrev.00021.2006
Nilius, B., Prenen, J., Tang, J., Wang, C., Owsianik, G., Janssens, A., et al. (2005). Regulation of the Ca2+ sensitivity of the nonselective cation channel TRPM4. J. Biol. Chem. 280, 6423–6433. doi: 10.1074/jbc.M411089200
Nishioka, K., Nishida, M., Ariyoshi, M., Jian, Z., Saiki, S., Hirano, M., et al. (2011). Cilostazol suppresses angiotensin II-induced vasoconstriction via protein kinase A-mediated phosphorylation of the transient receptor potential canonical 6 channel. Arterioscler. Thromb. Vasc. Biol. 31, 2278–2286. doi: 10.1161/ATVBAHA.110.221010
Niswender, C. M., and Conn, P. J. (2010). Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 50, 295–322. doi: 10.1146/annurev.pharmtox.011008.145533
Noorbakhsh, F., Vergnolle, N., Hollenberg, M. D., and Power, C. (2003). Proteinase-activated receptors in the nervous system. Nat. Rev. Neurosci. 4, 981–990. doi: 10.1038/nrn1255
Nortley, R., Mishra, A., Jaunmuktane, Z., Kyrargyri, V., Madry, C., Gong, H., et al. (2019). Amyloid? oligomers constrict human capillaries in Alzheimer's disease via signalling to pericytes. Science 365:300. doi: 10.1126/science.aav9518
Ohkita, M., Tawa, M., Kitada, K., and Matsumura, Y. (2012). Pathophysiological roles of endothelin receptors in cardiovascular diseases. J. Pharmacol. Sci. 119, 302–313. doi: 10.1254/jphs.12R01CR
Olah, M. E. (1997). Identification of A2a adenosine receptor domains involved in selective coupling to Gs: analysis of chimeric A1/A2a adenosine receptors. J. Biol. Chem. 272, 337–344. doi: 10.1074/jbc.272.1.337
Orkand, R. K., Nicholls, J. G., and Kuffler, S. W. (1966). Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol. 29, 788–806. doi: 10.1152/jn.1966.29.4.788
Owen, N. E. (1984). Regulation of Na/K/Cl cotransport in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 125, 500–508. doi: 10.1016/0006-291X(84)90568-0
Öz, G., DiNuzzo, M., Kumar, A., Moheet, A., and Seaquist, E. R. (2015). Revisiting glycogen content in the human brain. Neurochem. Res. 40, 2473–2481. doi: 10.1007/s11064-015-1664-4
Ozen, G., Benyahia, C., Amgoud, Y., Patel, J., Abdelazeem, H., Bouhadoun, A., et al. (2020). Interaction between PGI2 and ET-1 pathways in vascular smooth muscle from Group-III pulmonary hypertension patients. Prostaglandins Other Lipid Mediat. 146:106388. doi: 10.1016/j.prostaglandins.2019.106388
Paik, J. H., Skoura, A., Chae, S. S., Cowan, A. E., Han, D. K., Proia, R. L., et al. (2004). Sphingosine 1-phosphate receptor regulation of N-cadherin mediates vascular stabilization. Genes Dev. 18, 2392–2403. doi: 10.1101/gad.1227804
Palazzo, E., Marabese, I., de Novellis, V., Rossi, F., and Maione, S. (2016). Metabotropic glutamate receptor 7: from synaptic function to therapeutic implications. Curr. Neuropharmacol. 14, 504–513. doi: 10.2174/1570159X13666150716165323
Park, J. Y., Kang, H. W., Moon, H. J., Huh, S. U., Jeong, S. W., Soldatov, N. M., et al. (2006). Activation of protein kinase C augments T-type Ca2+ channel activity without changing channel surface density. J. Physiol. 577, 513–523. doi: 10.1113/jphysiol.2006.117440
Patel, C., Narayanan, S. P., Zhang, W., Xu, Z., Sukumari-Ramesh, S., Dhandapani, K. M., et al. (2014). Activation of the endothelin system mediates pathological angiogenesis during ischemic retinopathy. Am. J. Pathol. 184, 3040–3051. doi: 10.1016/j.ajpath.2014.07.012
Patton, N., Aslam, T., MacGillivray, T., Pattie, A., Deary, I. J., and Dhillon, B. (2005). Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 206, 319–348. doi: 10.1111/j.1469-7580.2005.00395.x
Paulino, C., Kalienkova, V., Lam, A. K. M., Neldner, Y., and Dutzler, R. (2017). Activation mechanism of the calcium-activated chloride channel TMEM16A revealed by cryo-EM. Nature 552, 421–425. doi: 10.1038/nature24652
Paulson, O. B., Hasselbalch, S. G., Rostrup, E., Knudsen, G. M., and Pelligrino, D. (2010). Cerebral blood flow response to functional activation. J. Cereb. Blood Flow Metab. 30, 2–14. doi: 10.1038/jcbfm.2009.188
Paulson, O. B., Strandgaard, S., and Edvinsson, L. (1990). Cerebral autoregulation. Cerebrovasc. Brain Metab. Rev. 2, 161–192.
Pearson-Leary, J., and McNay, E. C. (2016). Novel roles for the insulin-regulated glucose transporter-4 in hippocampally dependent memory. J. Neurosci. 36, 11851–11864. doi: 10.1523/JNEUROSCI.1700-16.2016
Pébay, A., and Wong, R. C. B. (2017). Lipidomics of Stem Cells. Cham: Humana Press. doi: 10.1007/978-3-319-49343-5
Pedersen, S. F., Owsianik, G., and Nilius, B. (2005). TRP channels: an overview. Cell Calcium 38, 233–252. doi: 10.1016/j.ceca.2005.06.028
Pelligrino, D. A., Vetri, F., and Xu, H. L. (2011). Purinergic mechanisms in gliovascular coupling. Semin. Cell Dev. Biol. 22, 229–236. doi: 10.1016/j.semcdb.2011.02.010
Peppiatt, C. M., Howarth, C., Mobbs, P., and Attwell, D. (2006). Bidirectional control of CNS capillary diameter by pericytes. Nature 443, 700–704. doi: 10.1038/nature05193
Perálvarez-Marín, A., Doñate-Macian, P., and Gaudet, R. (2013). What do we know about the transient receptor potential vanilloid 2 (TRPV2) ion channel? FEBS J. 280, 5471–5487. doi: 10.1111/febs.12302
Perez-Reyes, E. (2003). Molecular physiology of low-voltage-activated T-type calcium channels. Physiol. Rev. 83, 117–161. doi: 10.1152/physrev.00018.2002
Peterson, B. Z., DeMaria, C. D., and Yue, D. T. (1999). Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron 22, 549–558. doi: 10.1016/S0896-6273(00)80709-6
Pitt, S. J., Reilly-O'Donnell, B., and Sitsapesan, R. (2016). Exploring the biophysical evidence that mammalian two-pore channels are NAADP-activated calcium-permeable channels. J. Physiol. 594, 4171–4179. doi: 10.1113/JP270936
Platania, C. B. M., Giurdanella, G., Di Paola, L., Leggio, G. M., Drago, F., Salomone, S., et al. (2017). P2X7 receptor antagonism: implications in diabetic retinopathy. Biochem. Pharmacol. 138, 130–139. doi: 10.1016/j.bcp.2017.05.001
Poyner, D. R., Sexton, P. M., Marshall, I., Smith, D. M., Quirion, R., Born, W., et al. (2002). International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol. Rev. 54, 233–246. doi: 10.1124/pr.54.2.233
Pozzi, A., Yurchenco, P. D., and Iozzo, R. V. (2017). The nature and biology of basement membranes. Matrix Biol. 57–58, 1–11. doi: 10.1016/j.matbio.2016.12.009
Prakriya, M., and Lewis, R. S. (2015). Store-operated calcium channels. Physiol. Rev. 95, 1383–1436. doi: 10.1152/physrev.00020.2014
Praticò, D., and Dogné, J. M. (2005). Selective cyclooxygenase-2 inhibitors development in cardiovascular medicine. Circulation 112, 1073–1079. doi: 10.1161/CIRCULATIONAHA.104.524231
Proks, P., de Wet, H., and Ashcroft, F. M. (2010). Activation of the K(ATP) channel by Mg-nucleotide interaction with SUR1. J. Gen. Physiol. 136, 389–405. doi: 10.1085/jgp.201010475
Prossnitz, E. R., and Arterburn, J. B. (2015). International union of basic and clinical pharmacology. XCVII. G protein–coupled estrogen receptor and its pharmacologic modulators. Pharmacol. Rev. 67, 505–540. doi: 10.1124/pr.114.009712
Purves, G. I., Kamishima, T., Davies, L. M., Quayle, J. M., and Dart, C. (2009). Exchange protein activated by cAMP (Epac) mediates cAMP-dependent but protein kinase A-insensitive modulation of vascular ATP-sensitive potassium channels. J. Physiol. 587, 3639–3650. doi: 10.1113/jphysiol.2009.173534
Quayle, J. M., Nelson, M. T., and Standen, N. B. (1997). ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 77, 1165–1232. doi: 10.1152/physrev.1997.77.4.1165
Quayle, J. M., Turner, M. R., Burrell, H. E., and Kamishima, T. (2006). Effects of hypoxia, anoxia, and metabolic inhibitors on KATP channels in rat femoral artery myocytes. Am. J. Physiol. Hear. Circ. Physiol. 291, H71–H80. doi: 10.1152/ajpheart.01107.2005
Querques, G., Borrelli, E., Sacconi, R., De Vitis, L., Leocani, L., Santangelo, R., et al. (2019). Functional and morphological changes of the retinal vessels in Alzheimer's disease and mild cognitive impairment. Sci. Rep. 9:63. doi: 10.1038/s41598-018-37271-6
Quignard, J., Harley, E., Duhault, J., Vanhoutte, P., and Félétou, M. (2003). K+ channels in cultured bovine retinal pericytes: effects of β-adrenergic stimulation. J. Cardiovasc. Pharmacol. 42, 379–388. doi: 10.1097/00005344-200309000-00009
Quinn, K. V., Cui, Y., Giblin, J. P., Clapp, L. H., and Tinker, A. (2003). Do anionic phospholipids serve as cofactors or second messengers for the regulation of activity of cloned ATP-sensitive K+ channels? Circ. Res. 93, 646–655. doi: 10.1161/01.RES.0000095247.81449.8E
Quinn, K. V., Giblin, J. P., and Tinker, A. (2004). Multisite phosphorylation mechanism for protein kinase A activation of the smooth muscle ATP-sensitive K+ channel. Circ. Res. 94, 1359–1366. doi: 10.1161/01.RES.0000128513.34817.c4
Raifman, T. K., Kumar, P., Haase, H., Klussmann, E., Dascal, N., and Weiss, S. (2017). Protein kinase C enhances plasma membrane expression of cardiac L-type calcium channel, Cav1.2. Channels 11, 604–615. doi: 10.1080/19336950.2017.1369636
Ramos, D., Navarro, M., Mendes-Jorge, L., Carretero, A., López-Luppo, M., Nacher, V., et al. (2013). “The use of confocal laser microscopy to analyze mouse retinal blood vessels,” in Confocal Laser Microscopy - Principles and Applications in Medicine, Biology, and the Food Sciences, ed N. Lagali (London: IntechOpen), 19–37. doi: 10.5772/56131
Ranade, S. S., Qiu, Z., Woo, S. H., Hur, S. S., Murthy, S. E., Cahalan, S. M., et al. (2014). Piezo1, a mechanically activated ion channel, is required for vascular development in mice. Proc. Natl. Acad. Sci. U.S.A. 111, 10347–10352. doi: 10.1073/pnas.1409233111
Ratelade, J., Klug, N. R., Lombardi, D., Angelim, M. K. S. C., Dabertrand, F., Domenga-Denier, V., et al. (2020). Reducing hypermuscularization of the transitional segment between arterioles and capillaries protects against spontaneous intracerebral hemorrhage. Circulation 141, 2078–2094. doi: 10.1161/CIRCULATIONAHA.119.040963
Reichhart, N., Schöberl, S., Keckeis, S., Alfaar, A. S., Roubeix, C., Cordes, M., et al. (2019). Anoctamin-4 is a bona fide Ca2+-dependent non-selective cation channel. Sci. Rep. 9:2257. doi: 10.1038/s41598-018-37287-y
Robertson, B. E., and Nelson, M. T. (1994). Aminopyridine inhibition and voltage dependence of K+ currents in smooth muscle cells from cerebral arteries. Am. J. Physiol. Cell Physiol. 267, C1589–C1597. doi: 10.1152/ajpcell.1994.267.6.C1589
Ross, E. M., and Wilkie, T. M. (2000). GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 69, 795–827. doi: 10.1146/annurev.biochem.69.1.795
Rossier, M. F. (2016). T-type calcium channel: a privileged gate for calcium entry and control of adrenal steroidogenesis. Front. Endocrinol. 7:43. doi: 10.3389/fendo.2016.00043
Rouach, N., Koulakoff, A., Abudara, V., Willecke, K., and Giaume, C. (2008). Astroglial metabolic networks sustain hippocampal synaptic transmission. Science 322, 1551–1555. doi: 10.1126/science.1164022
Rouget, C. (1873). Mémoire sur le développement, la structure et les proprietés physiologiques des capillaires sanguins et lymphatiques. Arch. Physiol. Norm. Pathol. 5, 603–663.
Rungta, R. L., Chaigneau, E., Osmanski, B.-F., and Charpa, S. (2018). Vascular compartmentalization of functional hyperemia from the synapse to the pia. Neuron 99, 362–337. doi: 10.1016/j.neuron.2018.06.012
Russell, F. D., and Davenport, A. P. (1999). Secretory pathways in endothelin synthesis. Br. J. Pharmacol. 126, 391–398. doi: 10.1038/sj.bjp.0702315
Sadana, R., and Dessauer, C. W. (2009). Physiological roles for G protein-regulated adenylyl cyclase isoforms: insights from knockout and overexpression studies. NeuroSignals 17, 5–22. doi: 10.1159/000166277
Sakagami, K., Wu, D. M., and Puro, D. G. (1999). Physiology of rat retinal pericytes: modulation of ion channel activity by serum-derived molecules. J. Physiol. 521, 637–650. doi: 10.1111/j.1469-7793.1999.00637.x
Salomone, S., Soydan, G., Ip, P. C. T., Hopson, K. M. P., and Waeber, C. (2010). Vessel-specific role of sphingosine kinase 1 in the vasoconstriction of isolated basilar arteries. Pharmacol. Res. 62, 465–474. doi: 10.1016/j.phrs.2010.09.002
Sasaki, Y., Hoshi, M., Akazawa, C., Nakamura, Y., Tsuzuki, H., Inoue, K., et al. (2003). Selective expression of Gi/o-coupled ATP receptor P2Y12 in microglia in rat brain. Glia 44, 242–250. doi: 10.1002/glia.10293
Sassone-Corsi, P. (2012). The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol. 4:a011148. doi: 10.1101/cshperspect.a011148
Sawyer, I., Smillie, S. J., Bodkin, J. V., Fernandes, E., O'Byrne, K. T., and Brain, S. D. (2011). The vasoactive potential of kisspeptin-10 in the peripheral vasculature. PLoS ONE 6:e14671. doi: 10.1371/journal.pone.0014671
Scherer, D., Seyler, C., Xynogalos, P., Scholz, E. P., Thomas, D., Backs, J., et al. (2016). Inhibition of cardiac Kir current (IK1) by protein kinase C critically depends on PKCβ and Kir2.2. PLoS ONE 11:e0156181. doi: 10.1371/journal.pone.0156181
Schlingmann, K. P., Waldegger, S., Konrad, M., Chubanov, V., and Gudermann, T. (2007). TRPM6 and TRPM7–gatekeepers of human magnesium metabolism. Biochim. Biophys. Acta 1772, 813–821. doi: 10.1016/j.bbadis.2007.03.009
Schulte, G. (2010). International Union of Basic and Clinical Pharmacology. LXXX. The class frizzled receptors. Pharmacol. Rev. 62, 632–667. doi: 10.1124/pr.110.002931
Schwartz, E., Adamany, A. M., and Blumenfeld, O. O. (1981). Isolation and characterization of the internal elastic lamina from calf thoracic aorta. Exp. Mol. Pathol. 34, 299–306. doi: 10.1016/0014-4800(81)90047-2
Seino, S., and Miki, T. (2003). Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog. Biophys. Mol. Biol. 81, 133–176. doi: 10.1016/S0079-6107(02)00053-6
Sekiguchi, F., and Kawabata, A. (2013). T-type calcium channels: functional regulation and implication in pain signaling. J. Pharmacol. Sci. 122, 244–250. doi: 10.1254/jphs.13R05CP
Selim, S., Sunkara, M., Salous, A. K., Leung, S. W., Berdyshev, E. V., Bailey, A., et al. (2011). Plasma levels of sphingosine 1-phosphate are strongly correlated with haematocrit, but variably restored by red blood cell transfusions. Clin. Sci. 121, 565–572. doi: 10.1042/CS20110236
Sharif-Naeini, R., Folgering, J. H. A., Bichet, D., Duprat, F., Lauritzen, I., Arhatte, M., et al. (2009). Polycystin-1 and−2 dosage regulates pressure sensing. Cell 139, 587–596. doi: 10.1016/j.cell.2009.08.045
Sherwood, T. W., Frey, E. N., and Askwith, C. C. (2012). Structure and activity of the acid-sensing ion channels. Am. J. Physiol. Cell Physiol. 303, C699–C710. doi: 10.1152/ajpcell.00188.2012
Shi, Y., Chen, X., Wu, Z., Shi, W., Yang, Y., Cui, N., et al. (2008a). cAMP-dependent protein kinase phosphorylation produces interdomain movement in SUR2B leading to activation of the vascular KATP channel. J. Biol. Chem. 283, 7523–7530. doi: 10.1074/jbc.M709941200
Shi, Y., Cui, N., Shi, W., and Jiang, C. (2008b). A short motif in Kir6.1 consisting of four phosphorylation repeats underlies the vascular KATP channel inhibition by protein kinase C. J. Biol. Chem. 283, 2488–2494. doi: 10.1074/jbc.M708769200
Shi, Y., Wu, Z., Cui, N., Shi, W., Yang, Y., Zhang, X., et al. (2007). PKA phosphorylation of SUR2B subunit underscores vascular KATP channel activation by beta-adrenergic receptors. Am. J. Physiol. Regul. Integr. Comp. Physiol. 293, R1205–R1214. doi: 10.1152/ajpregu.00337.2007
Shih, A. Y., Rühlmann, C., Blinder, P., Devor, A., Drew, P. J., Friedman, B., et al. (2015). Robust and fragile aspects of cortical blood flow in relation to the underlying angioarchitecture. Microcirculation 22, 204–218. doi: 10.1111/micc.12195
Shima, Y., Kawaguchi, S. Y., Kosaka, K., Nakayama, M., Hoshino, M., Nabeshima, Y., et al. (2007). Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nat. Neurosci. 10, 963–969. doi: 10.1038/nn1933
Shyng, S. L., Ferrigni, T., and Nichols, C. G. (1997). Regulation of KATP channel activity by diazoxide and MgADP. Distinct functions of the two nucleotide binding folds of the sulfonylurea receptor. J. Gen. Physiol. 110, 643–654. doi: 10.1085/jgp.110.6.643
Shyng, S. L., and Nichols, C. G. (1998). Membrane phospholipid control of nucleotide sensitivity of KATP channels. Science 282, 1138–1141. doi: 10.1126/science.282.5391.1138
Silva, A. S., and Zanesco, A. (2010). Physical exercise, β-adrenergic receptors, and vascular response. J. Vasc. Bras. 9, 47–56. doi: 10.1590/S1677-54492010000200007
Simon, M. I., Strathmann, M. P., and Gautam, N. (1991). Diversity of G proteins in signal transduction. Science 252, 802–808. doi: 10.1126/science.1902986
Singh, A., Gebhart, M., Fritsch, R., Sinnegger-Brauns, M. J., Poggiani, C., Hoda, J. C., et al. (2008). Modulation of voltage- and Ca2+-dependent gating of Cav1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J. Biol. Chem. 283, 20733–20744. doi: 10.1074/jbc.M802254200
Singh, J., Wen, X., and Scales, S. J. (2015). The orphan G protein-coupled receptor Gpr175 (Tpra40) enhances Hedgehog signaling by modulating cAMP levels. J. Biol. Chem. 290, 29663–29675. doi: 10.1074/jbc.M115.665810
Smith, I. F., and Parker, I. (2009). Imaging the quantal substructure of single IP3R channel activity during Ca2+ puffs in intact mammalian cells. Proc. Natl. Acad. Sci. U.S.A. 106, 6404–6409. doi: 10.1073/pnas.0810799106
Smrcka, A. V. (2008). G protein βγ subunits: Central mediators of G protein-coupled receptor signaling. Cell. Mol. Life Sci. 65, 2191–2214. doi: 10.1007/s00018-008-8006-5
Soboloff, J., Spassova, M. A., Tang, X. D., Hewavitharana, T., Xu, W., and Gill, D. L. (2006). Orai1 and STIM reconstitute store-operated calcium channel function. J. Biol. Chem. 281, 20661–20665. doi: 10.1074/jbc.C600126200
Soh, U. J., Dores, M. R., Chen, B., and Trejo, J. (2010). Signal transduction by protease-activated receptors. Br. J. Pharmacol. 160, 191–203. doi: 10.1111/j.1476-5381.2010.00705.x
Sokolovsky, M. (1995). Endothelin receptor subtypes and their role in transmembrane signaling mechanisms. Pharmacol. Ther. 68, 435–471. doi: 10.1016/0163-7258(95)02015-2
Souza Bomfim, G. H., Costiniti, V., Li, Y., Idaghdour, Y., and Lacruz, R. S. (2020). TRPM7 activation potentiates SOCE in enamel cells but requires ORAI. Cell Calcium 87:102187. doi: 10.1016/j.ceca.2020.102187
Stirling, L., Williams, M. R., and Morielli, A. D. (2009). Dual roles for RhoA/Rho-kinase in the regulated trafficking of a voltage-sensitive potassium channel. Mol. Biol. Cell 20, 2991–3002. doi: 10.1091/mbc.e08-10-1074
Stölting, G., Teodorescu, G., Begemann, B., Schubert, J., Nabbout, R., Toliat, M. R., et al. (2013). Regulation of ClC-2 gating by intracellular ATP. Pflügers Arch. 465, 1423–1437. doi: 10.1007/s00424-013-1286-0
Stott, J. B., Povstyan, O. V., Carr, G., Barrese, V., and Greenwood, I. A. (2015). G-protein βγ subunits are positive regulators of Kv7.4 and native vascular Kv7 channel activity. Proc. Natl. Acad. Sci. U.S.A. 112, 6497–6502. doi: 10.1073/pnas.1418605112
Straub, A., Zeigler, A., and Isakson, B. (2014). The myoendothelial junction: connections that deliver the message. Physiology 29, 242–249. doi: 10.1152/physiol.00042.2013
Straub, S. V., Girouard, H., Doetsch, P. E., Hannah, R. M., Wilkerson, M. K., and Nelson, M. T. (2009). Regulation of intracerebral arteriolar tone by Kv channels: effects of glucose and PKC. Am. J. Physiol. Cell Physiol. 297, C788–C796. doi: 10.1152/ajpcell.00148.2009
Striessnig, J., Pinggera, A., Kaur, G., Bock, G., and Tuluc, P. (2014). L-type Ca2+ channels in heart and brain. Wiley Interdiscip. Rev. Membr. Transp. Signal. 3, 15–38. doi: 10.1002/wmts.102
Sugimura, R., He, X. C., Venkatraman, A., Arai, F., Box, A., Semerad, C., et al. (2012). Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 150, 351–365. doi: 10.1016/j.cell.2012.05.041
Sugiyama, T., Kawamura, H., Yamanishi, S., Kobayashi, M., Katsumura, K., and Puro, D. G. (2005). Regulation of P2X7-induced pore formation and cell death in pericyte-containing retinal microvessels. Am. J. Physiol. 288, C568–C576. doi: 10.1152/ajpcell.00380.2004
Sung, T. S., Jeon, J. P., Kim, B. J., Hong, C., Kim, S. Y., Kim, J., et al. (2011). Molecular determinants of PKA-dependent inhibition of TRPC5 channel. Am. J. Physiol. Cell Physiol. 301, C823–C832. doi: 10.1152/ajpcell.00351.2010
Suzuki, J., Umeda, M., Sims, P. J., and Nagata, S. (2010). Calcium-dependent phospholipid scrambling by TMEM16F. Nature 468, 834–838. doi: 10.1038/nature09583
Swärd, K., Mita, M., Wilson, D. P., Deng, J. T., Susnjar, M., and Walsh, M. P. (2003). The role of RhoA and Rho-associated kinase in vascular smooth muscle contraction. Curr. Hypertens. Rep. 5, 66–72. doi: 10.1007/s11906-003-0013-1
Tadross, M. R., Johny, M., Ben, and Yue, D. T. (2010). Molecular endpoints of Ca2+/calmodulin- and voltage-dependent inactivation of Cav1.3 channels. J. Gen. Physiol. 135, 197–215. doi: 10.1085/jgp.200910308
Tadross, M. R., and Yue, D. T. (2010). Systematic mapping of the state dependence of voltage- and Ca2+-dependent inactivation using simple open-channel measurements. J. Gen. Physiol. 135, 217–227. doi: 10.1085/jgp.200910309
Takezawa, R., Schmitz, C., Demeuse, P., Scharenberg, A. M., Penner, R., and Fleig, A. (2004). Receptor-mediated regulation of the TRPM7 channel through its endogenous protein kinase domain. Proc. Natl. Acad. Sci. U.S.A. 101, 6009–6014. doi: 10.1073/pnas.0307565101
Tanabe, K., Tucker, S. J., Ashcroft, F. M., Proks, P., Kioka, N., Amachi, T., et al. (2000). Direct photoaffinity labeling of Kir6.2 by [gamma-(32)P]ATP-[gamma]4-azidoanilide. Biochem. Biophys. Res. Commun. 272, 316–319. doi: 10.1006/bbrc.2000.2780
Tarasov, A., Dusonchet, J., and Ashcroft, F. (2004). Metabolic regulation of the pancreatic beta-cell ATP-sensitive K+ channel: a pas de deux. Diabetes 53, S113–S122. doi: 10.2337/diabetes.53.suppl_3.S113
Taylor, M. S., Bonev, A. D., Gross, T. P., Eckman, D. M., Brayden, J. E., Bond, C. T., et al. (2003). Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulates arterial tone and blood pressure. Circ. Res. 93, 124–131. doi: 10.1161/01.RES.0000081980.63146.69
Tejada, M. A., Stople, K., Bomholtz, S. H., Meinild, A.-K., Poulsen, A. N., and Klaerke, D. A. (2014). Cell volume changes regulate slick (Slo2.1), but not slack (Slo2.2) K+ channels. PLoS ONE 9:e110833. doi: 10.1371/journal.pone.0110833
Thiriet, M. (2013). Tissue Functioning and Remodeling in the Circulatory and Ventilatory Systems. Verlag; New York, NY: Springer. doi: 10.1007/978-1-4614-5966-8
Thorin, E., and Webb, D. J. (2010). Endothelium-derived endothelin-1. Pflugers Arch. 459, 951–958. doi: 10.1007/s00424-009-0763-y
Tilton, R. G., Kilo, C., and Williamson, J. R. (1979). Pericyte-endothelial relationships in cardiac and skeletal muscle capillaries. Microvasc. Res. 18, 325–335. doi: 10.1016/0026-2862(79)90041-4
Tobo, M., Tomura, H., Mogi, C., Wang, J. Q., Liu, J. P., Komachi, M., et al. (2007). Previously postulated “ligand-independent” signaling of GPR4 is mediated through proton-sensing mechanisms. Cell. Signal. 19, 1745–1753. doi: 10.1016/j.cellsig.2007.03.009
Tovey, S. C., Dedos, S. G., Rahman, T., Taylor, E. J. A., Pantazaka, E., and Taylor, C. W. (2010). Regulation of inositol 1,4,5-trisphosphate receptors by cAMP independent of cAMP-dependent protein kinase. J. Biol. Chem. 285, 12979–12989. doi: 10.1074/jbc.M109.096016
Trost, A., Lange, S., Schroedl, F., Bruckner, D., Motloch, K. A., Bogner, B., et al. (2016). Brain and retinal pericytes: Origin, function and role. Front. Cell. Neurosci. 10:20. doi: 10.3389/fncel.2016.00020
Tsai, P. S., Kaufhold, J. P., Blinder, P., Friedman, B., Drew, P. J., Karten, H. J., et al. (2009). Correlations of neuronal and microvascular densities in murine cortex revealed by direct counting and colocalization of nuclei and vessels. J. Neurosci. 29, 14553–14570. doi: 10.1523/JNEUROSCI.3287-09.2009
Tu, H., Wang, Z., Nosyreva, E., Smedt, H., De, and Bezprozvanny, I. (2005). Functional characterization of mammalian inositol 1,4,5-trisphosphate receptor isoforms. Biophys. J. 88, 1046–1055. doi: 10.1529/biophysj.104.049593
Tucker, S. J., Gribble, F. M., Zhao, C., Trapp, S., and Ashcroft, F. M. (1997). Truncation of Kir6.2 produces ATP-sensitive K+ channels in the absence of the sulphonylurea receptor. Nature 387, 179–183. doi: 10.1038/387179a0
Tung, R. T., and Kurachi, Y. (1991). On the mechanism of nucleotide diphosphate activation of the ATP-sensitive K+ channel in ventricular cell of guinea-pig. J. Physiol. 437, 239–256. doi: 10.1113/jphysiol.1991.sp018593
Ullrich, N. D., Voets, T., Prenen, J., Vennekens, R., Talavera, K., Droogmans, G., et al. (2005). Comparison of functional properties of the Ca2+-activated cation channels TRPM4 and TRPM5 from mice. Cell Calcium 37, 267–278. doi: 10.1016/j.ceca.2004.11.001
Upchurch, C., and Leitinger, N. (2019). “Biologically active lipids in vascular biology,” in Fundamentals of Vascular Biology. Learning Materials in Biosciences, ed M. Geiger (Cham: Springer), 171–193. doi: 10.1007/978-3-030-12270-6_9
Urtatiz, O., and Van Raamsdonk, C. D. (2016). Gnaq and Gna11 in the Endothelin signaling pathway and melanoma. Front. Genet. 7:59. doi: 10.3389/fgene.2016.00059
van de Kreeke, J. A., Nguyen, H. T., Konijnenberg, E., Tomassen, J., den Braber, A., ten Kate, M., et al. (2018). Retinal and cerebral microvasculopathy: Relationships and their genetic contributions. Investig. Ophthalmol. Vis. Sci. 59, 5025–5031. doi: 10.1167/iovs.18-25341
Vanderheyden, V., Devogelaere, B., Missiaen, L., De Smedt, H., Bultynck, G., and Parys, J. B. (2009). Regulation of inositol 1,4,5-trisphosphate-induced Ca2+ release by reversible phosphorylation and dephosphorylation. Biochim. Biophys. Acta 1793, 959–970. doi: 10.1016/j.bbamcr.2008.12.003
Vanlandewijck, M., He, L., Mäe, M. A., Andrae, J., Ando, K., Gaudio, F., et al. (2018). A molecular atlas of cell types and zonation in the brain vasculature. Nature 554, 475–480. doi: 10.1038/nature25739
Veldhuis, N. A., Poole, D. P., Grace, M., McIntyre, P., Bunnett, N. W., and Christopoulos, A. (2015). The G protein–coupled receptor–transient receptor potential channel axis: molecular insights for targeting disorders of sensation and inflammation. Pharmacol. Rev. 67, 36–73. doi: 10.1124/pr.114.009555
Venkatachalam, K., and Montell, C. (2007). TRP Channels. Annu. Rev. Biochem. 76, 387–417. doi: 10.1146/annurev.biochem.75.103004.142819
Venkatachalam, K., Wong, C.-O., and Zhu, M. X. (2015). The role of TRPMLs in endolysosomal trafficking and function. Cell Calcium 58, 48–56. doi: 10.1016/j.ceca.2014.10.008
Vergarajauregui, S., Oberdick, R., Kiselyov, K., and Puertollano, R. (2008). Mucolipin 1 channel activity is regulated by protein kinase A-mediated phosphorylation. Biochem. J. 410, 417–425. doi: 10.1042/BJ20070713
Vermassen, E., Fissore, R. A., Kasri, N. N., Vanderheyden, V., Callewaert, G., Missiaen, L., et al. (2004). Regulation of the phosphorylation of the inositol 1,4,5-trisphosphate receptor by protein kinase C. Biochem. Biophys. Res. Commun. 319, 888–893. doi: 10.1016/j.bbrc.2004.05.071
Vetri, F., Xu, H., Paisansathan, C., and Pelligrino, D. A. (2012). Impairment of neurovascular coupling in type 1 diabetes mellitus in rats is linked to PKC modulation of BKCa and Kir channels. Am. J. Physiol. Hear. Circ. Physiol. 302, H1274–H1284. doi: 10.1152/ajpheart.01067.2011
Vilardaga, J. P., Romero, G., Friedman, P. A., and Gardella, T. J. (2011). Molecular basis of parathyroid hormone receptor signaling and trafficking: a family B GPCR paradigm. Cell. Mol. Life Sci. 68, 1–13. doi: 10.1007/s00018-010-0465-9
Villari, A., Giurdanella, G., Bucolo, C., Drago, F., and Salomone, S. (2017). Apixaban enhances vasodilatation mediated by protease-activated receptor 2 in isolated rat arteries. Front. Pharmacol. 8:480. doi: 10.3389/fphar.2017.00480
von Bartheld, C. S., Bahney, J., and Herculano-Houzel, S. (2016). The search for true numbers of neurons and glial cells in the human brain: a review of 150 years of cell counting. J. Comp. Neurol. 524, 3865–3895. doi: 10.1002/cne.24040
von Beckerath, N., Nees, S., Neumann, F. J., Krebs, B., Juchem, G., and Schömig, A. (2000). An inward rectifier and a voltage-dependent K+ current in single, cultured pericytes from bovine heart. Cardiovasc. Res. 46, 569–578. doi: 10.1016/S0008-6363(00)00055-9
Wagner, T. F. J., Loch, S., Lambert, S., Straub, I., Mannebach, S., Mathar, I., et al. (2008). Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic β cells. Nat. Cell Biol. 10, 1421–1430. doi: 10.1038/ncb1801
Wang, B., Li, C., Huai, R., and Qu, Z. (2015). Overexpression of ANO1/TMEM16A, an arterial Ca2+-activated Cl− channel, contributes to spontaneous hypertension. J. Mol. Cell. Cardiol. 82, 22–32. doi: 10.1016/j.yjmcc.2015.02.020
Wang, Q., Leo, M. D., Narayanan, D., Kuruvilla, K. P., and Jaggar, J. H. (2016). Local coupling of TRPC6 to ANO1/TMEM16A channels in smooth muscle cells amplifies vasoconstriction in cerebral arteries. Am. J. Physiol. Cell Physiol. 310, C1001–C1009. doi: 10.1152/ajpcell.00092.2016
Wang, Y., and Venton, B. J. (2017). Correlation of transient adenosine release and oxygen changes in the caudate-putamen. J. Neurochem. 140, 13–23. doi: 10.1111/jnc.13705
Watson, N., Linder, M. E., Druey, K. M., Kehrl, J. H., and Blumer, K. J. (1996). RGS family members: GTPase-activating proteins for heterotrimeric G- protein α-subunits. Nature 383, 172–175. doi: 10.1038/383172a0
Watts, A. O., Verkaar, F., Van Der Lee, M. M. C., Timmerman, C. A. W., Kuijer, M., Offenbeek, J., et al. (2013). β-Arrestin recruitment and G protein signaling by the atypical human chemokine decoy receptor CCX-CKR. J. Biol. Chem. 288, 7169–7181. doi: 10.1074/jbc.M112.406108
Weiß, K. T., Fante, M., Köhl, G., Schreml, J., Haubner, F., Kreutz, M., et al. (2017). Proton-sensing G protein-coupled receptors as regulators of cell proliferation and migration during tumor growth and wound healing. Exp. Dermatol. 26, 127–132. doi: 10.1111/exd.13209
Wheeler, D. G., Groth, R. D., Ma, H., Barrett, C. F., Owen, S. F., Safa, P., et al. (2012). Cav1 and Cav2 channels engage distinct modes of Ca2+ signaling to control CREB-dependent gene expression. Cell 149, 1112–1124. doi: 10.1016/j.cell.2012.03.041
Wihlborg, A. K., Wang, L., Braun, O. Ö., Eyjolfsson, A., Gustafsson, R., Gudbjartsson, T., et al. (2004). ADP receptor P2Y12 is expressed in vascular smooth muscle cells and stimulates contraction in human blood vessels. Arterioscler. Thromb. Vasc. Biol. 24, 1810–1815. doi: 10.1161/01.ATV.0000142376.30582.ed
Winkler, E. A., Bell, R. D., and Zlokovic, B. V. (2011). Central nervous system pericytes in health and disease. Nat. Neurosci. 14, 1398–1405. doi: 10.1038/nn.2946
Woodward, D. F., Jones, R. L., and Narumiya, S. (2011). International union of basic and clinical pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacol. Rev. 63, 471–538. doi: 10.1124/pr.110.003517
Worzfeld, T., Wettschureck, N., and Offermanns, S. (2008). G12/G13-mediated signalling in mammalian physiology and disease. Trends Pharmacol. Sci. 29, 582–589. doi: 10.1016/j.tips.2008.08.002
Woszczek, G., Chen, L.-Y., Nagineni, S., Alsaaty, S., Harry, A., Logun, C., et al. (2007). IFN-γ induces cysteinyl leukotriene receptor 2 expression and enhances the responsiveness of human endothelial cells to cysteinyl leukotrienes. J. Immunol. 178, 5262–5270. doi: 10.4049/jimmunol.178.8.5262
Wroblewska, B., Santi, M. R., and Neale, J. H. (1998). N-acetylaspartylglutamate activates cyclic AMP-coupled metabotropic glutamate receptors in cerebellar astrocytes. Glia 24, 172–179. doi: 10.1002/(SICI)1098-1136(199810)24:2<172::AID-GLIA2>3.0.CO;2-6
Wu, D. M., Kawamura, H., Sakagami, K., Kobayashi, M., and Puro, D. G. (2003). Cholinergic regulation of pericyte-containing retinal microvessels. Am. J. Physiol. Hear. Circ. Physiol. 284, H2083–H2090. doi: 10.1152/ajpheart.01007.2002
Wu, J., Lewis, A. H., and Grandl, J. (2017). Touch, tension, and transduction – the function and regulation of piezo ion channels. Trends Biochem. Sci. 42, 57–71. doi: 10.1016/j.tibs.2016.09.004
Wu, Y., Shyng, S. L., and Chen, P. C. (2015). Concerted trafficking regulation of Kv2.1 and KATP channels by leptin in pancreatic β-cells. J. Biol. Chem. 290, 29676–29690. doi: 10.1074/jbc.M115.670877
Xi, Q., Adebiyi, A., Zhao, G., Chapman, K. E., Waters, C. M., Hassid, A., et al. (2009). IP3 constricts cerebral arteries via IP3 receptor-mediated TRPC3 channel activation and independently of sarcoplasmic reticulum Ca2+ release. Circ. Res. 105:e1. doi: 10.1161/CIRCRESAHA.108.173948
Xiao, Q., Yu, K., Perez-Cornejo, P., Cui, Y., Arreola, J., and Hartzell, H. C. (2011). Voltage- and calcium-dependent gating of TMEM16A/Ano1 chloride channels are physically coupled by the first intracellular loop. Proc. Natl. Acad. Sci. U.S.A. 108, 8891–8896. doi: 10.1073/pnas.1102147108
Xiao, X., Liu, H. X., Shen, K., Cao, W., and Li, X. Q. (2017). Canonical transient receptor potential channels and their link with cardio/cerebro-vascular diseases. Biomol. Ther. 25, 471–481. doi: 10.4062/biomolther.2016.096
Xu, W., and Lipscombe, D. (2001). Neuronal Cav1.3α1 L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J. Neurosci. 21, 5944–5951. doi: 10.1523/JNEUROSCI.21-16-05944.2001
Yamada, M., Isomoto, S., Matsumoto, S., Kondo, C., Shindo, T., Horio, Y., et al. (1997). Sulphonylurea receptor 2B and Kir6.1 form a sulphonylurea-sensitive but ATP-insensitive K+ channel. J. Physiol. 499, 715–720. doi: 10.1113/jphysiol.1997.sp021963
Yang, G., Xu, J., Li, T., Ming, J., Chen, W., and Liu, L. (2010). Role of V1a receptor in AVP-induced restoration of vascular hyporeactivity and its relationship to MLCP-MLC20 phosphorylation pathway. J. Surg. Res. 161, 312–320. doi: 10.1016/j.jss.2009.01.005
Yang, H., Kim, A., David, T., Palmer, D., Jin, T., Tien, J., et al. (2012). TMEM16F forms a Ca2+-activated cation channel required for lipid scrambling in platelets during blood coagulation. Cell 151, 111–122. doi: 10.1016/j.cell.2012.07.036
Yang, L., Liu, G., Zakharov, S. I., Morrow, J. P., Rybin, V. O., Steinberg, S. F., et al. (2005). Ser1928 is a common site for Cav1.2 phosphorylation by protein kinase C isoforms. J. Biol. Chem. 280, 207–214. doi: 10.1074/jbc.M410509200
Yang, Y. (2012). Wnt signaling in development and disease. Cell Biosci. 2:14. doi: 10.1186/2045-3701-2-14
Yang, Y. D., Cho, H., Koo, J. Y., Tak, M. H., Cho, Y., Shim, W. S., et al. (2008). TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature 455, 1210–1215. doi: 10.1038/nature07313
Yin, J., and Kuebler, W. M. (2010). Mechanotransduction by TRP channels: general concepts and specific role in the vasculature. Cell Biochem. Biophys. 56, 1–18. doi: 10.1007/s12013-009-9067-2
Yu, F. H., and Catterall, W. A. (2003). Overview of the voltage-gated sodium channel family. Genome Biol. 4:207. doi: 10.1186/gb-2003-4-3-207
Yuan, K., Orcholski, M. E., Panaroni, C., Shuffle, E. M., Huang, N. F., Jiang, X., et al. (2015). Activation of the wnt/planar cell polarity pathway is required for pericyte recruitment during pulmonary angiogenesis. Am. J. Pathol. 185, 69–84. doi: 10.1016/j.ajpath.2014.09.013
Yudin, Y., and Rohacs, T. (2018). Inhibitory Gi/o-coupled receptors in somatosensory neurons: potential therapeutic targets for novel analgesics. Mol. Pain 14:1744806918763646. doi: 10.1177/1744806918763646
Yue, R., Li, H., Liu, H., Li, Y., Wei, B., Gao, G., et al. (2012). Thrombin receptor regulates hematopoiesis and endothelial-to-hematopoietic transition. Dev. Cell 22, 1092–1100. doi: 10.1016/j.devcel.2012.01.025
Zamponi, G. W., and Currie, K. P. M. (2013). Regulation of Cav2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta 1828, 1629–1643. doi: 10.1016/j.bbamem.2012.10.004
Zamponi, G. W., Striessnig, J., Koschak, A., Dolphin, A. C., and Sibley, D. R. (2015). The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 67, 821–870. doi: 10.1124/pr.114.009654
Zeisel, A., Hochgerner, H., Lönnerberg, P., Johnsson, A., Memic, F., van der Zwan, J., et al. (2018). Molecular architecture of the mouse nervous system. Cell 174, 999.e22–1014.e22. doi: 10.1016/j.cell.2018.06.021
Zhang, H. L., and Bolton, T. B. (1996). Two types of ATP-sensitive potassium channels in rat portal vein smooth muscle cells. Br. J. Pharmacol. 118, 105–114. doi: 10.1111/j.1476-5381.1996.tb15372.x
Zhang, Q., Cao, C., Zhang, Z., Wier, W. G., Edwards, A., and Pallone, T. L. (2008). Membrane current oscillations in descending vasa recta pericytes. Am. J. Physiol. Ren. Physiol. 294, F656–F666. doi: 10.1152/ajprenal.00493.2007
Zhang, T., Wu, D. M., Xu, G., and Puro, D. G. (2011). The electrotonic architecture of the retinal microvasculature: modulation by angiotensin II. J. Physiol. 589, 2383–2399. doi: 10.1113/jphysiol.2010.202937
Zhang, Y., Ji, H., Wang, J., Sun, Y., Qian, Z., Jiang, X., et al. (2018). Melatonin-mediated inhibition of Cav3.2 T-type Ca2+ channels induces sensory neuronal hypoexcitability through the novel protein kinase C-eta isoform. J. Pineal Res. 64:e12476. doi: 10.1111/jpi.12476
Zhang, Y. J., Zhang, L., Ye, Y. L., Fang, S. H., Zhou, Y., Zhang, W. P., et al. (2006). Cysteinyl leukotriene receptors CysLT1 and CysLT2 are upregulated in acute neuronal injury after focal cerebral ischemia in mice. Acta Pharmacol. Sin. 27, 1553–1560. doi: 10.1111/j.1745-7254.2006.00458.x
Zhao, G., Joca, H. C., Nelson, M. T., and Lederer, W. J. (2020). ATP- And voltage-dependent electro-metabolic signaling regulates blood flow in heart. Proc. Natl. Acad. Sci. U. S. A. 117, 7461–7470. doi: 10.1073/pnas.1922095117
Zhao, Q., Wu, K., Geng, J., Chi, S., Wang, Y., Zhi, P., et al. (2016). Ion permeation and mechanotransduction mechanisms of mechanosensitive piezo channels. Neuron 89, 1248–1263. doi: 10.1016/j.neuron.2016.01.046
Zhao, Q., Zhou, H., Chi, S., Wang, Y., Wang, J., Geng, J., et al. (2018). Structure and mechanogating mechanism of the Piezo1 channel. Nature 554, 487–492. doi: 10.1038/nature25743
Zhu, J., Zhuo, C., Xu, L., Liu, F., Qin, W., and Yu, C. (2017). Altered coupling between resting-state cerebral blood flow and functional connectivity in schizophrenia. Schizophr. Bull. 43, 1363–1374. doi: 10.1093/schbul/sbx051
Zimmerli, D. (2018). Elucidating the Roles of Wnt-Secretion and β-Catenin's Interaction Partners in Development and Disease. University of Zurich.
Keywords: pericytes, ion channels, GPCRs (G protein coupled receptors), neurovascular coupling (NVC), cerebral blood flow (CBF), KATP channels, brain metabolism
Citation: Hariharan A, Weir N, Robertson C, He L, Betsholtz C and Longden TA (2020) The Ion Channel and GPCR Toolkit of Brain Capillary Pericytes. Front. Cell. Neurosci. 14:601324. doi: 10.3389/fncel.2020.601324
Received: 31 August 2020; Accepted: 13 November 2020;
Published: 18 December 2020.
Edited by:
Fabrice Dabertrand, University of Colorado, United StatesReviewed by:
Chiara Bianca Maria Platania, University of Catania, ItalyCopyright © 2020 Hariharan, Weir, Robertson, He, Betsholtz and Longden. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thomas A. Longden, dGhvbWFzLmxvbmdkZW5Ac29tLnVtYXJ5bGFuZC5lZHU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.