
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Cell. Neurosci., 05 August 2020
Sec. Cellular Neuropathology
Volume 14 - 2020 | https://doi.org/10.3389/fncel.2020.00229
This article is part of the Research TopicCellular Neuropathology Editor’s Pick 2021View all 24 articles
 Matija Fenrich1*
Matija Fenrich1* Stefan Mrdenovic2,3
Stefan Mrdenovic2,3 Marta Balog1
Marta Balog1 Svetlana Tomic4,5
Svetlana Tomic4,5 Milorad Zjalic1
Milorad Zjalic1 Alen Roncevic1
Alen Roncevic1 Dario Mandic6,7
Dario Mandic6,7 Zeljko Debeljak7,8
Zeljko Debeljak7,8 Marija Heffer1*
Marija Heffer1*Coronavirus disease (CoVID-19), caused by recently identified severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2), is characterized by inconsistent clinical presentations. While many infected individuals remain asymptomatic or show mild respiratory symptoms, others develop severe pneumonia or even respiratory distress syndrome. SARS-CoV-2 is reported to be able to infect the lungs, the intestines, blood vessels, the bile ducts, the conjunctiva, macrophages, T lymphocytes, the heart, liver, kidneys, and brain. More than a third of cases displayed neurological involvement, and many severely ill patients developed multiple organ infection and injury. However, less than 1% of patients had a detectable level of SARS-CoV-2 in the blood, raising a question of how the virus spreads throughout the body. We propose that nerve terminals in the orofacial mucosa, eyes, and olfactory neuroepithelium act as entry points for the brain invasion, allowing SARS-CoV-2 to infect the brainstem. By exploiting the subcellular membrane compartments of infected cells, a feature common to all coronaviruses, SARS-CoV-2 is capable to disseminate from the brain to periphery via vesicular axonal transport and passive diffusion through axonal endoplasmic reticula, causing multiple organ injury independently of an underlying respiratory infection. The proposed model clarifies a wide range of clinically observed phenomena in CoVID-19 patients, such as neurological symptoms unassociated with lung pathology, protracted presence of the virus in samples obtained from recovered patients, exaggerated immune response, and multiple organ failure in severe cases with variable course and dynamics of the disease. We believe that this model can provide novel insights into CoVID-19 and its long-term sequelae, and establish a framework for further research.
The ongoing pandemic of coronavirus disease (CoVID-19) has profoundly affected many aspects of our lives. The disease is caused by severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2), a positive-sense single-stranded RNA beta-coronavirus that uses angiotensin converting enzyme 2 (ACE2) to invade host cells (Hoffmann et al., 2020a). CoVID-19 exhibits variable clinical presentations, ranging from mild respiratory and/or gastrointestinal symptoms to acute respiratory distress syndrome and multiple organ failure (Hani et al., 2020; Jiang et al., 2020; Lai et al., 2020; Pan et al., 2020). A significant number of apparently asymptomatic individuals were also reported (Day, 2020).
So far, SARS-CoV-2 was has been shown to infect bronchial, alveolar, and conjunctival epithelia, alveolar macrophages (Bao et al., 2020; Hui et al., 2020), T-lymphocytes (Wang et al., 2020c), neurons (Moriguchi et al., 2020; Paniz-Mondolfi et al., 2020), cholangiocytes (Zhao et al., 2020a), vascular endothelium (Varga et al., 2020), gastrointestinal mucosa (Xiao et al., 2020), the heart, liver, and kidneys (Puelles et al., 2020). It has been suggested that brain involvement might contribute to more complicated clinical presentations (Li et al., 2020; Steardo et al., 2020). According to initial reports, more than a third of hospitalized patients exhibited symptoms and signs of neuronal involvement (Mao et al., 2020), and speculations on neuroinvasive potential of the virus were promptly made (Toljan, 2020). We would like to propose that SARS-CoV-2, after infecting the targeted brain nuclei, might be capable of spreading to multiple organs through peripheral nerves, precipitating multiple organ failure independently of an underlying respiratory infection.
S-glycoproteins, expressed on the surface of SARS-CoV-2 virions, engage the ACE2 on host cells, and invade the cells either by membrane fusion or endocytosis. In order to initiate the membrane fusion, S-glycoproteins need to undergo cleaving by endogenous proteases, which enables them to engage the ACE2 more avidly (Ou et al., 2020). This feature is absent in other coronaviruses, including SARS-CoV-1 (Jaimes et al., 2020). Some proteases involved in this process have already been identified, e.g., furin and TMPRSS2 (Hoffmann et al., 2020b; Walls et al., 2020), however, other proteases might be also involved. Additionally, the docking of SRAS-CoV-2 to the cell membrane is facilitated by heparan sulfate proteoglycans on the host cell, which interact with S-glycoproteins (Mycroft-West et al., 2020). In SARS-CoV-2 S-glycoprotein, three novel glycosaminoglycan-binding motifs have been recently described, one of which is located at S1/S2 cleavage site (Kim et al., 2020). This finding further implies involvement of host cell surface proteoglycans in the process of cell entry.
When the proteases are unavailable, membrane fusion cannot happen, and binding of SARS-CoV-2 to ACE2 would result in endocytosis instead. Moreover, even when the proteases are available, the virions still prefer entry via endocytosis (Ou et al., 2020). Endocytotic entry in Coronaviridae dependents on the localization of their receptors in membrane lipid rafts, since lipid rafts mediate this process. This mechanism shares the same activating principles with renin-angiotensin-aldosterone system, suggesting their common phylogenic origin (Chen et al., 2012). The initiation of ACE2-dependent endocytosis in SARS-CoV-2 was reported to be dependent on phosphatidylinositole biphosphate (Ou et al., 2020). The protease-independent and lipid-raft-mediated entry might mimic physiological activation of the receptor by angiotensin II, which results in its recruitment to the intracellular renin-angiotensin system (Escobales et al., 2019; Abassi et al., 2020). We still do not know much of this system, nonetheless, its involvement in the various aspects of metabolic regulation of subcellular compartments is gradually being elucidated (Villar-Cheda et al., 2017; Shi et al., 2018; Sotomayor-Flores et al., 2020).
After ACE2 endocytosis, lysosomal cathepsin L proteases are normally trafficked to the endosome. It has been recently demonstrated that cathepsin L is capable of cleaving S-glycoproteins, enabling virions to initiate fusion, escape endosomes and release their proteins and genetic material into cytosol (Mao et al., 2020). By preferably relying on endocytosis instead of membrane fusion, SARS-CoV-2 likely postpones its detection by the immune system, because in this way fewer antigenic viral proteins are left on the cell surface (Marsh and Helenius, 2006). The mechanisms of cell entry are summarized in the Figure 1A.
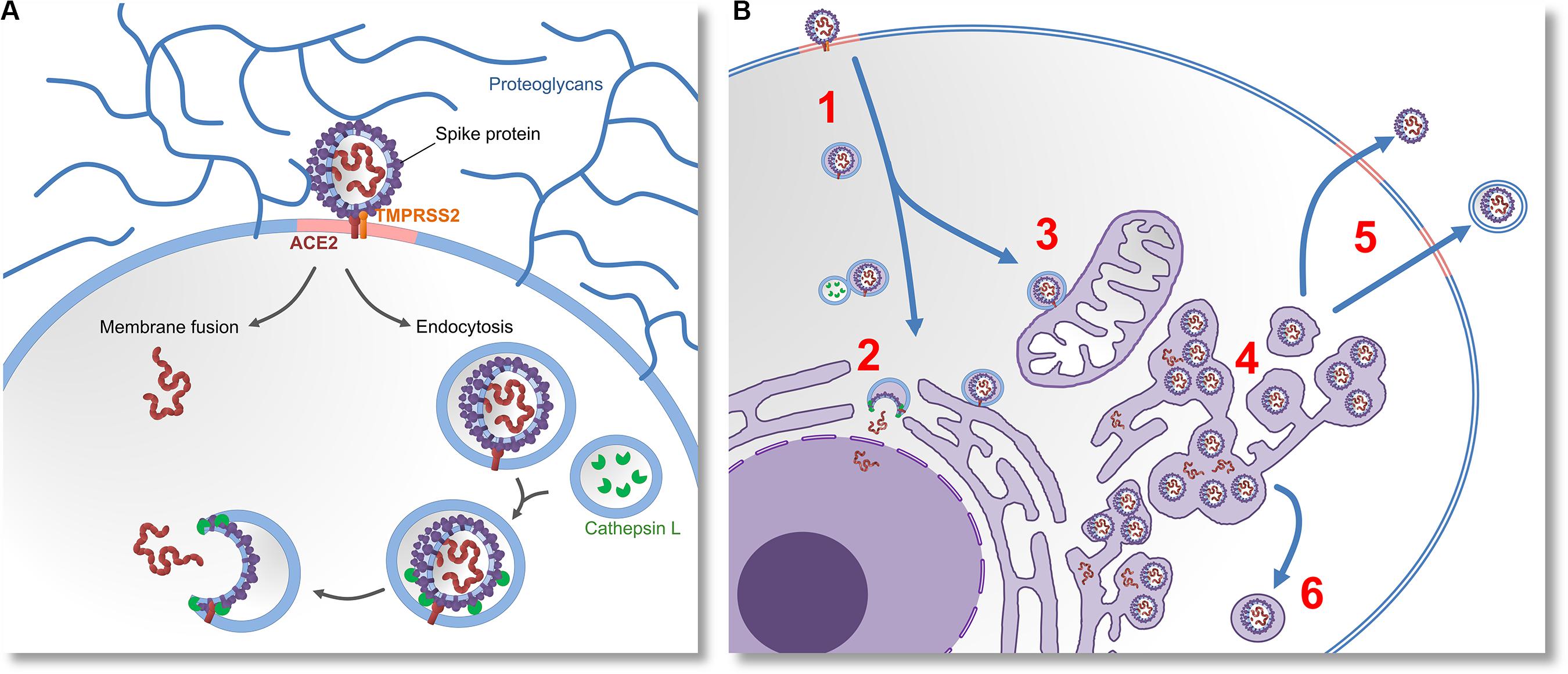
Figure 1. Molecular mechanisms of cell entry, replication and egression of SARS-CoV-2. (A) The virus invades the cell by docking on the cell-surface proteoglycans and engaging the ACE2 receptor with “Spike” (S) glycoprotein. If S glycoprotein is cleaved by host proteases (TMPRSS2), entry by membrane fusion or endocytosis would happen. If the cleavage does not occur, the virus would invade the cell via receptor-mediated endocytosis. Lysosomal proteases (Cathepsin L) eventually cleave the S glycoproteins. This enables them to induce membrane fusion and release the viral proteins and RNA into the cytoplasm. (B) After the virus enters the cell (1) it releases its genetic material (2), and replicates in the cell nucleus. Virions that do not get their S glycoproteins primed by lysosomal proteases would be further trafficked to various subcellular membrane compartments (3), which possibly modulates their metabolism and changes their morphology. This results in the emergence of a membranous system called reticulovesicular network. Translation of viral messenger RNA, synthesis of viral proteins and assembly of new virions takes place inside of this network and other intracellular vesicles (4). New virions leave the infected cell by budding through lipid rafts, either by membrane-fusion-mediated egression or by exosomes (5). Intracellular cleavage of S glycoprotein by furin or TMPRSS2 proteases would enable the virions to induce membrane fusion. Alternatively, assembly of virions inside of secretory vesicles would allow them to be transported to the apical cell membrane (6) or presynaptic membrane (in the case of neurons).
An important feature of coronaviruses is that their replication-transcription complexes are associated with double membrane vesicles built from modified Golgi apparatus and endoplasmic reticulum (Snijder et al., 2020). The viruses extensively remodel the membranes of subcellular compartments into organelle-like and web-like structures, known as reticulovesicular networks (Knoops et al., 2008). A recent preprint electron microscopy study confirmed the same for SARS-CoV-2 (Belhaouari et al., 2020). The viral replication-transcription domains and assembled virions were also reported in autophagosomes (Prentice et al., 2004) and secretory vesicles (Krijnse-Locker et al., 1994; Salanueva et al., 1999; Verheije et al., 2008), implying that assembly and transport of new virions might take place during vesicular trafficking. For their replication and neuronal dissemination, neuroinvasive viruses must express proteins that control vesicular traffic (Enquist, 2012). Angiotensin II increases and mediates neuronal vesicular trafficking (Wang et al., 2001; Aschrafi et al., 2019), and since the receptor binding site of S-glycoproteins in SARS-CoV-2 is structurally similar to angiotensin II, the virus might be capable of increasing and modulating the neuronal vesicular trafficking system in the same manner (see Figure 1B). Moreover, as coronaviruses modify and assembly inside of structures derived from endoplasmic reticulum, we further suggest that SARS-CoV-2 could also utilize continuous longitudinally spanning endoplasmic reticula, which were described in the myelinated axons, and which are likely a continuation of the somatic organelles (Gonzalez and Couve, 2014). Since SARS-CoV-2 is a neurotropic virus, we suggest that, by binding to ACE2, it is able to disseminate via both vesicular transport and passive diffusion through axonal endoplasmic reticulum over large distances and at a fast pace.
New virions that are assembled in a reticulovesicular network are not immediately released out of the infected cell. Instead, they are accumulating in dedicated areas of its lumen (Knoops et al., 2008). Their egression is most likely elicited by fusion of the vesicles derived from the reticulovesicular network and plasma membrane in a process that seems to be dependent on interaction with lipid rafts (Chazal and Gerlier, 2003; Baglivo et al., 2020; Fantini et al., 2020) and autophagosomal proteins (Tanida et al., 2009). Since the surfaces of lipid rafts are much smaller than the viral envelopes, egression has to happen on sites where many lipid rafts cluster into a lipid microdomain (Lorizate and Kräusslich, 2011). This egression mechanism might be crucial for the induction of syncytia. S-glycoproteins of SARS-CoV-2 induce syncytia by transcellular transfections dependent on TMPRSS2 proteolytic activity (Ou et al., 2020). Apparently, the virion cannot directly induce a syncytium without proteases, likely because membrane fusion cannot be initiated. In such cases the budding would likely result in an endocytic transfection, enabling the virus to spread in a cell-to-cell fashion. SARS-CoV-2 was reported to show superior in vitro cell-cell fusion capacity compared to SARS-CoV-1 (Xia et al., 2020). Additionally, in some coronaviruses, soluble S-glycoproteins are secreted out of the infected cell, and are shown to induce syncytia independently of transfection (Masters, 2006).
Axonal dissemination by vesicular transport and passive diffusion, syncytium induction and cell-to-cell spread could explain the unexpectedly low viral load in the blood – possibly less than 1% of PCR blood tests in CoVID-19 patients yield a positive result (Wang et al., 2020b; Wölfel et al., 2020; Yu et al., 2020), suggesting that viremia likely does not underlie the multi-organ dissemination.
ACE2 is expressed in neurons of many brain regions (Doobay et al., 2007). It can bind to integrins and modulate their signaling (Clarke et al., 2012). Integrins are transmembrane receptors responsible for signal transduction between a cell and extracellular matrix, and are abundantly expressed in synapses and terminals of sensory neurons that mediate pain (Dina et al., 2004), implying a possible colocalization with ACE2 at those sites. Furthermore, an integrin-binding motif in S-glycoprotein of SARS-CoV-2 was recently identified, suggesting that they might be alternative receptors for the virus, and an ACE2-independent infection in integrin-expressing cells might be possible (Sigrist et al., 2020). The presence of SARS-CoV-2 in the cerebrospinal fluid was recently reported in a case of viral encephalitis (Moriguchi et al., 2020), and the virus was directly observed in the brain cells of deceased CoVID-19 patients (Paniz-Mondolfi et al., 2020), confirming its neurotropic nature. Based on these findings and recent reports (Cheema et al., 2020; Colavita et al., 2020), we propose that nerve terminals in the oral and nasal mucosa, conjunctiva and eyes, as well as the olfactory nerves, might be potential entry sites for SARS-CoV-2 neurotropic infections. Post-mortem MRI findings revealed asymmetric olfactory bulbs in four deceased CoVID-19 patients, further implying that olfactory neuroepithelium might be an entry point for the virus (Coolen et al., 2020).
CoVID-19 patients frequently present with hyposmia and dysguesia (Bagheri et al., 2020; Lechien et al., 2020), and both ACE2 and TMPRSS2 proteases are expressed in olfactory neuroepithelium (Fodoulian et al., 2020). Moreover, Dubé et al. (2018) directly observed propagation of a human coronavirus to the brainstems of mice following the intranasal and intralingual inoculations, suggesting that SARS-CoV-2 might be able to spread to the brainstem either directly via olfactory nerves, or alternatively, through orofacial nerve fibers via cranial ganglia. A non-peer-reviewed report demonstrated the presence of SARS-CoV-2 in the trigeminal ganglia, olfactory epithelium, olfactory bulbs, brainstem, uvula, conjunctiva and cornea in some deceased patients (Meinhardt et al., 2020). Olfactory inoculation likely involves propagation to the piriform cortex and amygdala, and further spreading through the medial forebrain bundle to the brainstem (see Figure 2A). Lateral fiber stream of the medial forebrain bundle projects caudally to the solitary tract and dorsal vagal nuclei (Holstege, 1987). Replication of the virus in the solitary tract neurons may also explain the reported dysgeusia. Spreading through the orofacial sensory fibers would be especially convenient for the virus, since their pseudounipolar somata, which reside in the cranial ganglia, could be plausible persistent infection sites or intermediary replication posts. This could facilitate either further brainstem invasion by axonal transport or allow for an exocytosis-endocytosis-mediated transfection of other fibers passing through the ganglia. Such virion-containing endocytes could establish membrane contact sites with axonal endoplasmic reticulum (Eden, 2016), enabling the virion to freely diffuse along the axon using the organelle lumen. Passive diffusion of coronavirions in axons was reported both in vitro and in vivo (Dubé et al., 2018). However, it is possible that vesicular transport might prevail in vivo. Although hematologic dissemination to the brain cannot be excluded, the observed discrepancy between a significant incidence of neurological manifestations (Mao et al., 2020) and a low yield of positive blood tests (Wang et al., 2020b; Wölfel et al., 2020; Yu et al., 2020) suggests that viremia is unlikely to be a major contributor to the brain infection.
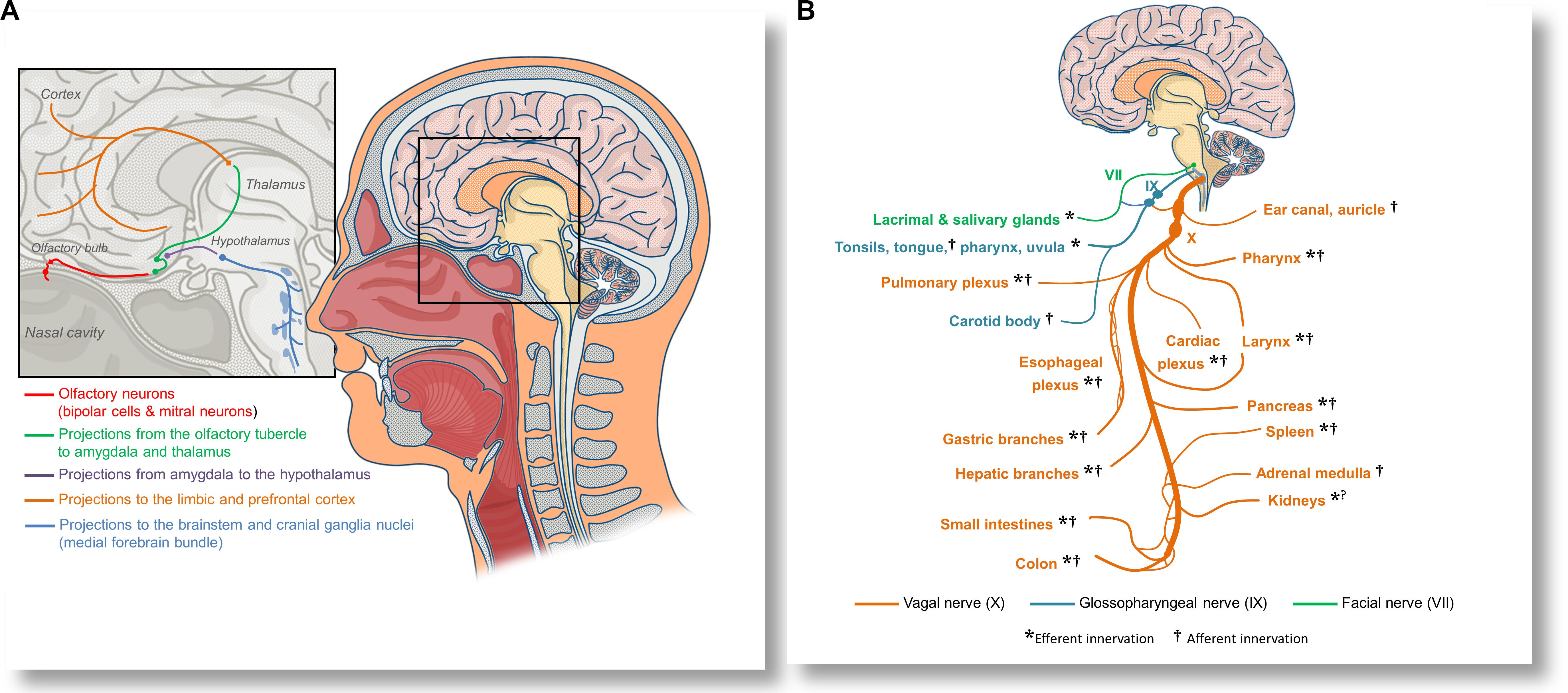
Figure 2. Anatomical overview of the proposed olfactory inoculation and axonal dissemination pathways of SARS-CoV-2. (A) Olfactory neurons are only a synapse away from the central nervous system. SARS-CoV-2 has been reported to infect olfactory neuroepithelium and to invade the olfactory bulbs via cribriform plate. By exploiting the anterograde axonal transport in the olfactory tract, the virus could infect neurons of the olfactory tubercle and spread to the amygdala and thalamus, from where it might further invade the cingular and orbitofrontal cortex. By exploiting the axonal transport in fibers projecting into the hypothalamus, the virus may infect cranial ganglia nuceli via the medial forebrain bundle. (B) SARS-CoV-2 could also disseminate to various organs and tissues by axonal transport in the vagal nerve (X). Immediately after leaving the skull, the vagus establishes anastomoses (connections) with the glossopharyngeal nerve (IX), allowing the virus to spread to the oropharyngeal mucosa, or alternatively, to use the same route for neuroinvasion. Glossopharyngeal fibers that cross to the facial nerve (VII) could be an additional pathway for dissemination or neuroinvasion. The vagal nerve innervates many tissues and organs that can be affected in CoVID-19, including the pharynx, larynx, lungs, the heart, esophagus, stomach, liver, gallbladder, pancreas, spleen, adrenal medulla, kidneys, muscles, and glands of a part of the intestines, as well as lymphatic tissue in the correspondent intestinal mucosa. By disrupting the vagal innervation, SARS-CoV-2 could also impair the activity of the cholinergic inflammatory reflex, and precipitate dysregulated immune responses in many organs.
Viral penetration into the central nervous system through peripheral fibers is a multi-step process. In order to reach neuronal soma from the periphery, the virus needs to exploit the retrograde axonal transport machinery. SARS-CoV-2 uses ACE2-mediated endocytotic pathway for internalization and intracellular transport, and in the case of SARS-CoV-1 infection, endosomes containing virion/ACE2 complexes are trafficked to the perinuclear area (Wang et al., 2008). The virus might use this intrinsic clathrin-independent intracellular ACE2 endocytic transport to reach the perikaryon. However, for a successful further invasion, it would also need to be able to cross synaptic membranes. Another beta-coronavirus was shown to be capable of trans-synaptic propagation by presynaptic exocytosis and postsynaptic endocytosis (Li et al., 2013), which suggests that SARS-CoV-2 could use the same mechanism. Anterograde axonal transport is mediated by kinesin molecular motors, and allows for trafficking of vesicles and organelles from the soma to the axon and synaptic terminals (Berth et al., 2009). Since the virus replicates and assembles inside of vesicles derived from the endoplasmic reticulum and Golgi apparatus, it could also exploit the already present kinesin-mediated anterograde transport to propagate further along the axons. Lateral transfections, i.e., cell-to-cell or axo-axonal spreading via exocytosis, could be also possible. Exosomal pathways are hypothesized to contribute to viral dissemination (Khan et al., 2017), and it was demonstrated that ACE2 trafficking could involve exosome-mediated cell-to-cell transfer (Wang et al., 2020a). Arguably, this mechanism could allow the infection to spread from neurons to cerebrovascular endothelial cells, and vice versa. The ways the virus might exploit intracellular vesicular trafficking in neurons are summarized in Figure 3.
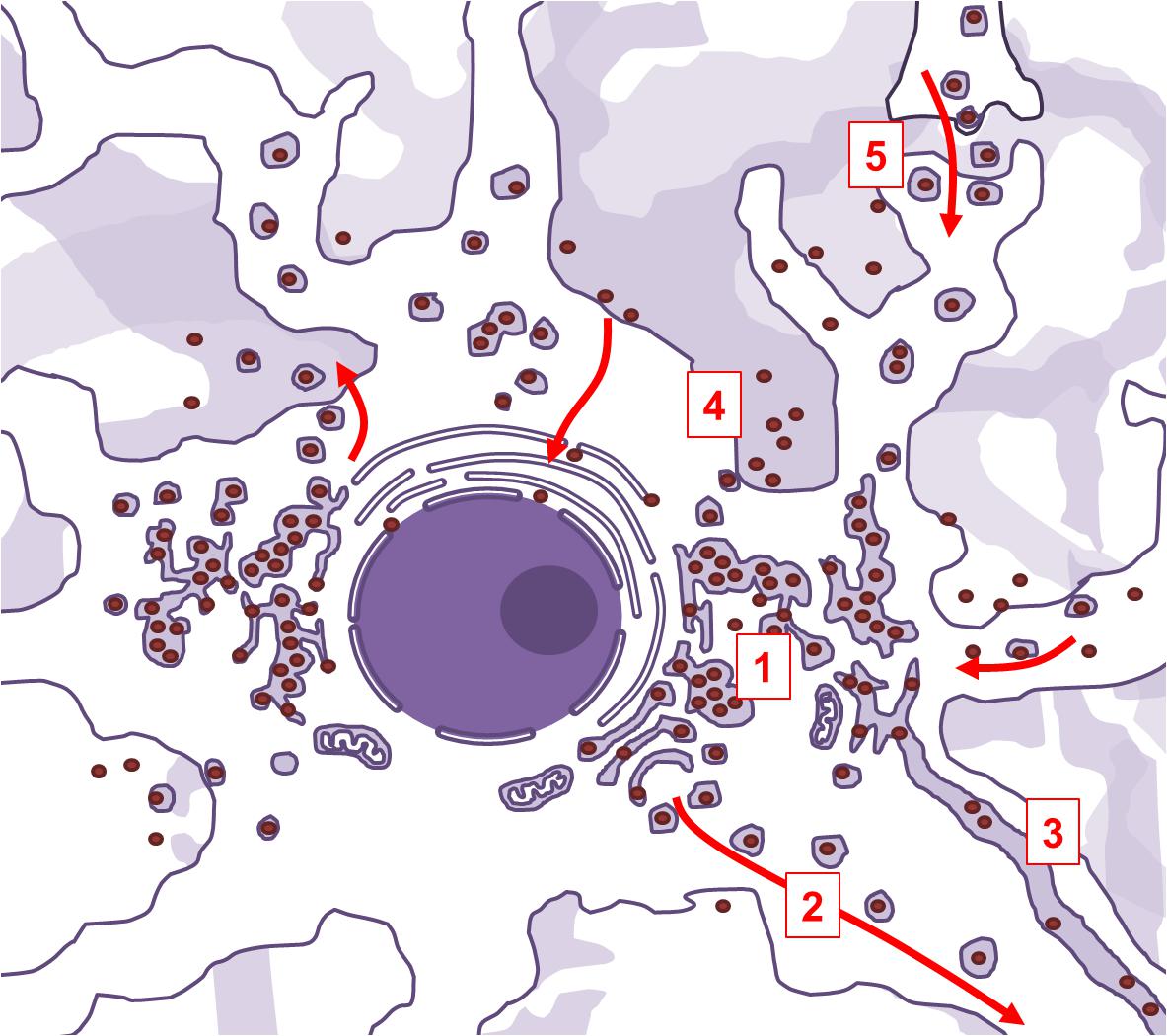
Figure 3. Aspects of intracellular vesicular trafficking that may be exploited by SARS-CoV-2 in infected neurons. Neurons are polarized cells with abundance of intracellular endocytic pathways. Life cycle of SARS-CoV-2 is compatible with the possibility of exploiting those pathways. The virus extensively modifies subcellular organelles into a reticulovesicular network, a structure where viral membrane-bound replication-transcription complexes are situated and where new virions are being assembled (1). This elaborate network is connected to secretory Golgi compartments, allowing the newly assembled virions to be trafficked to the synapse via kinesin-mediated anterograde axonal transport (2). The reticulovesicular network is also continuous with endoplasmic reticulum. In myelinated projection neurons, endoplasmic reticulum extends along the axon, which might enable the virions to freely diffuse inside its lumen (3). Newly assembled virions can also directly leave the infected neurons by membrane-fusion or by exosomes (4), and infect the nearby cells. Trans-synaptic spreading is confirmed in some beta-coronaviruses, and could be possible in SARS-CoV-2 as well (5).
Once the virus has reached the brainstem and spinal cord, it could access practically every organ system in the body. By infecting the vagal nuclei alone, the virus may be capable of dissemination to the lungs, heart, liver, intestines and kidneys, as well as of impairing the vagal activity (see Figure 2B). This might precipitate multiple organ injury independently of an underlying respiratory pathology. In a murine model of neuronal infection with human coronavirus OC43, the viral RNA was detected in the livers of three out of nine animals in spite of undetectable viral loads in the blood (Dubé et al., 2018). This finding supports the possibility of dissemination through vagal fibers. Additionally, viral shedding at the periphery could also be associated with activation of the integrin signalization on peripheral nerve terminals, which could enable the virus to attenuate local algesic and inflammatory response, hindering the immune reaction to its shedding (Dina et al., 2004; Moon et al., 2009; Hu et al., 2016). Alternatively, the virus may establish a persistent neuronal infection, and stay dormant for a certain period until eventual reactivation. This is a common strategy in some neuroinvasive viruses (Koyuncu et al., 2013).
Other manifestations that are considered atypical for a respiratory infection, such as coagulopathy (Iba et al., 2020), thrombosis (Helms et al., 2020; Leisman et al., 2020), vasculitis (Castelnovo et al., 2020; Sachdeva et al., 2020; Verdoni et al., 2020) and dysregulated inflammatory responses (Blanco-Melo et al., 2020; Leisman et al., 2020) were also reported in CoVID-19 patients. Previous studies showed that vagal activity is an important factor in anti-inflammatory modulation and inhibition of prothrombotic events in the innervated tissues (de Jonge et al., 2005; Westerloo et al., 2006; Koopman et al., 2016; Li et al., 2016; Cacho et al., 2020), and SARS-CoV-2 could be capable of hijacking axonal transport in the vagal nerves, impairing their signaling in the cholinergic anti-inflammatory pathway (Johnston and Webster, 2009; Pavlov and Tracey, 2012). We propose that vagal dysfunction might significantly contribute to exaggerated immune responses and thromboembolic incidents in some CoVID-19 patients. Interestingly, vagal neuropathies due to upper-respiratory viral infections are already clinically recognized as contributors to various para-infectious and post-infectious sequelae (Amin and Koufman, 2001; Rees et al., 2009; Chung et al., 2013; Niimi and Chung, 2015).
Due to the dynamics of the active transport and passive diffusion in axons, the brain infection might develop weeks after the virus exposure or development of primary respiratory infection, giving rise to the possibility that patients with severe clinical presentation and multiple organ affection might have contracted the virus earlier than assumed. The exact time needed for the virus to invade the brain in humans is unknown, and it certainly depends on the entry route and inocculation dose. In mice, a strain of human coronavirus was detected in the olfactory bulbs as early as 2 days after intranasal inoculation, in the cortex and brainstem 3 days after inoculation, and in the spinal cord 5 days after inoculation (Dubé et al., 2018).
Based on the presented concept, we would like to suggest that respiratory and neuronal types of CoVID-19 may be distinct clinical entities. These two types might present independently, as a respiratory infection without brain infection and vice versa, concomitantly or consecutively. Due to different entry routes, the two types would likely have different incubation periods and different occurrence rates of initial symptoms, which could explain the observed variability in both parameters (Day, 2020; Jiang et al., 2020; Wan et al., 2020). However, wide dispersion of reported values could be also due to limited sample sizes in the initial reports.
Increased susceptibility to a particular type of the disease might be driven by underlying conditions. ACE2 expression is increased in patients with morbidities associated with metabolic syndrome (Pinto et al., 2020), and those patients are also more likely to develop neurological manifestations (Mao et al., 2020). Patients with such conditions who develop CoVID-19 respiratory infections might be at risk of more serious CoVID-19 neuronal infections which could in turn result in the virus dissemination to multiple organs through peripheral nerves. Most patients, however, do not develop brain infection. It is important to note that the nasal mucosa possess mechanisms that efficiently prevent neuroinvasion via olfactory nerves, such as nasal secretion, mucus barrier formation, pathogen recognition receptors (Kalinke et al., 2011) and cyclic shedding and replacement of olfactory neurons with the new ones (Loseva et al., 2009). Another host protective response was reported to be apoptosis of olfactory neurons (Mori et al., 2004). Conditions that interfere with these mechanisms might compromise their protective roles against neuroinvasive infectious agents. Aging, diabetes, and hypertension are associated with less efficient nasal mucocilliary clearance (Selimoglu et al., 1999; Yue, 2007; Proença de Oliveira-Maul et al., 2013), and aging might also precipitate reduced olfactory nerve replenishment (Enwere et al., 2004; Brann and Firestein, 2014). This would additionally explain the observed higher incidence of neurological involvement in patients with these comorbidities. For most otherwise healthy and younger individuals, respiratory epithelium would be the primary and likely the only site of infection, whereas the aforementioned high-risk groups might be more susceptible to both neuronal and respiratory types of CoVID-19.
Theoretically, a primary lung infection could also progress to a brain or spinal cord infection via retrograde axonal transport through peripheral nerves. However, more aggressive immune responses to viral pathogens in peripheral tissues compared to the ones in the central nervous system would likely impede such a scenario. Due to irreplaceability of neurons, the immune reactions to viral infection in the brain do not include cytolytic responses, and are therefore less efficient in containing and clearing intracellular pathogens (Griffin, 2003). Olfactory neurons, although replaceable, are in an immediate proximity to the central nervous system, which makes them an anatomically and immunologically more plausible route for successful neuroinvasion. Nevertheless, a primary lung infection in some patients could still progress to acute respiratory distress syndrome without or independently of neuronal infection. Such lung injuries might be due to suboptimal host reaction to the infection, possibly characterized by a weak antiviral response and elevated expression of proinflammatory cytokines, as demonstrated by an in vitro study (Blanco-Melo et al., 2020). Still, many CoVID-19 patients who develop respiratory infection without neural involvement could have better clinical outcomes, whereas a combination of direct cytopathic effects, vagal neuropathy and centrally driven lung injuries could be associated with less favorable outcomes.
We propose that the original type of cell in which the virion assembly and budding took place could be identified based on the lipid profile of the viral particles. The lipid composition of retroviral envelopes corresponds to the lipid profile of the membrane lipid rafts at which the budding took place (Ono, 2010; Waheed and Freed, 2010). Since lipid rafts of the brain have a distinctive lipid profile rich in specific gangliosides (Vajn et al., 2013; Schnaar et al., 2014), by comparing it to the lipid profile of the virions, it could be possible to confirm the neuronal origin of SARS-CoV-2 in peripheral tissues.
A proportion of purportedly asymptomatic or oligosymptomatic carriers could suffer a less severe CoVID-19 neuronal infection, with subtle neuropsychiatric manifestations without respiratory involvement. RNA viruses are known to be able to persistently infiltrate CNS as well as to cause subacute psychiatric and neurological symptoms and post-infectious sequelae, such as cognitive impairment, seizures, ataxia, psychiatric illnesses, chronic fatigue syndrome, etc. (Klein et al., 2019; Bo et al., 2020). To the best of our knowledge, so far reported neurological manifestations in CoVID-19 patients include hyposmia, dysgeusia (Lechien et al., 2020), convulsions (Karimi et al., 2020), neurogenic syncope (Canetta et al., 2020), meningoencephalitis (Moriguchi et al., 2020), Guillain-Barré syndrome (Zhao et al., 2020b), intracerebral hemorrhage (Sharifi-Razavi et al., 2020), acute hemorrhagic necrotizing encephalopathy (Poyiadji et al., 2020), acute post-infectious myelitis (Zhao et al., 2020c), cerebrovascular diseases (Mao et al., 2020), vertigo, nausea, headaches (Mao et al., 2020; Nie et al., 2020), demyelination (Zanin et al., 2020), and cortical blindness (Kaya et al., 2020), but the causative or coincidental nature of these findings is yet to be determined. It is important to point out that some of the reported neurological symptoms could also be caused by hypoxia as a consequence of lung injury. However, not all CoVID-19 patients who developed neurological symptoms suffered pulmonary insufficiency, and the presence of subtle neuropsychiatric abnormalities in the subclinical cases might be actually underreported (Zhang et al., 2020b).
The fetus seems to be protected from the axonal invasion of SARS-CoV-2 from the infected mother by factors that inhibit nerve growth on the maternal side of the umbilicus and placenta (Marzioni et al., 2004). Both amniotic fluid and umbilical cord blood samples were reported to test negative to SARS-CoV-2, and no vertical transmission was reported (Chen et al., 2020a), except for a recent report of three cases of neonatal CoVID-19, in which vertical transmission could not be ruled out (Zeng et al., 2020). Since ACE2 is expressed in the uterus and placenta (Valdes et al., 2013), a possibility of viral interference with expression of the factors that mediate nerve growth inhibition must not be dismissed. In addition, CoVID-19-related thromboembolic placental injuries were recently described (Baergen and Heller, 2020).
Development of neuronal CoVID-19 infection might explain a growing number of positive PCR tests in recovered patients even weeks after hospital discharge (Lan et al., 2020; Xing et al., 2020; Zhang et al., 2020c). Viral shedding at nerve terminals of pulmonary epithelium and nasopharyngeal mucosa could explain the sustained presence of SARS-CoV-2 in throat and nasal swabs, implying that a carrier state could persist over a significant timespan. Although prolonged positivity could theoretically be explained by presence of remnants of unviable viral RNA, we believe this is an unlikely explanation. Physiological nasopharyngeal washing and, possibly, activity of certain canonical ribonucleases in the respiratory mucosa (Koczera et al., 2016) would not allow for a sustained presence of the viral RNA weeks after recovery. By analogy, viral shedding may be also possible on the enteric nerve terminals, maintaining the detectability of the virus in enterocytes and stool even after apparent recovery. Hu et al. (2020) have recently reported that SARS-CoV-2 can persist in stool samples longer than in the respiratory tract in recovered patients who were previously without gastrointestinal symptoms.
The damage to multiple organs in some patients may as well be explained by hypoxia and cytokine storm (Bonow et al., 2020; Mehta et al., 2020; Pei et al., 2020; Yang et al., 2020). Even so, hypoxia and cytokine storm do not accompany all cases of organ damage (Kochi et al., 2020; Zhang et al., 2020a), and the correlation of incidence of hypercytokinemia and presence of viral RNA in blood (Chen et al., 2020b), in spite of practically non-existent viremia, suggests that cytokine storm might be preceded and driven by organ damage and subsequent release of viral antigens from necrotic cells. As a matter of fact, the virus presence was confirmed in vascular endothelial cells (Varga et al., 2020) and multiple organs in deceased patients (Puelles et al., 2020), and different mechanisms of organ failure do not necessarily exclude each other. Detrimental pro-thrombotic and pro-inflammatory state could also be driven by hypothesized SARS-CoV-2-induced vagal neuropathy (Li et al., 2011; Huston, 2012), and eventual development of neurogenic pulmonary edema secondary to an infection-related cerebrovascular event might contribute to the ultimate cardiopulmonary failure (Davison et al., 2012).
It was also suggested that possible fecal-oral transmission may explain the gastrointestinal symptoms in CoVID-19 (Cha et al., 2020; Steardo et al., 2020; Tian et al., 2020), even though SARS-CoV-2 is not stable in the media with pH <3 (Chin et al., 2020). Nonetheless, SARS-CoV-2 was still detected in stool and gastrointestinal mucosa (Xiao et al., 2020), but stool tested positive even in patients who did not have gastrointestinal symptoms (Zhang et al., 2020c). Still, the proposed fecal-oral route does not exclude the possibility of axonal dissemination of SARS-CoV-2 to gastrointestinal tract via vagal fibers and spinal nerves. Another possibility might be an infection of the gallbladder or biliary ducts (Zhao et al., 2020a), in which case the virus in stool would be of biliary origin.
Finally, pharmacologic approaches that would hinder the exploitation of the neuronal endocytic trafficking by SARS-CoV-2 could be an effective treatment for the infection. Chloroquine and its derivatives disrupt endocytosis and vesicular trafficking by endosomal alkalization and inhibition of autophagy, also interfering with terminal glycosylation in ACE2, which hinders its interaction with S-glycoproteins (Liu et al., 2020). These medications are already being clinically used in CoVID-19 patients. Other autophagy inhibitors, such as azithromycin are also commonly used (Gautret et al., 2020). Therefore, we suggest that the treatment of CoVID-19, due to its neuroinvasive properties, should focus on interfering with viral hijacking of the cellular endocytic trafficking system and axonal transport. A study of rat primary superior cervical ganglia culture revealed that emetine (translation elongation inhibitor) may be used as inhibitory modulator of rabies virus axonal transport (MacGibeny et al., 2018), implying a possible therapeutic approach for SARS-CoV-2. In the case of poliomyelitis virus infection in rats, vinblastine (inhibitor of tubulin polymerization) was shown to hinder retrograde axonal transport of the virus when applied topically to infected peripheral nerves (Ohka et al., 2004). Microtubule-associated inhibitors, such as vinblastine, vincristine, paclitaxel, colchicine, nocodazole and other inhibitors of retrograde axonal transport, such as macrolide drug mycalolide B (Cavolo et al., 2015), could be used to investigate the mechanisms underlying retrograde axonal transport of SARS-CoV-2 in vivo. However, these drugs do not alter the redistribution and abundance of viral proteins, and do not influence the viral replication (Wu et al., 2019). Moreover, treatment with these agents was reported to induce reactivation of varicella-zoster virus infection along with their neurotoxic effects. HSP90 inhibitor geldanamycin is suggested as a potential drug in the treatment of CoVID-19 (Sultan et al., 2020), and SARS-CoV-2 proteases inhibitor quercetine is being studied as a prophylaxis and treatment option (Onal and Semerci, 2020). It also affects the cytoskeletal signaling by inhibiting protein kinase C. Another potential treatment option for CoVID-19 are rho-kinase inhibitors, such as fasudil, ripasudil, and netarsudil (Abedi et al., 2020; Calò et al., 2020). Interestingly, all these compounds share a quinoline backbone moiety. Additionally, since neurotropic viruses have to propagate across the synapses, neutralizing antibodies could be used to stop them from spreading from neuron to neuron, as it was demonstrated in animal models of West Nile virus neuronal infection (Oliphant et al., 2005; Samuel et al., 2007). Another group of potential axonal transport modulators could be bioactive compounds isolated from marine organisms. Some of them are reported to inhibit molecular motors underlying anterograde or retrograde axonal transport (kinesin and dynein, respectively), and several compounds are proposed to interfere with autophagosomal pathways in neurons (White et al., 2016).
The model we put forward clarifies a wide range of clinically observed phenomena in CoVID-19 patients (see Supplementary Table S1). Detection of viral particles in peripheral nerves, together with recent findings of brainstem and cranial ganglia infection, as well as other findings summarized in this paper, could confirm the axonal dissemination of SARS-CoV-2. If correct, this would significantly affect our understanding of this novel disease and its potential long-term sequelae. This would warrant modifications in many aspects of diagnostics, treatment and follow-up of CoVID-19 patients. The proposed model could also be utilized by many other viruses – chronic persistence in the host’s nervous system and eventual reactivations with shedding in the respiratory or gastrointestinal mucosa could be an effective survival and spreading strategy for a virus. Finally, the presence of antibodies to other coronaviruses in the cerebrospinal fluid of patients with Parkinson’s disease and some psychiatric disorders (Fazzini et al., 1992; Severance et al., 2009; Okusaga et al., 2011) points to the possibility that these and similar pathologies might be triggered by viral infections. Vagal atrophy observed in patients with Parkinson’s disease (Walter et al., 2018), might also be secondary to bulbar lesions caused by a coronavirus infection. The proposed model of axonal dissemination and vagal dysfunction could give us novel insights not only into CoVID-19, but also into hypothesized common viral etiology of certain neurodegenerative and psychiatric disorders and their systemic manifestations. Therefore, we believe this idea merits further investigation.
As a closing remark, it is important to add that most individuals diagnosed with CoVID-19 will likely convalesce without developing neuronal infection. Moreover, the sole presence of proviral genomes in the brain does not imply a definite corresponding clinical correlate. Many viruses have already left their genetic imprints in our DNA, and thus became a part of our evolutionary heritage, and a part of us.
All datasets generated for this study are included in the article/Supplementary Material.
MF, SM, and MH devised the main conceptual ideas. MF drafted the initial manuscript. MF, SM, MB, ST, MZ, AR, DM, ZD, and MH reviewed and revised the manuscript, and expanded the original concept. All authors contributed to the article and approved the submitted version.
This study was funded by the European Union through the European Regional Development Fund, Operational Programme Competitiveness and Cohesion, Grant Agreement No. KK.01.1.1.01.0007, the CoRE – Neuro, and by the Croatian Science Foundation (HRZZ IP-2014-09-2324).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2020.00229/full#supplementary-material
Abassi, Z., Assady, S., Khoury, E. E., and Heyman, S. N. (2020). Letter to the editor: angiotensin-converting enzyme 2: an ally or a Trojan horse? Implications to SARS-CoV-2-related cardiovascular complications. Am. J. Physiol. Heart Circul. Physiol. 318, H1080–H1083.
Abedi, F., Rezaee, R., and Karimi, G. (2020). Plausibility of therapeutic effects of rho kinase inhibitors against severe acute respiratory syndrome coronavirus 2 (COVID-19). Pharmacol. Res. 156:104808. doi: 10.1016/j.phrs.2020.104808
Amin, M. R., and Koufman, J. A. (2001). Vagal neuropathy after upper respiratory infection: a viral etiology? Am. J. Otolaryngol. 22, 251–256. doi: 10.1053/ajot.2001.24823
Aschrafi, A., Berndt, A., Kowalak, J. A., Gale, J. R., Gioio, A. E., and Kaplan, B. B. (2019). Angiotensin II mediates the axonal trafficking of tyrosine hydroxylase and dopamine beta-hydroxylase mRNAs and enhances norepinephrine synthesis in primary sympathetic neurons. J. Neurochem. 150, 666–677. doi: 10.1111/jnc.14821
Baergen, R. N., and Heller, D. S. (2020). Placental pathology in covid-19 positive mothers: preliminary findings. Pediatr. Dev. Pathol. 23, 177–180. doi: 10.1177/1093526620925569
Bagheri, S. H. R., Asghari, A. M., Farhadi, M., Shamshiri, A. R., Kabir, A., Kamrava, S. K., et al. (2020). Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak. medRxiv [Preprint] doi: 10.1101/2020.03.23.20041889
Baglivo, M., Baronio, M., Natalini, G., Beccari, T., Chiurazzi, P., Fulcheri, E., et al. (2020). Natural small molecules as inhibitors of coronavirus lipid-dependent attachment to host cells: a possible strategy for reducing SARS-COV-2 infectivity? SARS-COV-2 lipid-dependent attachment to host cells. Acta Bio Med. Atenei Parmensis 91, 161–164.
Bao, L., Deng, W., Huang, B., Gao, H., Liu, J., Ren, L., et al. (2020). The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature [Epub ahead of print].
Belhaouari, D. B., Fontanini, A., Baudoin, J.-P., Haddad, G., Le Bideau, M., Bou Khalil, J. Y., et al. (2020). The Strengths of Scanning Electron Microscopy in Deciphering SARSCoV-2 Infectious Cycle. Marseille: HU pre-publication site on COVID-19.
Berth, S. H., Leopold, P. L., and Morfini, G. N. (2009). Virus-induced neuronal dysfunction and degeneration. Front. Biosci. (Landmark Ed.) 14, 5239–5259.
Blanco-Melo, D., Nilsson-Payant, B. E., Liu, W. C., Uhl, S., Hoagland, D., Møller, R., et al. (2020). Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 181, 1036.e9–1045.e9.
Bo, H. X., Li, W., Yang, Y., Wang, Y., Zhang, Q., Cheung, T., et al. (2020). Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. [Epub ahead of print].
Bonow, R. O., Fonarow, G. C., O’Gara, P. T., and Yancy, C. W. (2020). Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiol. [Epub ahead of print].
Brann, J. H., and Firestein, S. J. (2014). A lifetime of neurogenesis in the olfactory system. Front. Neurosci. 8:182. doi: 10.3389/fnins.2014.00182
Cacho, B., Abdala, J., and Wilson, C. G. (2020). Anti-inflammatory effects of vagus nerve simulation in a newborn rat model of acute inflammation. FASEB J. 34:1. doi: 10.1096/fasebj.2020.34.s1.03202
Calò, L. A., Bertoldi, G., and Davis, P. A. (2020). Rho kinase inhibitors for SARS-CoV-2 induced acute respiratory distress syndrome: Support from Bartter’s and Gitelman’s syndrome patients. Pharmacol. Res. 158:104903. doi: 10.1016/j.phrs.2020.104903
Canetta, C., Accordino, S., Buscarini, E., Benelli, G., La Piana, G., Scartabellati, A., et al. (2020). Syncope at SARS-CoV-2 onset due to impaired baroreflex response. medRxiv [Preprint] doi: 10.1101/2020.05.29.20114751
Castelnovo, L., Capelli, F., Tamburello, A., Faggioli, P. M., and Mazzone, A. (2020). Symmetric cutaneous vasculitis in COVID-19 pneumonia. J. Eur. Acad. Dermatol. Venereol. doi: 10.1111/jdv.16589 [Epub ahead of print].
Cavolo, S. L., Zhou, C., Ketcham, S. A., Suzuki, M. M., Ukalovic, K., Silverman, M. A., et al. (2015). Mycalolide B dissociates dynactin and abolishes retrograde axonal transport of dense-core vesicles. Mol. Biol. Cell 26, 2664–2672. doi: 10.1091/mbc.e14-11-1564
Cha, M. H., Regueiro, M., and Sandhu, D. S. (2020). Gastrointestinal and hepatic manifestations of COVID-19: a comprehensive review. World J. Gastroenterol. 26, 2323–2332.
Chazal, N., and Gerlier, D. (2003). Virus entry, assembly, budding, and membrane rafts. Microbiol. Mol. Biol. Rev. 67, 226–237. doi: 10.1128/mmbr.67.2.226-237.2003
Cheema, M., Aghazadeh, H., Nazarali, S., Ting, A., Hodges, J., McFarlane, A., et al. (2020). Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19). Can. J. Ophthalmol. [Epub ahead of print].
Chen, H., Guo, J., Wang, C., Luo, F., Yu, X., Zhang, W., et al. (2020a). Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet (London, England) 395, 809–815. doi: 10.1016/s0140-6736(20)30360-3
Chen, X., Zhao, B., Qu, Y., Chen, Y., Xiong, J., Feng, Y., et al. (2020b). Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely associated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin. Infect. Dis. 17:ciaa449. doi: 10.1093/cid/ciaa449 [Epub ahead of print].
Chen, L., Lin, Y.-L., Peng, G., and Li, F. (2012). Structural basis for multifunctional roles of mammalian aminopeptidase N. Proc. Natl. Acad. Sci. U.S.A. 109, 17966–17971. doi: 10.1073/pnas.1210123109
Chin, A., Chu, J., Perera, M., Hui, K., Yen, H.-L., Chan, M., et al. (2020). Stability of SARS-CoV-2 in different environmental conditions. medRxiv [Preprint] doi: 10.1101/2020.03.15.20036673
Chung, K. F., McGarvey, L., and Mazzone, S. B. (2013). Chronic cough as a neuropathic disorder. Lancet Respir. Med. 1, 414–422. doi: 10.1016/s2213-2600(13)70043-2
Clarke, N. E., Fisher, M. J., Porter, K. E., Lambert, D. W., and Turner, A. J. (2012). Angiotensin converting enzyme (ACE) and ACE2 bind integrins and ACE2 regulates integrin signalling. PLoS ONE 7:e34747. doi: 10.1371/journal.pone.0034747
Colavita, F., Lapa, D., Carletti, F., Lalle, E., Bordi, L., Marsella, P., et al. (2020). SARS-CoV-2 isolation from ocular secretions of a patient with COVID-19 in Italy with prolonged viral RNA detection. Ann. Int. Med. M20–M1176.
Coolen, T., Lolli, V., Sadeghi, N., Rovai, A., Trotta, N., Taccone, F. S., et al. (2020). Early postmortem brain MRI findings in COVID-19 non-survivors. medRxiv [Preprint] doi: 10.1101/2020.05.04.20090316
Day, M. (2020). Covid-19: four fifths of cases are asymptomatic, China figures indicate. BMJ 369:m1375. doi: 10.1136/bmj.m1375
de Jonge, W. J., van der Zanden, E. P., The, F. O., Bijlsma, M. F., van Westerloo, D. J., Bennink, R. J., et al. (2005). Stimulation of the vagus nerve attenuates macrophage activation by activating the Jak2-STAT3 signaling pathway. Nat. Immunol. 6, 844–851. doi: 10.1038/ni1229
Dina, O. A., Parada, C. A., Yeh, J., Chen, X., McCarter, G. C., and Levine, J. D. (2004). Integrin signaling in inflammatory and neuropathic pain in the rat. Eur. J. Neurosci. 19, 634–642. doi: 10.1111/j.1460-9568.2004.03169.x
Doobay, M. F., Talman, L. S., Obr, T. D., Tian, X., Davisson, R. L., and Lazartigues, E. (2007). Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. physiol. Regul. Integr. Compar. Physiol. 292, R373–R381.
Dubé, M., Le Coupanec, A., Wong, A. H. M., Rini, J. M., Desforges, M., and Talbot, P. J. (2018). Axonal transport enables neuron-to-neuron propagation of human coronavirus OC43. J. Virol. 92, JVI.404–JVI.418. doi: 10.1128/JVI.00404-18
Eden, E. R. (2016). The formation and function of ER-endosome membrane contact sites. Biochim. Biophys. Acta (BBA) 1861, 874–879. doi: 10.1016/j.bbalip.2016.01.020
Enquist, L. W. (2012). Five questions about viral trafficking in neurons. PLoS Pathog. 8:e1002472. doi: 10.1371/journal.ppat.1002472
Enwere, E., Shingo, T., Gregg, C., Fujikawa, H., Ohta, S., and Weiss, S. (2004). Aging results in reduced epidermal growth factor receptor signaling, diminished olfactory neurogenesis, and deficits in fine olfactory discrimination. J. Neurosci. 24, 8354–8365. doi: 10.1523/jneurosci.2751-04.2004
Escobales, N., Nunez, R. E., and Javadov, S. (2019). Mitochondrial angiotensin receptors and cardioprotective pathways. Am. J. physiol. Heart Circul. Physiol. 316, H1426–H1438.
Fantini, J., Di Scala, C., Chahinian, H., and Yahi, N. (2020). Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS-CoV-2 infection. Int. J. Antimicrob Agents 55:105960. doi: 10.1016/j.ijantimicag.2020.105960
Fazzini, E., Fleming, J., and Fahn, S. (1992). Cerebrospinal fluid antibodies to coronavirus in patients with Parkinson’s disease. Movement Disord. 7, 153–158. doi: 10.1002/mds.870070210
Fodoulian, L., Tuberosa, J., Rossier, D., Landis, B. N., Carleton, A., and Rodriguez, I. (2020). SARS-CoV-2 receptor and entry genes are expressed by sustentacular cells in the human olfactory neuroepithelium. bioRxiv [Preprint] doi: 10.1101/2020.03.31.013268
Gautret, P., Lagier, J.-C., Parola, P., Hoang, V. T., Meddeb, L., Mailhe, M., et al. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 56:105949. doi: 10.1016/j.ijantimicag.2020.105949 [Epub ahead of print].
Gonzalez, C., and Couve, A. (2014). The axonal endoplasmic reticulum and protein trafficking: Cellular bootlegging south of the soma. Semin. Cell Dev. Biol. 27, 23–31. doi: 10.1016/j.semcdb.2013.12.004
Griffin, D. E. (2003). Immune responses to RNA-virus infections of the CNS. Nature Reviews Immunology. 3, 493–502. doi: 10.1038/nri1105
Hani, C., Trieu, N. H., Saab, I., Dangeard, S., Bennani, S., Chassagnon, G., et al. (2020). COVID-19 pneumonia: a review of typical CT findings and differential diagnosis. Diagn Interv. Imaging. 101, 263–268. doi: 10.1016/j.diii.2020.03.014
Helms, J., Tacquard, C., Severac, F., Leonard-Lorant, I., Ohana, M., Delabranche, X., et al. (2020). High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intens. Care Med. [Epub ahead of print].
Hoffmann, M., Kleine-Weber, H., Krüger, N., Müller, M., Drosten, C., and Pöhlmann, S. (2020a). The novel coronavirus 2019 (2019-nCoV) uses the SARS-coronavirus receptor ACE2 and the cellular protease TMPRSS2 for entry into target cells. bioRxiv [Preprint] doi: 10.1101/2020.01.31.929042
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020b). SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8. doi: 10.1016/j.cell.2020.02.052
Holstege, G. (1987). Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: an HRP and autoradiographic tracing study in the cat. J. Compar. Meurol. 260, 98–126. doi: 10.1002/cne.902600109
Hu, X., Han, C., Jin, J., Qin, K., Zhang, H., Li, T., et al. (2016). Integrin CD11b attenuates colitis by strengthening Src-Akt pathway to polarize anti-inflammatory IL-10 expression. Sci. Rep. 6:26252.
Hu, Y., Shen, L., Xu, Z., Zhou, J., and Zhou, H. (2020). SARS-CoV-2 may persist in digestive tract longer than respiratory tract. [Preprint]. doi: 10.20944/preprints202002.0354.v1
Hui, K., Cheung, M.-C., Perera, R. A. P. M., Ng, K.-C., Bui, C., Ho, J., et al. (2020). Tropism of the novel coronavirus SARS-CoV-2 in human respiratory tract: an analysis in ex vivo and in vitro cultures. SSRN Electron. J. 1:e10.
Huston, J. M. (2012). The vagus nerve and the inflammatory reflex: wandering on a new treatment paradigm for systemic inflammation and sepsis. Surg. Infect. 13, 187–193. doi: 10.1089/sur.2012.126
Iba, T., Levy, J. H., Levi, M., Connors, J. M., and Thachil, J. (2020). Coagulopathy of coronavirus disease 2019. Crit. Care Med. [Preprint]. doi: 10.1097/CCM.0000000000004458
Jaimes, J. A., André, N. M., Millet, J. K., and Whittaker, G. R. (2020). Structural modeling of 2019-novel coronavirus (nCoV) spike protein reveals a proteolytically-sensitive activation loop as a distinguishing feature compared to SARS-CoV and related SARS-like coronaviruses. bioRxiv [Preprint] doi: 10.1101/2020.02.10.942185
Jiang, F., Deng, L., Zhang, L., Cai, Y., Cheung, C. W., and Xia, Z. (2020). Review of the clinical characteristics of coronavirus disease 2019 (COVID-19). J. General Int. Med. 35, 1545–1549.
Johnston, G. R., and Webster, N. R. (2009). Cytokines and the immunomodulatory function of the vagus nerve. Br. J. Anaesth. 102, 453–462. doi: 10.1093/bja/aep037
Kalinke, U., Bechmann, I., and Detje, C. N. (2011). Host strategies against virus entry via the olfactory system. Virulence 2, 367–370. doi: 10.4161/viru.2.4.16138
Karimi, N., Sharifi Razavi, A., and Rouhani, N. (2020). Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran. Red. Crescent Med. J. 22:e102828.
Kaya, Y., Kara, S., Akinci, C., and Kocaman, A. S. (2020). Transient cortical blindness in COVID-19 pneumonia; a PRES-like syndrome: case report. J. Neurol. Sci. 413:116858. doi: 10.1016/j.jns.2020.116858
Khan, G., Ahmed, W., and Rony, P. (2017). Exosomes and their role in viral infections. Open Virol. J. 12, 134–148.
Kim, S. Y., Jin, W., Sood, A., Montgomery, D. W., Grant, O. C., Fuster, M. M., et al. (2020). Glycosaminoglycan binding motif at S1/S2 proteolytic cleavage site on spike glycoprotein may facilitate novel coronavirus (SARS-CoV-2) host cell entry. bioRxiv [Preprint] doi: 10.1101/2020.04.14.041459
Klein, R. S., Garber, C., Funk, K. E., Salimi, H., Soung, A., Kanmogne, M., et al. (2019). Neuroinflammation During RNA Viral Infections. Annu. Rev. Immunol. 37, 73–95. doi: 10.1146/annurev-immunol-042718-041417
Knoops, K., Kikkert, M., Worm, S. H., Zevenhoven-Dobbe, J. C., van der Meer, Y., Koster, A. J., et al. (2008). SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol. 6:e226. doi: 10.1371/journal.pbio.0060226
Kochi, A. N., Tagliari, A. P., Forleo, G. B., Fassini, G. M., and Tondo, C. (2020). Cardiac and arrhythmic complications in patients with COVID-19. J. Cardiovasc. Electrophysiol. 31, 1003–1008. doi: 10.1111/jce.14479
Koczera, P., Martin, L., Marx, G., and Schuerholz, T. (2016). The ribonuclease A superfamily in humans: canonical rnases as the buttress of innate immunity. Int. J. Mol. Sci. 17:1278. doi: 10.3390/ijms17081278
Koopman, F. A., Chavan, S. S., Miljko, S., Grazio, S., Sokolovic, S., Schuurman, P. R., et al. (2016). Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl. Acad. Sci. U.S.A. 113, 8284–8289. doi: 10.1073/pnas.1605635113
Koyuncu, O. O., Hogue, I. B., and Enquist, L. W. (2013). Virus infections in the nervous system. Cell Host Microbe. 13, 379–393.
Krijnse-Locker, J., Ericsson, M., Rottier, P. J., and Griffiths, G. (1994). Characterization of the budding compartment of mouse hepatitis virus: evidence that transport from the RER to the Golgi complex requires only one vesicular transport step. J. Cell Biol. 124, 55–70. doi: 10.1083/jcb.124.1.55
Lai, C.-C., Shih, T.-P., Ko, W.-C., Tang, H.-J., and Hsueh, P.-R. (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents 55:105924. doi: 10.1016/j.ijantimicag.2020.105924
Lan, L., Xu, D., Ye, G., Xia, C., Wang, S., Li, Y., et al. (2020). Positive RT-PCR test results in patients recovered from COVID-19. JAMA 323, 1502–1503.
Lechien, J. R., Chiesa-Estomba, C. M., De Siati, D. R., Horoi, M., Le Bon, S. D., Rodriguez, A., et al. (2020). Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur. Arch. Otorhinolaryngol. 277, 2251–2261. doi: 10.1007/s00405-020-05965-1
Leisman, D. E., Deutschman, C. S., and Legrand, M. (2020). Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Inten. Care Med. 46, 1105–1108. doi: 10.1007/s00134-020-06059-6
Li, D.-J., Evans, R., Yang, Z.-W., Song, S.-W., Wang, P., Ma, X.-J., et al. (2011). Dysfunction of the cholinergic anti-inflammatory pathway mediates organ damage in hypertension. Hypertension (Dallas, Tex: 1979) 57, 298–307. doi: 10.1161/hypertensionaha.110.160077
Li, P., Liu, H., Sun, P., Wang, X., Wang, C., Wang, L., et al. (2016). Chronic vagus nerve stimulation attenuates vascular endothelial impairments and reduces the inflammatory profile via inhibition of the NF-κB signaling pathway in ovariectomized rats. Exp. Gerontol. 74, 43–55. doi: 10.1016/j.exger.2015.12.005
Li, Y. C., Bai, W. Z., and Hashikawa, T. (2020). The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. [Epub ahead of print].
Li, Y. C., Bai, W. Z., Hirano, N., Hayashida, T., Taniguchi, T., Sugita, Y., et al. (2013). Neurotropic virus tracing suggests a membranous-coating-mediated mechanism for transsynaptic communication. J. Compar. Neurol. 521, 203–212. doi: 10.1002/cne.23171
Liu, J., Cao, R., Xu, M., Wang, X., Zhang, H., Hu, H., et al. (2020). Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 6:16.
Lorizate, M., and Kräusslich, H.-G. (2011). Role of lipids in virus replication. Cold Spring Harb. Perspect. Biol. 3:a004820–a.
Loseva, E., Yuan, T.-F., and Karnup, S. (2009). Neurogliogenesis in the mature olfactory system: a possible protective role against infection and toxic dust. Brain Res. Rev. 59, 374–387. doi: 10.1016/j.brainresrev.2008.10.004
MacGibeny, M. A., Koyuncu, O. O., Wirblich, C., Schnell, M. J., and Enquist, L. W. (2018). Retrograde axonal transport of rabies virus is unaffected by interferon treatment but blocked by emetine locally in axons. PLoS Pathog. 14:e1007188. doi: 10.1371/journal.ppat.1007188
Mao, L., Jin, H., Wang, M., Hu, Y., Chen, S., He, Q., et al. (2020). Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 77, 683–690.
Marsh, M., and Helenius, A. (2006). Virus entry: open sesame. Cell 124, 729–740. doi: 10.1016/j.cell.2006.02.007
Marzioni, D., Tamagnone, L., Capparuccia, L., Marchini, C., Amici, A., Todros, T., et al. (2004). Restricted innervation of uterus and placenta during pregnancy: evidence for a role of the repelling signal Semaphorin 3A. Dev. Dynam. 231, 839–848. doi: 10.1002/dvdy.20178
Masters, P. S. (2006). The molecular biology of coronaviruses. Adv. Virus Res. 66, 193–292. doi: 10.1016/s0065-3527(06)66005-3
Mehta, P., McAuley, D. F., Brown, M., Sanchez, E., Tattersall, R. S., and Manson, J. J. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet (Lond. Engl). 395, 1033–1034. doi: 10.1016/s0140-6736(20)30628-0
Meinhardt, J., Radke, J., Dittmayer, C., Mothes, R., Franz, J., Laue, M., et al. (2020). Olfactory transmucosal SARS-CoV-2 invasion as port of central nervous system entry in COVID-19 patients. bioRxiv [Preprint] doi: 10.1101/2020.06.04.135012
Moon, C., Han, J. R., Park, H. J., Hah, J. S., and Kang, J. L. (2009). Synthetic RGDS peptide attenuates lipopolysaccharide-induced pulmonary inflammation by inhibiting integrin signaled MAP kinase pathways. Respir. Res. 10:18.
Mori, I., Nishiyama, Y., Yokochi, T., and Kimura, Y. (2004). Virus-induced neuronal apoptosis as pathological and protective responses of the host. Rev. Med. Virol. 14, 209–216. doi: 10.1002/rmv.426
Moriguchi, T., Harii, N., Goto, J., Harada, D., Sugawara, H., Takamino, J., et al. (2020). A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 4, 55–58.
Mycroft-West, C., Su, D., Elli, S., Guimond, S., Miller, G., Turnbull, J., et al. (2020). The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. bioRxiv [Preprint] doi: 10.1101/2020.02.29.971093
Nie, S., Zhao, X., Zhao, K., Zhang, Z., Zhang, Z., and Zhang, Z. (2020). Metabolic disturbances and inflammatory dysfunction predict severity of coronavirus disease 2019 (COVID-19): a retrospective study. medRxiv [Preprint] doi: 10.1101/2020.03.24.20042283
Niimi, A., and Chung, K. F. (2015). Evidence for neuropathic processes in chronic cough. Pulmon. Pharmacol. Therap. 35, 100–104. doi: 10.1016/j.pupt.2015.10.004
Ohka, S., Matsuda, N., Tohyama, K., Oda, T., Morikawa, M., Kuge, S., et al. (2004). Receptor (CD155)-dependent endocytosis of poliovirus and retrograde axonal transport of the endosome. J. Virol. 78, 7186–7198. doi: 10.1128/jvi.78.13.7186-7198.2004
Okusaga, O., Yolken, R. H., Langenberg, P., Lapidus, M., Arling, T. A., Dickerson, F. B., et al. (2011). Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J. Affect. Disord. 130, 220–225. doi: 10.1016/j.jad.2010.09.029
Oliphant, T., Engle, M., Nybakken, G. E., Doane, C., Johnson, S., Huang, L., et al. (2005). Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat. Med. 11, 522–530. doi: 10.1038/nm1240
Onal, H., and Semerci, S. Y. (2020). Effect of Quercetin on Prophylaxis and Treatment of COVID-19: Kanuni Sultan Suleyman Training and Research Hospital Available online at: https://clinicaltrials.gov/ct2/show/NCT04377789 (accessed March 20, 2020).
Ono, A. (2010). Relationships between plasma membrane microdomains and HIV-1 assembly. Biol. Cell. 102, 335–350. doi: 10.1042/bc20090165
Ou, X., Liu, Y., Lei, X., Li, P., Mi, D., Ren, L., et al. (2020). Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 11:1620.
Pan, C., Chen, L., Lu, C., Zhang, W., Xia, J.-A., Sklar, M. C., et al. (2020). Lung recruitability in COVID-19-associated acute respiratory distress syndrome: a single-center observational study. Am. J. Respir. Crit. Care Med. 201, 1294–1297. doi: 10.1164/rccm.202003-0527le
Paniz-Mondolfi, A., Bryce, C., Grimes, Z., Gordon, R. E., Reidy, J., Lednicky, J., et al. (2020). Central nervous system involvement by severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2). J. Med. Virol. 92, 699–702. doi: 10.1002/jmv.25915
Pavlov, V. A., and Tracey, K. J. (2012). The vagus nerve and the inflammatory reflex–linking immunity and metabolism. Nat. Rev. Endocrinol. 8, 743–754. doi: 10.1038/nrendo.2012.189
Pei, G., Zhang, Z., Peng, J., Liu, L., Zhang, C., Yu, C., et al. (2020). Renal involvement and early prognosis in patients with COVID-19 pneumonia. J. Am. Soc. Nephrol. 31, 1157–1165. doi: 10.1681/asn.2020030276
Pinto, B. G., Oliveira, A. E., Singh, Y., Jimenez, L., Goncalves, A. N., Ogava, R. L., et al. (2020). ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. medRxiv [Preprint]. doi: 10.1101/2020.03.21.20040261.
Poyiadji, N., Shahin, G., Noujaim, D., Stone, M., Patel, S., and Griffith, B. (2020). COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 296:201187.
Prentice, E., McAuliffe, J., Lu, X., Subbarao, K., and Denison, M. R. (2004). Identification and characterization of severe acute respiratory syndrome coronavirus replicase proteins. J. Virol. 78, 9977–9986. doi: 10.1128/jvi.78.18.9977-9986.2004
Proença de Oliveira-Maul, J., Barbosa, de Carvalho, H., Goto, D. M., Maia, R. M., et al. (2013). Aging, diabetes, and hypertension are associated with decreased nasal mucociliary clearance. Chest 143, 1091–1097. doi: 10.1378/chest.12-1183
Puelles, V. G., Lütgehetmann, M., Lindenmeyer, M. T., Sperhake, J. P., Wong, M. N., Allweiss, L., et al. (2020). Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. doi: 10.1056/NEJMc2011400 [Epub ahead of print].
Rees, C. J., Henderson, A. H., and Belafsky, P. C. (2009). Postviral vagal neuropathy. Ann. Otol. Rhinol. Laryngol. 118, 247–252. doi: 10.1177/000348940911800402
Sachdeva, M., Gianotti, R., Shah, M., Lucia, B., Tosi, D., Veraldi, S., et al. (2020). Cutaneous manifestations of COVID-19: report of three cases and a review of literature. J. Dermatol. Sci. 98, 75–81. doi: 10.1016/j.jdermsci.2020.04.011
Salanueva, I. J., Carrascosa, J. L., and Risco, C. (1999). Structural maturation of the transmissible gastroenteritis coronavirus. J. Virol. 73, 7952–7964. doi: 10.1128/jvi.73.10.7952-7964.1999
Samuel, M. A., Wang, H., Siddharthan, V., Morrey, J. D., and Diamond, M. S. (2007). Axonal transport mediates West Nile virus entry into the central nervous system and induces acute flaccid paralysis. Proc. Natl. Acad. Sci. U.S.A. 104, 17140–17145. doi: 10.1073/pnas.0705837104
Schnaar, R. L., Gerardy-Schahn, R., and Hildebrandt, H. (2014). Sialic acids in the brain: gangliosides and polysialic acid in nervous system development, stability, disease, and regeneration. Physiol. Rev. 94, 461–518. doi: 10.1152/physrev.00033.2013
Selimoglu, M. A., Selimoglu, E., and Kurt, A. (1999). Nasal mucociliary clearance and nasal and oral pH in patients with insulin-dependent diabetes. Ear Nose Throat J. 78, 585–590. doi: 10.1177/014556139907800814
Severance, E., Dickerson, F., Bossis, Y., Stallings, C., Origoni, A., Sullens, A., et al. (2009). Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr. Bull. 37, 101–107. doi: 10.1093/schbul/sbp052
Sharifi-Razavi, A., Karimi, N., and Rouhani, N. (2020). COVID 19 and Intra cerebral hemorrhage: causative or coincidental. New Microbes New Infect. 35:100669. doi: 10.1016/j.nmni.2020.100669
Shi, T.-T., Yang, F.-Y., Liu, C., Cao, X., Lu, J., Zhang, X.-L., et al. (2018). Angiotensin-converting enzyme 2 regulates mitochondrial function in pancreatic β-cells. Biochem. Biophys. Res. Commun. 495, 860–866. doi: 10.1016/j.bbrc.2017.11.055
Sigrist, C. J., Bridge, A., and Le Mercier, P. (2020). A potential role for integrins in host cell entry by SARS-CoV-2. Antiv. Res. 177:104759. doi: 10.1016/j.antiviral.2020.104759
Snijder, E. J., Limpens, R. W. A. L., de Wilde, A. H., de Jong, A. W. M., Zevenhoven-Dobbe, J. C., Maier, H. J., et al. (2020). A unifying structural and functional model of the coronavirus replication organelle: tracking down RNA synthesis. bioRxiv [Preprint] doi: 10.1101/2020.03.24.005298
Sotomayor-Flores, C., Rivera-Mejias, P., Vasquez-Trincado, C., Lopez-Crisosto, C., Morales, P. E., Pennanen, C., et al. (2020). Angiotensin-(1-9) prevents cardiomyocyte hypertrophy by controlling mitochondrial dynamics via miR-129-3p/PKIA pathway. Cell Death Diff. [Epub ahead of print].
Steardo, L., Steardo, L. Jr., Zorec, R., and Verkhratsky, A. (2020). Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19. Acta Physiol. (Oxf. Engl.) 229:e13473.
Sultan, I., Howard, S., and Tbakhi, A. (2020). Drug repositioning suggests a role for the heat shock protein 90 inhibitor geldanamycin in treating COVID-19 infection. Res. Square [Preprint]. doi: 10.21203/rs.3.rs-18714/v1
Tanida, I., Fukasawa, M., Ueno, T., Kominami, E., Wakita, T., and Hanada, K. (2009). Knockdown of autophagy-related gene decreases the production of infectious hepatitis C virus particles. Autophagy 5, 937–945. doi: 10.4161/auto.5.7.9243
Tian, Y., Rong, L., Nian, W., and He, Y. (2020). Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol. Ther. 51, 843–851. doi: 10.1111/apt.15731
Toljan, K. (2020). Letter to the editor regarding the viewpoint Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanism. ACS Chem. Neurosci. 11, 1192–1194. doi: 10.1021/acschemneuro.0c00174
Vajn, K., Viljetic, B., Degmecic, I. V., Schnaar, R. L., and Heffer, M. (2013). Differential distribution of major brain gangliosides in the adult mouse central nervous system. PLoS ONE 8:e75720. doi: 10.1371/journal.pone.0075720
Valdes, G., Corthorn, J., Bharadwaj, M. S., Joyner, J., Schneider, D., and Brosnihan, K. B. (2013). Utero-placental expression of angiotensin-(1-7) and ACE2 in the pregnant guinea-pig. Reproduct. Biol. Endocrinol. 11:5. doi: 10.1186/1477-7827-11-5
Varga, Z., Flammer, A. J., Steiger, P., Haberecker, M., Andermatt, R., Zinkernagel, A. S., et al. (2020). Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 395, 1417–1418. doi: 10.1016/s0140-6736(20)30937-5
Verdoni, L., Mazza, A., Gervasoni, A., Martelli, L., Ruggeri, M., Ciuffreda, M., et al. (2020). An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet (London, Engl.) 395, 1771–1778. doi: 10.1016/s0140-6736(20)31103-x
Verheije, M. H., Raaben, M., Mari, M., Te Lintelo, E. G., Reggiori, F., van Kuppeveld, F. J., et al. (2008). Mouse hepatitis coronavirus RNA replication depends on GBF1-mediated ARF1 activation. PLoS Pathog. 4:e1000088. doi: 10.1371/journal.ppat.1000088
Villar-Cheda, B., Costa-Besada, M. A., Valenzuela, R., Perez-Costas, E., Melendez-Ferro, M., and Labandeira-Garcia, J. L. (2017). The intracellular angiotensin system buffers deleterious effects of the extracellular paracrine system. Cell Death Dis. 8:e3044. doi: 10.1038/cddis.2017.439
Waheed, A. A., and Freed, E. O. (2010). The role of lipids in retrovirus replication. Viruses 2, 1146–1180. doi: 10.3390/v2051146
Walls, A. C., Park, Y. J., Tortorici, M. A., Wall, A., McGuire, A. T., and Veesler, D. (2020). Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281–292.e6. doi: 10.1016/j.cell.2020.02.058
Walter, U., Tsiberidou, P., Kersten, M., Storch, A., and Lohle, M. (2018). Atrophy of the vagus nerve in Parkinson’s disease revealed by high-resolution ultrasonography. Front. Neurol. 9:805.
Wan, S., Xiang, Y., Fang, W., Zheng, Y., Li, B., Hu, Y., et al. (2020). Clinical features and treatment of COVID-19 patients in northeast Chongqing. J. Med. Virol. 92, 797–806. doi: 10.1002/jmv.25783
Wang, H., Yang, P., Liu, K., Guo, F., Zhang, Y., Zhang, G., et al. (2008). SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 18, 290–301. doi: 10.1038/cr.2008.15
Wang, J., Chen, S., and Bihl, J. (2020a). Exosome-mediated transfer of ACE2 (angiotensin-converting enzyme 2) from endothelial progenitor cells promotes survival and function of endothelial cell. Oxidat. Med. Cell. Long. 2020: 4213541.
Wang, W., Xu, Y., Gao, R., Lu, R., Han, K., Wu, G., et al. (2020b). Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323, 1843–1844. doi: 10.1001/jama.2020.3786
Wang, X., Xu, W., Hu, G., Xia, S., Sun, Z., Liu, Z., et al. (2020c). SARS-CoV-2 infects T lymphocytes through its spike protein-mediated membrane fusion. Cell. Mol. Immunol. [Epub ahead of print].
Wang, X., Yang, H., and Raizada, M. K. (2001). Angiotensin II increases vesicular trafficking in brain neurons. Hypertension (Dallas Tex: 1979) 37(2 Pt 2), 677–682. doi: 10.1161/01.hyp.37.2.677
Westerloo, D., Giebelen, I., Meijers, J., Daalhuisen, J., Vos, A., Levi, M., et al. (2006). Vagus nerve stimulation inhibits activation of coagulation and fibrinolysis during endotoxemia in rats. J. Thrombos. Haemostas. 4, 1997–2002. doi: 10.1111/j.1538-7836.2006.02112.x
White, J. A., Banerjee, R., and Gunawardena, S. (2016). Axonal Transport and neurodegeneration: how marine drugs can be used for the development of therapeutics. Mar. Drugs 14:102. doi: 10.3390/md14050102
Wölfel, R., Corman, V. M., Guggemos, W., Seilmaier, M., Zange, S., Müller, M. A., et al. (2020). Virological assessment of hospitalized patients with COVID-2019. Nature 581, 465–469. doi: 10.1038/s41586-020-2196-x
Wu, Y., Wei, F., Tang, L., Liao, Q., Wang, H., Shi, L., et al. (2019). Herpesvirus acts with the cytoskeleton and promotes cancer progression. J. Cancer 10, 2185–2193. doi: 10.7150/jca.30222
Xia, S., Liu, M., Wang, C., Xu, W., Lan, Q., Feng, S., et al. (2020). Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 30, 343–355. doi: 10.1038/s41422-020-0305-x
Xiao, F., Tang, M., Zheng, X., Li, C., He, J., Hong, Z., et al. (2020). Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 158, 1831–1833.e3. doi: 10.1053/j.gastro.2020.02.055
Xing, Y., Mo, P., Xiao, Y., Zhao, O., Zhang, Y., and Wang, F. (2020). Post-discharge surveillance and positive virus detection in two medical staff recovered from coronavirus disease 2019 (COVID-19), China. Euro Surveill. 25:2000191.
Yang, Y., Shen, C., Li, J., Yuan, J., Yang, M., Wang, F., et al. (2020). Exuberant elevation of IP-10, MCP-3 and IL-1ra during SARS-CoV-2 infection is associated with disease severity and fatal outcome. medRxiv [Preprint] doi: 10.1101/2020.03.02.20029975
Yu, F., Yan, L., Wang, N., Yang, S., Wang, L., Tang, Y., et al. (2020). Quantitative detection and viral load analysis of SARS-CoV-2 in infected patients. Clin. Infect. Dis. [Epub ahead of print]. doi: 10.1093/cid/ciaa345
Yue, W. L. (2007). Nasal mucociliary clearance in patients with diabetes mellitus. J. Laryngol. Otol. 103, 853–855. doi: 10.1017/s0022215100110291
Zanin, L., Saraceno, G., Panciani, P. P., Renisi, G., Signorini, L., Migliorati, K., et al. (2020). SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochirurg. [Epub ahead of print].
Zeng, L., Xia, S., Yuan, W., Yan, K., Xiao, F., Shao, J., et al. (2020). Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr.
Zhang, C., Shi, L., and Wang, F. S. (2020a). Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 5, 428–430. doi: 10.1016/s2468-1253(20)30057-1
Zhang, J., Lu, H., Zeng, H., Zhang, S., Du, Q., Jiang, T., et al. (2020b). The differential psychological distress of populations affected by the COVID-19 pandemic. Brain Behav. Immun. 87, 49–50. doi: 10.1016/j.bbi.2020.04.031
Zhang, J., Yan, K., Ye, H., Lin, J., Zheng, J., and Cai, T. (2020c). SARS-CoV-2 turned positive in a discharged patient with COVID-19 arouses concern regarding the present standard for discharge. Int. J. Infect. Dis. 97, 212–214. doi: 10.1016/j.ijid.2020.03.007
Zhao, B., Ni, C., Gao, R., Wang, Y., Yang, L., Wei, J., et al. (2020a). Recapitulation of SARS-CoV-2 infection and cholangiocyte damage with human liver organoids. Protein Cell [Epub ahead of print].
Zhao, H., Shen, D., Zhou, H., Liu, J., and Chen, S. (2020b). Guillain-barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 19, 383–384. doi: 10.1016/s1474-4422(20)30109-5
Keywords: SARS-CoV-2, neurotropic infection, axonal transport, peripheral nerves, neurological symptoms, multiple organ failure
Citation: Fenrich M, Mrdenovic S, Balog M, Tomic S, Zjalic M, Roncevic A, Mandic D, Debeljak Z and Heffer M (2020) SARS-CoV-2 Dissemination Through Peripheral Nerves Explains Multiple Organ Injury. Front. Cell. Neurosci. 14:229. doi: 10.3389/fncel.2020.00229
Received: 18 April 2020; Accepted: 30 June 2020;
Published: 05 August 2020.
Edited by:
Peter S. Steyger, Creighton University, United StatesReviewed by:
Andrew MacLean, Tulane University, United StatesCopyright © 2020 Fenrich, Mrdenovic, Balog, Tomic, Zjalic, Roncevic, Mandic, Debeljak and Heffer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Matija Fenrich, bWZlbnJpY2hAbWVmb3MuaHI=; Marija Heffer, bWhlZmZlckBtZWZvcy5ocg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.