
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Neurosci. , 28 February 2020
Sec. Cellular Neuropathology
Volume 14 - 2020 | https://doi.org/10.3389/fncel.2020.00021
This article is part of the Research Topic Neurodegeneration: From Genetics to Molecules, Volume II View all 18 articles
Neurodegenerative diseases are characterized by chronic progressive degeneration of the structure and function of the nervous system, which brings an enormous burden on patients, their families, and society. It is difficult to make early diagnosis, resulting from the insidious onset and progressive development of neurodegenerative diseases. The drugs on the market cannot cross the blood–brain barrier (BBB) effectively, which leads to unfavorable prognosis and less effective treatments. Therefore, there is an urgent demand to develop a novel detection method and therapeutic strategies. Recently, nanomedicine has aroused considerable attention for diagnosis and therapy of central nervous system (CNS) diseases. Nanoparticles integrate targeting, imaging, and therapy in one system and facilitate the entry of drug molecules across the blood–brain barrier, offering new hope to patients. In this review, we summarize the application of iron oxide nanoparticles (IONPs) in the diagnosis and treatment of neurodegenerative disease, including Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS). We focus on IONPs as magnetic resonance imaging (MRI) contrast agents (CAs) and drug carriers in AD. What most neurodegenerative diseases have in common is that hall marker lesions are represented by protein aggregates (Soto and Pritzkow, 2018). These diseases are of unknown etiology and unfavorable prognosis, and the treatments toward them are less effective (Soto and Pritzkow, 2018). Such diseases usually develop in aged people, and early clinical manifestations are atypical, resulting in difficulty in early diagnosis. Recently, nanomedicine has aroused considerable attention for therapy and diagnosis of CNS diseases because it integrates targeting, imaging, and therapy in one system (Gupta et al., 2019). In this review article, we first introduce the neurodegenerative diseases and commonly used MRI CAs. Then we review the application of IONPs in the diagnosis and treatment of neurodegenerative diseases with the purpose of assisting early theranostics (therapy and diagnosis).
The main characteristic of Alzheimer’s disease (AD) is the progressive deterioration of cognitive function, most commonly the loss of memory, increasingly influencing patients’ activity of daily living and leading to loss of independency (Tiwari et al., 2019). The hallmark histological abnormalities of AD comprise of the extracellular aggregation of amyloid plaques and fibrillar aggregates of the microtubule associated with tau protein (Tiwari et al., 2019). The deposition of amyloid plaque, which is caused by the mounting production, accumulation, and aggregation of the amyloid-β (Aβ), is the primary histopathological characteristic of AD (Tiwari et al., 2019). The most widely accepted AD etiology is the amyloid cascade hypothesis (Barage and Sonawane, 2015). The incorrect process of the Aβ protein precursor (AβPP) by γ-secretase, which gives rise to the pathological 40–42 amino acid long cleaved peptide, known as Aβ, is fundamental to this hypothesis (Chen and Mobley, 2019; Tiwari et al., 2019). The excess of Aβ finally results in the aggregated fibrils of plaques and neurotoxic oligomers (Chen and Mobley, 2019).
Currently for AD, the early diagnosis could provide treatment opportunities to high-risk groups, and the way to cure diseases under development seems to be proven effective only at the very early stages of the initiated amyloid deposition (Frisoni et al., 2017). For patients in the earlier stage of AD, the correct diagnosis and treatment would enable to delay cognitive impairment and irreversible neuronal damage (Frisoni et al., 2017). Therefore, there is a rising demand to develop reliable early detective tools for AD.
Parkinson’s disease (PD) is the second most common neurodegenerative disease in the whole world (Balestrino and Schapira, 2019). PD mainly affects the locomotor system by degenerating the neuron, finally leading to severe disability (Balestrino and Schapira, 2019). The clinical criteria for diagnosis of PD depend mainly on the observation of movement disorders such as cogwheel rigidity, bradykinesia, resting tremor, etc. (Balestrino and Schapira, 2019). In the dopaminergic neurons, phosphor-α-synuclein molecules can aggregate with one another easily to form Lewy body, and these degenerative dopaminergic neurons lose the function of expressing dopamine, finally leading to the damage of the motor cortex and movement disorders (Ikenaka et al., 2019). Thus, for PD, α-synuclein is the most acknowledged biomarker (Ikenaka et al., 2019). If the quantity of degenerative dopaminergic neurons in the basal ganglia is over 50%, the clinical features of PD can gradually present. The clinical symptoms of PD might overlap with other movement disorders such as progressive supranuclear palsy (PSP) and multiple system atrophy (MSA; Giagkou and Stamelou, 2018). Therefore, going by clinical manifestations only, early PD diagnosis is difficult and prone to misdiagnosis.
Another neurodegenerative disease, amyotrophic lateral sclerosis (ALS), is an adult-onset neurodegenerative disorder. The major pathological manifestations of ALS are progressive loss of upper motor neurons of the corticospinal tract, lower motor neurons of ventral roots of the spinal cord, and brainstem nuclei (Gagliardi et al., 2019).
For the moment, despite the fact that many kinds of treatment appeared, no treatment has been shown to be effective for neurodegenerative diseases. Mesenchymal stem cell (MSC) therapy is regarded as one of the most prospective approaches for the treatment of neurodegenerative diseases, including PD and ALS (Baloh et al., 2018; Staff et al., 2019). In addition to MSC, neural stem cells (NSCs) could turn to be an optimal selection to replace specific lost neurons in vitro and in vivo (Sugaya and Vaidya, 2018).
Currently for AD, there is no other confirmed diagnostic method except autopsy (Pillai et al., 2018). Although researchers use plenty of neuropsychological tests to make the clinical diagnosis, the diagnosis process could be affected by many other factors. So the definitive diagnosis still depends on autopsy with histological and pathological findings of sufficient numbers of plaques (Tiwari et al., 2019). But this method is an invasive test and is not usually accepted by patients.
Cerebrospinal fluid (CSF) is usually collected through the lumbar puncture, which is invasive and uncomfortable. The reduction of CSF Aβ42 and the elevation of CSF tau are the biomarkers of AD. But the elevated CSF tau is just a biomarker of neuronal injury, not specific to AD (Olsson et al., 2016).
Compared to CSF, blood sample is much easier to obtain in clinics (Yang et al., 2016). For PD, α-synuclein can be expressed in the peripheral blood system at very low amounts, but it is not applicable for clinical use due to the poor low-detection limit of blood test (Yang et al., 2016).
Nanotechnology can play an important role in increasing the sensitivity of detection of biomarkers (Gupta et al., 2019). Applications of the magnetic nanoparticles (MNPs) detecting biomarkers for AD and PD are mentioned later in the section The Application of Iron Oxide Nanoparticles (IONPs) in Neurodegenerative Diseases.
On positron emission tomography (PET), one of the biomarkers of AD-related synaptic dysfunction is the decreased fluorodeoxyglucose 18F (FDG) uptake, which indicates temporoparietal hypometabolism (Chandra et al., 2019). Thus, currently PET is being used to image the amyloid deposition in AD, mild cognitive impairment (MCI), and normal aged controls in human (Chandra et al., 2019). However, in terms of PET, the visualization of individual plaques may be limited by the low spatial resolution for it is not clear enough to detect the earliest stage of amyloid deposition (Wadghiri et al., 2013).
Compared with PET, magnetic resonance imaging (MRI) has a much higher spatial resolution especially for soft tissue and does not require radiotracer injection (Wadghiri et al., 2013). And the instrumentation operating at 1.5–3 T is widely used for patient and animal imaging (Wadghiri et al., 2013). MRI can be used not only for imaging Aβ aggregation in the murine brain but also for monitoring other AD-related morphological and functional alterations (Rotman et al., 2011).
Chamberlain et al. (2009) reported that the susceptibility-weighted imaging (SWI) method can provide high plaque contrast for ex vivo plaque imaging, which increases the sharpness of the image in about 1 h 30 min. But in vivo, the SWI method has been found impractical due to the magnetic susceptibility artifact from the surface vessels and the air-to-skull interface (Chamberlain et al., 2011).
Genetic sequence analysis might be a method for diagnosis of early-stage PD, whereas only about 10% of PD patients are hereditary (Yang et al., 2016).
The contrast agents (CAs) in MRI can enhance the image contrast of the tissue/regions where they are delivered by shortening the water protons spin−lattice T1 and/or spin−spin T2 relaxation times (Busquets et al., 2014). Therefore, the image is CA affection to the relaxivity of the adjacent water protons through the dipolar interaction predominantly, rather than the CA compound itself (Busquets et al., 2014).
Traditionally, there are two different categories for MRI CAs taking effect in T1 and T2, respectively. T1 CAs can increase the T1 relaxation time, resulting in high signal in T1-weighted images. T2 CAs could reduce the T2 relaxation time, which reduces both T2 and T2* signals and gives rise to dark contrast in T2-weighted images (De et al., 2011). MRI CAs are also divided into “positive” CAs, which are in the majority, and “negative” CAs. In brief, “positive” CAs are the contrasts made by a gadolinium-based compound (paramagnetic CAs) administration, which enhances the MRI intensity of the signal. “Negative” CAs mean iron oxide based on superparamagnetic CAs. Superparamagnetic CAs can commonly decrease the MRI signal of the regions where they are delivered (Busquets et al., 2014).
Currently, the most widely used MRI CAs are gadolinium (Gd3+) chelates (Marasini et al., 2020). By using high-field MRI, Gd3+ chelates can demonstrate the Aβ plaque imaging both ex vivo and in vivo (Sillerud et al., 2013). Due to the long electron spin-lattice relaxation time and abundant unpaired electrons (seven) per Gd3+ ion has, the relaxation rate of water hydrogen can be increased significantly. However, ionic gadolinium complexes can leak toxic Gd3+ ions with short half-life and it has been reported that Gd3+ chelates can lead to renal insufficiency (Rashid et al., 2016; Marasini et al., 2020). Fluorinated small molecules that bind to amyloid plaques can be detected by 19F MRI (Jirak et al., 2019). But for their low in vivo concentrations, this method might be difficult to be applied to human clinical medicine (Sillerud et al., 2013). Besides, gold nanoparticles (Au NPs) present a significant MRI signal change during Aβ self-aggregation, but the intrinsic cytotoxicity caused by Co in preparing the NPs remains to be thoroughly evaluated (Brambilla et al., 2011).
Among all these MRI CAs, pure iron oxides such as magnetite (Fe3O4) and maghemite (γ-Fe2O3) are the most common biocompatible magnetic nanomaterials (Ling and Hyeon, 2013). Iron oxides are benign, nontoxic, and tolerated biologically, and they can be injected into the human body and incorporated into human natural processes of metabolism, serving as MRI CAs or drug delivery system (Ling and Hyeon, 2013). Currently, some IONP-based MRI CAs have already been used in clinical trials. Apart from the benefits mentioned above, IONPs offer many important biomedical applications, such as cell tracking, protein separation, and hyperthermia (Ling and Hyeon, 2013).
IONPs usually accumulate in liver and spleen. They can be eliminated by liver, spleen, and kidney (Arami et al., 2015). The potential toxic effects of free iron ions released from IONPs can be blocked by maintaining iron homeostasis. Excess iron ions can combine with ferritin to maintain the iron store, which is involved in many biological processes, including hemoglobin synthesis (Arami et al., 2015). One study has showed that the release of iron ions from IONPs may result in iron accumulation, oxidative stress, and protein aggregation, which are toxic to the neural cell. However, the toxicity levels of IONPs are determined by their properties, including the size, concentration, surface charge, and the type of coating and functional groups (Yarjanli et al., 2017).
Besides, colloidal IONPs such as ultrasmall superparamagnetic iron oxide (USPIO) and superparamagnetic iron oxide (SPIO) have already been used as MRI CAs in targeting drug delivery (Busquets et al., 2014; Dulińska-Litewka et al., 2019). Superparamagnetic iron oxide nanoparticles (SPIONs), with the iron oxide core and magnetic coating, have fundamental features such as high saturation magnetic moment, relatively stable chemical properties, and minimized potential toxicity (Busquets et al., 2014; Dulińska-Litewka et al., 2019). They can improve the MRI sensitivity by serving as in vivo or in vitro CAs and thus have potential biomedical applications for MRI CAs (Busquets et al., 2014; Dulińska-Litewka et al., 2019). The commonly used MRI CAs are briefly summarized in Table 1.
The blood–brain barrier (BBB) is mainly formed by neuronal pericytes, perivascular astrocytes, and brain capillary endothelial cells (BCECs). The infrastructure that each BCEC tightly connects with all their neighboring cells can firmly constitute a physical, chemical, and immunological barrier, and thus can keep the central nervous system (CNS) separate from peripheral blood circulation (Teleanu et al., 2019). The BBB permits only a few percent of potential CNS drugs into the CNS (Teleanu et al., 2019). Take MNPs for example; atomic force microscopy demonstrated that when MNPs cross the BBB, they can be internalized by endothelial cells (Kong et al., 2012). The BBB is responsible for the accurate internal regulation of the CNS; thus, any damage of the BBB can be related to systemic inflammatory or immune dysfunction, which further leads to the initiation of several neurodegenerative pathways (Teleanu et al., 2019).
In general, there are two ways of carrying drugs: (1) directly immobilized on the MNPs surface; and (2) tethered via polyethylene glycol (PEG) coating or other organic/inorganic polymer layer. The problem is that the free drugs can be eliminated by metabolism (enzymatic mainly) easily before they reach the target (Ding et al., 2014).
There has been a study examining the biodistribution of IONPs using lysophosphatidic acid (LPA) to help the entry to the brain. IONPs without LPA coating were mainly deposited in liver and spleen, with a plasma half-life of 6 min. The accumulation amount of IONPs with LPA coating in the brain and spleen increased approximately 4-fold compared with the control. Mice treated with LPA and IONP showed no sign of peripheral immune cell passing through the BBB and minimal activation of microglia and astrocytes, indicating a safe and effective strategy for IONP delivery to the brain (Sun et al., 2016).
CAs or therapeutic drugs can be delivered from the olfactory mucosa to the brain by neuronal cells through the olfactory pathway, which consists of olfactory epithelium in nasal cavity, lamina propria, and olfactory bulb in the CNS (Khan et al., 2017). Since the nasal passage is the only direct connection between the external environment and the brain, it provides an applicable method for CAs or therapeutic drugs to enter the brain by bypassing the BBB rather than to cross it (Salama et al., 2017). Thus, the olfactory pathway is more efficient for reducing hepatic/renal clearance and the systemic exposure (Akilo et al., 2016; Khan et al., 2017).
For the carriers whose sizes fall within the range from 120 to 200 nm, they can pass the trap of the reticuloendothelial system (RES) easily and cannot be detected by the cells of liver and spleen. This can prolong the time that the carriers remain in contact with the BBB, thus increasing the odds for the drug to be absorbed by the CNS ultimately (Sachdeva et al., 2015). Carriers such as MNPs can be encapsulated in liposomes while carrying potential therapeutic molecules such as cDNA, siRNA, and polypeptides, which serves as the drug delivery system (Thomsen et al., 2015).
Of all the drug delivery strategies, SPIONs comprising of maghemite (Fe2O3) and magnetite (Fe3O4) prove their advantage in targeted drug delivery systems (Anwar et al., 2014). The characteristics of both CAs and the delivery system make SPIONs the optimal choice for targeting drug delivery systems (Anwar et al., 2014; Dulińska-Litewka et al., 2019).
In this section, we simply summarized the application of iron nanoparticles in diagnosis and treatment in neurodegenerative disease, including AD, PD, and ALS. The following parts related to AD are summarized in Figure 1.
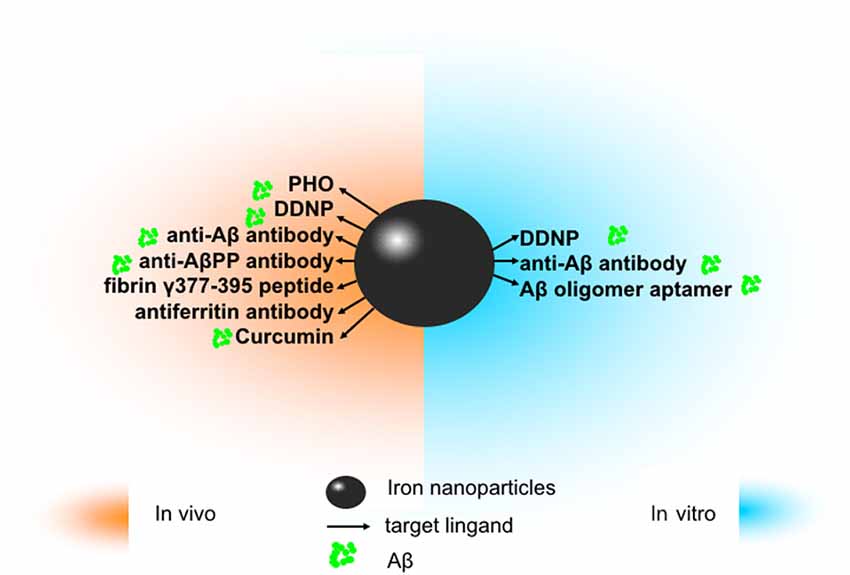
Figure 1. The application of iron oxide nanoparticles (IONPs) in diagnosis and treatment of Alzheimer’s disease (AD). Since amyloidβ (Aβ) has been identified as an ideal imaging biomarker of AD at present, with the help of magnetic resonance imaging (MRI), IONPs can be used for the detection of Aβ to assist in diagnosis and treatment of AD. In vitro, magnetic nanoparticles (MNPs) labeled with antibodies against Aβ-40 and Aβ-42 is applicable to detect Aβ in the blood. Conjugated with the Aβ oligomer aptamer and the complementary oligonucleotide of the Aβ oligomer aptamer, IONPs can be developed as a method to measure the Aβ oligomer in the artificial cerebrospinal fluid (CSF). DDNP-superparamagnetic iron oxide nanoparticles (SPIONs) with high affinities to Aβ(1–40) aggregates can be detected by fluorophotometry. In vivo, ultrasmall superparamagnetic iron oxide (USPIO)-PHO could mark on amyloid plaques in the NMRI mice brain; anti-Aβ protein precursor (AβPP) antibody-conjugated SPIONs can visualize the number of plaques in AβPP/PS1 transgenic mice. DDNP-SPIONs nanoparticles significantly decrease the signal intensity (SI) in the hippocampal area in the rat AD model. Curcumin-conjugated superparamagnetic iron oxides (SPIOs) can detect amyloid plaques in Tg2576 mice brains. Fibrin γ377–395 peptide-conjugatedγ-Fe2O3 nanoparticles could specifically inhibit the microglial cells in rTg4510 tau-mutant mice and thus provide a possible therapeutic strategy towards neurodegenerative tauopathies. In addition, magnetic IONPs bound to an antiferritin antibody were developed to detect ferritin protein in areas with a high amount of amyloid plaques in the brain of a transgenic AD mouse model. BBB, blood–brain barrier.
The deposition of Aβ is one of the primary histopathological characteristics of AD (Tiwari et al., 2019). With the relatively large size and being located intercellularly, Aβ can be regarded as an ideal imaging biomarker (Zhang et al., 2015). The Aβ deposition is earlier than clinical manifestations and increases gradually with the progress of the disease (Tiwari et al., 2019).
It has been reported that because of the limited brain uptake after the intravenous injection, the styrylbenzenes and the pathological staining dye analogs, such as X-34, ISB, IMSB, Congo Red, and Chrysamine G, are not suitable for the imaging of the Aβ plaque (Zhou et al., 2014). Since there are iron deposits in the amyloid plaques, in T2- and T2*-weighted MR images, the plaques showed a hypointense signal (Chamberlain et al., 2009). Thus, with the help of specific MRI sequences, the detection of amyloid plaques can use not the CAs but the iron content (Ansciaux et al., 2015). Since the detection of iron relies on the nature properties of the amyloid plaques and the stage of the disease (larger plaques contain higher amounts of iron, which makes the detection easier), it normally requires greater than 7-T magnetic field and several hours as acquisition time (Ansciaux et al., 2015). In AD transgenic mice, the levels of iron were high in thalamic plaques and low in cortical/hippocampal plaques, and by using different MRI sequences, the visibility of plaques in the cortex/hippocampus was different from those in the thalamus (Wengenack et al., 2011). The results showed that by T(2)SE, all plaques were detectable equally, but by T(2)*GE pulse sequences, only thalamic plaques were detected reliably (Wengenack et al., 2011). Human AD plaques are similar to cortical/hippocampal plaques of AD mice, and MRI methods that are less dependent on iron magnetic susceptibility effect might be suitable for imaging the human AD plaque (Wengenack et al., 2011). Studies have shown that CAs can conduce to Aβ plaques detecting under the condition of high field MRI both in vitro and in vivo (Yang et al., 2011a; Petiet et al., 2012).
The effective treatments for AD depend on the detection and quantitation of soluble AD biomarkers as early diagnosis, mainly by measuring the total tau protein and Aβ concentrations in CSF or plasma and detecting a suspected pathogenic biomarker. But being affected by low concentrations and other factors, these two strategies may lead to inconclusive imprecise results (Brambilla et al., 2011).
It has been reported that magnetic reagents that consist of MNPs magnetically labeled with antibodies against Aβ-40 and Aβ-42 can be applied to detect Aβ in the blood (Yang et al., 2011a). By applying the immunomagnetic reduction (IMR) assay, the concentration can be detected, which is lower than 10 pg/ml at Aβ-40 and 20 pg/ml at Aβ-42 and, furthermore, with high specificity (Yang et al., 2011a). Skaat et al. (2013) reported that the fixation of the aAβmAb clone BAM10 to near-infrared fluorescent maghemite nanoparticles enables to detect Aβ40 fibrils specifically ex vivo by both fluorescence and MRI.
1,1-Dicyano-2-[6-(dimethylamino)naphthalene-2-yl]propene (DDNP) carboxyl derivative-modified SPIONs (DDNP-SPIONs), synthetized by Zhou et al. (2014) have shown high binding affinities toward Aβ(1–40) aggregates by the investigation of fluorophotometry in vitro trials. Because of benefits such as versatile surface chemistry, relatively small size, and monodisperse size distribution, DDNP-SPIONs would provide opportunity for the molecular diagnosis of AD (Zhou et al., 2014).
With BaYF5:Yb, Er nanoparticles (UCNPs) as upconversion fluorescence labels, Fe3O4 MNPs as the recognition and concentration elements conjugated with the Aβ oligomer aptamer and the complementary oligonucleotide of the Aβ oligomer aptamer, respectively (Jiang et al., 2017). The developed method measured Aβ oligomer in artificial CSF successfully (Jiang et al., 2017).
With corresponding antibodies conjugated as targeting ligands, magnetic IONPs coated by antibiofouling polymer polyethylene glycol-block-allyl glycidyl ether (PEG-b-AGE) could capture Aβ40 and Aβ42 peptides and tau protein in CSF- and serum-mimicking samples, with high specificity (>90%) and sensitivity (>95%; Li et al., 2019). And the antibody-conjugated IONPs also detected Aβs and tau protein from the human whole blood samples, with significantly higher sensitivities than those of antibody-conjugated Dynabeads (Li et al., 2019).
It is reported that in AD transgenic mice, a gadolinium-loaded molecular probe can cross the BBB and specifically bind to the Aβ plaques after intravenous injection, and gained more than ninefold enhancement in the cortex and the hippocampus by using 7-T MRI (Poduslo et al., 2002). But as set forth, ionic gadolinium complexes can leak toxic Gd3+ ions with short half-life, and the Gd3+ chelates can lead to renal insufficiency.
Another research suggested that after introducing several magnetic CAs in AD transgenic mice to detect amyloid plaques, the USPIONs were able to commendably identify transgenic mice to the wild type by detecting amyloid plaques T2*-weighted in MRI (Yang et al., 2011b). Ansciaux et al. (2015) reported that a USPIO-PHO (USPIO coupled to peptide C-IPLPFYN-C) could cross the BBB of NMRI mice by intravenous injection and accumulates in the brain for 90 min, with high affinity (nanomolar binding affinity) and low toxicity. The half-life of USPIO-PHO was about 3 h. These MRI and histochemistry studies showed that USPIO-PHO had the potential to label amyloid plaques in the brain (Ansciaux et al., 2015). Sillerud et al. (2013) synthesized an anti-AβPP antibody-conjugated SPION, which can cross the BBB and act as an in vivo CA for MRI of Aβ plaques in AD. By MRI, the number of plaques visible per brain was about twice in AβPP/PS1 transgenic mice than that in control AD mice (Sillerud et al., 2013). DDNP-SPION nanoparticles were further tested by intrahippocampal injection of Aβ1–40 in a rat AD model (Zhang et al., 2015). After intravenous injection of DDNP-SPIONs in AD rats and testing by coronal T2*-weighted images, the signal intensity (SI) detected in the hippocampal area was decreased significantly, which indicated the binding of DDNP-SPIONs to the Aβ plaques (Zhang et al., 2015). Curcumin is a natural compound that can bind to amyloid plaques specifically (Cheng et al., 2015). Cheng et al. (2015) injected curcumin-conjugated SPIOs to Tg2576 mouse and nontransgenic mice; amyloid plaques were detectable in Tg2576 mice brains by ex vivo T2*-weighted MRI, but no plaques were found in the control group.
Growing evidence shows that vascular remodeling might be an important factor in the pathophysiologic mechanisms of AD (Klohs et al., 2012). Based on this, Klohs et al. (2012) used contrast-enhanced magnetic resonance microangiography (CE-μMRA) in wild-type control mice and arcAβ mice to estimate the density of the cortical microvasculature before and after the administration of SPIONs. CE-μMRA can be available for visualizing the cerebral arteries and veins whose diameter is less than 60 μm, the nominal pixel resolution (Klohs et al., 2012). The authors take the attitude that the deposition of Aβ and fibrin results in impaired perfusion and vascular occlusion, which may finally contribute to the density reduction of transcortical vessels (Klohs et al., 2012).
In addition to the experiments on animals mentioned above, medical researchers may consider using a new and broader approach, including T1ρ-weighting, macroscopic T2 mapping, SE imaging, FSE imaging, and gradient-echo (GRE) imaging, to detect the plaque in human clinical trials (Chamberlain et al., 2009).
By ex vivo MRI (11.7 T), anti-amyloid targeted superparamagnetic IONPs were capable of detecting deposition of amyloid β plaques and neuroinflammation activation by microglia in 3X AD transgenic mice (Tafoya et al., 2017).
Based on magnetic resonance molecular imaging (MRMI), ultrasmall particles of iron oxide (USPIO) functionalized with a disulfide constrained cyclic heptapeptide (PHO) was able to target Aβ plaques and cross the BBB in AD transgenic mice (André et al., 2017). The colocalization of USPIO-PHO with amyloid plaques on brain sections was demonstrated by immunohistochemistry and immunofluorescent experiment (André et al., 2017). Moreover, the amount of amyloid plaques detected by USPIO-PHO was in good correlation with that of plaques detected with anti-amyloid β antibody and Perls’-DAB staining (André et al., 2017).
In a recent study, magnetic IONPs bound to an antiferritin antibody were developed to detect ferritin protein in areas with a high amount of amyloid plaques, in particular the subiculum in the hippocampal area, in the brain of a transgenic mouse model with five familial AD mutations (Fernández et al., 2018). Functionalized IONPs were capable of recognizing and combining specifically to the ferritin protein accumulated in the subiculum area of the AD transgenic mice (Fernández et al., 2018).
For AD, drug therapy can improve the symptoms but cannot prevent the development of the disease (Busquets et al., 2014). For patients whose disease course beyond an average of 6 months, the benefits of drug therapy are not sustained (Corbett et al., 2012). The drug distribution spreading across the whole brain may decrease the amount of drug reaching the target, thus reducing the effectiveness of the therapeutic. Therefore, a delivery system must be developed to ensure that the drug reaches the exact lesion site. Major efforts have been directed toward developing molecules with high affinity for Aβ, which can reduce the Aβ level in the brain (Brambilla et al., 2011).
It is reported that prominent microglial activation precedes tangle formation, and elimination of tau-induced microglial activation could delay the progression of neurodegenerative tauopathies (Yoshiyama et al., 2007). Adams et al. (2007) conjugated fibrin γ377–395 peptide [one fibrin-derived peptide that can inhibit microglial activity in vivo specifically to iron oxide (γ-Fe2O3) nanoparticles with diameters 21 ± 3.5 nm] in order to counteract the short half-life of the peptide. The study showed that, compared to the free peptide of the same concentration, γ-Fe2O3 nanoparticles could specifically inhibit the microglial cells in rTg4510 tau-mutant mice, supporting the fact that the nanoparticles can be used for the delivery of substances to the brain and for providing a possible therapeutic strategy to neurodegenerative tauopathies (Glat et al., 2013).
Kouyoumdjian et al. (2013) reported a biomimetic path using glyconanoparticles-SPIO to detect Aβ. The superparamagnetic nature enabled the detection of Aβ both in vitro and in mouse brains by MRI. The glyconanoparticles not only can reduce Aβ mediated cytotoxicity damnification to cells greatly but also can highlight the detection and imaging potential of Aβ (Kouyoumdjian et al., 2013).
There are studies describing the effect of magnetic nanoparticulate on Aβ fibrillation. Depending on the size and the surface area, a dual effect on the Aβ fibrillation kinetics was observed, with lower concentrations of SPIONs decreasing the rate of Aβ fibrillation, while higher concentrations enhanced the rate in the aqueous solution (Mahmoudi et al., 2013). Consistent with the previous study, Mirsadeghi et al. (2016) showed that lower concentrations of SPIONs coated by PEG-NH2 inhibited the process of Aβ fibrillation under magnetic field, whereas high concentrations accelerated the process. Furthermore, the coating charge also exerts a considerable effect on the Aβ fibrillation process. In comparison with the negatively charged or uncharged SPIONs, lower concentrations of SPIONs with positive coating charge promoted the fibrillation (Mirsadeghi et al., 2016). By applying thioflavin-T fluorescence emission, the effect of SPIONs with different electric charges on both β-amyloid and α-synuclein fibrillation process was investigated. The negatively charged nanoparticles encoded to -COOH by dextran-coating decreased the binding level of thioflavin-T particles to β-sheets (Javdani et al., 2019).
Combining nerve growth factor (NGF) and quercetin with superparamagnetic IONPs promoted neurite outgrowth and increased the complexity of the neuronal branching trees in PC12 cells, as potential therapeutics for neurodegenerative diseases (Katebi et al., 2019).
Sonawane et al. (2019) screened out one kind of protein-capped (PC) metal nanoparticles that inhibit Tau aggregation in vitro for the first time. They proved that because of the increased reactive oxygen species production and the resulting oxidative stress, the uncapped CdS nanoparticles make themselves toxic to HeLa cells and bacteria, but by capping, these CdS can obtain an entirely different property and become more biocompatible; thus, the biosynthetic PC metal nanoparticles, particularly iron oxide, will not influence the viability of neuroblastoma cells (Sonawane et al., 2019).
Yang et al. (2016) invented a reagent for IMR consisting of antibodies against α-synuclein functionalized with MNPs. By using an ultrasensitive immunoassay utilizing IMR, the α-synuclein detection dynamic range is from 0.3 fg/ml to 310 pg/ml in plasma (Yang et al., 2016). The nanoparticles can differentiate PD patients, PDD (PD dementia) patients, and healthy subjects depending on the significantly different concentration of plasma α-synuclein (Yang et al., 2016).
Studies have conjugated multimodal IONPs to Rhodamine-B (MION-Rh), and then labeled with MSCs from umbilical cord blood (UC-MSC; Sibov et al., 2014). Labeled cells were infused into the striatum of PD adult male rats, and 15 days later by T2 MRI, the cells were observed migrating along the medial forebrain bundle to the substantia nigra as hypointense spots (Sibov et al., 2014).
Gene therapy, which targets the expression of α-synuclein in neurons, is of great concern, and shRNA (short hairpin RNA) has been identified as a promising treatment of PD (Niu et al., 2017). Therefore, researchers coated magnetic Fe3O4 nanoparticles with oleic acid molecules as a nanocarrier and absorbed shRNA. They demonstrated that these superparamagnetic nanoparticles can reduce the expression of α-synuclein and thus can prevent the toxic effects on the cell and suppress apoptosis by α-synuclein (Niu et al., 2017).
Salama et al. (2017) isolated MSCs from C57BL/6 mice, incubated MSCs with micrometer-sized iron oxide (referred to as MPIOs) particles, and finally administrated them in a PD mouse model by the way of the intranasal (IN) route. In the experiment, MPIO-labeled MSCs were used as stem cell tracking stained with Prussian blue (Salama et al., 2017). The following histopathological evaluation by positive Prussian blue staining revealed the successful delivery of MSCs (Salama et al., 2017). The neurobehavioral assessment was improved following MSC administration (Salama et al., 2017).
Studies have confirmed that after receiving human ventral mesencephalic NSC (hVM1) grafts, parkinsonian animals showed an amelioration in resting tremor and cognitive performance (Kouyoumdjian et al., 2013) Based on this, Ramos-Gómez et al. (2015) believed that hVM1 cells and their derivatives represented a helpful method for cell therapies focused on neurodegenerative diseases, PD in particular. In the study, they found that MNPs of different sizes (with a diameter of 50 and 100 nm) were labeled with hVM cells nearly 100% in defect of any transfection agents (Ramos-Gómez et al., 2015). After transplanting MNP-labeled hVM cells into the striatum of the PD rat model, MNPs were distributed evenly throughout the transplant region detected by histological analysis (Ramos-Gómez et al., 2015). The researchers used MNPs to label hNSCs (human NSCs) and injected them into hemiparkinsonian rats in order to follow stem cell fate over time to verify the efficient application of stem cells after transplantation (Ramos-Gómez et al., 2015). The result shows that by the use of MRI, the MNP-labeled hNSCs grafted into hemiparkinsonian rats can be successfully visualized up to 5 months after transplantation at different time points (Ramos-Gómez et al., 2015).
A recent study has demonstrated that dextran-coated IONPs (Dex-IO NPs) can improve the therapeutic effects of human MSCs in a mouse model of PD (Chung et al., 2018). The loss of dopaminergic neurons was decreased and the migration capacity and the differentiation of human MSCs to dopaminergic neurons were enhanced (Chung et al., 2018). Therefore, Dex-IO NPs can be considered as a promising carrier for MSC therapy for PD.
Evans et al. (2014) suggested that T2-weighted MRI offered a strong biomarker potential in superoxide dismutase (SOD1) G93A transgenic ALS mouse model. They put VCAM-1 (vascular cell adhesion molecule 1, one cellular adhesion molecule that can upregulate during endothelial activation) together with MPIO (microparticles of iron oxide; Evans et al., 2014). However, they concluded that VCAM-MPIO as a biomarker in SOD1 ALS is useless (Evans et al., 2014).
The therapeutic efficacy of SkmSCs (subpopulation of human skeletal muscle–derived stem cells) with MSC-like features was evaluated after intracerebroventricular injection to the Wr mouse (Wobbler mouse, the most typical model of spontaneous motor neuron degeneration) by Canzi et al. (2012). The research team confirmed that MRI can visualize stem cells and follow their migration after transplantation in the CNS of rodents (Canzi et al., 2012). Bigini et al. (2012) traced amniotic fluid cells (hAFCs) in a chronic neurodegenerative/inflammatory environment in a similar approach; after SPION loaded hAFC administration, the signal in the ventricles of the brain became hypointense in both healthy and Wr mice. All the abovementioned indicate that SPION can be a tracer to monitor the efficacy of stem cell therapy for ALS.
For neurodegenerative diseases such as AD, PD, and ALS, the early diagnosis toward these diseases is difficult. In order to improve the symptoms by drug therapy based on the diagnosis at the early stage of the disease, MRI CAs, especially SPIO and USPIO, have already been developed in imaging and targeting drug delivery. Despite the fact that regulatory bodies have already approved some SPIO agents for many years, the clinical application of these agents has been proven a difficult journey (Wáng and Idée, 2017). For instance, by the end of 2015, only five types of SPIO had been designed and applied in clinical settings as magnetic resonance CAs (Yang et al., 2016). Of these, one is available only in a few countries; the other four have already been terminated in the follow-up study or even pulled out of the market (Yang et al., 2016). The mandatory requirements of nanoparticles should be improved, including biocompatibility, biodegradability, biodistribution, stability under physiological conditions, accurate pharmacokinetics, and minimal side effects. Designing nanoparticles to pass through the BBB is even more challenging and complex than conventional drug delivery. Major efforts should be made to improve targeted drug release and therapeutic efficacy, and to increase sensitivity and specificity of noninvasive imaging. And the olfactory pathway provides a noninvasive route for nanoparticles to enter the CNS. Even if the noninvasive imaging examination method cannot be applied to the human body temporarily, they can be used to the mouse model to explore new diagnostic and therapeutic methods for neurodegenerative diseases. It is not too far off before IONPs hold promise for the development of early diagnosis and the accomplishment of the aim of personalized therapy toward neurodegenerative diseases.
SL, CM, M-QZ, and W-NJ searched the literature and drafted the manuscript. XW and YY critically revised the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81901083), Norman Bethune Program of Jilin University (Grant No. 2015335), the Key National Research Projects on Prevention and Control of Major Chronic Non-communicable Disease (2018YFC1312300), as well as from Fund of Science and Technology Development Project of Jilin Province (20180414041GH) and Jilin Health Technology Innovation Project (2019J003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Editage for English language editing.
Aβ, amyloid β; AD, Alzheimer’s disease; ALS, amyotrophic lateral sclerosis; BBB, blood–brain barrier; CA, contrast agent; CNS, central nervous system; CSF, cerebrospinal fluid; IONP, iron oxide nanoparticle; MNP, magnetic nanoparticle; MRI, magnetic resonance imaging; PET, positron emission tomography; PD, Parkinson’s disease; USPIO, ultrasmall superparamagnetic iron oxide; SPIO, superparamagnetic iron oxide; SPION, superparamagnetic iron oxide nanoparticle.
Adams, R. A., Bauer, J., Flick, M. J., Sikorski, S. L., Nuriel, T., Lassmann, H., et al. (2007). The fibrin-derived gamma377–395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J. Exp. Med. 204, 571–582. doi: 10.1084/jem.20061931
Akilo, O. D., Choonara, Y. E., Strydom, A. M., du Toit, L. C., Kumar, P., Modi, G., et al. (2016). AN in vitro evaluation of a carmustine-loaded Nano-co-Plex for potential magnetic-targeted intranasal delivery to the brain. Int. J. Pharm. 500, 196–209. doi: 10.1016/j.ijpharm.2016.01.043
André, S., Ansciaux, E., Saidi, E., Larbanoix, L., Stanicki, D., Nonclercq, D., et al. (2017). Validation by magnetic resonance imaging of the diagnostic potential of a heptapeptide-functionalized imaging probe targeted to amyloid-beta and able to cross the blood-brain barrier. J. Alzheimers Dis. 60, 1547–1565. doi: 10.3233/jad-170563
Ansciaux, E., Burtea, C., Laurent, S., Crombez, D., Nonclercq, D., Vander Elst, L., et al. (2015). In vitro and in vivo characterization of several functionalized ultrasmall particles of iron oxide, vectorized against amyloid plaques and potentially able to cross the blood-brain barrier: toward earlier diagnosis of Alzheimer’s disease by molecular imaging. Contrast Media Mol. Imaging 10, 211–224. doi: 10.1002/cmmi.1626
Anwar, M., Asfer, M., Prajapati, A. P., Mohapatra, S., Akhter, S., Ali, A., et al. (2014). Synthesis and in vitro localization study of curcumin-loaded SPIONs in a micro capillary for simulating a targeted drug delivery system. Int. J. Pharm. 468, 158–164. doi: 10.1016/j.ijpharm.2014.04.038
Arami, H., Khandhar, A., Liggitt, D., and Krishnan, K. M. (2015). In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 44, 8576–8607. doi: 10.1039/c5cs00541h
Balestrino, R., and Schapira, A. H. V. (2019). Parkinson disease. Eur. J. Neurol. doi: 10.1111/ene.14108 [Epub ahead of print].
Baloh, R. H., Glass, J. D., and Svendsen, C. N. (2018). Stem cell transplantation for amyotrophic lateral sclerosis. Curr. Opin. Neurol. 31, 655–661. doi: 10.1097/WCO.0000000000000598
Barage, S. H., and Sonawane, K. D. (2015). Amyloid cascade hypothesis: pathogenesis and therapeutic strategies in Alzheimer’s disease. Neuropeptides 52, 1–18. doi: 10.1016/j.npep.2015.06.008
Bigini, P., Diana, V., Barbera, S., Fumagalli, E., Micotti, E., Sitia, L., et al. (2012). Longitudinal tracking of human fetal cells labeled with super paramagnetic iron oxide nanoparticles in the brain of mice with motor neuron disease. PLoS One 7:e32326. doi: 10.1371/journal.pone.0032326
Brambilla, D., Le Droumaguet, B., Nicolas, J., Hashemi, S. H., Wu, L. P., Moghimi, S. M., et al. (2011). Nanotechnologies for Alzheimer’s disease: diagnosis, therapy, and safety issues. Nanomedicine 7, 521–540. doi: 10.1016/j.nano.2011.03.008
Busquets, M. A., Sabate, R., and Estelrich, J. (2014). Potential applications of magnetic particles to detect and treat Alzheimer’s disease. Nanoscale Res. Lett. 9:538. doi: 10.1186/1556-276x-9-538
Canzi, L., Castellaneta, V., Navone, S., Nava, S., Dossena, M., Zucca, I., et al. (2012). Human skeletal muscle stem cell antiinflammatory activity ameliorates clinical outcome in amyotrophic lateral sclerosis models. Mol. Med. 18, 401–411. doi: 10.2119/molmed.2011.00123
Chamberlain, R., Reyes, D., Curran, G. L., Marjanska, M., Wengenack, T. M., Poduslo, J. F., et al. (2009). Comparison of amyloid plaque contrast generated by T2-weighted, T2*-weighted, and susceptibility-weighted imaging methods in transgenic mouse models of Alzheimer’s disease. Magn. Reson. Med. 61, 1158–1164. doi: 10.1002/mrm.21951
Chamberlain, R., Wengenack, T. M., Poduslo, J. F., Garwood, M., and Jack, C. R. Jr. (2011). Magnetic resonance imaging of amyloid plaques in transgenic mouse models of Alzheimer’s disease. Curr. Med. Imaging Rev. 7, 3–7. doi: 10.2174/157340511794653522
Chandra, A., Valkimadi, P. E., Pagano, G., Cousins, O., Dervenoulas, G., and Politis, M. (2019). Applications of amyloid, tau, and neuroinflammation PET imaging to Alzheimer’s disease and mild cognitive impairment. Hum. Brain Mapp. 40, 5424–5442. doi: 10.1002/hbm.24782
Chen, X. Q., and Mobley, W. C. (2019). Alzheimer disease pathogenesis: insights from molecular and cellular biology studies of oligomeric Aβ and tau species. Front. Neurosci. 13:659. doi: 10.3389/fnins.2019.00659
Cheng, K. K., Chan, P. S., Fan, S., Kwan, S. M., Yeung, K. L., Wang, Y. X., et al. (2015). Curcumin-conjugated magnetic nanoparticles for detecting amyloid plaques in Alzheimer’s disease mice using magnetic resonance imaging (MRI). Biomaterials 44, 155–172. doi: 10.1016/j.biomaterials.2014.12.005
Chung, T. H., Hsu, S. C., Wu, S. H., Hsiao, J. K., Lin, C. P., Yao, M., et al. (2018). Dextran-coated iron oxide nanoparticle-improved therapeutic effects of human mesenchymal stem cells in a mouse model of Parkinson’s disease. Nanoscale 10, 2998–3007. doi: 10.1039/c7nr06976f
Corbett, A., Smith, J., and Ballard, C. (2012). New and emerging treatments for Alzheimer’s disease. Expert Rev. Neurother. 12, 535–543. doi: 10.1586/ern.12.43
De, M., Chou, S. S., Joshi, H. M., and Dravid, V. P. (2011). Hybrid magnetic nanostructures (MNS) for magnetic resonance imaging applications. Adv. Drug Deliv. Rev. 63, 1282–1299. doi: 10.1016/j.addr.2011.07.001
Ding, H., Sagar, V., Agudelo, M., Pilakka-Kanthikeel, S., Atluri, V. S., Raymond, A., et al. (2014). Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology 25:055101. doi: 10.1088/0957-4484/25/5/055101
Dulińska-Litewka, J., Łazarczyk, A., Hałubiec, P., Szafranski, O., Karnas, K., and Karewicz, A. (2019). Superparamagnetic iron oxide nanoparticles-current and prospective medical applications. Materials (Basel) 12:E617. doi: 10.3390/ma12040617
Evans, M. C., Serres, S., Khrapitchev, A. A., Stolp, H. B., Anthony, D. C., Talbot, K., et al. (2014). T(2)-weighted MRI detects presymptomatic pathology in the SOD1 mouse model of ALS. J. Cereb. Blood Flow Metab. 34, 785–793. doi: 10.1038/jcbfm.2014.19
Fernández, T., Martínez-Serrano, A., Cussó, L., Desco, M., and Ramos-Gómez, M. (2018). Functionalization and characterization of magnetic nanoparticles for the detection of ferritin accumulation in Alzheimer’s disease. ACS Chem. Neurosci. 9, 912–924. doi: 10.1021/acschemneuro.7b00260
Frisoni, G. B., Boccardi, M., Barkhof, F., Blennow, K., Cappa, S., Chiotis, K., et al. (2017). Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. Lancet Neurol. 16, 661–676. doi: 10.1016/S1474-4422(17)30159-X
Gagliardi, D., Meneri, M., Saccomanno, D., Bresolin, N., Comi, G. P., and Corti, S. (2019). Diagnostic and prognostic role of blood and cerebrospinal fluid and blood neurofilaments in amyotrophic lateral sclerosis: a review of the literature. Int. J. Mol. Sci. 20:E4152. doi: 10.3390/ijms20174152
Giagkou, N., and Stamelou, M. (2018). Therapeutic management of the overlapping syndromes of atypical Parkinsonism. CNS Drugs 32, 827–837. doi: 10.1007/s40263-018-0551-3
Glat, M., Skaat, H., Menkes-Caspi, N., Margel, S., and Stern, E. A. (2013). Age-dependent effects of microglial inhibition in vivo on Alzheimer’s disease neuropathology using bioactive-conjugated iron oxide nanoparticles. J. Nanobiotechnology 11:32. doi: 10.1186/1477-3155-11-32
Gupta, J., Fatima, M. T., Islam, Z., Khan, R. H., Uversky, V. N., and Salahuddin, P. (2019). Nanoparticle formulations in the diagnosis and therapy of Alzheimer’s disease. Int. J. Biol. Macromol. 130, 515–526. doi: 10.1016/j.ijbiomac.2019.02.156
Ikenaka, K., Suzuki, M., Mochizuki, H., and Nagai, Y. (2019). Lipids as trans-acting effectors for alpha-synuclein in the pathogenesis of Parkinson’s disease. Front. Neurosci. 13:693. doi: 10.3389/fnins.2019.00693
Javdani, N., Rahpeyma, S. S., Ghasemi, Y., and Raheb, J. (2019). Effect of superparamagnetic nanoparticles coated with various electric charges on α-synuclein and β-amyloid proteins fibrillation process. Int. J. Nanomedicine 14, 799–808. doi: 10.2147/ijn.s190354
Jiang, L. F., Chen, B. C., Chen, B., Li, X. J., Liao, H. L., Huang, H. M., et al. (2017). Detection of Aβ oligomers based on magnetic-field-assisted separation of aptamer-functionalized Fe3O4 magnetic nanoparticles and BaYF5:Yb,Er nanoparticles as upconversion fluorescence labels. Talanta 170, 350–357. doi: 10.1016/j.talanta.2017.04.021
Jirak, D., Galisova, A., Kolouchova, K., Babuka, D., and Hruby, M. (2019). Fluorine polymer probes for magnetic resonance imaging: quo vadis? MAGMA 32, 173–185. doi: 10.1007/s10334-018-0724-6
Katebi, S., Esmaeili, A., Ghaedi, K., and Zarrabi, A. (2019). Superparamagnetic iron oxide nanoparticles combined with NGF and quercetin promote neuronal branching morphogenesis of PC12 cells. Int. J. Nanomedicine 14, 2157–2169. doi: 10.2147/ijn.s191878
Khan, A. R., Liu, M., Khan, M. W., and Zhai, G. (2017). Progress in brain targeting drug delivery system by nasal route. J. Control Release 268, 364–389. doi: 10.1016/j.jconrel.2017.09.001
Klohs, J., Baltes, C., Princz-Kranz, F., Ratering, D., Nitsch, R. M., Knuesel, I., et al. (2012). Contrast-enhanced magnetic resonance microangiography reveals remodeling of the cerebral microvasculature in transgenic ArcAβ mice. J. Neurosci. 32, 1705–1713. doi: 10.1523/jneurosci.5626-11.2012
Kong, S. D., Lee, J., Ramachandran, S., Eliceiri, B. P., Shubayev, V. I., Lal, R., et al. (2012). Magnetic targeting of nanoparticles across the intact blood-brain barrier. J. Control Release 164, 49–57. doi: 10.1016/j.jconrel.2012.09.021
Kouyoumdjian, H., Zhu, D. C., El-Dakdouki, M. H., Lorenz, K., Chen, J., Li, W., et al. (2013). Glyconanoparticle aided detection of β-amyloid by magnetic resonance imaging and attenuation of β-amyloid induced cytotoxicity. ACS Chem. Neurosci. 4, 575–584. doi: 10.1021/cn3002015
Li, Y., Lim, E., Field, T., Wu, H., and Mao, H. (2019). Improving sensitivity and specificity of amyloid-β peptide and tau protein detection with anti-biofouling magnetic nanoparticles for liquid biopsy of Alzheimer’s disease. ACS Biomater. Sci. Eng. 5, 3595–3605. doi: 10.1021/acsbiomaterials.9b00086
Ling, D., and Hyeon, T. (2013). Chemical design of biocompatible iron oxide nanoparticles for medical applications. Small 9, 1450–1466. doi: 10.1002/smll.201202111
Mahmoudi, M., Quinlan-Pluck, F., Monopoli, M. P., Sheibani, S., Vali, H., Dawson, K. A., et al. (2013). Influence of the physiochemical properties of superparamagnetic iron oxide nanoparticles on amyloid β protein fibrillation in solution. ACS Chem. Neurosci. 4, 475–485. doi: 10.1021/cn300196n
Marasini, R., Thanh Nguyen, T. D., and Aryal, S. (2020). Integration of gadolinium in nanostructure for contrast enhanced-magnetic resonance imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 12:e1580. doi: 10.1002/wnan.1580
Mirsadeghi, S., Shanehsazzadeh, S., Atyabi, F., and Dinarvand, R. (2016). Effect of PEGylated superparamagnetic iron oxide nanoparticles (SPIONs) under magnetic field on amyloid β fibrillation process. Mater. Sci. Eng. C Mater. Biol. Appl. 59, 390–397. doi: 10.1016/j.msec.2015.10.026
Niu, S., Zhang, L. K., Zhang, L., Zhuang, S., Zhan, X., Chen, W. Y., et al. (2017). Inhibition by multifunctional magnetic nanoparticles loaded with α-synuclein RNAi plasmid in a Parkinson’s disease model. Theranostics 7, 344–356. doi: 10.7150/thno.16562
Olsson, B., Lautner, R., Andreasson, U., Ohrfelt, A., Portelius, E., Bjerke, M., et al. (2016). CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 15, 673–684. doi: 10.1016/S1474-4422(16)00070-3
Petiet, A., Santin, M., Bertrand, A., Wiggins, C. J., Petit, F., Houitte, D., et al. (2012). Gadolinium-staining reveals amyloid plaques in the brain of Alzheimer’s transgenic mice. Neurobiol. Aging 33, 1533–1544. doi: 10.1016/j.neurobiolaging.2011.03.009
Pillai, J. A., Appleby, B. S., Safar, J., and Leverenz, J. B. (2018). Rapidly progressive Alzheimer’s disease in two distinct autopsy cohorts. J. Alzheimers Dis. 64, 973–980. doi: 10.3233/jad-180155
Poduslo, J. F., Wengenack, T. M., Curran, G. L., Wisniewski, T., Sigurdsson, E. M., Macura, S. I., et al. (2002). Molecular targeting of Alzheimer’s amyloid plaques for contrast-enhanced magnetic resonance imaging. Neurobiol. Dis. 11, 315–329. doi: 10.1006/nbdi.2002.0550
Ramos-Gómez, M., Seiz, E. G., and Martinez-Serrano, A. (2015). Optimization of the magnetic labeling of human neural stem cells and MRI visualization in the hemiparkinsonian rat brain. J. Nanobiotechnology 13:20. doi: 10.1186/s12951-015-0078-4
Rashid, H. U., Martines, M. A. U., Jorge, J., de Moraes, P. M., Umar, M. N., Khan, K., et al. (2016). Cyclen-based Gd3+ complexes as MRI contrast agents: relaxivity enhancement and ligand design. Bioorg. Med. Chem. 24, 5663–5684. doi: 10.1016/j.bmc.2016.09.069
Rotman, M., Snoeks, T. J., and van der Weerd, L. (2011). Pre-clinical optical imaging and MRI for drug development in Alzheimer’s disease. Drug Discov. Today Technol. 8, e117–e125. doi: 10.1016/j.ddtec.2011.11.005
Sachdeva, A. K., Misra, S., Pal Kaur, I., and Chopra, K. (2015). Neuroprotective potential of sesamol and its loaded solid lipid nanoparticles in ICV-STZ-induced cognitive deficits: behavioral and biochemical evidence. Eur. J. Pharmacol. 747, 132–140. doi: 10.1016/j.ejphar.2014.11.014
Salama, M., Sobh, M., Emam, M., Abdalla, A., Sabry, D., El-Gamal, M., et al. (2017). Effect of intranasal stem cell administration on the nigrostriatal system in a mouse model of Parkinson’s disease. Exp. Ther. Med. 13, 976–982. doi: 10.3892/etm.2017.4073
Sibov, T. T., Pavon, L. F., Miyaki, L. A., Mamani, J. B., Nucci, L. P., Alvarim, L. T., et al. (2014). Umbilical cord mesenchymal stem cells labeled with multimodal iron oxide nanoparticles with fluorescent and magnetic properties: application for in vivo cell tracking. Int. J. Nanomedicine 9, 337–350. doi: 10.2147/ijn.s53299
Sillerud, L. O., Solberg, N. O., Chamberlain, R., Orlando, R. A., Heidrich, J. E., Brown, D. C., et al. (2013). SPION-enhanced magnetic resonance imaging of Alzheimer’s disease plaques in AβPP/PS-1 transgenic mouse brain. J. Alzheimers Dis. 34, 349–365. doi: 10.3233/jad-121171
Skaat, H., Corem-Slakmon, E., Grinberg, I., Last, D., Goez, D., Mardor, Y., et al. (2013). Antibody-conjugated, dual-modal, near-infrared fluorescent iron oxide nanoparticles for antiamyloidgenic activity and specific detection of amyloid-beta fibrils. Int. J. Nanomedicine 8, 4063–4076. doi: 10.2147/ijn.s52833
Soto, C., and Pritzkow, S. (2018). Protein misfolding, aggregation and conformational strains in neurodegenerative diseases. Nat. Neurosci. 21, 1332–1340. doi: 10.1038/s41593-018-0235-9
Sonawane, S. K., Ahmad, A., and Chinnathambi, S. (2019). Protein-capped metal nanoparticles inhibit tau aggregation in Alzheimer’s disease. ACS Omega 4, 12833–12840. doi: 10.1021/acsomega.9b01411
Staff, N. P., Jones, D. T., and Singer, W. (2019). Mesenchymal stromal cell therapies for neurodegenerative diseases. Mayo Clin. Proc. 94, 892–905. doi: 10.1016/j.mayocp.2019.01.001
Sugaya, K., and Vaidya, M. (2018). Stem cell therapies for neurodegenerative diseases. Adv. Exp. Med. Biol. 1056, 61–84. doi: 10.1007/978-3-319-74470-4_5
Sun, Z., Worden, M., Thliveris, J. A., Hombach-Klonisch, S., Klonisch, T., van Lierop, J., et al. (2016). Biodistribution of negatively charged iron oxide nanoparticles (IONPs) in mice and enhanced brain delivery using lysophosphatidic acid (LPA). Nanomedicine 12, 1775–1784. doi: 10.1016/j.nano.2016.04.008
Tafoya, M. A., Madi, S., and Sillerud, L. O. (2017). Superparamagnetic nanoparticle-enhanced MRI of Alzheimer’s disease plaques and activated microglia in 3X transgenic mouse brains: contrast optimization. J. Magn. Reson. Imaging 46, 574–588. doi: 10.1002/jmri.25563
Teleanu, D. M., Negut, I., Grumezescu, V., Grumezescu, A. M., and Teleanu, R. I. (2019). Nanomaterials for drug delivery to the central nervous system. Nanomaterials (Basel) 9:E371. doi: 10.3390/nano9030371
Thomsen, L. B., Thomsen, M. S., and Moos, T. (2015). Targeted drug delivery to the brain using magnetic nanoparticles. Ther Deliv 6, 1145–1155. doi: 10.4155/tde.15.56
Tiwari, S., Atluri, V., Kaushik, A., Yndart, A., and Nair, M. (2019). Alzheimer’s disease: pathogenesis, diagnostics and therapeutics. Int. J. Nanomedicine 14, 5541–5554. doi: 10.2147/IJN.S200490
Wadghiri, Y. Z., Li, J., Wang, J., Hoang, D. M., Sun, Y., Xu, H., et al. (2013). Detection of amyloid plaques targeted by bifunctional USPIO in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. PLoS One 8:e57097. doi: 10.1371/journal.pone.0057097
Wáng, Y. X., and Idée, J. M. (2017). A comprehensive literatures update of clinical researches of superparamagnetic resonance iron oxide nanoparticles for magnetic resonance imaging. Quant. Imaging Med. Surg. 7, 88–122. doi: 10.21037/qims.2017.02.09
Wengenack, T. M., Reyes, D. A., Curran, G. L., Borowski, B. J., Lin, J., Preboske, G. M., et al. (2011). Regional differences in MRI detection of amyloid plaques in AD transgenic mouse brain. NeuroImage 54, 113–122. doi: 10.1016/j.neuroimage.2010.08.033
Yang, C. C., Yang, S. Y., Chieh, J. J., Horng, H. E., Hong, C. Y., Yang, H. C., et al. (2011a). Biofunctionalized magnetic nanoparticles for specifically detecting biomarkers of Alzheimer’s disease in vitro. ACS Chem. Neurosci. 2, 500–505. doi: 10.1021/cn200028j
Yang, J., Wadghiri, Y. Z., Hoang, D. M., Tsui, W., Sun, Y., Chung, E., et al. (2011b). Detection of amyloid plaques targeted by USPIO-Aβ1–42 in Alzheimer’s disease transgenic mice using magnetic resonance microimaging. NeuroImage 55, 1600–1609. doi: 10.1016/j.neuroimage.2011.01.023.
Yang, S. Y., Chiu, M. J., Lin, C. H., Horng, H. E., Yang, C. C., Chieh, J. J., et al. (2016). Development of an ultra-high sensitive immunoassay with plasma biomarker for differentiating Parkinson disease dementia from Parkinson disease using antibody functionalized magnetic nanoparticles. J. Nanobiotechnology 14:41. doi: 10.1186/s12951-016-0198-5
Yarjanli, Z., Ghaedi, K., Esmaeili, A., Rahgozar, S., and Zarrabi, A. (2017). Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress and protein aggregation. BMC Neurosci. 18:51. doi: 10.1186/s12868-017-0369-9
Yoshiyama, Y., Higuchi, M., Zhang, B., Huang, S. M., Iwata, N., Saido, T. C., et al. (2007). Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron 53, 337–351. doi: 10.1016/j.neuron.2007.01.010
Zhang, D., Fa, H. B., Zhou, J. T., Li, S., Diao, X. W., and Yin, W. (2015). The detection of beta-amyloid plaques in an Alzheimer’s disease rat model with DDNP-SPIO. Clin. Radiol. 70, 74–80. doi: 10.1016/j.crad.2014.09.019
Zhou, J., Fa, H., Yin, W., Zhang, J., Hou, C., Huo, D., et al. (2014). Synthesis of superparamagnetic iron oxide nanoparticles coated with a DDNP-carboxyl derivative for in vitro magnetic resonance imaging of Alzheimer’s disease. Mater. Sci. Eng. C Mater. Biol. Appl. 37, 348–355. doi: 10.1016/j.msec.2014.01.005
Keywords: iron oxide nanoparticle, magnetic resonance imaging, Alzheimer’s disease, amyloid-β, neurodegeneration
Citation: Luo S, Ma C, Zhu M-Q, Ju W-N, Yang Y and Wang X (2020) Application of Iron Oxide Nanoparticles in the Diagnosis and Treatment of Neurodegenerative Diseases With Emphasis on Alzheimer’s Disease. Front. Cell. Neurosci. 14:21. doi: 10.3389/fncel.2020.00021
Received: 31 August 2019; Accepted: 24 January 2020;
Published: 28 February 2020.
Edited by:
Rocío Martínez De Pablos, University of Seville, SpainReviewed by:
Rachid El Fatimy, Harvard Medical School, United StatesCopyright © 2020 Luo, Ma, Zhu, Ju, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu Wang, c3VubnkudXVAMTYzLmNvbQ==; Yu Yang, bTE1NzU0MzA2MDkzQDE2My5jb20=
† These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.