
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cell. Neurosci., 09 October 2019
Sec. Non-Neuronal Cells
Volume 13 - 2019 | https://doi.org/10.3389/fncel.2019.00418
This article is part of the Research TopicDelivering Therapeutics to the Inner EarView all 20 articles
 Caroline R. Anderson1,2*†
Caroline R. Anderson1,2*† Carol Xie1,2†
Carol Xie1,2† Matthew P. Su1,2
Matthew P. Su1,2 Maria Garcia1,2
Maria Garcia1,2 Helen Blackshaw1,2
Helen Blackshaw1,2 Anne G. M. Schilder1,2*
Anne G. M. Schilder1,2*Background: Advances in the understanding of the genetic and molecular etiologies of inner ear disorders have enabled the identification of therapeutic targets and innovative delivery approaches to the inner ear. As this field grows, the need for knowledge about effective delivery of therapeutics to the inner ear has become a priority. This review maps all clinical and pre-clinical research published in English in the field to date, to guide both researchers and clinicians about local drug delivery methods in the context of novel therapeutics.
Methods: A systematic search was conducted using customized strategies in Cochrane, pubmed and EMBASE databases from inception to 30/09/2018. Two researchers undertook study selection and data extraction independently.
Results: Our search returned 12,200 articles, of which 837 articles met the inclusion criteria. 679 were original research and 158 were reviews. There has been a steady increase in the numbers of publications related to inner ear therapeutics delivery over the last three decades, with a sharp rise over the last 2 years. The intra-tympanic route accounts for over 70% of published articles. Less than one third of published research directly assesses delivery efficacy, with most papers using clinical efficacy as a surrogate marker.
Conclusion: Research into local therapeutic delivery to the inner ear has undergone a recent surge, improving our understanding of how novel therapeutics can be delivered. Direct assessment of delivery efficacy is challenging, especially in humans, and progress in this area is key to understanding how to make decisions about delivery of novel hearing therapeutics.
Recent advances in the understanding of the varied genetic and molecular etiologies of inner ear dysfunction have enabled the identification of multiple potential therapeutic targets (Müller and Barr-Gillespie, 2015; Mittal et al., 2017). This has accelerated the development of a range of novel therapeutics, from small molecule drugs to gene and cell therapies, several of which are starting to enter the clinical trial domain (Schilder et al., 2018)1,2. Whilst significant progress has been achieved with regards to therapeutic identification and development, the most effective mechanisms of therapeutic delivery to the inner ear have yet to be determined. The importance of delivery cannot be understated; the success of any novel therapeutic depends on selection of the most suitable method for the pharmacokinetic profile of the individual agent, and the balance of risks associated with delivery against the potential benefit of the treatment (Salt and Plontke, 2009; Plontke and Salt, 2018).
The inner ear poses a unique pharmacokinetic and pharmacodynamic challenge due to its anatomical location, epithelial barriers and the relatively unstirred nature of the perilymph (Salt, 2002). Local delivery is an attractive option as it overcomes concerns regarding toxicity or side effects associated with systemic administration (Plontke et al., 2014; Salt and Plontke, 2018), allowing higher concentrations to reach the inner ear (Liu et al., 2018). It can be broadly divided into intra-tympanic or intra-cochlear routes, each with many options for delivery method and therapeutic formulation (Liu et al., 2018; Peppi et al., 2018; Salt and Plontke, 2018). Middle ear approaches, such as transtympanic injection, rely on simple diffusion through the epithelial barriers of the round and/or oval window (Salt and Plontke, 2018). This can lead to the formation of concentration gradients, with variable concentrations reaching more apical regions of cochlea, and potentially insufficient levels of the therapeutic reaching the basal regions (Salt et al., 2007; Liu et al., 2014; Li et al., 2018; Salt and Plontke, 2018). Delivery using sustained release formulations, magnetically targeted delivery, and nanoparticles (Pyykkö and Jing Zou, 2013; Shapiro et al., 2014; Pyykkö et al., 2016) aims to overcome these problems, but they remain a significant concern. Intra-cochlear therapeutic delivery offers the best control of delivery at the cost of the highest risk to hearing, although the problem of base-apex gradient formation remains (Hahn et al., 2012; Salt et al., 2017). Cochlear implant (CI) associated delivery presents a unique opportunity to develop this route for a subset of patients (Hochmair et al., 2006; Budenz et al., 2012; Roemer et al., 2016; Plontke et al., 2017).
At present there is a limited understanding of how delivery method, therapeutic agent and formulation, and disease process interact. This makes choosing a delivery method for a given therapeutic a major challenge faced by all involved in the development, production and administration of novel therapeutics, including discovery scientists, clinicians, industry, regulators, and patients. There is little to guide these stakeholders as to the best delivery method for a given therapeutic in a given patient group. A systematic review of the available literature is therefore necessary in order to collate and make accessible the currently available information.
The aims of this paper are to map all research undertaken so far in the field of local delivery of therapeutics to the inner ear, and to signpost to information to support decisions about delivery methods in the context of novel therapeutics.
• To identify all relevant literature on local delivery of therapeutics to the inner ear.
• To understand the ways in which delivery methods are tested, and delivery efficacy assessed.
• To understand how the field of local therapeutic delivery to the inner ear has evolved, by exploring trends in publication type, experimental design, therapeutic classes and delivery methods.
• To identify which local delivery routes and methods have been tested in the pre-clinical and clinical research settings.
• To create searchable tables of the current literature on local delivery of therapeutics to the inner ear to signpost interested parties to the work that is most relevant to them.
A systematic review of the published scientific literature.
The review protocol was registered with PROSPERO and is available from http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018105903.
The search strategy was designed using key words for inner ear therapeutic delivery and customized for each database (available in Supplementary Material Section 1) with assistance provided by the University College London Ear Institute & Action on Hearing Loss Libraries. No restriction was placed on study design. The search was restricted to English language articles.
Pubmed, Embase and the Cochrane library were searched from inception to May 30th, 2017. The search was updated on September 30th 2018 and the results combined with the initial search.
Titles and abstracts were screened for inclusion independently by two review authors, with discrepancies resolved by full text review and discussion within the study team.
All studies and reviews of local delivery of therapeutics to the inner ear in human or animal models, where the architectural structure of the inner ear was maintained, were included. Studies that included only systemic therapeutic administration, data on in vitro experiments, animal explants, mechanical models and models utilizing computer-generated data were excluded. Inclusion and exclusion criteria are summarized in Table 1.
Two authors carried out full text review and data extraction independently, with discrepancies resolved by discussion within the study team. During full text review, papers were identified as original research or review articles. Data extracted from review articles was limited to the type of review; narrative, systematic, or systematic with meta-analysis. Data extracted from original research articles was year of publication, experimental model (animal or human, living or temporal bone), method of assessment of efficacy of delivery, delivery method(s), therapeutic agent, therapeutic formulation, and underlying disorder targeted or treated.
Extracted data was grouped as shown in Table 2.
Categorization of the method of assessment of delivery efficacy was designed to aid understanding of how research is designed. Studies directly investigating the efficacy of delivery (e.g., by concentration in perilymph, or presence or absence of a delivered substance in the target tissue) were classified as “direct assessment of delivery efficacy via pharmacokinetics.” Studies investigating the functional, toxic or clinical effects of a therapeutic agent delivered to the inner ear were classified as “indirect assessment of delivery efficacy via functional effects” (as an indirect marker of delivery method efficacy). Studies focusing on the practicalities and/or adverse effects of a proposed delivery method or therapeutic formulation, without measuring the efficacy of delivery, were classified as “assessing feasibility.”
Delivery methods were categorized by route, approach, and method, as shown in Table 2, and in schematic form in Figure 1.
Formulation was defined as the combination of therapeutic and any other substance that affects the pharmacokinetic profile of that therapeutic (Kulkarni and Shaw, 2016). They were categorized as solutions and suspensions, sustained release formulations, nano-scale formulations and viral vectors (Fukui and Raphael, 2013; Kulkarni and Shaw, 2016; Pyykkö et al., 2016; Kim, 2017; Liu et al., 2018). These categories are defined with examples in Table 3. Therapeutics were categorized as clinically used small molecules, experimental small molecules, biopharmaceuticals, other, multiple, and none, as defined in Table 4 (Nakagawa and Ito, 2005; Budenz et al., 2012; Fukui and Raphael, 2013; Liu, 2014)3.
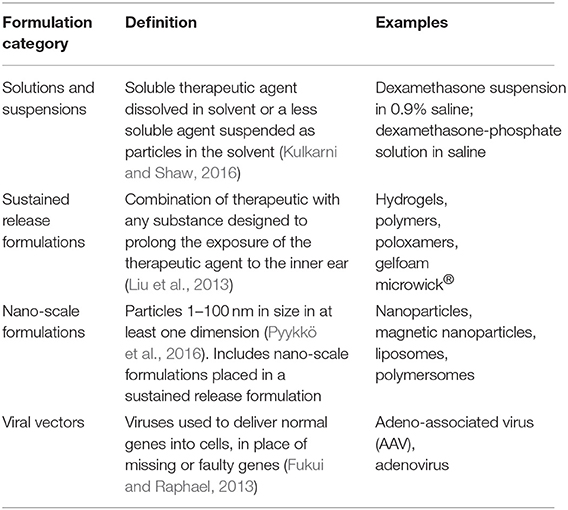
Table 3. Categorization of formulations used in therapeutic delivery to the inner ear with definitions and examples.
This categorization was designed to allow combinations of delivery methods, therapeutic agents and formulations to be considered together. For example, a study evaluating transtympanic injection of a liquid form drug and a study evaluating transtympanic injection of a gel containing nanoparticles containing the same drug would both be classed broadly as intra-tympanic injections, but would be differentiated in the formulation category to allow for the fact that this may alter delivery efficacy.
Data was managed in Microsoft Excel and analyzed in R. The annual number of publications in the field was counted as a whole and for each subcategory. Total numbers of publications across each subcategory were counted and displayed as both a total number and a list of references for those publications.
Combining the searches from May 30th, 2017 and September 30th 2018 yielded a total of 12,201 records. Removing duplicates left 6,468 unique entries. Title and abstract screening excluded 5,600 articles, with a further 31 excluded during full text screening, leaving a total of 837 papers included in this review (Figure 2).
Of the 837 papers, 679 were original research papers and 158 were reviews. Review papers were primarily narrative reviews (131/158, 82.9%). Systematic reviews accounted for 18 papers, and meta-analysis for 9 papers (Table 5). Of the original research papers, 315 (46.4%) studied humans and 364 (53.6%) studied animals.
Eight studies (1.2%) directly assessed delivery efficacy via pharmacokinetics to the live human inner ear, with a further three directly assessing delivery efficacy in human temporal bones. Animal experimental models were used in 177 (26.0%) papers to directly assess delivery efficacy via pharmacokinetics. The majority of papers used functional effects as an indirect marker of delivery efficacy; 296 (43.6%) studies in humans and 158 (23.2%) in animals. Feasibility of delivery methods, without assessment of delivery efficacy, was assessed in 8 (1.2%) human studies and 29 (4.3%) animal studies.
The most common disease studied was Meniere's disease (or endolymphatic hydrops) with 164 (24.2%) original research papers looking at local therapeutic delivery in this context. Next was idiopathic sudden sensorineural hearing loss (ISSNHL) in 98 (14.4%) studies, followed by ototoxicity with 87 (12.8%) studies.
Four hundred and eighty two (70.9%) original studies investigated intra-tympanic delivery methods, 175 (25.7%) intra-cochlear delivery methods, 19 (2.8%) a combination of the two, and 3 (0.4%) other delivery methods.
Solution and suspension formulations were used in 472 (69.5%) papers, sustained release formulations for 99 (14.6%), nanoscale in 41 (6.0%), and viral vectors in 50 (7.4%). Seventeen papers studied other or multiple formulations.
Clinically used small molecules were studied in 340 (50.1%) publications, experimental small molecules in 106 (15.6%), and biopharmaceuticals in 143 (21.1%). Fifty papers studied combinations or empty formulations.
The annual number of publications in the field of local therapeutic delivery to the inner ear has increased steadily over the last three decades, with a sharp increase over the last 5 years (Figure 3A). Of the 679 original research articles 226 have been published in the last 5 years. Remarkably, there have been 56 reviews published in the last 5 years.
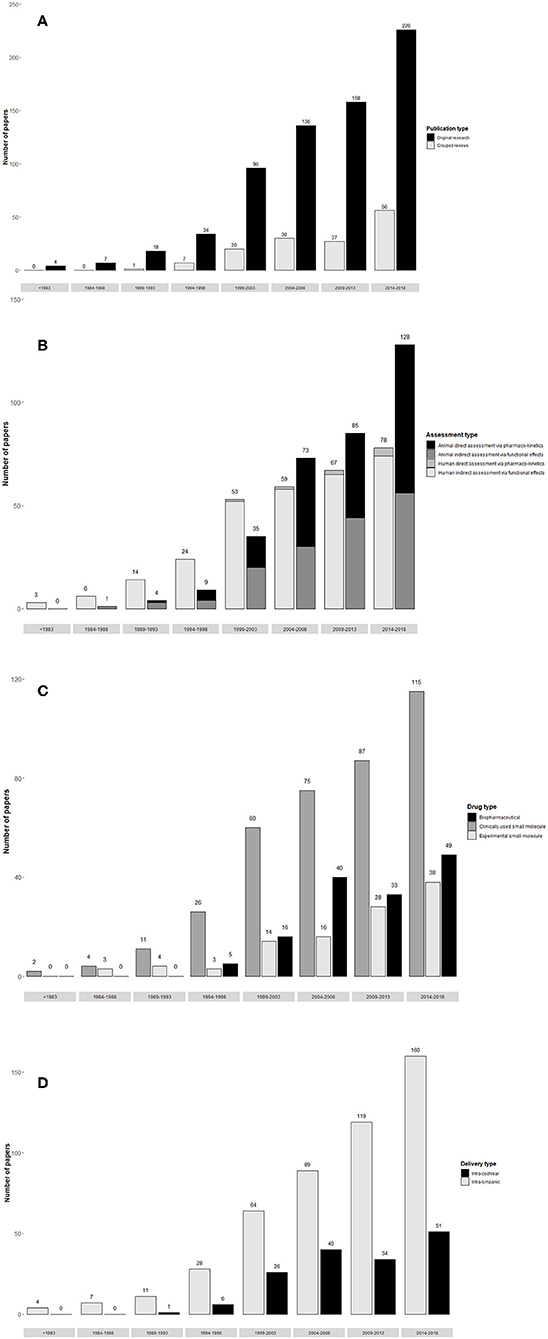
Figure 3. Trends in publication of studies on local delivery to the inner ear. (A) Publications per 5 years by study type (original research or review). (B) Original research publications of per 5 years by method of assessment of delivery efficacy (direct assessment via pharmacokinetics or indirect assessment via functional effects, excluding feasibility studies where delivery efficacy is not assessed). (C) Original research publications per 5 years by intratympanic and intracochlea delivery routes in both humans and animals (excluding “other” delivery routes). (D) Original research publications per 5 years by therapeutic class (clinically used small molecule, experimental small molecule, or biopharmaceutical) in both humans and animals. [n.b. (A–D) runs from top to bottom of figure panel].
Trends in experimental design are seen in Figure 3B. There has been a sharp increase in the number of animal studies published, while human studies have increased more gradually. The early human work indirectly assessing delivery via functional effects relates almost exclusively to transtympanic injections of corticosteroids and aminoglycosides. Direct assessment of delivery methods via pharmacokinetics accounts for around half of animal studies year on year.
Trends in delivery routes are seen in Figure 3C. Transtympanic delivery methods outnumber intracochlear methods year on year.
The trend in category of therapeutic administered is shown in Figure 3D. Clinically used small molecules (corticosteroids and aminoglycosides) account for the majority of publications each year, the majority of which are case series. The number of papers delivering experimental small molecules and biopharmaceuticals has increased steadily, reflecting development in the field.
The delivery methods used in original research papers identified by this study are seen in Table 6, with numbers of publications organized by therapeutic formulation and experimental model. An identical table is found in Supplementary Material giving the reference numbers of papers referred to within each cell of the table (Supplementary Table 1).
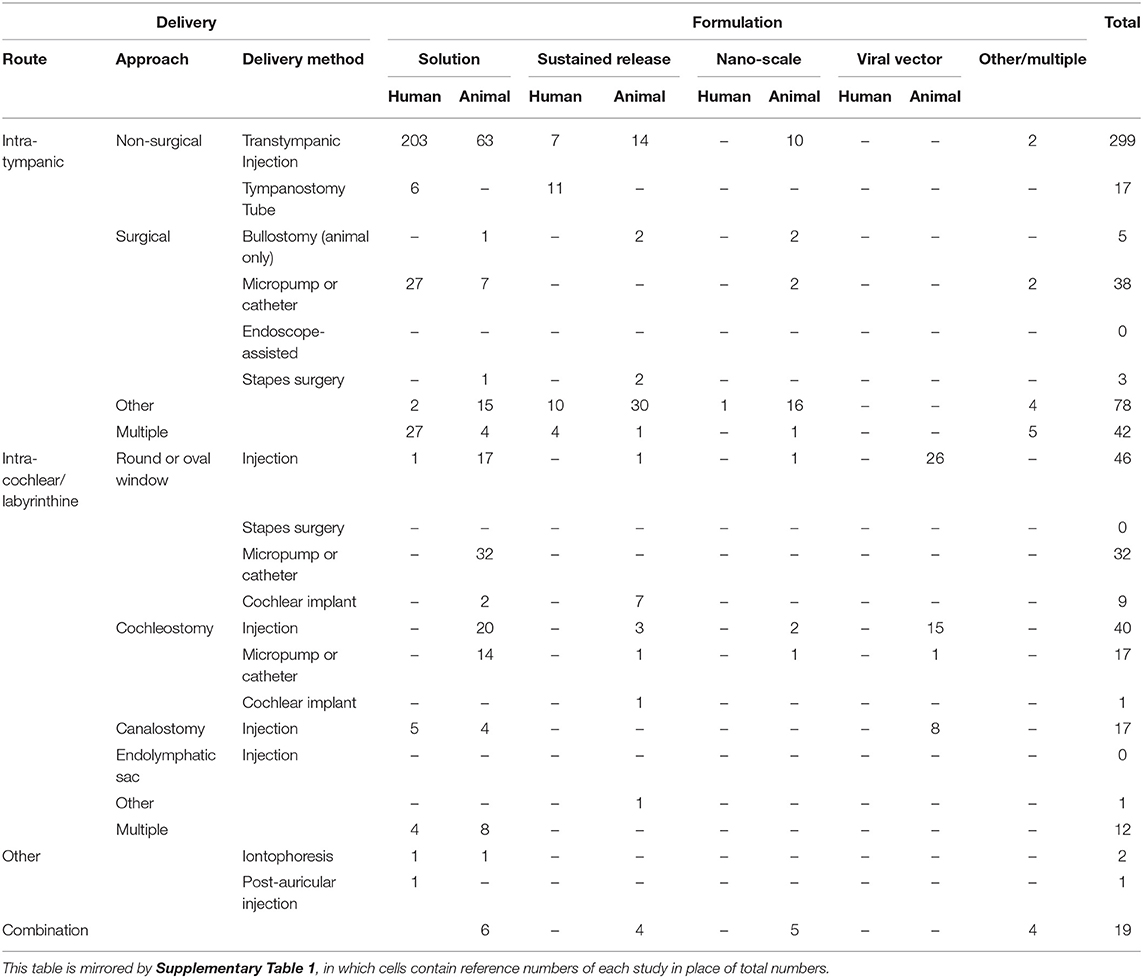
Table 6. Total numbers of original studies for each delivery method arranged by formulation, in humans (living and temporal bone models), and animals.
Cross-referenced tables have been formed to allow identification of papers looking at any combination of delivery method, therapeutic class/formulation, and disease model. Reviews are tabulated separately. All are available in Supplementary Material.
In this review we provide a comprehensive overview of all research activity in the field of local therapeutic delivery to the inner ear, and show how this activity has increased over the last two decades. Though our categorization systems, we reveal how delivery methods are developed and assessed, and our cross referenced tables highlight the complexity in the way that delivery methods combine with formulations and therapeutic agents.
Categorization of the studies by method of assessment of delivery has exposed for the first time a relative lack of research into direct delivery efficacy, especially in humans, with most studies looking at clinical efficacy. Direct assessment of delivery efficacy is critical to understanding how to deliver therapeutics. Without this understanding, it becomes impossible to tell if an absence of a clinical effect is due to therapeutic failure or delivery failure, which has significant implications for both pre-clinical work and trials of novel therapeutics. Given the difficulties in assessing delivery directly in humans (found in this review to be limited to imaging of compounds with a contrast effect, or tissue/perilymph sampling alongside operative management of a pathological ear), more work is needed into understanding how the animal work in this area translates to humans. Computer modeling has potential to assist with this but at present is limited to interpretation of experimental data (Salt, 2002).
Original research outputs have increased steeply over the last decade, reflecting increased interest and funding into hearing. Animal models have been used in over half of the original research studies included in this systematic review, with a growing trend. This reflects the increasing number of novel therapeutics in pre-clinical development, and to some extent, the challenges of locally delivering therapeutics in humans. Whilst animal models play a vital role in the development of delivery methods, and the testing of novel inner ear therapeutics (Frisina et al., 2018), their applicability to humans is unclear (Denayer et al., 2014; Le Prell et al., 2016; Frisina et al., 2018). There remain significant challenges in translating from animals to humans due to differences in anatomy (thinner bone overlying cochlea; Mikulec et al., 2009), size (smaller cochlea allows greater diffusion; Salt, 2008), and disease models (ISSNHL difficult to replicate).
There is a significant body of literature on aminoglycosides and steroids as intra-tympanic treatments for Meniere's disease and ISSNHL, respectively. Whilst this is expected for treatments in clinical use, the large number of papers that continue to be published investigating the efficacy of these drugs suggests that either published information is not of high enough quality to draw conclusions, or is not being disseminated widely enough. In either case, it emphasizes the need for appropriately powered studies with accessible results as novel therapeutics move to trials and beyond.
This review has gone beyond previous systematic reviews in the field, which have focused on assessing the efficacy of single delivery techniques and therapeutic agents in humans. Here we have mapped the large number of delivery methods available, which have been tested with a range of therapeutic agents and formulations, in order to signpost readers from different backgrounds to the information most relevant to them. Intra-tympanic delivery routes dominate the field. This in large part reflects clinical acceptance of intra-tympanic injections resulting from the work described above. Furthermore, small molecules account for the majority of therapeutics delivered locally to the inner ear (447/680, 65.7%), and these agents are mostly suitable for intra-tympanic administration. Intra-cochlear delivery, whilst currently almost essential for cell and gene based therapeutic modalities, carries much higher risks, and has only recently started to be tested in humans (Nakagawa and Ito, 2005)1. The observed imbalance in research across these delivery routes therefore indicates how the field has developed, and is developing, rather than representing a gap in research into intra-cochlear delivery methods.
Some limitations of this study merit discussion. Firstly, we did not set out to extract data on the methodological quality, or to extract and meta-analyze results of individual publications. We did not therefore aim to make recommendations on which delivery method should be used for a given experimental model, therapeutic, or disease, although we do enable studies containing this information to be identified.
Secondly, we did not include gray literature in this paper. In a fast moving field, this inevitably means we have not captured ongoing, or very recently completed studies.
Finally, the extraction of data about delivery method and formulation is highly complex, with multiple sub-categories required (e.g., intra-tympanic injection, through tympanostomy tube, of hydrogel) to ensure the exact nature of a delivery method was captured. This opens the possibility of misclassification, which we mitigated by ensuring two authors extracted all data independently, and that any discrepancies or difficult classifications were discussed within the review team.
Research into local therapeutic delivery to the inner ear has undergone a recent surge, improving our understanding of how novel therapeutics can be delivered. The way in which a therapeutic should be delivered, however, remains somewhat elusive, limited by the difficulty in directly researching the efficacy of delivery to the human inner ear.
The majority of research so far focuses on clinical efficacy of administered therapies. Our knowledge about the efficacy of delivery methods in humans remains limited, and this has the potential to limit how we assess the effectiveness of novel therapeutics.
As the field of inner ear therapeutics develops, it is crucial that research into delivery methods considers both the relationships between therapeutic agent, formulation, delivery method and disease, and the translational challenges that exist when moving from animal to human work. Collaboration between lab scientists, computer scientists, clinicians, industry, and patients will be key to overcoming these challenges, and tailoring delivery methods to novel therapeutics to maximize the chances of success in clinical trials.
All datasets generated for this study are included in the manuscript/Supplementary Files.
CA, CX, HB, and AS designed the study. CX ran the systematic searches. CA, CX, MS, MG, and HB determined study inclusion/exclusion and extracted data. CA, CX, and MS performed data analysis. CA, CX, MS, MG, HB, and AS wrote the manuscript.
This research was co-funded by the NIHR UCLH BRC Deafness and Hearing Problems Theme.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fncel.2019.00418/full#supplementary-material
1. ^Safety, Tolerability and Efficacy for CGF166 in Patients With Unilateral or Bilateral Severe-to-profound Hearing Loss—Full Text View—ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/ct2/show/NCT02132130 (accessed July 20, 2018).
2. ^ISRCTN-ISRCTN59733689: A First-in-Human Study of the Safety and Efficacy of a New Drug, a Gamma Secretase Inhibitor, to Treat People With Sensorineural Hearing Loss. Available online at: http://www.isrctn.com/ISRCTN59733689 (accessed December 23, 2018).
3. ^Documentation and Sources-DrugBank. Available online at: https://www.drugbank.ca/documentation#drug-cards (accessed January 26, 2019).
Budenz, C. L., Pfingst, B. E., and Raphael, Y. (2012). The use of neurotrophin therapy in the inner ear to augment cochlear implantation outcomes. Anat. Rec. 295, 1896–1908. doi: 10.1002/ar.22586
Denayer, T., Stöhr, T., and Van Roy, M. (2014). Animal models in translational medicine: validation and prediction. New Horiz. Transl. Med. 2, 5–11. doi: 10.1016/j.nhtm.2014.08.001
Frisina, R. D., Budzevich, M., Zhu, X., Martinez, G. V., Walton, J. P., and Borkholder, D. A. (2018). Animal model studies yield translational solutions for cochlear drug delivery. Hear. Res. 368, 67–74. doi: 10.1016/j.heares.2018.05.002
Fukui, H., and Raphael, Y. (2013). Gene therapy for the inner ear. Hear. Res. 297, 99–105. doi: 10.1016/j.heares.2012.11.017
Hahn, H., Salt, A. N., Biegner, T., Kammerer, B., Delabar, U., Hartsock, J. J., et al. (2012). Dexamethasone levels and base-to-apex concentration gradients in the scala tympani perilymph after intracochlear delivery in the guinea pig. Otol. Neurotol. 33, 660–665. doi: 10.1097/MAO.0b013e318254501b
Hochmair, I., Nopp, P., Jolly, C., Schmidt, M., Schößer, H., Garnham, C., et al. (2006). MED-EL cochlear implants: state of the art and a glimpse into the future. Trends Amplif. 10, 201–219. doi: 10.1177/1084713806296720
Kim, D.-K. (2017). Nanomedicine for inner ear diseases: a review of recent in vivo studies. Biomed. Res. Int. 2017:3098230. doi: 10.1155/2017/3098230
Kulkarni, V. S., and Shaw, C. (2016). “Chapter 1–Introduction,” in Essential Chemistry for Formulators of Semisolid and Liquid Dosages, eds V. S. Kulkarni and C. Shaw (Boston, MA: Academic Press), 1–4. Available online at: http://www.sciencedirect.com/science/article/pii/B9780128010242000017 (accessed January 22, 2019).
Le Prell, C. G., Fulbright, A., Spankovich, C., Griffiths, S. K., Lobarinas, E., Campbell, K. C., et al. (2016). Dietary supplement comprised of β-carotene, vitamin C, vitamin E, and magnesium: failure to prevent music-induced temporary threshold shift. Audiol. Neurotol. Extra 6, 20–39. doi: 10.1159/000446600
Li, W., Hartsock, J. J., Dai, C., and Salt, A. N. (2018). Permeation enhancers for intratympanically-applied drugs studied using fluorescent dexamethasone as a marker. Otol. Neurotol. 39, 639–647. doi: 10.1097/MAO.0000000000001786
Liu, H., Feng, L., Tolia, G., Liddell, M. R., Hao, J., and Li, S. K. (2014). Evaluation of intratympanic formulations for inner ear delivery: methodology and sustained release formulation testing. Drug Dev. Ind. Pharm. 40, 896–903. doi: 10.3109/03639045.2013.789054
Liu, H., Hao, J., and Li, K. S. (2013). Current strategies for drug delivery to the inner ear. Acta Pharm. Sinica B 3, 86–96. doi: 10.1016/j.apsb.2013.02.003
Liu, J. K. H. (2014). The history of monoclonal antibody development – Progress, remaining challenges and future innovations. Ann. Med. Surg. 3, 113–116. doi: 10.1016/j.amsu.2014.09.001
Liu, X., Li, M., Smyth, H., and Zhang, F. (2018). Otic drug delivery systems: formulation principles and recent developments. Drug Dev. Ind. Pharm. 44, 1395–1408. doi: 10.1080/03639045.2018.1464022
Mikulec, A. A., Plontke, S. K., Hartsock, J. J., and Salt, A. N. (2009). Entry of substances into perilymph through the bone of the otic capsule after intratympanic applications in guinea pigs: implications for local drug delivery in humans. Otol. Neurotol. 30, 131–138. doi: 10.1097/MAO.0b013e318191bff8
Mittal, R., Nguyen, D., Patel, A. P., Debs, L. H., Mittal, J., Yan, D., et al. (2017). Recent advancements in the regeneration of auditory hair cells and hearing restoration. Front. Mol. Neurosci. 10:236. doi: 10.3389/fnmol.2017.00236
Müller, U., and Barr-Gillespie, P. G. (2015). New treatment options for hearing loss. Nat. Rev. Drug Discov. 14, 346–365. doi: 10.1038/nrd4533
Nakagawa, T., and Ito, J. (2005). Cell therapy for inner ear diseases. Curr. Pharm. Des. 11, 1203–1207. doi: 10.2174/1381612053507530
Peppi, M., Marie, A., Belline, C., and Borenstein, J. T. (2018). Intracochlear drug delivery systems: a novel approach whose time has come. Expert Opin. Drug Deliv. 15, 319–324. doi: 10.1080/17425247.2018.1444026
Plontke, S. K., Glien, A., Rahne, T., Mäder, K., and Salt, A. N. (2014). Controlled release dexamethasone implants in the round window niche for salvage treatment of idiopathic sudden sensorineural hearing loss. Otol. Neurotol. 35, 1168–1171. doi: 10.1097/MAO.0000000000000434
Plontke, S. K., Götze, G., Rahne, T., and Liebau, A. (2017). Intracochlear drug delivery in combination with cochlear implants. HNO 65(suppl. 1), 19–28. doi: 10.1007/s00106-016-0285-9
Plontke, S. K., and Salt, A. N. (2018). Local drug delivery to the inner ear: principles, practice, and future challenges. Hear. Res. 368, 1–2. doi: 10.1016/j.heares.2018.06.018
Pyykkö, I., and Jing Zou, Y. Z. (2013). Nanoparticle based inner ear therapy. World J. Otorhinolaryngol. 3, 114–133. doi: 10.5319/wjo.v3.i4.114
Pyykkö, I., Zou, J., Schrott-Fischer, A., Glueckert, R., and Kinnunen, P. (2016). “An overview of nanoparticle based delivery for treatment of inner ear disorders,” in Auditory and Vestibular Research: Methods and Protocols (Methods in Molecular Biology), ed B. Sokolowski (New York, NY: Springer New York), 363–415.
Roemer, A., Köhl, U., Majdani, O., Klöß, S., Falk, C., Haumann, S., et al. (2016). Biohybrid cochlear implants in human neurosensory restoration. Stem Cell Res. Ther. 7:148. doi: 10.1186/s13287-016-0408-y
Salt, A., Hartsock, J., Gill, R., Smyth, D., Kirk, J., and Verhoeven, K. (2017). Perilymph pharmacokinetics of marker applied through a cochlear implant in guinea pigs. PLoS ONE 12:e0183374. doi: 10.1371/journal.pone.0183374
Salt, A. N. (2002). “Simulation of methods for drug delivery to the cochlear fluids,” in Advances in Oto-Rhino-Laryngology, eds D. Felix and E. Oestreicher (Basel: KARGER), 140–148. Available online at: https://www.karger.com/Article/FullText/59251 (accessed July 20, 2018).
Salt, A. N. (2008). Dexamethasone concentration gradients along scala tympani after application to the round window membrane. Otol. Neurotol. 29, 401–406. doi: 10.1097/MAO.0b013e318161aaae
Salt, A. N., and Plontke, S. K. (2009). Principles of local drug delivery to the inner ear. Audiol. Neurootol. 14, 350–360. doi: 10.1159/000241892
Salt, A. N., and Plontke, S. K. (2018). Pharmacokinetic principles in the inner ear: influence of drug properties on intratympanic applications. Hear. Res. 368, 28–40. doi: 10.1016/j.heares.2018.03.002
Salt, A. N., Sirjani, D. B., Hartsock, J. J., Gill, R. M., and Plontke, S. K. (2007). Marker retention in the cochlea following injections through the round window membrane. Hear. Res. 232:78–86. doi: 10.1016/j.heares.2007.06.010
Schilder, A. G. M., Blackshaw, H., Lenarz, T., Warnecke, A., Lustig, L. R., and Staecker, H. (2018). Biological therapies of the inner ear: what otologists need to consider. Otol. Neurotol. 39, 135–137. doi: 10.1097/MAO.0000000000001689
Keywords: drug delivery, inner ear, novel therapeutics, systematic review, intratympanic, intracochlear
Citation: Anderson CR, Xie C, Su MP, Garcia M, Blackshaw H and Schilder AGM (2019) Local Delivery of Therapeutics to the Inner Ear: The State of the Science. Front. Cell. Neurosci. 13:418. doi: 10.3389/fncel.2019.00418
Received: 02 April 2019; Accepted: 30 August 2019;
Published: 09 October 2019.
Edited by:
Stefan K. Plontke, Martin Luther University of Halle-Wittenberg, GermanyReviewed by:
Parisa Gazerani, Aalborg University, DenmarkCopyright © 2019 Anderson, Xie, Su, Garcia, Blackshaw and Schilder. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caroline R. Anderson, Y2Fyb2xpbmUuYW5kZXJzb25AdWNsLmFjLnVr; Anne G. M. Schilder, YS5zY2hpbGRlckB1Y2wuYWMudWs=
†Joint first authors
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.