
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Neurosci. , 09 April 2019
Sec. Cellular Neurophysiology
Volume 13 - 2019 | https://doi.org/10.3389/fncel.2019.00138
This article is part of the Research Topic Stress Disrupts GABA Signaling: Relevance for Stress-Related Psychiatric Disorders View all 5 articles
 Xenia Gonda1,2,3*†
Xenia Gonda1,2,3*† Peter Petschner2,4†
Peter Petschner2,4† Nora Eszlari3,4
Nora Eszlari3,4 Sara Sutori4
Sara Sutori4 Zsofia Gal4
Zsofia Gal4 Szabolcs Koncz4
Szabolcs Koncz4 Ian M. Anderson5
Ian M. Anderson5 Bill Deakin5,6
Bill Deakin5,6 Gabriella Juhasz2,4,5,7
Gabriella Juhasz2,4,5,7 Gyorgy Bagdy2,3,4
Gyorgy Bagdy2,3,4Environmental stress and its interaction with genetic variation are key contributors in the development of depression and anxiety, yet there is a failure to identify replicable genetic variants and gene-interaction effects in the background of these psychiatric symptoms. Recently it has been reported that 5-HTTLPR and NOSI interact with financial but not other types of recent stressors in the development of depression. In the present study we investigated the interaction of GABRA6 rs3219151 and CNR1 rs7766029 in interaction with different types of recent life events on the presence of depression and anxiety in a large general population sample. 2191 participants completed the List of Threatening Experiences questionnaire which covers four categories of stressful life events (financial problems, illness/personal problems, intimate relationships, and social network) experienced over the previous year and the Brief Symptom Inventory for depression and anxiety symptoms. Participants were genotyped for rs3219151 and rs7766029. Data were analyzed with linear regression models with age and gender as covariates. Results indicated that CNR1 rs7766029 interacted significantly with financial but not other types of life events both in case of depression and anxiety symptoms. In contrast, GABRA6 rs3219151 showed a significant interaction with social network related life events in case of anxiety and with illness/personal problem-related life events in case of depression. Our results suggest that the psychological impact of different types of recent stress may be differentially modulated by distinct molecular genetic pathways. Furthermore, in case of certain genetic variants, the occurring psychiatric symptom may depend on the type of stress experienced.
In spite of the ever-increasing interest in genetic mechanisms of depression, findings in candidate gene association studies have generally not been replicated and not detected in genome-wide association studies (Flint and Kendler, 2014; Dunn et al., 2015; Gonda et al., 2018c). Recently, a study investigating variation in 7 genes in pathways previously implicated in the neurobiology of depression found that none influenced depressive phenotype in the absence of exposure to recent stress (Gonda et al., 2018b). However, in those exposed to moderate and/or severe stress, the majority of variants showed “relevance,” a Bayesian measure, to phenotype. In some genes relevance was greatest for moderate rather than severe stress suggesting that may recruit different or additional genes and/or neurotransmitter systems. The 5-HTTLPR polymorphism is the most widely studied candidate mechanism of depression with the most contradictory findings in individual (Juhasz et al., 2015) and meta-analytic studies (Bleys et al., 2018; Culverhouse et al., 2018a,b), We recently reported that the polymorphism did not modify risk of depression either alone or in interaction with a unitary measure of life events (Gonda et al., 2016a,b, 2018a). However, the short allele of 5-HTTLPR was associated with increased depressive symptoms in those exposed to recent financial stressors, but not in those exposed to any other types of recent life events. Furthermore, in those aged 30 or younger the short allele showed an inverse impact on depression when exposed to social stress, reflecting a possible protective effect of this variant. These results suggest that the effect of the majority of genes involved in depression may be manifested only under certain environmental settings, and different types of stressors can exert their effects via divergent genetic and ultimately neurochemical pathways. Consequentially, different types of stressors should be analyzed separately in gene-environment interaction studies of depression.
Our currently used antidepressants (Gonda et al., 2018c), all of which – with the current exception of agomelatine and the possible exception of esketamine – act primarily on monoaminergic systems show only a frustratingly limited clinical efficacy. Therefore it is crucial to understand the involvement of other genes and neurochemical systems, and especially in interaction with possibly pro-depressive stress in the background of depression.
Recently, we reported that a variation of the GABRA6 gene encoding the alpha6 subunit of the GABA-A receptor has no main effect on depression but exerts a significant impact following exposure to recent stress on several depression- and anxiety-related symptoms, giving rise to a symptom constellation that significantly increases suicide risk (Gonda et al., 2017). In this study the different types of recent life stressors were not studied separately, nevertheless, according to previous studies recent social stress may be mediated by the GABRA6 gene (Uhart et al., 2004). Another system involved both in stress and in depression and anxiety, is the endocannabinoid system, with its relevance underlined by the withdrawal of the endocannabionoid-1 receptor antagonist rimonabant from the market due to severe psychological side effects including increased anxiety and depression (Christensen et al., 2008). While previously a significant interaction effect between stress and CNR1 receptor variation on depressive symptoms (Juhasz et al., 2009) has been reported, and an effect of CNR1 receptor variants on anxiety influenced also by serotonin receptor variants have been described (Lazary et al., 2009, 2011) the role of different stress types in this interaction has not been studied. Thus, the aim of the present study was to investigate the effect of the interaction between different types of recent life events and GABRA6 or CNR1 gene variants on recent depressive/anxiety symptoms in a large European general population sample.
Under the aegis of the NewMood study (New Molecules in Mood Disorders, LHSM-CT-2004-503474, Sixth Framework Program of the European Union) (Deakin et al., 2011), adult subjects were recruited from the general population through advertisements, a website and general practices. 2269 subjects (923 in Budapest, Hungary, and 1346 in Manchester, United Kingdom) provided self-reported data on gender, age, recent stress, current depression, and anxiety scores by filling out a questionnaire pack, and provided genetic data by a saliva sampling kit. All of them reported to be of European white ethnic origin, and none of them reported to have any relative participating in the study.
All of the participants signed the official informed consent form. The study was carried out in accordance with the Declaration of Helsinki, and it was approved both by the Scientific and Research Ethics Committee of the Medical Research Council, Budapest, Hungary, and by the North Manchester Local Research Ethics Committee, Manchester, United Kingdom.
Among these participants, 2193 (n = 902 in Budapest, and n = 1291 in Manchester) were successfully genotyped for CNR1 rs7766029, and n = 2206 (n = 902 in Budapest, and n = 1304 in Manchester) for GABRA6 rs3219151.
The Brief Symptom Inventory (BSI) was used to measure current levels of anxiety and depression experienced within the past 7 days (Derogatis, 1993). Each depression and anxiety item was scored 0–4 depending on the distress caused. Anxiety score was calculated as the sum of anxiety symptom item scores divided by the number of completed items, and depression score was calculated as the sum of depression and additional item scores divided by the number of completed items.
Four different types of recent stress were measured by the List of Threatening Experiences (Brugha et al., 1985), summing the number of recent negative life events (RLEs) pertaining to each subscale, occurring within the last year. These subscales have already been used in gene-by-environment (GxE) interaction models in our previous genetic association analyses (Gonda et al., 2016a). The RLE-relationship subscale encompasses problems in marriage or steady relationship (e.g., “separation due to marital problems” and “broke off steady relationship”). The RLE-financial subscale denotes for financial crisis, becoming unemployed or unsuccessfully seeking work (e.g., “became unemployed or seeking work for more than 1 month” and “major financial crisis”). The RLE-illness/personal problems subscale embodies illness, injury, assault, problems with the police, a relative, a close friend or neighbor, court appearance, and losing or being stolen something (“serious illness,” “injury,” or “assault to self” and “serious problems with close friend,” “neighbor,” or “relative”). Finally, the RLE-social (social network disturbances) subscale comprises items on death, illness, injury or assault of a relative or friend (“close friend” or “other relative died” and “serious illness,” “injury,” or “assault to close relative”). Intercorrelations between the four subscales have been reported in Gonda et al. (2016a).
Participants provided buccal mucosa cells collected by a cytology brush (Cytobrush plus C0012, Durbin PLC). Genomic DNA was extracted according to the protocol of Freeman et al. (2003). Genotyping was performed by the Sequenom’s MassARRAY technology (1San Diego, CA, United States) with the IplexTM assay. All laboratory work, carried out under the ISO 9001:2000 quality management requirements, was blinded regarding phenotypes.
IBM SPSS Statistics 21 was used to calculate descriptive statistics on the variables and their comparisons between the Budapest and Manchester subsamples, in addition, to run univariate general linear models for visualization purposes.
Plink v1.902 was used to calculate Hardy-Weinberg equilibrium and minor allele frequency (MAF), and to build additive linear regression models on BSI anxiety and depression scores as primary and secondary analyses, and on each RLE subscale to test gene-environment correlation. Analyses were supported by scripts individually written in R 3.0.2 (R Core Team, 2013).
In the linear regression models run either in Plink or in SPSS, gender and age were always covariates. In case of the combined Budapest + Manchester sample, population was an additional covariate. In case of the GxE models on the BSI scores, main effects of both G and E were also included as covariates.
Only in order to facilitate visualization in the general linear models, RLE scores were divided into three categories as described previously (Gonda et al., 2016a): 0 event, 1 event, 2 or more events.
P-values of the primary tests were entered into QVALUE v1.0 (Storey et al., 2004) to calculate false discovery rate (FDR) q-values (without robust method), with the aim of correction for multiple testing. To estimate the proportion of true null hypotheses, tuning parameter lambda was set to be from 0 to 0.99 by 0.05, and a bootstrap method was used for automatically choosing lambda. In case of the primary tests, we consider results with a q-value ≤ 0.05 as significant.
In case of secondary tests and all descriptive statistics, we consider a p-value ≤ 0.05 as significant, and a p-value ≤ 0.10 as trend.
To evaluate statistical power of the primary tests, Quanto v1.23 was used. Type I error rate was set to 0.05, and we assumed an RGE2 = 0.5% for the GxE term, an RG2 = 0% for the G term, and RE2 values, based on Pearson correlations and coefficients of determination (n = 2269), as the following. On BSI anxiety score, RLE-relationship has an RE2 of 0.0146, RLE-financial has an RE2 of 0.0562, RLE-illness/personal problems has an RE2 of 0.0324, and RLE-social has an insignificant RE2 of 0.0005. On BSI depression score, RLE-relationship has an RE2 of 0.0272, RLE-financial has an RE2 of 0.0655, RLE-illness/personal problems has an RE2 of 0.0396, and RLE-social has an insignificant RE2 of 0.0004. The MAF value of CNR1 rs7766029 was 0.4806, and that of GABRA6 rs3219151 was 0.4341 in the combined population.
Mean values or frequencies of the investigated variables, and their differences between the Budapest and Manchester subsamples are displayed in Table 1. We can see that subjects from Manchester are older, more depressed and more anxious, and have experienced more stressful events related to financial and illness/personal problems within the last year than subjects from Budapest, moreover they also show differences in CNR1 rs7766029 genotype distribution.
Hardy–Weinberg equilibrium did not deviate from the expected in case of either SNP (single nucleotide polymorphisms) in the combined sample or in any of the two subsamples. For CNR1 rs7766029 p-values were 0.967 in the combined sample, 0.337 in Budapest, and 0.402 in Manchester populations. For GABRA6 rs3219151, 0.115 in the combined, 0.090 in Budapest, and 0.536 in Manchester samples.
Gene-environment correlation results, calculated in additive linear regression models, are displayed in Table 2. GABRA6 rs3219151 is significantly related to RLE-illness/personal in Budapest, and CNR1 rs7766029 is significantly related to RLE-social in Manchester.
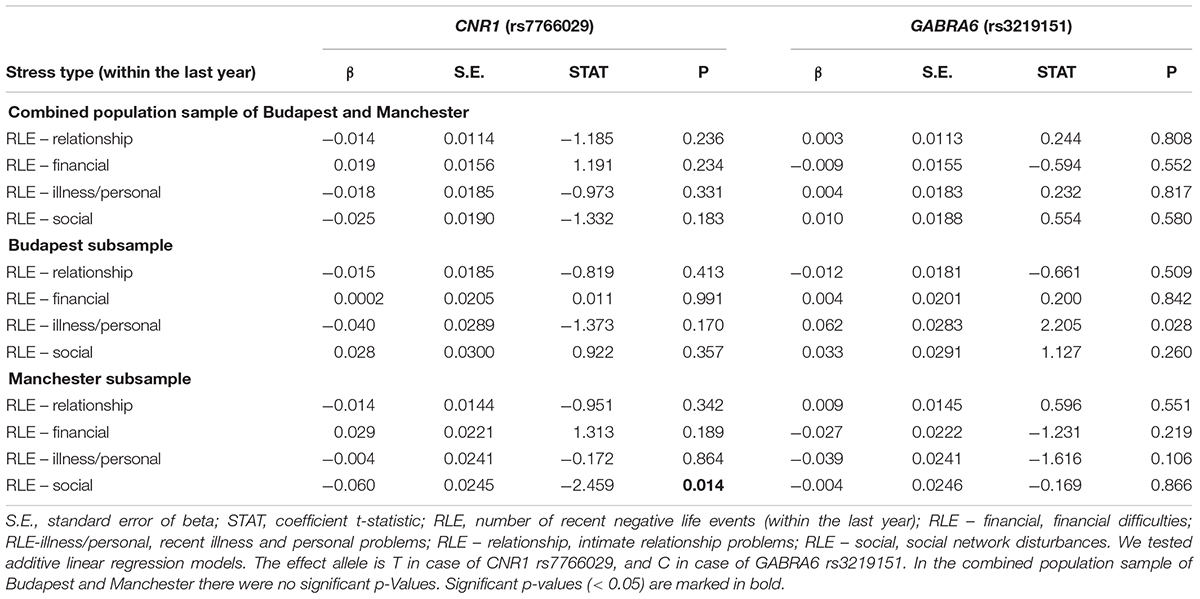
Table 2. Gene-environment correlation between CNR1 (rs7766029) and GABRA6 (rs3219151) polymorphisms and different types of recent negative life events.
Table 3 displays results of the 16 primary tests and shows that the interaction between CNR1 rs7766029 and RLE-financial is significant both on BSI anxiety score (Figure 1) and BSI depression score (Figure 2). However, GABRA6 rs3219151 exerts a significant interaction with RLE-social on BSI anxiety score (Figure 3), and with RLE-illness/personal on BSI depression score (Figure 4).
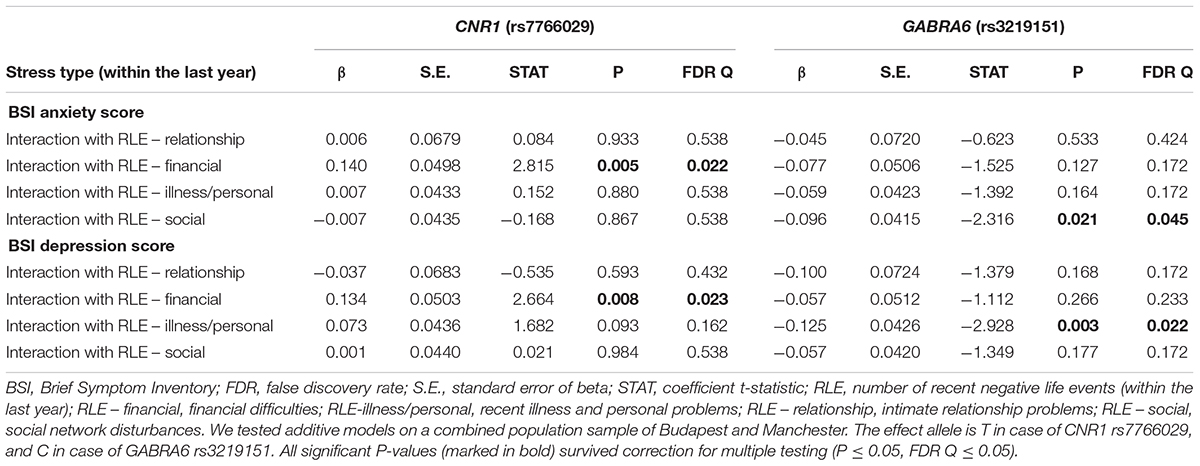
Table 3. Interactions of number of recent negative life events and CNR1 (rs7766029) or GABRA6 (rs3219151) polymorphisms on BSI anxiety and BSI depression scores as the outcome in the combined Budapest + Manchester sample.
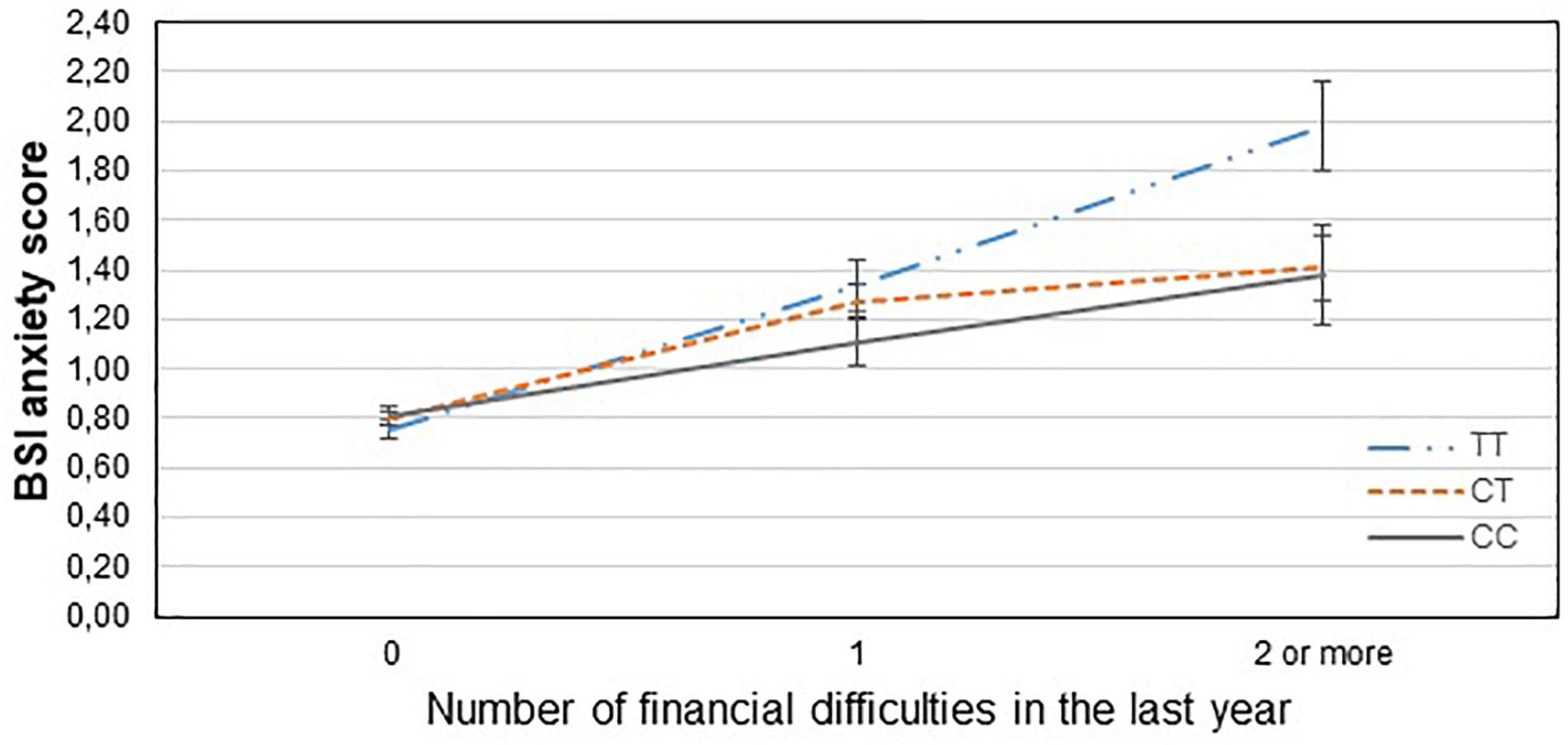
Figure 1. Significant interaction effect of financial difficulties and CNR1 rs7766029 on current anxiety scores in the total sample. Mean BSI anxiety scores are displayed with standard error bars, in function of genotype and the number of life events related to financial difficulties occurred within the last year (general linear model performed only for visualization purposes).
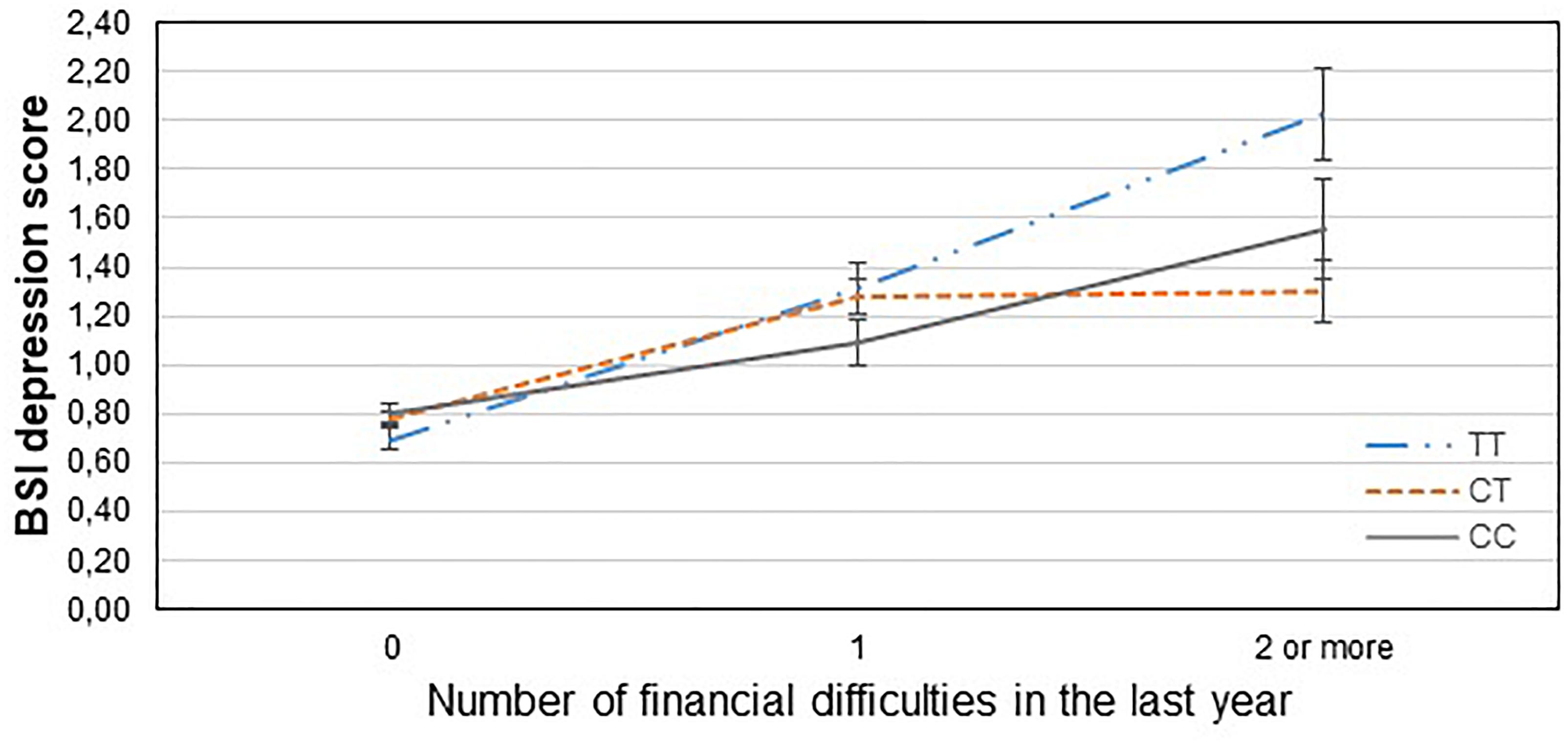
Figure 2. Significant interaction effect of financial difficulties and CNR1 rs7766029 on current depression scores in the total sample. Mean BSI depression scores are displayed with standard error bars, in function of genotype and the number of life events related to financial difficulties occurred within the last year (general linear model performed only for visualization purposes).
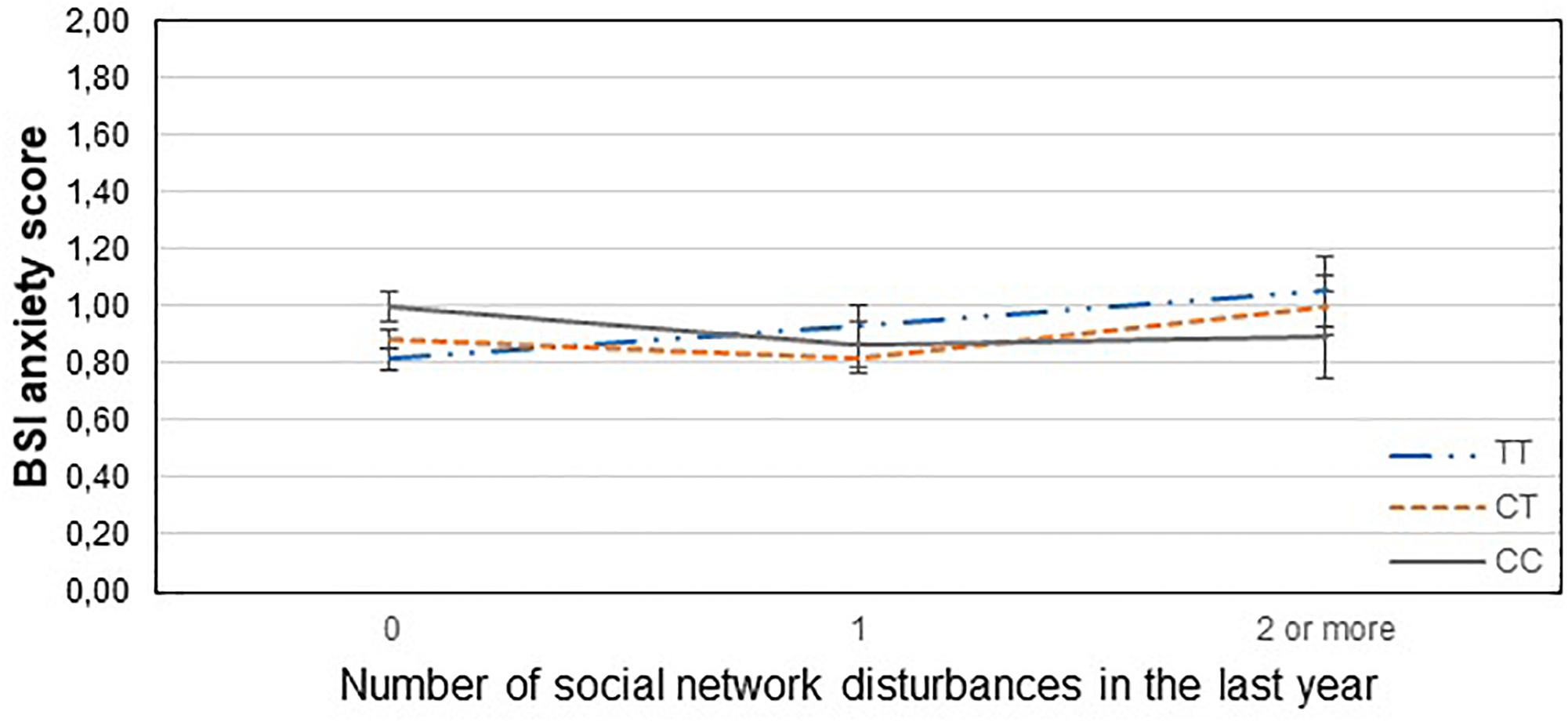
Figure 3. Significant interaction effect of social network disturbances and GABRA6 rs321915 on current anxiety scores in the total sample. Mean BSI anxiety scores are displayed with standard error bars, in function of genotype and the number of life events related to social network disturbances occurred within the last year (general linear model performed only for visualization purposes).
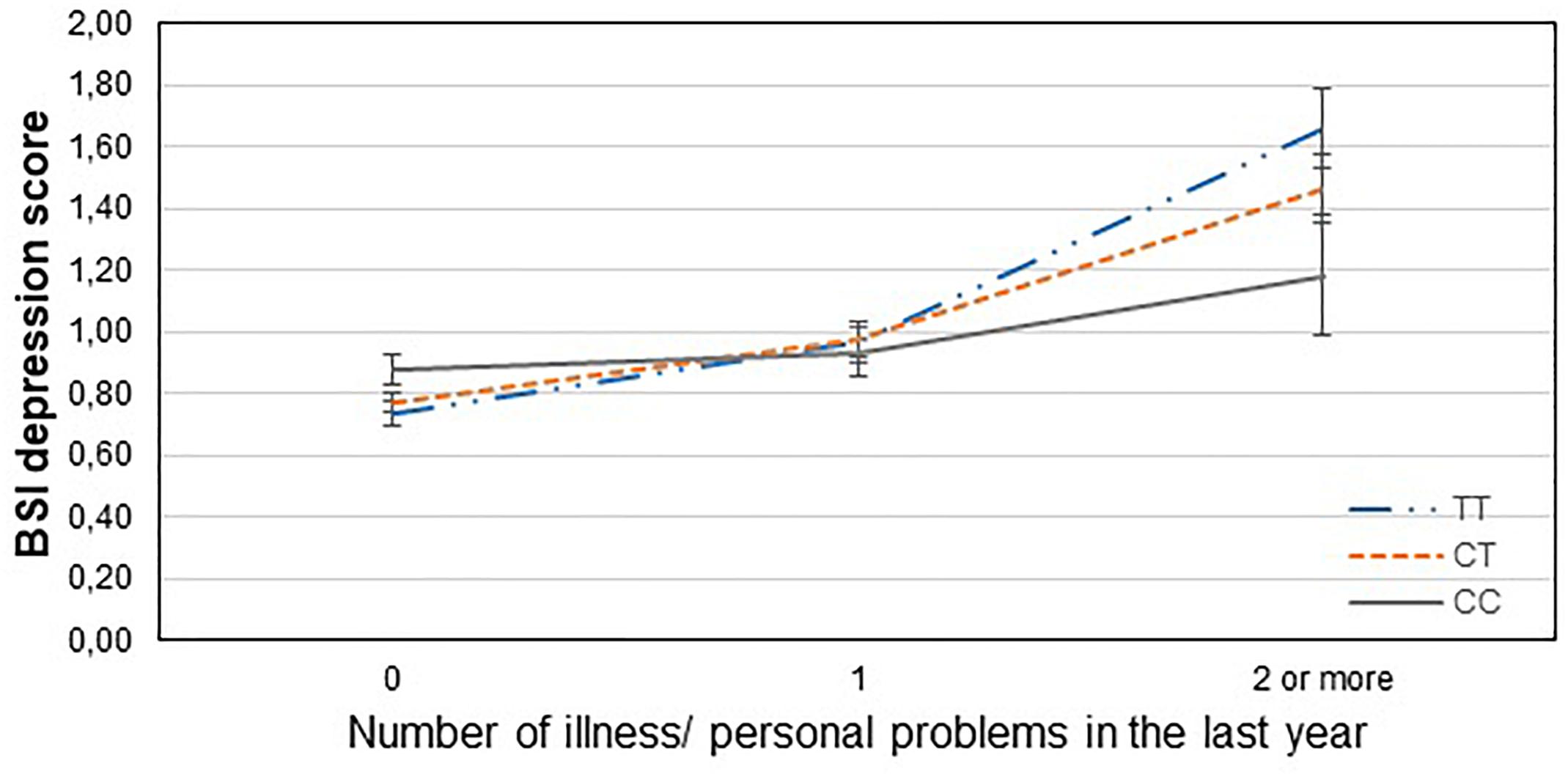
Figure 4. Significant interaction effect of illness/personal problems and GABRA6 rs3219151 on current depression scores in the total sample. Mean BSI depression scores are displayed with standard error bars, in function of genotype and the number of life events related to illness, injury or problems occurred within the last year (general linear model performed only for visualization purposes).
Table 4 shows replicability of significant primary results within the separate Budapest and Manchester subsamples. None of the results proven significant in the combined sample could be replicated in both of the subsamples. However, the interaction of GABRA6 rs3219151 and RLE-illness/personal is significant in Manchester and a trend in Budapest despite the differences in subpopulation characteristics demonstrated in Table 1, suggesting a rather generalizable effect. Nevertheless, GABRA6 rs3219151 has shown a gene-environment correlation with RLE-illness/personal in Budapest (Table 2), albeit with the opposite direction of effect compared to that of the GxE on BSI depression (Tables 3, 4).
In our present study we demonstrated that variants in GABRA6 and CNR1 genes, previously implicated in both stress and depression (Juhasz et al., 2009, 2017; Gonda et al., 2017), interact with particular types of recent life stressors in influencing depression and anxiety. These results suggest that different genes and neurochemical systems may mediate the effects of different types of recent stress. Furthermore, while stress is a significant factor associated with both depression and anxiety, which overlap significantly both genotypically and phenotypically, we found that the two investigated genetic variants interacted with different types of stress in the background of these phenotypes. Specifically, in case of anxiety CNR1 rs7766029 showed an interaction with recent financial stress and GABRA6 rs3219151 with recent social network-related stressors. In case of depression, CNR1 rs7766029 similarly interacted with recent financial stress, GABRA6 rs3219151, however, interacted with recent illness and personal problem stressors. Our findings, thus, also show that while certain genes uniformly mediate the effect of a given type of recent stressor in the development of various facets of psychopathology, others may transform different stressors into distinct phenotypes.
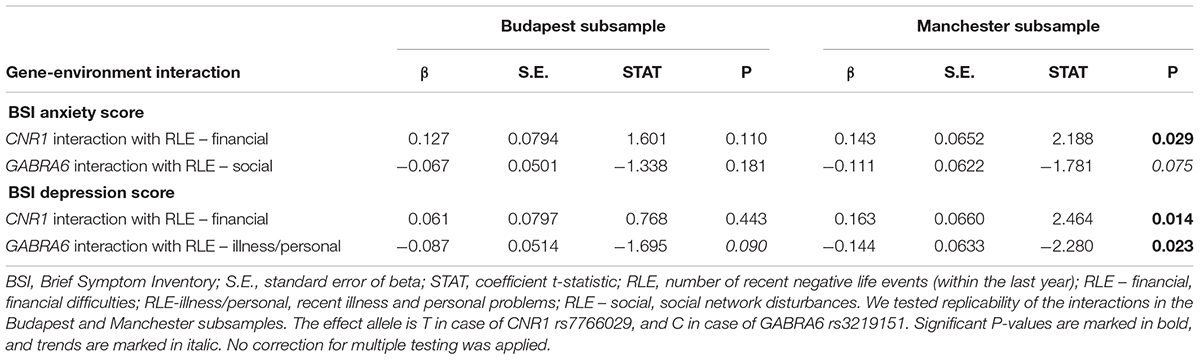
Table 4. Re-analysis of data split according to study site in the Budapest and Manchester subsamples.
Modulation of the stress response and stress adaptation is one of the major roles and effects of the central nervous endocannabinoid system (Patel and Hillard, 2008). The presynaptic CB1 endocannabinoid receptors with dense localizations in the prefrontal cortex, amygdala and hippocampus (Morena and Campolongo, 2014) play crucial roles in this process (McLaughlin et al., 2014). The two retrogradely acting endocannabinoids, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), modulate glutamate and GABA neurons following their release and, thereby, balance excitatory and inhibitory activity (Freund et al., 2003; Hill et al., 2010; McLaughlin et al., 2014). CB1 activation also enhances brainstem serotonin and noradrenaline activity and regulates the sensitivity and activity of the hypothalamus-pituitary-adrenal (HPA) axis in stress adaptation and habituation (Gorzalka et al., 2008; Steiner and Wotjak, 2008; Hill et al., 2010; McLaughlin et al., 2014). While decreased CB1 transmission contributes to a state resembling chronic stress (Lazary et al., 2011), chronic stress alters endocannabinoid transmission contributing to stress habituation (Patel and Hillard, 2008).
The role of the endocannabinoid system and the CB1 receptor in depression and anxiety is also supported by several lines of animal studies linking reduced CB1 signaling to increased depression and anxiety (Hill and Gorzalka, 2005). CB1 KO mice showed increased sensitivity toward developing learned helplessness, an animal model of depression, as well as anhedonia upon exposure to chronic mild stress (Gorzalka et al., 2008), and CB1 receptor mediated endocannabinoid transmission was found to play a role in influencing affective processing under stress exposure in both rodents (McLaughlin et al., 2012; Wang et al., 2012; Morena et al., 2016) and humans (Wirz et al., 2018). In addition, the importance of the cannabinoid signaling in mood disorders is specifically highlighted by the severe increase in depressive and anxiety symptoms following administration of the CB1 antagonist appetite suppressant rimonabant in previously non-depressed human subjects (Christensen et al., 2008; Goodwin et al., 2012). Such studies suggest that the endocannabinoid system buffers the emotional effects of stress exposure (Morena and Campolongo, 2014; Morena et al., 2016), and raise the possibility of variations in the CNR1 gene (encoding the CB1 receptor) altering the neural information processing of affective stimuli (Wirz et al., 2018). The endocannabinoid system has also been reported to play a role in stress-induced anxiety, with pharmacological or genetic disruption of CB1 signaling increasing anxiety moderately without, and dramatically following stress exposure (Haller et al., 2004; Hill et al., 2011; Morena et al., 2016). Indeed, in humans disrupted CB1 signaling related to CNR1 variation appears to be involved in stress vulnerability and manifestation of stress related psychiatric conditions (Morena et al., 2016) including schizophrenia (Fernandez-Espejo et al., 2009; Gouvea et al., 2017) as well as non-response to antipsychotics; substance abuse and addiction (Benyamina et al., 2011; Lopez-Moreno et al., 2012); eating disorders; autism (Hillard et al., 2012); and mood and anxiety disorders (Ashton and Moore, 2011; Hillard et al., 2012).
The CNR1 gene encoding the human endocannabinoid receptor 1 (CB1) is located on chromosome 6q14-15 and while the function of SNPs in the CNR1 gene and their effect on expression and activity has not yet been reported, they may decrease mRNA stability and thus contribute to reduced CB1 receptor expression (Domschke et al., 2008). A significant association was reported between CNR1 and high neuroticism, which leads to a propensity to both perceive and experience life events as more negative and stressful as well as to less adaptive coping in the face of stress further stressing the role of this variant in the emergence of stress-related psychopathology (Juhasz et al., 2009). CNR1 variation increases risk and vulnerability to depression upon exposure to both early and recent stress (Juhasz et al., 2009; Agrawal et al., 2012). In particular, the 3′-end polymorphism rs7766029 has been suggested to play a role in the development of depression by impacting the experience of negative life events through increased exposure by life choice or through response to or interpretation of negative life events (Bouchard and Mcgue, 2003; Juhasz et al., 2009; Aleksandrova et al., 2012) but it remained uninvestigated whether this gene uniformly mediates the effects of all types of stressors. Thus this is the first study to report that CNR1 rs7766029 selectively mediates the effects of financial stress, but not other types of recent life events in both depression and anxiety symptoms.
Similarly to our present results, we previously reported that in a general population sample, in males but not in females, and in those above, but not under thirty years of age, that the 5-HTTLPR polymorphism of the serotonin transporter gene mediated the effects of recent financial but not other types of stressors in the background of depression (Gonda et al., 2016a,b). Variation in the neural nitric oxide synthase (NOS I) gene also interacted with financial difficulty in the development of depression in another study (Sarginson et al., 2014). In addition, financial stress was found to predict both risk and persistence of depression and also associated with lower remission rates during antidepressant treatment with citalopram (Trivedi et al., 2006). It has also been previously reported that early childhood financial difficulties show a strong association with reduced connectivity in the default mode network, which remains observable in adulthood and it associated with increased stress sensitivity based on increased cortisol production during social stress anticipation (Sripada et al., 2014). This suggests a distinct effect of financial problems versus other types of stressors. Financial stress can arguably be viewed as a proxy for a threat for general safety of existence and thus can be considered a pervasive, and especially in extreme cases a severe and life-threatening stressor as opposed to other, non-existential types of environmental and life events. The importance of such stress and the vital threat linked with it may be one reason why the effect of this type of recent stress is mediated by multiple genes and neurochemical systems and argues for its strong role in the background of various manifestations of stress-related psychopathologies.
The GABA system plays an important role in acute stress reaction (Giordano et al., 2008) while stress exposure also exerts both short and long-term effects on GABA signaling, including changes in composition, sensitivity and availability of GABA-A receptors which in turn contribute to alterations in the stress response (Skilbeck et al., 2010; Gunn et al., 2011; Luscher et al., 2011). The GABA system plays a key role in the HPA-axis downregulation in response to stress as demonstrated by the strong inhibitory effect on the HPA axis by alprazolam, an anxiolytic agent enhancing GABA signaling by increasing the affinity of GABA for the GABA-A receptor (Giordano et al., 2006). Studies have shown that the GABA system in interaction with stress influences central nervous stress control through GABA-A receptors localized on hypothalamic CRH neurons in the paraventricular nuclei, inhibiting the HPA axis (Oquendo and Mann, 2000; Luscher et al., 2011) and attenuating response to stress (Gunn et al., 2011). Chronic stress, however, leads to reduced GABA activity and thus altered response during subsequent stress exposure (Hu et al., 2010; Gunn et al., 2011; Luscher et al., 2011).
Disrupted GABA signaling and GABA deficit is also hypothesized to play a role in the development of depression and anxiety (Luscher et al., 2011), while Gad2 mRNA, the synthesis enzyme of GABA, was upregulated after chronic treatment with the serotonin and noradrenaline reuptake inhibitor venlafaxine (Tamasi et al., 2015). Reciprocally, the GABA system is also involved in regulating and fine-tuning depression-relevant serotonergic and noradrenergic processes (Luscher et al., 2011; Pehrson and Sanchez, 2015). Furthermore, it appears that decreased HPA axis inhibition possibly related to GABA-A receptor variation may in turn lead to increased physiological stress response and higher risk of mental health disorders like depression (Uhart et al., 2004). These results again point to the possible involvement of GABA-A receptor variations in the modulation of stressful stimuli in psychiatric phenotypes, nevertheless, studies failed to investigate the roles related to specific stressors.
Rs3219151, located in the 3′ untranslated region in the GABRA6 gene encoding the alpha-6 subunit of the GABA-A receptor, and predicted to be in the target region of 4 microRNAs, has previously been demonstrated to play a significant role in modulating HPA-axis activity. Those carrying the T allele exhibited higher plasma cortisol levels both during resting conditions (Rosmond et al., 2002) as well as during stimulation in the Trier Social Stress Test (Uhart et al., 2004), showing that this allele is, indeed, associated with increased stress response. Other studies similarly reported association between GABA-A subunit variation and stress reactivity manifested in increased blood pressure, cortisol, and adrenocorticotropic hormone (ACTH) levels following stress in GABRA6 rs3219151 T allele carriers (Sen et al., 2004; Uhart et al., 2004; Inoue et al., 2015). In a recent study we found that while the T allele of GABRA6 rs3219151 was not directly associated with either depression or anxiety, a strong effect was observed in interaction with recent life stress on both anxiety and depression (Gonda et al., 2017).
In our present study we observed that GABRA6 rs3219151 mediated the effects of different types of life events in the background of depression and anxiety. While in case of depression the effects of rs3219151 were observable in case of exposure to recent illness- and personal problem related life events, in case of anxiety rs3219151 only interacted with recent social-network related stressors. In both cases, just as in case of our previous study (Gonda et al., 2017), presence of the T allele increased risk of depression and anxiety. This novel finding corroborates previous reporting that those carrying the T allele show a larger stress reactivity and expand these results with the information that this increased reactivity appears to be specific to certain life events.
In line with our previous study of 5-HTTLPR (Gonda et al., 2016a), the present results of CNR1 rs7766029 support a distinct role of financial stress among different life events in the development of depression and anxiety symptoms possibly related to the prominent and pervasive impact of this type of stress. However, our findings concerning GABRA6 rs3219151 indicate that other types of stressors may also be selectively mediated by distinct genetic elements, suggesting that the effects of different types of hardships are mediated by distinct neurochemical pathways. Furthermore, in case of GABRA6 rs3219151 we also observed that different types of recent stressors contribute to the emergence of different types of psychopathology: depress ion in case of illness and personal concerns and anxiety in case of social network-related problems. A further interesting aspect of these results is that in case of anxiety, a crossover pattern was observed with the “risk” T allele associated with lower anxiety without stress exposure and a higher anxiety in those exposed to severe stress. This is in part similar to a previous finding of 5-HTTLPR in a population aged younger than 30 years, we similarly found a crossover pattern in interaction with recent social-network related stress, however, in that case the “risk” s allele of 5-HTTLPR was associated with decreased depression when exposed to severe stress (Gonda et al., 2018a). Thus, our present findings also show that the same type of stressor may increase the risk or protect against different types of psychological symptoms depending on the interacting gene and the involved neurochemical system. Which, in part, may explain for the presence of these variations in the genome and the evolutionary maintenance of pro-depressive states.
The divergent genetic interaction patterns of distinct types of recent stress in the background of depression and anxiety has not been widely investigated so far, although in case of childhood traumas the differential genetic interaction effects of different types of maltreatment is already well-known (Cicchetti et al., 2007; Fisher et al., 2013). Our present results clearly show that recent stress is a heterogeneous phenomenon and different types of recent life events and stressors may exert their effect via different neurobiological pathways and mechanisms in the emergence of depression and anxiety. These findings also expand our previous results where we found that genes belonging to different neurochemical pathways mediate the effects of moderate or severe stress in depression, with some genetic variants being more relevant in case of moderate and others in case of severe recent stress exposure (Gonda et al., 2018b). In spite of these, there is little attention paid to subcategorization of life events according to type and severity in genetic studies of depression, and lack of consideration of the distinct genetic and biochemical pathways of different stressors may be an important contributor to the lack of positive findings and replicability in gene-environment interaction studies in depression (Gonda et al., 2018c). Furthermore if certain genetic variants increase risk of depression or anxiety only when exposed to specific stressors and certain stressors only lead to depression and anxiety in those carrying given genetic variants, this may contribute to a more sophisticated understanding of predicting, screening and preventing depression. Such findings may not only advance our understanding on the complex pattern of interaction between stressors and genes, but could also help in the refinement of subtyping affective disorders, pinpointing new pharmacological targets and advancing precision treatment of these illnesses.
There are several limitations of our present study. First of all, we would emphasize the exploratory nature of our analyses and urge replication of the results presented here in other cohorts. Second, our research is cross-sectional, thus it is possible that depressive symptoms developing with a greater latency following exposure or repeated exposure remained hidden. Third, recent life events occurring in the previous year were recorded retrospectively, and these, just as measures of depression and anxiety were based on self-report. Fourth, recall of negative life events is subject to recall bias, which is, on the one hand memory dependent, and on the other hand, state dependent, so it is possible that those less depressed recalled less, while those more depressed recalled more negative life events. Fifth, our study sample was a general population sample of volunteers, which contributes to a possibility of sampling bias with respect to depression. Sixth, we used two geographically different subsamples in our study, and ancestry was not assessed in the present study using molecular methods such as whole-genome SNP genotyping. Although to consider this we used population as a covariate in all our statistical analyses, there may exist subtle genetic differences both between the two subsamples and also within each sample due to population stratification which may lead to spurious effects. Finally, while our reported results were significant in the combined population, we could not replicate some of them separately in the two population subsamples.
In our study we demonstrated that genetic variation in two distinct neurochemical systems, namely, rs7766029 in the CNR1 and rs3219151 in GABRA6, mediate the effects of different types of recent stress in depression or anxiety. Interestingly, in case of GABRA6, the resulting psychological symptom may be the function of the type of stress experienced. Our findings thus show that stress is a heterogeneous phenomenon and different types of it may activate different neural pathways. Besides its possible clinical and pharmacological implications, our results suggest that the environmental context of psychiatric symptoms, disorders and relevant genes should be specified in a more detailed and multidimensional manner in order to be able to better predict, prevent and treat affective illnesses.
This study was carried out in accordance with the Declarations of Helsinki. All subjects gave written informed consent and the study protocol was approved both by the Scientific and Research Ethics Committee of the Medical Research Council, Budapest, Hungary, and the North Manchester Local Research Ethics Committee, Manchester, United Kingdom.
XG, PP, IA, BD, GJ, and GB conceived and designed the study. XG, PP, NE, and GJ participated in recruiting and evaluating the study sample and collecting the DNA samples. NE, SS, ZG, and SK participated in managing and analyzing the data. XG, PP, NE, SS, ZG, SK, IA, BD, GJ, and GB participated in interpreting the data. XG and PP wrote the first draft of the manuscript. All authors revised subsequent versions of the manuscript, contributed to and approved the final version of the manuscript.
This study was supported by the Hungarian Academy of Sciences (MTA-SE Neuropsychopharmacology and Neurochemistry Research Group); the Hungarian Brain Research Program (Grants: 2017-1.2.1-NKP-2017-00002; KTIA_13_NAPA-II/14); the National Development Agency (Grant: KTIA_NAP_13-1-2013-0001); by the Hungarian Academy of Sciences, Hungarian National Development Agency, Semmelweis University and the Hungarian Brain Research Program (Grant: KTIA_NAP_13-2-2015-0001) (MTA-SE-NAP B Genetic Brain Imaging Migraine Research Group) and by the National Institute for Health Research, Manchester Biomedical Research Centre. XG was supported by the Bolyai Research Fellowship Program of the Hungarian Academy of Sciences. XG was supported by ÚNKP-18-4-SE-33, PP by ÚNKP-17-4-I-SE-8, and NE by ÚNKP-16-3 and ÚNKP-17-3-III-SE-2 grants of the New National Excellence Program of the Ministry of Human Capacities. BD variously performed consultancy, speaking engagements and research for P1vital, 488 Autifony and AstraZeneca; fees are paid to the University of Manchester; he has share options 489 in P1vital. IMA has received consultancy fees from Servier, Alkermes, Lundbeck/Otsuka and 490 Janssen, an honorarium for speaking from Lundbeck and grant support from Servier and 491 AstraZeneca. All other authors report no financial relationships with commercial interests. The sponsors had no further role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
BD variously performed consultancy, speaking engagements and research for P1vital, Autifony, and AstraZeneca; fees are paid to The University of Manchester; he has share options in P1vital. IA has received consultancy fees from Servier, Alkermes, Lundbeck/Otsuka, and Janssen, an honorarium for speaking from Lundbeck and grant support from Servier and AstraZeneca.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Agrawal, A., Nelson, E. C., Littlefield, A. K., Bucholz, K. K., Degenhardt, L., Henders, A. K., et al. (2012). Cannabinoid receptor genotype moderation of the effects of childhood physical abuse on anhedonia and depression. Arch. Gen. Psychiatry 69, 732–740. doi: 10.1001/archgenpsychiatry.2011.2273
Aleksandrova, L. R., Souz, R. P., Bagby, M. R., Casey, D. M., Hodgins, D. C., Smith, G. J., et al. (2012). Genetic underpinnings of neuroticism: a replication study. J. Addict. Res. Ther. 3:119. doi: 10.4172/2155-6105.1000119
Ashton, C. H., and Moore, P. B. (2011). Endocannabinoid system dysfunction in mood and related disorders. Acta Psychiatr. Scand. 124, 250–261. doi: 10.1111/j.1600-0447.2011.01687.x
Benyamina, A., Kebir, O., Blecha, L., Reynaud, M., and Krebs, M. O. (2011). CNR1 gene polymorphisms in addictive disorders: a systematic review and a meta-analysis. Addict. Biol. 16, 1–6. doi: 10.1111/j.1369-1600.2009.00198.x
Bleys, D., Luyten, P., Soenens, B., and Claes, S. (2018). Gene-environment interactions between stress and 5-HTTLPR in depression: a meta-analytic update. J. Affect. Disord. 226, 339–345. doi: 10.1016/j.jad.2017.09.050
Bouchard, T. J., and Mcgue, M. (2003). Genetic and environmental influences on human psychological differences. J. Neurobiol. 54, 4–45. doi: 10.1002/neu.10160
Brugha, T., Bebbington, P., Tennant, C., and Hurry, J. (1985). The List of Threatening Experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol. Med. 15, 189–194. doi: 10.1017/S003329170002105X
Christensen, R., Kristensen, P. K., Bartels, E. M., Bliddal, H., and Astrup, A. (2008). Efficacy and safety of the weight-loss drug rimonabant: a meta analysis of randomised trials (vol 370, pg 1706, 2007). Lancet 371, 558–558.
Cicchetti, D., Rogosch, F. A., and Sturge-Apple, M. L. (2007). Interactions of child maltreatment and serotonin transporter and monoamine oxidase A polymorphisms: depressive symptornatology among adolescents from low socioeconomic status backgrounds. Dev. Psychopathol. 19, 1161–1180. doi: 10.1017/S0954579407000600
Culverhouse, R. C., Saccone, N. L., and Bierut, L. J. (2018a). The state of knowledge about the relationship between 5-HTTLPR, stress, and depression. J. Affect. Disord. 228, 205–206. doi: 10.1016/j.jad.2017.12.002
Culverhouse, R. C., Saccone, N. L., Horton, A. C., Ma, Y., Anstey, K. J., Banaschewski, T., et al. (2018b). Collaborative meta-analysis finds no evidence of a strong interaction between stress and 5-HTTLPR genotype contributing to the development of depression. Mol. Psychiatry 23, 133–142. doi: 10.1038/mp.2017.44
Deakin, J. F., Harro, J., and Anderson, I. M. (2011). NewMood: a productive European model of collaboration for translational research in depression. Eur. Neuropsychopharmacol. 21, 1–2. doi: 10.1016/j.euroneuro.2010.11.008
Derogatis, L. R. (1993). BSI: Brief Symptom Inventory: Administration, Scoring, and Procedures Manual. Minneapolis, MN: National Computer Systems Pearson, Inc.
Domschke, K., Danniowski, U., Ohrmann, P., Lawford, B., Bauer, J., Kugel, H., et al. (2008). Cannabinoid receptor 1 (CNR1) gene: impact on antidepressant treatment response and emotion processing in Major Depression. Eur. Neuropsychopharmacol. 18, 751–759. doi: 10.1016/j.euroneuro.2008.05.003
Dunn, E. C., Brown, R. C., Dai, Y., Rosand, J., Nugent, N. R., Amstadter, A. B., et al. (2015). Genetic determinants of depression: recent findings and future directions. Harv. Rev. Psychiatry 23, 1–18. doi: 10.1097/Hrp.0000000000000054
Fernandez-Espejo, E., Viveros, M. P., Nunez, L., Ellenbroek, B. A., and De Fonseca, F. R. (2009). Role of cannabis and endocannabinoids in the genesis of schizophrenia. Psychopharmacology 206, 531–549. doi: 10.1007/s00213-009-1612-6
Fisher, H. L., Cohen-Woods, S., Hosang, G. M., Korszun, A., Owen, M., Craddock, N., et al. (2013). Interaction between specific forms of childhood maltreatment and the serotonin transporter gene (5-HTT) in recurrent depressive disorder. J. Affect. Disord. 145, 136–141. doi: 10.1016/j.jad.2012.05.032
Flint, J., and Kendler, K. S. (2014). The genetics of major depression. Neuron 81, 484–503. doi: 10.1016/j.neuron.2014.01.027
Freeman, B., Smith, N., Curtis, C., Huckett, L., Mill, J., and Craig, I. W. (2003). DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav. Genet. 33, 67–72. doi: 10.1023/A:1021055617738
Freund, T. F., Katona, I., and Piomelli, D. (2003). Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 83, 1017–1066. doi: 10.1152/physrev.00004.2003
Giordano, R., Pellegrino, M., Picu, A., Bonelli, L., Balbo, M., Berardelli, R., et al. (2006). Neuroregulation of the hypothalamus-pituitary-adrenal (HPA) axis in humans: effects of GABA-, mineralocorticoid-, and GH-secretagogue-receptor modulation. Sci. World J. 6, 1–11. doi: 10.1100/tsw.2006.09
Giordano, R., Picu, A., Bonelli, L., Balbo, M., Berardelli, R., Marinazzo, E., et al. (2008). Hypothalamus-pituitary-adrenal axis evaluation in patients with hypothalamo-pituitary disorders: comparison of different provocative tests. Clin. Endocrinol. 68, 935–941. doi: 10.1111/j.1365-2265.2007.03141.x
Gonda, X., Eszlari, N., Anderson, I. M., Deakin, J. F. W., Juhasz, G., and Bagdy, G. (2018a). 5-HTTLPR ‘social sensitivity’ short allele may protect against depression after exposure to social network stressors in young people. Eur. Neuropsychopharmacol. 28, S581–S582.
Gonda, X., Hullam, G., Antal, P., Eszlari, N., Petschner, P., Hokfelt, T. G., et al. (2018b). Significance of risk polymorphisms for depression depends on stress exposure. Sci. Rep. 8:3946. doi: 10.1038/s41598-018-22221-z
Gonda, X., Petschner, P., Eszlari, N., Baksa, D., Edes, A., Antal, P., et al. (2018c). Genetic variants in major depressive disorder: from pathophysiology to therapy. Pharmacol. Ther. 194, 22–43. doi: 10.1016/j.pharmthera.2018.09.002
Gonda, X., Eszlari, N., Kovacs, D., Anderson, I. M., Deakin, J. F., Juhasz, G., et al. (2016a). Financial difficulties but not other types of recent negative life events show strong interactions with 5-HTTLPR genotype in the development of depressive symptoms. Transl. Psychiatry 6:e798. doi: 10.1038/tp.2016.57
Gonda, X., Eszlari, N., Kovacs, D., Anderson, I. M., Deakin, J. F. W., Juhasz, G., et al. (2016b). Distinct types of life events interact with 5-HTTLPR in the development of depressive symptoms in an age-dependent manner. Eur. Neuropsychopharmacol. 26, s164–s165. doi: 10.1016/S0924-977X(16)30987-7
Gonda, X., Sarginson, J., Eszlari, N., Petschner, P., Toth, Z. G., Baksa, D., et al. (2017). A new stress sensor and risk factor for suicide: the T allele of the functional genetic variant in the GABRA6 gene. Sci. Rep. 7:12887. doi: 10.1038/s41598-017-12776-8
Goodwin, R. S., Baumann, M. H., Gorelick, D. A., Schwilke, E., Schwope, D. M., Darwin, W. D., et al. (2012). CB1-cannabinoid receptor antagonist effects on cortisol in cannabis-dependent men. Am. J. Drug Alc. Abuse 38, 114–119. doi: 10.3109/00952990.2011.600398
Gorzalka, B. B., Hill, M. N., and Hillard, C. J. (2008). Regulation of endocannabinoid signaling by stress: implications for stress-related affective disorders. Neurosci. Biobehav. Rev. 32, 1152–1160. doi: 10.1016/j.neubiorev.2008.03.004
Gouvea, E. S., Santos, A. F., Ota, V. K., Mrad, V., Gadelha, A., Bressan, R. A., et al. (2017). The role of the CNR1 gene in schizophrenia: a systematic review including unpublished data. Rev. Bras. Psiquiatr. 39, 160–171. doi: 10.1590/1516-4446-2016-1969
Gunn, B. G., Brown, A., Lambert, J. L., and Belelli, D. (2011). Neurosteroids and GABA(A) receptor interactions: a focus on stress. Front. Neurosci. 5:131. doi: 10.3389/fnins.2011.00131
Haller, J., Varga, B., Ledent, C., and Freund, T. F. (2004). CB1 cannabinoid receptors mediate anxiolytic effects: convergent genetic and pharmacological evidence with CB1-specific agents. Behav. Pharmacol. 15, 299–304. doi: 10.1097/01.fbp.0000135704.56422.40
Hill, M. N., and Gorzalka, B. B. (2005). Is there a role for the endocannabinoid system in the etiology and treatment of melancholic depression? Behav. Pharmacol. 16, 333–352. doi: 10.1097/00008877-200509000-00006
Hill, M. N., Hillard, C. J., and Mcewen, B. S. (2011). Alterations in corticolimbic dendritic morphology and emotional behavior in cannabinoid CB1 receptor-deficient mice parallel the effects of chronic stress. Cereb. Cortex 21, 2056–2064. doi: 10.1093/cercor/bhq280
Hill, M. N., Mclaughlin, R. J., Bingham, B., Shrestha, L., Lee, T. T. Y., Gray, J. M., et al. (2010). Endogenous cannabinoid signaling is essential for stress adaptation. Proc. Nat. Acad. Sci. U.S.A. 107, 9406–9411. doi: 10.1073/pnas.0914661107
Hillard, C. J., Weinlander, K. M., and Stuhr, K. L. (2012). Contributions of endocannabinoid signaling to psychiatric disorders in humans: genetic and biochemical evidence. Neuroscience 204, 207–229. doi: 10.1016/j.neuroscience.2011.11.020
Hu, W., Zhang, M. Y., Czeh, B., Flugge, G., and Zhang, W. Q. (2010). Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology 35, 1693–1707. doi: 10.1038/npp.2010.31
Inoue, A., Akiyoshi, J., Muronaga, M., Masuda, K., Aizawa, S., Hirakawa, H., et al. (2015). Association of TMEM132D, COMT, and GABRA6 genotypes with cingulate, frontal cortex and hippocampal emotional processing in panic and major depressive disorder. Int. J. Psychiatr. Clin. Pract. 19, 192–200. doi: 10.3109/13651501.2015.1043133
Juhasz, G., Chase, D., Pegg, E., Downey, D., Toth, Z. G., Stones, K., et al. (2009). CNR1 gene is associated with high neuroticism and low agreeableness and interacts with recent negative life events to predict current depressive symptoms. Neuropsychopharmacology 34, 2019–2027. doi: 10.1038/npp.2009.19
Juhasz, G., Csepany, E., Magyar, M., Edes, A. E., Eszlari, N., Hullam, G., et al. (2017). Variants in the CNR1 gene predispose to headache with nausea in the presence of life stress. Genes Brain Behav. 16, 384–393. doi: 10.1111/gbb.12352
Juhasz, G., Gonda, X., Hullam, G., Eszlari, N., Kovacs, D., Lazary, J., et al. (2015). Variability in the effect of 5-HTTLPR on depression in a large European population: the role of age, symptom profile, type and intensity of life stressors. PLoS One 10:e0116316. doi: 10.1371/journal.pone.0116316
Lazary, J., Juhasz, G., Hunyady, L., and Bagdy, G. (2011). Personalized medicine can pave the way for the safe use of CB1 receptor antagonists. Trends Pharmacol. Sci. 32, 270–280. doi: 10.1016/j.tips.2011.02.013
Lazary, J., Lazary, A., Gonda, X., Benko, A., Molnar, E., Hunyady, L., et al. (2009). Promoter variants of the cannabinoid receptor 1 gene (CNR1) in interaction with 5-HTTLPR affect the anxious phenotype. Am. J. Med. Genet. B 150b, 1118–1127. doi: 10.1002/ajmg.b.31024
Lopez-Moreno, J. A., Echeverry-Alzate, V., and Buhler, K. M. (2012). The genetic basis of the endocannabinoid system and drug addiction in humans. J. Psychopharmacol. 26, 133–143. doi: 10.1177/0269881111416689
Luscher, B., Shen, Q., and Sahir, N. (2011). The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 16, 383–406. doi: 10.1038/mp.2010.120
McLaughlin, R. J., Hill, M. N., Bambico, F. R., Stuhr, K. L., Gobbi, G., Hillard, C. J., et al. (2012). Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur. Neuropsychopharmacol. 22, 664–671. doi: 10.1016/j.euroneuro.2012.01.004
McLaughlin, R. J., Hill, M. N., and Gorzalka, B. B. (2014). A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci. Biobehav. Rev. 42, 116–131. doi: 10.1016/j.neubiorev.2014.02.006
Morena, M., and Campolongo, P. (2014). The endocannabinoid system: an emotional buffer in the modulation of memory function. Neurobiol. Learn. Mem. 112, 30–43. doi: 10.1016/j.nlm.2013.12.010
Morena, M., Patel, S., Bains, J. S., and Hill, M. N. (2016). Neurobiological interactions between stress and the endocannabinoid system. Neuropsychopharmacology 41, 80–102. doi: 10.1038/npp.2015.166
Oquendo, M. A., and Mann, J. J. (2000). The biology of impulsivity and suicidality. Psychiatr. Clin. North Am. 23, 11–25. doi: 10.1016/S0193-953x(05)70140-4
Patel, S., and Hillard, C. J. (2008). Adaptations in endocannabinoid signaling in response to repeated homotypic stress: a novel mechanism for stress habituation. Eur. J. Neurosci. 27, 2821–2829. doi: 10.1111/j.1460-9568.2008.06266
Pehrson, A. L., and Sanchez, C. (2015). Altered gamma-aminobutyric acid neurotransmission in major depressive disorder: a critical review of the supporting evidence and the influence of serotonergic antidepressants. Drug Des. Dev. Ther. 9, 603–624. doi: 10.2147/Dddt.S62912
R Core Team (2013). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing.
Rosmond, R., Bouchard, C., and Bjorntorp, P. (2002). Association between a variant at the GABA(A)alpha 6 receptor subunit gene, abdominal obesity, and cortisol secretion. Ann. N. Y. Acad. Sci. 967, 566–570. doi: 10.1111/j.1749-6632.2002.tb04318.x
Sarginson, J. E., Deakin, J. F. W., Anderson, I. M., Downey, D., Thomas, E., Elliott, R., et al. (2014). Neuronal nitric oxide synthase (NOSI) polymorphisms interact with financial hardship to affect depression risk. Neuropsychopharmacology 39, 2857–2866. doi: 10.1038/npp.2014.137
Sen, S., Villafuerte, S., Nesse, R., Stoltenberg, S. F., Hopcian, J., Gleiberman, L., et al. (2004). Serotonin transporter and GABA(A) alpha 6 receptor variants are associated with neuroticism. Biol. Psychiatry 55, 244–249. doi: 10.1016/j.biopsych.2003.08.006
Skilbeck, K. J., Johnston, G. A. R., and Hinton, T. (2010). Stress and GABA(A) receptors. J. Neurochemistry 112, 1115–1130. doi: 10.1111/j.1471-4159.2009.06539.x
Sripada, R. K., Swain, J. E., Evans, G. W., Welsh, R. C., and Liberzon, I. (2014). Childhood poverty and stress reactivity are associated with aberrant functional connectivity in default mode network. Neuropsychopharmacology 39, 2244–2251. doi: 10.1038/npp.2014.75
Steiner, M. A., and Wotjak, C. T. (2008). Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog. Brain Res. 170, 397–432. doi: 10.1016/S0079-6123(08)00433-0
Storey, J. D., Taylor, J. E., and Siegmund, D. (2004). Strong control, conservative point estimation, and simultaneous conservative consistency of false discovery rates: a unified approach. J. R. Stat. Soc. B 66, 187–205. doi: 10.1111/j.1467-9868.2004.00439.x
Tamasi, V., Petschner, P., Adori, C., Kirilly, E., Ando, R. D., and Tothfalusi, L. (2015). Transcriptional evidence for the role of chronic venlafaxine treatment in neurotrophic signaling and neuroplasticity including also glutatmatergic- and insulin-mediated neuronal processes. PLoS One 9:e113662. doi: 10.1371/journal.pone.0123269
Trivedi, M. H., Rush, A. J., Wisniewski, S. R., Nierenberg, A. A., Warden, D., Ritz, L., et al. (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR∗D: implications for clinical practice. Am. J. Psychiatry 163, 28–40. doi: 10.1176/appi.ajp.163.1.28
Uhart, M., Mccaul, M. E., Oswald, L. M., Choi, L., and Wand, G. S. (2004). GABRA6 gene polymorphism and an attenuated stress response. Mol. Psychiatry 9, 998–1006. doi: 10.1038/sj.mp.4001535
Wang, M. N., Hill, M. N., Zhang, L. H., Gorzalka, B. B., Hillard, C. J., and Alger, B. E. (2012). Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J. Psychopharmacology 26, 56–70. doi: 10.1177/0269881111409606
Keywords: types of stress, depression, anxiety, gene-environment interaction, GABA, GABRA6, CNR1, endocannabinoid system
Citation: Gonda X, Petschner P, Eszlari N, Sutori S, Gal Z, Koncz S, Anderson IM, Deakin B, Juhasz G and Bagdy G (2019) Effects of Different Stressors Are Modulated by Different Neurobiological Systems: The Role of GABA-A Versus CB1 Receptor Gene Variants in Anxiety and Depression. Front. Cell. Neurosci. 13:138. doi: 10.3389/fncel.2019.00138
Received: 15 January 2019; Accepted: 20 March 2019;
Published: 09 April 2019.
Edited by:
Boldizsar Czeh, University of Pécs, HungaryReviewed by:
Urs Heilbronner, University of Munich, GermanyCopyright © 2019 Gonda, Petschner, Eszlari, Sutori, Gal, Koncz, Anderson, Deakin, Juhasz and Bagdy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xenia Gonda, Z29uZGEueGVuaWFAbWVkLnNlbW1lbHdlaXMtdW5pdi5odQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.