- 1Department of Neurology, David Geffen School of Medicine, University of California Los Angeles, Los Angeles, CA, USA
- 2Department of Neuroscience, Tufts University School of Medicine, Boston, MA, USA
Stress-derived steroid hormones regulate the expression and function of GABAA receptors (GABAARs). Changes in GABAAR subunit expression have been demonstrated under conditions of altered steroid hormone levels, such as stress, as well as following exogenous steroid hormone administration. In addition to the effects of stress-derived steroid hormones on GABAAR subunit expression, stress hormones can also be metabolized to neuroactive derivatives which can alter the function of GABAARs. Neurosteroids allosterically modulate GABAARs at concentrations comparable to those during stress. In addition to the actions of stress-derived steroid hormones on GABAARs, GABAARs reciprocally regulate the production of stress hormones. The stress response is mediated by the hypothalamic-pituitary-adrenal (HPA) axis, the activity of which is governed by corticotropin releasing hormone (CRH) neurons. The activity of CRH neurons is largely controlled by robust GABAergic inhibition. Recently, it has been demonstrated that CRH neurons are regulated by neurosteroid-sensitive, GABAAR δ subunit-containing receptors representing a novel feedback mechanism onto the HPA axis. Further, it has been demonstrated that neurosteroidogenesis and neurosteroid actions on GABAAR δ subunit-containing receptors on CRH neurons are necessary to mount the physiological response to stress. Here we review the literature describing the effects of steroid hormones on GABAARs as well as the importance of GABAARs in regulating the production of steroid hormones. This review incorporates what we currently know about changes in GABAARs following stress and the role in HPA axis regulation.
GABAARs are regulated by stress-derived steroid hormones and neurosteroids [for review see Belelli et al. (2009); Maguire and Mody (2009); Gunn et al. (2011)]. Conversely, the HPA axis, and thus the production of stress-derived steroid hormones and neurosteroids, is under robust GABAergic control [for review see Herman et al. (2004); Gunn et al. (2011)].
GABAergic Regulation of the HPA Axis
Stress induces a physiological response which is mediated by the HPA axis. CRH is released from the hypothalamus and acts in the pituitary to signal the release of adrenocorticotropic hormone (ACTH), which triggers the release of cortisol from the adrenal gland in humans (corticosterone in mice). The HPA axis is regulated by inputs from numerous different brain regions, involving multiple neurotransmitter systems, as well as the feedback of steroid hormones acting on mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs) [for review see Herman et al. (2003); Larsen et al. (2003); Ulrich-Lai and Herman (2009)]. These inputs impinge on CRH neurons in the paraventricular nucleus (PVN), which mediate the output of the HPA axis. Although CRH neurons receive a wide variety of inputs from diverse brain regions, their activity is ultimately regulated by GABAergic inhibition [for review see Decavel and van den Pol (1990); Herman et al. (2004)].
A role for GABA in HPA axis regulation has been well established. CRH neurons receive robust GABAergic inhibition (Decavel and van den Pol, 1990, 1992) [for review see Herman et al. (2004); Cullinan et al. (2008)]. It has been suggested that a third of the inputs onto CRH neurons are GABAergic and the density of GABAergic synapses in the parvocellular division of the PVN has been estimated to be above 20 × 106 synaptic contacts per mm3 (Miklos and Kovacs, 2002), highlighting the importance of GABAergic inhibition in the regulation of CRH neurons. In addition, microinjection of GABA antagonists, such as bicuculline, into the PVN activates the HPA axis (Cullinan et al., 2008; Marques de and Franci, 2008) and microinfusion of GABA agonists, such as the stress-derived neurosteroid, THDOC, into the PVN decreases circulating levels of stress hormones (Sarkar et al., 2011).
GABA inputs onto CRH neurons originate primarily from local interneurons surrounding the PVN (peri-PVN) as well as from the subparaventricular zone, the anterior hypothalamic area, dorsomedial hypothalamic nucleus, the medial preoptic area, lateral hypothalamic area, and from multiple nuclei within the bed nucleus of the stria terminalis (BNST) (Cullinan et al., 1993; Roland and Sawchenko, 1993) [for review see Herman et al. (2004); Cullinan et al. (2008)]. In addition to the direct inhibitory connections from these brain regions, CRH neurons also receive indirect inhibition from other regulatory brain regions including limbic and cortical regions which exert their influences on CRH neurons via interneuron mediators [for review see Herman et al. (2004); Cullinan et al. (2008)].
Despite the well-established role for GABAergic control of the HPA axis at the level of the PVN, very little is known about the GABAAR subtypes which mediate the GABAergic control over CRH neurons. GABAARs are members of the large “Cys-loop” super-family of evolutionarily related and structurally similar ligand-gated ion channels. To-date, 19 different subunits; α1-6, β1-3, γ1-3, δ, ε, θ, π, and ρ 1-3 have been identified (Barnard et al., 1998; Whiting et al., 1999), which form heteropentameric receptors predominantly composed of 2 αs, 2 βs, and either the γ2 or the δ subunit. Depending on their subunit composition, GABAARs have specific anatomical distributions (Pirker et al., 2000) including subcellular localization (Kittler et al., 2002), kinetics, and pharmacology (Hevers and Luddens, 1998; Mody and Pearce, 2004). GABAARs mediate two distinct forms of GABAergic inhibition, tonic, and phasic, which are mediated by GABAARs with unique subunit assemblies (Farrant and Nusser, 2005). Extrasynaptically localized δ subunit-containing receptors mediate tonic GABAergic inhibition in many brain regions and confer neurosteroid sensitivity (Mihalek et al., 1999; Belelli et al., 2002; Brown et al., 2002; Wohlfarth et al., 2002; Spigelman et al., 2003). Only recently has it been demonstrated that these neurosteroid-sensitive, δ subunit-containing GABAARs play a pivotal role in the regulation of stress reactivity (Sarkar et al., 2011).
Several GABAAR subunits have been identified within the PVN (Fritschy and Mohler, 1995). However, it has been historically difficult to conclusively determine which GABAAR subunits are expressed on the CRH neurons within the PVN due to the inability to specifically identify this subset of neurons within this heterogeneous nucleus. Dual hybridization histochemical studies have demonstrated mRNA expression of the GABAAR α1, α2, β1-3, and γ1-2 subunits in CRH neurons (Cullinan, 2000). Due to the sparse number of studies that have attempted to identify the specific GABAAR subtypes controlling CRH neurons, this list remains incomplete. Information regarding the GABAAR subtypes involved in regulation of CRH neurons will provide insight into pharmacological tools which may modulate HPA axis activity. It has recently been demonstrated that rostral ventrolateral medulla (RVLM)-projecting parvocellular neurons in the PVN are regulated by a THIP-sensitive tonic current (Park et al., 2007), indicating that neurosteroid-sensitive, extrasynaptic δ subunit-containing GABAARs may play a role in the regulation of these neurons (Boehm et al., 2006; Mortensen et al., 2010). Further, recent studies have demonstrated GABAAR δ subunit expression in the PVN and GABAAR δ subunit-mediated tonic GABAergic control of CRH neurons (Sarkar et al., 2011). These findings demonstrate that GABAAR δ subunit-containing receptors on CRH neurons play a role in the regulation of the HPA axis.
Stress-derived steroid hormones can be metabolized to neuroactive derivatives, termed neurosteroids, such as the stress-derived neurosteroid, 3α, 21-dihydroxy-5α-pregnan-20-one (THDOC), and the ovarian-derived neurosteroid, 3α-hydroxy-5α-pregnan-20-one (allopregnanolone). Neurosteroids are positive allosteric modulators of GABAARs (Barker et al., 1986; Majewska et al., 1986; Puia et al., 1990; Purdy et al., 1991; Lambert et al., 1995; Morrow et al., 1995; Hosie et al., 2006; Smith et al., 2007), acting on a neurosteroid binding site identified on GABAARs (Hosie et al., 2006). It has been demonstrated that neurosteroids act preferentially on GABAAR δ subunit-containing receptors (Mihalek et al., 1999; Belelli et al., 2002; Brown et al., 2002; Wohlfarth et al., 2002; Spigelman et al., 2003) at physiologically relevant concentrations (Stell et al., 2003). These data are consistent with previous findings demonstrating changes in GABAAR δ subunit expression in parvocellular neurons in the PVN following stress (Verkuyl et al., 2004), implicating these receptors in the regulation of the stress response. In response to stress, THDOC and allopregnanolone are released at levels which can potently modulate GABAARs (Barker et al., 1986; Majewska et al., 1986; Puia et al., 1990; Purdy et al., 1991; Lambert et al., 1995; Morrow et al., 1995; Barbaccia et al., 1996a, b; Hosie et al., 2006; Smith et al., 2007). Under basal conditions, neurosteroids can exert a negative feedback onto the HPA axis, decreasing CRH and ACTH levels (Patchev et al., 1994, 1996) [for review see Morrow (2007)]. Recent data demonstrate a role for neurosteroid actions on GABAAR δ subunit-containing receptors on CRH neurons in the regulation of the HPA axis (Sarkar et al., 2011), and thus, production of stress hormones. This study demonstrates a decrease in the firing rate of CRH neurons upon the addition of a low concentration of THDOC (10 nM) under basal conditions (Sarkar et al., 2011). Further, the role of the GABAAR δ subunit in the neurosteroid regulation of CRH neurons was confirmed by demonstrating the loss of this regulation in mice lacking the GABAAR δ subunit (Gabrd−/− mice). Together, there is ample evidence that under normal conditions, there is a basal GABAergic inhibition of CRH neurons.
Interestingly, the effects of GABA on CRH neurons are dramatically altered following stress. Stress activates GABAergic neurons which project to the PVN (Cullinan et al., 1995; Campeau and Watson, 1997), which would intuitively suggest inhibition of the HPA axis rather than activation. However, GABA agonists have been shown to increase stress-induced corticosterone levels (Borycz et al., 1992; Sarkar et al., 2011) and blocking production with finasteride has been shown to blunt the corticosterone response to stress (Sarkar et al., 2011). However, due to the fact that both THDOC and allopregnanolone levels are elevated following stress, it isn't clear which of these neurosteroids are responsible for activation of the HPA axis. The role of neurosteroids on GABAAR δ subunit-containing receptors in the activation of the HPA axis following stress (Sarkar et al., 2011), implicates excitatory actions of GABA in regulation of the HPA axis. Recent evidence suggests that there are deficits in GABAergic control of CRH neurons following stress due to a depolarizing shift in the reversal potential for chloride (Cl−) (Hewitt et al., 2009). The inhibitory effects of GABA require the maintenance of the Cl− gradient, which is primarily accomplished by the K+/Cl− co-transporter, KCC2, in the adult brain (Rivera et al., 1999; Payne et al., 2003; Rivera et al., 2005). The surface expression and activity of KCC2 is regulated by phosphorylation of KCC2 residue Ser940 (Lee et al., 2007). Dephosphorylation of KCC2 residue Ser940 and downregulation of KCC2 results in depolarizing and excitatory actions of GABA in vitro (Lee et al., 2011). Recently, it has been demonstrated that KCC2 plays a role in the regulation of the HPA axis (Sarkar et al., 2011). Following stress, there is a dephosphorylation of KCC2 residue Ser940 and downregulation of surface KCC2 expression in the PVN (Sarkar et al., 2011), resulting in excitatory actions of GABA on CRH neurons (Sarkar et al., 2011). Consistent with excitatory actions of GABA on CRH neurons following stress, recent data demonstrate that following acute restraint stress, THDOC increases the activity of CRH neurons and increases the corticosterone response to stress (Sarkar et al., 2011). The GABA-mediated activation of CRH neurons following acute stress is due to a collapse in the chloride gradient as previously demonstrated (Hewitt et al., 2009) and depolarizing and excitatory actions of GABA (Sarkar et al., 2011), overriding the inhibitory constraint of CRH neurons. These data demonstrate dramatic alterations in GABAergic control of CRH neurons following stress mediated by neurosteroids rather than the actions of steroid hormones on MRs or GRs. We propose a model in which rapid dephosphorylation and downregulation of KCC2 is the most efficient mechanism to overcome the robust GABAergic constraint of CRH neurons to mount a rapid, all-or-none stress response (Figure 1) (Sarkar et al., 2011). This model suggests that both downregulation of KCC2, resulting in excitatory actions of GABA and neurosteroid potentiation of GABAAR δ subunit-containing receptors is required to mount the full physiological response to stress.
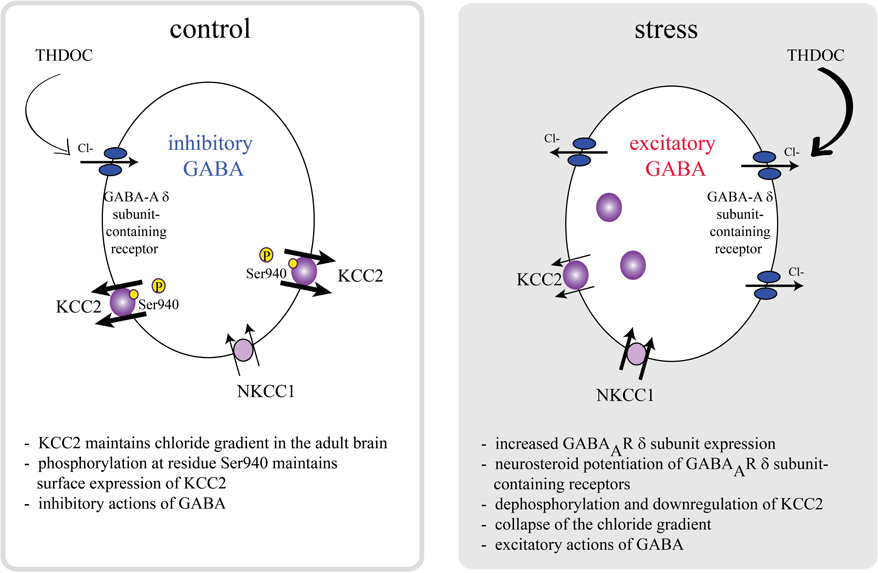
Figure 1. A model of HPA axis regulation. The activity of the HPA axis is regulated by CRH neurons in the PVN, which are under robust GABAergic control. Under normal conditions, KCC2 is phosphorylated at residue Ser940, maintaining a low intracellular Cl− concentration and inhibitory effects of GABA. Further, these neurons are regulated by a neurosteroid-sensitive tonic GABAergic inhibition mediated by GABAAR δ subunit-containing receptors. Following stress, KCC2 residue Ser940 is dephosphorylated and surface KCC2 expression is downregulated, resulting in a collapse in the chloride gradient and excitatory actions of GABA on CRH neurons. Neurosteroid actions on GABAAR δ subunit-containing receptors following stress potentiate the excitatory actions of GABA on CRH neurons. Both the downregulation of KCC2 and excitatory effects of neurosteroids on GABAAR δ subunit-containing receptors are required to mount the full physiological response to stress in a rapid, all-or-none fashion [adapted from Sarkar et al. (2011)].
Stress Hormone Regulation of GABAergic Inhibition
In addition to the well-established role of GABAergic transmission in the regulation of the HPA axis as outlined above, conversely, stress hormones can also alter GABAergic inhibition. This review will focus on changes that occur in adulthood and will not discuss the vast literature documenting changes in GABAergic inhibition resulting from early life stress. For a more in-depth review of the role of neurosteroids in stress, including prenatal stress, see (Gunn et al., 2011).
Acute and chronic stress has been shown to alter the expression of both GAD and GABA (Yoneda et al., 1983; Otero Losada, 1988; Maroulakou and Stylianopoulou, 1991; Acosta et al., 1993; Bowers et al., 1998) [for review see Cullinan et al. (2008)]. Increased GAD65 and GAD67 expression have been demonstrated following stress in brain regions associated with the regulation of the HPA axis, including the anterior hypothalamic area, dorsomedial nucleus, medial preoptic area, suprachiasmatic nucleus, anterior BST, perifornical nucleus, and peri-PVN region [Bowers et al., 1998; for review see Cullinan et al. (2008)]. Despite the upregulation of enzymes responsible for GABA synthesis, the frequency of spontaneous inhibitory postsynaptic currents (sIPSCs) has been shown to be decreased following stress (Verkuyl et al., 2004). Similarly, a high dose of exogenous corticosterone has been shown to decrease mIPSC frequency (Verkuyl et al., 2005) and adrenalectomy increases miniature inhibitory postsynaptic currents (mIPSC) frequency (Verkuyl and Joels, 2003) and the number of GABAergic synapses on CRH neurons (Miklos and Kovacs, 2002). Further, demonstrating presynaptic changes in GABAergic inhibition following stress, the expression of receptors for stress-derived steroid hormones (MRs and GRs) have been identified on GABAergic interneurons in the peri-PVN region and stress hormones have been shown to increase the burst firing of these neurons (Shin et al., 2011). These findings are in contrast with the decreased frequency of both mIPSCs and sIPSCs following stress (Verkuyl et al., 2004) and may represent a compensatory change to restore inhibition in this region following stress. In addition to potential changes in presynaptic GABAergic release suggested by changes in GAD expression and GABA levels, there is also abundant evidence of postsynaptic changes in GABAAR subunit expression associated with stress.
There is reduced [3H]GABA and [35S]TBPS binding following stress suggesting alterations in GABAA receptor (GABAAR) expression (Skerritt et al., 1981; Schwartz et al., 1987; Akinci and Johnston, 1993; Serra et al., 2000) [for review see Skilbeck et al. (2010)]. One thing is for certain, the changes in binding to GABAARs following stress is extremely variable and results differ according to gender, paradigm used, and laboratory where the experiments were conducted. These results leave little certainty regarding changes in radio-labeled ligand binding to GABAARs following stress. Pharmacological changes more consistently point to alterations in GABAAR expression following stress. For example, stress and adrenalectomy have both been shown to alter benzodiazepine binding (Majewska et al., 1985; De Souza et al., 1986; Goeders et al., 1986; Miller et al., 1987, 1988; Weizman et al., 1990; Smith et al., 1992). However complex, these data suggest that there are changes in GABAAR expression associated with stress.
Studies investigating changes in GABAAR subunit expression following stress have demonstrated specific changes in GABAAR subtypes. There are brain region-specific alterations in GABAAR subunit expression following stress, including decreased GABAAR β1 and β2 subunit expression in the PVN following stress, with no change in GABAAR α1, α3, γ1, or γ2 expression (Verkuyl et al., 2004). Consistent with a role of extrasynaptic GABAARs in the regulation of the HPA axis, a significant increase in GABAAR α5 subunit expression and a decrease in GABAAR δ subunit expression have been demonstrated in the PVN following stress (Verkuyl et al., 2004). In the hippocampus, GABAAR β1 and β2 subunit expression is increased (Cullinan and Wolfe, 2000) and GABAAR γ2 subunit expression is decreased (Maguire and Mody, 2007). Increased expression of the predominantly extrasynaptic GABAAR δ subunit was demonstrated in the hippocampus following stress (Maguire and Mody, 2007) [for review see Belelli et al. (2009); Maguire and Mody (2009)] and these changes can by mimicked by treatment with THDOC (Maguire and Mody, 2007). Although the exact mechanisms underlying alterations in GABAAR subunit expression associated with stress are not fully understood, it is thought that these changes are mediated by the actions of stress hormones and/or stress-derived neurosteroids.
Both steroid hormones and neurosteroids are elevated in response to acute stress (Majewska et al., 1985; Purdy et al., 1991; Barbaccia et al., 1996a, b). Acute stress induces an elevation in circulating levels of THDOC from 1–5 nM to 15–30 nM (Reddy and Rogawski, 2002) [for review see Reddy (2003)]. Stress can increase neurosteroid levels to concentrations which can act directly on GABAARs to both potentiate the effects of GABA (Purdy et al., 1991; Barbaccia et al., 1996b) as well as alter GABAAR subunit expression (Maguire and Mody, 2007). Neurosteroids can potentiate the tonic component of GABAergic inhibition via action on GABAAR δ subunit-containing receptors at low concentrations (Stell et al., 2003), can potentiate the phasic component of GABAergic inhibition at higher concentrations, and at very high concentrations have even been shown to directly gate the receptor [for review see Lambert et al. (2009)]. In addition to the potentiation of GABAergic transmission by neurosteroids, steroid hormones themselves can alter synaptic GABAergic transmission (Maggio and Segal, 2009). Corticosterone alters the frequency of spontaneous sIPSCs in the hippocampus via actions on MRs (Maggio and Segal, 2009) and increases the amplitude of sIPSCs via actions on GRs (Maggio and Segal, 2009). Neurosteroidogenesis has been demonstrated to be essential for steroid hormone-linked alterations in GABAAR subunit expression (Maguire and Mody, 2007). These alterations in GABAAR subunit expression following stress are likely mediated by neurosteroid-mediated effects on GABAAR phosphorylation (Brussaard and Koksma, 2003), which controls GABAAR expression [for review see Kittler and Moss (2003)]. These data demonstrate the complex actions of both steroid hormones and neurosteroids on GABAARs via direct modulation or by altering receptor expression.
The findings highlighted in this review demonstrate a reciprocal regulation of stress hormones and GABA receptors, in that GABAergic transmission plays a key role in the regulation of the HPA axis and the production of stress hormones and stress-derived neurosteroids can alter GABAAR subunit expression as well as directly modulate GABAergic transmission.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Acosta, G. B., Otero Losada, M. E., and Rubio, M. C. (1993). Area-dependent changes in GABAergic function after acute and chronic cold stress. Neurosci. Lett. 154, 175–178.
Akinci, M. K., and Johnston, G. A. (1993). Sex differences in acute swim stress-induced changes in the binding of MK-801 to the NMDA subclass of glutamate receptors in mouse forebrain. J. Neurochem. 61, 2290–2293.
Barbaccia, M. L., Concas, A., Roscetti, G., Bolacchi, F., Mostallino, M. C., Purdy, R. H., and Biggio, G. (1996a). Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol. Biochem. Behav. 54, 205–210.
Barbaccia, M. L., Roscetti, G., Trabucchi, M., Mostallino, M. C., Concas, A., Purdy, R. H., and Biggio, G. (1996b). Time-dependent changes in rat brain neuroactive steroid concentrations and GABA(A) receptor function after acute stress. Neuroendocrinology 63, 166–172.
Barker, J. L., Harrison, N. L., Meyers, D. E. R., and Majewska, M. D. (1986). Steroid modulation of Gaba-A receptor-coupled C1- conductance. Clin. Neuropharmacol. 9, 392–394.
Barnard, E. A., Skolnick, P., Olsen, R. W., Mohler, H., Sieghart, W., Biggio, G., Braestrup, C., Bateson, A. N., and Langer, S. Z. (1998). International union of pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol. Rev. 50, 291–313.
Belelli, D., Casula, A., Ling, A., and Lambert, J. J. (2002). The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology 43, 651–661.
Belelli, D., Harrison, N. L., Maguire, J., Macdonald, R. L., Walker, M. C., and Cope, D. W. (2009). Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 29, 12757–12763.
Boehm, S. L., Homanics, G. E., Blednov, Y. A., and Harris, R. A. (2006). delta-subunit containing GABAA receptor knockout mice are less sensitive to the actions of 4,5,6,7-tetrahydroisoxazolo-[5,4-c]pyridin-3-ol. Eur. J. Pharmacol. 541, 158–162.
Borycz, J., Borycz, J. A., and Bugajski, J. (1992). Effect of gamma-aminobutyric acid and muscimol on corticosterone secretion in rats. J. Physiol. Pharmacol. 43, 259–269.
Bowers, G., Cullinan, W. E., and Herman, J. P. (1998). Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J. Neurosci. 18, 5938–5947.
Brown, N., Kerby, J., Bonnert, T. P., Whiting, P. J., and Wafford, K. A. (2002). Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta GABA(A) receptors. Br. J. Pharmacol. 136, 965–974.
Brussaard, A. B., and Koksma, J. J. (2003). Conditional regulation of neurosteroid sensitivity of GABAA receptors. Ann. N.Y. Acad. Sci. 1007, 29–36.
Campeau, S., and Watson, S. J. (1997). Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J. Neuroendocrinol. 9, 577–588.
Cullinan, W. E. (2000). GABA(A) receptor subunit expression within hypophysiotropic CRH neurons: a dual hybridization histochemical study. J. Comp. Neurol. 419, 344–351.
Cullinan, W. E., Herman, J. P., Battaglia, D. F., Akil, H., and Watson, S. J. (1995). Pattern and time course of immediate early gene expression in rat brain following acute stress. Neuroscience 64, 477–505.
Cullinan, W. E., Herman, J. P., and Watson, S. J. (1993). Ventral subicular interaction with the hypothalamic paraventricular nucleus: evidence for a relay in the bed nucleus of the stria terminalis. J. Comp. Neurol. 332, 1–20.
Cullinan, W. E., and Wolfe, T. J. (2000). Chronic stress regulates levels of mRNA transcripts encoding beta subunits of the GABA(A) receptor in the rat stress axis. Brain Res. 887, 118–124.
Cullinan, W. E., Ziegler, D. R., and Herman, J. P. (2008). Functional role of local GABAergic influences on the HPA axis. Brain Struct. Funct. 213, 63–72.
De Souza, E. B., Goeders, N. E., and Kuhar, M. J. (1986). Benzodiazepine receptors in rat brain are altered by adrenalectomy. Brain Res. 381, 176–181.
Decavel, C., and van den Pol, A. N. (1990). GABA: a dominant neurotransmitter in the hypothalamus. J. Comp. Neurol. 302, 1019–1037.
Decavel, C., and van den Pol, A. N. (1992). Converging GABA- and glutamate-immunoreactive axons make synaptic contact with identified hypothalamic neurosecretory neurons. J. Comp. Neurol. 316, 104–116.
Farrant, M., and Nusser, Z. (2005). Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat. Rev. Neurosci. 6, 215–229.
Fritschy, J. M., and Mohler, H. (1995). GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J. Comp. Neurol. 359, 154–194.
Goeders, N. E., De Souza, E. B., and Kuhar, M. J. (1986). Benzodiazepine receptor GABA ratios: regional differences in rat brain and modulation by adrenalectomy. Eur. J. Pharmacol. 129, 363–366.
Gunn, B. G., Brown, A. R., Lambert, J. J., and Belelli, D. (2011). Neurosteroids and GABA(A) receptor interactions: a focus on stress. Front. Neurosci. 5, 131. doi: 10.3389/fnins.2011.00131
Herman, J. P., Figueiredo, H., Mueller, N. K., Ulrich-Lai, Y., Ostrander, M. M., Choi, D. C., and Cullinan, W. E. (2003). Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front. Neuroendocrinol. 24, 151–180.
Herman, J. P., Mueller, N. K., and Figueiredo, H. (2004). Role of GABA and glutamate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann. N.Y. Acad. Sci. 1018, 35–45.
Hevers, W., and Luddens, H. (1998). The diversity of GABA(A) receptors – pharmacological and electrophysiological properties of GABA(A) channel subtypes. Mol. Neurobiol. 18, 35–86.
Hewitt, S. A., Wamsteeker, J. I., Kurz, E. U., and Bains, J. S. (2009). Altered chloride homeostasis removes synaptic inhibitory constraint of the stress axis. Nat. Neurosci. 12, 438–443.
Hosie, A. M., Wilkins, M. E., da Silva, H. M., and Smart, T. G. (2006). Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature 444, 486–489.
Kittler, J. T., McAinsh, K., and Moss, S. J. (2002). Mechanisms of GABAA receptor assembly and trafficking: implications for the modulation of inhibitory neurotransmission. Mol. Neurobiol. 26, 251–268.
Kittler, J. T., and Moss, S. J. (2003). Modulation of GABA(A) receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr. Opin. Neurobiol. 13, 341–347.
Lambert, J. J., Belelli, D., HillVenning, C., and Peters, J. A. (1995). Neurosteroids and Gaba(A) receptor function. Trends Pharmacol. Sci. 16, 295–303.
Lambert, J. J., Cooper, M. A., Simmons, R. D., Weir, C. J., and Belelli, D. (2009). Neurosteroids: endogenous allosteric modulators of GABA(A) receptors. Psychoneuroendocrinology 34(Suppl. 1), S48–S58.
Larsen, P. J., Seier, V., Fink-Jensen, A., Holst, J. J., Warberg, J., and Vrang, N. (2003). Cocaine- and amphetamine-regulated transcript is present in hypothalamic neuroendocrine neurones and is released to the hypothalamic-pituitary portal circuit. J. Neuroendocrinol. 15, 219–226.
Lee, H. H., Deeb, T. Z., Walker, J. A., Davies, P. A., and Moss, S. J. (2011). NMDA receptor activity downregulates KCC2 resulting in depolarizing GABA(A) receptor-mediated currents. Nat. Neurosci. 14, 736–743.
Lee, H. H., Walker, J. A., Williams, J. R., Goodier, R. J., Payne, J. A., and Moss, S. J. (2007). Direct protein kinase C-dependent phosphorylation regulates the cell surface stability and activity of the potassium chloride cotransporter KCC2. J. Biol. Chem. 282, 29777–29784.
Maggio, N., and Segal, M. (2009). Differential corticosteroid modulation of inhibitory synaptic currents in the dorsal and ventral hippocampus. J. Neurosci. 29, 2857–2866.
Maguire, J., and Mody, I. (2007). Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J. Neurosci. 27, 2155–2162.
Maguire, J., and Mody, I. (2009). Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology 29, 9592–9601.
Majewska, M. D., Bisserbe, J. C., and Eskay, R. L. (1985). Glucocorticoids are modulators of GABAA receptors in brain. Brain Res. 339, 178–182.
Majewska, M. D., Harrison, N. L., Schwartz, R. D., Barker, J. L., and Paul, S. M. (1986). Steroid-hormone metabolites are barbiturate-like modulators of the Gaba receptor. Science 232, 1004–1007.
Maroulakou, I. G., and Stylianopoulou, F. (1991). The effects of adrenalectomy and thermal stress on glutamic acid decarboxylase activity in different regions of the rat brain. Neurochem. Res. 16, 1265–1268.
Marques de, S. L., and Franci, C. R. (2008). GABAergic mediation of stress-induced secretion of corticosterone and oxytocin, but not prolactin, by the hypothalamic paraventricular nucleus. Life Sci. 83, 686–692.
Mihalek, R. M., Banerjee, P. K., Korpi, E. R., Quinlan, J. J., Firestone, L. L., Mi, Z. P., Lagenaur, C., Tretter, V., Sieghart, W., Anagnostaras, S. G., Sage, J. R., Fanselow, M. S., Guidotti, A., Spigelman, I., Li, Z. W., DeLorey, T. M., Olsen, R. W., and Homanics, G. E. (1999). Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc. Natl. Acad. Sci. U.S.A. 96, 12905–12910.
Miklos, I. H., and Kovacs, K. J. (2002). GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy. Neuroscience 113, 581–592.
Miller, L. G., Greenblatt, D. J., Barnhill, J. G., Thompson, M. L., and Shaderh, R. I. (1988). Modulation of benzodiazepine receptor binding in mouse brain by adrenalectomy and steroid replacement. Brain Res. 446, 314–320.
Miller, L. G., Thompson, M. L., Greenblatt, D. J., Deutsch, S. I., Shader, R. I., and Paul, S. M. (1987). Rapid increase in brain benzodiazepine receptor binding following defeat stress in mice. Brain Res. 414, 395–400.
Mody, I., and Pearce, R. A. (2004). Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 27, 569–575.
Morrow, A. L. (2007). Recent developments in the significance and therapeutic relevance of neuroactive steroids – introduction to the special issue. Pharmacol. Ther. 116, 1–6.
Morrow, A. L., Devaud, L. L., Purdy, R. H., and Paul, S. M. (1995). Neuroactive steroid modulators of the stress response. Stress 771, 257–272.
Mortensen, M., Ebert, B., Wafford, K., and Smart, T. G. (2010). Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J. Physiol. 588, 1251–1268.
Otero Losada, M. E. (1988). Changes in central GABAergic function following acute and repeated stress. Br. J. Pharmacol. 93, 483–490.
Park, J. B., Skalska, S., Son, S., and Stern, J. E. (2007). Dual GABAA receptor-mediated inhibition in rat presympathetic paraventricular nucleus neurons. J. Physiol. 582, 539–551.
Patchev, V. K., Hassan, A. H., Holsboer, D. F., and Almeida, O. F. (1996). The neurosteroid tetrahydroprogesterone attenuates the endocrine response to stress and exerts glucocorticoid-like effects on vasopressin gene transcription in the rat hypothalamus. Neuropsychopharmacology 15, 533–540.
Patchev, V. K., Shoaib, M., Holsboer, F., and Almeida, O. F. (1994). The neurosteroid tetrahydroprogesterone counteracts corticotropin-releasing hormone-induced anxiety and alters the release and gene expression of corticotropin-releasing hormone in the rat hypothalamus. Neuroscience 62, 265–271.
Payne, J. A., Rivera, C., Voipio, J., and Kaila, K. (2003). Cation-chloride co-transporters in neuronal communication, development and trauma. Trends Neurosci. 26, 199–206.
Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W., and Sperk, G. (2000). GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850.
Puia, G., Santi, M. R., Vicini, S., Pritchett, D. B., Purdy, R. H., Paul, S. M., Seeburg, P. H., and Costa, E. (1990). Neurosteroids act on recombinant human Gaba-A receptors. Neuron 4, 759–765.
Purdy, R. H., Morrow, A. L., Moore, P. H., and Paul, S. M. (1991). Stress-induced elevations of gamma-aminobutyric-acid type-A receptor-active steroids in the rat-brain. Proc. Natl. Acad. Sci. U.S.A. 88, 4553–4557.
Reddy, D. S. (2003). Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol. Sci. 24, 103–106.
Reddy, D. S., and Rogawski, M. A. (2002). Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J. Neurosci. 22, 3795–3805.
Rivera, C., Voipio, J., and Kaila, K. (2005). Two developmental switches in GABAergic signalling: the K+-Cl- cotransporter KCC2 and carbonic anhydrase CAVII. J. Physiol. 562, 27–36.
Rivera, C., Voipio, J., Payne, J. A., Ruusuvuori, E., Lahtinen, H., Lamsa, K., Pirvola, U., Saarma, M., and Kaila, K. (1999). The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255.
Roland, B. L., and Sawchenko, P. E. (1993). Local origins of some GABAergic projections to the paraventricular and supraoptic nuclei of the hypothalamus in the rat. J. Comp. Neurol. 332, 123–143.
Sarkar, J., Wakefield, S., Mackenzie, G., Moss, S. J., and Maguire, J. (2011). Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J. Neurosci. 31, 18198–18210.
Schwartz, R. D., Wess, M. J., Labarca, R., Skolnick, P., and Paul, S. M. (1987). Acute stress enhances the activity of the Gaba receptor-gated chloride-ion channel in brain. Brain Res. 411, 151–155.
Serra, M., Pisu, M. G., Littera, M., Papi, G., Sanna, E., Tuveri, F., Usala, L., Purdy, R. H., and Biggio, G. (2000). Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J. Neurochem. 75, 732–740.
Shin, S. Y., Han, T. H., Lee, S. Y., Han, S. K., Park, J. B., Erdelyi, F., Szabo, G., and Ryu, P. D. (2011). Direct corticosteroid modulation of GABAergic neurons in the anterior hypothalamic area of GAD65-eGFP mice. Korean J. Physiol. Pharmacol. 15, 163–169.
Skerritt, J. H., Trisdikoon, P., and Johnston, G. A. (1981). Increased GABA binding in mouse brain following acute swim stress. Brain Res. 215, 398–403.
Skilbeck, K. J., Johnston, G. A., and Hinton, T. (2010). Stress and GABA receptors. J. Neurochem. 112, 1115–1130.
Smith, C. C., Hauser, E., Renaud, N. K., Leff, A., Aksentijevich, S., Chrousos, G. P., Wilder, R. L., Gold, P. W., and Sternberg, E. M. (1992). Increased hypothalamic [3H]flunitrazepam binding in hypothalamic-pituitary-adrenal axis hyporesponsive Lewis rats. Brain Res. 569, 295–299.
Smith, S. S., Shen, H., Gong, Q. H., and Zhou, X. (2007). Neurosteroid regulation of GABA(A) receptors: focus on the alpha4 and delta subunits. Pharmacol. Ther. 116, 58–76.
Spigelman, I., Li, Z. W., Liang, J., Cagetti, E., Samzadeh, S., Mihalek, R. M., Homanics, G. E., and Olsen, R. W. (2003). Reduced inhibition and sensitivity to neurosteroids in hippocampus of mice lacking the GABA(A) receptor delta subunit. J. Neurophysiol. 90, 903–910.
Stell, B. M., Brickley, S. G., Tang, C. Y., Farrant, M., and Mody, I. (2003). Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA(A) receptors. Proc. Natl. Acad. Sci. U.S.A. 100, 14439–14444.
Ulrich-Lai, Y. M., and Herman, J. P. (2009). Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 10, 397–409.
Verkuyl, J. M., Hemby, S. E., and Joels, M. (2004). Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur. J. Neurosci. 20, 1665–1673.
Verkuyl, J. M., and Joels, M. (2003). Effect of adrenalectomy on miniature inhibitory postsynaptic currents in the paraventricular nucleus of the hypothalamus. J. Neurophysiol. 89, 237–245.
Verkuyl, J. M., Karst, H., and Joels, M. (2005). GABAergic transmission in the rat paraventricular nucleus of the hypothalamus is suppressed by corticosterone and stress. Eur. J. Neurosci. 21, 113–121.
Weizman, A., Weizman, R., Kook, K. A., Vocci, F., Deutsch, S. I., and Paul, S. M. (1990). Adrenalectomy prevents the stress-induced decrease in in vivo [3H]Ro15-1788 binding to GABAA benzodiazepine receptors in the mouse. Brain Res. 519, 347–350.
Whiting, P. J., Bonnert, T. P., McKernan, R. M., Farrar, S., Le Bourdelles, B., Heavens, R. P., Smith, D. W., Hewson, L., Rigby, M. R., Sirinathsinghji, D. J. S., Thompson, S. A., and Wafford, K. A. (1999). Molecular and functional diversity of the expanding GABA-A receptor gene family. Molecular and functional diversity of ion channels and receptors. Ann. N.Y. Acad. Sci. 868, 645–653.
Wohlfarth, K. M., Bianchi, M. T., and Macdonald, R. L. (2002). Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J. Neurosci. 22, 1541–1549.
Keywords: GABA, stress, inhibition, corticosterone, CRH
Citation: Mody I and Maguire J (2012) The reciprocal regulation of stress hormones and GABAA receptors. Front. Cell. Neurosci. 6:4.doi: 10.3389/fncel.2012.00004
Received: 22 December 2011; Paper pending published: 10 January 2012;
Accepted: 13 January 2012; Published online: 30 January 2012.
Edited by:
Nicola Maggio, The Chaim Sheba Medical Center, IsraelReviewed by:
Menahem Segal, Weizman Institute for Science, IsraelNicola Maggio, The Chaim Sheba Medical Center, Israel
Copyright: © 2012 Mody and Maguire. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Jamie Maguire, Department of Neuroscience, Tufts University School of Medicine, 136 Harrison Ave., SC205, Boston, MA 02111, USA. e-mail:SmFtaWUuTWFndWlyZUB0dWZ0cy5lZHU=

