- 1Institut für Physiologie I, Westfälische Wilhelms-Universität Münster, Münster, Germany
- 2Institut für Physiologie, Otto-von-Guericke-Universität, Magdeburg, Germany
- 3Max-Planck-Institut für Herz- und Lungenforschung, Abteilung Pharmakologie, Bad Nauheim, Germany
- 4Department of Neurology, Inflammatory Disorders of the Nervous System and Neurooncology, Westfälische Wilhelms-Universität Münster, Münster, Germany
In thalamocortical relay (TC) neurons, G-protein-coupled receptors play an important part in the control of activity modes. A conditional Gαq knockout on the background of a constitutive Gα11 knockout (Gαq/Gα11−/−) was used to determine the contribution of Gq/G11 family G-proteins to metabotropic serotonin (5-HT) and glutamate (Glu) function in the dorsal part of the lateral geniculate nucleus (dLGN). In control mice, current clamp recordings showed that α-m-5-HT induced a depolarization of Vrest which was sufficient to suppress burst firing. This depolarization was concentration-dependent (100 μM: +6 ± 1 mV, n = 10; 200 μM: +10 ± 1 mV, n = 7) and had a conditioning effect on the activation of other Gαq-mediated pathways. The depolarization was significantly reduced in Gαq/Gα11−/− (100 μM: 3 ± 1 mV, n = 11; 200 μM: 5 ± 1 mV, n = 6) and was apparently insufficient to suppress burst firing. Activating Gαq-coupled muscarinic receptors affected the magnitude of α-m-5-HT-induced effects in a reciprocal manner. Furthermore, the depolarizing effect of mGluR1 agonists was significantly reduced in Gαq/Gα11−/− mice. Immunohistochemical stainings revealed binding of 5-HT2CR- and mGluR1α-, but not of 5-HT2AR-specific antibodies in the dLGN of Gαq/Gα11−/− mice. In conclusion, these findings demonstrate that transmitters of ascending brainstem fibers and corticofugal fibers both signal via a central element in the form of Gq/G11-mediated pathways to control activity modes in the TC system.
Introduction
The thalamocortical (TC) network takes up two states of activity: slow and highly synchronized oscillatory burst activity during slow wave sleep, and tonic generation of action potentials alongside fast oscillations during mental alertness and REM sleep. Slow oscillatory activity has a frequency of <15 Hz, while fast oscillations occur at ∼40 Hz (Steriade et al., 1997). Chemically-coded projections from the brainstem activate the forebrain by releasing acetylcholine (ACh), noradrenalin (NA), and serotonin (5-HT). These neurotransmitters mainly act on G-protein-coupled membrane receptors (McCormick, 1992a). In a similar way, the arousing action of glutamate, released from corticothalamic axons, is mediated by metabotropic glutamate receptors (mGluR) (Salt, 2002). A common action of these neurotransmitters is a depolarizing shift of the membrane potential of TC neurons, causing rhythmic bursting to cease and tonic activity to commence. Membrane depolarization is caused to a large part by the inhibition of a leak K+ conductance (IKL), the molecular correlate of which are the two pore-domain K+ (K2P) channels TASK-1 and TASK-3 (Meuth et al., 2003, 2006). The activation of muscarinic ACh receptors (mAChR) and mGluR1 coupled to Gq/G11 inhibits TASK-1 and TASK-3 (Exton, 1996; Chemin et al., 2003; Lopes et al., 2005; Chen et al., 2006) in a similar fashion as IKL (McCormick, 1992a; Salt, 2002).
Recently, it has been shown that the reduction of the standing outward current (ISO), and the depolarization induced by the activation of m1AChR and m3AChR, depends on the action of Gαq in TC neurons (Broicher et al., 2008b). Acetylcholine is one of several neurotransmitters that play a major role in the modulation of thalamic states of activity. Thus, we were interested in other neurotransmitter systems depending on the presence of Gαq and involved in Gαq-mediated signaling in TC neurons. We used the same genetic strategy to investigate the role of mGluR and metabotropic 5-HT receptors (5-HTR) in conditional forebrain-specific Gαq/Gα11-double-deficient mice (Gαq/Gα11−/−) (Wettschureck et al., 2004b).
Materials and Methods
Mice
All animal handling and procedures were approved by the local authorities. Generation of forebrain-specific Gαq/Gα11 deficient mice and genotyping of the gnaqflox allele, of gna11-wildtype and -knockout alleles, and of the Cre-transgene has been described previously (Wettschureck et al., 2001, 2004b). The genetic background was predominantly C57BL6/N (4th generation backcross). As controls, littermates with the genotype Camkcre4−/−; gnaqfl/fl; gna11−/− (named Gα11−/− in the following) were used. Former studies have shown that no significant differences exist between Camkcre4−/−; gnaqfl/fl; gna11−/− and non-littermate C57BL6/N mice (Offermanns et al., 1998; Broicher et al., 2008b). For this reason, we have also included control experiments with C57BL6/N mice.
Preparation of Brain Slices for Electrophysiological Experiments
At postnatal days 18–24 (Gα11−/− and Gαq/Gα11−/−) or 11–28 (C57BL6/N) mice were deeply anesthetized using isoflurane and decapitated as described earlier (Meuth et al., 2006). Briefly, thalamic slices were prepared as coronal sections on a vibratome (Series 1000 Classic, St. Louis, USA) in an ice-chilled solution containing (in mM): Sucrose, 200; PIPES, 20; KCl, 2.5; NaH2PO4, 1.25; MgSO4, 10; CaCl2, 0.5; dextrose, 10. The pH was adjusted to 7.35 with NaOH. Prior to recording, slices were kept at room temperature submerged in artificial cerebrospinal fluid (ACSF) that contained (in mM): NaCl, 125; KCl, 2.5; NaH2PO4, 1.25; NaHCO3, 24; MgSO4, 2; CaCl2, 2; dextrose, 10. The pH was adjusted to 7.35 by gassing with carbogen (95% O2, 5% CO2).
Patch-Clamp Recordings
Whole-cell recording pipettes (2–3 MΩ) were prepared from borosilicate glass capillaries [GT150T-10(F), Clark Electromedical Instruments, Pangbourne, UK] and filled with an intracellular solution containing (in mM): K-gluconate, 95; K3-citrate, 20; NaCl, 10; HEPES, 10; MgCl2, 1; CaCl2, 0.5; BAPTA, 3; Mg-ATP, 3; Na-GTP, 0.5. The pH was adjusted to 7.25 with KOH, the osmolality was 295 mOsm/kg. In the recording chamber, slices were continuously superfused with a solution containing (in mM): NaCl, 120; KCl, 2.5; NaH2PO4, 1.25; HEPES, 30; MgSO4, 2; CaCl2, 2; dextrose, 10. The pH was adjusted to 7.25 with HCl and osmolality was 305 mOsm/kg. Recordings were performed at room temperature. Whole-cell patch-clamp electrodes were attached to an EPC-10 amplifier (HEKA Elektronik, Lamprecht, Germany), and digitized signals were saved to a computer using Pulse software (HEKA). During current clamp recordings, the instantaneous frequency (fi) of action potential generation was determined by analyzing the first two action potentials that were generated upon a depolarizing current pulse.
Recordings were only performed on recombined TC neurons. We distinguished these from non-recombined GABAergic interneurons based on our previously established physiological and morphological criteria (Broicher et al., 2008a). All cells had a resting membrane potential negative of −60 mV, the access resistance was in the range of 5–15 MΩ and series resistance compensation of 30% or more was routinely applied. A liquid junction potential of 8 ± 2 mV (n = 10) was measured and taken into account.
All results are presented as mean ± SEM and differences were considered significant when p < 0.05. Substance effects were tested for statistical significance using a modified Student’s t-test for small samples.
Drugs
(±)-1-Aminocyclopentane-trans-1,3-dicarboxylic acid (t-ACPD), (RS)-3,5-Dihydroxyphenylglycine (DHPG) and α-methyl-5-hydroxytryptamine (α-m-5-HT) were obtained from Tocris (Biozol, Eching, Germany) or from Biotrend (Biotrend Chemikalien GmbH, Cologne, Germany). Muscarine was obtained from Sigma-Aldrich (Sigma, Deisenhofen, Germany) and CP 809101 was purchased from Biozol (Eching, Germany). Drugs were prepared as stock solutions in distilled water or directly added to the perfusion medium.
Immunofluorescence
Gαq/Gα11−/− mice (postnatal days 18–27) were deeply anesthetized using pentobarbital (50 mg/kg body weight) and transcardially perfused with PBS, followed by an ice-cold 4% PFA/PBS for 35–40 min. Brains were removed, postfixed for 4 h in 4% PFA/PBS and cryoprotected with 25% sucrose. Coronal sections (40 μm) were cut at the level of the dLGN, washed several times with TBS, and blocked with 10% normal horse serum (NHS), 2% BSA, and 0.3% Triton X-100 in TBS for 2 h to minimize non-specific binding before incubation of slices with primary antibodies in 2% NHS, 2% BSA, and 0.3% Triton X-100 in TBS at 4°C for 16–18 h. The following antibodies were used: rabbit anti-5-HT2A (1:500, ImmunoStar, Hudson, WI, USA), rabbit anti-5-HT2C (1:1000, ImmunoStar), rabbit anti-mGluR1a (1:1000, Novus Biologicals Inc., Littleton, CO, USA), mouse anti-NeuN (1:150, Millipore, Schwalbach, Germany), and mouse anti-MAP2 (1:200, Sigma). After washing (3 × 10 min with TBS), sections were exposed to Cy2- or Cy3-conjugated donkey-IgG (1:300, Dianova, Germany) for 1.5 h, washed again, and cover slipped with Immumount. For negative controls, occlusion of the primary antibody from the staining procedure was routinely performed with no positive immunological signal detected. Densitometric analysis of immunofluorescence was performed by using the fluorescence measuring function of ImageJ software (public domain, National Institutes of Health). One hundred square areas (30 μm × 30 μm) were placed on different positions of dLGN images and the mean fluorescence intensity was determined after background subtraction. This analysis was repeated for three slices taken from different animals.
Results
5-HT receptor Signaling and Competition between Different Receptor Classes in Gαq/Gα11−/− mice
In order to mimic an arousal by brainstem and cortical inputs to the thalamus, we have chosen the paradigm described in the following. A hyperpolarized membrane potential negative to −70 mV is necessary to achieve burst firing of TC neurons in response to a depolarizing current step in vivo (Steriade, 1991), and thus, cells were held at about −71 mV by DC current injection. Rather small sleep-related variations of the membrane potential (∼10 mV) found in TC neurons are sufficient to mediate a switch between burst and tonic firing in vivo (Hirsch et al., 1983). The experiments described below tested to what extent the knockout of Gαq has affected the ability of TC neurons to perform this switch.
Acetylcholine plays a major role in the modulation of thalamic states of activity and the function of this transmitter depends on Gαq-coupled muscarinic receptors. However, it is unknown which G-proteins are targeted by the other brainstem neurotransmitters. Thus, we tested neurotransmitter candidates expected to be connected to Gq/G11-mediated signaling pathways. Because of the known coupling of 5-HT2 receptors to Gq/G11 family G-proteins (Roth et al., 1998), we applied the 5-HT2 receptor agonist α-m-5-HT and monitored changes in TC neurons under current clamp conditions. A shift from burst to tonic firing relies on the initial membrane potential. In order to provide comparable conditions between neurons with a different resting membrane potential and to ensure robust bursting with two or more action potentials riding on top of a low-threshold Ca2+ spike (LTS), all TC neurons investigated here were set to a membrane potential of −70.7 ± 0.2 mV (n = 70) by DC offset. The resting membrane potential was −69.4 ± 0.9 mV and the applied DC offset current was −10 ± 2.4 pA (n = 70). Of 70 neurons investigated under these conditions, only 1 had a resting membrane potential positive to −65 mV (−63 mV) and almost all (68 of 70 TC neurons) were able to generate a LTS. Cells with a membrane potential positive to −65 mV and cells that could not generate a LTS were excluded from further analysis. Step depolarization resulted in high-frequency burst firing in both mice genotypes (Gα11−/−: fi = 151 ± 7 Hz, n = 17; Gαq/Gα11−/−: fi = 159 ± 8 Hz, n = 17; Figure 1A). In Gα11−/−, the application of 100 and 200 μM α-m-5-HT depolarized the resting membrane potential of TC neurons by 6 ± 1 mV (n = 10) and 10 ± 1 mV (n = 7), respectively (Figure 1B). We also tested the effect of a more specific agonist for 5-HT2C receptors (CP 809101) on the membrane potential in current clamp recordings (Siuciak et al., 2007). This agonist produced comparable depolarizations (10 μM: 8.3 ± 2.0 mV, n = 5; 100 μM: 17.5 ± 1.4 mV, n = 4). This suggests that 5-HT2C receptors in the dLGN could be responsible for a large portion of the observed effect. The response to α-m-5-HT was significantly reduced in Gαq/Gα11−/− (100 μM: 3 ± 1 mV, n = 11, p < 0.001; 200 μM: 5 ± 1 mV, n = 6, p < 0.01). In the presence of 200 μM α-m-5-HT, depolarizing current steps elicited tonic firing in Gα11−/− (fi = 69 ± 14 Hz, n = 7; data not shown). During application of 100 μM α-m-5-HT in Gαq/Gα11−/− burst firing was either preserved (fi = 126 ± 6 Hz, n = 4) or the LTS was crowned by a single action potential (n = 7, Figure 1A, lower right trace). For the other two recording conditions (100 μM in controls, 200 μM in Gαq/Gα11−/−) depolarizing current steps either evoked an intermediate response with slow bursting (fi ≈ 100 Hz) followed by 1–4 tonic action potentials (not shown) or passive membrane responses (Figure 1A, upper right trace). See also Sherman (1996).
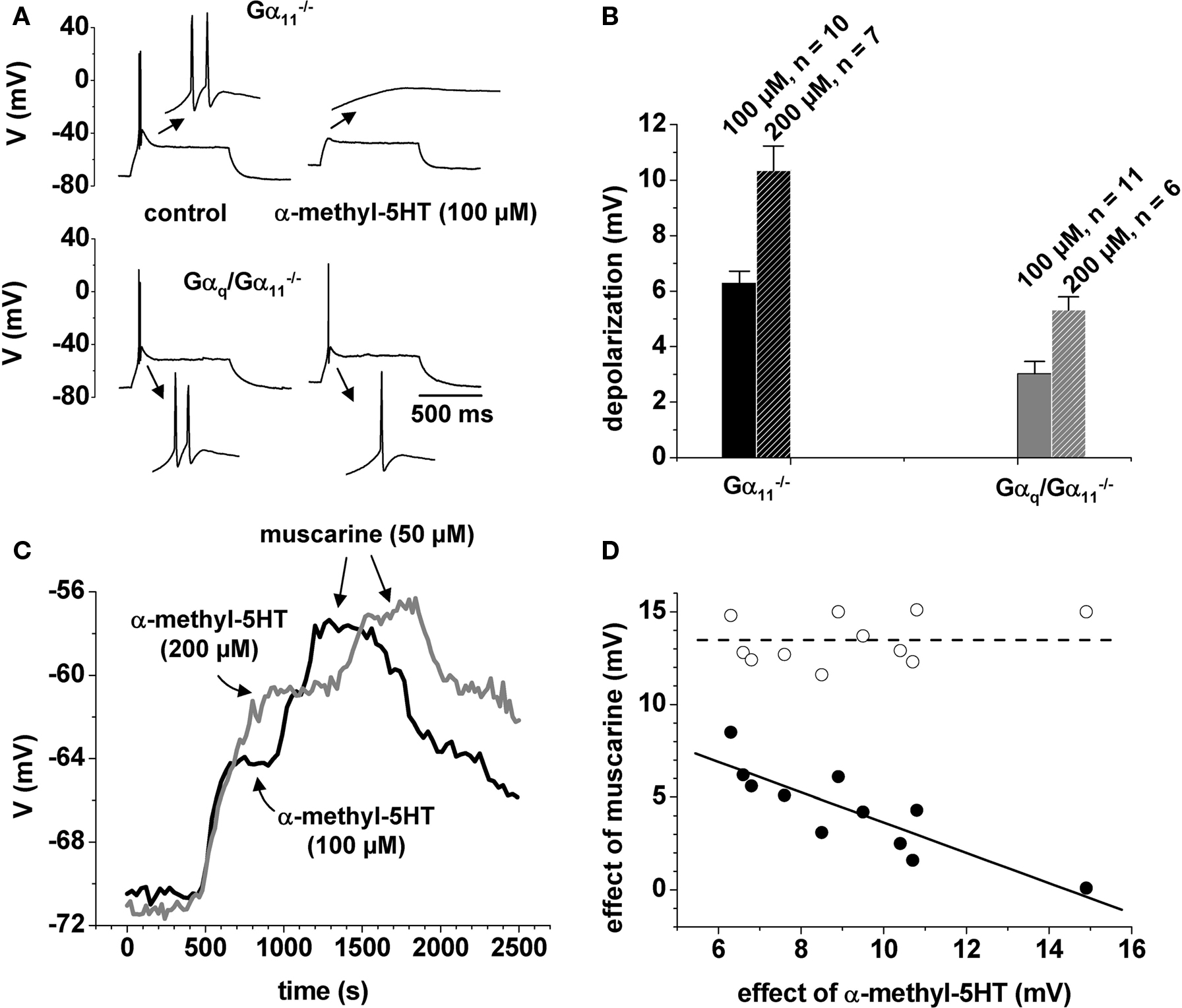
Figure 1 Membrane potential and muscarinic signaling are influenced by α-m-5-HT. (A) Firing pattern in a control mouse (upper panel) and in Gαq/Gα11−/− (lower panel) under control conditions (left traces) and in the presence of α-m-5-HT (right traces). The insets show the low-threshold Ca2+ potentials at a ten times expanded timescale. (B) Mean bar graph representation of the depolarization induced by two different substance concentrations as indicated. (C) Voltage vs. time plot of two individual cells from control animals (black line: application of 100 μM α-m-5-HT followed by application of 50 μM muscarine; gray line: application of 200 μM α-m-5-HT followed by application of 50 μM muscarine). Arrows illustrate plateau of the substance effects. Muscarine was applied in the continuous presence of α-m-5-HT. (D) Plot of the depolarization induced by α-m-5-HT vs. the depolarization induced by muscarine in individual cells from control animals (filled circles). Open circles represent the numerical sum of the two depolarizations. Straight black line = linear regression of the data points. Dashed line = mean value of the summed effects.
To further characterize Gq-dependent signaling in TC neurons we applied muscarine (50 μM) on top of different concentrations of α-m-5-HT in Gα11−/−. At the concentration we used, muscarine is known to depolarize Gα11−/− TC neurons by about 18 mV (Broicher et al., 2008b). 100 μM α-m-5-HT induced a depolarizing shift of the membrane potential of 7 ± 1 mV (n = 4; Figure 1C, black trace). The following muscarine-induced depolarization was only 7 ± 1 mV (n = 4; Figure 1C black trace). The combined depolarization was ∼14 mV. An increased concentration of α-m-5-HT (200 μM) induced a stronger depolarization of 10 ± 1 mV which was accompanied by a reduced muscarine effect averaging 3 ± 1 mV (n = 7; Figure 1C gray trace), so that the combined depolarization was similar to the previous experiment. Plotting the amplitude of the muscarine effect as a function of the amplitude of the α-m-5-HT effect revealed a clear linear dependency of the two parameters (Figure 1D; closed circles), i.e., the larger the effect of α-m-5-HT, the smaller the effect of muscarine. The sum of the combined substance effects (Figure 1D, open circles) was independent of the recording conditions and averaged 13.5 ± 0.4 mV (Figure 1D, dashed line). Obviously, this was an upper limit for a combined effect of muscarine and α-m-5-HT on VM. To verify this, we reversed the order of agonist application using C57BL6/N mice. Now, the initial muscarine effect (50 μM) was 15 ± 3 mV followed by a strongly reduced α-m-5-HT effect (100 μM) of 3 ± 2 mV, summing up to a combined effect of 18 ± 4 mV (n = 6). Agonist-induced depolarization was significantly reduced in Gαq/Gα11−/− (100 μM α-m-5-HT/50 μM muscarine: 4 ± 1 mV/2 ± 1 mV, p < 0.01/p < 0.001, n = 6; 200 μM α-m-5-HT/50 μM muscarine: 5 ± 1 mV/2 ± 1 mV, p < 0.01/p = 0.27, n = 5).
As shown in Figure 1D, the variability of a combined effect of the two agonists, namely α-m-5-HT and muscarine is rather small (open circles). Moreover, the variation in the effect of α-m-5-HT is comparable to the variation in the effect of muscarine. Still, when applied together, the combined effect does not exceed a given value. Or, in other words, both agonist effects vary, depending on how occupied the system is by the presence of the respective other agonist. This suggests that both mechanisms of activation involve a common and limiting mechanism.
In summary, these data demonstrate that serotonergic signaling depends on Gq-type G-proteins, and suggest that the same pool of G-proteins can be accessed by different transmitter pathways.
Metabotropic GluR-Mediated Signaling in Gαq/Gα11−/− Mice
Since glutamate and mGluR1 are also known to depolarize TC neurons by their action on IKL (McCormick and von Krosigk, 1992; Salt, 2002) and, moreover, are known to be coupled to Gq/G11 (Wettschureck et al., 2004a), we investigated the effect of the mGluR agonist t-ACPD on the TC neurons’ firing patterns. TC neurons set to a membrane potential of −71.2 ± 0.3 mV (n = 28) by DC offset showed burst responses to depolarizing current steps as described above (Gα11−/−: fi = 139 ± 5 Hz, n = 16; Gαq/Gα11−/−: fi = 146 ± 4 Hz, n = 12; Figures 2A,B, left panels). Application of t-ACPD resulted in a depolarizing shift of the membrane potential, the amplitude of which was significantly (p < 0.001) larger in Gα11−/− (50 μM: 23 ± 1 mV, n = 7; 100 μM: 17 ± 2 mV, n = 8) than in Gαq/Gα11−/− (50 μM: 11 ± 2 mV, n = 7; 100 μM: 8 ± 2 mV, n = 6; Figures 2C,D). The effects of 50 and 100 μM t-ACPD were not significantly different, indicating a saturating response. In both mouse strains, the t-ACPD-dependent depolarization was strong enough to induce a shift from burst to tonic firing of action potentials, which is also reflected in the firing frequency (Gα11−/−, 50 μM: fi = 32 ± 2 Hz, n = 7; Gα11−/−, 100 μM: fi = 44 ± 11 Hz, n = 8; Gαq/Gα11−/−, 50 μM: fi = 38 ± 3 Hz, n = 7; Figures 2A,B). In Gαq/Gα11−/− at 100 μM t-ACPD four out of six cells revealed an intermediate response with a slow burst (fi = 96 ± 9 Hz, n = 4) followed by 2–8 tonic action potentials (data not shown). Only one cell showed tonic firing (∼27 Hz), and the remaining cell was lost in the course of the experiment.
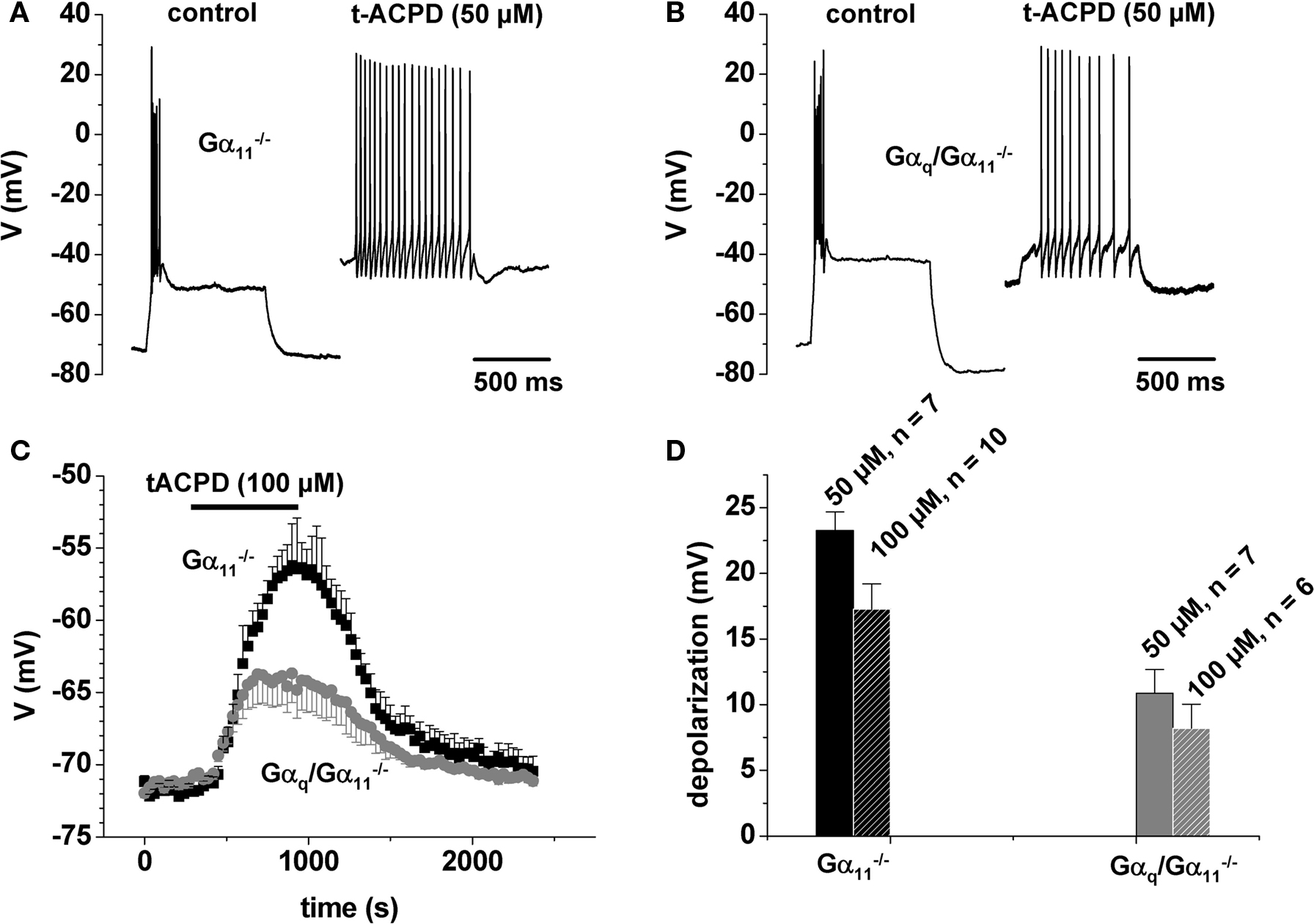
Figure 2 t-ACPD effect on firing properties. (A,B) Functional consequences of t-ACPD administration during current clamp recordings in a control mouse (A) and in Gαq/Gα11−/− (B). (C) Mean voltage vs. time plot (black squares, control animal; gray circles, Gαq/Gα11−/−). The horizontal bar indicates substance application. (D) Mean bar graph representation of the depolarization induced by two different substance concentrations as indicated.
To further elucidate the role of mGluR we used the selective group I agonist (RS)-3,5-DHPG (DHPG) (Ito et al., 1992). Application of DHPG (50 or 100 μM) induced a reversible depolarization of the membrane potential of 13 ± 2 mV (50 μM, n = 7) or 24 ± 1 mV (100 μM, n = 5) under current clamp conditions in Gα11−/− or C57BL6/N (Figures 3C,D). This effect was significantly (p < 0.01) reduced in Gαq/Gα11−/− (50 μM, 5 ± 1mV, n = 8; Figures 3C,D). In control mice (Figure 3A), but not in Gαq/Gα11−/− (Figure 3B), the DHPG-induced depolarization was accompanied by a switch to tonic firing of action potentials.
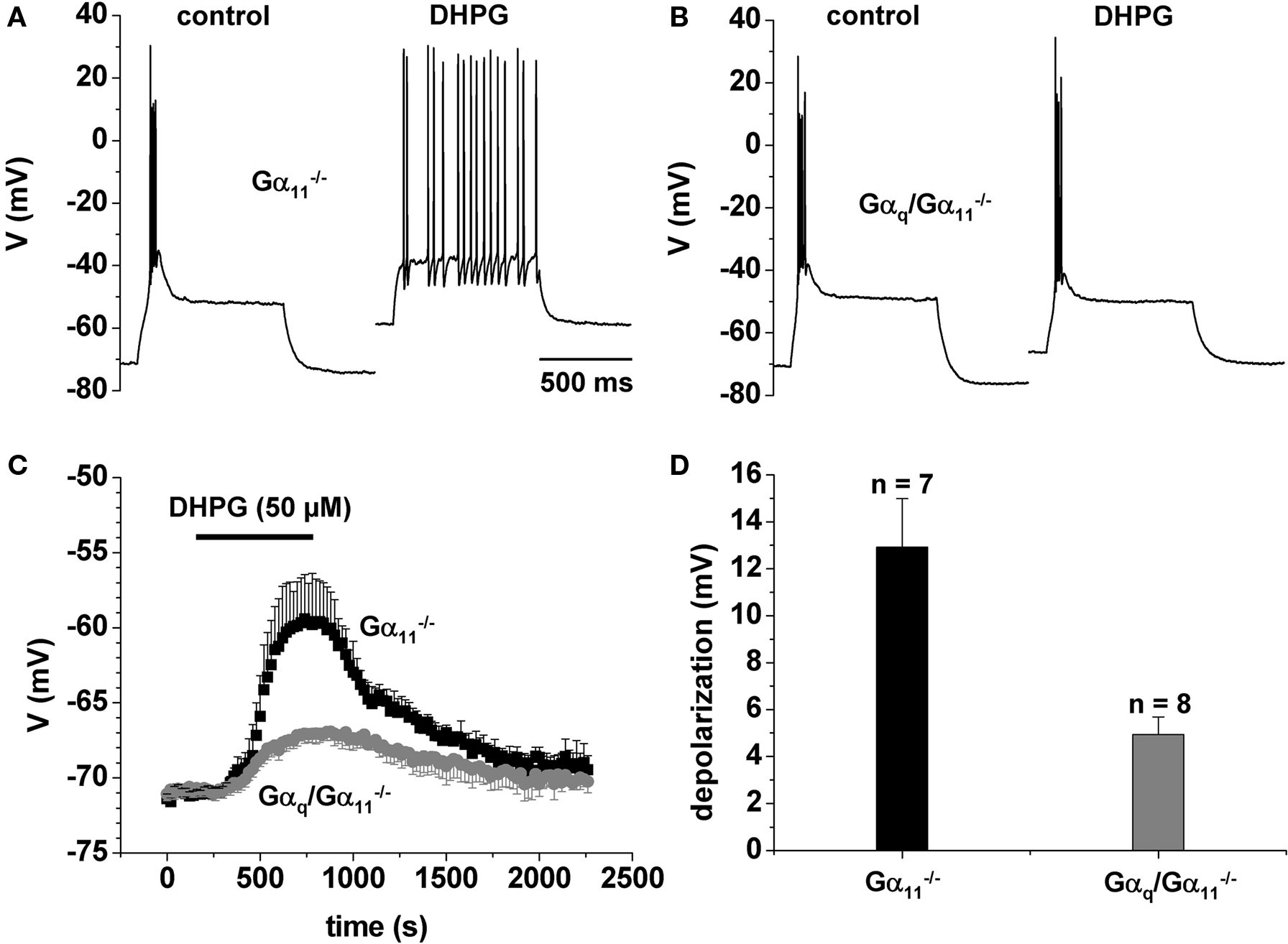
Figure 3 DHPG effect on firing properties. (A,B) Functional consequences of DHPG administration during current clamp recordings in a control mouse (A) and in Gαq/Gα11−/− (B). (C) Mean voltage vs. time plot (black squares, control animal; gray circles, Gαq/Gα11−/−). The horizontal bar indicates substance application. (D) Mean bar graph representation of the DHPG-induced depolarization.
It could be argued that the application of a neuromodulator could lead to changes in interneuron firing (Sherman, 2004; Munsch et al., 2005), which in turn could lead to changes in RMP unrelated to the activation of Gαq-coupled receptors of the recorded cell. To test for this, we applied 50 μM DHPG in the presence of 0.5 μM TTX, eliminating all action potential firing and excluding presynaptic effects. Under these conditions, the observed depolarization was very similar to control conditions (12 ± 3 mV, n = 3).
In summary, these data indicate that the action of glutamate in the thalamus critically depends on intact Gq-mediated intracellular signaling pathways.
Expression of 5-HT and mGlu Receptor Subtypes in dLGN
To provide further evidence that Gαq-coupled receptors are functionally expressed in TC neurons we performed immunohistochemical stainings by antibody labeling.
Application of 5-HT2AR-specific antibodies did not lead to staining by binding of the secondary antibody (Figure 4B, middle image). Strong staining was observed upon application of 5-HT2CR- (Figure 4C, middle image) and mGluR1α-specific antibodies (Figure 4D, middle image). We co-stained slices with the neuron-specific nucleus marker NeuN (Figures 4B–D left images; merged images to the right). This revealed that 5-HT2CR and mGluR1α were not somatically expressed in neurons of the LGN. We verified this by examining slices that were co-stained with a marker for microtubule associated protein 2 (MAP2) at a higher magnification. The images revealed that neither the staining for 5-HT2C (not shown) nor for mGluR1α clearly marked the outline of the soma (Figure S1 in Supplementary Material). We could not observe any differences between stainings in the dLGN of control and Gαq/Gα11−/− mice (Figure S2 in Supplementary Material).
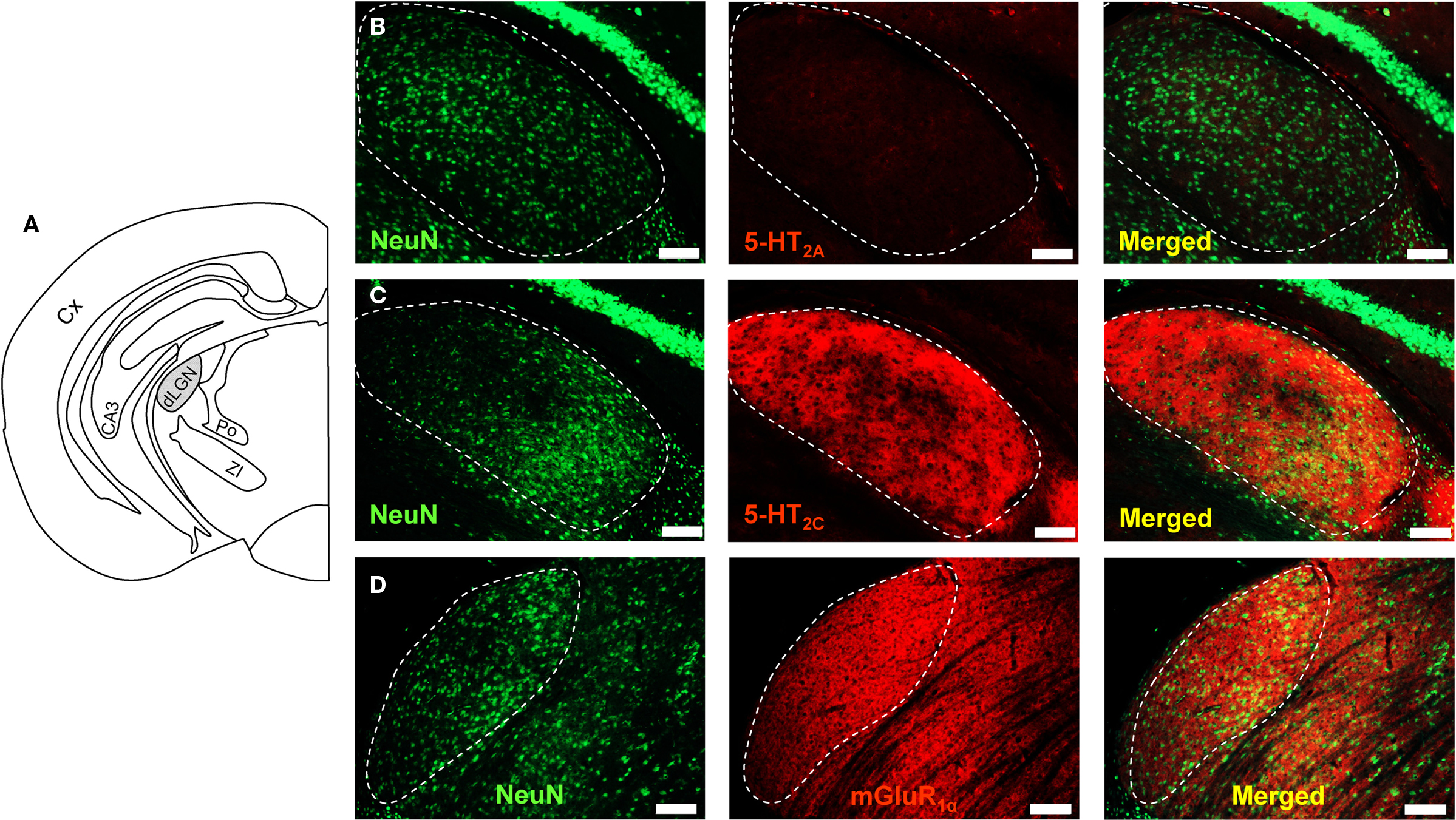
Figure 4 Immunohistochemical detection of mGluR and 5-HTR subtypes in dLGN of Gαq/Gα11−/−. (A) Schematic drawing of a frontal brain slice showing the position of the dLGN. In B-D the dLGN is marked by a dashed line. Specific antibodies for NeuN, 5-HT2AR, 5-HT2CR, and for mGluR1α were used and labeled with secondary antibodies conjugated to Cy2 or Cy3. 5-HT2AR-specific antibodies did not cause staining (B). 5-HT2CR- (C) and mGluR1α-specific antibodies (D) caused strong staining. 5-HT2CR and mGluR1α did not appear to be somatically expressed. Scale bars indicate 100 μm.
The densitometric analysis of 5-HT2CR-specific fluorescence revealed significant differences between control (mean fluorescence intensity = 32 ± 1 a.u.; n = 3 independent slices) and Gαq/Gα11−/− mice (mean fluorescence intensity = 41 ± 1 a.u.; n = 3; data not shown). No differences were found for mGluR1α-specific fluorescence (control: mean fluorescence intensity = 44 ± 1 a.u.; Gαq/Gα11−/−: mean fluorescence intensity = 42 ± 1 a.u.; n = 3 independent slices each; data not shown). These findings point to an attempt to compensate the loss of 5-HT2C receptor function by an increased number of receptors in Gαq/Gα11−/− mice.
Discussion
The results of the present study can be summarized as follows: (1) The action of 5-HT and glutamate in TC neurons depends on Gq-coupled intracellular signaling cascades. The effects of α-m-5-HT, t-ACPD, and DHPG under current clamp conditions were significantly reduced in Gαq/Gα11−/−. As a consequence, the stimulation of metabotropic 5-HTR and GluR could not induce a full shift from burst to tonic firing of action potentials in Gαq/Gα11−/− mice. (2) The amplitude of membrane potential depolarization by muscarine depends on the degree of prior utilization of the Gq-dependent pathway. (3) It is concluded that Gq/G11 family G-proteins play a central role in the state-dependent control of thalamic activity modes.
Gαq/Gα11-Dependent Signaling in the Thalamus
The flow of sensory information from the sensory organs to the cerebral cortex via the thalamus is highly regulated by inputs from the ascending activating brainstem system, releasing ACh, 5-HT, and noradrenalin (Steriade et al., 1997). These neuromodulators exert their influence onto the state of the thalamus by altering specific ion channels. This is achieved through the activation of G-protein-coupled membrane receptors (McCormick, 1992a). In addition, the excitatory transmitter glutamate exerts powerful activation of thalamic neurons, thereby driving the sensory relay in the thalamus (Salt, 2002). Corticofugal inputs, in particular, are connected to mGluR (Godwin et al., 1996b).
5-HT receptor-dependent signaling
Waking, but not REM sleep, is accompanied by increased serotonergic activity in the thalamus (Steriade et al., 1997). The role of 5-HT in the thalamus seems complex and is not yet fully understood: Cellular effects may be direct or indirect and show regional differences. In vitro studies demonstrated that 5-HT causes a small depolarization and a shift in voltage dependency of the hyperpolarization activated cation current, Ih. The latter is achieved via GS-proteins and cAMP production (McCormick and Pape, 1990; Lee and McCormick, 1996). Moreover, inhibition of an ISO component occurred, resembling the current mediated by TASK channels (S. G. Meuth and T. Budde, unpublished results). In consequence, oscillatory burst activity is suppressed. An indirect modulation of Ih via Gq-proteins may include IP3-induced Ca2+ release from intracellular stores and subsequent activation of a Ca2+-dependent adenylyl cyclase (Lüthi and McCormick, 1998). The findings of the present study indicate that a significant part of the 5-HT-induced depolarization is mediated by Gq/G11-coupled receptors. All receptors of the 5-HT2 subclass are coupled to Gq/G11-proteins. These in turn, activate PLC-β. 5-HT2A and 5-HT2C are widely distributed in the brain and are present in the rodent dLGN (Li et al., 2004). Thus, the strong reduction of the effect of α-m-5-HT in Gαq/Gα11−/− is in good agreement with a 5-HT2 expression in dLGN and is possibly connected to the modulation of TASK channels.
In the course of this study, we made the interesting observation that muscarinic and serotonergic receptors seem to compete for the same Gq-protein-coupled signaling pathway. The effect of activation of one receptor class is strongly and negatively correlated to the strength of prior activation of the other receptor class, thereby suggesting convergence onto the same – limited – pool of Gq-protein-coupled signaling pathways. This view is supported by results obtained from cat and guinea pig TC neurons that suggested acetylcholine- and noradrenalin-induced slow depolarizations to occur through the activation of the same second-messenger system (McCormick, 1992b).
mGluR-dependent signaling
Group I mGluRs consist of mGluR1 and mGluR5 that are positively coupled to PLC-β. Several types of mGluRs are expressed in the dLGN of different species, with retinal (mGluR5) and cortical (mGluR1) inputs accessing specific subtypes (Godwin et al., 1996b; Lourenco Neto et al., 2000). Application of t-ACPD and selective mGluR1 agonists depolarizes TC neurons and switches their activity mode from burst to tonic firing, thereby mediating TC transmission (McCormick and von Krosigk, 1992; Godwin et al., 1996a; Salt, 2002). The results of the present study are in line with these findings. However, the remaining t-ACPD effect in Gαq/Gα11−/− indicates that mGluRs not coupled to Gq/G11 contribute to the response. This conclusion is corroborated by the finding that mGluR3, mGluR4, and mGluR7 are expressed in rodent dLGN (Lourenco Neto et al., 2000). During postnatal development, specific changes in the subcellular location of mGluRs have been observed (Liu et al., 1998) which are the basis for the topographical association to different input systems (Godwin et al., 1996b; Turner and Salt, 2000).
Residual Effects of Neurotransmitters in Gαq/Gα11−/− Mice
While Gαq and Gα11 have very similar effector-coupling properties and may substitute each other (Offermanns, 1999), the remaining effect of receptor agonists in Gαq/Gα11−/− may result from different mechanisms:
(1) Other G-protein families may compensate for the lack of Gαq in deficient mice. Findings from hippocampal neurons of Gαo-deficient mice have shown that in the absence of Gαo, ion channels will be regulated by other G-proteins with different properties (Greif et al., 2000). In particular Gα15 and Gα16 can link a variety of predominantly Gαq-coupled receptors to the PLCβ pathway (Offermanns and Simon, 1995).
(2) Receptors, intracellular signaling proteins, and effectors of the Gαq-dependent pathways may be up-regulated. However, the Gαq/Gα11−/− mice develop a severe epileptic phenotype with a reduced life span, which argues against an effective compensation mechanism (Wettschureck et al., 2006; Broicher et al., 2008b). Indeed, mRNA expression of effector channels (TASK channels, HCN channels) and all mAChR subtypes were unchanged in dLGN tissue (Wettschureck et al., 2006; Broicher et al., 2008b) and a lack of strong plastic compensation has been noted before in TC neurons of mice deficient for HCN2 (Ludwig et al., 2003) and TASK-1 (Meuth et al., 2006). Nevertheless, the densitometric analysis of immunohistochemical staining in the present study indicated an increased expression of 5-HT2CR in Gαq/Gα11−/− mice, thereby possibly showing a futile attempt to compensate the loss of receptor function by an increased number of membrane receptors.
(3) G-protein signaling outside the canonical seven transmembrane domain receptors and G-protein independent pathways of these receptors may exist. Recent evidence indicates that G-proteins play important roles in receptor tyrosine kinase signaling and may be activated by accessory proteins (Marty and Ye, 2010; Sato and Ishikawa, 2010). Furthermore, increasing evidence indicates that ERK, JAK/STATs, Src-family tyrosine kinases, β-arrestins, and PDZ domain-containing proteins directly relay signals from seven transmembrane domain receptors, independent of G-proteins (Sun et al., 2007). Future studies have to show the coupling of metabotropic ACh, 5-HT, and Glu receptors to parallel pathways, activated by βγ-subunits, by G-proteins not belonging to the Gq/G11-family, and/or by G-protein-independent mechanisms.
(4) Gαq may be incompletely eliminated. The CaMKII-Cre mouse line has been shown to express Cre in forebrain principal neurons, but not in forebrain interneurons (Mantamadiotis et al., 2002; Marsicano et al., 2003). With respect to recombination efficiency within the population of forebrain principal neurons it is generally assumed that recombination is complete (Marsicano et al., 2003). Within a particular forebrain principal neuron, Cre expression always results in a complete inactivation of Gαq, since the CaMKII promoter chosen to drive Cre expression is very strong. We are therefore positive that we can rule out any partial Gαq inactivation (i.e., only one of the two Gαq alleles is recombined) in Cre-expressing principal neurons.
Expression of 5-HT and mGlu Receptors
Immunohistochemical stainings provided evidence that Gαq-coupled receptors are functionally expressed in TC neurons. However, the application of 5-HT2AR-specific antibodies failed to stain the tissue. Why this is the case remains unclear. The strong staining that was observed upon application of 5-HT2CR- and mGluR1α-specific antibodies was not reduced in Gαq/Gα11−/− mice, suggesting that the deletion of Gq does not lead to a down regulation of its upstream receptors (compare Figure 4 and Figure S2 in Supplementary Material). Co-staining slices with the neuron-specific nucleus marker NeuN revealed that 5-HT2CR and mGluR1α were not somatically expressed in neurons of the LGN. This was confirmed in a co-staining with MAP2.
The finding that a specific agonist for 5-HT2C receptors had the same effect on the membrane potential in current clamp recordings than α-m-5-HT in control mice suggests that 5-HT2C receptors in the dLGN could be the functionally dominant isoform.
Convergence of Transmitter Pathways
The strong convergence of several transmitter/receptor systems on Gq/G11 family G-proteins in TC neurons leads to the question of the degree of redundancy in their signaling. The finding (i) of differential subcellular locations of specific membrane receptors, (ii) of the topographical organization of different input systems, (iii) of different state-dependent releases of neurotransmitters, and (iv) of the formation of tight receptor/effector protein complexes in the forebrain indicate a separation of function between the different Gq/G11-coupled receptor classes in the same neuronal population. Thus, spatial separation and state-dependent activation allow divergent action of several metabotropic receptor classes, which seem to utilize the same pool of Gq/G11-proteins. These findings, therefore, suggest a scenario in which Gq/G11-proteins could function as a central element in thalamic physiology.
Conflict of Interest Statement:
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
Supported by the German Research Foundation (DFG): FOR1086, TP2 to Thomas Budde and Sven G. Meuth. Supported by the “Interdisziplinäres Zentrum für Klinische Forschung Münster”, Bud3/010/10 to Thomas Budde. The authors wish to thank A. Jahn, E. Nass, A. Markovic, and R. Ziegler for excellent technical assistance.
References
Broicher, T., Kanyshkova, T., Meuth, P., Pape, H. C., and Budde, T. (2008a). Correlation of T-channel coding gene expression, IT, and the low threshold Ca2+ spike in the thalamus of a rat model of absence epilepsy. Mol. Cell. Neurosci. 39, 384–399.
Broicher, T., Wettschureck, N., Munsch, T., Coulon, P., Meuth, S. G., Kanyshkova, T., Seidenbecher, T., Offermanns, S., Pape, H. C., and Budde, T. (2008b). Muscarinic ACh receptor-mediated control of thalamic activity via G(q)/G(11)-family G-proteins. Pflugers Arch. 456, 1049–1060.
Chemin, J., Girard, C., Duprat, F., Lesage, F., Romey, G., and Lazdunski, M. (2003). Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J. 22, 5403–5411.
Chen, X., Talley, E. M., Patel, N., Gomis, A., McIntire, W. E., Dong, B., Viana, F., Garrison, J. C., and Bayliss, D. A. (2006). Inhibition of a background potassium channel by Gq protein {alpha}-subunits. Proc. Natl. Acad. Sci. U. S.A.103, 3422–3427.
Exton, J. H. (1996). Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu. Rev. Pharmacol. Toxicol. 36, 481–509.
Godwin, D. W., Vaughan, J. W., and Sherman, S. M. (1996a). Metabotropic glutamate receptors switch visual response mode of lateral geniculate nucleus cells from burst to tonic. J. Neurophysiol. 76, 1800–1816.
Godwin, D. W., Van Horn, S. C., Eriir, A., Sesma, M., Romano, C., and Sherman, S. M. (1996b). Ultrastructural localization suggests that retinal and cortical inputs access different metabotropic glutamate receptors in the lateral geniculate nucleus. J. Neurosci. 16, 8181–8192.
Greif, G. J., Sodickson, D. L., Bean, B. P., Neer, E. J., and Mende, U. (2000). Altered regulation of potassium and calcium channels by GABAB and adenosine receptors in hippocampal neurons from mice lacking galpha o. J. Neurophysiol. 83, 1010–1018.
Hirsch, J. C., Fourment, A., and Marc, M. E. (1983). Sleep-related variations of membrane potential in the lateral geniculate body relay neurons of the cat. Brain Res. 259, 308–312.
Ito, I., Kohda, A., Tanabe, S., Hirose, E., Hayashi, M., Mitsunaga, S., and Sugiyama, H. (1992). 3,5-Dihydroxyphenyl-glycine: a potent agonist of metabotropic glutamate receptors. Neuroreport 3, 1013–1016.
Lee, K. H., and McCormick, D. A. (1996). Abolition of spindle oscillations by serotonin and norepinephrine in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuron 17, 309–321.
Li, Q. H., Nakadate, K., Tanaka-Nakadate, S., Nakatsuka, D., Cui, Y., and Watanabe, Y. (2004). Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: western blot and immunohistochemical analyses. J. Comp. Neurol. 469, 128–140.
Liu, X. B., Munoz, A., and Jones, E. G. (1998). Changes in subcellular localization of metabotropic glutamate receptor subtypes during postnatal development of mouse thalamus. J. Comp. Neurol. 395, 450–465.
Lopes, C. M., Rohacs, T., Czirjak, G., Balla, T., Enyedi, P., and Logothetis, D. E. (2005). PIP2 hydrolysis underlies agonist-induced inhibition and regulates voltage gating of two-pore domain K+ channels. J. Physiol. (Lond.) 564, 117–129.
Lourenco Neto, F., Schadrack, J., Berthele, A., Zieglgansberger, W., Tolle, T. R., and Castro-Lopes, J. M. (2000). Differential distribution of metabotropic glutamate receptor subtype mRNAs in the thalamus of the rat. Brain Res. 854, 93–105.
Ludwig, A., Budde, T., Stieber, J., Moosmang, S., Wahl, C., Holthoff, K., Langebartels, A., Wotjak, C., Munsch, T., Zong, X., Feil, S., Feil, R., Lancel, M., Chien, K. R., Konnerth, A., Pape, H. C., Biel, M., and Hofmann, F. (2003). Absence epilepsy and sinus dysrhythmia in mice lacking the pacemaker channel HCN2. EMBO J. 22, 216–224.
Lüthi, A., and McCormick, D. A. (1998). Periodicity of thalamic synchronized oscillations: the role of Ca2+-mediated upregulation of Ih. Neuron 20, 553–563.
Mantamadiotis, T., Lemberger, T., Bleckmann, S. C., Kern, H., Kretz, O., Villalba, A. M., Tronche, F., Kellendonk, C., Gau, D., Kapfhammer, J., Otto, C., Schmid, W., and Schutz, G. (2002). Disruption of CREB function in brain leads to neurodegeneration. Nat. Genet. 31, 47–54.
Marsicano, G., Goodenough, S., Monory, K., Hermann, H., Eder, M., Cannich, A., Azad, S. C., Cascio, M. G., Gutierrez, S. O., van der Stelt, M., Lopez-Rodriguez, M. L., Casanova, E., Schutz, G., Zieglgansberger, W., Di Marzo, V., Behl, C., and Lutz, B. (2003). CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science 302, 84–88.
Marty, C., and Ye, R. D. (2010). Heterotrimeric G protein signaling outside the realm of seven transmembrane domain receptors. Mol. Pharmacol. 78, 12–18.
McCormick, D. A. (1992a). Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog. Neurobiol. 39, 337–388.
McCormick, D. A. (1992b). Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J. Neurosci. 12, 278–289.
McCormick, D. A., and Pape, H.-C. (1990). Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J. Physiol. (Lond.) 431, 319–342.
McCormick, D. A., and von Krosigk, M. (1992). Corticothalamic activation modulates thalamic firing through glutamate “metabotropic” receptors. Proc. Natl. Acad. Sci. U.S.A. 89, 2774–2778.
Meuth, S. G., Aller, M. I., Munsch, T., Schuhmacher, T., Seidenbecher, T., Kleinschnitz, C., Pape, H. C., Wiendl, H., Wisden, W., and Budde, T. (2006). The contribution of TASK-1-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol. Pharmacol. 69, 1468–1476.
Meuth, S. G., Budde, T., Kanyshkova, T., Broicher, T., Munsch, T., and Pape, H.-C. (2003). Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J. Neurosci. 23, 6460–6469.
Munsch, T., Yanagawa, Y., Obata, K., and Pape, H. C. (2005). Dopaminergic control of local interneuron activity in the thalamus. Eur. J. Neurosci. 21, 290–294.
Offermanns, S. (1999). New insights into the in vivo function of heterotrimeric G-proteins through gene deletion studies. Naunyn Schmiedebergs Arch. Pharmacol. 360, 5–13.
Offermanns, S., and Simon, M. I. (1995). G and G couple a wide variety of receptors to phospholipase C. J. Biol. Chem. 270, 15175–15180.
Offermanns, S., Zhao, L. P., Gohla, A., Sarosi, I., Simon, M. I., and Wilkie, T. M. (1998). Embryonic cardiomyocyte hypoplasia and craniofacial defects in G alpha q/G alpha 11-mutant mice. EMBO J. 17, 4304–4312.
Roth, B. L., Willins, D. L., Kristiansen, K., and Kroeze, W. K. (1998). 5-Hydroxytryptamine2-family receptors (5-hydroxytryptamine2A, 5-hydroxytryptamine2B, 5-hydroxytryptamine2C): where structure meets function. Pharmacol. Ther. 79, 231–257.
Salt, T. E. (2002). Glutamate receptor functions in sensory relay in the thalamus. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 357, 1759–1766.
Sato, M., and Ishikawa, Y. (2010). Accessory proteins for heterotrimeric G-protein: implication in the cardiovascular system. Pathophysiology 17, 89–99.
Sherman, S. M. (1996). Dual reponse modes in lateral geniculate neurons: mechanisms and functions. Vis. Neurosci. 13, 205–213.
Sherman, S. M. (2004). Interneurons and triadic circuitry of the thalamus. Trends Neurosci. 27, 670–675.
Siuciak, J. A., McCarthy, S. A., Chapin, D. S., Reed, T. M., Vorhees, C. V., and Repaske, D. R. (2007). Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1B (PDE1B) enzyme. Neuropharmacology 53, 113–124.
Sun, Y., McGarrigle, D., and Huang, X.-Y. (2007). When a G protein-coupled receptor does not couple to a G protein. Mol. Biosyst. 3, 849–854.
Turner, J. P., and Salt, T. E. (2000). Synaptic activation of the group I metabotropic glutamate receptor mGlu1 on the thalamocortical neurons of the rat dorsal lateral geniculate nucleus in vitro. Neuroscience 100, 493–505.
Wettschureck, N., Moers, A., and Offermanns, S. (2004a). Mouse models to study G-protein-mediated signaling. Pharmacol. Ther. 101, 75–89.
Wettschureck, N., Moers, A., Hamalainen, T., Lemberger, T., Schutz, G., and Offermanns, S. (2004b). Heterotrimeric G proteins of the Gq/11 family are crucial for the induction of maternal behavior in mice. Mol. Cell. Biol. 24, 8048–8054.
Wettschureck, N., Rutten, H., Zywietz, A., Gehring, D., Wilkie, T. M., Chen, J., Chien, K. R., and Offermanns, S. (2001). Absence of pressure overload induced myocardial hypertrophy after conditional inactivation of Galphaq/Galpha11 in cardiomyocytes. Nat. Med. 7, 1236–1240.
Wettschureck, N., van der Stelt, M., Tsubokawa, H., Krestel, H., Moers, A., Petrosino, S., Schutz, G., Di Marzo, V., and Offermanns, S. (2006). Forebrain-specific inactivation of Gq/G11 family G proteins results in age-dependent epilepsy and impaired endocannabinoid formation. Mol. Cell. Biol. 26, 5888–5894.
Keywords: thalamic function, muscarinic receptor, metabotropic glutamate receptor, serotonin receptor, gene knockout, G-protein
Citation: Coulon P, Kanyshkova T, Broicher T, Munsch T, Wettschureck N, Seidenbecher T, Meuth SG, Offermanns S, Pape H-C and Budde T (2010) Activity modes in thalamocortical relay neurons are modulated by Gq/G11 family G-proteins – serotonergic and glutamatergic signaling. Front. Cell. Neurosci. 4:132. doi: 10.3389/fncel.2010.00132
Received: 06 July 2009;
Accepted: 28 September 2010;
Published online: 25 October 2010.
Edited by:
Barry W. Connors, Brown University, USAReviewed by:
Charles Cox, University of Illinois at Urbana-Champiagn, USA;Barry W. Connors, Brown University, USA
Copyright: © 2010 Coulon, Kanyshkova, Broicher, Munsch, Wettschureck, Seidenbecher, Meuth, Offermanns, Pape and Budde. This is an open-access article subject to an exclusive license agreement between the authors and the Frontiers Research Foundation, which permits unrestricted use, distribution, and reproduction in any medium, provided the original authors and source are credited.
*Correspondence: Thomas Budde, Institut für Physiologie I, Westfälische Wilhelms-Universität Münster, Robert-Koch-Street 27a, 48149 Münster, Germany. e-mail:dGJ1ZGRlQHVuaS1tdWVuc3Rlci5kZQ==









