- 1Department of Laboratory Medicine, Shengli Oilfield Central Hospital, Dongying, Shandong, China
- 2Medical Technology School, Xuzhou Medical University, Xuzhou, Jiangsu, China
- 3Guangdong Provincial People’s Hospital (Guangdong Academy of Medical Sciences), Southern Medical University, Guangzhou, Guangdong, China
- 4Intelligent Medical Engineering, School of Medical Informatics and Engineering, Xuzhou Medical University, Xuzhou, Jiangsu, China
Background: Helicobacter pylori (H. pylori), linked to gastric cancer, lacks regional data on infection rates, resistance, and risks in Dongying, China.
Methods: A pilot study (N = 314) using non-invasive string tests and qPCR assessed H. pylori prevalence and antibiotic resistance. Logistic regression identified risk factors from survey data.
Results: The H. pylori infection rate in Dongying was 24.84% (78/314, 95% CI: 20.06%-29.62%). Of the infected individuals, 60.25% (47/78, 95% CI: 49.40%-71.12%) and 46.15% (36/78, 95% CI: 35.09%-57.22%) showed resistance to clarithromycin and levofloxacin, respectively.
Conclusions: This pilot study provides preliminary data with valuable insights for preventing the transmission of H. pylori infection among the urban population in the East Coast region of China and offers guidance for the personalized treatment of drug-resistant H. pylori infections.
Introduction
In 1983, Marshall and J. Robin Warren successfully cultured and identified Helicobacter pylori (H. pylori) in gastric mucosa for the first time (Yamada et al., 1994). H. pylori is a Gram-negative, microaerophilic bacterium known for its ability to colonize the human stomach persistently. By producing urease, H. pylori can neutralize gastric acid, allowing it to adapt to the acidic environment of the stomach—a key feature of its pathogenic mechanism. Moreover, H. pylori uses a variety of virulence factors to target host cellular proteins. These factors finely regulate inflammatory responses and contribute to diverse damage to the gastric mucosa. H. pylori infection has been conclusively linked to the development of chronic gastritis, peptic ulcers, gastric cancer, and MALT lymphoma (Burucoa and Axon, 2017). The International Agency for Research on Cancer (IARC) has classified H. pylori as a Group I carcinogen (Yan et al., 2024). Eradicating H. pylori can significantly reduce the risk of gastric cancer, and many countries and regions have achieved lower incidence rates of gastric cancer by eradicating this bacterium (Hooi et al., 2017; Chiang et al., 2021). The prevalence of H. pylori infection is influenced by various factors such as geographical location, sanitary conditions, and socioeconomic status (Li et al., 2023). A multicenter study conducted by Wang et al. in 2024 found that the prevalence of H. pylori infection in the urban areas of China was 27.08%, with significant differences in infection rates across different regions (Wang et al., 2024).
The global issue of antibiotic resistance is becoming increasingly severe, posing significant challenges to the eradication of H. pylori (Hong et al., 2024). According to recent studies, the resistance rates of urban residents in China to clarithromycin and levofloxacin are as high as 50.83% and 47.17%, respectively (Wang et al., 2024). The resistance of H. pylori to these two antibiotics is mainly attributed to point mutations in key genes, which alter the drug targets and significantly reduce the effectiveness of the antibiotics. Clarithromycin resistance is closely associated with point mutations in the 23S rRNA gene (A2142G, A2143G, and A2142C), which prevent clarithromycin from binding to the bacterial ribosome’s 50S subunit, thereby blocking its ability to inhibit protein synthesis. On the other hand, levofloxacin resistance is typically related to mutations in the gyrA gene (such as N87K and D91G). The gyrA gene encodes the A subunit of DNA gyrase, and these mutations decrease the affinity of levofloxacin for DNA gyrase, hindering its ability to interfere with DNA replication. In some cases, overexpression of efflux pumps or changes in membrane permeability may further exacerbate resistance to levofloxacin. These molecular mechanisms significantly affect the therapeutic efficacy of clarithromycin and levofloxacin, influencing clinical treatment strategies and the rate of treatment failure (Greenberg et al., 2011; Molina–Infante et al., 2013). Therefore, detecting resistance gene mutations is crucial for personalized therapy. However, antibiotic resistance develops rapidly due to the widespread use of antibiotics like clarithromycin in the population and the high adaptability of H. pylori, making the treatment of H. pylori infections increasingly difficult (Thung et al., 2016). As a result, effectively controlling antibiotic resistance and successfully eradicating H. pylori has become an urgent public health issue.
When detecting H. pylori infection, understanding the patient’s antibiotic resistance is crucial for developing an effective treatment plan. Existing methods for detecting H. pylori are mainly divided into invasive and non-invasive categories (Mégraud and Lehours, 2007). Culture and molecular diagnostic methods can provide information on H. pylori infection and its antibiotic resistance. Gastric fluid is a sample that can provide real-time insights into the gastric environment. In most current studies, gastric fluid samples are obtained via gastroscopy. However, the invasiveness of this method makes gastric fluid more difficult to collect compared to other body fluids. Previous studies have reported a method for collecting gastric fluid samples using the string test (Domínguez-Bello et al., 2001; Wang et al., 2003, 2023). Introduced in 1970, the string test is a minimally invasive method in which a patient swallows a capsule containing a string, which is then retrieved to collect gastric fluid samples (Beal et al., 1970). Han et al (Han et al., 2023). conducted a single-center study combining the string test with qPCR to guiding H. pylori antibiotic sensitive treatment for H. pylori. Guided by the results of antibiotic resistance testing, patients receiving 14-day bismuth quadruple therapy achieved an H. pylori eradication rate of H. pylori. This further confirmed that the minimally invasive string test combined with qPCR is reliable for collecting gastric fluid samples containing H. pylori to diagnose infections and antibiotic resistance. This method reduces the need for invasive procedures (such as gastroscopy), makes large-scale screening feasible, and allows for the real-time acquisition of accurate information regarding infection status and antibiotic resistance.
As a burgeoning immigrant city, Dongying has maintained a steady population growth. In 1950, the population of Dongying was 21,504. The population of Dongying is expected to reach 1,410,790 by 2024. With the development of the petroleum industry, Dongying has gradually evolved from a small fishing village into an important industrial city. The first generation of oil workers came from all over the country, exhibiting different dietary habits and lifestyles. To the best of our knowledge, no detailed reports have been published regarding the prevalence, antibiotic resistance, and risk factors of H. pylori infection in Dongying. Therefore, it is essential to conduct screening and management of H. pylori infection in Dongying, Shandong, which may not only help reduce the infection rate but also effectively prevent the occurrence of serious diseases such as gastric cancer. In this study, we randomly recruited individuals aged 18 to 60 years from Dongying City, Shandong Province, and non-invasively collected gastric fluid samples from participants using the string test method. A combination of string tests with qPCR detected H. pylori infection status and antibiotic resistance profiles in the participating population. Additionally, we conducted univariate and multivariate logistic regression analyses based on the collected questionnaire data to identify risk factors associated with H. pylori infection. In conclusion, this study has the potential to help optimize prevention and eradication strategies for H. pylori infection and further improve public health outcomes in local areas.
Materials and methods
Study design
From May 8 to May 23, 2023, we conducted a cross-sectional survey to investigate the prevalence of Helicobacter pylori infection in Dongying City, Shandong Province, China. The survey was conducted through online promotion, inviting urban residents aged 18 to 60 to participate randomly and confirm their participation by providing their information. The following groups were excluded from the study: (1) those who had taken antibiotics, bismuth agents, or antibacterial traditional Chinese medicines within one month, or proton pump inhibitors, H2 receptor antagonists, or other drugs within two weeks; (2) those who had undergone H. pylori eradication therapy in the past three months; (3) pregnant and breastfeeding women; and (4) those with severe heart, liver, or kidney dysfunction, severe neuropathy, or mental illness. The study adhered to the ethical principles of the Declaration of Helsinki. All participants signed informed consent forms, and the study was approved by the Ethics Review Committee of Guangdong Provincial People’s Hospital (Ethics Approval No. KY-Q-2022-384-02).
Study data
Under the guidance of the on-site staff, each participant completed the questionnaire. The questionnaire covered the following aspects: (1) demographic characteristics, including sex, age (divided into <30, 30-39, 40-49, and 50-60 years), height, weight, marital status (unmarried and married), and education level (categorized as lower secondary education and below, Intermediate: high school or vocational training, and higher education); (2) lifestyle, including dietary habits, smoking, alcohol consumption, tea drinking, and sharing cups. BMI is defined as weight (kg) divided by the square of height (m2) and is used to reflect the obesity status. Based on WHO criteria, underweight was identified as BMI<18.5 kg/m2, normal weight as BMI 18.5-24.9 kg/m2, overweight as BMI 25.0-29.9 kg/m2, and obese as BMI≥30.0 kg/m2 (Consultation, 2000).
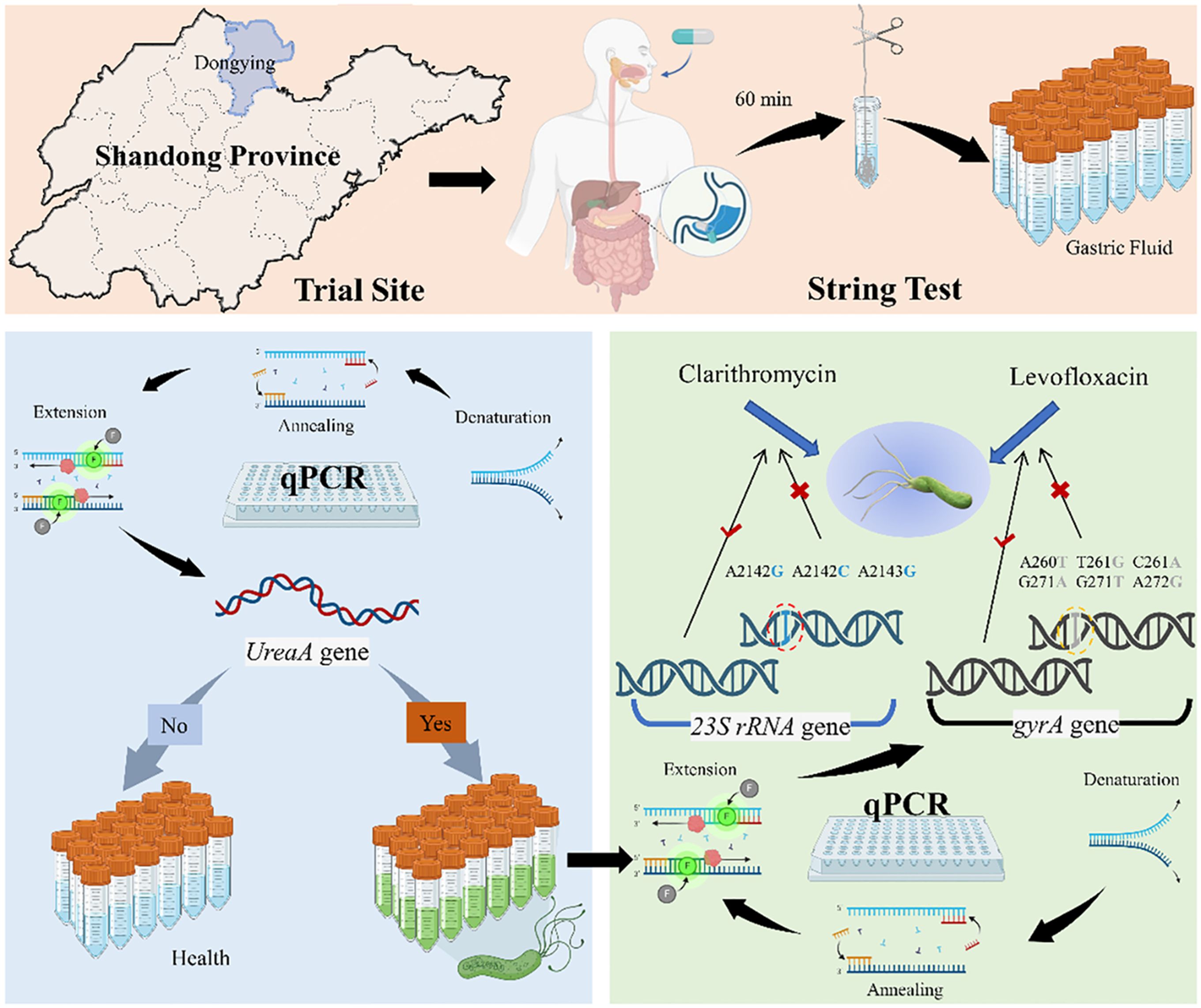
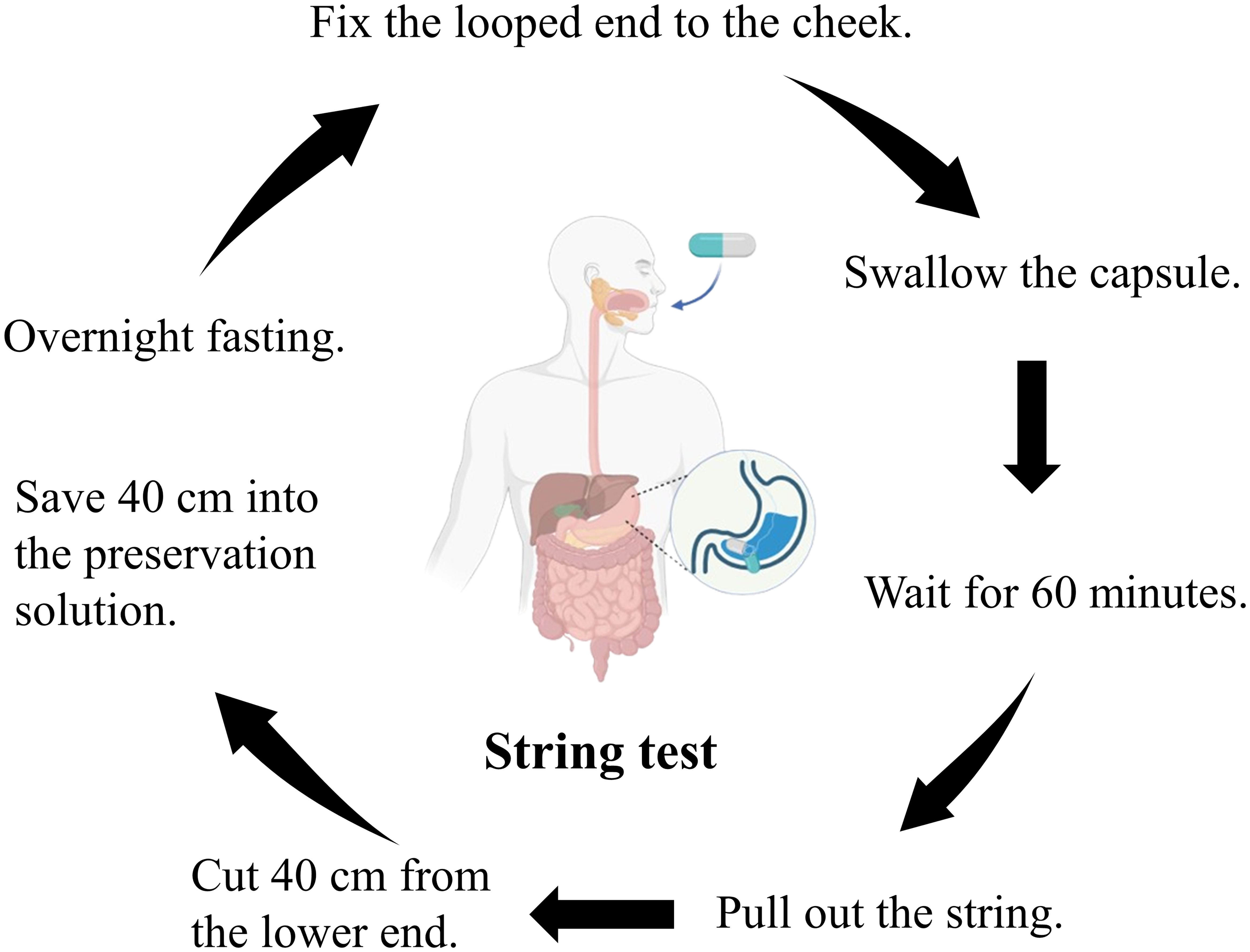
Non-invasive collection of gastric fluid via string test
After overnight fasting, gastric fluid was collected using a disposable string test kit (Hongmed-Infagen, Shenzhen, China). The kit included a weighted capsule with an absorbent cotton line. Participants swallowed the capsule, and after 60 minutes, the cotton line, saturated with gastric fluid, was retrieved and cut with sterile scissors. The distal end, which was 40 cm from the cut, was then transferred to a commercial preservative solution (Hongmed-Infagen, Shenzhen, China) (Figure 1). The collected gastric fluid samples were sent to the clinical laboratory at the Department of Laboratory Medicine, Guangdong Provincial People’s Hospital, for qPCR analysis.
Measurement of H. pylori infection and antibiotic resistance
DNA was extracted using a nucleic acid extraction kit (Daan Gene, Guangzhou, China) with a Stream SP96 Automated Extractor. The H. pylori nucleic acid detection kit (PCR-fluorescent probe method), H. pylori, 23S rRNA, and gyrA gene mutation detection kit (fluorescent PCR method) were provided by Shenzhen Hongmei Diagnostic Technology Co., Ltd. DNA (5 µL of DNA and PCR reaction mixture (20 µL) were added to the corresponding wells of an eight-tube strip. PCR conditions included 42°C for 2 min, 95°C for 2 min, and 40 cycles of 95°C for 10 s and 58°C for 45 s. A cycle threshold value ≤35 for ureA indicated H. pylori infection, and ≤30 for 23S rRNA and gyrA indicated resistance to clarithromycin and levofloxacin.
Statistical analysis
We used the chi-square test to analyze H. pylori infection and compare variables such as gender, age, BMI, education level, and marital status. Sample size calculation was based on the observed infection rate (24.84%), a margin of error of 5%, and a confidence level of 95%. We calculated 95% confidence intervals for all statistical measures, including infection and antibiotic resistance rates. Based on univariate logistic regression analysis, we included factors with a P-value less than 0.5 and those identified in previous studies (Zhou et al., 2023) as significantly associated with H. pylori infection in the multivariate logistic regression analysis. Although no factors with a P-value less than 0.5 were found in the univariate analysis, considering their relevance in prior research, we included gender, age, education level, and marital status in the multivariate analysis to further assess their relationship with H. pylori infection. Both univariate and multivariate logistic regression were used to identify and confirm independent risk factors, with odds ratios (OR) and 95% confidence intervals (CI) reflecting their impact (P < 0.05). All analyses were performed using R software (version 4.4.1).
Results
Basic information about the participants
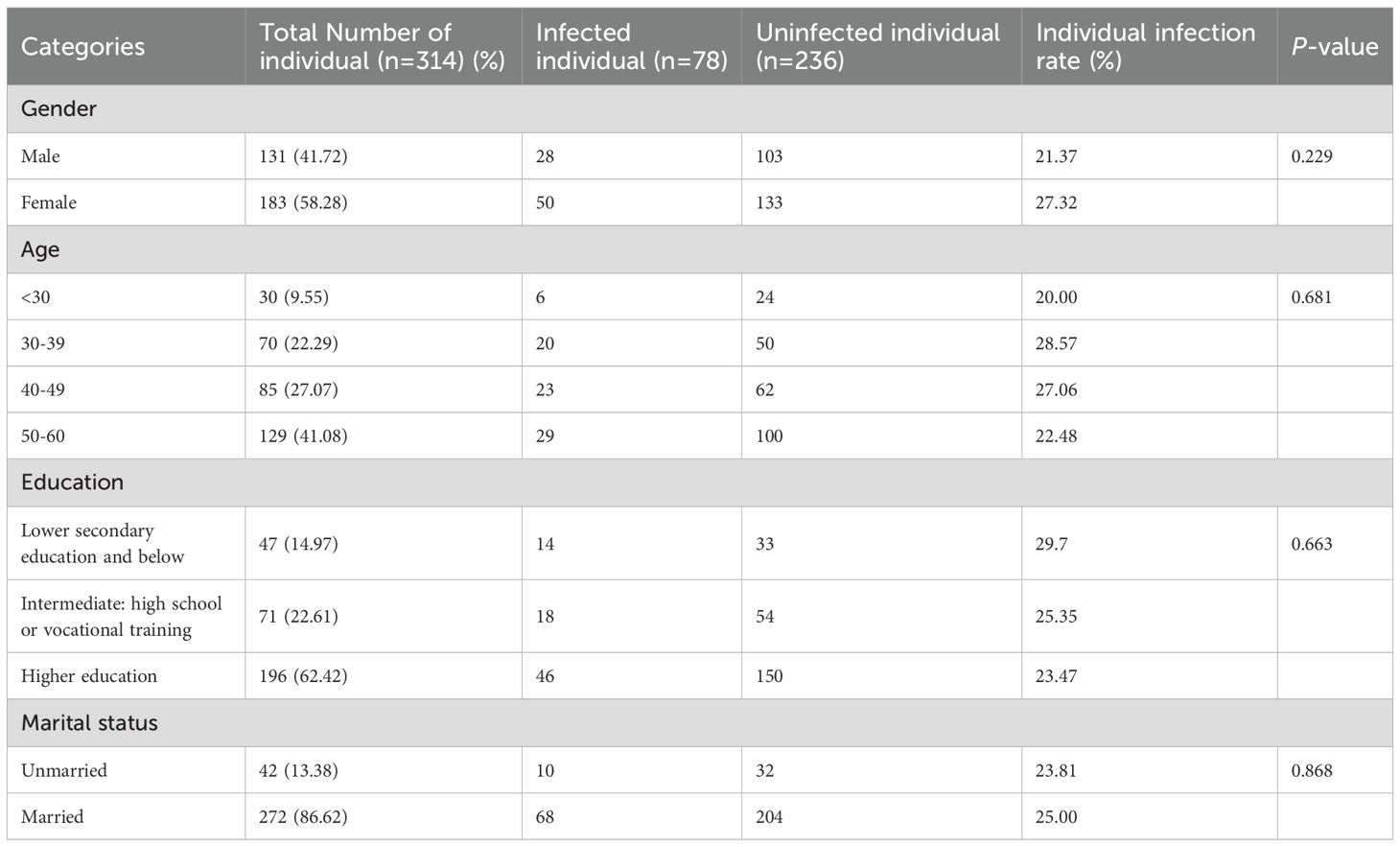
We randomly enrolled 314 participants for gastric fluid sampling via community recruitment. The average age of the participants was 44.83 ± 10.55 years, with 41.72% (n = 131) being male and 58.28% (n = 183) female. Of the participants, 56.36% had a normal weight, while over 40% were overweight or obese. More than half of the participants had a higher education level, with 62.42% having a higher education degree. There were 272 married individuals, accounting for 86.62% of the total population (Table 1).
H. pylori prevalence and antibiotic resistance in the Dongying City
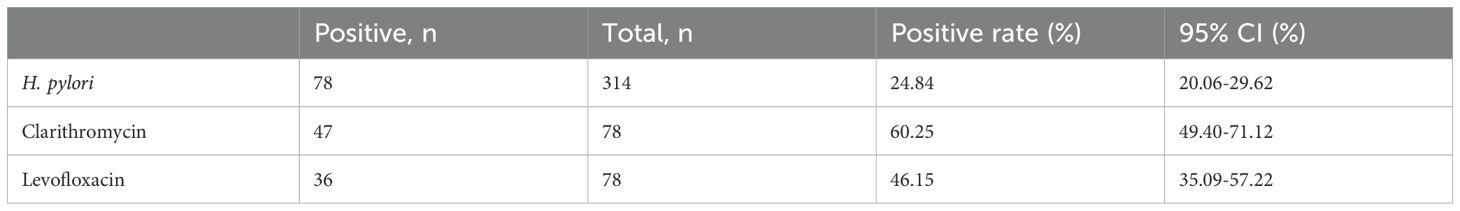
The survey showed that the prevalence of H. pylori infection in the urban area of Dongying City was 24.84% (78/314, 95% CI: 20.06%-29.62%). The resistance rates of H. pylori to clarithromycin and levofloxacin were 60.25% (47/78, 95% CI: 49.40%-71.12%) and 46.15% (36/78, 95% CI: 35.09%-57.22%), respectively (Table 2). These data indicate that H. pylori infection is relatively common in this region and that there is a significant problem with drug resistance, particularly with notable resistance to clarithromycin.
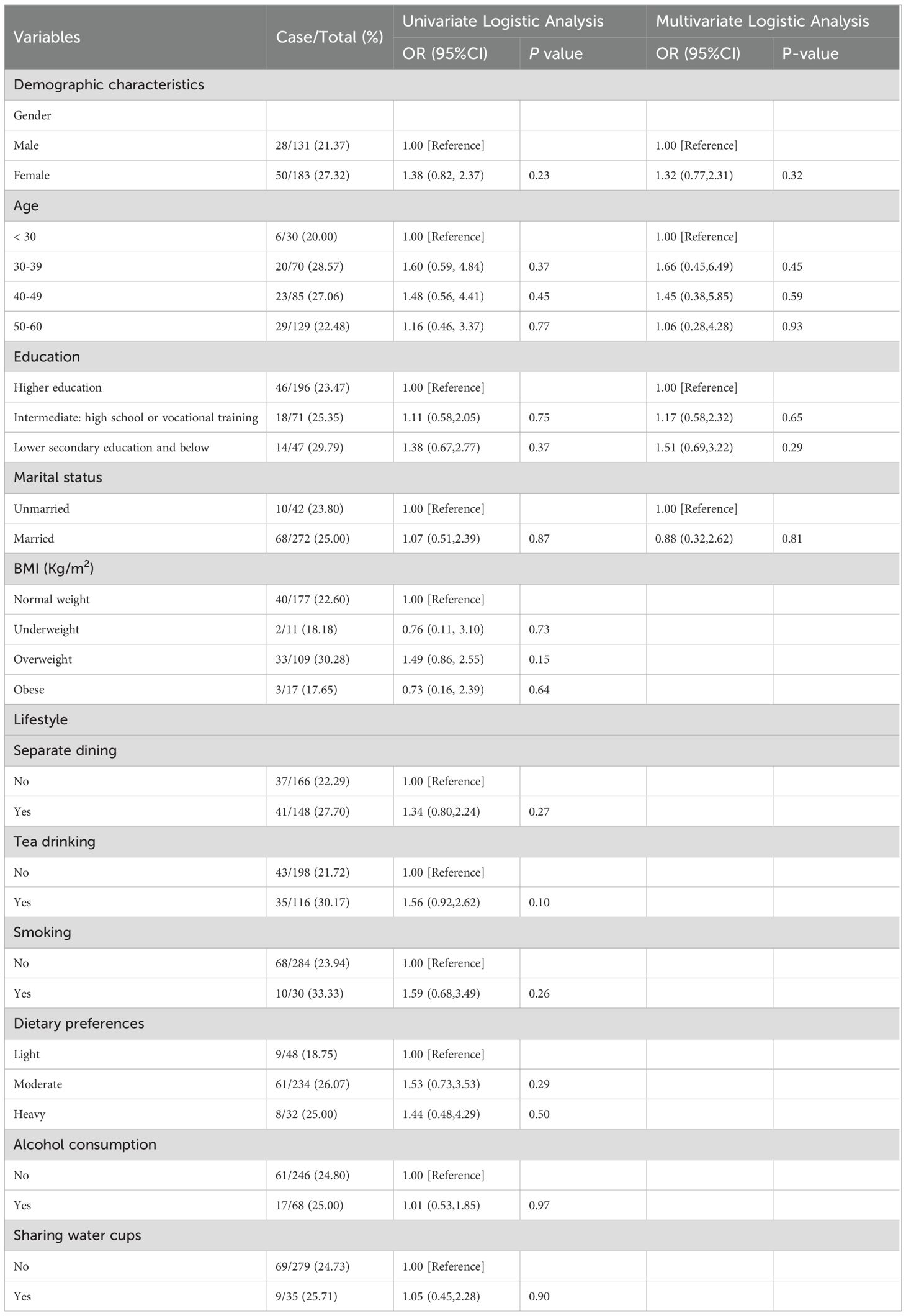
Risk factors associated with the prevalence of H. pylori in the Dongying cohort
Risk factors associated with H. pylori infection in the Dongying cohort are shown in Table 3. In this cohort, there was no significant difference in the infection rates between males (21.37%) and females (27.32%) (P = 0.23). Among the different age groups, the infection rate was highest in the 30–39 age group (OR 1.60, 95% CI 0.59-4.84, P = 0.37), followed by the 40–49 age group (OR 1.48, 95% CI 0.56–4.41, P = 0.45). Compared to individuals with normal weight (22.60%, 40/177), those who were overweight had a higher infection rate (OR 1.49, 95% CI 0.86–2.55, P = 0.15). The educational level also influenced the infection rate. Compared with the lower infection rate in the higher education group (23.47%, 46/196), the infection rates were higher in those with intermediate education (high school or vocational training, OR 1.11, 95% CI 0.58–2.05, P = 0.75) and lower education (secondary education and below, OR 1.38, 95% CI 0.67–2.77, P = 0.37). The infection rate among married individuals (OR 1.07, 95% CI 0.51–2.39, P = 0.87) was slightly higher than that among unmarried individuals.
In addition, we analyzed lifestyle habits such as separate dining, tea drinking, smoking, dietary preferences, alcohol consumption, and water cups. Although no significant association was found between these factors and H. pylori, we noticed that the infection rate of H. pylori was higher among people who practiced separate dining (OR 1.34, 95% CI 0.80–2.24, P = 0.27), tea drinking (OR 1.56, 95% CI 0.92–2.62, P = 0.10), and smoking (OR 1.59, 95% CI 0.68–3.49, P = 0.26). We included gender, age, education level, and marital status in the multivariate analysis to further assess their relationship with H. pylori infection. However, this study did not identify any risk factors significantly associated with H. pylori infection (see Table 3).
Discussion
This study aims to assess the prevalence, antibiotic resistance, and associated risk factors of H. pylori infection in the urban population of Dongying City, China. Non-invasive methods for detecting H. pylori infection status and antibiotic resistance are crucial. The study by Losurdo et al. demonstrated that non-invasive molecular detection in fecal samples is a feasible and effective method for accurately detecting H. pylori infection and its antibiotic resistance (Losurdo et al., 2020). However, considering the potential interference caused by the microbial diversity in fecal samples, we used the string test combined with qPCR to determine H. pylori infection status and antibiotic resistance in gastric fluid samples (Wang et al., 2024). The string test method effectively reduces the invasiveness of obtaining gastric mucosal tissue and fluid samples through endoscopy. Furthermore, using gastric fluid samples helps avoid sampling representativeness issues caused by localized H. pylori colonization in the gastric mucosa (Yang et al., 2023). The combination of qPCR enables precise detection of multiple targets by selecting different primers and probes, allowing for the simultaneous detection of H. pylori infection status and its antibiotic resistance (Pabinger et al., 2014).
The prevalence of H. pylori infection shows a declining trend. Li et al. reported that the global estimated prevalence of H. pylori decreased from 58.2% (95%CI: 50.7–65.8) between 1980 and 1990 to 43.1% (95%CI: 40.3–45.9) between 2011 and 2022 (Xie et al., 2024). Our results show that the infection rate of H. pylori in Dongying City, Shandong Province, is 24.84%, which is lower than the overall infection rate of 36.7% diagnosed by Kong et al. using the 13C-urea breath test on 1,173 participants in six regions of Shandong Province (Kong et al., 2022). The prevalence of H. pylori infection varies widely among countries and regions (Xu et al., 2014). Our study found a lower infection rate than the prevalence rates obtained using the same detection methods, such as 27.08% in urban areas of China (Wang et al., 2024). Specifically, when compared to other Chinese cities, such as Quanzhou, which reported a rate of 52.60% (Xie et al., 2025), the infection rate observed in our study was significantly lower.
Appropriate antibiotic selection and combination therapy are key to successfully eradicating H. pylori. Currently, guidelines in China recommend H. pylori eradication with bismuth quadruple therapy (BQT) and high-dose dual therapy (HDDT) (Ding et al., 2022). Bismuth quadruple therapy includes a proton pump inhibitor, bismuth, metronidazole, and either tetracycline or amoxicillin. High-dose dual therapy consists of high doses of a proton pump inhibitor and amoxicillin. In our study, H. pylori-positive patients from Dongying City in Shandong Province showed resistance rates to clarithromycin and levofloxacin above the 15% threshold. Existing data show that in most provinces, the primary and secondary resistance rates to clarithromycin, metronidazole, and levofloxacin exceed 15%, with clarithromycin at 30.72% (95% CI: 27.53%–33.99%), metronidazole at 70.14% (95% CI: 29.53%–37.46%), and levofloxacin at 32.98% (95% CI: 28.73%–37.37%). However, the resistance rates to amoxicillin, tetracycline, and furazolidone are generally below 15%, with amoxicillin at 2.41% (95% CI: 1.43%–3.60%), tetracycline at 2.53% (95% CI: 1.19%–4.28%), and furazolidone at 1.54% (95% CI: 0.28%–3.62%) (Zeng et al., 2024). Notably, resistance varies significantly across different regions. The clarithromycin resistance rate is relatively low in the eastern (24%) and southern regions (24%), but higher in the western regions. Similarly, the resistance to metronidazole has decreased in the northern regions, dropping from 71% to 62% over the years, but remains high in other areas (Chen et al., 2022). These regional differences may be related to various factors such as local antibiotic usage habits, distribution of healthcare resources, patient adherence to treatment, and regional microbial community variations.
Factors associated with H. pylori infection have not been established (Yan et al., 2013). Regional factors such as diet, sanitation, and healthcare access significantly influence H. pylori infection rates and resistance patterns. Although this study analyzed several risk factors for H. pylori infection, excluding socioeconomic status and family history may lead to confounding bias. According to previous studies (Zhou et al., 2023), these factors significantly impact H. pylori infection. Therefore, future studies should incorporate these factors to more comprehensively assess the risks. However, we could not identify any independent risk factors for H. pylori infection in this study. This result may be related to the study design, sample characteristics, or other uncontrolled variables and warrants further investigation. Our findings provide important insights into the prevalence of antibiotic resistance among H. pylori strains, particularly for commonly used antibiotics such as clarithromycin and levofloxacin. These data are essential for clinicians in tailoring treatment strategies that are both effective and aligned with local resistance patterns.
This study’s strength lies in using highly accurate methods to detect H. pylori. However, it has limitations. First, the cross-sectional design prevents establishing causal links between H. pylori and risk factors. Second, random enrollment without stratified sampling may limit population representativeness. Although Dongying’s residents come from diverse regions, the small sample size restricts generalizability. Third, this study did not conduct a detailed analysis of risk factors related to antibiotic resistance, such as demographic factors and previous antibiotic use. This limitation may affect the interpretation of the study results. Future research should further explore the impact of these factors on antibiotic resistance rates to guide targeted resistance mitigation strategies. Finally, the lack of methodological comparisons within this cohort may affect result reliability, and the method’s applicability to other populations requires further validation. Future research should aim to expand its scope, focusing on a comprehensive analysis of H. pylori resistance patterns. As commonly used antibiotics in H. pylori eradication regimens, metronidazole and amoxicillin resistance patterns merit in-depth investigation. Previous studies have reported that the resistance rate of metronidazole in China exceeds 50%, while the resistance rate of amoxicillin remains relatively low. Future studies should also consider increasing sample sizes and optimizing study designs, such as incorporating longitudinal studies to establish causal relationships and employing stratified sampling methods to enhance population representativeness. These efforts would further improve the findings’ scientific rigor and practical applicability.
Conclusion
Among the urban population on the eastern coast of Dongying, China, the infection rate of H. pylori was 24.84%, with clarithromycin resistance at 60.25% and levofloxacin resistance at 46.15%. Females are more susceptible to H. pylori infection than men. These findings will aid in formulating specific measures to prevent H. pylori infection in Dongying Province. At the same time, the updated resistance rates to Clarithromycin and Levofloxacin provide local guidance for the personalized clinical treatment of bacterial infections. This approach will help reduce antibiotic resistance and improve eradication success rates, ultimately lowering the overall prevalence of H. pylori infection in the region.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Review Committee of Guangdong Provincial People’s Hospital (Ethics Approval No. KY-Q-2022-384-02). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
H-JC: Data curation, Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. Y-TS: Formal Analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft. LL: Investigation, Methodology, Validation, Writing – original draft. J-XL: Formal Analysis, Methodology, Validation, Visualization, Writing – original draft. J-LW: Resources, Supervision, Writing – original draft. Y-RT: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. LW: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Research Foundation for Advanced Talents of Guangdong Provincial People’s Hospital (Grant No. KY012023293).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Beal, C. B., Viens, P., Grant, R. G., Hughes, J. M. (1970). A new technique for sampling duodenal contente. Demonstration of upper small-bowel pathogens. Am J Trop Med Hyg 19(2):349–52. doi: 10.4269/ajtmh.1970.19.349
Burucoa, C., Axon, A. (2017). Epidemiology of Helicobacter pylori infection. Helicobacter 22, e12403. doi: 10.1111/hel.2017.22.issue-S1
Chen, J., Li, P., Huang, Y., Guo, Y., Ding, Z., Lu, H. (2022). Primary antibiotic resistance of Helicobacter pylori in different regions of China: a systematic review and meta-analysis. Pathogens 11, 786. doi: 10.3390/pathogens11070786
Chiang, T. H., Chang, W. J., Chen, S. L., Yen, A. M., Fann, J. C., Chiu, S. Y., et al. (2021). Mass eradication of Helicobacter pylori to reduce gastric cancer incidence and mortality: a long-term cohort study on Matsu Islands. Gut 70, 243–250. doi: 10.1136/gutjnl-2020-322200
Consultation, W. (2000). Obesity: preventing and managing the global epidemic. World Health Organ. Tech. Rep. Ser. 894, 1–253.
Ding, S.-Z., Du, Y.-Q., Lu, H., Wang, W.-H., Cheng, H., Chen, S.-Y., et al. (2022). Chinese consensus report on family-based Helicobacter pylori infection control and management, (2021 edition). Gut 71, 238–253. doi: 10.1136/gutjnl-2021-325630
Domínguez-Bello, M. G., Cienfuentes, C., Romero, R., García, P., Gómez, I., Mago, V., et al. (2001). PCR detection of Helicobacter pylori in string-absorbed gastric juice. FEMS Microbiol. Lett. 198, 15–16. doi: 10.1016/S0378-1097(01)00120-3
Greenberg, E. R., Anderson, G. L., Morgan, D. R., Torres, J., Chey, W. D., Bravo, L. E., et al. (2011). 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet 378, 507–514. doi: 10.1016/S0140-6736(11)60825-8
Han, X., Yu, X., Gao, X., Wang, X., Tay, C. Y., Wei, X., et al. (2023). Quantitative PCR of string-test collected gastric material: A feasible approach to detect Helicobacter pylori and its resistance against clarithromycin and levofloxacin for susceptibility-guided therapy. Helicobacter 28, e12985. doi: 10.1111/hel.12985
Hong, T. C., El-Omar, E. M., Kuo, Y. T., Wu, J. Y., Chen, M. J., Chen, C. C., et al. (2024). Primary antibiotic resistance of Helicobacter pylori in the Asia-Pacific region between 1990 and 2022: an updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 9, 56–67. doi: 10.1016/S2468-1253(23)00281-9
Hooi, J. K. Y., Lai, W. Y., Ng, W. K., Suen, M. M. Y., Underwood, F. E., Tanyingoh, D., et al. (2017). Global prevalence of helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology 153, 420–429. doi: 10.1053/j.gastro.2017.04.022
Kong, Q., Li, Y., Li, R., Li, Z., Zheng, X., Wang, Z., et al. (2022). Low compliance to post-screening recommendations in a family-based Helicobacter pylori screening and treatment program: A prospective cohort study. Helicobacter 27, e12912. doi: 10.1111/hel.12912
Li, Y., Choi, H., Leung, K., Jiang, F., Graham, D. Y., Leung, W. K. (2023). Global prevalence of Helicobacter pylori infection between 1980 and 2022: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 8, 553–564. doi: 10.1016/S2468-1253(23)00070-5
Losurdo, G., Giorgio, F., Pricci, M., Girardi, B., Russo, F., Riezzo, G., et al. (2020). Helicobacter pylori primary and secondary genotypic resistance to clarithromycin and levofloxacin detection in stools: a 4-year scenario in southern Italy. Antibiotics 9, 723. doi: 10.3390/antibiotics9100723
Mégraud, F., Lehours, P. (2007). Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 20, 280–322. doi: 10.1128/CMR.00033-06
Molina–Infante, J., Romano, M., Fernandez–Bermejo, M., Federico, A., Gravina, A. G., Pozzati, L., et al. (2013). Optimized nonbismuth quadruple therapies cure most patients with Helicobacter pylori infection in populations with high rates of antibiotic resistance. Gastroenterology 145, 121–128.e121. doi: 10.1053/j.gastro.2013.03.050
Pabinger, S., Rödiger, S., Kriegner, A., Vierlinger, K., Weinhäusel, A. (2014). A survey of tools for the analysis of quantitative PCR (qPCR) data. Biomolecular Detection Quantification 1, 23–33. doi: 10.1016/j.bdq.2014.08.002
Thung, I., Aramin, H., Vavinskaya, V., Gupta, S., Park, J., Crowe, S., et al. (2016). the global emergence of Helicobacter pylori antibiotic resistance. Alimentary Pharmacol. Ther. 43, 514–533. doi: 10.1111/apt.2016.43.issue-4
Wang, L., Lai, J.-X., Si, Y.-T., Cui, X.-X., Umar, Z., Ru, X.-J., et al. (2023). Quantitative polymerase chain reaction (qPCR)-based rapid diagnosis of Helicobacter pylori infection and antibiotic resistance. JoVE (Journal Visualized Experiments) 197), e65689. doi: 10.3791/65689
Wang, L., Li, Z., Tay, C. Y., Marshall, B. J., Gu, B., Tian, Y., et al. (2024). Multicentre, cross-sectional surveillance of Helicobacter pylori prevalence and antibiotic resistance to clarithromycin and levofloxacin in urban China using the string test coupled with quantitative PCR. Lancet Microbe 5, e512–e513. doi: 10.1016/S2666-5247(24)00027-2
Wang, S.-W., Yu, F.-J., Lo, Y.-C., Yang, Y.-C., Wu, M.-T., Wu, I.-C., et al. (2003). The clinical utility of string-PCR test in diagnosing Helicobacter pylori infection. Hepato-gastroenterology 50, 1208–1213.
Xie, L., Liu, G.-W., Liu, Y.-N., Li, P.-Y., Hu, X.-N., He, X.-Y., et al. (2024). Prevalence of Helicobacter pylori infection in China from 2014-2023: A systematic review and meta-analysis. World J. Gastroenterol. 30, 4636–4656. doi: 10.3748/wjg.v30.i43.4636
Xie, D., Xu, W., Zhang, Z., Huang, F., Dai, X. (2025). Epidemiological surveys, antibiotic resistance, and related risk factors of Helicobacter pylori in Quanzhou, China: a cross-sectional study. Sci. Rep. 15, 4410. doi: 10.1038/s41598-025-89073-2
Xu, C., Yan, M., Sun, Y., Joo, J., Wan, X., Yu, C., et al. (2014). Prevalence of Helicobacter pylori infection and its relation with body mass index in a Chinese population. Helicobacter 19, 437–442. doi: 10.1111/hel.2014.19.issue-6
Yamada, T., Searle, J. G., Ahnen, D., Aipers, D. H., Greenberg, H. B., Gray, M., et al. (1994). Helicobacter pylori in peptic ulcer disease. Jama 272, 65–69. doi: 10.1001/jama.1994.03520010077036
Yan, T. L., Hu, Q. D., Zhang, Q., Li, Y. M., Liang, T. B. (2013). National rates of H elicobacter pylori recurrence are significantly and inversely correlated with human development index. Alimentary Pharmacol. Ther. 37, 963–968. doi: 10.1111/apt.2013.37.issue-10
Yan, X., Zeng, H., Li, H., Cao, M., Yang, F., He, S., et al. (2024). The current infection with Helicobacter pylori and association with upper gastrointestinal lesions and risk of upper gastrointestinal cancer: Insights from multicenter population-based cohort study. Int. J. Cancer 155(7):1203–1211. doi: 10.1002/ijc.34998
Yang, H., Wang, L., Zhang, M., Hu, B. (2023). The role of adhesion in Helicobacter pylori persistent colonization. Curr. Microbiol. 80, 185. doi: 10.1007/s00284-023-03264-6
Zeng, S., Kong, Q., Wu, X., Duan, M., Nan, X., Yang, X., et al. (2024). Antibiotic Resistance of Helicobacter pylori in Mainland China: A Focus on Geographic Differences through Systematic Review and Meta-analysis. Int. J. Antimicrobial Agents 64(5), 107325. doi: 10.1016/j.ijantimicag.2024.107325
Keywords: prevalence, antibiotic resistance, string test, H. pylori, qPCR
Citation: Chen H-J, Si Y-T, Luan L, Lai J-X, Wang J-L, Tang Y-R and Wang L (2025) Current rates of Helicobacter pylori infection and antibiotic resistance in the eastern coast of China: a single center study. Front. Cell. Infect. Microbiol. 15:1561778. doi: 10.3389/fcimb.2025.1561778
Received: 16 January 2025; Accepted: 13 March 2025;
Published: 01 April 2025.
Edited by:
Tales Fernando da Silva, Institute of Biological Sciences, BrazilReviewed by:
Giuseppe Losurdo, University of Bari Aldo Moro, ItalyDaniela Araújo, National Institute for Agricultural and Veterinary Research (INIAV), Portugal
Tamer A. Addissouky, University of Menoufia, Egypt
Xianzhu Zhou, Second Military Medical University, China
Copyright © 2025 Chen, Si, Luan, Lai, Wang, Tang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Wang, aGVhbHRoc2NpZW5jZUBmb3htYWlsLmNvbQ==; Yu-Rong Tang, enh5eWp5a3R5ckAxNjMuY29t
†These authors have contributed equally to this work
 Hui-Jin Chen
Hui-Jin Chen Yu-Ting Si2†
Yu-Ting Si2† Ji-Liang Wang
Ji-Liang Wang Liang Wang
Liang Wang