
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 10 April 2025
Sec. Bacteria and Host
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1553688
This article is part of the Research TopicComprehensive Insights into Microbial Infection: From Pathogenesis to Therapeutic solutionsView all articles
 Rahvia Alam Sthity1
Rahvia Alam Sthity1 Md. Zahidul Islam1
Md. Zahidul Islam1 Md. Ehsanul Kabir Sagar1
Md. Ehsanul Kabir Sagar1 Md. Amran Gazi1,2
Md. Amran Gazi1,2 Jafrin Ferdous1
Jafrin Ferdous1 Md. Mamun Kabir3
Md. Mamun Kabir3 Mustafa Mahfuz1
Mustafa Mahfuz1 Tahmeed Ahmed4,5
Tahmeed Ahmed4,5 Ishita Mostafa1,6*
Ishita Mostafa1,6*Introduction: Environmental enteric dysfunction (EED), a subclinical intestinal disorder, is characterized by chronic fecal-oral exposure to entero-pathogens and could be diagnosed by measuring non-invasive biomarkers. Escherichia coli is the one of the key bacterial enteric pathogens that drives EED, but there is a lack of information on the E. coli pathotypes in relation to the biomarkers of EED in malnourished adults. Here, we intended to measure the possible association of these pathotypes with EED biomarkers and nutritional status of adults residing in a slum in Bangladesh.
Method: Fecal samples were collected from 524 malnourished adults (BMI ≤18.5 kg/m2) living in a slum-setting in Dhaka from March 2016 to September 2019 and analyzed by TaqMan Array Card assays to evaluate the presence of E. coli pathotypes and other entero-pathogens. The multivariable linear regression model was used to assess the association.
Results: In these malnourished adults, the most prevalent pathotype of E. coli was EAEC (61.7%) and the least prevalent was STEC (6.7%). The prevalence of atypical EPEC, ETEC and Shigella/EIEC were 52%, 48.9% and 45.1% respectively. The infection with atypical EPEC had significant positive association with levels of Myeloperoxidase (b = 0.38; 95% CI = 0.11, 0.65; p-value = 0.006). Similarly, a significantly higher concentration of alpha-1-antitrypsin (b = 0.13; 95% CI = 0.03, 0.22; p-value = 0.011) was found in the STEC-infected adults. However, no notable association was found between the E. coli pathotypes and nutritional status of these adult participants. Moreover, Plesiomonas infected adults were more likely to be infected with EAEC (p-value = 0.017), ETEC (p-value <0.001) and STEC (pvalue = 0.002). Significant coinfection was also detected among the pathotypes and other entero-pathogens such as Giardia, Ascaris, Campylobacter, Salmonella, Enterocytozoon bieneusi, and Adenovirus.
Discussion: The study results imply that there is an influence of particular E. coli pathotypes (EPEC and STEC) on intestinal inflammation and gut permeability of the malnourished Bangladeshi adults, but no association with nutritional status is found. Potential pathogenicity of the E. coli pathotypes is also observed when co-infection with other pathogens exists in these adults.
Diarrheagenic Escherichia coli (DECs) poses a significant public health threat to both children and adults, leading to the occurrence of diarrhea. These pathogens are most commonly transferred from human or animal waste to vulnerable hosts by contact with food and various environmental sources (such as water, soil, hands and flies) (Navab-Daneshmand et al., 2018). The disease mostly affects the developing world, particularly Africa, Asia, and Latin America. The 2015 Global Burden of Disease (GBD) study aimed to identify the causes of deaths associated to diarrheal disease in Bangladesh. It found that E. coli was one of the main factors responsible for these deaths in the region (Troeger et al., 2017). Child malnutrition, inadequate sanitation, contaminated water sources, poor water quality, and improper cooking methods, among other factors, contribute to the majority of cases (Croxen et al., 2013; Troeger et al., 2018). These enteric pathogens activate the immune system in the gut, resulting in both systemic and local inflammation. These conditions disrupt the normal functioning of the gut by increasing the permeability, decreasing the intestinal barrier function and damaging the structure of the gut. The term used to describe these conditions is Environmental Enteric Dysfunction (EED) (Budge et al., 2019).
One study found a strong correlation between increased levels of E. coli in drinking water and ready-to-eat meals and higher concentrations of fecal biomarkers of EED, as well as a higher EED composite score in children (Gizaw et al., 2022). To diagnose EED, the gold standard is an intestinal biopsy; however, it is an invasive method (Syed et al., 2016). Hence, a combination of biomarkers, such as Myeloperoxidase (MPO), Neopterin (NEO), Alpha-1 anti-trypsin (AAT), Calprotectin, and regenerative family member 1 beta (Reg1B), can be used to evaluate EED (Bartelt et al., 2019; Tickell et al., 2019). MPO, a neutrophil-specific marker, reacts with hydrogen peroxide to release highly cytotoxic substances that aid in the elimination of foreign microbes. Nevertheless, these harmful products can also harm healthy tissue and lead to inflammation in the gastrointestinal tract (Hansberry et al., 2017). The selection of fecal NEO as a marker for intestinal cell-mediated inflammation is based on its release from phagocytic cells by an IFN-dependent manner under circumstances involving cell-mediated inflammation (Seki et al., 1996). The concentration of AAT in the gut rises when there is an increase in permeability or when the mucosal barrier of the gut is damaged. Extravasation of serum into the gut and subsequent presence in fecal matter make it a suitable biomarker for assessing intestinal permeability (Wang et al., 2015). Calprotectin is a protein present in white blood cells that becomes active in the presence of inflammation. This characteristic makes calprotectin an efficient biomarker for identifying inflammation in the gut (Kapel et al., 2010; Jukic et al., 2021). Reg1B functions as an indicator, signaling damage to the epithelial tissue and subsequent repair mechanisms in the small intestine (Syed et al., 2016).
Although EED is typically acquired in early childhood, it can also continue into adulthood, and it was initially identified in the adults (Crane et al., 2015). In addition, underweight, a persistent issue in many developing nations, stems from various factors such as diarrhea, inadequate sanitation, and starvation (Black et al., 2013). DEC pathotypes are a primary contributor to diarrhea in adults, especially in developing countries (Nataro and Kaper, 1998). DEC strains have been classified into separate groups according to their distinctive virulence factors and phenotypic features. These categories comprise Enteroaggregative E. coli (EAEC), Enteropathogenic E. coli (EPEC), Enterotoxigenic E. coli (ETEC), Enteroinvasive E. coli (EIEC) and shiga-like toxin (STEC) (Gomes et al., 2016). PCR tests now easily identify the specific genetic features that define these pathotypes. EPEC is primarily associated with childhood diarrhea, while ETEC and EAEC are commonly associated with traveler’s diarrhea. EIEC is typically associated with a more severe disease, often characterized by bloody diarrhea. STEC is also associated with bloody diarrhea and is frequently accompanied by thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS). Additionally, EAEC has been associated with persistent diarrhea and gastrointestinal disorders in developing countries (Croxen et al., 2013). EPEC consists of two different groups of organisms: typical EPEC (tEPEC) and atypical EPEC (aEPEC). The differentiation between tEPEC and aEPEC strains relies on the presence of the bundle-forming pilus (BFP), an adhesive structure essential for the adherence of bacterial strains to the epithelial cells of the intestine. Strains that exhibit BFP are categorized as tEPEC, whereas those that do not possess BFP are classified as aEPEC. aEPEC exhibits greater heterogeneity compared to tEPEC regarding phenotypic traits and virulence factors (Gomes and Pedrajo, 2012). tEPEC is more frequently isolated in diarrheal patients from developing countries, while aEPEC isolation is more prevalent in developed countries (Trabulsi et al., 2002; Kotloff et al., 2013). Prior studies conducted in different countries have examined the various DEC strains and their correlation with EED biomarkers. Researchers had previously reported an elevated plasma neopterin level in young Norwegians infected with ETEC (Rim et al., 2024).
Modgil et al. observed a notable rise in calprotectin levels in EAEC-infected mice isolated from symptomatic cases (Modgil et al., 2022). Gazi et al. conducted an analysis of various fecal markers of inflammation in children between the ages of 12 and 18 months. It was found that infection with specific strains of E. coli bacteria (EAEC and ETEC) had an impact on the gastrointestinal health of undernourished children in Bangladesh. ETEC infections resulted in elevated calprotectin levels and decreased NEO levels, whereas MPO levels appeared to remain unchanged (Gazi et al., 2022). The majority of existing studies have primarily examined the infection in newborns or children under five years of age, with less research available on adults. In addition, previous research has investigated potential correlations between specific E. coli pathotypes and EED biomarkers. However, to the best of our knowledge, no investigations have included all pathotypes in adults at the same time. Hence, our study aims to investigate the correlation between infections caused by different strains of E. coli and biomarkers indicating EED and nutritional status (body mass index, BMI) in undernourished adults residing in Bangladesh.
The data used in this study was obtained from the Bangladesh Environmental Enteric Dysfunction (BEED) project, which was carried out in a slum neighborhood in Dhaka, Bangladesh. The BEED project was a community-based nutrition intervention trial that included adults with malnutrition (BMI ≤18.5 kg/m2). The study spanned from March 2016 to September 2019, and the participants’ ages ranged from 18 to 45 years. Fecal samples were collected from 524 participants for this investigation. The study excluded individuals with severe anemia, tuberculosis, psychiatric disorder, and any chronic conditions. The study excluded pregnant and lactating women, as well as individuals diagnosed with cancer. During the enrollment process, our trained field workers collected socio-demographic information and anthropometric data. The BEED study aims to assess the effectiveness of nutrition intervention in enhancing the nutritional status of the participants, exploring the contribution of enteric pathogens to the development of EED and malnutrition, and creating a histological scoring system to detect EED and validate the score using non-invasive biomarkers of EED (Fahim et al., 2021). The detailed methodology of the BEED study was previously published (Mahfuz et al., 2017).
The laboratory experiments were conducted at the “Emerging Infections & Parasitology Laboratory” of icddr,b. Plasma was separated from blood samples using centrifuges at 4000 rotations per minute for 10 minutes. Plasma and stool samples were frozen at -80°C until analysis. Alpha-1-acid glycoprotein (AGP) (Alpco, Salem, NH), C-reactive protein (CRP) (Immundiagnostik, Bensheim, Germany), and ferritin (ORGENTEC Diagnostika GmbH, 55129 Mainz, Germany) were among the plasma biomarkers analyzed using ELISA. The plasma zinc concentration was quantified by atomic absorption spectrometry. Commercial ELISA kits were used to assess the fecal biomarkers for instance, MPO (Alpco, Salem, New Hampshire), AAT (Biovendo Chandler, North Carolina), NEO (GenWay Biotech, San Diego, California), Calprotectin (BUHLMANN fCAL, Schonenbuch, Switzerland) and Reg1B (TechLab, Blacksburg, Virginia). A quantitative PCR test employing TaqMan Array Cards (TAC) by Applied Biosystems (Life Technologies Corporation, Carlsbad, CA) was used to detect the presence of E. coli pathotypes and other enteropathogens in stool samples. This test is a 384-well singleplex probe-based real-time PCR. In this experiment, nucleic acid extraction was performed using a modified procedure that involved bead beating with the QIAamp Fast DNA Stool Mini Kit (Qiagen, Hilden, Germany) (Ijarotimi, 2013). Phocine herpes virus and MS2 bacteriophage were included as external controls during nucleic acid extraction to assess the efficacy of both the extraction and amplification processes. Contamination was assessed by monitoring extraction blanks and no-template controls. The cutoff for finding pathogens in the TAC test was a Ct-value of ≤35 which was seen as an indication of pathogen positivity. The previous literature has provided a detailed description of the performance and interlaboratory reproducibility of the TAC technique and the specifics on TAC design, including the primer sequences (Liu et al., 2013; Platts-Mills et al., 2017).
The main aim of this study was to assess the association of nutritional status (BMI) of Bangladeshi underweight adults and EED biomarkers with the presence of E. coli pathotypes in the feces. Accordingly, E. coli pathotypes were exposure variables and on the basis of the presence of E. coli pathotypes derived from TAC findings. In this study, our main outcome variables were the nutritional status of Bangladeshi adults (BMI) and fecal biomarkers (NEO, MPO, AAT, Reg1B and Calprotectin). For the nutritional status BMI model, age, sex, household monthly income, wash indices, EED biomarkers, zinc, and ferritin were considered covariates. On the other hand, for the EED biomarker model, the age, sex, household monthly income, wash indices (a score that was determined by answering five hygiene-related queries, e.g. handwashing, use of toilet paper etc.), inflammatory biomarkers (AGP, CRP), zinc, and ferritin were considered covariates.
The socioeconomic and demographic features were represented using frequency and percentage for categorical data, median with inter-quartile ranges (IQR) for asymmetric continuous data, and mean with standard deviation for symmetric continuous variables. The EED biomarkers were compared across the groups of E. coli pathotypes that tested positive and negative using either a t-test or a Mann-Whitney U test. The concentrations of fecal biomarkers (Reg1B, AAT, MPO, NEO and Calprotectin) were log transformed and made symmetric before being incorporated into the multivariate model. We applied multivariable linear regression to analyze the association between E. coli pathotypes and concentrations of EED biomarkers, as well as the relationship between E. coli pathotypes and the nutritional status indicator BMI in adults. All the models were adjusted by some covariates; covariates were selected based on available literature and their biological and clinical significance. A probability of 0.05 was considered to have statistical significance. The statistical studies were conducted using the STATA program, specifically version 15.0.
A total of 524 patients were included in the specific analyses conducted as part of the EDD study in Bangladesh (Modgil et al., 2022). Out of all the participants, the proportion of female participants was the highest at 72%. The average age of the participants was 23.8 years with a standard deviation of 6.9 years (Table 1).
Out of the many E. coli pathotypes, including EAEC, tEPEC, aEPEC, ETEC, ST_ETEC, LT_ETEC, Shigella/EIEC, and STEC, EAEC had the highest prevalence, accounting for 61.7% (Figure 1). The incidence of heat-stable ETEC (ST-ETEC) was greater at 38.7% compared to heat-labile ETEC (LT-ETEC) at 10.1%. In comparison, the prevalence of aEPEC was greater at 52%, whereas the prevalence of tEPEC was only 5.9%.
To investigate the coinfection of E. coli pathotypes with other enteric pathogens an analysis was conducted. Coinfections may affect disease severity, immunological responses, and intestinal inflammation, thereby altering the biomarker profiles identified in this investigation. The findings (Supplementary Table S1) indicate that EAEC was significantly coinfected with aEPEC (p = <0.001), ETEC (p = <0.001), and ST_ETEC (p = <0.001). Additionally, ETEC showed coinfection with aEPEC (p = 0.027), and aEPEC was found to be coinfected with ST_ETEC (p = 0.001). Blastocystis showed a considerable co-infection with aEPEC (p=0.001) and ST_ETEC (p=0.043), while Plesiomonas also exhibited significant co-infection with EAEC (p=0.014), ETEC (p=<0.001), and ST_ETEC (p=<0.001). Furthermore, there was a significant association between Campylobacter-Pan Genome and LT_ETEC (p = 0.024) as well as Shigella/EIEC (p = 0.016).
This investigation revealed that EED biomarker levels exhibited no significant differences between Bangladeshi adults infected with EAEC or tEPEC and those without such infections (Supplementary Table S2, Graph SG1 And Graph SG2), indicating that these pathogens may not substantially affect EED responses as assessed by MPO, NEO, AAT, Calprotectin, and REG1B in this population. However, MPO concentrations were significantly higher in aEPEC-positive samples relative to negative samples (p = 0.027). Considering that MPO serves as a biomarker for intestinal inflammation, this observation implies a potential role for aEPEC in triggering an immunological response. Similarly, the notable rise in Calprotectin levels in Shigella/EIEC-positive cases (p = 0.007) indicates that Shigella/EIEC infections can lead to intestinal inflammation. The increased AAT levels in STEC-positive samples (p = 0.006) may suggest a correlation between STEC infection and intestinal permeability. No additional pathotypes of E. coli shown a significant correlation with any other biomarkers of EED.
Multivariate linear regression analysis, adjusting for covariates and potential confounders, demonstrated a significant positive correlation between EPEC infection and the fecal biomarker MPO (β = 0.35; 95 percent CI = 0.09, 0.62; p-value = 0.008), indicating that EPEC may play a role in neutrophil activation and intestinal inflammation (Figure 2; Supplementary Table S3). Likewise, STEC infection had a significant association with increased levels of the fecal biomarker AAT (β = 0.15; 95 percent CI = 0.05, 0.24; p-value = 0.003), supporting its function in intestinal permeability. No additional infections were found to be associated with the remaining biomarkers.
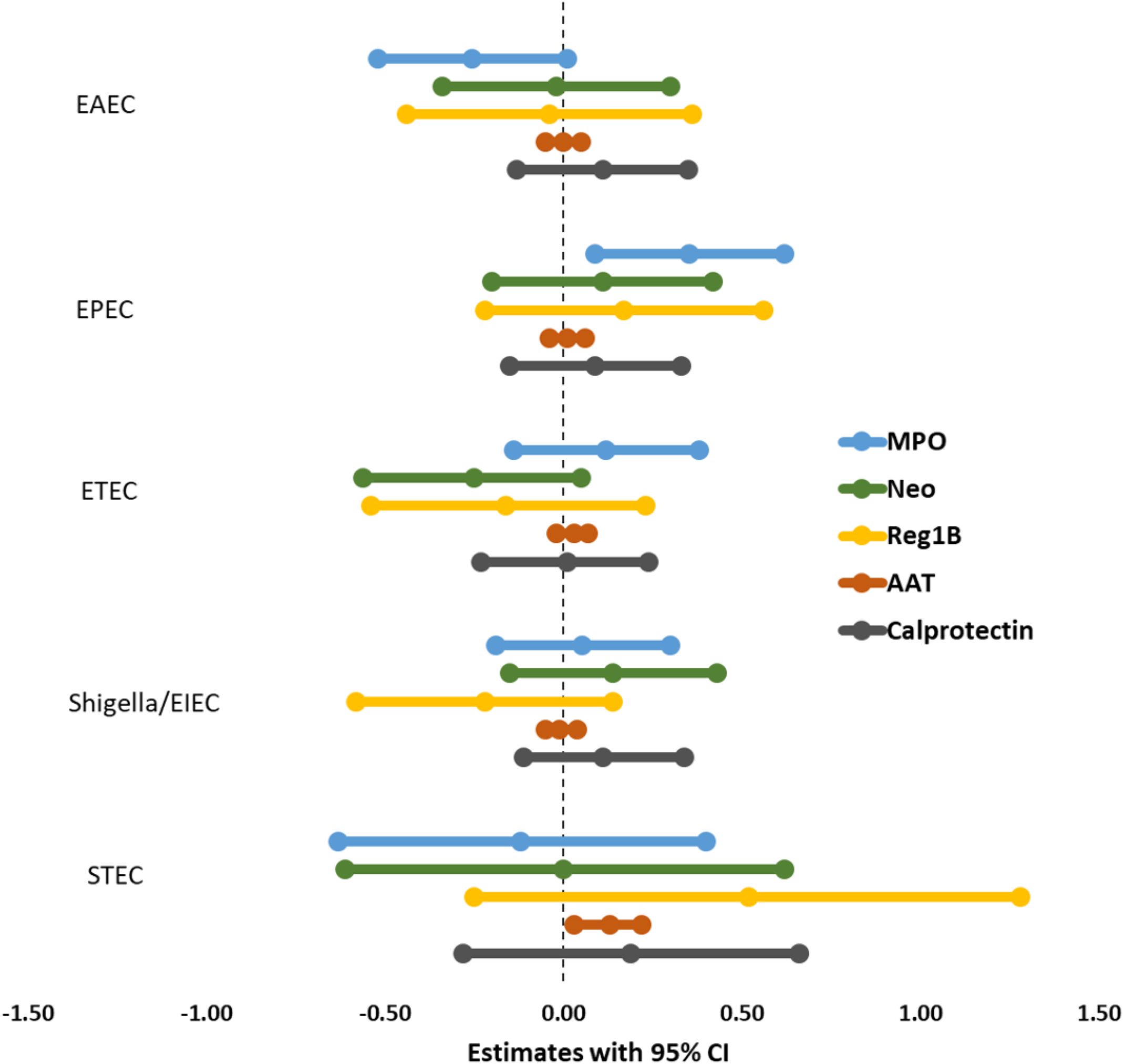
Figure 2. Association between E. coli pathotypes and fecal biomarkers of EED in malnourished adults.
Through a multivariate linear regression analysis that adjusted for covariates and potential confounders, we observed no significant association with BMI and E. coli, suggesting that E. coli pathotypes may not have a direct impact on BMI of this population (Supplementary Table S4; Figure 3)
The analysis of E. coli subtypes showed a notable positive correlation between aEPEC and the fecal biomarker MPO (β = 0.38; 95% CI = 0.11, 0.65; p = 0.006) (Supplementary Table S5). This finding indicates that aEPEC might trigger an intensified neutrophilic inflammatory response in the gastrointestinal tract, as MPO serves as a marker of neutrophil activity. Nonetheless, no other E. coli subtypes or additional EED biomarkers exhibited significant associations, suggesting possible variability in the inflammatory effects of different strains.
An alternative analysis was conducted using two subgroups: E. coli positive and negative (Supplementary Tables S6, S7), and the results were very similar to those in Supplementary Table S2. E. coli was defined as positive if any of the following were positive: EAEC, tEPEC, aEPEC, ETEC, ST_ETEC, LT_ETEC, Shigella/EIEC or STEC. The alternative was E. coli negative.
A multivariable regression analysis was performed separately for male and female participants to investigate potential variations in E. coli infections. However, the results were nearly identical (Supplementary Table S8).
While there are several publications on diarrheal disease in children under the age of five, this study stands out as one of the few that examines the presence of five distinct DEC pathotypes in adults. Diarrhea may be a clinical symptom of EED, indicating exposure to enteric pathogens. However, this exposure can occur with or without diarrhea (Budge et al., 2019). Prior to this investigation, little to nothing was known about the correlation between DEC strains and EED markers in Bangladeshi adults.
This study found that infection with specific pathotypes of E. coli (EPEC, Shigella/EIEC, and STEC) affected gut health biomarkers in adults from Bangladesh. However, there is no correlation between these infections and underweight individuals living in a slum. The findings of our study indicate that the highest prevalence of infection was observed in adults infected with EAEC (61.71%), followed by EPEC (52%), ETEC (48.85%), Shigella/EIEC (45%), and STEC (6.67%). Prior research has indicated that EAEC and EPEC are significant contributors to acute intestinal infections in people residing in industrialized countries (Makarova, 2023). In Chhatak and Mathabaria, Bangladesh, a study found that children under five years and adults aged 20 to 60 years were most susceptible to ETEC diarrhea (Chakraborty et al., 2024). EAEC, EPEC, and ETEC were the most commonly identified pathogens in children having EED, according to a recent study conducted in a slum in Dhaka, Bangladesh (Gazi et al., 2022). Moreover, the present analysis demonstrates a greater occurrence of aEPEC in comparison to tEPEC. Recent research indicates that aEPEC is more common than tEPEC in cases of chronic diarrhea, regardless of whether it occurs in developing or developed nations (Ochoa et al., 2008). Besides, this study reveals that the prevalence of ETEC that expresses the heat-stable enterotoxin was high.
The higher occurrence of E. coli pathotypes identified in our study may be linked to co-infection among E. coli pathotypes or with other intestinal pathogens. Shigella/EIEC showed a strong association with Salmonella, Campylobacter-Pan Genome, Enterocytozoon bieneusi, Trichuris, Adenovirus-Pan Genome, and Aeromonas. Conversely, Plesiomonas exhibited strong correlations with EAEC, ETEC, and STEC. The Campylobacter-Pan Genome showed a high degree of co-infection with ETEC. The presence of Ascaris was found to have a substantial association with EPEC, while the presence of Entamoeba histolytica was found to have a significant association with ETEC. Similarly, Blastocystis showed a significant co-infection with both EPEC and ETEC, whereas Isospora showed a significant co-infection with EPEC and STEC. Additionally, there were cases of coinfections among E. coli pathotypes. Numerous diarrheal studies conducted worldwide have recognized dual infections with intestinal pathogens (Zhang et al., 2016; Ledwaba et al., 2018; Pijnacker et al., 2019). Concurrent infections not only worsen the progression of diseases but also impact the host’s immunological responses to pathogens, undermining the efficiency of disease control programs at the state or country level (Cox, 2001; Read and Taylor, 2001; Li and Zhou, 2013). In any species, the presence of many pathogens can result in more severe diarrhea compared to an infection caused by a single pathogen alone (Grimprel et al., 2008). Nyholm et al. discovered that Diarrheagenic E. coli has the ability to obtain virulence genes from other pathogroups by horizontal gene transfer (Nyholm et al., 2015). This process leads to the development of intermediate pathogroups (Müller et al., 2007), also known as hybrid (Mellmann et al., 2011). A study conducted in Pakistani children under the age of five identified co-infection of EPEC with EHEC and EAEC (Zil-e-Huma et al., 2019). A study conducted in children from rural and peri-urban populations in South Africa revealed the co-infection of DECs with Campylobacter, Salmonella, Cryptosporidium parvum, Rotavirus, and Adenovirus (Potgieter et al., 2023). The presence of EAEC has been detected in individuals who are infected with the human immunodeficiency virus (HIV), particularly in children and travelers in developing nations (Zil-e-Huma et al., 2019).
This study found a strong and positive correlation between the amount of MPO in feces and the presence of atypical EPEC. EPEC infections are commonly linked to inflammatory enteropathy and/or diarrhea in populations with limited resources (Platts-Mills et al., 2015; Rogawski et al., 2018). The diarrhea caused by EPEC infection is a result of elevated ion secretion, increased permeability of the intestines, inflammation in the intestines, and loss of absorptive surface area due to microvillus effacement (Kaper et al., 2004). Both neutrophils and monocytes may become activated by lipopolysaccharides produced by bacteria (Kothari et al., 2011). Researchers suggest that during phagocytosis MPO regulates the respiratory activity of these polymorphonuclear leukocytes (PMNs) (Butterfield et al., 1998). MPO is considered a significant mechanism for O2-dependent microbicidal action. The activation of PMNs leads to an abrupt rise in oxygen consumption, along with the production of reactive oxygen species (ROS) and the secretion of enzymes including elastase and MPO (Woods et al., 2003). Multiple prior studies have demonstrated that solubilized MPO from inflammatory tissue correlates directly with the quantity of neutrophils present (Weissmann et al., 1980; Krawisz et al., 1984). Consequently, an elevated amount of MPO in stool samples indicates intestinal inflammation (Arndt et al., 2016). An earlier study confirmed that elevated levels of fecal MPO correlate with an increased prevalence of enteric pathogens in stool, and this biomarker has been utilized as an indicator of EED (George et al., 2018). Our current finding is consistent with a prior murine model, where MPO levels were elevated during the acute phase of EPEC infection (Ledwaba et al., 2020). LT-ETEC and MPO levels exhibited a significant association in the MAL-ED birth cohort studies (Khalil et al., 2021).
The findings also demonstrated a noteworthy positive correlation between AAT and STEC. The pathogenic processes of STEC are associated with both adherence and toxin generation. STEC attaches to gastrointestinal epithelial cells using the outer membrane protein intimin (Karmali et al., 2010), which is similar to the adherence mechanisms observed in EPEC. Subsequently, the STEC toxin is transported to the kidney either through the bloodstream or by the movement of neutrophils (Hurley et al., 2001). Neutrophils, during the initial stage of diarrhea-associated hemolytic uremic syndrome, are stimulated and become more adhesive than usual. They affect the endothelium by generating elastase complexed with AAT (Zoja et al., 2010). Multiple studies indicate that E. coli can compromise the intestinal barrier and increase permeability within the gastrointestinal tract (Spitz et al., 1995; Mangell et al., 2002). Moreover, Alpha-1-antitrypsin functions as a protease inhibitor, exhibiting increased levels during mucosal barrier disruption and enhanced gut permeability (Barekatain et al., 2020; O’Brien et al., 2022). This could explain the reason why participants who tested positive for STEC had elevated levels of AAT. Increased fecal AAT levels are also observed in cases of diarrhea caused by enterotoxigenic E. coli, Shigella, Salmonella, Rotavirus and Adenovirus. These pathogens damage the tight junctions in the gastrointestinal tract, resulting in the leakage of proteins (Aulia et al., 2009; Rahmat et al., 2023). Furthermore, Shigella/EIEC-positive participants showed a notable rise in Calprotectin levels. EIEC exhibits numerous similarities to Shigella, particularly in terms of their virulence mechanisms (Okeke, 2009). However, the multivariable model could not establish a significant association between Calprotectin and E. coli pathotypes. Elevated fecal calprotectin levels in samples with various enteropathogens were observed in a study involving participants aged between three months and four years conducted in Southern India (Praharaj et al., 2018). Additionally, the presence of ETEC in stool was associated with increased fecal calprotectin concentrations in children aged 6–30 months in rural Bangladesh (George et al., 2018).
This study observed no statistically significant association between E. coli pathotypes and the nutritional status (BMI) of the individuals. A recent study on Bangladeshi children aged 12 to 18 months revealed a notable adverse correlation between EPEC and linear growth, as indicated by the length-for-age z score (LAZ), as well as underweight, as indicated by the weight-for-age z score (WAZ) (Trabulsi et al., 2002). The MAL-ED study discovered that EAEC exhibited a negative association with linear growth at 24 months of age (Das et al., 2021).
This study has several limitations. Initially, our research solely included persons who had a low BMI. The lack of a group of healthy controls reduces the robustness of the results we obtained. Consequently, we conducted an exploratory study using two subgroups (E. coli-positive and E. coli-negative), and the findings demonstrated consistency with the bivariate analysis (Supplementary Table S2). In addition, there is a significant disparity in the ratio of female to male participation. This community-based intervention trial involved male participants who were required to visit the study site every day in the morning for two months to receive the nutritional intervention. The majority of eligible males were those who earned wages for their families and declined to miss work for an hour, leading to an increased proportion of female participants, mainly homemakers or part-time workers. Moreover, the BEED study only enrolled undernourished adults who were not experiencing any acute illness at the time of enrollment. Therefore, all participants were asymptomatic, which limits our ability to directly assess the relationship between E. coli pathotypes and clinical symptoms. Future studies that include both symptomatic and asymptomatic individuals could provide further insights into high-risk groups and the potential role of biomarkers in disease progression.
This research’s strength is the use of quantitative TAC PCR assays to identify E. coli pathotypes and a large array of other intestinal pathogens (Platts-Mills et al., 2017). The TAC assay is regarded as a very sensitive molecular diagnostic technique for detecting a broad spectrum of enteropathogens. Moreover, by incorporating a wide range of socio-demographic variables, we were able to mitigate the influence of possible confounding factors. Furthermore, the study benefits from a large sample size and an extensive questionnaire, both of which are important aspects of the research.
Only limited work has been conducted in Bangladeshi adults about the relationship between E. coli pathotypes and EED biomarkers. Therefore, this study offers current information and highlights the importance of E. coli pathotypes as significant diarrheagenic bacteria that have a negative effect on the gastrointestinal health of undernourished adults.
In conclusion, the study results indicate that the presence of E. coli pathotypes, such as EPEC and STEC, in the human intestine affects gut health. Additionally, all of these pathotypes may potentially have a pathogenic role when other organisms are present. The ongoing research can aid in the investigation of the mechanisms underlying EED with common E. coli pathotypes and potentially facilitate the development of vaccines. These findings can be used as evidence by public health services to inform the planning and development of therapeutic intervention programs for adults.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving humans were approved by ‘Institutional Review Board of the International Center for Diarrhoeal Disease Research, Bangladesh (icddr,b). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
RS: Methodology, Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Investigation. MI: Data curation, Software, Methodology, Writing – review & editing, Investigation. MS: Data curation, Methodology, Writing – review & editing. MG: Formal analysis, Validation, Writing – review & editing, Conceptualization, Supervision. JF: Methodology, Writing – review & editing. MK: Supervision, Writing – review & editing. MM: Project administration, Writing – review & editing, Resources. TA: Conceptualization, Funding acquisition, Resources, Writing – review & editing. IM: Investigation, Supervision, Writing – review & editing, Project administration.
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Bill and Melinda Gates Foundation. The investment ID was OPP1136751.
The authors express their sincere gratitude to the participants who wholeheartedly took part in this research study. icddr,b appreciates the dedication of the Bill and Melinda Gates Foundation to their research efforts and is also grateful to the Governments of Bangladesh and Canada for providing core/unrestricted support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1553688/full#supplementary-material
Arndt, M. B., Richardson, B. A., Ahmed, T., Mahfuz, M., Haque, R., John-Stewart, G. C., et al. (2016). Fecal markers of environmental enteropathy and subsequent growth in Bangladeshi children. Am. J. Trop. Med. Hyg. 95, 694–701. doi: 10.4269/ajtmh.16-0098
Aulia, D., Timan, I., Firmansyah, A. (2009). Fecal alpha-1 antitrypsin concentration in protein-losing enteropathies caused by Rotavirus and enteropathogenic bacteria infection. Paediatrica Indonesiana. 49, 315–321. doi: 10.14238/pi49.6.2009.315-21
Barekatain, R., Howarth, G. S., Willson, N. L., Cadogan, D., Wilkinson, S. (2020). Excreta biomarkers in response to different gut barrier dysfunction models and probiotic supplementation in broiler chickens. PloS One 15, e0237505. doi: 10.1371/journal.pone.0237505
Bartelt, L. A., Bolick, D. T., Guerrant, R. L. (2019). Disentangling microbial mediators of malnutrition: modeling environmental enteric dysfunction. Cell Mol. Gastroenterol. Hepatol. 7, 692–707. doi: 10.1016/j.jcmgh.2018.12.006
Black, R. E., Victora, C. G., Walker, S. P., Bhutta, Z. A., Christian, P., De Onis, M., et al. (2013). Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382, 427–451. doi: 10.1016/S0140-6736(13)60937-X
Budge, S., Parker, A. H., Hutchings, P. T., Garbutt, C. (2019). Environmental enteric dysfunction and child stunting. Nutr. Rev. 77, 240–253. doi: 10.1093/nutrit/nuy068
Butterfield, D. A., Koppal, T., Howard, B., Subramaniam, R., Hall, N., Hensley, K., et al. (1998). Structural and functional changes in proteins induced by free radical-mediated oxidative stress and protective action of the antioxidants N-tert-butyl-alpha-phenylnitrone and vitamin E. Ann. N Y Acad. Sci. 854, 448–462. doi: 10.1111/j.1749-6632.1998.tb09924.x
Chakraborty, S., Johura, F. T., Sultana, M., Zhang, X., Sadique, A., George, C. M., et al. (2024). Epidemiology of Enterotoxigenic Escherichia coli among Children and Adults Seeking Care at Hospitals in Two Geographically Distinct Rural Areas in Bangladesh. Microorganisms 12 (2), 359. doi: 10.3390/microorganisms12020359
Cox, F. E. (2001). Concomitant infections, parasites and immune responses. Parasitology. 122 Suppl, S23–S38. doi: 10.1017/S003118200001698X
Crane, R. J., Jones, K. D., Berkley, J. A. (2015). Environmental enteric dysfunction: an overview. Food Nutr. Bull. 36, S76–S87. doi: 10.1177/15648265150361S113
Croxen, M. A., Law, R. J., Scholz, R., Keeney, K. M., Wlodarska, M., Finlay, B. B. (2013). Recent advances in understanding enteric pathogenic Escherichia coli. Clin. Microbiol. Rev. 26, 822–880. doi: 10.1128/CMR.00022-13
Das, R., Palit, P., Haque, M. A., Mahfuz, M., Faruque, A. S. G., Ahmed, T. (2021). Site specific incidence rate of virulence related genes of enteroaggregative Escherichia coli and association with enteric inflammation and growth in children. Sci. Rep. 11, 23178. doi: 10.1038/s41598-021-02626-z
Fahim, S. M., Gazi, M. A., Hasan, M. M., Alam, M. A., Das, S., Mahfuz, M., et al. (2021). Infection with Blastocystis spp. and its association with enteric infections and environmental enteric dysfunction among slum-dwelling malnourished adults in Bangladesh. PloS Negl. Trop. Dis. 15, e0009684. doi: 10.1371/journal.pntd.0009684
Gazi, M. A., Alam, M. A., Fahim, S. M., Wahid, B. Z., Khan, S. S., Islam, M. O., et al. (2022). Infection with escherichia coli pathotypes is associated with biomarkers of gut enteropathy and nutritional status among malnourished children in Bangladesh. Front. Cell Infect. Microbiol. 12, 901324. doi: 10.3389/fcimb.2022.901324
George, C. M., Burrowes, V., Perin, J., Oldja, L., Biswas, S., Sack, D., et al. (2018). Enteric infections in young children are associated with environmental enteropathy and impaired growth. Trop. Med. Int. Health 23, 26–33. doi: 10.1111/tmi.2018.23.issue-1
Gizaw, Z., Yalew, A. W., Bitew, B. D., Lee, J., Bisesi, M. (2022). Fecal biomarkers of environmental enteric dysfunction and associated factors among children aged 24-59 months in east Dembiya district, northwest Ethiopia. BMC Gastroenterol. 22, 172. doi: 10.1186/s12876-022-02255-4
Gomes, T. A., Elias, W. P., Scaletsky, I. C., Guth, B. E., Rodrigues, J. F., Piazza, R. M., et al. (2016). Diarrheagenic escherichia coli. Braz. J. Microbiol. 47 Suppl 1, 3–30. doi: 10.1016/j.bjm.2016.10.015
Gomes, T. A., Pedrajo, B. G. (2012). “Enteropathogenic escherichia coli (EPEC),” in Pathogenic Escherichia coli in latin America (Bentham Science Publishers), 25–47.
Grimprel, E., Rodrigo, C., Desselberger, U. (2008). Rotavirus disease: impact of coinfections. Pediatr. Infect. Dis. J. 27, S3–S10. doi: 10.1097/INF.0b013e31815eedfa
Hansberry, D. R., Shah, K., Agarwal, P., Agarwal, N. (2017). Fecal myeloperoxidase as a biomarker for inflammatory bowel disease. Cureus. 9, e1004. doi: 10.7759/cureus.1004
Hurley, B. P., Thorpe, C. M., Acheson, D. W. (2001). Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect. Immun. 69, 6148–6155. doi: 10.1128/IAI.69.10.6148-6155.2001
Ijarotimi, O. S. (2013). Determinants of childhood malnutrition and consequences in developing countries. Curr. Nutr. Rep. 2, 129–133. doi: 10.1007/s13668-013-0051-5
Jukic, A., Bakiri, L., Wagner, E. F., Tilg, H., Adolph, T. E. (2021). Calprotectin: from biomarker to biological function. Gut. 70, 1978–1988. doi: 10.1136/gutjnl-2021-324855
Kapel, N., Campeotto, F., Kalach, N., Baldassare, M., Butel, M. J., Dupont, C. (2010). Faecal calprotectin in term and preterm neonates. J. Pediatr. Gastroenterol. Nutr. 51, 542–547. doi: 10.1097/MPG.0b013e3181e2ad72
Kaper, J. B., Nataro, J. P., Mobley, H. L. (2004). Pathogenic escherichia coli. Nat. Rev. Microbiol. 2, 123–140. doi: 10.1038/nrmicro818
Karmali, M. A., Gannon, V., Sargeant, J. M. (2010). Verocytotoxin-producing escherichia coli (VTEC). Vet. Microbiol. 140, 360–370. doi: 10.1016/j.vetmic.2009.04.011
Khalil, I., Walker, R., Porter, C. K., Muhib, F., Chilengi, R., Cravioto, A., et al. (2021). Enterotoxigenic Escherichia coli (ETEC) vaccines: Priority activities to enable product development, licensure, and global access. Vaccine. 39, 4266–4277. doi: 10.1016/j.vaccine.2021.04.018
Kothari, N., Keshari, R. S., Bogra, J., Kohli, M., Abbas, H., Malik, A., et al. (2011). Increased myeloperoxidase enzyme activity in plasma is an indicator of inflammation and onset of sepsis. J. Crit. Care 26, 435.e1–435.e7. doi: 10.1016/j.jcrc.2010.09.001
Kotloff, K. L., Nataro, J. P., Blackwelder, W. C., Nasrin, D., Farag, T. H., Panchalingam, S., et al. (2013). Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382, 209–222. doi: 10.1016/S0140-6736(13)60844-2
Krawisz, J. E., Sharon, P., Stenson, W. F. (1984). Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 87, 1344–1350. doi: 10.1016/0016-5085(84)90202-6
Ledwaba, S. E., Costa, D. V. S., Bolick, D. T., Giallourou, N., Medeiros, P., Swann, J. R., et al. (2020). Enteropathogenic escherichia coli infection induces diarrhea, intestinal damage, metabolic alterations, and increased intestinal permeability in a murine model. Front. Cell Infect. Microbiol. 10, 595266. doi: 10.3389/fcimb.2020.595266
Ledwaba, S., Kabue, J., Barnard, T., Traore, A., Potgieter, N. (2018). Enteric pathogen co-infections in the paediatric population from rural communities in the Vhembe District, South Africa. South Afr. J. Child Health 12, 170–174. doi: 10.7196/SAJCH.2018.v12i4.1550
Li, X. X., Zhou, X. N. (2013). Co-infection of tuberculosis and parasitic diseases in humans: a systematic review. Parasit Vectors. 6, 79. doi: 10.1186/1756-3305-6-79
Liu, J., Gratz, J., Amour, C., Kibiki, G., Becker, S., Janaki, L., et al. (2013). A laboratory-developed TaqMan Array Card for simultaneous detection of 19 enteropathogens. J. Clin. Microbiol. 51, 472–480. doi: 10.1128/JCM.02658-12
Mahfuz, M., Das, S., Mazumder, R. N., Masudur Rahman, M., Haque, R., Bhuiyan, M. M. R., et al. (2017). Bangladesh Environmental Enteric Dysfunction (BEED) study: protocol for a community-based intervention study to validate non-invasive biomarkers of environmental enteric dysfunction. BMJ Open 7, e017768. doi: 10.1136/bmjopen-2017-017768
Makarova, M. A. (2023). A modern view of diarrheagenic Escherichia coli—a causative agent of acute intestinal infections. J. microbiology Epidemiol. immunobiology. 100, 333–344. doi: 10.36233/0372-9311-410
Mangell, P., Nejdfors, P., Wang, M., Ahrné, S., Weström, B., Thorlacius, H., et al. (2002). Lactobacillus plantarum 299v inhibits Escherichia coli-induced intestinal permeability. Dig Dis. Sci. 47, 511–516. doi: 10.1023/A:1017947531536
Mellmann, A., Harmsen, D., Cummings, C. A., Zentz, E. B., Leopold, S. R., Rico, A., et al. (2011). Prospective genomic characterization of the German enterohemorrhagic Escherichia coli O104:H4 outbreak by rapid next generation sequencing technology. PloS One 6, e22751. doi: 10.1371/journal.pone.0022751
Modgil, V., Narayan, C., Kaur, H., Yadav, V. K., Chaudhary, N., Kant, V., et al. (2022). Analysis of the virulence and inflammatory markers elicited by Enteroaggregative Escherichia coli isolated from clinical and non-clinical sources in an experimental infection model, India. Microbiol. Res. 13, 882–897. doi: 10.3390/microbiolres13040062
Müller, D., Greune, L., Heusipp, G., Karch, H., Fruth, A., Tschäpe, H., et al. (2007). Identification of unconventional intestinal pathogenic Escherichia coli isolates expressing intermediate virulence factor profiles by using a novel single-step multiplex PCR. Appl. Environ. Microbiol. 73, 3380–3390. doi: 10.1128/AEM.02855-06
Nataro, J. P., Kaper, J. B. (1998). Diarrheagenic escherichia coli. Clin. Microbiol. Rev. 11, 142–201. doi: 10.1128/CMR.11.1.142
Navab-Daneshmand, T., Friedrich, M. N. D., Gächter, M., Montealegre, M. C., Mlambo, L. S., Nhiwatiwa, T., et al. (2018). Escherichia coli Contamination across Multiple Environmental Compartments (Soil, Hands, Drinking Water, and Handwashing Water) in Urban Harare: Correlations and Risk Factors. Am. J. Trop. Med. Hyg. 98, 803–813. doi: 10.4269/ajtmh.17-0521
Nyholm, O., Heinikainen, S., Pelkonen, S., Hallanvuo, S., Haukka, K., Siitonen, A. (2015). Hybrids of shigatoxigenic and enterotoxigenic escherichia coli (STEC/ETEC) among human and animal isolates in Finland. Zoonoses Public Health 62, 518–524. doi: 10.1111/zph.2015.62.issue-7
O’Brien, M. E., Murray, G., Gogoi, D., Yusuf, A., McCarthy, C., Wormald, M. R., et al. (2022). A review of alpha-1 antitrypsin binding partners for immune regulation and potential therapeutic application. Int. J. Mol. Sci. 23 (5), 2441. doi: 10.3390/ijms23052441
Ochoa, T. J., Barletta, F., Contreras, C., Mercado, E. (2008). New insights into the epidemiology of enteropathogenic Escherichia coli infection. Trans. R Soc. Trop. Med. Hyg. 102, 852–856. doi: 10.1016/j.trstmh.2008.03.017
Okeke, I. N. (2009). Diarrheagenic Escherichia coli in sub-Saharan Africa: status, uncertainties and necessities. J. Infect. Dev. Ctries. 3, 817–842. doi: 10.3855/jidc.586
Pijnacker, R., van Pelt, W., Vennema, H., Kortbeek, L. M., Notermans, D. W., Franz, E., et al. (2019). Clinical relevance of enteropathogen co-infections in preschool children-a population-based repeated cross-sectional study. Clin. Microbiol. Infect. 25, 1039.e7–.e13. doi: 10.1016/j.cmi.2018.11.029
Platts-Mills, J. A., Babji, S., Bodhidatta, L., Gratz, J., Haque, R., Havt, A., et al. (2015). Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3, e564–e575. doi: 10.1016/S2214-109X(15)00151-5
Platts-Mills, J. A., Taniuchi, M., Uddin, M. J., Sobuz, S. U., Mahfuz, M., Gaffar, S. A., et al. (2017). Association between enteropathogens and malnutrition in children aged 6-23 mo in Bangladesh: a case-control study. Am. J. Clin. Nutr. 105, 1132–1138. doi: 10.3945/ajcn.116.138800
Potgieter, N., Heine, L., Ngandu, J. P. K., Ledwaba, S. E., Zitha, T., Mudau, L. S., et al. (2023). High Burden of Co-infection with multiple enteric pathogens in children suffering with Diarrhoea from Rural and Peri-urban communities in South Africa. Pathogens. 12, 315. doi: 10.3390/pathogens12020315
Praharaj, I., Revathy, R., Bandyopadhyay, R., Benny, B., Azharuddin Ko, M., Liu, J., et al. (2018). Enteropathogens and gut inflammation in asymptomatic infants and children in different environments in southern India. Am. J. Trop. Med. Hyg. 98, 576–580. doi: 10.4269/ajtmh.17-0324
Rahmat, D., Firmansyah, A., Timan, I. S., Bardosono, S., Prihartono, J., Gayatri, P. (2023). Risk factors of prolonged diarrhea in children under 2 years old. Clin. Exp. Pediatr. 66, 538–544. doi: 10.3345/cep.2023.00668
Read, A. F., Taylor, L. H. (2001). The ecology of genetically diverse infections. Science. 292, 1099–1102. doi: 10.1126/science.1059410
Rim, S., Vedøy, O. B., Brønstad, I., McCann, A., Meyer, K., Steinsland, H., et al. (2024). Inflammation, the kynurenines, and mucosal injury during human experimental enterotoxigenic Escherichia coli infection. Med. Microbiol. Immunol. 213, 2. doi: 10.1007/s00430-024-00786-z
Rogawski, E. T., Liu, J., Platts-Mills, J. A., Kabir, F., Lertsethtakarn, P., Siguas, M., et al. (2018). Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 6, e1319–e1e28. doi: 10.1016/S2214-109X(18)30351-6
Seki, T., Sugie, N., Johl, K., Oh-ishi, T. (1996). The regulation of neopterin production by cytokines. Pteridines. 7, 5–9. doi: 10.1515/pteridines.1996.7.12.5
Spitz, J., Yuhan, R., Koutsouris, A., Blatt, C., Alverdy, J., Hecht, G. (1995). Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am. J. Physiology-Gastrointestinal Liver Physiol. 268, G374–G3G9. doi: 10.1152/ajpgi.1995.268.2.G374
Syed, S., Ali, A., Duggan, C. (2016). Environmental enteric dysfunction in children. J. Pediatr. Gastroenterol. Nutr. 63, 6–14. doi: 10.1097/MPG.0000000000001147
Tickell, K. D., Atlas, H. E., Walson, J. L. (2019). Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies. BMC Med. 17, 181. doi: 10.1186/s12916-019-1417-3
Trabulsi, L. R., Keller, R., Gomes, T. A. T. (2002). Typical and atypical enteropathogenic escherichia coli. Emerging Infect. Dis. 8, 508. doi: 10.321/eid0805
Troeger, C., Forouzanfar, M., Rao, P. C., Khalil, I., Brown, A., Reiner, R. C., et al. (2017). Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 17, 909–948. doi: 10.1016/S1473-3099(17)30276-1
Troeger, C., Blacker, B. F., Khalil, I. A., Rao, P. C., Cao, S., Zimsen, S. R., et al. (2018). Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect. Dis. 18, 1211–1228. doi: 10.1016/S1473-3099(18)30362-1
Wang, L., Llorente, C., Hartmann, P., Yang, A. M., Chen, P., Schnabl, B. (2015). Methods to determine intestinal permeability and bacterial translocation during liver disease. J. Immunol. Methods 421, 44–53. doi: 10.1016/j.jim.2014.12.015
Weissmann, G., Smolen, J. E., Korchak, H. M. (1980). Release of inflammatory mediators from stimulated neutrophils. New Engl. J. Med. 303, 27–34. doi: 10.1056/NEJM198007033030109
Woods, A. A., Linton, S. M., Davies, M. J. (2003). Detection of HOCl-mediated protein oxidation products in the extracellular matrix of human atherosclerotic plaques. Biochem. J. 370, 729–735. doi: 10.1042/bj20021710
Zhang, S. X., Zhou, Y. M., Xu, W., Tian, L. G., Chen, J. X., Chen, S. H., et al. (2016). Impact of co-infections with enteric pathogens on children suffering from acute diarrhea in southwest China. Infect. Dis. Poverty. 5, 64. doi: 10.1186/s40249-016-0157-2
Zil-e-Huma, Z.-e., AM, T., KU, K. U., TM, A., AS, A. S., AI, A. I., et al. (2019). Incidence of diarrheagenic Escherichia coli pathotypes in children suffering from diarrhea in tertiary care hospitals (Quetta, Pakistan: Pakistan Journal of Zoology).
Keywords: Escherichia coli, diarrhea, malnourished adults, environmental enteric dysfunction, Bangladesh
Citation: Sthity RA, Islam MZ, Sagar MEK, Gazi MA, Ferdous J, Kabir MM, Mahfuz M, Ahmed T and Mostafa I (2025) Association of Escherichia coli pathotypes with fecal markers of enteropathy and nutritional status among underweight adults in Bangladesh. Front. Cell. Infect. Microbiol. 15:1553688. doi: 10.3389/fcimb.2025.1553688
Received: 31 December 2024; Accepted: 24 March 2025;
Published: 10 April 2025.
Edited by:
Rahul Kumar Maurya, Washington University in St. Louis, United StatesReviewed by:
Suman Bharti, Washington University in St. Louis, United StatesCopyright © 2025 Sthity, Islam, Sagar, Gazi, Ferdous, Kabir, Mahfuz, Ahmed and Mostafa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ishita Mostafa, aXNoaXRhLm1vc3RhZmFAaWNkZHJiLm9yZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.