- 1Center for Yunnan Plateau Biological Resources Protection and Utilization, College of Biological Resource and Food Engineering, Qujing Normal University, Qujing, Yunnan, China
- 2School of Medical, Molecular and Forensic Sciences, Murdoch University, Perth, WA, Australia
- 3Center of Excellence in Microbial Diversity and Sustainable Utilization, Chiang Mai University, Chiang Mai, Thailand
- 4Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 5College of Biodiversity Conservation, Southwest Forestry University, Kunming, China
Members of the genus Fomitopsis are medicinal mushrooms and a rich source of bioactive compounds with significant pharmacological and biotechnological potential. This paper provides a comprehensive review of their secondary metabolites, including polysaccharides, terpenoids, and phenolic compounds. In addition, their chemical structures and biological activities are described in detail. These compounds exhibit antioxidant, antimicrobial, anti-inflammatory, and immunomodulatory properties, with promising applications in cancer therapy, cardiovascular health, and immune modulation. Beyond medicine, Fomitopsis plays a crucial role in biotechnology, contributing to bioremediation, biofuel production, pharmaceutical development, and functional food innovation. By integrating traditional medicinal knowledge with recent scientific advances, this review highlights the biomedical significance and industrial relevance of Fomitopsis, underscoring its expanding role in health and environmental sustainability.
1 Introduction
The genus Fomitopsis comprises highly diverse fungi and encompasses species with unique ecological distributions and bioactive properties (Liu S. et al., 2022; Flores et al., 2023; Spirin et al., 2024; Gáper et al., 2025). Species such as Fomitopsis betulina, F. cajanderi, F. feei, F. officinalis, F. palustris, and F. pinicola (Figure 1) have been extensively studied for their medicinal and industrial potential (Li et al., 2024; Shen et al., 2024; Nowotarska et al., 2024). Initially recognized for their role in wood decomposition, these fungi are now celebrated as a rich source of bioactive compounds with significant therapeutic applications (Blanchette et al., 1992; Grienke et al., 2014; Girometta, 2019; Blanchette et al., 2021; Turner and Cuerrier, 2022; Hobbs, 2023; Flores et al., 2025). Advances in analytical techniques have revealed a wide range of pharmacologically active compounds, including polysaccharides, terpenoids, phenolic compounds, and secondary metabolites, all of which contribute to their ecological functions and therapeutic potential (Hsiao et al., 2003; Choi et al., 2007; Pleszczyńska et al., 2017; Zhao et al., 2018; Sułkowska-Ziaja et al., 2018; Tai et al., 2019; Muszyńska et al., 2020; Bishop, 2020; Sofrenić et al., 2021; Verekar et al., 2021; Gafforov et al., 2023; Zhang et al., 2023; Krupodorova et al., 2024).

Figure 1. Some species of Fomitopsis. (a) Fomitopsis betulina (b) F. cajanderi (c) F. feei (d) F. officinalis (e) F. Palustaris (f) F. Pinicola (https://www.inaturalist.org/, the images are used under the license Attribution Non-Commercial-No Derivs 4.0).
Traditionally valued by indigenous cultures, species of Fomitopsis have been used for treating headache, nausea, and liver problems, as well as serving as haemostatics and anti-inflammatory agents due to their astringent effects. They were also employed for anti-fatigue, immune enhancement, cancer treatment, and as a styptic, antiseptic, and pain reliever across various regions., owing to the diverse bioactive compounds they produce (Hobbs, 1995; Grienke et al., 2014). These compounds exhibit potent antioxidant, antimicrobial, anti-inflammatory, and immunomodulatory effects, making them promising candidates for treating various medical conditions (Shamtsyan et al., 2004; Sulkowska-Ziaja et al., 2012; Li et al., 2013; Grienke et al., 2014; Peng et al., 2019; Fijałkowska et al., 2020; Kuo et al., 2021). In addition, Fomitopsis has shown significant potential in addressing chronic diseases such as cancer, cardiovascular disorders, and neurodegenerative conditions (Yoshikawa et al., 2005; Alresly et al., 2016, 2019; Bishop, 2020; Muszyńska et al., 2020; Badalyan et al., 2023). Members of this genus also offer innovative solutions to combat antibiotic-resistant pathogens, a pressing global health challenge (Girometta, 2019; Kumar and Prasher, 2022; Hashem et al., 2023). In addition, several Fomitopsis-derived products, including dietary supplements, functional foods, and extracts, are increasingly being developed and marketed for their purported health benefits, particularly in immune support and metabolic health.
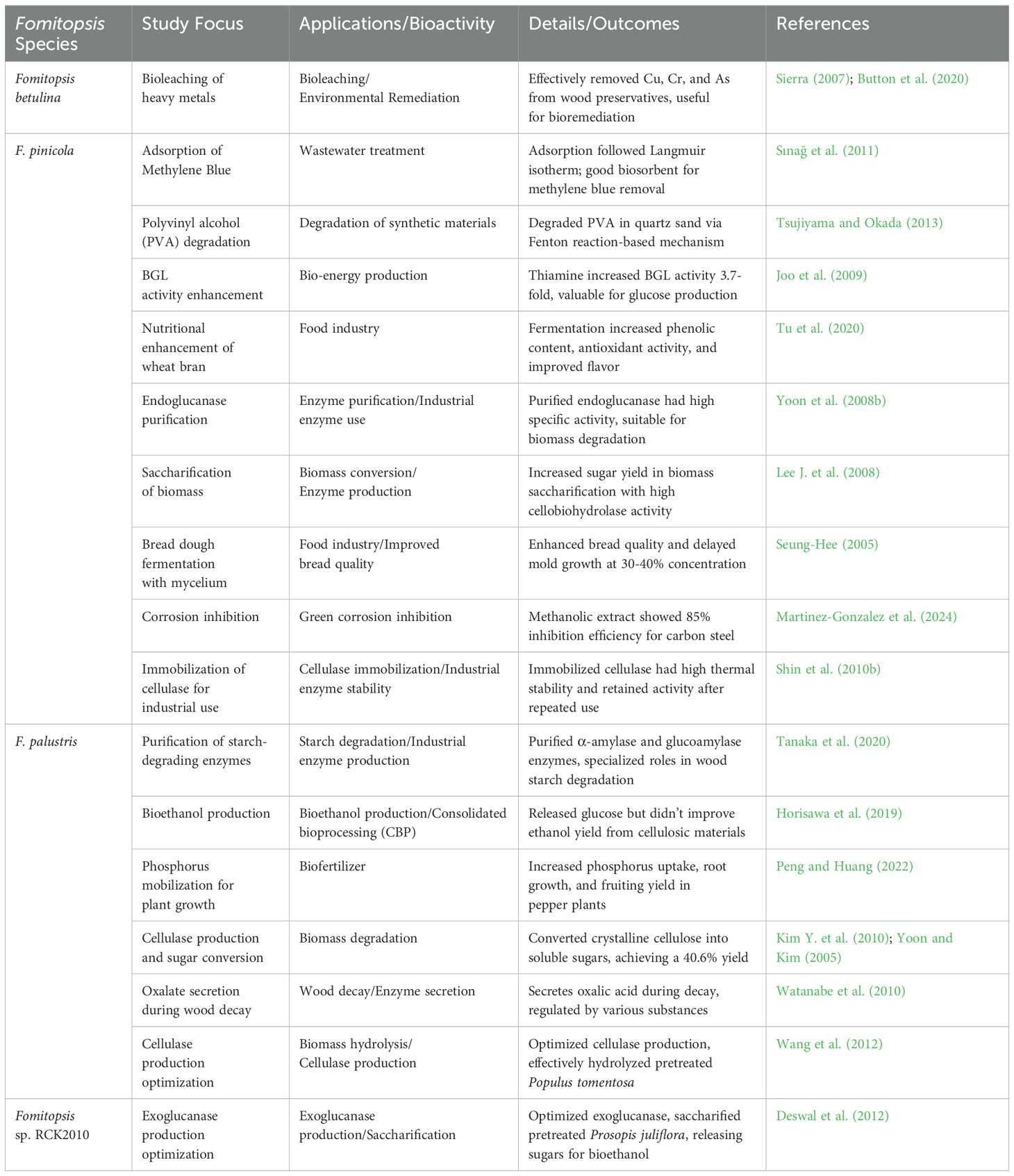
Beyond medicine, Fomitopsis holds immense biotechnological promise. Its applications span bioremediation, biofuel production, pharmaceutical development, and functional food innovation (Tsujiyama and Okada, 2013; Pleszczyńska et al., 2017; Purnomo et al., 2020, 2022; Rehman et al., 2020; Mahmood et al., 2023; Prajapati et al., 2023). Some of the enzymes produced by Fomitopsis are particularly effective in breaking down lignocellulosic materials, offering sustainable solutions for biofuel production and environmental remediation (Kim H. et al., 2010; Park et al., 2015; Stipniece-Jekimova et al., 2022; Tiwari et al., 2023). In addition, their bioactive compounds are increasingly recognized as natural additives in the food industry, enhancing product quality and providing health benefits (Bishop, 2020; Grosse et al., 2020; Kozarski et al., 2022; Goppa et al., 2023). The multifaceted potential of Fomitopsis, including its bioactive compounds, therapeutic applications, and expanding role in biotechnology, are highlighted in this review.
2 Taxonomy and evolution of Fomitopsis species
The Fomitopsidaceae Jülich is one of the largest families of polypores, with twenty-four accepted genera (Liu et al., 2022, 2023). Most species are placed in the genus Fomitopsis, with 233 taxa being recognized (http://www.indexfungorum.Org, accessed 06 February 2025). However, recent studies based on multigene phylogenetic analysis have led to the acceptance of three genera (Anthoporia, Antrodia, and Fomitopsis) within the family Fomitopsidaceae. Fomitopsis now encompasses 128 species, including those formerly placed in the genera Antrodia, Daedalea, and Laccocephalum (Han et al., 2015; Spirin et al., 2024). Fomitopsis P. Karst. was originally introduced by Karsten, 1881, with F. pinicola (Sw.) P. Karst. designated as the type species (Kirk et al., 2008). Fomitopsis is widely distributed worldwide, highly polyphyletic, and serves as the type genus within the Fomitopsidaceae (Ortiz-Santana et al., 2013; Han et al., 2016; Liu et al., 2019, 2021). The species within the genus Fomitopsis are associated with brown rot, a process of significant ecological importance involving the decomposition and alteration of wood in forest ecosystems (Wei and Dai, 2004; Soares et al., 2017; Shah et al., 2018; Liu et al., 2023). Species of Fomitopsis produce basidiomata that range from sessile to effused-reflexed, growth patterns that range from annual to perennial, a spectrum of colors from white to purple, and a hyphal system that can be di- to trimitic, and features clamped generative hyphae. In addition, the basidiospores of species of Fomitopsis are characterized as smooth, hyaline, thin-walled, and may be subglobose to cylindrical in shape (Gilbertson and Ryvarden, 1986; Ryvarden and Gilbertson, 1993; Dai, 2012a; Li et al., 2013; Han and Cui, 2015; Haight et al., 2019).
3 Pathogenicity and ecological impact of Fomitopsis in forest ecosystems
Fomitopsis includes several species such as F. nivosa (Berk.) Gilb. & Ryvarden and F. pinicola Sw.) P. Kars that are known for their pathogenicity in forest ecosystems (Dai, 2012b; Liu S. et al., 2022). These fungi are primarily saprotrophic but can act as pathogens, causing significant decay in timber and living trees, affecting forest productivity and ecosystem health (Gramss, 2020; Zmitrovich et al., 2023; Pawłowicz et al., 2024). Species of Fomitopsis predominantly target conifers, with F. pinicola (commonly known as the red belt fungus) being notorious for causing brown rot in a variety of forest trees such as spruce, fir, and pine (Glaeser and Smith, 2016; Spirin et al., 2024). The pathogenicity of this fungus arises from its ability to decompose lignin selectively, leaving behind cellulose-rich residues. This leads to severe structural weakening of trees, making them susceptible to wind breakage and other environmental stressor factors (Singh and Singh, 2016; Haq et al., 2022; Waszczuk et al., 2022). Moreover, the persistence of F. pinicola in decayed wood can reduce timber quality, causing economic losses in forest industries (Hu, 2022; Sun et al., 2024).
Infections by Fomitopsis are facilitated by wounds on host trees, which serve as entry points for fungal spores. Once inside, the fungus colonizes the heartwood, initiating decay through enzymatic degradation of wood components (Schwarze et al., 1999; Adarsh et al., 2015; Pleszczyńska et al., 2017; Hu, 2022). The species of Fomitopsis involved produce a variety of enzymes, including cellulases, hemicellulases, and lignin-modifying enzymes, that help them break down the complex structure of wood, contributing to rapid degradation (Shah et al., 2018; Civzele et al., 2023). Fomitopsis pinicola also can colonize standing dead trees, stumps, and fallen logs, making it a key player in forest decomposition dynamics (Adarsh et al., 2015; Kauserud et al., 2024). Species of Fomitopsis, while pathogenic, also play essential roles in nutrient cycling within forest ecosystems. By breaking down woody material, they help release nutrients into the soil, aiding in forest regeneration (Rayner & Boddy, 1988; Pawłowicz et al., 2024; Ngwogu and Ngwogu, 2025).
However, the pathogenicity of species of Fomitopsis often outweighs their ecological benefits, especially in managed forests where timber quality and tree health are priorities (Schwarze and Baum, 2000; Gramss, 2020, Liu S. et al., 2022). In some cases, species of Fomitopsis can cause large-scale tree mortality, as observed in forests of the Pacific Northwest, where F. pinicola has been implicated in the widespread destruction and decline of coniferous forests (Hennon et al., 2002; U.S. Forest Service, 2023a, 2023b). Managing Fomitopsis infections in forests requires an integrated approach, including silvicultural practices that reduce tree stress and wound management to prevent fungal entry (Schwarze and Baum, 2000; Roberts et al., 2020; Dahlsjö, 2023). Chemical treatments including fungicides are often used in forestry to protect timber from fungal decay. However, biological control methods, including antagonistic fungi or bacteria, have shown promise in reducing the spread of Fomitopsis in forest ecosystems (Lonsdale et al., 2008; Hu, 2022, Griffin, 2024).
4 Major bioactive compounds in Fomitopsis and their beneficial medicinal properties
Species of Fomitopsis produce a diverse array of bioactive compounds with significant biotechnological potential. These compounds include polysaccharides, triterpenoids, and phenolics. They play key roles in immune modulation, anticancer activity, antioxidant protection, neuroprotection, and aromatic applications. In addition, enzymatic activities contribute to such things as bioremediation and wastewater treatment. Together, these bioactivities support applications that involve pharmaceuticals, cosmetics, agriculture, and industrial waste management (Figure 2). The following section thoroughly explores these bioactive compounds, highlighting their specific properties and biotechnological applications.
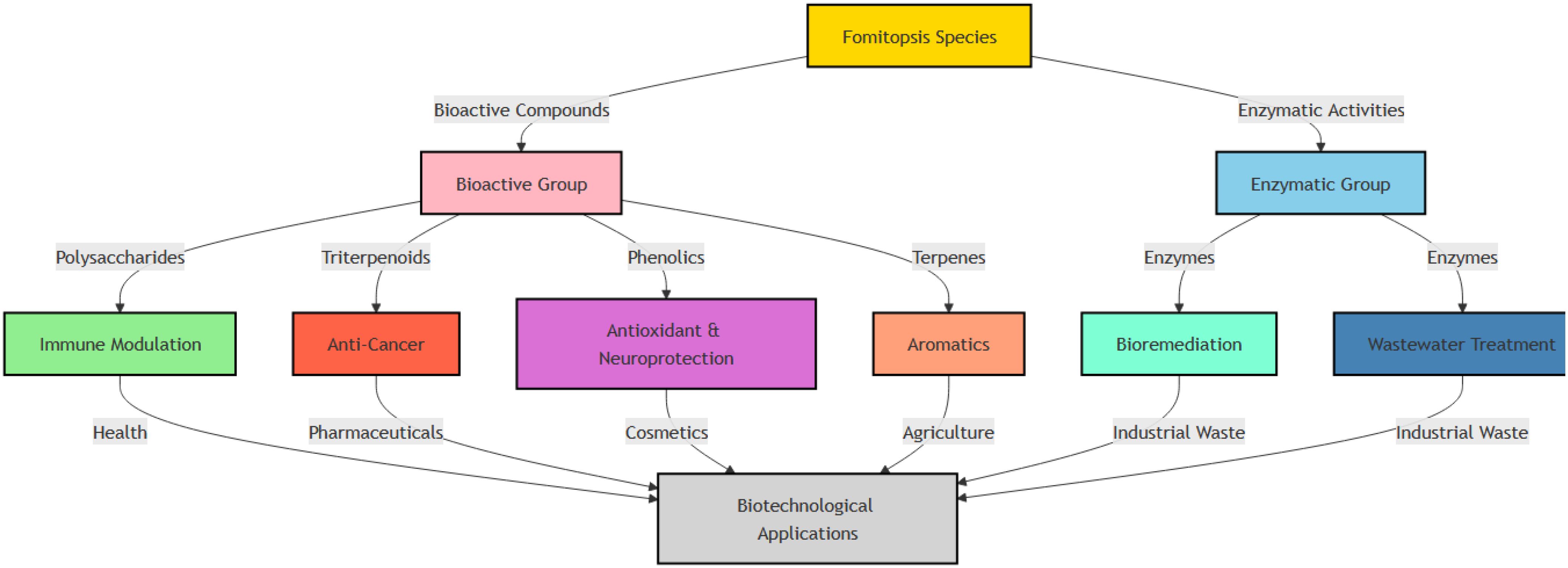
Figure 2. Bioactive compounds in species of Fomitopsis and their biotechnological applications in healthcare, cosmetics, agriculture, and industry.
4.1 Polysaccharides
4.1.1 Fomitopsis betulina
The cellular structure of F. betulina contains beneficial polysaccharides, notably α-glucans, which exhibit water insolubility but can be dissolved in alkaline solutions (Grün, 2003; Pleszczyńska et al., 2017). Wiater et al. (2011) isolated and characterized (1→3)-α-d-glucans, with the main chain comprising 84.6% (1→3)-linked α-d-glucopyranose units along with 6% (1→4)-linked units. Piptoporane I was extracted and purified by Olennikov et al. (2012). The α-glucan in F. betulina primarily consists of (1→3)-α-d-glucopyranose units, with occasional branching by β-d-glucopyranose at the C6 position (17.3%). This specific type of fungal α-glucan, present in F. betulina, is known to stimulate the production of microbial mutanases, which have the potential to prevent dental caries. Streptococci mutans produces (1→3), (1→6)-α-d-Glucans (mutans), crucial components of dental plaque matrix, making them a promising target for enzymatic anti-caries strategies (Pleszczyńska et al., 2015). Nonetheless, Streptococcal glucans pose challenges as inducers of mutanases due to their low yield and structural variability. Birch polypore α-glucan, which can constitute up to 44–53% of the dry weight of the cell wall of F. betulina (Grün, 2003), offers a potential alternative to replace S. glucans (Wiater et al., 2008). α-(1 → 3)-Glucooligosaccharides (α-(1 → 3)-GOS), sourced from F. betulina, underwent evaluation for their ability to combat cancer. They demonstrated the capacity to hinder the growth of colon cancer cells by reducing their proliferation and promoting apoptosis, all the while leaving normal colon cells unaffected. These results suggest that α-(1 → 3)-GOS has potential as a valuable dietary or therapeutic substance for restraining cancer cell proliferation, especially in colon carcinoma models (Czerwonka et al., 2019). Fomitopsis betulina was assessed for mycelial growth and exopolysaccharide production across 22 strains. The study found significant variability in growth rates (3.50 ± 0.33 to 8.75 ± 0.50 mm/day) and exopolysaccharide production (0.02 ± 0.00 to 2.20 ± 0.31 g/L), with maltose as the optimal carbon source for growth and dextrose and starch enhancing exopolysaccharide yield. Notably, strain F. betulina 311 demonstrated strong growth and high biopolymer production, highlighting its biotechnological potential (Kizitska et al., 2024).
4.1.2 Fomitopsis castaneus
The fermentation characteristics of exopolysaccharides (EPS) isolated from F. castaneus were studied in simulated human intestinal environments. The purified EPS, containing glucose, galactose, rhamnose, mannose, and arabinose, increased the production of short-chain fatty acids (SCFAs) in fecal extracts from both adults and children, with higher SCFA yields in children. Adding to the microbial flora, such as with Enterococcus fecalis and Lactobacillus rhamnosus, further enhanced SCFA production, highlighting the potential of EPS from F. castaneus to support gut health (Guo and Chi, 2017). FEPS, extracted from Fomitopsis castanea mycelia using ethanol precipitation, exhibited potent inhibition of mushroom tyrosinase with an IC50 of 16.5 mg/ml. It effectively reduced melanin production in human melanoma cells, diminished pigment density in embryos, and hindered NO production in macrophage cells with an IC50 of 42.8 ± 0.64 μg/ml (Jin et al., 2019).
4.1.3 Fomitopsis cytisina
Three types of polygalacturonases, including two endo-type (EndoPG I, II) and one exo-type (ExoPG), were purified from F. cytisina. EndoPG I and II had molecular weights of 38 kDa, while ExoPG ranged from 50-60 kDa, with optimal pH values around 5.0-5.5 and thermal stability up to 45°C. EndoPGs showed varying activity on oligo-galacturonic acids, while ExoPG displayed maximum activity on substrates with 9 GalUA and no activity on unsaturated oligo-galacturonic acids (Miyairi et al., 2001).
4.1.4 Fomitopsis officinalis
Mannofucogalactan, a major polysaccharide from F. officinalis fruiting bodies, was extracted using boiling water and purified, revealing a branched structure with a backbone of partially 3-O-methylated 1,6-O-linked α-D-galactopyranosyl residues. These residues were substituted at O-2 by 3-O-α-D-mannopyranosyl-α-L-fucopyranosyl and β-D-galactopyranosyl units, with α-L-fucopyranosyl units forming part of the side chains (Golovchenko et al., 2018).
Branched β-glucans from F. officinalis showed cytotoxic activity (Golovchenko et al., 2020). Fomitopsis officinalis yielded a purified heteropolysaccharide, FOBP50–1, with a molecular weight of 2.21 × 104 g/mol, composed of 3-O-methylfucose, fucose, mannose, glucose, and galactose in a ratio of 1:6.5:4.4:8.1:18.2. Its structure was elucidated using UV, FT-IR, GC–MS, and NMR analysis. FOBP50–1 demonstrated significant antitumor activity in zebrafish assays by interacting with TLR-4, PD-1, and VEGF, thereby activating immunity and inhibiting angiogenesis. These results highlight its potential as a tumor immunotherapy agent (Shen et al., 2024). A purified heteropolysaccharide, FOBP90-1, was isolated from F. officinalis to explore its anticancer potential. FOBP90-1, with a molecular weight of 2.87 × 104 g/mol, comprises several sugar residues, including α-d-Galp, α-l-Fucp, β-d-Glcp, α-d-Manp, and 3-O-Me-α-l-Fucp, as identified by UV, FT-IR, methylation analysis, and NMR. In zebrafish models, FOBP90-1 demonstrated anticancer activity by promoting immune activation and inhibiting angiogenesis. Mechanistic studies revealed that these effects were mediated through interactions with TLR-2, TLR-4, PD-L1, and VEGFR-2, suggesting FOBP90-1’s potential as a cancer treatment agent (Liu et al., 2024).
4.1.5 Fomitopsis palustris
An extracellular β-glucosidase was purified from the brown-rot F. palustris, with a molecular mass of approximately 138 kDa and high homology with fungal β-glucosidases from glycosyl hydrolase family 3. The enzyme exhibited optimal activity at pH 4.5 and 70°C, with significant activity against p-nitrophenyl-β-d-glucoside and cellobiose, while being competitively inhibited by glucose and gluconolactone. These findings classify the β-glucosidase as an aryl-β-glucosidase with cellobiase activity, demonstrating notable thermostability (Yoon et al., 2008a).
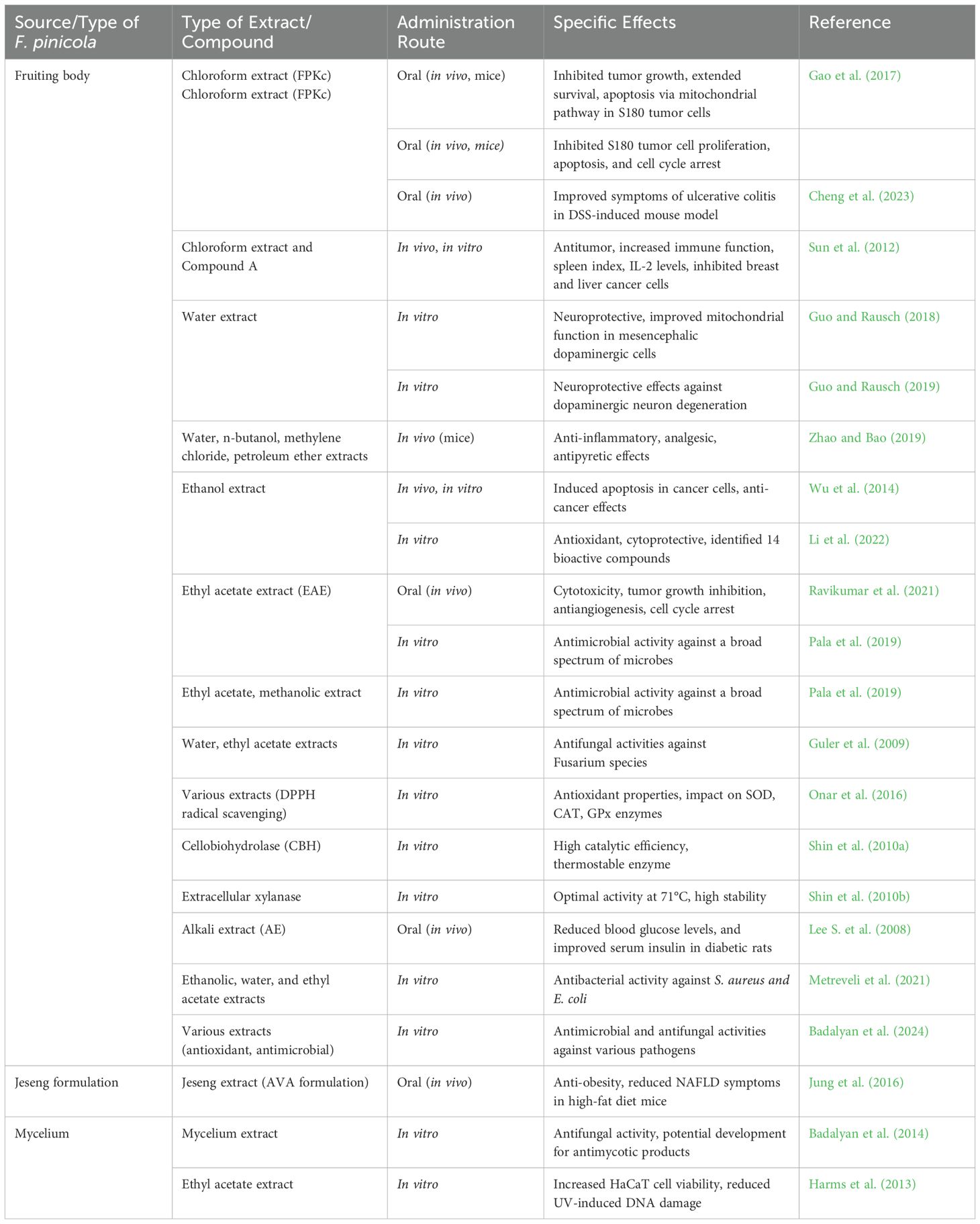
4.1.6 Fomitopsis pinicola
Fomitopsis pinicola is a traditional medicinal mushroom used in folk medicine in both China and Korea. Polysaccharides are the principal constituents of the fruiting body of F. pinicola. The extract (polysaccharide [FPP]) derived from F. pinicola was observed to lower fasting blood glucose levels while promoting increased body weight. In addition, FPP displayed a restorative impact on insulin levels in the bloodstream. Moreover, FPP demonstrated a noteworthy influence on lipid metabolism by reducing total cholesterol, triacylglycerol, and low-density lipoprotein cholesterol levels while elevating high-density lipoprotein cholesterol levels. Consequently, the FPP extract demonstrated beneficial properties for diabetes management, antioxidant effects, and regulation of lipid levels (Zahid et al., 2020a, 2020b, 2020c).
A heterogalactan was isolated from F. pinicola fruiting bodies and further separated into fucogalactan and mannofucogalactans using chromatography. Structural analysis revealed that all fractions are highly branched polysaccharides with a (1→6)-linked α-D-galactopyranosyl backbone, substituted with L-fucopyranosyl or mannopyranosyl-fucopyranose units (Usui et al., 1981). Polysaccharides from F. pinicola showed no toxicity to endothelial cells and had strong anti-angiogenic and anti-inflammatory effects (Cheng et al., 2008). Fomitopsis pinicola extract effectively lowered blood glucose levels by 77% after 20 days increasing HDL cholesterol by 73% and decreasing LDL cholesterol by 76%. This suggests its potential for atherosclerosis prevention and treatment, though more research is needed to understand the mechanisms involved (Cha et al., 2009). Researchers isolated F. pinicola, a potent ß-1, 4-glucosidase (BGL) producer through morphological and genetic analysis. They purified the BGL using a chromatographic process and found it belongs to glycoside hydrolase family 3, known for efficient enzymes. F. pinicola BGL stands out for its remarkable efficiency and specific substrate preferences (Joo et al., 2009). Fomitopsis pinicola polysaccharides comprise both extracellular and intracellular variants with distinct molecular weights, and both types of polysaccharides exhibited antioxidant properties, as evidenced by their ability to counteract DPPH and hydroxyl radicals in vitro and protect yeast cells from UV and hydrogen peroxide-induced oxidative damage. Importantly, intracellular polysaccharides displayed superior antioxidant activity compared to their extracellular counterparts (Hao et al., 2016).
Fomitopsis pinicola yielded twelve new sesquiterpenoids (fomitopins A–L [1–12]), through bioassay-guided purification. Their structures were determined using spectroscopic analyses and confirmed by ECD simulations. Ten compounds were tested for anti-inflammatory activity, with compound 11 showing the strongest inhibition of superoxide anion generation and elastase release (IC50 values of 0.81 ± 0.15 and 0.74 ± 0.12 μM). These sesquiterpenoids are promising candidates for further anti-inflammatory research (Tai et al., 2019). Fomitopsis pinicola exhibits protective effects against alcohol-induced liver injury through its mycelia polysaccharides (FPMPS). FPMPS improved serum lipid levels, maintained hepatic and cecal morphology, and modulated gut microbiota disrupted by alcohol. Mechanistically, FPMPS-regulated pathways, such as retinol metabolism, bile secretion, TRP channel inflammation, and the PI3K-Akt signaling pathway, play a key role in preventing liver damage. FPMPS shows promise as a potential therapeutic agent for acute alcoholic liver injury and as a functional health food (Wu et al., 2023). Table 1 lists polysaccharides from various species of Fomitopsis, along with their structures and therapeutic applications.
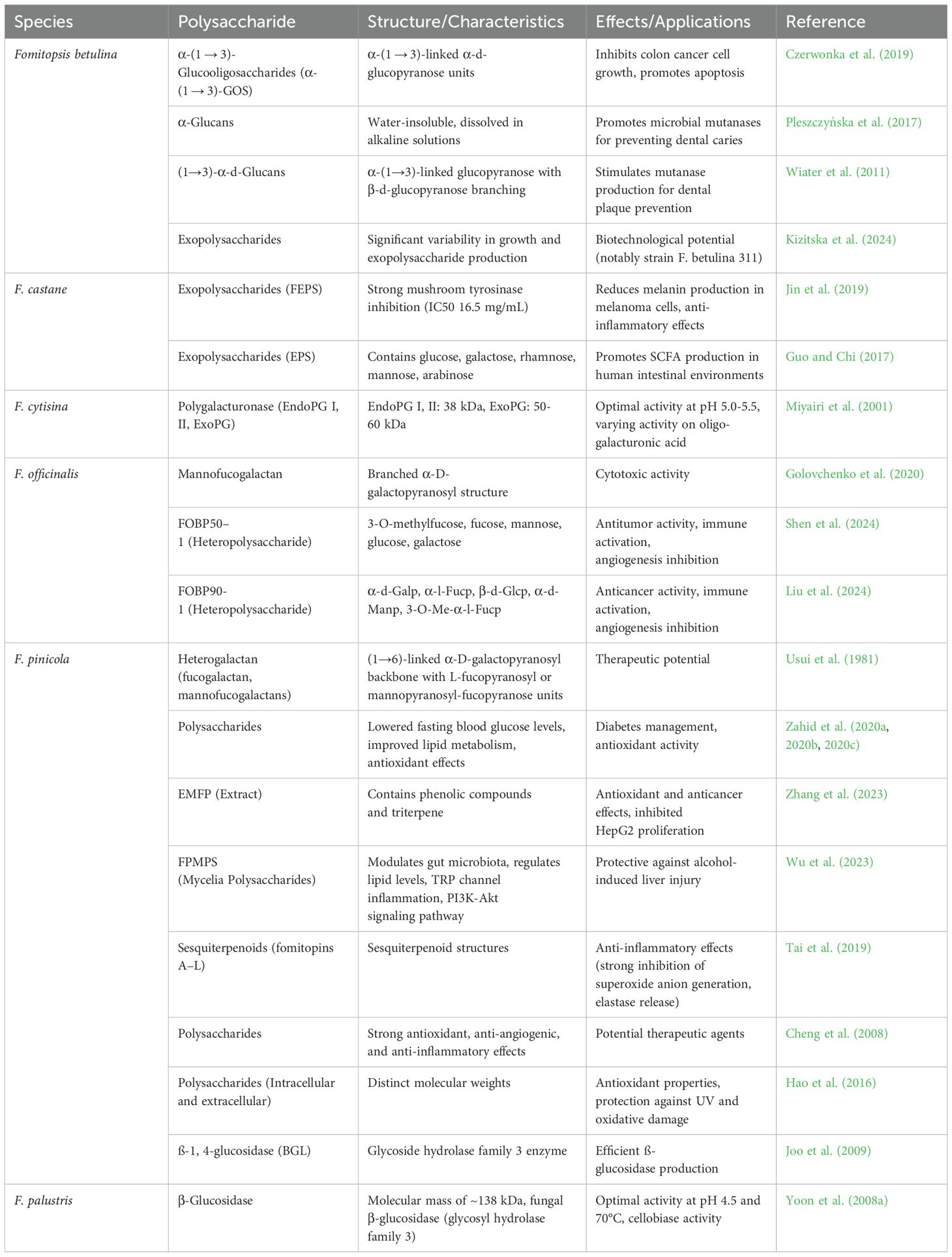
Table 1. Polysaccharides, structures, and therapeutic applications of various species of Fomitopsis.
4.2 Terpenoids
Terpenoids are a large and diverse class of natural compounds known for their structural variety and biological activities. In species of Fomitopsis, these compounds (Figure 3) are particularly notable for their antimicrobial, anti-inflammatory, and therapeutic properties. In this section, we discuss recent research on Fomitopsis terpenoids, highlighting their chemical diversity and potential applications in medicine and biotechnology.
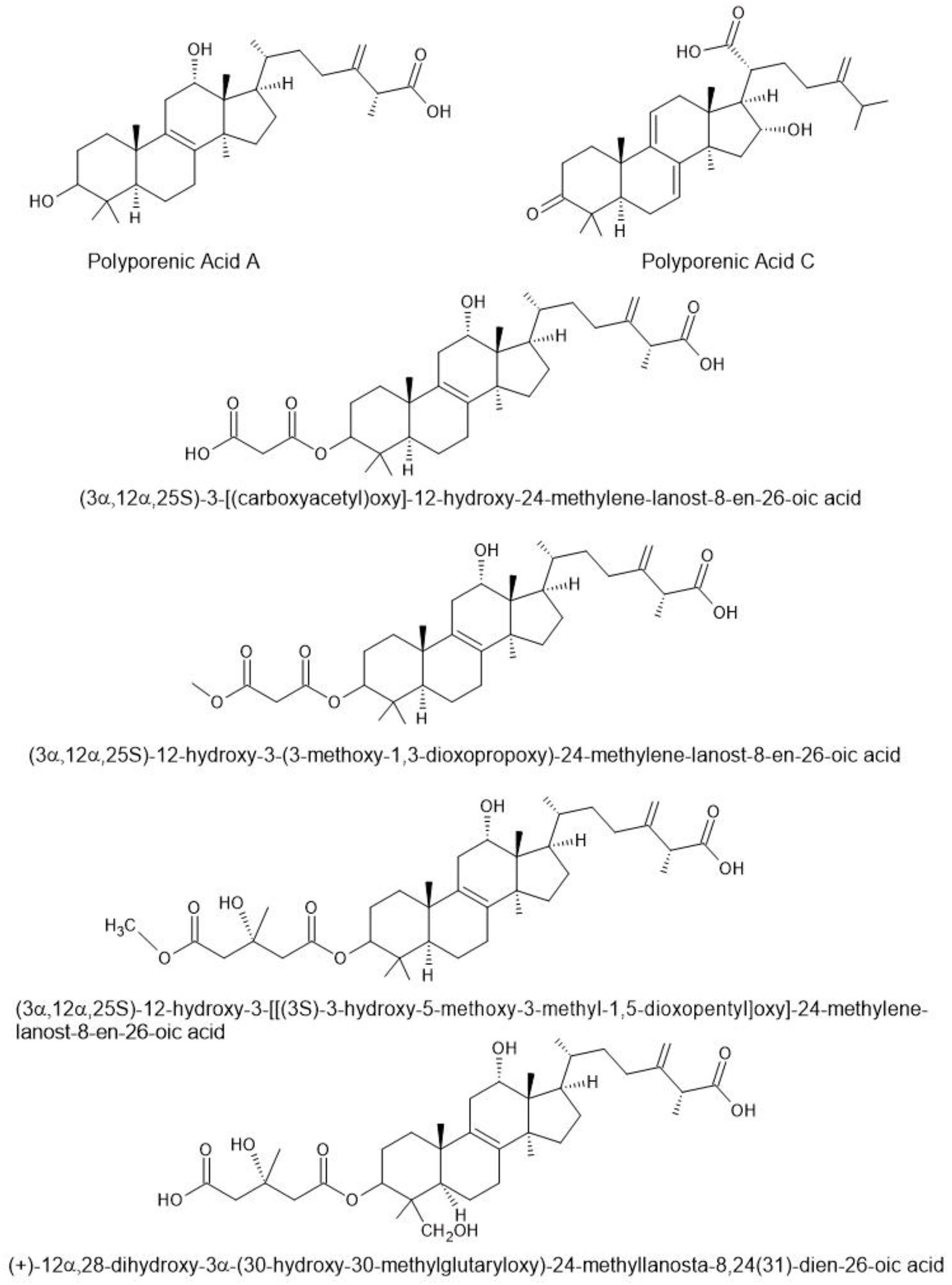
Figure 3. Triterpenoids found in species of Fomitopsis (Pleszczyńska et al., 2017).
4.2.1 Fomitopsis betulina
The mycelium of F. betulina was cultured via liquid fermentation, leading to the isolation of seven pimarane-type diterpenes from the fermentation broth. These were pipulinus A–D, pipulinus F, elaeicolasides B, and pipulinus E (Rapior et al., 1996). Twenty sesquiterpene compounds were identified from fresh F. betulina fruiting bodies, including (R)-trans-nerolidol, β-elemene, and α-chamigrene. Four of these—Isobazzanene, (S)-(−)-daucene, (−)-β-barbatene, and (+)-α-barbatene—were reported as fungal components for the first time. Subsequent research identified four additional monoterpenes such as linalool and α-terpineol, further expanding the understanding of F. betulina’s volatile constituents (Rösecke et al., 2000). Schlegel et al. (2000) found piptamine in F. betulina, which exhibited antimicrobial properties against bacteria and yeast, including C. albicans, at low concentrations. This discovery highlights the significance of compounds beyond polysaccharides in mushroom bioactivity. A novel phenolic compound, (E)-2-(4-hydroxy-3-methyl-2-butenyl)-hydroquinone, was isolated from the fresh fruiting bodies of F. betulina (Kawagishi et al., 2002).
Polyporenic acid C and three additional triterpenoids found in F. betulina have been shown to exhibit anti-inflammatory and antibacterial properties by proficiently blocking the activity of 3α hydroxysteroid dehydrogenase and bacterial hyaluronate lyase (Wangun et al., 2004). Two significant tetraterpene compounds, β-carotene, and lycopene, were successfully identified in the dried powder of F. betulina fruiting bodies. These compounds are recognized for their potent antioxidant properties. The known spectrum of phenolics in this species was expanded by detecting four tocopherols—α-tocopherol, β-tocopherol, γ-tocopherol, and δ-tocopherol—in a dried powder of F. betulina (Reis et al., 2011). Alresly et al. (2016) isolated one new and ten known triterpenes from fungal fruiting bodies of F. betulina. The new compound had antibacterial activity. Fomitopsis betulina showed high potential for antitumor activities (Sari et al., 2020). In a rich fermentation broth of F. betulina, three sesquiterpenes, including cryptosphaerolide B and two bicyclic sesquiterpenes, rel-(1S,4S,5R,7R,10R)-10-desmethyl-11-euduemene and 10,11-epoxyguaian-13-ol, were identified. In addition, seven pimarane-type diterpenes, pipulinus A–D, pipulinus F, elaeicolasides B, and pipulinus E, were isolated from the mycelium cultured via liquid fermentation. Two phenolic compounds, (3R)-5-carbomethoxymellein and 4-hydroxyphenethyl alcohol, were also identified in the fermentation broth (Sun, 2015). Five previously unreported lanostane-type triterpenoids, named piptolinic acids A–E, were identified from F. betulina, along with five known lanosterol-type triterpenoids: 3-epi-(3′-hydroxy-3′-methylglutaryloxyl)-dehydrotumulosic acid, dehydroeburiconic acid, 6α-hydroxypolyporenic acid C, and 3-epi-dehydropachymic acid (Tohtahon et al., 2017). Previously unreported 24-methyl-lanostane-type triterpenes (piptolinic acid F–J) were isolated from dried F. betulina fruiting bodies, along with seven known lanosterol-type triterpenoids (Khalilov et al., 2018). Eleven triterpenoids and betulin from F. betulina were tested for protective effects against chromosome aberrations in human lymphocytes using the CBMN assay. Most compounds reduced DNA damage more effectively than amifostine, with 2.0 µg/mL being the most effective concentration. D8-lanostanes exhibited better activity than those with a conjugated 7,9 (11)-diene system, while betulin showed the lowest protective activity, comparable to amifostine (Anđelković et al., 2021). Thirteen new 24-methylene lanostane triterpenoids and seventeen previously identified compounds were isolated from F. betulina. Fomitosides L and N exhibited cytotoxic effects on HL60 leukemia cells. Among the known compounds, dehydropachymic acid, pachymic acid, 3-epi-dehydrotumulosic acid, and 12α-hydroxy-3α-(3’-hydroxy-4’-methoxycarbonyl-3’-methylbutyryloxy)-24-methyllanosta-8,24(31)-dien-26-oic acid demonstrated significant cytotoxicity against HL60 leukemia cells while showing selectivity for MRC-5 healthy cells (Sofrenić et al., 2021). Researchers have identified 47 different lanostane-type triterpenoids in F. betulina (Li et al., 2024).
4.2.2 Fomitopsis officinalis
Fomitopsis officinalis fruiting bodies contain abundant triterpenoids, polysaccharides, organic acids, coumarins, and phenolic compounds. Scientific studies have shown that extracts and isolated components from F. officinalis offer diverse therapeutic advantages, encompassing anti-inflammatory, cytotoxic, and antimicrobial properties (Muszyńska et al., 2020). Antiviral effects of F. officinalis could prevent neuropathies associated with infections caused by the herpes viruses or hepatitis C (Stamets, 2011). Two chlorinated coumarins were isolated from the ethanol extract of F. officinalis and characterized. These compounds demonstrated specific antimicrobial activity against M. tuberculosis, including drug-resistant isolates, with minimum inhibitory concentrations (MICs) ranging from 22 to 50 µg/ml (Hwang et al., 2012). Eight compounds were isolated from F. officinalis, including 4, 6, 8 (14), 22 (23)-tetraen-3-one-ergostane, identified for the first time. Fomefficinic acids A and C and 3-keto-dehydrosulfurenic acid inhibited MCF-7 breast cancer cells, while fomefficinic acids A and C also suppressed SMMC-7721 liver cancer cells (Chi et al., 2014). Triterpene lactone, known as fomefficin, and the sesquiterpene extracted from F. officinalis have demonstrated notable anti-cancer effects (Feng and Yang, 2015). Similar properties have been observed in a group of triterpene compounds called the officimalonic acids A – H isolated from the methanol extract of F. officinalis. Their anti-inflammatory and cytotoxic activity in in vitro conditions was confirmed in contact with human cancer cells H460, HepG2, and BGC–823 (Wu et al., 2014). The exploration of the chemical composition of F. officinalis resulted in the isolation of four newly identified lanostane triterpenoids and four previously reported triterpenoids. Their capacity to inhibit Trypanosoma congolense, a pathogenic agent causing severe animal diseases, was assessed. Compounds 2-5 and 8 demonstrated moderate inhibitory activity, with IC50 values ranging from 7.0-27.1 µM (Naranmandakh et al., 2018). Flavonoids in F. officinalis can potentially mitigate oxidative stress in the aging mouse brain (Sha, 2016). Dehydrosulfurenic acid, a lanostane-type triterpenoid derived from F. officinalis, has been patented for its potential use as a pharmaceutical treatment for ischemic stroke (Saba et al., 2015; Simi and Prisco, 2018; Flores et al., 2023),. Moreover, eburicoid acid could further prove to have antidepressant effects (Yang et al., 2019; Muszyńska et al., 2020).
4.2.3 Fomitopsis pinicola
In this study, the fresh fruiting body of F. pinicola was freeze-dried, and 300g of the dried mushroom was extracted sequentially with dichloromethane and methanol. The dichloromethane extract, accounting for 13.7% of the total extract, exhibited antimicrobial activity in a TLC bioassay (Hamburger & Cordell, 1987). Phytochemical analysis of methanol and n-hexane extracts from F. pinicola identified triterpene derivatives and aromatic compounds reduced from lignin. New natural compounds such as pinicolol C and pinicolic acid E were discovered, along with steryl esters such as 3β-linoleyloxyergosta derivatives. HPLC and TLC confirmed that the surface of F. pinicola is rich in lanostane derivatives (Rösecke and König, 2000). Two newly discovered lanostane triterpenoids and ten previously unreported lanostane triterpene glycosides were found in F. pinicola (Yoshikawa et al., 2005). A novel lanostane triterpene, 3α-acetyloxylanosta-8,24-diene-21-ol, was obtained from an active fraction of the fungus F. pinicola extract. In addition, two known triterpenic acids, pinicolic acid A and 3α-acetoxylanosta-8, 24-dien-21-oic acid, were identified. These compounds demonstrate cytotoxic and antimicrobial activities (Petrova et al., 2007). Various lanostane triterpenoids and an ergostane compound obtained from American F. pinicola exhibited antimicrobial activity, particularly against B. cereus, with MIC values ranging from 16 to 128 μg/ml. Compounds A, B, C, and F demonstrated specific antimicrobial effects, while D and E displayed lower specificity (Liu et al., 2010). The effects of F. pinicola, a medicinal fungus with notable anti-tumor activity primarily due to 3α-acetoxylanosta-8,24-dien-21-oic acid, were assessed on immune function in mice. The study found increased phagocytic rate, serum hemolysin levels, lymphocyte conversion rate, and phagocytic index in the F. pinicola group compared to controls. The compound enhanced lymphocyte transformation at low concentrations, but the effect decreased at higher concentrations. Overall, dry mycelia of F. pinicola effectively enhanced immune competence in mice (Bao et al., 2015).
To identify the chemical constituents of the fruiting bodies of F. pinicola, researchers isolated a novel lanostane triterpene glycoside, named fomitoside K, from its methanolic extract (Lee et al., 2012). Three new 24-methyl-lanostane triterpenoids (fomitopsins D-F) and four known compounds were isolated from F. feei. Fomitopsins E and F showed antibacterial activity against B. cereus, while fomitopsin D exhibited antiviral activity against herpes simplex virus type 1 (HSV-1) (Isaka et al., 2017). Seven lanostane-type triterpenes isolated from F. pinicola and F. officinalis showed significant antitumor activity, particularly in MCF-7 cells. Compounds 2 and 4 effectively suppressed tumor growth in mice, influencing VEGF and cytokine expression. Acetyl or carbonyl at C-3 and hydroxy at C-15 enhanced their antitumor effects (Shi et al., 2017). A triterpenoid, 3 acetoxylanosta 8,24 dien 21 oic acid (FPOA), a triterpenoid obtained from the fruiting body of F. pinicola, exhibits cytotoxic properties, particularly targeting HepG2 hepatoma cells (Song et al., 2018). Fomitopsis pinicola yielded twelve new sesquiterpenoids, fomitopins A–L (1–12), through bioassay-guided purification. The structures were determined using spectroscopic analyses and confirmed by ECD simulations. Ten compounds were tested for anti-inflammatory activity, with compound 11 showing the strongest inhibition of superoxide anion generation and elastase release (IC50 values of 0.81 ± 0.15 and 0.74 ± 0.12 μM). These sesquiterpenoids are promising candidates for further anti-inflammatory research (Tai et al., 2019). A methanolic extract of F. pinicola yielded 35 lanostane-type triterpenoids, including 13 newly discovered compounds and 22 previously known ones. These compounds demonstrated cytotoxicity against various human tumor cell lines, such as HL-60, A549, SMMC-7721, MCF-7, and SW480. In addition, certain compounds exhibited selective inhibitory effects against specific cell lines, with some inducing apoptosis in HL-60 cells (Peng et al., 2019). Twelve previously unreported sesquiterpenoids, named fomitopins A–L (1–12), were isolated from F. pinicola, known for its antimicrobial and anti-inflammatory properties. These newly discovered sesquiterpenoids hold promise for further anti-inflammatory research (Tai et al., 2019). Fruiting bodies of F. pinicola yielded 28 lanostane triterpenoids, comprising 11 novel and 17 previously identified compounds. Some of these compounds reduced nitric oxide release, while others demonstrated notable PTP1B inhibitory properties. Kinetic analysis confirmed two compounds as competitive PTP1B inhibitors, and three were observed to enhance glucose uptake in insulin-resistant cells. These findings indicate the potential of F. pinicola as a functional food or medicine for diabetes management (Zhang et al., 2020).
Thirteen novel and nine known lanostane triterpenoids were isolated from F. pinicola fruiting bodies, with their structures confirmed through spectroscopic analysis and X-ray diffraction. Nor-pinicolic acids A−F, featuring unique C-25-C-27 nor-lanostane skeletons, were first identified in this species. Anti-inflammatory assays showed that pinicopsic acid F and 16α-hydroxy-3-oxolanosta-7,9(11),24-trien-21-oic acid exhibited moderate inhibition of LPS-induced NO production in RAW 264.7 cells, with IC50 values of 24.5 and 25.7 μM (Liu et al., 2022).
Twelve previously unreported lanostane-type triterpenes, along with twenty-two known triterpenes, were discovered and characterized in F. pinicola. Thirty-two triterpene compounds were assessed for their anti-inflammatory potential using neutrophils as a model, with pinicolasin J emerging as the most effective inhibitor of superoxide anion generation (Kuo et al., 2021). Volatile compounds from F. pinicola were analyzed, and during sporulation, F. pinicola released (R)- and (S)-oct-1-en-3-ol, octan-3-one, and sesquiterpenes. Chopping up the fruiting bodies released volatiles and they attracted wood-living beetles and moths, with rac-oct-1-en-3-ol being a key attractant (Fäldt et al., 1999). Phytochemical analysis of F. pinicola yielded a new lanostanoid derivative and seven known triterpenes. Five of these compounds showed antimicrobial activity against Bacillus subtilis (Keller et al., 1996).
4.2.4 Other Fomitopsis species
Chemical analysis of Fomitopsis carnea solid-state cultures led to the discovery of two new triterpenoid glycosides, forpiniosides B and C, and two already known compounds. Structural identification was carried out using HRESIMS and NMR techniques. Among the compounds, forpinioside B showed notable antimicrobial activity against Staphylococcus aureus and B. subtilis, with MIC values comparable to standard antibiotics gentamycin and oxytetracycline (Sum et al., 2023). A new lanostane triterpene glycoside, fomitoside-K, was isolated from Fomitopsis nigra and tested for anticancer activity against human oral squamous cell carcinoma (YD-10B) cells. Fomitoside-K induced apoptosis via mitochondrial dysfunction, increased ROS levels, and activated JNK and ERK pathways. Its effects were reduced by ROS scavengers and MAPK inhibitors. In addition, fomitoside-K showed synergy with adriamycin, suggesting its potential as a treatment for oral cancer through a ROS-dependent mitochondrial apoptosis pathway (Bhattarai et al., 2012). Hypercholesterolemia, a major risk factor for coronary heart disease, can be mitigated by inhibiting NPC1L1-mediated cholesterol absorption. A novel compound, fomiroid A, was discovered in F. nigra mushroom extracts, showing potent inhibition of ezetimibe glucuronide binding to NPC1L1. Fomiroid A, a lanosterone derivative (C30H48O3), dose-dependently blocked cholesterol uptake in Caco2 cells and acted as a pharmacological chaperone for the L1072T/L1168I mutant of NPC1L1, suggesting a unique mechanism of action compared to ezetimibe (Chiba et al., 2014).
The extract from Fomitopsis rosea yielded two novel lanostane triterpenes—3α-(3′-butylcarboxyacetoxy) oxepanoquercinic acid C 1 and 3α-hydroxy-24-methylene-23-oxolanost-8-en-26-carboxylic acid 2—in addition to three previously identified triterpenes and one epidioxy sterol derivative. While all these triterpenes demonstrated antibacterial effects against S. aureus, none exhibited anti-radical properties against DPPH radicals (Popova et al., 2009). Fomitopsis spraguei was investigated for its methanolic extract, leading to the isolation of five lanostane-type triterpenoids. These included three novel compounds named fomitopsins A–C, along with two known compounds: quercinic acid C and 3α-carboxyacetyl-12β-hydroxyquercinic acid (Quang et al., 2005). Two newly identified 24-methyl-lanostane triterpenoids, named fomitopsins I and J, were detected in Fomitopsis sp. along with seven previously recognized compounds. One known compound exhibited antibacterial effects against B. cereus (with a minimum inhibitory concentration of 6.25 μg/ml) and Enterococcus faecium (with a minimum inhibitory concentration of 12.5 μg/ml) (Isaka et al., 2019). The triterpenoid composition of various Fomitopsis species, highlighting their bioactivities, including anticancer, anti-inflammatory, and antimicrobial properties is summarized in Table 2.
Table 2. Summary of results from triterpenoid analysis and bioactivity findings related to various species of Fomitopsis.
4.3 Proteins/Enzymes
4.3.1 Fomitopsis meliae
A novel thermostable endoglucanase has been identified from the brown rot fungus F. meliae CFA 2, which was purified 34.18-fold and has a specific activity of 302.90 U/mg. The enzyme exhibits optimal activity at 70°C and a pH of 4.8, with a molecular weight of 37.87 kDa, making it promising for biomass hydrolysis due to its favorable kinetic properties and stability. Its activity is enhanced by Zn2+ and K+ ions, with a half-life of 11.36 h at 70°C (Patel and Shah, 2021). The cellulolytic-hemicellulolytic enzyme production of F. meliae CFA 2, a newly isolated brown rot fungus, was studied. Under solid-state fermentation with wheat bran, it produced 1391.12 U/g of endoglucanase. After statistical optimization, the endoglucanase yield increased by 1.83-fold. Enzymatic saccharification of alkali-treated wheat and rice straw released 190.8 and 318.8 mg/g of reducing sugars, respectively, highlighting its potential for biomass degradation (Patel et al., 2021). Cellulose can be broken down by cellulases for biofuel production. Fomitopsis meliae has been cultivated under solid-state fermentation (SSF) on wheat bran, achieving high cellulase yields, particularly CMCase. Using the One-Factor-at-a-Time (OFAT) approach, optimal SSF conditions were determined, including a temperature of 32–36°C, pH 4.0, and a 1:3 moisture ratio. These results highlight F. meliae as a promising cellulase producer for industrial applications (Jini and Singh, 2024). Enzymes and proteins isolated from species of Fomitopsis along with details on their activities, characteristics, and potential biotechnological applications are presented in Table 3.
Table 3. Activities, characteristics, and potential applications of enzymes and proteins isolated from species of Fomitopsis.
4.3.2 Fomitopsis palustris
Malate synthase, a key enzyme in the glyoxylate cycle, was purified from F. palustris. The enzyme, with a molecular mass of 520 kDa and composed of eight 65-kDa subunits, showed Km values of 45 μM for glyoxylate and 2.2 μM for acetyl-CoA. Its activity was inhibited by oxalate, glycolate, and coenzyme A, with p-chloromercuribenzoate indicating the presence of a sulfhydryl group at the active site. The enzyme required Mg2+ for full activation and stability, becoming inactive without metal ions (Munir et al., 2002). Isocitrate lyase, a key enzyme in the glyoxylate cycle, was purified 76-fold with a 23% yield from F. palustris grown on glucose. The enzyme, with a molecular mass of 186 kDa, consists of three 60-kDa subunits. It has a Km of 1.6 mM for isocitrate at pH 7.0 and requires Mg2+ and sulfhydryl compounds for optimal activity. The enzyme is strongly inhibited by oxalate and itaconate, with Ki values of 37 and 68 μM, respectively. These findings suggest that isocitrate lyase plays a regulatory role in fungal growth (Munir et al., 2002). One study investigated the roles of the glyoxylate and tricarboxylic acid cycles in oxalate biosynthesis during fungal development. Enzyme activities were higher during the vegetative stage, with isocitrate lyase contributing to oxalate synthesis early on, while isocitrate dehydrogenase played a key role in glutamate synthesis during F. palustris fruiting body formation (Yoon et al., 2002). NADP-linked isocitrate dehydrogenase (EC 1.1.1.42) was purified 672-fold from the copper-tolerant fungus F. palustris. The enzyme, with a molecular mass of 115 kDa, consists of two 55-kDa subunits and has Km values of 12.7, 2.9, and 23.9 μM for isocitrate, NADP, and Mg2+, respectively, at pH 9.0. Mg2+ enhances activity and prevents inactivation. The enzyme is competitively inhibited by 2-oxoglutarate (Ki, 127.0 μM) and strongly inhibited by oxaloacetate and glyoxylate through mixed inhibition (Yoon et al., 2003). Fomitopsis palustris has been shown to degrade crystalline cellulose (Avicel) by producing exoglucanases, endoglucanases, and β-glucosidase. After 14 days, the relative crystallinity of Avicel decreased from 83% to 78.5%. The optimal conditions for exoglucanase activity were pH 4.5 and 70°C. Hydrolysis yielded 1.6 mg/mL of glucose after 43 hours, with a cellulose conversion degree of 3.2%, highlighting F. palustris’ capability to break down crystalline cellulose (Jeong-Jun, 2005).
Fomitopsis palustris produces a 72 kDa extracellular enzyme, identified as a glucoamylase, when grown in cellulose culture with cellobiose. When the enzyme was purified, its amino acid sequence showed high similarity to fungal glycoside hydrolase family 15 glucoamylases. The kinetic activity of the enzyme increased with the substrate’s polymerization, and the glucoamylase gene (gla) was cloned via reverse transcriptase PCR (Yoon et al., 2006). Two endoglucanases produced by F. palustris were purified, with molecular masses of 47 kDa and 35 kDa. The 47-kDa enzyme resembled fungal glycoside hydrolase family 5, while the 35-kDa enzyme exhibited high cellulase activity despite no homology to known glycosylhydrolases. Both enzymes efficiently degraded Avicel, producing cellobiose as the primary product (Yoon et al., 2007a). An extracellular xylanase from the brown-rot fungus F. palustris was purified to a single protein band, showing a molecular mass of approximately 43 kDa on SDS-PAGE. The amino acid sequence of the enzyme indicated significant homology with fungal glycoside hydrolase family 10 xylanases. The optimal activity of the purified xylanase occurred at pH 4.0–5.0 and a temperature of 70∘C (Yoon et al., 2007b).
When cDNA and FpTRP26 were isolated from F. palustris through yeast transformant screening, they conferred specific resistance to OA. Transformants showed a 65% reduction in OA content when grown with 2 mM OA. FpTRP26 transcript levels increased with OA accumulation and remained high, even in the stationary phase, suggesting that FpTRP26 plays a key role in OA resistance in F. palustris (Watanabe et al., 2007). Two acidic β-glucosidases (βGI and βGII) from Fomitopsis palustris were purified, showing optimal activity at pH 2.5 and 55°C. Both enzymes effectively hydrolyzed cello-oligosaccharides to release glucose, and F. palustris produced high ethanol yields from various sugars, highlighting its potential for bioethanol production (Okamoto et al., 2011). Fomitopsis palustris produces two oxalate-generating enzymes, with oxaloacetate acetylhydrolase (FpOAH) playing a dominant role in oxalate biosynthesis. A cloned 1080-bp cDNA confirmed FpOAH activity, and its gene expression was significantly higher than that of glyoxylate dehydrogenase (FpGLOXDH), suggesting FpOAH’s primary role in oxalate production (Hisamori et al., 2013). Fomitopsis palustris produces cellulases to degrade cellulose, including a newly identified endoglucanase gene, cel12. This gene encodes a protein lacking a cellulose-binding domain but is highly conserved among GH family 12 cellulases. The expression of the gene increases during growth on cellulose, and recombinant cel12 shows endoglucanase activity on carboxymethyl cellulose, but not crystalline cellulose (Song et al., 2018).
4.3.3 Fomitopsis pinicola
The milk-clotting enzyme (BR) from F. pinicola was compared to commercial rennet (HR) for cheese-making. BR had higher activity at 35°C, greater heat stability, and more proteolytic activity than HR. Both enzymes acted similarly on κ-casein, and after 50 minutes, curds produced by BR and HR had the same tension. The study concluded that BR could be a viable substitute for commercial rennet in cheese production (Nakanishi and Itoh, 1969). The solid fermentation product of F. pinicola demonstrated notable anti-tumor and anti-oxidation effects in H22 tumor-bearing mice, with high and moderate doses achieving inhibition rates of 66.66% and 64.70%, respectively. Treatment increased serum levels of IL-2 and IFN-γ, reduced MDA levels, and enhanced antioxidant enzyme activities (SOD, CAT, and GSH-PX), highlighting its potential as a therapeutic agent (Sun et al., 2016).
Laccases from F. pinicola FP58527 SS1 were detected in the secretome when grown on poplar and spruce wood. Two laccases, FpLcc1 and FpLcc2, were produced and purified for testing. Both showed similar low pH-optima and moderate catalytic efficiency. Notably, FpLcc2 was significantly activated by acetic acid, especially at pH 5.0, suggesting a unique regulatory mechanism in brown rot fungi (Csarman et al., 2021). The heterologous expression and characterization of a GH45 endoglucanase from F. pinicola were reported, comparing it with a known GH45 from Phanerochaete chrysosporium. Both enzymes, expressed in Pichia pastoris, demonstrated an acidophilic nature with an optimal pH of 4 and a preference for β-1,4-glucans. No significant differences were observed between the enzymes from the two fungi (Amengual et al., 2022). Fomitopsis pinicola was identified as the wood decay pathogen affecting Korean pine (Pinus koraiensis) in this study through rDNA-ITS analysis and morphological observations (ITS accession number OQ880566.1). The cellulase enzymes, endoglucanase (CMCase) and β-glucosidase, were quantified using the DNS method, and enzyme activity was optimized using a single-factor and orthogonal test. The highest cellulase activity reached 116.94 U/mL under specific conditions, providing a foundation for improving cellulose degradation and advancing biotransformation research by brown-rot fungi (Sun et al., 2024).
5 Other bioactive compounds and beneficial medicinal properties
In addition to terpenoids, species of Fomitopsis are a rich source of diverse bioactive compounds (Figure 4) with significant medicinal properties. The following section discusses other bioactive compounds and their beneficial medicinal properties.
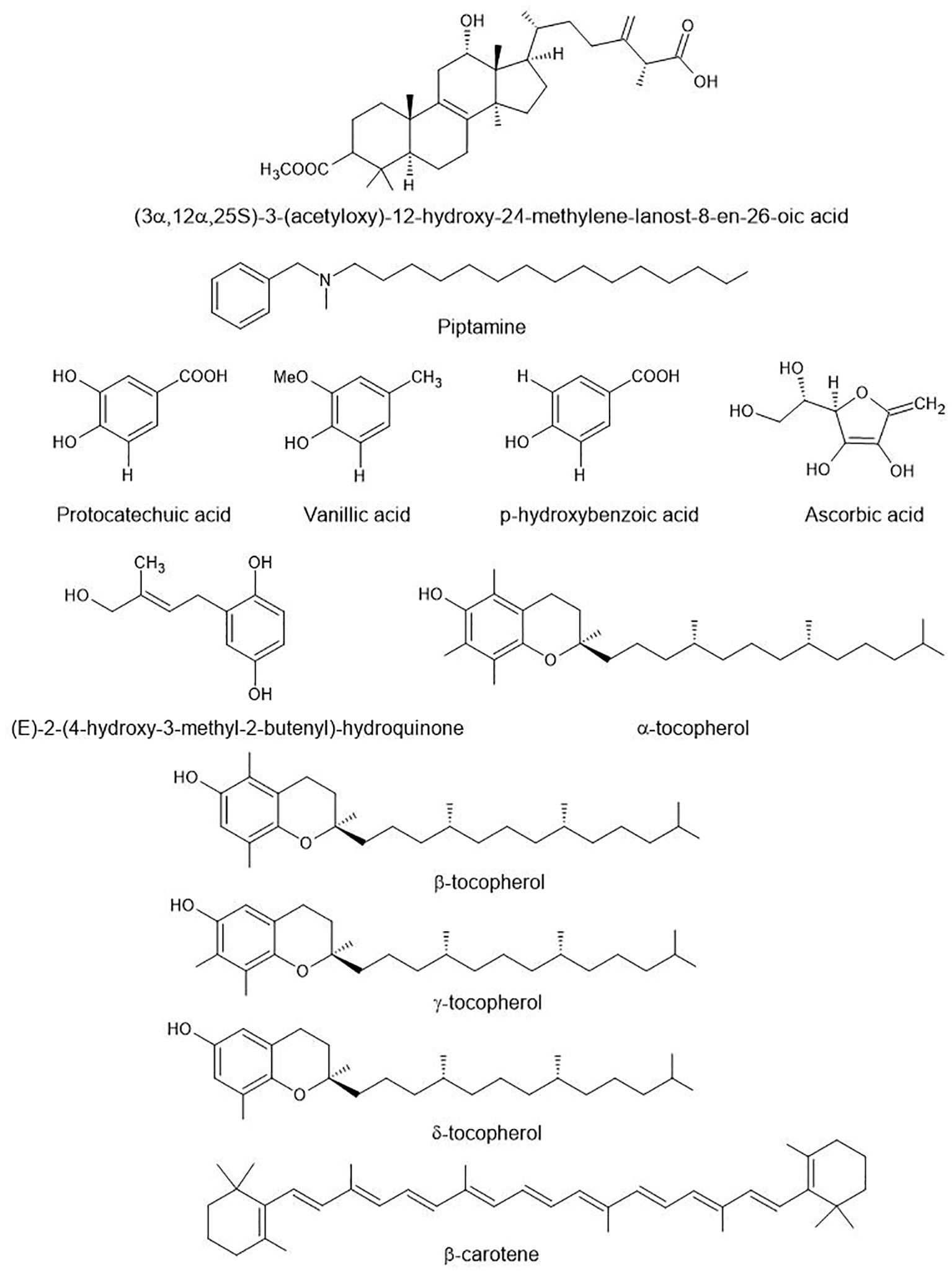
Figure 4. Other bioactive compounds found in species of Fomitopsis. These are aromatic amines (piptamine), phenolic acids (protocatechuic acid, vanillic acid, p-Hydroxybenzoic acid), hydroquinone derivatives, and vitamin-related compounds (ascorbic acid, α, β, γ, δ-tocopherol, β-carotene).
5.1 Fomitopsis betulina
The methanol and chloroform extracts from fruiting bodies of F. betulina exhibited antibacterial properties against B. subtilis and E. coli (Keller et al., 2002). The ethyl extract fraction derived from dried F. betulina fruiting bodies was examined for its impact on various cell lines, including A549, HT-29, murine lung carcinoma, colon adenocarcinoma, and C6 rat glioma. The extract exhibited significant reductions in cell viability, proliferation, and migration among tumor cells, while it also counteracted the stimulating effect of IGF-1, all without causing toxicity to normal cells (Lemieszek et al., 2009). Mycelia of in vitro-cultivated F. betulina were extracted with ether and ethanol and these extracts showed significant anti-cancer activity, reducing cancer cell viability, slightly inhibiting proliferation, and decreasing tumor cell adhesion. The effects were dependent on the duration and dosage of exposure (Cyranka et al., 2011). An ethyl acetate extract from Piptoporus betulinus mycelium increased HaCaT cell viability, reversed G1 cell cycle arrest induced by serum deprivation, and mitigated UV-induced DNA damage by 20% in HaCaT cells. Further, proteome analysis revealed elevated levels of cellular oxidoreductase after treatment with P. betulinus (Harms et al., 2013). The hot water extract obtained from the fruiting bodies of F. betulina demonstrated a moderate cytotoxic effect, as evidenced by an IC50 value of 0.1 mg/ml against HeLa cells. Furthermore, it significantly inhibited angiotensin-converting enzyme (ACE) activity. These alkali extracts also displayed antioxidant activity in the FRAP assay (Vunduk et al., 2015). Artificially cultivated fruiting bodies of F. betulina were used to produce water and ethanol extracts. These extracts exhibited activity against cancer cell lines A549, HT-29, and T47D (Pleszczyńska et al., 2016). In addition, ethanolic extracts of F. betulina significantly enhanced granulocyte phagocytosis by 158% (Doskocil et al., 2016). Fomitopsis betulina cultivated on agro-industrial by-products produced a pineapple-like aroma. Two compounds, (5E/Z,7E,9)-decatrien-2-ones, were identified as the source of the scent. These compounds were synthesized and confirmed by mass spectrometry, with (5Z,7E,9)-decatrien-2-one having the strongest pineapple-like odor. A specific structure, including 10 carbon atoms, was found to be essential for the aroma (Grosse et al., 2019). Fomitopsis betulina shows antibacterial activity against S. haemoliticus and A. baumannii. Concentrating and drying the cultural liquid improve its antibacterial properties, making it a potential candidate for pharmaceutical applications (Krupodorova et al., 2019).
5.2 F. cajanderi, F. feei, F. iberica and F. meliae
Hot ethanol and aqueous extracts of F. cajanderi were studied for their cytotoxic effects on human cancer cell lines and modulation of TNF secretion. The ethanol extract exhibited concentration-dependent cytotoxicity against MCF7 and U-937 cells, while the aqueous extract was non-cytotoxic to MCF7 cells but inhibited TNF secretion in LPS-stimulated U-937 cells due to its beta-glucan content. These results indicate the potential of F. cajanderi extracts as novel adjunctive anti-cancer and anti-inflammatory agents (Wenner et al., 2021). The effect of ten plant oils and eleven mineral chlorides on exopolysaccharide production from F. feei was tested in a broth medium. Groundnut oil and sodium chloride positively influenced exopolysaccharide production, providing a scientific foundation for optimizing the extraction of these compounds from F. feei, and enhancing its potential medicinal use (Bindu and Charya, 2018). Metabolic profiling of hydroalcoholic and organic extracts from F. iberica, A. biennis, and S. hirsutum mycelia was investigated using NMR methodology. The analysis revealed various amino acids, sugars, organic acids, and fatty acid chains. Fomitopsis iberica extracts were notable for the presence of galactose (GABA) and a high amount of ergosterol, highlighting the potential of Fomitopsis for developing nutritionally valuable food products (Goppa et al., 2023). Two antibacterial compounds, 5-hydroxymethyl-2-furoic acid methyl ester and 5-hydroxymethyl-2-furancarboxylic acid (HMFCA), were isolated from F. meliae. HMFCA demonstrated antibacterial activity against methicillin-susceptible S, aureus, while both compounds showed no activity against A549 cancer cells (Srisit et al., 2024).
5.3 Fomitopsis officinalis
Several strains of F. officinalis show strong antiviral activity, with F. officinalis I achieving a Selectivity Index (SI) of >20 against cowpox and F. officinalis IV a SI of >29 against the Vaccinia virus. The variation in potency underscores the importance of preserving F. officinalis biodiversity, which is threatened by habitat destruction. Collecting and studying more strains is vital for developing medicines against pox viruses. Research is ongoing to identify the active antiviral agents, their modes of action, and their impact on immune defense (Stamets, 2005). A family 12 endoglucanase (EG-II) with a molecular mass of 23,926 Da was purified and characterized from the brown-rot basidiomycete F. palustris. EG-II is believed to play a role in wood degradation by loosening the polysaccharide network in cell walls through the disentangling of hemicelluloses associated with cellulose (Shimokawa et al., 2008). Agaricinic acid, extracted from F. officinalis carpophores using ethanol, was identified through NMR spectroscopy and comparison to a standard sample. The extraction process involved ethanol, followed by purification with ether. The analysis revealed the presence of hydroxyl and various carboxy groups (Airapetova et al., 2010).
Fomitopsis officinalis is reported to exhibit broad-spectrum antibacterial and antiviral effects against various pathogens, including Mycobacterium tuberculosis, Yersinia pseudotuberculosis, S. aureus, and the Ortopox virus. Chlorinated coumarins from mycelia and lanostane triterpenoids from basidiomes have been linked to antiviral-antibacterial and trypanocidal activities, respectively. While there is significant in vitro potential with crude extracts, standardization remains a challenge (Sidorenko, 2009; Girometta, 2019). Fomitopsis officinalis EtOH extract produced two new chlorinated coumarins, identified through spectroscopy and chemical synthesis. Analogues were also synthesized. These compounds had limited antimicrobial activity, with the lowest MICs against M. tuberculosis complex (Hwang et al., 2013). Mycelium and fruiting bodies of F. officinalis contain various compounds such as L-tryptophan, phenolics (e.g., p-hydroxybenzoic acid, gallic acid), sterols (including ergosterol and ergosterol peroxide), and trace elements. These extracts showed antioxidant and growth-inhibitory effects on cancer cell lines like A549 lung cancer, DU145 prostate cancer, and A375 melanoma cells (Fijałkowska et al., 2020). The effects of adding zinc and magnesium salts to the culture medium in 10-L bioreactors were examined in this study. Results showed that mycelium grown on sulfate-enriched medium had higher levels of these minerals compared to the fruiting bodies. The enrichment increased the bioavailability of bioelements and organic compounds (indole, phenolic compounds, and L-phenylalanine), indicating that the method effectively produces fortified mycelium as a natural therapeutic material (Fijałkowska et al., 2021). The antimicrobial effects of F. officinalis were examined through culture fluid and mycelial mass extracts from various strains. The extracts exhibited high activity against S. aureus, particularly strains IBK-5004 and IBK-2498, and moderate activity against Klebsiella pneumoniae. However, no activity was observed against Escherichia coli, Pseudomonas aeruginosa, or B. subtilis (Mykchaylova and Poyedіnok, 2021). The biological activity of F. officinalis was evaluated for its antioxidant and anticancer effects using six extracts against hepatocellular carcinoma cells. All extracts showed antioxidant and anticancer potential. The chloroformic extract (Fo3) notably induced apoptosis, activated the G2/M cell cycle phase and selectively influenced NF-kB proteins. This highlights Fo3’s potential as a natural antitumor agent, warranting further research for its use in cancer treatment (Altannavch et al., 2022).
A study investigates the metabolic differences between the cap (median and apical parts) and hymenium of F. officinalis. Chromatographic analysis revealed that the apical part is richest in phenolic compounds. These extracts showed strong antifungal, antibacterial, and antiradical activity, especially against Gram+ bacteria and dermatophytic species, with MIC values below 100 µg/mL, suggesting F. officinalis as a valuable source for antioxidant and antimicrobial food supplements (Flores et al., 2023).
5.4 Fomitopsis palustris
Fomitopsis palustris primarily converts glucose to oxalic acid rather than fully oxidizing it through the TCA cycle. Key enzymes, including isocitrate lyase and oxaloacetase, connect the TCA and glyoxylate cycles to facilitate oxalate production, while malate dehydrogenase plays a crucial role in generating NADH. This process allows the fungus to obtain biochemical energy (Munir et al., 2001). A novel enzyme, FpPG28A, was isolated from F. palustris. It collaborates with oxalic acid to break down wood pectin. FpPG28A doesn’t affect esterified pectin, suggesting the involvement of a pectin esterase. This enzyme operates optimally at 60°C and pH 5.0 and efficiently degrades pectin in the presence of oxalate. Oxalate also enhances its thermostability at pH 3.0, highlighting its role in wood pectin degradation (Tanaka et al., 2018).
5.5 Fomitopsis pinicola
A cerebroside fraction was extracted from F. pinicola fruiting bodies, with the main cerebroside determined to have the structure (4E,8E,2S,3R,2’R)-N-2’-hydroxypalmityl-1-O-α-D-glucopyranosyl-9-methyl-4,8-sphingadienine (Striegler and Haslinger, 1996). N-hexane and methanol extracts from F. pinicola identified six new lanostanoid derivatives, confirmed using mass spectrometry and NMR (Rösecke and König, 1999). The fruiting body of F. pinicola has been analyzed for nutritional components, revealing high fiber (43%) and carbohydrate (23%) contents, along with 12% amino acids. Glutamate was the most abundant amino acid, and vitamin C was the dominant vitamin at 276 mg/100 g dry mushroom. Key minerals included potassium (16.86 mg/g) and calcium (16.19 mg/100 g) (Ding et al., 2006). Methanol extracts from F. pinicola showed strong inhibition against Helicobacter pylori, with maximum activity after 8 days of fermentation and a MIC of 0.25 mg. The most active fraction, Fp-T3, identified as an aminosugar, displayed significant inhibitory activity (14.4 mm ID) against H. pylori (Lee et al., 2006). The hypoglycemic effect of F. pinicola extracts was tested in alloxan-induced diabetic rats. Rats given the hot-water extract showed a significant decrease in blood glucose levels, while those given the ethanol extract experienced a minimal reduction compared to the control group with 600 mg/dl (Shin et al., 2007). Fomitopsis pinicola extracts possess significant anti-oxidant and anti-tumor activities comparable to the conventional extracts. However, further studies are necessary to elucidate the relationship between antioxidant and antitumor activities and the pharmacological activity of the F. pinicola extract. Also, as the growth of F. pinicola in nature is very slow. As such, isolation and artificial culture may be required for further progress in the mass production of this compound (Choi et al., 2007).
The alkali extract (AE) from F. pinicola significantly lowered blood glucose levels and improved weight gain in streptozotocin (STZ)-induced diabetic rats while also restoring serum insulin levels and reducing pancreatic damage. This study is the first to demonstrate the antihyperglycemic effects of F. pinicola in this model, suggesting its components may enhance insulin secretion during recovery or protect pancreatic tissue from STZ-induced damage (Lee S. et al., 2008). Fomitopsis pinicola extracts showed potential antifungal activities against Fusarium inflexum and F. heterosporium (Guler et al., 2009). Fomitopsis pinicola showed high cellobiohydrolase (CBH) activity. The purified CBH, a 64 kDa monomer, is highly stable at high temperatures and displays efficient catalysis. It differs from other CBHs due to its exceptional catalytic efficiency and thermostability (Shin et al., 2010a). Extracellular xylanase from F. pinicola was purified using chromatography techniques. The enzyme, with a molecular weight of 58 kDa, showed optimal activity at 71°C and pH 4.6, with a half-life of 33 hours at 70°C. It had a catalytic efficiency of kcat = 77.4 s−1 and kcat/km = 22.7 mg/ml/s. The amino acid sequence revealed homology with GH family 10 hydrolases, confirming F. pinicola as a member of this family (Shin et al., 2010b). The anti-tumor activities of F. pinicola extract (FP-I) were studied in vitro and in vivo. FP-I inhibited the proliferation of mouse hepatocellular carcinoma (H22) and sarcoma (S180) cells. In an S180 mouse model, FP-I significantly reduced tumor growth and improved immune function indicators, such as lymphocyte proportion and thymus index. The extract also induced apoptosis in tumor cells. These results suggest that F. pinicola extract possesses anti-tumor effects related to immune enhancement and apoptosis induction (Xiao et al., 2011). Chloroform, petroleum, water extracts, and Compound A were studied for antitumor activity. The chloroform extract boosted immune function, increasing the spleen index and IL-2 levels. One compound (Compound A) showed strong antitumor effects, with a 52.31% inhibition rate in vivo and significant inhibition of breast (MCF-7) and liver (SMMC-7721) cancer cells in vitro, suggesting it is the main antitumor agent in F. pinicola (Sun et al., 2012).
The effect of F. pinicola chloroform extract (FPKc) on SW-480 cancer cells involved inhibiting cell viability, reducing migration, and inducing ROS-mediated apoptosis. FPKc also caused G1 phase arrest and decreased MMP-2 and MMP-9 expression. Ergosterol (ES), a major component of FPKc, demonstrated similar effects, contributing to its anti-cancer activity (Wang et al., 2014). Fomitopsis pinicola mycelium extract produces extracellular antifungal metabolites and possesses antifungal activity against potentially pathogenic filamentous fungi. Further research on the antifungal activity of F. pinicola mycelial extract may aid in the development of antimycotic biotechnological products from this mushroom (Badalyan et al., 2014). Research indicated that F. pinicola ethanol extract possesses anti-cancer properties against S-180 malignant cells, both in laboratory and animal tests. It was observed to induce apoptosis in advanced stages of lung, colorectal, breast, and hepatoma cancer cells (Wu et al., 2014). Fomitopsis pinicola extracts were evaluated for their antioxidant properties, including DPPH radical scavenging activities and their impact on important antioxidant enzymes such as SOD, CAT, and GPx. These enzymes help protect against oxidative stress and related diseases like Alzheimer’s, Parkinson’s, cancer, and aging (Onar et al., 2016). AVA, a formulation containing F. pinicola Jeseng extract, significantly combats obesity and nonalcoholic fatty liver disease (NAFLD) in high-fat diet-induced obese mice. The anti-obesity effects may result from inhibiting specific genes and cholesterol synthesis. FAVA could be a promising dietary supplement to prevent obesity and NAFLD (Jung et al., 2016). The antioxidant and heavy metal content of F. pinicola from Kazdağı and Çınarcık in Turkey were examined. Higher oxidative stress index (OSI) and iron levels were found in Çınarcık samples. These fungi may serve as antioxidant sources, but elevated heavy metals could increase oxidative stress (Sevindik et al., 2017). The chloroform extract derived from F. pinicola effectively curbed the proliferation of S180 tumor cells and contributed to the extended survival of mice. In a laboratory setting, it was evident that FPKc induced apoptosis in S180 tumor cells and brought about cell cycle arrest, most likely through the mitochondrial pathway (Gao et al., 2017).
The antitumor effects of F. pinicola chloroform extract (FPKc) was investigated on S180 tumor cells, revealing its active components and significant inhibitory effects on cell proliferation, leading to apoptosis and cell cycle arrest. In vivo, FPKc inhibited tumor growth and extended the survival of tumor-bearing mice while sparing normal cells. The findings suggest that FPKc induces tumor cell apoptosis primarily through mitochondrial pathways. Neuroprotective effects of water extract from F. pinicola were assessed in mesencephalic dopaminergic cells exposed to MPP+. The extract improved survival and neurite growth of TH-immunoreactive neurons while enhancing mitochondrial respiratory chain complex I activity and reducing apoptosis rates at doses of 50 and 25 μg/mL. These findings indicate that F. pinicola protects dopaminergic cells from MPP+-induced damage (Guo and Rausch, 2018). Comparing mycelium and fruiting body extracts of Fomitopsis, the mycelium extract demonstrated high cytotoxicity to prostate cancer cells, while both extracts displayed potential anti-inflammatory effects. These findings indicate their biotechnological potential as sources of bioactive compounds (Sułkowska-Ziaja et al., 2018). The F. pinicola extract displayed weak antioxidant properties but effectively hindered the growth of human tumor cells in a dose-dependent manner. It induced apoptosis in one cell line but showed toxicity in another. No DNA damage was observed in normal human leukocytes exposed to the extract. The extract exhibited variable antifungal effects against pathogenic fungi. Overall, the extract demonstrated potent antimicrobial and chemo-preventive activities but had limited antioxidant capabilities (Angelini et al., 2018). The anti-inflammatory, analgesic, and antipyretic effects of F. pinicola fruiting body extracts were tested in mice. Water and n-butanol extracts had the strongest effects, reducing capillary permeability, pain, and fever. Methylene chloride and petroleum ether extracts reduced ear swelling and oxidative stress. The active compounds are concentrated in the highly polar extracts, showing significant anti-inflammatory and pain-relief properties (Zhao and Bao, 2019).
Ethyl acetate and methanolic extract of F. pinicola extracts showed significant antimicrobial activity against a broad spectrum of microbes (Pala et al., 2019). Neuroprotective effects of the water extract of F. pinicola were investigated using primary dopaminergic cell cultures from embryonic mouse mesencephala subjected to MPP+ toxicity. The extract demonstrated significant protection against dopaminergic neuron degeneration, exhibiting antioxidant and anti-inflammatory activities. The mechanism underlying its neuroprotective effect is likely related to inhibiting mitochondrial oxidative stress (Guo and Rausch, 2019). Treatment with F. pinicola extract did not exhibit a statistically significant influence on PC3 prostate cancer tumor progression in mice. However, it demonstrated notable growth-inhibitory properties in vitro using the same cell line. This underscores the continued potential of F. pinicola as a valuable reservoir of bioactive compounds with anti-cancer properties (Kao et al., 2020). The growth-inhibitory potential of eight wild British Columbian mushrooms, including F. pinicola, Phaeolus schweinitzii, and Phaeolus sp., was investigated in this thesis. Of the 28 crude extracts tested, 15 demonstrated significant inhibitory activity. Hispidin, a known anti-cancer compound, was purified from Phaeolus sp. through liquid-liquid extraction and HPLC-MS, while another compound with a mass-to-charge ratio of 283.2 was detected. This research provides a foundation for further studies on these species, including F. pinicola, as sources of bioactive compounds (Da, 2020).
Ethyl acetate extract (EAE) of F. pinicola exhibited significant cytotoxicity (IC50 of 100 µg/mL), inhibited tumor growth (at 500 mg/kg), antiangiogenesis, and halted cell cycle progression at the G1 phase. The chemical analysis identified 11-α-acetoxykhivorin as the major active component. This suggests that F. pinicola EAE has potent antineoplastic effects, possibly due to its key chemical constituents (Ravikumar et al., 2021). The antibacterial activity of F. pinicola BCC58 was evaluated under different cultivation conditions. Xylose, glucose, and mandarin squeeze showed the highest inhibition of S. aureus and E. coli. Supplementing with KNO3 or yeast extract enhanced ABA. Ethanolic extracts from biomass and culture liquid had the strongest ABA, especially against E. coli with an MIC of 0.5 mg/mL, while hot water and ethyl acetate extracts showed lower activity (Metreveli et al., 2021). The antioxidant and cytoprotective activities of ethanol extracts from F. pinicola (FPE) were evaluated. UPLC-MS/MS analysis identified 14 bioactive compounds in FPE, including 8 triterpenoids, 4 triterpene glycosides, 1 lanosterol, and 1 lanostanoid. FPE demonstrated potent in vitro antioxidative effects, with a DPPH scavenging rate of 91.76% at 1.4 mg/mL and an ABTS radical scavenging rate of 100% at 0.6 mg/mL. In addition, FPE effectively protected against AAPH-induced oxidative damage and inhibited cell aging in cytoprotection assays (Li et al., 2022). The potential benefits of the chloroform extract of F. pinicola (FPKc) on ulcerative colitis (UC) were explored in a study using a DSS-induced UC mouse model. Treatment with FPKc improved symptoms such as hematochezia and weight loss, reduced disease activity and colonic damage indices, and enhanced colon tissue structure. FPKc also lowered pro-inflammatory cytokines (IL-6, IL-8) and reduced AST and ALT levels, indicating its protective effects may be linked to immune regulation and inflammation reduction (Cheng et al., 2023). Fomitopsis pinicola extract (EMFP) showed strong antioxidant and anticancer properties. EMFP effectively scavenged free radicals, protected against protein oxidation, and inhibited HepG2 cell proliferation by increasing ROS, depleting mitochondrial membrane potential, and inducing apoptosis. It also altered oxidative stress markers and contained phenolic compounds and triterpenes, contributing to its therapeutic potential (Zhang et al., 2023).
Bioactive metabolite production can be enhanced by optimizing F. pinicola cultivation conditions. The highest biomass (8.5 g/L) was achieved at 20°C, while maximum antioxidant activity (78.2%) occurred at 30°C. Xylose and peptone promoted phenol synthesis, whereas galactose and yeast extract supported biomass growth. The fungus adapted to pH 2.5–7.5, with shaking conditions maximizing phenol yield (21.44 mg GAE/g). These findings highlight strategies for improving fungal cultivation to enhance bioactive compound production (Krupodorova et al., 2024). The antimicrobial activity of 14 dikaryotic strains of F. pinicola isolated from various trees in Russia, France, and Italy was evaluated against dermatophytes, species of Penicillium, and both Gram-negative and Gram-positive bacteria. Cultural broth samples demonstrated stronger antifungal and antibacterial effects than mycelial extracts, indicating the potential of F. pinicola as a source of antimicrobial compounds for future biotech applications. Further studies are needed to elucidate the underlying mechanisms of its antimicrobial effects (Badalyan et al., 2024). Table 4 summarizes the chemical analyses, bioactivities, and potential medicinal applications of various Fomitopsis species, while Table 5 highlights the diverse bioactivities and chemical compounds species of F. pinicola.
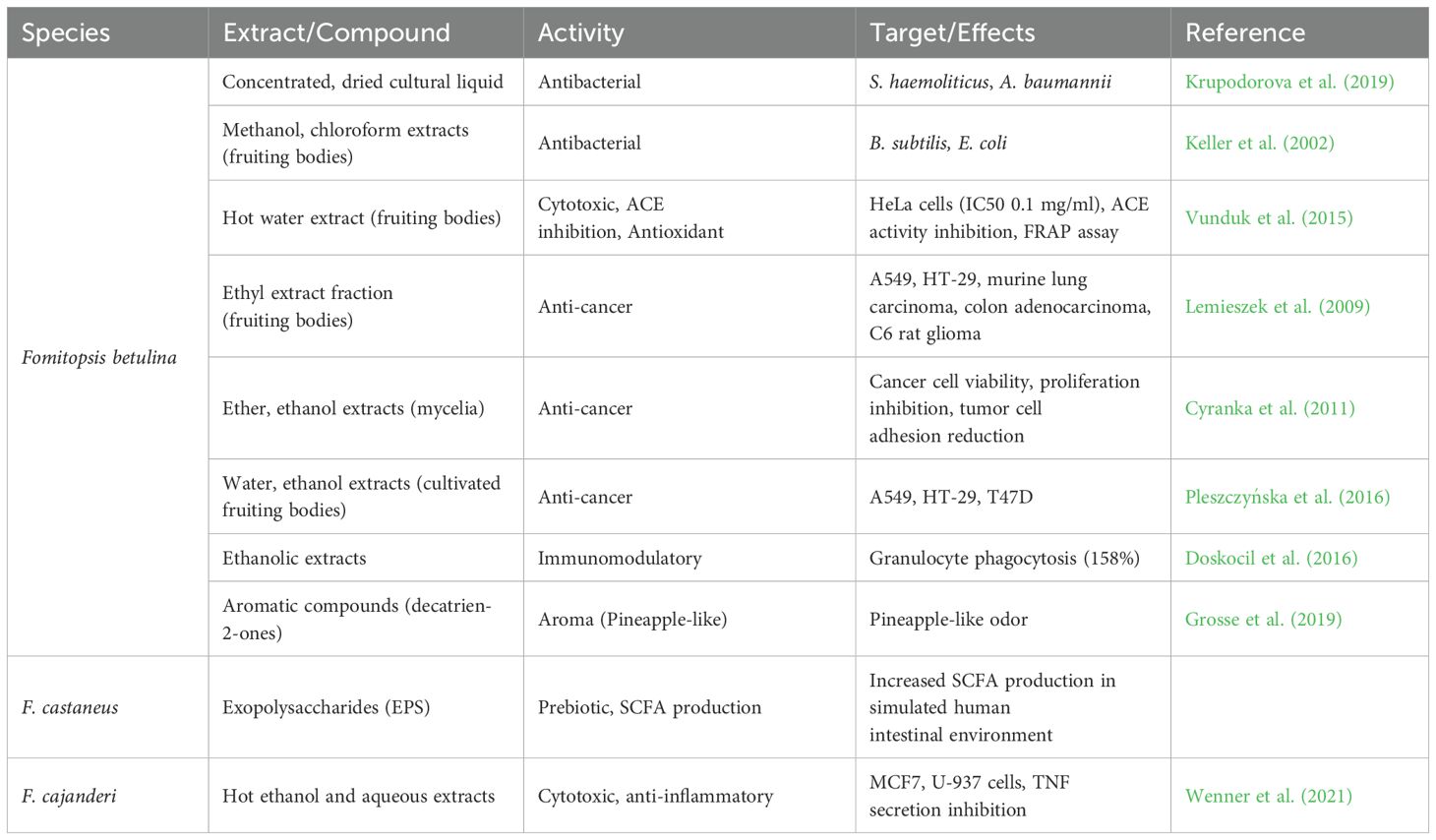
Table 4. Chemical analyses, bioactivities, and potential medicinal applications of various species of Fomitopsis.
6 Clinical trials and patents
Despite these promising bioactivities, human clinical trials on Fomitopsis remain scarce. The MACH19 study (NCT04667247) is currently evaluating the safety and feasibility of using FoTv—a combination of F. officinalis and T. versicolor—for treating mild-to-moderate COVID-19 in outpatients. This randomized, double-blind, placebo-controlled trial assesses safety, disease progression, and immune response markers. Similarly, another MACH19 study (NCT04951336) investigates the potential of FoTv mushrooms to enhance immune response and mitigate vaccine-related side effects in individuals receiving the COVID-19 vaccine. This study follows a similar design, focusing on immune parameters such as antibody titers. Both trials are ongoing, with completion expected by December 2024, but no published findings are available yet (Saxe, 2021a, 2021b).
A patent was disclosed for F. pinicola extracts, including both fruit body and mycelial extracts, which demonstrated the inhibition of renal and retinal aldose reductase activity. These extracts also reduced triglycerides, total cholesterol, and LDL cholesterol, suggesting their potential for developing functional foods aimed at preventing diabetes complications and managing diabetes-induced hyperlipidemia (Oh et al., 2007). A patent was granted for active ingredients from Polyporus officinalis (= F. officinalis), primarily eburicoic acid, dehydroeburicoic acid, and Versisponic acid D, with over 50% total triterpenoid acid. A purification method using macroporous resin and a medicinal preparation were also provided. Animal studies showed efficacy in preventing and treating tumors, particularly liver, stomach, and colon cancer (Zhou, 2012). A patent has been issued for dehydrosulfurenic acid, a compound unique to Fomes officinalis (=F. officinalis), as a potential treatment for ischemic stroke. When administered at 50 mg/kg just 10 minutes before an ischemic event, it significantly alleviated motor impairments and neuronal damage (Simi and Prisco, 2018). A patent by Sidorenko and Buzoleva (2012) was based on F. officinalis mycelia-based preparations against pseudotuberculosis, owing to their inhibitory effect on Y. pseudotuberculosis (Sidorenko and Buzoleva 2012).
Stamets has outlined several practical applications utilizing the antimicrobial properties of fungi, with a focus on the mycelial phase (Stamets, 2011). While the research includes numerous fungal species, F. officinalis is consistently emphasized for its distinct characteristics (Stamets, 2011). In one study on the antiviral properties of medicinal mushrooms, F. officinalis demonstrated activity against Cowpox and Vaccinia viruses in human foreskin fibroblast (HFF) cells (Stamets, 2011). Notably, the extract was found to be devoid of agaric acid, leaving its potential bioactivity unclear (Stamets, 2011). Furthermore, in research exploring both antiviral and antibacterial effects, the extract led to a significant reduction in colony-forming units (CFUs) of E. coli and S. aureus (Stamets, 2014). A 1–2% extract from F. officinalis demonstrated 50% inhibition of virus-induced cellular damage (EC50), with a 1:106 diluted crude extract still effective against influenza A, B, and herpes. The extract showed high selectivity and significant inhibition against M. tuberculosis (Stamets, 2018). The patent CA 2980173 covers the antiviral properties of medicinal mushrooms containing phenyl carboxylate/acrylate compounds. It includes a diverse group of medicinal mushrooms, potentially encompassing F. officinalis, which has been recognized for its antimicrobial and antiviral properties in other studies and patents by Stamets. The invention explores fungal-derived compounds for antiviral applications, emphasizing their therapeutic potential against various viral pathogens (Stamets, 2021).
7 Fomitopsis based products and their market potential
The market potential for Fomitopsis-based products, particularly F. officinalis (Agarikon), is increasing due to growing interest in natural health supplements and medicinal mushrooms (Figure 5). This species has been recognized for its potential antiviral, anti-inflammatory, and immune-boosting properties, making it a valuable ingredient in nutraceuticals, pharmaceuticals, and functional foods (Table 6). Products such as extracts, tinctures, capsules, and powders are in demand, with potential expansion into skincare and therapeutic applications (Out-grow.com). However, challenges such as slow natural growth and limited large-scale cultivation methods hinder commercial scalability. Efforts to improve cultivation techniques, such as liquid cultures and controlled growth environments, are being explored to enhance production and meet increasing demand (Out-grow.com). In addition, the commercialization of medicinal mushrooms is gaining traction in global markets, further supporting the economic potential of Fomitopsis-derived products (Businesswire.com). The growing demand for natural and sustainable health solutions has also driven research into optimizing extraction methods and bioactive compound yields from Fomitopsis. This has opened new opportunities for its use in personalized medicine and integrative healthcare approaches. Furthermore, the integration of advanced biotechnological tools, such as genetic engineering and metabolomics, is expected to accelerate the development of high-quality, standardized products.

Figure 5. Fomitopsis based products. (a) Agarikon powder capsules (www.Hostdefense.com), (b) Agarikon mushroom powder (www.goodrootsllc.com) (c) Fomitopsis officinalis drops (dropsukrainashop.com) (d) AGARIKON Ultra (https://longevitybotanicals.com) (e) Red belt tincture (https://www.herbal-goods.com/) (f) Mycomedia Agarikon capsules (www.mycomedica.eu).
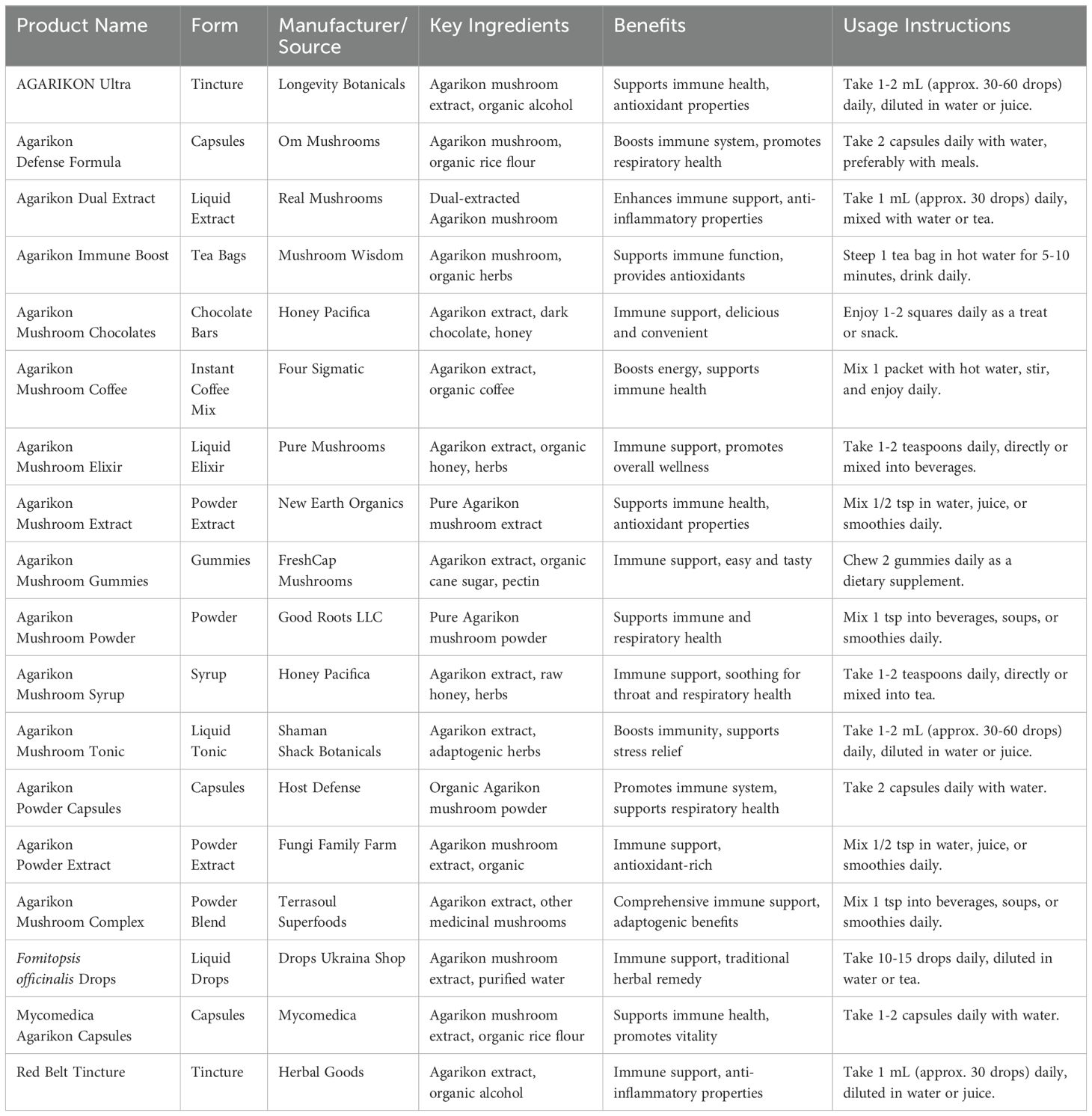
Table 6. A comparative analysis of Fomitopsis based-products based on form, key ingredients, benefits, and usage instructions.
8 Biotechnological applications
8.1 Bioremediation
Recently, various synthetic chemicals, including a wide range of solvents, pesticides, and plasticizers, have seen extensive use. Chemical pesticides, crucial for safeguarding crops, represent a prominent example, with the commonly used 1, 1, 1-trichloro-2, 2-bis(4-chlorophenyl) ethane, referred to as DDT, being a notable illustration (Hai et al., 2012). The presence of chlorine atoms in DDT contributes to its high toxicity towards complex organisms and is attributed to its partial solubility and propensity for partitioning into the lipophilic phase (Sudharshan et al., 2012). Its notable persistence and toxicity characteristics have led to its classification as a primary environmental pollutant by the United States Environmental Protection Agency (Foght et al., 2001).
Fomitopsis sp. IMER2 can treat black liquor through biological acidification, causing alkali lignin precipitation. Despite alkali lignin inhibiting fungal growth and acid production, it enhanced glucose consumption, suggesting a unique stress response. FTIR spectroscopy showed improvements in several functional groups of the resulting alkali lignin, aiding the fungus in utilizing available resources more effectively (Xiong et al., 2007). In the potato dextrose broth (PDB) medium, F. pinicola demonstrated a significant capacity for DDT degradation via different mechanisms (Purnomo et al., 2008, 2010). The addition of P. aeruginosa significantly enhanced the biodegradation of DDT by F. pinicola, achieving approximately 68% degradation after 7 days in Potato Dextrose Broth, compared to 42% by F. pinicola alone. The metabolites detected from this biodegradation included DDD, DDE, and DDMU (Sariwati and Purnomo, 2018). An intriguing discovery was found that a synergistic interaction between F. pinicola and the bacterium Ralstonia pickettii in degrading DDT, a highly toxic and recalcitrant pesticide that has been employed for eradicating malaria-carrying mosquitoes over an extended period (Purnomo et al., 2020). The co-culture of F. pinicola and B. subtilis for DDT degradation was explored, revealing that the addition of B. subtilis significantly enhanced the biodegradation of DDT by F. pinicola, resulting in the formation of DDE, DDD, and DDMU through distinct pathways (Sariwati et al., 2017). Research focusing on the bioremediation of wood contaminated with heavy metals highlighted the remarkable tolerance of F palustris to arsenic, copper, and chromium (Kartal et al., 2004; Hattori et al., 2015).
8.2 Wastewater treatment
Species of Fomitopsis have been shown to have potential environmental applications, particularly in wastewater treatment. Their enzymatic systems and bioactive compounds contribute to the degradation of pollutants and the bioremediation of contaminated water sources (Figure 6). Exopolysaccharides (EPS) isolated from F. castaneus were assessed for their fermentation characteristics in simulated human intestinal environments. The EPS increased short-chain fatty acid (SCFA) production in both adult and child fecal extracts under anaerobic conditions. Furthermore, the addition of specific lactic acid bacteria enhanced SCFA content, with differing effects observed between adult and child models. Fomitopsis palustris hosts a unique, thermostable endoglucanase, which displayed remarkable efficiency on β-1, 4-glucans and has promising industrial applications (Cha et al., 2018). Fomitopsis palustris, played a key role in the transformation, where isobutylene, a valuable compound often derived from petroleum processes, was produced using a biomass source, isobutanol, via a novel dehydration process. The maximum isobutylene production rate observed was approximately 5.9 times higher than in previous studies, highlighting the efficiency of this method (Kim et al., 2019).
Figure 6. Fomitopsis-based remediation in waste-water treatment (Malik et al., 2023).
Fomitopsis pinicola exhibited a high decolorization rate and metabolic product analysis proposed three transformation pathways—demethylation, desulfonylation, and hydroxylation (Purnomo et al., 2019). Solid-state fermentation by F. pinicola improved the bioactivity and baking performance of wheat bran. Hence, F. pinicola was an excellent potential starter culture for wheat bran fermentation to develop functional whole grain foods (Tu et al., 2020). Fomitopsis pinicola has the potential to serve as a cost-effective adsorbent for treating low-concentration Cr (VI) wastewater (Pertile and Zamarsky, 2020). A novel laccase from F. pinicola was isolated and the enzyme exhibited the highest specific activity with ABTS. It effectively degraded various recalcitrant αs at different time intervals, suggesting its industrial potential (Park and Park, 2014). Fomitopsis feei effectively decolorized triphenylmethane dyes, especially basic fuchsin (98%). Lignolytic activity didn’t correlate with dye decolorization, however, Triphenylmethane reductase was the key enzyme. This eco-friendly method shows promise for treating dye industry effluents (Nidadavolu et al., 2013). Fomitopsis rosea demonstrated a higher effectiveness in decolorizing methylene blue (Jayasinghe et al., 2008). The use of dead F. carnea has proven effective in the biosorption of three cationic dyes—Orlamar Red BG (ORBG), Orlamar Blue G (OBG), and Orlamar Red GTL (ORGTL)—with high saturation capacities of 503.1, 545.2, and 643.9 mg/g, respectively. This process represents an effective method for wastewater treatment, as it removes harmful pollutants from contaminated water (Mittal and Gupta, 1996).
8.3 Industrial waste treatment
Understanding the mechanisms underlying brown rot decay opens up exciting prospects for applying these fungi in biotechnology. The enzymatic and non-enzymatic systems breaking down lignocellulose offer promising avenues for biomass bioprocessing, targeting the production of fuels and chemicals (Ray et al., 2010; Giles and Parrow, 2011; Arantes et al., 2012). The adsorption of methylene blue by F. pinicola from Kızılcahamam Işık Mountain, Turkey, was studied. Factors like dye concentration, adsorbent dose, pH, and temperature were tested. The adsorption followed the Langmuir isotherm and fit a pseudo-second-order kinetic model. Thermodynamic parameters were calculated, and the results suggest that F. pinicola can serve as a low-cost biosorbent for wastewater treatment (Sınağ et al., 2011). Fomitopsis betulina was evaluated for its ability to bioleach heavy metals, including Cu, Cr, and As, from wood preservatives, facilitated by the accumulation of metal-complexing oxalic acid (Sierra, 2007), and has a potential for As bioremediation in contaminated soils (Button et al., 2020). In addition, research has derived into the production of biomass-degrading enzymes such as cellulases, hemicellulases, and amylases (Krupodorova et al., 2014; Valášková and Baldrian, 2006a, 2006b). Fomitopsis pinicola effectively degrades polyvinyl alcohol (PVA) in quartz sand but not in liquid culture. Gel permeation chromatography revealed a decrease in the high-molecular-weight PVA fraction, with increased coloration due to low-molecular-weight PVA. Spectral analysis indicated a Fenton reaction-based degradation, suggesting the potential of F. pinicola for PVA degradation in woody wastes (Tsujiyama and Okada, 2013). Fomitopsis pinicola, a high-efficiency BGL-producing strain, was isolated and improved using thiamine (20 mg/L) supplementation. This led to a 3.7-fold increase in BGL activity (114.4 μmol/min/mg protein). The BGL-specific activity is remarkable, making F. pinicola valuable for industrial use, particularly in bio-energy production. The purified BGL exhibited exceptional catalytic efficiency, enhancing glucose production through biological processes (Joo et al., 2009).
8.4 Other biotechnological applications
8.4.1 Fomitopsis palustris
Fomitopsis palustris degrades crystalline cellulose (Avicel) and produces cellulases capable of converting it into soluble sugars, with a cellulose conversion degree of 3.2% (Yoon and Kim, 2005). Fomitopsis palustris effectively performs sugar conversion, achieving a 40.6% yield within 72 hours when using rice straw as a substrate (Kim Y. et al., 2010). Fomitopsis palustris secretes oxalic acid during wood decay. Oxalate transport is ATP-dependent and inhibited by various substances. A cDNA, FpOAR, was isolated, and it appears to function as an oxalate transporter. FpOAR-transformants are resistant to oxalic acid, and FpOAR plays a crucial role in oxalate secretion during wood decay (Watanabe et al., 2010). Fomitopsis palustris was optimized for cellulase production from 11 wood rotting fungi. The optimized FPase activity reached 130.45 FPU/mL in an 8-day culture with 4.46 g/L of urea and 27.83 μL/L of Tween 80. The crude cellulase saccharified liquid hot water (LHW)-pretreated Populus tomentosa, released 25.15% reducing sugars after 72 hours, significantly higher than the 14.66% from untreated wood. This demonstrates the potential of F. palustris for cellulase production in woody biomass hydrolysis (Wang et al., 2012). The cost of bioethanol production from lignocellulosic materials is high due to delignification and saccharification processes. Consolidated bioprocessing (CBP) combines these with fermentation in one reactor, potentially reducing costs. The white rot fungus Schizophyllum commune was identified as a strong fermenter. When combined with Bjerkandera adusta and F. palustris, B. adusta enhanced ethanol production from cedar wood, while F. palustris released glucose but did not increase ethanol yield. The results suggest that using multiple fungi in CBP can effectively produce bioethanol from cellulosic materials (Horisawa et al., 2019). Two starch-degrading enzymes, FpAmy13A (α-amylase) and FpGLA15A (glucoamylase), were purified and characterized from F. palustris, and their corresponding genes were cloned. Variations in enzyme characteristics suggest distinct roles in wood starch degradation (Tanaka et al., 2020). Fomitopsis palustris CQ2018 effectively mobilized soil phosphorus, enhancing phosphorus availability in various soil types and promoting root growth in pepper (Capsicum annuum) plants. The fungal inoculation increased phosphorus uptake and fruit yield, improving fruit quality by raising levels of potassium and vitamin C while reducing nitrate content. This fungus shows potential as an environmentally friendly biofertilizer, with further research needed on its applications in different plants and soils (Peng and Huang, 2022).
8.4.2 Fomitopsis pinicola
Incorporating F. pinicola mycelium (CM) in bread dough fermentation improved its bioactive properties and bread quality. Concentrations of 30–40% CM maintained quality, reduced baking loss, and enhanced sensory characteristics, while 50% CM reduced loaf volume and crust brightness. In addition, CM delayed retrogradation and reduced mold growth during storage, with 30–40% CM identified as optimal for maintaining quality and offering potential anti-diabetic benefits (Seung-Hee, 2005). Cellulase from F. pinicola KMJ812, known for its high β-glucosidase activity, was immobilized on various resins, with Duolite A568 yielding the best results: 61.7% cellulase activity and 64.4% β-glucosidase activity. The optimal reaction conditions were 55°C and pH 4.0-4.5, slightly more stable than the unimmobilized enzyme. Notably, the immobilization improved the enzyme’s thermal stability, making it more suitable for industrial applications. The immobilized enzyme retained 98% of its activity after 72 hours at 50°C and 50% after eight uses at the same temperature (Shin et al., 2010b). Fomitopsis pinicola contains 30.11% chitin and yields 71.75% chitosan. The chitin is 72.5% acetylated, with a DTGmax of 341°C. Chitosan has a 73.1% deacetylation rate and a DTGmax of 265°C. Chitin exhibits a CrI of 52%, while chitosan has a CrI of 41%. Both show nano-fiber surface structures. Fomitopsis pinicola could serve as an alternative chitin and chitosan source due to its abundance (Kaya et al., 2015). Endoglucanase, with an apparent molecular weight of 32 kDa, was purified from F. pinicola). The bio-directed synthesis of titanium oxide and silver nanoparticles using F. pinicola was confirmed through various analytical techniques. These nanoparticles exhibited significant antibacterial and anticancer activities, with AgNPs demonstrating enhanced effects, highlighting their potential for biomedical applications (Rehman et al., 2020, Figure 7). The methanolic extract of F. pinicola was evaluated as a green corrosion inhibitor for 1018 carbon steel in 0.5 M sulfuric acid. The extract demonstrated 85% inhibition efficiency at 400 ppm, with thermodynamic analysis showing physical adsorption following the Langmuir isotherm. Solid-state fermentation of wheat bran by F. pinicola significantly improved its nutritional and sensory properties, increasing phenol, alkylresorcinol, and antioxidant content. The fermentation also enhanced the flavor, reduced phytic acid, and improved the texture of dough and bread, making it a promising method for enhancing wheat bran as a nutritious food ingredient (Tu et al., 2020). The extract formed a protective film on the steel surface, increasing polarization resistance at higher extract concentrations. GC-MS identified Dehydrergosterol and Phthalic acid as the main inhibitory compounds, supported by theoretical EHOMO and ELUMO values (Martinez-Gonzalez et al., 2024).
Figure 7. Benefits of Fomitopsis-based nanoparticles (Rehman et al., 2020).
Brown rot fungi (G. sepiarium, F. pinicola, and L. sulphureus) filtrate saccharified biomass. Fomitopsis pinicola had high cellobiohydrolase activity, and combining L. sulphureus and F. pinicola enzymes increased sugar yield (Lee J. et al., 2008). Exoglucanase production from Fomitopsis sp. RCK2010 was optimized, resulting in a significant increase. The crude cellulase was then applied to saccharify pretreated Prosopis juliflora, releasing reducing sugars for bioethanol production (Deswal et al., 2012). Table 7 outlines the biotechnological applications of various Fomitopsis species, focusing on their industrial, pharmaceutical, and environmental uses.
9 Conclusions
Species of Fomitopsis, with the diverse bioactive compounds they produce, hold significant promise in traditional and modern medicinal applications. This comprehensive review has highlighted the potent antioxidant, immunomodulatory, and anti-inflammatory properties of key compounds such as polysaccharides, triterpenoids, and phenolic substances found within Fomitopsis. These properties underscore the therapeutic potential of Fomitopsis in cancer treatment and immune system support and emphasize its role as a valuable source of natural antioxidant supplements. However, there is no documented evidence of significant toxicity in Fomitopsis species based on available literature. Comprehensive studies on their long-term safety and potential adverse effects are limited. Hence, more research is needed to fully understand their toxicity profile and ensure safe therapeutic use.
Moreover, the exploration of biotechnological approaches, including advanced cultivation, extraction, and purification techniques, is a fascinating area of study that reveals the growing interest in optimizing the production and utilization of Fomitopsis bioactive compounds. The challenges and prospects in this field underscore the importance of continued research and innovation, inviting the scientific community to contribute to unlocking the full potential of Fomitopsis in medicine and biotechnology. In conclusion, Fomitopsis stands out as a genus with substantial health benefits and biotechnological value. Future research should focus on further elucidating the molecular mechanisms of its bioactive compounds, enhancing biotechnological methods, and expanding its applications in various therapeutic areas, paving the way for new, effective treatments and health-promoting products.
Author contributions
SK: Writing – original draft, Writing – review & editing, Conceptualization, Investigation, Methodology, Project administration. NP: Methodology, Software, Writing – review & editing, Writing – original draft. JK: Writing – review & editing, Writing – original draft. KH: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. NS: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Samantha C. Karunarathna thanks the High-Level Talent Recruitment Plan of Yunnan Province (“High-End Foreign Experts” Program), the National Natural Science Foundation of China (Grant No. 32260004) and Key Laboratory of Yunnan Provincial Department of Education of the Deep-Time Evolution on Biodiversity from the Origin of the Pearl River, Qujing Normal University, Qujing, Yunnan 655011, China, for their support. Nakarin Suwannarach and Jaturong Kumla thank for the partial support from Chiang Mai University, Thailand.
Acknowledgments
Prof. Steven L. Stephenson, Department of Biological Sciences, University of Arkansas, Fayetteville, Arkansas 72701, USA, is thanked for English editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adarsh, C. K., Kumar, V., Vidyasagaran, K., Ganesh, P. N. (2015). Decomposition of wood by polypore fungi in tropics—biological, ecological and environmental factors—a case study. Res. J. Agric. For. Sci. 3, 15–37.
Airapetova, A. Y., Gavrilin, M. V., Dmitriev, A. B., Mezenova, T. D. (2010). Examination of the structure of agaricinic acid using 1H and 13C NMR spectroscopy. Pharm. Chem. J. 44, 510–513. doi: 10.1007/s11094-010-0475-9
Alresly, Z. (2019). Chemical and pharmacological investigations of Fomitopsis betulina (formerly: Piptoporus betulinus) and Calvatia gigantea (Greifswald, Germany: Faculty of Mathematics and Natural Sciences). doi: 10.13140/RG.2.2.27835.54561
Alresly, Z., Lindequist, U., Lalk, M., Porzel, A., Arnold, N., Wessjohann, L. A. (2016). Bioactive triterpenes from the fungus. Piptoporus betulinus. Rec. Nat. Prod. 10, 103–108. doi: 10.1080/19390211.2015.1089287
Altannavch, N., Zhou, X., Khan, M. A., Ahmed, A., Naranmandakh, S., Fu, J. J., et al. (2022). Antioxidant and anticancerous effect of Fomitopsis officinalis (Vill. ex Fr. Bond. et Sing.) mushroom on hepatocellular carcinoma cells in vitro through the NF-κB pathway. Anti-Cancer Agents Med. Chem. 22, 1561–1570. doi: 10.2174/1871520622666220418112045
Amengual, N. G., Csarman, F., Wohlschlager, L., Ludwig, R. (2022). Expression and characterization of a family 45 glycosyl hydrolase from Fomitopsis pinicola and comparison to Phanerochaete chrysosporium Cel45A. Enzyme Microb. Technol. 156, 110000. doi: 10.1016/j.enzmictec.2022.110000
Anđelković, B., Vujisić, L., Novaković, M., Knežević, A., Stanković, M., Milosavljević, S., et al. (2021). DNA protective activity of triterpenoids isolated from medicinal mushroom Fomitopsis betulina. J. Serb. Chem. Soc 86, 809–817. doi: 10.2298/JSC210121047A
Angelini, P., Tirillini, B., Bistocchi, G., Arcangeli, A., Rubini, A., Pellegrino, R. M., et al. (2018). Overview of the biological activities of a methanol extract from wild red belt conk, Fomitopsis pinicola (Agaricomycetes), fruiting bodies from Central Italy. Int. J. Med. Mushrooms 20, 1047–1063. doi: 10.1615/IntJMedMushrooms.2018027417
Arantes, V., Jellison, J., Goodell, B. (2012). Peculiarities of brown-rot fungi and biochemical Fenton reaction with regard to their potential as a model for bioprocessing biomass. Appl. Microbiol. Biotechnol. 94, 323–338. doi: 10.1007/s00253-012-3954-y
Badalyan, S. M., Gharibyan, N. G., Shnyreva, A. V., Shahbazyan, T. A. (2014). Antifungal activity of Fomitopsis pinicola collections against potentially pathogenic for humans and animals filamentous fungi. ICMBMP8 128, 93–94.
Badalyan, S. M., Morel, S., Barkhudaryan, A., Rapior, S. (2023). “Mushrooms as promising therapeutic resources: Review and future perspectives,” in Mushrooms with Therapeutic Potentials: Recent Advances in Research and Development (Singapore: Springer Nature Singapore Pte Ltd), vol. 25. , 1–54. doi: 10.1007/978-981-19-9550-7_1
Badalyan, S. M., Shnyreva, A. V., Barkhudaryan, A. (2024). Antimicrobial activity of different collections of medicinal polypore fungus Fomitopsis pinicola (Agaricomycetes). Int. J. Med. Mushrooms 26, 33–48. doi: 10.1615/IntJMedMushrooms.2024052920
Bao, H. Y., Sun, Q., Huang, W., Sun, X., Bau, T., Li, Y. (2015). Immunological regulation of fermentation mycelia of Fomitopsis pinicola on mice. Mycosystema 34, 287–292. doi: 10.13346/j.mycosystema.130248
Bhattarai, G., Lee, Y. H., Lee, N. H., Lee, I. K., Yun, B. S., Hwang, P. H., et al. (2012). Fomitoside-K from Fomitopsis nigra induces apoptosis of human oral squamous cell carcinomas (YD-10B) via mitochondrial signaling pathway. Biol. Pharm. Bull. 35, 1711–1719. doi: 10.1248/bpb.b12-00425
Bindu, S. H., Charya, M. S. (2018). Influence of plant oils and metals on exopolysaccharide production by Fomitopsis feei. PSM Microbiol. 3, 93–97.
Bishop, K. S. (2020). Characterization of extracts and anti-cancer activities of Fomitopsis pinicola. Nutrients 12, 609. doi: 10.3390/nu12030609
Blanchette, R. A., Compton, B. D., Turner, N. J., Gilbertson, R. L. (1992). Nineteenth-century shaman grave guardians are carved Fomitopsis officinalis sporophores. Mycologia 84, 119–124. doi: 10.2307/3760396
Blanchette, R. A., Haynes, D. T., Held, B. W., Niemann, J., Wales, N. (2021). Fungal mycelial mats used as textile by indigenous people of North America. Mycologia 113, 261–267. doi: 10.1080/00275514.2020.1858686
Businesswire.com Global medicinal mushroom market trends and opportunities. Available online at: https://www.businesswire.com (Accessed March 20, 2025).
Button, M., Koch, I., Watts, M. J., Reimer, K. J. (2020). Arsenic speciation in the bracket fungus Fomitopsis betulina from contaminated and pristine sites. Environ. Geochem. Health 42, 2723–2732. doi: 10.1007/s10653-019-00435-y
Cha, W. S., Ding, J. L., Shin, H. J., Kim, J. S., Kim, Y. S., Choi, D., et al. (2009). Effect of Fomitopsis pinicola extract on blood glucose and lipid metabolism in diabetic rats. Korean J. Chem. Eng. 26, 1696–1699. doi: 10.1007/s11814-009-0284-6
Cha, J. H., Yoon, J. J., Cha, C. J. (2018). Functional characterization of a thermostable endoglucanase belonging to glycoside hydrolase family 45 from Fomitopsis palustris. Appl. Microbiol. Biotechnol. 102, 6515–6523. doi: 10.1007/s00253-018-9128-9
Cheng, X., Ji, Y., Li, X., Wang, Z., Wang, B., He, F., et al. (2023). The beneficial effects of Fomitopsis pinicola chloroform extract on a dextran sulfate sodium-induced ulcerative colitis mice model. Ann. Transl. Med. 11, 35. doi: 10.21037/atm-22-5143
Cheng, J. J., Lin, C. Y., Lur, H. S., Chen, H. P., Lu, M. K. (2008). Properties and biological functions of polysaccharides and ethanolic extracts isolated from medicinal fungus Fomitopsis pinicola. Process Biochem. 43, 829–834. doi: 10.1016/j.procbio.2008.04.005
Chi, M., Jia, L., Bao, H. (2014). Isolation, identification and cancer cell anti-proliferative activity of Fomitopsis officinalis fruit body constituents. Acta Edulis Fungi 21 (2), 72–77.
Chiba, T., Sakurada, T., Watanabe, R., Yamaguchi, K., Kimura, Y., Kioka, N., et al. (2014). Fomiroid A, a novel compound from the mushroom Fomitopsis nigra, inhibits NPC1L1-mediated cholesterol uptake via a mode of action distinct from that of ezetimibe. PloS One 9, , e116162. doi: 10.1371/journal.pone.0116162
Choi, D., Park, S. S., Ding, J. L., Cha, W. S. (2007). Effects of Fomitopsis pinicola extracts on antioxidant and antitumor activities. Biotechnol. Bioprocess Eng. 12, 516–524. doi: 10.1007/BF02931350
Civzele, A., Stipniece-Jekimova, A. A., Mezule, L. (2023). Fungal ligninolytic enzymes and their application in biomass lignin pretreatment. J. Fungi 9, 780. doi: 10.3390/jof9070780
Csarman, F., Obermann, T., Zanjko, M. C., Man, P., Halada, P., Seiboth, B., et al. (2021). Functional expression and characterization of two laccases from the brown rot Fomitopsis pinicola. Enzyme Microb. Technol. 148, 109801. doi: 10.1016/j.enzmictec.2021.109801
Cyranka, M., Graz, M., Kaczor, J., Kandefer-Szerszen, M., Walczak, K., Kapka-Skrzypczak, L., et al. (2011). Investigation of the antiproliferative effect of ether and ethanol extracts of birch polypore medicinal mushroom, Piptoporus betulinus (Bull.: Fr.) P. Karst. (Higher Basidiomycetes) in in vitro grown mycelium. Int. J. Med. Mushrooms 13, 525–533. doi: 10.1615/IntJMedMushr.v13.i6.30
Czerwonka, A., Wiater, A., Komaniecka, I., Adamczyk, P., Rzeski, W., Pleszczyńska, M.. (2019). Antitumor effect of glucooligosaccharides obtained via hydrolysis of α-(1→3)-glucan from Fomitopsis betulina. Mol. Biol. Rep. 46, 5977–5982. doi: 10.1007/s11033-019-05032-x
Da, J. (2020). Purification and characterization of growth-inhibitory compounds from two genera of British Columbia wild mushrooms: Fomitopsis and Phaeolus. Canada: University of Northern British Columbia.
Dahlsjö, C. A. L. (2023). Strategies to manage tree pest and disease outbreaks: a balancing act. BMC Ecol. Evo. 23, 70. doi: 10.1186/s12862-023-02184-0
Dai, Y. C. (2012a). Polypore diversity in China with an annotated checklist of Chinese polypores. Mycoscience 53, 49–80. doi: 10.1007/s10267-011-0134-3
Dai, Y. C. (2012b). Pathogenic wood-decaying fungi on woody plants in China. Mycosystema 31, 493–509.
Deswal, D., Gupta, R., Kuhad, R. C. (2012). Enhanced exoglucanase production by brown rot fungus Fomitopsis sp. RCK2010 and its application for cellulose saccharification. Appl. Biochem. Biotechnol. 168, 2004–2016. doi: 10.1007/s12010-012-9901-7
Ding, J. L., Shin, H. J., Cha, W. S. (2006). Analysis of amino acids, vitamins, and minerals in the fruiting body of Fomitopsis pinicola. J. Life Sci. 16, 1123–1126. doi: 10.5352/JLS.2006.16.7.1123
Doskocil, I., Havlik, J., Verlotta, R., Tauchen, J., Vesela, L., Macakova, K., et al. (2016). In vitro immunomodulatory activity, cytotoxicity, and chemistry of some Central European polypores. Pharm. Biol. 54, 2369–2376. doi: 10.1080/13880209.2016.1197282
Fäldt, J., Jonsell, M., Nordlander, G., Borg-Karlson, A. K. (1999). Volatiles of bracket fungi Fomitopsis pinicola and Fomes fomentarius and their functions as insect attractants. J. Chem. Ecol. 25, 567–590. doi: 10.1023/A:1020989318168
Feng, W., Yang, J. S. (2015). A new drimane sesquiterpenoid and a new triterpene lactone from Fomes officinalis. J. Asian Nat. Prod. Res. 17, 1065–1072. doi: 10.1080/10286020.2015.1119126
Fijałkowska, A., Krakowska, A., Lazur, J., Włodarczyk, A., Zięba, P., Suchanek, M., et al. (2021). Fortified mycelium of Fomitopsis officinalis as a source of biologically active substances effective in the prevention of civilization diseases. Int. J. Med. Mushrooms 23, 29–44. doi: 10.1615/IntJMedMushrooms.2021040295
Fijałkowska, A., Muszyńska, B., Sułkowska-Ziaja, K., Kała, K., Pawlik, A., Stefaniuk, D., et al. (2020). Medicinal potential of mycelium and fruiting bodies of Fomitopsis officinalis in therapy of lifestyle diseases. Sci. Rep. 10, 20081. doi: 10.1038/s41598-020-76899-1
Flores, G. A., Cusumano, G., Ianni, F., Blasi, F., Angelini, P., Cossignani, L., et al. (2023). Fomitopsis officinalis: Spatial (Pileus and Hymenophore) metabolomic variations affect functional components and biological activities. Antibiotics 12, 766. doi: 10.3390/antibiotics12040766
Flores, G. A., Cusumano, G., Zengin, G., Venanzoni, R., Angelini, P. (2025). Fungal biomolecules for food and pharmaceutical application. eFood 6, e70033. doi: 10.1002/efd2.70033
Foght, J., April, T., Biggar, K., Aislabie, J. (2001). Bioremediation of DDT-contaminated soils: a review. Bioremediation J. 5, 225–246. doi: 10.1080/20018891079308
Gafforov, Y., Deshmukh, S. K., Verekar, S. A., Tomšovský, M., Yarasheva, M., Chen, J. J., et al. (2023). “Fomitopsis betulina (Bull.) B. K. Cui, M. L. Han & Y. C. Dai; Fomitopsis pinicola (Sw.) P. Karst. – FOMITOPSIDACEAE,” in Ethnobiology of Uzbekistan: Ethnomedicinal Knowledge of Mountain Communities (Springer International Publishing, Cham), 1085–1101. doi: 10.1007/978-3-031-23031-8_108
Gao, Y., Wang, P., Wang, Y., Wu, L., Wang, X., Zhang, K., et al. (2017). In vitro and in vivo activity of Fomitopsis pinicola chloroform extract against S180 tumor cells. Cell Physiol. Biochem. 44, 2042–2056. doi: 10.1159/000485944
Gáper, J., Gáperová, S., Pristaš, P., Šebesta, M., Kollárová, P., Gallay, I., et al. (2025). The geographical distribution, trophic modes, and host preferences of Fomitopsis pinicola in Central Europe: a comprehensive review. Cent. Eur. For. J. 71, 73–82. doi: 10.2478/forj-2024-0026
Gilbertson, R. L., Ryvarden, L. (1986). North American Polypores 2: Megasporoporia – Wrightoporia (Oslo: Fungiflora).
Giles, R., Parrow, M. (2011). Lignocellulosic treatments and applications thereof (USA: United States Patent Application Publication). US 20110008384 A1.
Girometta, C. (2019). Antimicrobial properties of Fomitopsis officinalis in the light of its bioactive metabolites: a review. Mycology 10, 32–39. doi: 10.1080/21501203.2018.1536680
Glaeser, J. A., Smith, K. T. (2016). “Wood decay fungi of subalpine conifer forests,” in Proceedings, 8th Western Hazard Tree Workshop (Western International Forest Disease Working Committee, Bend, OR), 21–47. October 17–20.
Golovchenko, V. V., Khramova, D. S., Shinen, N., Jamsranjav, G., Chizhov, A. O., Shashkov, A. S. (2018). Structure characterization of the mannofucogalactan isolated from fruit bodies of quinine conk Fomitopsis officinalis. Carbohydr. Polym. 199, 161–169. doi: 10.1016/j.carbpol.2018.07.018
Golovchenko, V. V., Naranmandakh, S., Ganbaatar, J., Prilepskii, A. Y., Burygin, G. L., Chizhov, A. O., et al. (2020). Structural investigation and comparative cytotoxic activity of water-soluble polysaccharides from fruit bodies of the medicinal fungus quinine conk. Phytochemistry 175, 112313. doi: 10.1016/j.phytochem.2020.112313
Goppa, L., Spano, M., Baiguera, R. M., Cartabia, M., Rossi, P., Mannina, L., et al. (2023). NMR-based characterization of wood decay fungi as promising novel foods: Abortiporus biennis, Fomitopsis iberica, and Stereum hirsutum mycelia as case studies. Foods 12, 2507. doi: 10.3390/foods12132507
Gramss, G. (2020). Aspects determining the dominance of Fomitopsis pinicola in the colonization of deadwood and the role of the pathogenicity factor oxalate. Forests 11, 290. doi: 10.3390/f11030290
Grienke, U., Zöll, M., Peintner, U., Rollinger, J. M. (2014). European medicinal polypores–A modern view on traditional uses. J. Ethnopharmacology 154, 564–583. doi: 10.1016/j.jep.2014.04.035
Griffin, G. (2024). Forest pathogens: Identification and treatment. Feesity. Available online at: https://forestry.com/forestry-management/forest-health/forest-pathogens/ (Accessed March 20, 2025).
Grosse, M., Pendzialeck, T., Fohrer, J., Berger, R. G., Krings, U. (2019). (5 E/Z, 7 E, 9)-Decatrien-2-ones, pineapple-like flavors from Fomitopsis betulina—Structure elucidation and sensorial properties. J. Agric. Food Chem. 68, 10329–10335. doi: 10.1021/acs.jafc.9b04287
Grosse, M., Wu, S., Krings, U., Berger, R. G. (2020). Formation of decatrienones with a pineapple-like aroma from 1-13c-acetate by cell cultures of the birch polypore, Fomitopsis betulina. J. Agric. Food Chem. 68, 1678–1683. doi: 10.1021/acs.jafc.9b07794
Grün, C. H. (2003). Structure and biosynthesis of fungal α-glucans. Netherlands: University of Utrecht.
Guler, P., Akata, I., Kutluer, F. (2009). Antifungal activities of Fomitopsis pinicola (Sw.: Fr) Karst and Lactarius vellereus (Pers.) Fr. Afr. J. Biotechnol. 8, 3811–3813. Available online at: http://www.academicjournals.org/AJB.
Guo, W., Chi, Y. (2017). Purification and fermentation characteristics of exopolysaccharide from Fomitopsis castaneus Imaz. Int. J. Biol. Macromolecules 105, 213–218. doi: 10.1016/j.ijbiomac.2017.07.028
Guo, S., Rausch, W. D. (2018). Study on neuroprotective effects of water extract of Fomitopsis pinicola on dopaminergic neurons in vitro. Chin. J. Pharmacovigilance 15, 582–586. Available online at: https://www.zgywjj.com/EN/Y2018/V15/I10/582.
Guo, S., Rausch, W. D. (2019). The study on the neuroprotective mechanism of water extract of Fomitopsis pinicola on mesencephalic dopaminergic neurons induced by MPP+. TMR Mod. Herb. Med. 2, 74–82.
Hai, F. I., Modin, O., Yamamoto, K., Fukushi, K., Nakajima, F., Nghiem, L. D. (2012). Pesticide removal by a mixed culture of bacteria and white-rot fungi. J. Taiwan Institute Chem. Engineers 43, 459–462. doi: 10.1016/j.jtice.2011.11.008
Haight, J. E., Nakasone, K. K., Laursen, G. A., Redhead, S. A., Taylor, D. L., Glaeser, J. A. (2019). Fomitopsis mounceae and F. schrenkii—two new species from North America in the F. pinicola complex. Mycologia 111, 339–357. doi: 10.1080/00275514.2018.1564449
Hamburger, M. O., Cordell, G. A. (1987). A direct bioautographic TLC assay for compounds possessing antibacterial activity. J. Nat. Prod. 50, 19–22. doi: 10.1021/np50049a003
Han, M. L., Cui, B. K. (2015). Morphological characters and molecular data reveal a new species of Fomitopsis (Polyporales) from southern China. Mycoscience 56, 168–176. doi: 10.1016/j.myc.2014.06.002
Han, M. L., Vlasák, J., Cui, B. K. (2015). Daedalea americana sp. nov. (Polyporales, Basidiomycota) evidenced by morphological characters and phylogenetic analysis. Phytotaxa 204, 277–286. doi: 10.11646/phytotaxa.204.4.3
Han, M.-L., Chen, Y.-Y., Sun, L.-L., Song, J., Vlasák, J., Dai, Y.-C., et al. (2016). Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. 80, 343–373. doi: 10.1007/s13225-016-0364-y
Hao, L., Sheng, Z., Lu, J., Tao, R., Jia, S. (2016). Characterization and antioxidant activities of extracellular and intracellular polysaccharides from Fomitopsis pinicola. Carbohydr. Polym 141, 54–59. doi: 10.1016/j.carbpol.2015.11.048
Haq, I. U., Hillmann, B., Moran, M., Willard, S., Knights, D., Fixen, K. R., et al. (2022). Bacterial communities associated with wood rot fungi that use distinct decomposition mechanisms. ISME Commun. 2, 26. doi: 10.1038/s43705-022-00108-5
Harms, M., Lindequist, U., Al-Resly, Z., Wende, K. (2013). Influence of the mushroom Piptoporus betulinus on human keratinocytes. Planta Med. 79, PC4. doi: 10.1055/s-0033-1351998
Hashem, A. H., Attia, M. S., Kandil, E. K., Fawzi, M. M., Abdelrahman, A. S., Khader, M. S., et al. (2023). Bioactive compounds and biomedical applications of endophytic fungi: a recent review. Microb. Cell Fact 22, 107. doi: 10.1186/s12934-023-02118-x
Hattori, T., Hisamori, H., Suzuki, S., Umezawa, T., Yoshimura, T., Sakai, H. (2015). Rapid copper transfer and precipitation by wood-rotting fungi can affect copper removal from copper sulfate-treated wood blocks during solid-state fungal treatment. Int. Biodeterior. Biodegradation 97, 195–201. doi: 10.1016/j.ibiod.2014.10.018
Hennon, P. E., McClellan, M. H., Palkovic, P. (2002). Comparing deterioration and ecosystem function of decay-resistant and decay-susceptible species of dead trees (Albany, CA: USDA Forest Service, Pacific Southwest Research Station). Gen. Tech. Rep. PSW-GTR-25.
Hisamori, H., Watanabe, T., Suzuki, S., Okawa, K., Sakai, H., Yoshimura, T., et al. (2013). Cloning and expression analysis of a cDNA encoding an oxaloacetate acetylhydrolase from the brown-rot fungus Fomitopsis palustris. Sustain. Humanosphere: Bull. Res. Institute Sustain. Humanosphere Kyoto Univ. 9, 57–64.
Hobbs, C. (1995). Medicinal mushrooms: An exploration of tradition, healing & culture. 2nd ed (Santa Cruz: Botanica Press), ISBN: 9781884360015.
Hobbs, C. (2023). The health and clinical benefits of medicinal fungi. Adv. Biochem. Eng. Biotechnol. (Springer, Cham) 184. doi: 10.1007/10_2023_230
Horisawa, S., Inoue, A., Yamanaka, Y. (2019). Direct ethanol production from lignocellulosic materials by mixed culture of wood rot fungi Schizophyllum commune, Bjerkandera adusta, and Fomitopsis palustris. Fermentation 5, 21. doi: 10.3390/fermentation5010021
Hsiao, G., Shen, M. Y., Lin, K. H., Lan, M. H., Wu, L. Y., Chou, D. S., et al. (2003). Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J. Agric. Food Chem. 51, 3302–3308. doi: 10.1021/jf0343395
Hu, J. (2022). A selective medium for the recovery and enumeration of Fomitopsis meliae, causing lemon canker and brown wood rot. Plant Health Prog. 23, 212–220. doi: 10.1094/PHP-02-22-0016-RS
Hwang, C. H., Jaki, B. U., Klein, L. L., Lankin, D. C., McAlpine, J., Napolitano, J. G., et al. (2012). Biological and chemical evaluation of anti-TB coumarins from the polypore mushroom, Fomitopsis officinalis. Planta Med. 78, PI470. doi: 10.1055/s-0032-1321157
Hwang, C. H., Jaki, B. U., Klein, L. L., Lankin, D. C., McAlpine, J. B., Napolitano, J. G., et al. (2013). Chlorinated coumarins from the polypore mushroom Fomitopsis officinalis and their activity against Mycobacterium tuberculosis. J. Nat. Prod 76, 1916–1922. doi: 10.1021/np4004335
Isaka, M., Chinthanom, P., Srichomthong, K., Thummarukcharoen, T. (2017). Lanostane triterpenoids from fruiting bodies of the bracket fungus Fomitopsis feei. Tetrahedron Lett. 58, 1758–1761. doi: 10.1016/j.tetlet.2017.03.070
Isaka, M., Chinthanom, P., Suvannakad, R., Thummarukcharoen, T., Feng, T., Liu, J. K. (2019). Fomitopsins I and J, 24-methyl-lanostane triterpenoids from fruiting bodies of the wood-rot basidiomycete Fomitopsis sp. Phytochem. Lett 29, 178–181. doi: 10.1016/j.phytol.2019.04.012
Jayasinghe, C., Imtiaj, A., Lee, G. W., Im, K. H., Hur, H., Lee, M. W., et al. (2008). Degradation of three aromatic dyes by white rot fungi and the production of ligninolytic enzymes. Mycobiology 36, 114–120. doi: 10.4489/MYCO.2008.36.2.114
Jeong-Jun, Y. (2005). Degradation of crystalline cellulose by the brown-rot basidiomycete Fomitopsis palustris. J. Microbiol. 43, 487–492. doi: 10.1007/BF03030582
Jini, G., Singh, R. K. (2024). Optimization of physical parameters for cellulase production by Fomitopsis meliae under solid-state fermentation. J. Bioresour. 11, 71–76. doi: 10.5281/zenodo.11318516
Joo, A. R., Lee, K. M., Sim, W. I., Jeya, M., Hong, M. R., Kim, Y. S., et al. (2009). Thiamine increases β-glucosidase production in the newly isolated strain of Fomitopsis pinicola. Lett. Appl. Microbiol. 49, 196–2203. doi: 10.1111/j.1472-765X.2009.02638.x
Jung, H. Y., Ji, Y., Kim, N. R., Kim, D. Y., Kim, K. T., Choi, B. H. (2016). A Fomitopsis pinicola jeseng formulation has an antiobesity effect and protects against hepatic steatosis in mice with high-fat diet-induced obesity. Evidence-Based Complementary Altern. Med. 2016, 7312472. doi: 10.1155/2016/7312472
Kao, C. H., Greenwood, D. R., Jamieson, S. M., Coe, M. E., Murray, P. M., Ferguson, L. R., et al. (2020). Anticancer characteristics of Fomitopsis pinicola extract in a xenograft mouse model—A preliminary study. Nutr. Cancer 72, 645–2652. doi: 10.1080/01635581.2019.1651348
Karsten, P. A. (1881). Enumeratio Boletinearum et Polyporearum Fennicarum, systemate novo dispositarum. Rev. Mycol Toulouse 3, 16–19.
Kartal, S. N., Munir, E., Kakitani, T., Imamura, Y. (2004). Bioremediation of CCA treated wood by brown-rot fungi Fomitopsis palustris, Coniophora puteana, and Laetiporus sulphureus. J. Wood Sci. 50, 182–188. doi: 10.1007/s10086-003-0551-9
Kauserud, H., Justad, T. A., Vindenes, Y., Methlie, I. S., Sønstebø, J. H., Skrede, I., et al. (2024). Limited evidence of local adaptation of growth and decomposition rates in the widespread wood-decay fungus Fomitopsis pinicola. Fungal Ecol. 70, 101353. doi: 10.1016/j.funeco.2024.101353
Kawagishi, H., Hamajima, K., Inoue, Y. (2002). Novel hydroquinone as a matrix metallo-proteinase inhibitor from the mushroom Piptoporus betulinus. Biosci. Biotechnol. Biochem. 66, 2748–2750. doi: 10.1271/bbb.66.2748
Kaya, M., Akata, I., Baran, T., Menteş, A. (2015). Physicochemical properties of chitin and chitosan produced from medicinal fungus (Fomitopsis pinicola). Food Biophysics 10, 162–2168. doi: 10.1007/s11483-014-9377-9
Keller, A. C., Maillard, M. P., Hostettmann, K. (1996). Antimicrobial steroids from the fungus Fomitopsis pinicola. Phytochemistry 41, 1041–21046. doi: 10.1016/0031-9422(95)00762-6
Keller, C., Maillard, M., Keller, J., Hostettmann, K. (2002). Screening of European fungi for antibacterial, antifungal, larvicidal, molluscicidal, antioxidant and free-radical scavenging activities and subsequent isolation of bioactive compounds. Pharm. Biol. 40, 518–525. doi: 10.1076/phbi.40.7.518.14684
Khalilov, Q., Li, L., Liu, Y., Tohtahon, Z., Chen, X., Aisa, H. A., et al. (2018). Piptolinic acids F–J, five new lanostane-type triterpenoids from Piptoporus betulinus. Nat. Prod. Res. 33, 3044–3051. doi: 10.1080/14786419.2018.1514400
Kim, Y., Cho, M., Shin, K., Kim, T., Kim, N., Kim, Y. (2010). Enzymatic hydrolysis of rice straw, a lignocellulosic biomass, by extracellular enzymes from Fomitopsis palustris. J. Korean Wood Sci. Technol. 38, 262–273. doi: 10.5658/WOOD.2010.38.3.262
Kim, H. J., Kim, Y. H., Shin, K., Kim, T. J., Kim, Y. S. (2010). Effect of carbon source on the hydrolytic ability of the enzyme from Fomitopsis pinicola for lignocellulosic biomass. J. Korean Wood Sci. Technol. 38, 429–438. doi: 10.5658/WOOD.2010.38.5.429
Kim, D. H., Lee, D. G., Park, J., Yang, Y. H., Park, J. H., Yoon, J. J. (2019). A wood-rot fungus-mediated production of isobutylene from isobutanol. Fuel. 253, 857–863. doi: 10.1016/j.fuel.2019.05.062
Kirk, P. M., Cannon, P. F., Minter, D. W. (2008). Dictionary of the Fungi. 10th edn (Wallingford: CAB International).
Kizitska, T., Barshteyn, V., Sevindik, M., Krupodorova, T. (2024). Evaluation of Fomitopsis betulina strains for growth on different media and exopolysaccharide production. Arch. Biol. Sci. 00), 18. doi: 10.2298/ABS240523018K
Kozarski, M., Klaus, A., Vunduk, J., Lazić, V., Spirović, Trifunović, B., et al. (2022). “Lignicolous mushroom Fomitopsis pinicola as a potent inhibitor of lipid peroxidation,” in Book of Abstracts of the 7th International Scientific Meeting: Mycology, Mycotoxicology, and Mycose (Matica Srpska, Novi Sad), 39. Available at: https://cer.ihtm.bg.ac.rs/handle/123456789/5142 (Accessed March 20, 2025).
Krupodorova, T., Barshteyn, V., Dzhagan, V., Pluzhnyk, A., Zaichenko, T., Blume, Y. (2024). Enhancement of antioxidant activity and total phenolic content of Fomitopsis pinicola mycelium extract. Fungal Biol. Biotechnol. 11, 18. doi: 10.1186/s40694-024-00178-1
Krupodorova, T., Barshteyn, V., Pokas, E. (2019). Antibacterial activity of Fomitopsis betulina cultural liquid. Eureka: Life Sci. 3, 10–16. doi: 10.21303/2504-5695.2019.001066
Krupodorova, T. A., Ivanova, T., Barshteyn, V. Y. (2014). Screening of extracellular enzymatic activity of macrofungi. J. Microbiol. Biotechnol. Food Sci. 3, 315–318.
Kumar, V., Prasher, I. B. (2022). Antimicrobial potential of endophytic fungi isolated from Dillenia indica L. and identification of bioactive molecules produced by Fomitopsis meliae (Undrew.) Murril. Nat. Prod Res. 36, 6064–6068. doi: 10.1080/14786419.2021.2022667
Kuo, P. C., Tai, S. H., Hung, C. C., Hwang, T. L., Kuo, L. M., Lam, S. H., et al. (2021). Antiinflammatory triterpenoids from the fruiting bodies of Fomitopsis pinicola. Bioorg Chem. 108, 104562. doi: 10.1016/j.bioorg.2021.104562
Lee, J. K., Choi, S. W., Hwang, Y. H., Park, H. K., Yoo, J. W. (2006). Isolation and characterization of inhibition Helicobacter pylori from culture media of Fomitopsis pinicola. KSBB J. 21, 422–2427. Available online at: https://koreascience.kr/article/JAKO200608508468208.page.
Lee, I. K., Jung, J. Y., Yeom, J. H., Ki, D. W., Lee, M. S., Yeo, W. H., et al. (2012). a new lanostane triterpene glycoside from the fruiting body of Fomitopsis nigra. Mycobiology. 40, 76–278. doi: 10.5941/MYCO.2012.40.1.076
Lee, J. W., Kim, H. Y., Koo, B. W., Choi, D. H., Kwon, M., Choi, I. G. (2008). Enzymatic saccharification of biologically pretreated Pinus densiflora using enzymes from brown rot fungi. J. Biosci. Bioeng. 106, 162–167. doi: 10.1263/jbb.106.162
Lee, S. I., Kim, J. S., Oh, S. H., Park, K. Y., Lee, H. G., Kim, S. D. (2008). Antihyperglycemic effect of Fomitopsis pinicola extracts in streptozotocin-induced diabetic rats. J. Med. Food. 11, 518–524. doi: 10.1089/jmf.2007.0615
Lemieszek, M. K., Langner, E., Kaczor, J., Kandefer-Szerszen, M., Sanecka, B., Mazurkiewicz, W., et al. (2009). Anticancer effect of fraction isolated from medicinal Birch polypore mushroom, Piptoporus betulinus (Bull.: Fr.) P. Karst. (Aphyllophoromycetideae): In vitro studies. Int. J. Med. Mushrooms. 11, 351–364. doi: 10.1615/IntJMedMushr.v11.i4.20
Li, H. J., Han, M. L., Cui, B. K. (2013). Two new Fomitopsis species from southern China based on morphological and molecular characters. Mycol Prog. 12, 709–718. doi: 10.1007/s11557-012-0879-x
Li, J., Li, Z., Duan, Y., Liu, C., Yan, M. (2024). Secondary metabolites of Fomitopsis betulina: Chemical structures, biological activity and application prospects. J. Fungi. 10, 616. doi: 10.3390/jof10090616
Li, X. Y., Li, S. Y., Yin, F., Chen, H. M., Yang, D. F., Liu, X. Q., et al. (2022). Antioxidative and cytoprotective effects of Ganoderma applanatum and Fomitopsis pinicola in PC12 adrenal phaeochromocytoma cells. Int. J. Med. Mushrooms. 24, 15–29. doi: 10.1615/IntJMedMushrooms.2022045050
Liu, S., Chen, Y. Y., Sun, Y. F., He, X. L., Song, C. G., Si, J., et al. (2023). Systematic classification and phylogenetic relationships of the brown-rot fungi within the Polyporales. Fungal Divers. 118, 1–94. doi: 10.1007/s13225-023-00515-6
Liu, S., Han, M. L., Xu, T. M., Wang, Y., Wu, D. M., Cui, B. K. (2021). Taxonomy and phylogeny of the Fomitopsis pinicola complex with descriptions of six new species from East Asia. Front. Microbiol. 12. doi: 10.3389/fmicb.2021.644979
Liu, Y., Liu, W., Li, M., Yuan, T. (2022). Lanostane triterpenoids from the fruiting bodies of Fomitopsis pinicola and their anti-inflammatory activities. Phytochemistry 193, 112985. doi: 10.1016/j.phytochem.2021.112985
Liu, W., Shen, Y., Hou, J., Jiang, H., Wang, Q., Zhang, L., et al. (2024). A fungal polysaccharide from Fomitopsis officinalis as a multi-target molecule to combat cancer. Int. J. Biol. Macromol. 272, 132543. doi: 10.1016/j.ijbiomac.2024.132543
Liu, S., Song, C. G., Cui, B. K. (2019). Morphological characters and molecular data reveal three new species of Fomitopsis (Basidiomycota). Mycological Prog. 18, 1317–1327. doi: 10.1007/s11557-019-01527-w
Liu, S., Song, C. G., Xu, T. M., Ji, X., Wu, D. M., Cui, B. K. (2022). Species diversity, molecular phylogeny, and ecological habits of Fomitopsis (Polyporales, Basidiomycota). Front. Microbiol. 13. doi: 10.3389/fmicb.2022.859411
Liu, X. T., Winkler, A. L., Schwan, W. R., Volk, T. J., Rott, M., Monte, A. (2010). Antibacterial compounds from mushrooms II: lanostane triterpenoids and an ergostane steroid with activity against Bacillus cereus isolated from Fomitopsis pinicola. Planta Med. 76, 464–466. doi: 10.1055/s-0029-1240621
Lonsdale, D., Pautasso, M., Holdenrieder, O. (2008). Wood-decaying fungi in the forest: conservation needs and management options. Eur. J. For. Res. 127, 1–22. doi: 10.1007/s10342-007-0182-6
Mahmood, R. T., Asad, M. J., Hadri, S. H., El-Shorbagy, M. A., Mousa, A. A., Dara, R. N., et al. (2023). Bioremediation of textile industrial effluents by Fomitopsis pinicola IEBL-4 for environmental sustainability. Hum. Ecol. Risk Assessment: Int. J. 29, 285–302. doi: 10.1080/10807039.2022.2137776
Malik, S., Bora, J., Nag, S., Sinha, S., Mondal, S., Rustagi, S., et al. (2023). Fungal-based remediation in the treatment of anthropogenic activities and pharmaceutical-pollutant-contaminated wastewater. Water 15, 2262. doi: 10.3390/w15122262
Martinez-Gonzalez, J. J., Tello-Salgado, I., Larios-Galvez, A. K., Lopez-Sesenes, R., Zahri, Z., Ramirez-Arteaga, A. M., et al. (2024). Electrochemical, thermodynamic and DFT studies of Fomitopsis pinicola as green corrosion inhibitor for carbon steel in sulfuric acid. J. Mol. Structure 10, 140014. doi: 10.1016/j.molstruc.2024.140014
Metreveli, E., Khardziani, T., Didebulidze, K., Elisashvili, V. I. (2021). Improvement of antibacterial activity of Red Belt Conk medicinal mushroom, Fomitopsis pinicola BCC58 (Agaricomycetes), in fermentation of lignocellulosic materials. Int. J. Medicinal Mushrooms 23, 27–37. doi: 10.1615/IntJMedMushrooms.2021038372
Mittal, A. K., Gupta, S. K. (1996). Biosorption of cationic dyes by dead macrofungus Fomitopsis carnea: batch studies. Water Sci. Technol. 34, 81–87. doi: 10.1016/0273-1223(96)00594-8
Miyairi, K., Toyoda, M., Okuno, T. (2001). Purification and characterization of endo- and exo-polygalacturonases from Fomitopsis cytisina. J. Appl. Glycoscience 48, 105–114. doi: 10.5458/jag.48.105
Munir, E., Hattori, T., Shimada, M. (2002). Purification and characterization of malate synthase from the glucose-grown wood-rotting basidiomycete Fomitopsis palustris. Bioscience Biotechnology Biochem. 66, 576–581. doi: 10.1271/bbb.66.576
Munir, E., Yoon, J. J., Tokimatsu, T., Hattori, T., Shimada, M. (2001). A physiological role for oxalic acid biosynthesis in the wood-rotting basidiomycete Fomitopsis palustris. Proc. Natl. Acad. Sci. 98, 11126–11130. doi: 10.1073/pnas.191389598
Muszyńska, B., Fijałkowska, A., Sułkowska-Ziaja, K., Włodarczyk, A., Kaczmarczyk, P., Nogaj, E., et al. (2020). Fomitopsis officinalis: a species of arboreal mushroom with promising biological and medicinal properties. Chem. Biodiversity 17, e2000213. doi: 10.1002/cbdv.202000213
Mykchaylova, O. B., Poyedіnok, N. L. (2021). Antimicrobial activity of Fomitopsis officinalis (Vill.) Bondartsev & Singer in pure culture. Ukrainian J. Ecol. 11, 1–5. doi: 10.15421/2021_100
Nakanishi, T., Itoh, M. (1968). Studies on the milk clotting enzyme produced by Fomitopsis pinicola. I. Some properties of the enzyme. Jpn. J. Dairy Sci. 17, A94–A101. doi: 10.5555/19690401399
Naranmandakh, S., Murata, T., Odonbayar, B., Suganuma, K., Batkhuu, J., Sasaki, K. (2018). Lanostane triterpenoids from Fomitopsis officinalis and their trypanocidal activity. J. Natural medicines. 72, 523–529. doi: 10.1007/s11418-018-1182-1
Nidadavolu, S. H., Gudikandula, K., Pabba, S. K., Maringanti, S. C. (2013). Decolorization of triphenyl methane dyes by Fomitopsis feei. Natural Science 5 (6), 30–35. doi: 10.4236/ns.2013.56A005
Ngwogu, A. C., Ngwogu, K. O. (2025). “Production of secondary metabolites by forest fungi,” in Forest Fungi: Biodiversity, Conservation, Mycoforestry and Biotechnology (USA: Academic Press), 255–270. doi: 10.1016/B978-0-443-18870-1.00011-1
Nowotarska, P., Janeczek, M., Wiatrak, B. (2024). Cytotoxic activity of Fomitopsis betulina against normal and cancer cells – a comprehensive literature review. Contemp. Oncology/Współczesna Onkologia 28, 191–200. doi: 10.5114/wo.2024.136947
Oh, S.-H., Kim, S.-D., Lee, S.-I., Lee, H.-G. (2007). Fomitopsis pinicola extracts and use thereof (WO2007078157A1) (South Korea: Eugene Bio.Farm Co. Ltd). Available at: https://patents.google.com/patent/WO2007078157A1/en (Accessed March 21, 2025).
Okamoto, K., Sugita, Y., Nishikori, N., Nitta, Y., Yanase, H. (2011). Characterization of two acidic β-glucosidases and ethanol fermentation in the brown rot fungus Fomitopsis palustris. Enzyme Microbial Technol. 48, 359–364. doi: 10.1016/j.enzmictec.2010.12.010
Olennikov, D. N., Agafonova, S. V., Rokhin, A. V., Penzina, T. A., Borovskii, G. B. (2012). Branched glucan from the fruiting bodies of Piptoporus betulinus (Bull.: Fr) Karst. Appl. Biochem. Microbiol. 48, 65–70. doi: 10.1134/S0003683812010121
Onar, O., Akata, I., Celep, G. S., Yildirim, O. (2016). Antioxidant activity of extracts from the red-belt conk medicinal mushroom, Fomitopsis pinicola (Agaricomycetes), and its modulatory effects on antioxidant enzymes. Int. J. Medicinal Mushrooms 18, 489–499. doi: 10.1615/IntJMedMushrooms.v18.i6.30
Ortiz-Santana, B., Lindner, D. L., Miettinen, O., Justo, A., Hibbett, D. S. (2013). A phylogenetic overview of the Antrodia clade (Basidiomycota, Polyporales). Mycologia 105, 1391–1411. doi: 10.3852/13-051
Out-grow.com Cultivation and market potential of medicinal mushrooms. Available online at: https://www.out-grow.com (Accessed March 20, 2025).
Pala, S. A., Wani, A. H., Ganai, B. A. (2019). Antimicrobial potential of some wild Macromycetes collected from Kashmir Himalayas. Plant Sci. Today 6, 137–146. doi: 10.14719/pst.2019.6.2.486
Park, N., Park, S. S. (2014). Purification and characterization of a novel laccase from Fomitopsis pinicola mycelia. Int. J. Biol. Macromolecules 70, 583–589. doi: 10.1016/j.ijbiomac.2014.07.023
Park, A. R., Park, J. H., Ahn, H. J., Jang, J. Y., Yu, B. J., Um, B. H., et al. (2015). Enhancement of β-glucosidase activity from a brown rot fungus Fomitopsis pinicola KCTC 6208 by medium optimization. Mycobiology 43, 57–62. doi: 10.5941/MYCO.2015.43.1.57
Patel, A., Divecha, J., Shah, A. (2021). Fomitopsis meliae CFA 2, a novel brown rot for endoglucanase: Emphasis towards enhanced endoglucanase production by statistical approach. Mycology 12, 325–340. doi: 10.1080/21501203.2021.1924373
Patel, A., Shah, A. (2021). Purification and characterization of novel, thermostable and non-processive GH5 family endoglucanase from Fomitopsis meliae CFA 2. Int. J. Biol. Macromolecules 182, 1161–1169. doi: 10.1016/j.ijbiomac.2021.04.162
Pawłowicz, T., Gabrysiak, K. A., Wilamowski, K. (2024). Investigating the potential of polypore fungi as eco-friendly materials in food industry applications. Forests 15, 1230. doi: 10.3390/f15071230
Peng, L., Huang, J. (2022). Mobilization of unavailable phosphorus and improvement of pepper P absorption, fruit yield and quality by the wood rot-fungus Fomitopsis palustris CQ2018. Soil Res. 60, 731–742. doi: 10.1071/SR21138
Peng, X. R., Su, H. G., Liu, J. H., Huang, Y. J., Yang, X. Z., Li, Z. R., et al. (2019). C30 and C31 triterpenoids and triterpene sugar esters with cytotoxic activities from edible mushroom Fomitopsis pinicola (Sw. Ex Fr.) Krast. J. Agric. Food Chem. 67, 10330–10341. doi: 10.1021/acs.jafc.9b04064
Pertile, E., Zamarsky, P. (2020). An alternative method of removing Cr (VI) from aquatic solution using chemically modified cone biomass and Fomitopsis pinicola. IOP Conf. Series: Earth Environ. Sci. 444, 12043. doi: 10.1088/1755-1315/444/1/012043
Petrova, A., Popov, S., Gjoshev, M., Bankova, V. (2007). A new triterpenic alcohol from Fomitopsis pinicola. Natural Product Res. 21, 401–405. doi: 10.1080/14786410601129679
Pleszczyńska, M., Lemieszek, M. K., Siwulski, M., Wiater, A., Rzeski, W., Szczodrak, J. (2017). Fomitopsis betulina (formerly Piptoporus betulinus): The Iceman’s polypore fungus with modern biotechnological potential. World J. Microbiol. Biotechnol. 33, 1–2. doi: 10.1007/s11274-016-2139-8
Pleszczyńska, M., Wiater, A., Janczarek, M., Szczodrak, J. (2015). (1→3)-α-d-Glucan hydrolases in dental biofilm prevention and control: A review. Int. J. Biol. Macromolecules 79, 761–778. doi: 10.1016/j.ijbiomac.2015.05.052
Pleszczyńska, M., Wiater, A., Siwulski, M., Lemieszek, M. K., Kunaszewska, J., Kaczor, J., et al. (2016). Cultivation and utility of Piptoporus betulinus fruiting bodies as a source of anticancer agents. World J. Microbiol. Biotechnol. 32, 151. doi: 10.1007/s11274-016-2114-4
Popova, M., Trusheva, B., Gyosheva, M., Tsvetkova, I., Bankova, V. (2009). Antibacterial triterpenes from the threatened wood-decay fungus Fomitopsis rosea. Fitoterapia 80, 263–266. doi: 10.1016/j.fitote.2009.03.002
Prajapati, D., Bhatt, A., Gupte, A. (2023). Evaluation of bioactive attributes and emulsification potential of exopolysaccharide produced by a brown-rot fungus Fomitopsis meliae AGDP-2. Appl. Biochem. Biotechnol. 195, 2974–2992. doi: 10.1007/s12010-022-04277-w
Purnomo, A. S., Kamei, I., Kondo, R. (2008). Degradation of 1, 1, 1-trichloro-2, 2-bis (4-chlorophenyl) ethane (DDT) by brown-rot fungi. J. Bioscience Bioengineering 105, 614–621. doi: 10.1263/jbb.105.614
Purnomo, A. S., Mauliddawati, V. T., Khoirudin, M., Yonda, A. F., Nawfa, R., Putra, S. R. (2019). Bio-decolorization and novel bio-transformation of methyl orange by brown-rot fungi. Int. J. Environ. Sci. Technol. 16, 7555–7564. doi: 10.1007/s13762-019-02362-y
Purnomo, A. S., Mori, T., Kondo, R. (2010). Involvement of Fenton reaction in DDT degradation by brown-rot fungi. Int. Biodeterioration Biodegradation 64, 560–565. doi: 10.1016/j.ibiod.2010.06.006
Purnomo, A. S., Rachmawati, N., Rizqi, H. D., Nawfa, R., Putra, S. R. (2022). Role of Fe2+-dependent reaction in biodecolorization of methyl orange by brown-rot fungus Fomitopsis pinicola. HAYATI J. Biosci. 29, 146–154. doi: 10.4308/hjb.29.2.146-154
Purnomo, A. S., Sariwati, A., Kamei, I. (2020). Synergistic interaction of a consortium of the brown-rot fungus Fomitopsis pinicola and the bacterium Ralstonia pickettii for DDT biodegradation. Heliyon 6, e04027. doi: 10.1016/j.heliyon.2020.e04027
Rayner, A. D. M., Boddy, L. (1988). Fungal Decomposition of Wood: Its Biology and Ecology. Amoebae Myxomycetes (Chichester) pp, 132–134).
Quang, D. N., Arakawa, Y., Hashimoto, T., Asakawa, Y. (2005). Lanostane triterpenoids from the inedible mushroom Fomitopsis spraguei. Phytochemistry 66, 1656–1661. doi: 10.1016/j.phytochem.2005.05.016
Rapior, S., Cavalié, S., Andary, C., Pélissier, Y., Marion, C., Bessière, J.-M. (1996). Investigation of some volatile components of seven fresh wild mushrooms (Basidiomycetes). J. Essential Oil Res. 8, 199–201. doi: 10.1080/10412905.1996.9700608
Ravikumar, K. S., Ramya, H., Ajith, T. A., Shah, M. A., Janardhanan, K. K. (2021). Bioactive extract of Fomitopsis pinicola rich in 11-α-acetoxykhivorin mediates anticancer activity by cytotoxicity, induction of apoptosis, inhibition of tumor growth, angiogenesis and cell cycle progression. J. Funct. Foods 78, 104372. doi: 10.1016/j.jff.2021.104372
Ray, M. J., Leak, D. J., Spanu, P. D., Murphy, R. J. (2010). Brown rot fungal early stage decay mechanism as a biological pretreatment for softwood biomass in biofuel production. Biomass Bioenergy 34, 1257–1262. doi: 10.1016/j.biombioe.2010.03.027
Rehman, S., Jermy, R., Asiri, S. M., Shah, M. A., Farooq, R., Ravinayagam, V., et al. (2020). Using Fomitopsis pinicola for bioinspired synthesis of titanium dioxide and silver nanoparticles, targeting biomedical applications. RSC Adv. 10, 32137–32147. doi: 10.1039/D0RA05599E
Reis, F. S., Pereira, E., Barros, L., Sousa, M. J., Martins, A., Ferreira, I. C. (2011). Biomolecule profiles in inedible wild mushrooms with antioxidant value. Molecules 16, 4328–4338. doi: 10.3390/molecules16054328
Roberts, M., Gilligan, C. A., Kleczkowski, A., Hanley, N., Whalley, A. E., Healey, J. R. (2020). The effect of forest management options on forest resilience to pathogens. Front. Forests Global Change 3. doi: 10.3389/ffgc.2020.00007
Rösecke, J., König, W. A. (1999). Steroids from the fungus Fomitopsis pinicola. Phytochemistry 52, 1621–1627. doi: 10.1016/S0031-9422(99)00236-5
Rösecke, J., König, W. A. (2000). Constituents of various wood-rotting basidiomycetes. Phytochemistry 54, 603–610. doi: 10.1016/S0031-9422(00)00134-8
Rösecke, J., Pietsch, M., König, W. A. (2000). Volatile constituents of wood-rotting basidiomycetes. Phytochemistry 54, 747–750. doi: 10.1016/S0031-9422(00)00135-X
Ryvarden, L., Gilbertson, R. L. (1993). European Polypores (2 vols)Synopsis Fungorum (Norway: Fungiflora), ISBN: 82-00724-12-8. Vol. 6 and 7.
Saba, E., Son, Y., Jeon, B. R., Kim, S. E., Lee, I. K., Yun, B. S., et al. (2015). Acetyl eburicoic acid from Laetiporus sulphureus var. miniatus suppresses inflammation in murine macrophage RAW 264.7 cells. Mycobiology 43, 131–136. doi: 10.5941/MYCO.2015.43.2.131
Sari, M., Toepler, K., Roth, C., Teusch, N., Hambitzer, R. (2020). The birch bracket medicinal mushroom, Fomitopsis betulina (Agaricomycetes) - bioactive source for beta-glucan fraction with tumor cell migration blocking ability. Int. J. Medicinal Mushrooms 22, 1–13. doi: 10.1615/IntJMedMushrooms.2019033291
Sariwati, A., Purnomo, A. S. (2018). The effect of Pseudomonas aeruginosa addition on 1, 1, 1-Trichloro-2, 2-bis (4-chlorophenyl) ethane (DDT) biodegradation by brown-rot fungus Fomitopsis pinicola. Indonesian J. Chem. 18, 75–81. doi: 10.22146/ijc.25303
Sariwati, A., Purnomo, A. S., Kamei, I. (2017). Abilities of co-cultures of brown-rot fungus Fomitopsis pinicola and Bacillus subtilis on biodegradation of DDT. Curr. Microbiol. 74, 1068–1075. doi: 10.1007/s00284-017-1283-1
Saxe, G. D. (2021a). Mushroom-based Product for COVID-19 (MACH19) (United States: ClinicalTrials.gov, National Library of Medicine, National Institutes of Health). Available at: https://clinicaltrials.gov/ct2/show/NCT04667247. NCT04667247 (Accessed March 21, 2025).
Saxe, G. D. (2021b). RCT of Mushroom-Based Natural Product to Enhance Immune Response to COVID-19 Vaccination (MACH19) (United States: ClinicalTrials.gov, National Library of Medicine, National Institutes of Health). Available at: https://clinicaltrials.gov/ct2/show/NCT04951336. NCT04951336 (Accessed March 21, 2025).
Schlegel, B., Luhmann, U., Hartl, A., Grafe, U. (2000). Piptamine, a new antibiotic produced by Piptoporus betulinus Lu 9–1. J. Antibiotics 53, 973–974. doi: 10.7164/antibiotics.53.973
Schwarze, F. W., Baum, S. (2000). Mechanisms of reaction zone penetration by decay fungi in wood of beech (Fagus sylvatica). New Phytol. 146, 129–140. doi: 10.1046/j.1469-8137.2000.00624.x
Schwarze, F. W. M. R., Engels, J., Mattheck, C. (1999). “Fungal strategies of wood decay in trees,” in Fungal Strategies of Wood Decay in Trees. Eds. Schwarze, F. W. M. R., Engels, J., Mattheck, C. (Germany: Springer), 179–221. doi: 10.1007/978-3-642-57302-6_4
Seung-Hee, O. (2005). Characteristics of dough fermentation and quality characteristics of bread using submerged-culture broth of Fomitopsis pinicola mycelium. Korean J. Food Preservation 12, 583–590. Available online at: https://koreascience.kr/article/JAKO200511722583355.page.
Sevindik, M., Akgul, H., Akata, I. L., Alli, H., Selamoglu, Z. (2017). Fomitopsis pinicola in healthful dietary approach and their therapeutic potentials. Acta Alimentaria 46, 464–469. doi: 10.1556/066.2017.46.4.9
Sha, A. L. (2016). Effects of the Fomes officinalis flavonoids on anti-senile action in the aging model mice. Chin. J. Appl. Physiol. 32, 121–123. doi: 10.13459/j.cnki.cjap.2016.02.007
Shah, F., Mali, T., Lundell, T. K. (2018). Polyporales brown rot species Fomitopsis pinicola: Enzyme activity profiles, oxalic acid production, and Fe³+-reducing metabolite secretion. Appl. Environ. Microbiol. 84, e02662–e02617. doi: 10.1128/AEM.02662-17
Shamtsyan, M., Konusova, V., Maksimova, Y., Goloshchev, A., Panchenko, A., Simbirtsev, A., et al. (2004). Immunomodulating and anti-tumor action of extracts of several mushrooms. J. Biotechnol. 113, 77–83. doi: 10.1016/j.jbiotec.2004.06.004
Shen, Y., Hou, J., Liu, W., Lin, Z., Ma, L., Xu, J., et al. (2024). An antitumor fungal polysaccharide from Fomitopsis officinalis by activating immunity and inhibiting angiogenesis. Int. J. Biol. Macromolecules 267, 131320. doi: 10.1016/j.ijbiomac.2024.131320
Shi, Z. T., Bao, H. Y., Feng, S. (2017). Antitumor activity and structure-activity relationship of seven lanostane-type triterpenes from Fomitopsis pinicola and F. officinalis. China J. Chin. Materia Med. 42, 915–922. doi: 10.19540/j.cnki.cjcmm.20170121.017
Shimokawa, T., Shibuya, H., Nojiri, M., Yoshida, S., Ishihara, M. (2008). Purification, molecular cloning, and enzymatic properties of a family 12 endoglucanase (EG-II) from Fomitopsis palustris: Role of EG-II in larch holocellulose hydrolysis. Appl. Environ. Microbiol. 74, 5857–5861. doi: 10.1128/AEM.00696-08
Shin, K., Kim, Y. H., Jeya, M., Lee, J. K., Kim, Y. S. (2010a). Purification and characterization of a thermostable cellobiohydrolase from Fomitopsis pinicola. J. Microbiol. Biotechnol. 20, 1681–1688. doi: 10.4014/jmb.1007.07015
Shin, K. S., Kim, T. J., Kim, Y. K., Kim, Y. S. (2010b). Immobilization of cellulases from Fomitopsis pinicola and their changes of enzymatic characteristics. Mokchae Konghak 38, 251–261. doi: 10.5658/WOOD.2010.38.3.251
Shin, H. J., Park, S. S., Cha, W. S. (2007). Hypoglycemic effect of hot-water and ethanol extract of Fomitopsis pinicola. Proc. Korean Soc. Life Sci. Conf., 79–81.
Sidorenko, M. (2009). Strain of basidial fungus Fomitopsis officinalis showing antibacterial activity against bacteria Yersinia pseudotuberculosis. Russia Patent 2375439.
Sidorenko, M. L., Buzoleva, L. S. (2012). Search for new types of raw materials for antibacterial drugs. Antibiotics and Chemoterapy [sic]. 57(5-6):7–10. (In Russian).
Sierra, A. R. (2007). Fungal bioleaching of metals in preservative-treated wood. Process Biochem. 42, 798–804. doi: 10.1016/j.procbio.2007.01.019
Singh, T., Singh, A. P. (2016). “White and brown rot fungi as decomposers of lignocellulosic materials and their role in waste and pollution control,” in Fungal Applications in Sustainable Environmental Biotechnology, vol. 14 . Ed. Purchase, D. Fungal Biology (Springer, Cham), 233–247. doi: 10.1007/978-3-319-42852-9_9
Sınağ, A., Akata, İ., İslek, C. (2011). Dye biosorption from aqueous solutions by Fomitopsis pinicola (Sw.) P. Karst. Gazi Univ. J. Sci. 24, 209–217.
Soares, A. M., Nogueira-Melo, G., Plautz, H. L., Jr., Gibertoni, T. B. (2017). A new species, two new combinations and notes on Fomitopsidaceae (Agaricomycetes, Polyporales). Phytotaxa 331, 75–83. doi: 10.11646/phytotaxa.331.1.7
Sofrenić, I., Anđelković, B., Todorović, N., Stanojković, T., Vujisić, L., Novaković, M., et al. (2021). Cytotoxic triterpenoids and triterpene sugar esters from the medicinal mushroom. Fomitopsis betulina. Phytochem. 181, 112580. doi: 10.1016/j.phytochem.2020.112580
Song, M., Bao, H., Bau, T. (2018). FPOA induces the apoptosis of HepG2 cells. Exp. Ther. Med. 15, 2649–2654. doi: 10.3892/etm.2018.5733
Spirin, V., Runnel, K., Vlasák, J., Viner, I., Barrett, M. D., Ryvarden, L., et al. (2024). The genus Fomitopsis (Polyporales, Basidiomycota) reconsidered. Stud. Mycology 107, 149–249. doi: 10.1016/j.simyco.2024.100149
Srisit, S., Bunloed, C., Soma, W., Panchompoo, J., Takpho, C., Rattarom, R., et al. (2024). The isolation of 5-hydroxymethylfuran metabolites from the broth extract of Fomitopsis meliae (Agaricomycetes). Int. J. Medicinal Mushrooms 26, 495–506. doi: 10.1615/IntJMedMushrooms.2024055584
Stamets, P. E. (2005). Antipox properties of Fomitopsis officinalis (Vill.: Fr.) Bond. Et Singer (Agarikon) from the Pacific Northwest of North America. Int. J. Medicinal Mushrooms 7, 495–506. doi: 10.1615/IntJMedMushr.v7.i3.60
Stamets, P. E. (2011). Inventor. Antiviral activity from medicinal mushrooms (USA:United States patent application). US 11/728,613.
Stamets, P. E. (2014). Antiviral and antibacterial activity from medicinal mushrooms (Washington (DC: U.S. Patent and Trademark Office). U.S. Patent No. 8,765,138.
Stamets, P. E. (2018). Antiviral activity from medicinal mushrooms and their active constituents, U.S. Patent Application No 15/918,082.
Stamets, P. E. (2021). Antiviral activity from medicinal mushrooms containing phenyl carboxylate/acrylate compounds (Canada: Canadian Intellectual Property Office). Available at: https://www.ic.gc.ca/opic-cipo/cpd/eng/patent/2980173/summary.html. Canadian Patent No. CA 2980173 (Accessed March 21, 2025).
Stipniece-Jekimova, A. A., Civzele, A., Mezule, L. (2022). Application of fungi for lignocellulosic biomass treatment and biofuel production. Bioenergy Technol. Biotechnol. 20, 8. Available online at: https://wrebl.rtu.lv/wp-content/uploads/sites/12/2023/04/Bioenergy_Technologies_and_Biotechnologies_2022.pdf#page=8.
Striegler, S., Haslinger, E. (1996). Cerebroside aus Fomitopsis pinicola (Sw. Ex Fr.) Karst. Monatshefte für Chemie/Chemical Monthly 127, 755–761. doi: 10.1007/BF00817267
Sudharshan, S., Naidu, R., Mallavarapu, M., Bolan, N. (2012). DDT remediation in contaminated soils: a review of recent studies. Biodegradation 23, 851–863. doi: 10.1007/s10532-012-9574-5
Sulkowska-Ziaja, K., Muszynska, B., Motyl, P., Pasko, P., Ekiert, H. (2012). Phenolic compounds and antioxidant activity in some species of polyporoid mushrooms from Poland. Int. J. Medicinal Mushrooms 14, 385–393. doi: 10.1615/IntJMedMushr.v14.i4.60
Sułkowska-Ziaja, K., Szewczyk, A., Galanty, A., Gdula-Argasińska, J., Muszyńska, B. (2018). Chemical composition and biological activity of extracts from fruiting bodies and mycelial cultures of Fomitopsis betulina. Mol. Biol. Rep. 45, 2535–2544. doi: 10.1007/s11033-018-4422-2
Sum, W. C., Ebada, S. S., Gonkhom, D., Decock, C., Teponno, R. B., Matasyoh, J. C., et al. (2023). Two new lanostanoid glycosides isolated from a Kenyan polypore Fomitopsis carnea. Beilstein J. Organic Chem. 19, 1161–1169. doi: 10.3762/bjoc.19.85
Sun, C. (2015). Studies on chemical constituents of four higher fungi. Anhui University of Chinese Medicine, Hefei, China.
Sun, Q., Huang, W., Bao, H. Y., Bau, T., Li, Y. (2016). Anti-tumor and antioxidation activities of solid fermentation products of Fomitopsis pinicola. Mycosystema 35, 965–974.
Sun, J., Yang, H., Ge-Zhang, S., Chi, Y., Qi, D. (2024). Identification of a Fomitopsis pinicola from Xiaoxing’an Mountains and optimization of cellulase activity. Forests 15, 1673. doi: 10.3390/f15091673
Sun, X., Zhao, X. H., Bao, H. Y. (2012). Antitumor active constituent in fruiting body of Fomitopsis pinicola. Shizhen Guoyi Guoyao 23, 1634–1637.
Tai, S. H., Kuo, P. C., Hung, C. C., Lin, Y. H., Hwang, T. L., Lam, S. H., et al. (2019). Bioassay-guided purification of sesquiterpenoids from the fruiting bodies of Fomitopsis pinicola and their anti-inflammatory activity. RSC Adv. 9, 34184–34195. doi: 10.1039/C9RA06400A
Tanaka, Y., Konno, N., Suzuki, T., Habu, N. (2020). Starch-degrading enzymes from the brown-rot fungus Fomitopsis palustris. Protein Expr Purif 170, 105609. doi: 10.1016/j.pep.2020.105609
Tanaka, Y., Suzuki, T., Nakamura, L., Nakamura, M., Ebihara, S., Kurokura, T., et al. (2018). A GH family 28 endo-polygalacturonase from the brown-rot fungus Fomitopsis palustris: Purification, gene cloning, enzymatic characterization and effects of oxalate. Biomacromolecules 19, 4288–4296. doi: 10.1021/acs.biomac.8b01150
Tiwari, A., Chen, C. W., Haldar, D., Patel, A. K., Dong, C. D., Singhania, R. R. (2023). Laccase in biorefinery of lignocellulosic biomass. Appl. Sci. 13, 4673. doi: 10.3390/app13084673
Tohtahon, Z., Xue, J., Han, J., Liu, Y., Hua, H., Yuan, T. (2017). Cytotoxic lanostane triterpenoids from the fruiting bodies of Piptoporus betulinus. Phytochemistry 143, 98–103. doi: 10.1016/j.phytochem.2017.08.003
Tsujiyama, S., Okada, A. (2013). Biodegradation of polyvinyl alcohol by a brown-rot fungus, Fomitopsis pinicola. Biotechnol. Lett. 35, 1907–1911. doi: 10.1007/s10529-013-1284-5
Tu, J., Zhao, J., Liu, G., Tang, C., Han, Y., Cao, X., et al. (2020). Solid state fermentation by Fomitopsis pinicola improves physicochemical and functional properties of wheat bran and the bran-containing products. Food Chem. 328, 127046. doi: 10.1016/j.foodchem.2020.127046
Turner, N. J., Cuerrier, A. (2022). [amp]]lsquo;Frog’s umbrella’ and ‘ghost’s face powder’: the cultural roles of mushrooms and other fungi for Canadian Indigenous Peoples. Botany 100, 183–205. doi: 10.1139/cjb-2021-0095
U.S. Forest Service (2023a). Region 10 - Forest & Grassland Health (USDA). Available at: https://www.fs.usda.gov (Accessed March 21, 2025).
U.S. Forest Service (2023b). Phylogeny of Fomitopsis pinicola: A Species Complex (U.S. Forest Service (USDA)). Available at: https://www.fs.usda.gov/research/ (Accessed March 21, 2025).
Usui, T., Hosokawa, S., Mizuno, T., Suzuki, T., Meguro, H. (1981). Investigation of the heterogeneity of heterogalactan from the fruit bodies of Fomitopsis pinicola, by employing concanavalin A-Sepharose affinity chromatography. J. Biochem. 89, 1029–1037. doi: 10.1093/oxfordjournals.jbchem.a133313
Valášková, V., Baldrian, P. (2006a). Degradation of cellulose and hemicelluloses by the brown rot fungus Piptoporus betulinus – production of extracellular enzymes and characterization of the major cellulases. Microbiology 152, 3613–3622. doi: 10.1099/mic.0.29149-0
Valášková, V., Baldrian, P. (2006b). Estimation of bound and free fractions of lignocellulose-degrading enzymes of wood-rotting fungi Pleurotus ostreatus, Trametes versicolor and Piptoporus betulinus. Res. Microbiol. 157, 119–124. doi: 10.1016/j.resmic.2005.06.004
Verekar, S. A., Gupta, M. K., Deshmukh, S. K. (2021). “Fomitopsis betulina: A rich source of diverse bioactive metabolites,” in Advances in Macrofungi (United States: CRC Press), 52–66.
Vunduk, J., Klaus, A., Kozarski, M., Petrovic, P., Zizak, Z., Nikšić, M., et al. (2015). Did the Iceman know better? Screening of the medicinal properties of the birch polypore medicinal mushroom, Piptoporus betulinus (Higher Basidiomycetes). Int. J. Med. Mushrooms 17, 1113–1125. doi: 10.1615/IntJMedMushrooms.v17.i12.50
Wang, Y., Cheng, X., Wang, P., Wang, L., Fan, J., Wang, X., et al. (2014). Investigating migration inhibition and apoptotic effects of Fomitopsis pinicola chloroform extract on human colorectal cancer SW-480 cells. PloS One 9, e101303. doi: 10.1371/journal.pone.0101303
Wang, W., Yuan, T., Wang, K., Cui, B., Dai, Y. (2012). Statistical optimization of cellulase production by the brown rot fungi, Fomitopsis palustris, and its application in the enzymatic hydrolysis of LHW-pretreated woody biomass. Process Biochem. 47, 2552–2556. doi: 10.1016/j.procbio.2012.09.018
Wangun, H. V., Berg, A., Hertel, W., Nkengfack, A. E., Hertweck, C. (2004). Anti-inflammatory and anti-hyaluronate lyase activities of lanostanoids from Piptoporus betulinus. J. Antibiot 57, 755–758. doi: 10.7164/antibiotics.57.755
Waszczuk, U., Zapora, E., Berezovska, D., Stocki, M., Wołkowycki, M., Malewski, T., et al. (2022). Use of secondary metabolites of wood-decaying fungi to reduce damping off disease. Forests 13, 1208. doi: 10.3390/f13081208
Watanabe, T., Shitan, N., Suzuki, S., Umezawa, T., Shimada, M., Yazaki, K., et al. (2010). Oxalate efflux transporter from the brown rot fungus Fomitopsis palustris. Appl. Environ. Microbiol. 76, 7683–7690. doi: 10.1128/AEM.01142-10
Watanabe, T., Shitan, N., Umezawa, T., Yazaki, K., Shimada, M., Hattori, T. (2007). Involvement of FpTRP26, a thioredoxin-related protein, in oxalic acid-resistance of the brown-rot fungus Fomitopsis palustris. FEBS Lett. 581, 1788–1792. doi: 10.1016/j.febslet.2007.03.068
Wei, Y. L., Dai, Y. C. (2004). Ecological function of wood-inhabiting fungi in forest ecosystem. J. Appl. Ecol. 15, 1935–1938. Available online at: https://europepmc.org/article/med/15624839.
Wenner, C. A., Froehlich, O. R., Sitkoff, A. (2021). Fomitopsis cajanderi extracts have differential tumoricidal actions on human MCF7 and U-937 cancer cells and inhibit TNF secretion by U-937 monocytes. J. Immunol. 206, 29.10. doi: 10.4049/jimmunol.206.Supp.29.10
Wiater, A., Paduch, R., Pleśczyńska, M., Próchniak, K., Choma, A., Kandefer-Szerszeń, M., et al. (2011). Alpha-(1→3)-β-glucans from fruiting bodies of selected macromycetes fungi and the biological activity of their carboxymethylated products. Biotechnol. Lett. 33, 787–795. doi: 10.1007/s10529-010-0492-y
Wiater, A., Szczodrak, J., Pleszczyńska, M. (2008). Mutanase induction in Trichoderma harzianum by cell wall of Laetiporus sulphureus and its application for mutan removal from oral biofilms. J. Microbiol. Biotechnol. 18, 1335–1341.
Wu, X., Wu, Y., Ye, L., Wu, L., Su, C., Fu, J. (2023). The protective effect and potential mechanism analysis of Fomitopsis pinicola mycelia polysaccharides (FPMPS) on acute alcoholic liver injury in mice. Authorea. doi: 10.22541/au.169744255.53501516/v1
Wu, H. T., Lu, F. H., Su, Y. C., Ou, H. Y., Hung, H. C., Wu, J. S., et al. (2014). In vivo and in vitro anti-tumor effects of fungal extracts. Molecules 19, 2546–2556. doi: 10.3390/molecules19022546
Xiao, X. L., Chen, W. G., Gao, J. S., You, H., Li, G. Z., Jiang, J. H., et al. (2011). Inhibitory effect of Fomitopsis pinicola extract on hepatocellular carcinoma H22 cells and sarcoma S180 cells. Chin. J. Cancer Biotherapy 18, 181–185.
Xiong, Z., Zhang, X., Wang, H., Ma, F., Li, L., Li, W. (2007). Application of brown-rot basidiomycete Fomitopsis sp. IMER2 for biological treatment of black liquor. J. Biosci. Bioeng 104, 446–450. doi: 10.1263/jbb.104.446
Yang, Z., Liu, B., Yang, L. E., Zhang, C. (2019). Platycodigenin as potential drug candidate for Alzheimer’s disease via modulating microglial polarization and neurite regeneration. Molecules 24, 3207. doi: 10.3390/molecules24183207
Yoon, J. J., Cha, C. J., Kim, Y. S., Kim, W. (2008b). Degradation of cellulose by the major endoglucanase produced from the brown-rot fungus Fomitopsis pinicola. Biotechnol. Lett. 30, 1373–1378. doi: 10.1007/s10529-008-9702-9
Yoon, J. J., Cha, C. J., Kim, Y. S., Son, D. W., Kim, Y. K. (2007a). The brown-rot basidiomycete Fomitopsis palustris has the endo-glucanases capable of degrading microcrystalline cellulose. J. Microbiol. Biotechnol. 17, 800–805. Available online at: https://europepmc.org/article/med/18051302.
Yoon, J. J., Hattori, T., Shimada, M. (2002). A metabolic role of the glyoxylate and tricarboxylic acid cycles for development of the copper-tolerant brown-rot fungus Fomitopsis palustris. FEMS Microbiol. Lett. 217, 9–14. doi: 10.1111/j.1574-6968.2002.tb11453.x
Yoon, J. J., Hattori, T., Shimada, M. (2003). Purification and characterization of NADP-linked isocitrate dehydrogenase from the copper-tolerant wood-rotting basidiomycete Fomitopsis palustris. Biosci. Biotechnol. Biochem. 67, 114–120. doi: 10.1271/bbb.67.114
Yoon, J. J., Igarashi, K., Kajisa, T., Samejima, M. (2006). Purification, identification and molecular cloning of glycoside hydrolase family 15 glucoamylase from the brown-rot basidiomycete Fomitopsis palustris. FEMS Microbiol. Lett. 259, 288–294. doi: 10.1111/j.1574-6968.2006.00278.x
Yoon, J. J., Kim, Y. K. (2005). Degradation of crystalline cellulose by the brown-rot basidiomycete Fomitopsis palustris. J. Microbiol. 43, 487–492. Available online at: https://www.researchgate.net/profile/Jeong-Jun Yoon/publication/7360991_Degradation_of_Crystalline_Cellulose_by_the_Brown rot_Basidiomycete/links/55af59c708ae98e661a70325/Degradation-of-Crystalline-Cellulose-by-the-Brown-rot-Basidiomycete.pdf.
Yoon, J. J., Kim, K. Y., Cha, C. J. (2008a). Purification and characterization of thermostable β-glucosidase from the brown-rot basidiomycete Fomitopsis palustris grown on microcrystalline cellulose. J. Microbiol. 46, 51–55. doi: 10.1007/s12275-007-0235-z
Yoon, J. J., Lee, Y. M., Choi, D. Y., Kim, Y. K., Kim, Y. S. (2007b). Purification and characterization of xylanase from Fomitopsis palustris in rice straw culture. J. Korean Wood Sci. Technol. 35, 159–165.
Yoshikawa, K., Inoue, M., Matsumoto, Y., Sakakibara, C., Miyataka, H., Matsumoto, H., et al. (2005). Lanostane triterpenoids and triterpene glycosides from the fruit body of Fomitopsis pinicola and their inhibitory activity against COX-1 and COX-2. J. Nat. Prod 68, 69–73. doi: 10.1021/np049730e
Zahid, M. T., Idrees, M., Abdullah, I., Ying, W., Zaki, A. H., Bao, H. (2020a). Antidiabetic properties of the red belt conk medicinal mushroom Fomitopsis pinicola (Agaricomycetes) extracts on streptozotocin-induced diabetic rats. Int. J. Med. Mushrooms 22, 731–741. doi: 10.1615/IntJMedMushrooms.2020035625
Zahid, M. T., Idrees, M., Farooq, U., Abdullah, I., Ying, W., Zaki, A. H., et al. (2020c). Anti-diabetic, anti-oxidant and anti-hyperlipidemic activity of Fomitopsis pinicola. Int. J. Biosci. 16, 102–112. doi: 10.1615/IntJMedMushrooms.2020035472
Zahid, M. T., Idrees, M., Ying, W., Zaki, A. H., Abdullah, I., Bao, H. (2020b). Review of chemical constituents and pharmacology of brown-rot fungus Fomitopsis pinicola. Cellulose 10, 58–68. doi: 10.7176/JNSR/10-2-07
Zhang, J., Chen, B., Liang, J., Han, J., Zhou, L., Zhao, R., et al. (2020). Lanostane triterpenoids with PTP1B inhibitory and glucose-uptake stimulatory activities from mushroom Fomitopsis pinicola collected in North America. J. Agric. Food Chem. 68, 10036–10049. doi: 10.1021/acs.jafc.0c04460
Zhang, Z. F., Wu, C., Wang, M., Chen, J. F., Lv, G. Y. (2023). Chemical fingerprinting and the biological properties of extracts from Fomitopsis pinicola. Arab J. Chem. 16, 104669. doi: 10.1016/j.arabjc.2023.104669
Zhao, X. H., Bao, H. Y. (2019). Study on anti-inflammatory, analgesic and antipyretic activities of extracts of Fomitopsis pinicola fruiting bodies. Acta Edulis Fungi 26, 82–90. doi: 10.16488/j.cnki.1005-9873.2019.03.010
Zhao, J., Yang, Y., Yu, M., Yao, K., Luo, X., Qi, H., et al. (2018). Lanostane-type C31 triterpenoid derivatives from the fruiting bodies of cultivated Fomitopsis palustris. Phytochemistry 152, 10–21. doi: 10.1016/j.phytochem.2018.04.012
Zhou, Y. (2012). Active ingredients of Fomes officinalis, preparation method thereof and use thereof (Patent No. CN101721434A) (China: Suzhou Green Growth Investment Center LP).
Keywords: bioactive compounds, traditional and modern medicine, therapeutic properties, enzymatic potential, biotechnological applications
Citation: Karunarathna SC, Patabendige NM, Kumla J, Hapuarachchi KK and Suwannarach N (2025) The bioactive compounds, beneficial medicinal properties, and biotechnological prospects of Fomitopsis: a comprehensive overview. Front. Cell. Infect. Microbiol. 15:1534617. doi: 10.3389/fcimb.2025.1534617
Received: 26 November 2024; Accepted: 11 March 2025;
Published: 22 April 2025.
Edited by:
Jianping Xu, McMaster University, CanadaReviewed by:
Jing Si, Beijing Forestry University, ChinaHariprasath Lakshmanan, JSS Academy of Higher Education and Research, India
Copyright © 2025 Karunarathna, Patabendige, Kumla, Hapuarachchi and Suwannarach. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kalani K. Hapuarachchi, a2FsYW5pZmlyc3RAeWFob28uY29t; Nakarin Suwannarach, c3V3YW5fNDYxQGhvdG1haWwuY29t
 Samantha C. Karunarathna
Samantha C. Karunarathna Nimesha M. Patabendige2
Nimesha M. Patabendige2 Jaturong Kumla
Jaturong Kumla Kalani K. Hapuarachchi
Kalani K. Hapuarachchi Nakarin Suwannarach
Nakarin Suwannarach