- 1Henan Provincial Engineering and Technology Center of Animal Disease Diagnosis and Integrated Control, Henan Provincial Engineering Laboratory of Insects Bio-reactor, Nanyang Normal University, Nanyang, China
- 2Henan Province Engineering Technology Research Center of Animal Disease Control and Prevention, Nanyang Vocational College of Agriculture, Nanyang, China
Staphylococcus aureus is a significant pathogen in dairy animals, particularly when it infects the mammary gland; however, its prevalence among dairy goats in China remains poorly understood. This study aimed to investigate the distribution and characteristics of S. aureus isolates in dairy goats across China. A total of 515 milk samples were collected from goats diagnosed with mastitis in 14 provinces. These samples underwent bacterial isolation and identification, capsular polysaccharides typing, spa typing, antimicrobial susceptibility testing, and assessment of antimicrobial resistance and virulence gene. The findings revealed the isolation of 61 S. aureus strains. The highest prevalence rate was recorded in 2018, at 20.4% (11 out of 54 samples), while the lowest prevalence rate was noted in 2023, at 5.2% (3 out of 58 samples). Among the five regions studied, southern China exhibited the highest prevalence rate of 17.5% (10 out of 57 samples), whereas northeastern China showed the lowest rate at 8.2% (8 out of 97 samples). Capsular polysaccharide type 5 emerged as the most prevalent, accounting for 52.5%, and spa type t521 was identified most frequently, at 19.7%. Notably, 52 isolates (85.2%) demonstrated multidrug resistance, displaying resistance to three or more antibiotics. The resistance rates of S. aureus isolates were significantly high to penicillin (95.1%), followed by enrofloxacin (82.0%), kanamycin (78.7%), and levofloxacin (77.0%). Trimethoprim-sulfamethoxazole exhibited the lowest resistance rate at 11.5%. Resistance rates varied across the five different regions. Additionally, eight genes associated with resistance to six classes of antimicrobials were detected, with the blaZ gene (93.4%) being the most prevalent at 93.4%. Furthermore, nine virulence-associated genes were identified, with clfA being the most common virulence gene, present in all isolates. In conclusion, most S. aureus isolates were multiresistant with diverse resistance patterns. Those diverse antimicrobial resistance profiles associated with corresponding resistance genes (p < 0.05) were reported for the first time in S. aureus from caprine mastitis. Sulfonamides could be prioritized preferentially for the treatment of S. aureus mastitis.
1 Introduction
Mastitis refers to the inflammation of the mammary glands, which can be attributed to various pathogenic factors. Clinical mastitis may result in a reduction in milk production, diminished milk quality, udder gangrene, loss of lactation capability, prolonged estrus, and decreased overall production performance, ultimately leading to significant economic losses (Olechnowicz and Jaśkowski, 2014). In China, the prevalence of mastitis in dairy goats has been reported to be 45.82% (Zhao et al., 2015). Bacterial infection is the primary etiological factor associated with mastitis, with Staphylococcus aureus being one of the most significant pathogens. S. aureus is a species of Gram-positive, grape-forming coccus. To date, the presence of S. aureus in dairy cattle suffering from mastitis has been documented in numerous reports within China (Gao et al., 2017; Cheng et al., 2019; Song et al., 2020; Song et al., 2024). A meta-analysis, based on 4,215 bovine mastitis samples in 33 studies, revealed a pooled prevalence of S. aureus at 36.23% (Wang et al., 2022). In contrast, the epidemiology of S. aureus in dairy goats with mastitis has been reported in only a limited number of studies (Zhao et al., 2015; Liu et al., 2023). It is noteworthy that China hosts over 1,290,000 dairy goats (Luo et al., 2019). In 2015, the prevalence rates of S. aureus in samples collected from dairy goats were reported to be 12.82%, 22.58%, and 14.85%, respectively, in three provinces (Shandong, Yunnan, and Shaanxi) in China (Zhao et al., 2015). Recently, in Shaanxi province, the prevalence of S. aureus was observed in 58.33% of raw milk samples (28/48) collected from dairy goats with clinical mastitis (Liu et al., 2023). However, the characteristics of S. aureus strains have not been documented in the aforementioned reports.
To date, the capsular polysaccharides (CP) of S. aureus have been classified into 11 types, of which only CP5 and CP8 types (encoded by the genes cap5 and cap8, respectively) are present in more than 80% of clinical strains (Rozemeijer et al., 2015). In addition, spa typing, another widely utilized typing method, relies on the tandem repeats and sequence variations in the protein A gene region X (Moodley et al., 2006). Spa typing employs a standardized international nomenclature based on single-base sequence analysis, which is convenient for probing molecular correlations between S. aureus from different hosts, environments, sources, and countries (Rodriguez et al., 2015). To our knowledge, no studies have been conducted on the prevalent types of S. aureus strains from dairy goats in China; thus, molecular epidemiology data are not available.
Currently, antimicrobial therapy is the primary method for preventing and treating S. aureus infections in dairy herds. Unfortunately, the extensive use of antimicrobial agents has led to the emergence of resistance to various antibiotics through phenotype changes, rendering these antibiotics increasingly ineffective (Bujňáková and Karahutová, 2024; Hutu et al., 2024; Shahzad et al., 2024; Zhu et al., 2024). In China, national antimicrobial resistance monitoring and surveillance programming in animals have been carried out for many years. However, information of the antibiotic resistance of S. aureus from dairy goats remains scarce. The resistance of S. aureus is closely related to drug resistance genes; for example, blaZ and mecA are considered the main genes responsible for β-lactam resistance, and tetK and tetM account for tetracycline resistance (Al-Ashmawy et al., 2016; Merz et al., 2016; Akkou et al., 2018). Moreover, since resistance genes may transfer from S. aureus to the indigenous microbiota in the human gut (Lee John, 2003), this undoubtedly increases the huge public health risk.
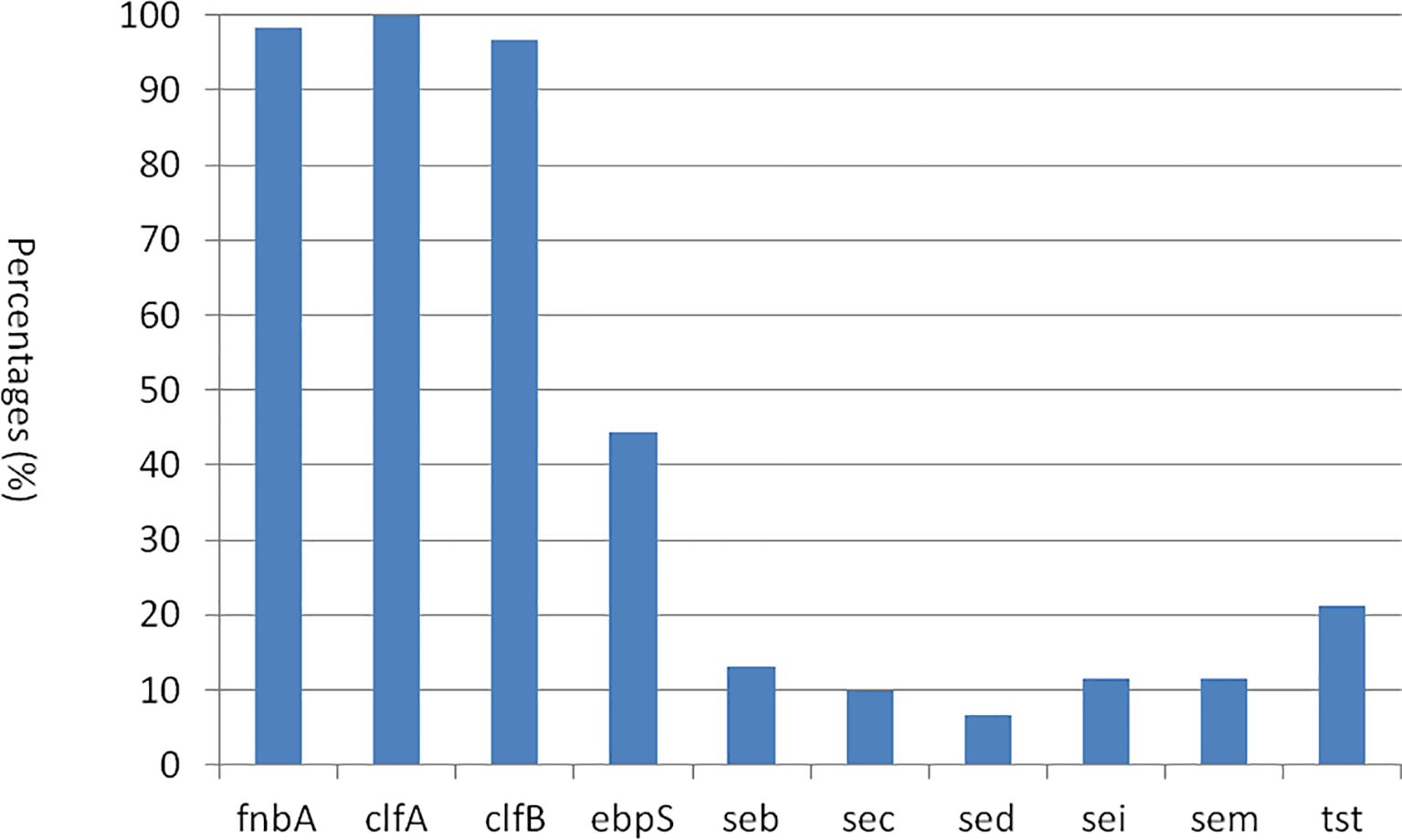
The pathogenicity of S. aureus multiplies, influenced by a variety of virulence factors, which included both structural components of the bacteria and secreted factors (Wang et al., 2018). For instance, bacterial adhesion to the epithelial cells of the teat canal is mediated by clumping factors A and B (clfA and clfB) as well as fibronectin-binding protein A (fnbA) (Bochniarz et al., 2016). Furthermore, the secreted virulence factors encompass over 20 different enterotoxins, which are traditionally categorized into classical genes from sea to sem, along with other significant factors, such as the toxic shock syndrome toxin (TSST-1) and staphylococcal exfoliative toxin (ET). These factors play a crucial role in the pathogenesis of mastitis caused by S. aureus (Costa et al., 2018; Mohseni et al., 2018).
The aim of the present study is to investigate the distribution of S. aureus isolates in cases of mastitis in dairy goat mastitis and to identify the presence of resistance and virulence genes in these isolates collected from 2015 to 2024. The findings of this study will contribute to the scientific foundation for the development of prevention and control strategies in the production of dairy goats in China.
2 Materials and methods
2.1 Sample collection, bacterial isolation, and identification
From 2015 to 2024, a total of 515 batches of raw milk were collected from dairy goats diagnosed with clinical mastitis across 32 farms located in 14 provinces in China (Table 1). The dairy goats involved in this study were raised in backyard farms utilizing a free-range system, with herd sizes ranging from 20 to 60 animals. Milking was predominantly conducted manually twice daily. It is noteworthy that teat disinfection practices on these farms were irregular; specific details regarding the cleaning agents used, their concentrations, mode of application, and frequency of cleaning are provided in Supplementary Table 1. All milk samples were collected under aseptic conditions, transferred to sterile tubes, and stored in an ice box at 4°C for transportation to the laboratory for bacterial analysis.
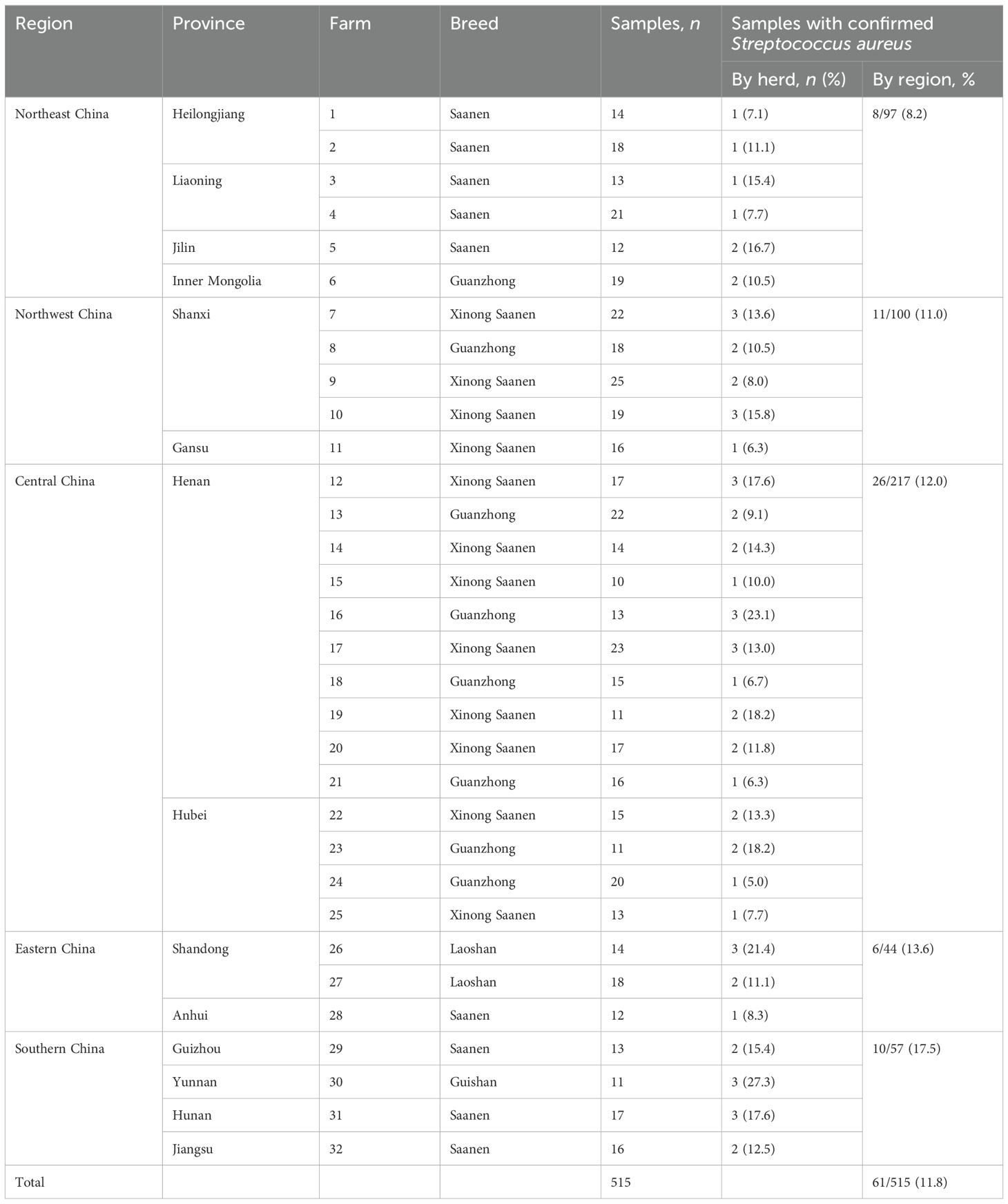
Table 1. Goat milk samples with clinical mastitis collected from backyard dairy goat farms in five regions of China from 2015 to 2024.
Milk samples were inoculated onto blood agar plates supplemented with 5% defibrinated sheep blood and incubated at 37°C for 24 to 48 h. Subsequently, typical colonies were identified through Gram staining and catalase testing. Suspected S. aureus isolates were confirmed by detecting the thermonuclease (nuc) gene, which is specific to this bacterium (Brakstad et al., 1992). In summary, DNA was extracted using a Bacteria Genomic DNA kit (TransGen Biotech, China) in accordance with the manufacturer’s instructions. The extracted DNA was dissolved in 100 µL of ultrapure water and stored at −70°C for subsequent PCR analysis. In addition, PCR products from five nuc positive strains were randomly selected for sequencing to ensure further verification. Following identification, one single nuc gene positive isolate from each sample was selected and stored for subsequent antimicrobial susceptibility testing. S. aureus ATCC25923 was used as a quality control strain. The prevalence rate was calculated as the following formula: prevalence rate = (number of existing cases/total sample number) × 100%.
2.2 Typing of S. aureus
Both capsular polysaccharide typing and spa typing were used to type all stored S. aureus strains. According to the previous study (Verdier et al., 2007), two primer sets were selected for capsular polysaccharide typing, namely, Cap5 k1 (5′-GTCAAAGATTATGTGATGCTACTGAG-3′) and Cap5 k2 (5′-ACTTCGAATATAAACTTGAATCAATGTTATACAG-3′) located in cap5k, specific for capsular type 5, and cap8 k1 (5′-GCCTTATGTTAGGTGATAAACC-3′) and cap8 k2 (5′-GGAAAAACACTATCATAGCAGG-3′) located in cap8I, specific for capsular type 8.
Molecular typing of spa types was also carried out via PCR using the primer set including spa-1113f (5′-TAAAGA CGATCC TTC GGTGAGC-3′) and spa-1514r (5′-CAG CAGTAGTGCCGTTTGCTT-3′). The results were submitted to the website for further detection. Then, these results were interpreted according to the guidelines described at the Ridom SpaServer database (www.spaserver.ridom.de).
2.3 Antimicrobial susceptibility testing
The antibiotic resistance patterns of each nuc-positive S. aureus isolate from the samples (selected and stored in Section 2.1) were determined by disk diffusion methods according to Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2018). The inhibition zones were measured and recorded, with breakpoints for various antibiotics determined based on CLSI guidelines (CLSI, 2018). In instances where specific antibiotic testing and interpretation guidelines were unavailable, the instructions for antibiotic-sensitive papers (Hangzhou Microbial Reagent Company, China) were used as a reference. Resistance to 18 antimicrobials belonging to eight antimicrobial categories was investigated in the drug susceptibility test. Each experiment was repeated at least three times to ensure reproducibility, and S. aureus ATCC25923 served as a quality control strain. Moreover, isolates exhibiting resistance to three or more antimicrobial categories were classified as multidrug resistant (Moodley et al., 2006).
2.4 Detection of resistance genes
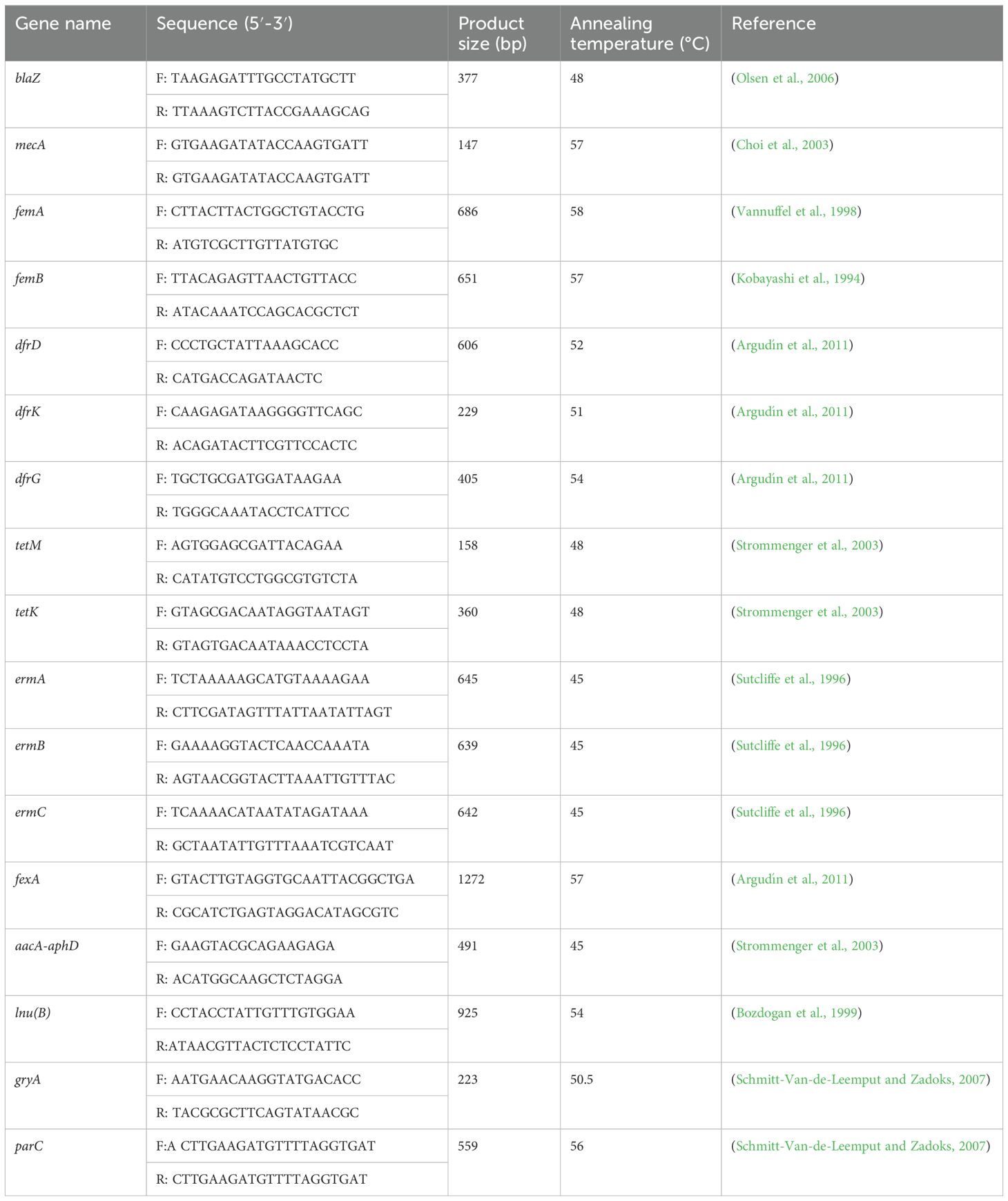
Bacterial genomic DNA was extracted as described in the section on bacterial identification. The detection of antibiotic resistance genes and virulence genes was performed using conventional PCR. In alignment with the categories of antimicrobials utilized in this work and the most commonly identified resistance genes in S. aureus as reported in previous research conducted in China (Zhang et al., 2022; Ning et al., 2023; Zhu et al., 2024), the following resistance genes were detected: β-lactam resistance genes mecA, blaZ, femA, and femB (Kobayashi et al., 1994; Vannuffel et al., 1998; Choi et al., 2003; Olsen et al., 2006); tetracycline resistance genes tetK and tetM (Strommenger et al., 2003); macrolide resistance genes ermA, ermB, and ermC (Sutcliffe et al., 1996); phenicols resistance gene fexA (Argudín et al., 2011); sulfonamide resistance gene dfrD (Argudín et al., 2011); aminoglycoside resistance genes aacA-aphD (Strommenger et al., 2003); the lincosamide resistance gene lnu(B) (formerly known as linB) (Bozdogan et al., 1999); and quinolone resistance genes gyrA and parC (Schmitt-Van-de-Leemput and Zadoks, 2007). Detailed information regarding all resistance gene primers, including product size and annealing temperature, is presented in Table 2.
An EasyTaq® PCR SuperMix kit (TransGen, Beijing, China) was used for PCR reactions. A total reaction mixture was 20 μL, comprising 10 μL of 2× EasyTaq® PCR SuperMix, 0.4 μM of each primer, and 20 ng of template DNA. Then, these mixtures were denatured at 94°C for 5 min, followed by 30 cycles of 30 s at 94°C, 30 s at an appropriate annealing temperature determined by the specific resistance and virulence gene primers, 30 s at 72°C, and a final extension at 72°C for 10 min. Meanwhile, samples with goat DNA or without genomic DNA were included as controls. The amplified products were subjected to electrophoresis through a 2% agarose gel (Solarbio, China) at 120 V for 60 min.
2.5 Detection of virulence genes
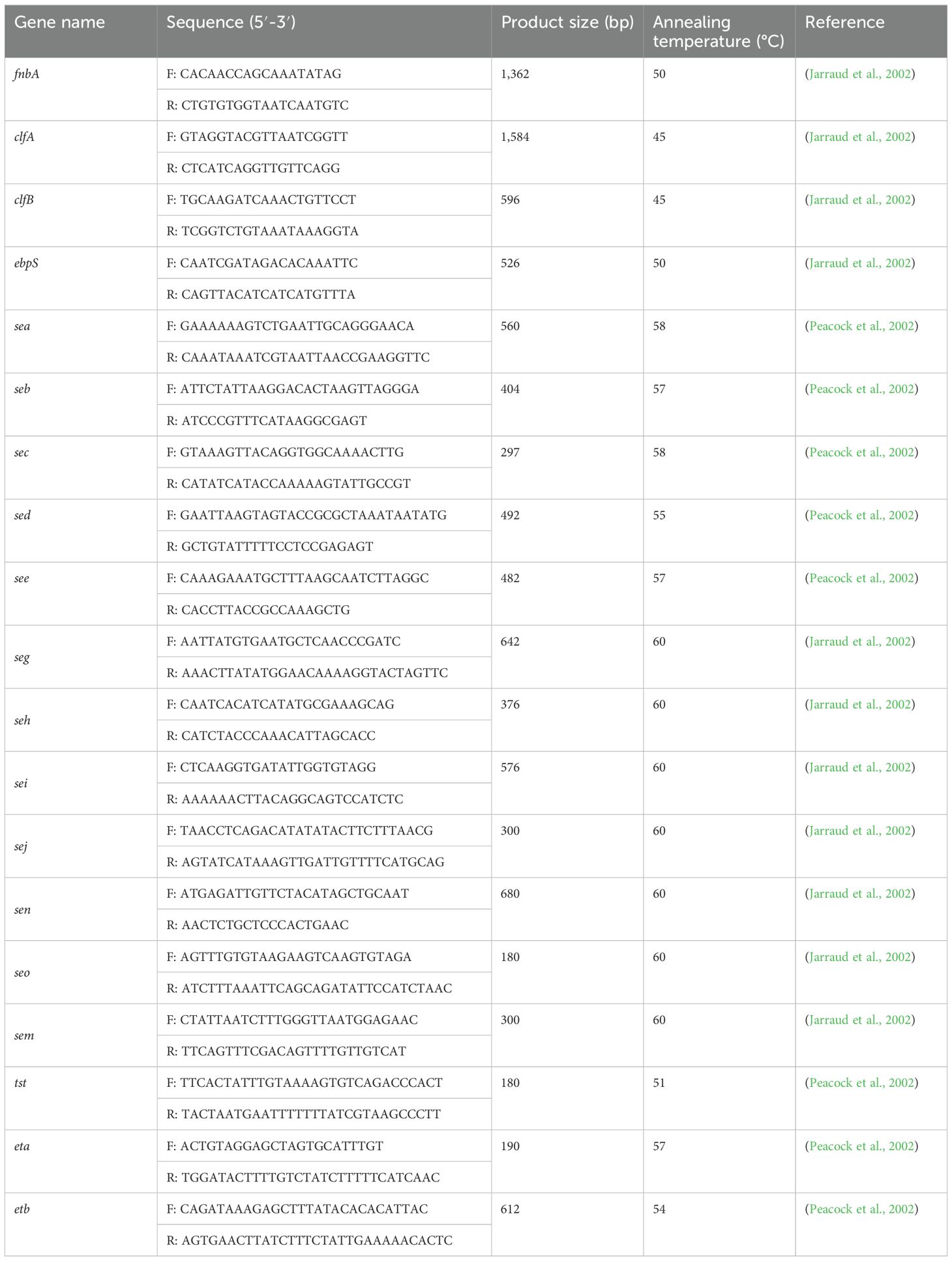
Several genes associated with virulence were screened via PCR, including the staphylococcal adhesion factors fnbA, clfA, clfB, and ebpS; enterotoxin (sea, seb, sec, sed, see, seg, seh, sei, sej, sen, seo, and sem); poisoning syndrome toxin (tst); and shedding toxin (eta, etb) (Jarraud et al., 2002; Peacock et al., 2002). The primers for all virulence genes, along with their respective product sizes and annealing temperatures, are presented in Table 3. The PCR protocols employed were consistent with the previously described protocols in Section 2.4.
2.6 Statistical analyses
The independent-samples method and a non-parametric test were used to estimate the correlation between S. aureus typing and various factors, including antimicrobial resistance, resistance genes, and virulence genes. Statistical analyses were performed using IBM SPSS Statistics (IBM Corp., Armonk, NY, USA) (p < 0.05 was considered to indicate statistical significance).
3 Results
3.1 Isolation and identification of S. aureus
In the present study, a total of 61 bacterial isolates were recovered in 515 milk samples and identified as S. aureus through Gram staining and species-specific PCR targeting the nuc gene. The nuc gene was successfully detected in all isolates. Upon sequencing, the homology of nuc gene sequences for five isolates in this study ranged from 98.6% to 100%, while the homology with other S. aureus strains available in GenBank ranged from 97.6% to 99.3%. The overall isolation rate was determined to be 11.8%. As shown in Figure 1, the highest prevalence rate was recorded in 2018, at 20.4% (11/54), followed by 17.2% (5/29) in 2015, and the lowest prevalence rate was observed in 2023, at 5.2% (3/58). Across five regions in China, the prevalence of the S. aureus infection rates was as follows (in descending order): 17.5% (10/57) in southern China, 13.6% (6/44) in eastern China, 12.0% (26/217) in central China, 11.0% (11/100) in northwestern China, and 8.2% (8/97) in northeastern China. Among the 14 provinces studied, Yunan province in southern China exhibited the highest isolation rate at 27.3%, while Gansu province in northwestern China had the lowest isolation rate at 6.3%. The details of the S. aureus isolates are documented in Table 1. The milking disinfection practices are shown in Supplementary Table 1. The cleaning agents, their concentrations, mode of application, time duration for each disinfection, and frequency of cleaning varied across different farms. Notably, some farms employing identical disinfection practices showed similar isolation rates (farms 3, 7, 8, 14, 20, 27, and 32), while others with the same disinfection practices demonstrated different isolation rates (farms 1, 2, 9, 11, 12, 16, 19, 21, 22, 26, and 28). Three farms (5, 10, and 17) utilizing low concentrations of disinfectants showed high isolation rates compared to those in farms 1, 9, 11, 21, and 28 that employed higher concentrations; however, these rates were comparable to those observed in three other farms (2, 12, and 19) using higher concentrations. Moreover, three farms (12, 22, and 31) that implemented a lower frequency of cleaning (twice per day) exhibited higher isolation rates than those in farms 1, 9, 11, 21, and 28 that maintained a higher frequency of cleaning (four times per day).
3.2 Bacterial typing
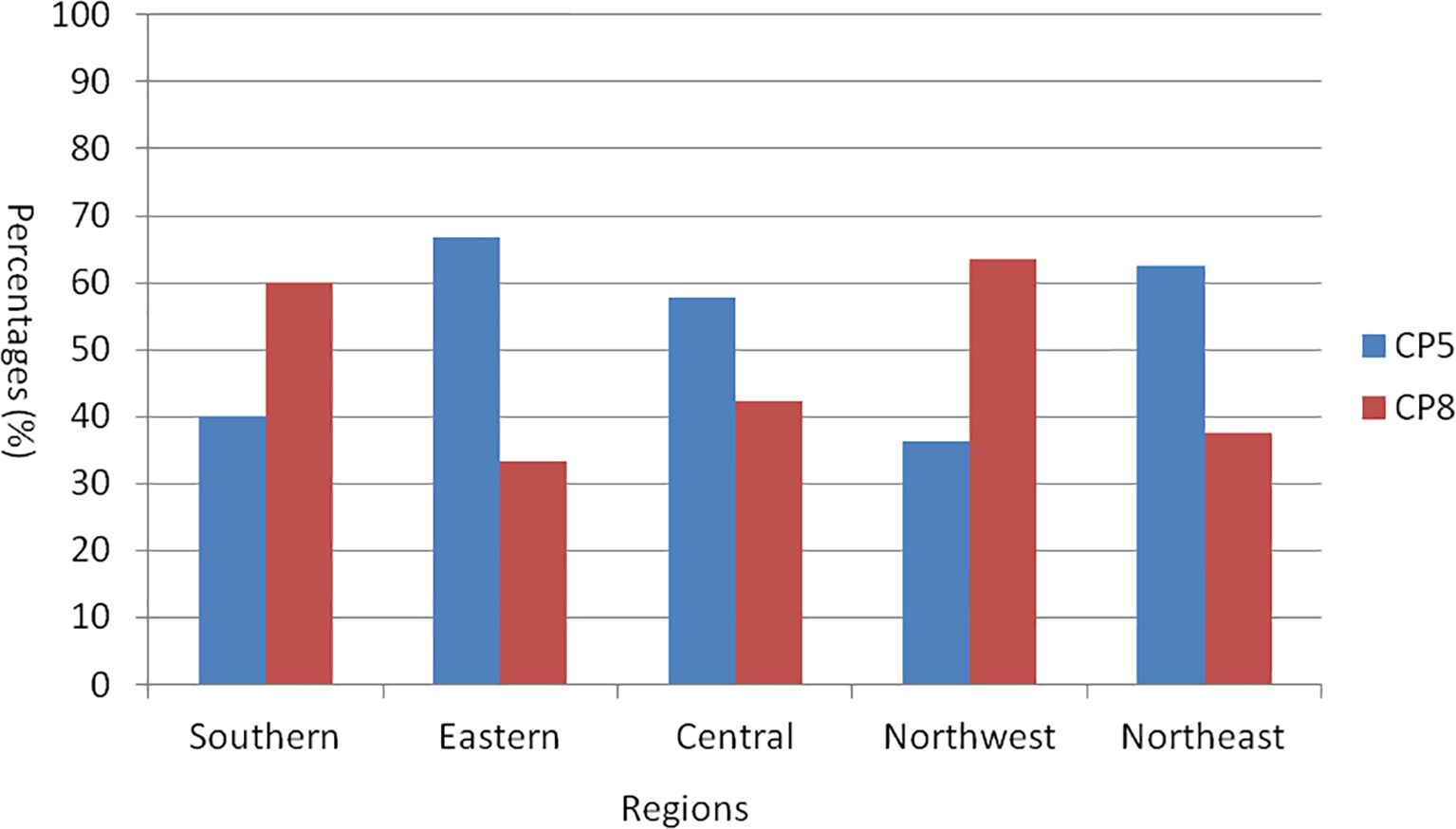
A total of 61 S. aureus isolates were classified into CP5 or CP8 types through PCR detection. The detection rates for CP5 in samples collected from various regions of China were as follows: southern China, 40.0% (4/10); eastern China, 66.7% (4/6); central China, 57.7% (15/26); northwestern China, 36.4% (4/11); and northeastern China, 62.5% (5/8). In contrast, the detection rates for CP8 in the same regions were 60.0% (6/10) in southern China, 33.3% (2/6) in eastern China, 42.3% (11/26) in central China, 63.6% (7/11) in northwest China, and 37.5% (3/8) in northeastern China (Figure 2).
The various spa types and their corresponding geographical distributions are illustrated in Figure 3. A total of 61 S. aureus isolates were differentiated into 18 distinct spa types, with no novel spa types identified in this work. Among the 18 spa types, t521 was the most prevalent, accounting for 12 out of 61 isolates (19.7%), followed by t899 (8/61, 13.1%), t529 (5/61, 8.2%), t172 (4/61, 6.6%), t2788 (4/61, 6.6%), t067 (3/61, 4.9%), t870 (3/61, 4.9%), t114 (3/61, 4.9%), t304 (3/61, 4.9%), t518 (2/61, 3.3%), t459 (2/61, 3.3%), t2844 (2/61, 3.3%), t224 (2/61, 3.3%), t246 (1/61, 1.6%), t267 (1/61, 1.6%), t803 (1/61, 1.6%), t2755 (1/61, 1.6%), and t808 (1/61, 1.6%). In particular, the spa types t521 and t899 were the most widely distributed among goats, being detected in at least three provinces in China, whereas the other spa types exhibited more localized distributions.
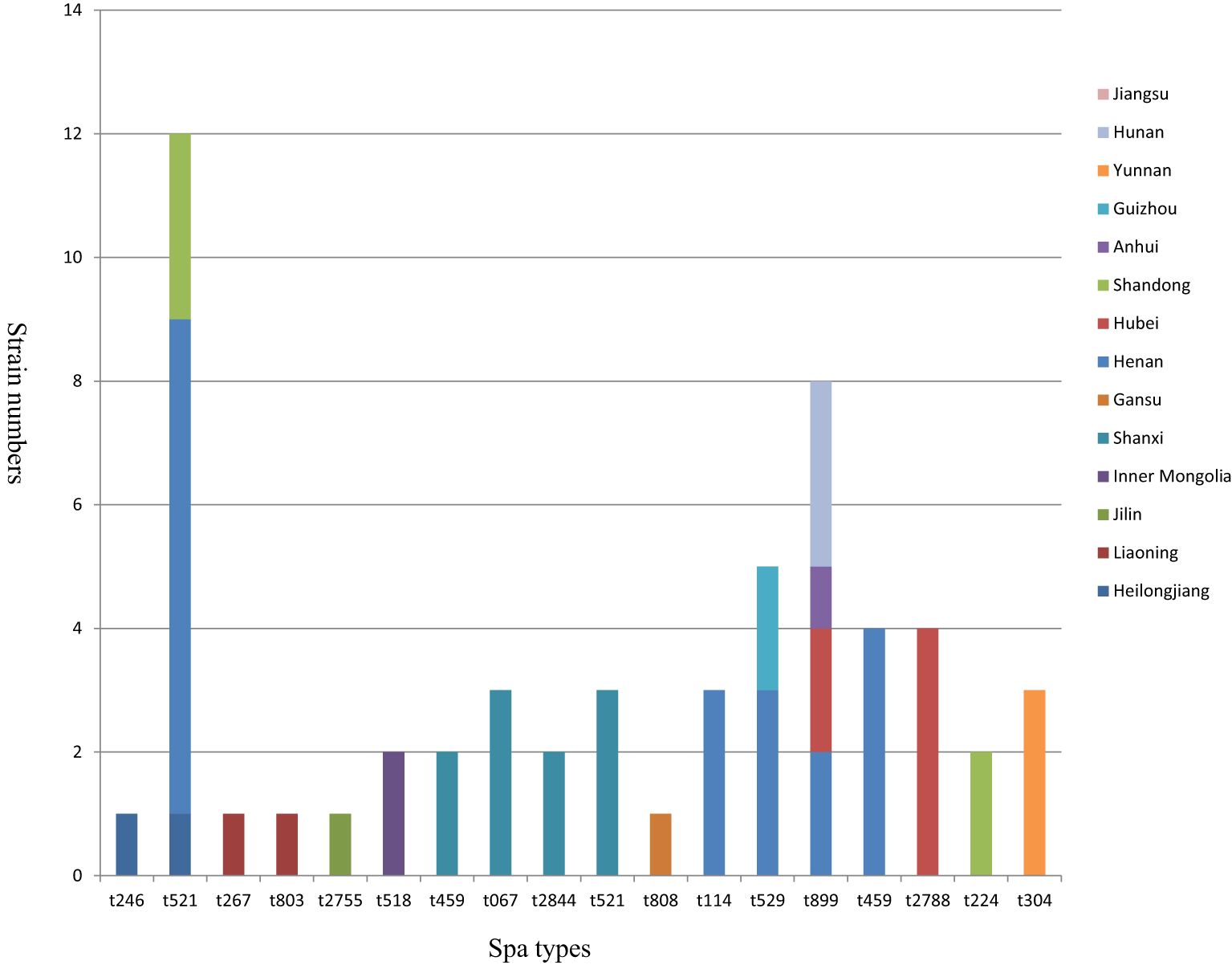
Figure 3. Distribution of 61 S. aureus strains typed by spa. Each column represents different spa types. The height of each column indicates the isolate number of the particular type. Each color represents a strain isolation site.
3.3 Antimicrobial susceptibility
The 61 S. aureus strains isolated from dairy goats in this work were categorized as susceptible, intermediate, or resistant to 18 antibiotics belonging to eight classes. As shown in Table 4, isolates from different provinces exhibited varying degrees of resistance to the antibiotics tested. The resistance rates, ranked from highest to lowest, were as follows: penicillin (95.1%), enrofloxacin (82.0%), kanamycin (78.7%), levofloxacin (77.0%), oxacillin (75.4%), amikacin (73.8%), gentamicin (68.9%), tetracycline (62.3%), lincomycin (59.0%), erythromycin (47.5%), clindamycin (41.0%), doxycycline (39.3%), cefalotin (37.7%), ceftiofur (36.1%), chloramphenicol (29.5%), azithromycin (26.2%), sulfisoxazole (24.6%), and trimethoprim-sulfamethoxazole (11.5%). Additionally, 52 isolates (85.2%) were found to be multiresistant to three or more antibiotics, while one isolate (1.6%) demonstrated resistance to all antibiotics tested.
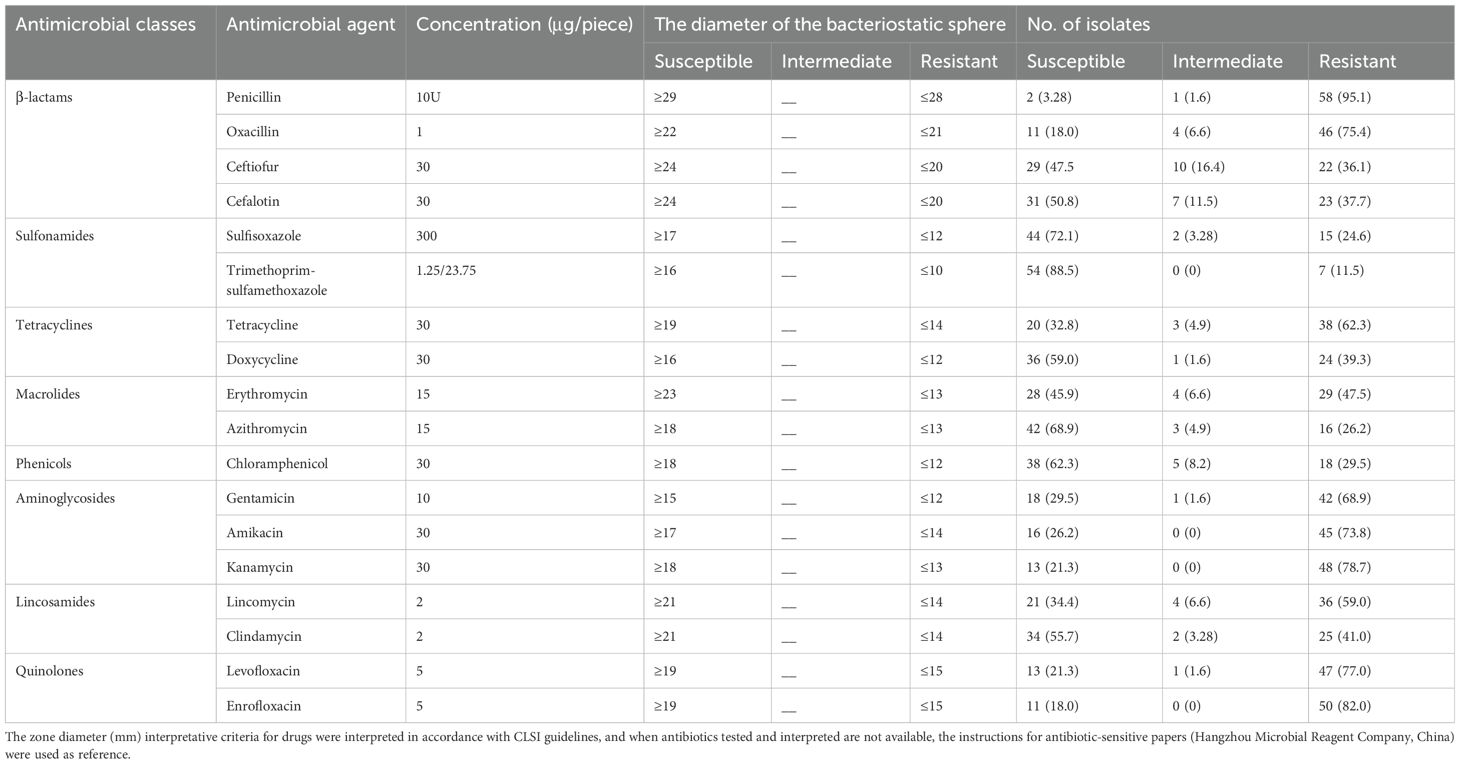
Table 4. The antimicrobial discs, breakpoints, and the distributions of antimicrobial resistances for 61 S. aureus strains isolated from goat milk samples with clinical mastitis.
In a further analysis from a geographical perspective, the average percentage of resistant strains to eight classes of antibiotics in five regions of China is recorded in Table 5. The results showed that isolates from all regions exhibited a higher sensitivity to sulfonamides, macrolides, and phenicols, while also being more resistant to quinolones and aminoglycosides. Notably, the average percentages were reobserved in the other four regions, with the overall average percentage of resistant strains across all antibiotic classes being the lowest at 46.1% (Table 5). In addition, in both CP5 and CP8 types, no significant difference was observed between S. aureus types and the antimicrobial resistance phenotypes among eight antimicrobial classes (p > 0.05).
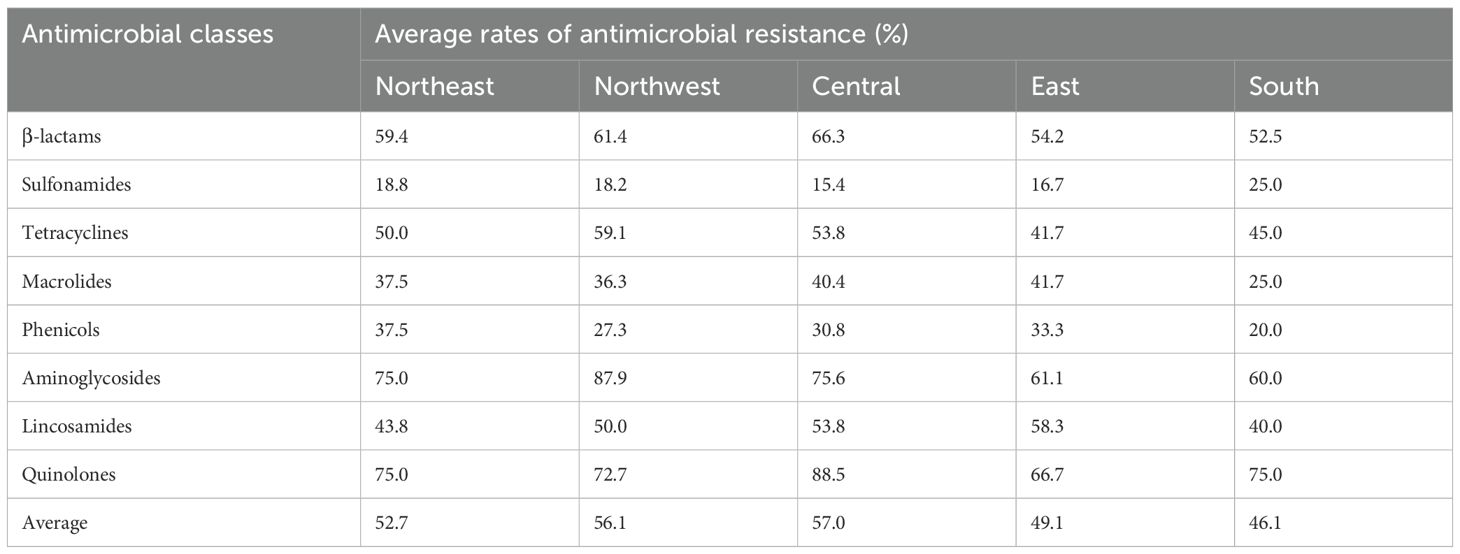
Table 5. Average rates of antimicrobial resistance of S. aureus isolated from goat milk samples in five regions of China.
3.4 Detection of antimicrobial resistance genes
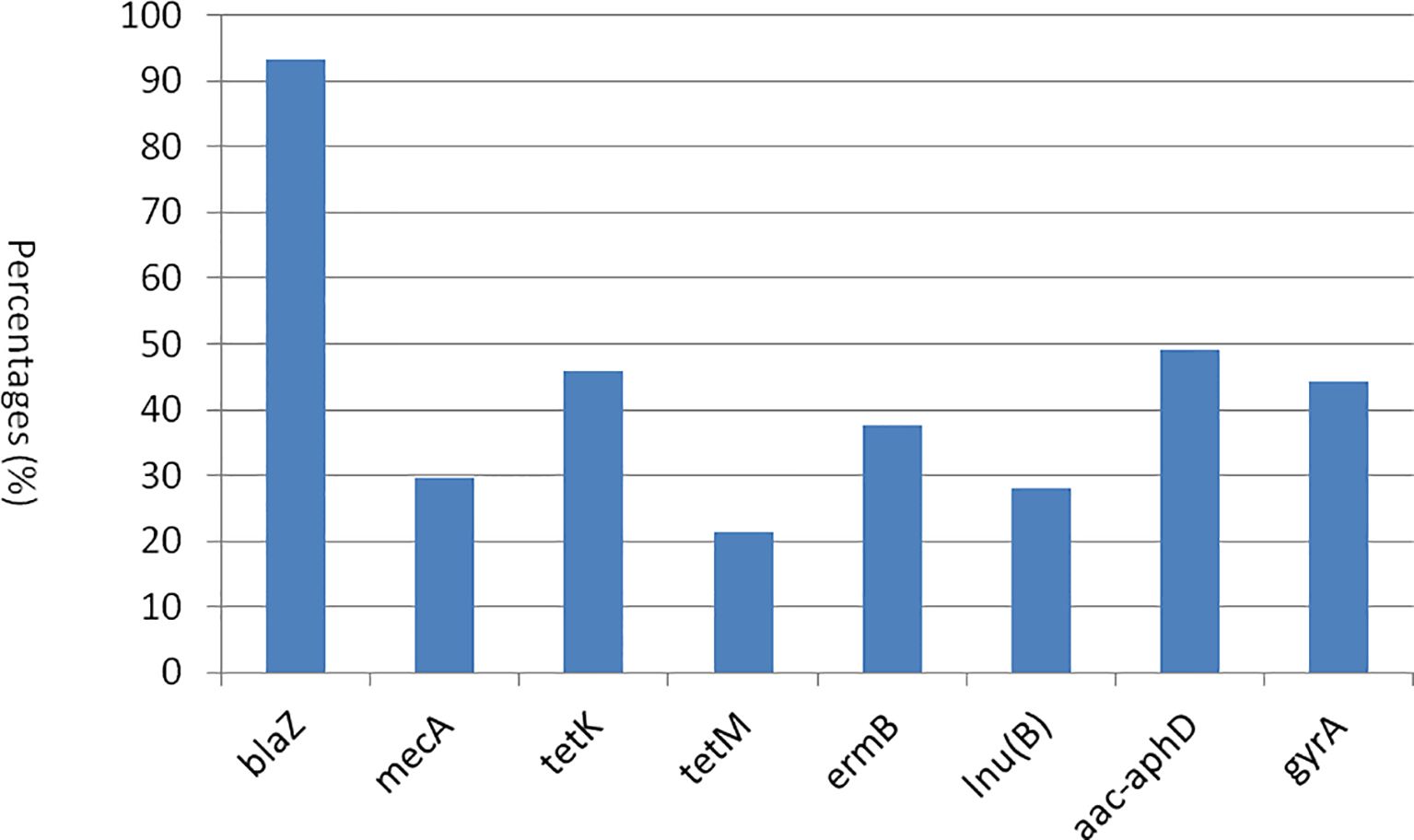
A total of 17 genes associated with resistance to eight distinct classes of antimicrobials were screened using PCR. Subsequently, eight genes from various classes, as well as multiple genes exhibiting a spectrum of resistance, were identified. Among the β-lactam resistance genes, blaZ was the most prevalent in 93.4% of the strains, followed by mecA at 29.5%. Notably, the femA and femB genes and dfrD genes were absent in the strains examined. In the context of tetracycline resistance, the tetK gene was more frequently observed (45.9%) compared to tetM gene (21.3%). Among the macrolide resistance genes, only the ermB gene (37.7%) was identified, with no detection of ermA and ermC genes. The fexA gene, which is associated with phenicol resistance, was absent in all strains analyzed. Furthermore, the lnu(B) gene, linked to lincosamide resistance, was found in 27.9% of the strains. The aac-aphD gene, associated with aminoglycoside resistance, was present in 49.2% of the samples. Moreover, among the quinolone resistance genes, only gyrA was detected (44.3%), while the parC gene was not found in any strains (Figure 4). As shown in Supplementary Table 2, 12 strains harbored only one resistance gene, whereas the remaining 49 strains possessed two or more resistance genes. Among the various patterns of multiple gene resistance, the combination of blaZ and tetK genes was the most prevalent, occurring in 6.6% of the strains. This was followed by the pattern comprising blaZ+mecA+tetK+lnu(B)+aac-aphD+gyrA, which was observed in 4.9% of the strains (3/61). Each of the seven additional patterns [blaZ+mecA+tetM, blaZ+tetK+lnu(B)+aac-aphD+gyrA, blaZ+tetK+ermB, blaZ+mecA+ermB+aac-aphD+gyrA, blaZ+mecA+tetK, blaZ+tetK+ermB+aac-aphD+gyrA, and blaZ+tetK+lnu(B)] was identified in two strains (3.3%). Most importantly, the remaining 28 strains exhibited unique patterns, each containing two or more antibiotic resistance genes. In this study, the detection of blaZ, mecA, tetK, tetM, ermB, lnu(B), aac-aphD, and gyrA in the corresponding antimicrobial resistance phenotypes was statistically significant (p < 0.05) (Table 6). Furthermore, in both CP5 and CP8 types, no significant difference was observed between S. aureus types and the antimicrobial resistance gene distributions among eight resistance genes (p > 0.05).
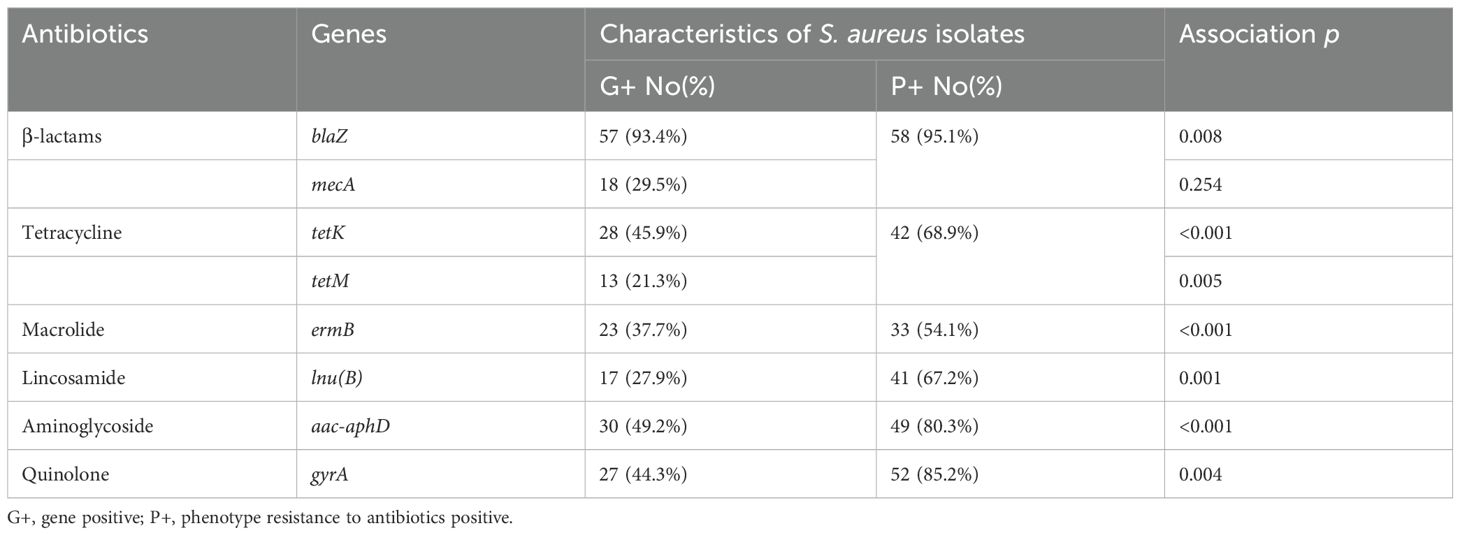
Table 6. Comparison of genotypic and phenotypic characteristics for antimicrobial resistance in S. aureus isolates.
3.5 Detection of virulence genes
The presence and distribution of virulence genes in 61 S. aureus strains are presented in Figure 5. All S. aureus isolates were found to possess the adhesin factor gene clfA, with a prevalence of 100% (61 strains). The majority of these strains also contained the fnbA gene (98.4%, n = 60) and clfB gene (96.7%, n = 59). In contrast, the ebpS gene was detected only in 44.3% (n = 27) of the strains. Regarding enterotoxin genes, the frequencies of seb, sec, sed, sei, and sem genes were observed in 13.1% (n = 8), 9.8% (n = 6), 6.6% (n = 4), 11.5% (n = 7), and 15.7% (n = 27) of the strains, respectively. Additionally, the toxic shock syndrome toxin gene tst was identified in 21.3% (n = 13) of the strains. Notably, none of the S. aureus isolates were positive for sea, see, seg, seh, sej, sen, seo, eta, and etb genes. In this study, in both CP5 and CP8 types, no significant difference was observed between S. aureus types and the virulence gene distributions among 10 positive virulence genes (p > 0.05).
4 Discussion
In this work, the nucleotide sequences of the nuc gene from the S. aureus isolates exhibited homology with other S. aureus strains in GenBank, ranging from 97.6% to 99.3%. Similar homology ranges were also observed in isolates from cows in China (Ning et al., 2023). The isolation rate of S. aureus was 11.8%, which is lower than the rates reported in goats from Shaanxi province (58.3%, 28/48) (Liu et al., 2023) and Yunnan province (15.24%, 32/209) (Zhao et al., 2015) in China, as well as lower than that found in goats in Indonesia (25.58%, 66/258) (Praja et al., 2023) and in Kenya (22.0%, 40/182) (Kabui et al., 2024). However, this rate is higher than that found in dairy goats in Portugal (6.9%, 35/508) (Andrade et al., 2021). Moreover, the isolation rate is comparable to those reported in four studies involving cows with mastitis in China, which reported rates of 10.1%, 11.1%, 10.1%, and 10.2%, respectively (Gao et al., 2017; Zhang et al., 2018; Zhang et al., 2022; Ning et al., 2023), and lower than the rate at 13.6% in Ningxia in China (Wang et al., 2017). The findings indicate that the prevalence of S. aureus in northeast and northwest China is lower than that observed in the other three regions and the overall average prevalence rate. Several management practices have been proven to be effective in controlling S. aureus mastitis, including post-milking teat dipping in antiseptic solutions and maintaining cows in a clean, dry, and suitable environment for the animals (Kerro Dego and Vidlund, 2024). These factors, as well as the use of antiseptic solutions, may influence the spread of S. aureus. Notably, isolation rates were low in most farms utilizing high concentrations of povidone iodine or sodium hypochlorite with extended disinfection times, with only a few exceptions exhibiting the opposite trend. These results suggest the necessity of selecting appropriate milking disinfection practices. Furthermore, other factors such as environmental temperature and humidity, the hygiene status of farms, and supplementation with vitamin E and selenium in goats (Ruegg, 2017) were not included in the analysis in this work. It is noteworthy that the prevalence of the pathogen has decreased to below 10% since 2022, likely due to the implementation of a national antimicrobial resistance monitoring and surveillance program. However, the limited number of samples in this study restricts the effectiveness of the evaluation, indicating that future investigations should include a larger sample size.
Capsular polysaccharide serotyping of S. aureus has been employed to predict and analyze the epidemiologic trends of isolates in dairy animals suffering from mastitis, with 11 serotypes documented in the literature (Poutrel et al., 1988). The detection rate of CP5 in our study was higher than previously reported in cattle in China, which were 42.1% (32/76) (Ning et al., 2023) and 35.57% (106/298) (Zhang et al., 2022), and also exceeded the rates observed in cattle in South Korea (Han et al., 2000). This information suggests that the distribution of CPs in S. aureus may be influenced by host species and geographical location. Most importantly, capsular polysaccharides are critical for the immunogenicity of S. aureus and have been proven to protect vaccinated mice from lethal challenges with the bacterium (Han et al., 2000). Our study provides a valuable reference for the development of vaccines against goat mastitis. Furthermore, among the 18 spa types in this study, t521 was the most prevalent, accounting for 19.7% (12/61) of the strains. This spa type has been previously detected in cattle at a rate of 2.01% in China, where it is considered a minor prevalent spa type (Zhang et al., 2022). Other notable spa types included t899 (13.1%, 8/61) and t529 (8.2%, 5/61), among others. These spa types have also been recorded in other reports involving isolates from cattle, at low detection rates (Zhang et al., 2022; Ning et al., 2023; Zhu et al., 2024). Additionally, isolates from cattle showed a high diversity of spa types. A national survey across 16 provinces identified 48 spa types among 298 S. aureus strains, with t459 as the predominant type (18.79%) (Zhang et al., 2022). In Hunan province, 20 spa types were identified among 76 S. aureus strains, with t796 as the most common type (23.68%) (Ning et al., 2023). Another investigation across 15 provinces found 17 spa types among 72 S. aureus strains, with t2734 as the main type (17.09%) (Zhu et al., 2024). Additionally, an investigation across nine provinces found 24 spa types among 103 S. aureus strains, with t359 as the main type (26.21%) (Zhang et al., 2018). However, among these three predominant types in goats, only t459 was detected at a rate of 3.3% in our study. In Greece, nine spa types were detected in 29 S. aureus strains, including t3586 (8), t4038 (6), t1534 (1), t306 (2), and t1773 (1) (Kotzamanidis et al., 2021); these types were not observed in the present study. Comparing these results, we speculate that S. aureus may spread between cattle and goat species, and isolates from different countries or regions often exhibit distinct molecular types. Notably, the dairy goats in this work were raised in backyard farms in a free-range style, sharing the same grassland with cattle, which underscores the necessity of controlling transmission between these two dairy species.
In China, antimicrobial therapy is the primary strategy for controlling goat mastitis; however, recent reports indicate an increase in bacterial resistance to antimicrobial agents among S. aureus isolates (Zhang et al., 2022; Ning et al., 2023; Zhu et al., 2024). Antimicrobial susceptibility testing is crucial for guiding the selection of appropriate antimicrobial agents for treating infections. In this work, the highest resistance was noted against penicillin, aligning with findings from several studies on S. aureus isolates in dairy cows with mastitis in China (Zhang et al., 2018; Cheng et al., 2019; Zhang et al., 2022). Given that penicillin is widely used in mastitis treatment, the selective pressure exerted on goats will likely accelerate the emergence of drug resistance. In contrast, studies conducted in Greece reported relatively low resistance rates for S. aureus isolates from goats, sheep, and cows, with rates of 6.9% (2/29), 16.5% (16/97), and 27.8% (10/36), respectively (Kotzamanidis et al., 2021). In Portugal, Staphylococcal isolates from goats and sheep exhibited a non-susceptible rate to penicillin of 27.7% (38/137) (Andrade et al., 2021). Our study indicated that the prevalence of penicillin-resistant S. aureus in dairy goat farms in China is higher than that observed in other countries. Additionally, in five regions, the isolates demonstrated high resistance to quinolones (ranging from 66.7% to 88.5%) and aminoglycosides (60.0% to 87.9%), which exceeded the resistance levels reported for bovine S. aureus in China (Zhang et al., 2022; Ning et al., 2023) and caprine S. aureus in Indonesia (Praja et al., 2023). This trend is not unexpected, as quinolones and aminoglycosides are among the most commonly used antibiotics in dairy animals in China (Wang, 2014). Their extensive use likely contributes to the increased resistance in S. aureus. Furthermore, the resistance rates of the isolates to the remaining five antimicrobial classes were lower than those observed in the three aforementioned antimicrobial classes, with sulfonamides exhibiting the lowest resistance rate. These findings underscore the urgency for judicious use of antimicrobials in the treatment of goat S. aureus mastitis. Therefore, clinical use of β-lactams, quinolones, and aminoglycosides should be minimized, unless guided by sensitive tests. Based on our results, sulfonamides should be considered as a preferential treatment option for S. aureus mastitis.
The two primary mechanisms employed by S. aureus to develop β-lactam resistance involve the synthesis of penicillinase associated with the blaZ gene and the production of penicillin-binding protein 2a linked to the mecA, femA, and femB genes (Kobayashi et al., 1994; Vannuffel et al., 1998; Choi et al., 2003; Olsen et al., 2006). In this work, the prevalence rates of blaZ and mecA genes were higher than the rates observed in water buffalo in Guangdong province, China (19.5% and 16.8%, respectively) (Zhang et al., 2023). These findings are, however, compared to those reported in cows in China, where the rates were 92.9% and 24.5%, respectively (Zhang et al., 2022), higher than the rates documented in cows in Pakistan (55.3% and 17.0%, respectively) (Haq et al., 2024). In S. aureus isolates, tetracycline resistance is mediated by the activation of efflux pumps encoded by the tetM and tetK genes (Al-Ashmawy et al., 2016; Merz et al., 2016; Akkou et al., 2018). Resistance to aminoglycosides is conferred by genes encoding aminoglycoside-modifying enzymes, such as aac-aphD gene (Strommenger et al., 2003). Quinolone resistance is associated with genes encoding the type II topoisomerase enzymes, specifically DNA gyrase (gyrA) and topoisomerase IV (parC) (Schmitt-Van-de-Leemput and Zadoks, 2007). Lincosamide resistance is mediated by the lnu(B) gene, which encodes a lincosamide inactivating nucleotidyl transferase (Bozdogan et al., 1999). As in this study, these resistance genes have also been detected in isolates from cows and water buffalo in China with varying prevalence rates (Zhang et al., 2022; Zhang et al., 2023). In isolates from goats in Pakistan, mecA, blaZ, tetK, aac-aphD, and gyrA genes were identified at rates of 35.9%, 45.6%, 32.5%, 50.49%, and 0%, respectively (Javed et al., 2024). To date, the documentation of antibiotic resistance genes in S. aureus isolates from goats in China is limited. Our data indicate that all isolates examined carry at least one resistance gene. Commonly, the correlation between antibiotic resistance genes and phenotypes was not significant (Bissong and Ateba, 2020), as several factors may affect bacterial antibiotic resistance phenotypes, including environmental changes, strain activity, and frequent use of antimicrobial agents. In contrast, genotypes are determined only by internal gene factors. However, in this study, the detection of blaZ, mecA, tetK, tetM, ermB, lnu(B), aac-aphD, and gyrA genes in the corresponding antimicrobial resistance phenotypes was statistically significant (p < 0.05). Similar correlations have also been observed in S. aureus isolates from cows in Hunan province in China (Ning et al., 2023). Although several isolates exhibited resistance to sulfonamides and phenicols, the corresponding resistance genes were not detected. This discrepancy may be attributed to the screening of only one or a few resistance genes. Consequently, a whole-genome sequencing approach in future research would provide a more comprehensive and unbiased antimicrobial resistance profile of these isolates. The high prevalence of antibiotic resistance genes among the 61 S. aureus isolates in this work underscores the issue of antibiotic misuse in dairy goats. It is imperative to exercise caution in antibiotic use and to monitor antibiotic-resistant genes in S. aureus isolates on farms.
Capsular polysaccharides have been identified as key pathogenicity factors associated with S. aureus (Han et al., 2000). The results of CP typing achieved a 100% positive rate. This finding is consistent with observations of S. aureus isolates from cows with mastitis in China (Zhang et al., 2022) and is notably higher than the detection rates reported in Jordan (91.3%) (Gharaibeh and Abu-Qatouseh, 2022), Australia (70.1%), and India (60.8%) (Gogoi-Tiwari et al., 2015). The lower detection rates in these regions may be attributed to the persistence of S. aureus in chronically infected hosts (Ambroggio et al., 2018). Adhesins are regarded as the most critical virulence factors during the initial stages of S. aureus infection, facilitating the invasion of host cells (Bien et al., 2011). In the present study, clfA, fnbA, clfB, and ebpS genes have been observed, which were also detected in isolates from cows (Zhang et al., 2018; Zhang et al., 2022) and from water buffalo (Zhang et al., 2023) in China. Enterotoxins are extracellular, low-molecular-weight proteins that exhibit resistance to heat, cold, and proteolytic enzymes (Suzuki et al., 2020), and they can elicit emetic and superantigenic activities in both animals and humans (Liu et al., 2022). In our research, the prevalence of seb, sec, sed, sei, and sem genes has been verified. A study involving strains from cows reported the prevalence of the sea, seb, sec, and sed genes at 17.5%, 16.4%, 7.4%, and 1.7%, respectively (Zhang et al., 2022). In Ningxia province, China, the prevalence of sei, sen, and seu was 44.0% each, followed by seo, tst, and etB (28.0% each), etA (24.0%), sem and sep (16.0% each), seb, sec, sed, and sek (12.0% each), and sea and seh genes (8.0% each); the seg, sej, and ser genes were present in 4.0% of the isolates (Wang et al., 2017). Another investigation of strains from water buffalo in Guangdong province, China, reported prevalence rates of 3.5% for sea, 15.9% for sec, and 9.7% for see gene (Zhang et al., 2023). These findings suggest that the enterotoxin gene content of S. aureus varies across different hosts and geographical regions. Toxic shock syndrome toxin, a prototypical superantigen encoded by the tsst gene, is known to induce toxic shock syndrome (Zhu et al., 2023). The prevalence of the tsst gene in S. aureus isolates from cows and water buffalo in China was reported to be 23.5% and 25%, respectively (Zhang et al., 2022; Zhang et al., 2023), which is higher than the rate observed in this study. Additionally, negative results for the virulence genes eta and etb were also noted in bovine isolates in China (Zhang et al., 2022). Therefore, it is imperative to monitor the epidemiology of virulence genes to mitigate public health risks.
5 Conclusions
The presence of S. aureus in goats with mastitis have been confirmed in 14 provinces. Notably, most S. aureus isolates exhibited multidrug resistance with diverse resistance patterns, and the diversity of antimicrobial resistance profiles associated with corresponding resistance genes (p < 0.05) was reported for the first time in S. aureus from caprine mastitis. Sulfonamides should be considered preferentially for the treatment of S. aureus mastitis. Additionally, spa types in this study have also been recorded in other reports involving isolates from cattle in China, suggesting potential cross-species transmissions of S. aureus between cattle and goats. Moreover, the high occurrence of the virulence genes clfA, fnbA, and clfB genes tested in this study in S. aureus underscores their pathogenic potential in causing mastitis in the studied area. Overall, this work provides a primary source for the molecular epidemiology of S. aureus in dairy goat herds in China.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The animal studies were approved by Animal Welfare and Ethics Committee of Nanyang Normal University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
HS: Conceptualization, Funding acquisition, Writing – original draft. GL: Data curation, Methodology, Resources, Validation, Writing – review & editing. DL: Software, Visualization, Writing – review & editing. HZ: Methodology, Resources, Writing – review & editing. SJ: Methodology, Resources, Writing – review & editing. YH: Methodology, Resources, Writing – review & editing. LW: Methodology, Resources, Writing – review & editing. TL: Methodology, Resources, Writing – review & editing. LY: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The Natural Science Foundation of Henan province (grant no. 242300421334), the Scientific and Technological Project of Henan Province (grant no. 222102110260), and the NanYang Science and Technology Research Project (KJGG136) supported the sample collection, analysis, and interpretation of data in this study.
Acknowledgments
We thank AJE (www.aje.com) for its linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1533844/full#supplementary-material
References
Akkou, M., Bentayeb, L., Ferdji, K., Medrouh, B., Bachtarzi, M.-A., Ziane, H., et al. (2018). Phenotypic characterization of Staphylococci causing mastitis in goats and microarray-based genotyping of Staphylococcus aureus isolates. Small Ruminant Res. 169, 29–33. doi: 10.1016/j.smallrumres.2018.10.015
Al-Ashmawy, M. A., Sallam, K. I., Abd-Elghany, S. M., Elhadidy, M., Tamura, T. (2016). Prevalence, molecular characterization, and antimicrobial susceptibility of methicillin-resistant Staphylococcus aureus isolated from milk and dairy products. Foodborne Pathog. Dis. 13, 156–162. doi: 10.1089/fpd.2015.2038
Ambroggio, M. B., Perrig, M. S., Camussone, C., Pujato, N., Bertón, A., Gianneechini, E., et al. (2018). Survey of potential factors involved in the low frequency of CP5 and CP8 expression in Staphylococcus aureus isolates from mastitis of dairy cattle from Argentina, Chile, and Uruguay. J. Appl. Genet. 59, 357–363. doi: 10.1007/s13353-018-0443-8
Andrade, N. C., Laranjo, M., Costa, M. M., Queiroga, M. C. (2021). Virulence factors in staphylococcus associated with small ruminant mastitis: biofilm production and antimicrobial resistance genes. Antibiotics (Basel) 10 (6), 633. doi: 10.3390/antibiotics10060633
Argudín, M. A., Tenhagen, B. A., Fetsch, A., Sachsenröder, J., Käsbohrer, A., Schroeter, A., et al. (2011). Virulence and resistance determinants of German Staphylococcus aureus ST398 isolates from nonhuman sources. Appl. Environ. Microbiol. 77, 3052–3060. doi: 10.1128/aem.02260-10
Bien, J., Sokolova, O., Bozko, P. (2011). Characterization of virulence factors of Staphylococcus aureus: novel function of known virulence factors that are implicated in activation of airway epithelial proinflammatory response. J. Pathog. 2011, 601905. doi: 10.4061/2011/601905
Bissong, M. E. A., Ateba, C. N. (2020). Genotypic and phenotypic evaluation of biofilm production and antimicrobial resistance in Staphylococcus aureus isolated from milk, North West Province, South Africa. Antibiotics (Basel) 9 (4), 156. doi: 10.3390/antibiotics9040156
Bochniarz, M., Adaszek, Ł, Dzięgiel, B., Nowaczek, A., Wawron, W., Dąbrowski, R., et al. (2016). Factors responsible for subclinical mastitis in cows caused by Staphylococcus chromogenes and its susceptibility to antibiotics based on bap, fnbA, eno, mecA, tetK, and ermA genes. J. Dairy Sci. 99, 9514–9520. doi: 10.3168/jds.2016-11723
Bozdogan, B., Berrezouga, L., Kuo, M. S., Yurek, D. A., Farley, K. A., Stockman, B. J., et al. (1999). A new resistance gene, linB, conferring resistance to lincosamides by nucleotidylation in Enterococcus faecium HM1025. Antimicrob. Agents Chemother. 43, 925–929. doi: 10.1128/AAC.43.4.925
Brakstad, O. G., Aasbakk, K., Maeland, J. A. (1992). Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 30, 1654–1660. doi: 10.1128/jcm.30.7.1654-1660.1992
Bujňáková, D., Karahutová, L. (2024). Molecular characteristics and antibiotic resistance of Staphylococcus aureus and Staphylococcus haemolyticus isolated from bovine mastitis. Res. Vet. Sci. 177, 105365. doi: 10.1016/j.rvsc.2024.105365
Cheng, J., Qu, W., Barkema, H. W., Nobrega, D. B., Gao, J., Liu, G., et al. (2019). Antimicrobial resistance profiles of 5 common bovine mastitis pathogens in large Chinese dairy herds. J. Dairy Sci. 102, 2416–2426. doi: 10.3168/jds.2018-15135
Choi, S. M., Kim, S. H., Kim, H. J., Lee, D. G., Choi, J. H., Yoo, J. H., et al. (2003). Multiplex PCR for the detection of genes encoding aminoglycoside modifying enzymes and methicillin resistance among Staphylococcus species. J. Korean Med. Sci. 18, 631–636. doi: 10.3346/jkms.2003.18.5.631
CLSI (2018). Performance Standards for Antimicrobial Susceptibility Testing. 29th Edition (Pennsylvania, USA: Clinical and Laboratory Standards Institute).
Costa, F. N., Belo, N. O., Costa, E. A., Andrade, G. I., Pereira, L. S., Carvalho, I. A., et al. (2018). Frequency of enterotoxins, toxic shock syndrome toxin-1, and biofilm formation genes in Staphylococcus aureus isolates from cows with mastitis in the Northeast of Brazil. Trop. Anim. Health Production 50, 1089–1097. doi: 10.1007/s11250-018-1534-6
Gao, J., Barkema, H. W., Zhang, L., Liu, G., Deng, Z., Cai, L., et al. (2017). Incidence of clinical mastitis and distribution of pathogens on large Chinese dairy farms. J. Dairy Sci. 100, 4797–4806. doi: 10.3168/jds.2016-12334
Gharaibeh, M. H., Abu-Qatouseh, L. F. (2022). First molecular characterization of capsule expression and antibiotic susceptibility profile of Staphylococcus aureus isolates from bovine mastitis in Jordan. Vet. World 15, 2269–2274. doi: 10.14202/vetworld.2022.2269-2274
Gogoi-Tiwari, J., Babra Waryah, C., Sunagar, R., Veeresh, H. B., Nuthanalakshmi, V., Preethirani, P. L., et al. (2015). Typing of Staphylococcus aureus isolated from bovine mastitis cases in Australia and India. Aust. Vet. J. 93, 278–282. doi: 10.1111/avj.12349
Han, H., Pak, S. I., Guidry, A. (2000). Prevalence of Capsular Polysaccharide (CP) Types of Staphylococcus aureus Isolated from Bovine Mastitic Milk and Protection of S. aureus Infection in Mice with CP Vaccine. J. Veterinary Med. Sci. 62, 1331–1333. doi: 10.1292/jvms.62.1331
Haq, I. U., Kamal, M., Swelum, A. A., Khan, S., Ríos-Escalante, P. R. L., Usman, T. (2024). Alarming multidrug resistance in Staphylococcus aureus isolated from raw milk of cows with subclinical mastitis: Antibiotic resistance patterns and occurrence of selected resistance genes. PloS One 19, e0301200. doi: 10.1371/journal.pone.0301200
Hutu, I., Lungu, B. C., Spataru, I. I., Torda, I., Iancu, T., Barrow, P. A., et al. (2024). Microbiological and molecular investigation of antimicrobial resistance in Staphylococcus aureus isolates from western Romanian dairy farms: an epidemiological approach. Anim. (Basel) 14 (15), 2266. doi: 10.3390/ani14152266
Jarraud, S., Mougel, C., Thioulouse, J., Lina, G., Meugnier, H., Forey, F., et al. (2002). Relationships between Staphylococcus aureus genetic background, virulence factors, agr groups (alleles), and human disease. Infect. Immun. 70, 631–641. doi: 10.1128/iai.70.2.631-641.2002
Javed, M. U., Ijaz, M., Durrani, A. Z., Ali, M. M. (2024). Molecular insights into antimicrobial resistant Staphylococcus aureus strains: A potential zoonosis of goat origin. Microb. Pathog. 196, 106961. doi: 10.1016/j.micpath.2024.106961
Kabui, S., Kimani, J., Ngugi, C., Kagira, J. (2024). Prevalence and antimicrobial resistance profiles of mastitis causing bacteria isolated from dairy goats in Mukurweini Sub-County, Nyeri County, Kenya. Vet. Med. Sci. 10, e1420. doi: 10.1002/vms3.1420
Kerro Dego, O., Vidlund, J. (2024). Staphylococcal mastitis in dairy cows. Front. Vet. Sci. 11. doi: 10.3389/fvets.2024.1356259
Kobayashi, N., Wu, H., Kojima, K., Taniguchi, K., Urasawa, S., Uehara, N., et al. (1994). Detection of mecA, femA, and femB genes in clinical strains of staphylococci using polymerase chain reaction. Epidemiol. Infect. 113, 259–266. doi: 10.1017/s0950268800051682
Kotzamanidis, C., Vafeas, G., Giantzi, V., Anastasiadou, S., Mygdalias, S., Malousi, A., et al. (2021). Staphylococcus aureus isolated from ruminants with mastitis in northern Greece dairy herds: genetic relatedness and phenotypic and genotypic characterization. Toxins (Basel) 13 (3), 176. doi: 10.3390/toxins13030176
Lee John, H. (2003). Methicillin (Oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl. Environ. Microbiol. 69, 6489–6494. doi: 10.1128/AEM.69.11.6489-6494.2003
Liu, C., Shen, Y., Yang, M., Chi, K., Guo, N. (2022). Hazard of Staphylococcal enterotoxins in food and promising strategies for natural products against virulence. J. Agric. Food Chem. 70, 2450–2465. doi: 10.1021/acs.jafc.1c06773
Liu, X., Zuo, J., Teng, J., Yang, L., Guo, J., Liu, L., et al. (2023). Antibiofilm potential of luteolin against multidrug-resistant Staphylococcus aureus isolated from dairy goats and farm environments. Environ. pollut. 335, 122274. doi: 10.1016/j.envpol.2023.122274
Luo, J., Shi, H., Wang, J., Zhang, F., Zhen, H., Ji, Z., et al. (2019). A summary of the development of chinese dairy goat industry-development trends and characteristics. China dairy cattle 09), 1–11. doi: 10.19305/j.cnki.11-3009/s.2019.09.001
Merz, A., Stephan, R., Johler, S. (2016). Staphylococcus aureus isolates from goat and sheep milk seem to be closely related and differ from isolates detected from bovine milk. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.00319
Mohseni, M., Rafiei, F., Ghaemi, E. A. (2018). High frequency of exfoliative toxin genes among Staphylococcus aureus isolated from clinical specimens in the north of Iran: Alarm for the health of individuals under risk. Iran J. Microbiol. 10, 158–165. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC6087700/pdf/IJM-10-158.pdf.
Moodley, A., Stegger, M., Bagcigil, A. F., Baptiste, K. E., Loeffler, A., Lloyd, D. H., et al. (2006). spa typing of methicillin-resistant Staphylococcus aureus isolated from domestic animals and veterinary staff in the UK and Ireland. J. Antimicrobial Chemotherapy 58, 1118–1123. doi: 10.1093/jac/dkl394
Ning, K., Zhou, R., Li, M. (2023). Antimicrobial resistance and molecular typing of Staphylococcus aureus isolates from raw milk in Hunan Province. PeerJ 11, e15847. doi: 10.7717/peerj.15847
Olechnowicz, J., Jaśkowski, J. M. (2014). Mastitis in small ruminants. Medycyna Weterynaryjna 70, 67–72. Available at: http://www.medycynawet.edu.pl/archives/summary/454-summary-2014/316-summary-2014-02/5072-summary-med-weter-70-2-67-72-2014.
Olsen, J. E., Christensen, H., Aarestrup, F. M. (2006). Diversity and evolution of blaZ from Staphylococcus aureus and coagulase-negative staphylococci. J. Antimicrob. Chemother. 57, 450–460. doi: 10.1093/jac/dki492
Peacock, S. J., Moore, C. E., Justice, A., Kantzanou, M., Story, L., Mackie, K., et al. (2002). Virulent combinations of adhesin and toxin genes in natural populations of Staphylococcus aureus. Infect. Immun. 70, 4987–4996. doi: 10.1128/iai.70.9.4987-4996.2002
Poutrel, B., Boutonnier, A., Sutra, L., Fournier, J. M. (1988). Prevalence of capsular polysaccharide types 5 and 8 among Staphylococcus aureus isolates from cow, goat, and ewe milk. J. Clin. Microbiol. 26 (1), 38–40. doi: 10.1128/jcm.26.1.38-40.1988
Praja, R. N., Yudhana, A., Saputro, A. L., Hamonangan, J. M. (2023). The first study on antimicrobial resistance of Staphylococcus aureus isolated from raw goat milk associated with subclinical mastitis in Siliragung Subdistrict, East Java, Indonesia. Vet. World 16, 786–791. doi: 10.14202/vetworld.2023.786-791
Rodriguez, M., Hogan, P. G., Satola, S. W., Crispell, E., Wylie, T., Gao, H., et al. (2015). Discriminatory indices of typing methods for epidemiologic analysis of contemporary Staphylococcus aureus strains. Medicine (Baltimore) 94 (37), e1534. doi: 10.1097/md.0000000000001534
Rozemeijer, W., Fink, P., Rojas, E., Jones, C. H., Pavliakova, D., Giardina, P., et al. (2015). Evaluation of approaches to monitor Staphylococcus aureus virulence factor expression during human disease. PloS One 10, e0116945. doi: 10.1371/journal.pone.0116945
Ruegg, P. L. (2017). A 100-Year Review: Mastitis detection, management, and prevention. J. Dairy Sci. 100, 10381–10397. doi: 10.3168/jds.2017-13023
Schmitt-Van-de-Leemput, E., Zadoks, R. N. (2007). Genotypic and phenotypic detection of macrolide and lincosamide resistance in Streptococcus uberis. J. Dairy Sci. 90, 5089–5096. doi: 10.3168/jds.2007-0101
Shahzad, M. A., Yousaf, A., Ahsan, A., Irshad, H., Riaz, A., Khan, A., et al. (2024). Virulence and resistance profiling of Staphylococcus aureus isolated from subclinical bovine mastitis in the Pakistani Pothohar region. Sci. Rep. 14, 14569. doi: 10.1038/s41598-024-65448-9
Song, X., Huang, X., Xu, H., Zhang, C., Chen, S., Liu, F., et al. (2020). The prevalence of pathogens causing bovine mastitis and their associated risk factors in 15 large dairy farms in China: An observational study. Vet. Microbiol. 247, 108757. doi: 10.1016/j.vetmic.2020.108757
Song, X., Wang, Y., Bai, R., Pei, X., Xu, H., Zhu, K., et al. (2024). Antimicrobial resistance profiles of common mastitis pathogens on large Chinese dairy farms. JDS Commun. 5, 185–189. doi: 10.3168/jdsc.2023-0413
Strommenger, B., Kettlitz, C., Werner, G., Witte, W. (2003). Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J. Clin. Microbiol. 41, 4089–4094. doi: 10.1128/jcm.41.9.4089-4094.2003
Sutcliffe, J., Grebe, T., Tait-Kamradt, A., Wondrack, L. (1996). Detection of erythromycin-resistant determinants by PCR. Antimicrob. Agents Chemother. 40, 2562–2566. doi: 10.1128/aac.40.11.2562
Suzuki, Y., Ono, H. K., Shimojima, Y., Kubota, H., Kato, R., Kakuda, T., et al. (2020). A novel staphylococcal enterotoxin SE02 involved in a staphylococcal food poisoning outbreak that occurred in Tokyo in 2004. Food Microbiol. 92, 103588. doi: 10.1016/j.fm.2020.103588
Vannuffel, P., Laterre, P. F., Bouyer, M., Gigi, J., Vandercam, B., Reynaert, M., et al. (1998). Rapid and specific molecular identification of methicillin-resistant Staphylococcus aureus in endotracheal aspirates from mechanically ventilated patients. J. Clin. Microbiol. 36, 2366–2368. doi: 10.1128/jcm.36.8.2366-2368.1998
Verdier, I., Durand, G., Bes, M., Taylor, K. L., Lina, G., Vandenesch, F., et al. (2007). Identification of the capsular polysaccharides in Staphylococcus aureus clinical isolates by PCR and agglutination tests. J. Clin. Microbiol. 45 (3), 725–729. doi: 10.1128/jcm.01572-06
Wang, W. (2014). Impact of antibiotic use in large scale dairy farming on the diversity of microorganisms isolated from quarter milk (master thesis). (Beijing, China: Chinese Center for Disease Control and Prevention).
Wang, K., Cha, J., Liu, K., Deng, J., Yang, B., Xu, H., et al. (2022). The prevalence of bovine mastitis-associated Staphylococcus aureus in China and its antimicrobial resistance rate: A meta-analysis. Front. Vet. Sci. 9. doi: 10.3389/fvets.2022.1006676
Wang, W., Lin, X., Jiang, T., Peng, Z., Xu, J., Yi, L., et al. (2018). Prevalence and characterization of Staphylococcus aureus cultured from raw milk taken from dairy cows with mastitis in Beijing, China. Front. Microbiol. 9, 1123. doi: 10.3389/fmicb.2018.01123
Wang, D., Zhang, L., Yong, C., Shen, M., Ali, T., Shahid, M., et al. (2017). Relationships among superantigen toxin gene profiles, genotypes, and pathogenic characteristics of Staphylococcus aureus isolates from bovine mastitis. J. Dairy Sci. 100, 4276–4286. doi: 10.3168/jds.2016-12405
Zhang, Z., Chen, Y., Li, X., Wang, X., Li, H. (2022). Detection of antibiotic resistance, virulence gene, and drug resistance gene of Staphylococcus aureus isolates from bovine mastitis. Microbiol. Spectr. 10, e0047122. doi: 10.1128/spectrum.00471-22
Zhang, L., Gao, J., Barkema, H. W., Ali, T., Liu, G., Deng, Y., et al. (2018). Virulence gene profiles: alpha-hemolysin and clonal diversity in Staphylococcus aureus isolates from bovine clinical mastitis in China. BMC Vet. Res. 14, 63. doi: 10.1186/s12917-018-1374-7
Zhang, D., Lu, X., Feng, X., Shang, X., Liu, Q., Zhang, N., et al. (2023). Molecular characteristics of Staphylococcus aureus strains isolated from subclinical mastitis of water buffaloes in Guangdong Province, China. Front. Vet. Sci. 10. doi: 10.3389/fvets.2023.1177302
Zhao, Y., Liu, H., Zhao, X., Gao, Y., Zhang, M., Chen, D. (2015). Prevalence and pathogens of subclinical mastitis in dairy goats in China. Trop. Anim. Health Production 47, 429–435. doi: 10.1007/s11250-014-0742-y
Zhu, Z., Hu, Z., Li, S., Fang, R., Ono, H. K., Hu, D. L. (2023). Molecular characteristics and pathogenicity of Staphylococcus aureus exotoxins. Int. J. Mol. Sci. 25 (1), 395. doi: 10.3390/ijms25010395
Keywords: Staphylococcus aureus, mastitis, goat, antimicrobial resistance, virulence gene
Citation: Shi H, Wang L, Li G, Li D, Zhai H, Ji S, Hu Y, Lv T and Yao L (2025) Characteristic profiles of molecular types, antibiotic resistance, antibiotic resistance genes, and virulence genes of Staphylococcus aureus isolates from caprine mastitis in China. Front. Cell. Infect. Microbiol. 15:1533844. doi: 10.3389/fcimb.2025.1533844
Received: 25 November 2024; Accepted: 23 January 2025;
Published: 18 February 2025.
Edited by:
Chuan Chiang-Ni, Chang Gung University, TaiwanReviewed by:
Babafela Awosile, Texas Tech University, United StatesTariq Ali, Veterinary Research Institute, Peshawar, Pakistan
Siti Salasia, Gadjah Mada University, Indonesia
Copyright © 2025 Shi, Wang, Li, Li, Zhai, Ji, Hu, Lv and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongfei Shi, a2NuMUAxNjMuY29t; Lunguang Yao, bHVuZ3Vhbmd5YW9AMTYzLmNvbQ==
 Hongfei Shi
Hongfei Shi Long Wang1
Long Wang1 Dandan Li
Dandan Li Lunguang Yao
Lunguang Yao