
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 26 February 2025
Sec. Antibiotic Resistance and New Antimicrobial drugs
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1533177
This article is part of the Research TopicSynergistic Approaches to Managing Gram-negative Bacterial ResistanceView all 14 articles
Objective: This study aimed to predict and evaluate the efficacy of various polymyxin B dosing regimens for Gram-negative bacteremia using Monte Carlo simulation, with a specific focus on assessing the efficacy in patients receiving continuous renal replacement therapy (CRRT). The goal was to optimize clinical dosing regimens and guide rational polymyxin B use in practice.
Methods: A total of 1,939 Gram-negative bacterial strains were analyzed, collected between April 2019 and December 2021 through the China Bloodstream Gram-negative Pathogens Antimicrobial Resistance and Virulence Surveillance Network (CARVIS-NET). Pharmacokinetic parameters of polymyxin B from existing literature were used to conduct a Monte Carlo simulation based on pharmacokinetic/pharmacodynamic (PK/PD) theory. The probability of target attainment (PTA) and cumulative fraction of response (CFR) were evaluated across various dosing regimens.
Results: The main pathogens of Gram-negative bacteremia were Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter baumannii, all of which demonstrated high susceptibility to polymyxin B. For pathogens with a minimum inhibitory concentration (MIC) ≤1 mg/L, all regimens achieved PTA >90%. However, when the MIC increased to 2 mg/L, the PTA for the 500,000 IU q12h regimen decreased to 77.53%, and at an MIC of 4 mg/L, none of the dosing regimens achieved a PTA >90%. For P. aeruginosa and K. pneumoniae with MIC ≤0.5 mg/L, all regimens demonstrated effectiveness. However, at MIC ≥1 mg/L, significant declines in PTA were observed, with the 500,000 IU q12h and 1.25 mg/kg q12h regimens yielding suboptimal outcomes. In CRRT patients, PTA values declined further, particularly against K. pneumoniae, raising concerns about potential treatment failure.
Conclusion: Polymyxin B demonstrates high efficacy for Gram-negative bacteremia with MIC ≤1 mg/L. However, efficacy diminishes as MIC increases, particularly for P. aeruginosa and K. pneumoniae, where 500,000 IU q12h and 1.25 mg/kg q12h regimens may result in suboptimal outcomes. For CRRT patients with K. pneumoniae bacteremia, therapeutic drug monitoring and dose adjustments are crucial to mitigate treatment failure risks.
Polymyxins, a class of non-ribosomal cyclic cationic polypeptide antibiotics, were first isolated in 1947 by Japanese researchers from Bacillus polymyxa cultures. The group primarily includes five variants: A, B, C, D, and E, with polymyxin B and colistin (polymyxin E) being the most commonly used agents in clinical practice. Polymyxins exert their antibacterial effect by binding to lipopolysaccharides on the outer membrane of Gram-negative bacteria, leading to leakage of intracellular contents (Zavascki et al., 2007). Although initially approved for clinical use in the 1950s, concerns over nephrotoxicity and neurotoxicity, along with the emergence of more potent antibiotics, led to a decline in their use. However, in recent years, with the global rise in antibiotic resistance, particularly the emergence of carbapenem-resistant Gram-negative bacilli (CRGNB), positioning polymyxins as a last-line treatment for multidrug-resistant Gram-negative infections.
Compared to colistin, polymyxin B has more favorable pharmacokinetic properties and superior renal safety, making it a preferable choice for the treatment of invasive infections (Tsuji et al., 2019). However, significant variability exists in the recommended dosing regimens across countries and clinical guidelines. For instance, the Chinese product label recommends a fixed dose of 500,000 to 1,000,000 IU/day (Shanghai First Biochemical Pharmaceutical Co. L, 2020), while the FDA recommends a weight-based dosing of 15,000–25,000 IU/kg/day in divided doses (U.S. National Library of Medicine, 2020). International guidelines propose a weight-based regimen with a loading dose of 2.0 to 2.5 mg/kg (1mg=10,000 units) followed by a maintenance dose of 1.25 to 1.5 mg/kg every 12 hours (Tsuji et al., 2019).These discrepancies highlight the challenge of establishing an optimal dosing regimens for polymyxin B.
Previous PK/PD studies (Sandri et al., 2013; Nelson et al., 2015) have demonstrated a correlation between increased polymyxin B dosing and improved clinical outcomes. Suboptimal dosing, particularly with polymyxin B <15,000 units/kg/day, has been linked to insufficient serum drug concentrations and inadequate AUC/MIC ratios, often leading to poor outcomes and treatment failure in critically ill patients (Ismail et al., 2018; Xia and Jiang, 2021).Conversely, higher doses (≥200 mg/day) of polymyxin B have been associated with reduced in-hospital mortality, but at the cost of increased nephrotoxicity. Several studies have confirmed that a daily dose of ≥200 mg/day is independently and significantly associated with a high risk of acute kidney injury (AKI) (Elias et al., 2010; Falagas et al., 2021).These findings underscore the need for individualized dosing strategies that account for both efficacy and safety.
Patients undergoing continuous renal replacement therapy (CRRT) represent a particularly complex population. Traditional practices suggest no need for polymyxin B dose adjustments in renal impairment or CRRT. However, recent studies indicate that CRRT increases the clearance of polymyxin B, potentially requiring higher doses to achieve therapeutic targets (Luo et al., 2022).This creates a challenge in selecting the appropriate dosing regimen for these patients.
Monte Carlo simulation analysis is a well-established approach for optimizing antibiotic dosing regimens. Previous studies, such as those by Sandri et al (Sandri et al., 2013), emphasized the importance of initiating therapy with a loading dose to achieve optimal drug exposure. Miglis et al (Miglis et al., 2018)further explored weight-based loading and fixed-dose regimens, demonstrating their potential in balancing efficacy and toxicity. Studies by Yu et al (Yu et al., 2021) and Wu et al. (2021) showed that adjusting polymyxin B doses in patients with renal insufficiency enhances the likelihood of achieving optimal drug exposure. However, studies on optimal dosing regimens for CRRT patients remain sparse, and the existing researches were primarily based on small, localized patient cohorts,often fail to incorporate bacterial resistance data or tailor treatment strategies for different pathogens (Luo et al., 2022; Wang et al., 2022b), which presents certain limitations.
Building on these findings, this study uses Monte Carlo simulation to evaluate different polymyxin B dosing regimens for treating Gram-negative bacteremia, including in CRRT patients, to provide clinical guidance on optimal dosing strategies. By integrating large-scale epidemiological data on bacterial resistance, our approach aims to optimize and personalize dosing strategies for critically ill patients with Gram-negative bacteremia caused by different pathogens, addressing a gap in the current literature and contributing to the development of more precise and effective treatment protocols.
This study utilized a publicly available dataset from the CARVIS-NET comprising 1,939 Gram-negative bacterial isolates. These isolates were consecutively and non-repetitively collected from individual patients with clinically and laboratory-confirmed bloodstream infections across 21 centers in 20 cities in China between April 2019 and December 2021. All organisms were considered clinically significant based on local hospital criteria and isolated from high-quality specimens of each patient’s first positive blood culture. The dataset includes community-associated and nosocomial bloodstream infections, with community-associated infections accounting for approximately 47.8%. Infection sources were classified based on clinical records into categories such as respiratory tract infections, urinary tract infections, alimentary tract infections, and others. Ethical approval for the initial data collection was obtained from the Human Research Ethics Committee of PUMCH (Ethics Approval Number: HS2755), as reported in the original study (Xi et al., 2022). No additional ethical approval was required for our analysis, as this study solely relies on publicly accessible data.
Antibiotic susceptibility testing for polymyxin B was performed using the broth microdilution method, as recommended by the Clinical and Laboratory Standards Institute (CLSI). The MICs were interpreted according to the CLSI M100-S32 guidelines or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standards. Specifically, MIC breakpoints for polymyxin B were as follows: For Enterobacterales and Acinetobacter, MICs ≤2 mg/L were considered susceptible, and MICs >2 mg/L as resistant. For Pseudomonas MICs ≤4 mg/L were categorized as susceptible, and MICs >4 mg/L as resistant (EUCAST, 2022). E. coli ATCC 25922, P. aeruginosa ATCC 27853 and K. pneumoniae ATCC 700603 were used as quality controls (Xi et al., 2022).
The PK/PD index for polymyxin B is the ratio of free drug area under the curve to MIC (f AUC/MIC) (Sandri et al., 2013; Tsuji et al., 2019).In this formula, AUC is calculated as Dose/CL where Dose represents the administered dose, CL refers to the drug clearance rate, and f denotes the fraction of unbound drug. The reference target value for this ratio is ≥10, with a range of 3.5–28.0 (Hanafin et al., 2023). For specific pathogens, the target values are 20.8 for P. aeruginosa, 28.0 for K. pneumoniae, and 13.9 for A. baumannii (Yang et al., 2021).
Based on the polymyxin B product insert, relevant guidelines, and common clinical practice, the following intravenous dosing regimens were evaluated. All regimens were administered over at least 1 hour.
1. 500,000 IU q12h;
2. 1,000,000 IU q12h;
3. 1.25 mg/kg q12h;
4. Loading dose: 2 mg/kg over 1 hour, followed by maintenance: 1.25 mg/kg q12h;
5. Loading dose: 2.5 mg/kg over 1 hour, followed by maintenance: 1.5 mg/kg q12h.
Polymyxin B PK parameters were derived from population pharmacokinetic (PPK) studies in different patient populations. For patients with normal renal function, polymyxin B CL was reported as 0.028 ± 0.007 L/h/kg (Yu et al., 2022), based on adult patients with endogenous creatinine clearance ranging from 60 to 120 mL/min and bloodstream infections caused by carbapenem-resistant K. pneumoniae, as detailed in Table 1; while in patients undergoing CRRT, polymyxin B CL was slightly higher, measured at 0.033 ± 0.003 L/h/kg (Luo et al., 2022). The PPK analysis for CRRT patients was based on adult intensive care unit (ICU) patients with confirmed or suspected infections caused by carbapenem-resistant organisms. The mean fraction of unbound drug in plasma was 0.42 (range: 0.26–0.64) (Sandri et al., 2013; Zheng et al., 2023).
Monte Carlo simulation were performed using Oracle Crystal Ball (version 11.1.1.4.400) software to evaluate the efficacy of different polymyxin B dosing regimens. A total of 10,000 simulations were conducted, assuming that CL follows a normal distribution and f follows a uniform distribution, with a 95% confidence interval. Based on the PK/PD parameter targets, the probability of target attainment (PTA) was calculated for each dosing regimen at various MIC values. Custom MIC values and probability distributions were input into the simulation model, and corresponding PK/PD target values were adjusted. The cumulative fraction of response (CFR) was then calculated to express the expected probability of each dosing regimen achieving the target threshold against a population of pathogens. Generally, dosing regimens with PTA or CFR ≥90% are considered appropriate for empirical antimicrobial therapy.
Among them, PTAi refers to the probability of target attainment for a specific MIC value, and Fi represents the percentage of each MIC distribution within the population of bacterial strains.
Among the 1,939 Gram-negative bacterial strains isolated, 1,724 (88.91%) belonged to the Enterobacterales family, while 207 (10.67%) were non-fermentative bacteria. The five most common pathogens identified were E. coli (896 strains, 46.21%), K. pneumoniae (612 strains, 31.56%), P. aeruginosa (95 strains, 4.90%), A. baumannii (82 strains, 4.23%), and E. cloacae (58 strains, 2.99%) (Table 2).
The susceptibility of these Gram-negative bacteria to polymyxin B varied across different infection sources, as shown in Table 3. Bloodstream infection isolates derived from catheter-related bloodstream infections exhibited the lowest susceptibility to polymyxin B (90.48%), whereas isolates from alimentary tract and cardiovascular system infections demonstrated 100% susceptibility.
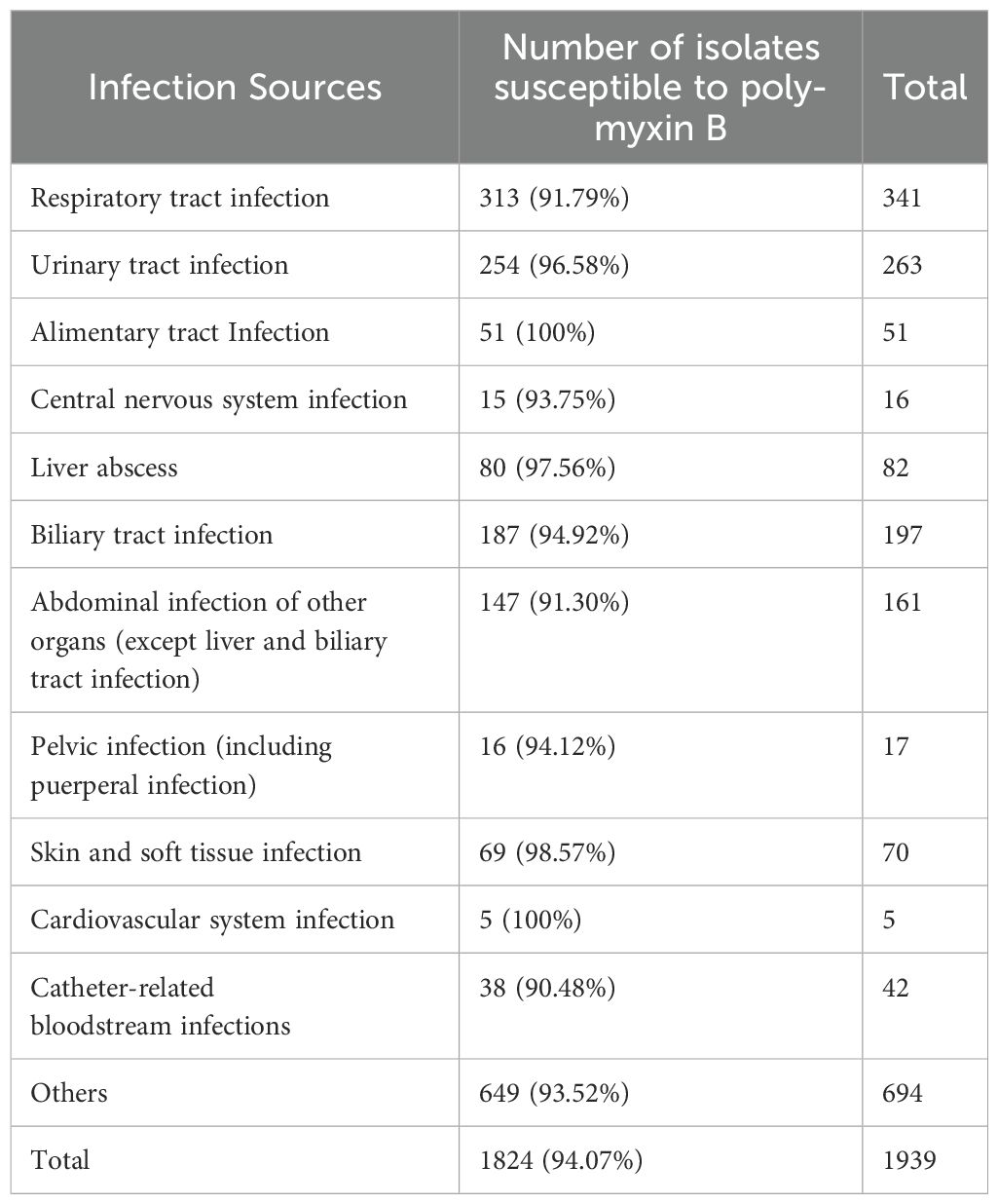
Table 3. Susceptibility of Gram-negative bacteria to polymyxin B across different infection sources.
The isolated Gram-negative bacteria demonstrated a high level of susceptibility to polymyxin B, with 94.07% of the isolates exhibiting an MIC ≤2 mg/L. E. coli showed the highest susceptibility, with MIC50 and MIC90 values of 0.25 mg/L and 0.5 mg/L, respectively. For K. pneumoniae, the MIC50 and MIC90 values were both 0.5 mg/L, while for P. aeruginosa, they were both 1 mg/L, and for A. baumannii, they were 0.5 mg/L and 1 mg/L, respectively (Table 4).
The PTA values of various polymyxin B dosing regimens for treating Gram-negative bacteremia are shown in Figure 1. As demonstrated in Figure 1A, all dosing regimens achieved PTA ≥90% for Gram-negative bacteremia isolates with MIC ≤1 mg/L. However, when the MIC increased to 2 mg/L, 500,000 IU q12h showed a sharp decline in PTA to 77.53%, falling below the target threshold, and when the MIC increased to 4 mg/L, none of the regimens could achieve a PTA >90%.
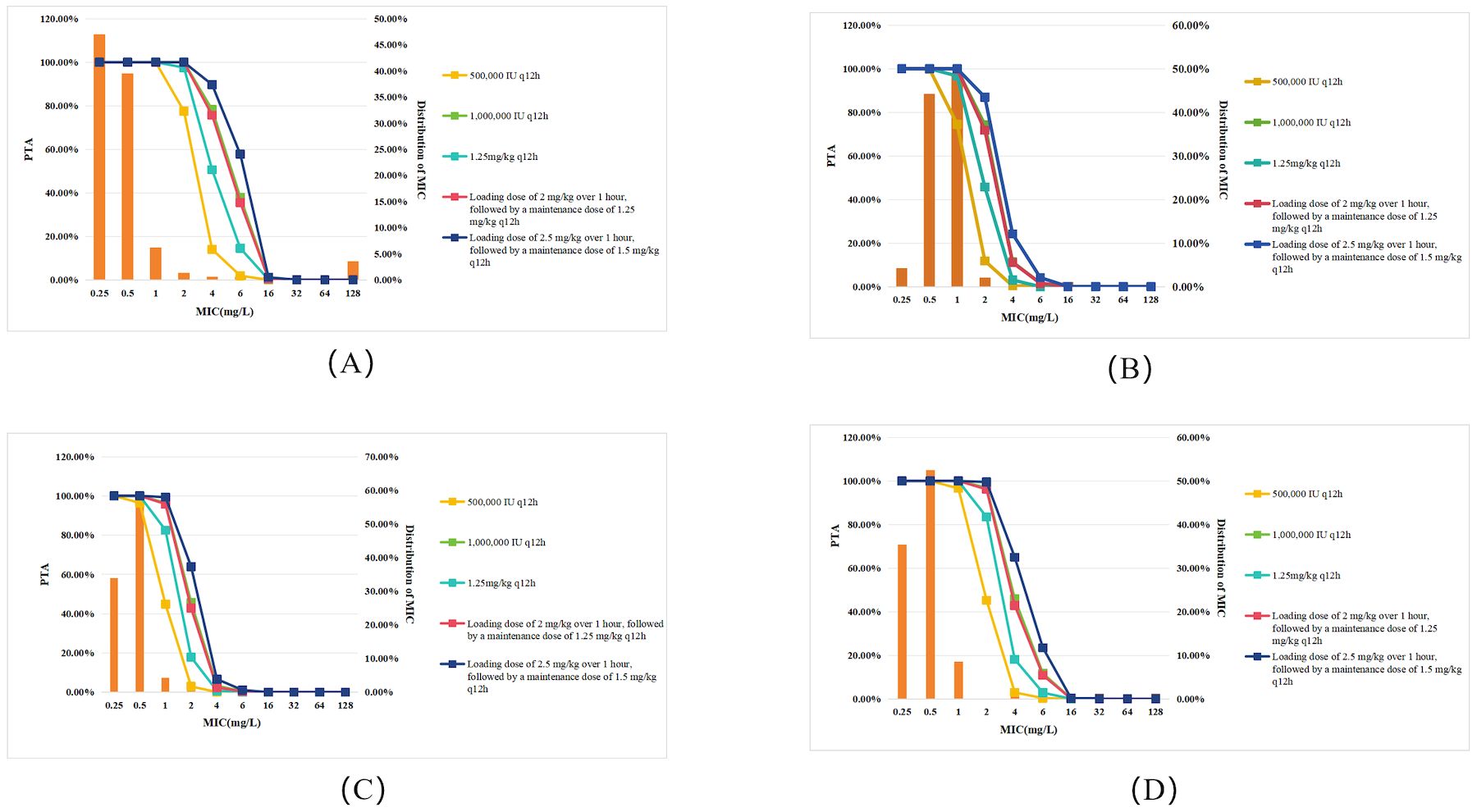
Figure 1. PTA for different polymyxin B dosing regimens against Gram-negative bacteremia in patients with normal renal function. Each panel represents PTA values at varying MICs (mg/L): (A) All Gram-negative bacteria; (B) P. aeruginosa; (C) K. pneumoniae; (D) A. baumannii. Legend represent dosing regimens as follows: 500,000 IU q12h (orange); 1,000,000 IU q12h (green); 1.25 mg/kg q12h (deep green); Loading dose 2 mg/kg, maintenance 1.25 mg/kg q12h (red); Loading dose 2.5 mg/kg, maintenance 1.5 mg/kg q12h (deep blue). The bars represent the MIC distribution of polymyxin B against Gram-negative bacteria.
For P. aeruginosa infections (Figure 1B), all dosing regimens achieved PTA ≥90% at MIC ≤0.5 mg/L. However, when the MIC increased to 1 mg/L, 500,000 IU q12h failed to reach the effective target, with PTA dropping to 74.52%. Notably, when MIC increased to 2 mg/L, no regimen achieved PTA >90%.
For K. pneumoniae infections (Figure 1C), the simulation indicated that when the MIC ≤0.5 mg/L, all regimens reached the target. However, at an MIC of 1 mg/L, the 500,000 IU q12h and 1.25 mg/kg q12h regimens failed to reach the effective target. When the MIC increased to 2 mg/L, none of the regimens achieved a PTA >90%.
For A. baumannii infections (Figure 1D), the simulation showed that when the MIC ≤1 mg/L, all regimens reached the target. However, at an MIC of 2 mg/L, the 500,000 IU q12h and 1.25 mg/kg q12h regimens failed to reach the effective target. When the MIC increased to 4 mg/L, none of the regimens achieved a PTA >90%.
Compared to patients with normal renal function, CRRT patients exhibited a decrease in PTA across all dosing regimens and target pathogens (Figure 2). As shown in Figure 2A, the PTA of 500,000 IU q12h regimen for all Gram-negative bacteria at MIC=2 mg/L declined by approximately 13.6% (from 77.53% to 63.97%) in CRRT patients compared to those with normal renal function (Figure 1A). Specifically, for P. aeruginosa (Figure 2B), the PTA declined from 11.80% to 0.07%; for K. pneumoniae (Figure 2C), it dropped from 2.88% to 0.01%; and for A. baumannii (Figure 2D), it declined from 45.26% to 24.21%. However, using a PTA ≥90% as the criterion for regimen selection, CRRT did not significantly impact treatment outcomes.
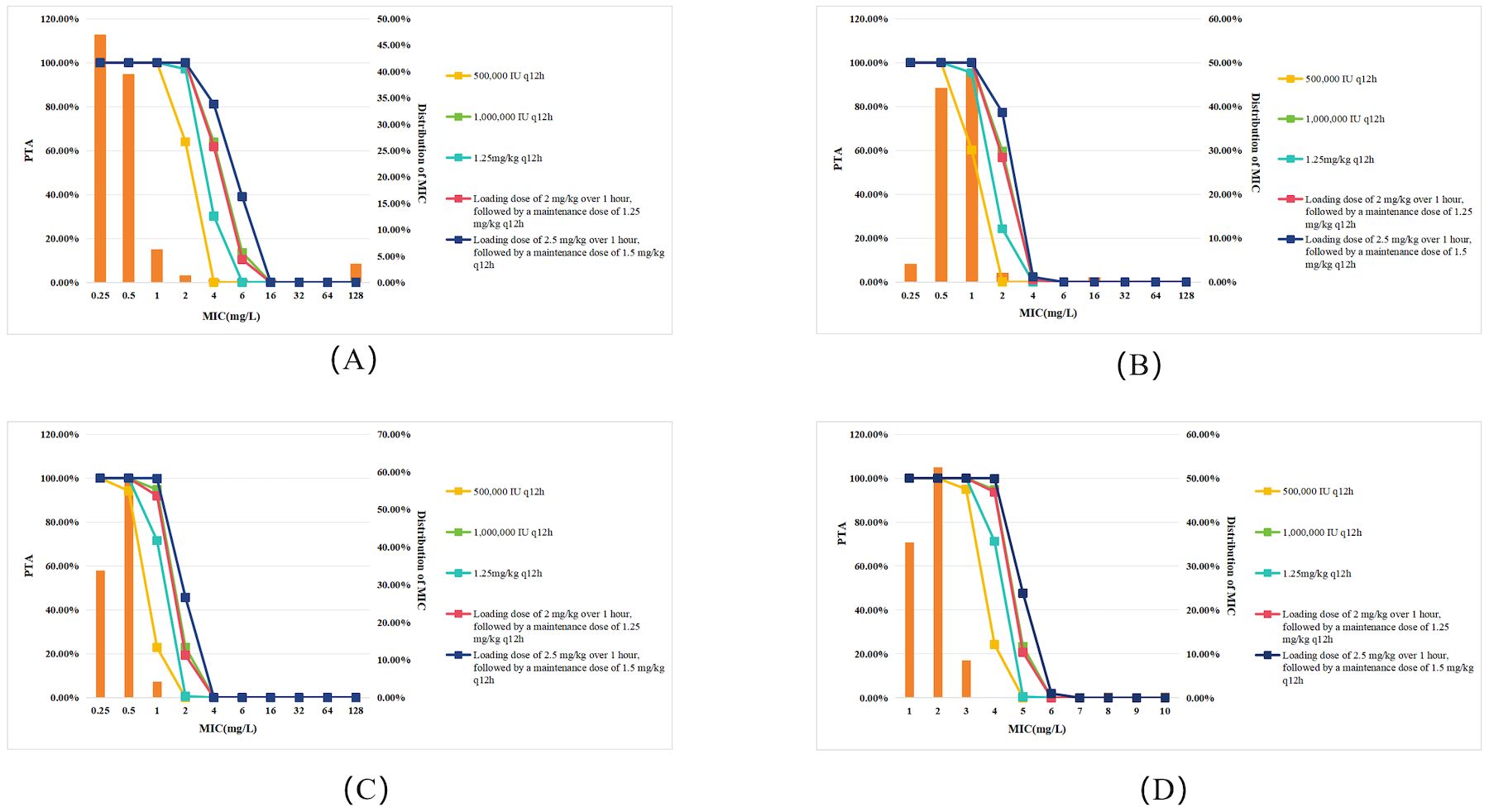
Figure 2. PTA for different polymyxin B dosing regimens against Gram-negative bacteremia in patients undergoing CRRT. Each panel represents PTA values at varying MICs (mg/L): (A) All Gram-negative bacteria; (B) P. aeruginosa; (C) K. pneumoniae; (D) A. baumannii. Legend represent dosing regimens as follows: 500,000 IU q12h (orange); 1,000,000 IU q12h (green); 1.25 mg/kg q12h (deep green); Loading dose 2 mg/kg, maintenance 1.25 mg/kg q12h (red); Loading dose 2.5 mg/kg, maintenance 1.5 mg/kg q12h (deep blue). The bars represent the MIC distribution of polymyxin B against Gram-negative bacteria.
Considering the higher therapeutic demands for severe infections caused by multidrug-resistant Gram-negative bacteria, PTA was also evaluated with a more stringent≥95% threshold for regimen selection (Paranos et al., 2022). Under this criterion, CRRT patients with K. pneumoniae infections (MIC≥0.5mg/L) could not achieve therapeutic efficacy with the 500,000 IU q12h regimen. Furthermore, neither the 1,000,000 IU q12h regimen nor the dosing strategy consisting of a loading dose of 2 mg/kg followed by a maintenance dose of 1.25 mg/kg q12h was effective when MIC ≥1mg/L. Similarly, for A. baumannii infections, both the 1,000,000 IU q12h regimen and the aforementioned loading and maintenance dose strategy failed to achieve the effective target when MIC≥2mg/L.
The CFR values for different polymyxin B dosing regimens in treating Gram-negative bacteremia in patients with normal renal function are shown in Table 5. The Monte Carlo simulation indicated that all simulated regimens achieved a CFR >90% for Gram-negative bacteremia, suggesting good clinical efficacy. However, when the pathogen was P. aeruginosa, the 500,000 IU q12h regimen had a CFR value of 85.12%, indicating a potential for treatment failure.
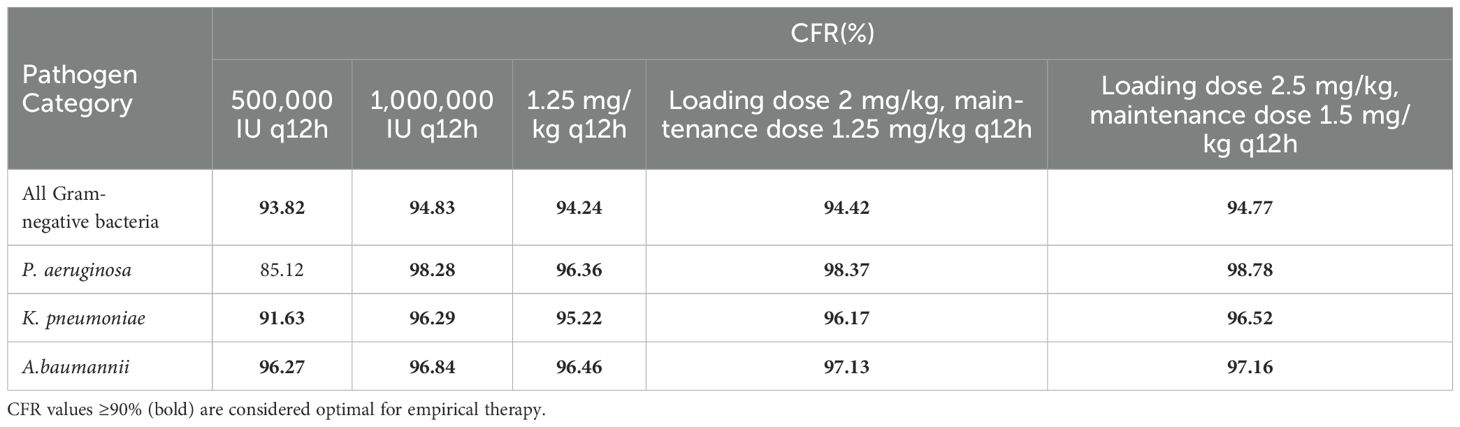
Table 5. CFR of polymyxin B dosing regimens for treating bacteremia in patients with normal renal function.
In CRRT patients, all regimens showed a decrease in CFR compared to those with normal renal function (Table 6). However, using CFR ≥90% as the criterion for regimen selection, CRRT only affected the treatment of K. pneumoniae. For CRRT patients with K. pneumoniae infections, the CFR for the 500,000 IU q12h regimen dropped to 89.59%, indicating a potential risk of suboptimal treatment.
With the widespread use of carbapenem antibiotics, CRGNB, which have emerged under the selective pressure of powerful antibiotics, have become a major global public health threat. Compared to other infections, bloodstream infections caused by CRGNB have a significantly higher mortality rate. A study by Falcone et al. (2023) showed that the 30-day mortality rate for bloodstream infections caused by carbapenem-resistant bacteria (26.6%~43.2%) was significantly higher than that for infections caused by carbapenem-sensitive bacteria (13.7%).
Treatment options for CRGNB are extremely limited, with sensitive drugs including tigecycline, polymyxins, and ceftazidime-avibactam. Polymyxin B has high sensitivity against common Gram-negative bacteria. In this study, the sensitivity rate of polymyxin B against common Gram-negative bacteria ranged from 96.3% to 99.0%, consistent with another surveillance report showing sensitivity rates between 95.1% and 99.3% (Liu and Ji, 2024). Even for carbapenem-resistant strains, polymyxin B exhibited high efficacy, with sensitivity rates of 89.7%, 88.3%, 95.0%, and 99.0% for carbapenem-resistant E. coli, K. pneumoniae, A. baumannii, and P. aeruginosa respectively. Despite its high sensitivity, several factors, including the expression of antimicrobial activity, outdated drug labels, and uncertainties in susceptibility testing, have contributed to inconsistent clinical application of polymyxin B (Tsuji et al., 2019). Therefore, determining the appropriate dosing strategy is a key aspect of optimizing polymyxin B treatment regimens.
In our study, polymyxin B effectively treated Gram-negative bacteremia when the MIC was ≤1 mg/L. However, the effectiveness of the treatment regimen decreased when the infecting pathogens were P. aeruginosa or K. pneumoniae. At an MIC of 1 mg/L, the 500,000 IU q12h may result in suboptimal clinical outcomes. The CFR values further indicate that the 500,000 IU q12h carries a significant risk of treatment failure, especially in CRRT patients. This may be attributed to the lower sensitivity of P. aeruginosa to polymyxin B compared to other bacteria and the higher target values for K. pneumoniae. To optimize targeted therapy for these pathogens, higher off-label doses should be considered to ensure effective treatment. However, when the MIC increases to 2 mg/L, none of the simulated dosing regimens can achieve the PTA target (≥90%) for bloodstream infections caused by P. aeruginosa or K. pneumoniae. According to EUCAST (2022) guidelines, the susceptibility breakpoint for polymyxins is 2 mg/L for K. pneumoniae, indicating that, in clinical practice, susceptibility testing may indicate sensitivity, but the clinical treatment outcomes might still be poor.It is important to note that the results of the Monte Carlo simulation are highly dependent on the input parameters and bacterial susceptibility. f AUC/MIC is considered the best PK/PD index for predicting the efficacy of polymyxin B, but there remains no consensus on the recommended target values, and the target values vary significantly across studies, leading to differences in study outcomes. For instance, Wang et al (Wang et al., 2022a)proposed a target of 50 and concluded that in elderly patients with multidrug-resistant Gram-negative bacterial infections, doses of 50 mg and 75 mg q12h achieve an optimal balance between nephrotoxicity and efficacy. Yu et al. (2022) adopted bacteria-specific target values and found that for isolates with MIC ≤1 mg/L, a maintenance dose of 1 mg/kg q12h could achieve a PTA >90%. In our study, we selected an fAUC/MIC target of 10, which is the average value required to reduce bacterial counts by 1 log10 in a mouse thigh infection model for nine Gram-negative bacteria. For K. pneumoniae, P. aeruginosa, and A. baumannii, the selected targets were the doses required to reduce bacterial counts by 1 log10, which were 28.0, 20.8, and 13.9, respectively (Landersdorfer et al., 2018; Society of Clinical Microbiology and Infection of China International Exchange and Promotion Association for Medical and Healthcare CMGotLMSotCMA and Clinical Microbiology Group of the Microbiology and Immunology Society of the Chinese Medical Association, 2020).
Critically ill patients often exhibit substantial pathophysiological changes, such as hepatic and renal dysfunction, hypoalbuminemia, extensive fluid resuscitation, and alterations in drug distribution volume, all of which can significantly impact the PK of antibiotics. In this study, the mean unbound fraction of polymyxin B was assumed to be 0.42, based on data derived from ICU patients. However, actual unbound fractions can vary considerably across individual patients, particularly in critically ill populations, potentially influencing the interpretation of therapeutic outcomes.
Polymyxin B is predominantly cleared through non-renal pathways, with less than 1% of the drug excreted unchanged in the urine (Zavascki et al., 2007). Consequently, dose adjustments are generally unnecessary in patients with renal insufficiency or those undergoing renal replacement therapy (RRT). However, CRRT, a vital intervention in critically ill patients, can significantly alter drug clearance, subsequently affecting PK/PD parameters and therapeutic outcomes. Evidence suggests that in CRRT patients, especially those receiving continuous venovenous hemodiafiltration (CVVHDF), polymyxin B clearance may increase (Luo et al., 2022; Hanafin et al., 2023; Pi et al., 2023). A study by Luo et al. (2022) showed that the clearance of polymyxin B in CRRT patients (1.95 L/h) was higher than in non-CRRT patients (1.5 L/h). Hanafin et al. (2023) reported that in CVVHDF patients, the steady-state AUC0-24h was 50% lower than in patients not receiving CRRT, underscoring the need to consider the impact of CRRT on therapeutic outcomes. Consistent with these observations, our study demonstrated that CRRT patients exhibited a reduced PTA for polymyxin B across multiple dosing regimens compared to non-CRRT patients. When using PTA or CFR ≥90% as the threshold for therapeutic effectiveness, CRRT had no significant impact on treatment outcomes except for K.pneumoniae infections. However, when applying the stricter criterion of PTA ≥95%, a notable shift in efficacy was observed. In CRRT patients, both the 1,000,000 IU q12h regimen and the dosing strategy of 2 mg/kg loading dose followed by a maintenance dose of 1.25 mg/kg q12h demonstrated reduced efficacy against K.pneumoniae and A.baumannii, particularly when the MIC values ranged from 0.5 to 2 mg/L. Therefore, for these patients, it is recommended to enhance TDM and adjust drug dosing based on the monitoring results to ensure treatment efficacy and minimize the risk of resistance.
Due to the limited sample size of bloodstream infections caused by carbapenem-resistant bacteria, the bacterial susceptibility data used in this study are based on bloodstream infections caused by all Gram-negative bacteria. Since polymyxin B is primarily used for infections caused by carbapenem-resistant bacteria, differences in sensitivity to polymyxin B may lead to varying clinical outcomes. Data from the China Bacterial Resistant Investigation Collaborative System(BRICS) showed (Liu and Ji, 2024) that the resistance rate of E. coli to polymyxin B is 1.6%, while the rate increases to 10.3% in carbapenem-resistant E. coli. For K. pneumoniae, the resistance rate of to polymyxin B is 4.5%, rising to 11.7% in carbapenem-resistant strains. The resistance rate of P. aeruginosa is 0.7%, while the carbapenem-resistant strain has a slightly higher rate of 1%. Similarly, for A. baumannii, the resistance rate of to polymyxin B is 4.9%, increasing slightly to 5.0% in carbapenem-resistant strains. Susceptibility testing results showed that carbapenem resistance significantly affected the sensitivity of E. coli and K. pneumoniae to polymyxin B, but had a smaller impact on P. aeruginosa and A. baumannii. Therefore, a more conservative approach should be taken when interpreting CFR values for E. coli and K. pneumoniae.
The safety of polymyxin B is also an important consideration when optimizing dosing regimens. Nephrotoxicity is the main dose-limiting toxicity of polymyxin B, affecting up to 30% of patients (Phe et al., 2014; Rigatto et al., 2015; Oliota et al., 2019). A study by Elias et al., 2010 showed that a polymyxin B dose ≥200 mg/day was associated with a higher incidence of severe renal injury. Our study showed that as the drug dose increased, the probability of achieving the target concentration also increased, but higher doses were associated with an increased risk of severe renal injury. Weight-adjusted dosing regimens may result in suboptimal drug concentrations in underweight patients (Miglis et al., 2018; Hanafin et al., 2023), or significant adverse reactions in overweight patients (Wang et al., 2022a). Our study showed that when the MIC of the pathogen was ≤0.5 mg/L, a fixed-dose regimen of 500,000 IU q12h had similar efficacy to weight-adjusted dosing regimens, with potentially better safety. When the MIC increased to 1 mg/L, the 1,000,000 IU q12h regimen had similar efficacy to the loading-dose plus maintenance-dose regimen, but potentially better safety. A fixed-dose regimen may be the better option after balancing efficacy and adverse reactions, but further studies are needed to determine the optimal regimen.
Although previous studies have explored the optimization of polymyxin B dosing regimens for the treatment of Gram-negative infections, these studies often focus on single-pathogen infections or lack considerations for the dynamic changes in critically ill patients, particularly those receiving CRRT. Furthermore, previous studies typically did not incorporate comprehensive bacterial resistance data or explore individualized treatment strategies for different pathogens. Our study addresses these gaps by combining large-scale epidemiological resistance data with Monte Carlo simulations to optimize polymyxin B dosing regimens for various Gram-negative pathogens, including those in CRRT patients. However, this study has several limitations that should be acknowledged. First, all polymyxin B dosing regimens in the simulation were based on monotherapy, without accounting for the potential synergistic effects or improved clinical outcomes associated with combination therapy. The rapid spread of plasmid-mediated MCR-1 resistance genes has significantly impacted the effectiveness of polymyxin antibiotics (Liu et al., 2016). Combination antibiotic therapy presents an attractive option for treatment. Additionally, factors such as the safety of high-dose polymyxin B administration, the presence of heteroresistance, and the link between colistin resistance and increased in-hospital mortality further highlight the advantages of combination therapy (Tsuji et al., 2019). However, our study did not explore the role or impact of combination regimens, which may limit the applicability of the findings in clinical practice. Additionally, the population pharmacokinetic data used in this study were derived from a relatively small cohort, necessitating further data collection and validation to improve model accuracy. Finally, it is important to note that critically ill patients often exhibit significant variability in PK/PD parameters due to dynamic physiological changes, which should be carefully considered when applying these results to clinical practice.
Our study showed that polymyxin B demonstrated good clinical efficacy for Gram-negative bacteremia, especially in pathogens with lower MICs. However, for infections caused by P. aeruginosa and K. pneumoniae with higher MIC values, lower-dose regimens, such as 500,000 IU q12h, may result in suboptimal treatment outcomes. In patients undergoing CRRT, both PTA and CFR values decrease across all dosing regimens compared to those with normal renal function, with a notable reduction in efficacy against K. pneumoniae. These findings highlight the importance of adjusting dosing regimens based on renal function and MIC values to optimize clinical outcomes. We recommend the routine measurement of MICs and individualized therapy to ensure effective treatment. Furthermore, integrating PK/PD modeling with local resistance patterns and TDM can assist clinicians in selecting the most appropriate antibiotic regimens for Gram-negative bacteremia, especially in complex patient populations such as those receiving CRRT.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The manuscript presents research on animals that do not require ethical approval for their study.
ZH: Validation, Writing – original draft. YY: Conceptualization, Validation, Writing – original draft. CW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Hospital Pharmacy Research Project of Shandong Pharmaceutical Association (yyyx2021qn-02).
We sincerely thank the China Bloodstream Gram-negative Pathogens Antimicrobial Resistance and Virulence Surveillance Network (CARVIS-NET) for providing access to the data on Gram-negative bacterial strains isolated from bloodstream infections between April 2019 and December 2021. This publicly available dataset, as published in Frontiers in Microbiology (https://doi.org/10.3389/fmicb.2022.1017488), has been invaluable in facilitating our research on optimizing Polymyxin B dosing regimens. We are grateful to the researchers and contributors who carried out the original data collection and made this valuable resource accessible to the scientific community.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Elias, L. S., Konzen, D., Krebs, J. M., Zavascki, A. P. (2010). The impact of polymyxin B dosage on in-hospital mortality of patients treated with this antibiotic. J. Antimicrobial Chemotherapy. 65, 2231–2237. doi: 10.1093/jac/dkq285
EUCAST (2022). Implementation and use of the 2022 revised Polymyxin Breakpoints. Available at: https://www.eucast.org/ast_of_bacteria/previous_versions_of_documents (Accessed February 16, 2025).
Falagas, M. E., Kyriakidou, M., Voulgaris, G. L., Vokos, F., Politi, S., Kechagias, K. S. (2021). Clinical use of intravenous polymyxin B for the treatment of patients with multidrug-resistant Gram-negative bacterial infections: An evaluation of the current evidence. J. Glob Antimicrob. Resist. 24, 342–359. doi: 10.1016/j.jgar.2020.12.026
Falcone, M., Tiseo, G., Carbonara, S., Marino, A., Di Caprio, G., Carretta, A., et al. (2023). Mortality attributable to bloodstream infections caused by different carbapenem-resistant gram-negative bacilli: results from a nationwide study in Italy (ALARICO network). Clin. Infect. Diseases: an Off. Publ. Infect. Dis. Soc. America. 76, 2059–2069. doi: 10.1093/cid/ciad100
Hanafin, P. O., Kwa, A., Zavascki, A. P., Sandri, A. M., Scheetz, M. H., Kubin, C. J., et al. (2023). A population pharmacokinetic model of polymyxin B based on prospective clinical data to inform dosing in hospitalized patients. Clin. Microbiol. Infect. 29, 1174–1181. doi: 10.1016/j.cmi.2023.05.018
Ismail, B., Shafei, M. N., Harun, A., Ali, S., Omar, M., Deris, Z. Z. (2018). Predictors of polymyxin B treatment failure in Gram-negative healthcare-associated infections among critically ill patients. J. Microbiol. Immunol. Infect. 51, 763–769. doi: 10.1016/j.jmii.2017.03.007
Landersdorfer, C. B., Wang, J., Wirth, V., Chen, K., Kaye, K. S., Tsuji, B. T., et al. (2018). Pharmacokinetics/pharmacodynamics of systemically administered polymyxin B against Klebsiella pneumoniae in mouse thigh and lung infection models. J. Antimicrobial Chemotherapy. 73, 462–468. doi: 10.1093/jac/dkx409
Liu, Z., Ji, J. (2024). National bloodstream infection bacterial resistance surveillance report (2022): Gram-negative bacteria. Chin. J. Clin. Infect. Dis. 17, 42–57. doi: 10.3760/cma.j.issn.1674-2397.2024.01.005
Liu, Y. Y., Wang, Y., Walsh, T. R., Yi, L. X., Zhang, R., Spencer, J., et al. (2016). Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect. Dis. 16, 161–168. doi: 10.1016/S1473-3099(15)00424-7
Luo, X., Zhang, Y., Liang, P., Zhu, H., Li, M., Ding, X., et al. (2022). Population pharmacokinetics of polymyxin B and dosage strategy in critically ill patients with/without continuous renal replacement therapy. Eur. J. Pharm. Sci. 175, 106214. doi: 10.1016/j.ejps.2022.106214
Miglis, C., Rhodes, N. J., Avedissian, S. N., Kubin, C. J., Yin, M. T., Nelson, B. C., et al. (2018). Population pharmacokinetics of polymyxin B in acutely ill adult patients. Antimicrobial Agents Chemotherapy. 62, e01475–e01417. doi: 10.1128/AAC.01475-17
Nelson, B. C., Eiras, D. P., Gomez-Simmonds, A., Loo, A. S., Satlin, M. J., Jenkins, S. G., et al. (2015). Clinical outcomes associated with polymyxin B dose in patients with bloodstream infections due to carbapenem-resistant Gram-negative rods. Antimicrob. Agents Chemother. 59, 7000–7006. doi: 10.1128/AAC.00844-15
Oliota, A. F., Penteado, S. T., Tonin, F. S., Fernandez-Llimos, F., Sanches, A. C. (2019). Nephrotoxicity prevalence in patients treated with polymyxins: a systematic review with meta-analysis of observational studies. Diagn. Microbiol. Infect. Dis. 94, 41–49. doi: 10.1016/j.diagmicrobio.2018.11.008
Paranos, P., Vourli, S., Pournaras, S., Meletiadis, J. (2022). Assessing clinical potential of old antibiotics against severe infections by multi-drug-resistant gram-negative bacteria using in silico modelling. Pharm. (Basel) 15, 1501. doi: 10.3390/ph15121501
Phe, K., Lee, Y., McDaneld, P. M., Prasad, N., Yin, T., Figueroa, D. A., et al. (2014). In vitro assessment and multicenter cohort study of comparative nephrotoxicity rates associated with colistimethate versus polymyxin B therapy. Antimicrobial Agents Chemotherapy. 58, 2740–2746. doi: 10.1128/AAC.02476-13
Pi, M. Y., Cai, C. J., Zuo, L. Y., Zheng, J. T., Zhang, M. L., Lin, X. B., et al. (2023). Population pharmacokinetics and limited sampling strategies of polymyxin B in critically ill patients. J. Antimicrob. Chemother. 78, 792–801. doi: 10.1093/jac/dkad012
Rigatto, M. H., Behle, T. F., Falci, D. R., Freitas, T., Lopes, N. T., Nunes, M., et al. (2015). Risk factors for acute kidney injury (AKI) in patients treated with polymyxin B and influence of AKI on mortality: a multicentre prospective cohort study. J. Antimicrobial Chemotherapy. 70, 1552–1557. doi: 10.1093/jac/dku561
Sandri, A. M., Landersdorfer, C. B., Jacob, J., Boniatti, M. M., Dalarosa, M. G., Falci, D. R., et al. (2013). Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin. Infect. Dis. 57, 524–531. doi: 10.1093/cid/cit334
Shanghai First Biochemical Pharmaceutical Co. L (2020). Prescribing Information for Polymyxini B Sulphas for Injection (Beijing, China).
Society of Clinical Microbiology and Infection of China International Exchange and Promotion Association for Medical and Healthcare CMGotLMSotCMA, Clinical Microbiology Group of the Microbiology and Immunology Society of the Chinese Medical Association (2020). Expert consensus on polymyxins, tigecycline and ceftazidime/avibactam susceptibility testing. Chin. J. Lab. Med. 43, 964–972. doi: 10.3760/cma.j.cn114452-20200719-00619
Tsuji, B. T., Pogue, J. M., Zavascki, A. P., Paul, M., Daikos, G. L., Forrest, A., et al. (2019). International consensus guidelines for the optimal use of the polymyxins: endorsed by the american college of clinical pharmacy (ACCP), european society of clinical microbiology and infectious diseases (ESCMID), infectious diseases society of america (IDSA), international society for anti-infective pharmacology (ISAP), society of critical care medicine (SCCM), and society of infectious diseases pharmacists (SIDP). Pharmacotherapy. 39, 10–39. doi: 10.1002/phar.2209
U.S. National Library of Medicine. (2020). Polymyxin B. DailyMed: Drug Information (FDA-approved drug labels). Available online at: https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=b56f18c0-ef5e-4ed9-a5af-f79f3cd189b6 (Accessed February 16, 2025).
Wang, P., Liu, D., Sun, T., Zhang, X., Yang, J. (2022a). Pharmacokinetics and pharmacodynamics of polymyxin B and proposed dosing regimens in elderly patients with multi-drug-resistant Gram-negative bacterial infections. Int. J. Antimicrobial Agents 60, 106693. doi: 10.1016/j.ijantimicag.2022.106693
Wang, P., Xing, H., Zhang, F., Liu, S., Lu, Y., Zhang, X., et al. (2022b). Population pharmacokinetics of polymyxin B in critically ill patients receiving continuous venovenous haemofiltration. Int. J. Antimicrob. Agents. 60, 106599. doi: 10.1016/j.ijantimicag.2022.106599
Wu, X., Huang, C., Wang, H., Ji, J., Ying, C., Xiao, Y. (2021). Optimal empiric polymyxin B treatment of patients infected with gram-negative organisms detected using a blood antimicrobial surveillance network in China. Drug Des. Devel Ther. 15, 2593–2603. doi: 10.2147/DDDT.S313714
Xi, J., Jia, P., Zhu, Y., Yu, W., Zhang, J., Gao, H., et al. (2022). Antimicrobial susceptibility to polymyxin B and other comparators against Gram-negative bacteria isolated from bloodstream infections in China: Results from CARVIS-NET program. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1017488
Xia, G. L., Jiang, R. L. (2021). Efficacy and safety of polymyxin B in carbapenem-resistant gram-negative organisms infections. BMC Infect. Dis. 21, 1034. doi: 10.1186/s12879-021-06719-y
Yang, Q. W., Ma, X. L., Hu, F. P., Zhang, J., Sun, T. W., Chen, B. Y., et al. (2021). Expert consensus on polymyxin antimicrobial susceptibility testing and clinical interpretation. Chin. Med. Sci. J. 36, 1–16. doi: 10.24920/003864
Yu, X. B., Jiao, Z., Zhang, C. H., Dai, Y., Zhou, Z. Y., Han, L., et al. (2021). Population pharmacokinetic and optimization of polymyxin B dosing in adult patients with various renal functions. Br. J. Clin. Pharmacol. 87, 1869–1877. doi: 10.1111/bcp.14576
Yu, Z., Liu, X., Du, X., Chen, H., Zhao, F., Zhou, Z., et al. (2022). Pharmacokinetics/pharmacodynamics of polymyxin B in patients with bloodstream infection caused by carbapenem-resistant Klebsiella pneumoniae. Front. In Pharmacol. 13. doi: 10.3389/fphar.2022.975066
Zavascki, A. P., Goldani, L. Z., Li, J., Nation, R. L. (2007). Polymyxin B for the treatment of multidrug-resistant pathogens: a critical review. J. Antimicrob. Chemother. 60, 1206–1215. doi: 10.1093/jac/dkm357
Zheng, Y., Xu, B., Chen, S., Liu, M., Huang, H., Wang, J., et al. (2023). Population pharmacokinetic modeling using polymyxin B free plasma concentrations from published reports and evaluation of dosage regimens based on monte carlo simulation in critically ill patients. J. Clin. Pharmacol. 63, 1036–1044. doi: 10.1002/jcph.2261
Keywords: polymyxin B, Monte Carlo simulation, Gram-negative bacteria, bacteremia, pharmacokinetics/pharmacodynamics (PK/PD)
Citation: Yu Y, He Z and Wang C (2025) Monte Carlo simulation to optimize polymyxin B dosing regimens for the treatment of Gram-negative bacteremia. Front. Cell. Infect. Microbiol. 15:1533177. doi: 10.3389/fcimb.2025.1533177
Received: 23 November 2024; Accepted: 06 February 2025;
Published: 26 February 2025.
Edited by:
Percy Schröttner, Technische Universität Dresden, GermanyReviewed by:
Marie Louise Guadalupe Attwood, North Bristol NHS Trust, United KingdomCopyright © 2025 Yu, He and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengcheng Wang, d2NjMDI4OTkwQHFseXlxZC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.