
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Cell. Infect. Microbiol. , 07 March 2025
Sec. Intestinal Microbiome
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1525609
Introduction: The gut microbiome, specifically enterotoxigenic Bacteroides fragilis (ETBF), has been reported to play a role in colorectal cancer development. We aimed to conduct a systematic review and meta-analysis of published studies to compare the prevalence of ETBF in patients with colorectal cancer and healthy controls as well as in various stages of colorectal cancer.
Methods: PubMed, EMBASE, and The Cochrane Library were systematically searched for studies published until May 2024. We utilized studies either comparing the prevalence of ETBF in patients with colorectal cancer and healthy control or examining its prevalence across different stages of colorectal cancer. The prevalence of ETBF colonization in biological samples from individuals with colorectal cancer compared to that in healthy controls or adjacent normal tissue as well as the association between the prevalence of ETBF and various stages of colorectal cancer were plotted using a random-effect or fixed-effect model.
Results: Fourteen relevant articles were identified. Meta-analyses revealed that patients with colorectal cancer had a higher likelihood of having ETBF than healthy controls (odds ratio [OR]: 2.54, 95% confidence interval [CI]: 1.63–3.98, I2 = 55%). Additionally, ETBF detection was lower in stage I/II than in stage III/IV colorectal cancer (OR: 0.61, 95% CI: 0.41–0.91, I2 = 41%).
Discussion: The prevalence of ETBF was consistently higher in the tissue and fecal samples of patients with colorectal cancer than in those of controls. A difference in ETBF prevalence between stage I/II and stage III/IV colorectal cancer was noted, but further analysis revealed that the conclusion is unreliable.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD 42024548325.
Colorectal cancer (CRC) is the third most commonly diagnosed cancer in both men and women and the second leading cause of cancer-related deaths worldwide (Sung et al., 2021). The vast majority of CRC cases (1.9 million cases per year) are sporadic and can be attributed to various environmental factors (Islami et al., 2018). Cancer incidence in the large intestine is estimated to be 12-fold higher than that in the small intestine, which has been partially attributed to the greater bacterial density in the large intestine (Sun and Kato, 2016). In addition to host genetic factors, the gut microbiota plays an important role in CRC. An imbalance in the normal intestinal microbiota can promote chronic inflammation and carcinogenic metabolite production, ultimately leading to neoplasia (Marchesi et al., 2011).
Several bacterial species, including Helicobacter pylori, Escherichia coli, Bacteroides fragilis, Salmonella enterica, and Fusobacterium nucleatum, have been implicated in the development of CRC (Sun and Kato, 2016). A meta-analysis revealed a consistent increase in the prevalence of F. nucleatum in the tissue and fecal samples of patients with CRC compared to controls. Moreover, a high abundance of F. nucleatum in colorectal tumors was associated with poorer overall survival (Gethings-Behncke et al., 2020).
The anaerobe B. fragilis is a colonic symbiote that prefers mucosal colonization and accounts for only a small proportion of fecal microbiota (approximately 0.5%–1%). There are two molecular subtypes, nontoxigenic B. fragilis (NTBF) and enterotoxigenic B. fragilis (ETBF). According to some studies, ETBF is associated with both colitis and CRC (Basset et al., 2004; Toprak et al., 2006a; Dadgar-Zankbar et al., 2023). A review summarizes existing evidence for the association between ETBF and CRC as well as the current state of knowledge about the molecular mechanisms by which the B. fragilis toxin (BFT) influences the etiology of CRC (Scott et al., 2022). However, despite the increasing research on the relationship between ETBF and CRC, its role in the development of colorectal cancer remains largely uncertain (Zamani et al., 2020; Oliero et al., 2022). To our knowledge, no systematic reviews with meta-analyses have fully investigated the potential role of ETBF in CRC development.
This systematic review and meta-analyses of the published scientific literature aimed to assess (1) the prevalence of ETBF colonization in biological samples from individuals with CRC compared to healthy controls or adjacent normal tissues and (2) the relationship between the prevalence of ETBF and various stages of CRC.
This study was conducted based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses reporting guidelines (Liberati et al., 2009). The review protocol has been registered with PROSPERO (CRD 42024548325). The need for ethical approval or informed consent was waived in this study.
Following recommendations of the Meta-analysis of Observational Studies in Epidemiology group (Stroup et al., 2000), we searched the following electronic databases for studies written in English from their inception until May 15, 2024: PubMed, Embase, and The Cochrane Library. The following search terms were used: (“colorectal” or “colon” or “rectal”) and (“Bacteroides fragilis” or “B. fragilis” or “enterotoxigenic B. fragilis” or “enterotoxigenic Bacteroides fragilis” or “ETBF”). The search strategy was implemented by combining index words with free text keywords. In addition, the reference lists in these articles were reviewed to include more comprehensive studies.
Study selection was performed independently, in duplicate, by two reviewers (SJX, LJM), with discrepancies resolved by a third reviewer (YL), using two levels of study screening.
Inclusion criteria were as follows: (1) cohort studies, (2) human studies, (3) studies involving patients with CRC, and (3) studies reporting the prevalence of ETBF in any biological sample.
Exclusion criteria were as follows: (1) studies involving participants with malignancies other than CRC, (2) those only recruiting patients with B. fragilis but no ETBF, and (3) those that could not obtain or calculate relevant data.
Furthermore, if duplicate articles were derived from the same or overlapping patient population, only the most recent and/or complete one was included in the meta-analysis. When there were multiple groups of useful data in the same article, only the data from the group with the largest sample size was used for the analysis.
Data extraction was conducted independently, by two reviewers (SJX, LCY), with discrepancies resolved by a third reviewer (HL). The data included were authors, year of publication, study location, study design, ETBF detection method, sample type (tissue or fecal), participant status (patients with CRC or healthy controls), number of samples, and prevalence of ETBF in each sample.
Study quality was assessed using the Newcastle Ottawa Scale. Our meta-analysis categorized the study quality as good (≥7 stars), fair (4–6 stars), or poor (<4 stars).
Regarding the prevalence of ETBF, meta-analyses were used to determine the pooled odds ratios (ORs) (the definition is provided in the Supplementary Data Sheet 1) and corresponding 95% confidence intervals (CIs) of ETBF prevalence in tissue and fecal samples, respectively, using published ORs, proportions, or numbers.
Review Manager version 5.3 (North Cochrane Center, Cochrane Collaboration, London, UK) was used to analyze data. Based on I2 values (the definition is provided in the Supplementary Data Sheet 1), four categories of heterogeneity were established: no heterogeneity (I2 < 25%), low heterogeneity (25% ≤ I2 < 50%), moderate heterogeneity (50% ≤ I2 < 75%), and high heterogeneity (I2 ≥ 75%). When the I2 value was <50%, a fixed-effects model was used, while a random-effects model was used for I2 > 50%.
After identifying 2126 references, we excluded 480 duplicate publications and 1595 irrelevant studies, leaving 51 potentially eligible studies (Figure 1). Finally, 14 cohort studies (Toprak et al., 2006b; Boleij et al., 2015; Viljoen et al., 2015; Keenan et al., 2016; Haghi et al., 2019; Jasemi et al., 2020; Zamani et al., 2020; Khodaverdi et al., 2021; Piciocchi et al., 2021; Shariati et al., 2021; Oliero et al., 2022; Périchon et al., 2022; Matsumiya et al., 2023; Zhou et al., 2023) conducted between 2006 and 2023 were considered for the meta-analysis. Table 1 summarizes the general characteristics of the included studies. A total of 1692 patients were involved in these studies, with trial sizes ranging from 30 to 197 participants. Among these studies, two were from Europe, two from North America, six from West Asia, two from East Asia, one from South Africa, and one from New Zealand. Regarding the sample type, five studies used fecal samples, while nine used tissue samples. The detection method used was real-time polymerase chain reaction (PCR) in two studies, PCR in four studies, and quantitative PCR (qPCR) in eight studies. According to the quality assessment criteria, 11 studies were rated as good quality and 3 as fair quality.
Thirteen studies examined the prevalence of ETBF in patients with CRC vs. healthy controls. As shown in Figure 2, a meta-analysis of ETBF prevalence indicated that the odds of ETBF detection were higher in patients with CRC than in healthy controls (OR: 2.54, 95% CI: 1.63–3.98, I2 = 55%).
Subgroup analyses were conducted based on country, sample type, and detection method (Table 2). A significant prevalence of ETBF was noted in both Europe, America, and Oceania region (OR: 1.95, 95% CI: 1.23–3.09, I2 = 7%) and West Asia region (OR: 5.09, 95% CI: 3.06–8.47, I2 = 0%); however, no difference in prevalence was noted between East Asia region (OR: 1.10, 95% CI: 0.47–2.55) and southern Africa region (OR: 0.89, 95% CI: 0.38–2.08). The results revealed significant associations in both colorectal tissue samples from separate individuals and fecal samples from separate individuals (OR: 4.42, 95% CI: 1.71–11.42, I2 = 60% and OR: 2.69, 95% CI: 1.67–4.35, I2 = 22%), but not in adjacent colorectal tissue samples [OR: 1.07, 95% CI: 1.61–1.87, I2 = 0%]. Regarding the detection method, the results showed a significant association in the use of both PCR and qPCR (OR: 4.95, 95% CI: 2.70–9.10, I2 = 0% and OR: 1.77, 95% CI: 1.13–2.79, I2 = 31%), but not in the use of real-time PCR (OR: 4.11, 95% CI: 0.26–65.83, I2 = 89%).
Eight studies compared the prevalence of ETBF in stage I/II CRC vs. stage III/IV CRC. As shown in Figure 3, a meta-analysis assessing ETBF prevalence revealed that the risk of ETBF being detected was lower in stage I/II CRC than in stage III/IV CRC (OR: 0.61, 95% CI: 0.41–0.91, I2 = 41%).
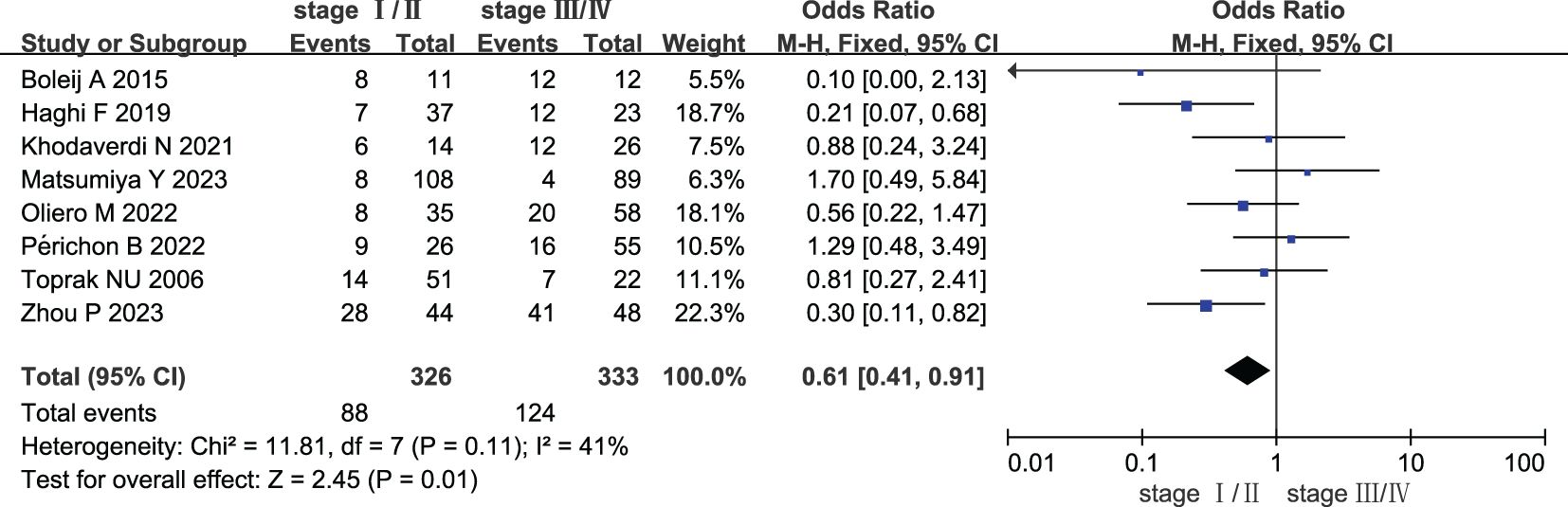
Figure 3. Forest plots of comparison between patients with stage I/II colorectal cancer and stage III/IV colorectal cancer.
Subgroup analyses were also conducted based on country, sample type, and detection method (Table 3). There was no significant prevalence of ETBF in Europe (OR: 0.70, 95% CI: 0.27–1.81, I2 = 37%), West Asia region (OR: 0.53, 95% CI: 0.21–1.30, I2 = 43%), and East Asia region (OR: 0.69, 95% CI: 0.13–3.77, I2 = 78%). The findings revealed that the association was not significant in either colorectal tissue or fecal samples (OR: 0.59, 95% CI: 0.32–1.09, I2 = 52% and OR: 0.63, 95% CI: 0.38–1.05, I2 = 46%). The results of the detection method showed that there was no significant association in both the use of qPCR and real-time PCR (OR: 0.63, 95% CI: 0.38–1.06, I2 = 32% and OR: 1.70, 95% CI: 0.49–5.84), but there was a significant association with the use of PCR (OR: 0.38, 95% CI: 0.18–0.80, I2 = 44%).
Sensitivity analysis was performed to assess the stability of the results, which resulted in the removal of one study from the meta-analysis at a time. The results revealed no change in the corresponding merged estimates of comparison between patients with colorectal cancer and healthy controls. Table 4 presents the results of the sensitivity analysis. The results revealed a change in the corresponding merged estimates of comparison between patients with stage I/II colorectal cancer and stage III/IV colorectal cancer, indicating that two studies influenced the results: Haghi (2019) and Zhou (2023). Further investigation is required to elucidate the discrepancies between these two studies and the remaining six studies to ascertain the underlying causes responsible for this observed influence, which is beyond the scope of this work. Table 5 presents the results of the sensitivity analysis.
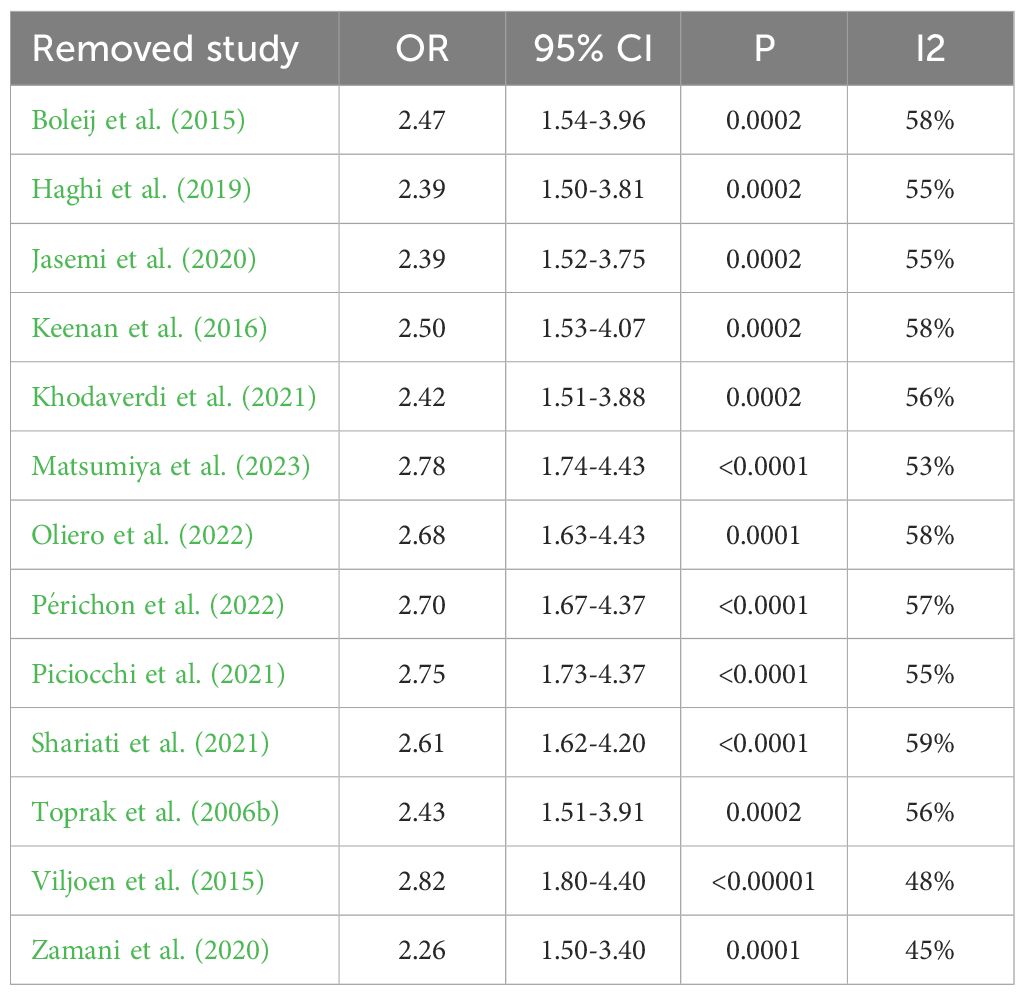
Table 4. Sensitivity analysis results after removing one study at a time of comparison between patients with colorectal cancer and healthy controls.
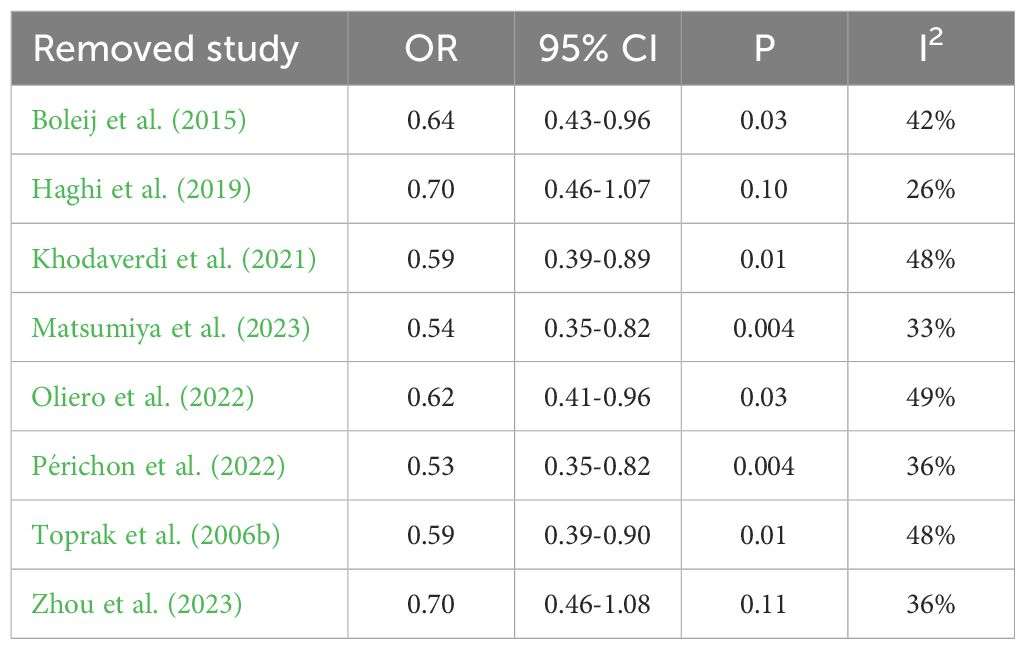
Table 5. Sensitivity analysis results after removing one study at a time of comparison between patients with stage I/II colorectal cancer and stage III/IV colorectal cancer.
Figures 4 and 5 show funnel plots with scatter points that were generally symmetrical within the CIs, each study was evenly distributed on both sides of the vertical line, indicating that there was no significant publication bias.
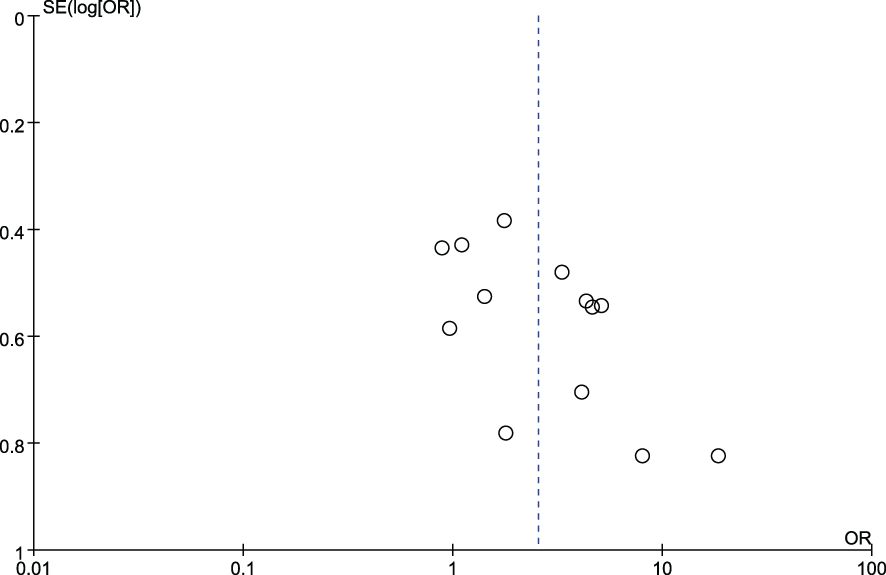
Figure 4. Funnel plot diagram of comparison between patients with colorectal cancer and healthy controls.
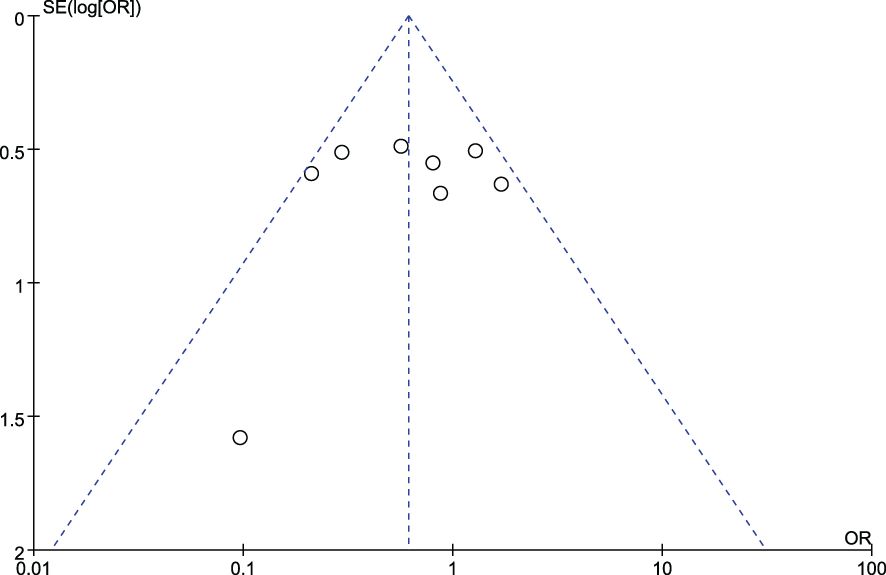
Figure 5. Funnel plot diagram of comparison between patients with stage I/II colorectal cancer and those with stage III/IV colorectal cancer.
The association between ETBF and CRC has attracted increasing interest. In this first comprehensive systematic review with meta-analyses of published literature, we aimed to investigate the relationship between ETBF and CRC, shedding light on its potential role in CRC development and progression.
Our findings suggest that ETBF is more prevalent in patients with CRC than in healthy controls, particularly in mucosal tissue or fecal samples from different individuals as shown by the subgroup analysis. The included studies revealed that ETBF colonization was more common in patients with CRC (6.1%–88.5%) than in healthy controls (3.8%–64.9%). This is consistent with previous research suggesting an association between ETBF colonization and CRC pathogenesis (Nouri et al., 2022). ETBF pathogenicity is attributed to BFT, a 20-kDa zinc-dependent metalloprotease toxin with three isotypes (BFT1, BFT-2, and BFT-3) (Sears, 2009). BFT binds to a specific colonic epithelial receptor, activating the Wnt and NF-κB signaling pathways, resulting in increased cell proliferation, epithelial release of proinflammatory mediators, and DNA damage (Sears, 2009; Goodwin et al., 2011), whereas ETBF promotes tumor formation in experimental animals (Wu et al., 2009; Goodwin et al., 2011).
Our analysis suggested that the detection rate of ETBF did not differ significantly between adjacent colorectal tissue samples and CRC tissue samples, but the next conclusion showed significant differences in ETBF prevalence between stage I/II and stage III/IV CRC. A previous study showed that ETBF supports the progression of malignancy as well as tumorigenesis (Kim and Lee, 2022). This suggests that ETBF may play a role in CRC initiation, and could possibly correlate with disease progression or severity. Therefore, this finding should be confirmed in larger cohorts.
Gut microbiota is a complex ecosystem that evolves in tandem with hosts and is influenced by their physiological environment. The composition and function of gut microbiota are closely associated with dietary habits and regional differences. Human dietary patterns have a direct impact on the abundance and diversity of gut microbiota. Diet is an important modifiable factor influencing the gut microbiome (Leeming et al., 2019). Furthermore, the proportion of plant-based and animal-based foods in the diet influences gut microbiota composition. The alteration in the abundance and diversity of gut microbiota caused by dietary changes has been associated with colorectal carcinogenesis (Appunni et al., 2021; Levy et al., 2021; Rebersek, 2021; Zygulska and Pierzchalski, 2022). Moreover, recent research has indicated that transitioning from a traditional to Western diet increases the abundance of CRC-associated bacteria (Ahmad Kendong et al., 2021). Another study demonstrated that switching from a traditional to Western diet increases the risk of CRC (Le Marchand and Kolonel, 1992). In our subgroup analysis of comparison between patients with CRC and healthy controls, the findings confirmed that individuals from different regions may have different outcomes due to differences in dietary habits.
These findings have two important implications. First, the increased prevalence of ETBF in the mucosal tissue or fecal samples of patients with CRC suggests that it can be used as a biomarker for CRC screening and diagnosis. Detection of ETBF may serve as an adjunctive tool in existing screening protocols to improve the sensitivity and specificity of CRC detection methods. Second, the consistent detection of ETBF in various stages of CRC highlights the need for additional research to determine its precise role in CRC pathogenesis. Understanding the mechanisms underlying ETBF-induced carcinogenesis may pave the way for targeted therapeutic interventions that disrupt the ETBF–CRC axis. However, a limitation is that the small number of studies prevented a formal assessment of publication or reporting bias, which may reduce the robustness of some meta-analyses involving subgroup analyses.
There is consistent evidence that ETBF is more prevalent in the fecal and tissue samples of patients with CRC than in healthy controls. Further prospective studies into the role of ETBF as a causal factor or predictive biomarker for CRC promotion and development are warranted.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
SX: Conceptualization, Data curation, Formal Analysis, Methodology, Software, Visualization, Writing – original draft, Writing – review & editing. LM: Conceptualization, Data curation, Formal Analysis, Writing – original draft. HL: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. YL: Conceptualization, Data curation, Writing – original draft. LY: Conceptualization, Data curation, Writing – original draft.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Funding was received for the construction of key clinical specialties in Futian District, Shenzhen.
The authors would like to thank HL, for her generous support during the preparation of this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1525609/full#supplementary-material
Ahmad Kendong, S. M., Raja Ali, R. A., Nawawi, K. N.M., Ahmad, H. F., Mokhtar, N. M., et al. (2021). Gut dysbiosis and intestinal barrier dysfunction: potential explanation for early-onset colorectal cancer. Front. Cell. Infect. Microbiol. 11, 1244. doi: 10.3389/fcimb.2021.744606
Appunni, S., Rubens, M., Ramamoorthy, V., Tonse, R., Saxena, A., McGranaghan, P., et al. (2021). Emerging evidence on the effects of dietary factors on the gut microbiome in colorectal cancer. Front. Nutr. 8, 752. doi: 10.3389/fnut.2021.718389
Basset, C., Holton, J., Bazeos, A., Vaira, D., Bloom, S. (2004). Are Helicobacter species and enterotoxigenic Bacteroides fragilis involved in inflammatory bowel disease? Dig. Dis. Sci. 49, 1425–1432. doi: 10.1023/b:ddas.0000042241.13489.88
Boleij, A., Hechenbleikner, E. M., Goodwin, A. C., Badani, R., Stein, E. M., Lazarev, M. G., et al. (2015). The Bacteroides fragilis toxin gene is prevalent in the colon mucosa of colorectal cancer patients. Clin. Infect. Dis. 60, 208–215. doi: 10.1093/cid/ciu787
Dadgar-Zankbar, L., Shariati, A., Bostanghadiri, N., Elahi, Z., Mirkalantari, S., Razavi, S., et al. (2023). Evaluation of enterotoxigenic Bacteroides fragilis correlation with the expression of cellular signaling pathway genes in Iranian patients with colorectal cancer. Infect. Agent Cancer 18, 48. doi: 10.1186/s13027-023-00523-w
Gethings-Behncke, C., Coleman, H. G., Jordao, H. W. T., Longley, D. B., Crawford, N., Murray, L. J., et al. (2020). Fusobacterium nucleatum in the colorectum and its association with cancer risk and survival: A systematic review and meta-analysis. Cancer Epidemiol. Biomarkers Prev. 29, 539–548. doi: 10.1158/1055-9965.EPI-18-1295
Goodwin, A. C., Shields, C. E. D., Wu, S., Huso, D. L., Wu, X., Murray-Stewart, T. R., et al. (2011). Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc. Natl. Acad. Sci. 108, 15354e15359. doi: 10.1073/pnas.1010203108
Haghi, F., Goli, E., Mirzaei, B., Zeighami, H. (2019). The association between fecal enterotoxigenic B. fragilis with colorectal cancer. BMC Cancer 19, 879. doi: 10.1186/s12885-019-6115-1
Islami, F., Goding Sauer, A., Miller, K. D., Siegel, R. L., Fedewa, S. A., Jacobs, E. J., et al. (2018). Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J. Clin. 68, 31–54. doi: 10.3322/caac.21440
Jasemi, S., Emaneini, M., Fazeli, M. S., Ahmadinejad, Z., Nomanpour, B., Sadeghpour Heravi, F., et al. (2020). Toxigenic and non-toxigenic patterns I, II and III and biofilm-forming ability in Bacteroides fragilis strains isolated from patients diagnosed with colorectal cancer. Gut Pathog. 12, 28. doi: 10.1186/s13099-020-00366-5
Keenan, J. I., Aitchison, A., Purcell, R. V., Greenlees, R., Pearson, J. F., Frizelle, F. A. (2016). Screening for enterotoxigenic Bacteroides fragilis in stool samples. Anaerobe 40, 50–53. doi: 10.1016/j.anaerobe.2016.05.004
Khodaverdi, N., Zeighami, H., Jalilvand, A., Haghi, F., Hesami, N. (2021). High frequency of enterotoxigenic Bacteroides fragilis and Enterococcus faecalis in the paraffin-embedded tissues of Iranian colorectal cancer patients. BMC Cancer 21, 1353. doi: 10.1186/s12885-021-09110-x
Kim, J., Lee, H. K. (2022). Potential role of the gut microbiome in colorectal cancer progression. Front. Immunol. 12. doi: 10.3389/fimmu.2021.807648
Leeming, E. R., Johnson, A. J., Spector, T. D., Le Roy, C. I. (2019). Effect of diet on the gut microbiota: rethinking intervention duration. Nutrients 11, 2862. doi: 10.3390/nu11122862
Le Marchand, L., Kolonel, L. (1992). Cancer in Japanese migrants to Hawaii: interaction between genes and environment. Rev. d’epidemiologie sante publique 40, 425–430.
Levy, B. T., Daly, J. M., Xu, Y., Crockett, S. D., Hoffman, R. M., Dawson, J. D., et al. (2021). Comparative effectiveness of five fecal immunochemical tests using colonoscopy as the gold standard: study protocol. Contemp. Clin. Trials 106, 106430. doi: 10.1016/j.cct.2021.106430
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PloS Med. 6,7, e1000100. doi: 10.1371/journal.pmed.1000100
Marchesi, J. R., Dutilh, B. E., Hall, N., Peters, W. H., Roelofs, R., Boleij, A., et al. (2011). Towards the human colorectal cancer microbiome. PloS One 6, e20447. doi: 10.1371/journal.pone.0020447
Matsumiya, Y., Suenaga, M., Ishikawa, T., Kudo, T., Nakagawa, T., Okamoto, K., et al. (2023). Clinical significance of Bacteroides fragilis as a potential prognostic factor in colorectal cancer. Anaerobe 84, 102784. doi: 10.1016/j.anaerobe.2023.102784
Nouri, R., Hasani, A., Asgharzadeh, M., Sefidan, F. Y., Hemmati, F., Rezaee, M. A. (2022). Roles of gut microbiota in colorectal carcinogenesis providing a perspective for early diagnosis and treatment. Curr. Pharm. Biotechnol. 23, 1569–1580. doi: 10.2174/1389201023666220307112413
Oliero, M., Hajjar, R., Cuisiniere, T., Fragoso, G., Calvé, A., Dagbert, F., et al. (2022). Prevalence of pks + bacteria and enterotoxigenic Bacteroides fragilis in patients with colorectal cancer. Gut Pathog. 14, 51. doi: 10.1186/s13099-022-00523-y
Périchon, B., Lichtl-Häfele, J., Bergsten, E., Delage, V., Trieu-Cuot, P., Sansonetti, P., et al. (2022). Detection of Streptococcus gallolyticus and Four Other CRC-Associated Bacteria in Patient Stools Reveals a Potential “Driver” Role for Enterotoxigenic Bacteroides fragilis. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.794391
Piciocchi, A., Germinario, E. A.P., Garcia Etxebarria, K., Rossi, S., Sanchez-Mete, L., Porowska, B., et al. (2021). Association of polygenic risk score and bacterial toxins at screening colonoscopy with colorectal cancer progression: A multicenter case-control study. Toxins (Basel). 13, 569. doi: 10.3390/toxins13080569
Rebersek, M. (2021). Gut microbiome and its role in colorectal cancer. BMC Cancer 21, 1–13. doi: 10.1186/s12885-021-09054-2
Scott, N., Whittle, E., Jeraldo, P., Chia, N. (2022). A systemic review of the role of enterotoxic Bacteroides fragilis in colorectal cancer. Neoplasia 29, 100797. doi: 10.1016/j.neo.2022.100797
Sears, C. L. (2009). Enterotoxigenic Bacteroides fragilis: a rogue among symbiotes. Clin. Microbiol. Rev. 22, 349e369. doi: 10.1128/CMR.00053-08
Shariati, A., Razavi, S., Ghaznavi-Rad, E., Jahanbin, B., Akbari, A., Norzaee, S., et al. (2021). Association between colorectal cancer and Fusobacterium nucleatum and Bacteroides fragilis bacteria in Iranian patients: a preliminary study. Infect. Agent Cancer 16, 41. doi: 10.1186/s13027-021-00381-4
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012. doi: 10.1001/jama.283.15.2008
Sun, J., Kato, I. (2016). Gut microbiota, inflammation and colorectal cancer. Genes Dis. 3, 130–143. doi: 10.1016/j.gendis.2016.03.004
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 71, 209–249. doi: 10.3322/caac.21660
Toprak, N. U., Yagci, A., Gulluoglu, B. M., Akin, M. L., Demirkalem, P., Celenk, T., et al. (2006a). A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 12, 782–786. doi: 10.1111/j.1469-0691.2006.01494.x
Toprak, N. U., Yagci, A., Gulluoglu, B. M., et al. (2006b). A possible role of Bacteroides fragilis enterotoxin in the aetiology of colorectal cancer. Clin. Microbiol. Infect. 12, 782–786. doi: 10.1111/j.1469-0691.2006.01494.x
Viljoen, K. S., Dakshinamurthy, A., Goldberg, P., Blackburn, J. M. (2015). Quantitative profiling of colorectal cancer-associated bacteria reveals associations between fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and clinicopathological features of colorectal cancer. PloS One 10, e0119462. doi: 10.1371/journal.pone.0119462
Wu, S., Rhee, K.-J., Albesiano, E., Rhee, K. J., Albesiano, E., Rabizadeh, S., Wu, X., Yen, H. R., et al. (2009). A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nat. Med. 15, 1016e1022. doi: 10.1038/nm.2015
Zamani, S., Taslimi, R., Sarabi, A., Jasemi, S., Sechi, L. A., Feizabadi, M. M. (2020). Enterotoxigenic bacteroides fragilis: A possible etiological candidate for bacterially-induced colorectal precancerous and cancerous lesions. Front. Cell Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00449
Zhou, P., Dai, Z., Xie, Y., Li, T., Xu, Z., Huang, Y., et al. (2023). Differences in tissue-associated bacteria between metastatic and non-metastatic colorectal cancer. Front. Microbiol. 14. doi: 10.3389/fmicb.2023.1133607
Keywords: enterotoxigenic Bacteroides fragilis, colorectal cancer, prevalence, systematic review, meta-analysis
Citation: Xia S, Ma L, Li H, Li Y and Yu L (2025) Prevalence of enterotoxigenic Bacteroides fragilis in patients with colorectal cancer: a systematic review and meta-analysis. Front. Cell. Infect. Microbiol. 15:1525609. doi: 10.3389/fcimb.2025.1525609
Received: 20 November 2024; Accepted: 19 February 2025;
Published: 07 March 2025.
Edited by:
Momchilo Vuyisich, Viome Life Sciences, Inc., United StatesCopyright © 2025 Xia, Ma, Li, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Li, bGlodWl0Y21AMTI2LmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.