
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 07 March 2025
Sec. Intestinal Microbiome
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1521754
This article is part of the Research Topic Influence of Maternal Dietary Patterns on Offspring Gut Microbiome and Immunity View all 3 articles
The gut microbiota is the collective term for the microorganisms that reside in the human gut. In recent years, advances in sequencing technology and bioinformatics gradually revealed the role of gut microbiota in human health. Dramatic changes in the gut microbiota occur during pregnancy due to hormonal and dietary changes, and these changes have been associated with certain gestational diseases such as preeclampsia (PE) and gestational diabetes mellitus (GDM). Modulation of gut microbiota has also been proposed as a potential treatment for these gestational diseases. The present article aims to review current reports on the association between gut microbiota and gestational diseases, explore possible mechanisms, and discuss the potential of probiotics in gestational diseases. Uncovering the link between gut microbiota and gestational diseases could lead to a new therapeutic approach.
Pregnancy is a normal physiological phenomenon, during which the major organs of the body undergo a series of adaptive changes to meet the needs of pregnancy (Ouzounian and U, 2012). Successful pregnancy is influenced by many factors such as environmental pollution, poor lifestyle habits, and others (Thiele et al., 2019). At the same time, pregnancy is considered an allograft and immune tolerance between maternal tissue, placenta and fetus is essential for a successful pregnancy (Goldstein et al., 2020). Extensive clinical and basic research has made great strides in understanding the various factors that contribute to a successful pregnancy. However, changes in the gut microbiota during pregnancy have been overlooked by clinicians and researchers for many years. In fact, the gut microbiota also plays an important role in successful pregnancy, and gut dysbiosis has been shown to be associated with many pregnancy complications (Ziętek et al., 2021).
Despite the current sophisticated healthcare measures during pregnancy, the incidence of diseases in pregnancy (especially metabolic diseases) continues to be high as living standards improve. For instance, the global prevalence of gestational diabetes mellitus (GDM), a classic metabolic disorder, is approximately 14% and has an impact on pregnancy outcomes (Wang et al., 2022). Gut microbiota is the general term for microbes that colonize the human gut. Benefiting from the Human Microbiome Project, the composition of gut microbiota and its role in human health has been gradually reported (Consortium., I H i R N, 2019). Indeed, the gut microbiota of healthy individuals always have higher diversity and predominant phyla are Firmicutes, Actinobacteria, Bacteroidetes, and Proteobacteria. Human body may suffer a series of immune- or metabolism-related diseases when gut dysbiosis occurs (G, 2020). Mechanistic research revealed that an increase in the relative abundance of opportunistic pathogenic bacteria and a decrease in the relative abundance of probiotic bacteria are thought to induce or promote disease progression by promoting inflammation, increasing toxic metabolites, increasing intestinal epithelial permeability, and altering crosstalk between the intestine and individual organs such as the brain and liver. Moreover, the gut microbiota was supposed to be associated with gestational diseases such as preeclampsia (Chen et al., 2020), gestational diabetes mellitus (Medici Dualib et al., 2021), and postpartum depression (Hou et al., 2020) in Figure 1. For example, the over proliferation of Proteobacteria and the progressive decrease of Firmicutes in the intestinal flora are thought to be associated with the development of GDM, and the relative abundance of Neiss./Lepto. and Prevo./Aeroc. has been shown to be positively correlated with OGTT test values (Wang et al., 2018). Likewise, higher abundance of Bacteroidetes, Proteobacteria, and Actinobacteria was observed in the intestinal flora of preeclampsia patients, and these alterations in intestinal bacteria are thought to be associated with higher serum concentrations of toxic bacterial products such as LPS and TMAO (Wang et al., 2019). These studies imply that gut microbiota is inextricably linked to gestational diseases and elucidating the involved pathogenic mechanisms is essential for their prevention and treatment. Given the important role that gut microbiota plays in health, this review aims to summarize the currently published literature on the relationship between gut microbiota and gestational diseases, and discuss the potential mechanism of microbe-host interaction during pregnancy. It is expected to provide a new perspective for future research on gut microbiota and pregnancy diseases as well as the clinical application of gut microbiota intervention.
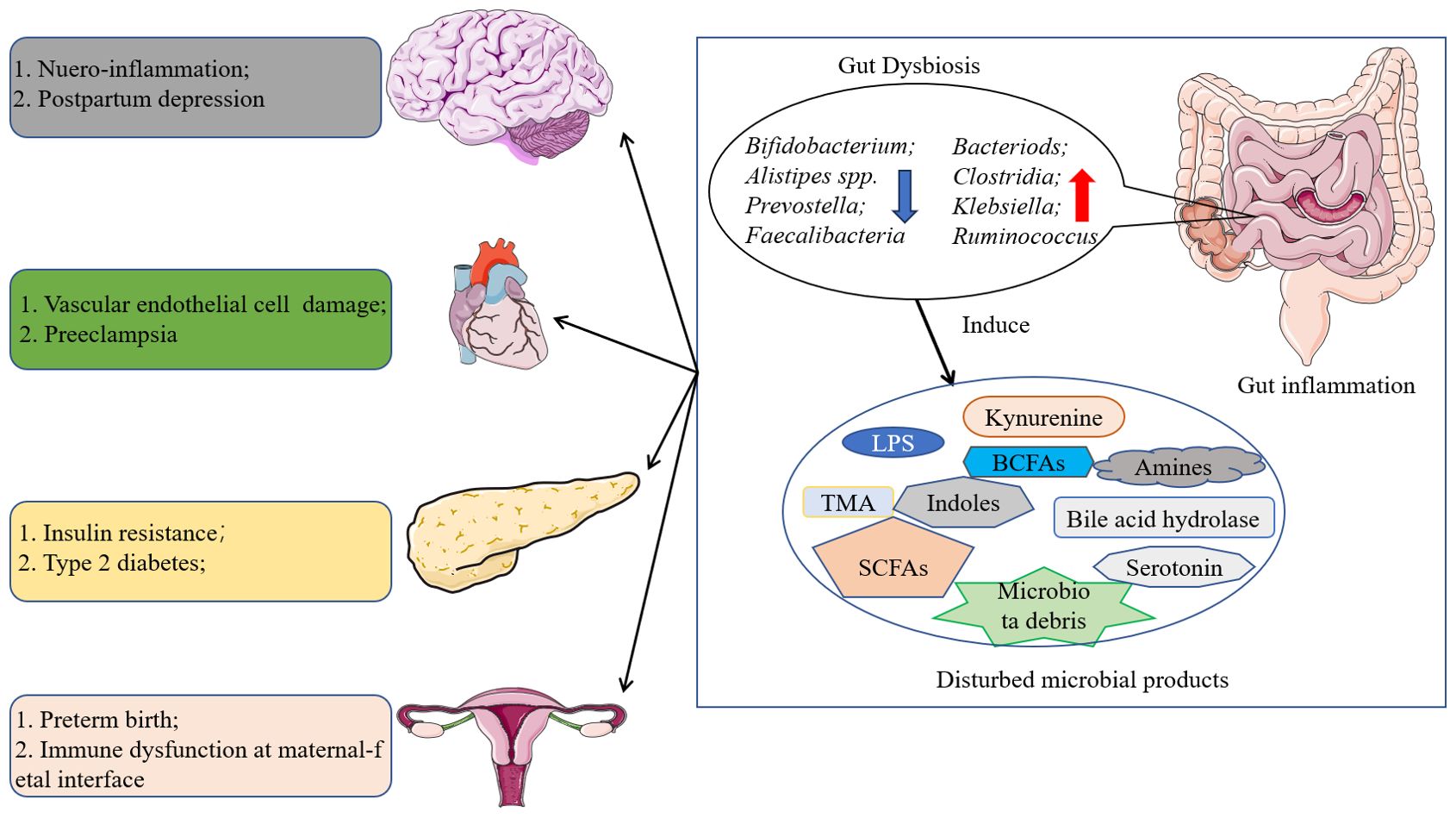
Figure 1. Role of gut microbiota in organs dysfunction. The disturbed gut microbiota may induce lot kind of diseases by modulating the metabolism of fatty acids and amino acids and so on, and further leading to auto-immune disease and metabolism related diseases.
In general, the gut microbiota of healthy adults can be mainly divided into four categories, namely Bacteroidetes, Firmicutes, Actinobacteria and Proteobacteria, which account for 23%, 64%, 3% and 8%, respectively (Adak and K, 2019). The structure of normal intestinal flora can be affected by changes in hormone levels. A study comparing the differences in gut microbial structure between uncastrated male mice, castrated male mice and female mice showed that the structure of gut flora was most similar in castrated male mice and female mice (Yurkovetskiy et al., 2013). Conversely, the intestinal flora also affects the level of hormones in the body, and this phenomenon is related to the deconjugation of food-derived hormones by β-glucuronidase synthesized by the intestinal flora (He et al., 2021). Nevertheless, the impact of fluctuations in estrogen and progesterone levels on the gut microbiota of women during the natural menstrual cycle remains somewhat controversial. Mihajlovic et al. compared gut microbiota diversity during the follicular phase (when serum sex hormone levels are relatively low) and the luteal phase (when serum sex hormone levels are relatively high) in 9 women aged 21-29. The results indicated that fluctuations in serum sex hormone levels throughout the menstrual cycle were insufficient to influence β-diversity of gut microbiota (Mihajlovic et al., 2021). Another study involving 20 Belgian women of reproductive age continuously sampled their gut microbiota over a period of six weeks and conducted microbiome analysis. The results showed that the majority of gut microbes exhibited significant temporal variation, with some bacterial genera experiencing over a 100-fold change in abundance during the study period. Furthermore, the diversity and evenness indices of the participants’ gut microbiota fluctuated considerably. However, researchers believe that these changes in the gut microbiota are unrelated to the fluctuations in sex hormone levels during the menstrual cycle (Vandeputte et al., 2021). Although the aforementioned studies all indicate that gut microbiota diversity does not change with hormonal fluctuations during the menstrual cycle, both studies note an increase in the relative abundance of Akkermansia during the luteal phase when hormone levels rise.
Similarly, changes in the gut microbiota during pregnancy have been reported (Nuriel-Ohayon et al., 2019; Yoon and K, 2021), with alterations of the intestinal flora occurring primarily during the second and third trimesters in response to increasing levels of relevant hormones (Sun et al; Smid et al., 2018). Studies have demonstrated that during early pregnancy, the gut microbiota remains predominantly dominated by Firmicutes, exhibiting consistent stability compared to the pre-pregnancy period (Koren et al., 2012). As pregnancy progresses, gut microbiota changes are primarily characterized by reduced α-diversity (within hosts), and increased dissimilarity between hosts (β-diversity) (Nuriel-Ohayon et al., 2016). The relative abundances of butyrate-producing Bifidobacteria and lactic acid-producing microorganisms increase (Ruebel et al., 2021), as do the relative abundance of Akkermansia (Qin et al., 2021), similar to changes during the menstrual cycle, potentially associated with elevated sex hormones during pregnancy. These changes in gut microbiota are thought to drive the host to store sufficient energy, maintain insulin resistance, and ensure relatively high blood glucose levels to meet the developmental needs of the fetus, placing the host in a state of metabolic conditions akin to diabetes (Koren et al., 2012; Gomez-Arango et al., 2016a; Ziętek et al., 2021). Researchers provided experimental evidence for this theory by transplanting the gut microbiota of late-term pregnant women into germ-free mice; glucose metabolism disorders were observed in the mice that received the microbiota transplant (Koren et al., 2012). Moreover, as pregnancy progresses, the abundance of Actinobacteria and Proteobacteria increases, and microbial communities associated with short-chain fatty acid and butyrate production decrease to some extent (Koren et al., 2012). Adaptive adjustments in the gut microbiota occur during normal pregnancy are thought to serve several important functions, including promoting fetal growth and development, reducing infection risk in the host, and optimizing the host’s nutritional metabolism (Sinha et al., 2023).
During pregnancy, various factors other than hormones can also influence the gut microbiota. Diet is a key factor that can impact the gut microbiota, whether pregnant or not (Barrett et al., 2018). A diet high in fat and salt, as well as the intake of trace elements and dietary fiber, can all influence gut microbiota (Gioia et al., 2020). Guo et al. studied the effects of a high-salt diet on gut microbiota and reported that a high-salt diet increased the relative abundance of Proteobacteria and Bacteroides (Guo et al., 2021). The effects of alcohol consumption on maternal gut microbiota during pregnancy were also studied, with alcohol consumption positively correlated with Phascolarctobacterium and Blautia and negatively correlated with Faecalibacterium (Wang et al., 2021). In addition, the ingestion of exogenous bacterial preparations during pregnancy can alter gut microbiota structure. A study of mice administered Akkermansia muciniphila during pregnancy showed that supplementation can alter diversity and composition of the gut microbiota and strengthen the gut barrier (Qi et al., 2022). Furthermore, immune changes (F, 2020), antibiotic intervention (Zhou et al., 2020; Benner et al., 2021), sleep factors (Yao et al., 2022), and psychological stress (Hechler et al., 2019) can all influence gut microbiota composition in humans and animals. It is clear that the gut microbiota of pregnant women can change during pregnancy.
Women experience major metabolic changes from preconception through pregnancy, primarily manifested as enhanced anabolic metabolism, elevated serum free fatty acid and steroid hormone levels, and decreasing amino acid levels. Additionally, fasting blood glucose decreases in the early stages of pregnancy, while a degree of insulin resistance may occur in the second and third trimesters (Hadden and M, 2009; Liang et al., 2020). All these pregnancy-related metabolic changes typically return to pre-pregnancy levels shortly after childbirth. Gestational diabetes mellitus (GDM) refers to a disorder of maternal blood glucose metabolism first diagnosed during pregnancy (Association, A D, 2019). In terms of pathophysiology, the main aberrations in GDM are chronic inflammation, insulin resistance, and pancreatic islet β cell dysfunction (Plows et al., 2018). It has numerous effects on the mother and fetus, including abnormal fetal development, polyhydramnios, infections, and fetal macrosomia and dystocia (Szmuilowicz et al., 2019). The impact of gestational diabetes mellitus on mothers does not appear to be limited to pregnancy. Studies have shown that patients with gestational diabetes mellitus have a 10-fold higher risk of developing type 2 diabetes later in life (Vounzoulaki et al., 2020), and it can also program the metabolism of offspring and severely affect their health (Chen et al., 2018). So far, insulin is still the most effective therapy, but it can sometimes cause side effects. Therefore, finding a new target to prevent it is of great importance.
In recent years, there has been an increase in research on the gut microbiota, revealing differences in the gut microbiota of patients with and without gestational diabetes mellitus (GDM) and their putative roles (shown in Table 1). Wei et al. compared the gut microbiota of GDM patients (n=15) with that of healthy controls (n=18), reporting differences in β diversity of the gut microbiota and a higher abundance of Ruminococcus bromii, Clostridium colinum, and Streptococcus infantis in GDM patients. Among these altered bacteria, Streptococcus infantis was positively associated with a higher risk of GDM (Wei et al., 2022). Another study also reported differences in β diversity while several altered bacteria including Bacteroides dorei and Bacteroides spp. 3_1_33FAA, were negatively associated with glucose tolerance, and Alistipes putredinis positively related to insulin sensitivity (Wu et al., 2020). Although similar studies have reported differences in the gut microbiota of GDM patients and healthy pregnant women, the specific altered bacteria have not been consistent, possibly due to different study methods and subjects (Medici Dualib et al., 2021). To better elucidate the causal relationship between gut microbiota and GDM, Sun et al. compared the gut microbiota of GDM patients (n=120) before disease onset with that of healthy pregnant women (n=120) of the same gestational age, reporting differences existed before disease onset. Compared to the control group, during pregnancy, the relative abundance of Ruminococcus bromii was consistently lower in patients with GDM. Moreover, Desulfovibrio and Bacteroides ovatus also remained lower at T1 and T2. The study also indicates that gut fiber fermentation metabolism is associated with blood glucose control in patients with gestational diabetes (Sun et al). Furthermore, transplantation of the gut microbiota of GDM patients to germ-free mice developed abnormal blood glucose metabolism, supporting a causal relationship (Liu et al., 2020). This result provides strong evidence for a causal relationship between gut microbiota and GDM, and demonstrate that the gut microbiota of different types exerts distinct impacts on the occurrence of GDM. Moreover, one study reported changes in the gut microbiota of GDM patients following metformin therapy, suggesting that this change may represent a therapeutic mechanism of metformin (Molina-Vega et al., 2022). Diet modification (Ponzo et al., 2019), exercise increases (Mahizir et al., 2020), probiotics consumption (Zheng et al., 2021), and trace elements supplementation (Zhang et al., 2021) have all been reported to improve glucose metabolism in GDM patients by modulating or restoring gut microbiota. Additionally, some researchers utilized mendelian randomization, a causal inference analytical technique, to investigate potential causal associations between differences in gut microbiota and GDM at the genetic level. Study results indicate that an increased relative abundance of Collinsella, Coprobacter, Olsenella, Lachnoclostridium, Prevotella 9, and Ruminococcus 2 may be associated with an increased risk of GDM. Conversely, the abundance of Oscillibacter and Methanobrevibacter is negatively correlated with the risk of gestational diabetes mellitus. In addition, this study reveals that gut microbiome metabolites significantly associated with an increased risk of GDM include serine, indole, acetate, adrenate, and phenylacetate; while metabolites such as pyruvate, pipecolate, glycodeoxycholate, and carnitine demonstrate potential protective effects against GDM (Wu et al., 2023b). Although the reported gut microbiota-GDM relationship offer a new perspective, the primary task remains clarifying potential mechanisms by which gut microbiota contributes to insulin resistance, thereby better exploiting the gut microbiota as a strategy for treating or preventing GDM.
Mechanistically, GDM is characterized by relative insulin insensitivity, which explains the tendency for individuals to develop type 2 diabetes later in life (Vounzoulaki et al., 2020), and may be at the core of the relationship between gut microbiota and GDM. Insulin receptor substrate-1 (IRS-1), a key link in the insulin signaling pathway (Yaribeygi et al., 2019), has been shown to be phosphorylated and inactivated when the NF-κB pathway is activated, resulting in attenuated insulin signaling (Zhang et al., 2018). This is the mechanism by which inflammation impairs tissue insulin sensitivity. Additionally, lipopolysaccharide (LPS), the major component of the cell wall of Gram-negative bacteria, also known as endotoxin, has been shown to activate the NF-κB pathway in type 2 diabetes (Andreasen et al., 2011). In the absence of infection, the gut is considered the primary source of circulating serum LPS. Dysbiosis of the gut microbiota in pregnancy-related disorders can lead to intestinal barrier damage, ultimately resulting in significantly increased serum LPS levels (Huang et al., 2021b). Therefore, it is plausible that excessive activation of the LPS/NF-κB pathway may be one mechanism by which gut dysbiosis during pregnancy contributes to GDM. Moreover, it has been gradually reported that the fermentation products of the gut microbiota play some role in GDM (see in Table 2) (Gao et al., 2020a). Short-chain fatty acids (SCFAs), the most studied fermentation products of carbohydrates derived from the gut microbiota, are mainly composed of acetate, butyrate and propionate, and play a vital role in metabolism (Morrison and P, 2016). Recent studies have reported that SCFAs promote the secretion of glucagon-like peptide 1 (GLP-1) and peptide YY (PYY) by activating intestinal receptors GPR41/43, thereby increasing tissue sensitivity to insulin (Kimura et al., 2014). SCFAs have also been shown to play a role in maintaining intestinal epithelial barrier function, and a deficiency of SCFAs can lead to an excessive influx of LPS that eventually causes inflammation, or directly result in decreased secretion of anti-inflammatory mediators, disrupting glucose metabolism (Martin-Gallausiaux et al., 2021). SCFAs can even alter energy expenditure by affecting the activity of uncoupling proteins (He et al., 2020). In addition to SCFAs, amino acid metabolism is also involved in the interaction between gut dysbiosis and insulin resistance (Zhang et al., 2019). A normal gut microbiota can convert aromatic amino acids to indoles, which has been reported to play a protective role in maintaining the intestinal barrier and promoting GLP-1 secretion, thereby improving tissue insulin sensitivity (Canfora et al., 2019). Trimethylamine n-oxide (TMAO), the product of choline fermentation by gut microbiota, has also been shown to contribute to the regulation of metabolic function (Day-Walsh et al., 2021). It can trigger endoplasmic reticulum stress and lead to tissue remodeling disorders (Govindarajulu et al., 2020). Further, Yang et al. performed cell assemblies and reported that TMAO promotes apoptosis of pancreatic acinar cells by activating the expression of endoplasmic reticulum stress signaling pathway (Yang and Z, 2021). However, Krueger et al. concluded to the opposite, that TMAO has some protective effect on pancreatic β cell damage and islet dysfunction caused by type 2 diabetes-like glucolipotoxicity (Krueger Es et al., 2021). Moreover, TMAO may also induce inflammation via the NF-κB pathway, which has been reported to block insulin signaling (Yang G et al., 2019). Combined with the above studies, despite inconsistent results, we still conclude that the gut microbiota plays a pivotal role in GDM (Figure 2).
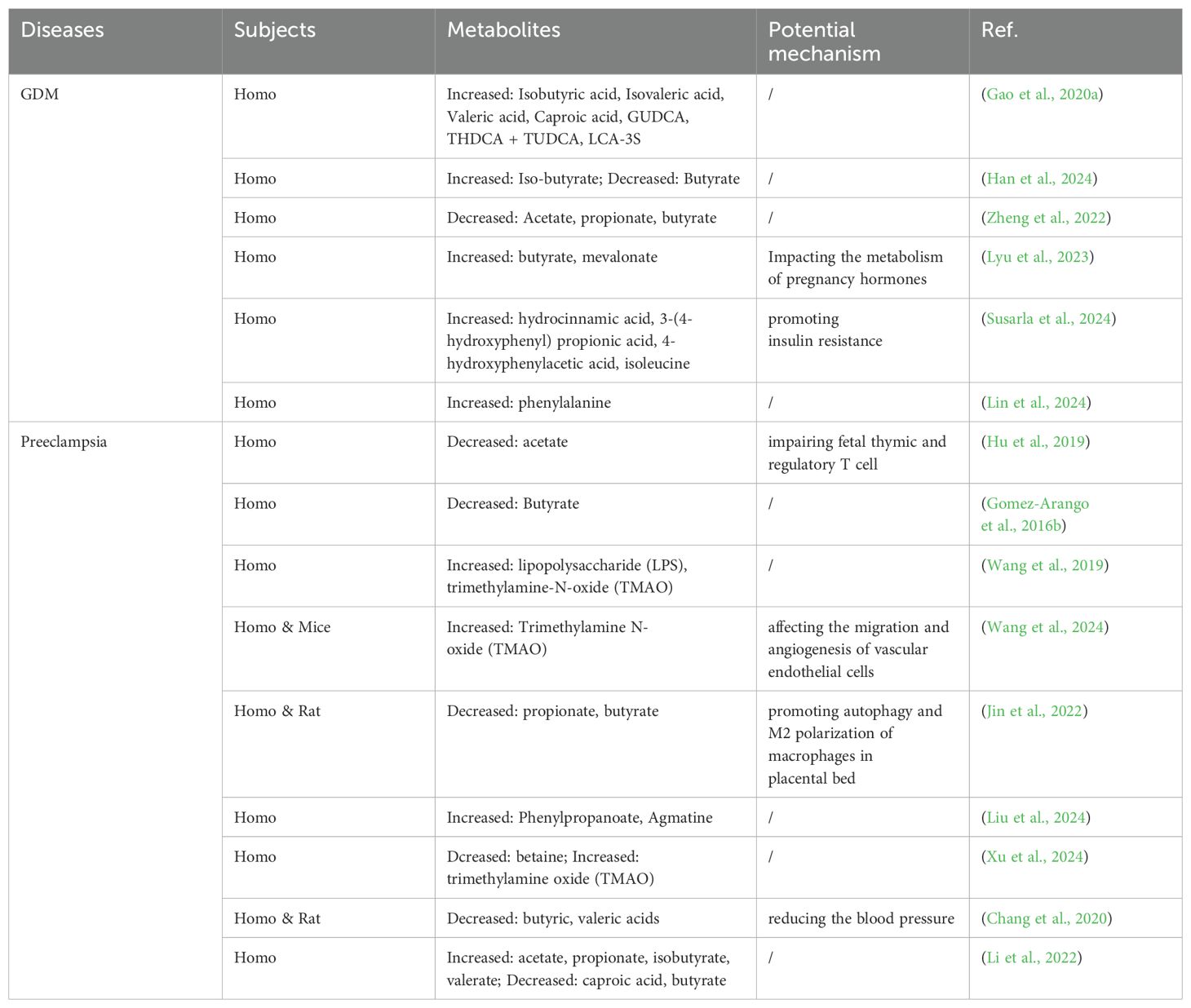
Table 2. Reported metabolites of gut microbiota associated with gestational diabetes mellitus and preeclampsia.

Figure 2. Mechanisms of gut dysbiosis related organ dysfunction. When gut microbiota was disturbed, their structure and biological function may change and produce more detrimental metabolites, such as TMA, indole, etc. which may lead to dysfunction of liver, pancreas, brain, blood vessel and intestine barrier. And then lead to PE, GDM, PPD and preterm labor in pregnancy. SCFAs, short chain fatty acids; LPS, lipopolysaccharide; TMA, trimethylamine; TMAO, trimethylamine N-oxide; GLP-1, glucagon-like peptide 1; PYY, peptide YY; ENS, enteric nervous system; IRS-1, Insulin receptor substrates-1; Ox-LDL, oxidized low density lipoprotein; ET-1, endothelin-1; NO, nitric oxide; GPR41/43, G protein coupled receptors 41/43.
Pre-eclampsia (PE), with a global prevalence of 3-5%, is a disorder of maternal blood pressure regulation during pregnancy, characterized by hypertension and proteinuria, posing serious threats to the life of the mother and fetus. Its pathogenesis remains unclear, and the current prevailing theory suggests that inadequate remodeling of the uterine spiral arterioles, excessive immune activation, vascular endothelial damage, nutritional deficiencies, and genetic factors may all be involved (Ramos et al., 2017). To better manage the disease, pathophysiological changes of preeclampsia are divided into two stages: the first due to dysregulated production of immunoregulatory cytokines and angiogenic factors leading to poor trophoblast invasion; the second characterized by a maternal inflammatory response, systemic inflammatory syndrome (Rana et al., 2019). Early diagnosis and treatment is key, but prevention and treatment based on currently established high-risk factor prediction systems has been ineffective, and it is crucial to identify other more sensitive biomedical markers (Ma’ayeh and C, 2020). Recently, research on gut microbiota-human health interaction has brought certain possibilities for preeclampsia diagnosis and treatment, with differences found in gut microbiota between preeclamptic and normal pregnant women suggesting the potential for the gut microbiota as an early diagnosis biomarker (Lv et al., 2019).
Miao et al. reported decreased relative abundance of Actinobacteria, Bifidobacteriaceae and Bifidobacterium at the phylum, family, and genus levels, respectively, and increased abundance of the genera Blautia and Ruminococcus in the gut microbiota of preeclampsia patients compared to controls. Correlation analyses confirmed that Blautia and Ruminococcus were positively correlated with obesity and dyslipidemia, high-risk factors for preeclampsia (Miao et al., 2021). In addition, higher abundance of Blautia, Ruminococcus, Bilophila, and Fusobacterium and relatively low abundance of Faecalibacterium, Gemmiger, Akkermansia, Dialister, and Methanobrevibacter were reported in the prenatal gut microbiota of patients with early-onset preeclampsia (occurring between 20 and 34 weeks of gestation) (Lv et al., 2019). Studies also reported other bacteria changes in preeclampsia (see in Table 1) (Liu et al., 2017; Wang et al., 2019; Chang et al., 2020; Wang et al., 2020; Huang et al., 2021a). To confirm the causal relationship between gut microbiota and preeclampsia, gut microbiota of preeclampsia patients was transplanted into mice pretreated with antibiotics; mice receiving transplantation had higher systolic blood pressure (Chen et al., 2020). This suggests that preeclampsia patient gut microbiota may disturb blood pressure regulation. Moreover, modulation of the gut microbiota by changing daily diet has been shown to play a role in preeclampsia relief (Torjusen et al., 2014). Therefore, despite inconsistent differential intestinal bacteria reported in existing studies, exploring the relationship between gut microbiota and the pathological mechanisms of preeclampsia remains of significant importance (H., 2020).
In terms of clinical presentation, preeclampsia is a disorder of maternal blood pressure regulation that occurs during pregnancy. The gut microbiota has been shown to influence blood pressure regulation primarily through modulation of its products (see in Table 2) (Hsu et al., 2021). And the major bacterial metabolites, SCFAs, have been reported to be altered in patients with pre-eclampsia (Gomez-Arango et al., 2016b; Chang et al., 2020). It has been reported that the relative abundance of butyrate-producing bacteria is reduced in the gut of patients with preeclampsia, while another study confirmed that butyrate supplementation can alleviate preeclampsia lesions in rats (Altemani et al., 2021; Yong et al., 2022). SCFAs have also been proved to bind to GPR41 and GPR43 and then induce vasodilation and upregulate energy expenditure, thereby preventing fat accumulation and reducing the risk of atherosclerosis; they may also activate Olfr-78 to regulate the activity of the renin-angiotensin system, thus preventing elevated blood pressure (Hsu et al., 2021). In addition, gut dysbiosis may trigger inflammation by increasing LPS levels in the vessels and lead to oxidation of low-density lipoprotein (LDL) to ox-LDL (D, 2021). A step further, increased ox-LDL can decrease the total amount of circulating NO by impairing the activity of nitric oxide synthase and decreasing the expression of endothelin-1(ET -1), leading to hypertension (Dulak et al., 1997) (Figure 2). Moreover, increased TMAO levels have been confirmed in preeclampsia patients, implying that TMAO may also play a role in PE (Wang et al., 2019). Indeed, TMAO may cause disturbances in glucose and lipid metabolism and promote phagocytosis of ox-LDL by leukocytes and macrophages, leading to the formation of foam cells and increasing the risk of atherosclerosis (Hoseini-Tavassol and H-R, 2021). In addition, TMAO can also activate protein kinase C (PKC) and NF-kB pathway, which promotes macrophage adhesion and impairs the self-repair process of vascular endothelial cells (Zhang et al., 2021). It can still increase the production of reactive oxygen species (ROS) and induce the formation of NLPR3 inflammasomes in human umbilical vein endothelial cells (HUVECs), which may lead to pyroptotic injury of HUVECs (Sun et al., 2016). TMAO further exacerbates angiotension-induced hypertension II and exerts its function through the PERK/ROS/CaMKII/PLCβ3 axis (Jiang et al., 2021), which play a role in the pathophysiology of preeclampsia (Fu et al., 2015). Gut microbiota can even regulate blood pressure by altering the secretion of intestinal hormones such as GLP-1 and gastrin, thereby regulating the activity of sodium-proton exchanger subtype 3 (NHE3) and nitric oxide synthase to modulate sodium ion reabsorption in distal renal tubules and NO concentration in vascular endothelial cells, respectively (Martins et al., 2020; Vallianou et al., 2020; Younes et al., 2020). However, the effect of the intestinal hormone gastrin is much more confusing. A study comparing the serum gastrin in pregnant women with and without preeclampsia came to a negative conclusion (Morán et al., 1996), although the negative correlation is likely due to the extremely small sample size.
Gut microbiota may also play a role in blood pressure regulation by altering the nervous system (Wang et al., 2021). The interaction between gut microbiota and the enteric nervous system (ENS) has also been gradually revealed. SCFAs can directly stimulate intestinal mucosal receptors, promote the secretion of enteric neurotransmitters such as PYY, and regulate the activity of vagus and sympathetic nerves (Vallianou et al., 2020). Gut microbiota also metabolizes sulfur-containing amino acids and tryptophan to hydrogen sulfide (H2S) and γ-aminobutyric acid (GABA); these molecules can act as messengers to influence nervous system function (S., 2018). In addition, studies have shown that gut dysbiosis can lead to increased permeability of the blood-brain barrier (BBB), and this change promotes the influx of LPS into the central nervous system, causing neuroinflammation (Tang et al., 2020). This may also contribute to neuroinflammation and blood-brain barrier disruption reported in preeclampsia patients (Bergman et al., 2021). Interestingly, in animal studies, injection of a proinflammatory factor into the brain increased sympathetic nervous system activity and blood pressure, whereas injection of an anti-inflammatory factor such as IL -10 had the opposite effect (Shi et al., 2010). H2S was also reported to exert a protective effect against neuroinflammation (Ghanbari et al., 2019). In addition, the gut microbiota has been reported to play a role in immune system dysregulation in preeclampsia. Studies have demonstrated that in preeclampsia patients, the ratio of Th1 and Th2 differs from that of normal pregnant women; and the imbalance in Th1/Th2 ratio leads to a pro-inflammatory microenvironment at the maternal-fetal interface and contributes to the occurrence of preeclampsia (Vargas-Rojas et al., 2016). Modulating the gut microbiota can affect the Th1/Th2 ratio and M2 macrophage polarization (Song et al., 2020); this may improve symptoms in preeclampsia patients and may be another effective treatment for preeclampsia, although it is still far from clinical application.
Based on the comprehensive analysis presented, it indicates that the gut microbiota plays a crucial role in the onset and progression of preeclampsia. Although the gut microbiota may influence blood pressure regulation through various mechanisms, the effect of gut microbiota on the depth of placental implantation at the initial onset of preeclampsia lacks corresponding reports, which requires further studies to more fully elucidate the relationship between gut microbiota and preeclampsia.
Preterm birth refers to delivery before 37 weeks of gestation, with a global incidence rate ranging from 5% to 18%. It is divided into spontaneous and iatrogenic preterm birth (W, 2020). Risk factors include lower genital tract infections, smoking, malnutrition, etc (Goldenberg et al., 2008). The current therapeutic strategy for preterm birth is to prolong gestation by inhibiting uterine contractions, and, if necessary, anti-infective treatment (Hoh et al., 2019). In fact, preterm birth is a complex disease with multiple etiologies and risk factors highly correlated with systemic inflammation and immune dysregulation (Gilman-Sachs et al., 2018). Studies have confirmed that dysbiosis in the oral cavity and lower genital tract has been shown to contribute to preterm birth (Chu et al., 2018). However, it is worth noting that the incidence of preterm birth did not always decrease after targeted treatment of oral or genital tract infections (Kim et al., 2012), suggesting that other factors might contribute to preterm birth. In fact, changes in the gut microbiota during pregnancy may influence infections, inflammation, and immune dysregulation, all of which are considered primary triggers for preterm birth.
Shiozaki et al. amplified the 16S rDNA of bacteria and used terminal restriction fragment length polymorphism (T-RFLP) to analyze differences in gut microbiota between preterm birth and term mothers; results showed lower abundance of Clostridium subcluster XVIII, IV and XIVa, as well as Bacteroides, while Lactobacillus was significantly increased in preterm birth mother (Shiozaki et al., 2014). Another study applying high-throughput sequencing technology reported reduced α-diversity of gut microbiota in pregnant women with preterm delivery (Hiltunen et al., 2021). In general, normal pregnancy is associated with decreasing serum LPS levels as progesterone rise and pregnancy progresses, which has been proposed as a form of self-protection (Zhou et al., 2019). And maternal serum anti-LPS IgG was negatively related to pregnancy length (Lauer et al., 2018). Mechanistically, LPS activates the uterine contraction system via increased serum prostaglandins and oxytocin levels (Okawa et al., 2001). Evidence from experimental animals showed that oral administration of Enterococcus faecium to pregnant mice confirmed gut microbes can migrate beyond the gut and colonize the maternal-fetal interface, altering local or systemic immunity (Jiménez et al., 2005). Gut microbiota translocation may benefit from increased intestinal permeability, which is facilitated by a normal gut microbiota maintaining gut function and permeability (Allam-Ndoul et al., 2020). Air pollution may also contribute to preterm birth via disrupting the maternal gut microbiota (Gan et al., 2022). High-throughput sequencing and non-targeted metabolomics combined revealed gut microbiota influences nutritional preterm birth and may be a potential factor for predicting preterm birth (Gershuni et al., 2021). Furthermore, gut dysbiosis may also promote preterm delivery by affecting bile acid metabolism (Ramírez-Pérez et al., 2017; You et al., 2020).
In fact, vaginal microbiota have gained more prominence in preterm birth research compared to gut microbiota, and their role in the process of preterm birth is more easily understood. During normal pregnancy, immune tolerance in the pregnant woman’s body allows for an increase in vaginal microbiota diversity in the early stages, while the relative proportion of Lactobacilli decreases. As pregnancy progresses, the levels of pregnancy-related hormones, particularly estrogen, rise rapidly, leading to an increase in glycogen content in the vaginal epithelium. This change helps restore the relative abundance of Lactobacilli to pre-pregnancy levels or higher, maintaining the acidic environment of the vagina and inhibiting the proliferation of pathogenic microorganisms (Shen et al., 2022). A study has revealed differences in the vaginal microbiota of 45 preterm and 90 term-born African American women. The results indicate that, in the preterm group, the overall diversity of the vaginal microbiota increased, with a structure more akin to bacterial vaginosis. Significant decreases in Lactobacillus crispatus levels and significant increases in bacterial vaginosis-associated bacterium 1 (BVAB1), Prevotella cluster 2, and Sneathia amnii were observed. In contrast, the vaginal microbiota of term-born women exhibited an absolute dominance of Lactobacillus crispatus (Fettweis et al., 2019). Additionally, there appears to be a certain level of crosstalk between gut microbiota and vaginal microbiota during pregnancy. A randomized controlled clinical trial conducted in Malaysia included 78 patients with recurrent vaginal candidiasis, and they were randomly assigned in a 1:1 ratio to either the oral probiotic supplementation group or the placebo control group. The results showed that 47% of the participants who received oral probiotic supplements did not experience a recurrence of vaginal candidiasis. This indicates that oral probiotic supplementation can influence the structure of the perineal and vaginal microbiota, although the study did not explore the potential mechanisms involved (Ang et al., 2022). Another study conducted in the United States on pregnant women with bacterial vaginosis who received probiotic supplementation showed significant results, despite the relatively small clinical sample size (n=16). Researchers observed that pregnant women with bacterial vaginosis had markedly different vaginal microbiome structures, while their gut microbiomes were relatively similar. However, through oral probiotic supplementation induced significant positive changes in both vaginal and gut microbial community structures. Notably, among subjects receiving oral probiotics, Prevotella copri abundance significantly increased in the vaginal microbiome, a phenomenon also observed in the gut microbiota (Vasundhara et al., 2021). Prevotella copri, found in the gut, plays a role in carbohydrate, particularly fiber, metabolism, producing short-chain fatty acids (Gong et al., 2024; Yang et al., 2024). This process is beneficial for maintaining gut microbiota balance and may influence the immune system. Bacterial vaginosis and vaginal candidiasis have been widely recognized as risk factors for preterm birth (Fettweis et al., 2019). The potential effects of oral probiotics on these conditions may, in part, result from their ability to improve and regulate the gut microbiota. However, given the limited number of relevant studies reported to date, we must maintain a cautious attitude and exercise prudent judgment regarding this conclusion.
In fact, changes in the gut microbiome may influence the occurrence of preterm birth by affecting the host’s immune system and metabolic processes. At the maternal-fetal interface, immune cells including regulatory T cells (Treg cells), natural killer cells (NK cells), and macrophages undergo significant changes and play crucial roles throughout early to late pregnancy (Giles et al., 2023). Research indicates that changes in the gut microbiota can influence the host’s immune homeostasis, including effects on the proliferation and function of Treg cells (Gao et al., 2020b). These effects are partially mediated by the metabolic byproducts of the gut microbiota—SCFAs. SCFAs possess the ability to regulate inflammatory response balance and induce immune tolerance, which appears to have a positive impact on maintaining pregnancy (Hu et al., 2019). And the reduction in serum levels of SCFAs during late pregnancy may be one of the critical factors leading to immune imbalance at the maternal-fetal interface. Conversely, the host’s intestinal mucosa regulates the secretion of substances such as mucus, immunoglobulin A (IgA), and defensins by recognizing microbial-associated molecular patterns (MAMPs) and bacterial metabolites, thereby maintaining the dynamic equilibrium of the gut microbiota (Wiertsema et al., 2021). As previously mentioned, the metabolic state during pregnancy transitions from early to late stages towards an energy storage state, maintaining higher levels of serum circulating fatty acids and glucose to ensure normal pregnancy progression. These metabolic shifts appear to be associated with increased abundances of microbial communities such as Akkermansia and Bifidobacterium (Qin et al., 2021). However, studies have indicated that excessive weight gain during pregnancy may be positively correlated with iatrogenic preterm birth (Faucher et al., 2016). Furthermore, excessive weight gain can increase abdominal pressure, potentially leading to premature cervical ripening and ultimately triggering preterm birth (Khan et al., 2023).
In summary, although current research on the relationship between gut microbiota and preterm birth is still limited, existing evidence suggests that gut microbiota may influence the occurrence of preterm birth by altering vaginal microecology, immune responses, and metabolic processes.
Postpartum depression (PPD), a severe form of depression that occurs after delivery, has an incidence as high as 17.22% worldwide (Wang et al., 2021). Pathophysiologically, PPD is associated with environmental factors, genetic factors, nervous system inflammation, reproductive hormone withdrawal, neuroendocrine changes, and metabolism (Payne and M, 2019). The role of gut microbiota in depression reported in recent years suggests a new direction in the study of PPD, although research in this area is limited. Zhou et al. reported lower abundance of Faecalibacterium, Phascolarctobacterium, Butyricicoccus, Lachnospiraceae and enrichment of Enterobacteriaceae in the gut microbiota of PPD patients (Hou et al., 2020). Furthermore, a clinical trial revealed a modest protective effect of probiotic supplementation and improved diet on postpartum depression (Hulkkonen et al., 2021). In a mouse model of obesity-induced PPD, it was found that increased intake of dietary fiber could alleviate symptoms by remodeling gut microbiota and increasing short-chain fatty acid production (Liu et al., 2020). These findings suggest that gut microbiota may impact PPD.
Recent studies suggest that the gut microbiota may have a multifaceted relationship with postpartum mental health. The gut microbiota is involved in the interaction by altering amino acid metabolism, regulating the vagus nervous system, and remodeling its metabolites, which has been termed the “microbiota-gut-brain axis” (Margolis et al., 2021). When the balance of the gut microbiota is disrupted, a shift in tryptophan metabolism can lead to an imbalance between serotonin and kynurenine, and deficiencies of serotonin and excesses of kynurenine have been implicated in postpartum depression (Duan et al., 2018). Additionally, Lactobacillus, Bifidobacterium, and Klebsiella have been shown to synthesize neurotransmitters such as dopamine, serotonin, Gamma-Aminobutyric Acid (GABA), and acetylcholine in the gut to mediate the microbiota’s interaction with the nervous system (S., 2018). Changes in these neurotransmitter-producing bacteria, can alter neurotransmitter levels and ratios, which may induce postpartum depression (Payne and M, 2019). Neuroinflammation is also involved in the “microbiota-gut-brain axis”. Gut dysbiosis has been shown to induce inflammatory factors such as IL-6 and TNF-α, which may contribute to postpartum depression’s pathophysiology (Beurel et al., 2020). Moreover, inflammation induced by gut dysbiosis may compromise the blood-brain barrier (BBB) and allow for an influx of LPS and peptidoglycan, which can bind to TLR4 in the brain, triggering an inflammatory cascade that promotes postpartum depression (Payne and M, 2019). Elevated LPS levels have also been implicated in upregulating central oxytocin levels and postpartum depression pathophysiology (Kong et al., 2015; Payne and M, 2019). It is worth noting that Th17 cells in the blood and IL-17A, which have long been reported to be regulated by the gut microbiota, are positively associated with postpartum depression (Liu et al., 2020; Min et al., 2022). The hypothalamic-pituitary-adrenal (HPA) axis, which refers to the feedback network between the hypothalamus, pituitary, and adrenal glands, has been reported to participate in the crosstalk between gut microbiota and postpartum depression by regulating cortisol secretion (Redpath et al., 2019). Disturbed gut microbiota has been associated with lower cortisol levels, which has been reported in depression patients, and an association with chronic postpartum depression has also been demonstrated (Seth et al., 2016). However, the specific mechanism of the gut microbiota in the HPA axis remains to be investigated (see Figure 2).
Although much of the evidence cited in this review focuses on depression, we can clearly conclude that the prevention of postpartum depression requires coordination between the gut microbiota, the nervous system, and the endocrine system.
Probiotics is a general term for bacteria that exert positive effects on their hosts when alive bacteria reach a certain threshold, namely at least 1×109 Colony-Forming Units (CFU) (Hill et al., 2014). Bacteria currently used as probiotics mainly include Lactobacillus, Bifidobacterium, Enterococcus faecalis and Streptococcus thermophilus (W., 2010). Numerous studies indicate that they can generate short-chain fatty acids and influence amino acid metabolism, thereby affecting energy metabolism and immune regulation in the body (Lee et al., 2018). In addition, some bacterial cell membrane components have been shown to have a regulatory effect on the immune system (Bae et al., 2022). Although the current clinical application of probiotics is relatively limited, potential beneficial effects of probiotics on the nervous system (Pluta et al., 2020), circulatory system (Liang et al., 2021), immune system (Frei et al., 2015) and even malignancies (Zheng et al., 2021) have been demonstrated. Likewise, the use of probiotics for pregnancy complaints has been studied in detail.
Preclinical studies have consistently demonstrated that probiotics have beneficial effects on pregnancy complications (see in Table 3). In animal models of pre-eclampsia, probiotics therapy has been shown to lower blood pressure by upregulating the level of nitric oxide (NO) and endothelin-1 (ET-1), and remodeling the gut microbiota with increased relative abundance of Bifidobacterium and Lactobacillus (Sun et al., 2020). However, clinical trial results have been inconsistent. A US trial found that Bifidobacteria and Lactobacilli supplementation improved fasting blood glucose and insulin resistance without affecting weight gain (Kijmanawat et al., 2019). Similarly, a randomized controlled trial showed probiotic (containing Lactobacillus acidophilus, Lactobacillus casei, Bifidobacterium bifidum, Lactobacillus fermentum) supplementation improved glucose and lipid metabolism in patients with gestational diabetes (Babadi et al., 2019). In contrast, a trial in Australia found oral probiotics (Lactobacillus rhamnosus and Bifidobacterium animalis subspecies lactis) did not prevent gestational diabetes in overweight/obese patients (Callaway et al., 2019). Differences may relate to probiotic selection, ethnicity, diet and culture, and publication bias. Furthermore, Lactobacillus casei has been shown to alleviate postpartum depression by regulating the microbiota-gut-brain axis (Yang et al., 2022). Recent reviews have concluded that single or combined probiotics reduce mortality in preterm and low birth weight neonates (Morgan et al., 2020). In general, despite the inherent variability among the subjects studied, the strategy of supplementing with probiotics to improve disease conditions appears to hold significant potential and promise.
The study of gut microbiota has opened new avenues for understanding the etiology and pathology of various diseases, including during pregnancy and the postpartum period. Although current research on the relationship between gut microbiota and disease during pregnancy shows promise and is popular, many findings may not have an immediate visible effect and further research is warranted.
First, gut microbiota composition exhibits significant heterogeneity, influenced by differences in genetic background and dietary habits. Second, various clinical studies differ in sample collection, sequencing technology, and sequencing depth, which may be primary drivers of inconsistent findings across studies on the same disease. In addition, current dietary and probiotic interventions are not effective for all patients, and trial results are inconsistent. This poses a challenge to the study of gut microbiota, highlighting the need for further experimental reviews with greater stability and reproducibility. One potential approach is to develop tailored probiotic strains for specific populations based on their dietary and cultural characteristics, thereby reducing variability in experimental results when probiotics are applied to different groups. Furthermore, to clarify the regulatory network of gut microbiota in multiple physiological systems, upcoming research should prioritize mechanistic exploration. This is crucial for effectively curbing disease progression and simultaneously reducing medical costs.
In summary, published literature suggests that gut microbiota plays a vital role in the initiation, development, prevention, and treatment of diseases during pregnancy. The application of probiotics in gestational diseases is promising. However, the use of gut flora modulation as a treatment strategy still requires comprehensive and multifaceted assessments of disease-specific bacterial strains to optimize interventions and maximize therapeutic outcomes.
YX: Conceptualization, Visualization, Writing – original draft. QC: Methodology, Writing – review & editing. DS: Software, Writing – review & editing. XP: Conceptualization, Methodology, Writing – review & editing. YH: Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. The present paper is funded by Sichuan Provincial Department of Science and Technology (granted NO.2022YFS0043), Sichuan University Zigong City School-land Science and Technology Cooperative R&D Project (granted NO.00402153A2153).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdullah, B., D, S., Aazmi, M. S., Idorus, M. Y., Mahamooth, M. I. J. (2022). Gut microbiota in pregnant Malaysian women: a comparison between trimesters, body mass index and gestational diabetes status. BMC Pregnancy Childbirth. 22, 152. doi: 10.1186/s12884-022-04472-x
Adak, A., K, M. (2019). An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 76, 473–493. doi: 10.1007/s00018-018-2943-4
Allam-Ndoul, B., C-P, S., Veilleux, A. (2020). Gut microbiota and intestinal trans-epithelial permeability. Int. J. Mol. Sci. 21, 6402–6015. doi: 10.3390/ijms21176402
Altemani, F., B, H., Gomez-Arango, L., Josh, P., David McIntyre, H., Callaway, L. K., et al. (2021). Pregnant women who develop preeclampsia have lower abundance of the butyrate-producer Coprococcus in their gut microbiota. Pregnancy Hypertens. 23, 211–219. doi: 10.1016/j.preghy.2021.01.002
Andreasen, A. S., K, M., Berg, R. M., Møller, K., Pedersen, B. K. (2011). Type 2 diabetes is associated with altered NF-κB DNA binding activity, JNK phosphorylation, and AMPK phosphorylation in skeletal muscle after LPS. PLoS One 6, e23999–e24006. doi: 10.1371/journal.pone.0023999
Ang, X. Y., C, F., Lee, B. K., Azhar, S. N. A., Sany, S., Roslan, N. S., et al. (2022). Lactobacilli reduce recurrences of vaginal candidiasis in pregnant women: a randomized, double-blind, placebo-controlled study. J. Appl. Microbiol. 132, 3168–3180. doi: 10.1111/jam.15158
Arai, E. N., Y, S., Yoneda, N., Ito, M., Tsuda, S., Shiozaki, A., et al. (2022). Probiotics including Clostridium butyricum, Enterococcus faecium, and Bacillus subtilis may prevent recurrent spontaneous preterm delivery. J. Obstet Gynaecol Res. 48, 688–693. doi: 10.1111/jog.15166
Association, A D (2019). Classification and diagnosis of diabetes: standards of medical care in diabetes-2019. Diabetes Care 42, S13–S28. doi: 10.2337/dc19-S002
Babadi, M., K, A., Aghadavood, E., Samimi, M., Kavossian, E., Bahmani, F., et al. (2019). The effects of probiotic supplementation on genetic and metabolic profiles in patients with gestational diabetes mellitus: a randomized, double-blind, placebo-controlled trial. Probiotics Antimicrob. Proteins. 11, 1227–1235. doi: 10.1007/s12602-018-9490-z
Bae, M., C, C., Liu, X., Park, S. M., Tusi, B. K., Chen, X., et al. (2022). Akkermansia muciniphila phospholipid induces homeostatic immune responses. Nature 608, 168–173. doi: 10.1038/s41586-022-04985-7
Barrett, H. L., G-A, L., Wilkinson, S. A., McIntyre, H. D., Callaway, L. K., Morrison, M., et al. (2018). A vegetarian diet is a major determinant of gut microbiota composition in early pregnancy. Nutrients 10, 890–902. doi: 10.3390/nu10070890
Benítez-Guerrero, T., V-I, J., Juárez-Castelán, C. J., Corona-Cervantes, K., Piña-Escobedo, A., Martínez-Corona, H., et al. (2022). Gut microbiota associated with gestational health conditions in a sample of mexican women. Nutrients 14, 4818. doi: 10.3390/nu14224818
Benner, M., L-R, A., Thijssen, S., Garssen, J., Ferwerda, G., Joosten, I., et al. (2021). Antibiotic intervention affects maternal immunity during gestation in mice. Front. Immunol. 12, 685742–685751. doi: 10.3389/fimmu.2021.685742
Bergman, L., H, R., Zetterberg, H., Blennow, K., Schell, S., Langenegger, E., et al. (2021). Evidence of neuroinflammation and blood-brain barrier disruption in women with preeclampsia and eclampsia. Cells 10, 3045–3055. doi: 10.3390/cells10113045
Beurel, E., T, M., Nemeroff, C. B. (2020). The bidirectional relationship of depression and inflammation: double trouble. Neuron 107, 234–256. doi: 10.1016/j.neuron.2020.06.002
Brantsaeter, A. L., M, R., Haugen, M., Myking, S., Sengpiel, V., Magnus, P., et al. (2011). Intake of probiotic food and risk of preeclampsia in primiparous women: the Norwegian Mother and Child Cohort Study. Am. J. Epidemiol. 174, 807–815. doi: 10.1093/aje/kwr168
Callaway, L. K., M, H., Barrett, H. L., Foxcroft, K., Tremellen, A., Lingwood, B. E., et al. (2019). Probiotics for the prevention of gestational diabetes mellitus in overweight and obese women: findings from the SPRING double-blind randomized controlled trial. Diabetes Care 42, 364–371. doi: 10.2337/dc18-2248
Canfora, E. E., M, R., Venema, K., Blaak, E. E. (2019). Gut microbial metabolites in obesity, NAFLD and T2DM. Nat. Rev. Endocrinol. 15, 261–273. doi: 10.1038/s41574-019-0156-z
Chang, Y., C, Y., Zhou, Q., Wang, C., Chen, L., Di, W., et al. (2020). Short-chain fatty acids accompanying changes in the gut microbiome contribute to the development of hypertension in patients with preeclampsia. Clin. Sci. (Lond). 134, 289–302. doi: 10.1042/CS20191253
Chen, Q., F, E., Hu, G., Chen, L. (2018). Metabolomic profiling of women with gestational diabetes mellitus and their offspring: Review of metabolomics studies. J. Diabetes Complications. 32, 512–523. doi: 10.1016/j.jdiacomp.2018.01.007
Chen, F., G, Y., Li, Y., He, W., Wu, W., Wang, K., et al. (2021). Association of gestational diabetes mellitus with changes in gut microbiota composition at the species level. BMC Microbiol. 21, 147. doi: 10.1186/s12866-021-02207-0
Chen, X., J, X., Huang, X., He, H., Zheng, J. (2019). Association between probiotic yogurt intake and gestational diabetes mellitus: A case-control study. Iran J. Public Health 48, 1248–1256.
Chen, X., L, P., Liu, M., Zheng, H., He, Y., Chen, M. X., et al. (2020). Gut dysbiosis induces the development of preeclampsia through bacterial translocation. Gut 69, 513–522. doi: 10.1136/gutjnl-2019-319101
Chen, T., Z, Y., Zhang, Y., Shan, C., Zhang, Y., Fang, K., et al. (2021). Relationships between gut microbiota, plasma glucose and gestational diabetes mellitus. J. Diabetes Investig. 12, 641–650. doi: 10.1111/jdi.13373
Chu, D. M., S, M., Pace, R. M., Aagaard, K. M. (2018). The microbiome in preterm birth. Best Pract. Res. Clin. Obstet Gynaecol. 52, 103–113. doi: 10.1016/j.bpobgyn.2018.03.006
Consortium., I H i R N (2019). The integrative human microbiome project. Nature 569, 641–648. doi: 10.1038/s41586-019-1238-8
Crusell, M. K. W., H, T., Nielsen, T., Allin, K. H., Rühlemann, M. C., Damm, P., et al. (2018). Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6, 89–107. doi: 10.1186/s40168-018-0472-x
D, A. K. (2021). Role of gut microbiota and their metabolites on atherosclerosis, hypertension and human blood platelet function: A review. Nutrients 13, 144–160. doi: 10.3390/nu13010144
Day-Walsh, P., S, E., Saha, S., Savva, G. M., Nemeckova, B., Speranza, J., et al. (2021). The use of an in-vitro batch fermentation (human colon) model for investigating mechanisms of TMA production from choline, L-carnitine and related precursors by the human gut microbiota. Eur. J. Nutr. 60, 3987–3999. doi: 10.1007/s00394-021-02572-6
Dolatkhah, N., H, M., Abbasalizadeh, F., Aghamohammadzadeh, N., Mehrabi, Y., Abbasi, M. M. (2015). Is there a value for probiotic supplements in gestational diabetes mellitus? A randomized clinical trial. J. Health Popul Nutr. 33, 25. doi: 10.1186/s41043-015-0034-9
Dong, L., H, L., Duan, T., Lin, S., Li, J., Liu, X. (2020). Integrated microbiome-metabolome analysis reveals novel associations between fecal microbiota and hyperglycemia-related changes of plasma metabolome in gestational diabetes mellitus. RSC Adv. 10, 2027–2036. doi: 10.1039/C9RA07799E
Dualib, P. M., T, C., Fernandes, G., Carvalho, C. R. S., Sparvoli, L. G., Silva, I. T., et al. (2022). Gut microbiota across normal gestation and gestational diabetes mellitus: A cohort analysis. Metabolites 12, 796. doi: 10.3390/metabo12090796
Duan, K. M., M, J., Wang, S. Y., Huang, Z., Zhou, Y., Yu, H. (2018). The role of tryptophan metabolism in postpartum depression. Metab. Brain Dis. 33, 647–660. doi: 10.1007/s11011-017-0178-y
Dulak, J., Polus, M., Guevara, I., Polus, A., Hartwich, J., Dembinskakiec, A. (1997). Regulation of inducible nitric oxide synthase (iNOS) and GTP cyclohydrolase I (GTP-CH I) gene expression by ox-LDL in rat vascular smooth muscle cells. J. Physiol. Pharmacol. Off. J. Polish Physiol. Society. 48, 689.
F, G. M. (2020). The immune system and microbiome in pregnancy. Best Pract. Res. Clin. Gastroenterol. 44-45, 101671–101701. doi: 10.1016/j.bpg.2020.101671
Faucher, M. A., H-T, M., Song, J. J., Willoughby, D. S., Bader, S. G. (2016). Gestational weight gain and preterm birth in obese women: a systematic review and meta-analysis. BJOG 123, 199–206. doi: 10.1111/bjo.2016.123.issue-2
Ferrocino, I., P, V., Gambino, R., Zarovska, A., Leone, F., Monzeglio, C., et al. (2018). Changes in the gut microbiota composition during pregnancy in patients with gestational diabetes mellitus (GDM). Sci. Rep. 8, 12216. doi: 10.1038/s41598-018-30735-9
Fettweis, J. M., S, M., Brooks, J. P., Edwards, D. J., Girerd, P. H., Parikh, H. I., et al. (2019). The vaginal microbiome and preterm birth. Nat. Med. 25, 1012–1021. doi: 10.1038/s41591-019-0450-2
Frei, R., A, M., O’Mahony, L. (2015). Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr. Opin. Gastroenterol. 31, 153–158. doi: 10.1097/MOG.0000000000000151
Fu, J., Z, L., Wang, L., Zhu, X. (2015). Expression of markers of endoplasmic reticulum stress-induced apoptosis in the placenta of women with early and late onset severe pre-eclampsia. Taiwan J. Obstet Gynecol. 54, 19–23. doi: 10.1016/j.tjog.2014.11.002
G, E. Z. (2020). Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek. 113, 2019–2040. doi: 10.1007/s10482-020-01474-7
Gan, Q., Y, W., Zhao, X., Teng, Y., Mei, S., Long, Y., et al. (2022). Mediating effects of gut microbiota in the associations of air pollutants exposure with adverse pregnancy outcomes. Ecotoxicol Environ. Saf. 234. doi: 10.1016/j.ecoenv.2022.113371
Gao, Y., C, H., Li, J., Ren, S., Yang, Z., Zhou, Y., et al. (2020a). Alterations of gut microbiota-derived metabolites in gestational diabetes mellitus and clinical significance. J. Clin. Lab. Anal. 36, e24333–e24347. doi: 10.1002/jcla.24333
Gao, Y., O H, M., Quinn, T. P., Ponsonby, A. L., Harrison, L. C., Frøkiær, H., et al. (2020b). Maternal gut microbiota during pregnancy and the composition of immune cells in infancy. Front. Immunol. 13, 986340. doi: 10.3389/fimmu.2022.986340
Gershuni, V., L, Y., Elovitz, M., Li, H., Wu, G. D., Compher, C. W. (2021). Maternal gut microbiota reflecting poor diet quality is associated with spontaneous preterm birth in a prospective cohort study. Am. J. Clin. Nutr. 113, 602–611. doi: 10.1093/ajcn/nqaa361
Ghanbari, F., K, M., Vaezi, G., Hojati, V., Shiravi, A. (2019). Hydrogen sulfide protects hippocampal neurons against methamphetamine neurotoxicity via inhibition of apoptosis and neuroinflammation. J. Mol. Neurosci. 67, 133–141. doi: 10.1007/s12031-018-1218-8
Giles, M. L., C, S., O’Bryan, J., Krishnaswamy, S., Ben-Othman, R., Amenyogbe, N., et al. (2023). The PRotective Effect of Maternal Immunisation on preTerm birth: characterising the Underlying mechanisms and Role in newborn immune function: the PREMITUR study protocol. Front. Immunol. 14, 1212320. doi: 10.3389/fimmu.2023.1212320
Gilman-Sachs, A., D, S., Salazar Garcia, M. D., Hussein, Y., Kwak-Kim, J., Beaman, K. (2018). Inflammation induced preterm labor and birth. J. Reprod. Immunol. 129, 53–58. doi: 10.1016/j.jri.2018.06.029
Gioia, C., L, B., Tarsitano, M. G., Iannuccelli, C., Di Franco, M. (2020). Dietary habits and nutrition in rheumatoid arthritis: can diet influence disease development and clinical manifestations? Nutrients 12, 1456–1480. doi: 10.3390/nu12051456
Goldenberg, R. L., C, J., Iams, J. D., Romero, R. (2008). Epidemiology and causes of preterm birth. Lancet 371, 75–84. doi: 10.1016/S0140-6736(08)60074-4
Goldstein, J. A., G, K., Beck, C., Kumar, R., Gernand, A. D. (2020). Maternal-fetal inflammation in the placenta and the developmental origins of health and disease. Front. Immunol. 11, 531543–531556. doi: 10.3389/fimmu.2020.531543
Gomez-Arango, L. F., B, H., McIntyre, H. D., Callaway, L. K., Morrison, M., Dekker Nitert, M., et al. (2016a). Connections between the gut microbiome and metabolic hormones in early pregnancy in overweight and obese women. Diabetes Care, 2214–2223. doi: 10.2337/db16-0278
Gomez-Arango, LF, Barrett, HL, McIntyre, HD, Callaway, LK, Morrison, M, Dekker Nitert, M, et al. (2016b). Increased systolic and diastolic blood pressure is associated with altered gut microbiota composition and butyrate production in early pregnancy. Hypertension. 68 (4), 974–981. doi: 10.1161/HYPERTENSIONAHA.116.07910
Gong, J., Z, Q., Hu, R., Yang, X., Fang, C., Yao, L., et al. (2024). Effects of Prevotella copri on insulin, gut microbiota and bile acids. Gut Microbes 16, 2340487. doi: 10.1080/19490976.2024.2340487
Govindarajulu, M., P, P., Steinke, I., Bloemer, J., Ramesh, S., Kariharan, T., et al. (2020). Gut metabolite TMAO induces synaptic plasticity deficits by promoting endoplasmic reticulum stress. Front. Mol. Neurosci. 13, 138–150. doi: 10.3389/fnmol.2020.00138
Guo, Q., T, Y., Li, Y., Xu, Z., Zhang, D., Liu, J., et al. (2021). Perinatal high-salt diet induces gut microbiota dysbiosis, bile acid homeostasis disbalance, and NAFLD in weanling mice offspring. Nutrients 13, 2135–2147. doi: 10.3390/nu13072135
Gupta, A., C, S., Toh, R., Low, J. M., Liu, I. M. Z., Lim, S. L., et al. (2024). Gestational diabetes-related gut microbiome dysbiosis is not influenced by different Asian ethnicities and dietary interventions: a pilot study. Sci. Rep. 14, 9855. doi: 10.1038/s41598-024-60386-y
H., T. (2020). Do gut bacteria play a role in preeclampsia? JAMA 323, 2120–2121. doi: 10.1001/jama.2020.4755
Hadden, D. R., M, C. (2009). Normal and abnormal maternal metabolism during pregnancy. Semin. Fetal Neonatal Med. 14, 66–71. doi: 10.1016/j.siny.2008.09.004
Hajifaraji, M., J, F., Abbasalizadeh, F., Aghamohammadzadeh, N., Abbasi, M. M., Dolatkhah, N. (2018). Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: a randomized clinical trial. Asia Pac J. Clin. Nutr. 27, 581–591. doi: 10.6133/apjcn.082017.03
Han, W., W, J., Yan, X., Liu, C., Huang, J., Zhang, L., et al. (2024). Butyrate and iso-butyrate: a new perspective on nutrition prevention of gestational diabetes mellitus. Nutr. Diabetes. 14, 24. doi: 10.1038/s41387-024-00276-4
Hasain, Z., R, A. R., Ahmad, H. F., Abdul Rauf, U. F., Oon, S. F., Mokhtar, N. M. (2022). The roles of probiotics in the gut microbiota composition and metabolic outcomes in asymptomatic post-gestational diabetes women: A randomized controlled trial. Nutrients 14, 3878. doi: 10.3390/nu14183878
He, S., L, H., Yu, Z., Zhang, F., Liang, S., Liu, H., et al. (2021). The gut microbiome and sex hormone-related diseases. Front. Microbiol. 12, 711137. doi: 10.3389/fmicb.2021.711137
He, J., Z, P., Shen, L., Niu, L., Tan, Y., Chen, L., et al. (2020). Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 21, 6356–6371. doi: 10.3390/ijms21176356
Hechler, C., B, K., Beijers, R., Saccenti, E., Riksen-Walraven, M., Smidt, H., et al. (2019). Association between psychosocial stress and fecal microbiota in pregnant women. Sci. Rep. 9, 4463–4472. doi: 10.1038/s41598-019-40434-8
Hill, C., G, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., et al. (2014). Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 11, 506–514. doi: 10.1038/nrgastro.2014.66
Hiltunen, H., C, M., Ollila, H., Kolari, T., Tölkkö, S., Isolauri, E., et al. (2021). Spontaneous preterm delivery is reflected in both early neonatal and maternal gut microbiota. Pediatr. Res. 91, 1804–1811. doi: 10.1038/s41390-021-01663-8
Hoh, J. K., L, M., Liu, C., Qiao, C., Pallavi, K., Takeda, J., et al. (2019). Preterm birth rate and dilemma of preterm labor treatment in Asia. Placenta 79, 68–71. doi: 10.1016/j.placenta.2019.01.005
Hoseini-Tavassol, Z., H-R, S. (2021). Targeting TMAO and its metabolic pathway for cardiovascular diseases treatment. J. Diabetes Metab. Disord. 20, 1095–1097. doi: 10.1007/s40200-021-00819-x
Hou, Y., C, C., Yu, H., Yang, Z. (2020). Fecal microbiota changes in patients with postpartum depressive disorder. Front. Cell Infect. Microbiol. 10, 567268–5672280. doi: 10.3389/fcimb.2020.567268
Hsu, C. N., C, J., Wu, K. L. H., Yu, H. R., Lee, W. C., Hou, C. Y., et al. (2021). Altered gut microbiota and its metabolites in hypertension of developmental origins: exploring differences between fructose and antibiotics exposure. Int. J. Mol. Sci. 22, 2674–2689. doi: 10.3390/ijms22052674
Hu, P., C, X., Chu, X., Fan, M., Ye, Y., Wang, Y., et al. (2021). Association of gut microbiota during early pregnancy with risk of incident gestational diabetes mellitus. J. Clin. Endocrinol. Metab. 106, e4128–e4141. doi: 10.1210/clinem/dgab346
Hu, M., E, D., Hsu, P., Mariño, E., Chidgey, A., Santner-Nanan, B., et al. (2019). Decreased maternal serum acetate and impaired fetal thymic and regulatory T cell development in preeclampsia. Nat. Commun. 10, 3031. doi: 10.1038/s41467-019-10703-1
Huang, L., C, M., Li, L., Zhang, X., Xu, Y., Xiao, J., et al. (2021a). Gut microbiota changes in preeclampsia, abnormal placental growth and healthy pregnant women. BMC Microbiol. 21, 265–273. doi: 10.1186/s12866-021-02327-7
Huang, L., T, C., Chattipakorn, N., Chattipakorn, S. C. (2021b). Impacts of gut microbiota on gestational diabetes mellitus: a comprehensive review. Eur. J. Nutr. 60, 2343–2360. doi: 10.1007/s00394-021-02483-6
Hulkkonen, P., K, E., Vahlberg, T., Koivuniemi, E., Houttu, N., Pellonperä, O., et al. (2021). The efficacy of probiotics and/or n-3 long-chain polyunsaturated fatty acids intervention on maternal prenatal and postnatal depressive and anxiety symptoms among overweight and obese women. J. Affect. Disord. 289, 21–30. doi: 10.1016/j.jad.2021.04.006
Jafarnejad, S., S, S., Jafarnejad, F., Arab, A. (2016). Effects of a multispecies probiotic mixture on glycemic control and inflammatory status in women with gestational diabetes: A randomized controlled clinical trial. J. Nutr. Metab. 2016, 5190846. doi: 10.1155/2016/5190846
Jiang, S., S, Y., Cui, Y., Tang, C., Wang, X., Qiu, X., et al. (2021). Gut microbiota dependent trimethylamine N-oxide aggravates angiotensin II-induced hypertension. Redox Biol. 46, 102115–102127. doi: 10.1016/j.redox.2021.102115
Jiménez, E., F, L., Marín, M. L., Martín, R., Odriozola, J. M., Nueno-Palop, C., et al. (2005). Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 51, 270–274. doi: 10.1007/s00284-005-0020-3
Jin, J., G, L., Zou, X., Zhang, Y., Zheng, Z., Zhang, X., et al. (2022). Gut dysbiosis promotes preeclampsia by regulating macrophages and trophoblasts. Circ. Res. 131, 492–506. doi: 10.1161/CIRCRESAHA.122.320771
Karamali, M., D, F., Sadrkhanlou, M., Jamilian, M., Ahmadi, S., Tajabadi-Ebrahimi, M., et al. (2016). Effects of probiotic supplementation on glycaemic control and lipid profiles in gestational diabetes: A randomized, double-blind, placebo-controlled trial. Diabetes Metab. J. 42, 234–241. doi: 10.1016/j.diabet.2016.04.009
Khan, S. S., P, L., Huang, X., Harrington, K., McNeil, R. B., Bello, N. A., et al. (2023). Body Mass Index, Adverse Pregnancy Outcomes, and Cardiovascular Disease Risk. Circ. Res. 133, 725–735. doi: 10.1161/CIRCRESAHA.123.322762
Kijmanawat, A., P, P., Reutrakul, S., Tangshewinsirikul, C. (2019). Effects of probiotic supplements on insulin resistance in gestational diabetes mellitus: A double-blind randomized controlled trial. J. Diabetes Investig. 10, 163–170. doi: 10.1111/jdi.2019.10.issue-1
Kim, A. J., L, A., Pullin, D. A., Thornton-Johnson, D. S., Karimbux, N. Y. (2012). Scaling and root planing treatment for periodontitis to reduce preterm birth and low birth weight: a systematic review and meta-analysis of randomized controlled trials. J. Periodontol. 83, 1508–1519. doi: 10.1902/jop.2012.110636
Kimura, I., I, D., Hirano, K., Tsujimoto, G. (2014). The SCFA receptor GPR43 and energy metabolism. Front. Endocrinol. (Lausanne). 5, 85–87. doi: 10.3389/fendo.2014.00085
Kong, L., W, R., Wang, L., Feng, W., Cao, Y., Tai, F. (2015). Postpartum repeated separation from pups affects the behavior and neuroendocrine parameters of mandarin vole fathers. Physiol. Behav. 139, 89–96. doi: 10.1016/j.physbeh.2014.11.017
Koren, O., G, J., Cullender, T. C., Spor, A., Laitinen, K., Bäckhed, H. K., et al. (2012). Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480. doi: 10.1016/j.cell.2012.07.008
Krueger Es, B. J., Russon, K. B., Elison, W. S., Davis, J. R., Hansen, J. M., Neilson, A. P., et al. (2021). Gut metabolite trimethylamine N-oxide protects INS-1 β-cell and rat islet function under diabetic glucolipotoxic conditions. Biomolecules 11, 1892–1911. doi: 10.3390/biom11121892
Kuang, Y. S., L, J., Li, S. H., Li, J. H., Yuan, M. Y., He, J. R., et al. (2017). Connections between the human gut microbiome and gestational diabetes mellitus. Gigascience 6, 1–12. doi: 10.1093/gigascience/gix058
Lauer, J. M., D, C., Ausman, L. M., Griffiths, J. K., Webb, P., Agaba, E., et al. (2018). Biomarkers of maternal environmental enteric dysfunction are associated with shorter gestation and reduced length in newborn infants in Uganda. Am. J. Clin. Nutr. 108, 889–896. doi: 10.1093/ajcn/nqy176
Lee, E. S., S, E., Nam, Y. D., Lee, S. Y. (2018). Probiotics in human health and disease: from nutribiotics to pharmabiotics. J. Microbiol. 56, 773–782. doi: 10.1007/s12275-018-8293-y
Li, J., W, L., Chen, H., Yang, Z., Chen, S., Wang, J., et al. (2022). The diagnostic potential of gut microbiota-derived short-chain fatty acids in preeclampsia. Front. Pediatr. 10, 878924. doi: 10.3389/fped.2022.878924
Liang, Y. Y., L, L., Jia, Y., Li, Y., Cai, J. N., Shu, Y., et al. (2022). Correlation between gut microbiota and glucagon-like peptide-1 in patients with gestational diabetes mellitus. World J. Diabetes. 13, 861–876. doi: 10.4239/wjd.v13.i10.861
Liang, L., R, M., Piening, B., Shen, X., Chen, S., Röst, H., et al. (2020). Metabolic dynamics and prediction of gestational age and time to delivery in pregnant women. Cell 181, 1680–1692.e15. doi: 10.1016/j.cell.2020.05.002
Liang, T., W, L., Xi, Y., Li, Y., Xie, X., Fan, C., et al. (2021). Probiotics supplementation improves hyperglycemia, hypercholesterolemia, and hypertension in type 2 diabetes mellitus: An update of meta-analysis. Crit. Rev. Food Sci. Nutr. 61, 1670–1688. doi: 10.1080/10408398.2020.1764488
Lin, H., L, C., Zhang, R. (2024). The association between gut microbiota and its metabolites in gestational diabetes mellitus. J. Microbiol. Biotechnol. 34, 1995–2004. doi: 10.4014/jmb.2403.03064
Lindsay, K. L., B, L., Kennelly, M. A., Maguire, O. C., Smith, T., Curran, S., et al. (2015). Impact of probiotics in women with gestational diabetes mellitus on metabolic health: a randomized controlled trial. Am. J. Obstet Gynecol. 212, 496.e1–496.11.
Liu, Z., L, L., Ma, S., Ye, J., Zhang, H., Li, Y., et al. (2020). High-dietary fiber intake alleviates antenatal obesity-induced postpartum depression: roles of gut microbiota and microbial metabolite short-chain fatty acid involved. J. Agric. Food Chem. 68, 13697–13710. doi: 10.1021/acs.jafc.0c04290
Liu, H., P, L., Lv, S., Yang, Q., Zhang, H., Chen, W., et al. (2019). Alterations of gut microbiota and blood lipidome in gestational diabetes mellitus with hyperlipidemia. Front. Physiol. 10, 1015. doi: 10.3389/fphys.2019.01015
Liu, Y., Q, S., Feng, Y., Song, Y., Lv, N., Liu, F., et al. (2020). Perturbations of gut microbiota in gestational diabetes mellitus patients induce hyperglycemia in germ-free mice. J. Dev. Orig Health Dis. 11, 580–588. doi: 10.1017/S2040174420000768
Liu, N., S, Y., Wang, Y., Ma, L., Zhang, S., Lin, H. (2023). Composition of the intestinal microbiota and its variations between the second and third trimesters in women with gestational diabetes mellitus and without gestational diabetes mellitus. Front. Endocrinol. (Lausanne). 14, 1126572. doi: 10.3389/fendo.2023.1126572
Liu, Y. J., T, B., Wang, F. C., Tang, L., Lei, Y. Y., Luo, Y., et al. (2020). Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 10, 5225–5241. doi: 10.7150/thno.43716
Liu, J., Y, H., Yin, Z., Jiang, X., Zhong, H., Qiu, D., et al. (2017). Remodeling of the gut microbiota and structural shifts in Preeclampsia patients in South China. Eur. J. Clin. Microbiol. Infect. Dis. 36, 713–719. doi: 10.1007/s10096-016-2853-z
Liu, X., Z, X., Li, X., Xin, S., Zhang, F., Liu, F., et al. (2024). Landscapes of gut bacterial and fecal metabolic signatures and their relationship in severe preeclampsia. J. Transl. Med. 22, 360. doi: 10.1186/s12967-024-05143-5
Lv, L. J., L, S., Li, S. C., Zhong, Z. C., Duan, H. L., Tian, C., et al. (2019). Early-onset preclampsia is associated with gut microbial alteration in antepartum and postpartum women. Front. Cell Infect. Microbiol. 9, 224–238. doi: 10.3389/fcimb.2019.00224
Lv, L. J., L, S., Wen, J. Y., Wang, G. Y., Li, H., He, T. W., et al. (2022). Deep metagenomic characterization of gut microbial community and function in preeclampsia. Front. Cell Infect. Microbiol. 12, 933523. doi: 10.3389/fcimb.2022.933523
Lyu, X., W, S., Zhong, J., Cai, L., Zheng, Y., Zhou, Y., et al. (2023). Gut microbiome interacts with pregnancy hormone metabolites in gestational diabetes mellitus. Front. Microbiol. 14, 1175065. doi: 10.3389/fmicb.2023.1175065
Ma, S., Y, Y., Huang, L., Long, S., Zhang, J., Guo, C., et al. (2020). Alteration in gut microbiota of gesational diabetes patients during the first trimester of pregnancy. Front. Cell Infect. Microbiol. 10, 58–71. doi: 10.3389/fcimb.2020.00058
Ma’ayeh, M., C, M. (2020). Prevention of preeclampsia. Semin. Fetal Neonatal Med. 25, 101123–101127. doi: 10.1016/j.siny.2020.101123
Mahizir, D., B, J., Wood, J. L., Anevska, K., Hill-Yardin, E. L., Jefferies, A. J., et al. (2020). Exercise improves metabolic function and alters the microbiome in rats with gestational diabetes. FASEB J. 34, 1728–1744. doi: 10.1096/fj.201901424R
Margolis, K. G., C, J., Mayer, E. A. (2021). The microbiota-gut-brain axis: from motility to mood. Gastroenterology 160, 1486–1501. doi: 10.1053/j.gastro.2020.10.066
Martin-Gallausiaux, C., M, L., Blottière, H. M., Larraufie, P., Lapaque, N. (2021). SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc 80, 37–49. doi: 10.1017/S0029665120006916
Martins, F. L., B, M., Girardi, A. C. C. (2020). Endogenous activation of glucagon-like peptide-1 receptor contributes to blood pressure control: role of proximal tubule Na+/H+ Exchanger isoform 3, renal angiotensin II, and insulin sensitivity. Hypertension 76, 839–848. doi: 10.1161/HYPERTENSIONAHA.120.14868
Medici Dualib, P., O, J., Mattar, R., Mariko Koga da Silva, E., Atala Dib, S., de Almeida Pititto, B. (2021). Gut microbiota and gestational Diabetes Mellitus: A systematic review. Diabetes Res. Clin. Pract. 180, 109078–109088. doi: 10.1016/j.diabres.2021.109078
Meijer, S., P, E., Renzi, S., Lavasani, S., Nouri, M., Erlandsson, L., et al. (2023). Gut micro- and mycobiota in preeclampsia: bacterial composition differences suggest role in pathophysiology. Biomolecules 13, 346. doi: 10.3390/biom13020346
Miao, T., Y, Y., Sun, J., Ma, A., Yu, J., Cui, M., et al. (2021). Decrease in abundance of bacteria of the genus Bifidobacterium in gut microbiota may be related to pre-eclampsia progression in women from East China. Food Nutr. Res. 65, 578–589. doi: 10.29219/fnr.v65.5781
Mihajlovic, J., L, M., Hausmann, B., Kohl, G., Schwarz, J., Röver, H., et al. (2021). Combined hormonal contraceptives are associated with minor changes in composition and diversity in gut microbiota of healthy women. Environ. Microbiol. 23, 3037–3047. doi: 10.1111/1462-2920.15517
Min, Z., L, Y., Ying, H. (2022). Blood T-helper 17 cells and interleukin-17A correlate with the elevated risk of postpartum depression and anxiety. J. Clin. Lab. Anal. 36, e24559. doi: 10.1002/jcla.24559
Molina-Vega, M., P-C, M., Gutiérrez-Repiso, C., Fernández-Valero, A., Lima-Rubio, F., González-Romero, S., et al. (2022). Metformin action over gut microbiota is related to weight and glycemic control in gestational diabetes mellitus: A randomized trial. BioMed. Pharmacother. 145, 112465–112463. doi: 10.1016/j.biopha.2021.112465
Morán, C., C-L, S., Ochoa, R., Martínez, J. C., Herrera, M., Fonseca, E., et al. (1996). Gastrin levels in mothers and neonates at delivery in various perinatal conditions. Acta Obstet Gynecol Scand. 75, 608–611. doi: 10.3109/00016349609054683
Morgan, R. L., P, G., Kashyap, P. C., Weizman, A. V., Sadeghirad, B., McMaster probiotic, prebiotic, and synbiotic work group (2020). probiotics reduce mortality and morbidity in preterm, low-birth-weight infants: A systematic review and network meta-analysis of randomized trials. Gastroenterology 159, 467–480. doi: 10.1053/j.gastro.2020.05.096
Morrison, D. J., P, T. (2016). Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200. doi: 10.1080/19490976.2015.1134082
Myhre, R., B, A., Myking, S., Gjessing, H. K., Sengpiel, V., Meltzer, H. M., et al. (2011). Intake of probiotic food and risk of spontaneous preterm delivery. Am. J. Clin. Nutr. 93, 151–157. doi: 10.3945/ajcn.110.004085
Nachum, Z., P, Y., Shavit, L. Y., Magril, G., Vitner, D., Zipori, Y., et al. (2024). The effect of oral probiotics on glycemic control of women with gestational diabetes mellitus-a multicenter, randomized, double-blind, placebo-controlled trial. Am. J. Obstet Gynecol MFM. 6, 101224. doi: 10.1016/j.ajogmf.2023.101224
Nordqvist, M., J, B., Brantsæter, A. L., Myhre, R., Nilsson, S., Sengpiel, V. (2018). Timing of probiotic milk consumption during pregnancy and effects on the incidence of preeclampsia and preterm delivery: a prospective observational cohort study in Norway. BMJ Open 8, e018021. doi: 10.1136/bmjopen-2017-018021
Nuriel-Ohayon, M., N, H., Koren, O. (2016). Microbial changes during pregnancy, birth, and infancy. Front. Microbiol. 7, 1031–1043. doi: 10.3389/fmicb.2016.01031
Nuriel-Ohayon, M., N, H., Ziv, O., Belogolovski, A., Barsheshet, Y., Bloch, N., et al. (2019). Progesterone increases bifidobacterium relative abundance during late pregnancy. Cell Rep. 27, 730–736. doi: 10.1016/j.celrep.2019.03.075
Okawa, T., S, H., Yaanagida, K., Sato, A., Vedernikov, Y., Saade, G., et al. (2001). Effect of lipopolysaccharide on uterine contractions and prostaglandin production in pregnant rats. Am. J. Obstet Gynecol. 184, 84–89. doi: 10.1067/mob.2001.108083
Ouzounian, J. G., U, E. (2012). Physiologic changes during normal pregnancy and delivery. Cardiol. Clin. 30, 317–329. doi: 10.1016/j.ccl.2012.05.004
Payne, J. L., M, J. (2019). Pathophysiological mechanisms implicated in postpartum depression. Front. Neuroendocrinol. 52, 165–180. doi: 10.1016/j.yfrne.2018.12.001
Pellonperä, O., M, K., Houttu, N., Vahlberg, T., Koivuniemi, E., Tertti, K., et al. (2019). Efficacy of fish oil and/or probiotic intervention on the incidence of gestational diabetes mellitus in an at-risk group of overweight and obese women: A randomized, placebo-controlled, double-blind clinical trial. Diabetes Care 42, 1009–1017. doi: 10.2337/dc18-2591
Pinto, Y., F, S., Turjeman, S., Eshel, A., Nuriel-Ohayon, M., Shrossel, O., et al. (2023). Gestational diabetes is driven by microbiota-induced inflammation months before diagnosis. Gut 72, 918–928. doi: 10.1136/gutjnl-2022-328406
Plows, J. F., S, J., Baker, P. N., Reynolds, C. M., Vickers, M. H. (2018). The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 19, 3342–3362. doi: 10.3390/ijms19113342
Pluta, R., U-K, M., Januszewski, S., Czuczwar, S. J. (2020). Gut microbiota and pro/prebiotics in Alzheimer’s disease. Aging (Albany NY). 12, 5539–5550. doi: 10.18632/aging.102930
Ponzo, V., F, D., Goitre, I., Leone, F., Lezo, A., Monzeglio, C., et al. (2019). Diet-gut microbiota interactions and gestational diabetes mellitus (GDM). Nutrients 11, 330–342. doi: 10.3390/nu11020330
Qi, Y., Y, L., Tian, F., Zhao, J., Zhang, H., Chen, W., et al. (2022). A. muciniphila Supplementation in Mice during Pregnancy and Lactation Affects the Maternal Intestinal Microenvironment. Nutrients 14, 390–406. doi: 10.3390/nu14020390
Qin, S., L, Y., Wang, S., Ma, J., Yang, H. (2021). Distribution characteristics of intestinal microbiota during pregnancy and postpartum in healthy women. J. Matern Fetal Neonatal Med. 35 (15), 2915–2922. doi: 10.1080/14767058.2020.1812571
Ramírez-Pérez, O., C-R, V., Chinchilla-López, P., Méndez-Sánchez, N. (2017). The role of the gut microbiota in bile acid metabolism. Ann. Hepatol. 16, s15–s20. doi: 10.5604/01.3001.0010.5672
Ramos, J. G. L., S, N., Costa, S. H. M. (2017). Preeclampsia. Rev. Bras. Ginecol Obstet. 39, 496–512. doi: 10.1055/s-0037-1604471
Rana, S., L, E., Granger, J. P., Karumanchi, S. A. (2019). Preeclampsia: pathophysiology, challenges, and perspectives. Circ. Res. 124, 1094–1112. doi: 10.1161/CIRCRESAHA.118.313276
Redpath, N., R, H., Kimmel, M. C. (2019). The relationship between perinatal mental health and stress: a review of the microbiome. Curr. Psychiatry Rep. 21, 18–26. doi: 10.1007/s11920-019-0998-z
Ruebel, M. L., G, S., Sims, C. R., Zhong, Y., Turner, D., Chintapalli, S. V., et al. (2021). Associations between maternal diet, body composition and gut microbial ecology in pregnancy. Nutrients 13, 3295–3312. doi: 10.3390/nu13093295
S., P. (2018). Neurotransmitter modulation by the gut microbiota. Brain Res. 1693, 128–133. doi: 10.1016/j.brainres.2018.03.015
Sahhaf Ebrahimi, F., R, A. H., Mosen, M., Abbasalizadeh, F., Tabrizi, A., Khalili, L. (2019). Effect of L. acidophilus and B. lactis on blood glucose in women with gestational diabetes mellitus: a randomized placebo-controlled trial. Diabetol. Metab. Syndr. 11, 75. doi: 10.1186/s13098-019-0471-5
Seth, S., L, A., Galbally, M. (2016). Perinatal maternal depression and cortisol function in pregnancy and the postpartum period: a systematic literature review. BMC Pregnancy Childbirth. 16, 124–143. doi: 10.1186/s12884-016-0915-y
Shen, W., C, Q., Lin, R., Hu, Z., Luo, M., Ren, Y., et al. (2024). Imbalance of gut microbiota in gestational diabetes. BMC Pregnancy Childbirth. 24, 226. doi: 10.1186/s12884-024-06423-0
Shen, L., Z, W., Yuan, Y., Zhu, W., Shang, A. (2022). Vaginal microecological characteristics of women in different physiological and pathological period. Front. Cell Infect. Microbiol. 12, 959793. doi: 10.3389/fcimb.2022.959793
Shi, P., D-F, C., Jun, J. Y., Qi, Y., Katovich, M. J., Li, Q., et al. (2010). Brain microglial cytokines in neurogenic hypertension. Hypertension 56, 297–303. doi: 10.1161/HYPERTENSIONAHA.110.150409
Shiozaki, A., Y, S., Yoneda, N., Yonezawa, R., Matsubayashi, T., Seo, G., et al. (2014). Intestinal microbiota is different in women with preterm birth: results from terminal restriction fragment length polymorphism analysis. PLoS One 9, e111374–111380. doi: 10.1371/journal.pone.0111374
Sinha, T., B, S., Prins, J., Zhernakova, A. (2023). The maternal gut microbiome during pregnancy and its role in maternal and infant health. Curr. Opin. Microbiol. 74, 102309. doi: 10.1016/j.mib.2023.102309
Slykerman, R. F., H, F., Wickens, K., Thompson, J. M. D., Barthow, C., Murphy, R., et al. (2017). probiotic in pregnancy study group. Effect of lactobacillus rhamnosus HN001 in pregnancy on postpartum symptoms of depression and anxiety: A randomised double-blind placebo-controlled trial. EBioMedicine 24, 159–165. doi: 10.1016/j.ebiom.2017.09.013
Smid, M. C., R, N., Panzer, A., Mccoy, A. N., Azcarate-Peril, M. A., Keku, T. O., et al. (2018). Maternal gut microbiome biodiversity in pregnancy. Am. J. Perinatol. 35, 24–30. doi: 10.1055/s-0037-1604412
Solgi, E., T-F, B., Badehnoosh, B., Khavandegar, A., Bakhtiyari, M. (2022). Vaginal and oral probiotics effect in the prevention of preterm delivery in patients visiting Kamali Hospital, Karaj, Iran in 2020. Eur. J. Obstet Gynecol Reprod. Biol. X. 16, 100169. doi: 10.1016/j.eurox.2022.100169
Song, J., L, Y., Li, J., Wang, H., Zhang, Y., Suo, H. (2020). Lactobacillus rhamnosus 2016SWU.05.0601 regulates immune balance in ovalbumin-sensitized mice by modulating expression of the immune-related transcription factors and gut microbiota. J. Sci. Food Agric. 100, 4930–4939. doi: 10.1002/jsfa.v100.13
Su, Y., W, H., Gan, X. P., Chen, L., Cao, Y. N., Cheng, D. C., et al. (2021). Alterations of gut microbiota in gestational diabetes patients during the second trimester of pregnancy in the Shanghai Han population. J. Transl. Med. 19, 366. doi: 10.1186/s12967-021-03040-9
Sun, X., J, X., Ma, Y., Liu, Y., Zhang, L., He, Y., et al. (2016). Trimethylamine N-oxide induces inflammation and endothelial dysfunction in human umbilical vein endothelial cells via activating ROS-TXNIP-NLRP3 inflammasome. Biochem. Biophys. Res. Commun. 481, 63–70. doi: 10.1016/j.bbrc.2016.11.017
Sun, B. M., M, L., Liu, H., Bao, D. (2020). Changes in intestinal flora in preeclampsia rats and effects of probiotics on their inflammation and blood pressure. Eur. Rev. Med. Pharmacol. Sci. 24, 10155–10161.
Sun, Z., Pan, X. F., Li, X., Jiang, L., Hu, P., Wang, Y., et al. (2023) The gut microbiome dynamically associates with host glucose metabolism throughout pregnancy: longitudinal findings from a matched case-control study of gestational diabetes mellitus. Adv. Sci. (Weinh). 10, e2205289. doi: 10.1002/advs.202205289
Susarla, S. M., F, O., Thiele, I., Ngo, A. L., Barupal, D. K., Chehab, R. F., et al. (2024). Microbiome-derived metabolites in early to mid-pregnancy and risk of gestational diabetes: a metabolome-wide association study. BMC Med. 22, 449. doi: 10.1186/s12916-024-03606-6
Szmuilowicz, E. D., J, J., Metzger, B. E. (2019). Gestational diabetes mellitus. Endocrinol. Metab. Clin. North Am. 48, 479–493. doi: 10.1016/j.ecl.2019.05.001
Tanaka, K., H, G., Miyazawa, K., He, F., Tanigaki, S., Kobayashi, Y. (2022). The gut microbiota of non-obese Japanese pregnant women with gestational diabetes mellitus. Biosci. Microbiota Food Health 41, 4–11. doi: 10.12938/bmfh.2021-025
Tang, W., Zhu, H., Feng, Y., Guo, R., Wan, D. (2020). The impact of gut microbiota disorders on the blood-Brain barrier. Infect. Drug Resist. 13, 3351–3363. doi: 10.2147/IDR.S254403
Thiele, K., A, L., Hecher, K., Arck, P. C. (2019). The mnemonic code of pregnancy comparative analyses of pregnancy success and complication risk in first and second human pregnancies. J. Reprod. Immunol. 134, 11–20. doi: 10.1016/j.jri.2019.06.003
Torjusen, H., B, A., Haugen, M., Alexander, J., Bakketeig, L. S., Lieblein, G., et al. (2014). Reduced risk of pre-eclampsia with organic vegetable consumption: results from the prospective Norwegian Mother and Child Cohort Study. BMJ Open 4, e006143–006144. doi: 10.1136/bmjopen-2014-006143
Vallianou, N. G., G, E., Kounatidis, D. (2020). Microbiome and hypertension: where are we now? J. Cardiovasc. Med. (Hagerstown). 21, 83–88. doi: 10.2459/JCM.0000000000000900
Vanda, R., D, T., Taghavi, S. A., Sadeghi, H., Lambert, N., Bazarganipour, F. (2024). Pregnancy outcomes in pregnant women taking oral probiotic undergoing cerclage compared to placebo: two blinded randomized controlled trial. BMC Pregnancy Childbirth. 24, 311. doi: 10.1186/s12884-024-06496-x
Vandeputte, D., D C, L., Tito, R. Y., Kathagen, G., Sabino, J., Vermeire, S., et al. (2021). Temporal variability in quantitative human gut microbiome profiles and implications for clinical research. Nat. Commun. 12, 6740. doi: 10.1038/s41467-021-27098-7
Vargas-Rojas, M. I., S-V, H., Soto-Vega, E. (2016). Th1, Th2, Th17 and Treg levels in umbilical cord blood in preeclampsia. J. Matern Fetal Neonatal Med. 29, 1642–1655. doi: 10.3109/14767058.2015.1057811
Vasundhara, D., R, V., Hemalatha, R., Nagpal, R., Kumar, M. (2021). Vaginal & gut microbiota diversity in pregnant women with bacterial vaginosis & effect of oral probiotics: An exploratory study. Indian J. Med. Res. 153, 492–502. doi: 10.4103/ijmr.IJMR_350_19
Vavreckova, M., G, N., Kostovcik, M., Krystynik, O., Ivanovova, E., Roubalova, R., et al. (2022). Specific gut bacterial and fungal microbiota pattern in the first half of pregnancy is linked to the development of gestational diabetes mellitus in the cohort including obese women. Front. Endocrinol. (Lausanne). 13, 970825. doi: 10.3389/fendo.2022.970825
Vicariotto, F., M, P., Torricelli, M., Lungaro, L., Caio, G., De Leo, V. (2023). Beneficial Effects of Limosilactobacillus reuteri PBS072 and Bifidobacterium breve BB077 on Mood Imbalance, Self-Confidence, and Breastfeeding in Women during the First Trimester Postpartum. Nutrients 15, 3513. doi: 10.3390/nu15163513
Vounzoulaki, E., K, K., Abner, S. C., Tan, B. K., Davies, M. J., Gillies, C. L. (2020). Progression to type 2 diabetes in women with a known history of gestational diabetes: systematic review and meta-analysis. BMJ 369, m1361–m1371. doi: 10.1136/bmj.m1361
W, S. R. (2020). Global burden of preterm birth. Int. J. Gynaecol Obstet. 150, 31–33. doi: 10.1002/ijgo.v150.1
Wang, X., Chen, Z., Geng, B., Cai, J. (2021). The bidirectional signal communication of microbiota-gut-brain axis in hypertension. Int. J. Hypertens. 2021, 8174789–8174797. doi: 10.1155/2021/8174789
Wang, J., Zheng, J, Shi, W, Du, N, Xu, X, Zhang, Y, et al. (2018). Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 67, 1614–1625. doi: 10.1136/gutjnl-2018-315988
Wang, J., G, Y., Ren, S., Li, J., Chen, S., Feng, J., et al. (2024). Gut microbiota-derived trimethylamine N-Oxide: a novel target for the treatment of preeclampsia. Gut Microbes 16, 2311888. doi: 10.1080/19490976.2024.2311888
Wang, J., G, X., Yang, J., Wei, Y., Zhao, Y. (2019). Gut microbiota dysbiosis and increased plasma LPS and TMAO levels in patients with preeclampsia. Front. Cell Infect. Microbiol. 9, 409–419. doi: 10.3389/fcimb.2019.00409
Wang, H., L, N., Chivese, T., Werfalli, M., Sun, H., Yuen, L., et al. (2022). IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by international association of diabetes in pregnancy study group’s criteria. Diabetes Res. Clin. Pract. 183, 109050. doi: 10.1016/j.diabres.2021.109050
Wang, X., L, H., Li, Y., Huang, S., Zhang, L., Cao, C., et al. (2020). Altered gut bacterial and metabolic signatures and their interaction in gestational diabetes mellitus. Gut Microbes 12, 1–13. doi: 10.1080/19490976.2020.1840765
Wang, Z., L, J., Shuai, H., Cai, Z., Fu, X., Liu, Y., et al. (2021). Mapping global prevalence of depression among postpartum women. Transl. Psychiatry 11, 543–566. doi: 10.1038/s41398-021-01663-6
Wang, J., S, Z., Yang, J., Wei, Y., Wang, X. Y., Zhao, Y. Y., et al. (2020). Gut microbiota dysbiosis in preeclampsia patients in the second and third trimesters. Chin (Engl) 133, 1057–1065. doi: 10.1097/CM9.0000000000000734
Wang, Y., X, T., Wu, Y., Liu, Y., Zou, Z., Bai, J. (2021). Impacts of maternal diet and alcohol consumption during pregnancy on maternal and infant gut microbiota. Biomolecules 11, 369–381. doi: 10.3390/biom11030369
Wei, J., Q, Y., Zhou, H., Liu, J., Qi, C., Gao, J. (2022). 16S rRNA gene amplicon sequencing of gut microbiota in gestational diabetes mellitus and their correlation with disease risk factors. J. Endocrinol. Invest. 45, 279–289. doi: 10.1007/s40618-021-01595-4
Wickens, K. L., B, C., Murphy, R., Abels, P. R., Maude, R. M., Stone, P. R., et al. (2017). Early pregnancy probiotic supplementation with Lactobacillus rhamnosus HN001 may reduce the prevalence of gestational diabetes mellitus: a randomised controlled trial. Br. J. Nutr. 117, 804–813. doi: 10.1017/S0007114517000289
Wiertsema, S. P., v B, J., Garssen, J., Knippels, L. M. J. (2021). The interplay between the gut microbiome and the immune system in the context of infectious diseases throughout life and the role of nutrition in optimizing treatment strategies. Nutrients 13, 886. doi: 10.3390/nu13030886
Wu, Y., B, P., Long, S., Ming, W. K., Ding, W., Long, Y., et al. (2020). Metagenomic analysis reveals gestational diabetes mellitusrelated microbial regulators of glucose tolerance. Acta Diabetol. 57, 569–581. doi: 10.1007/s00592-019-01434-2
Wu, Z., G, M., Liu, J., Chen, X., Cai, Z., Huang, H. (2024). The gut microbiota composition and metabolites are different in women with hypertensive disorders of pregnancy and normotension: A pilot study. J. Obstet Gynaecol Res. 50, 334–341. doi: 10.1111/jog.15844
Wu, X., L, Q., Cai, J., Huang, H., Ma, S., Tan, H. (2023). Longitudinal change of gut microbiota in hypertensive disorders in pregnancy: a nested case-control and Mendelian randomization study. Sci. Rep. 13, 16986. doi: 10.1038/s41598-023-43780-w
Wu, X., L, D., Li, Q., Cai, J., Huang, H., Xiang, T., et al. (2023). Investigating causal associations among gut microbiota, gut microbiota-derived metabolites, and gestational diabetes mellitus: a bidirectional Mendelian randomization study. Aging (Albany NY). 15, 8345–8366. doi: 10.18632/aging.204973
Wu, N., Z, J., Mo, H., Mu, Q., Su, H., Li, M., et al. (2022). The gut microbial signature of gestational diabetes mellitus and the association with diet intervention. Front. Cell Infect. Microbiol. 11, 800865. doi: 10.3389/fcimb.2021.800865
Xu, H., F, P., Sun, Y., Wu, D., Wang, D., Wu, L., et al. (2024). Plasma trimethylamine N-oxide metabolites in the second trimester predict the risk of hypertensive disorders of pregnancy: a nested case-control study. Hypertens. Res. 47, 778–789. doi: 10.1038/s41440-023-01563-w
Xu, Y., Z, M., Zhang, J., Sun, Z., Ran, L., Ban, Y., et al. (2020). Differential intestinal and oral microbiota features associated with gestational diabetes and maternal inflammation. Am. J. Physiol. Endocrinol. Metab. 319, E247–E253. doi: 10.1152/ajpendo.00266.2019
Yang, C., L, R., Zhao, L., Pu, J., Hu, D., Yang, J., et al. (2024). Prevotella copri alleviates hyperglycemia and regulates gut microbiota and metabolic profiles in mice. mSystems 9, e0053224. doi: 10.1128/msystems.00532-24
Yang, G., Z, X. (2021). TMAO promotes apoptosis and oxidative stress of pancreatic acinar cells by mediating IRE1α-XBP-1 pathway. Saudi J. Gastroenterol. 27, 361–369. doi: 10.4103/sjg.sjg_12_21
Yang, Y., Z, S., Yang, X., Li, W., Si, J., Yang, X. (2022). The antidepressant potential of lactobacillus casei in the postpartum depression rat model mediated by the microbiota-gut-brain axis. Neurosci. Lett. 774, 136474–136480. doi: 10.1016/j.neulet.2022.136474
Yang G, L. C., Yang, Y., Yuan, L., Wang, P., Wen, X., Pan, M. H., et al. (2019). Nobiletin prevents trimethylamine oxide-induced vascular inflammation via inhibition of the NF-κB/MAPK pathways. J. Agric. Food Chem. 67, 6169–6176. doi: 10.1021/acs.jafc.9b01270
Yao, Z. Y., L, X., Zuo, L., Xiong, Q., He, W. T., Li, D. X., et al. (2022). Maternal sleep deprivation induces gut microbial dysbiosis and neuroinflammation in offspring rats. Zool Res. 43, 380–390. doi: 10.24272/j.issn.2095-8137.2022.023
Yaribeygi, H., F, F., Butler, A. E., Sahebkar, A. (2019). Insulin resistance: Review of the underlying molecular mechanisms. J. Cell Physiol. 234, 8152–8161. doi: 10.1002/jcp.v234.6
Ye, D., H, J., Wu, J., Xie, K., Gao, X., Yan, K., et al. (2023). Integrative metagenomic and metabolomic analyses reveal gut microbiota-derived multiple hits connected to development of gestational diabetes mellitus in humans. Gut Microbes 15, 2154552. doi: 10.1080/19490976.2022.2154552
Yong, W., Z, Y., Jiang, X., Li, P. (2022). Sodium butyrate alleviates pre-eclampsia in pregnant rats by improving the gut microbiota and short-chain fatty acid metabolites production. J. Appl. Microbiol. 132, 1370–1383. doi: 10.1111/jam.v132.2
Yoon, K., K, N. (2021). Roles of sex hormones and gender in the gut microbiota. J. Neurogastroenterol Motil. 27, 314–325. doi: 10.5056/jnm20208
You, S., C, A., Hashmi, S. F., Zhang, X., Nadolny, C., Chen, Y., et al. (2020). Dysregulation of bile acids increases the risk for preterm birth in pregnant women. Nat. Commun. 11, 2111–2125. doi: 10.1038/s41467-020-15923-4
Younes, S. T., M, K., Sasser, J., Ryan, M. J. (2020). The glucagon-like peptide 1 receptor agonist liraglutide attenuates placental ischemia-induced hypertension. Am. J. Physiol. Heart Circ. Physiol. 318, H72–H77. doi: 10.1152/ajpheart.00486.2019
Yurkovetskiy, L., B, M., Khan, A. A., Graham, L., Volchkov, P., Becker, L., et al. (2013). Gender bias in autoimmunity is influenced by microbiota. Immunity 39, 400–412. doi: 10.1016/j.immuni.2013.08.013
Zhang, Y., C, T., Zhang, Y., Hu, Q., Wang, X., Chang, H., et al. (2021). Contribution of trace element exposure to gestational diabetes mellitus through disturbing the gut microbiome. Environ. Int. 153, 106520–106528. doi: 10.1016/j.envint.2021.106520
Zhang, X. Y., L, Y., He, T., Yang, T. T., Wu, J., Cianflone, K., et al. (2018). Anaphylatoxin C5a induces inflammation and reduces insulin sensitivity by activating TLR4/NF-kB/PI3K signaling pathway in 3T3-L1 adipocytes. BioMed. Pharmacother. 103, 955–964. doi: 10.1016/j.biopha.2018.04.057
Zhang, X., O, X., Zhuang, H., Wu, N., Cheng, S., Wiklund, P. (2019). Branched-chain and aromatic amino acids are associated with insulin resistance during pubertal development in girls. J. Adolesc. Health 65, 337–343. doi: 10.1016/j.jadohealth.2019.01.030
Zhang, W. Q., W, Y., Zhang, A., Ding, Y. J., Zhang, X. N., Jia, Q. J., et al. (2021). TMA/TMAO in hypertension: novel horizons and potential therapies. J. Cardiovasc. Transl. Res. 14, 1117–1124. doi: 10.1007/s12265-021-10115-x
Zhao, Y., W, B., Zhao, X., Cui, D., Hou, S., Zhang, H. (2023). The effect of gut microbiota dysbiosis on patients with preeclampsia. Front. Cell Infect. Microbiol. 12, 1022857. doi: 10.3389/fcimb.2022.1022857
Zheng, C., C, T., Lu, J., Wei, K., Tian, H., Liu, W., et al. (2021). Adjuvant treatment and molecular mechanism of probiotic compounds in patients with gastric cancer after gastrectomy. Food Funct. 12, 6294–6308. doi: 10.1039/D1FO01375K
Zheng, Q. X., J, X., Wang, H. W., Ge, L., Lai, Y. T., Jiang, X. Y., et al. (2021). Probiotic supplements alleviate gestational diabetes mellitus by restoring the diversity of gut microbiota a study based on 16S rRNA sequencing. J. Microbiol. 59, 827–839. doi: 10.1007/s12275-021-1094-8
Zheng, Q. X., W, H., Jiang, X. M., Ge, L., Lai, Y. T., Jiang, X. Y., et al. (2022). Changes in the gut metabolic profile of gestational diabetes mellitus rats following probiotic supplementation. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.779314
Zheng, W., X, Q., Huang, W., Yan, Q., Chen, Y., Zhang, L., et al. (2020). Gestational diabetes mellitus is associated with reduced dynamics of gut microbiota during the first half of pregnancy. mSystems 5, e00109–e00120. doi: 10.1128/mSystems.00109-20
Zhou, Z., B, C., Luo, Z., Guille, C., Ogunrinde, E., Wu, J., et al. (2019). Progesterone decreases gut permeability through upregulating occludin expression in primary human gut tissues and Caco-2 cells. Sci. Rep. 9, 8367–8379. doi: 10.1038/s41598-019-44448-0
Zhou, P., Z, Y., Liu, B., Jin, Z., Zhuang, X., Dai, W., et al. (2020). Perinatal antibiotic exposure affects the transmission between maternal and neonatal microbiota and is associated with early-onset sepsis. mSphere 5, e00984–e00919. doi: 10.1128/mSphere.00984-19
Keywords: gut microbiota, preeclampsia, insulin resistance, probiotics, postpartum depression, gestational diabetes mellitus
Citation: Xie Y, Chen Q, Shan D, Pan X and Hu Y (2025) Unraveling the role of the gut microbiome in pregnancy disorders: insights and implications. Front. Cell. Infect. Microbiol. 15:1521754. doi: 10.3389/fcimb.2025.1521754
Received: 02 November 2024; Accepted: 20 February 2025;
Published: 07 March 2025.
Edited by:
Julie Mirpuri, University of Texas Southwestern Medical Center, United StatesReviewed by:
Sakthivel Govindaraj, Emory University, United StatesCopyright © 2025 Xie, Chen, Shan, Pan and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yayi Hu, eWF5aS5odUAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.