- 1Department of Biotechnology, School of Engineering and Technology, Manav Rachna International Institute of Research and Studies, Faridabad, India
- 2Centre for Virology, School of Interdisciplinary Science and Technology, Jamia Hamdard, New Delhi, India
- 3Regional Centre for Biotechnology, NCR Biotech Science Cluster, Faridabad, India
Severe Acute Respiratory Syndrome Coronavirus (SARS-CoV) caused an outbreak in 2002-2003, spreading to 29 countries with a mortality rate of about 10%. Strict quarantine and infection control methods quickly stopped the spread of the disease. Later research showed that SARS-CoV came from animals (zoonosis) and stressed the possibility of a similar spread from host to human, which was clearly shown by the COVID-19 outbreak. The COVID-19 pandemic, instigated by SARS-CoV-2, has affected 776 million confirmed cases and more than seven million deaths globally as of Sept 15, 2024. The existence of animal reservoirs of coronaviruses continues to pose a risk of re-emergence with improved fitness and virulence. Given the high death rate (up to 70 percent) and the high rate of severe sickness (up to 68.7 percent in long-COVID patients), it is even more critical to identify new therapies as soon as possible. This study combines research on antivirals that target SARS coronaviruses that have been conducted over the course of more than twenty years. It is a beneficial resource that might be useful in directing future studies.
Introduction
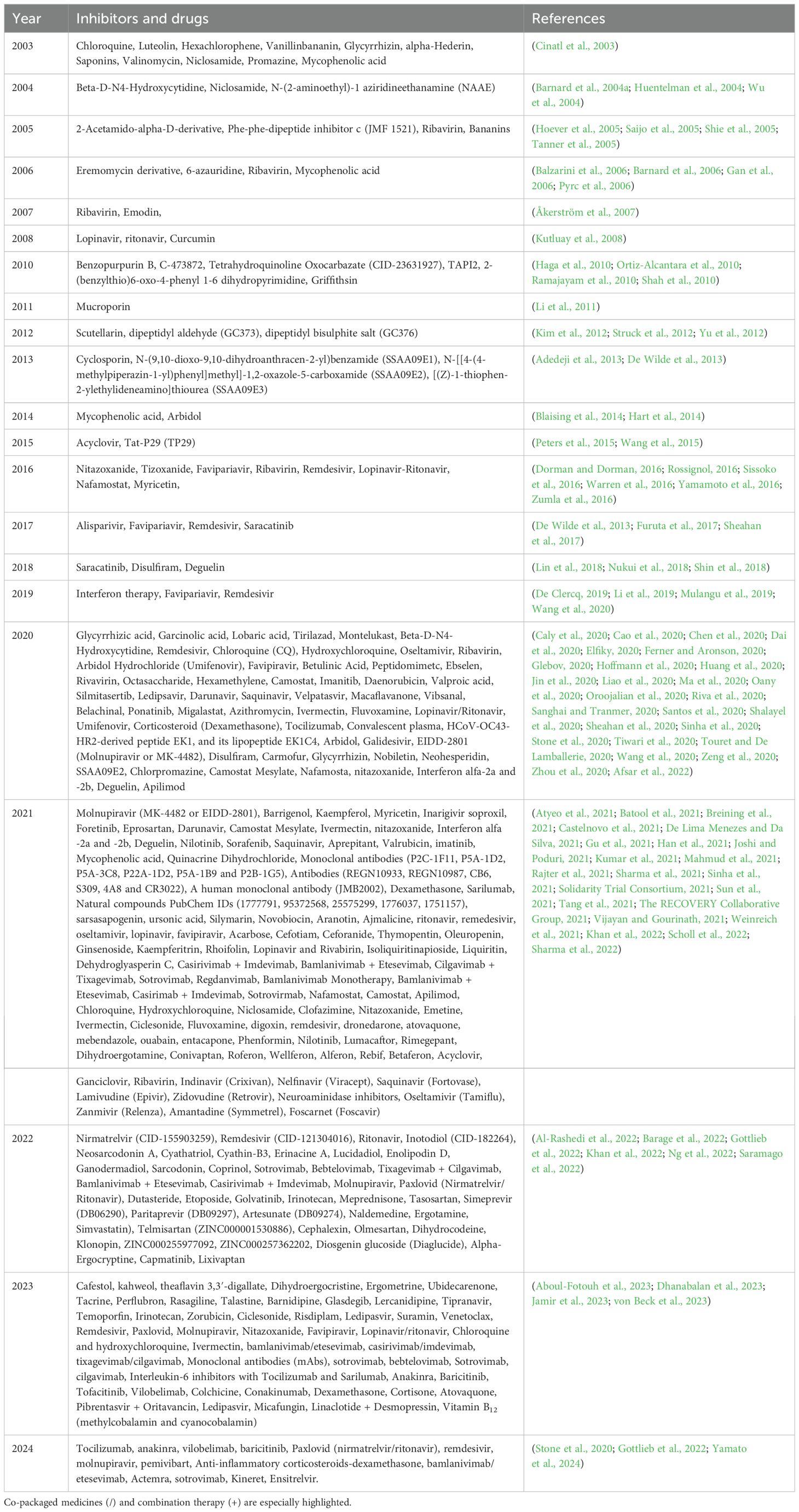
SARS-CoV-2, a betacoronavirus, emerged in Wuhan, China, in December 2019, causing a respiratory syndrome, COVID-19, which became a pandemic by March 2020 (Chan et al., 2020; Huang et al., 2020). Since then, it has spread to 206 countries and territories, causing 776 million confirmed cases and more than seven million deaths globally as of Sept 15 2024 (World Health Organization (WHO), 2024). COVID-19, caused by SARS-CoV-2, follows past outbreaks of related coronaviruses, including MERS-CoV in 2012 and SARS-CoV in 2003. SARS-CoV-2 and SARS-CoV share significantly high genomic similarity. Although, notable differences at specific amino acid residues in key proteins also exist (Figure 1). Despite high similarity, SARS-CoV-2’s global impact has been far more widespread and devastating (Baig et al., 2021; Gorkhali et al., 2021; Baig et al., 2023). However, due to the similarities among the two coronaviruses, antiviral research involving SARS-CoV has become relevant for SARS-CoV-2, as described in the following sections.
Figure 1. SARS-CoV-1 and SARS-CoV-2 belong to the genus betacoronavirus, showing notable differences in genomic sizes and features. (A) SARS-CoV-1 genome size is 29751 bases, (B) SARS-CoV-2 genome size is 29903 bases, slightly bigger than CoV-1. Both are positive-sense, single-stranded RNA (+ssRNA) and share a similar genomic architecture, including a 5’ untranslated region (UTR), coding regions (CDS) for nonstructural and structural proteins, and a 3’ untranslated region (UTR). Both genomes have a similar organisation, comprising around fourteen open reading frames (ORFs) encoding twenty-seven proteins. The genes are organised in the following order: 5’ UTR – ORF1ab (nonstructural proteins) – structural proteins (S, E, M, N) – 3’ UTR. The largest ORF, ORF1ab (light green), occupies two-thirds of the genome and encodes a polyprotein processed into sixteen nonstructural proteins (NSPs). ORF1ab CDS (yellow) indicates the coding sequence for the ORF1ab polyprotein, with regions labelled as ORF1ab peptide (dark green), denoting cleavage sites or functional peptides derived from the polyprotein. Both viruses have conserved structural protein genes encoding the envelope (E), spike (S), membrane (M), and nucleocapsid (N) proteins. Both viruses utilise a discontinuous transcription mechanism, which generates a set of subgenomic mRNAs. These subgenomic mRNAs are used for the translation of structural and accessory proteins. The transcriptional regulatory sequences (TRSs) upstream of each gene share similar structures in both viruses, allowing efficient transcription. SARS-CoV-1 and SARS-CoV-2 encode small accessory ORFs, such as ORF3a, ORF6, ORF7a, ORF7b, and ORF8. These smaller ORFs, located between structural protein genes, encode accessory proteins that modulate host immune responses and may contribute to immune evasion, host interactions, and virus replication. The ORF8 gene has undergone major changes between the two viruses. It is established that ORF8 in SARS-CoV-1 encodes two distinct accessory proteins, 8a and 8b, while a single accessory protein is produced by SARS-CoV-2 ORF8. Interestingly, SARS-CoV-2 has an additional ORF10 adjacent to the 3’ UTR encoding a single accessory protein. Meanwhile, the SARS-CoV-2 genome encodes characteristic “stem-loop” structures marked within ORF1ab, ORF10 and 3’UTR, which could represent a regulatory RNA secondary structure essential for viral replication and transcription.
Timeline of antiviral therapies for SARS coronaviruses
Over the past two decades, antiviral therapy research for SARS-CoV has progressed dramatically. The COVID-19 pandemic began in 2020, rapid advancements were made with drugs like Remdesivir and Favipiravir, alongside continued investigation into other repurposed and novel therapies. Various synthetic and natural compounds have been used to treat coronavirus infections throughout previous pandemics and early epidemics from 2003 to 2024. This paper provides a thorough evaluation of these compounds.
2003-2006
The 2003 SARS outbreak prompted quick research and evaluation of various existing pharmaceuticals. Because of their antiviral properties, chloroquine and nicosamide, initially prescribed as antimalarial and antihelminthic medications, respectively, were repurposed (Cinatl et al., 2003). The potential of luteolin, a naturally occurring flavonoid, glycyrrhizin, hexachlorophene, vanillinbananin, and alpha-Hederin to prevent SARS-CoV replication was investigated. Early attempts to diversify antiviral options were also made with the testing of saponins, valinomycin, and promazine (Cinatl et al., 2003). Another significant discovery from 2003 was the repurposing of penicillium-derived antibiotic mycophenolic acid (MPA), which has shown promise in preventing or minimising coronavirus infection. In 2004, further research led to the discovery of Beta-D-N4-Hydroxycytidine (NHC), which inhibited HCoV-NL63 (EC50 of 400 nM and CC50 of >100 μM) and SARS-CoV (EC50 of 10 μM and CC50 of >100 μM) (Barnard et al., 2004b). Also, an antihelminthic drug, Niclosamide, showed SARS-CoV inhibition at post-entry steps in Vero cells (EC50 of 1–3 μM and CC50 of 250 μM) (Wu et al., 2004). N-(2-aminoethyl)-1 aziridineethanamine (NAAE), a new small-molecule inhibitor (EC50 of 0.5 μM), represented a big step in targeting the SARS-CoV spike protein-mediated cell fusion capabilities (Huentelman et al., 2004). Researchers began to focus on structurally customised inhibitors, such as 2-acetamido-alpha-D- derivatives (Hoever et al., 2005) and Phe-phe-dipeptide inhibitor c(JMF 1521) (Shie et al., 2005), which emerged as potent inhibitors. 2-acetamido-α-D-glucopyranosylamine, benzylcysteine, and Gly-Leu peptide cause an increase of 10-fold to 70-fold in anti-SARS-CoV activity by inhibiting viral entry. Ribavirin (nucleoside analogue of guanosine), which has historically been used to treat Hepatitis C, was tested for its antiviral activity against SARS-CoV in vitro (EC50 of 20 μg/mL and CC50 of >200 μg/mL) (Saijo et al., 2005). Adamantane-based compounds such as Bananins, specifically engineered to inhibit helicase activities and replication of SARS Coronavirus (EC50 of <10 μM and CC50 of 390 μM), were also introduced as promising antiviral agents (Tanner et al., 2005). The emphasis on derivatives remained in 2006; for example, the Eremomycin derivative molecules 27 and 39 (Gan et al., 2006) showed efficacy in blocking viral proteases SARS-CoV (EC50 of 14 μM and CC50 of 22 μM) (Balzarini et al., 2006). In contrast, pyrimidine analogue 6-azauridine emerged as a nucleoside analogue for suppressing HCoV-NL63 replication (EC50 of 32 nM and CC50 of 80 μM) (Pyrc et al., 2006). The antiviral effects of mycophenolic acid (MPA) and ribavirin were confirmed by further characterisation (Barnard et al., 2006). One of the most important factors in preventing viral infections is the suppression of immunological responses, which MPA blocks in vivo by suppressing the apoptosis-inducing enzyme IMP dehydrogenase in the host (BALB/c mice) (Barnard et al., 2006) (Table 1).
2007-2010
Repurposing has been a significant trend throughout the years. A reevaluation of the effectiveness of ribavirin and emodin was performed, with an exploration of the prospect of interferon therapy to augment immune responses against the virus (Åkerström et al., 2007). Ritonavir and Lopinavir were part of the combo therapies offered for SARS-CoV in 2008. Curcumin also became a contender because of its antiviral and anti-inflammatory qualities (Kutluay et al., 2008). By 2010, several novel compounds were introduced, such as Benzopurpurin B, C-473872 and Tetrahydroquinoline Oxocarbazate (Ortiz-Alcantara et al., 2010). A plant-derived protein, Griffithsin, has shown strong efficacy in blocking viral entry (O’Keefe et al., 2010). It became clear that there was a paradigm shift toward exploring remedies that were more natural or drawn from biological sources. SARS-CoV and SARS-CoV-2 are constantly developing, and researchers are continuing their search for effective antiviral medicines. A significant focus exists on both synthetic and natural compounds that may enhance efficacy against the SARS-CoV and hCoV-2 strains (Table 1).
2011 to 2019
Between 2011 and 2019, the primary emphasis was on the development of new chemicals. This time signified substantial advancements in the comprehension of coronavirus pathophysiology and viral replication processes. This comprehension establishes a vital basis for formulating therapies for the ensuing SARS-CoV-2 epidemic. In 2011, researchers discovered mucroporin-M1, an antimicrobial peptide (AMP) exhibiting antiviral efficacy against the influenza virus (H5N1), measles virus (MeV), and SARS-CoV, signifying a potential foundation for further development (Li et al., 2011). They demonstrated that mucroporin-M1 could inhibit SARS coronavirus entry in HeLa-ACE2 cells with an EC50 of 14.46 μg/mL and a CC50 of 61.58 μg/mL with intense virucidal activity (Li et al., 2011).
By 2012, the antiviral focus had expanded to include molecules such as Scutellarin and Tyr-lys-Tyr-Arg-Tyr-Leu, hexapeptides that showed inhibitory activity against SARS-CoV, HCoV-NL63, and SARS-CoV-2 infection in Vero cells without any cytotoxic effects (Struck et al., 2012). The discovery of protease inhibitors, such as dipeptides dipeptidyl aldehyde (GC373) and dipeptidyl bisulphite adduct salt (GC376), was particularly significant as it targeted the SARS-CoV 3CLpro, with IC50 of 3.48 and 4.35 μM, respectively (Kim et al., 2012). In 2013, three derivatives of N-(9,10-dioxo-9,10-dihydroanthracen-2-yl)benzamide (SSAA09E1), N-[[4-(4-methylpiperazin-1-yl)phenyl]methyl]-1,2-oxazole-5-carboxamide (SSAA09E2), and [(Z)-1-thiophen-2-ylethylideneamino]thiourea (SSAA09E3) were developed. These inhibitors showed activity against SARS-CoV by blocking virus-host cell early interactions at EC50 varying from 3.1-9.7 μM and CC50 varying from 20- 100 μM (Adedeji et al., 2013). Additionally, a peptide, Cyclosporin A, was recognised for its broad-spectrum ability to inhibit diverse SARS-CoV, HCoV-229E and MHV replication or transcription, furthering the exploration of immunosuppressive drugs in antiviral therapy (De Wilde et al., 2011; Pfefferle et al., 2011; De Wilde et al., 2013). 2014 saw the re-emergence of Mycophenolic acid (MPA), a penicillium-derived antibiotic traditionally used as an immunosuppressant. MPA also inhibited MERS-CoV replication in Vero cells with an EC50 of 2.87 μM (Hart et al., 2014). Another compound, Arbidol and its derivatives, a broad-spectrum antiviral used in the treatment of influenza viruses, demonstrates the potential to reduce the SARS-CoV viral load in vitro at 50 μg/mL (Blaising et al., 2014; Hart et al., 2014).
This indicates a growing focus on utilising drugs that have already demonstrated safety for the treatment of SARS-CoV. In 2015, an innovative acyclic sugar scaffold of acyclovir was introduced, demonstrating structural modifications that improved its antiviral properties against coronaviruses, specifically MERS-CoV (EC50 and CC50 of 23 and 71 μM) and HCoV-NL63 (EC50 and CC50 of 8.8 and 120 μM) (Peters et al., 2015). A significant advancement was HIV Tat-protein-derived peptide (YGRKKRRQRRRGSG) known as Tat-P29 (TP29), which demonstrated strong broad-spectrum antiviral activity, effectively inhibiting in vitro replication of SARS-CoV (with an EC50 of 200 μM) and MHV (with an EC50 of 60 μM) (Wang et al., 2015). TP29 negatively affected the 2’-O-MTase activity of nsp10/nsp16, disrupting the replication process of the SARS-CoV genome.
Broad-spectrum coronavirus antiviral medication research advanced in 2016 with numerous promising candidates. Myricetin, a flavonoid, inhibited HCoV-229E and SARS-CoV-2 Mpro and 3CL-Pro enzymes, preventing in vitro replication (Dorman and Dorman, 2016). Nitazoxanide and its derivative, Tizoxanide, displayed broad-spectrum antiviral activities exploited for treating influenza viruses (A and B), as well as Ebola virus (EBOV) (Rossignol, 2016). In vitro studies on LLC-MK2 cells demonstrated that Nitazoxanide could inhibit MERS-CoV with an EC50 of 0.92 μg/mL. Favipiravir, Ribavirin, and Remdesivir have shown effectiveness in inhibiting viral RNA polymerase, indicating significant potential for treating coronavirus (Sissoko et al., 2016). The protease inhibitors Lopinavir-Ritonavir and Nafamostat demonstrated efficacy in inhibiting viral replication by acting on the viral protease (Zumla et al., 2016; Yamamoto et al., 2020).
In 2017, researchers continued to look for antiviral drugs that may stop coronaviruses from replicating. Remdesivir maintained its encouraging performance in preventing viral replication in both in vitro and animal models, whereas Alisparivir and Favipiravir were investigated further for their ability to target RNA polymerase (Furuta et al., 2017; Sheahan et al., 2017). In 2018, the identification of drugs like Saracatinib, a kinase inhibitor, and Disulfiram, typically used for alcohol dependence, broadened the range of repurposed drugs with possible antiviral activity (Shin et al., 2018). Deguelin (9,10-Dimethoxy-3,3-dimethyl-13,13a-dihydro-3H-chromeno[3,4-b]pyrano[2,3-h]chromen-7(7aH)-one) is a natural compound known for its ability to inhibit the HCV replication mechanism. Its potential application in combating SARS-CoV infection underscores the importance of plant-based antivirals (Nukui et al., 2018).
In 2019, these efforts reached a significant point with the additional use of Remdesivir (a nucleoside analogue- GS-5734) and Favipiravir (a purine nucleic acid analogue- T-705). Both compounds demonstrated notable potential in clinical trials and emerged as essential instruments in combating coronaviruses (Mulangu et al., 2019; Declercq et al., 2022). Exploring interference techniques such as interference RNAs (iRNAs) and short interference RNAs (siRNAs) for the treatment of HBV, HCV, HIV, and HTLV infections (Hernández and Sanan-Mishra, 2017) was also found to be a promising antiviral against structural proteins E, M, and N of SARS-CoV. This technique is also potent in antiviral therapies by modulating the host immune response (Li et al., 2011). Overall, the period from 2011 to 2019 laid a strong foundation for developing effective antiviral drugs against SARS-CoV. From 2003 to 2019, a heap of data of in vitro and in vivo antiviral activity assays for several molecules against CoVs was generated. It was high time to critically compare these molecules that could be further investigated for their clinical applicability. The research during these years expanded the antiviral arsenal and provided evidence of the mandatory need for further research and rapid development of broad pharmaceutical agents against emerging viral threats, including the global response to the SARS-CoV-2 pandemic in 2020.
2020-2024
The development of antivirals targeting SARS-CoV-2 had a record-breaking pace during this period (Table 1). Scientists all across the globe have been working hard to find ways to lessen the effects of the virus, develop new vaccinations, and finding new uses for old medications. In order to improve innate immunity against SARS-CoV-2 infections, the present research expanded the spectrum of holistic antiviral techniques by highlighting the relevance of peptide-based antivirals, immunosuppressive drugs, anti-inflammatory molecules, corticosteroids, and natural substances. Additionally, convalescent plasma therapy (CPT)- obtained from previously infected donors who developed antibodies- was further exploited to enhance immune responses in severe COVID-19 cases (Chen et al., 2020). The major challenge in the COVID-19 pandemic was the onset of evolving serotypes and clinical variants across the globe. Therefore, future innovative and diversified antiviral strategies are critically needed for sustained research to combat drug resistance.
Key antiviral agents included Remdesivir (GS-5734), one of the first drugs approved for emergency use, along with other repurposed treatments like Chloroquine, Hydroxychloroquine, Lopinavir/Ritonavir, and Favipiravir, which were tested based on their previous antiviral properties (Dehelean et al., 2020). New molecular candidates were also investigated, such as human monoclonal antibodies (mAbs) 47D11 H2L2-neutralizing Ab, which showed promise in neutralising the SARS-CoV-2 entry in Vero cells with an EC50 of 0.57 μg/mL (Wang et al., 2020). This research opens doors for mAbs that can be used alone or in association with other compounds to treat COVID-19. Peptidomimetic α,β-unsaturated esters, Ebselen, Betulinic acid, and Glycyrrhizic acid were explored for their potential to inhibit viral replication. Various inhibitors targeting the main protease (Mpro) and other essential viral enzymes were developed, including N3, 11a/11b, and Carmofur (Jin et al., 2020; Tiwari et al., 2020). The results of randomised, controlled, and multicenter clinical studies show that immune modulators such as dexamethasone, a corticosteroid, have emerged as essential therapies for decreasing inflammation in severe instances of COVID-19. These treatments have also been shown to considerably reduce death rates in hospitalised patients, according to Recovery 2020 (The RECOVERY Collaborative Group, 2021). Other immunomodulatory agents like tocilizumab, an IL-6 inhibitor, were employed to combat cytokine storms caused by SARS-CoV-2 (Stone et al., 2020; Zeng et al., 2020). The pan-CoV peptide-based fusion inhibitors EK1 and its lipopeptide EK1C4 were also effective against membrane fusion mediated by S proteins of the Omicron subvariants of SARS-CoV-2 and virus-like particle infection. HCoV-OC43’s HR2 region was used to produced peptide EK1 and lipopeptide EK1C4. EK1C4, a lipopeptide with cholesterol and GSGSG-PEG4 linker at its C-terminus, has much higher antiviral effectiveness than EK1.
Several other drugs impairing different stages of viral replication and interfering with nonstructural proteins (NSPs) were explored, such as Camostat Mesylate, Ivermectin, Arbidol Hydrochloride (Umifenovir), Oseltamivir, and Nitazoxanide (Dehelean et al., 2020). Ivermectin of 5 μM concentration inhibited up to 99% of SARS-CoV-2 replication in Vero cells and showed no cytotoxic effects (Caly et al., 2020). The clinical trial studies of Arbidol Hydrochloride (Umifenovir) on COVID-19 patients did not decrease viral load but were still used as an antiviral drug due to its antioxidant potential (Proskurnina et al., 2020). Because vitamin D was also investigated for its ability to strengthen immunological defences, it continued to be used as a medication to treat viral infections (Shalayel et al., 2020).
The year 2021 saw the development and scientific investigation of a number of different antiviral medications in order to combat the worldwide epidemic that was triggered by SARS-CoV-2. Among these medicines, EIDD-2801 (also known as Molnupiravir or MK-4482) has emerged as a potential nucleoside analogue showing considerable activity against SARS-CoV-2 in preclinical models (Wahl et al., 2022). Molnupiravir (EIDD-2801 or MK-4482), which started to treat RNA viruses, has attracted attention for its ability to significantly inhibit SARS-CoV-2 replication (within 48 hours of infection) and transmission, making it a contender for early COVID-19 therapy. Barrigenol, Kaempferol, and Myricetin, extracted from Camelia Sinensis (tea) were discovered in several research (Sharma et al., 2022), also indicated antiviral action. This was mostly due to their capacity to impede viral entrance or reproduction. In order to investigate the therapeutic potential of these plant-based compounds against SARS-CoV-2, the pool of naturally derived medicines that were investigated.
Additionally, throughout the year, continued research was conducted on existing drugs, such as Camostat Mesylate and Ivermectin, to investigate the possibility that these medications may be used to treat SARS-CoV-2 infections. The ability of Camostat Mesylate, a protease inhibitor, to inhibit TMPRSS2, a protein that is necessary for viral entry into host cells, was investigated in this study (Rajter et al., 2021). Likewise, Ivermectin, a standard antiparasitic drug, was examined for its in vitro antiviral properties, although clinical outcomes remained ambiguous (Rajter et al., 2021).
As of the year 2021, Monoclonal antibodies (mAbs) continued to be utilised and researched, marking a significant advancement. It was shown that the combination of REGN10933 and REGN10987, which is known as REGN-COV2, was able to successfully lower the viral load and avoid catastrophic outcomes in COVID-19 patients (Weinreich et al., 2021). Monoclonal antibody therapies continued to play a critical role in the treatment landscape, such as Sotrovimab (VIR-7831 or GSK-4182136)/Bebtelovimab (LY-CoV1404‖ LY3853113) and combinations like Tixagevimab/Cilgavimab and Bamlanivimab/Etesevimab remaining at the forefront. The combination of these MAbs effectively neutralised both the wide-type SARS-CoV-2 and the variants that are now causing concern, including Alpha (B.1.1.7), Beta (B.1.351), Gamma (P.1), and Delta (B.1.617.2). Additionally, they continued to have significant action against Omicron lineages, such as BA.2.75, BA.4, BA.4.6, and BA.5.
Other monoclonal antibody therapies, such as Casirivimab and Imdevimab, Sotrovimab, and Bamlanivimab with Etesevimab, were also evaluated and used as therapeutic interventions in high-risk patients. The investigation into repurposed drugs persisted in 2021, with compounds like Darunavir (an HIV protease inhibitor), Nitazoxanide (an antiparasitic), and Sorafenib (a cancer therapy) being subjected to clinical trials to assess their antiviral potential (Sun et al., 2021). In addition, Foretinib, Eprosartan, and Inarigivir soproxil were investigated for their capacity to suppress the replication of SARS-CoV-2 and to reduce the severity of the symptoms of infection (Joshi and Poduri, 2021). Dexamethasone and other more recent medications, such as Sarilumab, an IL-6 receptor antagonist, continued to be administered to strengthen the role that immunological modulation performs well against severe COVID-19 (Castelnovo et al., 2021). The findings highlighted the importance of addressing hyperinflammatory syndrome and acute respiratory failure, conditions that were often associated with unfavourable outcomes in patients with severe COVID-19. Innovative agents like P2C-1F11, a neutralising antibody, and CR3022, a monoclonal antibody aimed at the receptor-binding domain of SARS-CoV-2, have offered fresh perspectives on the efficacy of antibody-based therapies in the prevention or treatment of SARS-CoV infections (Atyeo et al., 2021). Finally, naturally derived chemicals such as Aranotin, Ajmalicine, Silymarin, and Ursonic Acid were investigated for their antiviral effects, increasing interest in plant-based and naturally occurring substances for medicinal development against SARS-CoV-2 (Kumar et al., 2021).
The breakthrough development of antiviral drugs for SARS-CoV-2 in 2022 was oral antiviral medicine Paxlovid, a combination of Nirmatrelvir (PF-07321332) with Ritonavir, which emerged as a prominent antiviral therapy for the treatment of COVID-19. This oral treatment significantly reduced the risk of hospitalisation and severe outcomes in high-risk patients when administered early in infection. The Paxlovid leverages the main protease (Mpro) inhibition by Nirmatrelvir and utilises Ritonavir to enhance its pharmacokinetics, with mild side effects. Nirmatrelvir was equally effective as remdesivir, which has been shown to reduce hospitalisation or mortality by 87% in patients with severe COVID-19 (Gottlieb et al., 2022). The efficacy of Paxlovid in lowering viral load established it as a cornerstone in the treatment of COVID-19 arsenal against COVID-19. This highlights a combination therapy trend in antiviral development toward optimising existing medications. Continuing the trend of repurposing existing drugs, the combination of parenteral polymerase inhibitor Remdesivir and Molnupiravir for oral treatment of COVID-19 in non-hospitalised patients was extensively evaluated in 2022. Remdesivir, an already established antiviral agent, demonstrated its ability to reduce the duration of hospitalisation in patients with severe COVID-19.
Inotodiol, neosarcodonin, Dutasteride, Etoposide, and Golvatinib were explored for their potential antiviral properties. Inotodiol, a triterpenoid derived from Inonotus obliquus (I. obliquus), a wild Chaga mushroom, was one of the new compounds whose antiviral and anti-inflammatory efficacy against SARS-CoV-2 was also investigated. Similarly, the well-established anti-inflammatory qualities of neosarcodonin A, B, and C—isolated from Sarcodon scabrosus bitter mushroom—led to its repurposing in light of the rising interest in alternate sources of antiviral compounds. The function of antiandrogens as agents of protection against COVID-19 was also investigated. Under this particular scenario, a double-blind, placebo-controlled randomised clinical trial (RCT) was conducted for dutasteride (DUTA) with early antiandrogen therapy (EAT) was recommended as a treatment for COVID-19 (EAT-DUTA AndroCoV). Etoposide, a medication that contains epipodophyllotoxin, has been used to treat immune-mediated inflammatory illnesses linked to cytokine storm syndrome for over 40 years. This molecule has been investigated for COVID-19 treatment. The most significant binding free energy is exhibited by golvatinib (IC50 around 1uM), indicating that it may be evaluated for anti-SARS-CoV-2 infection in vitro (Shen et al., 2023).
Simeprevir, a protease inhibitor and Paritaprevir authorised for managing HCV infection, has shown potential antiviral efficacy at EC50 of 1.41 ± 0.12 μM to suppress SARS-CoV-2 replication in Vero E6 cells (Muturi et al., 2022). Ivermectin, tipranavir, and paritaprevir have dose-dependent inhibitory action (IC50) against protease (3CLPro) at 21.5, 27.7, and 73.4 µM, indicating their potential as anti-SARS-CoV-2 inhibitors (Mody et al., 2021). Furthermore, in silico investigations on alpha-ergocryptine and diosgenin glucoside revealed inhibitory ability against SARS-CoV-2 endoribonuclease (NSP15) and potentially bioactive molecules.
Throughout year 2023, researchers found and analysed a wide range of compounds, demonstrating the changing landscape of antiviral development. Prominent among the antiviral agents in 2023 are combinations of drugs such as bamlanivimab/etesevimab, casirivimab/imdevimab, and tixagevimab/cilgavimab have been actively researched and administered to enhance the efficacy of COVID-19 treatment, especially in high-risk patients. Remdesivir, Paxlovid (nirmatrelvir/ritonavir), and molnupiravir are well-known medicines still important in COVID-19 care. In addition to these proven drugs, the year saw the development of many new compounds having antiviral characteristics. Natural compounds such as cafestol, kahweol, and theaflavin 3,3′-digallate showed inhibitory potential in suppressing SARS-CoV-2 replication. Other potential possibilities were tested for antiviral efficacy, including dihydroergocristine, ergometrine, and ubidecarenone. The year also witnessed a concerted effort to better understand the immunomodulatory landscape of COVID-19. This effort included the development of several interleukin-6 inhibitors (such as tocilizumab and salirumab) and other antiviral drugs (such as Nakinra, baricitinib, and tofacitinib) in order to develop treatments that would reduce the severity of the disease.
As of 2024, the prevalence of clinical variations of concern (VoC) of SARS-CoV-2 in the U.S. renders them resistant to several neutralizing monoclonal antibodies (mAbs). The US FDA is now not allowing or is revoking several products, including REGEN-COV (casirivimab and imdevimab), Sotrovimab, Bamlanivimab and Etesevimab, Bebtelovimab, and Evusheld (tixagevimab/cilgavimab) as of the end of 2023. They cannot be used for pre-exposure prophylaxis to prevent or treat COVID-19 under the EUA. Meanwhile, several existing molecules have received FDA approval for COVID-19 treatment, including tocilizumab administered intravenously or subcutaneously, remdesivir as an intravenous therapy, baricitinib as an oral immune modulator, and Paxlovid (nirmatrelvir/ritonavir) as an oral antiviral treatment for adults and long-COVID patients. Tocilizumab is an intravenous or subcutaneous immune modulator for hospitalized children aged 2 to under 18 years. Vilobelimab is an intravenous infusion immune modulator approved for treating hospitalized adults, provided it is administered within 48 hours of initiating invasive mechanical ventilation (IMV) or extracorporeal membrane oxygenation (ECMO). Anakinra is an immune modulator approved for the treatment of COVID-19 in hospitalized adults with pneumonia who require supplemental oxygen, whether low- or high-flow and are at risk of developing severe respiratory failure. Molnupiravir is an oral antiviral that has received FDA authorization for emergency use in treating mild-to-moderate COVID-19 in individuals aged 18 and older at high risk for progression to severe COVID-19. Baricitinib is an oral immune modulator approved for emergency use in hospitalized children aged 2 to under 18 years who require supplemental oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation (ECMO) due to COVID-19. Ensitrelvir, a 3C-like protease inhibitor, when administered orally once a day for five days, dramatically decreased viral RNA levels by day 4 and showed a significantly quicker resolution of the five typical symptoms of the Omicron SARS-CoV2 (Yamato et al., 2024). Based on such evidences, Ensitrelvir received emergency approval in Japan in 2022. However, larger trials are required for further confirmation.
Conclusion
As of now, the nucleoside analogues Ribavirin, Favipiravir, and Remdesivir; the HIV-1 protease inhibitors lopinavir and Ritonavir; and the SARS-CoV-2 main protease (Mpro/3CLpro) inhibitor Nirmatrelvir stand out as the pivotal antiviral agents based on their track record of use and effectiveness in coronavirus outbreaks. Despite how well these antivirals generally work in clinical settings, much work still needs to be done to make an antiviral that works well against coronaviruses.
Author contributions
MK: Writing – original draft, Writing – review & editing. MB: Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. KB: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aboul-Fotouh, S., Mahmoud, A. N., Elnahas, E. M., Habib, M. Z., Abdelraouf, S. M. (2023). What are the current anti-COVID-19 drugs? From traditional to smart molecular mechanisms. Virol. J. 20, 241. doi: 10.1186/s12985-023-02210-z
Adedeji, A. O., Severson, W., Jonsson, C., Singh, K., Weiss, S. R., Sarafianos, S. G. (2013). Novel inhibitors of severe acute respiratory syndrome coronavirus entry that act by three distinct mechanisms. J. Virol. 87, 8017–8028. doi: 10.1128/JVI.00998-13
Afsar, M., Narayan, R., Akhtar, M. N., Das, D., Rahil, H., Nagaraj, S. K., et al. (2022). Drug targeting Nsp1-ribosomal complex shows antiviral activity against SARS-CoV-2. eLife. 11, e74877. doi: 10.7554/eLife.74877
Åkerström, S., Mirazimi, A., Tan, Y. J. (2007). Inhibition of SARS-CoV replication cycle by small interference RNAs silencing specific SARS proteins, 7a/7b, 3a/3b and S. Antiviral Res. 73, 219–227. doi: 10.1016/j.antiviral.2006.10.008
Al-Rashedi, N. A. M., Munahi, M. G., Ah ALObaidi, L. (2022). Prediction of potential inhibitors against SARS-CoV-2 endoribonuclease: RNA immunity sensing. J. Biomolecular Structure Dynamics. 40, 4879–4892. doi: 10.1080/07391102.2020.1863265
Atyeo, C., Slein, M. D., Fischinger, S., Burke, J., Schäfer, A., Leist, S. R., et al. (2021). Dissecting strategies to tune the therapeutic potential of SARS-CoV-2–specific monoclonal antibody CR3022. JCI Insight 6, e143129. doi: 10.1172/jci.insight.143129
Baig, M. S., Deepanshu, Prakash, P., Alam, P., Krishnan, A. (2023). In silico analysis reveals hypoxia-induced miR-210-3p specifically targets SARS-CoV-2 RNA. J. Biomolecular Structure Dynamics. 41, 12305–12327. doi: 10.1080/07391102.2023.2175255
Baig, M. S., Reyaz, E., Selvapandiyan, A., Krishnan, A., Krishnan, A. (2021). Differential binding of SARS-CoV-2 Spike protein variants to its cognate receptor hACE2 using molecular modeling based binding analysis. Bioinformation. 17, 337–347. Available at: https://pmc.ncbi.nlm.nih.gov/articles/PMC8225600/.
Balzarini, J., Keyaerts, E., Vijgen, L., Egberink, H., De Clercq, E., Van Ranst, M., et al. (2006). Inhibition of feline (FIPV) and human (SARS) coronavirus by semisynthetic derivatives of glycopeptide antibiotics. Antiviral Res. 72, 20–33. doi: 10.1016/j.antiviral.2006.03.005
Barage, S., Karthic, A., Bavi, R., Desai, N., Kumar, R., Kumar, V., et al. (2022). Identification and characterisation of novel RdRp and Nsp15 inhibitors for SARS-COV2 using computational approach. J. Biomolecular Structure Dynamics. 40, 2557–2574. doi: 10.1080/07391102.2020.1841026
Barnard, D. L., Day, C. W., Bailey, K., Heiner, M., Montgomery, R., Lauridsen, L., et al. (2006). Enhancement of the infectivity of SARS-CoV in BALB/c mice by IMP dehydrogenase inhibitors, including ribavirin. Antiviral Res. 71, 53–63. doi: 10.1016/j.antiviral.2006.03.001
Barnard, D. L., Hubbard, V. D., Burton, J., Smee, D. F., Morrey, J. D., Otto, M. J., et al. (2004a). Inhibition of severe acute respiratory syndrome-associated coronavirus (SARSCoV) by calpain inhibitors and β-D-N 4 -hydroxycytidine. Antivir Chem. Chemother. 15, 15–22. doi: 10.1177/095632020401500102
Barnard, D. L., Hubbard, V. D., Smee, D. F., Sidwell, R. W., Watson, K. G. W., Tucker, S. P. T., et al. (2004b). In vitro activity of expanded-spectrum pyridazinyl oxime ethers related to pirodavir: novel capsid-binding inhibitors with potent antipicornavirus activity. Antimicrob. Agents Chemother. 48, 1766–1772. doi: 10.1128/AAC.48.5.1766-1772.2004
Batool, A., Bibi, N., Amin, F., Kamal, M. A. (2021). Drug designing against NSP15 of SARS-COV2 via high throughput computational screening and structural dynamics approach. Eur. J. Pharmacol. 892, 173779. doi: 10.1016/j.ejphar.2020.173779
Blaising, J., Polyak, S. J., Pécheur, E. I. (2014). Arbidol as a broad-spectrum antiviral: An update. Antiviral Res. 107, 84–94. doi: 10.1016/j.antiviral.2014.04.006
Breining, P., Frølund, A. L., Højen, J. F., Gunst, J. D., Staerke, N. B., Saedder, E., et al. (2021). Camostat mesylate against SARS-CoV-2 and COVID-19—Rationale, dosing and safety. Basic Clin. Pharma Tox. 128, 204–212. doi: 10.1111/bcpt.v128.2
Caly, L., Druce, J. D., Catton, M. G., Jans, D. A., Wagstaff, K. M. (2020). The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 178, 104787. doi: 10.1016/j.antiviral.2020.104787
Cao, Y., Su, B., Guo, X., Sun, W., Deng, Y., Bao, L., et al. (2020). Potent neutralising antibodies against SARS-coV-2 identified by high-throughput single-cell sequencing of convalescent patients’ B cells. Cell. 182, 73–84.e16. doi: 10.1016/j.cell.2020.05.025
Castelnovo, L., Tamburello, A., Lurati, A., Zaccara, E., Marrazza, M. G., Olivetti, M., et al. (2021). Anti-IL6 treatment of serious COVID-19 disease: A monocentric retrospective experience. Medicine. 100, e23582. doi: 10.1097/MD.0000000000023582
Chan, J. F. W., Kok, K. H., Zhu, Z., Chu, H., To, K. K. W., Yuan, S., et al. (2020). Genomic characterisation of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 9, 221–236. doi: 10.1080/22221751.2020.1719902
Chen, J., Xia, L., Liu, L., Xu, Q., Ling, Y., Huang, D., et al. (2020). Antiviral activity and safety of darunavir/cobicistat for the treatment of COVID-19. Open Forum Infect. Dis. 7, ofaa241. doi: 10.1093/ofid/ofaa241
Cinatl, J., Morgenstern, B., Bauer, G., Chandra, P., Rabenau, H., Doerr, H. (2003). Treatment of SARS with human interferons. Lancet 362, 293–294. doi: 10.1016/S0140-6736(03)13973-6
Dai, W., Zhang, B., Jiang, X. M., Su, H., Li, J., Zhao, Y., et al. (2020). Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 368, 1331–1335. doi: 10.1126/science.abb4489
De Clercq, E. (2019). New nucleoside analogues for the treatment of hemorrhagic fever virus infections. Chem. Asian J. 14, 3962–3968. doi: 10.1002/asia.201900841
Declercq, J., De Leeuw, E., Lambrecht, B. N. (2022). Inflammasomes and IL-1 family cytokines in SARS-CoV-2 infection: from prognostic marker to therapeutic agent. Cytokine. 157, 155934. doi: 10.1016/j.cyto.2022.155934
Dehelean, C. A., Lazureanu, V., Coricovac, D., Mioc, M., Oancea, R., Marcovici, I., et al. (2020). SARS-coV-2: repurposed drugs and novel therapeutic approaches—Insights into chemical structure—Biological activity and toxicological screening. J. Clin. Med. 9, 2084. doi: 10.3390/jcm9072084
De Lima Menezes, G., Da Silva, R. A. (2021). Identification of potential drugs against SARS-CoV-2 nonstructural protein 1 (nsp1). J. Biomolecular Structure Dynamics. 39, 5657–5667. doi: 10.1080/07391102.2020.1792992
De Wilde, A. H., Raj, V. S., Oudshoorn, D., Bestebroer, T. M., Van Nieuwkoop, S., Limpens, R. W. A. L., et al. (2013). MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J. Gen. Virology. 94, 1749–1760. doi: 10.1099/vir.0.052910-0
De Wilde, A. H., Zevenhoven-Dobbe, J. C., van der Meer, Y., Thiel, V., Narayanan, K., Makino, S., et al. (2011). Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virology. 92, 2542–2548. doi: 10.1099/vir.0.034983-0
Dhanabalan, A. K., Raghavan, S. S., Rajendran, S., Ramasamy, V., Abdul, S. A. A., Narayanasamy, N., et al. (2023). Evaluation of action of steroid molecules on SARS-CoV-2 by inhibiting NSP-15, an endoribonuclease. Mol. Divers. 27, 2715–2728. doi: 10.1007/s11030-022-10576-5
Dorman, C. J., Dorman, M. J. (2016). DNA supercoiling is a fundamental regulatory principle in the control of bacterial gene expression. Biophys. Rev. 8, 209–220. doi: 10.1007/s12551-016-0205-y
Elfiky, A. A. (2020). Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): a molecular docking study. Life Sci. 253, 117592. doi: 10.1016/j.lfs.2020.117592
Ferner, R. E., Aronson, J. K. (2020). Chloroquine and hydroxychloroquine in covid-19. BMJ, m1432. doi: 10.1136/bmj.m1432
Furuta, Y., Komeno, T., Nakamura, T. (2017). Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc. Japan Acad. Ser. B: Phys. Biol. Sci. 93, 449–463. doi: 10.2183/pjab.93.027
Gan, Y. R., Huang, H., Huang, Y. D., Rao, C. M., Zhao, Y., Liu, J. S., et al. (2006). Synthesis and activity of an octapeptide inhibitor designed for SARS coronavirus main proteinase. Peptides. 27, 622–625. doi: 10.1016/j.peptides.2005.09.006
Glebov, O. O. (2020). Understanding SARS-CoV-2 endocytosis for COVID-19 drug repurposing. FEBS J. 287, 3664–3671. doi: 10.1111/febs.v287.17
Gorkhali, R., Koirala, P., Rijal, S., Mainali, A., Baral, A., Bhattarai, H. K. (2021). Structure and function of major SARS-coV-2 and SARS-coV proteins. Bioinform. Biol. Insights 15, 11779322211025876. doi: 10.1177/11779322211025876
Gottlieb, R. L., Vaca, C. E., Paredes, R., Mera, J., Webb, B. J., Perez, G., et al. (2022). Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl. J. Med. 386, 305–315. doi: 10.1056/NEJMoa2116846
Gu, C., Cao, X., Wang, Z., Hu, X., Yao, Y., Zhou, Y., et al. (2021). A human antibody of potent efficacy against SARS-CoV-2 in rhesus macaques showed strong blocking activity to B.1.351. mAbs. 13, 1930636. doi: 10.1080/19420862.2021.1930636
Haga, S., Nagata, N., Okamura, T., Yamamoto, N., Sata, T., Yamamoto, N., et al. (2010). TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 85, 551–555. doi: 10.1016/j.antiviral.2009.12.001
Han, Y., Duan, X., Yang, L., Nilsson-Payant, B. E., Wang, P., Duan, F., et al. (2021). Identification of SARS-CoV-2 inhibitors using lung and colonic organoids. Nature. 589, 270–275. doi: 10.1038/s41586-020-2901-9
Hart, B. J., Dyall, J., Postnikova, E., Zhou, H., Kindrachuk, J., Johnson, R. F., et al. (2014). Interferon-β and mycophenolic acid are potent inhibitors of Middle East respiratory syndrome coronavirus in cell-based assays. J. Gen. Virology. 95, 571–577. doi: 10.1099/vir.0.061911-0
Hernández, Y., Sanan-Mishra, N. (2017). miRNA mediated regulation of NAC transcription factors in plant development and environment stress response. Plant Gene. 11, 190–198. doi: 10.1016/j.plgene.2017.05.013
Hoever, G., Baltina, L., Michaelis, M., Kondratenko, R., Baltina, L., Tolstikov, G. A., et al. (2005). Antiviral activity of glycyrrhizic acid derivatives against SARS–coronavirus. J. Med. Chem. 48, 1256–1259. doi: 10.1021/jm0493008
Hoffmann, M., Kleine-Weber, H., Schroeder, S., Krüger, N., Herrler, T., Erichsen, S., et al. (2020). SARS-coV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 181, 271–280. doi: 10.1016/j.cell.2020.02.052
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi: 10.1016/S0140-6736(20)30183-5
Huentelman, M. J., Zubcevic, J., Hernández Prada, J. A., Xiao, X., Dimitrov, D. S., Raizada, M. K., et al. (2004). Structure-based discovery of a novel angiotensin-converting enzyme 2 inhibitor. Hypertension. 44, 903–906. doi: 10.1161/01.HYP.0000146120.29648.36
Jamir, E., Sarma, H., Priyadarsinee, L., Kiewhuo, K., Nagamani, S., Sastry, G. N. (2023). Polypharmacology guided drug repositioning approach for SARS-CoV2. PloS One 18, e0289890. doi: 10.1371/journal.pone.0289890
Jin, Z., Du, X., Xu, Y., Deng, Y., Liu, M., Zhao, Y., et al. (2020). Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 582, 289–293. doi: 10.1038/s41586-020-2223-y
Joshi, G., Poduri, R. (2021). Selection of active antiviral compounds against COVID-19 disease targeting coronavirus endoribonuclease nendou/NSP15 via ligandbased virtual screening and molecular docking. LDDD. 18, 610–619. doi: 10.2174/1570180817999201211191445
Khan, R. J., Jha, R. K., Singh, E., Jain, M., Amera, G. M., Singh, R. P., et al. (2022). Identification of promising antiviral drug candidates against nonstructural protein 15 (NSP15) from SARS-CoV-2: anin silicoassisted drug-repurposing study. J. Biomolecular Structure Dynamics. 40, 438–448. doi: 10.1080/07391102.2020.1814870
Kim, Y., Lovell, S., Tiew, K. C., Mandadapu, S. R., Alliston, K. R., Battaile, K. P., et al. (2012). Broad-spectrum antivirals against 3C or 3C-like proteases of picornaviruses, noroviruses, and coronaviruses. J. Virol. 86, 11754–11762. doi: 10.1128/JVI.01348-12
Kumar, S., Sharma, P. P., Upadhyay, C., Kempaiah, P., Rathi, B., Poonam (2021). Multi-targeting approach for nsp3, nsp9, nsp12 and nsp15 proteins of SARS-CoV-2 by Diosmin as illustrated by molecular docking and molecular dynamics simulation methodologies. Methods. 195, 44–56. doi: 10.1016/j.ymeth.2021.02.017
Kutluay, S. B., Doroghazi, J., Roemer, M. E., Triezenberg, S. J. (2008). Curcumin inhibits herpes simplex virus immediate-early gene expression by a mechanism independent of p300/CBP histone acetyltransferase activity. Virology. 373, 239–247. doi: 10.1016/j.virol.2007.11.028
Li, K., Li, H., Bi, Z., Song, D., Zhang, F., Lei, D., et al. (2019). Significant inhibition of re-emerged and emerging swine enteric coronavirus in vitro using the multiple shRNA expression vector. Antiviral Res. 166, 11–18. doi: 10.1016/j.antiviral.2019.03.010
Li, Q., Zhao, Z., Zhou, D., Chen, Y., Hong, W., Cao, L., et al. (2011). Virucidal activity of a scorpion venom peptide variant mucroporin-M1 against measles, SARS-CoV and influenza H5N1 viruses. Peptides. 32, 1518–1525. doi: 10.1016/j.peptides.2011.05.015
Liao, W., Liu, X., Yang, Q., Liu, H., Liang, B., Jiang, J., et al. (2020). Deguelin inhibits HCV replication through suppressing cellular autophagy via down regulation of Beclin1 expression in human hepatoma cells. Antiviral Res. 174, 104704. doi: 10.1016/j.antiviral.2020.104704
Lin, M. H., Moses, D. C., Hsieh, C. H., Cheng, S. C., Chen, Y. H., Sun, C. Y., et al. (2018). Disulfiram can inhibit MERS and SARS coronavirus papain-like proteases via different modes. Antiviral Res. 150, 155–163. doi: 10.1016/j.antiviral.2017.12.015
Ma, C., Hu, Y., Townsend, J. A., Lagarias, P. I., Marty, M. T., Kolocouris, A., et al. (2020). Ebselen, disulfiram, carmofur, PX-12, tideglusib, and shikonin are nonspecific promiscuous SARS-coV-2 main protease inhibitors. ACS Pharmacol. Transl. Sci. 3, 1265–1277. doi: 10.1021/acsptsci.0c00130
Mahmud, S., Elfiky, A. A., Amin, A., Mohanto, S. C., Rahman, E., Acharjee, U. K., et al. (2021). Targeting SARS-coV-2 nonstructural protein 15 endoribonuclease: an in silico perspective. Future Virology. 16, 467–474. doi: 10.2217/fvl-2020-0233
Mody, V., Ho, J., Wills, S., Mawri, A., Lawson, L., Ebert, M. C. C. J. C., et al. (2021). Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 4, 93. doi: 10.1038/s42003-020-01577-x
Mulangu, S., Dodd, L. E., Davey, R. T., Tshiani Mbaya, O., Proschan, M., Mukadi, D., et al. (2019). A randomised, controlled trial of ebola virus disease therapeutics. N Engl. J. Med. 381, 2293–2303. doi: 10.1056/NEJMoa1910993
Muturi, E., Hong, W., Li, J., Yang, W., He, J., Wei, H., et al. (2022). Effects of simeprevir on the replication of SARS-CoV-2 in vitro and in transgenic hACE2 mice. Int. J. Antimicrobial Agents. 59, 106499. doi: 10.1016/j.ijantimicag.2021.106499
Ng, T. I., Correia, I., Seagal, J., DeGoey, D. A., Schrimpf, M. R., Hardee, D. J., et al. (2022). Antiviral drug discovery for the treatment of COVID-19 infections. Viruses. 14, 961. doi: 10.3390/v14050961
Nukui, M., O’Connor, C. M., Murphy, E. A. (2018). The natural flavonoid compound deguelin inhibits HCMV lytic replication within fibroblasts. Viruses. 10, 614. doi: 10.3390/v10110614
O’Keefe, B. R., Giomarelli, B., Barnard, D. L., Shenoy, S. R., Chan, P. K. S., McMahon, J. B., et al. (2010). Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family coronaviridae. J. Virol. 84, 2511–2521. doi: 10.1128/JVI.02322-09
Oany, A. R., Mia, M., Pervin, T., Junaid, M. D., Hosen, S. M. Z., Moni, M. A. (2020). Design of novel viral attachment inhibitors of the spike glycoprotein (S) of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) through virtual screening and dynamics. Int. J. Antimicrobial Agents. 56, 106177. doi: 10.1016/j.ijantimicag.2020.106177
Oroojalian, F., Haghbin, A., Baradaran, B., Hemmat, N., Shahbazi, M. A., Baghi, H. B., et al. (2020). Novel insights into the treatment of SARS-CoV-2 infection: an overview of current clinical trials. Int. J. Biol. Macromolecules. 165, 18–43. doi: 10.1016/j.ijbiomac.2020.09.204
Ortiz-Alcantara, J., Bhardwaj, K., Palaninathan, S., Frieman, M., Baric, R., Kao, C. (2010). Small molecule inhibitors of the SARS-CoV Nsp15 endoribonuclease. Virus Adaptation Treat. , 2:125–2:133. doi: 10.2147/VAAT.S12733
Peters, H. L., Jochmans, D., De Wilde, A. H., Posthuma, C. C., Snijder, E. J., Neyts, J., et al. (2015). Design, synthesis and evaluation of a series of acyclic fleximer nucleoside analogues with anti-coronavirus activity. Bioorganic Medicinal Chem. Letters. 25, 2923–2926. doi: 10.1016/j.bmcl.2015.05.039
Pfefferle, S., Schöpf, J., Kögl, M., Friedel, C. C., Müller, M. A., Carbajo-Lozoya, J., et al. (2011). The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. Denison MR editor. PloS Pathog. 7, e1002331. doi: 10.1371/journal.ppat.1002331
Proskurnina, E. V., Izmailov, D. Y., Sozarukova, M. M., Zhuravleva, T. A., Leneva, I. A., Poromov, A. A. (2020). Antioxidant potential of antiviral drug umifenovir. Molecules. 25, 1577. doi: 10.3390/molecules25071577
Pyrc, K., Bosch, B. J., Berkhout, B., Jebbink, M. F., Dijkman, R., Rottier, P., et al. (2006). Inhibition of human coronavirus NL63 infection at early stages of the replication cycle. Antimicrob. Agents Chemother. 50, 2000–2008. doi: 10.1128/AAC.01598-05
Rajter, J. C., Sherman, M. S., Fatteh, N., Vogel, F., Sacks, J., Rajter, J. J. (2021). Use of ivermectin is associated with lower mortality in hospitalised patients with coronavirus disease 2019. Chest. 159, 85–92. doi: 10.1016/j.chest.2020.10.009
Ramajayam, R., Tan, K. P., Liu, H. G., Liang, P. H. (2010). Synthesis, docking studies, and evaluation of pyrimidines as inhibitors of SARS-CoV 3CL protease. Bioorganic Medicinal Chem. Letters. 20, 3569–3572. doi: 10.1016/j.bmcl.2010.04.118
Riva, L., Yuan, S., Yin, X., Martin-Sancho, L., Matsunaga, N., Pache, L., et al. (2020). Discovery of SARS-CoV-2 antiviral drugs through large-scale compound repurposing. Nature. 586, 113–119. doi: 10.1038/s41586-020-2577-1
Rossignol, J. F. (2016). Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infection Public Health 9, 227–230. doi: 10.1016/j.jiph.2016.04.001
Saijo, M., Morikawa, S., Fukushi, S., Mizutani, T., Hasegawa, H., Nagata, N., et al. (2005). Inhibitory effect of mizoribine and ribavirin on the replication of severe acute respiratory syndrome (SARS)-associated coronavirus. Antiviral Res. 66, 159–163. doi: 10.1016/j.antiviral.2005.01.003
Sanghai, N., Tranmer, G. K. (2020). Taming the cytokine storm: repurposing montelukast for the attenuation and prophylaxis of severe COVID-19 symptoms. Drug Discovery Today 25, 2076–2079. doi: 10.1016/j.drudis.2020.09.013
Santos, I. D. A., Grosche, V. R., Bergamini, F. R. G., Sabino-Silva, R., Jardim, A. C. G. (2020). Antivirals against coronaviruses: candidate drugs for SARS-coV-2 treatment? Front. Microbiol. 11, 1818. doi: 10.3389/fmicb.2020.01818
Saramago, M., Costa, V. G., Souza, C. S., Bárria, C., Domingues, S., Viegas, S. C., et al. (2022). The nsp15 nuclease as a good target to combat SARS-coV-2: mechanism of action and its inactivation with FDA-approved drugs. Microorganisms. 10, 342. doi: 10.3390/microorganisms10020342
Scholl, A. R., Korentzelos, D., Forns, T. E., Brenneman, E. K., Kelm, M., Datto, M., et al. (2022). Characterisation of casirivimab plus imdevimab, sotrovimab, and bamlanivimab plus etesevimab-derived interference in serum protein electrophoresis and immunofixation electrophoresis. J. Appl. Lab. Med. 7, 1379–1387. doi: 10.1093/jalm/jfac064
Shah, P. P., Wang, T., Kaletsky, R. L., Myers, M. C., Purvis, J. E., Jing, H., et al. (2010). A Small-Molecule Oxocarbazate Inhibitor of Human Cathepsin L Blocks Severe Acute Respiratory Syndrome and Ebola Pseudotype Virus Infection into Human Embryonic Kidney 293T cells. Mol. Pharmacol. 78, 319–324. doi: 10.1124/mol.110.064261
Shalayel, M., Al-Mazaideh, G., Aladaileh, S., Alswailmi, F., Al-thiabat, M. (2020). Vitamin D is a potential inhibitor of COVID-19: In silico molecular docking to the binding site of SARS-CoV-2 endoribonuclease Nsp15. Pakistan J. Pharm. Sci. , 33:2179–86. doi: 10.36721/PJPS.2020.33.5.REG.2179-2186.1
Sharma, A., Goyal, S., Yadav, A. K., Kumar, P., Gupta, L. (2022). In-silico screening of plant-derived antivirals against main protease, 3CLpro and endoribonuclease, NSP15 proteins of SARS-CoV-2. J. Biomolecular Structure Dynamics. 40, 86–100. doi: 10.1080/07391102.2020.1808077
Sharma, J., Kumar Bhardwaj, V., Singh, R., Rajendran, V., Purohit, R., Kumar, S. (2021). An in-silico evaluation of different bioactive molecules of tea for their inhibition potency against non structural protein-15 of SARS-CoV-2. Food Chem. 346, 128933. doi: 10.1016/j.foodchem.2020.128933
Sheahan, T. P., Sims, A. C., Graham, R. L., Menachery, V. D., Gralinski, L. E., Case, J. B., et al. (2017). Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci. Transl. Med. 9, eaal3653. doi: 10.1126/scitranslmed.aal3653
Sheahan, T. P., Sims, A. C., Leist, S. R., Schäfer, A., Won, J., Brown, A. J., et al. (2020). Comparative therapeutic efficacy of remdesivir and combination lopinavir, Ritonavir, and interferon beta against MERS-CoV. Nat. Commun. 11, 222. doi: 10.1038/s41467-019-13940-6
Shen, M., Ding, P., Luan, G., Du, T., Deng, S. (2023). The antiviral activity of a small molecule drug targeting the NSP1-ribosome complex against Omicron, especially in elderly patients. Front. Cell Infect. Microbiol. 13, 1141274. doi: 10.3389/fcimb.2023.1141274
Shie, J. J., Fang, J. M., Kuo, T. H., Kuo, C. J., Liang, P. H., Huang, H. J., et al. (2005). Inhibition of the severe acute respiratory syndrome 3CL protease by peptidomimetic α,β-unsaturated esters. Bioorganic Medicinal Chem. 13, 5240–5252. doi: 10.1016/j.bmc.2005.05.065
Shin, J. S., Jung, E., Kim, M., Baric, R. S., Go, Y. Y. (2018). Saracatinib inhibits middle east respiratory syndrome-coronavirus replication in vitro. Viruses 10, 283. doi: 10.3390/v10060283
Sinha, S. K., Prasad, S. K., Islam, M. A., Gurav, S. S., Patil, R. B., AlFaris, N. A., et al. (2021). Identification of bioactive compounds from Glycyrrhiza glabra as possible inhibitor of SARS-CoV-2 spike glycoprotein and nonstructural protein-15: a pharmacoinformatics study. J. Biomolecular Structure Dynamics. 39, 4686–4700. doi: 10.1080/07391102.2020.1779132
Sinha, S. K., Shakya, A., Prasad, S. K., Singh, S., Gurav, N. S., Prasad, R. S., et al. (2020). An in-silico evaluation of different Saikosaponins for their potency against SARS-CoV-2 using NSP15 and fusion spike glycoprotein as targets. J. Biomolecular Structure Dynamics, 1–12. doi: 10.1080/07391102.2020.1762741
Sissoko, D., Laouenan, C., Folkesson, E., M’Lebing, A. B., Beavogui, A. H., Baize, S., et al. (2016). Experimental treatment with favipiravir for ebola virus disease (the JIKI trial): A historically controlled, single-arm proof-of-concept trial in Guinea. PloS Med. 13, e1001967. doi: 10.1371/journal.pmed.1001967
Solidarity Trial Consortium, W. H. O. (2021). Repurposed antiviral drugs for covid-19 — Interim WHO solidarity trial results. N Engl. J. Med. 384, 497–511. doi: 10.1056/NEJMoa2023184
Stone, J. H., Frigault, M. J., Serling-Boyd, N. J., Fernandes, A. D., Harvey, L., Foulkes, A. S., et al. (2020). Efficacy of tocilizumab in patients hospitalised with covid-19. N Engl. J. Med. 383, 2333–2344. doi: 10.1056/NEJMoa2028836
Struck, A. W., Axmann, M., Pfefferle, S., Drosten, C., Meyer, B. (2012). A hexapeptide of the receptor-binding domain of SARS corona virus spike protein blocks viral entry into host cells via the human receptor ACE2. Antiviral Res. 94, 288–296. doi: 10.1016/j.antiviral.2011.12.012
Sun, L., Li, P., Ju, X., Rao, J., Huang, W., Ren, L., et al. (2021). In vivo structural characterisation of the SARS-CoV-2 RNA genome identifies host proteins vulnerable to repurposed drugs. Cell. 184, 1865–1883. doi: 10.1016/j.cell.2021.02.008
Tang, J. Y., Tsigelny, I. F., Greenberg, J. P., Miller, M. A., Kouznetsova, V. L. (2021). Potential SARS-coV-2 nonstructural protein 15 inhibitors: repurposing FDA-approved drugs. J. Exploratory Res. Pharmacol. 6, 137–147. doi: 10.14218/JERP.2021.00032
Tanner, J. A., Zheng, B. J., Zhou, J., Watt, R. M., Jiang, J. Q., Wong, K. L., et al. (2005). The adamantane-derived bananins are potent inhibitors of the helicase activities and replication of SARS coronavirus. Chem. Biol. 12, 303–311. doi: 10.1016/j.chembiol.2005.01.006
The RECOVERY Collaborative Group (2021). Dexamethasone in hospitalized patients with covid-19. N Engl. J. Med. 384, 693–704. doi: 10.1056/NEJMoa2021436
Tiwari, V., Beer, J. C., Sankaranarayanan, N. V., Swanson-Mungerson, M., Desai, U. R. (2020). Discovering small-molecule therapeutics against SARS-CoV-2. Drug Discovery Today 25, 1535–1544. doi: 10.1016/j.drudis.2020.06.017
Touret, F., De Lamballerie, X. (2020). Of chloroquine and COVID-19. Antiviral Res. 177, 104762. doi: 10.1016/j.antiviral.2020.104762
Vijayan, R., Gourinath, S. (2021). Structure-based inhibitor screening of natural products against NSP15 of SARS-CoV-2 revealed thymopentin and oleuropein as potent inhibitors. J. Proteins Proteom. 12, 71–80. doi: 10.1007/s42485-021-00059-w
von Beck, T., Mena Hernandez, L., Zhou, H., Floyd, K., Suthar, M. S., Skolnick, J., et al. (2023). Atovaquone and pibrentasvir inhibit the SARS-coV-2 endoribonuclease and restrict infection in vitro but not in vivo. Viruses 15, 1841. doi: 10.3390/v15091841
Wahl, S., Steen-Larsen, H. C., Hughes, A. G., Dietrich, L. J., Zuhr, A., Behrens, M., et al. (2022). Atmosphere-snow exchange explains surface snow isotope variability. Geophysical Res. Lett. 49, e2022GL099529. doi: 10.1029/2022GL099529
Wang, M., Cao, R., Zhang, L., Yang, X., Liu, J., Xu, M., et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 30, 269–271. doi: 10.1038/s41422-020-0282-0
Wang, Y., Sun, Y., Wu, A., Xu, S., Pan, R., Zeng, C., et al. (2015). Coronavirus nsp10/nsp16 Methyltransferase can be targeted by nsp10-Derived peptide in vitro and in vivo to reduce replication and pathogenesis. J. Virol. 89, 8416–8427. doi: 10.1128/JVI.00948-15
Warren, T. K., Jordan, R., Lo, M. K., Ray, A. S., Mackman, R. L., Soloveva, V., et al. (2016). Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 531, 381–385. doi: 10.1038/nature17180
Weinreich, D. M., Sivapalasingam, S., Norton, T., Ali, S., Gao, H., Bhore, R., et al. (2021). REGN-COV2, a neutralizing antibody cocktail, in outpatients with covid-19. N Engl. J. Med. 384, 238–251. doi: 10.1056/NEJMoa2035002
World Health Organization (WHO). (2024). COVID-19 epidemiological update. Available at: https://www.who.int/publications/m/item/covid-19-epidemiological-update-edition-171 (Accessed 17 September 2024).
Wu, C. J., Jan, J. T., Chen, C. M., Hsieh, H. P., Hwang, D. R., Liu, H. W., et al. (2004). Inhibition of severe acute respiratory syndrome coronavirus replication by niclosamide. Antimicrob. Agents Chemother. 48, 2693–2696. doi: 10.1128/AAC.48.7.2693-2696.2004
Yamamoto, N., Matsuyama, S., Hoshino, T., Yamamoto, N. (2020). Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. Microbiology. 11(3):e0431122. doi: 10.1101/2020.04.06.026476
Yamamoto, M., Matsuyama, S., Li, X., Takeda, M., Kawaguchi, Y., Inoue, J. I., et al. (2016). Identification of nafamostat as a potent inhibitor of middle east respiratory syndrome coronavirus S protein-mediated membrane fusion using the split-protein-based cell-cell fusion assay. Antimicrob. Agents Chemother. 60, 6532–6539. doi: 10.1128/AAC.01043-16
Yamato, M., Kinoshita, M., Miyazawa, S., Seki, M., Mizuno, T., Sonoyama, T. (2024). Ensitrelvir in patients with SARS-CoV-2: A retrospective chart review. J. Infection Chemotherapy. 30(9), 946–950. doi: 10.1016/j.jiac.2024.02.015
Yu, M. S., Lee, J., Lee, J. M., Kim, Y., Chin, Y. W., Jee, J. G., et al. (2012). Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorganic Medicinal Chem. Letters. 22, 4049–4054. doi: 10.1016/j.bmcl.2012.04.081
Zeng, Y. M., Xu, X. L., He, X. Q., Tang, S. Q., Li, Y., Huang, Y. Q., et al. (2020). Comparative effectiveness and safety of ribavirin plus interferon-alpha, lopinavir/ritonavir plus interferon-alpha, and ribavirin plus lopinavir/ritonavir plus interferon-alpha in patients with mild to moderate novel coronavirus disease 2019: study protocol. Chin. Med. J. 133, 1132–1134. doi: 10.1097/CM9.0000000000000790
Zhou, P., Yang, X. L., Wang, X. G., Hu, B., Zhang, L., Zhang, W., et al. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 579, 270–273. doi: 10.1038/s41586-020-2012-7
Keywords: COVID-19, long COVID, SARS coronavirus, betacoronavirus, antivirals
Citation: Kumar M, Baig MS and Bhardwaj K (2025) Advancements in the development of antivirals against SARS-Coronavirus. Front. Cell. Infect. Microbiol. 15:1520811. doi: 10.3389/fcimb.2025.1520811
Received: 31 October 2024; Accepted: 02 January 2025;
Published: 23 January 2025.
Edited by:
Shih-Heng Chen, National Institute of Environmental Health Sciences (NIH), United StatesReviewed by:
Nan Zheng, Nanjing University, ChinaCopyright © 2025 Kumar, Baig and Bhardwaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanchan Bhardwaj, a2FuY2hhbi5zZXRAbXJpdS5lZHUuaW4=; Mirza Sarwar Baig, YmFpZ21pcnphc2Fyd2FyQGdtYWlsLmNvbQ==
 Mrityunjay Kumar
Mrityunjay Kumar Mirza Sarwar Baig
Mirza Sarwar Baig Kanchan Bhardwaj
Kanchan Bhardwaj