
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 17 February 2025
Sec. Parasite and Host
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1504741
This article is part of the Research TopicAddressing Contemporary Threats to Global Malaria Control: New Tools and StrategiesView all 8 articles
 Inke Nadia Diniyanti Lubis1*
Inke Nadia Diniyanti Lubis1* Irbah Rea Alvieda Nainggolan1
Irbah Rea Alvieda Nainggolan1 Meliani Meliani1
Meliani Meliani1 Beby Syofiani Hasibuan1
Beby Syofiani Hasibuan1 Kumuthamalar Sangaran2,3
Kumuthamalar Sangaran2,3 Luqman Samsudin4
Luqman Samsudin4 Sriwipa Chuangchaiya5
Sriwipa Chuangchaiya5 Paul Cliff Simon Divis6
Paul Cliff Simon Divis6 Ranti Permatasari1
Ranti Permatasari1 Zulkarnain Md Idris2*
Zulkarnain Md Idris2*Introduction: The incidence of malaria in Indonesia has declined significantly over the last few decades. Thus, a demand for more sensitive techniques to describe low levels of transmission in the country is important. This study was conducted to evaluate antibody response to Plasmodium falciparum and Plasmodium vivax in an area nearing elimination in North Sumatera Province, Indonesia.
Methods: A cross-sectional survey was conducted in Langkat district, North Sumatera Province, in June 2019. Basic demographic data and filter paper blood spots were collected from 339 participants. Antibody responses to two P. falciparum (PfAMA-1 and PfMSP-119) and two P. vivax (PvAMA-1 and PvMSP-119) antigens were measured using indirect enzyme-linked immunosorbent assay (ELISA). Seroconversion rates (SCR) were estimated by fitting a simple reversible catalytic model to seroprevalence data for each antibody. Multiple logistic regression was used to investigate factors associated with exposure.
Results: The overall malaria seroprevalence was 10.6% for PfAMA-1, 13% for PfMSP-119, 18.6% for PvAMA-1, and 7.4% for PvMSP-119. Seropositive individuals for P. falciparum (PfAMA-1/PfMSP-119) and P. vivax (PvAMA-1/PvMSP-119) were similar at 20.7%, with no significant differences observed between age groups (p > 0.05). Based on the reversible catalytic model, the calculated SCRs indicated a higher level of P. falciparum transmission than P. vivax using all tested antigens. In the adjusted model, only spending nights in the forest was associated with P. vivax seropositivity (odd ratio: 3.93, p < 0.001).
Conclusion: The analysis of community-based serological data helps describe the similar levels of P. falciparum and P. vivax transmission in the Langkat district. The use of a serological approach enhances the detection of past exposure, aiding in the identification of epidemiological risk factors and malaria surveillance in low transmission settings in Indonesia.
Malaria remains a significant global health concern, particularly in tropical and subtropical countries. Indonesia, one of nine malaria-endemic countries in tropical Southeast Asia, has set a goal to eliminate malaria by 2030 (WHO, 2015). The country has made remarkable progress in malaria control, reducing the incidence from 1.8 million cases in 2011 to 811,636 cases in 2021 (WHO, 2023). In 2022, it was estimated that only 6.4% of Indonesia’s population was at high risk of malaria with 8.2% still living in active foci areas (WHO, 2023). As malaria-endemic areas in Indonesia shrink and become more localized, ongoing efforts to control and monitor the disease are crucial to containing its transmission.
Measuring malaria transmission patterns is important for effectively targeting control strategies and evaluating their impact after implementation. One method to estimate transmission involves assessing the malaria-specific immune responses in local populations, which serve as indicators of exposure to infection (van den Hoogen and Drakeley, 2017). Serological markers offer an alternative to traditional surveillance methods, allowing for the effective measurement of malaria transmission through the detection of antimalarial antibodies developed in response to the parasite antigens (Zakeri et al., 2016; Idris et al., 2017a). In low malaria transmission settings, this validated approach not only serves as a proxy to conventional methods but also provides greater sensitivity and reliability (van den Hoogen and Drakeley, 2017). As such, it is a valuable tool for guiding tailored malaria control programs and monitoring changes in transmission following intervention (Cook et al., 2010; Dewasurendra et al., 2017; Idris et al., 2017b; Macalinao et al., 2023). Numerous studies conducted in low-endemicity regions have demonstrated positive outcomes in using seroepidemiological analysis in determining the malaria burden, leading to improved public health policy planning (Rosas-Aguirre et al., 2013; Zakeri et al., 2016; Idris et al., 2017a; Keffale et al., 2019; Rahim et al., 2022; Rahim et al., 2023; Pinedo-Cancino et al., 2024).
Investigating the application of serological metrics in order to understand the historical patterns of malaria transmission in a population is essential in Indonesia. Whilst several seroepidemiological studies have been conducted in provinces in Indonesia with a very low prevalence of Plasmodium falciparum and Plasmodium vivax namely Central Java (Bretscher et al., 2013), Lampung (Supargiyono et al., 2013), Aceh (Surendra et al., 2019) and Yogyakarta (Surendra et al., 2020), no such study has been carried out in North Sumatera Province where the prevalence of Plasmodium knowlesi and multispecies of human malaria have been reported (Lubis et al., 2017; Nainggolan et al., 2022). In the present study, antibody responses to P. falciparum and P. vivax blood-stage antigens apical membrane antigen 1 (AMA-1), and merozoite surface antigen-119 (MSP-119) were measured to assess malaria exposure and transmission in North Sumatera Province.
The study was conducted following the Declaration of Helsinki and was approved by the Ethics Committee of the Faculty of Medicine, Universitas Sumatera Utara (No. 179/TGL/KEPK FK-USU-RSUPHAM/2019). Participants were sensitized to the study objectives and procedures by the local health district personnel for the study participation.
A community-based cross-sectional survey using a convenience sampling strategy was conducted in Langkat district, North Sumatera Province, Indonesia, in June 2019 (Figure 1). The dominant ethnic group in the study area is Batak Karo; Karo dialect is primarily spoken, as well as the national language of Indonesia (Nainggolan et al., 2022). Langkat district covers 6,263 km² with an altitude ranging from 4 to 105 meters above sea level. In 2019, the population of Langkat was estimated at 1,041,775 inhabitants (Pemerintah kabupaten Langkat, 2014). All villages share similar environmental characteristics, as they are located in the middle of the forest, with forestry and agriculture being the primary economic activities. These forest activities by the local population could potentially increase malaria transmission to outdoor-biting and forest-dwelling mosquitoes. This study is the first to assess the seroprevalence of malaria in the area, building on previous knowledge of a very low malaria prevalence, including a 0.3% microscopic infection rate, zero rapid diagnostic test (RDT)-positive cases, and a 0.9% submicroscopic infection rate in the population (Nainggolan et al., 2022).
Figure 1. Map of the study area. (A) Map of the Republic of Indonesia. (B) Map of Sumatera Island in Indonesia showing the location of the study area (red circle) within Langkat district, North Sumatera Province (blue).
The sample size for study participation was calculated using Cochran’s formula: N = z²p(1 – p)/e², where z is the 95% confidence interval (z-value of 1.96), p is the expected prevalence of malaria (11.4% from a previous study by Surendra et al (Surendra et al., 2019)), and e is the allowed error margin (5%). Based on these considerations, a minimum size of 155 participants was calculated. The study protocol was explained to participants, and informed consent was documented, with provisions for illiterate participants and those under 18 years requiring parental or guardian consent. Participants were informed of their right to withdraw from the study at any time without prejudice. Village leaders and household heads were informed about the study’s objectives and procedures and asked to invite residents to the survey point. Inclusion criteria were individuals over 6 months old who had lived in the area for at least 6 months and consented to participate, while exclusion criteria included physically or mentally unfit individuals and incomplete examination data.
A standardized questionnaire was used to collect sociodemographic information from each participant. Finger prick blood samples were collected to prepare for dried blood spots (DBSs) on Whatman 3MM filter paper (Whatman, UK). Axillary body temperature was determined using a digital thermometer and fever was defined as a temperature exceeding 37.5°C. Hemoglobin (Hb) level was measured with the HemoCue Hb 201 analyzer (HemoCue, Sweden). Anemia was defined based on the concentrations of Hb in the blood according to WHO criteria (WHO, 2011). The DBS samples were air-dried and stored individually in sealed plastic bags. All DBS samples were transported under cold conditions to the laboratory in the Faculty of Medicine, Universitas Sumatera Utara, Medan, and stored at −20°C until further processing.
A serum elution from 6-mm diameter DBS punches was used as previously described (Idris et al., 2017a; Idris et al., 2017b; Rahim et al., 2022; Rahim et al., 2023). Briefly, proper elution of plasma from DBSs (i.e. equivalent to a 1:200 dilution of serum) was assessed by the color change of the spots (to white) as well as the elution (to red/brown) after soaking them for 1–2 nights in reconstitution solution at ambient temperature while on a horizontal shaker. If blood spots did not change color, still retaining the brownish blood color, spots were soaked further until a color change was observed or excluded from analyses as reported before (Corran et al., 2008). Antibody responses (immunoglobulin G) against apical membrane antigen-1 or the 19-kDa fragment of merozoite surface protein-1 for P. falciparum (PfAMA-1 and PfMSP-119, respectively) and P. vivax (PvAMA-1 and PvMSP-119) were tested using enzyme-linked immunosorbent assay (ELISA) as previously described (Idris et al., 2017a; Idris et al., 2017b; Rahim et al., 2022; Rahim et al., 2023). Briefly, sera from the reconstituted blood spot samples were added in duplicate at a final concentration of 1:1,000 for MSP-119 and 1:2,000 for AMA-1. In addition, four wells of malaria-naïve Malaysians sera as negative controls and a fivefold dilution series (starting at 1:100 for AMA-1 and 1:50 for MSP-119) of a hyper-immune plasma pool (n = 15) were added per plate. Optical density (OD) values were measured at 450 nm with a Multiskan Go ELISA microplate reader (Thermo Scientific, USA).
The gathered data were compiled into a Microsoft Excel spreadsheet and cross-checked for errors, with all further statistical analyses conducted in STATA version 13.1 (StataCorp, TX, USA). Continuous variables were presented using the median and interquartile range (IQR), while categorical variables were described using frequencies and percentages. Differences in proportions were tested using the chi-squared test or Fisher’s exact test. Duplicate ODs per individual were averaged, adjusted for background reactivity, and normalized against the positive control curve as previously described to adjust for plate variation (Corran et al., 2008). Seropositivity thresholds for separate antigens were calculated using a finite mixture model (Stewart et al., 2009), defining individuals as seropositive when their adjusted OD value exceeded the mean of the lower Gaussian distribution plus three times the standard deviation. The reversible catalytic model was employed to define the seroconversion rate (SCR) and plot corresponding seroconversion curves while fitting age-adjusted seropositivity to P. falciparum or P. vivax using maximum likelihood (Drakeley et al., 2005). Infants under one year of age were excluded from the reversible catalytic model to eliminate the influence of maternally derived antibodies (Drakeley et al., 2005). Factors associated with P. falciparum and P. vivax seropositivities were determined independently for each site using generalized estimating equations, adjusting for correlation between observations from the same variables. Variables significant at p < 0.10 in the univariate analyses were incorporated into the multivariate model and retained in the final model if their association with immune responses was statistically significant at p < 0.05.
A total of 339 individuals were sampled during a cross-sectional survey in Langkat district, Sumatera Utara Province, Indonesia, in June 2019 (Table 1). The median age was 39 years old (IQR 19-54) and were mostly female (68.1%). Individuals with fever and anemia at enrolment accounted for 0.6% and 36.9%, respectively. A total of 36.9% of the study participants reported having at least one bed net in their house, resulting in an overall usage of 42.2%. The majority of the participants were forest-goers (58.7%) and live within the forest fringes (86.1%). Approximately 35.7% of the study participants reported spending a night in the forest within the last 2 weeks and only 0.6% reported a history of malaria over the past 3 months.
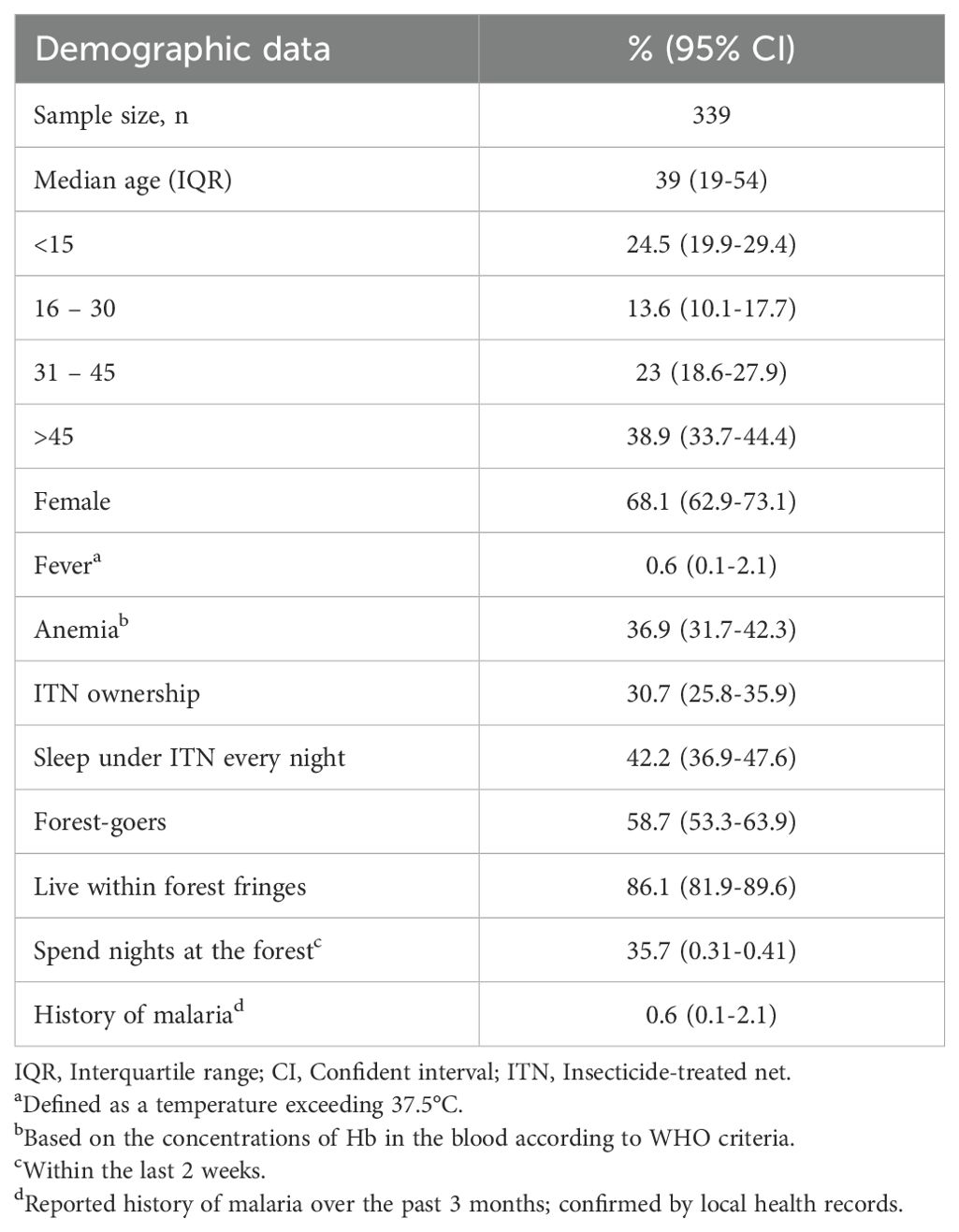
Table 1. General characteristics of the study population in Langkat district, North Sumatera Province, Indonesia in 2019.
Table 2 shows the age-specific seroprevalence and seroconversion rates of P. falciparum and P. vivax antigens among participants. Overall, malaria seroprevalence was 10.6% for PfAMA-1, 13% for PfMSP-119, 18.6% for PvAMA-1, and 7.4% for PvMSP-119. For all parasite antigens, no significant difference was observed in the proportion of seropositive individuals with increased age (all p > 0.05). Between species-specific antigens and age groups, the proportion of seropositive individuals was significantly higher for PvAMA-1 compared to PvMSP-119 (all p < 0.05), except for 16-30 years (p = 0.354). Furthermore, the proportion of participants who were seropositive for either P. falciparum antigens (PfAMA-1/PfMSP-119) or P. vivax antigens (PvAMA-1/PvMSP-119) were similar at 20.7%, with no significant differences observed between age groups (p > 0.05).
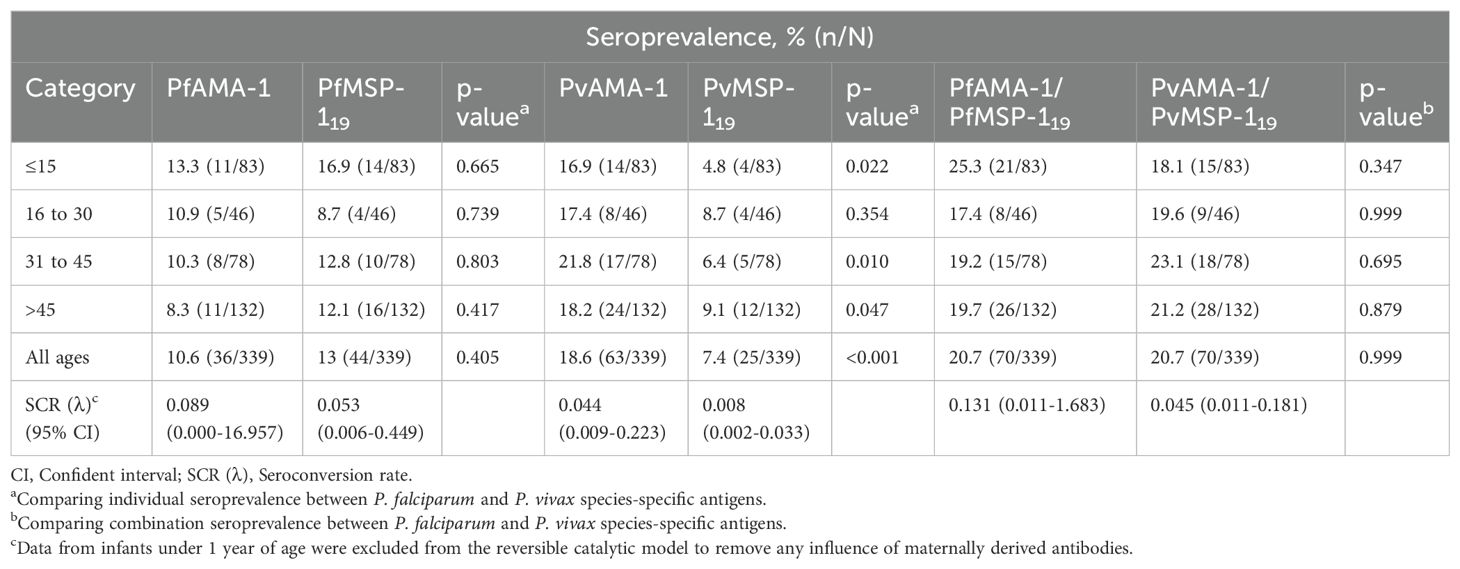
Table 2. Age-specific malaria seropositivity and seroconversion rates for participants in Langkat district, North Sumatera Province, Indonesia.
The relationship between seroprevalence and age was further examined using reversible catalytic conversion models. The SCR rates for parasite antigens are shown in Table 2; Figure 2. Based on the reversible catalytic model, the calculated SCRs indicated a higher level of P. falciparum transmission than P. vivax using all tested antigens. The P. falciparum SCR was 0.089 person-year (95% CI: 0.000–19.957), 0.053 (95% CI: 0.006–0.449) and 0.131 person-year (95% CI: 0.011–1.683) for PfAMA-1, PfMSP-119 and PfAMA-1/PfMSP-119, respectively. The P. vivax SCR was 0.053 person-year (95% CI: 0.006–0.449), 0.008 (95% C: 0.002-0.033) and 0.045 person-year (95% CI: 0.011–0.181) for PvAMA-1, PvMSP-119 and PvAMA-1/PvMSP-119, respectively. Nevertheless, the SCRs were not statistically significant between species-specific antigens, evidenced by the overlapping confidence intervals. Univariate and multivariate logistic regression analyses to identify factors associated with seropositivity to a combination of any P. falciparum– and P. vivax–specific antigens are shown in Table 3 and Table 4, respectively. Only spending nights in the forest was associated with P. vivax seropositivity in the adjusted model. The crude odd ratio (OR) of P. vivax seropositivity for those spending nights in the forest compared to those who are not was 3.89 (95% CI: 2.25–6.75, p < 0.001). The increased trend for P. vivax positivity remained apparent in the adjusted model (AOR: 3.93, 95% CI: 2.21–7.14, p < 0.001). For P. falciparum, no variables were significantly associated with seropositivity in the adjusted model (all p > 0.05).
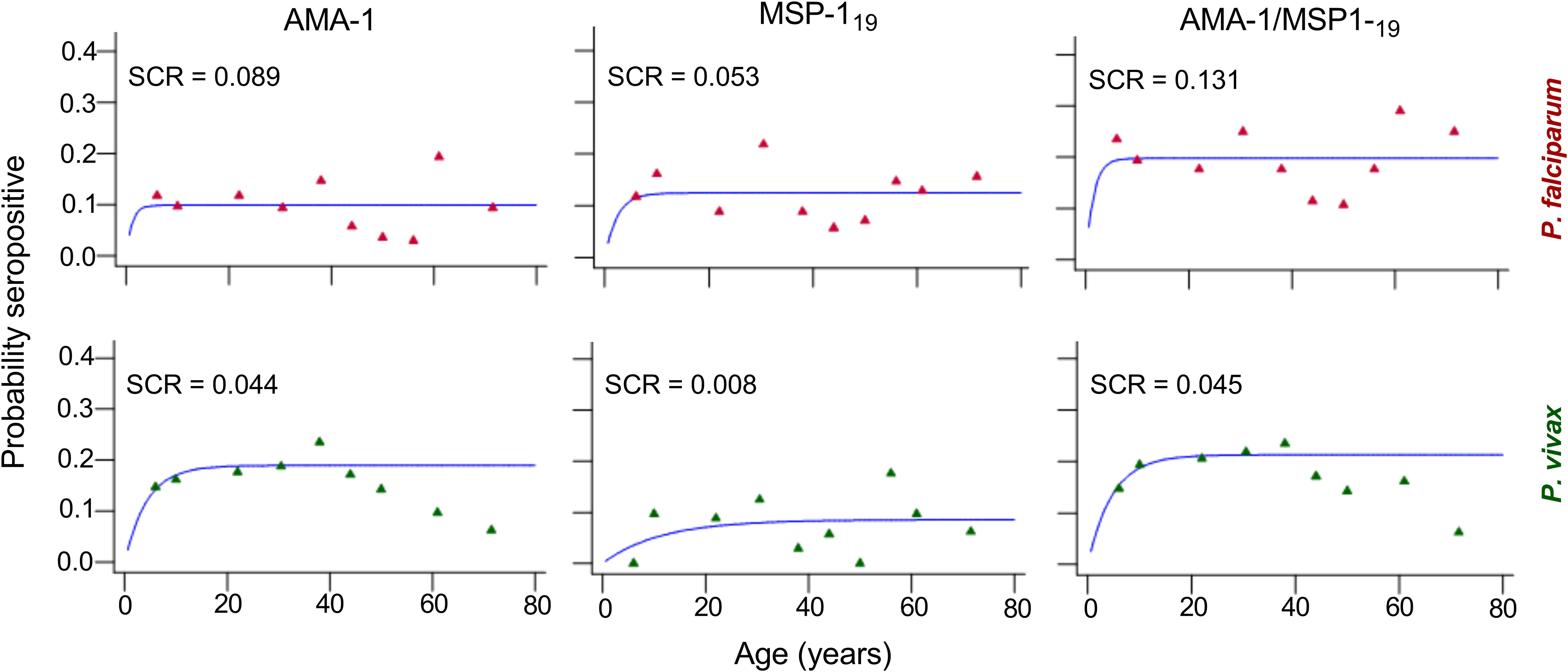
Figure 2. Annual probability of seroconversion rate (SCR) for specific malaria antigen by age in the community of Langkat district, North Sumatera Province, Indonesia. Seropositive data were obtained using age deciles and fitted to reversible catalytic seroconversion models. Points show the observed values within each age group for P. falciparum (red) and P. vivax (green) recombinant antigens and the blue line shows the fitted curve.
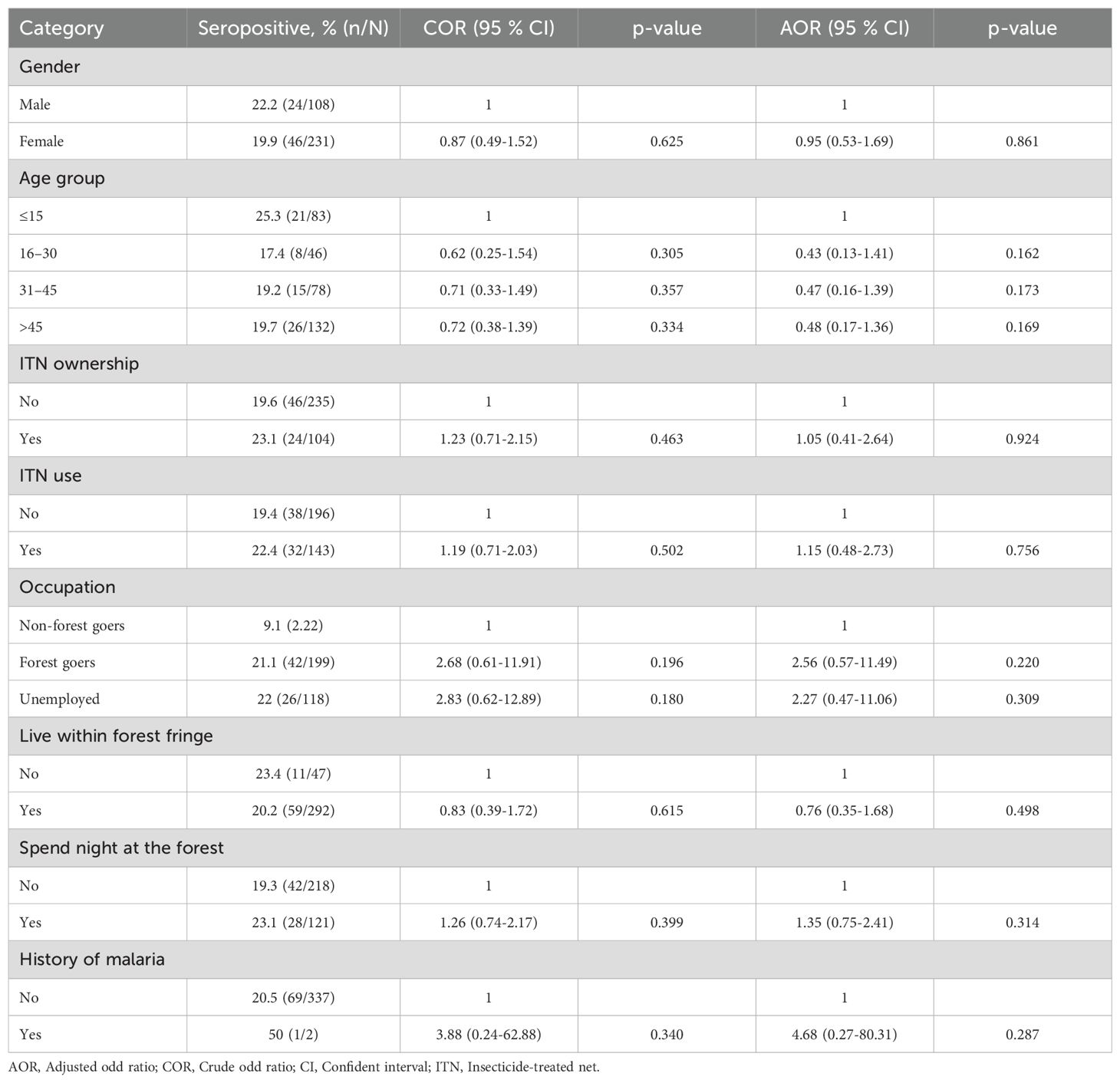
Table 3. Logistic regression analysis of explanatory factors for serological evidence of exposure to P. falciparum in Langkat district, North Sumatera Province, Indonesia.
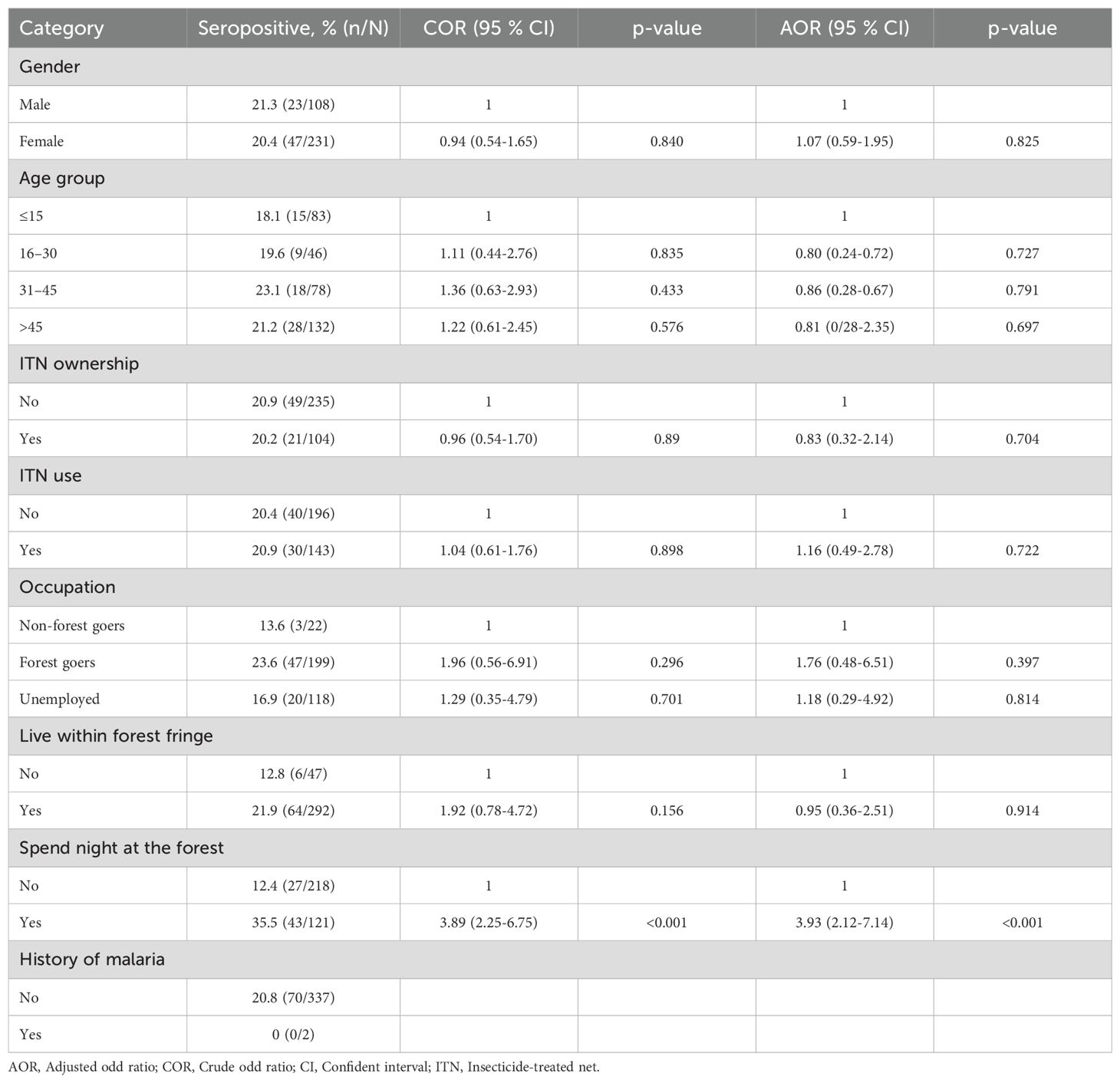
Table 4. Logistic regression analysis of explanatory factors for serological evidence of exposure to P. vivax in Langkat district, North Sumatera Province, Indonesia.
This cross-sectional study, nested within a prior epidemiological survey on submicroscopic malaria (Nainggolan et al., 2022), analyzed antibody responses to AMA-1 and MSP-119 of P. falciparum and P. vivax in samples collected from the population in Langkat district, North Sumatera Province, Indonesia. The serological outcomes revealed similarities in the seroprevalence of both species and a relationship between age groups and seroprevalence rates. The study also identified higher transmission levels of P. falciparum compared to P. vivax based on SCR and found that spending nights in the forest was a risk factor associated with P. vivax exposure. These findings could help inform malaria elimination efforts in Indonesia and support the potential integration of seroepidemiological methods into routine elimination programs.
The antigens AMA-1 and MSP-119 were selected because they are present in both species, represent the erythrocytic stage of the parasites, and have been widely used as markers of exposure in the past. The analysis of antibody responses among the study participants revealed a similar seroprevalence rate of 20.7%, with individuals responding to at least one antigen from either P. falciparum or P. vivax. Although no other serological surveys had been conducted previously in Langkat district, these rates were relatively higher compared to those found in similar epidemiological settings in Sumatera, such as a population survey in Aceh Province, Indonesia, in 2013 (6.9% for P. falciparum and 2%, for P. vivax) (Surendra et al., 2019). Nevertheless, these findings are inconsistent with reports from other countries with P. falciparum–P. vivax co-endemicity in the Southeast Asian region where antibodies against P. falciparum antigens were found to be higher than P. vivax antigens such as Malaysia (58% and 10%) (Rahim et al., 2023), Thailand (79% and 40%) (Rahim et al., 2022), Vietnam (38% and 31%) (San et al., 2022), and Myanmar (30% and 14%) (Edwards et al., 2021). The observed difference in seroprevalence rates could be attributed to the fact that antibody responses reflect cumulative exposure events, including past asymptomatic submicroscopic infections (Idris et al., 2017a; Macalinao et al., 2023), and the relatively long half-life of responses against Pf/PvAMA-1 and Pf/PvMSP-119 antigens generated after exposure (San et al., 2022). Additionally, the historical co-dominance of P. falciparum and P. vivax in the study area with similar prevalence of 13.8% and 13.6%, respectively (Lubis et al., 2017), may have contributed to the similar seroprevalence rates observed for both species in the population, highlighting the need for targeted malaria elimination strategies in Indonesia.
Seroprevalence reflects cumulative malaria exposure and modelling changes between seroprevalence and age (i.e. SCR), can be used to estimate transmission intensity in a population (Idris et al., 2017a; van den Hoogen and Drakeley, 2017). In this study, SCRs estimated from the age-adjusted seroprevalence curves for P. falciparum antigens were higher than the ones for P. vivax antigens, reflecting the more intense transmission of the former species in Langkat district. These differences in SCR estimates are likely to reflect the different ecological factors that affect malaria exposure and the acquisition of immunity to malaria in the area (Cunha et al., 2014). Unfortunately, data on the ecology of malaria parasitism in this survey were not recorded to enable the testing of these hypotheses. Additionally, different transmission patterns for falciparum and vivax as evidenced by data are likely to reflect the actual difference in transmission of the two species observed over the years. Furthermore, the higher seroconversion rate of P. falciparum compared to P. vivax may be due to its more frequent symptomatic infections, higher transmission intensity, and possibly longer persistence of antibodies post-infection (Kattenberg et al., 2018; Herman et al., 2023). P. falciparum also typically causes more severe disease, prompting a stronger and more detectable immune response over time (van den Hoogen et al., 2020).
The estimated SCR for the AMA-1 was higher than that of MSP-119 for both P. falciparum and P. vivax in Langkat District, aligning with results from other seroepidemiological studies (Cook et al., 2010; Tusting et al., 2014; Wong et al., 2014; Idris et al., 2017b; Kwenti et al., 2017; Surendra et al., 2019; van den Hoogen et al., 2020). The differences in transmission estimates between AMA-1 and MSP-119 may be attributed to variations in seroconversion and reversion rates, which are potentially influenced by differences in immunogenicity, subclass-dependent half-life, and antigen polymorphism (Badu et al., 2012). AMA-1, being more immunogenic and associated with higher antibody titers than MSP-119, likely exhibits faster seroconversion and seroreversion rates (Drakeley et al., 2005), which might also explain the observations. Furthermore, the absence of an age-related trend in seroprevalence for the AMA-1 and MSP-119 antigens of both P. falciparum and P. vivax in the present study limits their value when analyzed using the current modelling approach. Alternative serological estimates of malaria transmission intensity, such as the antibody acquisition model (Yman et al., 2016) and the unified mechanistic model (Kyomuhangi and Giorgi, 2021) can enhance the precision of transmission estimates.
Multivariate regression analyses identified that for P. falciparum, no variables were significantly associated with seropositivity in the adjusted model. However, spending nights in the forest was associated with P. vivax seropositivity. This observed association could be due to the distinct ecological and behavioral patterns of the vectors that transmit P. vivax, which are often more exophagic (outdoor-biting) and exophilic (outdoor-resting) compared to those transmitting P. falciparum (Baird, 2013). A recent study in Sumatera showed that predominant P. vivax vectors, such as Anopheles dirus (Aceh Province) and Anopheles kochi (North Sumatera Province), thrive in forested environments where they have greater access to humans sleeping outdoors or in forested areas (Permana et al., 2023), which could lead to higher exposure risk. This is consistent with the reported risk factors from a previous study, where 59.4% of individuals had forest-associated occupation, and 75.7% had a history of forest visits (Nainggolan et al., 2022). In contrast, P. falciparum vectors are generally more endophagic (indoor-biting), reducing the likelihood of transmission in forest settings (Cohen et al., 2012). The resilience of P. vivax to low-density environments and its ability to persist in temperate climates further increases its transmission potential in forested regions (Battle et al., 2012; Yman et al., 2016). Additionally, P. vivax hypnozoites can cause relapses, maintaining seropositivity even with intermittent exposure (Flannery et al., 2022).
This study had several limitations. The most significant was the insufficient sample size, which inevitably reduced the precision of the current SCR estimates (Sepúlveda et al., 2015). Additionally, the disproportionate sampling and overrepresentation of adult participants may have led to results that are not fully representative of the study population. This oversampling likely occurred due to the timing of surveys, as most were conducted on weekdays when children were in school. Furthermore, the study was restricted to a small geographical area in Langkat district, a region with very low malaria endemicity (Lubis et al., 2017; Nainggolan et al., 2022). This limitation raises concerns about the generalizability of the findings to other areas of Indonesia or beyond. Lastly, the study utilized only two recombinant antigens for each Plasmodium species. While these antigens are considered long-term markers of transmission (Drakeley et al., 2005), individual variations in immune responses to different parasite antigens suggest that using a broader range of antigens, including short-term markers, might improve the identification of seropositive individuals (Kerkhof et al., 2016). Despite these limitations, the study contributes to the collective body of knowledge on malaria antibodies and lays the groundwork for the potential future use of this tool in Indonesia, which is especially relevant for other countries also aiming for malaria elimination.
This study assessed antibody responses to AMA-1 and MSP-119 antigens of P. falciparum and P. vivax in Langkat District, Indonesia, revealing similarities in seroprevalence and significant age-related variations in seropositivity rates. The findings indicate higher transmission levels of P. falciparum compared to P. vivax, aligning with global patterns but diverging from observations in co-endemic settings in Southeast Asia. The association between spending nights in the forest and increased P. vivax seropositivity highlights the role of vector behavior and ecological factors in transmission dynamics. These insights emphasize the need for targeted interventions tailored to specific populations with varying demographics and risk factors, as well as the integration of seroepidemiological tools into malaria elimination strategies in Indonesia. By understanding the distinct transmission patterns and associated risk factors, tailored approaches can be developed to address the specific challenges of P. falciparum and P. vivax elimination.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Faculty of Medicine, Universitas Sumatera Utara. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
IL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. IN: Investigation, Methodology, Writing – original draft. MM: Investigation, Methodology, Writing – original draft. BH: Investigation, Methodology, Writing – original draft. KS: Data curation, Formal analysis, Software, Writing – original draft. LS: Data curation, Formal analysis, Software, Writing – original draft. SC: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, Investigation. PD: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, Investigation. RP: Investigation, Methodology, Writing – original draft. ZI: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the ASEAN Science Technology and Innovation Fund (ASTIF; FF-2019-124) from the ASEAN Secretariat and Geran Ganjaran Penerbitan (GP-K019336, TAP-K019336) from the Faculty of Medicine, Universiti Kebangsaan Malaysia.
We would like to extend our gratitude to the communities and community leaders for their support and participation in the survey. We wish to sincerely thank all members of the field team. We are grateful to Mohd Amirul Fitri A. Rahim, Nuraffini Ghazali and Noor Wanie Hassan for their assistance in the laboratory work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Badu, K., Afrane, Y. A., Larbi, J., Stewart, V. A., Waitumbi, J., Angov, E., et al. (2012). Marked variation in MSP-119 antibody responses to malaria in western Kenyan highlands. BMC Infect. Dis. 12, 50. doi: 10.1186/1471-2334-12-50
Baird, J. K. (2013). Evidence and implications of mortality associated with acute Plasmodium vivax malaria. Clin. Microbiol. Rev. 26, 36–57. doi: 10.1128/CMR.00074-12
Battle, K. E., Gething, P. W., Elyazar, I. R., Moyes, C. L., Sinka, M. E., Howes, R. E., et al. (2012). The global public health significance of Plasmodium vivax. Adv. Parasitol. 80, 1–111. doi: 10.1016/B978-0-12-397900-1.00001-3
Bretscher, M. T., Supargiyono, S., Wijayanti, M. A., Nugraheni, D., Widyastuti, A. N., Lobo, N. F., et al. (2013). Measurement of Plasmodium falciparum transmission intensity using serological cohort data from Indonesian schoolchildren. Malar J. 12, 21. doi: 10.1186/1475-2875-12-21
Cohen, J. M., Smith, D. L., Cotter, C., Ward, A., Yamey, G., Sabot, O. J., et al. (2012). Malaria resurgence: a systematic review and assessment of its causes. Malar J. 11, 122. doi: 10.1186/1475-2875-11-122
Cook, J., Reid, H., Iavro, J., Kuwahata, M., Taleo, G., Clements, A., et al. (2010). Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J. 9, 169. doi: 10.1186/1475-2875-9-169
Corran, P. H., Cook, J., Lynch, C., Leendertse, H., Manjurano, A., Griffin, J., et al. (2008). Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 7, 195. doi: 10.1186/1475-2875-7-195
Cunha, M. G., Silva, E. S., Sepúlveda, N., Costa, S. P., Saboia, T. C., Guerreiro, J. F., et al. (2014). Serologically defined variations in malaria endemicity in Pará state, Brazil. PloS One 9, e113357. doi: 10.1371/journal.pone.0260513
Dewasurendra, R. L., Dias, J. N., Sepulveda, N., Gunawardena, G. S., Chandrasekharan, N., Drakeley, C., et al. (2017). Effectiveness of a serological tool to predict malaria transmission intensity in an elimination setting. BMC Infect. Dis. 17, 49. doi: 10.1186/s12879-016-2164-0
Drakeley, C. J., Corran, P. H., Coleman, P. G., Tongren, J. E., McDonald, S. L., Carneiro, I., et al. (2005). Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc. Natl. Acad. Sci. 102, 5108–5113. doi: 10.1073/pnas.0408725102
Edwards, H. M., Dixon, R., Zegers de Beyl, C., Celhay, O., Rahman, M., Myint Oo, M., et al. (2021). Prevalence and seroprevalence of Plasmodium infection in Myanmar reveals highly heterogeneous transmission and a large hidden reservoir of infection. PloS One 16, e0252957. doi: 10.1371/journal.pone.0252957
Flannery, E. L., Kangwanrangsan, N., Chuenchob, V., Roobsoong, W., Fishbaugher, M., Zhou, K., et al. (2022). Plasmodium vivax latent liver infection is characterized by persistent hypnozoites, hypnozoite-derived schizonts, and time-dependent efficacy of primaquine. Mol. Ther. Methods Clin. Dev. 26, 427–440. doi: 10.1016/j.omtm.2022.07.016
Herman, C., Leonard, C. M., Uhomoibhi, P., Maire, M., Moss, D., Inyang, U., et al. (2023). Non-falciparum malaria infection and IgG seroprevalence among children under 15 years in Nigeria, 2018. Nat. Commun. 14, 1360. doi: 10.1038/s41467-023-37010-0
Idris, Z. M., Chan, C. W., Kongere, J., Hall, T., Logedi, J., Gitaka, J., et al. (2017a). Naturally acquired antibody response to Plasmodium falciparum describes heterogeneity in transmission on islands in Lake Victoria. Sci. Rep. 7, 9123. doi: 10.1038/s41598-017-09585-4
Idris, Z. M., Chan, C. W., Mohammed, M., Kalkoa, M., Taleo, G., Junker, K., et al. (2017b). Serological measures to assess the efficacy of malaria control programme on Ambae Island, Vanuatu. Parasit Vectors. 10, 204. doi: 10.1186/s13071-017-2139-z
Kattenberg, J. H., Erhart, A., Truong, M. H., Rovira-Vallbona, E., Vu, K. A. D., Nguyen, T. H. N., et al. (2018). Characterization of Plasmodium falciparum and Plasmodium vivax recent exposure in an area of significantly decreased transmission intensity in Central Vietnam. Malar J. 17, 180. doi: 10.1186/s12936-018-2326-1
Keffale, M., Shumie, G., Behaksra, S. W., Chali, W., Hoogen, L. L. V. D., Hailemeskel, E., et al. (2019). Serological evidence for a decline in malaria transmission following major scale-up of control efforts in a setting selected for Plasmodium vivax and Plasmodium falciparum malaria elimination in Babile district, Oromia, Ethiopia. Trans. R Soc. Trop. Med. Hyg. 113, 305–311. doi: 10.1093/trstmh/trz005
Kerkhof, K., Sluydts, V., Willen, L., Kim, S., Canier, L., Heng, S., et al. (2016). Serological markers to measure recent changes in malaria at population level in Cambodia. Malar J. 15, 529. doi: 10.1186/s12936-016-1576-z
Kwenti, T. E., Moye, A. L., Wiylanyuy, A. B., Njunda, L. A., Nkuo-Akenji, T. (2017). Variation in the immune responses against Plasmodium falciparum merozoite surface protein-1 and apical membrane antigen-1 in children residing in the different epidemiological strata of malaria in Cameroon. Malar J. 16, 453. doi: 10.1186/s12936-017-2105-4
Kyomuhangi, I., Giorgi, E. (2021). A unified and flexible modelling framework for the analysis of malaria serology data. Epidemiol. Infect. 149, e99. doi: 10.1017/S0950268821000753
Lubis, I. N. D., Wijaya, H., Lubis, M., Lubis, C. P., Divis, P. C. S., Beshir, K. B., et al. (2017). Contribution of Plasmodium knowlesi to multispecies human malaria infections in North Sumatera, Indonesia. J. Infect. Dis. 215, 1148–1155. doi: 10.1093/infdis/jix091
Macalinao, M. L. M., Fornace, K. M., Reyes, R. A., Hall, T., Bareng, A. P. N., Adams, J. H., et al. (2023). Analytical approaches for antimalarial antibody responses to confirm historical and recent malaria transmission: an example from the Philippines. Lancet Reg. Health West Pac. 37, 100792. doi: 10.1016/j.lanwpc.2023.100792
Nainggolan, I. R. A., Syafutri, R. D., Sinambela, M. N., Devina, C., Handayani, Hasibuan, B. S., et al. (2022). The presence of Plasmodium malariae and Plasmodium knowlesi in near malaria elimination setting in western Indonesia. Malar J. 21, 316. doi: 10.1186/s12936-022-04335-y
Pemerintah kabupaten Langkat (2014). “Langkat: kabupaten langkat; 2014,” in Iklim dan wilayah. Available at: https://www.langkatkab.go.id/page/14/iklim-dan-wilayah (Accessed June 10, 2024).
Permana, D. H., Hasmiwati, Suryandari, D. A., Rozi, I. E., Syahrani, L., Setiadi, W., et al. (2023). The potential for zoonotic malaria transmission in five areas of Indonesia inhabited by non-human primates. Parasit Vectors. 16, 267. doi: 10.1186/s13071-023-05880-4
Pinedo-Cancino, V., Arista, K. M., Baldeviano, G. C., Saavedra-Langer, R., Arana, A., Vásquez-Chasnamote, M. E., et al. (2024). Unravelling heterogeneous malaria transmission dynamics in the Peruvian Amazon: insights from a cross-sectional survey. Malar J. 23, 209. doi: 10.1186/s12936-024-05032-8
Rahim, M. A. F. A., Chuangchaiya, S., Chanpum, P., Palawong, L., Kantee, P., Dian, N. D., et al. (2022). Seroepidemiological surveillance, community perceptions and associated risk factors of malaria exposure among forest-goers in Northeastern Thailand. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.953585
Rahim, M. A. F. A., Munajat, M. B., Dian, N. D., Seri Rakna, M. I. M., Wahid, W., Ghazali, N., et al. (2023). Naturally acquired antibody response to Plasmodium falciparum and Plasmodium vivax among indigenous Orang Asli communities in Peninsular Malaysia. Front. Cell Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1165634
Rosas-Aguirre, A., Llanos-Cuentas, A., Speybroeck, N., Cook, J., Contreras-Mancilla, J., Soto, V., et al. (2013). Assessing malaria transmission in a low endemicity area of north-western Peru. Malar J. 12, 339. doi: 10.1186/1475-2875-12-339
San, N. N., Kien, N. X., Manh, N. D., Van Thanh, N., Chavchich, M., Binh, N. T. H., et al. (2022). Cross-sectional study of asymptomatic malaria and seroepidemiological surveillance of seven districts in Gia Lai province, Vietnam. Malar J. 21, 40. doi: 10.1186/s12936-022-04060-6
Sepúlveda, N., Paulino, C. D., Drakeley, C. (2015). Sample size and power calculations for detecting changes in malaria transmission using antibody seroconversion rate. Malar J. 14, 529. doi: 10.1186/s12936-015-1050-3
Stewart, L., Gosling, R., Griffin, J., Gesase, S., Campo, J., Hashim, R., et al. (2009). Rapid assessment of malaria transmission using age-specific sero-conversion rates. PloS One 4, e6083. doi: 10.1371/journal.pone.0006083
Supargiyono, S., Bretscher, M. T., Wijayanti, M. A., Sutanto, I., Nugraheni, D., Rozqie, R., et al. (2013). Seasonal changes in the antibody responses against Plasmodium falciparum merozoite surface antigens in areas of differing malaria endemicity in Indonesia. Malar J. 12, 444. doi: 10.1186/1475-2875-12-444
Surendra, H., Supargiyono, Ahmad, R. A., Kusumasari, R. A., Rahayujati, T. B., Damayanti, S. Y., et al. (2020). Using health facility-based serological surveillance to predict receptive areas at risk of malaria outbreaks in elimination areas. BMC Med. 18, 9. doi: 10.1186/s12916-019-1482-7
Surendra, H., Wijayanti, M. A., Murhandarwati, E. H., Irnawati, Yuniarti, T., Mardiati, et al. (2019). Analysis of serological data to investigate heterogeneity of malaria transmission: a community-based cross-sectional study in an area conducting elimination in Indonesia. Malar J. 18, 227. doi: 10.1186/s12936-019-2866-z
Tusting, L. S., Bousema, T., Smith, D. L., Drakeley, C. (2014). Measuring changes in Plasmodium falciparum transmission: precision, accuracy and costs of metrics. Adv. Parasitol. 84, 151–208. doi: 10.1016/B978-0-12-800099-1.00003-X
van den Hoogen, L., Drakeley, C. (2017). “Immunoepidemiology for the evaluation of malaria transmission patterns,” in Encyclopedia of Malaria. Eds. Kremsner, P., Krishna, S. (Springer, New York, NY).
van den Hoogen, L. L., Stresman, G., Présumé, J., Romilus, I., Mondélus, G., Elismé, T., et al. (2020). Selection of antibody responses associated with Plasmodium falciparum infections in the context of malaria elimination. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00928
WHO (2011). Hemoglobin Concentrations for The Diagnosis of Anemia and Assessment of Severity (Geneva: World Health Organization). Available at: https://apps.who.int/iris/handle/10665/85839 (Accessed May 14, 2024).
WHO (2015). Global technical strategy for malaria 2016–2030 (Geneva: World Health Organization). Available at: https://www.who.int/publications/i/item/9789240031357 (Accessed May 14, 2024).
WHO (2023). World Malaria Report (Geneva: World Health Organization). Available at: https://www.who.int/publications/i/item/9789240086173 (Accessed May 10, 2024).
Wong, J., Hamel, M. J., Drakeley, C. J., Kariuki, S., Shi, Y. P., Lal, A. A., et al. (2014). Serological markers for monitoring historical changes in malaria transmission intensity in a highly endemic region of Western Kenya, 1994-2009. Malar J. 13, 451. doi: 10.1186/1475-2875-13-451
Yman, V., White, M. T., Rono, J., Arcà, B., Osier, F. H., Troye-Blomberg, M., et al. (2016). Antibody acquisition models: A new tool for serological surveillance of malaria transmission intensity. Sci. Rep. 6, 19472. doi: 10.1038/srep19472
Keywords: malaria, P. falciparum, P. vivax, serology, transmission, Indonesia
Citation: Lubis IND, Nainggolan IRA, Meliani M, Hasibuan BS, Sangaran K, Samsudin L, Chuangchaiya S, Divis PCS, Permatasari R and Idris ZM (2025) Serology reveals comparable patterns in the transmission intensities of Plasmodium falciparum and Plasmodium vivax in Langkat district, North Sumatera Province, Indonesia. Front. Cell. Infect. Microbiol. 15:1504741. doi: 10.3389/fcimb.2025.1504741
Received: 01 October 2024; Accepted: 28 January 2025;
Published: 17 February 2025.
Edited by:
Louisa Alexandra Messenger, University of Nevada, Las Vegas, United StatesReviewed by:
Akira Kaneko, Karolinska Institutet (KI), SwedenCopyright © 2025 Lubis, Nainggolan, Meliani, Hasibuan, Sangaran, Samsudin, Chuangchaiya, Divis, Permatasari and Idris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Inke Nadia Diniyanti Lubis, aW5rZS5uYWRpYUB1c3UuYWMuaWQ=; Zulkarnain Md Idris, enVsa2FybmFpbi5tZGlkcmlzQHVrbS5lZHUubXk=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.