- 1Department of Biotechnology, Faculty of Science and Humanities, SRM Institute of Science and Technology, Tamil Nadu, India
- 2Department of Bioscience, Saveetha School of Engineering, Saveetha Institute of Medical and Technical Sciences (SIMATS), Saveetha University, Chennai, India
- 3Institute for Liver and Digestive Disease, Hallym University, Chuncheon, Republic of Korea
- 4Department of Microbiology, School of Basic Science, Central University of Punjab, Bathinda, Punjab, India
- 5Advanced Pharmacology and Neuroscience Laboratory, Department of Pharmacology, School of Health Sciences, Central University of Punjab, Bathinda, Punjab, India
- 6Neurochemistry and Neuroendocrinology Lab, Department of Zoology, Central University of Punjab, Bathinda, Punjab, India
- 7Department of Pediatrics, All India Institute of Medical Sciences (AIIMS), Bathinda, Punjab, India
- 8Human Cytogenetics and Stem Cell Laboratory, Department of Zoology, School of Basic Sciences, Central University of Punjab, Bathinda, Punjab, India
Significant changes in gut microbial composition are associated with chronic liver disease. Using preclinical models, it has been demonstrated that ethanol/alcohol-induced liver disease is transmissible through fecal microbiota transplantation (FMT). So, the survival rate of people with severe alcoholic hepatitis got better, which suggests that changes in the makeup and function of gut microbiota play a role in metabolic liver disease. The leaky intestinal barrier plays a major role in influencing metabolic-related liver disease development through the gut microbiota. As a result, viable bacteria and microbial products can be transported to the liver, causing inflammation, contributing to hepatocyte death, and causing the fibrotic response. As metabolic-related liver disease starts and gets worse, gut dysbiosis is linked to changes in the immune system, the bile acid composition, and the metabolic function of the microbiota in the gut. Metabolic-related liver disease, as well as its self-perpetuation, will be demonstrated using data from preclinical and human studies. Further, we summarize how untargeted treatment approaches affect the gut microbiota in metabolic-related liver disease, including dietary changes, probiotics, antibiotics, and FMT. It discusses how targeted therapies can improve liver disease in various areas. These approaches may improve metabolic-related liver disease treatment options.
Introduction
The gut microbiome influences liver function both directly and indirectly. Gut products reach the liver directly through the portal vein, which transports blood from the intestines. The composition and function of gut bacteria influence metabolites approaching the liver by affecting carbohydrate, protein, lipid, and bile acid metabolism (Caporaso et al., 2010; Oliphant and Allen-Vercoe, 2019; Visconti et al., 2019). Through the portal vein, bacteria, viruses, and fungi in the intestine affect immune cells and molecules that travel to the liver. Gut-liver connections are bidirectional (Madatali Abuwani et al., 2021) and the duodenum receives bile from the liver via bile ducts. Through detergent properties, antimicrobial peptide induction, and immune regulation, bile influences bacterial composition and function (Huttenhower et al., 2012; Wang et al., 2021).
Microbiome therapeutics in liver disease may also target metabolic and immune pathways shared between the gut microbiome and the liver (Gupta et al., 2022b). By entering the intestine through the biliary tree, the liver produces primary bile acids that are deconjugated by intestinal bacteria and further transformed (Qu et al., 2024; Wiefels et al., 2024). The composition of the intestinal bile acid pool, which is largely dictated by the microbiota, affects various aspects of intestinal barrier function, including the mucosal layer, immunological modulation, and tight junction protein integrity (Raimondi et al., 2008; Pavlidis et al., 2015). Short-chain fatty acids (SCFAs: acetic acid, propionic acid, and butyric acid) are products of carbohydrate fermentation and are an important energy source for colonic enterocytes (Wu et al., 2024). Both bile acids and SCFAs, which are products of bacterial metabolism, are important in the regulation of intestinal barrier function and therefore affect the substrates arriving in the liver via the portal circulation (Zeng et al., 2024).
Many molecules cross the intestinal barrier and enter the liver. This is one of many factors that allow pathogens to reach the liver, stimulate macrophages, and stimulate macrophages (Acuna and Olive, 2024). The Klebsiella pneumoniae and Lactobacilli play a significant role in liver metabolisms. Ammonia is a product of intestinal bacteria that reaches the liver, stimulates the pancreas, and can promote pancreatic growth. The bacterial microbiome of Klebsiella pneumoniae Lactobacilli plays a significant role in various liver diseases (Li et al., 2021; Meijnikman et al., 2022). Ammonia is a by-product of intestinal bacteria and may occur.
Several molecules have been implicated in liver disease once they have crossed the gut barrier. The lipopolysaccharide endotoxin is one of several pathogen-associated molecular patterns that can reach the liver, activate macrophages, and promote hepatic fibrosis. Human and animal studies have increasingly demonstrated endogenous alcohol production by microbiota (Cope et al., 2000; Zhu et al., 2013; Yuan et al., 2019).
Metabolomics platforms in various liver diseases
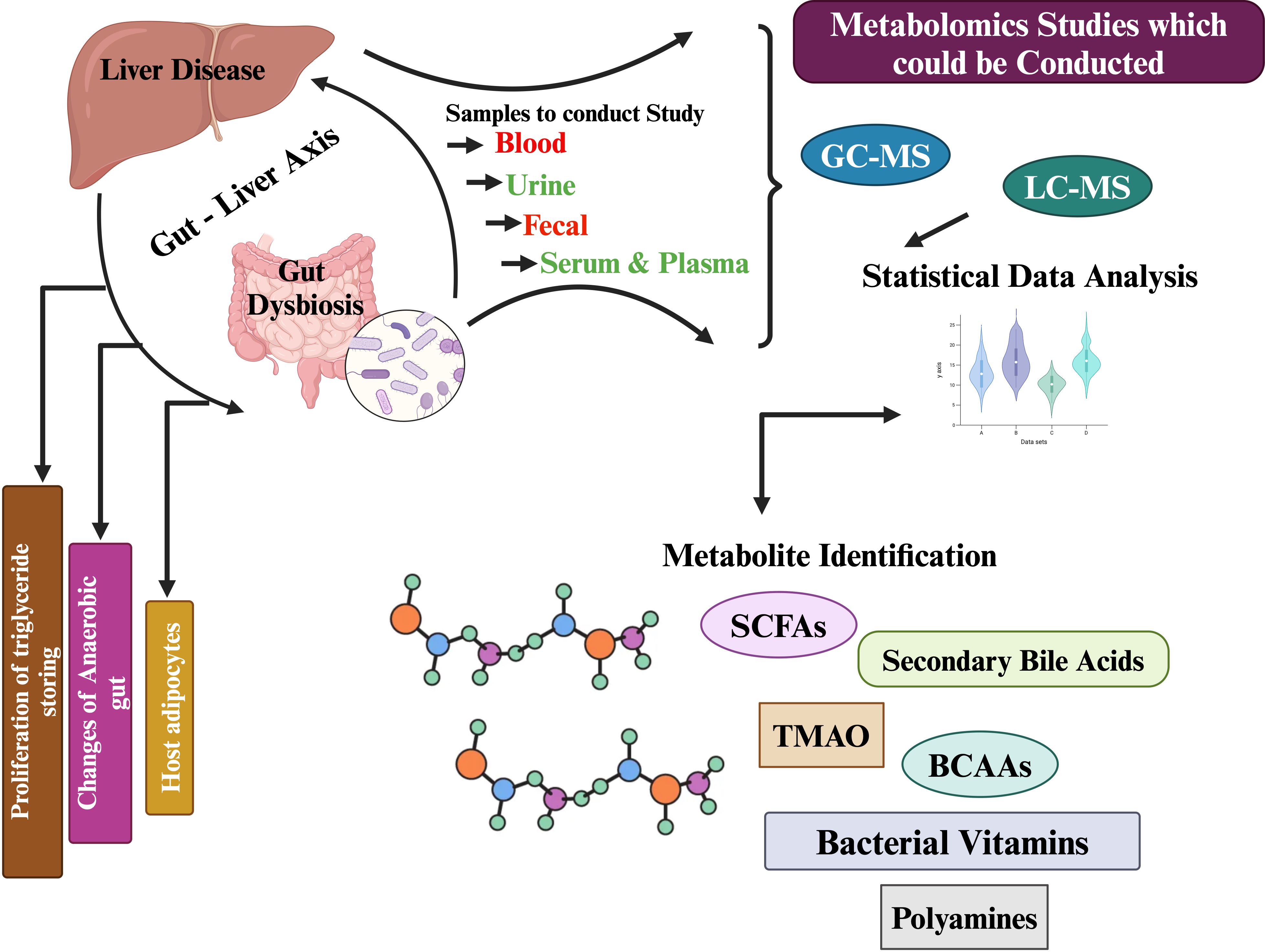
Biological samples can be analyzed using a variety of analytical platforms, including nuclear magnetic resonance (NMR) and mass spectrometry (MS), the latter coupled to either liquid or gas chromatography (GC-MS). NMR offers high reproducibility and requires less sample preparation than MS techniques, as it is a non-destructive analytical platform (Jaber et al., 2023; Lacalle-Bergeron et al., 2023; Luo et al., 2023; Pekkala, 2023). Using MS, metabolite discrimination and coverage are improved because of its selectivity and sensitivity. It is possible to separate metabolites in a complex matrix before detection, increasing the sensitivity and the sensitivity of detection when combined with a separation technique (Raja et al., 2021). Clinical studies use LC-MS more often than GC-MS because the sample is non-volatile. An individual platform cannot detect, identify, and quantify metabolites completely. Each analytical platform has its own advantages, as well as sensitivity, selectivity, and reliability (Emwas, 2015; Ganesan et al., 2023). A comprehensive metabolite analysis can be achieved by using a combination of NMR and MS, coupled to both liquid and gas chromatography (LC-MS/GC-MS) (Wang et al., 2023; Wang et al., 2023). Combining NMR and MS allows non-destructive analysis of metabolites while also improving the selectivity and sensitivity of analyses of complex matrix metabolites (Zeki Ö et al., 2020; Ganesan et al., 2022). By utilizing both analytical platforms, a more comprehensive understanding of the metabolite profile can be achieved, ensuring more accurate identification, detection, and quantification. Using NMR in metabolite analysis offers high reproducibility and requires less sample preparation, making it a non-destructive analytical platform. On the other hand, MS provides improved metabolite discrimination and coverage due to its selectivity and sensitivity. By combining both NMR and MS, a comprehensive metabolite analysis can be achieved, ensuring more accurate identification, detection, and quantification metabolites in (Figure 1) (Madatali Abuwani et al., 2021; Ye et al., 2023; Yu et al., 2023; Yu et al., 2024).
Gut microbiome and liver diseases
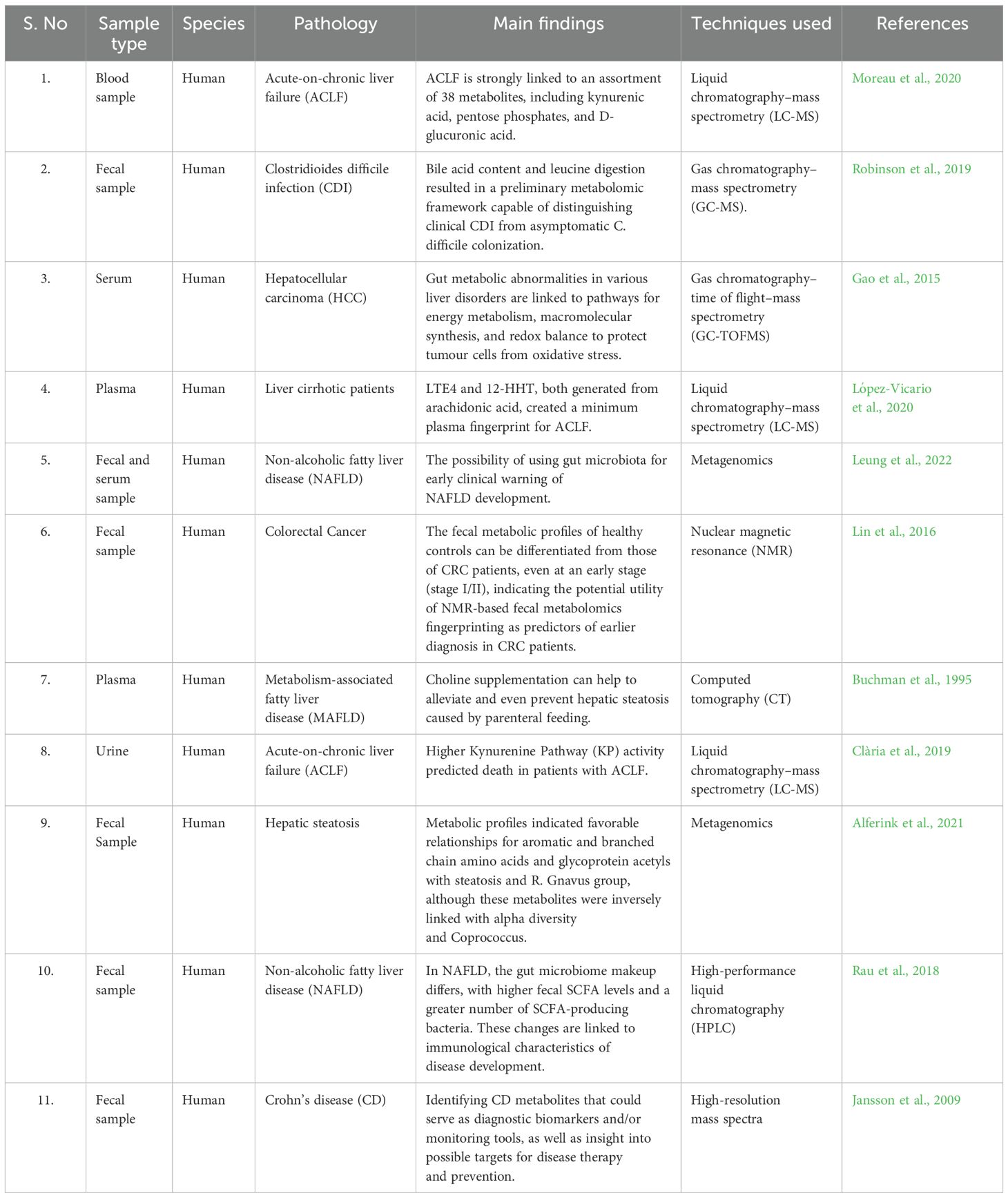
There has been a well-characterized gut microbiome associated with alcohol-related liver disease as well as other chronic liver diseases, showing a reduction in Bacteroides and Lactobacillus species and an increase in Proteobacteria and Fusobacteria (Zhu et al., 2023). A decrease in the Lachnospiraceae and Ruminococcaceae families was observed in cirrhosis, while significant increases in the Enterobacteriaceae, Alcaligenaceae, and Fusobacteriaceae families are noted. The increase in Enterobacteriaceae, Alcaligenaceae, and Fusobacteriaceae families in cirrhosis could be due to the disruption of gut microbiota caused by liver damage (Zheng et al., 2023). This disruption can lead to an overgrowth of harmful bacteria from these families, which can contribute to further inflammation and disease progression in cirrhosis. Bacterial microbiota can change with the progression and stage of liver disease, which can lead to further inflammation and disease progression in cirrhosis (Ribeiro et al., 2024). As cirrhosis progresses, harmful bacteria from these families can overgrow. Recent studies have also described fungal dysbiosis. Independent of the stage of liver disease, patients with alcohol use disorder have a decrease in fungal diversity and an increase in Candida species. Identifying the influence of gut microbiota on liver disease progression as well as the influence of progressive liver disease on gut microbiota would be worthwhile (Table 1) (Tu et al., 2023; Wang et al., 2024).
Prebiotics-based microbial diversity and human health
Health benefits are conferred by prebiotics, which are substrates exclusively used by host microorganisms (Gibson et al., 2017). Hematological stains are a common complication of decompensated cirrhosis, and lactulose is the primary prebiotic for treating it (Bloom and Tapper, 2023). A prebiotic is a substrate used exclusively by host microorganisms that confers health benefits (Gibson et al., 2017). Lactulose is the primary prebiotic for treating HE, a common complication of decompensated cirrhosis (Bloom and Tapper, 2023). In most cases, colonic bacteria ferment lactulose into SCFAs. There are multiple benefits to fermenting lactulose. SCFAs are produced by fermentation of lactulose, which provides nutrients to the intestinal epithelium and reduces gut translocation (Moratalla et al., 2017). A decrease in ammonia production from certain bacteria is attributed to SCFAs, which cause acidification of colonic contents (Sanders et al., 2019). By fermenting lactulose, bacteria can grow more rapidly, pushing other bacteria out of the ecological niche, such as bacteria-producing lipopolysaccharides (Vince and Burridge, 1980; Wang et al., 2019). Ammonia is a substrate used by probiotic taxa as a result of lactulose fermentation (Agostini et al., 1972; Weber, 1979; Vince et al., 1990). Ammonia may translocate across the intestinal epithelium into the colon lumen, be trapped as an ammonium ion, and be expelled in stool due to acidification of colonic contents.
Prebiotics impact gut-derived liver diseases by promoting the growth of beneficial bacteria in the gut. These beneficial bacteria produce metabolites that maintain a healthy gut lining and reduce inflammation, reducing the risk of liver diseases. Additionally, prebiotics stimulate the release of anti-inflammatory molecules and digestive enzymes, which further contribute to preventing and managing liver diseases. Prebiotics stimulate the release of anti-inflammatory molecules such as SCFAs, such as butyrate and acetate. Butyrate in particular has been shown to have potent anti-inflammatory effects, inhibiting the production of pro-inflammatory cytokines and promoting the growth of anti-inflammatory immune cells. These anti-inflammatory molecules help to calm and reduce chronic inflammation, which can be a contributing factor to liver diseases. However, it is important to note that while prebiotics can be beneficial in managing and preventing liver diseases, they are not a magical cure. In some cases, prebiotics may not significantly alter the course of the disease, and individuals may require other interventions or treatments in conjunction with prebiotics. Additionally, prebiotics can interact with other medications or medical conditions, so it is important to consult with a healthcare professional before starting a prebiotic regimen. Prebiotics stimulate the release of anti-inflammatory molecules and digestive enzymes, which further contribute to preventing and managing liver diseases. Specifically, prebiotics promote the growth of beneficial bacteria in the gut, which produce metabolites that maintain a healthy gut lining and reduce inflammation.
Prebiotics are a type of dietary fiber that feeds the beneficial bacteria in the gut. These bacteria play a crucial role in maintaining a balanced gut microbiome and overall health. By promoting a healthy gut microbiome, prebiotics can potentially reduce the risk of gut-derived metabolic liver diseases, such as non-alcoholic fatty liver disease (NAFLD) and cirrhosis (Ince Palamutoglu et al., 2024). Probiotics are defined as preparations or products containing viable, defined microorganisms in sufficient numbers, which alter the microflora of a host compartment by implantation or colonization, and by doing so exert positive health effects on the host (Summer et al., 2024; Teker et al., 2024). Emerging mechanisms probiotics have shown the potential to improve liver function through various mechanisms. Firstly, they can enhance bile acid metabolism, reducing its toxic metabolite levels and protecting the hepatocytes from damage. Secondly, probiotics can stimulate the production of short-chain fatty acids (SCFAs) through gut bacterial fermentation, which has shown hepatoprotective properties (Yoon et al., 2023; Zhang et al., 2023). Lastly, probiotics can modulate the gut microbiota, reducing the growth of harmful bacteria and improving the balance between beneficial and harmful bacteria, ultimately promoting liver health. Probiotics can enhance bile acid metabolism by promoting the growth of specific gut bacteria that can convert bile acids into less toxic forms (Cao et al., 2023). This conversion helps to reduce the risk of liver damage caused by toxic bile acid metabolites. Additionally, probiotics can lower cholesterol levels, which further contributes to improved bile acid metabolism and liver health. SCFAs such as butyric acid, are produced through the fermentation of dietary fiber by gut bacteria. These fatty acids have hepatoprotective properties and can reduce inflammation, oxidative stress, and insulin resistance in the liver. They can also enhance the regeneration of hepatocytes and improve the overall functioning and health of the liver (Zhang et al., 2023; Zhang et al., 2023).
Gut bacteria play a crucial role in the production of SCFAs. When dietary fiber is fermented by gut bacteria, it produces short-chain fatty acids, such as butyric acid. These fatty acids then have a range of beneficial health effects, including protecting the liver from damage and improving its functioning. SCFAs particularly butyric acid, protect the liver through multiple mechanisms. They reduce inflammation by suppressing the cytokines that cause liver inflammation (Ansari et al., 2023; Gao et al., 2023). Additionally, they reduce oxidative stress by neutralizing free radicals and inhibiting lipid peroxidation. They also improve insulin sensitivity, reducing the risk of insulin resistance-related liver diseases. The hepatoprotective properties of SCFAs make them potential candidates for the treatment of liver diseases (Giridhar et al., 2024). They have shown potential in reducing inflammation, oxidative stress, and insulin resistance in the liver, leading to improved hepatocyte regeneration and overall liver health. By targeting these mechanisms, SCFAs may hold promise in the development of novel treatments for liver diseases (Guo and Lv, 2023; He et al., 2023; Hojsak and Kolacek, 2024). Many inflammatory and metabolic disorders have been associated with probiotics.
Some potential applications of probiotics in treating specific medical conditions include:
1. Gastroesophageal Reflux Disease (GERD): Probiotics have been shown to reduce the frequency of GERD symptoms and improve quality of life.
2. Inflammatory Bowel Disease (IBD): Probiotics have been demonstrated to have some beneficial effects on symptoms of Crohn’s disease and ulcerative colitis, the two main forms of IBD.
3. Diarrhea: Probiotics are commonly used to treat and prevent diarrhea caused by a variety of factors, including antibiotics and viral infections.
4. An irritable bowel syndrome (IBS): Probiotics have shown promise in reducing symptoms and improving the quality of life for individuals suffering from IBS.
5. Skin Conditions: Certain probiotics have been used to promote skin health and reduce symptoms of conditions such as eczema and acne.
It is important to note that while there is evidence to suggest the potential benefits of these applications of probiotics, more research is needed to understand their efficacy and long-term outcomes fully.
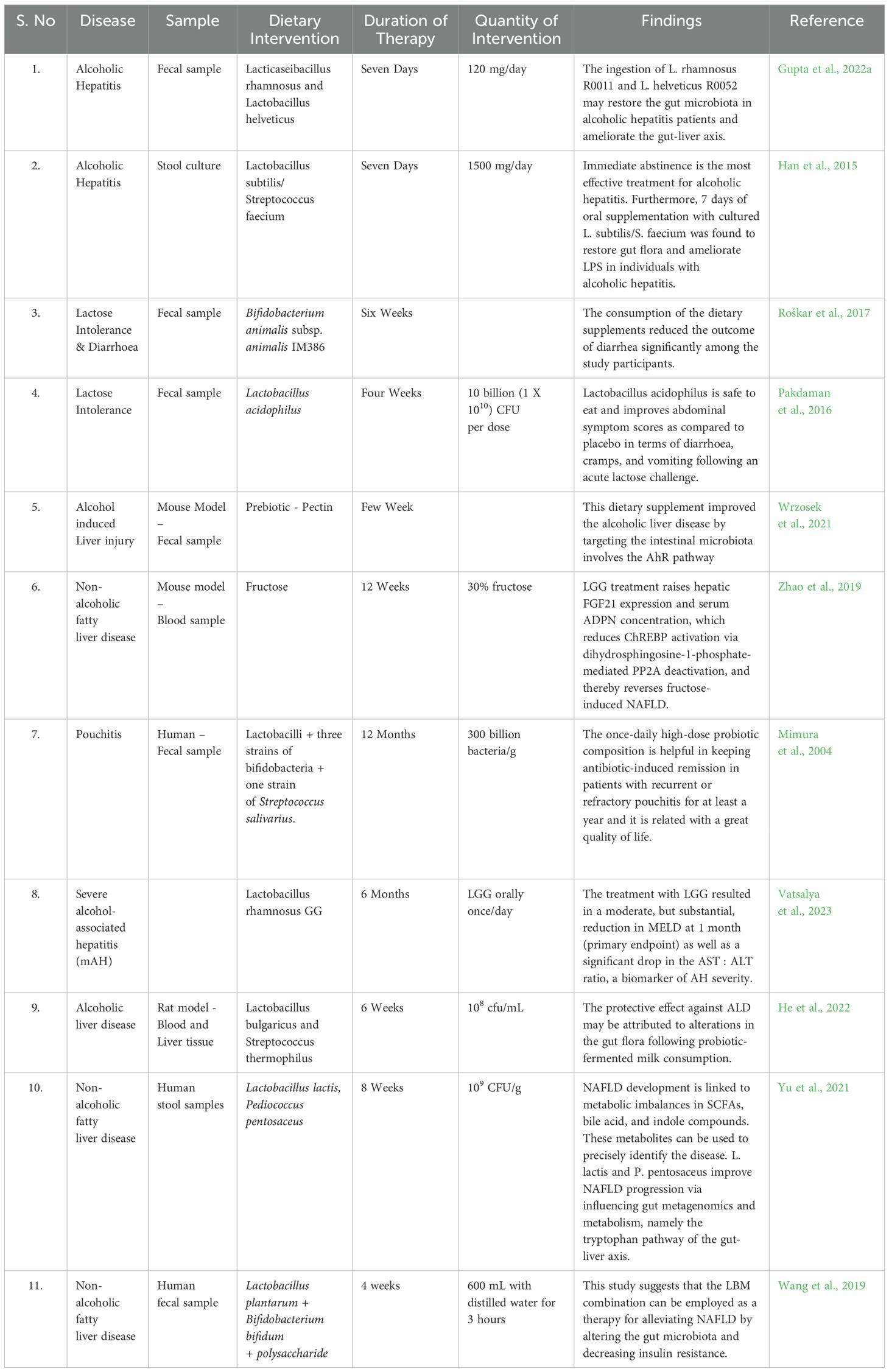
SCFAs and secondary bile acids are postbiotic products, which are bioactive products of bacteria. In other gastrointestinal conditions, postbiotics have been studied (Golob et al., 2019), but they don’t always produce consistent or uniform positive results (Mortensen and Clausen, 1996; Roda et al., 2007; Abbasi et al., 2022). As of now, there are no clinical trials on SCFAs supplementation in humans. SCFAs supplementation in humans could potentially offer several benefits. It can help improve gut health by promoting a healthy balance of gut bacteria, reducing inflammation, and enhancing intestinal barrier function. Additionally, SCFAs have metabolic benefits, such as reducing insulin resistance, improving weight management, and potentially reducing the risk of chronic diseases like obesity and diabetes (Hsu and Schnabl, 2023). In Table 2, we have depicted the therapeutic interventions due to the ingestion of prebiotics in various liver diseases.
Antibiotics-based microbial diversity and human health
An infant’s gut microbial colonization and resistive profile are influenced by perinatal and peripartum antibiotic use (Zou et al., 2018; Wong et al., 2020). To understand the potential impact of antibiotic administration on offspring during pregnancy, scientists examined the temporal effects of cefoperazone when administered during the peripartum period on the microbiota of both maternal and offspring in an interleukin-10 (IL‐10) ‐ deficient murine model of colitis (Miyoshi et al., 2017).
Cefoperazone-exposed dams had offspring with altered gut microbe communities who were more susceptible to spontaneous and chemically induced colitis (Miyoshi et al., 2017). Similar results were demonstrated by Schulfer et al., who inoculated germ‐free pregnant mice with an antibiotic‐altered microbial community. According to Schulfer et al., 2018 decreased IL-10 proliferates in the offspring after the altered microbial community is transmitted (Schulfer et al., 2018).
There has been some evidence that maternal antibiotic intake during pregnancy alters the composition of the microbial community (Azad et al., 2013; Coker et al., 2020). Fluoroquinolones (norfloxacin and ciprofloxacin), third-generation cephalosporins (G3) (ceftriaxone and cefotaxime), and trimethoprim-sulfamethoxazole (SXT) are recommended for preventing infections in patients with cirrhosis or liver failure. Spontaneous Bacterial Peritonitis (SBP) is a common bacterial infection in patients with cirrhosis. SBP in cirrhosis patients can be caused by the rupture of bacteria-containing ascite pockets or the spread of bacteria from the digestive tract (Puri, 2023; Petruzziello et al., 2024). Risk factors for SBP in cirrhosis patients include advanced cirrhosis, a history of gastrointestinal bleeding, portal hypertension, and the use of gastrointestinal prophylaxis. As well as bacterial overgrowth in small intestines, intestinal permeability increases, and intestinal motility decreases in patients with liver disease. Examples of preventive measures for SBP in patients with cirrhosis include regular monitoring of liver function, proper hygiene and sanitation practices, vaccination against bacterial infections, and antibiotic prophylaxis when necessary (Giridhar et al., 2024). Additionally, maintaining a healthy diet, avoiding alcohol, and managing underlying liver disease can help reduce the risk of SBP. The study conducted by Prado et al. (Prado et al., 2022) also examined whether patients with cirrhosis, who resistant bacteria had earlier colonized, were at greater risk of re-infection by the same strain in the future (Aziz et al., 2022). Increased intestinal permeability, commonly observed in liver disease, allows bacteria to translocate from the intestines to the peritoneal cavity, leading to SBP. The impaired intestinal motility further facilitates the spread of bacteria, making patients more susceptible to infections. Therefore, addressing intestinal permeability and motility is crucial in preventing and managing SBP in patients with cirrhosis. Increased intestinal permeability in cirrhosis patients can lead to bacterial translocation, where the harmful bacteria from the digestive tract enter the peritoneal cavity and cause spontaneous bacterial peritonitis (SBP). This increases the risk of bacterial infections and can lead to serious complications and even death in cirrhosis patients. Additionally, increased intestinal permeability can contribute to developing systemic infections, as harmful bacteria can spread throughout the bloodstream.
Infections caused by spontaneous processes, such as SBP, occur in about 36% of patients with liver cirrhosis (Tay et al., 2021). Third-generation cephalosporins are often used empirically to treat SBP, except in cases where multidrug-resistant organism risk factors apply, where piperacillin/tazobactam is prescribed.
Translational research in various liver diseases
Specific areas of focus for future translational research in hepatic diseases could include: 1) developing novel therapeutic strategies to target liver diseases, such as gene therapy or targeted drug delivery systems (Rungratanawanich et al., 2023). 2) Understanding the underlying molecular mechanisms of liver disease progression and the development of new diagnostic tools to predict and monitor disease activity. 3) Investigating the role of gut microbiome in liver diseases and its potential impact on the development and severity of liver diseases. 4) Exploring the potential of regenerative medicine approaches, such as stem cell therapy, for the treatment of liver diseases. Examples of liver diseases influenced by the gut microbiome include NAFLD and cirrhosis. Studies have shown that the gut microbiome can contribute to the development of NAFLD, as it can promote the accumulation of fat in the liver (Tanaka and Ui, 2010; Taner et al., 2020). Additionally, the gut microbiome has been implicated in the pathogenesis of cirrhosis, as it can contribute to inflammation and impaired liver function. Examples of gene therapy for liver diseases include the use of viral or non-viral vectors to deliver normal copies of genes that are mutated in liver diseases, or the use of gene therapy to silence the expression of genes involved in liver disease development (Koziel, 2008; Gottlieb et al., 2019). Targeted drug delivery systems, on the other hand, can involve the development of nanoparticles or bioconjugates that specifically target the liver and deliver therapeutic agents directly to the affected cells. Recent advancements in stem cell therapy for liver diseases have shown promising results (Nacif et al., 2018). Researchers have developed techniques to differentiate stem cells into liver cells, which are then transplanted into the liver to replace damaged cells (El-Serag, 2007; Gijbels et al., 2021). This approach has shown potential in the treatment of various liver diseases, including cirrhosis and hepatocellular carcinoma. Additionally, advancements in regenerative medicine have led to the development of bioengineered livers, using a combination of stem cells and biomaterials to create functional liver structures (Ma et al., 2023; Liu et al., 2024; Martini et al., 2024). While further research is needed to fully understand the therapeutic potential of stem cell therapy in liver diseases, these advancements offer hope for potential new treatments. One challenge of targeted drug delivery systems is ensuring the specificity and accuracy of the delivery system. The system must be able to target the right location in the body and avoid delivery to unintended areas, which can be challenging to achieve (El-Serag, 2007; Gijbels et al., 2021). Additionally, the stability and release of the therapeutic agent within the targeted cells can be problematic, as the desired therapeutic effect may be compromised if the drug is released too quickly or too slowly. Furthermore, the systemic delivery of targeted drug delivery systems may be limited by biodistribution and clearance, as the drug may be eliminated or distributed throughout the body before reaching its intended target (Neuman et al., 2015; Huang et al., 2024; Jamshidi et al., 2023).
Alcohol use disorder and treatment potential of liver diseases
FMT has shown potential in the treatment of alcohol use disorder (AUD). FMT involves the transplantation of a healthy donor’s stool into the recipient’s colon, which can alter the recipient’s gut microbiota and improve overall gut health. This, in turn, could potentially regulate alcohol consumption and withdrawal symptoms, making FMT a valuable tool in the treatment and management of AUD (Neuman et al., 2015). FMT alters gut microbiota by introducing a healthy donor’s stool, which contains a diverse population of microorganisms. These microorganisms establish a new balance in the recipient’s gut, impacting digestion, metabolism, and even brain function (Hwang et al., 2024; Kim et al., 2024). By modifying the gut microbiota, FMT can regulate alcohol consumption and withdrawal symptoms, offering a potential treatment option for AUD (Zafar et al., 2024). Further research should be conducted to examine the effectiveness of FMT at different stages of AUD, such as in the acute withdrawal phase, during ongoing recovery, and in long-term maintenance (McMillan et al., 2024). This would help to determine the optimal timing and frequency of FMT treatments and establish its role in the overall treatment plan for individuals with AUD. Additionally, exploring different delivery methods of FMT, such as capsules or nasal sprays, could further enhance its accessibility and effectiveness in addressing AUD. Further research should be conducted to explore the long-term effects of FMT treatment for AUD (Kim et al., 2024). This would involve tracking the sobriety and overall well-being of individuals who have received FMT treatment for an extended duration, to understand the sustainability of the treatment’s effects and its potential for relapse prevention. Additionally, investigating the effect of FMT on different subgroups within the AUD population, such as individuals with specific genetic traits or co-occurring mental health disorders, would provide more insights into the individualized benefits of FMT (Hu et al., 2023; Hediyal et al., 2024).
Immunity-based microbiome and metabolome alteration in liver diseases
Liver diseases can disrupt the immune system, leading to a weakened response to infections and an increased risk of infection. Additionally, the immune system can also play a role in the development and progression of liver diseases, as chronic inflammation and immune cells can attack the liver and contribute to liver damage (Won et al., 2021). Specific mechanisms by which liver diseases disrupt the immune system include: 1) Immunodeficiency: Liver diseases can lead to impaired production of immune cells, such as T cells and B cells, leading to a weakened response to infections. 2) Inflammation: Chronic inflammation in liver diseases can recruit immune cells to the liver, which can result in liver damage and impaired immune function. 3) Altered cytokine profile: Liver diseases can cause an imbalance in the production of cytokines, which are signaling molecules that regulate immune responses, leading to dysregulation of the immune system. In addition to the role of chronic inflammation and immune cells in attacking the liver, specific types of immune cells can also directly contribute to liver damage (Schneider et al., 9900). For example, natural killer cells (NKT) and Kupffer, which are both components of the liver’s innate immune system, can release inflammatory mediators and cytokines that can lead to liver damage. Furthermore, activation of T cells and B cells in response to liver antigens can result in autoimmune liver damage and the progression of liver diseases. NKT cells and Kupffer cells play crucial roles in the liver’s innate immune system. While Kupffer cells are responsible for engulfing and removing harmful substances from the liver, NKT cells can produce cytokines that can trigger immune responses (Behary et al., 2021). However, in liver diseases, both cell types can release excessive inflammatory mediators and cytokines, leading to liver damage and promoting the progression of liver diseases. The dysregulation of immune responses in liver diseases can have a profound impact on liver function. The inflammation caused by immune cells and cytokines can lead to liver damage and impaired immune function, making the liver more susceptible to infections. Additionally, the impaired production of immune cells can result in a weakened response to infections, further exacerbating the liver disease. Overall, the dysregulated immune responses contribute to the progression and severity of liver diseases, highlighting the importance of maintaining a balanced immune response for optimal liver health (Ganesan et al., 2022).
The potential consequences of impaired immune cell production include an increased risk of infections, as the body has a weakened ability to fight off pathogens. This can result in more frequent and severe infections, which can further worsen the liver disease (He et al., 2021). Furthermore, the impaired production of immune cells can lead to a compromised immune system, making the individual more susceptible to other types of infections and cancer. Overall, impaired immune cell production can have severe implications for both acute and chronic liver diseases (Wang et al., 2020).
FMT in liver transplantation recipients: clinical significance
Liver transplantation (LT) is universally acknowledged as the sole therapeutic choice for patients suffering from end-stage liver disease, acute liver failure, and HCC (Ancona et al., 2021). In recent decades, LT has become an established and standard surgical technique for treating liver disorders (Hübscher, 2011). Nevertheless, patients undergoing LT are particularly susceptible to many infections, including Clostridium difficile infection (CDI) (Becattini et al., 2016), cytomegalovirus (CMV) infection (Engelmann et al., 2020), fungal infections, and recurrent hepatitis B virus (HBV) infection. A prior cohort analysis indicated that around 19% of deaths that occurred five years after LT were attributed to diverse sources of infection (Annavajhala et al., 2019; Ancona et al., 2021). The primary reason for this is the administration of immunosuppressive medications following liver transplantation, which weakens the immune system’s ability to detect and fight off pathogens. This allows the pathogens to avoid natural immunity and increases the likelihood of infection. In addition, post-LT infection is also linked to pre-transplant infection and other risk factors (Abad et al., 2017). Furthermore, multiple investigations have shown that gut microbiota composition might undergo considerable alterations following LT (Bajaj et al., 2017). Therefore, liver transplant recipients must restore the balance of their gut microbiota by FMT. For example, Schneider et al. documented a case where FMT was performed on a liver transplant patient who had severe Clostridioides difficile infection that was further worsened by acute renal injury (Bajaj et al., 2017). Moreover, a meta-analysis of 44 trials (Shogbesan et al., 2018) has provided evidence of the safety of FMT in patients with weakened immune systems. Thus, FMT could serve as a promising treatment approach for treating Clostridioides difficile infection following liver transplantation. As far as we know, there has not been a clinical trial that has evaluated the suitability of FMT for treating infectious disorders.
The future of microbiome and healthy humans
Microbiomes play an important role in the nutrient metabolism and immune regulation in the human body and can directly affect the liver. Lactulose, rifampin, and certain antibiotics are currently being used in liver disease as microbiome-targeted therapeutics. Translational research in many areas is needed before microbiome-targeted therapeutics can be used to treat or prevent liver disease. We should move forward with rigorous randomized clinical trials for microbiome therapeutics such as FMT, consortium products, bacteriophages, and genetically engineered probiotics. These microbiome-targeted therapeutics need further research to better understand their efficacy, mechanism of action, and optimal delivery method.
Patients with HE have completed enrolment in a clinical trial (ClinicalTrials.gov NCT03796598), but results have not yet been released. A patient’s microbiome composition varies even within one liver disease. A prespecified microbiome analysis and stratification, or a post hoc analysis of the interaction between therapy and baseline microbiome, will be required for the design of trials and therapy selection to take account of this heterogeneity. One strategy could be to include microbiome analysis as a prespecified endpoint in future trials, allowing for a more comprehensive understanding of the role the microbiome plays in liver disease and response to therapy. Another strategy could be to conduct post hoc analyses of the interaction between therapy and baseline microbiome, allowing for a more targeted approach to therapy selection and customization based on individual patient characteristics. Additionally, utilizing standardized protocols for microbiome analysis and ensuring robust sample collection and storage can help minimize heterogeneity and improve the reliability of findings.
Conclusion
The importance of gut microbiota in host metabolism and immune functions has been summarized in this review, which includes immune development, colonization resistance, and cell signaling. With the help of advanced omics technologies, we are now beginning to understand how the host and microbiota interact complexly. As a result of antibiotics, the bacterial community and the host are disrupted, thereby disrupting the microbial balance. Overall, these approaches offer a promising new set of biomarkers for liver disease diagnosis and therapy. Fecal microbiota transplantation may also have potential as a treatment option for liver diseases. Further research is needed better to understand the safety and efficacy of this approach. Ultimately, these approaches have the potential to revolutionize the way we diagnose and treat liver diseases. As such, they hold great potential for improving the lives of patients with liver diseases.
Author contributions
RG: Funding acquisition, Writing – original draft, Writing – review & editing. DT: Resources, Writing – review & editing. SV: Data curation, Writing – review & editing. DK: Supervision, Writing – review & editing. KS: Supervision, Visualization, Writing – review & editing. MI: Data curation, Methodology, Writing – review & editing. MY: Data curation, Methodology, Writing – review & editing. DH: Validation, Writing – review & editing. JP: Validation, Writing – review & editing. AW: Data curation, Writing – review & editing. BV: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The SRM Institute of Science & Technology (SRMIST) has supported this work through Selective Excellence Research Initiative (No. SRMIST/R/AR(A)/SERI2024/174/53) grants to RG.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abad, C. L., Lahr, B. D., Razonable, R. R. (2017). Epidemiology and risk factors for infection after living donor liver transplantation. Liver transplantation: Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 23, 465–477. doi: 10.1002/lt.24739
Abbasi, A., Hajipour, N., Hasannezhad, P., Baghbanzadeh, A., Aghebati-Maleki, L. (2022). Potential in vivo delivery routes of postbiotics. Crit. Rev. Food Sci. Nutr. 62, 3345–3369. doi: 10.1080/10408398.2020.1865260
Acuna, A. M., Olive, M. F. (2024). Influence of gut microbiome metabolites on cocaine demand and cocaine-seeking behavior. Neuropsychopharmacology 49, 357–358. doi: 10.1038/s41386-023-01743-9
Agostini, L., Down, P. F., Murison, J., Wrong, O. M. (1972). Faecal ammonia and pH during lactulose administration in man: comparison with other cathartics. Gut 13, 859–866. doi: 10.1136/gut.13.11.859
Alferink, L. J. M., Radjabzadeh, D., Erler, N. S., Vojinovic, D., Medina-Gomez, C., Uitterlinden, A. G., et al. (2021). Microbiomics, metabolomics, predicted metagenomics, and hepatic steatosis in a population-based study of 1,355 adults. Hepatology. 73, 968–982. doi: 10.1002/hep.31417
Ancona, G., Alagna, L., Lombardi, A., Palomba, E., Castelli, V., Renisi, G., et al. (2021). The interplay between gut microbiota and the immune system in liver transplant recipients and its role in infections. Infection Immun. 89, e0037621. doi: 10.1128/IAI.00376-21
Annavajhala, M. K., Gomez-Simmonds, A., Macesic, N., Sullivan, S. B., Kress, A., Khan, S. D., et al. (2019). Colonizing multidrug-resistant bacteria and the longitudinal evolution of the intestinal microbiome after liver transplantation. Nat. Commun. 10, 4715. doi: 10.1038/s41467-019-12633-4
Ansari, F., Neshat, M., Pourjafar, H., Jafari, S. M., Samakkhah, S. A., Mirzakhani, E. (2023). The role of probiotics and prebiotics in modulating of the gut-brain axis. Front. Nutr. 10, 1173660. doi: 10.3389/fnut.2023.1173660
Azad, M. B., Konya, T., Maughan, H., Guttman, D. S., Field, C. J., Chari, R. S., et al. (2013). Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ: Can. Med. Assoc. J. = J. l'Association medicale Can. 185, 385–394. doi: 10.1503/cmaj.121189
Aziz, D. A., Abbas, A., Alam, A., Aziz, N. (2022). Role of inhaled antibiotics in children and adolescents with cystic fibrosis: Experience from the tertiary care center. Lung India 39, 274–278. doi: 10.4103/lungindia.lungindia_370_21
Bajaj, J. S., Fagan, A., Sikaroodi, M., White, M. B., Sterling, R. K., Gilles, H., et al. (2017). Liver transplant modulates gut microbial dysbiosis and cognitive function in cirrhosis. Liver transplantation: Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 23, 907–914. doi: 10.1002/lt.24754
Becattini, S., Taur, Y., Pamer, E. G. (2016). Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol. Med. 22, 458–478. doi: 10.1016/j.molmed.2016.04.003
Behary, J., Amorim, N., Jiang, X.-T., Raposo, A., Gong, L., McGovern, E., et al. (2021). Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 12, 187. doi: 10.1038/s41467-020-20422-7
Bloom, P. P., Tapper, E. B. (2023). Lactulose in cirrhosis: Current understanding of efficacy, mechanism, and practical considerations. Hepatol. Commun. 7 (11), e0295. doi: 10.1097/HC9.0000000000000295
Buchman, A. L., Dubin, M. D., Moukarzel, A. A., Jenden, D. J., Roch, M., Rice, K. M., et al. (1995). Choline deficiency: a cause of hepatic steatosis during parenteral nutrition that can be reversed with intravenous choline supplementation. Hepatology. 22, 1399–1403. doi: 10.1016/0270-9139(95)90143-4
Cao, X., Zolnikova, O., Maslennikov, R., Reshetova, M., Poluektova, E., Bogacheva, A., et al. (2023). Differences in fecal short-chain fatty acids between alcoholic fatty liver-induced cirrhosis and non-alcoholic (Metabolic-associated) fatty liver-induced cirrhosis. Metabolites 13 (7), 859. doi: 10.3390/metabo13070859
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Clària, J., Moreau, R., Fenaille, F., Amorós, A., Junot, C., Gronbaek, H., et al. (2019). CANONIC study investigators of the EASL clif consortium, grifols chair and the European foundation for the study of chronic liver failure (EF clif). Orchestration of tryptophan-kynurenine pathway, acute decompensation, and acute-on-chronic liver failure in cirrhosis. Hepatology. 69, 1686–1701. doi: 10.1002/hep.30363
Coker, M. O., Hoen, A. G., Dade, E., Lundgren, S., Li, Z., Wong, A. D., et al. (2020). Specific class of intrapartum antibiotics relates to maturation of the infant gut microbiota: a prospective cohort study. BJOG: an Int. J. obstetrics gynaecology 127, 217–227. doi: 10.1111/1471-0528.15799
Cope, K., Risby, T., Diehl, A. M. (2000). Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology 119, 1340–1347. doi: 10.1053/gast.2000.19267
El-Serag, H. B. (2007). Translational research: the study of community effectiveness in digestive and liver disorders. Gastroenterology 132, 8–10. doi: 10.1053/j.gastro.2006.11.038
Emwas, A. H. (2015). The strengths and weaknesses of NMR spectroscopy and mass spectrometry with particular focus on metabolomics research. Methods Mol. Biol. (Clifton N.J.) 1277, 161–193. doi: 10.1007/978-1-4939-2377-9_13
Engelmann, C., Sterneck, M., Weiss, K. H., Templin, S., Zopf, S., Denk, G., et al. (2020). Prevention and management of CMV infections after liver transplantation: current practice in german transplant centers. J. Clin. Med. 9 (8), 2352. doi: 10.3390/jcm9082352
Ganesan, R., Gupta, H., Jeong, J.-J., Sharma, S. P., Won, S.-M., Oh, K.-K., et al. (2023). A metabolomics approach to the validation of predictive metabolites and phenotypic expression in non-alcoholic fatty liver disease. Life Sci. 322, 121626. doi: 10.1016/j.lfs.2023.121626
Ganesan, R., Jeong, J.-J., Kim, D. J., Suk, K. T. (2022). Recent trends of microbiota-based microbial metabolites metabolism in liver disease. Front. Med. 9. doi: 10.3389/fmed.2022.841281
Gao, R., Cheng, J., Fan, C., Shi, X., Cao, Y., Sun, B., et al. (2015). Serum metabolomics to identify the liver disease-specific biomarkers for the progression of hepatitis to hepatocellular carcinoma. Sci. Rep. 5, 18175. doi: 10.1038/srep18175
Gao, T., Wang, X., Li, Y., Ren, F. (2023). The role of probiotics in skin health and related gut-skin axis: A review. Nutrients 15. doi: 10.3390/nu15143123
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature reviews. Gastroenterol. Hepatol. 14, 491–502. doi: 10.1038/nrgastro.2017.75
Gijbels, E., Pieters, A., De Muynck, K., Vinken, M., Devisscher, L. (2021). Rodent models of cholestatic liver disease: A practical guide for translational research. Liver international: Off. J. Int. Assoc. Study Liver 41, 656–682. doi: 10.1111/liv.14800
Giridhar, P., Pradhan, S., Dokania, S., Venkatesulu, B., Sarode, R., Welsh, J. S. (2024). Microbiome and abdominopelvic radiotherapy related chronic enteritis: A microbiome-based mechanistic role of probiotics and antibiotics. Am. J. Clin. Oncol. 47 (5), 246–252. doi: 10.1097/COC.0000000000001082
Golob, J. L., DeMeules, M. M., Loeffelholz, T., Quinn, Z. Z., Dame, M. K., Silvestri, S. S., et al. (2019). Butyrogenic bacteria after acute graft-versus-host disease (GVHD) are associated with the development of steroid-refractory GVHD. Blood Adv. 3, 2866–2869. doi: 10.1182/bloodadvances.2019000362
Gottlieb, A., Mosthael, W., Sowa, J. P., Canbay, A. (2019). Nonalcoholic-fatty-liver-disease and nonalcoholic steatohepatitis: successful development of pharmacological treatment will depend on translational research. Digestion 100, 79–85. doi: 10.1159/000493259
Guo, N., Lv, L. L. (2023). Mechanistic insights into the role of probiotics in modulating immune cells in ulcerative colitis. Immun. Inflammation Dis. 11, e1045. doi: 10.1002/iid3.v11.10
Gupta, H., Kim, S. H., Kim, S. K., Han, S. H., Kwon, H. C., Suk, K. T. (2022a). Beneficial Shifts in Gut Microbiota by Lacticaseibacillus rhamnosus R0011 and Lactobacillus helveticus R0052 in Alcoholic Hepatitis. Microorganisms. 10, 1474. doi: 10.3390/microorganisms10071474
Gupta, H., Min, B.-H., Ganesan, R., Gebru, Y. A., Sharma, S. P., Park, E., et al. (2022b). Gut microbiome in non-alcoholic fatty liver disease: from mechanisms to therapeutic role. Biomedicines 10, 550. doi: 10.3390/biomedicines10030550
Han, S. H., Suk, K. T., Kim, D. J., Kim, M. Y., Baik, S. K., Kim, Y. D., et al. (2015). Effects of probiotics (cultured Lactobacillus subtilis/Streptococcus faecium) in the treatment of alcoholic hepatitis: randomized-controlled multicenter study. Eur. J. Gastroenterol. Hepatol. 27, 1300–1306. doi: 10.1097/MEG.0000000000000458
He, Q., Yang, C., Kang, X., Chen, Y., Zhang, T., Zhang, H., et al. (2022). Intake of Bifidobacterium lactis Probio-M8 fermented milk protects against alcoholic liver disease. J. Dairy Sci. 105, 2908–2921. doi: 10.3168/jds.2021-21265
He, L. H., Yao, D. H., Wang, L. Y., Zhang, L., Bai, X. L. (2021). Gut microbiome-mediated alteration of immunity, inflammation, and metabolism involved in the regulation of non-alcoholic fatty liver disease. Front. Microbiol. 12, 761836. doi: 10.3389/fmicb.2021.761836
He, X., Zhang, W., Feng, P., Mai, Z., Gong, X., Zhang, G. (2023). Role of surface coverage of sessile probiotics in their interplay with pathogen bacteria investigated by digital holographic microscopy. Langmuir 39, 17308–17317. doi: 10.1021/acs.langmuir.3c02436
Hediyal, T. A., Vichitra, C., Anand, N., Bhaskaran, M., Essa, S. M., Kumar, P., et al. (2024). Protective effects of fecal microbiota transplantation against ischemic stroke and other neurological disorders: an update. Front. Immunol. 15, 1324018. doi: 10.3389/fimmu.2024.1324018
Hojsak, I., Kolacek, S. (2024). Role of probiotics in the treatment and prevention of common gastrointestinal conditions in children. Pediatr. Gastroenterol. Hepatol. Nutr. 27, 1–14. doi: 10.5223/pghn.2024.27.1.1
Hsu, C. L., Schnabl, B. (2023). The gut-liver axis and gut microbiota in health and liver disease. Nat. Rev. Microbiol. 21, 719–733. doi: 10.1038/s41579-023-00904-3
Hu, X., Jin, H., Yuan, S., Ye, T., Chen, Z., Kong, Y., et al. (2023). Fecal microbiota transplantation inhibited neuroinflammation of traumatic brain injury in mice via regulating the gut-brain axis. Front. Cell Infect. Microbiol. 13, 1254610. doi: 10.3389/fcimb.2023.1254610
Huang, X., He, X., Chen, X., Li, Y. (2024). Fecal microbiota transplantation alleviates severe PD-1 inhibitor-associated colitis caused by neoadjuvant therapy for esophageal cancer: A case report. Gastroenterol. Nurs. 47 (5), 331–337. doi: 10.1097/SGA.0000000000000794
Hübscher, S. G. (2011). What is the long-term outcome of the liver allograft? J. Hepatol. 55, 702–717. doi: 10.1016/j.jhep.2011.03.005
Huttenhower, C., Gevers, D., Knight, R., Abubucker, S., Badger, J. H., Chinwalla, A. T., et al. (2012). Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214. doi: 10.1038/nature11234
Hwang, S. J., Choi, Y. J., Wang, J. H., Son, C. G. (2024). Lactobacillus Casei-fermented Amomum Xanthioides Mitigates non-alcoholic fatty liver disease in a high-fat diet mice model. BioMed. Pharmacother. 172, 116250. doi: 10.1016/j.biopha.2024.116250
Ince Palamutoglu, M., Kose, G., Bas, M. (2024). Probiotics and prebiotics affecting mental and gut health. Healthcare (Basel) 12 (5), 510. doi: 10.3390/healthcare12050510
Jaber, M. A., Ghanim, B. Y., Al-Natour, M., Arqoub, D. A., Abdallah, Q., Abdelrazig, S., et al. (2023). Potential biomarkers and metabolomics of acetaminophen-induced liver injury during alcohol consumption: A preclinical investigation on C57/BL6 mice. Toxicol. Appl. Pharmacol. 465, 116451. doi: 10.1016/j.taap.2023.116451
Jamshidi, P., Farsi, Y., Nariman, Z., Hatamnejad, M. R., Mohammadzadeh, B., Akbarialiabad, H., et al. (2023). Fecal microbiota transplantation in irritable bowel syndrome: A systematic review and meta-analysis of randomized controlled trials. Int. J. Mol. Sci. 24 (19), 14562. doi: 10.3390/ijms241914562
Jansson, J., Willing, B., Lucio, M., Fekete, A., Dicksved, J., Halfvarson, J., et al. (2009). Metabolomics reveals metabolic biomarkers of crohn's disease. PLoS One 4 (7), e6386. doi: 10.1371/journal.pone.0006386
Kim, K. S., Hong, S., Han, K., Park, C. Y. (2024). Association of non-alcoholic fatty liver disease with cardiovascular disease and all cause death in patients with type 2 diabetes mellitus: nationwide population based study. BMJ 384, e076388. doi: 10.1136/bmj-2023-076388
Kim, J. S., Park, H., Lee, J. H., Shin, J., Cha, B., Kwon, K. S., et al. (2024). Effect of altered gene expression in lipid metabolism on cognitive improvement in patients with Alzheimer's dementia following fecal microbiota transplantation: a preliminary study. Ther. Adv. Neurol. Disord. 17, 17562864231218181. doi: 10.1177/17562864231218181
Koziel, M. J. (2008). Translational research in liver disease. Hepatol. (Baltimore Md.) 48, 1028–1029. doi: 10.1002/hep.22528
Lacalle-Bergeron, L., Izquierdo-Sandoval, D., Fernandez-Quintela, A., Portillo, M. P., Sancho, J. V., Hernandez, F., et al. (2023). LC-IMS-HRMS for identification of biomarkers in untargeted metabolomics: The effects of pterostilbene and resveratrol consumption in liver steatosis, animal model. Food Res. Int. 165, 112376. doi: 10.1016/j.foodres.2022.112376
Leung, H., Long, X., Ni, Y., Qian, L., Nychas, E., Siliceo, S. L., et al. (2022). Risk assessment with gut microbiome and metabolite markers in NAFLD development. Sci. Transl. Med. 14, eabk0855. doi: 10.1126/scitranslmed.abk0855
Li, N. N., Li, W., Feng, J. X., Zhang, W. W., Zhang, R., Du, S. H., et al. (2021). High alcohol-producing Klebsiella pneumoniae causes fatty liver disease through 2,3-butanediol fermentation pathway in vivo. Gut Microbes 13, 1979883. doi: 10.1080/19490976.2021.1979883
Lin, Y., Ma, C., Liu, C., Wang, Z., Yang, J., Liu, X., et al. (2016). NMR-based fecal metabolomics fingerprinting as predictors of earlier diagnosis in patients with colorectal cancer. Oncotarget. 7, 29454–29464. doi: 10.18632/oncotarget.8762
Liu, X., Wang, C., Li, Y., Wang, Y., Sun, X., Wang, Q., et al. (2024). Fecal microbiota transplantation revealed the function of folic acid on reducing abdominal fat deposition in broiler chickens mediated by gut microbiota. Poult Sci. 103, 103392. doi: 10.1016/j.psj.2023.103392
López-Vicario, C., Checa, A., Urdangarin, A., Aguilar, F., Alcaraz-Quiles, J., Caraceni, P., et al. (2020). Targeted lipidomics reveals extensive changes in circulating lipid mediators in patients with acutely decompensated cirrhosis. J. Hepatol. 73, 817–828. doi: 10.1016/j.jhep.2020.03.046
Luo, R., Chen, C., Shi, Y., Tao, Q., Bai, D., Li, A. (2023). Effects of overfeeding on liver lipid metabolism in mule ducks based on transcriptomics and metabolomics. Br. Poult Sci. 64, 143–156. doi: 10.1080/00071668.2022.2154638
Ma, L., Song, J., Chen, X., Dai, D., Chen, J., Zhang, L. (2023). Fecal microbiota transplantation regulates TFH/TFR cell imbalance via TLR/MyD88 pathway in experimental autoimmune hepatitis. Heliyon 9, e20591. doi: 10.1016/j.heliyon.2023.e20591
Madatali Abuwani, A., Priyadarshini Dash, S., Ganesan, R., Renu, K., Vellingiri, B., Kandasamy, S., et al. (2021). Gut microbiome and metabolic response in non-alcoholic fatty liver disease. Clinica Chimica Acta 523, 304–314. doi: 10.1016/j.cca.2021.10.014
Martini, S., Zaccaria, T., Gasbarrini, A., Cammarota, G., Romagnoli, R., Ianiro, G. (2024). Fecal microbiota transplantation before liver transplant in patient colonized with New Delhi metallo-beta-lactamase: Are we ready for a sequential approach? Transpl Infect. Dis. 26, e14248. doi: 10.1111/tid.14248
McMillan, A. S., Zhang, G., Dougherty, M. K., McGill, S. K., Gulati, A. S., Baker, E. S., et al. (2024). Metagenomic, metabolomic, and lipidomic shifts associated with fecal microbiota transplantation for recurrent Clostridioides difficile infection. mSphere. 9 (10), e0070624. doi: 10.1128/msphere.00706-24
Meijnikman, A. S., Davids, M., Herrema, H., Aydin, O., Tremaroli, V., Rios-Morales, M., et al. (2022). Microbiome-derived ethanol in nonalcoholic fatty liver disease. Nat. Med. 28, 2100–2106. doi: 10.1038/s41591-022-02016-6
Mimura, T., Rizzello, F., Helwig, U., Poggioli, G., Schreiber, S., Talbot, I. C., et al. (2004). Once daily high dose probiotic therapy (VSL3) for maintaining remission in recurrent or refractory pouchitis. Gut 53, 108–114. doi: 10.1136/gut.53.1.108
Miyoshi, J., Bobe, A. M., Miyoshi, S., Huang, Y., Hubert, N., Delmont, T. O., et al. (2017). Peripartum antibiotics promote gut dysbiosis, loss of immune tolerance, and inflammatory bowel disease in genetically prone offspring. Cell Rep. 20, 491–504. doi: 10.1016/j.celrep.2017.06.060
Moratalla, A., Ampuero, J., Bellot, P., Gallego-Durán, R., Zapater, P., Roger, M., et al. (2017). Lactulose reduces bacterial DNA translocation, which worsens neurocognitive shape in cirrhotic patients with minimal hepatic encephalopathy. Liver international: Off. J. Int. Assoc. Study Liver 37, 212–223. doi: 10.1111/liv.2017.37.issue-2
Moreau, R., Clària, J., Aguilar, F., Fenaille, F., Lozano, J. J., Junot, C., et al. (2020). Blood metabolomics uncovers inflammation-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J. Hepatol. 72, 688–701. doi: 10.1016/j.jhep.2019.11.009
Mortensen, P. B., Clausen, M. R. (1996). Short-chain fatty acids in the human colon: relation to gastrointestinal health and disease. Scandinavian J. gastroenterology. Supplement 216, 132–148. doi: 10.3109/00365529609094568
Nacif, L. S., Kim, V., Galvao, F., Ono, S. K., Pinheiro, R. S., Carrilho, F. J., et al. (2018). Translational medical research and liver transplantation: systematic review. Transl. Gastroenterol. Hepatol. 3, 91. doi: 10.21037/tgh.2018.10.14
Neuman, M. G., Malnick, S., Maor, Y., Nanau, R. M., Melzer, E., Ferenci, P., et al. (2015). Alcoholic liver disease: Clinical and translational research. Exp. Mol. Pathol. 99, 596–610. doi: 10.1016/j.yexmp.2015.09.001
Oliphant, K., Allen-Vercoe, E. (2019). Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome 7, 91. doi: 10.1186/s40168-019-0704-8
Pakdaman, M. N., Udani, J. K., Molina, J. P., Shahani, M. (2016). The effects of the DDS-1 strain of lactobacillus on symptomatic relief for lactose intolerance - a randomized, double-blind, placebo-controlled, crossover clinical trial. Nutr. J. 15, 56. doi: 10.1186/s12937-016-0172-y
Pavlidis, P., Powell, N., Vincent, R. P., Ehrlich, D., Bjarnason, I., Hayee, B. (2015). Systematic review: bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Alimentary Pharmacol. Ther. 42, 802–817. doi: 10.1111/apt.2015.42.issue-7
Pekkala, S. (2023). Fecal metagenomics and metabolomics identifying microbial signatures in non-alcoholic fatty liver disease. Int. J. Mol. Sci. 24 (5), 4855. doi: 10.3390/ijms24054855
Petruzziello, C., Saviano, A., Manetti, L. L., Macerola, N., Ojetti, V. (2024). The role of gut microbiota and the potential effects of probiotics in heart failure. Medicina (Kaunas) 60 (2), 271. doi: 10.3390/medicina60020271
Prado, V., Hernández-Tejero, M., Mücke, M. M., Marco, F., Gu, W., Amoros, A., et al. (2022). Rectal colonization by resistant bacteria increases the risk of infection by the colonizing strain in critically ill patients with cirrhosis. J. Hepatol. 76, 1079–1089. doi: 10.1016/j.jhep.2021.12.042
Puri, A. S. (2023). The role of yeast probiotics in gastrointestinal conditions: an overview. J. Assoc. Physicians India 71, 11–12. doi: 10.5005/japi-11001-0235
Qu, L., Ma, X., Wang, F. (2024). The roles of gut microbiome and metabolites associated with skin photoaging in mice by intestinal flora sequencing and metabolomics. Life Sci. 341, 122487. doi: 10.1016/j.lfs.2024.122487
Raimondi, F., Santoro, P., Barone, M. V., Pappacoda, S., Barretta, M. L., Nanayakkara, M., et al. (2008). Bile acids modulate tight junction structure and barrier function of Caco-2 monolayers via EGFR activation. Am. J. Physiol. Gastrointestinal liver Physiol. 294, G906–G913. doi: 10.1152/ajpgi.00043.2007
Raja, G., Gupta, H., Gebru, Y. A., Youn, G. S., Choi, Y. R., Kim, H. S., et al. (2021). Recent advances of microbiome-associated metabolomics profiling in liver disease: principles, mechanisms, and applications. Int. J. Mol. Sci. 22, 1160. doi: 10.3390/ijms22031160
Rau, M., Rehman, A., Dittrich, M., Groen, A. K., Hermanns, H. M., Seyfried, F., et al. (2018). Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United Eur. Gastroenterol. J. 6, 1496–1507. doi: 10.1177/2050640618804444
Ribeiro, D. M., Leclercqc, C. C., Charton, S. A. B., Costa, M. M., Carvalho, D. F. P., Sergeant, K., et al. (2024). The impact of dietary Laminaria digitata and alginate lyase supplementation on the weaned piglet liver: A comprehensive proteomics and metabolomics approach. J. Proteomics 293, 105063. doi: 10.1016/j.jprot.2023.105063
Robinson, J. I., Weir, W. H., Crowley, J. R., Hink, T., Reske, K. A., Kwon, J. H., et al. (2019). Metabolomic networks connect host-microbiome processes to human Clostridioides difficile infections. J. Clin. Invest. 129, 3792–3806. doi: 10.1172/JCI126905
Roda, A., Simoni, P., Magliulo, M., Nanni, P., Baraldini, M., Roda, G., et al. (2007). A new oral formulation for the release of sodium butyrate in the ileo-cecal region and colon. World J. Gastroenterol. 13, 1079–1084. doi: 10.3748/wjg.v13.i7.1079
Roškar, I., Švigelj, K., Štempelj, M., Volfand, J., Štabuc, B., Malovrh, Š., et al. (2017). Effects of a probiotic product containing Bifidobacterium animalis subsp. animalisIM386 and Lactobacillus plantarum MP2026 in lactose intolerant individuals: randomized, placebo-controlled clinical trial. J. Funct. Foods 35, 1–8. doi: 10.1016/j.jff.2017.05.020
Rungratanawanich, W., Ballway, J. W., Wang, X., Won, K. J., Hardwick, J. P., Song, B. J. (2023). Post-translational modifications of histone and non-histone proteins in epigenetic regulation and translational applications in alcohol-associated liver disease: Challenges and research opportunities. Pharmacol. Ther. 251, 108547. doi: 10.1016/j.pharmthera.2023.108547
Sanders, M. E., Merenstein, D. J., Reid, G., Gibson, G. R., Rastall, R. A. (2019). Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 16, 605–616. doi: 10.1038/s41575-019-0173-3
Schneider, K. M., Kummen, M., Trivedi, P. J., Hov, J. R. (2024). Role of microbiome in autoimmune liver diseases. Hepatol0gy 80(4), 965–987. doi: 10.1097/HEP.0000000000000506
Schulfer, A. F., Battaglia, T., Alvarez, Y., Bijnens, L., Ruiz, V. E., Ho, M., et al. (2018). Intergenerational transfer of antibiotic-perturbed microbiota enhances colitis in susceptible mice. Nat. Microbiol. 3, 234–242. doi: 10.1038/s41564-017-0075-5
Shogbesan, O., Poudel, D. R., Victor, S., Jehangir, A., Fadahunsi, O., Shogbesan, G., et al. (2018). A systematic review of the efficacy and safety of fecal microbiota transplant for clostridium difficile infection in immunocompromised patients. Can. J. Gastroenterol. Hepatol. 2018, 1394379. doi: 10.1155/2018/1394379
Summer, M., Sajjad, A., Ali, S., Hussain, T. (2024). Exploring the underlying correlation between microbiota, immune system, hormones, and inflammation with breast cancer and the role of probiotics, prebiotics and postbiotics. Arch. Microbiol. 206, 145. doi: 10.1007/s00203-024-03868-x
Tanaka, S., Ui, S. (2010). Translational research of molecular targeting drug for liver cancer. Gan To Kagaku Ryoho 37, 1874–1877.
Taner, T., Martins, P. N., Ling, Q., Ng, K. T., Huang, K. T., Eymard, C., et al. (2020). What is hot and new in basic and translational science in liver transplantation in 2019? Report of the basic and translational research committee of the international liver transplantation society. Transplantation 104, 516–521. doi: 10.1097/TP.0000000000003058
Tay, P. W. L., Xiao, J., Tan, D. J. H., Ng, C., Lye, Y. N., Lim, W. H., et al. (2021). An epidemiological meta-analysis on the worldwide prevalence, resistance, and outcomes of spontaneous bacterial peritonitis in cirrhosis. Front. Med. 8, 693652. doi: 10.3389/fmed.2021.693652
Teker, H. T., Ceylani, T., Keskin, S., Samgane, G., Allahverdi, H., Acikgoz, E., et al. (2024). Supplementing probiotics during intermittent fasting proves more effective in restoring ileum and colon tissues in aged rats. J. Cell Mol. Med. 28, e18203. doi: 10.1111/jcmm.18203
Tu, C., Guo, Z. J., Jiang, B. Q., Kang, Q. J., Wang, T. (2023). Difference in liver injury induced by dictamnine between males and females: based on untargeted metabolomics. Zhongguo Zhong Yao Za Zhi 48, 3317–3326. doi: 10.19540/j.cnki.cjcmm.20230119.401
Vatsalya, V., Feng, W., Kong, M., Hu, H., Szabo, G., McCullough, A., et al. (2023). The beneficial effects of lactobacillus GG therapy on liver and drinking assessments in patients with moderate alcohol-associated hepatitis. Am. J. Gastroenterol. 118, 1457–1460. doi: 10.14309/ajg.0000000000002283
Vince, A. J., Burridge, S. M. (1980). Ammonia production by intestinal bacteria: the effects of lactose, lactulose and glucose. J. Med. Microbiol. 13, 177–191. doi: 10.1099/00222615-13-2-177
Vince, A. J., McNeil, N. I., Wager, J. D., Wrong, O. M. (1990). The effect of lactulose, pectin, arabinogalactan and cellulose on the production of organic acids and metabolism of ammonia by intestinal bacteria in a faecal incubation system. Br. J. Nutr. 63, 17–26. doi: 10.1079/BJN19900088
Visconti, A., Le Roy, C. I., Rosa, F., Rossi, N., Martin, T. C., Mohney, R. P., et al. (2019). Interplay between the human gut microbiome and host metabolism. Nat. Commun. 10, 4505. doi: 10.1038/s41467-019-12476-z
Wang, J. Y., Bajaj, J. S., Wang, J. B., Shang, J., Zhou, X. M., Guo, X. L., et al. (2019). Lactulose improves cognition, quality of life, and gut microbiota in minimal hepatic encephalopathy: A multicenter, randomized controlled trial. J. digestive Dis. 20, 547–556. doi: 10.1111/1751-2980.12816
Wang, R., Chen, Y., Chen, J., Ma, M., Xu, M., Liu, S. (2023). Integration of transcriptomics and metabolomics analysis for unveiling the toxicological profile in the liver of mice exposed to uranium in drinking water. Environ. pollut. 335, 122296. doi: 10.1016/j.envpol.2023.122296
Wang, C., Fu, R. J., Xu, D. Q., Zuo, Q., Liu, J. P., Tang, Y. P. (2024). A study integrated metabolomics and network pharmacology to investigate the effects of Shicao in alleviating acute liver injury. J. Ethnopharmacol 319, 117369. doi: 10.1016/j.jep.2023.117369
Wang, Q., Shan, B., Cheng, W., He, T., Chen, K., Zhang, J., et al. (2023). Metabolomics study of the hepatoprotective effects and mechanism of aqueous extract of dendrobium nobile lindl. on alcoholic liver injury in rats. Comb Chem. High Throughput Screen 26, 2718–2729. doi: 10.2174/1386207326666230330150211
Wang, R., Tang, R., Li, B., Ma, X., Schnabl, B., Tilg, H. (2021). Gut microbiome, liver immunology, and liver diseases. Cell. Mol. Immunol. 18, 4–17. doi: 10.1038/s41423-020-00592-6
Wang, W., Xu, A. L., Li, Z. C., Li, Y., Xu, S. F., Sang, H. C., et al. (2020). Combination of Probiotics and Salvia miltiorrhizaPolysaccharide Alleviates Hepatic Steatosis via Gut Microbiota Modulation and Insulin Resistance Improvement in High Fat-Induced NAFLD Mice. Diabetes Metab. J. 44, 336–348. doi: 10.4093/dmj.2019.0042
Weber, F. L., Jr. (1979). The effect of lactulose on urea metabolism and nitrogen excretion in cirrhotic patients. Gastroenterology 77, 518–523. doi: 10.1016/0016-5085(79)90015-5
Wiefels, M. D., Furar, E., Eshraghi, R. S., Mittal, J., Memis, I., Moosa, M., et al. (2024). Targeting gut dysbiosis and microbiome metabolites for the development of therapeutic modalities for neurological disorders. Curr. Neuropharmacol 22, 123–139. doi: 10.2174/1570159X20666221003085508
Won, S. M., Park, E., Jeong, J. J., Ganesan, R., Gupta, H., Gebru, Y. A., et al. (2021). The gut microbiota-derived immune response in chronic liver disease. Int. J. Mol. Sci. 22 (15), 8309. doi: 10.3390/ijms22158309
Wong, W. S. W., Sabu, P., Deopujari, V., Levy, S., Shah, A. A., Clemency, N., et al. (2020). Prenatal and peripartum exposure to antibiotics and cesarean section delivery are associated with differences in diversity and composition of the infant meconium microbiome. Microorganisms 8, 179. doi: 10.3390/microorganisms8020179
Wrzosek, L., Ciocan, D., Hugot, C., Spatz, M., Dupeux, M., Houron, C., et al. (2021). Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut 70(7), 1299–1308. doi: 10.1136/gutjnl-2020-321565
Wu, H., Ma, W., Wang, Y., Wang, Y., Sun, X., Zheng, Q. (2024). Gut microbiome-metabolites axis: A friend or foe to colorectal cancer progression. BioMed. Pharmacother. 173, 116410. doi: 10.1016/j.biopha.2024.116410
Ye, Y., Zhang, B., Mai, W., Tan, Y., Feng, Z., Huang, Q. (2023). Metabolomics study of the hepatoprotective effect of total flavonoids of Mallotus apelta leaf in carbon tetrachloride-induced liver fibrosis in rats. BioMed. Chromatogr 37, e5711. doi: 10.1002/bmc.5711
Yoon, S. J., Yu, J. S., Min, B. H., Gupta, H., Won, S. M., Park, H. J., et al. (2023). Bifidobacterium-derived short-chain fatty acids and indole compounds attenuate nonalcoholic fatty liver disease by modulating gut-liver axis. Front. Microbiol. 14, 1129904. doi: 10.3389/fmicb.2023.1129904
Yu, Z., Cheng, M., Luo, S., Wei, J., Song, T., Gong, Y., et al. (2023). Comparative lipidomics and metabolomics reveal the underlying mechanisms of taurine in the alleviation of nonalcoholic fatty liver disease using the aged laying hen model. Mol. Nutr. Food Res. 67, e2200525. doi: 10.1002/mnfr.202200525
Yu, C., Xu, Y., Wei, Y., Guo, Y., Wang, Y., Song, P., et al. (2024). Gut microbiota and liver metabolomics reveal the potential mechanism of Lactobacillus rhamnosus GG modulating the liver toxicity caused by polystyrene microplastics in mice. Environ. Sci. pollut. Res. Int. 31, 6527–6542. doi: 10.1007/s11356-023-31564-8
Yu, J. S., Youn, G. S., Choi, J., Kim, C. H., Kim, B. Y., Yang, S. J., et al. (2021). Lactobacillus lactis and Pediococcus pentosaceus-driven reprogramming of gut microbiome and metabolome ameliorates the progression of non-alcoholic fatty liver disease. Clin. Transl. Med. 11, e634. doi: 10.1002/ctm2.634
Yuan, J., Chen, C., Cui, J., Lu, J., Yan, C., Wei, X., et al. (2019). Fatty liver disease caused by high-alcohol-producing klebsiella pneumoniae. Cell Metab. 30, 675–688.e7. doi: 10.1016/j.cmet.2019.08.018
Zafar, Y., Siddiqi, A. K., Shaikh, N., Imran, M., Javaid, S. S., Manzoor, L., et al. (2024). Alcohol relapse after early liver transplantation in patients with alcoholic liver disease: A meta-analysis. Gastroenterol. Res. 17, 10–14. doi: 10.14740/gr1674
Zeki Ö, C., Eylem, C. C., Reçber, T., Kır, S., Nemutlu, E. (2020). Integration of GC-MS and LC-MS for untargeted metabolomics profiling. J. Pharm. Biomed. Anal. 190, 113509. doi: 10.1016/j.jpba.2020.113509
Zeng, F., Su, X., Liang, X., Liao, M., Zhong, H., Xu, J., et al. (2024). Gut microbiome features and metabolites in non-alcoholic fatty liver disease among community-dwelling middle-aged and older adults. BMC Med. 22, 104. doi: 10.1186/s12916-024-03317-y
Zhang, J., Fan, J., Luo, H., Liang, Z., Guan, Y., Lei, X., et al. (2023). Alleviation of alcoholic fatty liver by dendrobium officinale flower extracts due to regulation of gut microbiota and short-chain fatty acids in mice exposed to chronic alcohol. Foods 12 (7), 1428. doi: 10.3390/foods12071428
Zhang, W., Mackay, C. R., Gershwin, M. E. (2023). Immunomodulatory effects of microbiota-derived short-chain fatty acids in autoimmune liver diseases. J. Immunol. 210, 1629–1639. doi: 10.4049/jimmunol.2300016
Zhao, C., Liu, L., Liu, Q., Li, F., Zhang, L., Zhu, F., et al. (2019). Fibroblast growth factor 21 is required for the therapeutic effects of Lactobacillus rhamnosus GG against fructose-induced fatty liver in mice. Mol. Metab. 29, 145–157. doi: 10.1016/j.molmet.2019.08.020
Zheng, X., Wang, R., Yin, C. (2023). An untargeted metabolomics investigation in liver of flaviviruses-infected mice. Virology 582, 12–22. doi: 10.1016/j.virol.2023.03.008
Zhu, L., Baker, S. S., Gill, C., Liu, W., Alkhouri, R., Baker, R. D., et al. (2013). Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatol. (Baltimore Md.) 57, 601–609. doi: 10.1002/hep.26093
Zhu, L., Li, D., Yang, X. (2023). Gut metabolomics and 16S rRNA sequencing analysis of the effects of arecoline on non-alcoholic fatty liver disease in rats. Front. Pharmacol. 14, 1132026. doi: 10.3389/fphar.2023.1132026
Keywords: microbiome, metabolomics, metabolic alterations, liver diseases, metabolites, hepatology, gastroenterology
Citation: Ganesan R, Thirumurugan D, Vinayagam S, Kim DJ, Suk KT, Iyer M, Yadav MK, HariKrishnaReddy D, Parkash J, Wander A and Vellingiri B (2025) A critical review of microbiome-derived metabolic functions and translational research in liver diseases. Front. Cell. Infect. Microbiol. 15:1488874. doi: 10.3389/fcimb.2025.1488874
Received: 20 September 2024; Accepted: 31 January 2025;
Published: 24 February 2025.
Edited by:
Francesca Guerrieri, INSERM U1052 Centre de Recherche en Cancerologie de Lyon, FranceReviewed by:
Seogsong Jeong, Korea University, Republic of KoreaSabariswaran Kandasamy, Bharathiar University, India
Copyright © 2025 Ganesan, Thirumurugan, Vinayagam, Kim, Suk, Iyer, Yadav, HariKrishnaReddy, Parkash, Wander and Vellingiri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Raja Ganesan, Z2FuZXNhbnIyQHNybWlzdC5lZHUuaW4=; Balachandar Vellingiri, YmFsYWNoYW5kYXIudmVsbGluZ2lyaUBjdXAuZWR1Lmlu
 Raja Ganesan
Raja Ganesan Durairaj Thirumurugan
Durairaj Thirumurugan Saranya Vinayagam2
Saranya Vinayagam2 Dong Joon Kim
Dong Joon Kim Ki Tae Suk
Ki Tae Suk Mahalaxmi Iyer
Mahalaxmi Iyer Arvinder Wander
Arvinder Wander Balachandar Vellingiri
Balachandar Vellingiri