- 1Clinical Laboratory, Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Clinical Laboratory, Beijing Ditan Hospital, Capital Medical University, Beijing, China
Objectives: The goal of this study is to evaluate the analytical and clinical performance of a new human immunodeficiency virus antigen and antibody (HIV Ag/Ab) test using light-initiated chemiluminescent assay (LiCA®) and compare it with the well-established Architect® HIV Ag/Ab combo assay in a clinical setting.
Methods: We used banked samples and national reference controls to identify the ability to detect HIV Ag/Ab and different viral subtypes. Thirteen seroconversion panels were tested to evaluate early detection of HIV. A total of 21,042 patient samples were collected to compare the diagnostic performance of LiCA® with Architect®. Screening-reactive results were confirmed by Western blotting and nucleic acid testing.
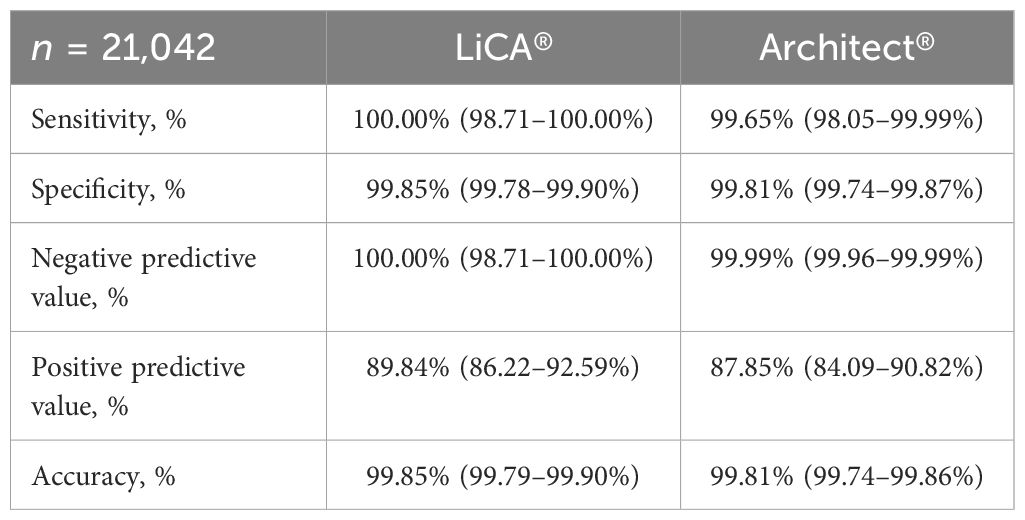
Results: Total imprecision was within 2.49%–6.56%. The C5–C95 interval was within −10.20%–7.67% away from C50. The limit of detection for p24 antigen was <1.00 IU/mL. Using national reference panels and banked sample pools, LiCA® successfully detected all negative and positive controls in line with the criteria, and all HIV-positive specimens containing different viral subtypes. In 13 seroconversion panels, LiCA® detected reactive results on average 5.73 days (95% CI: 3.42–8.04) after the initial RNA test results were confirmed positive, which was 1.27 days earlier (−3.75 to 1.21) compared to Architect®. Paired comparisons in 21,042 clinical patient samples demonstrated that LiCA® detected HIV Ag/Ab with a slightly better performance in sensitivity (100.00% vs. 99.65%), specificity (99.85% vs. 99.81%), negative predictive value (NPV, 100.00% vs. 99.99%), and positive predictive value (PPV, 89.84% vs. 87.85%) than Architect®. Total agreement between two assays was 99.67% with a kappa value of 0.89.
Conclusion: LiCA® HIV Ag/Ab is a precise and highly sensitive assay for measuring HIV-1 p24 antigen and HIV-1/2 antibodies with differentiated S/Co values of Ag/Ab. The assay is appropriate for use in the clinical routine test for the early detection of HIV.
1 Introduction
Acquired immune deficiency syndrome (AIDS), caused by the human immunodeficiency virus (HIV), is a global infectious disease that seriously endangers human health. HIV attacks the patient’s immune system, which causes a variety of complications and even death. In addition, the virus continues to be spread through blood transfusions, sexual contact, and drug use (Volberding and Deeks, 2010). As reported by the US Centers for Disease Control and Prevention (CDC), approximately 1.7 million people were newly infected with HIV worldwide in 2018, and 770,000 people died among those living with AIDS (UNAIDS, 2019). Of added concern is that several African countries and Middle East nations are far from controlling this epidemic (El-Sadr et al., 2019). Fortunately, continued access to antiretroviral therapy (ART) in the early stages of infection can have a major positive effect on reducing transmission and death among those living with HIV (Cohen et al., 2011; Rodger et al., 2019). Therefore, early diagnosis of the infection and linking the patients to the proper medicinal therapy are critical for the management of AIDS patients. This heavily relies on an effective viral screening strategy.
During recent decades, various methods for detecting HIV antibody (Ab), p24 antigen (Ag), and ribonucleic acid (RNA) in serum or plasma have been developed for the diagnosis of HIV infection (Alexander, 2016; Gray et al., 2018). The detection capabilities of various methods exhibit certain variations. For instance, the sensitivity of first-generation HIV-Ab reagents is 99%, with a specificity ranging from 95% to 98% (Alexander, 2016). Taking the Alere HIV Combo POCT test as an example for HIV-1 P24 antigen detection, its sensitivity is 88%, and its specificity is 100% (Fitzgerald et al., 2017). The detection capability of HIV RNA is primarily reflected in its sensitivity. Currently, the lower limit of detection for ordinary HIV RNA quantitative reagents is 100–200 copies/mL, while high-sensitivity quantitative reagents can achieve a lower limit of detection as low as 20 copies/mL. In general, a suspected subject is initially detected with the screening test for HIV Ag/Ab, and the screening-reactive assay is further confirmed by Western blot (WB) and/or nucleic acid test (NAT) to clarify the infection condition or even a false-positive result (Centers for Disease Control and Prevention, 2018; Branson, 2019). The Ag/Ab screening test with higher sensitivity and specificity can be more favorable in clinical practice due to its better detection capability for viral infection from first- to fifth-generation commercial kits (Alexander, 2016). Unlike the third-generation assay that detects Ab alone, the fourth- and fifth-generation assays detect both Ab and p24 Ag in combination, reducing the test-negative window to 8–14 days (Alexander, 2016; Qiu et al., 2017). Moreover, the fifth-generation assay can differentiate Ab and Ag reactivity instead of a single result by the fourth-generation kit, thus facilitating the confirmatory strategy for early detection of HIV (Muhlbacher et al., 2019; Yang et al., 2022).
Here, we introduce a new fifth-generation HIV Ag/Ab combination test that is based on the light-initiated chemiluminescent assay (LiCA®) (Bian et al., 2018). LiCA® provides a fully automatic homogeneous immunoassay platform, which has been widely developed for detection of various analytes with high sensitivity and specificity, such as hormones (Yu et al., 2021; Wang et al., 2022), cardiac proteins (Yang et al., 2022; Li et al., 2024), and Ag and Ab (Li et al., 2023; Yu et al., 2023). In this study, we aim to evaluate the performance of the LiCA® HIV Ag/Ab assay in analytical and clinical perspectives and compare it with the well-established Architect® HIV Ag/Ab combo test in clinical setting.
2 Materials and methods
2.1 Sample collection and HIV Ag/Ab serological assays
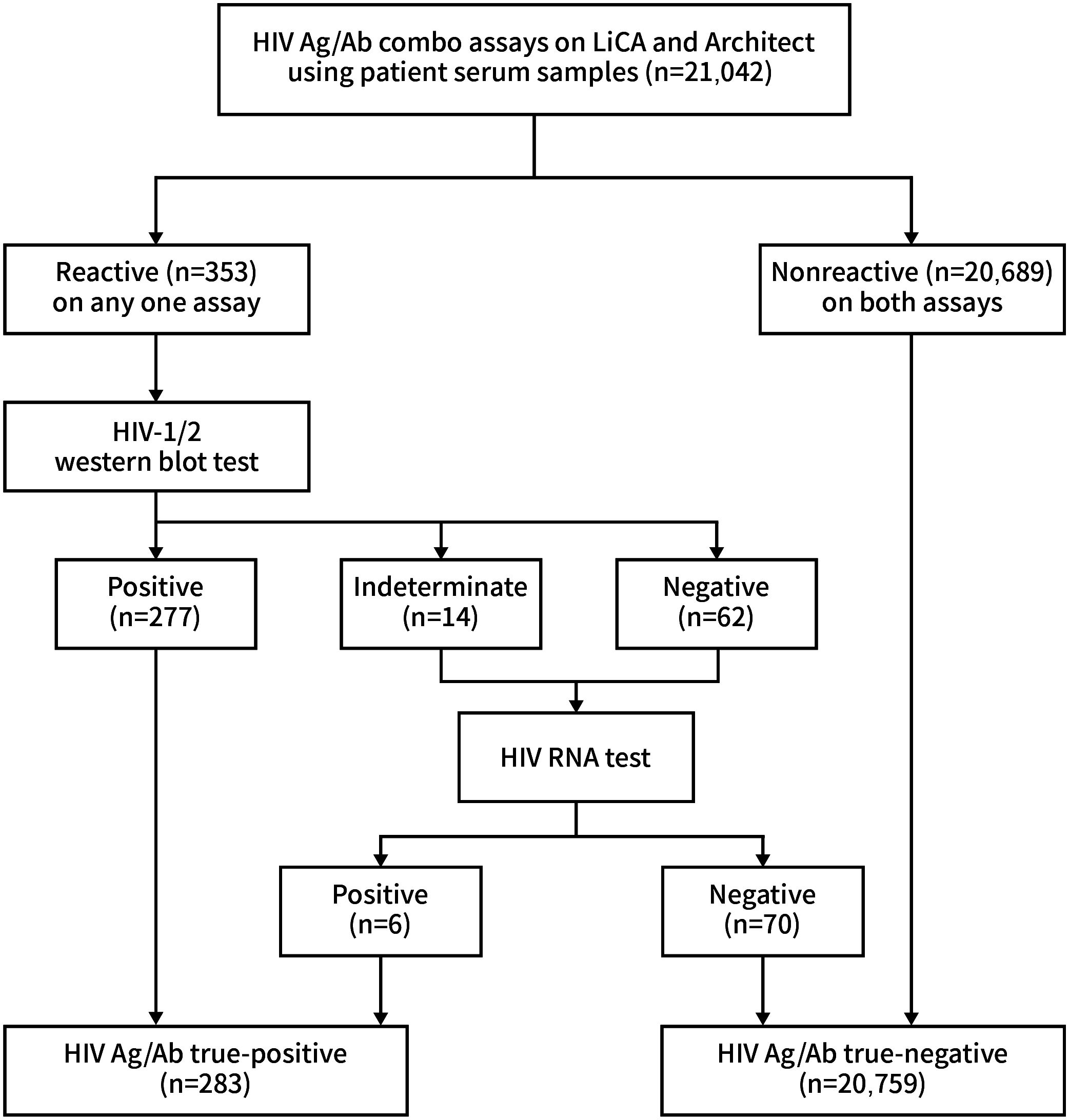
We recruited a total of 21,042 clinical serum specimens from inpatients and outpatients in Dongzhimen Hospital. HIV Ag/Ab screening tests were performed on the LiCA® 500 platform (Chemclin Diagnostics, Beijing, China) and the Architect® i2000SR system (Abbott Laboratories, IL, USA) in parallel (Figure 1). LiCA® HIV Ag/Ab is a one-step fully automatic homogeneous immunoassay. Serum samples were dispensed into two cuvettes for detecting antibodies to HIV-1/HIV-2 subtypes and HIV-1 p24 antigen, respectively. The Ag/Ab reactivity can be differentiated in results. A signal-to-cutoff (S/Co) ratio ≥1.0 in any cuvette is considered to be screening-reactive. Time to the first report is approximately 25 min. Architect® HIV Ag/Ab combo is a two-step indirect immunoassay and reports combined Ag/Ab reactivity in a single result. A ratio of S/Co ≥1.0 is regarded as screening-reactive. Any one assay with a reactive S/Co was retested in duplicate. The repeated screening-reactive assays were then allocated for antibody identification with the WB test of recomLine HIV-1/HIV-2 IgG (Mikrogen Diagnostics, Neuried, Germany). Subjects with WB-indeterminate and WB-negative results were further identified with the Cobas® AmpliPrep/Cobas® TagMan® HIV-1 RNA test (Roche Diagnostics, Mannheim, Germany). Finally, the HIV Ag/Ab true-positive group included both WB-positive and RNA-positive results, and the true-negative group included those with screening-negative results in both assays and RNA-negative results. The testing protocol was plotted as shown in Figure 2 (Alexander, 2016; Fitzgerald et al., 2017).
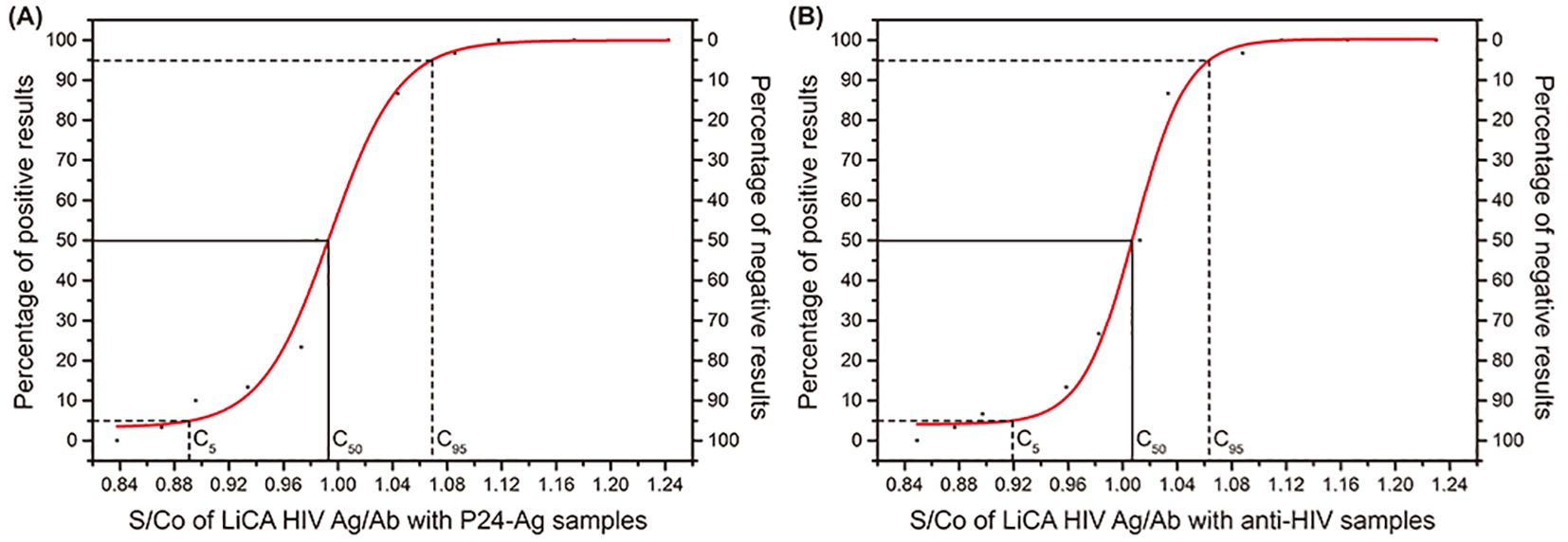
Figure 2. The S/Co of LiCA HIV Ag/Ab with P24-Ag samples and anti-HlV samples. (A) S/Co of LiCA HIV Ag/Ab with P24-Ag samples. (B) S/Co of LiCA HIV Ag/Ab with anti-HlV samples.
2.2 Precision study
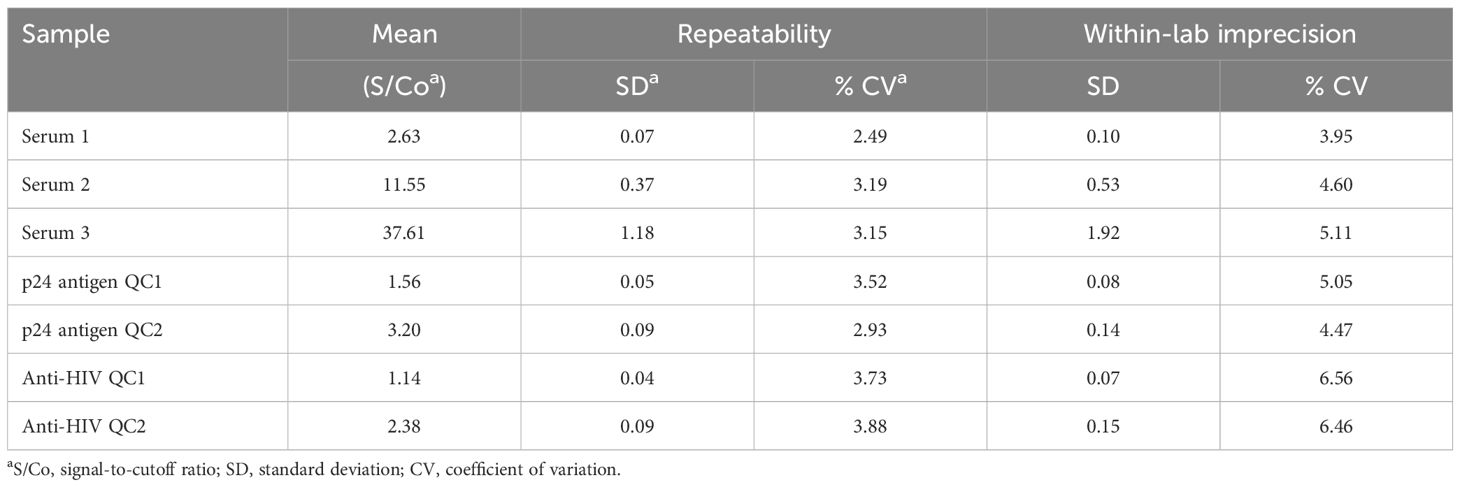
We performed precision analysis in S/Co ratios according to the EP15-A3 protocol of the Clinical and Laboratory Standards Institute (CLSI) (CLSI, 2014), using three levels of patient serum samples, and two levels of controls for HIV antibodies and p24 antigen, respectively. An acceptable coefficient of variation (CV) was ≤15%. In addition, we followed the guideline of EP12-A2 (CLSI, 2008) and prepared a series of dilutions with a positive sera to determine the C50 target and the C5–C95 interval for HIV antibodies and p24 antigen, respectively. An acceptable C5–C95 interval was within C50 ± 15%.
2.3 Detection capability
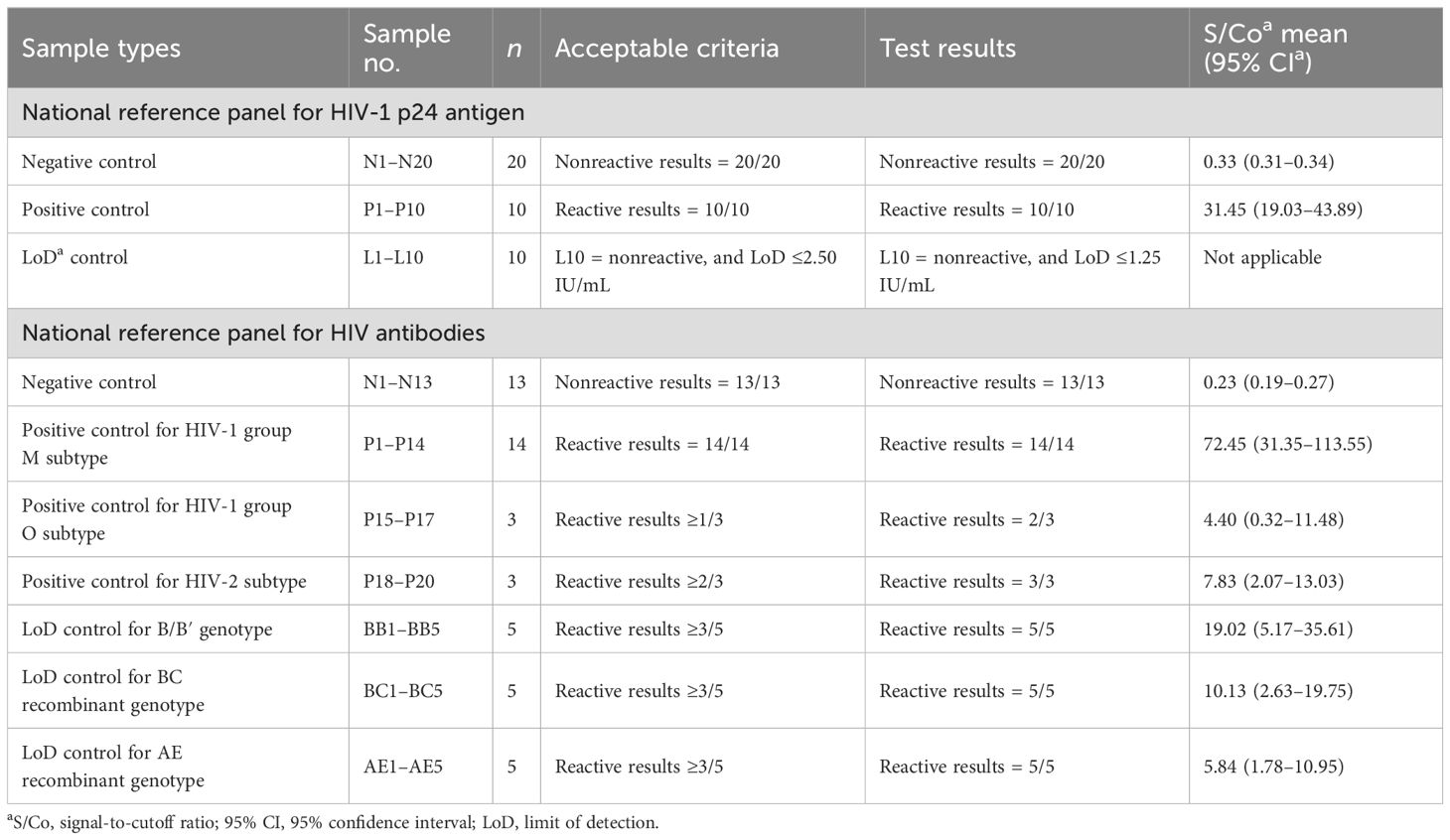
We evaluated the assay detection capability to HIV antibodies and p24 antigen with the reference panels of the China National Institutes for Food and Drug Control (NIFDC). The panel for HIV-1 p24 antigen (Lot 220015-201906) was composed of 20 negative controls, 10 positive controls, and 10 levels of serial dilutions for study of the limit of detection (LoD). The panel for HIV antibodies (Lot 370045-201901) contained different types of negative and positive controls for the detection of HIV-1/HIV-2 subtypes and controls for the LoD study of B/B′, BC, and AE genotypes. Furthermore, we prepared a series of doubling dilutions to quantify the assay LoD to HIV-1 p24 antigen using the NIFDC reference panel (Lot 220015-201906, baseline p24 concentration 20 IU/mL) and the World Health Organization (WHO) international standard from the National Institute for Biological Standards and Control (NIBSC, code 90/636, baseline p24 concentration 1,000 IU/mL).
2.4 Detection of seroconversion panels and HIV subtypes
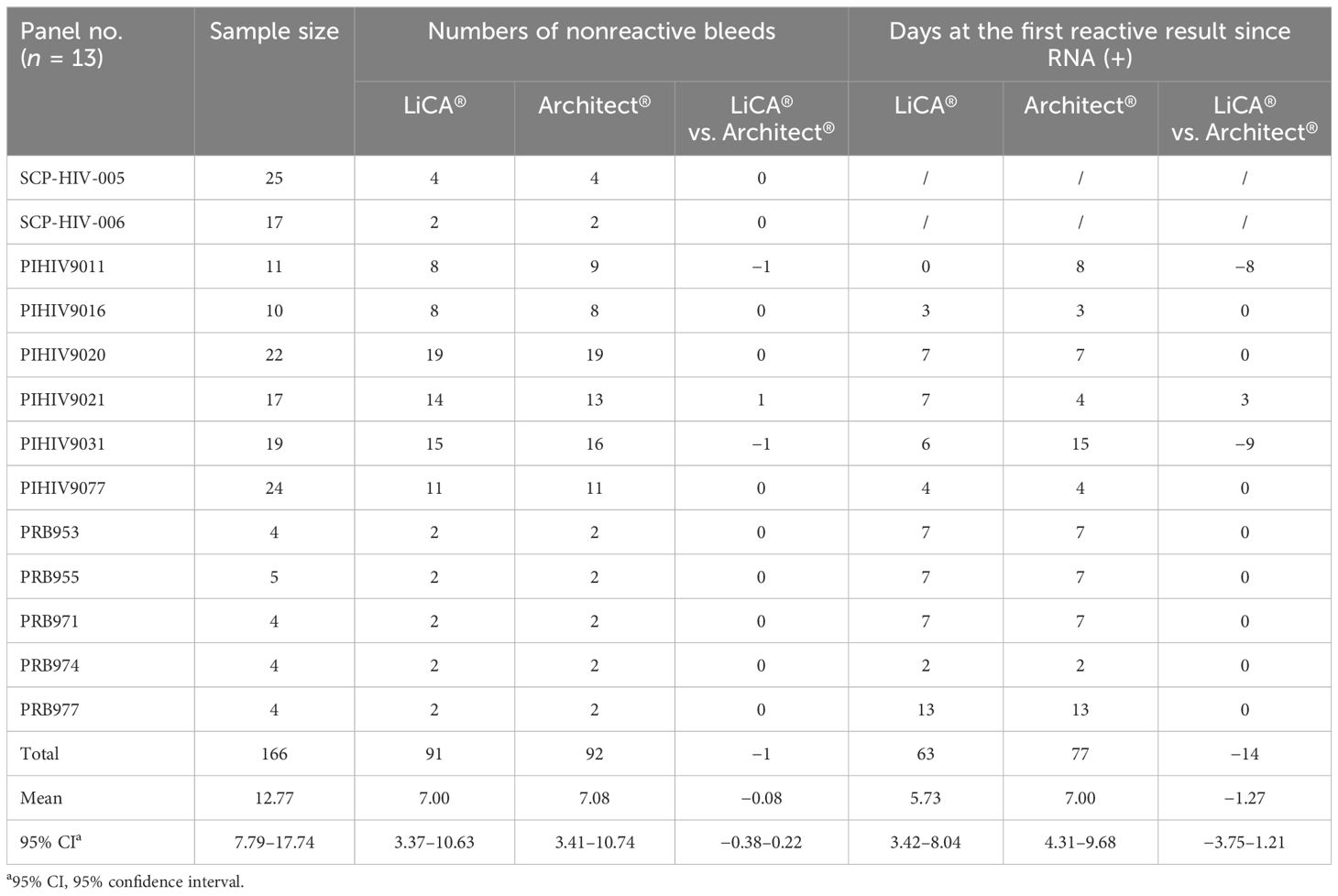
We used 74 banked clinical samples with identified HIV subtypes, 79 HIV-positive patient sera with a low level of S/Co in 1.0–35.0, and 13 commercial seroconversion panels for comparative detection between LiCA® and Architect® HIV Ag/Ab assays. Seroconversion panels were purchased from BioMex (n = 2, SCP-HIV 005–006, Heidelberg, Germany), ZeptoMetrix (n = 6, PIHIV 9011–9077, Franklin, MA, USA), and SeraCare (n = 5, PRB 953–977, Milford, MA, USA).
2.5 Potential interference and cross-reactivity
To evaluate potential interferences from bilirubin, triglycerides, hemoglobin, and biotin, two patient sera (baseline S/Co 0.72 and 5.68) were used as the diluent to prepare a pool of samples with a serial concentration of each interferent, respectively. The cross-reactivity study was performed using 169 serum specimens free of HIV but positive for potential interferents, such as auto-antibodies and other viral infection. All samples were tested in duplicate. A significant interference was considered when the recovery change of mean S/Co was ≥15% in the sample with a baseline S/Co >0.8 or a reactive result was recorded in the sample with a baseline S/Co <0.8.
2.6 Statistics
Statistical analyses were conducted using MedCalc (MedCalc Software, Mariakerke, Belgium) and Excel (Microsoft, WA, USA). Agreement between LiCA® and Architect® was analyzed based on the screening-reactive (S/Co ≥1.0) or -nonreactive (S/Co <1.0) assay results. Specifically, using the HIV Ag/Ab test kit, HIV-negative samples, HIV p24 antigen-positive samples, and HIV antibody-positive samples were tested. Through the ROC curve, with the maximum Youden index as the criterion, the optimal cutoff signal values corresponding to HIV p24 antigen and HIV antibody were obtained, respectively. The ratio of the sample detection signal value to the cutoff signal value was defined as S/Co. Since the signal value corresponding to CO represents the optimal threshold for distinguishing between negative and positive results, the S/Co value of 1 was used as the cutoff for this distinction.
Diagnostic performance parameters, such as sensitivity, specificity, negative predictive value (NPV), and positive predictive value (PPV) were determined with confirmatory results by WB and RNA tests. The t-test was used to evaluate the significant difference between paired samples. p-value <0.05 was considered as statistically significant.
3 Results
3.1 Precision analysis
A precision study on S/Co ratios presented that the assay CVs for repeatability and within-lab imprecision were 2.49%–5.11% on patient sera, 2.93%–5.05% on p24 antigen controls, and 3.73%–6.56% on anti-HIV controls (Table 1). The C50 target and the C5–C95 interval away from C50 were 0.99 S/Co and −10.20%–7.67% for p24 antigen and 1.01 S/Co and −8.79%–5.64% for HIV antibodies, respectively (Supplementary Figure 1).
3.2 Detection capability
Using China national reference control panels (Table 2), LiCA® presented nonreactive results in all negative controls for both HIV-1 p24 antigen and HIV antibodies. The mean S/Co ratios with 95% confidence interval (95% CI) were 0.33 (0.31–0.34) for p24 and 0.23 (0.19–0.27) for antibodies, respectively. All positive controls for p24 were detected to be reactive with a mean S/Co of 31.45 (19.03–43.89). One positive control for the antibody of HIV-1 group O (n = 3) was undetected (S/Co = 0.32). All other positive controls for HIV antibodies were measured with reactive S/Co ratios at a mean of 55.30 (22.84–87.76). The LoD for p24 antigen was identified to be ≤1.25 IU/mL. All positive controls for LoDs to B/B′, BC, and AE genotypes were recorded with reactive S/Co values at a mean of 11.66 (4.80–18.53).
To further clarify the assay LoD to p24 antigen, linear regression analyses were performed between the low range (0–10 IU/mL) of p24 antigen concentrations (Y) and assay S/Co values (X). For the NIFDC reference material, the regression equation was Y = 1.091X − 0.365 (R = 0.999) and LoD was calculated to be 0.73 IU/mL. For the WHO international standard, the equation was Y = 2.908X − 2.008 (R = 0.999) and LoD was assessed to be 0.90 IU/mL (Supplementary Table 1).
3.3 Detection of seroconversion panels and HIV subtypes
Thirteen seroconversion panels were measured to evaluate early detection of HIV (Table 3). Among them, LiCA® presented 2/13 with earlier, 1/13 with later, and 10/13 with equal detections in comparison to Architect®. In general, LiCA® detected the panels with an average of 5.73 (95% CI, 3.42–8.04) days at the first reactive result since the RNA-positive detection, which had a mean of −1.27 (−3.75 to 1.21) days earlier than Architect®. The relative sensitivity coefficient was −0.08 (−0.38 to 0.22).
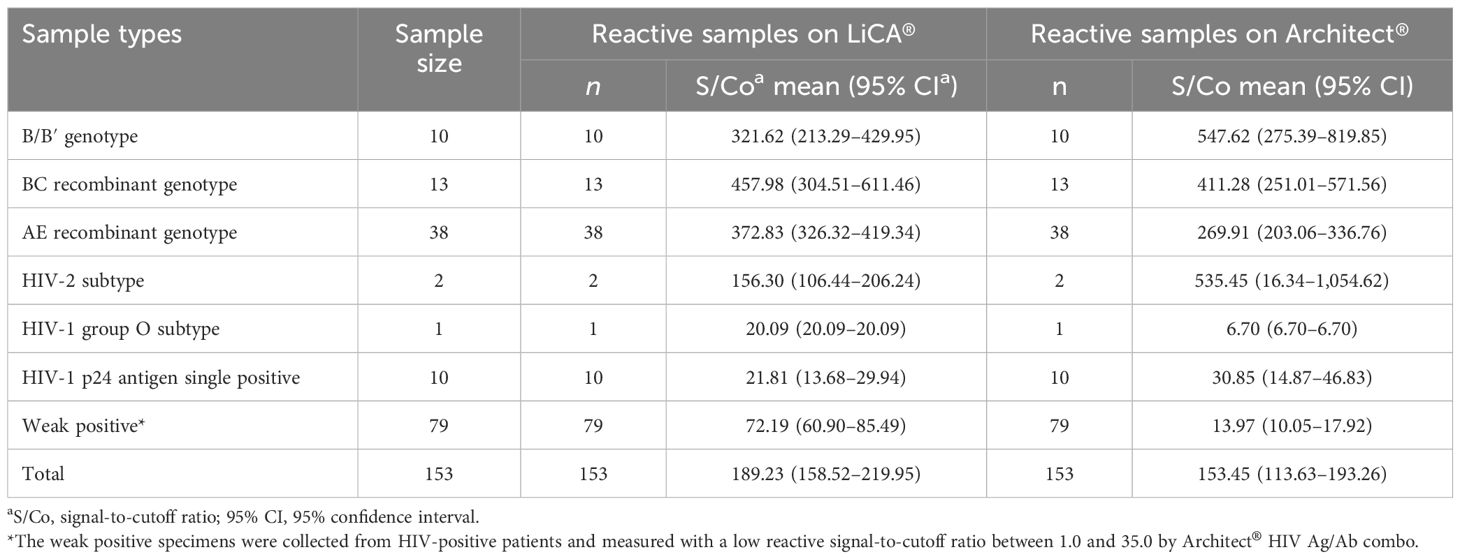
Detection of various HIV subtypes in clinical patient sera was evaluated with 64 specimens containing HIV antibodies to different types of HIV subtypes, 10 p24 antigen single positive samples, and 79 weak positive cases that were collected from HIV-confirmed patients and measured with low reactive S/Co values (1.0–35.0) by the Architect® HIV Ag/Ab combo assay. Both LiCA® and Architect® successfully recorded reactive results for all subjects studied (Table 4).
3.4 Cross-reactivity and interference
The assay recovery changes were determined to be −4.01%–4.76%, −4.91%–4.74%, −2.62%–4.79%, and −2.15%–6.98% on LiCA® by spiking potential interferents (up to 342.08 µmol/L bilirubin, 33.90 mmol/L triglycerides, 5.00 g/L hemoglobin, and 102.25 nmol/L biotin) into both low and high levels of specimens (baseline S/Co 0.72 and 5.68), respectively. In all 169 samples free of HIV but positive for potential interfering factors such as auto-antibodies, other viral infections, and multiple pregnancies, no test-reactive results were observed on both LiCA® and Architect® (Supplementary Table 2).
3.5 Comparison of diagnostic performance between LiCA® and Architect®
Among 21,042 clinical patient sera recruited, 283 (1.34%) were confirmed to be HIV-positive and 20,759 (98.66%) were HIV-negative (Figure 2). Compared to Architect®, LiCA® presented a slightly better but not significantly different (p > 0.05) performance in sensitivity (100.00% vs. 99.65%), specificity (99.85% vs. 99.81%), NPV (100.00% vs. 99.99%), PPV (89.84% vs. 87.85%), and overall accuracy (99.85% vs. 99.81%) for the diagnosis of HIV infection (Table 5).
With further analysis of the segmented S/Co values (Table 6), we found that LiCA® detected true-positive results with a portion of 10.34% (n = 29), 60.00% (n = 10), 88.89% (n = 9), 94.74% (n = 19) and 100% (n = 248), and 89.84% (n = 315) in an S/Co range of 1.00–4.99, 5.00–9.99, 10.00–29.99, 50.00–99.99 and ≥100.00, and in overall reactive S/Co ratios (≥1.00), respectively. In contrast, the corresponding true-positive detection portions on Architect® were 7.41% (n = 32), 44.44% (n = 9), 89.47% (n = 19), 94.29% (n = 35) and 100% (n = 226), and 87.85% (n = 321), respectively. One case, which was confirmed to be HIV-positive, was misdetected on Architect® (S/Co = 0.59) but strongly reactive on LiCA® (S/Co = 64.14).
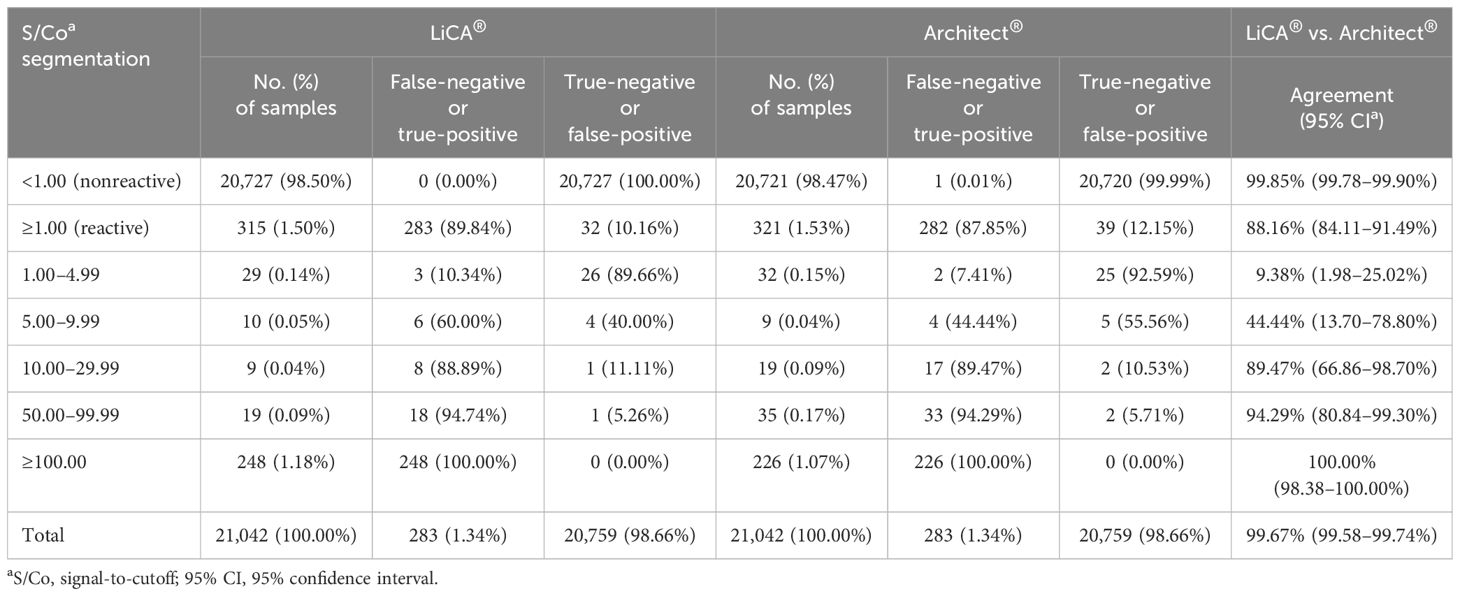
Table 6. Comparisons between LiCA® and Architect® HIV Ag/Ab assays in patient serum samples (n = 21,042).
3.6 Agreement between LiCA® and Architect®
Paired comparisons demonstrated that the overall agreement between LiCA® and Architect® was 99.67% (95% CI, 99.58–99.74%, n = 21,042) and the Cohen’s kappa was 0.89 (0.86–0.91). Agreements in nonreactive and reactive assays were 99.85% (99.78%–99.90%, n = 20,721) and 88.16% (84.11%–91.49%, n = 321), respectively (Table 6). The S/Co segmentation analysis for reactive results revealed that more detailed agreements in a range of 1.00–4.99, 5.00–9.99, 10.00–29.99, 50.00–99.99, and ≥100.00 were 9.38% (n = 32), 44.44% (n = 9), 89.47% (n = 19), 94.29% (n = 35), and 100% (n = 226), respectively. There were 70 (0.33%, n = 21,042) discrepant results between these two assays (Table 7). Architect® contributed 38 (54.29%) false-positive subjects and 1 (1.43%) false-negative subject. The remaining 31 (44.28%) cases had false-positive reactivity on LiCA®. Among these 70 discrepancies, 63 (90.00%) were identified as having false-positive reactivity in a low range of S/Co values (<10.00) either from Architect® (34/63, 53.97%) or from LiCA® (29/63, 46.03%).
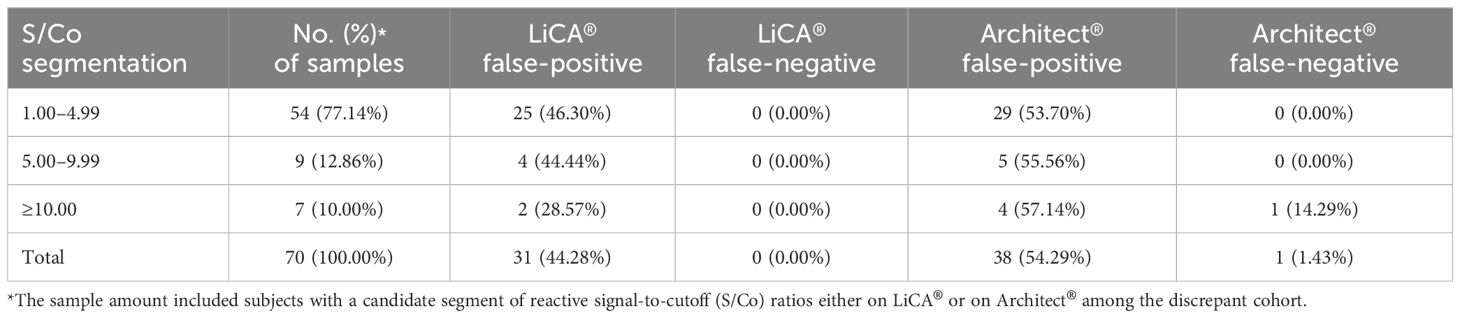
Table 7. Analysis of discrepant assays between LiCA® and Architect® HIV Ag/Ab combo in patient serum samples (n = 70).
4 Discussion
The screening test to HIV Ag/Ab is the initial step for the diagnosis of HIV infection (Centers for Disease Control and Prevention, 2018; Branson, 2019). The viral genetic diversity and geographic distribution change of the variants remain a great challenge to the detection of early HIV infection (Gaudy et al., 2004; Pyne et al., 2013; Xiao et al., 2017). Therefore, higher sensitivity and higher detection capability to various HIV subtypes are essential to the HIV screening assay. The current study included HIV-positive cohorts, with HIV-1 p24 antigen, group O and M, genotypes B/B′, BC, and AE, HIV-2, and low S/Co reactivity (1.0–35.0), to evaluate the sensitivity and detection capability of the LiCA® HIV Ag/Ab assay. LiCA® successfully detected all subtypes of HIV and weak positive samples were recruited. Using the national reference material and WHO international standard for p24 antigen, the LoD of LiCA® was estimated to be 0.73 and 0.90 IU/mL, respectively. These data are favorably comparable to other counterpart assays such as Architect® HIV Ag/Ab combo (LoD = 0.94–1.03 IU/mL), Elecsys® HIV combi PT (LoD = 1.05–1.10 IU/mL), and Centaur® HIV Ag/Ab combo (LoD = 1.89–1.90 IU/mL) (Ly et al., 2012; Muhlbacher et al., 2013).
Furthermore, the high sensitivity of LiCA® is explained with excellent detection of seroconversion panels in comparison to Architect®. In 13 panels tested on both assays, LiCA® presented an average of 5.73 (3.42–8.04) days at the first reactive detection after positive RNA and detected more positive samples with a of mean −1.27 (−3.75–1.21) days earlier than Architect®.
Paired comparisons in 21,042 clinical patient samples revealed that LiCA® detected HIV Ag/Ab with a slightly better performance in sensitivity (100.00% vs. 99.65%), specificity (99.85% vs. 99.81%), NPV (100.00% vs. 99.99%), and PPV (89.84% vs. 87.85%) than Architect®. Excellent assay concordance was observed in nonreactive results (99.85%) but decreased agreement occurred in reactive measurements (88.16%). Most discrepancies (90.00%) primarily resulted from false-positive assays in a low level of S/Co reactivity (<10.00). Previous studies have demonstrated that the Architect® HIV Ag/Ab combo assay yielded a high rate of false-positive results, especially in S/Co values <30.00 (Alonso et al., 2018; Wang et al., 2019). The false reactivity can be generated due to non-specific binding to the immune complex in the Ag–Ab combination assay (Mahajan et al., 2010). The testing discrepancies in the same cohort most likely result from the different Ag/Ab configuration in different assays (Stickle et al., 2002). Notably, there was one subject that was misdetected on Architect® (S/Co = 0.59) but strongly reactive on LiCA® (S/Co = 64.14). This case was from an AIDS inpatient who was at the late stage of ART during our study. The missed detection of Architect® could be explained by the less sensitivity to certain subtypes of HIV (Ly et al., 2012) or the influence on the assay due to viral mutation after treatment (Zuo et al., 2020).
It has been reported that false-positive HIV Ag/Ab screening tests can be caused by other viral infections, autoimmune diseases, and multiple pregnancies (Mahajan et al., 2010; Liu et al., 2016; Adhikari et al., 2018). Our study indicated that no significant cross-reactivity or interference was observed from any of 15 potential interference factors assessed for the LiCA® assay, including auto-antibodies, viral infections such as Epstein–Barr virus and hepatitis viruses, multiple pregnancies such as different stages of normal pregnancy and co-infection pregnancies, and various endogenous interferents. The essence of high specificity and high sensitivity can be attributed to the unique methodology and light-initiated multi-amplification signaling mechanism for the LiCA® assay (Li et al., 2023; Yu et al., 2023).
Combining HIV-1 p24 antigen with antibodies classifies the HIV screening test from the third-generation to the fourth-generation method, which enables the assay to achieve higher sensitivity, reducing the test-negative window to 8–14 days from approximately 3 weeks (Alexander, 2016; Qiu et al., 2017). However, the fourth-generation assay integrates the Ag/Ab reactivity together and can only report a single result in combination of the Ag/Ab S/Co values. In contrast, LiCA® performs immunoassays for detecting HIV p24 antigen and antibodies in two independent cuvettes and separates the S/Co value of p24 antigen from antibodies. Differentiation of the Ag/Ab reactivity can easily identify the preclinical infectious patient with the single-positive p24 antigen (Salmona et al., 2014) and thus facilitate the subsequent confirmatory process for early detection of HIV infection (Muhlbacher et al., 2019; Yang et al., 2022).
In this study, most of the HIV-positive samples were collected from the inpatients with AIDS and the outpatients with highly suspicious history. The positive detection rate was 1.34% (n = 21,042). This situation is different from the clinical conditions for screening populations and blood donors, in which the viral prevalence rate can be quite lower (Wang et al., 2019). Another investigation is valuable for further characterization of the assay performance in low prevalence of HIV.
5 Conclusion
LiCA® provides a precise and fully automatic platform for measuring HIV-1 p24 antigen and HIV-1/2 antibodies with high sensitivity and specificity. The assay performance is favorably comparable to the well-established Architect® HIV Ag/Ab combo assay in analytical and clinical perspectives. Additionally, LiCA® HIV Ag/Ab can differentiate the reactivity of p24 antigen from antibodies in a separate S/Co result. It is appropriate for use in the clinical routine test for the early detection of HIV.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Research involving human subjects complied with all relevant national regulation, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by Dongzhimen Hospital, Beijing University of Chinese Medicine (No. 2024DZMEC-363-01).
Author contributions
XY: Writing – review & editing. YW: Writing – review & editing. YJL: Writing – original draft, Writing – review & editing. FJ: Writing – original draft, Writing – review & editing. YHL: Writing – original draft, Writing – review & editing. YL: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1474127/full#supplementary-material
References
Adhikari, E. H., Macias, D., Gaffney, D., White, S., Rogers, V. L., McIntire, D. D., et al. (2018). Diagnostic accuracy of fourth-generation ARCHITECT HIV Ag/Ab Combo assay and utility of signal-to-cutoff ratio to predict false-positive HIV tests in pregnancy. Am. J. Obstet Gynecol 219, 408.e1–408.e9. doi: 10.1016/j.ajog.2018.06.008
Alexander, T. S. (2016). Human immunodeficiency virus diagnostic testing: 30 years of evolution. Clin. Vaccine Immunol. 23, 249–253. doi: 10.1128/CVI.00053-16
Alonso, R., Perez-Garcia, F., Gijon, P., Collazos, A., Bouza, E. (2018). Evaluation of the Architect HIV Ag/Ab Combo Assay in a low-prevalence setting: The role of samples with a low S/CO ratio. J. Clin. Virol. 103, 43–47. doi: 10.1016/j.jcv.2018.04.002
Bian, Y., Liu, C., She, T., Wang, M., Yan, J., Wei, D., et al. (2018). Development of a light-initiated chemiluminescent assay for the quantitation of sIgE against egg white allergens based on component-resolved diagnosis. Anal. Bioanal Chem. 410, 1501–1510. doi: 10.1007/s00216-017-0791-y
Branson, B. M. (2019). HIV diagnostics: current recommendations and opportunities for improvement. Infect. Dis. Clin. North Am. 33, 611–628. doi: 10.1016/j.idc.2019.04.001
Centers for Disease Control and Prevention (2018). 2018 Quick reference guide: recommended laboratory HIV testing algorithm for serum or plasma specimens. Available online at: https://stacks.cdc.gov/view/cdc/50872 (Accessed October 17, 2018).
CLSI (2008). “User protocol for evaluation of qualitative test performance; approved guideline-second edition,” in CLSI document EP12-A2 (Clinical and Laboratory Standards Institute, Wayne, PA).
CLSI (2014). “User verification of precision and estimation of bias; approved guidline-third edition,” in CLSI document EP15-A3 (Clinical and Laboratory Standards Institute, Wayne, PA).
Cohen, M. S., Chen, Y. Q., McCauley, M., Gamble, T., Hosseinipour, M. C., Kumarasamy, N., et al. (2011). Prevention of HIV-1 infection with early antiretroviral therapy. N Engl. J. Med. 365, 493–505. doi: 10.1056/NEJMoa1105243
El-Sadr, W. M., Mayer, K. H., Rabkin, M., Hodder, S. L. (2019). AIDS in America - back in the headlines at long last. N Engl. J. Med. 380, 1985–1987. doi: 10.1056/NEJMp1904113
Fitzgerald, N., Cross, M., O’Shea, S., Fox, J. (2017). Diagnosing acute HlV infection at point of care:a retrospective analysis of the sensitivity andspecificity of a fourth-generation point-of-care testfor detection of HlV core protein p24. Sexually Transmitted Infections 93, 100–101. doi: 10.1136/sextrans-2015-052491
Gaudy, C., Moreau, A., Brunet, S., Descamps, J. M., Deleplanque, P., Brand, D., et al. (2004). Subtype B human immunodeficiency virus (HIV) type 1 mutant that escapes detection in a fourth-generation immunoassay for HIV infection. J. Clin. Microbiol. 42, 2847–2849. doi: 10.1128/JCM.42.6.2847-2849.2004
Gray, E. R., Bain, R., Varsaneux, O., Peeling, R. W., Stevens, M. M., McKendry, R. A. (2018). p24 revisited: a landscape review of antigen detection for early HIV diagnosis. AIDS 32, 2089–2102. doi: 10.1097/QAD.0000000000001982
Li, H., Yang, S., Cao, D., Wang, Q., Zhang, S., Zhou, Y., et al. (2023). A new double-antigen sandwich test based on the light-initiated chemiluminescent assay for detecting anti-hepatitis C virus antibodies with high sensitivity and specificity. Front. Cell Infect. Microbiol. 13, 1222778. doi: 10.3389/fcimb.2023.1222778
Li, Z., Yang, S., Qiao, J., Tan, Y., Liu, Q., Yang, B., et al. (2024). Performance evaluation of a novel high-sensitivity cardiac troponin T assay: analytical and clinical perspectives. Clin. Chem. Lab. Med. 62, 979–987. doi: 10.1515/cclm-2023-0789
Liu, P., Jackson, P., Shaw, N., Heysell, S. (2016). Spectrum of false positivity for the fourth generation human immunodeficiency virus diagnostic tests. AIDS Res. Ther. 13, 1. doi: 10.1186/s12981-015-0086-3
Ly, T. D., Plantier, J. C., Leballais, L., Gonzalo, S., Lemee, V., Laperche, S. (2012). The variable sensitivity of HIV Ag/Ab combination assays in the detection of p24Ag according to genotype could compromise the diagnosis of early HIV infection. J. Clin. Virol. 55, 121–127. doi: 10.1016/j.jcv.2012.06.012
Mahajan, V. S., Pace, C. A., Jarolim, P. (2010). Interpretation of HIV serologic testing results. Clin. Chem. 56, 1523–1526. doi: 10.1373/clinchem.2009.139535
Muhlbacher, A., Sauleda, S., Piron, M., Rietz, R., Permpikul, P., Klinkicht, M., et al. (2019). A multicentre evaluation of the Elecsys HIV Duo assay. J. Clin. Virol. 112, 45–50. doi: 10.1016/j.jcv.2018.11.005
Muhlbacher, A., Schennach, H., van Helden, J., Hebell, T., Pantaleo, G., Burgisser, P., et al. (2013). Performance evaluation of a new fourth-generation HIV combination antigen-antibody assay. Med. Microbiol. Immunol. 202, 77–86.
Pyne, M. T., Hackett, J., Jr., Holzmayer, V., Hillyard, D. R. (2013). Large-scale analysis of the prevalence and geographic distribution of HIV-1 non-B variants in the United States. J. Clin. Microbiol. 51, 2662–2669. doi: 10.1128/JCM.00880-13
Qiu, X., Sokoll, L., Yip, P., Elliott, D. J., Dua, R., Mohr, P., et al. (2017). Comparative evaluation of three FDA-approved HIV Ag/Ab combination tests using a genetically diverse HIV panel and diagnostic specimens. J. Clin. Virol. 92, 62–68. doi: 10.1016/j.jcv.2017.05.005
Rodger, A. J., Cambiano, V., Bruun, T., Vernazza, P., Collins, S., Degen, O., et al. (2019). Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): final results of a multicentre, prospective, observational study. Lancet 393, 2428–2438. doi: 10.1016/S0140-6736(19)30418-0
Salmona, M., Delarue, S., Delaugerre, C., Simon, F., Maylin, S. (2014). Clinical evaluation of BioPlex 2200 HIV Ag-Ab, an automated screening method providing discrete detection of HIV-1 p24 antigen, HIV-1 antibody, and HIV-2 antibody. J. Clin. Microbiol. 52, 103–107. doi: 10.1128/JCM.02460-13
Stickle, D. F., Pirruccello, S. J., Swindells, S., Hinrichs, S. H. (2002). Discrepant results of 2 screening tests for anti-HIV antibody. Clin. Infect. Dis. 35, 773–4;author reply 774-5. doi: 10.1086/cid.2002.35.issue-6
UNAIDS (2019). 2018 Global HIV & AIDS statistics-fact sheet. Available online at: https://www.unaids.org/en/resources/fact-sheet (Accessed Febuary 13, 2019).
Volberding, P. A., Deeks, S. G. (2010). Antiretroviral therapy and management of HIV infection. Lancet 376, 49–62. doi: 10.1016/S0140-6736(10)60676-9
Wang, M., Li, J., Huang, Y., Chen, T., Dong, S., Zhang, R., et al. (2022). Analytical validation of the LiCA high-sensitivity human thyroid stimulating hormone assay. Clin. Biochem. 101, 42–49. doi: 10.1016/j.clinbiochem.2021.11.018
Wang, L., Xiao, Y., Tian, X. D., Ruan, J. X., Chen, W., Yu, Y. (2019). HIV infection in Xi’an, China: epidemic characterization, risk factors to false positives and potential utility of the sample-to-cutoff index to identify true positives using Architect HIV Ag/Ab combo. Antimicrob. Resist. Infect. Control 8, 9. doi: 10.1186/s13756-019-0463-0
Xiao, P., Li, J., Fu, G., Zhou, Y., Huan, X., Yang, H. (2017). Geographic distribution and temporal trends of HIV-1 subtypes through heterosexual transmission in China: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 14 (7), 830. doi: 10.3390/ijerph14070830
Yang, M., Yang, W., Shi, W., Tao, C. (2022). Clinical application evaluation of elecsys((R)) HIV duo assay in southwest China. Front. Cell Infect. Microbiol. 12, 877643. doi: 10.3389/fcimb.2022.877643
Yang, S., Zhang, Q., Yang, B., Li, Z., Sun, W., Cui, L. (2022). Analytical and clinical performance evaluation of a new high-sensitivity cardiac troponin I assay. Clin. Chem. Lab. Med. 60, 1299–1307. doi: 10.1515/cclm-2021-1136
Yu, M., Chen, D., Tang, X., Zhang, Y., Liang, P., Xiong, Y., et al. (2023). Evaluation of a high-sensitivity SARS-CoV-2 antigen test on the fully automated light-initiated chemiluminescent immunoassay platform. Clin. Chem. Lab. Med. 61, 1123–1130. doi: 10.1515/cclm-2022-1039
Yu, Y., She, T., Huang, L., Xu, J., Yan, J., Jiang, Q., et al. (2021). Establishment of a homogeneous immunoassay-light-initiated chemiluminescence assay for detecting anti-Mullerian hormone in human serum. J. Immunol. Methods 494, 113059. doi: 10.1016/j.jim.2021.113059
Keywords: human immunodeficiency virus, light-initiated chemiluminescent assay, homogeneous immunoassay, performance evaluation, LiCA®
Citation: Li Y, Jin F, Li Y, Li Y, Wang Y and Yang X (2025) Evaluation of a new human immunodeficiency virus antigen and antibody test using light-initiated chemiluminescent assay. Front. Cell. Infect. Microbiol. 15:1474127. doi: 10.3389/fcimb.2025.1474127
Received: 01 August 2024; Accepted: 06 January 2025;
Published: 31 January 2025.
Edited by:
Kamal El Bissati, The University of Chicago, United StatesReviewed by:
Haifei Jiang, Mayo Clinic, United StatesVictor Manuel Luna-Pineda, Hospital Infantil de México Federico Gómez, Mexico
Copyright © 2025 Li, Jin, Li, Li, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ximing Yang, ZHptbXlzQDE2My5jb20=; Yajie Wang, d2FuZ3lhamllQGNjbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Yijun Li1†
Yijun Li1† Yan Li
Yan Li Yajie Wang
Yajie Wang Ximing Yang
Ximing Yang