
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Cell. Infect. Microbiol., 05 March 2025
Sec. Antibiotic Resistance and New Antimicrobial drugs
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1456046
This article is part of the Research TopicEmerging Leaders in Antibiotic Resistance: Pioneering Research and Future DirectionsView all 10 articles
There is increasing demand for novel antimicrobial agents to tackle the antimicrobial resistance crisis. Here we report that two Enterobacteriaceae-produced siderophores, enterobactin and salmochelin S4, inhibit the growth of Staphylococcus aureus isolates, including methicillin-resistance S. aureus (MRSA) clinical isolates. The IC50 for different S. aureus isolates were 2-5 µM for salmochelin S4 and 5-10 µM for enterobactin. This inhibitory activity was partially repressed by adding Fe+3. These siderophores also inhibited the growth of Enterococcus strains, including vancomycin-resistant enterococci (VRE) clinical isolates, though less effectively than for S. aureus. The growth of various Gram-negative bacteria was barely affected by these siderophores. These results shed new light on the role of enterobactin and salmochelin in bacterial physiology and ecology and have potential for the development of novel strategies to combat the rapid rise of multidrug-resistant bacteria.
New antimicrobial molecules, especially with innovative modes of action, are urgently needed to tackle the antimicrobial resistance crisis (Miethke et al., 2021). The World Health Organization (WHO) classified antimicrobial resistance as one of the top-10 global health threats faced by humanity (https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance). Staphylococcus aureus is among the leading pathogens that accounts for the mortality rate associated with drug resistance, in particular by methicillin-resistant S. aureus (MRSA) strains (Antimicrobial Resistance, 2022). Vancomycin-resistant enterococci (VRE) are also Gram-positive resistant pathogens. MRSA and VRE are both ESKAPE pathogens which represent a global threat to human health and have been given high priority in efforts to develop new antibiotics (De Oliveira et al., 2020; Mancuso et al., 2021; Antimicrobial Resistance, 2022). The emergence of antibiotic-resistant strains has also been accelerated by the almost complete lack of new classes of clinically relevant antibiotics in the last few decades (Antimicrobial Resistance, 2022; Muteeb et al., 2023).
Iron is a mandatory nutrient for the growth of most bacteria due to its essential roles in several biological processes. However, its bioavailability is limited by the low solubility of ferric iron (Fe+³) at physiological pH levels (Abbaspour et al., 2014). Although the human body contains significant amounts of iron, its acquisition by pathogens is hindered by transport and storage proteins. During infection, the host’s innate immune system further restricts iron availability to pathogens through a process which involves reducing intestinal iron absorption and increasing the activity of neutrophils at infection sites. Neutrophils contribute to iron deprivation by enhancing the production of proteins like ferritin and lactoferrin, which sequester iron, as well as siderocalins, which bind and neutralize bacterial siderophores that would otherwise capture iron for bacterial use (Cassat and Skaar, 2013; Nairz et al., 2014; Marchetti et al., 2020; Ullah and Lang, 2023). However, microorganisms overcome this problem by developing highly efficient uptake systems for using the iron present in the host through low-molecular weight organic chelators (150 to 2000 Da) called siderophores. These metabolites are synthesized by bacteria and released into the environment, where they chelate iron with an extremely high affinity (Johnstone and Nolan, 2015; Page, 2019; Kramer et al., 2020). Since iron uptake is essential to bacterial pathogenesis, siderophore iron uptake pathways are useful gates for antibiotic treatment using Trojan horse delivery strategies (Johnstone and Nolan, 2015; Page, 2019; Kramer et al., 2020). The tris-catecholate siderophore enterobactin is an archetype of iron acquisition in Gram-negative bacteria. It has the highest affinity for ferric iron of all natural siderophore compounds and is produced by most members of Enterobacteriaceae and a few other bacteria (Raymond et al., 2003). Salmochelin is a C-glucosylated enterobactin which enable it to evade the host’s defense protein lipocalin-2, an enterobactin scavenger. Salmochelins are produced by some Salmonella, Escherichia coli and Klebsiella strains (Muller et al., 2009). Salmochelin S4 is a C5,C5’ diglucosylated enterobactin and is the key compound for the production of other salmochelins (Bister et al., 2004). The ferric complex of enterobactin binds to the specific outer membrane receptor FepA, whereas ferric salmochelin binds to the IroN receptor (which is also capable of binding ferric enterobactin) (Hantke et al., 2003). The extensive research on these siderophores, their high affinity, and the ability of a variety of Gram-negative bacteria to utilize them, make them a preferred target for the conjugation of known antibiotics (sideromycins), exploiting a Trojan horse delivery strategy (Mollmann et al., 2009; Johnstone and Nolan, 2015; Page, 2019). The Trojan horse method involves using the bacterial iron uptake system to transport antibiotics into cells that would typically be impermeable to these drugs. In this study, we show that these two siderophores (salmochelin and enterobactin), unexpectedly inhibit the growth of S. aureus (including MRSA clinical isolates).
Iron-free enterobactin was purchased from Sigma-Aldrich (E3910) and from EMC (Tübingen, Germany). Iron-free salmochelin S4 was purchased from EMC (Tübingen, Germany). Iron (III) chloride was purchased from Sigma-Aldrich, catalog 157740 – 100G. Cation-Adjusted Mueller-Hinton Broth (CAMHB) was purchased from BD-BBL (catalog 212322, Mueller-Hinton II Broth). Lincomycin hydrochloride was purchased from bioWORLD, Linezolid was purchased from Sigma-Aldrich (PZ0014).
The bacterial strains were kindly provided by the Clinical Microbiology Laboratory at Sheba Medical Center.
Antibacterial activity was determined using the broth microdilution method. The inhibitory effect was measured using broth microdilution based on Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2022), in a 96-well microtiter plate, with CAMHB, and the Tecan GENios plate reader at optical density (OD) at 590nm for 18-20 h, at 37 ± 1°C. The final bacterial inoculum was 5 x 105 colony-forming units ml-1. IC50 was defined as the lowest concentration that inhibited the bacterial growth to 50% of the control OD after 18 hours of incubation. Enterobactin and salmochelin were dissolved in 100% dimethylsulfoxide (DMSO) in a stock solution of 10 mM and kept at -20°C. The stock solutions were further diluted in double-distilled water (DDW) or directly using CAMHB to the final concentrations.
Enterobactin and salmochelin S4 effectively inhibited the growth of S. aureus ATCC 25923 in a dose-dependent manner (Figure 1). All the tested S. aureus strains were inhibited by these two siderophores with IC50 of 2-5 µM (2-5 µg/ml) for salmochelin S4 and 5-10 µM (3.3-6.7 µg/ml) for enterobactin (Table 1). Salmochelin S4 was two- to four- fold more potent than enterobactin. The growth inhibition by the two siderophores in rich media (CAMHB) at 37°C was detected after 4-5 hours of incubation and the effect was maintained for about 20 hours (Figure 1). Interestingly, a low concentration of salmochelin S4 (≤ 1.25 µM for strain ATCC 25923) or enterobactin (≤ 2.5 µM for strain ATCC 25923) enhanced the growth of the bacteria. The combination of salmochelin S4 and enterobactin displayed enhanced activity against S. aureus at concentrations as low as 0.5 µM for salmochelin S4 and 4 µM enterobactin as depicted in Figure 2.
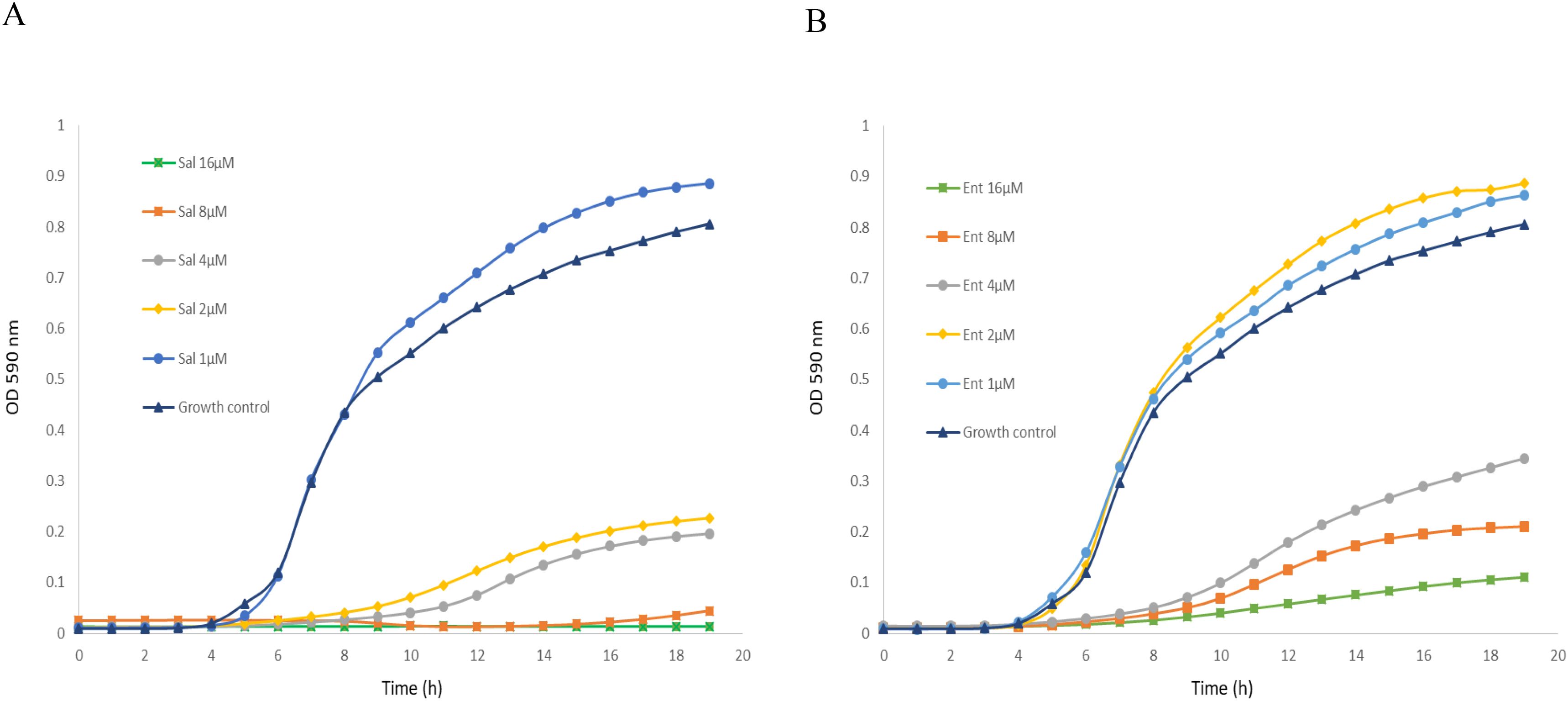
Figure 1. Inhibitory activity of salmochelin S4 (A) and enterobactin (B) on the growth of S. aureus strain ATCC 25923. Sal – salmochelin S4, Ent - enterobactin, Growth control - without siderophore. Inhibitory effect was measured using broth microdilution with CAMHB, and at optical density at 590nm for 18-20 h, at 37 ± 1°C. The initial bacterial inoculum was 5 x 105 colony-forming units ml-1. Figure 1 is a representative of three experiments.
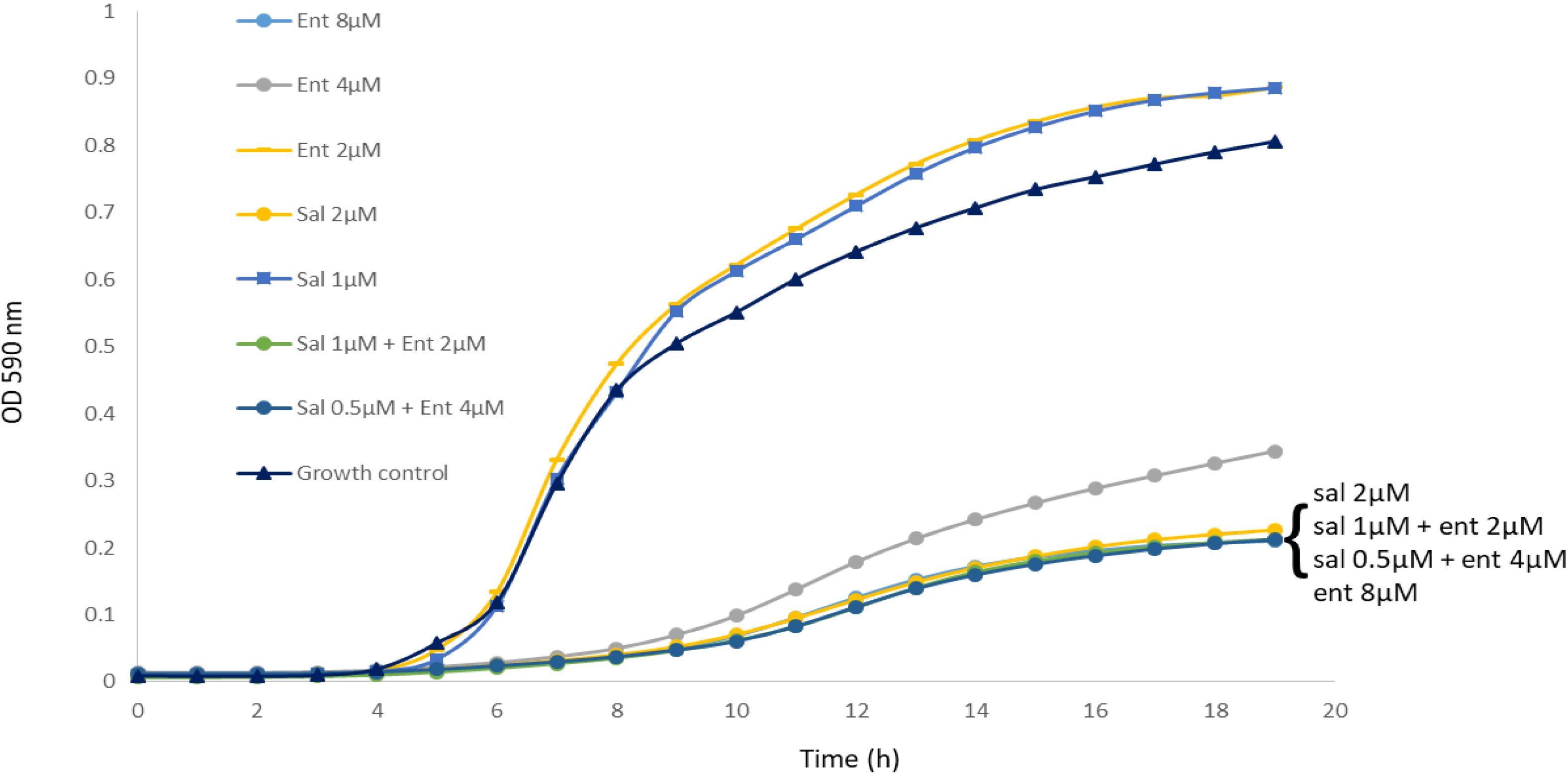
Figure 2. The combination of salmochelin S4 and enterobactin enhanced the inhibition of S. aureus strain ATCC 25923 growth. Sal - salmochelin S4, Ent - enterobactin, Growth control - without siderophore. Inhibitory effect was measured using broth microdilution with CAMHB, and at optical density at 590nm for 18-20 h, at 37 ± 1°C. The initial bacterial inoculum was 5 x 105 colony-forming units ml-1. Figure 2 is a representative of three experiments.
Next, combinations of siderophores and several antibiotics were tested. Lincomycin showed an additive effect to salmochelin S4 and enterobactin, whereas linezolid had an antagonistic effect to salmochelin S4 (Supplementary Figure S1). These effects were also found for MRSA USA300 strain 742 (data not shown).
The inhibitory activity of enterobactin and salmochelin S4 was further tested on other Gram-positive and Gram-negative bacteria. Enterococcus strains, including vancomycin-resistant enterococci (VRE) clinical isolates were inhibited though at higher concentrations than for S. aureus (Supplementary Figure S2). Gram-negative bacteria were not affected (Klebsiella pneumonia, Acinetobacter baumannii) or only slightly affected (Escherichia coli, Pseudomonas aeruginosa) at siderophore concentrations of up to 20 µM (Supplementary Figure S3).
We also tested siderophore activity with addition of different concentration of Fe+3. The addition of Fe+3 reduced the growth inhibition effect of the siderophores (Figure 3). For example, the addition of 20µM Fe+3 to 5 µM of salmochelin S4 significantly reduced its growth inhibitory effect, whereas 10µM Fe+3 only had a slight effect (Figure 3A). The addition of 10-80µM Fe+3 without the siderophore slightly enhanced the growth of the bacteria (not shown). Co-administration of an iso-molar concentration of Fe+3 and the siderophores generally only had a slight effect on the inhibitory activity (Figures 3B, C).
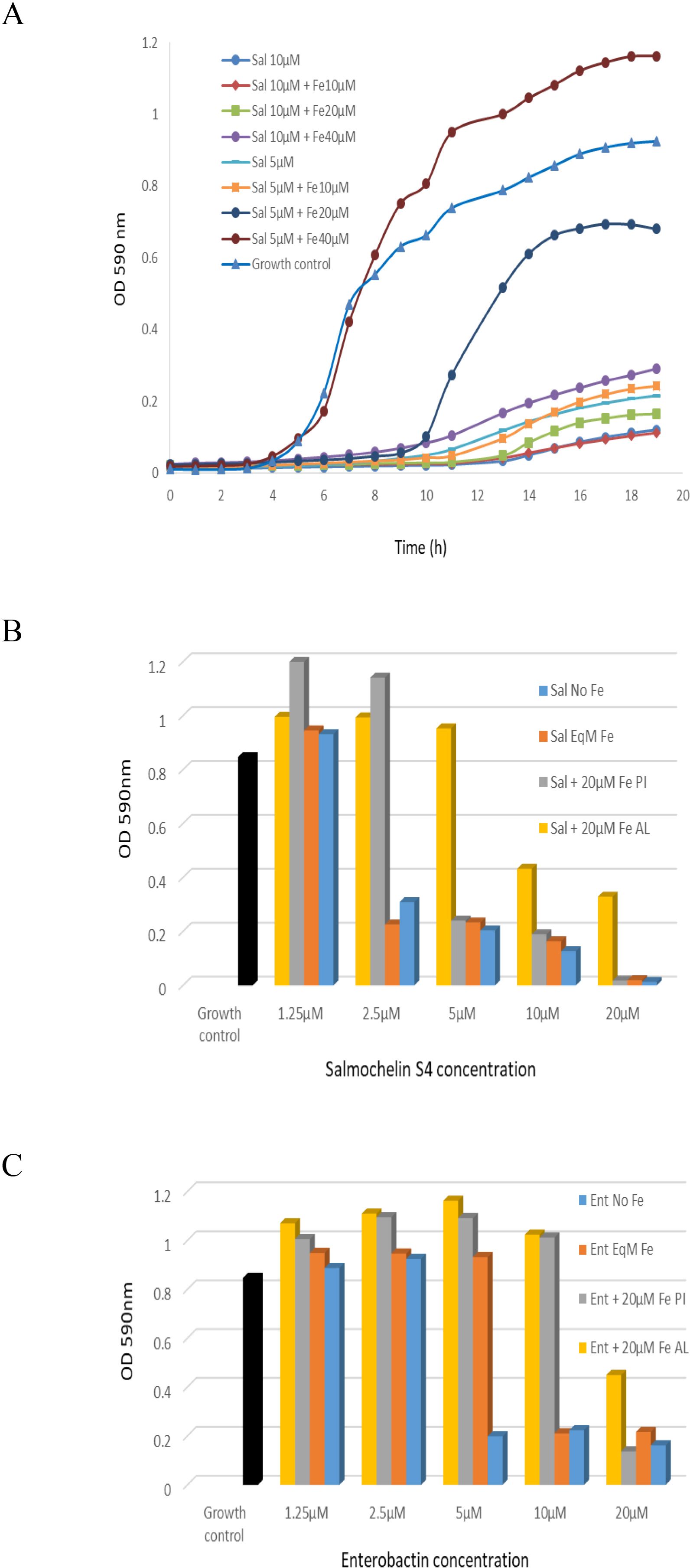
Figure 3. The inhibitory effect of salmochelin S4 and enterobactin with the addition of Fe+3 on the growth of S. aureus strain ATCC 25923. (A) Growth curve with the addition of 5 or 10 µM salmochelin S4 and 10, 20 or 40 µM of Fe+3. OD after 18 hours incubation with salmochelin S4 (B) or enterobactin (C). Growth control – without siderophore. Sal – salmochelin S4, Ent - enterobactin, Sal/Ent No Fe - siderophore without addition of Fe+3, Sal/Ent EqM Fe - Iso-molar concentration of the siderophore and Fe+3, Fe PI – 20µM Fe+3 pre-incubated with the siderophores for 30 minutes, Fe AL - 20µM Fe+3 added after pre-incubation of the siderophores with the bacteria for 30 minutes. Inhibitory effect was measured using optical density at 590nm, at 37 ± 1°C. The initial bacterial inoculum was 5 x 105 colony-forming units ml-1. This figure is representative of two independent experiments.
We then tested siderophore activity with addition of Fe+3 at different stages. The addition of 20µM Fe+3 when pre-incubated for 30 minutes with different concentrations of siderophores only had a slight effect on high concentrations of the siderophores (salmochelin ≥ 5 µM, enterobactin = 20 µM, for strain ATCC 25923), but a significant effect on lower siderophore concentrations (Figures 3B, C). When the bacteria were first incubated for 30 minutes with the siderophores and 20 µM Fe+3 were added later, the rescue effect of the iron was higher; i.e., there was less inhibition of the siderophore (Figures 3B, C). Similar results were observed for MRSA USA300 strain 742 (not shown).
The results demonstrate that both enterobactin and salmochelin effectively inhibited the growth of S. aureus, including methicillin-resistant S. aureus (MRSA) isolates. Salmochelin S4 exhibited greater potency than enterobactin, and their combination elicited enhanced activity. By contrast, low concentrations of the siderophores enhanced bacterial growth. Extensive research on enterobactin and salmochelin over many years has contributed to a better understanding of their importance within the bacteria that produce them, and for other organisms including Eukarya (Raymond et al., 2003; Muller et al., 2009; Qi and Han, 2018). The findings here contribute to furthering this field.
Several publications have described the effects of enterobactin and salmochelin on the growth of S. aureus. Although some of these studies have suggested that enterobactin promotes growth (Maskell, 1980), or that growth is promoted by both enterobactin and salmochelin (Beasley et al., 2011; Sebulsky et al., 2000; Sebulsky and Heinrichs, 2001), a recent study found slight growth inhibition by enterobactin for some of the S. aureus strains examined (Uranga et al., 2020). These inconsistencies in the impact on growth may be due to differences in media, iron availability, siderophore concentrations and other experimental conditions. Our findings make it clear that the concentrations of siderophores can either stimulate or suppress the growth of S. aureus.
The current data innovate by showing very effective growth inhibition for the first time of various S. aureus isolates including MRSA, in particular by salmochelin S4. Nolan and colleagues used conjugations of enterobactin and salmochelin S4 to enhance activity and selectivity of β–lactam antibiotics against the pathogens that produce these siderophores. They used co-cultures with S. aureus (ATCC 25923) to demonstrate the selectivity and the negligible effect of the conjugations on non-producers of these siderophores (Zheng and Nolan, 2014; Sargun et al., 2021a, 2021), thus also demonstrating the relatively limited capability of S. aureus to absorb these siderophores (although transport through the Sst system is possible; see below).
S. aureus, like many other bacteria, can activate a variety of mechanisms for iron acquisition that include the secretion of endogenous siderophores, and the ability to use siderophores produced by other bacteria (xenosiderophores) (Beasley et al., 2011; Sheldon and Heinrichs, 2012; Marchetti et al., 2020; van Dijk et al., 2022). Catechol-type xenosiderophores such as salmochelin and enterobactin can be transported into the S. aureus cell through the Sst system (Beasley et al., 2011). The affinity of the substrate binding protein SstD for the ferric enterobactin and ferric salmochelin were found to have a Kd of 0.29 and 0.35 µM, respectively. These affinities are orders of magnitude lower than the affinities of the endogenous S. aureus siderophores to their transporters; e.g., Hts and Sir (Grigg et al., 2010a, 2010; Beasley et al., 2011). This probably reflects a sacrifice in ligand affinity in the name of greater ligand diversity (Beasley et al., 2011; Marchetti et al., 2020). Transport through the Sst system may explain the growth promotion observed when low concentrations of the siderophores are used. CAMHB, the growth medium used here, is not controlled for iron concentration. However, according to Hackel et al. (2019) the medium we used (BD-BBL, catalog number: 212322) contains 4.3µM (0.24 µg/ml) of Fe+3 (Hackel et al., 2019). Our results that the addition of Fe+3 partially represses enterobactin and salmochelin growth inhibition suggest that iron depletion is involved in the inhibition process. This depletion may be the result of a combination of the high affinity of these siderophores to iron, along with the relatively low affinity of the ferric siderophores to the SstD binding protein, and the relatively low capacity of this system to import or utilize ferric xenosiderophores, thus curtailing the availability of iron to other more effective iron acquisition systems. However, the result that Fe+3 addition only reduced but did not eliminate the inhibition effect, and the much stronger effect of salmochelin S4 as compared to enterobactin, may suggest that iron depletion only partially explains the inhibitory modes of action of these siderophores, and salmochelin in particular.
The potential to use iron chelators in combination with existing antibiotics was recently highlighted (Vinuesa and McConnell, 2021). The current findings provide another example of possible combinations. However, as demonstrated here, each combination should be verified independently for its efficiency.
Overall, the findings here show that enterobactin and salmochelin can act as potent inhibitors that suppress the growth of other bacterial species, thus highlighting the dual impact of these iron chelating compounds and shedding light on a novel facet of their role in bacterial physiology and ecology. Mounting evidence suggests that siderophores possess other roles beyond iron acquisition, including antibiotic activity, and can serve as mediators for interactions within microbial communities (Johnstone and Nolan, 2015; Page, 2019; Tejman-Yarden et al., 2019; Kramer et al., 2020). The report of inhibitory activity of enterobactin and salmochelin presented in this study paves the way for exploring their therapeutic applications and highlights the need for further investigation into the intricate interplay between iron acquisition and antimicrobial activity as mediated by these siderophores, including the implementation of in vivo experiments. Many other issues such as the breadth of this antimicrobial activity, its mode/s of action, and why salmochelin is more potent than enterobaction have yet to be discovered.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
YD: Data curation, Writing – original draft, Writing – review & editing. NT-Y: Writing – original draft, Writing – review & editing. AR: Writing – review & editing. GR: Project administration, Supervision, Writing – review & editing. IN: Data curation, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
We would like to extend our gratitude to Dr. Gill Smolen from the Clinical Microbiology Laboratory at Sheba Medical Center, for her invaluable assistance in providing clinical isolates used in this experiment.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1456046/full#supplementary-material
Abbaspour, N., Hurrell, R., Kelishadi, R. (2014). Review on iron and its importance for human health. J. Res. Med. Sci. 19, 164–174.
Antimicrobial Resistance, C. (2022). Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655. doi: 10.1016/S0140-6736(21)02724-0
Beasley, F. C., Marolda, C. L., Cheung, J., Buac, S., Heinrichs, D. E. (2011). Staphylococcus aureus transporters Hts, Sir, and Sst capture iron liberated from human transferrin by Staphyloferrin A, Staphyloferrin B, and catecholamine stress hormones, respectively, and contribute to virulence. Infect. Immun. 79, 2345–2355. doi: 10.1128/IAI.00117-11
Bister, B., Bischoff, D., Nicholson, G. J., Valdebenito, M., Schneider, K., Winkelmann, G., et al. (2004). The structure of salmochelins: C-glucosylated enterobactins of Salmonella enterica. Biometals 17, 471–481. doi: 10.1023/b:biom.0000029432.69418.6a
Cassat, J. E., Skaar, E. P. (2013). Iron in infection and immunity. Cell Host Microbe 13, 509–519. doi: 10.1016/j.chom.2013.04.010
CLSI (2022). Performance Standards for Antimicrobial Susceptibility Testing. CLSI Supplement M100. 32nd edn (Malvern, PA: Clinical and Laboratory Standards Institute).
De Oliveira, D. M. P., Forde, B. M., Kidd, T. J., Harris, P. N. A., Schembri, M. A., Beatson, S. A., et al. (2020). Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 33, 10.1128/cmr.00181-19. doi: 10.1128/CMR.00181-19
Grigg, J. C., Cheung, J., Heinrichs, D. E., Murphy, M. E. (2010a). Specificity of Staphyloferrin B recognition by the SirA receptor from Staphylococcus aureus. J. Biol. Chem. 285, 34579–34588. doi: 10.1074/jbc.M110.172924
Grigg, J. C., Cooper, J. D., Cheung, J., Heinrichs, D. E., Murphy, M. E. (2010b). The Staphylococcus aureus siderophore receptor HtsA undergoes localized conformational changes to enclose staphyloferrin A in an arginine-rich binding pocket. J. Biol. Chem. 285, 11162–11171. doi: 10.1074/jbc.M109.097865
Hackel, M. A., Tsuji, M., Yamano, Y., Echols, R., Karlowsky, J. A., Sahm, D. F. (2019). Reproducibility of broth microdilution MICs for the novel siderophore cephalosporin, cefiderocol, determined using iron-depleted cation-adjusted Mueller-Hinton broth. Diagn. Microbiol. Infect. Dis. 94, 321–325. doi: 10.1016/j.diagmicrobio.2019.03.003
Hantke, K., Nicholson, G., Rabsch, W., Winkelmann, G. (2003). Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc. Natl. Acad. Sci. U.S.A. 100, 3677–3682. doi: 10.1073/pnas.0737682100
Johnstone, T. C., Nolan, E. M. (2015). Beyond iron: non-classical biological functions of bacterial siderophores. Dalton Trans. 44, 6320–6339. doi: 10.1039/c4dt03559c
Kramer, J., Ozkaya, O., Kummerli, R. (2020). Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 18, 152–163. doi: 10.1038/s41579-019-0284-4
Mancuso, G., Midiri, A., Gerace, E., Biondo, C. (2021). Bacterial antibiotic resistance: the most critical pathogens. Pathogens 10, 1310. doi: 10.3390/pathogens10101310
Marchetti, M., De Bei, O., Bettati, S., Campanini, B., Kovachka, S., Gianquinto, E., et al. (2020). Iron metabolism at the interface between host and pathogen: from nutritional immunity to antibacterial development. Int. J. Mol. Sci. 21, 2145. doi: 10.3390/ijms21062145
Maskell, J. P. (1980). The functional interchangeability of enterobacterial and staphylococcal iron chelators. Antonie Van Leeuwenhoek 46, 343–351. doi: 10.1007/BF00421981
Miethke, M., Pieroni, M., Weber, T., Bronstrup, M., Hammann, P., Halby, L., et al. (2021). Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 5, 726–749. doi: 10.1038/s41570-021-00313-1
Mollmann, U., Heinisch, L., Bauernfeind, A., Kohler, T., Ankel-Fuchs, D. (2009). Siderophores as drug delivery agents: application of the "Trojan Horse" strategy. Biometals 22, 615–624. doi: 10.1007/s10534-009-9219-2
Muller, S. I., Valdebenito, M., Hantke, K. (2009). Salmochelin, the long-overlooked catecholate siderophore of Salmonella. Biometals 22, 691–695. doi: 10.1007/s10534-009-9217-4
Muteeb, G., Rehman, M. T., Shahwan, M., Aatif, M. (2023). Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharm. (Basel) 16, 1615. doi: 10.3390/ph16111615
Nairz, M., Haschka, D., Demetz, E., Weiss, G. (2014). Iron at the interface of immunity and infection. Front. Pharmacol. 5. doi: 10.3389/fphar.2014.00152
Page, M. G. P. (2019). The role of iron and siderophores in infection, and the development of siderophore antibiotics. Clin. Infect. Dis. 69, S529–S537. doi: 10.1093/cid/ciz825
Qi, B., Han, M. (2018). Microbial siderophore enterobactin promotes mitochondrial iron uptake and development of the host via interaction with ATP synthase. Cell 175, 571–582 e511. doi: 10.1016/j.cell.2018.07.032
Raymond, K. N., Dertz, E. A., Kim, S. S. (2003). Enterobactin: an archetype for microbial iron transport. Proc. Natl. Acad. Sci. U.S.A. 100, 3584–3588. doi: 10.1073/pnas.0630018100
Sargun, A., Johnstone, T. C., Zhi, H., Raffatellu, M., Nolan, E. M. (2021a). Enterobactin- and salmochelin-beta-lactam conjugates induce cell morphologies consistent with inhibition of penicillin-binding proteins in uropathogenic Escherichia coli CFT073. Chem. Sci. 12, 4041–4056. doi: 10.1039/d0sc04337k
Sargun, A., Sassone-Corsi, M., Zheng, T., Raffatellu, M., Nolan, E. M. (2021b). Conjugation to Enterobactin and Salmochelin S4 Enhances the Antimicrobial Activity and Selectivity of beta-Lactam Antibiotics against Nontyphoidal Salmonella. ACS Infect. Dis. 7, 1248–1259. doi: 10.1021/acsinfecdis.1c00005
Sebulsky, M. T., Heinrichs, D. E. (2001). Identification and characterization of fhuD1 and fhuD2, two genes involved in iron-hydroxamate uptake in Staphylococcus aureus. J. Bacteriol 183, 4994–5000. doi: 10.1128/JB.183.17.4994-5000.2001
Sebulsky, M. T., Hohnstein, D., Hunter, M. D., Heinrichs, D. E. (2000). Identification and characterization of a membrane permease involved in iron-hydroxamate transport in Staphylococcus aureus. J. Bacteriol 182, 4394–4400. doi: 10.1128/JB.182.16.4394-4400.2000
Sheldon, J. R., Heinrichs, D. E. (2012). The iron-regulated staphylococcal lipoproteins. Front. Cell Infect. Microbiol. 2. doi: 10.3389/fcimb.2012.00041
Tejman-Yarden, N., Robinson, A., Davidov, Y., Shulman, A., Varvak, A., Reyes, F., et al. (2019). Delftibactin-A, a non-ribosomal peptide with broad antimicrobial activity. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.02377
Ullah, I., Lang, M. (2023). Key players in the regulation of iron homeostasis at the host-pathogen interface. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1279826
Uranga, C. C., Arroyo, P., Jr., Duggan, B. M., Gerwick, W. H., Edlund, A. (2020). Commensal oral rothia mucilaginosa produces enterobactin, a metal-chelating siderophore. mSystems 5, 10.1128/msystems.00161-20. doi: 10.1128/mSystems.00161-20
van Dijk, M. C., de Kruijff, R. M., Hagedoorn, P. L. (2022). The Role of Iron in Staphylococcus aureus Infection and Human Disease: A Metal Tug of War at the Host-Microbe Interface. Front. Cell Dev. Biol. 10. doi: 10.3389/fcell.2022.857237
Vinuesa, V., McConnell, M. J. (2021). Recent advances in iron chelation and gallium-based therapies for antibiotic resistant bacterial infections. Int. J. Mol. Sci. 22, 2876. doi: 10.3390/ijms22062876
Keywords: Staphylococcus aureus, salmochelin, enterobactin, siderophore, antibiotic
Citation: Davidov Y, Tejman-Yarden N, Robinson A, Rahav G and Nissan I (2025) Enterobactin and salmochelin S4 inhibit the growth of Staphylococcus aureus. Front. Cell. Infect. Microbiol. 15:1456046. doi: 10.3389/fcimb.2025.1456046
Received: 27 June 2024; Accepted: 12 February 2025;
Published: 05 March 2025.
Edited by:
Maria Teresa Mascellino, Sapienza University of Rome, ItalyReviewed by:
Zehava Eichenbaum, Georgia State University, United StatesCopyright © 2025 Davidov, Tejman-Yarden, Robinson, Rahav and Nissan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Israel Nissan, SXNyYWVsLm5pc3NhbkBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.