
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 18 March 2025
Sec. Microbes and Innate Immunity
Volume 15 - 2025 | https://doi.org/10.3389/fcimb.2025.1445660
This article is part of the Research TopicRespiratory Pathogen Infection and Host Innate Immune ResponseView all 5 articles
 Emily A. Wheeler1
Emily A. Wheeler1 Patricia M. Lenhart-Pendergrass2†
Patricia M. Lenhart-Pendergrass2† Noel M. Rysavy1
Noel M. Rysavy1 Katie R. Poch1
Katie R. Poch1 Silvia M. Caceres1
Silvia M. Caceres1 Kara M. Calhoun3
Kara M. Calhoun3 Karina A. Serban1,3†
Karina A. Serban1,3† Jerry A. Nick1,3
Jerry A. Nick1,3 Kenneth C. Malcolm1,3*
Kenneth C. Malcolm1,3*Mycobacterium abscessus is a nontuberculous mycobacterium emerging as a significant pathogen in individuals with chronic lung diseases, including cystic fibrosis and chronic obstructive pulmonary disease. Current therapeutics have poor efficacy. Strategies of bacterial control based on host defenses are appealing; however, antimycobacterial immunity remains poorly understood and is further complicated by the appearance of smooth and rough morphotypes, which elicit distinct host responses. We investigated the role of serum components in neutrophil-mediated clearance of M. abscessus morphotypes. M. abscessus opsonization with complement enhanced bacterial killing compared to complement-deficient opsonization. Killing of rough isolates was less reliant on complement. Complement C3 and mannose-binding lectin 2 (MBL2) were deposited on M. abscessus morphotypes in distinct patterns, with a greater association of MBL2 on rough M. abscessus. Killing was dependent on C3; however, depletion and competition experiments indicate that canonical complement activation pathways are not involved. Complement-mediated killing relied on natural IgG and IgM for smooth morphotypes and on IgG for rough morphotypes. Both morphotypes were recognized by complement receptor 3 in a carbohydrate- and calcium-dependent manner. These findings indicate a role for noncanonical C3 activation pathways for M. abscessus clearance by neutrophils and link smooth-to-rough adaptation to complement activation.
Mycobacterium abscessus (Mab) is a nontuberculous mycobacterium (NTM) and a significant pathogen in individuals with chronic pulmonary diseases such as cystic fibrosis (CF), non-CF bronchiectasis, and chronic obstructive pulmonary disease (Nick et al., 2021; Victoria et al., 2021), as well as soft tissue infections (Sepulcri et al., 2023). Subclinical infections of the lung may take years to progress to chronic pulmonary disease (Martiniano et al., 2013; Nick et al., 2022). Mab infections are highly resistant to most current therapies, which require prolonged multidrug regimens that often have poor tolerance and serious side effects (Daley et al., 2020). Infection occurs through environmental exposure or person-to-person transmission (Bryant et al., 2016; Honda et al., 2018), and is associated with neutrophilic inflammation.
Environmental M. abscessus strains exhibit a distinct smooth colony morphology when grown on mycobacterial solid media. The smooth morphotype possesses traits that may facilitate colonization, such as biofilm formation and sliding motility (Howard et al., 2006). However, it is generally less virulent and less frequently associated with disease. In vivo, the smooth morphotype can transition to a rough colony morphotype through a poorly understood mechanism, potentially influenced by the host environment. The rough morphotype is strongly associated with increased virulence, immunogenicity, pulmonary disease, and chronic infection (Byrd and Lyons, 1999; Sanguinetti et al., 2001; Howard et al., 2006; Rottman et al., 2007; Catherinot et al., 2009; Bernut et al., 2014; Hedin et al., 2023), though the underlying mechanisms remain unclear. These observations suggest that host factors alone are insufficient to control infection in at-risk individuals and may instead facilitate adaptation to the virulent phenotype. However, little is known about the host factors that protect healthy individuals or predispose others to Mab infection.
Cell envelope components of Mab are key determinants distinguishing the smooth and rough morphotypes. This morphological difference primarily results from the loss of glycopeptidolipid (GPL) in the smooth morphotype’s cell wall (Howard et al., 2006). The absence of GPL in the rough morphotype exposes mycobacterial-specific molecules, such as phosphatidylinositol mannosides (PIMs) and lipoproteins, leading to increased immune cell activation (Howard et al., 2006; Rhoades et al., 2009; Roux et al., 2011). Emerging insights into rough Mab infection are coming into focus, including enhanced cytokine production and inhibition of autophagy in macrophages (Howard et al., 2006; Rhoades et al., 2009; Roux et al., 2011; Roux et al., 2016; Kim et al., 2017). However, in neutrophils, cytokine production is similar between the morphotypes, and autophagy does not appear to be involved (Malcolm et al., 2013; Malcolm et al., 2018).
Plasma contains pattern-recognition molecules capable of binding and controlling pathogens. These opsonins converge on the activation of innate immune complement pathways. Canonical complement activation consists of the classical pathway (CP), the lectin pathway (LP), and the alternative pathway (AP). The CP is initiated by IgG and IgM binding to pathogens, leading to the binding of the C1 complex, a multiprotein protease complex consisting of C1q, C1r, and C1s. The LP is activated by binding of mannose-binding lectin-2 (MBL2) to mannose and other carbohydrates, including N-acetylglucosamine (GlcNAc), and is complexed with MBL2-associated serine proteases (MASPs) (Ip et al., 2009). The AP uses Factor B in an amplification mechanism to enhance the activity of C3 convertases, which are the common protease activities of all three pathways, and cleave C3 to reactive C3b and further to iC3b. Covalent association of the C3 fragments C3b and iC3b marks pathogens for recognition by the complement receptors CR1, CR3, and CR4 on neutrophils. In addition, specific antibodies can opsonize pathogens for recognition via Fc receptors, although lower-affinity nonimmune, or natural, antibodies can also act with and without complement to promote pathogen clearance (Schiller et al., 1979; Lutz et al., 1993; Schwartz et al., 2012; Lenhart-Pendergrass et al., 2023).
The role of plasma components in controlling NTM infections remains poorly understood. We recently demonstrated that neutrophil-mediated killing of the slow-growing NTM M. avium is enhanced by plasma complement C3 and IgM (Lenhart-Pendergrass et al., 2023). To extend these findings to rapidly growing NTM, we investigated the role of complement and immunoglobulins in neutrophil-mediated killing of Mab morphotypes.
M. abscessus ssp. abscessus (Mab; ATCC strain 19977) smooth and rough morphotypes were propagated from frozen aliquots in 7H9 broth supplemented with 0.5 g/l bovine albumin fraction V, 0.2 g/l dextrose, 0.3 mg/l catalase (ADC; BD Biosciences, Franklin Lakes, NJ), along with 2% glycerol and 0.05% Tween-80, at 37°C with shaking at 200 rpm for 3–4 days. Clinical isolates were obtained from the Cystic Fibrosis Foundation NTM National Resource Center at National Jewish Health. Washed cultures were sonicated using six 1-s bursts of a Fisher Sonic Dismembrator 100 at approximately 4 W output to obtain single cells and smaller aggregates, then adjusted to OD600 of 1.0 in PBS containing Ca2+ and Mg2+, corresponding to approximately 3 ×108 CFU/ml (Pohl et al., 2020).
Blood was drawn from healthy donors for neutrophil and plasma isolation. These studies were approved by the Biomedical Research Alliance of New York Institutional Review Board, and written informed consent was obtained from all donors. The study was conducted in accordance with the Declaration of Helsinki.
Neutrophils were isolated from healthy volunteers using the plasma Percoll method, as previously described (Young et al., 2011), from blood drawn into 13 mM sodium citrate. The isolated neutrophils were washed and resuspended in Krebs–Ringer phosphate-buffered dextrose (154 mM NaCl, 5.6 mM KCl, 1.1 mM MgSO4, 2.2 mM CaCl2, 0.85 mM NaH2PO4, 2.15 mM Na2HPO4, and 0.2% dextrose). The cells were confirmed to be > 98% pure by visual inspection of cytospins.
Citrated platelet-rich plasma from the neutrophil isolation was centrifuged at 2,000 × g for 15 min to obtain platelet-poor plasma and was then recalcified by adding 40 mM CaCl2 to allow clotting. Plasma from EDTA tubes was recalcified with 5 mM CaCl2 and clotted as with citrated plasma. The serum was clotted for > 1 h at room temperature, and all plasmas were isolated after centrifugation at 2,000 × g for 15 min. As serum tubes may contain components that inhibit complement-activating factors (Brady et al., 2014), blood was also drawn into 15 ml conical tubes without additives and clotted at room temperature for 1 h. In most cases, serum samples were recentrifuged for 5 min to remove residual red blood cells. Plasmas and sera were stored at − 20°C until ready for use. Plasma from citrated blood, designated whole plasma (WP), remained active when stored at 4°C for over 1 week. Plasmas were also incubated at 56°C for 30 min to inactivate complement, generating heat-inactivated plasma (HIP), and were likewise stored at − 20°C. Normal human serum and sera depleted of C2, C3, C4, C1q, and Factor B were purchased from Complement Technologies, Tyler, TX. The depletion of C3 and C1q had been demonstrated previously (Lenhart-Pendergrass et al., 2023).
WP and HIP were mixed with Protein A-agarose (GoldBio, Boulder, CO), Protein G-agarose (Invitrogen, Waltham, MA), and anti-IgM-agarose (Sigma, St Louis, MO, A9935) at an approximate ratio of 1 volume of plasma to 1 volume of packed beads. The mixtures were rotated for 1 h, pelleted by centrifugation, and the depleted supernatants removed from the bead pellet.
Washed Mab (~ 1 × 107) was incubated with 33% plasmas, serum, or complement-depleted sera for 20 min at 37°C (Lenhart-Pendergrass et al., 2023), then diluted in PBS to 12% plasma. The final plasma concentration was 1.2% when added to cells. In some experiments, mannan (1 mg/ml), GlcNAc (100 mM), Mg2+/EGTA (5 mM/10 mM), or EDTA (10 mM) was added to bacteria before the addition of WP. Opsonized Mab was briefly sonicated and added directly to neutrophils. For Western blotting, cells (~ 1 × 108) were opsonized for 30 min in 33% plasmas and washed three times in PBS before solubilization in the SDS sample buffer. In some cases, the supernatant was retained for analysis. Plasmas and sera used were from multiple five-donor pools.
Neutrophils were suspended in RPMI supplemented with 10 mM HEPES (pH 7.4) and 1%–2% pooled heat-inactivated platelet-poor plasma (complete RPMI). Sample tubes contained cells (1 × 106) suspended in complete RPMI and the respective bacteria at a multiplicity of infection (MOI) of approximately 1:1 in a 0.1-ml volume. Tubes were initially centrifuged at 2,000×g for 1 min, and pelleted cells were incubated at 37°C for 5 min to promote bacteria–neutrophil interaction, followed by resuspension and incubation for up to 2 h, as indicated (Pohl et al., 2020). These experimental conditions promote synchronous, close contact between mycobacteria and neutrophils (Malcolm, 2018). In some experiments, 10% WP and HIP were added to neutrophils immediately before infection with nonopsonized Mab. Inhibitor experiments were performed by the addition of mannan (1 mg/ml), GlcNAc (100 mM), GalNAc (100 mM), and EDTA (10 mM) immediately before bacterial inoculum. Triton X-100 (0.1%) in 0.9% NaCl was added to inactivate neutrophils and facilitate mycobacterial dispersion. Following vortex mixing and serial dilutions in saline, samples were plated on 7H10 agar supplemented with OADC and incubated at 37°C. Colonies were counted 3 to 5 days after plating, and compared to colony counts at initiation of infection. Cell-free killing bacteria were incubated with WP and HIP for the opsonization reactions, added to a complete medium for the indicated times, and enumerated as described above.
Neutrophils were pre-incubated for 15 min with 10 mg/ml anti-CR1 (GeneTex, Irvine, TX, GTX44217), 30 mg/ml anti-CR3 (BioLegend, San Diego, CA, clone M1/70, No. 101248), or the combination. Isotype antibody (BioLegend, No. 400644, IgG2b) was used in all experiments so that equal protein was present in all experiments.
Opsonized M. abscessus in SDS sample buffer were heated at 95°C for 5 min and loaded onto 10% polyacrylamide gels. The gels were transferred to nitrocellulose, and Mab-associated proteins were detected using anti-iC3b (BioLegend, 846302), anti-C1q (Novus, Centennial, CO, NB100-64420), anti-MBL2 (Abcam, Waltham, MA, ab189856), anti-IgG-HRP (Abcam, ab97175), anti-IgA-HRP (Southern Biotech, 2050-05), and anti-IgM-HRP (Southern Biotech, Homewood, AL, 2020-05) via enhanced chemiluminescence.
Statistical comparisons were performed using ANOVA and t-tests in Excel after confirming normal distributions with the Kolmogorov–Smirnov test (https://www.statskingdom.com/kolmogorov-smirnov-test-calculator.html).
We have previously shown that in the absence of complement, Mab is poorly recognized or killed by human neutrophils (Malcolm et al., 2013; Malcolm et al., 2018; Pohl et al., 2020). The role of complement as an opsonin was determined by measuring Mab survival in the presence of human neutrophils. We compared opsonization with naïve, complement-containing WP and HIP, which is devoid of complement activity. Opsonization of both smooth and rough Mab with WP led to rapid and efficient killing by neutrophils (Figures 1A, B). Killing with WP opsonization was more robust than with HIP opsonization (Figure 1C). A smaller difference between killing in the presence of WP and HIP was seen for the rough morphotype of Mab, and the difference was significantly different between morphotypes (two-way ANOVA, p = 1.5 × 10−4). Mock opsonization with BSA resulted in less neutrophil killing than opsonization with WP or HIP (Figure 1D). Efficient killing occurred at plasma concentrations as low as 5% (Figures 1E, F). Reaction of WP or HIP in the absence of neutrophils did not affect Mab survival over the times used in these studies (Supplementary Figure S1). The heat-labile nature of plasma components suggests a role for complement opsonization in the recognition and clearance of Mab.
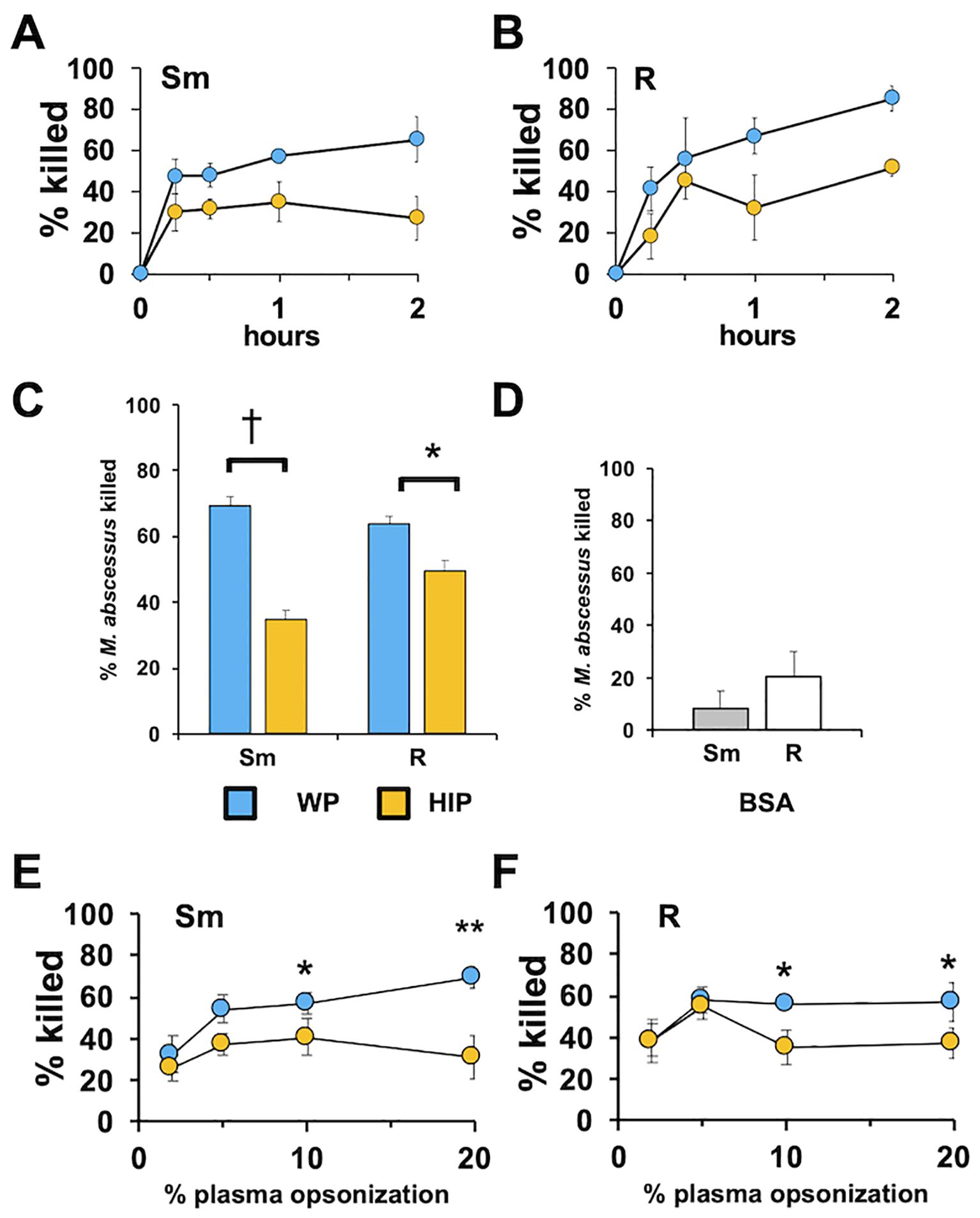
Figure 1. Whole plasma enhances the killing of Mab. (A) Smooth (Sm) Mab and (B) rough (R) Mab opsonized with whole plasma (WP; blue) or heat-inactivated plasma (HIP; orange) were added to human neutrophils at an MOI of 1 for the indicated times and killing determined; n = 3. (C) Smooth and rough Mab were opsonized in WP or HIP, or (D) incubated with BSA and killing determined after 1 h of incubation with neutrophils (n = 37 for Sm and R, and n = 4 for BSA). (E) Smooth and (F) rough Mab opsonized with the indicated percentage of WP or HIP and killing determined after 1 h of incubation with neutrophils (n = 5–6: *p < 0.05; **p < 0.01: *p < 0.05; †p < 0.001).
The need for pre-opsonization of Mab, as performed in the above experiments, was also addressed to determine if the effect of complement opsonization was influenced by the presence of neutrophils. When plasma was introduced to the neutrophils just before non-opsonized Mab was added, the killing of smooth Mab was reduced, but the killing of rough Mab was not changed when compared to pre-opsonized Mab (Supplementary Figure S2). Decreased killing when WP was added concurrently with nonopsonized Mab may reflect the activity of neutrophil-derived negative regulatory factors (e.g., CD55) or different morphotype kinetics of neutrophil recognition or of C3 activation. Drawing blood into different types of collection tubes can affect plasma components (Brady et al., 2014). Therefore, we compared the killing ability of serum and of plasmas from blood drawn into citrate, heparin, and EDTA. Citrate and EDTA WP were recalcified and clotted. Killing was similar in all conditions (Supplementary Figure S3), suggesting that plasma processing is not a major constraint in measuring complement function.
The plasma protein components associated with Mab under various opsonization conditions were analyzed by immunoblotting. Mab was opsonized with WP, washed, and Mab-associated proteins extracted in an SDS sample buffer. Blots were probed with antibodies against iC3b, C1q, MBL2, IgG, and IgA (Figures 2A–C). C3 fragments were deposited on smooth Mab, whereas little MBL2 and C1q deposition was observed. The C3-positive bands represented the iC3b fragments, including those covalently bound to Mab moieties through the reactive ester, with major bands at 70, 130, and a group at 260 kDa. Rough Mab exhibited a distinct pattern of iC3b association compared to smooth Mab; the major 70- and 130-kDa bands were minimal, while the 260-kDa bands were present at levels similar to those in smooth Mab. C1q deposition was weak. MBL2 predominantly deposited on rough Mab. Immunoblotting revealed that the immunoglobulin classes IgG, IgA, and IgM bound both Mab morphotypes (Figures 2B, C), with no clear distinction between smooth and rough Mab. When HIP was used to opsonize Mab, no loss of IgG was detected, while C3 binding was reduced, and C3 in plasma was degraded. Surprisingly, increased C1q and MBL2 levels were observed, suggesting nonspecific binding of the inactivated proteins (Supplementary Figure S4). Furthermore, the deposition of complement factors on two unrelated bacterial species, Pseudomonas aeruginosa, and Staphylococcus aureus, were significantly different than that of either Mab morphotype (Supplementary Figure S5), indicating that complement activation depends on bacterial species. These data indicate morphotype-specific interaction of C3 and MBL2.
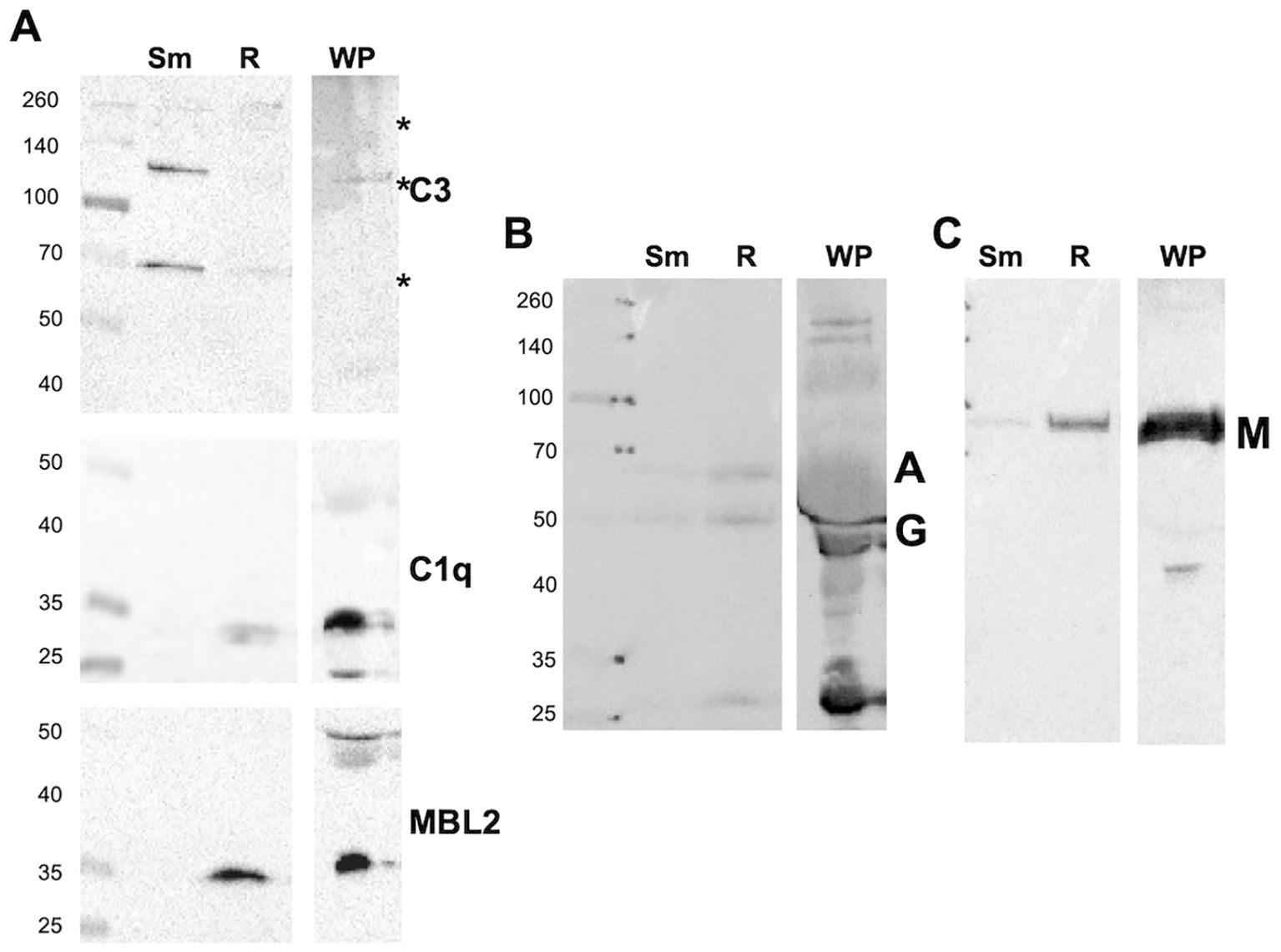
Figure 2. Whole plasma differently opsonizes Mab morphotypes. (A) Smooth (Sm) and rough (R) Mab opsonized with whole plasma were washed, proteins separated by SDS-PAGE, and deposited iC3b, C1q, and MBL2 were detected. A 1:20 dilution of WP was run as a positive control; Asterisk indicates iC3b reactivity at 70, 130, and 260 kDa. (B) Detection of IgG (G) and IgA (A). (C) Detection of IgM (M). The results are representative of over 10 separate experiments. Molecular weight markers are shown to the left of each blot.
To determine if morphotype differences between iC3b and MBL deposition were a general phenomenon, we measured complement deposition on genetically distinct CF clinical smooth and rough isolates from people with CF (Davidson et al., 2021). Like the type strains, iC3b deposition of the 70- and 130-kDa bands was predominant on the smooth strains. C1q was minimally detected. While in the two isogenic pairs (ATCC and CF016) MBL2 deposition was greater on the rough strains (Figure 3A), MBL2 deposition was variable overall among isolates. We observed more deposition of MBL2 on CF0123, a smooth strain, than on the genetically related, but not isogenic, rough strain CF0017-R. The effect of killing after opsonization of these clinical isolates was determined. Smooth isolates were more susceptible to heat-sensitive plasma components than rough isolates (Figure 3B), either individually or in composite (Supplementary Figure S6). These data suggest that, in general, smooth isolates are more sensitive to complement than rough isolates.
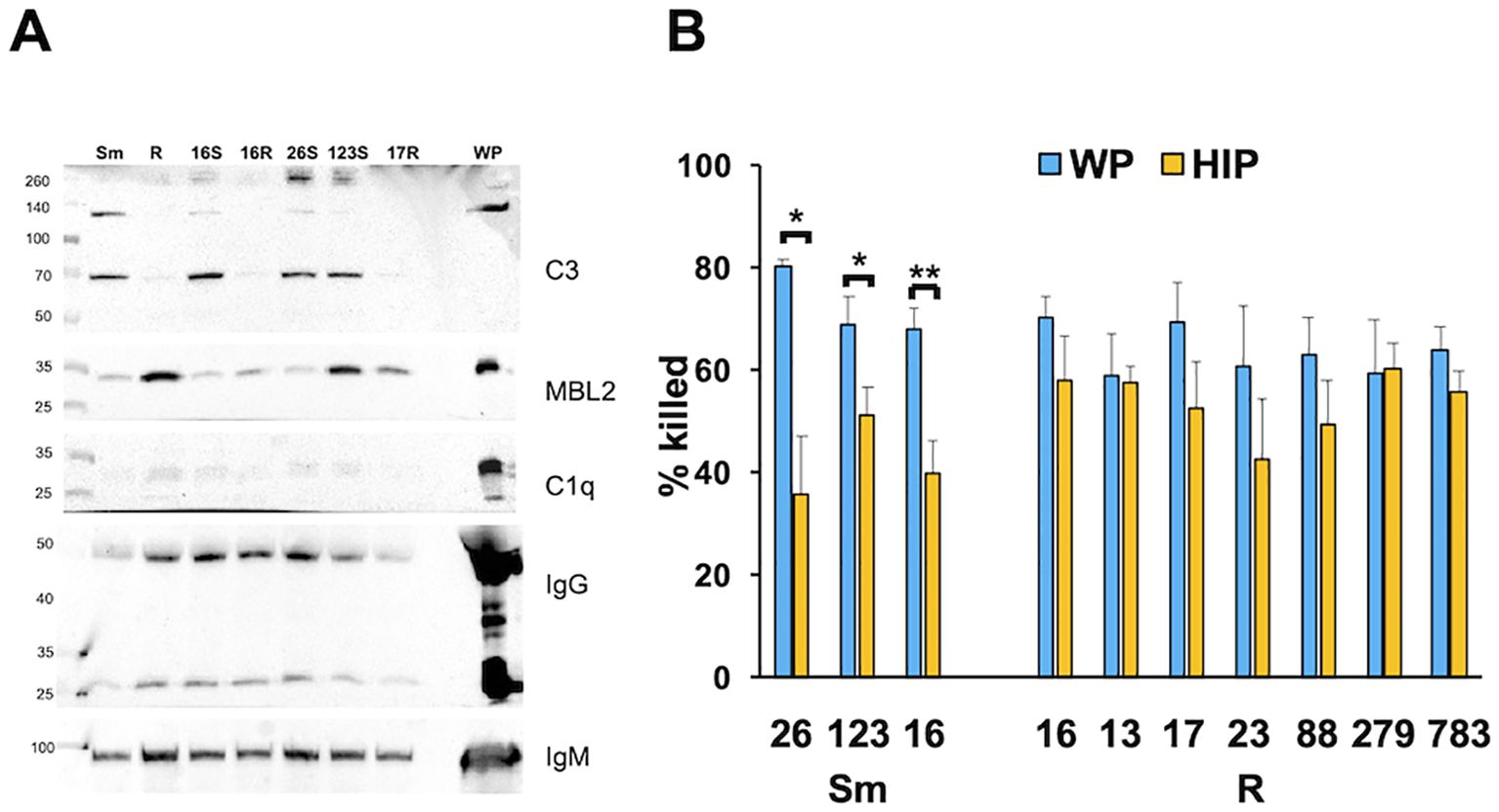
Figure 3. Killing of smooth Mab clinical isolates is more dependent on complement than rough Mab isolates. (A) Smooth (S) and rough (R) Mab clinical isolates (numbered by isolate) opsonized with WP were washed, proteins separated by SDS-PAGE, and deposited iC3b, C1q, MBL2, IgG, and IgM were detected. A 1:20 dilution of WP was run as a positive control. S and R designate the morphotype. *p < 0.05; **p < 0.01. (B) Smooth and rough Mab clinical isolates opsonized with WP (blue) or HIP (orange) were added to human neutrophils at an MOI of 1 for 1 h and killing determined; n = 4–11 per isolate.
The activation of C3 is a common feature of the three major complement activation pathways. Opsonization of Mab with C3-depleted serum resulted in reduced killing of both morphotypes (Figures 4A, B), with a greater inhibition observed for smooth Mab. The finding that rough Mab killing was dependent on C3, despite minimal iC3b deposition, could be attributed to reduced C3 activation or the loss of reacted C3 from the cell surface (Bhakdi et al., 1974; Laarman et al., 2011). To investigate this, we measured residual C3 in the supernatant after the opsonization reaction. Opsonization of rough Mab with WP preserved C3 in the postreaction supernatant at a similar size (~ 130 kDa) as unreacted C3 in WP (Figure 4C), consistent with reduced C3 activation. In contrast, the postopsonization supernatant of smooth Mab had minimal unreacted C3, indicating efficient reaction and deposition on its surface. These data confirm a role for C3 as a smooth Mab opsonin while highlighting reduced C3 activation in rough Mab.
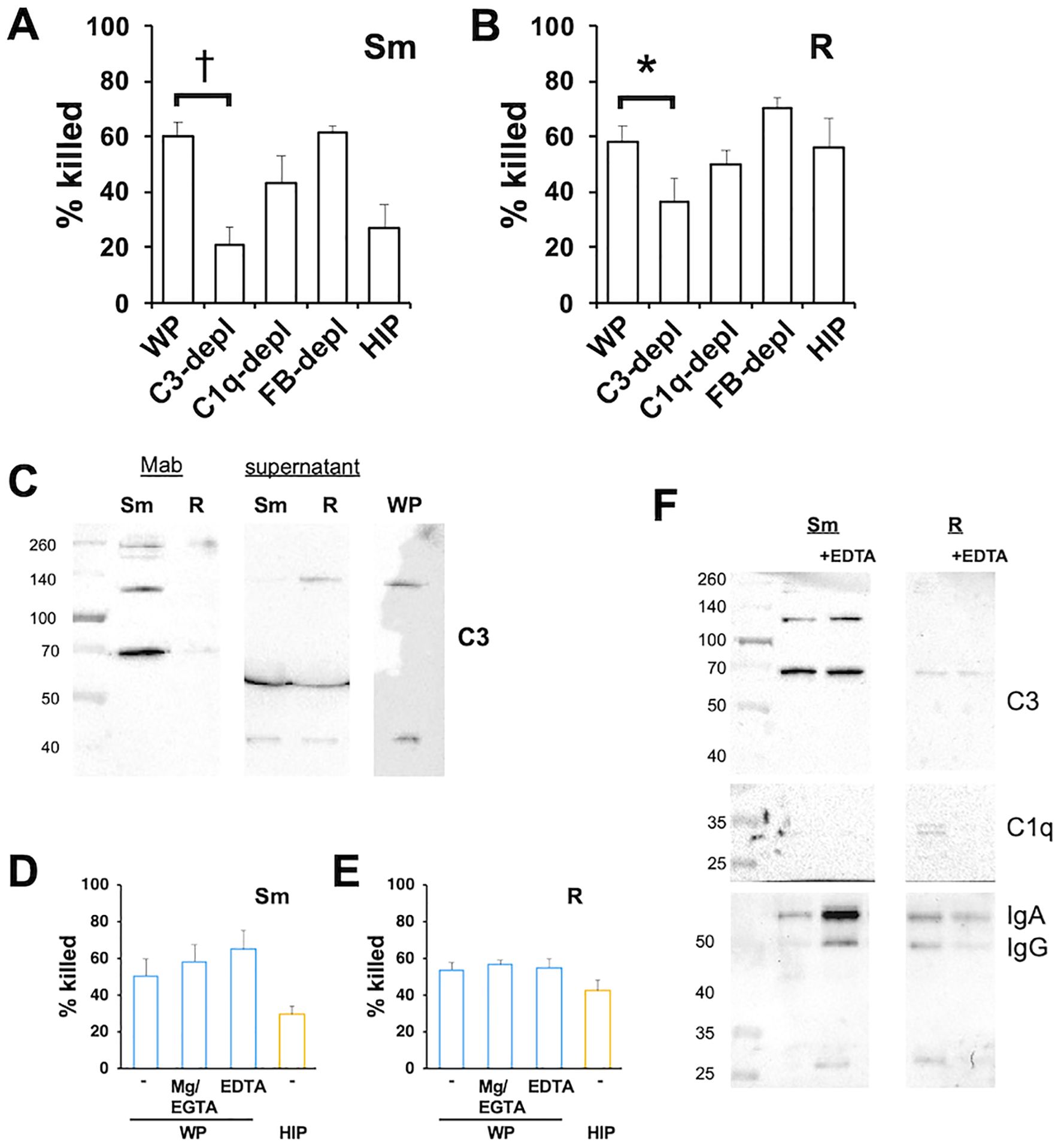
Figure 4. The opsonized killing of Mab requires C3 but is independent of CP and AP. (A) Smooth and (B) rough Mab opsonized with serum, sera depleted of C3, C1q, and Factor B, or HIP were added to human neutrophils at an MOI of 1 for 1 h and killing determined; n = 6–8. (C) Smooth and rough Mab were opsonized with WP and the supernatants retained. The washed cellular pellet and supernatant proteins were separated by SDS-PAGE, and deposited iCb3 was detected. A 1:20 dilution of WP was run as a positive control; n = 3–5. (D) Smooth and (E) rough Mab were opsonized with WP or HIP alone or in the presence of Mg2+/EDTA or EGTA before adding to neutrophils. Killing was determined after 1 h of incubation; n = 6. (F) Smooth and rough Mab were opsonized in WP or WP + EDTA, washed, and deposited iC3b, C1q, IgG, and IgA were detected; n = 3. *p < 0.05; †p < 0.001.
To determine the role of the CP and AP on C3 activation we optimized Mab with serum depleted of C1q and Factor B, mediators of the CP and AP, respectively. Depletion of C1q or Factor B did not reduce killing, indicating that opsonization occurred independently of the CP and AP (Figures 4A, B). In addition, killing was not affected when Mab was opsonized with C2- or C4-depleted sera (Supplementary Figure S7), conditions that inhibit CP and LP activation. To confirm these findings, Mab was opsonized with WP in the presence of Mg2+/EGTA, a condition that allows only AP activation, or EDTA to chelate Ca2+ and Mg2+ and inhibit activation of CP, LP, and AP (Figures 4D, E). Interestingly, these conditions did not reduce Mab killing. Opsonization in the presence of EDTA did not affect the deposition of iC3b on Mab (Figure 4F) or the deposition of IgG or IgA. These data suggest that canonical activation of the CP, LP, and AP are not essential for WP opsonization.
To further address the role of MBL2 in Mab opsonization, we performed the opsonization reaction in the presence of competitive soluble mannan (an a-linked mannose polymer) and GlcNAc (N-acetyl-d-glucosamine), two agents that bind MBL2 to activate the MASP2-dependent C3-convertase. Opsonization in the presence of mannan or GlcNAc did not affect the killing of Mab (Figure 5A). In the same conditions, deposition of iC3b and MBL2 was measured. Mannan did not inhibit MBL2 or iC3b association with either morphotype (Figure 5B). GlcNAc reduced association with MBL2 with little effect on iC3b deposition. Neither agent affected IgG and IgM deposition. These data suggest that C3 fixation can occur independently of MBL2.
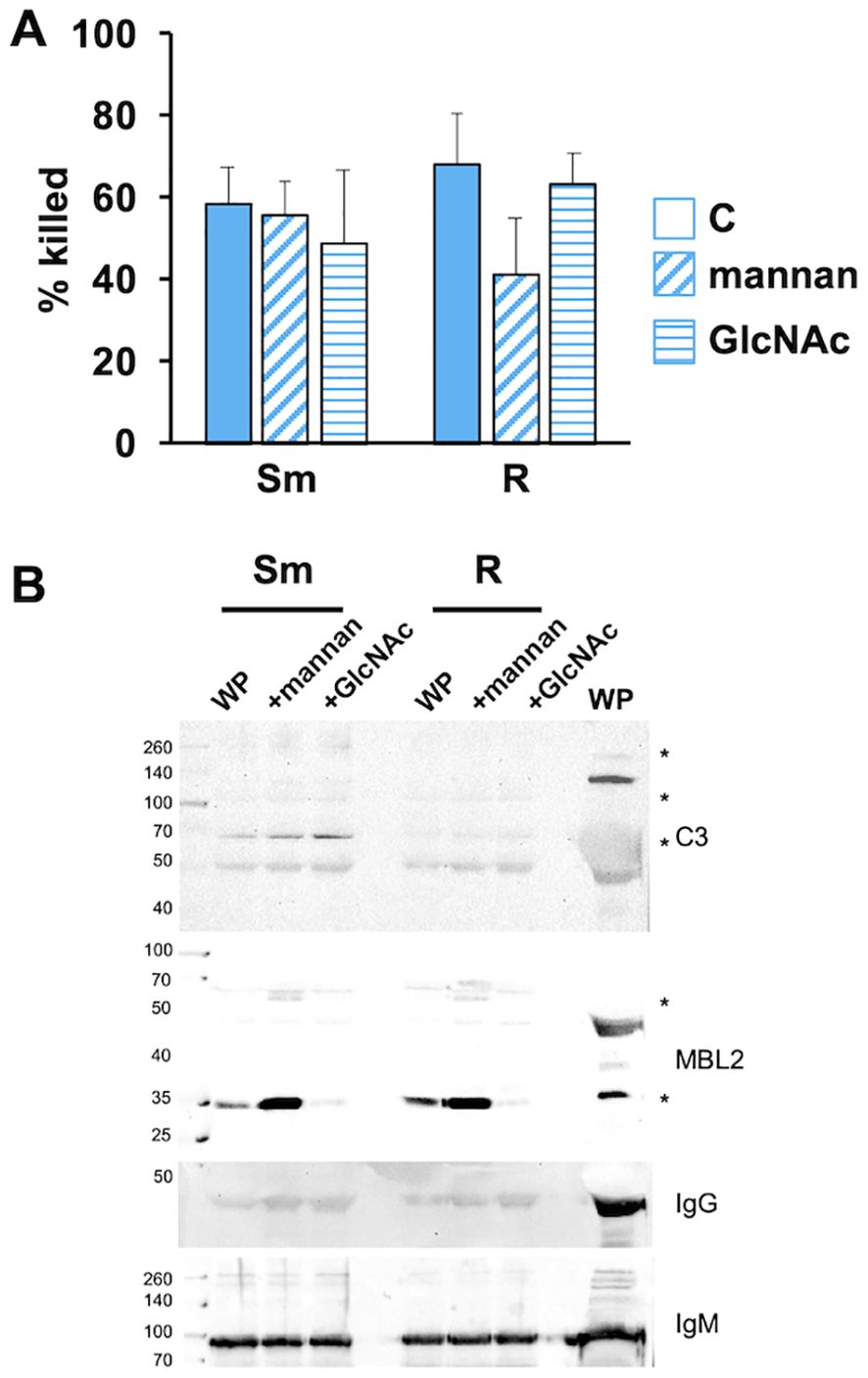
Figure 5. MBL2 ligands present during opsonization do not affect the killing of Mab. (A) Smooth and rough Mab opsonized with WP alone (solid) or in the presence of mannan (diagonal stripes; 1 mg/ml) or GlcNAc (horizontal stripes; 100 mM) were added to human neutrophils at an MOI of 1 for 1 h and killing determined; n = 5. (B) Mab opsonized as in (A) were washed, proteins separated by SDS-PAGE, and deposited iC3b, MBL2, IgG, and IgM were detected; n = 5. A 1:20 dilution of WP was run as a positive control. Asterisk indicates immunopositive bands.
In addition to activation of the CP, antibodies can act as opsonins to mediate phagocytosis and killing in the absence of complement. The role of immunoglobulins in the recognition and killing of Mab was explored using depleted plasmas. We previously observed a role of IgM in complement-dependent neutrophil M. avium killing (Lenhart-Pendergrass et al., 2023). To explore the role of IgM in the opsonization of Mab, we treated Mab with IgM-depleted WP. IgM-depleted WP reduced the killing of smooth, but not rough, Mab (Figures 6A, B). Depletion of IgM with anti-IgM resulted in reduced deposition of iC3b, MBL2, IgG, and IgM, although some MBL2 remained associated with rough Mab (Figure 6C). IgM depletion did not affect killing when Mab was opsonized with HIP, consistent with IgM lacking a direct opsonizing effect.
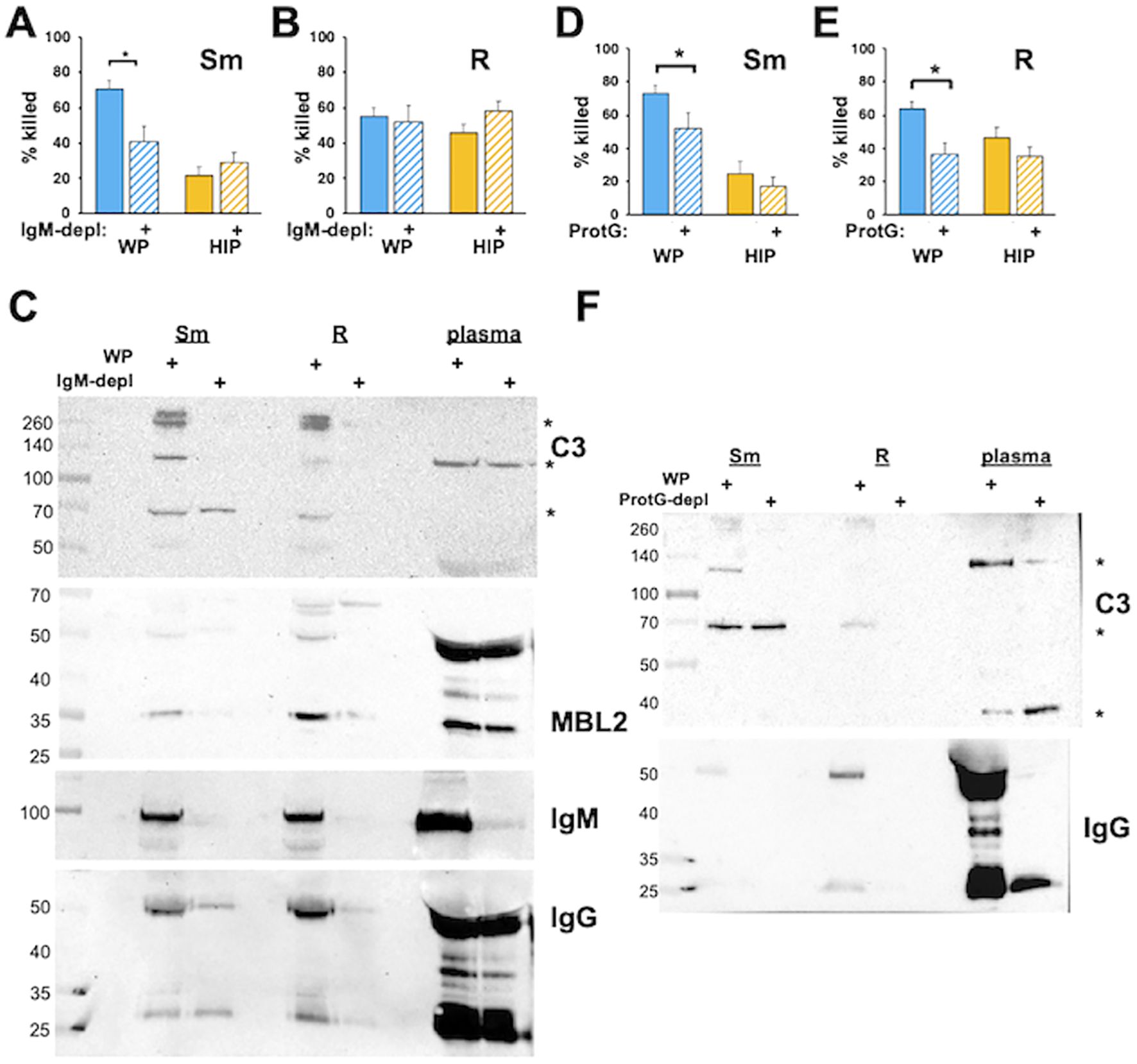
Figure 6. Immunoglobulins are required for the killing of Mab opsonized in WP. (A) Smooth and (B) rough Mab opsonized with WP or HIP alone (solid) or in WP or HIP depleted of IgM with anti-IgM agarose (striped bars) were added to human neutrophils at an MOI of 1 for 1 h and killing determined. (C) Mab opsonized as for (A, B) were washed, proteins separated by SDS-PAGE, and deposited iC3b, MBL2, IgG, and IgM were detected. A 1:20 dilution of WP or IgM-depleted WP (plasma) was run as a positive control. The asterisk indicates C3 fragments. (D) Smooth and (E) rough Mab opsonized with WP or HIP alone (solid) or in WP or HIP depleted of IgG with Protein G Sepharose (striped bars) were added to human neutrophils at an MOI of 1 for 1 h and killing determined; n = 5–10 independent experiments. (F) Mab opsonized as in (D, E) were washed, proteins separated by SDS-PAGE, and deposited iC3b and IgG were detected. A 1:20 dilution of WP or IgG-depleted WP (plasma) was run as a positive control. The asterisk indicates C3 fragments. n = 5. *p < 0.05.
To understand the role of IgG, the four IgG subclasses were selectively depleted using Protein G. Opsonization in IgG-depleted WP led to reduced Mab killing (Figures 6D, E). The incomplete reduction of killing in smooth Mab in Protein G-depleted WP suggests that additional factors, such as IgM, may be involved. In contrast, depleting IgG from HIP had no effect on Mab killing, indicating that IgG opsonization does not have a direct effect in this context. Consistent with these findings, iC3b deposition was reduced in IgG-depleted serum (Figure 6F), as was the association of IgG with Mab. These data suggest that the IgG and IgM serve as opsonins for smooth Mab, whereas IgG alone acts as an opsonin for rough Mab. However, immunoglobulins are necessary but not sufficient for optimal Mab killing in the presence of WP. Similar results were obtained using protein A depletion, which binds IgG (except for the IgG3 subclass), IgM, and, to a lesser extent, IgA (Supplementary Figure S8).
Receptors for C3 fragments include the integrin family of adhesion receptors, which require divalent cations for efficient binding (Ueda et al., 1994). Reducing Ca2+ levels using EGTA and depleting both Ca2+ and Mg2+ with EDTA during the killing assay led to decreased killing of Mab opsonized with WP and HIP (Figures 7A, B), suggesting that calcium-dependent recognition plays a crucial role in both complement- and noncomplement-mediated opsonization by neutrophils. To specifically examine the role of neutrophil complement receptors, we used blocking antibodies against CR1 (CD35), which binds C3b, and CR3 (CD11b/CD18), which binds iC3b. Anti-CD11b partially inhibited killing (Figures 7C, D), whereas anti-CD35 had no effect. The combination of anti-CD35 and anti-CD11 (Schwartz et al., 2012) produced a similar effect to anti-CD11b alone. However, inhibition by anti-CD11b did not reduce killing to the same extent observed for HIP-opsonized Mab. These data indicate that CR3 plays a role in recognizing WP-opsonized with Mab, and that divalent cations are important for both complement-dependent and complement-independent recognition by neutrophils.
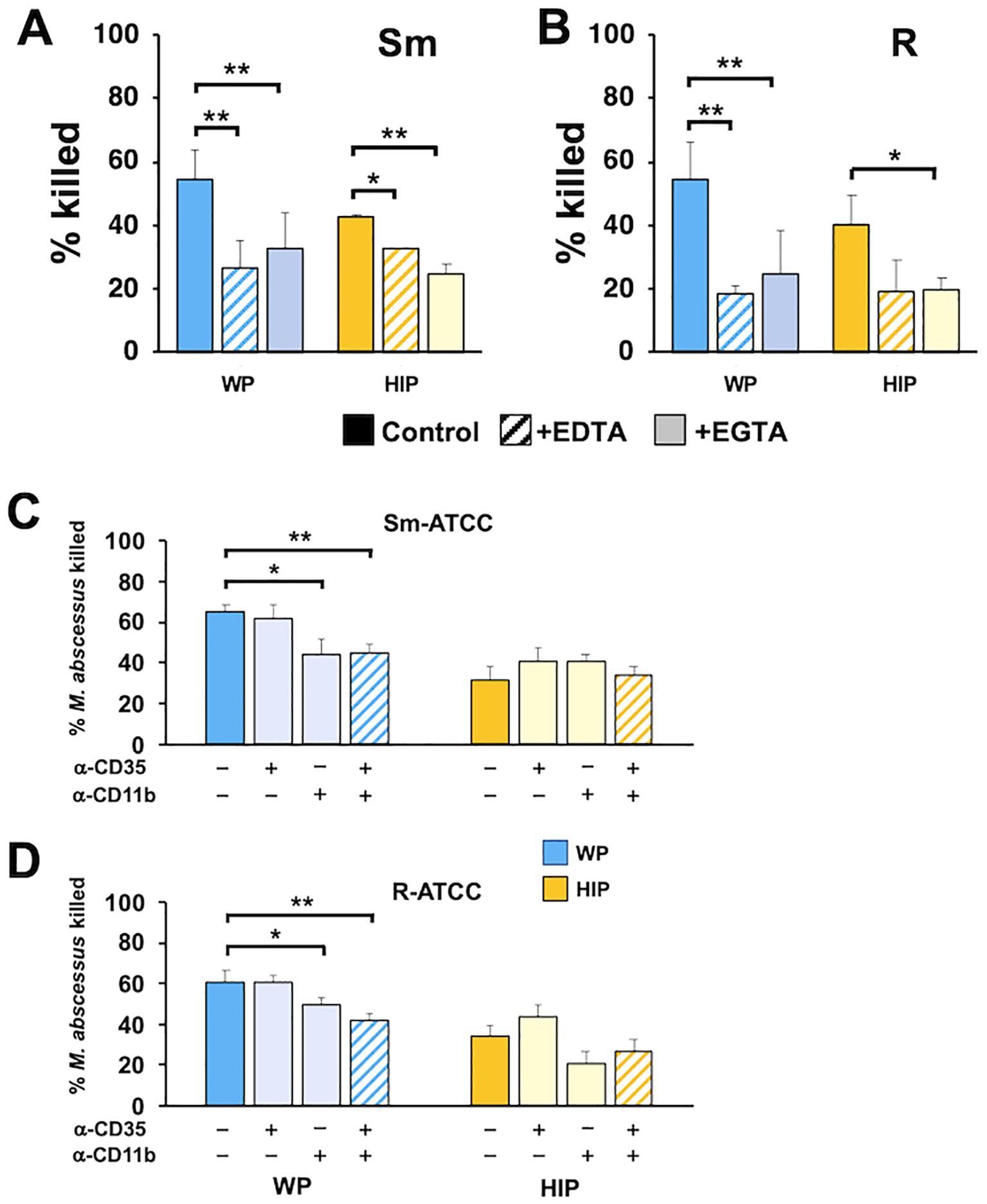
Figure 7. CR3/CD11b and cations play independent roles in the killing of opsonized Mab. (A) Smooth and (B) rough Mab opsonized with WP or HIP (solid) were added to human neutrophils in the presence of EDTA (striped bars) or EGTA (light shading) at an MOI of 1 for 1 h and killing determined; n = 4–6. (C) Smooth and (D) rough Mab opsonized with WP or HIP (solid) were added to human neutrophils preincubated with anti-CD35 (CR1) or anti-CD11b (CR3), as indicated, and killing determined; n = 5–6. *p < 0.05; **p < 0.01.
To further define complement recognition receptors, we explored the ability of free carbohydrates that mimic surface moieties to affect Mab killing. GlcNAc, GalNAc (N-acetyl-d-galactosamine), and mannose are sugars found on mycobacterial cell walls (Abrahams and Besra, 2021) and on host cells and can interact with host and pathogen receptors (Beuth et al., 1988; Kolbe et al., 2019; Sung et al., 2020). Incubation of neutrophils with either GlcNAc or GalNAc reduced the killing of Mab opsonized with either WP or HIP (Figures 8A, B). They had no effect on the minimal killing of Mab mock-opsonized with BSA. Incubation with mannan did not consistently affect Mab killing (Figures 8C, D); minimal inhibition of smooth Mab opsonized with WP was seen, but a small, enhanced killing of HIP-opsonized smooth Mab and WP-opsonized rough Mab was also observed. Therefore, recognition of N-acetylated sugars, but not mannose, is important for neutrophil killing of Mab, although these carbohydrate interactions likely involve both complement-dependent and complement-independent mechanisms.
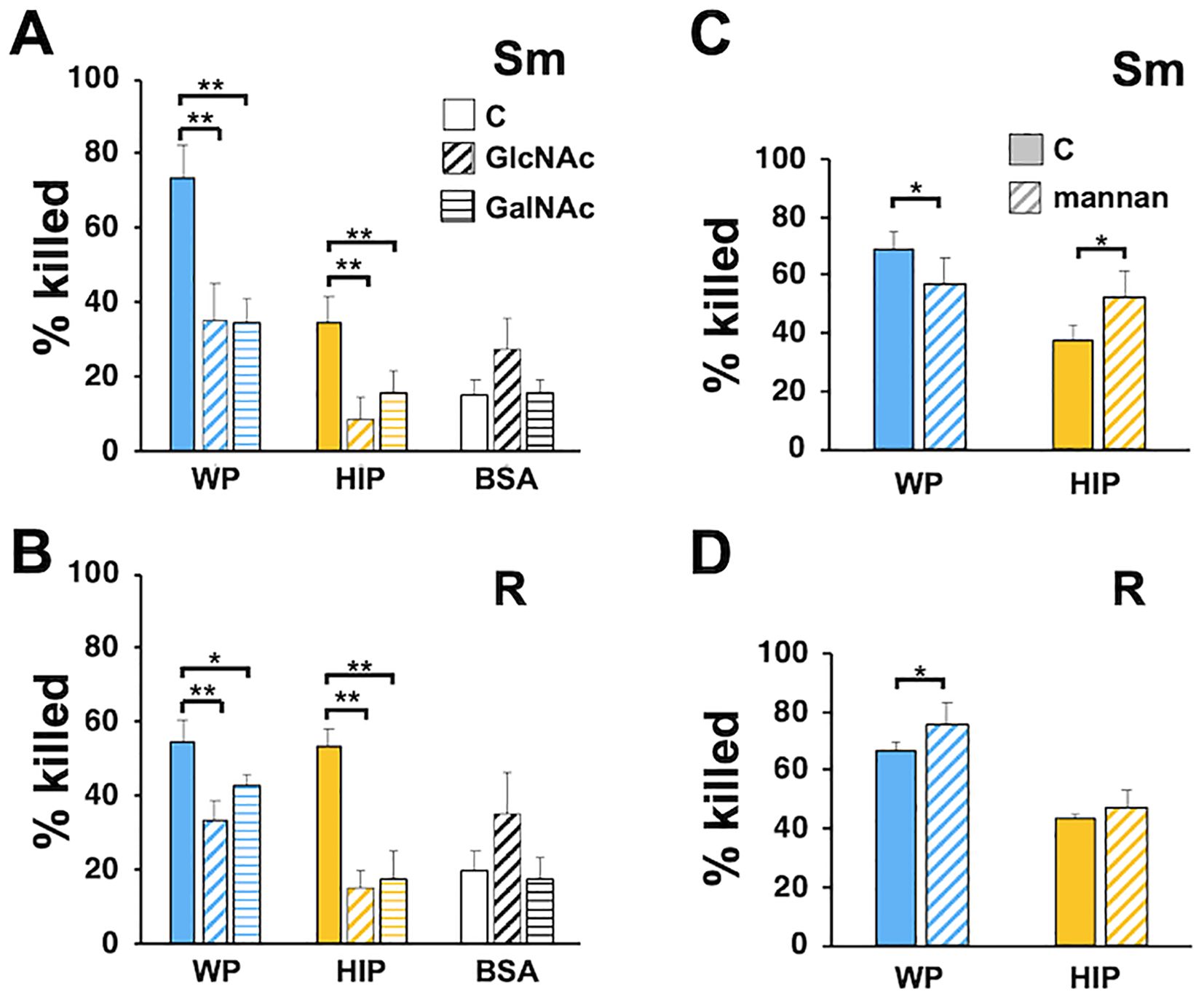
Figure 8. Inhibition of Mab killing by N-acetylated sugars. (A) Smooth and (B) rough Mab opsonized with WP or HIP alone (solid) or incubated with BSA were added to human neutrophils in the presence of GlcNAc (diagonal stripes; 100 mM) or GalNAc (horizontal stripes; 100 mM) for 1 h and killing determined; n = 5. (C) Smooth and (D) rough Mab opsonized with WP or HIP alone (solid) were added to human neutrophils in the presence of mannan (diagonal stripes; 1 mg/ml) for 1 h and killing determined; n = 6; *p < 0.05; **p < 0.01.
Host mechanisms for containing NTM infections remain poorly understood. While innate immune cells play a role in infection control, the precise mechanisms of clearance have not been thoroughly addressed. Furthermore, most studies have focused on the interaction of myeloid cells and NTM in isolation, without considering other host-derived factors. The uptake and killing of the slow-growing NTM M. avium by neutrophils is enhanced in a complement- and IgM-dependent manner (Hartmann et al., 2001; Lenhart-Pendergrass et al., 2023). However, the role of complement in controlling rapidly growing NTM has not yet been explored. Here, we demonstrate for the first time that rapidly growing NYM undergoes antibody-dependent complement opsonization.
Our findings are notable in that the adapted, rough form has developed mechanisms to evade two different innate immune defenses—C3 and IgM opsonization—resulting in MBL2 and IgG specificity. This suggests that C3 and IgM serve as in-host selective pressures for adaptation in the diseased lung. However, this adaptation comes at a cost, as it promotes MBL2 opsonization while preserving the effects of IgG. The observations that C3 facilitates Mab clearance and that more severe Mab pulmonary disease is linked to a slow conversion to the rough morphotype suggest that a delicate balance exists between adaptation and immune control of the two morphotypes. The emergence of specific morphotypes may depend on microenvironments with varying growth conditions, complement availability, or Ig class repertoires. For instance, vascular leakage may enrich complement levels in an injured lung compared to a healthy lung (Fidler et al., 2009). Killing of both morphotypes was reduced in C3-depleted serum, suggesting that even the minimal C3 deposition, as indicated by the 260-kDa bands on rough Mab, is sufficient to drive some level of killing. These data also imply that plasma-derived complement can effectively limit systemic infection.
Differences in complement activation and function between the morphotypes are further highlighted by clinical isolates. Killing of smooth Mab isolates was more dependent on heat-sensitive plasma components than that of rough Mab isolates. These data support the idea that the smooth morphotype may establish a foothold for sustained infection in infection sites that are limited or deficient in complement factors (Malcolm et al., 2013; Malcolm et al., 2018). The transition to the more virulent rough morphotype may occur under prolonged selective pressure in the presence of complement, possibly due to vascular leakage. Recent reports indicate reduced complement levels in the CF lung (Hayes et al., 2022), but not blood (Lenhart-Pendergrass et al., 2023), further supporting this idea. Whether MBL2 deposition on rough Mab is associated with virulence and disease progression remains to be studied. However, tissue injury by excessive complement activation is possible.
The dependence of complement activation on antibodies was unexpected. The plasma donors were healthy, and we previously demonstrated minimal anti-Mab-specific antibodies in healthy individuals (Malcolm et al., 2022). These findings implicate natural antibodies in complement activation, as previously reported (Schiller et al., 1979; Lutz et al., 1993; Schwartz et al., 2012; Khandelwal et al., 2018; Lenhart-Pendergrass et al., 2023). The antibody specificities required for full opsonization of the morphotypes may reflect changes in cell surface components. The loss of glycopeptidolipids (GPL)—a hallmark of rough Mab—is accompanied by exposure to PIMs (Rhoades et al., 2009) and overexpression of lipoproteins (Roux et al., 2011). These exposed molecules may serve as novel epitopes for nonimmune IgG. Other differences in cell surface composition may also play a role (Dubois et al., 2018; Daher et al., 2020; Palcekova et al., 2020). Additionally, differences in host Fc receptor utilization or activation may contribute to specificity. The interaction of CR3 (CD11b/CD18) and FcgRIII suggests one possible mechanism (Zhou et al., 1993; Galon et al., 1996), but the exact mechanism of antibody-complement interaction remains unclear and is expected to be complex.
Surprisingly, C3 activation by smooth Mab is IgG- and IgM-dependent but appears to be independent of the CP, as evidenced by the lack of killing inhibition after opsonization with C1q-depleted serum, minimal C1q deposition on Mab, and the inability of EDTA to block opsonization. Similarly, the AP does not seem to play a major role, as killing and iC3b deposition are not inhibited by EDTA, and Factor B-depleted serum does not reduce killing. A similar CP- and AP-independent mechanism has been observed for the killing of M. avium (Lenhart-Pendergrass et al., 2023). Reduced MBL2 association with smooth Mab, along with the lack effect of EDTA effects on iC3b deposition or killing, does not support a role for the LP. Furthermore, GlcNAc reduced MBL2 deposition, it did not affect killing. These data suggest that canonical complement activation is not a major driver of smooth Mab killing.
Opsonization of rough Mab also does not appear to involve CP and AP. However, the role of LP in the opsonization of rough Mab remains unclear. Rough Mab preferentially associates with MBL2; however, its killing was not blocked by EDTA, Mg2+/EGTA, mannan, or GlcNAc. Additionally, MBL2 deposition in the presence of these agents did not correlate with killing—reducing divalent cations or adding mannan did not affect MBL2 deposition, while GlcNAc significantly reduced MBL2 deposition. Killing of rough Mab was also unaffected by opsonization with C2- or C4-depleted sera, conditions that prevent MASP2-activated C3 convertase activity. These data suggest that Mab killing involves pathways distinct from canonical C3 activation. This discrepancy may be explained by direct MBL2 or C3 opsonization (Kuhlman et al., 1989; Polotsky et al., 1997; Neth et al., 2000), a mechanism previously demonstrated for M. avium. The surface exposure of PIMs or mannose-capped lipoarabinomannan in rough Mab may underlie the association of MBL2 with rough Mab. In addition, MBL2 can activate C3 in the absence of C2/C4 activation, through a process known as “C2/C4 bypass” (Dumestre-Perard et al., 2008). However, the interaction between C3 and MBL2 on rough Mab remains unresolved. Notably, C3 deposition on rough Mab, while reduced, is still detectable and functionally significant—particularly in high molecular weight conjugates. This observation explains the partial but not complete killing of rough Mab in C3-depleted serum and when CR3 is blocked.
Noncanonical plasma opsonins and complement activators could explain the morphotype-specific profiles of C3 activation. Alternative factors may include members of the pentraxin family (pentraxin 3, C-reactive protein, and serum amyloid P) or ficolins (FCNs), all of which are pattern-recognition receptors associated with complement activation (Haapasalo and Meri, 2019). Preliminary experiments showed little to no deposition of FCN1, FCN2, FCN3, or pentraxin 3 on Mab (data not shown); however, these results may have been limited by poor antibody recognition. In addition, because ficolin binding is Ca2+-dependent (Zacho et al., 2012) and EDTA did not reduce iC3b deposition or killing, it is unlikely that ficolins are involved. Further studies are warranted to clarify alternative pathways for C3 deposition.
Evidence supports the recognition of WP-opsonized Mab by CR3, an integrin receptor that plays a major role in the binding and phagocytosis of iC3b-opsonized pathogens (Dustin, 2016). Antibody blockade of CR3 reduced Mab killing, and the inhibition of killing by EGTA and EDTA further supports the role of integrins. The finding that blocking CR3 antibodies does not affect HIP-opsonized Mab suggests that CR3 does not recognize intrinsic Mab surface molecules. CR3 binds GlcNAc, which may interfere with the interaction of CD11b and FcgRIII (Galon et al., 1996). However, the profound inhibition of killing by GlcNAc and GalNAc in both WP- and HIP-opsonized Mab, when present with neutrophils, indicates that noncomplement lectins are also involved. The greater inhibition by acetylated sugars may also reflect the ability of bacterial lectins to recognize host GlcNAc (Beuth et al., 1988; Kolbe et al., 2019). The killing was not blocked by the neutralizing TLR2 antibody TL2.1 (not shown). Additionally, nonreceptor mycobacterial recognition has previously been described (Nakayama et al., 2016). These data suggest that both CR3 and noncomplement-dependent receptors play key roles in Mab recognition.
This study has limitations. We were unable to completely define the complement activation pathways or the recognition receptors. The deposition of C3 on Mab in the absence of CP, AP, or LP is unusual. Complement activation is activated by both positive and negative regulators, which were not explored. Our studies focused on the role of nonimmune plasma. While we have previously demonstrated the presence of anti-Mab IgG in NTM-infected individuals with CF (Malcolm et al., 2022), the impact of specific immunity on opsonization and killing remains a subject of future investigation. Finally, these studies focus on neutrophils; the role of complement in other immune cells and its in vivo effects require further exploration.
In summary, we have identified C3-dependent killing of Mab by neutrophils, which requires distinct opsonization patterns for the smooth and rough morphotypes. Opsonization of Mab involves noncanonical complement activation that depends on restricted natural antibody classes. Morphotype-specific shifts in complement and antibody use suggest that complement activity may be a disease risk factor and implicate complement in Mab adaptation during chronic infection.
The datasets presented in this article are not readily available. Requests to access the datasets should be directed tobWFsY29sbWtAbmpoZWFsdGgub3Jn.
The studies involving humans were approved by BRANY Institutional Review Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
EW: Data curation, Investigation, Methodology, Writing – review & editing. PL-P: Investigation, Methodology, Writing – review & editing, Conceptualization, Funding acquisition. NR: Writing – review & editing, Data curation, Resources. KP: Data curation, Resources, Writing – review & editing. SC: Data curation, Resources, Writing – review & editing. KC: Writing – review & editing, Investigation. KS: Investigation, Writing – review & editing, Methodology, Resources. JN: Resources, Writing – review & editing, Conceptualization, Funding acquisition. KM: Conceptualization, Funding acquisition, Data curation, Formal Analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing – original draft.
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded in part by grants from the Cystic Fibrosis Foundation to K.C.M. (MALCOL19I0), J.A.N. (NICK18P0, NICK17K0), T.M.L-P. (LENHAR20D0), and K.A.S. and K.C.M. (Alpha-1 Foundation) and was supported by resources provided by the CFF National Resource Center (NICK20Y2-OUT) and the NIH (R01HL146228) to J.A.N.
This manuscript was available as a preprint at https://www.biorxiv.org/content/10.1101/2023.05.15.540822v1.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2025.1445660/full#supplementary-material
Abrahams, K. A., Besra, G. S. (2021). Synthesis and recycling of the mycobacterial cell envelope. Curr. Opin. Microbiol. 60, 58–65. doi: 10.1016/j.mib.2021.01.012
Bernut, A., Herrmann, J. L., Kissa, K., Dubremetz, J. F., Gaillard, J. L., Lutfalla, G., et al. (2014). Mycobacterium abscessus cording prevents phagocytosis and promotes abscess formation. Proc. Natl. Acad. Sci. U S A. 111, E943–E952. doi: 10.1073/pnas.1321390111
Beuth, J., Ko, H. L., Pulverer, G. (1988). The role of staphylococcal lectins in human granulocyte stimulation. Infection. 16, 46–48. doi: 10.1007/BF01646932
Bhakdi, S., Knufermann, H., Fischer, H., Wallach, D. F. (1974). Interaction between erythrocyte membrane proteins and complement components. II. The identification and peptide composition of complement components C3 and C4 desorbed from erythrocyte membranes. Biochim. Biophys. Acta 373, 295–307. doi: 10.1016/0005-2736(74)90153-9
Brady, A. M., Spencer, B. L., Falsey, A. R., Nahm, M. H. (2014). Blood collection tubes influence serum ficolin-1 and ficolin-2 levels. Clin. Vaccine Immunol. 21, 51–55. doi: 10.1128/CVI.00607-13
Bryant, J. M., Grogono, D. M., Rodriguez-Rincon, D., Everall, I., Brown, K. P., Moreno, P., et al. (2016). Emergence and spread of a human-transmissible multidrug-resistant nontuberculous mycobacterium. Science. 354, 751–757. doi: 10.1126/science.aaf8156
Byrd, T. F., Lyons, C. R. (1999). Preliminary characterization of a Mycobacterium abscessus mutant in human and murine models of infection. Infect. Immun. 67, 4700–4707. doi: 10.1128/IAI.67.9.4700-4707.1999
Catherinot, E., Roux, A. L., Macheras, E., Hubert, D., Matmar, M., Dannhoffer, L., et al. (2009). Acute respiratory failure involving an R variant of Mycobacterium abscessus. J. Clin. Microbiol. 47, 271–274. doi: 10.1128/JCM.01478-08
Daher, W., Leclercq, L. D., Viljoen, A., Karam, J., Dufrene, Y. F., Guerardel, Y., et al. (2020). O-methylation of the glycopeptidolipid acyl chain defines surface hydrophobicity of mycobacterium abscessus and macrophage invasion. ACS Infect. Dis. 6, 2756–2770. doi: 10.1021/acsinfecdis.0c00490
Daley, C. L., Iaccarino, J. M., Lange, C., Cambau, E., Wallace, R. J., Andrejak, C., et al. (2020). Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Clin. Infect. Dis. 71, 905–913. doi: 10.1093/cid/ciaa1125
Davidson, R. M., Hasan, N. A., Epperson, L. E., Benoit, J. B., Kammlade, S. M., Levin, A. R., et al. (2021). Population genomics of mycobacterium abscessus from U.S. Cystic fibrosis care centers. Ann. Am. Thorac. Soc. 18, 1960–1969. doi: 10.1513/AnnalsATS.202009-1214OC
Dubois, V., Viljoen, A., Laencina, L., Le Moigne, V., Bernut, A., Dubar, F., et al. (2018). MmpL8(MAB) controls Mycobacterium abscessus virulence and production of a previously unknown glycolipid family. Proc. Natl. Acad. Sci. U S A. 115, E10147–E10E56. doi: 10.1073/pnas.1812984115
Dumestre-Perard, C., Lamy, B., Aldebert, D., Lemaire-Vieille, C., Grillot, R., Brion, J. P., et al. (2008). Aspergillus conidia activate the complement by the mannan-binding lectin C2 bypass mechanism. J. Immunol. 181, 7100–7105. doi: 10.4049/jimmunol.181.10.7100
Dustin, M. L. (2016). Complement receptors in myeloid cell adhesion and phagocytosis. Microbiol. Spectr. 4. doi: 10.1128/microbiolspec.MCHD-0034-2016
Fidler, K. J., Hilliard, T. N., Bush, A., Johnson, M., Geddes, D. M., Turner, M. W., et al. (2009). Mannose-binding lectin is present in the infected airway: a possible pulmonary defence mechanism. Thorax. 64, 150–155. doi: 10.1136/thx.2008.100073
Galon, J., Gauchat, J. F., Mazieres, N., Spagnoli, R., Storkus, W., Lotze, M., et al. (1996). Soluble Fcgamma receptor type III (FcgammaRIII, CD16) triggers cell activation through interaction with complement receptors. J. Immunol. 157, 1184–1192. doi: 10.4049/jimmunol.157.3.1184
Haapasalo, K., Meri, S. (2019). Regulation of the complement system by pentraxins. Front. Immunol. 10, 1750. doi: 10.3389/fimmu.2019.01750
Hartmann, P., Becker, R., Franzen, C., Schell-Frederick, E., Romer, J., Jacobs, M., et al. (2001). Phagocytosis and killing of Mycobacterium avium complex by human neutrophils. J. Leukoc. Biol. 69, 397–404. doi: 10.1189/jlb.69.3.397
Hayes, D., Jr., Shukla, R. K., Cheng, Y., Gecili, E., Merling, M. R., Szczesniak, R. D., et al. (2022). Tissue-localized immune responses in people with cystic fibrosis and respiratory nontuberculous mycobacteria infection. JCI Insight 7. doi: 10.1172/jci.insight.157865
Hedin, W., Froberg, G., Fredman, K., Chryssanthou, E., Selmeryd, I., Gillman, A., et al. (2023). A rough colony morphology of mycobacterium abscessus is associated with cavitary pulmonary disease and poor clinical outcome. J. Infect. Dis. 227, 820–827. doi: 10.1093/infdis/jiad007
Honda, J. R., Virdi, R., Chan, E. D. (2018). Global environmental nontuberculous mycobacteria and their contemporaneous man-made and natural niches. Front. Microbiol. 9, 2029. doi: 10.3389/fmicb.2018.02029
Howard, S. T., Rhoades, E., Recht, J., Pang, X., Alsup, A., Kolter, R., et al. (2006). Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology 152, 1581–1590. doi: 10.1099/mic.0.28625-0
Ip, W. K., Takahashi, K., Ezekowitz, R. A., Stuart, L. M. (2009). Mannose-binding lectin and innate immunity. Immunol. Rev. 230, 9–21. doi: 10.1111/j.1600-065X.2009.0078.x
Khandelwal, S., Ravi, J., Rauova, L., Johnson, A., Lee, G. M., Gilner, J. B., et al. (2018). Polyreactive IgM initiates complement activation by PF4/heparin complexes through the classical pathway. Blood. 132, 2431–2440. doi: 10.1182/blood-2018-03-834598
Kim, S. W., Subhadra, B., Whang, J., Back, Y. W., Bae, H. S., Kim, H. J., et al. (2017). Clinical Mycobacterium abscessus strain inhibits autophagy flux and promotes its growth in murine macrophages. Pathog. Dis. 75. doi: 10.1093/femspd/ftx107
Kolbe, K., Veleti, S. K., Reiling, N., Lindhorst, T. K. (2019). Lectins of Mycobacterium tuberculosis - rarely studied proteins. Beilstein J. Org Chem. 15, 1–15. doi: 10.3762/bjoc.15.1
Kuhlman, M., Joiner, K., Ezekowitz, R. A. (1989). The human mannose-binding protein functions as an opsonin. J. Exp. Med. 169, 1733–1745. doi: 10.1084/jem.169.5.1733
Laarman, A. J., Ruyken, M., Malone, C. L., van Strijp, J. A., Horswill, A. R., Rooijakkers, S. H. (2011). Staphylococcus aureus metalloprotease aureolysin cleaves complement C3 to mediate immune evasion. J. Immunol. 186, 6445–6453. doi: 10.4049/jimmunol.1002948
Lenhart-Pendergrass, P. M., Malcolm, K. C., Wheeler, E., Rysavy, N. M., Poch, K., Caceres, S., et al. (2023). Deficient complement opsonization impairs mycobacterium avium killing by neutrophils in cystic fibrosis. Microbiol. Spectr. 11, e0327922. doi: 10.1128/spectrum.03279-22
Lutz, H. U., Nater, M., Stammler, P. (1993). Naturally occurring anti-band 3 antibodies have a unique affinity for C3. Immunology. 80, 191–196.
Malcolm, K. C. (2018). Measuring neutrophil bactericidal activity. Methods Mol. Biol. 1809, 139–144. doi: 10.1007/978-1-4939-8570-8_12
Malcolm, K. C., Caceres, S. M., Pohl, K., Poch, K. R., Bernut, A., Kremer, L., et al. (2018). Neutrophil killing of Mycobacterium abscessus by intra- and extracellular mechanisms. PloS One 13, e0196120. doi: 10.1371/journal.pone.0196120
Malcolm, K. C., Nichols, E. M., Caceres, S. M., Kret, J. E., Martiniano, S. L., Sagel, S. D., et al. (2013). Mycobacterium abscessus induces a limited pattern of neutrophil activation that promotes pathogen survival. PloS One 8, e57402. doi: 10.1371/journal.pone.0057402
Malcolm, K. C., Wheeler, E. A., Calhoun, K., Lenhart-Pendergrass, P. M., Rysavy, N., Poch, K. R., et al. (2022). Specificity of immunoglobulin response to nontuberculous mycobacteria infection in people with cystic fibrosis. Microbiol. Spectr. 10, e0187422. doi: 10.1128/spectrum.01874-22
Martiniano, S. L., Sontag, M. K., Daley, C. L., Nick, J. A., Sagel, S. D. (2013). Clinical significance of a first positive nontuberculous mycobacteria culture in cystic fibrosis. Ann. Am. Thorac. Soc 11, 36–44. doi: 10.1513/AnnalsATS.201309-310OC
Nakayama, H., Kurihara, H., Morita, Y. S., Kinoshita, T., Mauri, L., Prinetti, A., et al. (2016). Lipoarabinomannan binding to lactosylceramide in lipid rafts is essential for the phagocytosis of mycobacteria by human neutrophils. Sci. Signal 9, ra101. doi: 10.1126/scisignal.aaf1585
Neth, O., Jack, D. L., Dodds, A. W., Holzel, H., Klein, N. J., Turner, M. W. (2000). Mannose-binding lectin binds to a range of clinically relevant microorganisms and promotes complement deposition. Infect. Immun. 68, 688–693. doi: 10.1128/IAI.68.2.688-693.2000
Nick, J. A., Daley, C. L., Lenhart-Pendergrass, P. M., Davidson, R. M. (2021). Nontuberculous mycobacteria in cystic fibrosis. Curr. Opin. Pulm Med. 27, 586–592. doi: 10.1097/MCP.0000000000000816
Nick, J. A., Dedrick, R. M., Gray, A. L., Vladar, E. K., Smith, B. E., Freeman, K. G., et al. (2022). Host and pathogen response to bacteriophage engineered against Mycobacterium abscessus lung infection. Cell. 185, 1860–74 e12. doi: 10.1016/j.cell.2022.04.024
Palcekova, Z., Gilleron, M., Angala, S. K., Belardinelli, J. M., McNeil, M., Bermudez, L. E., et al. (2020). Polysaccharide succinylation enhances the intracellular survival of mycobacterium abscessus. ACS Infect. Dis. 6, 2235–2248. doi: 10.1021/acsinfecdis.0c00361
Pohl, K., Grimm, X. A., Caceres, S. M., Poch, K. R., Rysavy, N., Saavedra, M., et al. (2020). Mycobacterium abscessus clearance by neutrophils is independent of autophagy. Infect. Immun. 88. doi: 10.1128/IAI.00024-20
Polotsky, V. Y., Belisle, J. T., Mikusova, K., Ezekowitz, R. A., Joiner, K. A. (1997). Interaction of human mannose-binding protein with Mycobacterium avium. J. Infect. Dis. 175, 1159–1168. doi: 10.1086/jid.1997.175.issue-5
Rhoades, E. R., Archambault, A. S., Greendyke, R., Hsu, F. F., Streeter, C., Byrd, T. F. (2009). Mycobacterium abscessus Glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J. Immunol. 183, 1997–2007. doi: 10.4049/jimmunol.0802181
Rottman, M., Catherinot, E., Hochedez, P., Emile, J. F., Casanova, J. L., Gaillard, J. L., et al. (2007). Importance of T cells, gamma interferon, and tumor necrosis factor in immune control of the rapid grower Mycobacterium abscessus in C57BL/6 mice. Infect. Immun. 75, 5898–5907. doi: 10.1128/IAI.00014-07
Roux, A. L., Ray, A., Pawlik, A., Medjahed, H., Etienne, G., Rottman, M., et al. (2011). Overexpression of proinflammatory TLR-2-signalling lipoproteins in hypervirulent mycobacterial variants. Cell Microbiol. 13, 692–704. doi: 10.1111/j.1462-5822.2010.01565.x
Roux, A. L., Viljoen, A., Bah, A., Simeone, R., Bernut, A., Laencina, L., et al. (2016). The distinct fate of smooth and rough Mycobacterium abscessus variants inside macrophages. Open Biol. 6. doi: 10.1098/rsob.160185
Sanguinetti, M., Ardito, F., Fiscarelli, E., La Sorda, M., D’Argenio, P., Ricciotti, G., et al. (2001). Fatal pulmonary infection due to multidrug-resistant Mycobacterium abscessus in a patient with cystic fibrosis. J. Clin. Microbiol. 39, 816–819. doi: 10.1128/JCM.39.2.816-819.2001
Schiller, N. L., Friedman, G. L., Roberts, R. B. (1979). The role of natural IgG and complement in the phagocytosis of type 4 Neisseria gonorrhoeae by human polymorphonuclear leukocytes. J. Infect. Dis. 140, 698–707. doi: 10.1093/infdis/140.5.698
Schwartz, J. T., Barker, J. H., Long, M. E., Kaufman, J., McCracken, J., Allen, L. A. (2012). Natural IgM mediates complement-dependent uptake of Francisella tularensis by human neutrophils via complement receptors 1 and 3 in nonimmune serum. J. Immunol. 189, 3064–3077. doi: 10.4049/jimmunol.1200816
Sepulcri, C., Vena, A., Bassetti, M. (2023). Skin and soft tissue infections due to rapidly growing mycobacteria. Curr. Opin. Infect. Dis. 36, 74-80. doi: 10.1097/QCO.0000000000000905
Sung, P. S., Chang, W. C., Hsieh, S. L. (2020). CLEC5A: A promiscuous pattern recognition receptor to microbes and beyond. Adv. Exp. Med. Biol. 1204, 57–73. doi: 10.1007/978-981-15-1580-4_3
Ueda, T., Rieu, P., Brayer, J., Arnaout, M. A. (1994). Identification of the complement iC3b binding site in the beta 2 integrin CR3 (CD11b/CD18). Proc. Natl. Acad. Sci. U S A. 91, 10680–10684. doi: 10.1073/pnas.91.22.10680
Victoria, L., Gupta, A., Gomez, J. L., Robledo, J. (2021). Mycobacterium abscessus complex: A Review of Recent Developments in an Emerging Pathogen. Front. Cell Infect. Microbiol. 11, 659997. doi: 10.3389/fcimb.2021.659997
Young, R. L., Malcolm, K. C., Kret, J. E., Caceres, S. M., Poch, K. R., Nichols, D. P., et al. (2011). Neutrophil extracellular trap (NET)-mediated killing of Pseudomonas aeruginosa: evidence of acquired resistance within the CF airway, independent of CFTR. PloS One 6, e23637. doi: 10.1371/journal.pone.0023637
Zacho, R. M., Jensen, L., Terp, R., Jensenius, J. C., Thiel, S. (2012). Studies of the pattern recognition molecule H-ficolin: specificity and purification. J. Biol. Chem. 287, 8071–8081. doi: 10.1074/jbc.M111.301044
Zhou, M., Todd, R. F., 3rd, van de Winkel, J. G., Petty, H. R. (1993). Cocapping of the leukoadhesin molecules complement receptor type 3 and lymphocyte function-associated antigen-1 with Fc gamma receptor III on human neutrophils. Possible role of lectin-like interactions. J. Immunol. 150, 3030–3041. doi: 10.4049/jimmunol.150.7.3030
Keywords: cystic fibrosis, natural antibodies, complement, neutrophils, adaptation
Citation: Wheeler EA, Lenhart-Pendergrass PM, Rysavy NM, Poch KR, Caceres SM, Calhoun KM, Serban KA, Nick JA and Malcolm KC (2025) Divergent host humoral innate immune response to the smooth-to-rough adaptation of Mycobacterium abscessus in chronic infection. Front. Cell. Infect. Microbiol. 15:1445660. doi: 10.3389/fcimb.2025.1445660
Received: 07 June 2024; Accepted: 20 February 2025;
Published: 18 March 2025.
Edited by:
Edith Porter, California State University, Los Angeles, United StatesReviewed by:
Thomas F. Byrd, University of New Mexico, United StatesCopyright © 2025 Wheeler, Lenhart-Pendergrass, Rysavy, Poch, Caceres, Calhoun, Serban, Nick and Malcolm. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kenneth C. Malcolm, bWFsY29sbWtAbmpoZWFsdGgub3Jn
†Present address: Patricia M. Lenhart-Pendergrass, Department of Pediatrics University of California San Diego, La Jolla, CA, United States Karina A. Serban, Department of Medicine, University of Florida, Gainesville, FL, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.