- 1Department of Diagnostic Sciences, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
- 2Faculty of Medicine, Catholic University of Bukavu, Bukavu, Democratic Republic of Congo
- 3Department of Obstetrics and Gynecology, Hôpital Provincial Général de Référence de Bukavu, Bukavu, Democratic Republic of Congo
- 4Department of Internal Medicine and Pediatrics, Faculty of Medicine and Health Sciences, Ghent University, Ghent, Belgium
Background: Gardnerella is a key pathogen in bacterial vaginosis (BV), but the role of the different Gardnerella species remains unclear. We investigated the role of four Gardnerella species, as well as Fannyhessea vaginae, Lactobacillus crispatus and L. iners in BV.
Methods: From 331 pregnant women from the Democratic Republic of the Congo, BV was diagnosed using Nugent scoring and a cervicovaginal lavage was used to quantify G. leopoldii, G. piotii, G. swidsinskii, G. vaginalis, F. vaginae, L. crispatus and L. iners by qPCR. Univariate associations between these species and clinical outcomes were assessed. A logistic regression model and ROC curves were calculated to determine the best diagnostic marker for BV.
Results: Here, L. iners (75.8%) was the most prevalent species and G. vaginalis (36.0%) the most common Gardnerella species. All investigated Gardnerella spp. were prevalent (50.9-57.9%) in women with (asymptomatic) BV. Univariate analysis revealed no significant associations with clinical symptoms of BV, while F. vaginae (positive Whiff test, high pH), G. vaginalis (high pH) and L. crispatus (low pH) were associated with signs of BV. G. piotii was associated with markers of urinary tract infection. Women with L. iners had higher odds of delivering preterm. ROC analyses showed that F. vaginae was the best marker for BV (AUC 0.81), and the combined model further increased the diagnostic performance (AUC 0.90).
Conclusion: All Gardnerella species were involved in BV, although none were associated with the most important clinical symptoms of BV and none emerged as a superior molecular marker for BV.
Introduction
Bacterial vaginosis (BV) is the most common gynecological condition among women of reproductive age worldwide (Allsworth and Peipert, 2007), with a prevalence among the general population of approximately 25% both globally (Peebles et al., 2019) and in sub-Saharan Africa (Park et al., 2024). Besides being a discomforting and often recurrent/chronic condition, BV is also considered a significant risk factor for adverse pregnancy outcomes such as preterm birth (PTB) and low birthweight (LBW) (Hillier et al., 1995). Furthermore, BV has been associated with an increased risk for the acquisition of sexually transmitted infections, such as human immunodeficiency virus (HIV) (Atashili et al., 2008), herpes simplex virus-2 (HSV-2) (Cherpes et al., 2005), Trichomonas vaginalis, Neisseria gonorrhoeae and Chlamydia trachomatis (Brotman et al., 2010).
Microbiologically, BV is characterized by a dysbiosis of the healthy vaginal microbiome (VMB) (Muzny et al., 2019), normally characterized by the predominance of only a single Lactobacillus species, mostly L. crispatus or L. iners (Vaneechoutte, 2017a). The vaginal microbiome can be categorized into community-state types (CSTs), with CST-I representing a vaginal microbiome dominated by L. crispatus, CSTI-II dominated by L. gasseri, CST-III dominated by L. iners, CST-IV representing a diverse microbiome and finally CST-V dominated by L. jensenii (Ravel et al., 2011). The lactobacilli-dominated environment has a low pH due to production of lactic acid by lactobacilli, making the vagina a hostile environment for many pathogens, although it is generally known that L. iners is less protective than L. crispatus (Vaneechoutte, 2017a). In BV, the VMB is replaced by a polymicrobial VMB with Gardnerella as the key marker (Muzny et al., 2018). However, also L. iners has been found among women with BV (Vaneechoutte, 2017b). Additionally, Gardnerella has also been isolated from healthy women in several studies (Hickey and Forney, 2014). G. vaginalis was long considered the single species within the Gardnerella genus. This is because the 16S rRNA gene, which forms the basis for bacterial taxonomy and microbiome studies (Clarridge, 2004), lacks resolution needed to distinguish the different Gardnerella species (Vaneechoutte et al., 2019). In 2019, Vaneechoutte and coworkers showed the existence of minimum thirteen genomospecies in the genus (Vaneechoutte et al., 2019), of which six have been named validly: Gardnerella greenwoodii, G. leopoldii, G. pickettii, G. piotii, G. swidsinskii and G. vaginalis (Vaneechoutte et al., 2019; Sousa et al., 2023).
Gardnerella species mostly cooccur in the VMB rather than being present as a single species (Hill and Albert, 2019; Munch et al., 2024; Schuster et al., 2024) and their clinical relevance has been under investigation for several years. In non-pregnant reproductive-aged Canadian women, Hill and coworkers (2019) already demonstrated, using a cpn60 sequencing approach, that G. vaginalis and G. swidsinskii, but not G. leopoldii and G. piotii, were associated with vaginal discharge and malodor, typical symptoms of BV (Hill and Albert, 2019). Turner and coworkers showed that, in American women with recurrent BV, persistently high concentrations of genomospecies 12 (as defined by Vaneechoutte and colleagues (Vaneechoutte et al., 2019)) were associated with refractory responses after metronidazole treatment of BV, and persistently low concentrations of genomospecies 12 and G. swidsinskii/G. leopoldii with remission (Turner et al., 2021). Munch and coworkers recently documented that American non-pregnant BV-negative women colonized with three or more Gardnerella species had higher chance for incident BV within 100 days compared to women colonized with fewer Gardnerella species (Munch et al., 2024). In a cohort of pregnant women at high risk of recurrent preterm birth, G. leopoldii, but not G. piotii, G. swidsinskii or G. vaginalis, was associated with spontaneous preterm birth (Schuster et al., 2024).
However, the different Gardnerella spp. have not yet been studied in African women, known to harbor a VMB that can differ significantly compared to Caucasian women (Fettweis et al., 2014). Therefore, we aimed to investigate the distribution of G. leopoldii, G. piotii, G. swidsinskii and G. vaginalis and the correlates with clinical signs and symptoms and adverse pregnancy outcomes using species-specific quantitative PCR (qPCR) assays in a population of pregnant women from the Democratic Republic of the Congo.
Materials and methods
Ethical approval
Ethical approval was obtained by the Internal Review Board of the Catholic University of Bukavu (reference number UCB/CIE/NC/016/2016), the Ministry of Public Health (reference number 062/CD/DPS/SK/2017) and the Ethical Committee of Ghent University Hospital (reference number PA2014/003). All pregnant women that participated in this study signed an informed consent form.
Study design and population
This research was part of the AVEONS (acronym for Angamiza Vizuri (Swahili for ‘stop’) Early Onset Neonatal Sepsis) study. The AVEONS project had the overall aim to study the prevalence and clinical correlates of vaginal infections in a population of pregnant women from Bukavu, Democratic Republic of the Congo (DRC). The prevalence (26.3%), risk factors and adverse pregnancy outcomes of second trimester BV [assessed by microscopy (Nugent score)], as well as the study design and population have been described elsewhere (Mulinganya et al., 2021). Briefly, the AVEONS study was a prospective observational study where pregnant women were seen between 16 and 20 weeks of gestation [visit 1 (V1)], between 36 and 38 weeks (V2) and at delivery. Participants were recruited from January to October 2017 at the Provincial Referral Hospital of Bukavu (PRHB). Pregnant women visiting the PRHB for antenatal care were asked whether they were interested in participation, whereafter eligible women were individually informed about the study details. Women were considered eligible when they (i) were between 16 and 20 weeks pregnant, (ii) accepted to be followed by a referral hospital team, (iii) were willing to deliver at PRHB, and (iv) agreed to be contacted by phone or other means. Women could not be included in the study (i) if they planned to move out of the study area during their pregnancy, (ii) in case of genital bleeding, (iii) in case of twin pregnancies or a visible malformation of the fetus at ultrasound examination, or (iv) in case they used antibiotics during the two weeks before recruitment. The research described here only reports data on the VMB and clinical correlates at V1, and pregnancy outcomes (such as PTB and LBW).
Routine antenatal care and delivery procedures
At V1, a questionnaire on sociodemographics, reproductive health history, sexual activity, vaginal practices and vaginal complaints was completed by each participant. A general physical examination, including anthropometric measurements, and a gynecological examination, including a speculum examination with a sterile non-moistened speculum, were performed. The vaginal mucosa and cervix were inspected for the presence of sores and tumors, and a diagnosis of vaginal infection was made according to the syndromic-based protocol for the management of pregnancy of the Ministry of Public Health of DRC (Ministère de la Santé RDdC, 2006), which is based on previous WHO recommendations (World Health, 2007). When a vaginal infection was diagnosed, women were treated empirically with a combination (in one vaginal ovule) of clotrimazole (200 mg) (against candidiasis) and clindamycin (100 mg) (against BV) once a day for six days, in accordance with the local protocol. In case a woman was allergic to clindamycin, this was replaced by metronidazole (in a vaginal ovule). During the gynecological examination, the vaginal pH was also determined by means of indicator pH papers (Hilo Indicator® pH paper, Sigma Aldrich). In addition, an ultrasound examination was performed to determine the viability of the fetus and to measure cervical length. Furthermore, five mL of total blood was collected in a VacuTube® (Becton Dickinson) red tube without anticoagulant for HIV, malaria and hemoglobin testing. Next, midstream urine was collected in a sterile container to investigate the presence of nitrite and white blood cells (indicative for urinary tract infections or bacteriuria) using dipsticks (Multistix dipsticks®, Siemens). Lastly, three vaginal swabs were taken from the midportion of the lateral vaginal wall, and a cervicovaginal lavage (CVL) sample was collected by rinsing the cervical mucosa with 5 mL of sterile physiological water and collecting as much lavage as possible into a VacuTube®. These CVL samples were stored at -20°C for shipment on dry ice to the Laboratory Bacteriology Research (LBR) (Ghent University, Ghent, Belgium) respecting the cold chain. All participants followed routine antenatal care and they received a single dose of mebendazole (500 mg) against intestinal worm infections and a single dose of sulfadiazine-pyrimethamine (500 mg) against malaria. At delivery, the labor was monitored, and delivery features and pregnancy outcomes were collected by nurses and the senior assistant.
Study specific laboratory procedures
Microscopic examinations
At the PRHB, a wet mount slide was prepared within 20 minutes after collection of a vaginal swab. Saline (0.5 mL) was added to the swab and one droplet of this was put on a glass slide and covered with a cover slip. The presence of Trichomonas vaginalis, Candida, white blood cells and clue cells was determined with microscopy.
A second vaginal swab was rolled on a glass slide and fixated by briefly passing the back of this slide through a flame. Subsequently, these smears were stored and shipped to the LBR, where they were Gram-stained at the Department of Laboratory Medicine (Ghent University Hospital, Ghent, Belgium) using an automated PolyStainer (IUL). These Gram-stained slides were used to diagnose BV according to Nugent as described previously (Mulinganya et al., 2021).
DNA extraction
A random selection was made of 331 CVLs to be analyzed by qPCR. DNA was extracted from CVLs using the RNeasy PowerMicrobiome Kit (Qiagen) according to manufacturer’s instructions, with minor modifications (i.e. DNase treatment was omitted). The DNA extracts were then stored at -20°C until use.
qPCR assays
Species-specific qPCR assays were used to quantify Fannyhessea (Atopobium) vaginae, G. leopoldii, G. piotii, G. swidsinskii, G. vaginalis, L. crispatus and L. iners. The species-specific qPCR assays for G. leopoldii, G. piotii, G. swidsinskii and G. vaginalis were designed and validated in-house (Latka et al., 2022). All reactions were carried out in a total volume of 10 µL, containing 1X LightCycler 480 SYBR Green I master mix (Roche), forward and reverse primer (listed in Table 1) and 2 µL of DNA extract, DNA standard (positive control and calibration) or molecular water (negative control). DNA was extracted from cultured F. vaginae (CCUG 38953T), G. leopoldii (UGent 09.48), G. piotii (UGent 18.01T), G. swidsinskii (GS10234), G. vaginalis (GvB LMG7832T), L. crispatus (LMG 9479T) and L. iners (FB123-CNA-4) using the High Pure PCR Template Preparation Kit (Roche) as previously described (De Keyser, 2020) and used to make a tenfold standard dilution series to generate qPCR standard curves.
Reactions were carried out on a LightCycler 480 (Roche). qPCRs were performed for F. vaginae by pre-incubation for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 20 s at 62°C and 40 s at 72°C, for Gardnerella species by pre-incubation 5 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 56°C and 30 s at 72°C, for L. crispatus by pre-incubation for 10 min at 95°C, followed by 40 cycles of 15 s at 95°C, 30 s at 60°C and 30 s at 72°C, and for L. iners by pre-incubation for 10 min at 95°C, followed by 40 cycles of 10s at 95°C, 20 s at 50°C and 4 s at 72°C.
High resolution melting curves were generated by first melting all amplified dsDNA at 95°C for 5 s, followed by renaturating the DNA for 30 s at 50°C (F. vaginae), 1 min at 55°C (Gardnerella species), or 1 min at 60°C (L. crispatus and L. iners), whereafter the temperature was increased to 97°C at a ramp rate of 0.02°C/s. Specific target amplification was defined based on a melting temperature within a range of 1°C of the mean of the standard dilution series of the respective assays. All raw data was analyzed with the standard LightCycler 480 Software Version 1.5 (Roche). All bacterial concentrations were expressed in genome equivalents/mL (GE/mL).
Data analysis
First, the presence of each bacterial species as assessed by qPCR was defined as a binary variable and was used to calculate prevalences and univariate associations. The prevalence of each bacterial species was documented together with the corresponding 95% confidence interval (CI), which was calculated using the Wilson method. The median and range of (log-transformed) concentrations of the different species among positive women were documented in boxplots. Next, the Pearson correlation coefficients between the different bacterial concentrations were calculated with the R programming language to document the correlations between the species and were illustrated in a correlation matrix created with heatmapper.ca (http://www.heatmapper.ca/pairwise/). The Pearson correlation coefficients were defined as negligible (0.00-0.10), weak (0.10-0.39), moderate (0.40-0.69), strong (0.70-0.89), or very strong (0.90-1.00) (Schober et al., 2018). To identify the best molecular markers for BV, receiver-operator characteristic (ROC) curves were created based on the log-transformed bacterial concentrations and the categorization of BV based on Nugent (healthy and intermediate scores seen as negative for BV) using the R package pROC (Robin et al., 2011). A logistic regression model was made to investigate the best combination of markers.
The log-transformed bacterial concentrations were used as continuous variables to create VMB profiles and were visualized in a heatmap using the R function pheatmap() (Kolde, 2019). On this heatmap, clusters of VMB profiles were visually defined after hierarchical clustering. Species were also hierarchically clustered on the heatmap. We defined clusters by cutting the tree at a height (indicating dissimilarity) of 110 using the function cutree(). This height was chosen because it yielded two expected clusters of healthy VMB (one dominated by L. crispatus and one dominated by L. iners).
Subsequently, univariate associations between the bacterial species and clusters on the one hand, and clinical signs and symptoms and pregnancy outcomes on the other hand were determined. For this, the presence of each bacterial species/cluster was defined as the independent variable in each univariate analysis, and clinical signs and symptoms of mother and neonate, as well as pregnancy outcomes, were defined as dependent variables. PTB was defined as birth before 37 completed weeks of gestation and low birthweight as a birthweight below 2500 g. Fisher exact tests were performed to determine which of these associations were significant (p-value <0.05) and odds ratios (OR) were reported for each binary variable. For the parameters that showed a significant p-value when comparing clusters, specific T-tests were performed to compare all clusters against a reference cluster. Here, cluster 6, dominated by L. crispatus and mainly containing women with a healthy VMB according to Nugent, was selected as reference cluster for these one-on-one comparative analyses. All statistical analyses described above were performed with R Studio (version 2024.09.1 + 394) or GraphPad Prism [version 10.0.3 (217)].
Results
Participant flow and sociodemographics
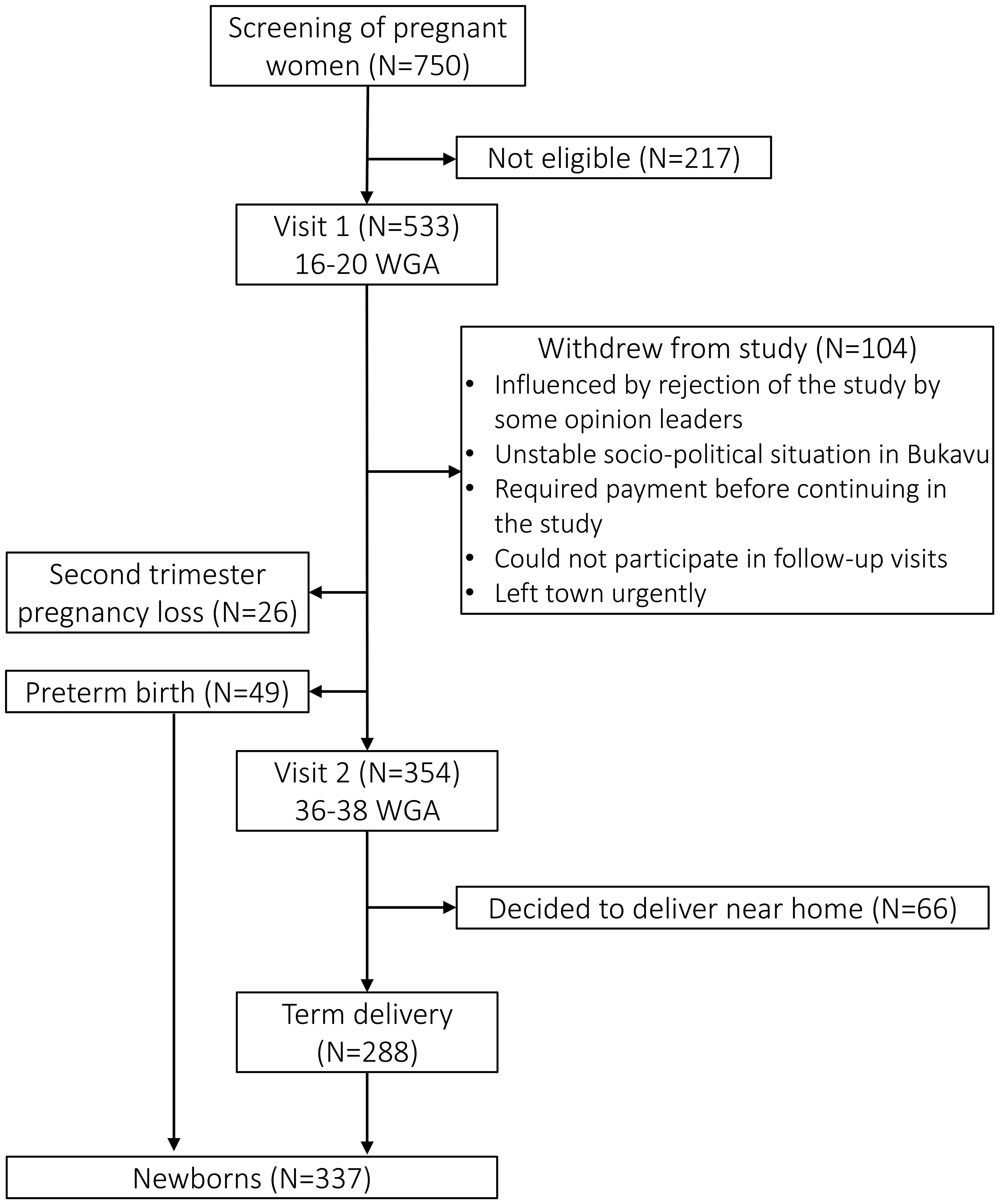
The flowchart depicted in Figure 1 describes the number of pregnant women and neonates withheld at each visit. A total of 750 women were screened, of which 533 were found eligible and included in the study (at V1). Roughly one fifth of these women withdrew from the study before V2, mostly due to rejection of the study by some opinion leaders (who wrongly believed participants were given a substantial imbursement) and the instable socio-political situation in Bukavu during the study period. Of the 533 women included, a second trimester pregnancy loss happened in 26 women (4.9%), and 49 neonates (9.2%) were born preterm. Of the 354 women who completed V2, 66 (18.6%) withdrew from the study because they decided to not deliver at PRHB. The 288 other neonates (81.4%) were all born at term at PRHB.
The complete AVEONS study population has previously been described in detail (Mulinganya et al., 2024). The demographics are summarized in Table 2. There were no statistically significant differences in terms of demographics and Nugent score at V1 in the 331 participants whose CVLs were selected for qPCR analysis compared to the overall study population (data not shown). Among these 331 women, 172 gave birth at term and 30 PTBs were observed.
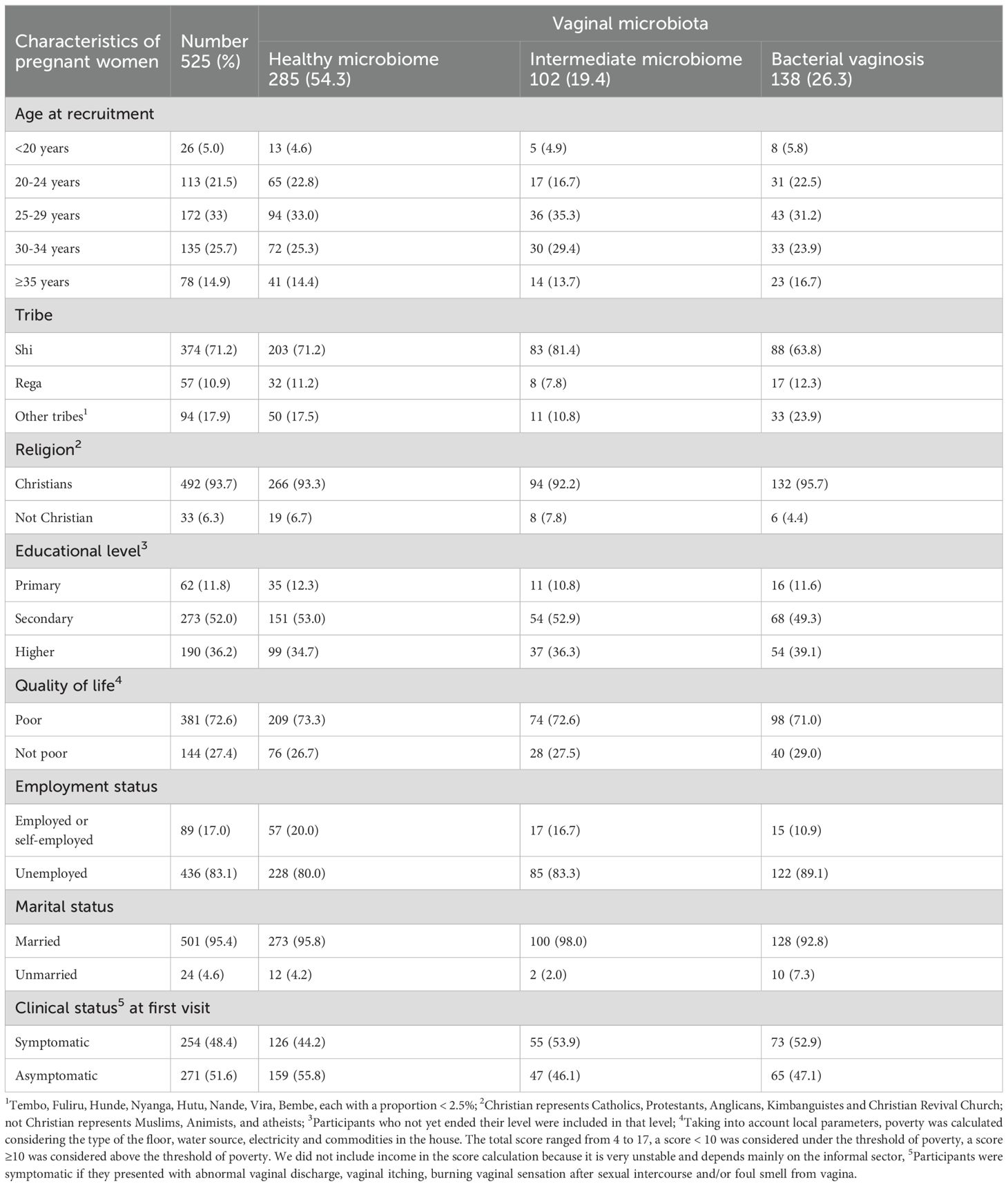
Table 2. Sociodemographic characteristics and pregnancy outcomes of the study population stratified by vaginal microbiota categorization.
Distribution and concentration of the bacterial species
The prevalence, overall and across VMB categories, and the median and range of the (log10-transformed) concentrations of each species among positive women are presented in Figure 2. Overall, L. iners was the most prevalent species (75.8%) and had the highest median concentration (8.31 log10 GE/ml). We found that 36.0% of women were positive for G. vaginalis, making it the most common Gardnerella species in this population. G. leopoldii was the least prevalent Gardnerella species, with a prevalence of 14.5%. G. swidsinskii had the highest median concentration among Gardnerella species (8.04 log10 GE/ml) while G. piotii had the lowest median concentration (6.48 log10 GE/ml).
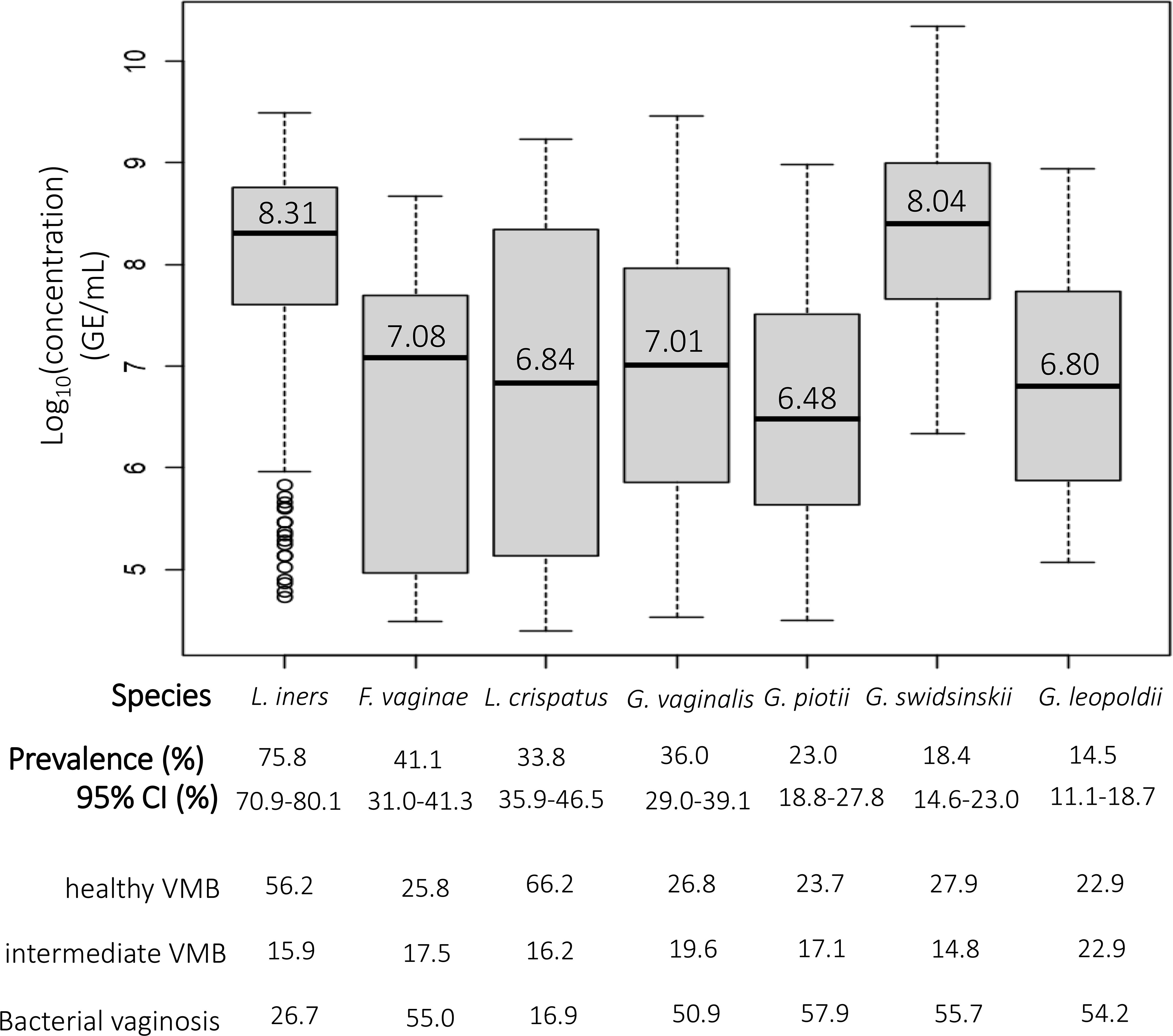
Figure 2. The prevalence and median concentrations of the different species. The black horizontal line in each boxplot represents the median concentration of each species among women positive for that species, which is also shown above this line; the bottom and top edge of the grey boxes represent the first (25% of the data is below this value) and third quartile (75% of the data is below this value), respectively; bottom and top end of the dotted line represent minimum and maximum, respectively; open circles represent outliers.
In total, 157 women (47.4%) were not colonized with Gardnerella species, 82 women (24.8%) were colonized with only a single Gardnerella species, 62 women (18.7%) were colonized with two Gardnerella species, 29 women (8.8%) were colonized with 3 Gardnerella species and one woman (0.3%) was colonized with all four Gardnerella species. Overall, Gardnerella and F. vaginae occurred mostly among women with BV, more specifically 54.2% of all G. leopoldii was found among women with BV, and this was 57.9% for G. piotii, 55.7% for G. swidsinskii, 50.9% for G. vaginalis and 55.0% for F. vaginae. In contrast, L. crispatus and L. iners were most abundant among women with a healthy VMB (66.2% and 56.2% of L. crispatus and L. iners, respectively).
Correlations between the different species
Figure 3 presents the Pearson correlation coefficients between the concentrations of the different species. The strongest correlation was seen between G. swidsinskii and F. vaginae (r = 0.403, moderate correlation). There was a significantly weak negative correlation between L. iners and L. crispatus (r = -0.172), and a significantly weak positive correlation between L. iners and G. piotii (r = 0.143). Furthermore, a significantly weak positive correlation was seen between G. swidsinskii and G. vaginalis (r = 0.155), G. swidsinskii and G. piotii (r = 0.234), F. vaginae and G. leopoldii (r = 0.139), and F. vaginae and G. piotii (r = 0.126).
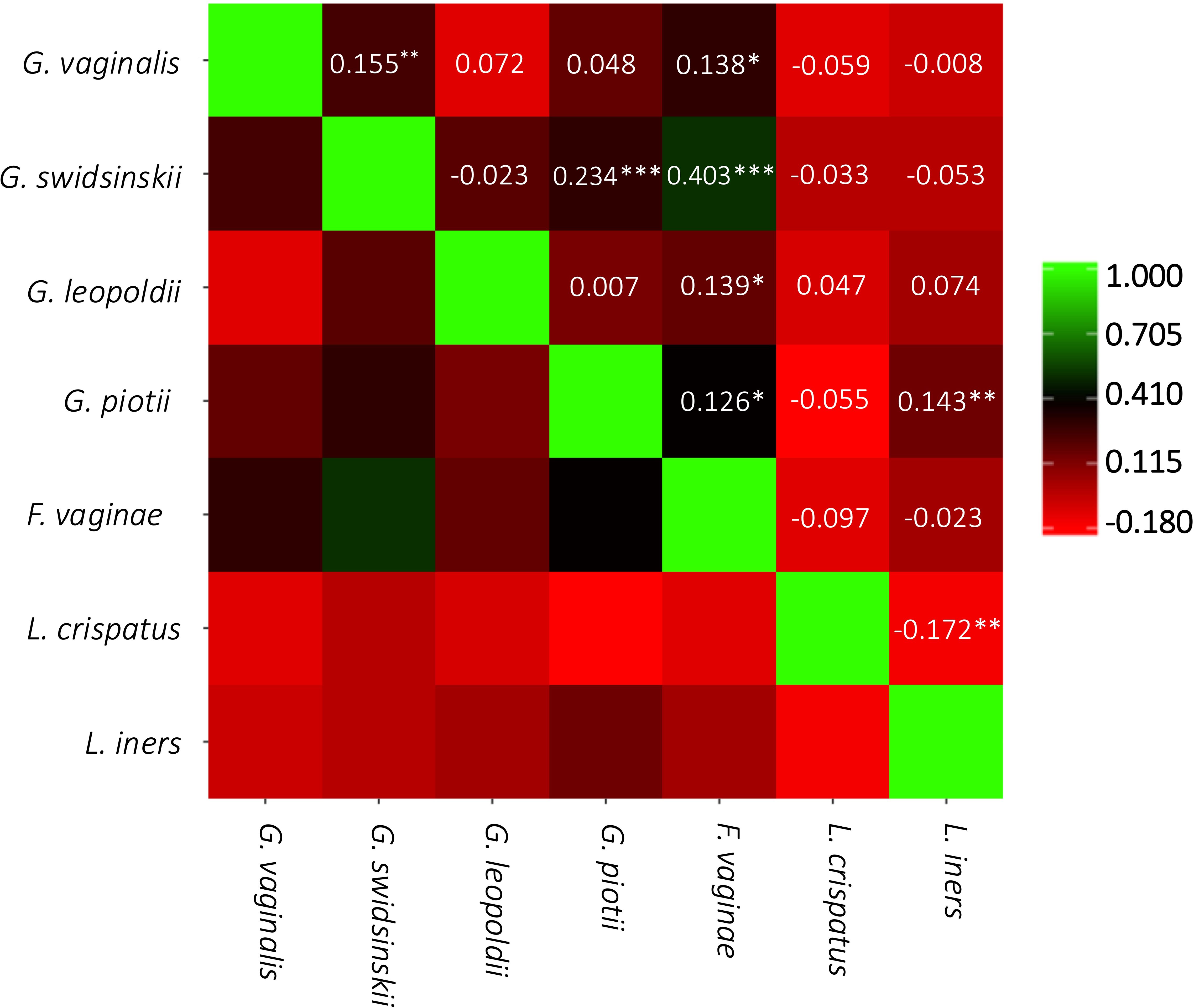
Figure 3. Pairwise correlation matrix. Pearson correlation coefficients are represented by the color scale and are shown in the squares. ***p<0.001; **p<0.01; *p<0.05.
ROC analysis and logistic regression model
In Figure 4 the ROC curves and in Table 3 the area under the curve (AUC), the Youden’s index, sensitivity and specificity are shown for each species and the multiple logistic regression model. Off all investigated species, F. vaginae had the largest area under the curve (0.81), followed by G. vaginalis (0.72), G. piotii (0.68), G. swidsinskii (0.64) and G. leopoldii (0.60) The logistic regression model gave the following combined result as optimal to diagnose BV: -1.46 + (0.14 × G. swidsinskii) + (0.10 × G. vaginalis) + (0.15 × G. leopoldii) + (0.15 × G. piotii) + (0.29 × F. vaginae) - (0.26 × L. crispatus) - (0.13 × L. iners).
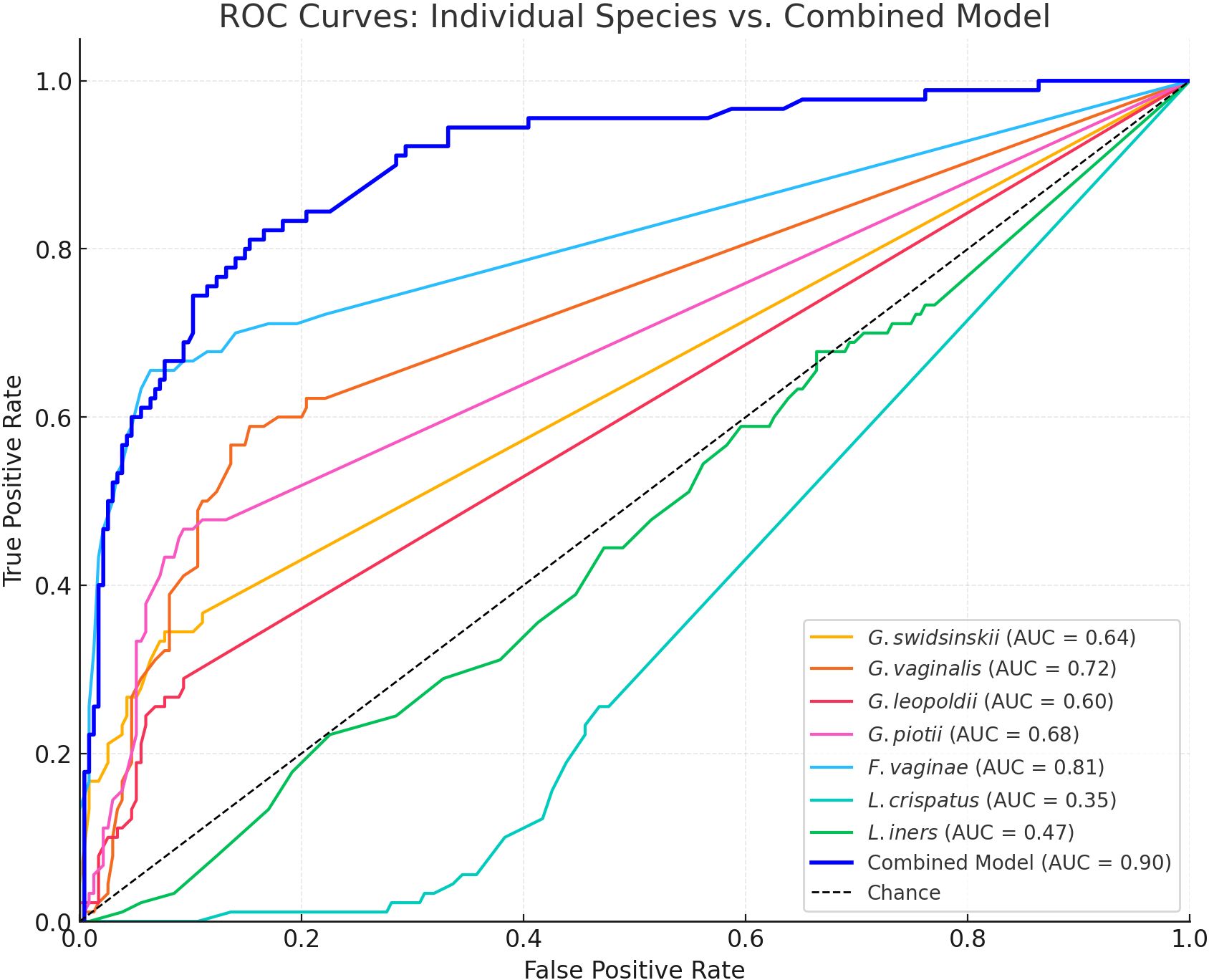
Figure 4. Receiver operator characteristics (ROC) curves for all investigated species and the multiple logistic regression model. AUC, area under the curve. The dashed diagonal line represents random performance (AUC = 0.50).
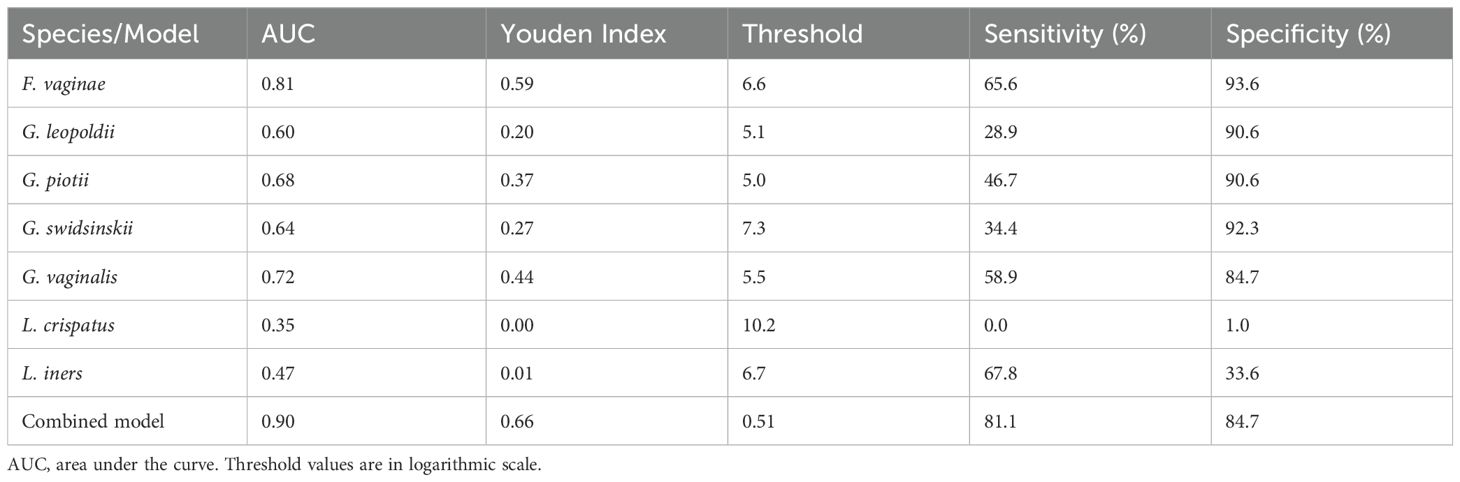
Table 3. Diagnostic performance of each investigated species and the multiple logistic regression model for bacterial vaginosis.
Characterization of clusters based on vaginal microbiome profiles
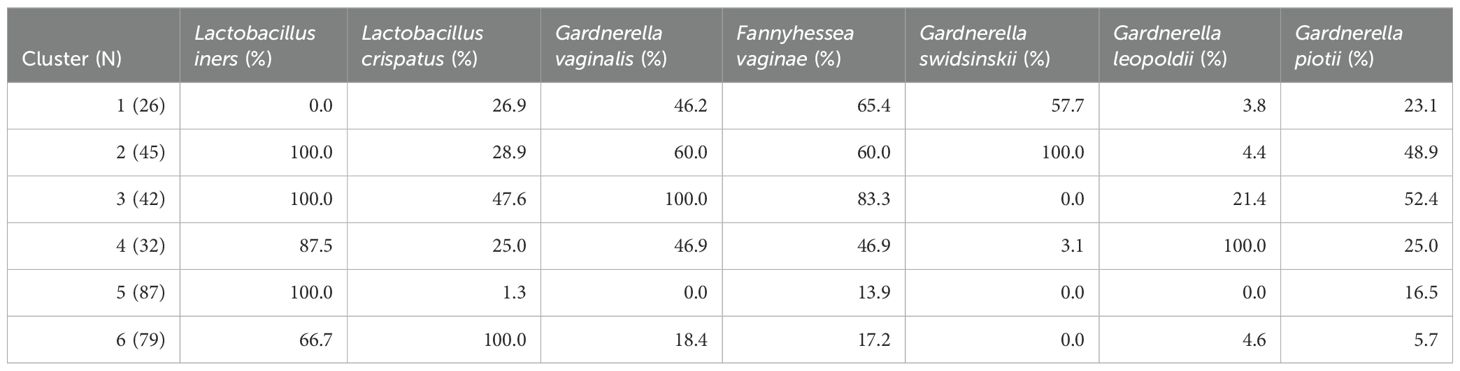
In Figure 5 a heatmap with hierarchical clustering of VMB profiles and bacterial species, annotated by Nugent score categorization, is shown. Six different clusters (1 to 6) were defined after cutting the dendrogram of these profiles at a height of 110. Four of these clusters mainly contained women with Nugent BV (cluster 1-4), while the other clusters contained mainly women with a healthy VMB according to Nugent (cluster 5-6). Each cluster can be characterized by a distinct distribution pattern of species. The prevalence of the species among the different clusters is listed in Table 4. Cluster 1 was the only cluster not containing L. iners. Also in cluster 1, almost no G. leopoldii was present. In cluster 2, also almost no G. leopoldii was present, while G. swidsinskii and L. iners were found in all women of this cluster. Cluster 3 was characterized by 100% abundance of G. vaginalis, and an absence of G. swidsinskii, while G. leopoldii was found among approximately one fifth of the women in this cluster. In cluster 4, G. leopoldii was 100% abundant, while G. swidsinskii was hardly present, and G. piotii and L. crispatus were found in one quarter of the women in this cluster. In cluster 5, L. iners was 100% abundant, with hardly any L. crispatus, and G. piotii as the only Gardnerella species. In cluster 6, both L. iners and L. crispatus were highly abundant, while (almost) no G. swidsinskii, G. leopoldii and G. piotii were found among this cluster.
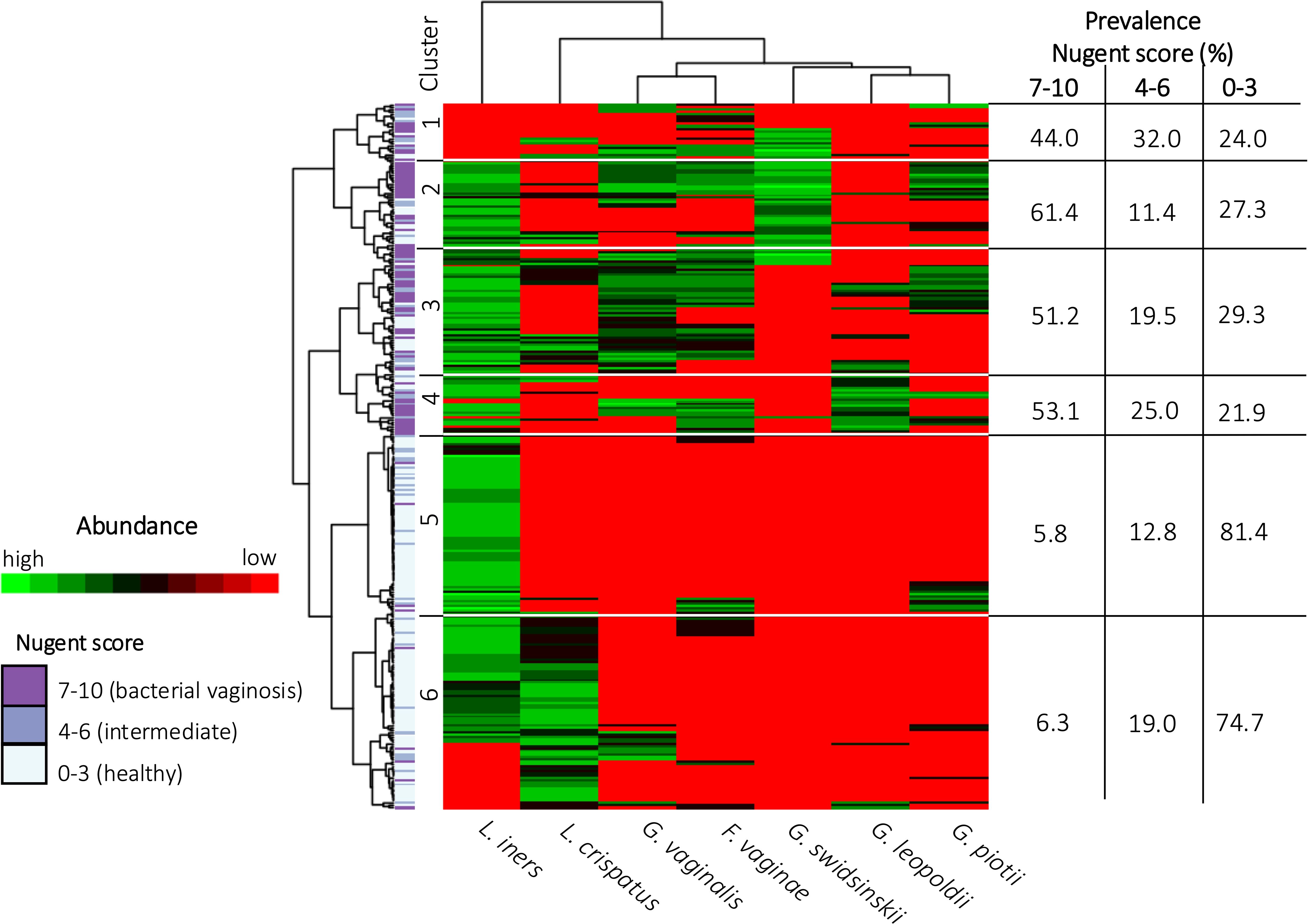
Figure 5. Heatmap of the vaginal microbiome. Each row depicts a woman’s vaginal microbiome profile and each column represents a bacterial species. The hierarchical clustering of vaginal microbiome profiles and bacterial species is based on log-transformed concentrations of bacterial species. The color scale ranges from red, indicating the absence of bacterial species, to green, indicating a high concentration of bacterial species. The categorization of the vaginal microbiome in healthy, intermediate or BV is shown on the left.
Univariate associations of the different species and clusters with laboratory findings and clinical signs and symptoms
The statistically significant univariate associations of the presence of the different species with laboratory findings and clinical signs and symptoms are summarized in Table 5. Further results of these univariate analyses (which were not statistically significant) for the different species are shown in Supplementary Information 1 (SI1) to SI7. The results of the univariate analysis across all clusters are shown in SI8.
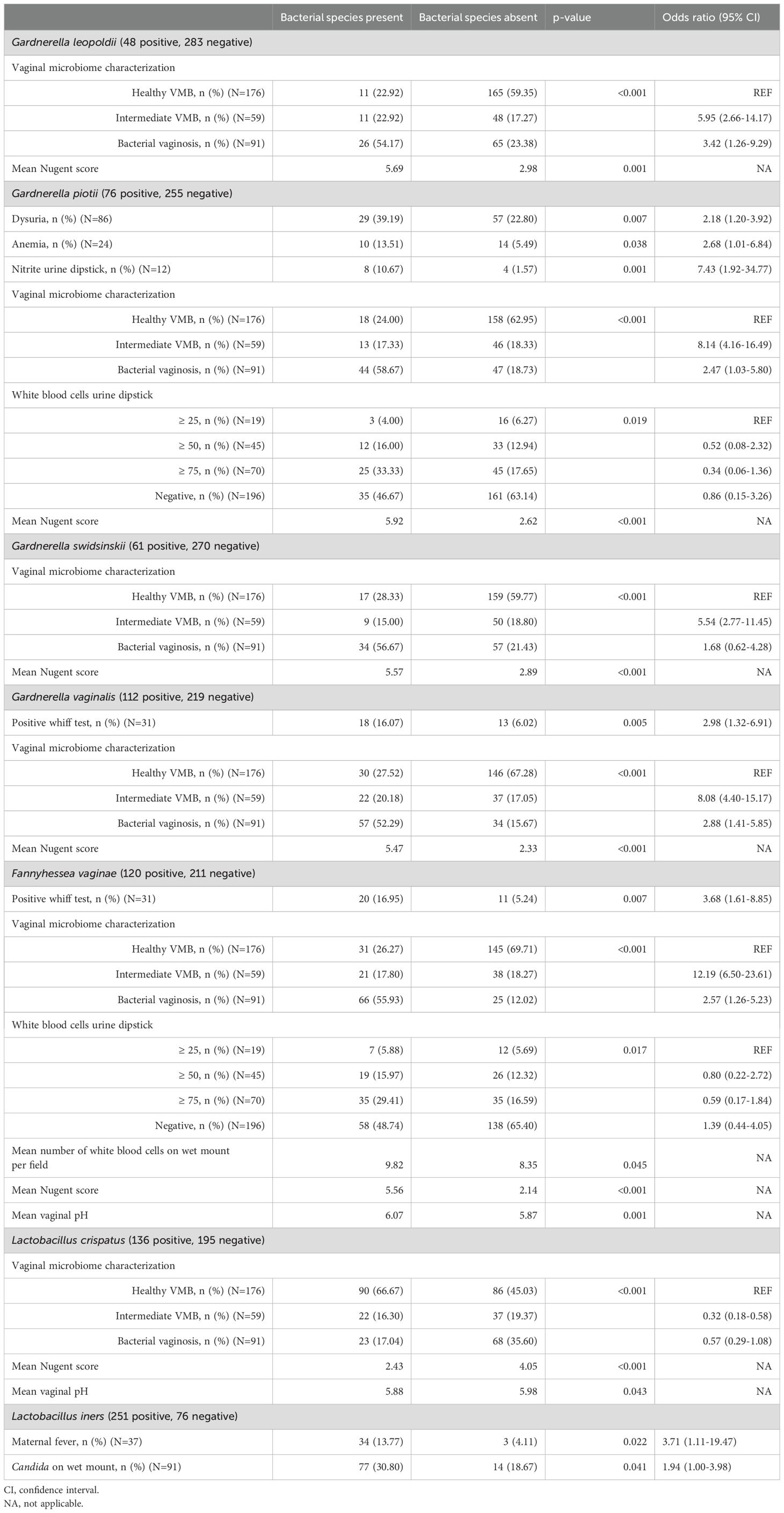
Table 5. Summary of univariate associations between bacterial species and clinical signs and symptoms.
All species except for L. iners were significantly associated with the Nugent score and the corresponding categorization of the VMB. Furthermore, G. piotii was positively associated with clinical symptoms and markers of urinary tract infections, i.e. dysuria (OR: 2.18; 95% CI: 1.20-3.92), nitrite levels measured with urine dipstick (OR: 7.43; 95% CI: 1.92-34.77) and white blood cell levels measured with urine dipstick. Also, women colonized with G. piotii had higher odds for anemia compared to women not colonized with G. piotii (OR: 2.68; 95% CI: 1.01-6.48). G. vaginalis showed a positive association with the Whiff test (OR: 2.98; 95% CI: 1.32-6.91). Women colonized with F. vaginae also showed increased odds for a positive Whiff test compared to women not colonized with F. vaginae (OR: 3.68; 95% CI: 1.61-8.85). Furthermore, F. vaginae was significantly associated with an increase in vaginal pH and white bloods cell levels determined on wet mount as well as with urine dipstick. L. crispatus, on the other hand, showed a significant association with a decrease in vaginal pH. For L. iners a significant positive association was found with maternal fever at V1 (OR: 3.71; 95% CI: 1.11-19.47) and Candida on wet mount (OR: 1.94; 95% CI: 1.00-3.98).
After one-on-one comparison with reference cluster 6, significant differences were found for Whiff test with cluster 2, with cluster 3 and with cluster 4. Cluster 6 and cluster 2 differed significantly with regard to Candida on wet mount. For Nugent score and VMB-categorization according to Nugent, cluster 1 to cluster 4 differed significantly from cluster 6. For fever the significant difference was between clusters other than reference cluster 6.
Univariate associations of the different species and clusters with pregnancy outcomes
The associations between the different species and pregnancy outcomes are shown in SI1-7. A significant positive association was found between LBW and two species, i.e. G. vaginalis (OR: inf; 95% CI: 3.03-inf, based on all seven women with a LBW baby being colonized with G. vaginalis) and F. vaginae (OR: 9.50; 95% CI: 1.12-444.16, based on six of the seven women with a LBW baby being positive for F. vaginae) (SI4 and SI5). Furthermore, women colonized with L. iners showed an almost four times higher odds for delivering a baby preterm compared to women not colonized with L. iners (OR: 3.73; 95% CI: 1.07-20.08) (SI7). However, also 70.6% of women with term birth were colonized by L. iners, although the mean L. iners concentration was twice as high in women with PTB compared to women who delivered at term (p<0.05) (data not shown). For the different clusters, no significant associations were found with any of the pregnancy outcomes.
Discussion
Prevalence and cooccurrence
In this study we investigated the distribution and clinical correlates of four Gardnerella species, L. crispatus, L. iners and F. vaginae related to women’s reproductive health. To our best knowledge, the different Gardnerella species have not yet been studied in African women. In our study population of Congolese pregnant women, G. vaginalis was the most prevalent Gardnerella species (33.8%), followed by G. piotii (23.0%), G. swidsinskii (18.4%), and G. leopoldii (14.5%). This is in line with a study among Dutch women where G. leopoldii was also the least prevalent species (22.2%) (Schuster et al., 2024). Also, if colonized with Gardnerella, the proportion of women harboring one Gardnerella species was approximately equal to the proportion of women carrying more than one Gardnerella species. This is in line with a study by Berman et al., who, based on sequences derived from vaginal swab samples from three demographically distinct cohorts of pregnant women, showed that metagenomes in which Gardnerella was present often contained more than one species (mean 3.92) (Berman et al., 2024), and with other previous work that has shown the majority of women are being colonized by more than one Gardnerella species (Munch et al., 2024; Schuster et al., 2024). Also noteworthy here is that G. swidsinskii had a median concentration around ten times higher compared to the other Gardnerella species.
In 2019, Hill and coworkers investigated the distribution of Gardnerella species among Gardnerella-positive non-pregnant Canadian women based on cpn60 deep sequencing (Hill and Albert, 2019). They reported G. vaginalis to be the most prevalent Gardnerella species (68.4%), followed by G. swidsinskii (49.2%), G. leopoldii (26.2%) and G. piotii (25.2%). These prevalences are considerably higher compared to ours, likely because only Gardnerella-positive women (72.9% of their total study population) were analyzed. In their study, they also reported a statistically significant co-occurrence between G. swidsinskii and G. vaginalis, and G. piotii and genomospecies 3, while G. swidsinskii and G. leopoldii significantly did not occur together. This is partly in line with findings reported here, since we also observed a positive correlation between G. swidsinskii and G. vaginalis. We did, however, also find a statistically significant correlation between G. swidsinskii and G. piotii.
In our study population, L. iners was the most prevalent species (75.8%), which is in line with results from numerous previous studies in African populations (Vaneechoutte, 2017a) (Vaneechoutte, 2017b). We found a negative correlation between L. iners and L. crispatus (r = -0.172), which is also in line with most literature, documenting single species dominance of lactobacilli in the healthy VMB (Vaneechoutte, 2017a). Only very weak correlations were seen between L. crispatus and Gardnerella species, while a positive correlation did exist between L. iners and G. piotii (r = 0.143). For F. vaginae a co-occurrence was reported with G. swidsinskii (r = 0.403), with G. leopoldii (r = 0.139) and with G. piotii (r = 0.126), which is in line with the general knowledge that F. vaginae often occurs together with Gardnerella (Bradshaw et al., 2006).
Diagnostic markers
BV-associated species such as G. vaginalis, F. vaginae, Megasphaera type 1, bacterial vaginosis-associated bacterium-2 (BVAB-2) and/or species associated with health, such as Lactobacillus spp., are commonly utilized as qPCR targets in both commercial [e.g. BD MAX™ Vaginal Panel (BD), Xpert Xpress MVP (Cepheid)] and in-house assays for BV detection. However, some of these markers, for example BVAB-2, have been shown to differ in prevalence in women found positive for BV depending on race (Srinivasan et al., 2012).
In our study population, we showed that F. vaginae was the best species marker for BV according to ROC analysis, with a sensitivity of 66% and specificity of 94%, using Nugent-BV as reference. This is in line with several previous studies that have shown that F. vaginae is a more specific marker for BV compared to the different Gardnerella species investigated (De Backer et al., 2007; Marconi et al., 2012; Vaneechoutte, 2017b). When considering combinations of species, our logistic regression model increased the AUC to 0.90, with a sensitivity and specificity of 81% and 85%, respectively.
Other studies using similar approaches of combining species markers to diagnose BV reported higher sensitivities and specificities (Fredricks et al., 2007; Hilbert et al., 2016; Munch et al., 2024). This might partly be due to the fact that we did not consider markers such as Megasphaera and BVAB-2, which have been found to have sensitivities and specificities of around 96% and 94%, respectively, compared to Nugent-BV (Fredricks et al., 2007). However, the same study also documented that Fannyhessea alone had a sensitivity and specificity of 96% and 85%, respectively, outperforming our combined model (Fredricks et al., 2007). Also in the study of Hilbert and coworkers, Fannyhessea alone had a sensitivity and specificity of 87% and 91%, respectively, and the overall model had a sensitivity and specificity of 92% and 95%, respectively (Hilbert et al., 2016). Taken together, above findings might suggest that optimal (combined) molecular BV-markers are population dependent.
The AUCs for the investigated Gardnerella species were similar (ranging from 0.60 for G. leopoldii to 0.72 for G. vaginalis), with G. vaginalis having the highest sensitivity but the lowest specificity. G. vaginalis was also suggested as the best marker among the different Gardnerella species by Munch and colleagues (Munch et al., 2024). Furthermore, all Gardnerella species and F. vaginae were positively, and L. crispatus negatively associated with the Nugent score and Nugent categorization, which confirms their role as key markers of (Nugent) BV also in our study population of women from Bukavu (DRC).
Univariate species associations
None of the seven investigated species showed a significant association with the symptoms typically associated with BV (i.e. discharge and malodor), or less typically associated with BV (itching and burning). Not stratifying our data for Candida carriage here might explain this observation, since this is a known confounder of BV and we previously showed that in Candida-negative women BV was significantly associated with malodor, while in Candida-positive women BV was not significantly associated to any typical symptom (De Keyser, 2020).
We did find a statistically significant association between G. piotii and both symptoms (dysuria) and laboratory markers (nitrite and white blood cells on dipstick) of urinary tract infections (UTIs). Gardnerella has previously been identified as a pathogen causing UTIs (Woolfrey et al., 1986) and, in a mouse model, G. piotii has been shown to facilitate Escherichia coli UTIs (O'Brien et al., 2020). G. piotii, in contrast to other Gardnerella spp., possesses the gene for extracellular sialidase activity (Kurukulasuriya et al., 2021), which in Bifidobacterium bifidum has been shown to enhance mucosal adhesion in in vitro experiments (Nishiyama et al., 2017). Hence, this trait could enhance bladder mucosal adhesion of G. piotii or other pathogens. Facilitation of adherence by other pathogens might be the more likely explanation, given that Gardnerella is thought to be negative for nitrate reduction (Taylor-Robinson, 1984), although this has not been investigated for the different species.
A significant positive association was also seen for G. piotii with anemia (hemoglobin levels <12.0 g/dL). Verstraelen and coworkers documented that subclinical iron-deficiency in Belgian pregnant women was an independent predictor for BV (Verstraelen et al., 2005) and it has also been demonstrated that Gardnerella can use human hemoglobin as a source of iron (Jarosik et al., 1998). Brabin and coworkers, in contrast, showed that iron-deficient women from Burkina Faso were more likely to have a normal VMB compared to iron-replete women, but also that the prevalence of vaginal discharge was significantly higher among iron-deficient women (Brabin et al., 2017).
Both G. vaginalis and F. vaginae were found to show a positive association with a positive Whiff test, one of the four Amsel criteria used for the clinical diagnosis of BV (Amsel et al., 1983). In a Whiff test, 10% potassium hydroxide (KOH) is added to vaginal discharge, and a fishy smell (positive Whiff test) is caused by aromatization of aromatic amines by anaerobes associated with BV. In vitro and bio-informatic analyses suggest that Prevotella but not Gardnerella is involved in the production of these amines (Nelson et al., 2015), but this has not yet been studied using various Gardnerella species.
Univariate cluster associations
All Gardnerella species were found among the clusters containing mainly Nugent-BV-positive women (i.e. cluster 1 to cluster 4), suggesting that not a single Gardnerella species but rather an interplay between different species plays a role in (asymptomatic) BV. This is in line with previous studies showing the majority of women are colonized by more than one Gardnerella species (Berman et al., 2024; Munch et al., 2024; Schuster et al., 2024). G. swidsinskii and G. leopoldii were not and nearly not (4.6%), respectively, observed among clusters with mainly women without Nugent-BV (i.e. cluster 5 and cluster 6), confirming the above described finding that these species are more specific markers for BV than G. vaginalis.
We hypothesized that distinct Gardnerella species specific clusters within women with Nugent BV existed and were associated to different degrees with clinical signs and symptoms and/or pregnancy outcomes. However, we showed that, although clusters could be defined based on the Gardnerella species composition, no signs and symptoms, with the exception of Whiff test, nor pregnancy outcomes were associated with these clusters. This is in line with the fact that we also did not find single Gardnerella species to be associated with clinical signs and symptoms and/or pregnancy outcomes (with the exception of G. vaginalis being associated with pH, and with LBW, albeit with a very broad 95% CI for the OR).
In cluster 5, which was dominated by L. iners and mostly contained women with a healthy Nugent score (74.7%), we saw no statistically significantly lower pH compared to the clusters 1-4, which is somewhat unexpected since this cluster represents mostly healthy women, but is in line with a report stating that the pH is not always lowered in case of L. iners predominance (Vaneechoutte, 2017b). Likewise, for cluster 6, which was dominated by L. crispatus and also mainly represented healthy women according to Nugent (81.4%), no statistically significant difference in pH was found compared to clusters 1-4.
A previous meta-analysis showed a twofold higher odd for PTB in women with BV (Vandenwyngaerden et al., 2020). In our study population, however, we previously showed that Nugent-BV was not significantly associated with PTB, but with LBW (Mulinganya et al., 2021). Here, using Gardnerella-species specific qPCR, offering absolute quantification and a higher taxonomic resolution compared to the Nugent scoring system, we showed that there was no association between different key markers of BV (Gardnerella, F. vaginae) and a healthy VMB (L. crispatus), and PTB. This is in contrast to a study in a cohort of pregnant Dutch women at high risk of recurrent preterm birth, where G. leopoldii was in fact associated with spontaneous preterm birth (Schuster et al., 2024).
In contrast, here, we did find that women with PTB had a significantly nearly four times higher odds of L. iners compared to women giving birth at term, and we see a higher concentration of L. iners among women with preterm birth than among the L. iners-carrying women with term birth. These findings are in line with results presented by Petricevic and colleagues, who studied pregnant Austrian women and reported a significantly higher prevalence of L. iners among women with PTB (85%) compared to women with term birth (16%) (Petricevic et al., 2014). In another study (among English women with an increased risk of PTB), L. iners dominance at 16 weeks of gestation, but not vaginal dysbiosis, was found to be significantly associated with PTB (<34 + 0 weeks) (Kindinger et al., 2017). Fettweis and colleagues, on the other hand, demonstrated that women who delivered at term showed a significant increase in the prevalence of L. iners (Fettweis et al., 2019).
Study limitations
Our study was subject to several limitations. First, approximately one third of study participants dropped out between the first visit and follow up, causing the number of women who delivered at the PRHB hospital to be less than foreseen. Second, women with complaints were treated after the first visit, which could have masked certain correlations. Lastly, we only studied a limited scope of seven key species, excluding other potential marker species such as Prevotella spp., L. gasseri, L. jensenii and L. vaginalis.
Conclusion
G. vaginalis was the most prevalent Gardnerella species in pregnant women from Bukavu (DRC), and G. leopoldii was the least prevalent species. Our results suggest that all Gardnerella species are involved in BV, although none were associated with the most clinically important BV symptoms. However, G. piotii was associated with markers of urinary tract infection. F. vaginae was the best single species diagnostic marker for BV.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Internal Review Board of the Catholic University of Bukavu (reference number UCB/CIE/NC/016/2016), Ministry of Public Health (reference number 062/CD/DPS/SK/2017) and Ethical Committee of Ghent University Hospital (reference number PA2014/003). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
LH: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. GM: Conceptualization, Data curation, Funding acquisition, Investigation, Writing – review & editing. TR: Investigation, Methodology, Validation, Writing – review & editing. GB: Investigation, Writing – review & editing. FK: Investigation, Writing – review & editing. YK: Investigation, Writing – review & editing. JM: Investigation, Writing – review & editing. IM: Investigation, Writing – review & editing. SC: Conceptualization, Funding acquisition, Writing – review & editing. MV: Conceptualization, Resources, Validation, Writing – review & editing. PC: Conceptualization, Data curation, Methodology, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Flemish Interuniversity Council (Vlaamse Interuniversitaire Raad (Flemish Interuniversities Council)-Universitaire Ontwikkelingssamenwerking (University Development Co-operation) (VLIR-UOS) (grant ZIUOS2012AP024). This work was published with the support of the University Foundation (Belgium). PC was supported through the Special Research Fund of Ghent University (BOF.COV.2020.0009.01) and LH by the Belgian Research Foundation Flanders (FWO) under a Ph.D. fellowship grant (1SB0225N).
Acknowledgments
The authors thank the staff of the Provincial General Reference Hospital of Bukavu neonatal intensive care unit, the staff of the HPGRB Department of Obstetrics and Gynecology, and all participants and their neonates for their helpful support of the study. The authors would also like to thank Katleen De Preter for her help with data analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1514884/full#supplementary-material
Abbreviations
BV, bacterial vaginosis; BVAB-2, bacterial vaginosis-associated bacterium 2; CI, confidence interval; CVL, cervicovaginal lavage; DRC, Democratic Republic of the Congo; GE/mL, genome equivalents per milliliter; LBW, low birthweight; OR, odds ratio; PRHB, Provincial Referral Hospital of Bukavu; PTB, preterm birth; qPCR, quantitative polymerase chain reaction; UTI, urinary tract infection; VMB, vaginal microbiome; WGA, weeks of gestational age.
References
Allsworth, J. E., Peipert, J. F. (2007). Prevalence of bacterial vaginosis: 2001-2004 National Health and Nutrition Examination Survey data. Obstet Gynecol. 109, 114–120. doi: 10.1097/01.AOG.0000247627.84791.91
Amsel, R., Totten, P. A., Spiegel, C. A., Chen, K. C., Eschenbach, D., Holmes, K. K. (1983). Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74, 14–22. doi: 10.1016/0002-9343(83)91112-9
Atashili, J., Poole, C., Ndumbe, P. M., Adimora, A. A., Smith, J. S. (2008). Bacterial vaginosis and HIV acquisition: a meta-analysis of published studies. Aids. 22, 1493–1501. doi: 10.1097/QAD.0b013e3283021a37
Berman, H. L., Goltsman, D. S. A., Anderson, M., Relman, D. A., Callahan, B. J. (2024). Gardnerella diversity and ecology in pregnancy and preterm birth. mSystems. 9, e0133923. doi: 10.1128/msystems.01339-23
Brabin, L., Roberts, S. A., Gies, S., Nelson, A., Diallo, S., Stewart, C. J., et al. (2017). Effects of long-term weekly iron and folic acid supplementation on lower genital tract infection - a double blind, randomised controlled trial in Burkina Faso. BMC Med. 15, 206. doi: 10.1186/s12916-017-0967-5
Bradshaw, C. S., Tabrizi, S. N., Fairley, C. K., Morton, A. N., Rudland, E., Garland, S. M. (2006). The association of Atopobium vaginae and Gardnerella vaginalis with bacterial vaginosis and recurrence after oral metronidazole therapy. J. Infect. Dis. 194, 828–836. doi: 10.1086/jid.2006.194.issue-6
Brotman, R. M., Klebanoff, M. A., Nansel, T. R., Yu, K. F., Andrews, W. W., Zhang, J., et al. (2010). Bacterial vaginosis assessed by gram stain and diminished colonization resistance to incident gonococcal, chlamydial, and trichomonal genital infection. J. Infect. Dis. 202, 1907–1915. doi: 10.1086/657320
Byun, R., Nadkarni, M. A., Chhour, K. L., Martin, F. E., Jacques, N. A., Hunter, N. (2004). Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J. Clin. Microbiol. 42, 3128–3136. doi: 10.1128/JCM.42.7.3128-3136.2004
Cherpes, T. L., Melan, M. A., Kant, J. A., Cosentino, L. A., Meyn, L. A., Hillier, S. L. (2005). Genital tract shedding of herpes simplex virus type 2 in women: effects of hormonal contraception, bacterial vaginosis, and vaginal group B Streptococcus colonization. Clin. Infect. Dis. 40, 1422–1428. doi: 10.1086/429622
Clarridge, J. E., 3rd (2004). Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin. Microbiol. Rev. 17, 840–862. doi: 10.1128/CMR.17.4.840-862.2004
De Backer, E., Verhelst, R., Verstraelen, H., Alqumber, M. A., Burton, J. P., Tagg, J. R., et al. (2007). Quantitative determination by real-time PCR of four vaginal Lactobacillus species, Gardnerella vaginalis and Atopobium vaginae indicates an inverse relationship between L. gasseri and L. iners. BMC Microbiol. 7, 115. doi: 10.1186/1471-2180-7-115
De Keyser, K. (2020). Vaginal carriage of Candida sp., Enterobacter cloacae complex and Klebsiella pneumoniae in pregnant women in Bukavu, Democratic Republic of the Congo: prevalence, risk factors, symptoms and adverse pregnancy outcomes. Available online at: https://libstore.ugent.be/fulltxt/RUG01/002/863/076/RUG01-002863076_2020_0001_AC.pdf2020https://libstore.ugent.be/fulltxt/RUG01/002/863/076/RUG01-002863076_2020_0001_AC.pdf.
Fettweis, J. M., Brooks, J. P., Serrano, M. G., Sheth, N. U., Girerd, P. H., Edwards, D. J., et al. (2014). Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiol. (Reading) 160, 2272–2282. doi: 10.1099/mic.0.081034-0
Fettweis, J. M., Serrano, M. G., Brooks, J. P., Edwards, D. J., Girerd, P. H., Parikh, H. I., et al. (2019). The vaginal microbiome and preterm birth. Nat. Med. 25, 1012–1021. doi: 10.1038/s41591-019-0450-2
Fredricks, D. N., Fiedler, T. L., Thomas, K. K., Oakley, B. B., Marrazzo, J. M. (2007). Targeted PCR for detection of vaginal bacteria associated with bacterial vaginosis. J. Clin. Microbiol. 45, 3270–3276. doi: 10.1128/JCM.01272-07
Hickey, R. J., Forney, L. J. (2014). Gardnerella vaginalis does not always cause bacterial vaginosis. J. Infect. Dis. 210, 1682–1683. doi: 10.1093/infdis/jiu303
Hilbert, D. W., Smith, W. L., Chadwick, S. G., Toner, G., Mordechai, E., Adelson, M. E., et al. (2016). Development and validation of a highly accurate quantitative real-time PCR assay for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 54, 1017–1024. doi: 10.1128/JCM.03104-15
Hill, J. E., Albert, A. Y. K. (2019). Resolution and Cooccurrence Patterns of Gardnerella leopoldii, G. swidsinskii, G. piotii and G. vaginalis within the Vaginal Microbiome. Infect. Immun. 87, e00532–19. doi: 10.1128/IAI.00532-19
Hillier, S. L., Nugent, R. P., Eschenbach, D. A., Krohn, M. A., Gibbs, R. S., Martin, D. H., et al. (1995). Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl. J. Med. 333, 1737–1742. doi: 10.1056/NEJM199512283332604
Jarosik, G. P., Land, C. B., Duhon, P., Chandler, R., Jr., Mercer, T. (1998). Acquisition of iron by Gardnerella vaginalis. Infect. Immun. 66, 5041–5047. doi: 10.1128/IAI.66.10.5041-5047.1998
Kindinger, L. M., Bennett, P. R., Lee, Y. S., Marchesi, J. R., Smith, A., Cacciatore, S., et al. (2017). The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome. 5, 6. doi: 10.1186/s40168-016-0223-9
Kolde, R. (2019). pheatmap: pretty heatmaps2019. Available online at: https://CRAN.R-project.org/package=pheatmap (Accessed January 24, 2020).
Kurukulasuriya, S. P., Patterson, M. H., Hill, J. E. (2021). Slipped-strand mispairing in the gene encoding sialidase nanH3 in gardnerella spp. Infect. Immun. 89, e00583–20. doi: 10.1128/IAI.00583-20
Latka, A., Van Simaey, L., Reynders, M., Cools, P., Rogier, T., Lebbe, B., et al. (2022). Optimization of propidium monoazide qPCR (Viability-qPCR) to quantify the killing by the gardnerella-specific endolysin PM-477, directly in vaginal samples from women with bacterial vaginosis. Antibiotics (Basel) 11 (1). doi: 10.3390/antibiotics11010111
Marconi, C., Cruciani, F., Vitali, B., Donders, G. G. (2012). Correlation of atopobium vaginae amount with bacterial vaginosis markers. J. Low Genit Tract Dis. 16, 127–132. doi: 10.1097/LGT.0b013e31823c79c4
Menard, J. P., Fenollar, F., Henry, M., Bretelle, F., Raoult, D. (2008). Molecular quantification of Gardnerella vaginalis and Atopobium vaginae loads to predict bacterial vaginosis. Clin. Infect. Dis. 47, 33–43. doi: 10.1086/588661
Ministère de la Santé RDdC (2006). Guide national de prise en charge des infections sexuellement transmissibles selon l’approche syndromique. Available online at: https://medbox.org/pdf/5e148832db60a2044c2d475f (Accessed May 1, 2020).
Mulinganya, M. G., De Keyser, K., Mongane, I. J., Kampara, M. F., De Vulder, A., Boelens, J., et al. (2024). Second trimester vaginal Candida colonization among pregnant women attending antenatal care in Bukavu, Democratic Republic of the Congo: prevalence, clinical correlates, risk factors and pregnancy outcomes. Front. Glob Womens Health 5, 1339821. doi: 10.3389/fgwh.2024.1339821
Mulinganya, G., De Vulder, A., Bisimwa, G., Boelens, J., Claeys, G., De Keyser, K., et al. (2021). Prevalence, risk factors and adverse pregnancy outcomes of second trimester bacterial vaginosis among pregnant women in Bukavu, Democratic Republic of the Congo. PLoS One 16, e0257939. doi: 10.1371/journal.pone.0257939
Munch, M. M., Strenk, S. M., Srinivasan, S., Fiedler, T. L., Proll, S., Fredricks, D. N. (2024). Gardnerella species and their association with bacterial vaginosis. J. Infect. Dis. 230 (1), e171–e181. doi: 10.1093/infdis/jiae026
Muzny, C. A., Blanchard, E., Taylor, C. M., Aaron, K. J., Talluri, R., Griswold, M. E., et al. (2018). Identification of key bacteria involved in the induction of incident bacterial vaginosis: A prospective study. J. Infect. Dis. 218, 966–978. doi: 10.1093/infdis/jiy243
Muzny, C. A., Taylor, C. M., Swords, W. E., Tamhane, A., Chattopadhyay, D., Cerca, N., et al. (2019). An updated conceptual model on the pathogenesis of bacterial vaginosis. J. Infect. Dis. 220, 1399–1405. doi: 10.1093/infdis/jiz342
Nelson, T. M., Borgogna, J. L., Brotman, R. M., Ravel, J., Walk, S. T., Yeoman, C. J. (2015). Vaginal biogenic amines: biomarkers of bacterial vaginosis or precursors to vaginal dysbiosis? Front. Physiol. 6, 253. doi: 10.3389/fphys.2015.00253
Nishiyama, K., Yamamoto, Y., Sugiyama, M., Takaki, T., Urashima, T., Fukiya, S., et al. (2017). Bifidobacterium bifidum extracellular sialidase enhances adhesion to the mucosal surface and supports carbohydrate assimilation. mBio 8, e00928–17. doi: 10.1128/mBio.00928-17
O'Brien, V. P., Joens, M. S., Lewis, A. L., Gilbert, N. M. (2020). Recurrent Escherichia coli Urinary Tract Infection Triggered by Gardnerella vaginalis Bladder Exposure in Mice. J. Vis. Exp. 166. doi: 10.3791/61967-v
Park, F., Rosca, A., Cools, P., Chico, R. M. (2024). Prevalence of bacterial vaginosis among pregnant women attending antenatal care in low- and middle-income countries between 2000 and 2020: A systematic review and meta-analysis. Reproductive Female Child Health 3 (2), e99. doi: 10.1002/rfc2.v3.2
Peebles, K., Velloza, J., Balkus, J. E., McClelland, R. S., Barnabas, R. V. (2019). High global burden and costs of bacterial vaginosis: A systematic review and meta-analysis. Sex Transm Dis. 46, 304–311. doi: 10.1097/OLQ.0000000000000972
Petricevic, L., Domig, K. J., Nierscher, F. J., Sandhofer, M. J., Fidesser, M., Krondorfer, I., et al. (2014). Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 4, 5136. doi: 10.1038/srep05136
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U S A. 108 Suppl 1, 4680–4687. doi: 10.1073/pnas.1002611107
Robin, X., Turck, N., Hainard, A., Tiberti, N., Lisacek, F., Sanchez, J. C., et al. (2011). pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinf. 12, 77. doi: 10.1186/1471-2105-12-77
Schober, P., Boer, C., Schwarte, L. A. (2018). Correlation coefficients: appropriate use and interpretation. Anesth. Analg. 126, 1763–1768. doi: 10.1213/ANE.0000000000002864
Schuster, H. J., Bos, A. M., Himschoot, L., van Eekelen, R., Matamoros, S. P. F., de Boer, M. A., et al. (2024). Vaginal microbiota and spontaneous preterm birth in pregnant women at high risk of recurrence. Heliyon. 10, e30685. doi: 10.1016/j.heliyon.2024.e30685
Sousa, M., Ksiezarek, M., Perovic, S. U., Antunes-Lopes, T., Grosso, F., Ribeiro, T. G., et al. (2023). Gardnerella pickettii sp. nov. (formerly Gardnerella genomic species 3) and Gardnerella greenwoodii sp. nov. (formerly Gardnerella genomic species 8) isolated from female urinary microbiome. Int. J. Syst. Evol. Microbiol. 73.
Srinivasan, S., Hoffman, N. G., Morgan, M. T., Matsen, F. A., Fiedler, T. L., Hall, R. W., et al. (2012). Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS One 7, e37818. doi: 10.1371/journal.pone.0037818
Taylor-Robinson, D. (1984). The bacteriology of Gardnerella vaginalis. Scand. J. Urol Nephrol. Suppl. 86, 41–55.
Turner, E., Sobel, J. D., Akins, R. A. (2021). Prognosis of recurrent bacterial vaginosis based on longitudinal changes in abundance of Lactobacillus and specific species of Gardnerella. PLoS One 16, e0256445. doi: 10.1371/journal.pone.0256445
Vandenwyngaerden, E.-A., Cools, P. C., Verstraelen, H. P. (2020). Bacterial vaginosis and preterm birth: a systematic review and meta-analysis 2020.
Vaneechoutte, M. (2017a). The human vaginal microbial community. Res. Microbiol. 168, 811–825. doi: 10.1016/j.resmic.2017.08.001
Vaneechoutte, M. (2017b). Lactobacillus iners, the unusual suspect. Res. Microbiol. 168, 826–836. doi: 10.1016/j.resmic.2017.09.003
Vaneechoutte, M., Guschin, A., Van Simaey, L., Gansemans, Y., Van Nieuwerburgh, F., Cools, P. (2019). Emended description of Gardnerella vaginalis and description of Gardnerella leopoldii sp. nov., Gardnerella piotii sp. nov. and Gardnerella swidsinskii sp. nov., with delineation of 13 genomic species within the genus Gardnerella. Int. J. Syst. Evol. Microbiol. 69, 679–687. doi: 10.1099/ijsem.0.003200
Verstraelen, H., Delanghe, J., Roelens, K., Blot, S., Claeys, G., Temmerman, M. (2005). Subclinical iron deficiency is a strong predictor of bacterial vaginosis in early pregnancy. BMC Infect. Dis. 5, 55. doi: 10.1186/1471-2334-5-55
Woolfrey, B. F., Ireland, G. K., Lally, R. T. (1986). Significance of Gardnerella vaginalis in urine cultures. Am. J. Clin. Pathol. 86, 324–329. doi: 10.1093/ajcp/86.3.324
Keywords: bacterial vaginosis, Gardnerella, molecular diagnosis, preterm birth, low birthweight, Democratic Republic of the Congo
Citation: Himschoot L, Mulinganya G, Rogier T, Bisimwa G, Kampara F, Kujirakwinja Y, Mongane J, Mubalama I, Callens S, Vaneechoutte M and Cools P (2025) Prevalence and clinical correlates of Gardnerella spp., Fannyhessea vaginae, Lactobacillus crispatus and L. iners in pregnant women in Bukavu, Democratic Republic of the Congo. Front. Cell. Infect. Microbiol. 14:1514884. doi: 10.3389/fcimb.2024.1514884
Received: 21 October 2024; Accepted: 20 December 2024;
Published: 17 January 2025.
Edited by:
António Machado, University of the Azores, PortugalReviewed by:
Valentina Margarita, University of Sassari, ItalyEduardo Costa, Escola Superior de Biotecnologia - Universidade Católica Portuguesa, Portugal
Copyright © 2025 Himschoot, Mulinganya, Rogier, Bisimwa, Kampara, Kujirakwinja, Mongane, Mubalama, Callens, Vaneechoutte and Cools. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Piet Cools, UGlldC5Db29sc0BVR2VudC5iZQ==
 Lisa Himschoot
Lisa Himschoot Guy Mulinganya2,3,4
Guy Mulinganya2,3,4 Mario Vaneechoutte
Mario Vaneechoutte Piet Cools
Piet Cools