
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol., 10 December 2024
Sec. Clinical Infectious Diseases
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1485825
This article is part of the Research TopicHIV/AIDS: Pathogenesis and VaccineView all 7 articles
Human immunodeficiency Virus (HIV) and Mycobacterium tuberculosis (Mtb) co-infection presents a significant public health challenge worldwide. Comprehensive assessment of the immune response in HIV/Mtb co-infection is complex and challenging. CD8+T cells play a pivotal role in the adaptive immune response to both HIV and Mtb. The differentiation of CD8+T cells follow a hierarchical pattern, with varying degrees of exhaustion throughout the process. Memory stem T cells (TSCM cells) is at the apex of the memory T lymphocyte system, which has recently emerged as a promising target in immunotherapy. In this context, we discuss the alterations of CD8+TSCM cells in HIV/Mtb mono- and co-infection, their implications and clinical significance, and potential for improving immunotherapy.
Human immunodeficiency Virus (HIV) and Mycobacterium tuberculosis (Mtb) co-infection has been an urgent public health problem worldwide. Coinfection with HIV accelerates the progression of Mtb infection and exacerbated its severity (Ajayi et al., 2022; World Health Organization, 2020; Seyoum et al., 2022; Sultana et al., 2021). Nowadays, tuberculosis (TB) remains the leading cause of death among people living with HIV (PLWH). According to the latest data released by the World Health Organization (WHO), TB accounts for approximately 27% of AIDS-related deaths worldwide (World Health Organisation, 2023). What’s more, comprehensive assessment of immune response turns to be complicated and challenging in HIV/Mtb co-infection (Manna et al., 2020).
Although CD4+T cells are traditionally regarded as the primary IFN-γ producers in TB, which is pivotal in host defense against Mtb, vaccine trial setbacks suggest a need for reevaluation and exploration of alternative immune targets. Recently, protective role of CD8+T cells was revealed in early control of Mtb infection (Winchell et al., 2023). At the same time, an extensive body of evidence indicates that CD8+T cells play a fundamental role in the adaptive immune response to HIV. Exploration of CD8+T cells as alternative immune targets is prospective, and figuring out the characteristics of CD8+T cells in mediating cellular immunity in HIV/Mtb co-infection would offer a rationale for harnessing long-term control to combat disease.
Memory stem T cells (TSCM cells), a newly defined memory T cells endowed with extreme longevity and robust potential for immune reconstitution (Gattinoni et al., 2017). TSCM cells are commonly generated during natural immune responses against foreign pathogens. Though not fully characterized, work in the context of HIV or Mtb infection has shown the pertinence between CD8+TSCM cells and both diseases, implying distinct role of this subsets in chronic infection. What’s more, functionally distinct from other memory subsets of T cells, CD8+TSCM cells demonstrate a promising outlook in immunotherapy (Marraco et al., 2015). Taking CD8+TSCM cells as a starting point to explore its regulatory mechanisms in HIV/Mtb co-infection may contribute to enhancing the efficacy of vaccines and adoptive T-cell therapies for Mtb infection in the context of HIV co-infection.
In this review, we discuss the alteration of CD8+TSCM cells in HIV/Mtb co-infection, implications and clinical significance, and its potential for improvement of immunotherapy. Given the limited research on CD8+TSCM cells in HIV/Mtb co-infection, we initially examined the patterns of CD8+T cells in both HIV and Mtb mono-infections as well as co-infections, aiming to gain insights that could contribute to the study of CD8+TSCM cells.
In the absence of antiretroviral therapy (ART), the initial burst of HIV replication is characterized by an increase in viral load in blood. Subsequently, the viral load decreases, and this temporal shift coincides with an elevation in HIV-specific CD8+T cells, which is crucial for eliminating HIV-infected T cells (Walker et al., 1987). In most people living with HIV without ART treatment, HIV-specific CD8+T cells maintain dysfunctional during chronic HIV infection, because of continuous HIV antigen burden (Trautmann et al., 2012). Recently, research has reported that long-term ART initiated in Fiebig stage I prevents residual dysfunction of HIV-specific CD8+T cells (Takata et al., 2022), but most patients are unable to initiate ART treatment promptly, and residual dysfunction of HIV-specific CD8+T cells maybe a common phenomenon among HIV patients. In HIV-infection, T cells specific to other pathogen also manifest immune abnormalities. Latent viruses, such as cytomegalovirus (CMV) and Epstein-Barr virus (EBV), reactivate more frequently during HIV-1 infection due to the depletion of T cells that control viral replication (Walton et al., 2013). It has been observed that perforin expression in EBV- and CMV-specific CD8+T cells is reduced in HIV-infected patients, and this defect is accompanied by a lower expression of granzyme B (Zhang et al., 2003). In HIV/HCV co-infection, HCV-specific CD8+T cells co-express Tim-3 and PD-1 were in significantly higher frequencies and positively correlated with a clinical parameter of liver disease progression (Vali et al., 2010). Despite ART-induced viral suppression, alterations of CD8+T cells from HIV-infected patients include: 1) persistently increased absolute counts but impaired proliferative capacity (Helleberg et al., 2015; Gaiha et al., 2014); 2) defect in cytotoxic program (Perdomo-Celis et al., 2019b); 3) persistent immune activation and systemic inflammation (Hunt et al., 2003; Olson et al., 2021);4) defect in differentiation into functional cells (Takata et al., 2023); 5) persistent exhausted status (Trautmann et al., 2006; Jin et al., 2010; Wang et al., 2020).
Furthermore, HIV significantly impacts the differentiation of CD8+T cells. CD8+T cells can differentiate into memory and effector subsets, with TSCM cells and central memory T cells (TCM cells) acting as “stem-like” precursors within the memory subset. Between the two types of T cell subsets, TSCM cells are phenotypically defined as naive T cells (TN cells) by the expression of TN cell markers, such as CD45RA and CCR7, but distinguishable from TN cells by two memory T cell markers: CD95, CD58 and CD122, and excelling in typical TCM cells cell traits, but less phenotypically differentiated than TCM cells and are overall less frequent (Figure 1) (Lugli et al., 2013; Gattinoni et al., 2011). Thus, they represent cells at an intermediate state of differentiation between TN and TCM cells. Commonly, after antigen priming, TN cells progressively differentiate into diverse memory T cell subpopulations, and ultimately into terminally differentiated effector T cells (Figure 1). During acute HIV infection, memory CD8+T cells are driven toward a more terminally differentiated status, along with a decrease frequency of long-lived T cell subsets, including TSCM cells and TCM cells, promoting the differentiation of CD8+T cells with short-lived transitional memory (TTM cells) and effector memory (TEM cells) subsets (Takata et al., 2022). TSCM cells and TCM cells are the fount to sustain persistent CD8+T cell responses, and a failure to generate proliferation-competent precursor cells in chronic infections results in the collapse of the T cell response (Zehn et al., 2022).
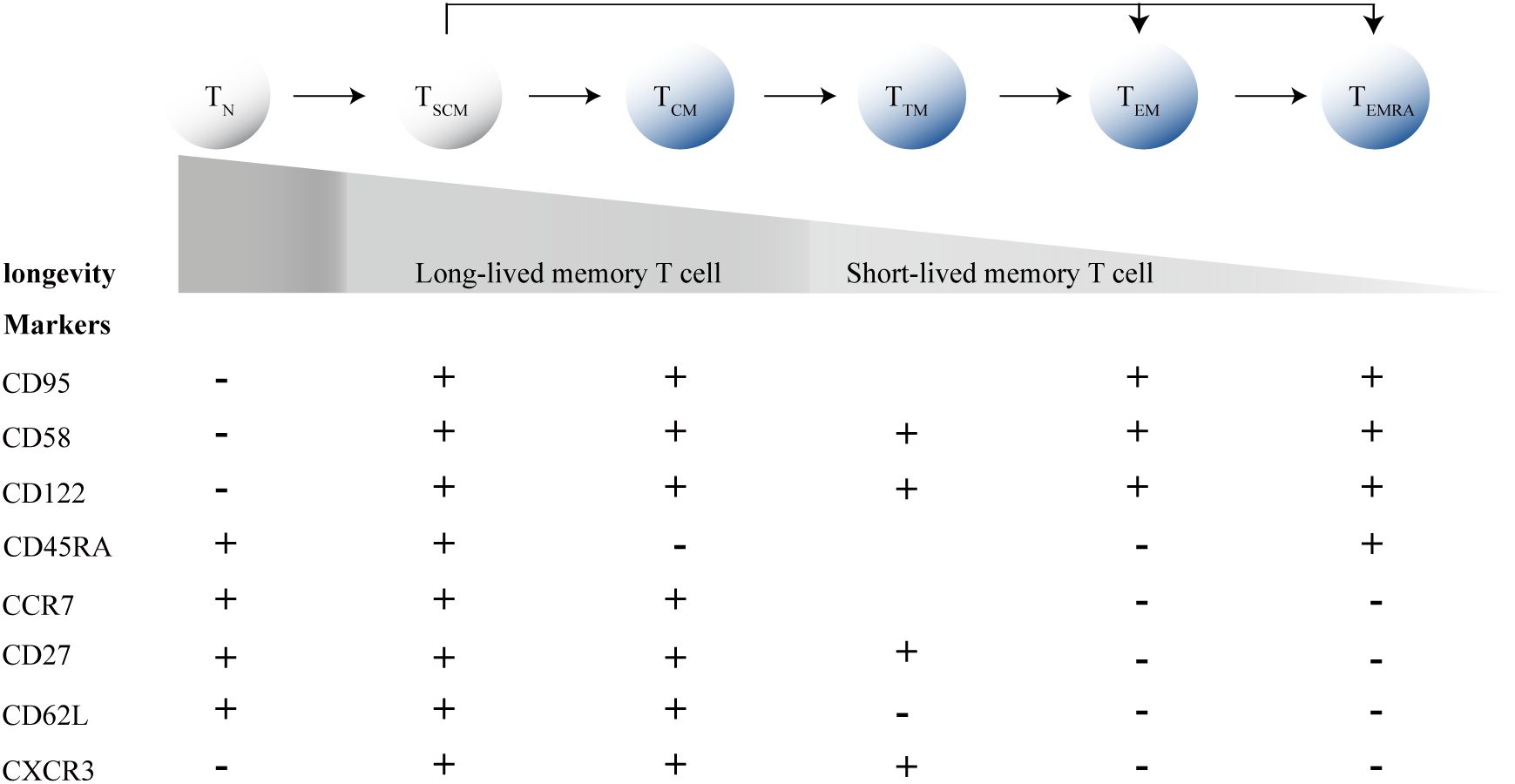
Figure 1. T cell differentiation process and marker expression profiles. TN, Naive T cells; TSCM cells, memory stem T cells; TCM cells, central memory T cells; TEM cells, effector memory T cells; TTM cells, transitional memory T-cells; TEM cells, effector memory T cells; TEMRA, terminally differentiated effector T cells.
As minimally differentiated cells at the apex of the hierarchical system of memory T lymphocytes, TSCM cells endowed with the stem cell-like ability to self-renew and had multipotent capacity to reconstitute the entire spectrum of memory and effector T cell subsets (Gattinoni et al., 2009; Ahmed et al., 2016). What’s more, TSCM cells have an exceptional capacity to persist long term proved in HIV infection (Vigano et al., 2015). HIV-specific CD8+TSCM cells represent a long-lasting component of the cellular immune response to HIV-1 and are detectable during all stages of HIV-1 infection (Vigano et al., 2015). In HIV-exposed seronegative individuals and HIV patients with treatment interruption, count and frequency of HIV-specific CD8+T cells with stem cell-like phenotypes elevated, which implies the antiviral role of TSCM cells in control of HIV infection (Ponnan et al., 2021; Sachdeva et al., 2023). Indeed, natural preservation of CD8+TSCM cells in the setting of untreated HIV-1 infection is associated with improved viral control and immune reconstitution (Ribeiro et al., 2014). In the CD8+T cell compartment of ART-naive pediatric slow progressors, an enrichment of TSCM cells were identified, whereas pediatric progressors and viremic adults had a terminally exhausted population (Vieira et al., 2023).
Although ART can result in an undetectable viral load in peripheral blood plasma and significantly reduce the HIV reservoir and CD8+T cell responses after 2 years of ART, the persistent viral reservoir continues to impact the differentiation status of HIV-specific CD8+T cells (Takata et al., 2023). The proportion of CD8+T cells increase during the acute phase of HIV infection, but there is a decrease in TN cells (Perdomo-Celis et al., 2019a). Defined by traditional T cell subset markers such as CD45RA and CD62L or CCR7, it did not distinguish TSCM cells from TN cells, meaning that the TN population in earlier studies actually included both TN and TSCM cells. As the precursor to other memory T cells, it can be speculated that the proportion of TSCM cells also decreases following viral stimulation during the acute phase of HIV infection. Indeed, a decline in frequency of TSCM cells can already be observed during Fiebig stages III and IV of HIV infection (Takata et al., 2022). TCF-1, a transcription factor important for self-renewal capacity, marks a population of stem-like CD8+T cells and sustain the immune response to chronic viral infections (Escobar et al., 2020; Utzschneider et al., 2016). The decrease in TCF-1 expression levels and the increase in PD-1 expression levels in CD8+T cells during HIV infection suggest that the loss of stem-like CD8+T cells including TSCM cells in HIV infection may be due to the functional impairment, specifically their sustained proliferative capacity and self-renewal ability.
Actually, ART had an immune restorative effect on CD8+TSCM cells (Tuluc et al., 2017), and the earlier the ART timing, the better effect the recovery. ART initiation in acute HIV infection promoted the persistence of HIV-specific CD8+TSCM cells, with high expansion and cytotoxic capacity, and mitigatory activated/exhausted phenotype, whereas ART initiation in chronic HIV infection led to more differentiated HIV-specific CD8+T cells with a higher combined frequency of short-lived T cells (Takata et al., 2022; Salido et al., 2018; Tartaro et al., 2022). In patients with ART, the proportion of CD8+TSCM cells rises to the level of healthy controls after 144 weeks of treatment (Song et al., 2017). On the contrary, the frequency of CD8+TSCM cells was decreased in all individuals with chronic, untreated HIV-1 infection (Ribeiro et al., 2014). Initiation of ART recovered the expression of TCF-1, but HIV-specific CD8+T cells from people treated during Fiebig stage I expressed significantly higher TCF-1 compared with people treated during Fiebig stages III and IV (Takata et al., 2022). Although current HIV treatment guidelines emphasize initiating ART as early as possible, detecting and treating PLWH at Fiebig stage 1 remains very challenging, thus, functional impairments in TSCM cells exist in most HIV-infected individuals.
The changes in the proportion of CD8+T cells in Mtb infection are still inconclusive. In various studies, the observed results regarding changes in the proportion of CD8+T cells due to Mtb infection are not consistent. Discrepancies exist in the alterations of the overall proportion of CD8+T cells across various studies on Mtb infection. Kudryavtsev I et al. found no differences in the CD8+T cells frequencies in peripheral blood between patients with pulmonary TB and healthy controls (Kudryavtsev et al., 2023). However, Chávez-Galán et al. found that TB patients had a higher frequency of CD8+T cells from same type samples (Chávez-Galán et al., 2019). When it comes to Mtb-specific CD8+T cells populations, divergent opinions persist across various studies. Cheryl L. Day et al. and Virginie Rozot et al. found no difference in percentage of Mtb-specific CD8+T cells between TB and latent Mtb infection (LTBI) patients (Day et al., 2014; Rozot et al., 2015). But subsequent studies found that TB patients had increased frequencies of Mtb-specific CD8+T cells, compared with LTBI (Pollock et al., 2013; Azgomi et al., 2022; Caccamo et al., 2015). Heterogeneity in results from different researches may be attributed to different methods employed to generate Mtb-specific CD8+T cell, including marking T cell by Mtb proteins tetramer, stimulating T cell by ESAT-6 and CFP-10 or stimulating by peptides pools covering a variety of antigen of Mtb. Considering immune response of Mtb-specific CD8+T cells is associated with Mtb and were predominantly found in patients with active TB compared to those with LTBI (Prezzemolo et al., 2014; Lancioni et al., 2019; Rozot et al., 2013), the observation that the proportion of Mtb-specific CD8+T cells increased in Mtb infection may be more reflective of the actual scenario. Considering the absolute changes, active TB led to reduced levels of CD3+ and CD4+T cells, but increased levels of CD8+T cells, confirming the rise in the CD8+T cells proportion (Li et al., 2020). Methodologically, it is more reliable to generate Mtb-specific CD8+T cell by using peptides pools covering a variety of antigen rather than just ESAT-6 and CFP-10, which is consistent with previous studies that adequate antigen is a prerequisite for the generation of Mtb-specific CD8+T cells (Lancioni et al., 2012).
What’s more, the proportion of CD8+T cells are subject to dynamic changes in Mtb infection. Compared with persons with LTBI, Mtb-specific CD8+T cells from TB diseased patients had significantly higher expression of Ki67, which is a cellular proliferation marker (Kudryavtsev et al., 2023). Indeed, TB patients had increased frequencies of Mtb-specific CD8+T cells compared with LTBI (Day et al., 2011). Significant changes in Ki67 expression of Mtb-specific CD8+T cells were observed two months after the initiation of anti-TB chemotherapy, accompanied by decreased frequency of Mtb-specific CD8+T cells, and to the comparable levels as healthy controls at the end of treatment (Day et al., 2014; Li et al., 2020; Day et al., 2011; Nyendak et al., 2013). The above study indicates that CD8+T cells are critical immunological players throughout the course of Mtb infection, including LTBI, active TB, and during anti-TB treatment.
The immune response of CD8+TSCM cells in Mtb infection shares many similarities with their precursor cells—CD8+T cells. Mtb-specific TSCM cells were not detected in a negative QuantiFERON Gold In-Tube (QFT) test persons. After QFT conversion, frequencies of TSCM cells increased to measurable levels and remained detectable thereafter, suggesting that primary Mtb infection induces TSCM cells (Mpande et al., 2018; Sun et al., 2024). For individuals with LTBI, the host sustains a complex interaction with Mtb through the regulation of nutrient availability, as well as the innate and adaptive immune responses, including the dynamic shifts in TSCM cells. This relationship can lead to the reversion of tuberculin skin tests (TSTs) and IFN-γ release assays (IGRAs) from positive to negative in some individuals (Drain et al., 2018). Among those with measurable responses, lower proportions of TSCM cells were observed in reverters, defined as adolescents with two positive QFT tests followed by two negative QFT tests 6 months apart, compared with non-converters (Mpande et al., 2021). These findings suggest that TSCM cells may not only be involved in the immune response induced by Mtb but also play a role in well-controlled or previously cleared Mtb infections.
In the process of Mtb infection, CD8+T cells play a role in fighting against Mtb, simultaneously, progressive impairment of Mtb-specific CD8+T cell responses was observed with increasing Mtb load (Day et al., 2011). Mtb-specific T cell population displaying significant bioenergetic insufficiencies, declining mitochondrial health, and limited cytokine production, all early indicators of T cell exhaustion during Mtb infection (Russell et al., 2019). Indeed, T cell exhaustion is a significant feature of Mtb infection, revealed by a single-cell transcriptome atlas, the immune landscape in severe TB patients was characterized by widespread immune exhaustion in CD8+T cells (Wang et al., 2023). Successful anti-TB treatment results in restoration of Mtb-specific CD8+T cell function, the proportions can return to normal levels, but its limited proliferative function, a part of T cell progressive development exhaustion, may not be fully restored with the progress of treatment (Day et al., 2014). As expected by exhausted T cells, CD8+T cells display reduced production of cytotoxic granule molecules expression levels of perforin and granulysin in Mtb infection (Shen et al., 2023), and increased expression levels of suppressive cytokines, such as IL-10 (Jalbert et al., 2023). Furthermore, numerous previous studies have confirmed that through the detection of increase exhaustion markers on Mtb-specific CD8+T cells, such as CD57, PD-1, CTLA-4, KLRG-1, BATF, NKG2A in Mtb infection (Day et al., 2014; Russell et al., 2019; Liu et al., 2019; Shen et al., 2023). Antibody-mediated blockade of inhibitory receptor signaling pathways has been shown to enhance Mtb-specific T cell function (Shen et al., 2016). Checkpoint blockade immunotherapy in the treatment of Mtb infection is promising to promote control of disease. But in the subset of granulomas with ongoing caspase 1 activation, PD-1 blockade resulted in the exacerbation of Mtb infection, accompanied by the significantly enhanced expansion and function of Mtb-specific CD8+T cells in granulomas, though there were no definite conclusions regarding the contributions of CD8+T cells to the detrimental outcome of PD-1 blockade (Kauffman et al., 2021). McCaffrey et al. showed that the few PD-1-expressing lymphocytes present are largely concentrated in neighboring tertiary lymphoid structures (TLSs) (Mccaffrey et al., 2022). This distribution may help explain how PD-1 blockade exacerbates immunopathology by activating TLS-resident and peripheral T cells, while failing to engage granuloma T cells. Furthermore, PD-L1 blockade is another widely used strategy in anti-PD-1/PD-L1 immunotherapy. Compared with PD-1 blockade, while PD-L1 blockade also enhances CD8+T cells function, it may have a broader impact on the microenvironment by affecting other immune cells that express PD-L1. This can lead to a more complex modulation of the immune response, potentially enhancing overall anti-Mtb immunity. The exhaustion of CD8+T cells diminish their ability to control Mtb infection and impede complete clearance of Mtb. However, it may also play a critical role in preventing the progression of chronic Mtb infection, and anti-PD-1-based therapy needs to be used cautiously in patients with cancer with a history of Mtb exposure. Additionally, further research is needed to explore the therapeutic effects of PD-L1 in the treatment of TB. The impact of CD8+T cell exhaustion in the Mtb infection is multifaceted, not simply beneficial or detrimental. Though the exhausted phenotype of Mtb-specific CD8+T cells can be restored by certain drugs in vitro experiments, it is crucial to observe their impact on disease progression under the complex microenvironment in vivo experiments.
In single-cell transcriptomic analysis, Mtb-specific TSCM cells possess unique phenotypic and functional profiles that share more similarities with bulk TCM and effector T cells (TEFF cells) than bulk TSCM cells. This suggest that TSCM are exposed to chronic antigen stimulation in Mtb infection (Mpande et al., 2018). A functionally impaired and exhausted state of TSCM cells may manifest in Mtb infection, similar to what is observed in HIV infection. Recently, a study indicated that HLA-E-restricted Mtb-specific TSCM cells are lost during Mtb infection and do not fully recover following anti-TB treatment, likely due to infection-induced cellular exhaustion (Azgomi et al., 2022). Studies have shown that there is a parallel differentiation program for human CD8+TSCM cells. Stem-like T cells (TSTEM) and progenitor exhausted-like T cells (TPEX) were two clonally, epigenetically and transcriptionally distinct subsets of TSCM and committed to parallel differentiation programs. Acute viral infections would preferentially generate antigen-specific TSTEM cells, whereas chronic viral infections would preferentially generate antigen-specific TPEX cells. These subsets were defined by core transcriptional signatures that could be distilled phenotypically into simple profiles, namely CCR7+PD-1-TIGIT- (TSTEM) and CCR7+PD-1+TIGIT+ (TPEX). TPEX cells are functionally inferior to TSTEM and committed to a terminally dysfunctional state but expressed memory-like features (Galletti et al., 2020). In Mtb infection, TSCM cells may primarily exist in the form of TPEX cells. Findings from animal models support this hypothesis, showing increased expression of GZMK on peripheral stem cell-like T cells in rhesus macaques infected with Mtb (Foreman et al., 2023). According to recent insights into TSCM cells differentiation programs, GZMK expression is a key feature of TSCM cells differentiation towards a functionally exhausted lineage (Galletti et al., 2020), suggesting that TSCM cells may predominantly exist in an exhausted state during Mtb infection.
Akin to adult stem cells, precursor exhausted T cells are hierarchically organized. Developmental trajectory for TPEX cell originates from long-lived CD62L+CD8+stem-like T cells, which are at a hierarchically superior level compared with their CD62L- counterparts. From CD62L+TPEX cells to CD62L-TPEX cells to terminally exhausted T cells (TEX cells), a progressive loss of multipotency and repopulation capacity were observed (Tsui et al., 2022) (Figure 2). Existence of TSTEM and TPEX have been proved in the human CD8+ memory T cell pool, and further research is needed to deeply understand of CD62L+ stem-like T cells biology and identification of their human counterpart.
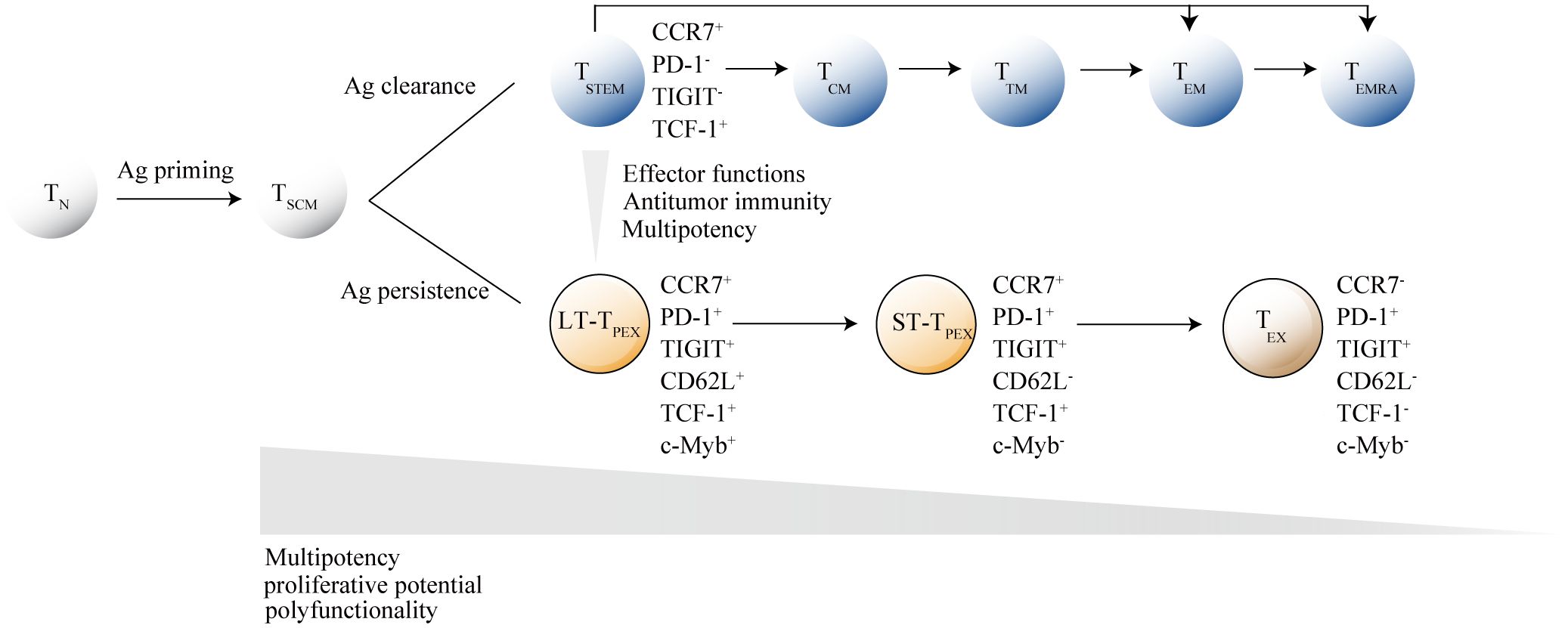
Figure 2. Hierarchical model of memory stem T cells differentiation. After antigen stimulation, naive T cells (TN cells) gradually differentiate into memory T cell subsets, with memory stem T cells (TSCM cells) at the apex of the memory T lymphocyte system. Under different antigen stimulation conditions, TSCM cells develop into either functional T cells or exhausted T cells. When the antigen is cleared, activated T cells differentiate into central memory T cells (TCM cells), transitional memory T-cells (TTM cells) or effector memory T cells (TEM cells), and ultimately into terminally differentiated effector T cells (TEMRA). When the antigen persists, TSCM cells differentiate into terminally exhausted T cells (TEX) through CD62L+ long-term precursor exhausted T cells (LT-TPEX) and CD62L- long-term precursor exhausted T cells (ST-TPEX). T cell subsets are distinguished by the combinatorial expression of key surface markers. The inhibitory receptor markers TIGIT and PD-1 are crucial for distinguishing between TSCM cells and TPEX cells, while CD62L+ and transcription factor c-Myb are the primary markers for identifying different levels in the exhaustion developmental branch. As TN cells gradually differentiate into their terminal states, they lose specific functions.
The percentage of Mtb-specific CD8+T cells identified by tetramers was significantly higher in the circulation of patients with HIV/Mtb co-infection compared to those with Mtb mono-infection (Manna et al., 2020). What’s more, Mtb-specific CD8+T cells exhibit further impairment of proliferative capability in co-infection (Manna et al., 2020; Kalokhe et al., 2015). It is possible that HIV- and Mtb-driven antigenic stimulation jointly determines the acquisition and maintenance of dysfunctional, exhausted-like traits in Mtb-specific CD8+T cells. Indeed, previous studies reported that PD-1 was significantly increased on Mtb-specific CD8+T cells in HIV/Mtb co-infection compared to Mtb mono-infection, with decreased expression of CD107a, IFN-γ and perforin, furthermore, level of PD-1 expression was associated with reduced IL-2 production capacity (Amelio et al., 2019; Kalokhe et al., 2015; Tan et al., 2023). Differences exist not only between patients with HIV/TB and TB, but also between those with HIV/LTBI and LTBI. However, the relevant studies are mainly conducted in ART-naïve individuals, they cannot explain why PLWH with sustained viral suppression still have a higher risk of Mtb infection. To prove whether dysregulation of Mtb-specific T cell functional homeostasis induced by HIV infection can potentially enhance the onset of TB in LTBI subjects, it is imperative to investigate that in long-term ART-treated aviremic HIV-infected patients.
Mtb-specific CD8+T cells identified by with ESAT-6 and/or CFP-10 peptide pools stimulation assays were mostly represented by TEM cells in TB patients (Rozot et al., 2013). Another study showed that mean 45% of Mtb-specific CD8+T cells restricted by HLA-E were composed of TEMRA cells in patients with active TB disease, and 70% of Mtb-specific CD8+T cells restricted by HLA-E in HIV/Mtb co-infected patients were composed of TEMRA cells. Thus, Mtb-specific CD8+T cells response in HIV/Mtb co-infection appears to be largely dominated by a differentiated effector-memory profile (Manna et al., 2020). This indicates that the persistent stimulation by HIV and Mtb antigens enhances the terminal differentiation of CD8+T cells, leading to a further decrease in the proportion of TSCM cells.
During Mtb infection, T cell metabolism and function deteriorate over time. This is manifested by bioenergetic insufficiency in Mtb-specific T cell populations, mitochondrial dysfunction, and restricted cytokine production, all early signs of T cell exhaustion (Russell et al., 2019). In HIV/Mtb co-infected individuals, this deterioration is exacerbated. Compared to patients with TB alone, markers of T cell exhaustion, such as PD-1 expression, are further elevated in Mtb-specific T cells of HIV/Mtb co-infected individuals. This is accompanied by declines in cytotoxicity and proliferation functions (Manna et al., 2020; Amelio et al., 2019). Similar differences are observed between LTBI with and without HIV infection (Manna et al., 2020; Amelio et al., 2019). In both HIV and Mtb mono-infections, CD8+TSCM cells undergo chronic stimulation, leading to exhaustion. This is characterized by increased expression of co-inhibitory molecules (PD-1) and exhaustion markers specific to TSCM cells (GZMK), diminished cytokine secretion capacity (IFN-γ, IL-2) and self-renewal marker TCF-1, and a reduced proportion of these cells (Ribeiro et al., 2014; Vieira et al., 2023; Foreman et al., 2023; Tuluc et al., 2017; Takata et al., 2022; Vigano et al., 2015) (Figure 3). In the context of HIV/Mtb co-infection, the chronic stimulation from dual pathogens is likely to further aggravate these effects. However, current analyses of T cells in human Mtb infection and HIV/Mtb co-infection have primarily relied on traditional markers such as CD45RA and CD62L or CCR7. These markers do not effectively distinguish TSCM cells, leaving the phenotype, functional differences, and mechanisms involved in TSCM cells during HIV/Mtb co-infection remain unresolved questions, requiring further research for exploration.
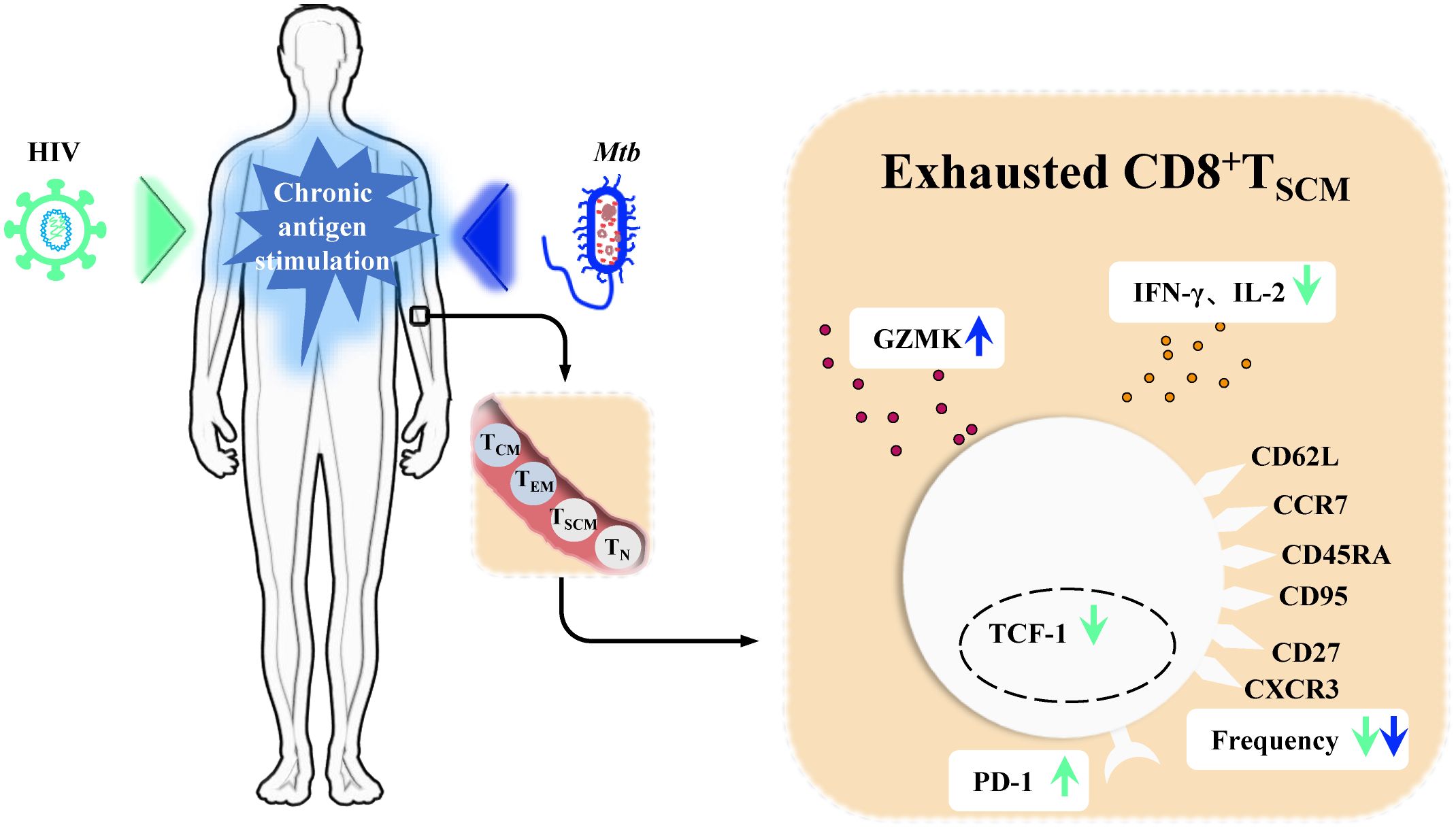
Figure 3. CD8+TSCM cells in chronic Human immunodeficiency Virus (HIV) infection and Mycobacterium tuberculosis (Mtb) infection. In chronic Human immunodeficiency Virus (HIV) infection and Mycobacterium tuberculosis (Mtb) infection, persistent antigenic stimulation drives memory stem T cells (TSCM cells) toward terminal exhaustion. In chronic HIV infection, CD8+TSCM cells exhibit increased expression of the inhibitory receptor PD-1, reduced levels of IFN-γ and IL-2, and decreased expression of the self-renewal marker TCF-1. Staining and quantification of their characteristic surface markers further reveal a decline in their frequency. During Mtb infection, CD8+TSCM cells show a decreased frequency, as seen in HIV infection, but exhibit elevated expression of GZMK.
CD8+T cell-intrinsic IL-27 signaling safeguards the ability of TCF1hi cells to maintain proliferation and avoid terminal differentiation or programmed cell death. Mechanistically, IL-27 endowed rapidly dividing cells with IRF1, a transcription factor that was required for sustained division in a cell-intrinsic manner (Huang et al., 2019). Single-cell transcriptomics and epigenomics approaches revealed that BACH2 establishes the transcriptional and epigenetic programs of stem-like CD8+T cells. BACH2 overexpression enforced stem-like cell fate, whereas BACH2 deficiency impaired stem-like CD8+T cell differentiation. BACH2 suppressed the molecular program driving terminal exhaustion through transcriptional repression and epigenetic silencing (Yao et al., 2021). NR4A1 was previously found to be important for T cell dysfunction. Hao et al. further demonstrate that NR4A1 regulates TPEX cells development and maintenance in the tumor microenvironment. NR4A1 inhibits effector cytokine production and fosters accumulation of TPEX cells by directly stimulating TPEX-related genes while repressing genes associated with terminal exhaustion (Tsui et al., 2022). FOXP1, a hub in the stem-like network, promoted expansion and stemness of chimeric antigen receptor (CAR)-T cells and limited excessive effector differentiation. In the effector network, KLF2 enhanced effector CD8+T cell differentiation and prevented terminal exhaustion (Zhu et al., 2024). In the hierarchical fashion of precursor exhausted T cells. c-Myb has a critical role in restraining exhausted T cell differentiation. The transcription factor MYB is not only essential for the development of CD62L+TPEX cells and maintenance of the antiviral CD8+T cell response, but also induces functional exhaustion and thereby prevents lethal immunopathology (Tsui et al., 2022). Although many studies have focused on elucidating the mechanism of CD8+TSCM cell differentiation since the discovery of parallel differentiation programs, the majority of these studies have been conducted in animal models. Due to significant physiological and immunological differences between animals and humans, the findings may not be directly applicable to human biology. Currently, a comprehensive understanding remains elusive.
Wnt/β-catenin signaling pathway, classically considered necessary for cell differentiation, effector functions and migration, is the canonical Wnt signaling pathway and the best understood and characterized pathway of Wnt signaling (Gattinoni et al., 2010). Activation of Wnt/β-catenin signaling pathway results in β-catenin accumulation and translocation to the nucleus where it drives the expression of T-cell factor/lymphoid enhancer-binding factor (TCF/LEF)-dependent genes, which are important for self-renewal capacity of CD8+TSCM cells (Lin et al., 2016) (Figure 4). CD8+T cells have both a cytolytic effect on infected cells before SIV integration, and a direct, non-cytolytic effect by suppressing viral production (Policicchio et al., 2023). Wnts expressed by CD8+T cells can mediate CD8+T cell noncytolytic anti-HIV-1 activity by canonical Wnt signaling in HIV-infected recipient cells (Wallace et al., 2020). Influenced by HIV, concomitant loss of active Wnt/β-catenin genetic signature at the single-cell level was observed during HIV infection (Kared et al., 2020). Indeed, decreased expression levels of TCF-1 and loss of CD8+TSCM cells have been proved in HIV infection (Takata et al., 2022). Furthermore, Similarly, in Mtb infection, key genes of Wnt/β-catenin signaling were impaired in blood cells of patients with severe pulmonary TB, furthermore, β-catenin expressions in CD8+T cells were significantly decreased in patients with severe pulmonary TB compared with those in mild diseases (Fan et al., 2017; Xiong et al., 2021). SIRT2, a class III HDAC, is overexpressed in Mtb-specific CD4+T cells. Inhibition of SIRT2 enhances could enhances CD4+TSCM cells response by activating b-catenin, and finally enhances the BCG vaccine efficacy during primary infection and TB recurrence (Bhaskar et al., 2023). As counterparts to CD4+T cells, SIRT2 regulation has also been proven in CD8+T cells (Jiang et al., 2020). Similar effects may occur in CD8+TSCM cells under SIRT2 inhibition. same effect may appear in CD8+TSCM under inhibition of SIRT2. Using single cells RNA sequencing and high-dimensional flow cytometry, Kared et al. demonstrate that TSCM heterogeneity results from differential engagement of Wnt signaling. In humans, aging is associated with the coupled loss of Wnt/β-catenin signature in TSCM cells (Kared et al., 2020). It hints that HIV and Mtb infection may cause a certain of caused a certain degree of immunosenescence, leading to disruptions in Wnt/β-catenin signaling, which in turn causes dysfunction in the immune function of CD8+TSCM cells. And it is reasonable to assume that Wnt/β-catenin signaling pathway deteriorates in HIV/Mtb co-infection than in mono-infection. In recent years, with the emergence of immunotherapy, the indispensable role of Wnt in regulating T cell development and differentiation has been recognized (Pai et al., 2017). Modifying the activity of Wnt/β-catenin signaling is an attractive therapeutic approach for infectious diseases. However, given the limited number of relevant studies, the regulation mechanism and alteration require further investigation and validation through more comprehensive omics studies.
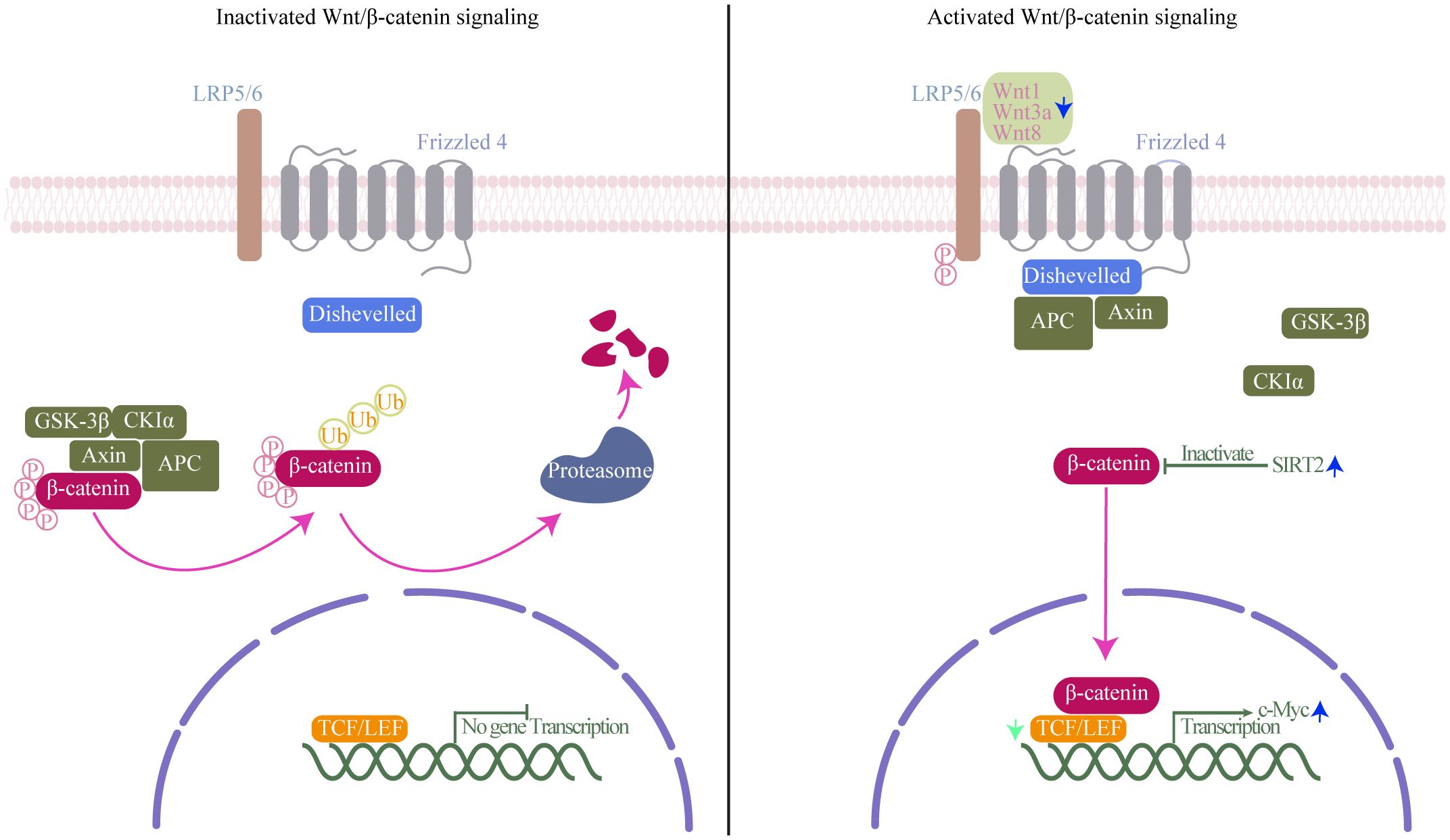
Figure 4. Wnt/β-catenin signaling mechanism. Left (inactivated Wnt/β-catenin signaling): In the absence of Wnt ligands, β-catenin interacts with a degradation complex composed of axis inhibition protein (axin), adenomatous polyposis coli (APC), glycogen synthase kinase-3β (GSK-3β), and casein kinase 1α (CK1α). Within this complex, β-catenin undergoes phosphorylation by GSK-3β and CK1α, followed by ubiquitination and degradation in the proteasome. Right (activated of Wnt/β-catenin signaling): Wnt ligands bind to the frizzled receptor and the co-receptor low-density lipoprotein receptor-related protein (LRP). This interaction induces the phosphorylation of LRP by GSK-3β and CK1α, leading to the recruitment of axin and disheveled to the LRP/Frizzled receptor complex, thereby releasing β-catenin. Subsequently, β-catenin accumulates in the nucleus, where it binds to lymphocyte enhancer factor-1 (LEF1) and T cell factor (TCF) to initiate the transcription of specific genes. Green arrow, TCF expression decreases in Human immunodeficiency Virus (HIV) infection; blue arrow, Wnt1, Wnt3a, and Wnt8 are downregulated, and class III HDAC SIRT2 and transcription factor c-Myc expression increases in Mycobacterium tuberculosis (Mtb) infection.
Many T cell subtypes have been shown to be key responders to various pathogen infections and are utilized to predict vaccine effectiveness. For instance, tissue-resident memory T cells (TRM) in the respiratory tract play a crucial role in limiting the severity of SARS coronavirus infections (Zheng and Wakim, 2022; Buggert et al., 2023b). Consequently, the induction of cytokine-secreting TRM cells has been widely employed to forecast improved clinical outcomes for patients and enhanced protective efficacy for vaccine recipients (Buggert et al., 2023a; Zheng and Wakim, 2022). While the primary assurance of infection prevention lies in the induction of neutralizing antibodies, the cytotoxic CD8+T cell responses are of particular importance in the elimination of pathogens (Plotkin, 2008). Report has shown the persistence of yellow fever specific CD8+TSCM cells for 25 years post vaccination (Marraco et al., 2015). Among HPV-specific CD8+T cells induced by vaccine, CD8+TSCM cells were found to be stronger and long-term anti-tumor function, highlighting its crucial role in the process of vaccine efficacy (Zhang et al., 2020). However, the efficacy of the vaccine in HIV-infected patients may be compromised. Impaired primary responses of CD8+T cells to vaccination exist in older individuals, and many of the immune alterations in HIV-infected individuals resemble the process of immune aging, which is characteristic of old age (Schulz et al., 2015; Chauvin and Sauce, 2022). In elderly individuals, BCG vaccination induced diminished frequencies of CD8+TN and TSCM cells (Kumar et al., 2021). The loss of CD8+TSCM cells may also occur in HIV patients given BCG vaccination. Of note, referring to the impact of aging on CD8+TN cells (Gustafson et al., 2019), CD8+TSCM cells may also undergo phenotypic, functional, transcriptional, and epigenetic deterioration in HIV infection. Impairment of CD8+TSCM cells immune function potentially account for a reduction in vaccine effectiveness in HIV-infected patients given Mtb vaccine. Fortunately, vaccines combined with adjuvant formulations that stimulate the generation of CD8+TSCM cells are promising to enhance the effectiveness of the vaccine. Generation of CD8+T cells response is regulated by T cell receptor (TCR) signaling, and investigation of TCR downregulation and manipulation of TCR signaling strength may help design vaccines to elicit CD8+TSCM cells, capable of surviving antigen restimulation to generate antiviral effects (Wu et al., 2017). Moreover, in the settings of circulating and evolving viruses, CD8+TSCM cells is a remarkably stable marker of long-term protection against evolving pathogen, thus, measuring vaccine-induced TSCM cells may be more accurate to predict the effectiveness of vaccines (Aleksova et al., 2023).
Cumulating evidence in mice indicates that the infusion of less-differentiated T cells results in greater cell expansion, persistence in adoptive immunotherapy (Hinrichs et al., 2009; Sommermeyer et al., 2016; Klebanoff et al., 2016). Quiescent memory T cells seem to be more susceptible to lentiviral transduction than their naive counterparts (Ghassemi et al., 2022). Thus, compared with TN and other memory subsets of T cells, TSCM cells type is an ideal cell population to improve CAR-T cell therapy’s time-dependent efficacy and stability for its extreme longevity, the robust proliferative potential and the capacity to reconstitute a wide-ranging diversity of the T cell compartment (Ahmed et al., 2016). CAR-modified CD8+TSCM cells mediated superior and durable responses in anti-tumor roles, CD8+TSCM cells might also provide an attractive approach for immunotherapy in the setting of chronic infection. However, T cell immunotherapy targeting TSCM cells is limited by the relatively small proportion of these cells. In peripheral blood, TSCM cells account for 2%∼4% of CD8+T cells (Lu et al., 2016). Many new regulators of CD8+TSCM cells have been found, such as gene encoding transcriptional repressor BACH2 (Yao et al., 2021), IL-33 (Marx et al., 2023), TGF-β (Hu et al., 2022), CXCR3 (Bangs et al., 2022), and HMGB2 (Neubert et al., 2023), which sheds light on future interventions that harness the differentiation of therapeutic T cells to treat chronic infection. IL-7 and IL-15 have been implicated in the generation and maintenance of TSCM cells (Cieri et al., 2013). Recently, a simplified protocol enabling efficient derivation of gene-modified CD8+TSCM cells from CD8+TN cells by culturing with IL-7 and IL-15 was presented which may facilitate improved adoptive immunotherapy (Kranz et al., 2022). A mechanistically novel peptide agonist of the IL-7 receptor, MDK-703, could induce pronounced expansion of memory T-cells, particularly the population of TSCM cells (Dower et al., 2023). The Wnt/β-catenin signaling pathway is one pathway which is likely to be involved in influencing whether TSCM cells undergoes self-renewal or differentiation (Gattinoni et al., 2009). Treatments such as β-catenin inhibitors would be useful for assisting in the treatment of HIV-1, acting as a prompt for the formation of CD8+TSCM cells (Denk et al., 2022).
Accumulating evidence has illuminated the significant role of CD8+T cells in both HIV and Mtb infections. Moreover, more pronounced alterations in CD8+T cells during co-infection have been observed, highlighting close associations with disease progression. Delving into the evolutionary characteristics, mechanisms, and functions of CD8+TSCM cells in co-infection contributes to a deeper understanding of immunological mechanisms. In the differentiation process of CD8+T cells, CD8+TSCM cells are at the apex in the hierarchical system of memory CD8+T lymphocytes, holding potential implications for the development of immunotherapies and vaccines. While research about CD8+TSCM cells in HIV/Mtb co-infection is currently limited, noteworthy changes identified in existing articles underscore the need for further studies to elucidate these mechanisms.
JX: Writing – original draft. FW: Writing – original draft. HY: Writing – review & editing. BW: Writing – review & editing. BS: Writing – review & editing. XL: Project administration, Writing – review & editing. TZ: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China (NSFC, 82072271 and 82241072 to TZ, 81501732 to XL), Beijing Hospital Authority's Third Phase "Sailing" Program for Clinical Technology Innovation (to TZ), the High-level Public Health Technical Personnel Construction Project (2022-1-007 to TZ, 2023-02-21 to XL), the Peak Talent Program of Beijing Hospital Authority (DFL20191701 to TZ), National Key Research and Development Program of China (2021YFC0122601 to TZ, 2022YFC2305004 to YL), the Capital’s Funds for Health Improvement and Research (2022-1-1151 to TZ), the Research and Translational Application of Clinical Characteristic Diagnostic and Treatment Techniques in Capital City (Z221100007422055 to TZ), Beijing You'an Hospital Construction of Talent Pool Program (YARCKB2022002 to XL), and the Beijing Key Laboratory for HIV/AIDS Research (BZ0089).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmed, R., Roger, L., Amo, P. C. D., Miners, K. L., Jones, R. E., Boelen, L., et al. (2016). Human stem cell-like memory T cells are maintained in a state of dynamic flux. Cell Rep. 17, 2811–2818. doi: 10.1016/j.celrep.2016.11.037
Ajayi, B. D., Ogunkoya, J. O., Onunu, A., Okwara, B., Ehondor, O., Ajayi, F. O. (2022). Latent tuberculosis among human immunodeficiency virus (HIV) positive patients: prevalence and correlates. West Afr. J. Med. 39, 670–677. doi: 10.1165/rcmb.2021-0311LE
Aleksova, M., Todorova, Y., Emilova, R., Baymakova, M., Yancheva, N., Andonova, R., et al. (2023). Virus-specific stem cell memory CD8+ T cells may indicate a long-term protection against evolving SARS-CoV-2. Diagnostics (Basel) 13, 1280. doi: 10.3390/diagnostics13071280
Amelio, P., Portevin, D., Hella, J., Reither, K., Kamwela, L., Lweno, O., et al. (2019). HIV infection functionally impairs mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses. J. Virol. 93, e01728–18. doi: 10.1128/jvi.01728-18
Azgomi, M. S., Manna, M. P. L., Sullivan, L. C., Brooks, A. G., Carlo, P. D., Dieli, F., et al. (2022). Permanent loss of human leukocyte antigen E-restricted CD8+ T stem memory cells in human tuberculosis. American journal of respiratory cell and molecular biology 67, 127–131. doi: 10.1165/rcmb.2021-0311LE
Bangs, D. J., Tsitsiklis, A., Steier, Z., Chan, S. W., Kaminski, J., Streets, A., et al. (2022). CXCR3 regulates stem and proliferative CD8+ T cells during chronic infection by promoting interactions with DCs in splenic bridging channels. Cell Rep. 38, 110266. doi: 10.1016/j.celrep.2021.110266
Bhaskar, A., Pahuja, I., Negi, K., Verma, A., Ghoshal, A., Mathew, B., et al. (2023). SIRT2 inhibition by AGK2 enhances mycobacteria-specific stem cell memory responses by modulating beta-catenin and glycolysis. iScience 26, 106644. doi: 10.1016/j.isci.2023.106644
Buggert, M., Price, D. A., Mackay, L. K., Betts, M. R. (2023a). Author Correction: Human circulating and tissue-resident memory CD8+ T cells. Nat. Immunol. 24, 1591. doi: 10.1038/s41590-023-01586-y
Buggert, M., Price, D. A., Mackay, L. K., Betts, M. R. (2023b). Human circulating and tissue-resident memory CD8+ T cells. Nat. Immunol. 24, 1076–1086. doi: 10.1038/s41590-023-01538-6
Caccamo, N., Pietra, G., Sullivan, L. C., Brooks, A. G., Prezzemolo, T., Manna, M. P. L., et al. (2015). Human CD8 T lymphocytes recognize Mycobacterium tuberculosis antigens presented by HLA-E during active tuberculosis and express type 2 cytokines. Eur. J. Immunol. 45, 1069–1081. doi: 10.1002/eji.201445193
Chauvin, M., Sauce, D. (2022). Mechanisms of immune aging in HIV. Clin. Sci. (London England: 1979) 136, 61–80. doi: 10.1042/cs20210344
Chávez-Galán, L., Illescas-Eugenio, J., Alvarez-Sekely, M., Baez-Saldaña, R., Chávez, R., Lascurain, R. (2019). Tuberculosis patients display a high proportion of CD8+ T cells with a high cytotoxic potential. Microbiol. Immunol. 63, 316–327. doi: 10.1111/1348-0421.12724
Cieri, N., Camisa, B., Cocchiarella, F., Forcato, M., Oliveira, G., Provasi, E., et al. (2013). IL-7 and IL-15 instruct the generation of human memory stem T cells from naive precursors. Blood 121, 573–584. doi: 10.1182/blood-2012-05-431718
Day, C. L., Abrahams, D. A., Lerumo, L., Rensburg, E. J. V., Stone, L., O'rie, T., et al. (2011). Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immunol. 187, 2222–2232. doi: 10.4049/jimmunol.1101122
Day, C. L., Moshi, N. D., Abrahams, D. A., Rooyen, M. V., O'rie, T., Kock, M. D., et al. (2014). Patients with tuberculosis disease have Mycobacterium tuberculosis-specific CD8 T cells with a pro-apoptotic phenotype and impaired proliferative capacity, which is not restored following treatment. PloS One 9, e94949. doi: 10.1371/journal.pone.0094949
Denk, D., Petrocelli, V., Conche, C., Drachsler, M., Ziegler, P. K., Braun, A., et al. (2022). Expansion of T memory stem cells with superior anti-tumor immunity by Urolithin A-induced mitophagy. Immunity 55, 2059–2073.e8. doi: 10.1016/j.immuni.2022.09.014
Dower, W. J., Park, A. I., Bakker, A. V., Cwirla, S. E., Pongtornpipat, P., Williams, B. M., et al. (2023). A mechanistically novel peptide agonist of the IL-7 receptor that addresses limitations of IL-7 cytokine therapy. PloS One 18, e0286834. doi: 10.1371/journal.pone.0286834
Drain, P. K., Bajema, K. L., Dowdy, D., Dheda, K., Naidoo, K., Schumacher, S. G., et al. (2018). Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin. Microbiol. Rev. 31, e00021–18. doi: 10.1128/cmr.00021-18
Escobar, G., Mangani, D., Anderson, A. C. (2020). T cell factor 1: A master regulator of the T cell response in disease. Sci. Immunol. 5, eabb9726. doi: 10.1126/sciimmunol.abb9726
Fan, L., Shen, H., Huang, H., Yang, R., Yao, L. (2017). Impairment of Wnt/β-catenin signaling in blood cells of patients with severe cavitary pulmonary tuberculosis. PloS One 12, e0172549. doi: 10.1371/journal.pone.0172549
Foreman, T. W., Nelson, C. E., Sallin, M. A., Kauffman, K. D., Sakai, S., Otaizo-Carrasquero, F., et al. (2023). CD30 co-stimulation drives differentiation of protective T cells during Mycobacterium tuberculosis infection. J. Exp. Med. 220, e20222090. doi: 10.1084/jem.20222090
Gaiha, G. D., Mckim, K. J., Woods, M., Pertel, T., Rohrbach, J., Barteneva, N., et al. (2014). Dysfunctional HIV-specific CD8+ T cell proliferation is associated with increased caspase-8 activity and mediated by necroptosis. Immunity 41, 1001–1012. doi: 10.1016/j.immuni.2014.12.011
Galletti, G., Simone, G. D., Mazza, E. M. C., Puccio, S., Mezzanotte, C., Bi, T. M., et al. (2020). Two subsets of stem-like CD8+ memory T cell progenitors with distinct fate commitments in humans. Nat. Immunol. 21, 1552–1562. doi: 10.1038/s41590-020-0791-5
Gattinoni, L., Ji, Y., Restifo, N. P. (2010). Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin. Cancer Res. 16, 4695–4701. doi: 10.1158/1078-0432.Ccr-10-0356
Gattinoni, L., Lugli, E., Ji, Y., Pos, Z., Paulos, C. M., Quigley, M. F., et al. (2011). A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297. doi: 10.1038/nm.2446
Gattinoni, L., Speiser, D. E., Lichterfeld, M., Bonini, C. (2017). T memory stem cells in health and disease. Nat. Med. 23, 18–27. doi: 10.1038/nm.4241
Gattinoni, L., Zhong, X.-S., Palmer, D. C., Ji, Y., Hinrichs, C. S., Yu, Z., et al. (2009). Wnt signaling arrests effector T cell differentiation and generates CD8+ memory stem cells. Nat. Med. 15, 808–813. doi: 10.1038/nm.1982
Ghassemi, S., Durgin, J. S., Nunez-Cruz, S., Patel, J., Leferovich, J., Pinzone, M., et al. (2022). Rapid manufacturing of non-activated potent CAR T cells. Nat. Biomed. Eng. 6, 118–128. doi: 10.1038/s41551-021-00842-6
Gustafson, C. E., Cavanagh, M. M., Jin, J., Weyand, C. M., Goronzy, J. J. (2019). Functional pathways regulated by microRNA networks in CD8 T-cell aging. Aging Cell 18, e12879. doi: 10.1111/acel.12879
Helleberg, M., Kronborg, G., Ullum, H., Ryder, L. P., Obel, N., Gerstoft, J. (2015). Course and clinical significance of CD8+ T-cell counts in a large cohort of HIV-infected individuals. J. Infect. Dis. 211, 1726–1734. doi: 10.1093/infdis/jiu669
Hinrichs, C. S., Borman, Z. A., Cassard, L., Gattinoni, L., Spolski, R., Yu, Z., et al. (2009). Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc. Natl. Acad. Sci. U. S. A. 106, 17469–17474. doi: 10.1073/pnas.0907448106
Hu, Y., Hudson, W. H., Kissick, H. T., Medina, C. B., Baptista, A. P., Ma, C., et al. (2022). TGF-β regulates the stem-like state of PD-1+ TCF-1+ virus-specific CD8 T cells during chronic infection. J. Exp. Med. 219, e20211574. doi: 10.1084/jem.20211574
Huang, Z., Zak, J., Pratumchai, I., Shaabani, N., Vartabedian, V. F., Nguyen, N., et al. (2019). IL-27 promotes the expansion of self-renewing CD8+ T cells in persistent viral infection. J. Exp. Med. 216, 1791–1808. doi: 10.1084/jem.20190173
Hunt, P. W., Martin, J. N., Sinclair, E., Bredt, B., Hagos, E., Lampiris, H., et al. (2003). T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J. Infect. Dis. 187, 1534–1543. doi: 10.1086/374786
Jalbert, E., Liu, C., Mave, V., Lang, N., Kagal, A., Valvi, C., et al. (2023). Comparative immune responses to Mycobacterium tuberculosis in people with latent infection or sterilizing protection. iScience 26, 107425. doi: 10.1016/j.isci.2023.107425
Jiang, C., Liu, J., Guo, M., Gao, X., Wu, X., Bai, N., et al. (2020). The NAD-dependent deacetylase SIRT2 regulates T cell differentiation involved in tumor immune response. Int. J. Biol. Sci. 16, 3075–3084. doi: 10.7150/ijbs.49735
Jin, H.-T., Anderson, A. C., Tan, W. G., West, E. E., Ha, S.-J., Araki, K., et al. (2010). Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc. Natl. Acad. Sci. U. S. A. 107, 14733–14738. doi: 10.1073/pnas.1009731107
Kalokhe, A. S., Adekambi, T., Ibegbu, C. C., Ray, S. M., Day, C. L., Rengarajan, J. (2015). Impaired degranulation and proliferative capacity of Mycobacterium tuberculosis-specific CD8+ T cells in HIV-infected individuals with latent tuberculosis. J. Infect. Dis. 211, 635–640. doi: 10.1093/infdis/jiu505
Kared, H., Tan, S. W., Lau, M. C., Chevrier, M., Tan, C., How, W., et al. (2020). Immunological history governs human stem cell memory CD4 heterogeneity via the Wnt signaling pathway. Nat. Commun. 11, 821. doi: 10.1038/s41467-020-14442-6
Kauffman, K. D., Sakai, S., Lora, N. E., Namasivayam, S., Baker, P. J., Kamenyeva, O., et al. (2021). PD-1 blockade exacerbates Mycobacterium tuberculosis infection in rhesus macaques. Sci. Immunol. 6, eabf3861. doi: 10.1126/sciimmunol.abf3861
Klebanoff, C. A., Scott, C. D., Leonardi, A. J., Yamamoto, T. N., Cruz, A. C., Ouyang, C., et al. (2016). Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J. Clin. Investig. 126, 318–334. doi: 10.1172/jci81217
Kranz, E., Kuhlmann, C. J., Chan, J., Kim, P. Y., Chen, I. S. Y., Kamata, M. (2022). Efficient derivation of chimeric-antigen receptor-modified TSCM cells. Front. Immunol. 13. doi: 10.3389/fimmu.2022.877682
Kudryavtsev, I., Zinchenko, Y., Serebriakova, M., Akisheva, T., Rubinstein, A., Savchenko, A., et al. (2023). A key role of CD8+ T cells in controlling of tuberculosis infection. Diagnostics (Basel Switzerland) 13, 2961. doi: 10.3390/diagnostics13182961
Kumar, N. P., Padmapriyadarsini, C., Rajamanickam, A., Bhavani, P. K., Nancy, A., Jayadeepa, B., et al. (2021). BCG vaccination induces enhanced frequencies of memory T cells and altered plasma levels of common γc cytokines in elderly individuals. PloS One 16, e0258743. doi: 10.1371/journal.pone.0258743
Lancioni, C., Nyendak, M., Kiguli, S., Zalwango, S., Mori, T., Mayanja-Kizza, H., et al. (2012). CD8+ T cells provide an immunologic signature of tuberculosis in young children. Am. J. Respir. Crit. Care Med. 185, 206–212. doi: 10.1164/rccm.201107-1355OC
Lancioni, C., Swarbrick, G. M., Park, B., Nyendak, M., Nsereko, M., Mayanja-Kizza, H., et al. (2019). Recognition of CD8+ T-cell epitopes to identify adults with pulmonary tuberculosis. Eur. Respir. J. 53, 1802053. doi: 10.1183/13993003.02053-2018
Li, G., Yang, F., He, X., Liu, Z., Pi, J., Zhu, Y., et al. (2020). Anti-tuberculosis (TB) chemotherapy dynamically rescues Th1 and CD8+ T effector levels in Han Chinese pulmonary TB patients. Microbes Environ. 22, 119–126. doi: 10.1016/j.micinf.2019.10.001
Lin, W.-H. W., Nish, S. A., Yen, B., Chen, Y.-H., Adams, W. C., Kratchmarov, R., et al. (2016). CD8+ T lymphocyte self-renewal during effector cell determination. Cell Rep. 17, 1773–1782. doi: 10.1016/j.celrep.2016.10.032
Liu, Q., Ou, Q., Shen, L., Qiu, C., Zhang, B., Zhang, W., et al. (2019). BATF potentially mediates negative regulation of PD-1/PD-ls pathway on T cell functions in mycobacterium tuberculosis infection. Front. Immunol. 10. doi: 10.3389/fimmu.2019.02430
Lu, X., Li, L., Xia, H., Wu, H., Su, B., Zhang, T. (2016). Advances in the role of stem memory T cells in HIV-1 infection. Bing du xue bao = Chin. J. Virol. 32, 796–799.
Lugli, E., Gattinoni, L., Roberto, A., Mavilio, D., Price, D. A., Restifo, N. P., et al. (2013). Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat. Protoc. 8, 33–42. doi: 10.1038/nprot.2012.143
Manna, M. P. L., Orlando, V., Prezzemolo, T., Carlo, P. D., Cascio, A., Delogu, G., et al. (2020). HLA-E-restricted CD8+ T lymphocytes efficiently control mycobacterium tuberculosis and HIV-1 coinfection. Am. J. Respir. Cell Mol. Biol. 62, 430–439. doi: 10.1165/rcmb.2019-0261OC
Marraco, S., Soneson, C., Cagnon, L., Gannon, P. O., Allard, M., Maillard, S. A., et al. (2015). Long-lasting stem cell-like memory CD8+ T cells with a naïve-like profile upon yellow fever vaccination. Sci. Transl. Med. 7, 282ra48. doi: 10.1126/scitranslmed.aaa3700
Marx, A.-F., Kallert, S. M., Brunner, T. M., Villegas, J. A., Geier, F., Fixemer, J., et al. (2023). The alarmin interleukin-33 promotes the expansion and preserves the stemness of Tcf-1+ CD8+ T cells in chronic viral infection. Immunity 56, 813–828.e10. doi: 10.1016/j.immuni.2023.01.029
Mccaffrey, E. F., Donato, M., Keren, L., Chen, Z., Delmastro, A., Fitzpatrick, M. B., et al. (2022). The immunoregulatory landscape of human tuberculosis granulomas. Nat. Immunol. 23, 318–329. doi: 10.1038/s41590-021-01121-x
Mpande, C., Dintwe, O. B., Musvosvi, M., Mabwe, S., Bilek, N., Hatherill, M., et al. (2018). Functional, antigen-specific stem cell memory (TSCM) CD4+ T cells are induced by human mycobacterium tuberculosis infection. Front. Immunol. 9. doi: 10.3389/fimmu.2018.00324
Mpande, C., Steigler, P., Lloyd, T., Rozot, V., Mosito, B., Schreuder, C., et al. (2021). Mycobacterium tuberculosis-specific T cell functional, memory, and activation profiles in quantiFERON-reverters are consistent with controlled infection. Front. Immunol. 12. doi: 10.3389/fimmu.2021.712480
Neubert, E. N., Derogatis, J. M., Lewis, S. A., Viramontes, K. M., Ortega, P., Henriquez, M. L., et al. (2023). HMGB2 regulates the differentiation and stemness of exhausted CD8+ T cells during chronic viral infection and cancer. Nat. Commun. 14, 5631. doi: 10.1038/s41467-023-41352-0
Nyendak, M. R., Park, B., Null, M. D., Baseke, J., Swarbrick, G., Mayanja-Kizza, H., et al. (2013). Mycobacterium tuberculosis specific CD8(+) T cells rapidly decline with antituberculosis treatment. PloS One 8, e81564. doi: 10.1371/journal.pone.0081564
Olson, A., Coote, C., Snyder-Cappione, J. E., Lin, N., Sagar, M. (2021). HIV-1 transcription but not intact provirus levels are associated with systemic inflammation. J. Infect. Dis. 223, 1934–1942. doi: 10.1093/infdis/jiaa657
Pai, S. G., Carneiro, B. A., Mota, J. M., Costa, R., Leite, C. A., Barroso-Sousa, R., et al. (2017). Wnt/beta-catenin pathway: modulating anticancer immune response. J. Hematol. Oncol. 10, 101. doi: 10.1186/s13045-017-0471-6
Perdomo-Celis, F., Taborda, N. A., Rugeles, M. T. (2019a). CD8+ T-cell response to HIV infection in the era of antiretroviral therapy. Front. Immunol. 10. doi: 10.3389/fimmu.2019.01896
Perdomo-Celis, F., Velilla, P. A., Taborda, N. A., Rugeles, M. T. (2019b). An altered cytotoxic program of CD8+ T-cells in HIV-infected patients despite HAART-induced viral suppression. PloS One 14, e0210540. doi: 10.1371/journal.pone.0210540
Plotkin, S. A. (2008). Vaccines: correlates of vaccine-induced immunity. Clin. Infect. diseases: an Off. Publ. Infect. Dis. Soc. America 47, 401–409. doi: 10.1086/589862
Policicchio, B. B., Cardozo-Ojeda, E. F., Xu, C., Ma, D., He, T., Raehtz, K. D., et al. (2023). CD8+ T cells control SIV infection using both cytolytic effects and non-cytolytic suppression of virus production. Nat. Commun. 14, 6657. doi: 10.1038/s41467-023-42435-8
Pollock, K. M., Whitworth, H. S., Montamat-Sicotte, D. J., Grass, L., Cooke, G. S., Kapembwa, M. S., et al. (2013). T-cell immunophenotyping distinguishes active from latent tuberculosis. J. Infect. Dis. 208, 952–968. doi: 10.1093/infdis/jit265
Ponnan, S. M., Thiruvengadam, K., Kathirvel, S., Shankar, J., Rajaraman, A., Mathaiyan, M., et al. (2021). Elevated numbers of HIV-specific poly-functional CD8+ T cells with stem cell-like and follicular homing phenotypes in HIV-exposed seronegative individuals. Front. Immunol. 12. doi: 10.3389/fimmu.2021.638144
Prezzemolo, T., Guggino, G., Manna, M. P. L., Liberto, D. D., Dieli, F., Caccamo, N. (2014). Functional signatures of human CD4 and CD8 T cell responses to mycobacterium tuberculosis. Front. Immunol. 5. doi: 10.3389/fimmu.2014.00180
Ribeiro, S. P., Milush, J. M., Cunha-Neto, E., Kallas, E. G., Kalil, J., Somsouk, M., et al. (2014). The CD8⁺ memory stem T cell (T(SCM)) subset is associated with improved prognosis in chronic HIV-1 infection. J. Virol. 88, 13836–13844. doi: 10.1128/jvi.01948-14
Rozot, V., Patrizia, A., Vigano, S., Mazza-Stalder, J., Idrizi, E., Day, C. L., et al. (2015). Combined use of Mycobacterium tuberculosis-specific CD4 and CD8 T-cell responses is a powerful diagnostic tool of active tuberculosis. Clin. Infect. Dis. 60, 432–437. doi: 10.1093/cid/ciu795
Rozot, V., Vigano, S., Mazza-Stalder, J., Idrizi, E., Day, C. L., Perreau, M., et al. (2013). Mycobacterium tuberculosis-specific CD8+ T cells are functionally and phenotypically different between latent infection and active disease. Eur. J. Immunol. 43, 1568–1577. doi: 10.1002/eji.201243262
Russell, S. L., Lamprecht, D. A., Mandizvo, T., Jones, T. T., Naidoo, V., Addicott, K. W., et al. (2019). Compromised Metabolic Reprogramming Is an Early Indicator of CD8+ T Cell Dysfunction during Chronic Mycobacterium tuberculosis Infection. Cell Rep. 29, 3564–3579.e5. doi: 10.1016/j.celrep.2019.11.034
Sachdeva, M., Sharma, A., Arora, S. K. (2023). High frequency of memory stem cells with a distinct gene signature in HIV patients with treatment interruption. Scand. J. Immunol. 97, e13262. doi: 10.1111/sji.13262
Salido, J., Ruiz, M. J., Trifone, C., Figueroa, M. I., Caruso, M. P., Gherardi, M. M., et al. (20182443). Phenotype, Polyfunctionality, and Antiviral Activity of in vitro Stimulated CD8+ T-Cells From HIV+ Subjects Who Initiated cART at Different Time-Points After Acute Infection. Front. Immunol. 9. doi: 10.3389/fimmu.2018.02443
Schulz, A. R., Mälzer, J. N., Domingo, C., Jürchott, K., Grützkau, A., Babel, N., et al. (2015). Low thymic activity and dendritic cell numbers are associated with the immune response to primary viral infection in elderly humans. J. Immunol. 195, 4699–4711. doi: 10.4049/jimmunol.1500598
Seyoum, E., Demissie, M., Worku, A., Mulu, A., Berhane, Y., Abdissa, A. (2022). Increased mortality in HIV infected individuals with tuberculosis: A retrospective cohort study, addis ababa, Ethiopia. HIV AIDS (Auckl.) 14, 143–154. doi: 10.2147/hiv.S354436
Shen, L., Gao, Y., Liu, Y., Zhang, B., Liu, Q., Wu, J., et al. (2016). PD-1/PD-L pathway inhibits M.tb-specific CD4+ T-cell functions and phagocytosis of macrophages in active tuberculosis. Sci. Rep. 6, 38362. doi: 10.1038/srep38362
Shen, X., Wu, T., Ji, X., Yang, K., Wang, L., Peng, Y., et al. (2023). Mycobacterium tuberculosis infection depressed cytotoxic T cells activity owing to decreasing NKG2C and increasing NKG2A expression. Mol. Immunol. 162, 133–142. doi: 10.1016/j.molimm.2023.08.014
Sommermeyer, D., Hudecek, M., Kosasih, P. L., Gogishvili, T., Maloney, D. G., Turtle, C. J., et al. (2016). Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity. vivo. Leukemia 30, 492–500. doi: 10.1038/leu.2015.247
Song, B., Lu, X., Weng, J., Su, B., Zhang, T., Gao, Y. (2017). Dynamic changes of CD8+ stem memory T cells and their effects on diseases progression in chronic HIV-1 infection. J. Capital Med. Univ. 38, 650–653.
Sultana, Z. Z., Hoque, F. U., Beyene, J., Akhlak-Ul-Islam, M., Khan, M. H. R., Ahmed, S., et al. (2021). HIV infection and multidrug resistant tuberculosis: a systematic review and meta-analysis. BMC Infect. Dis. 21, 51. doi: 10.1186/s12879-020-05749-2
Sun, M., Phan, J. M., Kieswetter, N. S., Huang, H., Yu, K. K. Q., Smith, M. T., et al. (2024). Specific CD4+ T cell phenotypes associate with bacterial control in people who 'resist' infection with Mycobacterium tuberculosis. Nat. Immunol. 25, 1411–1421. doi: 10.1038/s41590-024-01897-8
Takata, H., Kakazu, J. C., Mitchell, J. L., Kroon, E., Colby, D. J., Sacdalan, C., et al. (2022). Long-term antiretroviral therapy initiated in acute HIV infection prevents residual dysfunction of HIV-specific CD8+ T cells. EBioMedicine 84, 104253. doi: 10.1016/j.ebiom.2022.104253
Takata, H., Mitchell, J. L., Pacheco, J., Pagliuzza, A., Pinyakorn, S., Buranapraditkun, S., et al. (2023). An active HIV reservoir during ART is associated with maintenance of HIV-specific CD8+ T cell magnitude and short-lived differentiation status. Cell Host Microbe 31, 1494–1506.e4. doi: 10.1016/j.chom.2023.08.012
Tan, Y., Guo, W., Zhu, Q., Song, S., Xiang, Y., Wu, S., et al. (2023). Characterization of peripheral cytokine-secreting cells responses in HIV/TB co-infection. Front. Cell. infection Microbiol. 13. doi: 10.3389/fcimb.2023.1162420
Tartaro, D. L., Camiro-Zúñiga, A., Nasi, M., Biasi, S. D., Najera-Avila, M. A., Jaramillo-Jante, M. D. R., et al. (2022). Effective treatment of patients experiencing primary, acute HIV infection decreases exhausted/activated CD4+ T cells and CD8+ T memory stem cells. Cells 11, 2307. doi: 10.3390/cells11152307
Trautmann, L., Janbazian, L., Chomont, N., Said, E. A., Gimmig, S., Bessette, B., et al. (2006). Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12, 1198–1202. doi: 10.1038/nm1482
Trautmann, L., Mbitikon-Kobo, F.-M., Goulet, J.-P., Peretz, Y., Shi, Y., Grevenynghe, J. V., et al. (2012). Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood 120, 3466–3477. doi: 10.1182/blood-2012-04-422550
Tsui, C., Kretschmer, L., Rapelius, S., Gabriel, S. S., Chisanga, D., Knöpper, K., et al. (2022). MYB orchestrates T cell exhaustion and response to checkpoint inhibition. Nature 609, 354–360. doi: 10.1038/s41586-022-05105-1
Tuluc, F., Spitsin, S., Tustin, N. B., Murray, J. B., Tustin, R., Schankel, L. A., et al. (2017). Decreased PD-1 expression on CD8 lymphocyte subsets and increase in CD8 Tscm cells in children with HIV receiving raltegravir. AIDS Res. Hum. Retroviruses 33, 133–142. doi: 10.1089/aid.2016.0108
Utzschneider, D. T., Charmoy, M., Chennupati, V., Pousse, L., Ferreira, D. P., Calderon-Copete, S., et al. (2016). T cell factor 1-expressing memory-like CD8(+) T cells sustain the immune response to chronic viral infections. Immunity 45, 415–427. doi: 10.1016/j.immuni.2016.07.021
Vali, B., Jones, R. B., Sakhdari, A., Sheth, P. M., Clayton, K., Yue, F.-Y., et al. (2010). HCV-specific T cells in HCV/HIV co-infection show elevated frequencies of dual Tim-3/PD-1 expression that correlate with liver disease progression. Eur. J. Immunol. 40, 2493–2505. doi: 10.1002/eji.201040340
Vieira, V., Lim, N., Singh, A., Leitman, E., Dsouza, R., Adland, E., et al. (2023). Slow progression of pediatric HIV associates with early CD8+ T cell PD-1 expression and a stem-like phenotype. JCI Insight 8, e156049. doi: 10.1172/jci.insight.156049
Vigano, S., Negron, J., Ouyang, Z., Rosenberg, E. S., Walker, B. D., Lichterfeld, M., et al. (2015). Prolonged antiretroviral therapy preserves HIV-1-specific CD8 T cells with stem cell-like properties. J. Virol. 89, 7829–7840. doi: 10.1128/jvi.00789-15
Walker, B. D., Chakrabarti, S., Moss, B., Paradis, T. J., Flynn, T., Durno, A. G., et al. (1987). HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328, 345–348. doi: 10.1038/328345a0
Wallace, J., Narasipura, S. D., Sha, B. E., French, A. L., Al-Harthi, L. (2020). Canonical wnts mediate CD8+ T cell noncytolytic anti-HIV-1 activity and correlate with HIV-1 clinical status. J. Immunol. 205, 2046–2055. doi: 10.4049/jimmunol.1801379
Walton, S., Mandaric, S., Oxenius, A. (2013). CD4 T cell responses in latent and chronic viral infections. Front. Immunol. 4. doi: 10.3389/fimmu.2013.00105
Wang, Y., Sun, Q., Zhang, Y., Li, X., Liang, Q., Guo, R., et al. (2023). Systemic immune dysregulation in severe tuberculosis patients revealed by a single-cell transcriptome atlas. J. infection 86, 421–438. doi: 10.1016/j.jinf.2023.03.020
Wang, S., Zhang, Q., Hui, H., Agrawal, K., Karris, M., Rana, T. M. (2020). An atlas of immune cell exhaustion in HIV-infected individuals revealed by single-cell transcriptomics. Emerging Microbes infections 9, 2333–2347. doi: 10.1080/22221751.2020.1826361
Winchell, C. G., Nyquist, S. K., Chao, M. C., Maiello, P., Myers, A. J., Hopkins, F., et al. (2023). CD8+ lymphocytes are critical for early control of tuberculosis in macaques. J. Exp. Med. 220, e20230707. doi: 10.1084/jem.20230707
World Health Organisation (2023). Global tuberculosis report 2023. Available online at: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2023 (Accessed 2024).
World Health Organization (2020). Tuberculosis. Available online at: https://www.who.int/tb/areas-of-work/tb-hiv/en/ (Accessed 2024).
Wu, S., Zhu, W., Peng, Y., Wang, L., Hong, Y., Huang, L., et al. (2017). The antitumor effects of vaccine-activated CD8+ T cells associate with weak TCR signaling and induction of stem-like memory T cells. Cancer Immunol. Res. 5, 908–919. doi: 10.1158/2326-6066.Cir-17-0016
Xiong, K., Niu, J., Zheng, R., Liu, Z., Song, Y., Wang, L., et al. (2021). The role of β-catenin in th1 immune response against tuberculosis and profiles of expression in patients with pulmonary tuberculosis. J. Immunol. Res. 2021, 6625855. doi: 10.1155/2021/6625855
Yao, C., Lou, G., Sun, H.-W., Zhu, Z., Sun, Y., Chen, Z., et al. (2021). BACH2 enforces the transcriptional and epigenetic programs of stem-like CD8+ T cells. Nat. Immunol. 22, 370–380. doi: 10.1038/s41590-021-00868-7
Zehn, D., Thimme, R., Lugli, E., Almeida, G. P. D., Oxenius, A. (2022). 'Stem-like' precursors are the fount to sustain persistent CD8+ T cell responses. Nat. Immunol. 23, 836–847. doi: 10.1038/s41590-022-01219-w
Zhang, D., Shankar, P., Xu, Z., Harnisch, B., Chen, G., Lange, C., et al. (2003). Most antiviral CD8 T cells during chronic viral infection do not express high levels of perforin and are not directly cytotoxic. Blood 101, 226–235. doi: 10.1182/blood-2002-03-0791
Zhang, Y., Wang, N., Ding, M., Yang, Y., Wang, Z., Huang, L., et al. (2020). CD40 accelerates the antigen-specific stem-like memory CD8+ T cells formation and human papilloma virus (HPV)-positive tumor eradication. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01012
Zheng, M. Z. M., Wakim, L. M. (2022). Tissue resident memory T cells in the respiratory tract. Mucosal Immunol. 15, 379–388. doi: 10.1038/s41385-021-00461-z
Keywords: HIV, Mycobacterium tuberculosis, CD8 + T cells, memory stem T cells, exhaustion
Citation: Xiao J, Wang F, Yan H, Wang B, Su B, Lu X and Zhang T (2024) Memory stem CD8+T cells in HIV/Mtb mono- and co-infection: characteristics, implications, and clinical significance. Front. Cell. Infect. Microbiol. 14:1485825. doi: 10.3389/fcimb.2024.1485825
Received: 25 August 2024; Accepted: 13 November 2024;
Published: 10 December 2024.
Edited by:
Victor Hugo Aquino, National University of Asunción, ParaguayReviewed by:
Wei Jiang, Medical University of South Carolina, United StatesCopyright © 2024 Xiao, Wang, Yan, Wang, Su, Lu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tong Zhang, enRfZG9jQGNjbXUuZWR1LmNu; Xiaofan Lu, bHV4aWFvZmFuMjAwOGhrQGNjbXUuZWR1LmNu; Bin Su, Ymluc3VAY2NtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.