
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 09 January 2025
Sec. Clinical Infectious Diseases
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1482787
This article is part of the Research Topic Cellular and host immune responses in the context of dual viral infection View all 6 articles
 Xiaolin Ma1
Xiaolin Ma1 Yuting Wu1
Yuting Wu1 Ri De2
Ri De2 Hailan Yao3
Hailan Yao3 Feng He3
Feng He3 Yi Wang4
Yi Wang4 Wei Wang3
Wei Wang3 Chao Yan5
Chao Yan5 Qinwei Song6
Qinwei Song6 Chunjie Guo1
Chunjie Guo1 Li Wen1
Li Wen1 Linqing Zhao2*
Linqing Zhao2* Ling Cao1*
Ling Cao1* Chunmei Zhu1*
Chunmei Zhu1*Background: The pathogenic distribution of co-infections and immunological status of patients infected with human adenovirus serotypes 3 or 7 (HAdV-3 or HAdV-7) were poorly understood.
Methods: This study involved a retrospective analysis of respiratory specimens collected from enrolled children with lower respiratory tract infections (LRTIs), positive for HAdV-3 or HAdV-7 from January 2017 to December 2019. Demographic data, clinical features, laboratory and radiographic findings were compared to delineate the impact of co-infections, and immune responses on clinical severity of HAdV-3 or HAdV-7 infections.
Results: Among 1311cases enrolled, there were 66 infected with HAdV-3 and 58 with HAdV-7. HAdV-7-infected patients exhibited more prolonged fever (100% vs 89.4%, p=0.014), pneumonia (100% vs 89.4%, p=0.014), hypoxia (34.5% vs 12.1%, p=0.003), higher propensity for aspartate aminotransferase exceeding 80U/L (21.1% vs 4.7%, p=0.006), D-Dimer exceeding 1.65mg/L (64.9% vs 12.5%, p<0.001), consolidation (50.0% vs 27.4%, p=0.011), and pleural effusion (32.8% vs 6.5%, p<0.001), co-infections with Mycoplasma pneumoniae (77.1% vs 32.6%, p<0.001), and multiple infections (56.8% vs 41.3%, p=0.007), compared to those with HAdV-3 infections. Immune cell analysis indicated that HAdV-7 infections led to a more pronounced decrease in CD3+ T cells (1596.8 vs 2444.8 cells/𝛍l, p=0.042), CD8+ cytotoxic T cells (668.6 vs 774.0 cells/µl, p=0.045), and increased NK cell percentages (11.5% vs 9.0%, p=0.044) compared to HAdV-3 infections.
Conclusions: Hospitalized children with HAdV-7-associated LRTIs exhibit greater severity, multiple infections, and significant potential for greater cellular immune dysregulation compared to those with HAdV-3 infection, indicating a more severe clinical course and distinct pathogenic profiles.
Human adenoviruses (HAdV), specifically serotypes 3 and 7 (HAdV-3 and HAdV-7), are recognized as significant pathogens in the etiology of lower respiratory tract infections (LRTIs) among children (Lu et al., 2013; Chen et al., 2022). These infections pose substantial health concerns, particularly when they manifest in severe forms such as pneumonia (Jain et al., 2015; Xie et al., 2019). These infections not only lead to high rates of hospital admissions but also present a considerable challenge in terms of management and prevention, due to their rapid transmission, potential to cause large-scale outbreaks, and the absence of effective antiviral drugs or vaccines. The situation is further complicated by the variability in serotypes, clinical presentations and the potential for severe complications, including respiratory failure and death. This severity is notably pronounced in densely populated regions such as China, where frequent outbreaks have been highlighted critical areas for healthcare focus and intervention (Xie et al., 2019; Li et al., 2021; Zou et al., 2021; Wang et al., 2022; Liu et al., 2023a).
While the direct impact of these adenovirus serotypes on the severity of disease has been documented more and more (Lin et al., 2017; Liu et al., 2022; Wang et al., 2023), a critical gap remains in understanding how co-infections and variations in immune response influence the severity of these infections. This gap underscores the need for in-depth research focused on the interplay between co-infecting pathogens and the host’s immune system, which can significantly affect disease outcomes. Infections with HAdV not only result in primary disease manifestations but are also frequently complicated by secondary bacterial or viral infections (Mao et al., 2022; Liu et al., 2023b). These co-infections can exacerbate the severity of the respiratory illness, resulting in more severe symptoms, an increased risk of complications, and prolonged hospital stays (Gao et al., 2020; Chen et al., 2024). Moreover, the immune response to these co-infections, which can vary significantly among individuals, especially in pediatric patients, plays a crucial role in the progression and outcome of the disease. A robust immune response might control the spread of the virus and secondary infections more effectively, while an inadequate or overly aggressive response may lead to worse clinical outcomes (Nazir and Metcalf, 2005).
Currently, the pathogenic distribution of co-infections and the immunological status of patients infected with HAdV-3 or HAdV-7 remains poorly understood. This study aims to elucidate the role of co-infections and immune responses in shaping the clinical severity of HAdV-3 and HAdV-7 infections among hospitalized children with LRTIs. Through this research, we bridge a gap in the literature and lay the groundwork for a comprehensive investigation into the complex dynamics of adenovirus infections compounded by co-infecting organisms and the corresponding immunological responses.
This retrospective study was conducted at Capital Institute of Pediatrics between January 2017 and December 2019. The cohort included children under 18 years diagnosed with LRTIs and confirmed to have HAdV-3 or HAdV-7 infections.
Respiratory specimens, including throat swabs, nasopharyngeal swabs, nasopharyngeal aspirates, and bronchoalveolar lavage fluid (BALF), were collected from children admitted with LRTIs within two days of admission. Specimens were immediately transported to the laboratory. Two microliters of Hank’s solution were added for suspension prior to centrifugation at 1,200 g for ten minutes. Screening for common respiratory pathogens, including viruses, bacteria, and fungi, was conducted by the laboratory.
For multiple pathogen nucleic acid screening, nucleic acids were extracted using the QIAamp® Viral RNA Mini Kit (QIAGEN, Germany) from a 140 µl sample volume, with the total elution volume of 60µl stored at -80℃ from the supernatants, and used for screening encompassed influenza A and B, respiratory syncytial virus, HAdV, metapneumovirus, rhinovirus, bocavirus, parainfluenza virus, coronavirus, Mycoplasma pneumoniae (MP) and chlamydia pneumoniae by the NxTAGTM RPP assay (Luminex Molecular Diagnostics Inc., Toronto, Canada) according to the manufacturer’s protocol.
Respiratory bacteria and atypical pathogens including Streptococcus pneumoniae, Hemophilus influenzae, Staphylococcus aureus, Methicillin resistant staphylococcus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, Stenotrophomonas maltophilia, Legionella pneumoniae, Chlamydia pneumoniae, Mycobacterium tuberculosis complex and MP, were identified in specimens through the Pathogenic Bacteria Nucleic Acid Detection Kit adhering to the manufacturers’ protocols (CapitalBio Technology, Beijing, China). Cultures for bacteria and fungi were performed on sputum and BALF specimens.
HAdV-positive specimens underwent molecular genotyping for HAdV-3 and HAdV-7 using Polymerase chain reaction and sequencing of the penton base, hexon, and fiber genes, as described previously (Yang et al., 2021; Wu et al., 2022). Sequences were analyzed using the Sanger method on an ABI3730xl DNA analyzer (SinoGenoMax Co., Ltd, Beijing, China).
Chromas Lite 2.22 and NCBI BLAST facilitated initial sequence analysis. Editseq and Seqman (DNAStar) were utilized for sequence editing and alignment. Phylogenetic trees were constructed using MEGA X with Clustal W (Molecular Evolutionary Genetics Analysis Version X), employing the neighbor-joining method and bootstrap analysis with 1,000 replicates for node reliability assessment (Tamura et al., 2004; Kumar et al., 2018).
Lymphocyte percentages and counts were determined using flow cytometry. Peripheral blood specimens (50 μl) were collected into ethylenediaminetetraacetic acid anticoagulation tubes and transferred to BD Trucount™ Tubes using reverse sampling for absolute counting. This was followed by the addition of 450 μl hemolysin, which was incubated in the dark for 15 minutes. Cell subset counts were obtained using a BD FACSCanto™ II and analyzed with BD FACSCanto™ software version 3.1(BD, USA). All procedures adhered to applicable guidelines and regulations.
Using the fully-automated biochemical analyzer (Siemens, Germany, BN II machine), children’s serum samples were serially diluted with a sample dilution solution, and subsequently, anti-human immunoglobulins and complement antibodies were added. Turbidity measurements were taken at a wavelength of 340 nm using the immunoturbidimetric method, and ultimately, quantitative assessments of immunoglobulins (IgG, IgA, IgM, IgE) and complement levels (C3, C4) were conducted.
Demographic data, clinical features, laboratory findings, treatment outcomes, and radiographic features were retrospectively derived from electronic medical chart after the patients’ discharge from the hospital. LRTIs encompassed bronchitis, bronchiolitis, and pneumonia. Pneumonia was characterized by the presence of consolidation (a dense or fluffy opacity with or without air bronchograms), other infiltrates (linear and pathy alveolar or interstitial densities), or pleural effusion as observed on chest radiographs (Cherian et al., 2005). Severity categorization adhered to the American guidelines for community-acquired pneumonia in children, with cases classified as severe based on ≥1 major or ≥2 minor criteria (Bradley et al., 2011). Hypoxia was defined as sustained saturation of peripheral oxygen or pulse oximetry measurement <92% on room air. The criteria for respiratory distress in children with pneumonia were as follows: (1) any of the following signs, including tachypnea (respiratory rate >60 breaths/min for these aged 0-2 months, >50 breaths/min for these aged 2-12 months, >40 breaths/min for these aged 1-5 years, and >20 breaths/min for these aged more than 5 years), dyspnea, retractions (suprasternal, intercostal, or subcostal), grunting, nasal flaring, apnea, altered mental status and pulse oximetry measurement <92% on room air (Bradley et al., 2011); (2) The ratio of the partial pressure of arterial oxygen to fraction of inspired oxygen <300 mmHg according to arterial blood gas analysis. Due to the study’s retrospective nature, written informed consent was waived as no personally identifiable information was utilized. Patients coinfected with human coronaviruses were excluded from statistical analysis, accounting for missing data.
The Statistical Package for the Social Sciences (version 27.0) was employed for statistical analyses. Continuous variables are described as means ± standard deviation or medians (interquartile ranges), whereas categorical data are presented as frequencies and percentages. Differences between groups were assessed using chi-square or Fisher’s exact tests for categorical variables and t-tests or Mann-Whitney U tests for continuous variables.
Among 1311 respiratory specimens collected from children hospitalized with acute respiratory infection during the study period, 147 cases tested positive for HAdV (147/1311, 11.2%). Among specimens positive for HAdV, 126 clinical samples were successfully typed as HAdV-B3 and B7 by phylogenetic analyses of the penton base, hexon, and fiber genes (Figure 1; Supplementary Table 1). Of these, 67 cases were identified as HAdV-B3 (45.58%, 67/147) and 59 cases as HAdV-B7 (40.14%, 59/147). The phylogenetic analyses results were consistent with the BLAST results. Panels (A), (B), and (C) in Figure 1 displayed the phylogenetic relationships of the penton base, hexon, and fiber genes, respectively. Ultimately, 66 cases of HAdV-3 and 58 of HAdV-7 associated LRTIs with detailed clinical information were enrolled.
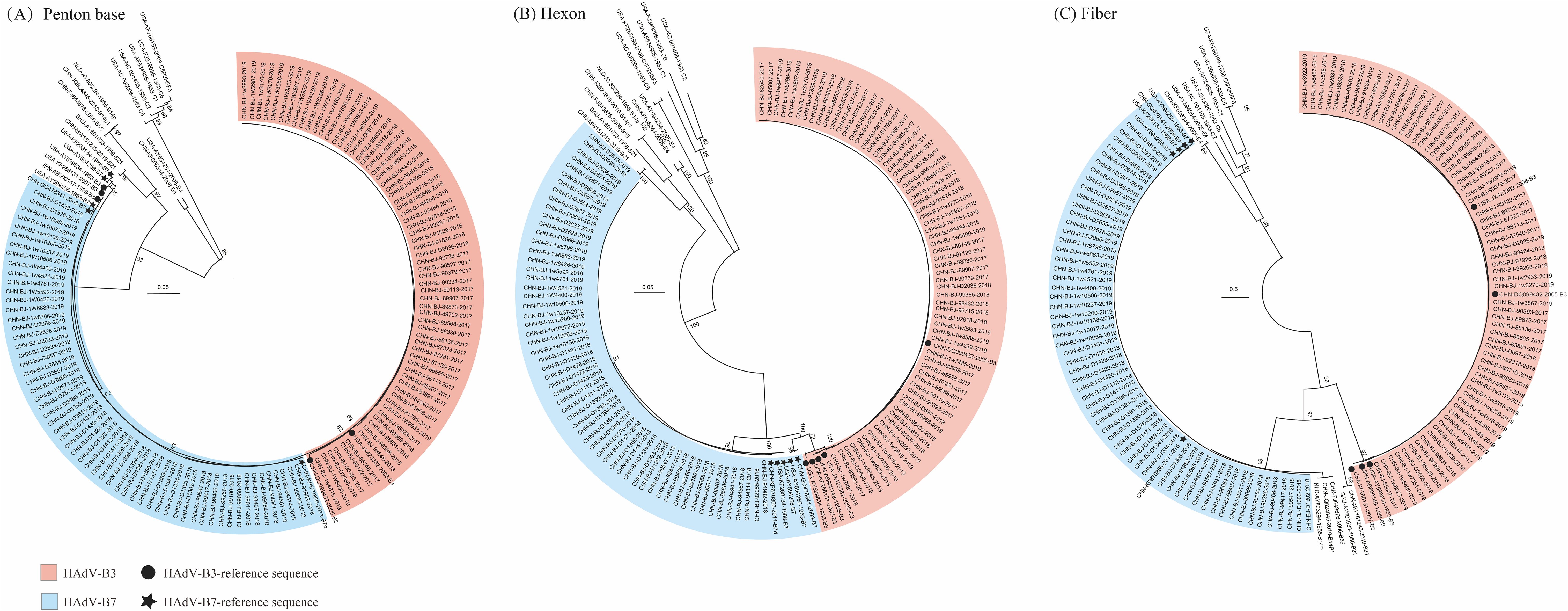
Figure 1. The phylogenetic trees of the penton base (A), hexon (B), and fiber genes (C) of the 126 identified HAdV-positive clinical specimens were generated using the General Time Reversible (GTR) model by MEGA X with the neighbor-joining method and 1,000 bootstrap replicates. Additional reference sequences were retrieved from GenBank for context and comparison.
As illustrated in Table 1, the median age of hospitalized patients with HAdV-7-associated LRTIs was 3.7 years (range: 0.3-9.7 years), whereas for HAdV-3-associated LRTIs, it was 3.3 years (range: 0.56-10.9 years). The demographic characteristics of patients infected with HAdV-3 and HAdV-7 were comparable, with no significant differences in age, gender distribution, or underlying medical conditions noted.
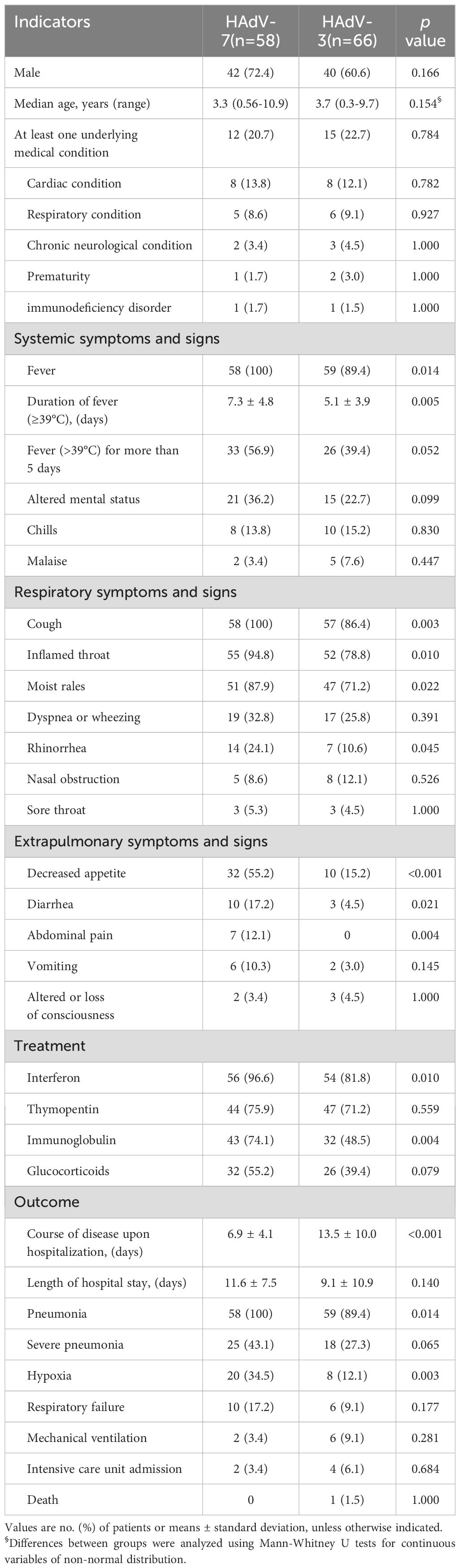
Table 1. Comparison of demographics, underlying medical conditions and clinical features in 124 hospitalized patients with HAdV-3 and HAdV-7 associated LRTIs.
Compared to those with HAdV-3 infections, patients with HAdV-7 were significantly more likely to experience an increased incidence of symptoms such as prolonged fever (100% vs 89.4%, p=0.014), cough (100% vs 86.4%, p=0.003), inflamed throat (94.8% vs 78.8%, p=0.010), moist rales (87.9% vs 71.2%, p=0.022), decreased appetite (55.2% vs 15.2%, p<0.001), rhinorrhea (24.1% vs 10.6%, p=0.045), diarrhea (17.2% vs 4.5%, p=0.021), and abdominal pain (12.1% vs 0, p=0.004) (Table 1). Pediatric patients infected with HAdV-7 experienced significantly longer fever durations exceeding 39°C than those with HAdV-3 infections (7.3 ± 4.8 vs 5.1 ± 3.9 days, p=0.005). The mean disease course upon hospitalization for patients with HAdV-7 was 6.9 ± 4.1 days, considerably shorter than that of the HAdV-3 group (13.5 ± 10.0 days, p<0.001). Both groups presented comparable rates of dyspnea or wheezing.
In terms of treatment and outcomes, patients with HAdV-7 infections notably required more frequent medications, such as interferon (96.6% vs 81.8%, p=0.010) and immunoglobulin (74.1% vs 48.5%, p=0.004), and demonstrated higher morbidity rates of pneumonia (100% vs 89.4%, p=0.014) and hypoxia (34.5% vs 12.1%, p=0.003) than those with HAdV-3 infections (Table 1). Despite a higher proportion of severe pneumonia diagnoses (43.1% vs 27.3%), glucocorticoids use (55.2% vs 39.4%) and occurrence of respiratory failure (17.2% vs 9.1%) among HAdV-7 patients, statistical significance was not reached. Unexpectedly, admission to the intensive care unit (3.4% vs 6.1%) and the use of mechanical ventilation (3.4% vs 9.1%) were more frequent in the HAdV-3 group, among which one child died due to complications.
Laboratory findings for patients infected with HAdV-7 differed significantly from those infected with HAdV-3, showing a greater likelihood of leukopenia (21.1% vs 3.1%, p=0.002), neutropenia (29.8% vs 14.1%, p=0.035), markers indicative of liver damage such as alanine aminotransferase >80 U/L (10.5% vs 1.6%, p=0.051), aspartate aminotransferase >80 U/L (21.1% vs 4.7%, p=0.006), lactate dehydrogenase >300 U/L (78.9% vs 59.4%, p=0.021), α1-hydroxybutyrate dehydrogenase >260 U/L (63.2% vs 43.8%, p=0.033), coagulation disorders such as D-Dimer>1.65 mg/L (64.9% vs 12.5%, p<0.001), fibrinogen degradation products [6.2 (3.0-12.2) vs 3.0 (2.3-5.1)g/L, p=0.002], as well as less likelihood of marker indicative of myocardial damage such as creatine kinase-MB >6.33 ng/ml (3.5% vs 14.1%, p=0.044) (Table 2). Despite these differences, no significant disparities were found in other common blood parameters.
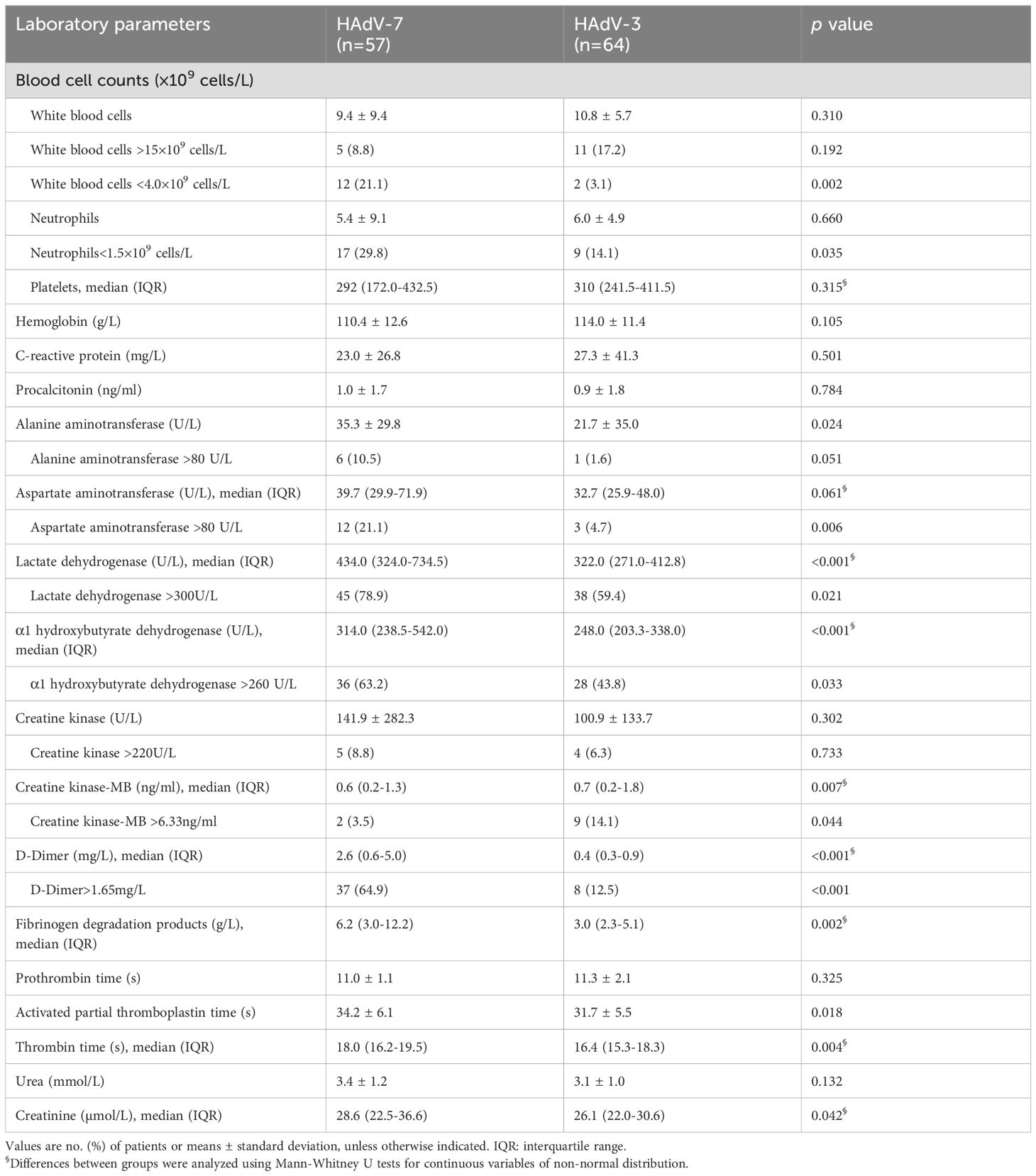
Table 2. Comparison of laboratory findings in 121 hospitalized patients with HAdV-3 and HAdV-7 associated with LRTIs.
Imaging of chest X-ray or computed tomography scan revealed a spectrum of radiological abnormalities (Table 3). Imaging studies revealed that HAdV-7 patients had a higher frequency of alveolar or interstitial infiltrates (98.3% vs 85.5%, p=0.017), consolidation (50.0% vs 27.4%, p=0.011) and pleural effusion (32.8% vs 6.5%, p<0.001) than those with HAdV-3. Unexpectedly, multilobe infiltrates were slightly more common in HAdV-3-infected patients (32.8% vs 43.5%, p=0.224). Both groups exhibited similar rates of atelectasis and small airway disease, with two HAdV-7-infected patients experiencing severe complications like pulmonary necrosis and emphysema.
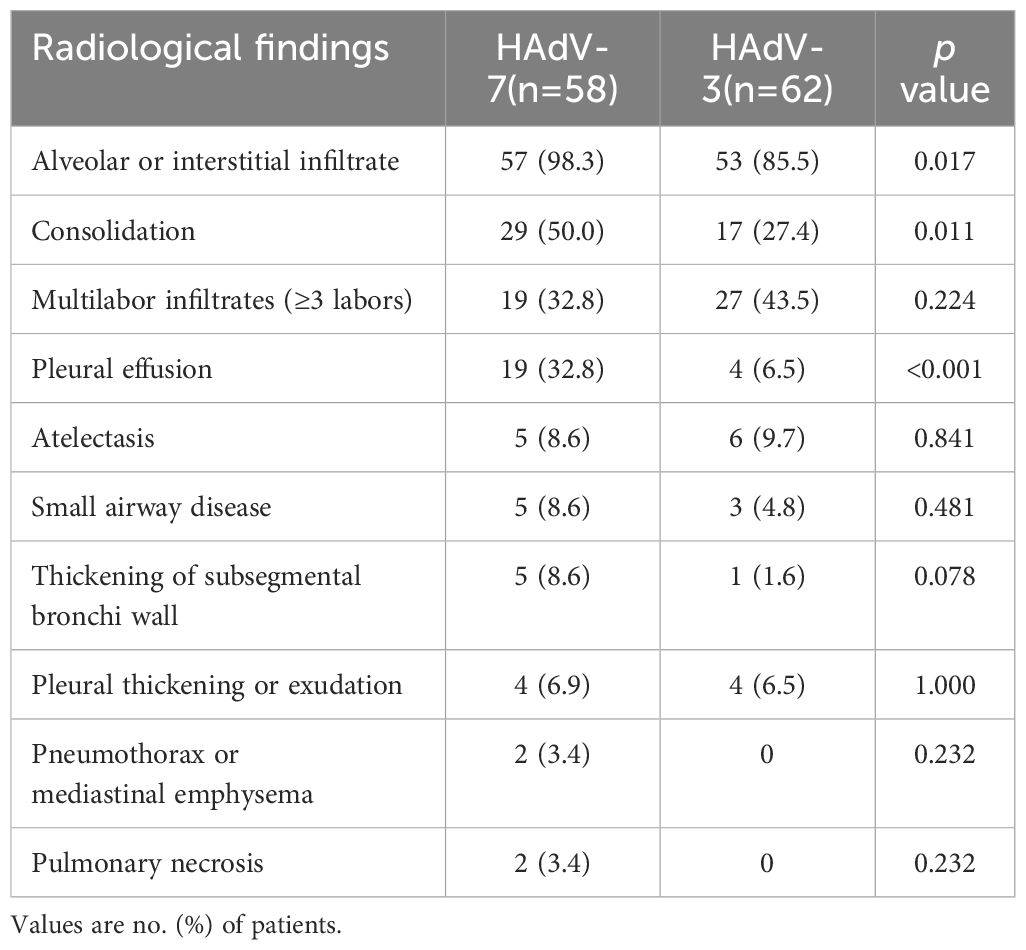
Table 3. Comparison of radiological findings in 120 hospitalized children of HAdV-3 and HAdV-7 associated LRTIs.
Among the HAdV-7 positive patients (n=35), 33 (89.5%) exhibited co-infections with other respiratory pathogens, compared to an 82.6% co-infection rate among the HAdV-3 positive patients (n=46). Patients infected with HAdV-3 demonstrated a higher propensity for dual infections (29.6% vs 41.3%, p=0.081; Supplementary Table 2). Conversely, patients infected with HAdV-7 exhibited a greater likelihood of multiple infections (56.8% vs 41.3%, p=0.007; Supplementary Table 3). Patients with HAdV-7 infection demonstrated significantly higher rates of co-infection with other respiratory viruses (48.6% vs 26.1%, p=0.035), atypical pathogens (80.0% vs 32.6%, p<0.001), and marginally higher rates with respiratory bacteria (62.9% vs 56.5%) compared to those with HAdV-3 infection (Table 4). Significantly higher frequencies of MP and parainfluenza virus co-infection were observed in patients with HAdV-7 compared to those with HAdV-3 (77.1% vs 32.6%, p<0.001; 20.0% vs 2.2%, p=0.018, respectively). Furthermore, HAdV-7 positive children were frequently co-infected with pathogens such as Staphylococcus aureus, respiratory syncytial virus, bocavirus, rhinovirus, Candida albicans or Aspergillus fumigatus, whereas HAdV-3 positive cases showed a higher propensity for co-infection with Hemophilus influenzae, Methicillin resistant staphylococcus, Acinetobacter baumannii, and exhibited higher positive sputum or BALF cultures, albeit these were not statistically significantly different. Co-infection rates with Streptococcus pneumoniae were similar between the two groups.
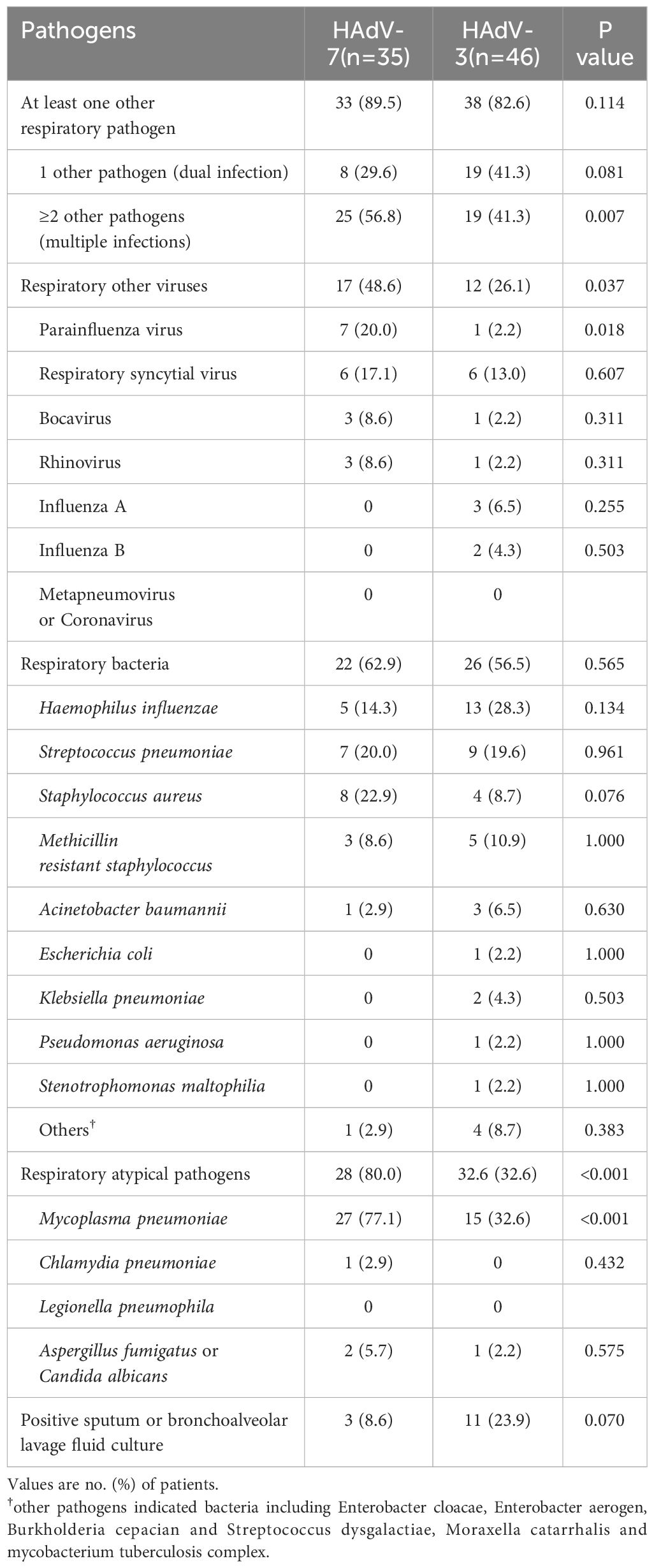
Table 4. Comparison of co-detections in 81 hospitalized children of etiologic HAdV-3 and HAdV-7 associated LRTIs.
The effects of HAdV-7 and HAdV-3 infections on major lymphocyte subset alterations, immunoglobulins and complement levels in peripheral blood were investigated among 67 children with LRTIs (Table 5, Figure 2). Patients with HAdV-7 infection exhibited a decrease in total lymphocyte counts in peripheral blood compared to those with HAdV-3 infection, though this difference was not statistically significant [2612.0 (1873.2-4057.3) vs 3528.1 (2104.1-5228.5) cells/l, p=0.080]. Among T cell subsets, HAdV-7-infected patients showed significant decreases in CD3+ and CD8+ T cell counts [1596.8 (1132.5-2521.9) vs 2444.8 (1171.0-3700.8) cells/l, p=0.042; 668.6 (378.3-1000.8) vs 774.0 (485.9-1624.5) cells/l, p=0.045, respectively] compared to those with HAdV-3 infection, suggesting an imbalance in T cell subsets and potential immune dysregulation. HAdV-7 positive patients had lower CD4+ T cell counts [861.6 (626.6-1305.6) vs 1456.0 (595.0-2064.2) cells/l], CD19+ B cell frequencies (654.7 ± 446.0 vs 711.9 ± 511.4 cells/l), and CD16+CD56+ NK cell numbers (358.2 ± 318.6 vs 390.5 ± 450.5 cells/l) than those with HAdV-3 infection, though these differences were not statistically significant. The HAdV-7 positive group exhibited a higher percentage of CD16+CD56+ NK cells compared to the HAdV-3 positive group [11.5% (7.0%-18.0%) vs 9.0% (5.0%-12.5%), p=0.044], with comparable percentages of other lymphocyte subsets between the groups. No significant differences were noted in the levels of complement 3 and 4, or immunoglobulins G, A, M, and E between patients infected with HAdV-7 and those with HAdV-3.
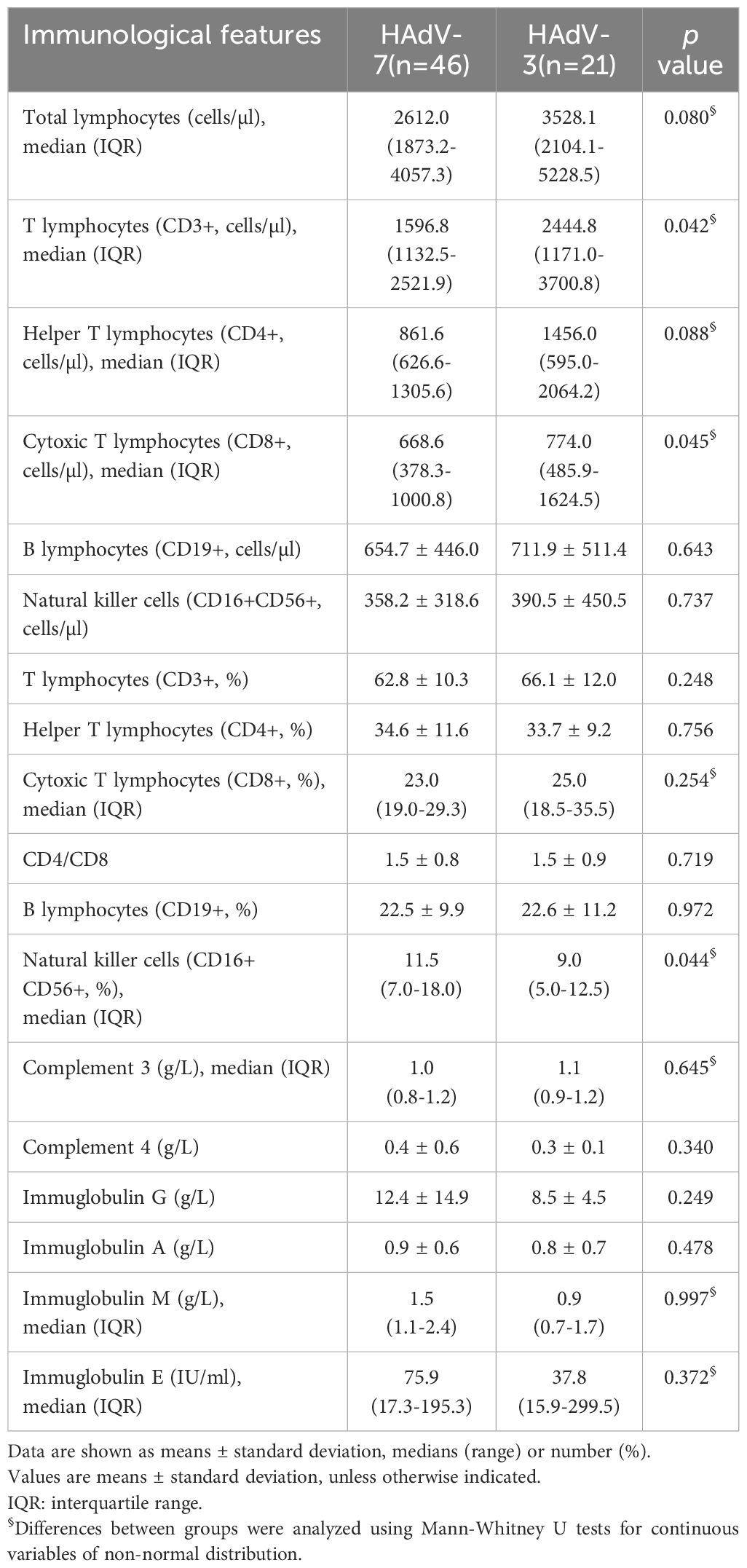
Table 5. Comparison of immunological features in 67 hospitalized children of HAdV-7 and HAdV-3 associated LRTIs.
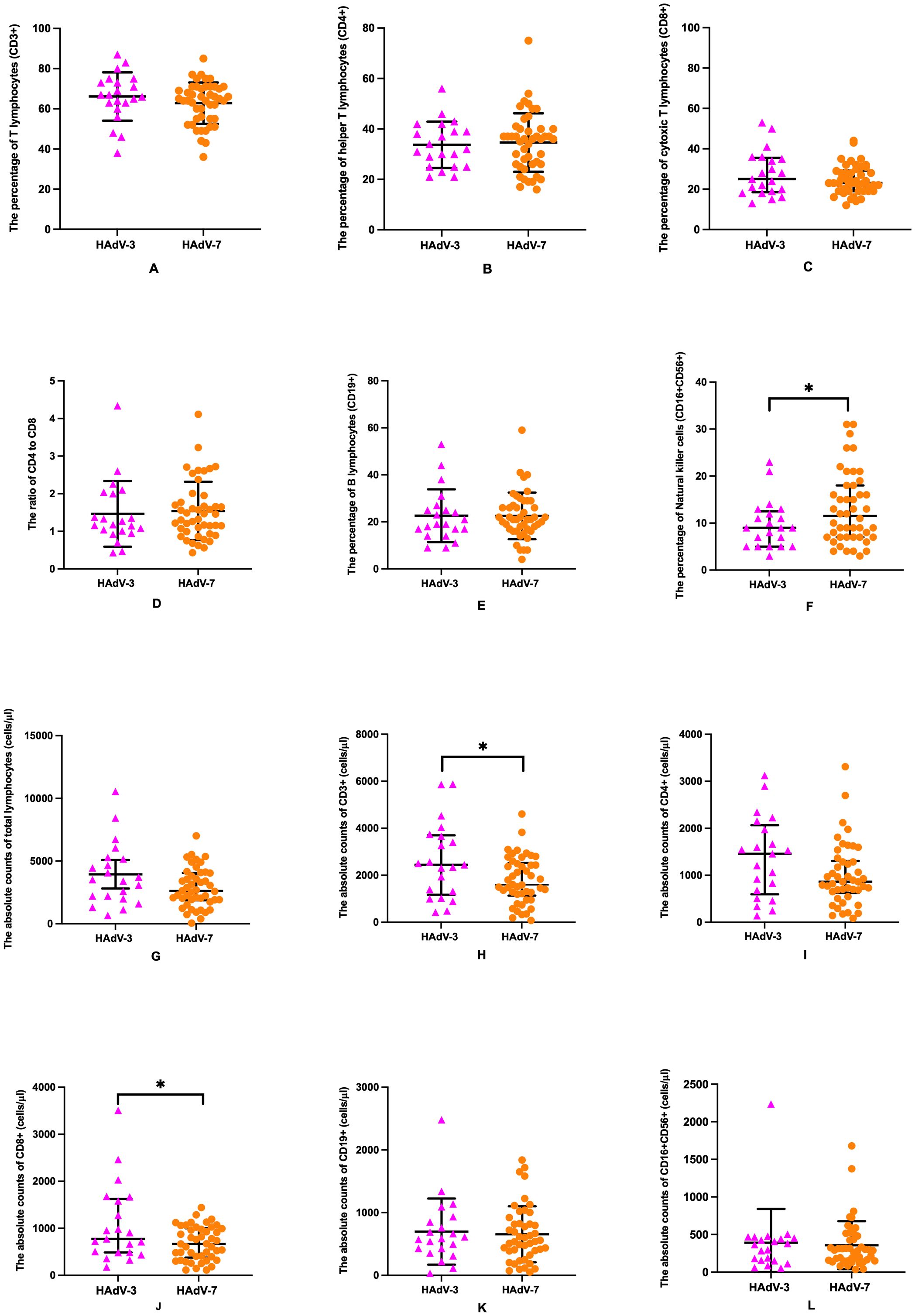
Figure 2. Comparative analysis of lymphocyte subsets in HAdV-3 (n=21) and HAdV-7 (n=46) infected patients with LRTIs. Percentages of CD3+ T lymphocytes (A) and CD8+ T lymphocytes (C), percentages and absolute counts of CD4+ T lymphocytes (B, I), CD19+ B lymphocytes (E, K), absolute counts of total lymphocytes (G) and CD16+CD56+ natural killer cells (L), and the ratio of CD4+ to CD8+ T lymphocytes (D) were shown no significant difference between HAdV-3 group and HAdV-7 group. Percentage of CD16+CD56+ natural killer cells (F) was with a significant increase in the HAdV-7 group, while absolute counts of CD3+ T lymphocytes (H) and CD8+ T lymphocytes (J) were with a significant reduction in the HAdV-7 group. Error bars represented the mean with standard deviation or median interquartile range. *: Statistically significant differences were indicated between two groups (p<0.05).
The current analysis highlights significant differences in clinical severity and outcomes between HAdV-7 and HAdV-3 infections, with HAdV-7 patients exhibiting a higher incidence of severe symptoms, including prolonged fever, pneumonia, hypoxia, liver damage, and coagulation disorders. Radiological findings further support the clinical observation of a more severe disease in HAdV-7 infections, evidenced by a higher frequency of alveolar or interstitial infiltrates, consolidation, and pleural effusion compared to those in HAdV-3 infections. This is consistent with the understanding that HAdV-7 can lead to more extensive lung involvement and damage, potentially explaining the increased severity and adverse clinical outcomes observed in these patients. Studies have demonstrated that HAdV-7 can lead to more severe respiratory illnesses, often characterized by higher mortality and requiring increased mechanical ventilation and intensive care unit admissions compared to HAdV-3 (Lin et al., 2017; Dai et al., 2020; Wang et al., 2023). Previous studies have indicated that HAdV-7 exhibits more robust replication and triggers an exacerbated cytokine response compared to HAdV-3, resulting in more severe airway inflammation (Fu et al., 2019; Siew et al., 2020). In the current study, higher frequencies of mechanical ventilation and intensive care unit admissions were observed in the HAdV-3 group; however, mortality remained low across both groups. The investigation in current study revealed a higher incidence of immunoglobulin and glucocorticoid use in treating individuals with HAdV-7 compared to those infected with HAdV-3. Intravenous immunoglobulin and/or glucocorticoid treatments are effective in preventing severe adenovirus pneumonia in children (Oliveira et al., 2020; Cai et al., 2023). Corroborating these observations in this study, Li L et al. (Li et al., 2018) demonstrated that strategic glucocorticoid and immunoglobulin use in adenovirus pneumonia treatment effectively reduces complication risks and enhances recovery prospects.
In this study, co-infection rates were notably higher in patients testing positive for HAdV-7 compared to those with HAdV-3. Furthermore, the current analysis indicated a greater propensity for patients infected with HAdV-3 to present with dual infections, whereas those with HAdV-7 infections were more commonly associated with multiple infections. This discrepancy suggests that HAdV-7 may predispose children to a broader spectrum of secondary infections, potentially owing to differences in pathogenic mechanisms and immune evasion strategies between these adenovirus serotypes. A notable aspect of this investigation is the remarkably high detection rate of MP infection in subjects infected with either HAdV-7 (77.1%) or HAdV-3 (32.6%), identifying MP as a predominant co-infecting agent with adenovirus. These findings underscore the importance of considering HAdV-MP co-infections in the initial empirical treatment strategy for pediatric severe community-acquired pneumonia, aiding in the prognosis of HAdV-infected patients. Previous investigations of Chinese children with LRTIs have noted HAdV-MP co-infection rates ranging from 10% to 16% among all children infected with adenovirus (Lu et al., 2013; Chen et al., 2016). A surge to 58% in co-infection rates has been documented in a recent study by Chen Q et al (Chen et al., 2024). The elevated detection rates of HAdV-7-MP co-infections could stem from the selection of more severe cases, an outbreak of MP and HAdV-7 during the study period enhanced sensitivity of molecular assays (Yang et al., 2021; Cheng et al., 2022), and the prolonged presence of MP nucleic acid in the airway (Liu et al., 2019). These findings in current study align with recent literatures that suggests HAdV-MP co-infections significantly impact the clinical severity and outcomes of patients with LRTIs. Individuals with concurrent HAdV and MP infections exhibit prolonged febrile responses and more acute illness than those afflicted by MP alone (Gao et al., 2020; Zhou et al., 2020; Zhang et al., 2021; Zhou et al., 2022; Chen et al., 2024). Co-infection with MP typically results in prolonged fever, indicating that co-infections may delay pathogen elimination and intensify the host’s immune response due to increased internal and external pyrogens (Gao et al., 2020). Co-infected cases of HAdV-MP exhibited more frequent occurrences of lung consolidation, atelectasis, pleural effusion, and multiple lung lobe lesions, indicating that co-infections can exacerbate pulmonary inflammation in children with adenovirus pneumonia through direct damage or indirect immune responses (Wei et al., 2022). HAdV-7 may prolong pathogen clearance duration and exacerbate the host’s immune response, resulting in prolonged inflammation (Chen et al., 2021). Although the precise mechanisms remain unclear, a potential synergistic interaction between MP and other pathogens is suggested (Cimolai et al., 1995). A study analyzing lung microbiota in BALF revealed that HAdV-MP co-infections increase the diversity within groups while maintaining similar species richness, offering insights into potential mechanisms (Zhou et al., 2022). Future research investigating the mechanisms underlying these observations and their implications for treatment strategies is essential. Addressing the challenges posed by co-infections necessitates a multifaceted approach that combines robust diagnostic strategies, comprehensive treatment regimens, and preventive measures to mitigate the burden of HAdV-associated respiratory diseases in children.
This study highlights the differential impact of HAdV-7 and HAdV-3 infections on the immune system’s cellular components in pediatric patients with LRTIs. Comparative analysis between HAdV-7 and HAdV-3 infected patients revealed a notable, albeit not statistically significant, decrease in total lymphocyte counts in the peripheral blood of HAdV-7 infections. The observed reduction in T cell frequencies in the bloodstream may stem from the demise of T cells engaged in combating HAdV-7 or from virus-induced inhibition of T cell production. This observation supports the hypothesis that viral mechanisms, such as direct lymphocyte destruction, impaired proliferation, or migration to infection sites, contribute to the observed lymphocytopenia (Sun et al., 2023). Regarding T cell subsets, current findings indicate a significant decrease in both CD3+ T cells and CD8+ cytotoxic T cells, as well as marginal decrease in CD4+ helper T cells among HAdV-7 infected patients compared to those with HAdV-3 infections. This disparity suggests a pronounced impact of HAdV-7 on the T cell compartment, potentially leading to an imbalance in T cell subsets and ensuing immune dysregulation. This might reflect a distinct immune response strategy against different adenovirus serotypes and suggest that the primary distinctions in immune response are confined to aspects of cellular immunity. These findings are supported by a broader understanding of adenovirus-specific T cell responses in humans, as detailed by Hutnick NA et al (Hutnick et al., 2010). Adenovirus-specific T cells, both CD4+ and CD8+, exhibit a wide range of functionality and can respond to various adenovirus serotypes due to cross-reactivity against conserved hexon regions. Sun J et al. (Sun et al., 2023) reported a significant decline in the percentages of CD3+ T cells and NK cells in patients with HAdV-7 infection, categorized into upper respiratory infection, common pneumonia, and severe pneumonia groups, with the decline being more pronounced in NK cells and CD4+ T cells, but not in CD8+ T cells. Chen WW et al. (Chen et al., 2014) found that patients with severe HAdV-55 infections exhibited significantly elevated levels of IL-17+CD4+ cells and reduced levels of IL-17+CD8+ cells compared to those with asymptomatic HAdV-55 infections. The notable reduction in T cell subsets, accompanied by increased NK cell percentages in HAdV-7 infections, may have implications for understanding the pathogenesis of these infections and developing targeted therapeutic strategies. Future research should focus on further elucidating the mechanisms behind these observations, potentially exploring the role of adenovirus-specific T cell cross-reactivity in shaping the immune response to different adenovirus infections.
To better understanding the molecular and immunological mechanisms underlying the differences between HAdV-7 and HAdV-3, the following research directions are proposed. First,
further investigation into the viral factors and host cell receptors involved in the differential immune response to these two adenoviruses could provide valuable insights. A comparative study of viral proteins and their interactions with immune cells may reveal key determinants of immune modulation. Second, a comprehensive exploration of the cytokine and chemokine profiles associated with HAdV-7 and HAdV-3 infections is essential, particularly in relation to their roles in driving inflammatory responses and exacerbating disease severity. Such studies will be crucial for elucidating the divergent clinical outcomes. Additionally, leveraging genomic and proteomic approaches to identify host genetic factors and protein interactions that contribute to the severity of HAdV-7 infections will be important. Finally, the development and use of animal models to investigate HAdV-7 and HAdV-3 infections will enable a more detailed examination of in vivo immune responses and the pathophysiological mechanisms contributing to the observed differences.
This study has several limitations. First, its retrospective nature. Second, the relatively small sample size may not fully represent the broader population affected by HAdV-3 and HAdV-7 associated LRTIs. Third, the inability to detect viral load and the presence of HAdV in blood samples limits understanding of the virus’s role in clinical pathogenicity. Particularly in cases of co-infection with other respiratory viruses, the precise contribution of HAdV to disease severity remains unclear. These limitations indicate the need for multicenter, longitudinal studies with larger and more diverse populations to confirm and expand upon these findings.
In conclusion, hospitalized children with HAdV-7-associated LRTIs exhibit greater severity, multiple infections, higher incidence of co-infections with MP, and significant potential for greater cellular immune dysregulation compared to those with HAdV-3 infection, indicating a more severe clinical course and distinct pathogenic profiles. These findings underscore the importance of continuous targeted surveillance, accurate diagnosis, innovative therapeutic interventions, and tailored clinical management strategies to mitigate the impact of these infections on pediatric populations, highlighting the potential for vaccine development to combat the significant health burden posed by HAdV infections. Future research should focus on elucidating the molecular mechanisms underlying the pathogenicity differences between HAdV serotypes and exploring targeted therapeutic interventions to enhance patient outcomes.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
This study was conducted in accordance with the ethical standards outlined by the Capital Institute of Pediatrics. All procedures involving human subjects were approved by the Institutional Review Board of the Capital Institute of Pediatrics (approval number: SHERLL2023076). Patient confidentiality was rigorously protected, and all data were handled anonymously. The studies were conducted in accordance with the local legislation and institutional requirements. Due to the study’s retrospective nature, informed consent was waived as no personally identifiable information was utilized. Patient confidentiality was strictly maintained throughout the research process, and all data were anonymized to ensure privacy. The study adhered to the principles outlined in the Declaration of Helsinki regarding research involving human subjects.
XM: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Resources, Writing – original draft. YTW: Data curation, Writing – review & editing. RD: Methodology, Writing – review & editing. HY: Funding acquisition, Methodology, Writing – review & editing. FH: Methodology, Writing – review & editing. YW: Methodology, Writing – review & editing. WW: Methodology, Writing – review & editing. CY: Methodology, Writing – review & editing. QS: Methodology, Writing – review & editing. CG: Data curation, Writing – review & editing. LW: Data curation, Writing – review & editing. LZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing. LC: Conceptualization, Methodology, Supervision, Writing – review & editing. CZ: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Beijing Municipal Administration of Hospitals Incubating Program (Grant No. PX20241306), Beijing Hospitals Authority Youth Programme (QML20191304), Research Foundation of Capital Institute of Pediatrics (Grant No. LCYJ-2023-06), the Foundation of Beijing High-level International Talent Returning to China in 2022 (Beijing), Pathogen spectrum and host marker analysis in respiratory tract infection of children (Grant No. 2024-0040), Beijing Research Ward Excellence Program (Grant No. BRWEP2024W102100101), Beijing Finance Bureau (Grant No. CIP2024-0040) and the National Key R&D Program of China (Grant No. 2023YFC2308200).
We gratefully acknowledge to Peipei Fu, Juan Zhao, Lei Chen and Wei Wang for providing support for the data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1482787/full#supplementary-material
BALF, Bronchoalveolar lavage fluid; HAdV, Human adenovirus; HAdV-3, Human adenovirus serotype 3; HAdV-7, Human adenovirus serotype 7; LRTIs, Lower respiratory tract infections; MP, Mycoplasma pneumoniae.
Bradley, J. S., Byington, C. L., Shah, S. S., Alverson, B., Carter, E. R., Harrison, C., et al. (2011). The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 53, e25–e76. doi: 10.1093/cid/cir531
Cai, S., Zhu, C. H., Chen, F. G., Liu, F., Gao, M. L., Xiong, Y. (2023). Establishment of a risk model for severe adenovirus pneumonia and prospective study of the timing of intravenous immunoglobulin therapy in children. Zhongguo Dang Dai Er Ke Za Zhi 25, 619–625. doi: 10.7499/j.issn.1008-8830.2211069
Chen, Q., Lin, L., Zhang, N., Yang, Y. (2024). Adenovirus and Mycoplasma pneumoniae co-infection as a risk factor for severe community-acquired pneumonia in children. Front. Pediatr. 12. doi: 10.3389/fped.2024.1337786
Chen, Q., Liu, J., Liang, W., Chen, Y., Dou, M., Liu, Z., et al. (2021). Clinical features, replication competence, and innate immune responses of human adenovirus type 7 infection. J. Infect. Dis. 223, 1390–1399. doi: 10.1093/infdis/jiaa524
Chen, W. W., Nie, W. M., Xu, W., Xie, Y. X., Tu, B., Zhao, P., et al. (2014). Cross-sectional study of the relationship of peripheral blood cell profiles with severity of infection by adenovirus type 55. BMC Infect. Dis. 14, 147. doi: 10.1186/1471-2334-14-147
Chen, Y., Lin, T., Wang, C. B., Liang, W. L., Lian, G. W., Zanin, M., et al. (2022). Human adenovirus (HAdV) infection in children with acute respiratory tract infections in Guangzhou, China 2010-2021: a molecular epidemiology study. World J. Pediatr. 18, 545–552. doi: 10.1007/s12519-022-00590-w
Cheng, Y., Cheng, Y., Dai, S., Hou, D., Ge, M., Zhang, Y., et al. (2022). The prevalence of mycoplasma pneumoniae among children in beijing before and during the COVID-19 pandemic. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.854505
Chen, Y., Liu, F., Wang, C., Zhao, M., Deng, L., Zhong, J., et al. (2016). Molecular identification and epidemiological features of human adenoviruses associated with acute respiratory infections in hospitalized children in southern China 2012-2013. PLoS One 11, e0155412. doi: 10.1371/journal.pone.0155412
Cherian, T., Mulholland, E. K., Carlin, J. B., Ostensen, H., Amin, R., de Campo, M., et al. (2005). Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ 83, 353–359.
Cimolai, N., Wensley, D., Seear, M., Thomas, E. T. (1995). Mycoplasma pneumoniae as a cofactor in severe respiratory infections. Clin. Infect. Dis. 21, 1182–1185. doi: 10.1093/clinids/21.5.1182
Dai, H., Xi, H., Huang, L., Yuan, Z., Liao, Y., Tang, Y., et al. (2020). Molecular epidemiology and clinical features analysis of respiratory adenovirus infections reveals correlations between genotype, inflammatory biomarkers, and disease severity. BioMed. Res. Int., 4357910. doi: 10.1155/2020/4357910
Fu, Y., Tang, Z., Ye, Z., Mo, S., Tian, X., Ni, K., et al. (2019). Human adenovirus type 7 infection causes a more severe disease than type 3. BMC Infect. Dis. 19, 36. doi: 10.1186/s12879-018-3651-2
Gao, J., Xu, L., Xu, B., Xie, Z., Shen, K. (2020). Human adenovirus Coinfection aggravates the severity of Mycoplasma pneumoniae pneumonia in children. BMC Infect. Dis. 20, 420. doi: 10.1186/s12879-020-05152-x
Hutnick, N. A., Carnathan, D., Demers, K., Makedonas, G., Ertl, H. C., Betts, M. R. (2010). Adenovirus-specific human T cells are pervasive, polyfunctional, and cross-reactive. Vaccine 28, 1932–1941. doi: 10.1016/j.vaccine.2009.10.091
Jain, S., Williams, D. J., Arnold, S. R., Ampofo, K., Bramley, A. M., Reed, C., et al. (2015). Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl. J. Med. 372, 835–845. doi: 10.1056/NEJMoa1405870
Kumar, S., Stecher, G., Li, M., Knyaz, C., Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35, 1547–1549. doi: 10.1093/molbev/msy096
Li, L., Woo, Y. Y., de Bruyne, J. A., Nathan, A. M., Kee, S. Y., Chan, Y. F., et al. (2018). Epidemiology, clinical presentation and respiratory sequelae of adenovirus pneumonia in children in Kuala Lumpur, Malaysia. PLoS One 13, e0205795. doi: 10.1371/journal.pone.0205795
Li, Z. J., Zhang, H. Y., Ren, L. L., Lu, Q. B., Ren, X., Zhang, C. H., et al. (2021). Etiological and epidemiological features of acute respiratory infections in China. Nat. Commun. 12, 5026. doi: 10.1038/s41467-021-25120-6
Lin, M. R., Yang, S. L., Gong, Y. N., Kuo, C. C., Chiu, C. H., Chen, C. J., et al. (2017). Clinical and molecular features of adenovirus type 2, 3, and 7 infections in children in an outbreak in Taiwa. Clin. Microbiol. Infect. 23, 110–116. doi: 10.1016/j.cmi.2016.11.004
Liu, J., Zhao, F., Lu, J., Xu, H., Liu, H., Tang, X., et al. (2019). High Mycoplasma pneumoniae loads and persistent long-term Mycoplasma pneumoniae DNA in lower airway associated with severity of pediatric Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. 19, 1045. doi: 10.1186/s12879-019-4667-y
Liu, S., Cai, J., Li, Y., Ying, L., Li, H., Zhu, A., et al. (2023a). Outbreak of acute respiratory disease caused by human adenovirus type 7 and human coronavirus-229E in Zhejiang Province, China. J. Med. Virol. 95, e28101. doi: 10.1002/jmv.28101
Liu, W., Qiu, S., Zhang, L., Wu, H., Tian, X., Li, X., et al. (2022). Analysis of severe human adenovirus infection outbreak in Guangdong Province, southern China in 2019. Virol. Sin. 37, 331–340. doi: 10.1016/j.virs.2022.01.010
Liu, Y. N., Zhang, Y. F., Xu, Q., Qiu, Y., Lu, Q. B., Wang, T., et al. (2023b). Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: a national surveillance study. Lancet Microbe 4, e330–e339. doi: 10.1016/S2666-5247(23)00031-9
Lu, M. P., Ma, L. Y., Zheng, Q., Dong, L. L., Chen, Z. M. (2013). Clinical characteristics of adenovirus associated lower respiratory tract infection in children. World J. Pediatr. 9, 346–349. doi: 10.1007/s12519-013-0431-3
Mao, N. Y., Zhu, Z., Zhang, Y., Xu, W. B. (2022). Current status of human adenovirus infection in China. World J. Pediatr. 18, 533–537. doi: 10.1007/s12519-022-00568-8
Nazir, S. A., Metcalf, J. P. (2005). Innate immune response to adenovirus. J. Investig. Med. 53, 292–304. doi: 10.2310/6650.2005.53605
Oliveira, F. R. C., Macias, K. M., Rolli, P. A., Colleti Junior, J., Carvalho, W. B. (2020). Management of acute respiratory distress syndrome in a child with adenovirus pneumonia: case report and literature review. Rev. Paul Pediatr. 38, e2018280. doi: 10.1590/1984-0462/2020/38/2018280
Siew, J. X., Seah, X. F. V., Chew, Y. R., Thoon, K. C., Chong, C. Y., Yung, C. F., et al. (2020). Epidemiology of adenovirus infections and outcomes of cidofovir treatment in severely ill children. Pediatr. Infect. Dis. J. 39, 907–913. doi: 10.1097/INF.0000000000002726
Sun, J., Ma, X., Zhang, M., Xie, M., Zhang, X., Han, X., et al. (2023). Comparisons of lymphocytes profiles and inflammatory cytokines levels in blood of patients with differed severity of infection by human adenovirus type 7. BMC Infect. Dis. 23, 174. doi: 10.1186/s12879-023-08132-z
Tamura, K., Nei, M., Kumar, S. (2004). Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. U.S.A. 101, 11030–11035. doi: 10.1073/pnas.0404206101
Wang, F. M., Yang, C. Y., Qian, Y., Li, F., Gu, L., Chen, D. M., et al. (2022). Clinical characteristics of human adenovirus infection in hospitalized children with acute respiratory infection in Beijing. Zhonghua Er Ke Za Zhi 60, 30–35. doi: 10.3760/cma.j.cn112140-20210809-00658
Wang, L., Hu, X., Huang, Z., Zhang, Y., Zhao, X., Liu, X., et al. (2023). Analysis of the typing of adenovirus and its clinical characteristics in children with acute respiratory tract infection. BMC Pediatr. 23, 25. doi: 10.1186/s12887-023-03840-6
Wei, J., Wu, S., Jin, X., Zhang, J., Pan, S. (2022). Association of Mycoplasma pneumoniae coinfection with adenovirus pneumonia severity in children. Allergol Immunopathol. (Madr) 50, 31–36. doi: 10.15586/aei.v50i1.476
Wu, X., Zhang, J., Lan, W., Quan, L., Ou, J., Zhao, W., et al. (2022). Molecular typing and rapid identification of human adenoviruses associated with respiratory diseases using universal PCR and sequencing primers for the three major capsid genes: penton base, hexon, and fiber. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.911694
Xie, L., Zhang, B., Xiao, N., Zhang, F., Zhao, X., Liu, Q., et al. (2019). Epidemiology of human adenovirus infection in children hospitalized with lower respiratory tract infections in Hunan, China. J. Med. Virol. 91, 392–400. doi: 10.1002/jmv.25333
Yang, C., Zhu, C., Qian, Y., Deng, J., Zhang, B., Zhu, R., et al. (2021). Application of human adenovirus genotyping by phylogenetic analysis in an outbreak to identify nosocomial infection. Virol. Sin. 36, 393–401. doi: 10.1007/s12250-020-00299-y
Zhang, R., Wang, H., Tian, S., Deng, J. (2021). Adenovirus viremia may predict adenovirus pneumonia severity in immunocompetent children. BMC Infect. Dis. 21, 213. doi: 10.1186/s12879-021-05903-4
Zhou, W., Chen, J., Xi, Z., Shi, Y., Wang, L., Lu, A. (2022). Characteristics of lung microbiota in children's refractory mycoplasma pneumoniae pneumonia coinfected with human adenovirus B. Can. J. Infect. Dis. Med. Microbiol., 7065890. doi: 10.1155/2022/7065890
Zhou, Y., Wang, J., Chen, W., Shen, N., Tao, Y., Zhao, R., et al. (2020). Impact of viral coinfection and macrolide-resistant mycoplasma infection in children with refractory Mycoplasma pneumoniae pneumonia. BMC Infect. Dis. 20, 633. doi: 10.1186/s12879-020-05356-1
Keywords: human adenovirus serotypes 3, human adenovirus serotypes 7, lower respiratory tract infections, co-infections, immune responses, children
Citation: Ma X, Wu Y, De R, Yao H, He F, Wang Y, Wang W, Yan C, Song Q, Guo C, Wen L, Zhao L, Cao L and Zhu C (2025) Impact of co-infections and immune responses on clinical severity of human adenovirus 3 and 7 infections in hospitalized children with lower respiratory tract infections: a comparative study. Front. Cell. Infect. Microbiol. 14:1482787. doi: 10.3389/fcimb.2024.1482787
Received: 18 August 2024; Accepted: 06 December 2024;
Published: 09 January 2025.
Edited by:
Srivastava Vartika, Cleveland Clinic, United StatesReviewed by:
Mingbin Liu, Nanchang University, ChinaCopyright © 2025 Ma, Wu, De, Yao, He, Wang, Wang, Yan, Song, Guo, Wen, Zhao, Cao and Zhu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunmei Zhu, emh1Y2h1bm1laTE5NzFAMTI2LmNvbQ==; Ling Cao, Y2FvbGluZzk5MTlAMTYzLmNvbQ==; Linqing Zhao, bGlucWluZ3o1MjVAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.