- 1College of Animal Science and Technology, Hunan Agricultural University, Key Laboratory for Quality Regulation of Livestock and Poultry Products of Hunan Province, Changsha, China
- 2Yuelushan Laboratory, Changsha, China
- 3Department of General Surgery, No. 924 Hospital of Joint Logistics Support Force of PLA, Guilin, China
This study investigated the effects of Enterococcus hirae (Eh) derived from Ningxiang pigs on growth performance, diarrhea incidence, and immune responses in ETEC-challenged piglets. The results showed that compared to the CON group, ETEC infection significantly reduced the average daily gain (ADG) and average daily feed intake (ADFI), increased rectal temperature, and resulted in a diarrhea rate of up to 24%. Additionally, ETEC infection significantly increased the spleen index and the expression of inflammatory cytokines in the spleen, serum and intestine, with decreasing serum sIgA and colonic SCFAs of piglets. Compared to the ETEC group, orally Eh significantly increased ADFI in ETEC-infected piglets, reduced the diarrhea rate to 11.53%, reduced the spleen index and the expression of inflammatory cytokines in the spleen, serum and intestine, with decreasing serum sIgA and colonic SCFAs of ETEC-infected piglets. Furthermore, correlation analysis revealed that the levels of SCFAs (particularly acetate) were significantly negatively correlated with the expression levels of inflammatory cytokines in colonic and splenic tissues, suggesting that acetate may be a key metabolite in the anti-inflammatory effects of Eh. These results indicate that Eh can enhance the protection of piglets against ETEC K88 via intestine-acetate-spleen axis, thereby alleviating diarrhea and improving growth performance in piglets.
1 Introduction
ETEC infections, particularly those caused by the K88 serotype, are a leading cause of diarrheal diseases in animals, leading to severe intestinal infections and related illnesses (Vidal et al., 2016; Sun and Kim, 2017; Yue et al., 2020; Zhang et al., 2022a). The intestine is not only crucial for nutrient digestion and absorption but also serves as the largest immune organ. ETEC K88 infection disrupts this vital system by inducing intestinal inflammation and compromising the intestinal barrier through bacterial attachment and enterotoxin secretion, which can trigger a systemic inflammatory response in the host (Zhang et al., 2022a). Extensive evidence indicates that ETEC K88 infection adversely affects growth and increases mortality in livestock, causing significant economic losses to the livestock industry (Augustino et al., 2022; Ntakiyisumba et al., 2022). Historically, antibiotics have been extensively used as growth promoters and antimicrobial agents in animals (Rahman et al., 2022). However, the rise of antibiotic-resistant bacteria and the banning of antibiotics in feed have shifted research focus towards finding viable alternatives to antibiotics to combat the challenges posed by ETEC K88 infections.
Lactic acid bacteria hold promise as potential alternatives to antibiotics due to their anti-inflammatory and antimicrobial properties and their ability to modulate the intestinal microbiota (Ayivi et al., 2020; Abedin et al., 2023). Zeyner et al. found that administering E. faecalis daily from birth to weaning reduced diarrhea rates (Zeyner and Boldt, 2006). Strompfova et al. reported that administering E. faecalis EK13 to neonatal piglets significantly reduced fecal staphylococcus counts on day 1. After 7 days of feeding, E. faecalis EK13 significantly reduced fecal E. coli counts (Strompfová et al., 2006). Additionally, E. faecium T-013 and other probiotic composites have been shown to improve the intestinal environment of pigs, reduce NH3 emissions, enhance the breeding environment, and boost the immune system (Franz et al., 2003).
As a novel strain of lactic acid bacteria, Enterococcus hirae (Eh) has been recognized as a potential probiotic in human diseases and food (Goubet et al., 2021; Li et al., 2024). Our previous study found that Eh HNAU0516, isolated from Ningxiang pigs with high resistance, could promote the intestinal development of weaned piglets, enhance intestinal barrier function, and improve gut microbiota (Zhang et al., 2024). Furthermore, it significantly inhibited E. coli activity in an in vitro bacteriostatic assay, suggesting its potential as an antibiotic alternative. Therefore, the present study utilized an ETEC K88-infected piglet model to validate the hypothesis that Ningxiang pig-derived Eh HNAU0516 could attenuate ETEC K88-induced inflammatory responses and intestinal health dysfunction.
2 Materials and methods
All experiments were conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee of Hunan Agricultural University.
2.1 Bacterial strain
The Eh HNAU0516 strain used in this study was initially isolated from feces of healthy Ningxiang piglets and conserved in the China Center for Type Culture Collection (CCTCC NO.M20221530, Wuhan, Hubei, China). Eh HNAU0516 was cultured in MRS medium (No. HB0384-1, Qingdao) for 12 h at 37°C. The counts of viable probiotic bacteria in the Eh -containing supplements were verified via a cultural method using a MRS agar medium (No. HB0384, Qingdao). ETEC k88 was kindly provided by Prof. Wenkai Ren, from South China Agricultural University (Guangzhou, China). And the counts of viable bacteria in the ETEC -containing supplements were verified via cultural method using a LB agar medium (No. HB0128, Qingdao) for 12 h at 37°C.
2.2 IPEC-J2 cells
The IPEC-J2 cells were maintained in DMEM/F12 medium (BI, Dibosi Biological Technology, Co., Ltd., Shanghai, China), supplemented with 100 U/mL penicillin, 100 μg/mL streptomycin, and 10% (vol/vol) heat-inactivated fetal bovine serum (FBS) (BS-1101, Inner Mongolia Opcel Biotechnology Co.,Ltd., Neimenggu, China), at 37°C and 5% CO2 in a humidified incubator. Cells (106 cells per well) were plated in 6-well plates and co-cultured with live Eh (106 CFU) for 2h and ETEC (105 CFU for 1 h).
2.3 Animals and experimental design
Twenty-two healthy Duroc × Landrace × Yorkshire (DLY) piglets, aged 24 days, were randomly divided into different group with similar body weights (BWs). From 0 to 14 d, Con group received a standard diet and daily gavage of 10 mL of sterile saline; Eh group received a standard diet and gavage of 109 CFU/mL (10 mL) Eh every day. At 15 d, all groups except the CON group received a daily gastric infusion of 109 CFU/mL (10 mL) ETEC, and the Con group received a gastric infusion of sterile saline using the same method. From 15 to 17 d, Eh+ETEC group continue received a standard diet and gavage of 109 CFU/mL (10 mL) Eh every day, while the CON group and ETEC group received a gastric infusion of sterile saline using the same method. Each consisting of 7 or 8 replicates with 1 piglet per replicate. No antibiotics were given to the animals throughout the trial for prophylactic or therapeutic reasons. All piglets had free access to feeding and drinking water. Room temperature was maintained at approximately 27-30 °C, and the humidity was controlled between 50%-60%. Piglets were sacrificed by exsanguination after electrical stunning, and serum, intestine and fecal samples were collected for further analysis. The experimental design diagram is shown in Figure 1. The basic formula and nutritional level are presented in Supplementary Table S1.
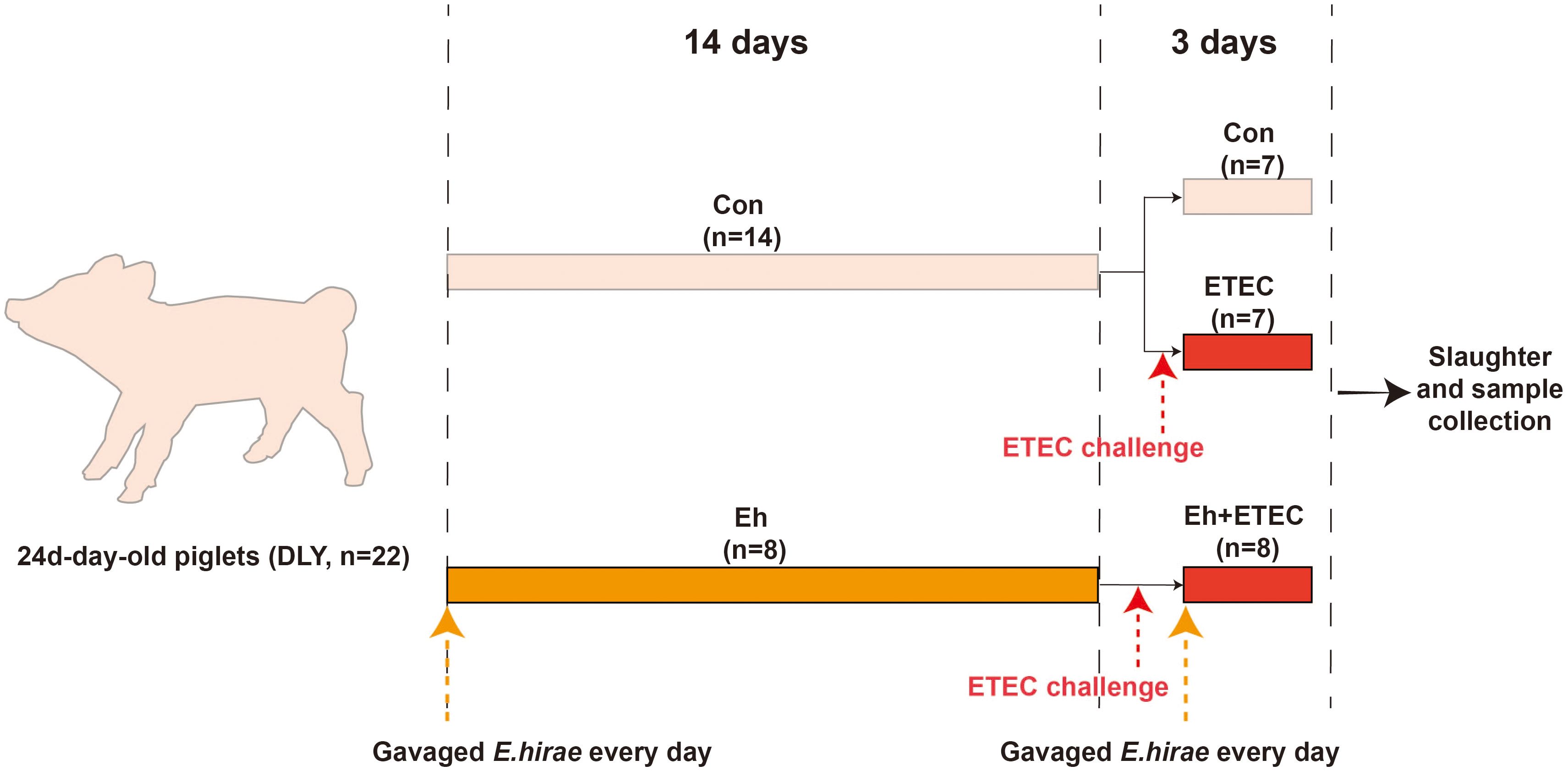
Figure 1. Experimental design diagram. From 0 to 14 d, CON group received a standard diet and daily gavage of 10 mL of sterile saline; Eh group received a standard diet and gavage of 109 CFU/mL (10 mL) Enterococcus hirae (E.hirae) every day. At 15 d, all groups except the CON group received a daily gastric infusion of 109 CFU/mL (10 mL) ETEC, and the CON group received a gastric infusion of sterile saline using the same method. From 15 to 17 d, Eh+ETEC group continue received a standard diet and gavage of 109 CFU/mL (10 mL) E.hirae every day, while the CON group and ETEC group received a gastric infusion of sterile saline using the same method.
2.4 Diarrhea rate, and relative organ weight
Visceral organs (liver and spleen) were removed and weighed. The relative weight of each organ = organ weight/final live body weight. Additionally, the fecal score (0, normal; 1, mild diarrhea; 2, medium diarrhea; 3, serious diarrhea) of each piglet was recorded daily and their diarrhea rate were also be calculated. Diarrhea rate was calculated as a percentage of the number of diarrheal piglets during the period divided by the total number of piglets (day × piglets).
2.5 Histopathology and mucus staining
For histologic analysis, the duodenum, jejunum and colon (mid-section) tissues were fixed in 10% formalin for 24 hours at room temperature, embedded in paraffin, and sectioned at a thickness of 3 mm for staining. As previously reported (Zhang et al., 2021), 10-μm-thick sections were stained with hematoxylin and eosin and analyzed under a microscope (BX53, Olympus, Tokyo, Japan). Histological analysis was performed using ImageJ software. Additionally, AB-PAS staining was performed to verify the function of mucous-secreting goblet cells. Sections (10-μm) from the tissue were stained with AB (Abcam, Cambridge, UK) and PAS (Millipore Sigma, Billerica, MA, USA) staining kits according to the manufacturer’s protocol. It is also visualized using an Olympus microscope (BX53, Olympus, Tokyo, Japan).
2.6 Total RNA extraction and real-time reverse transcription PCR
Total RNA was prepared using the RNeasy Kit (SENO Biotech Co., Ltd., Zhangjiakou, China). cDNA was synthesized from total RNA using the SuperScript IV First Strand Synthesis System (AG11711, Accurate Biotechnology Co., Ltd, Changsha, China) according to the manufacturer’s instructions. A 7500 Fast Real-Time PCR system (Applied Biosystems, Foster City, CA, USA) was used for quantitative PCR analysis. The primers used are listed in Supplementary Table S2.
2.7 Serum inflammatory biomarkers
Serums were centrifuged for 90 s at 15,000 g and serum aliquots were snap-frozen until further analysis. Quantification of cytokine levels in serum was assessed using a commercially available porcine cytokine multiplex immunoassay kit (RayBiotech, Norcross, GA). And the concentration of cytokines was calculated by QAP-CYT-1 software. Additionally, the sIgA concentrations were determined by ELISA assay (Ruixinbio Quanzhou, China). Briefly, serum was prepared according to the standard procedures. The enzyme-linked reaction was carried out according to the commercial kit, and the absorbance value at 450 nm was finally determined. Concentrations of sIgA were calculated from the standard curve.
2.8 SCFAs analysis
The concentrations of SCFAs including acetate, propionate, butyrate and valerate were analyzed using the gas chromatographic method. Briefly, as previously reported (Zhang et al., 2024), 1.0 g of colonic chyme was first fully homogenized with 1.5 mL of deionized water. The above fecal homogenate was centrifuged at 15 000 × g for 10 min at 4°C. The samples were acidified with 25% metaphosphoric acid at a ratio of 1:5 for 30 min on ice. Samples were injected into a gas chromatographic 8890 series gas chromatograph (Agilent, USA) for detection.
2.9 Statistical analysis
The data were analyzed using SPSS 26.0 statistical software (ver. 26.0 for Windows, SPSS Inc., Chicago, IL, USA). The diarrhea rates of piglets were compared with use of chi-square analysis. For the continuous variables with a normal distribution, one-way ANOVA was used to analyze the difference among groups, and the homogeneity of variance among groups was further tested using LSD multiple-range tests. And correlation test between the inflammatory biomarkers and SCFAs was explored by R package. And P-value < 0.05 was considered statistically significant, 0.05 ≤ P-value < 0.1 was considered statistically trend. All data were expressed as means with their standard errors.
3 Results
3.1 Growth performance
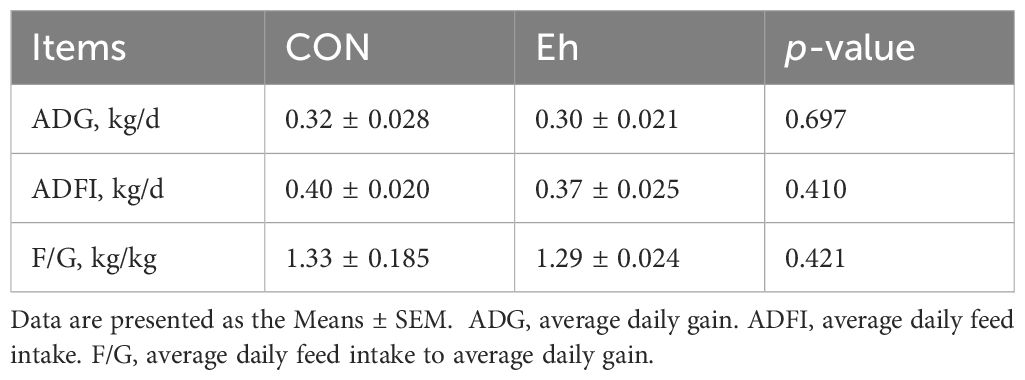
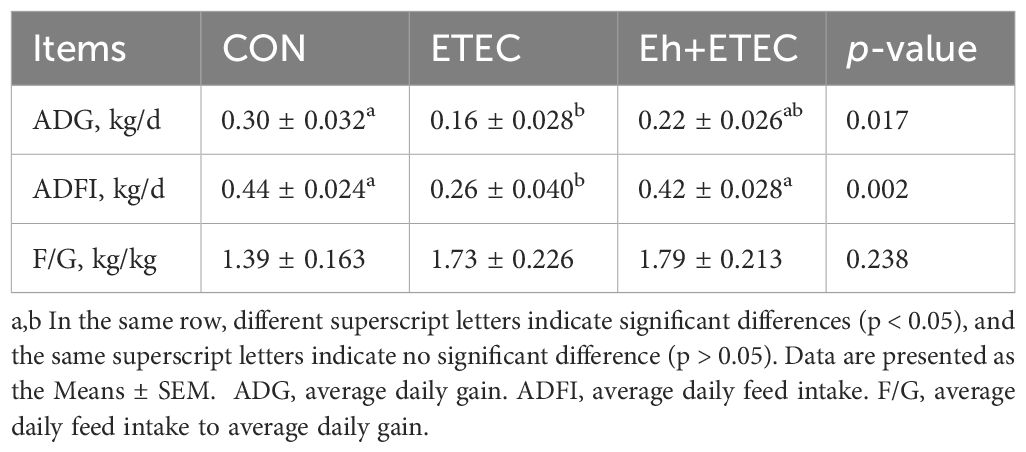
As showed in Table 1, there were no significant differences in the average daily gain (ADG), average daily feed intake (ADFI) or the ratio of feed to gain (F/G) among two group from days 1 to 14 of the experimental period. During days 15-17, ETEC challenge significantly decreased ADG and ADFI compared with CON group. Furthermore, the ADFI was numerically higher in Eh+ETEC group than in the ETEC group, with the ADG was no significant differences in two groups (Table 2). Additionally, there were no significant differences in the F/G among three groups (Table 2).
3.2 Diarrhea rate and rectal temperature
During days 1-14, there was no difference in the diarrhea rate between the Eh and CON groups (7.14% vs. 3.57%) (Supplementary Figure 1A). During days 15-17, although there was no significant difference in diarrhea rates, the ETEC group had the highest rate (CON vs. ETEC vs. Eh+ETEC: 8.00% vs. 24.00% vs. 11.53%) (Supplementary Figure 1B). Meanwhile, the rectal temperature of piglets was significantly increased after the ETEC challenge (Supplementary Figure 1C).
3.3 Viscera index and spleen inflammatory expression
Eh significantly reduced the spleen index in ETEC-challenged piglets, while there was no difference in the liver index (Figure 2A). Compared to the CON group, ETEC challenges significantly increased the levels of TNF-α, IL-6, and IL-8 in the spleen (Figures 2B, D, E). Oral administration of Eh significantly decreased the level of IL-8 in ETEC-challenged piglets (Figure 2E), while there was no difference in TNF-α and IL-6 levels (Figures 2B, D). Additionally, there was no difference in the levels of IL-1β and IL-10 in the spleen among the three groups (Figures 2C, F).
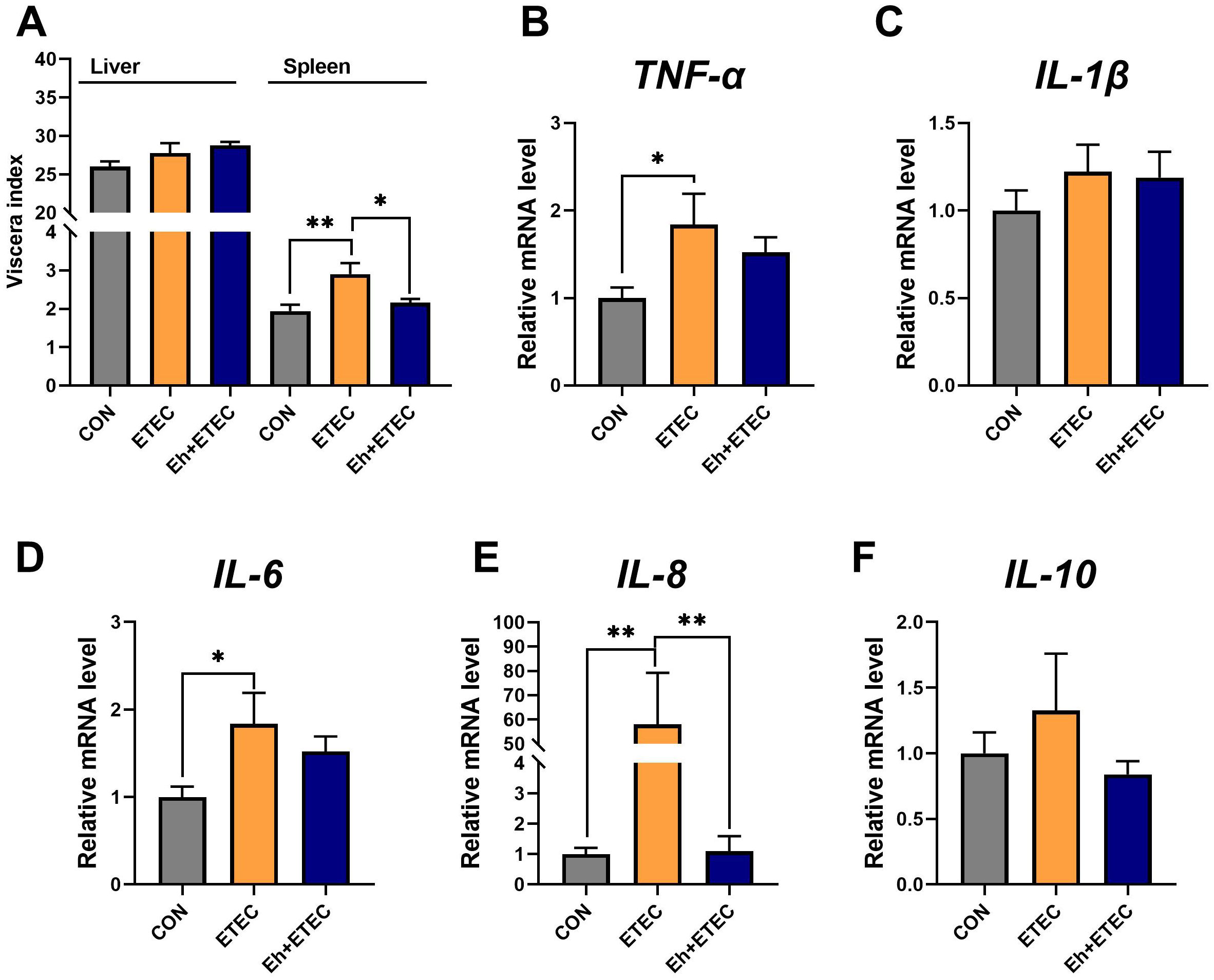
Figure 2. Effect of E.hirae on spleen inflammatory expression in ETEC-challenge piglets. (A) The viscera index of piglets, including liver and spleen. (B–F) The effects of Enterococcus hirae in spleen TNF-α (B), IL-1β (C), IL-6 (D), IL-8 (E) and IL-10 (F). Mean ± SEM are shown. * 0.01 ≤ p ≤ 0.05; **0.001 < p ≤ 0.01. CON group, ETEC group, n=7; Eh+ETEC, n=8.
3.4 Serum inflammatory expression
Additionally, ETEC challenges significantly decreased the levels of serum sIgA, and oral administration of Eh reversed this effect (Figure 3A). Compared with the CON group, ETEC challenges significantly increased the levels of TNF-α, IL-1β, and IL-6 in serum (Figures 3B–D). Oral administration of Eh significantly decreased the levels of IL-1β and IL-6 in ETEC-challenged piglets (Figures 3C, D), while there was no difference in TNF-α levels (Figure 3B). Additionally, there was no difference in the levels of IL-8 and IL-10 in serum among the three groups (Figures 3E, F).
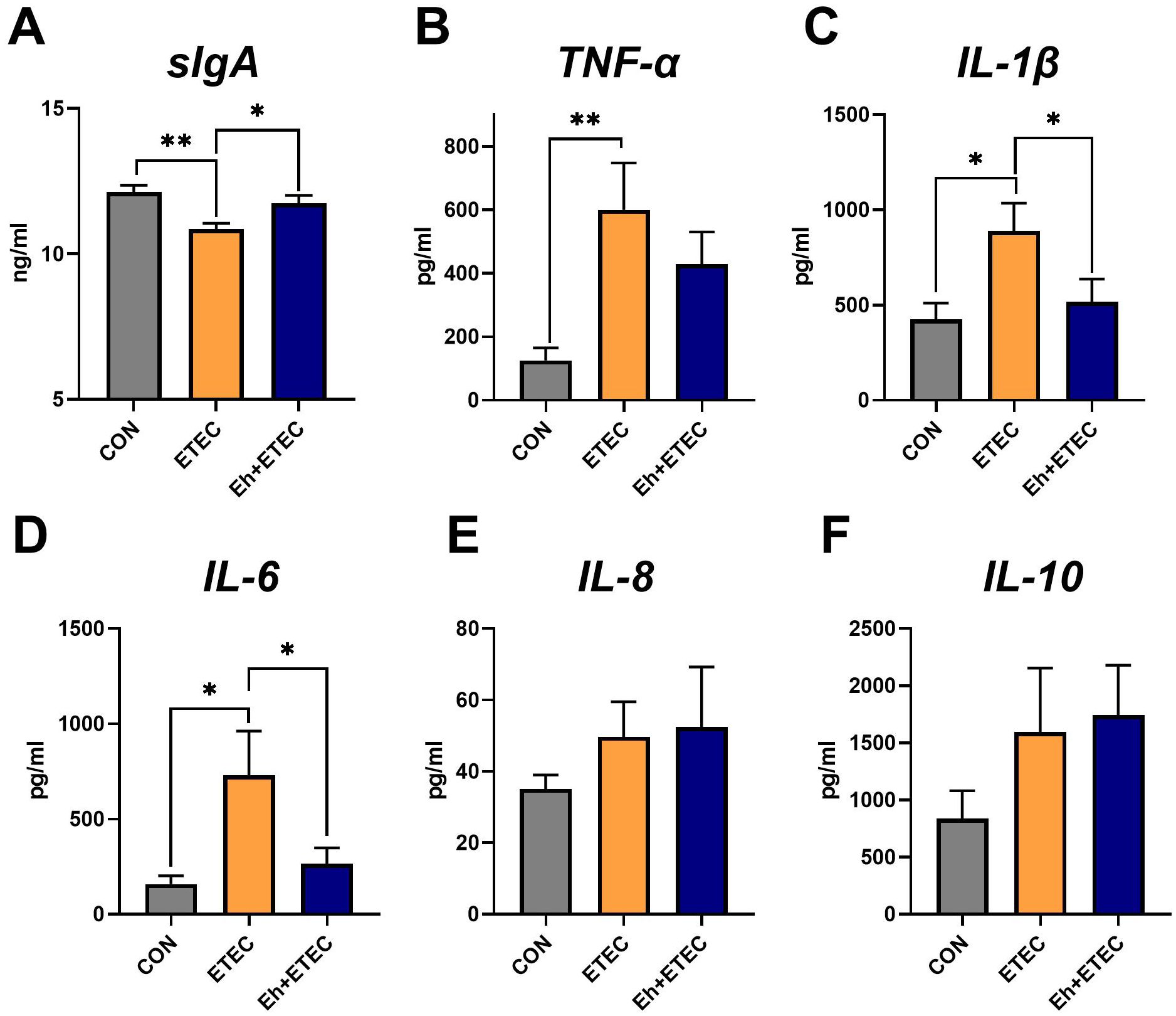
Figure 3. Effects of Enterococcus hirae on serum inflammatory expression in ETEC-challenged Piglets. (A) The sIgA levels in serum. (B–F) The effects of Enterococcus hirae in serum TNF-α (B), IL-1β (C), IL-6 (D), IL-8 (E) and IL-10 (F). Mean ± SEM are shown. * 0.01 ≤ p ≤ 0.05; **0.001 < p ≤ 0.01. CON group, ETEC group, n=7; Eh+ETEC, n=8.
3.5 Intestine morphology and intestinal inflammatory expression
As shown in Figure 4, jejunum and colon morphology were examined using H&E staining to determine the effects of Eh treatment on ETEC-challenged piglets. In the jejunum, compared with the CON group, ETEC challenges significantly decreased villi height and V/C, and increased crypt depth, whereas oral Eh increased villi height and V/C, and decreased crypt depth in ETEC-challenged piglets (Figures 4A–D). In the colon, ETEC challenges showed a trend of decreasing crypt depth, whereas oral Eh increased crypt depth in ETEC-challenged piglets (Figures 4D, E). Additionally, AB-PAS staining was used to show colonic goblet cell density. Compared with the CON group, ETEC challenges decreased the number of goblet cells, whereas oral Eh increased the number of goblet cells in ETEC-challenged piglets (Figure 4D).
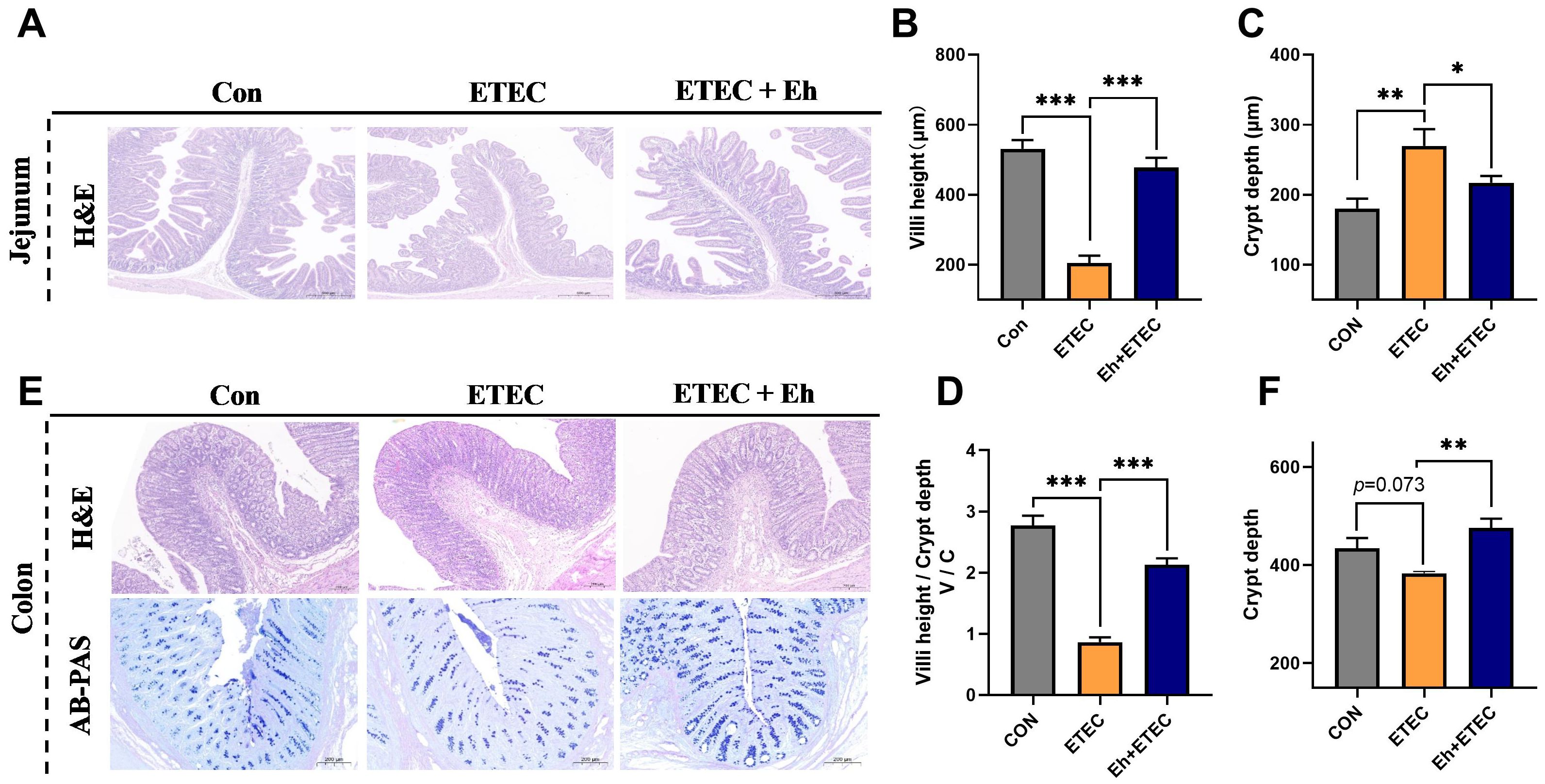
Figure 4. Effects of Enterococcus hirae on intestinal morphology in ETEC-challenged Piglets. (A) The H&E representative images of the jejunum. (B–D) The villi height (B), crypt depth (C) and villi height/crypt depth (V/C) (D) of jejunum. (E) The H&E and AB-PAS representative images of the colon. (F) The crypt depth of colon. * 0.01 ≤ p ≤ 0.05; **0.001 < p ≤ 0.01; *** p ≤ 0.001. Mean ± SEM are shown. CON group, ETEC group, n=7; Eh+ETEC, n=8.
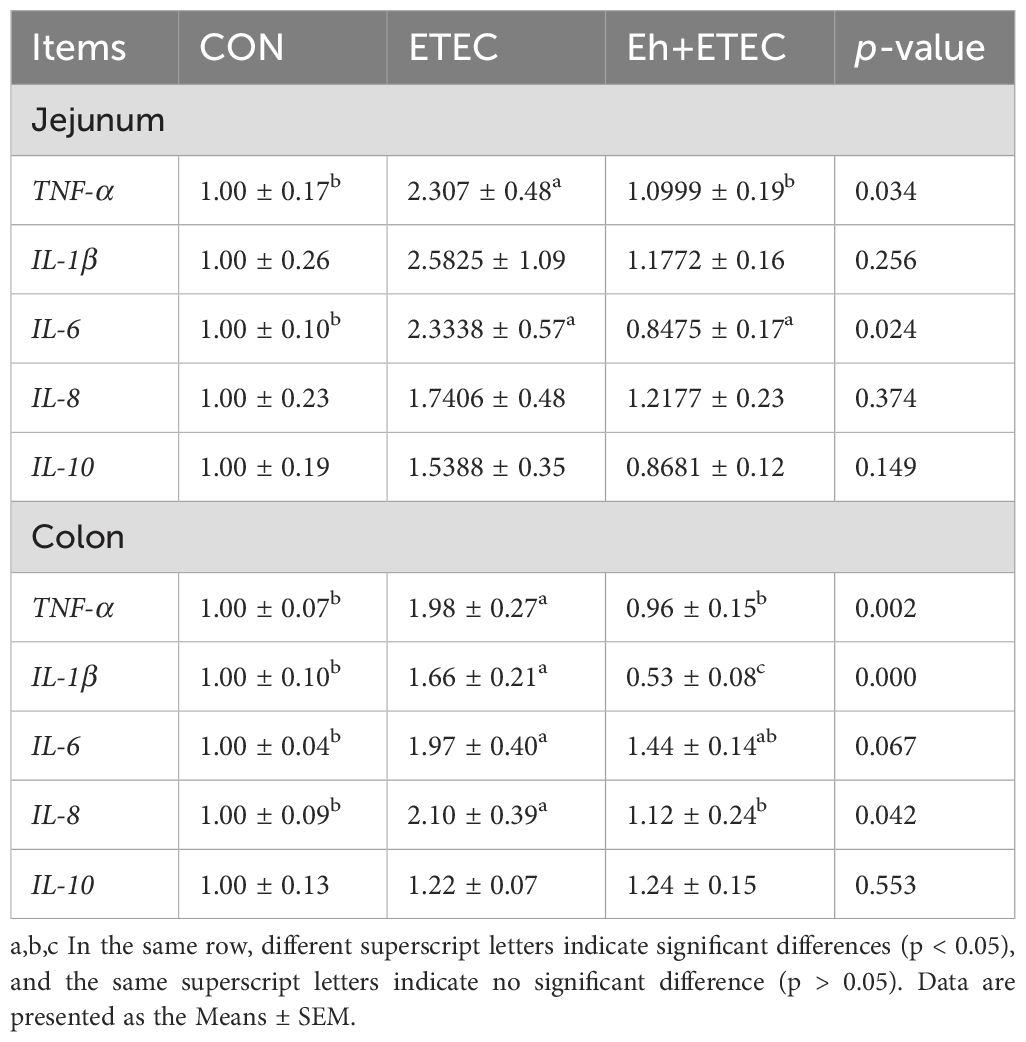
In the jejunum, compared with the CON group, ETEC challenges significantly increased the expression levels of TNF-α and IL-6, while oral administration of Eh significantly decreased these inflammatory factors (Table 3). In the colon, compared with the CON group, ETEC challenges significantly increased the expression levels of TNF-α, IL-1β, IL-6, and IL-8, while oral administration of Eh significantly decreased these inflammatory factors (Table 2). Furthermore, the IPEC-J2 cell model was used to investigate the effect of Eh on the expression of inflammation challenged by ETEC (Supplementary Figure S2A). Compared with the CON group, ETEC challenges significantly increased the expression levels of TNF-α, IL-1β, IL-6, and IL-8 and decreased the expression levels of IL-10, while administration of Eh significantly reversed these inflammatory factors (Supplementary Figure S2B).
3.6 SCFAs
As shown in Figure 5, colonic short-chain fatty acids were measured. The results showed that ETEC challenges significantly decreased the concentrations of acetate, propionate, butyrate, valerate, and total SCFAs, while oral administration of Eh significantly increased the concentrations of acetate, propionate, and total SCFAs in ETEC-challenged piglets (Figures 5A–E). The ratio of acetate, propionate, butyrate, and valerate in total SCFAs was calculated and shown in Figure 5F, suggesting that oral administration of Eh resulted in a higher proportion of acetate in ETEC-challenged piglets.
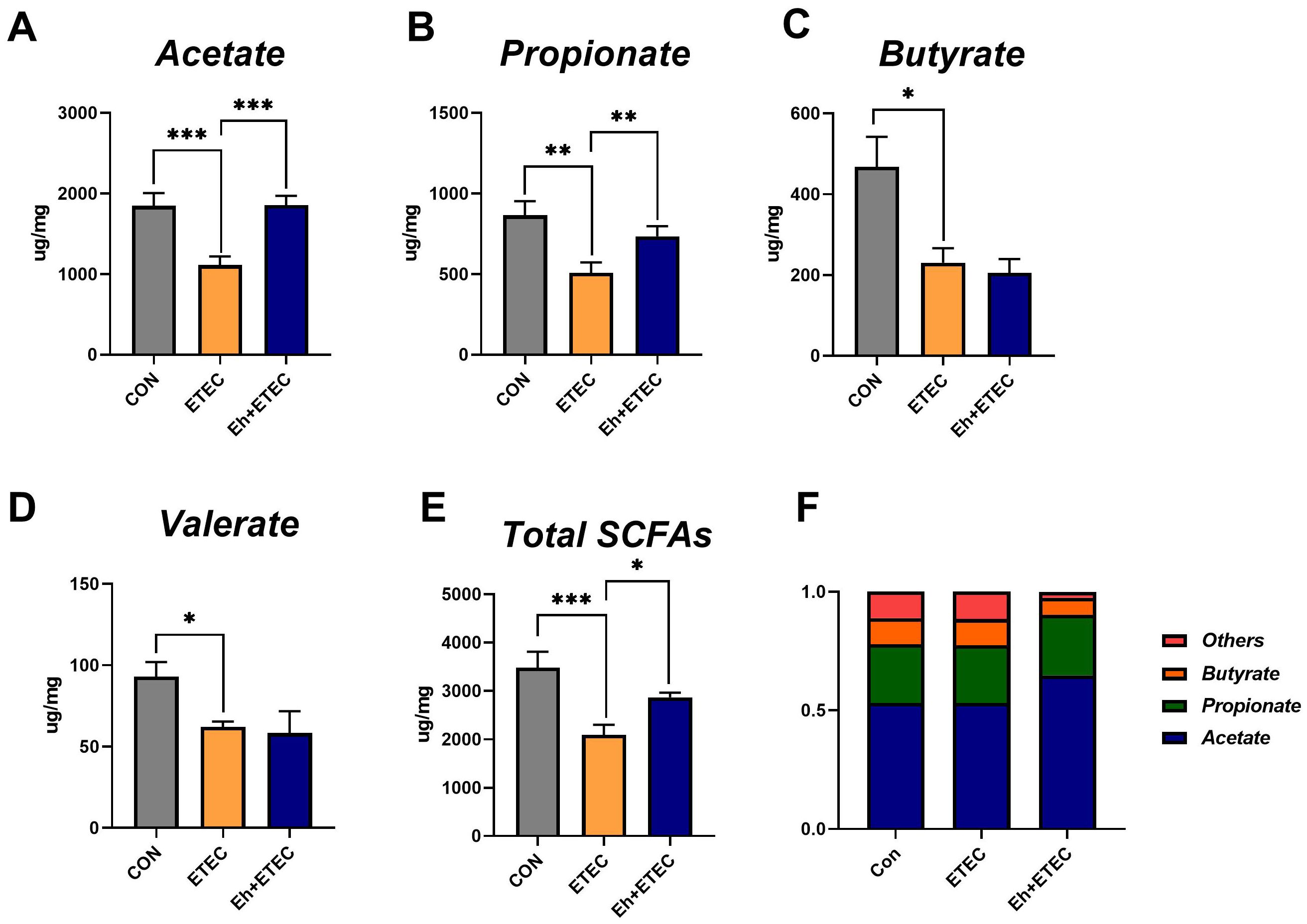
Figure 5. Effects of Enterococcus hirae on SCFAs in ETEC-challenged Piglets. (A–E) The concentration of acetate (A), propionate (B), butyrate (C), valerate (D), and total SCFAs (E) in colonic contents of piglets. (F) The relative content of each SCFA in colonic contents of piglets. The Mean ± SEM are shown. * 0.01 ≤ p ≤ 0.05; **0.001 < p ≤ 0.01; *** p ≤ 0.001. CON group, ETEC group, n=7; Eh+ETEC, n=8.
3.7 Correlation of SCFAs levels with inflammatory biomarker
A Spearman’s correlation was employed to investigate the correlation between inflammatory biomarkers and SCFAs in piglets (Figure 6). The results showed that the concentrations of acetate and total SCFAs were significantly negatively correlated with the expression of TNF-α, IL-1β, and IL-6 in the colon, and also significantly negatively correlated with the expression of IL-8 in the spleen (Figure 6). Additionally, the concentrations of propionate were significantly negatively correlated with the expression of IL-1β in the colon and IL-10 in serum, while the concentrations of valerate were significantly negatively correlated with the expression of IL-6 in the colon (Figure 6). These results suggest that acetate may be the key metabolite in the anti-inflammatory action of Eh.
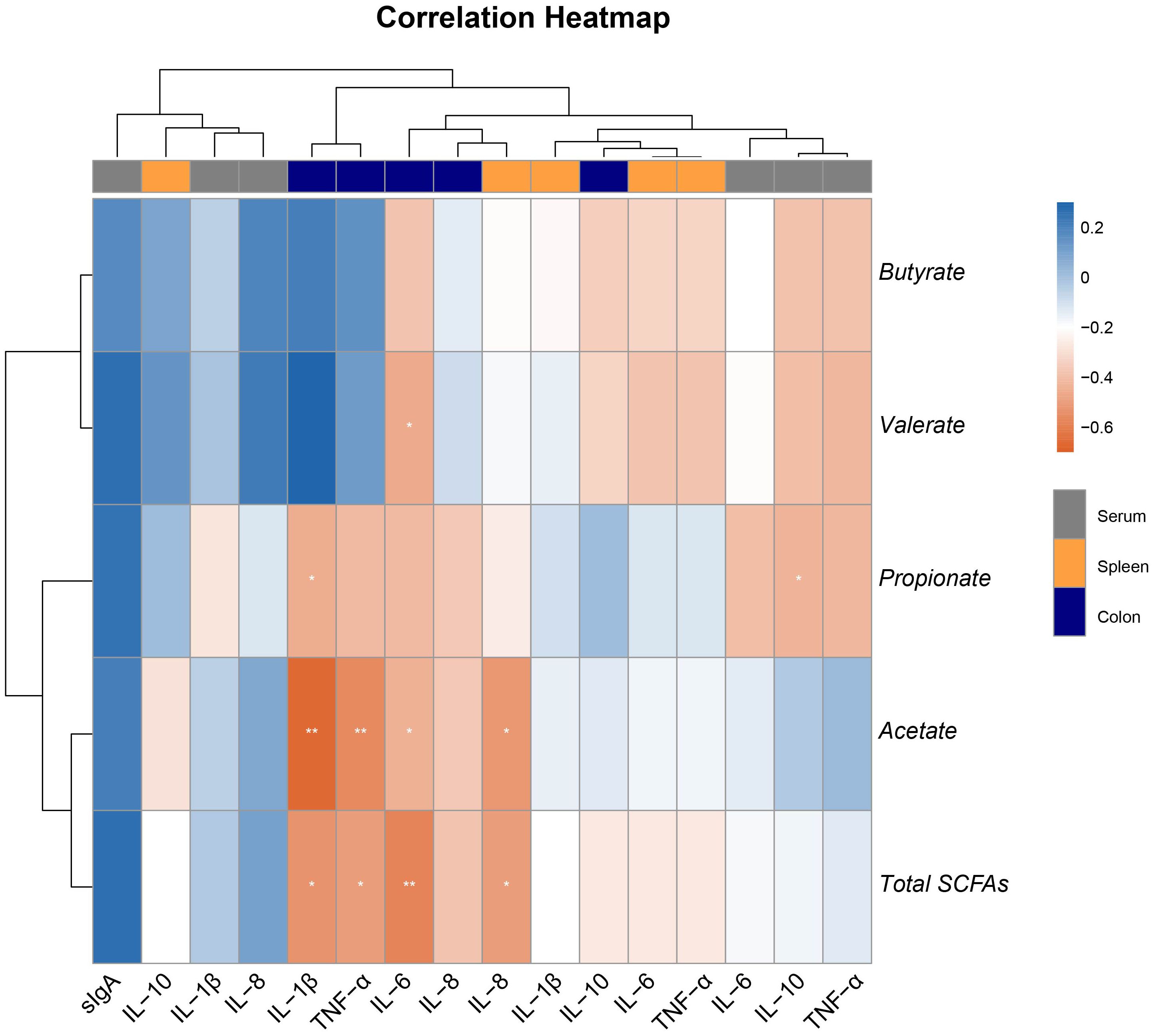
Figure 6. Heatmap of the Spearman’s r correlations between the colonic SCFAs and inflammatory biomarker in serum, spleen, and colon. Data are presented as means ± SEM. * 0.01 ≤ p ≤ 0.05; **0.001 < p ≤ 0.01 (following the correlation analysis).
4 Discussion
Due to the increase of drug-resistant bacteria and the ban on feed antibiotics, alternatives need to be found to deal with enterotoxin-producing Escherichia coli infections, represented by ETEC K88, which are the main cause of diarrheal diseases in animals, and are particularly harmful to the intestinal tract (Zeyner and Boldt, 2006; Zhang et al., 2022a). Studies have demonstrated that probiotics can promote the establishment of healthy gut microbiota in piglets, improve nutrient digestion and absorption, and enhance intestinal health (Ding et al., 2021; Ma et al., 2022). In this study, piglets infected with ETEC showed significantly reduced ADG and ADFI, with the highest diarrhea rate and rectal temperature. However, oral administration of the probiotic - Eh alleviated the high diarrhea rate and low ADFI induced by ETEC infection, indicating its effectiveness in mitigating growth performance decline and increased diarrhea caused by ETEC.
As a crucial component of the host digestive system, the intestine plays a vital role in food digestion, nutrient absorption, and metabolism (Abdallah et al., 2020; Zhang, 2022). Indicators such as villus height, crypt depth, and the V/C are important measures of digestive and absorptive capacity (Assis et al., 2021; Zhu et al., 2022). ETEC infection can damage the mucosa and the crypt-villus axis, leading to villus atrophy and increased crypt depth, thereby affecting intestinal absorption capacity (Augustino et al., 2022). In this study, ETEC infection significantly reduced jejunal villus length and V/C while increasing crypt depth. Orally Eh significantly increased jejunal villus length and V/C in ETEC-infected piglets and reduced crypt depth. Additionally, the number of goblet cells and crypt depth in the colon of ETEC-infected piglets also increased significantly after probiotic administration. These results indicate that Eh can improve the intestinal morphology of ETEC-infected piglets.
Following ETEC infection, piglets experience initial colonization of the small intestine epithelium, where the bacteria proliferate and secrete enterotoxins, disrupting the intestinal mucosa and increasing intestinal wall permeability, leading to inflammatory responses (Sun et al., 2021; Augustino et al., 2022). Levels of inflammatory cytokines are direct indicators of the host’s inflammatory response, playing a crucial role in regulating immune function and promoting rapid immune reactions following pathogenic infection (Paludan et al., 2021; Sierawska et al., 2022). Moreover, extensive research has confirmed that probiotics possess immunomodulatory effects, enhancing the host’s innate immune system to combat infections and suppress inflammation (Begum et al., 2021; Cristofori et al., 2021). In this study, ETEC infection elevated serum levels of pro-inflammatory factors (TNF-α, IL-1β, IL-6) and expression of intestinal tissue pro-inflammatory factors (TNF-α, IL-1β, IL-6, IL-8). And orally Eh reversed these effects. Using an ETEC-infected IPEC-J2 cell model, we further validated that Eh could improve intestinal inflammation levels. Additionally, we found that orally Eh significantly increased the levels of sIgA in ETEC-infected piglets. SIgA is a major antibody produced by B cells in the intestine, which plays a key role in host immunity (Li et al., 2020; Pabst and Slack, 2020). These results suggest that Eh enhances the host’s anti-inflammatory capacity to counteract immune responses induced by ETEC infection.
The spleen is the vital lymphoid organ an plays a crucial role in responding to oxidative stress and immune defense (Liu et al., 2022). It is also a central organ for nutrient metabolism and detoxification, making it highly susceptible to oxidative stress and inflammatory reactions from various environmental factors (Liu et al., 2022; Wang et al., 2023). Studies have shown that the immune-inflammatory status of the body can be reflected by the spleen index (Yan et al., 2020). In this study, ETEC infection significantly increased the spleen index of piglets and elevated the expression of pro-inflammatory factors such as TNF-α, IL-6, and IL-8. Orally Eh significantly reduced the spleen index and IL-8 expression. This further demonstrates that Eh can mitigate the host inflammatory response induced by ETEC infection, thereby reducing diarrhea.
As the major metabolites of intestinal microbiota, SCFAs provide energy to the body, regulate the intestinal microbiota, inhibit the production of harmful substances in the intestine, and suppress the release of inflammatory factors (Yao et al., 2022; Zhang et al., 2022b). Probiotics can produce large amounts of SCFAs by fermenting carbohydrates in the animal intestine, lowering intestinal pH, inhibiting pathogenic bacteria, and maintaining intestinal health. Research indicates that adding probiotic preparations during the piglet stage can significantly increase lactate and butyrate content in the colon (Li et al., 2021). In this study, Eh significantly increased the content of acetate, propionate, and total SCFAs in the colonic contents of ETEC-infected piglets. Interestingly, we also found that orally Eh in ETEC-infected piglets resulted in a higher proportion of acetate, and the content of acetate in the colon was associated with the expression of inflammatory factors in the colonic and splenic tissues of piglets. This suggests that Eh can alleviate host inflammatory responses by promoting the content of acetate and other SCFAs in the colon of ETEC-infected piglets, thereby reducing diarrhea.
In conclusion, Enterococcus hirae derived from Ningxiang pigs effectively alleviates ETEC-induced diarrhea in weaned piglets, improves their growth performance, and enhances intestinal morphology. Significantly, Eh modulates the intestine-acetate-spleen axis by its regulation of the spleen index and mitigation of inflammatory responses induced by ETEC infection through increased acetate content in colonic chyme (Figure 7). This study highlights the intestine-acetate-spleen axis as a critical mechanism and presents Eh as a viable alternative to antibiotics for addressing challenges posed by ETEC K88 infection.

Figure 7. Schematic diagram of Enterococcus hirae regulates the inflammatory function and enhances the protection of piglets against ETEC challenge via intestine-acetate-spleen axis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by The animal handling and all procedures of this study have received approval from the Animal Care and Use Ethics Committee of the Hunan Agricultural University (Ethic code: 20221027). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
LZ: Formal analysis, Methodology, Writing – original draft. ZW: Formal analysis, Methodology, Writing – review & editing. ZZ: Formal analysis, Writing – review & editing. RC: Formal analysis, Writing – review & editing. SP: Formal analysis, Writing – review & editing. JW: Funding acquisition, Project administration, Writing – review & editing. XB: Project administration, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study is funded by the National Natural Science Foundation of China (32102571), Natural Science Foundation of Hunan Province (2024JJ1004, 2022JJ20027), Science and Technology Major Project of Yunnan Province (202202AE090032).
Acknowledgments
The authors would like to express their sincere gratitude to the High quality medical specialty in the Southern Theater Command, Department of General Surgery, Joint Logistics Support Force 924th Hospital and the College of Animal Science and Technology, Hunan Agricultural University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1476564/full#supplementary-material
References
Abdallah, A., Elemba, E., Zhong, Q., Sun, Z. (2020). Gastrointestinal interaction between dietary amino acids and gut microbiota: with special emphasis on host nutrition. Curr. Protein Pept. Sci. 21, 785–798. doi: 10.2174/1389203721666200212095503
Abedin, M. M., Chourasia, R., Phukon, L. C., Sarkar, P., Ray, R. C., Singh, S. P., et al. (2023). Lactic acid bacteria in the functional food industry: Biotechnological properties and potential applications. Crit. Rev. Food Sci. Nutr. 7, 1–19. doi: 10.1080/10408398.2023.2227896
Assis, S. D., Mogyca Leandro, N. S., Arnhold, E., Café, M. B., de Carvalho, F. B., Stringhini, J. H., et al. (2021). Relative weight and length of digestive tract and intestinal histomorphometric measurements of slow-growing broilers of different genotypes. Semin. Agrar. 42, 319–334. doi: 10.5433/1679-0359.2021v42n1p319
Augustino, S. M. A., Ochi, E. B., Xu, Q., Jackson, V. M. L., Yu, Y. (2022). Enterotoxigenic E. coli (ETEC) and Porcine Epidemic Diarrhea Virus (PEDV) diarrheal infections and resistance in piglets. Arch. Environ. Sci. Environ. Toxicol. 5, 136. doi: 10.29011/2688-948X.100136
Ayivi, R. D., Gyawali, R., Krastanov, A., Aljaloud, S. O., Worku, M., Tahergorabi, R., et al. (2020). Lactic acid bacteria: food safety and human health applications. Dairy 1, 202–232. doi: 10.3390/dairy1030015
Begum, J., Buyamayum, B., Lingaraju, M. C., Dev, K., Biswas, A. (2021). Probiotics: Role in immunomodulation and consequent effects. Lett. Anim. Biol. 1, 01–06. doi: 10.62310/liab.v1i1.53
Cristofori, F., Dargenio, V. N., Dargenio, C., Miniello, V. L., Barone, M., Francavilla, R. (2021). Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 12, 578386. doi: 10.3389/fimmu.2021.578386
Ding, S., Yan, W., Ma, Y., Fang, J. (2021). The impact of probiotics on gut health via alternation of immune status of monogastric animals. Anim. Nutr. 7, 24–30. doi: 10.1016/j.aninu.2020.11.004
Franz, C. M. A. P., Stiles, M. E., Schleifer, K. H., Holzapfel, W. H. (2003). Enterococci in foods - A conundrum for food safety. Int. J. Food Microbiol. 88, 105–122. doi: 10.1016/S0168-1605(03)00174-0
Goubet, A. G., Wheeler, R., Fluckiger, A., Qu, B., Lemaître, F., Iribarren, K., et al. (2021). Multifaceted modes of action of the anticancer probiotic Enterococcus hirae. Cell Death Differ. 28, 2276–2295. doi: 10.1038/s41418-021-00753-8
Li, W., Lim, C. H., Zhao, Z., Wang, Y., Conway, P. L., Loo, S. C. J. (2024). In vitro profiling of potential fish probiotics, enterococcus hirae strains, isolated from jade perch, and safety properties assessed using whole genome sequencing. Probiotics Antimicrob. Proteins. doi: 10.1007/s12602-024-10244-0
Li, W., Xu, B., Wang, L., Sun, Q., Deng, W., Wei, F., et al. (2021). Effects of clostridium butyricum on growth performance, gut microbiota and intestinal barrier function of broilers. Front. Microbiol. 12, 777456. doi: 10.3389/fmicb.2021.777456
Li, Y., Jin, L., Chen, T., Pirozzi, C. J. (2020). The effects of secretory igA in the mucosal immune system. BioMed. Res. Int. 2020, 2032057. doi: 10.1155/2020/2032057
Liu, F., Li, X., Bello, B. K., Zhang, T., Yang, H., Wang, K., et al. (2022). Difenoconazole causes spleen tissue damage and immune dysfunction of carp through oxidative stress and apoptosis. Ecotoxicol. Environ. Saf. 237, 113563. doi: 10.1016/j.ecoenv.2022.113563
Ma, C., Azad, M. A. K., Tang, W., Zhu, Q., Wang, W., Gao, Q., et al. (2022). Maternal probiotics supplementation improves immune and antioxidant function in suckling piglets via modifying gut microbiota. J. Appl. Microbiol. 133, 515–528. doi: 10.1111/jam.15572
Ntakiyisumba, E., Lee, S., Won, G. (2022). Evidence-Based Approaches for Determining Effective Target Antigens to Develop Vaccines against Post-Weaning Diarrhea Caused by Enterotoxigenic Escherichia coli in Pigs: A Systematic Review and Network Meta-Analysis. Animals 12, 1–19. doi: 10.3390/ani12162136
Pabst, O., Slack, E. (2020). IgA and the intestinal microbiota: the importance of being specific. Mucosal Immunol. 13, 12–21. doi: 10.1038/s41385-019-0227-4
Paludan, S. R., Pradeu, T., Masters, S. L., Mogensen, T. H. (2021). Constitutive immune mechanisms: mediators of host defense and immune regulation. Nat. Rev. Immunol. 21, 137–150. doi: 10.1038/s41577-020-0391-5
Rahman, M. R. T., Fliss, I., Biron, E. (2022). Insights in the development and uses of alternatives to antibiotic growth promoters in poultry and swine production. Antibiotics 11, 1–29. doi: 10.3390/antibiotics11060766
Sierawska, O., Małkowska, P., Taskin, C., Hrynkiewicz, R., Mertowska, P., Grywalska, E., et al. (2022). Innate immune system response to burn damage—Focus on cytokine alteration. Int. J. Mol. Sci. 23, 716. doi: 10.3390/ijms23020716
Strompfová, V., Marciňáková, M., Simonová, M., Gancarčíková, S., Jonecová, Z., Sciranková, Ľ., et al. (2006). Enterococcus faecium EK13-an enterocin A-producing strain with probiotic character and its effect in piglets. Anaerobe 12, 242–248. doi: 10.1016/j.anaerobe.2006.09.003
Sun, Y., Duarte, M. E., Kim, S. W. (2021). Dietary inclusion of multispecies probiotics to reduce the severity of post-weaning diarrhea caused by Escherichia coli F18+ in pigs. Anim. Nutr. 7, 326–333. doi: 10.1016/j.aninu.2020.08.012
Sun, Y., Kim, S. W. (2017). Intestinal challenge with enterotoxigenic Escherichia coli in pigs, and nutritional intervention to prevent postweaning diarrhea. Anim. Nutr. 3, 322–330. doi: 10.1016/j.aninu.2017.10.001
Vidal, R. M., Chamorro, N. L., Girón, J. A. (2016). Enterotoxigenic escherichia coli. Escherichia Coli Am. 7, 10. doi: 10.1007/978-3-319-45092-6_1
Wang, X., Du, C., Subramanian, S., Turner, L., Geng, H., Bu, H. F., et al. (2023). Severe gut mucosal injury induces profound systemic inflammation and spleen-associated lymphoid organ response. Front. Immunol. 14, 1340442. doi: 10.3389/fimmu.2023.1340442
Yan, N., Xu, G., Zhang, C., Liu, X., Li, X., Sun, L., et al. (2020). Chronic arsenic exposure induces the time-dependent modulation of inflammation and immunosuppression in spleen. Cell Biosci. 10, 1–10. doi: 10.1186/s13578-020-00448-6
Yao, Y., Cai, X., Fei, W., Ye, Y., Zhao, M., Zheng, C. (2022). The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 62, 1–12. doi: 10.1080/10408398.2020.1854675
Yue, Y., He, Z., Zhou, Y., Ross, R. P., Stanton, C., Zhao, J., et al. (2020). Lactobacillus plantarum relieves diarrhea caused by enterotoxin-producing Escherichia coli through inflammation modulation and gut microbiota regulation. Food Funct. 11, 10362–10374. doi: 10.1039/d0fo02670k
Zeyner, A., Boldt, E. (2006). Effects of a probiotic Enterococcus faecium strain supplemented from birth to weaning on diarrhea patterns and performance of piglets. J. Anim. Physiol. Anim. Nutr. (Berl). 90, 25–31. doi: 10.1111/j.1439-0396.2005.00615.x
Zhang, P. (2022). Influence of foods and nutrition on the gut microbiome and implications for intestinal health. Int. J. Mol. Sci. 23, 9588. doi: 10.3390/ijms23179588
Zhang, Y., Tan, P., Zhao, Y., Ma, X. (2022a). Enterotoxigenic Escherichia coli: intestinal pathogenesis mechanisms and colonization resistance by gut microbiota. Gut Microbes 14, 2055943. doi: 10.1080/19490976.2022.2055943
Zhang, L., Wu, Z., Li, J., Li, H., Liu, Z., Wang, J., et al. (2024). Ningxiang pig-derived Enterococcus hirae HNAU0516 ameliorates post-weaning diarrhea by promoting intestinal health and modulating the gut microbiota in piglets. Animal 18, 101220. doi: 10.1016/j.animal.2024.101220
Zhang, L., Yao, X., Ma, M., Ding, Y., Zhang, H., He, X., et al. (2021). Protective Effect of l -Theanine against DSS-Induced Colitis by Regulating the Lipid Metabolism and Reducing Inflammation via the NF-κB Signaling Pathway. J. Agric. Food Chem. 69, 14192–14203. doi: 10.1021/acs.jafc.1c05839
Zhang, Z., Zhang, H., Chen, T., Shi, L., Wang, D., Tang, D. (2022b). Regulatory role of short-chain fatty acids in inflammatory bowel disease. Cell Commun. Signal. 20, 1–10. doi: 10.1186/s12964-022-00869-5
Zhu, X., Zhang, Y., Zhao, Y., Tao, L., Liu, H., Dong, W., et al. (2022). Effects of dietary supplementation with itaconic acid on the growth performance, nutrient digestibility, slaughter variables, blood biochemical parameters, and intestinal morphology of broiler chickens. Poult. Sci. 101, 101732. doi: 10.1016/j.psj.2022.101732
Keywords: ETEC, piglets, inflammatory cytokines, spleen, acetate
Citation: Zhang L, Wu Z, Zhang Z, Cai R, Pang S, Wang J and Bao X (2024) Ningxiang pigderived Enterococcus hirae regulates the inflammatory function and enhances the protection of piglets against ETEC challenge. Front. Cell. Infect. Microbiol. 14:1476564. doi: 10.3389/fcimb.2024.1476564
Received: 06 August 2024; Accepted: 16 September 2024;
Published: 17 October 2024.
Edited by:
Matthias Tröltzsch, Maxillofacial and Facial Reconstructive Surgery, GermanyReviewed by:
Ming Jiang, Guangdong Academy of Agricultural Sciences, ChinaNikhilesh Joardar, Washington University in St. Louis, United States
Copyright © 2024 Zhang, Wu, Zhang, Cai, Pang, Wang and Bao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiyuan Bao, YmFveGl5dWFuQDE2My5jb20=
†These authors have contributed equally to this work
 Longlin Zhang
Longlin Zhang Zichen Wu1,2†
Zichen Wu1,2†