- 1Department of Thoracic Medicine, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 2School of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Respiratory Therapy, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 4School of Medicine, National Tsing Hua University, Hsin-Chu, Taiwan
- 5Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Taoyuan, Taiwan
Introduction: Invasive pulmonary aspergillosis (IPA) increases the risk of mortality of critically ill patients. Diagnostic criteria specifically targeting patients in intensive care units(ICUs) have been developed to improve diagnostic sensitivity. This study investigated health outcomes among patients in ICUs with Aspergillus isolates identified using bronchoscopy.
Methods: This retrospective cohort study obtained data from the Chang Gung Research Database of Chang Gung Memorial Hospital. Patients admitted to the ICU between January 2017 and December 2022 who received bronchoalveolar lavage were enrolled. Patients with a fungus culture yielding Aspergillus spp. isolates or who had an Aspergillus galactomannan antigen index value of >1.0 were categorized into the Aspergillus-positive group.
Results: A total of 2372 patients were enrolled, and 146 patients (6.16%) tested positive for Aspergillus. Of the patients who tested positive for Aspergillus, 37.67% had a positive culture result, and 77.4% had a positive galactomannan antigen result. Patients with Aspergillus isolates were more likely to have a recent influenza infection, concurrent bacterial sepsis, and a cavitation and to die in hospital (in-hospital mortality rate 58.9% vs. 48.57%, P = 0.016).
Discussion: Identifying Aspergillus through bronchoscopy in the ICU is associated with higher mortality rates than in patients who test negative for Aspergillus. Galactomannan antigen from bronchoalveolar lavage may provide higher diagnostic sensitivity.
Introduction
Invasive pulmonary aspergillosis (IPA) frequently occurs as an opportunistic infection in intensive care units (ICUs) and has been associated with increased risks of morbidity and mortality, particularly in immunocompromised individuals. Previous research has shown that ICU patients diagnosed with IPA face high mortality rates (Meersseman et al., 2004; Ku et al., 2017; Lin et al., 2017), with IPA-associated tracheobronchitis mortality reached 93.5% (Lin et al., 2017). IPA even increases the risk of death among nonneutropenic patients (Ku et al., 2017).
Diagnostic criteria for IPA were established in 2002 by the European Organization for Research and Treatment of Cancer Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG). These criteria were then updated in 2008 and 2020 to enhance their utility in research and in clinical settings (Ascioglu et al., 2002; De Pauw et al., 2008; Donnelly et al., 2020). These criteria predominantly target immunocompromised populations.
Aspergillus spp. can cause invasive diseases in diverse patient groups (Coulon et al., 2020; Nyga et al., 2020; Kuo et al., 2022; Hatzl et al., 2024). Worldwide, an estimated 519,000 patients in ICUs may be affected by IPA (Denning, 2024). According to one study, mortality among patients in ICUs with IPA who did not receive treatment exceeded 95% (Denning, 2024). Diagnosing IPA is particularly challenging in clinical settings. Standard diagnostic definitions, developed primarily for patients with cancer or patients who have undergone hematopoietic stem cell transplant, may not apply to critically ill patients, who often lack the host factors specified in EORTC criteria. Obtaining histological diagnoses for critically ill patients is also difficult. Diagnostic criteria that can be effectively applied to this patient group are warranted.
Several algorithms—including AspICU, BM-AspICU, and Modified AspICU—have been developed for use as IPA diagnostic tools in ICUs (Blot et al., 2012; Schauwvlieghe et al., 2018; Hamam et al., 2021). These algorithms can be used to determine the risk of IPA in patients who have had influenza, patients with neutropenia, patients who have received systemic corticosteroid treatment, and patients who have undergone stem cell transplant; however, whether they can be used with other patient groups is uncertain. Our objective was to compare the prognosis of patients with Aspergillus isolates identified using bronchoscopy against other patient groups.
Method
Data source
Patients in ICUs between 2017 and 2022 were identified from the Chang Gung Research Database (CGRD), which belongs to the Chang Gung Medical Foundation. This foundation is the largest hospital system in Taiwan, comprising three medical centers (in Linkou, Taipei, and Kaohsiung) and four regional hospitals (in Taoyuan, Keelung, Chiayi, and Yunlin) located across Taiwan. The CGRD contains patients’ demographic data, inpatient and outpatient records, diagnostic codes, medication records, microbiological data, imaging study reports, and functional examination data (Lin et al., 2022). Disease diagnoses are coded in the database by using the International Classification of Diseases, Tenth Revision. This study received approval from the Institutional Review Board of the Chang Gung Memorial Foundation (IRB No. 202301837B0). Due to the retrospective nature of the study, the requirement for informed consent was waived.
Study design
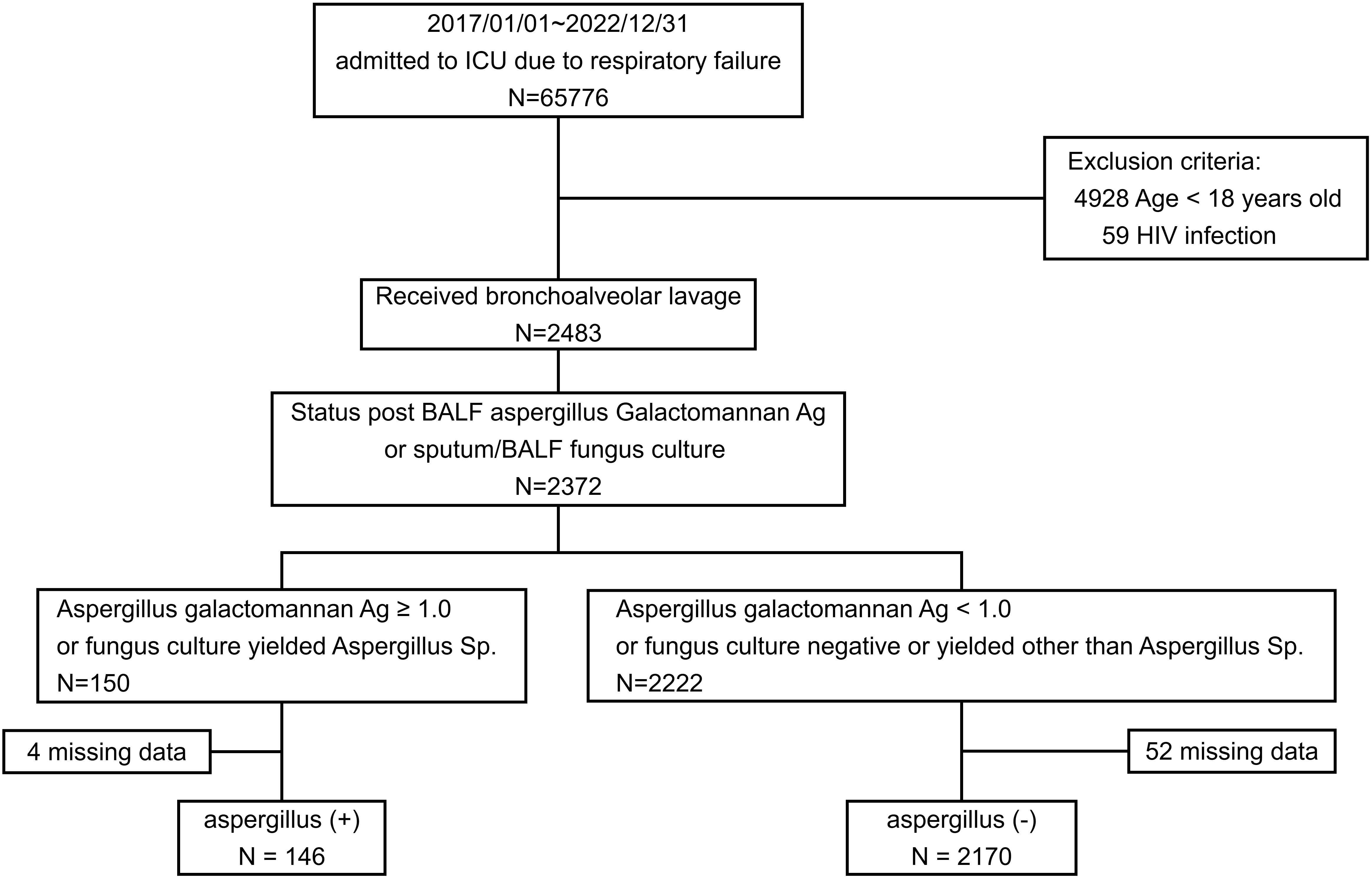
Patients admitted to an ICU between January 2017 and December 2022 who required mechanical ventilation and who underwent bronchoscopy and bronchoalveolar lavage (BAL) were enrolled and divided into two groups. Patients with a fungus culture from BAL fluid yielding Aspergillus spp. isolates or who had an Aspergillus galactomannan antigen index value of >1.0 were categorized into the Aspergillus-positive group. The remaining patients were categorized into the Aspergillus-negative group. Patients were excluded if they were aged <18 years, had human immunodeficiency virus, or did not have data on Acute Physiology and Chronic Health Evaluation II (APACHE II) scores obtained upon their ICU admission.
Covariates and outcomes
The following covariates were analyzed: age, sex, chronic comorbidities (d iabetes mellitus, heart failure, liver cirrhosis, chronic renal insufficiency, cancer, hematological malignancy, chronic obstructive pulmonary disease, prior tuberculosis infection, autoimmune disease, and organ transplant), and the following patient conditions: APACHE II score, acute kidney injury requiring renal replacement therapy, length of ICU stay, length of mechanical ventilation use, length of hospital stay, neutropenia prior to bronchoscopy (defined as absolute neutrophil count ≤500 cells/mm3), concurrent bacterial sepsis (defined as any positive bacterial culture of blood, BAL fluid, or sputum within the 1 week before and after entering or leaving the ICU), imaging reports from radiologists, serum and BAL fluid galactomannan antigen indices, fungus culture results, and antifungal treatment status. Patients were defined as having a comorbidity if they had at least two outpatient diagnoses or one inpatient diagnosis for that comorbidity prior to the index date. The patterns of image reports were defined by reports from radiologists. Antifungal treatment status was defined as adequate or inadequate on the basis of whether the patient was administered any dose of voriconazole, posaconazole, isavuconazole, caspofungin, amphotericin B, or liposomal amphotericin B.
Statistical analysis
Results are presented as means with standard deviations or as numbers and percentages. Student’s t test for independent samples was used to compare continuous variables that followed a normal distribution. Pearson’s chi-square test or Fisher’s exact test were used to compare categorical variables. The Mann–Whitney U test was employed to compare continuous variables that did not follow a normal distribution, which occurred in several subgroup analyses involving only a few patients. Statistical significance was set at a two-sided P value of < 0.05.
Results
Between January 1, 2017, and December 31, 2022, BAL procedures were conducted on 2483 patients. Results for either the Aspergillus galactomannan antigen index or sputum BAL fluid fungus culture were available for 2372 of these patients. In total, 146 patients tested positive for Aspergillus, and 2170 tested negative for Aspergillus (Figure 1). Overall, 6.16% of the patients with positive galactomannan antigen or fungus culture results tested positive for Aspergillus.
Baseline characteristics
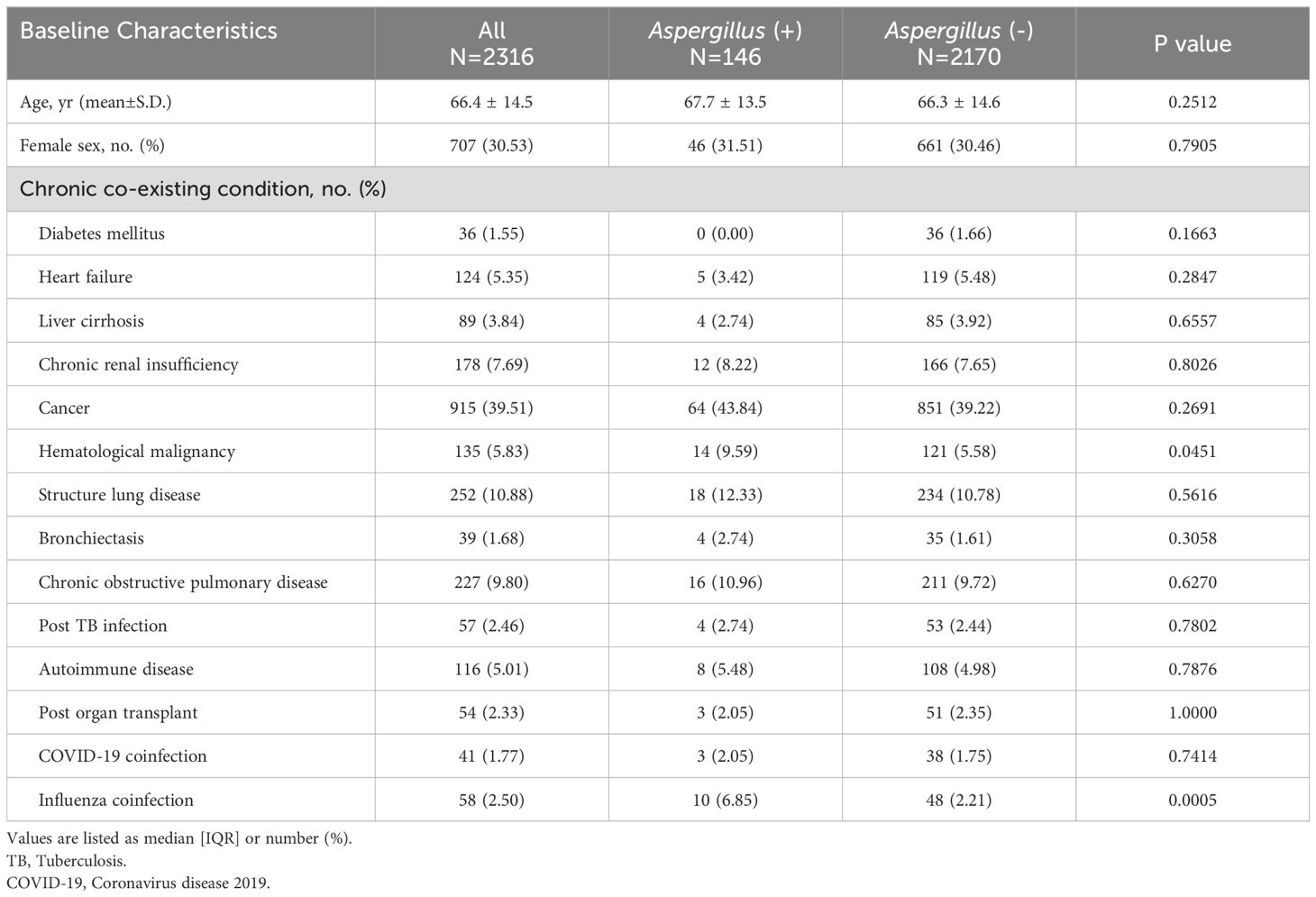
In the Aspergillus-positive group, 113 patients (77.4%) had a positive result for the Aspergillus galactomannan antigen index in BAL fluid. Additionally, 55 patients (54.79%) had a positive result from a fungal culture, while 22 patients (15.1%) tested positive in both the fungal culture and the galactomannan antigen index. The mean age of the patients in this study was 66.4 years, and approximately one-third (707, 30.53%) of the patients were women (Table 1). The most common chronic comorbidity was cancer (915, 39.51%). No differences in the prevalence of chronic comorbidities were discovered between the Aspergillus-positive and Aspergillus-negative groups except for hematological malignancy (9.85% in the Aspergillus-positive group and 5.58% in the Aspergillus-negative group, P = 0.045). The Aspergillus-positive group was more likely to experience postinfluenza infection than was the Aspergillus-negative group (6.85% vs. 2.21%, P = 0.005). No difference in the rate of post-COVID-19 infection was found between the groups.
Clinical outcomes
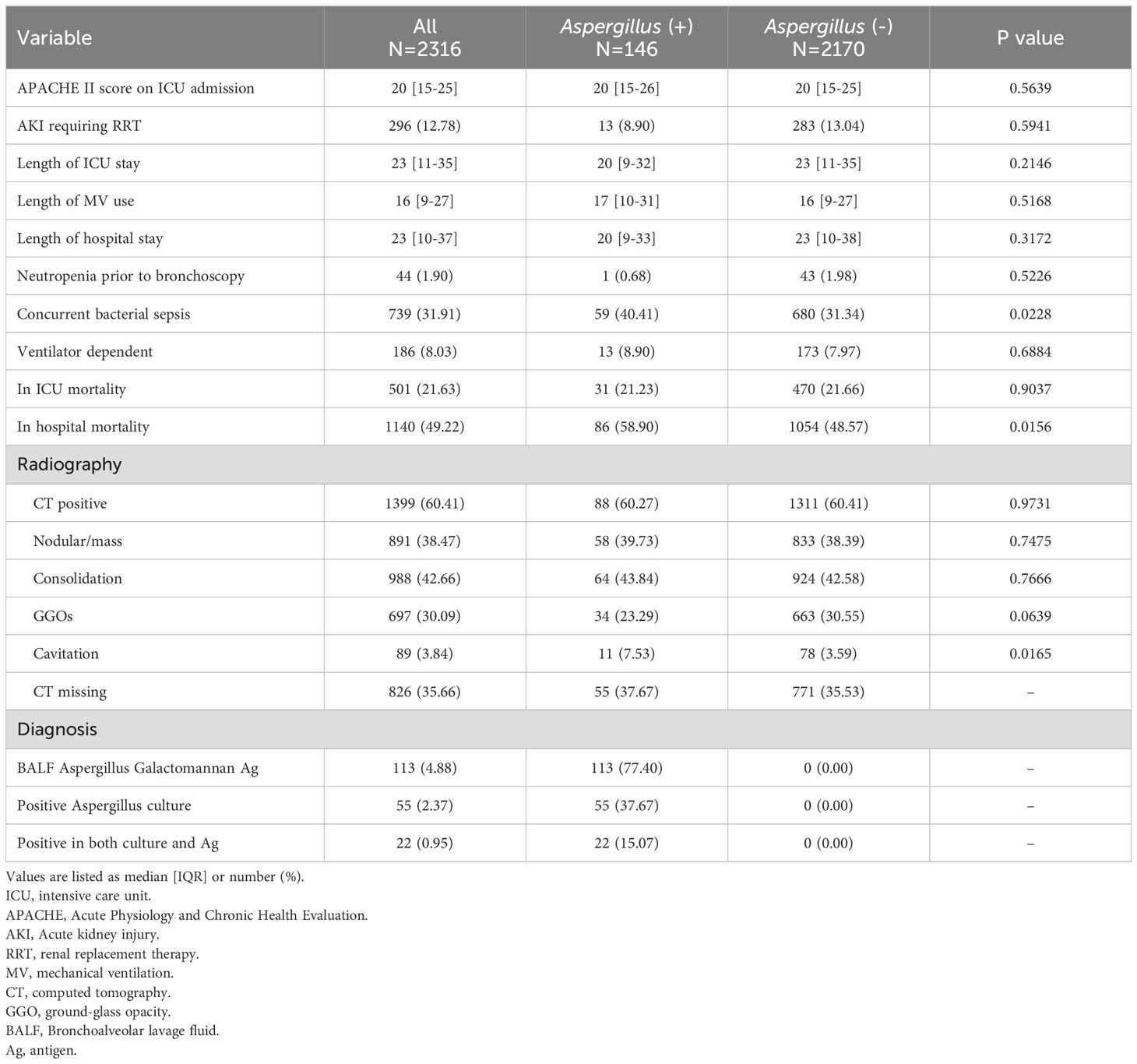
The overall mortality rate was 49.22% (Table 2). The mortality rate was significantly higher in the Aspergillus-positive group than in the Aspergillus-negative group (58.9% vs. 48.57%, P = 0.016). No differences in the incidence of acute kidney injury requiring renal replacement therapy, length of ICU stay, length of mechanical ventilation use, length of hospital stay, ICU mortality rate, or ventilator dependency were found. The Aspergillus-positive group had a higher risk of concurrent bacterial sepsis than did the Aspergillus-negative group (40.41% vs. 31.34%, P = 0.023). Computed tomography revealed a higher proportion of cavitation in the Aspergillus-positive group than in the Aspergillus-negative group (7.53% vs. 3.59%, P = 0.017). The treatment outcomes for the Aspergillus-positive group are listed in Table 3. Although patients who survived were more likely than were those who did not survive to have received effective antifungal treatment, the disparity was nonsignificant. Kaplan–Meier survival analysis was used to compare the mortality rates of the Aspergillus-positive and Aspergillus-negative groups (Figure 2); the in-hospital mortality rate was found to be significantly higher in the Aspergillus-positive group (hazard ratio: 1.38, reference: Aspergillus-negative group; 95% confidence interval: 1.07–1.78, log-rank test P = 0.002). The Aspergillus spp. identified in fungus cultures are listed in Table 4.
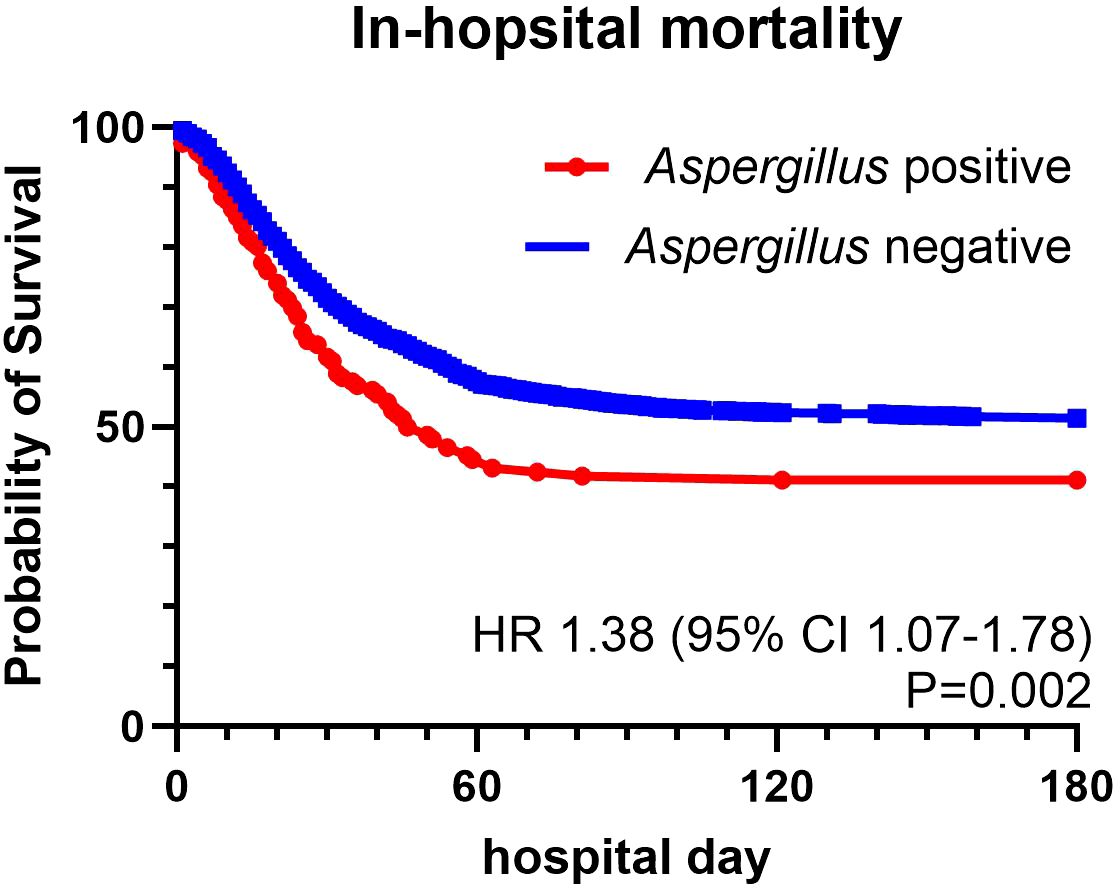
Figure 2. Survival curves for patients with or without aspergillus identification. hazard ratio: 1.38; 95% Confidence Interval: 1.07-1.78, Log-rank Test P =0.002.
Discussion
The present study represents a pioneering approach to examining the occurrence of Aspergillus spp. in BAL samples collected from ICU patients. In total, 6.16% of our study cohort tested positive for Aspergillus. Patients with postinfluenza infection were more likely to test positive for Aspergillus. In-hospital mortality was higher among the patients who tested positive for Aspergillus than among those who tested negative; however, in-ICU mortality was not correlated with Aspergillus status.
Other studies have reported IPA prevalence ranging from 5% to 7% in nonimmunocompromised patients in ICUs (Baddley et al., 2013; Tudesq et al., 2019), similar to the rate obtained in the present study. IPA was shown to occur in 12.5% of patients with acute respiratory distress syndrome, as determined by autopsy (Tudesq et al., 2019). Also, the positive and negative predictive values of AspICU were only 61% and 92%, respectively. Negative predictive values may be as low as 71% in nonimmunocompromised individuals (Blot et al., 2012). This suggests that the prevalence of IPA among nonimmunocompromised patients in ICUs may be somewhat underestimated.
The mortality rate in the present study was 58.9% and 48.6% in the Aspergillus-positive and Aspergillus-negative groups, respectively. IPA-associated mortality differs across patient categories. In one study, the in-hospital mortality rate among patients with influenza-associated pulmonary aspergillosis was 49% (Schauwvlieghe et al., 2018). In another study, which involved patients with acute respiratory distress syndrome and IPA, the in-ICU mortality rate was 60% (Contou et al., 2016). A study involving patients with hematological malignancies and IPA and receiving invasive mechanical ventilation reported a 90-day mortality rate of 80.4% (Pardo et al., 2019). A systemic review and meta-analysis performed in 2022 reported an IPA-associated mortality rate of 54% (Shi et al., 2022). An article recently published in Lancet Infectious Diseases revealed a large discrepancy in mortality rates among patients with IPA in ICUs between those who did and those who did not receive treatment for IPA (50% vs. 95%) (Denning, 2024). In the present study, the Aspergillus-positive group did not fully meet any single diagnostic criteria. Mortality rates significantly differed between patients with and without positive results for Aspergillus. Blot et al. developed the AspICU algorithm to calculate a 70% mortality rate in patients with putative IPA (Blot et al., 2012). Schauwvlieghe et al. used the modified-AspICU algorithm to calculate a 49% in-hospital mortality rate among patients with IPA (Schauwvlieghe et al., 2018). Our research revealed an in-hospital mortality rate of 58.9% for patients with positive results for Aspergillus. These findings suggest that existing diagnostic algorithms are insufficient when employed in ICU settings.
The optimal method for diagnosing IPA in nonimmunocompromised patients in ICUs remains unclear. Delayed diagnoses of IPA could be linked to higher mortality (Inoue et al., 2022). Postponing antifungal treatment for IPA could lead to higher likelihood of in-hospital mortality and a longer hospital stay (Baddley et al., 2013; Denning, 2024). For each day that antifungal therapy initiation is delayed, the length of hospital stay increases by 1.28 days and costs increase by 3.5% (Baddley et al., 2013). Therefore, implementing advanced diagnostic techniques is essential, particularly in cases involving patients with malignancy or recent viral infections, such as COVID-19 or influenza. The galactomannan antigen index proved to be an excellent diagnostic tool, demonstrating high sensitivity and specificity. A meta-analysis revealed sensitivity of 85% and specificity of 86% when employing a cutoff galactomannan antigen index of 1.0 (Zou et al., 2012). The latest EORTC/MSG guidelines recommend a cutoff index of 1.0 for the result of galactomannan antigen enzyme-linked immunosorbent assay for BAL samples. This cutoff yielded sensitivity between 75% and 86% and specificity between 94% and 95%. Sensitivity and specificity values were consistent regardless of the presence of hematological malignancies, with sensitivity ranging from 85% to 87% and specificity from 91% to 89% (Mercier et al., 2021). The AspICU diagnostic criteria do not incorporate galactomannan antigen index values (Blot et al., 2012). Similarly, the Invasive Fungal Diseases in Adult Patients in Intensive Care Unit (FUNDICU) criteria do not cover all ICU patients with predisposing conditions (Bassetti et al., 2024). The mortality rate in the present study’s Aspergillus-positive group was comparable to those in other studies and was significantly higher than that in the Aspergillus-negative group. Consequently, we propose that being an ICU patient be specified as a host factor in IPA diagnostic criteria. This would facilitate the timely diagnosis of IPA in critically ill patients and improve their health outcomes.
This study has several limitations. First, because the study was retrospective in nature, selection bias may have been present. This bias was potentially mitigated by including only patients who underwent BAL in the ICU. Second, BAL is potentially unsuitable for patients with high oxygen requirements and for those who are hemodynamically unstable. These conditions arise from IPA but also from other factors, possibly leading to an underestimation of the mortality rate in both groups. Third, we were unable to determine whether antifungal agents had been administered for prophylaxis or treatment purposes, nor could we verify whether patients had completed their prescribed courses of treatment. This uncertainty could have resulted in an underestimation of treatment effectiveness. Furthermore, specific immune traits associated with IPA, such as the length and dosage of glucocorticoid treatment and the period of neutropenia, were not discernible. Consequently, these results should be interpreted with caution. Finally, several novel tools for diagnosing IPA, such as polymerase chain reaction (Rath and Steinmann, 2018; Scharmann et al., 2021) and serum IL-8 testing (Heldt et al., 2018), were unavailable in the present study due to equipment constraints. Prospective, large-scale studies are warranted to validate our results.
Conclusion
Identifying Aspergillus through bronchoscopy in the ICU is linked to increased mortality. Existing diagnostic criteria may not be effective when applied to patients in ICUs. Regular evaluation of galactomannan antigen index values obtained through BAL may provide better diagnostic sensitivity, particularly in patients with a recent influenza infection.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: Applicants who fulfill one of the following requirements are eligible to apply: (1) A full-time attending physician of this hospital or a full-time, part-time attending physician, resident, clinical researcher, administrative, medical and technical, or nursing staff of Chang Gung University. (2) Teachers of Chang Gung University and Chang Gung University of Science and Technology who are at the rank of assistant professor or above are required to collaborate on research projects with attending physicians specializing in related disciplines or fields in the Hospital before submitting an application. Requests to access these datasets should be directed to Center for Big Data Analytics and Statistics, Y2hpYWxpbmdAY2dtaC5vcmcudHc=.
Ethics statement
The studies involving humans were approved by the Institutional Review Board (IRB) of the Chang Gung Memorial Foundation (IRB No. 202301837B0C601). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent from the patients/participants or patients/participants legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
HC: Conceptualization, Data curation, Investigation, Methodology, Writing – original draft. CL: Conceptualization, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. HL: Conceptualization, Validation, Writing – review & editing. SL: Conceptualization, Validation, Writing – review & editing. MH: Conceptualization, Validation, Writing – review & editing. YF: Conceptualization, Validation, Writing – review & editing. PC: Conceptualization, Validation, Writing – review & editing. WH: Conceptualization, Data curation, Investigation, Writing – review & editing. KC: Formal analysis, Investigation, Resources, Software, Writing – review & editing. CH: Conceptualization, Methodology, Project administration, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Chang Gung Memorial Hospital Research Project Grant (CGRPG3K0011). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Acknowledgments
The authors thank Ko Cheng for the statistical assistance and wish to acknowledge for statistical and data analysis assistance and interpretation support by the Center for Big Data Analytics and Statistics, Chang Gung Memorial Hospital, Linkou, Taiwan. This study is based in part on data from the Chang Gung Research Database provided by Chang Gung Memorial Hospital. The interpretation and conclusions contained herein do not represent the position of Chang Gung Memorial Hospital. This manuscript was edited by Wallace Academic Editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ascioglu, S., Rex, J. H., de Pauw, B., Bennett, J. E., Bille, J., Crokaert, F., et al. (2002). Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin. Infect. Dis. 34, 7–14. doi: 10.1086/323335
Baddley, J. W., Stephens, J. M., Ji, X., Gao, X., Schlamm, H.T., Tarallo, M. (2013). Aspergillosis in Intensive Care Unit (ICU) patients: epidemiology and economic outcomes. BMC Infect. Dis. 13, 29. doi: 10.1186/1471-2334-13-29
Bassetti, M., Giacobbe, D. R., Agvald-Ohman, C., Akova, M., Alastruey-Izquierdo, A., Arikan-Akdagli, S., et al. (2024). Invasive Fungal Diseases in Adult Patients in Intensive Care Unit (FUNDICU): 2024 consensus definitions from ESGCIP, EFISG, ESICM, ECMM, MSGERC, ISAC, and ISHAM. Intensive Care Med. 50, 502–515. doi: 10.1007/s00134-024-07341-7
Blot, S. I., Taccone, F. S., Van den Abeele, A. M., Bulpa, P., Meersseman, W., Brusselaers, N., et al. (2012). A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am. J. Respir. Crit. Care Med. 186, 56–64. doi: 10.1164/rccm.201111-1978OC
Contou, D., Dorison, M., Rosman, J., Schlemmer, F., Gibelin, A., Foulet, F., et al. (2016). Aspergillus-positive lower respiratory tract samples in patients with the acute respiratory distress syndrome: a 10-year retrospective study. Ann. Intensive Care 6, 52. doi: 10.1186/s13613-016-0156-2
Coulon, P., Cordier, C., Saint-Leger, P., Lambiotte, F., Loridant, S., Mazars, E. (2020). Invasive pulmonary aspergillosis in an ICU patient with Legionnaires' disease: A diagnostic challenge. J. Mycol Med. 30, 100985. doi: 10.1016/j.mycmed.2020.100985
Denning, D. W. (2024). Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 24, e428–e438. doi: 10.1016/S1473-3099(23)00692-8
De Pauw, B., Walsh, T. J., Donnelly, J. P., Stevens, D. A., Edwards, J. E., Calandra, T., et al. (2008). Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821. doi: 10.1086/588660
Donnelly, J. P., Chen, S.C., Kauffman, C. A., Steinbach, W. J., Baddley, J. W., Verweij, P. E., et al. (2020). Revision and update of the consensus definitions of invasive fungal disease from the european organization for research and treatment of cancer and the mycoses study group education and research consortium. Clin. Infect. Dis. 71, 1367–1376. doi: 10.1093/cid/ciz1008
Hamam, J., Navellou, J. C., Bellanger, A. P., Bretagne, S., Winiszewski, H., Scherer, E., et al. (2021). New clinical algorithm including fungal biomarkers to better diagnose probable invasive pulmonary aspergillosis in ICU. Ann. Intensive Care 11, 41. doi: 10.1186/s13613-021-00827-3
Hatzl, S., Scholz, L., Posch, F., Eller, P., Reisinger, A.C., Zacharias, M., et al. (2024). Invasive pulmonary aspergillosis in critically ill patients with hantavirus infection, Austria. Emerg. Infect. Dis. 30, 1275–1278. doi: 10.3201/eid3006.231720
Heldt, S., Prattesb, J., Eigla, S., Spiessd, B., Flicka, H., Rabensteiner, J., et al. (2018). Diagnosis of invasive aspergillosis in hematological Malignancy patients: Performance of cytokines, Asp LFD, and Aspergillus PCR in same day blood and bronchoalveolar lavage samples. J. Infect. 77, 235–241. doi: 10.1016/j.jinf.2018.05.001
Inoue, K., Muramatsu, K., Nishimura, T., Fujino, Y., Matsuda, S., Fushimi, K., et al. (2022). Association between early diagnosis of and inpatient mortality from invasive pulmonary aspergillosis among patients without immunocompromised host factors: a nationwide observational study. Int. J. Infect. Dis. 122, 279–284. doi: 10.1016/j.ijid.2022.05.048
Ku, Y. H., Chan, K. S., Yang, C. C., Tan, C. K., Chuang, Y. C., Yu, W. L. (2017). Higher mortality of severe influenza patients with probable aspergillosis than those with and without other coinfections. J. Formos Med. Assoc. 116, 660–670. doi: 10.1016/j.jfma.2017.06.002
Kuo, C. W., Wang, S. Y., Tsai, H. P., Su, P. L., Cia, C. T., Lai, C. H., et al. (2022). Invasive pulmonary aspergillosis is associated with cytomegalovirus viremia in critically ill patients - A retrospective cohort study. J. Microbiol. Immunol. Infect. 55, 291–299. doi: 10.1016/j.jmii.2021.03.005
Lin, C. Y., Liu, W. L., Chang, C. C., Chang, H. T., Hu, H. C., Kao, K. C., et al. (2017). Invasive fungal tracheobronchitis in mechanically ventilated critically ill patients: underlying conditions, diagnosis, and outcomes. Ann. Intensive Care 7, 9. doi: 10.1186/s13613-016-0230-9
Lin, C. Y., Huang, H. Y., Hsieh, M. H., Fang, Y. F., Lo, Y. L., Lin, S. M., et al. (2022). Impacts of nontuberculous mycobacteria isolates in non-cystic fibrosis bronchiectasis: A 16-year cohort study in Taiwan. Front. Microbiol. 13, 868435. doi: 10.3389/fmicb.2022.868435
Meersseman, W., Vandecasteele, S. J., Wilmer, A., Verbeken, E., Peetermans, W. E., Van Wijngaerden, E. (2004). Invasive aspergillosis in critically ill patients without Malignancy. Am. J. Respir. Crit. Care Med. 170, 621–625. doi: 10.1164/rccm.200401-093OC
Mercier, T., Castagnola, E., Marr, K. A., Wheat, L. J., Verweij, P. E., Maertens, J. A. (2021). Defining galactomannan positivity in the updated EORTC/MSGERC consensus definitions of invasive fungal diseases. Clin. Infect. Dis. 72, S89–S94. doi: 10.1093/cid/ciaa1786
Nyga, R., Maizel, J., Nseir, S., Chouaki, T., Milic, I., Roger, P. A., et al. (2020). Invasive tracheobronchial aspergillosis in critically ill patients with severe influenza. A Clin. Trial. Am. J. Respir. Crit. Care Med. 202, 708–716. doi: 10.1164/rccm.201910-1931OC
Pardo, E., Lemiale, V., Mokart, D., Stoclin, A., Moreau, A. S., Kerhuel, L., et al. (2019). Invasive pulmonary aspergillosis in critically ill patients with hematological Malignancies. Intensive Care Med. 45, 1732–1741. doi: 10.1007/s00134-019-05789-6
Rath, P. M., Steinmann, J. (2018). Overview of commercially available PCR assays for the detection of aspergillus spp. DNA Patient Samples. Front. Microbiol. 9, 740. doi: 10.3389/fmicb.2018.00740
Scharmann, U., Kirchhoff, L., Hain, A., Buer, J., Koldehoff, M., Steinmann, J., et al. (2021). Evaluation of three commercial PCR assays for the detection of azole-resistant aspergillus fumigatus from respiratory samples of immunocompromised patients. J. Fungi (Basel) 7. doi: 10.3390/jof7020132
Schauwvlieghe, A., Rijnders, B. J. A., Philips, N., Verwijs, R., Vanderbeke, L., Van Tienen, C., et al. (2018). Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir. Med. 6, 782–792. doi: 10.1016/S2213-2600(18)30274-1
Shi, C., Shan, Q., Xia, J., Wang, L., Wang, L., Qiu, L., et al. (2022). Incidence, risk factors and mortality of invasive pulmonary aspergillosis in patients with influenza: A systematic review and meta-analysis. Mycoses 65, 152–163. doi: 10.1111/myc.13410
Tudesq, J. J., Peyrony, O., Lemiale, V., Azoulay, E., et al. (2019). Invasive pulmonary aspergillosis in nonimmunocompromised hosts. Semin. Respir. Crit. Care Med. 40, 540–547. doi: 10.1055/s-0039-1696968
Keywords: Aspergillus, invasive pulmonary aspergillosis (IPA), galactomannan (GM), intensive care unit (ICU), broncho alveolar lavage (BAL)
Citation: Cheng H-I, Lin C-Y, Lin H-C, Lin S-M, Hsieh M-H, Fang Y-F, Chang P-J, Hung W-S, Cheng K and Huang C−C (2025) Aspergillus identification through bronchoscope in intensive care unit – a retrospective, databased cohort study. Front. Cell. Infect. Microbiol. 14:1471298. doi: 10.3389/fcimb.2024.1471298
Received: 31 July 2024; Accepted: 18 December 2024;
Published: 13 January 2025.
Edited by:
Ashraf S. Ibrahim, University of California, Los Angeles, United StatesReviewed by:
Tamás Papp, University of Szeged, HungaryNan Zheng, Nanjing University, China
Melda Turken, University of Health Sciences, Türkiye
Copyright © 2025 Cheng, Lin, Lin, Lin, Hsieh, Fang, Chang, Hung, Cheng and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chung−Chi Huang, Y2NoNDg0OEBjZ21oLm9yZy50dw==
 Hsin-I Cheng
Hsin-I Cheng Chun-Yu Lin1,2
Chun-Yu Lin1,2 Shu-Min Lin
Shu-Min Lin