- 1Department of Biochemistry and Pharmacology, Uzhhorod National University, Uzhhorod, Ukraine
- 2Department of Microbiology, Virology, and Immunology, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
Leptospirosis is an emerging illness presenting a broad range of clinical manifestations, ranging from asymptomatic or mild cases to severe and fatal outcomes. Early detection is crucial for effective treatment; however, similar clinical presentations in various febrile illnesses or co-infections, along with challenges in laboratory diagnostics, can lead to misdiagnosis and severe consequences. Identifying clinical predictors for severe forms of the disease is essential in mitigating complications and reducing mortality. Consequently, we conducted a retrospective case-control study to identify clinical markers indicative of severe disease in leptospirosis patients from the Transcarpathian region. The study focused on patients admitted with clinically suspected leptospirosis, involving a total of 51 diagnosed cases, with 13 resulting in severe outcomes and death. Categorical variables were analyzed using χ2, revealing a mean patient age of 50 years, predominantly male (n = 36, 70.5%). Oliguria emerged as a significant independent factor associated with mortality (odds ratio [OR], 13.5; 95% confidence interval [CI], 2.56–71.12; p = 0.001). Additionally, our analysis uncovered a noteworthy increase in leptospirosis notification rates in Transcarpathian compared to Ukraine, with 150 cases out of the total 433 in Ukraine. The highest notification rates were observed in Mukachevo District and Perechyn District. These findings highlight the importance of early recognition of key clinical markers, such as oliguria, which are critical for predicting severe outcomes in leptospirosis patients. The higher notification rates in Transcarpathian regions also underscore the need for enhanced surveillance, targeted public health interventions, and timely treatment to reduce mortality in endemic areas.
1 Introduction
Leptospirosis represents a re-emerging zoonotic disease with a global presence, spanning all continents except Antarctica (Ullmann and Langoni, 2011; Petakh and Nykyforuk, 2022; Petakh et al., 2022a; Petakh and Kamyshnyi, 2023). Its clinical manifestations range from mild to severe, posing life-threatening consequences. Symptoms often mimic those of other infectious diseases, such as influenza (Philip et al., 2021). Severe cases, affecting approximately 5% to 15% of individuals, manifest with acute renal failure, acute respiratory distress syndrome, pulmonary complications, hypotension, icterus, and altered mental status (Panaphut et al., 2002; Doudier et al., 2006; Spichler et al., 2008; Petakh et al., 2022c; Petakh et al., 2022b). A 2015 systematic review estimated that around one million cases of leptospirosis occur globally each year, resulting in approximately 60,000 deaths (Costa et al., 2015). Between 2010 and 2021, 23 EU countries reported 12,180 confirmed cases of leptospirosis, with an average annual rate of 0.24 per 100,000 population. Five countries—France, Germany, the Netherlands, Portugal, and Romania—accounted for 79% of cases. Slovenia had the highest rate at 0.82 per 100,000 (Beauté et al., 2024). In Ukraine, 4,873 cases were reported from 2009 to 2023, with an average annual rate of 0.75 per 100,000, among the highest in Europe (Petakh et al., 2024a; Petakh et al., 2024b).
In the Transcarpathian region, leptospirosis persists as a prevalent zoonotic disease. Between 2005 and 2015, 420 cases were reported, with an incidence three times higher than the national average. The case fatality rate (CFR) for leptospirosis in Transcarpathian region averages 12.5%, exceeding the national level of 9.8% (Markovych et al., 2019; Petakh et al., 2023b; Petakh et al., 2023a).
Geographical variations worldwide contribute to distinct presentations of leptospirosis. Divergent Leptospira species and serovars, coupled with socioeconomic and environmental factors, play pivotal roles in shaping the clinical presentation of the disease (Spichler et al., 2008). Proposed variations in intrinsic virulence among serovars and species may explain differences in disease severity (Philip et al., 2021).
The spectrum of leptospirosis, ranging from mild and subclinical to severe or fatal illness, underscores the importance of vigilant patient monitoring. Identifying prognostic markers is crucial for continuous surveillance, yet the factors predicting the transition from mild to severe/fatal illness remain unclear (Dupont et al., 1997; Spichler et al., 2008).
Despite extensive research on leptospirosis, there remains a significant gap in identifying specific clinical and epidemiological predictors that signal the progression from mild to severe forms of the disease. While geographic variations and differences in Leptospira species have been studied, the factors driving severe outcomes in leptospirosis—particularly in high-incidence regions like Transcarpathia—are still poorly understood. This study aims to address this gap by examining potential predictors of severe illness, which could improve early diagnosis and intervention, thereby reducing the associated morbidity and mortality.
2 Materials and methods
A retrospective case-control study was conducted at the Transcarpathian Regional Clinical Infectious Diseases Hospital, involving the review of 51 medical records of patients admitted between 2012 and 2018. The study aimed to investigate factors associated with severe outcomes of leptospirosis. To avoid selection bias, only patients meeting the laboratory-confirmed diagnostic criteria for leptospirosis were included. These criteria involved positive PCR or Microagglutination Testing (MAT), in conjunction with clinical symptoms indicative of the disease. Patients without laboratory confirmation were excluded to ensure diagnostic consistency across the sample.
A standardized questionnaire was employed to collect data on patient demographics (age and gender) and clinical symptoms, including fever (≥38 °C), myalgia, jaundice, arthralgia, oliguria, nausea, vomiting, headache, and abdominal pain. Oliguria was defined as urine production of less than 400 ml per day. The age range of the included patients was not limited, allowing for a comprehensive assessment of the disease across different age groups.
In Ukraine, leptospirosis is a notifiable disease as mandated by the Ministry of Health order dated July 30, 2020, No. 1726. When a case is identified, it must be reported to local epidemiological departments within two hours via telephone. An emergency notification is then prepared, with a paper copy delivered within 18 hours, following the Ministry of Health order dated July 30, 2020 (No. 1726). Medical professionals and other healthcare workers who receive health-related information are responsible for completing these notifications.
Medical staff gather comprehensive patient histories, including potential exposure to contaminated water, animals, or recent travel. According to the Ministry of Health order No. 905 of December 28, 2015, leptospirosis diagnosis criteria include fever or at least two of the following symptoms: chills, headache, muscle pain, conjunctival redness, skin or mucous membrane hemorrhages, rash, jaundice, myocarditis, meningitis, kidney failure, or respiratory complications such as hemoptysis. However, clinical and epidemiological data alone are insufficient for confirmation, necessitating PCR or MAT.
Blood samples from suspected cases are analyzed using the MAT test at the Especially Dangerous Infections (EDI) laboratories. Initially, samples are tested at dilutions of 1:5 and 1:50. If positive, further dilutions (1:10, 1:100, 1:200, etc.) are performed according to the Methodological Recommendations 9.1.1.098-02 for Anti-Epidemic Measures and Laboratory Diagnostics of Leptospirosis (Goris and Hartskeerl, 2015). Control culture diluted 1:2 in phosphate-buffered saline is used to validate results, defining the endpoint as the serum dilution with 50% agglutination. Results are reported as endpoint dilution or titer, with antibody titers of ≥1:100 plus clinical and epidemiological data indicating infection. A fourfold increase in antibody titers between paired samples strongly suggests acute infection. MAT testing involves 14 Leptospira spp. serovars per local protocols (Methodological Recommendations 9.1.1.098-02, 2002). For patients who died with clinical leptospirosis, PCR analysis of kidney tissue sections using lipL32 gene primers confirms the disease (Ahmed et al., 2020; Jayasundara et al., 2021).
Statistical analysis was performed using Jamovi version 2.2.5. Categorical variables were compared using χ² (Chi-square) tests to assess the association between various clinical characteristics and severe outcomes of leptospirosis. For each significant association identified, adjusted odds ratios (OR) were calculated to measure the strength of association between potential predictors and severe disease, along with their corresponding 95% confidence intervals (CI) to provide a range of uncertainty around the OR estimates. A Mann-Whitney U test was performed to compare the urea and serum creatinine levels between survivors and non-survivors of leptospirosis. Significance for all statistical tests was established at P < 0.05. Ethical approval for the study was granted by the Ethics Committee of Transcarpathian Regional Clinical Infectious Hospital.
3 Results
3.1 Leptospiral clinical signs as predictors of severe leptospirosis
Out of the 51 patients involved in the study, 38 were categorized in the survivors’ group, while 13 were part of the non-survivors’ group. The majority were males, accounting for 70.5% of the cases, and the average age was 50 years (Figures 1A, B).
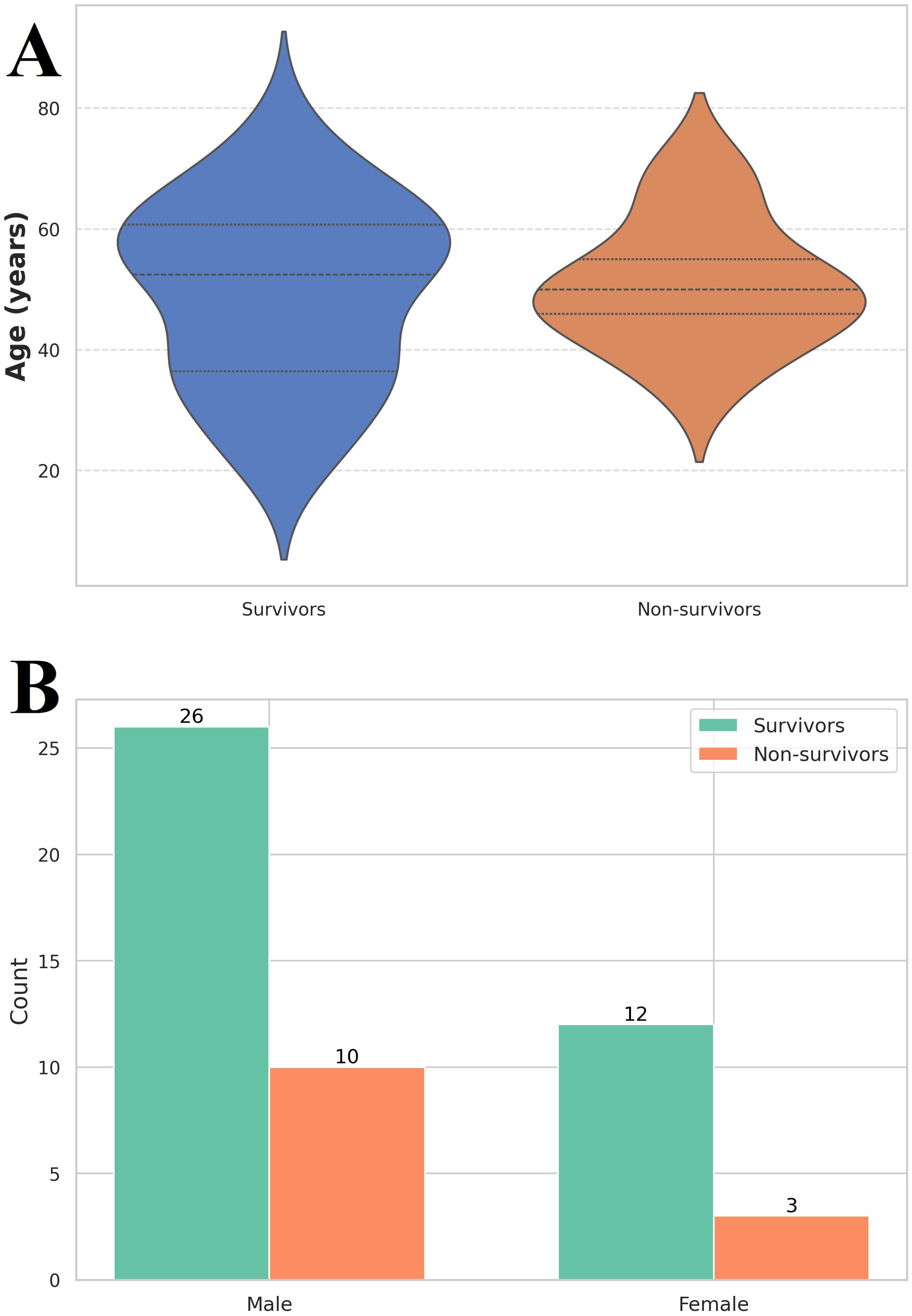
Figure 1. (A) Violin plot of age distribution among the investigated group. Survivors (n = 38) had a median age of 49 (36 – 61) years, while non-survivors (n = 13) had a median age of 50 (44 – 58) years. Label Y axe Age, years (B) Gender distribution among the investigated group. The vast majority of the 38 out of 51 patients were male (26 in the survivor’s group and 10 in the non-survivor’s group).
Upon admission to the hospital, the primary signs and symptoms observed in both groups were fever (78.4%), myalgia (76.4%), and jaundice (86.2%).
All individuals with severe leptospirosis (n = 13, 100%) reported experiencing jaundice, and 11 patients exhibited oliguria (84.6%). Myalgia symptoms were complained of by 10 patients (76.9%), and 8 patients presented with fever (61.5%). Nausea was reported by 5 patients (38.4%), and abdominal symptoms were noted by 2 patients (15.3%) (Figure 2).

Figure 2. Analysis of clinical signs in patients with leptospirosis (butterfly bar plot). On the left (depicted in red), the frequencies of clinical symptoms are shown for the survivor’s group, while on the right (depicted in orange), the frequencies are shown for the non-survivor’s group. Oliguria was observed in 84.6% of patients with severe leptospirosis who died, compared to 28.9% in the survivor’s group.
A chi-square test revealed a significant association between oliguria and severe outcomes, including mortality (p = 0.001). Oliguria was identified as the strongest predictor of severe leptospirosis, with an estimated odds ratio of 13.5, indicating a substantial risk increase for severe disease.
In our study, we also included an analysis of serum creatinine and urea levels upon admission. The analysis revealed significant differences in urea levels between the two groups. The median urea level among survivors was 10.2 mmol/L (interquartile range [IQR]: 7.4 - 15.7 mmol/L), whereas non-survivors exhibited a markedly elevated median level of 41.4 mmol/L (IQR: 34.0 - 55.0 mmol/L). Similarly, serum creatinine levels showed significant variation between the groups. Survivors had a median serum creatinine level of 112.3 µmol/L (IQR: 99.0 - 227.0 µmol/L), while non-survivors presented with a significantly higher median level of 510.0 µmol/L (IQR: 371.0 - 743.0 µmol/L). The analysis yielded a p-value of p < 0.001 (Figure 3A).
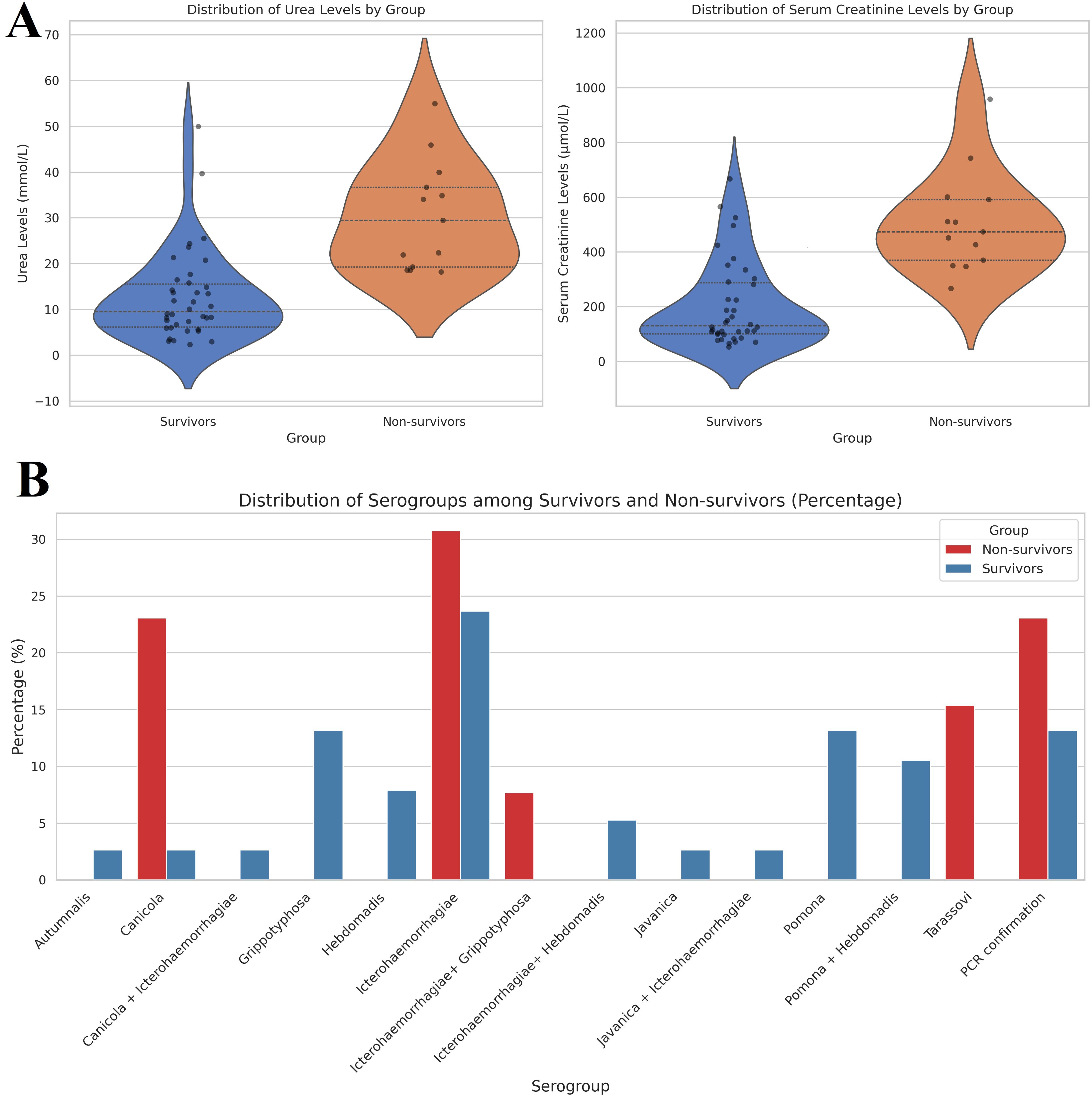
Figure 3. (A) Violin plot of distribution of serum creatine and urea levels among investigated groups. The median urea level among survivors was 10.2 mmol/L (interquartile range [IQR]: 7.4 - 15.7 mmol/L), whereas non-survivors exhibited a markedly elevated median level of 41.4 mmol/L (IQR: 34.0 - 55.0 mmol/L). Similarly, serum creatinine levels showed significant variation between the groups. Survivors had a median serum creatinine level of 112.3 µmol/L (IQR: 99.0 - 227.0 µmol/L), while non-survivors presented with a significantly higher median level of 510.0 µmol/L (IQR: 371.0 - 743.0 µmol/L). (B) Distribution of serogroups among investigated groups. Among non-survivors, Icterohaemorrhagiae was the most prevalent serogroup, accounting for 30.77%, followed by Canicola at 23.08% and Tarassovi at 15.38%. In contrast, survivors exhibited a higher prevalence of Grippotyphosa and Pomona, both at 13.16%. The analysis revealed that Icterohaemorrhagiae was significantly present in both groups, but it had a higher percentage in non-survivors (30.77%) compared to survivors (23.68%).
3.2 Distribution of Leptospira serogroups among survivors and non-survivors
The distribution of different serogroups was quantified as percentages for both survivors and non-survivors, as summarized in Figure 3B. Among non-survivors, Icterohaemorrhagiae (30.77%) was the most prevalent serogroup, followed by Canicola (23.08%) and Tarassovi (15.38%). In contrast, survivors showed a higher prevalence of Grippotyphosa (13.16%) and Pomona (13.16%). The analysis revealed that Icterohaemorrhagiae was significantly present in both groups but had a higher percentage in non-survivors (30.77%) compared to survivors (23.68%). Additionally, the presence of serogroups such as Grippotyphosa and Pomona was primarily observed in survivors, indicating a potential correlation between these serogroups and better clinical outcomes.
The results indicated a statistically significant difference in the distribution of serogroups between survivors and non-survivors, with a chi-square statistic of 22.60, a p-value of 0.0468, and 13 degrees of freedom. These findings suggest that the serogroups present in patients have an influence on disease severity and mortality.
3.3 Regional features of leptospirosis epidemiology
As depicted in Figure 2, the relative number of patients in Transcarpathian region has consistently been higher. For the first time in 2023, it reached 12.08 per 100,000 population, surpassing the Ukrainian average by 12 times (Figure 4). We also analyzed the incidence of leptospirosis by district and found that the highest incidence was observed, in all years in Mukachevo District at 5.58 cases per 100,000 persons. This rate is four times higher than the Transcarpathian average. Additionally, Perechyn District had an incidence rate of 7.8 cases per 100,000 persons.
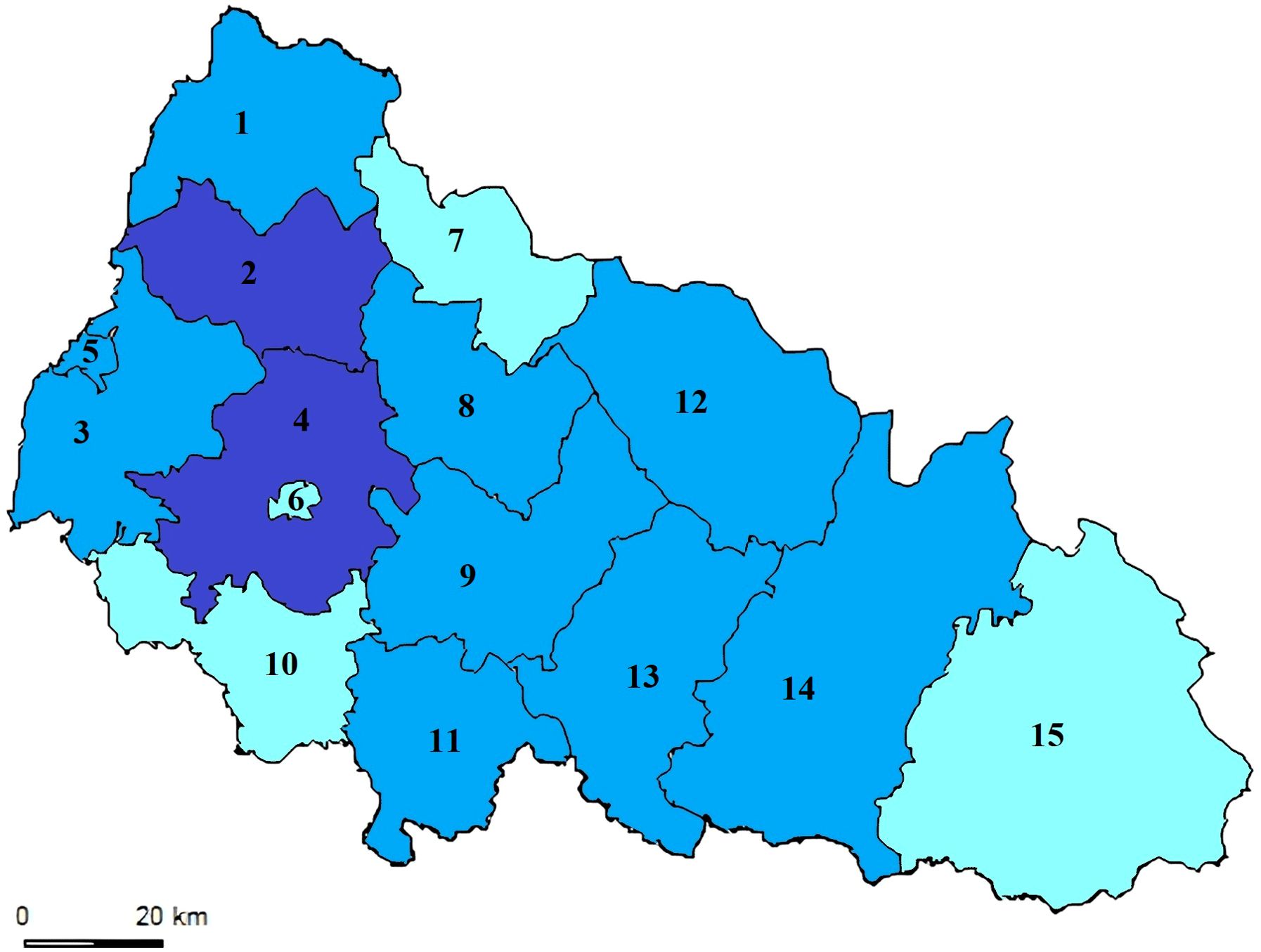
Figure 4. Leptospirosis Notification Rate (per 100K) in the Transcarpathian Region (2015-2022). The map illustrates the levels of leptospirosis incidence depicted in three color gradations. The blue districts indicate low notification rates (less than 0.72), which fall below the 25th percentile. Light blue represents districts with moderate notification rates, ranging between the 25th and 75th percentiles (specifically between 0.72 and 2.62). Dark blue signifies rates above 2.62, surpassing the 75th percentile. Districts and cities of the Transcarpathian region: 1. Velykobereznyansky District; 2. Perechyn District; 3. Uzhhorod District; 4. Mukachevo District; 5. Uzhhorod City; 6. Mukachevo City; 7. Volovetsky District; 8. Svalyavsky District; 9. Irshav District; 10. Berehiv District; 11. Vynogradivskyi District; 12. Mizhhirsky District; 13. Khust District; 14. Tyachiv District; 15. Rakhiv District.
4 Discussion
This study aimed to identify predictive factors for lethality and severe leptospirosis in the Transcarpathian region.
Our analysis reveals a significant increase in the notification rate of leptospirosis in Ukraine in 2023. This surge is primarily attributed to the rising incidence of leptospirosis in Transcarpathia, accounting for 150 cases out of the total 433 in Ukraine, and the Ivano-Frankivsk region, contributing 34 cases.1
Zakarpattia oblast (also known as Transcarpathian region), located on the western border of Ukraine, shares boundaries with Romania, Hungary, Poland, and Slovakia, while also bordering Ivano-Frankivsk and L’viv oblasts to the east. In comparison, the rates for Transcarpathian region are significantly higher than those in neighboring countries, with Romania at 0.34, Slovakia at 0.15, Hungary at 0.13, and Poland at 0.01 per 100,000 population (Beauté et al., 2024). Characterized by a moderate continental climate with ample moisture, and moderate summers and winters, Zakarpattia presents geographical, landscape, zoological, and parasitological conditions influencing various natural focal diseases, including leptospirosis. The region has above-average human population density, with 63% residing in rural areas. High leptospirosis incidence is often reported in areas with abundant surface freshwater. The dense river system in Zakarpattia, influenced by high humidity and mountain relief, experiences increased water levels, spring flooding, and occasional disasters, as witnessed in 2010 (Wasiński and Dutkiewicz, 2013).
Comparing the notification rate with EU countries, Ukraine’s leptospirosis incidence surpasses that of all EU nations. France and Slovenia reported the highest rates at 0.76 and 0.82, respectively (Beauté et al., 2024).
The findings suggest that oliguria serves as a risk factor for severe leptospirosis. Recognizing this sign upon admission allows for early identification and the management of diuresis can alert physicians to the potential development of severe leptospirosis, enabling prompt initiation of aggressive treatment. The kidneys are a primary target of Leptospira, with kidney damage observed in 20–85% of patients (Meneses et al., 2020). Renal failure, particularly in cases with oliguria, stands as a well-established predictor of death and is linked to more frequent pulmonary involvement (Bharti et al., 2003; Silva Junior et al., 2011).
Leptospirosis is frequently linked to acute kidney injury (AKI), characterized by acute interstitial nephritis and acute tubular necrosis (Chou et al., 2023). The clinical presentation of AKI in leptospirosis is notably marked by non-oliguric and hypokalemic conditions, observed in 41–45% of patients experiencing AKI related to leptospirosis (Seguro et al., 1990; Lin et al., 1999). Conversely, disseminated intravascular coagulation has been noted in patients with severe leptospirosis complications, with septic AKI often presenting oliguria and anuria, likely resulting from acute tubular necrosis (Chierakul et al., 2008). Several factors contribute to the pathogenesis of AKI in leptospirosis, including the direct nephrotoxic effects of Leptospira, immune responses induced by toxins, and secondary effects from dehydration and hypoxia (Rista et al., 2022).
The acute phase of leptospiral infection typically lasts about one week, followed by the development of symptoms five to fourteen days later, leading to an immunological phase (Brito Monteiro et al., 2021). Severe cases can trigger a cytokine storm, characterized by elevated levels of TNF-α, IL-6, and IL-10 in the bloodstream (Haake and Levett, 2015). A study conducted in Sri Lanka in 2021 indicated increased levels of monocyte chemoattractant protein (MCP)-1 and kidney injury molecule (KIM)-1 in the blood and urine of patients with AKI linked to leptospirosis, with KIM-1 showing greater specificity than MCP-1 (Nisansala et al., 2021).
In a mouse model of leptospiral infection during the acute phase, TNF-α, IL-6, IL-10, MCP-1, and KIM-1 exhibited high expression levels. Furthermore, notable increases in renal lipocalin 2 (LCN2, also known as neutrophil gelatinase-associated lipocalin [NGAL]) and IL-34 expression levels have been documented (Chou et al., 2018). Previous investigations have established a correlation between urinary NGAL levels and leptospirosis-associated AKI (Srisawat et al., 2015).
The data imply that risk stratification for leptospirosis patients should not solely rely on the presence of icterus. Jaundice results from damage to hepatic capillary vessels without hepatocellular necrosis. Retrospective studies both confirm (Durmaz Cetin et al., 2004; Daher et al., 2009), and contradict (Spichler et al., 2008; Daher et al., 2015) the role of jaundice as a predictor of death in this infectious disease.
Myalgia, commonly affecting the back and legs, is a frequent symptom of leptospirosis (Vijayachari et al., 2008). Most studies indicate that myalgia is not linked to mortality in cases of leptospirosis (Al-shere et al., 2012; Daher et al., 2015). Histologically, there is evidence of focal necrosis of muscle fibers, often accompanied by a modest elevation in creatinine kinase (CK) (Lim, 2011). A study conducted by Dupont et al. in France reported higher CK levels in patients with leptospirosis who did not survive (Dupont et al., 1997).
Headache is a frequently observed symptom in cases of leptospirosis, often characterized by its severity and association with vomiting (Bharti et al., 2003). While impaired consciousness in the initial stages may progress to meningitic symptoms in approximately a quarter of cases during the immunological phase, it is noteworthy that headache was not a prevalent presentation in this particular series.
To improve the generalizability of these findings, future studies should incorporate a broader range of geographical regions with varying epidemiological characteristics of leptospirosis. Conducting multicentric prospective studies, ideally with a larger sample size, could better account for the role of environmental and socioeconomic factors in disease progression. In addition, future research should aim to control for potential confounding variables, such as pre-existing conditions, immunocompromised status, and co-infections, to provide more precise insights into clinical predictors of severe leptospirosis.
Biomarkers such as kidney injury molecule-1 (KIM-1) and neutrophil gelatinase-associated lipocalin (NGAL), which have shown promise in other studies, could also be incorporated into future research to enhance the prediction of severe outcomes. Studies focused on the immunological mechanisms, particularly the cytokine storm phenomenon, may help identify early intervention strategies to prevent the progression to severe or fatal illness.
5 Limitations
First, the retrospective design relies on existing medical records, which can lead to potential biases due to incomplete or inconsistent data. In particular, the diagnosis of leptospirosis was based on clinical presentation and laboratory confirmation using MAT or PCR, but co-infections or other underlying health conditions that could have influenced the severity of the disease were not systematically recorded. For example, common co-infections such as dengue or malaria, particularly in regions with similar environmental conditions, may complicate the clinical picture and affect patient outcomes.
Additionally, while this study focused on the Transcarpathian region, external factors such as regional variations in healthcare quality, availability of early intervention, and environmental conditions unique to the region could limit the generalizability of our findings. The study region has high humidity, frequent flooding, and proximity to natural reservoirs, factors that may contribute to a higher incidence and severity of leptospirosis compared to other regions. Thus, further research is necessary to explore whether the identified clinical predictors, such as oliguria, hold similar significance in lower-incidence areas with different environmental and healthcare contexts.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Uzhhorod National University (protocol code 5/1 on 30 May 2024). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
PP: Conceptualization, Data curation, Investigation, Methodology, Visualization, Writing – original draft. OK: Formal analysis, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
- ^ Available online at: https://phc.org.ua/kontrol-zakhvoryuvan/inshi-infekciynizakhvoryuvannya/infekciyna-zakhvoryuvanist-naselennya-ukraini. (Accessed June 23, 2024).
References
Ahmed, A. A., Goris, M. G. A., Meijer, M. C. (2020). Development of lipL32 real-time PCR combined with an internal and extraction control for pathogenic Leptospira detection. PloS One 15, e0241584. doi: 10.1371/journal.pone.0241584
Al-shere, T. A., Ujiie, M., Suzuki, M., Salva, E., Belo, M. C. P., Koizumi, N., et al. (2012). Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg. Infect. Dis. 18, 91. doi: 10.3201/eid1801.101892
Beauté, J., Innocenti, F., Aristodimou, A., Špačková, M., Eves, C., Kerbo, N., et al. (2024). Epidemiology of reported cases of leptospirosis in the EU/EEA, 2010 to 2021. Euro. Surveill. 29, 2300266. doi: 10.2807/1560-7917.ES.2024.29.7.2300266
Bharti, A. R., Nally, J. E., Ricaldi, J. N., Matthias, M. A., Diaz, M. M., Lovett, M. A., et al. (2003). Leptospirosis: a zoonotic disease of global importance. J. Lancet Infect. Dis. 3, 757–771. doi: 10.1016/S1473-3099(03)00830-2
Brito Monteiro, M., Egídio de Sousa, I., Piteira, M., Coelho, S., Freitas, P. (2021). Leptospirosis, a re-emerging threat. Cureus 13, e14295. doi: 10.7759/cureus.14295
Chierakul, W., Tientadakul, P., Suputtamongkol, Y., Wuthiekanun, V., Phimda, K., Limpaiboon, R., et al. (2008). Activation of the coagulation cascade in patients with leptospirosis. Clin. Infect. Dis. 46, 254–260. doi: 10.1086/524664
Chou, L. F., Chen, T. W., Yang, H. Y., Chang, M. Y., Hsu, S. H., Tsai, C. Y., et al. (2018). Murine renal transcriptome profiles upon leptospiral infection: implications for chronic kidney diseases. J. Infect. diseases. 218, 1411–1423. doi: 10.1093/infdis/jiy339
Chou, L. F., Yang, H. Y., Hung, C. C., Tian, Y. C., Hsu, S. H., Yang, C. W. (2023). Leptospirosis kidney disease: Evolution from acute to chronic kidney disease. Biomed. J. 46, 100595. doi: 10.1016/j.bj.2023.100595
Costa, F., Hagan, J. E., Calcagno, J., Kane, M., Torgerson, P., Martinez-Silveira, M. S., et al. (2015). Global morbidity and mortality of leptospirosis: a systematic review. PloS Negl. Trop. diseases. 9, e0003898. doi: 10.1371/journal.pntd.0003898
Daher, E. F., Silva, G. B., Jr., Karbage, N. N., Carvalho, P. C., Jr., Kataoka, R. S., Silva, E. C., et al. (2009). Predictors of oliguric acute kidney injury in leptospirosis. Nephron Clin. Practice. 112, 25–30. doi: 10.1159/000210571
Daher, E. D. F., Soares, D. S., de Menezes Fernandes, A. T. B., Girão, M. M. V., Sidrim, P. R., Pereira, E. D. B., et al. (2015). Risk factors for intensive care unit admission in patients with severe leptospirosis: a comparative study according to patients’ severity. BMC Infect. diseases. 16, 1–7. doi: 10.1186/s12879-016-1349-x
Doudier, B., Garcia, S., Quennee, V., Jarno, P., Brouqui, P. J. C. M. (2006). “Infection,” in Prognostic factors associated with severe leptospirosis, vol. p. (Wiley Online Library), 299–300.
Dupont, H., Dupont-Perdrizet, D., Perie, J. L., Zehner-Hansen, S., Jarrige, B., Daijardin, J. B. (1997). Leptospirosis: prognostic factors associated with mortality. Clin. Infect. Diseases. 25, 720–724. doi: 10.1086/cid.1997.25.issue-3
Durmaz Cetin, B., Harmankaya, O., Hasman, H., Gunduz, A., Oktar, M., Seber, E. (2004). Acute renal failure: a common manifestation of leptospirosis. Renal failure. 26, 655–661. doi: 10.1081/JDI-200037154
Goris, M., Hartskeerl, R. (2015). Letter to the editor: Trends in human leptospirosis in Denmark, 1980 to 2012. Eurosurveillance 20, 21054. doi: 10.2807/1560-7917.ES2015.20.9.21053
Haake, D. A., Levett, P. N. (2015). Leptospirosis in humans. J. Leptospira leptospirosis 387, 65–97. doi: 10.1007/978-3-662-45059-8_5
Jayasundara, D., Gamage, C., Senavirathna, I., Warnasekara, J., Matthias, M. A., Vinetz, J. M., et al. (2021). Optimizing the microscopic agglutination test (MAT) panel for the diagnosis of Leptospirosis in a low resource, hyper-endemic setting with varied microgeographic variation in reactivity. PloS Negl. Trop. Dis. 15, e0009565. doi: 10.1371/journal.pntd.0009565
Lin, C. L., Wu, M. S., Yang, C. W., Huang, C. C. (1999). Leptospirosis associated with hypokalaemia and thick ascending limb dysfunction. Nephrology dialysis Transplant. 14, 193–195. doi: 10.1093/ndt/14.1.193
Markovych, O., Tymchyk, V., Kolesnikova, I. (2019). Leptospirosis in zakarpattia oblast (2005–2015). Vector-Borne Zoonotic Diseases. 19, 333–340. doi: 10.1089/vbz.2018.2356
Methodological Recommendations 9.1.1.098-02. (2002). Anti-Epidemic Measures and Laboratory Diagnostics of Leptospirosis approved by the Decree of the Chief State Sanitary Doctor of Ukraine No. 39 of 11 December 2002. (Kyiv: Ministry of Health of Ukraine) 43–49.
Meneses, G. C., Silva Junior, G., Tôrres, P. P. B. F., Castro, V., Lopes, R. L., Martins, A. M. C., et al. (2020). Novel kidney injury biomarkers in tropical infections: a review of the literature. Rev. Inst. Med. Trop. Sao Paulo 62. doi: 10.1590/s1678-9946202062014
Nisansala, T., Weerasekera, M., Ranasinghe, N., Marasinghe, C., Gamage, C., Fernando, N., et al. (2021). Importance of KIM-1 and MCP-1 in determining the leptospirosis-associated AKI: A Sri Lankan study. BioMed. Res. Int. 2021, 1752904. doi: 10.1155/2021/1752904
Panaphut, T., Domrongkitchaiporn, S., Thinkamrop, B. (2002). Prognostic factors of death in leptospirosis: a prospective cohort study in Khon Kaen, Thailand. Int. J. Infect. Diseases. 6, 52–59. doi: 10.1016/S1201-9712(02)90137-2
Petakh, P., Isevych, V., Griga, V., Kamyshnyi, A. (2022a). The risk factors of severe leptospirosis in the transcarpathian region of Ukraine–search for „Red flags”. Arch. Balk. Med. 57, 231–237. doi: 10.31688/ABMU.2022.57.3.02
Petakh, P., Isevych, V., Kamyshnyi, A., Oksenych, V. (2022b). Weil’s disease-immunopathogenesis, multiple organ failure, and potential role of gut microbiota. Biomolecules 12. doi: 10.3390/biom12121830
Petakh, P., Isevych, V., Mohammed, I. B., Nykyforuk, A., Rostoka, L. (2022c). Leptospirosis: prognostic model for patient mortality in the transcarpathian region, Ukraine. Vector borne zoonotic Dis. (Larchmont NY). 22, 584–588. doi: 10.1089/vbz.2022.0063
Petakh, P., Kamyshnyi, A. (2023). Risks of outbreaks: The health concerns of internally displaced persons in Transcarpathia, Ukraine. New Microbes New infections. 52, 101106. doi: 10.1016/j.nmni.2023.101106
Petakh, P., Kamyshnyi, A., Tymchyk, V., Armitage, R. (2023a). Infectious diseases during the Russian-Ukrainian war - Morbidity in the Transcarpathian region as a marker of epidemic danger on the EU border. Public Health Practice. 6, 100397. doi: 10.1016/j.puhip.2023.100397
Petakh, P., Nykyforuk, A. (2022). Predictors of lethality in severe leptospirosis in Transcarpathian region of Ukraine. Le infezioni medicina. 30, 272–276. doi: 10.53854/liim-3002-13
Petakh, P., Rostoka, L., Isevych, V., Kamyshnyi, A. (2023b). Identifying risk factors and disease severity in leptospirosis: A meta-analysis of clinical predictors. Trop. doctor. 53, 464–469. doi: 10.1177/00494755231187673
Petakh, P., Tymchyk, V., Kamyshnyi, O. (2024a). Surveillance of human leptospirosis infections in Ukraine between 2018 and 2023. Front. Public Health 12, 1394781. doi: 10.3389/fpubh.2024.1394781
Petakh, P., Tymchyk, V., Kamyshnyi, O. (2024b). Communicable diseases in Ukraine during the period of 2018-2023: Impact of the COVID-19 pandemic and war. Travel Med. Infect. Dis. 102733. doi: 10.1016/j.tmaid.2024.102733
Philip, N., Lung Than, L. T., Shah, A. M., Yuhana, M. Y., Sekawi, Z., Neela, V. K. (2021). Predictors of severe leptospirosis: a multicentre observational study from Central Malaysia. J. BMC Infect. Diseases. 21, 1–6. doi: 10.1186/s12879-021-06766-5
Rista, E., Puca, E., Cadri, V., Saliaj, K., Akshija, I., Duraku, A., et al. (2022). Acute kidney injury in leptospirosis: A country-level report. Travel Med. Infect. disease. 49, 102359. doi: 10.1016/j.tmaid.2022.102359
Seguro, A. C., Lomar, A. V., Rocha, A. S. (1990). Acute renal failure of leptospirosis: nonoliguric and hypokalemic forms. Nephron 55, 146–151. doi: 10.1159/000185943
Silva Junior, G. B., Abreu, K. L. S., Mota, R. M., Barreto, A. G., Araujo, S. M., Rocha, H. A., et al. (2011). RIFLE and Acute Kidney Injury Network classifications predict mortality in leptospirosis-associated acute kidney injury. J. Nephrology. 16, 269–276. doi: 10.1111/j.1440-1797.2010.01391.x
Spichler, A. S., Vilaça, P. J., Athanazio, D. A., Albuquerque, J. O., Buzzar, M., Castro, B., et al. (2008). Predictors of lethality in severe leptospirosis in urban Brazil. Am. J. Trop. Med. hygiene. 79, 911. doi: 10.4269/ajtmh.2008.79.911
Srisawat, N., Praditpornsilpa, K., Patarakul, K., Techapornrung, M., Daraswang, T., Sukmark, T., et al. (2015). Neutrophil gelatinase associated lipocalin (NGAL) in leptospirosis acute kidney injury: A multicenter study in Thailand. PloS One 10, e0143367. doi: 10.1371/journal.pone.0143367
Ullmann, L., Langoni, H. (2011). Interactions between environment, wild animals and human leptospirosis. J. Venomous Anim. Toxins including Trop. Diseases. 17, 119–129. doi: 10.1590/S1678-91992011000200002
Vijayachari, P., Sugunan, A., Shriram, A. (2008). Leptospirosis: an emerging global public health problem. J. J. biosciences. 33, 557–569. doi: 10.1007/s12038-008-0074-z
Keywords: mortality association, oliguria, acute kidney injury, leptospirosis, predictor
Citation: Petakh P and Kamyshnyi O (2024) Oliguria as a diagnostic marker of severe leptospirosis: a study from the Transcarpathian region of Ukraine. Front. Cell. Infect. Microbiol. 14:1467915. doi: 10.3389/fcimb.2024.1467915
Received: 21 July 2024; Accepted: 11 October 2024;
Published: 31 October 2024.
Edited by:
Rahul Kumar Maurya, Washington University in St. Louis, United StatesReviewed by:
Nazan Tuna, Namik Kemal University, TürkiyeSuman Bharti, Washington University in St. Louis, United States
Copyright © 2024 Petakh and Kamyshnyi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pavlo Petakh, cGF2bG8ucGV0YWtoQHV6aG51LmVkdS51YQ==; Oleksandr Kamyshnyi, YWxleGthbXlzaG55aUBnbWFpbC5jb20=
 Pavlo Petakh
Pavlo Petakh Oleksandr Kamyshnyi
Oleksandr Kamyshnyi