- 1The Institute of Brain Science and Brain-inspired Research, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, China
- 2College of Environmental and Life Sciences, Murdoch University, Perth, WA, Australia
- 3College of Life Sciences, Qingdao Agricultural University, Qingdao, China
- 4State Key Laboratory of Animal Nutrition, Institute of Animal Sciences, Chinese Academy of Agricultural Sciences, Beijing, China
Background: Ammonia (NH3) and hydrogen sulfide (H2S) are produced during digestion in the human gut, yet the impact of these internally generated gases on male reproduction have received limited attention in scientific research.
Methods: We systematically reviewed 935 scientific publications, spanning from 1947 to 2023, focusing on external or internal NH3 and/or H2S, male infertility, and gut microbiota. Meta-analysis was conducted to evaluate the summary relative risk (RR) and 95% confidence intervals (CIs) of combined studies.
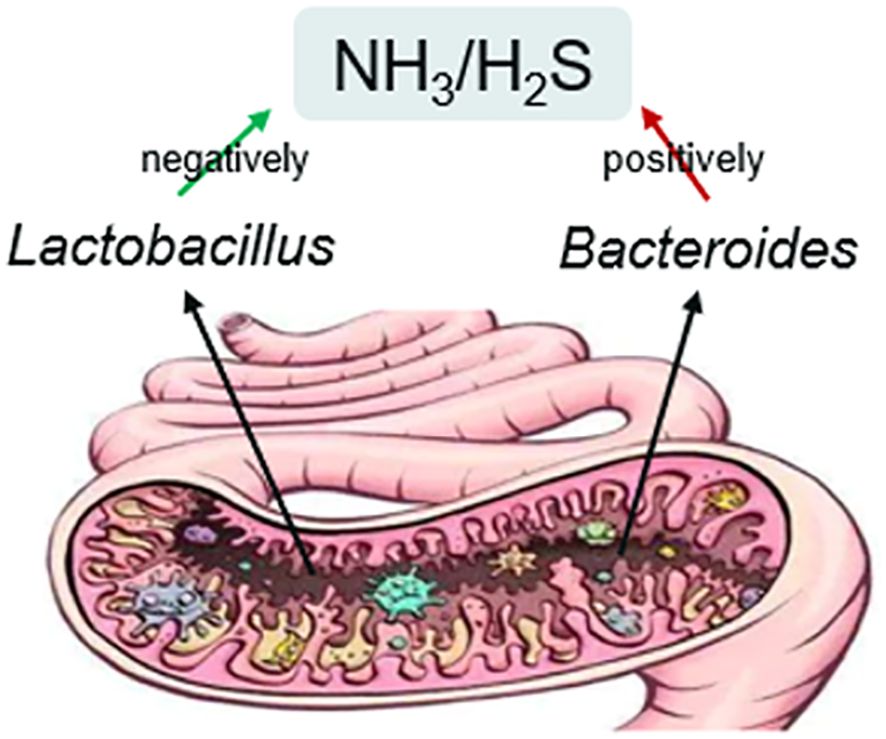
Results: Our findings revealed that the internal NH3 and/or H2S were negatively related to the Lactobacillus, which is beneficial to male fertility, whereas NH3 and H2S were positively related to Bacteroides, which showed negative effects on male fertility. The meta-analysis comparing Lactobacillus and Bacteroides levels with NH3 showed statistically significant results (p<0.001).
Conclusions: The meta-analysis is the first to confirm these facts and explored the potential existence of a gut microbiota-inner gases-male fertility axis in the human gut.
1 Introduction
Exposure to gaseous NH3 and H2S has detrimental effects on human health (Jankowski et al., 2014). These gases are emitted by various sources, including industry processes (Norizan et al., 2022), landfills (Jiang et al., 2021), livestock operations (Barrasa et al., 2012; Kim et al., 2008; Liu et al., 2014; Lu et al., 2020; Song et al., 2013; Wu et al., 2020; Zicari et al., 2013), and agriculture activities (Geiser et al., 2008; Li et al., 2023). NH3 and/or H2S not only adversely affect humans and livestock (Zicari et al., 2013) but also wild ecosystems (Dordevic´ et al., 2021; Geiser et al., 2008). As inorganic solutes, these gases exert multiple negative influences on the blood, breath, stools, and the gastrointestinal tract, including protein binding (Jankowski et al., 2014). Hydrogen sulfide (H2S) can cause eye damage even after brief exposure to low concentration (Lambert et al., 2006). Moreover, exposure to NH3 and H2S diminishes male reproductive ability by reducing sperm motility through AMPK/AKT-related pathways, and this damage may be heritable (Zhao et al., 2016; Zhang et al., 2018). Research has shown that external NH3 and H2S disrupt sperm parameters (e.g., sperm motility, sperm concentration) and the expression of spermatogenetic proteins via the energy metabolic pathway.
Apart from external sources, NH3 and H2S are also produced within the body. The generation of these gases in the body is related to the gut microbiota responsible for protein metabolization (Cai et al., 2022). Studies have revealed that the production of these gases can induce intestinal diseases (Wang et al., 2020; Yang et al., 2020), diabetes (Yang et al., 2020), obesity, central nervous system diseases, and cardiovascular diseases (Cai et al., 2022; Li et al., 2009). The gut microbiota performs crucial functions in the human body’s immunological, metabolic, structural, and neurological landscapes (Adak and Khan, 2019). Moreover, the gut microbiota plays a significant part in male fertility. Zhang et al. (2021a) and Zhang et al. (2021b) provided evidence that gut dysbiosis influences the fertility of male mice. The species profile of gut microbiota in infertile mice differs from that of fertile ones. The infertile group showed decreases in ‘beneficial’ bacteria and increases in ‘harmful’ bacteria. Grande et al. (2024) discovered a relationship between male tract microbiota and male infertility, highlighting interactions between the seminal and vaginal microbiota. According to Ashonibare et al. (2024) and Wang and Xie (2022), gut microbiota influences male reproductive function and behaviors in several ways, including alterations in ROS and sex hormones generation, and activation of cytokine accumulation and the immune system.
Therefore, a link between external NH3 and/or H2S and male fertility, as well as gut microbiota and male fertility, has been proven, but studies on the effects of internally generated NH3 and/or H2S gases are limited. NH3 and/or H2S is produced by the decomposition or metabolism of intestinal bacteria (Han et al., 2021; Wang et al., 2021), which is primarily influenced by daily diet (Kalantar-Zadeh et al., 2019), even the intestinal gas status, abdominal symptoms, and gastrointestinal disease state. Consequently, the results of existing studies are often inconsistent, and there needs to be more statistical power in the performed trials.
The rising cases of infertility worldwide have attracted more and more attention, and male factors are estimated to contribute to 30%-50% of the cases (Eisenberg et al., 2023). This study aims to evaluate the correlation between internally produced NH3 and/or H2S gases and gut microbiota related to male infertility through a systematic review and a meta-analytic approach. This study will provide novel insights into the production of internal body gases in relation to gut microbiota and male fertility.
2 Methods
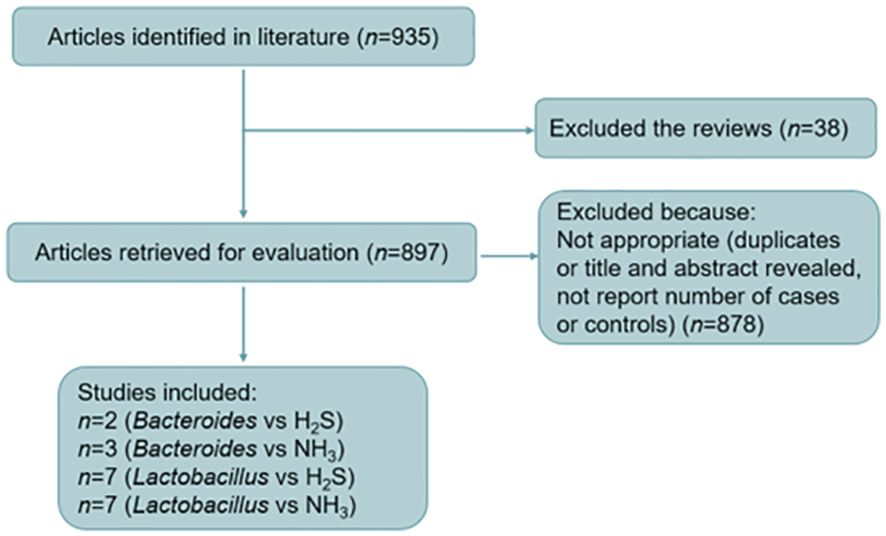
In September 2022, a fuzzy inquiry was conducted on PubMed (www.ncbi.nlm.nih.gov/pubme) to gather literature on male infertility responses, gut microbiota, and NH3 and H2S. The search terms utilized included male infertility or sterility or reproductive problems or reproductive issues and gut microbiota, Lactobacillus, Bacteroides, and NH3 or ammonia / H2S or hydrogen sulfide. These terms were used both as free text and as subject headings, with the language restriction set to English. After the fuzzy inquiry searching, the published papers were narrowed down to 1947-2023, and 935 papers were recovered from PubMed data. The titles and abstracts of these papers were then screened, and papers with information relating to gut dysbiosis in male infertility or bacteria with gas emission were selected. After this screening process, 33 papers related to male infertility and gut microbiota, 141 papers on Lactobacillus and NH3 and/or H2S, and 36 papers on Bacteroides and NH3 and/or H2S were identified. Patients or the public were not involved in our research’s design, conduct, reporting, or dissemination plans.
Duplicate papers were removed, and the remainder assessed for the following criteria:
i. Prospective, randomized, double-blind trials.
ii. A source of primary data, not a review.
iii. The sample size is given.
iv. Appropriate control and treatment groups.
v. The aim of the study was closely related to our searched keywords.
After screening, 19 research papers fulfilled all five criteria listed above. Meta-analyses were carried out in according to the published Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Page et al., 2021). Descriptive statistical analysis was applied to summarize the total events from the 19 studies through RevMan v5.4 (Cochrane, London, UK) (Figure 1). Response to treatment was defined as the number of individuals with overall symptoms divided by the total number of individuals studied, reported as ratio (R) and 95% confidence interval (95% CI) and relative ratio (RR) and 95% CI for Lactobacillus and Bacteroides, respectively. Fixed- and random-effects models were used when I square ≤50% and I square >50%, respectively. The results were displayed as forest plots.
3 Results
3.1 Levels of Lactobacillus and Bacteroides changed in infertile males
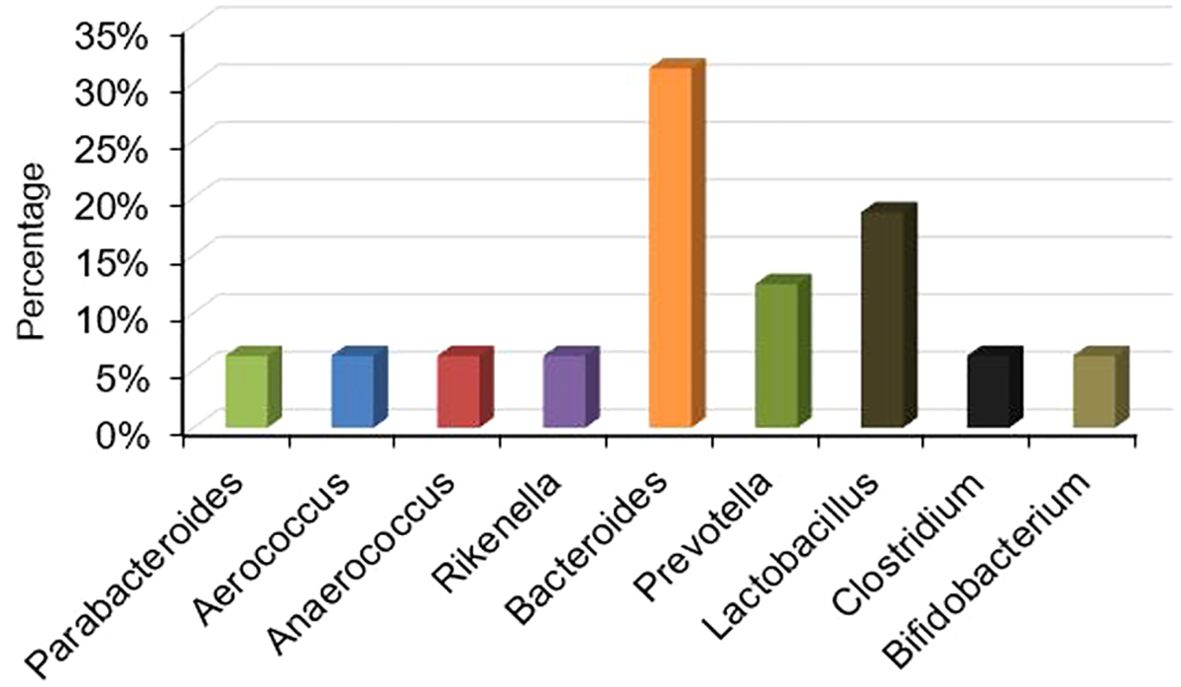
Gut dysbiosis has been associated with male infertility. In order to identify the critical variable bacteria in the infertile male, we summarized the results of altered bacterial profiles in sterile males (Supplementary Table 1). No matter how much the bacteria increased or decreased, we calculated the ratio of changed bacteria in 12 out of 33 studies. Notably, Lactobacillus and Bacteroides were the most frequently investigated bacteria in those research papers (Figure 2).
Since Lactobacillus and Bacteroides have been extensively studied in fertile and infertile males (19% and 31% respectively), we did a meta-analysis of the available literature on these two bacteria to further investigate their relationship with the production of NH3 and H2S in the gut.
3.2 Results of the meta-analysis of Lactobacillus vs. NH3 and/or H2S
We performed a meta-analysis to gain insight into the correlation between Lactobacillus species and NH3 and/or H2S in the gut. Seven studies related to Lactobacillus vs. H2S (Supplementary Table 2), and seven studies related to Lactobacillus vs. NH3 (Supplementary Table 3) were included in this analysis. The following is the formula of the random-effect model in this method:
Where: Yi is the observed effect in the study.
μ is the overall mean.
θi is the true mean of the studies.
ξ is the random variable.
The forest plot depicted in Figure 3 reveals a statistically significant meta-analysis result (p<0.00001) comparing Lactobacillus to NH3, with a total effect indicated by a risk ratio (RR) of159.75 and a 95% confidence interval (CI) ranging from 56.31 to 453.18. According to the 1938 individuals in the analysis, it is suggested that there is less NH3 in the Lactobacillus-rich group. The I square showed that the included studies had a low heterogeneity (I square=0%). The forest plot result of the meta-analysis of 2114 individuals on Lactobacillus vs. H2S suggested less H2S in the Lactobacillus-rich group with RR=122.12 (95% CI 25.16-592.78). Although two studies showed no significant statistical analysis (Liu et al., 2022; Zolnowski et al., 2022), the total result was significant (Chi square=14.04, df=6, I square=57%, Z-test p<0.00001). Furthermore, the p-value of the Z-test was smaller than 0.00001, indicating that the analysis was highly significant. Based on the different Z-values of the subgroups (Z=9.54 (NH3)< 5.96 (H2S)), the NH3 has a higher probability of negative relationship with Lactobacillus than H2S. Even though there is slightly higher heterogeneity in Lactobacillus vs H2S, the total heterogeneity is less than 50% (I square=31%). These data indicated that Lactobacillus exhibits a significantly negative relationship with both NH3 and H2S in the gut.
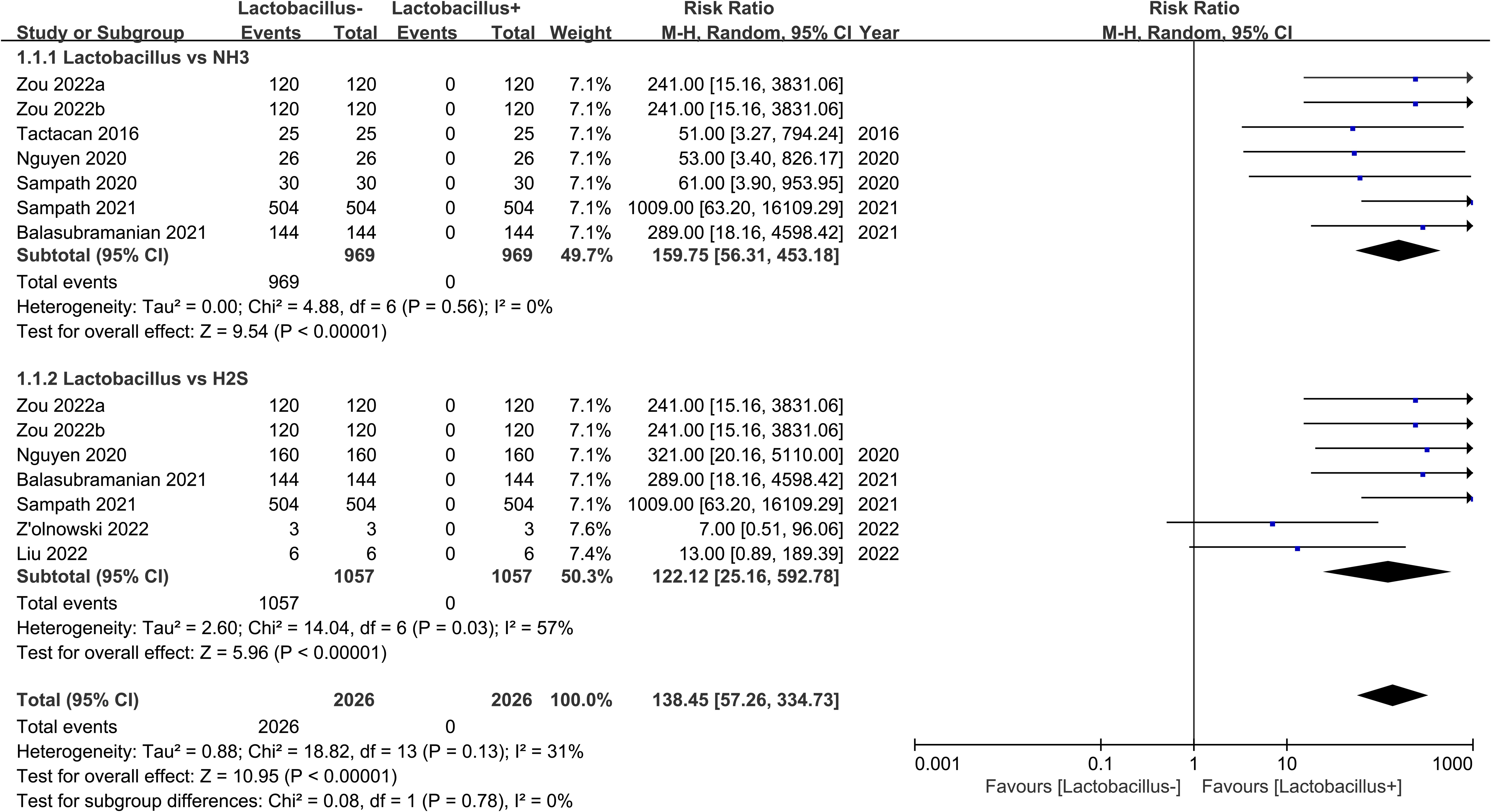
Figure 3. Meta-analysis of Lactobacillus vs. NH3 and H2S. Events means the number of the cases in which an event occurred. Total means the sample size for each group. + means rich, - means poor. M-H means effect amount.
This is the formula of Z-test in this study:
Z=(x-μ)/ ( ) Where: x is the sample mean.
μ is the overall mean.
σ is the overall standard deviation.
n is the sample size.
Notes: Z value represents the different probability (Z=~-∞: 0%; Z=~0: 50%; Z=~+∞: 100%)
3.3 Results of the meta-analysis of Bacteroides vs. NH3 and H2S
We used a meta-analytic approach to understand if there was a correlation between Bacteroides levels and NH3 and H2S production in the gut. Two studies related to Bacteroides vs. H2S (143 individuals) (Supplementary Table 4) and three studies related to Bacteroides vs. NH3 (243 individuals) (Supplementary Table 5). The following is the formula of the fixed model in this method:
Where: Yi is the observed effect in the study
Θ is the true effect in the study
ϵi is the difference between the true effect and the observed effect.
According to the forest plot (Figure 4), the meta-analysis of the Bacteroides vs. NH3 showed significant statistical results (p<0.001), the total RR=0.35 (95% CI 0.23-0.51). There is more NH3 in the Bacteroides-rich group. However, the I square indicated that the included studies had a higher heterogeneity (I square=92%). Furthermore, the forest plot result of meta-analysis on Bacteroides vs. H2S suggested that there are more H2S in the Bacteroides-rich group with RR=0.01 (95% CI 0.00-0.10). The p-value of the Z-test was smaller than 0.0001, which suggested that the analysis showed significant statistical results. Based on the different Z-values of the subgroup (Z=5.25 (NH3) > 4.31 (H2S)), the NH3 has a higher probability of a positive relationship with Bacteroides than the H2S. Despite the high heterogeneity among the included studies, the Z-test for the overall effect of Bacteroides vs. NH3 and Bacteroides vs. H2S demonstrated that Bacteroides showed a dramatic positive relationship with NH3 and H2S in the gut.
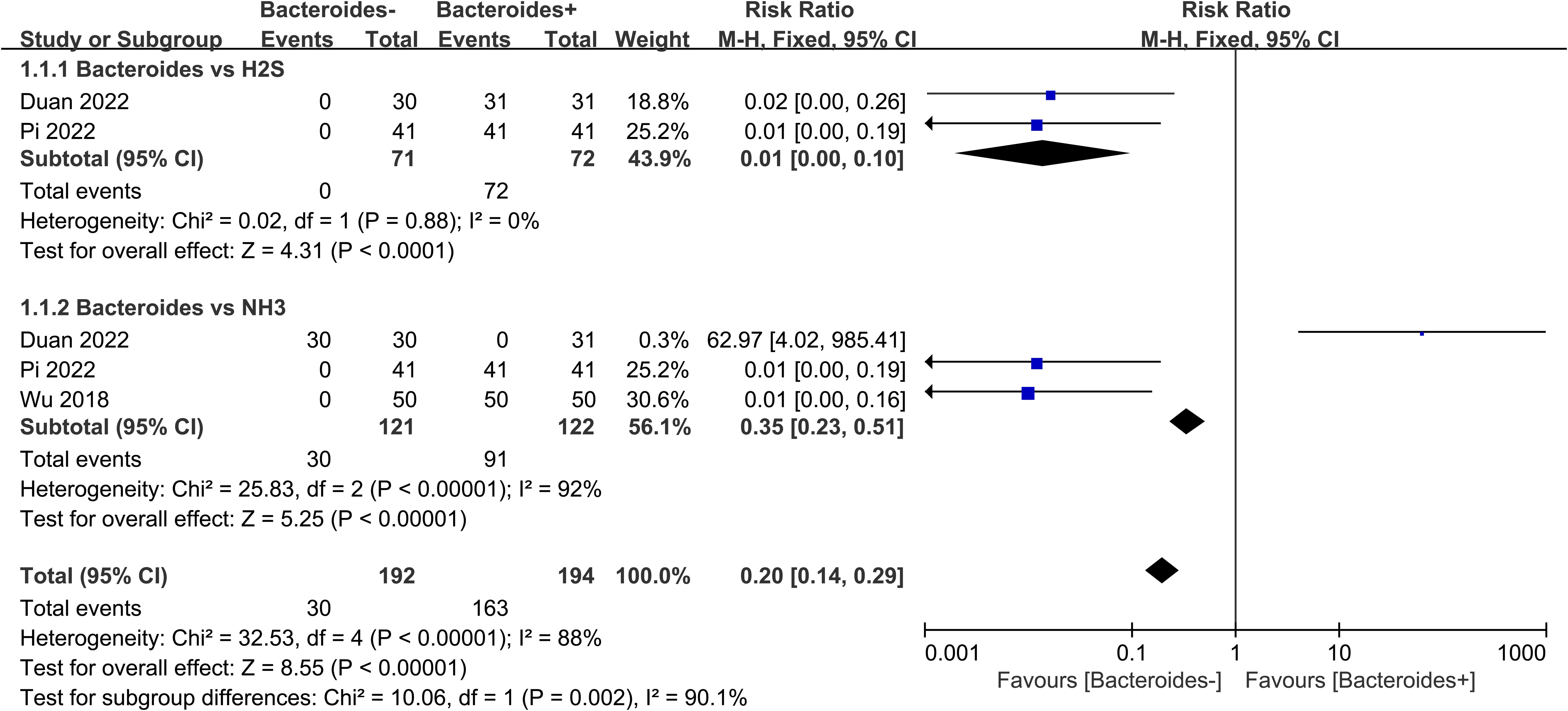
Figure 4. Meta-analysis of Bacteroides vs. NH3 and H2S. Events means the number of the cases in which an event occurred. Total means the sample size for each group. + means rich, - means poor. M-H means effect amount.
4 Discussion
This paper first proposed the potential axis linking gut microbiota, inner gases (NH3 or/and H2S produced by gut microbiota), and male fertility based on a comprehensive meta-analysis. Our investigation is the first meta-analysis study to investigate the NH3 and/or H2S in the gut involving male infertility.
The gut microbiota is a term that describes all microorganisms that inhabit the intestine (Chen et al., 2021; Pushpanathan et al., 2019). The gut microbiota engages in many important activities, such as maintaining physiological homeostasis and contributing to the physical performance of the host (Campaniello et al., 2022; Marttinen et al., 2020; Pushpanathan et al., 2019). Furthermore, gut flora communities play a pivotal role in diseases processes (Paoli et al., 2019). Gut dysbiosis shows adverse effects on the body (Chen et al., 2021), leading to increased permeability, endotoxemia, insulin resistance, and metabolic disorders (Pushpanathan et al., 2019). Besides that, the gut microbiota interacts with male fertility (Cai et al., 2022; Zhao et al., 2020; Zhang et al., 2021a and b) potentially contributing to testicular dysfunction by disrupting polyamine metabolism (Zhao et al., 2021). (). In this study, we synthesized the interaction between gut disorder and male infertility through a summary statistic. Among the variable bacteria in the included studies, those that changed noticeably were Lactobacillus (19%) and Bacteroides (31%). Interestingly, this finding aligns our previous study (Zhao et al., 2020; Zhang et al., 2021a and b), which reported significant alterations in Lactobacillus and Bacteroides in the gut of infertile male mice.
External sources of NH3 and H2S have been proven to induce male infertility (Zhang et al., 2018; Zhao et al., 2016). They diminish male fertility by reducing sperm motility through the AMPK/AKT pathway and could influence the offspring. It is known that the gut plays a crucial role in internal gas emissions (Kim et al., 2022). Our study first proposed the correlation between gut microbiota and internal NH3 and H2S by meta-analysis. As it was found that gut microbiota closely related to male fertility in our laboratory previous study by mice (Zhang et al., 2021a and b), this study further verified the interaction among gut microbiota, NH3 and H2S, and male fertility. The studies analyzed in this paper covered seven papers related to Lactobacillus and NH3 and seven papers related to Lactobacillus and H2S. The meta-analysis showed significant statistical data, indicating a negative association between Lactobacillus and these gases. Specifically, higher levels of Lactobacillus were associated with lower NH3 and H2S levels in the gut, which the outcomes are consistent with our previous work. Zhang et al. (2018) and Zhao et al. (2016) have demonstrated that NH3 and H2S adversely affect the fertility of male mice and boars. In humans, Lactobacillus sp. are the overwhelmingly dominant bacteria within reproductive tissues (Poole et al., 2023). Interestingly, Lactobacillus sp. have been found associated with improvements in semen parameters (Farahani et al., 2021). Lactobacillus could extend the male mating time and result in higher short-term offspring production in females (Morimoto et al., 2017). In addition, Lactobacillus could ameliorate the total and progressive motility and acrosome integrity (Mahiddine et al., 2022) and the testicular function (Çiftci and Tuna, 2021). Lactobacillus is not only beneficial for male fertility but also better for restraining the negative influence of Pseudomonas (Zhang et al., 2020). This study confirmed previous findings that showed a negative correlation between Lactobacillus and gut NH3 and H2S production further demonstrating the benefits of Lactobacillus on male fertility through mediating gas generation.
This study showed that the amount of Bacteroides is positively related to the concentration of NH3 and H2S. The heterogeneity of the meta-analysis of Bacteroides vs. NH3 is high because of the differences in the research analyzed in this study. Duan et al. (2022) showed data that was opposite to Pi et al. (2022) and Wu et al. (2018). Unlike the later studies, Duan et al. (2022) suggested more NH3 in the low Bacteroides group. However, the final Z-test of the published studies included in this analysis showed that increased levels of Bacteroides sp. are positively related to NH3. Combining the two subgroup results of meta-analysis, Bacteroides positively related to NH3 and H2S. Some investigations have proved the beneficial role of the Bacteroides in the gut (Singh, 2019). Microbiotas contribute substantially to the well-being of the host, including the immune response and nervous system (Strandwitz et al., 2019); however, different locations of the bacteria presented could also be pathogenic (Zafar and Saier, 2021). While Bacteroides species are part of the normal gastrointestinal microbiota they are also the most common anaerobic infective bacteria with an associated mortality of 19%. Bacteroides species have high resistance to antibiotics and contain antibiotic resistance mechanisms (Jasemi et al., 2021; Wexler, 2007). Quaglio et al. (2022) stated that Bacteroides species are associated with chronic tissue inflammation and the release of pro-inflammatory and carcinogenic mediators, upregulating the chance of developing colorectal cancer and irritable bowel syndrome. Ding et al. (2020) indicated that Bacteroides are related to metabolic endotoxemia, revealing a strong negative relationship with sperm motility and a positive correlation with blood endotoxin. Our study showed that Bacteroides has a positive relationship with the levels of NH3 and H2S. Based on our previous study on the external NH3 and H2S inducing male infertility (Zhang et al., 2018; Zhao et al., 2016), we hypothesize that Bacteroides might induce male sterility through increasing NH3 and H2S levels in the gut. However, the underlying mechanisms require further experimental verification. pH status has been shown to influence male fertility (Dai et al., 2024), and the intestinal pH closely correlates with the bacteria (Cotter and Hill, 2003). Herein, one hypothesis is that the internal NH3 and H2S produced by bacteria might affect male fertility by regulating the pH environment.
It is known that the gut environment is complex, and its composition is dynamic throughout the life of the host (Adak and Khan, 2019). The human microbiome plays a pivotal role in digestion, vitamin synthesis, and immune system training (Newcomb). Gut dysbiosis, characterized by an imbalance between the beneficial and harmful flora, has been linked to many diseases (Quaglio et al., 2022). Our investigation confirmed the interaction between gut microbiota and NH3 and H2S production in the gut based on meta-analysis, which gives fresh insights into gut flora-related diseases. Although some studies focused on gas emission after feeding the microbe (Payling et al., 2017) or the composition of human intestinal gases (Modesto et al., 2022), this study is first to propose an interaction between NH3 and H2S, and gut microbiota through a meta-analysis approach (Figure 5). Due to the limited number of studies included, we did not perform statistical tests to assess for publication bias. However, our findings further demonstrated the correlation among inner NH3 and H2S, the gut microbiota, and male infertility. This concept has been validated through meta-analysis for the first time. Oue meta-analysis indicated that lower NH3 and H2S levels are associated with a Lactobacillus-rich gut, whereas higher NH3 and H2S levels are linked to a Bacteroides-rich gut. Our meta-analysis concluded that Lactobacillus sp. might be beneficial bacteria for male fertility, while Bacteroides sp. may be harmful. This indicates that gut microbiota affects male fertility through small molecule chemicals, such as NH3 and H2S. These may further disrupt sperm motility by AMPK/AKT pathway to damage male fertility (Zhao et al., 2016).
Our study indicated a potential connection among animals ‘internal’ NH3 and/or H2S (produced in the gut by the microbiota), gut microbiota composition and male infertility via meta-analysis. This study provides a new perspective on the relationship between gut bacteria and male reproduction, highlighting the potential involvement of Bacteroide and Lactobacillus in male fertility via small molecule chemicals NH3 and H2S produced in the gut. Notably, our focus was on meta-analysis and did not encompass specific animal experiments. However, this new finding could lead to the development of novel methods or designs for future research and provide a novel insight for the future animal experiments. Indeed, this underscores the strengths and significance of conducting a meta-analysis. Satisfying data based on these would decrease the heterogeneity of the observation.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YH: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. XD: Conceptualization, Writing – original draft. CC: Writing – review & editing. YZ: Supervision, Writing – review & editing. YR: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1449453/full#supplementary-material
References
Adak, A., Khan, M. R. (2019). An insight into gut microbiota and its functionalities. Cell Mol. Life Sci. 76, 473–493. doi: 10.1007/s00018-018-2943-4
Ashonibare, V. J., Akorede, B. A., Ashonibare, P. J., Akhigbe, T. M., Akhigbe, R. E. (2024). Gut microbiota-gonadal axis: the impact of gut microbiota on reproductive functions. Front. Immunol. 15, 134035. doi: 10.3389/fimmu.2024.1346035
Barrasa, M., Lamosa, S., Fernandez, M. D., Fernandez, E. (2012). Occupational exposure to carbon dioxide, ammonia and hydrogen sulphide on livestock farms in north-west Spain. Ann. Agric. Environ. Med. 19, 17–24. doi: 10.1007/s12182-011-0111-7
Cai, H., Cao, X. H., Qin, D. Z., Liu, Y. D., Liu, Y., Hua, J. L., et al. (2022). Gut microbiota supports male reproduction via nutrition, immunity, and signaling. Front. Microbiol. 13, 977574. doi: 10.3389/fmicb.2022.977574
Campaniello, D., Corbo, M. R., Sinigaglia, M., Speranza, B., Racioppo, A., Altieri, C., et al. (2022). How diet and physical activity modulate gut microbiota: evidence, and perspectives. Nutrients 14, 2456. doi: 10.3390/nu14122456
Chen, Y. W., Zhou, J. H., Wang, L. (2021). Role and mechanism of gut microbiota in human disease. Front. Cell. Infection Microbiol. 11, 625913. doi: 10.3389/fcimb.2021.625913
Çiftci, G., Tuna, E. (2021). Effects of cholesterol and Lactobacillus acidophilus on testicular function. Clin. Exp. Reprod. Med. 48, 229–235. doi: 10.5653/cerm.2020.04322
Cotter, P. D., Hill, C. (2003). Surviving the acid test: responses of gram-positive bacteria to low Ph. Microbiol. Mol. Biol. Rev. 67, 429–453. doi: 10.1128/MMBR.67.3.429-453.2003
Dai, P. Y., Zou, M., Cai, Z. Y., Zeng, X. H., Zhang, X. N., Liang, M. (2024). pH homeodynamics and male fertility: A coordinated regulation of acid-based balance during sperm journey to fertilization. Biomolecules 14, 685. doi: 10.3390/biom14060685
Ding, N., Zhang, X., Zhang, X. D., Jing, J., Liu, S. S., Mu, Y. P., et al. (2020). Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut 69, 1608–1619. doi: 10.1136/gutjnl-2019-319127
Dordevic´, D., Jancˇíková, S., Víteˇzová, M., Kushkevych, I. (2021). Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Advanced Res. 27, 55–69. doi: 10.1016/j.jare.2020.03.003
Duan, Y. F., Wu, X. L., Yang, Y. N., Gu, L. Q., Liu, L., Yang, Y. F., et al. (2022). Marked shifts in gut microbial structure and neurotransmitter metabolism in fresh inmates revealed a close link between gut microbiota and mental health: A case-controlled study. Int. J. Clin. Health Psychol. 22, 100323. doi: 10.1016/j.ijchp.2022.100323
Eisenberg, M. L., Esteves, S. C., Lamb, D. J., Hotaling, J. M., Giwercman, A., Hwang, K., et al. (2023). Male infertility. Nat. Rev. 9, 49. doi: 10.1038/s41572-023-00459-w
Farahani, L., Tharakan, T., Yap, T., Ramsay, J. W., Jayasena, C. N., Minhas, S. (2021). The semen microbiome and its impact on sperm function and male fertility: A systematic review and meta-analysis. Andrology 9, 115–144. doi: 10.1111/andr.12886
Geiser, L. H., Ingersoll, A. R., Bytnerowicz, A., Copeland, S. A. (2008). Evidence of enhanced atmospheric ammoniacal nitrogen in Hells Canyon national recreation area: implications for natural and cultural resources. J. Air Waste Manage. Assoc. 58, 1223–1234. doi: 10.3155/1047-3289.58.9.1223
Grande, G., Graziani, A., Toni, L. D., Garolla, A., Ferlin, A. (2024). Male track microbiota and male infertility. Cells 13, 1275. doi: 10.3390/cells13151275
Han, S., Treuren, W. V., Fischer, C. R., Merrill, B. D., DeFelice, B. C., Sanchez, J. M., et al. (2021). A metabolomics pipeline for the mechanistic interrogation of the gut microbiome. Nature 595, 415–420. doi: 10.1038/s41586-021-03707-9
Jankowski, J., Westhof, T., Vaziri, N. D., Ingrosso, D., Perna, A. F. (2014). Gases as uremic toxins: is there something in the air? Semin. Nephrol. 34, 135–150. doi: 10.1016/j.semnephrol.2014.02.006
Jasemi, S., Emaneini, M., Ahmadinejad, Z., Fazeli, M. S., Sechi, L. A., Heravi, F. S., et al. (2021). Antibiotic resistance pattern of Bacteroides fragilis isolated from clinical and colorectal specimens. Annuals Clin. Microbiol. Antimicrobials 20, 27. doi: 10.1186/s12941-021-00435-w
Jiang, J., Wang, F., Wang, J., Li, J. H. (2021). Ammonia and hydrogen sulphide odour emissions from different areas of a landfill in Hangzhou, China. Waste Manage. Res. 39, 360–367. doi: 10.1177/0734242X20960225
Kalantar-Zadeh, K., Berean, K., Burgell, R. E., Muir, J. G., Gibson, P. R. (2019). Intestinal gases: influence on gut disorders and the role of dietary manipulations. Nat. Rev. 16, 733–747. doi: 10.1038/s41575-019-0193-z
Kim, K. Y., Ko, H. J., Kim, H. T., Kim, Y. S., Roh, Y. M., Lee, C. M., et al. (2008). Quantification of ammonia and hydrogen sulfide emitted from pig buildings in Korea. J. Environ. Manage. 88, 195–202. doi: 10.1016/j.jenvman.2007.02.003
Kim, J. H., Ko, G. P., Son, K. H., Ku, B. H., Bang, M. A., Kang, M. J., et al. (2022). Arazyme in combination with dietary carbohydrolases influences odor emission and gut microbiome in growing-finishing pigs. Sci. Total Environ. 848, 157735. doi: 10.1016/j.scitotenv.2022.157735
Lambert, T. W., Goodwin, V. M., Stefani, D., Strosher, L. (2006). Hydrogen sulfide (H2S) and sour gas effects on the eye. A historical perspective. Sci. Total Environ. 367, 1–22. doi: 10.1016/j.scitotenv.2006.01.034
Li, X. L., Bazer, F. W., Gao, H. J., Jobgen, W. J., Johnson, G. A., Li, P., et al. (2009). Amino acids and gaseous signaling. Amino Acids 37, 65–78. doi: 10.1007/s00726-009-0264-5
Li, M. H., Li, S. Y., Chen, S. G., Meng, Q. Y., Wang, Y., Yang, W. J., et al. (2023). Measures for controlling gaseous emissions during composting: A review. Int. J. Environ. Res. Public Health 20, 3587. doi: 10.3390/ijerph20043587
Liu, Z., Powers, W., Murphy, J., Maghirang, R. (2014). Ammonia and hydrogen sulfide emissions from swine production facilities in North America: a meta-analysis. J. Anim. Sci. 92, 1656–1665. doi: 10.2527/jas.2013-7160
Liu, J., Zhao, W., Gao, Z. W., Liu, N., Zhang, W. H., Ling, H. (2022). Effects of exogenous hydrogen sulfide on diabetic metabolic disorders in db/db mice are associated with gut bacterial and fungal microbiota. Front. Cell. Infection Microbiol. 12, 801331. doi: 10.3389/fcimb.2022.801331
Lu, D. D., Mi, J. D., Wu, Y. B., Liang, J. B., Liao, X. D., Wang, Y. (2020). Effects of different laying hen species on odour emissions. Animals 10, 2172. doi: 10.3390/ani10112172
Mahiddine, F. Y., You, I., Park, H., Kim, M. J. (2022). Commensal lactobacilli enhance sperm qualitative parameters in dogs. Front. Veterinary Sci. 9, 888023. doi: 10.3389/fvets.2022.888023
Marttinen, M., Ala-Jaakkola, R., Laitila, A., Lehtinen, M. J. (2020). Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients 12, 2936. doi: 10.3390/nu12102936
Modesto, A., Cameron, N. R., Varghese, C., Peters, N., Stokes, B., Phillips, A., et al. (2022). Meta-analysis of the composition of human intestinal gases. Digestive Dis. Sci. 67, 3842–3859. doi: 10.1007/s10620-021-07254-1
Morimoto, J., Simpson, S. J., Ponton, F. (2017). Direct and trans-generational effects of male and female gut microbiota in Drosophila melanogaster. Biol. Lett. 13, 20160966. doi: 10.1098/rsbl.2016.0966
Norizan, M. N., Abdullah, N., Halim, N. A., Demon, S. Z. N., Mohamad, I. S. (2022). Heterojunctions of rGO/metal oxide nanocomposites as promising gas-sensing materials-A review. Nanomaterials 12, 2278. doi: 10.3390/nano12132278
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. PloS Med. 18, e1003583. doi: 10.1371/journal.pmed.1003583
Paoli, A., Mancin, L., Bianco, A., Thomas, E., Mota, J. F., Piccini, F. (2019). Ketogenic diet and microbiota: friends or enemies? Genes 10, 534. doi: 10.3390/genes10070534
Payling, L., Kim, I. H., Walsh, M. C., Kiarie, E. (2017). Effects of a multi-strain Bacillus spp. direct-fed microbial and a protease enzyme on growth performance, nutrient digestibility, blood characteristics, fecal microbiota, and noxious gas emissions of grower pigs fed corn-soybean-meal-based diets-A meta-analysis. J. Anim. Sci. 95, 4018–4029. doi: 10.2527/jas.2017.1522
Pi, X. E., Yu, Z. C., Yang, X. X., Du, Z., Liu, W. (2022). Effects of Zymosan on Short-Chain Fatty Acid and Gas Production in in vitro Fermentation Models of the Human Intestinal Microbiota. Front. Nutr. 9, 921137. doi: 10.3389/fnut.2022.921137
Poole, R. K., Soffa, D. R., McAnally, B. E., Smith, M. S., Hickman-Brown, K. J., Stockland, E. L. (2023). Reproductive microbiomes in domestic livestock: insights utilizing 16S rRNA gene amplicon community sequencing. Animals 13, 485. doi: 10.3390/ani13030485
Pushpanathan, P., Mathew, G. S., Selvarajan, S., Seshadri, K. G., Srikanth, P. (2019). Gut microbiota and its mysteries. Indian J. Med. Microbiol. 37, 268–277. doi: 10.4103/ijmm.IJMM_19_373
Quaglio, A. E. V., Grillo, T. G., Oliveira, E. C. S. D., Stasi, L. C. D., Sassaki, L. Y. (2022). Gut microbiota, inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 28, 4053–4060. doi: 10.3748/wjg.v28.i30.4053
Singh, R. P. (2019). Glycan utilisation system in Bacteroides and Bifidobacteria and their roles in gut stability and health. Appl. Microbiol. Biotechnol. 103, 7287–7315. doi: 10.1007/s00253-019-10012-z
Strandwitz, P., Kim, K. H., Terekhova, D., Liu, J. K., Sharma, A., Levering, J., et al. (2019). GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 4, 396–403. doi: 10.1038/s41564-018-0307-3
Song, J. I., Park, K. H., Jeon, J. H., Choi, H. L., Barroga, A. J. (2013). Dynamics of air temperature, velocity and ammonia emissions in enclosed and conventional pig housing systems. Asian-Australas. J. Anim. Sci. 26, 433–442. doi: 10.5713/ajas.2011.11515
Wang, S. C., Wang, W., Li, X. J., Zhao, X., Wang, Y., Zhang, H. F., et al. (2020). Cooperative application of transcriptomics and ceRNA hypothesis: LncRNA-107052630/miR-205a/G0S2 crosstalk is involved in ammonia-induced intestinal apoptotic injury in chicken. J. Hazardous Materials 396, 122605. doi: 10.1016/j.jhazmat.2020.122605
Wang, Y., Xie, Z. G. (2022). Exploring the role of gut microbiome in male reproduction. Andrology 10, 441–450. doi: 10.1111/andr.13143
Wang, S., Zhang, L. L., Wang, D., Huang, M., Zhao, J. Y., Malik, V., et al. (2021). Gut microbiota composition is associated with responses to peanut intervention in multiple parameters among adults with metabolic syndrome risk. Mol. Nutr. Food Res. 65, e2001051. doi: 10.1002/mnfr.202001051
Wexler, H. M. (2007). Bacteroides: the good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 20, 593–621. doi: 10.1128/CMR.00008-07
Wu, C. D., Yang, F., Brancher, M., Liu, J. M., Qu, C., Piringer, M., et al. (2020). Determination of ammonia and hydrogen sulfide emissions from a commercial dairy farm with an exercise yard and the health-related impact for residents. Environ. Sci. pollut. Res. Int. 27, 37684–37698. doi: 10.1007/s11356-020-09858-y
Wu, Z. Y., Zhou, H. L., Li, F. C., Zhang, N. B., Zhu, Y. L. (2018). Effect of dietary fiber levels on bacterial composition with age in the cecum of meat rabbits. Microbiol. Open 8, e00708. doi: 10.1002/mbo3.708
Yang, N., Liu, Y., Li, T. P., Tuo, Q. H. (2020). Role of hydrogen sulfide in chronic diseases. DNA Cell Biol. 39, 187–196. doi: 10.1089/dna.2019.5067
Zolnowski, C., Bakuła, T., Rolka, E., Klasa., A. (2022). Effect of mineral–microbial deodorizing preparation on the value of poultry manure as soil amendment. Int. J. Environ. Res. Public Health 19, 16639. doi: 10.3390/ijerph192416639
Zafar, H., Saier, M. (2021). Gut Bacteroides species in health and disease. Gut Microbes 13, 1–20. doi: 10.1080/19490976.2020.1848158
Zhang, P. F., Feng, Y. N., Li, L., Ge, W., Yu, S., Hao, Y., et al. (2021a). Improvement in sperm quality and spermatogenesis following faecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut 70, 222–225. doi: 10.1136/gutjnl-2020-320992
Zhang, J., Liu, H., Yang, Q. Z., Li, P. F., Wen, Y., Han, X. J., et al. (2020). Genomic sequencing reveals the diversity of seminal bacteria and relationships to reproductive potential in boar sperm. Front. Microbiol. 11, 1873. doi: 10.3389/fmicb.2020.01873
Zhang, C., Xiong, B. H., Chen, L., Ge, W., Yin, S., Feng, Y. N., et al. (2021b). Rescue of male fertility following fecal microbiota transplantation from alginate oligosaccharide dosed mice. Gut 70, 1608–1619. doi: 10.1136/gutjnl-2020-323593
Zhang, W. D., Zhao, Y., Zhang, P. F., Hao, Y. N., Yu, S., Min, L. J., et al. (2018). Decrease in male mouse fertility by hydrogen sulfide and/or ammonia can Be inheritable. Chemosphere 194, 147–157. doi: 10.1016/j.chemosphere.2017.11.164
Zhao, Q., Huang, J. F., Cheng, Y., Dai, M. Y., Zhu, W. F., Yang, X. W., et al. (2021). Polyamine metabolism links gut microbiota and testicular dysfunction. Microbiome 9, 224. doi: 10.1186/s40168-021-01157-z
Zhao, Y., Zhang, P. F., Ge, W., Feng, Y. N., Li, L., Sun, Z. Y., et al. (2020). Alginate oligosaccharides improve germ cell development and testicular microenvironment to rescue busulfan disrupted spermatogenesis. Theranostics 10, 3308–3324. doi: 10.7150/thno.43189
Zhao, Y., Zhang, W. D., Liu, X. Q., Zhang, P. F., Hao, Y. N., Li, L., et al. (2016). Hydrogen Sulfide and/or Ammonia Reduces Spermatozoa Motility through AMPK/AKT Related Pathways. Sci. Rep. 6, 37884. doi: 10.1038/srep37884
Keywords: ammonia, hydrogen sulfide, gut microbiota, male infertility, internal gases
Citation: Hao Y, Du X, Cai C, Zhao Y and Ren Y (2025) Ammonia and hydrogen sulfide - new insights into gut microbiota and male infertility through meta-analysis. Front. Cell. Infect. Microbiol. 14:1449453. doi: 10.3389/fcimb.2024.1449453
Received: 18 June 2024; Accepted: 09 December 2024;
Published: 06 January 2025.
Edited by:
George Seghal Kiran, Pondicherry University, IndiaReviewed by:
Orlando Borras-Hidalgo, Yota Bio-Engineering Co., Ltd., ChinaPing Li, Guizhou University, China
Bin Du, Hebei Normal University of Science and Technology, China
Copyright © 2025 Hao, Du, Cai, Zhao and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonglin Ren, WS5SZW5AbXVyZG9jaC5lZHUuYXU=
 Yanan Hao
Yanan Hao Xin Du2
Xin Du2 Chang Cai
Chang Cai Yong Zhao
Yong Zhao Yonglin Ren
Yonglin Ren