
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol. , 16 August 2024
Sec. Clinical Microbiology
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1436509
This article is part of the Research Topic Application and Reliability Assessment of Next Generation Sequencing (NGS) and targeted NGS (tNGS) in the Diagnosis of Infectious Diseases-Volume III View all 38 articles
A correction has been applied to this article in:
Corrigendum: Distribution characteristics of human herpes viruses in the lower respiratory tract and their impact on 30-day mortality in Community-Acquired Pneumonia patients
Human herpes viruses (HHVs) are commonly detected in community-acquired pneumonia (CAP) patients, particularly those with complex complications, attracting increased attention from clinical practitioners. However, the significance of detecting HHVs in bronchoalveolar lavage fluid (BALF) with CAP patients is still unclear. This study retrospectively analyzed BALF samples from 64 CAP patients at the Kunming Third People’s Hospital between August 2021 and December 2023. Metagenomic next generation sequencing (mNGS) was conducted on BALF samples during CAP onset. Multivariate Cox regression models were used to identify independent risk factors for 30-day all-cause mortality in CAP. HHVs were found in 84.4% of CAP patients, which were the most common pathogens (45.1%), followed by bacteria (30.2%) and fungi (11.5%). Bacterial-viral co-infections were most common, occurring in 39 patients. Notably, there was no significant difference in HHV presence between severe and non-severe CAP patients (EBV: P = 0.431, CMV: P = 0.825), except for HHV-7 (P = 0.025). In addition, there was no significant difference in the 30-day mortality between HHV positive and HHV negative groups (P = 0.470), as well as between the HHV-7 positive and HHV-7 negative groups (P = 0.910). However, neither HHVs nor HHV-7 was independent risk factors for 30-day mortality in CAP patients (HHVs: HR 1.171, P = 0.888; HHV-7: HR 1.947, P = 0.382). In summary, among the prevalent presence of multiple HHVs, EBV and CMV were the most prevalent in CAP patients. Patients with sCAP were more susceptible to HHV-7 than those with non-sCAP. These results provide valuable insights for clinicians in guiding appropriate interventions for CAP treatment.
Community-acquired pneumonia (CAP) is a prevalent infection of the lower respiratory tract that has a significant effect on the health of adults, with an annual incidence ranging from 0.1% to 2.5% (Lu et al., 2023; Martin-Loeches et al., 2023). Notably, about 5.0-10.0% of CAP patients progress to severe community-acquired pneumonia (sCAP), leading to substantial morbidity, mortality, and economic costs (Liapikou, 2020; Niederman and Torres, 2022). This incidence increases with age, males, immunosuppressed status, and number of comorbidities (The Lancet Healthy Longevity, 2021; Niederman and Torres, 2022; Martin-Loeches et al., 2023).
Approximately 130 species of infectious herpesviridae have been identified, of which only eight are capable of infecting humans and known as human herpes viruses (HHVs) (Costa et al., 2019). HHVs including herpes simplex virus 1 (HSV-1), herpes simplex virus 2 (HSV-2), varicella-zoster virus (VZV), Epstein-Barr virus (EBV), human cytomegalovirus (CMV), human herpes virus 6 (HHV-6A, HHV-6B), human herpes virus 7 (HHV-7) and Kaposi’s sarcoma-associated herpes virus (KSHV) (Cohen, 2020; Connolly et al., 2021).
Human herpes viruses (HHVs) are widespread in CAP patients, once the host is infected, the virus can remain dormant in the host cells. Therefore, all HHVs can cause lifelong infection in the host, and cause disease when the virus is reactivated (Quach et al., 2023). Herpesviridae such as HSV-1, EBV, CMV and HHV-7 are commonly identified as primary pathogens in immunosuppressed patients or those with acute respiratory distress syndrome (ARDS) (Hraiech et al., 2019; Saura et al., 2022). HHVs had been extensively documented, which were associated with higher mortality in severe pneumonia patients (Huang et al., 2023; Liu et al., 2023b). HHV-7 has been observed in severe pneumonia patients, which were often in combination with co-infection of EBV and CMV (Xu et al., 2023). Additionally, studies have shown that EBV, CMV, HHV-7, and HHV-8 significantly increasing the risk of interstitial pneumonia or idiopathic pulmonary fibrosis (Sheng et al., 2020; De Rose et al., 2021). However, the significance of detecting HHVs in bronchoalveolar lavage fluid (BALF) from CAP patients remains unclear.
Due to the complex characteristics of CAP pathogens, there is an urgent need for metagenomic next generation sequencing (mNGS) as a rapid and effective diagnostic tool to identify pathogens in the lower respiratory tract, specifically in BALF. mNGS has proven to be more accurate and comprehensive compared to conventional detection methods, it can identify both known and unknown pathogens, leading to an improved detection rate and demonstrating its advantage in identifying multi-pathogenic CAP (Lv et al., 2023). This technology provides valuable insights to reduce delays in disease diagnosis and management (Sun et al., 2021; Lin et al., 2023).
The aim of this research was to improve the detection efficiency of pathogens in BALF by mNGS. Additionally, the study also aimed to assess the distribution characteristics of HHVs in CAP patients and their impact on 30-day mortality. Consequently, the findings offer important information for clinicians to guide timely interventions in treatment.
This study retrospectively analyzed data from 82 patients admitted to Kunming Third People’s Hospital between August 2021 and December 2023. All patients had been diagnosed with CAP, and BALF samples were detected using mNGS within 24 hours of collection. Patients meeting the sCAP primary criteria or at least three sCAP secondary criteria were considered for potential admission to the intensive care unit (ICU) (Metlay et al., 2019).
An expert consensus on managing CAP in immunosuppressed patients was published in Chest 2020 (Ramirez et al., 2020). This study included immunosuppressed patients meeting the defined criteria. The exclusion criteria for the study were as follows: 1. Patients under 18 years of age, 2. Patients received mNGS after 30 days of admission, 3. Patients lost to follow-up, 4. Patients with incomplete clinical trial parameters.
This study collected data from the electronic medical record system, which included demographic information such as gender, age, smoking history, drinking history, mechanical ventilation, length of stay (LOS), and underlying diseases. Additionally, various clinical trial parameters were collected 24 hours during the mNGS detection. These parameters included white blood cells, lymphocyte percentage, red blood cells, hematocrit, procalcitonin, hypersensitive C-reactive protein, bilirubin, urea, and creatinine. The severity and risk factors for death in CAP patients were assessed by calculating the SOFA score and the APACHE II score based on these parameters.
The Shapiro-Wilk test was used to assess the normality of the distribution of continuous variables. Variables that followed a normal distribution were represented by the means and standard deviations, while those not following a normal distribution were described using the median and quartiles. The data were classified utilizing either the χ2 test or the Fisher exact probability test. Frequency counts and percentages were used to summarize the results. Univariate comparisons between groups were conducted employing either the t-test or the Wilcoxon rank sum test for continuous variables. Kaplan-Meier analysis was used to compare the 30-day mortality of different groups after admission. Multivariate Cox regression models were utilized to identify independent risk factors for 30-day mortality, with continuous adjustment for variables. Cox.zph function were used to calculated the Schoenfeld residuals for each covariate to test the proportional hazards assumption of Cox regression. The hypothesis of parallelism was tested and the C-index was calculated. Sensitivity analysis was performed using different predictors in the Cox model to evaluate the robustness of the results. The statistical analysis and data visualization were performed using R software version 4.3.2. Significance levels were reported with double-tailed P values, with P < 0.05 deemed statistically significant. Pathogen stack was generated using Sangerbox3.0, while the pathogen proportion pie chart was created using SR plot (Tang et al., 2023).
Initially, this study included a total of 82 CAP patients. Exclusions were made for patients under 18 years old (5 patients), those who received mNGS after 30 days of admission (3 patients), those lost to follow-up (4 patients), and those with incomplete clinical trial parameters (6 patients). The final analysis focused on 64 CAP patients, whose BALF samples were subjected to mNGS. Each patient was matched with one BALF sample. Based on the diagnostic criteria for sCAP, the CAP patients were divided into 36 sCAP patients and 28 non-sCAP patients (Figure 1).
In Table 1, it was observed that sCAP patients were older than non-sCAP patients (61.6 vs. 46.6, P = 0.002). Mechanical ventilation proved to be an effective respiratory support therapy for sCAP patients (72.2% vs. 25.0%, P < 0.001). The prevalence of underlying diseases such as respiratory insufficiency (P < 0.001), sepsis (P < 0.001), and hypertension (P < 0.001) was significantly higher in sCAP patients. While symptoms like fever, cough, and sputum are typically indicative of CAP, no significant contrast was observed between sCAP and non-sCAP patients in this regard. Clinical trial parameters varied significantly between the two groups (P < 0.001), including white blood cell count, lymphocyte percentages, procalcitonin levels, hypersensitive C-reactive protein levels, and urea levels. These parameters may help to distinguish patients who are not responding to treatment from those who are responding slowly, emphasizing the importance of closely monitoring sCAP patients. Moreover, the SOFA score (P < 0.001) and APACHE II score (P < 0.001) were notably higher in sCAP patients compared to non-sCAP patients.
To further analyze the distribution characteristics of pathogens identified through mNGS, Figure 2A showed that 56 infectious pathogens were detected among the 64 CAP patients. These multiple pathogens included bacteria, fungi, viruses, mycoplasmas, and parasites. Specifically, 18 bacteria, 5 fungi, 16 viruses, 1 mycoplasma and 1 parasite were detected in sCAP patients, while 19 bacteria, 4 fungi, 15 viruses, 1 mycoplasma and 1 parasite were detected in non-sCAP patients. In addition, 12 bacteria, 3 fungi and 10 viruses were detected in both sCAP and non-sCAP patients. Notably, Mycobacterium abscessus (n=9) was the most common bacterial infection, Pneumocystis jirovecii (n=13) was the most common fungal infection, while EBV (n=33) and CMV (n=33) were the most common viral infections in CAP patients.

Figure 2. Compares and overlaps the pathogens in sCAP and non-sCAP groups. (A) Pathogens of lower respiratory tract infections in CAP patients. (B) Mixed infections in CAP patients. (C, D) The big pie chart on the left shows the distribution of pathogens detected by mNGS, and the small chart on the right shows the distribution of HHV detected by mNGS. (C) sCAP patients. (D) non-sCAP patients.
As shown in Figure 2B, co-infections were observed in 46 cases, with the remaining 18 cases associated with a single pathogen infection. The most common co-infections among the 64 CAP patients were bacterial-viral (39 cases), followed by fungal-viral (24 cases) and bacterial-fungal-viral (17 cases).
This study found that in 36 sCAP patients, the most prevalent pathogens were HHVs (48.9%), bacteria (30.9%), and fungi (7.9%), as shown in Figure 2C. Similarly, Figure 2D illustrated that in 28 non-sCAP patients, the most common pathogens were HHVs (39.6%), bacteria (29.2%), and fungi (16.7%). However, according to Table 2, no significant differences were observed between sCAP and non-sCAP patients in the presence of bacteria (P = 0.906), fungi (P = 0.309), HHVs (P = 0.090), other viruses (P = 0.622), mycoplasmas (P = 0.688) and parasites (P = 1.000).
At least one HHV infection was detected in 84.4% of CAP patients. Among all pathogens detected, multiple HHVs were prevalent, including HSV-1, HSV-2, EBV, CMV, HHV-6B, and HHV-7. Only HSV-2 was detected in sCAP patients. There was no significant difference in HHVs between sCAP and non-sCAP patients (91.7% vs. 75.0%, P = 0.090) (Table 2).
Analysis in Table 2 revealed that EBV (47.2% vs. 57.1%, P = 0.431) and CMV (52.8% vs. 50.0%, P = 0.825) were prevalent in both sCAP and non-sCAP patients, followed by HSV-1 and HHV-6B. The HHV-7 detection rate in sCAP patients was higher than that in non-sCAP patients (44.4% vs. 17.9%, P = 0.025).
To investigate the 30-day mortality of different groups, we plotted a survival curve. Figure 3A revealed a significantly higher 30-day mortality in sCAP patients compared to non-sCAP patients. Specifically, the 30-day mortality was 30.6% in sCAP patients, whereas it was only 3.6% in non-sCAP patients. In Figure 3B, although there was no statistically significant difference in 30-day mortality between HHV positive and negative groups (P = 0.470), the 30-day mortality was higher in the HHV positive group. Finally, Figure 3C showed no significant variance in the 30-day mortality between HHV-7 positive and HHV-7 negative groups (P = 0.910), which may have been influenced by the fact that there were fewer patients in the HHV-7 positive group than in the HHV-7 negative groups.
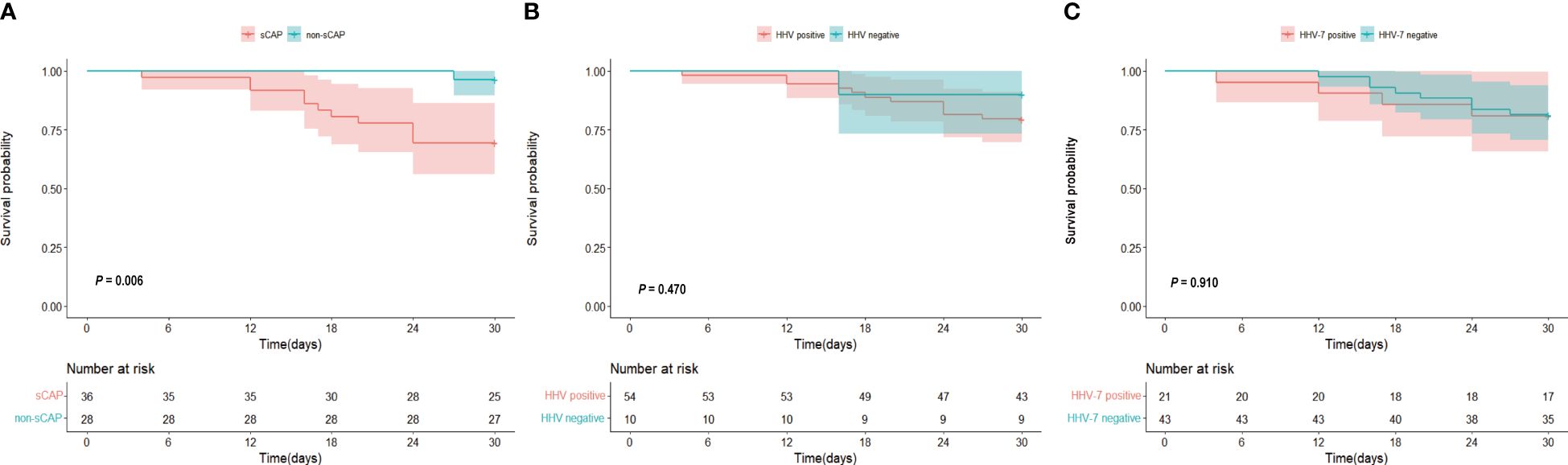
Figure 3. (A–C) Kaplan-Meier curve 30 days of (A) sCAP and non-sCAP groups, (B) HHV positive and HHV negative groups, (C) HHV-7 positive and HHV-7 negative groups.
A Cox multivariate regression analysis was conducted to investigate the influence of gender, age, LOS, HHVs, HHV-7, and APACHE II score on the 30-day mortality in CAP patients. The results indicated that neither HHVs (HR 1.171, P = 0.888) nor HHV-7 (HR 1.947, P = 0.382) was independent risk factors for 30-day mortality in CAP patients (Figure 4).
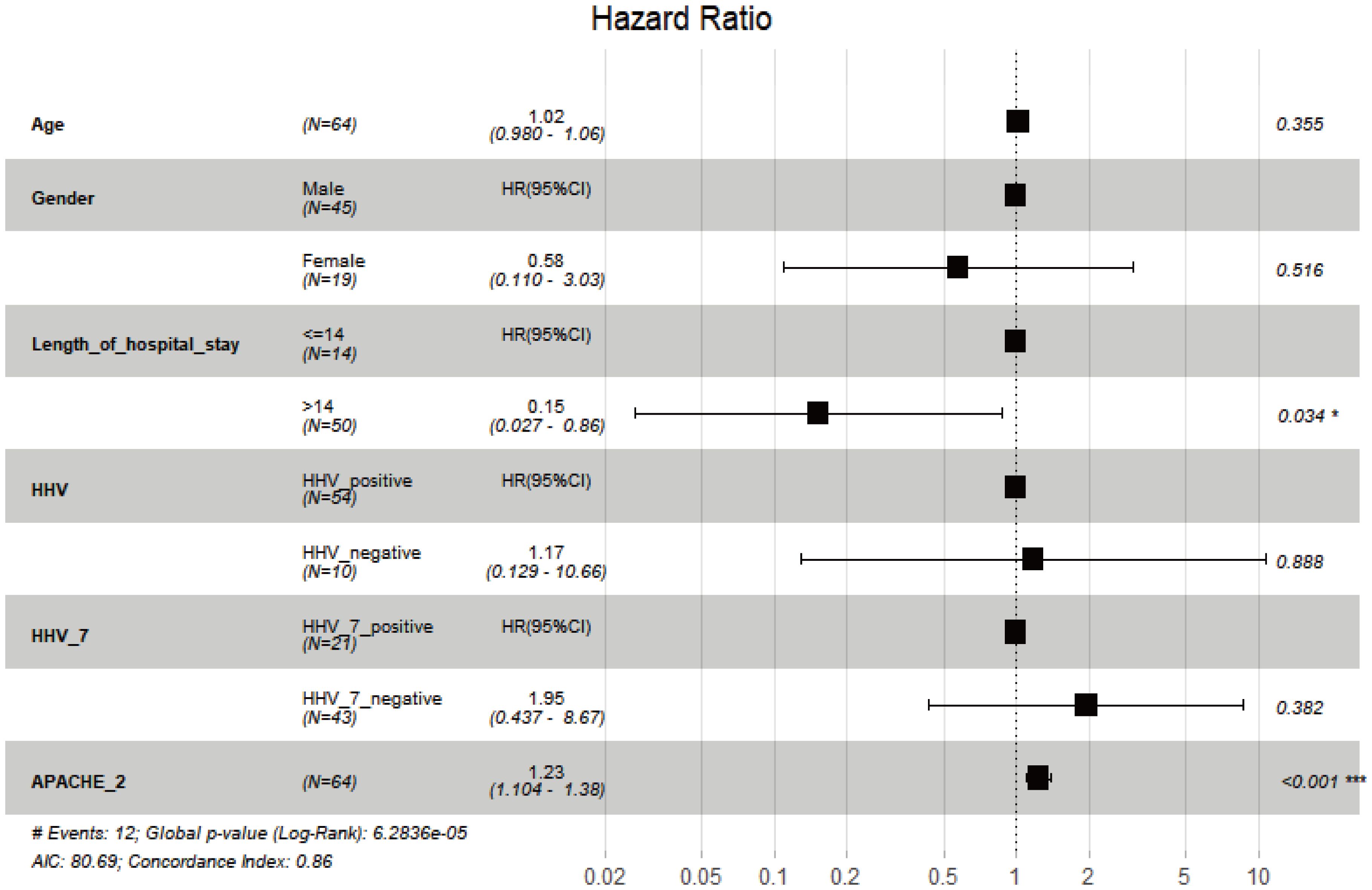
Figure 4. Multivariate analysis of 30-day all-cause mortality of CAP using Cox regression model. *, P < 0.05; ***, P < 0.001.
The proportional hazards assumption for Cox regression was evaluated. The P values for both the individual variable test and the global variable test were all greater than 0.05, indicating that the proportional hazards assumption was not violated. We calculated the Harrell’s C-index (0.791), indicating model fitting is better. Additionally, we performed a sensitivity analysis on the Cox regression model, and we observed that the hazard ratio (HR) value for HHV-7 remained largely unchanged even when other covariates were added or modified.
This study discovered that the older individuals were more vulnerable to sCAP, which aligns with previous research (Lin et al., 2023). The global issue of aging is becoming increasingly critical (Covino et al., 2020; Fan et al., 2024). CAP has emerged as a significant disease affecting individuals aged 65 and above, posing a serious health risk and a major public health concern (Arnold et al., 2020). Consequently, enhancing the awareness about CAP among older individuals, especially in sCAP cases, and prioritizing comprehensive treatment and quality care are crucial for improving the prognosis of CAP in this demographic (Li and Chu, 2023).
HHVs were the predominant infection in CAP patients, followed by bacteria and fungi. Interestingly, the number of viral pathogens detected in this study exceeded the combined number of bacterial and fungal pathogens, reflecting a similar trend observed in CAP patients admitted to hospitals in the United States (Jain et al., 2015). Among the HHVs, EBV and CMV were the most common viruses causing lower respiratory tract infections in CAP patients, which aligned with the findings of a previous multi-center retrospective study (Huang et al., 2023). These findings indicated the accuracy of our CAP pathogen detection rates.
The most prevalent bacterial infection in the lower respiratory tract was Mycobacterium abscessus, with Pneumocystis jirovecii being the most common fungal infection, consistent with previous research (Wei et al., 2020; Wu et al., 2020; Schwartz et al., 2023). Previous studies have identified Streptococcus pneumoniae as the main pathogen causing CAP (Jain et al., 2015; Ferrer et al., 2018; Liu et al., 2023a). It is important to highlight that these studies excluded immunosuppressed patients and did not use mNGS to detect BALF samples, making comparisons with our study challenging. Our research focused on CAP patients from infectious disease hospitals, considering complex complications like immunosuppression, AIDS, renal disease, respiratory insufficiency, and sepsis. Notably, these complications can impact the infectious pathogens. This may be related to the fact that after HHVs infection, the virus replicates and spreads in the body, leading to a decline in the immune system, which increases susceptibility to other pathogens. Immunosuppression is a common risk factor for CAP (Brands et al., 2020), which is why we intentionally included immunosuppressed patients in our study. Interestingly, we did not find a significant difference between sCAP and non-sCAP patients, in line with previous research on CAP caused by respiratory syncytial virus (Di Pasquale et al., 2019; Bahabri et al., 2023).
Patients with sCAP were more susceptible to HHV-7 than those with non-sCAP. Nonetheless, HHV-7 did not independently contribute to the risk of 30-day mortality, as observed in other severe pneumonia cases (Xu et al., 2023). This may be due to the fact that HHV-7 infection is asymptomatic in most adults, so its direct lethality may be relatively low. While a vaccine exists for varicella-zoster virus (VZV) (Curtis et al., 2021), there is currently no efficient method for preventing and treating HHV-7. Nucleoside analogues like acyclovir, ganciclovir, and valaciclovir may be utilized, although ganciclovir proves to be less effective against HHV-7 in comparison to HHV-6 and CMV (Ärlemalm et al., 2022; Sureram et al., 2022). Notably, the presence of HHV-7 in sCAP may potentially exacerbate lung inflammation and systemic inflammatory responses in conjunction with other viruses and bacteria. Thus, it is imperative to investigate whether HHV-7 poses a pathogenic threat to sCAP patients, emphasizing the necessity for the development of targeted anti-HHV medications and safe, effective vaccines. Targeted anti-infective therapy for individuals infected with HHVs play a crucial role in preventing recurrence.
There were several limitations to our research that must be recognized. Firstly, the follow-up period for mortality was limited to 30 days in our study, which was consistent with existing research (Simonetti et al., 2016; Jones et al., 2020). Given that CAP as a lung parenchymal infection, almost all patients showed HHVs by day 28 (Ong et al., 2017). There was a possibility that patients with COVID-19 pneumonia had HSV-1 reactivation within 30 days in BALF samples (Giacobbe et al., 2022). Current international guidelines stressed the importance of prioritizing the 30-day mortality outcome in studies (Martin-Loeches et al., 2023). Secondly, our study was cross-sectional, and mNGS detection of BALF samples was only conducted at the onset of CAP. Therefore, our results can only reflect the characteristics of HHVs distribution during this specific period and can not be generalized to the entire of CAP. Previous researches have shown that HHVs significantly increased with the length of hospital stay (Ong et al., 2017; Huang et al., 2023). Lastly,our study was a single retrospective analysis with a limited sample size, which impeded the generalizability of our findings. Nonetheless, it is crucial to emphasize that the application of mNGS technology facilitated the identification of all pathogens in CAP patients objectively, ensuring a uniform approach to the diagnosis and treatment of mixed infections.
Among the prevalent presence of multiple HHVs, EBV and CMV were the most prevalent in CAP patient. Patients with sCAP were more susceptible to HHV-7 than those with non-sCAP. However, neither HHVs nor HHV-7 was independent risk factors for 30-day mortality in CAP patients. The findings offer important information for clinicians to guide timely interventions in treatment.
The sequencing raw data for this study is public and can be found here: NCBI SRA under project ID: PRJNA1131635.
The studies involving humans were approved by Ethics Committee of Kunming Third People’s Hospital (No. KSLL20230711009). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YD: Data curation, Formal analysis, Methodology, Resources, Writing – original draft, Writing – review & editing. GL: Data curation, Formal analysis, Investigation, Writing – original draft, Writing – review & editing. QL: Data curation, Investigation, Resources, Writing – original draft, Writing – review & editing. LZ: Data curation, Formal analysis, Methodology, Software, Writing – review & editing. JD: Supervision, Validation, Writing – original draft, Writing – review & editing. VC: Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was financially supported by Prince of Songkla University and Ministry of Higher Education, Science, Research and Innovation under the Reinventing University Project (Grant Number REV65022), and Kunming Science and Technology Bureau (2023-1-NS-007).
We thank the study subject and collaborating clinicians for their participation and contribution to the work. Additionally, we thank Dr Jun Zhao, a statistician, from Hubei University of Medicine for statistical evaluation, and provided targeted revisions to ensure the utmost accuracy of these methodologies.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ärlemalm, A., Helldén, A., Karlsson, L., Carlsson, B. (2022). Rapid determination of acyclovir, its main metabolite 9-carboxymethoxymethylguanine, ganciclovir, and penciclovir in human serum using LC-MS/MS. Biomed. Chromatogr. 36, e5315. doi: 10.1002/bmc.5315
Arnold, F. W., Reyes Vega, A. M., Salunkhe, V., Furmanek, S., Furman, C., Morton, L., et al. (2020). Older adults hospitalized for pneumonia in the United States: incidence, epidemiology, and outcomes. J. Am. Geriatr. Soc. 68, 1007–1014. doi: 10.1111/jgs.16327
Bahabri, I., Abdulaal, A., Alanazi, T., Alenazy, S., Alrumih, Y., Alqahtani, R., et al. (2023). Characteristics, management, and outcomes of community-acquired pneumonia due to respiratory syncytial virus: A retrospective study. Pulm. Med. 2023, 4310418. doi: 10.1155/2023/4310418
Brands, X., Haak, B. W., Klarenbeek, A. M., Otto, N. A., Faber, D. R., Lutter, R., et al. (2020). Concurrent immune suppression and hyperinflammation in patients with community-acquired pneumonia. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00796
Connolly, S. A., Jardetzky, T. S., Longnecker, R. (2021). The structural basis of herpesvirus entry. Nat. Rev. Microbiol. 19, 110–121. doi: 10.1038/s41579-020-00448-w
Costa, T., Ribeiro, A., MaChado, R., Ribeiro, C., Lanceros-Mendez, S., Cavaco-Paulo, A., et al. (2019). Polymeric electrospun fibrous dressings for topical co-delivery of acyclovir and omega-3 fatty acids. Front. Bioeng. Biotechnol. 7. doi: 10.3389/fbioe.2019.00390
Covino, M., Piccioni, A., Bonadia, N., Onder, G., Sabia, L., Carbone, L., et al. (2020). Early procalcitonin determination in the emergency department and clinical outcome of community-acquired pneumonia in old and oldest old patients. Eur. J. Intern. Med. 79, 51–57. doi: 10.1016/j.ejim.2020.04.055
Curtis, J. R., Cofield, S. S., Bridges J.R., S. L., Bassler, J., Deodhar, A., Ford, T. L., et al. (2021). The safety and immunologic effectiveness of the live varicella-zoster vaccine in patients receiving tumor necrosis factor inhibitor therapy: A randomized controlled trial. Ann. Intern. Med. 174, 1510–1518. doi: 10.7326/M20-6928
De Rose, D. U., Auriti, C., Lozzi, S., Coltella, L., Piccioni, L., Rossi, S., et al. (2021). Severe herpes virus 6 interstitial pneumonia in an infant with three variants in genes predisposing to lung disease. J. Med. Virol. 93, 5182–5187. doi: 10.1002/jmv.27016
Di Pasquale, M. F., Sotgiu, G., Gramegna, A., Radovanovic, D., Terraneo, S., Reyes, L. F., et al. (2019). Prevalence and etiology of community-acquired pneumonia in immunocompromised patients. Clin. Infect. Dis. 68, 1482–1493. doi: 10.1093/cid/ciy723
Fan, G., Zhou, Y., Zhou, F., Yu, Z., Gu, X., Zhang, X., et al. (2024). The mortality and years of life lost for community-acquired pneumonia before and during COVID-19 pandemic in China. Lancet Reg. Health West. Pac. 42, 100968. doi: 10.1016/j.lanwpc.2023.100968
Ferrer, M., Travierso, C., Cilloniz, C., Gabarrus, A., Ranzani, O. T., Polverino, E., et al. (2018). Severe community-acquired pneumonia: Characteristics and prognostic factors in ventilated and non-ventilated patients. PLoS. One 13, e0191721. doi: 10.1371/journal.pone.0191721
Giacobbe, D. R., Di Bella, S., Dettori, S., Brucci, G., Zerbato, V., Pol, R., et al. (2022). Reactivation of herpes simplex virus type 1 (HSV-1) detected on bronchoalveolar lavage fluid (BALF) samples in critically ill COVID-19 patients undergoing invasive mechanical ventilation: preliminary results from two Italian centers. Microorganisms. 10, 362. doi: 10.3390/microorganisms10020362
Hraiech, S., Bonnardel, E., Guervilly, C., Fabre, C., Loundou, A., Forel, J. M., et al. (2019). Herpes simplex virus and Cytomegalovirus reactivation among severe ARDS patients under veno-venous ECMO. Ann. Intensive Care 9, 142. doi: 10.1186/s13613-019-0616-6
Huang, L., Zhang, X., Pang, L., Sheng, P., Wang, Y., Yang, F., et al. (2023). Viral reactivation in the lungs of patients with severe pneumonia is associated with increased mortality, a multicenter, retrospective study. J. Med. Virol. 95, e28337. doi: 10.1002/jmv.28337
Jain, S., Self, W. H., Wunderink, R. G., Fakhran, S., Balk, R., Bramley, A. M., et al. (2015). Community-acquired pneumonia requiring hospitalization among U.S. Adults. N. Engl. J. Med. 373, 415–427. doi: 10.1056/NEJMoa1500245
Jones, B. E., Ying, J., Stevens, V., Haroldsen, C., He, T., Nevers, M., et al. (2020). Empirical anti-MRSA vs standard antibiotic therapy and risk of 30-day mortality in patients hospitalized for pneumonia. JAMA. Intern. Med. 180, 552–560. doi: 10.1001/jamainternmed.2019.7495
Li, N., Chu, W. (2023). Development and validation of a survival prediction model in elder patients with community-acquired pneumonia: a MIMIC-population-based study. BMC. Pulm. Med. 23, 23. doi: 10.1186/s12890-023-02314-w
Liapikou, A. (2020). The burden of community-acquired pneumonia requiring admission to an ICU in the United States. Chest. 158, 841–843. doi: 10.1016/j.chest.2020.06.017
Lin, T., Tu, X., Zhao, J., Huang, L., Dai, X., Chen, X., et al. (2023). Microbiological diagnostic performance of metagenomic next-generation sequencing compared with conventional culture for patients with community-acquired pneumonia. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1136588
Liu, Y., Wen, Z., Fang, Y., Wang, T., Wu, F., Zhang, H., et al. (2023b). Herpesvirus reactivation in respiratory tract is associated with increased mortality of severe pneumonia patients and their respiratory microbiome dysbiosis. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1294142
Liu, Y. N., Zhang, Y. F., Xu, Q., Qiu, Y., Lu, Q. B., Wang, T., et al. (2023a). Infection and co-infection patterns of community-acquired pneumonia in patients of different ages in China from 2009 to 2020: a national surveillance study. Lancet Microbe. 4, e330–e339. doi: 10.1016/S2666-5247(23)00031-9
Lu, D., Abudouaini, M., Kerimu, M., Leng, Q., Wu, H., Aynazar, A., et al. (2023). Clinical evaluation of metagenomic next-generation sequencing and identification of risk factors in patients with severe community-acquired pneumonia. Infect. Drug Resist. 16, 5135–5147. doi: 10.2147/IDR.S421721
Lv, M., Zhu, C., Zhu, C., Yao, J., Xie, L., Zhang, C., et al. (2023). Clinical values of metagenomic next-generation sequencing in patients with severe pneumonia: a systematic review and meta-analysis. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1106859
Martin-Loeches, I., Torres, A., Nagavci, B., Aliberti, S., Antonelli, M., Bassetti, M., et al. (2023). ERS/ESICM/ESCMID/ALAT guidelines for the management of severe community-acquired pneumonia. Intensive Care Med. 61, 2200735. doi: 10.1183/13993003.00735-2022
Metlay, J. P., Waterer, G. W., Long, A. C., Anzueto, A., Brozek, J., Crothers, K., et al. (2019). Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American thoracic society and infectious diseases society of America. Am. J. Respir. Crit. Care Med. 200, e45–e67. doi: 10.1164/rccm.201908-1581ST
Niederman, M. S., Torres, A. (2022). Respiratory infections. Eur. Respir. Rev. 31, 220150. doi: 10.1183/16000617.0150-2022
Ong, D. S. Y., Bonten, M. J. M., Spitoni, C., Verduyn Lunel, F. M., Frencken, J. F., Horn, J., et al. (2017). Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin. Infect. Dis. 64, 1204–1210. doi: 10.1093/cid/cix120
Quach, D. H., Lulla, P., Rooney, C. M. (2023). Banking on virus-specific T cells to fulfill the need for off-the-shelf cell therapies. Blood. 141, 877–885. doi: 10.1182/blood.2022016202
Ramirez, J. A., Musher, D. M., Evans, S. E., Dela Cruz, C., Crothers, K. A., Hage, C. A., et al. (2020). Treatment of community-acquired pneumonia in immunocompromised adults: A consensus statement regarding initial strategies. Chest. 158, 1896–1911. doi: 10.1016/j.chest.2020.05.598
Saura, O., Chommeloux, J., Levy, D., Assouline, B., Lefevre, L., Luyt, C. E. (2022). Updates in the management of respiratory virus infections in ICU patients: revisiting the non-SARS-CoV-2 pathogens. Expert. Rev. Anti Infect. Ther. 20, 1537–1550. doi: 10.1080/14787210.2022.2134116
Schwartz, B., Dupont, V., Dury, S., Carsin-Vu, A., Guillard, T., Caillard, S., et al. (2023). Aetiology, clinical features, diagnostic studies, and outcomes of community-acquired pneumonia in kidney transplant recipients admitted to hospital: a multicentre retrospective French cohort study. Clin. Microbiol. Infect. 29, 542.e1–542.e5. doi: 10.1016/j.cmi.2022.12.014
Sheng, G., Chen, P., Wei, Y., Yue, H., Chu, J., Zhao, J., et al. (2020). Viral infection increases the risk of idiopathic pulmonary fibrosis: A meta-analysis. Chest. 157, 1175–1187. doi: 10.1016/j.chest.2019.10.032
Simonetti, A. F., Garcia-Vidal, C., Viasus, D., García-Somoza, D., Dorca, J., Gudiol, F., et al. (2016). Declining mortality among hospitalized patients with community-acquired pneumonia. Clin. Microbiol. Infect. 22, 567.e1–567.e5677. doi: 10.1016/j.cmi.2016.03.015
Sun, T., Wu, X., Cai, Y., Zhai, T., Huang, L., Zhang, Y., et al. (2021). Metagenomic next-generation sequencing for pathogenic diagnosis and antibiotic management of severe community-acquired pneumonia in immunocompromised adults. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.661589
Sureram, S., Arduino, I., Ueoka, R., Rittà, M., Francese, R., Srivibool, R., et al. (2022). The peptide A-3302-B isolated from a marine bacterium micromonospora sp. Inhibits HSV-2 infection by preventing the viral egress from host cells. Int. J. Mol. Sci. 23, 947. doi: 10.3390/ijms23020947
Tang, D., Chen, M., Huang, X., Zhang, G., Zeng, L., Zhang, G., et al. (2023). SRplot: A free online platform for data visualization and graphing. PLoS One. 18, e0294236. doi: 10.1371/journal.pone.0294236
The Lancet Healthy Longevity (2021). Older people and political instability. Lancet Healthy. Longev. 2, e528. doi: 10.1016/S2666-7568(21)00210-5
Wei, M., Zhao, Y., Qian, Z., Yang, B., Xi, J., Wei, J., et al. (2020). Pneumonia caused by Mycobacterium tuberculosis. Microbes Infect. 22, 278–284. doi: 10.1016/j.micinf.2020.05.020
Wu, X., Li, Y., Zhang, M., Li, M., Zhang, R., Lu, X., et al. (2020). Etiology of severe community-acquired pneumonia in adults based on metagenomic next-generation sequencing: A prospective multicenter study. Infect. Dis. Ther. 9, 1003–1015. doi: 10.1007/s40121-020-00353-y
Keywords: community-acquired pneumonia, human herpes viruses, metagenomic next generation sequencing, human herpes virus 7, mortality
Citation: Ding Y, Liu G, Li Q, Zou L, Dai J and Chongsuvivatwong V (2024) Distribution characteristics of human herpes viruses in the lower respiratory tract and their impact on 30-day mortality in community-acquired pneumonia patients. Front. Cell. Infect. Microbiol. 14:1436509. doi: 10.3389/fcimb.2024.1436509
Received: 22 May 2024; Accepted: 15 July 2024;
Published: 16 August 2024.
Edited by:
Li Ang, First Affiliated Hospital of Zhengzhou University, ChinaReviewed by:
Tejabhiram Yadavalli, University of Illinois Chicago, United StatesCopyright © 2024 Ding, Liu, Li, Zou, Dai and Chongsuvivatwong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingyi Dai, ZGFpamluZ3lpZGp5QDE2My5jb20=; Virasakdi Chongsuvivatwong, Y3ZpcmFzYWtAbWVkaWNpbmUucHN1LmFjLnRo
†These authors have contributed equally to this work and share first authorship
‡ORCID: Yadi Ding, orcid.org/0009-0001-8350-9659
Qiujing Li, orcid.org/0009-0005-6817-9240
Lingqing Zou, orcid.org/0009-0000-3678-6234
Jingyi Dai, orcid.org/0000-0002-9210-8902
Virasakdi Chongsuvivatwong, orcid.org/0000-0002-9850-4463
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.