
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 30 September 2024
Sec. Bacteria and Host
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1435745
 Toby I. Maidment1
Toby I. Maidment1 Elise S. Pelzer1
Elise S. Pelzer1 Danielle J. Borg2,3
Danielle J. Borg2,3 Eddie Cheung1
Eddie Cheung1 Jake Begun4,5
Jake Begun4,5 Marloes Dekker Nitert6
Marloes Dekker Nitert6 Kym M. Rae2
Kym M. Rae2 Vicki L. Clifton2,3
Vicki L. Clifton2,3 Alison J. Carey1*
Alison J. Carey1*Group B Streptococcus (GBS) asymptomatically colonises the vagina of up to 40% of pregnant women and can transmit to neonates during birth, causing neonatal pneumonia, sepsis, meningitis, and significant mortality. Vaginal GBS colonisation can be attributed to a range of host and bacterial factors, which may include the composition of the vaginal microbial community. There are few studies that have examined the vaginal community composition in relation to GBS colonisation throughout pregnancy. Here, we performed 16S rRNA sequencing (V3-V4) on vaginal swabs from women at 24- and 36-weeks’ gestation, who were GBS culture-negative or GBS culture-positive at either 24 weeks or 36 weeks’ gestation or at both timepoints. Vaginal swabs from 93 women were analysed; 46 women were culture-negative, 11 women GBS culture-positive at 24 weeks only, 21 women GBS culture-positive at 36 weeks only and 15 women GBS culture-positive at both timepoints on Brilliance GBS agar. V3-V4 16S rRNA gene amplicon sequencing demonstrated that in women that were GBS culture-positive at 36 weeks gestation only, G. vaginalis was significantly more abundant at 24-weeks’ gestation despite a lack of significant changes in community richness between the 24- and 36-week samples. The vaginal microbial communities of women persistently colonised with GBS, had a significantly higher abundance of Lactobacillus iners, compared to other groups where L. crispatus, L. gasseri or L. jensenii were dominant. We have characterised the vaginal microbial community composition during pregnancy in relation to GBS colonisation status, in a longitudinal study for the first time. The most interesting finding was that in women that were persistently colonised with GBS throughout pregnancy, there was a significant increase in L. iners and significant reduction in L. crispatus abundance. Given the lack of detail of the role that the vaginal microbial community plays in GBS colonisation in the literature, it is imperative that the relationship between L. iners and GBS in this unique environmental niche is further investigated.
Streptococcus agalactiae (Group B Streptococcus; herein GBS) is a significant cause of global neonatal and perinatal morbidity and mortality (Brokaw et al., 2021). GBS is a gastrointestinal commensal in adults, and up to 40% of pregnant women demonstrate asymptomatic vaginal GBS colonisation (Brokaw et al., 2021). Vaginal colonisation during pregnancy can lead to life-threatening invasive GBS infection of neonates through vertical transmission during delivery (Brokaw et al., 2021; Chaguza et al., 2022). Early onset disease (EOD) due to invasive GBS infection (< 7 days post-partum) can cause severe pneumonia and meningitis. GBS is the leading cause of neonatal sepsis, accounting for approximately 35% of global neonatal mortality cases (Sweeney et al., 2020; Alotaibi et al., 2023). In addition to EOD, ascending GBS infection prior to delivery is associated with an increased risk of preterm birth, stillbirth, premature rupture of membranes and fetal organ damage (Bianchi-Jassir et al., 2017; Brokaw et al., 2021; Mei and Silverman, 2023).
GBS colonisation of the vagina can be attributed to a range of host and bacterial factors. These include biological factors such as a history of ruptured membranes, age, diet, ethnicity, and gastrointestinal colonisation, as well as socioeconomic factors such as hygiene practices, occupation, illiteracy, and sexual activity (Stapleton et al., 2005; Le Doare and Heath, 2013; Capan-Melser et al., 2015; Brokaw et al., 2021). It is important to consider that GBS colonisation occurs within a distinct and typically hostile microbial ecosystem, which likely plays a determining role in facilitating or inhibiting persistent colonisation during pregnancy. Persistent GBS colonisation of the vagina occurs through the expression of several bacterial factors such as adhesins (Baron et al., 2004; Sheen et al., 2011; Jiang and Wessels, 2014), biofilm formation (D’Urzo et al., 2014), immune evasion (Jones et al., 2003; Carey et al., 2014; Kolar et al., 2015), and competitive antimicrobial defences (Gori et al., 2020). However, women can also be transiently colonised by GBS throughout pregnancy (Hansen et al., 2004) and the effects this has on pregnancy outcomes are unknown.
The vaginal microbial community exhibits hormone-dependent changes across the menstrual cycle and during pregnancy a loss of community diversity occurs, decreasing in species richness as pregnancy progresses (Ravel et al., 2011; Romero et al., 2014; Pace et al., 2021; Romero et al., 2023). A healthy vaginal microbial community is dominated by a small number of vaginal lactobacillus species (L. crispatus, L. gasseri, L. iners, and L. jensenii) (Ravel et al., 2011), representing community state types I, II, III and V respectively These lactobacilli populations maintain a low-pH environment (pH ~4.5) through production of lactic acid, which offers protection against colonising pathogens and is thereby an important facet of reproductive health (Ravel et al., 2011; O’Hanlon et al., 2013). Conversely, increased species diversity in the vaginal microbial community and/or dominance of other bacteria such as Gardnerella spp., Prevotella spp., Bifidobacterium spp., or Atopobium spp., can negatively alter the vaginal environment, causing vaginal dysbiosis (Ravel et al., 2011; Coudray and Madhivanan, 2020). Vaginal dysbiosis is associated with adverse maternal and neonatal sequelae; however, causality is difficult to assign to specific microorganisms (Brooks et al., 2017). GBS represents an opportunistic pathogen, present in the vaginal community of a significant number of women throughout pregnancy but is implicated in adverse outcomes only when invasion occurs (Brokaw et al., 2021). To date, characterisation of the vaginal microbial community in health and disease has primarily focussed on taxonomic classification of lactobacilli to species level; however, changes in the vaginal microbial community in the context of lactobacilli alone do not explain GBS colonisation and adverse pregnancy outcomes. Increased taxonomic diversity, Gardnerella vaginalis abundance, and loss of Lactobacillus dominance appear as possible drivers and regulators of GBS colonisation and invasion risk during pregnancy (Sroka-Oleksiak et al., 2020). In healthy, non-pregnant women GBS is not considered a pathogen, and in pregnant women GBS is only considered significant if invasion occurs. Thus, GBS has likely been disregarded in studies examining vaginal microbial communities. Indeed, the less than 30% of studies in this field reporting on GBS, highlight that this opportunistic pathogen is under-reported (Brooks et al., 2015; Lim et al., 2021).
Several cultivation-dependent investigations of the vaginal microbial community have previously identified compositional features associated with GBS colonisation similar to those in bacterial vaginosis (BV) (Bayo et al., 2002; Ronnqvist et al., 2006; Brzychczy-Wеloch et al., 2014; Tano et al., 2021). These include reductions in lactobacilli and increased populations of several BV-associated taxa such as Gardnerella vaginalis, Prevotella spp., and Atopobium spp., in addition to frequent co-colonisation with the opportunistic yeast Candida albicans (Bayo et al., 2002; Ronnqvist et al., 2006; Brzychczy-Wеloch et al., 2014; Pidwill et al., 2018; Tano et al., 2021). Using cultivation-independent analysis of the vaginal microbiota of 44 Egyptian women during the third trimester of pregnancy (Shabayek et al., 2022), Shabayek et al. (2022) similarly found GBS positive women to have significantly increased community diversity and lower abundances of Lactobacillus spp., compared to GBS negative women (Shabayek et al., 2022). Despite these findings however, our understanding of how GBS impacts the vaginal microbial community remains extremely limited. A considerable knowledge gap remains regarding community-wide changes in the vaginal microbial community throughout pregnancy that are associated with transient versus persistent GBS colonisation.
In this study, we used 16S rRNA gene amplicon sequencing to characterise the vaginal microbial communities of GBS culture-positive and -negative women at both 24- and 36-weeks’ gestation. We defined community-wide patterns in the vaginal microbiota associated with transient (culture-positive at one time point only) and persistent GBS carriage (culture-positive at both timepoints) during pregnancy, to better understand risk factors associated with GBS colonisation during pregnancy.
Participants, who freely gave informed consent, were recruited as part of the Queensland Family Cohort (QFC) prospective, longitudinal, and observational pilot study. The details of this study are published (Borg et al., 2021) and has full ethical approval by Mater Misericordiae Research Ethics committee (HREC/16/MHS/113).
At the 24- and 36- weeks’ gestation appointments the participants completed surveys on medication and lifestyle substance use. Medication use during labour and delivery, and delivery details including complications, as well as neonatal outcomes were also recorded and available. Participants were excluded from this study if they received antibiotics in the two weeks prior to vaginal swab collection, if they had diabetes (or developed gestational diabetes), or indicated they were taking probiotics. These time points represent sampling from the second and third trimester of gestation and occur prior to full term gestation (<37 weeks), where GBS may contribute to premature birth.
Upon enrolment participants were given detailed instructions on how to self-collect the vaginal swab and provided with the E-swab (Copan, CA, USA). Briefly, participants were instructed to insert the swab 3-5 centimetres into the vagina and while rolling the swab, wipe the vaginal wall in 3 full circles, ensuring the swab was kept in the vagina for a minimum of 20 seconds. Participants were instructed to not use vaginal douches, feminine sprays, genital wipes, vaginal medications/suppositories, or have sexual intercourse 48 hours prior to swab collection. Swabs were collected at the participant’s 24- and 36-week gestation maternal appointments and stored at -80°C until use. Placentae were observed at the time of birth and classified as normal/abnormal by the collector at the time.
All swabs were thawed and cultured on Brilliance GBS agar plates (ThermoFisher, Seventeen Mile Rocks QLD, Australia) using the 16-streak dilution technique as per Australian diagnostic standards. Plates were incubated at 37°C, atmospheric conditions (O2) for 24-48 hours and the growth of GBS was recorded as per the manufacturer’s instructions. Well isolated, individual GBS colonies were sub-cultured onto 5% Horse blood agar containing Colistin and Nalidixic Acid (CNA; ThermoFisher). Colony characteristics were recorded and well isolated colonies from the CNA were used to determine the serotype of the GBS using the ImmuLex Streptococcus-B kit (SSI Diagnostica, Denmark) as per manufacturer’s instructions.
Based on the GBS culture results participants were grouped into either GBS culture-negative, GBS culture-positive at 24 weeks or 36 weeks’ gestation only, or GBS culture-positive at both 24- and 36-weeks’ gestation. Vaginal swabs were thawed on ice, vortexed vigorously to suspend the bacterial cells into the liquid Amies solution and 500 μL of the liquid Amies was transferred to a sterile 2 mL tube on ice. For cell lysis, 50 μL of lysozyme (10 mg/ml stock; Sigma-Aldrich, NSW, Australia), 6 μL of mutanolysin (25,000 U/ml stock; Sigma-Aldrich), 3 μL of lysostaphin (4,000 U/ml in sodium acetate stock; Sigma-Aldrich), and 41 μL of TE50 buffer (10 mM Tris-HCl, 50 mM EDTA [pH 8.0]) were added to each sample. Samples were incubated at 37°C for 1 hr, then 10 μL of proteinase K (20 mg/mL stock; Qiagen, VIC, Australia), 100 μL of 10% sodium dodecyl sulfate, and 20 μL of RNase A (20 mg/ml stock; ThermoFisher) to each sample. Samples were incubated for 1 hr at 55°C. Following enzymatic lysis, samples were mechanically disrupted and homogenized using a Biospec Mini-BeadBeater 16 (Biospec, Oklahoma, USA). DNA was extracted using the QIAamp DNA mini kit (Qiagen) as per manufacturer’s instructions, omitting the recommended lysis step, as enzymatic and physical lysis was completed above. DNA was eluted using the molecular grade water provided with the Qiagen kit, pre-warmed to 56°C.
Library preparation and 16S rRNA gene amplicon sequencing was performed at the Australian Genome Research Facility (Melbourne, Victoria, Australia) using the Illumina MiSeq platform with 2x300bp chemistry. Sequencing was performed using a modified 319F (5’-CCTACGGGAGGCAGCAGT-3’) primer and 806R (5’-GGACTACHVGGGTWTCTAAT-3’) primer targeting the V3-V4 hypervariable region, which were selected due to use in prior investigations of the vaginal microbial communities (Graspeuntner et al., 2018; Van Der Pol et al., 2019; Romero et al., 2023).
Demultiplexed, 300-bp paired-end reads were imported into Quantitative Insights into Microbial Ecology-2 (QIIME2; v2021.11) (Bolyen et al., 2019), after which adapter sequences were removed using Cutadapt (Kechin et al., 2017) and reads quality checked using Q2-Demux. Denoising and amplicon sequence variant (ASV) assignment were performed on quality-filtered reads using the Deblur ‘denoise-16S’ tool (Amir et al., 2017). Denoised representative sequence outputs were then assigned taxonomy using a region-specific V3-V4 taxonomic classifier with the Classify-sklearn tool (Bokulich et al., 2018), which was built using q2-rescript (Robeson et al., 2021) with the SILVA database (SSUr138, NR_99; https://www.arb-silva.de/), and trained using the fit-classifier-naïve-bayes tool prior to use. Following this, the feature table was filtered to remove rare taxa (< 2 samples), taxa unassigned past the domain level, chloroplast sequences, and mitochondrial sequences. As contamination is commonplace in 16S rRNA gene amplicon data, taxonomic data was screened for putative contaminants using Decontam (Davis et al., 2018), with suspected contaminant ASVs identified and filtered from feature data based on their prevalence in negative controls (threshold = 0.5). Assignment of vaginal community state types (CSTs) to individual samples was performed manually based on the relative abundance of important taxa outlined by France et al. (2020), including sub-types for CSTs I, III, and IV (France et al., 2020). Subsequent visualisation of taxonomic and CST data was performed using QIIME2 (v2021.11) in addition to the R packages ggplot2 (v3.4.1), Microbiome (v1.16.0), and Phyloseq (v1.38.0) (Lahti and Shetty, [[NoYear]]; Wickham et al., [[NoYear]]; McMurdie and Holmes, 2013).
Rooted and unrooted phylogenetic trees used for subsequent analysis were then produced using the align-to-tree-mafft-fasttree QIIME2 command, after which generic alpha and beta diversity calculations were generated using the q2-diversity core-metrics phylogenetic tool from a feature table rarefied to 17,000 reads per sample. Statistically significant differences in Shannon entropy between sample groups were identified via Kruskal-Wallis test, using the q2-diversity alpha-group-significance tool. Statistical differences in community structure were calculated using Analysis of Similarity (ANOSIM) testing on unweighted Unifrac distances with 4000 permutations. Differential abundance testing was performed with DeSeq2 (Love et al., 2014) via Phyloseq (McMurdie and Holmes, 2013), using the geometric means of CLR-transformed count data. The Wald test with Benjamini-Hochberg multiple test corrections was used to identify taxa which significantly differed in abundance between groups, with an FDR-adjusted P-value <0.05 deemed statistically significant.
To ensure the validity of the low biomass vaginal samples, several extraction controls were included. This included a negative swab control where the swab was removed from the sterile transport tube exposed to the air where extractions were to be performed and sample lysis and DNA extraction was performed as described above. There was a DNA extraction kit control, where the water used to elute the DNA was used as a ‘sample’ and underwent DNA extraction. These controls were completed to account for possible kit and environmental contamination. A Gram-positive and Gram-negative positive control were also included and consisted of a combination of Enterococcus faecalis (ATCC29212) and Escherichia coli (ATCC8739), respectively (Maidment et al., 2023).
Women were recruited to the pilot QFC study at Mater Hospital and at their 24- and 36-weeks’ gestation appointment, completed surveys and provided a self-collected vaginal swab (n = 209). Vaginal swabs from 93 women were analysed and based on the culture of vaginal swabs were grouped as follows: GBS culture-negative at both 24- and 36-weeks’ gestation (GBS NEG; n = 46 women), GBS culture-positive at 24 weeks’ only (GBS 24 wk; n = 11 women), GBS culture-positive at 36 weeks’ only (GBS 36 wk; n = 21 women) and GBS culture-positive at both 24- and 36-weeks’ gestation (GBS 24/36 wk; n = 15 women). There was no difference in the maternal age or gestational age at birth between the groups and women were primarily of Caucasian decent (Table 1). Interestingly in those women that were GBS culture-positive at either or both collection timepoints, there was a 1.5 – 2-fold increase in observed placental abnormality, albeit not significant (Table 1).
16s rRNA gene amplicon sequencing of the V3-V4 variable region was performed on DNA extracted from vaginal swabs. This yielded 11,350,333 reads across 202 samples (range 53 - 96,455; Supplementary Table 1). Following denoising and ASV assignment, we identified 727 features at a total frequency of 6,681,294 across 201 samples (median frequency – 33,790 per sample; full range - 27 to 66,127 per sample), of which 41 were deemed to be contaminant artifacts and subsequently removed. One negative control sample (ddH2O only control) was filtered from the dataset during denoising. After filtering remaining control samples (n=11) and two samples of unknown GBS status (ID 119) from the dataset, 706 features remained across 188 samples, at a total frequency of 4,708,210 (median frequency - 25,472.5 per sample).
Figure 1A displays the relative abundance of the top 12 most abundant bacterial species. This highlights the high abundance of L. crispatus in the vaginal microbial community of GBS negative pregnant women but not in women who were persistently colonised with GBS at both collection timepoints.

Figure 1. Relative abundance of bacterial species in the vagina of women GBS culture negative and culture positive throughout pregnancy and the distribution of vaginal community state types. (A) shows a heat-map representation of bacterial relative abundance in individual vaginal samples, grouped by GBS culture status and gestational timepoint. (B) displays the proportional composition of vaginal community state types within each group at 24- and 36- weeks’ gestation. CST – Community state type; GBS – Group-B streptococcus (Streptococcus agalactiae). CST I-A: Higher % L. crispatus; CST I-B: Lower % L. crispatus; CST II: L. gasseri dominant; CST III-A: Higher % L. iners; CST III-B: Lower % L. iners; CST IV-A: moderate Gardnerella vaginalis and BV-associated bacteria-1; CST IV-B: moderate Atopobium vaginae and G. vaginalis; CST IV-C0: Diverse with Prevotella spp. present; CST IV-C1: Streptococcus spp. present; CST IV-C2: Enterococcus spp. present; CST IV-C3: Bifidobacterium spp. dominant; CST IV-C4: Staphylococcus spp. present; CST V: L. jensenii dominant.
To further characterise the vaginal microbial communities, we examined the community state types at both timepoints (CSTs; Figure 1B). Full taxonomic composition in each table can be found in Supplementary Tables 2–5. In the GBS negative group (n = 46), CST-I (L. crispatus dominant) represented the most prevalent CST (60%), followed by CST-III (L. iners dominant; 20%), CST-V (L. jensenii dominant; 7.37%), and CST-II (L. gasseri dominant; 5.26%). A non-Lactobacillus CST (-IV) was also identified in 7.4% of samples, with subtypes consisting of IV-B (Gardnerella vaginalis dominant; 1 sample at 24 weeks, 2 at 36 weeks), IV-C0 (even community with Prevotella spp.; 1 sample at each timepoint), and IV-C3 (Bifidobacterium dominant; 1 sample at each timepoint). Overall, the vaginal microbial community of GBS negative women remained stable between the 24- and 36-week timepoints, displaying no significant changes in community richness, and no significantly differentially abundant taxa (Figure 1B).
In the GBS positive 24-week group (n = 11), CST-I was the most prevalent vaginal CST at both gestational timepoints (43.5% total) followed by CST-III (26.1%), and CST-IV (30.4%; Figure 1B). Despite cultivation results, GBS was identified in just one individual sample at 24-weeks’ gestation (relative abundance – 59.2%) and one at 36-weeks (0.3% relative abundance). All samples containing Streptococcus spp. presented either CST-III or IV, with none exhibiting L. crispatus (CST-I) dominance. A decrease in the number of samples exhibiting CST-IV was observed at the 36-week timepoint in this group (GBS 24 wk group).
The GBS positive 36-week group contained 21 paired samples and CST-I represented the most common Lactobacillus-dominated vaginal CST in this group at both 24- and 36-weeks’ gestation (45% samples at both), followed by CST-III (24 weeks - 10%, 36 weeks – 30%), CST-V (24 weeks - 10%, 36 weeks – 0%), and CST-II (5% both; Figure 1B). A non-lactobacilli dominated CST-IV was identified in 30% and 25% of subjects at 24 weeks and 36 weeks’, respectively. These CST-IV samples were comprised of the subtypes IV-B (24 week – 10%, 36 week – 15%), IV-C0 (24 week – 20%, 36 week – 0%), and IV-C3 (24 week – 0%, 36 week – 5%). While there were no significant changes in community richness between 24- and 36-week samples in this group, the abundance of G. vaginalis was significantly more abundant at 24-weeks’ gestation compared to 36 weeks’ (Wald P-adj < 0.01). Interestingly, the 24-week timepoint samples in this group (GBS 36 wk), which were GBS culture negative, had a higher frequency of participants that had a diverse microbial community (CST-IV).
The most prevalent vaginal microbial CST in the GBS positive 24/36-week group (n = 15) was CST-III (L. iners dominated) which was identified in 46.7% of samples at both gestational timepoints (Figure 1B). CST-IV represented the next most prevalent compositional profile in this group with a prevalence of 26.7% at both timepoints, followed by CST-I (20% - both timepoints), and CST-II (6.7% - both timepoints). GBS was detected by 16S rRNA gene amplicon sequencing in the vaginal microbiota of 11/15 subjects in this group for at least one gestational timepoint, seven of which were positive at both, three at 24 weeks’ only, and one at 36 weeks’ only (Figure 2A; Table 2). As observed in other groups, vaginal microbial community richness remained stable between gestational time points, with only three individuals exhibiting shifts between CSTs (excluding changes within Lactobacillus-dominant CST subtypes).
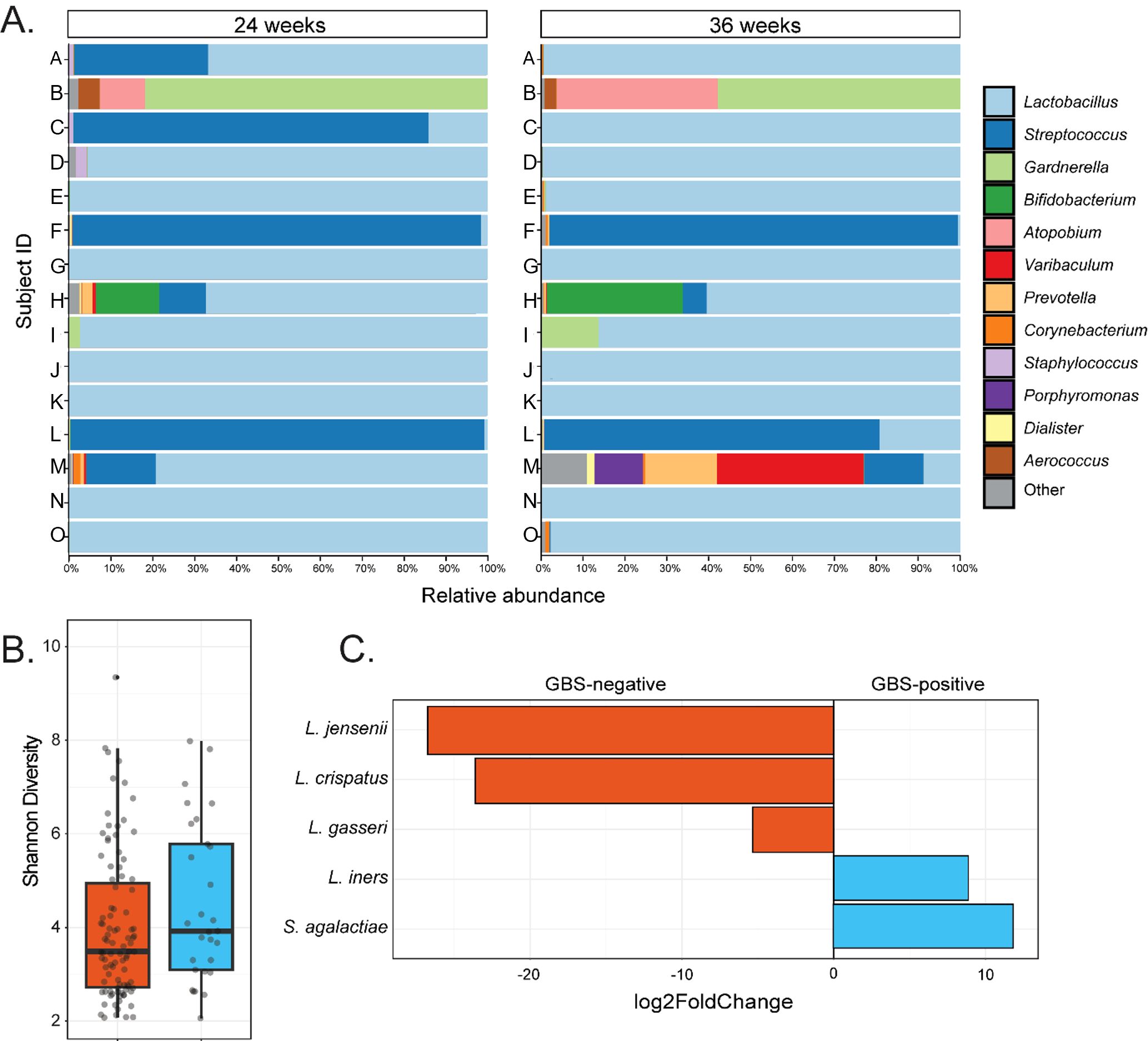
Figure 2. Taxonomic and compositional features of the vaginal microbiota in women GBS culture positive at both 24- and 36-weeks’ gestation. (A) Shows genus-level taxonomic bar plots for all individuals in the GBS 24/36 wk group, faceted by gestational timepoint. (B) Shows difference in Shannon diversity (alpha diversity) between GBS culture negative and the GBS positive at 24- and 36-week groups. (C) Displays taxa which significantly (Wald P-adj < 0.05) differed in abundance between GBS culture negative samples and the samples that were culture positive at both gestational timepoints, with degree of difference displayed on the X-axis as log2foldChange.
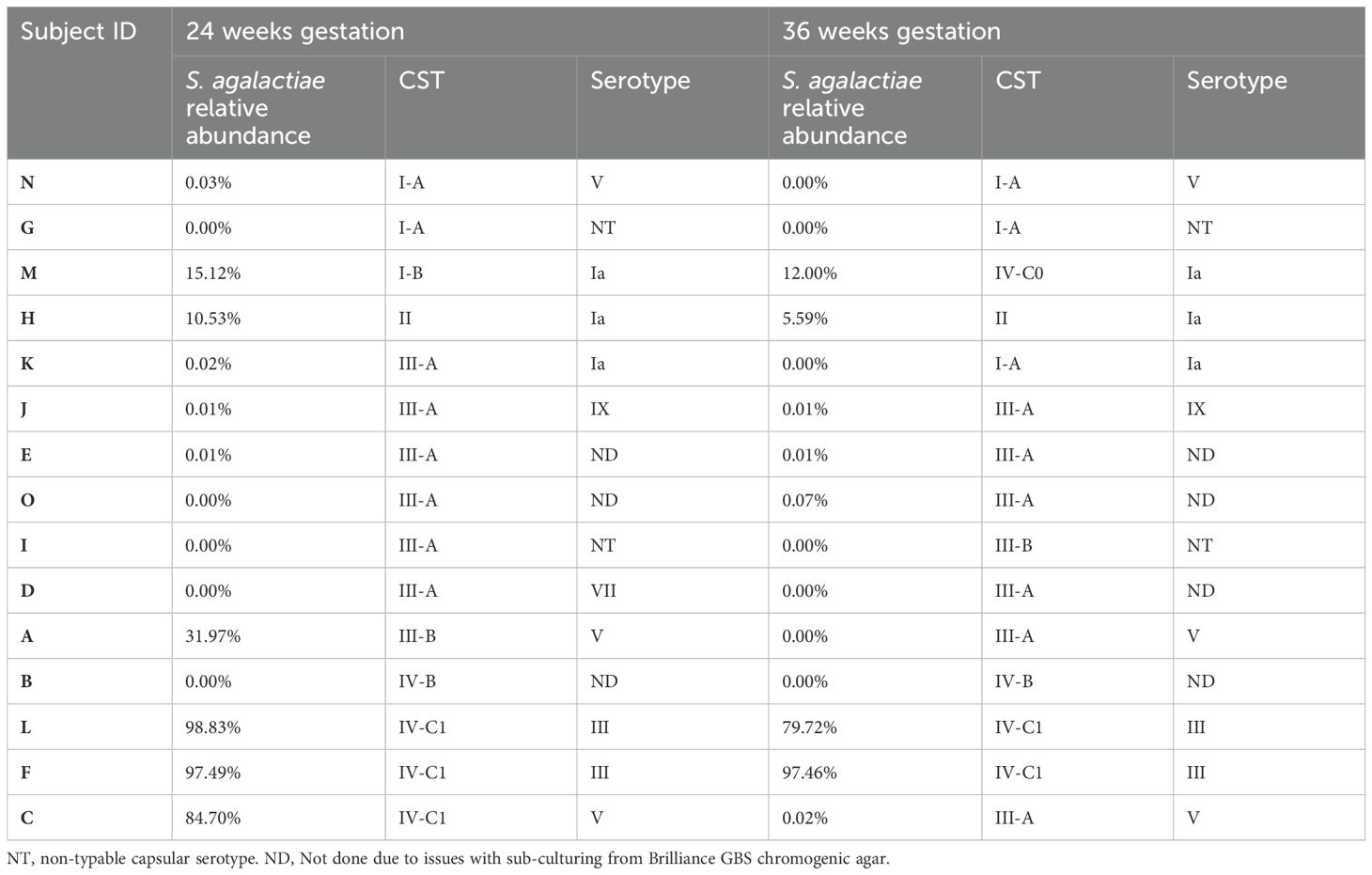
Table 2. Relative abundances of S. agalactiae and serotype in the vaginal microbiota of culture-positive samples, ordered based on their corresponding community state type at 24- and 36-weeks gestation.
The relative abundance of GBS in samples that were culture-positive at both gestational timepoints varied greatly between individuals, ranging from 98.8% to 0.01% (median – 8.1%; Figure 2A). In subjects where GBS was detected via 16S rRNA sequencing in the vaginal microbiota in at least one timepoint, the median relative abundance of GBS was higher at 24 weeks’ gestation (median = 10.5%) than was observed at 36 weeks’ (median – 0.05%), with an average decrease in GBS relative abundance of 11% from 24 to 36 weeks’ gestation (Table 2). Interestingly, in those women persistently colonised with GBS, the same serotype was detected in 60% of women at both timepoints (20% serotype 1a (3/15); 13% serotype III (2/15); 20% serotype V (3/15); 7% serotype IX (1/15); 13% non-typable (i.e. GBS growth, but no serotype confirmed; 2/15)). There were a small number (20%; 3/15) of samples that were unable to be serotyped as they would not successfully subculture from the chromogenic agar and the colonies taken from the chromogenic agar would not react with the serotyping kit, possibly due to the proprietary chromogens in the agars affecting viability and capsular expression. One sample (7%) was only able to be serotyped at one of the timepoints.
To identify differences in the vaginal microbial communities associated with persistent GBS colonisation, we performed group comparisons between GBS positive at 24- and 36-week group and GBS negative group’s vaginal samples with respect to phylogenetic diversity, community structure, and differential abundance testing. We identified no significant differences in community richness (Shannon diversity; Kruskal-Wallis P > 0.05; Figure 2B) or structure (Unweighted UniFrac; ANOSIM P > 0.05) between GBS culture-negative and culture-positive groups at both single and grouped timepoints. However, differential abundance testing identified significantly higher abundances of L. iners (Wald P-adj < 0.05), and S. agalactiae (Wald P-adj < 0.001), as well as significantly lower abundances of L. crispatus (Wald P-adj < 0.001), L. gasseri (Wald P-adj < 0.05), and L. jensenii (Wald P-adj < 0.001) in GBS positive samples compared to GBS negative samples (Figure 2C).
This study shows the dynamics of the vaginal microbial community during the second and third trimesters of pregnancy, in relation to GBS colonisation. The relationship between GBS and other bacterial taxa in the vagina is complex and poorly understood. Here we have demonstrated that the vaginal microbial community profile in women persistently colonised with GBS had a greater abundance of L. iners, compared to other Lactobacillus spp. in women not colonised with GBS. The vaginal microbial community tended to be more diverse in women exhibiting transient vaginal GBS colonisation, though not significant. The strength of our study is the longitudinal nature, in relation to GBS colonisation.
The composition of the vaginal microbial community changes throughout pregnancy, with increased abundance of Lactobacillus species and decreased abundance of anaerobic species (Romero et al., 2014). A longitudinal study from four timepoints during pregnancy demonstrated a pronounced shift in the CST composition with advancing gestational age, with those that were originally CST IV (diverse bacteria with no Lactobacillus spp.) becoming CST I (L. crispatus dominated) or CST III (L. iners dominated) by the end of the pregnancy (Romero et al., 2023). In line with previous studies, while we saw slight variations in the abundance of L. crispatus or L. iners between the two timepoints, each group of women had a relatively stable L. crispatus/L. iners dominance throughout pregnancy.
Vaginal metagenomic sequencing confirms GBS has a positive co-occurrence with L. iners and negative co-occurrence with L. crispatus (Pace et al., 2021). Culture of vaginal swabs has also shown GBS presence to be inversely related to any Lactobacillus species, with detection of L. crispatus being particularly uncommon in GBS positive samples (Starc et al., 2022). Accordingly, we observed that women persistently colonised with GBS had significantly decreased representation of L. crispatus compared to women with no or transient colonisation. Women who were GBS positive at 24 weeks only, tended to have more diverse vaginal microbial communities compared to GBS negative women, though not significant, and by 36 weeks, when GBS was not detected, the vaginal microbial community had shifted and was dominated by a combination of L. crispatus and L. iners. In contrast to our findings, a 16S rRNA vaginal microbiome study of pregnant women in Egypt showed that L. iners was predominant in GBS culture-negative women (Shabayek et al., 2022). However, GBS positive women did have a more diverse, less homogenous vaginal microbial communities, with a significant decrease in Lactobacillus spp. abundance and significantly higher Ureaplasma, Gardnerella, Streptococcus, Corynebacterium, Staphylococcus, and Peptostreptococcus genera (Rosen et al., 2017; Shabayek et al., 2022), similar to what we observed in women GBS positive at 24 weeks’ only. It is also well established that ethnicity and geographic location can influence vaginal microbiota composition, with differences in predominant CST commonly observed between regions (Roachford et al., 2022).
Reduced taxonomic diversity and a Lactobacillus spp. dominance in the vaginal niche are a possible mechanism for protection against GBS colonisation (Brokaw et al., 2021). Reduced lactic acid producing-Lactobacillus spp. dominance causes an increase in the vaginal pH (Ronnqvist et al., 2006; Ravel et al., 2011). In vitro studies have demonstrated that GBS binds to vaginal epithelial cells at a 4-fold higher rate when the pH is elevated (Park et al., 2012). Additionally, fluctuations in pH, from acidic to neutral, cause an upregulation of expression of numerous GBS virulence factors, which in turn can change GBS from an asymptomatic carriage state to a virulent invasive state (Brokaw et al., 2021).
L. iners is commonly detected in specimens from women with BV and is often considered a transitional species that provides less protection against pathogens than other Lactobacillus spp (Ferris et al., 2007; Zozaya-Hinchliffe et al., 2010).. For example, L. iners is only able to produce L-lactic acid, compared to L. crispatus, L. gasseri and L. jensenii which can make both L-lactic acid and D-lactic acid (France et al., 2016). The production of D-lactic acid is suggested to have greater inhibitory effect on exogenous bacteria than L-lactic acid (Zheng et al., 2021). L. crispatus can also generate antibacterial hydrogen peroxide, where L. iners cannot, again highlighting that L. iners is not as effective at protecting the vaginal environment against opportunistic pathogens such as GBS (France et al., 2016; Zheng et al., 2021). In cases of BV, L. iners often coexists with other potentially harmful bacteria associated with poor pregnancy outcomes and is not easily displaced by pathogens (Ferris et al., 2007; Zozaya-Hinchliffe et al., 2010). Co-infection with G. vaginalis, the most abundant member of a dysbiotic microbiota, and GBS, led to a 10-fold higher risk of GBS vaginal colonisation in a pregnant mouse model, with 40% of co-infected mice exhibiting ascending GBS infection of the uterus and placenta (Gilbert et al., 2021). In our study, women who were GBS positive at any or all timepoints examined displayed an increase in placental abnormalities detected. GBS infection of the uterus and placenta are associated with poor pregnancy outcomes including premature rupture of membranes, pre-term birth, clinical chorioamnionitis and neonatal infection (Bianchi-Jassir et al., 2017; Brokaw et al., 2021; Mei and Silverman, 2023). This highlights the importance of understanding how GBS can colonise a unique environmental niche, such as the vagina, and subsequently ascend to cause such sequalae.
In women that were persistently colonised with GBS there was an average decrease of 11% of GBS abundance as pregnancy progressed. It is established that the diversity of the vaginal microbial community decreases as pregnancy progresses (Romero et al., 2014; Romero et al., 2023). It has been suggested that this is hormonally driven, with increasing lactobacilli abundance associated with an increase in oestrogen (Prince et al., 2015). However, this is a field that is also lacking and requires far more detailed research to determine what drives the decrease in microbial diversity or increases in lactobacilli abundance as pregnancy progresses.
Our study has several strengths and limitations that need to be acknowledged. The cohort that the samples used here were collected from were a group of mostly healthy women, where extensive data such as patient demographics, medical usage and neonatal outcomes were collected (Borg et al., 2021). Importantly, these samples were able to be collected in a longitudinal manner, allowing us to examine changes in the vaginal microbial communities throughout pregnancy, in relation to GBS colonisation. In the state of Queensland, Australia, GBS screening is not recommended as part of the standard perinatal care, and a risk-based approach is used instead (Guidelines QC, 2022). This means that there are no official diagnostic records for GBS colonisation status. We did however use the diagnostic standard agar culture to differentiate our groups. Furthermore, we completed PCRs on the samples targeting the highly conserved sip gene (data not shown), confirming our culture results. As with any study involving human participants, larger sample sizes are always preferred. We included all eligible patient samples in this study and have examined the vaginal microbial community in relation to GBS colonisation, longitudinally throughout pregnancy. There are numerous V3-V4 primer sets that have been used for vaginal microbiome determination (Graspeuntner et al., 2018; Van Der Pol et al., 2019; Hugerth et al., 2020), with little consistency between studies. The primers used here were chosen based on broad coverage of diverse vaginal taxa (Graspeuntner et al., 2018; Van Der Pol et al., 2019). The primers that we used were unfortunately not highly specific towards clinical GBS isolates, which meant that for some samples where GBS was cultured, the sequencing was not able to specifically identify all isolates of GBS (Table 2). The primers were originally selected because of their ability to identify a wide range of bacterial species in the vaginal niche when compared to the use of V1-V2 primers (Graspeuntner et al., 2018).
Very few studies have examined vaginal microbial communities in relation to GBS colonisation during pregnancy (Shabayek et al., 2022), and this has only been done at a single timepoint during pregnancy, usually in the last trimester of pregnancy, preventing temporal changes from being detected (Shabayek et al., 2022; McCoy et al., 2023). The relationship between GBS and other bacterial taxa in the vagina is complex and poorly understood. Here, we have shown for the first time how the vaginal microbial communities change throughout pregnancy with changes in GBS colonisation status, and that GBS colonisation may be associated with a reduction in L. crispatus, L. gasseri and L. jensenii dominance in comparison to women who were not colonised with GBS at either timepoints. In women that were persistently colonised with GBS throughout pregnancy, we demonstrated a significant increase in L. iners and significant reduction in L. crispatus abundance. Given the lack of understanding of how the vaginal microbial community contributes to or prevents GBS colonisation, it is imperative to further investigate how L. iners and GBS interact in this unique environmental niche.
The datasets presented in this article are not readily available as the participants of this study did not give written consent for their clinical data to be shared publicly, so due to the sensitive nature of the research, supporting data is not publicly available. Access to raw sequencing reads and metadata may be achieved upon request to the corresponding author and consultation with the QFC governance committee.
The studies involving humans were approved by Mater Research Institute, University of Queensland. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
TM: Data curation, Formal analysis, Investigation, Software, Visualization, Writing – original draft, Writing – review & editing. EP: Writing – original draft, Writing – review & editing, Conceptualization, Funding acquisition, Methodology, Project administration. DB: Writing – review & editing, Data curation, Project administration. EC: Writing – review & editing, Formal analysis, Investigation, Methodology. JB: Writing – review & editing, Conceptualization. MD: Conceptualization, Writing – review & editing, Methodology. KR: Conceptualization, Writing – review & editing, Project administration. VC: Conceptualization, Writing – review & editing, Investigation, Resources, Writing – original draft, Data curation, Methodology, Project administration. AC: Conceptualization, Investigation, Resources, Writing – original draft, Writing – review & editing, Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Software, Supervision, Validation, Visualization.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by funding provided to AC from the QUT Women in Science Fund and the Institute of Health & Biomedical Innovation Mid-career researcher grant.
The authors would like to acknowledge the families that agreed to participate in the QLD Family Cohort Study and the midwives and staff involved in recruitment and participant follow-up.
Author JB was employed by Mater Misericordiae Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1435745/full#supplementary-material
Alotaibi, N. M., Alroqi, S., Alharbi, A., Almutiri, B., Alshehry, M., Almutairi, R., et al. (2023). Clinical characteristics and treatment strategies for group B Streptococcus (GBS) infection in pediatrics: A systematic review. Medicina (Kaunas) 59, 1279. doi: 10.3390/medicina59071279
Amir, A., McDonald, D., Navas-Molina, J. A., Kopylova, E., Morton, J. T., Zech Xu, Z., et al. (2017). Deblur rapidly resolves single-nucleotide community sequence patterns. mSystems 2, e00191-16. doi: 10.1128/mSystems.00191-16
Baron, M. J., Bolduc, G. R., Goldberg, M. B., Auperin, T. C., Madoff, L. C. (2004). Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J. Biol. Chem. 279, 24714–24723. doi: 10.1074/jbc.M402164200
Bayo, M., Berlanga, M., Agut, M. (2002). Vaginal microbiota in healthy pregnant women and prenatal screening of group B streptococci (GBS). Int. Microbiol. 5, 87–90. doi: 10.1007/s10123-002-0064-1
Bianchi-Jassir, F., Seale, A. C., Kohli-Lynch, M., Lawn, J. E., Baker, C. J., Bartlett, L., et al. (2017). Preterm birth associated with group B streptococcus maternal colonization worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 65, S133–Ss42. doi: 10.1093/cid/cix661
Bokulich, N. A., Kaehler, B. D., Rideout, J. R., Dillon, M., Bolyen, E., Knight, R., et al. (2018). Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6, 90. doi: 10.1186/s40168-018-0470-z
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Borg, D., Rae, K., Fiveash, C., Schagen, J., James-McAlpine, J., Friedlander, F., et al. (2021). Queensland Family Cohort: a study protocol. BMJ Open 11, e044463. doi: 10.1136/bmjopen-2020-044463
Brokaw, A., Furuta, A., Dacanay, M., Rajagopal, L., Adams Waldorf, K. M. (2021). Bacterial and host determinants of group B streptococcal vaginal colonization and ascending infection in pregnancy. Front. Cell. infection Microbiol. 11, 720789. doi: 10.3389/fcimb.2021.720789
Brooks, J. P., Buck, G. A., Chen, G., Diao, L., Edwards, D. J., Fettweis, J. M., et al. (2017). Changes in vaginal community state types reflect major shifts in the microbiome. Microbial Ecol. Health disease. 28, 1303265. doi: 10.1080/16512235.2017.1303265
Brooks, J. P., Edwards, D. J., Harwich, M. D., Jr., Rivera, M. C., Fettweis, J. M., Serrano, M. G., et al. (2015). The truth about metagenomics: quantifying and counteracting bias in 16S rRNA studies. BMC Microbiol. 15, 66. doi: 10.1186/s12866-015-0351-6
Brzychczy-Wеloch, M., Pabian, W., Majewska, E., Zuk, M. G., Kielbik, J., Gosiewski, T., et al. (2014). Dynamics of colonization with group B streptococci in relation to normal flora in women during subsequent trimesters of pregnancy. New Microbiol. 37, 307–319.
Capan-Melser, M., Mombo Ngoma, G., Akerey-Diop, D., Basra, A., Wurbel, H., Groger, M., et al. (2015). Evaluation of intermittent preventive treatment of malaria against group B Streptococcus colonization in pregnant women: a nested analysis of a randomized controlled clinical trial of sulfadoxine/pyrimethamine versus mefloquine. J. Antimicrob. Chemother. 70, 1898–1902. doi: 10.1093/jac/dkv041
Carey, A. J., Tan, C. K., Mirza, S., Irving-Rodgers, H., Webb, R. I., Lam, A., et al. (2014). Infection and cellular defense dynamics in a novel 17beta-estradiol murine model of chronic human group B Streptococcus genital tract colonization reveal a role for hemolysin in persistence and neutrophil accumulation. J. Immunol. (Baltimore Md: 1950). 192, 1718–1731. doi: 10.4049/jimmunol.1202811
Chaguza, C., Jamrozy, D., Bijlsma, M. W., Kuijpers, T. W., van de Beek, D., van der Ende, A., et al. (2022). Population genomics of Group B Streptococcus reveals the genetics of neonatal disease onset and meningeal invasion. Nat. Commun. 13, 4215. doi: 10.1038/s41467-022-31858-4
Coudray, M. S., Madhivanan, P. (2020). Bacterial vaginosis-A brief synopsis of the literature. Eur. J. Obstet Gynecol Reprod. Biol. 245, 143–148. doi: 10.1016/j.ejogrb.2019.12.035
D’Urzo, N., Martinelli, M., Pezzicoli, A., De Cesare, V., Pinto, V., Margarit, I., et al. (2014). Acidic pH strongly enhances in vitro biofilm formation by a subset of hypervirulent ST-17 Streptococcus agalactiae strains. Appl. Environ. Microbiol. 80, 2176–2185. doi: 10.1128/AEM.03627-13
Davis, N. M., Proctor, D. M., Holmes, S. P., Relman, D. A., Callahan, B. J. (2018). Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6, 226. doi: 10.1186/s40168-018-0605-2
Ferris, M. J., Norori, J., Zozaya-Hinchliffe, M., Martin, D. H. (2007). Cultivation-independent analysis of changes in bacterial vaginosis flora following metronidazole treatment. J. Clin. Microbiol. 45, 1016–1018. doi: 10.1128/JCM.02085-06
France, M. T., Ma, B., Gajer, P., Brown, S., Humphrys, M. S., Holm, J. B., et al. (2020). VALENCIA: a nearest centroid classification method for vaginal microbial communities based on composition. Microbiome 8, 166. doi: 10.1186/s40168-020-00934-6
France, M. T., Mendes-Soares, H., Forney, L. J. (2016). Genomic Comparisons of Lactobacillus crispatus and Lactobacillus iners Reveal Potential Ecological Drivers of Community Composition in the Vagina. Appl. Environ. Microbiol. 82, 7063–7073. doi: 10.1128/AEM.02385-16
Gilbert, N. M., Foster, L. R., Cao, B., Yin, Y., Mysorekar, I. U., Lewis, A. L. (2021). Gardnerella vaginalis promotes group B Streptococcus vaginal colonization, enabling ascending uteroplacental infection in pregnant mice. Am. J. Obstet Gynecol. 224, 530.e1–530e17. doi: 10.1016/j.ajog.2020.11.032
Gori, A., Harrison, O. B., Mlia, E., Nishihara, Y., Chan, J. M., Msefula, J., et al. (2020). Pan-GWAS of Streptococcus agalactiae highlights lineage-specific genes associated with virulence and niche adaptation. mBio 11, e00728-20. doi: 10.1128/mBio.00728-20
Graspeuntner, S., Loeper, N., Kunzel, S., Baines, J. F., Rupp, J. (2018). Selection of validated hypervariable regions is crucial in 16S-based microbiota studies of the female genital tract. Sci. Rep. 8, 9678. doi: 10.1038/s41598-018-27757-8
Guidelines QC (2022). Early onset Group B Streptococcal disease. Ed. Health, Q. (Brisbane: Queensland Health).
Hansen, S. M., Uldbjerg, N., Kilian, M., Sorensen, U. B. (2004). Dynamics of Streptococcus agalactiae colonization in women during and after pregnancy and in their infants. J. Clin. Microbiol. 42, 83–89. doi: 10.1128/JCM.42.1.83-89.2004
Hugerth, L. W., Pereira, M., Zha, Y., Seifert, M., Kaldhusdal, V., Boulund, F., et al. (2020). Assessment of in vitro and in silico protocols for sequence-based characterization of the human vaginal microbiome. mSphere 5, e00448-20. doi: 10.1128/mSphere.00448-20
Jiang, S., Wessels, M. R. (2014). BsaB, a novel adherence factor of group B Streptococcus. Infection Immun. 82, 1007–1016. doi: 10.1128/IAI.01014-13
Jones, A. L., Needham, R. H., Clancy, A., Knoll, K. M., Rubens, C. E. (2003). Penicillin-binding proteins in Streptococcus agalactiae: a novel mechanism for evasion of immune clearance. Mol. Microbiol. 47, 247–256. doi: 10.1046/j.1365-2958.2003.03297.x
Kechin, A., Boyarskikh, U., Kel, A., Filipenko, M. (2017). cutPrimers: A new tool for accurate cutting of primers from reads of targeted next generation sequencing. J. Comput. Biol. 24, 1138–1143. doi: 10.1089/cmb.2017.0096
Kolar, S. L., Kyme, P., Tseng, C. W., Soliman, A., Kaplan, A., Liang, J., et al. (2015). Group B Streptococcus evades host immunity by degrading hyaluronan. Cell Host Microbe 18, 694–704. doi: 10.1016/j.chom.2015.11.001
Lahti, L., Shetty, S. microbiome R package. 2012-2019. Available online at: https://bioconductor.org/packages/release/bioc/html/microbiome.html (Accessed March 22, 2023).
Le Doare, K., Heath, P. T. (2013). An overview of global GBS epidemiology. Vaccine 31 Suppl 4, D7–12. doi: 10.1016/j.vaccine.2013.01.009
Lim, S., Rajagopal, S., Jeong, Y. R., Nzegwu, D., Wright, M. L. (2021). Group B Streptococcus and the vaginal microbiome among pregnant women: a systematic review. PeerJ 9, e11437. doi: 10.7717/peerj.11437
Love, M. I., Huber, W., Anders, S. (2014). Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. doi: 10.1186/s13059-014-0550-8
Maidment, T. I., Bryan, E. R., Pyne, M., Barnes, M., Eccleston, S., Cunningham, S., et al. (2023). Characterisation of the koala (Phascolarctos cinereus) pouch microbiota in a captive population reveals a dysbiotic compositional profile associated with neonatal mortality. Microbiome 11, 75. doi: 10.1186/s40168-023-01527-9
McCoy, J. A., Burris, H. H., Gerson, K. D., McCarthy, C., Ravel, J., Elovitz, M. A. (2023). Cervicovaginal microbial-immune state and group B Streptococcus colonization in pregnancy. Am. J. Perinatol. 41, e2539–2546. doi: 10.1055/s-0043-1772226
McMurdie, P. J., Holmes, S. (2013). phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PloS One 8, e61217. doi: 10.1371/journal.pone.0061217
Mei, J. Y., Silverman, N. S. (2023). Group B streptococcus in pregnancy. Obstet Gynecol Clin. North Am. 50, 375–387. doi: 10.1016/j.ogc.2023.02.009
O’Hanlon, D. E., Moench, T. R., Cone, R. A. (2013). Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PloS One 8, e80074. doi: 10.1371/journal.pone.0080074
Pace, R. M., Chu, D. M., Prince, A. L., Ma, J., Seferovic, M. D., Aagaard, K. M. (2021). Complex species and strain ecology of the vaginal microbiome from pregnancy to postpartum and association with preterm birth. Med 2, 1027–1049. doi: 10.1016/j.medj.2021.06.001
Park, S. E., Jiang, S., Wessels, M. R. (2012). CsrRS and environmental pH regulate group B streptococcus adherence to human epithelial cells and extracellular matrix. Infection immunity. 80, 3975–3984. doi: 10.1128/IAI.00699-12
Pidwill, G. R., Rego, S., Jenkinson, H. F., Lamont, R. J., Nobbs, A. H. (2018). Coassociation between group B streptococcus and Candida albicans promotes interactions with vaginal epithelium. Infection immunity. 86, e00669-17. doi: 10.1128/IAI.00669-17
Prince, A. L., Chu, D. M., Seferovic, M. D., Antony, K. M., Ma, J., Aagaard, K. M. (2015). The perinatal microbiome and pregnancy: moving beyond the vaginal microbiome. Cold Spring Harb. Perspect. Med. 5, a023051. doi: 10.1101/cshperspect.a023051
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. United States America. 108 Suppl 1, 4680–4687. doi: 10.1073/pnas.1002611107
Roachford, O. S. E., Alleyne, A. T., Nelson, K. E. (2022). Insights into the vaginal microbiome in a diverse group of women of African, Asian and European ancestries. PeerJ 10, e14449. doi: 10.7717/peerj.14449
Robeson, M. S., 2nd, O’Rourke, D. R., Kaehler, B. D., Ziemski, M., Dillon, M. R., Foster, J. T., et al. (2021). RESCRIPt: Reproducible sequence taxonomy reference database management. PloS Comput. Biol. 17, e1009581. doi: 10.1371/journal.pcbi.1009581
Romero, R., Hassan, S. S., Gajer, P., Tarca, A. L., Fadrosh, D. W., Bieda, J., et al. (2014). The vaginal microbiota of pregnant women who subsequently have spontaneous preterm labor and delivery and those with a normal delivery at term. Microbiome 2, 18. doi: 10.1186/2049-2618-2-18
Romero, R., Theis, K. R., Gomez-Lopez, N., Winters, A. D., Panzer, J. J., Lin, H., et al. (2023). The vaginal microbiota of pregnant women varies with gestational age, maternal age, and parity. Microbiol. spectrum. 11, e0342922. doi: 10.1128/spectrum.03429-22
Ronnqvist, P. D., Forsgren-Brusk, U. B., Grahn-Hakansson, E. E. (2006). Lactobacilli in the female genital tract in relation to other genital microbes and vaginal pH. Acta Obstet Gynecol Scand. 85, 726–735. doi: 10.1080/00016340600578357
Rosen, G. H., Randis, T. M., Desai, P. V., Sapra, K. J., Ma, B., Gajer, P., et al. (2017). Streptococcus and the vaginal microbiota. J. Infect. Dis. 16), 744–751. doi: 10.1093/infdis/jix395
Shabayek, S., Abdellah, A. M., Salah, M., Ramadan, M., Fahmy, N. (2022). Alterations of the vaginal microbiome in healthy pregnant women positive for group B Streptococcus colonization during the third trimester. BMC Microbiol. 22, 313. doi: 10.1186/s12866-022-02730-8
Sheen, T. R., Jimenez, A., Wang, N. Y., Banerjee, A., van Sorge, N. M., Doran, K. S. (2011). Serine-rich repeat proteins and pili promote Streptococcus agalactiae colonization of the vaginal tract. J. Bacteriol. 193, 6834–6842. doi: 10.1128/JB.00094-11
Sroka-Oleksiak, A., Gosiewski, T., Pabian, W., Gurgul, A., Kapusta, P., Ludwig-Słomczyńska, A. H., et al. (2020). Next-generation sequencing as a tool to detect vaginal microbiota disturbances during pregnancy. Microorganisms 8, 1813. doi: 10.3390/microorganisms8111813
Stapleton, R. D., Kahn, J. M., Evans, L. E., Critchlow, C. W., Gardella, C. M. (2005). Risk factors for group B streptococcal genitourinary tract colonization in pregnant women. Obstet Gynecol. 106, 1246–1252. doi: 10.1097/01.AOG.0000187893.52488.4b
Starc, M., Lucovnik, M., Vrlic, P., Jeverica, S. (2022). Protective Effect of Lactobacillus crispatus against Vaginal Colonization with Group B Streptococci in the Third Trimester of Pregnancy. Pathogens 11, 980. doi: 10.3390/pathogens11090980
Sweeney, E. L., Gardiner, S., Tickner, J., Trim, L., Beagley, K. W., Carey, A. J. (2020). Group B Streptococcus serotypes Ia and V induce differential vaginal immune responses that may contribute to long term colonization of the female reproductive tract. Am. J. Reprod. Immunol. (New York NY: 1989). 83, e13199. doi: 10.1111/aji.13199
Tano, S., Ueno, T., Mayama, M., Yamada, T., Takeda, T., Uno, K., et al. (2021). Relationship between vaginal group B streptococcus colonization in the early stage of pregnancy and preterm birth: a retrospective cohort study. BMC pregnancy childbirth. 21, 141. doi: 10.1186/s12884-021-03624-9
Van Der Pol, W. J., Kumar, R., Morrow, C. D., Blanchard, E. E., Taylor, C. M., Martin, D. H., et al. (2019). In silico and experimental evaluation of primer sets for species-level resolution of the vaginal microbiota using 16S ribosomal RNA gene sequencing. J. Infect. diseases. 219, 305–314. doi: 10.1093/infdis/jiy508
Wickham, H., Chang, W., Henry, L., Pedersen, T., Takahashi, K., Wilke, C., et al. Available online at: https://ggplot2.tidyverse.org/ (Accessed March 22, 2023).
Zheng, N., Guo, R., Wang, J., Zhou, W., Ling, Z. (2021). Contribution of Lactobacillus iners to vaginal health and diseases: A systematic review. Front. Cell. infection Microbiol. 11, 792787. doi: 10.3389/fcimb.2021.792787
Keywords: Group B Streptococcus, vaginal microbiome, pregnancy, Lactobacillus sp., Lactobacillus iners
Citation: Maidment TI, Pelzer ES, Borg DJ, Cheung E, Begun J, Nitert MD, Rae KM, Clifton VL and Carey AJ (2024) Group B Streptococcus vaginal colonisation throughout pregnancy is associated with decreased Lactobacillus crispatus and increased Lactobacillus iners abundance in the vaginal microbial community. Front. Cell. Infect. Microbiol. 14:1435745. doi: 10.3389/fcimb.2024.1435745
Received: 20 May 2024; Accepted: 28 August 2024;
Published: 30 September 2024.
Edited by:
Jose A. Garcia-Salcedo, Andalusian Autonomous Government of Genomics and Oncological Research (GENYO), SpainReviewed by:
Valentina Margarita, University of Sassari, ItalyCopyright © 2024 Maidment, Pelzer, Borg, Cheung, Begun, Nitert, Rae, Clifton and Carey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alison J. Carey, YWxpc29uLmNhcmV5QHF1dC5lZHUuYXU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.