- 1Department of Biochemistry and Molecular Biology, School of Basic Medicine, Harbin Medical University, Harbin, Heilongjiang, China
- 2Medical Microbiology Department, Faculty of Applied Science, Hajjah University, Hajjah, Yemen
- 3Medical Laboratory Department, Faculty of Medical Sciences, Al-Razi University, Sana’a, Yemen
- 4Medical Microbiology Department, Faculty of Medical Sciences, Ibb University, Ibb, Yemen
- 5Department Oral Medicine, Harbin Medical University, Harbin, Heilongjiang, China
- 6Department of Gastroenterology and Hepatology, Second Affiliated Hospital of Harbin Medical University, Harbin, Heilongjiang, China
- 7Department of Physiology, School of Biomedical Sciences, Harbin Medical University, Harbin, Heilongjiang, China
Introduction: Candida species, opportunistic yeast, are the second most common cause of female vulvovaginal candidiasis. This study aimed to evaluate the antifungal susceptibility profile of the isolated Candida species in pregnant women in Hajjah governorate, Yemen.
Methods: A hospital-based cross-sectional study was conducted among 396 pregnant women attending Authority AL-Gumhorri Hospital Hajjah between February and July 2023. Vaginal swabs were collected, and Candida species were isolated and identified based on the standard laboratory method. Furthermore, the antifungal drug susceptibility of Candida species was determined by the Kirby-Bauer technique.
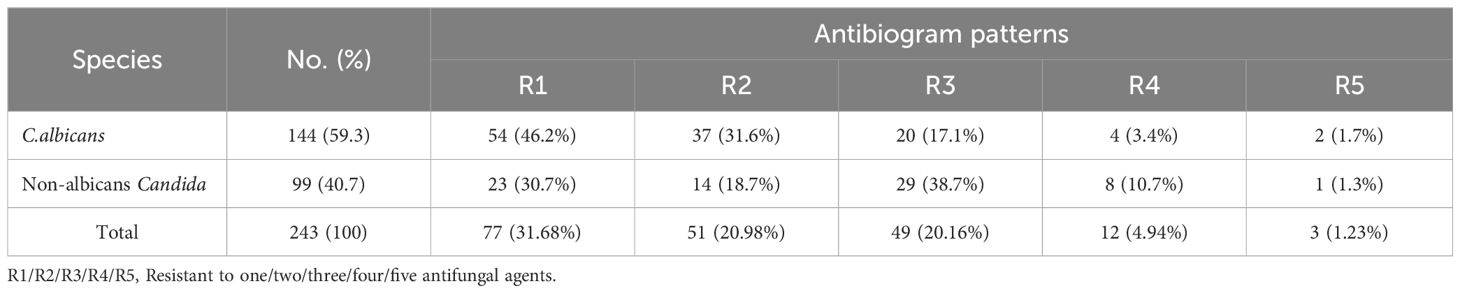
Results and discussion: The prevalence of vaginal Candida infection among pregnant women was 61.4%. Candida albicans was the most predominant species (59.26%), followed by Candida krusei(13.58%), Candida Tropicalis (11.12%), Candida Grabata (9.87%), and Candida dubliniensis (6.17%). The highest rate of Candida infections was among women aged 24–30 years (71.9%) who finished primary school (77.8%), with the third trimester (80%), multigravida (66.1%), and recurrent infection (67.7%) showing significant differences (P < 0.05). The Candida albicans isolates were resistant to clotrimazole and itraconazole at 34.7% and 23.6%, respectively.In addition, the resistance of Candida krusei, Candida tropicalis, Candida glabrata, and Candida dublinensis isolates to fluconazole, voriconazole, voriconazole, and nystatin was 57.6%, 63%, 43.8%, and 60%, respectively. Additionally, approximately 46.2% of isolated Candida albicans exhibited one kind of antifungal drug resistance, whereas 38.7% of isolated non-albicans exhibited resistance to three different antifungal agents. According to the above findings, Candida infection is highly prevalent in Yemen and quite widespread. Interventions in health education are advised to increase women’s knowledge of vaginitis and its prevention. The antifungal susceptibility test may also be helpful in determining the best medication for each patient.
Introduction
Vulvovaginal candidiasis (VVC) commonly infects the mucous membrane of the lower genital tract in females. It is usually caused by Candida albicans but may occasionally be caused by other Candida species or yeasts. It is also known to have severe or persistent daily symptoms and is linked to an increased rate of antifungal resistance (Central for Diseases Control and Prevention (CDC), 2021).
Furthermore, it is common for Candida species, which are a part of the vulvovagina’s natural flora, to produce opportunistic infections when the host’s immune system is impaired. Because Candida sp. coexist in harmony with the vaginal microbiota, asymptomatic colonization is frequent and can last for years. Young women without symptoms have a genital Candida colonization rate of 20%, whereas pregnant women have a rate of up to 30% (Ghaddar et al., 2020; Sule-Odu et al., 2020).
Pruritus, vaginal discomfort, dyspareunia, external dysuria, and atypical vaginal discharge are typical symptoms of VVC. According to estimates, 75% of women will experience at least one episode of VVC, and 40%–45% of them may experience two or more episodes. VVC is characterized as both complicated and uncomplicated. A complicated VVC will affect 10%–20% of women, necessitating particular diagnostic and treatment considerations (Sule-Odu et al., 2020; Central for Diseases Control and Prevention (CDC), 2021).
The predominant species historically has been Candida albicans, which accounts for 85–95% of Candida vaginal infections. However, non-albicans Candida (NAC) species have been reported more frequently globally, with Candida glabrata, Candida tropicalis, Candida parapsilosis, Candida krusei, and Candida dupliniesis being the most common (Makanjuola et al., 2018; Sule-Odu et al., 2020).
A key risk factor is the age at which sexual engagement begins. Host-related factors include immunologic alterations, increased estrogen levels, diabetes mellitus, immunodeficiency states, antibiotic use, treatment with glucocorticoids, genetic predispositions, and behavioral factors such as birth control pills, intrauterine devices, spermicides, and condoms. Hygiene practices, tight-fitting clothing, and sexual behavior are thought to be risk factors linked to an increased rate of VVC (Disha and Haque, 2022).
The issue is particularly significant during pregnancy since Candida colonization is linked to infant mortality and premature delivery and because pregnant women can contaminate their unborn children to a degree of up to 65%, leading to invasive neonatal candidiasis. Due to the emergence of Candida species that are resistant to routinely used antifungal medications and repeated infections, the prevalence of vaginal candidiasis has recently substantially increased (Roberts et al., 2011; Masri et al., 2015).
Increased Candida species resistance to various antifungal treatments is a result of improper use of antifungal medications and a lack of adequate strategies to govern their usage, particularly in the treatment of vulvovaginal candidiasis. Azoles are the first-line medications for treating VVC initially, but fluconazole’s prolonged usage has led to the emergence of multidrug-resistant infections and recurring infections, which is a serious healthcare issue. To combat the medication resistance issue, it is urgently necessary to find novel compounds with antifungal capabilities due to various restrictions on the accessibility of current antifungal agents, ineffective therapy, high toxicity, low tolerability, and drug interaction (Lírio et al., 2019; Rosati et al., 2020; Nikoomanesh et al., 2023; Zaman et al., 2024).
One of the Yemeni governorates, Hajjah, lacks information on the antifungal susceptibility pattern of Candida species that cause vulvovaginal candidiasis in pregnant women. Therefore, the objective of this study was to evaluate the antifungal susceptibility profile of the isolated Candida species in pregnant women in Hajjah governorate, Yemen.
Materials and methods
Study design and period
A hospital-based cross-sectional study was conducted in obstetrics and gynecology clinics at Authority Al-Gumhorri Hospital-Hajjah, which is situated in the Hajjah City. Hajjah is located in the northwest of Sana’a capital with a distance of 123 kilometers and an elevation of almost 1800 meters. Additionally, it is divided into 31 administrative districts on its border and consists of 2,630,678 inhabitants (Figure 1). The period of study was 6 months, from February to July 2023, and the laboratory analysis was performed at the hospital laboratory.
Population size
The population size consisted of 396 pregnant women attending obstetrics and gynecology clinics with symptoms suggestive of vaginitis. The age of the participants ranged between 17 and 44 years, with an average age of 28.6 years.
Data collection
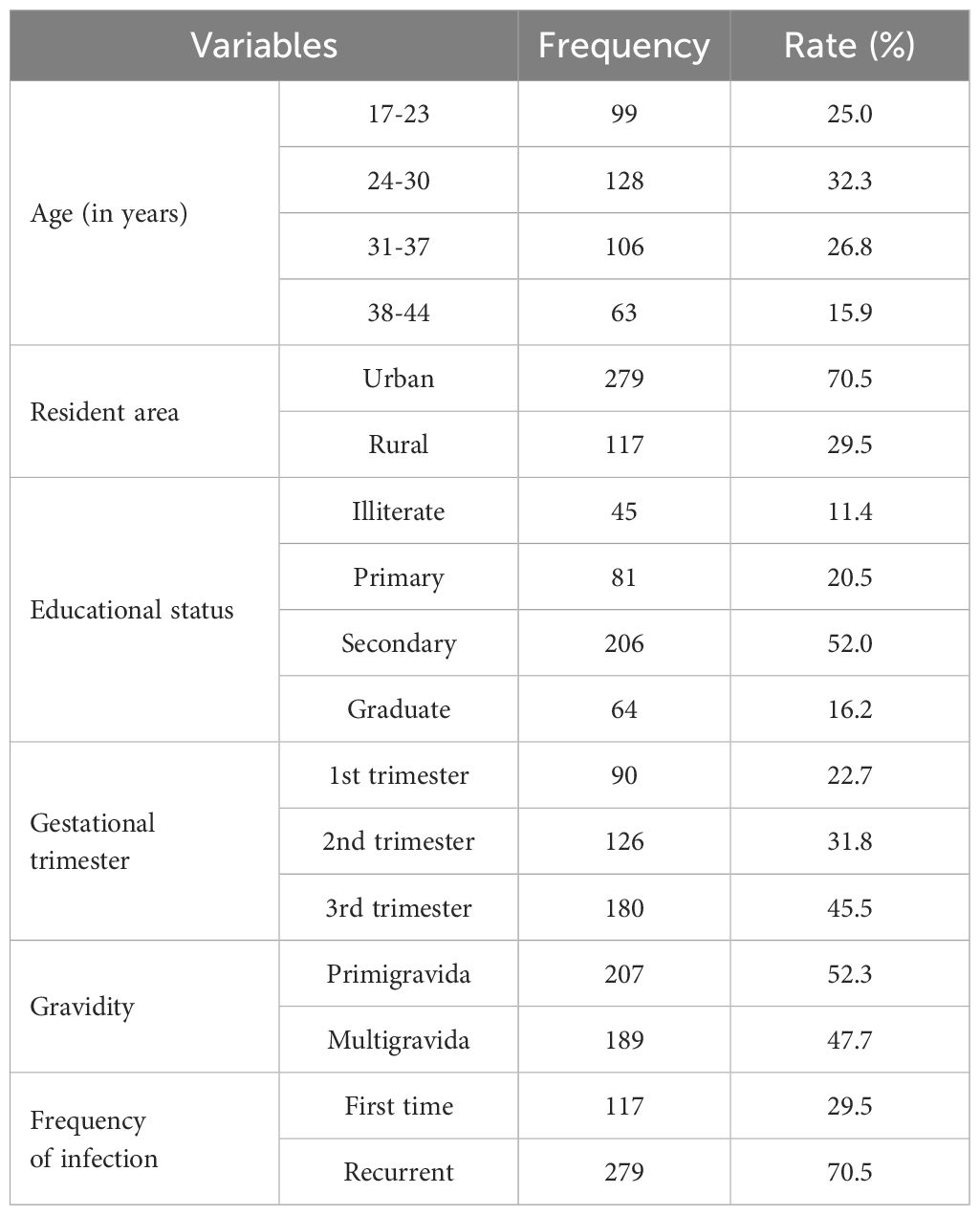
A pretested questionnaire was developed based on multiple past studies (Khan et al., 2018; Abdul-Aziz et al., 2019), and some adjustments were made to gather the necessary data separately from each participant. Age, residence area, educational level, trimester, gravidity, frequency of infection, and medical signs and symptoms of vaginal abnormalities (burning, itching, and vaginal discharge) were all information that was included in the questionnaire. Research teams completed the questionnaire. The age categories were divided into four groups: 18–23 years, 24–30 years, 31–37 years, and 38–44 years.
Inclusion and exclusion criteria
Women who gave vaginal specimens and had vaginal inflammation with discharge, burning, itching, and other symptoms during pregnancy were included. On the other hand, those who were currently receiving antimicrobial medicine treatment or refused to provide vaginal samples were not included in the study.
Sample collection and examination
A well-qualified obstetrician collected vaginal swabs from each pregnant woman by using a sterile cotton swab. The swab was inserted approximately 5 cm into the vaginal opening, gently turned for approximately 30 seconds, and rubbed against the wall of the vagina. The collected specimens were labelled with patient information and sent directly to the medical laboratory for further examination (Mahon et al., 2015; Tille, 2017).
Culture and identification of Candida species
The vaginal swabs were streaked individually under aseptic conditions onto Sabouraud Dextrose Agar (SDA) plate agar (HiMedia Lab., India) supplemented with chloramphenicol (250 mg/L). The inoculated plates were incubated aerobically at 37°C for 48 h. The growth colonies were inoculated onto HiCrome agar (HiMedia Lab., India) and incubated at 37°C for 48 h. In addition, the identification of Candida species was performed based on the Candida colony’s characterization of the medium. In addition, all isolates were further confirmed using the API 20C AUX strip, as recommended by the manufacturer (BioMereaux, Marcy-l`Étoile, France). The green colonies were identified as C. albicans, the purple colonies as C. glabrata, the pink colonies as C. krusei, the blue colonies as C. tropicalis, and the darker green colonies as C. dublinensis. Additionally, the germ tube test was used to distinguish between C. albicans and non-albicans (Mahon et al., 2015; Tille, 2017).
Antifungal susceptibility testing
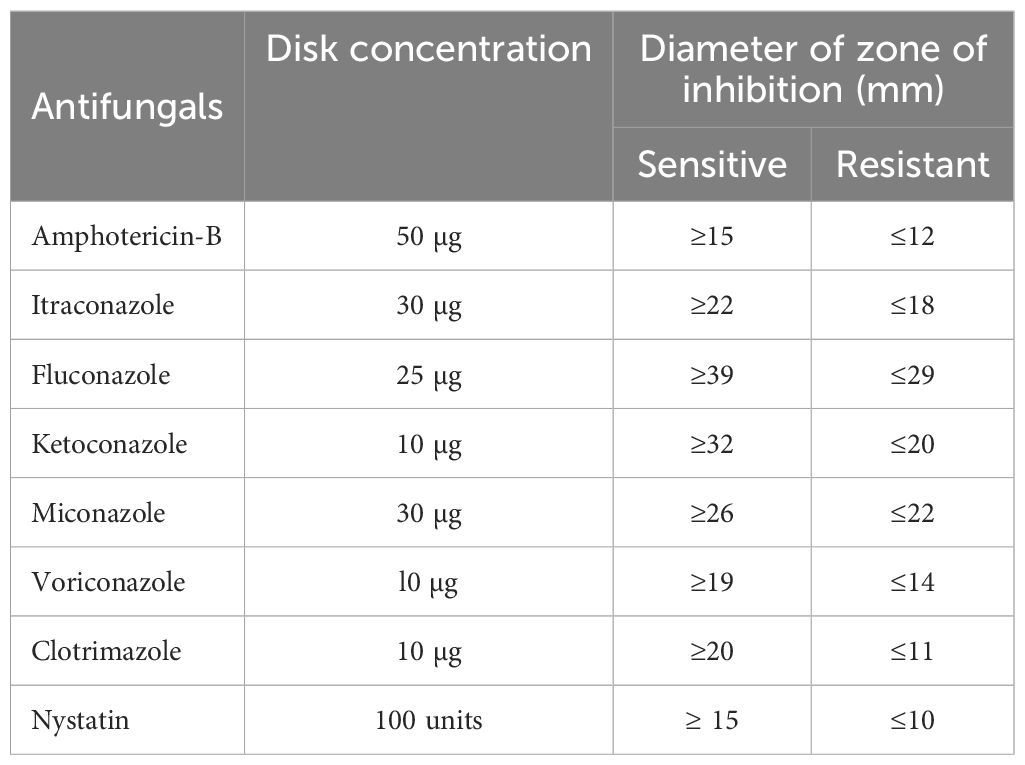
The disk diffusion test of the Kirby–Bauer technique was used to determine the antifungal drug susceptibility profiles of Candida species on Mueller Hinton agar (MHA) medium (HiMedia Lab., India) containing glucose (2%) and methylene blue dye (0.5 µg/mL) according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (2021). A suspension of overnight cultures of C. albicans, C. krusei, C. tropicalis, C. glabrata, and C. dublinensis were prepared separately in 5 mL of sterile 0.85% saline. The turbidity containing 1-5×106 CFU/mL of isolated Candida species was adjusted to McFarland 0.5 standard by using a DensiChek (bioMerieux) turbidity meter. A sterile cotton swab was moistened in the prepared suspension, and excess fluid was removed. Under aseptic conditions, the surface of Müller-Hinton agar (MHA) plate was streaked by the swab for three times and kept at room temperature for 15 minutes to dry. The antifungal discs, namely, amphotericin-B (50 µg), itraconazole (30 µg), fluconazole (25 µg), ketoconazole (10 µg), miconazole (30 µg), voriconazole (l0 µg), clotrimazole (10 µg), and nystatin (100 units) (HiMedia Lab., India), were placed over the surface of the MHA plate and incubated at 37°C for 48 h. According to CLSI (2021) recommendations, the zone of inhibition was measured in millimeters, and the results were interpreted as sensitive (S) and resistant (S) (Table 1) (Clinical and Laboratory Standards Institute (CLSI), 2021).
Quality control
The reference strains of Candida albicans ATCC 10231 and Candida krusei ATCC 6258 were used for quality control. In addition, the validity of the results was guaranteed through the implementation of standards of quality control during the pre-analytical, analytical, and post-analytical stages. All prepared culture media (plates and tubes) were checked for sterility and performance before being used for the isolation and identification of the studied Candida species. Positive controls included standard fungi and patient specimens. while negative control plates were un-inoculated. The positive and negative controls were employed to validate the quality of the isolation and identification results and to avoid the possibility of cross-contamination.
Ethics approval
The Ethical Review Board of the Faculty of Applied Sciences at Hajjah University granted ethical clearance on January 24, 2023. Additionally, the Faculty of Applied Sciences presented a written document to the Authority Al-Gumhorri Hospital’s administration to approve the collection and analysis of specimens. Before collecting data and specimens, study volunteers were fully informed of the objectives and justification for their participation in the study. The informed consent form was obtained from educated individuals by taking their signatures, while the illiterate participants took a fingerprint instead of a signature. Additionally, the study’s confidentiality of data was maintained.
Statistical analysis
The statistical analysis was performed using SPSS version 20 (Statistical Package for Social Science). Descriptive statistics, numbers, frequencies, percentages, and tables were used to describe the findings. Moreover, chi-square (χ2), confidence interval (95% CI), and odds ratio (OR) were used to assess the association between dependent and independent variables. Finally, a p value less than 0.05 was considered statistically significant.
Results
This research included approximately 396 pregnant women. Table 2 lists the participants’ characteristics, including the fact that 128 (32.3%) of them were between the ages of 24 and 30 years, 279 (70.5%) were from urban areas, 206 (52%) had completed their secondary education, 180 (45.5%) were in the third trimester, 207 (52.3%) were primigravida, and 279 (70.5%) were in the first time of infection.
Figure 2A shows that out of 396 vaginal swabs, 243 (61.4%) were positive for growth in culture media, while 153 (38.6%) were negative. Additionally, the frequency rates of isolates of C. albicans were 144 (59.26%) followed by C. krusei 33 (13.58%), C. tropicalis 27 (11.12%), C. glabrata 24 (9.87%), and C. dublinensis 15 (6.17%) as figured in Figure 2B.
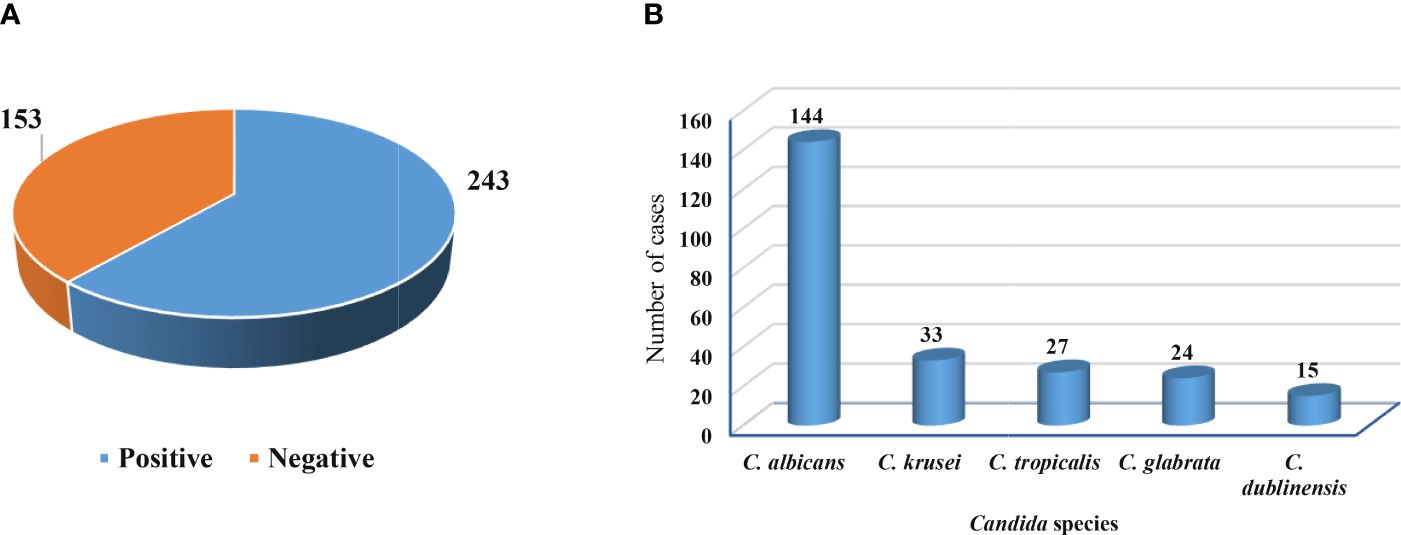
Figure 2 Growth in culture media and isolated Candida species. (A) Positive and negative growth of vaginal swabs in culture media. (B) Frequency of Candida species isolated from pregnant women.
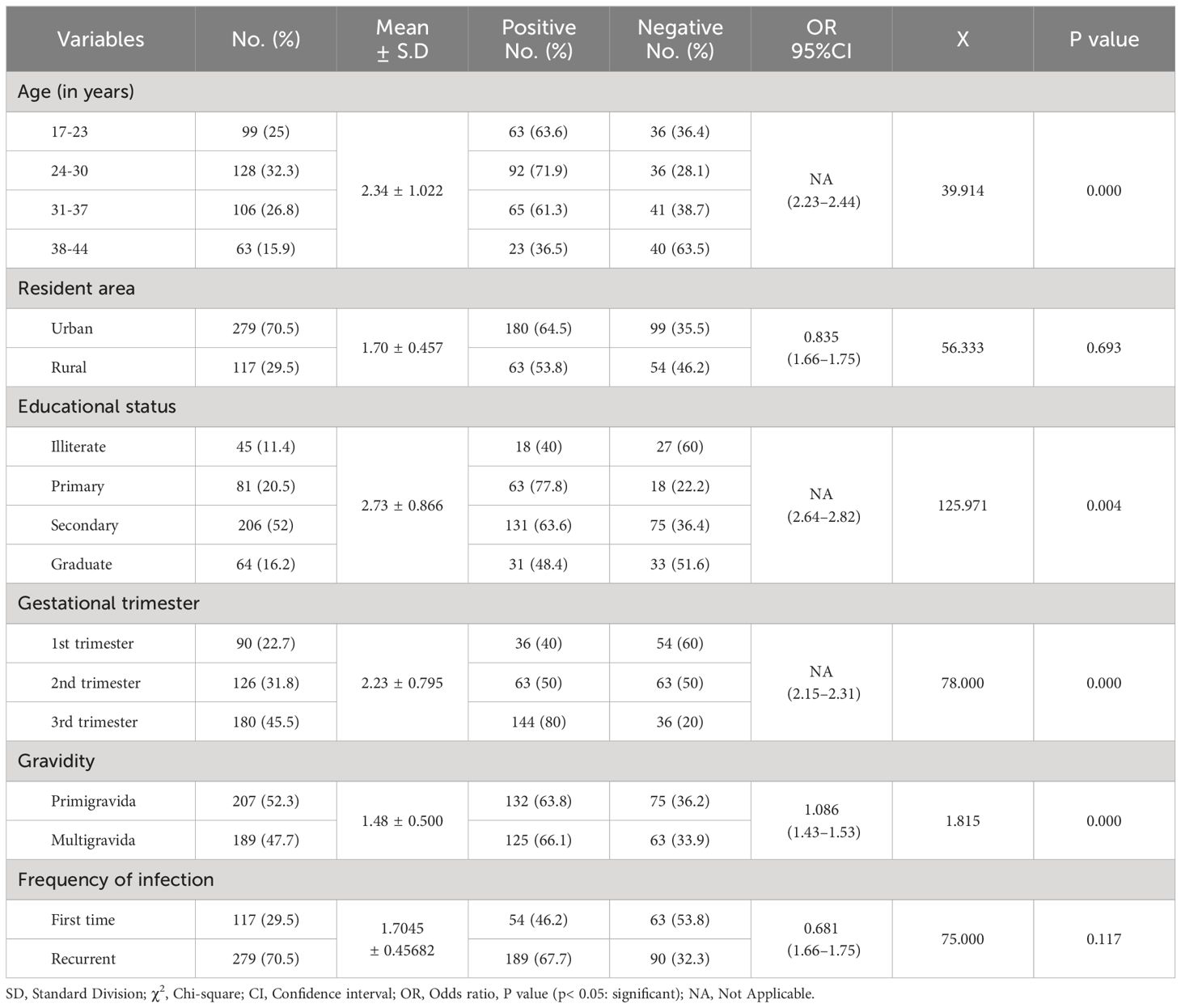
According to this finding, the highest rate of Candida infections was recorded among pregnant women aged between 24 and 30 years (71.9%), and the lowest rate was among individuals aged 38–44 years (36.5%), with a statistically significant difference (95% CI =2.23–2.44; P = 0.000). Similarly, the participants who lived in urban areas had the highest rate (64.5%) compared with those who lived in rural areas (53.8%). Furthermore, the study subjects who completed primary school had a higher rate of Candida infections (77.8%), whereas the uneducated participants had a lower rate (40.0%), with a statistically significant difference (P = 0.000). Additionally, a high rate of infection was significantly observed among pregnant women in the third trimester (80.0%) compared to others, with a statistically significant difference (P = 0.004). Furthermore, the multigravida mothers were found to be more infected (66.1%) than primigravida participants (63.8%), with a statistically significant difference (P = 0.000). Moreover, recurrent infections showed a high rate among participants (67.7%; P = 0.117) compared to the first time of infection (46.2%), as listed in Table 3.
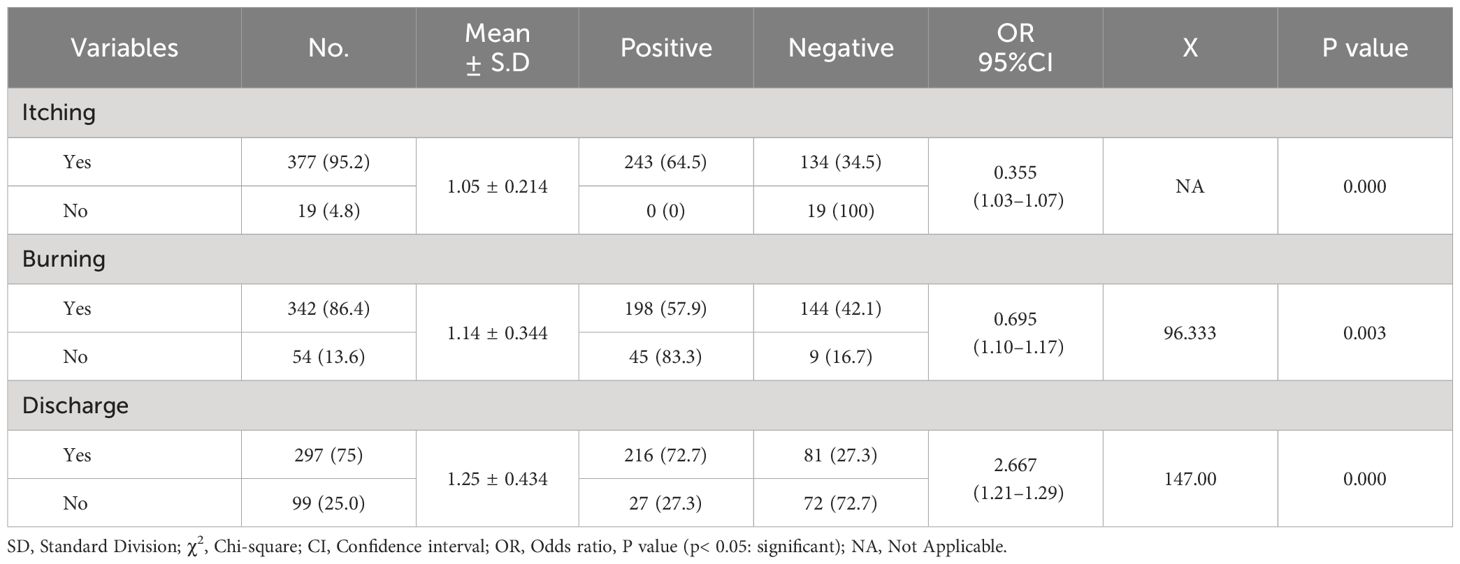
The current research found that participants’ clinical signs and symptoms and Candida sp. prevalence were statistically significant (P < 0.05). Additionally, as shown in Table 4, pregnant women who experienced discharge (72.7%; OR= 2.667; 95% CI=1.21–1.29) and itching (64.5%; OR= 0.335; 95% CI=1.03–1.07) had the greatest proportion of Candida sp. positivity.
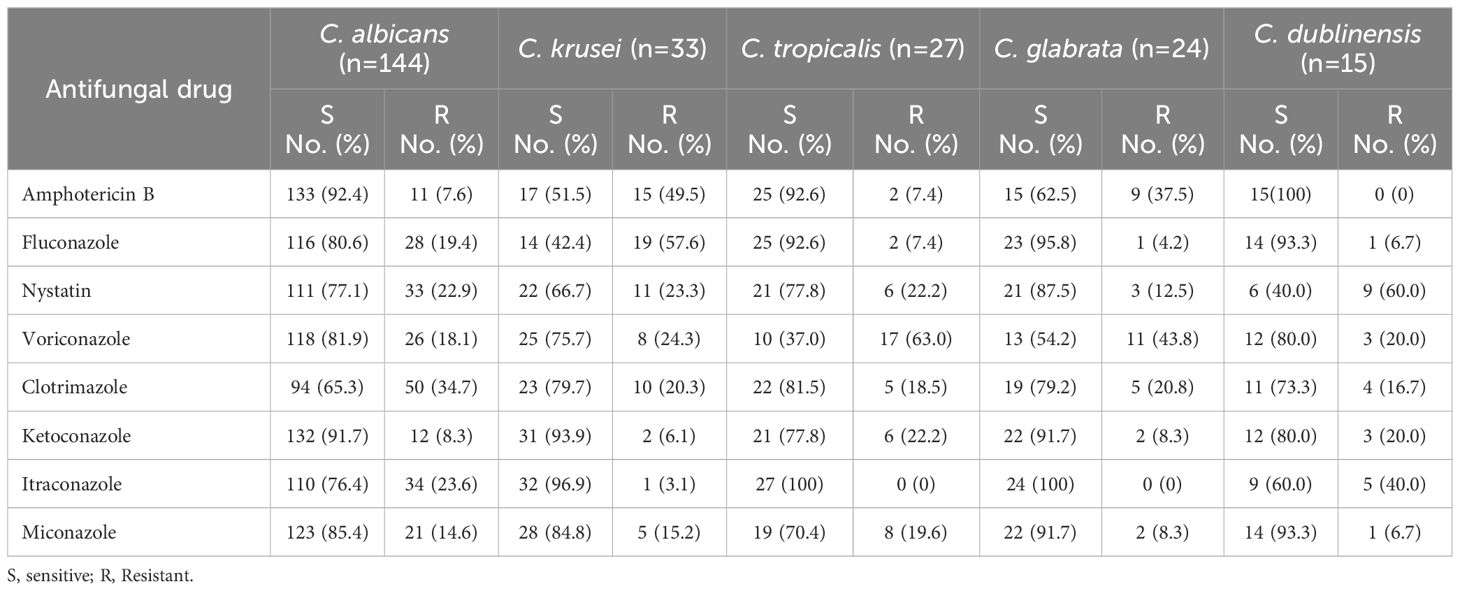
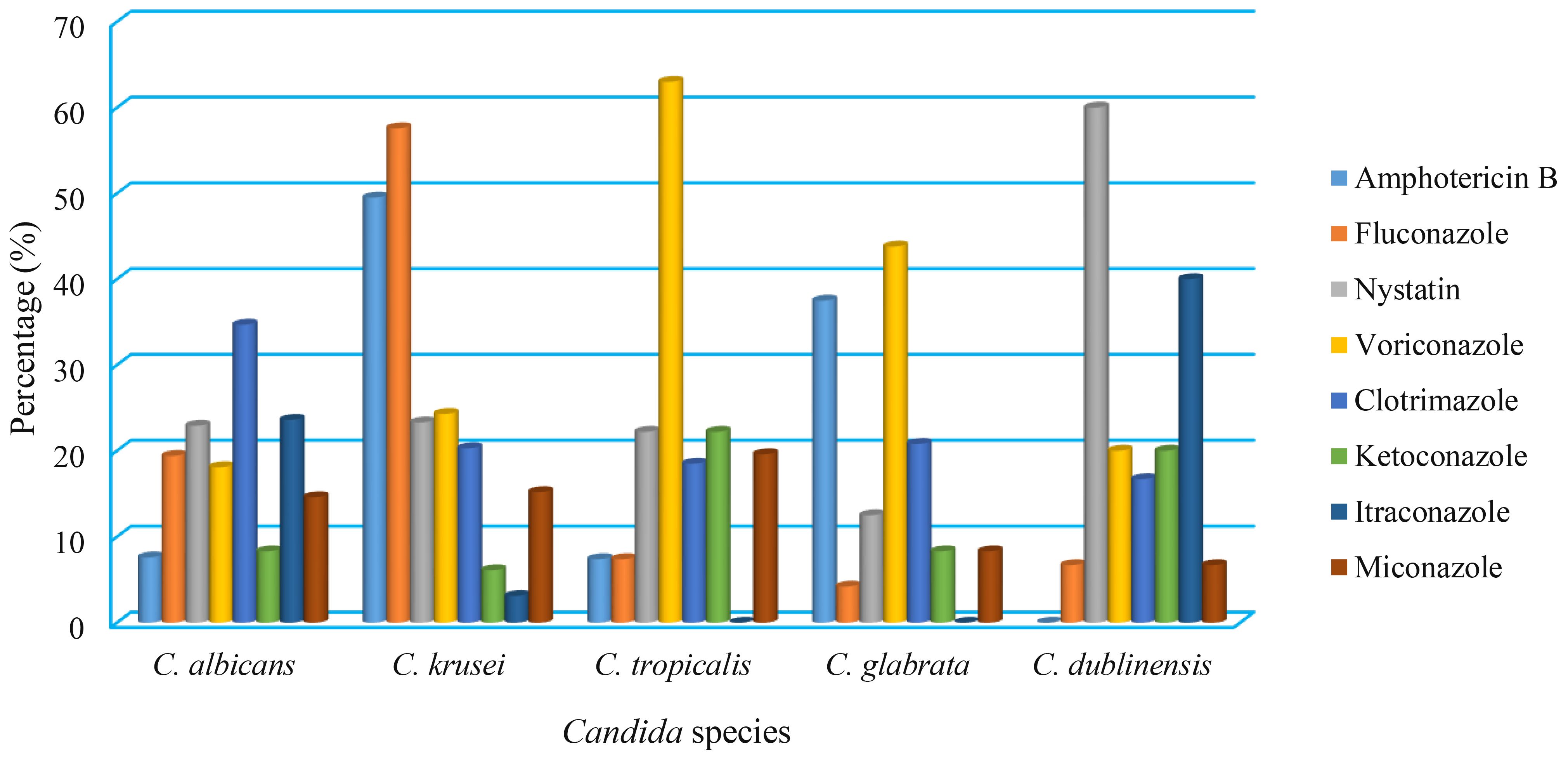
According to the antifungal susceptibility data, C. albicans isolates were highly susceptible to amphotericin B, which had a 92.4% sensitivity rate, followed by ketoconazole (91.7%), miconazole (85.4%), voriconazole (81.9%), and fluconazole (80.6%). In contrast, clotrimazole and itraconazole were both ineffective against 34.7% and 23.6% of C. albicans isolates, respectively. In addition, the C. krusei, C. tropicalis, C. glabrata, and C. dublinensis isolates were resistant to fluconazole (57.6%), voriconazole (63%), voriconazole (43.8%), and nystatin (60%), respectively, (Table 5, Figure 3).
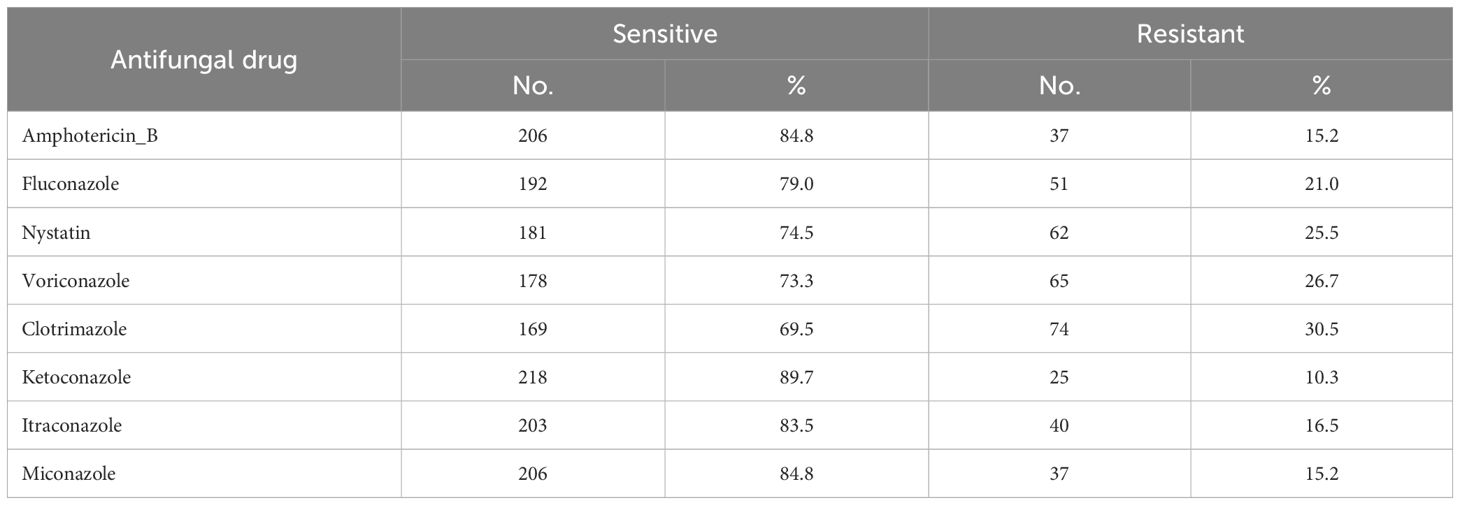
Of the nine tested antifungal drugs, the high-sensitivity proportion of isolated Candida sp. was to ketoconazole at 218 (89.7%), while the highest rate of Candida species resistance was for clotrimazoleat 74 (30.5%), followed by Voriconazole 65 (26.7%), Nystatin 62 (25.5%), and lowest resistant was for Ketoconazole 25 (10.3%) (Table 6).
According to Table 7, the majority of isolated Candida albicans (46.2%) exhibited only one kind of antifungal drug resistance, compared to 38.7% of isolated non-albicans Candida, which exhibited resistance to three different antifungal drug types (38.7%).
Discussion
The present finding revealed that the prevalence proportion of Candida sp. was 61.4% among pregnant women, which is close to the finding reported among pregnant women in Ibb City (Edrees et al., 2020). This outcome is less than a report conducted in Kenya, which found that 90.38% of pregnant women were infected by Candida sp (Nelson et al., 2013). In contrast, the current finding is lower than several reports that showed that the prevalence rate of Candida species among pregnant women was 55.4% in Cameroon (Toua et al., 2013), 60.8% in Egypt (Abbas et al., 2016), 51.6% in Sana’a, Yemen (Al-Rukeimi et al., 2020), 34% in Saudi Arabia (Venugopal et al., 2021), and 25% in north-western Ethiopia (Tsega and Mekonnen, 2019). The great variation in frequency rate might be due to the differences in geographical locations, study population, sample size, hygienic conditions, socioeconomic status, and diagnostic methods employed by the participants.
The present study revealed that the frequency rates of C. albicans, C. krusei, C. tropicalis, C. glabrata, and C. dublinensis were reported to be 59.26%, 13.58%, 11.12%, 9.87%, and 6.17%, respectively. This result is similar to some reports performed in different countries (Tsega and Mekonnen, 2019; Ghaddar et al., 2020; Venugopal et al., 2021). The widespread use of antifungal drugs over-the-counter, inappropriate use, incomplete description of treatment, longer treatment for recurrent candidiasis, and use of effective agents to eliminate C. albicans have all been proposed as potential explanations for the increased isolation of non-albicans Candida species from vulvovaginitis patients. Given the high frequency of VVC among women in the study area, it is important to screen or treat pregnant women for VVC to prevent its detrimental health effects (Sule-Odu et al., 2020; Zaman et al., 2024).
Remarkably, the highest rate of Candida infections in this study was noticed in pregnant women aged between 24 and 30 years (71.9%). Similar reports documented that a high frequency of Candida species was found at 60% among the ages 26–35 (Nelson et al., 2013), 38.5% in the ages 34–40 (Tsega and Mekonnen, 2019), 37.4% in the ages 20–34 (Konadu et al., 2019), and 44.4% in the ages 26–35 (Waikhom et al., 2020).
The cause of this might be explained by the fact that women in this age range release many reproductive hormones, which can inhibit the immune system and foster Candida infection. The use of antibiotics, which kill bacteria, including natural flora, is another factor that might be involved. This will give Candida a chance to attack the vaginal wall (Tsega and Mekonnen, 2019). Additionally, this high proportion is caused by the vagina’s higher glycogen content and high estrogen hormone levels. It offers a reliable source of carbon, which helps with Candida proliferation.
The present outcome showed that the highest rate of Candida infections was recorded among those living in urban areas (64.5%). The outcome, consistent with Abdul-Aziz et al. (Abdul-Aziz et al., 2019), revealed that the highest distribution of VVC was among pregnant women in the urban area, at 88.44%. The prevalence of the infection was higher in uneducated women than in patients with basic school education and above, and there was a statistically significant correlation between vulvovaginal candidiasis and educational level (P = 0.004). This finding was compatible with earlier reports (Konadu et al., 2019; Waikhom et al., 2020) that revealed a high frequency of Candida sp. among those with basic education. The difference in infection rates between illiterate individuals and those with more education could be explained by improvements in personal cleanliness and/or economic position brought on by education (Bitew and Abebaw, 2018).
Compared to primigravidae (63.8%), multigravidae (66.1%) women exhibited a higher rate of Candida colonization, with significant differences (P = 0.000) in the present study. Similarly, research conducted in Pakistan found that multigravidae women experienced the condition more frequently than primigravidae women, with results of 60% and 40%, respectively (Aslam et al., 2008). Further research from Nepal (Shrestha et al., 2011) supports our findings. The rationale is that the rate of infection rises with the frequency of pregnancies (3rd> 2nd> 1st), which lowers immunity and may lead to extensive Candida colonization. Additionally, a study by Tsega and Mekonnen (2019) showed that Candida infection was more common among women with multigravidae (61.5%) than among those with primigravidae (38.5%).
According to these data, females with recurrent infections had a greater prevalence rate of vulvovaginal candidiasis (67.7%) than those who had it for the first time (29.5%). This result conflicts with research performed in Nigeria by Sobel (2007). The significantly rising rate of recurrent vaginal candidiasis infections in this research may be due to an increase in Candida species that are resistant to widely used antifungal medications.
The current results revealed that there were significant differences between the gestational period and Candida colonization (80%; P = 0.000). The highest rate of vaginal Candida species was detected in the third-trimester participants at 80%, while the lowest was in the first trimester (40%). A similar finding was further reported: the highest rate of Candida species was in the third trimester, at 68.09% in Kenya (Nelson et al., 2013) and 57.4% in Ghana (Waikhom et al., 2020). According to research, third-trimester pregnant women were most likely to develop an illness. Pregnancy raises the risk of VVC, and the risk might reach 50% during the final trimester (Konadu et al., 2019; Tsega and Mekonnen, 2019; Waikhom et al., 2020).
This is because during pregnancy, particularly in the third trimester, elevated estrogen levels generate larger glycogen stores in the vagina, which serve as an excellent source of carbon and encourage the growth of Candida sp. Additionally, estrogen makes vaginal epithelial cells’ yeast cytosol receptors more attractive to Candida species (Babić and Hukić, 2010). So, to prevent vaginal microorganisms’ infections, particularly in the last trimester, it is imperative that health education interventions be enhanced and women’s awareness raised about the disease (Abruquah, 2012; Holzer et al., 2017).
The current findings showed that 92.4% of C. albicans were sensitive to amphotericin B. This finding was similar to reports that recorded 93.8% in Ethiopia (Tsega and Mekonnen, 2019) and 87.2% in Ghana (Kan et al., 2023). Amphotericin-B is more sensitive than other antibiotics because it is not frequently given and used extensively due to its high cost, difficulties in administration, and severe renal toxicity. Therefore, the less a drug is used, the less likely it is to develop resistance to it (Tsega and Mekonnen, 2019). Consequently, to avoid the emergence and spread of drug-resistant bacterial strains, it is critical to conduct periodic surveillance of antimicrobial susceptibility testing and proper management of pregnant women (Abruquah, 2012).
The overall rate of Candida species in the present investigation was highly resistant to clotrimazole (30.5%), voriconazole (26.7%), nystatin (25.5%), and fluconazole (21.0%). These results are lower than the results of Khan et al (Khan et al., 2018), who detected a high resistance rate of Candida sp. against fluconazole (62%), clotrimazole (59.3%), itraconazole (40.7%), and voriconazole (10.2%). Another study by Tsega and Mekonnen (2019) revealed that a high resistance rate of Candida sp. (57.3%) was found for ketoconazole and itraconazole. In addition, approximately 17.2% and 5.7% of Candida species were resistant to fluconazole and flucytosine, respectively (Bitew and Abebaw, 2018). Hence, more research on family health strategies is required for developing a better understanding of how drug-resistant microbes spread and their role in health-related infections.
The current study showed that ketoconazole was the most effective antifungal drug when compared to tested antifungal agents that had 89.7% effectiveness. In different reports, Bitew and Abebaw (2018) reported that fluconazole was the most effective antifungal drug, while Tsega and Mekonnen (2019) documented clotrimazole as an effective antifungal agent. Earlier studies demonstrated that the resistance rate in Candida sp. to voriconazole and fluconazole has remained constant over a decade (Pfaller et al., 2007; Lyon et al., 2010; Bitew and Abebaw, 2018). According to Gualco et al., (2007), C. albicans exhibited resistance to fluconazole and itraconazole at rates of 0.7% and 2.7%, respectively. To determine the most effective antibiotic, establishing a national antibiotic policy is crucial for regulating the administration of antibiotics to patients prior to conducting an antibiotic sensitivity test, which aims to identify the most optimal antibiotic.
Furthermore, the isolated C. krusei, C. tropicalis, C. glabrata, and C. dublinensis in this finding were resistant to fluconazole (57.6%), voriconazole (63%), voriconazole (43.8%), and nystatin (60%), respectively. A report by Luo et al. (Luo et al., 2016) found that the resistance levels of C. albicans, C. glabrata, and C. tropicalis to amphotericin B, voriconazole, fluconazole, 5-fluorocytosine, and itraconazole were found to be 0.5% to 6.4%, 0% to 7.7%, and 0% to 9.6%, respectively. In addition, itraconazole (24.1%) and posaconazole (14.5%) were the only two drugs for which resistance rates were noted to C. glabrata. Whereas C. krusei exhibited the highest resistance rates to itraconazole at 81.5% (Peman et al., 2012). Variations in the research populations and geographic settings, as well as variations in the usage of antifungals, could be an explanation for the varying resistance rates observed in this investigation.
According to the multidrug resistance results, a high proportion of Candida albicans (46.2%) were resistant to one type of antifungal drug, while 38.7% of isolated non-albicans Candida were resistant to three types of antifungal drugs. A study by Tsega and Mekonnen (2019) revealed that 46.29% of isolated C. albicans were resistant to three types of antifungal agents, and non-albicans Candida, including C. glabrata (35.29%) and C. krusei (57.17%), were resistant to one type of antifungal. Therefore, healthcare settings should strongly consider implementing an antibiogram as part of their infection control programs to guide their decision-making on appropriate empirical treatment and infection control programs (Brandão et al., 2018).
Strength and limitation of the study
This study is the first to try to shed some light on the prevalence of Candida species and their antifungal susceptibility profiles among pregnant women in Hajjah governorate, Yemen, which is an important public health issue that has not been previously studied in this region. Additionally, the results of this investigation are expected to yield valuable insights that will provide decision makers with antifungal susceptibility profiles in the study area and encourage other researchers to conduct further studies in this field. However, the limitations of this work include the fact that modern and advanced techniques such as polymerase chain reaction (PCR) for genotypes identifying the isolated Candida species are lacking due to limited resources. In addition, this study is unable to cover the behavioral risk factors that may be associated with the prevalence of Candida species among study participants.
Conclusion
The greater frequency of Candida species isolates in this study is considered a serious health problem for pregnant women. The high humidity in Hajjah may have contributed to the spread of Candida species among women. Therefore, to increase women’s knowledge of vaginal candidiasis and its prevention, health education interventions are highly recommended. Furthermore, screening women for vaginal infections prior to treatment is recommended, as well as educating spouses and partners on the transmission and prevention of sexually transmitted illnesses. The consequences of candidiasis can be avoided with proper and immediate treatment and diagnosis. Moreover, the antifungal susceptibility test may help determine the best drug therapy for each patient. Accordingly, future investigations should focus on the occurrence of drug-resistant Candida strains and their emergence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Authority AL-Gumhorri Hospital Hajjah. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
MA: Writing – original draft, Writing – review & editing, Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Funding acquisition, Project administration, Resources, Software, Supervision, Validation, Visualization. WE: Conceptualization, Data curation, Writing – review & editing, Methodology, Formal analysis, Resources, Validation. WA-S: Investigation, Resources, Software, Writing – review & editing. GX: Writing – review & editing, Supervision. SH: Writing – review & editing, Formal analysis, Software. EQ: Writing – review & editing, Resources. RC: Investigation, Visualization, Writing – review & editing, Software. NL: Writing – review & editing, Data curation, Investigation, Visualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We are appreciative of the females who agreed to voluntarily enroll in this study. We want to express our thanks to the laboratory staff of the Authority Al-Gumhorri Hospital-Hajjah for their technical assistance.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CDC, Centre for Disease Control and Prevention; CI, Confidence Interval; CLSI, Clinical and Laboratory Standard Institute; MHA, Mueller Hinton Agar; OR, Odds Ratio; SD, Standard Division; SPSS, Statistical Package for the Social Sciences; VVC, Vulvovaginal Candidiasis; χ2, Chi-square.
References
Abbas, A. M., Shaaban, O. M., Badran, S. M., Shaltout, A. S., Nasr, A., Abdullah, S. A. (2016). Risk factors and health hazards of vaginal infections in upper Egypt: A cross sectional study. Thai J. Obstet Gynecol. 30, 50–56. doi: 10.14456/tjog.2016.14
Abdul-Aziz, M., Mahdy, M. A. K., Abdul-Ghani, R., Alhilali, N. A., Al-Mujahed, L. K.A., Alabsi, S. A., et al. (2019). Bacterial vaginosis, vulvovaginal candidiasis and Trichomonal vaginitis among reproductive-aged women seeking primary healthcare in Sana’a city, Yemen. BMC Infect. Dis. 19, 1–10. doi: 10.1186/s12879-019-4549-3
Abruquah, H. H. (2012). Prevalence and antifungal susceptibility of Candida species isolated from women attending a gynecological clinic in Kumasi, Ghana. J. Sci. Techno. 32, 39–45. doi: 10.4314/just.v32i2.6
Al-Rukeimi, A. D. A., Al-Hatami, S. M. M., AL-Danany, D. A., Al-Shamahy, H. A., Al Rukeimi, R. A. A. (2020). Prevalence and risk factors associated with vulvovaginal candidiasis during pregnancy in Sana'a, Yemen. Universal J. Pharm. Res. 5, 1–5. doi: 10.22270/ujpr.v5i3.407
Aslam, M., Hafeez, R., Ijaz, S., Tahir, M. (2008). Vulvovaginal candidiasis in pregnancy. Biomedica 24, 54–56. Available at: https://citeseerx.ist.psu.edu/document?repid=rep1&type=pdf&doi=357bef2e32f8ac66f7290b06df4e dbe4db4385a6.
Babić, M., Hukić, M. (2010). Candida albicans and non-albicans species as etiological agent of vaginitis in pregnant and nonpregnant women. Bosnian J. Basic Med. Sci. 10, 89–97. doi: 10.17305/bjbms.2010.2744
Bitew, A., Abebaw, Y. (2018). Vulvovaginal candidiasis: Species distribution of Candida and their antifungal susceptibility pattern. BMC Women's Health 18, 94. doi: 10.1186/s12905-018-0607-z
Brandão, L. S., Boniek, D., Stoianoff, M. A.R., Da Mata, F. M.R., De Azevedo, P. R.M., Fernandes, J. V., et al. (2018). Prevalence and antifungal susceptibility of Candida species among pregnant women attending a school maternity at Natal, Brazil. Lett. Appl. Microbiol. 67, 285–291. doi: 10.1111/lam.2018.67.issue-3
Central for Diseases Control and Prevention (CDC) (2021). Sexually transmitted infections (STI) treatment guidelines, 2021 (Atlanta, US: Vulvovaginal Candidiasis (VVC). Available at: https://www.cdc.gov/std/treatment-guidelines/candidiasis.htm.
Clinical and Laboratory Standards Institute (CLSI) (2021). Performance standards for antimicrobial susceptibility testing. 31st ed (Wayne, PA: Clinical and Laboratory Standards Institute).
Disha, T., Haque, F. (2022). Prevalence and risk factors of vulvovaginal candidosis during pregnancy: A review. Infect. Dis. Obstet Gynecol. 20, 6195712. doi: 10.1155/2022/6195712
Edrees, W. H., Al-Asbahi, A. A., Al-Shehari, W. A., Qasem, E. A. (2020). Vulvovaginal candidiasis prevalence among pregnant women in different hospitals in Ibb, Yemen. Universal J. Pharm. Res. 5, 1–5. doi: 10.22270/ujpr.v5i4.431
Ghaddar, N., Anastasiadis, E., Halimeh, R., Ghaddar, A., Dhar, R., AlFouzan, W., et al. (2020). Prevalence and antifungal susceptibility of Candida albicans causing vaginal discharge among pregnant women in Lebanon. BMC Infect. Dis. 20, 32. doi: 10.1186/s12879-019-4736-2
Gualco, L., Debbia, E. A., Bandettini, R., Pescetto, L., Cavallero, A., Ossi, M. C., et al. (2007). Antifungal resistance in Candida spp. isolated in Italy between 2002 and 2005 from children and adults. Int. J. Antimicrob. Agents 29, 179–184. doi: 10.1093/jac/dks019
Holzer, I., Farr, A., Kiss, H., Hagmann, M., Petricevic, L. (2017). The colonization with Candida species is more harmful in the second trimester of pregnancy. Arch. Gynecol. Obstet 295, 891–895. doi: 10.1007/s00404-017-4331-y
Kan, S., Song, N., Pang, Q., Mei, H., Zheng, H., Li, D., et al. (2023). In vitro antifungal activity of azoles and other antifungal agents against pathogenic yeasts from vulvovaginal candidiasis in China. Mycopathologia 188, 99–109. doi: 10.1007/s11046-022-00687-w
Khan, M., Ahmed, J., Gul, A., Ikram, A., Lalani, F. K. (2018). Antifungal susceptibility testing of vulvovaginal Candida species among women attending antenatal clinic in tertiary care hospitals of Peshawar. Infection Drug Resistance 11, 447–456. doi: 10.2147/IDR.S153116
Konadu, D. G., Owusu-Ofori, A., Yidana, Z., Boadu, F., Iddrisu, L. F., Adu-Gyasi, D., et al. (2019). Prevalence of vulvovaginal candidiasis, bacterial vaginosis and Trichomoniasis in pregnant women attending antenatal clinic in the middle belt of Ghana. BMC Pregnancy Childbirth 19, 1–10. doi: 10.1186/s12884-019-2488-z
Lírio, J., Giraldo, P. C., Amaral, R. L., Sarmento, A. C. A., Costa, A. P. F., Gonçalves, A. K., et al. (2019). Antifungal (oral and vaginal) therapy for recurrent vulvovaginal candidiasis: A systematic review protocol. BMJ Open 9, e027489. doi: 10.1136/bmjopen-2018-027489
Luo, X., Dong, X., Pen, Z. (2016). Distribution and drug susceptibility of Candida spp. associated with female genital tract infection, Chongqing, China. Jundishapur. J. Microbiol. 9, e19386. doi: 10.5812/jjm.19386
Lyon, G. M., Karatela, S., Sunay, S., Adiri, Y. (2010). Antifungal susceptibility testing of Candida isolates from the Candida surveillance study. J. Clin. Microbiol. 48, 1270–1275. doi: 10.1128/JCM.02363-09
Mahon, R. C., Lehman, C. D., Manuselis, G. (2015). Textbook of diagnostic microbiology. 5th Edition (USA: Saunders, An Imprint of Elsevier, Inc), 37–115.
Makanjuola, O., Bongomin, F., Fayemiwo, S. A. (2018). An update on the roles of non-albicans Candida species in Vulvovaginitis. J. Fungi (Basel) 4, 121. doi: 10.3390/jof4040121
Masri, S. N., Noor, S. M., Nor, L. A., Osman, M., Rahman, M. M. (2015). Candida isolates from pregnant women and their antifungal susceptibility in a Malaysian tertiary-care hospital. Pak J. Med. Sci. 31, 658–661. doi: 10.12669/pjms.313.7072
Nelson, M., Wanjiru, W., Margaret, M. W. (2013). Prevalence of vaginal candidiasis and determination of the occurrence of Candida species in pregnant women attending the antenatal Clinic of Thika District Hospital, Kenya. OJMM 3, 264–272. doi: 10.4236/ojmm.2013.34040
Nikoomanesh, F., Falahatinejad, M., Černáková, L., dos Santos, A. L., Mohammadi, S. R., Rafiee, M., et al. (2023). Combination of Farnesol with common antifungal drugs: Inhibitory effect against Candida species isolated from women with RVVC. Medicina 59, 743. doi: 10.3390/medicina59040743
Peman, J., Canton, E., Quindos, G., Eraso, E., Alcoba, J., Guinea, J., et al. (2012). Epidemiology, species distribution and in vitro antifungal susceptibility of fungaemia in a Spanish multicentre prospective survey. J. Antimicrobial. Chemother. 67, 1181–1187. doi: 10.1093/jac/dks019
Pfaller, M. D., Diekema, D., Gibbs, V., Newell, J., Meis, I., Gould, W., et al. (2007). Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2005: An 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI 30 standardized disk diffusion testing. J. Clin. Microbiol. 45, 1735–1745. doi: 10.1128/JCM.00409-07
Roberts, C. L., Rickard, K., Kotsiou, G., et al. (2011). Treatment of asymptomatic vaginal candidiasis in pregnancy to prevent preterm birth: an open-label pilot randomized controlled trial. BMC Pregnancy Childbirth 11, 1–6. doi: 10.1186/1471-2393-11-18
Rosati, D., Bruno, M., Jaeger, M., ten Oever, J., Netea, M. G. (2020). Recurrent vulvovaginal candidiasis: An Immunological Perspective. Microorganisms 8, 2, 144. doi: 10.3390/microorganisms8020144
Shrestha, S., Tuladhar, N. R., Basnyat, S., Acharya, G. P., Shrestha, P., Kumar, P. (2011). Prevalence of vaginitis among pregnant women attending Paropakar maternity and Women's hospital, Thapathali, Kathmandu, Nepal. Nepal. Med. Coll. J. 13, 293–296.
Sobel, J. D. (2007). Vulvovaginal candidosis. Lancet 369, 1961–1971. doi: 10.1016/S0140-6736(07)60917-9
Sule-Odu, A., Akadri, A. A., Oluwole, A. A., Osinupebi, O. A., Andu, B. A., Akiseku, A. K., et al. (2020). Vaginal Candida infection in pregnancy and its implications for fetal well-being. Afr. J. Reprod. Health 24, 33–40. Available at Vaginal Candida infection in pregnancy and its implications for fetal well-being on JSTOR.
Tille, P. M. (2017). Bailey and Scott’s diagnostic microbiology. 14th edition (St. Louis, Missouri: Elsevier).
Toua, V., Djaouda, M., Gaké, B., Menye, D. E., Christie, E. A., Tambe, E., et al. (2013). Prevalence of vulvovaginal candidiasis amongst pregnant women in Maroua (Cameroon) and the sensitivity of Candida albicans to extracts of six locally used antifungal plants. Int. Res. J. Microbiol. 4, 89–97.
Tsega, A., Mekonnen, F. (2019). Prevalence, risk factors and antifungal susceptibility pattern of Candida species among pregnant women at Debre Markos Referral Hospital, Northwest Ethiopia. BMC Pregnancy Childbirth 19, 1–8. doi: 10.1186/s12884-019-2494-1
Venugopal, D., Husain, K., Mustafa, S. A., Sabeen, S. (2021). Epidemiology, risk factors and antimicrobial profile of vulvovaginal candidiasis (VVC): A study among women in the central region of Saudi Arabia. J. Mycol. Med. 31, 101049. doi: 10.17305/bjbms.2010.2744
Waikhom, S. D., Afeke, I., Kwawu, G. S., Mbroh, H. K., Osei, G. Y., Louis, B., et al. (2020). Prevalence of vulvovaginal candidiasis among pregnant women in the Ho municipality, Ghana: species identification and antifungal susceptibility of Candida isolates. BMC Pregnancy Childbirth 20, 266. doi: 10.1186/s12884-020-02963-3
Keywords: antifungal, Candida albicans, pregnant women, vulvo vaginal candidiasis, Yemen
Citation: Ali M, Edrees WH, Al-Shehari WA, Xue G, Al-Hammadi S, Qasem EA, Chaulagain RP and Lal N (2024) Antifungal susceptibility pattern of Candida species isolated from pregnant women. Front. Cell. Infect. Microbiol. 14:1434677. doi: 10.3389/fcimb.2024.1434677
Received: 18 May 2024; Accepted: 10 July 2024;
Published: 07 August 2024.
Edited by:
Kunlong Yang, Jiangsu Normal University, ChinaCopyright © 2024 Ali, Edrees, Al-Shehari, Xue, Al-Hammadi, Qasem, Chaulagain and Lal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maqsood Ali, YmlvY2hlbWlzdC5tYXFzb29kYWxpQGdtYWlsLmNvbQ==
 Maqsood Ali
Maqsood Ali Wadhah Hassan Edrees
Wadhah Hassan Edrees Wadee Abdullah Al-Shehari
Wadee Abdullah Al-Shehari Gao Xue1
Gao Xue1 Ram Prasad Chaulagain
Ram Prasad Chaulagain