- 1Department of Parasitology, Faculty of Veterinary Medicine, University of Firat, Elazig, Türkiye
- 2Department of Parasitology, Faculty of Veterinary Medicine, University of Selcuk, Konya, Türkiye
- 3Animal Disease Research Unit, Agricultural Research Service, US Department of Agriculture, Pullman, WA, United States
- 4Department of Veterinary Microbiology and Pathology, College of Veterinary Medicine, Washington State University, Pullman, WA, United States
Babesia ovis, transmitted by Rhipicephalus bursa ticks, is the causative agent of ovine babesiosis, a disease characterized by fever, anemia, hemoglobinuria, and high mortality in sheep. This study investigates whether sheep that survived babesiosis without treatment can serve as a source of infection for B. ovis-free host-seeking R. bursa larvae in a later season. Three donor sheep were experimentally infected with B. ovis, and after six months, persistence of B. ovis was assessed through blood and tick transmission experiments. Blood from donor sheep was intravenously injected into three recipient sheep, while donor sheep were also infested with B. ovis-free R. bursa larvae. Engorged nymphs molted to adults, and new recipient sheep were infested with these ticks. All recipient sheep were monitored for B. ovis for 100 days using microscopic, serological, and molecular approaches. The presence of B. ovis was confirmed in the recipient sheep that received blood, leading to clinical infection in two. However, no B. ovis was detected in recipient sheep infested with ticks. These results suggest that sheep recovering from B. ovis infection do not serve as a source of infection for R. bursa larvae in subsequent seasons.
Introduction
Babesiosis is a zoonotic protozoal disease caused by Babesia species, commonly observed in domestic and wild animals (Iqbal et al., 2011; Schnittger et al., 2022). Species within the Babesia genus, such as B. ovis, B. motasi, B. crassa, B. foliata, and B. taylori have been reported to cause babesiosis in sheep and goats (Schnittger et al., 2022). Additionally, new species or genotypes, including Babesia sp. Xinjiang and Babesia sp. BQ1 (Lintan) in sheep, as well as Babesia aktasi n. sp. in goats, have been reported (Guan et al., 2009, 2010; Ozubek and Aktas, 2017; Ozubek et al., 2023a). Babesia ovis, the primary causative agent of babesiosis in small ruminants, causes significant economic losses in sheep in tropical and subtropical regions. As an endemic ovine pathogen in South Europe, Africa, the Middle East, and Asia, B. ovis infection manifests clinically with clinical signs characterized by fever, hemolytic anemia, and hemoglobinuria, often leading to fatalities (Yeruham et al., 1998; Ceylan et al., 2021). The epidemiology of B. ovis is closely linked to the biology and ecology of Rhipicephalus ticks, particularly R. bursa (Yeruham et al., 1998). Both immature (larvae, nymphs) and adult stages of R. bursa primarily prefer sheep and goats as their hosts (Yeruham et al., 1995; Altay et al., 2008). Rhipicephalus bursa, a two-host tick that completes only one generation per year, derives its name from the Latin term “bursa” referring to the bloated pouch-like appearance of engorged females (Walker et al., 2000). This tick species exhibits a life cycle ranging from 99 to 214 days under laboratory conditions. The extended lifespan may be associated with the survival capacity of unfed larvae in warm and dry climate conditions. Adult R. bursa ticks become active when the average daytime and nighttime temperatures exceed 18°C and 12°C, respectively (Yeruham et al., 2000). Rhipicephalus bursa larvae remain inactive during the 5-6 months of warm and dry summer. Infestation on their hosts begins in October, peaks in December, and shows a slow decline until February. Unfed larvae and engorged nymphs of R. bursa, respectively, enter their states of inactivity or diapause during both the summer and winter. Sheep babesiosis cases are known to peak between May and July when adult R. bursa ticks are most abundant, followed by a decline and complete disappearance by the end of July (Yeruham et al., 1989, 1995). Babesia ovis, belonging to the Babesia sensu stricto group, is transmitted by vector ticks both transtadially and transovarially (Jalovecka et al., 2019). In competent vector ticks, acting as definitive hosts, B. ovis parasites undergo sexual reproduction, ultimately leading to transovarial transmission. Thus, different life stages of the parasite, including gametocytes, zygotes, kinetes, sporoblasts, and sporozoites can be present in infected ticks. Rhipicephalus bursa acquires the pathogen during the larval and nymphal stages (Erster et al., 2016) and transmits the pathogen to susceptible hosts during the adult stage (Yeruham et al., 1998; Erster et al., 2016). Transovarial transmission occurs upon invasion of ovary and eggs by the parasite in engorged female ticks (Buscher et al., 1988). A study on the transmission of B. ovis by R. bursa (Erster et al., 2016) reported that infected R. bursa larvae and nymphs, despite feeding on susceptible hosts, do not transmit B. ovis, and the parasite was only transmitted to the susceptible host by adult ticks. Therefore, clinical infections resulting from B. ovis are expected to be prevalent during the peak activity of adult R. bursa ticks, which occurs between May and July (Yeruham et al., 1998).
Despite numerous studies on the prevalence of B. ovis, several aspects of the transmission dynamics of the disease and factors influencing its epidemiology remain unknown. The objective of this study is to investigate whether sheep that have naturally overcome B. ovis infection without treatment during the peak clinical period (May to July) can serve as a source of infection for R. bursa larvae during the active host-seeking period between October and December (Fall season). This research aims to elucidate aspects of the transmission dynamics of B. ovis and contribute to the understanding of factors influencing its epidemiology, particularly the potential for sheep to act as reservoirs for the pathogen outside the peak infection season.
Materials and methods
Ethics statement
This study was carried out according to the regulations of animal and welfare issued by the Turkish legislation for the protection of animals. All animal experiments were approved by the Firat University, Animal Experiment Ethic Committee, protocol number 2023/12-05.
Sheep
Babesia-, Anaplasma-, and Theileria-free sheep were used in this study. To select experimental animals, blood samples (serum and EDTA tubes) were collected from apparently healthy 5-8-month-old sheep (n=10). Nested PCR with general primers, including Ec9/Ec12A (Kawahara et al., 2006)-16S8FE/B-GA1B (Bekker et al., 2002) for Anaplasma, and Nbab1F/Nbab1R (Oosthuizen et al., 2008)-RLBF2/RLBR2 (Georges et al., 2001) for Babesia and Theileria, were used to investigate the presence of these species. Samples that tested negative by nested PCR were subsequently subjected to indirect ELISA (iELISA) using BoSA1 protein for B. ovis specific antibody detection (Sevinc et al., 2015). Ten sheep determined to be negative by both nPCR and iELISA were included in the study. During the experiment, the sheep were relocated to a separate compartment at the Elazig Veterinary Control Institute Directorate. They were kept in a tick-free environment and provided with feed and water ad libitum.
Experimental infection
B. ovis/Alacakaya stabilates stored in a cryobank were utilized for experimental infection. The stabilates were thawed and promptly administered intravenously to one splenectomized sheep. The splenectomy was carried out using standard surgical, anesthetic, and analgesic procedures, as described by Sevinc et al. (2007). Once the parasitemia level reached 5%, 30 ml of infected fresh blood obtained from the donor splenectomized sheep were intravenously inoculated into each of the three donor spleen-intact sheep (#021, #668, and #671), as previously described (Guan et al., 2009; Ozubek et al., 2023b). Blood was collected from experimental donor animals for microscopy, nPCR and iELISA analyses daily during the first 40 days after experimental infection and then weekly up to 6 months after infection.
Microscopic detection of piroplasm
Blood smears were subjected to staining using a 10% Giemsa solution. The stained smears were then observed under a 100X objective lens to identify intraerythrocytic parasites. The percentage of parasitized erythrocytes (PPE) was determined by analyzing a minimum of 20 microscopic fields, as outlined in the methodology by Sevinc et al. (2007).
Babesia ovis nested PCR
Genomic DNA was extracted from 200 µL of EDTA anticoagulated blood samples obtained from sheep, as well as from R. bursa ticks used in experimental infestations, utilizing the PureLinkTM Genomic DNA Mini Kit (Invitrogen Corporation, Carlsbad, USA) following the manufacturer’s guidelines. Subsequently, the isolated DNA was stored at −20°C until further use. To identify B. ovis DNA, a nPCR assay was conducted utilizing the following sets of primers, Nbab1F/Nbab1R (Oosthuizen et al., 2008) and BboF/BboR (Aktas et al., 2005). To check the DNA of R. bursa, PCR was conducted using 16S + 1 and 16S – 1 primers (Black and Piesman, 1994), as previously described.
Babesia ovis indirect ELISA
Expression and purification of recombinant B. ovis Secreted Antigen-1 (rBoSA1) were carried out following the procedure outlined by Sevinc et al. (2015). Briefly, 50 µl of the reconstituted rBoSA1, prepared at a concentration of 2 μg/ml in carbonate-bicarbonate buffer (pH=9.6), was added to individual wells in a 96-well ELISA microplate. The subsequent test procedures were conducted in accordance with the methodologies previously detailed by Sevinc et al. (2015). The cutoff value was determined by adding the average value to three times the standard deviation of the optical density (OD) measurements obtained from the negative sheep sera.
Investigation of in vivo persistence of B. ovis following experimental infection
Following experimental infection, sheep were monitored for clinical signs of ovine babesiosis, including increased body temperature, anemia, jaundice, and hemoglobinuria. Additionally, the presence of B. ovis was tracked in the infected sheep by nPCR and iELISA by a period of 200 days (Aktas et al., 2005; Sevinc et al., 2015).
Transmission by direct blood inoculation
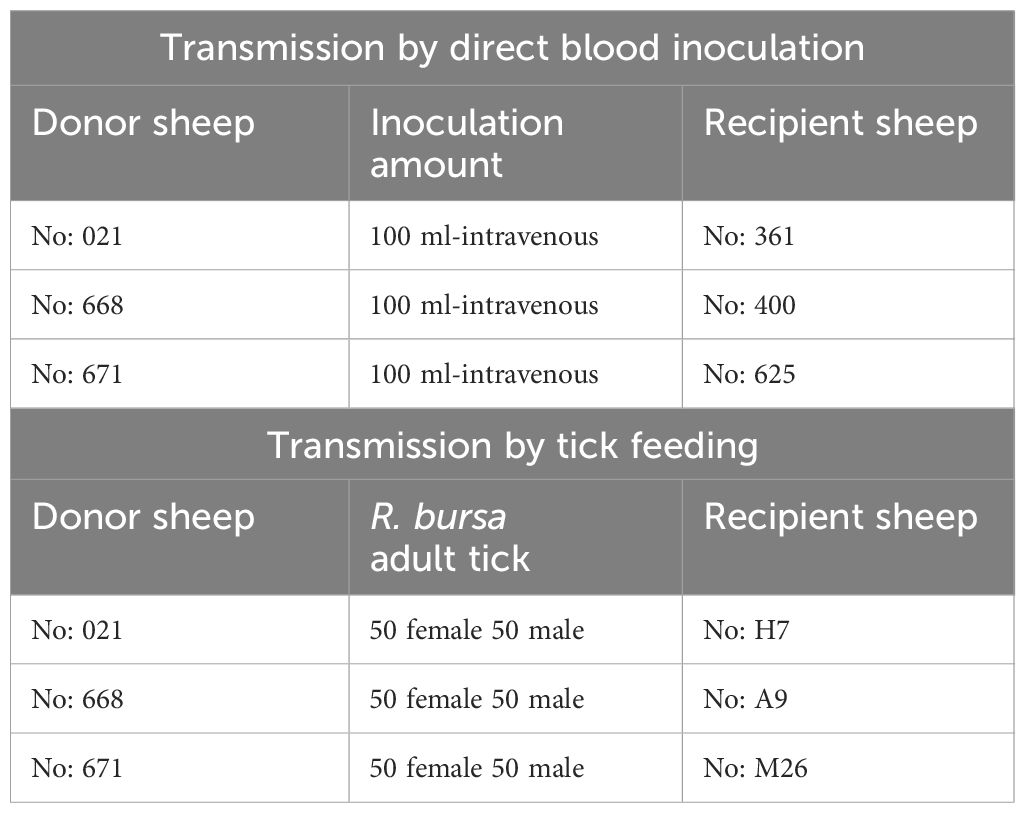
Six months after the experimental infection, 100 mL of blood was collected from each donor sheep into EDTA tubes, and the individual blood was transfused into individual recipient sheep free from tick-borne pathogens (Schwint et al., 2009). Specific identification of donor (#021, #668, and #671) and recipient (#361, #400, and #625) sheep are described in Figure 1 and Table 1. Sheep infected through blood transfusion (#361, #400, and #625) were monitored for babesiosis through microscopy, iELISA, and nPCR methods for 100 days post-infection (Schwint et al., 2009).
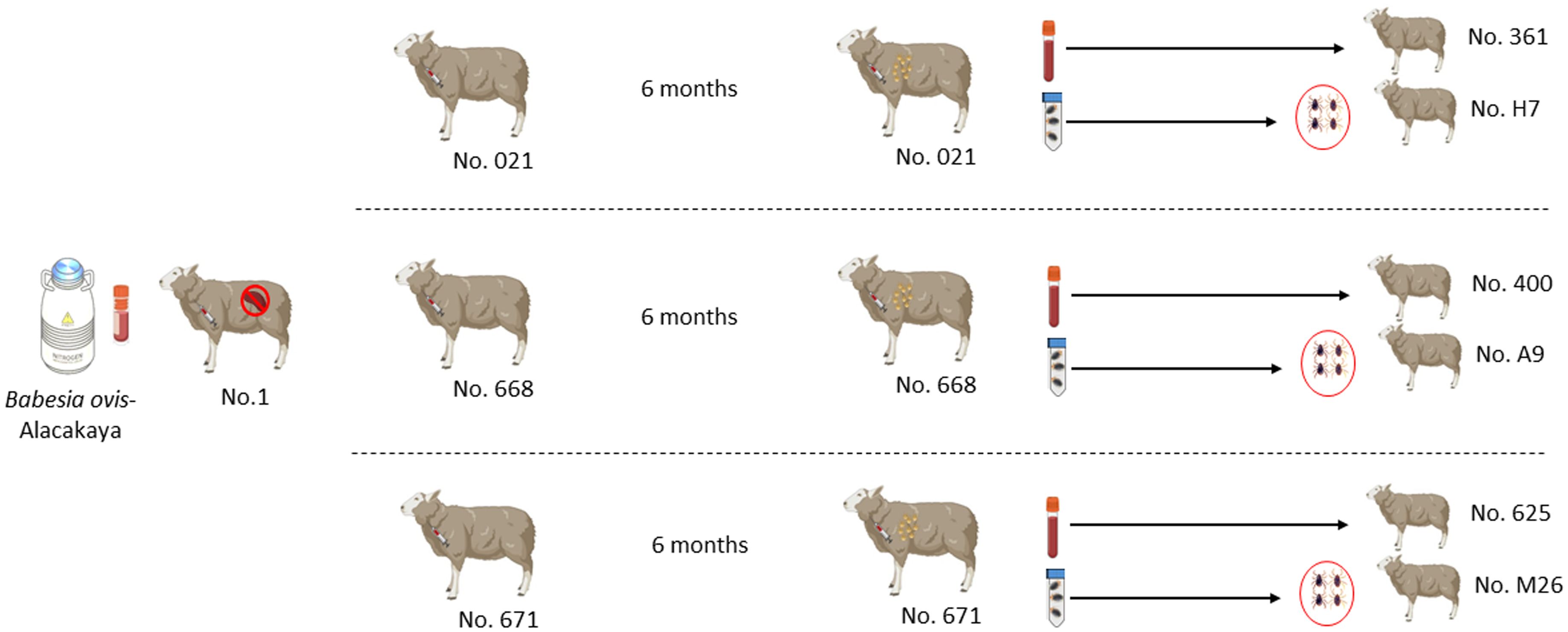
Figure 1 Schematic representation of the experimental design sheep used in experimental infection with blood and ticks.
Transmission by tick feeding
pRhipicephalus bursa ticks employed for experimental infestations were obtained from a colony regularly cultivated within our laboratory. Briefly, to establish the colony, we collected engorged female R. bursa ticks from cattle and placed them in an incubator to lay eggs. After the oviposition process was completed, the egg cluster was divided into two parts, with one half used for DNA extraction. The eggs were examined for the presence of Babesia/Anaplasma using nPCR (Ozubek et al. 2023b), and the clusters found to be negative were used to obtain larvae. These larvae were then used to infest a splenectomized sheep. Engorged nymphs collected from the splenectomized sheep were placed in an incubator to obtain unfed adults. These unfed adults were then used to infest another splenectomized sheep, and the engorged females were incubated to produce larvae. This process was repeated for three generations, and each time the larval clusters were examined for the presence of Babesia/Anaplasma using nPCR. After these procedures, the colony was considered Babesia-free. The ticks utilized in the transmission experiments were derived from eight successive generations bred and raised under laboratory conditions in our facility. Additionally, this colony has been extensively employed in our laboratory to transmit B. ovis (Sevinc et al., 2023). Six months after experimental infection, B. ovis-free unfed larvae (0.1 g for each sheep) were administered to the donor sheep (#021, #668, and #671) within a capsule. Engorged nymphs were collected from each sheep in separate tubes, placed in an incubator to molt into adults. The obtained unfed adult ticks (50 females and 50 males for each sheep) were transferred to recipient sheep free from tick-borne pathogens (#H7, #A9, and #M26). (Figure 1 and Table 1). For transstadial transmission, sheep (#H7, #A9, and #M26) were monitored for babesiosis through microscopy, iELISA, and nPCR methods for 100 days post-infestation (Schwint et al., 2009).
Results
Long-term monitoring and infection dynamics of B. ovis infection in sheep
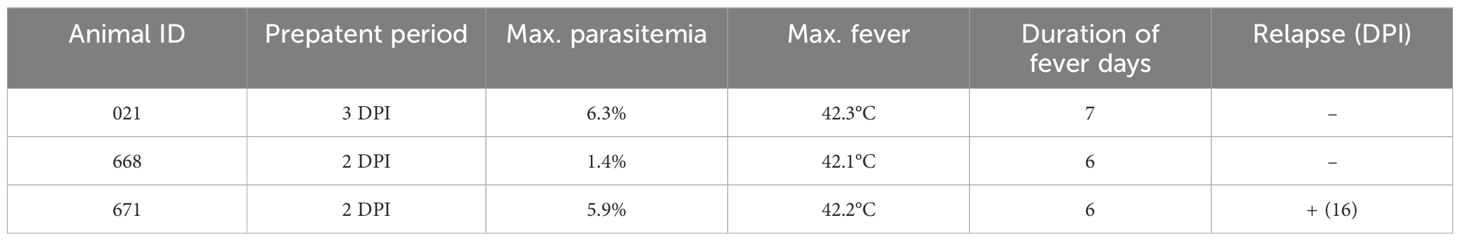
Parasites were identified in peripheral blood between the second and third days following the experimental infection of sheep with B. ovis/Alacakaya stabilate. As the infection progressed, all sheep exhibited fever, reaching peak temperatures between 42.0°C and 42.3°C, which corresponded to an increase in parasitemia. Microscopic examination of peripheral blood smears indicated parasitemia rates ranging from 5.9% to 6.3%. Severe clinical signs of babesiosis, such as hemoglobinuria, jaundice, decreased appetite, and lethargy, were observed in all experimentally infected sheep. Additionally, these sheep exhibited a significant decrease of >20% in the hematocrit levels. Parasitemia persisted for 6-7 days in all three sheep. Remarkably, despite severe babesiosis clinical signs, all sheep successfully recovered from the disease without treatment. In one sheep (#671), 9 days after the end of parasitemia, the temperature rose to 41.2°C, and piroplasms were observed in the blood smear. The following day, the fever in this sheep decreased to 39.0°C, and no piroplasms were detected under the microscope (Figure 2; Table 2).
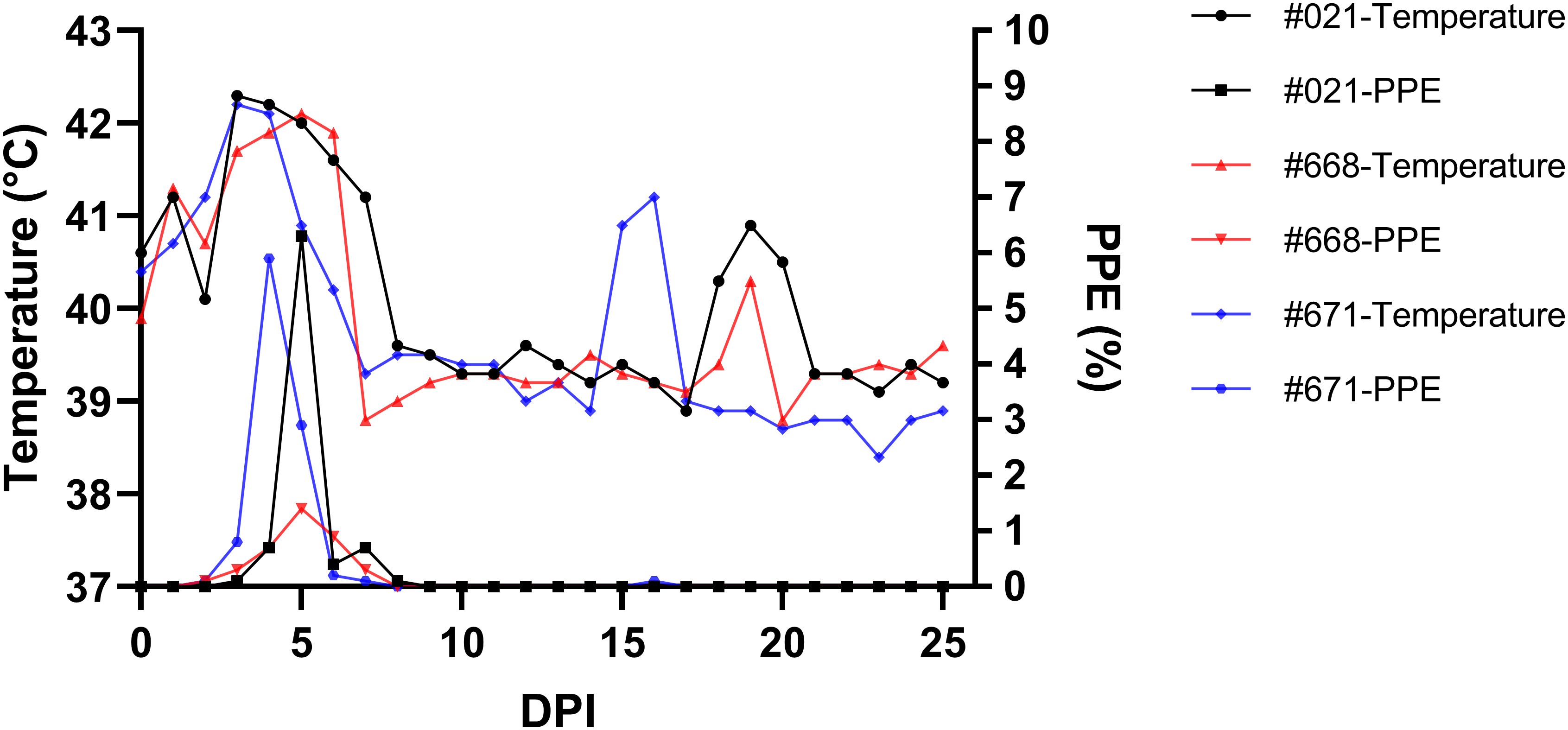
Figure 2 Monitoring parasitemia (PPE) and body temperature in sheep (#021, #668, #671) during the day post-infection period.
One day after experimental infection, the presence of the parasite in circulation was demonstrated through nPCR performed on DNA isolated from experimentally infected sheep’s blood. In sheep #021, #668, and #671, the presence of the parasite varied, continuing until days 59, 200, and 80, respectively, following experimental infection. Furthermore, in each of the three sheep, the parasite showed a fluctuating pattern of testing either as positive or negative after 50 days post-infection (DPI) (Figure 3).
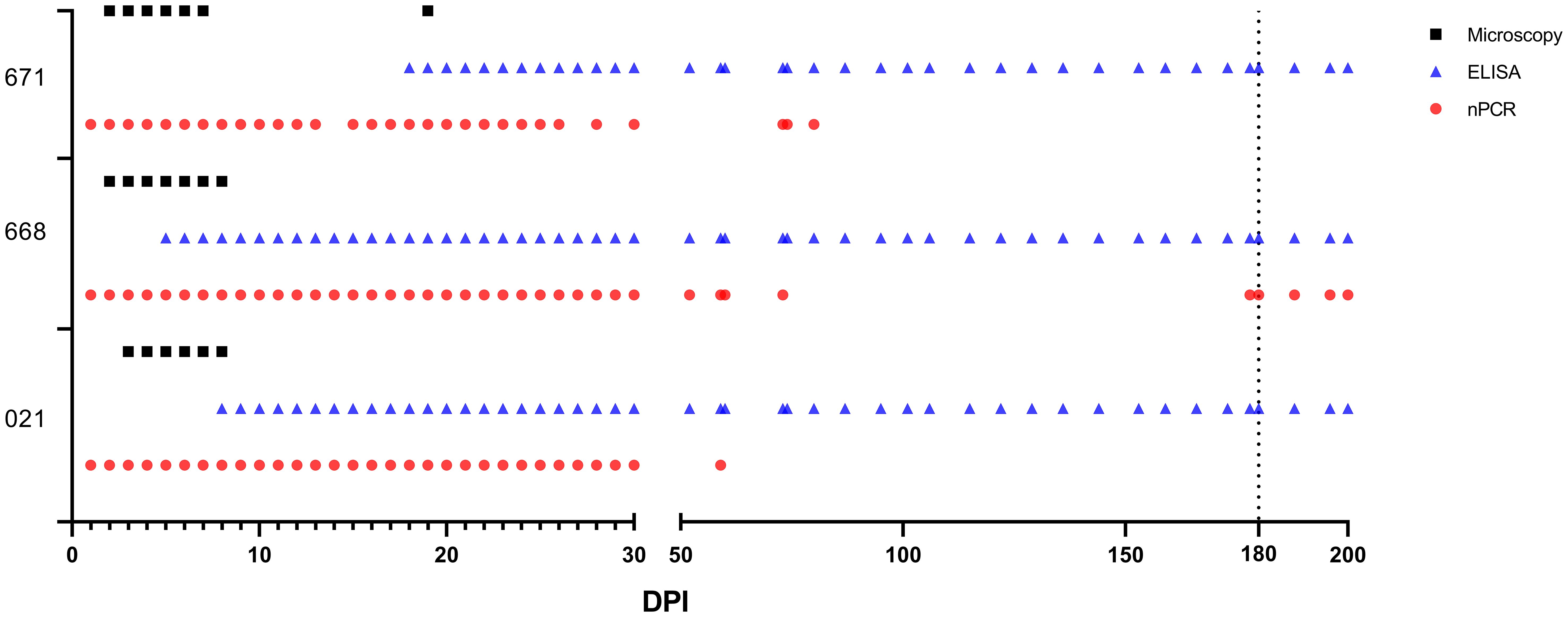
Figure 3 Babesia ovis infection dynamics by microscopy, nPCR and iELISA. On day 180, experimental animals were infested with sterile R. bursa larvae, and the blood taken from these animals was administered to the recipient animals.
Antibodies were detectable in experimentally infected sheep starting from 5-18 DPI. Following the initial detection, specific antibody responses to B. ovis persisted in all three sheep for more than 6 months after experimental infection (Figure 4). Fluctuations observed in nPCR were absent in the rBoSA1 iELISA.
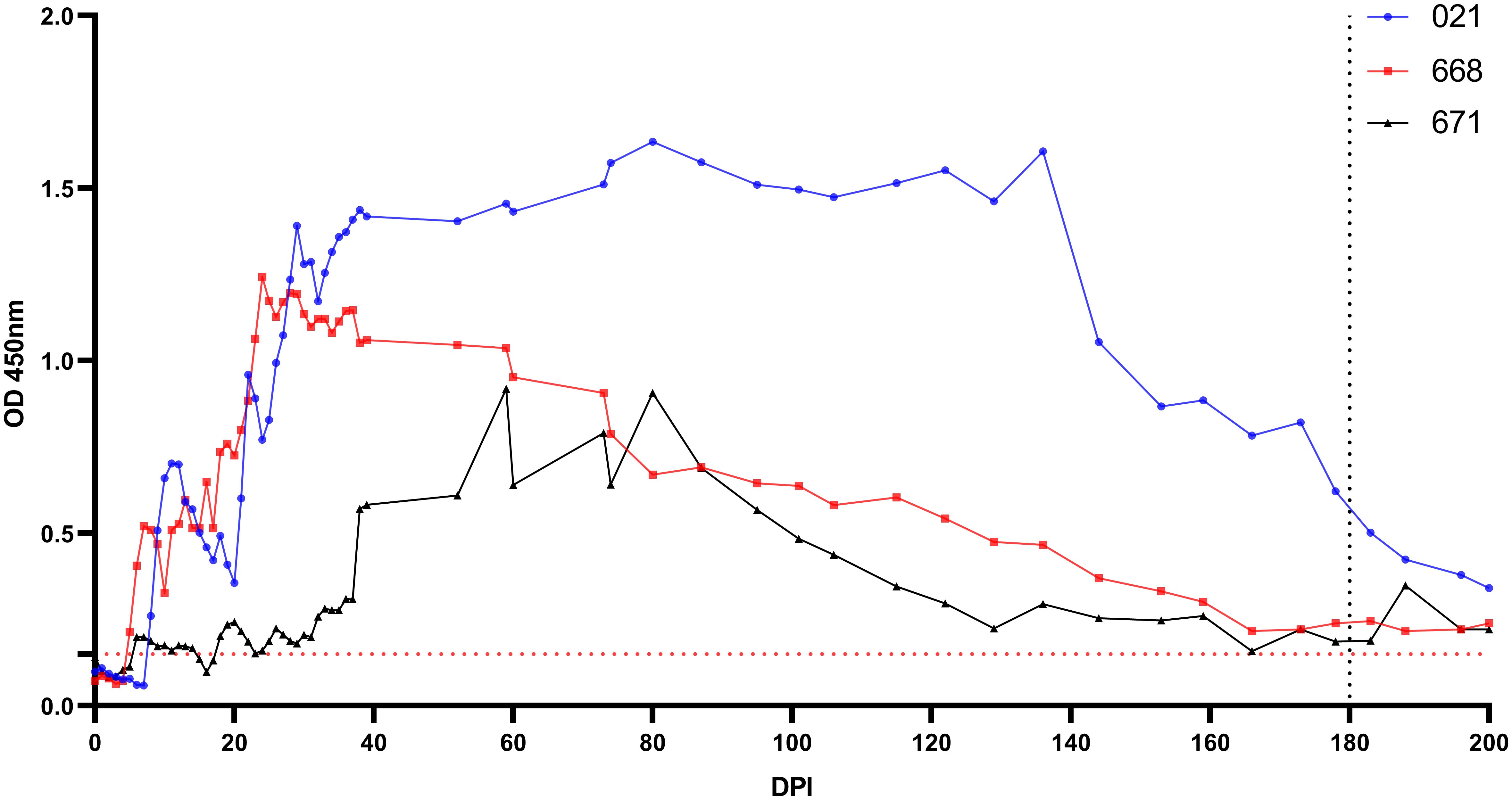
Figure 4 Antibody detection by iELISA for the detection of B ovis using rBoSA1 antigen on experimental animals (#021, #668, and #671). The Y and X axes represent absorbance at 450 nm and days post experimental infection (DPI) (days 0–200), respectively. Day 180 shows experimental infections. The red dashed line represents the cutoff value of the iELISA, calculated as the OD of non-infected sheep sera plus 3 standard deviations.
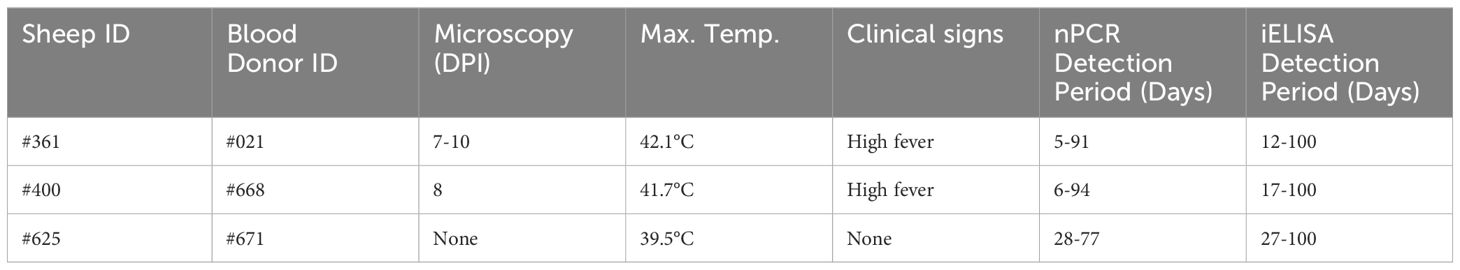
Babesia ovis transmission via blood inoculation from chronically infected sheep was performed as depicted in Figure 2. At the end of 6 months, nPCR results indicated the presence of B. ovis DNA only in sheep #668. In addition, blood transfusions were conducted to demonstrate the presence of B. ovis in the inoculated sheep. For this purpose, 100 ml of blood samples from #021, #668, and #671 were sequentially administered to sheep #361, #400, and #625, respectively, six months after experimental infection. Subsequently, the sheep receiving blood transfusion were monitored for 100 days using microscopic examination, nPCR, and iELISA. Following experimental infection, the initial presence of the B. ovis was observed in blood smears from #361 on the 7th day, and parasitemia persisted until day 10 post-infection. The maximum temperature measured on #361 was 42.1°C, and the high fever endured for 3 days, with no clinical signs other than fever noted. Babesia ovis was detected by nPCR between days 5 and 91 post-infection, showing fluctuations during this period. Antibodies against B. ovis were first detected on day 12 in sample #361 and continued to be present until day 100. In #400, B. ovis was observed microscopically at 8 DPI, and parasitemia lasted only for one day. A maximum temperature was recorded as 41.7°C, and like #361, no clinical signs other than high fever were observed. Following infection, the presence of B. ovis was detected by nPCR between days 6 and 94, with fluctuations during this period. In #625, the first positive reaction with iELISA started on day 17 and continued until day 100 post-infection. Unlike the other two sheep, in #625, no microscopic or clinical signs were observed after blood transfusion. Following infection, the presence of B. ovis was demonstrated by nPCR between days 28 and 77, with fluctuations during this period. The first positive reaction with iELISA started on day 27 and continued until day 100 (Figures 5–7; Table 3).
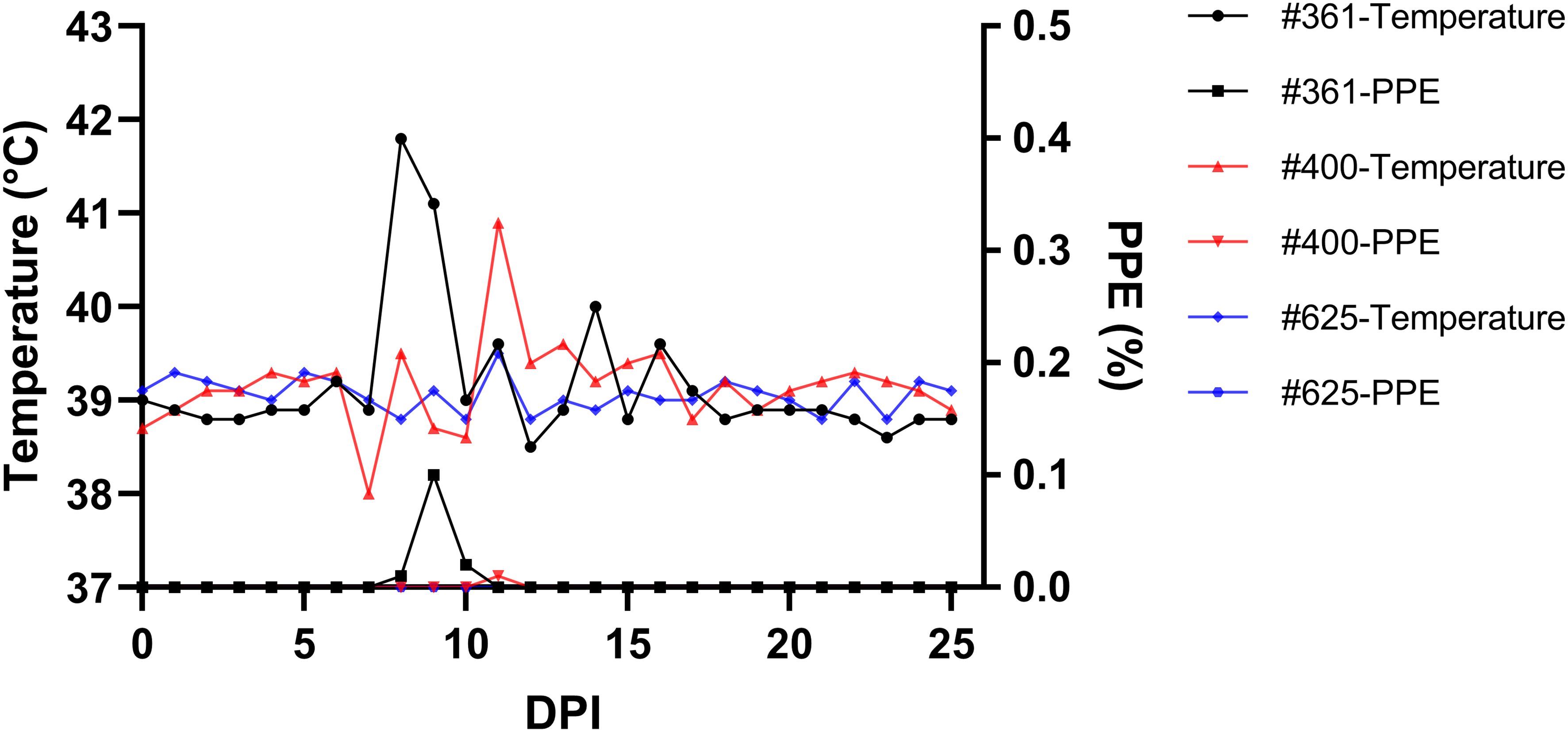
Figure 5 Monitoring parasitemia (PPE) and body temperature in sheep (#361, #400, #625) during the day post-infection period.
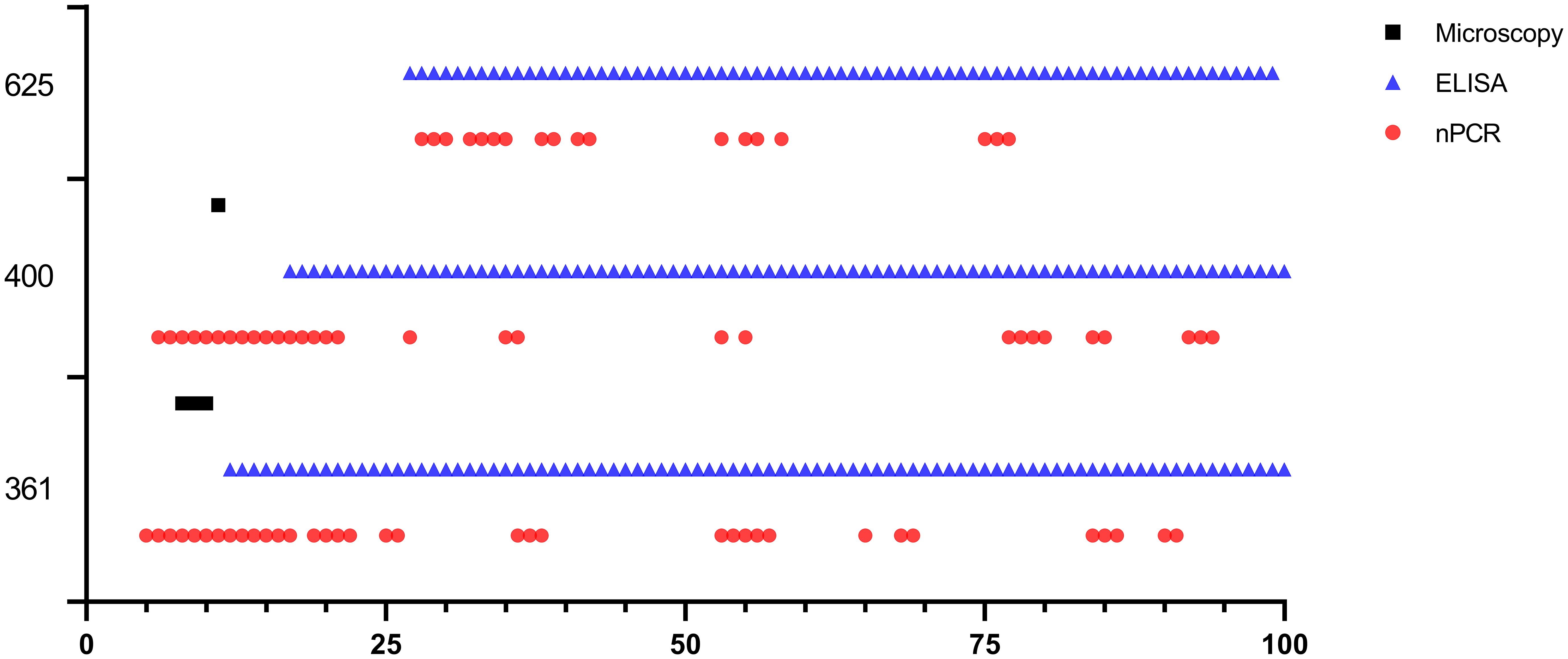
Figure 6 Long-term monitoring of sheep #361, #400, and #625 after experimental infection by microscopic examination, nPCR and iELISA.
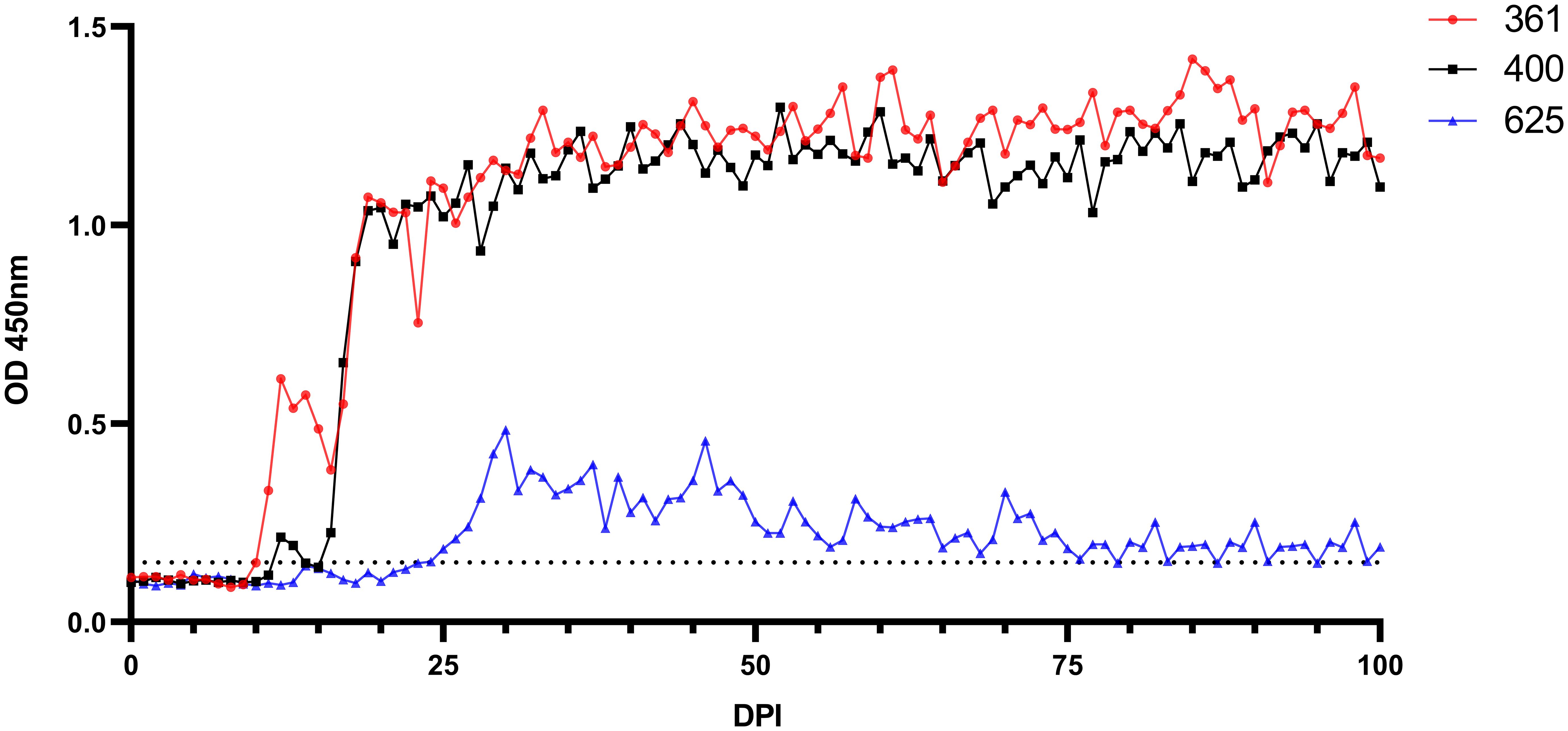
Figure 7 Serological analysis by iELISA for the detection of anti-B. ovis antibodies using rBoSA1 antigen on experimental animals (#361, #400, and #625). The Y and X axes represent absorbance at 450 nm and days after experimental infection (days 0–100), respectively. The black dashed line represents the cutoff value of the iELISA, calculated as the OD of non-infected sheep sera plus 3 standard deviations.
Sheep that survived B. ovis infection are not source of parasites for R. bursa transstadial transmission
Six months after experimental infection, sheep #021, #668, and #671 were infested with 0.1 grams of sterile R. bursa larvae, and after that, tick development was monitored daily. Twelve to eighteen days after experimental infestation, all engorged nymphs were collected. A total of 375, 190, and 150 engorged nymphs were collected from sheep #021, #668, and #671, respectively. From these nymphs, 302, 141, and 118 unfed adult R. bursa were obtained, respectively. Unfed adult ticks from individual sheep were segregated into separate pools of females and males, resulting in 12 pools (6 female, 6 male pools), each containing 5 females and 5 males (30 female, 30 male). These pools underwent nPCR analysis to detect the presence of B. ovis, with all pools yielding negative results. Following that, adult ticks, consisting of 50 females and 50 males per sheep, were applied to sheep #H7, #A9, and #M26 to investigate transmission. All ticks were subsequently collected from these sheep between 8 and 16 days after infestation. The transmission sheep (#H7, #A9, and #M26) were monitored for 100 days the presence of B. ovis using microscopic examination, nPCR, and iELISA, and the results were negative. Additionally, engorged male and female ticks collected from sheep #H7, #A9, and #M26 were individually examined with nPCR, revealing that these ticks were also negative. Collectively, these findings suggest no evidence of tick acquisition, nor transmission, of B. ovis from chronically infected sheep (6 months of infection) (#021, #668, and #671) to naive sheep (#H7, #A9, and #M26).
Discussion
Ovine babesiosis, caused by B. ovis, is a highly significant tick-borne disease prevalent in the Mediterranean region and other endemic areas worldwide where the vector tick R. bursa is present. This tick species, a two-host tick, is the exclusive known vector responsible for transmitting B. ovis among sheep populations (Yeruham et al., 1995, 2001; Erster et al., 2016). Extensive research has been conducted on the prevalence of B. ovis, yet significant gaps remain regarding the dynamics of the disease transmission and factors influencing its epidemiology. Morbidity and mortality caused by B. ovis typically occur once a year during the peak of adult R. bursa tick activity in the Spring months (April-June). Peak incidence is in May, lasting through April to July when adult ticks are active. No cases of babesiosis occur during the Autumn (September-November) when the immature stages of R. bursa (larvae and nymphs) are active (Yeruham et al., 1995). In this study, we utilized a sheep experimental model to investigate whether sheep infected with B. ovis during the peak transmission season could serve as reservoirs, potentially transmitting infection to host-seeking larval and nymphal stages of R. bursa.
The experimental infection of sheep with B. ovis/Alacakaya stabilate resulted in parasitemia in peripheral blood between 2 and 3 DPI, which was accompanied by fever. Severe clinical signs of babesiosis, including hemoglobinuria, icterus, reduced appetite, and lethargy, were also observed, leading to a substantial decline of 20% in the hematocrit level. Remarkably, the three experimentally infected sheep recovered from the acute disease without treatment, with parasitemia persisting for 6-7 days in all cases. These findings are consistent with previous results in sheep experimentally infected using the B. ovis/Extremadura isolate (Habela et al., 1990). However, in another study, B. ovis/Israel strain, parasitemia remained for 16 days in experimentally infected sheep (Erster et al., 2016). Microscopic analysis of peripheral blood smears revealed parasitemia levels ranging from 5.9% to 6.3%, indicating a high parasitemia rate based on the classification by Sevinc et al., 2013. Additionally, one sheep showed a transient rise in temperature and the presence of piroplasms in blood smear 9 days after parasitemia had finished. This result was expected, considering that parasite relapses in babesiosis have been documented in humans (Renard and Ben Mamoun, 2021), cattle (Mahoney and Mirre, 1977; Mahoney et al., 1979), and dogs (Ayoob et al., 2010). Furthermore, relapses have been reported in B. ovis despite imidocarb dipropionate treatment (Sevinc et al., 2007). Studies indicate that B. bovis infected cattle may also experience episodes of subclinical recrudescence that can happen up to 4 years after infection, likely attributed to antigenic variation (Mahoney et al., 1973). Additionally, stress associated with animal management has been identified as a potential trigger for these relapses (Stuen, 2020).
Notably, nPCR demonstrated the presence of the parasite in peripheral blood of the three sheep inoculated with blood from the donor animal, with parasitemia varying to up to 200 DPI, characterized by intermittent negative and positive results. Similar study, following experimental infection of bovines with B. bovis, the parasite presence was tracked using nPCR, revealing fluctuations akin to those observed in our investigation (Suarez et al., 2012; Chung et al., 2017). It was underscored that diagnostic methods reliant on molecular techniques, such as PCR, demonstrate fluctuations in parasitemia which can be attributed to parasite sequestration and antigenic variation (Suarez et al., 2012; Chung et al., 2017). While sequestration is not recognized as a characteristic of B. ovis, a gap on research addressing this aspect exists. Furthermore, in experimental infections conducted through blood inoculation and infected ticks, B. ovis was detected by qPCR for up to 180 and 395 DPI, respectively (Erster et al., 2016). Specific antibody responses to BoSA1 were detectable in all infected sheep starting at 5 to 18 DPI, consistently persisting for over 6 months post-infection. These results are in line with high antibody titers reported between days 7 and 75 after experimental infection in the rBoSA1 iELISA (Sevinc et al., 2015). The specific antibody titer, determined through IFAT, remained positive for up to 180-200 days post exposure in sheep infected with blood or tick infestation (Erster et al., 2016). Similarly, it is known that the antibody response persists for 330 days after experimental infection with B. ovis (Habela et al., 1990).
In the present study, B. ovis was exclusively detected in one sheep by nPCR six months after infection. Subsequent blood transfusions from donor sheep to recipient sheep were performed to demonstrate B. ovis infection. Notably, one recipient sheep exhibited parasitemia and high fever from day 7 to day 10, with B. ovis detected by nPCR and iELISA. Another recipient sheep exhibited a shorter parasitemia duration (one day) and a fever response similar to the first recipient. Conversely, a third recipient sheep showed no clinical signs, although B. ovis presence was confirmed by nPCR and antibodies to BoSA1 between days 28-77 and 27-100 post-infection, respectively. These results highlight the diverse responses among the transfused sheep following B. ovis infection, underscoring the intricate dynamics of this infection and suggesting that individual variability may significantly influence our findings. Several potential explanations for the distinct responses in recipient animals can be considered. First, variations in the number of parasites inoculated during stabilate or primary infection (dose) could play a crucial role in pathogenesis. Second, the animal remained persistently infected after six months may exhibit antigenic and phenotypic differences compared to the initial stabilate population. This variation could arise due to selective pressures exerted by the host’s immune system during the infection, potentially leading to the emergence of attenuated strains. These strains might already exist as a subpopulation within the original strain, subsequently selected during infection and immunity, or could arise through other processes, including epigenetic mechanisms. Additionally, the presence of genes, such as Babesia “vesa-like” in the genome of the parasite, could enable parasites to persist through antigenic variation mechanisms, although the specifics of how this mechanism operates in B. ovis remain unclear. Ultimately, whether such selection results in attenuated or phenotypically distinct parasites also remains an open question. While ticks are the main vectors for babesiosis, the disease can also spread to humans through blood transfusion. For instance, Babesia microti is reported as the leading pathogen transmitted via blood transfusion in the United States. Donors should be PCR screened for babesiosis before blood collection, but a negative result doesn’t rule out infection as parasite levels may be below detectable levels (Levin and Krause, 2016). Findings from our study provide supporting evidence for this phenomenon. Our research substantiates the notion that babesiosis can be transmitted despite negative nPCR results. Although blood transfusion has made significant advancements in recent years in veterinary medicine (Kumar, 2017), it is not widespread in practical terms for sheep.
It has been experimentally shown that immature R. bursa can acquire the agent from sheep acutely infected with B. ovis and transmit B. ovis transstadially to other sheep at the adult stage (Erster et al., 2016). However, in our study, considering natural conditions, sheep (#021, #668, #671) chronically infected with B. ovis six months post-acute infection were infested with immature R. bursa, and it was observed in vivo that the pathogen was not transmitted to the next generation. Several factors could explain the observed failure of transstadial transmission in our study. Firstly, the donor animals (#021, #668, #671) exhibited parasite loads that were undetectable even with nPCR, which may have contributed to the unsuccessful transstadial transmission. Experimental studies have demonstrated a direct correlation between the level of parasitemia in sheep and the number of kinetes in the tick’s hemolymph (Yeruham et al., 2001). This suggests that insufficient parasitemia in the donor animals could directly impact the ability of ticks to acquire and transmit the pathogen. Furthermore, the low parasite load in the host may influence the survival and replication of the pathogen within the tick due to interactions with the tick’s immune system. The innate immune responses of the tick, including antimicrobial peptides and phagocytic cells, could actively inhibit the transmission of B. ovis (de la Fuente et al., 2017). Overall, the combination of low parasitemia in donor animals, and the complex interactions between the pathogen, tick immune responses, and host immune responses appears to significantly hinder the transstadial transmission of B. ovis under natural conditions.
In conclusion, our study provides valuable insights into the epidemiology of B. ovis in sheep, particularly regarding the potential role of recovered chronically infected sheep as sources of infection for R. bursa larvae. While our blood transmission experiment demonstrated the presence of the parasite in recipient sheep, the tick transmission experiment indicated a lack of successful transmission from experimentally infected hosts to sterile R. bursa larvae and nymphs. Although generated in an experimental model, these findings suggest that sheep surviving babesiosis without treatment between May and July may not significantly contribute to the transmission of B. ovis to host-seeking R. bursa larvae in November-December. However, it should be remarked that these observations may not apply to the case of sheep acutely infected with B. ovis, where tick acquisition and transstadial mechanisms are more likely to occur due to a higher number of parasites in the blood intake by the ticks, which adds to the lack of selection of “attenuated” strains by the action of the immune system of the hosts at this stage of infection. Altogether, the data reported hereby emphasizes the significance of transovarial transmission in the dissemination of B. ovis, as the parasite demonstrates notable adaptation to the vector and persists within the tick for numerous successive generations (Markov and Abramov, 1970). Further research is warranted to explore additional factors influencing the transmission dynamics of B. ovis and to develop targeted control ,strategies to mitigate the impact of this disease on sheep populations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
This study was carried out according to the regulations of animal and welfare issued by the Turkish legislation for the protection of animals. All animal experiments were approved by the Firat University, Animal Experiment Ethic Committee, protocol number 2023/12-05. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
RF: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MU: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. MA: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. OC: Methodology, Project administration, Writing – original draft, Writing – review & editing. FS: Methodology, Writing – original draft, Writing – review & editing. RB: Formal analysis, Writing – original draft, Writing – review & editing. CS: Formal analysis, Writing – original draft, Writing – review & editing. SO: Conceptualization, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Scientific and Technological Council of Türkiye (TUBITAK) Grant Program (project number: 123O295). The sheep designated as #021, #668, and #671 utilized in this study were supported by a separate TÜBİTAK Grant Program (project number: 222O123).
Acknowledgments
We are grateful to Aleyna Karoglu, Arda Eyvaz, Havva Nur Cevik and Halil Unal for the excellent technical and administrative support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aktas, M., Altay, K., Dumanli, N. (2005). Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet. Parasitol. 133, 277–281. doi: 10.1016/j.vetpar.2005.05.057
Altay, K., Aktas, M., Dumanli, N. (2008). Detection of Babesia ovis by PCR in Rhipicephalus bursa collected from naturally infested sheep and goats. Res. Vet. Sci. 85, 116–119. doi: 10.1016/j.rvsc.2007.08.002
Ayoob, A. L., Hackner, S. G., Prittie, J. (2010). Clinical management of canine babesiosis. J. Vet. Emerg. Crit. Care (San Antonio) 20, 77–89. doi: 10.1111/vec.2010.20.issue-1
Bekker, C. P., de Vos, S., Taoufik, A., Sparagano, O. A., Jongejan, F. (2002). Simultaneous detection of Anaplasma and Ehrlichia species in ruminants and detection of Ehrlichia ruminantium in Amblyomma variegatum ticks by reverse line blot hybridization. Vet. Microbiol. 89, 223–238. doi: 10.1016/S0378-1135(02)00179-7
Black, W. C., Piesman, J. (1994). Phylogeny of hard- and soft-tick taxa (Acari: Ixodida) based on mitochondrial 16S rDNA sequences. Proc. Natl. Acad. Sci. U.S.A. 91, 10034–10038. doi: 10.1073/pnas.91.21.10034
Buscher, G., Friedhoff, K. T., EL-Allawy, T. A. A. (1988). Quantitative description of the development of Babesia ovis in Rhipicephalus bursa (hemolymph, ovary, eggs). Parasitol. Res. 74, 331–339. doi: 10.1007/BF00539454
Ceylan, O., Xuan, X., Sevinc, F. (2021). Primary tick-borne protozoan and rickettsial infections of animals in Turkey. Pathogens 10, 231. doi: 10.3390/pathogens10020231
Chung, C. J., Suarez, C. E., Bandaranayaka-Mudiyanselage, C. L., Bandaranayaka-Mudiyanselage, C. B., Rzepka, J., Heiniger, T. J., et al. (2017). A novel modified-indirect ELISA based on spherical body protein 4 for detecting antibody during acute and long-term infections with diverse Babesia bovis strains. Parasitol. Vectors 10, 77. doi: 10.1186/s13071-017-2016-9
de la Fuente, J., Antunes, S., Bonnet, S., Cabezas-Cruz, A., Domingos, A. G., Estrada-Peña, A., et al. (2017). Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. Cell Infect. Microbiol. 7. doi: 10.3389/fcimb.2017.00114
Erster, O., Roth, A., Wolkomirsky, R., Leibovich, B., Savitzky, I., Shkap, V. (2016). Transmission of Babesia ovis by different Rhipicephalus bursa developmental stages and infected blood injection. Ticks Tick. Borne. Dis. 7, 13–19. doi: 10.1016/j.ttbdis.2015.07.017
Georges, K., Loria, G. R., Riili, S., Greco, A., Caracappa, S., Jongejan, F., et al. (2001). Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 99, 273–286. doi: 10.1016/S0304-4017(01)00488-5
Guan, G., Ma, M., Moreau, E., Liu, J., Lu, B., Bai, Q., et al. (2009). A new ovine Babesia species transmitted by Hyalomma anatolicum anatolicum.. Exp. Parasitol 122, 261–267. doi: 10.1016/j.exppara.2009.05.001
Guan, G., Moreau, E., Liu, J., Hao, X., Ma, M., Luo, J., et al. (2010). Babesia sp. BQ1 (Lintan): molecular evidence of experimental transmission to sheep by Haemaphysalis qinghaiensis and Haemaphysalis longicornis. Parasitol. Int. 59, 265–267. doi: 10.1016/j.parint.2009.12.002
Habela, M., Reina, D., Nieto, C., Navarrete, I. (1990). Antibody response and duration of latent infection in sheep following experimental infection with Babesia ovis. Vet. Parasitol. 35, 1–10. doi: 10.1016/0304-4017(90)90111-N
Iqbal, F., Fatima, M., Shahnawaz, S., Naeem, M., Shaikh, R., Ali, M., et al. (2011). A study on the determination of risk factors associated with babesiosis and prevalence of Babesia sp., by PCR amplification, in small ruminants from Southern Punjab (Pakistan). Parasite 18, 229–234. doi: 10.1051/parasite/2011183229
Jalovecka, M., Sojka, D., Ascencio, M., Schnittger, L. (2019). Babesia life cycle - when phylogeny meets biology. Trends Parasitol. 35, 356–368. doi: 10.1016/j.pt.2019.01.007
Kawahara, M., Rikihisa, Y., Lin, Q., Isogai, E., Tahara, K., Itagaki, A., et al. (2006). Novel genetic variants of Anaplasma phagocytophilum, Anaplasma bovis, Anaplasma centrale, and a novel Ehrlichia sp. in wild deer and ticks on two major islands in Japan. Appl. Environ. Microbiol. 72, 1102–1109. doi: 10.1128/AEM.72.2.1102-1109.2006
Kumar, R. (2017). Blood transfusion in veterinary medicine. Hematol. Transfus. Int. J. 4, 116−122. doi: 10.15406/htij.2017.04.00093
Levin, A. E., Krause, P. J. (2016). Transfusion-transmitted babesiosis: is it time to screen the blood supply? Curr. Opin. Hematol. 23, 573–580. doi: 10.1097/MOH.0000000000000287
Mahoney, D. F., Kerr, J. D., Goodger, B. V., Wright, I. G. (1979). The immune response of cattle to Babesia bovis (syn. B. argentina). Studies on the nature and specificity of protection. Int. J. Parasitol. 9, 297–306. doi: 10.1016/0020-7519(79)90078-x
Mahoney, D. F., Mirre, G. B. (1977). The selection of larvae of Boophilus microplus infected with Babesia bovis (syn B argentina). Res. Vet. Sci. 23, 126–127. doi: 10.1016/S0034-5288(18)33242-9
Mahoney, D. F., Wright, I. G., Mirre, G. B. (1973). Bovine babesiasis: the persistence of immunity to Babesia argentina and B. bigemina in calves (Bos taurus) after naturally acquired infection. Ann. Trop. Med. Parasitol. 67, 197–203. doi: 10.1080/00034983.1973.11686877
Markov, A. A., Abramov, J. V. (1970). Results of a 20 years observation of repeated cycles of Babesia ovis in 44 generations of Rhipicephaus bursa (in Russian). Tr. V.ses. Inst. Eksp. Vet. 38, 5.
Oosthuizen, M. C., Zweygarth, E., Collins, N. E., Troskie, M., Penzhorn, B. L. (2008). Identification of a novel Babesia sp. from a sable antelope (Hippotragus Niger Harris 1838). J. Clin. Microbiol. 46, 2247–2251. doi: 10.1128/JCM.00167-08
Ozubek, S., Aktas, M. (2017). Molecular evidence of a new Babesia sp. in goats. Vet. Parasitol. 233, 1–8. doi: 10.1016/j.vetpar.2016.11.016
Ozubek, S., Ulucesme, M. C., Aktas, M. (2023a). Discovery of a novel species infecting goats: morphological and molecular characterization of Babesia aktasi n. sp. Pathogens 12, 113. doi: 10.3390/pathogens12010113
Ozubek, S., Ulucesme, M. C., Bastos, R. G., Alzan, H. F., Laughery, J. M., Suarez, C. E., et al. (2023b). Experimental infection of non-immunosuppressed and immunosuppressed goats reveals differential pathogenesis of Babesia aktasi n. sp. Front. Cell. Infect. Microbiol. 13. doi: 10.3389/fcimb.2023.1277956
Renard, I., Ben Mamoun, C. (2021). Treatment of human babesiosis: then and now. Pathogens 10, 1120. doi: 10.3390/pathogens10091120
Schnittger, L., Ganzinelli, S., Bhoora, R., Omondi, D., Nijhof, A. M., Florin-Christensen, M. (2022). The Piroplasmida Babesia, Cytauxzoon, and Theileria in farm and companion animals: species compilation, molecular phylogeny, and evolutionary insights. Parasitol. Res. 121, 1207–1245. doi: 10.1007/s00436-022-07424-8
Schwint, O. N., Ueti, M. W., Palmer, G. H., Kappmeyer, L. S., Hines, M. T., Cordes, R. T., et al. (2009). Imidocarb dipropionate clears persistent Babesia caballi infection with elimination of transmission potential. Antimicrob. Agents Chemother. 53, 4327–4332. doi: 10.1128/AAC.00404-09
Sevinc, F., Cao, S., Xuan, X., Sevinc, M., Ceylan, O. (2015). Identification and expression of Babesia ovis secreted antigen 1 and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay. J. Clin. Microbiol. 53, 1531–1536. doi: 10.1128/JCM.03219-14
Sevinc, F., Sevinc, M., Ekici, O. D., Yildiz, R., Isik, N., Aydogdu, U. (2013). Babesia ovis infections: detailed clinical and laboratory observations in the pre- and post-treatment periods of 97 field cases. Vet. Parasitol. 191, 35–43. doi: 10.1016/j.vetpar.2012.07.025
Sevinc, F., Turgut, K., Sevinc, M., Ekici, O. D., Coskun, A., Koc, Y., et al. (2007). Therapeutic and prophylactic efficacy of imidocarb dipropionate on experimental Babesia ovis infection of lambs. Vet. Parasitol. 149, 65–71. doi: 10.1016/j.vetpar.2007.07.014
Sevinc, F., Zhou, M., Cao, S., Ceylan, O., Ulucesme, M. C., Ozubek, S., et al. (2023). Babesia ovis secreted antigen-1 is a diagnostic marker during the active Babesia ovis infections in sheep. Front. Cell. Infect. Microbiol. 16 (13), 1238369. doi: 10.3389/fcimb.2023.1238369
Stuen, S. (2020). Haemoparasites-challenging and wasting infections in small ruminants: A review. Animals (Basel) 10, 2179. doi: 10.3390/ani10112179
Suarez, C. E., Laughery, J. M., Schneider, D. A., Sondgeroth, K. S., McElwain, T. F. (2012). Acute and persistent infection by a transfected Mo7 strain of Babesia bovis. Mol. Biochem. Parasitol. 185, 52–57. doi: 10.1016/j.molbiopara.2012.05.003
Walker, J. B., Keirans, J. E., Horak, I. G. (2000). The Genus Rhipicephalus (Acari,Ixodidae): A Guide to the Brown Ticks of the World (United Kingdom: Cambridge University Press).
Yeruham, I., Hadani, A., Galker, F. (1998). Some epizootiological and clinical aspects of ovine babesiosis caused by Babesia ovis–a review. Vet. Parasitol. 74, 153–163. doi: 10.1016/S0304-4017(97)00143-X
Yeruham, I., Hadani, A., Galker, F. (2000). The life cycle of Rhipicephalus bursa Canestrini and Fanzago 1877 (Acarina: ixodidae) under laboratory conditions. Vet. Parasitol. 89, 109–116. doi: 10.1016/S0304-4017(99)00225-3
Yeruham, I., Hadani, A., Galker, F. (2001). The effect of the ovine host parasitaemia on the development of Babesia ovis (Babes 1892) in the tick Rhipicephalus bursa (Canestrini and Fanzago 1877). Vet. Parasitol. 96, 195–202. doi: 10.1016/S0304-4017(00)00433-7
Yeruham, I., Hadani, A., Galker, F., Rosen, S. (1989). Notes on the biology of the tick Rhipicephalus bursa (Canestrini and Fanzago 1877) in Israel. Rev. Elev. Med. Vet. Pays.w Trop. 42, 233–235. doi: 10.19182/remvt.8845
Keywords: Babesia ovis, ELISA, experimental infection, PCR, Rhipicephalus bursa, sheep
Citation: Firat R, Ulucesme MC, Aktas M, Ceylan O, Sevinc F, Bastos RG, Suarez CE and Ozubek S (2024) Role of Rhipicephalus bursa larvae in transstadial transmission and endemicity of Babesia ovis in chronically infected sheep. Front. Cell. Infect. Microbiol. 14:1428719. doi: 10.3389/fcimb.2024.1428719
Received: 06 May 2024; Accepted: 12 July 2024;
Published: 26 July 2024.
Edited by:
Ana Gonçalves Domingos, New University of Lisbon, PortugalReviewed by:
Jixu Li, Qinghai University, ChinaHuarrisson Santos, Federal Rural University of Rio de Janeiro, Brazil
Consuelo Almazán, Autonomous University of Queretaro, Mexico
Copyright © 2024 Firat, Ulucesme, Aktas, Ceylan, Sevinc, Bastos, Suarez and Ozubek. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sezayi Ozubek, c296dWJla0BmaXJhdC5lZHUudHI=
 Recep Firat
Recep Firat Mehmet Can Ulucesme
Mehmet Can Ulucesme Munir Aktas
Munir Aktas Onur Ceylan
Onur Ceylan Ferda Sevinc
Ferda Sevinc Reginaldo G. Bastos
Reginaldo G. Bastos Carlos E. Suarez
Carlos E. Suarez Sezayi Ozubek
Sezayi Ozubek