
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 04 November 2024
Sec. Parasite and Host
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1418500
Alarmin cytokines including IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) function as danger signals to trigger host immunity in response to tissue injury caused by pathogenic factors such as parasitic infections. Parasitic diseases also provide an excellent context to study their functions and mechanisms. Numerous studies have indicated that alarmin cytokine released by non-immune cells such as epithelial and stromal cells induce the hosts to initiate a type 2 immunity that drives parasite expulsion but also host pathology such as tissue injury and fibrosis. By contrast, alarmin cytokines especially IL-33 derived from immune cells such as dendritic cells may elicit an immuno-suppressive milieu that promotes host tolerance to parasites. Additionally, the role of alarmin cytokines in parasite infections is reported to depend on species of parasites, cellular source of alarmin cytokines, and immune microenvironment, all of which is relevant to the parasitic sites or organs. This narrative review aims to provide information on the crucial and diverse role of alarmin cytokines in parasitic infections involved in different organs including intestine, lung, liver and brain.
Parasitic infections are a major global health and social burden, impacting over 25% of the global population with many more at risk of infection (Ryan et al., 2020). The interplay between parasites and the host immune responses determines the outcome of such diseases. Parasite infections can elicit a type 1 immune response characterized by the elevation of T helper 1 (Th1) cytokines such as interferon-γ, or a type 2 immune response featured by the production of Th2 cytokines such as interleukin 4 (IL-4), IL-5, and IL-13 (Engwerda et al., 2014; Zaiss et al., 2024). Notably, some parasites simultaneously induce both type 1 and type 2 responses (Muñoz-Carrillo et al., 2017; Muñoz-Carrillo et al., 2021). Host immune response is crucial to drive parasite expulsion or killing, whereas strong and long-lasting immune response can contribute to the development of host pathology such as tissue injury and fibrosis (Muñoz-Carrillo et al., 2021). However, the mechanism of the initiation and maintenance of host immunity induced by parasitic infections remains largely unknown.
Alarmins are endogenous molecules which function as danger signals such as parasitic infections and are rapidly released into the extracellular milieu in response to tissue injury to trigger defensive immune responses (Maizels, 2020). Among them, IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) are the first cytokines which are shown to activate group 2 innate lymphoid cell (ILC2) and have been conceptually grouped together ever since (El-Naccache et al., 2021). IL-25 (also known as IL-17E) belongs to the IL-17 cytokine family, which is produced by epithelial and immune cells. Its receptor IL-25R is a heterodimer consist of the common IL-17RA chain and specific IL-17RB chain (Wu et al., 2022). IL-33 is a member of the IL-1 family of cytokines, which is primarily expressed by non-immune cells such as epithelial cells, endothelial cells, and fibroblasts. Unlike the other cytokines, IL-33 is localized in the cell nucleus through its N-terminal domain which contains a chromatin-binding motif. The nuclear IL-33 can be passively released as alarmins from necrotic cells during tissue damage and unconventionally secreted from living cells. After released from cells, IL-33 binds to its receptor, suppressor of tumorigenesis 2 (ST2), on target cells (Dwyer et al., 2022). TSLP belongs to the IL-2 family of cytokines, which is mainly expressed by the epithelial cells of the gut, lung, and skin. Its receptor is a heterodimer of the common IL-7RA, which is shared with IL-7, and the specific TSLP receptor (TSLPR) (Corren and Ziegler, 2019). The importance of these alarmin cytokines in immune response especially the type 2 immunity is widely documented and extends beyond their role in ILC2 biology, because they are key regulators of Th2 cells, macrophage, mast cells, basophils, dendritic cells (DCs), regulatory T cells (Tregs), and more (Stanbery et al., 2022).
Recent works have highlighted the release of alarmin cytokines from damaged or stimulated epithelial and stromal cells is crucial for the initiation and maintenance of host protection but also tissue pathology after parasite infections (Maizels et al., 2012; Hung et al., 2013). Parasitic diseases also provide an excellent context to study the functions and mechanisms of alarmin cytokines (Henry et al., 2017; He and Pan, 2018). By understanding how they instruct hosts to activate innate and adaptive immune cells in response to parasitic invasions, we may be able to uncover novel strategies to prevent or treat parasitic diseases and other diseases. In addition, growing evidence has indicated that the role of alarmin cytokines in parasite infections is dependent upon species of parasites, cellular source of alarmin cytokines, and immune microenvironment, all of which is relevant to the parasitic sites or organs (McSorley and Smyth, 2021). Therefore, this narrative review aims to provide information on the crucial and diverse role of alarmin cytokines in parasitic infections involved in different organs including intestine, lung, liver and brain. In the literature review process, we performed a systematic search on Google Scholar and PubMed with the keywords including “IL-25”, “IL-33”, “TSLP”, and “parasite”.
It well documented that alarming cytokines are important regulators of type 2 inflammatory diseases triggered by parasitic worms and allergens (Liew et al., 2016; Gauvreau et al., 2023). In this review, we mainly summarized their crucial and diverse roles of in parasitic infections (Table 1), which will be addressed in detail in the subsequent sections. Since its link to atopic dermatitis, TSLP has widely been regarded as a promoter of type 2 inflammatory diseases, encompassing asthma, chronic rhinosinusitis, allergic rhinoconjunctivitis, and eosinophilic esophagitis (Rothenberg et al., 2010; Harada et al., 2011; Miyake et al., 2015; Ebina-Shibuya and Leonard, 2023). Multiple studies have highlighted the significance of IL-33 in airway allergic diseases. IL-33 levels are associated with the clinical severity of asthma, and genetic variants of IL-33 have been linked to susceptibility to allergic rhinitis and asthma risk (Sakashita et al., 2008; Kurowska-Stolarska et al., 2009). Additionally, IL-25 has been implicated in various models of allergic inflammation, such as asthma and chronic rhinosinusitis (Divekar and Kita, 2015; Patel et al., 2019). On the other hand, emerging data indicate that alarmin cytokines are not only involved in canonical type 2 responses but are also important in the context of various human diseases and even homeostasis. The involved human diseases include viral and bacterial infections, cancers, metabolic diseases, fibrotic diseases, and etc. They influence these diseases through both type 2– and non–type 2–mediated mechanisms (Monteleone et al., 2010; Rostan et al., 2015; Nie et al., 2016; Kotsiou et al., 2018; Tu and Yang, 2019).
Intestine is the most common colonized site for parasites. It is well documented that alarmin cytokines, especially for IL-33 and IL-25, are crucial protective regulators for hosts infected by intestinal helminths or protists. These intestinal helminths include Nippostrongylus brasiliensis (N. brasiliensis) (Stanbery et al., 2022; Varyani et al., 2022), Heligmosomoides polygyrus (H. polygyrus) (Zaiss et al., 2013; Coakley et al., 2017), Trichuris muris (Owyang et al., 2006; Chen et al., 2021), Strongyloides ratti (S. ratti) (Meiners et al., 2020), Trichinella spiralis (T. spiralis) (Angkasekwinai et al., 2017), and Echinostoma caproni (E. caproni) (Muñoz-Antoli et al., 2016). The intestinal protist includes Entamoeba histolytica (E. histolytica) (Noor et al., 2017; Uddin et al., 2022). Global IL-33 or IL-25 signaling deficiency resulted in delayed intestinal parasite expulsion, while administration of exogenous IL-33 or IL-25 led to accelerated intestinal parasite expulsion (Owyang et al., 2006; Zaiss et al., 2013; Muñoz-Antoli et al., 2016; Angkasekwinai et al., 2017; Coakley et al., 2017; Noor et al., 2017; Meiners et al., 2020; Chen et al., 2021; Stanbery et al., 2022; Uddin et al., 2022; Varyani et al., 2022). The extent of functional redundancy between IL-33 and IL-25 for intestinal parasitic infections is still unclear, but experiment evidence indicated that IL-33 and IL-25 may largely exert a co-operative action (Neill et al., 2010). The absence of both IL-33 and IL-25 signaling severely impaired N. brasiliensis expulsion, and their effector cells, ILC2, failed to expand in either the mesenteric lymph nodes (MLNs) or peritoneal lavage (Neill et al., 2010). However, TSLP is only essential for the expulsion of Trichuris muris and T. spiralis (Massacand et al., 2009; Giacomin et al., 2012).
The protective effects of IL-33 and IL-25 for intestinal parasitic infections are largely dependent on the activation of ILC2 which express IL-33 receptor ST2 and IL-25 receptor IL17RB (Mathä et al., 2022). It has been observed that, in the mouse model (C57BL/6) ILC2 is widely distributed at the tissue level, particularly in MLNs, spleen, and liver. These cells expand robustly after infection with intestinal parasites such as N. brasiliensis or in response to exogenous IL-33 or IL-25, and they are the major innate IL-13 resources under these conditions (Price et al., 2010). Activation of ILC2s is sufficient for N. brasiliensis clearance in the mouse model (C57BL/6), even in the absence of adaptive immunity (Stanbery et al., 2022). ILC2 is a functional heterogeneous cell population, which can be grouped into natural ILC2 (nILC2) and inflammatory ILC2 (iILC2). nILC2 locates at barrier tissues which are crucial for tissue homeostasis and primarily IL-33-responsive, while iILC2 is not resided in peripheral tissues in the steady state but can be elicited at many sites by intestinal parasite infection or IL-25/IL-33 treatment (Huang et al., 2015; Miller and Reinhardt, 2020). IL-13 is believed to be the main downstream effector of ILC2. The accumulation of IL-13 production from ILC2 instructs epithelial cells to produce resistin-like molecule beta (RELMβ) and recruits eosinophils, which together result in parasite destruction (Stanbery et al., 2022). In addition, ILC2 may also collaborate with Th2 and Th9 cells for hosts to better clear the intestinal parasites such as T. spiralis (Angkasekwinai et al., 2017). These cells together instruct the gut mucosa to mount a “weep and sweep” response to expel gut parasites through IL4Rα signaling (Bąska and Norbury, 2022) (Figure 1). TSLP promotes for the development of protective Th2 responses upon infection with Trichuris muris through inhibition of IL-12p40 production from dendritic cell (DC). Excretory-secretory (ES) products from H. polygyrus and N. brasiliensis, but not Trichuris muris, were capable of directly suppressing DC production of IL-12p40, thus bypassing the need for TSLP (Massacand et al., 2009).
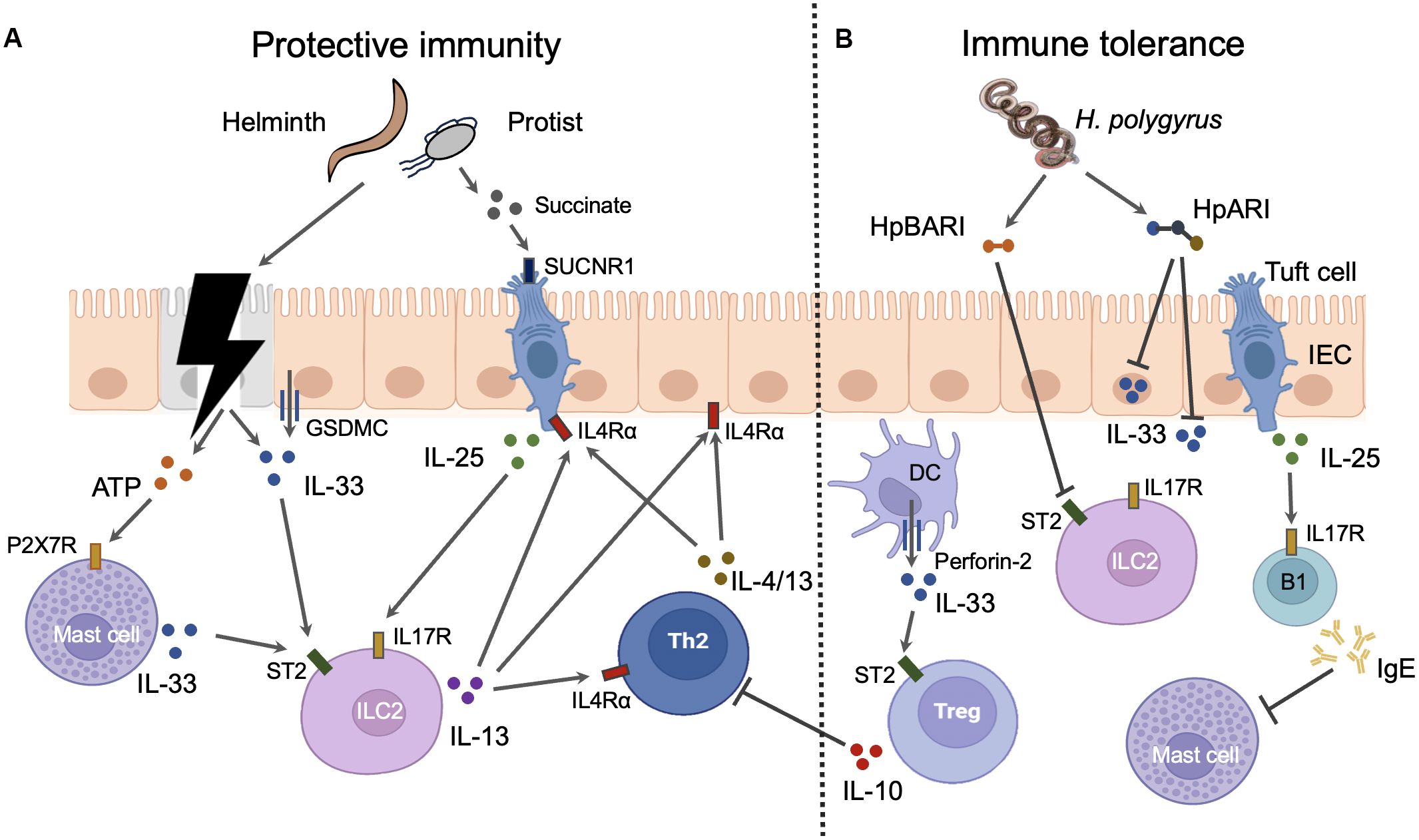
Figure 1. The roles and mechanisms of alarmin cytokines in intestinal parasitic infections. IL-25 and IL-33 can elicit both protective immunity and immune tolerance in intestine during infections. (A) Protective immunity. Parasites can cause the damage of intestinal epithelial cells (IECs) which results in the release of IL-33 from these cells. The injured IECs also release ATP which induce the mast cells to secret IL-33 through binding to P2X7R. Moreover, the intact IECs can actively secret IL-33 via GSDMC membrane pores after the activation of IL4Rα signaling. Epithelial tuft cells are the sole source of IL-25 in the gut. The metabolites such as succinate from intestinal parasites especially some protist can trigger the tuft cells to secret IL-25 through interaction with taste receptors such as SUCNR1. Both IL-25 and IL-33 can activate group 2 innate lymphoid cells (ILC2s) which express IL-25 receptor IL17R and IL-33 receptor ST2. IL-13 released from activated ILC2s promotes the activation of Th2 cells which produce Th2 cytokines such as IL-4 and IL-13. Th2 cytokines from both ILC2s and Th2 cells instruct the gut mucosa to mount a “weep and sweep” response to expel gut parasites through IL4Rα signaling. Th2 cytokines can also amplify the response of IECs and tuft cell to parasite invasion through IL4Rα signaling. Thus, the positive feed forward loop comprised of IECs, tuft cells, ILC2s, and Th2 cells is the main host defense mechanism against intestinal parasites. (B) Immune tolerance. Myeloid cells such as DCs release IL-33 through the inducible transmembrane pore-forming protein perforin-2. DC-derived IL-33 activates the ST2 positive intestinal Tregs which suppress the activation of Th2 cells. IL-25 induces B1 cell IgE production which blocks the activation of mast cells. H. polygyrus has evolved several mechanisms to negate the function of IL-33. Soluble excretory protein H. polygyrus alarmin release inhibitor (HpARI) can blocks the function of IL-33 by binding the intracellular and extracellular IL-33. Another soluble excretory protein H. polygyrus binds alarmin receptor and inhibits (HpBARI) can bind membrane ST2 which prevents IL-33-ST2 interactions.
Intestinal epithelial cells (IECs) are the dominant sources of IL-33 in the gut, while epithelial tuft cells are the sole source of IL-25 in the gut. As a nuclear cytokine, the IL-33 release from IECs is proposed to be resulted from cell necrosis induced by intestinal nematodes. The necrotic IECs also release ATPs which activate mast cell to produce IL-33 through P2X7 ATP receptor (Shimokawa et al., 2017). Mast cells are a potent source of IL-33 during infection with intestinal nematodes such as H. polygyrus (Shimokawa et al., 2017). However, IL-33 can also be actively released from IECs in the absence of cell death during infection (Zhao et al., 2022). This process requires the O-linked N-Acetylglucosamine transferase which mediates the O-GlcNAcylation of STAT6. This modification allows STAT6 to facilitate the unconventional IL-33 secretion from IECs via GSDMC membrane pores which also mediate the secretion of IL-1β and IL-18 from myeloid cells after inflammasome activation (Zhao et al., 2022). Besides, modified STAT6 also drives the differentiation of IL-25-producing tuft cells through promoting Pou2f3 transcription (Zhao et al., 2022). Thus, the type 2 cytokines can amplify the response of IECs and tuft cell to parasite invasion through IL4Rα/STAT6 signaling. The positive feed forward loop comprised of IECs, tuft cells, ILC2, and Th2 cells is the main host defense mechanism against intestinal parasites. Besides, the taste receptor signaling plays a crucial role in directing tuft cells to release IL-25. As a group of taste-chemosensory cells, tuft cells are the primary IEC subset expressing taste receptors such as succinate receptor (SUCNR1) and bitter-taste receptors (Tas2rs), which detect the presence of Tritrichomonas muris and T. spiralis (Schneider et al., 2018; Luo et al., 2019) (Figure 1).
In order to allow persistence of the parasite in the host, intestinal parasites, especially H. polygyrus, have evolved several mechanisms to negate the function of alarmin cytokines (Figure 1). It was noted early that soluble excretory/secretory products of H. polygyrus (HES) potently suppress type 2 inflammation (McSorley et al., 2012). Nowadays, two immunomodulatory proteins from HES have been identified by LC-MS/MS, which include H. polygyrus alarmin release inhibitor (HpARI) and H. polygyrus binds alarmin receptor and inhibits (HpBARI). Both HpARI and HpBARI contain complement control protein (CCP) domains which are present in different phyla including chordates and nematodes. HpARI cannot enter intact cells but can only gain access to the nucleus of necrotic cells, where it binds directly to IL-33 and nuclear DNA via its CCP domains, tethering IL-33 within necrotic cells. HpARI can also directly bind to both mouse and human extracellular IL-33 to prevent IL-33 interact with ST2 on target cells (Osbourn et al., 2017; Chauché et al., 2020). HpBARI binds ST2 and inhibits cell surface detection of ST2, which prevents IL-33-ST2 interactions and blocks IL-33 responses (Vacca et al., 2020).
Alarmin cytokines can also drive immune tolerance of host to the intestinal parasites. The functional response of IL-33 is dictated by its cellular source. Whereas IL-33 derived from IECs elicits host-protective immune responses, IL-33 released from myeloid cells such as DCs suppresses these responses in mice infected with N. brasiliensis. DCs use the inducible transmembrane pore-forming protein perforin-2 to deliver IL-33 from the cytoplasm into the extracellular space. DC-derived IL-33 functions to activate the intestinal Treg population through interaction with ST2 (Hung et al., 2020). Moreover, IL-25 induces B1 cell IgE production which blocks N. brasiliensis clearance through inhibition of mucosal mast cell activation (Martin et al., 2018).
Alarmin cytokines not only modulate the parasite colonization in intestine, but also drives the adaptive remodeling of intestine after parasite colonization. It is reported that the length of small bowel from mice colonized with H. polygyrus or Tritrichomonas muris was significantly longer as compared with non-colonized controls. The increased length of intestine was accompanied by a process of crypt fission resulting in duplication of stem cell niches that was prominent in areas in proximity to the colonized parasites. Mechanically, IL-25 dependent amplification of tuft cell-ILC2 circuit is necessary and sufficient to induce adaptive remodeling of the small bowel. Interestingly, succinate, an end product of Tritrichomonas muris metabolism, is a potent inducer of this circuit (Schneider et al., 2018).
Various nematode larvae need to pass through the lungs before they develop into adults in the guts or other parasitic sites. Such nematodes include N. brasiliensis, S. venezuelensis, Litomosoides sigmodontis (L. sigmodontis), and etc. Lung is the major site of larvae killing in anamnestic immunity against N. brasiliensis. This process needs pulmonary IL-13-producing ILC2 and Th2 cells working in concert to ensure maintenance of M2 macrophages (Bouchery et al., 2015). Th2 cell requirement can be bypassed by administration of IL-2 or IL-33, resulting in expansion of IL-13-producing ILC2 and larval killing (Bouchery et al., 2015). The prior presence of H. polygyrus or T. muris in the intestines also protects their hosts against migrating N. brasiliensis larvae in the lungs by a process involving IL-33-activated Th2 cells that releases IL-5 and recruits activated eosinophils. Importantly, lung immunity remains intact in mice cleared of prior H. polygyrus or T. muris infection (Filbey et al., 2019). For L. sigmodontis, larvae are rapidly cleared from peripheral blood and retained in the lung tissue of mice sensitized with dead larvae in an IL-33-driving eosinophil-dependent manner (Reichwald et al., 2022; Lenz et al., 2024). Besides playing a crucial role in anamnestic immunity, IL-33 is also involved in larvae killing in the primary infection of nematodes. It is reported that IL-33 from type II alveolar epithelial cells aids to killing the infected larvae of S. venezuelensis in the lungs by inducing the proliferation of ILC2 and production of IL-5 and IL-13 during the primary infection (Yasuda et al., 2012).
On the other hand, alarmin cytokines are associated with the development of parasite infection-related pulmonary pathology. As described above, IL-33 is a protective factor for hosts to eliminate migrating larvae of S. venezuelensis and L. sigmodontis in lungs. By contrast, IL-33 also contributes to the development of the airway hyperresponsiveness induced by S. venezuelensis and eosinophilic lung disease induced L. sigmodontis through the initiation of strong type 2 immune response (Araujo et al., 2016; Lenz et al., 2024). Parasite egg-induced pulmonary granuloma model is a widely used animal model to study the mechanism of schistosome-induced pathology. Using this model, Hams et al. demonstrate a role for IL-25 in the generation of pulmonary granuloma and fibrosis through the induction of IL-13 release from ILC2 (Hams et al., 2014).
Liver is another common targeted organ for parasite infection, such as Echinococcus granulosus (E. granulosus), Echinococcus multilocularis (E. multilocularis), Clonorchis sinensis (C. sinensis), Schistosoma mansoni (S. mansoni), Schistosoma japonicum (S. japonicum) and Leishmania donovani (L. donovani). Emerging evidence suggests that alarmin cytokines may promote the survival or growth of parasites colonized in the livers. It is reported that IL-33 accelerates the growth of larval stage of E. multilocularis in the liver putatively through the induction of a tolerogenic microenvironment (Autier et al., 2023). Administration of exogenous IL-33 increases the burdens of S. japonicum in infected mice, whereas worm burdens are decreased when blocking IL-33 using neutralizing antibodies (Yu et al., 2015). In experimental models of visceral leishmaniasis, transgenic BALB/c mice with ST2 deficiency exhibit better control of parasite load in the liver. This is associated with an early infiltration of monocytes and neutrophils, as well as a Th1-polarized immune response. Conversely, administering recombinant IL-33 to BALB/c mice leads to a high parasite burden in the liver, accompanied by a suppressed Th1 response and limited infiltration of monocytes and neutrophils (Rostan et al., 2013).
By contrast, alarmin cytokines may contribute to the development of liver injury and fibrosis induced by parasite infections. In the animal models, it is documented that IL-33 contributes to C. sinensis induced biliary injuries and fibrosis potentially through orchestration of type 2 immunity (Yan et al., 2022). The role of alarm cytokines in the liver pathology induced by schistosome infection is intensively investigated, however the conclusion is still controversy. ST2 deficiency or blockade of IL-33 using soluble ST2 treatment or neutralizing antibodies resulted in obvious less Th2 cytokine production and Th2-mediated pathology in livers, including marked decreases in granuloma size and fibrosis extent (McHedlidze et al., 2013). The pathogenic effects of IL-33 in hepatic schistosomiasis largely derived from the promotion of the expansion and IL-13 production of ILC2, or induction of the differentiation of M2 macrophages (McHedlidze et al., 2013; Peng et al., 2016). Our group uncovered that hepatic stellate cell is the primary source of IL-33 and that ILC2 is the primary source of IL-13 in the Schistosoma japonicum infected mouse livers (He et al., 2018). On the contrary, there were also several evidence which proved that alarmin cytokines had protective effects or had no impacts in schistosomiasis (Bai et al., 2021; Maggi et al., 2021; Mukendi et al., 2021; Maggi et al., 2023). Blocking IL-33 signaling by knockout of St2 or Il33 gene is reported to enhance host mortality by modulating granuloma-mediated pathology. The protective effects of IL-33 could stem from inducing thymic involution-associated naive T cell aging, or upregulation of Treg and downregulation of Th17 (Bai et al., 2021; Xu et al., 2022). Furthermore, an independent group showed that individual ablation of TSLP, IL-25, or IL-33/ST2 had no impact on the progression of Schistosoma mansoni induced type 2 inflammation or fibrosis, whereas simultaneous disruption of all three mediators resulted in significant reductions in granuloma-associated eosinophils, fibrosis, and IL-13–producing ILC2 (Vannella et al., 2016). These studies seemed to imply that the roles of alarmin cytokines in the initiation of type 2 immunity and pathology induced by schistosome infection were redundant, and single alarmin cytokine might have limited impacts on the disease progression of schistosomiasis.
Several parasites can effectively infiltrate and infect the human brain, such as Plasmodium falciparum (P. falciparum), and Toxoplasma gondii (T. gondii). In the case of cerebral malaria, while the sequestration of parasite-infected red blood cells has been associated with its pathology, it remains uncertain whether this is the direct or sole cause of the clinical syndrome. The development of cerebral malaria likely involves a multifaceted process, encompassing sequestration, inflammation, and endothelial dysfunction within the brain’s microvasculature (Luzolo and Ngoyi, 2019). Owing to their distinct metabolic and immunological characteristics, neurons are frequently susceptible to attack by T. gondii. The parasite multiplies inside neurons, leading to neuronal damage through the release of cytokines and chemokines, ultimately resulting in further neurological impairment and disruption of brain metabolism (Elsheikha et al., 2020). In humans and mice, IL-33 is constitutively expressed at high levels within the brain, particularly in stromal cells such as astrocytes and oligodendrocytes (Gadani et al., 2015; Abd Rachman Isnadi et al., 2018). This alarmin is reported to play a crucial role in cerebral malaria and toxoplasmosis. Children with cerebral malaria have elevated plasma and cerebrospinal fluid sST2 levels, which well correlate with serum markers of endothelial activation and neuronal damage and predict neurocognitive impairment (Fernander et al., 2022). In experimental cerebral malaria, infection triggered a dramatic increase of IL-33 expression in oligodendrocytes via ST2 pathway, and ST2-deficient mice were resistant to parasite induced neuropathology associated with attenuated neuroinflammation and exacerbated neurogenesis (Palomo et al., 2015; Reverchon et al., 2017). Surprisingly, administration of exogenous IL-33 prevented the development of cerebral malaria by orchestrating a protective immune response through ILC2, M2 macrophages and Tregs (Besnard et al., 2015). In experimental cerebral toxoplasmosis, IL-33 is expressed by oligodendrocytes and astrocytes during infection and is required for control of parasite burden. Specifically, astrocytes are capable of directly responding to IL-33, thus engaging the peripheral immune system to initiate a Th1 response in controlling the parasites (Jones et al., 2010; Still et al., 2020). Collectively, IL-33 may initiate a protective or detrimental role in cerebral parasite infection dependent on parasite species and IL-33 source. Nevertheless, it is worth pointing out that although IL-25 and TSLP are involved in type 2 immunity and diseases in the brain (Mamuladze and Kipnis, 2023), their functions in cerebral parasitic infections have not been characterized.
The roles of alarmin cytokines in parasitic infections are complex. On the one hand, alarmin cytokines are crucial initiator of Th2 immune response primarily through activating ILC2. This mechanism is important for the expulsion for the adult parasites in intestines and the killing for migrating larvae in lungs. However, strong and long-lasting Th2 immunity induced by alarmin cytokines is also contributes to the development of host tissue injury and fibrosis in lung, liver and brain. In these circumstances, alarmin cytokines are mainly derived from damaged or stimulated epithelial and stromal cells. However, it is still unknown how these cells sense the invasion of parasites and subsequently release the alarmin cytokines. On the other hand, alarmin cytokines especially IL-33 may elicit an immuno-suppressive milieu through inducing the Treg activation or T cell aging. This function promotes host tolerance to parasites but also may inhibit host liver pathology. In this case, IL-33 is released from immune cells such as DCs through an unconventional way (Figure 2). Collectively, recent studies have greatly extended our knowledge about the critical and diverse role of alarmin cytokines in parasite infections. These advances may help us to develop novel strategies to prevent or treat parasitic diseases and other diseases. Further studies will be also required to verify the animal model findings in human patients.
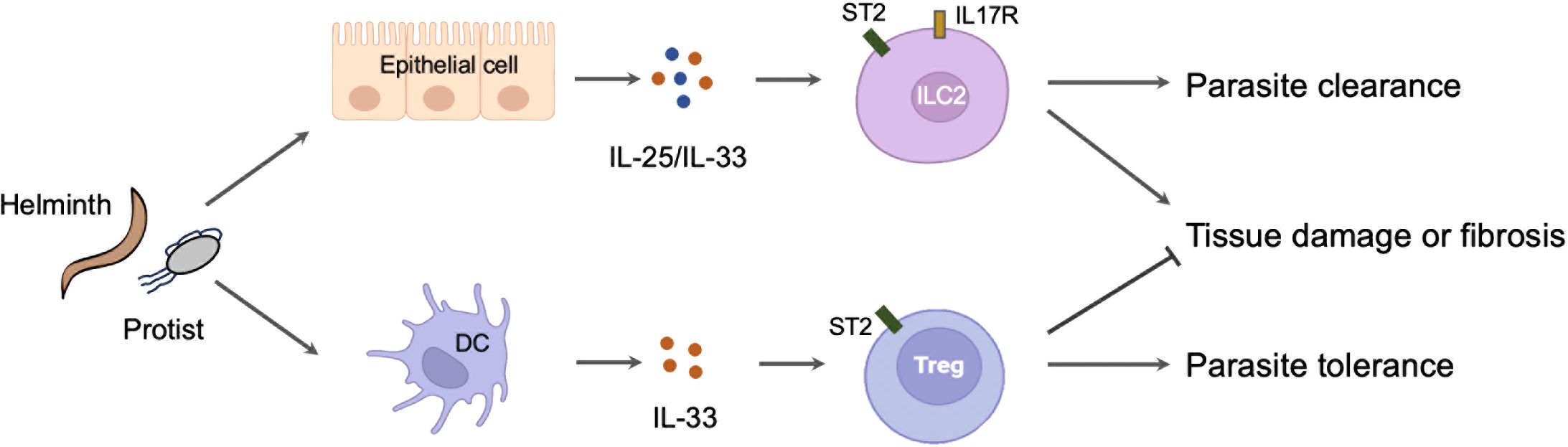
Figure 2. The diverse role of alarmin cytokines in parasitic infections. The invasion of parasites leads to the release of alarmin cytokines from the damaged or stimulated epithelial cells. These cytokines activate ILC2 to mount a protective immunity but also cause host tissue injury or fibrosis. By contrast, the invasion of parasites may simultaneously stimulate the immune cells such as DC to release IL-33. The IL-33 derived from DC activates Treg to mount a tolerance immunity but also prevent host pathology.
ZX: Writing – original draft. SL: Writing – review & editing, Writing – original draft. XH: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (No. 81871678, 82173640, and 82322061).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abd Rachman Isnadi, M. F., Chin, V. K., Abd Majid, R., Lee, T. Y., Atmadini Abdullah, M., Bello Omenesa, R., et al. (2018). Critical roles of IL-33/ST2 pathway in neurological disorders. Mediators Inflammation 2018, 5346413. doi: 10.1155/2018/5346413
Angkasekwinai, P., Sodthawon, W., Jeerawattanawart, S., Hansakon, A., Pattanapanyasat, K., Wang, Y. H. (2017). ILC2s activated by IL-25 promote antigen-specific Th2 and Th9 functions that contribute to the control of Trichinella spiralis infection. PloS One 12, e0184684. doi: 10.1371/journal.pone.0184684
Araujo, E. S., de Jesus Pereira, C. A., de Moura Pereira, A. T., Moreira, J. M., de Rezende, M. C., Rodrigues, J. L., et al. (2016). The role of IL-33/ST2, IL-4, and eosinophils on the airway hyperresponsiveness induced by Strongyloides venezuelensis in BALB/c mice. Parasitol. Res. 115, 3107–3117. doi: 10.1007/s00436-016-5066-6
Autier, B., Manuel, C., Lundstroem-Stadelmann, B., Girard, J. P., Gottstein, B., Gangneux, J. P., et al. (2023). Endogenous IL-33 accelerates metacestode growth during late-stage alveolar echinococcosis. Microbiol. Spectr. 11, e0423922. doi: 10.1128/spectrum.04239-22
Bai, Y., Guan, F., Zhu, F., Jiang, C., Xu, X., Zheng, F., et al. (2021). IL-33/ST2 axis deficiency exacerbates hepatic pathology by regulating Treg and Th17 cells in murine schistosomiasis japonica. J. Inflammation Res. 14, 5981–5998. doi: 10.2147/JIR.S336404
Bąska, P., Norbury, L. J. (2022). The role of the intestinal epithelium in the “weep and sweep” response during gastro-intestinal helminth infections. Anim. (Basel) 12, 175. doi: 10.3390/ani12020175
Besnard, A. G., Guabiraba, R., Niedbala, W., Palomo, J., Reverchon, F., Shaw, T. N., et al. (2015). IL-33-mediated protection against experimental cerebral malaria is linked to induction of type 2 innate lymphoid cells, M2 macrophages and regulatory T cells. PloS Pathog. 11, e1004607. doi: 10.1371/journal.ppat.1004607
Bouchery, T., Kyle, R., Camberis, M., Shepherd, A., Filbey, K., Smith, A., et al. (2015). ILC2s and T cells cooperate to ensure maintenance of M2 macrophages for lung immunity against hookworms. Nat. Commun. 6, 6970. doi: 10.1038/ncomms7970
Chauché, C., Vacca, F., Chia, S. L., Richards, J., Gregory, W. F., Ogunkanbi, A., et al. (2020). A truncated form of HpARI stabilizes IL-33, amplifying responses to the cytokine. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01363
Chen, Z., Luo, J., Li, J., Kim, G., Stewart, A., Urban, J. F., Jr, et al. (2021). Interleukin-33 promotes serotonin release from enterochromaffin cells for intestinal homeostasis. Immunity 54, 151–163.e6. doi: 10.1016/j.immuni.2020.10.014
Coakley, G., McCaskill, J. L., Borger, J. G., Simbari, F., Robertson, E., Millar, M., et al. (2017). Extracellular vesicles from a helminth parasite suppress macrophage activation and constitute an effective vaccine for protective immunity. Cell Rep. 19, 1545–1557. doi: 10.1016/j.celrep.2017.05.001
Corren, J., Ziegler, S. F. (2019). TSLP: from allergy to cancer. Nat. Immunol. 20, 1603–1609. doi: 10.1038/s41590-019-0524-9
Divekar, R., Kita, H. (2015). Recent advances in epithelium-derived cytokines (IL-33, IL-25, and thymic stromal lymphopoietin) and allergic inflammation. Curr. Opin. Allergy Clin. Immunol. 15, 98–103. doi: 10.1097/ACI.0000000000000133
Dwyer, G. K., D’Cruz, L. M., Turnquist, H. R. (2022). Emerging functions of IL-33 in homeostasis and immunity. Annu. Rev. Immunol. 40, 15–43. doi: 10.1146/annurev-immunol-101320-124243
Ebina-Shibuya, R., Leonard, W. J. (2023). Role of thymic stromal lymphopoietin in allergy and beyond. Nat. Rev. Immunol. 23, 24–37. doi: 10.1038/s41577-022-00735-y
El-Naccache, D. W., Haskó, G., Gause, W. C. (2021). Early events triggering the initiation of a type 2 immune response. Trends Immunol. 42, 151–164. doi: 10.1016/j.it.2020.11.006
Elsheikha, H. M., Marra, C. M., Zhu, X. Q. (2020). Epidemiology, pathophysiology, diagnosis, and management of cerebral toxoplasmosis. Clin. Microbiol. Rev. 34, e00115–e00119. doi: 10.1128/CMR.00115-19
Engwerda, C. R., Ng, S. S., Bunn, P. T. (2014). The regulation of CD4(+) T Cell responses during protozoan infections. Front. Immunol. 5. doi: 10.3389/fimmu.2014.00498
Fernander, E. M., Adogamhe, P., Datta, D., Bond, C., Zhao, Y., Bangirana, P., et al. (2022). Elevated plasma soluble ST2 levels are associated with neuronal injury and neurocognitive impairment in children with cerebral malaria. Pathog. Immun. 7, 60–80. doi: 10.20411/pai.v7i1.499
Filbey, K. J., Camberis, M., Chandler, J., Turner, R., Kettle, A. J., Eichenberger, R. M., et al. (2019). Intestinal helminth infection promotes Il-5- and Cd4(+) T cell-dependent immunity in the lung against migrating parasites. Mucosal Immunol. 12, 352–362. doi: 10.1038/s41385-018-0102-8
Gadani, S. P., Walsh, J. T., Smirnov, I., Zheng, J., Kipnis, J. (2015). The glia-derived alarmin IL-33 orchestrates the immune response and promotes recovery following CNS injury. Neuron 85, 703–709. doi: 10.1016/j.neuron.2015.01.013
Gauvreau, G. M., Bergeron, C., Boulet, L. P., Cockcroft, D. W., Côté, A., Davis, B. E., et al. (2023). Sounding the alarmins-The role of alarmin cytokines in asthma. Allergy 78, 402–417. doi: 10.1111/all.15609
Giacomin, P. R., Siracusa, M. C., Walsh, K. P., Grencis, R. K., Kubo, M., Comeau, M. R., et al. (2012). Thymic stromal lymphopoietin-dependent basophils promote Th2 cytokine responses following intestinal helminth infection. J. Immunol. 189, 4371–4378. doi: 10.4049/jimmunol.1200691
Hams, E., Armstrong, M. E., Barlow, J. L., Saunders, S. P., Schwartz, C., Cooke, G., et al. (2014). IL-25 and type 2 innate lymphoid cells induce pulmonary fibrosis. Proc. Natl. Acad. Sci. U.S.A. 111, 367–372. doi: 10.1073/pnas.1315854111
Harada, M., Hirota, T., Jodo, A. I., Hitomi, Y., Sakashita, M., Tsunoda, T., et al. (2011). Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am. J. Respir. Cell Mol. Biol. 44, 787–793. doi: 10.1165/rcmb.2009-0418OC
He, X., Pan, W. (2018). Role of alarmin cytokines and microRNAs in the host-schistosome interaction. F1000Res 7. doi: 10.12688/f1000research.15695.1
He, X., Xie, J., Wang, Y., Fan, X., Su, Q., Sun, Y., et al. (2018). Down-regulation of microRNA-203-3p initiates type 2 pathology during schistosome infection via elevation of interleukin-33. PloS Pathog. 14, e1006957. doi: 10.1371/journal.ppat.1006957
Henry, E. K., Inclan-Rico, J. M., Siracusa, M. C. (2017). Type 2 cytokine responses: regulating immunity to helminth parasites and allergic inflammation. Curr. Pharmacol. Rep. 3, 346–359. doi: 10.1007/s40495-017-0114-1
Huang, Y., Guo, L., Qiu, J., Chen, X., Hu-Li, J., Siebenlist, U., et al. (2015). IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential ‘inflammatory’ type 2 innate lymphoid cells. Nat. Immunol. 16, 161–169. doi: 10.1038/ni.3078
Hung, L. Y., Lewkowich, I. P., Dawson, L. A., Downey, J., Yang, Y., Smith, D. E., et al. (2013). IL-33 drives biphasic IL-13 production for noncanonical Type 2 immunity against hookworms. Proc. Natl. Acad. Sci. U.S.A. 110, 282–287. doi: 10.1073/pnas.1206587110
Hung, L. Y., Tanaka, Y., Herbine, K., Pastore, C., Singh, B., Ferguson, A., et al. (2020). Cellular context of IL-33 expression dictates impact on anti-helminth immunity. Sci. Immunol. 5, eabc6259. doi: 10.1126/sciimmunol.abc6259
Jones, L. A., Roberts, F., Nickdel, M. B., Brombacher, F., McKenzie, A. N., Henriquez, F. L., et al. (2010). IL-33 receptor (T1/ST2) signalling is necessary to prevent the development of encephalitis in mice infected with Toxoplasma gondii. Eur. J. Immunol. 40, 426–436. doi: 10.1002/eji.200939705
Kotsiou, O. S., Gourgoulianis, K. I., Zarogiannis, S. G. (2018). IL-33/ST2 axis in organ fibrosis. Front. Immunol. 9. doi: 10.3389/fimmu.2018.02432
Kurowska-Stolarska, M., Stolarski, B., Kewin, P., Murphy, G., Corrigan, C. J., Ying, S., et al. (2009). IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J. Immunol. 183, 6469–6477. doi: 10.4049/jimmunol.0901575
Lenz, B., Ehrens, A., Ajendra, J., Risch, F., Gal, J., Neumann, A. L., et al. (2024). Repeated sensitization of mice with microfilariae of Litomosoides sigmodontis induces pulmonary eosinophilia in an IL-33-dependent manner. PloS Pathog. 20, e1012071. doi: 10.1371/journal.ppat.1012071
Liew, F. Y., Girard, J. P., Turnquist, H. R. (2016). Interleukin-33 in health and disease. Nat. Rev. Immunol. 16, 676–689. doi: 10.1038/nri.2016.95
Luo, X. C., Chen, Z. H., Xue, J. B., Zhao, D. X., Lu, C., Li, Y. H., et al. (2019). Infection by the parasitic helminth Trichinella spiralis activates a Tas2r-mediated signaling pathway in intestinal tuft cells. Proc. Natl. Acad. Sci. U.S.A. 116, 5564–5569. doi: 10.1073/pnas.1812901116
Luzolo, A. L., Ngoyi, D. M. (2019). Cerebral malaria. Brain Res. Bull. 145, 53–58. doi: 10.1016/j.brainresbull.2019.01.010
Maggi, L., Camelo, G. M. A., Rocha, I. C., Pereira Alves, W., Moreira, J. M. P., Almeida Pereira, T., et al. (2023). Role of the IL-33/ST2 activation pathway in the development of the hepatic fibrosis induced by Schistosoma mansoni granulomas in mice. Int. J. Mol. Sci. 24, 10237. doi: 10.3390/ijms241210237
Maggi, L., Rocha, I. C., Camelo, G. M. A., Fernandes, V. R., Negrão-Corrêa, D. (2021). The IL-33/ST2 pathway is not essential to Th2 stimulation but is key for modulation and survival during chronic infection with Schistosoma mansoni in mice. Cytokine 138, 155390. doi: 10.1016/j.cyto.2020.155390
Maizels, R. M. (2020). Regulation of immunity and allergy by helminth parasites. Allergy 75, 524–534. doi: 10.1111/all.13944
Maizels, R. M., Hewitson, J. P., Smith, K. A. (2012). Susceptibility and immunity to helminth parasites. Curr. Opin. Immunol. 24, 459–466. doi: 10.1016/j.coi.2012.06.003
Mamuladze, T., Kipnis, J. (2023). Type 2 immunity in the brain and brain borders. Cell Mol. Immunol. 20, 1290–1299. doi: 10.1038/s41423-023-01043-8
Martin, R. K., Damle, S. R., Valentine, Y. A., Zellner, M. P., James, B. N., Lownik, J. C., et al. (2018). B1 cell IgE impedes mast cell-mediated enhancement of parasite expulsion through B2 IgE blockade. Cell Rep. 22, 1824–1834. doi: 10.1016/j.celrep.2018.01.048
Massacand, J. C., Stettler, R. C., Meier, R., Humphreys, N. E., Grencis, R. K., Marsland, B. J., et al. (2009). Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc. Natl. Acad. Sci. U.S.A. 106, 13968–13973. doi: 10.1073/pnas.0906367106
Mathä, L., Takei, F., Martinez-Gonzalez, I. (2022). Tissue resident and migratory group 2 innate lymphoid cells. Front. Immunol. 13. doi: 10.3389/fimmu.2022.877005
McHedlidze, T., Waldner, M., Zopf, S., Walker, J., Rankin, A. L., Schuchmann, M., et al. (2013). Interleukin-33-dependent innate lymphoid cells mediate hepatic fibrosis. Immunity 39, 357–371. doi: 10.1016/j.immuni.2013.07.018
McSorley, H. J., O’Gorman, M. T., Blair, N., Sutherland, T. E., Filbey, K. J., Maizels, R. M. (2012). Suppression of type 2 immunity and allergic airway inflammation by secreted products of the helminth Heligmosomoides polygyrus. Eur. J. Immunol. 42, 2667–2682. doi: 10.1002/eji.201142161
McSorley, H. J., Smyth, D. J. (2021). IL-33: A central cytokine in helminth infections. Semin. Immunol. 53, 101532. doi: 10.1016/j.smim.2021.101532
Meiners, J., Reitz, M., Rüdiger, N., Turner, J. E., Heepmann, L., Rudolf, L., et al. (2020). IL-33 facilitates rapid expulsion of the parasitic nematode Strongyloides ratti from the intestine via ILC2- and IL-9-driven mast cell activation. PloS Pathog. 16, e1009121. doi: 10.1371/journal.ppat.1009121
Miller, M. M., Reinhardt, R. L. (2020). The heterogeneity, origins, and impact of migratory iILC2 cells in anti-helminth immunity. Front. Immunol. 11. doi: 10.3389/fimmu.2020.01594
Miyake, Y., Hitsumoto, S., Tanaka, K., Arakawa, M. (2015). Association between TSLP polymorphisms and eczema in Japanese women: the Kyushu Okinawa maternal and child health study. Inflammation 38, 1663–1668. doi: 10.1007/s10753-015-0143-z
Monteleone, G., Pallone, F., Macdonald, T. T. (2010). Interleukin-25: a two-edged sword in the control of immune-inflammatory responses. Cytokine Growth Fact. Rev. 21, 471–475. doi: 10.1016/j.cytogfr.2010.05.001
Mukendi, J. P. K., Nakamura, R., Uematsu, S., Hamano, S. (2021). Interleukin (IL)-33 is dispensable for Schistosoma mansoni worm maturation and the maintenance of egg-induced pathology in intestines of infected mice. Parasit Vectors 14, 70. doi: 10.1186/s13071-020-04561-w
Muñoz-Antoli, C., Cortés, A., Santano, R., Sotillo, J., Esteban, J. G., Toledo, R. (2016). Interleukin-25 induces resistance against intestinal trematodes. Sci. Rep. 6, 34142. doi: 10.1038/srep34142
Muñoz-Carrillo, J. L., Contreras-Cordero, J. F., Muñoz-López, J. L., Maldonado-Tapia, C. H., Muñoz-Escobedo, J. J., Moreno-García, M. A. (2017). Resiniferatoxin modulates the Th1 immune response and protects the host during intestinal nematode infection. Parasite Immunol. 39, 115. doi: 10.1111/pim.12448
Muñoz-Carrillo, J. L., Gutiérrez-Coronado, O., Muñoz-Escobedo, J. J., Contreras-Cordero, J. F., Maldonado-Tapia, C., Moreno-García, M. A. (2021). Resiniferatoxin promotes adult worm expulsion in Trichinella spiralis-infected rats by Th2 immune response modulation. Parasite Immunol. 43, e12840. doi: 10.1111/pim.12840
Neill, D. R., Wong, S. H., Bellosi, A., Flynn, R. J., Daly, M., Langford, T. K., et al. (2010). Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367–1370. doi: 10.1038/nature08900
Nie, Y., Yang, D., Oppenheim, J. J. (2016). Alarmins and antitumor immunity. Clin. Ther. 38, 1042–1053. doi: 10.1016/j.clinthera.2016.03.021
Noor, Z., Watanabe, K., Abhyankar, M. M., Burgess, S. L., Buonomo, E. L., Cowardin, C. A., et al. (2017). Role of eosinophils and tumor necrosis factor alpha in interleukin-25-mediated protection from amebic colitis. mBio 8, e02329–e02316. doi: 10.1128/mBio.02329-16
Osbourn, M., Soares, D. C., Vacca, F., Cohen, E. S., Scott, I. C., Gregory, W. F., et al. (2017). HpARI protein secreted by a helminth parasite suppresses interleukin-33. Immunity 47, 739–751. doi: 10.1016/j.immuni.2017.09.015
Owyang, A. M., Zaph, C., Wilson, E. H., Guild, K. J., McClanahan, T., Miller, H. R., et al. (2006). Interleukin 25 regulates type 2 cytokine-dependent immunity and limits chronic inflammation in the gastrointestinal tract. J. Exp. Med. 203, 843–849. doi: 10.1084/jem.20051496
Palomo, J., Reverchon, F., Piotet, J., Besnard, A. G., Couturier-Maillard, A., Maillet, I., et al. (2015). Critical role of IL-33 receptor ST2 in experimental cerebral malaria development. Eur. J. Immunol. 45, 1354–1365. doi: 10.1002/eji.201445206
Patel, N. N., Kohanski, M. A., Maina, I. W., Workman, A. D., Herbert, D. R., Cohen, N. A. (2019). Sentinels at the wall: epithelial-derived cytokines serve as triggers of upper airway type 2 inflammation. Int. Forum Allergy Rhinol. 9, 93–99. doi: 10.1002/alr.22206
Peng, H., Zhang, Q., Li, X., Liu, Z., Shen, J., Sun, R., et al. (2016). IL-33 contributes to Schistosoma japonicum-induced hepatic pathology through induction of M2 macrophages. Sci. Rep. 6, 29844. doi: 10.1038/srep29844
Price, A. E., Liang, H. E., Sullivan, B. M., Reinhardt, R. L., Eisley, C. J., Erle, D. J., et al. (2010). Systemically dispersed innate IL-13-expressing cells in type 2 immunity. Proc. Natl. Acad. Sci. U.S.A. 107, 11489–11494. doi: 10.1073/pnas.1003988107
Reichwald, J. J., Risch, F., Neumann, A. L., Frohberger, S. J., Scheunemann, J. F., Lenz, B., et al. (2022). ILC2s control microfilaremia during Litomosoides sigmodontis infection in Rag2-/- Mice. Front. Immunol. 13. doi: 10.3389/fimmu.2022.863663
Reverchon, F., Mortaud, S., Sivoyon, M., Maillet, I., Laugeray, A., Palomo, J., et al. (2017). IL-33 receptor ST2 regulates the cognitive impairments associated with experimental cerebral malaria. PloS Pathog. 13, e1006322. doi: 10.1371/journal.ppat.1006322
Rostan, O., Arshad, M. I., Piquet-Pellorce, C., Robert-Gangneux, F., Gangneux, J. P., Samson, M. (2015). Crucial and diverse role of the interleukin-33/ST2 axis in infectious diseases. Infect. Immun. 83, 1738–1748. doi: 10.1128/IAI.02908-14
Rostan, O., Gangneux, J. P., Piquet-Pellorce, C., Manuel, C., McKenzie, A. N., Guiguen, C., et al. (2013). The IL-33/ST2 axis is associated with human visceral leishmaniasis and suppresses Th1 responses in the livers of BALB/c mice infected with Leishmania donovani. mBio 4, e00383–e00313. doi: 10.1128/mBio.00383-13
Rothenberg, M. E., Spergel, J. M., Sherrill, J. D., Annaiah, K., Martin, L. J., Cianferoni, A., et al. (2010). Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat. Genet. 42, 289–291. doi: 10.1038/ng.547
Ryan, N., Anderson, K., Volpedo, G., Varikuti, S., Satoskar, M., Satoskar, S., et al. (2020). The IL-33/ST2 axis in immune responses against parasitic disease: potential therapeutic applications. Front. Cell Infect. Microbiol. 10. doi: 10.3389/fcimb.2020.00153
Sakashita, M., Yoshimoto, T., Hirota, T., Harada, M., Okubo, K., Osawa, Y., et al. (2008). Association of serum interleukin-33 level and the interleukin-33 genetic variant with Japanese cedar pollinosis. Clin. Exp. Allergy 38, 1875–1881. doi: 10.1111/j.1365-2222.2008.03114.x
Schneider, C., O’Leary, C. E., von Moltke, J., Liang, H. E., Ang, Q. Y., Turnbaugh, P. J., et al. (2018). A metabolite-triggered tuft cell-ILC2 circuit drives small intestinal remodeling. Cell 174, 271–284.e14. doi: 10.1016/j.cell.2018.05.014
Shimokawa, C., Kanaya, T., Hachisuka, M., Ishiwata, K., Hisaeda, H., Kurashima, Y., et al. (2017). Mast cells are crucial for induction of group 2 innate lymphoid cells and clearance of helminth infections. Immunity 46, 863–874. doi: 10.1016/j.immuni.2017.04.017
Stanbery, A. G., Smita, S., von Moltke, J., Tait, W. E. D., Ziegler S, F. (2022). TSLP, IL-33, and IL-25: Not just for allergy and helminth infection. J. Allergy Clin. Immunol. 150, 1302–1313. doi: 10.1016/j.jaci.2022.07.003
Still, K. M., Batista, S. J., O’Brien, C. A., Oyesola, O. O., Früh, S. P., Webb, L. M., et al. (2020). Astrocytes promote a protective immune response to brain Toxoplasma gondii infection via IL-33-ST2 signaling. PloS Pathog. 16, e1009027. doi: 10.1371/journal.ppat.1009027
Tu, L., Yang, L. (2019). IL-33 at the crossroads of metabolic disorders and immunity. Front. Endocrinol. (Lausanne) 10. doi: 10.3389/fendo.2019.00026
Uddin, M. J., Leslie, J. L., Burgess, S. L., Oakland, N., Thompson, B., Abhyankar, M., et al. (2022). The IL-33-ILC2 pathway protects from amebic colitis. Mucosal Immunol. 15, 165–175. doi: 10.1038/s41385-021-00442-2
Vacca, F., Chauché, C., Jamwal, A., Hinchy, E. C., Heieis, G., Webster, H., et al. (2020). A helminth-derived suppressor of ST2 blocks allergic responses. Elife 9, e54017. doi: 10.7554/eLife.54017
Vannella, K. M., Ramalingam, T. R., Borthwick, L. A., Barron, L., Hart, K. M., Thompson, R. W., et al. (2016). Combinatorial targeting of TSLP, IL-25, and IL-33 in type 2 cytokine-driven inflammation and fibrosis. Sci. Transl. Med. 8, 337ra65. doi: 10.1126/scitranslmed.aaf1938
Varyani, F., Löser, S., Filbey, K. J., Harcus, Y., Drurey, C., Poveda, M. C., et al. (2022). The IL-25-dependent tuft cell circuit driven by intestinal helminths requires macrophage migration inhibitory factor (MIF). Mucosal Immunol. 15, 1243–1256. doi: 10.1038/s41385-022-00496-w
Wu, J., Zhang, F., Tao, H., Nawaz, W., Chen, D., Wu, Z. (2022). The potential roles of interleukin-25 in infectious diseases. Front. Immunol. 13. doi: 10.3389/fimmu.2022.986118
Xu, L., Wei, C., Chen, Y., Wu, Y., Shou, X., Chen, W., et al. (2022). IL-33 induces thymic involution-associated naive T cell aging and impairs host control of severe infection. Nat. Commun. 13, 6881. doi: 10.1038/s41467-022-34660-4
Yan, C., Xu, N., Liu, M., Jiang, Z., Wu, J., Koda, S., et al. (2022). Interleukin-33 deficiency prevents biliary injuries and repairments caused by Clonorchis Sinensis via restraining type 2 cytokines. Parasit Vectors 15, 386. doi: 10.1186/s13071-022-05490-6
Yasuda, K., Muto, T., Kawagoe, T., Matsumoto, M., Sasaki, Y., Matsushita, K., et al. (2012). Contribution of IL-33-activated type II innate lymphoid cells to pulmonary eosinophilia in intestinal nematode-infected mice. Proc. Natl. Acad. Sci. U.S.A. 109, 3451–3456. doi: 10.1073/pnas.1201042109
Yu, Y., Deng, W., Lei, J. (2015). Interleukin-33 promotes Th2 immune responses in infected mice with Schistosoma japonicum. Parasitol. Res. 114, 2911–2918. doi: 10.1007/s00436-015-4492-1
Zaiss, M. M., Maslowski, K. M., Mosconi, I., Guenat, N., Marsland, B. J., Harris, N. L. (2013). IL-1β suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PloS Pathog. 9, e1003531. doi: 10.1371/journal.ppat.1003531
Zaiss, D. M. W., Pearce, E. J., Artis, D., McKenzie, A. N. J., Klose, C. S. N. (2024). Cooperation of ILC2s and TH2 cells in the expulsion of intestinal helminth parasites. Nat. Rev. Immunol. 24, 294–302. doi: 10.1038/s41577-023-00942-1
Keywords: IL-25, IL-33, TSLP, type 2 immunity, parasitic infection
Citation: Xing Z, Liu S and He X (2024) Critical and diverse role of alarmin cytokines in parasitic infections. Front. Cell. Infect. Microbiol. 14:1418500. doi: 10.3389/fcimb.2024.1418500
Received: 16 April 2024; Accepted: 17 October 2024;
Published: 04 November 2024.
Edited by:
Debanjan Mukhopadhyay, Presidency University, IndiaReviewed by:
José Luis Muñoz-Carrillo, University of Guadalajara, MexicoCopyright © 2024 Xing, Liu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing He, eGluZy5oZUBhbGl5dW4uY29t; Suiyi Liu, bGl1c3VpeWk4MUAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.