- 1Department of Respiratory and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chao-Yang Hospital, Capital Medical University, Beijing, China
- 2Department of Respiratory, Beijing Huairou Hospital, Beijing, China
- 3Department of Pulmonary and Critical Care Medicine, Center of Respiratory Medicine, China-Japan Friendship Hospital, National Center for Respiratory Medicine, National Clinical Research Center for Respiratory Diseases, Beijing, China
Background: Parvimonas micra (P. micra) has been identified as a pathogen capable of causing lung abscesses; however, its identification poses challenges due to the specialized culture conditions for anaerobic bacterial isolation. Only a few cases of lung abscesses caused by P. micra infection have been reported. Therefore, we describe the clinical characteristics of lung abscesses due to P. micra based on our case series.
Methods: A retrospective analysis was conducted on eight patients who were diagnosed with lung abscesses attributed to P. micra. Detection of P. micra was accomplished through target next-generation sequencing (tNGS). A systematic search of the PubMed database using keywords “lung abscess” and “Parvimonas micra/Peptostreptococcus micros” was performed to review published literature pertaining to similar cases.
Results: Among the eight patients reviewed, all exhibited poor oral hygiene, with four presenting with comorbid diabetes. Chest computed tomography (CT) showed high-density mass shadows with necrosis and small cavities in the middle. Bronchoscopic examination revealed purulent sputum and bronchial mucosal inflammation. Thick secretions obstructed the airway, leading to the poor drainage of pus, and the formation of local abscesses leading to irresponsive to antibiotic therapy, which finally protracted recovery time. P. micra was successfully identified in bronchoalveolar lavage fluid (BALF) samples from all eight patients using tNGS; in contrast, sputum and BALF bacterial cultures yielded negative results, with P. micra cultured from only one empyema sample. Following appropriate antibiotic therapy, seven patients recovered. In previously documented cases, favorable outcomes were observed in 77.8% of individuals treated with antibiotics and 22.2% were cured after surgical interventions for P. micra lung abscesses.
Conclusions: This study enriches our understanding of the clinical characteristics associated with lung abscesses attributed to P. micra. Importantly, tNGS has emerged as a rapid and effective diagnostic test in scenarios where traditional sputum cultures are negative. Encouragingly, patients with lung abscesses caused by P. micra infection exhibit a favorable prognosis with effective airway clearance and judicious anti-infective management.
Introduction
Primary lung abscesses typically result from infections leading to necrosis and cavitation of the lung tissue. Aspiration of oral-derived anaerobic bacteria represents the predominant etiology of primary lung abscesses (Bansal et al., 2013). Individuals with periodontal disease are at an elevated risk of developing anaerobic lung abscesses. Since anaerobic organisms necessitate specific culture conditions, conventional sputum culture often yields negative results in the detection of anaerobic bacteria. Anaerobic organisms were isolated only in a limited number of lung abscess cases (Yazbeck et al., 2014).
Parvimonas micra (P. micra) is a gram-positive anaerobic coccus prevalent in the oral cavity, particularly in the gingival crevices. P. micra has been associated with aspiration pneumonia (Yu et al., 2021), lung abscesses (Zhang et al., 2021), and empyema (Vilcarromero et al., 2023). Nevertheless, clinical insights into P. micra-related lung abscesses remain rare.
Given the challenges in culturing and identifying anaerobic bacteria due to their vulnerability to atmospheric exposure, innovative approaches utilizing next-generation sequencing of metagenomes (NGS) have shown promise in enhancing the positive detection rate of anaerobic bacteria (Zhang et al., 2021).
In this study, we present findings from eight cases of lung abscess caused by P. micra, with the pathogenic bacterium identified through genomic sequencing. Additionally, a systematic review of prior cases detailed in the published literature was conducted to delineate the clinical features of lung abbesses attributed to this pathogen, which may be unfamiliar to pulmonary healthcare providers.
Patients and methods
Patients
A retrospective analysis was conducted on patients who were diagnosed with lung abscesses and admitted to Beijing Chao-Yang Hospital between July 2022 and December 2023. The results from bronchoalveolar lavage fluid (BALF) pathogen testing using target next-generation sequencing (tNGS) indicated P. Micra as the predominant bacterium, consistent with the clinical presentation. Two physicians independently confirmed the association of lung abscess with P. micra. Comprehensive patient medical records, laboratory tests, examinations, and treatment modalities were compiled for review.
tNGS testing
Bronchoscopies were performed within 5 days of hospital admission, with BALF samples obtained from the segmental bronchus corresponding to the identified lesions. Immediately following collection, 5 ml of BALF was aseptically preserved at −20°C and transported to Beijing KingMed Diagnostics Laboratory within 4 h. Cellular material within the samples was enriched, lysed, and subjected to DNA extraction from 500 μl of fluid as per established protocols. Subsequent to polymerase chain reaction amplification and purification, sequencing was performed utilizing a genetic sequencing platform (KM MiniSeqDx-CN) and an automatic nucleic acid-protein analyzer (Qsep100, BiOptic, Taiwan). Sequence data interpretation was facilitated by the Pathogenic Microbial Data Analysis and Management System 1.0 (KingMed Diagnostics, Co., Ltd., China), which comprises a bacterial minimal genome database consisting of 198 respiratory pathogens.
Literature review
A systematic search of the PubMed database was conducted to identify journal articles, employing the search terms “lung abscess” and “Parvimonas micra/Peptostreptococcus micros”. The inclusion criteria included papers published from 01/01/1980, to 31/12/2023. Five articles documenting pulmonary abscesses associated with P. micra, with a collective total of nine patients, were contained in the review.
Results
Characteristics
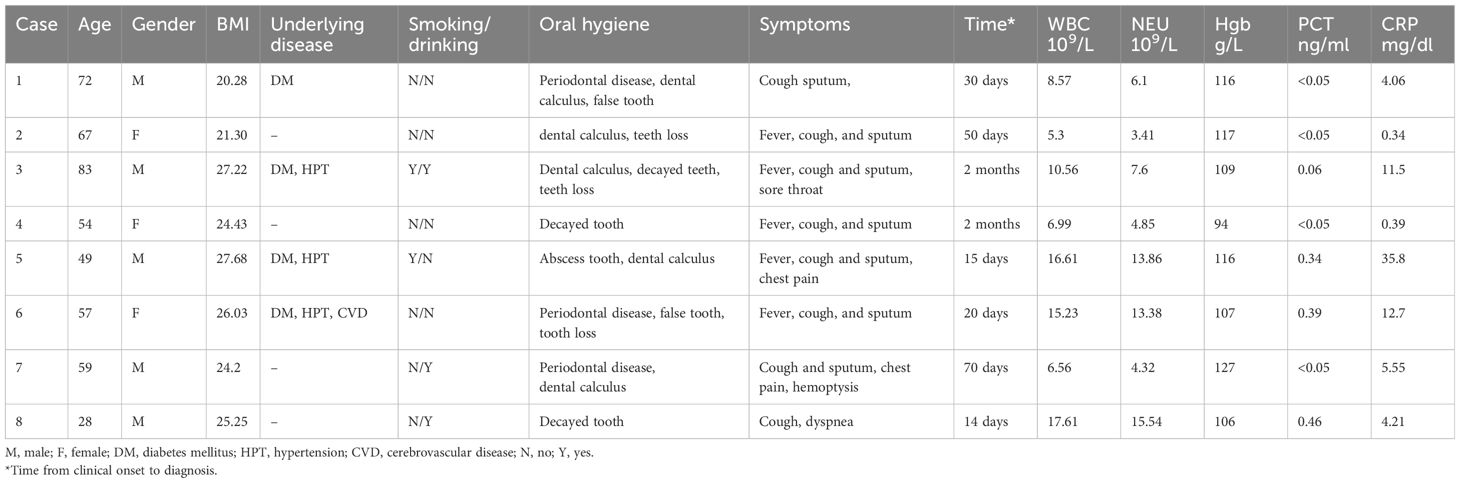
The general characteristics of the eight patients are outlined in Table 1. Their ages ranged from 28 to 83 years, with four patients having diabetes and none presenting HIV infection or receiving immunosuppressive treatment. The median duration from symptom onset to definitive diagnosis was approximately 40 days. Notably, all eight patients exhibited poor oral hygiene, with manifestations including periodontal disease and dental disease.
Primary symptoms were non-specific, yet common manifestations included fever (n = 5), cough (n = 8), and sputum production (n = 7). Additional complaints included sore throat, dyspnea, chest pain, and hemoptysis.
Laboratory tests, chest CT, and bronchoscopy
Upon admission, routine blood tests revealed a median white blood cell count of 9.57 × 109/L, with half of the patients exhibiting normal procalcitonin (PCT) levels.
Chest CT findings demonstrated large-scale consolidation with necrosis and small cavities (Figure 1), devoid of classical air-fluid levels. The boundaries of the focusing area were unclear and blurred. The lesions were located in the right upper lobe (n = 2), left upper lobe (n = 2), right middle lobe (n = 1), and inferior lobe of the right lung (n = 3). One patient was complicated with liver abscesses and empyema.
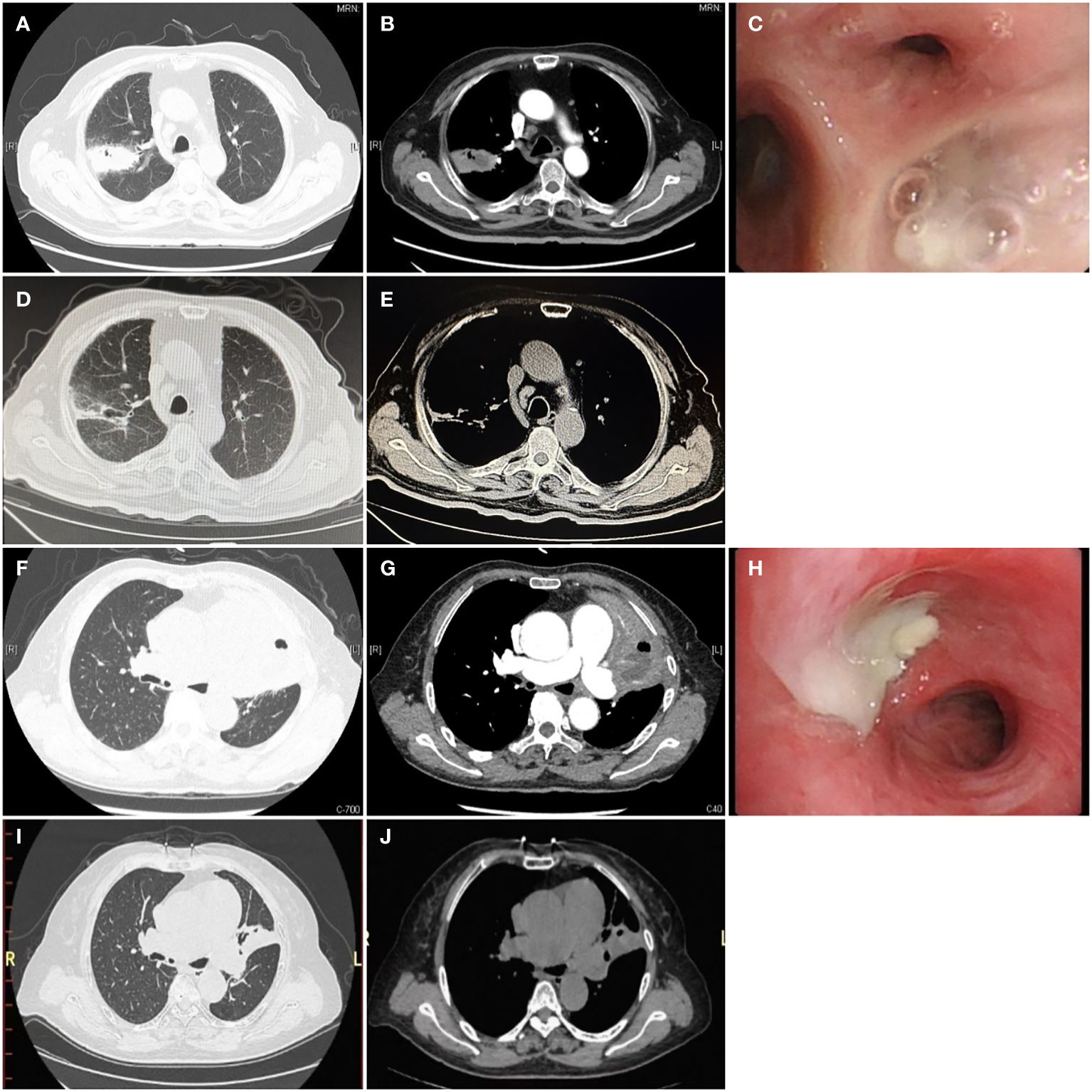
Figure 1 Chest computed tomography and bronchoscopy of P. micra lung abscess Computed tomography is displayed in the lung window (A, D, F, I), soft tissue window (B, E, G, J), and bronchoscopy (C, H). (A, B, F, G) The initial CT scan showed large consolidations with necrosis and small cavities (white →) without a clear air-liquid level, which can be described as a “bubble levitation sign”. (C, H) The bronchial segment was obstructed by purulent secretions (→). (D, E, I, J) After treatment for 3 months, the follow-up chest CT showed significant absorption of the lesions.
Bronchoscopy revealed purulent sputum (Figure 1) and inflammatory changes in the bronchial mucosa at the affected sites.
CT-guided pulmonary biopsy was performed on three patients to differentiate from lung cancer. Pathology indicated inflammation of the lung tissue.
Microbiological examinations
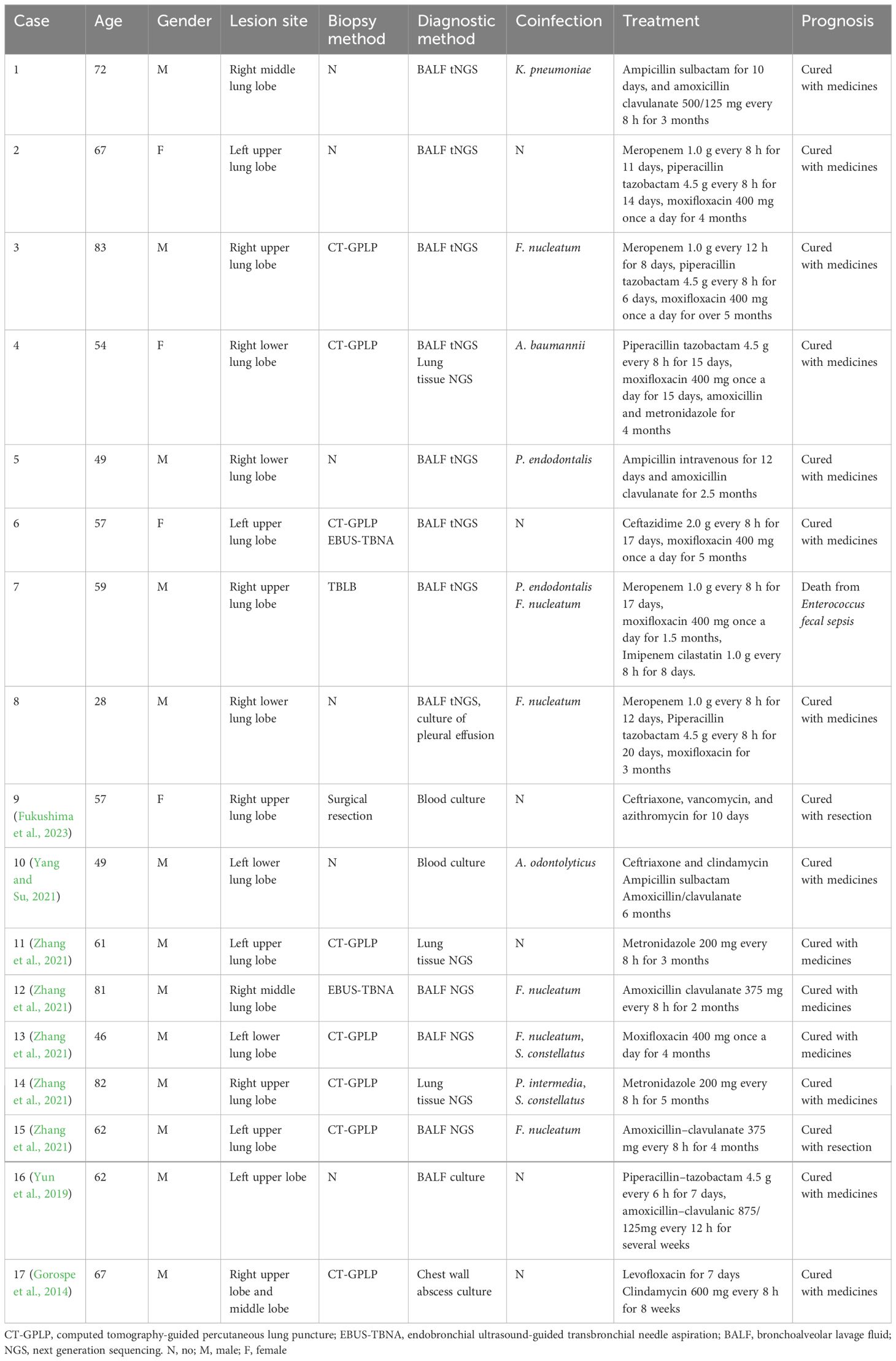
Although gram-positive cocci were identified in five cases via sputum or BALF smears, bacterial cultures yielded negative results for all eight patients. However, tNGS analysis of BALF samples confirmed P. micra as the predominant bacterium. Anaerobic culture of the pleural effusion identified P. micra in one patient complicated with concomitant empyema. Additionally, tNGS reports indicated the presence of other bacteria including Fusbacterium nucleatum (n = 5), Klebsiella pneumoniae (n = 2), Escherichia coli (n = 1), Streptococcus pneumoniae (n = 1), and Acinetobacter baumannii (n = 1).
Treatment and outcomes
Following diagnosis, patients received tailored anti-infection therapies such as piperacillin–tazobactam (n = 3), ampicillin–sulbactam (n = 2), moxifloxacin (n = 1), meropenem (n = 1), and imipenem–cilastatin (n = 1). Mucolytic drugs and airway clearance techniques were also employed to facilitate sputum expectoration.
Their symptoms improved, and chest CT scans indicated resolution of the lung abscesses. Subsequent to initial therapy, oral antibiotics were administered for 2 to 5 months. Seven patients achieved satisfactory outcomes, with almost no symptoms and almost completely absorbed lesions on chest CT. Two months later, one patient developed massive hemoptysis attributed to secondary Enterococcus faecium infection, leading to fatal pneumonia and sepsis.
Literature review
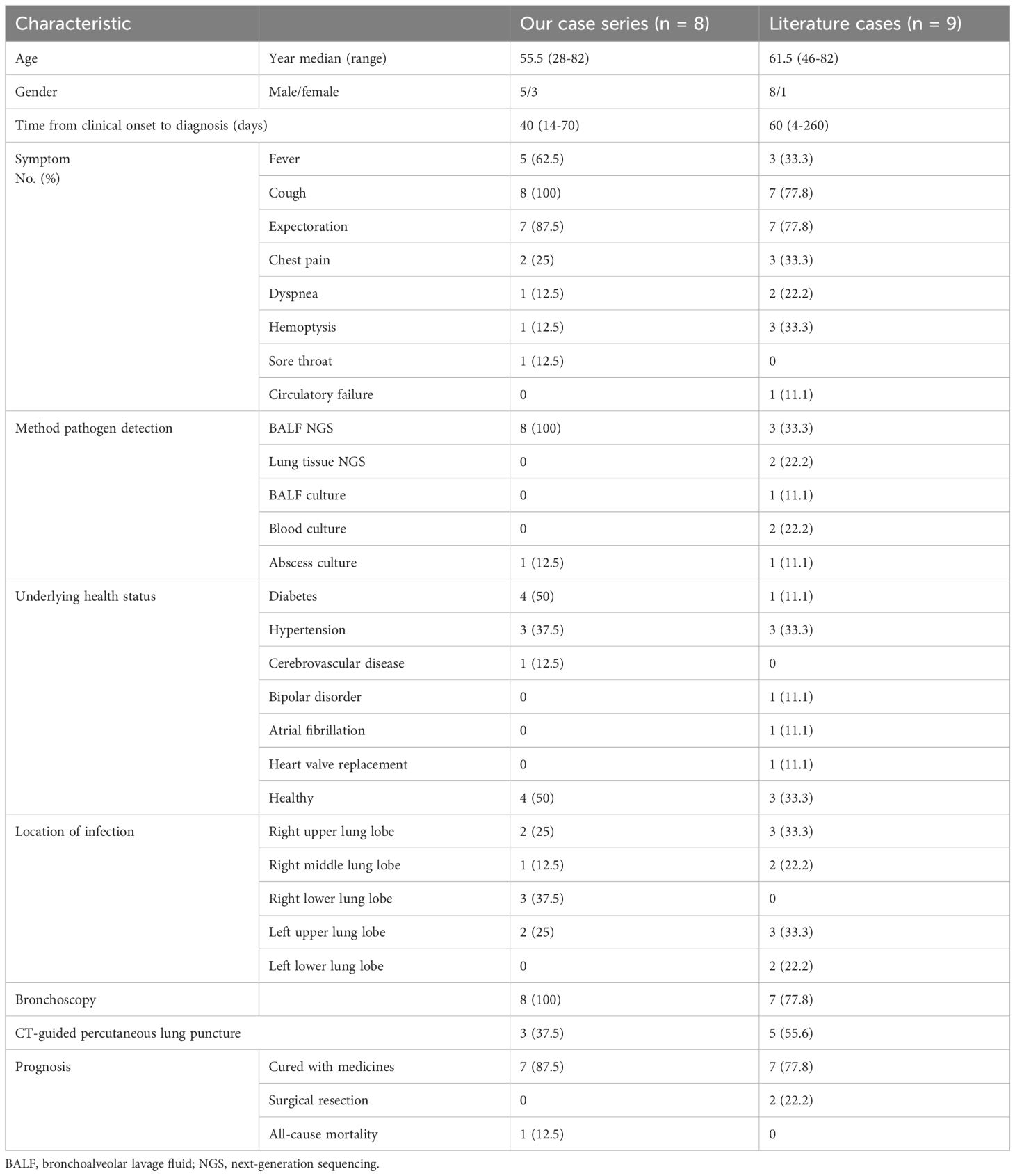
A review of the literature identified other nine reported cases (Table 2), revealing cough (77.8%) and expectoration (77.8%) as the most common symptoms. Additional data indicated smoking history, alcohol consumption, periodontal conditions, and dental issues. The median time taken from clinical onset to make a diagnosis was 2 months. CT scan showed irregular mass shadows which had no obvious improvement after short-term initial empirical treatment. It is difficult to distinguish from malignant lesions. Thus, CT-guided percutaneous lung biopsies were performed in 55.6% of these patients. In published cases, metagenomics next-generation sequencing (mNGS) facilitated pathogen identification in more than half of the cases. The details of coinfections and treatment are shown in Table 3. A majority of P. micra lung abscess cases reported in the literature also displayed favorable outcomes, with 77.8% of patients cured via medication and 22.2% benefitting from surgical intervention.
Discussion
The majority of lung abscesses are believed to be caused by the aspiration of anaerobic bacteria from the oral cavity, particularly from gingival crevices. P. micra is one of the most prevalent anaerobic microorganisms in the human oral cavity (Badri et al., 2019). However, due to challenges in laboratory culture, there are limited published data on the clinical characteristics of P. micra lung abscess, mainly in the last 5 years (Yun et al., 2019; Yang and Su, 2021; Zhang et al., 2021; Fukushima et al., 2023; Zhijun et al., 2023).
Anaerobic lung infections are often associated with poor oral hygiene conditions and inadequate airway protection (Hata et al., 2020). Zhang et al (Zhang et al., 2021). reported that patients with a long smoking history and poor oral hygiene are susceptible to P. micra lung abscesses. However, in our study, smoking was not identified as a significant risk factor, as only two male patients had a long history of smoking, whereas the other patients were non-smokers. Lai et al. reported four cases of childhood pneumonia and abscesses caused by oral obligate anaerobes, primarily P. micra, where poor oral hygiene was a crucial risk factor (Zhijun et al., 2023). Therefore, poor oral health is an important risk factor for P. micra–induced lung abscesses. In addition, alcoholism should also be noted as a common predisposing condition for aspiration (Kuhajda et al., 2015). Among our patients, three male patients had a history of regular alcohol consumption.
The symptoms observed in these patients were non-specific. All patients presented with a productive cough with sputum, and most experienced fever. Radiologically, chest CT scans revealed mass of lung consolidation with liquefactive necrosis and small cavities within the consolidation, without a distinct liquid–gas plane. Given the presence of small cavities with thick walls of consolidations on lung CT scan, along with non-specific symptoms, many patients underwent lung puncture biopsy for the differentiation of lung cancer. The lung biopsy pathology from these lung puncture biopsies revealed chronic inflammation of the lung tissue (Zhang et al., 2021). Gorospe et al. reported a case of a chest wall abscess of P. micra following CT-guided needle lung biopsy of the right lung consolidation (Gorospe et al., 2014).
In our case series, bronchoscopy revealed sticky secretion plugging in the bronchi as a feature of P. micra infection. For patients with lung consolidation and necrosis, bronchoscopy could be performed initially for pathogenic analysis. When empyema secretion plugging in the bronchial region was detected, without neoplasm, P. micra was isolated, and symptoms and radiological findings improved following targeted antibiotic therapy, percutaneous lung biopsy could be avoided.
The mNGS markedly improves pathogenic diagnosis, but it is expensive and involves procedural DNA and RNA sequencing, respectively. Target NGS is a more economical technology with a cost of approximately one-fifth of that of mNGS, with the similar advantages of rapid speed and high accuracy. The target detection of 198 pathogens include 80 bacteria, 79 DNA and RNA viruses, 32 fungi, and 7 mycoplasmas and chlamydia, which account for 95% respiratory infections (Li et al., 2022). Clinical data have shown that tNGS is effective and economical for diagnosis of respiratory infection (Li et al., 2022). However, NGS technology cannot achieve antibiotic sensitivity of the pathogen, and anaerobic culture remains important, particularly for determining antibiotic resistance. Additionally, as NGS often detects multiple bacterial species, clinicians must carefully ascertain the true pathogenic bacteria, by integrating patients’ symptoms, laboratory tests, and radiological findings. Compared with traditional culture, tNGS had a shorter turnaround time for positive pathogen detection (1 day vs. 4 days). Finally, for detected pathogens with high NGS sequencing read numbers, especially in RNA sequencing tests, this approach could be helpful for the differentiation of colonization and infection.
tNGS is only a method for detecting microbes rather than a diagnostic method. However, tNGS is designed to target highly suspected microbes when they are commonly recognized as pathogens that cause lower respiratory tract infections. Furthermore, after its routine application in the clinical diagnostic process in clinical practice for pathogen identification in our hospital, its role as an examination for diagnostic purposes becomes increasingly important. Therefore, we could have a positive view of its promising diagnostic usage in the future. Finally, the results from tNGS could not be held as the only diagnostic evidence. To establish a precise diagnosis for an infection, tNGS should be combined with other clinical information such as medical history, symptoms, laboratory, and radiological findings.
In bronchoscopy, if bronchial blockage by purulent secretions is observed, which leads to local hypoxia and creates conditions for anaerobic bacterial growth, promoting sputum excretion and maintaining airway patency are critical treatment procedures on the base of drug therapy. Chest physiotherapy by respiratory therapists, including the use of devices such as the Acapella valve and high-frequency chest wall oscillation devices, are helpful in clearing airway secretions (Polverino et al., 2017)
P. micra is susceptible to many antibiotics, including penicillin G, ampicillin, cefepime, vancomycin, and metronidazole (Boattini et al., 2018; Fukushima et al., 2023). In the case of lung abscess, intravenous antibiotics were administered for 2 to 4 weeks, followed by oral antibiotic treatment for 2 to 3 months until the chest radiographic lesions resolved. Proper drainage helps with absorption and hastens recovery. Although lung abscesses associated with P. micra have rarely been reported, it has a benign prognosis, as most patients recover after antibiotic treatment.
In patients who do not respond to antibiotic therapy, catheter drainage or surgical resection should still be considered (Herth et al., 2005). Both percutaneous tube drainage (Lee et al., 2022) and endoscopic drainage (Unterman et al., 2017) have been shown to effectively reduce abscess size and improve clinical outcomes. However, catheter placement in P. micra-related lung abscesses with small cavities can be challenging (Herth et al., 2005). Surgical resection may be necessary if antibiotic therapy is ineffective or in cases of life-threatening hemoptysis. In cases reported previously, two patients were cured after surgery.
This study has several limitations. First, this study has a small sample size, which may lead to bias. Furthermore, sputum or BALF cultures were negative for all patients. Finally, tNGS results do not cover all pathogens, which may be inaccurate in finding rare coinfected pathogens. To avoid missing important pathogens, retesting or conversion to mNGS would be considered, if the tNGS results not align with the clinical situation or if patients exhibited a poor response to treatment.
Conclusion
In conclusion, our study systemically reports the characteristics of P. micra–related lung abscesses in adults based on the largest number of cases to date. Imaging features included mass consolidation with necrosis without a clear liquid–gas plane on chest CT, and sticky secretion plugging in the bronchial region on bronchoscopy. tNGS is an effective and cost-efficient tool for rapidly detecting pathogens. The lung abscesses caused by P. micra have a good prognosis with appropriate treatment. Improving oral health, promoting sputum excretion, and following an appropriate extended course of antibiotic treatment are crucial for recovery from P. micra-induced anaerobic lung abscesses.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethics Review Committee of the Beijing Chao-Yang Hospital, Capital Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
DZ: Validation, Writing – original draft, Conceptualization. BF: Data curation, Investigation, Writing – original draft. YY: Data curation, Investigation, Writing – original draft. CJ: Data curation, Investigation, Writing – review & editing. LA: Conceptualization, Supervision, Writing – review & editing. XW: Data curation, Investigation, Writing – review & editing. HH: Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Scientific Technology Support Project of the Xizang Autonomous Region (XZ202201ZY0037G).
Conflict of interest
The authors declare that the research was conducted in the absence of commercial or financial relationships and without conflicts of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Badri, M., Nilson, B., Ragnarsson, S., Senneby, E., Rasmussen, M. (2019). Clinical and microbiological features of bacteraemia with Gram-positive anaerobic cocci: a population-based retrospective study. Clin.Microbiol. Infect. 25, 760.e1–760.e6. doi: 10.1016/j.cmi.2018.09.001
Bansal, M., Khatri, M., Taneja, V. (2013). Potential role of periodontal infection in respiratory diseases - a review. J. Med. Life 6, 244–248.
Boattini, M., Bianco, G., Cavallo, R., Costa, C. (2018). Parvimonasmicra bacteremia following endoscopic retrograde cholangiopancreatography: A new route of infection. Anaerobe 54, 136–139. doi: 10.1016/j.anaerobe.2018.09.003
Fukushima, S., Hagiya, H., Naito, H., Otsuka, F. (2023). Furious lung abscess due to Parvimonasmicra. Respirol Case Rep. 11, e01161. doi: 10.1002/rcr2.1161
Gorospe, L., Bermudez-Coronel-Prats, I., Gomez-Barbosa, C. F., Olmedo-Garcia, M. E., Ruedas-Lopez, A., Gomez delOlmo, V. (2014). Parvimonasmicra chest wall abscess following transthoracic lung needle biopsy. Korean J. Intern. Med. 29, 834–837. doi: 10.3904/kjim.2014.29.6.834
Hata, R., Noguchi, S., Kawanami, T., Yamasaki, K., Akata, K., Ikegami, H., et al. (2020). Poor oral hygiene is associated with the detection of obligate anaerobes in pneumonia. J. Periodontol. 91, 65–73. doi: 10.1002/JPER.19-0043
Herth, F., Ernst, A., Becker, H. D. (2005). Endoscopic drainage of lung abscesses: technique and outcome. Chest 127, 1378–1381. doi: 10.1378/chest.127.4.1378
Kuhajda, I., Zarogoulidis, K., Tsirgogianni, K., Tsavlis, D., Kioumis, I., Kosmidis, C., et al. (2015). Lung abscess-etiology, diagnostic and treatment options. Ann. Transl. Med. 3, 183. doi: 10.3978/j.issn.2305-5839.2015.07.08
Lee, J. H., Hong, H., Tamburrini, M., Park, C. M. (2022). Percutaneous transthoracic catheter drainage for lung abscess: a systematic review and meta-analysis. EurRadiol 32, 1184–1194. doi: 10.1007/s00330-021-08149-5
Li, S., Tong, J., Liu, Y., Shen, W., Hu, P. (2022). Targeted next generation sequencing is comparable with metagenomic next generation sequencing in adults with pneumonia for pathogenic microorganism detection. J. Infect. 85, e127–e129. doi: 10.1016/j.jinf.2022.08.022
Polverino, E., Goeminne, P. C., McDonnell, M. J., Aliberti, S., Marshall, S. E., Loebinger, M. R., et al. (2017). European Respiratory Society guidelines for the management of adult bronchiectasis. Eur. Respir. J. 50, 1700629. doi: 10.1183/13993003.00629-2017
Unterman, A., Fruchter, O., Rosengarten, D., Izhakian, S., Abdel-Rahman, N., Kramer, M. R. (2017). Bronchoscopic drainage of lung abscesses using a pigtail catheter. Respiration 93, 99–105. doi: 10.1159/000453003
Vilcarromero, S., Small, M., Lizarzaburu, A., Rivadeneyra-Rodriguez, A. (2023). Pleural empyema by Parvimonasmicra in an immunocompetent patient: a case report. Rev. Peruana medicina Exp. y saludpublica 40, 99–104. doi: 10.17843/rpmesp.2023.401.11956
Yang, H. W., Su, Y. J. (2021). Cavitary lung mass presenting in an outpatient. Am. J. Med. 134, 1113–1114. doi: 10.1016/j.amjmed.2021.02.017
Yazbeck, M. F., Dahdel, M., Kalra, A., Browne, A. S., Pratter, M. R. (2014). Lung abscess: update on microbiology and management. Am. J. Ther. 21, 217–221. doi: 10.1097/MJT.0b013e3182383c9b
Yu, Q., Sun, L., Xu, Z., Fan, L., Du, Y. (2021). Severe pneumonia caused by Parvimonasmicra: a case report. BMC Infect. Dis. 21, 364. doi: 10.1186/s12879-021-06058-y
Yun, S. S., Cho, H. S., Heo, M., Jeong, J. H., Lee, H. R., Ju, S., et al. (2019). Lung abscess by Actinomycesodontolyticus and Parvimonasmicra co-infection presenting as acute respiratory failure: A case report. Med. (Abingdon) 98, e16911. doi: 10.1097/MD.0000000000016911
Zhang, Y., Song, P., Zhang, R., Yao, Y., Shen, L., Ma, Q., et al. (2021). Clinical characteristics of chronic lung abscess associated with parvimonasmicra diagnosed using metagenomic next-generation sequencing. Infect. Drug Resist. 14, 1191–1198. doi: 10.2147/IDR.S304569
Keywords: Parvimonas micra, lung abscess, target next-generation sequencing, TNGS, diagnosis
Citation: Zhang D, Fan B, Yang Y, Jiang C, An L, Wang X and He H (2024) Target next-generation sequencing for the accurate diagnosis of Parvimonas micra lung abscess: a case series and literature review. Front. Cell. Infect. Microbiol. 14:1416884. doi: 10.3389/fcimb.2024.1416884
Received: 13 April 2024; Accepted: 26 June 2024;
Published: 11 July 2024.
Edited by:
Jiemin Zhou, Vision Medicals Co, Ltd, ChinaReviewed by:
Jiajia Zheng, Peking University, ChinaYang Jiao, Second Military Medical University, China
Yong Liu, First Affiliated Hospital of Jilin University, China
Copyright © 2024 Zhang, Fan, Yang, Jiang, An, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hangyong He, eW9uZ2hhbmcyMDA0QHNpbmEuY29t
†These authors have contributed equally to this work
 Dongmei Zhang
Dongmei Zhang Boyang Fan
Boyang Fan Yuan Yang
Yuan Yang Chunguo Jiang1
Chunguo Jiang1 Hangyong He
Hangyong He