- 1The First School of Clinical Medicine, Gannan Medical University, Ganzhou, China
- 2Department of Laboratory Medicine, First Affiliated Hospital of Gannan Medical University, Ganzhou, China
Bacterial extracellular vesicles (EVs) are crucial mediators of information transfer between bacteria and host cells. Macrophages, as key effector cells in the innate immune system, have garnered widespread attention for their interactions with bacterial EVs. Increasing evidence indicates that bacterial EVs can be internalized by macrophages through multiple pathways, thereby influencing their immune functions. These functions include inflammatory responses, antimicrobial activity, antigen presentation, and programmed cell death. Therefore, this review summarizes current research on the interactions between bacterial EVs and macrophages. This will aid in the deeper understanding of immune modulation mediated by pathogenic microorganisms and provide a basis for developing novel antibacterial therapeutic strategies.
1 Introduction
Intercellular communication is an indispensable process in living systems, with various cells transmitting information and coordinating behavior by releasing extracellular signaling molecules. Bacteria, as single-celled organisms, exchange information with other bacteria and host cells by secreting extracellular vesicles (EVs), thereby participating in the regulation of various physiological and pathological processes (Tiku and Tan, 2021). Bacterial EVs are small membrane vesicles rich in bioactive molecules such as proteins, lipids, and nucleic acids, capable of serving as carriers for signaling molecules and playing crucial roles in biofilm formation, stress responses, quorum sensing, and nutrient acquisition (Kim et al., 2015; Huang et al., 2023).
In recent years, research has revealed that bacterial EVs not only affect the behavior of bacteria themselves but also play significant roles in the interactions between microbes and hosts, especially impacting the host immune system (Kaparakis-Liaskos and Ferrero, 2015). As the main effector cells of the innate immune system, the interactions between macrophages and bacterial EVs have garnered considerable attention. Macrophages recognize, phagocytose, and digest invading pathogens, playing a frontline role in immune defense (Locati et al., 2020). However, how various molecular signals carried by bacterial EVs affect the immune functions of macrophages remains a hot and challenging field. On one hand, certain pathogenic bacteria can interfere with and evade macrophage immune surveillance through EVs (Kuehn and Kesty, 2005; Bielaszewska et al., 2017). on the other hand, commensal bacteria can enhance macrophage antimicrobial capacity via EVs, playing a role in regulating host-microbe balance (Shen et al., 2012). Additionally, bacterial EVs can influence other physiological processes of macrophages, such as metabolic state and cell death (Dhital et al., 2021). Therefore, fully elucidating the molecular mechanisms of the interactions between bacterial EVs and macrophages is of great significance and value for understanding the immune regulatory networks mediated by microbes.
2 Types and biogenesis of bacterial EVs
First, compared to EVs produced by eukaryotic cells, bacterial EVs exhibit more diverse types and biogenesis mechanisms. Taking Gram-negative bacteria as an example, current research indicates that they can produce EVs through two mechanisms: blebbing and explosive cell lysis (Toyofuku et al., 2023). Specifically, EVs produced through blebbing include outer membrane vesicles (OMVs) and outer-inner membrane vesicles (OIMVs). OMVs are formed due to the disturbance in the Gram-negative bacterial cell envelope, leading to the outer membrane blebbing outward and eventually shedding off (Kadurugamuwa et al., 1993; Kulp and Kuehn, 2010; Schwechheimer and Kuehn, 2015). In contrast, OIMVs are formed when the peptidoglycan layer of Gram-negative bacteria undergoes autolysin-mediated weakening, resulting in the outer and inner membranes blebbing outward together (Perez-Cruz et al., 2013; Perez-Cruz et al., 2015). Additionally, Gram-negative bacteria can produce explosive outer membrane vesicles (EOMVs) and explosive outer-inner membrane vesicles (EOIMVs) through the explosive cell lysis mechanism. This occurs when phage-encoded endolysins in Gram-negative bacteria are activated, degrading the peptidoglycan layer and causing explosive cell lysis, followed by spontaneous assembly and fusion of the fragmented membrane segments (Turnbull et al., 2016; Baeza et al., 2021).
As for Gram-positive bacterial EVs, current research indicates that the peptidoglycan cell wall of Gram-positive bacteria can form pores under the action of endolysins, autolysins, or certain antibiotics that inhibit peptidoglycan synthesis, ultimately leading to the cytoplasmic membrane protruding outward and forming cytoplasmic membrane vesicles (CMVs) (Mitchell et al., 2013; Toyofuku et al., 2017; Andreoni et al., 2019; Abe et al., 2021; Liu et al., 2022). However, throughout this process, Gram-positive bacterial cells do not undergo explosive lysis. Nonetheless, the disruption of cytoplasmic membrane integrity still results in bacterial cell death. Therefore, this mechanism is termed “bubbling cell death” (Jiang et al., 2024).
3 Macrophage internalization of bacterial EVs
Endocytosis is the main pathway through which bacterial EVs enter macrophages. Endocytosis refers to the process by which small molecules cross the bilayer cell membrane and enter the cell (Doherty and McMahon, 2009). Due to the differences in the surface and cargo of bacterial EVs, the pathways of endocytosis can vary. For instance, Staphylococcus aureus EVs can be internalized by macrophages through a dynamin-dependent endocytic pathway (Wang et al., 2020). Klebsiella pneumoniae EVs can enter macrophages through a lectin-like oxidized low-density lipoprotein receptor-dependent endocytic pathway (Ye et al., 2021). Additionally, clathrin-mediated endocytosis is also closely related to the entry of bacterial EVs into macrophages. Clathrin-mediated endocytosis involves the formation of a clathrin-coated pit, which generates vesicles to absorb target substances (Vercauteren et al., 2010). This mode of endocytosis plays a crucial role in the uptake of external substances by host cells, but it is usually tightly regulated by cell surface receptors, allowing only substances that specifically bind to the receptors to enter the cell (McMahon and Boucrot, 2011; Kaksonen and Roux, 2018; Mettlen et al., 2018). However, most current studies on EV internalization pathways focus on Gram-negative bacteria. Future research could further investigate the pathways of Gram-positive bacteria EV internalization.
4 The impact of bacterial EVs on macrophage immune function
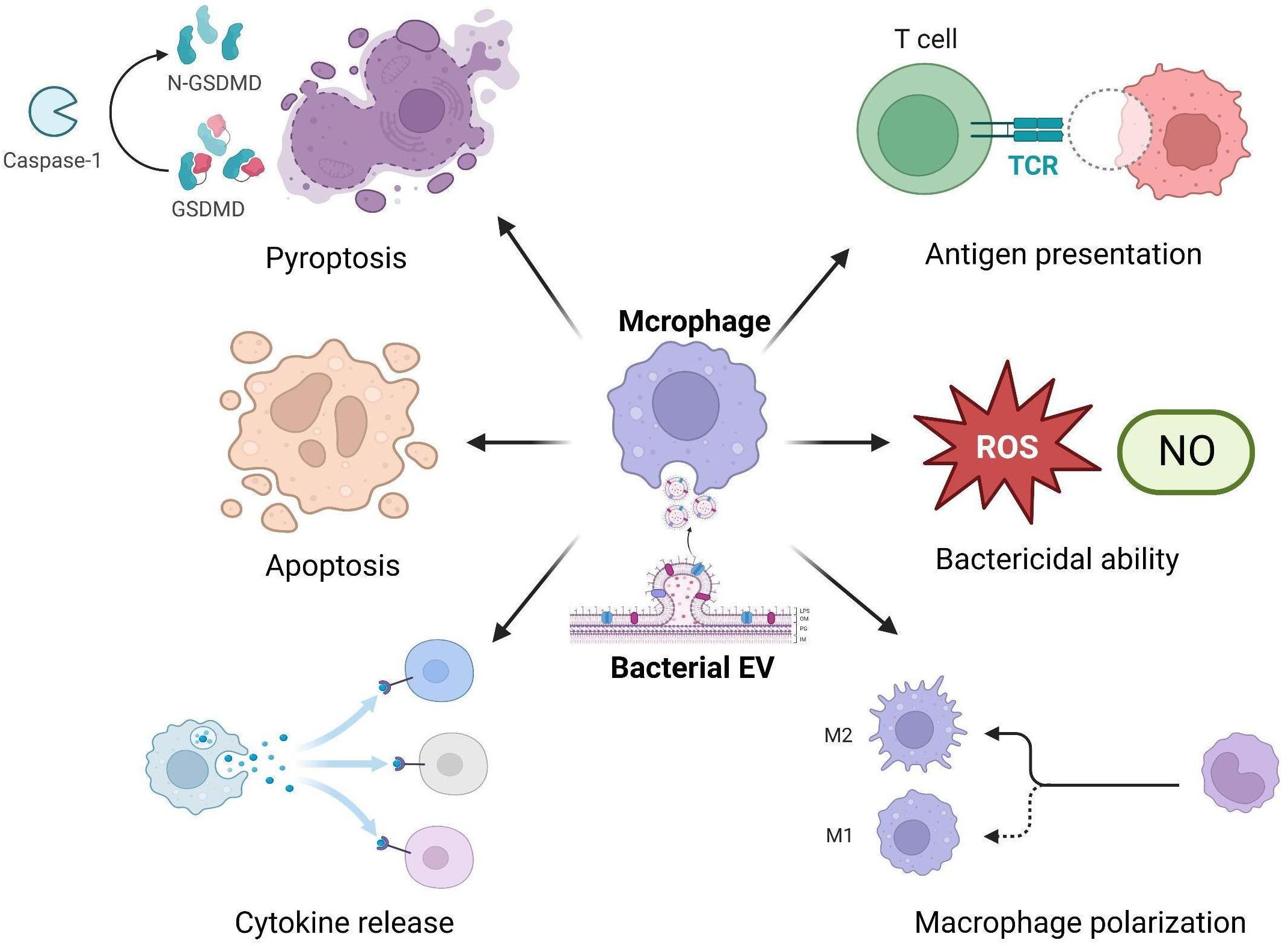
Increasing evidence suggests that bacterial EVs can significantly impact the immune functions of macrophages, including inflammatory responses, antibacterial activity, antigen presentation capacity, and programmed cell death (Ellis et al., 2010; Dhital et al., 2021; Hu et al., 2021) (Figure 1).
4.1 Bacterial EVs can modulate macrophage-mediated inflammatory responses
First, when bacterial EVs come into contact with or are phagocytosed by macrophages, the pathogen-associated molecular patterns (PAMPs) they carry interact with the pattern recognition receptors (PRRs) of macrophages (Asano et al., 2021). This interaction induces macrophages to produce various inflammatory factors (Ellis et al., 2010; Asano et al., 2021; Xu et al., 2022). For example, lipoproteins in S. aureus EVs can stimulate macrophages to release IL-6 and TNF-α (Kopparapu et al., 2021). LPS and flagellin in Pseudomonas aeruginosa EVs can stimulate macrophages to release MIP-2, TNF-α, and IL-6 (Ellis et al., 2010). Extracellular RNA in Aggregatibacter actinomycetemcomitans EVs can promote TNF-α production in macrophages (Han et al., 2019). These examples show that bacterial EVs can significantly promote macrophage-mediated inflammatory responses. However, in addition to their pro-inflammatory effects, EVs from some bacteria, such as Escherichia coli, Francisella tularensis, and Clostridium butyricum, can stimulate macrophages to release the anti-inflammatory cytokine IL-10 (Hu et al., 2020; Pavkova et al., 2021; Guangzhang et al., 2023). This indicates that EVs from certain bacteria have complex dual effects on macrophage-mediated inflammatory responses, capable of inducing the production of both pro-inflammatory and anti-inflammatory cytokines by macrophages. Moreover, besides directly inducing the production of various cytokines, studies have found that EVs from E. coli can induce macrophages to release pro-inflammatory EVs after interacting with them. This implies that EVs derived from E. coli have the ability to transmit inflammatory signals to macrophage EVs (Imamiya et al., 2023). Interestingly, although Porphyromonas gingivalis EVs can stimulate macrophages to release various inflammatory cytokines, the Arg- and Lys-gingipains enzymes in these EVs can degrade macrophage inflammatory cytokines, thereby achieving fine-tuned regulation of the immune response (Barth and Genco, 2016; Castillo et al., 2022).
Additionally, it is important to note that not all bacterial EVs exhibit pro-inflammatory effects. Current research indicates that EVs produced by some commensal bacteria, such as Lactobacillus plantarum, C. butyricum, and Bacteroides thetaiotaomicron, can induce macrophages to polarize towards the M2 type, leading to the release of the anti-inflammatory cytokine IL-10, thereby inhibiting inflammatory responses (Kim et al., 2020; Muller et al., 2021; Liang et al., 2022). Moreover, studies have found that certain mycobacteria, such as Mycobacterium avium and Mycobacterium kansasii, also produce EVs with anti-inflammatory properties that can induce macrophages to produce IL-10 (Kamran-Sarkandi et al., 2018; Tavassol et al., 2020). Therefore, these studies demonstrate that EVs from different bacterial sources have varying effects on macrophage-mediated inflammatory responses.
4.2 Bacterial EVs can influence the antibacterial activity of macrophages
Additionally, as immune cells, macrophages possess strong antibacterial capabilities. When pathogens are recognized by macrophages, they form phagosomes on their cell membranes to engulf the pathogens, which then fuse with lysosomes to form phagolysosomes, thereby eliminating the pathogens (Hirayama et al., 2017). However, it is worth noting that bacterial EVs have varying effects on the antibacterial activity of macrophages. For example, Blancá et al (Blanca et al., 2022). investigated the effects of Bordetella pertussis-derived EVs on the antimicrobial activity of macrophages. The study found that B. pertussis EVs contain various toxins, including adenylate cyclase (CyaA). It was shown that pre-incubating macrophages with EVs significantly weakened their phagocytic and bactericidal activities against B. pertussis. This is because the CyaA in B. pertussis EVs inhibits the antibacterial capacity of macrophages by reducing the level of complement receptor 3 on their surface. Furthermore, Jung et al. (2016) studied how EVs from Legionella pneumophila affect the immune response of macrophages and promote bacterial replication within macrophages. Their research revealed that macrophages pre-treated with L. pneumophila EVs responded differently to subsequent Legionella infection. Initially, the EVs enhanced the immune defense response of macrophages and reduced bacterial replication. However, as the infection progressed, this effect gradually shifted, making macrophages more tolerant of bacterial replication. These findings suggest that L. pneumophila EVs have a complex dual role in macrophages, initially activating immune defenses and then creating an environment conducive to bacterial survival and replication. Interestingly, studies have shown that while EVs from Streptococcus pneumoniae can enhance macrophage phagocytosis of S. pneumoniae, this makes macrophages a “reservoir” for the bacteria, negatively impacting their antibacterial activity (Yerneni et al., 2021). On the other hand, bacterial EVs can also promote the antibacterial activity of macrophages. For example, EVs from P. gingivalis and E. coli can induce macrophages to produce reactive oxygen species (ROS) and nitric oxide (NO) upon interaction (Imayoshi et al., 2011; Guangzhang et al., 2023). These substances are important antibacterial weapons of macrophages, capable of exerting toxic effects directly on pathogens (Nathan and Hibbs, 1991; Canton et al., 2021; Herb and Schramm, 2021). Additionally, EVs from E. coli Nissle can increase the activity of acid phosphatase in macrophages (Hu et al., 2020). This enzyme is located in lysosomes and is closely related to the digestive functions of macrophages (Xu et al., 2012).
4.3 Bacterial EVs can influence the antigen-presentation function of macrophages
Additionally, macrophages possess important antigen-presenting functions. After pathogens are digested by macrophages, they are degraded into multiple small antigen fragments. These antigen fragments bind to major histocompatibility complex (MHC) molecules in macrophages, thereby triggering specific immune responses (Guerriero, 2019). However, it should be noted that some studies have indicated that EVs secreted by certain bacteria have the ability to affect the antigen-presenting function of macrophages. For example, Armstrong et al. (2020) investigated the impact of EVs secreted by P. aeruginosa on the expression of MHC-related molecules in human lung macrophages. The study found that P. aeruginosa EVs significantly inhibited the expression of 11 different MHC-related molecules in lung macrophages, including several subunits of MHC class II molecules (HLA-DRA, HLA-DPA1, etc.). Additionally, three CpG sites within the CFB gene showed significant hypomethylation under the influence of EVs, which was closely associated with the downregulation of MHC gene expression. These results suggest that P. aeruginosa EVs may regulate the antigen-presenting function of macrophages by altering the epigenetic state of their genes. Moreover, another study found that EVs from E. coli can promote the expression of the co-stimulatory molecule CD86 on the surface of macrophages (Guangzhang et al., 2023). This molecule plays a crucial role in the activation of T cells induced by macrophages (Smith et al., 2016; Nordlohne et al., 2021). However, current research on the impact of bacterial EVs on the antigen-presenting function of macrophages is still limited, and it has not been clearly identified which components in EVs interfere with this function. This area warrants further exploration in future research.
4.4 Bacterial EVs can induce programmed cell death in macrophages
Programmed cell death is an orderly and controlled process of cell death, which includes apoptosis, pyroptosis, and autophagy (Newton et al., 2024). First, pyroptosis is an inflammation-related form of cell death characterized by the release of cellular contents and the subsequent induction of an inflammatory response (Rao et al., 2022). Current studies indicate that bacterial EVs can deliver a series of bacterial molecules to macrophages to induce pyroptosis. For instance, E. coli EVs can transport LPS into the cytoplasm, directly activating caspase-11 to induce pyroptosis in macrophages (Vanaja et al., 2016; Finethy et al., 2017). Similarly, lipoproteins and pore-forming toxins in S. aureus EVs can activate caspase-1 through the NLRP3 inflammasome, thereby inducing pyroptosis in macrophages (Wang et al., 2020). Additionally, bacterial EVs containing flagellin can induce pyroptosis in macrophages by activating the NLRC4 inflammasome rather than the NLRP3 inflammasome (Bitto et al., 2018). Unlike the NLRP3 inflammasome, the NLRC4 inflammasome specifically recognizes bacterial flagellin and flagellin-like molecules, leading to a more specific and rapid activation (Gram et al., 2021; Chebly et al., 2022). For example, Salmonella EVs containing flagellin can quickly activate the NLRC4 inflammasome in a shorter time compared to flagellin-deficient E. coli and Salmonella EVs (Yang et al., 2020). Interestingly, P. gingivalis itself cannot activate inflammasome formation in macrophages, but its EVs can, as observed in a study by Fleetwood et al. (2017). This might be due to the enrichment of gingipain in P. gingivalis EVs, which helps P. gingivalis survive within cells (Sheets et al., 2008; Mantri et al., 2015). After being phagocytosed by macrophages, P. gingivalis downregulates gingipain levels (Xia et al., 2007). Moreover, the activation of inflammasomes in macrophages by bacterial EVs requires the involvement of guanylate-binding proteins (GBPs) (Finethy et al., 2017; Santos et al., 2018). GBPs are a class of large GTPases that play a crucial role in inflammasome activation (Shenoy et al., 2012).
Additionally, bacterial EVs can also induce apoptosis in macrophages. Apoptosis is a non-inflammatory form of cell death that can occur via intrinsic and extrinsic pathways (Kiraz et al., 2016). Initial studies using Neisseria gonorrhoeae EVs to treat bone marrow-derived macrophages found that the toxin protein PorB in N. gonorrhoeae EVs was sufficient to cause mitochondrial dysfunction in macrophages, leading to the release of cytochrome c and inducing apoptosis (Deo et al., 2018). However, recent research has discovered that EVs from N. gonorrhoeae, uropathogenic E. coli, and P. aeruginosa cause mitochondrial dysfunction in macrophages, which inhibits intracellular protein synthesis without inducing rapid apoptosis (Deo et al., 2020). Nonetheless, prolonged inhibition of protein synthesis can regulate members of the BCL-2 family, thereby activating mitochondrial apoptosis (Speir et al., 2016). Consequently, over time, EVs indirectly activate the pro-apoptotic member BAK, leading to apoptosis (Czabotar et al., 2014).
Moreover, autophagy, as a form of programmed cell death, is also an important antibacterial mechanism in macrophages (Wu and Lu, 2019). However, to date, only one study by David et al. has reported on the effects of bacterial EVs on macrophage autophagy. This study found that EVs produced by E. coli expressing HlyF can inhibit autophagic flux by preventing the fusion of autophagosomes with lysosomes, thereby hindering the formation of acidic autolysosomes and the clearance of autophagosomes. This suggests that EVs from E. coli expressing HlyF can enhance bacterial virulence by inhibiting autophagy in macrophages (David et al., 2022).
4.5 Bacterial EVs can influence the antiviral immune response of macrophages
It is worth noting that, in addition to the aforementioned impacts, current research indicates that bacterial EVs can also affect the antiviral immune response of macrophages. For instance, Bierwagen et al. (2023) explored the interaction between bacterial EVs and human macrophages and how this interaction influences viral replication, particularly concerning influenza virus. This study focused on EVs from different Gram-negative bacteria (such as Klebsiella, E. coli, and Salmonella) and found that these EVs can activate the antiviral immune response in macrophages via the TLR4-TRIF signaling axis. The research demonstrated that these bacterial EVs can induce macrophages to produce type I interferons and other antiviral molecules like Mx1, effectively inhibiting the replication of influenza virus A. Additionally, Bhar et al. (2022) investigated how commensal bacterial EVs influence murine norovirus (MNV) infection by modulating the antiviral immune response of macrophages. The study found that commensal bacterial EVs can bind to MNV and promote the co-inoculation of the virus and vesicles into host cells. This co-inoculation method significantly reduced viral replication in host cells compared to infection with the virus alone. Furthermore, these EVs increased the production of pro-inflammatory cytokines such as IL-6 and TNFα in macrophages during MNV infection, suggesting that EVs control viral infection by enhancing the immune response of host cells.
4.6 Bioengineered bacterial EVs can modulate the anti-tumor effects of macrophages
It is noteworthy that engineered bacterial EVs have also been reported to affect the antitumor immune effects of macrophages. Some tumor cells overexpress the CD47 molecule, which can inhibit the phagocytic function of macrophages, thus avoiding clearance (Kaur et al., 2016). Therefore, effectively activating tumor-associated macrophages is crucial in tumor therapy. In this regard, Feng et al. (2022) developed engineered bacterial OMVs designed to activate the phagocytic function of macrophages in the tumor microenvironment. These engineered vesicles were fused with CD47 nanobodies (CD47nb) on their surface and encapsulated with a layer of selenium-bonded polyethylene glycol (PEG/Se), enabling controlled release in the tumor site triggered by radiation. The results showed that OMV-CD47nb significantly enhanced the phagocytic ability of macrophages against tumor cells, primarily by inducing M1 polarization and blocking the “don’t eat me” signal on tumor cells. Additionally, Guo et al. (2021) developed a drug delivery system based on bacterial OMVs for sequential targeting of tumor cells and macrophages. This system utilized OMVs from Gram-negative bacteria loaded with paclitaxel (PTX) and siRNA targeting Redd1 in macrophages, achieving sequential drug release through a pH-sensitive linkage. The system first released PTX in the acidic environment of the tumor site, followed by siRNA delivery to M2 macrophages, enhancing glycolysis and promoting their polarization to the antitumor M1 type. The results showed that this OMV system demonstrated significant antitumor activity both in vitro and in vivo, effectively regulating macrophage metabolism and inhibiting tumor cell migration and invasion. These studies indicate that engineered bacterial EVs can achieve antitumor effects by influencing macrophages, providing new strategies for future cancer therapies.
5 Conclusion
Bacterial EVs, as crucial information carriers between bacteria and the host, have a profound impact on key immune cells in the host immune system, particularly macrophages. EVs from different bacterial sources can carry various bioactive molecules that regulate macrophage inflammatory responses, antibacterial activity, antigen presentation functions, and induce different forms of programmed cell death, thus reshaping microbe-host interactions at the microscopic level. On one hand, certain pathogenic bacterial EVs can stimulate macrophages to release pro-inflammatory factors, inhibit their bactericidal capabilities, interfere with antigen presentation functions, and induce cell death. On the other hand, some commensal bacterial EVs can induce macrophages to release anti-inflammatory factors, enhance bactericidal activity, and activate antiviral immune responses, thereby providing protective effects for the host. In addition to these impacts, engineered bacterial EVs can also modulate macrophage metabolic states and polarization types, influencing their antitumor immune effects. Moreover, they can serve as novel drug carriers targeting tumors and regulating macrophages, demonstrating significant potential in the field of cancer therapy.
Author contributions
BJ: Writing – original draft. JH: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We would like to express our profound gratitude to all participants for their invaluable contributions to this research. Additionally, we appreciate the assistance of BioRender.com in creating the figure for this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abe, K., Toyofuku, M., Nomura, N., Obana, N. (2021). Autolysis-mediated membrane vesicle formation in bacillus subtilis. Environ. Microbiol. 23, 2632–2647. doi: 10.1111/1462-2920.15502
Andreoni, F., Toyofuku, M., Menzi, C., Kalawong, R., Mairpady Shambat, S., Francois, P., et al. (2019). Antibiotics stimulate formation of vesicles in staphylococcus aureus in both phage-dependent and -independent fashions and via different routes. Antimicrob. Agents Chemother. 63 (2). doi: 10.1128/AAC.01439-18
Armstrong, D. A., Lee, M. K., Hazlett, H. F., Dessaint, J. A., Mellinger, D. L., Aridgides, D. S., et al. (2020). Extracellular vesicles from pseudomonas aeruginosa suppress mhc-related molecules in human lung macrophages. Immunohorizons 4, 508–519. doi: 10.4049/immunohorizons.2000026
Asano, K., Hirose, S., Narita, K., Subsomwong, P., Kawai, N., Sukchawalit, R., et al. (2021). Extracellular vesicles from methicillin resistant staphylococcus aureus stimulate proinflammatory cytokine production and trigger ige-mediated hypersensitivity. Emerg. Microbes Infect. 10, 2000–2009. doi: 10.1080/22221751.2021.1991239
Baeza, N., Delgado, L., Comas, J., Mercade, E. (2021). Phage-mediated explosive cell lysis induces the formation of a different type of O-imv in shewanella vesiculosa M7(T). Front. Microbiol. 12, 713669. doi: 10.3389/fmicb.2021.713669
Barth, K., Genco, C. A. (2016). Microbial degradation of cellular kinases impairs innate immune signaling and paracrine tnfalpha responses. Sci. Rep. 6, 34656. doi: 10.1038/srep34656
Bhar, S., Zhao, G., Bartel, J. D., Sterchele, H., Del Mazo, A., Emerson, L. E., et al. (2022). Bacterial extracellular vesicles control murine norovirus infection through modulation of antiviral immune responses. Front. Immunol. 13, 909949. doi: 10.3389/fimmu.2022.909949
Bielaszewska, M., Ruter, C., Bauwens, A., Greune, L., Jarosch, K. A., Steil, D., et al. (2017). Host cell interactions of outer membrane vesicle-associated virulence factors of enterohemorrhagic escherichia coli O157: intracellular delivery, trafficking and mechanisms of cell injury. PloS Pathog. 13, e1006159. doi: 10.1371/journal.ppat.1006159
Bierwagen, J., Wiegand, M., Laakmann, K., Danov, O., Limburg, H., Herbel, S. M., et al. (2023). Bacterial vesicles block viral replication in macrophages via tlr4-trif-axis. Cell Commun. Signal 21, 65. doi: 10.1186/s12964-023-01086-4
Bitto, N. J., Baker, P. J., Dowling, J. K., Wray-McCann, G., De Paoli, A., Tran, L. S., et al. (2018). Membrane vesicles from pseudomonas aeruginosa activate the noncanonical inflammasome through caspase-5 in human monocytes. Immunol. Cell Biol. 96, 1120–1130. doi: 10.1111/imcb.12190
Blanca, B., Alvarez Hayes, J., Surmann, K., Hugo, V., Hentschker, C., Lamberti, Y., et al. (2022). Bordetella pertussis outer membrane vesicles as virulence factor vehicles that influence bacterial interaction with macrophages. Pathog. Dis. 80 (1). doi: 10.1093/femspd/ftac031
Canton, M., Sanchez-Rodriguez, R., Spera, I., Venegas, F. C., Favia, M., Viola, A., et al. (2021). Reactive oxygen species in macrophages: sources and targets. Front. Immunol. 12, 734229. doi: 10.3389/fimmu.2021.734229
Castillo, Y., Castellanos, J. E., Lafaurie, G. I., Castillo, D. M. (2022). Porphyromonas gingivalis outer membrane vesicles modulate cytokine and chemokine production by gingipain-dependent mechanisms in human macrophages. Arch. Oral. Biol. 140, 105453. doi: 10.1016/j.archoralbio.2022.105453
Chebly, H., Marvaud, J. C., Safa, L., Elkak, A. K., Kobeissy, P. H., Kansau, I., et al. (2022). Clostridioides difficile flagellin activates the intracellular nlrc4 inflammasome. Int. J. Mol. Sci. 23 (20). doi: 10.3390/ijms232012366
Czabotar, P. E., Lessene, G., Strasser, A., Adams, J. M. (2014). Control of apoptosis by the bcl-2 protein family: implications for physiology and therapy. Nat. Rev. Mol. Cell Biol. 15, 49–63. doi: 10.1038/nrm3722
David, L., Taieb, F., Penary, M., Bordignon, P. J., Planes, R., Bagayoko, S., et al. (2022). Outer membrane vesicles produced by pathogenic strains of escherichia coli block autophagic flux and exacerbate inflammasome activation. Autophagy 18, 2913–2925. doi: 10.1080/15548627.2022.2054040
Deo, P., Chow, S. H., Han, M. L., Speir, M., Huang, C., Schittenhelm, R. B., et al. (2020). Mitochondrial dysfunction caused by outer membrane vesicles from gram-negative bacteria activates intrinsic apoptosis and inflammation. Nat. Microbiol. 5, 1418–1427. doi: 10.1038/s41564-020-0773-2
Deo, P., Chow, S. H., Hay, I. D., Kleifeld, O., Costin, A., Elgass, K. D., et al. (2018). Outer membrane vesicles from neisseria gonorrhoeae target porb to mitochondria and induce apoptosis. PloS Pathog. 14, e1006945. doi: 10.1371/journal.ppat.1006945
Dhital, S., Deo, P., Stuart, I., Naderer, T. (2021). Bacterial outer membrane vesicles and host cell death signaling. Trends Microbiol. 29, 1106–1116. doi: 10.1016/j.tim.2021.04.003
Doherty, G. J., McMahon, H. T. (2009). Mechanisms of endocytosis. Annu. Rev. Biochem. 78, 857–902. doi: 10.1146/annurev.biochem.78.081307.110540
Ellis, T. N., Leiman, S. A., Kuehn, M. J. (2010). Naturally produced outer membrane vesicles from pseudomonas aeruginosa elicit a potent innate immune response via combined sensing of both lipopolysaccharide and protein components. Infect. Immun. 78, 3822–3831. doi: 10.1128/IAI.00433-10
Feng, Q., Ma, X., Cheng, K., Liu, G., Li, Y., Yue, Y., et al. (2022). Engineered bacterial outer membrane vesicles as controllable two-way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv. Mater 34, e2206200. doi: 10.1002/adma.202206200
Finethy, R., Luoma, S., Orench-Rivera, N., Feeley, E. M., Haldar, A. K., Yamamoto, M., et al. (2017). Inflammasome activation by bacterial outer membrane vesicles requires guanylate binding proteins. mBio 8 (5). doi: 10.1128/mBio.01188-17
Fleetwood, A. J., Lee, M. K. S., Singleton, W., Achuthan, A., Lee, M. C., O’Brien-Simpson, N. M., et al. (2017). Metabolic remodeling, inflammasome activation, and pyroptosis in macrophages stimulated by porphyromonas gingivalis and its outer membrane vesicles. Front. Cell Infect. Microbiol. 7, 351. doi: 10.3389/fcimb.2017.00351
Gram, A. M., Wright, J. A., Pickering, R. J., Lam, N. L., Booty, L. M., Webster, S. J., et al. (2021). Salmonella flagellin activates naip/nlrc4 and canonical nlrp3 inflammasomes in human macrophages. J. Immunol. 206, 631–640. doi: 10.4049/jimmunol.2000382
Guangzhang, C., Fangfang, F., Siqian, D., Xinyi, X., Xiaochuan, B., Yihan, R., et al. (2023). Outer membrane vesicles from escherichia coli are efficiently internalized by macrophage cells and alter their inflammatory response. Microb. Pathog. 175, 105965. doi: 10.1016/j.micpath.2022.105965
Guerriero, J. L. (2019). Macrophages: their untold story in T cell activation and function. Int. Rev. Cell Mol. Biol. 342, 73–93. doi: 10.1016/bs.ircmb.2018.07.001
Guo, Q., Li, X., Zhou, W., Chu, Y., Chen, Q., Zhang, Y., et al. (2021). Sequentially triggered bacterial outer membrane vesicles for macrophage metabolism modulation and tumor metastasis suppression. ACS Nano 15, 13826–13838. doi: 10.1021/acsnano.1c05613
Han, E. C., Choi, S. Y., Lee, Y., Park, J. W., Hong, S. H., Lee, H. J. (2019). Extracellular rnas in periodontopathogenic outer membrane vesicles promote tnf-alpha production in human macrophages and cross the blood-brain barrier in mice. FASEB J. 33, 13412–13422. doi: 10.1096/fj.201901575R
Herb, M., Schramm, M. (2021). Functions of ros in macrophages and antimicrobial immunity. Antioxidants (Basel) 10 (2). doi: 10.3390/antiox10020313
Hirayama, D., Iida, T., Nakase, H. (2017). The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 19 (1). doi: 10.3390/ijms19010092
Hu, R., Lin, H., Li, J., Zhao, Y., Wang, M., Sun, X., et al. (2020). Probiotic escherichia coli nissle 1917-derived outer membrane vesicles enhance immunomodulation and antimicrobial activity in raw264.7 macrophages. BMC Microbiol. 20, 268. doi: 10.1186/s12866-020-01953-x
Hu, R., Lin, H., Wang, M., Zhao, Y., Liu, H., Min, Y., et al. (2021). Lactobacillus reuteri-derived extracellular vesicles maintain intestinal immune homeostasis against lipopolysaccharide-induced inflammatory responses in broilers. J. Anim. Sci. Biotechnol. 12, 25. doi: 10.1186/s40104-020-00532-4
Huang, J., Wang, X., Wang, Z., Deng, L., Wang, Y., Tang, Y., et al. (2023). Extracellular vesicles as a novel mediator of interkingdom communication. Cytokine Growth Factor Rev. 73, 173–184. doi: 10.1016/j.cytogfr.2023.08.005
Imamiya, R., Shinohara, A., Yakura, D., Yamaguchi, T., Ueda, K., Oguro, A., et al. (2023). Escherichia coli-derived outer membrane vesicles relay inflammatory responses to macrophage-derived exosomes. mBio 14, e0305122. doi: 10.1128/mbio.03051-22
Imayoshi, R., Cho, T., Kaminishi, H. (2011). No production in raw264 cells stimulated with porphyromonas gingivalis extracellular vesicles. Oral. Dis. 17, 83–89. doi: 10.1111/j.1601-0825.2010.01708.x
Jiang, B., Lai, Y., Xiao, W., Zhong, T., Liu, F., Gong, J., et al. (2024). Microbial extracellular vesicles contribute to antimicrobial resistance. PloS Pathog. 20, e1012143. doi: 10.1371/journal.ppat.1012143
Jung, A. L., Stoiber, C., Herkt, C. E., Schulz, C., Bertrams, W., Schmeck, B. (2016). Legionella pneumophila-derived outer membrane vesicles promote bacterial replication in macrophages. PloS Pathog. 12, e1005592. doi: 10.1371/journal.ppat.1005592
Kadurugamuwa, J. L., Clarke, A. J., Beveridge, T. J. (1993). Surface action of gentamicin on pseudomonas aeruginosa. J. Bacteriol 175, 5798–5805. doi: 10.1128/jb.175.18.5798-5805.1993
Kaksonen, M., Roux, A. (2018). Mechanisms of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 19, 313–326. doi: 10.1038/nrm.2017.132
Kamran-Sarkandi, M., Behrouzi, A., Fateh, A., Vaziri, F., Mirsaeidi, M., Siadat, S. D. (2018). Mycobacterium avium complex extracellular vesicles attenuate inflammation via inducing il-10. Int. J. Mol. Cell Med. 7, 241–250. doi: 10.22088/IJMCM.BUMS.7.4.241
Kaparakis-Liaskos, M., Ferrero, R. L. (2015). Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 15, 375–387. doi: 10.1038/nri3837
Kaur, S., Elkahloun, A. G., Singh, S. P., Chen, Q. R., Meerzaman, D. M., Song, T., et al. (2016). A function-blocking cd47 antibody suppresses stem cell and egf signaling in triple-negative breast cancer. Oncotarget 7, 10133–10152. doi: 10.18632/oncotarget.v7i9
Kim, W., Lee, E. J., Bae, I. H., Myoung, K., Kim, S. T., Park, P. J., et al. (2020). Lactobacillus plantarum-derived extracellular vesicles induce anti-inflammatory M2 macrophage polarization in vitro. J. Extracell Vesicles 9, 1793514. doi: 10.1080/20013078.2020.1793514
Kim, J. H., Lee, J., Park, J., Gho, Y. S. (2015). Gram-negative and gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 40, 97–104. doi: 10.1016/j.semcdb.2015.02.006
Kiraz, Y., Adan, A., Kartal Yandim, M., Baran, Y. (2016). Major apoptotic mechanisms and genes involved in apoptosis. Tumor Biol. 37, 8471–8486. doi: 10.1007/s13277-016-5035-9
Kopparapu, P. K., Deshmukh, M., Hu, Z., Mohammad, M., Maugeri, M., Gotz, F., et al. (2021). Lipoproteins are responsible for the pro-inflammatory property of staphylococcus aureus extracellular vesicles. Int. J. Mol. Sci. 22 (13). doi: 10.3390/ijms22137099
Kuehn, M. J., Kesty, N. C. (2005). Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 19, 2645–2655. doi: 10.1101/gad.1299905
Kulp, A., Kuehn, M. J. (2010). Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 64, 163–184. doi: 10.1146/annurev.micro.091208.073413
Liang, L., Yang, C., Liu, L., Mai, G., Li, H., Wu, L., et al. (2022). Commensal bacteria-derived extracellular vesicles suppress ulcerative colitis through regulating the macrophages polarization and remodeling the gut microbiota. Microb. Cell Fact 21, 88. doi: 10.1186/s12934-022-01812-6
Liu, Y., Tempelaars, M. H., Boeren, S., Alexeeva, S., Smid, E. J., Abee, T. (2022). Extracellular vesicle formation in lactococcus lactis is stimulated by prophage-encoded holin-lysin system. Microb. Biotechnol. 15, 1281–1295. doi: 10.1111/1751-7915.13972
Locati, M., Curtale, G., Mantovani, A. (2020). Diversity, mechanisms, and significance of macrophage plasticity. Annu. Rev. Pathol. 15, 123–147. doi: 10.1146/annurev-pathmechdis-012418-012718
Mantri, C. K., Chen, C. H., Dong, X., Goodwin, J. S., Pratap, S., Paromov, V., et al. (2015). Fimbriae-mediated outer membrane vesicle production and invasion of porphyromonas gingivalis. Microbiologyopen 4, 53–65. doi: 10.1002/mbo3.221
McMahon, H. T., Boucrot, E. (2011). Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533. doi: 10.1038/nrm3151
Mettlen, M., Chen, P. H., Srinivasan, S., Danuser, G., Schmid, S. L. (2018). Regulation of clathrin-mediated endocytosis. Annu. Rev. Biochem. 87, 871–896. doi: 10.1146/annurev-biochem-062917-012644
Mitchell, G. J., Wiesenfeld, K., Nelson, D. C., Weitz, J. S. (2013). Critical cell wall hole size for lysis in gram-positive bacteria. J. R Soc. Interface 10, 20120892. doi: 10.1098/rsif.2012.0892
Muller, L., Kuhn, T., Koch, M., Fuhrmann, G. (2021). Stimulation of probiotic bacteria induces release of membrane vesicles with augmented anti-inflammatory activity. ACS Appl. Bio Mater 4, 3739–3748. doi: 10.1021/acsabm.0c01136
Nathan, C. F., Hibbs, J. B., Jr. (1991). Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr. Opin. Immunol. 3, 65–70. doi: 10.1016/0952-7915(91)90079-G
Newton, K., Strasser, A., Kayagaki, N., Dixit, V. M. (2024). Cell death. Cell 187, 235–256. doi: 10.1016/j.cell.2023.11.044
Nordlohne, J., Hulsmann, I., Schwafertz, S., Zgrajek, J., Grundmann, M., von Vietinghoff, S., et al. (2021). A flow cytometry approach reveals heterogeneity in conventional subsets of murine renal mononuclear phagocytes. Sci. Rep. 11, 13251. doi: 10.1038/s41598–021-92784-x
Pavkova, I., Klimentova, J., Bavlovic, J., Horcickova, L., Kubelkova, K., Vlcak, E., et al. (2021). Francisella tularensis outer membrane vesicles participate in the early phase of interaction with macrophages. Front. Microbiol. 12, 748706. doi: 10.3389/fmicb.2021.748706
Perez-Cruz, C., Carrion, O., Delgado, L., Martinez, G., Lopez-Iglesias, C., Mercade, E. (2013). New type of outer membrane vesicle produced by the gram-negative bacterium shewanella vesiculosa M7t: implications for DNA content. Appl. Environ. Microbiol. 79, 1874–1881. doi: 10.1128/AEM.03657-12
Perez-Cruz, C., Delgado, L., Lopez-Iglesias, C., Mercade, E. (2015). Outer-inner membrane vesicles naturally secreted by gram-negative pathogenic bacteria. PloS One 10, e0116896. doi: 10.1371/journal.pone.0116896
Rao, Z., Zhu, Y., Yang, P., Chen, Z., Xia, Y., Qiao, C., et al. (2022). Pyroptosis in inflammatory diseases and cancer. Theranostics 12, 4310–4329. doi: 10.7150/thno.71086
Santos, J. C., Dick, M. S., Lagrange, B., Degrandi, D., Pfeffer, K., Yamamoto, M., et al. (2018). Lps targets host guanylate-binding proteins to the bacterial outer membrane for non-canonical inflammasome activation. EMBO J. 37 (6). doi: 10.15252/embj.201798089
Schwechheimer, C., Kuehn, M. J. (2015). Outer-membrane vesicles from gram-negative bacteria: biogenesis and functions. Nat. Rev. Microbiol. 13, 605–619. doi: 10.1038/nrmicro3525
Sheets, S. M., Robles-Price, A. G., McKenzie, R. M., Casiano, C. A., Fletcher, H. M. (2008). Gingipain-dependent interactions with the host are important for survival of porphyromonas gingivalis. Front. Biosci. 13, 3215–3238. doi: 10.2741/2922
Shen, Y., Giardino Torchia, M. L., Lawson, G. W., Karp, C. L., Ashwell, J. D., Mazmanian, S. K. (2012). Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 12, 509–520. doi: 10.1016/j.chom.2012.08.004
Shenoy, A. R., Wellington, D. A., Kumar, P., Kassa, H., Booth, C. J., Cresswell, P., et al. (2012). Gbp5 promotes nlrp3 inflammasome assembly and immunity in mammals. Science 336, 481–485. doi: 10.1126/science.1217141
Smith, T. D., Tse, M. J., Read, E. L., Liu, W. F. (2016). Regulation of macrophage polarization and plasticity by complex activation signals. Integr. Biol. (Camb) 8, 946–955. doi: 10.1039/c6ib00105j
Speir, M., Lawlor, K. E., Glaser, S. P., Abraham, G., Chow, S., Vogrin, A., et al. (2016). Eliminating legionella by inhibiting bcl-xl to induce macrophage apoptosis. Nat. Microbiol. 1, 15034. doi: 10.1038/nmicrobiol.2015.34
Tavassol, Z. H., Aziziraftar, S. K., Behrouzi, A., Ghazanfari, M., Masoumi, M., Fateh, A., et al. (2020). Evaluation of mycobacterium kansasii extracellular vesicles role in balb/C mice immune modulatory. Int. J. Mycobacteriol 9, 58–61. doi: 10.4103/ijmy.ijmy_212_19
Tiku, V., Tan, M. W. (2021). Host immunity and cellular responses to bacterial outer membrane vesicles. Trends Immunol. 42, 1024–1036. doi: 10.1016/j.it.2021.09.006
Toyofuku, M., Carcamo-Oyarce, G., Yamamoto, T., Eisenstein, F., Hsiao, C. C., Kurosawa, M., et al. (2017). Prophage-triggered membrane vesicle formation through peptidoglycan damage in bacillus subtilis. Nat. Commun. 8, 481. doi: 10.1038/s41467-017-00492-w
Toyofuku, M., Schild, S., Kaparakis-Liaskos, M., Eberl, L. (2023). Composition and functions of bacterial membrane vesicles. Nat. Rev. Microbiol. 21, 415–430. doi: 10.1038/s41579-023-00875-5
Turnbull, L., Toyofuku, M., Hynen, A. L., Kurosawa, M., Pessi, G., Petty, N. K., et al. (2016). Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat. Commun. 7, 11220. doi: 10.1038/ncomms11220
Vanaja, S. K., Russo, A. J., Behl, B., Banerjee, I., Yankova, M., Deshmukh, S. D., et al. (2016). Bacterial outer membrane vesicles mediate cytosolic localization of lps and caspase-11 activation. Cell 165, 1106–1119. doi: 10.1016/j.cell.2016.04.015
Vercauteren, D., Vandenbroucke, R. E., Jones, A. T., Rejman, J., Demeester, J., De Smedt, S. C., et al. (2010). The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol. Ther. 18, 561–569. doi: 10.1038/mt.2009.281
Wang, X., Eagen, W. J., Lee, J. C. (2020). Orchestration of human macrophage nlrp3 inflammasome activation by staphylococcus aureus extracellular vesicles. Proc. Natl. Acad. Sci. U.S.A. 117, 3174–3184. doi: 10.1073/pnas.1915829117
Wu, M. Y., Lu, J. H. (2019). Autophagy and macrophage functions: inflammatory response and phagocytosis. Cells 9 (1). doi: 10.3390/cells9010070
Xia, Q., Wang, T., Taub, F., Park, Y., Capestany, C. A., Lamont, R. J., et al. (2007). Quantitative proteomics of intracellular porphyromonas gingivalis. Proteomics 7, 4323–4337. doi: 10.1002/pmic.200700543
Xu, Z., Hao, X., Li, M., Luo, H. (2022). Rhodococcus equi-derived extracellular vesicles promoting inflammatory response in macrophage through tlr2-nf-kappab/mapk pathways. Int. J. Mol. Sci. 23 (17). doi: 10.3390/ijms23179742
Xu, X., Huang, Q., Mao, Y., Cui, Z., Li, Y., Huang, Y., et al. (2012). Immunomodulatory effects of bacillus subtilis (Natto) B4 spores on murine macrophages. Microbiol. Immunol. 56, 817–824. doi: 10.1111/j.1348-0421.2012.00508.x
Yang, J., Hwang, I., Lee, E., Shin, S. J., Lee, E. J., Rhee, J. H., et al. (2020). Bacterial outer membrane vesicle-mediated cytosolic delivery of flagellin triggers host nlrc4 canonical inflammasome signaling. Front. Immunol. 11, 581165. doi: 10.3389/fimmu.2020.581165
Ye, C., Li, W., Yang, Y., Liu, Q., Li, S., Zheng, P., et al. (2021). Inappropriate use of antibiotics exacerbates inflammation through omv-induced pyroptosis in mdr klebsiella pneumoniae infection. Cell Rep. 36, 109750. doi: 10.1016/j.celrep.2021.109750
Keywords: extracellular vesicles, bacterial, macrophage, immune, influences
Citation: Jiang B and Huang J (2024) Influences of bacterial extracellular vesicles on macrophage immune functions. Front. Cell. Infect. Microbiol. 14:1411196. doi: 10.3389/fcimb.2024.1411196
Received: 03 April 2024; Accepted: 20 May 2024;
Published: 30 May 2024.
Edited by:
Xihui Shen, Northwest A&F University, ChinaReviewed by:
Rahman Khaleda Qazi, Stockholm University, SwedenRuoxi Zhao, Chinese Academy of Sciences (CAS), China
Jinshui Lin, Yan’an University, China
Copyright © 2024 Jiang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junyun Huang, MTM4Nzk3ODk2NjZAMTYzLmNvbQ==
 Bowei Jiang
Bowei Jiang Junyun Huang2*
Junyun Huang2*