- Department of Cardiology, Ganzhou People’s Hospital, Ganzhou, Jiangxi, China
Background: The Hepatitis C virus (HCV) infection is strongly associated with cardiovascular disease risk factors, but the relationship with blood pressure (BP) remains unclear.
Objectives: To assess the association between HCV infection status and BP in US adults.
Methods: Data for the study were obtained from the National Health and Nutrition Examination Survey (NHANES) between 1999 and 2012. The association of HCV infection status (including HCV infection, current HCV infection, and past HCV infection) with hypertension, systolic blood pressure (SBP), and diastolic blood pressure (DBP) were explored using logistic or linear regression analyses respectively.
Results: A total of 25,850 participants (age≥18 years) were enrolled in the current study, including 14,162 participants with hypertension. After adjusting for all covariates, HCV infection/current HCV infection was not associated with hypertension and SBP compared to participants with non-HCV infection (OR: 1.34,95% CI 0.96–1.87/1.31 95% CI 0.91,1.91, β: -0.92, 95% CI -2.7–0.86/-0.35 95% CI -2.51,1.81, respectively). HCV infection/current HCV infection was only associated with elevated DBP (β: 4.1,95% CI 2.57–5.63/4.24,95% CI 2.27–6.21). However, there was no correlation with past HCV infection in participants with hypertension, SBP, and DBP compared to those with non-HCV infection (OR: 1.23,95% CI 0.59–2.54; β: -3.79, 95% CI -7.67–0.08 and 2.28 95% CI -0.36–4.92, respectively).
Conclusion: In a representative sample of US adults, it was found that both HCV infection and current HCV infection were independently linked to higher DBP. However, there was no association between past HCV infection and DBP.
1 Introduction
Hepatitis C virus (HCV) infection refers to a viral hepatitis caused by an infection called HCV (Yeung et al., 2014; El-Ghitany et al., 2015), which brings about chronic inflammatory necrosis and fibrosis of the liver, and part of the patients may worsen into cirrhosis and eventually hepatocellular carcinoma (Udompap et al., 2016; Kanda et al., 2019). It is estimated that approximately 400,000 people die each year from HCV-related diseases (Pietschmann and Brown, 2019). Patients with HCV infection were often found to have hypertension, diabetes, and kidney disease, and mortality from cardiovascular disease caused by HCV infection was significantly increased (Petta et al., 2014; Lazo et al., 2017; Kuo et al., 2018; Sicras-Mainar et al., 2018). Therefore, HCV infection is closely related to cardiovascular diseases. Among US adults, hypertension is one of the most important diseases, leading to cardiovascular disease and premature death, which places a severe economic and social burden (Murray et al., 2013; Forouzanfar et al., 2017). More and more evidence shows that hypertension is one of the most common combined diseases in patients with HCV infection (Ruzicka et al., 2018; Sicras-Mainar et al., 2018; Chung et al., 2021), which has attracted the attention of clinicians in recent years.
The previous study suggested that HCV infection leads to liver damage and inflammation, which in turn may cause abnormal liver function (Sevastianos et al., 2020). The liver plays an important metabolic role in the body, including blood pressure (BP) regulation (Guzik and Touyz, 2017; Güven, 2023). Abnormal liver function may lead to dysregulation of angiotensin in the blood, which in turn may lead to increased BP (Morales et al., 2012). HCV infection may lead to functional impairment of endothelial cells in the vascular wall. Vascular endothelial cells play an important role in the regulation of BP, and their abnormal function may lead to disturbances in the regulation of vasodilation and contraction, which in turn may cause hypertension (González-Reimers et al., 2016; Konukoglu and Uzun, 2017). A study by Z M Younossi et al. showed that HCV antibody positivity was a risk factor for hypertension (Younossi et al., 2013). A recent retrospective study found that the prevalence of hypertension was significantly higher in patients with chronic HCV infection than in non-infected individuals (Butt et al., 2007). However, an analysis of the National Population Survey in Egypt found that HCV status (Past exposure and Chronic infection) was not associated with hypertension after adjusting for confounders such as BMI, smoking, age, and gender (Gadallah et al., 2018). Considering that the relationship between HCV and hypertension is controversial, more in-depth studies are needed to elucidate the correlation between HCV infection and hypertension. In addition, HCV infection status is classified as HCV antibody positive, current HCV infection, and past HCV infection, and it is unclear whether the association of different HCV infection status on BP (hypertension, systolic blood pressure [SBP], and diastolic blood pressure [DBP]) is consistent. In this study, we analyzed data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2012 to assess the association between HCV infection and hypertension, SBP, and DBP for clinical practice.
2 Methods
2.1 Study population
The National Center for Health Statistics/Centers for Disease Control and Prevention conducted NHANES, a program to assess the health and nutritional status of adults and children in the United States. Data on NHANES were obtained by the management of standardized questionnaires and medical evaluations conducted in mobile check-up clinics, including interviews conducted at home, physical examinations conducted in mobile check-up centers, and other data, including blood collection. NHANES included non-institutionalized civilians aged over two months, with complex, multi-stage, stratified, and grouped samples. The study was mandated by the CDC Institutional Review Board and written informed consent was obtained from all participants. All participants provided written informed consent, and all methods were performed according to the relevant guidelines and regulations (NHANES, 2022).
For this study, a total of 41,243 adults (aged ≥ 18 years) were included from NHANES 1999–2012. After excluding missing variables, 25,850 participants were finally included in this study (Figure 1).
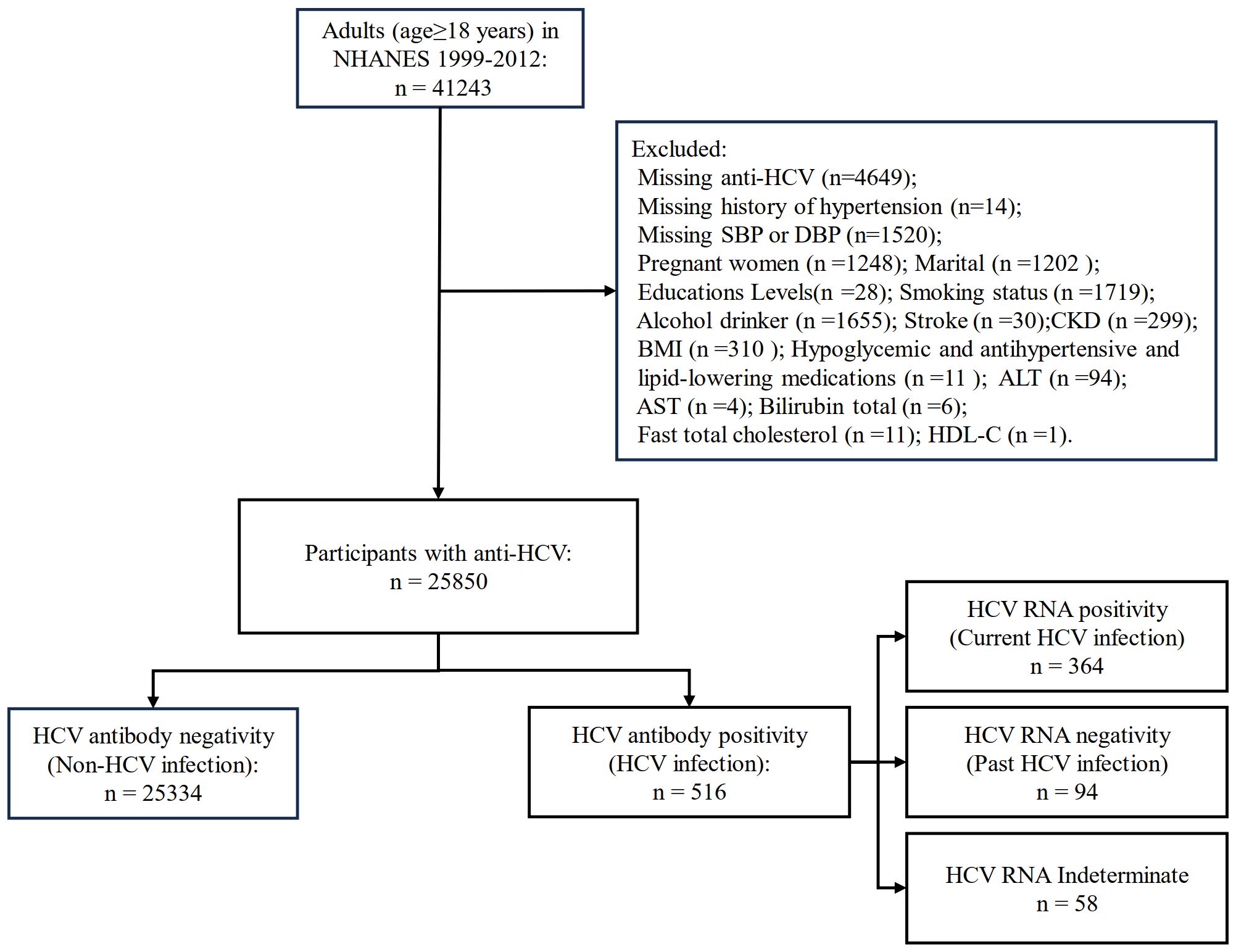
Figure 1 Flow diagram of inclusion criteria and exclusion criteria. NHANES, National Health and Nutrition Examination Survey; HCV, hepatitis C virus; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL-C, high-density lipoprotein cholesterol.
2.2 Definition of HCV infection
NHANES performs anti-HCV screening for participants 6 years of age and older. Serum specimens will be appropriately processed, stored, and shipped frozen at -30°C to the National Centers for Disease Control and Prevention for testing (Laboratory Procedures Manual). Detailed instructions for sample collection and processing can be found on the NHANES website (NHANES, 2011–2012).
The RIBA confirmatory test was utilized to test for the presence of anti-HCV. The Chiron RIBA 3.0 Strip Immunoblot Assay, developed by Chiron Corporation, Inc., is an in vitro qualitative enzyme immunoassay designed to detect anti-HCV in human serum or plasma. The presence of anti-HCV reactivity in specimens is visualized by utilizing anti-human IgG enzyme conjugates in combination with a colorimetric enzyme substrate. This enables the detection of individual HCV-encoded proteins. Samples with a positive RIBA result are reported as confirmed positive for anti-HCV. Samples that yield a negative outcome in the RIBA test are documented as negative for anti-HCV, whereas samples with indeterminate RIBA results are reported as being indeterminate. RIBA-positive and indeterminate specimens were further tested for HVC RNA, and HCV RNA in human serum or plasma was quantified on a COBAS AMPLICOR analyzer to confirm the participant’s infection status (NHANES, 2013–2014).
Participants were defined as HCV infection if they were anti-HCV positive; anti-HCV positive & HCV RNA positive were defined as current HCV infection and anti-HCV positive & HCV RNA negative were defined as past HCV infection. However, some participants with anti-HCV positive from the 1999–2012 NHANES were not tested for HCV RNA, which we defined as indeterminate HCV.
2.3 Measurements of blood pressure
BP was evaluated during 1999–2012 using the same protocol. BP values were measured by a trained clinician using an appropriately sized BP cuff and a mercury sphygmomanometer. Get the readings after resting in a seated position for 5 minutes. Three BP measurements were obtained at 30-second intervals. The average value of all available measurements was used to define SBP and DBP levels (Ostchega et al., 2003). Hypertension was defined as SBP ≥ 130 mmHg or DBP ≥ 80 mmHg, a self-reported history of hypertension, or the use of antihypertensive medications (Muntner et al., 2018).
2.4 Other covariate data acquisition methods
Data was collected by managing standardized questionnaires and performing medical evaluations in mobile check-up clinics. In combination with previous studies on hypertension and chronic HCV infection and NHANES dataset variables, the following variables were collected: sex (male or female), age, race/ethnicity (Non-Hispanic White, Non-Hispanic Black, Mexican American or Other Race), education levels (below high school, college/above), marital (widowed or divorced or separated, never married, married or living with partner), alcohol drinker (never, former or now), smoking status (current smoker, former smoker or never smoker), diabetes, stroke, atherosclerotic cardiovascular disease (ASCVD), chronic kidney disease (CKD), and hyperlipidemia. Diabetes was defined as fasting blood glucose ≥126 mg/dl or glycated hemoglobin ≥ 6.5%, or the use of hypoglycemia medications (Wang et al., 2021). Estimated glomerular filtration rate (eGFR) was calculated by the Modification of Diet in Renal Disease Study equation (Levey et al., 2006). Subjects with eGFR < 60ml/min/1.73m2 were considered to have CKD (Murphy et al., 2016). Body mass index (BMI, calculated as weight in kilograms divided by height in meters squared), and biochemical indicators (glutamic-oxaloacetic transaminase [AST], alanine aminotransferase [ALT], alkaline phosphatase, albumin, bilirubin total, fast total cholesterol, and high-density lipoprotein cholesterol [HDL-C]).
2.5 Statistical analysis
Data in this study were weighted using interview sample weights provided by NHANES to account for the complexity of the NHANES database survey design (NHANES). All statistical analyses were performed using SAS 9.4 (version 9.4, SAS Institute) and R Studio software (4.2.1), and a two-sided P<0.05 was considered statistically significant.
Continuous variables are expressed by mean standard (standard error [SE]) and classified variables are expressed by percentage (%). Continuous variables were analyzed for differences between groups using the ANOVA (NHANES). Categorical variables were analyzed for differences between groups using the chi-square test. Logistic regression analysis was used to assess the association between HCV infection/current HCV infection and past HCV infection and hypertension. Results were expressed as odds ratios (ORs) and 95% confidence intervals (95% CIs). Linear regression analysis was used to assess the association between HCV infection/current HCV infection and past HCV infection and DBP, and SBP. Results were presented as standardized coefficients (β) and 95% CIs. We constructed three models (Model 1 was adjusted for age, and sex. Model 2 was adjusted for age, sex, race, and education levels, marital, smoking status, alcohol drinker. Model 3 was adjusted for the variables in model 2 plus hypoglycemic medications, antihypertensive medications, lipid-lowering medications, diabetes, stroke, ASCVD, CKD, BMI, ALT, AST, albumin, bilirubin total, alkaline phosphatase, fast total cholesterol, and HDL-C. We also examined the association between HCV infection status and DBP by stratifying analyses by age (≤65 and >65 years), race/ethnicity (Non-Hispanic white, Non-Hispanic black, Mexican American, and Other Race), education levels (below high school and college/above), smoking status, alcohol drinker, and BMI (≤25 and >25kg/m2), and stratification interactions were tested.
3 Results
3.1 Baseline characteristics of participants with different HCV infection status
A total of 25,850 participants were enrolled in the current study, including 516 individuals infected with hepatitis C (current infection: 364, past infection: 94, and HCV RNA undetermined: 58). The age of HCV infection, current HCV infection and past HCV infection was 47.93, 48.09 and 47.42, respectively. The participants with HCV infection were more likely to be men, had more education, were more likely to be smokers, obesity and stroke, had higher levels of alkaline phosphatase, ALT, and AST, and lower levels of fast total cholesterol. Compared with those with current HCV infection, past HCV infection were more likely to be female, have stroke and hyperlipidemia, and have lower ALT, AST, alkaline phosphatase, and higher fast total cholesterol (Table 1).
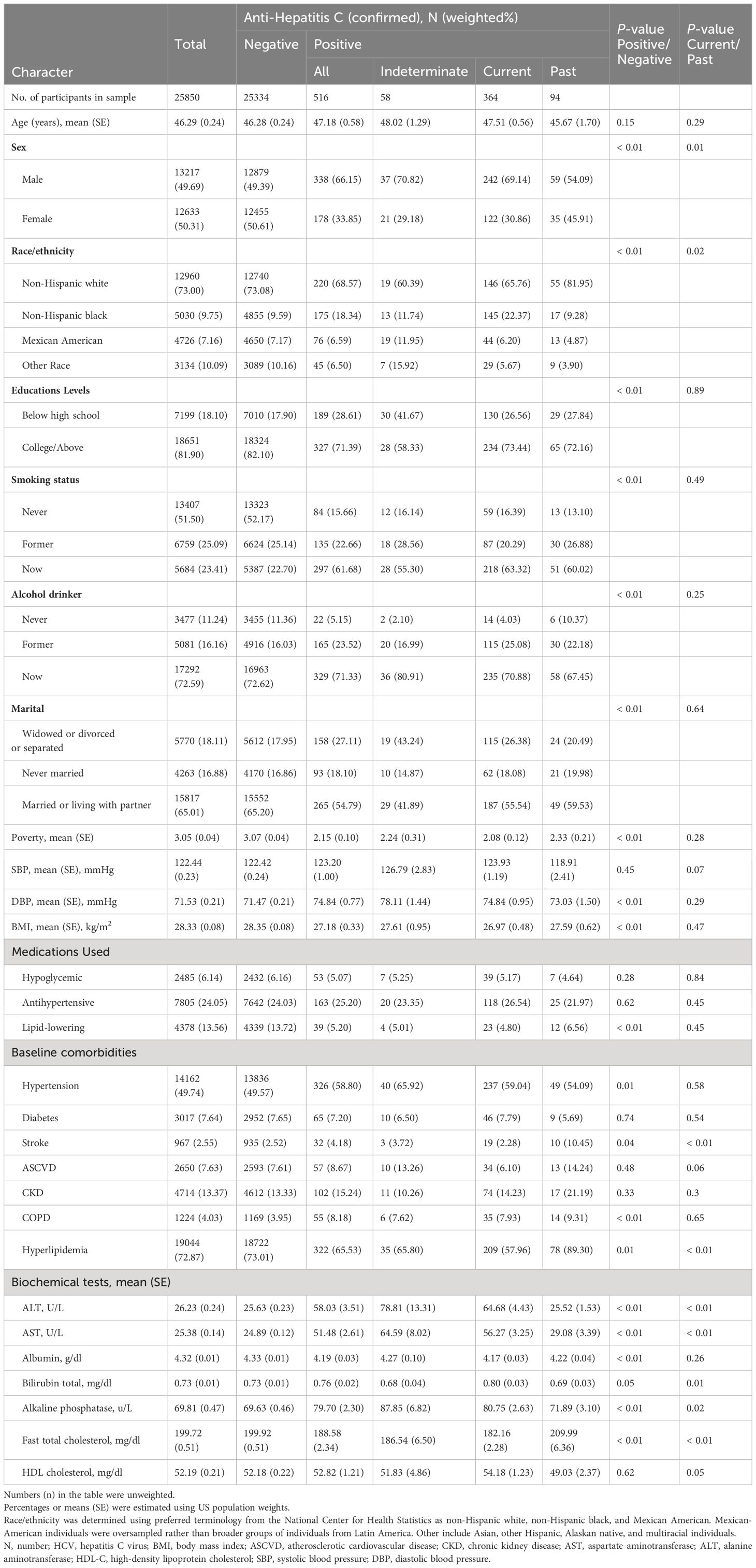
Table 1 The participant baseline characteristics by HCV infection status among US adults in NHANES (1999–2012).
3.2 The association between HCV infection status and hypertension, SBP and DBP
We first assessed the effect of HCV infection on hypertension (Table 2). After adjusting for age, and sex (Model 1), HCV infection was not associated with hypertension and SBP compared with non-HCV participants (OR: 1.25, 95% CI 0.92–1.7 and β: -0.67, 95% CI -2.57–1.24). After adjusting for age, sex, race, and education levels, marital, smoking status, alcohol drinker, hypoglycemic medications, antihypertensive medications, lipid-lowering medications, diabetes, stroke, ASCVD, CKD, BMI, ALT, AST, albumin, bilirubin total, alkaline phosphatase, fast total cholesterol, and HDL-C (Model 3), HCV infection remained unrelated to hypertension and SBP (OR: 1.34, 95% CI 0.96–1.87 and β: -0.92, 95% CI -2.7–0.86). However, HCV infection was associated with increased DBP compared to non-HCV participants (β: 4.1,95% CI 2.57–5.63) in Model 3).
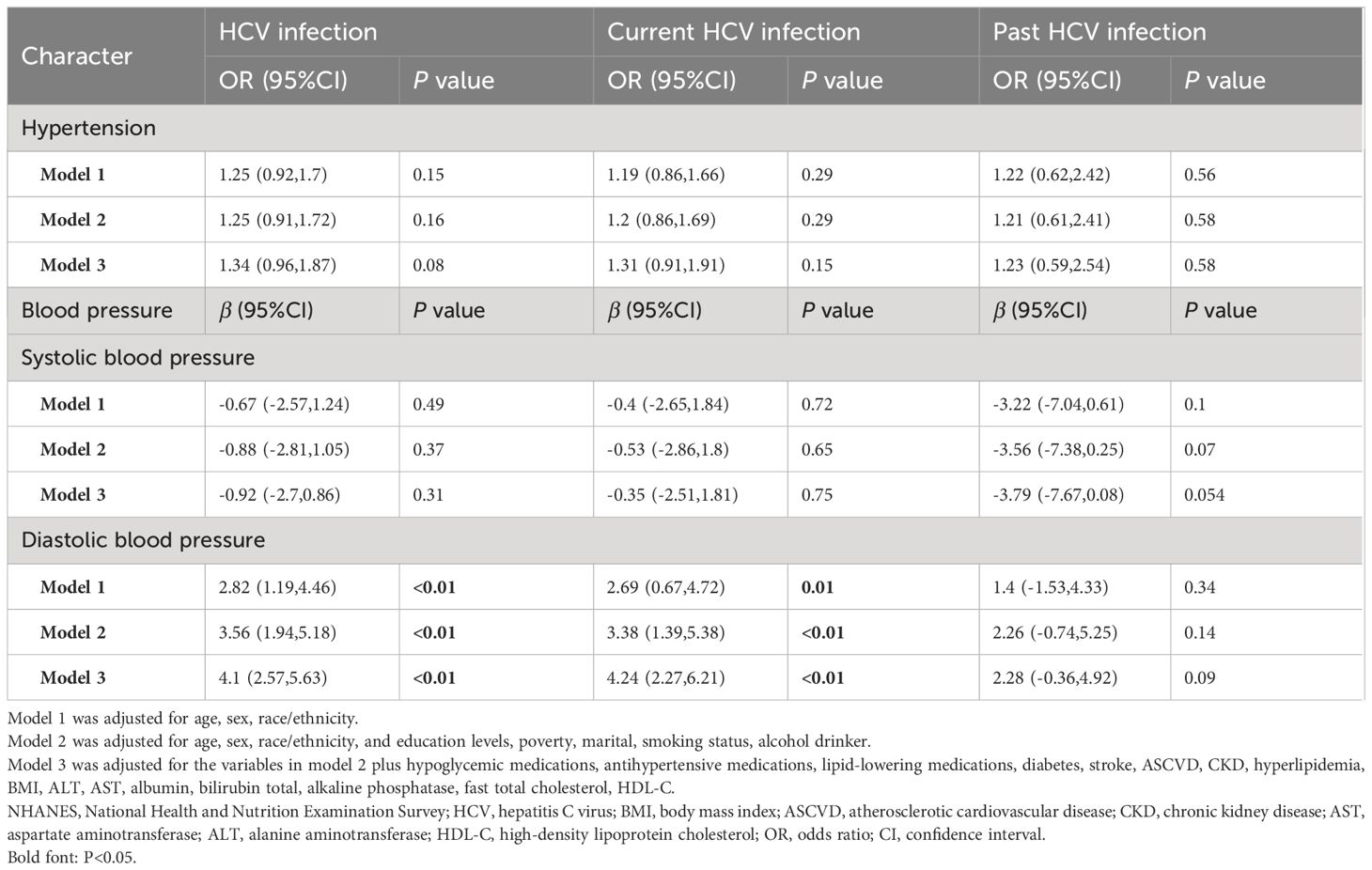
Table 2 The association between HCV infection state and hypertension, systolic blood pressure and diastolic blood pressure among US adults in NHANES (1999–2012).
The association between current and past HCV infection and BP was also assessed (Table 2), A similar relationship was observed. HCV current infection was not associated with hypertension and SBP compared with non-HCV participants, with OR: 1.31 (95% CI 0.91,1.91) and β: -0.35 (95% CI -2.51,1.81) in Model 3, respectively. DBP was higher in HCV current infection (β: 4.24,95% CI 2.27–6.21 in Model 3). Interestingly, there was no association with past HCV infection in participants with hypertension, SBP, and DBP compared to those with non-HCV infection (OR: 1.23,95% CI 0.59–2.54; β: -3.79, 95% CI -7.67–0.08 and 2.28 95% CI -0.36–4.92, respectively).
3.3 Stratified analysis
Stratified analyses were performed for age (≤65 and >65 years), races/ethnicity (Non-Hispanic white, Non-Hispanic black, Mexican American, Other Race), education levels (below high school, college/above), smoking status, alcohol drinker, and BMI (≤25kg/m2, >25kg/m2) as shown in Figure 2. Results indicated heterogeneity in the association between HCV infection status and DBP between Races/ethnicity. The association between HCV infection and current HCV infection and DBP was stronger in non-Hispanic blacks. No association between current HCV infection and DBP was observed in Non-Hispanic white or Other Race.
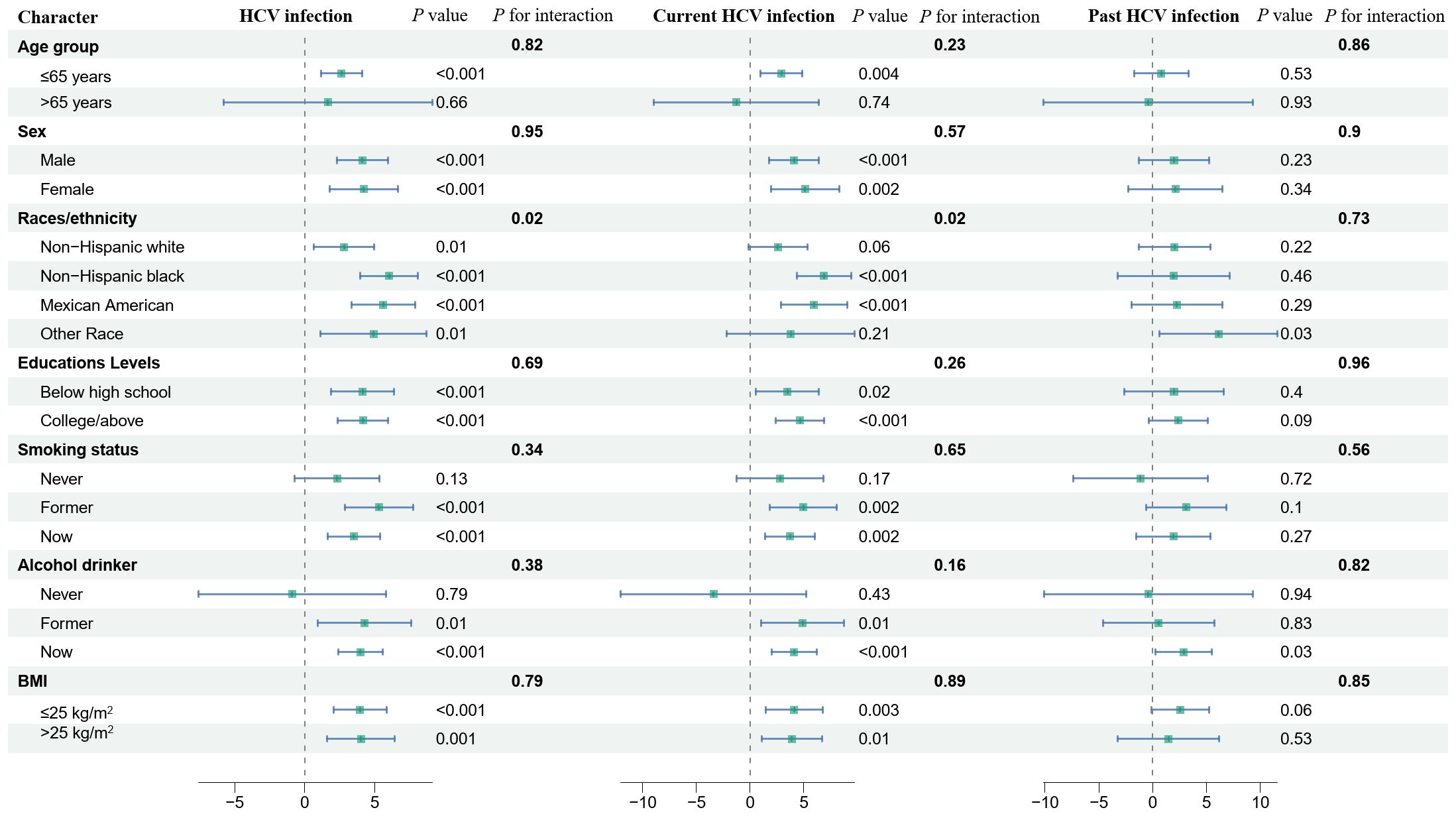
Figure 2 Association between HCV infection and hypertension, systolic blood pressure, and diastolic blood pressure exposure in stratified analyses. The model was adjusted for age, sex, race/ethnicity, and education levels, marital, smoking status, alcohol drinker, hypoglycemic medications, antihypertensive medications, lipid-lowering medications, diabetes, stroke, ASCVD, CKD, hyperlipidemia, BMI, ALT, AST, albumin, bilirubin total, alkaline phosphatase, fast total cholesterol, HDL-C. NHANES, National Health and Nutrition Examination Survey; HCV, hepatitis C virus; SBP, systolic blood pressure; DBP, diastolic blood pressure; BMI, body mass index; ASCVD, atherosclerotic cardiovascular disease; CKD, chronic kidney disease; AST, aspartate aminotransferase; ALT, alanine aminotransferase; HDL-C, high-density lipoprotein cholesterol; OR, odds ratio; CI, confidence interval.
4 Discussion
In this large cross-sectional study, we investigated the association between HCV infection status and BP in US adults. Our study found that HCV infection (anti-HCV positive) and current HCV infection were associated with increased DBP but not with hypertension or SBP among US adults, after adjusting for important potential confounders. However, past HCV infection was not associated with hypertension, SBP, or DBP. Our study suggests that HCV infection increases DBP, but clearance of the virus reversed the association.
Our study showed that HCV infection was not associated with hypertension. This conclusion is supported by the findings of Gadallah M (Gadallah et al., 2018). However, another study by Z.M. Younososi et al. came to the opposite conclusion (Chronic HCV patients have a 106% increased risk of hypertension), which may be related to the different adjusted variables (Younossi et al., 2013). The study only adjusted for the variables of age, race, and obesity. However, other confounding factors, such as total bilirubin, ALT, alkaline phosphatase, and AST may also affect blood pressure. Studies have shown that total bilirubin, ALT, alkaline phosphatase, and AST in people with HCV infection are significantly different from those without HCV infection (Arase et al., 2003; Eminler et al., 2014) and that these factors are also associated with BP (Ramsay, 1977; Kunutsor et al., 2017; Kuchulakanti et al., 2020). Our study added these confounders to make the results more reliable. In the study, we also found a correlation between HCV infection and DBP. The mechanism of correlation between HCV infection and DBP is currently unknown. HCV infection can also cause hepatic necroinflammation, atherosclerosis, and vascular cirrhosis (Di Pietro et al., 2017; Sevastianos et al., 2020). This metabolic change may affect blood pressure by altering the function and structure of blood vessels (Wang et al., 2024). In addition, a cross-sectional study showed that HCV-infected individuals had a higher 24-hour mean heart rate than non-HCV infection (Poliwczak et al., 2020). Higher heart rate, arteriosclerosis, and vascular sclerosis have been associated with increased DBP (Koskela et al., 2013; Wen et al., 2015).
Although some studies have suggested that some of the drugs used to treat HCV may lead to increased BP (Gamal Eldeen et al., 2022). However, the results of our study found no difference in BP (hypertension, SBP, and DBP) between those with past HCV infection and non-HCV infection. This one is an important finding. DBP elevation can be improved after virus clearance in HCV patients. Previous studies have shown that the prevalence of hypertension is reduced in patients with antiviral therapy compared to patients with HCV infection who do not use antiviral therapy (Carrat et al., 2019). The following possibilities exist for the potential mechanisms by which hepatitis C patients can reduce DBP after clearance of the virus. Firstly, one possible reason to consider is the reduction of HCV-induced liver inflammation and fibrosis after HCV antiviral therapy, which improves liver function (Shigefuku et al., 2016). Healthy liver function is essential for maintaining normal BP, as it is involved in the regulation of water and salt balance in the kidneys and in the release of some important in vivo regulators such as angiotensin (Henriksen et al., 2006; Møller and Henriksen, 2008). In addition, the treatment of HCV usually requires patients to follow a healthy lifestyle that includes abstaining from alcohol and smoking, eating a balanced diet, and exercising in moderation (Nobili et al., 2011; Sublette et al., 2013). These lifestyle changes may lead to an improvement in the patient’s overall health, thereby reducing the risk of elevated BP (Samadian et al., 2016; Valenzuela et al., 2021).
The results of this study have important implications. Firstly, the health benefits of clearing the HCV for hepatitis C patients were confirmed, especially the reduction of DBP. Secondly, in patients with hypertension, and especially co-infected with HCV, the treatment of HCV may have a positive impact on BP management and mitigate their cardiovascular risk. Moreover, this result also highlights the important role of the liver in regulating BP, furthering the understanding of the interrelationship between the liver and the circulatory system.
There are many strengths in this study. Strengths of the current study include a prospective study design, a relatively large sample size, and the use of a nationally representative sample of adults in the US, which helps in the generalizability of our findings. Despite this, there are limitations to our study. Although we were able to adjust for the major risk factors identified in the previous studies for HCV infection and hypertension, the possibility of residual confounding cannot be ruled out. A small proportion of HCV antibody-positive participants were not tested for HCV RNA, and this may have had an impact on our assessment of the association between current HCV and past HCV infection and BP. The NHANES does not sample institutionalized or homeless individuals, who are expected to have higher HCV prevalence. This research is a cross-sectional study, so a causal association between elevated DBP and HCV infection was not demonstrated.
4.1 Conclusions
In conclusion, we found a correlation between HCV infection and current HCV infection with increased DBP, but not with hypertension and SBP in US adults. Hypertension, as a major co-morbidity in HCV-infected patients (Sicras-Mainar et al., 2018), contributes to rising costs for the healthcare system and society. Our findings provide a basis for education and interventions to prevent and better control hypertension.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: Research data can be obtained from https://www.cdc.gov/nchs/nhanes/.
Ethics statement
The studies involving humans were approved by This study does not contain any studies with human participants or animals performed by any of the authors. This study only involved secondary data analysis of the existing NHANES database, which is publicly available and has been de-identified, thus there is no need to apply an IRB approval from our institution.. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
FY: Data curation, Formal Analysis, Investigation, Methodology, Software, Visualization, Writing – original draft. JL: Data curation, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Arase, Y., Ikeda, K., Tsubota, A., Suzuki, Y., Saitoh, S., Kobayashi, M., et al. (2003). Serum levels of gamma-globulin and total bilirubin influence the prevalence of multiple extrahepatic complication in patients with hepatitis C virus infection. Hepatol. Res. 25, 14–21. doi: 10.1016/S1386-6346(02)00149-3
Butt, A. A., Khan, U. A., McGinnis, K. A., Skanderson, M., Kent Kwoh, C. (2007). Co-morbid medical and psychiatric illness and substance abuse in HCV-infected and uninfected veterans. J. Viral Hepat. 14, 890–896. doi: 10.1111/j.1365-2893.2007.00885.x
Carrat, F., Fontaine, H., Dorival, C., Simony, M., Diallo, A., Hezode, C., et al. (2019). Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet 393, 1453–1464. doi: 10.1016/S0140-6736(18)32111-1
Chung, J. W., Choi, H. Y., Ki, M., Jang, E. S., Jeong, S. H. (2021). Comorbidities and prescribed medications in Korean patients with chronic hepatitis C: A nationwide, population-based study. Gut Liver. 15, 295–306. doi: 10.5009/gnl19387
Di Pietro, M., Filardo, S., Falasca, F., Turriziani, O., Sessa, R. (2017). Infectious agents in atherosclerotic cardiovascular diseases through oxidative stress. Int. J. Mol. Sci. 18, 2459. doi: 10.3390/ijms18112459
El-Ghitany, E. M., Abdel Wahab, M. M., Abd El-Wahab, E. W., Hassouna, S., Farghaly, A. G. (2015). A comprehensive hepatitis C virus risk factors meta-analysis (1989–2013): do they differ in Egypt? Liver Int. 35, 489–501. doi: 10.1111/liv.12617
Eminler, A. T., Irak, K., Ayyildiz, T., Keskin, M., Kiyici, M., Gurel, S., et al. (2014). The relation between liver histopathology and GGT levels in viral hepatitis: more important in hepatitis B. Turk J. Gastroenterol. 25, 411–415. doi: 10.5152/tjg
Forouzanfar, M. H., Liu, P., Roth, G. A., Ng, M., Biryukov, S., Marczak, L., et al. (2017). Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990–2015. JAMA 317, 165–182. doi: 10.1001/jama.2016.19043
Gadallah, M., Kandil, S., Mohsen, A. (2018). Association between hepatitis C infection and cerebro-cardiovascular disease: analysis of a national population-based survey in Egypt. Trop. Med. Int. Health 23, 738–747. doi: 10.1111/tmi.13068
Gamal Eldeen, H., Hassany, M., Elakel, W., AbdAllah, M., Abdel-Razek, W., Elshazly, Y., et al. (2022). Seroprevalence of HBV/HCV coinfection among patients with HCV screened during the national campaign for HCV eradication in Egypt. Arab J. Gastroenterol. 23, 259–262. doi: 10.1016/j.ajg.2022.06.006
González-Reimers, E., Quintero-Platt, G., Martín-González, C., Pérez-Hernández, O., Romero-Acevedo, L., Santolaria-Fernández, F. (2016). Thrombin activation and liver inflammation in advanced hepatitis C virus infection. World J. Gastroenterol. 22, 4427–4437. doi: 10.3748/wjg.v22.i18.4427
Güven, A. T. (2023). Evaluation of the relationship between inflammatory, metabolic, and liver-related indexes and blood pressure dipping ratios: A retrospective study. Niger J. Clin. Pract. 26, 1886–1894. doi: 10.4103/njcp.njcp_510_23
Guzik, T. J., Touyz, R. M. (2017). Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension 70, 660–667. doi: 10.1161/HYPERTENSIONAHA.117.07802
Henriksen, J. H., Fuglsang, S., Bendtsen, F., Møller, S. (2006). Arterial hypertension in cirrhosis: arterial compliance, volume distribution, and central haemodynamics. Gut 55, 380–387. doi: 10.1136/gut.2005.064329
Kanda, T., Goto, T., Hirotsu, Y., Moriyama, M., Omata, M. (2019). Molecular mechanisms driving progression of liver cirrhosis towards hepatocellular carcinoma in chronic hepatitis B and C infections: A review. Int. J. Mol. Sci. 20, 1358. doi: 10.3390/ijms20061358
Konukoglu, D., Uzun, H. (2017). Endothelial dysfunction and hypertension. Adv. Exp. Med. Biol. 956, 511–540. doi: 10.1007/5584_2016_90
Koskela, J. K., Tahvanainen, A., Haring, A., Tikkakoski, A. J., Ilveskoski, E., Viitala, J., et al. (2013). Association of resting heart rate with cardiovascular function: a cross-sectional study in 522 Finnish subjects. BMC Cardiovasc. Disord. 13, 102. doi: 10.1186/1471-2261-13-102
Kuchulakanti, P. K., Chaudhuri, J. R., Annad, U., Samala, N. R., Tallapaneni, L., Balaraju, B., et al. (2020). Association of serum 25-hydroxyvitamin D levels with primary hypertension: a study from south India. Hypertens. Res. 43, 389–395. doi: 10.1038/s41440-020-0394-4
Kunutsor, S. K., Kieneker, L. M., Burgess, S., Bakker, S. J. L., Dullaart, R. P. F. (2017). Circulating total bilirubin and future risk of hypertension in the general population: the prevention of renal and vascular end-stage disease (PREVEND) prospective study and a Mendelian randomization approach. J. Am. Heart Assoc. 6, e006503. doi: 10.1161/JAHA.117.006503
Kuo, S. H., Hung, W. T., Tang, P. L., Huang, W. C., Yang, J. S., Lin, H. C., et al. (2018). Impact of hepatitis C virus infection on long-term mortality after acute myocardial infarction: a nationwide population-based, propensity-matched cohort study in Taiwan. BMJ Open 8, e017412. doi: 10.1136/bmjopen-2017-017412
Laboratory Procedures Manual. Available online at: https://idghocbbahafpfhjnfhpbfbmpegphmmp/assets/pdf/web/viewer.html?file=https%3A%2F%2Fwwwn.cdc.gov%2Fnchs%2Fdata%2Fnhanes%2F2009–2010%2Fmanuals%2Flab.pdf.
Lazo, M., Nwankwo, C., Daya, N. R., Thomas, D. L., Mehta, S. H., Juraschek, S., et al. (2017). Confluence of epidemics of hepatitis C, diabetes, obesity, and chronic kidney disease in the United States population. Clin. Gastroenterol. Hepatol. 15, 1957–1964.e7. doi: 10.1016/j.cgh.2017.04.046
Levey, A. S., Coresh, J., Greene, T., Stevens, L. A., Zhang, Y. L., Hendriksen, S., et al. (2006). Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann. Intern. Med. 145, 247–254. doi: 10.7326/0003-4819-145-4-200608150-00004
Møller, S., Henriksen, J. H. (2008). Cardiovascular complications of cirrhosis. Gut 57, 268–278. doi: 10.1136/gut.2006.112177
Morales, J. M., Kamar, N., Rostaing, L. (2012). Hepatitis C and renal disease: epidemiology, diagnosis, pathogenesis and therapy. Contrib Nephrol. 176, 10–23. doi: 10.1159/issn.0302-5144
Muntner, P., Carey, R. M., Gidding, S., Jones, D. W., Taler, S. J., Wright, J. T., et al. (2018). Potential U.S. Population impact of the 2017 ACC/AHA high blood pressure guideline. J. Am. Coll. Cardiol. 71, 109–118. doi: 10.1016/j.jacc.2017.10.073
Murphy, D., McCulloch, C. E., Lin, F., Banerjee, T., Bragg-Gresham, J. L., Eberhardt, M. S., et al. (2016). Trends in prevalence of chronic kidney disease in the United States. Ann. Intern. Med. 165, 473–481. doi: 10.7326/M16-0273
Murray, C. J. L., Atkinson, C., Bhalla, K., Birbeck, G., Burstein, R., Chou, D., et al. (2013). The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA 310, 591–608. doi: 10.1001/jama.2013.13805
NHANES Tutorials - Weighting Module. Available online at: https://wwwn.cdc.gov/nchs/nhanes/tutorials/weighting.aspx.
NHANES Tutorials - Variance Estimation Module. Available online at: https://wwwn.cdc.gov/nchs/nhanes/tutorials/VarianceEstimation.aspx.
NHANES (2011–2012) Hepatitis C: Confirmed Antibody, RNA (HCV-RNA), & Genotype Data Documentation, Codebook, and Frequencies. Available online at: https://wwwn.cdc.gov/Nchs/Nhanes/2011–2012/HEPC_G.htm#LBDHCV.
NHANES (2013–2014) Hepatitis C virus RNA in Serum. Available online at: https://idghocbbahafpfhjnfhpbfbmpegphmmp/assets/pdf/web/viewer.html?file=https%3A%2F%2Fwwwn.cdc.gov%2Fnchs%2Fdata%2Fnhanes%2F2013–2014%2Flabmethods%2FHEPC_H_met_Hepatitis_C_virus_RNA.pdf.
NHANES (2022) NCHS Research Ethics Review Board Approval. Available online at: https://www.cdc.gov/nchs/nhanes/irba98.htm.
Nobili, V., Carter-Kent, C., Feldstein, A. E. (2011). The role of lifestyle changes in the management of chronic liver disease. BMC Med. 9, 70. doi: 10.1186/1741-7015-9-70
Ostchega, Y., Prineas, R. J., Paulose-Ram, R., Grim, C. M., Willard, G., Collins, D. (2003). National Health and Nutrition Examination Survey 1999–2000: effect of observer training and protocol standardization on reducing blood pressure measurement error. J. Clin. Epidemiol. 56, 768–774. doi: 10.1016/S0895-4356(03)00085-4
Petta, S., Macaluso, F. S., Craxì, A. (2014). Cardiovascular diseases and HCV infection: a simple association or more? Gut 63, 369–375. doi: 10.1136/gutjnl-2013-306102
Pietschmann, T., Brown, R. J. P. (2019). Hepatitis C virus. Trends Microbiol. 27, 379–380. doi: 10.1016/j.tim.2019.01.001
Poliwczak, A. R., Białkowska, J., Woźny, J., Koziróg, M., Bała, A., Jabłkowski, M. (2020). Cardiovascular risk assessment by electrocardiographic Holter monitoring in patients with chronic hepatitis C. Arch. Med. Sci. 16, 1031–1039. doi: 10.5114/aoms.2020.96600
Ramsay, L. E. (1977). Liver dysfunction in hypertension. Lancet 2, 111–114. doi: 10.1016/S0140-6736(77)90121-0
Ruzicka, D. J., Tetsuka, J., Fujimoto, G., Kanto, T. (2018). Comorbidities and co-medications in populations with and without chronic hepatitis C virus infection in Japan between 2015 and 2016. BMC Infect. Dis. 18, 237. doi: 10.1186/s12879-018-3148-z
Samadian, F., Dalili, N., Jamalian, A. (2016). Lifestyle modifications to prevent and control hypertension. Iran J. Kidney Dis. 10, 237–263.
Sevastianos, V. A., Voulgaris, T. A., Dourakis, S. P., Hepatitis, C. (2020). systemic inflammation and oxidative stress: correlations with metabolic diseases. Expert Rev. Gastroenterol. Hepatol. 14, 27–37. doi: 10.1080/17474124.2020.1708191
Shigefuku, R., Takahashi, H., Nakano, H., Watanabe, T., Matsunaga, K., Matsumoto, N., et al. (2016). Correlations of hepatic hemodynamics, liver function, and fibrosis markers in nonalcoholic fatty liver disease: comparison with chronic hepatitis related to hepatitis C virus. Int. J. Mol. Sci. 17, 1545. doi: 10.3390/ijms17091545
Sicras-Mainar, A., Navarro-Artieda, R., Sáez-Zafra, M. (2018). Comorbidity, concomitant medication, use of resources and healthcare costs associated with chronic hepatitis C virus carriers in Spain. Gastroenterol. Hepatol. 41, 234–244. doi: 10.1016/j.gastrohep.2017.11.008
Sublette, V. A., Douglas, M. W., McCaffery, K., George, J., Perry, K. N. (2013). Psychological, lifestyle and social predictors of hepatitis C treatment response: a systematic review. Liver Int. 33, 894–903. doi: 10.1111/liv.12138
Udompap, P., Mannalithara, A., Heo, N. Y., Kim, D., Kim, W. R. (2016). Increasing prevalence of cirrhosis among U.S. adults aware or unaware of their chronic hepatitis C virus infection. J. Hepatol. 64, 1027–1032. doi: 10.1016/j.jhep.2016.01.009
Valenzuela, P. L., Carrera-Bastos, P., Gálvez, B. G., Ruiz-Hurtado, G., Ordovas, J. M., Ruilope, L. M., et al. (2021). Lifestyle interventions for the prevention and treatment of hypertension. Nat. Rev. Cardiol. 18, 251–275. doi: 10.1038/s41569-020-00437-9
Wang, T. J., Chen, M. Y., Lin, Y. C., Chiu, W. N., Huang, T. J., Weng, H. H. (2024). High prevalence of fatty liver and its association with metabolic syndrome among rural adults with chronic hepatitis C: Implications for primary healthcare. BMC Public Health 24, 532. doi: 10.1186/s12889-024-17851-0
Wang, L., Li, X., Wang, Z., Bancks, M. P., Carnethon, M. R., Greenland, P., et al. (2021). Trends in prevalence of diabetes and control of risk factors in diabetes among US adults, 1999–2018. JAMA 326, 1–13. doi: 10.1001/jama.2021.9883
Wen, W., Luo, R., Tang, X., Tang, L., Huang, H. X., Wen, X., et al. (2015). Age-related progression of arterial stiffness and its elevated positive association with blood pressure in healthy people. Atherosclerosis 238, 147–152. doi: 10.1016/j.atherosclerosis.2014.10.089
Yeung, C. Y., Lee, H. C., Chan, W. T., Jiang, C. B., Chang, S. W., Chuang, C. K. (2014). Vertical transmission of hepatitis C virus: Current knowledge and perspectives. World J. Hepatol. 6, 643–651. doi: 10.4254/wjh.v6.i9.643
Keywords: hepatitis C virus, hypertension, diastolic blood pressure, systolic blood pressure, NHANES (National Health and Nutrition Examination Survey)
Citation: Yang F and Luo J (2024) The association between hepatitis C virus infection status and blood pressure in adults in the United States: NHANES 1999–2012. Front. Cell. Infect. Microbiol. 14:1401323. doi: 10.3389/fcimb.2024.1401323
Received: 15 March 2024; Accepted: 22 May 2024;
Published: 04 June 2024.
Edited by:
Jiabin Tu, Longyan First Hospital Affiliated to Fujian Medical University, ChinaReviewed by:
Enmin Xie, Guangdong Provincial People’s Hospital, ChinaJian Li, Fudan University, China
Copyright © 2024 Yang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianping Luo, bHVvamlhbnBpbmdAbWFpbC5nenNybXl5LmNvbQ==
 Feng Yang
Feng Yang Jianping Luo
Jianping Luo