
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 06 November 2024
Sec. Molecular Bacterial Pathogenesis
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1396762
This article is part of the Research TopicMetabolomics in Bacterial InfectionsView all 5 articles
 Zhen Zeng1
Zhen Zeng1 Meng Li1
Meng Li1 Simin Zhu1
Simin Zhu1 Ke Zhang1
Ke Zhang1 Yifan Wu1
Yifan Wu1 Minzi Zheng1
Minzi Zheng1 Yang Cao1
Yang Cao1 Zhenyu Huang1
Zhenyu Huang1 Qinping Liao1*
Qinping Liao1* Lei Zhang1,2*
Lei Zhang1,2*Introduction: GBS (group B streptococcus) is an opportunistic pathogen that can colonize healthy individuals but presents significant challenges in clinical obstetrics and gynecology, as it can cause miscarriage, preterm birth, and invasive infections in newborns. To develop specific and personalized preventative strategies, a better understanding of the epidemiological characteristics and pathogenic features of GBS is essential.
Methods: We conducted a comprehensive strain-level genomic analysis of GBS, examining serotype and genotype distributions, as well as the composition and correlations of virulence genes using the blastn-short mode of the BLAST program(v2.10.0+), mlstsoftware (https://github.com/tseemann/mlst), Snippy (v4.6.0), FastTree (v2.1.11) and iTOL. The coding sequence region of virulence factors was annotated by Prodigal (v2.6.3) and Glimmer(v3.02b). We further identified host protein interacting with Srr2 by mass spectrometry analysis.
Results: While certain genotypes showed strong serotype consistency, there was no significant association between overall serotypes and genotypes. However, the composition of virulence genes was more closely related to the phylogeny of GBS, among which simultaneous presence of Srr2 and HygA exhibit significant association with hypervirulence. Tubulin emerged as the most distinct and abundant hit. The specific interaction of Tubulin with Srr2-BR, rather than Srr1-BR, was further confirmed by immunoblotting.
Discussion: Considering the impact of cytoskeleton rearrangement on GBS pathogenesis, this observation offers a plausible explanation for the hypervirulence triggered by Srr2. Collectively, our findings indicate that in the future clinical practice, virulence gene detection should be given more attention to achieve precise GBS surveillance and disease prevention.
Streptococcus agalactiae, commonly referred to as Lancefield group B streptococcus (GBS), is a Gram-positive coccus that was initially recognized as a causative agent of bovine mastitis. It is commonly found in the gastrointestinal and genital tracts of healthy individuals. GBS can cause various infections, particularly in newborns, pregnant women, the elderly, and immunocompromised individuals (Verani et al., 2010). During pregnancy and childbirth, it is important to consider the presence of GBS in the vaginal and rectal regions, as it can increase the risk of ascending infection, potentially leading to preterm birth and stillbirth (Hillier et al., 1991; Allen et al., 1999). Furthermore, GBS may cause serious infections such as neonatal sepsis, pneumonia, and meningitis through mother-to-child transmission during delivery. GBS is also implicated in a number of other infections, including soft-tissue and urinary tract infections, arthritis, sepsis, endometritis and aerobic vaginitis (Jackson et al., 1995; Krohn et al., 1999; Zeng et al., 2023).
GBS typing can elucidate differences in pathogenicity, antibiotic resistance, and genetic diversity among strains, facilitating epidemiological surveillance and the development of more effective interventions (Furfaro et al., 2018). Based on the composition of capsular polysaccharide, a total of 10 serotypes, namely Ia, Ib, and II-IX, have been identified (Cieslewicz et al., 2005). Approximately 98% of the serotypes identified during maternal colonization and 97% of those identified during neonatal disease are estimated to be encompassed by six serotypes (Ia, Ib, II, III, IV and V). Among them, serotype-III is relatively clearly associated with invasive disease in newborns while serotypesIa and V are the dominant invasive isolates in non-pregnant cases (Alhhazmi et al., 2016). The prevalence of serotypes in maternal colonization or neonatal disease varies significantly by regions on a global scale and shows no clear pattern (Madrid et al., 2017). With multilocus sequence typing (MLST), GBS can be classified into more than five hundred of sequence types (STs) and then can be clustered into clonal complexes (CCs) (Furfaro et al., 2018). The most prevalent STs include ST-17, ST-1, ST-23, ST-19, ST-10, ST-8, ST-484, ST-182, ST-28, ST-12, ST-24, and ST-7, which corresponds 5 CCs (CC-17, CC-1, CC-23, CC-19, CC-10) (Furfaro et al., 2018). ST-1, ST-17, and ST-19 were reported to be more frequently associated with invasive neonatal disease (Bisharat et al., 2005; Kang et al., 2017).
A series of virulence factors are encoded by GBS to facilitate vaginal colonization and the occurrence of ascending infection. These virulence factors mainly function in adhesion, invasion, immune evasion, and environmental adaptation (Vornhagen et al., 2017). Adherence and invasion can be mediated by interactions between surface adhesin protein of GBS and host cell and extracellular matrix components (ECMs) (Landwehr-Kenzel and Henneke, 2014; Shabayek and Spellerberg, 2018). Functionally characterized adhesins that mediate GBS adhesion and/or host invasion include fibrinogen binding protein (Fbs), laminin binding protein (Lmb), group B streptococcal C5a peptidase (ScpB), streptococcal fibronectin protein binding protein A (SfbA), GBS immunogenic bacterial adhesin (BibA), host cell surface glycosaminoglycan binding protein (Alpha C protein), and pili (Shabayek and Spellerberg, 2018). These interactions may also promote GBS resistance to mechanical clearance, avoidance of immune surveillance, and paracellular transmigration. In addition to adhesion-related virulence factors, hyaluronidase (HylB), hemolysin, and capsule play significant roles as virulence factors in GBS. These factors have been reported to contribute to GBS colonization in the vagina, ascending infection, immune evasion, placental invasion, and fetal injury (Whidbey et al., 2013; Kolar et al., 2015; Vornhagen et al., 2016).
Currently, the primary clinical strategy against GBS involves universal culture-based screening for maternal colonization at 35 to 37 weeks of gestation and prophylactic antibiotic administration (Prevention of perinatal group B streptococcal disease: a public health perspective. Centers for Disease Control and Prevention, 1996; Schrag et al., 2002; Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion, number 782, 2019). There are also studies exploring the use of nucleic acid amplification tests (NAAT) for GBS screening. Compared to culture methods, NAAT offers comparable sensitivity and specificity. However, concerns remain about its high cost and inability to perform antibiotic susceptibility testing. Consequently, NAAT has not been widely adopted or validated in clinical practice (Church et al., 2017). With current strategies to screen for GBS colonization status, the incidence of neonatal GBS early-onset disease (EOD) was reduced to significantly. However, the biological characteristics of GBS infection are not well characterized by this approach, and the relationship between GBS and normal colonization and infection is not well established (Vornhagen et al., 2017). Currently, the comprehensive burden of GBS infection, including GBS late-onset disease (LOD), is not fully addressed. Furthermore, intrapartum antibiotic prophylaxis faces challenges such as antibiotic resistance and the increased incidence of non-GBS neonatal pathogens (Castor et al., 2008).
In this study, we analyzed the composition of GBS virulence genes and their correlation with various GBS genotyping methods on a global scale. Our goal is to provide references for clinical monitoring and disease prevention of GBS, as well as uncover insights into the pathogenesis and process of GBS. Through the analysis of GBS virulence genes, we summarized the distribution of different virulence genes in GBS, and 78% of the 1,552 genomes were categorized into 14 groups. We found that the distribution of virulence genes in GBS serotypesIII and ST-17 was obviously homogeneous, and compared with non-human GBS, especially fish-derived GBS, human GBS had more virulence genes. Besides, to augment the biological evidence for current study, we purified the binding regions of Srr1 and Srr2, and compared their host interactors by mass spectrometry analysis. Significantly, the microtubule cytoskeleton protein Tubulin was found to specifically interact with Srr2-BR, suggesting that the observed binding affinity of Tubulin may provide a mechanistic explanation for the specific induction of hypervirulence by Srr2-BR. Taken together, this information holds potential benefits in clinical settings, providing valuable insights into the characteristics of GBS infection and informing disease prevention strategies.
Genome sequences was collected from the NCBI Reference Sequence Database. The average nucleotide identity (ANI) to type strains (S. agalactiae NCTC 8181, GCF_900458965.1) was analyzed using fastANI (v1.33) to confirm the taxonomic status of the genomes (Jain et al., 2018). The quality of genome assemblies was evaluated using BUSCO (v5.4.7) (Manni et al., 2021).
Refer to the previous study of Breeding et al. to obtain the specific target sequences of different GBS serotypes(Ia, Ib, II, III, IV, V, VI, VII, VIII). Then these sequences were aligned to the genome using the blastn-short mode of the BLAST program(v2.10.0+) for serotyping (Breeding et al., 2016). MLST and single nucleotide polymorphism (SNP) were conducted using mlstsoftware (https://github.com/tseemann/mlst) and Snippy (v4.6.0), respectively (Jolley and Maiden, 2010; Silva et al., 2018). Phylogenetic trees based on SNP and annotations were constructed with FastTree (v2.1.11) and iTOL (Price et al., 2010; Letunic and Bork, 2021).
Fifty-eight coding genes associated with fifteen virulence factors were used as reference sequences for downstream analysis (Table 1) (Jerlstrom et al., 1996; Bohnsack et al., 1997; Gravekamp et al., 1998; Areschoug et al., 2002; Spellerberg et al., 2002; Baron et al., 2004; Gutekunst et al., 2004; Brown et al., 2005; Pietrocola et al., 2005; Bolduc and Madoff, 2007; Forquin et al., 2007; Pannaraj et al., 2007; Tenenbaum et al., 2007; Carlin et al., 2009; Klinzing et al., 2009; Devi and Ponnuraj, 2010; Tazi et al., 2010; Al Safadi et al., 2011; Sheen et al., 2011; Pezzicoli et al., 2012; Seo et al., 2012; Seo et al., 2013; Buscetta et al., 2014; Chang et al., 2014a; Chang et al., 2014b; Rosa-Fraile et al., 2014; Maeland et al., 2015; Six et al., 2015; Gabrielsen et al., 2017; Benito-Jardon et al., 2020; Dobrut and Brzychczy-Wloch, 2021; Creti et al., 2023). These virulence factors were reported to be related to GBS colonization, immune evasion and invasion. The coding sequence region was annotated by Prodigal (v2.6.3) and Glimmer(v3.02b), respectively (Delcher et al., 2007; Hyatt et al., 2010). The predicted amino acid and nucleic acid sequence were aligned to the reference virulence gene sequence by Diamond (v2.1.9) and Abricate (v1.0.1) (https://github.com/tseemann/abricate) (Buchfink et al., 2015). When comparing the annotation results of virulence genes obtained by different methods, consistent results were directly interpreted as positive. Inconsistent results were manually corrected to provide the final annotation.
Vaginal and anal swabs were collected from 43 pregnant women at 36~37 + 6 weeks of pregnancy, and samples were taken at the same time. After sampling, GBS was detected by NAAT and selective culture medium. NAAT was performed using registered 7500 real-time fluorescence quantitative PCR kits following the relevant instructions, and the CT value is set to less than 35, which is considered as GBS colonization.
DNA fragments spanning the Srr1-BR and Srr2-BR with Flag tag were synthesized by Sangon Biotech (Shanghai, China), and cloned into pET28a expression vectors. Proteins were expressed in E. coli BL21 Chemically Competent Cell (TransGen Biotech, Beijing, CHN). After treated with 0.5 mM IPTG in 16°C overnight, cells were harvested with buffer A (20 mM Tris–HCl and 50 mM NaCl) and clarified by ultrasonication. Lysates were centrifuged at 12,000 g for 30 min in 4°C. Then the supernatant of the lysates was harvested and incubated with Ni2+ Sepharose for 4 h at 4°C. Subsequently, proteins were purified by Ni2+ sepharose, then concentrated by Amicon Ultra-10 at 4°C. Protein concentration was determined using the Bradford assay with BSA as the standard.
To identify Srr1-BR and Srr2-BR-interacting proteins, two of 10 cm dishes HEK293T cells were lysed in 4 ml of ice-cold buffer containing 20 mM Hepes (pH 7.4), 150 mM NaCl, 0.3% Triton-X-100 and Complete Mini protease inhibitors (EDTAfree, Roche) for a duration of 10 minutes. The resulting lysate was subjected to centrifugation at 5,000 × g for 5 minutes to remove nuclei and insoluble cellular components. The supernatant was collected thereafter. Then 10 μg of Srr1-BR or Srr2-BR along with anti-FLAG M2 agarose were then added to the supernatant in 4°C overnight. Following this step, washing procedures were performed using ice-cold buffer containing 20 mM Hepes (pH 7.4), 150 mM NaCl and 0.1% Triton-X-100. Subsequently, Srr1-BR or Srr2-BR-interacting proteins were eluted using 3x FLAG peptide (150 ng/μl).
The protein bands were subjected to in-gel trypsin digestion, followed by extraction of peptides from the gel matrix and resuspension in an aqueous buffer for subsequent LC-MS/MS analysis.
The eluted proteins that interacted with Srr1-BR or Srr2-BR were resolved on 12% SDS-PAGE gels. Subsequently, the transferred proteins onto PVDF membranes were incubated with specific antibodies (β-Tubulin: proteintech, 10068-1-AP; Flag: Sigma, F1804) and visualized using the super sensitive ECL luminescence kit (# MA0186-1, Meilunbio, Meilun Biotechnology co. Ltd, Dalian, China).
A total of 1,552 genomes with ANI greater than 98% to the reference and genome completeness exceeding 95% were included in the analysis. The GC content of these genomes ranges from 35% to 40%, and their sizes vary between 1.68 Mb and 2.48 Mb. Among these 1,552 genomes, 1,201 GBS strains were isolated from human sources, while 351 genomes originated from non-human sources. The non-human GBS strains were primarily derived from bovine and fish hosts. The primary geographical regions included in this study are Portugal, the United States, France, China, and Korea (Figure 1).
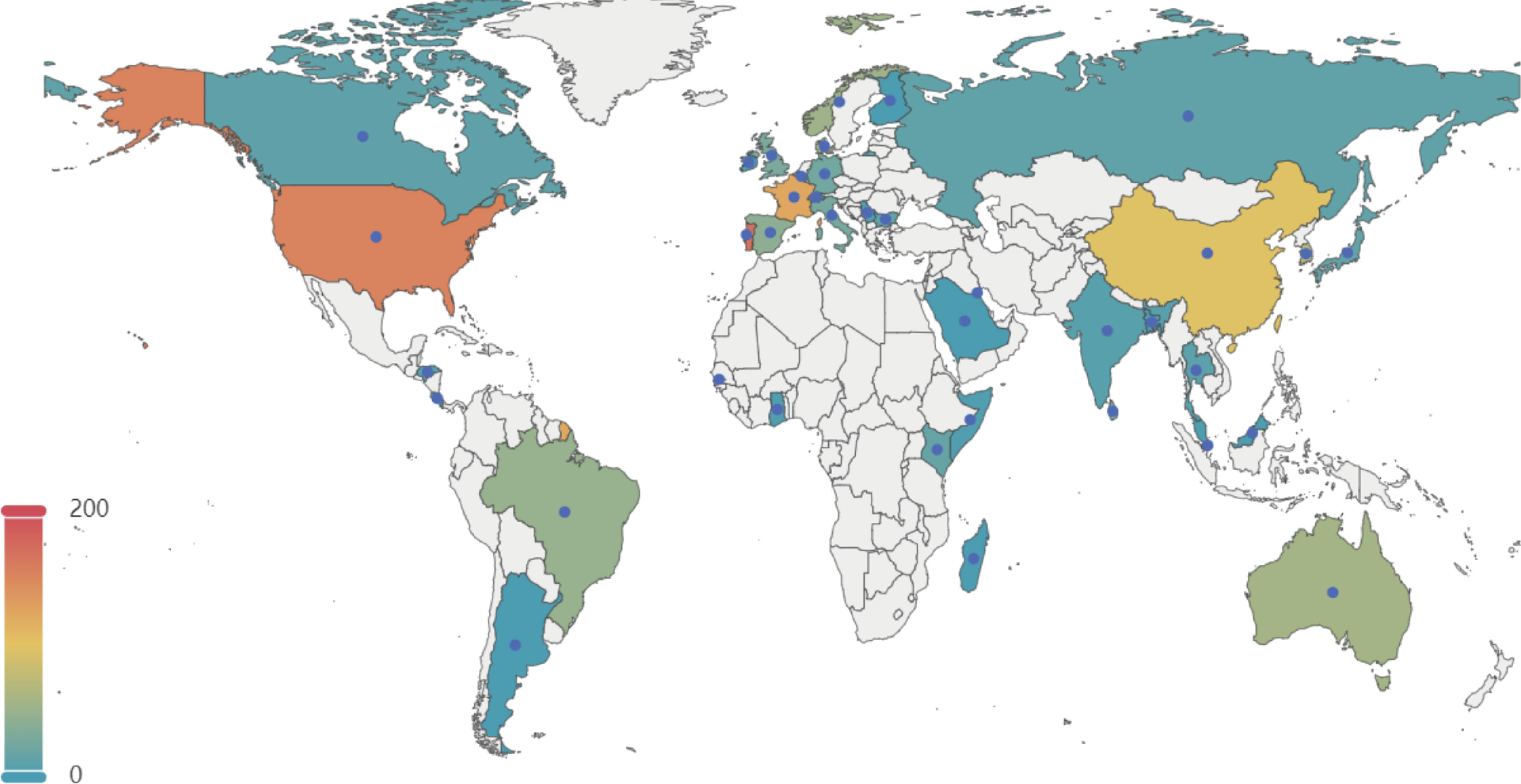
Figure 1. Geographical distribution of 1, 552 GBS strains selected in this study. This map illustrates the global distribution of the 1,552 GBSstrains analyzed in the study. The color gradient represents the number of strains from each country, with warmer colors indicating higher numbers. Key regions of interest include the United States, Portugal, France, China, and Korea, among others. The data points help to visualize the geographical diversity of the GBS strains included in the analysis.
The typing results revealed that 97% (1,500/1,552) of the genomes were classified into nine distinct serotypes, and 92% (1399/1552) were classified into 115 different genotypes. In the GBS strains collected from human sources, the most common serotypes, ranked from highest to lowest, were III, V, Ia, II, and Ib. The most commonly identified genotypes in these strains were ST-1, ST-17, ST-23, ST-19 and ST-12 (Figure 2A). Conversely, the GBS strains from non-human sources most frequently fell into serotypes II, Ib, Ia, III, V and most common genotypes were ST-61, ST-554, ST-261, ST-260, and ST-2 (Figure 2B).
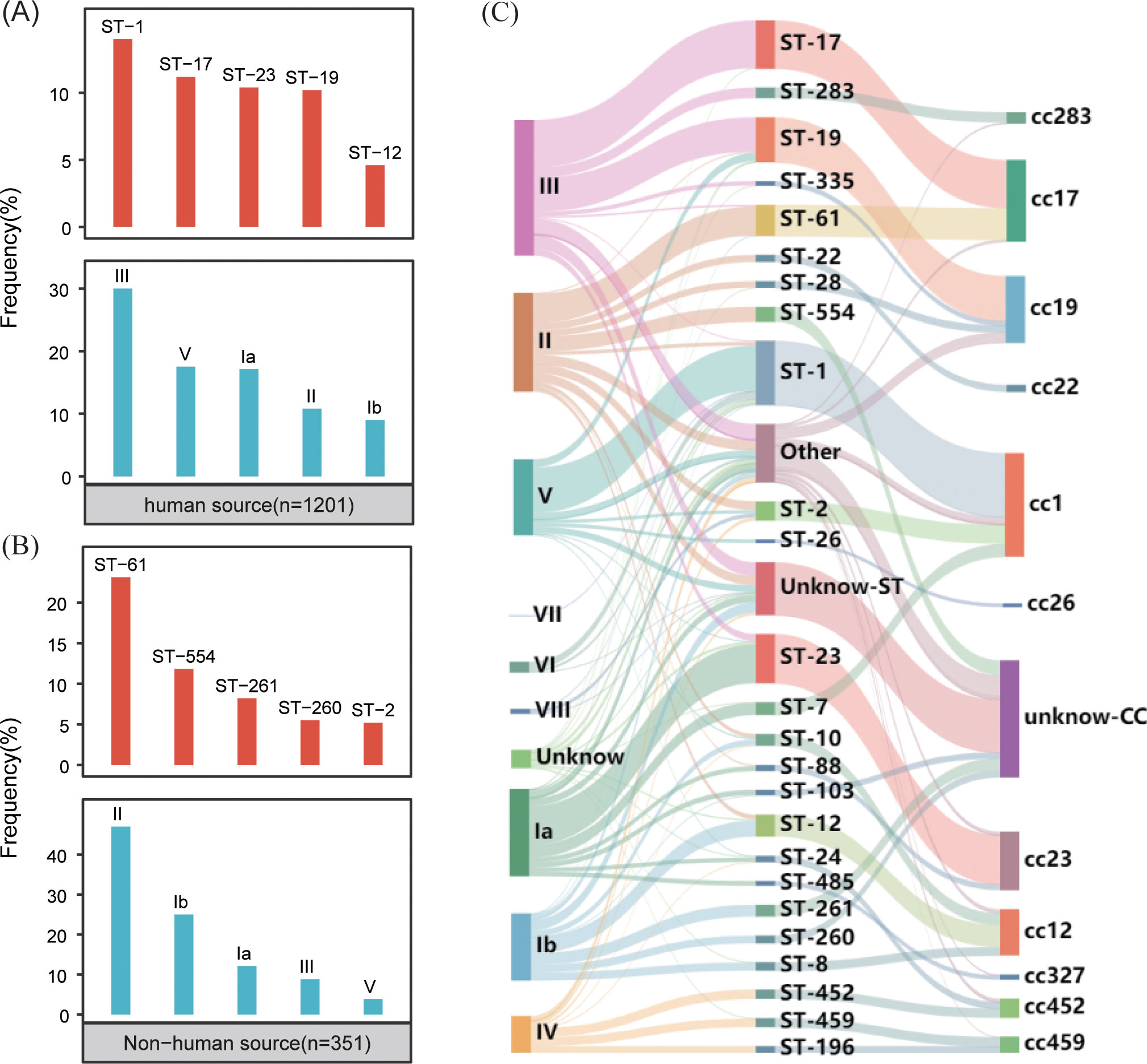
Figure 2. Distribution of GBS serotypes and genotypes. (A) The frequency distribution of the top five sequence types (STs) and serotypes in human-source GBS strains (n=1201). (B) The frequency distribution of the top five sequence types (STs) and serotypes in non-human-source GBS strains (n=351). (C) The correspondence between serotypes and sequence types (STs) in human-source GBS strains, illustrating the relationship between specific serotypes and genotypes.
The correlation analysis across all GBS serotypes and genotypes reveals that GBS type III is primarily associated with ST-17 and ST-19, collectively making up 35.0% (138/394) and 31.2% (123/394) of the total strains respectively. Type II is predominantly linked with ST-61 and ST-554, contributing to 29.4% (84/286) and 15.0% (43/286) respectively. Serotype Ia is mainly associated with ST-23, constituting 44.7% (113/253). Serotype Ib is largely connected with ST-12 and ST-261, together accounting for 24.2% (47/194) and 17.5% (34/194) respectively. Type V is primarily tied to ST-1, making up 70.9% (156/220). While some MLST genotypes such as ST-17 exhibit significant serotype homogeneity and belong to serotypes III, the overall distribution of serotypes and genotypes shows a cross-relationship, as illustrated in Figure 2C.
A phylogenetic analysis based on genome-wide SNPs was performed on all GBS strains. The findings, as illustrated in Figure 3, demonstrated that MLST displayed superior phylogenetic consistency in comparison to serotyping. Regarding geographical dispersion, GBS strains isolated from non-human hosts (primarily bovine) in Portugal exhibited a strong correlation with geographical consistency. However, no discernible new patterns within the geographical distribution of GBS were identified.

Figure 3. Proportional distribution of different virulence genes in human-source GBS and nonhuman-source GBS. This bar graph displays the frequency (%) of various virulence genes in GBS strains from three sources: all genomes (n=1,552), human-source (n=1,201), and non-human-source (n=351). The virulence genes are listed along the x-axis, and their corresponding frequencies are represented on the y-axis. The different colors indicate the source of the GBS strains: blue for all genomes, orange for human-source, and green for non-human-source.
The examination of virulence gene composition across 1,552 genomes has demonstrated variations in the distribution proportions of distinct virulence genes within GBS. As depicted in Figure 4, the genes hylB, cfb, sfbA,fbsC, cyl clusters, fbsA, srr1, scpB, and lmb were ubiquitously distributed in GBS, with the least prevalent gene still being found in over 70% of cases. Intriguingly, the genes hylB, cfb, sfbA, and fbsC were annotated across all genomes analysed. However, a smaller proportion of genomes contained the genes cba, fbsB, hvgA, and srr2, with distribution rates ranging from 10.6% to 26.3%.
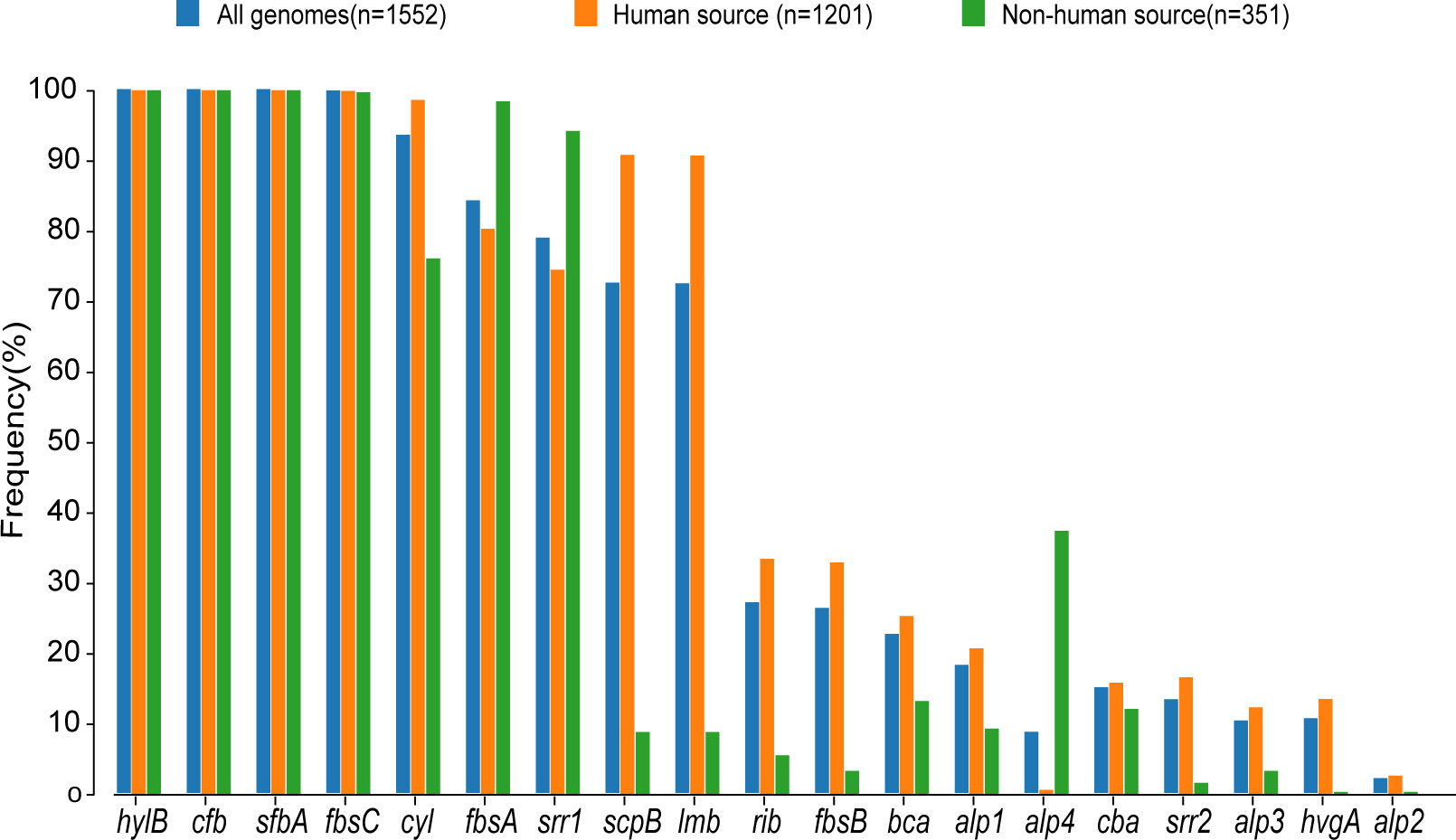
Figure 4. Distribution of CPS gene clusters in different serotypes. This heatmap illustrates the distribution of capsular polysaccharide (CPS) gene clusters across various GBS serotypes. The rows represent different CPS genes, while the columns represent individual GBS strains. The color intensity in each cell reflects the presence (red) or absence (yellow) of a specific CPS gene in a given strain.
Additionally, a comparison between human and non-human hosts revealed disparities in the prevalence of GBS virulence genes. The genes fbsB, hvgA, lmb, scpB, and ssr2 were significantly more common in strains from human hosts, with distribution frequencies exceeding ten folds those in non-human strains. Interestingly, the cyl gene cluster is universally present in genomes from human sources, whereas 30% of genomes from non-human sources lacked this gene cluster. Among these non-human sources, 74% were from fish hosts. A notable finding is that 86% of the GBS strains harbored a single ALP family protein-coding gene, with bca, rib, and alp1 being the most prevalent. Notably, the distribution ratio of rib is higher in human GBS than in non-human GBS, with a discrepancy of more than nine-fold. Moreover, alp4 was predominantly found in non-human GBS. The composition of capsular polysaccharides (CPS) forms the basis for serotyping and represents a significant type of virulence factor. An analysis of the CPS-encoding gene composition across different serotypes -Ia, Ib, II, III, IV, V, VI, and VII - presented in Figure 5, reveals that genes cpsA, cpsB, cpsC,cpsD, cpsE,cpsF, cpsL, neuB, neuC, neuD, and neuA were found and conserved in each GBS serotype. Although the genescpsG, cpsH, cpsJ, and cpsK were also present in various serotypes, their sequences were not conserved. The gene cpsM was found in serotypes IV, V, and VII, but its sequences were not conserved. Similarly, cpsN was found in serotypes IV and V, and its sequences weren’t conserved. The genes cpsP and cpsQ were unique to serotype II, and cpsO was only found in serotype V.
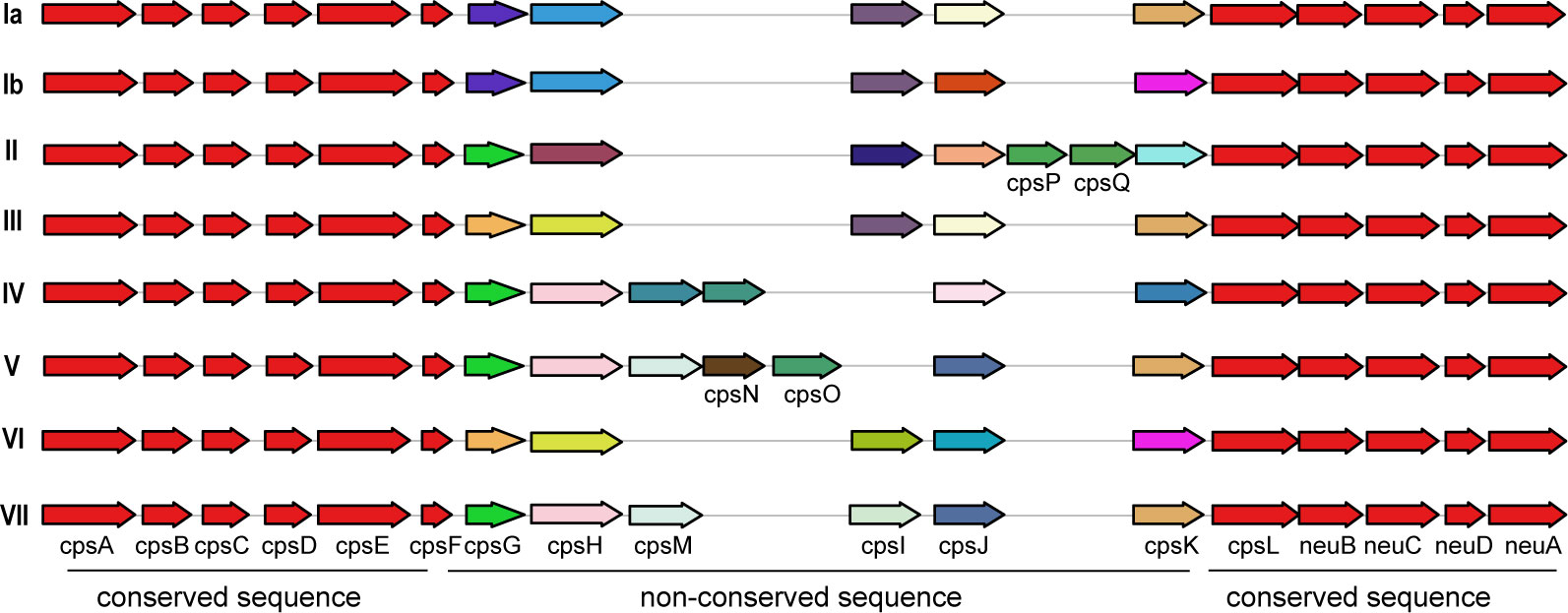
Figure 5. The correlation between the distribution of virulence genes and serotypes and genotypes. This diagram depicts the arrangement of capsular polysaccharide gene clusters in different GBS serotypes (Ia, Ib, II, III, IV, V, and VI). Each row represents a specific serotype, with arrows indicating the direction and identity of individual genes within the CPS gene cluster. Genes that are conserved across all serotypes are shown in red and purple on the left and right ends of each row. Non-conserved sequence: Genes that vary between serotypes are shown in various colors in the middle of each row. The arrangement of these genes highlights the genetic diversity and structural differences in the CPS gene clusters among the GBS serotypes.
A deeper analysis of the connection between the distribution of virulence genes, serotypes, and genotypes was conducted (as shown in the Figure 6). The study found marked differences in the annotations of certain genes in strains from human and non-human hosts. The annotation results of GBS virulence genes in human hosts seemed to significantly outnumber those from non-human hosts. The genes lmb and scpB did not appear to be present in non-human GBS. It’s particularly noteworthy that in ST-17 GBS, the distribution of hvgA and srr2 was clearly specific to this sequence type. Furthermore, the genes cba and bcaappeared to be more readily annotated concurrently in the GBS genome, yet these strains lacked noticeable consistency in the distribution of serotypes or genotypes.
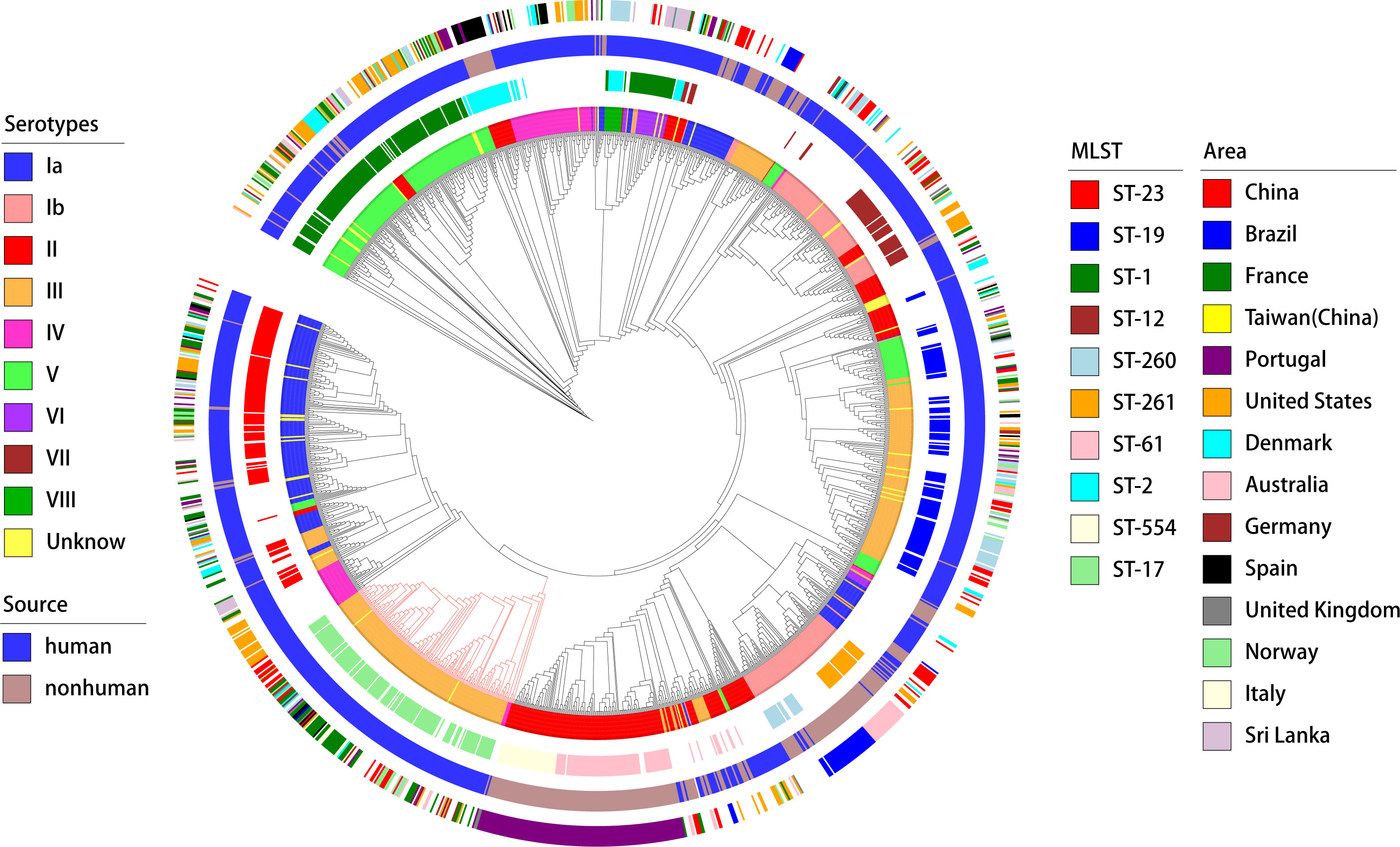
Figure 6. Phylogenetic tree and distribution of GBS strains based on serotypes, sources, sequence types, and geographical areas. This circular phylogenetic tree illustrates the genetic relationships among the GBS strains analyzed in this study. The tree is annotated with multiple concentric rings representing various attributes. Innermost ring (Serotypes): Different colors represent the serotypes of the GBS strains (Ia, Ib, II, III, IV, V, VI, VII, VIII, and Unknown);Second ring (MLST): Multi-locus sequence typing is shown with distinct colors representing different sequence types (e.g., ST-23, ST-19, ST-1, ST-17, ST-54, ST-261, and others);Third ring (Source): The source of the GBS strains is indicated by color, with blue representing human sources and green representing non-human sources; Outermost ring (Area): Geographical regions from which the GBS strains were isolated are indicated by different colors, including China, Brazil, France, Taiwan (China), Portugal, United States, Denmark, Australia, Germany, Spain, United Kingdom, Norway, Italy, and Sri Lanka. The phylogenetic tree provides a comprehensive overview of the genetic diversity and distribution of GBS strains across different serotypes, sources, sequence types, and geographical areas.
Among the 43 patients, the NAAT was positive in 15 cases (34.9%), while the culture method was positive in 3 cases (7.0%). Both methods were positive in 3 cases, and the NAAT was positive but the culture method negative in 12 cases. Notably, GBS was not detected by the culture method in one patient; however, the NAAT was positive, and the newborn was subsequently transferred to the pediatric department due to infection. In total, 3 out of 43 newborns were transferred to the pediatric department.
In order to explore host factors responsible for the hypervirulence of GBS strains with Srr2 compared to those with Srr1, we identified and compared potential host proteins interacting with them. To achieve this, we expressed and purified the binding regions of Srr1 (Srr1-BR) and Srr2 (Srr2-BR) (Figure 7A), which have been demonstrated to response for dock, lock, and latch (DLL) mechanism for GBS-host binding (Seo et al., 2013). Afterward, the HEK293T cell lysate was subjected to incubate with purified Srr1-BR or Srr2-BR, followed by affinity purification and identification through mass spectrometry (LC-MS/MS) analysis (Figure 7B).
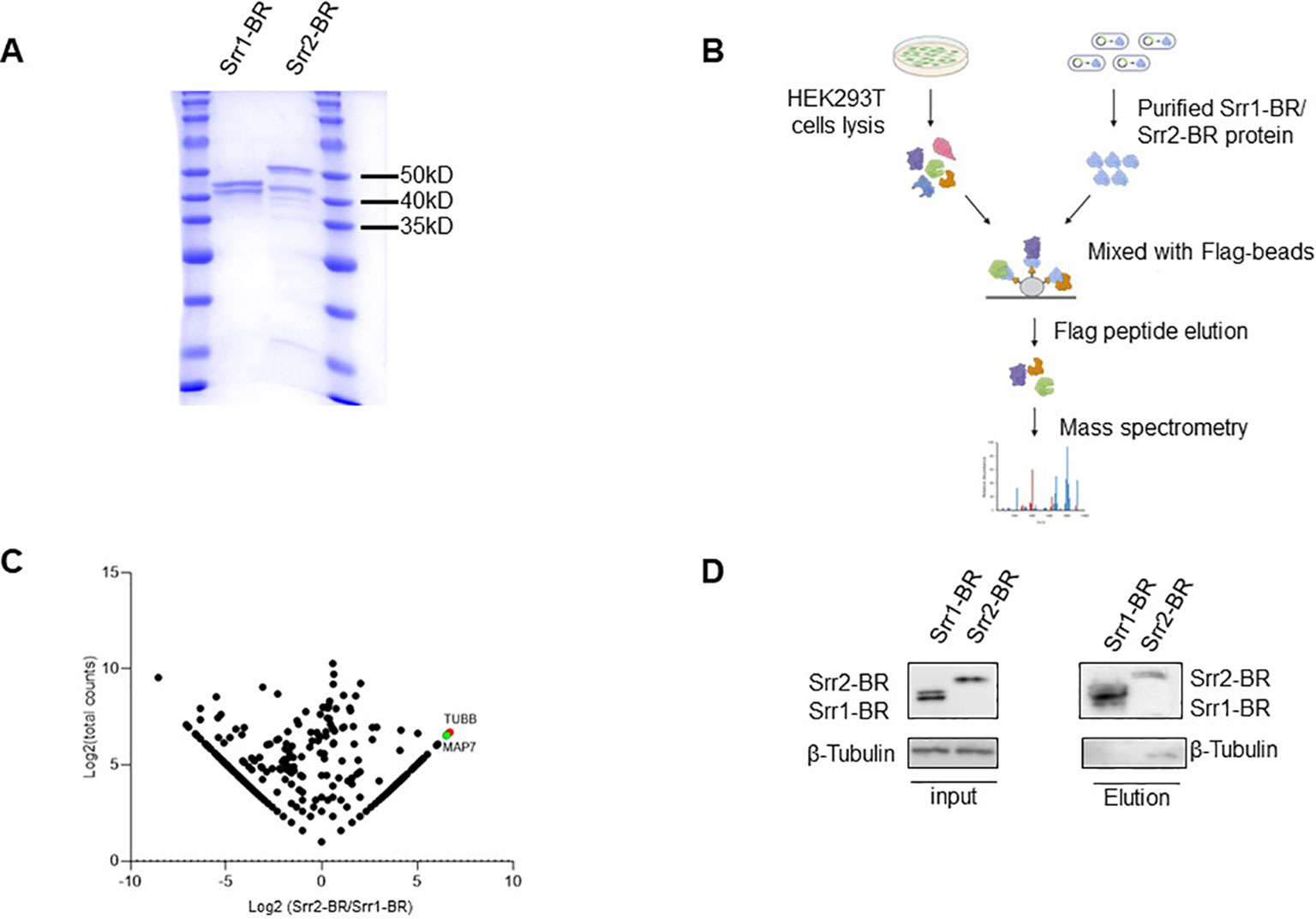
Figure 7. Identification of host factors interacting with GBS Srr1-BR and Srr2-BR. (A) Purification of GBS Srr1-BR and Srr2-BR. (B) Schematic diagram of workflow to identify host factors interacting with Srr1 and Srr2. (C) Comparison of Srr1-BR and Srr2-BR binding proteins identified by mass spectrometry. (D) Identification of Tubulin as a Srr2-BR specially binding protein.
As shown in Figure 7C, the comparison of Srr1-BR and Srr2-BR binding host proteins was conducted using a previously described method (Fu et al., 2024). There are 202 and 411 proteins specifically enriched by Srr2-BR and Srr1-BR, respectively. Among the proteins specifically interacting with Srr2-BR, Tubulin, a microtubule cytoskeleton protein, exhibited the most distinct and abundant interaction with Srr2-BR compared to Srr1-BR. To further validate the interaction between Tubulin and Srr2-BR, elution containing Srr1-BR or Srr2-BR binding proteins as in Figure 7B was subjected to immunoblotting with antibody against Tubulin. Notably, cellular endogenous Tubulin was found to specifically interact with Srr2-BR, whereas there was no binding of Tubulin and Srr1-BR (Figure 7D), suggesting that microtubule cytoskeleton Tubulin binding ability is Srr2-BR specific. Cytoskeleton rearrangement triggered by bacteria was essential for its phagocytosis and pathogenesis, this distinction of Tubulin binding affinity observed between Srr2-BR and Srr1-BR may provide an explanation for the hypervirulence induced specifically by Srr2-BR.
GBS is the only microorganism specifically targeted for screening in the third trimester, underscoring its significant risk to newborn health. However, GBS, as an opportunistic pathogen, can also colonize the intestinal tract and reproductive tract of healthy people, indicating that the GBS pathogenicity may be affected by host immunity, and may also be related to pathogenicity differences at the strain level. Compared with culture-based screening, the high sensitivity of molecular methods results in a higher positive rate. In previous studies, the colonization rate of GBS in late pregnancy women has been reported to range from 11-35% (Furfaro et al., 2018).In our results, the detection rate of NAAT was higher (34.9%), approaching the upper limit reported in the literature. However, the detection rate of selective culture medium was significantly lower than the lower limit (7%) reported in the literature. These differences indicate that current GBS screening methods require improvement. We hope to find a method that can immediately detect the colonization status of the GBS, and can accurately identify those with high virulence. Specific patterns of GBS virulence gene combinations seem to be a solution to make NAAT tests more targeted on a fundamental basis.
This study confirmed that although certain MLST-based genotypes of GBS exhibit homogeneity in serotypes, there is no overall significant relationship correlation between them. The serotypes of GBS are, in fact, a reflection of the composition of capsular polysaccharide virulence factors. However, previous epidemiological research has shown that both serotypes and genotypes do not provide a good indication of the incidence of clinical GBS and its relationship with neonatal infection. Therefore, we analyzed the composition of virulence genes in GBS and compared them with serotypes and genotypes. It was found that GBS strains derived from human sources tend to possess a greater number of virulence factors. There is a great difference between human and non-human GBS, while the GBS classification features of human are not obvious in the world, this is simply because that the distribution of GBS in the population is not affected by the region. This phenomenon may be due to the widespread nature of human GBS strains and their ability to colonize diverse populations regardless of regional differences. The concentration of non-human GBS features is also observed, potentially attributed to the relatively small number of researchers uploading non-human GBS, and the lack of migration and communication among host populations.
GBS is an important pathogen threatening maternal and fetal health in obstetrics, in which ST-17 strains (capsular serotype III) is considered to be the most virulent and harmful strain. In our study, it was also confirmed that the simultaneous existence of srr2 and hygA, which are highly virulent strain. The GBS Srr family of glycoproteins as surface-associated fibrinogen binding proteins (Fbs) binds to a single tandem repeat region of human fibrinogen via a ‘lock, dock and latch’ mechanism (Seo et al., 2013). The large locus encoding Srr1 and Srr2 are located in different chromosomal locations, but their Srr1 and Srr2 exhibit structural similarity with similar genetic organization. Srr1, which binds to fibrinogen and keratin 4 and thereby mediates adhesion to the vaginal and cervical epithelium, while Srr2 can bind plasminogen and plasmin, which is the characteristic surface glycoprotein of ST-17 strains (Six et al., 2015). In most cases of our study, the ssr1 and ssr2 genes are not simultaneously present in a single GBS strain. Only in a few instances are both genes either missing or present together. Highly virulent GBS can cause serious infection outcomes, and the different virulence genes play a series of roles in colonization and invasion at different stages. There may be a potential correlation between different virulence genes, which deserves further study. To investigate the mechanism driving the hypervirulence induced by the concurrent presence srr2 and hygA, we attempted to perform biological experiments aimed at unraveling the molecular pathways and host regulatory factors involved in their functionality. However, purification of hygA proved unsuccessful. Thus, we adopted a compromise strategy to compare the host factors enriched by Srr2-BR and Srr1-BR. The lack of co-existence between Srr2-BR and Srr1-BR, along with the increased virulence of GBS strains carrying Srr2, highlights the importance of comparing host factors that interact with these two variants, providing valuable insights for their distinction. Here, by conducting mass spectrometry analysis, microtubule cytoskeleton Tubulin was found to be the most significant factor interacting with Srr2-BR, and their interaction was further validated by immunoblotting. Cytoskeleton rearrangement represents a commonly employed strategy by diverse pathogens to facilitate their life cycle and pathogenesis (Dramsi and Cossart, 1998; Guiney and Lesnick, 2005). For GBS, it has been shown that cytoskeleton rearrangement is complicated for its invasion (Shin et al., 2006). Given the crucial role of Tubulin in microtubule dynamics and cellular fate, discerning its specific binding preference for Srr2-BR rather than Srr1-BR may offer insights into their distinct virulence mechanisms. In addition, microtubule-associated protein 7 (MAP7), a cofactor for microtubule-associated transport (Ferro et al., 2022) and has been proved to be novel host binding partners factors of pathogenic Escherichia coli (Law et al., 2016), was also among the most abundant interactors of Srr2-BR. Collectively, our findings suggested that hijacking of microtubes and their associated proteins may be involved in the hypervirulence of Srr2.
FbsA, FbsB, and FbsC are three members of the family of fibrinogen binding proteins encoded by GBS, and they adhere to human epithelial cells to promote vaginal colonization (Buscetta et al., 2014).It is reported that FbsC was not expressed in those clinical GBS isolates belonging to the highly pathogenic lineage ST17. But according to our results, all GBS strains poses a FbsC protein (Tazi et al., 2010). HvgA is currently considered to be the most relevant virulence gene for neonatal injury. The GBS hypervirulent adhesin (HvgA) is a novel cell wall anchored protein that is specific for the hypervirulent clone ST-17. It was initially described (Tazi et al., 2010) as being strongly associated with ST-17 causing neonatal meningitis in LOD. It was suggested to promote meningeal tropism in neonates through efficient intestinal colonization and subsequent translocation across the intestinal and the blood brain barriers. The strains isolated from pregnant women were found to encode surface protein virulence factors including HvgA, which helps it to acquire high virulence (Awwad et al., 2022). The specific combination of virulence factors predicts the high virulence of GBS. Highly virulent GBS can cause serious infection outcomes, and the different virulence genes play a series of roles in colonization and invasion at different stages. There may be a potential correlations between different virulence genes, which deserves further study.
In addition to hvgA and srr2, cba and fbsB may also be important virulence factors, one is because their prevalenceis relatively low, and the other is because the GBS serotypes corresponding to these two genes can better include types Ia and Ib. This corresponds to the presence of Ia and Ib in GBS isolated from newborns in addition to serotype III in clinical practice. The β protein encoded by cba is able to bind to the Fc of host IgA, and complement factor H (FH) (Pleass et al., 2001). The deposition of C3b suggests that the β protein plays an important role in GBS evading host immune system attack (Morgan et al., 1999).
Alpha-like proteins in Streptococcus agalactiae are multifunctional surface proteins that play critical roles in adherence, immune evasion, antigenic variation, and virulence, contributing significantly to the pathogenesis of GBS infections. Alps are immunogenic, give rise to protective antibodies, and are potential vaccine candidates (Stalhammar-Carlemalm et al., 1993; Gravekamp et al., 1997; Kling et al., 1997; Stalhammar-Carlemalm et al., 2007). Hundreds of human GBS strains from various geographical areas have been tested for alp4 possession, but only very few seems to be the only isolate that possesses alp4, the alp4-encoding gene (Kong et al., 2002; Moyo et al., 2002; Mavenyengwa et al., 2008; Maeland et al., 2015).In our study the GBS that detected alp-4 were all non-human sources. Based on antiserum against a serotype III/Rib GBS strain and antiserum against purified Rib, immunological testing proved that Rib is specific (Maeland et al., 2005)and in our result, the presence of rib had high coincidence with the highly independent group carrying hygA and srr2, which suggested that rib was of great significance for vaccine preparation.
In fact, the biological effects of GBS virulence factors in the treatment of GBS are not fully understood. The CAMP factor encoded by the cfb is a pore-forming toxin, but it is currently believed not to be essential for GBS pathogenicity. The FbsC protein has been shown to promote GBS adhesion and invasion of epithelial cells and endothelial cells. SfbA can bind to fibronectin, facilitating GBS binding to and invasion of human brain microvascular endothelial cells. The HylB protein of GBS degrades host proinflammatory hyaluronic acid fragments into disaccharide components and exerts immunosuppressive effects by binding to TLR2/TLR4 receptors. GBS lacking HylB exhibits reduced capacity for ascending from the vagina to the uterus. Whether the presence of these four genes (cfb, fbsC, sfbA, and hylB) in all GBS strains constitutes the fundamental pathogenicity of GBS at the species level still requires further confirmation.
In summary, the analysis of published typing and virulence gene annotations of GBS genomes revealed no significant correlation between serotypes and genotypes. In contrast, the virulence genes of GBS strains with different phylogenetic relationships showed certain patterns. In future clinical practice, greater emphasis should be placed on the detection of virulence genes to achieve precise GBS surveillance and disease prevention.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
ZZ: Conceptualization, Investigation, Methodology, Writing – original draft, Visualization. ML: Methodology, Writing – review & editing. SZ: Writing – review & editing, Methodology, Investigation. KZ: Writing – review & editing, Investigation. YW: Investigation, Writing – review & editing. MZ: Writing – review & editing, Visualization. YC: Writing – review & editing, Visualization. ZH: Writing – review & editing. QL: Writing – review & editing. LZ: Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (Grant no.61927819); Beijing Municipal Administration of Hospitals Incubation Program (px2022039); Tsinghua University Initiative Scientific Research Program of Precision Medicine (405-100010703) and Tsinghua University Chunfeng Research Program, Domestic Special (405-10001000221).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alhhazmi, A., Hurteau, D., Tyrrell, G. J. (2016). Epidemiology of invasive group B streptococcal disease in alberta, Canada, from 2003 to 2013. J. Clin. Microbiol. 54, 1774–1781. doi: 10.1128/JCM.00355-16
Allen, U., Nimrod, C., Macdonald, N., Toye, B., Stephens, D., Marchessault, V. (1999). Relationship between antenatal group B streptococcal vaginal colonization and premature labour. Paediatr. Child Health 4, 465–469. doi: 10.1093/pch/4.7.465
Al Safadi, R., Mereghetti, L., Salloum, M., Lartigue, M. F., Virlogeux-Payant, I., Quentin, R., et al. (2011). Two-component system RgfA/C activates the fbsB gene encoding major fibrinogen-binding protein in highly virulent CC17 clone group B Streptococcus. PloS One 6, e14658. doi: 10.1371/journal.pone.0014658
Areschoug, T., Stålhammar-Carlemalm, M., Karlsson, I., Lindahl, G. (2002). Streptococcal beta protein has separate binding sites for human factor H and IgA-Fc. J. Biol. Chem. 277, 12642–12648. doi: 10.1074/jbc.M112072200
(2019). Prevention of group B streptococcal early-onset disease in newborns: ACOG committee opinion, number 782. Obstet. Gynecol. 134, 1.
(1996). Prevention of perinatal group B streptococcal disease: a public health perspective. Centers for Disease Control and Prevention. MMWR. Recomm. Rep. 45, 1–24.
Awwad, E., Srour, M., Hasan, S., Khatib, S. (2022). Molecular determination, serotyping, antibiotic profile and virulence factors of group B Streptococcus isolated from invasive patients at Arabcare Hospital Laboratory, Palestine. Am. J. Infect. Control. 50, 934–940. doi: 10.1016/j.ajic.2021.12.006
Baron, M. J., Bolduc, G. R., Goldberg, M. B., Aupérin, T. C., Madoff, L. C. (2004). Alpha C protein of group B Streptococcus binds host cell surface glycosaminoglycan and enters cells by an actin-dependent mechanism. J. Biol. Chem. 279, 24714–24723. doi: 10.1074/jbc.M402164200
Benito-Jardon, M., Strohmeyer, N., Ortega-Sanchís, S., Bharadwaj, M., Moser, M., Müller, D. J., et al. (2020). alphav-Class integrin binding to fibronectin is solely mediated by RGD and unaffected by an RGE mutation. J. Cell Biol. 219:e202004198. doi: 10.1083/jcb.202004198
Bisharat, N., Jones, N., Marchaim, D., Block, C., Harding, R. M., Yagupsky, P., et al. (2005). Population structure of group B streptococcus from a low-incidence region for invasive neonatal disease. Microbiol. (Reading). 151, 1875–1881. doi: 10.1099/mic.0.27826-0
Bohnsack, J. F., Widjaja, K., Ghazizadeh, S., Rubens, C. E., Hillyard, D. R., Parker, C. J., et al. (1997). A role for C5 and C5a-ase in the acute neutrophil response to group B streptococcal infections. J. Infect. Dis. 175, 847–855. doi: 10.1086/jid.1997.175.issue-4
Bolduc, G. R., Madoff, L. C. (2007). The group B streptococcal alpha C protein binds alpha1beta1-integrin through a novel KTD motif that promotes internalization of GBS within human epithelial cells. Microbiol. (Reading). 153, 4039–4049. doi: 10.1099/mic.0.2007/009134-0
Breeding, K. M., Ragipani, B., Lee, K. D., Malik, M., Randis, T. M., Ratner, A. J. (2016). Real-time PCR-based serotyping of Streptococcus agalactiae. Sci. Rep. 6, 38523. doi: 10.1038/srep38523
Brown, C. K., Gu, Z. Y., Matsuka, Y. V., Purushothaman, S. S., Winter, L. A., Cleary, P. P., et al. (2005). Structure of the streptococcal cell wall C5a peptidase. Proc. Natl. Acad. Sci. U.S.A. 102, 18391–18396. doi: 10.1073/pnas.0504954102
Buchfink, B., Xie, C., Huson, D. H. (2015). Fast and sensitive protein alignment using DIAMOND. Nat. Methods 12, 59–60. doi: 10.1038/nmeth.3176
Buscetta, M., Papasergi, S., Firon, A., Pietrocola, G., Biondo, C., Mancuso, G., et al. (2014). FbsC, a novel fibrinogen-binding protein, promotes Streptococcus agalactiae-host cell interactions. J. Biol. Chem. 289, 21003–21015. doi: 10.1074/jbc.M114.553073
Carlin, A. F., Chang, Y. C., Areschoug, T., Lindahl, G., Hurtado-Ziola, N., King, C. C., et al. (2009). Group B Streptococcus suppression of phagocyte functions by protein-mediated engagement of human Siglec-5. J. Exp. Med. 206, 1691–1699. doi: 10.1084/jem.20090691
Castor, M. L., Whitney, C. G., Como-Sabetti, K., Facklam, R. R., Ferrieri, P., Bartkus, J. M., et al. (2008). Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect. Dis. Obstet. Gynecol. 2008, 727505. doi: 10.1155/2008/727505
Chang, Y. C., Olson, J., Beasley, F. C., Tung, C., Zhang, J., Crocker, P. R., et al. (2014a). Group B Streptococcus engages an inhibitory Siglec through sialic acid mimicry to blunt innate immune and inflammatory responses in vivo. PloS Pathog. 10, e1003846. doi: 10.1371/journal.ppat.1003846
Chang, Y. C., Olson, J., Louie, A., Crocker, P. R., Varki, A., Nizet, V. (2014b). Role of macrophage sialoadhesin in host defense against the sialylated pathogen group B Streptococcus. J. Mol. Med. (Berl). 92, 951–959. doi: 10.1007/s00109-014-1157-y
Church, D. L., Baxter, H., Lloyd, T., Larios, O., Gregson, D. B. (2017). Evaluation of strepBSelect chromogenic medium and the fast-track diagnostics group B streptococcus (GBS) real-time PCR assay compared to routine culture for detection of GBS during antepartum screening. J. Clin. Microbiol. 55, 2137–2142. doi: 10.1128/JCM.00043-17
Cieslewicz, M. J., Chaffin, D., Glusman, G., Kasper, D., Madan, A., Rodrigues, et al. (2005). Structural and genetic diversity of group B streptococcus capsular polysaccharides. Infect. Immun. 73, 3096–3103. doi: 10.1128/IAI.73.5.3096-3103.2005
Creti, R., Imperi, M., Stanziale, A., Giuliani, G., Fazii, P., Savini, V., et al. (2023). Group B streptococci (GBS) strains evading molecular diagnostics showed novel chromosomal deletions encompassing the CAMP-factor (cfb) encoding gene. Eur. J. Clin. Microbiol. Infect. Dis. 42, 913–916. doi: 10.1007/s10096-023-04620-x
Delcher, A. L., Bratke, K. A., Powers, E. C., Salzberg, S. L. (2007). Identifying bacterial genes and endosymbiont DNA with Glimmer. Bioinformatics 23, 673–679. doi: 10.1093/bioinformatics/btm009
Devi, A. S., Ponnuraj, K. (2010). Cloning, expression, purification and ligand binding studies of novel fibrinogen-binding protein FbsB of Streptococcus agalactiae. Protein Expr. Purif. 74, 148–155. doi: 10.1016/j.pep.2010.07.004
Dobrut, A., Brzychczy-Wloch, M. (2021). Immunogenic proteins of group B streptococcus-potential antigens in immunodiagnostic assay for GBS detection. Pathogens 11, 43. doi: 10.3390/pathogens11010043
Dramsi, S., Cossart, P. (1998). Intracellular pathogens and the actin cytoskeleton. Annu. Rev. Cell Dev. Biol. 14, 137–166. doi: 10.1146/annurev.cellbio.14.1.137
Ferro, L. S., Fang, Q., Eshun-Wilson, L., Fernandes, J., Jack, A., Farrell, D. P., et al. (2022). Structural and functional insight into regulation of kinesin-1 by microtubule-associated protein MAP7. Science 375, 326–331. doi: 10.1126/science.abf6154
Forquin, M. P., Tazi, A., Rosa-Fraile, M., Poyart, C., Trieu-Cuot, P., Dramsi, S. (2007). The putative glycosyltransferase-encoding gene cylJ and the group B Streptococcus (GBS)-specific gene cylK modulate hemolysin production and virulence of GBS. Infect. Immun. 75, 2063–2066. doi: 10.1128/IAI.01565-06
Fu, J., Li, S., Guan, H., Li, C., Zhao, Y. B., Chen, T. T., et al. (2024). Legionella maintains host cell ubiquitin homeostasis by effectors with unique catalytic mechanisms. Nat. Commun. 15, 5953. doi: 10.1038/s41467-024-50311-2
Furfaro, L. L., Chang, B. J., Payne, M. S. (2018). Perinatal streptococcus agalactiae epidemiology and surveillance targets. Clin. Microbiol. Rev. 31, 10–1128. doi: 10.1128/CMR.00049-18
Gabrielsen, C., Mæland, J. A., Lyng, R. V., Radtke, A., Afset, J. E. (2017). Molecular characteristics of Streptococcus agalactiae strains deficient in alpha-like protein encoding genes. J. Med. Microbiol. 66, 26–33. doi: 10.1099/jmm.0.000412
Gravekamp, C., Kasper, D. L., Michel, J. L., Kling, D. E., Carey, V., Madoff, L. C. (1997). Immunogenicity and protective efficacy of the alpha C protein of group B streptococci are inversely related to the number of repeats. Infect. Immun. 65, 5216–5221. doi: 10.1128/iai.65.12.5216-5221.1997
Gravekamp, C., Rosner, B., Madoff, L. C. (1998). Deletion of repeats in the alpha C protein enhances the pathogenicity of group B streptococci in immune mice. Infect. Immun. 66, 4347–4354. doi: 10.1128/IAI.66.9.4347-4354.1998
Guiney, D. G., Lesnick, M. (2005). Targeting of the actin cytoskeleton during infection by Salmonella strains. Clin. Immunol. 114, 248–255. doi: 10.1016/j.clim.2004.07.014
Gutekunst, H., Eikmanns, B. J., Reinscheid, D. J. (2004). The novel fibrinogen-binding protein FbsB promotes Streptococcus agalactiae invasion into epithelial cells. Infect. Immun. 72, 3495–3504. doi: 10.1128/IAI.72.6.3495-3504.2004
Hillier, S. L., Krohn, M. A., Kiviat, N. B., Watts, D. H., Eschenbach, D. A. (1991). Microbiologic causes and neonatal outcomes associated with chorioamnion infection. Am. J. Obstet. Gynecol. 165, 955–961. doi: 10.1016/0002-9378(91)90447-Y
Hyatt, D., Chen, G. L., Locascio, P. F., Land, M. L., Larimer, F. W., Hauser, L. J. (2010). Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinf. 11, 119. doi: 10.1186/1471-2105-11-119
Jackson, L. A., Hilsdon, R., Farley, M. M., Harrison, L. H., Reingold, A. L., Plikaytis, et al. (1995). Risk factors for group B streptococcal disease in adults. Ann. Intern. Med. 123, 415–420. doi: 10.7326/0003-4819-123-6-199509150-00003
Jain, C., Rodriguez-R, L. M., Phillippy, A. M., Konstantinidis, K. T., Aluru, S. (2018). High throughput ANI analysis of 90K prokaryotic genomes reveals clear species boundaries. Nat. Commun. 9, 5114. doi: 10.1038/s41467-018-07641-9
Jerlstrom, P. G., Talay, S. R., Valentin-Weigand, P., Timmis, K. N., Chhatwal, G. S. (1996). Identification of an immunoglobulin A binding motif located in the beta-antigen of the c protein complex of group B streptococci. Infect. Immun. 64, 2787–2793. doi: 10.1128/iai.64.7.2787-2793.1996
Jolley, K. A., Maiden, M. C. (2010). BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinf. 11, 595. doi: 10.1186/1471-2105-11-595
Kang, H. M., Lee, H. J., Lee, H., Jo, D. S., Lee, H. S., Kim, T. S., et al. (2017). Genotype characterization of group B streptococcus isolated from infants with invasive diseases in South Korea. Pediatr. Infect. Dis. J. 36, e242–e247. doi: 10.1097/INF.0000000000001531
Kling, D. E., Gravekamp, C., Madoff, L. C., Michel, J. L. (1997). Characterization of two distinct opsonic and protective epitopes within the alpha C protein of the group B Streptococcus. Infect. Immun. 65, 1462–1467. doi: 10.1128/iai.65.4.1462-1467.1997
Klinzing, D. C., Madoff, L. C., Puopolo, K. M. (2009). Genomic analysis identifies a transcription-factor binding motif regulating expression of the alpha C protein in Group B Streptococcus. Microb. Pathog. 46, 315–320. doi: 10.1016/j.micpath.2009.03.003
Kolar, S. L., Kyme, P., Tseng, C. W., Soliman, A., Kaplan, A., Liang, J., et al. (2015). Group B streptococcus evades host immunity by degrading hyaluronan. Cell Host Microbe 18, 694–704. doi: 10.1016/j.chom.2015.11.001
Kong, F., Gowan, S., Martin, D., James, G., Gilbert, G. L. (2002). Molecular profiles of group B streptococcal surface protein antigen genes: relationship to molecular serotypes. J. Clin. Microbiol. 40, 620–626. doi: 10.1128/JCM.40.2.620-626.2002
Krohn, M. A., Hillier, S. L., Baker, C. J. (1999). Maternal peripartum complications associated with vaginal group B streptococci colonization. J. Infect. Dis. 179, 1410–1415. doi: 10.1086/jid.1999.179.issue-6
Landwehr-Kenzel, S., Henneke, P. (2014). Interaction of streptococcus agalactiae and cellular innate immunity in colonization and disease. Front. Immunol. 5, 519. doi: 10.3389/fimmu.2014.00519
Law, R. J., Law, H. T., Scurll, J. M., Scholz, R., Santos, A. S., Shames, S. R., et al. (2016). Quantitative mass spectrometry identifies novel host binding partners for pathogenic escherichia coli type III secretion system effectors. J. Proteome Res. 15, 1613–1622. doi: 10.1021/acs.jproteome.6b00074
Letunic, I., Bork, P. (2021). Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296. doi: 10.1093/nar/gkab301
Madrid, L., Seale, A. C., Kohli-Lynch, M., Edmond, K. M., Lawn, J. E., Heath, P. T., et al. (2017). Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin. Infect. Dis. 65, S160–S172. doi: 10.1093/cid/cix656
Maeland, J. A., Bevanger, L., Lyng, R. V. (2005). Immunological markers of the R4 protein of Streptococcus agalactiae. Clin. Diagn. Lab. Immunol. 12, 1305–1310. doi: 10.1128/CDLI.12.11.1305-1310.2005
Maeland, J. A., et al. (2015). Survey of immunological features of the alpha-like proteins of Streptococcus agalactiae. Clin. Vaccine Immunol. 22, 153–159. doi: 10.1128/CVI.00643-14
Manni, M., Berkeley, M. R., Seppey, M., Zdobnov, E. M. (2021). BUSCO: assessing genomic data quality and beyond. Curr. Protoc. 1, e323. doi: 10.1002/cpz1.v1.12
Mavenyengwa, R. T., Maeland, J. A., Moyo, S. R. (2008). Distinctive features of surface-anchored proteins of Streptococcus agalactiae strains from Zimbabwe revealed by PCR and dot blotting. Clin. Vaccine Immunol. 15, 1420–1424. doi: 10.1128/CVI.00112-08
Moyo, S. R., Maeland, J. A., Bergh, K. (2002). Typing of human isolates of Streptococcus agalactiae (group B streptococcus, GBS) strains from Zimbabwe. J. Med. Microbiol. 51, 595–662. doi: 10.1099/0022-1317-51-7-595
Pannaraj, P. S., Kelly, J. K., Madoff, L. C., Rench, M. A., Lachenauer, C. S., Edwards, M. S., et al. (2007). Group B Streptococcus bacteremia elicits beta C protein-specific IgM and IgG in humans. J. Infect. Dis. 195, 353–356. doi: 10.1086/510627
Pezzicoli, A., Ruggiero, P., Amerighi, F., Telford, J. L., Soriani, M. (2012). Exogenous sialic acid transport contributes to group B streptococcus infection of mucosal surfaces. J. Infect. Dis. 206, 924–931. doi: 10.1093/infdis/jis451
Pietrocola, G., Schubert, A., Visai, L., Torti, M., Fitzgerald, J. R., Foster, T. J., et al. (2005). FbsA, a fibrinogen-binding protein from Streptococcus agalactiae, mediates platelet aggregation. Blood 105, 1052–1059. doi: 10.1182/blood-2004-06-2149
Pleass, R. J., Areschoug, T., Lindahl, G., Woof, J. M. (2001). Streptococcal IgA-binding proteins bind in the Calpha 2-Calpha 3 interdomain region and inhibit binding of IgA to human CD89. J. Biol. Chem. 276, 8197–8204. doi: 10.1074/jbc.M009396200
Price, M. N., Dehal, P. S., Arkin, A. P. (2010). FastTree 2–approximately maximum-likelihood trees for large alignments. PloS One 5, e9490. doi: 10.1371/journal.pone.0009490
Rosa-Fraile, M., Dramsi, S., Spellerberg, B. (2014). Group B streptococcal haemolysin and pigment, a tale of twins. FEMS Microbiol. Rev. 38, 932–946. doi: 10.1111/1574-6976.12071
Schrag, S., Gorwitz, R., Fultz-Butts, K., Schuchat, A. (2002). Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR. Recomm. Rep. 51, 1–22.
Seo, H. S., Mu, R., Kim, B. J., Doran, K. S., Sullam, P. M. (2012). Binding of glycoprotein Srr1 of Streptococcus agalactiae to fibrinogen promotes attachment to brain endothelium and the development of meningitis. PloS Pathog. 8, e1002947. doi: 10.1371/journal.ppat.1002947
Seo, H. S., Minasov, G., Seepersaud, R., Doran, K. S., Dubrovska, I., Shuvalova, L., et al. (2013). Characterization of fibrinogen binding by glycoproteins Srr1 and Srr2 of Streptococcus agalactiae. J. Biol. Chem. 288, 35982–35996. doi: 10.1074/jbc.M113.513358
Shabayek, S., Spellerberg, B. (2018). Group B streptococcal colonization, molecular characteristics, and epidemiology. Front. Microbiol. 9, 437. doi: 10.3389/fmicb.2018.00437
Sheen, T. R., Jimenez, A., Wang, N. Y., Banerjee, A., van Sorge, N. M., Doran, K. S. (2011). Serine-rich repeat proteins and pili promote Streptococcus agalactiae colonization of the vaginal tract. J. Bacteriol. 193, 6834–6842. doi: 10.1128/JB.00094-11
Shin, S., Paul-Satyaseela, M., Lee, J. S., Romer, L. H., Kim, K. S. (2006). Focal adhesion kinase is involved in type III group B streptococcal invasion of human brain microvascular endothelial cells. Microb. Pathog. 41, 168–173. doi: 10.1016/j.micpath.2006.07.003
Silva, M., Machado, M. P., Silva, D. N., Rossi, M., Moran-Gilad, J., Santos, S., et al. (2018). chewBBACA: A complete suite for gene-by-gene schema creation and strain identification. Microb. Genom. 4:e000166. doi: 10.1099/mgen.0.000166
Six, A., Bellais, S., Bouaboud, A., Fouet, A., Gabriel, C., Tazi, A., et al. (2015). Srr2, a multifaceted adhesin expressed by ST-17 hypervirulent Group B Streptococcus involved in binding to both fibrinogen and plasminogen. Mol. Microbiol. 97, 1209–1222. doi: 10.1111/mmi.2015.97.issue-6
Spellerberg, B., Rozdzinski, E., Martin, S., Weber-Heynemann, J., Lütticken, R. (2002). rgf encodes a novel two-component signal transduction system of Streptococcus agalactiae. Infect. Immun. 70, 2434–2440. doi: 10.1128/IAI.70.5.2434-2440.2002
Stalhammar-Carlemalm, M., Waldemarsson, J., Johnsson, E., Areschoug, T., Lindahl, G. (2007). Nonimmunodominant regions are effective as building blocks in a streptococcal fusion protein vaccine. Cell Host Microbe 2, 427–434. doi: 10.1016/j.chom.2007.10.003
Stalhammar-Carlemalm, M., Stenberg, L., Lindahl, G. (1993). Protein rib: a novel group B streptococcal cell surface protein that confers protective immunity and is expressed by most strains causing invasive infections. J. Exp. Med. 177, 1593–1603. doi: 10.1084/jem.177.6.1593
Tazi, A., Disson, O., Bellais, S., Bouaboud, A., Dmytruk, N., Dramsi, S., et al. (2010). The surface protein HvgA mediates group B streptococcus hypervirulence and meningeal tropism in neonates. J. Exp. Med. 207, 2313–2322. doi: 10.1084/jem.20092594
Tenenbaum, T., Spellerberg, B., Adam, R, Vogel, M., Kim, K. S., Schroten, H. (2007). Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 9, 714–720. doi: 10.1016/j.micinf.2007.02.015
Verani, J. R., et al. (2010). Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR. Recomm. Rep. 59, 1–36.
Vornhagen, J., Adams Waldorf, K. M., Rajagopal, L. (2017). Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol. 25, 919–931. doi: 10.1016/j.tim.2017.05.013
Vornhagen, J., Quach, P., Boldenow, E., Merillat, S., Whidbey, C., Ngo, L. Y., et al. (2016). Bacterial hyaluronidase promotes ascending GBS infection and preterm birth. mBio 7, 10–1128. doi: 10.1128/mBio.00781-16
Whidbey, C., Harrell, M. I., Burnside, K., Ngo, L., Becraft, A. K., Iyer, L. M., et al. (2013). A hemolytic pigment of Group B Streptococcus allows bacterial penetration of human placenta. J. Exp. Med. 210, 1265–1281. doi: 10.1084/jem.20122753
Zeng, Z., Wang, N., Sui, L., Zhang, R., Zhang, Q., Wang, Y., et al. (2023). Characteristics and potential diagnostic ability of vaginal microflora in patients with aerobic vaginitis using 16S Ribosomal RNA sequencing. Diagn. Microbiol. Infect. Dis. 105, 115806. doi: 10.1016/j.diagmicrobio.2022.115806
Keywords: group B streptococcus (GBS), surveillance, genotype, serotype, virulence gene
Citation: Zeng Z, Li M, Zhu S, Zhang K, Wu Y, Zheng M, Cao Y, Huang Z, Liao Q and Zhang L (2024) Strain-level genomic analysis of serotype, genotype and virulence gene composition of group B streptococcus. Front. Cell. Infect. Microbiol. 14:1396762. doi: 10.3389/fcimb.2024.1396762
Received: 06 March 2024; Accepted: 09 October 2024;
Published: 06 November 2024.
Edited by:
Yong Zhang, Jilin University, ChinaReviewed by:
Rui Yang, Peking University Third Hospital, ChinaCopyright © 2024 Zeng, Li, Zhu, Zhang, Wu, Zheng, Cao, Huang, Liao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Zhang, emxhMDA5NjlAYnRjaC5lZHUuY24=; Qinping Liao, THFwYTAwNTk0QGJ0Y2guZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.