- Department of Nephrology, First Medical Center of Chinese PLA General Hospital, Nephrology Institute of the Chinese People’s Liberation Army, State Key Laboratory of Kidney Diseases, National Clinical Research Center for Kidney Diseases, Beijing Key Laboratory of Kidney Disease Research, Beijing, China
Introduction: The impact of coronavirus disease 2019 (COVID-19) on diabetic kidney disease (DKD) patients in China is not fully understood. This study aimed to investigate infection status in a DKD cohort post-renal biopsy and analyze vaccination and infection rates, as well as symptom severity, across various renal pathologies in DKD patients.
Methods: This epidemiological survey, centered on COVID-19, employed a Chinese DKD and renal puncture follow-up cohort. A customized questionnaire enabled standardized data gathering. It collected data on clinical characteristics, vaccination and infection statuses, and diverse pathological types. The study analyzed the relationship between vaccination and infection statuses across various pathological types, evaluating characteristics and treatment outcomes in patients with infections.
Results: In total, 437 patients with DKD from 26 Chinese provinces were followed up for a median of 44.6 ± 20 months. COVID-19 infection, vaccination, and novel coronavirus pneumonia (NCP) rates were 73.68%, 59.3%, and 6.63%, respectively. Ten patients with NCP had severe pneumonia or died of COVID-19. Renal pathology revealed that 167 (38.22%) patients had diabetic nephropathy (DN), 171 (39.13%) had non-diabetic renal disease (NDRD), and 99 had DN and NDRD (22.65%). The DN group had the lowest vaccination (54.5%), highest all-cause mortality (3.6%), and highest endpoint rates (34.10%). Compared to patients who were not vaccinated pre-infection (117 cases), vaccinated patients (198 cases) had reduced NCP (6.6% vs. 13.7%), severity (1.0% vs. 3.4%), and endpoint (9.10% vs. 31.60%) rates.
Conclusion: Vaccination can prevent infection and diminish COVID-19 severity in patients with DKD; therefore, increasing vaccination rates is particularly important.
Clinical Trial registration: ClinicalTrails.gov, NCT05888909.
Introduction
The novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus began spreading in December 2019, initiating the coronavirus disease 2019 (COVID-19) pandemic that has impacted many aspects of human society (Chaw et al., 2020; Wiersinga et al., 2020). In 2022, COVID-19 was either the primary cause or a contributing factor in 0.24 million deaths in the United States (Ahmad et al., 2023). As of July 7, 2024, the World Health Organization (WHO) has reported 775.67 million confirmed cases of COVID-19, including 7.05 million deaths (W.H.O, 2023). In China, from January 3, 2020, to July 7, 2024, there were 99.4 million confirmed cases of COVID-19, resulting in 0.12 million deaths (W.H.O, 2023).
Patients with diabetes are at a high risk of infection with SARS-CoV-2. Older adult patients with comorbidities, including hypertension, diabetes, asthma, chronic obstructive pulmonary disorder, and chronic kidney disease, are at increased risk of developing severe COVID-19 and adverse clinical outcomes (Akhtar et al., 2021; Salinas-Aguirre et al., 2022). Age (Yang et al., 2020) and underlying diseases (including hypertension and diabetes) (Li et al., 2020) are the most important risk factors for COVID-19-related death. Type 2 diabetes mellitus-associated diabetic nephropathy (DN) and severe COVID-19 are known to be closely associated (Mourad et al., 2021), with DN increasing the risk of COVID-19-related death (Hadjadj et al., 2023). Patients with diabetic kidney disease (DKD) have increased rates of COVID-19, intensive care unit admission, and death compared to patients with chronic kidney disease (CKD) alone. Further, lung infection and intubation rates of those with DKD are twice those of patients with CKD (Abdulaziz Al-Muhanna et al., 2022).
In the early stages of the epidemic, SARS-CoV-2 was highly pathogenic, with pneumonia commonly seen in the clinic. Variations in the virus weakened its virulence, particularly after the Omicron viral strain predominated (Fan et al., 2022; Qu et al., 2023). Human infection manifestations currently include cough, fever, and sore throat, with few developing pneumonia. Over 3 years, China’s government has implemented protective public health policies. On January 6, 2023, China’s National Health Commission and the National Administration of Traditional Chinese Medicine released the updated Diagnosis and Treatment Plan for Novel Coronavirus Infection, in line with Class B COVID-19 management and epidemic control measure revisions. This plan renamed the disease from “novel coronavirus pneumonia” (NCP) to “novel coronavirus infection” and incorporated a positive COVID-19 antigen test into the diagnostic criteria (NHC, NATCM, 2023).
Since December 2022, China’s epidemic prevention has shifted from infection prevention to health protection and severe case prevention. Efforts have been made to administer medical treatment, enhance COVID-19 cure rates, and lower mortality rates (NHC, NATCM, 2023). The effect of COVID-19 on DKD patients in China post-policy change is yet to be determined. To understand this, an epidemiological survey was conducted on a follow-up cohort of patients diagnosed with DKD via renal biopsy, covering 26 provinces.
Materials and methods
Participants and survey design
This epidemiological study investigated 437 patients with type 2 diabetes who underwent renal biopsy at the First Medical Center of the People’s Liberation Army General Hospital between April 1, 2017, and November 30, 2022. The inclusion criteria were as follows: aged 18 years or older at renal biopsy for both sexes; diagnosis of type 2 diabetes; and diagnosis of renal lesions. Exclusion criteria included the following: incomplete data, unclear medical history, unclear pathological diagnoses, secondary diabetes, or tumors. The survey was conducted from January to March 2023, with a dedicated survey questionnaire designed for the collection of standardized data from the cohort of patients. Questionnaire data were collected both over the telephone and at outpatient follow-up. This study was approved by the Ethics Committee of the Chinese People’s Liberation Army General Hospital (No. S2022-482-01). Written informed consent was obtained from all participants.
Patients with pathological kidney results were categorized into three groups: DN, non-diabetic renal disease (NDRD), and DN combined with NDRD. They were further classified into infected and uninfected subgroups based on SARS-CoV-2 infection, with infected patients subdivided into vaccinated and unvaccinated. The study compared COVID-19 vaccination and infection status across different DKD pathological types and examined the impact of clinical characteristics on infection status.
Data collection and evaluation
Standardized data collection tables (Supplementary Material), tailored for this research, systematically organized and recorded data. Baseline clinical data, COVID-19 infection status, and vaccination status of renal biopsy patients were collected. Each patient’s detailed medical history, encompassing age, sex, residence, examination results, laboratory tests during renal biopsy, and pathology results, was gathered.
The questionnaire contained: follow-up endpoint status, SARS-CoV-2 infection status, vaccination status (dose times), COVID-19 diagnostic method, pneumonia diagnosis, and COVID-19 severity with symptoms and treatment details. COVID-19 severity was determined mainly based on the Diagnosis and Treatment Plan for Novel Coronavirus Infection (Tenth Edition). Based on a comprehensive analysis of epidemiological history, clinical manifestations, laboratory tests, and other factors, a clinical diagnosis and classification (severity) assessment were made. The included patients met the diagnostic criteria for severe and critically severe clinical classifications and were considered to be severely infected (NHC, NATCM, 2023). Symptoms included fever, cough, sore throat, nasal congestion, headache, muscle pain, shortness of breath, difficulty breathing, nausea, chills, vomiting, and diarrhea (shapeless or watery stools). Each symptom could be marked as nonexistent (score 0) or present (score 1–3). COVID-19 severity: mild (score 1), moderate (score 2), severe (score 3) (Supplementary Material).
Diagnosis and outcome assessment
Kidney disease diagnosis relied on renal pathology. Follow-up endpoints included renal replacement therapy or death from any cause. COVID-19 diagnosis encompassed relevant clinical symptoms and one or more positive tests: COVID-19 nucleic acid, antigen, pSARS-CoV-2 isolation/culture, or a convalescent stage COVID-19-specific IgG antibody level quadruple that of the acute stage (NHC, NATCM, 2023). NCP was designated if the requirements of COVID-19 diagnosis were met and characteristic pneumonia manifestations on lung imaging were visible.
Statistical analyses
Statistical analysis employed IBM SPSS Statistics for Windows, version 25.0. The Kolmogorov-Smirnov test assessed normality in continuous variables. Normally distributed variables are shown as means ± standard deviation, compared via t-test. Non-normally distributed continuous variables are depicted as medians and interquartile ranges and compared using non-parametric tests. Categorical variables are represented as frequencies and percentages and compared with chi-square tests. To compare the overall distribution of composition ratios of multiple groups, R is used × C, which is composed of multiple samples without a sequence-linked-table χ 2 inspection. Statistical significance was established at P < 0.05.
Results
General characteristics of patients
In total, 437 patients with DKD from 26 provinces and municipalities in China were followed for an average duration of 44.6 ± 20 months. Throughout this period, 322 patients (73.68%) contracted COVID-19, whereas 115 (26.32%) did not. Additionally, 266 patients (60.9%) received the COVID-19 vaccine. Among those with COVID-19, 29 patients (6.63%) were diagnosed with NCP, and 10 (2.29%) had severe pneumonia or died.
The distribution of patients across geographical regions of China was as follows: northeast (109, 24.9%), north (248, 56.8%), east (26, 5.9%), central (23, 5.3%), northwest (22, 5.0%), and southwest (9, 2.1%); the vaccination rates across regions were 66.1%, 59.3%, 57.7%, 56.5%, 77.3%, and 22.2%, respectively, and COVID-19 detection rates were 78.0%, 72.2%, 69.2%, 73.9%, 72.7%, and 77.8%, respectively. Infection and vaccination rates among regions did not significantly differ.
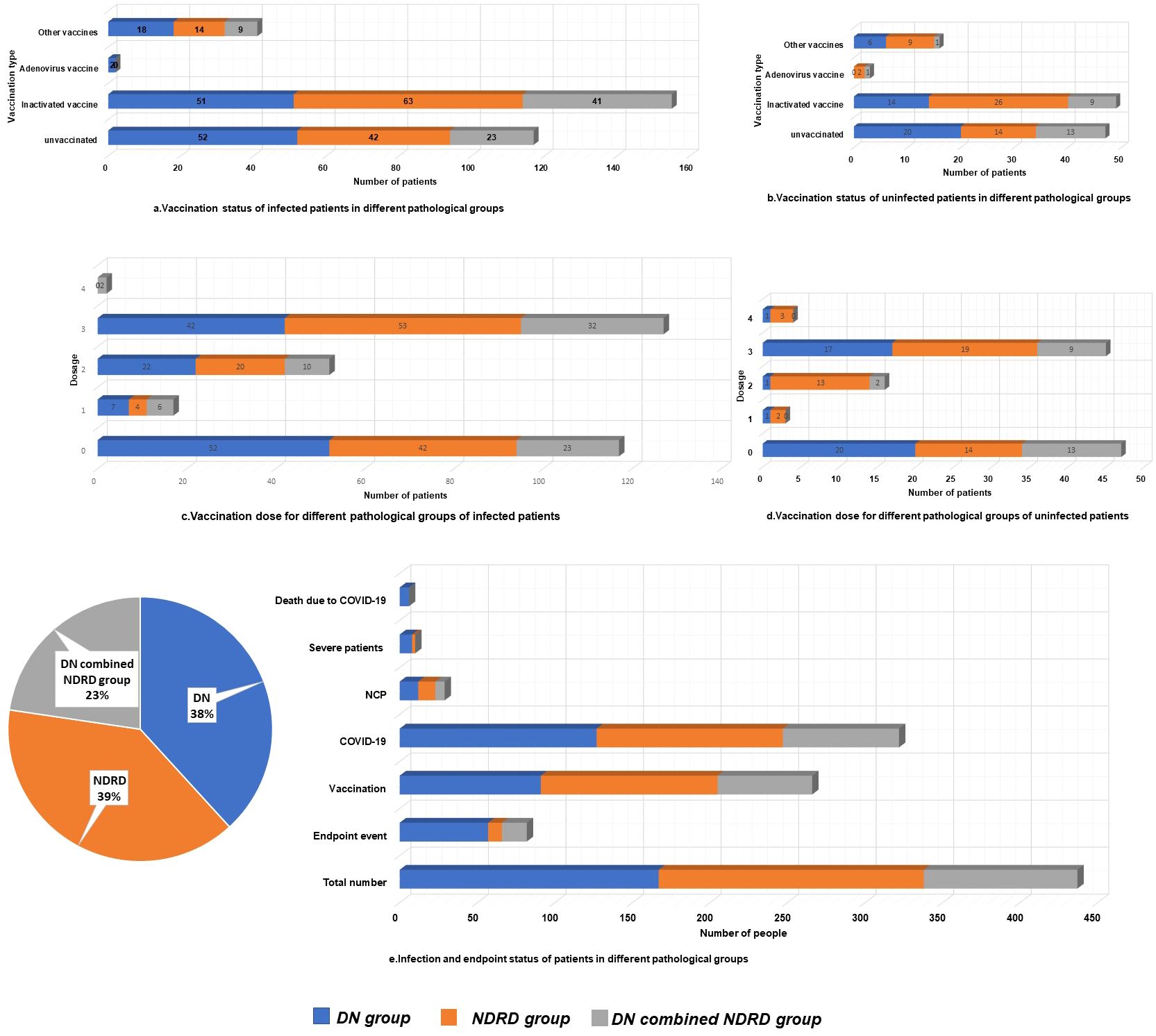
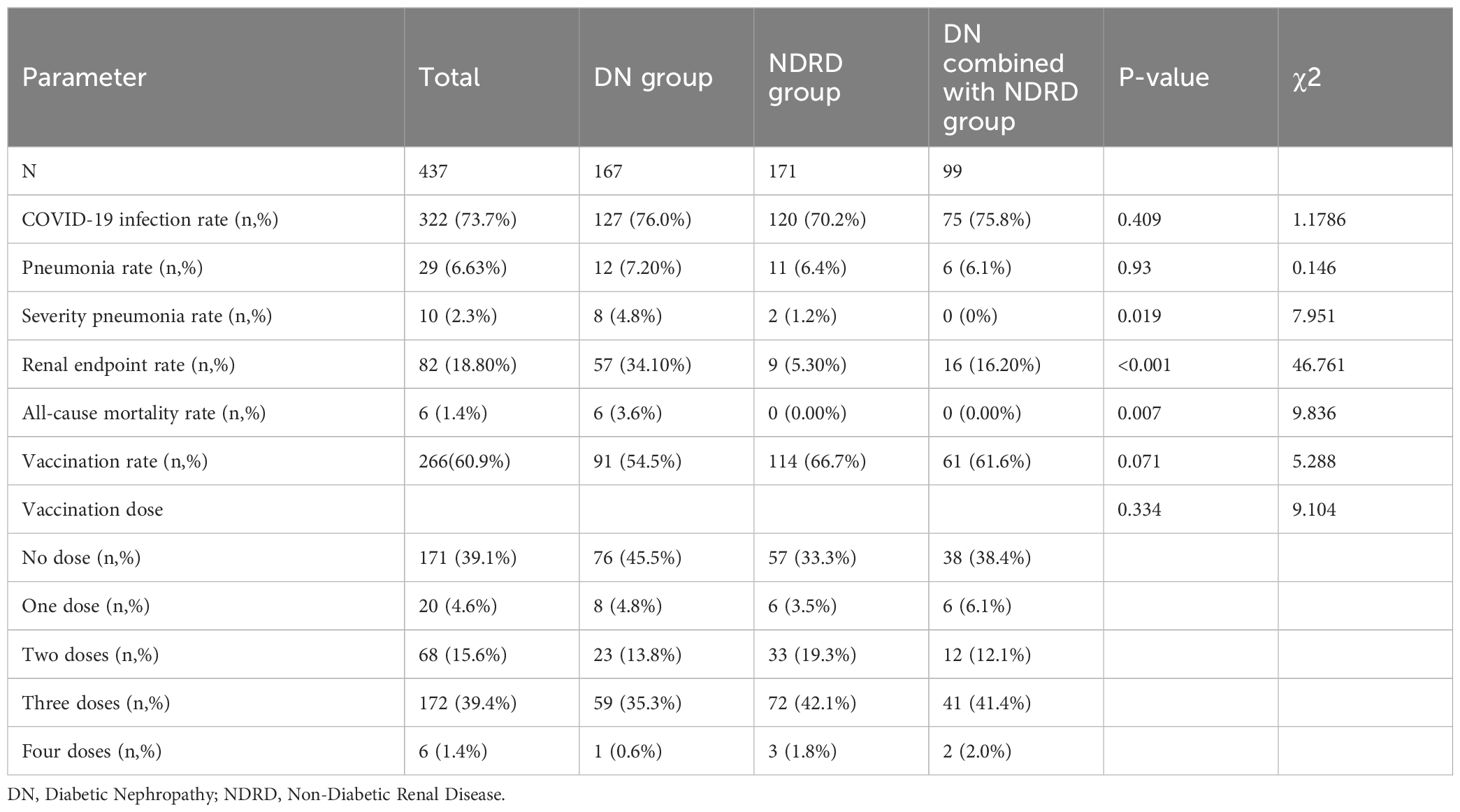
Patients grouped by SARS-CoV-2 infection status exhibited comparable characteristics (Table 1). Clinical and renal pathological features, along with vaccination rates and dosages, were similar across both groups (Figures 1a–d). Predominant renal pathologies differed—DN in the infected group (127 patients, 39.4%) and NDRD in the uninfected group (51 patients, 44.3%)—yet the subtype distribution showed no significant difference. Additionally, all-cause mortality and follow-up endpoint rates were consistent between groups.
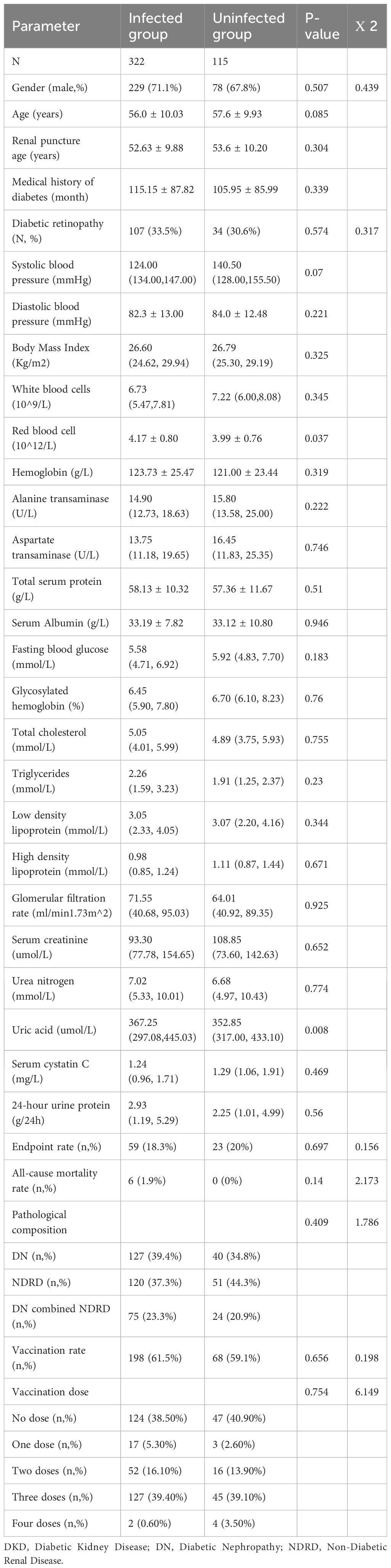
Table 1. Clinical characteristics and vaccination status of 437 DKD patients with or without infection.
Vaccination and infection status among pathological groups
Patients were grouped based on renal pathological subtype, as follows: DN (167, 38.22%), NDRD (171, 39.13%), and DN combined with NDRD (99, 22.65%) (Figure 1). In total, 82 patients reached the endpoint of follow-up (18.80%), with significant differences in rates of reaching the renal endpoint among patients with different renal pathologies. Among those reaching the endpoint of follow-up, 57 were in the DN group (34.10%), nine were in the NDRD group (5.30%), and 16 were in the DN combined with NDRD group (16.20%). Among them, a total of six patients (1.40%) died, all of whom were in the DN group (P=0.007). Rates of vaccination for the DN, NDRD, and combined DN and NDRD groups were 54.5% (91 cases), 66.7% (114 cases), and 61.6% (61 cases), respectively (P=0.071).
The COVID-19 infection rates for the groups were 76.0% (127 cases), 70.2% (120 cases), and 75.8% (75 cases), respectively. The NCP infection rates for the three groups were 7.20% (12 cases), 6.4% (11 cases), and 6.1% (6 cases), respectively. Infection and pneumonia rates did not significantly differ among the groups. Importantly, 10 patients developed severe pneumonia, and the rate of severe pneumonia in the DN group was 4.8% (eight cases), which was significantly higher than that in the NDRD group (1.2%, two cases) and the DN combined with NDRD group (0%) (P=0.019). The vaccination rate in the DN group was lower at 54.5% compared to that of the other groups. Additionally, the DN group had the highest all-cause mortality rates (3.6%, P=0.007), with 34.10% of patients reaching the follow-up endpoint (P<0.001). No pathological subtype-based differences in infection rates, vaccine coverage rates, or dosages received were observed; however, there were significant differences in disease severity and rates of reaching the follow-up endpoint (Table 2, Figure 1e).
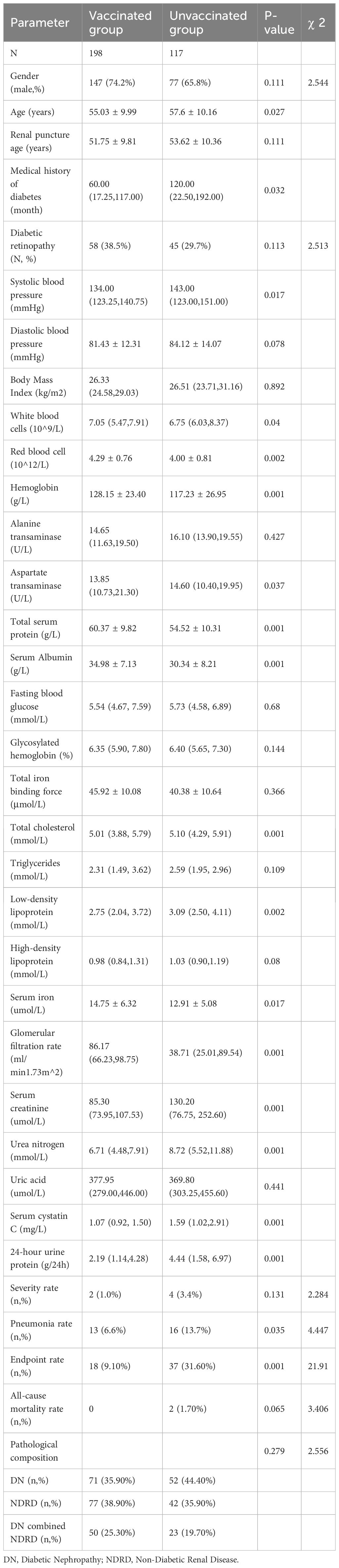
Clinical characteristics of infected patients and factors influencing vaccination
Of 322 infected patients, 114 (35.40%) had a positive nucleic acid test, 180 (55.90%) had a positive antigen test, and 28 (8.70%) were diagnosed with COVID-19 clinically without specified criteria. The vaccinated group comprised 198 patients, whereas the unvaccinated group included 117. No significant differences were observed in renal pathology between the groups. Notably, the vaccinated group exhibited a shorter diabetes history, lower mean systolic blood pressure, and higher hemoglobin, albumin, serum iron, and estimated glomerular filtration rate (eGFR) levels compared to the unvaccinated group. The opposite was found for lower serum creatinine, cystatin C, and proteinuria levels, suggesting better overall health (Table 3). Binary logistic regression identified serum albumin (OR=1.075, 95% CI: 1.034–1.117) and eGFR (OR=1.016, 95% CI: 1.006–1.026) as key factors influencing vaccination status among infected patients.
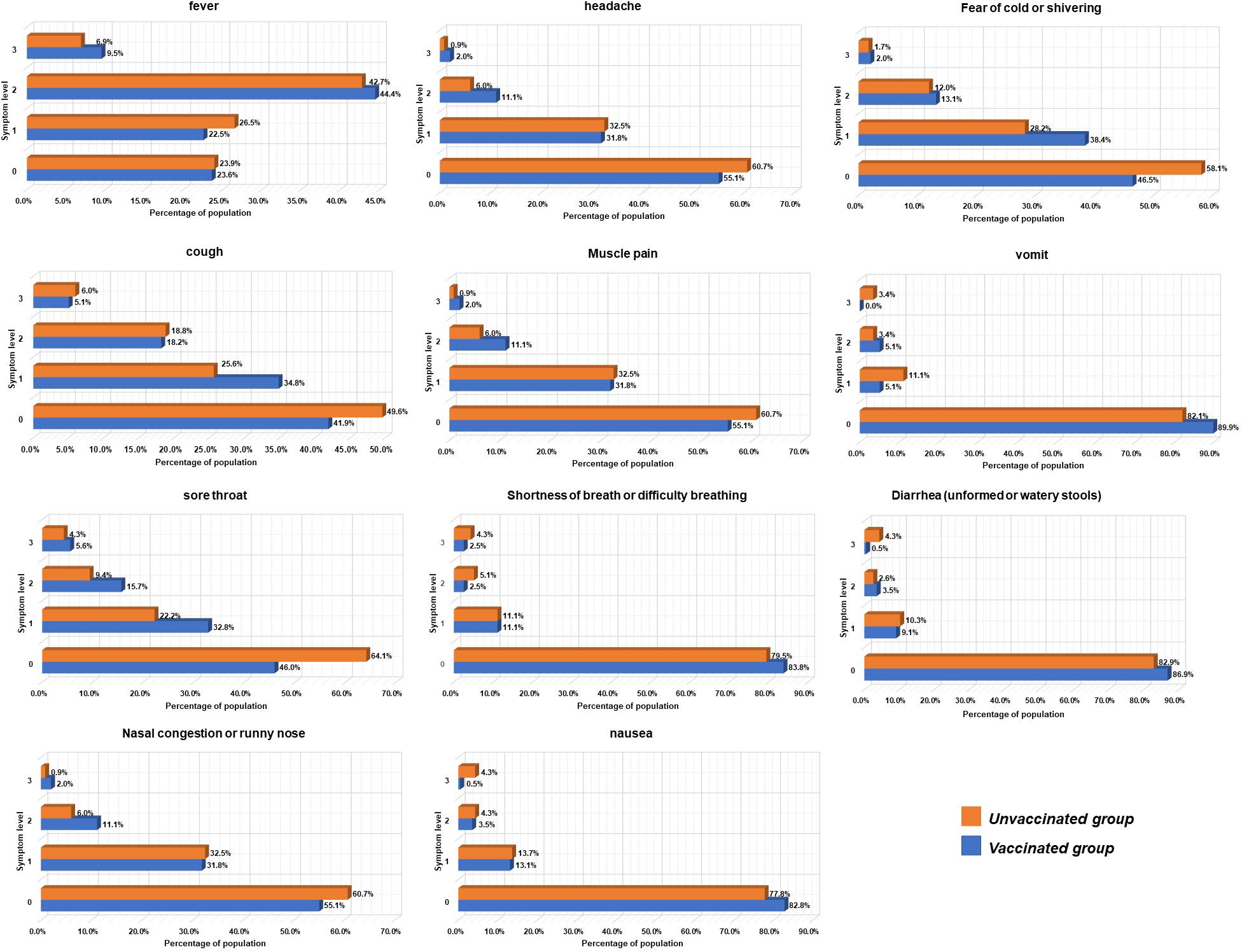
The analysis of COVID-19 symptoms in unvaccinated and vaccinated patients is depicted in Figure 2. No significant differences were observed in the primary symptoms among infected individuals, which predominantly included fever (72.59%), cough (52.41%), chills (46.69%), sore throat (44.88%), and muscle pain (44.58%). The average body temperature was 38.5°C, and the average fever duration was 2 days in infected individuals. Vaccinated patients experienced a shorter duration of upper respiratory symptoms compared to non-vaccinated patients (5.0 vs. 7.0 days, respectively).
The NCP (6.6% vs. 13.7%), severity (1.0% vs. 3.4%), and endpoint (9.10% vs. 31.60%) rates in the vaccinated group were significantly lower than those in the unvaccinated group, with notable differences. Notably, 83.84% of vaccinated patients received treatment at home, while 28.21% of unvaccinated patients relied on community and hospital treatment. Usage rates of antiviral drugs (1.01% vs. 7.69%), antibiotics (17.17% vs. 35.04%), and respiratory support and oxygen therapy (2.53% vs. 11.11%) in the vaccinated group were significantly lower than in the unvaccinated group (P < 0.001).
Discussion
This study elucidates the epidemiological traits and COVID-19 vaccination status of DKD patients. Our analysis showed that patients with DKD experienced a high SARS-CoV-2 infection rate (73.68%) but a low NCP rate (6.63%), with severe pneumonia or COVID-19-related deaths constituting only 2.29%. Among renal pathological subtypes, the DN group registered the lowest vaccination uptake (54.5%), highest all-cause mortality (3.6%), and endpoint of follow-up rates (34.10%). Vaccinated individuals typically exhibited improved kidney function and nutrition. The study further identified that vaccination does not significantly alter infection symptoms but effectively reduces the incidence and severity of COVID-19 pneumonia.
According to WHO COVID-19 dashboard data, at the time that the survey was conducted, the global SARS-CoV-2 infection rate is 10.01%, and the COVID-19 mortality rate is 0.91%. In contrast, China’s SARS-CoV-2 infection rate is 7.04%, and the COVID-19 mortality rate is 0.12% (W.H.O, 2023). Between December 31, 2021, and March 16, 2022, 96.26 million people in Hong Kong (12.96%) had confirmed SARS-CoV-2 infection, with severe disease occurring in 0.9% and deaths occurring in 0.7% of cases (McMenamin et al., 2022). The risk of COVID-19-related death was very low among the population of Qatar during the COVID-19 pandemic, with only 0.13 deaths among every 1,000 individuals per year (AlNuaimi et al., 2023). In a December 21, 2022, teleconference, China’s National Health Commission reported 248 million viral infections from December 1–20, 2022, with a national infection rate of 17.56% and that of some provinces above 50%. Our DKD cohort showed a higher infection rate than did the general population, yet a lower pneumonia rate (6.63%) and a higher mortality rate (1.37%). Diabetes impairs immune function, increasing COVID-19 susceptibility. Both diabetes and CKD are risk factors for SARS-CoV-2 infection and mortality, justifying our cohort’s higher mortality rate. However, it is significantly lower than previously reported mortality rates in China in 2020 [4.3% (Wang et al., 2020), 28.27% (Zhou et al., 2020), 51.7% (Yang et al., 2020)] and for 208 countries distributed globally (5.4%).
Compared with those of patients with other kidney diseases reported domestically and internationally, pneumonia and mortality rates of our cohort of patients were relatively low. A retrospective cohort study of COVID-19 in individuals with CKD in England showed that of those with prevalent and incident CKD, 6.7% and 7.8% were infected, 1.8% and 1.7% died from COVID-19, and 8.9% and 7.0% died from all causes, respectively (Dashtban et al., 2022). The COVID-19 infection rate among a hemodialysis cohort in Turkey was 13.12%, and the mortality rate was 2.87% (Kahvecioglu et al., 2023). Infection and mortality rates of the dialysis cohort in Turkey were higher than those of our population. In another study that considered patients on hemodialysis in Tirana, Albania, the prevalence of COVID-19 infection was 30.5%, and the mortality rate was 19.2%, a value higher than that for patients with DN (Rista et al., 2023). The COVID-19 pneumonia and mortality rates in our diabetic nephropathy cohort were lower than those reported previously for CKD and dialysis patients.
Diabetes has been reported to be associated with an increased risk of death among patients with COVID-19 (Alissa et al., 2023). Diabetes mortality increased significantly in the United States during the pandemic (Yao et al., 2023). In Ontario, 24% of adults with diabetes died compared to 15% of adults without diabetes. In Denmark, 16% of adults with diabetes die in the hospital compared to 13% of those without diabetes (Bogler et al., 2023).
COVID-19 morbidity and mortality are increased via unknown mechanisms in patients with diabetes and kidney disease (Menon et al., 2020). Bornstein et al. (Bornstein et al., 2020) proposed that COVID-19 infection may reduce the expression of angiotensin-converting enzyme 2 (ACE2), causing cell damage, excessive inflammation, and respiratory failure. Acute hyperglycemia upregulates ACE2 expression in cells, enhancing viral entry, while chronic hyperglycemia downregulates it, increasing vulnerability to viral inflammation and destruction. Diabetes predisposes individuals to severe COVID-19. Moreover, infection may precipitate new glucose-related complications. Elevated ACE2 expression and cellular susceptibility to SARS-CoV-2 in diabetic environments are evident in human kidney organoids and patient cells (Menon et al., 2020; Garreta et al., 2022). In addition, neuropilin-1 seems to play an important role in mechanisms linking COVID-19 to diabetic nephropathy (Mourad et al., 2021). Pre-existing endothelial dysfunction and microvascular disease in diabetes exacerbate vascular insults associated with COVID-19, leading to COVID-19 of increased severity (Basra et al., 2022).
Our study identified fever (72.59%), cough (52.41%), chills (46.69%), sore throat (44.88%), and muscle pain (44.58%) as the most prevalent symptoms in infected patients. Less common were shortness of breath, nausea, vomiting, and diarrhea. The mean age of patients was 56 years, with 23.6% being older adults (>60 years). These results align with global studies but show slight variations. A similar retrospective observational study in Pakistan reported fever (87.9%), cough (79.1%), and shortness of breath (55.1%) as primary symptoms upon hospital admission in patients with COVID-19 (Akhtar et al., 2021). In another study conducted at a screening clinic during the early outbreak period, common symptoms were fever (72%), cough (59.5%), and shortness of breath (57%). Our study found that 9.32% of patients were asymptomatic, possibly due to vaccine development and a decrease in viral strain pathogenicity.
Vaccination is a priority among patients with CKD, and the management of long-term conditions is important during and after the pandemic (Dashtban et al., 2022). A population-based study in Israel considered real-world data to evaluate the effectiveness of Paxlovid, revealing that 75.1% of patients had an adequate COVID-19 vaccination status (Najjar-Debbiny et al., 2023). At the time that the survey was conducted, China has provided 3.49 billion doses of the COVID-19 vaccine to a total of 1.3 billion people, with 1.27 billion people receiving a complete primary series of a COVID-19 vaccine. Nationally, the percentage of those receiving the first vaccine dose and that of those undergoing a complete vaccination program have reached 93.0% and 90.6%, respectively (Chinese CDC, 2023). Our study revealed a 59.3% vaccination rate in the DKD population and 54.5% in patients diagnosed with DN. Vaccinated individuals typically exhibit enhanced blood pressure, renal function, and nutritional status, unlike the unvaccinated. However, vaccine status shows a weak correlation with general clinical manifestations. Further, vaccine status is influenced by multiple factors, including willingness, infrastructure and health systems, legal and political influences (Terry et al., 2022), sociodemographic indices, and risk perception (Bayati et al., 2022).
Vaccination not only protects vulnerable individuals from SARS-CoV-2 infection but may also diminish the disease severity risk of COVID-19-related death (Zhang et al., 2023). A case-control analysis showed that unvaccinated patients accounted for 91.0% of COVID-19-related deaths, indicating that the COVID-19 vaccine may significantly reduce mortality and mechanical ventilation rates (Tenforde et al., 2021). In a study conducted in the Kingdom of Saudi Arabia, only 2.8% of mortality occurred in patients vaccinated against the SARS-CoV-2 virus (Alissa et al., 2023). Vaccination, while not significantly impacting infection rates, notably decreased pneumonia and mortality rates and lessened the need for antiviral drugs and respiratory support in vaccinated patients compared to unvaccinated ones. This suggests vaccination’s effectiveness in preventing pneumonia and reducing mortality. The similar infection rates between vaccinated and unvaccinated groups might stem from lifestyle factors, such as more cautious behavior (social distancing, mask-wearing, hand sanitization) among apprehensive patients with type 2 diabetes mellitus during the COVID-19 pandemic (Kaplan Serin and Bülbüloğlu, 2023).
Patients with DKD are closely associated with severe COVID-19, and those with varying renal pathologies exhibit different prognoses and risks of severe disease. Previous studies have not addressed COVID-19 infection in patients with DKD based on a clear pathological diagnosis. For the first time globally, we have elucidated the infection rates, vaccination statuses, and clinical profiles of patients with DKD, rooted in definitive pathological diagnoses. This provides crucial insights into how COVID-19 impacts this vulnerable group and underscores the importance of vaccination in mitigating infection severity.
The limitation of this study lies in deriving data solely from follow-up questionnaires, hence omitting neocoronavirus antibody titer, immune inflammation, and other infection markers, as well as lung CT results. The severity of COVID-19 may be associated with IgG antibody levels (Garcia-Beltran et al., 2021). Patients with severe COVID-19 exhibit elevated IgG antibody concentrations during the recovery phase (Yuan et al., 2021; Chiu et al., 2022). In these patients, the initial antibody response is delayed; however, it is notably enhanced in the middle and later stages. Specifically, IgG antibody levels in severe cases increase significantly in these stages, being 1.5 times higher than in patients exhibiting mild to moderate symptoms (Li et al., 2020). This phenomenon likely occurs because more severe infections elicit stronger immune responses. Notably, the presence of COVID-19 antibodies can serve as an indicator of disease severity and survival rates (Garcia-Beltran et al., 2021; Joyner et al., 2021).
With the global epidemic stabilizing and societal activities resuming, it is crucial to recognize that COVID-19 may hasten nephropathy progression in DN patients with compromised renal function and weakened immunity. Emphasis should be placed on infection prevention and implementing comprehensive diabetes management for these patients (Bornstein et al., 2020).
In conclusion, patients with diabetic nephropathy are vulnerable to COVID-19. Vaccination significantly reduces SARS-CoV-2 infection and lessens COVID-19 severity in DKD patients; thus, enhancing vaccination efforts and protective measures is crucial.
Data availability statement
The datasets generated and analyzed during the present study are available from the corresponding author on reasonable request.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Chinese People’s Liberation Army General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW: Data curation, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. ZD: Funding acquisition, Supervision, Writing – review & editing. WZ: Formal analysis, Methodology, Validation, Writing – review & editing. YZ: Data curation, Investigation, Visualization, Writing – review & editing. QL: Data curation, Formal analysis, Investigation, Writing – review & editing. RZ: Investigation, Writing – review & editing. HH: Investigation, Writing – review & editing. FL: Investigation, Writing – review & editing. YW: Resources, Writing – review & editing. LZ: Resources, Writing – review & editing. XCa: Resources, Writing – review & editing. JW: Resources, Writing – review & editing. JhZ: Resources, Writing – review & editing. GC: Resources, Writing – review & editing. XCh: Funding acquisition, Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Science & Technology Project of Beijing, China (No. Z221100007422121), Beijing Natural Science Foundation (Nos. L222133, L232122), Capital’s Funds for Health Improvement and Research(No. CFH 2024-1-5021)and National Natural Science Foundation of China (No.32141005, and 62250001).
Acknowledgments
We thank the patients involved in the study. We also thank Editage for assistance with editing and proofreading.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1388260/full#supplementary-material
References
Abdulaziz Al-Muhanna, F., Ibraham Ali Albakr, W., Subbarayalu, A. V., Cyrus, C., Ahmed Aljenaidi, H., Ali Alayoobi, L., et al. (2022). Impact of COVID-19 on kidney of diabetic patients. Medicina (Kaunas Lithuania) 58, 644. doi: 10.3390/medicina58050644
Ahmad, F. B., Cisewski, J. A., Xu, J., Anderson, R. N. (2023). COVID-19 mortality update - United States, 2022. MMWR. Morbidity mortality weekly Rep. 72, 493–496. doi: 10.15585/mmwr.mm7218a4
Akhtar, H., Khalid, S., Rahman, F. U., Umar, M., Ali, S., Afridi, M., et al. (2021). Comorbidities, and outcomes among patients with COVID-19 hospitalized in Pakistan: Retrospective observational study. JMIR Public Health surveillance 7, e32203. doi: 10.2196/32203
Alissa, D. A., Aburas, W., Almasuood, R., Almudaiheem, H. Y., Al Aseri, Z., Alrabiah, F., et al. (2023). Prevalence and epidemiological trends in mortality due to COVID-19 in Saudi Arabia. Public Health 215, 31–38. doi: 10.1016/j.puhe.2022.07.014
AlNuaimi, A. A., Chemaitelly, H., Semaan, S., AlMukdad, S., Al-Kanaani, Z., Kaleeckal, A. H., et al. (2023). All-cause and COVID-19 mortality in Qatar during the COVID-19 pandemic. BMJ Global Health 8, e012291. doi: 10.1136/bmjgh-2023-012291
Basra, R., Whyte, M., Karalliedde, J., Vas, P. (2022). What is the impact of microvascular complications of diabetes on severe COVID-19? Microvascular Res. 140, 104310. doi: 10.1016/j.mvr.2021.104310
Bayati, M., Noroozi, R., Ghanbari-Jahromi, M., Jalali, F. S. (2022). Inequality in the distribution of Covid-19 vaccine: a systematic review. Int. J. equity Health 21, 122. doi: 10.1186/s12939-022-01729-x
Bogler, O., Raissi, A., Colacci, M., Beaman, A., Biering-Sørensen, T., Cressman, A., et al. (2023). Association between diabetes and mortality among adult patients hospitalized with COVID-19: A cohort study of hospitalized adults in Ontario, Canada, and Copenhagen, Denmark. Can. J. Diabetes. 47, 352–358. doi: 10.1016/j.jcjd.2023.02.005
Bornstein, S. R., Rubino, F., Khunti, K., Mingrone, G., Hopkins, D., Birkenfeld, A. L., et al. (2020). Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 8, 546–550. doi: 10.1016/S2213-8587(20)30152-2
Chaw, S. M., Tai, J. H., Chen, S. L., Hsieh, C. H., Chang, S. Y., Yeh, S. H., et al. (2020). The origin and underlying driving forces of the SARS-CoV-2 outbreak. J. Biomed. Sci. 27, 73. doi: 10.1186/s12929-020-00665-8
Chiu, C. H., Chang, Y. H., Chang, F. Y., Hung, Y. J., Liao, C. L., Chiu, K. C., et al. (2022). Humoral, cellular and cytokine immune responses against SARS-CoV-2 variants in COVID-19 convalescent and confirmed patients with different disease severities. Front. Cell Infect. Microbiol. 12, 862656. doi: 10.3389/fcimb.2022.862656
Dashtban, A., Mizani, M. A., Denaxas, S., Nitsch, D., Quint, J., Corbett, R., et al. (2022). A retrospective cohort study predicting and validating impact of the COVID-19 pandemic in individuals with chronic kidney disease. Kidney Int. 102, 652–660. doi: 10.1016/j.kint.2022.05.015
Fan, Y., Li, X., Zhang, L., Wan, S., Zhang, L., Zhou, F. (2022). SARS-CoV-2 Omicron variant: recent progress and future perspectives. Signal transduction targeted Ther. 7, 141. doi: 10.1038/s41392-022-00997-x
Garcia-Beltran, W. F., Lam, E. C., Astudillo, M. G., Yang, D., Miller, T. E., Feldman, J., et al. (2021). COVID-19-neutralizing antibodies predict disease severity and survival. Cell 184, 476–488.e11. doi: 10.1016/j.cell.2020.12.015
Garreta, E., Prado, P., Stanifer, M. L., Monteil, V., Marco, A., Ullate-Agote, A., et al. (2022). A diabetic milieu increases ACE2 expression and cellular susceptibility to SARS-CoV-2 infections in human kidney organoids and patient cells. Cell Metab. 34, 857–873.e9. doi: 10.1016/j.cmet.2022.04.009
Hadjadj, S., Saulnier, P. J., Ruan, Y., Zhu, X., Pekmezaris, R., Marre, M., et al. (2023). Associations of microvascular complications with all-cause death in patients with diabetes and COVID-19: The CORONADO, ABCD COVID-19 UK national audit and AMERICADO study groups. Diabetes Obes. Metab. 25, 78–88. doi: 10.1111/dom.14845
Joyner, M. J., Carter, R. E., Senefeld, J. W., Klassen, S. A., Mills, J. R., Johnson, P. W., et al. (2021). Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl. J. Med. 384, 1015–1027. doi: 10.1056/NEJMoa2031893
Kahvecioglu, S., Bilen, N., Celik, H., Gul, C. B., Usta, M., Aktaş, N., et al. (2023). Comparison of all renal replacement therapy modalities in terms of COVID-19 infection rate & mortality in the COVID-19 pandemic and importance of home therapies. Ther. apheresis dialysis: Off. peer-reviewed J. Int. Soc. Apheresis Japanese Soc. Apheresis Japanese Soc. Dialysis Ther. 27, 402–411. doi: 10.1111/1744-9987.13930
Kaplan Serin, E., Bülbüloğlu, S. (2023). The effect of attitude to death on self-management in patients with type 2 diabetes mellitus during the COVID-19 pandemic. Omega. 87, 448–468. doi: 10.1177/00302228211020602
Li, K., Huang, B., Wu, M., Zhong, A., Li, L., Cai, Y., et al. (2020). Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 11, 6044. doi: 10.1038/s41467-020-19943-y
Li, X., Wang, L., Yan, S., Yang, F., Xiang, L., Zhu, J., et al. (2020). Clinical characteristics of 25 death cases with COVID-19: A retrospective review of medical records in a single medical center, Wuhan, China. Int. J. Infect. diseases: IJID: Off. Publ. Int. Soc. Infect. Dis. 94, 128–132. doi: 10.1016/j.ijid.2020.03.053
McMenamin, M. E., Nealon, J., Lin, Y., Wong, J. Y., Cheung, J. K., Lau, E. H. Y., et al. (2022). Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect. Dis. 22, 1435–1443. doi: 10.1016/S1473-3099(22)00345-0
Menon, R., Otto, E. A., Sealfon, R., Nair, V., Wong, A. K., Theesfeld, C. L., et al. (2020). SARS-CoV-2 receptor networks in diabetic and COVID-19-associated kidney disease. Kidney Int. 98, 1502–1518. doi: 10.1016/j.kint.2020.09.015
Mourad, D., Azar, N. S., Azar, S. T. (2021). Diabetic nephropathy and COVID-19: the potential role of immune actors. Int. J. Mol. Sci. 22, 7762. doi: 10.3390/ijms22157762
Najjar-Debbiny, R., Gronich, N., Weber, G., Khoury, J., Amar, M., Stein, N., et al. (2023). Effectiveness of paxlovid in reducing severe coronavirus disease 2019 and mortality in high-risk patients. Clin. Infect. diseases: an Off. Publ. Infect. Dis. Soc. America 76, e342–e349. doi: 10.1093/cid/ciac443
NHC, NATCM. (2023). China National Health Commission, Diagnosis and Treatment Plan for novel coronavirus Infection, Tenth Edition.
Qu, P., Evans, J. P., Faraone, J. N., Zheng, Y. M., Carlin, C., Anghelina, M., et al. (2023). Enhanced neutralization resistance of SARS-CoV-2 Omicron subvariants BQ.1, BQ.1.1, BA.4.6, BF.7, and BA.2.75.2. Cell Host Microbe 31, 9–17.e3. doi: 10.1016/j.chom.2022.11.012
Rista, E., Dervishi, D., Cadri, V., Akshija, I., Saliaj, K., Bino, S., et al. (2023). Predictors of mortality in hemodialysis patients with COVID-19: A single-center experience. J. infection developing countries 17, 454–460. doi: 10.3855/jidc.17065
Salinas-Aguirre, J. E., Sánchez-García, C., Rodríguez-Sanchez, R., Rodríguez-Muñoz, L., Díaz-Castaño, A., Bernal-Gómez, R. (2022). [Clinical characteristics and comorbidities associated with mortality in patients with COVID-19 in Coahuila (Mexico)]. Rev. clinica espanola 222, 288–292. doi: 10.1016/j.rce.2020.12.006
Tenforde, M. W., Self, W. H., Adams, K., Gaglani, M., Ginde, A. A., McNeal, T., et al. (2021). Association between mRNA vaccination and COVID-19 hospitalization and disease severity. Jama 326, 2043–2054. doi: 10.1001/jama.2021.19499
Terry, E., Cartledge, S., Damery, S., Greenfield, S. (2022). Factors associated with COVID-19 vaccine intentions during the COVID-19 pandemic; a systematic review and meta-analysis of cross-sectional studies. BMC Public Health 22, 1667. doi: 10.1186/s12889-022-14029-4
Wang, D., Hu, B., Hu, C., Zhu, F., Liu, X., Zhang, J., et al. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. Jama 323, 1061–1069. doi: 10.1001/jama.2020.1585
Wiersinga, W. J., Rhodes, A., Cheng, A. C., Peacock, S. J., Prescott, H. C. (2020). Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): A review. Jama 324, 782–793. doi: 10.1001/jama.2020.12839
Yang, L., Liu, J., Zhang, R., Li, M., Li, Z., Zhou, X., et al. (2020). Epidemiological and clinical features of 200 hospitalized patients with corona virus disease 2019 outside Wuhan, China: A descriptive study. J. Clin. virology: Off. Publ. Pan Am. Soc. Clin. Virol. 129, 104475. doi: 10.1016/j.jcv.2020.104475
Yang, X., Yu, Y., Xu, J., Shu, H., Xia, J., Liu, H., et al. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481. doi: 10.1016/S2213-2600(20)30079-5
Yao, X. I., Han, L., Sun, Y., He, D., Zhao, S., Ran, J. (2023). Temporal variation of excess deaths from diabetes during the COVID-19 pandemic in the United States. J. infection Public Health 16, 483–489. doi: 10.1016/j.jiph.2023.01.018
Yuan, Y., Wang, H., Zhao, J., Jing, N., Xu, J., Li, W., et al. (2021). Severe acute respiratory syndrome coronavirus 2 viral RNA load status and antibody distribution among patients and asymptomatic carriers in central China. Front. Cell Infect. Microbiol. 11, 559447. doi: 10.3389/fcimb.2021.559447
Zhang, J. J., Dong, X., Liu, G. H., Gao, Y. D. (2023). Risk and protective factors for COVID-19 morbidity, severity, and mortality. Clin. Rev. Allergy Immunol. 64, 90–107. doi: 10.1007/s12016-022-08921-5
Keywords: coronavirus disease, diabetic kidney disease, diabetic nephropathy, infection rate, vaccination
Citation: Wang Q, Dong Z, Zhang W, Zheng Y, Lyu Q, Zhang R, Huang H, Liu F, Wang Y, Zhang L, Cao X, Wu J, Zhou J, Cai G and Chen X (2024) COVID-19 epidemic investigation study of a follow-up cohort of patients with diabetic kidney disease. Front. Cell. Infect. Microbiol. 14:1388260. doi: 10.3389/fcimb.2024.1388260
Received: 19 February 2024; Accepted: 31 July 2024;
Published: 20 August 2024.
Edited by:
Zisis Kozlakidis, International Agency For Research On Cancer (IARC), FranceReviewed by:
Benjamin Florian Koch, Goethe University Frankfurt, GermanyDebjani Taraphdar, Sodani Hospital & Diagnostics Pvt Ltd, India
Copyright © 2024 Wang, Dong, Zhang, Zheng, Lyu, Zhang, Huang, Liu, Wang, Zhang, Cao, Wu, Zhou, Cai and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiangmei Chen, eG1jaGVuMzAxQDEyNi5jb20=; Zheyi Dong, c2hlbmdkYWkyNkAxNjMuY29t
†These authors have contributed equally to this work
‡ORCID: Zheyi Dong, orcid.org/0000-0001-7426-1666
 Qian Wang
Qian Wang Zheyi Dong
Zheyi Dong Weiguang Zhang
Weiguang Zhang Ruimin Zhang
Ruimin Zhang Hui Huang
Hui Huang Fang Liu
Fang Liu Jianhui Zhou
Jianhui Zhou Guangyan Cai
Guangyan Cai Xiangmei Chen
Xiangmei Chen