
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 10 July 2024
Sec. Clinical Infectious Diseases
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1380494
Background: Compared with Human Immunodeficiency Virus (HIV) patients, non-HIV patients with Pneumocystis pneumonia (PCP) have more rapid onset, more rapid progression, and higher mortality.
Objectives: To investigate the predictive value of variables obtained upon hospital admission for in-hospital death and 90-day outcomes in non-HIV-PCP patients with respiratory failure (RF).
Methods: This was a single center retrospective study in a tertiary care institution over 15 years. It included all adults inpatients (≥18 years old) with laboratory confirmed non-HIV-PCP with RF who were discharged or died from Peking University First Hospital between April 1st, 2007 and November 1st, 2022. Epidemiological, clinical, laboratory, imaging and outcome data were collected from patient records.
Results: In this study, a total of 146 non-HIV-PCP patients with RF were included. There were 57 patients (39%) died during hospitalization, 44 patients (53%) died in Intensive care unit (ICU). A total of 137 patients completed 90 days of follow-up, of which 58 (42.3%) died. The multivariable regression analysis revealed that a CD8+ T cell count <115/μl (P=0.009), bronchoalveolar lavage fluid (BALF)-neutrophil percentage ≥50% (P=0.047), the time from corticosteroids withdrawal to symptom onset ≤5 days (P=0.012), and the time from visit to initiation of sulfonamides ≥2 days (P=0.011) were independent risk factors for in-hospital death. Furthermore, a CD8+ T cell count < 115/μl (P=0.001) and the time from visit to initiation of sulfonamides therapy ≥2 days (P=0.033) was independently associated with 90-day all-cause death.
Conclusions: A low CD8+ T cell count in peripheral blood, a high percentage of BALF-neutrophils, a short time from corticosteroids withdrawal to symptom onset, and a long time from visit to initiation of sulfonamides are associated with poor prognosis in non-HIV-PCP patients with RF.
Pneumocystis pneumonia (PCP) is a serious fungal infection that primarily affects immunocompromised individuals, such as those with Human Immunodeficiency Virus (HIV)/acquired immune deficiency syndrome (AIDS), cancer, or organ transplant recipients. With the extensive clinical application of tumor chemoradiotherapy, corticosteroids and immunosuppressants, the susceptible population of Pneumocystis jirovecii has gradually shifted from HIV infected individuals to non-HIV infected individuals (Buchacz et al., 2016; Pegorie et al., 2017; Xue et al., 2023). Respiratory failure (RF) in non-HIV related PCP patients is the most common cause of intensive care unit (ICU) admission and an independent risk factor for death (Maschmeyer et al., 2016), with a mortality rate of 50%-80% (Festic et al., 2005; Limper et al., 2017).
The clinical course of PCP varies between HIV and non-HIV infected patients. Generally, non-HIV-PCP patients exhibit more abrupt RF, while HIV infected patients have more insidious clinical manifestations (Monnet et al., 2008; Ko et al., 2014). This is related to the pathogen burden and inflammatory response in the lungs of HIV and non-HIV infected patients (Ziefer and Abramowitz, 1989). Studies have indicated that at least two-thirds of PCP patients require mechanical ventilation, which is associated with higher in-hospital mortality (Curtis et al., 2000; Mansharamani et al., 2000b; Festic et al., 2005; Miller et al., 2006). Consequently, non-HIV-infected patients with RF requiring mechanical ventilation have worse clinical outcomes than HIV-infected patients (Gaborit et al., 2019). Previous studies on prognostic risk factors in non-HIV-PCP patients with RF have identified high acute physiology and chronic health evaluation III (APACHE III) score, corticosteroid use before diagnosis of PCP, severity of disease, underlying disease, initial anti-PCP treatment failure, delayed intubation, elevated end-expiratory positive pressure on day 3, long duration of positive airway pressure ventilation, and pneumothorax were independent risk factors for poor prognosis (Festic et al., 2005; Boonsarngsuk et al., 2009; Ko et al., 2014; Burghi et al., 2021). These studies mainly focused on the influence of patient factors, laboratory indicators and treatment on the clinical outcome of patients (Kim et al., 2014). When patients develop adverse events such as RF, tracheal intubation, and ICU admission, the management of patients with PCP will be more difficult, and it is particularly important to pay attention to the occurrence of critical node events and to find risk factors affecting the prognosis. At present, there are few studies on the prognosis of non-HIV-PCP patients with RF, and the relationship between important event nodes in the clinical course and prognosis is not clear.
Therefore, we described and analyzed the clinical data of 146 non-HIV-PCP patients with RF in detail, aiming to find the risk factors affecting the prognosis of patients with RF through the clinical course of such patients and the changes in the time nodes of relevant important events, so as to optimize the clinical diagnosis and treatment, and provide the basis for improving the diagnosis and treatment of the disease and reducing the mortality.
We performed a retrospective analysis of adult patients with non-HIV-PCP who were admitted to Peking University First Hospital requiring treatment for RF from April 2007 to October 2022. Patients who met the following criteria were enrolled in the study (Xue et al., 2023): HIV test is negative (Pegorie et al., 2017); Diagnostic criteria for PCP (Enomoto et al., 2010; Ainoda et al., 2012; Vogel et al., 2012; Ricciardi et al., 2017): ① Cough, fever, dyspnea or hypoxemia and other clinical manifestations; ② Imaging findings consistent with PCP, such as ground-glass changes in both lungs or diffuse interstitial infiltration; ③ Pneumocystis jirovecii was detected in bronchoalveolar lavage fluid (BALF), sputum, bronchial flushing fluid, bronchial secretions and lung tissue (Buchacz et al., 2016); The definition of RF (Roussos and Koutsoukou, 2003).
Clinical data on each patient’s demographic characteristics, past history, underlying disease, laboratory indicators, microbiology, radiology, treatment and prognosis were collected by consulting electronic medical records. The date of diagnosis was defined as the date of microbial confirmation. The time from presentation to initiation of sulfonamides therapy referred to the time interval between outpatient, emergency treatment or direct admission to trimethoprim-sulfamethoxazole (TMP-SMX). A history of corticosteroids use referred to the use of corticosteroids within one month prior to admission for PCP. All available information related to dosage and duration need to be documented, and the dosage of different types of corticosteroids should be converted to equivalent doses of prednisone.
In the study, measurement data conforming to normal distribution were expressed as mean ± standard deviation (x ± s), and t test was used to compare differences between groups. Measurement data of non-normal distribution were expressed as median (IQR), and comparison between two groups was performed by non-parametric Mann-Whitney U test. The rate of counting data (%) was expressed by chi-square test or Fisher exact test. The optimal cut-off value was determined by Receiver Operating Characteristic (ROC) curve, univariate and multivariate logistic regression was used to analyze the relationship between variables and poor prognosis. Kaplan-Meier survival curve was used to evaluate the 90-day survival of variables. Univariate and multivariate cox proportional hazard regression models were used to analyze the relationship between variables and 90-day poor prognosis. When P < 0.05, the difference was statistically significant. SPSS 23.0 and Graphpad Prism 8 were used for data statistics and analysis.
A total of 269 patients diagnosed with PCP in Peking University First Hospital from April 2007 to October 2022 were retrieved through the electronic medical record system, excluding 5 HIV-positive patients, 22 patients under the age of 18, 34 patients with insufficient etiological evidence, 7 patients with incomplete clinical data, 1 patient with colonization, and 54 patients with non-RF. Finally, 146 patients meeting the criteria were included (Figure 1). In the overall population, the median age was 59 years (range 45.8-66 years), of which 98 (67.1%) were male and 48 (32.9%) were female. The median length of hospitalization was 22.5 days (range 12-34 days), and the total number of deaths during hospitalization was 57 (39%). Of the 83 patients admitted to ICU (56.8%), 44 (53%) died. In descending order, 58 cases (36.7%) suffered from autoimmune diseases, 42 cases (26.6%) from kidney diseases, 13 cases (8.2%) from organ transplantation and so on. There were 138 patients (94.5%) with a history of corticosteroid use and 84 patients (60.9%) with a history of corticosteroid withdrawal. The median corticosteroid use was 45 mg/d and the median duration of corticosteroid use was 71 days. There were 103 patients (70.5%) who had taken other immunosuppressive drugs.
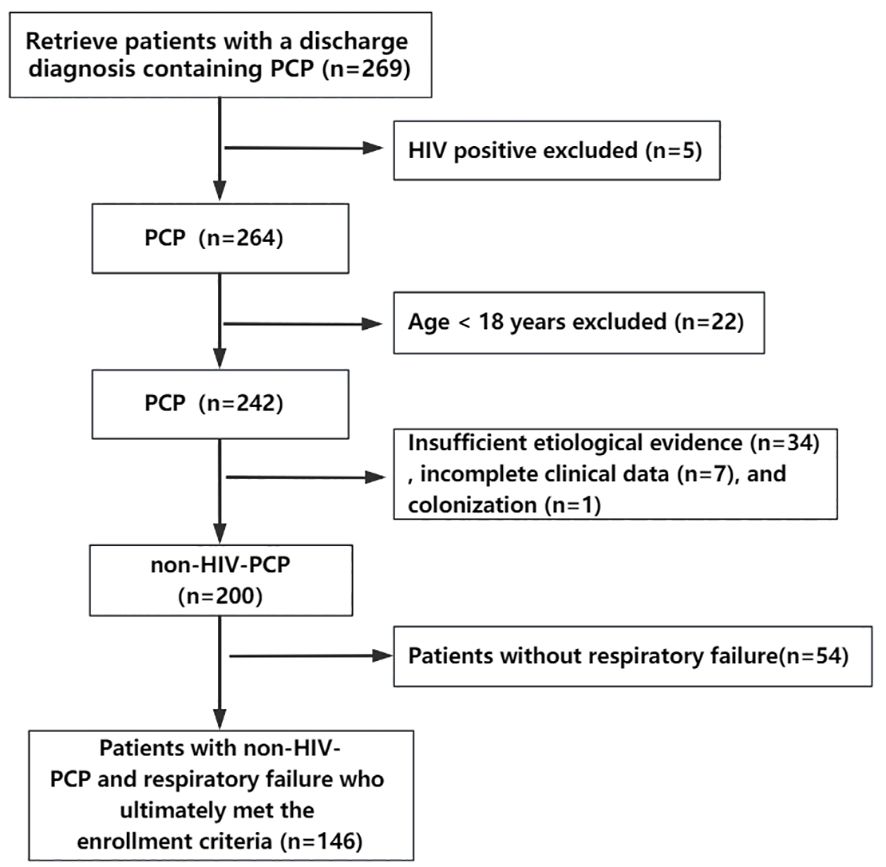
Figure 1 Flow chart of the study. A total of 269 patients diagnosed with PCP from April 2007 to October 2022 were retrieved through the electronic medical record system, excluding 5 HIV-positive patients, 22 patients under the age of 18, 34 patients with insufficient etiological evidence, 7 patients with incomplete clinical data, 1 patient with colonization, and 54 patients with non-respiratory failure. Finally, 146 patients meeting the criteria were included.
The patients were divided into non-survivor (n=57, 39%) and survivor (n=89, 61%) according to death during hospitalization. Patients in the non-survivor group had a higher median age (62 vs 53, P < 0.001) and a lower percentage of patients with a history of chemotherapy (3.5% vs 14.6%, P=0.031). There were no statistically significant differences between the two groups in gender, underlying diseases, and treatment before onset of disease (Table 1).
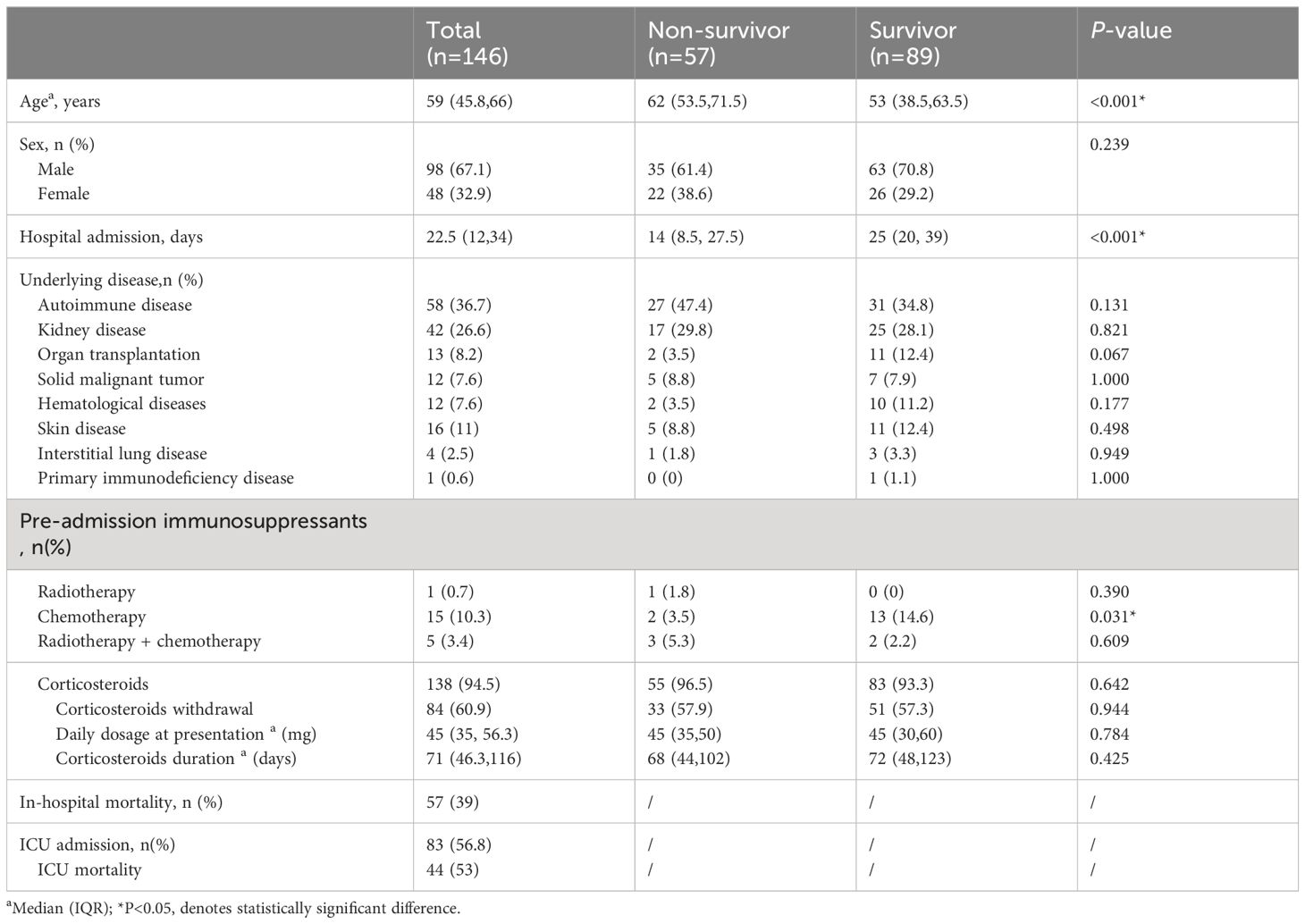
Table 1 Demographics of non-Human Immunodeficiency Virus -Pneumocystis Pneumonia patients with respiratory failure patients.
In terms of clinical symptoms of non-HIV-PCP patients with RF, the proportions of dyspnea, fever, cough, sputum, chest pain and hemoptysis were not statistically different between the two groups, as shown in Table 2.
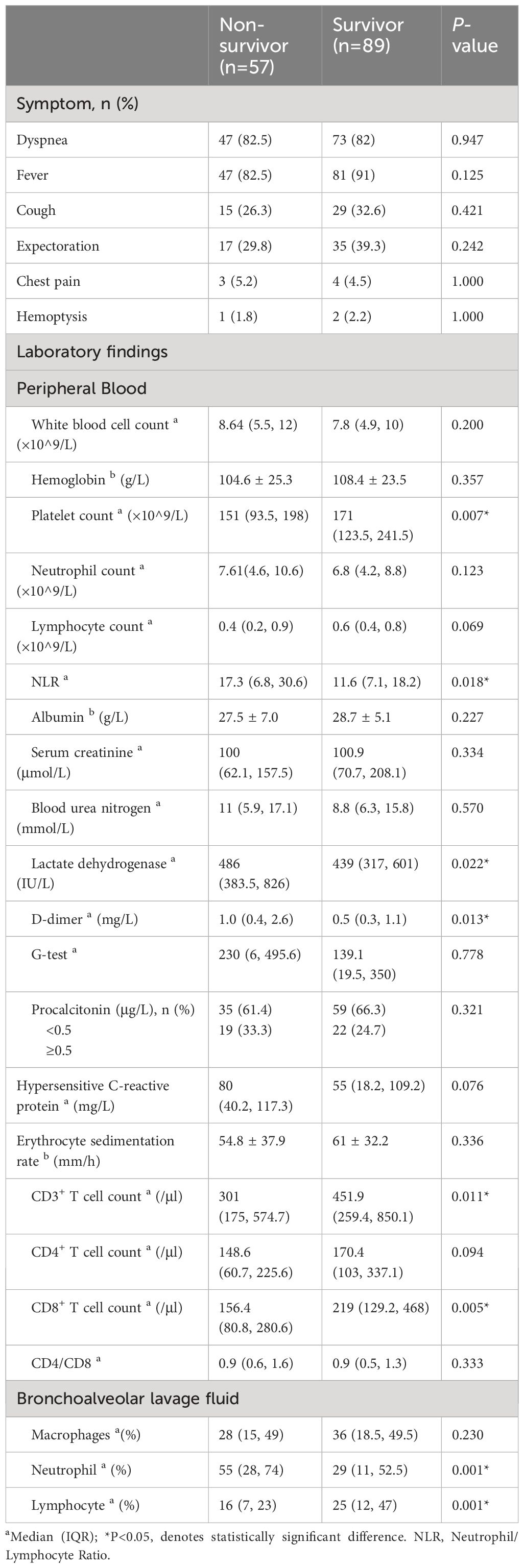
Table 2 Clinical features of non-Human Immunodeficiency Virus -Pneumocystis Pneumonia with respiratory failure patients.
In terms of laboratory measures at admission, patients in the non-survivor group had lower platelet counts (151 vs 171, P=0.007), neutrophil-lymphocyte ratio (NLR) (17.3 vs 11.6, P=0.018), lactate dehydrogenase (LDH) (486 vs 439, P=0.022), and D-dimer (1.0 vs 0.5, P=0.013) was higher, and there was no statistical difference in inflammatory indexes between the two groups. In terms of immunological indicators, peripheral blood CD3+ T cell count (301 vs 451.9, P=0.011) and CD8+ T cell count (156.4 vs 219, P=0.005) were lower in the non-survivors group than in the survivor group, as shown in Figure 2. In BALF, patients in the non-survivor group had a higher percentage of neutrophils (55% vs 29%, P=0.001) and a lower percentage of lymphocytes (16% vs 25%, P=0.001), as shown in Figure 3.
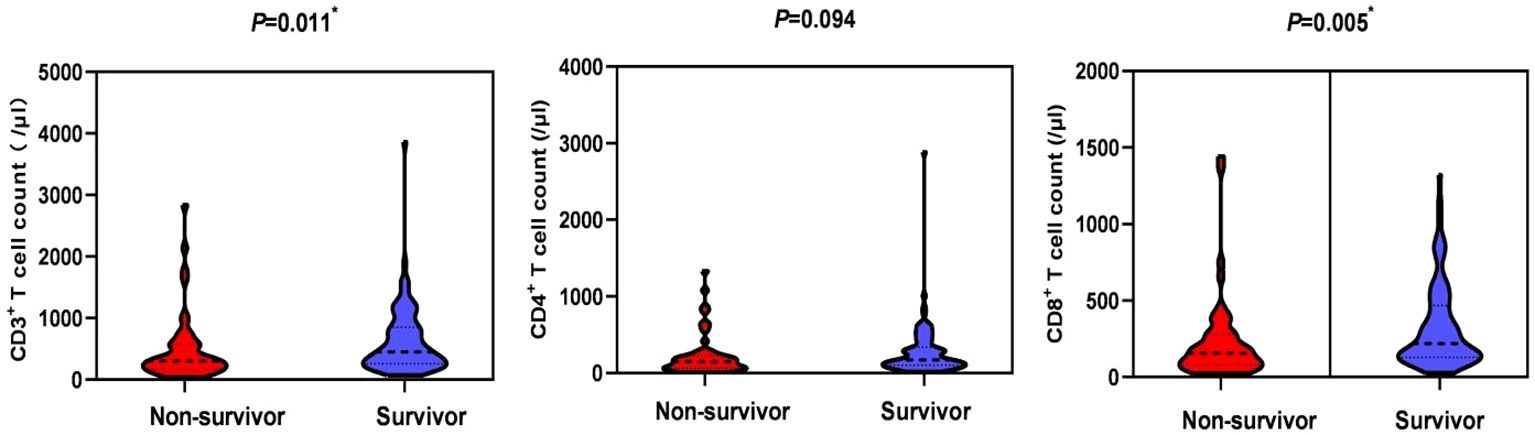
Figure 2 Comparison of peripheral blood lymphocyte subsets in the non-survival group and the survival group. *P < 0.05, indicating statistically significant difference.
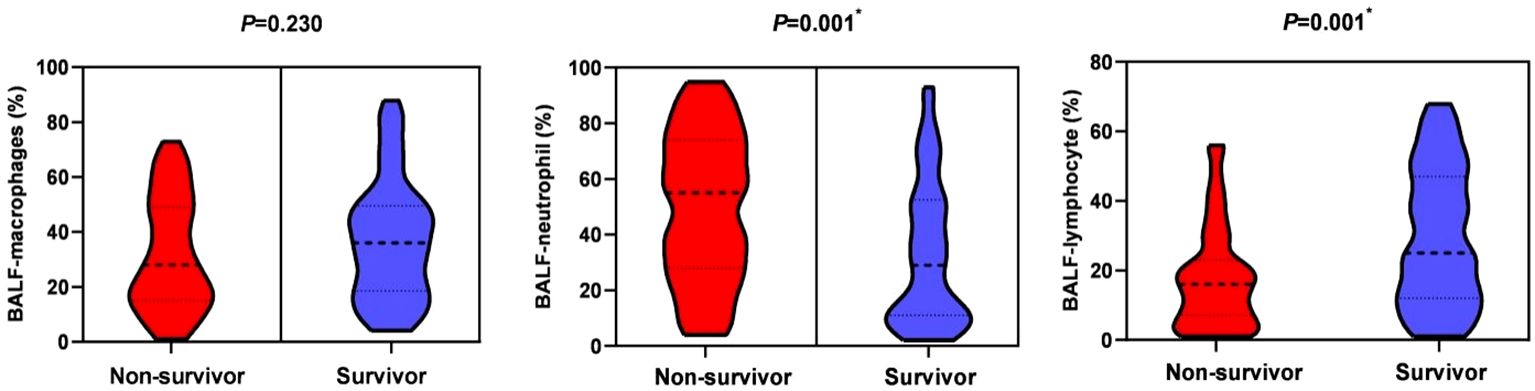
Figure 3 Bronchoalveolar lavage fluid-cytological classification in the non-survival group and the survival group. *P < 0.05, indicating statistically significant difference.
Etiological examination results showed that patients in the non-survivor group had a higher proportion of co-infection with pathogens than those in the survivor group (82.5% vs 58.4%, P=0.002) and a higher proportion of co-infection with two or more pathogens (47.4% vs 19.1%, P<0.001) and a higher proportion of hospital-acquired pneumonia-associated pathogens (42.1% vs. 11.2%, P<0.001). There was no significant difference in the proportion of Cytomegalovirus (CMV) infection and Aspergillus infection between the two groups.
In terms of imaging, patients in the death group had a higher proportion of pneumothorax than those in the survivor group (17.5% vs 4.5%, P=0.020), while there was no statistical difference between the two groups in the proportion of ground glass density shadows, solid shadows, mesh shadows, cellular shadows, and mediastinal emphysema, as shown in Table 3.
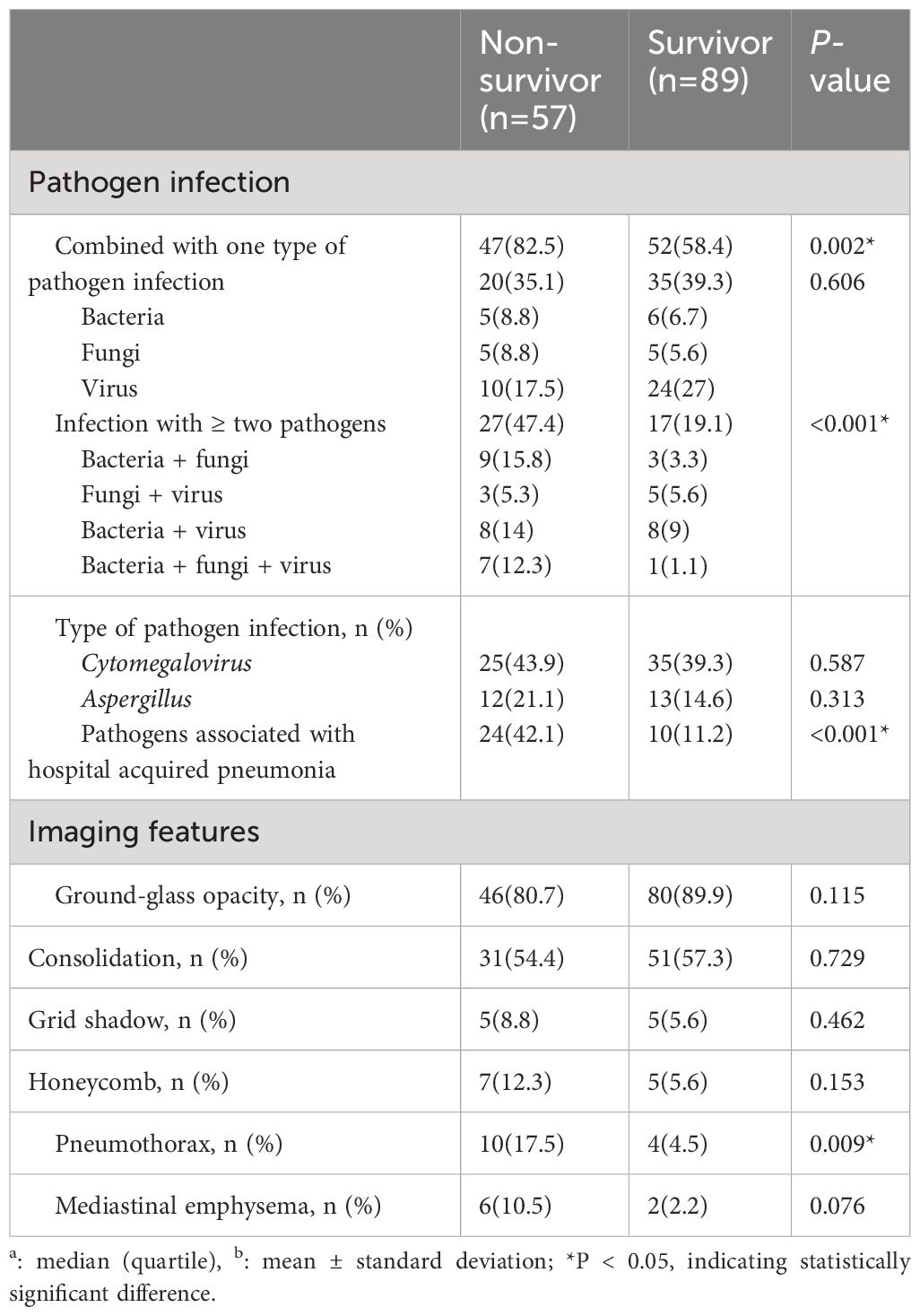
Table 3 Clinical features of non-Human Immunodeficiency Virus -Pneumocystis Pneumonia with respiratory failure patients.
In terms of treatment, the proportion of second-line treatment in the non-survivor group was significantly higher than that in the survivor group (36.8% vs 11.2%, P < 0.001), and the proportion of Caspofungin (56.1% vs 38.2%, P=0.034) and the proportion of high-dose corticosteroids (≥1mg/(kg·d)) (56.6% vs 31.3%, P=0.003) were higher than those in the survivor group, as shown in Supplementary Figure S1. The rates of high-flow oxygen therapy, non-invasive mechanical ventilation, invasive mechanical ventilation and ICU admission in the death group were significantly higher than those in the survivor group (P<0.001), but the length of ICU stay was not statistically different between the two groups, as shown in Table 4.
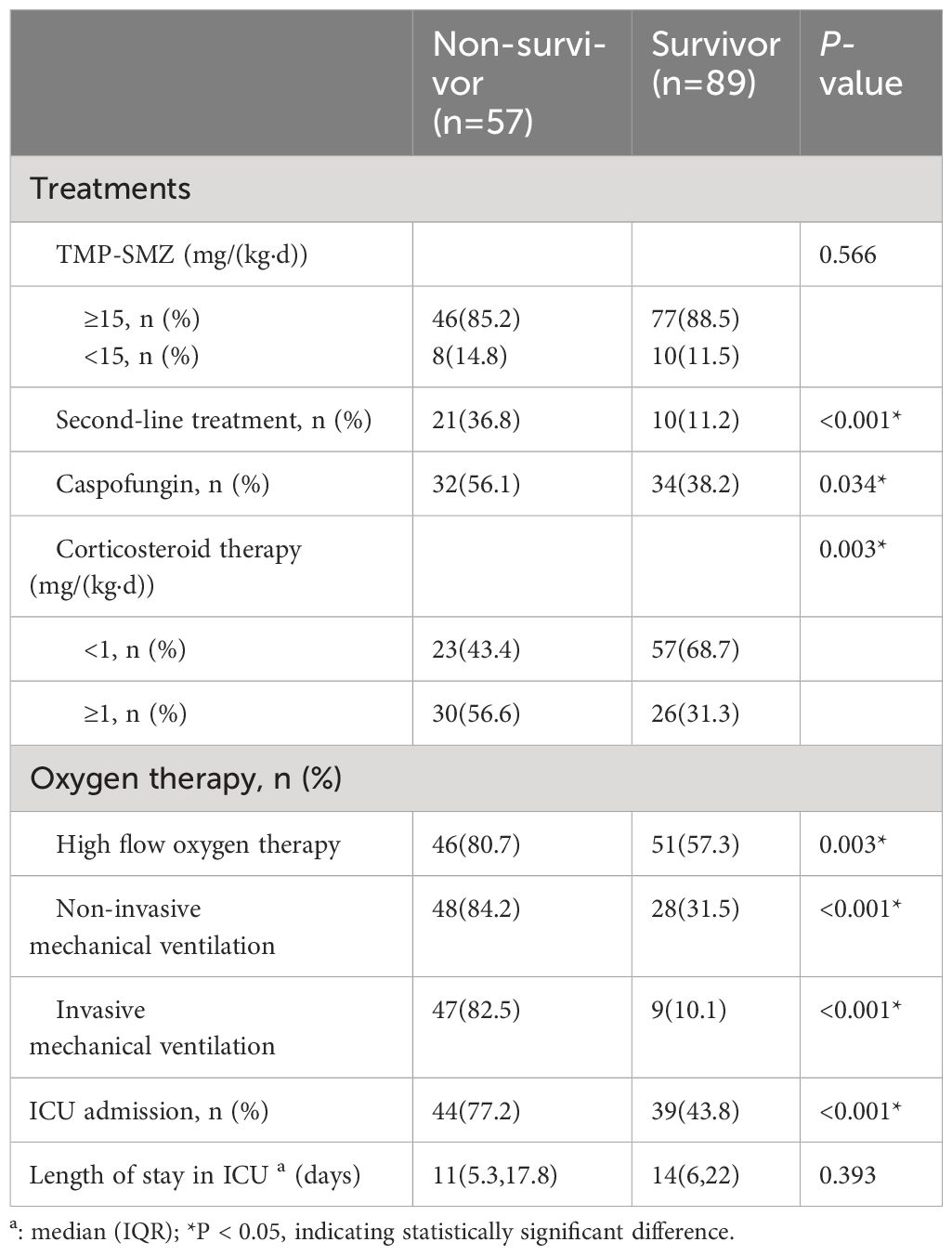
Table 4 Treatments of non-Human Immunodeficiency Virus -Pneumocystis Pneumonia with respiratory failure patients.
Compared with the survival group, a higher proportion of patients in the death group had less than 5 days of withdrawal from corticosteroids to onset of symptoms (57.6% vs 25.5%, P=0.003), and the time between treatment and initiation of sulfonamides therapy was longer (3 vs 2, P=0.019), as shown in Table 5 and Figure 4. The effect of prolonged treatment on in-hospital mortality was observed according to the interquartile segment of sulfonamides treatment interval, and the results showed that in-hospital death of non-HIV-PCP patients with RF increased with prolonged medication duration (P=0.021).
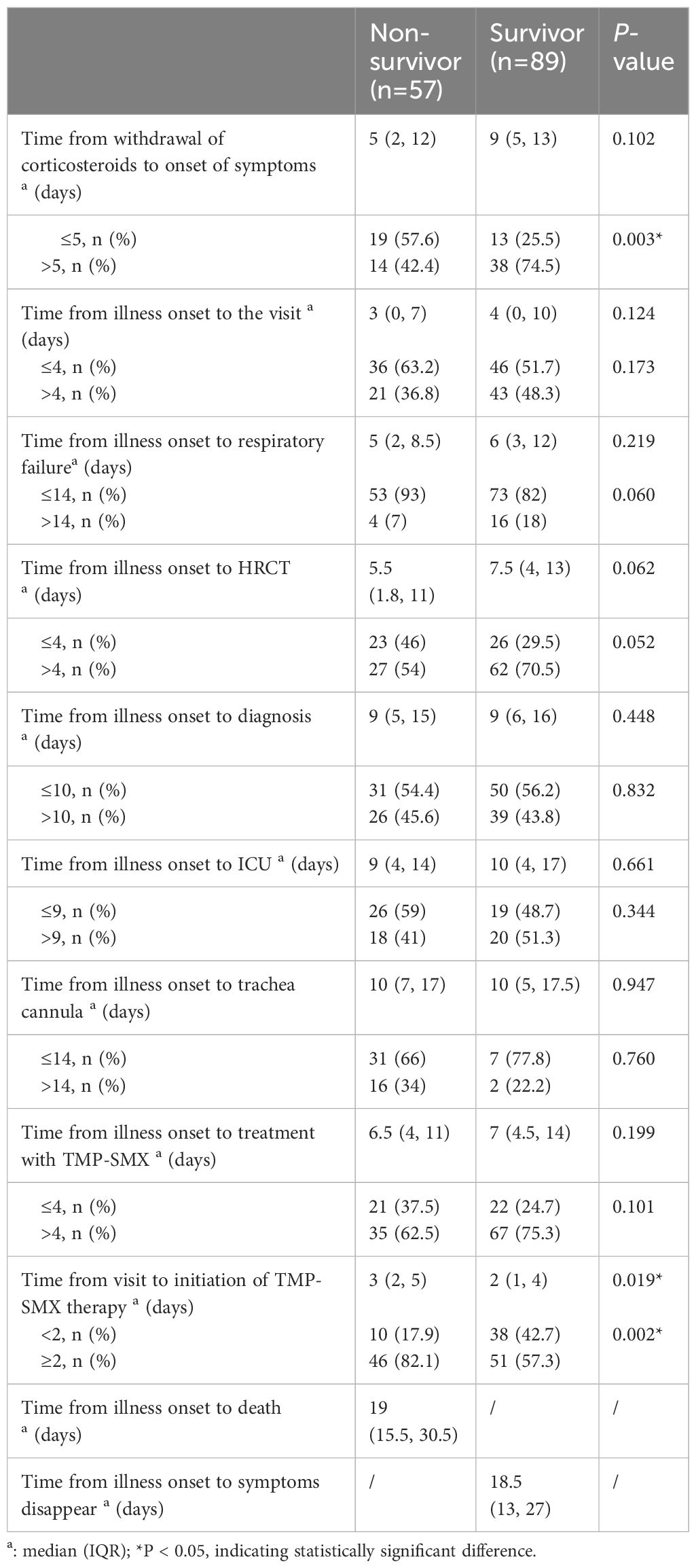
Table 5 Comparison of critical event nodes in non-Human Immunodeficiency Virus -Pneumocystis Pneumonia with respiratory failure patients.
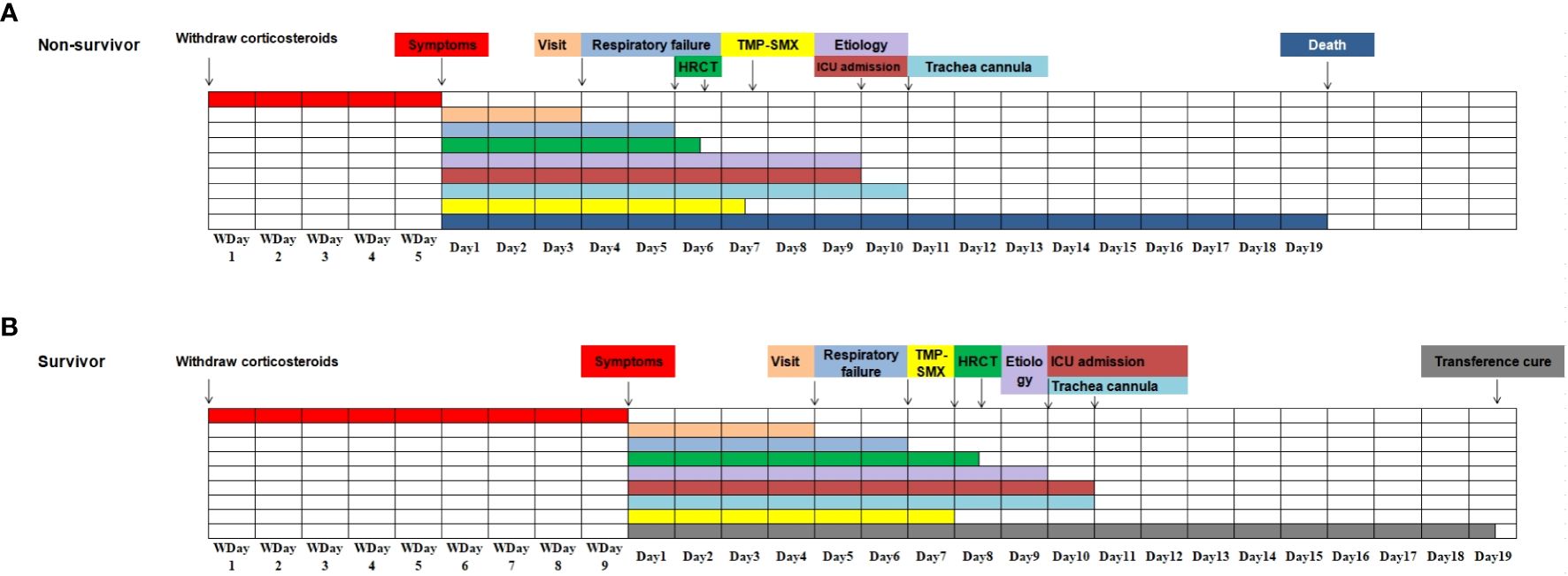
Figure 4 Progression and associated event notes in non-Human Immunodeficiency Virus -Pneumocystis Pneumonia patients with respiratory failure (all event notes are represented by median). (A). The progression of the disease and related important events in the non-survivor group; (B). The progression of the disease and related important events in the survivor group.
The optimal thresholds for CD4+ T cell count and CD8+ T cell count were determined by ROC curve to be 232/μl and 115/μl, respectively, as shown in Supplementary Figure S2. In univariable analysis, age ≥60 years old, chemotherapy history, platelet count <100×10^9/L, NLR≥20, LDH≥454 IU/L, CD4+ T cell count < 232/μl, CD8+ T cell count <115/μl, percentage of BALF-neutrophils ≥50%, percentage of BALF-lymphocytes <20%, infection with hospital-acquired pneumonia-associated pathogens, pneumothorax, withdrawal of corticosteroids to the onset of symptoms ≤5 days, and time from visit to initiation of sulfonamides therapy ≥2 days were associated with prognosis in non-HIV-PCP patients with RF (P < 0.05).
Factors with P <0.1 were incorporated into the logistic model for multivariate analysis, and the results showed that CD8+ T cell count <115/μl (OR=15.803, 95%CI 1.988-125.611, P=0.009), BALF-neutrophils percentage ≥50%(OR=7.678, 95%CI 1.025-57.502, P=0.047), withdrawal of corticosteroids to the onset of symptoms ≤5 days (OR=14.831, 95%CI 1.827-120.381, P=0.012), and the time from presentation to initiation of sulfonamides ≥2 days (OR=16.313, 95%CI 1.890-140.813, P=0.011) was an independent risk factor for in-hospital death in PCP patients with RF, as shown in Figure 5.

Figure 5 Analysis of risk factors for death during hospitalization in non-Human Immunodeficiency Virus -Pneumocystis Pneumonia patients with respiratory failure - Forest map. Blue represents the single factor analysis index, red represents the multi-factor analysis index; CI, Confidence Interval; OR, Odds Ratio.
In this study, 146 non-HIV-PCP patients with RF were followed up for 90 days, 9 were lost to follow-up and 58 were died (57 of them died during hospitalization and 1 died during follow-up). A total of 137 patients completed follow-up, and the 90-day survival rate was 57.7%. The survival analysis curve is shown in Supplementary Figure S3. Univariable analysis was performed for T cell count, BALF-neutrophil percentage, BALF-lymphocyte percentage, hospital-acquired pneumonia-associated pathogen infection, pneumothorax, time from withdrawal of corticosteroids to onset of symptoms, and time from visit to initiation of sulfonamides use.
Factors with P < 0.1 were included in cox regression model for multivariate analysis, and the results showed that a CD8+ T cell count <115/μl (HR=4.418, 95%CI 1.867-10.457, P=0.001) and the time from visit to initiation of sulfonamides therapy ≥2 days (HR=5.304, 95%CI 1.139-24.687, P=0.033) were independent risk factors for 90-day all-cause death in non-HIV-PCP patients with RF, as shown in Figure 6.
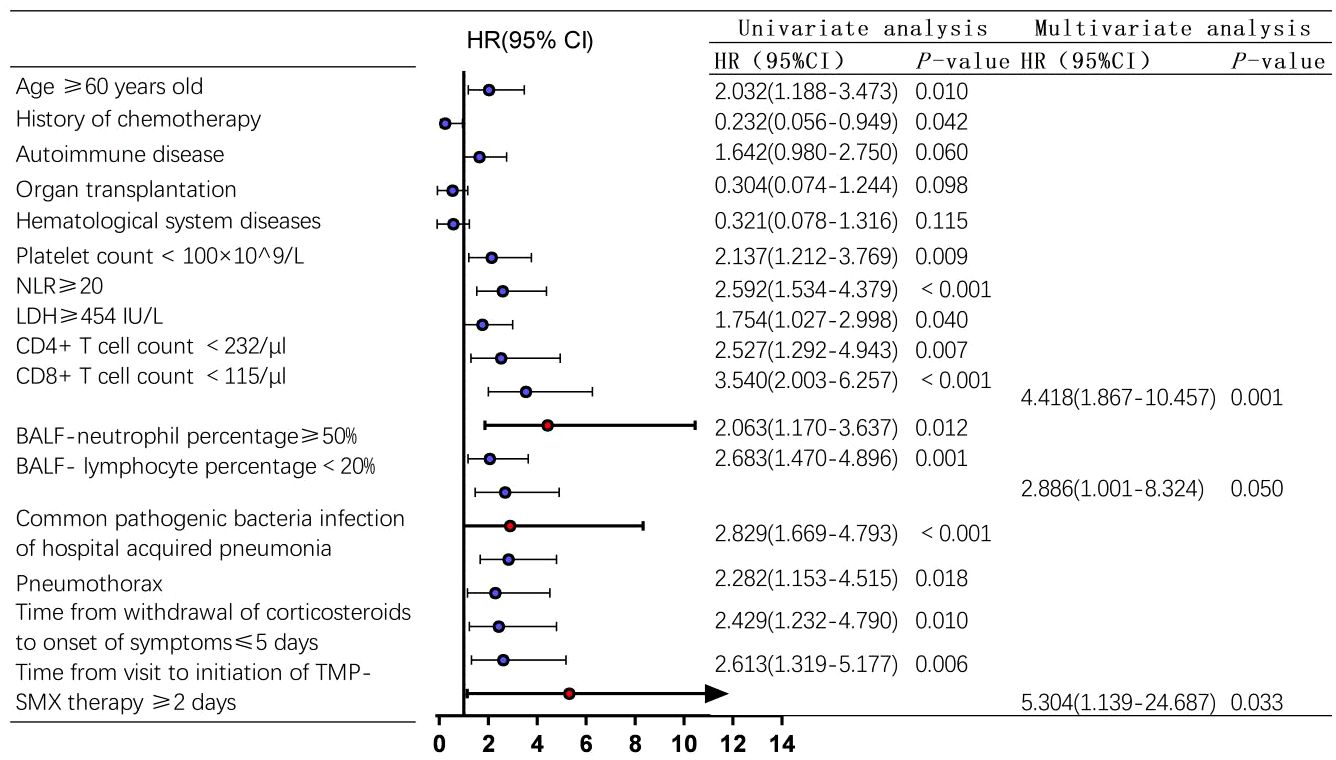
Figure 6 Analysis of 90-day risk factors for all-cause death in non-Human Immunodeficiency Virus -Pneumocystis Pneumonia patients with respiratory failure-Forest map. blue represents the single factor analysis index, red represents the multi-factor analysis index; CI, Confidence Interval; HR, Hazard Ratio.
In non-HIV patients, PCP-related RF remains a highly lethal disease, with a mortality rate of 50-80% (Festic et al., 2005; Limper et al., 2017). In this study, the mortality rate of non-HIV-PCP patients with RF was 39%, which was lower than that reported in previous literature. The main reason was considered that the enrolled population in the previous study was mostly RF patients who required mechanical ventilation and were admitted to ICU (Festic et al., 2005; Boonsarngsuk et al., 2009; Ko et al., 2014). Studies have shown that patients requiring mechanical ventilation have a higher mortality rate (Festic et al., 2005; Ko et al., 2014), which increases the overall mortality rate. By analyzing the clinical course of non-HIV-PCP patients with RF, this study innovatively explored the relationship between important event nodes and prognosis in PCP patients with RF, and screened out the risk factors affecting prognosis.
T lymphocyte subsets are important prognostic markers for non-HIV-PCP patients with RF. Previously, most of the studies were related to T lymphocyte subsets and PCP, and the focus was on CD4+ T cells. Previous studies have shown that approximately 90% of PCP occurs in patients with a CD4+ T cell count <200/μl (Helweg-Larsen et al., 1998), and the HIV-related guidelines have clearly recommended prophylactic treatment of PCP with a CD4+ T cell count < 200/μl as a level IA recommendation. However, the role of CD4+ T cells in non-HIV-PCP patients is not as prominent, and they are mainly valuable for identifying specific individuals at particularly high clinical risk of PCP, such as recipients of allogeneic hematopoietic stem cell (Castagnola et al., 1995), patients with solid malignancies undergoing chemotherapy (Sepkowitz et al., 1992), organ transplantation patients, and individuals with hematological malignancies (Mansharamani et al., 2000a). Tang et al. conducted a study on immunological indicators for immunocompromised patients and found that the decrease in the number of CD3+ T, CD4+ T and CD8+ T cells was the most typical feature of PCP patients (Tang et al., 2021). Brakemeier and colleagues observed similar results in laboratory measurements of PCP patients who received organ transplants, supporting CD3+ T and CD8+ T cell counts as prominent predictors of the development of non-HIV-PCP (Brakemeier et al., 2018). Furthermore, in patients with autoimmune diseases, a CD3+ T cell count <625/μl has been identified as an independent predictor of PCP, while a CD8+ T cell count <160/μl is a risk factor for death in PCP patients (Li et al., 2017). In our study, the majority of the subjects were patients with inflammatory diseases. The results showed that CD4+ T cell count was associated with the prognosis of non-HIV-PCP patients with RF, but was not an independent risk factor. On the other hand, a CD8+ T cell count < 115/μl was identified as an independent risk factor for all-cause death during hospitalization and 90 days in non-HIV-PCP patients with RF. This highlights the important role of CD8+ T cells in the host immune response. Previous studies have suggested that the pathogenesis of CD8+ T cells and non-HIV-PCP is as follows:
In the absence of CD4+ T cells, CD8+ T cells will play a role in eliminating Pneumocystis and constitute a secondary defense against the fungus. The protective factor reacts to Pneumocystis in absence of CD4+ T cells (Beck et al., 1996; Ruan et al., 2016). In cases of PCP, a large number of CD8+ T cells are recruited to the lungs, especially when CD4+ T cell depletion is significant (Meissner et al., 2005). While the removal of CD8+ T cells did not affect fungal load in immunocompetent mice, removing of CD8+ T cells in the absence of CD4+ T cells led to an increase in Pneumocystis load (Beck et al., 1996; Wright et al., 1999). Conversely, the increasing the number of CD8+ T cells through IL-7 in mice with depleted CD4+ T cells further reduced fungal load (Ruan et al., 2016). Studies have shown that CD8+ T cells eliminate Pneumocystis through cytokines such as TNF-α, TNF-β or IFN-γ (Mosmann and Sad, 1996; Mosmann et al., 1997), as well as cytotoxic efflux molecules like perforin or granase. CD8+ T cells also exhibit a pattern similar to helper T cell (Th) 1 and Th2, called cytotoxic T cells (Tc) 1 and Tc2. McAllister et al. discovered that in hosts with deficient CD4+ T cells, Tc1 is crucial for the clearance of Pneumocystis. In contrast, CD8+ T lymphocytes without the Tc1 phenotype (Tc2) seem to cause tissue damage (McAllister et al., 2004). The mechanism is complex, and further research on CD8+ T cell subsets is necessary to determine the specific effects of different subsets of CD8+ T cells on PCP. Our results also indicate a correlation between CD4+ T and CD8+ T cell levels and PCP outcomes. This serves as a reminder that patients with chronic inflammatory or autoimmune diseases should not only focus on monitoring CD4+ T cell count but also pay attention to CD8+ T cell count. This may have significant implications for disease prevention, treatment, and prognosis assessment.
Several studies have indicated that the percentage of neutrophils in BALF can serve as a prognostic marker for non-HIV-PCP patients (Smith et al., 1988; Mason et al., 1989). Lee et al. discovered that a 10% increase in BALF-neutrophil levels correlated with a 15% and 21% increase in 30-day and 60-day mortality, respectively (Lee et al., 2015). Tamai et al. found that BALF-neutrophils ≥31% were significantly associated with in-hospital mortality in non-HIV-PCP patients (Tamai et al., 2014). In our study, we observed that BALF-neutrophils ≥50% were associated with in-hospital death in non-HIV-PCP patients with RF, which is similar to previous findings suggesting that neutrophils may be associated with lung injury, RF and death (Thomas and Limper, 2004). Furthermore, our study demonstrated that BALF-lymphocytes were linked to 90-day all-cause death during hospitalization for non-HIV-PCP patients with RF in the univariate analysis. Some studies have only explored the relationship between BALF-lymphocytes and the prognosis of PCP. Kim et al. reported that BALF-lymphocyte ≤45% is associated with failure of first-line therapy using TMP-SMX (Kim et al., 2018). Additionally, Chung et al. demonstrated a significant correlation between BALF-lymphocyte percentage ≤30% and 90-day all-cause mortality in PCP patients (Chung et al., 2022). Hirasawa et al. reported that BALF-lymphocyte percentage ≥20% was significantly associated with increased 90-day and 1-year survival in patients with acute respiratory failure (ARF) caused by interstitial lung disease or non-interstitial lung disease (Hirasawa et al., 2021). These findings suggested that BALF-lymphocytes may play a vital role in the prognosis of PCP patients with RF, and the usefulness of BALF-lymphocytes warrants further investigation.
In addition to, it is also crucial to pay attention to important clinical events during the diagnosis and treatment of the disease, as well as identify any gaps in the diagnostic process. Previous studies have demonstrated that non-HIV patients experience a longer time interval between admission and initiation of PCP treatment compared to HIV-infected patients. The delay in treatment may be attributed to the non-specific clinical manifestations of PCP, leading to a delay in diagnosis (Li et al., 2014). Roux and colleagues also reported that non-HIV-PCP patients had a longer time between admission to initial TMP-SMX treatment and a higher 90-day mortality rate compared to HIV-PCP patients (Roux et al., 2014). KO et al. found that age and initial anti-PCP treatment failure were independently associated with increased mortality (Ko et al., 2019). Song et al. found that in patients with rheumatic disease and PCP, initiating treatment with TMP-SMX 7 days after the onset of symptoms was significantly linked to a higher 90-day mortality (Song et al., 2022). These findings highlight the fact that delayed diagnosis and treatment are major contributors to increased mortality and the need for mechanical ventilation in PCP patients. Additionally, early empiric anti-PCP treatment remains a topic of debate. We found that the median difference in the time to start TMP-SMX treatment between non-survivors and survivors was only 1 day, but this difference was significantly associated with in-hospital death and 90-day death in non-HIV-PCP patients with RF, and anti-PCP treatment within 2 days of admission was associated with improved outcomes. This finding is of significant importance for clinicians, emphasizing the need for immediate treatment of suspected PCP, rather than waiting for a confirmed diagnosis.
In the cohort of non-HIV-PCP patients with RF, 94.5% of the patients received corticosteroids within the previous 1 month, and 60.9% of the patients developed symptoms during corticosteroids withdrawal. We innovatively found that the time from corticosteroids withdrawal to symptom onset was correlated with disease prognosis, and when the time from corticosteroids withdrawal to symptom onset was less than 5 days, the prognosis was worse than that of patients > 5 days. In the process of managing clinical patients, most of the focus is on the dosage and duration of corticosteroids use, with little attention paid to corticosteroids withdrawal. The importance of prophylactic use of TMP-SMX for 4 weeks or more with a prednisone equivalent dose of corticosteroids ≥20mg/day and other conditions leading to immunosuppression is now recognized (Sepkowitz, 2002; Thomas and Limper, 2004; Huang et al., 2006; Limper et al., 2011). However, in addition to monitoring the dosage and time of corticosteroids use, attention should also be given to the situation of corticosteroids withdrawal. Our study demonstrated that the faster the disease onset after corticosteroids withdrawal, the worse the prognosis for patients. This finding may indirectly reflect the host immunity of PCP patients and assist doctors in understanding the progression of the disease and choosing more effective treatment options. Currently, the occurrence and development patterns of past corticosteroids use, corticosteroids withdrawal in non-HIV-PCP patients are not clear. This study innovatively explored the effects of clinically important event nodes on in-hospital death and 90-day prognosis of non-HIV-PCP patients with RF.
However, this study has some limitations. Firstly, due to its the retrospective study, not all patients were tested for relevant laboratory indicators, such as inflammatory markers and T lymphocyte subsets. Therefore, the role of these indicators in predicting in-hospital death and 90-day death may have been underestimated. Secondly, late presentation to the hospital and poor patient compliance may have contributed to poor clinical outcomes for some patients. Therefore, in the future, we will conduct prospective studies to further explore the factors that influence patient prognosis.
In conclusion, several factors indicate a poor prognosis in non-HIV-PCP patients with RF. These factors include a low CD8+ T cell count in peripheral blood, a high percentage of BALF-neutrophils, a short time from corticosteroids withdrawal to symptom onset, and a long time from visit to initiation of TMP-SMX therapy.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The study involving human participants was reviewed and approved by the Ethics Committee of Peking University First Hospital.
JL: Data curation, Formal analysis, Writing – original draft. XM: Supervision, Writing – review & editing. HL: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. XL: Project administration, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Beijing Clinical Key Specialty Project (XKB2022B1002).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcimb.2024.1380494/full#supplementary-material
Ainoda, Y., Hirai, Y., Fujita, T., Isoda, N., Totsuka, K. (2012). Analysis of clinical features of non-HIV Pneumocystis jirovecii pneumonia. J. Infect. Chemother. 18, 722–728. doi: 10.1007/s10156-012-0408-5
Beck, J. M., Newbury, R. L., Palmer, B. E., Warnock, M. L., Byrd, P. K., Kaltreider, H. B. (1996). Role of CD8+ lymphocytes in host defense against Pneumocystis carinii in mice. J. Lab. Clin. Med. 128, 477–487. doi: 10.1016/S0022-2143(96)90044-X
Boonsarngsuk, V., Sirilak, S., Kiatboonsri, S. (2009). Acute respiratory failure due to Pneumocystis pneumonia: outcome and prognostic factors. Int. J. Infect. Dis. 13, 59–66. doi: 10.1016/j.ijid.2008.03.027
Brakemeier, S., Pfau, A., Zukunft, B., Budde, K., Nickel, P. (2018). Prophylaxis and treatment of Pneumocystis Jirovecii pneumonia after solid organ transplantation. Pharmacol. Res. 134, 61–67. doi: 10.1016/j.phrs.2018.06.010
Buchacz, K., Lau, B., Jing, Y., Bosch, R., Abraham, A. G., Gill, M. J., et al. (2016). Incidence of AIDS-defining opportunistic infections in a multicohort analysis of HIV-infected persons in the United States and Canada, 2000-2010. J. Infect. Dis. 214, 862–872. doi: 10.1093/infdis/jiw085
Burghi, G., Biard, L., Roux, A., Valade, S., Robert-Gangneux, F., Hamane, S., et al. (2021). Characteristics and outcome according to underlying disease in non-AIDS patients with acute respiratory failure due to Pneumocystis pneumonia. Eur. J. Clin. Microbiol. Infect. Dis. 40, 1191–1198. doi: 10.1007/s10096-020-04118-w
Castagnola, E., Dini, G., Lanino, E., Tasso, L., Dallorso, S., Garaventa, A., et al. (1995). Low CD4 lymphocyte count in a patient with P. carinii pneumonia after autologous bone marrow transplantation. Bone Marrow Transplant. 15, 977–978.
Chung, C., Lim, C. M., Oh, Y. M., Hong, S. B., Choi, C. M., Huh, J. W., et al. (2022). Prognostic implication of bronchoalveolar lavage fluid analysis in patients with Pneumocystis jirovecii pneumonia without human immunodeficiency virus infection. BMC Pulm Med. 22, 251. doi: 10.1186/s12890-022-02041-8
Curtis, J. R., Yarnold, P. R., Schwartz, D. N., Weinstein, R. A., Bennett, C. L. (2000). Improvements in outcomes of acute respiratory failure for patients with human immunodeficiency virus-related Pneumocystis carinii pneumonia. Am. J. Respir. Crit. Care Med. 162, 393–398. doi: 10.1164/ajrccm.162.2.9909014
Enomoto, T., Azuma, A., Kohno, A., Kaneko, K., Saito, H., Kametaka, M., et al. (2010). Differences in the clinical characteristics of Pneumocystis jirovecii pneumonia in immunocompromized patients with and without HIV infection. Respirology 15, 126–131. doi: 10.1111/j.1440-1843.2009.01660.x
Festic, E., Gajic, O., Limper, A. H., Aksamit, T. R. (2005). Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection: outcome and associated features. Chest 128, 573–579. doi: 10.1378/chest.128.2.573
Gaborit, B. J., Tessoulin, B., Lavergne, R. A., Morio, F., Sagan, C., Canet, E., et al. (2019). Outcome and prognostic factors of Pneumocystis jirovecii pneumonia in immunocompromised adults: a prospective observational study. Ann. Intensive Care 9, 131. doi: 10.1186/s13613-019-0604-x
Helweg-Larsen, J., Tsolaki, A. G., Miller, R. F., Lundgren, B., Wakefield, A. E. (1998). Clusters of Pneumocystis carinii pneumonia: analysis of person-to-person transmission by genotyping. QJM 91, 813–820. doi: 10.1093/qjmed/91.12.813
Hirasawa, Y., Nakada, T. A., Shimazui, T., Abe, M., Isaka, Y., Sakayori, M., et al. (2021). Prognostic value of lymphocyte counts in bronchoalveolar lavage fluid in patients with acute respiratory failure: a retrospective cohort study. J. Intensive Care 9, 21. doi: 10.1186/s40560-021-00536-w
Huang, L., Morris, A., Limper, A. H., Beck, J. M. (2006). An Official ATS Workshop Summary: Recent advances and future directions in pneumocystis pneumonia (PCP). Proc. Am. Thorac. Soc. 3, 655–664. doi: 10.1513/pats.200602-015MS
Kim, S. J., Lee, J., Cho, Y. J., Park, Y. S., Lee, C. H., Yoon, H. I., et al. (2014). Prognostic factors of Pneumocystis jirovecii pneumonia in patients without HIV infection. J. Infect. 69, 88–95. doi: 10.1016/j.jinf.2014.02.015
Kim, T., Sung, H., Chong, Y. P., Kim, S. H., Choo, E. J., Choi, S. H., et al. (2018). Low Lymphocyte Proportion in Bronchoalveolar Lavage Fluid as a Risk Factor Associated with the Change from Trimethoprim/sulfamethoxazole used as First-Line Treatment for Pneumocystis jirovecii Pneumonia. Infect. Chemother. 50, 110–119. doi: 10.3947/ic.2018.50.2.110
Ko, R. E., Na, S. J., Huh, K., Suh, G. Y., Jeon, K. (2019). Association of time-to-treatment with outcomes of Pneumocystis pneumonia with respiratory failure in HIV-negative patients. Respir. Res. 20, 213. doi: 10.1186/s12931-019-1188-6
Ko, Y., Jeong, B. H., Park, H. Y., Koh, W. J., Suh, G. Y., Chung, M. P., et al. (2014). Outcomes of Pneumocystis pneumonia with respiratory failure in HIV-negative patients. J. Crit. Care 29, 356–361. doi: 10.1016/j.jcrc.2013.12.005
Lee, J. Y., Park, H. J., Kim, Y. K., Yu, S., Chong, Y. P., Kim, S. H., et al. (2015). Cellular profiles of bronchoalveolar lavage fluid and their prognostic significance for non-HIV-infected patients with Pneumocystis jirovecii pneumonia. J. Clin. Microbiol. 53, 1310–1316. doi: 10.1128/JCM.03494-14
Li, M. C., Lee, N. Y., Lee, C. C., Lee, H. C., Chang, C. M., Ko, W. C. (2014). Pneumocystis jiroveci pneumonia in immunocompromised patients: delayed diagnosis and poor outcomes in non-HIV-infected individuals. J. Microbiol. Immunol. Infect. 47, 42–47. doi: 10.1016/j.jmii.2012.08.024
Li, Y., Ghannoum, M., Deng, C., Gao, Y., Zhu, H., Yu, X., et al. (2017). Pneumocystis pneumonia in patients with inflammatory or autoimmune diseases: Usefulness of lymphocyte subtyping. Int. J. Infect. Dis. 57, 108–115. doi: 10.1016/j.ijid.2017.02.010
Limper, A. H., Adenis, A., Le, T., Harrison, T. S. (2017). Fungal infections in HIV/AIDS. Lancet Infect. Dis. 17, e334–e343. doi: 10.1016/S1473-3099(17)30303-1
Limper, A. H., Knox, K. S., Sarosi, G. A., Ampel, N. M., Bennett, J. E., Catanzaro, A., et al. (2011). An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am. J. Respir. Crit. Care Med. 183, 96–128. doi: 10.1164/rccm.2008-740ST
Mansharamani, N. G., Balachandran, D., Vernovsky, I., Garland, R., Koziel, H. (2000a). Peripheral blood CD4 + T-lymphocyte counts during Pneumocystis carinii pneumonia in immunocompromised patients without HIV infection. Chest 118, 712–720. doi: 10.1378/chest.118.3.712
Mansharamani, N. G., Garland, R., Delaney, D., Koziel, H. (2000b). Management and outcome patterns for adult Pneumocystis carinii pneumonia, 1985 to 1995: comparison of HIV-associated cases to other immunocompromised states. Chest 118, 704–711. doi: 10.1378/chest.118.3.704
Maschmeyer, G., Helweg-Larsen, J., Pagano, L., Robin, C., Cordonnier, C., Schellongowski, P. (2016). ECIL guidelines for treatment of Pneumocystis jirovecii pneumonia in non-HIV-infected haematology patients. J. Antimicrob. Chemother. 71, 2405–2413. doi: 10.1093/jac/dkw158
Mason, G. R., Hashimoto, C. H., Dickman, P. S., Foutty, L. F., Cobb, C. J. (1989). Prognostic implications of bronchoalveolar lavage neutrophilia in patients with Pneumocystis carinii pneumonia and AIDS. Am. Rev. Respir. Dis. 139, 1336–1342. doi: 10.1164/ajrccm/139.6.1336
McAllister, F., Steele, C., Zheng, M., Young, E., Shellito, J. E., Marrero, L., et al. (2004). T cytotoxic-1 CD8+ T cells are effector cells against pneumocystis in mice. J. Immunol. 172, 1132–1138. doi: 10.4049/jimmunol.172.2.1132
Meissner, N. N., Lund, F. E., Han, S., Harmsen, A. (2005). CD8 T cell-mediated lung damage in response to the extracellular pathogen pneumocystis is dependent on MHC class I expression by radiation-resistant lung cells. J. Immunol. 175, 8271–8279. doi: 10.4049/jimmunol.175.12.8271
Miller, R. F., Allen, E., Copas, A., Singer, M., Edwards, S. G. (2006). Improved survival for HIV infected patients with severe Pneumocystis jirovecii pneumonia is independent of highly active antiretroviral therapy. Thorax 61, 716–721. doi: 10.1136/thx.2005.055905
Monnet, X., Vidal-Petiot, E., Osman, D., Hamzaoui, O., Durrbach, A., Goujard, C., et al. (2008). Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit. Care 12, R28. doi: 10.1186/cc6806
Mosmann, T. R., Li, L., Sad, S. (1997). Functions of CD8 T-cell subsets secreting different cytokine patterns. Semin. Immunol. 9, 87–92. doi: 10.1006/smim.1997.0065
Mosmann, T. R., Sad, S. (1996). The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 17, 138–146. doi: 10.1016/0167-5699(96)80606-2
Pegorie, M., Denning, D. W., Welfare, W. (2017). Estimating the burden of invasive and serious fungal disease in the United Kingdom. J. Infect. 74, 60–71. doi: 10.1016/j.jinf.2016.10.005
Ricciardi, A., Gentilotti, E., Coppola, L., Maffongelli, G., Cerva, C., Malagnino, V., et al. (2017). Infectious disease ward admission positively influences P. jiroveci pneumonia (PjP) outcome: A retrospective analysis of 116 HIV-positive and HIV-negative immunocompromised patients. PloS One 12, e176881. doi: 10.1371/journal.pone.0176881
Roussos, C., Koutsoukou, A. (2003). Respiratory failure. Eur. Respir. J. Suppl. 47, 3s–14s. doi: 10.1183/09031936.03.00038503
Roux, A., Canet, E., Valade, S., Gangneux-Robert, F., Hamane, S., Lafabrie, A., et al. (2014). Pneumocystis jirovecii pneumonia in patients with or without AIDS, France. Emerg. Infect. Dis. 20, 1490–1497. doi: 10.3201/eid2009.131668
Ruan, S., Samuelson, D. R., Assouline, B., Morre, M., Shellito, J. E. (2016). Treatment with interleukin-7 restores host defense against pneumocystis in CD4+ T-lymphocyte-depleted mice. Infect. Immun. 84, 108–119. doi: 10.1128/IAI.01189-15
Sepkowitz, K. A. (2002). Opportunistic infections in patients with and patients without Acquired Immunodeficiency Syndrome. Clin. Infect. Dis. 34, 1098–1107. doi: 10.1086/339548
Sepkowitz, K. A., Brown, A. E., Telzak, E., Gottlieb, S., Armstrong, D. (1992). Pneumocystis carinii pneumonia among patients without AIDS at a cancer hospital. JAMA 267, 832–837. doi: 10.1001/jama.1992.03480060078034
Smith, R. L., El-Sadr, W. M., Lewis, M. L. (1988). Correlation of bronchoalveolar lavage cell populations with clinical severity of Pneumocystis carinii pneumonia. Chest 93, 60–64. doi: 10.1378/chest.93.1.60
Song, S., Zhang, Y., Yu, J., Xie, C., Chen, Y., Zhang, X. (2022). Time to trimethoprim/sulfamethoxazole initiation among patients with rheumatic disease complicated by Pneumocystis jirovecii pneumonia: impact on 90-day mortality. BMC Infect. Dis. 22, 961. doi: 10.1186/s12879-022-07940-z
Tamai, K., Tachikawa, R., Tomii, K., Nagata, K., Otsuka, K., Nakagawa, A., et al. (2014). Prognostic value of bronchoalveolar lavage in patients with non-HIV pneumocystis pneumonia. Intern. Med. 53, 1113–1117. doi: 10.2169/internalmedicine.53.0520
Tang, G., Tong, S., Yuan, X., Lin, Q., Luo, Y., Song, H., et al. (2021). Using routine laboratory markers and immunological indicators for predicting pneumocystis jiroveci pneumonia in immunocompromised patients. Front. Immunol. 12, 652383. doi: 10.3389/fimmu.2021.652383
Thomas, C. J., Limper, A. H. (2004). Pneumocystis pneumonia. N. Engl. J. Med. 350, 2487–2498. doi: 10.1056/NEJMra032588
Vogel, M. N., Vatlach, M., Weissgerber, P., Goeppert, B., Claussen, C. D., Hetzel, J., et al. (2012). HRCT-features of Pneumocystis jiroveci pneumonia and their evolution before and after treatment in non-HIV immunocompromised patients. Eur. J. Radiol. 81, 1315–1320. doi: 10.1016/j.ejrad.2011.02.052
Wright, T. W., Gigliotti, F., Finkelstein, J. N., McBride, J. T., An, C. L., Harmsen, A. G. (1999). Immune-mediated inflammation directly impairs pulmonary function, contributing to the pathogenesis of Pneumocystis carinii pneumonia. J. Clin. Invest. 104, 1307–1317. doi: 10.1172/JCI6688
Xue, T., Kong, X., Ma, L. (2023). Trends in the epidemiology of pneumocystis pneumonia in immunocompromised patients without HIV infection. J. Fungi (Basel) 9. doi: 10.3390/jof9080812
Keywords: Pneumocystis pneumonia, non-human immunodeficiency virus, respiratory failure, prognostic factor, risk factor
Citation: Li J, Mu X, Li H and Liu X (2024) Clinical course and prognostic factors of Pneumocystis pneumonia with respiratory failure in non-HIV patients. Front. Cell. Infect. Microbiol. 14:1380494. doi: 10.3389/fcimb.2024.1380494
Received: 01 February 2024; Accepted: 18 June 2024;
Published: 10 July 2024.
Edited by:
Guillaume Desoubeaux, Université de Tours, FranceReviewed by:
Retno Wahyuningsih, Universitas Kristen Indonesia, IndonesiaCopyright © 2024 Li, Mu, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haichao Li, bGhjaDkxNzY3QHNpbmEuY29t; Xinmin Liu, bHhtMjEyOEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.