- 1College of Pharmacy, Inner Mongolia Medical University, Hohhot, Inner Mongolia Autonomous Region, China
- 2Department of Obstetrics and Gynecology, Hohhot Maternal and Child Health Care Hospital, Hohhot, Inner Mongolia Autonomous Region, China
- 3Department of Oncology, Inner Mongolia Traditional Chinese Medicine Hospital, Hohhot, Inner Mongolia Autonomous Region, China
- 4College of Traditional Chinese Medicine, Inner Mongolia Medical University, Hohhot, Inner Mongolia Autonomous Region, China
- 5Department of Obstetrics and Gynecology, Affiliated Hospital of Inner Mongolia Medical University, Hohhot, Inner Mongolia Autonomous Region, China
In recent years, a growing body of research has confirmed that the gut microbiota plays a major role in the maintenance of human health and disease. A gut microbiota imbalance can lead to the development of many diseases, such as pregnancy complications, adverse pregnancy outcomes, polycystic ovary syndrome, endometriosis, and cancer. Short-chain fatty acids are metabolites of specific intestinal bacteria and are crucial for maintaining intestinal homeostasis and regulating metabolism and immunity. Endometriosis is the result of cell proliferation, escape from immune surveillance, and invasive metastasis. There is a strong correlation between the anti-proliferative and anti-inflammatory effects of short-chain fatty acids produced by gut microbes and the development of endometriosis. Given that the mechanism of action of gut microbiota and Short-chain fatty acids in endometriosis remain unclear, this paper aims to provide a comprehensive review of the complex interactions between intestinal flora, short-chain fatty acids and endometriosis. In addition, we explored potential microbial-based treatment strategies for endometriosis, providing new insights into the future development of diagnostic tests and prevention and treatment methods for endometriosis.
1 Introduction
Endometriosis (EMS) is a disease of the endometrium or endometrial tissue that is active outside the uterine cavity, such as in the peritoneal cavity, ovary, bladder or ureter, or even in the distal organs (Smolarz et al., 2021). The growth of endometrial implants and associated inflammation lead to increased production of pro-inflammatory cytokines (Tai et al., 2018). These processes eventually lead to chronic inflammation, the formation of pelvic adhesions and EMS, which impairs reproductive function (Jiang et al., 2021). In addition to gynecological symptoms, up to 90% of EMS patients experience gastrointestinal symptoms (Bloski and Pierson, 2008; Maroun et al., 2009; DiVasta et al., 2018). The researchers found that the human gut microbiota (GM) and the development of EMS are bidirectional (Jiang et al., 2021). The human gut microbiome is composed of bacteria, fungi, protozoa, archaea and some viruses, whose encoding genes are approximately 150 times the total number of human genes; bacteria play a dominant role and include Firmicutes, Bacteroidetes, Proteobacteria and Actinobacteria, four key bacteria (Senchukova, 2023). The type and quantity of the gut microbiota (GM) are affected by the environment, diet, genetics, drugs, disease and other factors. Studies have shown that the gut microbiota plays an important role in various kinds of inflammation (Qin et al., 2022). A study in monkeys found that monkeys with endometriosis had lower levels of lactobacillus and higher levels of gram-negative bacteria, and a higher incidence of intestinal inflammation than healthy controls (Bailey and Coe, 2002). Another study showed that mice with endometriosis showed a general decrease in bacteroides levels (Yuan et al., 2018). The mechanism by which gut microbiota affects EMS may involve estrogen metabolism, immune dysregulation and inflammatory response (Hantschel et al., 2019; Salliss et al., 2021).
In addition, one mechanism by which mammalian gut bacteria influence host physiological and immune processes is fermentation to produce hundreds of metabolites that regulate key host functions, including short-chain fatty acids (SCFAs) such as butyrate, propionate, and acetate, which are involved in maintaining intestinal barrier function, improving host metabolism, and regulating the immune system and inflammatory response (Martin-Gallausiaux et al., 2021). SCFAs is used by intestinal cells as an energy source or transported into the bloodstream and can have anti-proliferative and anti-inflammatory effects on distant organs (Maslowski et al., 2009; den Besten et al., 2013; Semaan et al., 2020). For example, n-butyrate inhibits the proliferation of colorectal cancer cells (Cao et al., 2019). N-butyrate can inhibit the expression of pro-inflammatory cytokines tumor necrosis factor α (TNF-α) and IL-6 in lipopolysaccharide-induced macrophages (Chen et al., 2018). Chadchan et al. reported that SCFAs can inhibit the growth of EMS ectopic lesions (Chadchan et al., 2021). SCFAs exerts anti-inflammatory and regulatory effects on immune cells mainly by activating G-protein-coupled receptors GPR43, GPR41 and GPR109A and inhibiting histone deacetylase, thereby inhibiting the development of EMS (Licciardi et al., 2011; Smith et al., 2013; Park et al., 2015). Future studies on the functional characterization of the microbiome will greatly assist in the development of microbiome-based therapeutics for the treatment of EMS. This review conducted a computer search for articles related to the gut microbiota, short-chain fatty acids and the endometriosis published in the PubMed (https://pubmed.ncbi.nlm.nih.gov/advanced/), Medline databases (https://ovidsp.ovid.com/autologin.cgi), Wan Fang Data Knowledge Service Platform (https://www.wanfangdata.com.cn), CNKI (https://www.cnki.net) and China Biomedical Literature Service System (http://www.sinomed.ac.cn/zh/). The search time limit was from the establishment of each database until March 2024. This paper describes the specific mechanisms involved and the future clinical application of the gut microbiota and its metabolites SCFAs in affecting EMS.
2 Endometriosis
EMS is a chronic inflammatory gynecological disease characterized by abnormal growth of the endometrial epithelium or stroma outside the uterus (Zondervan et al., 2018). Endometriosis often occurs in the pelvic area, including the ovaries, fallopian tubes, peritoneal surface, bladder, rectovaginal septum, and gastrointestinal tract. Distal organ endometriosis has also been reported, such as liver EMS, brain EMS, and chest EMS (da Costa et al., 2022; Elefante et al., 2022; Topbas Selcuki et al., 2022; Posses Bridi et al., 2023). The symptoms of EMS are often pelvic pain, dysmenorrhea, and infertility (Zondervan et al., 2018). The incidence of EMS in women of reproductive age is as high as 10% globally, seriously affecting the physical health of patients, and causing a huge economic burden (Simoens et al., 2012; Zondervan et al., 2020). EMS is a multifactorial disease whose pathogenesis has not been fully elucidated, and many factors have been scientifically investigated (Burney and Giudice, 2012). The most widely accepted pathogenesis of EMS is the theory of menstruation retrogression, in which endometrial tissue returns to the pelvic cavity through the fallopian tube during menstruation, and after reaching the pelvic cavity, endometrial stromal cells adhere to the peritoneal surface and proliferate, developing into invasive lesions (Halme et al., 1984; Yovich et al., 2020). Tai et al. suggested a link between EMS and pelvic infection. They included data from more than 14,000 patients to assess the association between pelvic inflammatory disease and endometriosis, showing a three-fold increased risk of EMS in patients with pelvic inflammatory disease (Tai et al., 2018). A growing number of studies have found links between EMS and chronic as well as autoimmune diseases (Kvaskoff et al., 2015; Zhang et al., 2018). Studies of the occurrence of endometriosis in identical twins suggest that EMS has a genetic basis (Hadfield et al., 1997; Hansen and Eyster, 2010). In addition, low birth weight, earlier age of menarche, and shorter menstrual cycles were all found to be significantly associated with a higher risk of EMS (Matalliotakis et al., 2008; Nnoaham et al., 2012; Borghese et al., 2015). There are many other indirect risk factors for EMS, including diet (e.g., alcohol and caffeine intake), environment (e.g., polychlorinated biphenyl and dioxin), and lifestyle (e.g., night shift work), but further research is needed to find a definitive association (Rier et al., 1993; Hemmings et al., 2004; Chiaffarino et al., 2014; Smarr et al., 2016; Jurkiewicz-Przondziono et al., 2017; Shafrir et al., 2018).
Currently, EMS is mainly confirmed through invasive procedures such as laparoscopy and histopathology EMS (Agarwal et al., 2019). Because EMS is often associated with gynecological, pain, and gastrointestinal disorders, its non-specific manifestations and highly limited diagnostic methods lead to delayed diagnosis and treatment of EMS (Arruda et al., 2003; McGrath et al., 2023). Conventional treatment with EMS (such as surgery and hormone therapy) can relieve associated symptoms (Bedaiwy et al., 2017). However, the recurrence rate is high, and there are side effects of hormone therapy and surgical risks (Vercellini et al., 2008). Therefore, it is necessary to search for non-invasive biomarkers to diagnose and treat EMS. It has been reported that some diagnostic markers in the urine, serum, menstrual blood, and other body fluids of women with endometriosis can circumvent unnecessary invasive techniques and have the advantages of being simple, rapid, and non-invasive (Anastasiu et al., 2020). Therefore, biomarkers have become an important direction in the diagnosis of this disease. There is a close relationship between gastrointestinal and reproductive tract microecology and EMS (Sobstyl et al., 2023). One study showed that the vaginal microbiome is less important as a predictor than the gut microbiome in diagnosing endometriosis (Huang et al., 2021b). This brings a new idea for the research of EMS. Recent studies have shown that intestinal microbiota and its small molecule metabolite SCFAs can affect the occurrence and development of EMS, which is expected to become a new biomarker of EMS (Chadchan et al., 2021).
3 Gut microbiota metabolites: SCFAs
One of the ways in which the gut microbiota affects host physiology may be through the production of the metabolite SCFAs. The critical role of microbiota in the production of SCFAs has been reported in a comparison of germ-free and normal mice (Høverstad and Midtvedt, 1986). SCFAs are saturated fatty acids with no more than six carbon atoms that are produced by specific bacteria in the cecum and colon through the fermentation of undigested dietary fiber (Cong et al., 2022). The main SCFAs in the intestine are acetate, propionate and butyrate, accounting for more than 95% of all SCFAs (Cummings et al., 1987). SCFAs is not only present in the gut, but can also be transported through the gut into the blood circulation (Pomare et al., 1985). Studies have shown that changes in SCFAs concentrations in the blood or tissues can cause inflammatory, immune, and metabolic diseases (Morris et al., 2017). SCFAs affects inflammation and immunity by regulating the production of immune mediators, cytokines and chemokines, as well as the differentiation, recruitment and activation of immune cells such as neutrophils, macrophages, DC and T lymphocytes (Corrêa-Oliveira et al., 2016). A growing body of evidence highlights the role of SCFAs in the gut as well as in the tissues and organs outside the gut (Figure 1). Low levels of butyrate producing bacteria and short-chain fatty acids have been found in patients with colorectal cancer (Wang et al., 2019). High levels of propionate in the blood prevent allergic inflammation in the lungs by enhancing the production of macrophages and DC precursors by GPR4 (Trompette et al., 2014). Clostridium butyricum may reduce microglia-mediated neuroinflammation in Alzheimer’s disease by regulating the metabolite butyrate (Sun et al., 2020). In addition, recent studies have found a complex relationship between SCFAs and EMS. Metabolomics showed reduced short-chain fatty acids in a mouse model of endometriosis (Alghetaa et al., 2023). Studies by Chadchan et al. also found that short-chain fatty acids were reduced in the feces of mice with endometriosis, and treatment with n-butyrate reduced the growth of endometriosis lesions in mice (Chadchan et al., 2021). A case-control study of 137 women found that the serum metabolite 2-hydroxybutyric acid was associated with endometriosis risk (Lefebvre et al., 2024). These studies have proved that SCFAs can affect the occurrence and development of EMS.
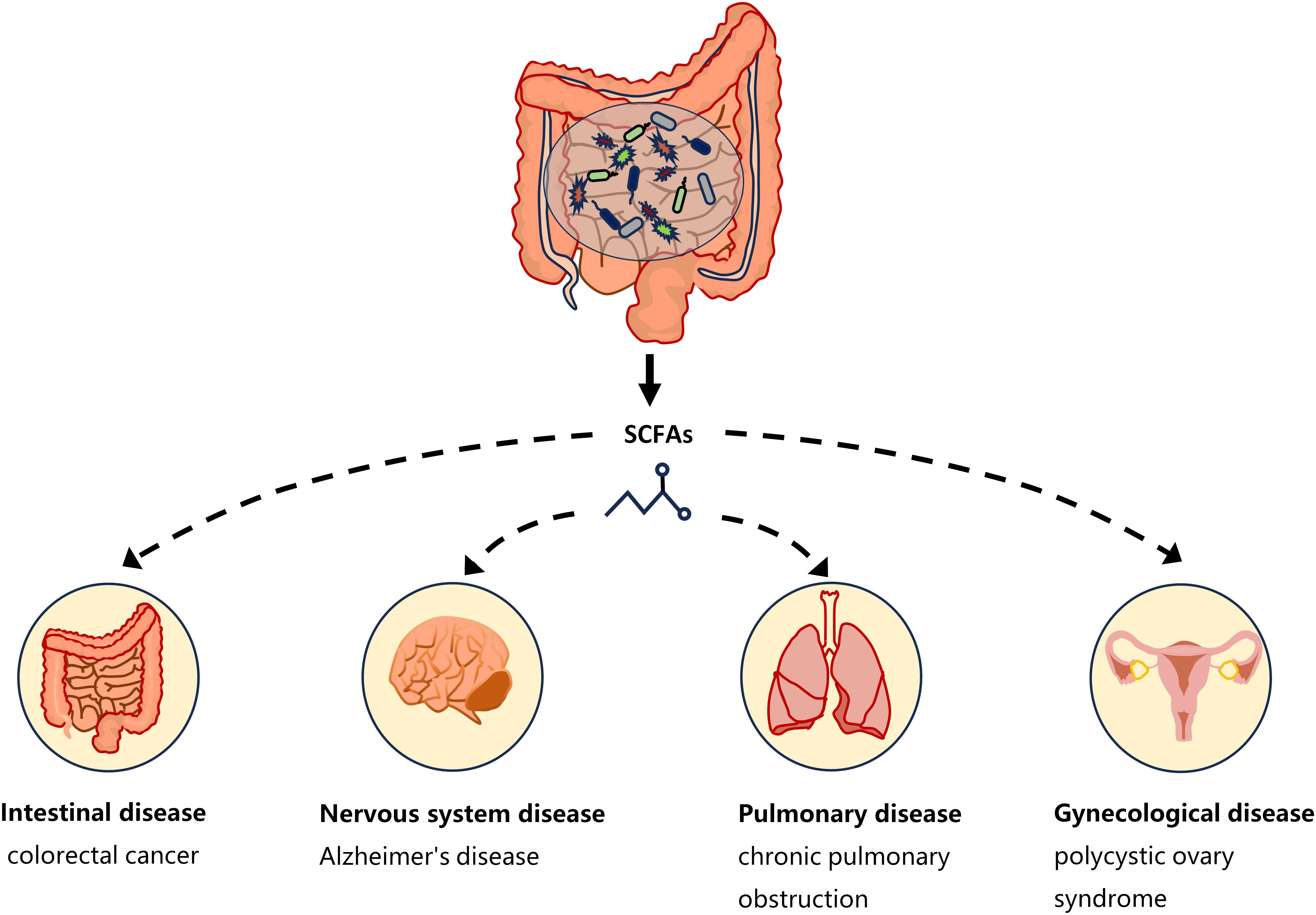
Figure 1 SCFAs and disease types. Short-chain fatty acids produced by gut microbiota have a complex relationship with intestinal diseases (such as colorectal cancer), neurological diseases (such as Alzheimer’s disease), gynecological diseases (such as polycystic ovary syndrome), and lung diseases (such as chronic pulmonary obstruction). The continuous lines indicate the direct action, and the fragmented lines indicate the indirect action.
4 Relationship between gut microbiota imbalance and EMS
A number of studies have shown that changes in the gut microbiota are associated with the pathogenesis of EMS (Table 1). Chadchan et al. gavaged metronidazole-treated mice with the feces of endometriosis mice and found that the growth of lesions was restored, indicating that gut bacteria are involved in the progression of endometriosis lesions (Chadchan et al., 2019). Possible explanations for the effect of gut microbiota dysbiosis on endometriosis include the bacterial contamination theory, estrogen metabolism, cytokines and impaired immune function (Zizolfi et al., 2023). Long-term inflammation induced by EMS may also impair intestinal barrier function and further promote an imbalance in the intestinal microbiota and aggravate EMS inflammation, thus forming a vicious cycle (Figure 2). The gut microbiota may be used as a biomarker for the early diagnosis of EMS in the future (Huang et al., 2021b).
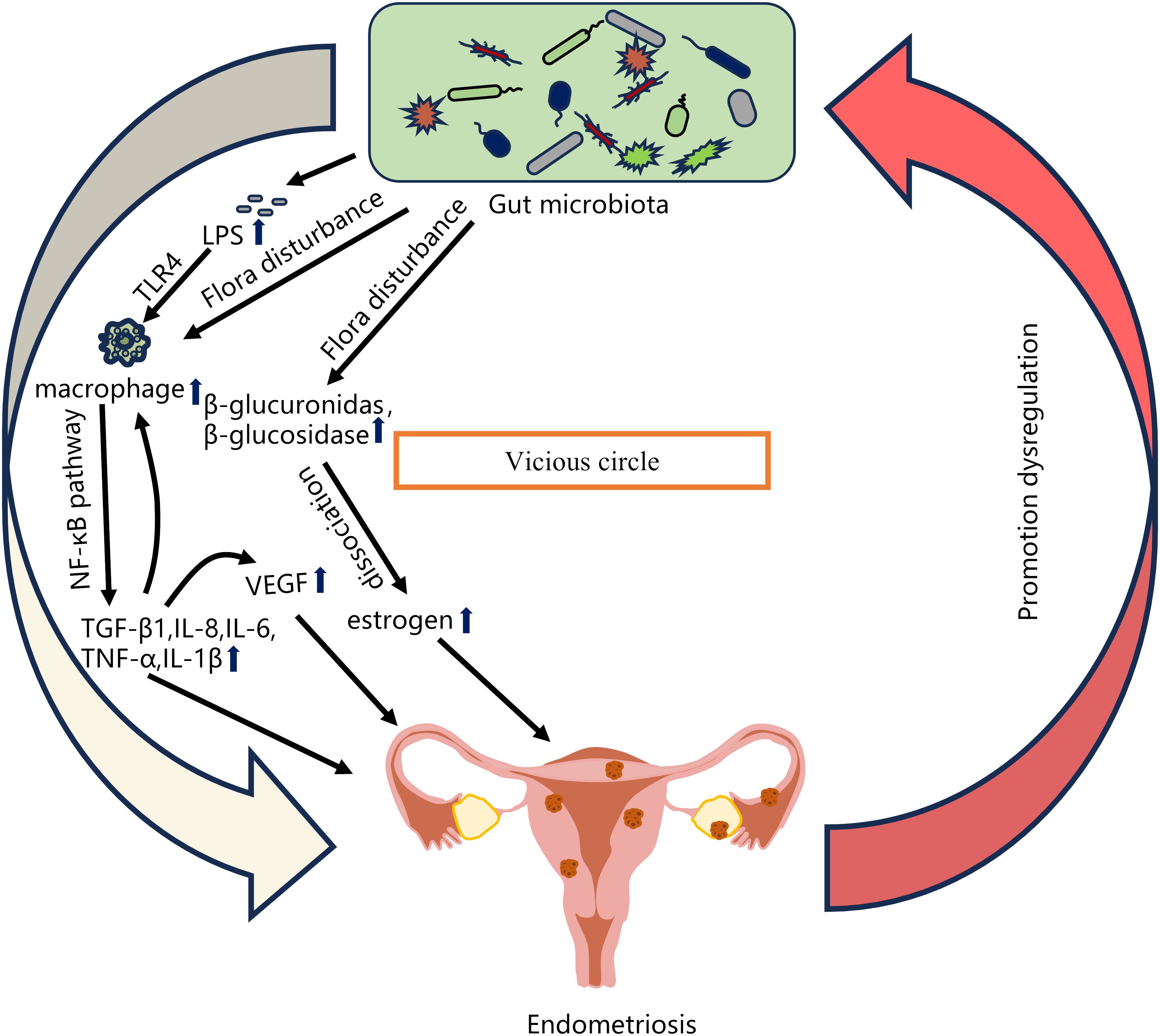
Figure 2 Relationship between gut microbiota imbalance and EMS. Possible explanations for the effect of gut microbiota dysbiosis on endometriosis include the bacterial contamination theory (Dysregulation of the gut microbiota leads to increased LPS levels, and LPS-induced primary inflammation promotes the secretion of the NF-κB mediator in the peritoneal cavity through TLR4 present on macrophages and other immune cells, and produces significant levels of TNF-α and IL-8), estrogen metabolism (When intestinal microbiota is disturbed, β-glucuronidase and β-glucosidase secreted by estrogen group can degrade bound estrogen, resulting in excessive free estrogen), cytokines and impaired immune function (The imbalance of intestinal microbiota leads to the increase of pro-inflammatory cytokines such as TGF-β1, IL-8, IL-6, TNF-α and IL-1β. The local surge of cytokines further promotes the recruitment of immune cells and the secretion of more cytokines, and stimulates the production of VEGF, thereby inducing endometrial cell proliferation). EMS long-term inflammation will further promote intestinal microbiota imbalance, thus forming a vicious cycle.
4.1 Bacterial contamination theory
The human lower reproductive tract harbors a diverse microbiota, which can ascend to the upper reproductive tract through direct migration and potentially cause infections (Cheng et al., 2021). Khan et al. proposed that some Gram-negative bacteria, such as E. coli, infect the uterine wall after migrating up from the vagina to contaminate menstrual blood. Their study found that women with endometriosis had higher levels of E. coli in their menstrual blood than the control group (Khan et al., 2010). Combined with the theory of menstrual reflux, menstrual blood may flow from the fallopian tubes into the pelvic cavity (Halme et al., 1984). Bacterial lipopolysaccharide (LPS) is the main component of the cell wall of Gram-negative bacteria such as E. coli. LPS is constantly shed by living bacteria and is released when the bacteria die (Raetz and Whitfield, 2002). Khan et al. considered that E. coli contamination in her menstrual blood was a continuous source of LPS in the peritoneal cavity. Elevated LPS levels in peritoneal fluid may promote Toll-like receptor 4 (TLR4)-mediated progression of endometriosis (Khan et al., 2010). Another possible explanation for the residual accumulation of bacterial endotoxins in the pelvic environment is the transfer of E. coli or endotoxins from the intestine through intestinal cells and into the pelvic cavity (Deitch et al., 1989; Alexander et al., 1990). Dysbiosis of the intestinal microbiota can lead to an increase in Gram-negative bacteria, causing a large amount of LPS to enter the circulatory system inducing chronic inflammation throughout the body (He et al., 2019). Studies have shown that patients with endometriosis have elevated intestinal and serum LPS levels (Ni et al., 2020). Primary inflammation caused by LPS promotes the secretion of the NF-κB mediator in the peritoneal cavity through TLR4 present on macrophages, neutrophils, and other immune cells, followed by a cascade of reactions such as the production of significant levels of tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8), thus promoting the development of endometriosis (Khan et al., 2018; Jeljeli et al., 2020). Although this theory provides a new strategy for treating EMS by targeting bacterial endotoxin or TLR4 to suppress inflammation, it is currently not feasible for clinical application.
4.2 Estrogen metabolism
Another possible mechanism by which the gut microbiota is involved in the development of endometriosis is through the regulation of estrogen (Baker et al., 2017). Estrogen plays an important role in maintaining the development of the female reproductive system and is closely associated with the development of endometriosis (Jeon et al., 2016; Galvankar et al., 2017). Studies have found that estrogen can induce proliferative diseases such as endometriosis, endometrial cancer, and hysteromyoma by stimulating the proliferation of female genital epithelial cells (Xu et al., 2019; Somasundaram et al., 2020). Endometriosis has been associated with alterations in estrogen signaling (Laux-Biehlmann et al., 2015). Endometriotic women have a heightened proinflammatory and anti-apoptotic response to estradiol (Reis et al., 2013). This attributed to changes in nuclear estrogen receptor expression. Endometriotic lesions express higher levels of estrogen receptor-β (ER-β), whose signaling promotes lesion growth by inhibiting TNF-α-induced apoptosis, activating an inflammasome which increases IL-1β, and enhancing cellular adhesion and proliferation (Han et al., 2015). The gut microbiota plays an important role in estrogen metabolism, and the gene pool in the gut microbiota capable of metabolizing estrogen is called the “estrogen group” (Salliss et al., 2021). It has been observed that the use of antibiotics leads to a decrease in estrogen levels (Chadchan et al., 2019). Estrogen metabolism takes place mainly in the liver. Estrogens are inactivated in the liver into less active estrone and estriol and bind to glucuronic acid to promote their excretion in bile, urine, and feces (Adlercreutz, 1974). However, beta-glucuronidase secreted by gut microbes can metabolize estrogen from the bound form to the unbound form (Ervin et al., 2019; Sui et al., 2021). Free estrogens can be absorbed into the circulatory system, resulting in increased levels of free estrogens, and can be transported through the blood to distal mucosal sites, such as the endometrium. Estrogen binds to estrogen receptors, which are subsequently internalized and function as transcription factors promoting the transcription of genes involved in cellular growth (Dabek et al., 2008; Baker et al., 2017; Salliss et al., 2021). Studies have shown that multiple genera in the gut microbiota, including Bifidobacteria, Bacteroidetes, Escherichia coli and Lactobacillus, can encode β-glucuronidase (Leonardi et al., 2020). When the intestinal flora is dysregulated, the flora producing β-glucuronidase in the gut increases, leading to elevated circulating estrogen levels and promoting the proliferation of ectopic endometrial lesions (Baker et al., 2017). Some studies have found that the levels of Bifidobacteria and Escherichia in the endometriosis group are higher than those in the control group (Yuan et al., 2018). In addition, elevated levels of β-glucuronidase expression were observed in endometriosis lesions compared to normal endometrium (Wei et al., 2023). Metabolites produced by the gut microbiota also affect estrogen metabolism and affect endometriosis. For example, short-chain fatty acids produced by some bacteria can affect estrogen metabolism by regulating the expression of estrogen-metabolizing enzymes in the liver and other tissues, affecting estrogen levels and hormone balance in endometriosis (Ervin et al., 2019). In summary, the gut microbiota can lead to increased circulating estrogen levels, which can promote the development of endometriosis (Qi et al., 2021). However, whether specific gut microbiota constituents stimulate β-glucuronidase production and whether the gut microbiota has synergistic effects on the pathogenesis of EMS remain to be studied further.
4.3 Immunity and inflammation
Immunity and inflammation play an integral role in the pathogenesis of endometriosis. The results of the sustained growth of endometrial lesions in ovariectomized animals suggest that the innate immune system in the pelvic environment can also influence the growth of endometriosis lesions in addition to the influence of sex hormones (Novella-Maestre et al., 2012). One study demonstrated that the number of macrophages, monocytes, and cytokines in the peritoneal fluid of women with endometriosis was increased, and the levels of the pro-inflammatory factors IL-1β, IL-18 and TGF-β are increased (Zhou et al., 2019; Agostinis et al., 2020). Increased numbers of macrophages help to secrete proinflammatory cytokines, such as transforming growth factor-β1 (TGF-β1), IL-8, IL-6, TNF-α, and IL-1β (Jeljeli et al., 2020). These cytokines are expressed mainly by macrophages in the peritoneal cavity through increasing the activation of the NF-κB pathway. A local cytokine surge can further promote the recruitment of immune cells and the secretion of additional cytokines, and stimulate the production of vascular endothelial growth factor (VEGF), which is conducive to angiogenesis (Becker and D'Amato, 2007; Symons et al., 2018). VEGF is a major regulator of vascularization and can induce endometrial cell proliferation (Young et al., 2015). In addition, when the inflammatory activity in the peritoneal fluid decreases, the level of the anti-inflammatory factor IL-37 increases. When inflammatory substances cannot be removed, an inflammatory response may occur, which spreads throughout the body, with long-term effects on the immune system (Symons et al., 2018; Zhou et al., 2019).
At present, many studies have confirmed the close relationship between gut microbiota and inflammation (Buford, 2017; Shi et al., 2017; Clemente et al., 2018; Brandsma et al., 2019). Jiang et al. hypothesized that changes in the gut and genital tract microbiota would lead to changes in the immune response. Gut microbiota dysbiosis destroys normal immune function and leads to the elevation of proinflammatory cytokines, which impairs immune surveillance in the pelvic region and changes the immune cell spectrum, creating an inflammatory microenvironment to promote the persistence of endometriosis (Jiang et al., 2021). Yuan et al. showed that the gut microbiota may interact with cytokines abnormally expressed in endometriotic lesions, peritoneal fluid and peripheral blood (Yuan et al., 2018). The effect of gut microbiota on host inflammatory response is mediated by a variety of mechanisms. Dysregulation of the gut microbiome can digest the protective mucus layer of the gut and interact directly with intestinal cells, leading to increased local and systemic inflammation (Blander et al., 2017). For example, segmented filamentous bacteria can directly stimulate T lymphocytes to differentiate into helper T lymphocytes (Th17) to drive autoimmune arthritis (Wu et al., 2010). Many studies have shown that Th17 cells and their cytokines are significantly increased in the peritoneal fluid of women with endometriosis (Gogacz et al., 2016; Tarokh et al., 2019). As previously mentioned, intestinal microbiota imbalance can also lead to LPS leakage by increasing intestinal permeability, which activates the innate immune system through TLR4 and increases the expression of pro-inflammatory cytokines, thus promoting the occurrence and development of endometriosis (Khan et al., 2010; Bruner-Tran et al., 2013). In addition, short-chain fatty acids act as a medium of communication between the gut microbiome and the immune system, serving as an energy source for intestinal cells or being transported into the blood, not only acting in the gut, but also regulating immune responses in distant tissues (Parada Venegas et al., 2019; Chadchan et al., 2021). These are described in detail later in Chapter 6. However, the molecular mechanisms that explain the microbiome’s interaction with immune inflammation are not fully understood, and more research is needed to explore the complex role of the gut microbiome in the multi-factorial etiology of EMS, including impaired cytokine and immune function.
5 Relationship between the gut microbiota and SCFAs interaction
5.1 The gut microbiota regulates SCFAs metabolism in the host
The literature clearly highlights that gut microbes play a key role in the metabolism of short-chain fatty acids (Mirzaei et al., 2022). In the gut, SCFAs are the main beneficial metabolites produced by gut microbes through the metabolism of indigestible dietary fiber. The most abundant SCFA is acetate, which is produced mainly by Bacteroides, one of the largest flora in the gut. Acetate can be produced from pyruvate via the acetyl-CoA or Wood-Ljungdahl pathways (Ragsdale and Pierce, 2008). Another major SCFA, propionate, is produced by a few dominant bacterial genera, such as Ruminococcus bromii, Veillonella parvula and Bacteroides (van der Hee and Wells, 2021). Propionate is mainly produced from succinate via the succinate pathway or from lactate via the acrylate pathway (Koh et al., 2016). The third major SCFA, butyrate, is mostly produced by Firmicutes, such as Faecalibacterium, Eubacterium rectale and Eubacterium hallii (Vital et al., 2014). Butyrate is formed from acetyl-CoA and butyryl-CoA (Koh et al., 2016). There are metabolic linkages between different types of bacteria. For example, acetate produced by Bacteroides can be utilized by Firmicutes to produce butyrate. The diversity and quantity of host gut microbes are the main factors affecting the production of SCFAs. One randomized trial revealed that resistant starch was able to regulate the gut microbiome, such as increased Bifidobacteria, altered Firmicutes to bacteria ratio, and observed an increase in the relative abundance of butyrate (Alfa et al., 2018). In addition, a new study examined whether Bifidobacteria can synthesize SCFAs in vivo by LC−MS/MS and revealed that Bifidobacteria mice and mice without a specific pathogen exhibit high concentrations of acetic acid and propionate. Bifidobacteria colonization and changes in the gut microbiota can affect the abundance of intestinal short-chain fatty acids (Horvath et al., 2022). The above studies showed that diet and the gut microbiota are the influencing factors of SCFAs metabolism.
5.2 SCFAs inhibit the growth of harmful bacteria
While gut microbes regulate the metabolism of short-chain fatty acids, SCFAs produced in the gut can inhibit the growth of harmful intestinal bacteria, which play an important role in maintaining the stability of the gut microbiota. Studies have shown that SCFAs can remodel antibiotic-induced disordered gut microbiota by increasing the number of beneficial bacteria and reducing the number of harmful bacteria. After the administration of SCFAs, the relative abundance of Firmicutes and Bacteroides in septic mice increased significantly, and the number of Enterobacteriaceae, such as Proteobacteria and Escherichia Shigella, was significantly reduced and returned to levels comparable to those in healthy mice (Lou et al., 2022). Oral administration of butyrate to mice can significantly enhance the antibacterial activity of colonic macrophages, for example, by conferring better killing effects against Salmonella enterica and Citrobacterrodentium (Flemming, 2019). SCFAs inhibit the growth of harmful bacteria mainly by releasing H+, reducing the intestinal pH, competing for energy, blocking the biosynthesis of harmful bacteria, and forming antibacterial peptides to achieve intestinal microecological balance (Table 2). In the intestine, some SCFAs are protonated by the carboxyl and phosphate groups of lipopolysaccharides on the bacterial outer membrane, destroying the integrity of the bacterial outer membrane; in addition, another part of SCFAs dissociates in bacterial cells to produce large amounts of RCOO- and H+. H+ disrupts intracellular pH homeostasis; RCOO- increases intracellular osmotic pressure, forcing these cells to excrete precursors or accessory substances necessary for their growth to balance osmotic pressure, thus inhibiting the growth of harmful bacteria (Ma et al., 2022). Moreover, the continuous accumulation of RCOO- changes the homeostatic environment in bacterial cells, thereby interfering with a series of physiological and biochemical characteristics inside bacteria and thereby blocking the biosynthesis of harmful bacteria. In addition, SCFAs trigger host cells to produce certain antibacterial peptides by activating and regulating the host immune system, which in turn achieves bacterial inhibition by lysing the bacterial cell membrane, resulting in leakage of the contents and death of the bacteria (Raqib et al., 2006; Jakubowski, 2019).
6 Inhibition of EMS by SCFAs relevant mechanisms
In recent years, with increasing research on the interaction between gut microbiota and EMS, it has been proposed that gut microbiota metabolites (such as short-chain fatty acids and bile acids) can affect EMS occurrence and development (Chadchan et al., 2021; Li et al., 2022; Chadchan et al., 2023). SCFAs, key signaling molecules involved in the interaction between the gut microbiota and host, mediate the NF-κB signaling pathway and MAPK signaling mainly by activating G protein-coupled receptors (GPCRs) and inhibiting histone deacetylase (HDAC). Therefore, SCFAs play an anti-inflammatory and regulatory role in immune cells, inhibiting the survival of endometriotic cells and the growth of lesions, thereby exerting an inhibitory effect on EMS (Chadchan et al., 2021, 2023).
The inflammatory environment and local and systemic immune cell dysfunction are characteristics of EMS (Riccio et al., 2018; Symons et al., 2018; Zhang et al., 2018; Viganò et al., 2020). The levels of proinflammatory cytokines (such as TNF-α, IL-1β, IL-6, and IL-17A) in ectopic lesions in patients with endometriosis increase to promote inflammation and the development of EMS (Ramírez-Pavez et al., 2021; Chen et al., 2023). The LPS concentration is a diagnostic marker of inflammatory diseases. LPSs act on TLRs on macrophages or neutrophils to promote the secretion of the proinflammatory cytokines TNF-α, IL-1β and IL-6, resulting in inflammation (Park and Lee, 2013). The two main inflammation-related signaling pathways in cells, the NF-κB and MAPK pathways, were activated after TLR activation. Two subunits of NF-κB, P65 and P50, are acetylated and translocated from the cytoplasm to the nucleus to promote the secretion of proinflammatory cytokines (Vallabhapurapu and Karin, 2009). Currently, NF-κB has been found to mediate a variety of cytokines (TNF-α, IL-1β, IL-2, IL-6, IL-8, and IL-12) and chemokines (MIP-2 and MCP-1) transcription (Liu and Malik, 2006). The MAPK signaling pathway is involved in the regulation of various key functions in the body and plays an important role in cell proliferation, differentiation and apoptosis. Phosphorylated mitogen-activated protein kinase (MAPK) regulates the ERK, JNK, and P38MAPK signal transduction pathways; gene transcription; secretion of the proinflammatory cytokines TNF-α and IL-6; and the expression of iNOS and nitric oxide (NO) (Arthur and Ley, 2013). Abnormal activation of the MAPK cascade can enhance the proliferation and survival of endometrial cells in EMS patients (Yotova et al., 2011). In vivo administration of a p38 MAPK inhibitor can significantly inhibit endometriotic lesions and reduce the levels of proinflammatory factors (IL-1β and TNF-α) and chemokines (MMP2 and MMP9) in peritoneal fluid (Zhou et al., 2010). Many studies have shown that LPS-mediated proinflammatory cytokines (such as IL-6) can be inhibited by SCFAs and that SCFAs can suppress LPS-induced inflammation by activating GPCR receptors and inhibiting HDACs (Vinolo et al., 2011) (Figure 3).
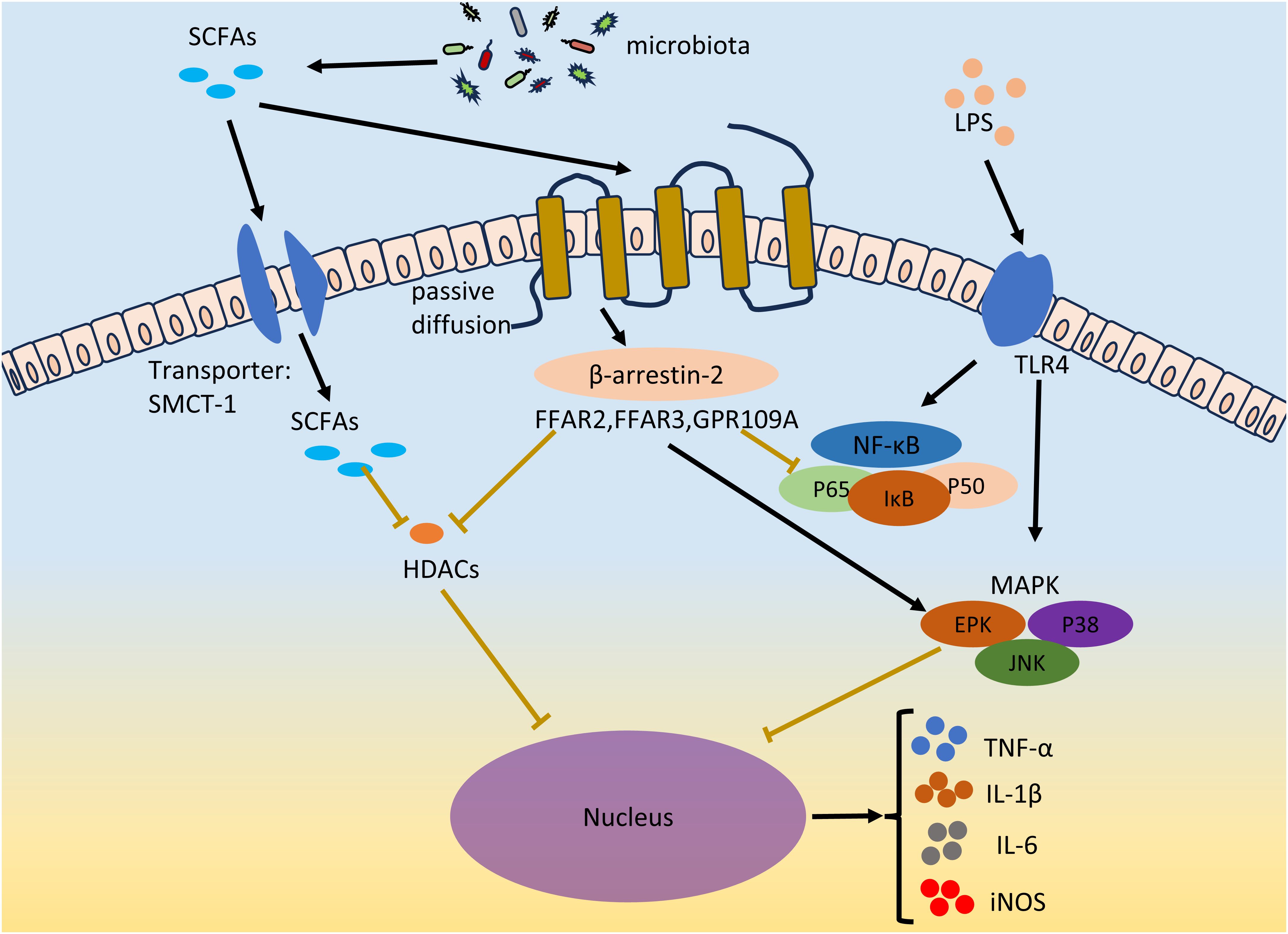
Figure 3 SCFAs inhibits EMS inflammation. LPS acts on TLR4 on immune cells, and promotes the secretion of pro-inflammatory factors TNF-α, IL-1β and IL-6 and the expression of iNOS by activating two inflammatory signaling pathways, NF-κB and MAPK. Gut microbiota derived SCFAs inhibit LPS-induced inflammation by activating GPCRs receptors and inhibiting HDACs in both ways. SCFAs enters through passive diffusion and then reduces NF-κB levels and the amount of p65 and p50 subunits of NF-κB entering the nucleus via the β-arrestin-2 signaling pathway or FFAR2, FFAR3, and GPR109A receptors. SCFAs regulates MAPK signaling pathways, including ERK, JNK and P38MAPK signal transduction pathways. SCFAs can inhibit HDACs through GPCRs, or directly inhibit HDACs through the entry of the transporter protein (SMCT-1) into cells. The secretion of TNF-α, IL-1β and IL-6 and the expression of iNOS were inhibited. The black lines indicate activation and the brown lines indicate inhibition.
6.1 Activation of GPCRs by SCFAs
SCFAs can exert anti-inflammatory effects on many cell types through the activation of G protein-coupled receptors, thereby inhibiting the development of EMS. Among them, GPR43 (also known as FFAR2), GPR41 (also known as FFAR3) and GPR109A are the most important receptors for SCFAs in the GPCR family. FFAR2 is widely expressed in neutrophils, monocytes, intestinal Treg cells, eosinophils and other immune cells (Smith et al., 2013). The FFAR2 receptor reduces NF-κB levels through the β-arrestin-2 signaling pathway and reduces the number of NF-κB subunits, p65 and p50, in the nucleus, thereby inhibiting the transcription of proinflammatory cytokines (IL-1β and IL-6) (Lee et al., 2013). SCFAs can inhibit the expression of IL-6, IL-1β and TNF-α through FFAR2, thereby exerting their anti-inflammatory effects (Nakajima et al., 2017; Pirozzi et al., 2018; Mizuta et al., 2019). Consistent with its function in FFAR2, FFAR3 also plays important roles in inflammation. C3 can inhibit the expression of IL-4, IL-5 and IL-17A through FFAR3, and C4 can inhibit the expression of nitric oxide synthase (iNOS), TNF-α, IL-6 and monocyte chemoattractant protein-1 (MCP-1) through FFAR3 (Li et al., 2018). In contrast to FFAR2 and FFAR3, GPR109A is activated by longer SCFAs, mainly C4 (Offermanns, 2017). Li et al. reported that GPR109A exerts an anti-inflammatory effect by inhibiting the Akt/mTOR signaling pathway in MIN6 pancreatic β cells (Priyadarshini et al., 2018).
6.2 Inhibition of HDACs by SCFAs
As a protease, histone deacetylases (HDACs) play important roles in the regulation of gene expression and the modification of chromosome structure. SCFAs are considered to be natural inhibitors of HDACs. Among all short-chain fatty acids, butyrate is the most effective inhibitor of HDAC activity. SCFAs may directly act on HDACs to enter cells through transporters or indirectly act on HDACs through GPCR activation. For example, C4 inhibits LPS-induced production of nitric oxide and inflammatory cytokines (such as IL-6 and IL-12) but directly inhibits HDACs and is unrelated to GPCRs (Chang et al., 2014). The authors of this study believe that C4 acts directly on HDACs. This is because SCFAs can be coupled to monocarboxylate transporter 1 (SMCT-1) through sodium transporter proteins rather than through membrane receptors, thereby entering cells, occupying the active sites of HDACs and inhibiting HDACs (Sun et al., 2017). In another report, the authors found that activation of FFAR3 could repress histone acetylation in a Chinese hamster ovary cell line through the inhibition of HDACs (Wu et al., 2012). In addition, FFAR3, FFAR2 and GPR109 may all be involved in the inhibition of HDACs by SCFAs. However, how SCFAs inhibit HDAC activity through GPCRs or directly has not been determined, and additional studies are needed to determine the underlying mechanisms involved.
6.3 Regulation of immune cells by SCFAs
SCFAs produced by the gut microbiota are transported throughout the body and can maintain systemic homeostasis by inhibiting inflammatory immune cells and promoting the differentiation of immune cells. We describe in detail how SCFAs regulate various immune cells, including macrophages (Macs), neutrophils, Tregs, and dendritic cells (DCs), thereby suppressing EMS-related inflammation.
6.3.1 Macs
The phenotype of Macs changes according to the surrounding microenvironment, and their function also changes with the phenotype. Macrophages are usually divided into two phenotypes: inflammatory macrophages (iMacs) and tolerant macrophages (tMacs). IMacs can participate in the inflammatory immune response, while tMacs suppress inflammation and maintain homeostasis by producing large amounts of anti-inflammatory factors, such as IL-10 and TGF-β. Macrophages play a major role in the development and expansion of EMS. Endometrial lesions are composed mainly of anti-inflammatory M2-polarized macrophages (Artemova et al., 2021). SCFAs can significantly reduce HDAC mRNA expression in Macs (Eslick et al., 2022). SCFAs, especially butyrate, inhibit the activation of Macs by negatively regulating NLRP3-mediated inflammatory signaling pathways (Shao et al., 2021). Furthermore, only butyrate can reprogram Macs metabolism to oxidative phosphorylation, converting Macs to an anti-inflammatory resistant phenotype (Scott et al., 2018).
6.3.2 Neutrophils
Neutrophils play an important role in the progression of EMS, and together with other leukocytes, they are involved in the regulation of EMS, including inflammation and repair (Huang et al., 2022). Several studies have reported increased neutrophil concentrations in plasma and peritoneal fluid during EMS. Moreover, the phagocytic ability of neutrophils is significantly reduced (Lukács et al., 2022). Studies have shown that SCFAs can enhance the phagocytosis of neutrophils at sites of inflammation by activating the GPR43 receptor (Vinolo et al., 2009). In addition, butyrate and propionate can also suppress the secretion of the proinflammatory factor TNFα and the activity of NF-κB and upregulate the expression of the anti-inflammatory factor IL-10 in LPS-activated monocytes and neutrophils through the inhibition of HDACs (Aoyama et al., 2010).
6.3.3 Tregs
Tregs play a key role in maintaining EMS immune homeostasis. The differentiation and function of Tregs can be regulated by SCFAs. Treg cells are present in large quantities in endometriotic lesions (Abramiuk et al., 2022). In some studies, the complete elimination of Tregs in EMS mice led to an increased volume of ectopic lesions (Tanaka et al., 2017). However, the role of Tregs in the progression of endometriosis has not been determined. SCFAs can promote the release of IL-10 from Treg cells through FFAR2. The main mechanism by which SCFAs regulate Tregs is to mediate signal transduction by activating GPCRs on the surface of target cells and inhibit HDACs to regulate gene expression (Smith et al., 2013). Butyrate increases histone acetylation to promote the differentiation of Tregs or promote the differentiation of Tregs by regulating dendritic cells (DCs) (Chung et al., 2009; Furusawa et al., 2013). In addition, SCFAs promote IL-10 production in Th1 and Th17 cells (Park et al., 2015).
6.3.4 DCs
DCs are antigen-presenting cells necessary for the activation of naive T cells. After DCs acquire tolerance, they participate in the elimination of the immune response. Furthermore, tolerable DCs (tDCs) mainly contribute to Treg differentiation and homeostasis. Butyrate and propionate inhibit the activation of bone marrow-derived DCs (BMDCs) by inhibiting the LPS-mediated expression of costimulatory molecules (such as CD40) and the production of cytokines such as IL-6 and IL-12 (Nastasi et al., 2015).
7 Treatment strategies
At present, the clinical treatments for EMS are mainly surgical treatment, drug treatment, or a combination of the two. It is necessary to find safe and effective methods to prevent and treat EMS (3). In recent years, an increasing number of researchers have shifted their focus to the gut microbiota and its metabolites. An imbalance in the gut microbiota strongly impacts the occurrence and development of EMS. Regulating the gut microbiota to restore it to a normal state could open up new avenues for the treatment of EMS. We reviewed the current potential interventions targeting the gut microbiota and SCFAs for the treatment of EMS, including probiotic and prebiotics, fecal transplantation (FMT), gut microbiota metabolite therapy and diet regulation, which may provide new ideas into the clinical treatment of EMS (Figure 4).
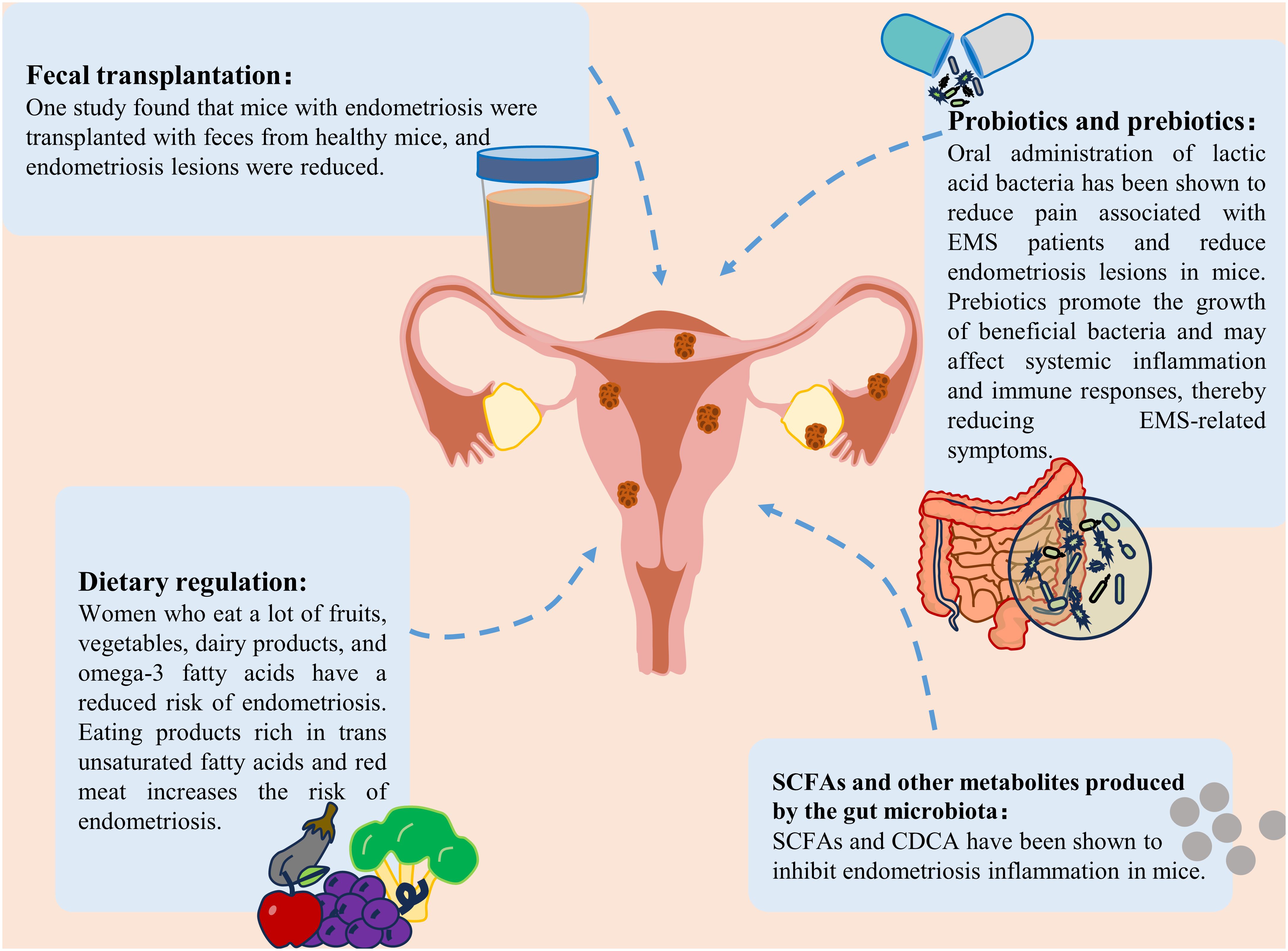
Figure 4 Treatment strategies for endometriosis. Treatment strategies for endometriosis include the use of probiotics and prebiotics, fecal bacterial transplantation, gut microbiota metabolite supplementation and dietary modification.
7.1 Probiotics and prebiotics
Probiotics and prebiotics are two components of new therapies based on the gut microbiota. Probiotic intervention is an effective method for treating EMS. The potential effects of probiotics on immune regulation, inflammation and intestinal flora imbalance are all associated with the development of EMS (Bodke and Jogdand, 2022; Hashem and Gonzalez-Bulnes, 2022). Oral lactic acid bacteria have been shown to reduce pain associated with endometriosis (Khodaverdi et al., 2019). It also reduces endometriotic lesions in mice by enhancing NK cell activity and increasing the IL-12 concentration (Itoh et al., 2011). Dysbiota and endometriosis-related inflammation lead to impaired NK cell activity, and probiotic treatment reversed this immune dysregulation. In a rat model, probiotic treatment was also found to inhibit the growth of endometriosis (Uchida and Kobayashi, 2013). In addition, as mentioned previously, SCFAs can inhibit endometriosis. In vitro and in vivo studies have confirmed the positive effect of probiotics on increasing short-chain fatty acids (Markowiak-Kopeć and Śliżewska, 2020). The use of different types of probiotics has been found to increase SCFAs levels in patients with different diseases (such as colorectal cancer and respiratory diseases) (Hou et al., 2022; Williams et al., 2022). It was speculated that probiotics may also inhibit EMS by increasing the production of SCFAs.
To increase the effectiveness of probiotics, they can be combined with prebiotics. Although studies specifically focusing on the effects of prebiotics on endometriosis are limited, prebiotics can promote the growth of beneficial bacteria, reduce the translocation of harmful bacteria and their products into the blood, and help maintain the ecological balance of the intestinal microbiota. Subsequently, it may affect systemic inflammation and the immune response, thereby affecting EMS-associated symptoms (You et al., 2022). In addition, prebiotics are widely used to increase SCFAs levels (Gibson et al., 2017). One study showed that the use of high doses of arabinoxylan oligosaccharides could increase butyrate, acetate, propionate, and total SCFAs (François et al., 2012). Therefore, prebiotics may have potential benefits for treating endometriosis. Although probiotics and prebiotics have shown good potential in treating EMS, there is still a lack of guidelines outlining or supporting the standard use of probiotics and prebiotics in EMS management, and additional studies are needed to evaluate the effects of probiotics and prebiotics on EMS in the future.
7.2 Fecal microbe transplantation
FMT involves the transfer of feces from healthy people to the gastrointestinal tract of patients with imbalanced flora to reconstruct the gut microbiota and treat related diseases (Nigam et al., 2022). Currently, FMT has been applied to treat many diseases, such as gastrointestinal diseases, neuropsychiatric diseases, and blood diseases (Kim and Gluck, 2019). In addition, FMT has become a mature therapy for recurrent Clostridium difficile infection (Cammarota et al., 2015). Recently, studies have shown that FMT can be used to treat female reproductive tract diseases (Quaranta et al., 2019; Huang et al., 2021a). A study by Chadchan et al. revealed that after endometriosis mice were transplanted with feces from healthy mice, the number of uterine lesions decreased (Chadchan et al., 2021, 2023). Ni et al. recently reported similar findings that ZO-2 expression was reduced after the administration of feces from endometriotic mice to healthy mice (Ni et al., 2021). These preliminary studies highlighted the potential of FMT to relieve EMS through the reinforcement of barrier integrity. In addition, several pieces of evidence suggest that an increase in SCFAs may be a key determinant in the treatment of different diseases by FMT (Chen et al., 2019; El-Salhy et al., 2021; Xu et al., 2023). Taken together, these findings indicate that FMT might be a potential treatment for EMS. However, this strategy is limited by the presence of unknown microorganisms and pathogenic bacteria in the transplanted feces.
7.3 SCFAs and other metabolites produced by the gut microbiota
One study revealed that SCFAs play an important role in maintaining the intestinal barrier and suppressing immune function (Rekha et al., 2022). Among them, butyric acid played a particularly significant role. Butyrate can also suppress the immune response caused by intestinal microbial imbalance (Ma et al., 2020). A recent study showed that gut microbiota-derived butyrate could inhibit endometriosis in mice through the regulation of G protein-coupled receptors (Chadchan et al., 2021). In addition, another type of microbiota metabolite that has therapeutic effects on EMS is secondary bile acids. A study on the correlation between fecal metabolomics and the gut microbiota in endometriosis mice revealed that bile acid and chenodeoxycholic acid (CDCA) levels were increased in the intestines of endometriosis mice (Ni et al., 2020). The above studies showed that changes in the metabolite levels of the gut microbiota may play an important role in the development of EMS. Although several metabolites of the gut microbiota are maypotentially useful for treating EMS, the functional diversity of these metabolites needs to be overcome to design effective treatment strategies.
7.4 Dietary adjustment
Dietary intervention may lead to different changes in the gut microbiota and SCFAs levels. Diet provides substrates that enable the manipulation of microbial composition. For example, the Mediterranean diet can increase the availability of substrates available for specific bacteria, thereby increasing the formation of SCFAs (Wagenaar et al., 2021; Yalçın Bahat et al., 2022). A fiber-rich diet can increase the levels of SCFAs, especially acetate and butyrate (So et al., 2018). Rinninella et al. evaluated the use of a ketogenic diet and reported that the total number and abundance of several beneficial bacteria and bacteria, were reduced. A ketogenic diet can significantly affect the characteristics of the gut microbiota and may lead to a reduction in short-chain fatty acids (Rew et al., 2022; Rinninella et al., 2023). A plant-based diet has been shown to lead to the dominance of SCFAs-producing Firmicutes, while an animal-based diet increases the number of Bacteroides (Tomova et al., 2019). Recently, a large number of studies have shown that diet is a potential risk factor for endometriosis (Jurkiewicz-Przondziono et al., 2017). Women who consume large amounts of fruits, vegetables, dairy products, or omega-3 fatty acids (PUFAs) have a reduced risk of developing endometriosis (Samaneh et al., 2019). A high intake of omega-3 polyunsaturated fatty acids can reduce the risk of endometriosis in women, which may be attributed to potential changes in the gut microbiota (Watson et al., 2018). A diet rich in ω-3 polyunsaturated fatty acids can reduce inflammation in a mouse model of endometriosis, as indicated by reduced TNF-α and IL-6 inflammatory marker levels (Pereira et al., 2019). In addition, some studies have shown that linolenic acid (ALA) can reduce inflammation by inhibiting the accumulation of prostaglandin E2 (PGE2) and nitrite. ALA can also reduce LPS levels and improve the abdominal inflammatory environment in endometriosis mice (Ni et al., 2021). Moreover, it can inhibit the inflammatory response of M1 macrophages (Pauls et al., 2018). However, the consumption of products and red meat rich in trans-unsaturated fatty acids can increase the risk of endometriosis (Marcinkowska and Górnicka, 2023). In conclusion, reasonable diet adjustment may help prevent EMS.
In summary, the gut microbiota and SCFAs can be modulated by probiotics and prebiotics, fecal microbiota transplantation, gut microbiota metabolites, or dietary regimens. These methods can greatly increase the SCFAs produced in the colon, inhibiting EMS. However, the clinical efficacy of these methods needs further study.
8 Conclusions
In conclusion, gut microbes and their metabolite SCFAs can regulate the development of EMS. A gut microbiota imbalance promotes the development of EMS through mechanisms such as bacterial contamination, estrogen metabolism, cytokine production, and impaired immune function. The normal gut microbiota inhibits the development of EMS by increasing the production of SCFAs in the host. The specific underlying mechanisms include the inhibition of HDACs and the activation of GPCRs. In addition, this review summarizes the current potential interventions targeting the gut microbiota and SCFAs for the treatment of EMS, including probiotics and prebiotics, FMT, gut microbiota metabolites, and dietary regulation, which may be new directions in the clinical treatment of EMS. Provide new thinking.
Diagnostic delay is one of the main difficulties faced by EMSs. Studying the different characteristics of the microbiota and its metabolites in EMS patients will aid in noninvasive diagnosis, reduce the incidence of delayed diagnosis, and enable prevention. Notably, prevention is more important than treatment. At present, a large number of studies have confirmed the function of the gut microbiota in EMS. In recent years, studies have also reported the important role of microbiota metabolites (especially SCFAs and bile acids) in the pathogenesis of EMS. However, the treatment of EMS based on this therapy is still at the experimental research stage and has not yet been clinically applied. The experimental data may be affected by various factors, including race, diet, or specimen quality, and further research is needed to address these issues. Nevertheless, we should focus on the effect of the gut microbiota-SCFAs-EMS axis on the EMS. Understanding the key microbiota-derived metabolites associated with EMS is important for the future design of microbiome-based therapeutics. In addition, with the advancement of molecular techniques and at the genetic level, new biomarkers are expected to be obtained, and new treatment options are expected to be developed in the future; however, additional studies are needed.
Author contributions
ML: Writing – original draft, Writing – review & editing. RP: Writing – original draft, Writing – review & editing. CT: Writing – original draft, Writing – review & editing. JS: Conceptualization, Writing – review & editing. JM: Visualization, Writing – review & editing. RS: Writing – review & editing. XQ: Writing – review & editing, Conceptualization. RZ: Funding acquisition, Writing – review & editing. HG: Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the “Trinity” college students innovation and entrepreneurship cultivation program of Inner Mongolia Medical University (No.SWYT2022010), the Nature Science Foundation of Inner Mongolia Autonomous Region (No.2023LHMS08001, 2020MS08106, 2022MS08060), the Unite program of Inner Mongolia Medical University (No.YKD2023LH060), the National Natural Science Foundation of China (No.82160703), the Key Program of Inner Mongolia Medical University (No.YKD2022ZD013), the Program for Young Talents of Science and Technology in Universities of Inner Mongolia Autonomous Region (No.NJYT23114), the Health Science and Technology Program of Inner Mongolia Health Commission (No. 202202158, 202201337), the PhD Initial Funding Project of the Affiliated Hospital of Inner Mongolia Medical University (No. NYFY BS 202120) and the General program of Inner Mongolia Medical University (No.YKD2022MS045), the Inner Mongolia Medical University student innovation and entrepreneurship project (NO.S202410132009, S202410132012), the 2022 Inner Mongolia Health Science and Technology Plan Project (NO.202201184), the 2023 Hohhot Science and Technology Plan Project (NO.2023-S-20), the 2023 Hohhot Health Youth Talent Science and Technology Project (NO.HHHY-2023015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abramiuk, M., Grywalska, E., Małkowska, P., Sierawska, O., Hrynkiewicz, R., Niedźwiedzka-Rystwej, P. (2022). The role of the immune system in the development of endometriosis. Cells 11, 2028. doi: 10.3390/cells11132028
Adlercreutz, H. (1974). Hepatic metabolism of estrogens in health and disease. N. Engl. J. Med. 290, 1081–1083. doi: 10.1056/NEJM197405092901913
Agarwal, S. K., Chapron, C., Giudice, L. C., Laufer, M. R., Leyland, N., Missmer, S. A., et al. (2019). Clinical diagnosis of endometriosis: a call to action. Am. J. Obstet. Gynecol. 220, 354.e1–354.e12. doi: 10.1016/j.ajog.2018.12.039
Agostinis, C., Balduit, A., Mangogna, A., Zito, G., Romano, F., Ricci, G., et al. (2020). Immunological basis of the endometriosis: the complement system as a potential therapeutic target. Front. Immunol. 11. doi: 10.3389/fimmu.2020.599117
Alexander, J. W., Boyce, S. T., Babcock, G. F., Gianotti, L., Peck, M. D., Dunn, D. L., et al. (1990). The process of microbial translocation. Ann. Surg. 212, 496–510; discussion 511-2. doi: 10.1097/00000658-199010000-00012
Alfa, M. J., Strang, D., Tappia, P. S., Graham, M., Van Domselaar, G., Forbes, J. D., et al. (2018). A randomized trial to determine the impact of a digestion resistant starch composition on the gut microbiome in older and mid-age adults. Clin. Nutr. 37, 797–807. doi: 10.1016/j.clnu.2017.03.025
Alghetaa, H., Mohammed, A., Singh, N. P., Bloomquist, R. F., Chatzistamou, I., Nagarkatti, M., et al. (2023). Estrobolome dysregulation is associated with altered immunometabolism in a mouse model of endometriosis. Front. Endocrinol. (Lausanne) 14. doi: 10.3389/fendo.2023.1261781
Anastasiu, C. V., Moga, M. A., Elena Neculau, A., Bălan, A., Scârneciu, I., Dragomir, R. M., et al. (2020). Biomarkers for the noninvasive diagnosis of endometriosis: state of the art and future perspectives. Int. J. Mol. Sci. 21, 1750. doi: 10.3390/ijms21051750
Aoyama, M., Kotani, J., Usami, M. (2010). Butyrate and propionate induced activated or non-activated neutrophil apoptosis via HDAC inhibitor activity but without activating GPR-41/GPR-43 pathways. Nutrition 26, 653–661. doi: 10.1016/j.nut.2009.07.006
Arruda, M. S., Petta, C. A., Abrão, M. S., Benetti-Pinto, C. L. (2003). Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum. Reprod. 18, 756–759. doi: 10.1093/humrep/deg136
Artemova, D., Vishnyakova, P., Khashchenko, E., Elchaninov, A., Sukhikh, G., Fatkhudinov, T. (2021). Endometriosis and cancer: exploring the role of macrophages. Int. J. Mol. Sci. 22, 5196. doi: 10.3390/ijms22105196
Arthur, J. S., Ley, S. C. (2013). Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 13, 679–692. doi: 10.1038/nri3495
Bailey, M. T., Coe, C. L. (2002). Endometriosis is associated with an altered profile of intestinal microflora in female rhesus monkeys. Hum. Reprod. 17, 1704–1708. doi: 10.1093/humrep/17.7.1704
Baker, J. M., Al-Nakkash, L., Herbst-Kralovetz, M. M. (2017). Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 103, 45–53. doi: 10.1016/j.maturitas.2017.06.025
Bauşic, A. I. G., Creţoiu, S. M., Bauşic, V., Matasariu, D. R., Stănculescu, R. V., Brătilă, E. (2023). The role of gut dysbiosis in endometriosis' diagnosis and treatment approaches - case report. Rom. J. Morphol. Embryol. 64, 263–269. doi: 10.47162/rjme.64.2.17
Becker, C. M., D'Amato, R. J. (2007). Angiogenesis and antiangiogenic therapy in endometriosis. Microvasc. Res. 74, 121–130. doi: 10.1016/j.mvr.2007.04.008
Bedaiwy, M. A., Alfaraj, S., Yong, P., Casper, R. (2017). New developments in the medical treatment of endometriosis. Fertil. Steril. 107, 555–565. doi: 10.1016/j.fertnstert.2016.12.025
Blander, J. M., Longman, R. S., Iliev, I. D., Sonnenberg, G. F., Artis, D. (2017). Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 18, 851–860. doi: 10.1038/ni.3780
Bloski, T., Pierson, R. (2008). Endometriosis and chronic pelvic pain: unraveling the mystery behind this complex condition. Nurs. Womens Health 12, 382–395. doi: 10.1111/j.1751-486X.2008.00362.x
Bodke, H., Jogdand, S. (2022). Role of probiotics in human health. Cureus 14, e31313. doi: 10.7759/cureus.31313
Borghese, B., Sibiude, J., Santulli, P., Lafay Pillet, M. C., Marcellin, L., Brosens, I., et al. (2015). Low birth weight is strongly associated with the risk of deep infiltrating endometriosis: results of a 743 case-control study. PLoS One 10, e0117387. doi: 10.1371/journal.pone.0117387
Brandsma, E., Kloosterhuis, N. J., Koster, M., Dekker, D. C., Gijbels, M. J. J., van der Velden, S., et al. (2019). A proinflammatory gut microbiota increases systemic inflammation and accelerates atherosclerosis. Circ. Res. 124, 94–100. doi: 10.1161/CIRCRESAHA.118.313234
Bruner-Tran, K. L., Herington, J. L., Duleba, A. J., Taylor, H. S., Osteen, K. G. (2013). Medical management of endometriosis: emerging evidence linking inflammation to disease pathophysiology. Minerva Ginecol. 65, 199–213.
Buford, T. W. (2017). (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 5, 80. doi: 10.1186/s40168-017-0296-0
Burney, R. O., Giudice, L. C. (2012). Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 98, 511–519. doi: 10.1016/j.fertnstert.2012.06.029
Cammarota, G., Ianiro, G., Magalini, S., Gasbarrini, A., Gui, D. (2015). Decrease in surgery for clostridium difficile infection after starting a program to transplant fecal microbiota. Ann. Intern. Med. 163, 487–488. doi: 10.7326/L15-5139
Cao, M., Zhang, Z., Han, S., Lu, X. (2019). Butyrate inhibits the proliferation and induces the apoptosis of colorectal cancer HCT116 cells via the deactivation of mTOR/S6K1 signaling mediated partly by SIRT1 downregulation. Mol. Med. Rep. 19, 3941–3947. doi: 10.3892/mmr.2019.10002
Chadchan, S. B., Cheng, M., Parnell, L. A., Yin, Y., Schriefer, A., Mysorekar, I. U., et al. (2019). Antibiotic therapy with metronidazole reduces endometriosis disease progression in mice: a potential role for gut microbiota. Hum. Reprod. 34, 1106–1116. doi: 10.1093/humrep/dez041
Chadchan, S. B., Naik, S. K., Popli, P., Talwar, C., Putluri, S., Ambati, C. R., et al. (2023). Gut microbiota and microbiota-derived metabolites promotes endometriosis. Cell Death Discov. 9, 28. doi: 10.1038/s41420-023-01309-0
Chadchan, S. B., Popli, P., Ambati, C. R., Tycksen, E., Han, S. J., Bulun, S. E., et al. (2021). Gut microbiota-derived short-chain fatty acids protect against the progression of endometriosis. Life Sci. Alliance 4, e202101224. doi: 10.26508/lsa.202101224
Chang, P. V., Hao, L., Offermanns, S., Medzhitov, R. (2014). The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. U.S.A. 111, 2247–2252. doi: 10.1073/pnas.1322269111
Chen, G., Ran, X., Li, B., Li, Y., He, D., Huang, B., et al. (2018). Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. EBioMedicine 30, 317–325. doi: 10.1016/j.ebiom.2018.03.030
Chen, R., Xu, Y., Wu, P., Zhou, H., Lasanajak, Y., Fang, Y., et al. (2019). Transplantation of fecal microbiota rich in short chain fatty acids and butyric acid treat cerebral ischemic stroke by regulating gut microbiota. Pharmacol. Res. 148, 104403. doi: 10.1016/j.phrs.2019.104403
Chen, S., Liu, Y., Zhong, Z., Wei, C., Liu, Y., Zhu, X. (2023). Peritoneal immune microenvironment of endometriosis: Role and therapeutic perspectives. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1134663
Cheng, L., Gao, Y., Xia, Q., Wang, H., Xie, X., Liu, Y., et al. (2021). Reproductive tract microbiota of women in childbearing age shifts upon gynecological infections and menstrual cycle. BMC Microbiol. 21, 252. doi: 10.1186/s12866-021-02300-4
Chiaffarino, F., Bravi, F., Cipriani, S., Parazzini, F., Ricci, E., Viganò, P., et al. (2014). Coffee and caffeine intake and risk of endometriosis: a meta-analysis. Eur. J. Nutr. 53, 1573–1579. doi: 10.1007/s00394-014-0662-7
Chung, D. J., Rossi, M., Romano, E., Ghith, J., Yuan, J., Munn, D. H., et al. (2009). Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood 114, 555–563. doi: 10.1182/blood-2008-11-191197
Clemente, J. C., Manasson, J., Scher, J. U. (2018). The role of the gut microbiome in systemic inflammatory disease. Bmj 360, j5145. doi: 10.1136/bmj.j5145
Cong, J., Zhou, P., Zhang, R. (2022). Intestinal microbiota-derived short chain fatty acids in host health and disease. Nutrients 14, 1977. doi: 10.3390/nu14091977
Corrêa-Oliveira, R., Fachi, J. L., Vieira, A., Sato, F. T., Vinolo, M. A. (2016). Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 5, e73. doi: 10.1038/cti.2016.17
Cummings, J. H., Pomare, E. W., Branch, W. J., Naylor, C. P., Macfarlane, G. T. (1987). Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227. doi: 10.1136/gut.28.10.1221
Dabek, M., McCrae, S. I., Stevens, V. J., Duncan, S. H., Louis, P. (2008). Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 66, 487–495. doi: 10.1111/fem.2008.66.issue-3
da Costa, A. C., Martins, C. R., Habib, N. (2022). Hepatic endometriosis. J. Gastrointest Surg. 26, 2396–2398. doi: 10.1007/s11605-021-05156-1
Deitch, E. A., Ma, W. J., Ma, L., Berg, R., Specian, R. D. (1989). Endotoxin-induced bacterial translocation: a study of mechanisms. Surgery 106, 292–9; discussion 299-300.
den Besten, G., van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D. J., Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
DiVasta, A. D., Vitonis, A. F., Laufer, M. R., Missmer, S. A. (2018). Spectrum of symptoms in women diagnosed with endometriosis during adolescence vs adulthood. Am. J. Obstet. Gynecol. 218, 324.e1–324.e11. doi: 10.1016/j.ajog.2017.12.007
Elefante, C., Brancati, G. E., Oragvelidze, E., Lattanzi, L., Maremmani, I., Perugi, G. (2022). Psychiatric symptoms in patients with cerebral endometriosis: A case report and literature review. J. Clin. Med. 11, 7212. doi: 10.3390/jcm11237212
El-Salhy, M., Valeur, J., Hausken, T., Gunnar Hatlebakk, J. (2021). Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol. Motil. 33, e13983. doi: 10.1111/nmo.13983
Ervin, S. M., Li, H., Lim, L., Roberts, L. R., Liang, X., Mani, S., et al. (2019). Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 294, 18586–18599. doi: 10.1074/jbc.RA119.010950
Eslick, S., Williams, E. J., Berthon, B. S., Wright, T., Karihaloo, C., Gately, M., et al. (2022). Weight loss and short-chain fatty acids reduce systemic inflammation in monocytes and adipose tissue macrophages from obese subjects. Nutrients 14, 765. doi: 10.3390/nu14040765
Flemming, A. (2019). Butyrate boosts microbicidal macrophages. Nat. Rev. Immunol. 19, 135. doi: 10.1038/s41577-019-0132-9
François, I. E., Lescroart, O., Veraverbeke, W. S., Marzorati, M., Possemiers, S., Evenepoel, P., et al. (2012). Effects of a wheat bran extract containing arabinoxylan oligosaccharides on gastrointestinal health parameters in healthy adult human volunteers: a double-blind, randomised, placebo-controlled, cross-over trial. Br. J. Nutr. 108, 2229–2242. doi: 10.1017/S0007114512000372
Furusawa, Y., Obata, Y., Fukuda, S., Endo, T. A., Nakato, G., Takahashi, D., et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. doi: 10.1038/nature12721
Galvankar, M., Singh, N., Modi, D. (2017). Estrogen is essential but not sufficient to induce endometriosis. J. Biosci. 42, 251–263. doi: 10.1007/s12038-017-9687-4
Gibson, G. R., Hutkins, R., Sanders, M. E., Prescott, S. L., Reimer, R. A., Salminen, S. J., et al. (2017). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 14, 491–502. doi: 10.1038/nrgastro.2017.75
Gogacz, M., Winkler, I., Bojarska-Junak, A., Tabarkiewicz, J., Semczuk, A., Rechberger, T., et al. (2016). Increased percentage of Th17 cells in peritoneal fluid is associated with severity of endometriosis. J. Reprod. Immunol. 117, 39–44. doi: 10.1016/j.jri.2016.04.289
Guinan, J., Wang, S., Hazbun, T. R., Yadav, H., Thangamani, S. (2019). Antibiotic-induced decreases in the levels of microbial-derived short-chain fatty acids correlate with increased gastrointestinal colonization of Candida albicans. Sci. Rep. 9, 8872. doi: 10.1038/s41598-019-45467-7
Hadfield, R. M., Mardon, H. J., Barlow, D. H., Kennedy, S. H. (1997). Endometriosis in monozygotic twins. Fertil. Steril. 68, 941–942. doi: 10.1016/S0015-0282(97)00359-2
Halme, J., Hammond, M. G., Hulka, J. F., Raj, S. G., Talbert, L. M. (1984). Retrograde menstruation in healthy women and in patients with endometriosis. Obstet. Gynecol. 64, 151–154.
Han, S. J., Jung, S. Y., Wu, S. P., Hawkins, S. M., Park, M. J., Kyo, S., et al. (2015). Estrogen receptor β Modulates apoptosis complexes and the inflammasome to drive the pathogenesis of endometriosis. Cell 163, 960–974. doi: 10.1016/j.cell.2015.10.034
Hansen, K. A., Eyster, K. M. (2010). Genetics and genomics of endometriosis. Clin. Obstet. Gynecol. 53, 403–412. doi: 10.1097/GRF.0b013e3181db7ca1
Hantschel, J., Weis, S., Schäfer, K. H., Menger, M. D., Kohl, M., Egert, M., et al. (2019). Effect of endometriosis on the fecal bacteriota composition of mice during the acute phase of lesion formation. PloS One 14, e0226835. doi: 10.1371/journal.pone.0226835
Hashem, N. M., Gonzalez-Bulnes, A. (2022). The use of probiotics for management and improvement of reproductive Eubiosis and function. Nutrients 14, 902. doi: 10.3390/nu14040902
He, C., Deng, J., Hu, X., Zhou, S., Wu, J., Xiao, D., et al. (2019). Vitamin A inhibits the action of LPS on the intestinal epithelial barrier function and tight junction proteins. Food Funct. 10, 1235–1242. doi: 10.1039/C8FO01123K
Hemmings, R., Rivard, M., Olive, D. L., Poliquin-Fleury, J., Gagné, D., Hugo, P., et al. (2004). Evaluation of risk factors associated with endometriosis. Fertil. Steril. 81, 1513–1521. doi: 10.1016/j.fertnstert.2003.10.038
Horvath, T. D., Ihekweazu, F. D., Haidacher, S. J., Ruan, W., Engevik, K. A., Fultz, R., et al. (2022). Bacteroides ovatus colonization influences the abundance of intestinal short chain fatty acids and neurotransmitters. iScience 25, 104158. doi: 10.1016/j.isci.2022.104158
Hou, H., Chen, D., Zhang, K., Zhang, W., Liu, T., Wang, S., et al. (2022). Gut microbiota-derived short-chain fatty acids and colorectal cancer: Ready for clinical translation? Cancer Lett. 526, 225–235. doi: 10.1016/j.canlet.2021.11.027
Høverstad, T., Midtvedt, T. (1986). Short-chain fatty acids in germfree mice and rats. J. Nutr. 116, 1772–1776. doi: 10.1093/jn/116.9.1772
Huang, Y., Li, Q., Hu, R., Li, R., Yang, Y. (2022). Five immune-related genes as diagnostic markers for endometriosis and their correlation with immune infiltration. Front. Endocrinol. (Lausanne) 13. doi: 10.3389/fendo.2022.1011742
Huang, L., Liu, B., Liu, Z., Feng, W., Liu, M., Wang, Y., et al. (2021b). Gut microbiota exceeds cervical microbiota for early diagnosis of endometriosis. Front. Cell Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.788836
Huang, J., Shan, W., Li, F., Wang, Z., Cheng, J., Lu, F., et al. (2021a). Fecal microbiota transplantation mitigates vaginal atrophy in ovariectomized mice. Aging (Albany NY) 13, 7589–7607. doi: 10.18632/aging.v13i5
Itoh, H., Sashihara, T., Hosono, A., Kaminogawa, S., Uchida, M. (2011). Lactobacillus gasseri OLL2809 inhibits development of ectopic endometrial cell in peritoneal cavity via activation of NK cells in a murine endometriosis model. Cytotechnology 63, 205–210. doi: 10.1007/s10616-011-9343-z
Jakubowski, H. (2019). Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 99, 555–604. doi: 10.1152/physrev.00003.2018
Jeljeli, M., Riccio, L. G. C., Chouzenoux, S., Moresi, F., Toullec, L., Doridot, L., et al. (2020). Macrophage immune memory controls endometriosis in mice and humans. Cell Rep. 33, 108325. doi: 10.1016/j.celrep.2020.108325
Jeon, S. Y., Hwang, K. A., Choi, K. C. (2016). Effect of steroid hormones, estrogen and progesterone, on epithelial mesenchymal transition in ovarian cancer development. J. Steroid Biochem. Mol. Biol. 158, 1–8. doi: 10.1016/j.jsbmb.2016.02.005
Jeong, S., Kim, H. Y., Kim, A. R., Yun, C. H., Han, S. H. (2019). Propionate ameliorates staphylococcus aureus skin infection by attenuating bacterial growth. Front. Microbiol. 10. doi: 10.3389/fmicb.2019.01363
Jiang, I., Yong, P. J., Allaire, C., Bedaiwy, M. A. (2021). Intricate connections between the microbiota and endometriosis. Int. J. Mol. Sci. 22, 5644. doi: 10.3390/ijms22115644
Jurkiewicz-Przondziono, J., Lemm, M., Kwiatkowska-Pamuła, A., Ziółko, E., Wójtowicz, M. K. (2017). Influence of diet on the risk of developing endometriosis. Ginekol Pol. 88, 96–102. doi: 10.5603/GP.a2017.0017
Khan, K. N., Fujishita, A., Hiraki, K., Kitajima, M., Nakashima, M., Fushiki, S., et al. (2018). Bacterial contamination hypothesis: a new concept in endometriosis. Reprod. Med. Biol. 17, 125–133. doi: 10.1002/rmb2.12083
Khan, K. N., Kitajima, M., Hiraki, K., Yamaguchi, N., Katamine, S., Matsuyama, T., et al. (2010). Escherichia coli contamination of menstrual blood and effect of bacterial endotoxin on endometriosis. Fertil. Steril. 94, 2860–3.e1-3. doi: 10.1016/j.fertnstert.2010.04.053
Khodaverdi, S., Mohammadbeigi, R., Khaledi, M., Mesdaghinia, L., Sharifzadeh, F., Nasiripour, S., et al. (2019). Beneficial effects of oral lactobacillus on pain severity in women suffering from endometriosis: A pilot placebo-controlled randomized clinical trial. Int. J. Fertil. Steril. 13, 178–183. doi: 10.22074/ijfs.2019.5584
Kim, K. O., Gluck, M. (2019). Fecal microbiota transplantation: an update on clinical practice. Clin. Endosc. 52, 137–143. doi: 10.5946/ce.2019.009
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Kvaskoff, M., Mu, F., Terry, K. L., Harris, H. R., Poole, E. M., Farland, L., et al. (2015). Endometriosis: a high-risk population for major chronic diseases? Hum. Reprod. Update 21, 500–516. doi: 10.1093/humupd/dmv013
Lamas, A., Regal, P., Vázquez, B., Cepeda, A., Franco, C. M. (2019). Short chain fatty acids commonly produced by gut microbiota influence salmonella enterica motility, biofilm formation, and gene expression. Antibiotics (Basel) 8, 265. doi: 10.3390/antibiotics8040265
Laux-Biehlmann, A., d'Hooghe, T., Zollner, T. M. (2015). Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol. Sci. 36, 270–276. doi: 10.1016/j.tips.2015.03.004
Lee, S. U., In, H. J., Kwon, M. S., Park, B. O., Jo, M., Kim, M. O., et al. (2013). β-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-κB. Biol. Pharm. Bull. 36, 1754–1759. doi: 10.1248/bpb.b13-00312
Lefebvre, T., Campas, M., Matta, K., Ouzia, S., Guitton, Y., Duval, G., et al. (2024). A comprehensive multiplatform metabolomic analysis reveals alterations of 2-hydroxybutyric acid among women with deep endometriosis related to the pesticide trans-nonachlor. Sci. Total Environ. 918, 170678. doi: 10.1016/j.scitotenv.2024.170678
Leonardi, M., Hicks, C., El-Assaad, F., El-Omar, E., Condous, G. (2020). Endometriosis and the microbiome: a systematic review. Bjog 127, 239–249. doi: 10.1111/1471-0528.15916
Li, M., van Esch, B., Wagenaar, G. T. M., Garssen, J., Folkerts, G., Henricks, P. A. J. (2018). Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharmacol. 831, 52–59. doi: 10.1016/j.ejphar.2018.05.003
Li, Y., Wang, K., Ding, J., Sun, S., Ni, Z., Yu, C. (2022). Influence of the gut microbiota on endometriosis: Potential role of chenodeoxycholic acid and its derivatives. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.954684
Licciardi, P. V., Ververis, K., Karagiannis, T. C. (2011). Histone deacetylase inhibition and dietary short-chain Fatty acids. ISRN Allergy 2011, 869647. doi: 10.5402/2011/869647
Liu, S. F., Malik, A. B. (2006). NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am. J. Physiol. Lung Cell Mol. Physiol. 290, L622–l645. doi: 10.1152/ajplung.00477.2005
Lou, X., Xue, J., Shao, R., Yang, Y., Ning, D., Mo, C., et al. (2022). Fecal microbiota transplantation and short-chain fatty acids reduce sepsis mortality by remodeling antibiotic-induced gut microbiota disturbances. Front. Immunol. 13. doi: 10.3389/fimmu.2022.1063543
Lukács, L., Kovács, A. R., Pál, L., Szűcs, S., Lampé, R. (2022). Evaluating the phagocytic index of peripheral leukocytes in endometriosis by plasma experiments. Medicina (Kaunas) 58, 925. doi: 10.3390/medicina58070925
Ma, J., Piao, X., Mahfuz, S., Long, S., Wang, J. (2022). The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Anim. Nutr. 9, 159–174. doi: 10.1016/j.aninu.2021.09.012
Ma, X., Zhou, Z., Zhang, X., Fan, M., Hong, Y., Feng, Y., et al. (2020). Sodium butyrate modulates gut microbiota and immune response in colorectal cancer liver metastatic mice. Cell Biol. Toxicol. 36, 509–515. doi: 10.1007/s10565-020-09518-4
Marcinkowska, A., Górnicka, M. (2023). The role of dietary fats in the development and treatment of endometriosis. Life (Basel) 13, 654. doi: 10.3390/life13030654
Markowiak-Kopeć, P., Śliżewska, K. (2020). The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 12, 1107. doi: 10.3390/nu12041107
Maroun, P., Cooper, M. J., Reid, G. D., Keirse, M. J. (2009). Relevance of gastrointestinal symptoms in endometriosis. Aust. N Z J. Obstet. Gynaecol. 49, 411–414. doi: 10.1111/j.1479-828X.2009.01030.x
Martin-Gallausiaux, C., Marinelli, L., Blottière, H. M., Larraufie, P., Lapaque, N. (2021). SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80, 37–49. doi: 10.1017/S0029665120006916
Maslowski, K. M., Vieira, A. T., Ng, A., Kranich, J., Sierro, F., Yu, D., et al. (2009). Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286. doi: 10.1038/nature08530
Matalliotakis, I. M., Cakmak, H., Fragouli, Y. G., Goumenou, A. G., Mahutte, N. G., Arici, A. (2008). Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch. Gynecol. Obstet. 277, 389–393. doi: 10.1007/s00404-007-0479-1
McGrath, I. M., Montgomery, G. W., Mortlock, S. (2023). Genomic characterisation of the overlap of endometriosis with 76 comorbidities identifies pleiotropic and causal mechanisms underlying disease risk. Hum. Genet. 142, 1345–1360. doi: 10.1007/s00439-023-02582-w
Mirzaei, R., Dehkhodaie, E., Bouzari, B., Rahimi, M., Gholestani, A., Hosseini-Fard, S. R., et al. (2022). Dual role of microbiota-derived short-chain fatty acids on host and pathogen. BioMed. Pharmacother. 145, 112352. doi: 10.1016/j.biopha.2021.112352
Mizuta, K., Matoba, A., Shibata, S., Masaki, E., Emala, C. W. (2019). Obesity-induced asthma: Role of free fatty acid receptors. Jpn Dent. Sci. Rev. 55, 103–107. doi: 10.1016/j.jdsr.2019.07.002
Morris, G., Berk, M., Carvalho, A., Caso, J. R., Sanz, Y., Walder, K., et al. (2017). The role of the microbial metabolites including tryptophan catabolites and short chain fatty acids in the pathophysiology of immune-inflammatory and neuroimmune disease. Mol. Neurobiol. 54, 4432–4451. doi: 10.1007/s12035-016-0004-2
Nakajima, A., Nakatani, A., Hasegawa, S., Irie, J., Ozawa, K., Tsujimoto, G., et al. (2017). The short chain fatty acid receptor GPR43 regulates inflammatory signals in adipose tissue M2-type macrophages. PloS One 12, e0179696. doi: 10.1371/journal.pone.0179696
Nastasi, C., Candela, M., Bonefeld, C. M., Geisler, C., Hansen, M., Krejsgaard, T., et al. (2015). The effect of short-chain fatty acids on human monocyte-derived dendritic cells. Sci. Rep. 5, 16148. doi: 10.1038/srep16148
Ni, Z., Ding, J., Zhao, Q., Cheng, W., Yu, J., Zhou, L., et al. (2021). Alpha-linolenic acid regulates the gut microbiota and the inflammatory environment in a mouse model of endometriosis. Am. J. Reprod. Immunol. 86, e13471. doi: 10.1111/aji.13471
Ni, Z., Sun, S., Bi, Y., Ding, J., Cheng, W., Yu, J., et al. (2020). Correlation of fecal metabolomics and gut microbiota in mice with endometriosis. Am. J. Reprod. Immunol. 84, e13307. doi: 10.1111/aji.13307
Nigam, M., Panwar, A. S., Singh, R. K. (2022). Orchestrating the fecal microbiota transplantation: Current technological advancements and potential biomedical application. Front. Med. Technol. 4. doi: 10.3389/fmedt.2022.961569
Nnoaham, K. E., Webster, P., Kumbang, J., Kennedy, S. H., Zondervan, K. T. (2012). Is early age at menarche a risk factor for endometriosis? A systematic review and meta-analysis of case-control studies. Fertil. Steril. 98, 702–712.e6. doi: 10.1016/j.fertnstert.2012.05.035
Novella-Maestre, E., Herraiz, S., Vila-Vives, J. M., Carda, C., Ruiz-Sauri, A., Pellicer, A. (2012). Effect of antiangiogenic treatment on peritoneal endometriosis-associated nerve fibers. Fertil. Steril. 98, 1209–1217. doi: 10.1016/j.fertnstert.2012.07.1103
Offermanns, S. (2017). Hydroxy-carboxylic acid receptor actions in metabolism. Trends Endocrinol. Metab. 28, 227–236. doi: 10.1016/j.tem.2016.11.007
Parada Venegas, D., de la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10. doi: 10.3389/fimmu.2019.00277
Park, T., Im, J., Kim, A. R., Lee, D., Jeong, S., Yun, C. H., et al. (2021). Short-chain fatty acids inhibit the biofilm formation of Streptococcus gordonii through negative regulation of competence-stimulating peptide signaling pathway. J. Microbiol. 59, 1142–1149. doi: 10.1007/s12275-021-1576-8
Park, T., Im, J., Kim, A. R., Lee, D., Jeong, S., Yun, C. H., et al. (2022). Propionate attenuates growth of oral streptococci through enhancing methionine biosynthesis. J. Microbiol. Biotechnol. 32, 1234–1244. doi: 10.4014/jmb.2205.05037
Park, J., Kim, M., Kang, S. G., Jannasch, A. H., Cooper, B., Patterson, J., et al. (2015). Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 8, 80–93. doi: 10.1038/mi.2014.44
Park, B. S., Lee, J. O. (2013). Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 45, e66. doi: 10.1038/emm.2013.97
Pauls, S. D., Rodway, L. A., Winter, T., Taylor, C. G., Zahradka, P., Aukema, H. M. (2018). Anti-inflammatory effects of α-linolenic acid in M1-like macrophages are associated with enhanced production of oxylipins from α-linolenic and linoleic acid. J. Nutr. Biochem. 57, 121–129. doi: 10.1016/j.jnutbio.2018.03.020
Pereira, F., Medeiros, F. D. C., Rocha, H. A. L., Silva, K. S. D. (2019). Effects of omega-6/3 and omega-9/6 nutraceuticals on pain and fertility in peritoneal endometriosis in rats. Acta Cir Bras. 34, e201900405. doi: 10.1590/s0102-865020190040000005
Pirozzi, C., Francisco, V., Guida, F. D., Gómez, R., Lago, F., Pino, J., et al. (2018). Butyrate modulates inflammation in chondrocytes via GPR43 receptor. Cell Physiol. Biochem. 51, 228–243. doi: 10.1159/000495203
Pomare, E. W., Branch, W. J., Cummings, J. H. (1985). Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J. Clin. Invest. 75, 1448–1454. doi: 10.1172/JCI111847
Posses Bridi, G. D., de Oliveira, M. R., Carvalho, C. R. R., do Nascimento, E. C. T., Baldi, B. G. (2023). Thoracic endometriosis presenting as diffuse cystic lung disease: a rare case report. Pulmonology 30, 195–197. doi: 10.1016/j.pulmoe.2023.04.005
Priyadarshini, M., Kotlo, K. U., Dudeja, P. K., Layden, B. T. (2018). Role of short chain fatty acid receptors in intestinal physiology and pathophysiology. Compr. Physiol. 8, 1091–1115. doi: 10.1002/cphy.c170050
Qi, X., Yun, C., Pang, Y., Qiao, J. (2021). The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut Microbes 13, 1–21. doi: 10.1080/19490976.2021.1894070
Qin, R., Tian, G., Liu, J., Cao, L. (2022). The gut microbiota and endometriosis: From pathogenesis to diagnosis and treatment. Front. Cell Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.1069557
Quaranta, G., Sanguinetti, M., Masucci, L. (2019). Fecal microbiota transplantation: A potential tool for treatment of human female reproductive tract diseases. Front. Immunol. 10. doi: 10.3389/fimmu.2019.02653
Raetz, C. R., Whitfield, C. (2002). Lipopolysaccharide endotoxins. Annu. Rev. Biochem. 71, 635–700. doi: 10.1146/annurev.biochem.71.110601.135414
Ragsdale, S. W., Pierce, E. (2008). Acetogenesis and the Wood-Ljungdahl pathway of CO(2) fixation. Biochim. Biophys. Acta 1784, 1873–1898. doi: 10.1016/j.bbapap.2008.08.012
Ramírez-Pavez, T. N., Martínez-Esparza, M., Ruiz-Alcaraz, A. J., Marín-Sánchez, P., MaChado-Linde, F., García-Peñarrubia, P. (2021). The role of peritoneal macrophages in endometriosis. Int. J. Mol. Sci. 22, 10792. doi: 10.3390/ijms221910792
Raqib, R., Sarker, P., Bergman, P., Ara, G., Lindh, M., Sack, D. A., et al. (2006). Improved outcome in shigellosis associated with butyrate induction of an endogenous peptide antibiotic. Proc. Natl. Acad. Sci. U.S.A. 103, 9178–9183. doi: 10.1073/pnas.0602888103
Reis, F. M., Petraglia, F., Taylor, R. N. (2013). Endometriosis: hormone regulation and clinical consequences of chemotaxis and apoptosis. Hum. Reprod. Update 19, 406–418. doi: 10.1093/humupd/dmt010
Rekha, K., Venkidasamy, B., Samynathan, R., Nagella, P., Rebezov, M., Khayrullin, M., et al. (2022). Short-chain fatty acid: An updated review on signaling, metabolism, and therapeutic effects. Crit. Rev. Food Sci. Nutr. 64, 2461–2489. doi: 10.1080/10408398.2022.2124231
Rew, L., Harris, M. D., Goldie, J. (2022). The ketogenic diet: its impact on human gut microbiota and potential consequent health outcomes: a systematic literature review. Gastroenterol. Hepatol. Bed Bench 15, 326–342. doi: 10.22037/ghfbb.v15i4.2600
Riccio, L., Santulli, P., Marcellin, L., Abrão, M. S., Batteux, F., Chapron, C. (2018). Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 50, 39–49. doi: 10.1016/j.bpobgyn.2018.01.010
Rier, S. E., Martin, D. C., Bowman, R. E., Dmowski, W. P., Becker, J. L. (1993). Endometriosis in rhesus monkeys (Macaca mulatta) following chronic exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fundam Appl. Toxicol. 21, 433–441. doi: 10.1006/faat.1993.1119
Rinninella, E., Tohumcu, E., Raoul, P., Fiorani, M., Cintoni, M., Mele, M. C., et al. (2023). The role of diet in shaping human gut microbiota. Best Pract. Res. Clin. Gastroenterol. 62–63, 101828. doi: 10.1016/j.bpg.2023.101828
Salliss, M. E., Farland, L. V., Mahnert, N. D., Herbst-Kralovetz, M. M. (2021). The role of gut and genital microbiota and the estrobolome in endometriosis, infertility and chronic pelvic pain. Hum. Reprod. Update 28, 92–131. doi: 10.1093/humupd/dmab035
Samaneh, Y., ShahidehJahanian, S., Azadeh, M., Anoshirvan, K. (2019). The association of food consumption and nutrient intake with endometriosis risk in Iranian women: A case-control study. Int. J. Reprod. BioMed. 17, 661–670. doi: 10.18502/ijrm.v17i9.5102
Scott, N. A., Andrusaite, A., Andersen, P., Lawson, M., Alcon-Giner, C., Leclaire, C., et al. (2018). Antibiotics induce sustained dysregulation of intestinal T cell immunity by perturbing macrophage homeostasis. Sci. Transl. Med. 10, eaao4755. doi: 10.1126/scitranslmed.aao4755
Semaan, J., El-Hakim, S., Ibrahim, J. N., Safi, R., Elnar, A. A., El Boustany, C. (2020). Comparative effect of sodium butyrate and sodium propionate on proliferation, cell cycle and apoptosis in human breast cancer cells MCF-7. Breast Cancer 27, 696–705. doi: 10.1007/s12282-020-01063-6
Senchukova, M. A. (2023). Microbiota of the gastrointestinal tract: Friend or foe? World J. Gastroenterol. 29, 19–42. doi: 10.3748/wjg.v29.i1.19
Shafrir, A. L., Farland, L. V., Shah, D. K., Harris, H. R., Kvaskoff, M., Zondervan, K., et al. (2018). Risk for and consequences of endometriosis: A critical epidemiologic review. Best Pract. Res. Clin. Obstet. Gynaecol. 51, 1–15. doi: 10.1016/j.bpobgyn.2018.06.001
Shan, J., Ni, Z., Cheng, W., Zhou, L., Zhai, D., Sun, S., et al. (2021). Gut microbiota imbalance and its correlations with hormone and inflammatory factors in patients with stage 3/4 endometriosis. Arch. Gynecol. Obstet. 304, 1363–1373. doi: 10.1007/s00404-021-06057-z
Shao, X., Sun, S., Zhou, Y., Wang, H., Yu, Y., Hu, T., et al. (2021). Bacteroides fragilis restricts colitis-associated cancer via negative regulation of the NLRP3 axis. Cancer Lett. 523, 170–181. doi: 10.1016/j.canlet.2021.10.002
Shi, N., Li, N., Duan, X., Niu, H. (2017). Interaction between the gut microbiome and mucosal immune system. Mil Med. Res. 4, 14. doi: 10.1186/s40779-017-0122-9
Simoens, S., Dunselman, G., Dirksen, C., Hummelshoj, L., Bokor, A., Brandes, I., et al. (2012). The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod. 27, 1292–1299. doi: 10.1093/humrep/des073
Smarr, M. M., Kannan, K., Buck Louis, G. M. (2016). Endocrine disrupting chemicals and endometriosis. Fertil. Steril. 106, 959–966. doi: 10.1016/j.fertnstert.2016.06.034
Smith, P. M., Howitt, M. R., Panikov, N., Michaud, M., Gallini, C. A., Bohlooly, Y. M., et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. doi: 10.1126/science.1241165
Smolarz, B., Szyłło, K., Romanowicz, H. (2021). Endometriosis: epidemiology, classification, pathogenesis, treatment and genetics (Review of literature). Int. J. Mol. Sci. 22, 10554. doi: 10.3390/ijms221910554
So, D., Whelan, K., Rossi, M., Morrison, M., Holtmann, G., Kelly, J. T., et al. (2018). Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 107, 965–983. doi: 10.1093/ajcn/nqy041
Sobstyl, A., Chałupnik, A., Mertowska, P., Grywalska, E. (2023). How do microorganisms influence the development of endometriosis? Participation of genital, intestinal and oral microbiota in metabolic regulation and immunopathogenesis of endometriosis. Int. J. Mol. Sci. 24, 10920. doi: 10.3390/ijms241310920
Somasundaram, A., Rothenberger, N. J., Stabile, L. P. (2020). The impact of estrogen in the tumor microenvironment. Adv. Exp. Med. Biol. 1277, 33–52. doi: 10.1007/978-3-030-50224-9_2
Sui, Y., Wu, J., Chen, J. (2021). The role of gut microbial β-glucuronidase in estrogen reactivation and breast cancer. Front. Cell Dev. Biol. 9. doi: 10.3389/fcell.2021.631552
Sun, M., Wu, W., Liu, Z., Cong, Y. (2017). Microbiota metabolite short chain fatty acids, GPCR, and inflammatory bowel diseases. J. Gastroenterol. 52, 1–8. doi: 10.1007/s00535-016-1242-9
Sun, J., Xu, J., Yang, B., Chen, K., Kong, Y., Fang, N., et al. (2020). Effect of Clostridium butyricum against Microglia-Mediated Neuroinflammation in Alzheimer's Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol. Nutr. Food Res. 64, e1900636. doi: 10.1002/mnfr.201900636
Sunkara, L. T., Achanta, M., Schreiber, N. B., Bommineni, Y. R., Dai, G., Jiang, W., et al. (2011). Butyrate enhances disease resistance of chickens by inducing antimicrobial host defense peptide gene expression. PloS One 6, e27225. doi: 10.1371/journal.pone.0027225
Svensson, A., Brunkwall, L., Roth, B., Orho-Melander, M., Ohlsson, B. (2021). Associations between endometriosis and gut microbiota. Reprod. Sci. 28, 2367–2377. doi: 10.1007/s43032-021-00506-5
Symons, L. K., Miller, J. E., Kay, V. R., Marks, R. M., Liblik, K., Koti, M., et al. (2018). The immunopathophysiology of endometriosis. Trends Mol. Med. 24, 748–762. doi: 10.1016/j.molmed.2018.07.004
Tai, F. W., Chang, C. Y., Chiang, J. H., Lin, W. C., Wan, L. (2018). Association of pelvic inflammatory disease with risk of endometriosis: A nationwide cohort study involving 141,460 individuals. J. Clin. Med. 7, 379. doi: 10.3390/jcm7110379
Tanaka, Y., Mori, T., Ito, F., Koshiba, A., Takaoka, O., Kataoka, H., et al. (2017). Exacerbation of endometriosis due to regulatory T-cell dysfunction. J. Clin. Endocrinol. Metab. 102, 3206–3217. doi: 10.1210/jc.2017-00052
Tarokh, M., Ghaffari Novin, M., Poordast, T., Tavana, Z., Nazarian, H., Norouzian, M., et al. (2019). Serum and peritoneal fluid cytokine profiles in infertile women with endometriosis. Iran J. Immunol. 16, 151–162. doi: 10.22034/iji.2019.80258
Tomova, A., Bukovsky, I., Rembert, E., Yonas, W., Alwarith, J., Barnard, N. D., et al. (2019). The effects of vegetarian and vegan diets on gut microbiota. Front. Nutr. 6. doi: 10.3389/fnut.2019.00047
Topbas Selcuki, N. F., Yilmaz, S., Kaya, C., Usta, T., Kale, A., Oral, E. (2022). Thoracic endometriosis: A review comparing 480 patients based on catamenial and noncatamenial symptoms. J. Minim Invasive Gynecol. 29, 41–55. doi: 10.1016/j.jmig.2021.08.005
Trompette, A., Gollwitzer, E. S., Yadava, K., Sichelstiel, A. K., Sprenger, N., Ngom-Bru, C., et al. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166. doi: 10.1038/nm.3444
Uchida, M., Kobayashi, O. (2013). Effects of Lactobacillus gasseri OLL2809 on the induced endometriosis in rats. Biosci. Biotechnol. Biochem. 77, 1879–1881. doi: 10.1271/bbb.130319
Vallabhapurapu, S., Karin, M. (2009). Regulation and function of NF-kappaB transcription factors in the immune system. Annu. Rev. Immunol. 27, 693–733. doi: 10.1146/annurev.immunol.021908.132641
van der Hee, B., Wells, J. M. (2021). Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 29, 700–712. doi: 10.1016/j.tim.2021.02.001
Vercellini, P., Somigliana, E., Viganò, P., Abbiati, A., Daguati, R., Crosignani, P. G. (2008). Endometriosis: current and future medical therapies. Best Pract. Res. Clin. Obstet. Gynaecol. 22, 275–306. doi: 10.1016/j.bpobgyn.2007.10.001
Viganò, P., Ottolina, J., Bartiromo, L., Bonavina, G., Schimberni, M., Villanacci, R., et al. (2020). Cellular components contributing to fibrosis in endometriosis: A literature review. J. Minim Invasive Gynecol. 27, 287–295. doi: 10.1016/j.jmig.2019.11.011
Vinolo, M. A., Hatanaka, E., Lambertucci, R. H., Newsholme, P., Curi, R. (2009). Effects of short chain fatty acids on effector mechanisms of neutrophils. Cell Biochem. Funct. 27, 48–55. doi: 10.1002/cbf.1533
Vinolo, M. A., Rodrigues, H. G., Hatanaka, E., Sato, F. T., Sampaio, S. C., Curi, R. (2011). Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 22, 849–855. doi: 10.1016/j.jnutbio.2010.07.009
Vital, M., Howe, A. C., Tiedje, J. M. (2014). Revealing the bacterial butyrate synthesis pathways by analyzing (meta) genomic data. mBio 5, e00889. doi: 10.1128/mBio.00889-14
Wagenaar, C. A., van de Put, M., Bisschops, M., Walrabenstein, W., de Jonge, C. S., Herrema, H., et al. (2021). The effect of dietary interventions on chronic inflammatory diseases in relation to the microbiome: A systematic review. Nutrients 13, 3208. doi: 10.3390/nu13093208
Wang, G., Yu, Y., Wang, Y. Z., Wang, J. J., Guan, R., Sun, Y., et al. (2019). Role of SCFAs in gut microbiome and glycolysis for colorectal cancer therapy. J. Cell Physiol. 234, 17023–17049. doi: 10.1002/jcp.28436
Watson, H., Mitra, S., Croden, F. C., Taylor, M., Wood, H. M., Perry, S. L., et al. (2018). A randomised trial of the effect of omega-3 polyunsaturated fatty acid supplements on the human intestinal microbiota. Gut 67, 1974–1983. doi: 10.1136/gutjnl-2017-314968
Wei, Y., Tan, H., Yang, R., Yang, F., Liu, D., Huang, B., et al. (2023). Gut dysbiosis-derived β-glucuronidase promotes the development of endometriosis. Fertil. Steril. 120, 682–694. doi: 10.1016/j.fertnstert.2023.03.032
Williams, L. M., Stoodley, I. L., Berthon, B. S., Wood, L. G. (2022). The effects of prebiotics, synbiotics, and short-chain fatty acids on respiratory tract infections and immune function: A systematic review and meta-analysis. Adv. Nutr. 13, 167–192. doi: 10.1093/advances/nmab114
Wu, H. J., Ivanov, I. I., Darce, J., Hattori, K., Shima, T., Umesaki, Y., et al. (2010). Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity 32, 815–827. doi: 10.1016/j.immuni.2010.06.001
Wu, J., Zhou, Z., Hu, Y., Dong, S. (2012). Butyrate-induced GPR41 activation inhibits histone acetylation and cell growth. J. Genet. Genomics 39, 375–384. doi: 10.1016/j.jgg.2012.05.008
Xu, Z., Mak, J. W. Y., Lin, Y., Yang, K., Liu, Q., Zhang, F., et al. (2023). Mixed-donor faecal microbiota transplantation was associated with increased butyrate-producing bacteria for obesity. Gut 73, 875–878. doi: 10.1136/gutjnl-2022-328993
Xu, Z., Zhang, L., Yu, Q., Zhang, Y., Yan, L., Chen, Z. J. (2019). The estrogen-regulated lncRNA H19/miR-216a-5p axis alters stromal cell invasion and migration via ACTA2 in endometriosis. Mol. Hum. Reprod. 25, 550–561. doi: 10.1093/molehr/gaz040
Yalçın Bahat, P., Ayhan, I., Üreyen Özdemir, E., İnceboz, Ü., Oral, E. (2022). Dietary supplements for treatment of endometriosis: A review. Acta BioMed. 93, e2022159. doi: 10.23750/abm.v93i1.11237
Yonezawa, H., Osaki, T., Hanawa, T., Kurata, S., Zaman, C., Woo, T. D. H., et al. (2012). Destructive effects of butyrate on the cell envelope of Helicobacter pylori. J. Med. Microbiol. 61, 582–589. doi: 10.1099/jmm.0.039040-0
Yotova, I. Y., Quan, P., Leditznig, N., Beer, U., Wenzl, R., Tschugguel, W. (2011). Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Hum. Reprod. 26, 885–897. doi: 10.1093/humrep/der010
You, S., Ma, Y., Yan, B., Pei, W., Wu, Q., Ding, C., et al. (2022). The promotion mechanism of prebiotics for probiotics: A review. Front. Nutr. 9. doi: 10.3389/fnut.2022.1000517
Young, V. J., Ahmad, S. F., Brown, J. K., Duncan, W. C., Horne, A. W. (2015). Peritoneal VEGF-A expression is regulated by TGF-β1 through an ID1 pathway in women with endometriosis. Sci. Rep. 5, 16859. doi: 10.1038/srep16859
Yovich, J. L., Rowlands, P. K., Lingham, S., Sillender, M., Srinivasan, S. (2020). Pathogenesis of endometriosis: Look no further than John Sampson. Reprod. BioMed. Online 40, 7–11. doi: 10.1016/j.rbmo.2019.10.007
Yuan, M., Li, D., Zhang, Z., Sun, H., An, M., Wang, G. (2018). Endometriosis induces gut microbiota alterations in mice. Hum. Reprod. 33, 607–616. doi: 10.1093/humrep/dex372
Zhang, T., De Carolis, C., Man, G. C. W., Wang, C. C. (2018). The link between immunity, autoimmunity and endometriosis: a literature update. Autoimmun Rev. 17, 945–955. doi: 10.1016/j.autrev.2018.03.017
Zhang, S., Dogan, B., Guo, C., Herlekar, D., Stewart, K., Scherl, E. J., et al. (2020). Short chain fatty acids modulate the growth and virulence of pathosymbiont Escherichia coli and host response. Antibiotics (Basel) 9, 462. doi: 10.3390/antibiotics9080462
Zhou, W. J., Yang, H. L., Shao, J., Mei, J., Chang, K. K., Zhu, R., et al. (2019). Anti-inflammatory cytokines in endometriosis. Cell Mol. Life Sci. 76, 2111–2132. doi: 10.1007/s00018-019-03056-x
Zhou, W. D., Yang, H. M., Wang, Q., Su, D. Y., Liu, F. A., Zhao, M., et al. (2010). SB203580, a p38 mitogen-activated protein kinase inhibitor, suppresses the development of endometriosis by down-regulating proinflammatory cytokines and proteolytic factors in a mouse model. Hum. Reprod. 25, 3110–3116. doi: 10.1093/humrep/deq287
Zizolfi, B., Foreste, V., Gallo, A., Martone, S., Giampaolino, P., Di Spiezio Sardo, A. (2023). Endometriosis and dysbiosis: State of art. Front. Endocrinol. (Lausanne) 14. doi: 10.3389/fendo.2023.1140774
Zondervan, K. T., Becker, C. M., Koga, K., Missmer, S. A., Taylor, R. N., Viganò, P. (2018). Endometriosis. Nat. Rev. Dis. Primers 4, 9. doi: 10.1038/s41572-018-0008-5
Keywords: gut microbiota, short-chain fatty acids, endometriosis, mechanism of action, treatment strategy
Citation: Liu M, Peng R, Tian C, Shi J, Ma J, Shi R, Qi X, Zhao R and Guan H (2024) Effects of the gut microbiota and its metabolite short-chain fatty acids on endometriosis. Front. Cell. Infect. Microbiol. 14:1373004. doi: 10.3389/fcimb.2024.1373004
Received: 19 January 2024; Accepted: 31 May 2024;
Published: 13 June 2024.
Edited by:
Sangappa B. Chadchan, Baylor College of Medicine, United StatesReviewed by:
Praveen Kumar, Banaras Hindu University, IndiaMartha Legorreta-Herrera, National Autonomous University of Mexico, Mexico
Copyright © 2024 Liu, Peng, Tian, Shi, Ma, Shi, Qi, Zhao and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haibin Guan, MjAwNzAwOTVAaW1tdS5lZHUuY24=; Rongwei Zhao, enJ3YXp5ZkBzaW5hLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Menghe Liu1†
Menghe Liu1† Haibin Guan
Haibin Guan