- 1Department of Endocrinology, the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University, Huai’an, Jiangsu, China
- 2Department of Gastroenterology, the Affiliated Huai’an No.1 People’s Hospital of Nanjing Medical University, Huai’an, Jiangsu, China
Diabetes mellitus (DM) refers to a group of chronic diseases with global prevalence, characterized by persistent hyperglycemia resulting from various etiologies. DM can harm various organ systems and lead to acute or chronic complications, which severely endanger human well-being. Traditional treatment mainly involves controlling blood sugar levels through replacement therapy with drugs and insulin; however, some patients still find a satisfactory curative effect difficult to achieve. Extensive research has demonstrated a close correlation between enteric dysbacteriosis and the pathogenesis of various types of DM, paving the way for novel therapeutic approaches targeting the gut microbiota to manage DM. Fecal microbiota transplantation (FMT), a method for re-establishing the intestinal microbiome balance, offers new possibilities for treating diabetes. This article provides a comprehensive review of the correlation between DM and the gut microbiota, as well as the current advancements in FMT treatment for DM, using FMT as an illustrative example. This study aims to offer novel perspectives and establish a theoretical foundation for the clinical diagnosis and management of DM.
1 Introduction
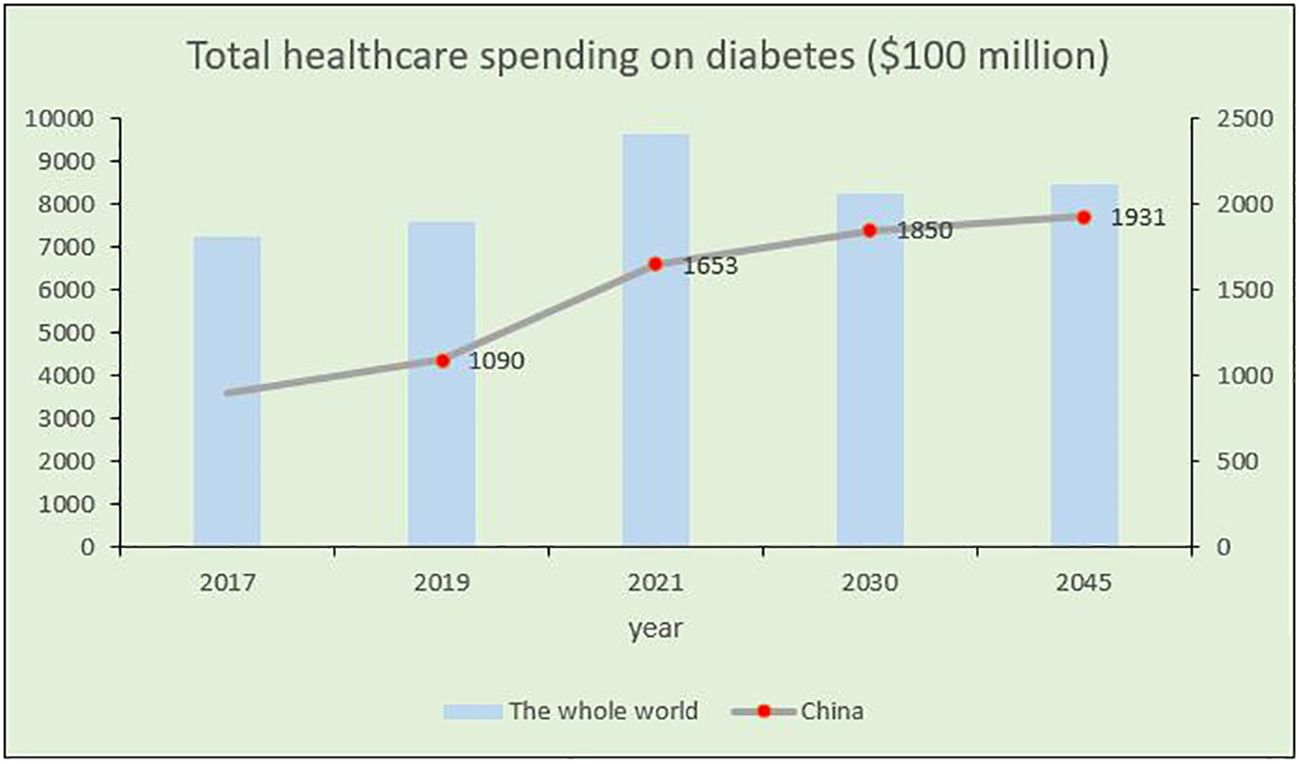
DM is a metabolic disorder characterized by persistent high blood glucose levels resulting from the combined influence of genetic and environmental factors (Sapra and Bhandari, 2023). Prolonged hyperglycemia leads to chronic progressive lesions, functional decline, and failure in various organs and tissues, causing irreversible damage to multiple systems in the human body and threatening human health (Kolarić et al., 2022). Despite extensive research, DM, a typical chronic progressive disease, has no definitive cure. Current large public health and medical investments and a series of new diabetes drugs have not significantly improved DM control. According to the latest International Diabetes Federation (IDF) Diabetes Atlas, approximately 537 million adults (aged 20–79 years) worldwide are expected to develop DM by 2021. This number is expected to increase to 643 million by 2030 and 783 million by 2045 (Bommer et al., 2018; GBD 2021 Diabetes Collaborators, 2023; Zhong et al., 2023) (Figure 1A). The number of patients with diabetes in China will account for a quarter of the world’s diabetic population and may increase at a rate of 3%–5% every year (Figure 1B), thus imposing a serious social and economic burden (Bommer et al., 2018). Global diabetes-related healthcare spending is anticipated to reach $1.054 trillion by 2045, with complications and mortality accounting for approximately 5%–10% of the global economic burden of DM (Figure 2). Currently, the conventional treatment for DM involves controlling blood glucose levels with pharmacological and insulin replacement therapies (Ceriello et al., 2022; Fralick et al., 2022). However, these are only palliative and can result in adverse reactions and limitations. Furthermore, patient compliance, supply and quality of drugs, and time of diagnosis crucially impact the treatment of diabetes. Therefore, development of new and effective therapies to supplement existing clinical methods is essential.
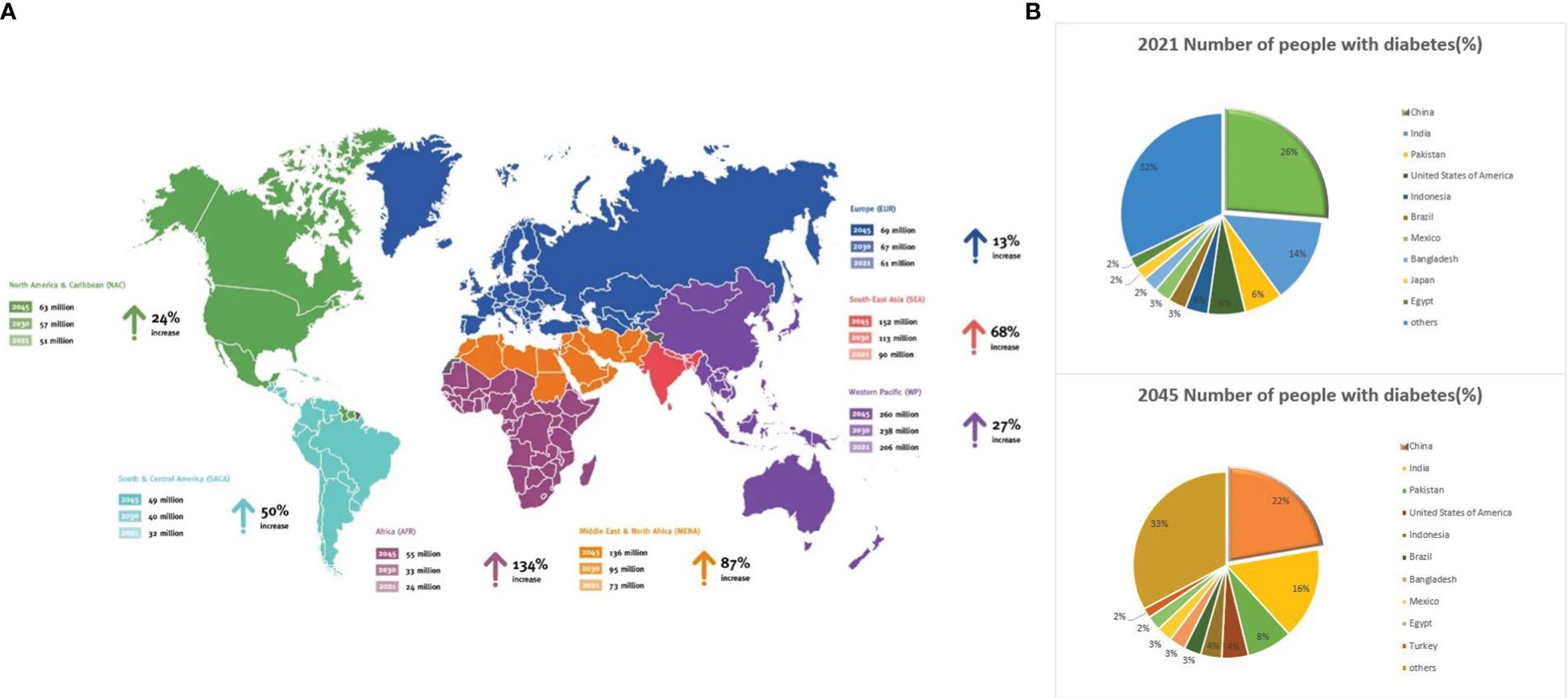
Figure 1 Global diabetes data. (A) Map 1: Number of people aged 20–79 years with diabetes worldwide and per IDF Region from 2021–2045. (B) Share of the global population with diabetes in 2021 and 2024. Source: https://www.diabetesatlas.org.
The human microbiome comprises fungi, yeast, bacteria, archaea, and viruses. The intestine, which is one of the biggest organs in contact with the external environment after the skin, is home to a host of microorganisms, earning it the name of the “second human genome” (Gilbert et al., 2018; Aggarwal et al., 2023). However, evidence of symbiotic interactions between gut microbes and their hosts remains inconclusive. Homeostasis of the gut microbiota can benefit the host through immunomodulation, nutrient exchange, and metabolism of pathogenic microbes (Adak and Khan, 2019; Wang et al., 2023). Conversely, dysregulation of the gut microbiota can lead to various diseases. Numerous studies have indicated that the gut microbiota plays a crucial role in the development of various diseases through its metabolites and byproducts (Zhou et al., 2020; Fu et al., 2023b; Wang et al., 2023). The gut microbiota is the most abundant group of antigen-presenting cells (APCs) (Adak and Khan, 2019), and their imbalance disrupts immunity and homeostasis. By introducing environmental antigens into the gastrointestinal tract, the gut microbiota interferes with the reaction between antigens and the host immune system, resulting in increased intestinal permeability and the development of diabetes (Del Chierico et al., 2022; Guo et al., 2022; Zhou et al., 2022; Crudele et al., 2023; Wu et al., 2023).
The prevailing forms of diabetes include type 1 DM (T1DM), type 2 DM (T2DM), specific variations of diabetes, and gestational DM (GDM) (Sapra and Bhandari, 2023), which are categorized as chronic inflammatory diseases. Recent research has provided substantial evidence suggesting that the development of diabetes is influenced by environmental and genetic factors, as well as the gut microbiota. Microbiota crucially participate in mediating oxidative stress, insulin resistance, and chronic inflammation, thereby facilitating the progression of diabetes and its associated complications. Moreover, the gut microbiota of patients with diabetes show distinct degrees of disorder, and the abundance of Firmicutes and Bacteroidetes is particularly significant. Disorders in the gut microbiota can reduce insulin sensitivity and energy metabolism by altering the host intestinal mucosal barrier, thus affecting short-chain fatty acid (SCFA) synthesis, bile acid metabolism, and other pathways, ultimately leading to diabetes.
Therefore, re-establishing immune homeostasis by altering the form and number of the gut microbiota may be a potential intervention for diabetes. Fecal microbiota transplantation (FMT) is an intervention method that involves the transfer of the whole gut microbiota of a healthy donor into the intestine of a patient and has shown great clinical application owing to its safety, stability, convenience, and low incidence of side effects (Wang et al., 2022b; Porcari et al., 2023; Yang et al., 2023). Patients treated with FMT typically show reduced microbial diversity, abundance, and richness, compared with patients with normal gut microbiomes. In contrast, FMT can lead to the continued colonization of the intestine, thereby establishing a new microbiome. FMT has been extensively used in DM and its complications and is considered a promising treatment for DM when conventional hypoglycemic regimens remain ineffective. Consequently, a systematic review is needed to explore the mechanism of FMT in the treatment of diabetes and to improve its therapeutic effect.
2 Gut microbiota and DM
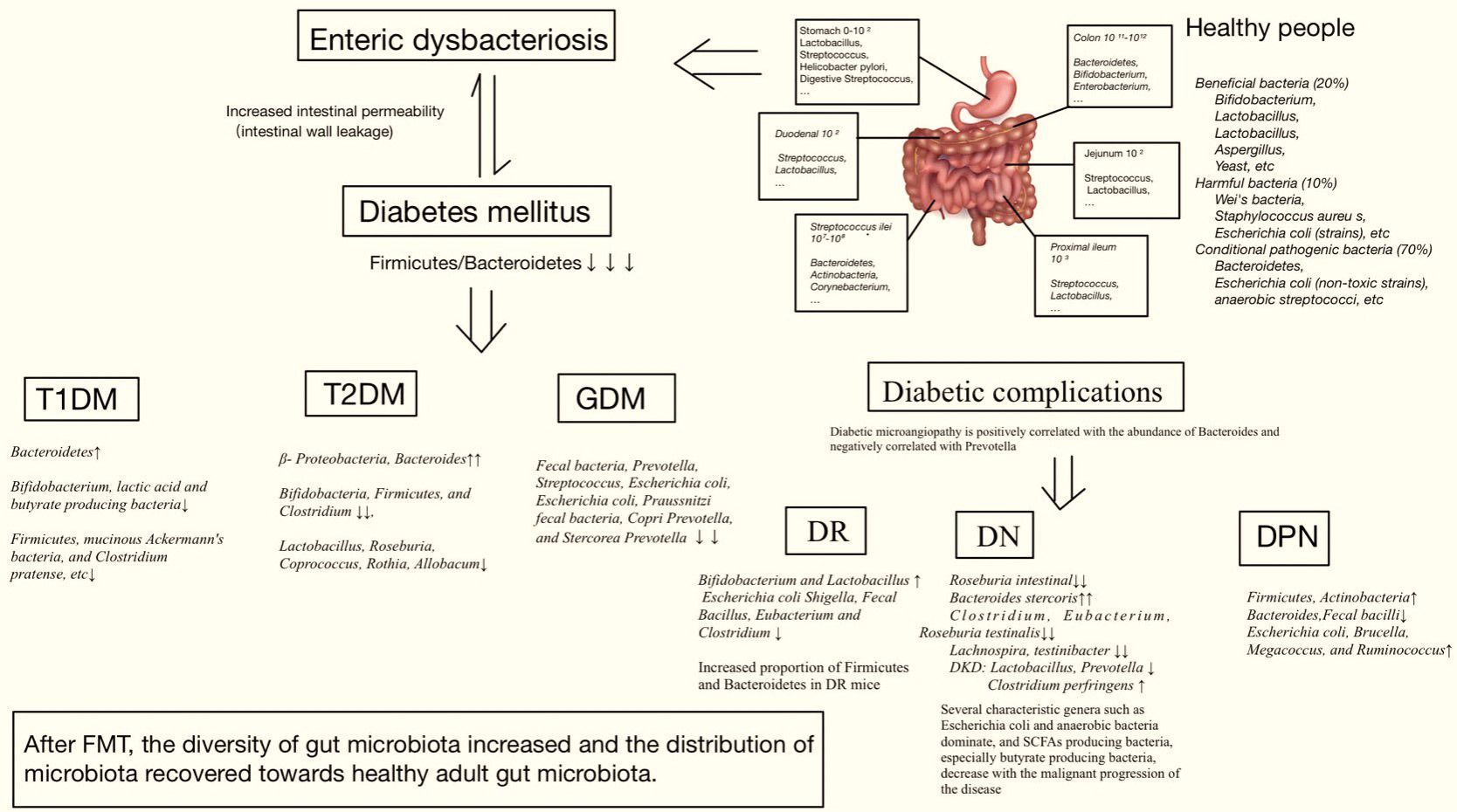
Through the analysis of human fecal samples collected using metagenomic sequencing (Schloissnig et al., 2013), dozens of phyla have been identified, including Firmicutes, Bacteroidetes, Actinomyces, Proteobacteria, Fusobacteria, Micrococcus verrucosa, Cyanobacteria, and Spirochaeta (de Vos et al., 2022). Actinobacteria, Firmicutes, Proteobacteria, and Bacteroidetes comprise 99% of the human bowel bacteria. The gut microbiota maintains host health and has been shown to be altered in various types of DM and its complications. Therefore, DM is associated with enteric dysbacteriosis, which damages the intestinal mucosal barrier and increases intestinal permeability. When the metabolite lipopolysaccharide (LPS) is translocated and released into the blood through a leaky intestinal mucosal barrier, a large amount of endotoxin is produced, which damages the function of islet β-cells, produces immune inflammation, activates macrophages, leads to vascular inflammation, and participates in the occurrence of DM (Zhang et al., 2021; Del Chierico et al., 2022; Guo et al., 2022; Lin et al., 2022; Zhou et al., 2022; Crudele et al., 2023; Wang et al., 2023; Wu et al., 2023) (Figure 3).
2.1 Gut microbiota and T1DM
T1DM is an autoimmune disease commonly observed in children and adolescents. It is mediated by T cells and characterized by the destruction of islet β-cells, absolute insulin deficiency, and hyperglycemia (Syed, 2022). Multiple studies have shown that, in contrast to the gut microbiota of healthy individuals, that of patients with T1DM is disordered in structure with decreased diversity (Diviccaro et al., 2023; Moreira et al., 2023), ultimately manifesting as an increase in the quantity of Bacteroides and a reduction in the quantity of Bifidobacterium, lactic acid, and butyrate-producing bacteria (Ma et al., 2020; Diviccaro et al., 2023; Moreira et al., 2023). Other studies (Demirci et al., 2020; Yuan et al., 2022) have found that, in contrast to those of non-diabetic groups, the proportions of Ackermannia mucosus, Firmicutes, and Clostridium praxobacter, as well as the proportions of Firmicutes and Bacteroides (F/B), were decreased in the T1DM group. After FMT, the numbers of Bifidobacteria and Escherichia coli increased, and with an increase in Firmicutes and a decrease in Bacteroides increasing the F/B ratio along with an increase in floral diversity, the distribution of gut microbiota approached normal (de Groot et al., 2021; He et al., 2022; Xie et al., 2022). Although T1DM is an autoimmune disease, patients may exhibit abnormal plasma metabolism (Zheng et al., 2022). In a longitudinal study, the early development of T1DM in infants was associated with reduced levels of sugar derivatives, amino acids, and fatty acids (including SCFAs), compared with controls (Huang et al., 2020). In addition, endogenous metabolites, including pyruvate, indoleacetic acid, and phenylacetylglycine, have been identified as potential biomarkers of T1DM (Wang et al., 2022).
2.2 Gut microbiota and T2DM
T2DM is an intricate polygenic disease with core pathological mechanisms involving islet dysfunction and insulin resistance (Mojsak et al., 2021; Sapra and Bhandari, 2023). Larsen et al. (2010) discovered that T2DM is associated with changes in the gut microbiota. Among them, β-Proteobacteria increased significantly, whereas Bifidobacteria, Firmicutes, and Clostridium decreased significantly, and the scale of Bacteroides and Firmicutes, as well as the proportion of Prevobacteria and Clostridium globosum, were positively correlated with blood glucose concentration. Existing research has indicated that Bifidobacteria, Bacteroides, Fecalobacteria, Akkermansia muciniphila, and Rosanobacteria are negatively correlated, whereas Ruminococcus, Clostridium, and Cyanobacteria are positively correlated with T2DM (Liu et al., 2022; Zhou et al., 2022; Liu et al., 2024; Pan et al., 2024). Studies suggest significant dissimilarities in the constitution and proportion of gut microbiota between patients with T2DM and those with normal glucose tolerance; overall, a reduced number of Firmicutes in participants with T2DM was observed (Fassatoui et al., 2019). In a controlled trial (Su et al., 2022), with the exacerbation of symptoms, the expression of Bacteroides in the gut microbiota of T2DM mice was high, whereas the expressions of Lactobacillus, Roseburia, Coprococcus, Rothia, and Allobacum decreased. In contrast, FMT-treated rats demonstrated a remarkable increase in this ratio. In addition, intestinal flora metabolites, such as bile acids, SCFAs, trimethylamine, tryptophan, and indole, to a certain extent, affected the reduced insulin sensitivity associated with T2DM dysfunction and regulated metabolism and immune homeostasis (Liu et al., 2022). Song et al. explored the relationship between the intestinal flora and differential metabolites in T2DM mice by identifying changes in urine metabolites (Song et al., 2023). Furthermore, 31 different metabolites, including phosphatidylcholine, that are identified by urine metabolomics may be closely related to the glycerophospholipid and arachidonic acid pathways. The results showed that the species diversity and abundance of intestinal flora in T2DM mice decreased, the levels of beneficial bacteria such as lactobacillus significantly decreased, and the levels of harmful bacteria such as Helicobacter pylori significantly increased. Common diabetes biomarkers, such as intravenous plasma glucose and hemoglobin A1c, are subject to individual limitations; therefore, identifying new specific metabolites can serve as important predictors of T2DM development. For example, serum indole propionic acid is negatively correlated with serum highly sensitive C-reactive protein levels, whereas indole propionic acid levels are lower in patients with impaired glucose tolerance, which is directly related to insulin secretion (Slouha et al., 2023). Therefore, in T2DM cynomolgus monkeys, four common serum lipids, phosphatidylcholine (18:0_22:4), lysophosphatidylcholine(14:0), phosphatidylethanolamine (PE) (16:1_18:2), and PE (18:0_22:4), were downregulated and identified as potential biomarkers, indicating that the glycerophospholipid pathway is related to its pathogenesis (Tian et al., 2024).
2.3 Gut microbiota and GDM
GDM refers to the progression of impaired glucose tolerance and diabetes during pregnancy (Kautzky-Willer et al., 2023). Previous studies (Koren et al., 2012; Sun et al., 2023) found that the abundances of Proteobacteria, Actinobacteria, and Roseburia in the intestines of healthy pregnant women changed throughout pregnancy. Throughout the gestational cycle, Tyzzerella 4 and Eisenbergiella were enriched in the guts of patients with GDM during the first trimester. Eisenbergiella and Taiseria showed a positive correlation with fasting blood glucose levels (Ma et al., 2020). During the third trimester in patients with GDM, high abundances of Collinsella, Actinomycetes, and Rothia were identified, and alterations in the gut microbiota persisted, even 8 months after childbirth (Crusell et al., 2018). Compared with the offspring of healthy pregnant women, patients with GDM had a lower alpha diversity of gut microbiota. The abundances of Firmicutes, Bacteroidetes, Prevotella, and Lactobacillus were also low (Su et al., 2018). Through comprehensive metagenomic and metabolomic analyses of a group of women with GDM and pregnant women with normal glucose tolerance, changes in the gut microbiome associated with both GDM and changes in circulating metabolites were identified (Ye et al., 2023). Compared with the control group, patients with GDM had significantly lower levels of SCFA-producing genera, including Faecalis, Prevotella, and Streptococcus, as well as faecalis, Eubacterium Schlierii, prausnitzi, Prevotella copri, and Prevotella stercorea. Additionally, 2-hydroxybutyric acid and L-alpha-aminobutyric acid were significantly increased, but methionine sulfoxide, allantoin, dopamine, and dopaminergic synapses were significantly decreased. These results suggest that insufficient dopamine circulation, unbalanced SCFA production, and excessive metabolic inflammation are important pathways for the gut microbiota to drive GDM development.
2.4 Gut microbiota and diabetic microvascular complications
Long-term elevated blood sugar can cause vascular disease, which can endanger the tissues and organs such as the eyes, heart, kidneys, brain, and nerves. According to World Health Organization statistics, up to 100 types of diabetic microvascular complications have occurred. Once diabetic complications occur, reverse treatment becomes difficult. The prevalent clinical complications of diabetic microvascular diseases include diabetic retinopathy (DR), diabetic peripheral vascular disease, diabetic nephropathy (DN), and diabetic neuropathy (Li et al., 2023; Sapra and Bhandari, 2023; Jia et al., 2024; Kanbour et al., 2024). Diabetic microangiopathy negatively correlates with Prevotella abundance and positively correlates with Bacteroides abundance, with changes in floral abundance, diversity, and proportion. The abundances of Lactobacillus and Prevotella decreased in patients with diabetic kidney disease, whereas the abundance of Fusobacterium prevotella increased. The F/B ratio was increased in DR mice. Metabolites such as LPS and SCFA affect neuronal homeostasis and participate in diabetic peripheral neuropathy (DPN). In addition, dysregulation of the metabolic pathways of gut-related products, such as tryptophan, vitamin B6, and purines, may be involved in the mechanism of diabetic complications associated with glucose homeostasis. In addition to sharing the metabolic pathways of DM, researchers have found that diabetic complications are associated with specific metabolite levels (Ma et al., 2024). Therefore, the microbiota influences variations in intestinal microecology in DM microangiopathy and is involved in the progression and prognosis of disease (Hong et al., 2022).
2.4.1 Diabetic retinopathy
Diabetic retinopathy (DR) is a major cause of visual impairment and blindness in patients with advanced T2DM (Shukla and Tripathy, 2023; Gupta and Thool, 2024). It is a microvascular complication of DM associated with oxidative stress, activation of inflammatory response pathways, microvascular injury, and dysfunction. DR is also related to the abnormal regeneration of small blood vessels and changes in the structure and function of glial components (Madonna et al., 2017). The use of 16S rRNA gene sequencing to analyze the stool samples of patients with DM, patients with DR, and healthy controls (HC group) without retinopathy revealed differences in microbial structures and compositions among the three groups. Compared with that of the HC group, the diversity of α and β in the DR and DM groups was decreased, and the amounts of Lactobacillus and Bifidobacterium were increased. Notably, Pasteurelliaceae was reduced in the DR group, whereas it was increased in the DM group, suggesting that Pasteurelliaceae bacteria can be used as a microbial feature to distinguish between DR and DM (Huang et al., 2021; Hasani et al., 2024).
2.4.2 Diabetic nephropathy
Diabetic nephropathy (DN) is a serious complication of diabetes and an important cause of death in patients (Varghese and Jialal, 2023; Das et al., 2024). Its clinical manifestations include proteinuria, hypertension, and edema. Studies have found that gut microbiota disorders are predisposing factors for DN. Patients with DN are characterized by an overgrowth of intestinal bacteria, accumulation of toxic compounds connected with the flora, disruption of intestinal barrier function, and chronic inflammatory response (Chi et al., 2021). The stool analysis results of 16S rRNA gene sequencing in patients with DN and healthy adults indicated that the multiplicity of patients’ flora was decreased. Escherichia-Shigella and anaerobic bacteria were dominant, whereas SCFA-producing bacteria, especially butyrate-producing bacteria, decreased with the malignant progression of the disease (Du et al., 2021). Using the meta-genomic sequencing of stool samples from an HC group and patients with T2DM with or without DN, Zhang et al (Zhang et al., 2022). identified gut microbiota imbalances in the stool samples from patients with DN, with the abundance of Roseburia consequentially decreasing and that of Bacteroides stercoris significantly increasing. Although the gut microbiota in these groups did not show any obvious distinction, the relative abundance of potential probiotics was visibly reduced in patients with diabetes. Renal function tests in DN often reveal decreased urinary albumin levels and glomerular filtration rates. Li et al. (2020) compared the consistency of the gut microbiota in mice with different outcomes during DN induction and found that aerobic and anaerobic Bacillus may exacerbate renal function deterioration and that Blautia may have a protective effect against DN. Therefore, the gut microbiota contributes to the regulation of renal function in this model.
2.4.3 Diabetic peripheral neuropathy
Diabetic peripheral neuropathy (DPN) (Bodman and Varacallo, 2023; Zhu et al., 2024) is a type of diabetic neuropathy that mainly affects the distal lower extremities and is often characterized by sensory loss, numbness, pain, gait disturbances, and amputation. A study on the characteristics of the gut microbiota in DPN showed that the abundance of Actinomycetes and Firmicutes is increased in DPN, whereas that of Bacteroides is decreased. Specifically, the abundances of Bacteroides and Faecalis is decreased in DPN, whereas those of Escherichia-Shigella, Braxella, Macrococcus, and Ruminococcus are increased (Wang et al., 2020). The products of the gut microbiota are closely associated with nervous system homeostasis. Although relevant research is currently being conducted, the influence of FMT on DPN needs to be explored further.
2.5 Fecal microbiota transplantation
Chronic low-grade inflammation, abnormal SCFA levels, and impaired bile acid metabolism caused by enteric dysbacteriosis play important roles in the progression of metabolic diseases. Therefore, regulating and restoring homeostasis of the gut microbiota is a novel intestinal microecological therapy for treating diabetes (Okubo et al., 2018; Agus et al., 2021). While these methods include FMT (Zheng et al., 2022), prebiotics, probiotics, and antibiotics, FMT is the most direct and effective.
Approximately 3,000 years ago, India first used cow dung for the treatment of gastrointestinal diseases (Hoh and Dhanashree, 2017). In the Eastern Jin Dynasty (AD 317–420), Ge Hong recorded a therapeutic method similar to FMT, also known as “Yellow Long Tang,” for the treatment of food poisoning and diarrhea (Zheng et al., 2022). During World War II (Bárcena et al., 2019), German soldiers used camel feces to treat diarrhea. Currently, FMT is used to treat bacterial infections, and several clinical trials have validated FMT as a viable therapeutic option.
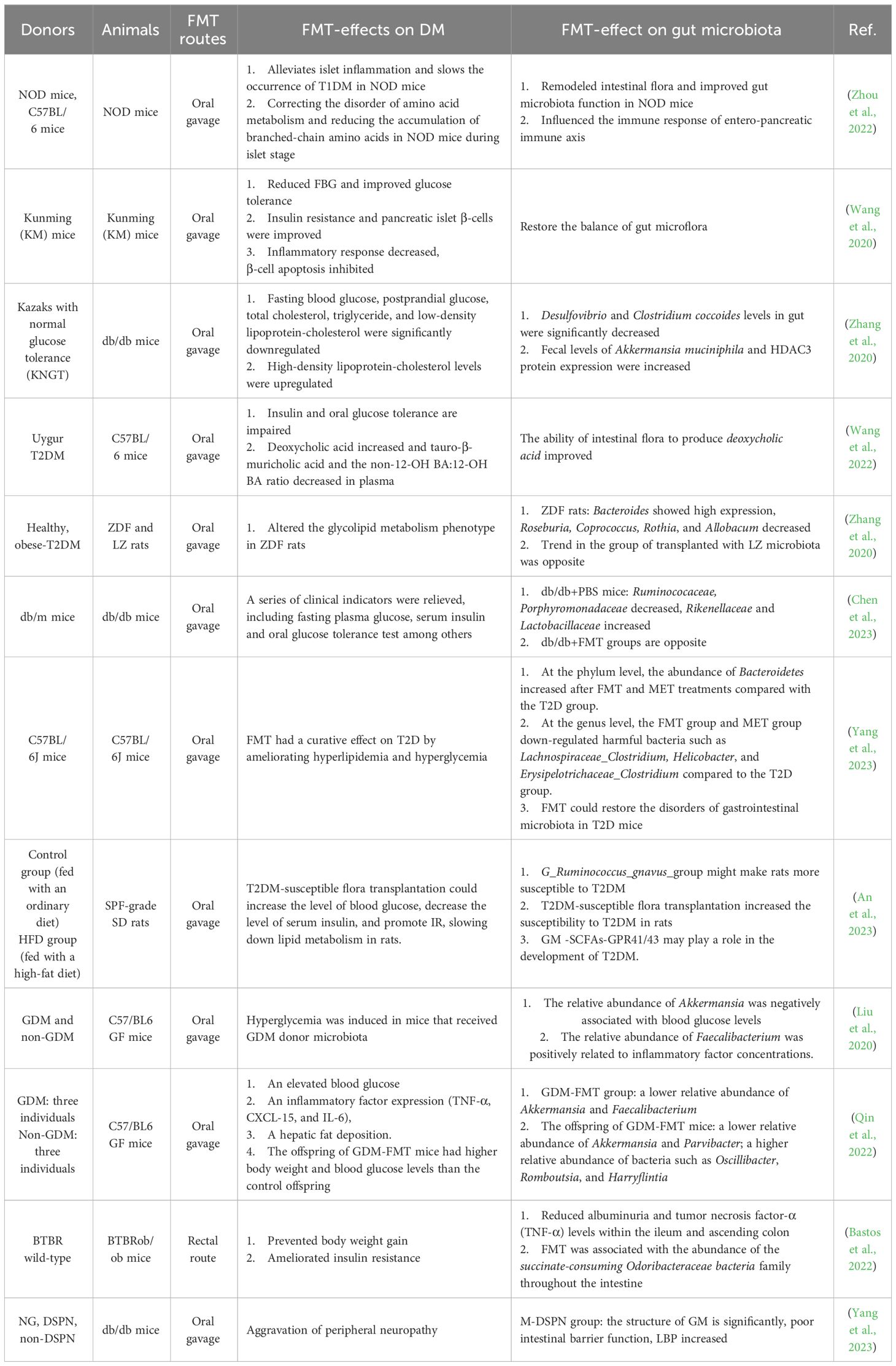
FMT generally delivers fecal microbiota from a thoroughly screened healthy donor into the small intestine via an oral capsule or duodenal tube, but it can also enter the large intestine via an enema or colonoscopy. Studies have compared the upper and lower digestive tract pathways of FMT and found no significant differences in cure rates between the two modes of administration (Youngster et al., 2014). However, the clinical resolution rate of the lower gastrointestinal pathway is higher than that of the upper gastrointestinal pathway in patients with Clostridioides difficile infection (CDI) (Quraishi et al., 2017). Oral bacterial liquid capsules and nasal feeding tubes have primarily been used (Green et al., 2020; Allegretti et al., 2021), and donor sources include autologous and allogeneic floral transplantations. In clinical studies on FMT in patients with diabetes (Aron-Wisnewsky et al., 2019; Belvoncikova et al., 2022; Hou et al., 2022; Zhang et al., 2022), a healthy human fecal donor who meets the relevant requirements is most commonly selected. When traditional hypoglycemic drugs are ineffective or cannot be tolerated, FMT, as a way to intervene in the gut microbiota, has a satisfactory overall effect with advantages such as high clinical safety and few adverse reactions (Wang et al., 2022a). Table 1 displays the clinical studies related to the treatment of DM with FMT.
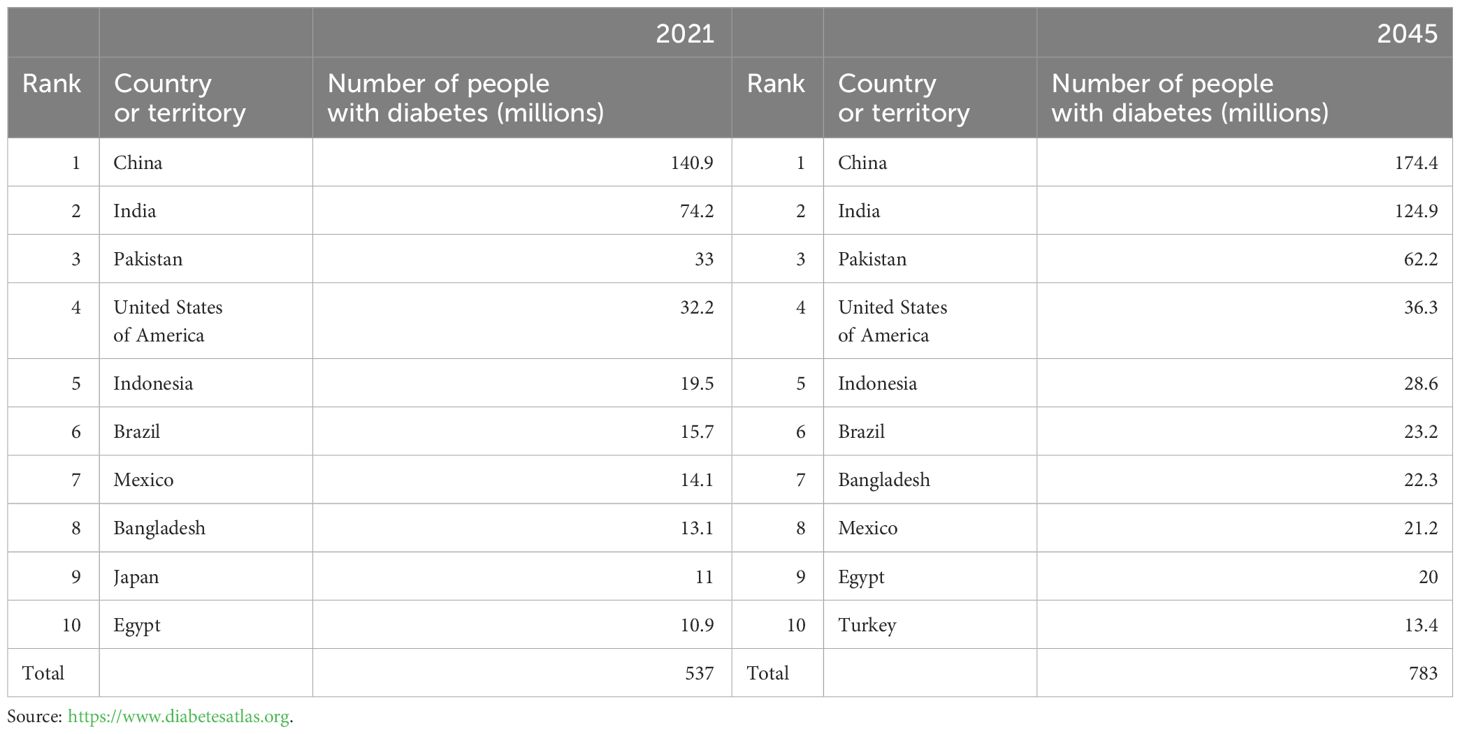
Table 1 Top 10 countries or territories for number of adults aged 20–79 years with diabetes in 2021 and 2045.
3 Role of gut microbiota in the pathophysiology of DM
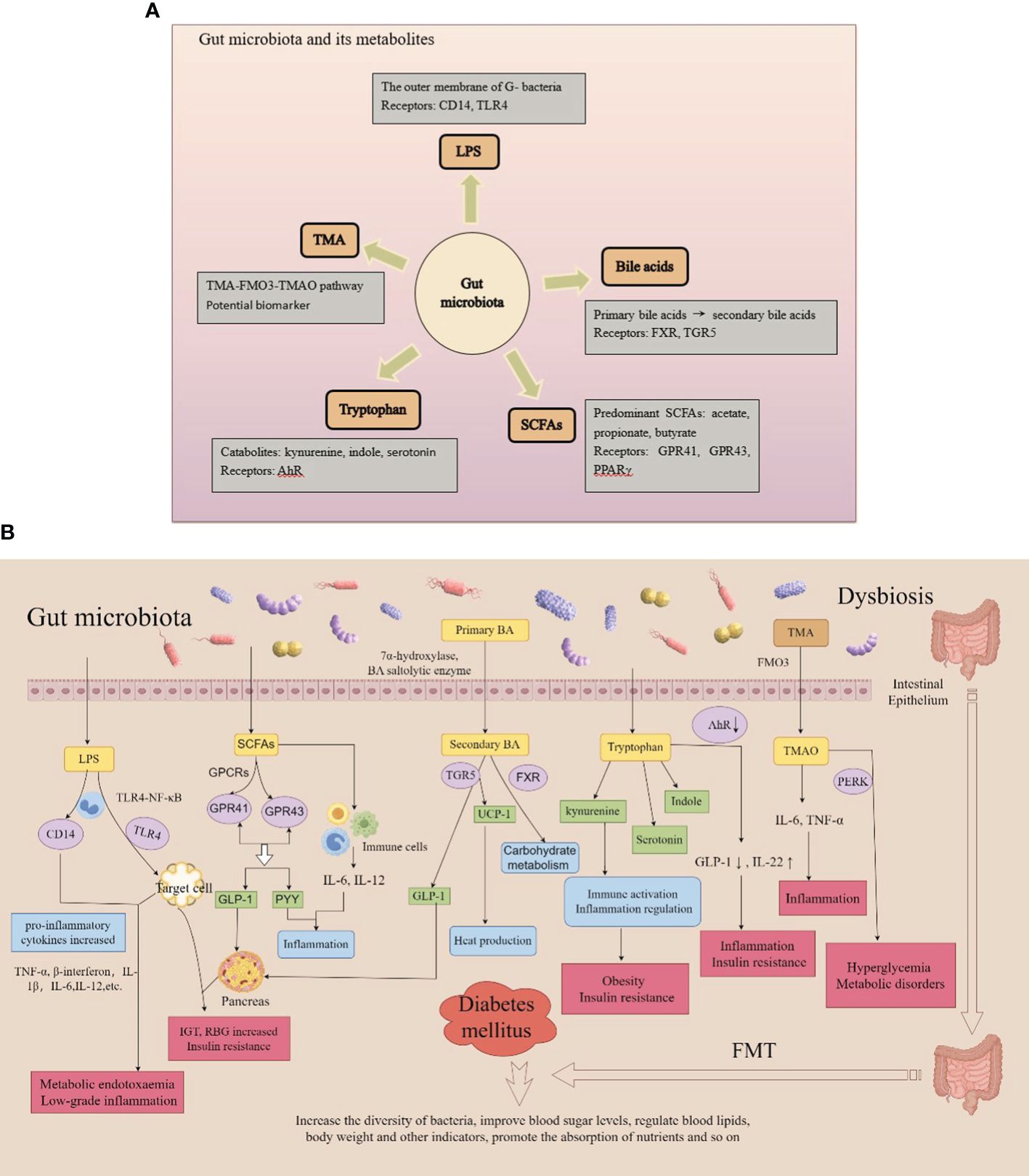
Recently, studies on various types of DM and its complications along with gut microbiota have been conducted (Yan et al., 2019; Arora et al., 2021; Ng et al., 2022). The gut microbiota of patients with diabetes differs from that of healthy counterparts. Specifically, the number of probiotic colonies, such as Bifidobacterium and Firmicutes, is decreased, and the number of pathogenic bacteria, such as Enterococcus and Enterobacter, is increased, resulting in an increase in inflammatory factors. In addition, these variations can induce the production of LPS, activate inflammatory responses, and affect the metabolism of SCFAs, bile acids, tryptophan, and trimethylamine N-oxide, thus promoting the development of DM (Fu et al., 2023a; Wu et al., 2023) (Figures 4A, B).
3.1 Gut microbiota: LPSs and DM
Endotoxins, also called LPSs, are a main component of the outer membrane of gram-negative bacteria and are involved in increasing pro-inflammatory cytokine levels and impairing pancreatic function. Variations in the gut microbiota in DM lead to various alterations, including an increase in the number of gram-negative bacteria, further release of LPS, and production of endotoxin, which damages the intestinal mucosa and results in leakage of the intestinal wall (Zhang et al., 2021; Christovich and Luo, 2022; Girdhar et al., 2022; Aleman et al., 2023) and endotoxemia (Lai et al., 2015; Velasquez, 2018). Subsequently, LPS can activate CD14 and toll-like receptor 4 (TLR4) on the surface of mononuclear macrophages, causing an increase in pro-inflammatory cytokines such as tumor necrosis factor α (TNF-α), β-interferon, and interleukin, and induce a series of chronic low-level inflammatory reactions in the body. The inflammation, in turn, interferes with insulin signaling, damaging islet β-cells and leading to absolute insufficiency of insulin secretion and insulin resistance (Siljander et al., 2019; Dedrick et al., 2020; Del Chierico et al., 2022; Girdhar et al., 2022; Luo et al., 2023). Cani et al. (2007) injected LPS subcutaneously into mice for 4 consecutive weeks to induce metabolic endotoxemia and chronic inflammation. After 4 weeks, the mice exhibited metabolic disorders characterized by impaired glucose tolerance, random blood glucose increases, and insulin resistance. Under the action of lipid polyglycoproteins, LPS binds to serum CD14 and promotes metabolic endotoxemia and low-grade inflammation (Zanoni and Granucci, 2013; Lee et al., 2019; Ciesielska et al., 2021; Mohammad and Thiemermann, 2021; Blanks et al., 2022). In addition, LPS activates the TLR4-nuclear factor-κB signal transduction pathway by combining with TLR4 on the surface of the cell membrane of host cells, thus causing target cells to secrete various inflammatory factors such as interleukin and TNF-α, which damage β-cells and reduce insulin secretion (Candelli et al., 2021; Ciesielska et al., 2021; Kim et al., 2022; Zhang et al., 2022; Li et al., 2023). Therefore, LPS-mediated chronic low-level inflammatory responses may be involved in the mechanisms underlying DM pathogenesis.
3.2 Gut microbiota: SCFAs and DM
SCFAs are a class of organic acids, including acetic acid, propionic acid, and butyric acid, generated by the fermentation of cellulose by the gut microbiota. The gut microbiota, especially Bifidobacterium, Lactobacillus, Clostridium, and Bacteroidetes, can produce abundant polysaccharide lyases and glycoside hydrolases that ferment carbohydrates into metabolic SCFAs. SCFAs regulate the gut microbiota, maintain fluid balance, inhibit the formation of intestinal inflammatory factors, and participate in intestinal immune responses and energy conversion. The G-protein-coupled receptors GPR41 and GPR43 are important regulatory factors that regulate glucose and lipid metabolism and can be directly activated by SCFAs to induce the formation of glucagon-like peptide 1 (GLP-1) and gastrointestinal polypeptide YY (PYY), which inhibit cellular inflammation and related immune diseases (Brar and Kohn, 2019; Roy et al., 2020; Zhang et al., 2023). Acetic acid can increase GLP-1 by binding to GPR43, thereby promoting insulin secretion. Propionic acid regulates gluconeogenesis via GPR41 and affects glucose metabolism. Butyrate stimulates the secretion of GLP-1 by activating peroxisome proliferator-activated receptor γ (PPARγ) and increases the number and activity of regulatory T-lymphocytes by inhibiting the recruitment of neutrophils and effector T-cells, ultimately reducing inflammation and enhance insulin sensitivity (He et al., 2020; Palmnäs-Bédard et al., 2022; Portincasa et al., 2022; Solar et al., 2023). Butyrate and acetate supplementation protects nonobese diabetic (NOD) mice from developing autoimmune diabetes (Mariño et al., 2017). Huang et al. (2020) compared the gut microbiota of healthy people and patients with T1DM and found that the gut microbiota of patients with diabetes could promote different IgA-mediated immune responses. SCFA treatment reduced the IgA response caused by intestinal bacteria as well as the severity of islet cell damage in NOD mice. SCFAs can stimulate intestinal L-cells to discharge the intestinal hormones GLP and PYY, thereby improving metabolic levels and increasing the absorption of energy substances (Wu et al., 2021). In contrast, SCFAs effectively enhance intestinal repair by repressing the activation of inflammatory cells and related responses (Salamone et al., 2021). Therefore, the gut microbiota is disturbed, and SCFA levels are significantly reduced, which can lead to diabetes.
3.3 Gut microbiota: Bile acids and DM
The gut microbiota and liver are involved in bile acid metabolism. Under the action of 7α-hydroxylase and bile acid saltolytic enzyme, primary bile acids transform into secondary bile acids while simultaneously activating a range of nuclear receptors, including the nuclear farnesoid X receptor (FXR) and G-protein-coupled bile acid receptor 1 (TGR5), thus facilitating both hepatic synthesis and intestinal reabsorption of bile acid. They additionally play significant roles in glucose, lipid, and energy metabolism (Liu et al., 2020; Zhao et al., 2020; Gao et al., 2022; Chen et al., 2023; Collins et al., 2023). Traussnigg et al. (2021) administered the non-steroidal FXR agonist PX-104 for four weeks in 12 non-diabetic patients with non-alcoholic fatty liver disease and found that the area under the C-peptide curve measured by oral glucose tolerance testing was significantly reduced, and 92% of the patients had improved insulin sensitivity. Therefore, FXR may be involved in glucose metabolism. Chen et al. discovered that the bile acid glycoursodeoxycholic acid (GUDCA) actively affects the gut microbiota by altering bile acid circulation. After eight weeks of GUDCA administration, blood glucose and lipid levels (total cholesterol and triglycerides) were significantly reduced in mice. Furthermore, the glucose and insulin tolerance test results, insulin levels, and HOMA-IR index of experimental mice were reduced. GUDCA increases the levels of taurine cholic acid and the abundance of Bacteroides vulgaris in mice, activating TGR5 and upregulating the expression of UCP-1, which leads to increased thermogenesis in white adipose tissue and induction of intestinal GLP-1 secretion (Chen et al., 2023). Therefore, disturbances in the gut microbiota can reduce the formation of bile acids, hinder the production of free and secondary bile acids (Fang et al., 2022), and weaken the activation of the FXR, resulting in abnormal glucose metabolism. Furthermore, the activation of the TGR5 receptor is weakened, which leads to a decrease in thyroid hormone thermogenesis, GLP-1 levels, and insulin secretion and an increase in blood sugar levels (Massey and Brown, 2021; Xia et al., 2021; Chen et al., 2023; Makki et al., 2023). As a metabolite, the reduction of bile acid (Fang et al., 2022) aggravates this enteric dysbacteriosis and forms a cycle that seriously affects the normal metabolic signaling pathways and leads to the occurrence of DM (Canfora et al., 2022).
3.4 Gut microbiota: tryptophan and DM
Tryptophan is an aromatic amino acid and one of the eight essential amino acids found in humans and animals. As a biosynthetic precursor of a large number of microbial and host metabolic pathways, various derivatives can be generated through the kynurenine, indole metabolic, and serotonin pathways, which exert biological effects. The kynurenine pathway is involved in immune activation and inflammatory regulation and is associated with obesity and insulin resistance (Vangipurapu et al., 2020). Tryptophan is catabolized by intestinal microbes and converted into various indole derivatives such as indole acetic acid, indole lactic acid, and indole propionic acid. Tryptophan, kynurenine pathway metabolites, and indole lactate are positively associated with T2DM risk, whereas indole propionate is negatively associated with T2DM risk (Tuomainen et al., 2018). In addition, some compounds produced by tryptophan metabolism are ligands for aromatic receptors (AhRs), which can cause changes in the AhR conformation. Under activation of the AhR pathway reduces GLP-1 and interleukin (IL)-22 production and increases intestinal permeability and LPS translocation, leading to inflammation and insulin resistance. An article published in GUT in 2022, based on an epidemiological cohort, examined prospective associations between circulating levels of host and microbial tryptophan metabolites and the incidence of DM, elucidating the relationship between metabolite-related host genetics, diet, and the gut microbiome (Qi et al., 2022). These results indicated that the transformation of tryptophan metabolism to intestinal microbial indole propionate production through host-microbial interactions could improve the progression of DM.
3.5 Gut microbiota: trimethylamine N-oxide and DM
Common symbiotic bacteria produce volatile trimethylamine (TMA), which is oxidized by flavin monooxygenases, primarily FMO3, to trimethylamine N-oxide (TMAO) in the liver. The TMA-FMO3-TMAO pathway is associated with T2DM. The addition of TMAO to the diet increased glucose tolerance in mice fed a high-fat diet. High levels of TMAO bind to and activate PERK, thereby promoting hyperglycemia and metabolic disorders. Previous studies have shown that the circulating levels of TMAO are significantly elevated under diabetic conditions in both human and animal models. Researchers have also found that TMAO is closely associated with adverse events such as diabetes-related retinopathy, coronary heart disease, and kidney disease (Zhang et al., 2021; Yakar et al., 2022; Huang et al., 2023; Yu et al., 2024). Jiang et al. found that TMAO increases vascular permeability and endothelial cell dysfunction under diabetic conditions (Jiang et al., 2024). By analyzing the bone mineral density of 254 patients with T2DM, Zhao et al. found a significant linear correlation between TMAO level and bone mineral density in patients with T2DM, suggesting that increased TMAO levels are associated with osteoporosis and osteoporotic fractures in this patient population (Yuan et al., 2024). Serum creatinine doubles in patients with T2DM and elevated circulating TMAO levels, suggesting that elevated serum TMAO levels are positively associated with the risk of Diabetic Kidney Disease in these patients (Huang et al., 2023; Yu et al., 2024). Therefore, elevated serum levels of trimethylamine oxide may serve as a potential biomarker of diabetes progression.
3.6 Brief summary
The preceding discussion introduces the mechanism by which gut microbiota metabolites participate in DM. However, the gut microbiota also influences the incidence of T1DM as an autoimmune disease by altering the immune response. Structural variations in the gut flora damage the integrity of the intestinal barrier, causing increased intestinal permeability and ectopic distribution of certain metabolites throughout the body. This process directly destroys islet β-cells and triggers systemic inflammation and autoimmune processes (Yan et al., 2019; Arora et al., 2021; Zhang et al., 2021; Ng et al., 2022; Fu et al., 2023a; Wu et al., 2023). Furthermore, innate immunity plays a crucial role in the etiology of T1DM. TLRs are important factors in innate immunity and are essential for maintaining intestinal homeostasis. Simon et al. (2020) recently found that compared with NOD TLR4+/+ mice, NOD TLR4-/- mice had an increased risk of diabetes before developing T1DM. In their study, the abundance of Bacteroides in the large intestine was higher, whereas that of Firmicutes was lower, suggesting that TLR4 expression affected the varieties and quantities of the gut microbiota, influencing the incidence of T1DM. Finally, T cells are significant players in adaptive immune responses, both in fighting pathogens and regulating immune responses to maintain immune homeostasis. Therefore, most studies suggest that enteric dysbacteriosis changes intestinal permeability and the intestinal immune response in the pathogenesis of T1DM (Demirci et al., 2020; Huang et al., 2020; Ma et al., 2020; de Groot et al., 2021; He et al., 2022; Syed, 2022; Wang et al., 2022; Xie et al., 2022; Yuan et al., 2022; Zheng et al., 2022; Diviccaro et al., 2023; Moreira et al., 2023).
In summary, FMT can improve obesity and blood sugar levels in diabetic mice; the mechanism may involve changes in microflora and metabolites, improvement of insulin resistance, and diabetes treatment.
4 FMT used to treat DM
4.1 Animal experiment
Relatively few studies exist on the treatment of T1DM using FMT. Previous studies have shown that FMT can significantly improve insulin resistance in non-obese diabetic mice. Subsequently, some scholars explored the relevant mechanism and found that the incidence of T1DM in mice in the FMT group was 40.9%, compared with 72.7% in the control group (Zhou et al., 2022). FMT alleviated the islets of NOD mice and slowed the development of T1DM. The mechanism may be related to the remodeling of the intestinal flora of NOD mice, improving intestinal barrier function, affecting the immune response of the gut-pancreas immune axis, concurrently modifying the disorder of amino acid metabolism, and reducing the accumulation of branch-chain amino acids in NOD mice during the islet stage. Researchers are continuing to gain insight into the advantages of FMT in T2DM treatment regimens through animal experiments. Wang et al (Wang et al., 2020). demonstrated that rebuilding the microbiota of T2DM mice using FMT could alleviate hyperglycemia, reverse insulin resistance, and repair damaged islets. For the Kazakh population in China, a healthy human fecal sample was collected after meeting the inclusion criteria for intervention in db/db mice. Desulfovibrio and Clostridium coccoides levels in the intestines of FMT-treated mice were significantly reduced, whereas Akkermansia levels in the feces and histone deacetylase-3 (HDAC3) protein expression in the colon were elevated. Both intestinal target bacteria and HDAC3 correlated with glycolipid levels, and Myxophilus levels positively correlated with HDAC3 protein expression (r = +0.620, p = 0.037). Correspondingly, the levels of fasting blood glucose, postprandial blood glucose, total cholesterol, triglycerides, and low-density lipoprotein cholesterol were significantly reduced in FMT-treated db/db mice, whereas the levels of high-density lipoprotein cholesterol were upregulated. Finally, the results of this study suggest that fecal bacteria from KNGT may be used to treat patients with diabetes (Zhang et al., 2020). In contrast, Wang et al. collected stool from Uyghur patients with T2DM to intervene with C57BM/6 mice and found that Uyghur T2DM fecal microbiota transplantation disrupted glucose metabolism by altering the ability of intestinal flora to metabolize BIS and the BAS/GLP-1 pathway (Wang et al., 2022). Zhang et al. transplanted the gut microbiota of healthy or obese T2DM rats into the gastrointestinal tracts of Zucker diabetic fatty (ZDF) and lean Zucker (LZ) rats (Zhang et al., 2020). Using 16S rRNA sequencing and macrometabolomics techniques, diabetic rats transplanted with normal gut microbiota exhibited significant weight loss, lower HbA1c levels, improved glucose tolerance, and enhanced insulin tolerance, compared with the control group. These findings provide evidence that FMT effectively enhances body weight and blood glucose modification in T2DM rats. To explore the impact of FMT on glycometabolism and insulin secretion in mice, a study conducted by Chen et al (Chen et al., 2023). revealed that FMT treatment in db/db mice resulted in significant improvements in various clinical indicators, such as serum insulin levels, fasting blood glucose levels, and oral glucose tolerance, compared with that in the control group. Yang et al (Yang et al., 2023). evaluated the curative effect of FMT, compared with that of metformin (MET) in 28 mice randomly divided into four groups (control, T2DM, MET, and FMT). Fasting blood glucose and low-density lipoprotein levels were reduced in both the MET- and FMT-treated diabetic mice, suggesting that FMT can be used to treat T2DM by improving hyperlipidemia and hyperglycemia. In the study by An et al. (2023), 32 SPF-grade SD rats were used as donors and divided into three groups (control, T2DM, and non-T2DM). Feces were collected, and the supernatant was retrieved after centrifugation. Subsequently, 79 SPF-grade SD rats were divided into a normal saline group (NS group) and an antibiotic group (ABX group). The ABX group was further randomly divided into five groups, and the NS group was divided into two groups according to diet and FMT intervention. In the ABX group, Ruminococcus gnavus in the T2DM fecal supernatant group after a high-fat diet was more abundant than that in the transplant control fecal supernatant group and FMT non-intervention group. The levels of blood glucose, serum insulin, total cholesterol, triglycerides, and low-density lipoproteins in rats were higher than those in high-fat diet intervention group. After FMT intervention, the levels of acetic acid and butyric acid were increased, and the expression of GPR41/43 was also significantly increased, indicating that Ruminococcus gnavus may increase the risk of T2DM in rats. The gut microbiota, SCFAs-GPR41/43, may play a role in the development of T2DM.
In a nested case-control study, the transfer of different gestational fecal samples from GDM and non-GDM donors to germ-free mice resulted in different patterns of intestinal microbiota colonization and induced hyperglycemia in mice that received GDM donor microbiota (Liu et al., 2020). Similarly, feces from patients with GDM and healthy pregnant women were transplanted into germ-free mice to establish a gestational mouse model, and data from mice at different gestational stages were recorded. The weight and blood glucose levels of the offspring of GDM-FMT mice were higher than those of control offspring (Qin et al., 2022). FMT further suggested that GDM is closely related to abnormal intestinal flora and that the offspring of these women have an increased risk of diabetes. Changes in maternal flora also affect the occurrence of GDM.
FMT was used for the first time in a preclinical model of DN (BTBR ob/ob mice) (Bastos et al., 2022) which revealed that FMT was a safe treatment that prevented weight gain, reduced proteinuria, reduced local expression of TNF-α in the intestine, and potentially improved insulin resistance.
4.2 Clinical research
The effect of FMT on the progression of T1DM was investigated by de Groot et al. by assessing the retention of stimulated C-peptide release in a 12-month mixed meal trial (de Groot et al., 2021). The results indicated that pancreatic β-cell function and stimulation levels were retained at 12 months in the FMT group, suggesting that FMT can protect pancreatic β-cell function in patients with diabetes diagnosed 12 months after onset and avoid a drop in endogenous insulin production. Additionally, the study revealed significant alterations in plasma metabolites, specifically MA-GPC (p = 0.02, MWU) and A-GPC (p = 0.02), between the allogeneic FMT group and autogenous FMT group (control group). In another clinical trial, researchers conducted a 1-year therapeutic study involving two teenagers with T1DM who received one to three rounds of FMT and were followed up for up to 30 weeks (He et al., 2022; Zhang et al., 2022). After transplantation, the diabetes-related clinical indicators of both patients showed significant improvement, indicating that FMT was effective and could be maintained to control blood glucose levels in patients with diabetes. FMT notably enhanced insulin resistance in two patients and effectively extended the function of residual β-cells in patients with diabetes receiving FMT. In addition to the therapeutic effects of FMT on T1DM, FMT also ameliorates DM-related complications. A malnourished patient with T1DM who was unable to control blood glucose levels with insulin and experienced severe gastrointestinal symptoms accepted FMT treatment via nasojejunal tube implantation (Xie et al., 2022). No obvious adverse reactions were observed after FMT, and vomiting, nausea, and other symptoms were alleviated. Additionally, body weight and body mass index increased, and trophic status, constipation, and glycemic control (fasting plasma glucose and glycated hemoglobin [HbA1c]) gradually improved. Other clinical indicators, including total protein, albumin, and hemoglobin levels, returned to normal.
In related clinical trials, the health of patients with T2DM improved after FMT. Researchers have found that FMT changes the gut microbiome more quickly than diet alterations, and patients with T2DM show further improvements in blood sugar, blood pressure, lipids, and body mass index. Ding et al. (2022) recruited 20 healthy individuals as baseline controls and 17 patients with T2DM to receive FMT from healthy donors for 12 weeks and evaluated their HbA1c% and metabolic parameter changes. Uric acid, blood sugar, and HbA1c% levels decreased, whereas postprandial C-peptide levels increased significantly after 12 weeks. Therefore, researchers believe that FMT can ameliorate insulin function and glycometabolism in T2DM and that some patients may benefit from FMT. In a randomized trial (Ng et al., 2022), 61 participants with T2DM were divided into three groups for 24 weeks of FMT and lifestyle intervention; combining the two methods led to more favorable variations in the participants’ microbiomes and improved lipid and liver stiffness. Wu et al (Wu et al., 2023). compared the effect of FMT alone, MET alone, and FMT plus MET on patients with T2DM and their gut microbiota. In their study, 31 newly diagnosed patients with T2DM were randomized to receive MET, FMT, or FMT plus MET and were followed up from week four, with blood and stool samples collected periodically. Compared with those of MET alone, blood glucose levels, HbA1c levels, and insulin resistance were clearly decreased after FMT treatment. Additionally, uric acid and triglyceride levels were significantly decreased, and fasting blood sugar and HbA1c levels were decreased when FMT was combined with MET treatment.
Cai et al. (2018) performed FMT in a patient with DPN combined with hypertension, poor blood sugar control, and anxiety caused by long-term pain. After the first FMT, the patient’s pain was alleviated; blood sugar levels, blood pressure, and lipid levels decreased significantly; and weight loss was observed. Although nutritional neuropathy and painkillers are the main treatment methods for diabetic neuropathy, the patient did not use any analgesic drugs after FMT. Furthermore, the patient’s HbA1c, blood sugar, and uric acid levels decreased significantly, while C-peptide levels significantly increased after meals. Therefore, the fecal microbiota provided by healthy donors was crucial for improving glycometabolism in T2DM. FMT also improved insulin resistance and mitigated islet dysfunction. In a randomized, double-blind trial of diabetic distal symmetrical polyneuropathy (DSPN), 22 patients with DSPN who received fecal microbiota transplants from healthy individuals showed significant improvements in symptoms, independent of blood glucose control, compared with 10 patients who received placebos (Yang et al., 2023).
These experiments suggest that re-establishing intestinal microecological balance through FMT may be an alternative remedy for DM and its associated diseases (Tables 2, 3)
4.3 Role of gut microbiota in the therapeutic approach for treating diabetes
The gut microbiota can be broadly classified into three categories: beneficial, harmful, and neutral bacteria (Gilbert et al., 2018; Adak and Khan, 2019; Zhou et al., 2020; Aggarwal et al., 2023; Fu et al., 2023b; Wang et al., 2023). Beneficial bacteria, also known as probiotics, primarily include various Bifidobacterium and lactobacillus, which synthesize various vitamins, participate in food digestion, promote intestinal peristalsis, inhibit the growth of pathogenic bacteria, and decompose harmful toxic substances. During the long-term evolutionary process of the gut microbiota, different types of flora; the flora and host; and the flora, host, and environment have always maintained a dynamic balance. Therefore, when the floral structure is relatively stable, it does not cause disease in the host. Existing literature shows that gut microbiota diversity in patients with diabetes is reduced, and the ratio of firmicutes to bacteroides is significantly reduced (Schloissnig et al., 2013; Zhang et al., 2021; Del Chierico et al., 2022; de Vos et al., 2022; Guo et al., 2022; Zhou et al., 2022; Crudele et al., 2023; Wu et al., 2023). Human health is closely related to the structure of probiotics in the gut, such as bifidobacterium, which is negatively correlated with blood sugar, blood pressure, lipids, and body mass index. Specifically, Bifidobacteria, Bacteroides, and Coprologis are negatively correlated with DM, whereas Ruminococcus, Fusobacterium, and Cyanobacteria are positively correlated. Diabetic microangiopathosis is positively correlated with Bacteroides abundance and negatively correlated with Prevotella abundance (Hong et al., 2022; Li et al., 2023; Sapra and Bhandari, 2023; Jia et al., 2024; Kanbour et al., 2024; Ma et al., 2024). Pasteurella bacteria can be used as a microbial feature to distinguish DR from DM, and Blautia may be a protective factor against DN. In addition, the gut microbiota plays a crucial role in the human metabolic system, and various metabolites are produced by the gut microbiota in different ways. As research deepens, some metabolites may not only participate in the pathogenesis of DM, but may also serve as important potential biomarkers indicating the complications of diabetes. The increase in LPS from gram-negative bacteria, SCFAs, secondary bile acids, trimethylamine, indole, tryptophan, and other gut microbiota derivatives aggravates inflammation and metabolic dysfunction and promotes the onset and progression of diabetes. The progression of diabetes also leads to gut microbiota disorders, manifested as the reduction of probiotics, increase of harmful bacteria, and introduction of disease caused by neutral bacteria; the interaction between these three aggravates disease. Generally, intestinal flora, especially probiotics, can improve the absorption of sugars in the intestine, regulate blood sugar, inhibit inflammation, protect islet cells, maintain the integrity of the intestinal barrier, reduce the entry of harmful substances into the blood, and reduce systemic inflammatory responses in patients. They can regulate the host metabolic pathway, improve insulin resistance, increase insulin sensitivity, affect intestinal pH and enzyme activity, promote the absorption and utilization of hypoglycemic drugs, promote nutrient absorption, and participate in lipid metabolism, thereby controlling blood sugar levels, reducing the risk of complications, and improving the prognosis and quality of life of patients with diabetes (Figures 5A, B).
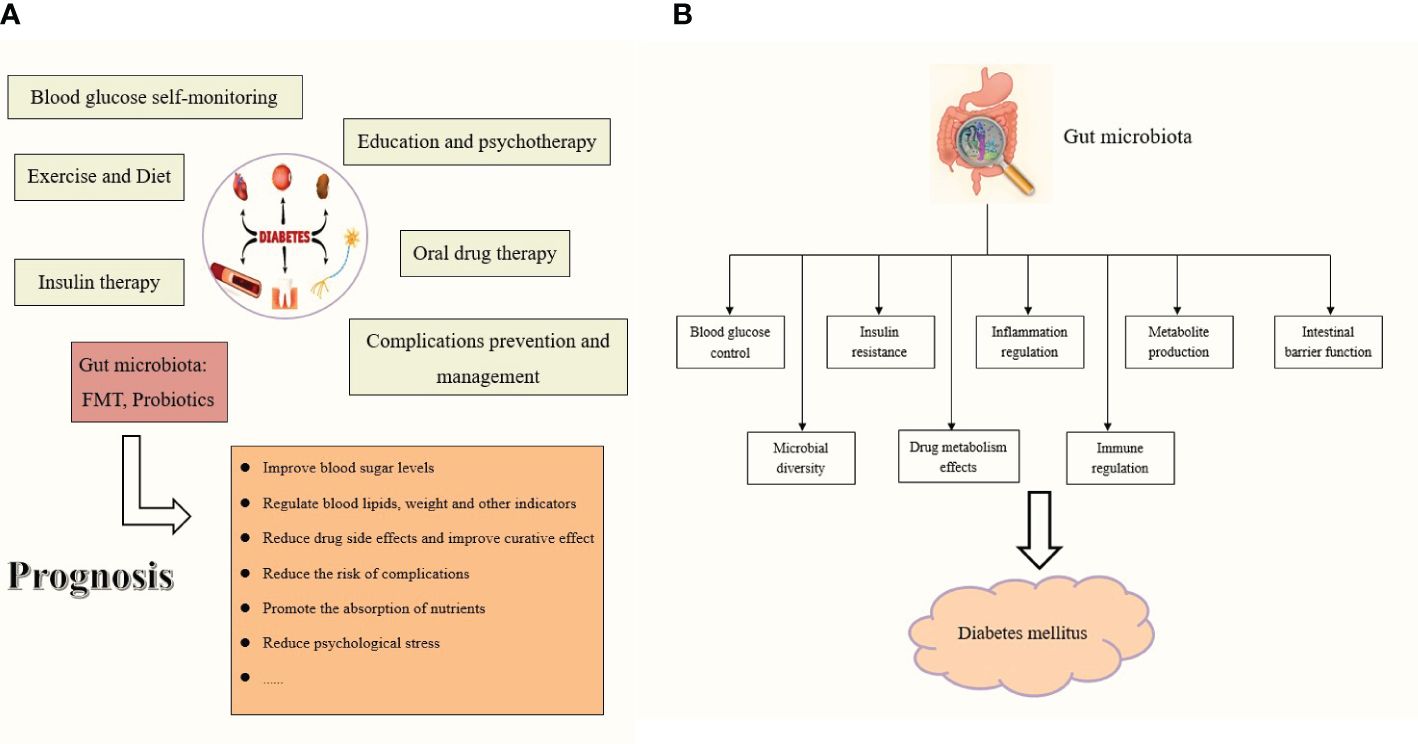
Figure 5 (A) The role of gut microbiota in DM prognosis. (B) The role of gut microbiota as a therapeutic to treat the diabetes mellitus.
While the mainstream treatment for diabetes currently relies on insulin replacement and hypoglycemic drugs, a paradigm unlikely to change in the immediate or distant future, the application of FMT introduces new perspectives and directions for the treatment of DM. Dietary habits, lifestyle modifications, pharmaceuticals, and supplementation with exogenous probiotics can all regulate gut microbiota, with FMT being the most direct and effective intervention. Initially, FMT served as a tool to isolate probiotics and enhance the gut microbiota ecology in diabetes. Subsequently, in animal experiments, FMT directly augmented the diversity of the gut microbiota, restored intestinal function, improved the in vivo metabolic status, delayed the progression of diabetes, and exerted a positive feedback effect. Regarding current symptomatic treatment of diabetes, FMT is anticipated to address the root cause of diabetes by adjusting the intestinal microecological balance and improving insulin resistance, implicating multiple mechanisms such as inflammation and metabolism. By examining the changes in metabolites before and after FMT, we can gain a deeper understanding of the role of potential markers and further elucidate the pathogenesis of gut microbiota in DM. Finally, the safety and feasibility of FMT were verified using animal models. Considering drug allergies, intolerance, and poor response to traditional treatment in some patients, FMT was administered one to three times. Subsequently, clinical indicators such as blood glucose level, hemoglobin level, BMI, and islet function showed significant improvement, thereby affirming that FMT is a viable option. Additionally, the long-term effects of FMT may be more stable and have fewer adverse effects than those of traditional drug therapy, which may necessitate constant dose adjustments or drug changes.
5 Clinical safety
No obvious adverse events have been reported in recent studies regarding the use of FMT for DM. The majority of diabetic patients do not experience adverse reactions following FMT treatment, which appears to improve HbA1c and blood glucose levels and islet function, and does not induce hypoglycemia or hypolipidemia. One study (Zhang et al., 2020) indicated that the initial transplantation of gut microbiota caused a temporary inflammatory response in patients. However, once patients adapted to the transplanted microbiota, the inflammatory markers returned to normal levels. Concurrently, no significant abnormalities were observed in certain indices before and after treatment, further suggesting that FMT did not inflict damage to the kidneys, liver, or other organs. When a patient with diabetes and gastrointestinal symptoms (Xie et al., 2022) experienced recurrent nausea and vomiting, existing drug treatments were effective. After FMT, the patient’s gastrointestinal discomfort decreased.
6 Conclusion
Previous studies have underscored the therapeutic potential of FMT for DM; however, several issues remain unresolved. Currently, large-sample randomized controlled trials investigating FMT for the treatment of DM are insufficient. Therefore, further research is required to ascertain long-term efficacy and safety for this treatment. Additionally, the mechanisms underlying the effects of FMT on DM and their broader impact on DM are discussed.
As a direct and potent intervention for gut microbiota disorders, FMT holds promise in predicting the clinical treatment of DM. It can restructure the gut microbiota in patients with diabetes, restore the diversity of the original gut microbiota, inhibit inflammatory responses, and modulate immune responses. FMT can also stabilize the metabolic state of the body. Clinical indicators related to DM, including blood glucose, insulin, C-peptide, and HbA1c levels, also improved. Although research on FMT in relation to GDM and other specific types of diabetes is limited, significant progress has been made. Ongoing studies aim to further explore these complications. FMT not only regulates the metabolic levels of diabetic patients but also treats other DM-related diseases, such as obesity, metabolic syndrome, and neuropsychiatric diseases, thereby achieving comprehensive intervention and management for these patients.
FMT represents a novel concept and technology for treating gut microbiota-related diseases. Despite these limitations, FMT remains a crucial tool for investigating the role of microorganisms in the pathogenesis of chronic diseases. As a new form of individualized microecological treatment, it can regulate blood glucose levels and metabolic status; reduce patient dependence on drugs and their side effects; improve patient prognosis and quality of life; and provide a more effective, safer, and longer-lasting treatment program.
Research has indicated a correlation between individual flora, blood sugar, and other indicators. Therefore, the feasibility of single-flora transplantation therapy is worth exploring. Currently, significant challenges inhibit the clinical promotion of FMT for the treatment of DM. The selection of FMT donors, transplantation mode, dose frequency, patient acceptance, and need for dietary intervention after FMT should be further studied. Overall, based on existing studies, DM treatment has been proven to be effective and feasible, and we believe that standard, high-quality, and safe FMT will provide hope to patients with DM.
Author contributions
JZ: Writing – original draft. HW: Writing – review & editing. YL: Writing – review & editing. MS: Writing – review & editing. MZ: Writing – review & editing. HZ: Writing – review & editing. JC: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adak, A., Khan, M. R. (2019). An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. CMLS 76, 473–493. doi: 10.1007/s00018-018-2943-4
Aggarwal, N., Kitano, S., Puah, G. R. Y., Kittelmann, S., Hwang, I. Y., Chang, M. W. (2023). Microbiome and human health: Current understanding, engineering, and enabling technologies. Chem. Rev. 123, 31–72. doi: 10.1021/acs.chemrev.2c00431
Agus, A., Clément, K., Sokol, H. (2021). Gut microbiota-derived metabolites as central regulators in metabolic disorders. Gut 70, 1174–1182. doi: 10.1136/gutjnl-2020-323071
Aleman, R. S., Moncada, M., Aryana, K. J. (2023). Leaky gut and the ingredients that help treat it: A review. Molecules (Basel Switzerland) 28, 619. doi: 10.3390/molecules28020619
Allegretti, J. R., Kassam, Z., Hurtado, J., Marchesi, J. R., Mullish, B. H., Chiang, A., et al. (2021). Impact of fecal microbiota transplantation with capsules on the prevention of metabolic syndrome among patients with obesity. Hormones (Athens Greece) 20, 209–211. doi: 10.1007/s42000-020-00265-z
An, Y., Dai, H., Duan, Y., Cheng, L., Shi, L., He, C., et al. (2023). The relationship between gut microbiota and susceptibility to type 2 diabetes mellitus in rats. Chin. Med. 18, 49. doi: 10.1186/s13020-023-00717-9
Aron-Wisnewsky, J., Clément, K., Nieuwdorp, M. (2019). Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes? Curr. Diabetes Rep. 19, 51. doi: 10.1007/s11892-019-1180-z
Arora, A., Behl, T., Sehgal, A., Singh, S., Sharma, N., Bhatia, S., et al. (2021). Unravelling the involvement of gut microbiota in type 2 diabetes mellitus. Life Sci. 273, 119311. doi: 10.1016/j.lfs.2021.119311
Bárcena, C., Valdés-Mas, R., Mayoral, P., Garabaya, C., Durand, S., Rodríguez, F., et al. (2019). Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 25, 1234–1242. doi: 10.1038/s41591-019-0504-5
Bastos, R. M. C., Simplício-Filho, A., Sávio-Silva, C., Oliveira, L. F. V., Cruz, G. N. F., Sousa, E. H., et al. (2022). Fecal microbiota transplant in a pre-clinical model of type 2 diabetes mellitus, obesity and diabetic kidney disease. Int. J. Mol. Sci. 23, 3842. doi: 10.3390/ijms23073842
Belvoncikova, P., Maronek, M., Gardlik, R. (2022). Gut dysbiosis and fecal microbiota transplantation in autoimmune diseases. Int. J. Mol. Sci. 23, 10729. doi: 10.3390/ijms231810729
Blanks, A. M., Pedersen, L. N., Caslin, H. L., Mihalick, V. L., Via, J., Canada, J. M., et al. (2022). LPS differentially affects expression of CD14 and CCR2 in monocyte subsets of Post-STEMI patients with hyperglycemia. Diabetes Res. Clin. Pract. 191, 110077. doi: 10.1016/j.diabres.2022.110077
Bodman, M. A., Varacallo, M. (2023). Peripheral Diabetic Neuropathy (StatPearls. StatPearls Publishing).
Bommer, C., Sagalova, V., Heesemann, E., Manne-Goehler, J., Atun, R., Bärnighausen, T., et al. (2018). Global economic burden of diabetes in adults: Projections from 2015 to 2030. Diabetes Care 41, 963–970. doi: 10.2337/dc17-1962
Brar, P. C., Kohn, B. (2019). Use of the microbiome in the management of children with type 2 diabetes mellitus. Curr. Opin. Pediatr. 31, 524–530. doi: 10.1097/MOP.0000000000000781
Cai, T. T., Ye, X. L., Yong, H. J., Song, B., Zheng, X. L., Cui, B. T., et al. (2018). Fecal microbiota transplantation relieve painful diabetic neuropathy: A case report. Medicine 97, e13543. doi: 10.1097/MD.0000000000013543
Candelli, M., Franza, L., Pignataro, G., Ojetti, V., Covino, M., Piccioni, A., et al. (2021). Interaction between lipopolysaccharide and gut microbiota in inflammatory bowel diseases. Int. J. Mol. Sci. 22, 6242. doi: 10.3390/ijms22126242
Canfora, E. E., Hermes, G. D. A., Müller, M., Bastings, J., Vaughan, E. E., van Den Berg, M. A., et al. (2022). Fiber mixture-specific effect on distal colonic fermentation and metabolic health in lean but not in prediabetic men. Gut Microbes 14, 2009297. doi: 10.1080/19490976.2021.2009297
Cani, P. D., Neyrinck, A. M., Fava, F., Knauf, C., Burcelin, R. G., Tuohy, K. M., et al. (2007). Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxemia. Diabetologia 50, 2374–2383. doi: 10.1007/s00125-007-0791-0
Ceriello, A., Prattichizzo, F., Phillip, M., Hirsch, I. B., Mathieu, C., Battelino, T. (2022). Glycemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol. 10, 75–84. doi: 10.1016/S2213-8587(21)00245-X
Chen, B., Bai, Y., Tong, F., Yan, J., Zhang, R., Zhong, Y., et al. (2023). Glycoursodeoxycholic acid regulates bile acids level and alters gut microbiota and glycolipid metabolism to attenuate diabetes. Gut Microbes 15, 2192155. doi: 10.1080/19490976.2023.2192155
Chen, L., Guo, L., Feng, S., Wang, C., Cui, Z., Wang, S., et al. (2023). Fecal microbiota transplantation ameliorates type 2 diabetes via metabolic remodeling of the gut microbiota in db/db mice. BMJ Open Diabetes Res. Care 11, e003282. doi: 10.1136/bmjdrc-2022-003282
Chi, M., Ma, K., Wang, J., Ding, Z., Li, Y., Zhu, S., et al. (2021). The immunomodulatory effect of the gut microbiota in kidney disease. J. Immunol. Res. 2021, 5516035. doi: 10.1155/2021/5516035
Christovich, A., Luo, X. M. (2022). Gut microbiota, leaky gut, and autoimmune diseases. Front. Immunol. 13. doi: 10.3389/fimmu.2022.946248
Ciesielska, A., Matyjek, M., Kwiatkowska, K. (2021). TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. CMLS 78, 1233–1261. doi: 10.1007/s00018-020-03656-y
Collins, S. L., Stine, J. G., Bisanz, J. E., Okafor, C. D., Patterson, A. D. (2023). Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat. Rev. Microbiol. 21, 236–247. doi: 10.1038/s41579-022-00805-x
Crudele, L., Gadaleta, R. M., Cariello, M., Moschetta, A. (2023). Gut microbiota in the pathogenesis and therapeutic approaches of diabetes. EBioMedicine 97, 104821. doi: 10.1016/j.ebiom.2023.104821
Crusell, M. K. W., Hansen, T. H., Nielsen, T., Allin, K. H., Rühlemann, M. C., Damm, P., et al. (2018). Gestational diabetes is associated with change in the gut microbiota composition in third trimester of pregnancy and postpartum. Microbiome 6, 89. doi: 10.1186/s40168-018-0472-x
Das, S., Devi Rajeswari, V., Venkatraman, G., Elumalai, R., Dhanasekaran, S., Ramanathan, G. (2024). Current updates on metabolites and its interlinked pathways as biomarkers for diabetic kidney disease: A systematic review. Trans. Res. J. Lab. Clin. Med. 265, 71–87. doi: 10.1016/j.trsl.2023.11.002
Dedrick, S., Sundaresh, B., Huang, Q., Brady, C., Yoo, T., Cronin, C., et al. (2020). The role of gut microbiota and environmental factors in type 1 diabetes pathogenesis. Front. Endocrinol. 11. doi: 10.3389/fendo.2020.00078
de Groot, P., Nikolic, T., Pellegrini, S., Sordi, V., Imangaliyev, S., Rampanelli, E., et al. (2021). Fecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomized controlled trial. Gut 70, 92–105. doi: 10.1136/gutjnl-2020-322630
Del Chierico, F., Rapini, N., Deodati, A., Matteoli, M. C., Cianfarani, S., Putignani, L. (2022). Pathophysiology of type 1 diabetes and gut microbiota role. Int. J. Mol. Sci. 23, 14650. doi: 10.3390/ijms232314650
Demirci, M., Bahar Tokman, H., Taner, Z., Keskin, F. E., Çağatay, P., Ozturk Bakar, Y., et al. (2020). Bacteroidetes and Firmicutes levels in gut microbiota and effects of hosts TLR2/TLR4 gene expression levels in adult type 1 diabetes patients in Istanbul, Turkey. J. Diabetes its complications 34, 107449. doi: 10.1016/j.jdiacomp.2019.107449
de Vos, W. M., Tilg, H., Van Hul, M., Cani, P. D. (2022). Gut microbiome and health: mechanistic insights. Gut 71, 1020–1032. doi: 10.1136/gutjnl-2021-326789
Ding, D., Yong, H., You, N., Lu, W., Yang, X., Ye, X., et al. (2022). Prospective study reveals host microbial determinants of clinical response to fecal microbiota transplant therapy in type 2 diabetes patients. Front. Cell. infection Microbiol. 12. doi: 10.3389/fcimb.2022.820367
Diviccaro, S., Falvo, E., Piazza, R., Cioffi, L., Herian, M., Brivio, P., et al. (2023). Gut microbiota composition is altered in a preclinical model of type 1 diabetes mellitus: Influence on gut steroids, permeability, and cognitive abilities. Neuropharmacology 226, 109405. doi: 10.1016/j.neuropharm.2022.109405
Du, X., Liu, J., Xue, Y., Kong, X., Lv, C., Li, Z., et al. (2021). Alteration of gut microbial profile in patients with diabetic nephropathy. Endocrine 73, 71–84. doi: 10.1007/s12020-021-02721-1
Fang, X., Miao, R., Wei, J., Wu, H., Tian, J. (2022). Advances in multi-omics study of biomarkers of glycolipid metabolism disorder. Comput. Struct. Biotechnol. J. 20, 5935–5951. doi: 10.1016/j.csbj.2022.10.030
Fassatoui, M., Lopez-Siles, M., Díaz-Rizzolo, D. A., Jmel, H., Naouali, C., Abdessalem, G., et al. (2019). Gut microbiota imbalances in Tunisian participants with type 1 and type 2 diabetes mellitus. Bioscience Rep. 39, BSR20182348. doi: 10.1042/BSR20182348
Fralick, M., Jenkins, A. J., Khunti, K., Mbanya, J. C., Mohan, V., Schmidt, M. I. (2022). Global accessibility of therapeutics for diabetes mellitus. Nat. Rev. Endocrinol. 18, 199–204. doi: 10.1038/s41574-021-00621-y
Fu, Y., Li, S., Xiao, Y., Liu, G., Fang, J. (2023a). A metabolite perspective on the involvement of the gut microbiota in type 2 diabetes. Int. J. Mol. Sci. 24, 14991. doi: 10.3390/ijms241914991
Fu, Y., Lyu, J., Wang, S. (2023b). The role of intestinal microbes on intestinal barrier function and host immunity from a metabolite perspective. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1277102
Gao, R., Meng, X., Xue, Y., Mao, M., Liu, Y., Tian, X., et al. (2022). Bile acids-gut microbiota crosstalk contributes to the improvement of type 2 diabetes mellitus. Front. Pharmacol. 13. doi: 10.3389/fphar.2022.1027212
GBD 2021 Diabetes Collaborators. (2023). Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet 402 (10397), 203–234. doi: 10.1016/S0140-6736(23)01301-6
Gilbert, J. A., Blaser, M. J., Caporaso, J. G., Jansson, J. K., Lynch, S. V., Knight, R. (2018). Current understanding of the human microbiome. Nat. Med. 24, 392–400. doi: 10.1038/nm.4517
Girdhar, K., Huang, Q., Chow, I. T., Vatanen, T., Brady, C., Raisingani, A., et al. (2022). A gut microbial peptide and molecular mimicry in the pathogenesis of type 1 diabetes. Proc. Natl. Acad. Sci. United States America 119, e2120028119. doi: 10.1073/pnas.2120028119
Green, J. E., Davis, J. A., Berk, M., Hair, C., Loughman, A., Castle, D., et al. (2020). Efficacy and safety of fecal microbiota transplantation for the treatment of diseases other than Clostridium difficile infection: a systematic review and meta-analysis. Gut Microbes 12, 1–25. doi: 10.1080/19490976.2020.1854640
Guo, Z., Pan, J., Zhu, H., Chen, Z. Y. (2022). Metabolites of gut microbiota and possible implication in development of diabetes mellitus. J. Agric. Food Chem. 70, 5945–5960. doi: 10.1021/acs.jafc.1c07851
Gupta, S., Thool, A. R. (2024). A narrative review of retinopathy in diabetic patients. Cureus 16, e52308. doi: 10.7759/cureus.52308
Hasani, M., Asadi Pilerud, Z., Kami, A., Abbas Vaezi, A., Sobhani, S., Ejtahed, H. S., et al. (2024). Association between gut microbiota compositions with microvascular complications in individuals with diabetes: A systematic review. Curr. Diabetes Rev. doi: 10.2174/0115733998280396231212114345
He, L., Chen, R., Zhang, B., Zhang, S., Khan, B. A., Zhu, D., et al. (2022). Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes mellitus. Front. Immunol. 13. doi: 10.3389/fimmu.2022.930872
He, J., Zhang, P., Shen, L., Niu, L., Tan, Y., Chen, L., et al. (2020). Short-chain fatty acids and their association with signaling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 21, 6356. doi: 10.3390/ijms21176356
Hoh, J. M., Dhanashree, B. (2017). Antifungal effect of cow's urine distillate on Candida species. J. Ayurveda Integr. Med. 8, 233–237. doi: 10.1016/j.jaim.2017.04.009
Hong, J., Fu, T., Liu, W., Du, Y., Min, C., Lin, D. (2022). Specific alterations of gut microbiota in diabetic microvascular complications: A systematic review and meta-analysis. Front. Endocrinol. 13. doi: 10.3389/fendo.2022.1053900
Hou, K., Zhang, S., Wu, Z., Zhu, D., Chen, F., Lei, Z. N., et al. (2022). Reconstruction of intestinal microecology of type 2 diabetes by fecal microbiota transplantation: Why and how. Bosnian J. basic Med. Sci. 22, 315–325. doi: 10.17305/bjbms.2021.6323
Huang, J., Pearson, J. A., Peng, J., Hu, Y., Sha, S., Xing, Y., et al. (2020). Gut microbial metabolites alter IgA immunity in type 1 diabetes. JCI Insight 5, e135718. doi: 10.1172/jci.insight.135718
Huang, Y., Wang, Z., Ma, H., Ji, S., Chen, Z., Cui, Z., et al. (2021). Dysbiosis and implication of the gut microbiota in diabetic retinopathy. Front. Cell. infection Microbiol. 11. doi: 10.3389/fcimb.2021.646348
Huang, Y., Zhu, Z., Huang, Z., Zhou, J. (2023). Elevated serum trimethylamine oxide levels as potential biomarker for diabetic kidney disease. Endocrine connections 12, e220542. doi: 10.1530/EC-22-0542
Jia, G., Bai, H., Mather, B., Hill, M. A., Jia, G., Sowers, J. R. (2024). Diabetic vasculopathy: Molecular mechanisms and clinical insights. Int. J. Mol. Sci. 25, 804. doi: 10.3390/ijms25020804
Jiang, J. Y., Liu, W. M., Zhang, Q. P., Ren, H., Yao, Q. Y., Liu, G. Q., et al. (2024). Trimethylamine N-oxide aggravates vascular permeability and endothelial cell dysfunction under diabetic condition: in vitro and in vivo study. Int. J. Ophthalmol. 17, 25–33. doi: 10.18240/ijo.2024.01.04
Kanbour, S., Harris, C., Lalani, B., Wolf, R. M., Fitipaldi, H., Gomez, M. F., et al. (2024). Machine learning models for prediction of diabetic microvascular complications. J. Diabetes Sci. Technol. 18, 273–286. doi: 10.1177/19322968231223726
Kautzky-Willer, A., Winhofer, Y., Kiss, H., Falcone, V., Berger, A., Lechleitner, M., et al. (2023). Gestationsdiabetes (GDM) (Update 2023) [Gestational diabetes mellitus (Update 2023)]. Wiener klinische Wochenschrift 135, 115–128. doi: 10.1007/s00508-023-02181-9
Kim, A., Gwon, M. H., Lee, W., Moon, H. R., Yun, J. M. (2022). Zerumbone suppresses high glucose and LPS-induced inflammation in THP-1-derived macrophages by inhibiting the NF-κB/TLR signaling pathway. Nutr. Res. (New York N.Y.) 100, 58–69. doi: 10.1016/j.nutres.2022.01.002
Kolarić, V., Svirčević, V., Bijuk, R., Zupančič, V. (2022). CHRONIC COMPLICATIONS OF DIABETES AND QUALITY OF LIFE. Acta clinica Croatica 61, 520–527. doi: 10.20471/acc.2022.61.03.18
Koren, O., Goodrich, J. K., Cullender, T. C., Spor, A., Laitinen, K., Bäckhed, H. K., et al. (2012). Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480. doi: 10.1016/j.cell.2012.07.008
Lai, L., Chen, Y., Tian, X., Li, X., Zhang, X., Lei, J., et al. (2015). Artesunate alleviates hepatic fibrosis induced by multiple pathogenic factors and inflammation through the inhibition of LPS/TLR4/NF-κB signaling pathway in rats. Eur. J. Pharmacol. 765, 234–241. doi: 10.1016/j.ejphar.2015.08.040
Larsen, N., Vogensen, F. K., van den Berg, F. W., Nielsen, D. S., Andreasen, A. S., Pedersen, B. K., et al. (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5, e9085. doi: 10.1371/journal.pone.0009085
Lee, M. K. S., Al-Sharea, A., Shihata, W. A., Bertuzzo Veiga, C., Cooney, O. D., Fleetwood, A. J., et al. (2019). Glycolysis is required for LPS-induced activation and adhesion of human CD14+CD16- monocytes. Front. Immunol. 10. doi: 10.3389/fimmu.2019.02054
Li, W., Cai, Z., Schindler, F., Bahiraii, S., Brenner, M., Heiss, E. H., et al. (2023). Norbergenin prevents LPS-induced inflammatory responses in macrophages through inhibiting NFκB, MAPK and STAT3 activation and blocking metabolic reprogramming. Front. Immunol. 14. doi: 10.3389/fimmu.2023.1117638
Li, Y., Liu, Y., Liu, S., Gao, M., Wang, W., Chen, K., et al. (2023). Diabetic vascular diseases: molecular mechanisms and therapeutic strategies. Signal transduction targeted Ther. 8, 152. doi: 10.1038/s41392-023-01400-z
Li, Y., Su, X., Gao, Y., Lv, C., Gao, Z., Liu, Y., et al. (2020). The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim. Biophys. Acta Mol. basis Dis. 1866, 165764. doi: 10.1016/j.bbadis.2020.165764
Lin, J. R., Wang, Z. T., Sun, J. J., Yang, Y. Y., Li, X. X., Wang, X. R., et al. (2022). Gut microbiota and diabetic kidney diseases: Pathogenesis and therapeutic perspectives. World J. Diabetes 13, 308–318. doi: 10.4239/wjd.v13.i4.308
Liu, Y., Qin, S., Feng, Y., Song, Y., Lv, N., Liu, F., et al. (2020). Perturbations of gut microbiota in gestational diabetes mellitus patients induce hyperglycemia in germ-free mice. J. Dev. origins Health Dis. 11, 580–588. doi: 10.1017/S2040174420000768
Liu, T., Song, X., Khan, S., Li, Y., Guo, Z., Li, C., et al. (2020). The gut microbiota at the intersection of bile acids and intestinal carcinogenesis: An old story, yet mesmerizing. Int. J. Cancer 146, 1780–1790. doi: 10.1002/ijc.32563
Liu, N., Yan, X., Lv, B., Wu, Y., Hu, X., Zheng, C., et al. (2024). A study on the association between gut microbiota, inflammation, and type 2 diabetes. Appl. Microbiol. Biotechnol. 108, 213. doi: 10.1007/s00253-024-13041-5
Liu, L., Zhang, J., Cheng, Y., Zhu, M., Xiao, Z., Ruan, G., et al. (2022). Gut microbiota: A new target for T2DM prevention and treatment. Front. Endocrinol. 13. doi: 10.3389/fendo.2022.958218
Luo, M., Sun, M., Wang, T., Zhang, S., Song, X., Liu, X., et al. (2023). Gut microbiota and type 1 diabetes: a two-sample bidirectional Mendelian randomization study. Front. Cell. infection Microbiol. 13. doi: 10.3389/fcimb.2023.1163898
Ma, Q., Li, Y., Wang, J., Li, P., Duan, Y., Dai, H., et al. (2020). Investigation of gut microbiome changes in type 1 diabetic mellitus rats based on high-throughput sequencing. Biomedicine pharmacotherapy = Biomedecine pharmacotherapie 124, 109873. doi: 10.1016/j.biopha.2020.109873
Ma, L., Liu, J., Deng, M., Zhou, L., Zhang, Q., Xiao, X. (2024). Metabolomics analysis of serum and urine in type 1 diabetes patients with different time in range derived from continuous glucose monitoring. Diabetol. Metab. syndrome 16, 21. doi: 10.1186/s13098-024-01257-4
Ma, S., You, Y., Huang, L., Long, S., Zhang, J., Guo, C., et al. (2020). Alterations in gut microbiota of gestational diabetes patients during the first trimester of pregnancy. Front. Cell. infection Microbiol. 10. doi: 10.3389/fcimb.2020.00058
Madonna, R., Balistreri, C. R., Geng, Y. J., De Caterina, R. (2017). Diabetic microangiopathy: Pathogenetic insights and novel therapeutic approaches. Vasc. Pharmacol. 90, 1–7. doi: 10.1016/j.vph.2017.01.004
Makki, K., Brolin, H., Petersen, N., Henricsson, M., Christensen, D. P., Khan, M. T., et al. (2023). 6α-hydroxylated bile acids mediate TGR5 signaling to improve glucose metabolism upon dietary fiber supplementation in mice. Gut 72, 314–324. doi: 10.1136/gutjnl-2021-326541
Mariño, E., Richards, J. L., McLeod, K. H., Stanley, D., Yap, Y. A., Knight, J., et al. (2017). Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562. doi: 10.1038/ni.3713
Massey, W., Brown, J. M. (2021). The gut microbial endocrine organ in type 2 diabetes. Endocrinology 162, bqaa235. doi: 10.1210/endocr/bqaa235
Mohammad, S., Thiemermann, C. (2021). Role of metabolic endotoxemia in systemic inflammation and potential interventions. Front. Immunol. 11. doi: 10.3389/fimmu.2020.594150
Mojsak, P., Miniewska, K., Godlewski, A., Adamska-Patruno, E., Samczuk, P., Rey-Stolle, F., et al. (2021). A Preliminary Study Showing the Impact of Genetic and Dietary Factors on GC-MS-Based Plasma Metabolome of Patients with and without PROX1-Genetic Predisposition to T2DM up to 5 Years Prior to Prediabetes Appearance. Curr. Issues Mol. Biol. 43, 513–528. doi: 10.3390/cimb43020039
Moreira, L. A. A., da Paz Lima, L., de Oliveira Falcão, M. A., Rosado, E. L. (2023). Profile of gut microbiota of adults with diabetes mellitus type 1: A systematic review. Curr. Diabetes Rev. 19, e280322202706. doi: 10.2174/1573399818666220328150044
Ng, S. C., Xu, Z., Mak, J. W. Y., Yang, K., Liu, Q., Zuo, T., et al. (2022). Microbiota engraftment after fecal microbiota transplantation in obese subjects with type 2 diabetes: a 24-week, double-blind, randomized controlled trial. Gut 71, 716–723. doi: 10.1136/gutjnl-2020-323617
Okubo, H., Nakatsu, Y., Kushiyama, A., Yamamotoya, T., Matsunaga, Y., Inoue, M. K., et al. (2018). Gut microbiota as a therapeutic target for metabolic disorders. Curr. medicinal Chem. 25, 984–1001. doi: 10.2174/0929867324666171009121702
Palmnäs-Bédard, M. S. A., Costabile, G., Vetrani, C., Åberg, S., Hjalmarsson, Y., Dicksved, J., et al. (2022). The human gut microbiota and glucose metabolism: a scoping review of key bacteria and the potential role of SCFAs. Am. J. Clin. Nutr. 116, 862–874. doi: 10.1093/ajcn/nqac217
Pan, Y., Bu, T., Deng, X., Jia, J., Yuan, G. (2024). Gut microbiota and type 2 diabetes mellitus: a focus on the gut-brain axis. Endocrine 84 (1), 1–15. doi: 10.1007/s12020-023-03640-z
Porcari, S., Benech, N., Valles-Colomer, M., Segata, N., Gasbarrini, A., Cammarota, G., et al. (2023). Key determinants of success in fecal microbiota transplantation: From microbiome to clinic. Cell Host Microbe 31, 712–733. doi: 10.1016/j.chom.2023.03.020
Portincasa, P., Bonfrate, L., Vacca, M., De Angelis, M., Farella, I., Lanza, E., et al. (2022). Gut microbiota and short chain fatty acids: Implications in glucose homeostasis. Int. J. Mol. Sci. 23, 1105. doi: 10.3390/ijms23031105
Qi, Q., Li, J., Yu, B., Moon, J. Y., Chai, J. C., Merino, J., et al. (2022). Host and gut microbial tryptophan metabolism and type 2 diabetes: an integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut 71, 1095–1105. doi: 10.1136/gutjnl-2021-324053
Qin, S., Wang, Y., Wang, S., Ning, B., Huai, J., Yang, H. (2022). Gut microbiota in women with gestational diabetes mellitus has potential impact on metabolism in pregnant mice and their offspring. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.870422
Quraishi, M. N., Widlak, M., Bhala, N., Moore, D., Price, M., Sharma, N., et al. (2017). Systematic review with meta-analysis: the efficacy of fecal microbiota transplantation for the treatment of recurrent and refractory Clostridium difficile infection. Alimentary Pharmacol. Ther. 46, 479–493. doi: 10.1111/apt.14201
Roy, R., Nguyen-Ngo, C., Lappas, M. (2020). Short-chain fatty acids as novel therapeutics for gestational diabetes. J. Mol. Endocrinol. 65, 21–34. doi: 10.1530/JME-20-0094
Salamone, D., Rivellese, A. A., Vetrani, C. (2021). The relationship between gut microbiota, short-chain fatty acids and type 2 diabetes mellitus: the possible role of dietary fibre. Acta diabetologica 58, 1131–1138. doi: 10.1007/s00592-021-01727-5
Schloissnig, S., Arumugam, M., Sunagawa, S., Mitreva, M., Tap, J., Zhu, A., et al. (2013). Genomic variation landscape of the human gut microbiome. Nature 493, 45–50. doi: 10.1038/nature11711
Siljander, H., Honkanen, J., Knip, M. (2019). Microbiome and type 1 diabetes. EBioMedicine 46, 512–521. doi: 10.1016/j.ebiom.2019.06.031
Simon, M. C., Reinbeck, A. L., Wessel, C., Heindirk, J., Jelenik, T., Kaul, K., et al. (2020). Distinct alterations of gut morphology and microbiota characterize accelerated diabetes onset in nonobese diabetic mice. J. Biol. Chem. 295, 969–980. doi: 10.1016/S0021-9258(17)49908-X
Slouha, E., Rezazadah, A., Farahbod, K., Gerts, A., Clunes, L. A., Kollias, T. F. (2023). Type-2 diabetes mellitus and the gut microbiota: Systematic review. Cureus 15, e49740. doi: 10.7759/cureus.49740
Solar, I., Ribeiro, F. B., Barbosa, M. G., de Oliveira Nascimento Freitas, R. G. B., Hanada, A. S., de Oliveira Ramos, C., et al. (2023). Short-chain fatty acids are associated with adiposity, energy and glucose homeostasis among different metabolic phenotypes in the Nutritionists' Health Study. Endocrine 80, 529–540. doi: 10.1007/s12020-023-03356-0
Song, Z., Yan, A., Guo, Z., Zhang, Y., Wen, T., Li, Z., et al. (2023). Targeting metabolic pathways: a novel therapeutic direction for type 2 diabetes. Front. Cell. infection Microbiol. 13. doi: 10.3389/fcimb.2023.1218326
Su, L., Hong, Z., Zhou, T., Jian, Y., Xu, M., Zhang, X., et al. (2022). Health improvements of type 2 diabetic patients through diet and diet plus fecal microbiota transplantation. Sci. Rep. 12, 1152. doi: 10.1038/s41598-022-05127-9
Su, M., Nie, Y., Shao, R., Duan, S., Jiang, Y., Wang, M., et al. (2018). Diversified gut microbiota in newborns of mothers with gestational diabetes mellitus. PLoS One 13, e0205695. doi: 10.1371/journal.pone.0205695
Sun, Z., Pan, X. F., Li, X., Jiang, L., Hu, P., Wang, Y., et al. (2023). The gut microbiome dynamically associates with host glucose metabolism throughout pregnancy: Longitudinal findings from a matched case-control study of gestational diabetes mellitus. Advanced Sci. (Weinheim Baden-Wurttemberg Germany) 10, e2205289. doi: 10.1002/advs.202205289
Syed, F. Z. (2022). Type 1 diabetes mellitus. Ann. Internal Med. 175, ITC33–ITC48. doi: 10.7326/AITC202203150
Tian, C. Y., Yang, Q. H., Lv, H. Z., Yue, F., Zhou, F. F. (2024). Combined untargeted and targeted lipidomics approaches reveal potential biomarkers in type 2 diabetes mellitus cynomolgus monkeys. J. Med. primatology 53, e12688. doi: 10.1111/jmp.12688
Traussnigg, S., Halilbasic, E., Hofer, H., Munda, P., Stojakovic, T., Fauler, G., et al. (2021). Open-label phase II study evaluating safety and efficacy of the non-steroidal farnesoid X receptor agonist PX-104 in non-alcoholic fatty liver disease. Wiener klinische Wochenschrift 133, 441–451. doi: 10.1007/s00508-020-01735-5
Tuomainen, M., Lindström, J., Lehtonen, M., Auriola, S., Pihlajamäki, J., Peltonen, M., et al. (2018). Associations of serum indolepropionic acid, a gut microbiota metabolite, with type 2 diabetes and low-grade inflammation in high-risk individuals. Nutr. Diabetes 8, 35. doi: 10.1038/s41387-018-0046-9
Vangipurapu, J., Fernandes Silva, L., Kuulasmaa, T., Smith, U., Laakso, M. (2020). Microbiota-related metabolites and the risk of type 2 diabetes. Diabetes Care 43, 1319–1325. doi: 10.2337/dc19-2533
Velasquez, M. T. (2018). Altered gut microbiota: A link between diet and the metabolic syndrome. Metab. syndrome related Disord. 16, 321–328. doi: 10.1089/met.2017.0163
Wang, X. S., Hu, M. X., Guan, Q. X., Men, L. H., Liu, Z. Y. (2022). Metabolomics analysis reveals the renal protective effect of Panax ginseng C. A. Mey in type 1 diabetic rats. Chin. J. Natural Medicines 20, 378–386. doi: 10.1016/S1875-5364(22)60175-4
Wang, Q., Lin, H., Shen, C., Zhang, M., Wang, X., Yuan, M., et al. (2023). Gut microbiota regulates postprandial GLP-1 response via ileal bile acid-TGR5 signaling. Gut Microbes 15, 2274124. doi: 10.1080/19490976.2023.2274124
Wang, H., Lu, Y., Yan, Y., Tian, S., Zheng, D., Leng, D., et al. (2020). Promising treatment for type 2 diabetes: Fecal microbiota transplantation reverses insulin resistance and impaired islets. Front. Cell. infection Microbiol. 9. doi: 10.3389/fcimb.2019.00455
Wang, C., Wang, Y., Yang, H., Tian, Z., Zhu, M., Sha, X., et al. (2022). Uygur type 2 diabetes patient fecal microbiota transplantation disrupts blood glucose and bile acid levels by changing the ability of the intestinal flora to metabolize bile acids in C57BL/6 mice. BMC endocrine Disord. 22, 236. doi: 10.1186/s12902-022-01155-8
Wang, Y., Yang, Z., Tang, H., Sun, X., Qu, J., Lu, S., et al. (2022a). Fecal microbiota transplantation is better than probiotics for tissue regeneration of type 2 diabetes mellitus injuries in mice. Arch. Physiol. Biochem., 1–9. doi: 10.1080/13813455.2022.2080229
Wang, Y., Ye, X., Ding, D., Lu, Y. (2020). Characteristics of the intestinal flora in patients with peripheral neuropathy associated with type 2 diabetes. J. Int. Med. Res. 48, 300060520936806. doi: 10.1177/0300060520936806
Wang, Y., Zhang, S., Borody, T. J., Zhang, F. (2022b). Encyclopedia of fecal microbiota transplantation: a review of effectiveness in the treatment of 85 diseases. Chin. Med. J. 135, 1927–1939. doi: 10.1097/CM9.0000000000002339
Wang, J., Zhu, N., Su, X., Gao, Y., Yang, R. (2023). Gut-microbiota-derived metabolites maintain gut and systemic immune homeostasis. Cells 12, 793. doi: 10.3390/cells12050793
Wu, J., Wang, K., Wang, X., Pang, Y., Jiang, C. (2021). The role of the gut microbiome and its metabolites in metabolic diseases. Protein Cell 12, 360–373. doi: 10.1007/s13238-020-00814-7
Wu, J., Yang, K., Fan, H., Wei, M., Xiong, Q. (2023). Targeting the gut microbiota and its metabolites for type 2 diabetes mellitus. Front. Endocrinol. 14. doi: 10.3389/fendo.2023.1114424
Wu, Z., Zhang, B., Chen, F., Xia, R., Zhu, D., Chen, B., et al. (2023). Fecal microbiota transplantation reverses insulin resistance in type 2 diabetes: A randomized, controlled, prospective study. Front. Cell. infection Microbiol. 12. doi: 10.3389/fcimb.2022.1089991
Xia, F., Wen, L. P., Ge, B. C., Li, Y. X., Li, F. P., Zhou, B. J. (2021). Gut microbiota as a target for prevention and treatment of type 2 diabetes: Mechanisms and dietary natural products. World J. Diabetes 12, 1146–1163. doi: 10.4239/wjd.v12.i8.1146
Xie, Y. C., Jing, X. B., Chen, X., Chen, L. Z., Zhang, S. H., Cai, X. B. (2022). Fecal microbiota transplantation treatment for type 1 diabetes mellitus with malnutrition: a case report. Ther. Adv. chronic Dis. 13, 20406223221117449. doi: 10.1177/20406223221117449
Yakar, B., Onalan, E., Kaymaz, T., Donder, E., Gursu, M. F. (2022). The role of trimethylamine-N-oxide level in the diagnosis of diabetic retinopathy and the differential diagnosis of diabetic and nondiabetic retinopathy. Arquivos brasileiros oftalmologia 87, 527. doi: 10.5935/0004-2749.2021-0527
Yan, F., Li, N., Shi, J., Li, H., Yue, Y., Jiao, W., et al. (2019). Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 10, 5804–5815. doi: 10.1039/C9FO01062A
Yang, R., Chen, Z., Cai, J. (2023). Fecal microbiota transplantation: Emerging applications in autoimmune diseases. J. Autoimmun. 141, 103038. doi: 10.1016/j.jaut.2023.103038
Yang, W., Xia, Z., Zhu, Y., Tang, H., Xu, H., Hu, X., et al. (2023). Comprehensive study of untargeted metabolomics and 16S rRNA reveals the mechanism of fecal microbiota transplantation in improving a mouse model of T2D. Diabetes Metab. syndrome Obes. Targets Ther. 16, 1367–1381. doi: 10.2147/DMSO.S404352
Yang, J., Yang, X., Wu, G., Huang, F., Shi, X., Wei, W., et al. (2023). Gut microbiota modulate distal symmetric polyneuropathy in patients with diabetes. Cell Metab. 35, 1548–1562.e7. doi: 10.1016/j.cmet.2023.06.010
Ye, D., Huang, J., Wu, J., Xie, K., Gao, X., Yan, K., et al. (2023). Integrative metagenomic and metabolomic analyses reveal gut microbiota-derived multiple hits connected to development of gestational diabetes mellitus in humans. Gut Microbes 15, 2154552. doi: 10.1080/19490976.2022.2154552
Youngster, I., Sauk, J., Pindar, C., Wilson, R. G., Kaplan, J. L., Smith, M. B., et al. (2014). Fecal microbiota transplant for relapsing Clostridium difficile infection using a frozen inoculum from unrelated donors: a randomized, open-label, controlled pilot study. Clin. Infect. Dis. an Off. Publ. Infect. Dis. Soc. America 58, 1515–1522. doi: 10.1093/cid/ciu135
Yu, P. S., Wu, P. H., Hung, W. W., Lin, M. Y., Zhen, Y. Y., Hung, W. C., et al. (2024). Association between trimethylamine N-oxide and adverse kidney outcomes and overall mortality in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab., dgae009. doi: 10.1210/clinem/dgae009
Yuan, Y., Gan, C., Wang, M., Zou, J., Wang, Z., Li, S., et al. (2024). Association of serum trimethylamine N-oxide levels and bone mineral density in type 2 diabetes mellitus. Endocrine. doi: 10.1007/s12020-024-03699-2
Yuan, X., Wang, R., Han, B., Sun, C., Chen, R., Wei, H., et al. (2022). Functional and metabolic alterations of gut microbiota in children with new-onset type 1 diabetes. Nat. Commun. 13, 6356. doi: 10.1038/s41467-022-33656-4
Zanoni, I., Granucci, F. (2013). Role of CD14 in host protection against infections and in metabolism regulation. Front. Cell. infection Microbiol. 3. doi: 10.3389/fcimb.2013.00032
Zhang, S., Deng, F., Chen, J., Chen, F., Wu, Z., Li, L., et al. (2022). Fecal microbiota transplantation treatment of autoimmune-mediated type 1 diabetes: A systematic review. Front. Cell. infection Microbiol. 12. doi: 10.3389/fcimb.2022.1075201
Zhang, D., Jian, Y. P., Zhang, Y. N., Li, Y., Gu, L. T., Sun, H. H., et al. (2023). Short-chain fatty acids in diseases. Cell communication Signaling CCS 21, 212. doi: 10.1186/s12964-023-01219-9
Zhang, P. P., Li, L. L., Han, X., Li, Q. W., Zhang, X. H., Liu, J. J., et al. (2020). Fecal microbiota transplantation improves metabolism and gut microbiome composition in db/db mice. Acta pharmacologica Sin. 41, 678–685. doi: 10.1038/s41401-019-0330-9
Zhang, Y., Liang, X., Bao, X., Xiao, W., Chen, G. (2022). Toll-like receptor 4 (TLR4) inhibitors: Current research and prospective. Eur. J. medicinal Chem. 235, 114291. doi: 10.1016/j.ejmech.2022.114291
Zhang, H., Qi, C., Zhao, Y., Lu, M., Li, X., Zhou, J., et al. (2021). Depletion of gut secretory immunoglobulin A coated Lactobacillus reuteri is associated with gestational diabetes mellitus-related intestinal mucosal barrier damage. Food Funct. 12, 10783–10794. doi: 10.1039/D1FO02517A
Zhang, Y. N., Wang, S. M., Yu, X., Zhang, W. D., Wang, X. Y., Yang, R. Y., et al. (2021). Zhonghua xin xue guan bing za zhi. Zhonghua Xue Ye Xue Za Zhi 49 (7), 680–686. doi: 10.3760/cma.j.cn112148-20200902-00696
Zhang, L., Wang, Z., Zhang, X., Zhao, L., Chu, J., Li, H., et al. (2022). Alterations of the gut microbiota in patients with diabetic nephropathy. Microbiol. Spectr. 10, e0032422. doi: 10.1128/spectrum.00324-22
Zhang, L., Zhou, W., Zhan, L., Hou, S., Zhao, C., Bi, T., et al. (2020). Fecal microbiota transplantation alters the susceptibility of obese rats to type 2 diabetes mellitus. Aging 12, 17480–17502. doi: 10.18632/aging.v12i17
Zhao, L., Lou, H., Peng, Y., Chen, S., Fan, L., Li, X. (2020). Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res. Clin. Pract. 169, 108418. doi: 10.1016/j.diabres.2020.108418
Zheng, L., Ji, Y. Y., Wen, X. L., Duan, S. L. (2022). Fecal microbiota transplantation in the metabolic diseases: Current status and perspectives. World J. Gastroenterol. 28, 2546–2560. doi: 10.3748/wjg.v28.i23.2546
Zheng, S. J., Luo, Y., Xiao, J. H. (2022). The impact of intestinal microorganisms and their metabolites on type 1 diabetes mellitus. Diabetes Metab. syndrome Obes. Targets Ther. 15, 1123–1139. doi: 10.2147/DMSO.S355749
Zhong, V. W., Yu, D., Zhao, L., Yang, Y., Li, X., Li, Y., et al. (2023). Achievement of guideline-recommended targets in diabetes care in China : A nationwide cross-sectional study. Ann. Internal Med. 176, 1037–1046. doi: 10.7326/M23-0442
Zhou, Y., Li, Y. Y., Liu, Y. (2022). Zhonghua yi xue za zhi. Zhonghua Xue Ye Xue Za Zhi 102, 16, 1224–1231. doi: 10.3760/cma.j.cn112137-20210907-02043
Zhou, Z., Sun, B., Yu, D., Zhu, C. (2022). Gut microbiota: An important player in type 2 diabetes mellitus. Front. Cell. infection Microbiol. 12. doi: 10.3389/fcimb.2022.834485
Zhou, B., Yuan, Y., Zhang, S., Guo, C., Li, X., Li, G., et al. (2020). Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front. Immunol. 11. doi: 10.3389/fimmu.2020.00575
Keywords: fecal microbiota transplantation, diabetes mellitus, diabetes complications, gastrointestinal microbiome, metabolite, metabolic disease
Citation: Zhang J, Wang H, Liu Y, Shi M, Zhang M, Zhang H and Chen J (2024) Advances in fecal microbiota transplantation for the treatment of diabetes mellitus. Front. Cell. Infect. Microbiol. 14:1370999. doi: 10.3389/fcimb.2024.1370999
Received: 22 January 2024; Accepted: 27 March 2024;
Published: 10 April 2024.
Edited by:
Valeriy Poroyko, Laboratory Corporation of America Holdings (LabCorp), United StatesReviewed by:
Sidharth Prasad Mishra, University of South Florida, United StatesBowen Li, Jiangnan University, China
Copyright © 2024 Zhang, Wang, Liu, Shi, Zhang, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Chen, Y2hlbmp1YW4yMDE2MDQxOEAxNjMuY29t; Hong Zhang, emhoNzkzMThAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Juan Zhang
Juan Zhang Honggang Wang
Honggang Wang Ying Liu1†
Ying Liu1† Minna Zhang
Minna Zhang Juan Chen
Juan Chen