
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Cell. Infect. Microbiol. , 01 March 2024
Sec. Biofilms
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1367233
This article is part of the Research Topic Vaginal Dysbiosis and Biofilms, volume II View all 12 articles
Bacterial vaginosis (BV) is an infection of the genital tract characterized by disturbance of the normally Lactobacilli-dominated vaginal flora due to the overgrowth of Gardnerella and other anaerobic bacteria. Gardnerella vaginalis, an anaerobic pathogen and the major pathogen of BV, produces sialidases that cleave terminal sialic acid residues off of human glycans. By desialylation, sialidases not only alter the function of sialic acid-containing glycoconjugates but also play a vital role in the attachment, colonization and spread of many other vaginal pathogens. With known pathogenic effects, excellent performance of sialidase-based diagnostic tests, and promising therapeutic potentials of sialidase inhibitors, sialidases could be used as a biomarker of BV. This review explores the sources of sialidases and their role in vaginal dysbiosis, in aims to better understand their participation in the pathogenesis of BV and their value in the diagnosis and treatment of BV.
Bacterial vaginosis (BV) is caused by a disturbance to the vaginal flora in which Gardnerella and other anaerobic bacteria replace the normal vaginal microbiota dominated by lactobacilli (Ravel et al., 2011). Lactic acid, H2O2, bacteriocins, and biosurfactants, which are antimicrobial and anti-inflammatory products produced by lactobacilli, decreases along with the health-promoting lactobacilli. The increased pH of the vagina creates advantages for the proliferation of facultative and obligate anaerobes, including Gardnerella, Atopobium, Mobiluncus, Prevotella, Streptococcus, Ureaplasma, Megasphaera etc (Amabebe and Anumba, 2018). Meanwhile, the concentrations of short chain fatty acids (SCFAs, such as acetate, malonate and succinate) and amines (such as putrescine, cadaverine, and tyramine) produced by the overgrown anaerobes increase in parallel with bacterial abundance and species biodiversity (Srinivasan et al., 2015; Vitali et al., 2015). A recent study found that a combination of vaginal microbiota metabolites representing BV increased basal and toll-like receptor (TLR) -induced production of TNF-α, demonstrating their immune regulatory effects (Delgado-Diaz et al., 2020).
As a major obstetrical and gynecological concern, BV is associated with many negative health outcomes, such as infertility (Ravel et al., 2021), preterm delivery (Honest et al., 2004; Cauci and Culhane, 2011; Manns-James, 2011), pelvic inflammatory disease (Taylor et al., 2013; Ravel et al., 2021), and sexually transmitted infections (Bautista et al., 2016; Armstrong and Kaul, 2021). Pathogenesis of BV involves degradation of the mucus layer on the surface of vaginal epithelium, exfoliation and detachment of the epithelial cells (Cauci et al., 2003), which in turn facilitates bacterial adhesion and biofilm formation (Swidsinski et al., 2005; Varki, 2009; Varki and Gagneux, 2012). Sialidases play a key role in the processes mentioned above, making sialidase activity measurement useful in the diagnosis and management of BV (Javed et al., 2019; Mabugana et al., 2023). Of course, mucus degradation is such a complex process that there are other glycosidases, proteases, and sulphatases involved in (Wiggins, 2001). For example, prolidase is a kind of proteolytic enzymes associated with BV, which shows a negative association with interleukin (IL)–8 levels in female CVF (Cauci et al., 2002) and can predict low birth weight and preterm birth with combination of vaginal pH and vaginal sialidase (Cauci et al., 2005).
As a major virulence factor of Gardnerella spp (Schellenberg et al., 2016; Kurukulasuriya et al., 2021), sialidases are important glycoside hydrolases that cleave sialic acid residues off of terminal glycans (Lewis et al., 2013; Robinson et al., 2019). Sialic acids are 9-carbon monosaccharides found in glycoconjugates such as glycoproteins and glycolipids, as well as at the distal end of N- and O-linked carbohydrate chains, also named glycans (Ghosh, 2020). As a part of glycoconjugates and substrates of sialidases, glycans have been found in human cervicovaginal fluid (CVF) (Moncla et al., 2015; Moncla et al., 2016; Wang et al., 2015) and surface of vaginal epithelial cells (Agarwal et al., 2023). Glycans heavily coat the surface of mammalian epithelial cells (Ochs et al., 2020; Argüeso et al., 2021), making them the frequent primary point of interaction between microorganisms and mucosal barriers (Poole et al., 2018). Through hydrolysis of sialic acids, which are highly electronegative carbohydrates, sialidases participate in many physiological and pathological pathways by lowering the surface charge of the whole cell, exposing glycoconjugates’ binding sites, changing the conformation of the glycoproteins, and eventually altering the functions of sialic acid-containing glycoconjugates (Pshezhetsky and Ashmarina, 2013).
Sialic acids support the defense barriers through a delicate balance between sialylation and desialylation (Cohen and Varki, 2010; Cao and Chen, 2012). Sialylation, mediated by sialyltransferases, is the addition of sialic acids to the end of oligosaccharides and glycoproteins, while desialylation, mediated by sialidases, is the removal of sialic acids. Sialoglycoproteins, composed of glycoproteins and sialic acids, are important defense components of the mucosal surface that create a physical barrier against pathogens (Lewis and Lewis, 2012). With a weight percentage of almost 16% sialic acids, mucins provide a dense physical barrier that disrupt the interactions between pathogens and epithelial cells (Slomiany et al., 1996; Moran et al., 2011). Moreover, sialylation also plays a role in immune response by altering the functions of immunoglobulins and regulating inflammation (Yoo and Morrison, 2005; Anthony and Ravetch, 2010).
Sialidases, also known as neuraminidases, have been detected in CVF and elevated level of sialidase activity is associated with BV (Briselden et al., 1992; Myziuk et al., 2003). In a 1992 study, women with BV had higher levels of sialidase activity in their vaginal secretions than those without (Briselden et al., 1992). Over the next three decades, many more studies produced similar results (Howe et al., 1999; Smayevsky et al., 2001; Cauci et al., 2003; Lewis et al., 2012). A recent study suggests that women with BV have higher sialic acid depletion and lower levels of sialylation (Agarwal et al., 2023), which could be explained by elevated sialidase activity as sialylation breaks down and depletes sialoglycans. Another study also detected roughly 3-fold lower amounts of total sialic acids and 3.5-fold greater amounts of free sialic acids in BV samples compared with normal samples using high-performance liquid chromatography (HPLC) (Lewis et al., 2012). However, the exact mechanism of sialidases causing BV is not fully understood, as the current understanding of the roles of vaginal epithelial glycans is still limited.
Besides BV, sialidases are involved in a broad spectrum of diseases within the human body as they can be produced by not only bacteria but also viruses, mammals, and protozoa. Bacterial sialidases also participate in host-bacteria interactions, coinfections, and dysbiosis in oral cavity, gastrointestinal tract and respiratory system (Siegel et al., 2014; Huang et al., 2015; Wong et al., 2018). Influenza A and B viruses can also produce sialidases, which in turn facilitates the development of influenza (Zambon, 2001). In mammals, sialidases are involved in a wide range of health issues, including cancers (SoÈnmez et al., 1999; Zhou et al., 2020), diabetes (Natori et al., 2013), neurodegenerative disorders (Liao et al., 2020; Khan et al., 2021), fibrosing diseases (Karhadkar et al., 2022) and heart diseases (Zhang et al., 2018; Chen et al., 2021).
As the catalytic activity of sialidases is essential to the colonization and dissemination of several pathogenic microorganisms, sialidases could be used as a promising diagnostic marker for BV (Briselden et al., 1992; Smayevsky et al., 2001). This article aims to review relevant literature to explore the characteristics of sialidases in CVF, their contributions to vaginal dysbiosis, and their clinical use in BV diagnosis and treatment.
So far research has reported in vitro sialidase activity in some BV-associated bacteria (BVAB), such as isolates of Prevotella, Bacteroides, and Gardnerella (Briselden et al., 1992). Studies have illustrated the ability to produce sialidases by every strain of Prevotella bivia, while only some G.vaginalis isolates produce sialidases (Briselden et al., 1992; Lopes Dos Santos Santiago et al., 2011). However, G.vaginalis is able to produce higher levels of sialidases, demonstrated in a study of C57BL/6 mouse models where Prevotella models showed similar levels of sialidase activities with G.vaginalis in a 100 times infection titer compared to Gardnerella-colonized models (Gilbert et al., 2019). Apart from the abundance of bacteria themselves, other factors, such as sialidase expression levels, individual heterogeneity, and interactions between bacteria, might also affect sialidase activity in the CVF. Furthermore, sialidase produced by possible viruses and the host should be taken into consideration though there are few studies about this.
Among genotypes of G.vaginalis, the expression levels of sialidases are highly heterogeneous (Schellenberg et al., 2016). Based on quantitative polymerase chain reaction (qPCR) targeting clade-specific genes, Gardnerella is divided into four clades, clade 1 (encoding putative a-L-fucosidase), clade 2 (encoding a hypothetical protein), clade 3 (encoding thioredoxin) and clade 4 (encoding CIC family chloride transporter) (Balashov et al., 2014). They are different in sialidase activity: clade 2 have the highest activity followed by clade 1, clade3, and clade 4 (Qin and Xiao, 2022). In clade 4, the proposed sialidase encoding gene sialidase A gene is not detected (Shipitsyna et al., 2019).
Three sialidase homologs, NanH1 (also known as sialidase A), NanH2, and NanH3, have been identified in G.vaginalis (Janulaitiene et al., 2018; Robinson et al., 2019). Sialidase activity in G.vaginalis was initially thought to derive from sialidase encoding gene nanH1 (Lopes Dos Santos Santiago et al., 2011), while a more recent study concludes that nanH2 and nanH3 are the primary sources of sialidase activity in G.vaginalis (Robinson et al., 2019). Schellenberg et al (Schellenberg et al., 2016). found that using a filter spot assay, the presence of nanH1 was not indicator of sialidase activity: only 36 of the 77 G.vaginalis isolates that tested positive for nanH1 actually produced sialidases. Meanwhile in another test done by polymerase chain reaction (PCR), sialidase activity in a collection of 34 isolated G.vaginalis strains was consistent with the detection of nanH2 or nanH3 (Robinson et al., 2019). The main functional distinction between NanH2 and NanH3 is that, NanH2 cleaves 9-O-acetylated sialic acid substrates far more efficiently than NanH3, either in vitro or in vivo (Robinson et al., 2019). In addition, nanH3 is more commonly present than nanH2 (Cauci et al., 2003). These results suggest that NanH2 and NanH3 are more likely to be the primary sources of sialidase activity in G.vaginalis in human CVF, whereas NanH1 contributes little.
Studies propose that the absence of sialidase activity by nanH1 could be due to transcriptional regulation (Janulaitiene et al., 2018) and a lack of signal sequence, suggesting an intracellular localization of nanH1 (Kurukulasuriya et al., 2021). However, limited evidence supports these hypotheses. Additionally, elevated nanH1 gene levels have been found to be associated with both high-risk human papillomavirus (HPV) (Di Paola et al., 2017) and BV (Hardy et al., 2017). Thus, more research is needed to better understand the roles of the sialidase encoding genes besides sialidases expression.
The host mucosal defense barrier, which is important in the identification, integration, and elimination of pathogens, can be destroyed by desialylation of glycoconjugates such as mucins, cellular receptors, and immunoglobulins, which in turn facilitates bacterial adherence, colonization, invasion, and tissue breakdown (Briselden et al., 1992; Cauci et al., 2003, 1998; Cauci and Culhane, 2011). Sialidases’ participation in the pathogenesis of G.vaginalis and BV is discussed below (Figure 1).
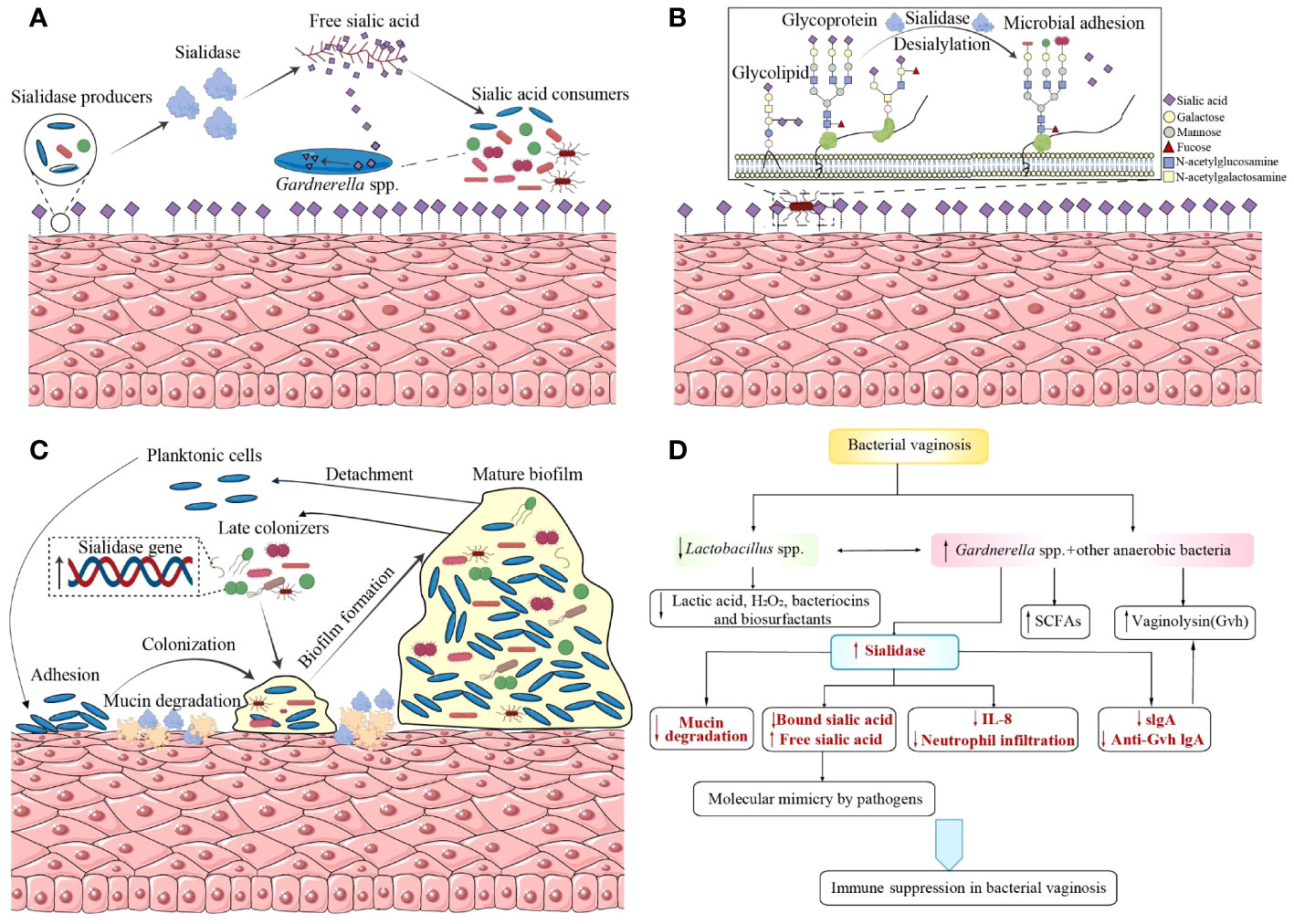
Figure 1 Sialidases’ participation in the pathogenesis of Gardnerella vaginalis and bacterial vaginosis. (A) Sialidase producers catalyze sialic acids from glycoconjugates as nutrition source for sialic acid consumers. (B) Desialylation of glycoconjugates by sialidases exposes new glycan epitopes for bacterial recognition and adhesion. (C) G.vaginalis and BVAB bacteria establish synergistic interactions based on sialidases during the formation of a polymicrobial biofilm. (D) Sialidases participate in the immune regulation of BV, supported by other hydrolytic enzymes, virulence, and immunomodulatory metabolites. BVAB, bacterial vaginosis-associated bacteria; SCFAs, short chain fatty acids; Gvh, Gardnerella vaginalis hemolysin; IL, interleukin; Anti-Gvh IgA, immunoglobulin A against Gardnerella vaginalis hemolysin.
Bacteria can use free sialic acids, a hydrolysate of glycoconjugates catalyzed by sialidases, as a source of carbon for their nutrition and colonization (Figure 1A) (Lewis et al., 2013; Agarwal et al., 2020; Agarwal and Lewis, 2021). Evidence from mouse models shows that free sialic acids released by sialidases promote the growth of group B Streptococcus and the spread of ascending vaginal tract infections (Pezzicoli et al., 2012; Gilbert et al., 2013). Bacteria lacking sialidase encoding genes can also benefit from sialoglycan in the vagina via sialidase producers such as G.vaginalis (Agarwal et al., 2020). Some bacteria, such as Fusobacterium nucleatum (Haines-Menges et al., 2015; Agarwal et al., 2020) and group B Streptococcus (Pezzicoli et al., 2012), have sialic acid transport or catabolic pathways despite being sialidase-negative themselves. Moreover, F.nucleatum can reinforce sialidase activity produced by G.vaginalis in both ex vivo and in vitro coculture studies. G.vaginalis titers exhibit a dose-dependent increase with higher inocula of F.nucleatum or increasing proportions of its cell-free supernatant in an in vitro coculture system of F.nucleatum and G.vaginalis, in which G.vaginalis could not survive itself. This suggests that F.nucleatum may secrete factors to facilitate G.vaginalis growth. Additionally, in comparison to cocultures with F.nucleatum, monocultures of G.vaginalis needed at least a 20,000-fold greater inoculum to be viable after an overnight incubation (Agarwal et al., 2020). Therefore, F.nucleatum and G.vaginalis form a mutually beneficial relationship based on their glycan cross-feeding mode, which promotes their colonization and contributes to vaginal dysbiosis.
There have also been reports of the cross-feeding between commensal bacteria in the gut. For example, Bifidobacterium breve UCC2003, which contains a functional Nan cluster for sialic consumption, can use the sialic acid produced by Bifidobacterium bifidum PRL201048 (Egan et al., 2014). Similarly, in the oral cavity, Streptococcus gordonii employs sialic acids as their only carbon source (Byers et al., 1996). During the coinfection of influenza and Streptococcus pneumoniae in the respiratory tract, sialic acids produced by influenza accelerate bacterial replication in vivo and stimulate pneumococcal proliferation (Siegel et al., 2014).
Sialidases can also promote infections by damaging the protective physical and biochemical barriers against pathogens through exposure of receptor binding sites for adhesins and toxins. In the oral cavity, adhesion of S.gordonii to oral epithelial cells is greatly increased by the presence of Streptococcus oralis in a sialidase-dependent manner through exposure of cryptic receptors binding sites (Beighton and Whiley, 1990; Wong et al., 2018).
Sialic acids are typically found at the terminal position of glycans. They can shield the underlying sugars (mostly galactose residues) from recognition, and then breakdown or adherence. Sialidases in the vagina may reveal glycan epitopes by the depletion of sialic acids and the exposure of underlying sugars to the surface (Figure 1B). In both N- and O-linked glycans, sialic acids cap Gal residues bound to N-acetylglucosamine (GlcNAc) or N-acetylgalactosamine (GalNAc), which is not accessible on the epithelial surface unless treated with exogenous sialidases or using cells from BV-positive specimens (Agarwal et al., 2023). Desialylation of glycoconjugates by sialidases causes loss of or reveal of new glycan epitopes, affecting microbe binding and host immunological recognition (Varki and Gagneux, 2012). Bacterial adhesion occurs when terminal sugars are exposed with the degradation of glycans, in which process carbohydrate-binding proteins like lectins, previously predicted in Gardnerella, serve as mediums (Bonnardel et al., 2021). According to genome screening, a greater repertory of carbohydrate-binding proteins is produced by vaginal bacterial species that are linked to infection and inflammation, which may allow them to bind a greater variety of glycans in the vagina. Compared with commensals like Lactobacillus crispatus, the mean number of lectins per strain is approximately 2-fold higher among those regarded as potential and confirmed pathogens (including Lactobacillus iners, G.vaginalis, Prevotella, group B Streptococcus, and Escherichia coli) (Bonnardel et al., 2021). With the deepening of research on the surface polysaccharide structure of the vagina and the bacterial carbohydrate-binding proteins, comprehensive insights into host–microbe interactions will be reached.
A biofilm is an organized community of microorganisms encased in a extracellular matrix made of proteins, polysaccharides, and nucleic acids, that attaches to a biological surface (Flemming et al., 2016; Jung et al., 2017) and contributes to the survival of bacterial infections (Del Pozo, 2018). Vaginal biofilms contribute to the persistence and recurrence of BV, as well as antibiotic resistance (Swidsinski et al., 2008; He et al., 2021). According to a recent research, 11 of the 24 G.vaginalis strains were able to form biofilms, providing themselves with advantages to evade host defense mechanisms and survive against antibiotics (Ma et al., 2022). An in vitro study suggests that most of the BVAB have a tendency to grow biofilms, and G.vaginalis has greater propensity to form a biofilm, enhancing its virulence potential through increased adhesion and cytotoxicity of epithelial cells compared to other anaerobes (Alves et al., 2014).
The lifecycle of biofilm formation is considered to include several stages: (i) adhesion to a surface, (ii) production of extracellular matrix, bacterial aggregation and biofilm accumulation until the development of a mature biofilm structure, and finally (iii) detachment (Joo and Otto, 2012). The initial adherence to vaginal epithelial cells has been acknowledged to be a necessary process to elicit BV (Swidsinski et al., 2005). As a dominant component of BV biofilm, Gardnerella spp. replaces pre-dominant L.crispatus, initiate bacterial colonization on vaginal epithelium and then serve as a scaffold for the attachment of other BVAB, including Atopobium vaginae (found in 80% of the samples and compromises 40% of the biofilm mass) and other heterogeneously mixed bacteria belonging to the Bacteroides, Corynebacterium, Lactobacillus, Staphylococcus, Streptococcus genera and so on (Castro et al., 2015; Castro et al., 2019; Swidsinski et al., 2005; Verstraelen and Swidsinski, 2013; Schwebke et al., 2014). The process is known as coaggregation (Figure 1C). Sialidases serve as a trigger at this initial stage of colonization. By means of its mucinase activity, the enzymes alter the characteristics of mucus discharges, catalyze them as a meal for bacteria and expose adhesion receptors on polysaccharides to promote bacterial colonization, increasing the potential for G.vaginalis to contact closely with the epithelium. Then early biofilm forms with the aggregation of other BVAB and the accumulation of extracellular matrix (Verstraelen and Swidsinski, 2013). Though sialidase A gene is not found to be associated with sialidase activity, it has been found to be associated with the presence of G.vaginalis biofilms, suggesting its possible contribution to biofilm formation (Hardy et al., 2017). There is still a lack of research comparing the expression levels of sialidase in biofilms and planktonic cells, which can provide us with deeper insights into the role of sialidase in biofilm formation. What’s more, interactions between the microorganisms within vaginal biofilms are worth investigating as sialidase activity may be affected by those sialidase-negative bacteria. Besides the finding that F.nucleatum and G.vaginalis benefit from each other, an in vitro dual-species biofilm model demonstrates that other BVAB, such as Actinomyces neuii and Enterococcus faecalis, can upregulate sialidase and vaginolysin expression in G.vaginalis to reinforce its virulence (Castro et al., 2019).
Similar findings of the involvements of sialidases in biofilm formation also presents in infections of other systems. In the early phases of pulmonary infection, Pseudomonas aeruginosa and its sialidases, existing on the highly sialylated surfaces of the upper respiratory tract, can target bacterial glycoconjugates, promote cell-cell interactions, and initiate biofilm formation (Soong, 2006). Viral sialidase inhibitors have demonstrated the ability to block the process of biofilm formation in clinical in vitro, suggesting a potential novel pharmacological target in bacterial pneumonia prevention (Soong, 2006). In Porphyromonas gingivalis, the main pathogenic bacterium in chronic periodontitis, the sialidase encoding gene shows a higher expression level than that in planktonic cells (Lo et al., 2009). Sialidase-deficient strains also demonstrates less and discontinuous biofilm formation compared with wild-type P.gingivalis strains (Xu et al., 2017).
The host-mucosa-sialidase can be regarded as a whole because sialidase functions on the mucosa. Sialidase is central to the suppression and overwhelm of host immune response. Meanwhile, it is also supported by other hydrolytic enzymes, vaginolysin, and immunomodulatory metabolites (Figure 1D) (Amabebe and Anumba, 2022). Sialylation of glycoconjugates, such as mucins, immunoglobulins (especially secretory immunoglobulin A, sIgA), and cytokines, cleave the molecules’ terminal sialic acids and uncover their carbohydrate residues to all kinds of glycosidases, thus making them more susceptible to proteolytic degradation and hampering immune response against bacteria (Cauci et al., 2003; Cauci, 2004). For example, during the incubation of sIgA and BV vaginal specimens, the release of products with lower molecular weight into the extracellular environment are observed and the phenomenon can be reproduced by adding three exogenous enzymes: sialidase, β-galactosidase and hexosaminidase, which suggests the deglycosylation and proteolysis of sIgA in BV (Lewis et al., 2012).
In BV-positive women with a specific IgA immune response against G.vaginalis hemolysin (Gvh, vaginolysin), increased cleavage of IgA and a 5-fold higher sialidase activity is observed compared to those with a weaker IgA response (Cauci et al., 1998). Later, another study reconfirmed that elevated sialidase and prolidase levels reduce this mucosal adaptive immune response. Vaginolysin, another virulence factor of G.vaginalis, is a cholesterol-dependent cytolysin (CDC) which forms pores on cell membranes, free host intracellular contents and disrupts genital epithelial cells (Morrill et al., 2023). The immunosuppression allows vaginolysin to fully carry out its cytolytic action, which results in the detachment and destruction of the vaginal epithelial cells that eventually produce clue cells (Castro et al., 2019).
High sialidases and prolidases levels are also associated with elevated vaginal IL-1β, leading to tissue damage and increased susceptibility to sexually transmitted infections (STIs) (Cauci et al., 2008). Despite that IL-1β stimulates IL-8 secretion, sialidase level is also inversely correlated to vaginal IL-8 and neutrophils, which inhibits neutrophil infiltration and the proinflammatory cascade (Cauci et al., 2008). According to in vivo research, BVAB can evade the immune response by either secreting molecules that aid in the breakdown of IL-8 or by suppressing the generation and stability of IL-8 (Santos et al., 2018). These findings suggest that in BV-positive women, sialidases contribute to the suppression of innate mucosal immunity.
However, BVAB induced the secretion of IL-6, IL-8, G-CSF, IP-10, MIP-1β, RANTES, and Gro-α, while lactobacilli did not in another study that used a coculture model to characterize the response of vaginal epithelial cells to a series of vaginal bacteria, including commensal lactobacilli and BVAB such as G.vaginalis, A.vaginae, Mobiluncis curtisii, and P.bivia (Eade et al., 2012). The results is consistent with that A.vaginae induces a robust proinflammatory response by elevating transcript levels of IL-6, IL-8, and antimicrobial peptide β-defensin 4 (Libby et al., 2008). It seems that BVAB trigger mucosal innate immune response, increasing production of cytokines and defensins to eliminate pathogens. But excessive inflammatory response might lead to a disturbance of the vaginal immunological barrier and increasing susceptibility to STIs (Doerflinger et al., 2014).
Furthermore, bacterial surface sialylation may serve as an immunological mask (Ram et al., 2017; Vimr and Lichtensteiger, 2002). It has been proposed that bacteria might be passed for host cells and evade the host’s immune system by incorporating the cleaved sialic acids into their cell surface structures (Varki and Gagneux, 2012). Differentiation between self-sialic-acids and close mimics is achieved through intrinsic lectins such as sialic acid-binding immunoglobulin-like lectins (Siglecs) anchored on most immune cells (Duan and Paulson, 2020). By engaging inhibitory Siglec‐5 and Siglec‐9, group B Streptococcus can escape from host immune responses (Carlin et al., 2007). Neisseria gonorrhoeae transfers sialic acid residues to its surface lipooligosaccharide (LOS) to achieve molecular simulation, which contributes to its serum resistance and complement resistance in all three pathways (classical, lectin, and alternative) (Ram et al., 2017). A study reports that vaginolysin is able to release the contents of cervical epithelial cells, promote gonococcal LOS acquisition of sialic acids, and evade complement attack through increased binding of the regulatory protein factor H (Morrill et al., 2023), suggesting that sialidases and vaginolysin are both crucial in the regulation of the LOS sialylation level and its pathogenic ability. Meanwhile, another study reports that desialylation of gonococcal LOS by sialidases in women promotes increased transmission of infection to men (Ketterer et al., 2016). These findings suggest that sialylation and desialylation may to have unique functions during the invasion of pathogens.
Elevated sialidase activity has been observed in BV CVF, suggesting that sialidases could be used as a promising biomarker for BV (Briselden et al., 1992). The presence of sialidase A gene was detected in all 24 G.vaginalis samples in a recent study (Ma et al., 2022), while another study reports an association between sialidase activity in molecular-BV (community state type IV, CST IV) and changes in the bacterial components of the local microbiome, assessed by using V3–V4 16S rRNA sequencing (Ferreira et al., 2022). Gardnerella, Atopobium, and Prevotella were among BV-associated the genera that were more prevalent in women with high sialidase activity (Ng et al., 2021). Increased sialidase may be attributed to the higher abundance of some BVAB that can produce sialidases by themselves, such as Prevotella (Briselden et al., 1992). At the same time, sialidases can impair the vaginal mucosal immune system, which creates a beneficial environment for the overgrowth of BVAB over the Lactobacillus spp. and increases bacterial diversity (Lewis et al., 2013).
As a biomarker for BV, sialidases could be used to develop new diagnostic tests as cheaper and quicker alternatives to the current standard clinical diagnostic tools. Current clinical diagnosis of BV is often based on the Nugent scoring system (Nugent et al., 1991) or the Amsel criteria (Amsel et al., 1983), both of which require microscopy and trained professionals. On the contrary, enzyme-based simple assays may be cheaper and quicker (Robinson et al., 2019; Wu et al., 2019; Cortés-Sarabia et al., 2020; Rodríguez-Nava et al., 2021; Avila-Huerta et al., 2023; Liu et al., 2023). Several new tests have been developed to detect sialidases. A comparison of their clinical diagnostic performance is shown in Table 1. The most widely used is BVBlue test, a microscopy-independent bedside test that detects sialidase activity using ≥7.8 U as the cut-off value for diagnosis of BV (Myziuk et al., 2003; Bradshaw et al., 2005; Permsak et al., 2005; Madhivanan et al., 2014; Foessleitner et al., 2021). OSOM® BVBLUE® Test is a commercial chromogenic test that can rapidly detect elevated vaginal fluid sialidase activity, with excellent sensitivity and specificity compared to Gram Stain, and it is widely used in many parts of the world. Similarly, a sensitive colorimetric bioactive paper that changes its color from white to dark purple in the presence of sialidases demonstrates a quick reaction time and strong storage stability (Zhang and Rochefort, 2013), though its clinical performance in BV diagnosis was not evaluated. Although sialidase activity tests are performed clinically, the results are currently only used as references and not as a diagnostic criterion.
The nanH3 gene expression could be used for PCR detection of BV as its level differs in normal microbiota and BV cervicovaginal fluid samples (Novak et al., 2023). PCR detection of nanH2 or nanH3 has a sensitivity of 80.95% and a specificity of 78.26% in differentiating between Lactobacillus-dominance and BV, as determined by Nugent scoring (Robinson et al., 2019). However, the test only detects sialidase produced by G.vaginalis, limiting its applicability to other BV pathogens.
Fluorescence could also be used to visualize sialic acids on cell membranes. The first test developed and adopted for BV diagnosis was turn-on tetravalent sialic acid-coated tetraphenylethene luminogen (Liu et al., 2018). Later on, a biochemiluminescent sialidase assay using a firefly luciferin derived substrate was developed, in which luciferins released by cleavage of the substrate subsequently oxidize and generate a light signal indicating relative sialidase concentration (Wu et al., 2019). More recently, a novel boron and nitrogen codoped fluorescent carbon dots (BN-CDs) was developed based on fluorescence spectrometry, in which sialidases can restore the fluorescence by interfering with the selective recognition interaction between the sialic acid and phenylboronic acid groups on the surface of BN-CDs, limiting fluorescence emission (Liu et al., 2023). The probe is comparable to Amsel criteria in its diagnosis of BV, indicating promising use for clinical diagnosis and therapy (Liu et al., 2023).
A new microfluidic paper-based analytical tool based on a monoclonal antibody that has a high specificity for sialidase recognition for BV diagnosis was described (Avila-Huerta et al., 2023). Taking advantage of a surface coated with graphene oxide as a fluorescence quencher, they developed a Y-shaped strip, consisting of an entrance, a control, and a test zone (Avila-Huerta et al., 2023). The apparatus can achieve a prompt and sensitive response within 20 minutes for the identification of BV, making it economically accessible and convenient for large scale use (Avila-Huerta et al., 2023).
Another research team recently designed and manufactured a monoclonal antibody (mAb) targeted against G.vaginalis sialidases (Cortés-Sarabia et al., 2020). They further developed a single-step quantitative biosensing system for BV diagnosis, using graphene oxide-coated microwells and mAb-decorated quantum dots (Rodríguez-Nava et al., 2021). Sialidase activity in vaginal swab samples detected by this method has a 96.29% specificity and 96.29% sensitivity, using Amsel criteria for the identification of BV (Rodríguez-Nava et al., 2021).
With the understanding of the molecular mechanism of sialidases and its association with the pathogenesis of BV, sialidases can be used as not only a promising diagnostic marker but also a pharmaceutical target through activity blockage using inhibitors (Keil et al., 2022). Sialidase inhibitors include transition-state analogue inhibitors, mechanism-based inhibitors, suicide substrate inhibitors, product analogue inhibitors, and natural product inhibitors (Keil et al., 2022), which can act on virus, bacteria, human and protozoa sialidases. Numerous natural compounds have been identified and examined for their ability to inhibit sialidases from human, bacteria and influenza viruses. As for bacteria sialidases, three novel compounds as potent inhibitors are isolated from Lespedeza bicolor and effect in a dose-dependent manner, among them the best inhibitor has an IC50 (represents the compound concentration that causes 50% enzyme activity loss) of 0.09 μM (Woo et al., 2011). A recent discovery is a curcumin analogue against S.pneumoniae Nan A, whose IC50 value is 0.2 ± 0.1 μM, exhibiting a 3-fold increase in inhibitory efficacy compared to curcumin (Kim et al., 2018). Natural products provide us with an alternate source for creating novel bacterial sialidases inhibitors and treating sepsis caused by bacteria infections, which are worth exploring for BV treatment. In our discussion of potential treatment options for BV, with G.vaginalis being the major pathogen, we will be focusing on bacterial sialidase inhibitors (Keil et al., 2022) and salic acid analogs (Agarwal and Lewis, 2021).
Among them the most studied is the influenza virus sialidase inhibitors. Influenza sialidase (usually called neuraminidase) is required for the infection cycle to continue because it releases the freshly generated virus from the host cell, contributing to its spreading and preventing self-aggregation of the viral particles (Glanz et al., 2018). Currently, there are three antiviral drugs that target the glycoprotein neuraminidase on the surface of the influenza virus, including oseltamivir, zanamivir, and peramivir. They are essentially transition-state analogue inhibitors and work by inhibiting the neuraminidase enzyme’s activity and preventing the virus from exiting the infected cells (Javanian et al., 2021).
As bacterial and viral sialidases share the same sialic acid interaction sites, the ASP boxes (Roggentin et al., 1989), influenza virus sialidase inhibitors can be used to block the bacterial sialidase active site and prevent the formation of biofilms (Hayden et al., 1999). Evidence shows that influenza virus sialidase inhibitors oseltamivir and peramivir can block P.aeruginosa biofilm formation in a dose-dependent manner (Soong, 2006). Similarly, the desialylation of sIgA during incubations with BV samples and can be inhibited by deoxy-dehydro-sialic acid (DDSia), a synthetic sialidase inhibitor (Lewis et al., 2012). Zanamivir impairs the virulence of the BV-associated pathogen G.vaginalis through a reduction of 30% in G.vaginalis sialidase activity and 50% in its ability to invade host cells (Govinden et al., 2018). It’s interesting that the medicine for influenza treatment associates with BV. Anyway, they provide us with a new prospective to treat BV, despite neuraminidase inhibitor sensitivity varies throughout mammalian, microbial, and viral neuraminidases.
The two major forms of sialic acid in mammals, N-acetylneuraminic acid (Neu5Ac) and N-glycolylneuraminic acid (Neu5Gc), differ by a single oxygen atom, with Neu5Ac being the most prevalent form of sialic acid in mammalian cells (Schauer and Kamerling, 2018). The enzyme needed to synthesize Neu5Gc from Neu5Ac is called CMP-N-acetylneuraminic acid (CMP-NeuAc) hydroxylase, which is inactive in human, so Neu5Gc is a non-human derived sialic acid (Lewis et al., 2013). Once Neu5Ac is released by sialidases in the vagina, transport, uptaking and catabolism of them are proceeded within cells. The intracellular process is mediated by Neu5Ac lyase/aldolase and the substrates are catalyzed into N-acetylmannosamine (ManNAc) and pyruvate without accumulation (Lewis et al., 2013). An inherent biological mechanism for regulating enzyme processes is feedback inhibition through end-product inhibition of upstream enzymes. Through feedback inhibition, free Neu5Ac is a weak inhibitor of sialidases (Schauer and Kamerling, 1997). While a high-affinity transport mechanism in G.vaginalis has a preference for Neu5Ac, G.vaginalis sialidase does not appear to have strong preferences between Neu5Ac and Neu5Gc as substrates (Byers et al., 1999). This means that the uptake and breakdown of sialic acids are substrate-dependent and occur far more slowly and incompletely, when there is a substantial concentration of Neu5Gc (Figure 2). Later a study confirmed that G.vaginalis could liberate free Neu5Ac from IgA but fails to consume them with the presence of Neu5Gc, which further indicates Neu5Gc’s potential as an inhibitor to reduce Neu5Ac transport into G.vaginalis (Lewis et al., 2013). These findings are consistent with a prior discovery that in the bacterium S.oralis, Neu5Gc inhibits the uptake of Neu5Ac (Byers et al., 1999). Despite that Neu5Gc shows sialidase inhibitory activity, its effects for anti-BV are not verified and its effectiveness and safety still need experimental verification.
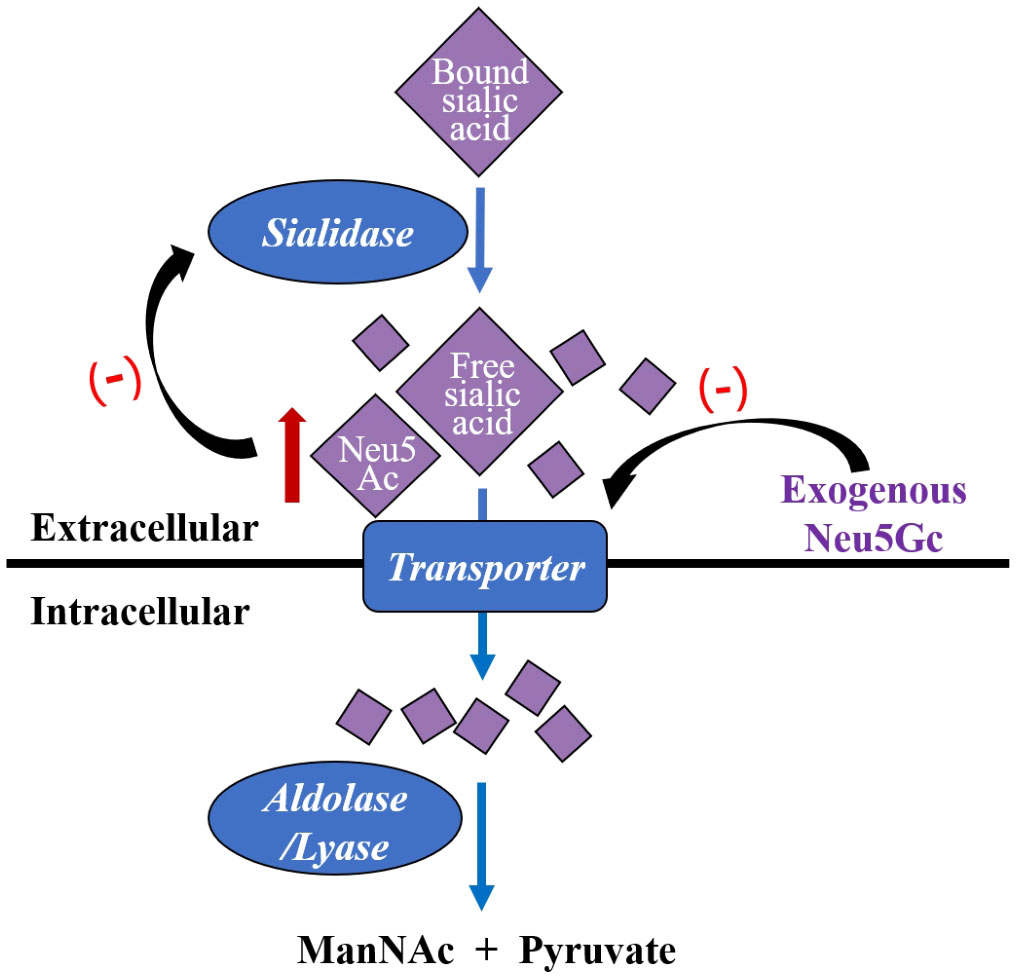
Figure 2 Gardnerella vaginalis captures free Neu5Ac hydrolyzed by sialidases, pumps them into the cell by a transporter, and then catalyze them into ManNac and pyruvate by intracellular aldolase/lyase. Exogenous Neu5Gc is a kind of sialic acid analogues, which inhibits G.vaginalis transporter and results in extracellular Neu5Ac accumulation. Neu5Ac is a weaker inhibitor of sialidases base on feedback mechanism. Neu5Ac, N-acetylneuraminic acid; Neu5Gc, N-glycolylneuraminic acid; ManNac, N-acetylmannosamine.
This study explores the role of sialidases in vaginal dysbiosis, pathogenesis of BV, and promising diagnostic and treatment options for BV. Although the composition and dynamics of the human vaginal microbiome are being studied more and more, we still know little about the mechanisms underlying the development of vaginal dysbiosis and the critical factors that influence it. As a main virulence factor of Gardnerella spp. and an important glycoside hydrolase enzyme, sialidases cleave sialic acid from terminal glycans, also known as desialylation. The process facilitates the destruction of mucosal defense barrier, as well as bacterial adhesion, colonization, and invasion into the vaginal epithelia through provision of nutrient sources, exposure of receptor binding sites, biofilms formation, and immunity regulation. However, not all G.vaginalis strains can produce sialidases and the contribution of sialidases to BV is just part of the pathogenesis of G.vaginalis. There are still other BVAB, virus, and even the human body itself can produce sialidases. Moreover, the use of sialidases as a biomarker for predicting treatment outcomes and the prognosis of BV still needs to be tested in clinical studies. Future research should focus on understanding the pathogenesis of sialidases produced by different strains of G.vaginalis and other sources, as well as the association between sialidases and the persistence and recurrence of BV, to provide new insights to improve diagnosis and treatment of BV.
LC: Writing – original draft, Writing – review & editing. JL: Writing – review & editing. BX: Conceptualization, Writing – review & editing, Funding acquisition.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This present work was funded by the grants of the National Key Research and Development Program of China (2021YFC2301000), the National Natural Science Foundation of China (81971342) and the Peking University First Hospital Interdisciplinary clinical research program (2022CR46).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Agarwal, K., Choudhury, B., Robinson, L. S., Morrill, S. R., Bouchibiti, Y., Chilin-Fuentes, D., et al. (2023). Resident microbes shape the vaginal epithelial glycan landscape. Sci. Trans. Med. 15, eabp9599. doi: 10.1126/scitranslmed.abp9599
Agarwal, K., Lewis, A. L. (2021). Vaginal sialoglycan foraging by Gardnerella vaginalis : mucus barriers as a meal for unwelcome guests? Glycobiology 31, 667–680. doi: 10.1093/glycob/cwab024
Agarwal, K., Robinson, L. S., Aggarwal, S., Foster, L. R., Hernandez-Leyva, A., Lin, H., et al. (2020). Glycan cross-feeding supports mutualism between Fusobacterium and the vaginal microbiota. PloS Biol. 18, e3000788. doi: 10.1371/journal.pbio.3000788
Alves, P., Castro, J., Sousa, C., Cereija, T. B., Cerca, N. (2014). Gardnerella vaginalis outcompetes 29 other bacterial species isolated from patients with bacterial vaginosis, using in an in vitro biofilm formation model. J. Infect. Dis. 210, 593–596. doi: 10.1093/infdis/jiu131
Amabebe, E., Anumba, D. O. C. (2018). The vaginal microenvironment: the physiologic role of lactobacilli. Front. Med. 5. doi: 10.3389/fmed.2018.00181
Amabebe, E., Anumba, D. O. C. (2022). Mechanistic insights into immune suppression and evasion in bacterial vaginosis. Curr. Microbiol. 79, 84. doi: 10.1007/s00284-022-02771-2
Amsel, R., Totten, P. A., Spiegel, C. A., Chen, K. C. S., Eschenbach, D., Holmes, K. K. (1983). Nonspecific vaginitis. Am. J. Med. 74, 14–22. doi: 10.1016/0002-9343(83)91112-9
Anthony, R. M., Ravetch, J. V. (2010). A novel role for the igG fc glycan: the anti-inflammatory activity of sialylated igG fcs. J. Clin. Immunol. 30, 9–14. doi: 10.1007/s10875-010-9405-6
Argüeso, P., Woodward, A. M., AbuSamra, D. B. (2021). The epithelial cell glycocalyx in ocular surface infection. Front. Immunol. 12. doi: 10.3389/fimmu.2021.729260
Armstrong, E., Kaul, R. (2021). Beyond bacterial vaginosis: vaginal lactobacilli and HIV risk. Microbiome 9, 239. doi: 10.1186/s40168-021-01183-x
Avila-Huerta, M. D., Leyva-Hidalgo, K., Cortés-Sarabia, K., Estrada-Moreno, A. K., Vences-Velázquez, A., Morales-Narváez, E. (2023). Disposable device for bacterial vaginosis detection. ACS Meas. Sci. Au 3, 355–360. doi: 10.1021/acsmeasuresciau.3c00007
Balashov, S. V., Mordechai, E., Adelson, M. E., Gygax, S. E. (2014). Identification, quantification and subtyping of Gardnerella vaginalis in noncultured clinical vaginal samples by quantitative PCR. J. Med. Microbiol. 63, 162–175. doi: 10.1099/jmm.0.066407-0
Bautista, C. T., Wurapa, E., Sateren, W. B., Morris, S., Hollingsworth, B., Sanchez, J. L. (2016). Bacterial vaginosis: a synthesis of the literature on etiology, prevalence, risk factors, and relationship with chlamydia and gonorrhea infections. Military Med. Res. 3, 4. doi: 10.1186/s40779-016-0074-5
Beighton, D., Whiley, R. A. (1990). Sialidase activity of the “Streptococcus milleri group” and other viridans group streptococci. J. Clin. Microbiol. 28, 1431–1433. doi: 10.1128/jcm.28.6.1431-1433.1990
Bonnardel, F., Haslam, S. M., Dell, A., Feizi, T., Liu, Y., Tajadura-Ortega, V., et al. (2021). Proteome-wide prediction of bacterial carbohydrate-binding proteins as a tool for understanding commensal and pathogen colonisation of the vaginal microbiome. NPJ Biofilms Microbiomes 7, 49. doi: 10.1038/s41522-021-00220-9
Bradshaw, C. S., Morton, A. N., Garland, S. M., Horvath, L. B., Kuzevska, I., Fairley, C. K. (2005). Evaluation of a point-of-care test, BVBlue, and clinical and laboratory criteria for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 43, 1304–1308. doi: 10.1128/JCM.43.3.1304-1308.2005
Briselden, A. M., Moncla, B. J., Stevens, C. E., Hillier, S. L. (1992). Sialidases (neuraminidases) in bacterial vaginosis and bacterial vaginosis-associated microflora. J. Clin. Microbiol. 30, 663–666. doi: 10.1128/jcm.30.3.663-666.1992
Byers, H. L., Homer, K. A., Beighton, D. (1996). Utilization of sialic acid by viridans streptococci. J. Dent. Res. 75, 1564–1571. doi: 10.1177/00220345960750080701
Byers, H. L., Homer, K. A., Tarelli, E., Beighton, D. (1999). N-Acetylneuraminic acid transport by Streptococcus oralis strain AR3. J. Med. Microbiol. 48, 375–381. doi: 10.1099/00222615-48-4-375
Cao, H., Chen, X. (2012). “General consideration on sialic acid chemistry,” in Carbohydrate microarrays, methods in molecular biology. Ed. Chevolot, Y. (Humana Press, Totowa, NJ), 31–56. doi: 10.1007/978-1-61779-373-8_3
Carlin, A. F., Lewis, A. L., Varki, A., Nizet, V. (2007). Group B streptococcal capsular sialic acids interact with siglecs (Immunoglobulin-like lectins) on human leukocytes. J. Bacteriol 189, 1231–1237. doi: 10.1128/JB.01155-06
Castro, J., Alves, P., Sousa, C., Cereija, T., França, Â., Jefferson, K. K., et al. (2015). Using an in-vitro biofilm model to assess the virulence potential of Bacterial Vaginosis or non-Bacterial Vaginosis Gardnerella vaginalis isolates. Sci. Rep. 5, 11640. doi: 10.1038/srep11640
Castro, J., MaChado, D., Cerca, N. (2019). Unveiling the role of Gardnerella vaginalis in polymicrobial Bacterial Vaginosis biofilms: the impact of other vaginal pathogens living as neighbors. ISME J. 13, 1306–1317. doi: 10.1038/s41396-018-0337-0
Cauci, S. (2004). Vaginal immunity in bacterial vaginosis. Curr. Infect. Dis. Rep. 6, 450–456. doi: 10.1007/s11908-004-0064-8
Cauci, S., Culhane, J. F. (2011). High sialidase levels increase preterm birth risk among women who are bacterial vaginosis–positive in early gestation. Am. J. Obstet. Gynecol. 204, 142.e1–142.e9. doi: 10.1016/j.ajog.2010.08.061
Cauci, S., Culhane, J. F., Di Santolo, M., McCollum, K. (2008). Among pregnant women with bacterial vaginosis, the hydrolytic enzymes sialidase and prolidase are positively associated with interleukin-1β. Am. J. Obstet. Gynecol. 198, 132.e1–132.e7. doi: 10.1016/j.ajog.2007.05.035
Cauci, S., Driussi, S., Monte, R., Lanzafame, P., Quadrifoglio, F. (1998). Immunoglobulin A response against Gardnerella vaginalis hemolysin and sialidase activity in bacterial vaginosis. Am. J. Obstet. Gynecol. 178, 511–515. doi: 10.1016/S0002-9378(98)70430-2
Cauci, S., Guaschino, S., Driussi, S., De Santo, D., Lanzafame, P., Quadrifoglio, F. (2002). Correlation of Local Interleukin-8 with Immunoglobulin A against Gardnerella vaginalis Hemolysin and with Prolidase and Sialidase Levels in Women with Bacterial Vaginosis. J. Infect. Dis. 185, 1614–1620. doi: 10.1086/340417
Cauci, S., McGregor, J., Thorsen, P., Grove, J., Guaschino, S. (2005). Combination of vaginal pH with vaginal sialidase and prolidase activities for prediction of low birth weight and preterm birth. Am. J. Obstet. Gynecol. 192, 489–496. doi: 10.1016/j.ajog.2004.07.023
Cauci, S., Thorsen, P., Schendel, D. E., Bremmelgaard, A., Quadrifoglio, F., Guaschino, S. (2003). Determination of Immunoglobulin A against Gardnerella vaginalis Hemolysin, Sialidase, and Prolidase Activities in Vaginal Fluid: Implications for Adverse Pregnancy Outcomes. J. Clin. Microbiol. 41, 435–438. doi: 10.1128/JCM.41.1.435-438.2003
Chen, Q.-Q., Ma, G., Liu, J.-F., Cai, Y.-Y., Zhang, J.-Y., Wei, T.-T., et al. (2021). Neuraminidase 1 is a driver of experimental cardiac hypertrophy. Eur. Heart J. 42, 3770–3782. doi: 10.1093/eurheartj/ehab347
Cohen, M., Varki, A. (2010). The sialome—Far more than the sum of its parts. OMICS: A J. Integr. Biol. 14, 455–464. doi: 10.1089/omi.2009.0148
Cortés-Sarabia, K., Rodríguez-Nava, C., Medina-Flores, Y., Mata-Ruíz, O., López-Meza, J. E., Gómez-Cervantes, M. D., et al. (2020). Production and characterization of a monoclonal antibody against the sialidase of Gardnerella vaginalis using a synthetic peptide in a MAP8 format. Appl. Microbiol. Biotechnol. 104, 6173–6183. doi: 10.1007/s00253-020-10691-z
Delgado-Diaz, D. J., Tyssen, D., Hayward, J. A., Gugasyan, R., Hearps, A. C., Tachedjian, G. (2020). Distinct immune responses elicited from cervicovaginal epithelial cells by lactic acid and short chain fatty acids associated with optimal and non-optimal vaginal microbiota. Front. Cell. Infect. Microbiol. 9. doi: 10.3389/fcimb.2019.00446
Del Pozo, J. L. (2018). Biofilm-related disease. Expert Rev. Anti-infective Ther. 16, 51–65. doi: 10.1080/14787210.2018.1417036
Di Paola, M., Sani, C., Clemente, A. M., Iossa, A., Perissi, E., Castronovo, G., et al. (2017). Characterization of cervico-vaginal microbiota in women developing persistent high-risk Human Papillomavirus infection. Sci. Rep. 7, 10200. doi: 10.1038/s41598-017-09842-6
Doerflinger, S. Y., Throop, A. L., Herbst-Kralovetz, M. M. (2014). Bacteria in the vaginal microbiome alter the innate immune response and barrier properties of the human vaginal epithelia in a species-specific manner. J. Infect. Dis. 209, 1989–1999. doi: 10.1093/infdis/jiu004
Duan, S., Paulson, J. C. (2020). Siglecs as immune cell checkpoints in disease. Annu. Rev. Immunol. 38, 365–395. doi: 10.1146/annurev-immunol-102419-035900
Eade, C. R., Diaz, C., Wood, M. P., Anastos, K., Patterson, B. K., Gupta, P., et al. (2012). Identification and characterization of bacterial vaginosis-associated pathogens using a comprehensive cervical-vaginal epithelial coculture assay. PloS One 7, e50106. doi: 10.1371/journal.pone.0050106
Egan, M., O’Connell Motherway, M., Ventura, M., van Sinderen, D. (2014). Metabolism of sialic acid by bifidobacterium breve UCC2003. Appl. Environ. Microbiol. 80, 4414–4426. doi: 10.1128/AEM.01114-14
Ferreira, C. S. T., Marconi, C., Parada, C. M. G. L., Ravel, J., Silva, M. G. D. (2022). Sialidase activity in the cervicovaginal fluid is associated with changes in bacterial components of lactobacillus-deprived microbiota. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.813520
Flemming, H.-C., Wingender, J., Szewzyk, U., Steinberg, P., Rice, S. A., Kjelleberg, S. (2016). Biofilms: an emergent form of bacterial life. Nat. Rev. Microbiol. 14, 563–575. doi: 10.1038/nrmicro.2016.94
Foessleitner, P., Kiss, H., Deinsberger, J., Ott, J., Zierhut, L., Rosta, K., et al. (2021). Screening pregnant women for bacterial vaginosis using a point-of-care test: A prospective validation study. JCM 10, 2275. doi: 10.3390/jcm10112275
Ghosh, S. (2020). Sialic acid and biology of life: An introduction, in Sialic acids and sialoglycoconjugates in the biology of life, health and disease (Amsterdam: Elsevier), 1–61. doi: 10.1016/B978-0-12-816126-5.00001-9
Gilbert, N. M., Lewis, W. G., Lewis, A. L. (2013). Clinical features of bacterial vaginosis in a murine model of vaginal infection with gardnerella vaginalis. PloS One 8, e59539. doi: 10.1371/journal.pone.0059539
Gilbert, N. M., Lewis, W. G., Li, G., Sojka, D. K., Lubin, J. B., Lewis, A. L. (2019). Gardnerella vaginalis and Prevotella bivia Trigger Distinct and Overlapping Phenotypes in a Mouse Model of Bacterial Vaginosis. J. Infect. Dis. 220, 1099–1108. doi: 10.1093/infdis/jiy704
Glanz, V. Y., Myasoedova, V. A., Grechko, A. V., Orekhov, A. N. (2018). Inhibition of sialidase activity as a therapeutic approach. DDDT Volume 12, 3431–3437. doi: 10.2147/DDDT.S176220
Govinden, G., Parker, J. L., Naylor, K. L., Frey, A. M., Anumba, D. O. C., Stafford, G. P. (2018). Inhibition of sialidase activity and cellular invasion by the bacterial vaginosis pathogen Gardnerella vaginalis. Arch. Microbiol. 200, 1129–1133. doi: 10.1007/s00203-018-1520-4
Haines-Menges, B. L., Whitaker, W. B., Lubin, J. B., Boyd, E. F. (2015). Host sialic acids: A delicacy for the pathogen with discerning taste. Microbiol. Spectr. 3, 3.4.07. doi: 10.1128/microbiolspec.MBP-0005-2014
Hardy, L., Jespers, V., Van Den Bulck, M., Buyze, J., Mwambarangwe, L., Musengamana, V., et al. (2017). The presence of the putative Gardnerella vaginalis sialidase A gene in vaginal specimens is associated with bacterial vaginosis biofilm. PloS One 12, e0172522. doi: 10.1371/journal.pone.0172522
Hayden, F. G., Treanor, J. J., Fritz, R. S., Lobo, M., Betts, R. F., Miller, M., et al. (1999). Use of the oral neuraminidase inhibitor oseltamivir in experimental human influenza: randomized controlled trials for prevention and treatment. JAMA 282, 1240. doi: 10.1001/jama.282.13.1240
He, Y., Na, R., Niu, X., Xiao, B., Yang, H. (2021). Lactobacillus rhamnosus and Lactobacillus casei Affect Various Stages of Gardnerella Species Biofilm Formation. Front. Cell. Infect. Microbiol. 11. doi: 10.3389/fcimb.2021.568178
Honest, H., Bachmann, L. M., Knox, E. M., Gupta, J. K., Kleijnen, J., Khan, K. S. (2004). The accuracy of various tests for bacterial vaginosis in predicting preterm birth: a systematic review. BJOG 111, 409–422. doi: 10.1111/j.1471-0528.2004.00124.x
Howe, L., Wiggins, R., Soothill, P. W., Millar, M. R., Horner, P. J., Corfield, A. P. (1999). Mucinase and sialidase activity of the vaginal microflora: implications for the pathogenesis of preterm labour. Int. J. STD AIDS 10, 442–447. doi: 10.1258/0956462991914438
Huang, Y.-L., Chassard, C., Hausmann, M., Von Itzstein, M., Hennet, T. (2015). Sialic acid catabolism drives intestinal inflammation and microbial dysbiosis in mice. Nat. Commun. 6, 8141. doi: 10.1038/ncomms9141
Janulaitiene, M., Gegzna, V., Baranauskiene, L., Bulavaitė, A., Simanavicius, M., Pleckaityte, M. (2018). Phenotypic characterization of Gardnerella vaginalis subgroups suggests differences in their virulence potential. PloS One 13, e0200625. doi: 10.1371/journal.pone.0200625
Javanian, M., Barary, M., Ghebrehewet, S., Koppolu, V., Vasigala, V., Ebrahimpour, S. (2021). A brief review of influenza virus infection. J. Med. Virol. 93, 4638–4646. doi: 10.1002/jmv.26990
Javed, A., Parvaiz, F., Manzoor, S. (2019). Bacterial vaginosis: An insight into the prevalence, alternative treatments regimen and it’s associated resistance patterns. Microbial Pathogenesis 127, 21–30. doi: 10.1016/j.micpath.2018.11.046
Joo, H.-S., Otto, M. (2012). Molecular basis of in vivo biofilm formation by bacterial pathogens. Chem. Biol. 19, 1503–1513. doi: 10.1016/j.chembiol.2012.10.022
Jung, H.-S., Ehlers, M. M., Lombaard, H., Redelinghuys, M. J., Kock, M. M. (2017). Etiology of bacterial vaginosis and polymicrobial biofilm formation. Crit. Rev. Microbiol. 43, 651–667. doi: 10.1080/1040841X.2017.1291579
Karhadkar, T. R., Chen, W., Pilling, D., Gomer, R. H. (2022). Inhibitors of the sialidase NEU3 as potential therapeutics for fibrosis. IJMS 24, 239. doi: 10.3390/ijms24010239
Keil, J. M., Rafn, G. R., Turan, I. M., Aljohani, M. A., Sahebjam-Atabaki, R., Sun, X.-L. (2022). Sialidase inhibitors with different mechanisms. J. Med. Chem. 65, 13574–13593. doi: 10.1021/acs.jmedchem.2c01258
Ketterer, M. R., Rice, P. A., Gulati, S., Kiel, S., Byerly, L., Fortenberry, J. D., et al. (2016). Desialylation of neisseria gonorrhoeae lipooligosaccharide by cervicovaginal microbiome sialidases: the potential for enhancing infectivity in men. J. Infect. Dis. 214, 1621–1628. doi: 10.1093/infdis/jiw329
Khan, A., Das, S., Sergi, C. (2021). Therapeutic potential of neu1 in alzheimer’s disease via the immune system. Am. J. Alzheimers Dis. Other Demen 36, 153331752199614. doi: 10.1177/1533317521996147
Kim, B. R., Park, J.-Y., Jeong, H. J., Kwon, H.-J., Park, S.-J., Lee, I.-C., et al. (2018). Design, synthesis, and evaluation of curcumin analogues as potential inhibitors of bacterial sialidase. J. Enzyme Inhibition Medicinal Chem. 33, 1256–1265. doi: 10.1080/14756366.2018.1488695
Kurukulasuriya, S. P., Patterson, M. H., Hill, J. E. (2021). Slipped-strand mispairing in the gene encoding sialidase nanH3 in gardnerella spp. Infect. Immun. 89, e00583–e00520. doi: 10.1128/IAI.00583-20
Lewis, A. L., Lewis, W. G. (2012). Host sialoglycans and bacterial sialidases: a mucosal perspective. Cell. Microbiol. 14, 1174–1182. doi: 10.1111/j.1462-5822.2012.01807.x
Lewis, W. G., Robinson, L. S., Gilbert, N. M., Perry, J. C., Lewis, A. L. (2013). Degradation, foraging, and depletion of mucus sialoglycans by the vagina-adapted actinobacterium gardnerella vaginalis. J. Biol. Chem. 288, 12067–12079. doi: 10.1074/jbc.M113.453654
Lewis, W. G., Robinson, L. S., Perry, J., Bick, J. L., Peipert, J. F., Allsworth, J. E., et al. (2012). Hydrolysis of secreted sialoglycoprotein immunoglobulin A (IgA) in ex vivo and biochemical models of bacterial vaginosis. J. Biol. Chem. 287, 2079–2089. doi: 10.1074/jbc.M111.278135
Liao, H., Klaus, C., Neumann, H. (2020). Control of innate immunity by sialic acids in the nervous tissue. IJMS 21, 5494. doi: 10.3390/ijms21155494
Libby, E. K., Pascal, K. E., Mordechai, E., Adelson, M. E., Trama, J. P. (2008). Atopobium vaginae triggers an innate immune response in an in vitro model of bacterial vaginosis. Microbes Infection 10, 439–446. doi: 10.1016/j.micinf.2008.01.004
Liu, G., Wang, B., Zhang, Y., Xing, G., Yang, X., Wang, S. (2018). A tetravalent sialic acid-coated tetraphenylethene luminogen with aggregation-induced emission characteristics: design, synthesis and application for sialidase activity assay, high-throughput screening of sialidase inhibitors and diagnosis of bacterial vaginosis. Chem. Commun. 54, 10691–10694. doi: 10.1039/C8CC06300A
Liu, X., Zhang, Y., Yu, W., Zhang, W., Jiang, J., Gu, Q., et al. (2023). Evaluating the activity of neuraminidase in bacterial vaginosis microflora and imaging sialic acid on the cell membrane by boron and nitrogen codoped fluorescent carbon dots. ACS Sens. 8, 2556–2562. doi: 10.1021/acssensors.3c00219
Lo, A. W., Seers, C. A., Boyce, J. D., Dashper, S. G., Slakeski, N., Lissel, J. P., et al. (2009). Comparative transcriptomic analysis of Porphyromonas gingivalisbiofilm and planktonic cells. BMC Microbiol. 9, 18. doi: 10.1186/1471-2180-9-18
Lopes Dos Santos Santiago, G., Deschaght, P., El Aila, N., Kiama, T. N., Verstraelen, H., Jefferson, K. K., et al. (2011). Gardnerella vaginalis comprises three distinct genotypes of which only two produce sialidase. Am. J. Obstet. Gynecol. 204, 450.e1–450.e7. doi: 10.1016/j.ajog.2010.12.061
Ma, X., Wang, X., Ye, S., Liu, J., Yuan, H., Wang, N. (2022). Biofilm and pathogenic factor analysis of Gardnerella vaginalis associated with bacterial vaginosis in Northeast China. Front. Microbiol. 13. doi: 10.3389/fmicb.2022.1033040
Mabugana, M. C., Dias, B. D. C., Muller, E. E., Kufa, T., Gumede, L., Mahlangu, M. P., et al. (2023). The evaluation of the AllplexTM BV molecular assay for the diagnosis of bacterial vaginosis in symptomatic South African females. Diagn. Microbiol. Infect. Dis. 106, 115924. doi: 10.1016/j.diagmicrobio.2023.115924
Madhivanan, P., Krupp, K., Li, T., Ravi, K., Selezneva, J., Srinivas, V., et al. (2014). Performance of BVBlue rapid test in detecting bacterial vaginosis among women in mysore, India. Infect. Dis. Obstet. Gynecol. 2014, 1–7. doi: 10.1155/2014/908313
Manns-James, L. (2011). Bacterial vaginosis and preterm birth. J. Midwife Womens Health 56, 575–583. doi: 10.1111/j.1542-2011.2011.00086.x
Moncla, B. J., Chappell, C. A., Debo, B. M., Meyn, L. A. (2016). The effects of hormones and vaginal microflora on the glycome of the female genital tract: cervical-vaginal fluid. PloS One 11, e0158687. doi: 10.1371/journal.pone.0158687
Moncla, B. J., Chappell, C. A., Mahal, L. K., Debo, B. M., Meyn, L. A., Hillier, S. L. (2015). Impact of bacterial vaginosis, as assessed by nugent criteria and hormonal status on glycosidases and lectin binding in cervicovaginal lavage samples. PloS One 10, e0127091. doi: 10.1371/journal.pone.0127091
Moran, A. P., Gupta, A., Joshi, L. (2011). Sweet-talk: role of host glycosylation in bacterial pathogenesis of the gastrointestinal tract. Gut 60, 1412–1425. doi: 10.1136/gut.2010.212704
Morrill, S. R., Saha, S., Varki, A. P., Lewis, W. G., Ram, S., Lewis, A. L. (2023). Gardnerella vaginolysin potentiates glycan molecular mimicry by neisseria gonorrhoeae. J. Infect. Dis. 228, 1610–1620. doi: 10.1093/infdis/jiad391
Myziuk, L., Romanowski, B., Johnson, S. C. (2003). BVBlue test for diagnosis of bacterial vaginosis. J. Clin. Microbiol. 41, 1925–1928. doi: 10.1128/JCM.41.5.1925-1928.2003
Natori, Y., Ohkura, N., Nasui, M., Atsumi, G., Kihara-Negishi, F. (2013). Acidic sialidase activity is aberrant in obese and diabetic mice. Biol. Pharm. Bull. 36, 1027–1031. doi: 10.1248/bpb.b12-00995
Ng, S., Chen, M., Kundu, S., Wang, X., Zhou, Z., Zheng, Z., et al. (2021). Large-scale characterisation of the pregnancy vaginal microbiome and sialidase activity in a low-risk Chinese population. NPJ Biofilms Microbiomes 7, 89. doi: 10.1038/s41522-021-00261-0
Novak, J., Belleti, R., da Silva Pinto, G. V., do Nascimento Bolpetti, A., da Silva, M. G., Marconi, C. (2023). Cervicovaginal Gardnerella sialidase-encoding gene in persistent human papillomavirus infection. Sci. Rep. 13, 14266. doi: 10.1038/s41598-023-41469-8
Nugent, R. P., Krohn, M. A., Hillier, S. L. (1991). Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J. Clin. Microbiol. 29, 297–301. doi: 10.1128/jcm.29.2.297-301.1991
Ochs, M., Hegermann, J., Lopez-Rodriguez, E., Timm, S., Nouailles, G., Matuszak, J., et al. (2020). On top of the alveolar epithelium: surfactant and the glycocalyx. IJMS 21, 3075. doi: 10.3390/ijms21093075
Permsak, S., Chananan, K., Suthee, P. (2005). BVBLUE test for diagnosis of bacterial vaginosis in pregnant women attending antenatal care at Phramongkutklao Hospital. J. Med. Assoc. Thai. 88 Suppl 3, S7–13.
Pezzicoli, A., Ruggiero, P., Amerighi, F., Telford, J. L., Soriani, M. (2012). Exogenous sialic acid transport contributes to group B streptococcus infection of mucosal surfaces. J. Infect. Dis. 206, 924–931. doi: 10.1093/infdis/jis451
Poole, J., Day, C. J., Von Itzstein, M., Paton, J. C., Jennings, M. P. (2018). Glycointeractions in bacterial pathogenesis. Nat. Rev. Microbiol. 16, 440–452. doi: 10.1038/s41579-018-0007-2
Pshezhetsky, A. V., Ashmarina, L. I. (2013). Desialylation of surface receptors as a new dimension in cell signaling. Biochem. Moscow 78, 736–745. doi: 10.1134/S0006297913070067
Qin, H., Xiao, B. (2022). Research progress on the correlation between gardnerella typing and bacterial vaginosis. Front. Cell. Infect. Microbiol. 12. doi: 10.3389/fcimb.2022.858155
Ram, S., Shaughnessy, J., De Oliveira, R. B., Lewis, L. A., Gulati, S., Rice, P. A. (2017). Gonococcal lipooligosaccharide sialylation: virulence factor and target for novel immunotherapeutics. Pathog. Dis. 75, ftx049. doi: 10.1093/femspd/ftx049
Ravel, J., Gajer, P., Abdo, Z., Schneider, G. M., Koenig, S. S. K., McCulle, S. L., et al. (2011). Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. U. S. A. 108, 4680–4687. doi: 10.1073/pnas.1002611107
Ravel, J., Moreno, I., Simón, C. (2021). Bacterial vaginosis and its association with infertility, endometritis, and pelvic inflammatory disease. Am. J. Obstet. Gynecol. 224, 251–257. doi: 10.1016/j.ajog.2020.10.019
Robinson, L. S., Schwebke, J., Lewis, W. G., Lewis, A. L. (2019). Identification and characterization of NanH2 and NanH3, enzymes responsible for sialidase activity in the vaginal bacterium Gardnerella vaginalis. J. Biol. Chem. 294, 5230–5245. doi: 10.1074/jbc.RA118.006221
Rodríguez-Nava, C., Cortés-Sarabia, K., Avila-Huerta, M. D., Ortiz-Riaño, E. J., Estrada-Moreno, A. K., Alarcón-Romero, L. D. C., et al. (2021). Nanophotonic sialidase immunoassay for bacterial vaginosis diagnosis. ACS Pharmacol. Transl. Sci. 4, 365–371. doi: 10.1021/acsptsci.0c00211
Roggentin, P., Rothe, B., Kaper, J. B., Galen, J., Lawrisuk, L., Vimr, E. R., et al. (1989). Conserved sequences in bacterial and viral sialidases. Glycoconjugate J. 6, 349–353. doi: 10.1007/BF01047853
Santos, C. M. A., Pires, M. C. V., Leão, T. L., Silva, A. K. S., Miranda, L. S., Martins, F. S., et al. (2018). Anti-inflammatory effect of two Lactobacillus strains during infection with Gardnerella vaginalis and Candida albicans in a HeLa cell culture model. Microbiology 164, 349–358. doi: 10.1099/mic.0.000608
Schauer, R., Kamerling, J. P. (1997). Chemistry, biochemistry and biology of sialic acids, in New comprehensive biochemistry (Amsterdam: Elsevier), 243–402. doi: 10.1016/S0167-7306(08)60624-9
Schauer, R., Kamerling, J. P. (2018). Exploration of the sialic acid world, in Advances in carbohydrate chemistry and biochemistry (Amsterdam: Elsevier), 1–213. doi: 10.1016/bs.accb.2018.09.001
Schellenberg, J. J., Paramel Jayaprakash, T., Withana Gamage, N., Patterson, M. H., Vaneechoutte, M., Hill, J. E. (2016). Gardnerella vaginalis Subgroups Defined by cpn60 Sequencing and Sialidase Activity in Isolates from Canada, Belgium and Kenya. PloS One 11, e0146510. doi: 10.1371/journal.pone.0146510
Schwebke, J. R., Muzny, C. A., Josey, W. E. (2014). Role of gardnerella vaginalis in the pathogenesis of bacterial vaginosis: A conceptual model. J. Infect. Dis. 210, 338–343. doi: 10.1093/infdis/jiu089
Shipitsyna, E., Krysanova, A., Khayrullina, G., Shalepo, K., Savicheva, A., Guschin, A., et al. (2019). Quantitation of all Four Gardnerella vaginalis Clades Detects Abnormal Vaginal Microbiota Characteristic of Bacterial Vaginosis More Accurately than Putative G. vaginalis Sialidase A Gene Count. Mol. Diagn. Ther. 23, 139–147. doi: 10.1007/s40291-019-00382-5
Siegel, S. J., Roche, A. M., Weiser, J. N. (2014). Influenza promotes pneumococcal growth during coinfection by providing host sialylated substrates as a nutrient source. Cell Host Microbe 16, 55–67. doi: 10.1016/j.chom.2014.06.005
Slomiany, B. L., Murty, V. L. N., Piotrowski, J., Slomiany, A. (1996). Salivary mucins in oral mucosal defense. Gen. Pharmacology: Vasc. System 27, 761–771. doi: 10.1016/0306-3623(95)02050-0
Smayevsky, J., Canigia, L. F., Lanza, A., Bianchini, H. (2001). Vaginal microflora associated with bacterial vaginosis in nonpregnant women: reliability of sialidase detection. Infect. Dis. Obstet. Gynecol. 9, 17–22. doi: 10.1155/S1064744901000047
SoÈnmez, H., SuÈer, S., GuÈngoÈr, Z., BalogÏlu, H., KoÈkogÏlu, E. (1999). Tissue and serum sialidase levels in breast cancer. Cancer Lett. 136, 75–78. doi: 10.1016/S0304-3835(98)00295-X
Soong, G. (2006). Bacterial neuraminidase facilitates mucosal infection by participating in biofilm production. J. Clin. Invest. 116, 2297–2305. doi: 10.1172/JCI27920
Srinivasan, S., Morgan, M. T., Fiedler, T. L., Djukovic, D., Hoffman, N. G., Raftery, D., et al. (2015). Metabolic signatures of bacterial vaginosis. mBio 6, e00204–e00215. doi: 10.1128/mBio.00204-15
Swidsinski, A., Mendling, W., Loening-Baucke, V., Ladhoff, A., Swidsinski, S., Hale, L. P., et al. (2005). Adherent biofilms in bacterial vaginosis. Obstet. Gynecol. 106, 1013–1023. doi: 10.1097/01.AOG.0000183594.45524.d2
Swidsinski, A., Mendling, W., Loening-Baucke, V., Swidsinski, S., Dörffel, Y., Scholze, J., et al. (2008). An adherent Gardnerella vaginalis biofilm persists on the vaginal epithelium after standard therapy with oral metronidazole. Am. J. Obstet. Gynecol. 198, 97.e1–97.e6. doi: 10.1016/j.ajog.2007.06.039
Taylor, B. D., Darville, T., Haggerty, C. L. (2013). Does bacterial vaginosis cause pelvic inflammatory disease? Sexually Transmitted Dis. 40, 117–122. doi: 10.1097/OLQ.0b013e31827c5a5b
Varki, A. (2009). Multiple changes in sialic acid biology during human evolution. Glycoconj J. 26, 231–245. doi: 10.1007/s10719-008-9183-z
Varki, A., Gagneux, P. (2012). Multifarious roles of sialic acids in immunity. Ann. New York Acad. Sci. 1253, 16–36. doi: 10.1111/j.1749-6632.2012.06517.x
Verstraelen, H., Swidsinski, A. (2013). The biofilm in bacterial vaginosis: implications for epidemiology, diagnosis and treatment. Curr. Opin. Infect. Dis. 26, 86. doi: 10.1097/QCO.0b013e32835c20cd
Vimr, E., Lichtensteiger, C. (2002). To sialylate, or not to sialylate: that is the question. Trends Microbiol. 10, 254–257. doi: 10.1016/S0966-842X(02)02361-2
Vitali, B., Cruciani, F., Picone, G., Parolin, C., Donders, G., Laghi, L. (2015). Vaginal microbiome and metabolome highlight specific signatures of bacterial vaginosis. Eur. J. Clin. Microbiol. Infect. Dis. 34, 2367–2376. doi: 10.1007/s10096-015-2490-y
Wang, L., Koppolu, S., Chappell, C., Moncla, B. J., Hillier, S. L., Mahal, L. K. (2015). Studying the effects of reproductive hormones and bacterial vaginosis on the glycome of lavage samples from the cervicovaginal cavity. PloS One 10, e0127021. doi: 10.1371/journal.pone.0127021
Wiggins, R. (2001). Mucinases and sialidases: their role in the pathogenesis of sexually transmitted infections in the female genital tract. Sex. Transm. Infect. 77, 402–408. doi: 10.1136/sti.77.6.402
Wong, A., Grau, M. A., Singh, A. K., Woodiga, S. A., King, S. J. (2018). Role of neuraminidase-producing bacteria in exposing cryptic carbohydrate receptors for streptococcus gordonii adherence. Infect. Immun. 86, e00068–e00018. doi: 10.1128/IAI.00068-18
Woo, H. S., Kim, D. W., Curtis-Long, M. J., Lee, B. W., Lee, J. H., Kim, J. Y., et al. (2011). Potent inhibition of bacterial neuraminidase activity by pterocarpans isolated from the roots of Lespedeza bicolor. Bioorganic Medicinal Chem. Lett. 21, 6100–6103. doi: 10.1016/j.bmcl.2011.08.046
Wu, S., Lin, X., Hui, K. M., Yang, S., Wu, X., Tan, Y., et al. (2019). A biochemiluminescent sialidase assay for diagnosis of bacterial vaginosis. Sci. Rep. 9, 20024. doi: 10.1038/s41598-019-56371-5
Xu, X., Tong, T., Yang, X., Pan, Y., Lin, L., Li, C. (2017). Differences in survival, virulence and biofilm formation between sialidase-deficient and W83 wild-type Porphyromonas gingivalis strains under stressful environmental conditions. BMC Microbiol. 17, 178. doi: 10.1186/s12866-017-1087-2
Yoo, E. M., Morrison, S. L. (2005). IgA: An immune glycoprotein. Clin. Immunol. 116, 3–10. doi: 10.1016/j.clim.2005.03.010
Zambon, M. C. (2001). The pathogenesis of influenza in humans. Rev. Med. Virol. 11, 227–241. doi: 10.1002/rmv.319
Zhang, Y., Rochefort, D. (2013). Fast and effective paper based sensor for self-diagnosis of bacterial vaginosis. Analytica Chimica Acta 800, 87–94. doi: 10.1016/j.aca.2013.09.032
Zhang, L., Wei, T.-T., Li, Y., Li, J., Fan, Y., Huang, F.-Q., et al. (2018). Functional metabolomics characterizes a key role for N -acetylneuraminic acid in coronary artery diseases. Circulation 137, 1374–1390. doi: 10.1161/CIRCULATIONAHA.117.031139
Keywords: bacterial vaginosis, Gardnerella vaginalis, sialidase, vaginal dysbiosis, pathogenesis
Citation: Chen L, Li J and Xiao B (2024) The role of sialidases in the pathogenesis of bacterial vaginosis and their use as a promising pharmacological target in bacterial vaginosis. Front. Cell. Infect. Microbiol. 14:1367233. doi: 10.3389/fcimb.2024.1367233
Received: 08 January 2024; Accepted: 19 February 2024;
Published: 01 March 2024.
Edited by:
Antonella Marangoni, University of Bologna, ItalyReviewed by:
Barbara Giordani, University of Bologna, ItalyCopyright © 2024 Chen, Li and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bingbing Xiao, ZG9jdG9yeGJiQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.