- Department of Endocrinology, Shengjing Hospital of China Medical University, Shenyang, Liaoning, China
Autoimmune thyroiditis (AITD) is a T-cell-mediated, organ- specific autoimmune disease caused by interactions between genetic and environmental factors. Patients with AITD show thyroid lymphocyte infiltration and an increase in the titer of thyroid autoimmune antibodies, thereby altering the integrity of thyroid follicle epithelial cells and dysregulating their metabolism and immune function, leading to a decrease in multi-tissue metabolic activity. Research has shown that patients with AITD have a significantly higher risk of adverse pregnancy outcomes, such as infertility and miscarriage. Levothyroxine(LT4) treatment can improve the pregnancy outcomes of normal pregnant women with thyroid peroxidase antibodies(TPOAb) positivity, but it is not effective for invitro fertilization embryo transfer (IVF-ET) in women with normal thyroid function and positive TPOAb. Other factors may also influence pregnancy outcomes of patients with AITD. Recent studies have revealed that the gut microbiota participates in the occurrence and development of AITD by influencing the gut-thyroid axis. The bacterial abundance and diversity of patients with Hashimoto thyroiditis (HT) were significantly reduced, and the relative abundances of Bacteroides, fecal Bacillus, Prevotella, and Lactobacillus also decreased. The confirmation of whether adjusting the composition of the gut microbiota can improve pregnancy outcomes in patients with AITD is still pending. This article reviews the characteristics of the gut microbiota in patients with AITD and the current research on its impact in pregnancy.
1 Background
Autoimmune thyroiditis (AITD) is a T-cell-mediated, organ-specific autoimmune disease that mainly manifests as Hashimoto’s thyroiditis (HT) and Graves’ disease (GD) (Antonelli et al., 2015). The incidence rate of AITD is approximately 5%, and is more common in women of childbearing age (Lee et al., 2015). It is accompanied by lymphocyte infiltration and elevated titers of thyroid autoimmune antibodies, such as thyroid peroxidase antibodies (TPOAb) and thyroid globulin antibodies (TgAb) (Fröhlich and Wahl, 2017), which are associated with varying degrees of hypothyroidism (Caturegli et al., 2014). Infiltrating lymphocytes can directly produce cytotoxicity in thyroid follicular cells or may indirectly affect their vitality and function through cytokines; this alters cell integrity and dysregulates their metabolism and immune function, leading to thyroid gland enlargement, gland fibrosis, decreased thyroid hormone (TH) levels, and ultimately reduced metabolic activity in multiple tissues (Ajjan and Weetman, 2015; Mori et al., 2012). It can cause a decrease in cardiovascular contractility and intestinal activity, coronary artery disease, hyperlipidemia, infertility, and neurosensory and musculoskeletal changes (Chaker et al., 2017).Therefore, it is crucial to reduce the incidence of AITD.
The etiology of AITD remains unclear. Epidemiological studies have shown that AITD is caused by interactions between genetic and environmental factors (Taylor et al., 2018). Genetic susceptibility plays a crucial role in autoimmune disorders, and immune modification genes (such as human leukocyte antigen classes I and II) and sites related to cytotoxic T lymphocyte-associated protein 4 (CTLA-4) may be involved in the autoimmune process. The interactions between these gene loci and environmental factors may affect the phenotype and severity of HT (Ajjan and Weetman, 2015). Environmental factors that may trigger the development of AITD include excessive iodine intake; deficiencies in selenium, iron, zinc, and vitamin D; intake of gluten (Liontiris and Mazokopakis, 2017), and alcohol; excessive stress; pregnancy; and the use of interferon, key immune modulators, such as iprimumab and alenzumab (Topliss, 2016).However, a study has found that smoking can reduce the risk of AITD (Effraimidis and Wiersinga, 2014). Recently, extensive research has indicated that the gut microbiota may play an important role in triggering AITD (Köhling et al., 2017), thus providing new ideas for treating AITD.
2 The correlation between AITD and gut microbiota
2.1 Gut microbiota
Gut microbiota is a general term for the microorganisms that parasitize the human intestine. It comprises bacteria, fungi, viruses, and archaea, with bacteria accounting for the majority. There are approximately 2000 species of gut microbiota, and more than 100 species have been identified by phylum classification. The main phylum categories include Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, and Verrucomycetes (Hardin et al., 2019). Among them, Firmicutes and Bacteroidetes account for > 90% of gut microbiota. The Firmicutes phylum has the highest number of bacteria, consisting of over 200 genera, including Lactobacillus, Mycoplasma, Bacillus, and Clostridium. The phylum Bacteroidetes includes more than 20 genera (Benson et al., 2010).
The gut microbiota undergoes corresponding changes owing to factors such as host genetics, diet, and environment, which can promote the growth of pathogenic bacteria (Kashtanova et al., 2016). Dysfunction of the gut microbiota not only causes a variety of gastrointestinal diseases, such as diarrhea, constipation, and enteritis, but can also induce chronic diseases, such as obesity, cardiovascular disease, diabetes, and metabolic syndrome (Marchesi et al., 2016; Cho and Blaser, 2012). Recent research has also shown that the intestinal flora and its metabolites may play a key role in the regulation of the immune system response and the development of autoimmune diseases, such as rheumatoid arthritis (RA) (Sun et al., 2019),multiple sclerosis (MS) (Cantoni et al., 2022), systemic lupus erythematosus(SLE) (Luo et al., 2018), type 1 diabetes(T1D) (Knip and Honkanen, 2017), and HT (Belvoncikova et al., 2022). The abundance of Prevotella in the feces of RA patients is higher (Alpizar-Rodriguez et al., 2019), and the genera Faecalibacterium and Bacteroides are reduced (Maeda and Takeda, 2019). Prevotella and Pseudomonas typically shows a decrease in the feces of patients with MS (Miyake et al., 2015), while the Akkermansia muciniphila typically increase (Ventura et al., 2019). Gut microbial diversity is significantly lower in patients with SLE with active disease than in non-SLE controls (Luo et al., 2018). In SLE patients, the relative abundance of Firmicutes decreased compared to the non-SLE controls, while Bacteroidetes increased (Hevia et al., 2014). A study conducted by Knip et al., to explore the relationship between gut microbiota and T1D, showed that children with positive islet-autoantibodies had a higher Bacteroidetes/Firmicutes ratio and lower Shannon diversity in the gut microbiota (Knip and Honkanen, 2017).
2.2 Characteristics of gut microbiota in patients with AITD
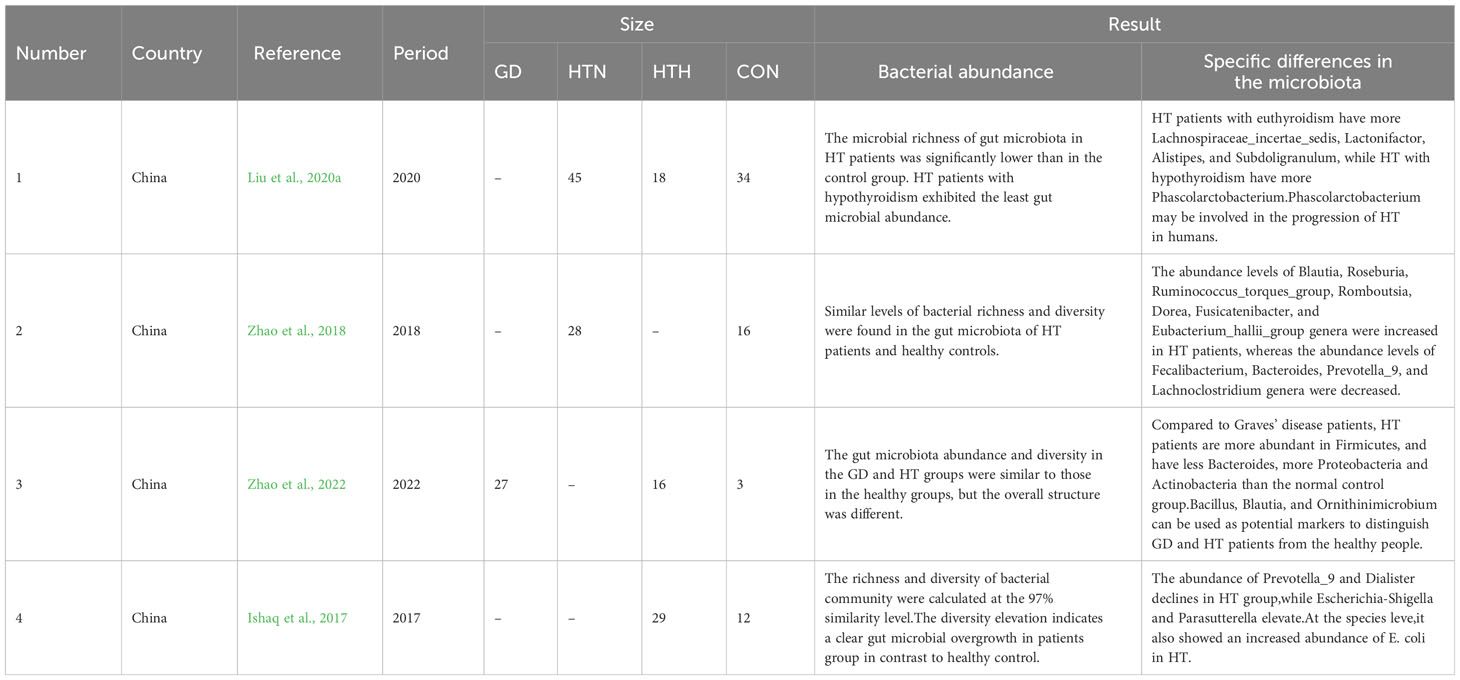
As shown in Table 1, some studies have proposed compositional modifications and bacterial ecological imbalances arise in the gut microbiota of patients with AITD, indicating that specific bacterial overgrowth and its impact on the gut-thyroid axis may play key roles in the occurrence and progression of AITD (Knezevic et al., 2020). This cross-sectional study compared 45 patients with HT of normal thyroid function (HTN), 18 patients with HT of hypothyroid status (HTH), and 34 healthy controls (CON). The bacterial abundance and diversity in patients with HTN and HTH were significantly lower than those in the healthy group, and patients with HTH showed the lowest intestinal microbial abundance (Liu et al., 2020a).
Sequencing analysis by Zhao et al. identified specific differences in the microbiota. The feces of patients with HT showed an increase in Firmicutes and Actinobacteria levels, whereas Bacteroides and Proteobacteria decreased. The ratio of Firmicutes to Bacteroides was significantly increased, and patients with HTN had a higher abundance and diversity of gut microbiota than the CON group (Zhao et al., 2018). A recent study found that compared to patients with Graves’ disease, patients with HT had more abundant Firmicutes, fewer Bacteroidetes, and more Proteobacteria and Actinobacteria levels than the normal control group (Zhao et al., 2022). Ishaq et al. also proposed that the relative abundance of Proteobacteria in the feces of patients with HT was significantly increased, whereas the relative abundance of Firmicutes and Bacteroidetes was decreased (Ishaq et al., 2017). These three studies found that the HT group had high levels of Spirochaetaceae, Enterobacteriaceae, Alcaligenaceae, Trichocomaceae, Erythrobacteraceae, and Bacteroidaceae. In contrast, the levels of Prevotella, Ruminococcus, and Vibrio were decreased in the HT group (Zhao et al., 2018; Zhao et al., 2022; Ishaq et al., 2017).
At the genus level, the relative abundances of Bacteroides, fecal Bacillus, Prevotella, and Lactobacillus in the fecal samples of patients with HT decreased, while the relative abundances of Blautia, Ruminococcus, Rose, Clostridium, Longbuti, Dorea, and Eubacterium increased significantly (Zhao et al., 2018). Studies have also suggested a decrease in Prevotella levels in the feces of patients with HT (Ishaq et al., 2017). A meta-analysis showed that the abundance of Firmicutes, Bifidobacteria, and Lactobacillus in patients with AITD was lower than that in healthy controls; patients with HT having slightly higher levels of Bacteroides than in other bacteria. These taxa are associated with clinical indicators, such as an altered host metabolism or TPOAb and TgAb positivity in the host (Gong et al., 2021). A cross-sectional study of 22 patients with HT and 11 healthy individuals conducted by Zhao et al. showed that 18 genera in the microbiota of patients with HT were positively correlated with TPOAb or TgAb, whereas six genera were negatively correlated. In addition, the Heterobacteria genus is positively correlated with free thyroxine, Clostridium genus is negatively correlated with free thyroxine, and Pleurotus genus is negatively correlated with serum thyrotropin (TSH) (Zhao et al., 2018).
2.3 The mechanism of gut microbiota affecting the development of AITD
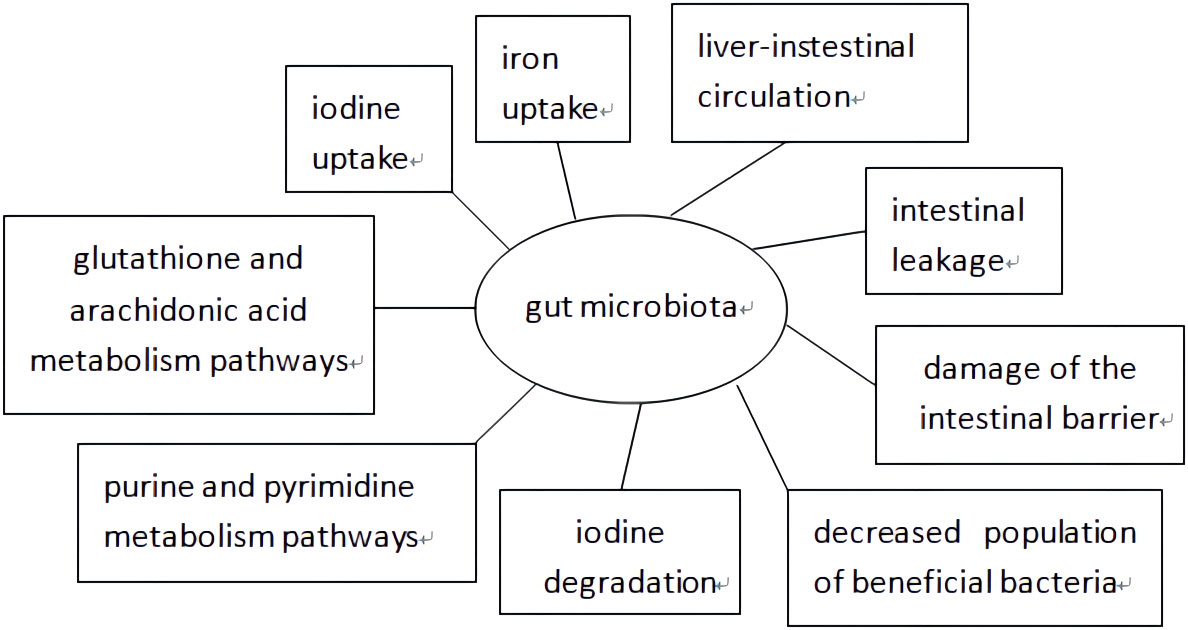
As shown in Figure 1, extensive research has been conducted on the mechanism by which the gut microbiota affects AITD development. Minerals such as selenium, iron, and zinc have a significant impact on the interactions between the host and gut microbiota (Knezevic et al., 2020), which affect TH levels by regulating iodine uptake, degradation, and hepatic-intestinal circulation (Fröhlich and Wahl, 2019). The gut microbiota produces its own antigens through protein post-translational modifications, activates Toll-like receptor 4 induced by lipopolysaccharide (LPS), induces T helper cell translocation from type 1 (Th1) to type 2 (Th2), reduces the integrity of intercellular connections, and promotes AITD development through intestinal leakage (Lerner et al., 2017). Some scholars also believe that changes in gut microbiota occur through post-translational modifications of luminal proteins, the transition of the intestinal mucosa to a pro-inflammatory environment, intestinal ecological imbalances leading to damage of the intestinal barrier, antigen entry into the circulation, activation of the immune system antibodies in the circulation, which react with bacterial antigens to enhance inflammatory body activations in the thyroid gland, and excessive bacterial growth that participates in the development of autoimmune thyroiditis (Mu et al., 2017; Cayres et al., 2021; Tomasello et al., 2015). Another theory suggests that a decreased population of beneficial bacteria such as Lactobacillus and Bifidobacterium is related to the development of AITD. Lactobacillus has been proven to protect TH17 cells and support their barrier integrity by secreting IL-22 and IL-17. The Th17/Treg imbalance may cause inflammatory disorders, indicating that Lactobacillus participates in the immune system balance. Bifidobacterium and Lactobacillus exhibit anti-inflammatory effects and protect the body from pathogens. Moreover, increased Bacteroides fragilis may account for the upregulation of IL-18, IL-1β, and caspase-1, promoting an inflammatory response (Kiseleva et al., 2011). It has been proposed that bacterial strains participate in the development of HT by influencing glutathione and arachidonic acid metabolism, and purine and pyrimidine metabolism pathways; however, further validation is still needed (Zhao et al., 2022).
3 The impact of AITD on pregnancy
Numerous studies have shown that AITD increases the risk of adverse pregnancy outcomes. Women may experience changes in hormone levels and metabolic needs during pregnancy, such as an increase demand for THs to meet the needs of fetal growth and brain development. Therefore, thyroid diseases are frequently observed during pregnancy (Krassas et al., 2010). Thyroid dysfunction during pregnancy can include overt hypothyroidism (OH) and subclinical hypothyroidism (SCH). The relative incidence rates of OH and SCH are approximately 0.3–1.0% and 4.0–17.8%, respectively (Shan and Wang, 2022). AITD is the main cause of hypothyroidism in pregnant women, with an average incidence of 7.8% (Krassas et al., 2015).
3.1 AITD and infertility
The incidence of infertility in women with AITD is high, with a prevalence of 52.3% in patients with GD and 47.0% in patients with HT (Quintino-Moro et al., 2014). In a prospective study, 438 women with infertility and 100 healthy women in postpartum were compared, and it was found that the prevalence of TPOAb positivity was significantly higher among women with infertility factors than those of the healthy group (Poppe et al., 2002).
3.2 AITD and miscarriage
AITD is associated with recurrent miscarriage (RM). Some scholars believe that autoimmunity in women with AITD affects embryo implantation by inducing endometrial receptive defects (Kim et al., 2011; Liu et al., 2020b; Wu et al., 2019), leading to an increase in fetal miscarriages. Some scholars also believe that, in women affected by thyroid autoimmunity, the thyroid may have insufficient TH release in the early stages of pregnancy, and their increased miscarriage rate may be due to TH deficiency rather than a systemic overreaction of the immune system (Abalovich et al., 2007). The local effects of TH on female reproductive organs and embryos during embryo implantation are crucial for successful pregnancies (Stavreus Evers, 2012).
A prospective cohort study conducted in women with infertility found that the median serum TSH levels were significantly higher in TPOAb- and TgAb-positive women than in women without AITD (Unuane et al., 2013). The TSH level is a sensitive indicator of thyroid function during pregnancy (Tortosa, 2011). The upper limit of the normal value of TSH in early pregnancy should be 4.0 mU/L, and 2.5 mU/L≤ TSH< 4.0 mU/L is called the normal high value of TSH. Women with positive thyroid antibodies or those undergoing assisted reproduction require levothyroxine (LT4) (Shan and Wang, 2022). Therefore, some scholars used LT4 intervention as adjuvant therapy in 227 women with AITD who suffered from RM and it was found that low-dose LT4 treatment can, to some extent, prevent miscarriage (Dal Lago et al., 2021). Another study also showed that administering LT4 treatment to pregnant women with a history of hypothyroidism and TPOAb-positivity can improve their live birth rates and reduce miscarriages (Leng et al., 2022). However, some studies have found that LT4 treatment did not increase live birth rates in women with RM, normal thyroid function, and positive TPOAb (van Dijk et al., 2022). Hong et al. also confirmed that LT4 treatment did not reduce miscarriage rates or increase live birth rates in women undergoing in vitro fertilization embryo transfer (IVF-ET) with intact thyroid function and positive TPOAb (Wang et al., 2017). The use of glucocorticoids and aspirin as adjunctive therapies in euthyroid women with AITD undergoing IVF-ET may not improve pregnancy or live birth rates either (Zhou et al., 2022).
3.3 AITD and other adverse pregnancy outcomes
After analyzing 35 studies, we found that TPOAb-positive women had a higher risk of premature birth than TPOAb-negative women. The relationship between TPOAb positivity and premature birth appears to be related to TSH concentration. TPOAb-positive women with TSH concentrations higher than 4.0 mU/L have a higher risk of premature birth (Korevaar et al., 2019). Tang et al. found that with an increase in TPOAb and TgAb (in early and mid-pregnancy), the maternal risk of gestational diabetes mellitus (GDM) significantly increased. Therefore, the presence of thyroid antibodies can predict postpartum glucose abnormalities in individuals with GDM (Tang et al., 2021). Some studies have evaluated the impact of LT4 on the risk of miscarriage, premature birth, preeclampsia, placental abruption, birth weight, gestational age at delivery, and neonatal admission rate in TPOAb-positive pregnant women with normal thyroid function; nevertheless, no significant differences between the LT4 administrated and control groups were found. However, there has been a downward trend in premature births and miscarriages.
4 Gut microbiota and pregnancy
In recent years, increasing evidence has shown that sex hormones can affect the structure of gut microbiota, and sex hormones act through steroid receptors directly regulate the metabolism of bacteria (Yoon and Kim, 2021). Autonomous diseases are typically more prevalent in women than in men (Quintero et al., 2012). A role for gut microbiota in the sex bias in autoimmunity has been revealed by different studies in animal models. This bias is at least partially mediated by the microbial metabolism of sex hormones (Ortona et al., 2016). Pregnancy is a special period for women, as the body undergoes various physiological changes, which provides the fetus with the best growth and development conditions (Costantine, 2014).Changes of hormones in pregnancy can alter the gut microbiota structure of pregnant women (Koren et al., 2012). As pregnancy progresses, there is a significant enrichment of Neisseria, Brautia, Collins, and Bifidobacterium genera. The increase in relative abundance of Bifidobacterium is highly likely mediated by progesterone (Nuriel-Ohayon et al., 2019). Throughout pregnancy, significant changes occur in the gut microbiota of mothers, which subsequently affect the gut microbiota of infants. Changes in microbiome composition occur between the first and third trimesters of pregnancy (Gorczyca et al., 2022). Scholars transplanted fecal microbiota from the first and third trimesters of pregnancy into sterile mice. Compared with mice transplanted with the first trimester of pregnancy microbiota, mice transplanted with the third trimester of microbiota showed significant weight gain, insulin resistance, and greater inflammatory response (Koren et al., 2012). Akkermansia, Bifidobacteria, and Firmicutes populations increase, which is related to an increase in energy storage requirements. Proteobacteria and Actinobacteria levels increase, owing to their pro-inflammatory properties, and have protective effects on both mothers and fetuses (Rodríguez et al., 2015). The mechanism of these changes involves the regulation of the brain and intestinal axes by production of maternal estrogen and progesterone, as well as immune activation of the intestinal mucosa (Mulak et al., 2014; Stanislawski et al., 2017).
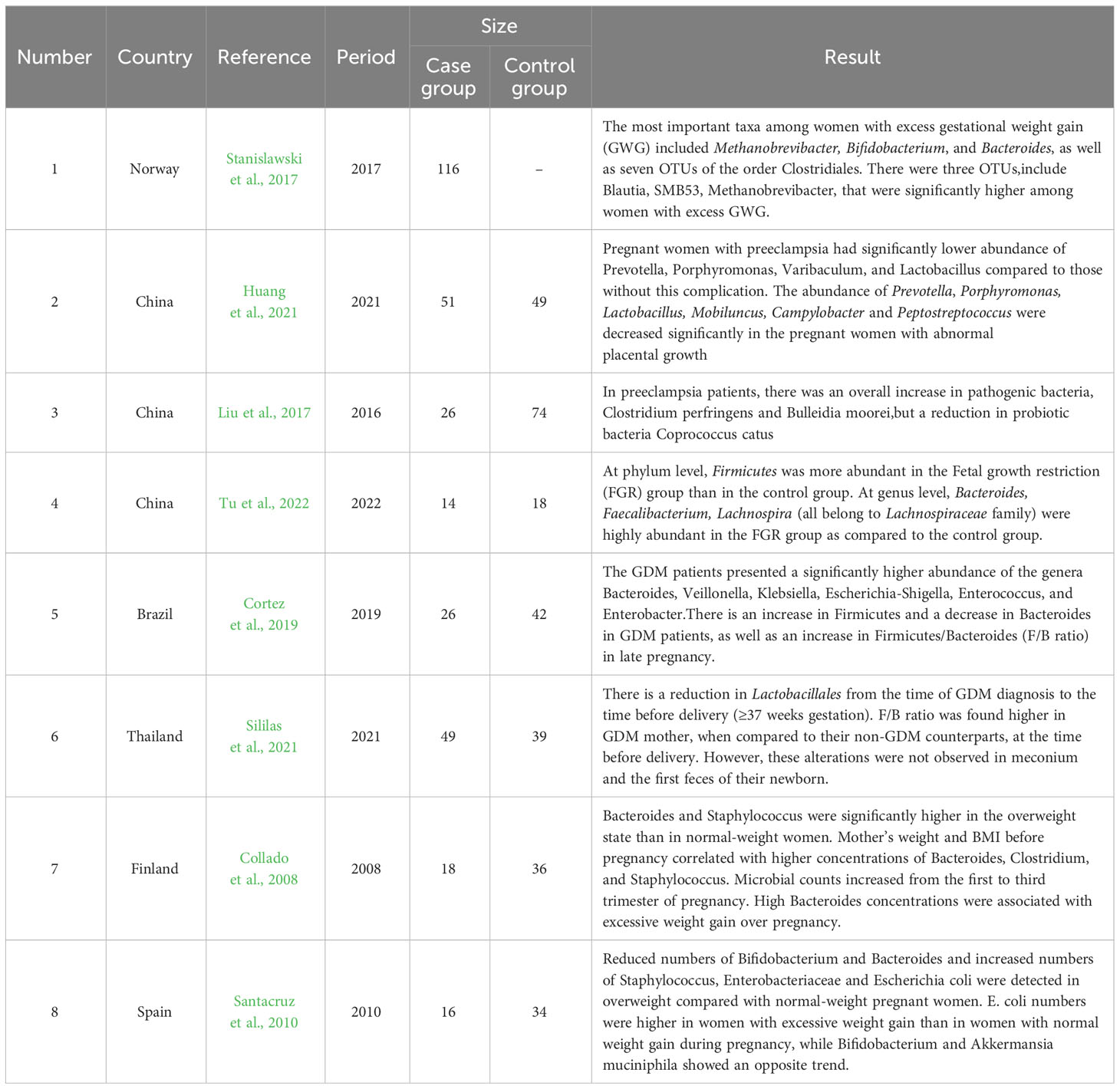
As shown in Table 2, many studies have demonstrated that the gut microbiota is associated with many diseases during pregnancy. A study conducted among 100 women showed that 26 pregnant women with preeclampsia had a significantly lower abundance of Prevotella, Porphyromonas, Varibaculum, and Lactobacillus than pregnant women without this complication (Huang et al., 2021). Liu also reported significant structural changes in the gut microbiota of patients with preeclampsia. In these patients, there was an overall increase in the pathogenic bacteria Clostridium perfringens and Bulleidia moorei, but a reduction in the probiotic bacteria Coprococcus catus (Liu et al., 2017). Fetal growth restriction (FGR) is a common obstetric complication and also known as intrauterine growth restriction (IUGR) (Sharma et al., 2016). By 16S rDNA amplicon sequencing of samples, collected from pregnant women in the FGR and control groups, it was revealed that the genera Bacteroides, Faecalibacterium, and Lachnospira were highly abundant in the FGR group (Tu et al., 2022). GDM is one of the most common metabolic complications of pregnancy and its prevalence has significantly increased over the last few years (Filardi et al., 2019). Cortez et al. found an increase in Firmicutes and a decrease in Bacteroides levels in patients with GDM, as well as an increase in Firmicutes/Bacteroides (F/B) ratio in late pregnancy (Cortez et al., 2019). The increase in the F/B ratio is associated with low-grade inflammation, insulin resistance, and obesity (Pascale et al., 2019). Sililas et al. also found that the F/B ratio in the third trimester of pregnancy was higher in patients with GDM than in those of the control group (Sililas et al., 2021). Specific shifts in microbial composition were also associated with maternal factors such as BMI, weight, and weight gain during pregnancy. A higher number of Bifidobacterium organisms and lower levels of Staphylococcus may protect the mother from developing excess weight (Collado et al., 2008; Santacruz et al., 2010). A study found that overweight participants had significantly higher fecal concentrations of the genus Bacteroides and a lower F/B ratio (Schwiertz et al., 2010).
5 Summary
AITD increases the risk of infertility, miscarriage, and other adverse pregnancy and neonatal outcomes. The use of LT4 intervention can reduce adverse outcomes in patients with normally high TSH levels. However, it is not effective in euthyroid patients with AITD who undergo IVF-ET assisted pregnancy. It is not clear whether other factors affect adverse pregnancy outcomes in patients with AITD (van Dijk et al., 2022). Therefore, a new interventional approach is required to reduce adverse outcomes. Some researchers have found differences in the composition of the gut microbiota between patients with AITD and the normal population. Specific bacterial overgrowth and its impact on the gut-thyroid axis may promote thyroid antibody production. Currently, little research has explored the relationship between specific differences in gut microbiota composition in patients with AITD, and especially of those who are pregnant. It is unclear how the gut microbiota contributes to adverse pregnancy outcomes in TPOAb-positive women. Whether it is possible to improve the pregnancy outcomes of patients with AITD by regulating the composition of the gut microbiota still needs to be confirmed.
Author contributions
YS: Writing – original draft, Writing – review & editing. YB: Writing – review & editing. CL: Writing – review & editing. XZ: Writing – review & editing. LZ: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This article was supported by “345 talent project plan” of Shengjing Hospital of China Medical University.
Acknowledgments
Thanks Shengjing Hospital of China Medical University for giving financial support for this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abalovich, M., Amino, N., Barbour, L. A., Cobin, R. H., De Groot, L. J., Glinoer, D., et al. (2007). Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 92, S1–47. doi: 10.1210/jc.2007-0141
Ajjan, R. A., Weetman, A. P. (2015). The pathogenesis of hashimoto’s thyroiditis: further developments in our understanding. Horm. Metab. Res. 47, 702–710. doi: 10.1055/s-0035-1548832
Alpizar-Rodriguez, D., Lesker, T. R., Gronow, A., Gilbert, B., Raemy, E., Lamacchia, C., et al. (2019). Prevotella copri in individuals at risk for rheumatoid arthritis. Ann. Rheum Dis. 78, 590–593. doi: 10.1136/annrheumdis-2018-214514
Antonelli, A., et al. (2015). Autoimmune thyroid disorders. Autoimmun Rev. 14, 174–180. doi: 10.1016/j.autrev.2014.10.016
Belvoncikova, P., Maronek, M., Gardlik, R. (2022). Gut dysbiosis and fecal microbiota transplantation in autoimmune diseases. Int. J. Mol. Sci. 23 (18), 10729. doi: 10.3390/ijms231810729
Benson, A. K., Kelly, S. A., Legge, R., Ma, F., Low, S. J., Kim, J., et al. (2010). Individuality in gut microbiota composition is a complex polygenic trait shaped by multiple environmental and host genetic factors. Proc. Natl. Acad. Sci. U.S.A. 107, 18933–18938. doi: 10.1073/pnas.1007028107
Cantoni, C., Lin, Q., Dorsett, Y., Ghezzi, L., Liu, Z., Pan, Y., et al. (2022). Alterations of host-gut microbiome interactions in multiple sclerosis. EBioMedicine 76, 103798. doi: 10.1016/j.ebiom.2021.103798
Caturegli, P., De Remigis, A., Rose, N. R. (2014). Hashimoto thyroiditis: clinical and diagnostic criteria. Autoimmun Rev. 13, 391–397. doi: 10.1016/j.autrev.2014.01.007
Cayres, L. C. F., de Salis, L. V. V., Rodrigues, G. S. P., Lengert, A. V. H., Biondi, A. P. C., Sargentini, L. D. B., et al. (2021). Detection of alterations in the gut microbiota and intestinal permeability in patients with hashimoto thyroiditis. Front. Immunol. 12, 579140. doi: 10.3389/fimmu.2021.579140
Chaker, L., Bianco, A. C., Jonklaas, J., Peeters, R. P. (2017). Hypothyroidism. Lancet 390, 1550–1562. doi: 10.1016/S0140-6736(17)30703-1
Cho, I., Blaser, M. J. (2012). The human microbiome: at the interface of health and disease. Nat. Rev. Genet. 13, 260–270. doi: 10.1038/nrg3182
Collado, M. C., Isolauri, E., Laitinen, K., Salminen, S. (2008). Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88, 894–899. doi: 10.1093/ajcn/88.4.894
Cortez, R. V., Taddei, C. R., Sparvoli, L. G., Ângelo, A. G. S., Padilha, M., et al. (2019). Microbiome and its relation to gestational diabetes. Endocrine 64, 254–264. doi: 10.1007/s12020-018-1813-z
Costantine, M. M. (2014). Physiologic and pharmacokinetic changes in pregnancy. Front. Pharmacol. 5, 65. doi: 10.3389/fphar.2014.00065
Dal Lago, A., Galanti, F., Miriello, D., Marcoccia, A., Massimiani, M., Campagnolo, L., et al. (2021). Positive impact of levothyroxine treatment on pregnancy outcome in euthyroid women with thyroid autoimmunity affected by recurrent miscarriage. J. Clin. Med. 10, 10 2105. doi: 10.3390/jcm10102105
Effraimidis, G., Wiersinga, W. M. (2014). Mechanisms in endocrinology: autoimmune thyroid disease: old and new players. Eur. J. Endocrinol. 170, R241–R252. doi: 10.1530/EJE-14-0047
Filardi, T., Panimolle, F., Crescioli, C., Lenzi, A., Morano, S. (2019). Gestational diabetes mellitus: the impact of carbohydrate quality in diet. Nutrients 11 (7), 1549. doi: 10.3390/nu11071549
Fröhlich, E., Wahl, R. (2017). Thyroid autoimmunity: role of anti-thyroid antibodies in thyroid and extra-thyroidal diseases. Front. Immunol. 8, 521. doi: 10.3389/fimmu.2017.00521
Fröhlich, E., Wahl, R. (2019). Microbiota and thyroid interaction in health and disease. Trends Endocrinol. Metab. 30, 479–490. doi: 10.1016/j.tem.2019.05.008
Gong, B., Wang, C., Meng, F., Wang, H., Song, B., Yang, Y., et al. (2021). Association between gut microbiota and autoimmune thyroid disease: A systematic review and meta-analysis. Front. Endocrinol. (Lausanne) 12, 774362. doi: 10.37766/inplasy2021.4.0135
Gorczyca, K., Obuchowska, A., Kimber-Trojnar, Ż., Wierzchowska-Opoka, M., Leszczyńska-Gorzelak, B. (2022). Changes in the gut microbiome and pathologies in pregnancy. Int. J. Environ. Res. Public Health 19. doi: 10.3390/ijerph19169961
Hardin, S. J., Singh, M., Eyob, W., Molnar, J. C., Homme, R. P., George, A. K., et al. (2019). Diet-induced chronic syndrome, metabolically transformed trimethylamine-N-oxide, and the cardiovascular functions. Rev. Cardiovasc. Med. 20, 121–128. doi: 10.31083/j.rcm.2019.03.518
Hevia, A., Milani, C., López, P., Cuervo, A., Arboleya, S., Duranti, S., et al. (2014). Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 5, e01548–e01514. doi: 10.1128/mBio.01548-14
Huang, L., Cai, M., Li, L., Zhang, X., Xu, Y., Xiao, J., et al. (2021). Gut microbiota changes in preeclampsia, abnormal placental growth and healthy pregnant women. BMC Microbiol. 21, 265. doi: 10.1186/s12866-021-02327-7
Ishaq, H. M., Mohammad, I. S., Guo, H., Shahzad, M., Hou, Y. J., Ma, C., et al. (2017). Molecular estimation of alteration in intestinal microbial composition in Hashimoto’s thyroiditis patients. BioMed. Pharmacother. 95, 865–874. doi: 10.1016/j.biopha.2017.08.101
Kashtanova, D. A., Popenko, A. S., Tkacheva, O. N., Tyakht, A. B., Alexeev, D. G., Boytsov, S. A. (2016). Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 32, 620–627. doi: 10.1016/j.nut.2015.12.037
Kim, N. Y., Cho, H. J., Kim, H. Y., Yang, K. M., Ahn, H. K., Thornton, S., et al. (2011). Thyroid autoimmunity and its association with cellular and humoral immunity in women with reproductive failures. Am. J. Reprod. Immunol. 65, 78–87. doi: 10.1111/aji.2010.65.issue-1
Kiseleva, E. P., Mikhailopulo, K. I., Sviridov, O. V., Novik, G. I., Knirel, Y. A., Szwajcer Dey, E. (2011). The role of components of Bifidobacterium and Lactobacillus in pathogenesis and serologic diagnosis of autoimmune thyroid diseases. Benef Microbes 2, 139–154. doi: 10.3920/BM2010.0011
Knezevic, J., Starchl, C., Tmava Berisha, A., Amrein, K. (2020). Thyroid-gut-axis: how does the microbiota influence thyroid function? Nutrients 12 (2), 1769. doi: 10.3390/nu12061769
Knip, M., Honkanen, J. (2017). Modulation of type 1 diabetes risk by the intestinal microbiome. Curr. Diabetes Rep. 17, 105. doi: 10.1007/s11892-017-0933-9
Köhling, H. L., Plummer, S. F., Marchesi, J. R., Davidge, K. S., Ludgate, M. (2017). The microbiota and autoimmunity: Their role in thyroid autoimmune diseases. Clin. Immunol. 183, 63–74. doi: 10.1016/j.clim.2017.07.001
Koren, O., Goodrich, J. K., Cullender, T. C., Spor, A., Laitinen, K., Bäckhed, H. K., et al. (2012). Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 150, 470–480. doi: 10.1016/j.cell.2012.07.008
Korevaar, T. I. M., Derakhshan, A., Taylor, P. N., Meima, M., Chen, L., Bliddal, S., et al. (2019). Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: A systematic review and meta-analysis. Jama 322, 632–641. doi: 10.1001/jama.2019.10931
Krassas, G., Karras, S. N., Pontikides, N. (2015). Thyroid diseases during pregnancy: a number of important issues. Hormones (Athens) 14, 59–69. doi: 10.1007/BF03401381
Krassas, G. E., Poppe, K., Glinoer, D. (2010). Thyroid function and human reproductive health. Endocr. Rev. 31, 702–755. doi: 10.1210/er.2009-0041
Lee, H. J., Li, C. W., Hammerstad, S. S., Stefan, M., Tomer, Y. (2015). Immunogenetics of autoimmune thyroid diseases: A comprehensive review. J. Autoimmun 64, 82–90. doi: 10.1016/j.jaut.2015.07.009
Leng, T., Li, X., Zhang, H. (2022). Levothyroxine treatment for subclinical hypothyroidism improves the rate of live births in pregnant women with recurrent pregnancy loss: a randomized clinical trial. Gynecol Endocrinol. 38, 488–494. doi: 10.1080/09513590.2022.2063831
Lerner, A., Jeremias, P., Matthias, T. (2017). Gut-thyroid axis and celiac disease. Endocr. Connect 6, R52–r58. doi: 10.1530/EC-17-0021
Liontiris, M. I., Mazokopakis, E. E. (2017). A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients.Points that need more investigation. Hell J. Nucl. Med. 20, 51–56. doi: 10.1967/s002449910507
Liu, J., Yang, H., Yin, Z., Jiang, X., Zhong, H., Qiu, D., et al. (2017). Remodeling of the gut microbiota and structural shifts in Preeclampsia patients in South China. Eur. J. Clin. Microbiol. Infect. Dis. 36, 713–719. doi: 10.1007/s10096-016-2853-z
Liu, S., An, Y., Cao, B., Sun, R., Ke, J., Zhao, D. (2020a). The composition of gut microbiota in patients bearing hashimoto’s thyroiditis with euthyroidism and hypothyroidism. Int. J. Endocrinol. 2020 p, 5036959. doi: 10.1155/2020/5036959
Liu, S., Xu, F., Wei, H., Huang, C., Chen, X., Lian, R., et al. (2020b). The correlation of thyroid autoimmunity and peripheral and uterine immune status in women with recurrent miscarriage. J. Reprod. Immunol. 139, 103118. doi: 10.1016/j.jri.2020.103118
Luo, X. M., Edwards, M. R., Mu, Q., Yu, Y., Vieson, M. D., Reilly, C. M., et al. (2018). Gut microbiota in human systemic lupus erythematosus and a mouse model of lupus. Appl. Environ. Microbiol. 84 (4), e02288-17. doi: 10.1128/AEM.02288-17
Maeda, Y., Takeda, K. (2019). Host-microbiota interactions in rheumatoid arthritis. Exp. Mol. Med. 51, 1–6. doi: 10.1038/s12276-019-0283-6
Marchesi, J. R., Adams, D. H., Fava, F., Hermes, G. D., Hirschfield, G. M., Hold, G., et al. (2016). The gut microbiota and host health: a new clinical frontier. Gut 65, 330–339. doi: 10.1136/gutjnl-2015-309990
Miyake, S., Kim, S., Suda, W., Oshima, K., Nakamura, M., Matsuoka, T., et al. (2015). Dysbiosis in the gut microbiota of patients with multiple sclerosis, with a striking depletion of species belonging to clostridia XIVa and IV clusters. PloS One 10, e0137429. doi: 10.1371/journal.pone.0137429
Mori, K., Nakagawa, Y., Ozaki, H. (2012). Does the gut microbiota trigger Hashimoto’s thyroiditis? Discovery Med. 14, 321–326.
Mu, Q., Kirby, J., Reilly, C. M., Luo, X. M. (2017). Leaky gut as a danger signal for autoimmune diseases. Front. Immunol. 8, 598. doi: 10.3389/fimmu.2017.00598
Mulak, A., Taché, Y., Larauche, M. (2014). Sex hormones in the modulation of irritable bowel syndrome. World J. Gastroenterol. 20, 2433–2448. doi: 10.3748/wjg.v20.i10.2433
Nuriel-Ohayon, M., Neuman, H., Ziv, O., Belogolovski, A., Barsheshet, Y., Bloch, N., et al. (2019). Progesterone increases bifidobacterium relative abundance during late pregnancy. Cell Rep. 27, 730–736.e3. doi: 10.1016/j.celrep.2019.03.075
Ortona, E., Pierdominici, M., Maselli, A., Veroni, C., Aloisi, F., Shoenfeld, Y. (2016). Sex-based differences in autoimmune diseases. Ann. Ist Super Sanita 52, 205–212. doi: 10.4415/ANN_16_02_12
Pascale, A., Marchesi, N., Govoni, S., Coppola, A., Gazzaruso, C. (2019). The role of gut microbiota in obesity, diabetes mellitus, and effect of metformin: new insights into old diseases. Curr. Opin. Pharmacol. 49, 1–5. doi: 10.1016/j.coph.2019.03.011
Poppe, K., Glinoer, D., Van Steirteghem, A., Tournaye, H., Devroey, P., Schiettecatte, J., et al. (2002). Thyroid dysfunction and autoimmunity in infertile women. Thyroid 12, 997–1001. doi: 10.1089/105072502320908330
Quintero, O. L., Amador-Patarroyo, M. J., Montoya-Ortiz, G., Rojas-Villarraga, A., Anaya, J. M. (2012). Autoimmune disease and gender: plausible mechanisms for the female predominance of autoimmunity. J. Autoimmun 38, J109–J119. doi: 10.1016/j.jaut.2011.10.003
Quintino-Moro, A., Zantut-Wittmann, D. E., Tambascia, M., Machado Hda, C., Fernandes, A. (2014). High prevalence of infertility among women with graves’ Disease and hashimoto’s thyroiditis. Int. J. Endocrinol. 2014, 982705. doi: 10.1155/2014/982705
Rodríguez, J. M., Murphy, K., Stanton, C., Ross, R. P., Kober, O. I., Juge, N., et al. (2015). The composition of the gut microbiota throughout life, with an emphasis on early life. Microb. Ecol. Health Dis. 26, 26050. doi: 10.3402/mehd.v26.26050
Santacruz, A., Collado, M. C., García-Valdés, L., Segura, M. T., Martín-Lagos, J. A., Anjos, T., et al. (2010). Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 104, 83–92. doi: 10.1017/S0007114510000176
Schwiertz, A., Taras, D., Schäfer, K., Beijer, S., Bos, N. A., Donus, C., et al. (2010). Microbiota and SCFA in lean and overweight healthy subjects. Obes. (Silver Spring) 18, 190–195. doi: 10.1038/oby.2009.167
Shan, Z. Y., Wang, L. H. (2022). Guidelines for prevention and management of thyroid diseases during pregnancy and perinatal period. China J. Endocrinol. Metab. 38 (7). doi: 10.3760/cma.j.cn311282-20220416-00234
Sharma, D., Shastri, S., Sharma, P. (2016). Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 10, 67–83. doi: 10.4137/CMPed.S40070
Sililas, P., Huang, L., Thonusin, C., Luewan, S., Chattipakorn, N., Chattipakorn, S., et al. (2021). Association between gut microbiota and development of gestational diabetes mellitus. Microorganisms 9 (8), 1686. doi: 10.3390/microorganisms9081686
Stanislawski, M. A., Dabelea, D., Wagner, B. D., Sontag, M. K., Lozupone, C. A., Eggesbø, M. (2017). Pre-pregnancy weight, gestational weight gain, and the gut microbiota of mothers and their infants. Microbiome 5, 113. doi: 10.1186/s40168-017-0332-0
Stavreus Evers, A. (2012). Paracrine interactions of thyroid hormones and thyroid stimulation hormone in the female reproductive tract have an impact on female fertility. Front. Endocrinol. (Lausanne) 3, 50. doi: 10.3389/fendo.2012.00050
Sun, Y., Chen, Q., Lin, P., Xu, R., He, D., Ji, W., et al. (2019). Characteristics of gut microbiota in patients with rheumatoid arthritis in Shanghai, China. Front. Cell Infect. Microbiol. 9, 369. doi: 10.3389/fcimb.2019.00369
Tang, L., Li, P., Zhou, H., Li, L. (2021). A longitudinal study of thyroid markers during pregnancy and the risk of gestational diabetes mellitus and post-partum glucose metabolism. Diabetes Metab. Res. Rev. 37, e3441. doi: 10.1002/dmrr.3441
Taylor, P. N., Albrecht, D., Scholz, A., Gutierrez-Buey, G., Lazarus, J. H., Dayan, C. M., et al. (2018). Global epidemiology of hyperthyroidism and hypothyroidism. Nat. Rev. Endocrinol. 14, 301–316. doi: 10.1038/nrendo.2018.18
Tomasello, G., Tralongo, P., Amoroso, F., Damiani, P., Sinagra, E., Noto, M., et al. (2015). Dysmicrobism, inflammatory bowel disease and thyroiditis: analysis of the literature. J. Biol. Regul. Homeost Agents 29, 265–272.
Topliss, D. J. (2016). Clinical update in aspects of the management of autoimmune thyroid diseases. Endocrinol. Metab. (Seoul) 31, 493–499. doi: 10.3803/EnM.2016.31.4.493
Tortosa, F. (2011). [Subclinical thyroid dysfunction in pregnancy]. Endocrinol. Nutr. 58, 255–257. doi: 10.1016/j.endonu.2011.05.001
Tu, X., Duan, C., Lin, B., Li, K., Gao, J., Yan, H., et al. (2022). Characteristics of the gut microbiota in pregnant women with fetal growth restriction. BMC Pregnancy Childbirth 22, 297. doi: 10.1186/s12884-022-04635-w
Unuane, D., Velkeniers, B., Anckaert, E., Schiettecatte, J., Tournaye, H., Haentjens, P., et al. (2013). Thyroglobulin autoantibodies: is there any added value in the detection of thyroid autoimmunity in women consulting for fertility treatment? Thyroid 23, 1022–1028. doi: 10.1089/thy.2012.0562
van Dijk, M. M., Vissenberg, R., Fliers, E., van der Post, J. A. M., van der Hoorn, M. P., de Weerd, S., et al. (2022). Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 10, 322–329. doi: 10.1016/S2213-8587(22)00045-6
Ventura, R. E., Iizumi, T., Battaglia, T., Liu, M., Perez-Perez, G. I., Herbert, J., et al. (2019). Gut microbiome of treatment-naïve MS patients of different ethnicities early in disease course. Sci. Rep. 9, 16396. doi: 10.1038/s41598-019-52894-z
Wang, H., Gao, H., Chi, H., Zeng, L., Xiao, W., Wang, Y., et al. (2017). Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: A randomized clinical trial. Jama 318, 2190–2198. doi: 10.1001/jama.2017.18249
Wu, Z., Cai, Y., Xia, Q., Liu, T., Yang, H., Wang, F., et al. (2019). Hashimoto’s thyroiditis impairs embryo implantation by compromising endometrial morphology and receptivity markers in euthyroid mice. Reprod. Biol. Endocrinol. 17, 94. doi: 10.1186/s12958-019-0526-3
Yoon, K., Kim, N. (2021). Roles of sex hormones and gender in the gut microbiota. J. Neurogastroenterol Motil. 27, 314–325. doi: 10.5056/jnm20208
Zhao, F., Feng, J., Li, J., Zhao, L., Liu, Y., Chen, H., et al. (2018). Alterations of the gut microbiota in hashimoto’s thyroiditis patients. Thyroid 28, 175–186. doi: 10.1089/thy.2017.0395
Zhao, H., Yuan, L., Zhu, D., Sun, B., Du, J., Wang, J. (2022). Alterations and mechanism of gut microbiota in graves’ Disease and hashimoto’s thyroiditis. Pol. J. Microbiol. 71, 173–189. doi: 10.33073/pjm-2022-016
Keywords: autoimmune thyroiditis, AITD, TPOAb, gut microbiota, pregnancy outcomes
Citation: Song Y, Bai Y, Liu C, Zhai X and Zhang L (2024) The impact of gut microbiota on autoimmune thyroiditis and relationship with pregnancy outcomes: a review. Front. Cell. Infect. Microbiol. 14:1361660. doi: 10.3389/fcimb.2024.1361660
Received: 26 December 2023; Accepted: 19 February 2024;
Published: 05 March 2024.
Edited by:
Xiangtian Yu, Shanghai Jiao Tong University, ChinaReviewed by:
Shigefumi Okamoto, Osaka University, JapanXin Sun, The First Affiliated Hospital of Soochow University, China
Copyright © 2024 Song, Bai, Liu, Zhai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Le Zhang, emhhbmdsZTE5ODYyNkAxNjMuY29t
 Yu Song
Yu Song Yu Bai
Yu Bai