
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Cell. Infect. Microbiol., 08 March 2024
Sec. Virus and Host
Volume 14 - 2024 | https://doi.org/10.3389/fcimb.2024.1360075
 Amirhossein Talebzadeh1
Amirhossein Talebzadeh1 Hadi Ghaffari2
Hadi Ghaffari2 Kazem Ghaffari3
Kazem Ghaffari3 Sorur Yazdanpanah4
Sorur Yazdanpanah4 Bahman Yousefi Goltappeh5
Bahman Yousefi Goltappeh5 Majid Eslami2
Majid Eslami2 Ali Ghasemi1,6*†
Ali Ghasemi1,6*†Introduction: Since there is very little information about the relationship between platelet parameters and vitamin D concentration in patients with COVID-19, the aim of this study is to investigate the relationship between serum vitamin D level and platelet parameters in patients with COVID-19 and to compare these parameters in patients with COVID-19 without vitamin D deficiency and, subsequently, the prognostic value of these parameters in cases of vitamin D deficiency.
Methods: Seven hundred and forty-three patients diagnosed with COVID-19 were enrolled in this study. Patients were divided into two groups: those with and without vitamin D deficiency. The associations between platelet indices and vitamin D levels were analyzed by Pearson’s correlation analysis and a one-way ANOVA test.
Results: Platelet count and mean platelet volume (MPV) were significantly higher in the patients with vitamin D deficiency than in the patients without vitamin D deficiency. There was a significant negative correlation between platelet count and MPV with vitamin D levels in patients with vitamin D deficiency (r = -0.835, P = 0.001 & r = -0.324, P = 0.042, respectively). Vitamin D levels in COVID-19 patients can determine the platelet count and MPV of the patients.
Discussion: The aforementioned results imply that maintaining an elevated concentration of vitamin D in COVID-19 patients is important because it is associated with a decrease in MPV, which in turn reduces susceptibility to diseases such as coronary artery disease.
In December 2019, a new coronavirus named “SARS-CoV2” was declared by the World Health Organization as the cause of the outbreak of COVID-19 (Li et al., 2021). In fact, COVID-19 is the third epidemic disease with respiratory manifestations after Sars and MERS syndromes (Li et al., 2021), which, along with higher contagiousness (Yu et al., 2020), was able to infect most countries of the world, including Iran (Zhang et al., 2020).
Vitamin D deficiency has been widely reported in different countries as a factor that may cause excessive inflammation and dysfunction of the immune system and may also have adverse effects on hemostasis and thrombosis (Bodnar et al., 2014). Recently, the vitamin D receptor has been found in PLTs, which plays an essential role in anti-thrombogenicity. In addition, vitamin D deficiency has been found to be associated with changes in PLT size (Korzonek-Szlacheta et al., 2018). Vitamin D deficiency is considered a serious public health problem that affects all populations of all ages in developed and developing countries. There is very little published information about the relationship between PLT function and changes in its indices with vitamin D concentration in patients with COVID-19. However, in previous studies, the relationship between high MPV and low vitamin D levels has been shown in patients with stable coronary artery disease, patients without chronic disease, and pregnant women with gestational diabetes mellitus (Cure et al., 2014; Gur et al., 2015; Park et al., 2017; Korzonek-Szlacheta et al., 2018). In some studies, it has been suggested that PLT parameters such as MPV can be used as a prognostic marker in sepsis, severe diseases and also in the disease of COVID-19 (Liu et al., 2020; Zhong and Peng, 2021).
Prognostic importance as well as changes in platelet (PLT) indices have been shown in some diseases such as metabolic syndrome, cardiovascular attacks, ischemic attacks, myocardial infarction, and hepatitis C infection (Pohl et al., 2001; Coban et al., 2006; Wojszel et al., 2008; Ateş et al., 2009; Meng et al., 2016).
Since there is very little information about the relationship between PLT parameters and vitamin D concentration in patients with COVID-19, the aim of this study is to investigate the relationship between serum vitamin D level and PLT parameters in patients with COVID-19 and to compare these parameters in patients with COVID-19 without vitamin D deficiency and, subsequently, the prognostic value of these parameters in cases of vitamin D deficiency. Increased understanding of PLT functions in COVID-19 has undoubtedly improved our knowledge about clinical management and treatment options and may lead to the development of more accurate treatment strategies.
Seven hundred and forty-three patients with diagnosed COVID-19 who were referred to the Khordad Hospital, Varamin, Iran, were included in this matched case-control study. Finally, patients with diagnosed COVID-19 were divided into two groups with and/or without vitamin D deficiency. Vitamin D levels were dichotomized between normal vitamin D levels and vitamin D deficiency according to the standard cut point of 20 ng/mL (Meltzer et al., 2020). A control group of outpatients from the same hospital was selected in the same time period to compare the results with the patient group.
Cases and controls were frequency matched on gender, body mass index (BMI), systolic/diastolic blood pressure, total cholesterol, fasting glucose, triglycerides, white blood cell, hemoglobin, smoking status, race, and C-reactive protein.
The result of the COVID-19 test in the control group was negative. All patients who had a complete initial blood count and biochemical tests were included in the study.
PLT count, PLT distribution width (PDW), MPV, plateletcrit (PCT), and lymphocyte count were automatically determined by using an automated blood cell counter (Mindray, BC-6800, Mindray Biomedical Electronics, Nanchang, Shenzhen, China). Aspartate aminotransferase (AST) was measured by spectrophotometric technique via the Pars Azmun kit (Karaj, Iran). The serum level of 25-OH-vitamin D (ng/mL) was measured with the enzyme‐linked immunosorbent assay method (EUROIM-MUN®, D-23560 Lubeck, Germany). AST-to-PLT ratio index (APRI = [AST/upper limit of normal] × [100/PLTs, ×109/L]), vitamin D*MPV, vitamin D*PLT, vitamin D*PDW, PLT-lymphocyte ratio (PLR), MPV/PLT count ratio (MPR), MPV to lymphocyte ratio (MLR), MPV/PCT, PDW/PCT, PDW/PLT and BMI; [weight/height squared (kg/m2)] were calculated. The variables of the control group were matched with the control group.
The inclusion criteria for the study included patients with COVID-19 with a definitive diagnosis by molecular method (PCR) without any history of disease leading to vitamin D deficiency. Exclusion criteria: (1) patients who had a primary sclerosing cholangitis, primary biliary cirrhosis, Wilson’s disease, hemochromatosis, pancreatitis, pancreatic cancer, bile duct cancer, ovarian cancer, endometrial cancer, breast cancer, lung cancer, esophageal and stomach cancer, patients with simultaneous infection of two or more types of hepatitis, a history of heart disease that leads to the use of antiplatelet drugs, pregnant women with a history of preeclampsia, and chronic and incurable diseases that affect the PLT count or PLT indices; (2) patients with uncontrolled diabetic mellitus; (3) comorbidity and (4) use of antiplatelet drugs such as aspirin and non-steroidal anti-inflammatory drugs.
Statistical analyses were performed using SPSS 25.0 software (Inc., Chicago, IL, USA) and a genetic analyzer (ABI PRISM 310, Applied Biosystems). The mean and standard deviation (mean ± SD), Pearsonʼs χ2 test and one-way ANOVA test were used to compare the two groups’ characteristics. Additionally, in order to evaluate the association between PLT parameters and serum vitamin D levels, the parameters that demonstrated a P-value less than 0.1 in the bivariable correlation analysis were incorporated into the binary logistic regression analysis model. Pearson’s correlation coefficient was used for assessing the relationships between PLT parameters and vitamin D levels. P < 0.05 was considered a statistical difference.
Biochemical tests and the complete blood count of 36 patients were incomplete; thus, these subjects were excluded from the study. A total of 707 patients were included in the study. Patients were divided into two groups: 345 COVID-19-infected patients with vitamin D deficiency and 362 COVID-19-infected patients without vitamin D deficiency. The mean ± SD age of the patients was 54.8 ± 13.2 years. Three hundred and forty-one patients (48.2%) were male, and 366 patients (51.8%) were female. The gender distribution was similar in the studied groups. About 91% of all studied patients were vaccinated.
The mean ± SD vitamin D level of the patients was 24.1 ± 9.3 ng/mL, ranging from 11.2–52.1 ng/mL. The mean level of vitamin D in females and males was 33.4 ± 10.6 ng/mL and 38.8 ± 11.3 ng/mL, respectively, which showed a significant difference (P < 0.001), so that the mean level of vitamin D in females was lower than that of males. In total, thrombocytopenia occurred in 11.8% of patients with COVID-19. The demographic characteristics of three study groups are summarized in Table 1.
There was no clinically significant difference between the study groups in gender, weight, body mass index, Eastern Cooperative Oncology Group, fever, or thorax computed tomography (P > 0.05); however, there was a significant difference in serum vitamin D levels between the groups. In the control subjects, serum vitamin D levels were significantly higher compared to the COVID-19-infected patients (Table 1).
The values of the studied variables in all three groups are shown in Table 2. Based on laboratory findings regarding PDW and AST, there were no significant differences between groups. The MPR, MLR, PCT, PDW/PCT, and lymphocytes showed no difference between the groups of COVID-19-infected patients with and without vitamin D deficiency (P > 0.05).
Using Pearson’s correlation coefficient test, we investigated the correlation between PLT count and MPV with vitamin D levels in the group of patients with vitamin D deficiency. As shown in Figures 1A, B, there was a significant negative correlation between PLT count and MPV with vitamin D levels in patients with vitamin D deficiency (r = -0.835, P = 0.001, & r = -0.324, P = 0.042, respectively).

Figure 1 (A) The negative correlation between platelet count and vitamin D levels. (B) The negative correlation between MPV and vitamin D levels. P-value was calculated by Pearson’s correlation.
The PLT count displayed significant differences between the groups, being greatest in the COVID-19-infected patients with vitamin D deficiency and least in the COVID-19-infected patients without vitamin D deficiency; COVID-19-infected patients with vitamin D deficiency vs. control subjects [378.2 ± 48.4 vs. 266.1 ± 45.9, P = 0.042], and COVID-19-infected patients with vitamin D deficiency vs. COVID-19-infected patients without vitamin D deficiency [378.2± 48.4 vs. 219.4± 56.6, P = 0.021] (Figure 2A).
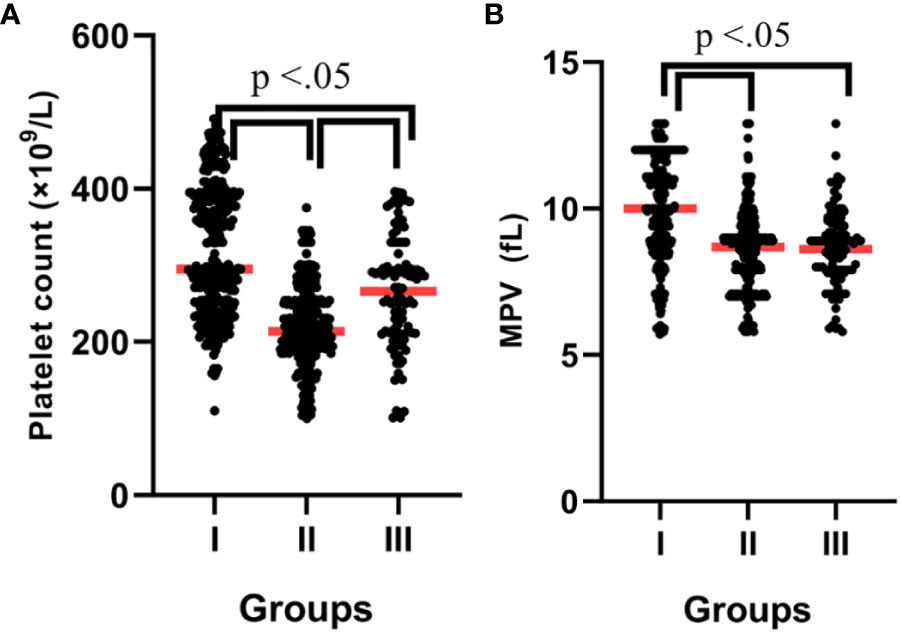
Figure 2 (A) Platelet count according to groups. (B) MPV according to groups. P-values were calculated by ANOVA. I; Patients with vitamin D deficiency, II; Patients without vitamin D deficiency, III; Control subjects.
As shown in Figure 2B, the MPV was significantly higher in the patients with vitamin D deficiency than in the patients without vitamin D deficiency (10.0 ± 0.6 vs. 8.6 ± 0.5, P = 0.038). Also, the MPR was significantly higher in the patients without vitamin D deficiency than in the patients with vitamin D deficiency (0.39 vs. 0.26, P = 0.023). The PDW/PCT and MLR were significantly lower in the control subjects than in the patients (P > 0.05). In addition, several complex parameters caused by serum vitamin D levels with MPV, PLT, and PDW are shown in Table 2.
Two sets of multiple linear regressions were performed, considering PLT parameters as the dependent variables and serum vitamin D levels and age as the independent variables. The first linear regression model (Table 3), using PLT count and vitamin D and age, exhibited that there is a relationship between PLT count with age and vitamin D serum level. But no relationship was found between PLT count and gender. Also, the first linear regression model, using MPV as a dependent variable and vitamin D as a regressor, showed that there is a relationship between MPV and vitamin D serum levels. A second adjusted linear regression model was performed, confirming the existence of an independent association between PLT count and MPV and serum vitamin D levels, even after adjusting for age (Table 3).
In this study, it was observed that the PLT count in COVID-19-infected patients with vitamin D deficiency was significantly higher than the other two groups. In other words, vitamin D deficiency increased the PLT count. Also, we showed in this study that there was a significant negative correlation between PLT count and MPV with vitamin D levels in COVID-19-infected patients.
Consistent with our results, in a study on Korean adults, the authors reported that the subjects with vitamin D deficiency had a significantly lower PLT and MPV than the sufficiency group (Park et al., 2017). In another study, Alanli et al. showed that PLT counts increased in people with low vitamin D levels (Alanli et al., 2020).
Vitamin D deficiency is a worldwide problem, with billions of people suffering from vitamin D deficiency (Amrein et al., 2020). Also, the prevalence of vitamin D deficiency in Iranian men and women is very high, especially in old age (Vatandost et al., 2018).
The correlation results between MPV and vitamin D levels have been inconsistent in different studies. Some studies have documented an inverse relationship between MPV and levels of vitamin D in different diseases, while others have failed to observe any association between these two variables. For example, a study on gestational diabetes mellitus found that low 25-hydroxyvitamin D3 levels and high MPV were observed in pregnant women with gestational diabetes mellitus (Gur et al., 2015). On the other hand, Cumhur et al (Chu et al., 2022). & Alanli et al. (2020) reported that there was no significant relationship between vitamin D deficiency and MPV levels in healthy participants.
Aihara et al. (2004) provided evidence indicating that the vitamin D receptor (VDR) system may have a significant role in the prevention of blood clotting in vivo. They demonstrated this by observing that mice lacking the VDR gene (VDRKO mice) experienced an intensified formation of blood clots in multiple organs after being injected with lipopolysaccharide, regardless of their levels of calcium. On the other hand, the activation of vitamin D increased the gene expression of antithrombotic factors and thrombomodulin in monocytic cells while decreasing the expression of the thrombogenic factor gene. In the VDRKO mice, the opposite effect was observed. Therefore, the vitamin D-VDR system promotes the expression of antithrombotic factors and inhibits the expression of thrombogenic factors.
Megakaryocytes, which serve as precursors for PLTs, possess VDRs as well. The activation of these receptors plays a role in governing cell maturation and the proliferation of megakaryocytes. In the context of vitamin D deficiency, the promotion of megakaryocyte maturation and the elevation of PLT counts can be observed (Silvagno et al., 2010).
Furthermore, low levels of vitamin D have been associated with increased levels of proinflammatory cytokines such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α), which in turn may lead to increased MPV (Cure et al., 2014). Increased levels of IL-6 and TNF-α can stimulate megakaryopoiesis and platelet production, and they may have effects on hematopoietic stem cells (Kaser et al., 2001; Chu et al., 2022). On the other hand, studies have shown that IL-6 and TNF-α levels increase in COVID-19 patients (Coomes and Haghbayan, 2020).
Hence, it is reasonable to propose that one of the reasons for the increase in MPV and PLT counts may potentially be attributed to the severe manifestation of the cytokine storm in COVID-19 patients who show vitamin D deficiency.
In some studies, it has been suggested that PLT parameters can be used as a prognostic marker in sepsis, critical illnesses, and also in COVID-19 (Liu et al., 2020; Zhong and Peng, 2021), but despite this, contradictory results are seen in different studies. By evaluating different studies, it was found that two factors can be mentioned among the reasons for observing these contradictions: one is the difference in measurement techniques, and the other is the clinical conditions of the patients. Among the differences in measurement techniques, we can mention the anticoagulants used to collect the sample and the duration of sample storage until the test. All these factors may affect PLT parameters, including MPV levels (Harrison and Goodall, 2016). Among the differences in the clinical conditions of the patients, we can mention the deficiency or decrease in the level of vitamin D in the patients, gender, and HPA polymorphisms (Gur et al., 2015; Alanli et al., 2020; Ghaffari et al., 2023).
There have been no studies that clearly show an increase in PC in COVID-19 patients with vitamin D deficiency. In this study, it was shown that although the mean PLT count in COVID-19 patients without vitamin D deficiency did not show thrombocytopenia, the mean PLT count in these patients was significantly lower than that of normal individuals, which can be due to various reasons, including abnormal hematopoiesis and the initiation of an auto-immune response against PLTs in COVID-19 patients (Jolicoeur and Lamontagne, 1995; Yang et al., 2005).
Our study had several limitations. The evidence suggests that calcium & magnesium deficiency may contribute to low vitamin D status (Dai et al., 2018; Khazai et al., 2008). Therefore, calcium & magnesium deficiency can indirectly cause an increase in MPV and PLT counts. Hence, it is suggested to measure calcium & magnesium levels as well. Also, we did not measure the level of pro-inflammatory cytokines in this study, while measuring their level can help us understand the mechanisms involved in the increase of MPV and PLT counts.
An increased number of platelets has been associated with an elevated likelihood of thrombosis in COVID-19 patients. In a study of 3915 hospitalized COVID-19 patients, it was found that patients with an elevated platelet count (>400 × 10^9/L) had an increased risk of critical illness and all-cause mortality (Barrett et al., 2021).
This study is simply a report of retrospective clinical data. However, it has nothing new to offer about mechanisms and interrelations. The interpretation of platelet role in COVID-19 is complicated. Platelets are key regulator of immune system in viral infections. COVID-19 patients platelets show higher activity. However complex pathophysiology of COVID-19 and cytokine storm in infection background makes it harder to study immune cells (including platelets) role in COVID-19 infection. It becomes more complicated when we think about relationship of vitamin D with platelets.
Vitamin D levels in COVID-19 patients can determine the platelet count and MPV of the patients. Vitamin D deficiency increased the number of PLT. Also, there is a significant negative correlation between the number of PLT and MPV with the level of vitamin D in patients with Covid-19.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical principles were followed based on the ethical protocol approved by the Ethics Committee at Semnan University of Medical Sciences, Semnan, Iran (IR.SEMUMS.REC.1401.309). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
AT: Data curation, Investigation, Writing – original draft. HG: Conceptualization, Methodology, Visualization, Writing – original draft. KG: Software, Validation, Writing – original draft. SY: Formal analysis, Methodology, Software, Writing – original draft. BY: Project administration, Resources, Supervision, Writing – original draft. ME: Funding acquisition, Validation, Writing – original draft. AG: Conceptualization, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Semnan University of Medical Sciences, Semnan, Iran.
The present study was supported by Semnan University of Medical Sciences, which has provided funding for this research.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aihara, K.-I., Azuma, H., Akaike, M., Ikeda, Y., Yamashita, M., Sudo, T., et al. (2004). Disruption of nuclear vitamin D receptor gene causes enhanced thrombogenicity in mice. J. Biol. Chem. 279, 35798–35802. doi: 10.1074/jbc.M404865200
Alanli, R., Küçükay, M. B., Yalçin, K. S. (2020). Relationship between vitamin D levels and platelet count: A retrospective study. Gulhane Med. J. 62 (3), 174–178. doi: 10.4274/gulhane
Amrein, K., Scherkl, M., Hoffmann, M., Neuwersch-Sommeregger, S., Köstenberger, M., Tmava Berisha, A., et al. (2020). Vitamin D deficiency 2.0: an update on the current status worldwide. Eur. J. Clin. Nutr. 74, 1498–1513. doi: 10.1038/s41430-020-0558-y
Ateş, O., Kiki, I., Bilen, H., Keleş, M., Koçer, I., Kulaçoğlu, D. N., et al. (2009). Association of mean platelet volume with the degree of retinopathy in patients with diabetes mellitus. Eur. J. Gen. Med. 6, 99–102. doi: 10.29333/ejgm/82648
Barrett, T. J., Bilaloglu, S., Cornwell, M., Burgess, H. M., Virginio, V. W., Drenkova, K., et al. (2021). Platelets contribute to disease severity in COVID-19. J. Thromb. Haemostasis. 19, 3139–3153. doi: 10.1111/jth.15534
Bodnar, L. M., Simhan, H. N., Catov, J. M., Roberts, J. M., Platt, R. W., Diesel, J. C., et al. (2014). Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiol. (Cambridge Mass). 25, 207. doi: 10.1097/EDE.0000000000000039
Chu, T., Hu, S., Qi, J., Li, X., Zhang, X., Tang, Y., et al. (2022). Bifunctional effect of the inflammatory cytokine tumor necrosis factor α on megakaryopoiesis and platelet production. J. Thromb. Haemostasis. 20, 2998–3010. doi: 10.1111/jth.15891
Coban, E., Bostan, F., Ozdogan, M. (2006). The mean platelet volume in subjects with impaired fasting glucose. Platelets. 17, 67–69. doi: 10.1080/09537100500220729
Coomes, E. A., Haghbayan, H. (2020). Interleukin-6 in COVID-19: a systematic review and meta-analysis. Rev. Med. virology. 30, 1–9. doi: 10.1002/rmv.2141
Cure, C., Cure, E., Yuce, S., Yazici, T., Karakoyun, I., Efe, H. (2014). Mean platelet volume and vitamin D level. Ann. Lab. Med. 34, 98–103. doi: 10.3343/alm.2014.34.2.98
Dai, Q., Zhu, X., Manson, J. E., Song, Y., Li, X., Franke, A. A., et al. (2018). Magnesium status and supplementation influence vitamin D status and metabolism: results from a randomized trial. Am. J. Clin. Nutr. 108, 1249–1258. doi: 10.1093/ajcn/nqy274
Ghaffari, K., Rad, M. A., Moradi Hasan-Abad, A., Khosravi, M., Benvidi, A., Iraji, M., et al. (2023). Association of the human platelet antigens polymorphisms with platelet count in patients with COVID-19. Front. Med. 10. doi: 10.3389/fmed.2023.1265568
Gur, E. B., Karadeniz, M., Genc, M., Eskicioglu, F., Yalcin, M., Hepyilmaz, I., et al. (2015). Relationship between mean platelet volume and vitamin D deficiency in gestational diabetes mellitus. Arch. Endocrinol. Metab. 59, 448–454. doi: 10.1590/2359-3997000000063
Harrison, P., Goodall, A. H. (2016). Studies on mean platelet volume (MPV)-new editorial policy. Platelets. 27, 605–606. doi: 10.1080/09537104.2016.1225467
Jolicoeur, P., Lamontagne, L. (1995). Impairment of bone marrow pre-B and B cells in MHV3 chronically-infected mice. Corona-and Related Viruses: Current Concepts in Molecular Biology and Pathogenesis. Adv. Exp. Med. Biol. 1995, 193–5. doi: 10.1007/978-1-4615-1899-0_33
Kaser, A., Brandacher, G., Steurer, W., Kaser, S., Offner, F. A., Zoller, H., et al. (2001). Interleukin-6 stimulates thrombopoiesis through thrombopoietin: role in inflammatory thrombocytosis. Blood J. Am. Soc. Hematology. 98, 2720–2725. doi: 10.1182/blood.V98.9.2720
Khazai, N., Judd, S. E., Tangpricha, V. (2008). Calcium and vitamin D: skeletal and extraskeletal health. Curr. Rheumatol. Rep. 10, 110–117. doi: 10.1007/s11926-008-0020-y
Korzonek-Szlacheta, I., Hudzik, B., Nowak, J., Szkodzinski, J., Nowak, J., Gąsior, M., et al. (2018). Mean platelet volume is associated with serum 25-hydroxyvitamin D concentrations in patients with stable coronary artery disease. Heart Vessels. 33, 1275–1281. doi: 10.1007/s00380-018-1182-9
Li, J., Huang, D. Q., Zou, B., Yang, H., Hui, W. Z., Rui, F., et al. (2021). Epidemiology of COVID-19: A systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J. Med. virology. 93, 1449–1458. doi: 10.1002/jmv.26424
Liu, Y., Sun, W., Guo, Y., Chen, L., Zhang, L., Zhao, S., et al. (2020). Association between platelet parameters and mortality in coronavirus disease 2019: Retrospective cohort study. Platelets. 31, 490–496. doi: 10.1080/09537104.2020.1754383
Meltzer, D. O., Best, T. J., Zhang, H., Vokes, T., Arora, V., Solway, J. (2020). Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA network Open 3, e2019722–e. doi: 10.1001/jamanetworkopen.2020.19722
Meng, X., Wei, G., Chang, Q., Peng, R., Shi, G., Zheng, P., et al. (2016). The platelet-to-lymphocyte ratio, superior to the neutrophil-to-lymphocyte ratio, correlates with hepatitis C virus infection. Int. J. Infect. Diseases. 45, 72–77. doi: 10.1016/j.ijid.2016.02.025
Park, Y. C., Kim, J., Seo, M. S., Hong, S. W., Cho, E. S., Kim, J.-K. (2017). Inverse relationship between vitamin D levels and platelet indices in Korean adults. Hematology. 22, 623–629. doi: 10.1080/10245332.2017.1318334
Pohl, A., Behling, C., Oliver, D., Kilani, M., Monson, P., Hassanein, T. (2001). Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am. J. gastroenterology. 96, 3142–3146. doi: 10.1111/j.1572-0241.2001.05268.x
Silvagno, F., De Vivo, E., Attanasio, A., Gallo, V., Mazzucco, G., Pescarmona, G. (2010). Mitochondrial localization of vitamin D receptor in human platelets and differentiated megakaryocytes. PLoS One 5, e8670. doi: 10.1371/journal.pone.0008670
Vatandost, S., Jahani, M., Afshari, A., Amiri, M. R., Heidarimoghadam, R., Mohammadi, Y. (2018). Prevalence of vitamin D deficiency in Iran: a systematic review and meta-analysis. Nutr. Health 24, 269–278. doi: 10.1177/0260106018802968
Wojszel, J., Czyzewska, J., Dymicka-Piekarska, V., Matowicka-Karna, J., Jakubowska, I., Kemona, H. (2008). Platelets activation in depending on glycaemic control in diabetes type 2. Polski Merkuriusz Lekarski: Organ Polskiego Towarzystwa Lekarskiego. 25, 335–339.
Yang, M., Ng, M. H., Li, C. K. (2005). Thrombocytopenia in patients with severe acute respiratory syndrome. Hematology. 10, 101–105. doi: 10.1080/10245330400026170
Yu, F., Jia, R., Tang, Y., Liu, J., Wei, B. (2020). SARS-CoV-2 infection and stem cells: Interaction and intervention. Stem Cell Res. 46, 101859. doi: 10.1016/j.scr.2020.101859
Zhang, T., Wu, Q., Zhang, Z. (2020). Probable pangolin origin of SARS-CoV-2 associated with the COVID-19 outbreak. Curr. Biol. 30, 1346–51.e2. doi: 10.1016/j.cub.2020.03.022
Keywords: platelets, COVID-19, vitamin D, thrombocytosis, mean platelet volume
Citation: Talebzadeh A, Ghaffari H, Ghaffari K, Yazdanpanah S, Yousefi Goltappeh B, Eslami M and Ghasemi A (2024) The effect of vitamin D deficiency on platelet parameters in patients with COVID-19. Front. Cell. Infect. Microbiol. 14:1360075. doi: 10.3389/fcimb.2024.1360075
Received: 22 December 2023; Accepted: 26 February 2024;
Published: 08 March 2024.
Edited by:
Antoinette van der Kuyl, University of Amsterdam, NetherlandsReviewed by:
Rizaldy Taslim Pinzon, Duta Wacana Christian University, IndonesiaCopyright © 2024 Talebzadeh, Ghaffari, Ghaffari, Yazdanpanah, Yousefi Goltappeh, Eslami and Ghasemi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Ghasemi, YS5xYXNlbWkyMDEyQHlhaG9vLmNvbQ==
†ORCID: Ali Ghasemi, orcid.org/0000-0002-4996-7656
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.