- Scientific Affairs, Luminex, A DiaSorin Company, Austin, TX, United States
Combination and polyvalent vaccines not only provide protection against several different pathogens at the same time but can also increase vaccine protection against pathogens that have closely related pathogenic strains or serotypes. Multiplexed serological testing is a preferred method for determining the efficacy of combination and polyvalent vaccines, as it reduces the need for conducting multiple individual assays to confirm immune responses and cross-reactivity, uses less sample, and can be faster, more reliable, and more cost-effective. Bead-based suspension array technologies, such as the Luminex® xMAP® Technology, are often used for development of multiplexed serological assays for various vaccine trials and for routine testing in clinical laboratories to determine immune status of vaccinated individuals. This article reviews publications describing the development and implementation of bead-based multiplexed serological assays for detection of immune responses to polyvalent polysaccharide and conjugate vaccines against Streptococcus pneumoniae. Many of these serological assays on the bead array platform have been further optimized and expanded over time and are still widely used today.
Introduction
Combination and polyvalent vaccines not only provide protection against several different pathogens at the same time but can also increase vaccine protection against pathogens that have closely related pathogenic strains or serotypes. In particular, the use of polyvalent conjugate vaccines for Streptococcus pneumoniae is important due to the organism’s many serotypes, each with a distinct polysaccharide capsule (Hausdorff et al., 2000). In conjugate vaccines, the polysaccharide is coupled to a carrier protein which increases the immunogenicity and helps elicit a stronger response (Pichichero, 2013). However, serotype variability and evolution of new or altered serotypes makes it difficult to create a vaccine that can provide comprehensive and long-lasting coverage. Thus the polyvalent pneumococcal vaccines have also changed over time from the first 7-valent vaccine licensed in 2000, to the 14-valent vaccine, and the 23-valent vaccine licensed in 1983 (Daniels et al., 2016). With such broad coverage, testing the vaccine immune response to each serotype can be extremely time-consuming and laborious if each component must be assessed in an individual serological immunoassay. Therefore, multiplexed serological testing methods have been developed over the past two decades and are preferred for determining the efficacy of combination and polyvalent vaccines. Multiplexed immunoassays reduce the number of assays needed to confirm immune responses and cross-reactivity, use less serum, and can be performed faster. Testing in multiplex reduces the variables that must be controlled when performing individual tests and can thus be more reliable, and ultimately are more cost-effective than single-plex methods.
Bead-based suspension array technologies are often used for development of multiplexed serological assays to simultaneously assess immune responses to multiple antigens and have been used in vaccine trials, testing in clinical laboratories, epidemiological studies, and in basic immunological research. In particular, the xMAP® microsphere technology from Luminex has been used extensively for multiplexed serological assays to detect immune responses to a variety of antigens, including pathogens, autoimmune markers, as well as human leukocyte antigen (HLA) and alloantigens, which is important for donor and recipient testing in transplantation (Das and Dunbar, 2020).
xMAP microspheres are polystyrene beads of the same size and with the same physical properties and surface composition but are internally dyed with two or three spectrally distinct fluorochromes (Graham et al., 2019). These internal classification dyes are excited at the same wavelength but have unique emission profiles that provide a unique “spectral address” for each individual microsphere region, or bead set, allowing each bead set to be differentiated from all others in the multiplexed reaction. Bead sets are covalently coupled with capture molecules specific to the target of interest and are then combined into a single multiplexed reaction. After binding the analytes present in a sample, a reporter fluorochrome is added to measure the binding that has occurred on the bead surface. Completed reactions are interrogated in a flow analysis instrument where the fluorescence of the internal dyes allows distinct analysis of the multiplex data, and the reporter fluorescence quantifies the result.
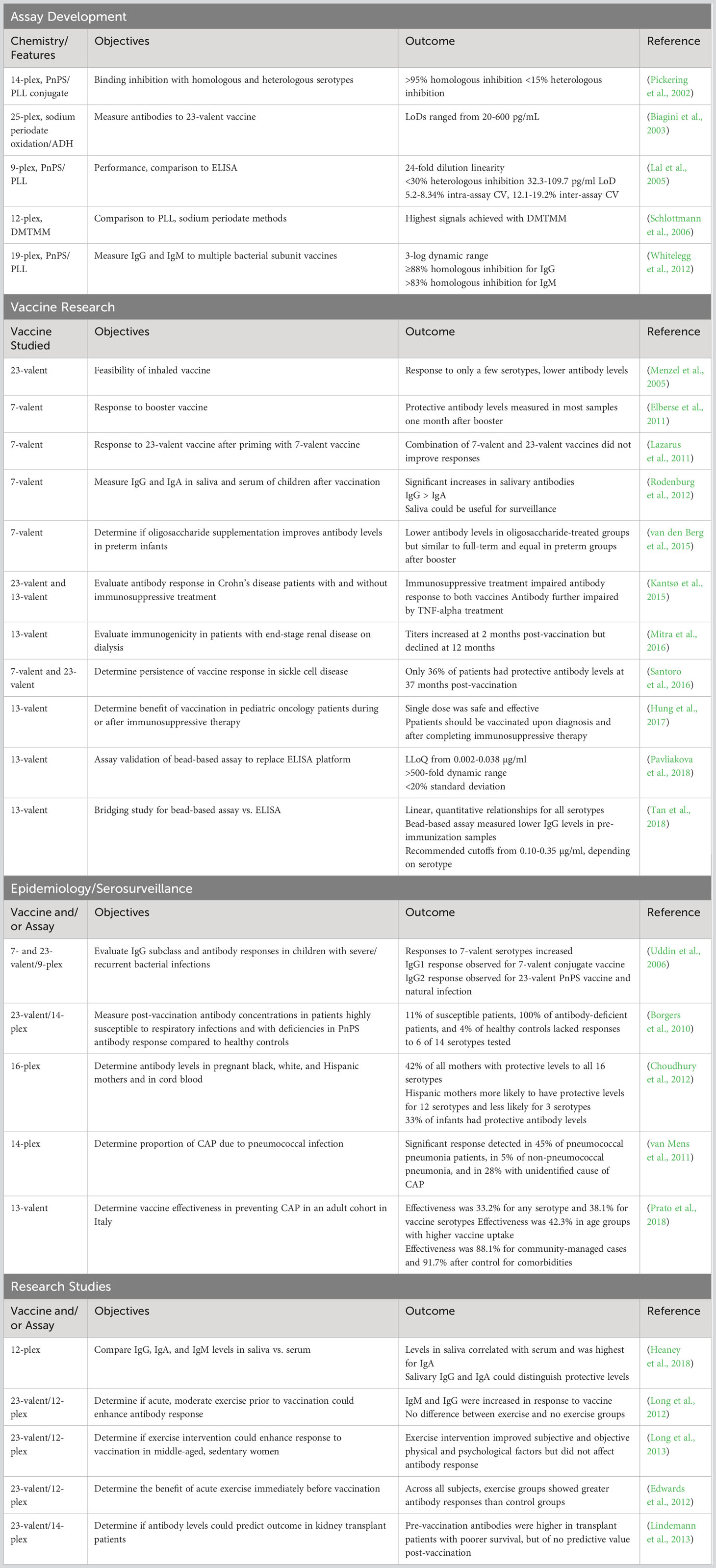
This article reviews selected publications describing the development and implementation of bead-based multiplexed serological assays for detection of immune responses to polyvalent vaccines against Streptococcus pneumoniae (Table 1). Many of the assays described have been further optimized and expanded over time or have been modified to have broad coverage for multiple vaccines used against different organisms, and many of these are still in use today. While initial studies focused on assay development and optimization, subsequent work has allowed implementation of these assays in clinical and research laboratories to characterize the immune responses to pneumococcal vaccines in various populations and to determine stability of immunity after vaccination and/or natural infection over time.
Multiplexed pneumococcal serological assay development
The first multiplexed microsphere-based immunoassay for measuring IgG antibodies to S. pneumoniae was described in 2002 by Pickering et al. (2002). The investigators developed a multiplexed indirect immunoassay to simultaneously measure serum IgG to 14 pneumococcal polysaccharides (PnPS). The PnPS were covalently bound to poly-L-lysine (PLL) using cyanuric chloride as the coupling agent. Each PnPS/PLL conjugate was coupled to a unique color-coded microsphere set using a two-step 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) and sulfo-N-hydroxysulfosuccinimide (sulfo-NHS) procedure (Fulton et al., 1997). Binding inhibition studies were performed using serum samples from adult vaccinated individuals. With the exception of serotypes 9V and 9N (which are closely related), they achieved >95% inhibition by homologous serotypes. Less than 15% inhibition was found with heterologous serotypes for all 14 serotypes. Cross-reacting antibodies caused ≥50% heterologous inhibition with some serotypes and could not be removed by preabsorption with pneumococcal C-polysaccharide. However, they could be removed by an additional preabsorption with serotype 22F PnPS. The multiplexed assay showed good overall agreement with the enzyme-linked immunosorbent assay (ELISA) that was recommended at the time for evaluating pneumococcal vaccine immunogenicity. Subsequently, Pickering and colleagues described false positive reactivity that could occur due to nonspecific binding to the microspheres or reactivity to bovine serum albumin (BSA) present in the bead storage buffer (Pickering et al., 2010). They found that the nonspecific reactivity could be eliminated by replacing the BSA with BSA-free StabliGuard immunoassay stabilizer (SurModics, Inc., Eden Prairie, MN) and might be applicable to other bead-based serological assays.
In 2003 Biagini et al., developed a 25-plex PnPS assay for simultaneous measurement of antibodies to the 23 serotypes included in the PNEUMOVAX 23 vaccine (Merck & Co., Inc., North Wales, PA) (Biagini et al., 2003). This assay also included an internal control to evaluate pneumococcal cell wall polysaccharide preadsorption and a control using serotype 25 (not included in the vaccine), to assess inter-assay reproducibility. For conjugation, PnPS were first oxidized with sodium periodate to generate reactive aldehyde groups suitable for coupling to molecules that contain amine or hydrazide (Hermanson, 2013). The carboxylated microspheres were then modified by coupling adipic acid dihydrazide (ADH) using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) to provide a 10-atom spacer with an active amine group for subsequent covalent coupling to the oxidized PnPS. The assay was standardized using a reference anti-pneumococcal serotype standard. The investigators found that the limits of detection (LoD) ranged from 20 pg/ml for PnPS 3 to 600 pg/ml for PnPS 14. The authors concluded that compared to standard ELISAs, the method had several advantages for measuring anti-PnPS IgG levels, such as faster time-to-result, less sample volume required, and an equal or better sensitivity with an increased dynamic range.
Lal and colleagues developed a multiplexed serological assay for simultaneous quantitation of IgG to nine pneumococcal serotypes (1, 4, 5, 6B, 9V, 14, 18C, 19F, 23F) (Lal et al., 2005). Pneumococcal polysaccharides were conjugated to poly-L-lysine (PLL) and covalently attached to fluorescent microspheres as previously described (Pickering et al., 2002). The coupled beads were assessed using 89-SF standard serum. The multiplexed assay was linear over a 24-fold range of serum dilution and comparison of the individual single-plex assays to the nine-plex assay demonstrated no interference between bead sets. The assay was specific with <30% heterologous inhibition, highly sensitive with LoDs from 32.3 to 109.7 pg/ml, and very reproducible with intra-assay coefficient of variation (CV) 5.2% to 8.34% and inter-assay CV at 12.1% to 19.2%. Results for clinical samples showed good correlation between the multiplexed assay and single-plex ELISAs. Correlation coefficients (R) were 0.91 to 0.96 and the slopes ranged from 0.82 to 1.07. The authors also added four additional, previously developed bead sets coupled with meningococcal polysaccharides to the PnPS multiplex and found no interference with the detection of pneumococcal or meningococcal IgG levels.
Schlottmann and coworkers reported a novel chemistry for conjugating PnPS to carboxylated microspheres (Schlottmann et al., 2006). In this method, a set of 12 PnPS were modified with 4-(4,6-dimethoxy (Hausdorff et al., 2000; Daniels et al., 2016; Graham et al., 2019)triazin-2-yl)-4-methyl-morpholinium (DMTMM) for subsequent conjugation to the microspheres. Several conjugation methods were compared: PLL/cyanuric chloride (Pickering et al., 2002), sodium periodate oxidation (Biagini et al., 2003), passive adsorption to carboxylated microspheres, DMTMM conjugation to carboxylated microspheres, and DMTMM conjugation to microspheres that were amino-modified with adipic dihydrazide (ADH). The authors evaluated each of these methods for robustness, reproducibility, and the effect on PnPS antigenicity in a multiplexed assay format. While they found that the PLL method had better overall coupling efficiency and better specificity compared to the oxidation method, it was not reproducible across different coupling episodes. PnPS did bind to the microspheres through passive adsorption, but the signals were less than those obtained with DMTMM, and the signals obtained for DMTMM with amino-modified microspheres was less than that with the carboxylated microspheres. When compared to PLL, the DMTMM method using carboxylated microspheres achieved the highest signals and the results were reproducible across multiple operators. Analytical sensitivities ranged from 0.6 to 53.2 ng/ml, depending on the particular PnPS, and the assay was quantitative across a 3.5 to 4 log dynamic range. Additionally, the method did not appear to affect antigenicity and was specific with ≥95% homologous inhibition for all PnPS types and 15-20% heterologous inhibition in only three instances.
Whitelegg et al. subsequently developed a multiplexed bead-based assay for measuring IgG and IgM antibodies to 19 antigens contained in groups of bacterial subunit vaccines including pneumococcal, meningococcal, and Haemophilus polysaccharides, and tetanus and diphtheria toxoids (Whitelegg et al., 2012). Polysaccharides were conjugated using the PLL method (Pickering et al., 2002; Lal et al., 2005) and the assay was used to measure specific IgG and IgM antibody levels in serum from 193 healthy adult donors. IgG and pneumococcal IgM concentrations could be measured across a 3-log dynamic range that included the protective threshold of IgG levels for each antigen. Comparison to the single-plex assays showed little interference in antibody measurements in the multiplexed assay. Specificity was determined by preincubating control serum with each of the 19 antigens before performing the assay. There was no cross-reactive IgG detected and the signal was reduced to ≤12% of the unadsorbed level for homologous antigen but the majority remained at >60% when adsorbed with the heterologous antigen. Cross-reactive IgM was found for three of the antigens, but signal was reduced to <17% for all others when preadsorbed with homologous antigen. They concluded that the assay was suitable for adoption by clinical laboratories to assess immune response to vaccinations and measure absolute antibody levels to these antigens.
Vaccine research using multiplexed pneumococcal polysaccharide assays
These well-characterized assays have been deployed in numerous studies to explore the safety and immunogenicity of PnPS vaccines in a variety of patient populations and vaccination protocols. In 2005, Menzel and colleagues used a 9-plex bead-based PnPS assay to evaluate the feasibility of PnPS vaccination by inhalation (Menzel et al., 2005). Patients were randomized to compare alveolar vaccination, bronchial vaccination, and the standard intramuscular vaccination. They used an IgG enzyme immunoassay (EIA) to measure total pneumococcal-specific IgG and selected four responders from each cohort to determine serotype-specific IgG using the multiplexed bead array. The data showed that responses were restricted to a few serotypes in the inhalation groups (1 to 4 of 9 serotypes) and was broader for the intramuscular group (6 to 9 of 9 serotypes). Overall, the authors found that the vaccine could be administered safely by controlled inhalation but induced lower serum antibody responses as compared to intramuscular vaccination.
Elberse et al. used an optimized 13-plex PnPS assay to study the response to booster vaccination following immunization to the seven-valent PnPS vaccine (Elberse et al., 2011). Antibody concentrations were measured in 188 serum samples obtained pre- and post-booster vaccination at 11 months after a primary series of the vaccine. The results of the bead-based assay were compared with those of the ELISA for the serotypes included in the vaccine and for a non-vaccine serotype (6A). The concentrations of the antibodies as determined by the bead-based assay were slightly higher than those determined by ELISA but the correlations between the assays were good, with R2 values ranging from 0.84 to 0.91 for all serotypes except 19F (R2 = 0.70). Most of the serum samples had antibody concentrations above the protective concentration for the vaccine serotypes one month after booster. However, based on the differences in the concentrations determined by ELISA and the bead-based assay, the authors suggested a new protective cutoff concentration may need to be determined and may need to be adjusted for each serotype.
Lazarus and coworkers used the assay described by Lal (Lal et al., 2005) in a randomized study of adults 50-70 years of age to determine if priming with the 7-valent pneumococcal conjugate vaccine (PCV7) could improve the immunogenicity of 23-valent polysaccharide vaccine (23vP) for PCV7 serotypes, and to investigate whether PCV7 could be used to reverse reduced responsiveness to 23vP (Lazarus et al., 2011). The investigators conducted a randomized study that compared three vaccine schedules, consisting of two doses of PCV7 and one dose of 23vP administered over one year. Blood samples were obtained before and one month after each vaccination. The results showed that vaccination with 23vP after priming with two doses of PCV7 produced significantly higher antibody concentrations for three of the PCV7 serotypes as compared to vaccination with one dose of 23vP. The same immunogenicity was also achieved with a single dose of PCV7. Previous vaccination with 23vP weakened the antibody response to subsequent PCV7 vaccination and could not be restored by additional doses of PCV7. The authors concluded that vaccination schedules combining PCV7 and 23vP do not improve immune responses in adults compared to a single dose of 23vP for most of the PCV7 serotypes.
Rodenburg et al. used the multiplexed PnPS assay described by Pickering (Pickering et al., 2002) to measure anti-pneumococcal IgG and IgA in saliva and serum of children who received 2 doses (2 and 4 months of age) or 3 doses (2, 4, and 11 months of age) of PCV7 (Rodenburg et al., 2012). Paired saliva samples were collected from 188 children at 12 and 24 months of age and serum samples were also collected from 15 children. At 12 months, both vaccine groups had higher levels of IgG against vaccine serotypes in serum and saliva as compared with unvaccinated controls. Antibody levels were sustained until 24 months for most serotypes and although salivary IgG was 10- to 20-fold lower than in serum, the serum and saliva levels were highly correlated. Serum and salivary IgA levels were higher in both vaccine groups at 12 months with the exception of serotype 19F. Higher salivary IgA levels remained for 24 months for most serotypes in the 3-dose group, but not in the 2-dose group. PCV7 vaccination resulted in significant increases in pneumococcal-specific salivary IgG and IgA but were more prominent for IgG compared to the control group. Since the salivary antibody levels correlated well serum IgG, the authors suggest that saliva testing could also prove useful for surveillance of pneumococcal antibody levels.
van den Berg and colleagues used the multiplexed PnPS assay described by Elberse (Elberse et al., 2011) to determine the effect of oligosaccharide supplementation on antibody levels in preterm infants (<32 weeks gestational age or birth weight <1500 g) after vaccination with the Prevenar-7® pneumococcal conjugate vaccine from Pfizer, Inc. (Pearl River, New York) (van den Berg et al., 2015). Following three primary vaccinations in 113 preterm infants, the oligosaccharide-treated groups had lower antibody levels than the placebo group at 5 months, but these levels were similar to those in a full-term control group. After receiving a booster vaccination at 11 months, antibody levels were the same between the preterm oligosaccharide-treated and the preterm placebo group. The authors suggested that lower IgG levels to vaccine serotypes in the oligosaccharide supplemented infants at 5 months might reflect either a direct or indirect (through alteration of microbiota) immunomodulation by neutral and acidic oligosaccharides in the first months of life. In addition, they observed that the transplacental transport of pneumococcal antibodies was lower in preterm infants than in full term infants.
These assays have also been used in many studies to evaluate immune responses to PnPS vaccines in a variety of different patient populations with conditions that can impair the immune response, such as autoimmune diseases, cancer, and transplantation. Kantsø et al. evaluated specific antibody response to two PnPS vaccines (23- and 13-valent) in Crohn’s disease patients with and without immunosuppressive treatment four weeks after vaccination (Kantsø et al., 2015). They used a laboratory-developed bead-based serological assay following the PnPS/PLL method (Pickering et al., 2002; Lal et al., 2005). Comparison between treatment groups showed that immunosuppressive treatment impaired the antibody response to both vaccines and that TNF-alpha treatment further impaired the response as compared to untreated patients.
Mitra and colleagues evaluated the immunogenicity of the 13-valent pneumococcal conjugate vaccine in patients with end-stage renal disease who were on dialysis, as these patients are at risk of pneumococcal disease (Mitra et al., 2016). Patients were given a single dose of vaccine and the serum antibody concentrations were measured using the multiplexed bead-based platform at baseline, and at 2 and 12 months after vaccination. Increased concentrations of antibodies to the vaccine serotypes were demonstrated at 2 months post-vaccination but declined by 38 to 72% at 12 months. They proposed further study to understand the reduced titers after 12 months.
Santoro et al. conducted a study to determine persistence of vaccine responses in patients with sickle cell disease (Santoro et al., 2016). The study assessed the vaccine response in sickle cell patients who were vaccinated at 2 and 5 years of age and then every 5 years. Anti-pneumococcal antibody titers were measured using the multiplexed bead assay described by Pickering (Pickering et al., 2002). The results showed a significant percentage of patients did not maintain a sufficient vaccine response for 5 years. Only 36% had protective levels of anti-pneumococcal antibody at 37 months post-vaccination and of these, 64% demonstrated a vaccine response to fewer than 25% of the tested serotypes. The authors recommended further investigation to determine an optimal vaccine schedule and to monitor anti-pneumococcal antibody titers in patients who are at risk for pneumococcal disease.
Hung and colleagues conducted a prospective study in pediatric oncology patients to determine the benefit of pneumococcal vaccination in patients receiving immunosuppressive therapy or who were within one year of completing therapy (Hung et al., 2017). IgG titers to 12 PnPS serotypes were measured in blood samples taken before and 4 weeks after vaccination using the bead-based multiplex platform. Prior to vaccination, ≤50% of the 82 patients had protective antibody titers for 8 serotypes in the completed treatment group (36 patients) and 10 serotypes in the active treatment group (46 patients) before vaccination. Post-vaccination, ≥70% had protective antibody titers for 9 and 11 serotypes in the active and completed groups, respectively. They concluded that a single dose of the 13-valent pneumococcal vaccine was safe and effective in pediatric oncology patients, and they should receive the vaccine upon cancer diagnosis and then again after completing immunosuppressive therapy.
In 2018, Pavliakova and colleagues at Pfizer reported the validation of a multiplexed direct bead-based immunoassay to replace the standard pneumococcal ELISA platform (Pavliakova et al., 2018). The multiplexed assay simultaneously measures the concentration of serum IgG antibodies specific for pneumococcal capsular polysaccharide serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F. This assay was developed using the PnPS/PLL method (Pickering et al., 2002). Assay validation was performed using residual human serum samples obtained from vaccine clinical studies. The lower limit of quantitation (LLoQ) for all serotypes covered in the 13-plex assay ranged from 0.002 to 0.038 µg/ml serum IgG. The dynamic range of the assay was >500-fold and the variability was <20% relative standard deviation for all serotypes. The assay is capable of generating up to 143 test results in a single 96-well plate and the authors concluded it was a suitable replacement for ELISA in evaluating vaccine clinical trials.
Tan et al. described the results of a study to bridge the World Health Organization (WHO) pneumococcal ELISA platform to the validated bead-based 13-plex direct immunoassay platform developed by Pfizer (Tan et al., 2018). Serum samples were selected from four pneumococcal vaccine clinical trials on the basis of serotype-specific IgG concentrations determined by ELISA. A comparison of assay results from the two platforms on 1,528 samples showed clear and robust linear quantitative relationships across all 13 serotypes. Lower IgG antibody concentrations in pre-immunization samples were measured in the bead-based assay and allowed better differentiation between pre-immunization and post-immunization samples with low titers. The results showed that the established protective threshold concentration of 0.35 µg/ml of serotype-specific serum IgG antibodies was appropriate for the multiplexed assay for 10 of the serotypes; however they recommended using the assay cutoff values of 0.23, 0.10, and 0.12 µg/ml for 3 serotypes.
Epidemiology and serosurveillance using multiplexed pneumococcal polysaccharide assays
Uddin and collaborators evaluated the IgG subclass and pneumococcal serotype-specific antibody responses in vaccinated children with history of severe or recurrent bacterial infections (Uddin et al., 2006). Subjects were vaccinated with two doses of the PCV7 conjugate vaccine and one dose of the 23vP vaccine. Using the 9-plex assay described Lal (Lal et al., 2005), the investigators found that the responses to the serotypes in PCV7 increased significantly post-vaccination but responses to two serotypes only included in 23vP were low. ELISA assays showed that children mounted an IgG1 antibody response after immunization with the PCV7 conjugate vaccine, whereas IgG2 responses are commonly found with PnPS vaccines and in natural infection with S. pneumoniae. IgG1 is the dominant antibody produced in response to vaccines containing protein antigens, such as conjugate vaccines, and is associated with strong, specific responses that can provide targeted and long-lasting immunity whereas IgG2 antibodies are more common in response to carbohydrate antigens (Vidarsson et al., 2014).
Borgers et al. used a multiplexed bead-based serological assay to measure post-vaccination antibody concentrations in healthy adults and children, patients with high susceptibility to respiratory infection, and patients with deficient anti-PnPS antibody response (Borgers et al., 2010). The cutoff values for serotype-specific antibody levels were determined in healthy controls. Of the susceptible group, 11% failed to respond to at least 6 of 14 serotypes tested, compared to 4% of the controls. In the antibody-deficient group, 100% failed to respond to at least 6 of the 14. They concluded that the multiplexed assay can be used to identify individuals with low responses to unconjugated pneumococcal vaccines.
Choudhury et al. used a 16-plex bead-based assay to determine S. pneumoniae antibody levels in pregnant non-Hispanic black, non-Hispanic white, and Hispanic mothers, and in cord blood specimens (Choudhury et al., 2012). For all mothers, 42% had protective antibody levels to all 16 serotypes. Hispanic mothers were more likely than non-Hispanic mothers to have protective antibody levels for 12 of the serotypes, but less likely to have protective levels for 3 of the serotypes (9V, 12F, and 18C). Cord blood analysis revealed that 33% of infants had protective antibody levels. Hispanic infants had higher occurrence of protective levels to all serotypes except 11A, 14, 18C, and 23F, while non-Hispanic black infants had higher prevalence of protective immunity to 11A, 14, and 18C, and non-Hispanic white infants only had a higher protective level to serotype 23F.
A longitudinal analysis of anti-pneumococcal antibodies during community-acquired pneumonia (CAP) was conducted by van Mens and colleagues to determine the proportion of CAP due to pneumococcal infections (van Mens et al., 2011). A 14-plex bead-based assay was used to measure pneumococcal antibodies in the serum of hospitalized CAP patients. A significant pneumococcal immune response was defined as a ≥2-fold increase in antibody concentrations against a single serotype between day 1 and day 30 and a final concentration above >0.35 µg/ml. A significant immune response was detected in 45% of pneumococcal pneumonia patients, in 5% of non-pneumococcal pneumonia patients, and in 28% of patients where the causative agent for CAP was not identified. The study showed that a substantial portion of pneumococcal pneumonia patients do not elicit a serotype-specific immune response.
Prato et al. conducted a case-control study in a 2-year prospective cohort in Italy to determine effectiveness of the 13-valent vaccine in preventing CAP in adults (Prato et al., 2018). The overall vaccine effectiveness was 33.2%, irrespective of serotype, and 38.1% for vaccine serotypes. The effectiveness was higher at 42.3% for the vaccine serotypes in age groups with higher vaccine uptake. For a subgroup of cases that were managed in the community, the overall vaccine effectiveness due to any pneumococcal strain was 88.1% and 91.7% when they controlled for underlying comorbidities. The results obtained here were not statistically significant however, the authors recommend larger studies to verify the direct benefits of vaccination.
Research studies using multiplexed pneumococcal polysaccharide assays
The multiplexed bead-based PnPS assay platform has also proven useful for measuring serotype-specific antibodies in a variety of clinical research studies. Heaney and coworkers conducted a study to determine if anti-pneumococcal antibody levels in saliva mirrored concentrations in serum (Heaney et al., 2018). They used the 12-plex assay described by Whitelegg (Whitelegg et al., 2012) to measure IgG, IgA and IgM levels in paired saliva and serum samples in 72 healthy adults. They found that the antibody levels in saliva were positively correlated with serum across immunoglobulin classes and was strongest for IgA. Individuals with protective serum antibody levels showed significantly higher IgG and IgA in saliva. Salivary IgG and IgA antibodies were able to distinguish between those with and without protective levels in serum for most serotypes. The findings suggested that anti-pneumococcal IgG and IgA in saliva may be suitable surrogate markers for antibody in serum.
Long et al. examined whether an acute moderate intensity aerobic exercise (walking) prior to vaccination could enhance pneumococcal antibody response (Long et al., 2012). Sixty young adults and 60 older adults participated in the study. A 12-plex assay using the PnPS/PLL chemistry (Pickering et al., 2002) was used to measure serotype-specific antibody levels prior to 45 min of exercise intervention and in resting controls. After exercise, all participants received a full-dose pneumococcal vaccination and a half-dose influenza vaccination. Antibody titers were determined again after four weeks. Both IgM and IgG antibody titers were increased to the pneumococcal vaccine. However, there was no significant difference between the groups. The authors suggest that higher intensity exercise would be needed to enhance antibody response to vaccination.
A subsequent study reported by the same group assessed whether exercise intervention could enhance the antibody response to pneumococcal vaccination in middle-aged, sedentary women (Long et al., 2013). Eighty-nine subjects participated in the study where 44 completed a 16-week walking exercise program and 45 served as controls. The 23vP vaccine was administered at 12 weeks and antibody levels were determined pre-vaccination and at 1- and 6-months post-vaccination. Physical and psychological factors were measured before and after intervention and participants in the exercise group had better subjective and objective physical activity levels and reported a better quality of life. However, no significant effects on antibody response to pneumococcal vaccination demonstrated in this study.
Edwards and collaborators conducted a similar study to determine the benefit of acute exercise immediately prior to vaccination to enhance the immune response (Edwards et al., 2012). They evaluated the effect of exercise on response to either a full- or half-dose of the 23vP vaccine in 133 young, healthy adults. Subjects were randomized according to exercise or control groups receiving full- or half-dose vaccination. Exercise groups completed a 15 min arm and shoulder exercise routine while control groups rested quietly. Antibody levels to pneumococcal serotypes were determined at baseline and one month using the 12-plex bead-based assay described by Whitelegg (Whitelegg et al., 2012). Across all subjects, the exercise groups showed greater antibody responses than the control groups and significantly higher responses for serotypes 1, 3, 4, and 9V. The group-by-time interaction effect was significant in the half-dose group, confirming the greater responses observed in the exercise group, but the main effect was similar for the full-dose group. The investigators concluded that the data indicate that exercise is useful as a vaccine adjuvant, particularly in weaker responses, and the potential benefit to improve protective immunity indicates acute exercise prior to vaccination should be evaluated in at-risk populations.
Lindemann et al. reported a study using bead-based multiplexed serological tests to determine if anti-pneumococcal and anti-HLA antibody levels could predict outcome in kidney transplant patients (Lindemann et al., 2013). Blood was collected from 49 patients pre-vaccination and at 1- and 15-months post-vaccination. Kidney function was unchanged at month 1 post-vaccination compared to pre-vaccination, and no rejection was observed within 5 years after vaccination. The median time between the transplant and vaccination was 6.5 years and the follow-up post-vaccination was 13 years. The data showed the pre-vaccination antibodies against pneumococci were significantly higher in transplant recipients with poorer patient survival but at months 1 and 15 post-vaccination, pneumococcal antibodies were of no predictive value, suggesting that only naturally acquired antibodies could be important for assessing outcomes. However, Class I HLA antibodies were significantly associated with patient survival both prior to and at 15 months post-vaccination.
Conclusion
Since the first report in 2002 of the development of a multiplexed bead-based serological assay for PnPS-specific antibody detection, more than 80 articles have been published describing use of this method for the study of vaccine immune responses to multi-valent polysaccharide and conjugate pneumococcal vaccines. The literature highlights the advances in technology that now allow multiplexed analysis of immune responses to multiple pneumococcal serotypes simultaneously. This change in methodology also triggered some challenges to be overcome in the transition away from the traditional single-plex ELISA to the multiplexed format. Antibody concentrations measured by the bead-based assay tend to be higher than that measured by ELISA, suggesting that new serotype-specific cutoffs for protective levels were needed (Elberse et al., 2011). While a single cutoff may be suitable for many serotypes, some serotypes may require different cutoffs (Tan et al., 2018). It would in fact be extremely challenging to assign a single universal protective threshold, as the predominant serotypes evolve and change over time and therefore the cutoffs need to change as new data become available to inform vaccine strategy.
The Luminex xMAP bead-based suspension array platform is considered a benchmark for multiplexed serological testing for many infectious diseases, autoimmune disorders, and in transplantation for screening donors and monitoring recipients (Das and Dunbar, 2020). This paper reviews selected studies on multiplexed bead based PnPS serological assays and includes publications describing the history of the assay development, optimization, and validation, and how these assays have been used in vaccine studies and to evaluate pneumococcal vaccine responses in various patient populations. These assays are also used for epidemiological studies and for conducting serosurveillance of pneumococcal infections in various settings. Finally, studies showing how these assays have been deployed in biological and clinical immunology research are also featured. The large body of published literature on these multiplexed bead array assays proves the importance of this technology to support the understanding of the safety, immunogenicity, and efficacy of vaccines against S. pneumoniae.
Author contributions
SD: Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
I am an employee of Luminex Corporation which manufactures the reagents and equipment used by the authors of the papers I reviewed in this manuscript.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Biagini, R. E., Schlottmann, S. A., Sammons, D. L., Smith, J. P., Snawder, J. C., Striley, C. A. F., et al. (2003). Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin. Diagn. Lab. Immunol. 10 (5), 744–750. doi: 10.1128/CDLI.10.5.744-750.2003
Borgers, H., Moens, L., Picard, C., Jeurissen, A., Raes, M., Sauer, K., et al. (2010). Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin. Immunol. 134 (2), 198–205. doi: 10.1016/j.clim.2009.10.006
Choudhury, S. A., Ladson, G., Kabir, M. S. (2012). Evaluation of serotype-specific immunity to Streptococcus pneumoniae in pregnant women and cord blood of infants: impact of race and ethnicity. J. Natl. Med. Assoc. 104 (5-6), 251–257. doi: 10.1016/S0027-9684(15)30160-7
Daniels, C. C., Rogers, P. D., Shelton, C. M. (2016). A review of pneumococcal vaccines: current polysaccharide vaccine recommendations and future protein antigens. J. Pediatr. Pharmacol. Ther. 21 (1), 27–35. doi: 10.5863/1551-6776-21.1.27
Edwards, K. M., Pung, M. A., Tomfohr, L. M., Ziegler, M. G., Campbell, J. P., Drayson, M. T., et al. (2012). Acute exercise enhancement of pneumococcal vaccination response: a randomised controlled trial of weaker and stronger immune response. Vaccine 30 (45), 6389–6395. doi: 10.1016/j.vaccine.2012.08.022
Elberse, K. E., de Greeff, S. C., Wattimena, N., Chew, W., Schot, C. S., van de Pol, J. E., et al. (2011). Seroprevalence of IgG antibodies against 13 vaccine Streptococcus pneumoniae serotypes in the Netherlands. Vaccine 29 (5), 1029–1035. doi: 10.1016/j.vaccine.2010.11.054
Fulton, R. J., McDade, R. L., Smith, P. L., Kienker, L. J., Kettman, JR, Jr. (1997). Advanced multiplexed analysis with the FlowMetrix system. Clin. Chem. 43 (9), 1749–1756. doi: 10.1093/clinchem/43.9.1749
Graham, H., Chandler, D. J., Dunbar, S. A. (2019). The genesis and evolution of bead-based multiplexing. Methods 158, 2–11. doi: 10.1016/j.ymeth.2019.01.007
Hausdorff, W. P., Bryant, J., Kloek, C., Paradiso, P. R., Siber, G. R. (2000). The contribution of specific pneumococcal serogroups to different disease manifestations: implications for conjugate vaccine formulation and use, part II. Clin. Infect. Dis. 30 (1), 122–140. doi: 10.1086/313609
Heaney, J. L. J., Phillips, A. C., Carroll, D., Drayson, M. T. (2018). The utility of saliva for the assessment of anti-pneumococcal antibodies: investigation of saliva as a marker of antibody status in serum. Biomarkers 23 (2), 115–122. doi: 10.1080/1354750X.2016.1265009
Hermanson, G. T. (2013). The Reactions of Bioconjugation, in Bioconjugate Techniques. Ed. Hermanson, G. T. (Cambridge, MA, USA: Academic Press), 229–258.
Hung, T. Y., Kotecha, R. S., Blyth, C. C., Steed, S. K., Thornton, R. B., Ryan, A. L., et al. (2017). Immunogenicity and safety of single-dose, 13-valent pneumococcal conjugate vaccine in pediatric and adolescent oncology patients. Cancer 123 (21), 4215–4223. doi: 10.1002/cncr.30764
Kantsø, B., Halkjær, S. I., Thomsen, O. Ø., Belard, E., Gottschalck, I. B., Jørgensen, C. S., et al. (2015). Immunosuppressive drugs impairs antibody response of the polysaccharide and conjugated pneumococcal vaccines in patients with Crohn's disease. Vaccine 33 (41), 5464–5469. doi: 10.1016/j.vaccine.2015.08.011
Lal, G., Balmer, P., Stanford, E., Martin, S., Warrington, R., Borrow, R. (2005). Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J. Immunol. Methods 296 (1-2), 135–147. doi: 10.1016/j.jim.2004.11.006
Lazarus, R., Clutterbuck, E., Yu, L-M, Bowman, J., Bateman, E. A., Diggle, L., et al. (2011). A randomized study comparing combined pneumococcal conjugate and polysaccharide vaccination schedules in adults. Clin. Infect. Dis. 52 (6), 736–742. doi: 10.1093/cid/cir003
Lindemann, M., Heinemann, F. M., Heinold, A., Zaslavskaya, M., Horn, P. A., Witzke, O. (2013). Pneumococcal antibodies in kidney transplant recipients are predictive of patient survival. Scand. J. Immunol. 78 (6), 554–556. doi: 10.1111/sji.12111
Long, J. E., Ring, C., Drayson, M., Bosch, J., Campbell, J. P., Bhabra, J. (2012). Vaccination response following aerobic exercise: can a brisk walk enhance antibody response to pneumococcal and influenza vaccinations? Brain Behav. Immun. 26 (4), 680–687. doi: 10.1016/j.bbi.2012.02.004
Long, J. E., Ring, C., Bosch, J., Eves, F., Drayson, M. T., Calver, R., et al. (2013). A life-style physical activity intervention and the antibody response to pneumococcal vaccination in women. Psychosom Med. 75 (8), 774–782. doi: 10.1097/PSY.0b013e3182a0b664
Menzel, M., Muellinger, B., Weber, N., Haeussinger, K., Ziegler-Heitbrock, L. (2005). Inhalative vaccination with pneumococcal polysaccharide in healthy volunteers. Vaccine 23 (43), 5113–5119. doi: 10.1016/j.vaccine.2005.05.040
Mitra, S., Stein, G. E., Bhupalam, S., Havlichek, D. H. (2016). Immunogenicity of 13-valent conjugate pneumococcal vaccine in patients 50 years and older with end-stage renal disease and on dialysis. Clin. Vaccine Immunol. 23 (11), 884–887. doi: 10.1128/CVI.00153-16
Pavliakova, D., Giardina, P. C., Moghazeh, S., Sebastian, S., Koster, M., Pavliak, V., et al. (2018). Development and validation of 13-plex luminex-based assay for measuring human serum antibodies to streptococcus pneumoniae capsular polysaccharides. mSphere 3 (4), 24. doi: 10.1128/mSphere.00128-18
Pichichero, M. E. (2013). Protein carriers of conjugate vaccines: characteristics, development, and clinical trials. Hum. Vaccin Immunother. 9 (12), 2505–2523. doi: 10.4161/hv.26109
Pickering, J. W., Martins, T. B., Greer, R. W., Schroder, M. C., Astill, M. E., Litwin, C. M., et al. (2002). A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol. 117 (4), 589–596. doi: 10.1309/LMCH-C4Q2-VFL9-3T1A
Pickering, J. W., Larson, M. T., Martins, T. B., Copple, S. S., Hill, H. R. (2010). Elimination of false-positive results in a luminex assay for pneumococcal antibodies. Clin. Vaccine Immunol. 17 (1), 185–189. doi: 10.1128/CVI.00329-09
Prato, R., Fortunato, F., Cappelli, M. G., Chironna, M., Martinelli, D. (2018). Effectiveness of the 13-valent pneumococcal conjugate vaccine against adult pneumonia in Italy: a case-control study in a 2-year prospective cohort. BMJ Open 8 (3), e019034. doi: 10.1136/bmjopen-2017-019034
Rodenburg, G. D., Sanders, E. A.M., van Gils, E. J. M., Veenhoven, R. H., Zborowski, T., Germie, P. J. M., van den Dobbelsteen, G. P. J. M., et al. (2012). Salivary immune responses to the 7-valent pneumococcal conjugate vaccine in the first 2 years of life. PloS One 7 (10), e46916. doi: 10.1371/journal.pone.0046916
Santoro, J. D., Myers, L., Kanter, J. (2016). Assessing the immunogenic response of a single center's pneumococcal vaccination protocol in sickle cell disease. J. Pediatr. Hematol. Oncol. 38 (3), e102–e106. doi: 10.1097/MPH.0000000000000510
Schlottmann, S. A., Jain, N., Chirmule, N., Esser, M. T. (2006). A novel chemistry for conjugating pneumococcal polysaccharides to Luminex microspheres. J. Immunol. Methods 309 (1-2), 75–85. doi: 10.1016/j.jim.2005.11.019
Tan, C. Y., Immermann, F. W., Sebastian, S., Pride, M. W., Pavliakova, D., Belanger, K. A., et al. (2018). Evaluation of a validated luminex-based multiplex immunoassay for measuring immunoglobulin G antibodies in serum to pneumococcal capsular polysaccharides. mSphere 3 (4), 13. doi: 10.1128/mSphere.00127-18
Uddin, S., Borrow, R., Haeney, M. R., Moran, A., Warrington, R., Balmer, P., et al. (2006). Total and serotype-specific pneumococcal antibody titres in children with normal and abnormal humoral immunity. Vaccine 24 (27-28), 5637–5644. doi: 10.1016/j.vaccine.2006.03.088
van den Berg, J. P., Westerbeek, E. A. M., van der Klis, F. R. M., Sanders, E. A. M., Berbers, G. A.M., van Elburg, R. M. (2015). Response on pneumococcal vaccine in preterm infants after neutral and acidic oligosaccharides supplementation. Pediatr. Infect. Dis. J. 34 (9), 976–982. doi: 10.1097/INF.0000000000000766
van Mens, S. P., Meijvis, S. C.A., Endeman, H., van Velzen-Blad, H., Biesma, D. H., Grutters, J. C., et al. (2011). Longitudinal analysis of pneumococcal antibodies during community-acquired pneumonia reveals a much higher involvement of Streptococcus pneumoniae than estimated by conventional methods alone. Clin. Vaccine Immunol. 18 (5), 796–801. doi: 10.1128/CVI.00007-11
Vidarsson, G., Dekkers, G., Rispens, T. (2014). IgG subclasses and allotypes: from structure to effector functions. Front. Immunol. 5, 520. doi: 10.3389/fimmu.2014.00520
Whitelegg, A. M., Birtwistle, J., Richter, A., Campbell, J. P., Turner, J. E., Ahmed, T. M., et al. (2012). Measurement of antibodies to pneumococcal, meningococcal and haemophilus polysaccharides, and tetanus and diphtheria toxoids using a 19-plexed assay. J. Immunol. Methods 377 (1-2), 37–46. doi: 10.1016/j.jim.2012.01.007
Keywords: Streptococcus pneumoniae (pneumococcus), multiplex, serology, immunoassay, vaccine, Luminex, xMAP
Citation: Dunbar SA (2023) Multiplexed suspension array immunoassays for detection of antibodies to pneumococcal polysaccharide and conjugate vaccines. Front. Cell. Infect. Microbiol. 13:1296665. doi: 10.3389/fcimb.2023.1296665
Received: 18 September 2023; Accepted: 30 October 2023;
Published: 15 November 2023.
Edited by:
Ying Xu, First Affiliated Hospital of Chengdu Medical College, ChinaReviewed by:
Rajna Minic, Institute of Virology, Vaccines and Sera “Torlak”, SerbiaKalyan K. Dewan, University of Georgia, United States
Copyright © 2023 Dunbar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sherry A. Dunbar, c2R1bmJhckBsdW1pbmV4Y29ycC5jb20=
 Sherry A. Dunbar
Sherry A. Dunbar