- 1Department of Parasitology and Tropical Medicine, School of Medicine, Pusan National University, Yangsan, Republic of Korea
- 2Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Yangsan, Republic of Korea
Background: Obesity is an inducible factor for the cause of chronic diseases and is described by an increase in the size and number of adipocytes that differentiate from precursor cells (preadipocytes). Parasitic helminths are the strongest natural trigger of type 2 immune system, and several studies have showed that helminth infections are inversely correlated with metabolic syndromes.
Methodology/Principal findings: To investigate whether helminth-derived molecules have therapeutic effects on high-fat diet (HFD)-induced obesity, we isolated total lysates from Trichinella spiralis muscle larvae. We then checked the anti-obesity effect after intraperitoneal administration and intraoral administration of total lysate from T. spiralis muscle larvae in a diet-induced obesity model. T. spiralis total lysates protect against obesity by inhibiting the proinflammatory response and/or enhancing M2 macrophages. In addition, we determined the effects of total lysates from T. spiralis muscle larvae on anti-obesity activities in 3T3-L1 preadipocytes by investigating the expression levels of key adipogenic regulators, including peroxisome proliferator-activated receptor gamma (PPARγ), CCAAT-enhancer-binding protein alpha (C/EBPα) and adipocyte protein 2 (aP2). Oil Red O staining showed that the total lysates from T. spiralis muscle larvae decreased the differentiation of 3T3-L1 preadipocytes by decreasing the number of lipid droplets. In addition, the production levels of proinflammatory cytokines IL-1β, IL-6, IFN-γ and TNF-α were examined by enzyme-linked immunosorbent assay (ELISA). T. spiralis total lysates decreased intracellular lipid accumulation and suppressed the expression levels of PPARγ, C/EBPα and aP2.
Conclusion/Significance: These results show that T. spiralis total lysate significantly suppresses the symptoms of obesity in a diet- induced obesity model and 3T3-L1 cell differentiation and suggest that it has potential for novel anti-obesity therapeutics.
Introduction
Obesity is a prevalent health problem worldwide and can cause diverse diseases such as cardiovascular disease, type 2 diabetes and cancer (Valenzuela et al., 2023). The prevail of obesity and the number of patients with metabolic disorders induced by obesity are increasing in most countries (Han and Lean, 2016) But, the development of anti-obesity medications progresses and some have been come into market, most of these drugs have been withdrawn because of serious side effects (Scheen et al., 2023). Thus, it is necessary to develop alternative medications to treat obesity without causing adverse effects.
Adipogenesis is the process through which cells differentiate into adipocytes. Pre-adipocytes transform maturing adipocytes with intracellular lipid accumulation (Cao et al., 2023). It is controlled by pivotal adipogenic transcription factors such as CCAAT-enhancer-binding protein alpha (C/EBPα), and peroxisome proliferator activated receptor gamma (PPARγ). C/EBPα and PPARγ also boost adipogenesis by inducing the expression of adipokines including adipocyte fatty acid-binding protein (aP2) (Giby and Ajith, 2014). Since adipocytes play a pivotal role in controlling adipokine secretion, which induces adipogenesis, elucidating the molecular mechanisms that control adipogenesis is important for discovering anti-obesity therapy (Kiernan and MacIver, 2020).
Trichinella spiralis is a zoonotic parasite that has broad mammalian host range and is responsible for the disease trichinellosis. T. spiralis plays an important role in immune regulation. Parasitic infection drives Th2-biased immune responses and upregulates regulatory T (Treg) cells. In a previous study, T. spiralis infection activated type 2 immune responses, including M2 macrophages within the adipose tissue, which is important for ameliorating obesity (Kang et al., 2021). T. spiralis infection ameliorated glucose and lipid metabolism and reduced lipid accumulation in the liver and adipose tissues. Understandably, instigating a T. spiralis infection in the process of developing obesity-improving drugs would be highly dangerous as well as unethical. Therefore, we wanted to check whether there was an obesity-improving effect by isolating parasite-derived molecules (T. spiralis total lysates). We also investigated the antiadipogenic effects underlying the action of T. spiralis total lysates in 3T3-L1 adipocytes.
Materials and methods
Mice
Six-week-old female C57BL/6 mice were purchased from Orient Bio, Inc. (Seongnam, Korea). The mice housed at a specific pathogen-free facility at the Institute for Laboratory Animals of Pusan National University, where all animal studies were conducted. In line with “The Act for the Care and Use of Laboratory Animals” of the Ministry of Food and Drug Safety of Korea, the Pusan National University Animal Care and Use Committee registered and approved the experiments (approval no. PNU- 2021-3149). They were bred in-house at the Pusan National University specific pathogen-free animal facility and accommodated according to regulations. The animals were maintained at a relative humidity of 50–60% and temperature of 25°C ± 2°C under natural light and dark condition (12 h light-dark cycle) with standard diet for 1 week prior to experimentation.
Study design
Mice were fed research diets, which were a normal low-fat diet with 10 kcal% fat [LFD], or a high-fat diet with 60kcal% fat [HFD] (New Brunswick, NJ, USA) to induce obesity during 6weeks. We determined the effects of HFD-induced obesity after T. spiralis total lysates treatment. After 6 weeks, T. spiralis total lysates (50ug) was injected intraperitoneally [HFD+TS IP] or intraorally [HFD+TS IO] once every 3 days for a period of 5 weeks in HFD group. The dietary treatments of LFD and HFD group were maintained throughout the experimental period (Figure 1).
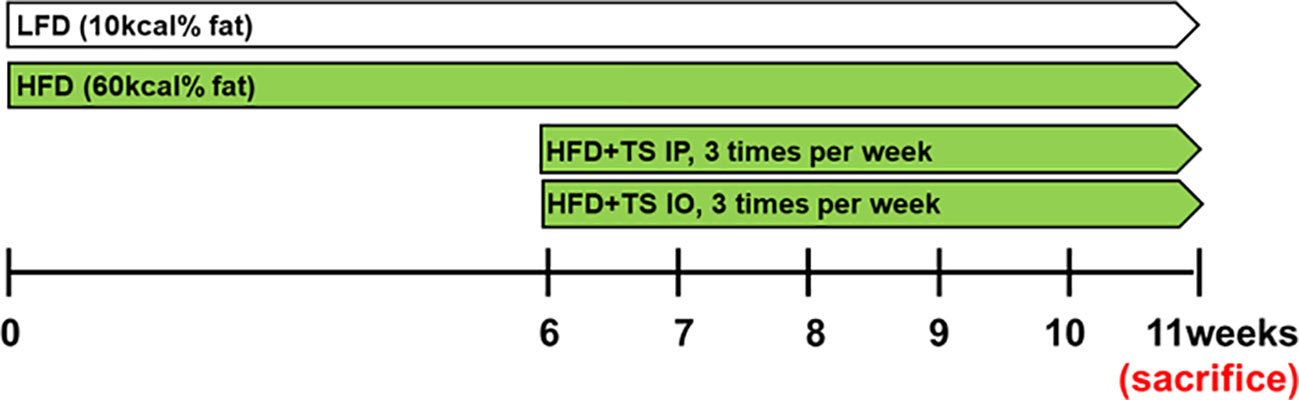
Figure 1 Experimental model. Schematic design illustrates the treatment of T. spiralis total lysates in a diet induced obesity model. HFD+TS IP, high-fat diet and intraperitoneal treatment of total lysates; HFD+TS IO, high-fat diet and intraoral treatment of total lysates.
Preparation of Trichinella spiralis total lysate
T. spiralis was propagated as previously described (Kang et al., 2012). T. spiralis larvae were collected as described previously (Kang et al., 2012). T. spiralis larvae were homogenized using a disposable Biomasher homogenizer (Nippi, Japan).
Measurement of body weight and food uptake
Food intake and body weight were weighed weekly, and the food was replaced weekly. The intake was measured by subtracting the remaining food, including any spilled food, from a weighed aliquot every week.
Measurement of lipids, glucose and insulin in the blood
Plasma total cholesterol, triglyceride, and glucose levels were measured using a Fuji Dri-Chem system (FUJIFILM Corp., Tokyo, Japan). Plasma insulin levels were measured using a mouse insulin enzyme-linked immunosorbent assay (ELISA) kit (SHIBAYAGI Co., Ltd. Gunma, Japan). Plasma insulin levels were determined by ELISA (mouse insulin ELISA, ALPCO, NH, USA).
Oral glucose tolerance test
In addition, for OGTT, 16-hour fasted mice were administered 20% aqueous glucose solution (fasting body mass (g) × 10 = volume (µL) 20% glucose solution) by gavage through a gastric tube (outer diameter 1.2 mm) that was inserted in the stomach. Blood samples were collected at 0, 15, 30, 60, and 120 min after the administration of glucose. Blood glucose levels were measured using a glucometer (Accu-Chek Active [Model GB] Kit; Roche Diabetes, Mannheim, Germany). The area under the curve (AUC) during OGTT represents glucose levels. To estimate the baseline-corrected AUC, basal glucose levels (time point 0) were taken away all subsequently obtained blood glucose levels for each mouse individually, followed by the calculation of the individual AUCs. Statistical analysis was performed using Student’s two-tailed t-test.
Measurement of cytokine production
ELISA was used to measure the culture supernatants of the spleen using according to the manufacturer’s instructions (eBioscience, San Diego, CA, USA). Serum concentrations of T helper cell-derived cytokines IL-1β, IL-6 and TNF-α were determined using a ELISA assay (eBioscience, San Diego, CA, USAA).
Flow cytometry
To investigate macrophage polarization during infection, live cells were isolated from the adipose tissue. The cell surfaces were stained with FITC rat anti-mouse F4/80, PE rat anti-mouse CD11c and APC rat anti-mouse CD206 antibodies (eBioscience) according to the manufacturer’s recommendations. During sample gating, cells were first gated for macrophages. The macrophage gate was used to determine the number of F4/80-positive cells, and CD11c or CD206 surface expression was then determined from the gated population.
RNA extraction and cDNA synthesis
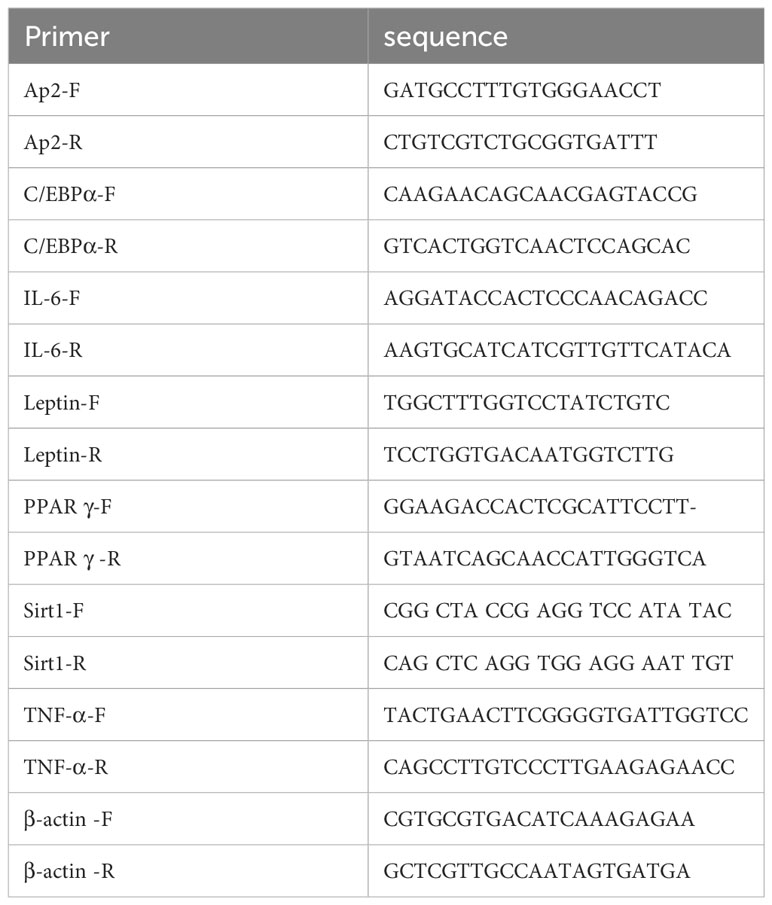
Gene expression was analyzed as previously described. Briefly, total RNA was isolated using TRIzol Reagent® (Invitrogen, Burlington, ON, Canada). Quantitative reverse transcription-PCR assays were performed by using SYBR™ Select Master Mix (Thermo Fisher Scientific, Waltham, MA, USA) in a QuantStudio 3 Real-Time PCR Instrument (Thermo Fisher Scientific). β- actin was used as a reference gene. The primer sequences are listed in Table 1.
Isolation of adipocytes and stromal vascular fraction from adipose tissue
Gonadal white adipose tissues (gWAT) were collected from infected and uninfected mice, minced, and digested for 1 h at 37°C in HEPES buffer (pH 7.4) containing 0.5 g/L type 1 collagenase from Clostridium histolyticum (Sigma-Aldrich, St. Louis, MO, USA) and 2% (w/v) dialyzed bovine serum albumin (Fraction V; Sigma-Aldrich). Disaggregated adipose tissue was filtered through a 100 mm nylon mesh. The residue of the gonadal adipose tissue homogenate was used to isolate the SVF cells for flow cytometry. The sample was centrifuged for 10 min at 350 g, the supernatant was removed and the pellet was treated with ACK lysis buffer (Fujisaka et al., 2009).
Cell culture
Cells from SVF (5x106 cells/mL) were cultured in 5% CO2 incubator at 37°C for 24 hours in the presence or absence of Ionomycin (5 mg/mL) and PMA (0.4 mg/mL). After incubation, the supernatant was collected and stored at -80°C until use. Cytokine production were determined by ELISA.
3T3-L1 cell differentiation
Mouse 3T3-L1 preadipocytes were purchased from ATCC, Manassas, VI, USA. 3T3-L1 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Thermo Fisher Scientific) containing 10% bovine calf serum (ATCC) and 1% penicillin/streptomycin (Thermo Fisher Scientific) and maintained at 37°C in a humidified atmosphere with 5% CO2. Cells were differentiated 2 days after confluence, and then were stimulated with DMEM (4.5 g/L glucose) containing 10% fetal bovine serum (FBS) (Corning, Corning, NY, USA), 1 g/mL insulin, 0.5 mM isobutylmethylxanthine (IBMX), 1 μM dexamethasone, and 1, 5, or 10 µg of total lysate, or PBS (for control cells) for 2 days. On day 2, the differentiation medium was replaced with DMEM (4.5 g/L glucose) containing 10% FBS, 1 μg/mL insulin, and changed every two days from days two to ten.
Oil red O staining
The Oil Red O staining protocol was modified from that described by Pacifici et al. (Pacifici et al., 2020). The differentiated cells were washed with phosphate-buffered saline (PBS, pH 7.4, Sigma-Aldrich) and fixed in 4% formalin (Sigma-Aldrich) for 20-30 min at room temperature. The cells were then washed with double-distilled water and incubated with 60% isopropanol for 5 min at room temperature (Sigma-Aldrich). After that, the cells were stained with Oil Red O solution (0.5 g/L, Sigma Aldrich) for 30 min at room temperature and washed completely with double-distilled water. Images were acquired using an optical microscope. Oil Red O dye retained in the cells was eluted in pure isopropanol and quantified at 490 nm using a microplate reader.
Western blot analysis
3T3-L1 adipocytes were lysed using PRO PREP™ Protein Extraction Solution (iNtRON Biotechnology, Seangnam, Korea). Proteins (30 µg) were separated on 10% sodium dodecyl sulfate polyacrylamide gels (SDS-PAGEs). Next, proteins were transferred to nitrocellulose membranes, blocked in 5% skim milk in TBST (10 mM Tris pH 8.0, 150 mM NaCl, and 0.05% Tween 20) for 1 h, and incubated overnight with primary antibodies against PPARγ, C/EBPα and actin (Cell Signaling Technology, Danvers, MA, USA) at 4°C. After washing with TBST, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h. Proteins were detected using an enhanced chemiluminescence reagent (GE Healthcare, Chicago, IL, USA).
Statistical analysis
Statistical analysis was performed using GraphPad Prism software (version 5.0; GraphPad, Inc., La Jolla, CA, USA), with Student’s t-test or analysis of variance (ANOVA). Multivariate data analysis and group differences were assessed using one-way or two-way ANOVA, followed by Bonferroni’s post-hoc test. All experiments were conducted in triplicates and showed similar results. The results are expressed as the mean ± SD (standard deviation) from each experiment.
Results
Trichinella spiralis total lysates treatment reduces obesity in HFD-fed obese mice
To determine the effect of T. spiralis total lysates on HFD-induced obesity, C57BL/6 female mice were fed a HFD for 6 weeks to induce obesity. T. spiralis total lysate was administered either intraorally or intraperitoneally three times a week for five weeks (Figure 1). All the groups were assayed for body weight, food efficiency ratio [FER= total body weight gain (g)/total food intake (g)], final fat mass, and blood biochemical parameters. Figure 2A shows a protein electrophoresis image of T. spiralis total lysates. Several bands over 40 kDa were detected, but no single bands. Figures 2B, C show photographs of representative mice from each group (LFD, HFD, HFD+TS IP, and HFD+TS IO) after six weeks. As expected, HFD feeding lead to a significant increase in body weight and fat mass in the total lysate treatment group. However, significant differences were observed in body and fat weight of mice treated with T. spiralis total lysates (HFD+TS IP and HFD+TS IO) and non-treated HFD-fed mice (HFD) (*p < 0.005) (Figures 2D, E). According to the food efficiency ratio (FER) equation, a change in body weight was the most important factor influencing the FER, as there were no significant changes in the food intake of the various groups of mice. Thus, it is possible to apply the FER as an indicator; a small FER value can denote obesity prevention. In our experiments, food intake did not differ significantly between T. spiralis total lysate-treated and non-treated HFD-fed mice. FER was significantly higher in the HFD-fed mice than in the LFD-fed mice (***p < 0.001). T. spiralis total lysate-treated HFD-fed mice had significantly lower FER than non-treated HFD-fed mice (*p < 0.005) (Figure 2F).
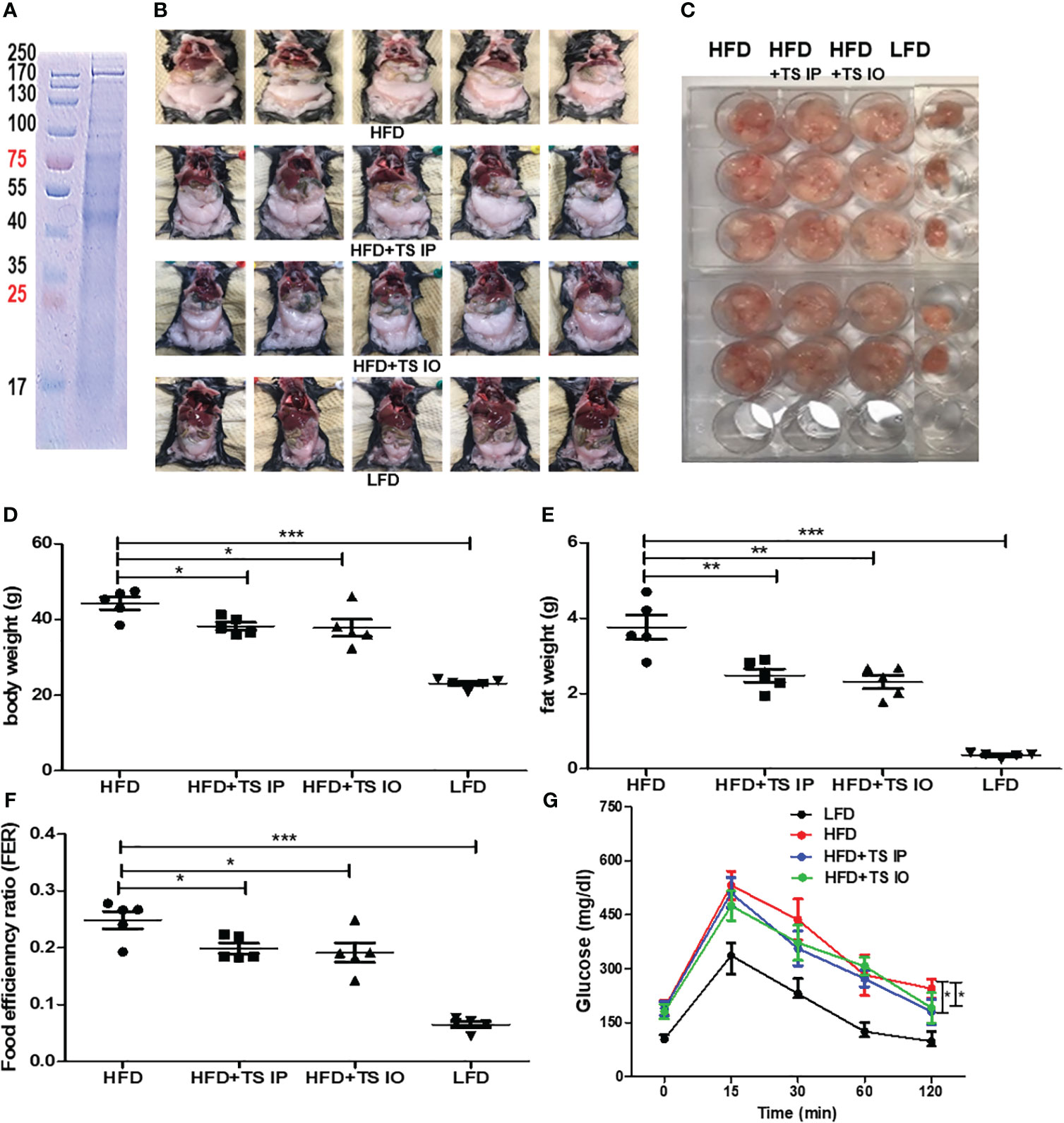
Figure 2 The effect of T. spiralis total lysates on body weight and food efficiency ratio (FER) in high-fat diet (HFD)-fed mice. Total lysates were isolated T. spiralis muscle larvae. (A) Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) of total lysates from T. spiralis muscle larvae. Obesity was induced in mice by feeding them a HFD for six weeks. The mice were then intraperitoneally injected with T. spiralis total lysate three times per week for five weeks. (B) Images of representative mice from each group (LFD, HFD, HFD+TS IP, and HFD+TS IO) after five weeks. (C) Representative image of gonadal adipose tissue. (D, E) Body weight and gonadal fat. (F) Food efficiency ratio (FER) = increased body weight (g)/food intake (g). (G) Overnight-fasted mice were administered 20% glucose by gastric gavage. Blood samples were collected at 0, 15, 30, 60, 90, and 120 min. Blood glucose levels measured using a glucometer. (*; p < 0. 05, **; p < 0.01 ***; p < 0.001, n = 5 mice per group, three independent experiments). LFD, low-fat diet; HFD, high-fat diet; HFD+TS IP, high-fat diet and intraperitoneal treatment of total lysates; HFD+TS IO, high-fat diet and intraoral treatment of total lysates.
Trichinella spiralis total lysates treatment could ameliorate glucose and lipid metabolism
Obesity induces abnormal glucose and lipid metabolism. To assess glucose metabolism, we examined whether T. spiralis-mediated attenuation of obesity improved the harmful effects of obesity. In a previous study, infection of T. spiralis ameliorated glucose and lipid metabolism. In all groups of mice, maximum plasma glucose levels were reached 15 min after the glucose challenge. In contrast, few glucose eliminations were monitored between 15 and 30 min in high-fat diet–fed mice, suggesting severe glucose intolerance. Glucose tolerance tests showed that when HFD mice were challenged with glucose, they had higher blood glucose levels, whereas T. spiralis total lysate-treated mice showed improved glucose tolerance at various time points in the tolerance test. The 60- and 120-minute insulin response to the intraorally glucose challenge were significantly weaken after high-fat feeding compared with those in T. spiralis total lysate-treated mice (*p < 0.001) (Figure 2G). T. spiralis total lysates-treated mice showed significantly decreased total cholesterol (Figure 3A), triglyceride (Figure 3B) and insulin (Figure 3D) (*p < 0.005) levels in HFD-fed mice, however this difference was not significant for glucose levels (Figure 3C).
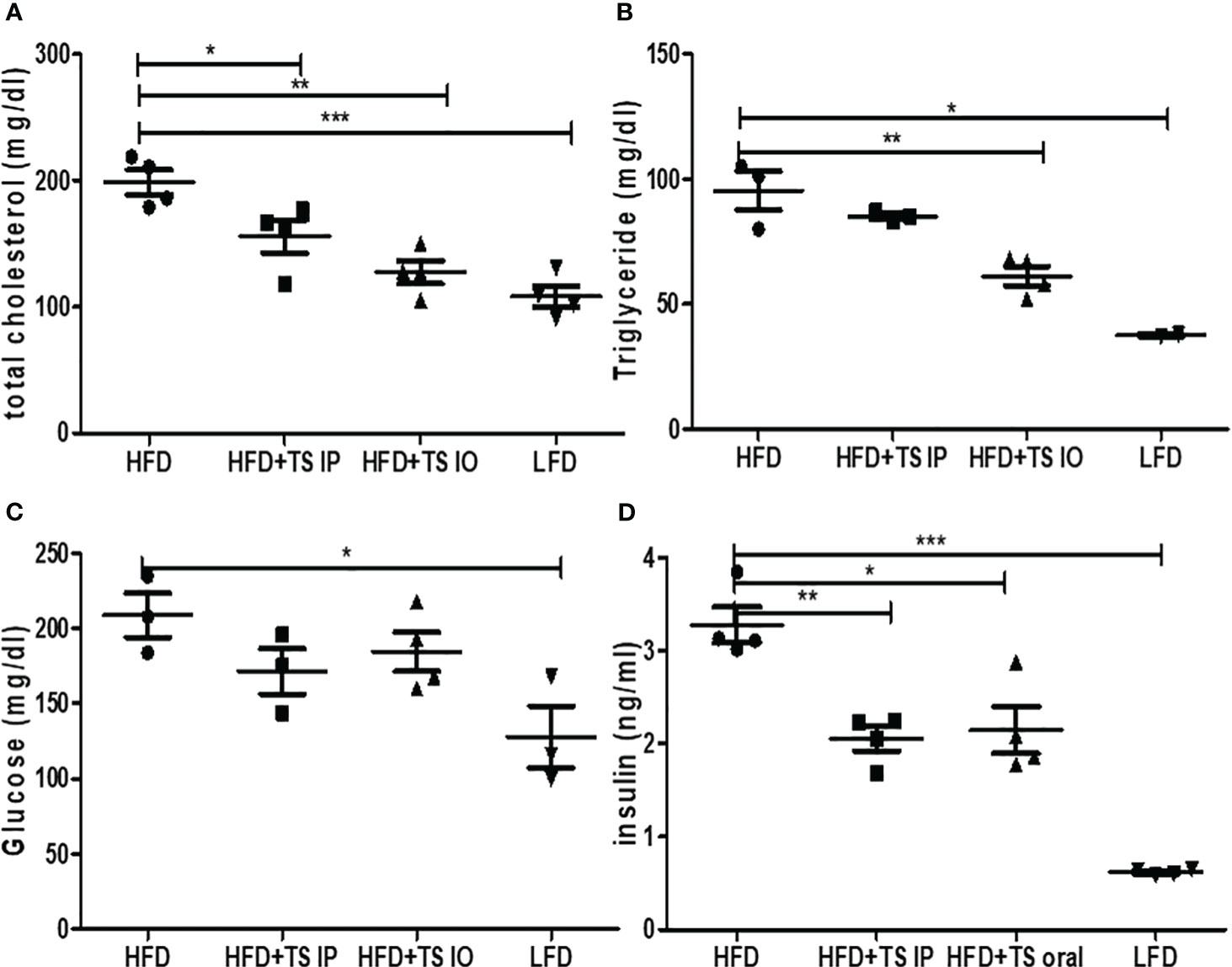
Figure 3 The Effect of T. spiralis total lysates on serum lipid profiles, glucose and insulin levels of mice fed an HFD. (A) Serum total cholesterol. (B) Serum triglyceride. (C) Serum glucose. (D) Serum insulin. To evaluate the effects of T. spiralis total lysates on serum lipid profiles, glucose and insulin. The serum biochemical parameters were assessed in mice of each group (LFD, HFD, HFD+IP, HFD+IO). (*; p < 0.05, **; p < 0.01, ***; p < 0.001, n = 5 mice/group, three independent experiments) LFD, low-fat diet; HFD, high-fat diet; HFD+TS IP, high-fat diet and intraperitoneal treatment of total lysates; HFD+TS IO, high-fat diet and intraoral treatment of total lysates.
Treatment of Trichinella spiralis affected immune response in the HFD-fed obese mice
To evaluate the effects of T. spiralis treatment on cytokine profiles, ELISA was conducted for IL-1β, L-6, TNF-α, IL-4, IL-17A, and IL-10. Figure 4A shows the levels of the six cytokines in the spleen. As expected, the HFD group mice had higher proinflammatory cytokines such as TNF-α, IL-1β and IL-6. Moreover, T. spiralis total lysates-treated mice (HFD+TS IP, HFD+TS IO) had significantly decreased proinflammatory cytokines, such as IL-1β and TNF-α, compared to the HFD group (*p < 0.001). In contrast, the production of the Th2 cytokine (IL-4), Treg-related cytokines (IL-10) and Th17 cytokines (IL-17A) was not significantly increased compared to that in the HFD group. We check the m-RNA expression level of adipokines including IL-6, TNF-α and leptin in gonadal white adipose tissue (Figure 4B). The results showed that adipokines including IL-6, TNF-α and leptin are significantly increased due to diet- induced obesity, but the increased adipokines are significantly decreased due to T. spiralis total lysates treatment.
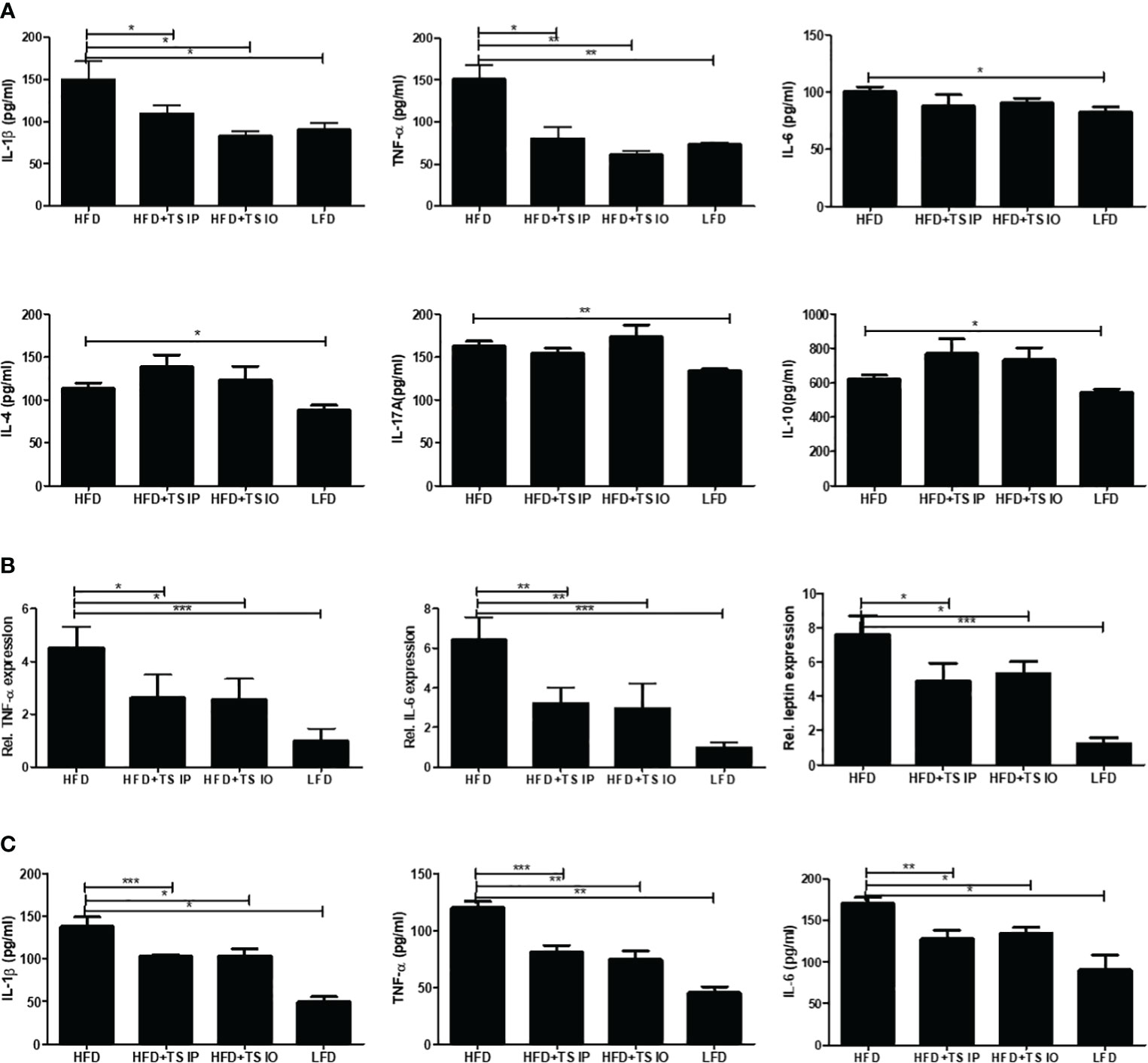
Figure 4 The production of cytokines in the spleen and the m-RNA expression levels in white adipose tissue. (A) Cytokine production was measured in lymphocytes isolated from the mouse spleen. Lymphocytes were activated using anti-CD3 antibody. The wells were incubated with 0.5 mg/mL of anti-CD3 for 16 h at 4 °C, and then the lymphocytes isolated from the spleen were added to the well and incubated for 3 days. After activation, the cytokine concentrations in the supernatant were measured using ELISA kits in accordance with the manufacturer’s instructions. (B) RNA isolated form gonadal white adipose tissue. Relative mRNA expression confirmed the expression of adipokine genes. The results show that T. spiralis total lysates treatment clearly decreased the expression of IL-6, leptin and TNF-α, as well as the mRNA of their target genes, including aP2. (C) Serum concentrations of T helper cell-derived cytokines IL-1β, IL-6 and TNF-α were determined using using ELISA kits in accordance with the manufacturer’s instructions. HFD induction increases the secretion of systemic cytokines into the serum. T. spiralis total lysates treatment significantly decreased the expression of IL-1β, IL-6 and TNF-α. Bars represent the means ± SD from three independent experiments. (*; p < 0. 05, ** p < 0.01 *** p < 0.001, n = 5 mice/group, three independent experiments) LFD, low-fat diet; HFD, high-fat diet; HFD+TS IP, high-fat diet and intraperitoneal treatment of total lysates; HFD+TS IO, high-fat diet and intraoral treatment of total lysates.
We used an ELISA assay to measure the levels of T helper cell-derived cytokines IL-1β, IL-6 and TNF-α in serum (Figure 4C). HFD mice exhibit increased levels of systemic (IL-1β, IL-6 and TNF-α) inflammatory cytokines. We detected a significant decrease in immune and AT-mediated cytokines in the group of mice fed HFD compared to those treated T. spiralis total lysates. These data suggest that T. spiralis total lysates downregulate the chronic inflammation, at least in part decreasing circulating levels of systemic cytokines. These results suggest an evident impact of parasite-derived molecule treatment on cytokine production profiles and effector T cell differentiation in HFD-fed mice by suppressing Th1 cell responses.
Treatment of Trichinella spiralis induces the ‘anti-inflammatory’ M2 levels by a balance transit in M1/M2 macrophage ratio
Obesity is related to macrophage accumulation in the adipose tissue. The number and population of macrophages in adipose tissue differs according to the metabolic state. We assessed whether helminth-derived molecules induced an imbalance in the ratio of M1/M2 macrophages in gonadal fat tissue. The results from our FACS analysis showed that HFD feeding of untreated obese mice led to an increase in M1 cells (F4/80+ CD11C+) and this increase was prevented by treatment with T. spiralis total lysate (***p < 0.01) (Figures 5A, B). In contrast, HFD feeding of non-treated obese mice lead to decreased M2 cells (F4/80+ CD206+) and T. spiralis total lysate treatment group (HFD+TS IP, HFD+TS IO) induced increase of M2 macrophages (F4/80+ CD206+) in the gonadal fat tissue (**p < 0.05). These results suggest that treatment with T. spiralis total lysate may protect against diet-induced obesity by promoting macrophage polarization to anti-inflammatory M2 macrophages in the gonadal fat of obese mice. Adipose tissues also produce cytokines known as adipokines that are involved in the development of metabolic diseases (Hotamisligil, 2017). Because of the importance of these cytokines in metabolism, we analyzed their production in gWAT. SVF cells were isolated from the adipocytes, cultured for 24 h, and cytokine levels were measured in the supernatants. We found no difference in the production of Th2 cytokines (IL-4), Th17 cytokine (IL-17A) and anti-inflammatory cytokine (TGF-β) by SVF cells when comparing HFD and T. spiralis total lysates treatment group (data not shown). On the other hand, the production of proinflammatory adipocytokines like IL-6 and TNF-α, by SVF cells, was increased in the HFD group when compared to T. spiralis total lysates treatment group (HFD+TS IP, HFD+TS IO) (Figure 6). These data suggest that the effect of T. spiralis total lysates treatment on metabolic parameters might be associated with a reduction in pro-inflammatory cytokines rather than the production of anti-inflammatory or homeostatic adipocytokines.
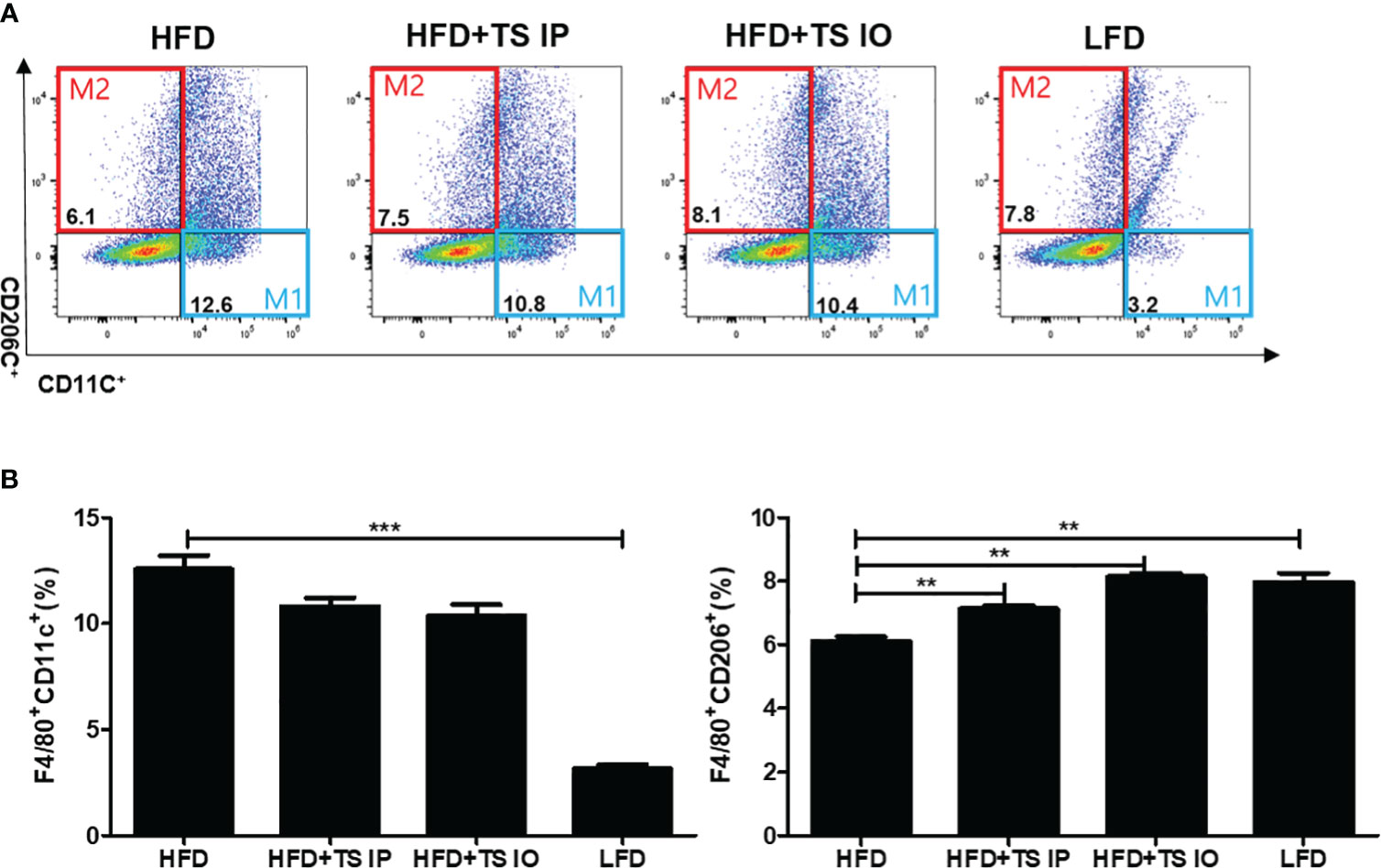
Figure 5 Expression of M1 adipose tissue macrophages (F40/80+CD11c+) and M2 adipose tissue macrophages (F40/80+CD206c+). To evaluate the population of macrophages after the administration of T. spiralis total lysate (IO and intraperitoneal injections), we evaluated the adipose tissue macrophage marker (CD11c+, CD206+) levels in the adipose tissue macrophages of mice. Gonadal adipose tissue from mice was digested with collagenase, and adipose tissue macrophages were isolated. Cells were stained with FITC rat anti-mouse F4/80, PE rat anti-mouse CD11c, and APC rat anti-mouse CD206 antibodies. (A) After staining, the plots indicate the expression levels of the proinflammatory M1 adipose tissue macrophage marker (CD11c+) and M2 adipose tissue macrophage marker (CD206+) in gated F4/80 positive cells. (B). (** p < 0.01, *** p < 0.05, n = 5 mice/group, three independent experiments). LFD, low-fat diet; HFD, high-fat diet; HFD+TS IP, high-fat diet and intraperitoneal treatment of total lysates; HFD+TS IO, high-fat diet and intraoral treatment of total lysates.
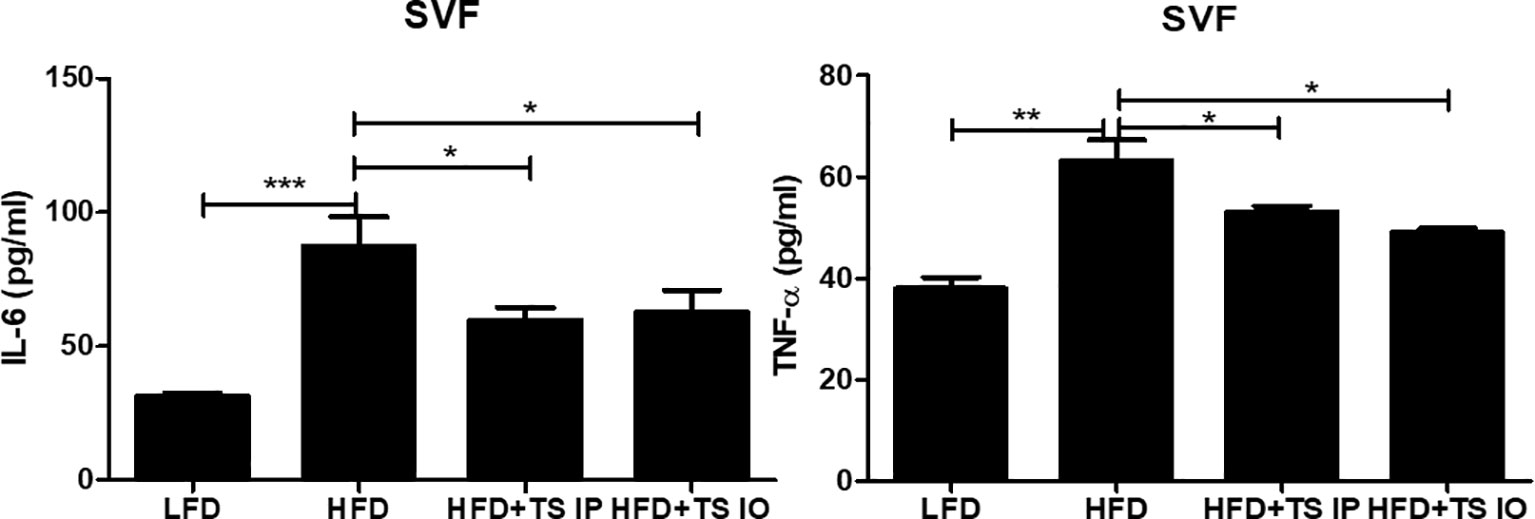
Figure 6 Cytokine production of in the SVFs. Cytokine production was measured in lymphocytes isolated from the stromal vascular fraction of the gonadal white adipose tissue (gWAT). Cells from SVF (5x106 cells/mL) were cultured in 5% CO2 incubator at 37°C for 24 hours in the presence or absence of Ionomycin (5 mg/mL) and PMA (0.4 mg/mL). After incubation, the supernatant was collected and stored at -80°C until use. Cytokine production were determined by ELISA. (*; p < 0. 05, ** p < 0.01 *** p < 0.001, n = 5 mice/group, three independent experiments). LFD, low-fat diet; HFD, high-fat diet; HFD+TS IP, high-fat diet and intraperitoneal treatment of total lysates; HFD+TS IO, high-fat diet and intraoral treatment of total lysates.
Liver histopathology
Histopathological analysis of the liver was assessed by hematoxylin and eosin (H&E) staining (Figure 7A). Hepatic H&E staining revealed that HFD increased hepatocellular vacuolation and the frequency of lipid droplets. Especially, these histopathological changes were attenuated by T. spiralis total lysate (Figure 7B). Hepatic H&E staining showed that the high fat diet increased hepatocellular vacuolation due to cellular lipid accumulation, enlarged hepatocytes, shifted nuclei toward the plasma membrane, and narrowed sinusoidal channels. In summary, we suggest that treatment with T. spiralis total lysate could regulate diet-induced lipid accumulation in white adipose tissue and the liver, which could reduce the risk of fatty liver disease and excessive adipocyte lipid accumulation, which could induce severe hepatic steatosis.
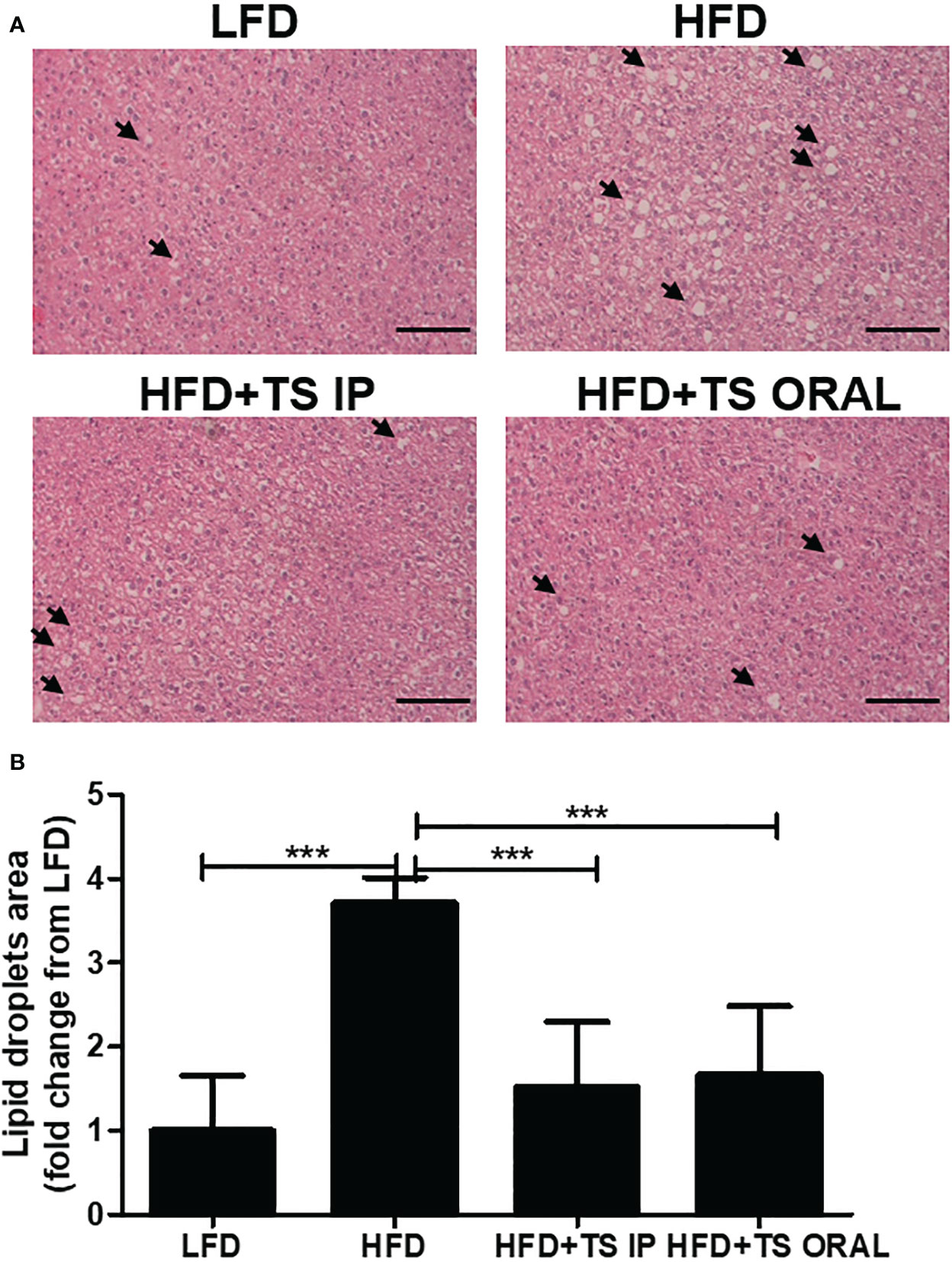
Figure 7 Histopathological alterations in the liver of mice from each group. Histopathological alterations in the livers of mice in each group. Hematoxylin and eosin (H&E) staining of the liver (200× magnification). Arrows indicate lipid droplets. Scale bar = 50 µm. (A) Representative images of one section per mouse are shown (B). Area of lipid droplets was quantified by microscopy (***p < 0.001, n = five mice/group, in three independent experiments). LFD, low-fat diet; HFD, high-fat diet; HFD+TS IP, high-fat diet and intraperitoneal treatment of total lysates; HFD+TS IO, high-fat diet and intraoral treatment of total lysates.
Trichinella spiralis total lysates regulates 3T3-L1 differentiation
To investigate whether T. spiralis total lysates regulate adipocyte maturation, the differentiation of 3T3-L1 cells was induced, and the lipids intracellular storage was monitored by performing Oil Red O staining on day 10. Treatment with T. spiralis total lysates significantly reduced lipid accumulation at 1, 5, 10 μg, compared with control cells (Figure 8A). It was confirmed by measuring the absorbance of Oil Red O at 490 nm. Lipid accumulation in 3T3-L1 cells was significantly decreased by 35% following T. spiralis total lysates, compared to the control (p < 0.01) (Figures 8B, C). Taken together, these results highlight the related ability of T. spiralis total lysate in regulating adipocyte differentiation, which may highly contribute to reduced adipose cell mass accumulation.
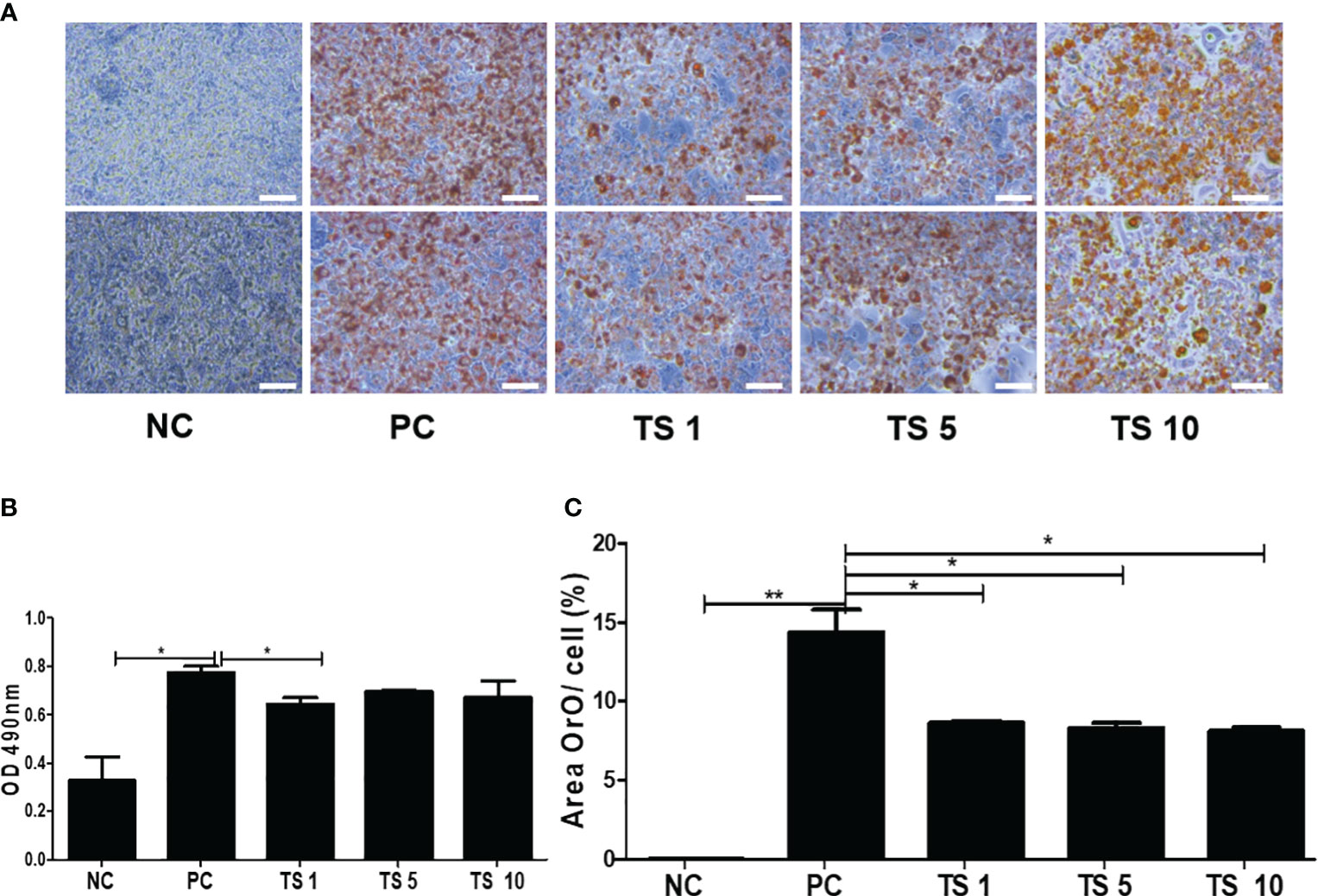
Figure 8 The effects of T. spiralis total lysates on adipocyte differentiation of 3T3-L1 cells. Preadipocyte 3T3-L1 cells were induced to differentiate by the addition of MDI (IBMX, DEX, and insulin). 3T3-L1 cells were treated with 1, 5, or 10 μg T. spiralis total lysates during differentiation. (A) The effect of T. spiralis total lysates on lipid accumulation in 3T3-L1 adipocytes. Oil Red O staining images of 3T3-L1 adipocytes at the terminal stage of differentiation. (B) Oil Red O was extracted from cells with 100% isopropanol and absorbance was determined spectrophotometrically at 490 nm. (C) 3T3-L1 adipocytes positive for Oil Red O staining were quantified. Scale bars represent 100 μm. (*; p < 0. 05, **; p < 0.01, n = 4 samples per group, three independent experiments). NC, negative control, undifferentiation; PC, positive control, differentiation; TS 1, treatment of T. spiralis total lysates 1 µg; TS 5, treatment of T. spiralis total lysates 5 µg; TS 10, treatment of T. spiralis total lysates 10 µg.
Trichinella spiralis total lysates exerts anti-inflammatory effects
Adipogenesis is involved in enhancing the proinflammatory state (Okuno et al., 2018). We evaluated whether T. spiralis total lysate reduced the proinflammatory state typically of adipocyte differentiation. In particular, we detected the levels of proinflammatory cytokine such as IL-1β, IL-6, IFN-γ, and TNF-α in supernatants of fully differentiated adipocytes treated with T. spiralis total lysates. Although they did not decrease in a concentration-dependent manner, all cytokine (IL-1β, IL-6, IFN-γ, TNF-α) levels were significantly reduced by T. spiralis total lysates (p < 0.01) compared to those in control cells. We found no difference in the production of other cytokines (IL-4, IL-10, IL-17A) when comparing differentiation and T. spiralis total lysates treatment group (data not shown) (Figure 9). T. spiralis total lysates may contribute to enhancing the changes in obesity-induced systemic conditions through their anti-inflammatory effect.
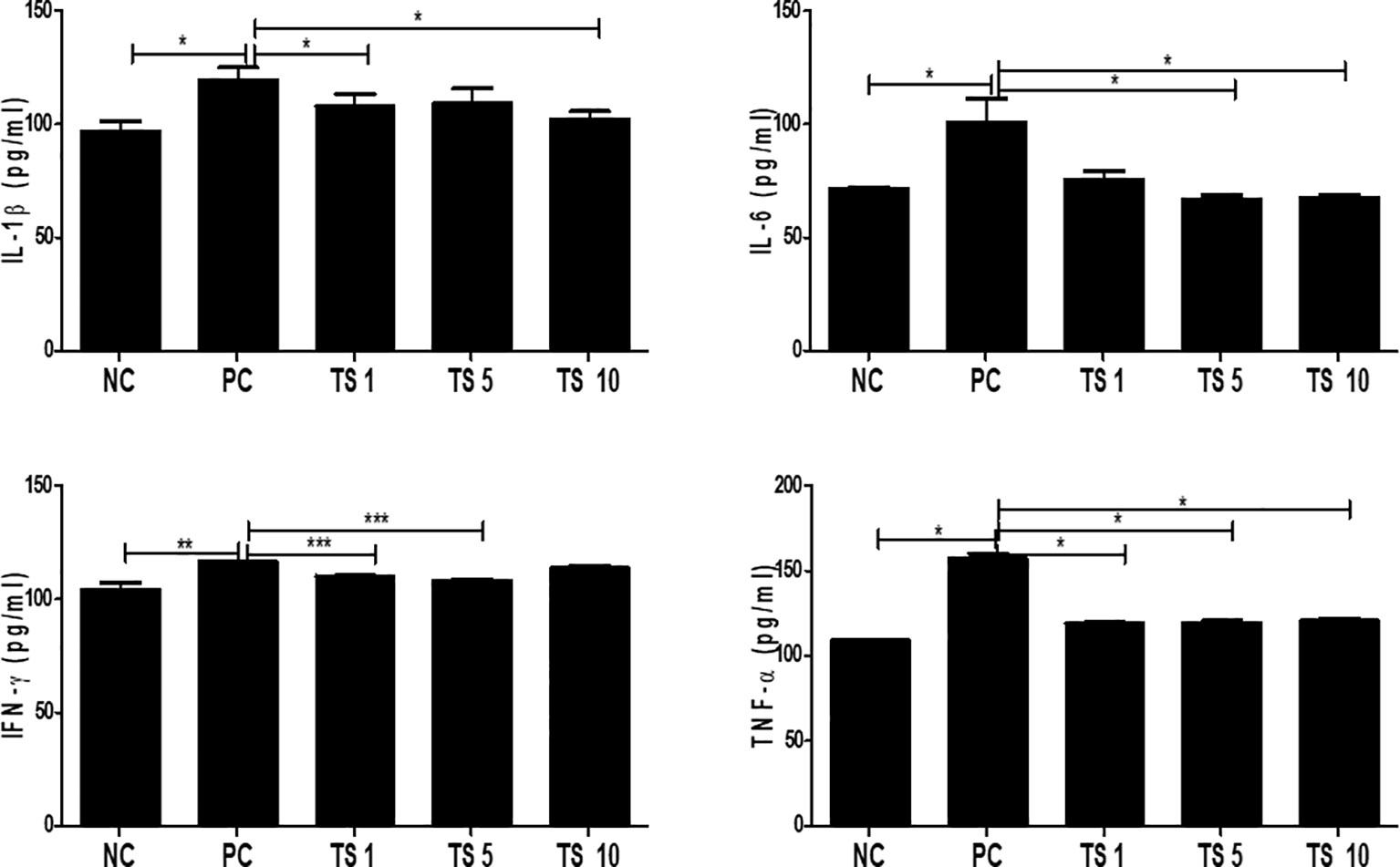
Figure 9 Effects of T. spiralis total lysates on the levels of IL-1β, IL-6, IFN-γ, and TNF-α in 3T3-L1 Adipocytes. Cytokines were detected in culture supernatants by ELISA. The cytokine concentrations in the supernatants of 3T3L1 cells were measured using ELISA kits in accordance with the manufacturer’s instructions. Values are expressed as mean ± SD. As expected, the differentiation group had higher proinflammatory cytokine such as IL-1β, IL-6, INF-γ and TNF-compared to non-differentiation group. Treatment of T. spiralis total lysates were significantly reduced proinflammatory cytokine such as IL-1β, IL-6, INF-γ and TNF-(*; p < 0. 05, ** p < 0.01, *** p < 0.001, n = 4 samples per group, three independent experiments). NC, negative control, undifferentiation; PC, positive control, differentiation; TS 1, treatment of T. spiralis total lysates 1 µg; TS 5, treatment of T. spiralis total lysates 5 µg; TS 10, treatment of T. spiralis total lysates 10 µg.
Trichinella spiralis total lysates weaken adipogenesis by controlling the C/EBPα and Sirt1/PPARγ pathway
To investigate the molecular mechanisms underlying the antiadipogenic effect of T. spiralis total lysates, we evaluated mRNA expression levels of C/EBPα, and PPARγ as key regulatory factors of adipogenesis (Jackson et al., 2017). As expected, after 10 days, T. spiralis total lysates treatment during cellular differentiation significantly decreased expression of PPARγ and C/EBPα, compared to control cells (Figure 10). In particular, we examined PPARγ and C/EBPα protein levels in completely differentiated adipocytes treated with T. spiralis total lysates. PPARγ and C/EBPα levels were significantly reduced by T. spiralis total lysates compared to control cells in a concentration dependent manner (Figure 11) (p < 0.05). In addition, inhibition in adipocyte differentiation was also evaluated by measuring aP2 levels, which are specific markers of adipose tissue differentiation (Ertunc et al., 2015; Palhinha et al., 2019). T. spiralis total lysates significantly reduced the levels of aP2, confirming the effect of T. spiralis total lysates as an antiadipogenic compound (Figure 10). Particularly, Sirt1 inactivation or deletion induces metabolic dysfunction and an increase in adipose tissue (Chalkiadaki and Guarente, 2012). Accordingly, a significant improvement in Sirt1 expression was present following T. spiralis total lysates (p < 0.05), compared to untreated cells, suggesting that the antiadipogenic effect of T. spiralis total lysates may be mediated by the Sirt1/PPARγ pathway. Taken together, these results highlight the role of T. spiralis total lysates in reducing adipocyte differentiation, which may markedly reduce the accumulation of adipose cell mass.
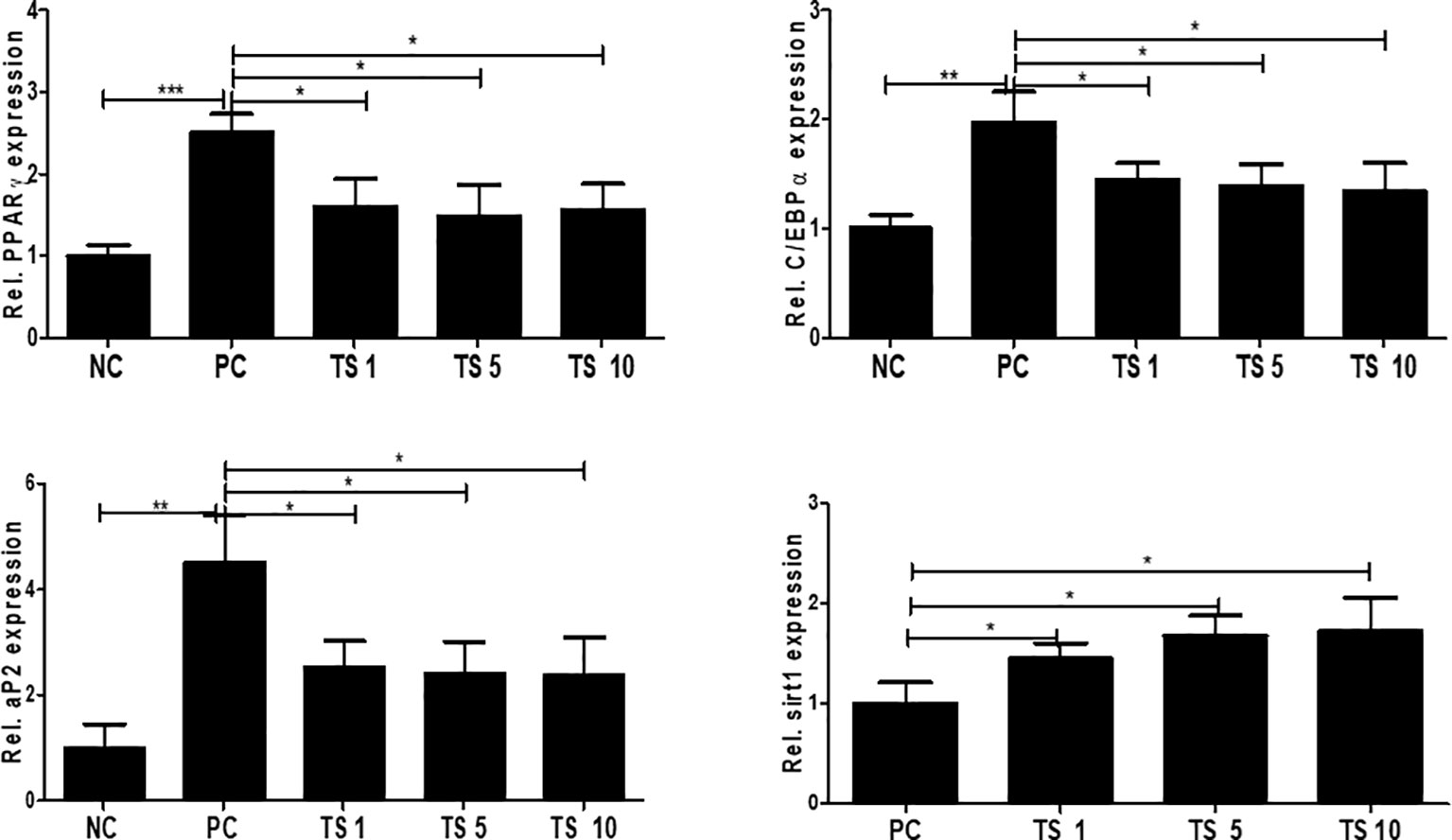
Figure 10 The effect of T. spiralis total lysates on adipogenic genes mRNA and protein expression. 3T3-L1 adipocytes were treated with 1, 5, or 10 μg T. spiralis total lysates during differentiation. RNA and proteins were isolated from the cells at the end-stage of differentiation. Relative mRNA expression confirmed the expression of adipogenic genes. The results show that T. spiralis total lysates treatment clearly decreased the expression of PPARγ, C/EBPα, and aP2 mRNA. Bars represent the means ± SD from three independent experiments. (*; p < 0. 05, ** p < 0.01 ***; p < 0.001*** n = 4; samples per group, two independent experiments). NC, negative control, undifferentiation; PC, positive control, differentiation; TS 1, treatment of T. spiralis total lysates 1 µg; TS 5, treatment of T. spiralis total lysates 5 µg; TS 10, treatment of T. spiralis total lysates 10 µg.
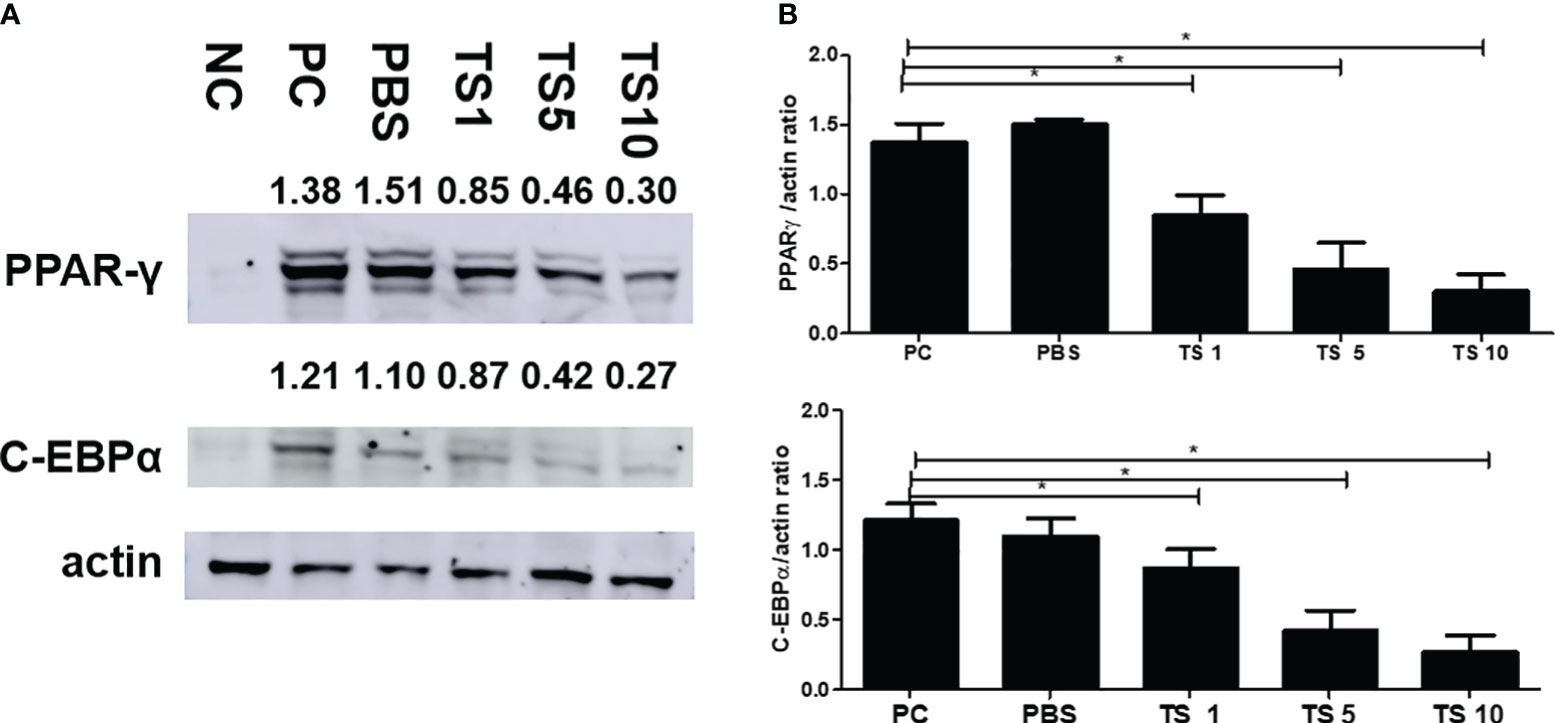
Figure 11 Effect of T. spiralis total lysates on adipogenic factors. The cells were seeded at a density of 8 × 104 cells/well in a 6-well plate. Differentiation was induced with or without T. spiralis total lysate for up to 10 days. (A) Steady-state levels of PPARγ, C/EBPα and actin were evaluated by western blot analysis. Results are expressed as the mean ± SEM. ∗p < 0.05. (B) Graphs show the results of three separate experiments. NC, negative control, undifferentiation; PC, positive control, differentiation; PBS, treatment of PBS; TS 1, treatment of T. spiralis total lysates 1 µg; TS 5, treatment of T. spiralis total lysates 5 µg; TS 10, treatment of T. spiralis total lysates 10 µg.
Discussion
Parasites have evolved to regulate the host immune system and tissue repair responses to induce survival by modulating inflammation that would otherwise induce worm expulsion (Gomes et al., 2016). Parasitic infections generally drive a type 2 immune response, thereby maintaining an anti-inflammatory immune microenvironment and improving host metabolic diseases including obesity (van der Zande et al., 2019). Our research group has focused on the infective immunity of T. spiralis for a long time. We previously reported the accumulation of immunomodulatory cells in the host following infection with this parasite (Kang et al., 2012; Kang et al., 2014; Kang et al., 2019; Kang and Yu, 2021; Kang et al., 2021; Park et al., 2022). Moreover, parasite-derived excretory-secretory products can suppress pro-inflammatory responses in vivo and in vitro (34, 36). Recently, we found that parasitic infections ameliorated diet-induced obesity in mice (Kang et al., 2021).
The relationship between the metabolism and immune response has become an important issue. Adipose tissue is a major immune organ in metabolism. It has been shown that changes in the metabolic state of an individual later result in changes in the immune balance. Obesity mainly weaken Th2 responses, while strengthening type 1 inflammatory responses. Whereas, hunger and malnutrition result in a type 2 biased immune response (Schmidt et al., 2022).
Th2 cells are beneficial to the homeostatic environment of adipose tissue in lean individuals. A question may be raised as to whether the induction of Th2 cells during obesity has an effect in improving obesity. Several studies reveals that helminth infections to induce a Th2 and regulatory condition that may impact the seriousness of irrelevant inflammation and illness (Ryan et al., 2020).
Previous studies, including ours, have shown that parasitic infection changes the differentiation of adipose tissues and metabolism in mice (Lu et al., 2020). Based on our previous data from murine models, T. spiralis infection significantly induces Th2 immune response and M2 macrophage polarization, which ameliorates diet-induced obesity. Several studies have revealed that infection of diet-induced obese mice with Heligmosomoides polygyrus Schistosoma mansoni and Nippostrongylus brasiliensis alleviates whole-body glucose tolerance and insulin sensitivity (Weng et al., 2007; Yang et al., 2013; Su et al., 2018). These results show an inverse relation between helminth infection and the incidence of metabolic syndrome (Wiria et al., 2012).
This study aimed to determine the effect of T. spiralis total lysate treatment on diet-induced obesity (in vivo) and adipocyte differentiation (in vitro). First, we investigated the effects of T. spiralis total lysates treatment in diet-induced obese mice. Secondly, we investigated whether the in vitro anti-obesity effects were retained. The present study investigated the antiadipogenic effects underlying the action of T. spiralis total lysate on 3T3-L1 adipocytes.
Our results showed that T. spiralis total lysates treatment significantly promotes M2 macrophage polarization and inhibits the proinflammatory cytokine as IL-1β and TNF-α.
Our results are consistent with prior reports that helminth infection alleviate obesity (Weng et al., 2007; Yang et al., 2013; Su et al., 2018; Yao et al., 2022) T cell derived cytokines play a pivotal role for generation of proinflammatory M1 macrophages during obesity. Therefore, the decreased production of IL-4 and IL-10 within the adipose tissue results in a relative decrease of anti- inflammatory M2 macrophages. Whereas, the increase of Th1 cells promotes M1 macrophages (Winer et al., 2009). Our results showed that there was no significant increase of IL-4 and IL-10, but T. spiralis total lysate treatment significantly inhibited the production of Th1 cells. Leptin is an adipokine produced primarily by adipocytes, although it is produced by various cells, such as immune cells. Therefore, increasing adiposity result in increased systemic concentrations of leptin. Studies provided evidence that leptin has been identified as a critical immune modulator in an obesity-associated inflammation by promoting pro-inflammatory immune response (Kiernan and MacIver, 2020; Perakakis et al., 2021). Our results showed that T. spiralis total lysate treatment reduced the amount of fat mass and the expression level of leptin in a white adipose tissues. Preadipocytes differentiate into mature adipocytes. Hyperplasia and hypertrophy of these cells can result in an increase in adipose tissue mass (Kim et al., 2013; Chen et al., 2014). During adipocyte differentiation, intracellular lipid droplets build up in mature adipocytes (Kim et al., 2013). Intracellular lipid content was measured using spectrophotometry and lipid accumulation was evaluated using Oil Red O staining. The results demonstrate that T. spiralis total lysates suppress the differentiation of 3T3-L1 adipocytes. Specifically, 3T3-L1 adipocytes treated with T. spiralis total lysate showed decreased lipid accumulation. Adipocyte differentiation is related to mainly by a cascade reaction entailing transcription factors such as C/EBPα and PPARγ (Farmer, 2006). The differentiation of preadipocytes into mature adipocytes is divided into early and late stages.
In the early stages, the transcription factors C/EBPβ and C/EBPδ are caused by specific hormones. Subsequently, PPARγ and C/EBPα are caused by these factors (Cao et al., 1991). PPARγ and C/EBPα then induce adipogenesis by modulating in a positive manner (Cao et al., 1991; Kim et al., 2018). Consistent with the data from the Oil Red O staining, the results of the present study revealed that T. spiralis total lysates significantly decreased the expression of PPARγ and C/EBPα mRNA and proteins, which are pivotal adipogenic transcription factors related to the final stage of adipocyte differentiation. Additionally, the expression levels of adipogenic marker genes including aP2 were decreased by T. spiralis total lysate treatment. These findings suggest that T. spiralis lysates reduce adipogenesis by regulating C/EBPα and PPARγ pathways. Further, Sirt1 is a major modulator of adipogenesis and lipid metabolism; it belongs to the 7 sirtuins, which are class III histone deacetylases that regulate senescent process and metabolic homeostasis (Li and Kazgan, 2011). Further, Sirt1 weakens adipogenesis by impairing PPARγ expression (Picard et al., 2004).
We used two methods for T. spiralis total lysate treatment: IO and IP administration. However, no significant differences were observed between the two administration methods. There was a difference in the absorption time between the two methods, but there was no significant difference in the anti-obesity effect.
In conclusion, the present study demonstrated that changes in immune cell composition caused by T. spiralis total lysate treatment improved glucose tolerance in mice fed a high-fat diet. T. spiralis total lysates inhibit the expression of adipogenic marker genes and lipid accumulation through down-regulation of C/EBPα and PPARγ. Further studies are required to identify specific molecules involved in the prevention and treatment of obesity through such pathways. Taken together, results from our study raise the interesting possibility of using parasite lysates as effective and novel therapeutics in the treatment of obesity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics statement
The animal study was approved by Pusan National University Animal Care and Use Committee. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HY: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – review & editing. SK: Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2017R1D1A3B05035680) supported this research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Cao, Y., Chen, Y., Miao, K., Zhang, S., Deng, F., Zhu, M., et al. (2023). PPARgamma as a potential target for adipogenesis induced by fine particulate matter in 3T3-L1 preadipocytes. Environ. Sci. Technol. 57 (20), 7684–7697. doi: 10.1021/acs.est.2c09361
Cao, Z., Umek, R. M., McKnight, S. L. (1991). Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 5 (9), 1538–1552. doi: 10.1101/gad.5.9.1538
Chalkiadaki, A., Guarente, L. (2012). High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16 (2), 180–188. doi: 10.1016/j.cmet.2012.07.003
Chen, H., Mo, D., Li, M., Zhang, Y., Chen, L., Zhang, X., et al. (2014). miR-709 inhibits 3T3-L1 cell differentiation by targeting GSK3beta of Wnt/beta-catenin signaling. Cell Signal. 26 (11), 2583–2589. doi: 10.1016/j.cellsig.2014.07.017
Ertunc, M. E., Sikkeland, J., Fenaroli, F., Griffiths, G., Daniels, M. P., Cao, H., et al. (2015). Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J. Lipid Res. 56 (2), 423–434. doi: 10.1194/jlr.M055798
Farmer, S. R. (2006). Transcriptional control of adipocyte formation. Cell Metab. 4 (4), 263–273. doi: 10.1016/j.cmet.2006.07.001
Fujisaka, S., Usui, I., Bukhari, A., Ikutani, M., Oya, T., Kanatani, Y., et al. (2009). Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 58 (11), 2574–2582. doi: 10.2337/db08-1475
Giby, V. G., Ajith, T. A. (2014). Role of adipokines and peroxisome proliferator-activated receptors in nonalcoholic fatty liver disease. World J. Hepatol. 6 (8), 570–579. doi: 10.4254/wjh.v6.i8.570
Gomes, P. S., Bhardwaj, J., Rivera-Correa, J., Freire-De-Lima, C. G., Morrot, A. (2016). Immune escape strategies of malaria parasites. Front. Microbiol. 7. doi: 10.3389/fmicb.2016.01617
Han, T. S., Lean, M. E. (2016). A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 5, 2048004016633371. doi: 10.1177/2048004016633371
Hotamisligil, G. S. (2017). Inflammation, metaflammation and immunometabolic disorders. Nature. 542 (7640), 177–185. doi: 10.1038/nature21363
Jackson, R. M., Griesel, B. A., Gurley, J. M., Szweda, L. I., Olson, A. L. (2017). Glucose availability controls adipogenesis in mouse 3T3-L1 adipocytes via up-regulation of nicotinamide metabolism. J. Biol. Chem. 292 (45), 18556–18564. doi: 10.1074/jbc.M117.791970
Kang, S. A., Cho, M. K., Park, M. K., Kim, D. H., Hong, Y. C., Lee, Y. S., et al. (2012). Alteration of helper T-cell related cytokine production in splenocytes during Trichinella spiralis infection. Vet. Parasitol. 186 (3-4), 319–327. doi: 10.1016/j.vetpar.2011.12.002
Kang, S. A., Choi, J. H., Baek, K. W., Lee, D. I., Jeong, M. J., Yu, H. S. (2021). Trichinella spiralis infection ameliorated diet-induced obesity model in mice. Int. J. Parasitol. 51 (1), 63–71. doi: 10.1016/j.ijpara.2020.07.012
Kang, S. A., Park, M. K., Cho, M. K., Park, S. K., Jang, M. S., Yang, B. G., et al. (2014). Parasitic nematode-induced CD4+Foxp3+T cells can ameliorate allergic airway inflammation. PloS Negl. Trop. Dis. 8 (12), e3410. doi: 10.1371/journal.pntd.0003410
Kang, S. A., Park, M. K., Park, S. K., Choi, J. H., Lee, D. I., Song, S. M., et al. (2019). Adoptive transfer of Trichinella spiralis-activated macrophages can ameliorate both Th1- and Th2-activated inflammation in murine models. Sci. Rep. 9 (1), 6547. doi: 10.1038/s41598-019-43057-1
Kang, S. A., Yu, H. S. (2021). Acceleration of Trichinella spiralis worm expulsion by leukotriene B4 receptor binding inhibition. Parasite Immunol. 43 (9), e12843. doi: 10.1111/pim.12843
Kiernan, K., MacIver, N. J. (2020). The role of the adipokine leptin in immune cell function in health and disease. Front. Immunol. 11. doi: 10.3389/fimmu.2020.622468
Kim, H. L., Jeon, Y. D., Park, J., Rim, H. K., Jeong, M. Y., Lim, H., et al. (2013). Corni fructus containing formulation attenuates weight gain in mice with diet-induced obesity and regulates adipogenesis through AMPK. Evid Based Complement Alternat Med. 2013, 423741. doi: 10.1155/2013/423741
Kim, J. S., Lee, S. G., Min, K., Kwon, T. K., Kim, H. J., Nam, J. O. (2018). Eupatilin inhibits adipogenesis through suppression of PPAR gamma activity in 3T3-L1 cells. Biomedicine Pharmacotherapy. 103, 135–139. doi: 10.1016/j.biopha.2018.03.073
Li, X., Kazgan, N. (2011). Mammalian sirtuins and energy metabolism. Int. J. Biol. Sci. 7 (5), 575–587. doi: 10.7150/ijbs.7.575
Lu, Y., Liu, H., Yang, X. Y., Liu, J. X., Dai, M. Y., Wu, J. C., et al. (2020). Microarray Analysis of lncRNA and mRNA Reveals Enhanced Lipolysis Along With Metabolic Remodeling in Mice Infected With Larval Echinococcus granulosus. Front. Physiol. 11. doi: 10.3389/fphys.2020.01078
Okuno, Y., Fukuhara, A., Hashimoto, E., Kobayashi, H., Kobayashi, S., Otsuki, M., et al. (2018). Oxidative stress inhibits healthy adipose expansion through suppression of SREBF1-mediated lipogenic pathway. Diabetes. 67 (6), 1113–1127. doi: 10.2337/db17-1032
Pacifici, F., Farias, C. L. A., Rea, S., Capuani, B., Feraco, A., Coppola, A., et al. (2020). Tyrosol may prevent obesity by inhibiting adipogenesis in 3T3-L1 preadipocytes. Oxid. Med. Cell Longev. 2020, 4794780. doi: 10.1155/2020/4794780
Palhinha, L., Liechocki, S., Hottz, E. D., Pereira, J., de Almeida, C. J., Moraes-Vieira, P. M. M., et al. (2019). Leptin induces proadipogenic and proinflammatory signaling in adipocytes. Front. Endocrinol. (Lausanne). 10. doi: 10.3389/fendo.2019.00841
Park, M. K., Kang, S. A., Cho, M. K., Yu, H. S. (2022). Trichinella spiralis nurse cell formation is regulated via CCR7(+) dendritic cells. Parasite Immunol. 44 (9), e12938. doi: 10.1111/pim.12938
Perakakis, N., Farr, O. M., Mantzoros, C. S. (2021). Leptin in leanness and obesity: JACC state-of-the-art review. J. Am. Coll. Cardiol. 77 (6), 745–760. doi: 10.1016/j.jacc.2020.11.069
Picard, F., Kurtev, M., Chung, N., Topark-Ngarm, A., Senawong, T., MaChado De Oliveira, R., et al. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 429 (6993), 771–776. doi: 10.1038/nature02583
Ryan, S. M., Eichenberger, R. M., Ruscher, R., Giacomin, P. R., Loukas, A. (2020). Harnessing helminth-driven immunoregulation in the search for novel therapeutic modalities. PloS Pathog. 16 (5), e1008508. doi: 10.1371/journal.ppat.1008508
Scheen, A., De Flines, J., Paquot, N. (2023). [Anti-obesity drugs : from previous disappointments to new hopes]. Rev. Med. Liege. 78 (3), 147–152.
Schmidt, V., Hogan, A. E., Fallon, P. G., Schwartz, C. (2022). Obesity-mediated immune modulation: one step forward, (Th)2 steps back. Front. Immunol. 13. doi: 10.3389/fimmu.2022.932893
Su, C. W., Chen, C. Y., Li, Y., Long, S. R., Massey, W., Kumar, D. V., et al. (2018). Helminth infection protects against high fat diet-induced obesity via induction of alternatively activated macrophages. Sci. Rep. 8 (1), 4607. doi: 10.1038/s41598-018-22920-7
Valenzuela, P. L., Carrera-Bastos, P., Castillo-Garcia, A., Lieberman, D. E., Santos-Lozano, A., Lucia, A. (2023). Obesity and the risk of cardiometabolic diseases. Nat. Rev. Cardiol. 20 (7), 475–494. doi: 10.1038/s41569-023-00847-5
van der Zande, H. J. P., Zawistowska-Deniziak, A., Guigas, B. (2019). Immune regulation of metabolic homeostasis by helminths and their molecules. Trends Parasitol. 35 (10), 795–808. doi: 10.1016/j.pt.2019.07.014
Weng, M., Huntley, D., Huang, I. F., Foye-Jackson, O., Wang, L., Sarkissian, A., et al. (2007). Alternatively activated macrophages in intestinal helminth infection: effects on concurrent bacterial colitis. J. Immunol. 179 (7), 4721–4731. doi: 10.4049/jimmunol.179.7.4721
Winer, S., Chan, Y., Paltser, G., Truong, D., Tsui, H., Bahrami, J., et al. (2009). Normalization of obesity-associated insulin resistance through immunotherapy. Nat. Med. 15 (8), 921–929. doi: 10.1038/nm.2001
Wiria, A. E., Djuardi, Y., Supali, T., Sartono, E., Yazdanbakhsh, M. (2012). Helminth infection in populations undergoing epidemiological transition: a friend or foe? Semin. Immunopathol. 34 (6), 889–901. doi: 10.1007/s00281-012-0358-0
Yang, Z., Grinchuk, V., Smith, A., Qin, B., Bohl, J. A., Sun, R., et al. (2013). Parasitic nematode-induced modulation of body weight and associated metabolic dysfunction in mouse models of obesity. Infect. Immun. 81 (6), 1905–1914. doi: 10.1128/IAI.00053-13
Keywords: obesity, Trichinella spiralis, adipocytes, adipogenesis, M1 macrophage
Citation: Kang SA and Yu HS (2024) Anti-obesity effects by parasitic nematode (Trichinella spiralis) total lysates. Front. Cell. Infect. Microbiol. 13:1285584. doi: 10.3389/fcimb.2023.1285584
Received: 30 August 2023; Accepted: 11 December 2023;
Published: 08 January 2024.
Edited by:
Ivana Klun, University of Belgrade, SerbiaReviewed by:
Oscar Medina-Contreras, Mexico Children’s Hospital, MexicoChia-Kwung Fan, Taipei Medical University, Taiwan
Copyright © 2024 Kang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hak Sun Yu, aHN5dUBwdXNhbi5hYy5rcg==
 Shin Ae Kang1
Shin Ae Kang1 Hak Sun Yu
Hak Sun Yu